Abstract
Background
Dopamine-β-hydroxylase (DBH, EC 1.14.17.1), which converts dopamine to norepinephrine, is a candidate gene in neuropsychiatric diseases.
Aim
To assess the effect of regulatory variants in DBH on schizophrenia and its endophenotypes –cognition and tardive dyskinesia.
Methods
We tested association of functional variants 19bp Ins/Del, rs1989787 and rs1611115 in DBH with i) schizophrenia (1236 cases, 1136 controls), ii) tardive dyskinesia (83 positive, 162 negative) and iii) performance functions of cognition (357 cases, 306 controls) estimated by the Penn Computerized Neurocognitive Battery.
Results
A modest haplotypic (Ins-C; 19bp Ins/Del – rs1989787 C>T; p=0.04) association was observed with schizophrenia. We observed ~39% reduction in activity of 19bp Del allele on luciferase assay. Analysis of covariance revealed interactions of tardive dyskinesia status and: i) 19bp Ins/Del (genotypic, p=0.04) and ii) rs1989787 and rs1611115 (combined genotypic, p=0.004) on Abnormal Involuntary Movement Scale total score. Association of rs1611115 with positive and negative syndrome scale (PANSS) total score (p=0.05) and allelic/genotypic association with lower positive (p=0.03/0.04), general psychopathology (p=0.01/0.01) PANSS scales in tardive dyskinesia-positive; and allelic/genotypic (p=0.02/0.05) with higher score of depressive factors in tardive dyskinesia-negative subgroups were observed. Analysis of covariance with continuous variable of cognition showed interaction of health status with: i) rs1989787 on accuracy and efficiency (p=0.03) of abstraction and mental flexibility; ii) rs1611115 on accuracy of working memory and emotion (p=0.05); iii) 19bp Ins/Del on processing speed of emotion (p=0.03). Allelic/genotypic association of rs1989787 with spatial ability (p=0.02–0.05) among healthy controls; association of rs1611115 with Global Assessment Scale scores in the past month (p=0.05) among schizophrenia subjects of cognition cohort was also observed.
Conclusions
With modest genotype–phenotype correlations available for DBH variants, personalized treatment regimens based on DBH activity for ameliorating tardive dyskinesia and cognitive symptoms may be plausible.
Keywords: Dopamine-β-hydroxylase, regulatory variants, 19bp Ins/Del (rs141116007), rs1611115, rs1989787, genetic interaction, schizophrenia, cognition, tardive dyskinesia, dual luciferase reporter assay
Introduction
Notable disturbances in thought process, emotion and loss of perception of reality are characteristic features of schizophrenia, a debilitating neuropsychiatric disorder. Dysfunctional dopaminergic and noradrenergic neurotransmission are implicated in the pathophysiology of schizophrenia (Davis et al., 1991; Klimek et al., 1999). Dopamine-β-hydroxylase (DBH) is an oxidoreductive enzyme in the norepinephrine biosynthetic pathway, a subpathway for catecholamine biosynthesis (Lamouroux et al., 1987), and catalyses the conversion of neurotransmitter dopamine to norepinephrine (Kaufman and Friedman, 1965; Levin et al., 1960). Several coding and regulatory variants (minor allele frequency (MAF) >0.05)) in this gene have been reported to confer susceptibility to a wide range of brain disorders including schizophrenia (Cubells and Zabetian, 2004; Gonzalez-Lopez and Vrana, 2019), Attention Deficit Hyperactivity Disorder (Kopeckova et al., 2006) and as expression quantitative trait loci (eQTLs) (Cubells et al., 1998; Mustapic et al., 2014; Zabetian et al., 2001). With specific reference to schizophrenia, there was association of the Del/Del genotype of 19bp Ins/Del with first episode schizophrenia (Hui et al., 2012); lower immediate memory in first episode schizophrenia (Hui et al., 2013); higher positive and negative syndrome scale (PANSS) score (Hui et al., 2012); higher excited symptoms score in tardive dyskinesia subjects (Hui et al., 2015a); lower attention scores in tardive dyskinesia-negative subjects (Hui et al., 2015b); lower attention scores in chronic schizophrenia (Hui et al., 2016). The two regulatory single nucleotide polymorphisms (SNPs) in this study, rs1989787 (p=0.11, 0.12) and rs1611115 (p=0.67, 0.9), were not associated with schizophrenia in the two genome-wide association studies (GWASs) (Pardinas et al., 2018; Schizophrenia Working Group of the Psychiatric Genomics, 2014). However, such observations are not uncommon considering the polygenic/genetically heterogeneous nature of schizophrenia. It is likely not captured in a GWAS at a genome-wide significance level, but may be relevant for a subset of the cohort. In this context, DBH is of considerable pharmacological relevance in schizophrenia/tardive dyskinesia and is also one of the few genes wherein a few regulatory variants have been demonstrated to be of notable functional relevance, warranting its detailed analysis in schizophrenia and related phenotypes. Needless to say, such studies may be insightful for personalized medicine. A haplotype of 19bp Ins/Del and exonic rs1108580 variants was found to be more common among schizophrenia patients not responding to neuroleptics (Yamamoto et al., 2003). In some conditions, low DBH activity could be rate limiting for norepinephrine synthesis, leading to a hike in the dopamine/norepinephrine ratio (Parasuraman et al., 2012), and this was hypothesized to be a risk factor for psychiatric diseases such as schizophrenia (Cubells et al., 1998; Meltzer et al., 1976).
The Del allele of 19bp variant was speculated to decrease promoter activity leading to lower DBH levels and enzyme activity (Tang et al., 2007). Furthermore, a single SNP (rs1611115) was found to be the main predictor of plasma DBH activity, accounting for ~29% variation in plasma activity while 19bp Ins/Del was shown to account for ~6.5% (Tang et al., 2007). However, the activity status of 19bp Ins/Del has not been experimentally demonstrated to date. Another SNP (rs1989787) was also found to be functional and was shown to alter transcription, enzyme secretion and blood pressure (Chen et al., 2011). SNPs in this gene may result in a quantitative or a qualitative change. Defective secretion of DBH due to non-synonymous SNPs was found in DBH/norepinephrine deficiency (Kim et al., 2011) and in the case of rs6271 (Punchaichira et al., 2017) associated with bipolar disorder (Ates et al., 2013).
By targeted sequencing of all the exons and 10kb upstream region of the DBH gene combined with enzyme phenotyping in subjects of Indian origin we have recently demonstrated that rs1611115 accounts for ~31% of the variance in DBH activity (Punchaichira et al., 2016). Though a large number of association studies of DBH variants were reported, studies on genetically distinct Indian populations are negligible (Srivastava et al., 2010). Furthermore, contribution of DBH variants to tardive dyskinesia, an important iatrogenic disorder in schizophrenia, and to cognition has been poorly investigated. As mentioned above, functional validation of the common 19bp Ins/Del regulatory variant and its contribution to enzyme activity in a cellular model has not been deciphered to date. Therefore, in this study we focused on identifying associations, if any, of the three common upstream regulatory variants (19bp Ins/Del, rs1989787 and rs1611115) with schizophrenia, tardive dyskinesia and cognition in a schizophrenia cohort of north Indian ancestry. We also performed dual luciferase assays to demonstrate for the first time the functional implications of the 19bp Ins/Del variant in vitro using the SH-SY5Y cell line, which endogenously expresses DBH (Biedler et al., 1978).
Materials and methods
Recruitment of study subjects
Schizophrenia subjects and healthy controls in this study were recruited as described previously (Kukshal et al., 2013; Tiwari et al., 2005a, 2007). Briefly, subjects with schizophrenia or schizoaffective disorder were diagnosed according to Diagnostic and Statistical Manual of Mental Disorders-IV criteria at the Postgraduate Institute of Medical Education and Research–Dr. Ram Manohar Lohia Hospital, New Delhi. Assessment of all the participants was done using the Hindi version of the Diagnostic Interview for Genetic Studies and the Family Interview from Genetic Studies (Deshpande et al., 1998; Nurnberger et al., 1994). In addition to healthy adults, cord blood samples collected from anonymous discarded placenta served as controls. Venous blood (5 mL), was collected from participants for genetic analysis. Isolation of genomic DNA was done using phenol chloroform extraction method. Experiments were undertaken after procuring an informed consent from the participants or parents/guardian of this study and this study conforms with the code of Ethics of the World Medical Association Declaration of Helsinki. Further institutional ethical committee clearance was obtained from the participating hospital.
