Abstract
This chapter provides an introduction to animals that are commonly used for research. It presents information on basic care topics such as biology, behavior, housing, feeding, sexing, and breeding of these animals. The chapter provides some insight into the reasons why these animals are used in research. It also gives an overview of techniques that can be utilized to collect blood or to administer drugs or medicine. Each section concludes with a brief description of how to recognize abnormal signs, in addition to lists of various diseases.
Keywords: Animal models, Basic biology, Behavior, Biomethodology, Breeding, Diseases, Reproductive biology
7.1. The Mouse
7.1.1. Introduction
The mouse is a small mammal that belongs to the order Rodentia (Fig. 7.1 ). The house mouse of North America and Europe, Mus musculus, is the species most commonly used for biomedical research. It is likely that the mouse originated in Eurasia and utilized its commensal relationship with humans to spread through to other continents as humans explored and colonized. Mouse fanciers around the turn of the 20th century are the source of the majority of the laboratory mice that are in use today. A summary of the overarching categories of mouse models that are available is presented in Table 7.1 .
Figure 7.1.

Laboratory Mouse.
Photo used with permission of American Association for Laboratory Animal Science.
Table 7.1.
Summary of the Various Categories of Mouse Types That Are Available for Use in Research
| Model | Generation | Uses | Examples |
|---|---|---|---|
| Inbred strains | 20 or more consecutive generations of sister × brother or parent × offspring matings | Studies that require genetically identical animals | BALB/c, C3H, C57BL/6, CBA, DBA/2, C57BL/10, AKR, A, 129, SJL |
| Outbred stocks | Deliberate mating of unrelated animals | Studies that require outbred vigor | Swiss Webster, CD-1, ICR |
| Spontaneous mutant | Strains that have been bred to conserve phenotypical characteristics that were due to spontaneous genetic mutations | Studies of disease processes associated with the spontaneous mutation | Athymic nude, nonobese diabetic (NOD) |
| Genetically engineered mice/“Knock-in”/“Knock-out” | Mice where genes have been turned on or off | Studies that are seeking to identify the effects of specific genes | |
| Transgenic | Mice where a gene from an unrelated species has been inserted into the genome | Studies that require a mouse model of human disease and toxicology |
7.1.2. Uses in Research
Mice and rats make up approximately 95% of all laboratory animals, with mice the most commonly used animal in biomedical research. Mice are a commonly selected animal model for a variety of reasons, including small size (facilitating housing and maintenance); short reproductive cycle and lifespan; generally mild-tempered and docile; wealth of information regarding their anatomy, genetics, biology, and physiology; and the possibility for breeding genetically manipulated mice and mice that have spontaneous mutations.
Mice have been used as research subjects for studies ranging from biology to psychology to engineering. They are used to model human diseases for the purpose of finding treatments or cures. Some of the diseases they model include: hypertension, diabetes, cataracts, obesity, seizures, respiratory problems, deafness, Parkinson's disease, Alzheimer's disease, various cancers, cystic fibrosis, and acquired immunodeficiency syndrome (AIDS), heart disease, muscular dystrophy, and spinal cord injuries. Mice are also used in behavioral, sensory, aging, nutrition, and genetic studies. This list is in no way complete as geneticists, biologists, and other scientists are rapidly finding new uses for the domestic mouse in research.
7.1.3. Normative Biology
Mice are mammals and their organ systems are very similar to organ systems in humans in terms of shape, structure, and physiology. Basic physiologic data are presented in Table 7.2 .
Table 7.2.
Basic Mouse Biological Data
| Adult weight | |
| Male | 20–40 g |
| Female | 25–40 g |
| Life span | 1–3 years |
| Body temperature | 97.7°F–100.4°F (36.5°C–38.0°C) |
| Heart rate | 310–840/min |
| Respiration rate | 163 breaths/min |
| Water consumption | 5–8 mL/day |
| Food consumption | 3–5 g/day |
Mice have very long loops of Henle in the kidneys, thus allowing for maximal concentration of their urine. As a result, urine output in mice usually consists of only a drop or two of highly concentrated urine at a time. They also excrete large amounts of protein in their urine with sexually mature male mice excreting the largest levels of protein possibly as pheromones.
Mice have only two types of teeth, incisors and molars. The incisors are open-rooted and erupt (i.e., grow) continuously throughout their lives. This predisposes mice to malocclusion if not given feeds or objects such as nylon bones to help wear down the teeth during mastication. The molars are rooted and, thus, do not continuously erupt.
The stomach has two compartments with the proximal portion completely keratinized and the distal portion entirely glandular. Their intestines are simple, but the rectum is very short (1–2 mm) and hence is prone to prolapse, especially if the animal has colitis. The gastrointestinal flora consists of more than 100 species of bacteria that form a complex ecosystem that aids digestion and health of the mouse.
Mice have no sweat glands but have a relatively large surface area per gram of body weight. This results in dramatic changes in physiology and behavior in response to fluctuations in ambient temperature. When too cold, mice will respond by nonshivering thermogenesis (i.e., metabolism of brown adipose tissue). In addition to the lack of sweat glands, they cannot pant or produce large amounts of saliva to aid in cooling their body temperature. Therefore, when exposed to very hot situations, mice increase the blood flow to their ears to maximize heat loss; and in the wild, they move into their burrows which are at cooler temperatures. The thermoneutral zone, the range of ambient temperatures at which the mouse does not have to perform regulatory changes in metabolic heat production or evaporative heat loss to maintain its core temperature, is about 85.3°F–86.9°F (29.6°C–30.5°C).
7.1.4. Reproductive Physiology
The female reproductive system is comprised of paired ovaries and oviducts, uterus, cervix, vagina, clitoris, and paired clitoral glands. Pregnant female mice have hemochorial placentation, similar to humans (i.e., maternal blood is in direct contact with the chorion, the outermost layer of the fetal placental membranes). The female mouse also has five pairs of mammary glands. The male reproductive system consists of paired testes, penis, and associated sexual ducts and glands. The inguinal canals are open in the male mouse, and the testes can retract easily into the abdominal cavity. Both sexes have well-developed preputial glands, which can become infected. Males have a number of accessory sex glands, including large seminal vesicles, coagulating glands, and a prostate. Secretions from these glands make up a large part of the mouse's ejaculate. When mice ejaculate, the semen forms a coagulum or copulatory plug.
Mice breed continuously throughout the year unless conditions are very unfavorable to them (e.g., lack of food). Their reproductive potential can be affected by a number of external influences such as noise, diet, light cycles, population density, or cage environment. Genotype also can affect reproductive performance as it is common knowledge that some inbred strains of mice are poor breeders, and if pups are born they may receive poor maternal care. Additional reproductive physiologic data are presented in Table 7.3 .
Table 7.3.
Basic Mouse Reproductive Reference Values
| Puberty | |
| Male | 4–7 weeks |
| Female | 4–8 weeks |
| Estrus cycle | 4–5 days |
| Gestation | 19–21 days |
| Litter size | 9–12 pups |
| Weaning | 21 days |
| Breeding duration | 7–9 months |
Mice can be bred using a one-on-one system (one male to one female; monogamous) or in a harem mating system (polygamous mating). In a monogamous system, the male and female are always left together, but at weaning the pups are removed from the cage. This system allows for maximal use of the postpartum estrus and the maximum number of litters for the females involved, and facilitates record-keeping and monitoring of specific breeders in the colony. In a harem mating system, multiple males are placed with multiple females, usually at a ratio of one male to two to six females. Usually, females are removed to separate cages just before parturition and the postpartum estrus is underutilized.
Mouse pups are born hairless, blind, and deaf and require extensive parental care that is provided mainly by the mother. Due to the ruddy coloration of the skin of the hairless pups, they are also known as “pinkies.” While mouse pups can increase their body temperature through the metabolism of brown fat stores, they are unable to adequately conserve body heat until they develop an adequate fur coat. Thus, inclusion of nesting materials in the cage is highly recommended as huddling inside the nest can provide much needed warmth and safeguard against temperature-associated neonatal losses.
The most reliable method for determining the sex of a mouse is by measuring the length of the anogenital distance, i.e., the distance from the anus to the genitalia. This distance can be measured with a ruler or animals assessed side by side with the rear ends held up by their tails. The anogenital distance is longer in males than females. In sexually mature animals one can also determine the sex of mice by the presence or absence of testicles in a testicular sac.
7.1.5. Mouse Behavior
Mice are social creatures and can be group housed easily. Their main method of communication is via pheromones. They use these olfactory cues to establish a pecking order (i.e., a hierarchical system of social organization). These chemicals are so important that when cage environments are changed, such as by simple cleaning or with bedding changes, a bout of fighting may occur until scent marking of the cage is completed as a way to reestablish the pecking order and social organization in that cage.
Pheromones also play a vital role in reproduction of these animals. This is demonstrated by the Whitten effect and the Bruce effect. The Whitten effect occurs when a group of female mice that are not cycling are exposed to male urine, which contains a large quantity of pheromones. The females will all resume cycling as a group soon after the introduction of the male. In contrast, the Bruce effect is characterized by abortion of litters when pregnant females are exposed to the urine of a strange male.
As with most rodents, mice are nocturnal animals exhibiting peak levels of activity at night. Because mice are a prey species, they display thigmotactic behavior or wall hugging. They avoid open spaces where they might be easily caught by predators. Despite this, mice are very curious about any new objects in their territory and will often examine them at length.
Mice not only have poorly developed eyesight but they are also color blind. A number of inbred strains (e.g., FVB/N and C3H/He) are functionally blind by weaning. They rely on their very sensitive hearing to escape detection and on their sense of smell and taste to detect food (and possibly avoid poisons). Mice can hear over a range of frequencies between 0.5 and 120 kHz; however, normal mice are most sensitive to frequencies of 12–24 kHz. It is important to note here that some inbred strains of mice, e.g., C57BL/6, suffer significant hearing loss before 1 year of age.
Mice can climb, swim, and jump (up to a foot), though they normally prefer to avoid swimming, if possible. Under certain conditions they display stereotypies, which are obsessive–compulsive behaviors. The behaviors may be strain-related, environment-related, or study-related and include wire gnawing, circling, jumping, and aggression. The use of environmental enrichment items such as cardboard tubes or other structures offer the animal an area for retreat from cage mates and add complexity to the environment.
Aggression is another important behavior that commonly occurs in group-housed male mice. It can also occur in group-housed females and mixed-sex cages. Indications that there is an aggressive animal in the cage include bite wounds on the tail, rump, ears, and shoulders of mice (Fig. 7.2 ). The wounds can be so severe as to cause significant blood loss and abscess formation at bite sites. Aggression has been shown to be influenced by strain, age, and prior encounters. In terms of strains, the more aggressive strains are BALB/c, C57BL/10, C57BL/6, DBA/2, and outbred Swiss. Methods to prevent or reduce aggression include use of properly designed enrichment devices; provision of adequate space and shelter for each animal; grouping of mice before they reach puberty; use of docile strains; and removal of dominant animals as soon as possible.
Figure 7.2.
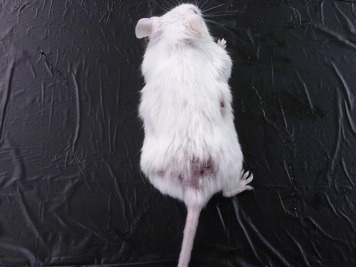
Lesions caused by aggression in mice.
Photograph provided by Jenelle Johnson.
A common manifestation of social organization in group-housed mice is barbering, a behavior in which a dominant mouse will trim, by chewing, the hair or whiskers of other mice in the cage. Barbering is also sometimes referred to as the Dalila effect. It is usually instigated by a dominant male or female mouse. The dominant animals retain their whiskers and full hair coats, while their cage mates have “shaved faces and bodies” (Fig. 7.3 ) (Garner et al., 2004). Although barbering does not generally result in any physical harm to the animal, removing the dominant mouse (the nonbarbered one) from the cage is a good approach to control.
Figure 7.3.
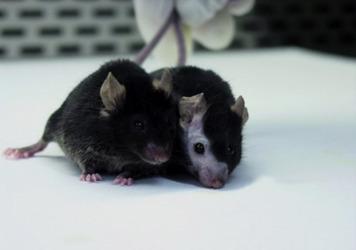
An example of barbering of one mouse (right) by the other mouse (left).
Photograph provided by Jenelle Johnson.
7.1.6. Housing and Handling
General types of housing mice in a laboratory setting include: conventional, specific pathogen free (SPF), and germ free. In conventional housing, no attempt is made to keep out adventitious microbial and parasitic organisms. Mice housed in this manner can be found in open-topped cages. Room air, along with any airborne contaminants, is allowed to freely circulate into the mouse's cage. In addition, the food and water are not sterilized, though it should be noted that microbial contaminants may enter into the mouse population in this way.
SPF mice are raised in barrier conditions to ensure that they remain free of a specific list of pathogens. Care is taken to ensure that adventitious microbes and parasites are excluded from the animals. SPF mice are typically raised in specialized caging such as microisolator cages. These cages contain a 0.22 μm filter top that aids in the exclusion of microbes and parasites. Individually ventilated caging systems include a rack of microisolator cages, each of which receives a filtered air supply (Fig. 7.4 ). Under SPF conditions, everything that comes into contact with the animal should be sterilized or disinfected. This includes, but is not limited to, the water, food, bedding, and caging. Special care must be taken by anyone handling the mice, including researchers, to ensure that handling and experimental procedures do not introduce potential pathogens into the colony. Thus, all handling and procedures done on SPF mice are often performed under HEPA-filtered air conditions, such as within a biosafety cabinet. Placing the mouse in an unfiltered environment (“room” air), even for a moment, is enough to potentially colonize the mouse with a whole host of adventitious microorganisms, thus destroying its SPF status. Once a contaminated mouse is placed back into the colony, the entire colony is at risk for infection.
Figure 7.4.
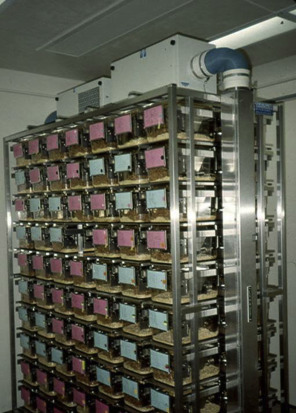
Individually ventilated caging system for mice.
Photo provided by Kay Stewart.
Germ-free, or axenic, mice are raised to contain no microbes or parasites whatsoever. Raising germ-free mice requires strict barrier maintenance. Usually, this requires the use of flexible film isolators which provide HEPA-filtered air to the mice within the isolator. Additionally, any materials (e.g., food, water, and bedding) must be sterilized or thoroughly disinfected prior to being moved into the isolator unit so as not to contaminate the living space of the germ-free rodents with adventitious microorganisms. All procedures performed within the flexible film isolator must utilize strict aseptic technique for the same reason.
In addition to the microbiological environment of the animal's housing systems, mice need to be housed at specific environmental parameters otherwise they may experience stress. The Guide for the Care and Use of Laboratory Animals, 8th edition (National Research Council, 2011) is an internationally accepted document that outlines and discusses globally accepted environmental parameters for housing different species of animals including the mouse. Table 7.4 outlines the specific environmental requirements listed in this document for housing mice.
Table 7.4.
Environmental Parameters for Mice
| Dry-bulb macroenvironmental temperature | 20–26°C (68–79°F) |
| Relative humidity | 30–70% |
| Air changes per hour in the animal rooms | 10–15 ACH |
| Light | 130–325 lux 1 m (3.3 ft.) above the floor |
| Floor space requirements | |
| Weight: <10 g |
|
| Up to 15 g |
|
| Up to 25 g |
|
| >25 g |
|
| Female and litter |
|
| Cage height requirements | 5 in. (12.7 cm) |
Mice are omnivorous and coprophagic with at least one-third of their diet being the consumption of their feces. In the laboratory setting mice are fed a clean, wholesome, and nutritious pelleted rodent diet ad lib. There are many commercially formulated diets for the various stages of life and for animals with specific induced diseases such as diabetes mellitus or hypertension. These diets may be available as autoclavable or irradiated forms to prevent transmission of disease via contaminated feed. There are also a variety of “pet” treats available for mice. However, the treats should not make up more than 5–10% of the daily diet.
Mice should be provided with a continuous supply of water daily. If the animals do not get enough water daily, their food consumption will decrease. The animals will also look scruffy and unhealthy. Mice can be provided with water from water bottles or pouches, automatic watering systems with nipples, or water-based gel packs.
7.1.7. Diseases
Some general signs of ill health include: weight loss, depression or lethargy, anorexia, obesity, diarrhea, scruffiness or ruffled coats, abnormal breathing, sneezing, weakness, dehydration, enlarged abdomen, discolorations (e.g., yellow for jaundiced animals or very pale for anemic animals), masses or swellings, and abnormal posture or gait. Body condition scoring is an objective measure to truly assess how fat or thin the animal is and can be used for accurate determination of endpoints in studies where animals are expected to lose or gain weight (Fig. 7.5 ) (Ullman-Cullere and Foltz, 1999). Some of the more commonly found diseases of mice are presented in Table 7.5 . Noninfectious disorders are presented in Table 7.6 .
Figure 7.5.
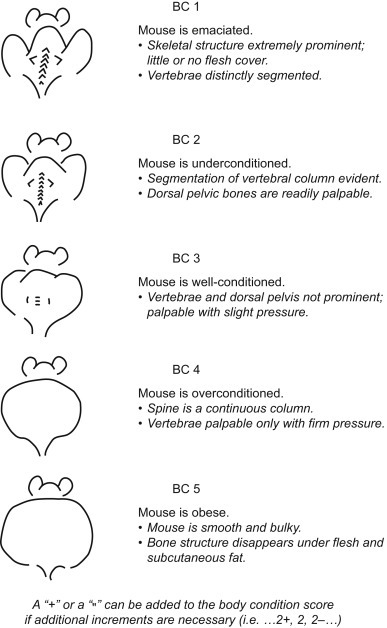
Mouse body condition (BC) scoring chart (Ullman-Cullere and Foltz, 1999).
Table 7.5.
Infectious Diseases of Mice
| Pathogen | Common Names of Diseases | Fatal/Self-Limiting | Effects on Research |
|---|---|---|---|
| Parvoviruses | Minute virus of mice (MVM) Mouse parvovirus (MPV) |
Self-limiting Asymptomatic |
Can distort biological responses that depend on cell proliferation, primarily on immune cells such as cytotoxic T cells. |
| Lymphocytic Choriomeningitis virus (LCMV) | IC—self-limiting and asymptomatic In others it can lead to clinical signs and death |
Death of animals on research Affects immunological function Zoonotic |
|
| Reoviruses | Reovirus 1, 2, or 3 | Disease and mortality in suckling mice | High mortality in young mice May be oncolytic Interference with organ systems |
| Rotavirus (Epizootic diarrhea of Infant mice) | Infection primarily in young mice but self-limiting | Clinical illness leads to diarrhea and retarded growth. | |
| Coronaviruses | Mouse hepatitis virus (Mouse coronaviruses) | IC—asymptomatic IDS and suckling mice—clinical disease and death |
Numerous effects including affecting immunological responses, contaminate transplantable neoplasms, altering tissue enzymes, and causing clinical disease. |
| Mycoplasmosis |
Mycoplasma pulmonis Mycoplasma arthritidis Mycoplasma neurolyticum Mycoplasma collis |
Rhinitis Otitis media Chronic pneumonia Infertility Can be fatal |
Cause clinical disease Compromise experiments involving respiratory tract Alter immunological responses Contamination of cell lines and tumors |
| Cilia-associated respiratory (CAR) bacillus | Bacillus sp. | Respiratory disease Clinical disease— rare |
|
| Helicobacteriosis |
Helicobacter hepaticus Helicobacter bilis, Helicobacter muridarum, Helicobacter rodentium, Helicobacter typhlonius |
IC— asymptomatic IDS— inflammatory bowel disease, Diarrhea, rectal prolapse |
Affects liver enzymes in some strains of mice Morbidity and mortality in mice |
| Alter immune function | |||
| Mycobacteriosis | Mycobacteria avium-intracellulare | Granulomatous pneumonia and microgranulomas in other major organs | Morbidity |
| Mycobacteria leprae-murium | |||
| Pneumocystosis | Pneumocystis carinii | IC—asymptomatic IDS— severe disease; pneumonia May be fatal |
Pneumonia in IDS mice; may be fatal |
| Nematodes (Mouse pinworms) |
Syphacia obvelata Aspicularis tetraptera |
Asymptomatic with gastrointestinal lesions such as enteritis and fecal impaction | Unthriftiness in mice Alter the immune responses in animal |
| Acariasis (Mite infestation) |
Myobia musculi Myocoptes musculinus Radfordia affinis |
Skin lesions, excessive pruritus (scratching), hair loss, dermatitis | Disrupt immunological responses Severe clinical disease |
IC, immune competent; IDS, immune compromised.
Table 7.6.
Noninfectious Disorders of Mice
| Disorder | Cause | Effects on Research |
|---|---|---|
| Hypothermia | Room environment too cold | Can distort biological responses of the mouse due to cold stress |
| Hyperthermia | Room environment too hot | Death of animals |
| Ringtail | Relative humidity of less than 30%; causes circular constrictions of the tail | Can lead to loss of tail |
| Malocclusion | Genetic; poor alignment of the incisors leads to overgrown teeth | Inability to eat |
Based on their genetic and physiologic makeup, mice can be either immunocompetent or immunodeficient. Immunocompetent means that the mouse has a normal functioning immune system and can stage an immune response to any insult or injury. In contrast, immunodeficient means that some component or components of the mouse's immune system is not working or functioning normally, and so they cannot stage an adequate immune response and are more susceptible to infectious disease. Immunosuppressed mice are mice that have a complete immune system but because of a drug or chemical or disease state, the immunological response is attenuated.
7.2. The Rat
7.2.1. Introduction
Rats and humans have a long history of coexistence. The origins of the laboratory rat, also known as the Norway rat, stretch back centuries to the areas of modern day China and Mongolia (Burt, 2006, Song et al., 2014). The dispersal of the Norway rat has occurred across the centuries and its natural habitat stretches from the Mediterranean across Southeast Asia and down into Australia and New Guinea (Song et al., 2014). Unfortunately, most people associate rats with disease and destruction. Throughout history, outbreaks of bubonic plague, typhus, and hantaviruses have had an unwitting accomplice in the rat (Zinsser, 1935, Benedictow, 2004, Firth et al., 2014). Over the centuries, rats have also been used for food (e.g., in Imperial China), companionship, and sport (Gorn and Goldstein, 2004, Hopkins et al., 2004, Burt, 2006). Ratting, a vicious blood sport where people laid bets on the dog that could kill the most rats in a given period of time was especially popular in both the Victorian England and American Underworld (Thomas and Mayhew, 1998, Gorn and Goldstein, 2004). At the turn of the 20th century, breeding rats as a hobby or for companionship (i.e., “fancy rats”) was recognized by the addition of “rat” to both the name and mission of National Mouse Club in the United Kingdom (American Fancy Rat and Mouse Association, 2014). However, as interest in pet rats waned over the following years, the club reorganized and dropped “rat” from its name. A similar club, the American Fancy Rat and Mouse Association, was founded in the United States in 1983 (American Fancy Rat and Mouse Association, 2014).
7.2.2. Uses in Research
The first recorded use of rats as research subjects occurred in 1828 (Hedrich, 2000), and the first known rat breeding experiments occurred in the late 1800s (Lindsey and Baker, 2006). The first major effort to perform research in the United States using laboratory rats occurred at the Wistar Institute of Philadelphia, the oldest independent research institute in the United States, in 1894 (Lindsey and Baker, 2006).
Rattus norvegicus constitutes one of the most commonly used laboratory species (Fig. 7.6 ), second only to the laboratory mouse. Because rats and mice are not included under the Animal Welfare Act Regulations, the precise number of these species used per year within the United States is unavailable. However, examining the data collected within the European Union can give some indication of their use relative to other common laboratory animal species. In 2011, rats accounted for just fewer than 14% (1.6 million) of the total animals (11.5 million) used in research within the European Union (European Commission, 2013). This contrasts to mice, which constituted 61% (6.9 million) of the total animals used within the European Union (European Commission, 2013).
Figure 7.6.

