Abstract
This chapter deals with small molecule modulators of the ubiquitin–proteasome system (UPS). They are designed to restore its impaired capacity to dispose of soluble, dysfunctional protein copies, and to fight its pathological impairment in proteinopathies in general and in tauopathies in particular. Two specific molecular targets belonging to the U-box E3 ligase family (C-terminus of Hsc70 interacting protein, CHIP) and to the proteasome-associated cysteine protease DUB family (USP14) are selected for their putative role against NDDs and tauopathies. The limited available structural information for the two targets, and for their interactions with members of UPS-driven protein complexes, is described. A small number of known modulators for each target (or even for structurally related targets, possibly to provide translatable examples) are portrayed in terms of their biological profile, and of their development potential as disease-modifying drugs against NDDs.
Keywords: protein–protein interaction inhibitors, proteasome, E3 ligases, deubiquitinases, intrinsically disordered proteins, CHIP USP14
3.1. UPS-mediated degradation of misfolded proteins
The elimination of misfolded protein copies is an appealing option to tackle neurodegenerative diseases (NDDs) and tauopathies. The ubiquitin– proteasome system (UPS) [1], [2] is the most important cellular pathway to dispose of soluble cytosolic proteins.
UPS is responsible for most regulated proteolytic events, and depends on the 76-mer protein ubiquitin (UBQ) [3], [4]. UBQ is a stable, compact protein with the six C-terminus amino acids (AAs) arranged as a flexible tail [4]. A first UBQ molecule is anchored to the ɛ-NH2 group of a Lys residue in the substrate proteins through an isopeptide bond involving the C-terminus of UBQ. Mono-ubiquitination may target a specific Lys residue [5], or a domain [6] on the substrate protein. Multiple mono- ubiquitination on different Lys residues of the substrate protein (multi- mono-ubiquitination) is also observed [7]. Ubiquitinated proteins are usually tagged with polyUBQ chains. UBQ chain elongation implies the formation of an isopeptide bond between one of seven Lys residues (K6, K11, K27, K29, K33, K48, and K63), or between the Met1 residue of a substrate-anchored proximal UBQ molecule and the C-terminus of a free, distal UBQ protein. Different UBQ chain elongation enzymes bind to interaction surfaces on UBQ with diverse specificities, and promote UBQ elongation on a specific anchoring point.
Once a di-UBQ chain is formed, the two UBQ molecules assume an anchoring residue-dependent conformation. Five out of the eight UBQ connections are structurally characterized by X-ray crystallography and/or nuclear magnetic resonance (NMR) [8]. Among them, K48-linked di-UBQ chains adopt a “closed,” compact conformation, where the two UBQ proteins interact with each other [9]. K63-linked di-UBQ chains adopt an “open” and more flexible conformation, where the two UBQ molecules do not interact beyond their isopeptide bond connection [10]. Highly flexible open conformations of di-UBQ chains provide even more alternative binding modes with their protein partners.
K48-linked polyUBQ chains are the most abundant homotypic polyUBQ species [11], acting as labels on proteins to be degraded through the UPS [12]. Proteasome inhibition causes a fast increase of K48-polyUBQ proteins [13], and mutation of Lys residues in yeast UBQ show that K48 is the only essential residue among them [14].
There are eight Lys/Met anchoring points on UBQ, UBQ chain lengths varying between 1 (mono-UBQ) and >10 polyUBQs on each anchoring point, and >700 enzymes involved in a multi-step process (including UBQ activation, conjugation, transfer to a protein substrate, and chain trimming) [8]. The combinations of UBQ codes easily match the experimental observation of thousands of UBQ-labeled protein substrates on multiple sites, and ensure an exquisitely specific UBQ/UPS-dependent regulation of the functions reconducible to ubiquitinated proteins. But how can the UBQ machinery select the anchoring point, the UBQ chain length and nature, and the specific substrate to be ubiquitinated or deubiquitinated in a dynamic cellular environment?
UBQ is activated by two UBQ-activating (E1) enzymes through the formation of a high energy Cys–UBQ thioester bond [15], [16]. E1 enzymes are relieved of their UBQ cargo by ≈40 UBQ-conjugating (E2) enzymes through a trans-thiolation reaction [17]. E2 enzymes contain a highly conserved 150–200 AA UBQ-conjugating (UBC) catalytic fold that acts as a scaffold for E1 enzymes, E3 UBQ ligases, and activated UBQ [18]. More than 600 E3 ligases receive UBQ from E2 enzymes and transfer it to substrates through three main mechanisms [19]. Really interesting new gene (RING) E3 ligases directly transfer UBQ from E2–UBQ complexes to RING E3-bound protein substrates [20]. Homologous to the E6AP carboxyl terminus (HECT) E3 ligases first bind UBQ onto a Cys residue of the HECT domain and release E2 enzymes, then bind protein substrates and transfer UBQ to them [21]. RING-in-between-RING (RBR) E3 ligases [22] act through a RING/HECT hybrid mechanism. Mono- and polyUBQ chains can be disassembled by a ≈100-membered class of isopeptide-specific deubiquitinating enzymes (DUBs) [23] that are essential to ensure proper processing of ubiquitinated proteins. Finally, the 26S proteasome complex is the protein degradation terminal for UBQ-tagged proteins in eukaryotes [24]. It is made by a 20S barrel-shaped catalytic core particle (CP) composed by 28 subunits, structurally arranged in four stacked seven membered rings [25], and by two 19S regulatory particles (RPs), composed each by 19 subunits (a 9 subunit lid, and a 10 subunit base structure [26]). The UBQ activation–conjugation–ligation-trimming cycle is extensively described in the biology- oriented companion book [27].
Any E1, E2, E3, and DUB enzyme may be considered a suitable target to restore the UPS activity in an impaired cellular environment. Proteasome activity may also be targeted for a direct effect on UPS. NDDs and tauopathies require the potentiation/restoring of cellular mechanisms leading to the elimination of misfolded/aggregated proteins. UPS inhibition may appear to lead in the opposite direction—decreasing UPS-mediated elimination of tau and other misfolded proteins. Certain enzymes in the UPS system, though, contribute to the elimination of proteins hindering the rescuing/refolding of misfolded proteins. Their inhibition, thus, should be beneficial.
In particular, the carboxy-terminus of Hsp70-interacting protein (CHIP) [28] is an E3 UBQ ligase, due to a U-box domain at its C-terminus [29], and a Hsp70/Hsp90 co-chaperone, due to its three tandem tetratricopeptide (TPR) domains at the N-terminus [30]. CHIP is a key player in cellular management of misfolded proteins. Its role as an Hsp70-dependent, tau-ubiquitinating enzyme has been known for a decade [31], [32]. The ubiquitin-specific protease USP14 is a UBQ-trimming, proteasome-bound DUB that frees and recycles UBQ before substrate protein degradation by the UPS [33]. The role of USP14 in physiological and pathological events of the CNS is well known [34], [35]. The next two sections describe small molecule modulators acting on each selected target.
3.2. CHIP
The role of CHIP in cellular management of misfolded proteins is due to its three N-terminal TPR/chaperone-binding domain repeats, and to its C-terminal U-box/UBQ-binding domain. Modulation of the whole set of CHIP functions would likely impact on many physiological processes. Specificity may be targeted through chaperone-mediated CHIP-misfolded protein interactions (through the TPR domains), aiming to regulate the target/substrate protein clearance (i.e., to increase it in NDDs) [36]. Specificity may also be obtained by targeting CHIP–E2-conjugating enzyme interactions (through the U-box domain), aiming to regulate the clearance of misfolded proteins through the specific CHIP–E2 couple that ubiquitinates a particular target/substrate protein [37].
NDDs often depend on pro-aggregation misfolded proteins. CHIP may contribute to their refolding, through complexation with Hsp90. When either neurotoxic protein oligomers exceed the refolding capacity of neurons, or the PQC machinery is impaired, CHIP may promote the aggregation of protein oligomers into insoluble, less toxic aggregates to be cleared via autophagy (see autophagy and aggrephagy, Chapters 4 and 545 here and in the biology-oriented companion book [27]). Either Hsp90 inhibition or CHIP overexpression increase the amount of protein clearance-directing Hsp70–CHIP complexes. Hsp90 inhibitors are described in Chapter 2, while small molecules capable of promoting CHIP induction in cells are unknown.
