Systemic diseases commonly cause associated ocular lesions and signs in all domestic species as well as in humans. Recognition of ocular signs assists both ocular and systemic diagnosis, because the eye can be examined readily. Such recognition allows earlier and more accurate diagnosis of systemic disorders as well as more effective evaluation of treatment. Ocular signs of some of the less common systemic diseases are poorly documented. Therefore this chapter focuses on the ocular manifestations of the more common systemic diseases. Ocular manifestations of neoplastic, nutritional, and dermatologic conditions as well as uncommon diseases are not discussed in this chapter; the reader is referred to standard internal medicine, oncology, and dermatology texts for discussion of these diseases.
Ocular examination is an essential part of a complete physical examination.
This chapter is divided into three sections. The first deals with ocular manifestations of systemic diseases in the dog and cat. Most of the diseases are discussed separately for these two species. For some diseases, however, the discussion of both species has been combined because the interspecies differences are minute; the heading of each subsection indicates the orientation of the discussion. The first section also includes a number of tables that provide systemic differential diagnosis for ocular signs in the two species. These tables are arranged in the anatomic order of the ocular structures to which they refer (i.e., disorders of the eyelids, conjunctiva, cornea, sclera, uvea, etc.) in order to facilitate finding the list of differential diagnosis for a given disorder. The following two sections, also containing similar tables, are devoted to ocular manifestations of systemic diseases in horses and ruminants.
It should be noted that for each systemic disease, the ocular manifestations and their treatment are described rather briefly. For detailed discussion of these manifestations, the reader is referred to the respective chapters in this book. Systemic pathogenesis, signs, diagnosis, and treatment of the diseases are discussed in greater detail. However, this discussion is not intended to replace the relevant textbooks. Rather, it is intended as a teaching and diagnostic aid to students and practitioners, who are also urged to consult the numerous tables in this chapter for lists of systemic differential diagnosis of the various ocular disorders.
OCULAR MANIFESTATIONS OF SYSTEMIC DISEASES IN DOGS AND CATS (Table 18-1, Table 18-2, Table 18-3, Table 18-4, Table 18-5, Table 18-6, Table 18-7, Table 18-8, Table 18-9, Table 18-10, Table 18-11, Table 18-12, Table 18-13, Table 18-14, Table 18-15, Table 18-16)
Table 18-1.
Systemic Causes of Eyelid Disorders in the Dog and Cat
| DISORDER | DOG | CAT |
|---|---|---|
| Infectious blepharitis* |
|
|
| Parasitic blepharitis* |
|
|
| Immune-mediated blepharitis* |
|
|
| Toxic blepharitis* | Sulfonamide/trimethoprim toxicity (in Doberman pinschers) | — |
| Allergic blepharitis* |
|
|
| Miscellaneous causes of blepharitis* | Zinc responsive dermatosis | — |
| Eyelid masses |
|
|
| Ptosis |
|
— |
The signs of blepharitis are generalized (i.e., not cause-specific); they include dermatitis, alopecia, scales, crusts, ulcers of the skin, and conjunctivitis, chemosis, and congestion of the palpebral conjunctiva.
Table 18-2.
Systemic Causes of Conjunctivitis* in the Dog and Cat
| CAUSES | DOG | CAT |
|---|---|---|
| Viral diseases |
|
|
| Bacterial and rickettsial diseases |
|
|
| Protozoal diseases |
|
— |
| Parasitic diseases | Ophthalmomyiasis (Diptera spp.) | — |
| Immune-mediated diseases | Canine idiopathic granulomatous disease | — |
| Dermal diseases |
|
|
| Miscellaneous diseases |
|
— |
Associated ocular signs include ocular discharge/secretion, chemosis, congestion, and follicular hyperplasia.
Table 18-3.
Systemic Causes of Miscellaneous Conjunctival Disorders in the Dog
| DISORDER | CAUSES |
|---|---|
| Conjunctival hyperemia |
|
| Conjunctival/subconjunctival hemorrhage |
|
Table 18-4.
Systemic Causes of Corneal Diseases in the Dog and Cat
| DISEASE | DOG | CAT |
|---|---|---|
| Infectious causes of keratitis*/keratoconjunctivitis |
|
|
| Corneal ulcers |
|
Feline rhinotracheitis (feline herpesvirus 1 [FHV-1]) has been implicated in the pathogenesis of corneal sequestrum |
| Primary corneal edema† |
|
— |
| Nonedematous corneal opacities |
|
|
| Keratoconjunctivitis sicca |
|
Feline dysautonomia (Key-Gaskell syndrome) |
| Symblepharon | — |
|
Associated ocular signs include epiphora and discharge, blepharospasm, conjunctival congestion, corneal edema, vascularization, infiltration, ulceration, and pigmentation.
Associated signs include corneal opacity, bullous keratopathy, keratoconus, and impairment of vision.
Table 18-5.
Systemic Causes of Scleral and Episcleral Diseases in the Dog and Cat
| DISEASE | DOG | CAT |
|---|---|---|
| Scleritis/episcleritis |
|
— |
| Scleral/episcleral granulomas |
|
Ophthalmomyiasis (Cuterebra spp.) |
Table 18-6.
Systemic Causes of Uveitis in the Dog and Cat*
| CAUSES | DOG | CAT |
|---|---|---|
| Viral diseases |
|
|
| Mycotic diseases |
|
|
| Bacterial diseases |
|
— |
| Protozoal diseases |
|
|
| Parasitic diseases |
|
|
| Neoplastic diseases |
|
|
| Other systemic causes | Periarteritis nodosa |
Associated ocular signs include corneal edema, flare, keratic precipitates, hypopyon and/or hyphema, hypotony, miosis, ciliary injection, blepharospasm, iris congestion, and photophobia. Secondary glaucoma and lens luxation are possible sequelae.
Has been reported to cause secondary glaucoma.
Table 18-7.
Systemic Causes of Cataract in the Dog and Cat
| CAUSES | DOG | CAT |
|---|---|---|
| Infectious diseases | Infectious canine hepatitis in neonates ([ICH] canine adenovirus 1 [CAV-1]) | — |
| Metabolic diseases |
|
— |
| Nutritional |
|
|
| Toxic causes |
|
— |
| Other systemic causes |
|
Chédiak-Higashi syndrome |
Table 18-8.
Systemic Diseases Causing Posterior Uveitis* in the Dog and Cat
| CAUSES | DOG | CAT |
|---|---|---|
| Viral diseases | Canine distemper |
|
| Bacterial diseases |
|
|
| Fungal diseases |
|
|
| Protozoal diseases |
|
Toxoplasmosis (T. gondii) |
| Parasitic diseases | Ocular larval migrans (Toxocara canis) |
|
| Neoplastic diseases |
|
Lymphoma |
| Other systemic causes |
|
Periarteritis nodosa |
Includes chorioretinitis and choroiditis. Associated signs include diffuse or multifocal retinal edema and hemorrhage, subretinal effusion and hemorrhage, vascular cuffing, and loss of vision. Retinal detachment and retinal atrophy are possible sequelae. Retinochoroiditis, which has a similar clinical presentation, is caused by canine distemper virus.
Table 18-9.
Systemic Noninfectious Causes of Retinal/Chorioretinal Scarring and Atrophy in the Dog and Cat*
| CAUSES | DOG† | CAT† |
|---|---|---|
| Nutritional causes | Chronic vitamin E deficiency | Taurine deficiency |
| Cardiovascular diseases |
|
|
| Toxic causes | Sulfonamide/trimethoprim toxicity in Doberman pinschers |
|
| Other systemic causes |
|
|
Associated signs include multifocal scarring, pigment clumping, depigmentation, tapetal hyperreflectivity, and attenuation of retinal blood vessels.
May be caused by any systemic disease causing posterior uveitis.
Table 18-10.
Systemic Causes of Lipemia Retinalis in the Dog and Cat
| DOG | CAT |
|---|---|
|
|
Table 18-11.
Systemic Causes of Retinal Hemorrhage in the Dog and Cat
| CAUSES | DOG | CAT |
|---|---|---|
| Infectious diseases |
|
|
| Parasitic diseases | Ophthalmomyiasis interna (Diptera spp.) | Ophthalmomyiasis interna |
| Cardiovascular diseases |
|
|
| Metabolic diseases | Diabetes mellitus* | Diabetes mellitus |
| Toxic causes | Anticoagulant poisoning | Megestrol acetate (may induce diabetes mellitus) |
| Neoplastic diseases |
|
|
| Other systemic causes |
|
— |
Has also been associated with increased tortuosity and/or dilatation of retinal blood vessels.
Table 18-12.
Systemic Causes of Retinal Detachment in the Dog and Cat*
| CAUSES | DOG | CAT |
|---|---|---|
| Infectious diseases |
|
|
| Parasitic diseases | Dirofilariasis (Dirofilaria immitis) | Ophthalmomyiasis interna |
| Cardiovascular diseases |
|
|
| Neoplastic diseases |
|
— |
| Toxic causes | — |
|
| Other systemic causes | — | Periarteritis nodosa |
Associated signs include anterior displacement of the retina and its vessels, loss of vision and pupillary light reaction, and focal/multifocal/diffuse retinal folds. Retinal detachment may also be caused by any disease causing retinal hemorrhage, as listed in Table 18-11.
Table 18-13.
Systemic Causes of Optic Neuritis in the Dog and Cat*
| CAUSES | DOG | CAT |
|---|---|---|
| Infectious diseases |
|
|
| Cardiovascular diseases |
|
|
| Neoplastic diseases | Intracranial neoplasia | — |
| Other systemic causes |
|
— |
Associated signs include papillary edema, optic nerve head congestion, hemorrhage of optic nerve vessels, and loss of vision and pupillary light reaction.
Table 18-14.
Systemic Causes of Disorders of the Globe in the Dog and Cat
| CAUSES | DOG | CAT |
|---|---|---|
| Exophthalmos |
|
|
| Enophthalmos |
|
|
Table 18-15.
Systemic Causes of Endophthalmitis/Panuveitis in the Dog and Cat
| CAUSES | DOG | CAT |
|---|---|---|
| Infectious diseases |
|
|
| Parasitic diseases | — |
|
| Neoplastic diseases |
|
|
| Other systemic causes | Uveodermatologic syndrome | Periarteritis nodosa |
Table 18-16.
Systemic Disorders Causing Blindness in the Dog and Cat
| CAUSES | DOG | CAT |
|---|---|---|
| Acute blindness |
|
|
| Progressive blindness |
|
|
Infectious Diseases
Canine Viral Diseases
CANINE DISTEMPER.
Distemper is a disease of wo rldwide prevalence afflicting many canids, including the dog. It is caused by a paramyxovirus (Morbillivirus) designated canine distemper virus (CDV) that is spread by aerosol and droplet exposure.
The systemic and ocular clinical signs vary with the stages of the disease and depend on the immune status of the dog (i.e., age, vaccination status, individual variation), the virulence of the virus, and environmental conditions. Most (50% to 70%) of the infections are subclinical.
The ocular signs are the earliest manifestations of the systemic disease; they include acute, mild to severe, bilateral, serous to mucopurulent conjunctivitis, mostly with involvement of the palpebral conjunctiva. With the progression of disease, respiratory and/or gastrointestinal signs appear. CDV may also cause lacrimal gland adenitis with decreased tear production leading to blepharospasm, keratoconjunctivitis sicca (KCS), and possible corneal ulceration. Corneal ulceration may be severe and may not respond well to routine therapy. KCS may resolve after recovery from the systemic disease.
Anterior and posterior uveitis often accompany distemper encephalomyelitis and may be observed even if the dog is clinically asymptomatic for the latter. A high incidence (41%) of multifocal, nongranulomatous chorioretinitis has been found in the neurologic forms of canine distemper. Choroidal exudation may induce retinal detachment. Retinal atrophy and scarring are the chronic sequelae of chorioretinitis. In the tapetal fundus they are characteristically observed as circumscribed, hyperreflective areas with clumps of pigment in the center, whereas the nontapetal lesions are characterized by depigmentation (see Chapter 15, Figure 15-34).
CDV has a predilection for the central nervous system (CNS), including the central visual pathways. It may cause inflammation or demyelinization of the optic nerve and tract, lateral geniculate nucleus, optic radiations, and visual cortex. Patients may present with actue, bilateral blindness and fixed, dilated pupils due to severe optic neuritis (Figure 18-1 ). The inflammation may be isolated, prodromal, or concurrent with other neurologic signs of canine distemper.
Figure 18-1.
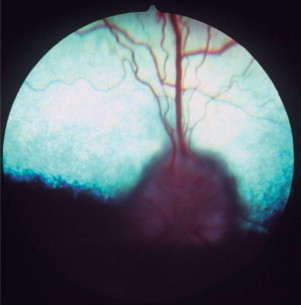
Optic neuritis in a dog. Note the blurry disc margins, hemorrhages, and loss of detail on the disc surface caused by edema of the nerve head.
(Courtesy University of California, Davis, Veterinary Ophthalmology Service Collection.)
The diagnosis of canine distemper is complicated because many dogs are infected but not clinically ill. Cytoplasmic inclusion bodies may be present in conjunctival epithelial cells 5 to 21 days after exposure and may be demonstrated in cytologic smears. Immunofluorescence (IF) techniques for the detection of these inclusion bodies may be used on different cytologic smears, including conjunctival epithelial smears. Recently, CDV amplicons were detected by reverse transcriptase– polymerase chain reaction (RT-PCR) of conjunctival swabs of all dogs experimentally with CDV, from day 3 to 14 after infection. The detection rate of these amplicons in conjunctival swabs was significantly higher during most of the experimental period compared to other tissue samples. Ocular treatment, which is essentially symptomatic, consists of topical ophthalmic antibacterial preparations for conjunctivitis and corneal ulcers. Cases of KCS may be treated with artificial tears, topical antibiotics, and lacromimetics. Treatment of severe corneal ulceration may require surgical intervention. Systemic and topical steroids as well as topical atropine are indicated in cases of uveitis. However, atropine should be used with extreme caution if the animal is also suffering from KCS, and steroids may not be used if the cornea is ulcerated. Systemic administration of antiinflammatory dosages of glucocorticosteroids is indicated in an animal with acute optic neuritis following confirming diagnosis of distemper, even if there is no other sign of clinical disease.
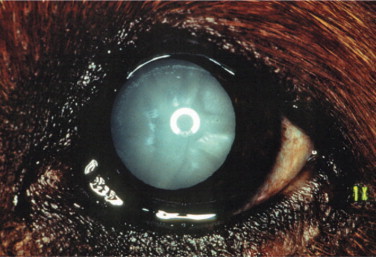
INFECTIOUS CANINE HEPATITIS.
Caused by canine adenovirus 1 (CAV-1), infectious canine hepatitis affects dogs and foxes. The virus is shed in the feces and urine of infected animals, and dogs are exposed through the oronasal route. After an incubation period of 4 to 7 days, seronegative animals infected by CAV-1 exhibit systemic clinical signs that range from those of a mild upper respiratory disease to those of a severe systemic disease, including hepatomegaly, icterus, and bleeding that may progress to disseminated intravascular coagulation. The prevalence of the disease has been dramatically reduced with the introduction of vaccination. Immunization with attenuated CAV-1 and, to a lesser extent, CAV-2 strains led to ocular signs of anterior uveitis and corneal edema in some animals. Dogs are currently vaccinated mostly with attenuated strains of CAV-2.
Ocular signs of infectious canine hepatitis are seen within 7 to 21 days of infection or vaccination. The signs are due to the presence of immune complexes in the eye and occur during convalescence. The initial signs include blepharospasm, miosis, hypotonicity, and anterior chamber flare (Figure 18-2 ) due to anterior uveitis. Corneal edema (“blue eye”) may develop within 1 to 2 days, although it is bilateral in only 12% to 28% of cases. The edema may be severe and lead to formation of keratoconus. Such cases may progress and cause corneal scarring and pigmentation. Persistent or long-lasting corneal edema may also occur, and the Afghan hound has been described as predisposed to chronic edema and glaucoma. However, in most cases the edema is transient, and animals recover spontaneously within a few days to 2 to 3 weeks.
Figure 18-2.
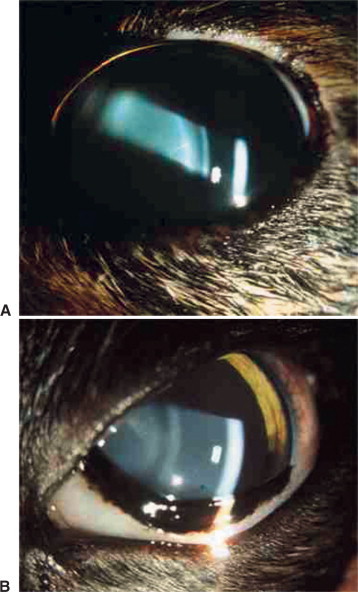
Slit-lamp photography is used to illustrate aqueous flare characteristic of uveitis. A, Two beams of light, on the corneal and anterior lens surfaces, are visible. B, The aqueous humor between these two beams is translucent owing to the presence of inflammatory material. This results in light scattering similar to that observed while driving on a foggy night.
(Courtesy Paul E. Miller.)
The diagnosis of the ocular disease is based on the signalment, history, and clinical signs. Treatment is symptomatic, including topical glucocorticoids or nonsteroidal antiinflammatory drugs (NSAIDs) and atropine. Hypertonic solutions and ointments may be used to resolve severe corneal edema.
Feline Viral Diseases
FELINE HERPESVIRUS INFECTION.
Feline herpesvirus 1 (FHV-1) infection, also called feline rhinotracheitis (FRV), is caused by a member of the Alphaherpesvirinae subfamily that affects all members of the Felidae, and all isolates belong to the same serotype. The virus is widespread in the domestic cat population, especially in colonies and catteries. Cats are infected after direct and indirect contact with sick and carrier animals; the infection occurs through the oronasal and conjunctival routes. Cats that recover from the disease probably remain persistent carriers, a state characterized by latent infection and intermittent periods of virus shedding.
Secondary bacterial infections are common complications, especially with Chlamydophila felis. Unilateral or bilateral conjunctivitis with hyperemia, ocular discharge, chemosis, and blepharospasm are the most common lesions in adult cats with no respiratory disease. Other ocular signs are dendritic (Figure 18-3 ) or geographic corneal ulcers, KCS, and stromal keratitis. Symblepharon is a common sequel of infection (Figure 18-4 ), and FHV-1 may also play a role in the pathogenesis of corneal sequestration and eosinophilic keratitis. Vascularization of the cornea and pain may be severe or absent.
Figure 18-3.
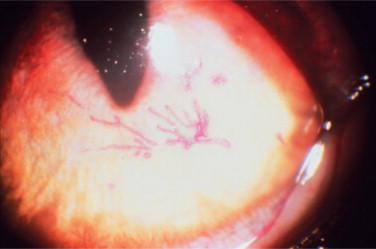
Rose bengal staining used to demonstrate dendritic corneal ulceration, typical of feline herpesvirus 1 infection.
(Courtesy Mark Nasisse.)
Figure 18-4.
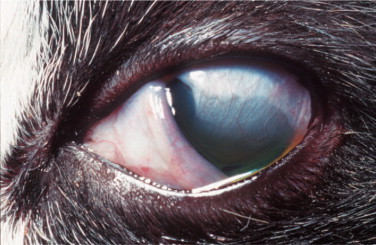
Symblepharon (adhesions of the conjunctiva to the cornea) following feline herpesvirus 1 infection in a cat. Note that the dorsotemporal part of the cornea (and inner ocular structures) is obscured by the adherent conjunctiva and its blood vessels.
(Courtesy David J. Maggs.)
Confirmatory diagnosis of FHV-1 can be made through virus isolation in feline cell cultures. Serology is not very useful owing to the presence of antibodies from vaccination; however, immunofluorescent antibody (IFA) techniques can be used on cytologic and histologic specimens. PCR analysis has been used successfully to identify infected cats, but it is of limited use in a clinical setting because of the high prevalence of the infection in the general feline population.
In vitro sensitivity studies have identified several effective antiviral drugs—in decreasing order of potency, they are trifluridine, 5-iododeoxyuridine, and vidarabine. However, treatment is hampered by drug irritancy and availability. Trifluridine is commercially available but is topically irritating and needs to be administered at high frequency. The other two drugs are less irritating and administered less frequently but are difficult to obtain because they are not available commercially. Bromovinyl-deoxyuridine and acyclovir are not effective against FHV-1, whereas valacyclovir is toxic in felines. Promising in vitro results have been reported with ganciclovir, cidofovir, and penciclovir, but large-scale clinical studies with these drugs are still lacking.
Use of human recombinant interferon, administered topically or orally, has shown synergism in vitro and has decreased the severity of clinical signs in experimentally infected cats when given 1 to 2 days after inoculation. l-Lysine, administered orally, may also inhibit viral replication. Treatment of deep corneal ulcers and necrosis includes surgical intervention. The use of glucocorticoids is contraindicated, as it may induce shedding of viral particles in the latent stage. Topical tetracycline is frequently added because coinfections with Mycoplasma spp. and/or Chlamydophila felis (formerly Chlamydia psittaci) are common. Topical treatments are frequently continued for several weeks after resolution of clinical signs to prevent recurrence.
Stress is a very important factor in the pathogenesis of the clinical disease, and events such as the introduction of a new animal to the household or traveling to cat shows may exacerbate the symptoms. For this reason, frequent treatment with multiple drugs may sometimes aggravate the clinical signs of the disease. If worsening of signs is noted, the clinician is advised to carefully consider reducing treatment rather than increasing it.
FELINE CALICIVIRUS INFECTION.
Feline calicivirus (FCV), which belongs to the family of caliciviruses, affects only members of the Felidae family. The genus consists of one serotype and many different strains varying in antigenicity and pathogenicity. It is widespread in the domestic cat population, especially in crowded conditions. The epidemiology is very similar to that of FHV-1, and despite extensive vaccinations, many cats are carriers of FCV. Some of these cats remain carriers for life and shed the virus continuously. Feline immunodeficiency virus (FIV) infection may potentiate FCV shedding from carriers. Infection by FCV occurs through the oronasal and conjunctival routes. The clinical signs may vary owing to differences in virulence and tropism of the different virus strains. They include fever, anorexia, oral and tongue ulceration, and mild respiratory signs (sneezing, nasal discharge). Certain FCV infections may manifest as shifting lameness and pyrexia for 24 to 48 hours, and oral and respiratory signs may be absent. FCV is also involved in chronic gingivitis. Recently, highly virulent strains of FCV have emerged that are associated with high mortality and a new range of clinical signs (FCV-associated virulent systemic disease). The ocular lesions of FCV include mainly conjunctivitis, but the disease is milder than that induced by FHV-1.
The diagnosis of FCV infection is based mostly on the clinical signs. The virus can be isolated in feline cell cultures from oropharyngeal swabs. These samples may serve for PCR analysis that allows identification of the virus and its strains. Conjunctivitis should be treated symptomatically.
FELINE LEUKEMIA VIRUS INFECTION.
A retrovirus with worldwide distribution, feline leukemia virus (FeLV) is transmitted primarily through the saliva, although it can be present in any body secretion. Infected cats become viremic and may be persistently infected or clear the infection. Latent infections and carrier states are common. The virus is responsible for a third of feline cancer-related deaths through cell transformation and may also lead to anemia and immunosuppression. The prevalence of FeLV-related diseases has been declining over the past 10 years owing to the introduction of a protective vaccine. The clinical signs of FeLV infection vary with the virus subtype and the body system involved.
The ocular disease in FeLV-infected cats may relate to lymphoma, and transformed lymphocytes invade the globe through the uvea, leading initially to a mild uveitis characterized by corneal precipitates. Small masses may be observed on the iris (Figure 18-5 ), and with progression they will lead to thickening and distortion of the iris. Secondary glaucoma is a common complication because of infiltration and obstruction of the iridocorneal angle by tumor cells.
Figure 18-5.
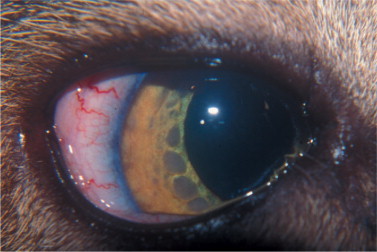
Multifocal gray masses on the surface of the iris of a 12-year-old male cat seropositive for feline leukemia virus. Histopathology confirmed the diagnosis of lymphoma.
The diagnosis of FeLV infection in cats can be made by serologic testing (enzyme-linked immunosorbent assay [ELISA], IFA) and PCR analysis. The latter can be used to detect viral material in tissues, including the cornea, when blood samples and immunohistochemistry of tissues are negative.
The treatment of lymphoma in cats usually requires a multidrug chemotherapy protocol. FeLV-positive cats with lymphoma treated chemotherapeutically were found to have significantly shorter remission and survival times compared with FeLV-negative cats with lymphoma treated with the same chemotherapeutic protocols. Other systemic conditions, including the ocular disease, are treated symptomatically. However, frequently the uveitis may be unresponsive to treatment or may cause secondary glaucoma, thus necessitating enucleation.
FELINE IMMUNODEFICIENCY VIRUS INFECTION.
Feline immunodeficiency virus is a lentivirus with worldwide distribution. At least four subtypes (A to D) have been isolated in different regions of the world, and cats can be concurrently infected with more than one subtype. The seroprevalence of FIV varies among countries, approaching 30% where the free-roaming cat population is large. It is higher in sick than in healthy cats. The virus is known to infect other Felidae. The primary mode of transmission is through bite wounds, because the virus is present in the blood and saliva of infected cats. Thus, intact outdoor male cats are at the highest risk of infection. Other important modes of transmission are the in utero route and through infected queens' milk to suckling kittens.
The disease has three main phases—acute, asymptomatic, and terminal. With the beginning of the terminal phase consisting of the acquired immunodeficiency syndrome (AIDS)– related complex (ARC), cats exhibit nonspecific signs that reflect opportunistic infections (e.g., toxoplasmosis, feline infectious peritonitis virus, systemic mycoses, exacerbation of FHV-1) in different body systems.
The ocular disease manifests mainly as conjunctivitis and anterior uveitis. Pars planitis has been observed in four of nine cats with natural FIV infection. Many FIV-positive cats may exhibit a concurrent FIV- and Toxoplasma-induced ocular disease that manifests mainly as an anterior uveitis and chorioretinitis. Other ocular abnormalities reported are glaucoma (Figure 18-6 ) with or without uveitis, focal retinal degeneration, and retinal hemorrhages.
Figure 18-6.
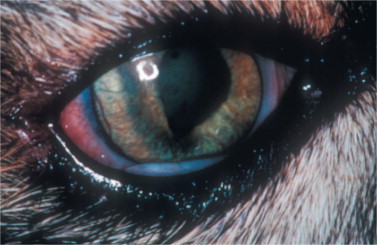
Glaucoma (secondary to posterior synechia) in domestic shorthair cat positive for feline immunodeficiency virus. Note swelling of the iris due to increased aqueous pressure in the posterior chamber, typical of the iris bombè syndrome. Color changes in the iris and ciliary congestion are indicative of uveitis.
The diagnosis of an FIV infection in cats relies mostly on serologic tests for antibody detection, including ELISA (most commonly) and IFA as well as Western blot and immunoblot techniques. Cats in the acute phase of the disease may be seronegative; so if the disease is suspected in a seronegative animal, a second test should be performed after 6 to 8 weeks.
Treatment of the ocular disease in FIV- and Toxoplasma-positive cats should include topical glucocorticoids and atropine In cats, it is advised to use atropine ointment rather than solution, because the latter may drain through the nasolacrimal duct and induce profound salivation due to its bitter taste. Topical tropicamide may substitute atropine. Cats may require a prolonged topical glucocorticoid therapy for control of the anterior uveitis; however, pars planitis responds poorly to such therapy. In cases of posterior uveitis, systemic clindamycin and glucocorticoids are indicated.
FELINE INFECTIOUS PERITONITIS.
Feline infectious peritonitis (FIP) viruses (FIPVs) are biotypes (or strains) of feline corona virus (FCoV), along with the feline enteric corona viruses (FECV), and have a worldwide distribution. In contrast to FECV that infects and replicates only in enterocytes and leads to diarrhea, FIPV has an additional tropism to macrophages and can replicate within these cells, eventually causing FIP. Macrophages carry FIPV to the tissues and viscera. The exact mechanism responsible for the higher virulence of FIPV compared with FECV is currently unknown. It is postulated, however, that in immunosuppressed cats under a heavy FECV infection and replication load, mutations of FECV are more likely to occur, leading to its increased virulence and transformation to FIPV. Cats become infected with FCoV mainly through ingestion, and the virus replicates in enterocytes and is shed through the feces. It may also replicate in the tonsils, in which case it is shed in the saliva.