Two juvenile Lobund-Wistar laboratory rats.
Photo provided by Kay Stewart.
Rats possess a number of qualities which make them a highly suitable and much preferred animal model. Like mice, these traits include relatively small size; known genetic background; short generation time; similarities to human disease conditions; and known microbial status. Their tractable nature makes them easier to handle in a laboratory setting than many other rodents. Rats rarely bite their handlers unless extremely stressed or in pain.
Rats have been used as animal models in numerous areas of research from space exploration to answering more basic scientific questions regarding nutrition, genetics, immunology, neurology, infectious disease, metabolic disease, and behavior. Perhaps their largest use is in drug discovery, efficacy, and toxicity studies. In the United States, the approval of any new drug for use in humans or animals usually necessitates that toxicity testing be done in at least one small animal species (e.g., rodents) and one large animal or target species (e.g., dog, nonhuman primate).
7.2.3. Normative Biology
There are known physiologic differences between the numerous outbred stocks and inbred strains of rats. The Rat Genome Database (RGD) is an extensive, free resource filled with information regarding the different phenotypes, models, and genomic tools used in rat research (Laulederkind et al., 2013). Vendors of commercially available rat stocks and strains are often good resources for normal physiologic data of these strains. Many provide stock- and strain-specific data directly on their websites such as growth curves, complete blood count and serum biochemistry panels, and spontaneous lesions seen on histopathology. A summary table of normal physiologic references is in Table 7.7 .
Table 7.7.
Basic Rat Biological Data
| Adult weight | |
| Male | 260–1000 g |
| Female | 225–500 g |
| Life span | 1–3 years |
| Body temperature | 97.7°F–100.4°F (36.5°C–38.0°C) |
| Heart rate | 310–493/min |
| Respiration rate | 145 breaths/min |
| Water consumption | 15–20 mL/day |
| Food consumption | 22–33 g/day |
7.2.4. Reproductive Biology
Sexual dimorphism exists between male and female rats. Sexing of adult rats is most easily done by examining the perineal area of the rat and identifying the external reproductive structures such as the penis, testes, or vagina. In addition, male rats are typically larger and weigh significantly more than their age- and strain-matched female counterparts. Sexing of rat pups is most easily performed by examining the distance between the anus and genital opening in the pup. Males have a greater anogenital distance than females.
Male rats possess paired testicles that descend from the abdomen into the scrotal sac at approximately 15 days of age (Russell, 1992). Due to the lack of closure of the inguinal rings, the testes may be retracted into the abdominal cavity even as an adult. The male rat also possesses a number of accessory sex glands. A four-lobed prostate is present along with four other paired glands, including: the seminal vesicles, coagulating glands, ampullary glands, and bulbourethral glands (noted in some texts by the older name, Cowper's gland) (Popesko et al., 1992). Due to the unusual bihorned shape of the closely associated coagulating gland and seminal vesicles, individuals unfamiliar with rodent male anatomy may initially mistake these structures for the female uterus. However, the apices of these glands are freely mobile and easily exteriorized from the abdominal cavity unlike the uterus, which is attached to the dorsal body wall bilaterally via paired ovaries and their respective ovarian ligaments.
The reproductive anatomy of the female rat contains some distinct features. The uterus of the female rat is classified as a duplex uterus, because the vagina is separated from the uterus by two individual cervices with each cervix leading to a separate uterine horn (Popesko et al., 1992). The placentation of the pregnant rat is hemotrichorial (three layers) rather than the hemomonochorial (single layer) placentation present in humans (Wooding and Burton, 2008). The rest of the reproductive anatomy (e.g., ovary, oviduct) is structurally and functionally similar to other mammals. A summary of basic reproductive physiology is presented in Table 7.8 .
Table 7.8.
Basic Rat Reproductive Reference Values
| Puberty | |
| Male | 45 days |
| Female | 33–42 days |
| Estrus cycle | 4–5 days |
| Gestation | 21–23 days |
| Litter size | 9–12 pups |
| Weaning | 21–28 days |
| Breeding duration | 7–9 months |
Rat pups are born hairless, blind, and deaf and require extensive parental care that is provided mainly by the mother. As with mice, the skin of the hairless rat pups has a pink coloration, thus they are also referred to as “pinkies.” The inclusion of nesting materials in the cage is recommended to assist the rat pups with thermal regulation until they have a full hair coat (Whishaw and Kolb, 2005).
7.2.5. Normal Behavior
Like other rodents, Rattus norvegicus is a nocturnal species with the highest level of activity occurring during the dark phase. Behaviors exhibited by rats include grooming, nesting, eating, and other social behaviors. Nesting behavior serves several purposes among rats and mice (Gaskill et al., 2012, Gaskill et al., 2013a, Gaskill et al., 2013b, Gaskill et al., 2013c). Nests allow for better thermoregulatory control within a given environment as well as protection against predation (Gaskill et al., 2013c). Recent work in mice suggests that energy not diverted to thermoregulation can be shunted to other activities as seen via improved feed conversion and breeding performance (Gaskill et al., 2013c). However, anecdotal evidence suggests that nest building in rats is largely a learned behavior, and it appears that there is a developmental period in young rats whereupon if exposed to nesting materials during this time they will begin using the materials to build at least rudimentary nests (Gaskill, 2014). At a minimum, rats benefit from having a structural shelter or nest box into which they may rest away from prying eyes (Fig. 7.7 ).
Figure 7.7.