Protein regulators of CHIP, capable of orienting the fate of misfolded proteins, are well known. Members of the Bcl-2-associated athanogene co-chaperone family (BAG) may mediate the docking of a CHIP–chaperone–target protein complex at the proteasome, facilitating proteasomal degradation (BAG-1 [38]). They may suppress CHIP-mediated ubiquitination and degradation of a target protein by abrogation of the CHIP–E2 interaction (BAG-2 [39]). They may promote autophagic clearance when complexed with Hsp70 and CHIP in aging and/or protein aggregate-rich tissues (BAG-3 [40]). Finally, they may inhibit CHIP-mediated ubiquitination and degradation of a target protein through unclarified molecular mechanisms (BAG-5) [41]. The Hsp70-binding protein 1 (HspBP1) co-chaperone causes a conformational change in CHIP–chaperone complexes and prevents the ubiquitination of Hsc70-bound client proteins [42]. The S100A2 and S100P proteins inhibit CHIP-mediated ubiquitination and proteasomal degradation in a Ca2+-dependent manner [43]. Stimulation of BAG-1- and BAG-3-promoted effects, and inhibition/prevention of CHIP negative regulation by BAG-2 and BAG-5 co-chaperones would be desirable in NDDs. Unfortunately, limited structural information on CHIP-containing chaperone complexes is available [44] to drive rational drug design projects.
Examples of selective modulation of CHIP–target protein complexes in the presence of Hsp chaperones are scarce [30]. Small molecules acting on TPR-mediated interactions of CHIP with chaperones, co-chaperones, and misfolded proteins are uncommon, and their selectivity is at best questionable. Thioflavin S (3.1a,b, Figure 3.1 , see also 2.45a,b, Figure 2.9 and section 2.3.2) is a mixture of benzothiazolylammonium salts showing sub-μM potency in preventing the Hsc/Hsp70–BAG-1 interaction [45]. BAG-1–CHIP interactions could be modulated by 3.1a,b, although the cellular activity of thioflavin S could be due to Hsc/Hsp70–BAG-1-unrelated mechanisms.
Figure 3.1.
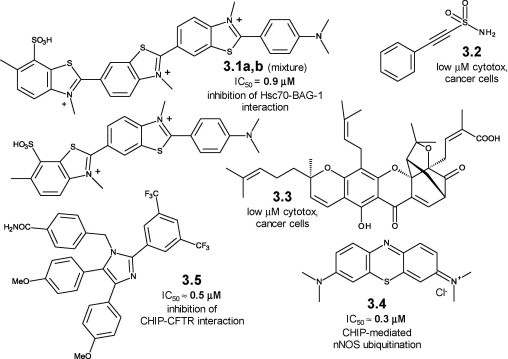
Small molecule modulators of chaperone-dependent CHIP activity: chemical structures, 3.1a–3.5.
The sulfonamide pifithrin-μ (PES, 3.2, see also 2.44, Figure 2.8 and section 2.3.1) shows multiple actions on the Hsp70 machinery [46]. Complexes between Hsp70 and CHIP, BAG-1 and other J-domain proteins are affected by PES. Its multiple and potent anticancer effects may be caused in part by the triple bond chemical reactivity, but probably are not entirely amenable to the Hsp70 chaperone network [46]. Naturally occurring gambogic acid (GA, 3.3) causes the UPS-dependent degradation of mutant p53 [47]. GA decreases the Hsp90–mutant p53 interaction and in parallel increases the levels of the ternary, UPS-oriented Hsp70–CHIP–mutant p53 complex through molecular interactions that are not yet elucidated. UPS-mediated degradation of polyUBQ mutant p53 via selective CHIP ubiquitination may be a GA-driven effect shared by other misfolded proteins [47]. The tricyclic phenothiazine methylene blue (MB, 3.4), currently in clinical trials in AD patients as a tau aggregation inhibitor [48], negatively modulates polyQ protein degradation through Hsp70 binding and subsequent sub-μM inhibition of CHIP-mediated polyQ protein ubiquitination [49]. Its multi-targeted activity profile is discussed in detail in Chapter 6. Imidazole-based apoptozole (3.5, Figure 3.1) restores the defective cellular processing of ΔF508-CFTR (cystic fibrosis transmembrane conductance regulator), a mutant, misfolded protein involved in cystic fibrosis [50]. Its rescuing effect at sub-μM concentrations is likely due to the disruption of the tertiary Hsp70–CHIP–mutant protein complex, and to the prevention of CHIP-mediated ubiquitination and degradation of ΔF508-CFTR [50].
Small molecule modulators of chaperone-independent E3 ligase–target protein complexes are known [51]. Although they do not target CHIP-containing complexes, they indirectly prove the druggability of this protein–protein interaction (PPI) and must be mentioned. Two RING E3 ligases are targeted by candidates in clinical evaluation. The tetrasubstituted imidazolidine nutlin-3 (3.6, Figure 3.2 ) [52] and indole-based serdemetan (3.7) [53] are clinically tested PPI inhibitors/anticancer agents targeting the p53-human double minute 2 (HDM2) PPI [54]. HDM2 is a RING E3 ligase that modulates UPS-mediated degradation of p53, and compounds 3.6 and 3.7 inhibit HDM2-mediated ubiquitination of p53 [55].
Figure 3.2.

Small molecule modulators of chaperone-independent E3 ligase–target protein complexes in clinical trials: chemical structures, 3.6–3.11.
Bicyclic (AT-406, 3.8) [56], thiadiazole-, and thiazole-based monomeric (respectively GDC-0152 [57], 3.9 and LCL-161 [58], 3.10) and dimeric antagonists of inhibitor of apoptosis proteins (IAPs) (TL32711, 3.11, Figure 3.2) [59] are in clinical trials as anticancer agents. Their structure is inspired by the N-terminal AVPI sequence of the second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/DIABLO), an endogenous ligand of IAPs [60]. They act as PPI inhibitors/Smac mimetics/IAP antagonists, preventing the interaction between IAP proteins and caspases [61]. They also bind to RING domain-containing cellular IAPs (cIAPs), activate their E3 ligase activity, and induce their auto-ubiquitination and rapid proteasomal degradation [62].
A few E3 ligase-containing complexes are targeted by small molecules at an earlier development stage. Such molecules are often discovered through high throughput screening (HTS) campaigns and assay formats that detect variations of E3 ligase activity [63]. The S-phase kinase-associated protein/Skp-cullin-F-box-containing (SCF) is the largest multi-protein RING E3 ligase family [64]. Diamino compound A (3.12, Figure 3.3 ) is identified through an HTS campaign targeted against inhibitors of the ubiquitination of p27Kip1 [65]. Enhanced UPS degradation of p27Kip1 is associated with poor prognosis in a variety of tumors, and SCFSkp2 is the E3 ligase that degrades p27Kip1 [66]. Compound A moderately increases p27Kip1 levels in cells through the exclusion of Skp2 from the SCFSkp2 E3 ligase complex, probably by inhibiting its binding with another complex member [65].
Figure 3.3.

Small molecule modulators of chaperone-independent E3 ligase–target protein complexes in preclinical studies: chemical structures, 3.12–3.18.