Kittens are more prone than adult cats to development of FIP, and 50% of the cats with FIP are younger than 2 years. The incidence of FIP is higher in cats from catteries, shelters, and multiple cat households. Stress may predispose cats to the disease. FIP is an immune complex disease resulting from interactions between the virus, or its antigens, and specific antiviral antibodies, complement, and inflammatory cells. The reaction leads to a pyogranulomatous vasculitis that affects the organs supplied by these blood vessels, including the retina (see Chapter 15, Figure 15-35). Cats with clinical FIP may exhibit an effusive (wet) or a noneffusive (dry) disease. The effusive disease is usually the more acute form. The noneffusive form develops over a longer period and is postulated to result from partial immunity to the virus.
Ocular lesions are very common in dry FIP, and the disease was found to be the most prevalent post-mortem finding in cats with uveitis. The ocular signs include iritis with color changes in the iris, bilateral anterior uveitis with aqueous flare, keratic precipitates (Figure 18-7 ), fibrinous exudates in the anterior chamber, hemorrhage into the anterior chamber, chorioretinitis, retinal hemorrhages and detachment, and optic neuritis. Neurologic signs may also be present due to focal, multifocal, or diffuse CNS involvement.
Figure 18-7.
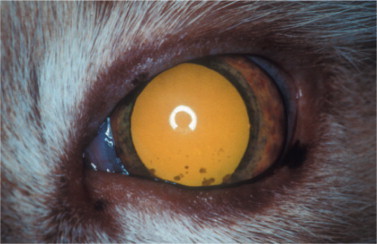
The anterior segment of a 2-year-old cat with anterior uveitis, presumably caused by feline infectious peritonitis. Inflammatory material that is prevalent in the aqueous humor is deposited on the interior (endothelial) aspect of the cornea and is seen as the ventral brown stains, a phenomenon known as keratic precipitates. Iridal congestion and fibrin deposition on the anterior lens capsule also indicate anterior uveitis.
No single diagnostic test can confirm the presence of FIP. Rather, it is the combination of many data that leads to the final diagnosis of the disease—the history, clinical signs, hematologic and serum biochemistry abnormalities, ultrasonography findings, serologic results, cytologic and biochemical findings in effusion samples, histopathology and immunohistochemistry of biopsy and fluid samples, and RT-PCR analysis results. Each finding is given a “likelihood for FIP” grade, and a scale for the total score has been suggested, in that the higher the score the greater the likelihood of the disease.
The prognosis of cats with FIP is poor despite therapy. Treatment, which is essentially symptomatic and supportive, includes immunosuppressive drugs (i.e., glucocorticoids, cyclophosphamide, melphalan, chlorambucil), human interferon-α, vitamins (A, thiamine, C), aspirin, anabolic steroids, and antibiotics. Ocular FIP is treated with glucocorticoids (topical or subconjunctival) and atropine ointment.
Canine Bacterial Diseases
BRUCELLOSIS.
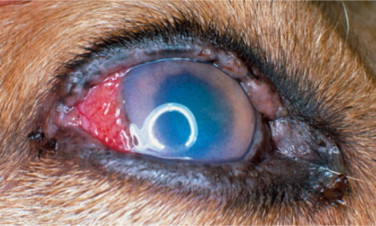
Brucellosis is a venereal disease of Canidae, including the dog, caused by the gram-negative intracellular coccobacillary bacterium Brucella canis. Transmission may occur via contact with contaminated body fluids. B. canis causes a long-lasting bacteremia and is spread hematogenously to the eyes, where it commonly leads to unilateral uveitis (Figure 18-8 ) or endophthalmitis. Owing to the insidious nature of the disease, ocular signs are sometimes the only presenting signs of infection. Other Brucella species (e.g., Brucella melitensis, Brucella abortus) may also infect dogs and cats through contaminated milk products and infected aborted fetuses. Clinical signs of canine brucellosis may often be absent or may vary and include listlessness, fatigue, lethargy, exercise intolerance, weight loss, lymphadenopathy, back pain (due to diskospondylitis), lameness (due to arthritis), neurologic and behavioral abnormalities (due to meningoencephalitis), infertility (in both genders), painful scrotal enlargement (due to orchitis), and testicular atrophy.
Figure 18-8.
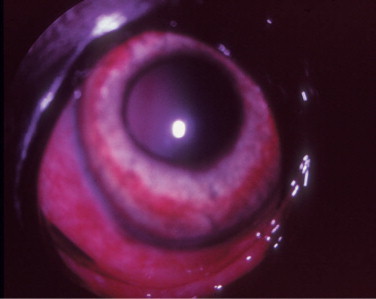
Anterior uveitis in a 3-year-old female German shepherd diagnosed with Brucella canis. Conjunctival and ciliary injection, corneal edema, and iridal congestion and petechiae are present.
Diagnosis relies mostly on serologic testing, with the rapid slide agglutination test (RSAT) as the screening test, followed by the tube agglutination test (TAT) as a confirmatory and quantifying test when the RSAT result is positive. TAT titers of 200 or higher often correlate with positive blood culture results and are presumptive indications of active infections. An agar gel immunodiffusion test for B. canis is a sensitive serodiagnostic test for the detection of infection. Recently, PCR testing of whole blood and semen samples has been shown to have equal or higher sensitivity, compared with blood culture or the RSAT, in the diagnosis of canine brucellosis.
Owners need to be aware of the zoonotic potential of the disease, and its persistent nature, before therapy is attempted. Treatment includes a long course of a systemic antibiotic of the tetracycline group, such as doxycycline or minocycline, with serologic or PCR monitoring for its efficacy. Relapses are common once antibiotic therapy is discontinued, and male dogs rarely recover from infection. Ocular treatment consists of topical glucocorticoids and atropine for uveitis. However, intractable cases of endophthalmitis may require enucleation.
BORRELIOSIS (CANINE LYME DISEASE).
Lyme borreliosis is a worldwide tick-borne disease caused by the spirochete Borrelia burgdorferi. It is transmitted to dogs mainly by ticks of the Ixodes ricinus complex, including Ixodes scapularis. Systemic clinical signs include fever, inappetence, lymphadenopathy, and shifting lameness due to polyarthritis. Nevertheless, ocular signs can be the presenting signs. They include conjunctivitis, anterior uveitis, chorioretinitis, and retinal petechiae (Figure 18-9 ) and detachment.
Figure 18-9.
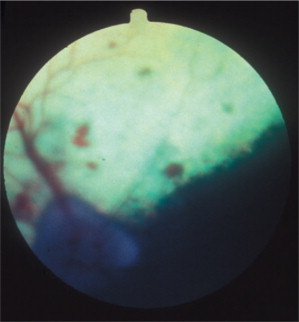
Preretinal petechiae on the fundus of a 7-year-old bloodhound diagnosed with canine Lyme disease.
Infection may be suspected from the clinical signs in an endemic area. Definitive diagnosis can be made through PCR analysis or by growing the spirochete in a culture from body fluids, although the latter is more challenging. Serologic testing is nonspecific because of persistence of antibodies, cross-reactivity with other bacteria, and exposure of healthy animals in endemic areas. Systemic treatment for 10 to 14 days with a variety of antibiotics (e.g., tetracyclines, ampicillin, ceftriaxone) has been shown to be effective. Uveitis is treated symptomatically with NSAIDs or glucocorticoids, and atropine.
RICKETTSIOSIS (EHRLICHIOSIS AND ROCKY MOUNTAIN SPOTTED FEVER).
Rickettsiae and Ehrlichiae are two tribes within the family Rickettssiales, which include many pathogenic, obligate intracellular, gram-negative, coccobacilli bacteria.
Rocky Mountain Spotted Fever.
Rocky Mountain spotted fever (RMSF) affects humans and dogs. It is caused by Rickettsia rickettsii, which is transmitted mainly by the wood tick Dermacentor variabilis and the American dog tick Dermacentor andersoni. However, the brown dog tick Rhipicephalus sanguineus and Amblyomma spp. can also transmit RMSF, and the former has recently been involved in the transmission of the disease in Arizona. The disease is seen in most parts of America, although the majority of cases in the United States occur in the Southwest. The systemic signs of RMSF are variable, resulting from endothelial damage and vasculitis. They include fever, anorexia, depression, tachypnea, coughing, and polyarthritis.
Ocular abnormalities occurred 14 to 21 days after an experimental infection, and were described in 9% to 11% of dogs in natural cases. The abnormalities include subconjunctival hemorrhage, conjunctivitis, chemosis, anterior uveitis (Figure 18-10 ), retinal petechiae, and focal retinal edema.
Figure 18-10.
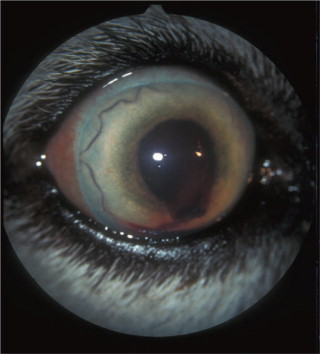
Anterior uveitis in the left eye of a 9-year-old mixed breed dog seropositive to Rickettsia rickettsii. Iridal congestion, blood and fibrin in the anterior chamber, and secondary glaucoma (iris bombè) can be seen.
RMSF may be suspected on the basis of the seasonal occurrence, history of tick infestation, and clinical signs. Thrombocytopenia is the most consistently observed hematologic abnormality. Confirmation of the diagnosis is based on results of PCR analysis or serologic tests such as IFA in tissue biopsy specimens. A fourfold increase in indirect IFA antibody titer between acute and convalescent sera is also diagnostic. Culture may also be used for the confirmation of the diagnosis, although it is not readily available.
The treatment of choice for RMSF is tetracycline, 22mg/kg q8h, or doxycycline, 5mg/kg q12h, for 14 days. The ocular disease is treated with topical or subconjunctival glucocorticoids and topical atropine.
CANINE MONOCYTIC EHRLICHIOSIS.
A worldwide tick-borne disease of dogs, canine monocytic ehrlichiosis (CME) is most prevalent in tropical and subtropical regions. It is caused by Ehrlichia canis, and is transmitted by R. sanguineus. A clinically and serologically indistinguishable disease is caused by Ehrlichia chaffeensis; however, its pathogenic importance and mode of transmission are currently unclear.
E. canis infection leads to acute, subclinical, and chronic disease phases. The acute phase, which lasts 2 to 4 weeks, is characterized by lymphoid hyperplasia and vasculitis with subsequent thrombocytopenia. The subclinical phase follows, consisting of persistence of thrombocytopenia, neutropenia, and anemia. The chronic phase of CME is characterized by hyperglobulinemia and bone marrow suppression with resultant pancytopenia.
The ocular disease may be present in up to 50% of the dogs in the acute phase of experimental infections. Under natural conditions, ocular signs were reported in 10% to 15% of dogs. Resulting from thrombocytopenia and vasculitis, ocular signs include hemorrhagic uveitis, hyphema, retinal hemorrhages leading to retinal detachment (Figure 18-11 ), and optic neuritis. Blindness may occur from ocular hemorrhage, and glaucoma is not an uncommon complication.
Figure 18-11.
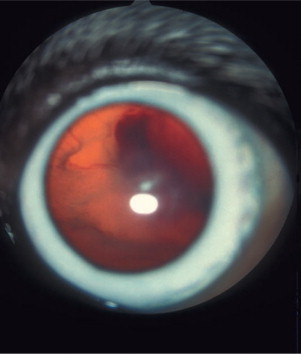
Hemorrhagic retinal detachment in a 4-year-old male Alaskan malamute diagnosed with ehrlichiosis. The retinal blood vessels as well as hemorrhage on the retinal surface may be clearly seen through the pupil without the use of an ophthalmoscope. Acute blindness was the presenting complaint in this case.
CME can be suspected in dogs with a history of tick infestation that manifest the preceding clinical signs and hematologic abnormalities. Confirmation of the disease is based on detection of the typical morulae within monocytes in peripheral blood smears or by PCR analysis, cell culture, or serologic antibody testing (IFA, Western blot technique, ELISA). The latter is most useful for the diagnosis in nonendemic areas.
The treatment of choice for CME is with tetracycline antibiotics (tetracycline, 22mg/kg q8h, or doxycycline, 10mg/kg q24h, for a minimum of 21 days). Imidocarb dipropionate may be added (5mg/kg IM twice in 14-day interval); however, it has been associated with failure to clear the organism when used as a single agent. Systemic glucocorticoid therapy is controversial but has been suggested by some clinicians for the acute disease phase. Treatment of the ocular disease is the same as for RMSF.
Bacterial Diseases of Dogs and Cats
BARTONELLOSIS.
Species of Bartonella are small, hemotropic, gram-negative bacteria, and they have been isolated from apparently healthy and ill dogs and cats. Bartonella henselae, Bartonella clarridgeiae (in cats), and Bartonella vinsonii ssp. berkoffii (in dogs) were established as infectious agents in companion animals. B. henselae is the primary cause of cat-scratch disease in people and is prevalent in most of the temperate regions of the world. The overall seroprevalence in the United States is 28%, and positive bacterial cultures have been reported in 8% to 53% of cats tested as well as in up to 89% of cats owned by Bartonella-infected people. Seropositivity was higher in outdoor cats and in younger cats and was associated with flea infestations. PCR studies have shown that 17% of tested cat fleas (Ctenocephalides felis) were positive for B. henselae. Prairie dogs and their fleas in Colorado were also positive for B. henselae when evaluated with PCR. B. clarridgeiae can occur in asymptomatic cats either as a sole organism or concurrently with B. henselae, and has also been reported to cause cat-scratch disease in people. B. vinsonii ssp. berkoffii is transmitted by the brown dog tick (R. sanguineus) and has been identified as a cause of canine endocarditis and granulomatous lymphadenitis.
Despite the persistence of bacteremia in naturally Bartonella-infected cats, it is a subclinical infection, and a cause-and-effect relationship between infection and disease in cats has not been established. Cats may have a mild febrile disease upon infection, and a transient neurologic disease was described in a naturally infected cat. Little is known of the ocular disease in dogs and cats. It has been reported that feline bartonellosis is associated with anterior blepharitis, conjunctivitis, keratitis, corneal ulcers, uveitis, and chorioretinitis. In one study of cats chronically infected with Toxoplasma gondii, inoculation with B. henselae and later with FHV-1 failed to reactivate ocular toxoplasmosis. B. vinsonii ssp. berkoffii was implicated as a cause of canine anterior uveitis and choroiditis.
The diagnosis of bartonellosis can be made with serologic testing (IFA), blood and tissue cultures, and PCR analysis.
Feline bartonellosis can be treated with amoxicillin, amoxicillin–clavulanic acid, doxycycline, and enrofloxacin, but the doses required to suppress bacteremia are higher than recommended doses. Addition of rifampin to doxycycline has led to bacterial clearance. Infection with B. vinsonii ssp. berkoffii in dogs can be treated with doxycycline, enrofloxacin, and rifampin. The duration of therapy is controversial, but it should be at least 14 to 21 days. Culture specimens should be collected at least 3 weeks after antibiotic discontinuation to verify treatment effectiveness. Treatment of the ocular disease in cats and dogs is essentially symptomatic.
Feline Bacterial Diseases
CHLAMYDIOSIS (CHLAMYDOPHILOSIS).
Chlamydiae are obligate intracellular bacteria that also have an extracellular form during their development cycle. They are commensals of ocular, gastrointestinal, respiratory and genitourinary mucosae. Chlamydophila felis (formerly Chlamydia psittaci) is the only important chlamydial species in cats, and several strains with genetic similarity have been isolated. In the cytoplasm of susceptible cells, the organism forms an initial body that proliferates through budding and fission; later, through a phase of rapid division, these bodies form a large population of elementary bodies that are released from the cell and infect other cells. As many as 45% of healthy cats are seropositive for Chlamydophila psittaci; however, the organism was isolated from conjunctival swabs only in 6%. The isolation rates and seropositivity rise up to 30% and 69%, respectively, in cats with conjunctivitis, and a similar trend was observed through PCR analysis of conjunctival specimens.
Cats infected with Chlamydophila psittaci rarely show systemic signs, although some may have mild upper respiratory signs. The ocular signs are those of conjunctivitis, including conjunctival hyperemia, chemosis (Figure 18-12 ), serous to mucopurulent ocular discharge, and blepharospasm. Cats may become chronically ill. Concurrent FIV or FHV-1 infection prolongs the conjunctivitis.
Figure 18-12.
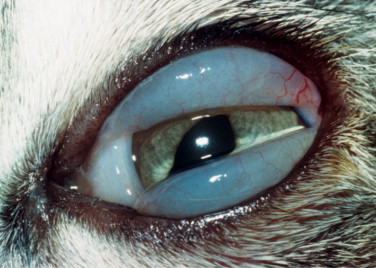
Severe chemosis (conjunctival edema) characteristic of Chlamydia infection in a cat.
(Courtesy David J. Maggs.)
The diagnosis of chlamydiosis can be made through cell culture, cytologic analysis of conjunctival swabs (Giemsa stain and IFA), serologic testing (IFA, ELISA) of patient specimens, and PCR analysis. Therapy of chlamydial infection consists of oral tetracyclines (doxycycline, tetracycline) and, in cases of multiple-cat households and catteries, should be continued for 6 weeks. Ocular infections respond well to tetracycline ophthalmic ointment. Modified live vaccines provide the best protection against the organism but do not prevent colonization of the mucosae or shedding of the organism. Nevertheless, they lead to reduction of the clinical signs in infected cats.
MYCOPLASMOSIS.
Mycoplasmas are small gram-negative bacteria. Mycoplasma felis, Mycoplasma gateae, and Mycoplasma arginini have been isolated from healthy and ill cats. Most mycoplasmas are normal inhabitants of the upper respiratory tract but do not appear in the lungs of healthy cats.
Mycoplasma organisms can be secondary opportunistic pathogens in virus infections and complicated pneumonia cases, mostly in kittens. They may also be isolated from visceral organs of seriously ill and debilitated animals. Mycoplasmosis has a controversial role in feline conjunctivitis, because the organisms have been isolated from healthy cats as well as from cats with conjunctivitis. Although Mycoplasma spp. lead to conjunctivitis in young cats, experimental infections in adult cats failed to induce the disease. Mycoplasma spp. can probably complicate cases of conjunctivitis caused by primary pathogens such as FHV-1 or Chlamydophila psittaci.
The diagnosis of mycoplasmosis can be made through culture and observation of the cytoplasmic inclusion bodies in epithelial cells (in cytologic preparations). PCR analysis of nasal swabs is more sensitive than culture in cats.
Mycoplasma conjunctivitis can be treated with most ocular antimicrobial preparations, although tetracycline is the drug of choice.
Mycotic Diseases of Dogs and Cats
See also Table 18-17 . It should be noted that in addition to the ocular signs described here, fungal infections may also cause focal or multifocal (granulomatous) lesions in the CNS, leading to various signs of neurologic or neuroophthalmic dysfunction. Blindness due to involvement of the central visual pathways, including the optic nerve (i.e., optic neuritis) and chiasm, may also occur.
Table 18-17.
Systemic and Ocular Granulomatous Diseases of Dogs and Cats
| DISEASE | GEOGRAPHIC DISTRIBUTION | BREED PREDISPOSITION | SYSTEMIC SIGNS | OCULAR SIGNS | DIAGNOSTIC TESTING | SEROLOGIC TESTING | TREATMENT |
|---|---|---|---|---|---|---|---|
| Blastomycosis (Blastomyces dermatitidis) | North America (endemic in central Atlantic states, Mississippi, Missouri, Ohio River valleys), Canada, Central America, Africa | Young, male, large-breed dogs, hunting and sporting dogs, Doberman pinschers |
|
Uveitis, secondary glaucoma, corneal edema, focal granulomatous chorioretinitis, retinal detachment, vitreal hemorrhage, periorbital cellulitis |
|
|
|
| Coccidioidomycosis (Coccidioides immitis) | Lower Sonoran life zone (Southwest United States, Mexico, Central and South America) | Boxers, Doberman pinschers |
|
Keratitis, granulomatous panuveitis, chorioretinitis, orbital cellulitis, acute blindness |
|
|
|
| Cryptococcosis (Cryptococcus neoformans) | Worldwide |
|
|
Cats/Dogs: Blindness with dilated, unresponsive pupils, granulomatous chorioretinitis, exudative retinal detachment, optic neuritis, papilledema, retinal hemorrhage, occasionally orbital abscess, cellulitis, rarely anterior uveitis |
|
|
|
| Histoplasmosis (Histoplasma capsulatum) | Endemic in temperate and subtropical regions of the world | Young cats and dogs (<4 yrs), overrepresentation of pointers, Weimaraners, Brittany spaniels |
|
|
|
AGID, CF (not reliable tests) |
|
| Aspergillosis (Aspergillus spp.) | Worldwide | Young to middle-aged German shepherd dogs, cats with concurrent immunosuppressive disease (FIP, FeLV) |
|
|
|
|
|
| Protothecosis (Prototheca spp.) | Ubiquitous in North America, Asia, Oceania, Europe | Dogs and cats with immunosuppression, collies, female dogs |
|
Ocular involvement in more than 50% of infected animals: granulomatous posterior uveitis, panuveitis, retinal detachment, blindness |
|
— |
|
| Toxoplasmosis (Toxoplasma gondii) | Worldwide | Domestic cat and other Felidae: definitive hosts |
|
|
|
|
|
| Tuberculosis (Mycobacterium bovis, Mycobacterium tuberculosis, Mycobacterium avium complex) | Worldwide |
|
Bronchopneumonia, pulmonary nodule formation, hilar lymphadenopathy, fever, anorexia, weight loss, anemia, diarrhea, harsh nonproductive cough, dysphagia, retching, hypersalivation, tonsilar enlargement | Granulomatous uveitis (Mycobacterium spp.), corneal granuloma (M. avium) |
|
|
|
AGID, Agar gel immunodiffusion; CF, complement fixation test; CNS, central nervous system; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; IgM, immunoglobulin M; PCR, polymerase chain reaction; tiw, three times a week.
DIC, Disseminated intravascular coagulation; FA, fluorescent antibodies; FeLV, feline leukemia virus; FIP, feline infectious peritonitis.
BLASTOMYCOSIS.
Blastomycosis is a systemic infection caused by the dimorphic fungus Blastomyces dermatitidis. It affects dogs and humans most commonly, but cats are also affected. The disease is prevalent in North America and it has been reported in Africa and Central America. The endemic distribution in North America includes the Mississippi, Missouri, and Ohio River valleys, the Mid-Atlantic States, and the Canadian provinces of Manitoba, Ontario, and Quebec. The reservoir for the fungus is the soil, and proximity to water and rain facilitates the release of infectious organisms. The spores are acquired mostly by inhalation, leading to establishment of the fungus in the lung tissue, but there are rare reports of invasion through skin wounds in dogs. The organism disseminates in the body through the hematogenous route or via the lymphatics to preferred sites, including the eyes. Most dogs (85%) with blastomycosis have pulmonary lesions. Recently, cardiovascular lesions and signs such as inflammatory myocarditis endocarditis, heart block, heart base or intracardiac mass lesions, and syncope have been described in dogs from endemic areas.
Ocular signs have been reported in up to 40% of the dogs with the disease, and in 50% of cases the ocular lesions were bilateral. They include mainly granulomatous anterior (Figure 18-13 ) and/or posterior uveitis that may be difficult to observe owing to severe corneal edema. Obstruction of the iridocorneal angle with inflammatory material may lead to secondary glaucoma and potential loss of vision. Periorbital cellulitis also occurs. In cats, the main ocular signs are chorioretinitis, retinal detachment, and panophthalmitis.
Figure 18-13.
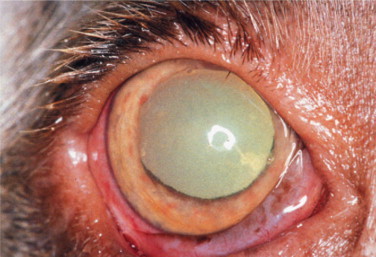
Anterior uveitis in a 1-year-old female Weimaraner diagnosed with blastomycosis. Conjunctival and ciliary injection, corneal edema, iridal congestion, and fibrin in the anterior chamber may be seen. The dog was subsequently euthanized because of progressive central nervous system signs.
(Courtesy Renee Carter.)
Diagnosis of the ocular disease is based on identification of the fungus in cytologic (e.g., vitreous aspiration) or histologic (e.g., enucleated eye) preparations. Serologic testing and thoracic radiography may support the diagnosis if the history and clinical signs are compatible and when microscopic identification of the fungus has failed.
Treatment of blastomycosis includes systemic antifungals (i.e., itraconazole, amphotericin B, and ketoconazole) for at least 60 days, and for at least 1 month after all signs of the disease have resolved. Such long-term treatment may be expensive, and relapses are common. Ocular signs are treated with topical atropine and antiinflammatory therapy. Glaucoma may be treated with carbonic anhydrase inhibitors, but a nonresponsive case may require enucleation of the affected eye.
COCCIDIOIDOMYCOSIS.
Caused by the geophilic, saprophytic, dimorphic fungus Coccidioides immitis, coccidioidomycosis is endemic in the southwest desert areas of the United States, Mexico, and South America. It affects virtually all mammalian species, including humans, dogs, and cats, as well as some reptiles; however, cats are more resistant to infection than dogs. Young (less than 4 years), medium to large dogs are most commonly affected. Rainy weather followed by dry environmental conditions promotes the spread of the arthrospores. These are inhaled into the lung and to the subpleural tissue, where spherules and subsequently endospores are formed. The disease is disseminated via the hematogenous and lymphogenous routes to many tissues, including the eyes. Almost 50% of affected dogs show no systemic signs of the disease.
The ocular disease is unilateral in 75% of the dogs, affecting mainly the posterior segment. The prevalence of ocular signs in cats is 10%. Ocular lesions include keratitis, uveitis (Figure 18-14 ), and chorioretinitis and may lead to retinal detachment and glaucoma.
Figure 18-14.
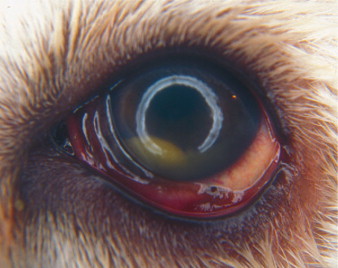
Hypopyon (precipitating inflammatory debris in the ventral aspect of the anterior chamber) in a 4-year-old mixed breed dog diagnosed with coccidioidomycosis.
The disease can be suspected in animals presented with the preceding clinical signs in endemic areas. Confirmation of the diagnosis can be made through identification of the organism in cytologic smears from infected organs, including the vitreous, or in histopathologic specimens from biopsy. Culturing of the fungus with inoculation into animals is possible; however, because of the highly infective nature of the arthrospores and the risk to laboratory personnel, it is not performed routinely. Serologic tests include the agar gel immunodiffusion (AGID) for the detection of precipitin immunoglobulin (Ig) M antibodies, and complement fixation (CF), which detects IgG.
The treatment of coccidioidomycosis is identical to that of blastomycosis.
CRYPTOCOCCOSIS.
Cryptococcosis is an opportunistic disease of worldwide distribution that affects people and animals, including the dog and the cat. It is caused by the saprophytic, round, yeastlike fungus Cryptococcus neoformans (var. neoformans and var. gatti). The disease is the most common systemic mycosis in the cat, in which it is much more common than in dogs. Pigeons are considered the main vectors of the organism, and high numbers of the organism are found in pigeon roosts, habitats, and droppings, where it can survive up to 2 years.
Inhalation of the organism is the most common route of infection, leading to nasal lesions. Smaller, desiccated, encapsulated organisms may reach the alveoli, where they may cause granulomas. The disease is disseminated through direct local extension or hematogenous spread and affects mainly the CNS, eyes, and skin. Natural infections in dogs and cats have been reported to worsen and accelerate owing to the immunosuppression due to glucocorticoid therapy.
Ocular lesions in cats include anterior uveitis, granulomatous chorioretinitis (Figure 18-15 ) frequently leading to retinal detachment, optic neuritis, and exophthalmos. In dogs, the lesions are similar; however, optic nerve leptomeningitis is common and is probably the route of ocular infection from the CNS.
Figure 18-15.