Assessment of tardive dyskinesia
Tardive dyskinesia was assessed in a subset of schizophrenia cases included in this study using the Schooler and Kane criteria (Schooler and Kane, 1982) for items 1 to 7 of the Abnormal Involuntary Movement Scale (AIMS) (Guy, 1976) as described previously (Tiwari et al., 2005b). This cohort comprised tardive dyskinesia-positive (TD+ve) (males n=51 (age=35±12 years); females n=32 (age=32±12 years); AIMS score=6.2±3.4) and tardive dyskinesia negative (TD−ve) (males n=77 (age=30±10 years); females n=85 (age=31±9.2 years); AIMS score=0.7±1.0) samples and is described elsewhere (Tiwari et al., 2005a, 2005b). Two mild or one moderate or higher rating in any of the symptoms was used as a research diagnostic criterion to classify schizophrenia patients as TD+ve or TD−ve. While the AIMS total score was used as continuous variable, tardive dyskinesia status was used as a dichotomous variable for testing association. In addition, PANSS (Kay et al., 1987) was estimated for subjects in the tardive dyskinesia cohort (TD+ve, PANSS total score=61.4±18.5; TD−ve, PANSS total score = 56.4±18.9). Disorganized/concrete (P2+N5+G11), excited (P4+P7+G8+G14) and depressed factors (G2+G3+G6) were derived from the PANSS scores as described elsewhere (Wallwork et al., 2012).
Neurocognitive assessment using Penn Computerized Neurocognitive Battery (PennCNB)
A Hindi version of PennCNB was used for cognitive assessment as described in previous studies (Bhatia et al., 2012; Kukshal et al., 2013). The PennCNB measures neurobehavioural functions of eight domains, namely abstraction and flexibility, attention, working memory, face memory, spatial memory, spatial processing, sensorimotor dexterity, and emotional processing (Gur et al., 2001a, 2001b, 2007). Three performance functions, namely accuracy, processing speed and efficiency were calculated for each domain. PennCNB was administered to 306 adult controls comprising 192 males (age=40±16 years) and 114 females (age=36±11 years) and 357 schizophrenia cases comprising 247 males (age=33±9.4 years) and 110 females (age=32±9.0 years) at the time of recruitment. The scores obtained from the PennCNB repository were transformed to confirm to near normality and were used for association testing. Scale for the Assessment of Positive Symptoms (SAPS, 22±16), Scale for the Assessment of Negative Symptoms (SANS, 45 ±28) (Andreasen and Olsen, 1982) and Global Assessment Scale during the previous month (GAS, 36±14) (Endicott et al., 1976) were estimated for schizophrenia subjects in the cognition cohort.
Genetic analysis
Selection of variants and genotyping
Three variants, namely 19bp Ins/Del, rs1989787 and rs1611115, in the 5′ upstream region of DBH (Supplementary Figure 1 online) were selected for genotyping. The selection was based on their: i) reported association with a wide range of neuropsychiatric disorders in the literature (Cubells and Zabetian, 2004; Kopeckova et al., 2006); ii) functional evidence or genotype × phenotype correlation for rs1611115 and rs1989787 (Chen et al., 2010, 2011) respectively; and iii) common nature with MAF >0.05. Primers were designed online using Primer 3 (v. 0.4.0) (http://bioinfo.ut.ee/primer3-0.4.0/). The restriction fragment length polymorphism (RFLP) patterns for two markers were generated using WatCut (http://watcut.uwaterloo.ca/template.php?act=snp_new). Polymerase chain reaction (PCR)-RFLP method was used to genotype two markers (rs1611115, rs1989787) and the 19bp Ins/Del variant was genotyped by size separation of the PCR product on a 4% TAE-agarose gel (primers used for amplification and RFLP details are provided in Supplementary Table 1). PCR amplification was done using 3B DNA polymerase (Biotools B&M Labs, SA, Madrid, Spain) and constituents are shown in Supplementary Table 1. PCR was performed on a Veriti 96-Well Thermal Cycler (Thermo Fisher Scientific Waltham, Massachusetts, USA) using the thermal cycling conditions given in Supplementary Table 2. Restriction digestion conditions for markers rs1989787 and rs1611115 are given in Supplementary Table 2. Sanger sequence confirmed DNA samples for each of the three genotypes were used as PCR and digestion controls for each of the 96 well plates for genotyping rs1611115 and rs1989787. Enzyme digested samples were electrophoresed on a 2% TAE-agarose gel, visualized on Gel Doc-It™ Imaging system (Ultra-Violet Products Ltd, Upland, California, USA) and genotypes were called.
Statistical analysis
Deviation, if any, of the three variants from Hardy–Weinberg equilibrium (HWE) were assessed using PLINK 1.07 (Purcell et al., 2007). MAF and linkage disequilibrium measures for these markers were also assessed. The power of the entire sample set was calculated with Quanto v. 1.2.4 software (Gauderman and Morrison, 2006).
Test of association with schizophrenia and tardive dyskinesia
Associations of the three variants with schizophrenia and tardive dyskinesia were tested using chi-squared (χ2) tests in PLINK 1.07. For analysing the effect of DBH genotypes on tardive dyskinesia, a full factorial model under analyses of covariance (ANCOVA) was done with tardive dyskinesia status and DBH genotypes as fixed factors and AIMS total score as dependent variable, with age and gender as covariates using IBM® SPSS® Statistics Subscription (IBM Corp., Armonk, New York, USA). Main effects of tardive dyskinesia status and genotype independently and tardive dyskinesia status × genotype on AIMS score were tested in the model. On identification of significant main effect or an interaction with AIMS total score, Fisher’s least significant difference (LSD) multiple comparisons were done to assess significantly different groups. Linear regression was done for TD+ve and TD−ve sub cohorts for genotypes of DBH variants with positive, negative, general psychopathology sub scales, total score, disorganized/concrete, excited and depressed factors of PANSS with age and gender as covariates using gPLINK v. 2.050 separately. If significant associations were found, Fisher’s LSD or Bonferroni multiple comparisons were done to assess significantly different groups.
Test of association with cognition
Cognition scores were age adjusted and transformed by skew power transformation (z = zbcn,u(y, λ, γ)) (Hawkins and Weisberg, 2017), if data were not conforming to normal distribution as implemented in the car package in R (Fox and Weisberg, 2011). Association of the DBH variants with cognitive scores in healthy adult controls was done by linear regression with age and gender as covariates using gPLINK v. 2.050. An ANCOVA with full factorial model was performed with health status and DBH genotypes as fixed factors; transformed cognition scores corresponding to performance functions (accuracy, processing speed and efficiency) for eight cognitive domains assessed through PennCNB as dependent variable, with gender as covariate using IBM® SPSS® Statistics Subscription (IBM Corp., Armonk, New York, USA). Main effects of health status and genotypes independently and health status × genotype were tested in each model. If a significant main effect or an interaction with cognitive scores was identified, Fisher’s LSD multiple comparisons were done to assess significantly different groups. The inverse BCN transformation was used to estimate the marginal means and confidence intervals where q = 2(λz+1)1/λ for λ ≠ 0, and y = (q2 – γ2)/(2q). Linear regression was done for DBH variants with SANS, SAPS and GAS scores with age and gender as covariates for schizophrenia subjects in the cognition cohort using gPLINK v. 2.050 separately.
Principal component analysis (PCA)
A Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity was done to estimate whether PCA needs to be done on the neurocognitive measures. A scree plot with eigenvalue versus component number was done to estimate the number of components which explain maximum amount of variance in the trait. PCA with direct oblimin rotation method was done to reduce neurocognitive measures of accuracy, processing speed and efficiency to their principal components. ANCOVA was performed with health status and DBH genotypes as fixed factors and each principal component of accuracy, processing speed and efficiency with gender as covariate.
K-means cluster analysis
A K-means clustering for all the 24 cognitive scores (eight each for accuracy, processing speed and efficiency) with 10 iterations was done using IBM® SPSS® Statistics Subscription to assess the variables that contribute the maximum to differentiate the study cohort into cases and controls.