Inhibitors of SCFSkp2 ligase activity stem from structure-based virtual screening of a 315K compound data set against an Skp2–Csk1–p27 ternary complex [67]. An alkylidene thiazolidine (compound C1, 3.13) reduces Skp2-mediated ubiquitination of p27 in vitro at low μM concentration, and increases p27 levels by decreasing its SCFSkp2-mediated degradation in melanoma cells [67]. The diacid SCF-I2 (3.14) is identified as an inhibitor of SCFCdc4 in an HTS campaign on a 50K member collection [68]. SCF-I2 binds to the WP40 domain of the F-box protein cell division control protein 4 (Cdc4), as shown by the X-ray structure of the Cdc4–Skp1–SCF-12 complex. The conformational change induced in Cdc4 by SCF-I2 allosterically inhibits substrate recognition and ubiquitination by SCFCdc4. Although WP40 domains are shared by F-box proteins in several SCF RING E3 ligases, SCF-I2 appears to be selective against SCFCdc4. SCF-I2 is not active in cellular assays, as its two carboxylates prevent cellular permeability [68]. LS-101 and LS-102 (respectively benzodiazepindione-based 3.15 and triazine-based 3.16) are identified from an ≈4M compound collection in an HTS campaign [69] targeted against the auto-ubiquitination of synoviolin, a RING E3 ligase highly expressed in synoviocytes of patients suffering of rheumatoid arthritis [70]. Both compounds show moderate μM potency against synoviolin auto-ubiquitination. LS-102 shows complete specificity vs. three other RING E3 ligases, while LS-101 is non-selective. Both compounds show in vivo activity in collagene-induced arthritis models [69]. Tetracyclic SMER3 (3.17) is identified in a phenotype-based HTS looking for small molecule enhancers of the therapeutic effects of rapamycin (see also autophagy, Chapter 4 here and in the biology-oriented companion book [27]) [71]. SMER3 inhibits the RING E3 ligase SCFMet30. It binds to the F-box motif in the Met30 protein, and it prevents its interaction with the SKC core protein Skp1. It is selective, as it is completely inactive against SCFCdc4 [71]. Finally, the bis-imide thalidomide (3.18, Figure 3.3) binds to cereblon (CRBN), a component of a RING E3 ligase complex, and inhibits its interaction with damaged DNA binding protein 1 (DDBP1) and Cullin 4 (Cul4) [72]. It inhibits auto-ubiquitination in cells and in vivo, both in zebrafish and chicken models. Teratogenicity of thalidomide is at least partially due to CRBN binding and E3 ligase inhibition [72].
Interactions between CHIP and E2 conjugating enzymes are extensively studied. The X-ray structures of murine CHIP complexed with the E2 enzymes Ubc13 [73], and of zebrafish CHIP and E2D1/UbcH5 [74] are available. The conformational dynamics of the human CHIP–Ubc13 and CHIP–UbcH5 complexes, studied by amide hydrogen exchange mass spectrometry (HX-MS), highlight their differences and suggest that CHIP–E2 complexes in protein ubiquitination and chaperone interaction can be selectively modulated [75]. A systematic study [76] identifies a subset of E2 enzymes that bind CHIP through a common Ser-Pro-Ala motif, and promote target protein ubiquitination via activation of E2–UBQ conjugates. Ubiquitination of target proteins depends on the E2 conjugating enzyme in terms of point of attachment (K48, K63, others) and UBQ chain length [76]. For example, CHIP–UbcH5 preferentially catalyzes the mono-ubiquitination of target proteins through any Lys residue, while CHIP–Ubc13 seems to be a polyUBQ-introducing complex with K63 specificity [77]. Finally, the tertiary E3:E2∼UBQ complexes containing either breast cancer type 1 (BRCA1)/BRCA1–associated RING domain protein (BARD) or E4B/UFD2a as E3 ligases, and UbcH5c as E2 conjugating enzymes are studied by NMR [78]. The study provides valuable information on the role of RING/U-box E3 ligases such as CHIP in facilitating UBQ transfer and in promoting allosteric activation of E2∼UBQ complexes [78]. The wealth of structural information is not yet translated into small molecules as regulators/modulators of CHIP:E2 binary, or CHIP:E2∼UBQ tertiary complexes, and of their functions.
3.3. USP14
USP14 is a member of the largest USP/ubiquitin-specific protease subfamily, which contains ≈60 characterized family members [79], [80]. Several USP enzymes are validated targets against various diseases [81], and CNS diseases in particular [34], [35]. USP DUB inhibitors with varying degrees of inter-class selectivity are known [81], [82], [83]. UBQ analogs capable of specifically and irreversibly inactivating the thiol protease function of USPs include UBQ aldehyde [84] and UBQ vinyl sulfone [85]. They are useful probes to identify and characterize cysteine protease DUBs, but their peptidic nature and aspecificity hinder their use either as such, or as structural models for drug discovery efforts [81].
Aspecific USP inhibitors include electrophilic dienones, resulting from a computational pharmacophoric search on the National Cancer Institute (NCI) chemical database [86]. Curcumin (3.19, Figure 3.4 ), shikoccin (3.20), and Δ12-PGJ2 (3.21) are cytotoxic compounds whose cellular activity is at least partially due to DUB inhibition [86]. Δ12-PGJ2 is the most potent representative among electrophilic prostaglandins [87], whose activity against UCH DUBs is also reported [88]. Curcumin is covered in detail for its anti-aggregating properties on amyloidogenic peptides in Chapter 6 here and in the biology-oriented companion book [27].
Figure 3.4.
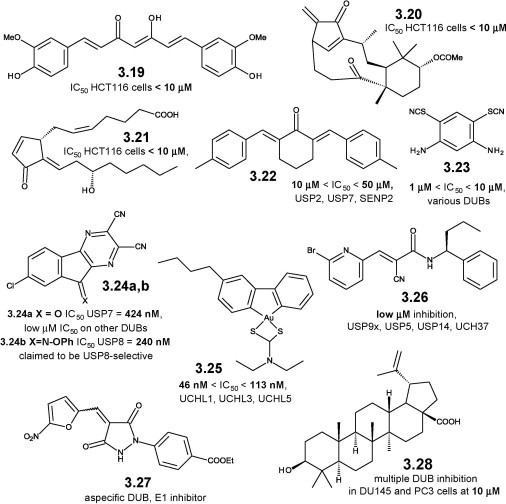
Small molecule inhibitors of USP14 and other deubiquitinases: chemical structures, 3.19–3.28.
The dienone NSC 632839 (3.22) shows similar, aspecific DUB inhibition [89]. Bis-isothiocyanate PR-619 (3.23) is an aspecific DUB inhibitor isolated from a pan-DUB-targeted HTS campaign [90]. It causes protein aggregation in neuronal cells and stabilizes microtubules (MTs), possibly with some effects on tau [91].
The tricyclic dinitrile HBX-41,108 (3.24a) results from structural optimization of hits from an HTS campaign targeted against the USP family member USP7 [92]. It stabilizes p53 and induces p53-dependent apoptosis in cancer cells through inhibition of the p53-deubiquitinating enzyme USP7 [92]. It inhibits at least five other USPs, and an UCH DUB family member [93]. Compound 3.24b is reported as a selective USP8 inhibitor [82], [94] but its structural similarity with 3.24a induces to suspect a limited selectivity against other DUBs. Gold complexes such as 3.25 potently inhibit DUBs and are endowed with cytotoxic activity [95].
The cyanoamide WP-1130 (3.26) is a member of a synthetic tyrphostin-like library [96]. It is active in a cell-based screen targeted towards the Janus-activated kinase (JAK)/signal transducer and activator of transcription (STAT) pathway [97]. WP-1130 is a partially selective, cell permeable USP inhibitor, active against USP5, USP9x, USP14, UCH-L1, and UCH37 [97]. It shows pro-apototic effects through up-regulation of p53 and down-regulation of myeloid cell leukemia sequence 1 (MCL-1) levels, and promotes aggresome formation in cancer cells [97]. The cellular effects of WP-1130 are due to inhibition of the unknown DUB responsible for JAK-2 deubiquitination [98]. It also enhances bacterial killing via localization of inducible nitric oxide synthase (iNOS) to the macrophage phagosome [99], and shows antiviral activity through USP14-mediated induction of the unfolded protein response (UPR) [100].
The alkyliden-pyrazolidindione PYR-41 (3.27), originally reported as a selective and irreversible E1 UBQ-activating enzyme [101], inhibits several DUBs, and even unrelated Cys-containing enzymes, through displacement of their nitro function by Cys residues [102]. Betulinic acid (3.28, Figure 3.4) shows multiple DUB-inhibiting activities and cytotoxicity on proliferating cancer cells, while it does not have similar effects on normal cells [103]. This may be due to a general increase of DUB levels in proliferating vs. non-proliferating cells, or to partially selective inhibition by betulinic acid of a subset of DUBs that are highly overexpressed/much more active in cancer cells [103].