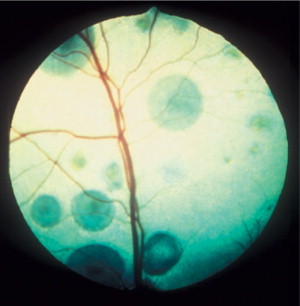
Multifocal chorioretinal granulomas, causing retinal detachments, in a cat diagnosed with cryptococcosis. The organism was identified in an aqueous humor sample. Note that the retinal blood vessels are coursing over some of the granulomas, indicating their intraretinal or subretinal nature.
The diagnosis of cryptococcosis is based on identification of the typical thickly encapsulated yeasts that show narrow base budding. These organisms can be seen in nasal and skin exudates, cerebrospinal fluid (CSF), and in fine-needle aspirates from affected tissues, including the aqueous humor or vitreous. Gram and new methylene blue stains are superior to Romanowsky stains for their detection. Serologic tests are very rapid and useful in suspected cases in which yeasts are not detected by cytology; such tests include latex agglutination of cryptococcal capsular antigen and ELISA. Both tests detect all known serotypes and can be conducted in serum, urine, or CSF samples. Histopathologic analysis of biopsy specimens may also help if cytology has failed to reveal the organism. The organism can be cultured from exudates, body fluids, and tissues, although growth may take up to 6 weeks.
The treatment of cryptococcosis in both cats and dogs involves a long (1 to 10 months) course of a systemic antifungal drug—amphotericin B with or without flucytosine, ketoconazole, itraconazole, or fluconazole. The last has been recommended for the CNS disease because of its better distribution in the system, including the CSF. Nevertheless, itraconazole has been used successfully in cases of feline cryptococcal meningitis. Recently, the success rate of treatment of cryptococcosis in dogs and cats has been reported to be 55 and 76%, respectively. The median duration of treatment required to effect a cure at first attempt was significantly shorter for fluconazole (4 months; range 1 to 8 months) than for itraconazole (9 months; range 3 to 24 months). Cats with neurologic involvement, disseminated disease, or refractory disease treated with protocols containing amphotericin B did as well, on average, as cats with less severe disease treated with azole monotherapy. Treatment of the ocular disease is similar to that provided in cases of blastomycosis.
HISTOPLASMOSIS.
Histoplasmosis is a systemic mycosis caused by the soil-borne dimorphic fungus Histoplasma capsulatum. The disease has been reported in North and South America. In the United States, most cases occur in the regions of the Ohio, Mississippi, and Missouri rivers. In the mycelium stage, the fungus is present in soil and produces microconidia that are infectious to mammals. Both dogs and cats can be affected; however, cats are more susceptible to infection than dogs. In both species young (less than 4 years) adults are mostly affected. The microconidia are inhaled into the lung and transformed to the yeast phase, which multiplies by budding. The disease usually starts with respiratory signs, although some cases of gastrointestinal histoplasmosis, with no respiratory involvement, have been described. Dissemination of the organism from the respiratory system occurs through hematogenous and lymphatic routes.
Ocular lesions in dogs and cats include anterior uveitis, granulomatous chorioretinitis, and optic neuritis. Blindness may result from retinal detachment or optic neuritis.
Histoplasmosis can be suspected in dogs and cats with the above clinical signs exhibiting a normocytic normochromic anemia and radiographic evidence of a linear to diffuse pulmonary interstitial pattern associated with granulomatous fungal pneumonia. A definitive diagnosis can be made with fine-needle aspiration of affected tissues and effusions, and identification of the organism within macrophages in routine Romanowsky stains. Histopathology of tissue biopsy specimens, including endoscopic samples, may be attempted when cytologic samples fail to demonstrate the organism. Culture is possible, although it is not recommended because of the potential hazard of the organism to humans. Serologic testing is unreliable owing to many false-positives and false-negative results.
Histoplasmosis is best treated with oral itraconazole, and in fulminating cases amphotericin B is added for a more rapid control of the disease. Fluconazole may be preferred in CNS or ocular disease because of its better distribution, and ketoconazole may be used. The latter is not recommended as a first-choice drug owing to its toxicity, although it may be considered when cost is a concern. The duration of treatment should be 4 to 6 months at least. Treatment of the ocular disease is similar to that provided in cases of blastomycosis.
Parasitic Diseases of Dogs and Cats
TOXOPLASMOSIS
(see Table 18-17). Toxoplasmosis is a zoonotic disease of worldwide distribution that affects all mammals and is caused by the obligate intracellular coccidian Toxoplasma gondii. Cats, the definitive hosts, shed oocysts in their feces. Other mammals, including cats and dogs, may ingest sporulated oocysts. Cats are infected mostly by ingesting intermediate hosts infected with tissue cysts and can also be infected by ingestion of oocysts; however, only 20% of cats fed oocysts have a patent infection. Congenital infection due to transplacental infection or through the queen's milk has been reported in kittens. The incidence of congenital infection in dogs is unknown.
The brain, liver, lungs, skeletal muscle, and eyes are common sites of cyst formation, initial replication, and persistence of chronic infection.
In cats, ocular signs were observed in 81.5% of cases, most commonly consisting of bilateral anterior and/or posterior uveitis. Secondary lens luxation, glaucoma, and retinal detachment were also described. Ocular manifestations in dogs are (in decreasing order) anterior uveitis, retinitis, choroiditis, scleritis, optic neuritis, and episcleritis.
Cytologic preparations of effusions may reveal the tachyzoites, which occasionally may be observed in other samples (i.e., CSF, transtracheal or bronchoalveolar lavage, and fine-needle aspirates from tissues). There are multiple serologic tests for antibody detection; however, no one test is confirmatory, and 30% of dogs and cats in the United States are seropositive. A positive IgM titer or a fourfold increase in IgG or IgA titer can verify a recent infection; positive IgM titers can persist for months after infection, however, and high IgG titers have been detected 6 years after inoculation in cats. Serologic tests cannot be used accurately to predict the oocyst shedding period. Simultaneous measurement of Toxoplasma-specific antibody titers in the CSF or aqueous humor and in the serum of animals presented with a neurologic and/or an ocular disease, along with measurement of other agent-specific antibody titers in these same samples, may help discriminate between local production of Toxoplasma-specific antibodies and serum antibodies passively leaking through damaged endothelial barriers. High ratios of Toxoplasma-specific antibody titers in the CSF or aqueous humor to serum titers in comparison with other agent-specific antibody titers are evidence of local antibody production of antibodies and, thus, suggest the presence of active infection in the CNS or eye, respectively. The presence of the organism can be confirmed by inoculation of laboratory mice or cell culture, with detection of tachyzoites or specific antibodies. The presence of T. gondii in tissue and body fluid samples can also be confirmed by PCR analysis, although a positive result cannot confirm the presence of an active disease.
Systemic toxoplasmosis can be treated with a variety of antimicrobials. However, treatment results in suppression, rather than killing, of the organism. Clindamycin (25mg/kg PO q12h for 21 to 30 days) is the drug of choice for treatment of clinical disease. Combinations of sulfonamides, pyrimethamine, and trimethoprim act synergistically in suppressing the parasite, although supplementation with folic acid is advisable, especially in cats. Other drugs that have shown in vitro and/or in vivo activity against T. gondii include doxycycline, minocycline, newer macrolides (roxithromycin, azithromycin, clarithromycin) and several other antibacterial drugs; however, these should be further evaluated in dogs and cats.
The ocular disease is treated with topical glucocorticoids and atropine, and in cases of posterior uveitis, systemic clindamycin and glucocorticoids are indicated.
Canine Parasitic Diseases
NEOSPOROSIS.
Canine neosporosis has worldwide distribution. It is caused by the apicomplexan protozoan Neospora caninum, which has a similar morphology to T. gondii. Its life cycle is at present incompletely understood. The definitive host is probably a carnivore that sheds oocysts in the feces; the oocysts are ingested by herbivores, in which tissue cysts are formed. As many as 20% of dogs are seropositive, probably infected subclinically and, supposedly, transplacentally during gestation.
Ocular lesions of neosporosis include mild anterior uveitis, retinitis and retinochoroiditis.
Serologic testing (IFA) of serum and CSF is the most commonly used diagnostic method for neosporosis. Most animals with the clinical disease show an increase in IgG titers within 1 to 2 weeks of initial signs. The organism may be observed in cytologic preparations of CSF and tissue fine-needle aspirates. Biopsy specimens examined histopathologically may demonstrate the tachyzoites or the typical thick-walled cysts. PCR analysis may prove the presence of the organism and helps in its differentiation from related organisms.
Treatment of neosporosis is similar to that described for toxoplasmosis. Young affected animals have a guarded to poor prognosis, whereas adult dogs respond better to therapy.
VISCERAL LEISHMANIASIS.
Leishmaniasis is a disease caused by the dimorphic protozoans of the genus Leishmania that affects humans and animals worldwide. The natural reservoir of the parasite, dogs have clinical disease. Visceral leishmaniasis is transmitted in the Old World by sandflies of the genus Phlebotomus and is caused primarily by Leishmania infantum. New World visceral leishmaniasis is transmitted by sandflies of the genus Lutzomyia and is caused by Leishmania chagasi, which is considered identical to L. infantum. In recent years, foci of leishmaniasis caused by L. infantum have been reported in the United States, mainly in foxhounds, and the disease has been transmitted to dogs accidentally through transfusion of contaminated blood products. In mammalian hosts the parasite is seen as the intracellular nonflagellate form, the amastigote, within macrophages. The disease incubation period before the appearance of clinical signs may last months to years, during which the parasite disseminates in the body. Some dogs develop clinical disease, whereas others remain asymptomatic carriers that are infectious to sandflies and can thus transmit the disease to other dogs and humans. Systemic signs of visceral leishmaniasis may include dermal abnormalities (e.g., exfoliative dermatitis, mainly involving the head and ears), lymphadenopathy, splenomegaly, signs of renal insufficiency, epistaxis, and musculoskeletal abnormalities.
The ocular signs include blepharitis (Figure 18-16 ), lid granulomas (Figure 18-17 ), conjunctivitis, scleritis, keratitis, anterior uveitis, panophthalmitis, and secondary glaucoma.
Figure 18-16.
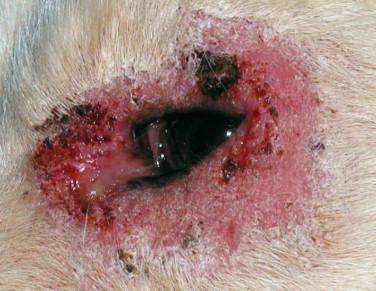
Severe blepharitis in a dog diagnosed with leishmaniasis.
(Courtesy Gad Baneth.)
Figure 18-17.
Lid granulomas (especially on the nasal aspect of the upper eyelid) and anterior uveitis (note the conjunctival and ciliary congestion and the corneal edema) in a dog diagnosed with leishmaniasis.
(Courtesy Teresa M. Pena and Xavier Roura.)
Serologic tests can confirm the presence of antileishmanial antibodies but cannot prove the presence of an active disease. Many highly specific and sensitive serologic tests are available, including IFA, ELISA, direct antiglobulin test, and CF. The presence of a high titer in a dog with the characteristic signs is highly suggestive of active disease. Ten percent to 20% of the seropositive dogs may eliminate the parasite spontaneously and may be apparently healthy; however, dogs may be clinically healthy and still harbor active infection. The amastigotes may be detected in cytologic and histologic preparations, within macrophages. Culture or PCR analysis of splenic or bone marrow aspirates may also confirm the presence of the organism. Recently, PCR studies have shown that conjunctival swabs were the most reliable source for parasitic DNA in dogs experimentally infected with L. infantum.
Treatment of infected dogs rarely achieves complete elimination of the parasite. Traditional treatment consists of daily injections of pentavalent antimonials (meglumine antimonate, sodium stibogluconate) for 3 to 4 weeks. Adverse effects and relapses are common. Oral allopurinol results in suppression of the parasite and clinical improvement but does not cure the disease. It has very few side effects and can be used as a sole agent or in combination with pentavalent antimonials. Lipid-associated amphotericin B has been shown to lead to clinical improvement in sick dogs but not to eliminate the infection.
Ocular treatment is directed against the inflammatory reaction and consists of topical atropine as well as glucocorticoids or NSAIDs.
Endocrine Diseases
Endocrine Diseases of Dogs and Cats
DIABETES MELLITUS.
Diabetes mellitus (DM), the most common endocrine disease in dogs and cats, is similar in incidence for dogs and cats, with reported frequencies of 1:100 to 1:500. The disease is classified to two subtypes, type I (insulin-dependent diabetes mellitus [IDDM]) and type II (non–insulin-dependent diabetes mellitus [NIDDM]). IDDM, the more common form, is seen in almost all dogs and in 50% of 70% of the cats with DM. This type is characterized by loss of pancreatic beta cells and subsequent insulin deficiency, leading to hyperglycemia. In contrast, NIDDM is uncommon in dogs, is observed more commonly in cats, and is characterized by insulin resistance. Consequently, insulin concentrations are variable and can be normal, decreased, or increased; however, hyperglycemia is always present. Ketoacidosis is seen more commonly in IDDM than in NIDDM. A transient form of DM has been described in cats, and rarely in dogs, in which some factor predisposes the animals to insulin antagonism and resistance, resulting in persistent hyperglycemia. The elevated glucose concentrations may lead to beta cell refractoriness and even to glucose toxicity and irreversible lesions in beta cell functions. Treatment of the underlying disease and the diabetic state may lead to transition to subclinical DM and euglycemia, with no requirement for insulin or other oral hypoglycemic drug therapy.
The most prevalent ocular sign of DM in dogs is bilateral cataracts. Initial changes include vacuole formation along the equatorial cortex that progresses to the anterior and posterior cortex. Diabetic cataracts progress very rapidly and may reach maturity, which may develop in a short time (days to weeks), and owners may present with a complaint of relatively acute blindness (Figure 18-18 ). Cataract formation depends on the age of the animal as well as on the magnitude and duration of hyperglycemia. Cataracts are detected in a high proportion of diabetic dogs and lead to lens-induced uveitis. The tendency for development of a diabetic cataract depends on the activity of aldose reductase in lenticular cells. Aldose reductase is the key enzyme in the formation and accumulation of sorbitol, fructose, and dulcitol in the lens. The resulting hyperosmolarity of the lens leads to fluid ingress with subsequent swelling, fiber rupture, and eventual cataract formation. Activity of aldose reductase in dogs is high in the lens regardless of age, whereas in cats it is significantly higher in those younger than 4 years than in older cats. Because DM occurs primarily in older cats, the relatively low aldose reductase activity protects the feline lens from cataract formation. Blindness due to diabetic cataracts can be corrected only with surgical removal of the lens, although experimental work using aldose reductase inhibitors to prevent the development of diabetic cataracts has shown promising results. The prognosis for successful outcome of surgery is reportedly unaffected by the presence of DM, although perioperative medical management has to be modified to include NSAIDs instead of glucocorticoids, and the incidence of long-term complications may be higher. See Chapter 13 for additional discussion of diabetic cataracts.
Figure 18-18.
Diabetic (mature) cataract in a dog. Note the prominent anterior Y sutural clefts.
(Courtesy Paul E. Miller.)
Diabetic cataracts may progress to maturity within a few weeks. The disease should be considered in patients presented with rapid-onset cataracts.
DM has also been associated with retinal and vitreal hemorrhages, as well as retinal detachment. This involvement of the posterior segment is more common in the cat and rare in the dog. The presentation is similar to diabetic retinopathy, a blinding disease in humans. However, microaneurysms and proliferative changes in retinal vasculature, which are the hallmarks of the human disease, have not been documented in animals.
Diabetic dogs have significantly reduced corneal sensitivity compared with nondiabetic normoglycemic dogs. It has been suggested that trigeminal nerve dysfunction may be associated with recurrent or nonhealing ulcers in diabetic dogs for which no other underlying cause can be found.
The diagnosis of DM is based on detection of persistent fasting hyperglycemia (or glycosuria). In addition, ketonemia and/or ketonuria are present in ketoacidosis. Serum fructosamine or glycosylated hemoglobin concentrations may help in differentiating stress-induced hyperglycemia from DM. Some diabetic dogs and cats have concurrent hyperadrenocorticism, and diabetic cats may have concurrent hyperthyroidism. Diagnostic procedures should include specific tests to exclude these diseases in cases where there is a high index of suspicion for them. Acute pancreatitis may lead to destruction of islet cells, with subsequent DM, that may be permanent or transient; therefore screening for presence of concurrent pancreatitis is advisable. A urine culture is recommended if the urinalysis yields results consistent with urinary tract infection.
Treatment of uncomplicated DM is primarily aimed at normalizing the glucose concentration. This is achieved principally with insulin therapy, although in certain animals with NIDDM, mostly cats, oral hypoglycemic drugs (e.g., glipizide and glyburide) can be used as sole agents or in conjunction with insulin. Oral vanadium therapy may also be useful in treatment of DM. Dietary modification should always be a part of the therapy of DM. Its aims are to minimize postprandial glucose concentration fluctuations and treat or prevent obesity. Modified diets for DM are limited in simple carbohydrates, include complex carbohydrates, and contain high fiber; some diets, mostly feline, are high in protein. Acarbose may be used in diabetic animals whose glucose concentration is poorly controlled despite insulin therapy and dietary modification. For treatment of diabetic ketoacidosis, the reader is referred to textbooks of veterinary internal medicine.
Canine Endocrine Diseases
HYPERADRENOCORTICISM.
Hyperadrenocorticism (HAC, Cushing's syndrome) is a common canine endocrinopathy characterized by glucocorticoid excess. The disease can be caused by an adrenocorticotropic hormone (ACTH)–secreting hyperplastic or neoplastic pituitary gland (pituitary-dependent HAC) or a cortisol-secreting adrenocortical tumor or may be iatrogenic, due to chronic excessive glucocorticoid therapy. Dogs with HAC may have concurrent DM.
The ocular surface lesions associated with canine HAC include progressive, nonhealing corneal ulceration, corneal calcification, and KCS. Corneal ulceration is not the direct result of HAC, but the high levels of endogenous glucocorticoids may delay healing of a corneal ulcer from other mechanisms. Cataracts are usually observed in dogs that suffer from concurrent DM. Intraocular manifestations include lipid accumulation in the aqueous, lipemia retinalis, and hypertensive retinopathy. Hyperlipidemia, commonly observed in dogs with HAC, and concurrent uveitis are responsible for the development of lipemia retinalis and lipids in the aqueous. Dogs with HAC also suffer from a relatively high incidence of sudden acquired retinal degeneration (see Chapter 16).
The diagnosis of canine HAC requires endocrine tests that include urinary cortisol-to-creatinine ratio, ACTH stimulation (with measurements of cortisol, with or without 17-hydroxyprogesterone) and low-dose dexamethasone suppression. Differentiation between pituitary-dependent HAC and adrenocortical tumor may require additional testing (i.e., measurement of endogenous ACTH concentration and high-dose dexamethasone suppression).
The most commonly used drug in the treatment of canine HAC is mitotane (op ã-DDD); recently, however, trilostane has been shown to be as effective as mitotane in the treatment of canine pituitary-dependent HAC. The ocular lesions are treated symptomatically. Corneal ulcers may heal once control of HAC is achieved.
HYPOTHYROIDISM.
Hypothyroidism is a common canine endocrinopathy, with a prevalence of 0.2%, but is extremely rare in cats. It leads to decreased production of thyroxine (T4) and triiodothyronine (T3). The disease can be the result of a hypothalamic disorder leading to deficiency of thyrotropin-releasing hormone (TRH) (tertiary hypothyroidism), a pituitary disorder leading to thyrotropin deficiency (secondary hypothyroidism) or a thyroid gland disorder (primary hypothyroidism). Most canine cases are primary hypothyroidism and result from lymphocytic thyroiditis or idiopathic thyroid atrophy.
The ocular manifestations of canine hypothyroidism are primarily the result of hyperlipidemia that may lead to lipid dystrophy (corneal lipidosis) (Figure 18-19 ) with secondary ulceration and uveitis, lipid deposition in the aqueous, and lipemia retinalis with retinal bleeding and detachment. Secondary glaucoma has been reported in canine hypothyroidism. There is an association between KCS and canine hypothyroidism, and 20% of dogs with the disease have been reported to be diagnosed with concurrent KCS. This association is probably an indirect one, likely resulting from a multiple glandular, immune-mediated inflammation.
Figure 18-19.
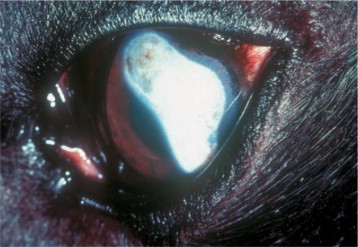
Corneal lipidosis in a dog with hypothyroidism. Extensive depositions of lipids in the cornea, such as this case, obviously affect the dog's vision and may warrant surgical removal (superficial keratectomy).
The diagnosis of canine hypothyroidism is mainly based on evaluation of total and/or free T4 along with canine thyroid-stimulating hormone (TSH) measurement. Other, more advanced tests are responses to TSH or TRH, measurement of antithyroglobulin or of anti-T3 and anti-T4antibodies, scintigraphy, and thyroid biopsy. Advanced brain imaging (computed tomography, magnetic resonance imaging) may be needed to diagnose hypothalamic or pituitary lesions leading to tertiary and secondary hypothyroidism, respectively.
Treatment of canine hypothyroidism essentially comprises oral levothyroxine (l-thyroxine) supplementation (22µg/kg q12-24h). Restriction of cholesterol and lipids in the diet is indicated. KCS should be treated, and topical glucocorticoids or NSAIDS (based on presence of corneal ulcers) should be used to treat the secondary keratitis and uveitis. Surgical removal of moderate corneal lipid plaques is contraindicated because recurrence of such plaques is often more severe than the initial lesion. Surgery is reserved for cases where significant visual deficits occur (see Figure 18-19).
Metabolic Diseases of Dogs and Cats
HYPERLIPIDEMIA.
Defined as excess blood lipids, hyperlipidemia can result from an increase in fasting serum triglycerides, in cholesterol, or both. A serum (or plasma) that appears grossly milky or turbid, referred to as hyperlipemic, results from triglyceride excess; hypercholesterolemia does not lead to increased serum turbidity and hyperlipemia. The term hyperlipoproteinemia is sometimes used interchangeably with hyperlipidemia, because lipoproteins carry both triglycerides and cholesterol in the plasma, and often there is a concurrent increase in serum lipoproteins when hyperlipidemia is present. However, this term should be reserved to cases in which laboratory tests confirm an increase in the concentrations of serum lipoproteins. The incidence of hyperlipidemia in the canine population was approximately 14% in one study. Hyperlipidemia may be primary (usually hereditary or familial) or secondary, and both forms have been described in dogs and cats, although secondary hyperlipidemia is much more common. In dogs secondary hyperlipidemia may result from hypothyroidism, DM, hyperadrenocorticism, glomerulonephropathy, pancreatitis, or cholestasis or may be due to a high-fat diet. Similar mechanisms lead to feline hyperlipidemia. In cats, excessive administration of megestrol acetate and glucocorticoids may lead to DM and secondary hyperlipidemia.
The ocular manifestations of hyperlipidemia include lipemia of the ocular blood vessels, corneal lipid keratopathy, lipemic aqueous, and lipid infiltration of the globe, most noticeably in the peripheral cornea and the uveal tract. When hyperlipidemia is associated with hypertriglyceridemia, visible changes may be observed in the conjunctival and retinal blood vessels, which look pink and engorged. Lipemic retinal blood vessels, lipemia retinalis, are more easily visualized over the nontapetal fundus (Figure 18-20 ). Lipids may also be observed in the anterior chamber, usually as a result of uveitis allowing leakage from vessels.
Figure 18-20.
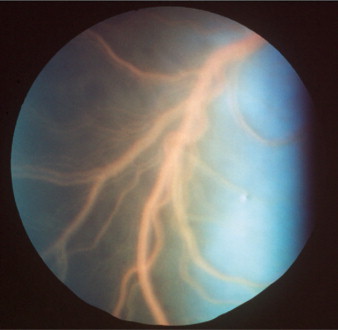
Lipemia retinalis in a cat with hyperlipidemia. The pink color of the blood vessels is easily appreciated against the dark background of the nontapetum.
(Courtesy David J. Maggs.)
The diagnosis of hyperlipidemia relies on the demonstration of fasting hypertriglyceridemia and/or hypercholesterolemia. In secondary hyperlipidemia, depending on the primary disease, further tests include urinalysis, urine protein-to-creatinine ratio, liver function tests, serum lipase–like immunoreactivity, hormonal assays, abdominal ultrasonography, and a careful analysis of the diet. In suspected cases of primary hyperlipidemia, every effort should be made to rule out the presence of another primary disease, and additional testing includes lipoprotein profiling (e.g., electrophoresis, densitometry, precipitation techniques, and ultracentrifugation).
Treatment of secondary hyperlipidemia is directed at the primary disease, whereas primary hyperlipidemia is usually treated with low-fat diets. Treatment of the secondary ocular complications is essentially symptomatic. Anterior uveitis treatment should be provided in cases of lipemic aqueous humor. Cases of corneal lipidosis in which vision is affected may be treated surgically, although recurrences should be considered.
Cardiovascular Diseases of Dogs and Cats
Ocular signs associated with hematologic disorders are summarized in Table 18-18 .
Table 18-18.
Ocular Signs of Hematologic Disorders in Dogs and Cats
| DISORDER | OCULAR SIGNS |
|---|---|
| Monoclonal gammopathy and hyperviscosity |
|
| Thrombocytopenia and thrombopathy |
|
| Severe anemia |
|
| Polycythemia | Dilated and tortuous dark red to brown conjunctival and retinal blood vessels, retinal hemorrhages and detachment, uveitis and chorioretinitis |
| von Willebrand's disease |
|
THROMBOCYTOPENIA AND THROMBOPATHY (THROMBASTHENIA).
Thrombocytopenia is a very common hematologic disorder in the dog but is less frequent in cats. In dogs the most common causes of thrombocytopenia are infectious diseases (e.g., RMSF, monocytic ehrlichiosis, infectious cyclic thrombocytopenia, babesiosis), neoplasia (e.g., many carcinomas and sarcomas, myeloproliferative and lymphoproliferative disorders), immune-mediated disorders (e.g., immune-mediated thrombocytopenia, systemic lupus erythematosus), and toxicities due to drugs (e.g., trimethoprim-sulfamethoxazole, many cytotoxic drugs) and other substances (e.g., snakebites). Vasculitis, neoplasia, protein-losing enteropathy/nephropathy, and disseminated intravascular coagulation may lead to platelet activation and consumption, and subsequently to thrombocytopenia. In cats, immune-mediated thrombocytopenia is rare; however, FeLV infection and neoplasia have been associated with thrombocytopenia.
Thrombopathy (thrombasthenia), a functional defect of platelets, can be inherited or acquired. Inherited thrombopathies have been reported mostly in dogs, and include von Willebrand's disease (vWD, also reported in cats), basset hound hereditary thrombopathia, canine thrombasthenic thrombopathy, Glanzmann's thrombasthenia, cyclic hematopoiesis, and storage pool disease. In cats the Chèdiak-Higashi syndrome is associated with platelet function defects. Acquired thrombopathy may accompany thrombocytopenia and can result from drug toxicity, paraproteinemia, immune-mediated mechanisms (e.g., antiplatelet antibodies, circulating immune complexes and vasculitis), disseminated intravascular coagulation (fibrinogen degradation products excess), uremia, neoplasia (e.g., hemangiosarcoma), and liver failure.
The ocular manifestations include both extraocular and intraocular bleeding, such as subconjunctival hemorrhage, hyphema, iridal petechias, and preretinal, intraretinal, and subretinal hemorrhages. The latter may also lead to retinal detachment and blindness. Depending on the primary cause of the platelet disease, anterior uveitis may also be present (e.g., in canine monocytic ehrlichiosis).
The diagnosis of thrombocytopenia is based on hematologic examination. Bleeding diathesis usually does not occur until platelet numbers fall below 50,000 cells/µL and is more common in acute than in chronic conditions. Very commonly, automated platelet counts in cats are falsely decreased, necessitating a manual count or a blood smear evaluation. The presence of thrombopathy can be confirmed by measurement of buccal mucosal bleeding time (provided that there is no concurrent thrombocytopenia). However, the diagnosis of the specific disorder requires special laboratory tests. Canine von Willebrand's factor assays are available (electroimmunoassay, ELISA).