Functional analysis of 19bp Ins/Del (rs141116007)
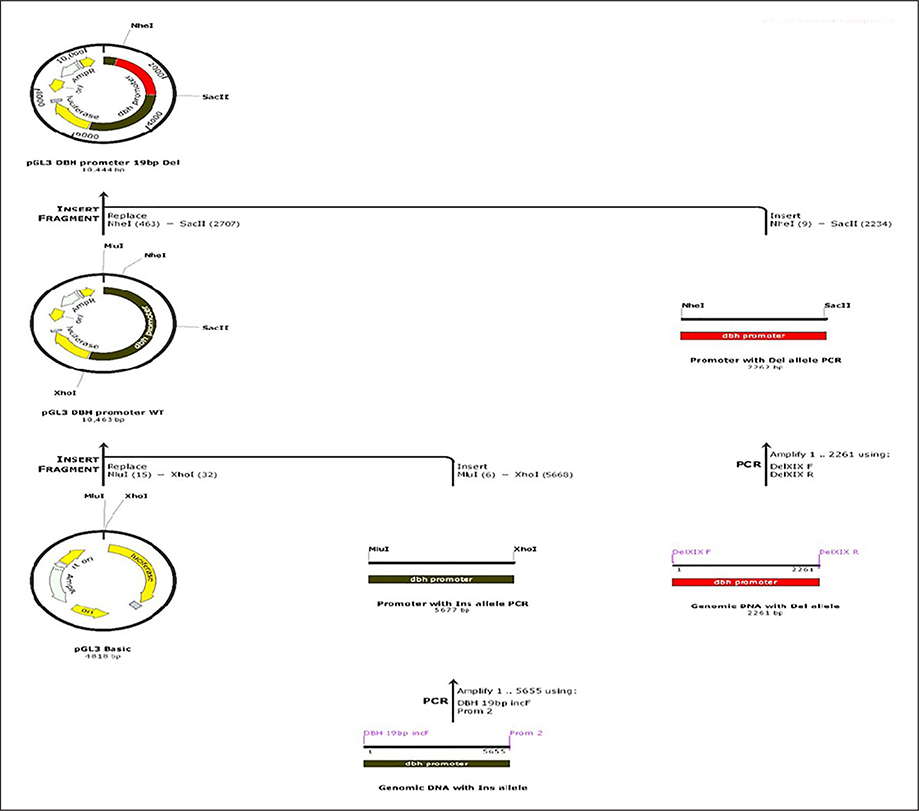
Functional characterization of this upstream 19bp Ins/Del variant was done using DBH promoter construct and with the aid of a non-commercial dual luciferase assay in this study. DBH promoter region (Supplementary Figure 1) was amplified from genomic DNA of two different individuals previously sequenced (Punchaichira et al., 2016) and identified to be homozygous for Ins/Ins and Del/Del respectively. Amplification was performed (5677bp; amplicon I) with primers (DBH 19bp incF and Prom 2) having Mlu I HF and Xho I overhangs (Supplementary Table 1) using LA Taq with GC buffer I (Clontech laboratories, Mountain View, California, USA) and appropriate cycling conditions (Supplementary Table 2). PCR product and pGL3 Basic vector (Promega, Madison, Wisconsin, USA) were restriction digested with Mlu I HF and Xho I (New England Biolabs, Ipswich, Massachusetts, USA), gel extracted using Wizard®SV Gel and PCR clean-up system (Promega, Madison, Wisconsin, USA) and ligated into pGL3 Basic vector using T4 DNA ligase (Promega, Madison, Wisconsin, USA) yielding pGL3 DBH promoter WT (Supplementary Figure 2). In this construct we identified two unique restriction enzymes (Nhe I and Sac II) that flank the region of 19bp Ins/Del polymorphism to be used for subsequent cloning steps. Another amplicon encompassing the region of Ins/Del polymorphism (2261bp; amplicon II) was amplified from the second individual who had Del/Del genotype of 19bp Ins/Del marker using primers Del XIX F and Del XIX R with suitable cycling conditions (Supplementary Table 2). The pGL3 DBH promoter WT construct (with amplicon I) and amplicon II were digested with Nhe I HF and Sac II (New England Biolabs, Ipswich, Massachusetts, USA) gel extracted using Wizard®SV Gel and PCR clean-up system (Promega, Madison, Wisconsin, USA) and ligated into the resulting pGL3 Basic vector backbone devoid of the region of Ins allele using T4 DNA ligase (Promega, Madison, Wisconsin, USA) resulting in construct pGL3 DBH Promoter 19bp Del. The scheme for construct creation is given in Figure 1.
Figure 1:
Scheme of creation of plasmids used for luciferase assay. DBH Ins allele was cloned into pGL3 Basic between Mlu I and Xho I to generate pGL3 DBH promoter WT. The Ins allele was removed by restriction digestion of the region of Ins allele with two flanking restriction sites (Nhe I and Sac II) and an amplified fragment with Del allele was cloned to generate pGL3 DBH promoter 19bp Del.
DBH: dopamine-β-hydroxylase; PCR: polymerase chain reaction
Cell culture and transfection
SH-SY5Y cells were grown on DMEM high glucose medium (Life Technologies, Carlsbad, California, USA) supplemented with 10% foetal bovine serum and 1% antibiotic and antimycotic. Cells were confirmed to be mycoplasma free with MycoAlert® mycoplasma detection kit (LT07–418, Lonza, Rockland, ME, USA). SH-SY5Y cells (2×104) in antibiotic free media were plated in white flat bottom tissue culture treated 96 well plates (Costar® Assay plate, Corning Incorporated, Kennebunk, Maine, USA). Three independent transfections of 100 ng of pGL3 basic vector, pGL3 DBH promoter WT and pGL3 DBH promoter 19bp Del each were carried out and co-transfected with 30 ng of pRL TK vector using 0.3 μL of Lipofectamine 3000 (Life Technologies, Carlsbad, California, USA) per transfection in four technical replicates. Mock transfection with the transfection reagent alone was also done as above. The cells were incubated for 48 h post transfection until luciferase assay was performed as described below. These experiments were performed three times.
Luciferase assay
The cells were washed with Dulbecco’s phosphate-buffered saline and lysed in 1× passive lysis buffer (Promega, Madison, Wisconsin, USA). A non-commercial dual luciferase assay as described previously (Dyer et al., 2000) was adopted for estimating Firefly and Renilla luciferase activity. Enzyme assays were done by injecting 100 μL of luciferase assay buffer into each well and assessed for Firefly luciferase activity followed by injection of Renilla assay buffer (100 μL) for assessing Renilla luciferase activity using the multimode reader (POLARstar Omega, BMG Labtech, Offenburg, Germany). The multimode reader was set for a continuous reading with 5 s delay followed by a 5 s reading for each luminescence with 0.5 s intervals. An unpaired one tailed t test was done to check for significant differences in expression between constructs with 19bp Ins and Del alleles using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, California, USA).
Results
Demographic data of the study cohort are shown in Table 1. All three variants, namely 19bp Ins/Del (rs141116007 Ins>Del, MAF=0.5), rs1989787 (C>T, MAF=0.14) and rs1611115 (C>T, MAF=0.24) were found to be in HWE (p=0.6, 0.09 and 0.5 respectively).
Table 1.
Study cohort composition.
| Gender | Case-control |
Cognition samples |
TD samples |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males |
Females |
Males |
Females |
Males |
Females |
|||||||
| Case | Controla | Case | Controla | Case | Control | Case | Control | TD+ve | TD−ve | TD+ve | TD−ve | |
| Samples (n) | 682 | 566 | 554 | 570 | 247 | 192 | 110 | 114 | 51 | 77 | 32 | 85 |
| Age in years (mean ± SD) | 31±9.0 | 41±14 | 31±10 | 35±12 | 33±9.4 | 40±16 | 32±9.0 | 36±11 | 35±12 | 30±10 | 32±12 | 31±9.2 |
Of the controls 507 were cord blood controls.