A chalcone-based library contains cytotoxic, UPS-inhibiting representatives [104]. Some of its members, such as RA-9 (3.29, Figure 3.5 ), are partially selective DUB inhibitors, inhibiting >50% overall DUB activity in cervical cancer HeLa cells at 10 μM [105]. UPS2, UPS5, UPS8, UCH-L1, and UCH-L3 are among the DUBs inhibited by RA-9, while USP7 is not affected. RA-9 and its analogs induce polyUBQ accumulation, deplete the free UBQ pool, and promote apoptosis in cancer cells, while being non-toxic to normal cells [105].
Figure 3.5.
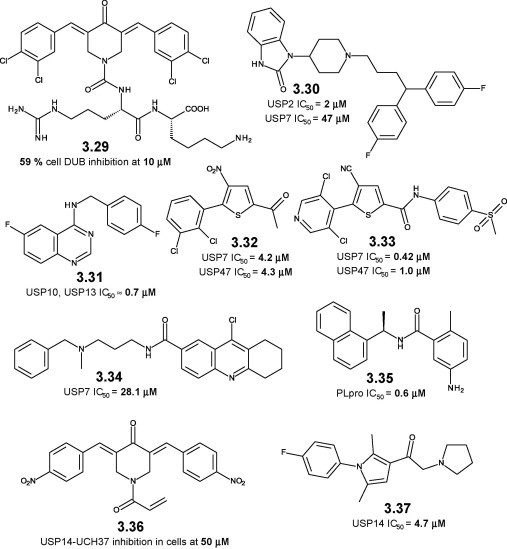
Small molecule inhibitors of USP14 and other deubiquitinases: chemical structures, 3.29–3.37.
The anti-psychotic, phthalimide-based pimozide (3.30) is a selective, allosteric, low μM inhibitor of the DUB enzyme USP1–USP1 associated factor 1 (UAF1) complex [106]. Pimozide weakly inhibits USP7, and is selective against a wide set of cysteine proteases. It restores cisplatin sensitivity to cisplatin-resistant small lung cancer cells [106]. A phenotype screen aimed towards autophagy inhibitors identifies the specific and potent autophagy inhibitor-1 (spautin-1, 3.31) [107]. Spautin-1 is a selective nM inhibitor of USP10 and USP13. USP10 and USP13 are the DUBs acting on beclin-1, a key component of the autophagy-regulating kinase vacuolar protein sorting 34 (Vps34) complex (see also autophagy, Chapter 4 here and in the biology-oriented companion book [27]). Conversely, beclin-1 and the Vsp34 complex regulate the levels of USP10 and USP13. Spautin-1-caused autophagy inhibition increases p53 levels and may represent a novel anticancer approach [107].
Thiophene-based P5091 (3.32) is a USP7–USP47-selective DUB inhibitor with low μM potency identified in a pan-DUB-targeted HTS campaign [90]. P5091 increases ubiquitinated HDM2, which is then degraded and leads to p53-mediated cytotoxicity in cancer cells [108]. P5091 restores sensitivity to bortezomib-resistant cancer cells, and shows synergistic effects when combined with proteasome and histone deacetylase (HDAC) inhibitors [108]. A structurally related analog (3.33) is more potent in cellular assays, causing an increase in p53 levels and an induction of p21 [109]. The aminotetrahydroacridine HBX-19,818 (3.34) is a cell permeable, selective, moderately potent USP7 inhibitor identified through an HTS campaign [93]. HDM2-p53 regulation and subsequent cytotoxicity are observed in human colon carcinoma (HCT116) cancer cells [93].
The naphthylamide GRL0617 (3.35) is an nM, non-covalent inhibitor of the papain-like protease (PLpro) from the severe acute respiratory syndrome (SARS)-causing coronavirus [110]. PLpro acts as a DUB, and the antiviral properties of GRL0617 (the result of structural optimization on an HTS hit) stem from inhibition of the deubiquitinase activity of PLpro. GRL0617 does not inhibit human DUBs, and its specificity is explained by the X-ray structure of the PLpro–GRL0617 complex [110].
Two USP14-targeted small molecule inhibitors are known. The electrophilic dienone NSC687852/b-AP15 (3.36) is a pro-apoptotic compound identified in an HTS campaign on an NCI chemical collection [111]. It induces caspase-dependent apoptosis, and increases the levels of polyUBQ proteins through DUB inhibition [112]. b-AP15 selectively inhibits with moderate potency two structurally unrelated, proteasomal DUBs, USP14 and UCH37, probably because of their common association with the proteasome [113]. It shows in vivo potency in animal models of leukemia, colon, lung, and breast carcinoma [113], [114]. Pyrrole-based IU1 (3.37, Figure 3.5) is a selective, low μM inhibitor of USP14. It enhances in vitro UBQ–transactive response/TAR DNA binding protein 43 (TDP-43), UBQ– ataxin-3, and UBQ–tau levels, and increases their UPS-mediated proteolysis in murine embryonic fibroblasts (MEFs) [115]. It shows antiviral properties against Dengue virus, most likely through UPS enhancement [116]. The tau- and ataxin-3-regulating role of USP14 (and consequently the putative therapeutic effect of IU1) is questionable [117], but a compensatory increase of USP14 activity is observed in elderly cells [118].
Additional basic studies and potent, selective compounds are needed to elucidate the potential of “clean” and “mixed” USP14 inhibitors against neurodegeneration, as even small selectivity profile changes may induce major alterations in cellular effects. For example, USP14–UCH37-targeted bAP15 regulates caspase-1 activation and interleukin (IL)-1 release in an inflammation model, while IU1, a “clean” USP14 inhibitor, is inactive in the same model [119]. The existence of an additional, unknown DUB target for bAP15 cannot be ruled out, but the importance of cell-permeable DUB inhibitors with finely tuned poly-DUB pharmacology is evident. The available information regarding the structure of DUBs [79], and in particular the crystal structure of the 45 kDa catalytic domain of USP14 in isolation and complexed with UBQ aldehyde [120], should assist the rational design and synthesis of USP14 inhibitors with varying selectivity profiles.
3.4. Recap
This chapter deals with small molecule modulators of neuropathological alterations caused by protein misfolding and aggregation in general, and by tau and/or tau-connected events in particular. A potential therapeutic mechanism was examined in detail in the biology-oriented companion book [27], and two targets were arbitrarily chosen. Thirty-eight scaffolds shown in Figure 3.1, Figure 3.2, Figure 3.3, Figure 3.4, Figure 3.5, acting on selected targets, are described in detail in this chapter, and are briefly summarized in Table 3.1 . The chemical core of each scaffold/compound is structurally defined; its molecular target is mentioned; the developing laboratory (either public or private) is listed; and the development status—according to publicly available information—is finally provided.
Table 3.1.