Thrombocytopenia may be corrected by treatment of the primary disease but this may prove difficult. Acute life-threatening thrombocytopenia is treated with fresh whole blood or platelet-rich plasma transfusions. Von Willebrand's disease is treated with desmopressin and cryoprecipitate or fresh-frozen plasma transfusions. Intraocular hemorrhage is treated with topical steroids, and systemic glucocorticoid treatment should be considered if the animal's systemic condition allows it. Mydriatics should be considered to prevent possible posterior synechia, as should prophylactic antiglaucoma treatment.
SYSTEMIC HYPERTENSION.
Systemic hypertension occurs in both dogs and cats. It is more common in cats because this species has a relatively higher incidence of chronic kidney disease. Systemic hypertension has been described in chronic kidney disease (cats, dogs) due to several renal disorders (e.g., chronic interstitial nephritis, amyloidosis, glomerulonephritis, pyelonephritis, polycystic kidney disease, renal dysplasia), HAC (dogs, 60%), pheochromocytoma (dogs, 50%), DM (dogs, 51%), hyperthyroidism (cats, 87%), primary aldosteronism (dogs) and hypothyroidism (dogs), hyperkinetic cardiac syndrome (e.g., anemia, polycythemia, fever, arteriovenous fistula), hypercalcemia (dogs), and hyperestrogenism. Physiologic hypertension is present in gazing hounds, in which it is probably a normal phenomenon. Essential (primary, idiopathic) hypertension is probably an extremely rare condition in dogs and has not been described in cats. Obesity has been described as a risk factor for systemic hypertension in dogs. Most (77%) of the hypertensive dogs are males.
The ocular lesions in systemic hypertension include retinal and papillary edema, tortuous retinal blood vessels, and preretinal, intraretinal, and subretinal hemorrhage. Secondary retinal degeneration, probably due to ischemia and/or inflammation, is a common sequel. Animals with systemic hypertension may be presented with a complaint of acute blindness (with fixed, dilated pupils) caused by bullous retinal detachment due to subretinal effusion (Figure 18-21 ).
Figure 18-21.
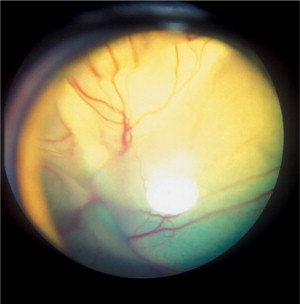
Retinal detachment in a cat with systemic hypertension. The retina is clearly visible as a vascularized membrane (note the folds of the “membrane”) seen through the pupil.
The diagnosis of systemic hypertension requires measurement of systolic or, preferably, systolic and diastolic blood pressures (BPs). Fractious animals may exhibit erroneously elevated BP. Therefore acclimation and several repeated measurements in a quiet, stress-free environment are advised. BP values (systolic/diastolic) exceeding 180/100 mm Hg are considered abnormally high, and values higher than 200/110 mm Hg to have clinical significance (Table 18-19 ).
Table 18-19.
Normal Arterial Blood Pressure Values (mm Hg) in the Dog and Cat
| DOG | CAT | |
|---|---|---|
| Systolic | 148 ±16 | 171 ± 22 |
| Diastolic | 87 ± 8 | 123 ± 17 |
Data from Gordon DB, Goldblatt H (1967): Direct percutaneous determination of systemic blood pressure and production of renal hypertension in the cat. Proc Soc Exp Biol Med 125:177; and Cowgill LCD, Kallet AJ (1986): Systemic hypertension, in Kirk RW (editor): Current Veterinary Therapy IX, 9th ed. Saunders, Philadelphia.
Treatment of systemic hypertension is primarily aimed at the underlying disorder. Commonly used drugs are listed in Table 18-20 . Antihypertensive drug therapy of systemic hypertension should lower the BP to a level that is not associated with the appearance of new lesions and probably should not attempt to normalize the BP. Drug therapy may consist of one or several agents, depending on the clinical signs and the underlying disease. These drugs include oral angiotensin-converting enzyme (ACE) inhibitors (e.g., enalapril, benazepril), calcium channel blockers (e.g., amlodipine), cardioselective adrenergic β-blockers (e.g., atenolol), vasodilators (e.g., hydralazine), and diuretics (e.g., furosemide). In emergency cases intravenous administration of nitroprusside or hydralazine may be advised; however, BP and urine production should be constantly monitored with this treatment because it may lead to a sharp drop in glomerular filtration.
Table 18-20.
Antihypertensive Drugs
| DOSAGE |
||
|---|---|---|
| GENERIC NAME | DOG | CAT |
| ANGIOTENSIN-CONVERTING ENZYME INHIBITORS | ||
| Enalapril | 0.5 mg/kg PO q12-24h | 0.25-0.5 mg/kg PO q12-24h |
| Captopril | 0.5-2.0 mg/kg PO q8-12h | 3.1-6.25 mg/cat PO q8-12h |
| Lisinopril | 0.5 mg/kg PO q24h | — |
| Benazepril | 0.25-0.5 mg/kg PO q24h | 0.25-0.5 mg/kg PO q24h |
| CALCIUM CHANNEL BLOCKERS | ||
| Diltiazem | 0.5-1.5 mg/kg PO q8-12h to maximum of 200 mg/day | 1.75-2.4 mg/kg q8h |
| Diltiazem sustained release | 10 mg/kg PO q24h | 10 mg/kg PO q24h |
| Amlodipine | 0.1 mg/kg PO q24h or 2.5 mg/dog | 0.625-1.25 mg/cat PO q24h |
| β-ADRENERGIC BLOCKERS | ||
| Propranolol | 0.2-1 mg/kg PO q8 to maximum of 200 mg/day | 0.4-1.2 mg/kg PO q8h or 2.5-5 mg/cat PO q8-12h |
| Atenolol | 0.2-2 mg/kg PO q12-24h or 6.25-12.5 mg/dog PO q12h | 2-3 mg/kg PO q12h or 6.25-12.5 mg/cat PO q12h |
| β-ADRENERGIC BLOCKERS | ||
| Prazosin | 0.065 mg/kg (1 mg/15 kg) PO q8-12h | 0.065 mg/kg (1 mg/15 kg) PO q8-12h |
| Phenoxybenzamine | 0.2-1.5 mg/kg PO q8-12h | 2.5-7.5 mg/cat PO q8-12h or 0.5 mg/kg PO q12h |
| VASODILATORS | ||
| Hydralazine | 0.5-2 mg/kg PO q12h | 2.5 mg/cat PO q12-24h |
| Nitroprusside | 1-10 μg/kg/min IV CRI | 1-10 μg/kg/min IV CRI |
| DIURETICS | ||
| Chlorothiazide | 20-40 mg/kg PO q12-24h | 20-40 mg/kg PO q12-24h |
| Hydrochlorothiazide | 2-4 mg/kg PO q12h | 2-4 mg/kg PO q12h |
| Furosemide | 1-4 mg/kg PO, IM, IV or SC q8-12h (or as needed) | 1-4 mg/kg PO, IM, IV or SC q8-24h |
CRI, Constant infusion rate.
Ocular treatment may be given to prevent secondary glaucoma and uveitis. Topical steroids may be prescribed, and systemic antiinflammatory treatment can be considered if the animal's systemic condition allows. Some clinicians advocate therapy with systemic carbonic anhydrase inhibitors.
POLYCYTHEMIA.
Polycythemia is an increase in the red blood cell mass above the reference range. Relative polycythemia is also referred to as erythrocytosis; this term is usually reserved for milder elevations of the hematocrit, most commonly due to hemoconcentration secondary to dehydration, and is a transient condition. True polycythemia can be primary or secondary. Primary polycythemia (polycythemia rubra vera) is a rare myeloproliferative disorder more commonly observed in cats (associated with FeLV infection) than in dogs. It is characterized by an abnormal proliferation of erythrocytes, platelets, and granulocytes; serum erythropoietin (EPO) concentrations are usually normal or mildly decreased. Secondary polycythemia may be appropriate and inappropriate and is associated with general hypoxemia or hypoxia, respectively. Chronic cardiopulmonary disorders, such as pulmonary neoplasia and right-to-left cardiac shunts, lead to hypoxemia, whereas renal neoplasia or neoplastic disorders of other abdominal organs may cause renal arterial blood flow obstruction and, consequently, renal hypoxia and a resultant increase in EPO concentrations. Hemoglobin disorders may also cause hypoxemia but are extremely rare in dogs and cats. All of these mechanisms lead to increases in production of EPO and its release from the hypoxic kidney, resulting in higher serum EPO concentrations, and, subsequently, in greater erythropoiesis and secondary polycythemia. EPO-secreting tumors have been reported rarely in humans and dogs (leiomyosarcoma, schwannoma) and were associated with secondary inappropriate polycythemia. Polycythemia leads to hyperviscosity, which reduces blood flow in the microcirculation resulting in local tissue hypoxia.
The ocular signs include dilated and tortuous dark red to brown conjunctival vessels, and the owner may complain of a “red eye.” Similar changes are seen in the retinal vasculature, and retinal vessels are described as having a “boxcar appearance” (i.e., intermittent dilatation and constriction of vessels). With progression, retinal hemorrhages and detachment occur. Uveitis and chorioretinitis were observed in dogs with polycythemia vera.
Examinations to diagnose polycythemia include hematologic profile, serum biochemistry, and urinalysis to differentiate relative (erythrocytosis) from absolute polycythemia. Further tests, such as arterial blood gas measurement, thoracic radiography, abdominal ultrasonography, and serum EPO concentration measurements, should be considered. In secondary polycythemia, the serum EPO concentration will be increased, whereas in primary polycythemia it will be low normal to decreased. Bone marrow cytologic examination shows erythroid hyperplasia and a decrease in the myeloid-to-erythroid (M:E) ratio in all polycythemic patients and thus is not diagnostically useful.
Relative polycythemia is treated with fluid therapy. Severe polycythemia is treated with repeated phlebotomies (10 to 20 mL/kg per treatment, until reaching a hematocrit of 50% to 55%). The treatment of secondary absolute polycythemia is aimed at the primary disease whenever possible. In polycythemia rubra vera and in cases of secondary absolute polycythemia in which correction of the primary disease is not possible (e.g., cyanotic heart disease), repeated phlebotomies and oral hydroxyurea (30 to 50 mg/kg q24h for 7 days, and then titration of dosage to effect) are recommended. Resolution of the ocular signs has been observed in cases in which polycythemia was resolved.
HYPERVISCOSITY SYNDROME.
In dogs and cats hyperviscosity syndrome is most commonly associated with malignancies such as multiple myeloma, chronic lymphocytic leukemia, lymphoma, and plasmacytoma (solitary osseous or extramedullary); however, it may also occur in certain infectious inflammatory diseases (e.g., canine ehrlichiosis and leishmaniasis). The increase of serum viscosity, which is due to the production and greater serum concentration of paraproteins, occurs more commonly with IgM class paraproteins (i.e., macroglobulinemia); however, IgA and IgG paraproteins have also been reported to lead to hyperviscosity syndrome in both dogs and cats. Clinical signs appear when serum viscosity rises to four to five times the normal level. Approximately 20% of the dogs with multiple myeloma have hyperviscosity syndrome.
The ocular signs of hyperviscosity syndrome include dilated, congested, tortuous retinal blood vessels, kinking of retinal blood vessels, papillary edema, retinal hemorrhages, intraretinal cysts, bullous retinal detachment, retinal degeneration, and blindness. Uveitis and secondary glaucoma have also been reported.
The diagnosis of hyperviscosity syndrome is based on serum viscosity measurement, although additional tests are needed to diagnose the specific causative disease. The diagnosis of multiple myeloma (and macroglobulinemia) may require serum electrophoresis and immunoelectrophoresis, skeletal survey radiographs, urinary heat precipitation test, and immunoelectrophoresis, whereas the definitive diagnosis usually requires a bone marrow aspirate or core biopsy. The diagnosis of lymphosarcoma may call for fine-needle aspirates or biopsies from lymphoid and visceral organs, thoracic radiography, and abdominal ultrasonography. The diagnosis of chronic lymphocytic leukemia is based on hematologic tests (e.g., complete blood count and peripheral blood cytology) and bone marrow cytology.
Multiple myeloma is best treated with melphalan or with other alkylating agents such as cyclophosphamide and chlorambucil. Some texts recommend the use of glucocorticoids, especially in presence of hypercalcemia and during the initial phase of treatment. Lymphoma is treated with a multidrug chemotherapeutical protocol, and chronic lymphocytic leukemia is usually treated with glucocorticoids and chlorambucil. Plasmapheresis is the preferred mode of therapy to treat hyperviscosity. Ocular treatment is symptomatic.
Immune-Mediated Diseases in Dogs
UVEODERMATOLOGIC SYNDROME (VOGT-KOYANAGI-HARADA–LIKE SYNDROME).
The uveodermatologic syndrome has been described in humans as well as in several dog breeds, including Samoyed, old English sheepdog, Siberian husky, Saint Bernard, Akita, Irish setter, chow chow, Shetland sheepdog, golden retriever, and Australian shepherd. The mean reported age was 3 years, and the ocular signs most commonly preceded the dermatologic lesions. The syndrome is a combination of several dermatologic signs (i.e., poliosis, vitiligo, and sometimes ulceration) and ocular signs. Meningitis or meningoencephalitis has been reported in the human disease; however, these complications are extremely rare in dogs. The cutaneous manifestations are most commonly restricted to the head area, occurring in the planum nasale (Figure 18-22 ), eyelids, and lips, but the footpads and the scrotum may be affected. The immunohistochemical findings in a recent study have suggested that the skin lesions were mediated by T cells and macrophages (Th1 immunity), whereas the ocular lesions were more consistent with a B-cell and macrophage response (Th2 immunity). These immune reactions are directed against melanocytes in the skin and in the (anterior and posterior) uvea.
Figure 18-22.
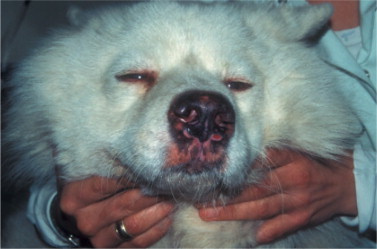
Depigmentation of the nasal planum in a 4-year-old Samoyed with uveodermatologic syndrome. Severe anterior uveitis and secondary glaucoma led to loss of vision in both eyes despite treatment with azathioprine.
The ocular disease is manifested as a bilateral uveitis—anterior uveitis or both anterior and posterior uveitis (panuveitis). Secondary ocular lesions, including cataract, posterior synechia, glaucoma, bullous retinal detachment, retinal and optic nerve atrophy, and acute blindness, are common. There is progressive depigmentation of the retinal pigment epithelium in the nontapetum, tapetal hyperreflectivity, and attenuation of the retinal blood vessels.
The diagnosis is based mainly on signalment and clinical presentation—in other words, on a combination of the ocular and dermatologic lesions. Histopathologic examination of the cutaneous lesions demonstrates a lichenoid dermatosis with dermal infiltration of histiocytes and lymphocytes and some giant cells as well as decreased melanin in the dermis and hair follicles.
Medical treatment consists of oral glucocorticoids and azathioprine for long periods (up to lifelong), combined with topical corticosteroids, NSAIDs, and atropine. In humans, an intravitreal triamcinolone-acetonide injection has led to improvement in vision and uveitis. Topical cyclosporine therapy has also been suggested. Treatment of the secondary complications of the syndrome is essentially symptomatic, and some complications (e.g., glaucoma, cataract) may require surgical intervention. Long-term prognosis for vision is usually poor. Recently, successful results were reported in a dog with use of oral and topical prednisone, along with 1% indomethacin eyedrops, methylprednisone (twice via the subconjunctival route at an interval of 15 days), and dorzolamide and timolol eyedrops, to prevent the development of secondary glaucoma due to posterior synechiae. Both dermatologic and ophthalmic signs showed good improvement, vision was preserved, and some repigmentation of the skin and hair occurred.
OCULAR MANIFESTATIONS OF SYSTEMIC DISEASES IN HORSES (Table 18-21, Table 18-22, Table 18-23, Table 18-24, Table 18-25, Table 18-26, Table 18-27, Table 18-28, Table 18-29, Table 18-30, Table 18-31, Table 18-32)
Table 18-21.
Systemic Causes of Conjunctivitis* in Horses and Cattle
| CAUSES | HORSE | COW |
|---|---|---|
| Viral diseases |
|
|
| Bacterial and related diseases | — | |
| Protozoal diseases | — | |
| Fungal diseases | Histoplasmosis§ | — |
| Parasitic diseases |
|
Gedoelstiasis† |
| Immune-mediated diseases | Pemphigus foliaceus | — |
| Neoplastic diseases | Lymphoma/lymphosarcoma§ | — |
| Toxic diseases | Generalized granulomatous disease | Vetch poisoning |
Associated ocular signs include ocular discharge/secretion, chemosis, congestion, and follicular hyperplasia.
Causes disease in both bovine and ovine species.
Causes disease in ovine species.
May cause ulceration of the conjunctiva and thereby lead to secondary corneal irritation.
Table 18-22.
Systemic Causes of Miscellaneous Conjunctival Disorders in Horses and Cattle
| DISORDER | HORSE | COW |
|---|---|---|
| Conjunctival/subconjunctival hemorrhage |
|
|
| Conjunctival icterus |
|
|
Table 18-23.
Systemic Causes of Keratitis/Keratoconjunctivitis* in Horses and Cattle
| CAUSES | HORSE | COW |
|---|---|---|
| Viral diseases |
|
|
| Bacterial and related diseases | Leptospirosis | |
| Protozoal diseases | Trypanosoma spp.† | |
| Fungal diseases | Histoplasmosis | — |
| Parasitic diseases |
|
|
| Immune-mediated diseases | Combined immunodeficiency | — |
| Toxic diseases | Generalized granulomatous disease |
|
Associated ocular signs include epiphora and discharge, blepharospasm, conjunctival congestion, and corneal edema, vascularization, infiltration, ulceration, and pigmentation.
Causes disease in both bovine and ovine species.
Causes disease in ovine species.
Table 18-24.
Systemic Causes of Anterior Uveitis* in Horses and Cattle
| CAUSES | HORSE | COW |
|---|---|---|
| Viral diseases | Equine adenovirus–microscopic panuveitis | Malignant catarrhal fever |
| Bacterial and related diseases |
|
|
| Protozoal diseases |
|
|
| Parasitic diseases |
|
Elaeophorosis |
| Neoplastic diseases | Lymphoma/lymphosarcoma | — |
| Other systemic causes | Multiple myeloma‡ | — |
| Foal diseases | — |
Associated ocular signs include corneal edema, flare (hypopyon/hyphema), hypotony, miosis, ciliary injection, blepharospasm, iris congestion, and photophobia. Secondary glaucoma and lens luxation are possible sequelae. Photophobia can also be caused by equine herpesvirus 2, equine viral arteritis, Leptospira, and Onchocerca in the horse.
Has been implicated as a potential cause of equine recurrent uveitis.
Has been shown to cause hyphema.
Table 18-25.
Systemic Diseases Causing Posterior Uveitis* in Horses and Cattle
| CAUSES | HORSE | COW |
|---|---|---|
| Viral diseases | Equine herpesvirus (experimental infection) | Malignant catarrhal fever |
| Bacterial and related diseases |
|
|
| Protozoal diseases | Toxoplasmosis | Toxoplasmosis |
| Parasitic diseases |
|
Elaeophorosis |
| Immune-mediated diseases | Combined immunodeficiency | — |
| Foal/calf diseases | Sepsis and failure of passive transfer | Septicemia |
| Toxic diseases | Generalized granulomatous disease | — |
Includes chorioretinitis and choroiditis. Associated signs include retinal edema and hemorrhage, subretinal effusion and hemorrhage, vascular cuffing, and loss of vision. Retinal detachment and retinal atrophy are possible sequelae.
Table 18-26.
Systemic Causes of Retinal Detachment, Hemorrhage, Atrophy, and Choroidal Depigmentation in Horses and Cattle
| DISORDER | HORSE | COW |
|---|---|---|
| Retinal detachment |
|
|
| Retinal hemorrhage |
|
|
| Retinal atrophy | Lyme disease |
|
| Choroidal/nontapetal depigmentation |
|
— |
Table 18-27.
Systemic Causes of Optic Nerve Disease in Horses and Cattle
| DISEASE | HORSE | COW |
|---|---|---|
| Optic neuropathy |
|
|
| Optic neuritis |
|
|
Table 18-28.
Systemic Causes of Central Blindness in Horses and Cattle*
| CAUSES | HORSE | COW |
|---|---|---|
| Viral diseases |
|
|
| Bacterial diseases | Strangles (Streptococcus equi) |
|
| Protozoal diseases | Equine protozoal myeloencephalitis | — |
| Parasitic diseases | — | Coenurosis |
| Neurologic diseases |
|
— |
| Metabolic diseases | — |
|
| Poisonings | Thiamine deficiency | Lead toxicity |
| Foal diseases |
|
— |
Optic nerve and retinal disorders may also cause blindness.
Table 18-29.
Systemic Causes of Neuroophthalmic Disorders in Horses and Cattle
| DISORDER | HORSE | COW |
|---|---|---|
| Nystagmus |
|
|
| Strabismus |
|
|
Table 18-30.
Systemic Causes of Orbital Disorders in Horses and Cattle
| DISORDER | HORSE | COW |
|---|---|---|
| Exophthalmos |
|
|
| Periorbital distention/edema |
|
— |
| Orbital cellulitis | Actinomycosis |
Table 18-31.
Systemic Causes of Pupillary Disorders in the Horse
| DISORDER | CAUSES |
|---|---|
| Horner's syndrome |
|
| Abnormal pupillary light reaction |
|
Table 18-32.
Systemic Causes of Adnexal Abnormalities in Horses and Cattle
| ABNORMALITY | CAUSES IN HORSE | CAUSES IN COW |
|---|---|---|
| Facial nerve paralysis* |
|
Listeriosis |
| Eyelid edema, infiltration, ulcers, alopecia, or crusting |
|
— |
| Eyelid protrusion |
|
— |
| Transient keratoconjunctivitis sicca | Strangles (Streptococcus equi) | — |
May lead to decreased tear production, secondary keratoconjunctivitis sicca, secondary corneal ulceration, exposure keratitis, and periorbital muscular atrophy.
Infectious Diseases
Viral Respiratory Diseases
EQUINE HERPESVIRUS (RHINOTRACHEITIS OR RHINOPNEUMONITIS).
Rhinotracheitis or rhinopneumonitis is a contagious viral respiratory disease caused by a member of the Alphaherpesvirinae subfamily (equine herpesvirus [EHV]-1 or EHV-4). It is difficult to differentiate clinically from signs due to other respiratory viruses. Infection is transmitted by aerosol, and the incubation period is 3 to 7 days. The disorder may also recur from a latent phase. The virus was successfully recovered from horses experimentally treated with corticosteroids, and it has also been found in lymph nodes of horses with no clinical signs of the disease. As with other viral respiratory diseases, rhinotracheitis is characterized by a high fever (up to 41°C) that is often biphasic and a serous to mucopurulent nasal discharge. In experimentally infected pony foals, mandibular lymph nodes were more significantly enlarged and coughs were less prominent with herpesvirus infection than with influenza infection. Leukopenia is the typical hematologic response. Conjunctivitis or keratitis may be observed, and a purulent ocular discharge could develop. Experimental infection of six foals resulted in bilateral chorioretinitis with mononuclear cell infiltration in one foal. Rhinotracheitis or rhinopneumonitis is usually a self-limiting disease, but topical antibiotics may be used to control secondary bacterial infections.
EHV-1 may also cause abortions or weakness in neonatal foals within the first week of life, as well as neurologic disease, which is apparently due to an immune complex vasculitis. Horses with neurologic disease may demonstrate nystagmus or blindness, depending on the location of the CNS lesions. Exposure keratitis or KCS secondary to facial paralysis and ulcerative lesions due to prolonged recumbency have also been documented.
EQUINE VIRAL ARTERITIS.
Equine viral arteritis is an arterivirus infection that causes vasculitis leading to abortion, respiratory disease, and even death. It can be transmitted by inhalation or venereally. There is a chronic carrier state in stallions. Clinical signs of the respiratory disease include pyrexia (up to 40.5°C) for 1 to 5 days, anorexia, depression, serous nasal discharge, lacrimation, and coughing. Edema of the limbs, eyelids, and scrotum is characteristic but is not seen in all cases. Leukopenia is found on hematologic evaluation. Neonatal foals may die acutely or may show severe respiratory signs. One ocular sign is serous to mucoid ocular discharge, as in the other respiratory viruses, but periorbital edema may also be seen. Corneal opacity and photophobia have been described.
EQUINE INFLUENZA.
Equine influenza is a contagious viral respiratory disease caused by the orthomyxovirus known as Equine influenza, particularly subtype 2 (AE-2). Outbreaks are more common in cooler, humid weather, as in winter and spring, depending on the climate. Horses 1 to 3 years of age are more susceptible during outbreaks. Infection occurs by aerosol, and the incubation time is 1 to 3 days. The damage to respiratory epithelial cells reduces the mucociliary clearance rate, apparently leading to secondary bacterial infections. Clinical signs include elevated rectal temperatures (40°to 41°C) that may be biphasic, reduced appetite, serous to mucoid nasal discharge, enlarged mandibular lymph nodes, and a cough. The cough may be very deep and last for several weeks. Detection of viral antigen up to 3 weeks after infection within vacuoles of alveolar macrophages has been reported. Hematologic changes include lymphopenia and eosinopenia followed by monocytosis a few days later. Ocular signs include epiphora and conjunctivitis (serous and erythematous) or keratoconjunctivitis, which usually resolve with resolution of the respiratory signs and do not require local treatment.
Bacterial Diseases
STRANGLES.
Streptococcus equi causes the disease known as “strangles” in horses. It affects primarily younger horses but may affect older horses that are immunologically naive. S. equi infection is transmitted by direct contact or via fomites such as water troughs, feed bunks, pastures, and stalls. The organism can survive at least 3 months in the environment. The disease usually causes fever and respiratory signs. The name is derived from the propensity of the organism to produce abscesses of lymph nodes, particularly around the head and upper neck, which can lead to suffocation through obstruction of the pharynx. Other lymph nodes, such as the mesenteric nodes, may also be involved; this condition is known as “bastard strangles.” Pharyngeal lymph nodes often drain before the horse recovers. The eyes may be involved in a mild inflammatory reaction, including dacryocystitis and transient KCS. The ocular discharge is often serous initially and mucopurulent later. Intraocular manifestations include anterior uveitis, panuveitis, chorioretinitis, retinal detachment, vitreous abscess, and optic neuritis. Ocular discharge and chorioretinal depigmentation in the nontapetal fundus of several horses have been described in one report. The depigmentation resolved spontaneously. In one case, anterior uveitis developed 10 days after strangles and subsequently progressed to corneal stromal abscesses and panophthalmitis. The organism was cultured from the eye. In a case of a brain abscess due to S. equi, the horse was blind.
SALMONELLOSIS.
Salmonellosis typically causes an acute colitis characterized by profuse watery diarrhea, endotoxemia, and coagulopathies with many severe sequelae, such as laminitis and acute renal failure. Horses are often depressed, dehydrated, febrile, and tachycardic and may exhibit abdominal pain. Recovered horses may shed Salmonella for months. Other manifestations of salmonella infections are chronic colitis and abortions in adult horses and respiratory infections, spinal abscesses, and septic arthritis in neonates. Signs of ocular involvement include anterior uveitis and hypopyon. The organism has been cultured from the anterior chamber.
LEPTOSPIROSIS.
Leptospira interrogans serovar pomona, a spirochete, has been documented in several foals and a stallion over the past decade. Pathogenetically, infection with the bacteria primarily causes a vasculitis and endotheliitis in multiple organs, particularly the kidneys and liver. Clinical signs include fever, depression, and partial anorexia. Azotemia is common. Gross hematuria has been observed in one foal, and leptospiruria detected in one foal. Leptospirosis should be considered in cases of acute renal failure with no obvious etiology. Leptospirosis also causes abortions, although less commonly in mares than in cows. Mares may show fever, depression and anorexia, and icterus for 3 to 4 days. Abortions occur 1 to 3 weeks later. Abortions are more common from the seventh month of pregnancy to term. Placentitis via ascending infections through the cervix is the primary cause.