A multilevel cage with an intracage shelter. This style of caging provides opportunities for exercise for the rats.
Photo provided by Melissa Swan.
Rats emit alarm vocalizations during times of distress. However, these negatively associated vocalizations typically register in the ultrasonic wavelengths (approximately 22 kHz), well outside of the human hearing range (Burman et al., 2007, Parasana et al., 2012). Rodents may also emit high-pitched audible vocalizations when extremely alarmed, distressed, or in pain (Jourdan et al., 1995, Hans et al., 2005).
As discussed in Chapter 5, rats exhibiting abnormal behaviors and stereotypies can create variables in the research findings and should not be used in a study unless abnormal behavior is the object of the study subjects (Baenninger, 1967, Callard et al., 2000, Garner and Mason, 2002, Cabib, 2005, Ibi et al., 2008). Examples of stereotypies seen in rats include: bar-gnawing, pawing behavior, repetitive circling, and backflipping. It is critical that rats should be provided some form of environmental enrichment to stimulate positive species-typical behaviors.
7.2.6. Housing
The housing of rats in a laboratory setting is similar to that described previously for mice: conventional, SPF, and germ free. As rats are social animals, at minimum, they should be housed in pairs whenever possible. There is a preponderance of evidence that shows the differences in affiliative versus aggressive behavior, biochemical changes, and changes in learning between rats raised and housed in social isolation versus those housed in social groups (Baenninger, 1967, Einon and Morgan, 1977, Robbins et al., 1996).
Enrichment items, such as a hut, nesting box, or similar type of shelter may be included in the cage to provide a visual barrier between the rat and the rest of the animal room. Evidence suggests that rats prefer shelters made from opaque plastic (Patterson-Kane, 2003). Rats also spend a lot of time in the wild chewing either for eating or for manipulating objects for nest building. Providing objects made of safe materials in the cage allows the rats to exhibit this natural behavior and encourage the normal wear of the rat's incisors, minimizing the incidence of malocclusion of the teeth.
Rodents can benefit from frequent gentle handling by the researcher and animal care staff. This concept is also known as “gentling” and has been demonstrated to reduce the stress experienced by rats during experimental handling and procedures (Hirsjarvi et al., 1990, Van Bergeijk et al., 1990). Another positive interaction between humans and rats is found in the “tickling” of rodents. Based on ultrasonic vocalization data, rodents find tickling a pleasurable experience (Burgdorf and Panksepp, 2001, Panksepp, 2007, Hori et al., 2014). Tickling may also decrease the stress response seen in rodents after experimental manipulations like intraperitoneal injections (Cloutier et al., 2014).
7.2.7. Diseases
As for mice, some general signs of ill health would include: weight loss, depression or lethargy, anorexia, obesity, diarrhea, scruffiness or ruffled coats, abnormal breathing, sneezing, weakness, dehydration, enlarged abdomen, discolorations (e.g., yellow for jaundiced animals or very pale for anemic animals), masses or swellings, and abnormal posture or gait. When assessing animals, a body condition score can be used as an objective measure or scale to truly assess how fat or thin the animal is; and it allows for the accurate determination of endpoints in studies where animals are expected to lose or gain weight (Hickman and Swan, 2010) (Fig. 7.8 ). Some of the more commonly found diseases of rats are presented in Table 7.9 .
Figure 7.8.
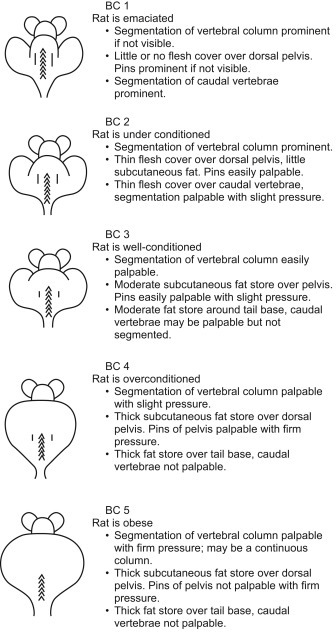
Rat body condition (BC) scoring chart (Hickman and Swan, 2010).
Table 7.9.
Infectious Diseases of Rats
| Pathogen | Common Names of Diseases | Fatal/Self-Limiting | Effects on Research |
|---|---|---|---|
| Parvovirus | Kilham rat virus; rat parvovirus | Asymptomatic | Immunomodulatory |
| Staph infection | Staphylococcus aureus | Dermatitis; septicemia | Significant clinical illness can result in necessity for euthanasia of the animal |
| Mycoplasmosis |
Mycoplasma pulmonis Mycoplasma arthritidis Mycoplasma neurolyticum Mycoplasma collis |
Rhinitis Otitis media Chronic pneumonia Infertility Can be fatal |
Cause clinical disease Compromise experiments involving respiratory tract Alter immunological responses Contamination of cell lines and tumors |
| Cilia-associated respiratory (CAR) bacillus | Bacillus sp. | Respiratory disease Clinical disease—rare |
|
| Helicobacteriosis |
Helicobacter hepaticus Helicobacter bilis, Helicobacter muridarum, Helicobacter rodentium, Helicobacter typhlonius |
IC—asymptomatic IDS— inflammatory bowel disease, diarrhea, rectal prolapse |
Affects liver enzymes in some strains of rats Morbidity and mortality in rats Alter immune function |
| Mycobacteriosis |
Mycobacteria avium-intracellulare Mycobacteria leprae-murium |
Granulomatous pneumonia and microgranulomas in other major organs | Morbidity |
| Pneumocystosis | Pneumocystis carinii | IC—asymptomatic IDS—severe disease; pneumonia May be fatal |
Pneumonia in IDS rats; may be fatal |
| Nematodes (Mouse pinworms) |
Syphacia obvelata Aspicularis tetraptera |
Asymptomatic with gastrointestinal lesions such as enteritis and fecal impaction | Unthriftiness in mice Alter the immune responses in animal |
| Acariasis (Mite Infestation) |
Myobia musculi Myocoptes musculinus Radfordia affinis |
Skin lesions, excessive pruritus (scratching), hair loss, dermatitis | Disrupt immunological responses Severe clinical disease |
IC, immune competent; IDS, immune compromised.
7.3. The Rabbit
7.3.1. Introduction
The ancestral home of the European rabbit (Oryctolagus cuniculus) is the Iberian Peninsula (Hardy et al., 1995). The earliest archeological evidence of the coexistence of humans and rabbits can be found in excavation sites dated at approximately 120,000 years BCE in Nice, France (Dickenson, 2013). In antiquity, Romans used rabbits as a food source and are thought to be responsible for their dispersal throughout Europe, although there is no evidence that they attempted to actually domesticate them (Dickenson, 2013). Domestication and selective breeding is thought to have begun in France in the Middle Ages where monks began to breed rabbits in their monasteries (Dickenson, 2013). The rabbits were kept confined in enclosures called “clapiers”(Dickenson, 2013). They were kept largely as a source of food for the monks especially since 600 CE when Pope Gregory I officially classified them as “fish” and thus eligible to being eaten during Lent. However, rabbit wool production soon became a welcome by-product of these domestication efforts.
7.3.2. Uses in Research
European rabbits have been used in research since the middle of the 19th century. Early work with the species was concentrated on the comparative anatomy of the rabbit with other species, such as the frog, and the unique features of the rabbit's heart and circulatory system (Champneys, 1874, Roy, 1879, Smith, 1891). Louis Pasteur used rabbits in a series of experiments that led to the development of the world's first rabies vaccine (Rappuoli, 2014).
While there are numerous so-called “fancy” breeds of rabbits available in the pet trade, the list of breeds routinely used in research is much shorter. The New Zealand White (NZW) rabbit is the most frequent breed of used in research (Fig. 7.9 ). The California and Dutch-belted rabbit breeds are also occasionally used. Researchers have developed genetically inbred rabbit strains for particular research applications. For example, the Watanabe heritable hyperlipidemic (WHHL) and the myocardial infarction-prone WHHL rabbit (WHHLMI), both developed by researchers in Japan, are used to explore diseases associated with dyslipidemia such as atherosclerosis (Shiomi et al., 2003, Shiomi and Ito, 2009).
Figure 7.9.

New Zealand White rabbits are commonly used in research.
Photo provided by Kay Stewart.
Rabbits have been used as a model of human pregnancy and for the production of polyclonal antibodies for use in immunology research (Hanly et al., 1995, Ema et al., 2010, Ito et al., 2011, Fischer et al., 2012). Rabbits are routinely used in atherosclerosis, osteoporosis, ocular, and immunology research (Southard et al., 2000, Arslan et al., 2003, McMahon et al., 2005, Castaneda et al., 2008, Habjanec et al., 2008, Manabe et al., 2008, Xiangdong et al., 2011, Panda et al., 2014, Sriram et al., 2014, Wei et al., 2014, Zhou et al., 2014). The production of polyclonal antibodies is preferentially performed in the rabbit due to its relatively large blood volume compared to rodents (Hanly et al., 1995). Their tractable nature and larger body size also make them suitable for surgical implantation of biomedical devices (Gotfredsen et al., 1995, Swindle et al., 2005, Ronisz et al., 2013). Additionally, rabbits are a favored model in pharmacologic studies for teratogenicity testing of novel pharmaceutic compounds (Gibson et al., 1966, Lloyd et al., 1999, Foote and Carney, 2000, Jiangbo et al., 2009, Oi et al., 2011).
7.3.3. Normative Biology
While much of the anatomy of the rabbit is similar to other mammalian species, it should be noted that there are a number of key differences. For example, the skin of the rabbit is quite thin and fragile. Care should be taken when restraining a rabbit or shaving a rabbit's fur (e.g., in preparation for surgery) to avoid tearing the skin. Unlike rodents and other laboratory animals, rabbits do not have pads on their feet; rather, the plantar surface is covered with fur (Quesenberry and Carpenter, 2012).
The long ears of the rabbit serve several purposes. The most obvious is for hearing. In addition, the central ear artery and marginal ear veins are easily accessible for both intravenous administration and blood sampling (Diehl et al., 2001, Parasuraman et al., 2010) (Fig. 7.10 ). The ears also serve as a means of thermoregulation, as excess heat may be exchanged across the large surface area of the ears (Sohn and Couto, 2012). The skin of rabbits lacks sweat glands and is therefore unable to sweat; panting is insufficient to dissipate the excess heat (Sohn and Couto, 2012). Thus, the ears play a vital role in maintaining proper body temperature. Other unique features of the skin and adnexa are the presence of chin and inguinal glands used in scent marking. Additionally, the female rabbit (doe) is noted by the presence of a large skin fold filled with subcutaneous fat just under the chin (the dewlap) (Sohn and Couto, 2012).
Figure 7.10.
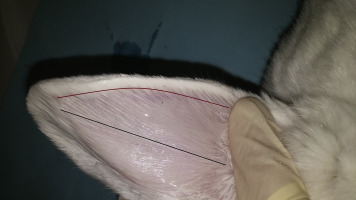
Blood collection sites include the central ear artery (black line) and marginal ear vein (burgundy line) of a New Zealand White rabbit.
Photo provided by Deb Hickman.
The skeleton of rabbits makes up only 8% of the body weight by mass (Brewer, 2006). This is in contrast to other similarly sized mammals. For example, the cat skeleton makes up 12–13% of body weight (Brewer, 2006). The small skeletal mass of the rabbit coupled with strong back muscles mean that the back is prone to traumatic fracture (Meredith and Richardson, 2015). Proper holding and restraint techniques are necessary to avoid this undesirable outcome.
There are several unique features of both the respiratory and cardiovascular systems of rabbits. For example, rabbits are obligate nose breathers (Varga, 2014). This is especially important during procedures involving anesthesia and placement of an endotracheal tube. With respect to the cardiovascular system, the rabbit heart is unique in that the right atrioventricular (AV) valve has only two leaflets instead of three (Brewer, 2006). Additionally, due to the similarity to humans with respect to the neural anatomy of the ventricles, the rabbit is the species of choice for Purkinje fiber research (Brewer, 2006).
Rabbit teeth are “open-rooted” meaning that they continue to erupt and grow throughout life. This applies to all of the teeth in the rabbit dental arcade (i.e., incisors, premolars, and molars; rabbits do not have canines). This contrasts with rodents, where the incisors are the only open-rooted (or hypsodontic) teeth (Sohn and Couto, 2012). Thus, rabbit teeth are subject to overgrowth. Another unique feature of rabbit dentition that sets them apart from rodents is the presence of a second set of incisors just behind the first set of upper incisors known as “peg” teeth (Sohn and Couto, 2012). They are thought to aid in tearing off the succulent leaves of plants while grazing.
As an obligate herbivore, the gastrointestinal tract of rabbits differs greatly from that of carnivores and omnivores. Rabbits require a high fiber diet of between 14 and 20% (Sohn and Couto, 2012). The small intestine is divided up into three main sections: the duodenum, jejunum, and ileum. The ileum connects to the cecum via a structure called the sacculus rotundus. The presence of lymph follicles suggests that the sacculus rotundus has immunological functions. It is sometimes referred to as the ileocecal “tonsil” (Jenkins, 2000). In the rabbit, gastric associated lymphoid tissue is also present in the small intestine and the vermiform appendix (Lanning et al., 2000).
The cecum, a large distensible outpouching of the large intestine, holds up to an estimated 40% of the total ingesta (Sohn and Couto, 2012). Rabbits are considered to be “hindgut fermenters.” Bacteria present in the cecum ferment the digestible fiber found within the diet. The product of this fermentative process becomes cecotrophs (also known as “night feces”). Cecotrophs are excreted roughly 8 h after the initial foodstuffs are ingested (Sohn and Couto, 2012). They are softer and more mucoid in appearance than the hard, dry “day feces” produced just 4 h after consuming food (Sohn and Couto, 2012). The bulk of day feces consists of the indigestible fiber found in the diet. The sorting of foodstuffs destined to become either day feces or cecotrophs and the timing of their relative excretion is largely dependent upon the neural input of the fusus coli, also termed the “pacemaker” of the colon (Sohn and Couto, 2012). The fusus coli is anatomically located between the ascending and transverse colons of the rabbit (Popesko, 1992). Consumption of cecotrophs by rabbits is an important part of the digestive process in rabbits as they are rich in B vitamins, such as niacin and B12, and vitamin K (Hörnicke, 1981). While cecotrophs are known colloquially as “night feces,” rabbits produce and eat them at all hours of the day (Sohn and Couto, 2012). Rabbits are agile enough to eat these night feces directly from their anus (Sohn and Couto, 2012). Those researchers performing digestive research (e.g., fecal collection via metabolism cages) should keep this in mind.
7.3.4. Reproductive Biology
Sexing of adult rabbits is aided by the sexual dimorphism present in the species. Mature females are readily identified by the presence of the dewlap (Sohn and Couto, 2012). Females have 8–10 nipples, while in males these nipples are present, but rudimentary (Sohn and Couto, 2012). NZW rabbits reach sexual maturity between 5 and 7 months (Suckow et al., 2002). Reproductive data are summarized in Table 7.10 .
Table 7.10.
Basic Rabbit Reproductive Reference Values
| Puberty | |
| Male | 22–52 weeks |
| Female | 22–53 weeks |
| Gestation | 29–35 days |
| Litter size | 4–12 kits |
| Weaning | 5–8 weeks |
| Breeding duration | |
| Male | 60–72 months |
| Female | 24–36 months |
Mature bucks have paired testicles enclosed in paired hairless scrotal sacs (Sohn and Couto, 2012). Like rodents, the inguinal rings do not close. Accessory sex glands include several bilobed organs: the seminal vesicle, vesicular gland, prostate, and paraprostatic gland. The bulbourethral glands of bucks are paired (Sohn and Couto, 2012).
Female rabbits are induced ovulators (Dal Bosco et al., 2011, Sohn and Couto, 2012). That is, the egg does not ovulate spontaneously from the ovary, rather manual stimulation via copulation is required. Ovulation occurs approximately 10 h postcopulation (Sohn and Couto, 2012). Because they are induced ovulators, does do not have a defined estrous cycle. Rather, they have periods of sexual receptivity lasting approximately 14–16 days followed by 1–2 days of nonreceptivity. Nonfertile matings may result in a period of pseudopregnancy of up to 15–16 days (Sohn and Couto, 2012). Fertile matings result in pregnancy lasting 31–32 days (Sohn and Couto, 2012). The placenta of rabbits is classified as hemodichorial; this is in contrast to humans which have a hemomonochorial placenta (Furukawa et al., 2014).
Birthing (i.e., parturition; also known as “kindling”) occurs most often during the early morning hours (Sohn and Couto, 2012). Kits are born deaf and blind. By 7 and 10 days of age they can hear and see, respectively (Quesenberry and Carpenter, 2012). Amazingly, does suckle their young ones daily, usually during the dark phase, and for approximately only 5–6 min (Sohn and Couto, 2012). The kits are able to drink about 30% of their entire body weight in that time. Wild and domesticated does both follow this nursing behavior. Rabbit kits may be weaned between 5 and 8 weeks of age (Suckow et al., 2002). Earlier weaning should not be attempted as there may be profound detrimental effects on the functioning of the gastrointestinal system (Bivolarski and Vachkova, 2014).
7.3.5. Behavior
Rabbits are very social, nocturnal creatures. Scent marking is a normal and important part of their behavior repertoire. Rabbits will rub the secretions from their chin scent glands against inanimate objects, other rabbits, and human handlers in a process called “chinning” (Sohn and Couto, 2012). Dominance hierarchies are established behaviorally. Dominant animals may mount, “barber,” or scent-mark subordinates (Sohn and Couto, 2012). Barbering is the act of chewing the hair of a subordinate animal, usually on the neck and back in the case of rabbits, very close to the skin giving the appearance of having been cut or “barbered” (Bays, 2006). Rabbits will “thump” one or both back feet on the ground when frightened or as an alarm call to other rabbits (Bays, 2006). Highly stressed rabbits may actually emit a loud, piercing scream, especially when roughly caught by an untrained individual (Bays, 2006). It is important to approach rabbits calmly and quietly. Relaxed, content rabbits may be heard making a purring sound (Bays, 2006). Rabbits benefit by repeated, positive interactions with people similar to the concept of “gentling” in rats (see Section 7.2).
Changes in behavior are often first indication that an animal is in pain. Given that rabbits are a prey species, it is evolutionarily speaking not in a rabbit's best interest to display signs of pain. Thus, these behaviors are most often quite subtle in nature and may be easily missed if particular attention is not paid. The first sign often seen in a rabbit experiencing pain is a decreased appetite resulting in little to no food intake (Sohn and Couto, 2012). Rabbits will often grind their teeth (i.e., bruxism) when experiencing pain (Sohn and Couto, 2012). Other rabbits may simply appear very dull and inactive.
As with rodents, rabbits can develop stereotypies. Due to the sensitivity of the rabbit's nose and lips many stereotypies involve chewing behaviors. Bar chewing, chewing on the water bottle, and self-barbering are all stereotypical behaviors seen in rabbits (Gunn and Morton, 1995, Chu et al., 2004). In addition, rabbits may engage in “nose sliding” against solid surfaces like the cage walls and head swaying (Sohn and Couto, 2012). Animals that exhibit stereotypies do not make good research animals. Efforts should be made, where possible, to prevent these behaviors through the use of environmental enrichment. Enrichment may be in the form of chew-resistant objects (such as plastic dumbbells and stainless steel rattles) and food treats (Poggiagliolmi et al., 2011). As a prey species, rabbits benefit from the inclusion of a hut in the cage or at least a visual barrier into which they may retreat when psychologically stressed (Baumans, 2005). Breeding females should always have access to a nest box to allow for the necessary expression of normal nesting behavior (Baumans, 2005).
7.3.6. Housing and Management
Being social creatures, ideally rabbits should be housed in compatible pairs or trios unless contraindicated by the research objectives or by incompatibility of the animals (Sohn and Couto, 2012). Stable social groups formed shortly after weaning, where animals are not added or removed, is most beneficial (Boers et al., 2002). Structurally, rabbits benefit from housing that has both adequate vertical and horizontal space (Boers et al., 2002). One recommendation on the space requirements of laboratory rabbits stipulates 16 in. as the minimum vertical cage height (National Research Council, 2011). At a minimum, rabbits must be able to comfortably sit upright in the cage without their ears bending over (National Research Council, 2011).
Laboratory rabbits are typically housed in easily sanitized stainless steel cage racks (Fig. 7.11 ). Slatted flooring allows for urine and feces to fall through the slats onto special pans fixed below the cage, thus providing for easier sanitation of the cages. However, care must be taken that the slats are of sufficient width so as to prevent a condition known as bumblefoot (see Section 7.3.7). Dog runs with elevated, slatted flooring or a solid floor with bedding have also been used by some investigators in group-housed rabbits with success (personal observations). Again, attention should be paid to the flooring and its effect on foot health.
Figure 7.11.