Compounds 3.1–3.37: Chemical Class, Target, Developing Organization, Development Status
| Number | Chemical cpd./class | Target | Organization | Dev. status |
|---|---|---|---|---|
| 3.1a,b | Thioflavin S | Hsp70–BAG-1 | Cancer Research, UK | DD |
| 3.2 | Pifithrin-m, PES | Hsp70, plus others | University of Pennsylvania | LO |
| 3.3 | Gambogic acid, GA | Hsp70–, Hsp90–CHIP regulation | Jangsu University, China | TM |
| 3.4 | Methylene blue, MB | Hsp70–CHIP regulation | TauRX Therapeutics | Ph III |
| 3.5 | Apoptozole | Hsp70, ATPase inhib. | Yonsei University, South Korea | LO |
| 3.6 | Nutlin-3 | HDM2–p53 | Roche | Ph I |
| 3.7 | Serdemetan | HDM2–p53 | Johnson & Johnson | Ph I |
| 3.8 | AT-406 | IAPs | Ascenta | Ph I |
| 3.9 | GDC-0152 | IAPs | Genentech | Ph I |
| 3.10 | LCL-161 | IAPs | Novartis | Ph II |
| 3.11 | TL32711 | IAPs | Tetralogic | Ph II |
| 3.12 | Diamines, compound A | Skp2 | University of North Carolina | DD |
| 3.13 | Alkylidene thiazolidines, compound C1 | Skp2 | NY University | DD |
| 3.14 | Diacids, SKP-I2 | Cdc4 | Mount Sinai Hosp., Toronto, Canada | DD |
| 3.15 | Benzodiazepindiones, LS-101 | Synoviolin | Tokyo Medical Univ. | LO |
| 3.16 | Triazines, LS-102 | RING E3 ligases | Tokyo Medical Univ. | LO |
| 3.17 | Tetracycles, SMER3 | Met30 | UCLA | LO |
| 3.18 | Thalidomide | Cereblon | Tokyo Institute of Technology | Ph III |
| 3.19 | Curcumin | Pan-DUB inhibition | University of Utah | TM |
| 3.20 | Shikoccin | Pan-DUB inhibition | University of Utah | DD |
| 3.21 | Δ12-PGJ2 | Pan-DUB inhibition | Karolinska Institute | DD |
| 3.22 | Dienones, NSC 632839 | Pan-DUB inhibition | Progenra | DD |
| 3.23 | Bis-isothiocyanate, PR-419 | Pan-DUB inhibition | Oldenburg University, Germany | DD |
| 3.24a,b | Tricyclic dinitriles, HBX-41,108 (3.24a) | USP DUBs | Hybrigenics | LO |
| 3.25 | Gold complexes | Pan-DUB inhibition | University of Hong Kong | DD |
| 3.26 | Tyrposthin-like WP-1130 | USP5, USP9x, USP14, UCH-L1, UCH37 | University of Michigan | LO |
| 3.27 | Alkyliden-pyrazolidindiones, PYR41 | Cys DUBs | University of Michigan | DD |
| 3.28 | Betulinic acid | Pan-DUB inhibition | University of Miami | PE |
| 3.29 | Chalcones, RA-9 | UPS2, UPS5, UPS8, UCH-L1, UCH-L3 | University of Minnesota | DD |
| 3.30 | Phthalimide based, pimozide | UAF1, USP7 | University of Delaware | DD |
| 3.31 | Spautin-1 | USP10, USP13 | Chinese Academy of Sciences | DD |
| 3.32 | Thiophene based, P5091 | USP7, USP47 | Harvard Med. School | LO |
| 3.33 | Thiophene based | USP7, USP47 | Progenra | LO |
| 3.34 | Aminotetrahydroacridines, HBX-19,818 | USP7 | Hybrigenics | Ph I |
| 3.35 | Naphthylamides, GRL0617 | PLpro | University of Illinois | LO |
| 3.36 | Electrophilic dienones, NSC687852/b-AP15 | USP14 | Karolinska Institute | LO |
| 3.37 | Pyrrole based, IU1 | USP14 | Harvard Med. School | LO |
Not progressed, NP; early discovery, DD; lead optimization, LO; preclinical evaluation, PE; clinical Phase I-II-III, Ph I–Ph III; marketed, MKTD; traditional medicine, TM. Please note that the most advanced status for NDD-targeted experiments is listed: for example, candidates in clinical trials for non-CNS indications with early in vitro characterization against proteinopathies/tauopathies are classified as DD.
References
- 1.Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Weissman A.M., Shabek N., Ciechanover A. The predator becomes the prey: regulating the ubiquitin system by ubiquitylation and degradation. Nat. Rev. Mol. Cell Biol. 2011;12:605–620. doi: 10.1038/nrm3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein G., Scheid M., Hammerling U., Boyse E.A., Schlesinger D.H., Niall H.D. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc. Natl. Acad. Sci. U.S.A. 1975;72:11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vijay-Kumar S., Bugg C.E., Cook W.J. Structure of ubiquitin refined at 1.8 A resolution. J. Mol. Biol. 1987;194:531–544. doi: 10.1016/0022-2836(87)90679-6. [DOI] [PubMed] [Google Scholar]
- 5.Hoege C., Pfander B., Moldovan G.L., Pyrowolakis G., Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 6.Carter S., Bischof O., Dejean A., Vousden K.H. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat. Cell Biol. 2007;9:428–435. doi: 10.1038/ncb1562. [DOI] [PubMed] [Google Scholar]
- 7.Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P.P., Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 8.Komander D., Rape R. The ubiquitin code. Annu. Rev. Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 9.Cook W.J., Jeffrey L.C., Carson M., Chen Z., Pickart C.M. Structure of a diubiquitin conjugate and a model for interaction with ubiquitin conjugating enzyme (E2) J. Biol. Chem. 1992;267:16467–16471. doi: 10.2210/pdb1aar/pdb. [DOI] [PubMed] [Google Scholar]
- 10.Varadan R., Assfalg M., Haririnia A., Raasi S., Pickart C., Fushman D. Solution conformation of Lys63-linked di-ubiquitin chain provides clues to functional diversity of polyubiquitin signaling. J. Biol. Chem. 2004;279:7055–7063. doi: 10.1074/jbc.M309184200. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser S.E., Riley B.E., Shaler T.A., Trevino R.S., Becker C.H., Schulman H., Kopito R.R. Protein standard absolute quantification (PSAQ) method for the measurement of cellular ubiquitin pools. Nat. Methods. 2011;8:691–696. doi: 10.1038/nmeth.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim W., Bennett E.J., Huttlin E.L., Guo A., Li J., Possemato A. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finley D. Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Mol. Cell. Biol. 1994;14:5501–5509. doi: 10.1128/mcb.14.8.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni M., Smith H.E. E1 ubiquitin-activating enzyme UBA-1 plays multiple roles throughout C. elegans development. PLoS Genet. 2008;4:e1000131. doi: 10.1371/journal.pgen.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavin J.M., Chen J.J., Liao H., Rollins N., Yang X., Xu Q. Mechanistic studies on activation of ubiquitin and di-ubiquitin-like protein, FAT10, by ubiquitin-like modifier activating enzyme 6, Uba6. J. Biol. Chem. 2012;287:15512–15522. doi: 10.1074/jbc.M111.336198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedford L., Lowe J., Dick L.R., Mayer R.J., Brownell J.E. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat. Rev. Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burroughs A.M., Jaffee M., Iyer L.M., Aravind L. Anatomy of the E2 ligase fold: implications for enzymology and evolution of ubiquitin/Ub-like protein conjugation. J. Struct. Biol. 2008;162:205–218. doi: 10.1016/j.jsb.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W., Bengtson M.H., Ulbrich A., Matsuda A., Reddy V.A., Orth A. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS ONE. 2008;3:e1487. doi: 10.1371/journal.pone.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budhidarmo R., Nakatani Y., Day C.L. RINGs hold the key to ubiquitin transfer. Trends Biochem. Sci. 2012;37:58–65. doi: 10.1016/j.tibs.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Rotin D., Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell. Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 22.Wenzel D.M., Klevit R.E. Following Ariadne’s thread: a new perspective on RBR ubiquitin ligases. BMC Biol. 2012;10:24. doi: 10.1186/1741-7007-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraile J.M., Quesada V., Rodrıguez D., Freije J.M.P., Lopez-Otın C. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene. 2012;31:2373–2388. doi: 10.1038/onc.2011.443. [DOI] [PubMed] [Google Scholar]
- 24.Gallastegui N., Groll M. The 26S proteasome: assembly and function of a destructive machine. Trends Biochem. Sci. 2010;35:634–642. doi: 10.1016/j.tibs.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Groll M., Dtizel L., Lowe J., Stock D., Bochtler M., Wolf D.H., Huber R. Structure of the 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 26.Glickman M.H., Rubin D.M., Coux O., Wefes I., Pfeifer G., Cjeka Z. A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell. 1998;94:615–623. doi: 10.1016/s0092-8674(00)81603-7. [DOI] [PubMed] [Google Scholar]