Uveitis has been associated with leptospirosis but primarily weeks to months after the acute disease. Uveitis was not seen until 18 to 24 months after the acute outbreak of leptospirosis in one account. In an experimental infection the uveitis appeared no earlier than 1 year after infection and as late as 2 years later. Leptospira has been implicated as causing corneal opacities, anterior uveitis, equine recurrent uveitis, peripapillary chorioretinitis (Figure 18-23 ), and optic neuropathies, at least in experimentally infected horses. The association with equine recurrent uveitis may be directly due to bacterial infection or secondary to an immunologic reaction to infection. Even when antigen to Leptospira was found in the eye, antibiotic treatment did not decrease the inflammation. Serum titers for leptospira organisms were similar in horses with or without uveitis, but there were significant vitreous titers in 67% of eyes with uveitis and 0% in eyes without uveitis, indicating probably intraocular synthesis of antibodies. Direct culture of Leptospira from vitreous material taken from horses affected by equine recurrent uveitis was first reported in 1998 (9% of cases). Brem et al. (1998) report that two serovars were isolated, Leptospira grippotyphosa in three cases and a serovar out of the serogroup Australis in one case.
Figure 18-23.
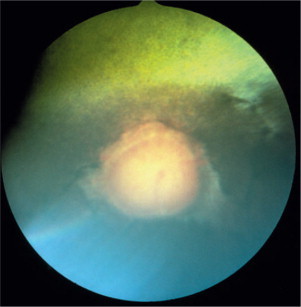
Peripapillary chorioretinal changes in a horse with chronic equine recurrent uveitis that was also seropositive for leptospirosis. The pale lesions around the optic disc, called “butterfly lesions,” are pathognomonic for the chorioretinitis that characterizes the disease.
(Courtesy Paul E. Miller.)
LYME DISEASE.
B. burgdorferi has been reported to cause polyarthritis in horses. One case report described organisms cultured from the anterior chamber. Spirochetes of B. burgdorferi were identified within the eye of a pony with arthritis and panuveitis.
Severe panuveitis with hyphema may occur, resulting in ocular hypotony or secondary glaucoma. Chronic inflammation may lead to rubeosis iridis (preiridal fibrovascular membranes, which appear clinically as congestion of the iris vessels), iridal hyperpigmentation, posterior synechiae, cataract, retinal atrophy and detachment, and blindness.
Protozoal Diseases
BABESIOSIS (PIROPLASMOSIS).
Babesiosis (piroplasmosis) is a tick-borne protozoan parasitic disease affecting red blood cells. Horses may be infected with Babesia caballi or Theileria equi (formerly known as Babesia equi). Typical signs of acute babesiosis include fever (39° to 42°C), hemolytic anemia, and jaundice. Hemoglobinuria and death can occur. B. caballi can be recognized as large intraerythrocytic organisms on blood smears, whereas T. equi is smaller and is often seen as four organisms in one erythrocyte, forming a “Maltese cross.” B. caballi can be passed vertically from one tick generation to the next, but T. equi is considered to be more pathogenic and tends to produce a carrier state in the horse. The ocular sign most commonly seen is icterus of the conjunctiva and sclera. Petechial hemorrhages of the conjunctiva, swelling of the periorbital fossa and eyelids as well as serous ocular discharge have also been reported with varying frequencies.
POTOMAC HORSE FEVER.
Neorickettsia risticii (formerly Ehrlichia risticii) is a known cause of Potomac horse fever (PHF), a disease characterized by enterocolitis in horses. The disease causes clinical signs of acute colitis similar to those of salmonellosis. The diarrhea can be profuse and watery and may be accompanied by endotoxemia. Endotoxemia is characterized by fever, leukopenia, congested mucous membranes, and hypercoagulability. Complications include sequelae typical of endotoxemia, but hypoproteinemia and laminitis are more commonly seen. N. risticii has also been associated with abortion between 6 and 8 months of pregnancy, but the incidence is not known. The organism may directly infect the eye, or ocular complications may result from systemic reaction to the disease. Hemorrhages may occur on the ocular surfaces, and infection of the eye may result in anterior uveitis and hyphema.
Parasitic Diseases
HABRONEMIASIS.
The larvae of the nematodes Habronema muscae, Habronema majus, and Draschia megastoma cause ulcerative cutaneous granulomas in horses. The adult nematodes inhabit the stomach. The eggs and larvae pass through the feces and are ingested by the maggots of the intermediate hosts (Musca domestica and Stomoxys calcitrans). The adult flies then deposit the larvae onto the mucous membranes, abraded skin, or open wounds in the horse. The disease occurs in the summer. Affected horses are predisposed to yearly recurrences. The infected area develops either proliferative, exuberant granulation tissue or ulcerative, nodular, and tumorous masses, which may have the characteristic yellow (sulfur) granules. Lesions may be seen on limbs, ventral body, prepuce, urethral process of the penis, commissure of the lips, and any other area of traumatized skin, but also in the conjunctiva and medial canthus of the eye. The granulation tissue may be a hypersensitivity reaction to dead or dying larvae.
When 63 cases were reviewed, ocular lesions were the most common, being seen either at the medial canthus (17 cases) or in the third eyelid (8 cases). Lesions were described as raised, proliferative, nonhealing wounds or granulation tissue with sulfur granules, mucopurulent discharge, chemosis, and injection of conjunctival vessels. The lesions may be friable and pruritic and may bleed easily. Fistulous tracts and subdermal nodules may develop below the medial canthus (Figure 18-24 ). The sulfur granules often seen are 1 to 2 mm in size. Corneal vascularization and edema can occur as a result of irritation of the cornea and altered lid function. Occasionally, corneal ulcers (2/17 in one study) and blepharospasm have been reported. Compared with the control population, Arabian horses were overrepresented, and thoroughbreds underrepresented. Color distribution may be a confounder, however, because horses of lighter colors are overrepresented compared with those of darker color, and Arabians tend to be lighter in color than thoroughbreds. There were no cases in horses younger than 1 year.
Figure 18-24.
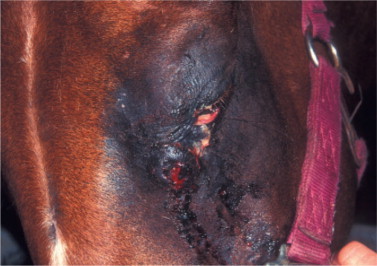
Habronemiasis in a horse. The nematode infestation caused an ulcer at the medial canthus, which drained into a fistulous tract.
CUTANEOUS ONCHOCERCIASIS.
Cutaneous onchocerciasis, a dermatitis caused by the microfilariae of Onchocerca cervicalis, is seen primarily in adult horses. The adult parasites are found in the funicular part of the ligamentum nuchae. The females produce microfilariae that migrate to the superficial dermis. The larvae are ingested by the vector, Culicoides, and transmitted to other horses after development of the larvae within the vector. Cutaneous lesions include diffuse or patchy alopecia, erythema, and scaling. Focal cutaneous depigmentation is common. Most of the lesions are found on the ventral midline, the lower eyelid, and the lateral limbus of the eye. Some are also found at the base of the mane as well as on the dorsomedial proximal forelimbs and cranial pectoral region. A bull's-eye lesion in the center of the forehead is characteristic. The lesions are nonseasonal and nonpruritic in most cases.
Ocular involvement is common in cutaneous onchocerciasis, being seen in 10% to 50% of cases. Initially there is chemosis and hyperemia of the conjunctiva accompanied by increased lacrimation and blepharospasm. Later, conjunctivitis, keratitis, depigmentation of the lateral limbus, and intraocular lesions are observed. Small, raised, white nodules (0.5 to 2mm in diameter) in the limbal conjunctiva and punctate, subepithelial corneal opacities of similar size are commonly present. Corneal lesions are often wedge-shaped with the base of the triangle at the limbus and are characterized by varying degrees of superficial and deep neovascularization and cellular stromal infiltrates. Lesions may progressively enlarge. With chronicity, patches of depigmentation occur. Recurrent episodes of keratoconjunctivitis are common. Both anterior and posterior uveitis are also observed. The former is characterized by photophobia, epiphora, miosis, aqueous flare, iris congestion, and hypotony. The latter is seen funduscopically as hyporeflective areas representing retinal edema. Inflammation around the optic papilla in a butterfly-shaped pattern may be present (see Figure 18-23) but is often hard to see owing to vitreous and aqueous opacification. Intraocular changes usually occur together with the eyelid lesions, suggesting that the initial invasion is in the eyelids. Intraocular filariae have been reported within the anterior chamber. O. cervicalis has also been implicated as a cause of equine recurrent uveitis.
Setaria INFECTION.
Setaria are primarily filarial parasites of cattle, which may aberrantly infect horses intraabdominally or in the spinal cord where they cause clinical signs of CNS disease. Reported cases included clinical signs of a hypotonic tail, bladder paralysis, ataxia, and conscious proprioceptive deficits. Setaria digitata and Setaria equina occasionally invade the eye, causing severe intraocular inflammation. They are the most common intraocular nematodes in the horse, particularly S. digitata. Successful surgical removal of the parasites from the anterior chamber has been reported.
Neurologic Diseases
EQUINE PROTOZOAL MYELOENCEPHALITIS.
Equine protozoal myeloencephalitis (EPM) is a multifocal, progressive disease of the CNS, most commonly caused by Sarcocystis neurona, although there have been reports of Neospora spp. as a cause. Clinical signs vary with the areas of the CNS affected. Originally the disease was described as causing asymmetric ataxia and associated muscle atrophy; however, involvement of cranial nerves and lesions of the cerebrum has also been reported. Ocular changes, including exposure keratopathy secondary to facial paralysis and decreased tear production, have been reported. Ptosis, enophthalmos, and prominence of the supraorbital process due to muscle denervation atrophy, Horner's syndrome, nystagmus, and blindness have also been reported. Horner's syndrome is a neurologic condition relating to an interruption of the ocular sympathetic pathways. In horses, the signs of Horner's syndrome primarily include ptosis, sweating, and warmth on the denervated side. Other signs seen in small animals with Horner's syndrome, such as miosis and enophthalmos with elevation of the third eyelid, are not prominent in the horse.
VIRAL ENCEPHALITIS.
The viruses of the Togaviridae family of arboviruses cause encephalitides in horses. The most prominent are members of the alphaviruses, which cause eastern, western, and venezuelan equine encephalitides. flaviviridae can also cause encephalitis, such as west nile fever, and japanese, california, st. louis, and murray valley encephalitides as well as cache valley, main drain, and borna fever. the clinical signs of all of the encephalitides are similar. fever is often reported early in the disease. neurologic signs related to diffuse encephalitis—depression, constant walking, head-pressing, constant chewing movements and ataxia—have been reported with eastern and western equine encephalitis. Additional signs, such as blindness, circling, excitement, and aggressive behavior, may also develop. As cortical damage worsens, paralysis of larynx, pharynx, and tongue may develop along with loss of brainstem function, leading to head tilt, nystagmus, strabismus, and pupil dilation. Signs of Venezuelan equine encephalitis may be similar to those of the other encephalitis viruses or may be unrelated, such as epistaxis, pulmonary hemorrhage, oral ulcers, and diarrhea. Horses with Venezuelan equine encephalitis occasionally appear blind. Seizures may occur with all three encephalitides.
Ocular signs are secondary to CNS disorders. They include blindness, nystagmus, strabismus, pupillary dilation, and facial nerve paralysis with secondary exposure keratopathy. Also, recumbency in horses may cause injuries to the eye or periorbital tissue owing to direct pressure, abrasion, chemical contact (e.g., urine), or foreign bodies such as shavings, dirt, and straw. These can cause conjunctivitis, keratitis, corneal ulcers, and secondary uveitis.
Clinical signs of Borna disease are similar to those of other equine encephalitides. Visual impairment and blindness may occur with CNS signs. Blindness is reportedly regularly observed in acute Borna disease. Nystagmus, strabismus, and miosis, due to involvement of the cranial nerves, have been reported.
West Nile virus meningomyeloencephalitis is a mosquito-borne virus closely related to St. Louis, Japanese, and Murray Valley encephalitides. A febrile response may occur with the onset of clinical disease. Initial signs, such as depression, listlessness, ataxia, and paresis, occur abruptly. Other signs progress over 1 to 3 days. These may include head shaking, incessant chewing, paralysis of the lower lip or tongue, severe ataxia, ascending paralysis, and terminal recumbency. Ocular signs, predominantly blindness, have been reported in horses with West Nile virus, particularly in the year 2000. This is a zoonotic disease, although horses are not a source of human infection. In humans, occlusive vasculitis, uveitis, chorioretinitis, and optic neuritis have been reported.
MENINGITIS.
Meningitis occurs either by direct extension of infectious agents into the calvarium (as with skull fractures or osteomyelitis from sinusitis or otitis, or as a sequel to surgical removal of progressive ethmoidal hematomas) or from hematogenous infection. Infection may be fungal, as with C. neoformans, but are more commonly bacterial. Bacteria involved in equine meningitis include Streptococcus zooepidemicus and Streptococcus suis in foals and Actinomyces spp. in adults. Meningitis of hematogenous origin in neonates commonly involves gram-negative bacteria such as Escherichia coli and Salmonella spp. and is associated with sepsis. Signs of meningitis include fever, anorexia, stiff neck, and hyperesthesia. It may be accompanied by diarrhea. The patient may be extremely depressed or hyperexcitable. Various other neurologic signs can be seen in addition to cranial nerve dysfunctions. Signs may progress to coma or status epilepticus. Ophthalmic signs are primarily due to cranial nerve dysfunction and include ptosis, strabismus, nystagmus, anisocoria, optic neuritis, and blindness.
PHOTIC HEAD SHAKING IN HORSES.
Photic head shaking in horses is stimulated by exposure to light and is exacerbated by exercise. The onset of the condition is usually in the spring. Affected horses often seek a darkened area. The mechanism is proposed to be an optic trigeminal summation via the infraorbital or facial sensory branch of the trigeminal nerve, with nasal stimulation or, alternatively, some as yet unidentified damage to the peripheral maxillary branch of the trigeminal nerve. Some success has been reported following treatment with cyproheptadine (0.3mg/kg bid), a histamine and serotonin blocking agent or with carbemazepine, a sodium channel blocking drug, or both. Attempts to control the signs have also included use of tinted contact lenses. Many cases do not respond to treatment.
Neuromuscular Diseases
TETANUS.
Tetanus is a neuromuscular disease caused by the toxin of the bacterium Clostridium tetani. It is characterized by muscular rigidity and death from respiratory arrest or convulsions. Usually, isolated cases occur when wounds are contaminated with the bacterium. Signs usually appear 2 to 4 weeks from the time of the injury. Within the first 24 hours, horses may show signs of colic. Additional early signs may include stiffness or lameness in the infected limb. The signs then progress to generalized spasticity with extended head posture. The hypertonia is most evident in the extensor muscles, so that the characteristic posture resembles that of a sawhorse (“sawhorse stance”). The tail becomes elevated, and eventually the lips and ears are pulled back. The jaws are tightly shut. The rigidity can be worsened by auditory, ocular, or tactile stimulation. The mortality is high, and death is usually due to hypoxia from paralysis of the respiratory muscles. Survivors begin to improve after 2 weeks, but the disease may take a month to resolve and the signs may not disappear completely. A classic sign of the disease is called “haws”; it involves the flashing of the third eyelid due to retraction of the eye that can be induced by sudden noises or sudden movement or contact such as a menacing gesture or a sharp blow to the lower jaw or neck.
BOTULISM (SHAKER FOAL OR FORAGE POISONING).
Botulism is a neuromuscular disease caused by the toxin of the bacterium Clostridium botulinum. Most commonly, the disease develops in adults through direct ingestion of the toxin, and in foals through ingestion of the spores or by contamination of wounds, as with tetanus. Signs of botulism are generalized muscle tremors and progressive weakness that can lead to recumbency. The animals remain bright and alert. Constipation and ileus are consistent signs that may cause colic. Dysphagia is common, and a characteristic sign is weakness of the tongue, which often appears relatively early. Death may occur from respiratory failure. In the case of recovery, the process is slow, requiring 10 to 14 days to resolve. Moderate mydriasis is an early sign of the disease and the pupillary light response may be sluggish. Ptosis has also been described.
EQUINE MOTOR NEURON DISEASE.
Equine motor neuron disease is presumed to be an oxidative condition of horses deprived of adequate dietary vitamin E. Clinical signs include muscle weakness and fasciculations with prolonged recumbency. Ocular involvement is common, being identified in 40 of 42 horses in one report. The fundic changes notes consisted of a dense mosaic of brown to black discoloration in lesions that were either widespread or found primarily in the transitional zone from tapetal to nontapetal fundus. Electroretinographic recordings showed a dramatic reduction in response to light stimulation, though behavioral visual defects are inconsistent.
Neoplastic Diseases
LYMPHOMA (LYMPHOSARCOMA).
Lymphoma is a sporadic but relatively common neoplasm in horses of all ages. Many organs can be involved. Leukemia is rare, although anemia is relatively common. The anemia may be due to bone marrow suppression or infiltration and therefore may be nonregenerative. Alternatively, blood loss may be the cause of the anemia or immune-mediated hemolysis may occur, particularly in the alimentary form of the disease. Lymphoma can affect horses of all ages, although the alimentary type appears to be more common in younger horses. Clinical signs include depression, weight loss, and lymphadenopathy. There may also be fever, respiratory distress, neurologic disease, mild colic, diarrhea, or ventral edema, depending on the tissues involved. Lymphadenopathy may be generalized or may involve only a few regional lymph nodes that may be internal, as occurs with the alimentary form. Splenic enlargement may be palpated rectally.
Rebhun and Del Piero (1998) describe involvement of the eye in 21 of 79 horses with lymphosarcoma seen over a 20-year period at the New York State College of Veterinary Medicine. The most common ocular manifestation was infiltration of the eyelids and palpebral conjunctivae (11 horses). Consistent findings included conjunctival thickening, hyperemia, and edema causing chemosis easily seen from a distance (Figure 18-25 ). Persistent serous or mucopurulent ocular discharge was observed in all 11 horses. Two horses had lesions limited to the third eyelid. Two horses had unilateral exophthalmos caused by diffuse orbital infiltration of lymphosarcoma, and two horses had corneoscleral masses.
Figure 18-25.
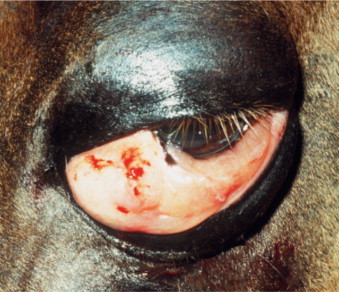
Orbital lymphoma with involvement of the bulbar and palpebral conjunctiva.
(Courtesy David J. Maggs.)
Malignant lymphoma frequently also invades the uvea and induces anterior uveitis (four horses in the 1998 study). Uveitis causes corneal edema, thickening of the iris, miosis, aqueous flare, and possible intraocular hemorrhage. Intraocular pressure usually decreases but may rise if glaucoma develops. If the vitreous is invaded by neoplastic cells, hemorrhage and retinal detachment can occur, thus leading to blindness. If the conjunctivae are invaded, then conjunctival ulceration can be seen. One would expect to find pale mucous membranes or petechiae in these cases because anemia and thrombocytopenia are common findings in horses with lymphoma in general (multicentric lymphoma, intestinal or thoracic).
Neonatal Diseases
ACQUIRED ENTROPION.
Entropion may be congenital or acquired in the neonate. Acquired entropion occurs with prematurity (see following section), with dehydration and the lack of periorbital fat, and with conditions associated with various disease processes, such as sepsis. Entropion leads to mechanical corneal abrasions or ulcers (Figure 18-26 ), which in turn cause conjunctivitis, lacrimation, and corneal edema.
Figure 18-26.
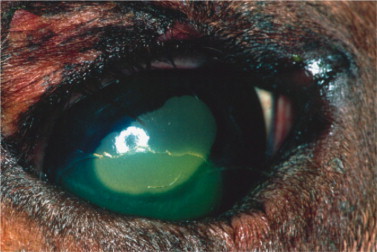
A 3-day-old Westphalian foal. Dehydration caused enophthalmia, which led to entropion of the lower eyelid. Mechanical abrasion by the inverted eyelid caused a corneal ulcer seen here stained with fluorescein.
(Courtesy Paul E. Miller.)
PREMATURITY.
Prematurity is a condition in which small or immature-appearing foals are born. A common definition is a foal born before 320 days of gestation. Signs of prematurity are small body size, short and silky haircoat, increased range of motion of joints, and immature skeletal ossification. Problems typical of premature foals include musculoskeletal problems, failure of passive transfer (see following section), and pulmonary dysfunction. Pulmonary dysfunction may be due to lung immaturity, as a primary or secondary surfactant deficiency. Retinal hemorrhages can be observed in hypoxic foals. Acquired entropion also occurs in premature foals (see Figure 18-26).
SEPSIS AND FAILURE OF PASSIVE TRANSFER.
Bacterial infections are an important cause of morbidity and mortality in neonates. Infections may be acquired prenatally through the placenta, from the mare's genital tract, or from the environment after birth. Infections most commonly involve gram-negative organisms normally present in the genital tract, skin, or environment. E. coli is most commonly isolated. There seems to be a trend recently, however, to more gram-positive isolates, such as Streptococcus, Staphylococcus, Enterococcus, and Clostridium spp. Portals of entry include the respiratory and gastrointestinal tracts and the umbilicus. Failure of passive transfer of immunoglobulins is assumed to be the predisposing cause of sepsis in foals. The infection leads to septicemia, which precipitates multiple organ failure. Later, the infection localizes in various organs, causing acute sepsis. Organs such as the lung, bones, joints, CNS, gastrointestinal system, and eyes are involved. Decreased pulmonary perfusion with sepsis can lead to dyspnea, or alternatively secondary pneumonia may develop either hematogenously or because of milk aspiration due to weak suckle reflex. Infections acquired in utero can also lead to pneumonia, which can result in hypoxia. Retinal hemorrhages can be observed in hypoxic foals.
Sepsis may cause anterior uveitis (Figure 18-27 ), which may be due to bacterial infection in the eye or to a sterile immunologic reaction. When due to bacterial infection, vitreous abscesses may develop. Ocular signs of anterior uveitis include corneal edema, iris congestion, hypototony, miosis, aqueous flare with fibrin deposition, hypopyon or hyphema, and, in severe cases, panophthalmitis may be seen. Chorioretinitis, retinal detachment, blindness, and neuroophthalmologic signs due to CNS involvement may also occur.
Figure 18-27.
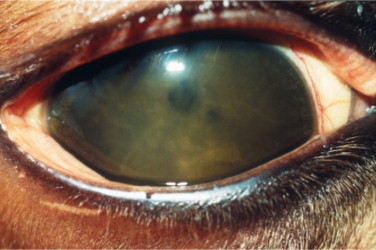
Anterior uveitis after septicemia in a foal. Note the diffuse corneal edema, the fibrin in the anterior chamber, and the miotic pupil. The large amount of fibrin in the anterior chamber is very indicative of foal septicemia.
(Courtesy David J. Maggs.)
NEONATAL MALADJUSTMENT SYNDROME.
Neonatal maladjustment syndrome (NMS) is a noninfectious CNS disorder of newborn foals of normal gestational age. Synonyms for the condition include “perinatal asphyxia syndrome,” “barkers,” “dummies,” and “wanderers.” The time of onset of signs varies from immediately at birth to around 24 hours of age. Affected foals primarily have signs of cerebral dysfunction or spinal cord deficits or both. Cerebral signs include loss of suckle reflex, aimless wandering, hyperexcitability or depression, extensor spasms or clonic convulsions, excessive chewing and salivation, abnormal vocalization, abnormal respiratory patterns, and apparent central blindness. Spinal cord signs include limb weakness, ataxia, and depressed spinal reflexes. The etiology is unknown, but birth asphyxia has been proposed. Many foals diagnosed as having neonatal maladjustment syndrome make a complete recovery with no residual neurologic deficits. The prognosis is less optimistic if sepsis occurs concomitantly, or if the signs began with birth or with dystocia, or if there is a history of asphyxia.
Complete or partial blindness may be seen in neonatal maladjustment syndrome. Subconjunctival hemorrhage, anisocoria, retinal hemorrhages, and papilledema have also been reported. As with adults, however, secondary ocular findings in cases of CNS disease include keratoconjunctivitis and corneal ulcers due to trauma during recumbency and entropion, which may be caused by spasm from corneal pain but commonly results from dehydration (see Figure 18-26).
Pulmonary lesions may develop secondary to neonatal maladjustment syndrome due to sepsis or aspiration pneumonia. If lung lesions lead to hypoxia, retinal hemorrhages can be observed.
NEONATAL ISOERYTHROLYSIS.
Neonatal isoerythrolysis is characterized by the destruction of red blood cells in the circulation of a foal by alloantibodies of the mother absorbed by the foal from the mare's colostrum. Because the antibodies are not naturally occurring, the disease does not appear until the mare is sensitized either by exposure during a previous pregnancy or through blood transfusion, or transplacentally during the current pregnancy, which is rare. The foals are normal at birth, developing signs 24 to 36 hours after ingesting colostrum. Early signs are those of progressive lethargy and weakness. Mucous membranes may be pale initially, but icterus develops. Hemoglobinemia and hemoglobinuria may be seen. Breathing becomes difficult, and seizures may occur as the anemia becomes more severe.
The predominant ocular sign is icterus of the conjunctiva; together with icterus of other mucous membranes, it is considered the cardinal sign of neonatal isoerythrolysis. However, conjunctival, episcleral, and intraocular hemorrhage can also occur.
Rhodococcus equi INFECTION.
Rhodococcus equi (formerly Corynebacterium equi) is a pleomorphic gram-positive rod that is a normal inhabitant of soil and can be cultured from horse feces. It causes a pyogranulomatous pneumonia in foals aged 2 to 6 months that are living on endemic farms. Infection is apparently transmitted through aerosolization of the bacteria and entry via the respiratory tract. Because the organism can live and multiply in alveolar macrophages, prolonged treatment with appropriate antibiotics is required. Clinical signs of R. equi infection are similar to those of pneumonia from other causes: fever, mucopurulent nasal discharge, tachypnea, dyspnea, and abnormal lung sounds on auscultation. Joint effusion that may be sterile, diarrhea, peritonitis, subcutaneous abscessation, and septic osteomyelitis and arthritis can also occur. Ocular signs described include hyphema and fibrin in the anterior chamber due to uveitis. The organism was cultured from the eye of one foal, indicating that the uveitis may be septic and not simply immune-mediated, as is often seen with the joint effusion. When large amounts of fibrin are found in the anterior chamber (see Figure 18-27), intracameral tissue-plasminogen activator treatment may be considered to prevent posterior synechia or traction retinal detachment.
OCULAR MANIFESTATIONS OF SYSTEMIC DISEASES IN RUMINANTS (see Table 18-21, Table 18-22, Table 18-23, Table 18-24, Table 18-25, Table 18-26, Table 18-27, Table 18-28, Table 18-29, Table 18-30, Table 18-31, Table 18-32)
Infectious Diseases
Bacterial Diseases
LISTERIOSIS.
Listeriosis is a bacterial disease of the brain caused by Listeria monocytogenes. Fever, anorexia, and depression are frequently observed. The organisms have a predilection for the brainstem, producing foci of necrosis and inflammation. The multiple neurologic signs include unilateral facial nerve paresis or paralysis, abducent nerve paralysis, trigeminal nerve motor paralysis, possible paresis or paralysis of the tongue, and pharyngeal paralysis. Signs of alterations in consciousness, circling, and paresis or paralysis of the limbs indicate that the lesion is confined to the CNS. Vestibular signs often accompany the lesion because of the involvement of vestibular nuclei in the medulla. Progression of the disease is associated with decreased consciousness, coma, and convulsions. CSF is often abnormal, with changes characteristic of nonsuppurative disease (despite the fact that this is a bacterial disease).
Ophthalmic signs include exposure keratitis (Figure 18-28 ) and, in chronic cases, KCS, anterior uveitis, and panophthalmitis. The disease may also cause lacrimation, photophobia, conjunctival hyperemia, and corneal edema. Neuroophthalmic signs are ptosis, medial strabismus, nystagmus, amaurosis, and blindness. Medial strabismus, together with other cranial nerve dysfunctions, strongly suggests listeriosis (see Chapter 16, Figure 16-16).
Figure 18-28.