Example of rabbit caging for a laboratory setting.
Photo provided by Deb Hickman.
Commercially available research diets specifically formulated for rabbits are available. These diets are preferred to so-called “natural diets” and feeding individual vegetables. This is because rabbits tend to be very selective eaters which can lead to nutritional imbalances (Fraser and Girling, 2009). Additionally, the use of fresh vegetables may lead to the introduction of unwanted pathogens like Salmonella (Varga, 2014). Commercial diets are available in maintenance and reproductive-performance dietary formulations as well as presterilized diets for rabbits housed under SPF conditions.
Rabbits are very easily heat stressed and thus must be kept at significantly lower temperatures than other laboratory animals like rats and mice. Noise is another significant stressor to rabbits (Verga et al., 2007). Sudden, high-pitched, sharp noises are most disruptive. However, in general, noise within the animal rooms should be avoided as much as possible. For this reason, rabbits should not be housed, even temporarily for short procedures, near areas of high noise.
7.3.7. Diseases
Problems in rabbits related to the gastrointestinal system are relatively common. These problems can become serious very quickly. Therefore, it is critical that abnormalities seen (e.g., rabbit not eating or abnormal feces) be reported to the veterinary care staff immediately. Even if a researcher is unsure if there is a problem, it is best to report suspicions because without prompt intervention, seemingly minor problems can escalate to potentially life-threatening conditions. Some of the most commonly seen clinical conditions in rabbits are summarized in Table 7.11 .
Table 7.11.
Commonly Seen Clinical Conditions in Rabbits
| Dysbiosis | Gastrointestinal upset generally secondary to the use of broad spectrum antibiotics, such as penicillin. |
| Malocclusion | Misalignment and subsequent overgrowth of the continuously growing teeth |
| Pododermatitis (“bumblefoot”) | Infection of the underside of the feet |
| Pasteurella multocida | Common cause of respiratory infections and abscesses |
7.4. Zebrafish
7.4.1. Introduction
The zebrafish, Danio rerio of the Cyprinidae family, is a small, dark blue and yellow striped, shoaling, teleost fish, popular among aquarium enthusiasts, and increasingly among the research community (Fig. 7.12 ). The adult fish are 4–5 cm in length, with an incomplete lateral line and two pairs of barbels (Laale, 1977). Males have larger anal fins and more yellow coloration; females have a small genital papilla just rostral to the anal fin (Laale, 1977, Creaser, 1934).
Figure 7.12.

Male zebrafish have a more stream-lined body with darker blue strips while the females have a white protruding belly.
Photo provided by Kay Stewart.
Zebrafish are hardy, fresh water fish originating from a tropical region with an annual monsoon season. The fish are generally found among slow moving waters of rivers, streams, and wetlands, across the South Asia region of India, Bangladesh, and Nepal (Engeszer et al., 2007, Spence et al., 2008). The waters tend to be shallow, relatively clear with substrates of clay, silt, or stone of varying size (McClure et al., 2006, Engeszer et al., 2007). The fish feed mostly on insects and plankton, with evidence of feeding along the water column as well as water surface (McClure et al., 2006, Engeszer et al., 2007, Spence et al., 2008).
7.4.2. Uses in Research
The small size of zebrafish, the ease of keeping large numbers, frequent spawning, large egg clutches, translucent nonadherent eggs, rapid development and complex sequencing of the zebrafish genome are all key components that make the zebrafish an attractive research model. Interestingly, approximately 70% of zebrafish genes have at least one orthologous human gene (Howe et al., 2013). Publications on the use of zebrafish in research are cited as early as the 1930s (Creaser, 1934). Until the early 1970s, the use of zebrafish stayed fairly low, with the number of articles published staying below 20 per year. In the mid-1970s, publications increased to about 40 per year, doubling again in the 1980s, increasing to almost 200 articles per year in the early 1990s, and rapidly expanding to 1929 publications by 2012.
Developmental biology was the initial focus of zebrafish research use. However, in recent years, use of the zebrafish in research related to biochemistry and molecular biology, cell biology, neurological sciences, and genetics has been rapidly increasing.
7.4.3. Biology
Zebrafish are known to live for only a year in the wild (Spence et al., 2008). For most of the year, the fish reside in shallow streams. With the onset of monsoon rains, they move to flooded, highly vegetated shallow wetlands and floodplains, including rice paddies, with little to no current and often silt bottoms for spawning (Engeszer et al., 2007). The offspring then develop in these waters until the seasonal waters diminish (Engeszer et al., 2007). Zebrafish rapidly mature, reaching sexual maturity as early as 2 months postfertilization (Lawrence et al., 2012). The zebrafish continues to grow throughout life, which is much longer in captivity, with a mean lifespan of 3.5 years in captivity (Gerhard et al., 2002).
In nature, spawning behavior occurs within small groups of three to seven fish. Males within the group pursue females, with spawning occurring along the substrate (Spence et al., 2008). Similar behaviors are noted in laboratory zebrafish, with spawning often occurring with the first light of day. Courtship behavior involves a rapid chase of the female, the male swimming around the female, nudging her, or swimming back and forth working the female to the spawning site. Interestingly, zebrafish prefer spawning near artificial plants. Once there, the male remains close to the female, extending his fins to bring his genital pore in line with the female. The male may also rapidly undulate his tail against the side of the female to initiate egg release by the female, coinciding with sperm discharge by the male. The female produces eggs in batches of 5–20 over several encounters with the male for up to an hour. Most eggs are released within the first 30 min, with a peak in production during the first 10 min (Darrow and Harris, 2004, Spence et al., 2008).
Zebrafish produce large clutches of eggs, from 150 to 400 eggs per clutch (Laale, 1977). The eggs, approximately 0.7 mn in diameter, are transparent and protected within a chorionic membrane (Kimmel et al., 1995). First body movements and beginning stages of organ development occur 10–24 h postfertilization (Kimmel et al., 1995). As development continues, the larva hatches from the egg two to three days postfertilization (Kimmel et al., 1995). The early larva has special secretory cells within multicellular regions of the head epidermis that allow the larvae to attach to various hard surfaces and plants until the swim bladder inflates 4 or 5 days postfertilization (Laale, 1977, Kimmel et al., 1995). Once the air bladder inflates, the fish can maneuver through the water column.
In captivity, zebrafish can breed year round. The presence of males, or even just the male pheromones, is needed to induce ovulation (Gerlach, 2006). If females are housed away from males for an extended period, they can retain the eggs resulting in egg-associated inflammation, which can be lethal (Kent et al., 2012a).
7.4.4. Housing and Management
To accommodate the fish life cycle, zebrafish are typically housed in static spawning cages to allow for fertilized egg production. Spawning cages include a housing tank containing a clear slotted bottom insert, and a plastic plant. The insert is often placed in the holding tank at an angle to create a shallow region for spawning and the slotted bottom of the insert allows for ease of egg collection (Lawrence and Mason, 2012, Nasiadka and Clark, 2012) (Fig. 7.13 ). The embryos are then incubated at around 28.5°C in a petri dish for at least 3–4 days postfertilization (Wilson, 2012). The fish are then kept in static or slow water flow containment and can be fed Paramecium, rotifer, a powdered food, or a combination of these feed types.
Figure 7.13.

Example of a zebrafish spawning system. The system is designed to allow eggs to fall beneath a slotted insert to the bottom of the tank as a way to prevent the adult fish from consuming the eggs.
Photo provided by Robin Crisler.
Unfortunately, other than the need for essential fatty acids in their diet, little is yet known on the nutritional requirements of zebrafish. Zebrafish in research settings are typically fed live feed like Artemia (brine shrimp), rotifers, bloodworms (Chironomid larvae), commercial feed, or a combination of all (Lawrence, 2007). The size of the feed is necessary to suit the gape size of the larvae, approximately 100 μm (Lawrence, 2007, Wilson, 2012). Water flow and feed size increase with development, with transition of the feed to Artemia (brine shrimp), and/or use of a larger particle commercial feed during the 8–15 days postfertilization (Wilson, 2012).
Once the juvenile stage is reached, around 29 days postfertilization, the fish are housed more like adult fish, with more frequent feeding and slower water flow to accommodate their remaining development and smaller size, respectively (Wilson, 2012). As early as 2 months of age the fish are sexually mature (Lawrence et al., 2012).
Adult zebrafish can be housed in traditional glass aquaria or elaborate computerized and automated systems that monitor and control water quality parameters such as temperature (typically 28.5°C), pH, water hardness, salinity, dissolved oxygen, and nitrogenous wastes (Lawrence, 2007, Lawrence and Mason, 2012). Whether maintained manually or computerized, these parameters are important to monitor and maintain at appropriate levels to maximize the health of the fish. Poor water quality can lead to disease in the fish (Kent et al., 2012b).
7.4.5. Diseases
Many of the organisms that cause disease in zebrafish are opportunists in the environment and remain subclinical until the fish is stressed, often due to problems with husbandry.
Appropriately maintained housing, combined with a healthy water quality, avoidance of overcrowding, and a functional quarantine and health surveillance program are key components to avoiding stress and disease. To date, there are currently no known viruses documented in zebrafish as naturally occurring disease concerns (Kent et al., 2012b). Mycobacterium infections are the most frequently documented bacterial infections (Kent et al., 20122, Kent et al., 2012b).
7.5. Amphibians and Reptiles
7.5.1. Introduction
Class Reptilia is made up of four orders classified as Chelonia, Rhynchocephalia, Squamata, and Crocodilia (Frye, 1991). In class Amphibia, animals more commonly encountered in research setting are in the order Anura, containing frogs and toads (such as Xenopus, Bufo, Rana, Hyla, and Dendrobates spp.); and in the order Caudata, containing salamanders such as the tiger salamander, Ambystoma tigrinum and the axolotl, Ambystoma mexicanum (National Research Council, 1974) (Figure 7.14, Figure 7.15 ). Snakes and lizards are in class Reptilia, order Squamata; chelonians (turtles, tortoises, terrapins) are in order Chelonia; and alligators, caimans, and crocodiles are in order Crocodilia.
Figure 7.14.