- 27.Seneci P. Academic Press; 2014,. Protein misfolding and neurodegenerative diseases: Focus on disease-modifying targets. 278 pages. [Google Scholar]
- 28.Ballinger C.A., Connell P., Wu Y., Hu Z., Thompson L.J., Yin L.Y., Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murata S., Minami Y., Minami M., Chiba T., Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2:1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connell P., Ballinger C.A., Jiang J., Wu Y., Thompson L.J., Hohfeld J., Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 31.Petrucelli L., Dickson D., Kehoe K., Taylor J., Snyder H., Grover A. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Human Mol. Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 32.Hatakeyama S., Matsumoto M., Kamura T., Murayama M., Chui D.-H., Planel E. U-box protein carboxyl terminus of Hsc70-interacting protein (CHIP) mediates polyUbiquitylation preferentially on four-repeat Tau and is involved in neurodegeneration of tauopathy. J. Neurochem. 2004;91:299–307. doi: 10.1111/j.1471-4159.2004.02713.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu C.-W., Jacobson A.D. Functions of the 19S complex in proteasomal degradation. Trends Biochem. Sci. 2013;38:103–110. doi: 10.1016/j.tibs.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todi S.V., Paulson H.L. Balancing act: deubiquitinating enzymes in the nervous system. Tr. Neurosci. 2011;34:370–382. doi: 10.1016/j.tins.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowalski J.R., Juo P. The role of deubiquitinating enzymes in synaptic function and nervous system diseases. Neural Plastic. 2012;2012:892749. doi: 10.1155/2012/892749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook C., Petrucelli L. Tau triage decisions mediated by the chaperone network. J. Alzheimer’s Dis. 2012;30:1–7. doi: 10.3233/JAD-2012-129008. [DOI] [PubMed] [Google Scholar]
- 37.Deshaies R.J., Joazeiro C.A.P. Ring domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 38.Alberti S., Demand J., Esser C., Emmerich N., Schild H., Hohfeld J. Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J. Biol. Chem. 2002;277:45920–45927. doi: 10.1074/jbc.M204196200. [DOI] [PubMed] [Google Scholar]
- 39.Arndt V., Daniel C., Nastainczyk W., Alberti S., Höhfeld J. BAG-2 acts as an inhibitor of the chaperone-associated ubiquitin ligase CHIP. Mol. Biol. Cell. 2005;16:5891–5900. doi: 10.1091/mbc.E05-07-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gamerdinger M., Carra S., Behl C. Emerging roles of molecular chaperones and co-chaperones in selective autophagy: focus on BAG proteins. J. Mol. Med. 2011;89:1175–1182. doi: 10.1007/s00109-011-0795-6. [DOI] [PubMed] [Google Scholar]
- 41.Kalia L.V., Kalia S.K., Chau H., Lozano A.M., Hyman B.T., McLean P.J. Ubiquitinylation of α-synuclein by carboxyl terminus Hsp70-interacting protein (CHIP) is regulated by Bcl-2-associated athanogene 5 (BAG5) PLoS ONE. 2011;6:e14695. doi: 10.1371/journal.pone.0014695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alberti S., Böhse K., Arndt V., Schmitz A., Höhfeld J. The cochaperone HspBP1 inhibits the CHIP ubiquitin ligase and stimulates the maturation of the cystic fibrosis transmembrane conductance regulator. Mol. Biol. Cell. 2004;15:4003–4010. doi: 10.1091/mbc.E04-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimamoto S., Kubota Y., Yamaguchi F., Tokumitsu H., Kobayashi R. Ca2+/S100 proteins act as upstream regulators of the chaperone-associated ubiquitin ligase CHIP (C Terminus of Hsc70-interacting Protein) J. Biol. Chem. 2013;288:7158–7168. doi: 10.1074/jbc.M112.436758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cichero E., Basile A., Turco M.C., Mazzei M., Fossa P. Scouting new molecular targets for CFTR therapy: the HSC70/BAG-1 complex. A computational study. Med. Chem. Res. 2012;21:4430–4436. [Google Scholar]
- 45.Sharp A., Crabb S.J., Johnson P.W.M., Hague A., Cutress R., Townsend P.A. Thioflavin S (NSC71948) interferes with Bcl-2-associated athanogene (BAG-1)-mediated protein-protein interactions. J. Pharmacol. Exp. Ther. 2009;331:680–689. doi: 10.1124/jpet.109.153601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leu J.I., Pimkina J., Frank A., Murphy M.E., George D.L. A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell. 2009;36:15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J., Zhao Q., Qi Q., Gu H.Y., Rong J.J., Mu R. Gambogic acid-induced degradation of mutant p53 is mediated by proteasome and related to CHIP. J. Cell. Biochem. 2011;112:509–519. doi: 10.1002/jcb.22941. [DOI] [PubMed] [Google Scholar]
- 48.Wischik, C. TauRX Therapeutics, Sept 10th, 2012. Press release announcing the initiation of a global Phase 3 clinical trial in a type of Frontotemporal Dementia (FTD) also known as Pick’s Disease.
- 49.Wang A.M., Morishima Y., Clapp K.M., Peng H.-M., Pratt W.B., Gestwicki J.E. Inhibition of Hsp70 by methylene blue affects signaling protein function and ubiquitination and modulates polyglutamine protein degradation. J. Biol. Chem. 2010;285:15714–15723. doi: 10.1074/jbc.M109.098806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho H.J., Gee H.Y., Baek K.-H., Ko S.-K., Park J.-M., Lee H. A small molecule that binds to an ATPase domain of Hsc70 promotes membrane trafficking of mutant cystic fibrosis transmembrane conductance regulator. J. Am. Chem. Soc. 2011;133:20267–20276. doi: 10.1021/ja206762p. [DOI] [PubMed] [Google Scholar]
- 51.Wang L., Liu Y.T., Hao R., Chen L., Chang Z., Wang H.R. Molecular mechanism of the negative regulation of Smad1/5 protein by carboxyl terminus of Hsc70-interacting protein (CHIP) J. Biol. Chem. 2011;286:15883–15894. doi: 10.1074/jbc.M110.201814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tovar C., Rosinski J., Filipovic Z., Higgins B., Kolinsky K., Hilton H. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: Implications for therapy. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabernero J., Dirix L., Schoffski P., Cervantes A., Lopez-Martin J.A., Capdevila J. A Phase I first-in-human pharmacokinetic and pharmacodynamic study of serdemetan in patients with advanced solid tumors. Clin. Cancer Res. 2011;17:6313–6321. doi: 10.1158/1078-0432.CCR-11-1101. [DOI] [PubMed] [Google Scholar]
- 54.Millard M., Pathania D., Grande F., Xu S., Neamati N. Small molecule inhibitors of p53-MDM2 interaction: the 2006-2010 update. Curr. Pharm. Des. 2011;17:536–559. doi: 10.2174/138161211795222649. [DOI] [PubMed] [Google Scholar]
- 55.Haupt Y., Maya R., Kazaz A., Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 56.Cai Q., Sun H., Peng Y., Lu J., Nikolovska-Coleska Z., McEachern D. A potent and orally active antagonist (SM-406/AT-406) of multiple Inhibitor of Apoptosis Proteins (IAPs) in clinical development for cancer treatment. J. Med. Chem. 2011;54:2714–2726. doi: 10.1021/jm101505d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flygare J.A., Beresini M., Budha N., Chan H., Chan I.T., Cheeti S. Discovery of a potent small-molecule antagonist of inhibitor of apoptosis (IAP) proteins and clinical candidate for the treatment of cancer (GDC-0152) J. Med. Chem. 2012;55:4101–4113. doi: 10.1021/jm300060k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weisberg E., Ray A., Barrett R., Nelson E., Christie A.L., Porter D. Smac mimetics: implications for enhancement of targeted therapies in leukemia. Leukemia. 2011;24:2100–2109. doi: 10.1038/leu.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Condon, S.M.; Deng, Y.; LaPorte, M.G.; Rippin, S.R. Smac mimetic. US 2011/0003877 A1.