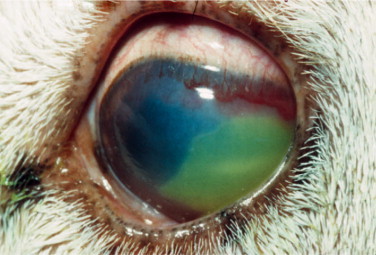
Corneal ulcer (stained with fluorescein) due to exposure keratitis in a sheep with listeriosis. The bacteria causes facial nerve paralysis, leading to this condition. Note the corneal edema and vascularization around the ulcer.
(Courtesy David J. Maggs.)
Other syndromes seen with L. monocytogenes are abortions and neonatal septicemia. No ocular signs have been associated with listerial abortion. Infected lambs may have spinal myelitis without brainstem disease. Some animals are depressed, and some not. Clinical signs include tetraparesis, tetraplegia, paraparesis, paraplegia, conscious proprioceptive deficits, and recumbency.
OVINE CHLAMYDIAL POLYARTHRITIS AND CONJUNCTIVITIS.
Chlamydophila psittaci (formerly Chlamydia psittaci) causes lameness and swollen joints in lambs. It is associated with high fever as well as with respiratory and, occasionally, neurologic disease. High morbidity and mortality are common. Up to 85% of lambs may show polyarthritis with lameness, stiff gait, and pyrexia. Ocular signs associated with keratoconjunctivitis may be an accompanying feature. Epiphora, conjunctival hyperemia, follicular hyperplasia and conjunctivitis, keratitis with peripheral edema, especially dorsally, and neovascularization may be seen in association with the lameness and swollen joints. C. psittaci is also a major cause of abortion in sheep and goats. Usually abortion occurs from placentitis in the fourth or fifth month of gestation. The dam is rarely ill. Other animals in the herd may have pneumonia or arthritis, although the serotype is perhaps not the same. The abortion serotype may not be associated with polyarthritis or keratoconjunctivitis. Topical tetracycline may be administered in addition to the systemic treatment, but the disease is usually self-limiting.
MYCOPLASMAL KERATOCONJUNCTIVITIS IN GOATS AND SHEEP.
Mycoplasma conjunctivae has been isolated from epidemics of keratoconjunctivitis, respiratory disease, and/or arthritis in goats and sheep (Figure 18-29 ). Mycoplasma and Ureaplasma have been isolated from cattle with conjunctivitis and mild respiratory signs. Mycoplasma mycoides var. mycoides has been isolated from an epidemic of mastitis, arthritis, and keratoconjunctivitis in goats. Mycoplasma agalactiae and Mycoplasma arginini have also been described as causing keratoconjunctivitis and systemic disease.
Figure 18-29.
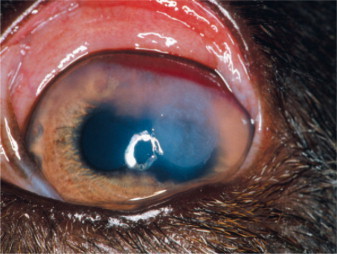
Keratoconjunctivitis in a goat with Mycoplasma spp. infection. Note the conjunctival congestion, severe vascular reaction, and diffuse stromal infiltration.
(Courtesy University of Wisconsin–Madison Veterinary Ophthalmology Service Collection.)
THROMBOEMBOLIC MENINGOENCEPHALITIS.
Thromboembolic meningoencephalitis (TEME) in cattle is due to Hemophilus somnus infection. It occurs in feedlots of yearlings in North America, especially during early winter. The infection produces vasculitis with thrombosis. H. somnus also causes yearling calf pneumonia, vulvitis, vaginitis, endometritis, and abortion in cattle. Death may occur 36 hours after appearance of the first neurologic signs in cattle with TEME.
Clinical signs include pyrexia, holding of the head up and forward, stupor, opisthotonos, ataxia, weakness, and paralysis. Circling may also be present. The classic ophthalmic sign is retinal exudates with hemorrhages (retinitis) (Figure 18-30 ), although nystagmus, strabismus, and blindness may also occur.
Figure 18-30.
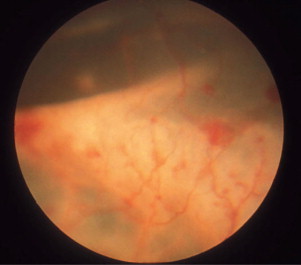
Retinal detachment and multifocal hemorrhages, characteristic of retinitis, in a cow with thromboembolic meningoencephalitis.
(Courtesy Drs. G.A. Severin and Julie Gionfriddo, Colorado State University.)
In later stages of the disease, quadriplegia and cranial nerve deficits reflect focal brain lesions. CSF has a high protein content and neutrophilia but is usually sterile.
Thromboembolic meningoencephalitis may be tentatively diagnosed ophthalmoscopically.
Peracute deaths with neurologic and ophthalmoscopic signs are suggestive of thromboembolic meningoencephalitis. The diagnosis is confirmed by the histologic lesions. A vaccine is available. Early treatment before recumbency may be implemented, but residual joint and neurologic disease may limit long-term growth and performance.
Mannheimia (Pasteurella) PNEUMONIA.
Severe Mannheimia (Pasteurella) haemolytica pneumonia in calves may cause conjunctivitis resulting in a mucopurulent discharge. The disease also affects sheep and goats. Topical antibiotics may be indicated for treatment of the conjunctivitis.
TUBERCULOSIS.
Mycobacterium bovis is the most common cause of tuberculosis in cattle and goats. Sheep are relatively resistant. Clinical signs are often inapparent; however, weight loss, variable appetite, and fluctuating fever may occur. Signs related to the respiratory system are relatively common, but gastrointestinal signs and reproductive disorders may also be seen. Granulomatous lesions in the eyes of cattle have been reported. The uvea is initially affected in both the anterior and posterior sections. Keratitis, anterior uveitis, chorioretinitis, and retinal detachment may be seen.
SEPTICEMIA.
Septicemia is the most common cause of uveitis in calves. Ophthalmic signs include conjunctival and ciliary injection, miosis, iris congestion, hypotony, and fibrin or hypopyon in one or both eyes (Figure 18-31 ). Chorioretinitis may also occur, and panophthalmitis has been described in severe cases. Typical embolic lesions of multifocal hemorrhages, exudates, and focal retinal detachments (see Figure 18-30) may also be present but may not be observed owing to the changes in the anterior chamber. Uveitis associated with septicemia is less common in adult cattle than in calves but does occur. Adult cattle are susceptible to septic mastitis, septic metritis, peritonitis, and endocarditis. Therapy should include treatment of the uveitis. Septicemia may also result in chorioretinitis. Funduscopically, the lesions appear as focal or multifocal exudative lesions, often perivascular (see Chapter 15, Figure 15-50, A). Inactive retinal lesions from prior septicemia may be observed as hyperreflective areas in the tapetal region and depigmented gray areas in the nontapetal region of the retina. The scarred lesions in the tapetum may be hyperpigmented centrally. In most cases the lesions do not cause blindness. In overwhelming septicemia, thrombocytopenia can occur from the excessive consumption of platelets, which can lead to disseminated intravascular coagulation. In this situation, conjunctival hemorrhages may be seen as petechiae or ecchymoses.
Figure 18-31.
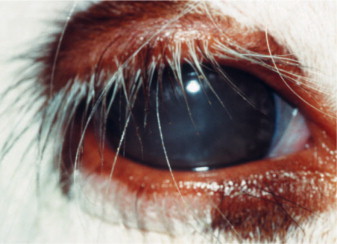
Anterior uveitis in a calf with septicemia. Note the diffuse corneal edema and miotic pupil.
(Courtesy David J. Maggs.)
Viral Diseases
ARTHROGRYPOSIS-HYDRENCEPHALY.
Akabane virus is known to cause arthrogryposis (permanent joint contracture) and hydrencephaly (replacement of missing cerebral tissue) in sheep. Calves born to affected cows show arthrogryposis and hydranencephaly as well. These conditions frequently cause dystocia at birth. Those surviving can be blind and mentally deranged. Ocular lesions include attenuation of retinal vessels, tapetal hyperreflectivity, pigmentary changes, and optic atrophy. Diagnosis is confirmed by a rising titer to the virus in serum.
BLUETONGUE.
Bluetongue is an arthropod-borne viral disease that infects ruminants. Clinical signs are most commonly seen in sheep, but cattle and goats occasionally show signs of the disease. Bluetongue causes a vasculitis and may cause a reproductive syndrome leading to abortion, embryonic death, and fetal anomalies. Vaccination of pregnant ewes with attenuated bluetongue virus in the first half of pregnancy leads to necrotizing retinopathy and CNS malformations. During the last half of pregnancy the fetus is resistant.
Ewes should not be vaccinated for bluetongue in the first half of pregnancy.
In cattle infection is usually asymptomatic, although severe conjunctivitis with serous or mucopurulent discharge may be seen, particularly in chronically infected animals. Systemic signs include mucosal lesions, edema of the lips, and laminitis. Infection of a pregnant cow can lead to hydranencephaly in the fetus, abortion, arthrogryposis, and other defects.
INFECTIOUS BOVINE RHINOTRACHEITIS.
Bovine herpesvirus 1 infections occur in four forms—the conjunctival form, in which no other signs are present, and the more common respiratory form, often referred to as infectious bovine rhinotracheitis or rednose. In this form, conjunctivitis is sometimes absent. Infectious pustular vulvovaginitis and an abortive form also occur, depending on the strain of virus. In the conjunctival and respiratory forms conjunctivitis is acute, erythematous, and serous with profuse lacrimation. White plaques may be present on the conjunctiva (Figure 18-32 ). Chemosis is sometimes present, but corneal lesions are rare. In the respiratory form, anorexia, fever, hyperemia of the nasal mucosa, nasal discharge, and salivation occur. In the early acute stages, ocular lesions can be distinguished from those of infectious bovine keratoconjunctivitis (pink eye) by the lack of corneal involvement. In later stages, nonulcerative keratitis with corneal vascularization and opacity, spreading toward the center of the cornea, may occur.
Figure 18-32.
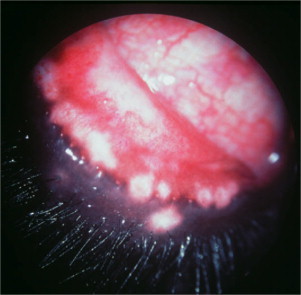
Severe conjunctivitis in a cow with infectious bovine rhinotracheitis. Note the white plaques that characterize the conjunctival form of the disease.
(Courtesy Cecil Moore.)
Early infectious bovine rhinotracheitis is distinguished from infectious bovine keratoconjunctivitis by lack of corneal involvement.
Goats are also susceptible to infectious bovine keratoconjunctivitis. Ocular signs include conjunctivitis and keratitis, which occur after onset of respiratory illness. Infectious bovine keratoconjunctivitis virus has been isolated from ocular and nasal discharge in goats.
MALIGNANT CATARRHAL FEVER.
Malignant catarrhal fever, also known as bovine malignant catarrh, is a highly fatal viral disease of cattle that may cause sporadic outbreaks or epizootics. The disease in cattle is caused by a herpesvirus, and the sheep disease may be caused by a sheep herpesvirus. Ocular lesions are seen in the “head and eye” form of the disease, although four other syndromes have been described. The catarrhal inflammation of upper respiratory and alimentary mucous membranes aids in differentiating the disease from other fulminating bovine viral diseases. Keratoconjunctival exanthema and lymph node enlargement also occur. Ocular lesions distinguish malignant catarrhal fever from mucosal disease, rinderpest, muzzle disease, and infectious stomatitis.
The corneal lesions of malignant catarrhal fever start at the limbus and progress toward the center of the cornea, distinguishing them from infectious bovine keratoconjunctivitis, which usually begins in the center of the cornea. In addition to the classic corneal lesions, severe bilateral uveitis and panophthalmitis occur (Figure 18-33 ) together with the high fever (40.5° to 42°C), depression, and mucosal erosions. The disease is almost always fatal over 24 to 96 hours. Ocular manifestations include severe bilateral uveitis, leading to ciliary injection, corneal edema, hypotony, miosis, iris congestion, and fibrin or hypopyon in the anterior chamber (see Figure 18-33). The choroid is usually spared, but retinal vasculitis is often present and blindness is possible. It is difficult to observe the retinal lesions in the living animal because of the lesions in the anterior segment. Histopathologic examination shows severe vasculitis in all major organs and all parts of the eye except the choroid.
Figure 18-33.
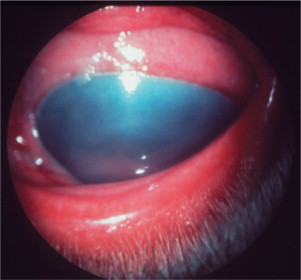
Anterior uveitis, with severe corneal edema, in a cow with malignant catarrhal fever.
(Courtesy Cecil Moore.)
BOVINE VIRAL DIARRHEA.
Bovine viral diarrhea virus is a pestivirus RNA virus of the Flaviviridae family. It causes a widespread contagious viral disease of cattle, sheep, goats, and wild ruminants, occurring in mild, acute, and chronic forms. In its mucosal disease syndrome it causes diarrhea outbreaks and can be a fatal disease in persistently infected cattle from in utero exposure. A hemorrhagic syndrome is characterized by marked thrombocytopenia, bloody diarrhea, epistaxis, hemorrhages on mucosal surfaces such as the conjunctiva, hyphema, bleeding from injection sites, pyrexia, leukopenia, and death. This syndrome is associated only with the noncytopathic isolate of bovine viral diarrhea virus. Bovine viral diarrhea also plays a role in the respiratory disease complex, together with M. haemolytica and viruses such as bovine herpesvirus 1 (BHV-1) and bovine respiratory syncytial virus (BRSV), by virtue of the immunosuppressive effects it produces. It has also been implicated in hydranencephaly, abortion and early embryonic death, and fetal anomalies.
Bovine viral diarrhea causes corneal opacity in adult cattle. Exposure of the fetus to the virus causes cataract, retinal atrophy, optic neuritis, microphthalmia with retinal dysplasia, and cerebellar hypoplasia. A gray optic disc due to optic atrophy, vascular attenuation, tapetal hyperreflectivity, pigment clumping, and multifocal depigmentation of the nontapetal fundus characterize the funduscopic lesions. Calves with ocular signs may be blind, with or without pupillary light response, and there may be ocular discharge in acute or chronic cases.
MAEDI-VISNA.
Maedi-visna is a chronic progressive encephalitis of sheep caused by a retrovirus (subfamily Lentivirinae). Nervous system signs of the disease are characteristic of diffuse encephalitis. They include ataxia, twitching of the facial muscles, conscious proprioceptive deficits, staggering or stumbling when turned, circling, and blindness. PCR analysis, immunohistochemistry, and in situ PCR examination have been used to detect the virus in third eyelids of infected sheep with typical maedi-visna pulmonary lesions.
SCRAPIE.
Scrapie is a transmissible form of spongiform encephalopathy that causes degenerative CNS disease in sheep and, less commonly, goats. The disease occurs in animals 1 to 5 years old. It has a slow clinical course. Nervousness, restlessness, weight loss, and pruritus have been described. In both sheep and goats scrapie causes multifocal, round retinal detachments in the tapetal fundus owing to accumulations of subretinal fluid. Finding of these lesions in association with chronic neurologic signs suggests a diagnosis of scrapie.
Protozoal Diseases
BABESIOSIS.
As mentioned in the section on horses, babesiosis is a tick-borne intraerythrocytic disease. The acute disease is characterized by fever, hemolytic anemia, icterus, hemoglobinuria, and death. At least six species of Babesia infect cattle, and two infect sheep and goats. Cerebral babesiosis, characterized by hyperexcitability, convulsions opisthotonos, coma, and death, may be observed in cattle, particularly those infected with Babesia bovis. Babesiosis due to Babesia spp. also causes conjunctival injection and icterus in affected cattle. The signs resolve with treatment of the systemic disease. Trypanosoma brucei causes keratoconjunctivitis, uveitis, and optic neuritis in sheep. Other Trypanosoma spp. cause edema, hyperemia and petechiation of the conjunctiva in ruminants.
Toxoplasma gondii INFECTION.
The ubiquitous protozoan. T. gondii is a major abortifacient in sheep and goats. It rarely causes disease in ruminants, and infection with the protozoan is often asymptomatic. Ocular signs are rare, but T. gondii may infiltrate the retina and uvea, causing retinitis and chorioretinitis due to a primary posterior segment lesion. Anterior uveitis may also be present.
Parasitic Diseases
COENUROSIS.
Coenurosis, also known as gid and sturdy, is a disease caused by invasion of the ovine brain by intermediate stages of Taenia multiceps and Taenia serialis. The disease is most commonly seen in sheep but can occur in cattle and goats as well. The initial clinical signs include frenzy, convulsions and salivation. They are followed by dullness, head pressing, head deviation, and circling as well as by ophthalmic manifestations, including papilledema and blindness.
Neoplasia
LYMPHOSARCOMA.
Lymphosarcoma should be suspected in a cow with exophthalmos, because the tumor may involve the retrobulbar lymphoid tissue. Unilateral presentation is most common. In the absence of other enlarged lymph nodes or other areas of lymphocytic infiltration, differential diagnoses include orbital cellulitis, orbital trauma, retrobulbar hemorrhage, and chronic sinusitis with orbital extension. The cornea on the affected side can be expected to undergo rapid desiccation and ulceration (Figure 18-34 ). A syndrome consisting of solid infiltration of the conjunctiva is also observed and must be distinguished from chemosis. Affected animals are sent to slaughter.
Figure 18-34.
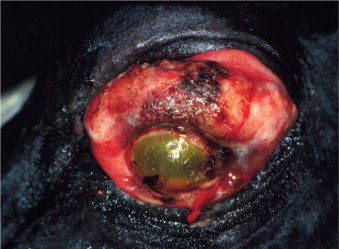
Orbital lymphosarcoma, with extensive conjunctival involvement, in a cow. The tumor also caused exposure keratitis and corneal desiccation.
(Courtesy Paul E. Miller.)
Metabolic Diseases
POLIOENCEPHALOMALACIA (PEM).
Polioencephalomalacia, also called cerebrocortical necrosis, occurs in pigs, sheep, and cattle and may be related to thiamine deficiency. Lambs between 2 and 4 months of age and 6-month-old calves are most commonly affected. Polioencephalomalacia occurs at pasture and in feedlots.
The initial ocular sign in sheep is trochlear nerve paralysis, which causes dorsomedial strabismus. Initial clinical signs in sheep include head pressing, aimless wandering or motionless standing, and cortical blindness. These initial signs progress to recumbency, opisthotonus, hyperesthesia, tonic-clonic convulsions, and nystagmus.
Initial clinical signs in cattle include cortical blindness, muscle tremor (head especially), salivation, opisthotonus, convulsions, head pressing, depression, and anorexia. These signs are followed by recumbency, nystagmus, and papilledema.
Blindness is often the first sign to appear and the last to resolve and may be the only sign, other than depression, in adult cattle. It may take up to one week after the resolution of the other signs for vision to return. Despite the fact that the blindness is central, papilledema and decreased pupillary light reflexes may occur and bilateral dorsomedial strabismus may be present.
Toxic Diseases
TOXIC PLANTS.
Various toxic plants have been described as causing ocular lesions. Among them are the following:
-
•
Male fern (Dryopteris spp.), which causes blindness due to optic nerve atrophy
-
•
Bracken fern, which can cause conjunctival hemorrhages and outer retinal degeneration
-
•
Helichrysum argyrosphaerum, causing blindness with retinal lesions in sheep and cattle
-
•
Veratrum in sheep, which leads to cyclopia or anophthalmia in lambs when the ewes ingest the plant during pregnancy
-
•
Locoweed, causing blindness with various intraocular, histopathologic changes
-
•
Many plants that cause liver damage, which manifests as jaundice of the conjunctiva and sclera
VETCH TOXICITY.
Vetch (Vicia spp.) poisoning has been reported in cattle. The following three clinical manifestations have been described:
-
•
An acute neurologic manifestation
-
•
A fatal form leading to death in 12 to 15 days and causing signs of weakness and loss of appetite, alopecia, subcutaneous swellings, herpetiform eruptions of the oral mucous membranes, purulent nasal discharge, abnormal lung sounds, cough, and cyanosis of mucous membranes
-
•
A systemic granulomatous disease, causing dermatitis, pruritus, diarrhea, dehydration, weight loss, decreased milk yield, cough, dyspnea, and conjunctivitis
A study of 10 cows with high suspicion of vetch toxicity reported moderate ocular and nasal discharge, which was usually serous but in some cases mucopurulent, in 7 animals. These researchers did not mention necropsy findings of ocular changes; however, ocular lesions in other species with vetch toxicity include conjunctivitis, ulcerative keratitis, and diffuse granulomatous inflammation of the choroid.