A commonly used amphibians in research is the axolotl, Ambystoma mexicanum.
Provided by Chris Konz.
Figure 7.15.
African clawed frogs, Xenopus laevis, a commonly used amphibian.
Provided by Randalyn Shepherd.
7.5.2. Uses in Research
In contrast to research in mammals, there is a tendency for reptile and amphibian research to be more oriented to studying evolution and ecology as opposed to basic science evaluating models of human disease (Pough, 1991). Salamanders and frogs are important for studying embryonic development, metamorphosis, regeneration, physiology, and climate change (Burggren and Warburton, 2007, Hopkins, 2007, Pough, 2007). Reptiles are often studied because of their more simple cardiovascular systems as well as for evaluating mechanisms of immune responses, hormonal controls, and unique reproduction methods such as parthenogenesis (Frye, 1991). Of the amphibians, Xenopus laevis (South African clawed frog) and Xenopus tropicalis (Western clawed frog) are commonly studied in the research setting. X. laevis is a prominent research model in comparative medicine and developmental studies, and is the most commonly studied species in the genus Xenopus (Denardo, 1995, Schultz and Dawson, 2003, O'Rourke, 2007). Advantages include large-sized eggs for ease of observing embryo development, as well as the wealth of published literature in areas of research such as evolution, neurobiology, regeneration, endocrinology, and toxicology (Koustubhan et al., 2008, Gibbs et al., 2011). Rana catesbeiana (bullfrogs) have been used for developmental and toxicological studies, and for infectious disease study of the chytrid fungus Batrachochytrium dendrobatidis (Alworth and Vazquez, 2009). A. mexicanum, in particular, is studied to understand the regenerative ability of the blastema of amputated limbs at the molecular level (Gresens, 2004, Rao et al., 2014). Ambystoma tigrarium has been studied in regard to general amphibian decline in North America, environmental contaminants such as pesticides and effects of infection with A. tigrarium virus (Sheafor et al., 2008, Kerby and Storfer, 2009, Chen and Robert, 2011, Kerby et al., 2011). A variety of snakes, crocodiles, lizards, and turtles have been studied in research. For example, Anolis carolinensis (the green anole) has been used for the study of reproduction biology (Lovern et al., 2004). Caiman crocodilus and Alligator mississippiensis (crocodiles), Trachemys scripta elegans (red-eared sliders) represent a few other examples of reptiles used in research (O'Rourke and Schumacher, 2002).
7.5.3. Biology
Amphibians and reptiles are considered to be ectotherms (Greene, 1995). Unlike mammals and birds, ectotherms are unable to internally regulate body temperatures above that of the ambient environment through metabolism and require complex behavioral and thermoregulatory adaptations to regulate temperature (Pough, 1991, Seebacher and Franklin, 2005). In captivity, ectotherms typically require supplemental sources of heat to mimic the thermoregulatory effects of basking in the sun.
Some amphibians and reptiles are aquatic (Xenopus frog spp.) whereas others are semiaquatic or terrestrial. X. laevis and X. tropicalis are from geographically distinct areas and have different temperature requirement depending on life stage, with X. tropicalis adults generally around 25°C in their natural habitat versus about 20°C for X. laevis (Tinsley et al., 2010). The skin of amphibians is permeable to water and some adults (semiterrestrial tree frogs in family Hylidae, arboreal and terrestrial toads in Bufonidae) may receive a significant portion of their daily water requirement via absorption through a vascular-rich area on the pelvic area termed the pelvic patch (Pough, 2007, Ogushi et al., 2010). The skin of some amphibians contains toxins which can cause arrhythmias in human handlers, for example, alkaloids from dendrobatid frogs, and bufotoxins from toads of the genus Bufo (DeNardo, 1995). The toxins serve to keep predators away but, as with Xenopus, may harm the animals themselves by continued direct contact or diffusion through the water (Tinsley, 2010, Chum et al., 2013). The skin of amphibians is easily damaged thus to protect the animal during handling powder-free gloves should be worn (Gentz, 2007).
7.5.4. Behavior
Researchers and animal care providers should investigate the natural environment of each species within their care and critically evaluate what features are required for normal behavior and physiology to provide the essential elements in the research setting (Pough, 1991). In the wild, amphibians and reptiles live in ecological environments that span a range of diversity from topical forest areas to dry desert. They may be arboreal, aquatic, or terrestrial. They are often secretive and hide when in natural habitats, preferring to hide under vegetation or in crevices. Parameters from the natural habitat to evaluate include temperature, humidity, nutritional requirements, natural diet, nocturnal versus diurnal behavior, and housing density. Temperature and lighting gradients should be established so animals can choose to move toward or away from the heat source as a way to avoid overheating. Most amphibian species in the wild are nocturnal (Pough, 2007, Tinsley, 2010).
7.5.5. Housing and Management
Amphibians and reptiles are sensitive to chemicals in the environment. Water quality parameters (such as pH, hardness, ammonia, nitrate/nitrite, salinity, conductivity) should be regularly monitored. Chloramine and chloramines are often present in municipal water supplies and are toxic to aquatic species. Water should be treated prior to use for aquatic species with an agent like sodium thiosulfate, since chloramine does not readily dissipate (Browne et al., 2007). Ammonia is a breakdown product between the chloramine and sodium thiosfulfate reaction and is a concern for aquatic animals (Browne et al., 2007, Koustubhan et al., 2008, O'Rourke and Schultz, 2002).
A wide variety of caging materials may be used for housing such as glass, plastic, stainless steel, or fiberglass but should be free of contaminants or harmful chemicals like bisphenol A that could leach from the caging into the water (Levy et al., 2004, Browne et al., 2007, Bhandari et al., 2015). Agents used to sanitize caging should be chosen to minimize likelihood of harmful residues.
Environmental enrichment should be provided to encourage natural behaviors and can include providing cage mates for social interaction, cage accessories that serve as hiding spots or shelters (Fig. 7.16 ) as well as providing a variety of food treats in changing locations for foraging opportunities (Hurme et al., 2003). Scents, sounds, and color choices may also be incorporated into enrichment strategies provided that they are carefully evaluated to ensure that they are beneficial and do not cause stress. For example, the tortoise, Chelonoidis denticulata, may show a color preference for red-colored enrichment items (Passos et al., 2014). PVC tubes are another example of enrichment that has been provided to X. laevis for use as hiding cover (Koustubhan et al., 2008). Some species may require haul-out ramps, areas for sun basking, floating rest areas, or enrichment devices along the water's surface to help prevent drowning. One should consider the possibility of ingestion, as reptiles and amphibians may attempt to consume the substrates provided to them.
Figure 7.16.

Use of a rabbit feeder for Xenopus enrichment.
Photo by Randalyn Shepherd.
The degree to which amphibians are social varies significantly depending on the species and is not always well understood. They use visual and olfactory discrimination to help them find food, forage, and avoid predators (Vitt and Caldwell, 2014). Both in the wild and in captivity, reptiles and amphibians may exhibit excitatory behavior when fed (sometimes described as a “feeding frenzy”) which may result in animal injury where animals are in close proximity (Divers and Mader, 2006, Tinsley, 2010). Overcrowded tanks can result in competition for food and subsequent trauma. Thus, when placed together for the first time, animals should always be observed for compatibility; and only members of the same species should be housed together.
Many reptiles and amphibians are escape artists and prevention of escape and injury is a critical factor when considering housing design. Species that are prone to jumping must have secured lids on their enclosures.
The diets of amphibians and reptiles are highly variable in the wild and are species dependent. Commercially prepared pelleted diets may be available and accepted by reptiles and aquatic amphibians, however, terrestrial amphibians and many reptiles may prefer live diets (Pough, 2007). It is not unusual for some species to go several days of fasting between meals in nature (Pough, 1991). Consultation with those experienced at successful housing and feeding the species in question (zoos, nutritionists, herpetologists) is recommended.
7.5.6. Diseases
There are many different types of infectious agents such as bacteria, viruses, fungi, and parasites that can cause health problems in amphibians and reptiles in addition to noninfectious conditions such as those resulting from nutritional imbalances, metabolic disease, neoplasia, trauma, and other spontaneous maladies. Although significant advances in knowledge have been made over the past 100 years regarding disease in these species, much still remains unknown. It is not possible to go into detail here, but there are excellent reference texts for diseases in amphibians and reptiles that can be consulted (Jacobson, 2007, Frye, 1991, Wright and Whitaker, 2001).
7.6. Birds
7.6.1. Introduction
From a taxonomic standpoint, birds are placed into class Aves which includes multiple orders based on anatomical, physiological, and genetic characteristics. Passeriformes is the largest order and contains songbirds and perching birds such as the finch, canary, and cardinal (Fig. 7.17 ). Order Columbiformes contains pigeons and doves; order Psittaciformes contains budgies and parrots such as the African gray; and order Galliformes contains domestic fowl such as the chicken and quail (Proctor and Lynch, 1993, Ritchie et al., 1994) (Fig. 7.18 ).
Figure 7.17.

The zebra finch is a common avian species used in research.
From http://www.redorbit.com/news/science/1112751282/male-zebra-finches-fake-song-121912/.
Figure 7.18.

The domesticated chicken commonly used in research.
Provided by Kay Stewart.
7.6.2. Uses in Research
Birds have been used as research models of human disease and are important in evaluation of aging, memory, parasitology, atherosclerosis, reproduction, and infectious disease among other topics (Austad, 1997, Austad, 2011, Maekawa et al., 2014). The genomes of several avian species have now been sequenced (Jarvis et al., 2014). Historically, chickens (Gallus domesticus) are the most common bird species studied in biomedical and agricultural research and are a classic model in areas such as immunology, virology, infectious disease, embryology, and toxicology (Scanes and McNabb, 2003, Kaiser, 2012). Chickens are also studied to evaluate reproductive development and retinal disease. Embryonated chicken eggs have been used to commercially produce vaccines (such as for human influenza), studied for developmental analysis, and are now being treated with viral vectors like lentivirus to produce transgenic embryos. Inbred lines with improved disease resistance are being developed and transgenic technology in the future may allow embryos to be used as bioreactors to produce therapeutic proteins of interest and potentially to generate transgenic chickens which have improved resistance to pathogens (Bacon et al., 2000, Scott et al., 2010). Because chickens develop spontaneous ovarian cancers at an incidence of up to 35%, they are also a prominent model of ovarian cancer in humans (Bahr and Wolf, 2012, Hawkridge, 2014). Quail (Coturnix coturnix and Coturnix japonica) have been studied in many of the same research disciplines as chickens, but offer advantages because of their smaller size and because they are among the shortest-lived bird species (Austad, 1997). Japanese quail (C. japonica) have been selected as a model to evaluate reproductive biology and social behaviors such as mate selection because they readily show sexual behavior in captivity (Ball and Balthazart, 2010). As with the chicken, methods to study transgenic quail are now becoming available and offer a useful tool to study gene function (Seidl et al., 2013).
Of the Psittaciformes, Amazon parrots and budgies (Melopsittacus undulates) are among the most commonly studied, with research topics including veterinary medicine, diagnostics, behavioral, cognition, aging, and sensory studies (Austad, 2011, Kalmar et al., 2010). The African gray parrot has been studied for its cognition and communication abilities (Hesse and Potter, 2004, Harrington, 2014).
Of the passerines studied in laboratory research, the most commonly evaluated include the zebra finch (Taeniopygia guttata), European starling (Sturnus vulgaris and Sturnus roseus), and house sparrow (Passer domesticus) (Bateson and Feenders, 2010). Zebra finches and other songbirds are commonly studied in regard to aging and neurogenesis in addition to speech, learning, and memory because of their ability to learn and communicate intricate bird songs (Harding, 2004, Scott et al., 2010, Austad, 2011, Mello, 2014). The most popular songbird species for neurobiological research include the zebra finch, canary, and other types of small finches such as Lonchura striata domestica (Schmidt, 2010). Zebra finches are favored in research settings since they are easy to house due to their small size, for their compatibility in groups, and proclivity for breeding. They are also studied for their biologic features such as sexual dimorphism, year-round singing in captivity, age-dependent period of song-learning propensity, and for ease of measurement with respect to their bird song (Fee and Scharff, 2010, Mello, 2014). Pigeons (Columba livia) have been evaluated in areas such as comparative psychology, neuroanatomy, neuroendocrinology, and atherosclerosis (Santerre et al., 1972, Austad, 1997, Shanahan et al., 2013). They are studied to understand their navigational skills and memory which allow homing, vision and discrimination ability. Barn owls (Tyto alba) are an example of a nocturnal avian species and are studied for neuroanatomy, vision, hearing, and for understanding learning mechanisms during auditory space mapping (Pena and Debello, 2010, Rosania, 2014).
7.6.3. Biology
Birds are warm-blooded vertebrates that have feathers for the purpose of flight and plumage. Their respiratory system includes avascular air sacs, some of which attach to the lung and bronchi, but do not serve as sites for gas exchange as does the lung (Maina, 2006, Ritchie et al., 1994). Air sacs serve as internal compartments which hold air and facilitate internal air passage to allow birds to have a continuous flow of large volumes of air through the lungs as a way to increase oxygen exchange capacity and efficiency. Birds lack a functional diaphragm and use muscles of the thorax to assist with respiration (Ritchie et al., 1994). Care must be taken to ensure that use of physical restraint does not interfere with respiratory movement, cause the bird to struggle, or become stressed. The skeletal system includes pneumatic bones which are lined with air sac epithelium and are considered pneumatized by connection to the respiratory system (Frandson et al., 2009). The specific bones which are pneumatized depend on the species but typically include the humerus, cervical vertebrae, sternum, sternal ribs, and sometimes the femur (Ritchie et al., 1994).
The esophagus in birds leads to the crop, which is an outpocketing where food is held temporarily, and then continues to the proventriculus (also called the true stomach) which produces enzymes to break down food. Food travels from the proventriculus to the ventriculus (gizzard) and then on into the small and large intestines. The presence or absence of a gallbladder is species dependent (Tully et al., 2009, Kalmar et al., 2010). The rectum and urinary tract terminate in the cloaca, resulting in excreta where the fecal portion of waste is mixed with urate (white and/or creamy component). There are many additional unique and complex anatomic and physiologic adaptations of birds. Other excellent references are available in the literature (Scanes, 2015).
7.6.4. Behavior and Housing
Housing requirements of birds held in captivity vary significantly depending on the particular species. Basic parameters that apply to all birds include the necessity to provide an enclosure which is safe and permits species-specific behaviors to the greatest extent possible. Consideration should be given to ensure that the type of structure is nontoxic, as some birds such as parrots have a powerful beak with the ability to chew through substrates. Enclosures may be made of metals or durable plastic, but it is important to note that zinc wire, as well as leaded paint, can be toxic to birds and is best avoided. Bar spacing on caging should be appropriate to prevent escape and injury based on the size of the bird. Caging size varies and can include large aviaries where full short-distance flight is possible, to individual housing in smaller sized cages where flight may not be feasible. Use of environmental enrichment and provision of opportunity for interaction is important to include as part of the cage structure, complexity, and social dynamic. Some types of birds are considered social, polygamous, and benefit from group housing, whereas others such as those that pair-bond (such as New World quail) may prefer housing with a single mate (Ritchie et al., 1994). Some species, genders, or individuals show aggression and may not be compatible. For example, sexually active male quail may injure each other and are generally considered incompatible (Huss et al., 2008). To help reduce aggression, housing densities should be kept low and multiple points of access to resources, such as feed and perches, should be provided.
Enrichment in the form of manipulanda can take the form of toys and food items. Some types of birds may demonstrate foraging behavior in nature and may like to manipulate their feed. Parrots, for example, typically grasp their food with their feet and may peel or strip the outer portion of the foodstuff prior to ingesting. Toys should be size appropriate for the species, easily sanitized, free from sharp edges, and replaced once wear shows. Birds can become easily caught in items that hang from the cage and as toys deteriorate they can become a hazard. For example, rope toys may begin to fray and become a hazard, causing entrapment; and some types of toys contain weights which pose a choking hazard or may be made of toxic materials such as lead. Some types of birds spend considerable time perching and require perches, which vary in diameter, for comfort and to prevent pressure sores from developing on their feet.
The respiratory system of the bird is very sensitive and caution must be taken by animal care staff to avoid exposure of birds to aerosols from chemicals that may arise from disinfectants used in the laboratory animal facility. Scented cleaners, perfumes, hairspray, and emissions from Teflon-coated materials are all examples of products which can be especially harmful to birds and may cause death.
Feeding requirements vary by species and life stage, but commercial pelleted diets designed to meet the nutritional needs can generally be provided. Although many birds are seed eaters, a diet of seeds alone is unlikely to provide adequate or balanced nutrition. Many birds have a requirement for dietary calcium, especially those that are reproductively active, and should be provided with calcium supplementation in the form of soluble grit such as cuttlebone or crushed oyster shells (Sandmeier and Coutteel, 2006, Tully et al., 2009). Birds often display neophobic behavior and may require long acclimation periods before fully accepting novel foodstuffs. For this reason, dietary changes should not be made abruptly and daily intake should be closely monitored. For birds in the laboratory setting, clean, fresh water should be provided daily either by use of nonbreakable bowls or sipper tubes. Water intake will vary by species and environmental housing conditions.
7.6.5. Diseases
Birds can mask disease and are easily stressed. It is best to first observe the bird in its normal home environment whenever possible and only perform restraint for physical exam or collection procedures when indicated. General indications of sickness may include decreased appetite, depressed behavior, loose stools, distended abdomen, ruffled feathers or unkempt appearance, skin lesions, open-mouth breathing, abnormal respiratory sounds such as wheezing or sneezing, or signs of dehydration such as reduced skin turgor and sunken eyes. A healthy bird should have well-groomed feathers, appear alert, active and inquisitive, and should show species-typical behaviors. Its eyes should be clear and bright. No evidence of discharge should be present from the eyes, nares, mouth, or urogenital area. Numerous types of infectious (example, Fig. 7.19 ) and noninfectious disease presentations are described in birds. Additional reference resources should be consulted for in-depth information (Ritchie et al., 1994, Tully et al., 2009, Doneley, 2010).
Figure 7.19.