TD: tardive dyskinesia; TD+ve: TD-positive; TD−ve: TD-negative
Association with schizophrenia
The study cohort (N = 1236 cases, 1136 controls) had 99.4, 90.5 and 97.7% of power under a log additive mode of inheritance, with genetic effect of 1.3 and disease population risk of 0.01 to detect associations, if any, at each of the three markers. Of note, none of the markers were associated with schizophrenia (19bp Ins/Del, χ2=2.7, p=0.1; rs1989787, χ2=1.3, p=0.2 and rs1611115, χ2=0.004, p=0.9). However, a two-marker haplotype Ins-C (19bp Ins/Del – rs1989787 C>T) showed modest association with schizophrenia (χ2=4.4, p=0.04).
Association with tardive dyskinesia
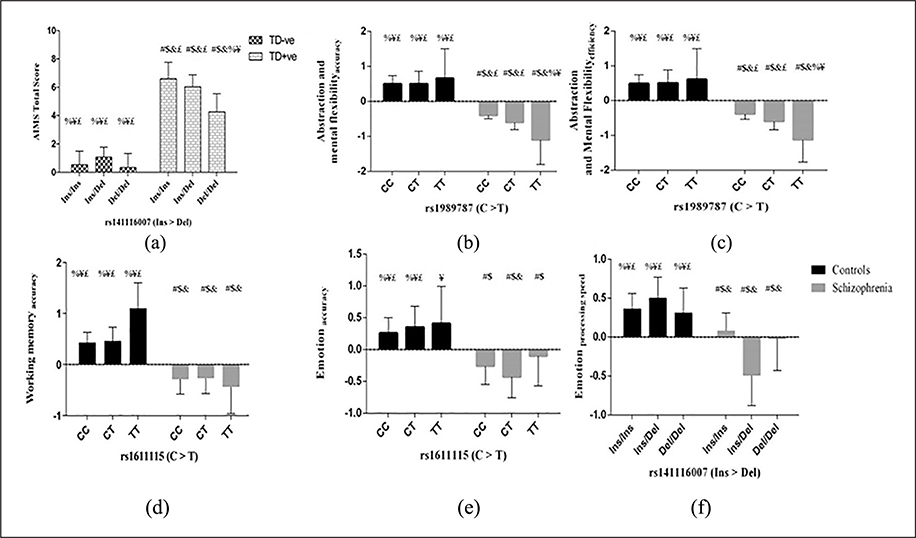
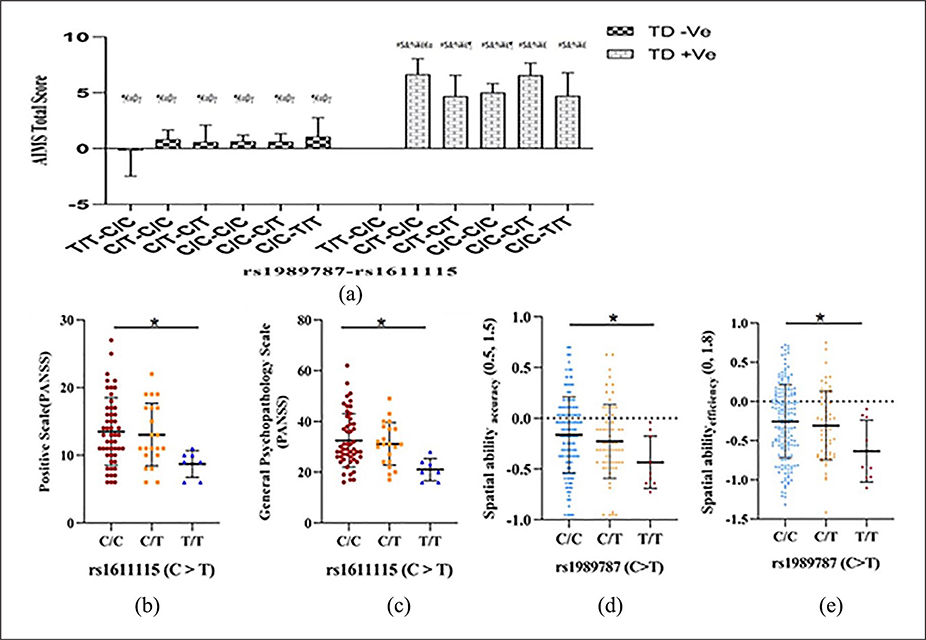
No association of any of the markers (19bp Ins/Del, χ2=1.6, p=0.2; rs1989787, χ2=2.4, p=0.1 and rs1611115, χ2=0.3, p=0.6) was seen with tardive dyskinesia. However, ANCOVA revealed a significant interaction of tardive dyskinesia status and genotypes of 19bp Ins/Del with AIMS total score (F(2, 174)=3.3, p=0.04), but with small effect size (0.20) and marginal means of AIMS total score adjusted for gender and age (Figure 2(a)). Furthermore, a significant interaction between tardive dyskinesia status and genotypes of rs1989787 and rs1611115 (with 83% power) with AIMS total score (Figure 3(a)) was also identified (F(1, 174)= 8.7, p=0.004), with small effect size (0.22). Test of interaction between tardive dyskinesia status and the three markers performed considering only orofacial (1–4 items of AIMS) or limb-truncal (5–7 items of AIMS) scores showed significant effect of tardive dyskinesia status, rs1611115 and rs1989787 interaction on limb-truncal scores only (F(1,174)=8.3, p=0.004).
Figure 2:
Estimated marginal means of (a) Abnormal Involuntary Movement Scale (AIMS) total score for the interaction of tardive dyskinesia status and DBH genotypes and (b to f) cognitive scores for the interaction of health status and DBH genotypes. An analysis of covariance with full factorial model was done to assess the effect of interaction of (a) tardive dyskinesia status and genotypes of 19bp Ins/Del on AIMS total score and (b to f) health status and genotypes of rs141116007, rs1989787 and rs1611115 on the transformed cognitive scores. Marginal means of AIMS total score adjusted for age and gender where a significant effect of interaction of tardive dyskinesia status and genotypes (p<0.05) is presented. In the tardive dyskinesia-positive (TD+ve) cohort, subjects with Ins/Ins genotype had higher AIMS score than subjects with Ins/Del or Del/Del genotype. While in the tardive dyskinesia-negative (TD−ve) cohort, subjects with the Ins/Del had higher AIMS score than those with Ins/Ins and Del/Del (a). The inverse BCN transformed marginal means adjusted for gender for the domains that had significant effect of interaction of health status and genotypes (p<0.05) is presented. One single nucleotide polymorphism (SNP; rs1989787) had different effect on accuracy and efficiency of abstraction and mental flexibility depending on health status (b and c). Accuracy and efficiency of abstraction and mental flexibility of the T/T genotype of rs1989787 was significantly lower than those with C/C and C/T genotypes in schizophrenia subjects. Another SNP (rs1611115) had differential effect on accuracy working memory (d) and emotion (e) depending on health status. The Ins/Del polymorphism (rs141116007) also had a differential effect on processing speed of emotion (f). Error bars indicate 95% confidence interval. #, $, &, %, ¥ and £ denote significantly different from the first to sixth bars (p<0.05) in the graph after Fisher’s least significant difference multiple comparisons.
BH: dopamine-β-hydroxylase
Figure 3:
Effect of (a) interaction of rs1989787 and rs1611115 with tardive dyskinesia status on Abnormal Involuntary Movement Scale (AIMS) total score of tardive dyskinesia in the tardive dyskinesia cohort; rs1611115 on (b) positive scale, (c) general psychopathology subscale scores of the Positive and Negative Syndrome Scale (PANSS) in the tardive dyskinesia-positive (TD+ve) sub cohort, (d) rs1989787 on accuracy and (e) efficiency of spatial ability in healthy subjects. In the (a) interaction of genotypes of rs1989787-rs1611115 with tardive dyskinesia status, the genotypic combination C/T–C/C had higher AIMS tardive dyskinesia scores than C/T–C/T. Further, the genotypic combination C/T–C/C had higher AIMS tardive dyskinesia scores than C/C–C/C. Since the T allele of rs1989787 and the C allele of rs1611115 were earlier associated with higher dopamine-β-hydroxylase (DBH) activity and earlier luciferase reporter assays involving promoter constructs containing these variants have confirmed these, these results show a direct correlation between DBH activity and AIMS tardive dyskinesia scores. Error bars denote 95% confidence interval. #, $, &, %, ¥, £, ¶, €, α, β and γ depict significantly different from first to 11th bars post Fisher’s least significant difference (LSD) multiple comparisons. The T/T genotype of rs1611115 had significantly lower (b) positive scale and (c) general psychopathology sub scores of PANSS in TD+ve sub cohort post Bonferroni multiple comparisons. Healthy subjects with (d) C/C genotype of rs1989787 had significantly higher spatial abilityaccuracy scores than those with the T/T genotype post LSD multiple comparison. Subjects with (e) the T/T genotype had lower spatial abilityefficiency scores than those with C/C genotype in healthy controls post Bonferroni multiple comparisons. (b) to (e) Error bars denote SD.