BIBLIOGRAPHY
Ocular Manifestations of Systemic Disease in Dogs and Cats
Canine Distemper
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Greene CE, Appel MJ. Canine distemper. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 25. [Google Scholar]
- Koutinas AF. Relation of clinical signs to pathological changes in 19 cases of canine distemper encephalomyelitis. J Comp Pathol. 2002;126:47. doi: 10.1053/jcpa.2001.0521. [DOI] [PubMed] [Google Scholar]
- Raw ME. Canine distemper infection associated with acute nervous signs in dogs. Vet Rec. 1992;130:291. doi: 10.1136/vr.130.14.291. [DOI] [PubMed] [Google Scholar]
- Sellon RK. Canine viral disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 646. [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Tipold A. Diagnosis of inflammatory and infectious diseases of the central nervous systemic dogs: a retrospective study. J Vet Intern Med. 1995;9:304. doi: 10.1111/j.1939-1676.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Willis AM. Canine viral infections. Vet Clin North Am Small Anim Pract. 2000;30:1119. doi: 10.1016/s0195-5616(00)05010-5. [DOI] [PubMed] [Google Scholar]
Infectious Canine Hepatitis
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Greene CE. Infectious canine hepatitis and canine acidophil cell hepatitis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 41. [Google Scholar]
- Sellon RK. Canine viral disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 646. [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Willis AM. Canine viral infections. Vet Clin North Am Small Anim Pract. 2000;30:1119. doi: 10.1016/s0195-5616(00)05010-5. [DOI] [PubMed] [Google Scholar]
Feline Herpesvirus (Feline Rhinotracheitis Virus, FHV-1, FRV) Infection
- Gaskell RM, Dawson S. Other feline viral diseases. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 667. [Google Scholar]
- Gaskell R. Feline respiratory disease. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 145. [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Lappin MR. Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats. J Am Vet Med Assoc. 2002;220:38. doi: 10.2460/javma.2002.220.38. [DOI] [PubMed] [Google Scholar]
- Nasisse MP. Clinical and laboratory findings in chronic conjunctivitis in cats: 91 cases (1983-1991) J Am Vet Med Assoc. 1993;203:834. [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Stiles J. Feline herpesvirus. Clin Tech Small Anim Pract. 2003;18:178. doi: 10.1016/s1096-2867(03)90014-4. [DOI] [PubMed] [Google Scholar]
- Stiles J. Treatment of cats with ocular disease attributable to herpesvirus infection: 17 cases (1983-1993) J Am Vet Med Assoc. 1995;207:599. [PubMed] [Google Scholar]
- Stiles J. Comparison of nested polymerase chain reaction, virus isolation, and fluorescent antibody testing for identifying feline herpesvirus in cats with conjunctivitis. Am J Vet Res. 1997;58:804. [PubMed] [Google Scholar]
Feline Calicivirus Infection
- Dawson S. Acute arthritis of cats associated with feline calicivirus infection. Res Vet Sci. 1994;56:133. doi: 10.1016/0034-5288(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Gaskell R. Feline respiratory disease. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 145. [Google Scholar]
- Gaskell RM, Dawson S. Other feline viral diseases. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 667. [Google Scholar]
- Hurley KF, Sykes JE. Update on feline calicivirus: new trends. Vet Clin North Am Small Anim Pract 2003. 2003;33:759. doi: 10.1016/s0195-5616(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Knowles JO. Studies on the role of feline calicivirus in chronic stomatitis in cats. Vet Microbiol. 1991;27:205. doi: 10.1016/0378-1135(91)90148-9. [DOI] [PubMed] [Google Scholar]
- Radford AD. Feline calicivirus. Vet Res. 2007;38:319. doi: 10.1051/vetres:2006056. [DOI] [PubMed] [Google Scholar]
- Ramsey DT. Feline chlamydia and calicivirus infections. Vet Clin North Am Small Anim Pract. 2000;30:1015. doi: 10.1016/s0195-5616(00)05004-x. [DOI] [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Sykes JE. Detection and strain differentiation of feline calicivirus in conjunctival swabs by RT-PCR of the hypervariable region of the capsid protein gene. Arch Virol. 1998;143:1321. doi: 10.1007/s007050050378. [DOI] [PubMed] [Google Scholar]
Feline Leukemia Virus Infection
- Arjona A. Seroepidemiological survey of infection by feline leukemia virus and immunodeficiency virus in Madrid and correlation with some clinical aspects. J Clin Microbiol. 2000;38:3448. doi: 10.1128/jcm.38.9.3448-3449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Hartmann K. Feline leukemia virus infection. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 105. [Google Scholar]
- Lappin MR. Opportunistic infections associated with retroviral infections in cats. Semin Vet Med Surg (Small Anim) 1995;10:244. [PubMed] [Google Scholar]
- Levy JK, Crawford PC. Feline leukemia virus. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 653. [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Vail DM. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med. 1998;12:349. doi: 10.1111/j.1939-1676.1998.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Willis AM. Feline leukemia virus and feline immunodeficiency virus. Vet Clin North Am Small Anim Pract. 2000;30:971. doi: 10.1016/s0195-5616(00)05001-4. [DOI] [PubMed] [Google Scholar]
Feline Immunodeficiency Virus Infection
- Arjona A. Seroepidemiological survey of infection by feline leukemia virus and immunodeficiency virus in Madrid and correlation with some clinical aspects. J Clin Microbiol. 2000;38:3448. doi: 10.1128/jcm.38.9.3448-3449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty JA. Feline immunodeficiency virus (FIV)-associated lymphoma: a potential role for immune dysfunction in tumourigenesis. Vet Immunol Immunopathol. 1998;65:309. doi: 10.1016/s0165-2427(98)00164-0. [DOI] [PubMed] [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Hartmann K. Feline immunodeficiency virus infection and related diseases. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 659. [Google Scholar]
- Hartmann K. Feline immunodeficiency virus infection: an overview. Vet J. 1998;155:123. doi: 10.1016/S1090-0233(98)80008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin MR. Primary and secondary Toxoplasma gondii infection in normal and feline immunodeficiency virus-infected cats. J Parasitol. 1996;82:733. [PubMed] [Google Scholar]
- Lappin MR. Serologic prevalence of selected infectious diseases in cats with uveitis. J Am Vet Med Assoc. 1992;201:1005. [PubMed] [Google Scholar]
- Malik R. Prevalences of feline leukaemia virus and feline immunodeficiency virus infections in cats in Sydney. Aust Vet J. 1997;75:323. doi: 10.1111/j.1751-0813.1997.tb15701.x. [DOI] [PubMed] [Google Scholar]
- Nasisse MP. Clinical and laboratory findings in chronic conjunctivitis in cats: 91 cases (1983-1991) J Am Vet Med Assoc. 1993;203:834. [PubMed] [Google Scholar]
- Sellon RK, Hartmann K. Feline immunodeficiency virus infection. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 131. [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Willis AM. Feline leukemia virus and feline immunodeficiency virus. Vet Clin North Am Small Anim Pract. 2000;30:971. doi: 10.1016/s0195-5616(00)05001-4. [DOI] [PubMed] [Google Scholar]
Feline Infectious Peritonitis Infection
- Addie DD. Risk of feline infectious peritonitis in cats naturally infected with feline coronavirus. Am J Vet Res. 1995;56:429. [PubMed] [Google Scholar]
- Addie DD, Jarret O. Feline coronavirus virus infection. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 88. [Google Scholar]
- Addie DD, Jarrett O. Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Vet Rec. 2001;148:649. doi: 10.1136/vr.148.21.649. [DOI] [PubMed] [Google Scholar]
- Foley JE. Patterns of feline coronavirus infection and fecal shedding from cats in multiple-cat environments. J Am Vet Med Assoc. 1997;210:1307. [PubMed] [Google Scholar]
- Foley JE. Risk factors for feline infectious peritonitis among cats in multiple-cat environments with endemic feline enteric coronavirus. J Am Vet Med Assoc. 1997;210:1313. [PubMed] [Google Scholar]
- Gamble DA. Development of a nested PCR assay for detection of feline infectious peritonitis virus in clinical specimens. J Clin Microbiol. 1997;35:673. doi: 10.1128/jcm.35.3.673-675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Legendre AM. Diagnosis and prevention of feline infectious peritonitis. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 291. [Google Scholar]
- Li X, Scott FW. Detection of feline coronaviruses in cell cultures and in fresh and fixed feline tissues using polymerase chain reaction. Vet Microbiol. 1994;42:65. doi: 10.1016/0378-1135(94)90078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CW. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet Microbiol. 1993;36:1. doi: 10.1016/0378-1135(93)90126-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
Brucellosis (Dogs)
- Carmichael LE, Greene CE. Canine brucellosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 369. [Google Scholar]
- Carmichael LE, Shin SJ. Canine brucellosis: a diagnostician's dilemma. Semin Vet Med Surg. 1996;11:161. doi: 10.1016/s1096-2867(96)80028-4. [DOI] [PubMed] [Google Scholar]
- Dziezyc J. Canine systemic bacterial infections. Vet Clin North Am Small Anim Pract. 2000;30:1103. doi: 10.1016/s0195-5616(00)05009-9. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Greene CE. Diseases caused by systemic bacterial infections. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 616. [Google Scholar]
- Hollett RB. Canine brucellosis: outbreaks and compliance. Theriogenol. 2006;66:575. doi: 10.1016/j.theriogenology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Kerwin SC. Diskospondylitis associated with Brucella canis infection in dogs: 14 cases (1980-1991) J Am Vet Med Assoc 1992. 1992;201:1253. [PubMed] [Google Scholar]
- Mateu-de-Antonio EM, Martin M. In vitro efficacy of several antimicrobial combinations against Brucella canis and Brucella melitensis strains isolated from dogs. Vet Microbiol. 1995;45:1. doi: 10.1016/0378-1135(94)00122-d. [DOI] [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; Philadelphia: 2006. p. 974. [Google Scholar]
- Vinayak A. Clinical resolution of Brucella canis-induced ocular inflammation in a dog. J Am Vet Med Assoc. 2004;224:1804. doi: 10.2460/javma.2004.224.1804. [DOI] [PubMed] [Google Scholar]
Borreliosis (Canine Lyme Disease)
- Appel MJG. Ocular CVT update: canine Lyme disease. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XII: Small Animal Practice. 12th ed. Saunders; Philadelphia: 1995. p. 303. [Google Scholar]
- Fritz CL, Kjemtrup AM. Lyme borreliosis. J Am Vet Med Assoc. 2003;223:1261. doi: 10.2460/javma.2003.223.1261. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Greene CE. Diseases caused by systemic bacterial infections. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 616. [Google Scholar]
- Littman MP. Canine borreliosis. Vet Clin North Am Small Anim Pract. 2003;33:827. doi: 10.1016/s0195-5616(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
Rocky Mountain Spotted Fever (Dogs) and Canine Monocytic Ehrlichiosis
- Belanger M. Comparison of serological detection methods for diagnosis of Ehrlichia canis infections in dogs. J Clin Microbiol 2002. 2002;40:3506. doi: 10.1128/JCM.40.9.3506-3508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB. Obligate intracellular bacterial pathogens. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 631. [Google Scholar]
- Davidson MG. Vascular permeability and coagulation during Rickettsia rickettsii infection in dogs. Am J Vet Res. 1990;51:165. [PubMed] [Google Scholar]
- Gasser AM. Canine Rocky Mountain spotted fever: a retrospective study of 30 cases. J Am Anim Hosp Assoc. 2001;37:41. doi: 10.5326/15473317-37-1-41. [DOI] [PubMed] [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Gould DJ. Canine monocytic ehrlichiosis presenting as acute blindness 36 months after importation into the UK. J Small Anim Pract. 2000;41:263–265. doi: 10.1111/j.1748-5827.2000.tb03937.x. [DOI] [PubMed] [Google Scholar]
- Greene CE, Breitschwerdt EB. Rocky Mountain spotted fever, Murine typhuslike disease, Rickettsialpox, Typhus, and Q fever. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 232. [Google Scholar]
- Greig B. Canine granulocytotropic ehrlichiosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 217. [Google Scholar]
- Harrus S. Recent advances in determining the pathogenesis of canine monocytic ehrlichiosis. J Clin Microbiol. 1999;37:2745. doi: 10.1128/jcm.37.9.2745-2749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrus S. Canine monocytic ehrlichiosis: an update. Compend Contin Educ Pract Vet. 1997;19:431. [Google Scholar]
- Harvey JW. Thrombocytotropic anaplasmosis (A. platys [E. Platys] infection) In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 229. [Google Scholar]
- Komnenou AA. Ocular manifestations of natural canine monocytic ehrlichiosis (Ehrlichia canis): a retrospective study of 90 cases. Vet Ophthalmol. 2007;10:137. doi: 10.1111/j.1463-5224.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Neer TM. Canine monocytotropic ehrlichiosis and neorickettsiosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 203. [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Stiles J. Canine rickettsial infections. Vet Clin North Am Small Anim Pract. 2000;30:1135. doi: 10.1016/s0195-5616(00)05011-7. [DOI] [PubMed] [Google Scholar]
- Warner RD, Marsh WW. Rocky Mountain spotted fever. J Am Vet Med Assoc. 2002;221:1413. doi: 10.2460/javma.2002.221.1413. [DOI] [PubMed] [Google Scholar]
Bartonellosis
- Birtles RJ. Prevalence of Bartonella species causing bacteraemia in domesticated and companion animals in the United Kingdom. Vet Rec. 2002;151:225. doi: 10.1136/vr.151.8.225. [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB. The immunologic response of dogs to Bartonella vinsonii subspecies berkhoffii antigens: as assessed by Western immunoblot analysis. J Vet Diagn Invest. 2003;15:349. doi: 10.1177/104063870301500408. [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB. Bartonellosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 511. [Google Scholar]
- Chomel BB. Aortic valve endocarditis in a dog due to Bartonella clarridgeiae. J Clin Microbiol. 2001;39:3548. doi: 10.1128/JCM.39.10.3548-3554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaus T. Seroprevalence of Bartonella henselae infection and correlation with disease status in cats in Switzerland. J Clin Microbiol. 1997;35:2883. doi: 10.1128/jcm.35.11.2883-2885.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptill L. Bartonellosis. Vet Clin North Am Small Anim Pract. 2003;33:809. doi: 10.1016/s0195-5616(03)00024-x. [DOI] [PubMed] [Google Scholar]
- Guptill L. Prevalence, risk factors, and genetic diversity of Bartonella henselae infections in pet cats in four regions of the United States. J Clin Microbiol. 2004;42:652–659. doi: 10.1128/JCM.42.2.652-659.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketring KL. Bartonella: a new etiological agent of feline ocular disease. J Am Anim Hosp Assoc. 2004;40:6. doi: 10.5326/0400006. [DOI] [PubMed] [Google Scholar]
- Lappin MR, Black JC. Bartonella spp. infection as a possible cause of uveitis in a cat. J Am Vet Med Assoc. 1999;214:1205. [PubMed] [Google Scholar]
- Luria BJ. Prevalence of infectious diseases in feral cats in Northern Florida. J Feline Med Surg. 2004;6:287. doi: 10.1016/j.jfms.2003.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mexas AM. Bartonella henselae and Bartonella elizabethae as potential canine pathogens. J Clin Microbiol. 2002;40:4670. doi: 10.1128/JCM.40.12.4670-4674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CC. Inoculation with Bartonella henselae followed by feline herpesvirus 1 fails to activate ocular toxoplasmosis in chronically infected cats. J Feline Med Surg. 2002;4:107. doi: 10.1053/jfms.2001.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolain JM. Prevalence of Bartonella clarridgeiae and Bartonella henselae in domestic cats from France and detection of the organisms in erythrocytes by immunofluorescence. Clin Diagn Lab Immunol. 2004;11:423. doi: 10.1128/CDLI.11.2.423-425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Gallego L. Bartonella henselae IgG antibodies are prevalent in dogs from southeastern USA. Vet Res. 2004;35:585. doi: 10.1051/vetres:2004034. [DOI] [PubMed] [Google Scholar]
Chlamydiosis (Chlamydophilosis) (Cats)
- Greene CE, Sikes JE. Chlamydial infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 245. [Google Scholar]
- McDonald M. A comparison of DNA amplification, isolation and serology for the detection of Chlamydia psittaci infection in cats. Vet Rec. 1998;143:97. doi: 10.1136/vr.143.4.97. [DOI] [PubMed] [Google Scholar]
- Nasisse MP. Clinical and laboratory findings in chronic conjunctivitis in cats: 91 cases (1983-1991) J Am Vet Med Assoc. 1993;203:834. [PubMed] [Google Scholar]
- Ramsey DT. Feline chlamydia and calicivirus infections. Vet Clin North Am Small Anim Pract. 2000;30:1015. doi: 10.1016/s0195-5616(00)05004-x. [DOI] [PubMed] [Google Scholar]
- Sparkes AH. The clinical efficacy of topical and systemic therapy for the treatment of feline ocular chlamydiosis. J Feline Med Surg. 1999;1:31. doi: 10.1016/S1098-612X(99)90007-4. [DOI] [PubMed] [Google Scholar]
- Sykes JE. Detection of feline calicivirus, feline herpesvirus 1 and Chlamydia psittaci mucosal swabs by multiplex RT-PCR/PCR. Vet Microbiol. 2001;81:95. doi: 10.1016/s0378-1135(01)00340-6. [DOI] [PubMed] [Google Scholar]
- Sykes JE. Comparison of the polymerase chain reaction and culture for the detection of feline Chlamydia psittaci in untreated and doxycycline-treated experimentally infected cats. J Vet Intern Med. 1999;13:146. doi: 10.1892/0891-6640(1999)013<0146:cotpcr>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Sykes JE. Prevalence of feline Chlamydia psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease. J Vet Intern Med. 1999;13:153. doi: 10.1892/0891-6640(1999)013<0153:pofpaf>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- von Bomhard W. Detection of novel chlamydiae in cats with ocular disease. Am J Vet Res. 2003;64:1421. doi: 10.2460/ajvr.2003.64.1421. [DOI] [PubMed] [Google Scholar]
Mycoplasmosis (Cats)
- Chalker VJ. Development of a polymerase chain reaction for the detection of Mycoplasma felis in domestic cats. Vet Microbiol. 2004;100:77. doi: 10.1016/j.vetmic.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Chandler JC. Mycoplasmal respiratory infections in small animals: 17 cases (1988-1999) J Am Anim Hosp Assoc. 2002;38:111. doi: 10.5326/0380111. [DOI] [PubMed] [Google Scholar]
- Foster SF. Lower respiratory tract infections in cats: 21 cases (1995-2000) J Feline Med Surg. 2004;6:167. doi: 10.1016/j.jfms.2003.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SF. A retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995-2000) J Feline Med Surg. 2004;6:189. doi: 10.1016/j.jfms.2003.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene CE. Mycoplasmal, ureaplasmal and L-forms infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 260. [Google Scholar]
- Rosendal S. Mycoplasma infections of dogs and cats. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XII: Small Animal Practice. 12th ed. Saunders; Philadelphia: 1995. p. 301. [Google Scholar]
Blastomycosis
- Arceneaux KA. Blastomycosis in dogs: 115 cases (1980-1995) J Am Vet Med Assoc. 1998;213:658. [PubMed] [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Gionfriddo JR. Feline systemic fungal infections. Vet Clin North Am Small Anim Pract. 2000;30:1029. doi: 10.1016/s0195-5616(00)05005-1. [DOI] [PubMed] [Google Scholar]
- Hendrix DV. Comparison of histologic lesions of endophthalmitis induced by Blastomyces dermatitidis in untreated and treated dogs: 36 cases (1986-2001) J Am Vet Med Assoc. 2004;224:1317. doi: 10.2460/javma.2004.224.1317. [DOI] [PubMed] [Google Scholar]
- Kerl ME. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract. 2003;33:721. doi: 10.1016/s0195-5616(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Krohne SG. Canine systemic fungal infections. Vet Clin North Am Small Anim Pract. 2000;30:1063. doi: 10.1016/s0195-5616(00)05007-5. [DOI] [PubMed] [Google Scholar]
- Legendre AM. Blastomycosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 569. [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Taboada J, Grooters AM. Systemic mycosis. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 671. [Google Scholar]
Coccidioidomycosis
- Davies C, Troy GC. Deep mycotic infections in cats. J Am Anim Hosp Assoc. 1996;32:380. doi: 10.5326/15473317-32-5-380. [DOI] [PubMed] [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Gionfriddo JR. Feline systemic fungal infections. Vet Clin North Am Small Anim Pract. 2000;30:1029. doi: 10.1016/s0195-5616(00)05005-1. [DOI] [PubMed] [Google Scholar]
- Greene RT. Cocidioidomycosis and paracoccidioidomycosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 598. [Google Scholar]
- Greene RT, Troy GC. Coccidioidomycosis in 48 cats: a retrospective study (1984-1993) J Vet Intern Med. 1995;9:86. doi: 10.1111/j.1939-1676.1995.tb03277.x. [DOI] [PubMed] [Google Scholar]
- Johnson LR. Clinical, clinicopathologic, and radiographic findings in dogs with coccidioidomycosis: 24 cases (1995-2000) J Am Vet Med Assoc. 2003;222:461. doi: 10.2460/javma.2003.222.461. [DOI] [PubMed] [Google Scholar]
- Kerl ME. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract. 2003;33:721. doi: 10.1016/s0195-5616(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Krohne SG. Canine systemic fungal infections. Vet Clin North Am Small Anim Pract. 2000;30:1063. doi: 10.1016/s0195-5616(00)05007-5. [DOI] [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Taboada J, Grooters AM. Systemic mycosis. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 671. [Google Scholar]
Cryptococcosis
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Gionfriddo JR. Feline systemic fungal infections. Vet Clin North Am Small Anim Pract. 2000;30:1029. doi: 10.1016/s0195-5616(00)05005-1. [DOI] [PubMed] [Google Scholar]
- Kerl ME. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract. 2003;33:721. doi: 10.1016/s0195-5616(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Krohne SG. Canine systemic fungal infections. Vet Clin North Am Small Anim Pract. 2000;30:1063. doi: 10.1016/s0195-5616(00)05007-5. [DOI] [PubMed] [Google Scholar]
- Lester SJ. Clinicopathologic features of an unusual outbreak of cryptococcosis in dogs, cats, ferrets, and a bird: 38 cases (January to July 2003) J Am Vet Med Assoc. 2004;225:1716. doi: 10.2460/javma.2004.225.1716. [DOI] [PubMed] [Google Scholar]
- Malik R. Cryptococcosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 584. [Google Scholar]
- Malik R. Serum antibody response to Cryptococcus neoformans in cats, dogs and koalas with and without active infection. Med Mycol. 1999;37:43. [PubMed] [Google Scholar]
- Malik R. Nasopharyngeal cryptococcosis. Aust Vet J. 1997;75:483. doi: 10.1111/j.1751-0813.1997.tb14377.x. [DOI] [PubMed] [Google Scholar]
- O'Brien CR. Long-term outcome of therapy for 59 cats and 11 dogs with cryptococcosis. Aust Vet J. 2006;84:384. doi: 10.1111/j.1751-0813.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- O'Brien CR. Retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases. Med Mycol. 2004;42:449. doi: 10.1080/13693780310001624547. [DOI] [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Taboada J, Grooters AM. Systemic mycosis. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 671. [Google Scholar]
- Tiches D. A case of canine central nervous system cryptococcosis: management with fluconazole. J Am Anim Hosp Assoc. 1998;34:145. doi: 10.5326/15473317-34-2-145. [DOI] [PubMed] [Google Scholar]
Histoplasmosis
- Davies C, Troy GC. Deep mycotic infections in cats. J Am Anim Hosp Assoc. 1996;32:380. doi: 10.5326/15473317-32-5-380. [DOI] [PubMed] [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Gionfriddo JR. Feline systemic fungal infections. Vet Clin North Am Small Anim Pract. 2000;30:1029. doi: 10.1016/s0195-5616(00)05005-1. [DOI] [PubMed] [Google Scholar]
- Greene CE. Histoplasmosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 577. [Google Scholar]
- Hodges RD. Itraconazole for the treatment of histoplasmosis in cats. J Vet Intern Med. 1994;8:409. doi: 10.1111/j.1939-1676.1994.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Johnson LR. Histoplasmosis infection in two cats from California. J Am Anim Hosp Assoc. 2004;40:165. doi: 10.5326/0400165. [DOI] [PubMed] [Google Scholar]
- Kerl ME. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract. 2003;33:721. doi: 10.1016/s0195-5616(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Krohne SG. Canine systemic fungal infections. Vet Clin North Am Small Anim Pract. 2000;30:1063. doi: 10.1016/s0195-5616(00)05007-5. [DOI] [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Taboada J, Grooters AM. Systemic mycosis. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 671. [Google Scholar]
Toxoplasmosis
- Bresciani KD. Experimental toxoplasmosis in pregnant bitches. Vet Parasitol. 1999;86:143. doi: 10.1016/s0304-4017(99)00136-3. [DOI] [PubMed] [Google Scholar]
- Davidson MG. Toxoplasmosis. Vet Clin North Am Small Anim Pract. 2000;30:1051. doi: 10.1016/s0195-5616(00)05006-3. [DOI] [PubMed] [Google Scholar]
- Davidson MG, English RV. Feline ocular toxoplasmosis. Vet Ophthalmol. 1998;1:71. doi: 10.1046/j.1463-5224.1998.00033.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis—waterborne zoonosis. Vet Parasitol. 2004;126:57. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lappin MR. Toxoplasmosis and neosporosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 754. [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Lappin MR. Protozoal and miscellaneous infections. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 638. [Google Scholar]
- Lappin MR. Feline infectious uveitis. J Feline Med Surg. 2000;2:159. doi: 10.1053/jfms.2000.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin MR. CVT update: feline toxoplasmosis. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XII: Small Animal Practice. 12th ed. Saunders; Philadelphia: 1995. p. 309. [Google Scholar]
- Lappin MR. Serologic prevalence of selected infectious diseases in cats with uveitis. J Am Vet Med Assoc. 1992;201:1005. [PubMed] [Google Scholar]
- Powell CC, Lappin MR. Clinical ocular toxoplasmosis in neonatal kittens. Vet Ophthalmol. 2001;4:87. doi: 10.1046/j.1463-5224.2001.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg SJ. Use of a multiplex polymerase chain reaction assay in the antemortem diagnosis of toxoplasmosis and neosporosis in the central nervous system of cats and dogs. Am J Vet Res. 2003;64:1507. doi: 10.2460/ajvr.2003.64.1507. [DOI] [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
Neosporosis (Dogs)
- Buxton D. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002;18:546. doi: 10.1016/s1471-4922(02)02414-5. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Neosporosis—the first decade of research. Int J Parasitol. 1999;29:1485. doi: 10.1016/s0020-7519(99)00134-4. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Recent advances in Neospora and neosporosis. Vet Parasitol. 1999;84:349. doi: 10.1016/s0304-4017(99)00044-8. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lappin MR. Toxoplasmosis and neosporosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 754. [Google Scholar]
- Dubey JP, Lindsay DS. Neosporosis. Parasitol Today. 1993;9:452. doi: 10.1016/0169-4758(93)90099-2. [DOI] [PubMed] [Google Scholar]
- Lappin MR. Protozoal and miscellaneous infections. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 638. [Google Scholar]
- Ortuno A. Seroprevalence of antibodies to Neospora caninum in dogs from Spain. J Parasitol. 2002;88:1263. doi: 10.1645/0022-3395(2002)088[1263:SOATNC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Schatzberg SJ. Use of a multiplex polymerase chain reaction assay in the antemortem diagnosis of toxoplasmosis and neosporosis in the central nervous system of cats and dogs. Am J Vet Res. 2003;64:1507. doi: 10.2460/ajvr.2003.64.1507. [DOI] [PubMed] [Google Scholar]
Canine Visceral Leishmaniasis
- Alvar J. Canine leishmaniasis. Adv Parasitol. 2004;57:1. doi: 10.1016/S0065-308X(04)57001-X. [DOI] [PubMed] [Google Scholar]
- Baneth G. Leishmaniasis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 685. [Google Scholar]
- Baneth G, Shaw SE. Chemotherapy of canine leishmaniosis. Vet Parasitol. 2002;106:315. doi: 10.1016/s0304-4017(02)00115-2. [DOI] [PubMed] [Google Scholar]
- Cortadellas O. Initial and long-term efficacy of a lipid emulsion of amphotericin B desoxycholate in the management of canine leishmaniasis. J Vet Intern Med. 2003;17:808. doi: 10.1111/j.1939-1676.2003.tb02518.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso M. Immunopathology of the uveitis in canine leishmaniasis. Parasite Immunol. 1996;18:617. doi: 10.1046/j.1365-3024.1996.d01-39.x. [DOI] [PubMed] [Google Scholar]
- Gaskin AA. Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med. 2002;16:34. doi: 10.1892/0891-6640(2002)016<0034:vliany>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Giger U. Leishmania donovani transmission by packed RBC transfusion to anemic dogs in the United States. Transfusion. 2002;42:381. doi: 10.1046/j.1537-2995.2002.00061.x. [DOI] [PubMed] [Google Scholar]
- Gilger BC. Ocular manifestations of systemic infectious diseases. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 276. [Google Scholar]
- Grosjean NL. Seroprevalence of antibodies against Leishmania spp. among dogs in the United States. J Am Vet Med Assoc. 2003;222:603. doi: 10.2460/javma.2003.222.603. [DOI] [PubMed] [Google Scholar]