Example of skin pox on the feet of a dark-eyed Junco (Junco hyemalis).
Photo from Randalyn Shepherd.
7.7. Other Small Mammals
To provide the reader a broader view of animal use in research, descriptions of some less commonly used small mammal models follow.
7.7.1. Guinea Pigs
Guinea pigs (Cavia porcellus) are rodents, related to porcupines and chinchillas in the suborder Hystricomorpha (Fig. 7.20 ). They originate from the mountain and grassland regions along the mid-range of the Andes Mountains in South America. They are small, stocky, nonburrowing, crepuscular herbivores with short legs and little to no tail, ranging from 700 to 1200 g, females being smaller than males (Harkness et al., 2010). Guinea pigs have a long-standing historical role in research stretching as far back as the 1600s, when they were first used in anatomical studies (Pritt, 2012). Further, they were used by Louis Pasteur and Robert Koch in their investigations of infectious disease, and have contributed to the work of several Nobel Prize worthy studies (Pritt, 2012). Specifically, the guinea pig has been used as a model for infectious diseases such as tuberculosis, Legionnaires disease, sexually transmitted diseases such as chlamydia and syphilis, and one of the more common causes of nosocomial infections in people, Staphylococcus aureus (Padilla-Carlin et al., 2008). Guinea pigs have also been useful tools in researching cholesterol metabolism, asthma, fetus and placental development and aspects of childbirth, as well as Alzheimer's disease (Bahr and Wolf, 2012).
Figure 7.20.

Juvenile guinea pig.
Photo from Randalyn Shepherd.
Guinea pigs have many similarities to humans hormonally, immunologically, and physiologically. Unlike other rodents, and more like primates (including people), guinea pigs are prone to scurvy if they do not receive adequate vitamin C, typically in their diet (Gresham et al., 2012). Guinea pigs are housed similarly to other rodents, although they require more room than the smaller rodents.
7.7.2. Hamsters
Hamsters are of the Rodentia order, suborder Myomorpha along with the mouse and the rat. There are over 24 species of hamsters described in the literature, with the most common hamster used in research being the Golden or Syrian hamster, Mesocricetus auratus (Harkness et al., 2010) (Fig. 7.21 ). Originating from the northwest region of Syria, Golden hamsters are thought to be descendants of only three or four littermates collected from Syria in 1930 (Adler, 1948, Smith, 2012). As their name implies, the typical wild-type coat is reddish gold along their dorsum, with a gray underside. They are granivores and insectivores, weighing 85–150 g, females weighing more than males, with short legs and short tail, and large cheek pouches (Harkness et al., 2010).
Figure 7.21.

Syrian hamster.
Photo from Randalyn Shepherd.
Specific anatomical and physiological features including their susceptibility to disease and infection make them a useful model for study. Initially hamsters were utilized in studies of infectious disease, parasitology and dental disease, transitioning into cancer research in the 1960s (Smith, 2012). Hamsters are still used in many areas of research, including investigations into metabolic diseases like diabetes mellitus (Hein et al., 2013), cardiovascular disease (Russell and Proctor, 2006), reproductive endocrinology (Ancel et al., 2012), and oncology (Tysome et al., 2012). Guinea pigs have also been used as models for infectious disease associated with bacteria, parasites, and viruses, such as leptospirosis (Harris et al., 2011), leishmaniasis (Gomes-Silva et al., 2013), and severe acute respiratory syndrome (SARS) and Ebola viruses (Roberts et al., 2010, Wahl-Jensen et al., 2012).
Other species of hamsters used have been used in research. For example, Chinese and African hamsters have been used for investigations into diabetes mellitus (Kumar et al., 2012); European and Turkish hamsters have been useful to evaluate aspects of hibernation (Batavia et al., 2013); and Siberian and Turkish hamsters have been used to study circadian rhythm and pineal gland activity (Butler et al., 2008) (Fig. 7.22 ).
Figure 7.22.

Siberian hamsters.
Photo from Greg Demas.
7.7.3. Chinchillas
Chinchillas (Fig. 7.23 ) are in the order Rodentia, suborder Hystricomorpha, as are the guinea pig and the degu. There are the long-tailed chinchilla,Chinchilla lanigera, and the short-tailed chinchilla, Chinchilla chinchilla. Chinchillas originate from the Andes Mountains of South America (Martin et al., 2012). They are 400–800 g in size, females weighing more than males, with compact bodies and long, strong hind limbs and dense fur coats (Alworth et al., 2012). The lushness of the coat is what led them close to extinction in the wild due to excessive hunting in the early to mid-1900s (Jimenez, 1996). The chinchilla has a large head, large eyes and ears. The large inner ear anatomy is of specific note as chinchillas are the traditional model for auditory studies (Shofner and Chaney, 2013) and otitis media (Morton et al., 2012).
Figure 7.23.

Chinchilla.
Photo from Bill Shofner Jr.
7.7.4. Gerbils
The gerbil is a rodent, suborder Myomorpha, used in research. There are over 100 species of gerbil-like rodents documented, but the Mongolian gerbil (Meriones unguiculatus) is the species most commonly used in the United States (Fig. 7.24 ). Mongolian gerbils originate from a desert terrain in Mongolia and northeast China. They are long-tailed, burrowing, herbivorous rodents, 55–130 g in size, males being larger than females (Harkness et al., 2010). Due to anatomical variations in the blood supply to the brain in an anatomical region known as the “Circle of Willis,” gerbils have been used most notably as a model for cerebral ischemia or stroke (Small and Buchan, 2000).
Figure 7.24.