*denotes significantly different groups (p<0.05).
TD−ve: tardive dyskinesia-negative
Allelic association of the T allele of the proximal promoter variant rs1611115 with positive (allelic (β=−1.8, p=0.03)), general psychopathology subscales (allelic (β=−4.27, p=0.01)) and PANSS total score (allelic (β=−6.5, p=0.05)) was identified in the TD+ve sub cohort in the additive model. Genotypic association of the same variant with positive (p=0.04) and general psychopathology (p=0.01) was also identified in the TD+ve sub cohort (Figure 3(b) and (c)). Further, there was allelic association of the T allele of rs1611115 with depressive factors in the TD−ve sub cohort (allelic (β=0.81, p=0.02)). Though there was genotypic association of rs1611115 (p=0.05) with depressive factors, only subjects with the C/T genotype were significantly different from those with the C/C genotype post LSD multiple comparisons (p=0.03).
Association with cognition
Transformations using the recent skew power approach were performed by estimating the λ and γ from the linear model with cognitive domains as dependent variable and health status and DBH genotypes as independent variables. In healthy controls, rs1989787C >T was found to be an eQTL for spatial abilityaccuracy (β=−0.10, p=0.02) and spatial abilityefficiency (β=−0.13, p=0.02) in an additive model. This SNP also exhibited genotypic association (Figure 3(d) and (e)) with spatial abilityaccuracy (p=0.05 and spatial abilityefficiency (p=0.03). Transformation parameters (λ and γ) for cognitive domains, where an association was observed, are described as follows. Abstraction and mental flexibilityaccuracy with z = zbcn,u (y, −0.34, 2.5); abstraction and mental flexibilityefficiency with z = zbcn,u (y, −0.53, 3.3); attentionaccuracy with z = zbcn,u (y, 0.94, 1.0); attentionefficiency with z = zbcn,u (y, 0.95, 1.2); working memoryaccuracy with z = zbcn,u (y, 0.58, 1.9); emotionaccuracy with z = zbcn,u (y, 0.31, 2.5) and emotionprocessing speed with z = zbcn,u (y, 0.82, 2.1). A significant effect of the interaction of health status and genotypes of rs1989787 on the abstraction and mental flexibilityaccuracy (F(2, 608) =3.7, p= 0.03) and abstraction and mental flexibilityefficiency (F (2, 601) =3.5, p=0.03) was observed. The effect size of these interactions on these domains was found to be small (0.11 and 0.10 respectively). The T/T genotype of rs1989787 had significantly lower scores of accuracy and efficiency of abstraction and mental flexibility than those with C/C and C/T genotypes in schizophrenia subjects (Figure 2(b) and (c)). Further, there was significant interaction between health status and genotypes of rs1611115 on working memoryaccuracy (F(2, 583)=3.1, p=0.048), with an effect size of 0.1. A significant interaction of health status and genotypes of rs1611115 on emotionaccuracy (F (2, 603) =2.9, p=0.05) was also identified. The effect size of this interaction on emotionaccuracy was found to be small (0.10). There was also a significant interaction of health status and genotypes of 19bp Ins/Del (rs141116007) on emotionprocessing speed (F(2, 598) =3.4; p=0.03). The effect size of this interaction was small (0.1). Another significant interaction of health status with markers rs1989787 and rs1611115 on attentionaccuracy (F(1, 529)=4.5; p=0.03), emotionaccuracy (F(1, 603)=4.4; p=0.04) and attention efficiency (F(1, 527)=4.1; p=0.04) was identified with a small effect size (0.1). The gender adjusted marginal means for significant interactions of DBH variants with health status are presented (Figure 2(b) to (f)). A summary of interactions of DBH variants that had an effect on performance functions of cognitive scores of accuracy, processing speed and efficiency is given in Supplementary Tables 3–5 respectively. Further, there was allelic association of the T allele of rs1611115 with GAS during the past month (β=2.7, p=0.05) in schizophrenia subjects of the cognition cohort.
PCA
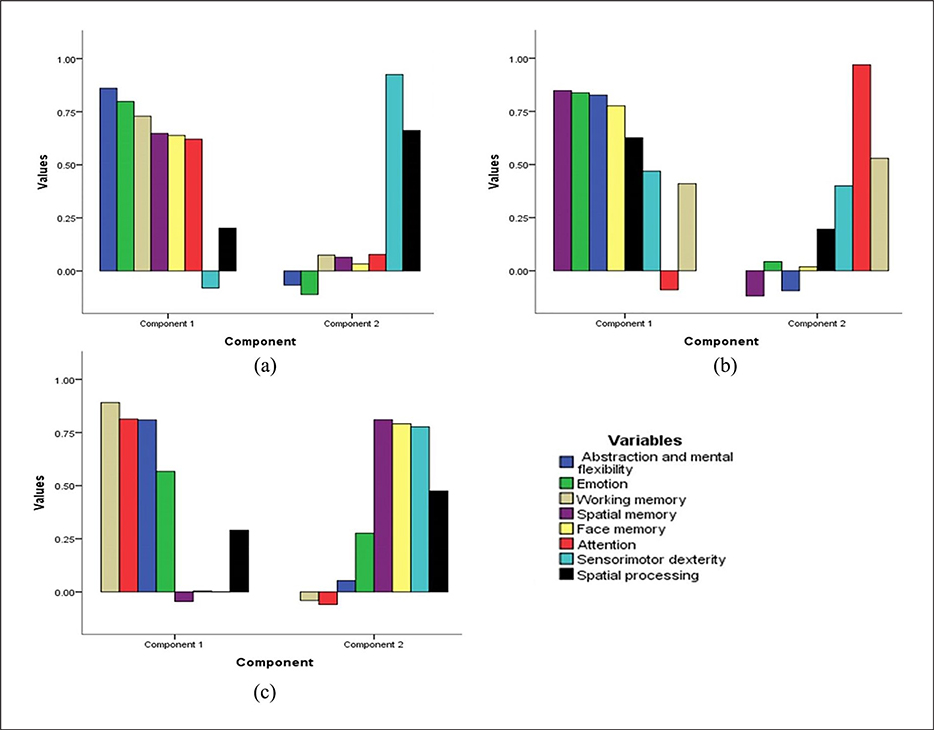
PCA was conducted on the neurocognitive measures of accuracy, processing speed and efficiency separately with oblique rotation (direct oblimin). Verification of the sampling adequacy for the analysis was done with the KMO measure. The KMO values for the cognitive scores of accuracy, processing speed and efficiency were found to be ⩾0.87, above the acceptable score of 0.5. For the cognitive measures, Bartlett’s test of sphericity was assessed (accuracy, χ2(28)=1237.6, p<0.001; processing speed, χ2(28)=1781.3, p<0.001 and efficiency, χ2(28)=1587.4, p<0.001) indicating high correlation between cognitive scores and a PCA could be done on the cognitive measures. Eigenvalues were obtained for each component by running an initial analysis on data. Only the first component of accuracy, processing speed and efficiency had Eigenvalues over Kaiser’s criterion of 1 and explained 46.1%, 53.7% and 53.1% of the variance for accuracy, processing speed and efficiency respectively. The second component explained 11%, 11.5% and 9.4% of variance. The Eigenvalues for the second principal component for the same measures were 0.9, 0.9 and 0.8 respectively. Scree plots showed inflections and the first component explained most of the variance in cognitive scores. Though two components were extracted, because of correlation between first and second components only the first component was retained in the final analysis. The factor loadings after rotation are given in Figure 4. Health had a significant effect on the two principal components of accuracy, processing speed and efficiency (p<0.05). The interaction of health and genotypes at rs1989787 was found to have a significant effect on the first principal component of efficiency of the neurocognitive scores (F(2, 460)=3.0, p=0.05).