- 60.Wu G., Chai J., Suber T.L., Wu J.-W., Du C., Wang X., Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 61.Sun H., Nikolovska-Coleska Z., Lu J., Qiu S., Yang C.-Y., Gao W. Design, synthesis, and evaluation of a potent, cell-permeable, conformationally constrained second mitochondria derived activator of caspase (Smac) mimetic. J. Med. Chem. 2006;49:7916–7920. doi: 10.1021/jm061108d. [DOI] [PubMed] [Google Scholar]
- 62.Varfolomeev E., Blankenship J.W., Wayson S.M., Fedorova A.V., Kayagaki N., Garg P. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 63.Goldenberg S.J., Marblestone J.G., Mattern M.R., Nicholson B. Strategies for the identification of ubiquitin ligase inhibitors. Biochem. Soc. Trans. 2010;38:132–136. doi: 10.1042/BST0380132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakayama K.I., Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin. Cell. Dev. Biol. 2005;16:323–333. doi: 10.1016/j.semcdb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Chen Q., Xie W., Kuhn D.J., Voorhees P.M., Lopez-Girona A., Mendy D. Targeting the p27 E3 ligase SCFSkp2 results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood. 2008;111:4690–4699. doi: 10.1182/blood-2007-09-112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Filipits M., Pohl G., Stranzl T., Kaufmann H., Ackermann J., Gisslinger H. Low p27Kip1 expression is an independent adverse prognostic factor in patients with multiple myeloma. Clin. Cancer Res. 2003;9:820–826. [PubMed] [Google Scholar]
- 67.Wu L., Grigoryan A.V., Li Y., Hao B., Pagano M., Cardozo T.J. Specific small molecule inhibitors of Skp2-mediated p27 degradation. Chem. Biol. 2012;19:1515–1524. doi: 10.1016/j.chembiol.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orlicky S., Tang X., Neduva V., Elowe N., Brown E.D., Sicheri F., Tyers M. An allosteric inhibitor of substrate recognition by the SCF(Cdc4) ubiquitin ligase. Nat. Biotechnol. 2010;28:733–737. doi: 10.1038/nbt.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yagishita N., Aratani S., Leach C., Amano T., Yamano Y., Nakatani K. RING-finger type E3 ubiquitin ligase inhibitors as novel candidates for the treatment of rheumatoid arthritis. Int. J. Mol. Med. 2012;30:1281–1286. doi: 10.3892/ijmm.2012.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amano T., Yamasaki S., Yagishita N., Tsuchimochi K., Shin H., Kawahara K.-I. Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev. 2003;17:2436–2449. doi: 10.1101/gad.1096603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aghajan M., Jonai N., Flick K., Fu F., Luo M., Cai X. Chemical genetics screen for enhancers of rapamycin identifies a specific inhibitor of an SCF family E3 ubiquitin ligase. Nat. Biotech. 2010;28:738–742. doi: 10.1038/nbt.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ito T., Ando H., Suzuki T., Ogura T., Hotta K., Imamura Y. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 73.Zhang M., Windheim M., Roe S.M., Peggie M., Cohen P., Prodromou C., Pearl L.H. Chaperoned ubiquitylation-crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol. Cell. 2005;20:525–538. doi: 10.1016/j.molcel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 74.Xu Z., Kohli E., Devlin K.I., Bold M., Nix J.C., Misra S. Interactions between the quality control ubiquitin ligase CHIP and ubiquitin conjugating enzymes. BMC Struct. Biol. 2008;8:26. doi: 10.1186/1472-6807-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graf C., Stankiewicz M., Nikolay R., Mayer M.P. Insights into the conformational dynamics of the E3 ubiquitin ligase CHIP in complex with chaperones and E2 enzymes. Biochemistry. 2010;49:2121–2129. doi: 10.1021/bi901829f. [DOI] [PubMed] [Google Scholar]
- 76.Soss S.E., Yue Y., Dhe-Paganon S., Chazin W.J. E2 conjugating enzyme selectivity and requirements for function of the E3 ubiquitin ligase CHIP. J. Biol. Chem. 2011;286:21277–21286. doi: 10.1074/jbc.M111.224006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Windheim M., Peggie M., Cohen P. Two different classes of E2 ubiquitin-conjugating enzymes are required for the monoubiquitination of proteins and elongation by polyubiquitin chains with a specific topology. Biochem. J. 2008;409:723–729. doi: 10.1042/BJ20071338. [DOI] [PubMed] [Google Scholar]
- 78.Pruneda J.N., Littlefield P.J., Soss S.E., Nordquist K.A., Chazin W.J., Brzovic P.S., Klevit R.E. Structure of an E3:E2∼Ub complex reveals an allosteric mechanism shared among RING/U-box ligases. Mol. Cell. 2012;47:933–942. doi: 10.1016/j.molcel.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Komander D., Clague M.J., Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 80.Faesen A.C., Luna-Vargas M.P.A., Sixma T.K. The role of UBL domains in ubiquitin-specific proteases. Biochem. Soc. Trans. 2012;40:539–545. doi: 10.1042/BST20120004. [DOI] [PubMed] [Google Scholar]
- 81.Daviet L., Colland F. Targeting ubiquitin specific proteases for drug discovery. Biochimie. 2008;90:270–283. doi: 10.1016/j.biochi.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 82.Colland F. The therapeutic potential of deubiquitinating enzyme inhibitors. Biochem. Soc. Trans. 2010;38:137–143. doi: 10.1042/BST0380137. [DOI] [PubMed] [Google Scholar]
- 83.Mattern M.R., Wu J., Nicholson B. Ubiquitin-based anticancer therapy: Carpet bombing with proteasome inhibitors vs surgical strikes with E1, E2, E3, or DUB inhibitors. Biochim. Biophys. Acta. 1823;2012:2014–2021. doi: 10.1016/j.bbamcr.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marchenko N.D., Wolff S., Erster S., Becker K., Moll U.M. Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J. 2007;26:923–934. doi: 10.1038/sj.emboj.7601560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ovaa H., Kessler B.M., Rolen U., Galardy P.J., Ploegh H.L., Masucci M.G. Activity-based ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2253–2258. doi: 10.1073/pnas.0308411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mullally J.E., Fitzpatrick F.A. Pharmacophore model for novel inhibitors of ubiquitin isopeptidases that induce p53-independent cell death. Mol. Pharmacol. 2002;62:351–358. doi: 10.1124/mol.62.2.351. [DOI] [PubMed] [Google Scholar]
- 87.Mullally J.E., Moos P.J., Edes K., Fitzpatrick F.A. Cyclopentenone prostaglandins of the J series inhibit the ubiquitin isopeptidase activity of the proteasome pathway. J. Biol. Chem. 2001;276:30366–30373. doi: 10.1074/jbc.M102198200. [DOI] [PubMed] [Google Scholar]
- 88.Li Z., Melandri F., Berdo I., Jansen M., Hunter L., Wright S. Delta12-Prostaglandin J2 inhibits the ubiquitin hydrolase UCH-L1 and elicits ubiquitin-protein aggregation without proteasome inhibition. Biochem. Biophys. Res. Commun. 2004;319:1171–1180. doi: 10.1016/j.bbrc.2004.05.098. [DOI] [PubMed] [Google Scholar]
- 89.Nicholson B., Leach C.A., Goldenberg S.J., Francis D.M., Kodrasov M.P., Tian X. Characterization of ubiquitin and ubiquitin-like-protein isopeptidase activities. Prot. Sci. 2008;17:1035–1043. doi: 10.1110/ps.083450408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Altun M., Kramer H.B., Willems L.I., McDermott J.L., Leach C.A., Goldenberg S.J. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem. Biol. 2011;18:1401–1412. doi: 10.1016/j.chembiol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 91.Seiberlich V., Goldbaum O., Zhukareva V., Richter-Landsberg C. The small molecule inhibitor PR-619 of deubiquitinating enzymes affects the microtubule network and causes protein aggregate formation in neural cells: Implications for neurodegenerative diseases. Biochim. Biophys. Acta. 1823;2012:2057–2068. doi: 10.1016/j.bbamcr.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 92.Colland F., Formstecher E., Jacq X., Reverdy C., Planquette C., Conrath S. Small molecule inhibitor of USP7/HAUSP ubiquitin protease stabilizes and activates p53 in cells. Mol. Cancer Ther. 2009;8:2286–2295. doi: 10.1158/1535-7163.MCT-09-0097. [DOI] [PubMed] [Google Scholar]