Gerbil.
Photo used with permission of American Association for Laboratory Animal Science.
7.7.5. Armadillo
An interesting animal model to note among the small mammals is the nine-banded armadillo (Dasypus novemcinctus), a new world mammal ranging from the southeastern half of North America, extending south through the Americas to the northern region of Argentina (Balamayooran et al., 2015). Armadillos have a banded carapace, and, importantly, a low core body temperature of 33–35°C. The breeding season is in the summer, but embryo implantation is delayed until late fall, at which point identical quadruplicates are always formed (Balamayooran et al., 2015). The armadillo's low body temperature, and susceptibility and physiologic response to the infectious organism, Mycobacterium leprae, have made it an ideal model for studying leprosy (Balamayooran et al., 2015). The consistent polyembryony of the species has also made the animal a model of interest in understanding various aspects of twinning (Blickstein and Keith, 2007).
7.8. Summary
Choosing the correct animal model is an essential component to the success of biomedical research. Each species used in biomedical research must be provided with adequate housing and care to ensure the well-being of the animals. Because good science and good animal care go hand in hand, it is important to understand and address the biological and behavioral needs of the animals being studied.
References
- Adler S. Origin of the golden hamster Cricetus auratus as a laboratory animal. Nature. 1948;162:256–257. doi: 10.1038/162256b0. [DOI] [PubMed] [Google Scholar]
- Alworth L.C., Harvey S.B. Chinchillas: anatomy, physiology and behavior. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press/Elsevier; London: 2012. [Google Scholar]
- Alworth L.C., Vazquez V.M. A novel system for individually housing bullfrogs. Lab. Anim. (NY) 2009;38:329–333. doi: 10.1038/laban1009-329. [DOI] [PubMed] [Google Scholar]
- American Fancy Rat and Mouse Association 2014. http://www.afrma.org/ Available from:
- Ancel C., Bentsen A.H., Sebert M.E., Tena-Sempere M., Mikklesen J.D., Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinology. 2012;153:1352–1363. doi: 10.1210/en.2011-1622. [DOI] [PubMed] [Google Scholar]
- Arslan H., Ketani A., Gezici A., Kapukaya A., Necmioglu S., Kesemenli C., Subasi M. The effects of osteoporosis on distraction osteogenesis: an experimental study in an ovariectomised rabbit model. Acta Orthop. Belg. 2003;69:67–73. [PubMed] [Google Scholar]
- Austad S.N. Birds as models of aging in biomedical research. ILAR J. 1997;38:137–141. doi: 10.1093/ilar.38.3.137. [DOI] [PubMed] [Google Scholar]
- Austad S.N. Candidate bird species for use in aging research. ILAR J. 2011;52:89–96. doi: 10.1093/ilar.52.1.89. [DOI] [PubMed] [Google Scholar]
- Bacon L.D., Hunt H.D., Cheng H.H. A review of the development of chicken lines to resolve genes determining resistance to diseases. Poult. Sci. 2000;79:1082–1093. doi: 10.1093/ps/79.8.1082. [DOI] [PubMed] [Google Scholar]
- Baenninger L.P. Comparison of behavioural development in socially isolated and grouped rats. Anim. Behav. 1967;15:312–323. doi: 10.1016/0003-3472(67)90018-8. [DOI] [PubMed] [Google Scholar]
- Bahr A., Wolf E. Domestic animal models for biomedical research. Reprod. Domest. Anim. 2012;47(Suppl. 4):59–71. doi: 10.1111/j.1439-0531.2012.02056.x. [DOI] [PubMed] [Google Scholar]
- Balamayooran G., Pena M., Sharma R., Truman R.W. The armadillo as an animal model and reservoir host for Mycobacterium leprae. Clin. Dermatol. 2015;33:108–115. doi: 10.1016/j.clindermatol.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Ball G.F., Balthazart J. Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR J. 2010;51:310–325. doi: 10.1093/ilar.51.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batavia M., Nguyen G., Zucker I. The effects of day length, hibernation, and ambient temperature on incisor dentin in the Turkish hamster (Mesocricetus brandti) J. Comp. Physiol. B. 2013;183:557–566. doi: 10.1007/s00360-012-0729-9. [DOI] [PubMed] [Google Scholar]
- Bateson M., Feenders G. The use of passerine bird species in laboratory research: implications of basic biology for husbandry and welfare. ILAR J. 2010;51:394–408. doi: 10.1093/ilar.51.4.394. [DOI] [PubMed] [Google Scholar]
- Baumans V. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. ILAR J. 2005;46:162–170. doi: 10.1093/ilar.46.2.162. [DOI] [PubMed] [Google Scholar]
- Bays T.B. Rabbit behavior. In: Mayer T.B.B.L., editor. Exotic Pet Behavior. W.B. Saunders; St. Louis: 2006. (Chapter 1) [Google Scholar]
- Benedictow O.J. Boydell Press; Rochester: 2004. The Black Death, 1346–1353: The Complete History. [Google Scholar]
- Bhandari R.K., Deem S.L., Holliday D.K., Jandegian C.M., Kassotis C.D., Nagel S.C., Tillitt D.E., Vom Saal F.S., Rosenfeld C.S. Effects of the environmental estrogenic contaminants bisphenol A and 17alpha-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen. Comp. Endocrinol. 2015;214:195–219. doi: 10.1016/j.ygcen.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Bivolarski B.L., Vachkova E.G. Morphological and functional events associated to weaning in rabbits. J. Anim. Physiol. Anim. Nutr. (Berl) 2014;98:9–18. doi: 10.1111/jpn.12058. [DOI] [PubMed] [Google Scholar]
- Blickstein I., Keith L.G. On the possible cause of monozygotic twinning: lessons from the 9-banded armadillo and from assisted reproduction. Twin Res. Hum. Genet. 2007;10:394–399. doi: 10.1375/twin.10.2.394. [DOI] [PubMed] [Google Scholar]
- Boers K., Gray G., Love J., Mahmutovic Z., McCormick S., Turcotte N., Zhang Y. Comfortable quarters for laboratory rabbits. In: Reinhardt V., Reinhardt A., editors. Comfortable Quarters for Laboratory Animals. ninth ed. Animal Welfare Institute; Washington, DC: 2002. [Google Scholar]
- Brewer N.R. Biology of the rabbit. J. Am. Assoc. Lab. Anim. Sci. 2006;45:8–24. [PMC free article] [PubMed] [Google Scholar]
- Browne R.K., Odum R.A., Herman T., Zippel K. Facility design and associated services for the study of amphibians. ILAR J. 2007;48:188–202. doi: 10.1093/ilar.48.3.188. [DOI] [PubMed] [Google Scholar]
- Burgdorf J., Panksepp J. Tickling induces reward in adolescent rats. Physiol. Behav. 2001;72:167–173. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- Burggren W.W., Warburton S. Amphibians as animal models for laboratory research in physiology. ILAR J. 2007;48:260–269. doi: 10.1093/ilar.48.3.260. [DOI] [PubMed] [Google Scholar]
- Burman O.H.P., Ilyat A., Jones G., Mendl M. Ultrasonic vocalizations as indicators of welfare for laboratory rats (Rattus norvegicus) Appl. Anim. Behav. Sci. 2007;104:116–129. [Google Scholar]
- Burt J. 2006. Rat Reaktion Books. (London) [Google Scholar]
- Butler M.P., Turner K.W., Zucker I. A melatonin-independent seasonal timer induces neuroendocrine refractoriness to short day lengths. J. Biol. Rhythms. 2008;23:242–251. doi: 10.1177/0748730408317135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S. The neurobiology of stereotypy II: the role of stress. In: Mason G., Rushen J., editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. second ed. CABI Publishing; Cambridge, MA: 2005. [Google Scholar]
- Callard M.D., Bursten S.N., Price E.O. Repetitive backflipping behaviour in captive roof rats (Rattus rattus) and the effects of cage enrichment. Anim. Welfare. 2000;9:139–152. [Google Scholar]
- Castaneda S., Calvo E., Largo R., Gonzalez-Gonzalez R., De La Piedra C., Diaz-Curiel M., Herrero-Beaumont G. Characterization of a new experimental model of osteoporosis in rabbits. J. Bone Miner. Metab. 2008;26:53–59. doi: 10.1007/s00774-007-0797-1. [DOI] [PubMed] [Google Scholar]
- Champneys F. The Septum atriorum of the frog and the rabbit. J. Anat. Physiol. 1874;8:340–352. [PMC free article] [PubMed] [Google Scholar]
- Chen G., Robert J. Antiviral immunity in amphibians. Viruses. 2011;3:2065–2086. doi: 10.3390/v3112065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L.-R., Garner J.P., Mench J.A. A behavioral comparison of New Zealand White rabbits (Oryctolagus cuniculus) housed individually or in pairs in conventional laboratory cages. Appl. Anim. Behav. Sci. 2004;85:121–139. [Google Scholar]
- Chum H., Felt S., Garner J., Green S. Biology, behavior, and environmental enrichment for the captive African clawed frog (Xenopus spp.) Appl. Anim. Behav. Sci. 2013;143:150–156. [Google Scholar]
- Cloutier S., Wahl K., Baker C., Newberry R.C. The social buffering effect of playful handling on responses to repeated intraperitoneal injections in laboratory rats. J. Am. Assoc. Lab. Anim. Sci. 2014;53:168–173. [PMC free article] [PubMed] [Google Scholar]
- Creaser C.W. The technic of handling the zebra fish (Brachydanio rerio) for the production of eggs which are favorable for embryological research and are available at any specified time throughout the year. Copeia. 1934;4:159–161. [Google Scholar]
- Dal Bosco A., Rebollar P.G., Boiti C., Zerani M., Castellini C. Ovulation induction in rabbit does: current knowledge and perspectives. Anim. Reprod. Sci. 2011;129:106–117. doi: 10.1016/j.anireprosci.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Darrow K.O., Harris W.A. Characterization and development of courtship in zebrafish, Danio rerio. Zebrafish. 2004;1:40–45. doi: 10.1089/154585404774101662. [DOI] [PubMed] [Google Scholar]
- Denardo D. Amphibians as laboratory animals. ILAR J. 1995;37:173–181. doi: 10.1093/ilar.37.4.173. [DOI] [PubMed] [Google Scholar]
- Dickenson V. 2013. Rabbit. Reaktion Books. (London) [Google Scholar]
- Diehl K.H., Hull R., Morton D., Pfister R., Rabemampianina Y., Smith D., Vidal J.M., Van De Vorstenbosch C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- Divers S.J., Mader D.R. second ed. Saunders Elsevier; St. Louis: 2006. Reptile Medicine and Surgery. [Google Scholar]
- Doneley B. Manson Publishing, Ltd.; London: 2010. Avian Medicine and Surgery in Practice. [Google Scholar]
- Einon D.F., Morgan M.J. A critical period for social isolation in the rat. Dev. Psychobiol. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Ema M., Naya M., Yoshida K., Nagaosa R. Reproductive and developmental toxicity of hydrofluorocarbons used as refrigerants. Reprod. Toxicol. 2010;29:125–131. doi: 10.1016/j.reprotox.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Engeszer R.E., Patterson L.B., Rao A.A., Parichy D.M. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 2007;4:21–38. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- European Commission . European Union; Brussels: 2013. Seventh Report on the Statistics on the Number of Animals Used for Experimental and Other Scientific Purposes in the Member States of the European Union. [Google Scholar]
- Fee M.S., Scharff C. The songbird as a model for the generation and learning of complex sequential behaviors. ILAR J. 2010;51:362–377. doi: 10.1093/ilar.51.4.362. [DOI] [PubMed] [Google Scholar]
- Firth C., Bhat M., Firth M.A., Williams S.H., Frye M.J., Simmonds P., Conte J.M., Ng J., Garcia J., Bhuva N.P., Lee B., Che X., Quan P.L., Lipkin W.I. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. MBio. 2014;5 doi: 10.1128/mBio.01933-14. e01933–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., Chavatte-Palmer P., Viebahn C., Naverrete Santos A., Duranthon V. Rabbit as a reproductive model for human health. Reproduction. 2012;144:1–10. doi: 10.1530/REP-12-0091. [DOI] [PubMed] [Google Scholar]
- Foote R.H., Carney E.W. The rabbit as a model for reproductive and developmental toxicity studies. Reprod. Toxicol. 2000;14:477–493. doi: 10.1016/s0890-6238(00)00101-5. [DOI] [PubMed] [Google Scholar]
- Frandson R.D., Wilke W.L., Fails A.D. Wiley-Blackwell; Ames: 2009. Anatomy and Physiology of Farm Animals. [Google Scholar]
- Fraser M.A., Girling S. Wiley-Blackwell; Ames: 2009. Rabbit Medicine and Surgery for Veterinary Nurses. [Google Scholar]
- Frye F.L. Krieger Publishing Co.; Malabar: 1991. Biomedical and Surgical Aspects of Captive Reptile Husbandry. [Google Scholar]
- Furukawa S., Kuroda Y., Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014;27:11–18. doi: 10.1293/tox.2013-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner J.P., Dufour B., Gregg L.E., Weisker S.M., Mench J.A. Social and husbandry factors affecting the prevalence and severity of barbering (’whisker trimming') by laboratory mice. Appl. Anim. Behav. Sci. 2004;89:263–282. [Google Scholar]
- Garner J.P., Mason G.J. Evidence for a relationship between cage stereotypies and behavioural disinhibition in laboratory rodents. Behav. Brain Res. 2002;136:83–92. doi: 10.1016/s0166-4328(02)00111-0. [DOI] [PubMed] [Google Scholar]
- Gaskill B.N. 2014. Personal Communication. [Google Scholar]
- Gaskill B.N., Gordon C.J., Pajor E.A., Lucas J.R., Davis J.K., Garner J.P. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One. 2012;7:e32799. doi: 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill B.N., Gordon C.J., Pajor E.A., Lucas J.R., Davis J.K., Garner J.P. Impact of nesting material on mouse body temperature and physiology. Physiol. Behav. 2013;110–111:87–95. doi: 10.1016/j.physbeh.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Gaskill B.N., Karas A.Z., Garner J.P., Pritchett-Corning K.R. Nest building as an indicator of health and welfare in laboratory mice. J. Vis. Exp. 2013:51012. doi: 10.3791/51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill B.N., Pritchett-Corning K.R., Gordon C.J., Pajor E.A., Lucas J.R., Davis J.K., Garner J.P. Energy reallocation to breeding performance through improved nest building in laboratory mice. PLoS One. 2013;8:e74153. doi: 10.1371/journal.pone.0074153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz E.J. Medicine and surgery of amphibians. ILAR J. 2007;48:255–259. doi: 10.1093/ilar.48.3.255. [DOI] [PubMed] [Google Scholar]
- Gerhard G.S., Kauffman E.J., Wang X., Stewart R., Moore J.L., Kasales C.J., Demidenko E., Cheng K.C. Life spans and senescent phenotypes in two strains of Zebrafish (Danio rerio) Exp. Gerontol. 2002;37:1055–1068. doi: 10.1016/s0531-5565(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Gerlach G. Pheromonal regulation of reproductive success in female zebrafish: female suppression and male enhancement. Anim. Behav. 2006;72:1119–1124. [Google Scholar]
- Gibbs K.M., Chittur S.V., Szaro B.G. Metamorphosis and the regenerative capacity of spinal cord axons in Xenopus laevis. Eur. J. Neurosci. 2011;33:9–25. doi: 10.1111/j.1460-9568.2010.07477.x. [DOI] [PubMed] [Google Scholar]
- Gibson J.P., Staples R.E., Newberne J.W. Use of the rabbit in teratogenicity studies. Toxicol. Appl. Pharmacol. 1966;9:398–407. [Google Scholar]
- Gomes-Silva A., Valverde J.G., Ribeiro-Romao R.P., Placido-Pereira R.M., Da-Cruz A.M. Golden hamster (Mesocricetus auratus) as an experimental model for Leishmania (Viannia) braziliensis infection. Parasitology. 2013;140:771–779. doi: 10.1017/S0031182012002156. [DOI] [PubMed] [Google Scholar]
- Gorn E.J., Goldstein W. University of Illinois Press; Urbana: 2004. A Brief History of American Sports. [Google Scholar]
- Gotfredsen K., Wennerberg A., Johansson C., Skovgaard L.T., Hjorting-Hansen E. Anchorage of TiO2-blasted, HA-coated, and machined implants: an experimental study with rabbits. J. Biomed. Mater. Res. 1995;29:1223–1231. doi: 10.1002/jbm.820291009. [DOI] [PubMed] [Google Scholar]
- Greene H.W. Nonavian reptiles as laboratory animals. ILAR J. 1995;37:182–186. doi: 10.1093/ilar.37.4.182. [DOI] [PubMed] [Google Scholar]
- Gresens J. An introduction to the Mexican axolotl (Ambystoma mexicanum) Lab. Anim. (NY) 2004;33:41–47. doi: 10.1038/laban1004-41. [DOI] [PubMed] [Google Scholar]
- Gresham V.C., Haines V.L. Guinea pigs: managment, husbandry and colony health. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press/Elsevier; London: 2012. [Google Scholar]