Figure 4.
The factor loadings of individual cognitive domain scores onto the principal components of (a) accuracy, (b) processing speed and (c) efficiency. Notably abstraction and mental flexibilityaccuracy, abstraction and mental flexibilityprocessing speed and abstraction and mental flexibilityefficiency loaded onto the first component of performance functions of accuracy, processing speed and efficiency respectively. Accuracy, processing speed and efficiency of cognitive scores of abstraction and flexibility, attention, working memory, face memory, spatial memory, spatial processing, sensorimotor dexterity and emotional processing are illustrated.
K-means cluster analysis
K-means clustering was done in two clusters based on the health status. Convergence of change in cluster centres was achieved by the fourth iteration. The final cluster centres are given in Supplementary Figure 3. A general decrease in all the cognitive scores in subjects with schizophrenia was observed as compared with healthy controls. Of all the cognitive scores, abstraction and mental flexibilityefficiency, abstraction and mental flexibilityaccuracy, emotionefficiency and working memoryefficiency contributed the most in separating subjects into these two clusters.
Functional characterization of 19bp Ins/Del marker
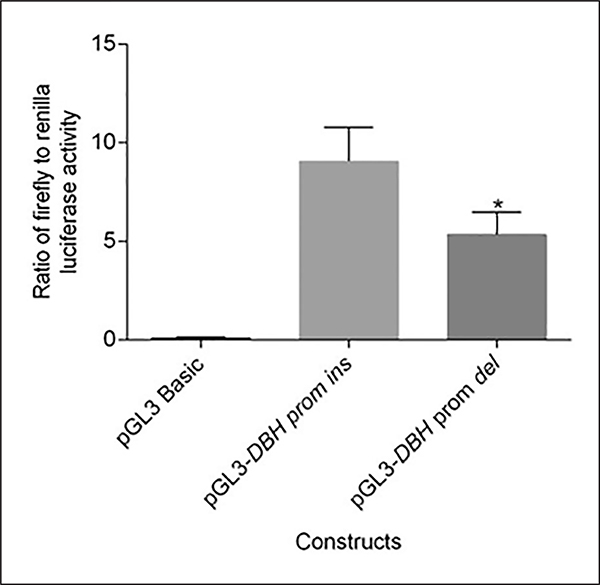
A significant 39% decrease in luciferase activity was observed with the construct having 19bp Del allele as compared with the wild type (p=0.03). This clearly provides the first ever evidence for functional significance of this common variant in DBH promoter (Figure 5).
Figure 5:
Differential expression of the DBH 19bp Del allele as compared with the Ins allele. The ratio of Firefly to Renilla luciferase activity was calculated from the luminescence measurements. A one tailed t-test as implemented in GraphPad Prism 6.0 was done to check for differences in expression of Ins and Del alleles. A significant decrease in expression of the Del allele as compared with the Ins allele was observed (*denotes p<0.05).
Discussion
Association of coding and regulatory genetic variants at DBH locus across brain disorders (Kopeckova et al., 2006), including schizophrenia, have been well reported (Cubells and Zabetian, 2004). Correlation of exonic variants with qualitative (Ishii et al., 1991) and with quantitative enzyme phenotypes (Kim et al., 2011; Punchaichira et al., 2017) has also been documented. Functional characterization of two commonly investigated regulatory variants, namely rs1989787 (Chen et al., 2011) and rs1611115 (Chen et al., 2010), has also been carried out. Of note, association of 19bp Ins/Del, yet another regulatory variant, has been well reported but not functionally validated. Furthermore, contribution of these three significant regulatory variants to phenotypes such as tardive dyskinesia and cognition are seldom documented. In this study, we investigated the likely contribution of three variants, namely 19bp Ins/Del, rs1989787 and rs1611115, in the 5′UTR of the gene to schizophrenia, tardive dyskinesia and cognition. To strengthen our association findings, we also established for the first time the functional significance of the Ins and Del alleles of 19bp marker based on high and low reporter activity respectively (Figure 5).
Genetic associations
Schizophrenia
None of the three variants were associated with schizophrenia despite the well powered (>90%) study cohort. Only a two-marker haplotype Ins-C of 19bp Ins/Del-rs1989787 showed modest association (p=0.04).
Tardive dyskinesia
No association of the three markers was observed with tardive dyskinesia per se. However, ANCOVA on TD+ve and TD−ve schizophrenia cohorts identified two differential genotypic effects in TD+ve subjects: i) higher AIMS score with 19bp Ins allele (Figure 2(a)); and ii) interaction of genotypes at rs1989787 and rs1611115 on AIMS score wherein those with the genotypic combination C/T–C/C(intermediate/high activity) had higher scores than those with C/T–C/T and C/C–C/C (Figure 3(a)). The former could be explained by the high activity of the Ins allele of 19bp marker as reported previously (Tang et al., 2007) and indirectly by the high reporter activity demonstrated in our study (Figure 5). The second differential genotypic effect involving rs1989787 and rs1611115 could also be explained by the higher DBH activity reported for their T and C alleles previously (Punchaichira et al., 2016). In addition, subjects with C/C and C/T genotypes of rs1989787 had lower DBH activity compared with those with T/T genotype (Chen et al., 2011) and the C allele of rs1611115 was found to increase plasma DBH activity and epinephrine excretion, and predict a higher basal blood pressure (Chen et al., 2010). These correlations imply that in the TD+ve subjects, higher DBH activity may lead to higher AIMS scores (Figures 2(a) and 3 (a)). Previous studies have also reported that schizophrenic subjects with tardive dyskinesia had higher DBH activity compared with those without tardive dyskinesia (Jeste et al., 1981; Kaufmann et al., 1986; Wagner et al., 1982). Furthermore, among TD+ve subjects those with C/C genotype (high activity) at rs1611115 were found to have higher positive scale and general psychopathology scale of PANSS (Figure 3(b) and (c)). This implies that among TD+ve subjects those with higher DBH activity have more severe disease. However, in the TD−ve cohort the variant allele (T) at this marker was associated with depressive factors.
Cognition
Substantial neurocognitive deficits have been documented in large scale studies of schizophrenia subjects (Greenwood et al., 2007; Heinrichs and Zakzanis, 1998; Toulopoulou et al., 2010). A computerized neurocognitive battery analyses accuracy, response time and efficiency of neurocognitive domains and have been used in genetic studies (Gur et al., 2001a, 2010). In healthy controls, accuracy and efficiency of spatial ability was higher in those with the C/C genotype of rs1989787 as compared with those with T/T genotype (Figure 3(d) and (e)). From this it may be inferred that this SNP is an eQTL for spatial abilityaccuracy and spatial abilityefficiency. The most notable association of the three DBH markers in this study was with different domains of cognition (Figure 2(b) to (f) and Supplementary Tables 3–5). We observed differential cognitive effect of rs1989787 on abstraction and mental flexibility in controls and schizophrenia subjects. The T/T genotypes (high activity) attributed to higher cognitive scores in controls, but to lowest scores among the three genotypes in schizophrenia subjects (Figure 2(b) and (c)). On the other hand, there was allelic association of T allele (low activity) of rs1611115 with increase in GAS scores in the past month among schizophrenia subjects in the cognition cohort. Hence, those with the T allele had lower severity of the disease as compared with those with the C allele. These results corroborate with the general psychopathology subscale of PANSS in the TD+ve cohort (Figure 3(c)) and suggest that rs1611115 may be an important SNP influencing disease severity.
PCA was done to reduce the dimensionality of cognitive variables. The interaction of health status and rs1989787 was significant with the first principal component of efficiency of cognitive scores. This could be explained in light of the fact that abstraction and mental flexibilityefficiency was loaded onto the first component of efficiency (Figure 4(c)) and the interaction was still significant even after multivariate corrections. Clustering analysis confirmed that all the cognitive variables showed a substantial reduction in schizophrenia subjects as compared with healthy controls and cognitive scores of abstraction and mental flexibilityefficiency, abstraction and mental flexibilityaccuracy, emotionefficiency followed by working memoryefficiency contributed the most in segregating schizophrenia subjects from healthy controls. Overall these observations derive notable support from previous studies. An extended promoter haplotype spanning seven common SNPs (six in the promoter region, and one in intron-A: rs1076151, rs1076152, rs1076150, rs1989787, rs1611114, rs1611115, rs2797849) was reported to predict DBH activity. Haplotype 1 (GCTCCTG) decreased DBH activity whereas haplotype 2 (GCCTCCC) increased the activity (Chen et al., 2011). In addition, dopamine and other neurotransmitters have been reported to have implications for cognition in schizophrenia subjects (Braver et al., 1999; Condray and Yao, 2011; Friedman et al., 1999; Sakurai et al., 2013). Of note, one of the limitations of our study is that subjects recruited in this study were not drug naïve. This may be kept in mind while considering genotype–phenotype correlations in cognition reported in this study.