- 93.Reverdy C., Conrath S., Lopez R., Planquette C., Atmanene C., Collura V. Discovery of specific inhibitors of human USP7/HAUSP deubiquitinating enzyme. Chem. Biol. 2012;19:467–477. doi: 10.1016/j.chembiol.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Colombo M., Vallese S., Peretto I., Jacq X., Rain J.-C., Colland F., Guedat P. Synthesis and biological evaluation of 9-oxo-9H-indeno[1,2-b]pyrazine-2,3-dicarbonitrile analogues as potential inhibitors of deubiquitinating enzymes. ChemMedChem. 2010;5:552–558. doi: 10.1002/cmdc.200900409. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J.-J., Ng K.-M., Lok C.-N., Wai-Yin Sun R., Che C.M. Deubiquitinases as potential anti-cancer targets for gold(III) complexes. Chem. Commun. 2013;49:5153–5155. doi: 10.1039/c3cc41766b. [DOI] [PubMed] [Google Scholar]
- 96.Peng Z., Pal A., Han D., Wang S., Maxwell D., Levitzki A. Tyrphostin-like compounds with ubiquitin modulatory activity as possible therapeutic agents for multiple myeloma. Bioorg. Med. Chem. 2011;19:7194–7204. doi: 10.1016/j.bmc.2011.09.057. [DOI] [PubMed] [Google Scholar]
- 97.Kapuria V., Peterson L.F., Fang D., Bornmann W.G., Talpaz M., Donato N.J. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010;70:9265–9276. doi: 10.1158/0008-5472.CAN-10-1530. [DOI] [PubMed] [Google Scholar]
- 98.Kapuria V., Levitzki A., Bornmann W.G., Maxwell D., Priebe W., Sorenson R.J. A novel small molecule deubiquitinase inhibitor blocks Jak2 signaling through Jak2 ubiquitination. Cell. Signalling. 2011;23:2076–2085. doi: 10.1016/j.cellsig.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Burkholder K.M., Perry J.W., Wobus C.E., Donato N.J., Showalter H.D., Kapuria V., O’Riordan M.X.D. A small molecule deubiquitinase inhibitor increases localization of inducible nitric oxide synthase to the macrophage phagosome and enhances bacterial killing. Infect. Immun. 2011;79:4850–4857. doi: 10.1128/IAI.05456-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perry J.W., Ahmed M., Chang K.-O., Donato N.J., Showalter H.D., Wobus C.E. Antiviral activity of a small molecule deubiquitinase inhibitor occurs via induction of the unfolded protein response. PLoS Pathogens. 2012;8:e1002783. doi: 10.1371/journal.ppat.1002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Y., Kitagaki J., Dai R.-M., Tsai Y.C., Lorick K.L., Ludwig R.L. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 2007;67:9472–9481. doi: 10.1158/0008-5472.CAN-07-0568. [DOI] [PubMed] [Google Scholar]
- 102.Kapuria V., Peterson L.F., Showalter H.D.H., Kirchhoff P.D., Talpaz M., Donato N.J. Protein cross-linking as a novel mechanism of action of a ubiquitin-activating enzyme inhibitor with anti-tumor activity. Biochem. Pharmacol. 2011;82:341–349. doi: 10.1016/j.bcp.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 103.Reiner T., Parrondo R., de las Pozas A., Palenzuela D., Perez-Stable C. Betulinic acid selectively increases protein degradation and enhances prostate cancer-specific apoptosis: Possible role for inhibition of deubiquitinase activity. PLoS ONE. 2013;8:e56234. doi: 10.1371/journal.pone.0056234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bazzaro M., Anchoori R.K., Mudiam M.K.R., Issaenko O., Kumar S., Karanam B. α,β-Unsaturated carbonyl system of chalcone-based derivatives is responsible for broad inhibition of proteasomal activity and preferential killing of human papilloma virus (HPV) positive cervical cancer cells. J. Med. Chem. 2011;54:449–456. doi: 10.1021/jm100589p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Issaenko O.A., Amerik A.Y. Chalcone-based small-molecule inhibitors attenuate malignant phenotype via targeting deubiquitinating enzymes. Cell Cycle. 2012;11:1804–1817. doi: 10.4161/cc.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen J., Dexheimer T.S., Ai Y., Liang Q., Villamil M.A., Inglese J. Selective and cell-active inhibitors of the USP1/UAF1 deubiquitinase complex reverse cisplatin resistance in non-small cell lung cancer cells. Chem. Biol. 2011;18:1390–1400. doi: 10.1016/j.chembiol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu J., Xia H., Kim M., Xu L., Li Y., Zhang L. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chauhan D., Tian Z., Nicholson B., Suresh Kumar K.G., Zhou B., Carrasco R. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22:345–358. doi: 10.1016/j.ccr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weinstock J., Wu J., Cao P., Kingsbury W.D., McDermott J.L., Kodrasov M.P. Selective dual inhibitors of the cancer-related deubiquitylating proteases USP7 and USP47. ACS Med. Chem. Lett. 2012;3:789–792. doi: 10.1021/ml200276j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ratia K., Pegan S., Takayama J., Sleeman K., Coughlin M., Baliji S. A noncovalent class of papain-like protease/deubiquitinase inhibitors blocks SARS virus replication. Proc. Natl. Acad. Sci. U.S.A. 2008;105:16119–16124. doi: 10.1073/pnas.0805240105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Erdal H., Berndtsson M., Castro J., Brunk U., Shoshan M.C., Linder S. Induction of lysosomal membrane permeabilization by compounds that activate p53-independent apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:192–197. doi: 10.1073/pnas.0408592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berndtsson M., Beaujouin M., Rickardson L., Mandic Havelka A., Larsson R., Westman J. Induction of the lysosomal apoptosis pathway by inhibitors of the ubiquitin-proteasome system. Int. J. Cancer. 2009;124:1463–1469. doi: 10.1002/ijc.24004. [DOI] [PubMed] [Google Scholar]
- 113.D’Arcy P., Brnjic S., Hägg Olofsson M., Fryknäs M., Lindsten K., De Cesare M. Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat. Med. 2011;17:1636–1640. doi: 10.1038/nm.2536. [DOI] [PubMed] [Google Scholar]
- 114.D’Arcy P., Linder S. Proteasome deubiquitinases as novel targets for cancer therapy. Int. J. Biochem. Cell Biol. 2012;44:1729–1738. doi: 10.1016/j.biocel.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 115.Lee B.-H., Lee M.J., Park S., Oh D.-C., Elsasser S., Chen P.C. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nag D.K., Finley D. A small-molecule inhibitor of deubiquitinating enzyme USP14 inhibits Dengue virus replication. Virus Res. 2012;165:103–106. doi: 10.1016/j.virusres.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 117.Jin Y.N., Chen P.-C., Watson J.A., Walters B.J., Phillips S.E., Green K. USP14 deficiency increases tau phosphorylation without altering tau degradation or causing tau-dependent deficits. PLoS ONE. 2012;7:e47884. doi: 10.1371/journal.pone.0047884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ponnappan S., Palmieri M., Sullivan D.H., Ponnappan U. Compensatory increase in USP14 activity accompanies impaired proteasomal proteolysis during aging. Mechan. Ageing Dev. 2013;134:53–59. doi: 10.1016/j.mad.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lopez-Castejon G., Luheshi N.M., Compan V., High S., Whitehead R.C., Flitsch S. Deubiquitinases regulate the activity of caspase-1 and interleukin-1β secretion via assembly of the inflammasome. J. Biol. Chem. 2013;288:2721–2733. doi: 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu M., Li P., Song L., Jeffrey P.D., Chernova T.A., Wilkinson K.D. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J. 2005;24:3747–3756. doi: 10.1038/sj.emboj.7600832. [DOI] [PMC free article] [PubMed] [Google Scholar]