- Lappin MR. Protozoal and miscellaneous infections. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 638. [Google Scholar]
- Manna L. Comparison of different tissue sampling for PCR-based diagnosis and follow-up of canine visceral leishmaniosis. Vet Parasitol. 2004;125:251. doi: 10.1016/j.vetpar.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Rosypal AC. Canine visceral leishmaniasis and its emergence in the United States. Vet Clin North Am Small Anim Pract. 2003;33:921. doi: 10.1016/s0195-5616(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Stiles J. Ocular infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; St. Louis: 2006. p. 974. [Google Scholar]
- Strauss-Ayali D. Polymerase chain reaction using noninvasively obtained samples, for the detection of Leishmania infantum DNA in dogs. J Infect Dis. 2004;189:1729. doi: 10.1086/383281. [DOI] [PubMed] [Google Scholar]
Diabetes Mellitus
- Bennett N. Monitoring techniques for diabetes mellitus in the dog and the cat. Clin Tech Small Anim Pract. 2002;17:65. doi: 10.1053/svms.2002.33044. [DOI] [PubMed] [Google Scholar]
- Cusick M. Effects of aldose reductase inhibitors and galactose withdrawal on fluorescein angiographic lesions in galactose-fed dogs. Arch Ophthalmol. 2003;121:1745. doi: 10.1001/archopht.121.12.1745. [DOI] [PubMed] [Google Scholar]
- Feldman EC, Nelson RW. Canine diabetes mellitus. In: Feldman EC, Nelson RW, editors. Canine and Feline Endocrinology and Reproduction. 3rd ed. Saunders; St. Louis: 2004. p. 486. [Google Scholar]
- Feldman EC, Nelson RW. Feline diabetes mellitus. In: Feldman EC, Nelson RW, editors. Canine and Feline Endocrinology and Reproduction. 3rd ed. Saunders; St. Louis: 2004. p. 539. [Google Scholar]
- Fleeman LM, Rand JS. Management of canine diabetes. Vet Clin North Am Small Anim Pract. 2001;31:855. doi: 10.1016/s0195-5616(01)50003-0. [DOI] [PubMed] [Google Scholar]
- Good KL. Corneal sensitivity in dogs with diabetes mellitus. Am J Vet Res. 2003;64:7. doi: 10.2460/ajvr.2003.64.7. [DOI] [PubMed] [Google Scholar]
- Hess RS. Concurrent disorders in dogs with diabetes mellitus: 221 cases (1993-1998) J Am Vet Med Assoc. 2000;217:1166. doi: 10.2460/javma.2000.217.1166. [DOI] [PubMed] [Google Scholar]
- Hoenig M. Comparative aspects of diabetes mellitus in dogs and cats. Mol Cell Endocrinol. 2002;197:221. doi: 10.1016/s0303-7207(02)00264-2. [DOI] [PubMed] [Google Scholar]
- Neuenschwander H. Dose-dependent reduction of retinal vessel changes associated with diabetic retinopathy in galactose-fed dogs by the aldose reductase inhibitor M79175. J Ocul Pharmacol Ther. 1997;13:517. doi: 10.1089/jop.1997.13.517. [DOI] [PubMed] [Google Scholar]
- Peikes H. Dermatologic disorders in dogs with diabetes mellitus: 45 cases (1986-2000) J Am Vet Med Assoc. 2001;219:203. doi: 10.2460/javma.2001.219.203. [DOI] [PubMed] [Google Scholar]
- Richter M. Aldose reductase activity and glucose-related opacities in incubated lenses from dogs and cats. Am J Vet Res. 2002;63:1591. doi: 10.2460/ajvr.2002.63.1591. [DOI] [PubMed] [Google Scholar]
Hyperlipidemia
- Barrie J, Watson TDG. Hyperlipidemia. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XII: Small Animal Practice. 12th ed. Saunders; Philadelphia: 1995. p. 430. [Google Scholar]
- Bauer JE. Evaluation and dietary considerations in idiopathic hyperlipidemia in dogs. J Am Vet Med Assoc. 1995;206:1684. [PubMed] [Google Scholar]
- Elliott DA. Dietary and medical considerations in lipidemia. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 592. [Google Scholar]
- Gunn-Moore DA. Transient hyperlipidaemia and anaemia in kittens. Vet Rec. 1997;140:355. doi: 10.1136/vr.140.14.355. [DOI] [PubMed] [Google Scholar]
- Johnstone AC. The pathology of an inherited hyperlipoproteinaemia of cats. J Comp Pathol. 1990;102:125. doi: 10.1016/s0021-9975(08)80118-1. [DOI] [PubMed] [Google Scholar]
- Jones BR. Peripheral neuropathy in cats with inherited primary hyperchylomicronaemia. Vet Rec. 1986;119:268. doi: 10.1136/vr.119.11.268. [DOI] [PubMed] [Google Scholar]
- Jones BR. Occurrence of idiopathic, familial hyperchylomicronaemia in a cat. Vet Rec. 1983;112:543. doi: 10.1136/vr.112.23.543. [DOI] [PubMed] [Google Scholar]
- Peritz LN. Characterization of a lipoprotein lipase class III type defect in hypertriglyceridemic cats. Clin Invest Med. 1990;13:259. [PubMed] [Google Scholar]
- Sato K. Hypercholesterolemia in Shetland sheepdogs. J Vet Med Sci. 2000;62:1297. doi: 10.1292/jvms.62.1297. [DOI] [PubMed] [Google Scholar]
- Watson P. Hypercholesterolaemia in briards in the United Kingdom. Res Vet Sci. 1993;54:80. doi: 10.1016/0034-5288(93)90015-8. [DOI] [PubMed] [Google Scholar]
- Whitney MS. Evaluation of hyperlipidemias in dogs and cats. Semin Vet Med Surg (Small Anim) 1992;7:292. [PubMed] [Google Scholar]
- Wisselink MA. Hyperlipoproteinaemia associated with atherosclerosis and cutaneous xanthomatosis in a cat. Vet Q. 1994;16:199. doi: 10.1080/01652176.1994.9694448. [DOI] [PubMed] [Google Scholar]
Hyperadrenocorticism (Dogs)
- Behrend EN, Kemppainen RJ. Diagnosis of canine hyperadrenocorticism. Vet Clin North Am Small Anim Pract. 2001;31:985. doi: 10.1016/s0195-5616(01)50009-1. [DOI] [PubMed] [Google Scholar]
- Braddock JA. Trilostane treatment in dogs with pituitary-dependent hyperadrenocorticism. Aust Vet J. 2003;81:600. doi: 10.1111/j.1751-0813.2003.tb12498.x. [DOI] [PubMed] [Google Scholar]
- Chapman PS. Adrenal necrosis in a dog receiving trilostane for the treatment of hyperadrenocorticism. J Small Anim Pract. 2004;45:307. doi: 10.1111/j.1748-5827.2004.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Feldman EC, Nelson RW. Canine hyperadrenocorticism (Cushing's syndrome) In: Feldman EC, Nelson RW, editors. Canine and Feline Endocrinology and Reproduction. 3rd ed. Saunders; St. Louis: 2004. p. 252. [Google Scholar]
- Laus JL. Combined corneal lipid and calcium degeneration in a dog with hyperadrenocorticism: a case report. Vet Ophthalmol. 2002;5:61. doi: 10.1046/j.1463-5224.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- Neiger R. Trilostane treatment of 78 dogs with pituitary-dependent hyperadrenocorticism. Vet Rec. 2002;150:799. doi: 10.1136/vr.150.26.799. [DOI] [PubMed] [Google Scholar]
- Reusch CE. Hyperadrenocorticism. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 1592. [Google Scholar]
- Ristic JM. The use of 17-hydroxyprogesterone in the diagnosis of canine hyperadrenocorticism. J Vet Intern Med. 2002;16:433. doi: 10.1892/0891-6640(2002)016<0433:tuoitd>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Wenger M. Effect of trilostane on serum concentrations of aldosterone, cortisol, and potassium in dogs with pituitary-dependent hyperadrenocorticism. Am J Vet Res. 2004;65:1245. doi: 10.2460/ajvr.2004.65.1245. [DOI] [PubMed] [Google Scholar]
Hypothyroidism (Dogs)
- Dixon RM. Epidemiological, clinical, haematological and biochemical characteristics of canine hypothyroidism. Vet Rec. 1999;145:481. doi: 10.1136/vr.145.17.481. [DOI] [PubMed] [Google Scholar]
- Feldman EC, Nelson RW. Hypothyroidism. In: Feldman EC, Nelson RW, editors. Canine and Feline Endocrinology and Reproduction. 3rd ed. Saunders; St. Louis: 2004. p. 86. [Google Scholar]
- Graham PA. Lymphocytic thyroiditis. Vet Clin North Am Small Anim Pract. 2001;31:915. doi: 10.1016/s0195-5616(01)50005-4. [DOI] [PubMed] [Google Scholar]
- Hess RS. Association between diabetes mellitus, hypothyroidism or hyperadrenocorticism, and atherosclerosis in dogs. J Vet Intern Med. 2003;17:489. doi: 10.1111/j.1939-1676.2003.tb02469.x. [DOI] [PubMed] [Google Scholar]
- Jaggy A. Neurologic manifestation of canine hypothyroidism. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 716. [Google Scholar]
- Kemppainen RJ, Behrend EN. Diagnosis of canine hypothyroidism: perspectives from a testing laboratory. Vet Clin North Am Small Anim Pract. 2001;31:951. doi: 10.1016/s0195-5616(01)50007-8. [DOI] [PubMed] [Google Scholar]
- Nachreiner RF. Prevalence of serum thyroid hormone autoantibodies in dogs with clinical signs of hypothyroidism. J Am Vet Med Assoc. 2002;220:466. doi: 10.2460/javma.2002.220.466. [DOI] [PubMed] [Google Scholar]
- Panciera D. Cardiovascular complications of thyroid disease. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 716. [Google Scholar]
- Panciera D. Complications and concurrent conditions associated with hypothyroidism in dogs. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XIII: Small Animal Practice. 13th ed. Saunders; Philadelphia: 2000. p. 327. [Google Scholar]
- Panciera DL. Conditions associated with canine hypothyroidism. Vet Clin North Am Small Anim Pract. 2001;31:935. [PubMed] [Google Scholar]
- Scott-Moncrieff JC, Guptil-Yoran L. Hypothyroidism. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 1535. [Google Scholar]
Thrombocytopenia and Thrombopathy (Thrombasthenia)
- Boozer AL, Macintire DK. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2003;33:885. doi: 10.1016/s0195-5616(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Boreaux MK. Acquired platelet dysfunction. In: Feldman BF, editor. Schalm's Veterinary Hematology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. p. 496. [Google Scholar]
- Brooks MB, Catalfano JL. Platelet disorders and von Willebrand disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 1918. [Google Scholar]
- Davidson MG. Vascular permeability and coagulation during Rickettsia rickettsii infection in dogs. Am J Vet Res. 1990;51:165. [PubMed] [Google Scholar]
- Grindem CB. Epidemiologic survey of thrombocytopenia in dogs: a report on 987 cases. Vet Clin Pathol. 1991;20:38. doi: 10.1111/j.1939-165x.1991.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Hackett TB. Clinical findings associated with prairie rattlesnake bites in dogs: 100 cases (1989-1998) J Am Vet Med Assoc. 2002;220:1675. doi: 10.2460/javma.2002.220.1675. [DOI] [PubMed] [Google Scholar]
- Harrus S. Acute blindness associated with monoclonal gammopathy induced by Ehrlichia canis infection. Vet Parasitol. 1998;78:155. doi: 10.1016/s0304-4017(98)00132-0. [DOI] [PubMed] [Google Scholar]
- Hisasue M. Hematologic abnormalities and outcome of 16 cats with myelodysplastic syndromes. J Vet Intern Med. 2001;15:471. doi: 10.1892/0891-6640(2001)015<0471:haaooc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Kirby R, Rudolf E. Acquired coagulopathy VI: disseminated intravascular coagulation. In: Feldman BF, editor. Schalm's Veterinary Hematology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. p. 579. [Google Scholar]
- Scott MA. Immune-mediated thrombocytopenia. In: Feldman BF, editor. Schalm's Veterinary Hematology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. p. 478. [Google Scholar]
- Segev G. Vipera palaestinae envenomation in 327 dogs: a retrospective cohort study and analysis of risk factors for mortality. Toxicon. 2004;43:691. doi: 10.1016/j.toxicon.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Topper MJ, Welles EG. Hemostasis. In: Latimer KS, editor. Duncan and Prasse's Veterinary Laboratory Medicine Clinical Pathology. 4th ed. Iowa State Press; Ames: 2003. p. 99. [Google Scholar]
- Trepanier LA. Clinical findings in 40 dogs with hypersensitivity associated with administration of potentiated sulfonamides. J Vet Intern Med. 2003;17:647. doi: 10.1111/j.1939-1676.2003.tb02495.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman KL. Drug-induced thrombocytopenias. In: Feldman BF, editor. Schalm's Veterinary Hematology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. p. 472. [Google Scholar]
Systemic Hypertension
- Bartges JW. Hypertension and renal disease. Vet Clin North Am Small Anim Pract. 1996;26:1331. doi: 10.1016/s0195-5616(96)50131-2. [DOI] [PubMed] [Google Scholar]
- Chetboul V. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med. 2003;17:89. doi: 10.1892/0891-6640(2003)017<0089:sfhcae>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Crispin SM, Mould JR. Systemic hypertensive disease and the feline fundus. Vet Ophthalmol. 2001;4:13. doi: 10.1046/j.1463-5224.2001.00190.x. [DOI] [PubMed] [Google Scholar]
- Elliott J. Feline hypertension: clinical findings and response to antihypertensive treatment in 30 cases. J Small Anim Pract. 2001;42:122. doi: 10.1111/j.1748-5827.2001.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Komaromy AM. Hypertensive retinopathy and choroidopathy in a cat. Vet Ophthalmol. 2004;7:3. doi: 10.1111/j.1463-5224.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- Maggio F. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985-1998) J Am Vet Med Assoc. 2000;217:695. doi: 10.2460/javma.2000.217.695. [DOI] [PubMed] [Google Scholar]
- Mertz BP. Rhegmatogenous retinal detachment in a hypertensive dog. Lens Eye Toxic Res. 1990;7:67. [PubMed] [Google Scholar]
- Miller RH. Effect of enalapril on blood pressure, renal function, and the renin-angiotensin-aldosterone system in cats with autosomal dominant polycystic kidney disease. Am J Vet Res. 1999;60:1516. [PubMed] [Google Scholar]
- Pedersen KM. Increased mean arterial pressure and aldosterone-to-renin ratio in Persian cats with polycystic kidney disease. J Vet Intern Med. 2003;17:21. [PubMed] [Google Scholar]
- Polzin DJ. Chronic kidney disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Saunders; St. Louis: 2005. p. 1756. [Google Scholar]
- Sansom J. Blood pressure assessment in healthy cats and cats with hypertensive retinopathy. Am J Vet Res. 2004;65:245. doi: 10.2460/ajvr.2004.65.245. [DOI] [PubMed] [Google Scholar]
- Snyder PS. Effect of amlodipine on echocardiographic variables in cats with systemic hypertension. J Vet Intern Med. 2001;15:52. doi: 10.1892/0891-6640(2001)015<0052:eoaoev>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Syme HM. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc. 2002;220:1799. doi: 10.2460/javma.2002.220.1799. [DOI] [PubMed] [Google Scholar]
- Van Boxtel SA. Hypertensive retinopathy in a cat. Can Vet J. 2003;44:147. [PMC free article] [PubMed] [Google Scholar]
Polycythemia
- Cote E, Ettinger SJ. Long-term clinical management of right-to-left (“reversed”) patent ductus arteriosus in 3 dogs. J Vet Intern Med. 2001;15:39. doi: 10.1892/0891-6640(2001)015<0039:lcmorp>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Couto CG. Tumor-associated erythrocytosis in a dog with nasal fibrosarcoma. J Vet Intern Med. 1989;3:183. doi: 10.1111/j.1939-1676.1989.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Crow SE. Concurrent renal adenocarcinoma and polycythemia in a dog. J Am Anim Hosp Assoc. 1995;31:29. doi: 10.5326/15473317-31-1-29. [DOI] [PubMed] [Google Scholar]
- Evans LM, Caylor KB. Polycythemia vera in a cat and management with hydroxyurea. J Am Anim Hosp Assoc. 1995;31:434. doi: 10.5326/15473317-31-5-434. [DOI] [PubMed] [Google Scholar]
- Foster ES, Lothrop CD., Jr Polycythemia vera in a cat with cardiac hypertrophy. J Am Vet Med Assoc. 1988;192:1736. [PubMed] [Google Scholar]
- Gorse MJ. Polycythemia associated with renal fibrosarcoma in a dog. J Am Vet Med Assoc. 1988;192:793. [PubMed] [Google Scholar]
- Gray HE. Polycythemia vera in a dog presenting with uveitis. J Am Anim Hosp Assoc. 2003;39:355. doi: 10.5326/0390355. [DOI] [PubMed] [Google Scholar]
- Hasler AH, Giger U. Serum erythropoietin values in polycythemic cats. J Am Anim Hosp Assoc. 1996;32:294. doi: 10.5326/15473317-32-4-294. [DOI] [PubMed] [Google Scholar]
- Henry CJ. Primary renal tumours in cats: 19 cases (1992-1998) J Feline Med Surg. 1999;1:165. doi: 10.1016/S1098-612X(99)90205-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HP. Polycythaemia vera in a dog treated by repeated phlebotomies. Vet Q. 1993;15:108. doi: 10.1080/01652176.1993.9694385. [DOI] [PubMed] [Google Scholar]
- Moore KW, Stepien RL. Hydroxyurea for treatment of polycythemia secondary to right-to-left shunting patent ductus arteriosus in 4 dogs. J Vet Intern Med. 2001;15:418. [PubMed] [Google Scholar]
- Sato K. Secondary erythrocytosis associated with high plasma erythropoietin concentrations in a dog with cecal leiomyosarcoma. J Am Vet Med Assoc. 2002;220:486. doi: 10.2460/javma.2002.220.486. [DOI] [PubMed] [Google Scholar]
- van Vonderen IK. Polyuria and polydipsia and disturbed vasopressin release in 2 dogs with secondary polycythemia. J Vet Intern Med. 1997;11:300. doi: 10.1111/j.1939-1676.1997.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Watson ADJ. Erythrocytosis and polycythemia. In: Feldman BF, editor. Schalm's Veterinary Hematology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. p. 216. [Google Scholar]
Hyperviscosity Syndrome
- Boone LI. Bence-Jones proteins. In: Feldman BF, editor. Schalm's Veterinary Hematology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. p. 925. [Google Scholar]
- Forrester SD. Serum hyperviscosity syndrome associated with multiple myeloma in two cats. J Am Vet Med Assoc. 1992;200:79. [PubMed] [Google Scholar]
- Forrester SD, Rogers KS. Hyperviscosity syndromes. In: Feldman BF, editor. Schalm's Veterinary Hematology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. p. 929. [Google Scholar]
- Fox LE. The paraneoplastic disorders. In: Bonagura JD, editor. Kirk's Current Veterinary Therapy XII: Small Animal Practice. 12th ed. Saunders; Philadelphia: 1995. p. 530. [Google Scholar]
- Harrus S. Acute blindness associated with monoclonal gammopathy induced by Ehrlichia canis infection. Vet Parasitol. 1998;78:155. doi: 10.1016/s0304-4017(98)00132-0. [DOI] [PubMed] [Google Scholar]
- Hawkins EC. Immunoglobulin A myeloma in a cat with pleural effusion and serum hyperviscosity. J Am Vet Med Assoc. 1986;188:876. [PubMed] [Google Scholar]
- Hohenhaus AE. Kirk's Current Veterinary Therapy XII: Small Animal Practice. 12th ed. Saunders; Philadelphia: 1995. Syndromes of hyperglobulinemia: diagnosis and therapy; p. 523. [Google Scholar]
- Hoskins JD. Serum hyperviscosity syndrome associated with Ehrlichia canis infection in a dog. Am Vet Med Assoc. 1983;183:1011. [PubMed] [Google Scholar]
- Ward DA. Orbital plasmacytoma in a cat. J Small Anim Pract. 1997;38:576. doi: 10.1111/j.1748-5827.1997.tb03323.x. [DOI] [PubMed] [Google Scholar]
- Weber NA, Tebeau CS. An unusual presentation of multiple myeloma in two cats. J Am Anim Hosp Assoc. 1998;34:477. doi: 10.5326/15473317-34-6-477. [DOI] [PubMed] [Google Scholar]
Uveodermatologic Syndrome (Vogt-Koyanagi-Harada–Like Syndrome) (Dogs)
- Bedford PG. Uveodermatological syndrome in the Japanese Akita. Vet Rec. 1986;118:134. doi: 10.1136/vr.118.5.134. [DOI] [PubMed] [Google Scholar]
- Bussanich MN. Granulomatous panuveitis and dermal depigmentation. J Am Anim Hosp Assoc. 1982;18:131. [Google Scholar]
- Carter WJ. An immunohistochemical study of uveodermatologic syndrome in two Japanese Akita dogs. Vet Ophthalmol. 2005;8:17. doi: 10.1111/j.1463-5224.2005.04059.x. [DOI] [PubMed] [Google Scholar]
- Cottrell BD, Barnett KC. Harada's disease in the Japanese Akita. J Small Anim Pract. 1986;28:517. [Google Scholar]
- Kern TJ. Uveitis associated with poliosis and vitiligo in six dogs. J Am Vet Med Assoc. 1985;187:408. [PubMed] [Google Scholar]
- Laus JL. Uveodermatologic syndrome in a Brazilian fila dog. Vet Ophthalmol. 2004;7:193. doi: 10.1111/j.1463-5224.2004.04023.x. [DOI] [PubMed] [Google Scholar]
- Lindley DM. Ocular histopathology of Vogt-Koyanagi-Harada–like syndrome in an Akita dog. Vet Pathol. 1990;27:294. doi: 10.1177/030098589002700415. [DOI] [PubMed] [Google Scholar]
- Muller RS. The uveodermatologic syndrome in dogs. Tierarztl Prax. 1992;20:632. [PubMed] [Google Scholar]
- Read RW. Vogt-Koyanagi-Harada disease. Ophthalmol Clin North Am. 2002;15:333. doi: 10.1016/s0896-1549(02)00025-1. [DOI] [PubMed] [Google Scholar]
Ocular Manifestations of Systemic Disease in Horses
- Aleman M. Juvenile idiopathic epilepsy in Egyptian Arabian foals: 22 cases (1985-2005) J Vet Intern Med. 2006;20:1443. doi: 10.1892/0891-6640(2006)20[1443:jieiea]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Divers TJ. Systemic granulomatous inflammation in a horse grazing hairy vetch. J AmVet Med Assoc. 1983;183:569. [PubMed] [Google Scholar]
- Anninger WV. West Nile virus-associated optic neuritis and chorioretinitis. Am J Ophthalmol. 2003;136:1183. doi: 10.1016/s0002-9394(03)00738-4. [DOI] [PubMed] [Google Scholar]
- Blogg JR. Blindness caused by Rhodococcus equi in a foal. Equine Vet J Suppl. 1983;2:25. [Google Scholar]
- Brem S. Intraocular leptospira isolation in 4 horses suffering from equine recurrent uveitis (ERU) Berl Münch Tierärtztl Wochenschr. 1998;111:415. [PubMed] [Google Scholar]
- Browning GF. Latency of equine herpesvirus-4 (equine rhinopneumonitis virus) Vet Rec. 1988;123:518. doi: 10.1136/vr.123.20.518. [DOI] [PubMed] [Google Scholar]
- Collinson PN. Isolation of equine herpesvirus-2 (equine gammaherpesvirus-2) from foals with keratoconjunctivitis. J Am Vet Med Assoc. 1994;205:329. [PubMed] [Google Scholar]
- Curnutte JT. Chronic granulomatous disease: the solving of a clinical riddle at the molecular level. Clin Immunol Immunopathol. 1993;67:S2. doi: 10.1006/clin.1993.1078. [DOI] [PubMed] [Google Scholar]
- Cutler TJ. Ophthalmic findings in the geriatric horse. Vet Clin North Am Equine Pract. 2002;18:545. doi: 10.1016/s0749-0739(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Edington N. Experimental reactivation of equid herpesvirus-1 (EHV-1) following the administration of corticosteroids. Equine Vet J. 1985;17:369. doi: 10.1111/j.2042-3306.1985.tb02524.x. [DOI] [PubMed] [Google Scholar]
- Ellis PM. Japanese encephalitis. Vet Clin North Am Equine Pract. 2000;16:565. doi: 10.1016/s0749-0739(17)30096-2. [DOI] [PubMed] [Google Scholar]
- Griffith G. Pemphigus foliaceus in a Welsh pony. Compend Contin Educ Pract Vet. 1987;9:347. [Google Scholar]
- Heath SE. Idiopathic granulomatous disease involving the skin in a horse. J Am Vet Med Assoc. 1990;197:1033. [PubMed] [Google Scholar]
- Henry M. Hemorrhagic diatheses caused by multiple myeloma in a three-month-old foal. J Am Vet Med Assoc. 1989;194:392. [PubMed] [Google Scholar]
- Jones TC. Clinical and pathologic features of equine viral arteritis. J Am Vet Med Assoc. 1969;155:35. [PubMed] [Google Scholar]
- Kaiser PK. Occlusive vasculitis in a patient with concomitant West Nile virus infection. Am J Ophthalmol. 2003;136:928. doi: 10.1016/s0002-9394(03)00866-3. [DOI] [PubMed] [Google Scholar]
- Kuchtey RW. Uveitis associated with West Nile virus infection. Arch Ophthalmol. 2003;121:1648. doi: 10.1001/archopht.121.11.1648. [DOI] [PubMed] [Google Scholar]
- Lavach JD. Ocular manifestations of systemic diseases. Vet Clin North Am Equine Pract. 1992;8:627. doi: 10.1016/s0749-0739(17)30445-5. [DOI] [PubMed] [Google Scholar]
- Leiffson PS. Ocular tuberculosis in a horse. Vet Rec. 1997;141:651. [PubMed] [Google Scholar]
- Madigan JE. Photic head shaking in the horse: 7 cases. Equine Vet J. 1995;27:306. doi: 10.1111/j.2042-3306.1995.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Martin CL. Ocular manifestations of systemic disease. In: Gelatt KN, editor. Veterinary Ophthalmology. 3rd ed. Lippincott Williams & Wilkins; Baltimore: 1999. [Google Scholar]
- McConnico RS. Supportive medical care of recumbent horses. Compend Contin Educ Pract Vet. 1991;13:1287. [Google Scholar]
- Miller TR. Herpetic keratitis in a horse. Equine Vet J Suppl. 1990;10:15. doi: 10.1111/j.2042-3306.1990.tb04703.x. [DOI] [PubMed] [Google Scholar]
- Newton SA. Headshaking in horses: possible aetiopathogenesis suggested by the results of diagnostic tests and several treatment regimes used in 20 cases. Equine Vet J. 2000;32:208. doi: 10.2746/042516400776563617. [DOI] [PubMed] [Google Scholar]
- Ostlund EN. Equine West Nile encephalitis, United States. Emerg Infect Dis. 2001;7:665. doi: 10.3201/eid0704.010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusterla N. Cutaneous and ocular habronemiasis in horses: 63 cases (1988-2002) J Am Vet Med Assoc. 2003;222:978. doi: 10.2460/javma.2003.222.978. [DOI] [PubMed] [Google Scholar]
- Rames DS. Ocular Halicephalobus (syn. Micronema) deletrix in a horse. Vet Pathol. 1995;32:540. doi: 10.1177/030098589503200514. [DOI] [PubMed] [Google Scholar]
- Rebhun WC, Del Piero F. Ocular lesions of horses with lymphosarcoma: 21 cases (1977-1997) J Am Vet Med Assoc. 1998;212:852. [PubMed] [Google Scholar]
- Richt JA. Borna disease in horses. Vet Clin North Am Equine Pract. 2000;16:579. doi: 10.1016/s0749-0739(17)30097-4. [DOI] [PubMed] [Google Scholar]
- Rico-Hesse R. Venezuelan equine encephalomyelitis. Vet Clin North Am Equine Pract. 2000;16:553. doi: 10.1016/s0749-0739(17)30095-0. [DOI] [PubMed] [Google Scholar]
- Savage CJ. Lymphoproliferative and myeloproliferative disorders. Vet Clin North Am Equine Pract. 1998;14:563. doi: 10.1016/s0749-0739(17)30187-6. [DOI] [PubMed] [Google Scholar]
- Sippel WL. Equine piroplasmosis in the United States. J Am Vet Med Assoc. 1962;141:694. [PubMed] [Google Scholar]
- Smith BP, editor. Large Animal Internal Medicine. 3rd ed. Mosby; St. Louis: 2002. [Google Scholar]
- Sutton GA. Pathogenesis and clinical signs of equine herpesvirus-1 in experimentally infected ponies in vivo. Can J Vet Res. 1998;62:49. [PMC free article] [PubMed] [Google Scholar]
- Sutton GA. Study of the duration and distribution of equine influenza virus subtype 2 (H3N8) antigens in experimentally infected ponies in vivo. Can J Vet Res. 1997;61:113. [PMC free article] [PubMed] [Google Scholar]
- Wada S. Nonulcerative keratouveitis as a manifestation of leptospiral infection in a horse. Vet Ophthalmol. 2003;6:191. doi: 10.1046/j.1463-5224.2003.00288.x. [DOI] [PubMed] [Google Scholar]
- Woods LW. Systemic granulomatous disease in a horse grazing pasture containing vetch (Vicia sp.) J Vet Diagn Invest. 1992;4:356. doi: 10.1177/104063879200400327. [DOI] [PubMed] [Google Scholar]
Ocular Manifestations of Systemic Diseases in Ruminants
- Abdelbaki YA, Davis RN. Ophthalmoscopic findings in elaeophorosis of domestic sheep. Vet Med. 1972;67:69. [PubMed] [Google Scholar]
- Adcock J, Hibler CP. Vascular and neuroophthalmic pathology of elaeophorosis in elk. Pathol Vet. 1969;6:185. doi: 10.1177/030098586900600301. [DOI] [PubMed] [Google Scholar]
- Bistner SI. The ocular lesions of bovine viral diarrhea-mucosal disease. Pathol Vet. 1970;7:275. doi: 10.1177/030098587000700306. [DOI] [PubMed] [Google Scholar]
- Capucchio MT. Maedi-visna virus detection in ovine third eyelids. J Comp Pathol. 2003;129:37. doi: 10.1016/s0021-9975(02)00158-5. [DOI] [PubMed] [Google Scholar]
- Collier LC. Ocular manifestations of the Chédiak-Higashi syndrome in four species of animals. J Am Vet Med Assoc. 1979;175:587. [PubMed] [Google Scholar]
- Dukes TW. The ocular lesions of thromboembolic meningoencephalitis in cattle. Can Vet J. 1971;12:180. [PMC free article] [PubMed] [Google Scholar]
- Fighera RA, Barros CSL. Systemic granulomatous disease in Brazilian cattle grazing pasture containing vetch (Cicia spp) Vet Human Toxicol. 2004;46:62. [PubMed] [Google Scholar]
- George L. Enhancement of infectious bovine keratoconjunctivitis by modified live infectious bovine rhinotracheitis virus vaccine. Am J Vet Res. 1988;49:1800. [PubMed] [Google Scholar]
- Hopkins JB. Conjunctivitis associated with chlamydial polyarthritis in lambs. J Am Vet Med Assoc. 1973;163:1157. [PubMed] [Google Scholar]
- Martin CL. Ocular manifestations of systemic disease. In: Gelatt KN, editor. Veterinary Ophthalmology. 3rd ed. Lippincott Williams & Wilkins; Baltimore: 1999. [Google Scholar]
- Mushi EZ. Isolation of bovine malignant catarrhal fever virus from ocular and nasal secretions of wildebeest calves. Res Vet Sci. 1980;29:168. [PubMed] [Google Scholar]
- Pierson RE. Clinical and clinicopathologic observations in induced malignant catarrhal fever of cattle. J Am Vet Med Assoc. 1973;173:833. [PubMed] [Google Scholar]
- Pierson RE. An epizootic of malignant catarrhal fever in feedlot cattle. J Am Vet Med Assoc. 1973;163:349. [PubMed] [Google Scholar]
- Radostits O. Veterinary Medicine. 10th ed. Saunders; London: 2007. [Google Scholar]
- Rebhun WC. Diseases of the bovine orbit and globe. J Am Vet Med Assoc. 1986;190:171. [PubMed] [Google Scholar]
- Rebhun WC. Ocular manifestations of systemic diseases in cattle. Vet Clin North Am Large Anim Pract. 1984;6:623. doi: 10.1016/s0196-9846(17)30014-9. [DOI] [PubMed] [Google Scholar]
- Rebhun WC. Orbital lymphosarcoma in cattle. J Am Vet Med Assoc. 1982;180:149. [PubMed] [Google Scholar]
- Rebhun WC, Del Piero F. Ocular lesions in horses with lymphosarcoma: 21 cases (1977-1997) J Am Vet Med Assoc. 1998;212:852. [PubMed] [Google Scholar]
- Rebhun WC, de Lahunta A. Diagnosis and treatment of bovine listeriosis. J Am Vet Med Assoc. 1982;180:395. [PubMed] [Google Scholar]
- Roeder PL. Pestivirus fetopathogenicity in cattle: changing sequelae with fetal maturation. Vet Rec. 1986;118:44. doi: 10.1136/vr.118.2.44. [DOI] [PubMed] [Google Scholar]
- Silverstein AM. An experimental virus-induced retinal dysplasia in the fetal lamb. Am J Ophthalmol. 1971;72:22. [Google Scholar]
- Slatter DH. Effects of experimental hyperlipoproteinemia on the canine eye. Exp Eye Res. 1979;29:437. doi: 10.1016/0014-4835(79)90061-7. [DOI] [PubMed] [Google Scholar]
- Smith BP, editor. Large Animal Internal Medicine. 3rd ed. Mosby; St. Louis: 2002. [Google Scholar]
- Smith MO. Diseases of the nervous system. In: Smith BP, editor. Large Animal Internal Medicine. 3rd ed. Mosby; St. Louis: 2002. p. 873. [Google Scholar]
- Stephens LR. Infectious thromboembolic meningoencephalitis in cattle: a review. J Am Vet Med Assoc. 1981;178:378. [PubMed] [Google Scholar]
- van der Lugt JJ. Status spongiosus, optic neuropathy, and retinal degeneration in Helichrysum argyrosphaerum poisoning in sheep and a goat. Vet Pathol. 1996;33:495. doi: 10.1177/030098589603300503. [DOI] [PubMed] [Google Scholar]
- Whiteley HE. Ocular lesions of bovine malignant catarrhal fever. Vet Pathol. 1985;22:219. doi: 10.1177/030098588502200304. [DOI] [PubMed] [Google Scholar]