The role of DBH levels in cognition as evidenced above also derives support from the documented role of norepinephrine in cognition (Chamberlain and Robbins, 2013). This is further substantiated by the documented improvement/decline of cognitive scores by augmenting (Gamo et al., 2010; Jentsch et al., 2009; Tzavara et al., 2006) or depleting (Cai et al., 1993; Sontag et al., 2008) norepinephrine levels through a range of pharmacological interventions. In addition, cerebrospinal fluid DBH levels were found to be lower in schizophrenia subjects who became nonpsychotic than in those who remained psychotic following neuroleptic treatment (Sternberg et al., 1982). These observations reiterate the importance of norepinephrine signalling in schizophrenia, tardive dyskinesia and cognition.
Taken together, investigation of the contribution of the three common regulatory variants in DBH to tardive dyskinesia and cognition among schizophrenia subjects in this study has provided useful insights with pharmacogenetic implications. However, considering that tardive dyskinesia and cognitive symptoms are observed with higher severity in subjects with high DBH activity, estimation of DBH activity (Punchaichira et al., 2018) prior to use of DBH inhibitors along with neuroleptics may be of therapeutic relevance.
Supplementary Material
Acknowledgements
SH-SY5Y cells were kindly provided by Dr Beena Pillai of Institute of Genomics and Integrative Biology (IGIB). pGL3 Basic and pRL-TK vectors were kindly given by Dr Akhil C Banerjea of National Institute of Immunology, New Delhi. Skew power transformations using car package in R was done with help from Prof. Sanford Weisberg, School of Statistics, University of Minnesota. Generous help was given by Prof. Ron Rodriguez, Department of Urology, University of Texas Health, San Antonio in sharing the details of non-commercial luciferase assay.
Infra structural support from central instrumentation facility, University of Delhi South Campus; Department of Science and Technology, Government of India, New Delhi (DST-FIST Level II; DST-PURSE grant phase-II); University Grants Commission, New Delhi (UGC-SAP (DRS III)) to Department of Genetics, University of Delhi South Campus) are gratefully acknowledged.
Informed consent: informed consent was obtained from all individual participants included in the study or parents or guardians as the case may be. For cord blood samples, informed consent was taken from mothers.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Biotechnology (DBT), Government of India, New Delhi (grants number BT/PR/2425/MED/13/089/2001; number BT/IC-2/Israel/Deshpande/2002; number BT/IC-2/00/Smita/99 to BKT and SND); Department of Science and Technology (DST) – Science and Engineering Research Board (SERB) (SR/S2/JCB-44/2011-JC Bose fellowship phase II to BKT)).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Statement of human rights: Ethical approval: all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (ethical committee at Dr. Ram Manohar Lohia Hospital, no. 18-15/2002-RMLH(HA-I)/3140 dated 5 March 2004 for tardive dyskinesia cohort; no. 18-62/06-RMLH(HA-I)/vol. II/63 dated 30 November 2008 for cognition cohort; no. 18-62/06-RMLH(HA-I)/1088 dated 15 January 2008 for schizophrenia cases and adult controls; no. 18-9/2002-RMLH(HA-I)/5262 dated 9 May 2005 for cord blood samples) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals: this article does not contain any studies with animals performed by any of the authors.
Supplemental material
Supplemental material for this article is available online.
References
- Andreasen NC and Olsen S (1982) Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry 39: 789–794. [DOI] [PubMed] [Google Scholar]
- Ates O, Celikel FC, Taycan SE, et al. (2013) Association between 1603C>T polymorphism of DBH gene and bipolar disorder in a Turkish population. Gene 519: 356–359. [DOI] [PubMed] [Google Scholar]
- Bhatia T, Agarwal A, Shah G, et al. (2012) Adjunctive cognitive remediation for schizophrenia using yoga: An open, non-randomized trial. Acta Neuropsychiatr 24: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler JL, Roffler-Tarlov S, Schachner M, et al. (1978) Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 38: 3751–3757. [PubMed] [Google Scholar]
- Braver TS, Barch DM and Cohen JD (1999) Cognition and control in schizophrenia: A computational model of dopamine and prefrontal function. Biol Psychiatry 46: 312–328. [DOI] [PubMed] [Google Scholar]
- Cai JX, Ma YY, Xu L, et al. (1993) Reserpine impairs spatial working memory performance in monkeys: Reversal by the alpha 2-adrenergic agonist clonidine. Brain Res 614: 191–196. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR and Robbins TW (2013) Noradrenergic modulation of cognition: Therapeutic implications. J Psychopharmacol 27: 694–718. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wen G, Rao F, et al. (2010) Human dopamine beta-hydroxylase (DBH) regulatory polymorphism that influences enzymatic activity, autonomic function, and blood pressure. J Hypertens 28: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang K, Wen G, et al. (2011) Human dopamine β-hydroxylase promoter variant alters transcription in chromaffin cells, enzyme secretion, and blood pressure. Am J Hypertens 24: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condray R and Yao JK (2011) Cognition, dopamine and bioactive lipids in schizophrenia. Front Biosci (Schol Ed) 3: 298–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF and Zabetian CP (2004) Human genetics of plasma dopamine beta-hydroxylase activity: Applications to research in psychiatry and neurology. Psychopharmacology (Berl) 174: 463–476. [DOI] [PubMed] [Google Scholar]
- Cubells JF, van Kammen DP, Kelley ME, et al. (1998) Dopamine beta-hydroxylase: Two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Hum Genet 102: 533–540. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, et al. (1991) Dopamine in schizophrenia: A review and reconceptualization. Am J Psychiatry 148: 1474–1486. [DOI] [PubMed] [Google Scholar]
- Deshpande SN, Mathur MN, Das SK, et al. (1998) A Hindi version of the diagnostic interview for genetic studies. Schizophr Bull 24: 489–493. [DOI] [PubMed] [Google Scholar]
- Dyer BW, Ferrer FA, Klinedinst DK, et al. (2000) A noncommercial dual luciferase enzyme assay system for reporter gene analysis. Anal Biochem 282: 158–161. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, et al. (1976) The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 33: 766–771. [DOI] [PubMed] [Google Scholar]
- Fox J and Weisberg S (2011) An R Companion to Applied Regression. Los Angeles, CA; London: SAGE Publications. [Google Scholar]
- Friedman JI, Adler DN and Davis KL (1999) The role of norepinephrine in the pathophysiology of cognitive disorders: Potential applications to the treatment of cognitive dysfunction in schizophrenia and Alzheimer’s disease. Biol Psychiatry 46: 1243–1252. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Wang M and Arnsten AF (2010) Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry 49: 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ and Morrison JM (2006) QUANTO 1.1: A Computer Program for Power and Sample Size Calculations for Genetic-epidemiology Studies. Available at: http://biostats.usc.edu/Quanto.html (accessed 13 September 2019).
- Gonzalez-Lopez E and Vrana KE (2019) Dopamine beta-hydroxylase and its genetic variants in human health and disease. J Neurochem. Epub ahead of print. DOI: 10.1111/jnc.14893 [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, et al. (2007) Initial heritability analyses of endophenotypic measures for schizophrenia: The consortium on the genetics of schizophrenia. Arch Gen Psychiatry 64: 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, et al. (2001a) Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology 25: 766–776. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, et al. (2001b) Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology 25: 777–788. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, et al. (2010) A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods 187: 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, et al. (2007) Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry 164: 813–819. [DOI] [PubMed] [Google Scholar]
- Guy W (1976) Early Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare, 534–537. [Google Scholar]
- Hawkins DM and Weisberg S (2017) Combining the box-cox power and generalised log transformations to accommodate nonpositive responses in linear and mixed-effects linear models. S Afr Stat J 51: 317–328. [Google Scholar]
- Heinrichs RW and Zakzanis KK (1998) Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 12: 426–445. [DOI] [PubMed] [Google Scholar]
- Hui L, Han M, Huang XF, et al. (2015a) Possible association between DBH 19 bp insertion/deletion polymorphism and clinical symptoms in schizophrenia with tardive dyskinesia. J Neural Transm (Vienna) 122: 907–914. [DOI] [PubMed] [Google Scholar]
- Hui L, Han M, Huang XF, et al. (2016) Association between DbetaH 5’-insertion/deletion polymorphism and cognition in patients with chronic schizophrenia. J Clin Psychiatry 77: 379–385. [DOI] [PubMed] [Google Scholar]
- Hui L, Han M, Yin GZ, et al. (2015b) Association between DBH 19bp insertion/deletion polymorphism and cognition in schizophrenia with and without tardive dyskinesia. Schizophr Res 182: 104–109. [DOI] [PubMed] [Google Scholar]
- Hui L, Zhang X, Huang XF, et al. (2012) The dopamine b-hydroxylase 19 bp insertion/deletion polymorphism was associated with first-episode but not medicated chronic schizophrenia. J Psychiatr Res 46: 733–737. [DOI] [PubMed] [Google Scholar]
- Hui L, Zhang X, Yu YQ, et al. (2013) Association between DBH 19 bp insertion/deletion polymorphism and cognition in first-episode schizophrenic patients. Schizophr Res 147: 236–240. [DOI] [PubMed] [Google Scholar]
- Ishii A, Kobayashi K, Kiuchi K, et al. (1991) Expression of two forms of human dopamine-beta-hydroxylase in COS cells. Neurosci Lett 125: 25–28. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Aarde SM and Seu E (2009) Effects of atomoxetine and methylphenidate on performance of a lateralized reaction time task in rats. Psychopharmacology (Berl) 202: 497–504. [DOI] [PubMed] [Google Scholar]
- Jeste DV, DeLisi LE, Zalcman S, et al. (1981) A biochemical study of tardive dyskinesia in young male patients. Psychiatry Res 4: 327–331. [DOI] [PubMed] [Google Scholar]
- Kaufman S and Friedman S (1965) Dopamine-Beta-Hydroxylase. Pharmacol Rev 17: 71–100. [PubMed] [Google Scholar]
- Kaufmann CA, Jeste DV, Shelton RC, et al. (1986) Noradrenergic and neuroradiological abnormalities in tardive dyskinesia. Biol Psychiatry 21: 799–812. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A and Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Kim CH, Leung A, Huh YH, et al. (2011) Norepinephrine deficiency is caused by combined abnormal mRNA processing and defective protein trafficking of dopamine beta-hydroxylase. J Biol Chem 286: 9196–9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek V, Rajkowska G, Luker SN, et al. (1999) Brain noradrenergic receptors in major depression and schizophrenia. Neuropsychopharmacology 21: 69–81. [DOI] [PubMed] [Google Scholar]
- Kopeckova M, Paclt I and Goetz P (2006) Polymorphisms and low plasma activity of dopamine-beta-hydroxylase in ADHD children. Neuro Endocrinol Lett 27: 748–754. [PubMed] [Google Scholar]
- Kukshal P, Bhatia T, Bhagwat AM, et al. (2013) Association study of neuregulin-1 gene polymorphisms in a North Indian schizophrenia sample. Schizophr Res 144: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouroux A, Vigny A, Faucon Biguet N, et al. (1987) The primary structure of human dopamine-beta-hydroxylase: Insights into the relationship between the soluble and the membrane-bound forms of the enzyme. EMBO J 6: 3931–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin EY, Levenberg B and Kaufman S (1960) The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem 235: 2080–2086. [PubMed] [Google Scholar]
- Meltzer HY, Goode DJ, Fang VS, et al. (1976) Dopamine and schizophrenia. Lancet 2: 1142. [DOI] [PubMed] [Google Scholar]
- Mustapic M, Maihofer AX, Mahata M, et al. (2014) The catecholamine biosynthetic enzyme dopamine beta-hydroxylase (DBH): First genome-wide search positions trait-determining variants acting additively in the proximal promoter. Hum Mol Genet 23: 6375–6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI Jr, Blehar MC, Kaufmann CA, et al. (1994) Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 51: 849–859; discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, de Visser E, Lin MK, et al. (2012) Dopamine beta hydroxylase genotype identifies individuals less susceptible to bias in computer-assisted decision making. PLoS One 7: e39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardinas AF, Holmans P, Pocklington AJ, et al. (2018) Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet 50: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punchaichira TJ, Deshpande SN and Thelma BK (2018) Determination of dopamine-beta-hydroxylase activity in human serum using UHPLC-PDA detection. Neurochem Res 43: 2324–2332. [DOI] [PubMed] [Google Scholar]
- Punchaichira TJ, Dey SK, Mukhopadhyay A, et al. (2017) Characterization of SNPs in the dopamine-beta-hydroxylase gene providing new insights into its structure-function relationship. Neurogenetics 18: 155–168. [DOI] [PubMed] [Google Scholar]
- Punchaichira TJ, Prasad S, Deshpande SN, et al. (2016) Deep sequencing identifies novel regulatory variants in the distal promoter region of the dopamine-beta-hydroxylase gene. Pharmacogenet Genomics 26: 311–323. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, et al. (2007) PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Bies RR, Stroup ST, et al. (2013) Dopamine D2 receptor occupancy and cognition in schizophrenia: Analysis of the CATIE data. Schizophr Bull 39: 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler NR and Kane JM (1982) Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry 39: 486–487. [DOI] [PubMed] [Google Scholar]
- Sontag TA, Hauser J, Kaunzinger I, et al. (2008) Effects of the noradrenergic neurotoxin DSP4 on spatial memory in the rat. J Neural Transm (Vienna) 115: 299–303. [DOI] [PubMed] [Google Scholar]
- Srivastava V, Deshpande SN and Thelma BK. (2010) Dopaminergic pathway gene polymorphisms and genetic susceptibility to schizophrenia among north Indians. Neuropsychobiology 61: 64–70. [DOI] [PubMed] [Google Scholar]
- Sternberg DE, VanKammen DP, Lerner P, et al. (1982) Schizophrenia: Dopamine beta-hydroxylase activity and treatment response. Science 216: 1423–1425. [DOI] [PubMed] [Google Scholar]
- Tang YL, Epstein MP, Anderson GM, et al. (2007) Genotypic and haplotypic associations of the DBH gene with plasma dopamine beta-hydroxylase activity in African Americans. Eur J Hum Genet 15: 878–883. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Deshpande SN, Lerer B, et al. (2007) Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: V. Association of CYP1A2 1545 C>T polymorphism. Pharmacogenomics J 7: 305–311. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Deshpande SN, Rao AR, et al. (2005a) Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: III. Lack of association of CYP3A4 and CYP2D6 gene polymorphisms. Schizophr Res 75: 21–26. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Deshpande SN, Rao AR, et al. (2005b) Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: I. Association of CYP1A2 gene polymorphism. Pharmacogenomics J 5: 60–69. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Goldberg TE, Mesa IR, et al. (2010) Impaired intellect and memory: A missing link between genetic risk and schizophrenia? Arch Gen Psychiatry 67: 905–913. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Overshiner CD, et al. (2006) Procholinergic and memory enhancing properties of the selective norepinephrine uptake inhibitor atomoxetine. Mol Psychiatry 11: 187–195. [DOI] [PubMed] [Google Scholar]
- Wagner RL, Jeste DV, Phelps BH, et al. (1982) Enzyme studies in tardive dyskinesia. I. One-year biochemical follow-up. J Clin Psychopharmacol 2: 312–314. [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, et al. (2012) Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res 137: 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Cubells JF, Gelernter J, et al. (2003) Dopamine beta-hydroxylase (DBH) gene and schizophrenia phenotypic variability: A genetic association study. Am J Med Genet B Neuropsychiatr Genet 117B: 33–38. [DOI] [PubMed] [Google Scholar]
- Zabetian CP, Anderson GM, Buxbaum SG, et al. (2001) A quantitativetrait analysis of human plasma-dopamine beta-hydroxylase activity: Evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet 68: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.