- Gunn D., Morton D.B. Inventory of the behaviour of New Zealand White rabbits in laboratory cages. Appl. Anim. Behav. Sci. 1995;45:277–292. [Google Scholar]
- Habjanec L., Halassy B., Vdovic V., Balija M.L., Tomasic J. Comparison of mouse and rabbit model for the assessment of strong PGM-containing oil-based adjuvants. Vet. Immunol. Immunopathol. 2008;121:232–240. doi: 10.1016/j.vetimm.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Hans J.S., Bird G.C., Li W., Jones J., Neugbauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J. Neurosci. Meth. 2005;141:261–269. doi: 10.1016/j.jneumeth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Hanly W.C., Artwohl J.E., Bennett B.T. Review of polyclonal antibody production procedures in mammals and poultry. ILAR J. 1995;37:93–118. doi: 10.1093/ilar.37.3.93. [DOI] [PubMed] [Google Scholar]
- Harding C.F. Learning from bird brains: how the study of songbird brains revolutionized neuroscience. Lab. Anim. (NY) 2004;33:28–33. doi: 10.1038/laban0504-28. [DOI] [PubMed] [Google Scholar]
- Hardy C., Callou C., Vigne J.D., Casane D., Dennebouy N., Mounolou J.C., Monnerot M. Rabbit mitochondrial DNA diversity from prehistoric to modern times. J. Mol. Evol. 1995;40:227–237. doi: 10.1007/BF00163228. [DOI] [PubMed] [Google Scholar]
- Harkness J.E., Turner P.V., VandeWoude S., Wheler C.L. fifth ed. Wiley; Ames: 2010. Harkness and Wagner's Biology and Medicine of Rabbits and Rodents. [Google Scholar]
- Harrington M. Speaking of psittacine research. Lab. Anim. (NY) 2014;43:343. doi: 10.1038/laban.630. [DOI] [PubMed] [Google Scholar]
- Harris B.M., Blatz P.J., Hinkle M.K., McCall S., Beckius M.L., Mende K., Robertson J.L., Griffith M.E., Murray C.K., Hospenthal D.R. In vitro and in vivo activity of first generation cephalosporins against Leptospira. Am. J. Trop. Med. Hyg. 2011;85:905–908. doi: 10.4269/ajtmh.2011.11-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkridge A.M. The chicken model of spontaneous ovarian cancer. Proteomics Clin. Appl. 2014;8:689–699. doi: 10.1002/prca.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich H.J. History, strains, and models. In: Krinke G., editor. The Laboratory Rat. Academic Press; San Diego: 2000. [Google Scholar]
- Hein G.J., Baker C., Hsieh J., Farr S., Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes. 2013;62:373–381. doi: 10.2337/db12-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse B.E., Potter B. A behavioral look at the training of Alex: a review of Pepperberg's the Alex studies: cognitive and communicative abilities of grey parrots. Anal. Verbal Behav. 2004;20:141–151. doi: 10.1007/BF03393001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman D.L., Swan M. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J. Am. Assoc. Lab. Anim. Sci. 2010;49:155–159. [PMC free article] [PubMed] [Google Scholar]
- Hirsjarvi P.A., Junnila M.A., Valiaho T.U. Gentled and non-handled rats in a stressful open-field situation; differences in performance. Scand. J. Psychol. 1990;31:259–265. doi: 10.1111/j.1467-9450.1990.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Hopkins J., Bourdain A., Freeman M. Tuttle Publishing; North Clarendon: 2004. Extreme Cuisine: The Weird & Wonderful Foods that People Eat. [Google Scholar]
- Hopkins W.A. Amphibians as models for studying environmental change. ILAR J. 2007;48:270–277. doi: 10.1093/ilar.48.3.270. [DOI] [PubMed] [Google Scholar]
- Hori M., Yamada K., Ohnishi J., Sakamoto S., Furuie H., Murakami K., Ichitani Y. Tickling during adolescence alters fear-related and cognitive behaviors in rats after prolonged isolation. Physiol. Behav. 2014;131:62–67. doi: 10.1016/j.physbeh.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Hörnicke H. Utilization of caecal digesta by caecotrophy (soft faeces ingestion) in the rabbit. Livestock Prod. Sci. 1981;8:361–366. [Google Scholar]
- Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M., Collins J.E., Humphray S., McLaren K., Matthews L., McLaren S., Sealy I., Caccamo M., Churcher C., Scott C., Barrett J.C., Koch R., Rauch G.J., White S., Chow W., Kilian B., Quintais L.T., Guerra-Assuncao J.A., Zhou Y., Gu Y., Yen J., Vogel J.H., Eyre T., Redmond S., Banerjee R., Chi J., Fu B., Langley E., Maguire S.F., Laird G.K., Lloyd D., Kenyon E., Donaldson S., Sehra H., Almeida-King J., Loveland J., Trevanion S., Jones M., Quail M., Willey D., Hunt A., Burton J., Sims S., McLay K., Plumb B., Davis J., Clee C., Oliver K., Clark R., Riddle C., Elliot D., Threadgold G., Harden G., Ware D., Begum S., Mortimore B., Kerry G., Heath P., Phillmore B., Tracey A., Corby N., Dunn M., Johnson C., Wood J., Clark S., Pelan S., Griffiths G., Smith M., Glithero R., Howden P., Barker N., Lloyd C., Stevens C., Harley J., Holt K., Panagiotidis G., Lovell J., Beasley H., Henderson C., Gordon D., Auger K., Wright D., Collins J., Raisen C., Dyer L., Leung K., Robertson L., Ambridge K., Leongamornlert D., McGuire S., Gilderthorp R., Griffiths C., Manthravadi D., Nichol S., Barker G. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurme K., Gonzalez K., Halvorsen M., Foster B., Moore D., Chepko-Sade B.D. Environmental enrichment for dendrobatid frogs. J. Appl. Anim. Welf. Sci. 2003;6:285–299. doi: 10.1207/s15327604jaws0604_3. [DOI] [PubMed] [Google Scholar]
- Huss D., Poynter G., Lansford R. Japanese quail (Coturnix japonica) as a laboratory animal model. Lab. Anim. (NY) 2008;37:513–519. doi: 10.1038/laban1108-513. [DOI] [PubMed] [Google Scholar]
- Ibi D., Takuma K., Koike H., Mizoguchi H., Tsuritani K., Kuwahara Y., Kamei H., Nagai T., Yoneda Y., Nabeshima T., Yamada K. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J. Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Ito T., Ando H., Handa H. Teratogenic effects of thalidomide: molecular mechanisms. Cell. Mol. Life Sci. 2011;68:1569–1579. doi: 10.1007/s00018-010-0619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E.R. CRC Press; Boca Raton: 2007. Infectious Diseases and Pathology of Reptiles: Color Atlas and Text. [Google Scholar]
- Jarvis E.D., Mirarab S., Aberer A.J., Li B., Houde P., Li C., Ho S.Y., Faircloth B.C., Nabholz B., Howard J.T., Suh A., Weber C.C., Da Fonseca R.R., Li J., Zhang F., Li H., Zhou L., Narula N., Liu L., Ganapathy G., Boussau B., Bayzid M.S., Zavodovych V., Subramanian S., Gabaldon T., Capelle-Gutierrez S., Huerta-Cepas J., Rekepalli B., Munch K., Schierup M., Lindow B., Warren W.C., Ray D., Green R.E., Bruford M.W., Zhan X., Dixon A., Li S., Li N., Huang Y., Derryberry E.P., Bertelsen M.F., Sheldon F.H., Brumfield R.T., Mello C.V., Lovell P.V., Wirthlin M., Schneider M.P., Prosdocimi F., Samaniego J.A., Velazquez A.M.V., Alfaro-Nunez A., Campos P.F., Petersen B., Sicheritz-Ponten T., Pas A., Bailey T., Scofield P., Bunch M., Lambert D.M., Zhou Q., Perelman P., Driskell A.C., Sharpiro B., Xiong Z., Zeng Y., Liu S., Li Z., Liu B., Wu K., Xiao J., Yinqi X., Zheng Q., Zhang Y., Yang H., Wang J., Smeds L., Rheindt F.E., Braun M., Fjeldsa J., Orlando L., Barker F.K., Jonsson K.A., Johnson W., Koepfli K.P., O'Brien S., Haussler D., Ryder O.A., Rahbek C., Willerslev E., Graves G.R., Glenn T.C., McCormack J., Burt D., Ellegren H., Alstrom P., Edwards S.V., Stamatakis A., Mindell D.P., Cracraft J. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science. 2014;346:1320–1331. doi: 10.1126/science.1253451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J.R. Rabbit and ferret liver and gastrointestinal testing. In: Fudge A.M., editor. Laboratory Medicine: Avian and Exotic Pets. Saunders; Philadelphia: 2000. [Google Scholar]
- Jiangbo Z., Xuying W., Yuping Z., Xili M., Yiwen Z., Tianbao Z. Effect of astragaloside IV on the embryo-fetal development of Sprague-Dawley rats and New Zealand White rabbits. J. Appl. Toxicol. 2009;29:381–385. doi: 10.1002/jat.1422. [DOI] [PubMed] [Google Scholar]
- Jimenez J.E. The extirpation and current status of wild chinchillas Chinchilla lanigera and C-brevicaudata. Biol. Conserv. 1996;77:1–6. [Google Scholar]
- Jourdan D., Ardid D., Chapuy E., Eschalier A., Le Bars D. Audible and ultrasonic vocalization elicited by single electrical nociceptive stimuli to the tail in the rat. Pain. 1995;63:237–249. doi: 10.1016/0304-3959(95)00049-X. [DOI] [PubMed] [Google Scholar]
- Kaiser P. The long view: a bright past, a brighter future? Forty years of chicken immunology pre- and post-genome. Avian Pathol. 2012;41:511–518. doi: 10.1080/03079457.2012.735359. [DOI] [PubMed] [Google Scholar]
- Kalmar I.D., Janssens G.P., Moons C.P. Guidelines and ethical considerations for housing and management of psittacine birds used in research. ILAR J. 2010;51:409–423. doi: 10.1093/ilar.51.4.409. [DOI] [PubMed] [Google Scholar]
- Kent M.L., Harper C., Wolf J.C. Documented and potential research impacts of subclinical diseases in zebrafish. ILAR J. 2012;53:126–134. doi: 10.1093/ilar.53.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent M.L., Spitsbergen J.M., Matthews J.M., Fournie J.W., Westerfield M. 2012. Diseases of Zebrafish in Research Facilities.https://zebrafish.org/wiki/health/disease_manual/start [Google Scholar]
- Kerby J.L., Hart A.J., Storfer A. Combined effects of virus, pesticide, and predator cue on the larval tiger salamander (Ambystoma tigrinum) Ecohealth. 2011;8:46–54. doi: 10.1007/s10393-011-0682-1. [DOI] [PubMed] [Google Scholar]
- Kerby J.L., Storfer A. Combined effects of atrazine and chlorpyrifos on susceptibility of the tiger salamander to Ambystoma tigrinum virus. Ecohealth. 2009;6:91–98. doi: 10.1007/s10393-009-0234-0. [DOI] [PubMed] [Google Scholar]
- Kimmel C.B., Ballard W.W., Kimel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Koustubhan P., Sorocco D., Levin M.S., Conn P.M. Establishing and maintaining a Xenopus laevis colony for research laboratories. In: Conn P.M., editor. Sourcebook of Models for Biomedical Research. Humana Press; Totowa: 2008. [Google Scholar]
- Kumar S., Singh R., Vasudeva N., Sharma S. Acute and chronic animal models for the evaluation of anti-diabetic agents. Cardiovasc. Diabetol. 2012;11:9. doi: 10.1186/1475-2840-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laale H.W. The biology and use of zebrafish, Brachydanio rerio in fisheries research. A literature review. J. Fish Biol. 1977;10:121–176. [Google Scholar]
- Lanning D., Zhu X., Zhai S.K., Knight K.L. Development of the antibody repertoire in rabbit: gut-associated lymphoid tissue, microbes, and selection. Immunol. Rev. 2000;175:214–228. [PubMed] [Google Scholar]
- Laulederkind S.J., Hayman G.T., Wang S.J., Smith J.R., Lowry T.F., Nigam R., Petri V., De Pons J., Dwinell M.R., Shimoyama M., Munzenmaier D.H., Worthey E.A., Jacob H.J. The rat genome database 2013–data, tools and users. Brief Bioinform. 2013;14:520–526. doi: 10.1093/bib/bbt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture. 2007;269:1–20. [Google Scholar]
- Lawrence C., Adatto I., Best J., James A., Maloney K. Generation time of zebrafish (Danio rerio) and medakas (Oryzias latipes) housed in the same aquaculture facility. Lab. Anim. (NY) 2012;41:158–165. doi: 10.1038/laban0612-158. [DOI] [PubMed] [Google Scholar]
- Lawrence C., Mason T. Zebrafish housing systems: a review of basic operating principles and considerations for design and functionality. ILAR J. 2012;53:179–191. doi: 10.1093/ilar.53.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G., Lutz I., Kruger A., Kloas W. Bisphenol A induces feminization in Xenopus laevis tadpoles. Environ. Res. 2004;94:102–111. doi: 10.1016/s0013-9351(03)00086-0. [DOI] [PubMed] [Google Scholar]
- Lindsey J.R., Baker H.J. Historical perspectives. In: Suckow M.A., Weisbroth S.H., Franklin C.L., editors. The Laboratory Rat. second ed. Elsevier; Boston: 2006. [Google Scholar]
- Lloyd M.E., Carr M., McElhatton P., Hall G.M., Hughes R.A. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551–563. doi: 10.1093/qjmed/92.10.551. [DOI] [PubMed] [Google Scholar]
- Lovern M.B., Holmes M.M., Wade J. The green anole (Anolis carolinensis): a reptilian model for laboratory studies of reproductive morphology and behavior. ILAR J. 2004;45:54–64. doi: 10.1093/ilar.45.1.54. [DOI] [PubMed] [Google Scholar]
- Maekawa F., Tsukahara S., Kawashima T., Nohara K., Ohki-Hamazaki H. The mechanisms underlying sexual differentiation of behavior and physiology in mammals and birds: relative contributions of sex steroids and sex chromosomes. Front. Neurosci. 2014;8:242. doi: 10.3389/fnins.2014.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina J.N. Development, structure, and function of a novel respiratory organ, the lung-air sac system of birds: to go where no other vertebrate has gone. Biol. Rev. Camb. Philos. Soc. 2006;81:545–579. doi: 10.1017/S1464793106007111. [DOI] [PubMed] [Google Scholar]
- Manabe Y.C., Kesavan A.K., Lopez-Molina J., Hatem C.L., Brooks M., Fujiwara R., Hochstein K., Pitt M.L., Tufariello J., Chan J., McMurray D.N., Bishai W.R., Dannenberg A.M., Jr., Mendez S. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis (Edinb) 2008;88:187–196. doi: 10.1016/j.tube.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B.J. Chinchillas: taxonomy and history. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press; London: 2012. [Google Scholar]
- McClure M.M., McIntyre P.B., McCune A.R. Notes on the natural diet and habitat of eight danionin fishes, including the zebrafish Danio rerio. J. Fish Biol. 2006;69:553–570. [Google Scholar]
- McMahon A.C., Kritharides L., Lowe H.C. Animal models of atherosclerosis progression: current concepts. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005;5:433–440. doi: 10.2174/156800605774962031. [DOI] [PubMed] [Google Scholar]
- Mello C.V. The zebra finch, Taeniopygia guttata: an avian model for investigating the neurobiological basis of vocal learning. Cold Spring Harb. Protoc. 2014;2014:1237–1242. doi: 10.1101/pdb.emo084574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith A.L., Richardson J. Neurological diseases of rabbits and rodents. J. Exot. Pet Med. 2015;24:21–33. [Google Scholar]
- Morton D.J., Hempel R.J., Seale T.W., Whitby P.W., Stull T.L. A functional tonB gene is required for both virulence and competitive fitness in a chinchilla model of Haemophilus influenzae otitis media. BMC Res. Notes. 2012;5:327. doi: 10.1186/1756-0500-5-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiadka A., Clark M.D. Zebrafish breeding in the laboratory environment. ILAR J. 2012;53:161–168. doi: 10.1093/ilar.53.2.161. [DOI] [PubMed] [Google Scholar]
- National Research Council . eighth ed. National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- National Research Council (U.S.) National Academies Press; Washington, DC: 1974. Amphibians: Guidelines for the Breeding, Care, and Management of Laboratory Animals; a Report. [PubMed] [Google Scholar]
- O'Rourke D.P. Amphibians used in research and teaching. ILAR J. 2007;48:183–187. doi: 10.1093/ilar.48.3.183. [DOI] [PubMed] [Google Scholar]
- O'Rourke D.P., Schultz T.W. Biology and diseases of amphibians. In: Fox J.G., Anderson L.C., Loew F.M., Quimby F.W., editors. Laboratory Animal Medicine. second ed. Academic Press; Amsterdam: 2002. [Google Scholar]
- O'Rourke D.P., Schumacher J. Biology and diseases of reptiles. In: Fox J.G., Anderson L.C., Loew F.M., Quimby F.W., editors. Laboratory Animal Medicine. second ed. Academic Press; Amsterdam: 2002. [Google Scholar]
- Ogushi Y., Tsuzuki A., Sato M., Mochida H., Okada R., Suzuki M., Hillyard S.D., Tanaka S. The water-absorption region of ventral skin of several semiterrestrial and aquatic anuran amphibians identified by aquaporins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R1150–R1162. doi: 10.1152/ajpregu.00320.2010. [DOI] [PubMed] [Google Scholar]
- Oi A., Morishita K., Awogi T., Ozaki A., Umezato M., Fujita S., Hosoki E., Morimoto H., Ishiharada N., Ishiyama H., Uesugi T., Miyatake M., Senba T., Shiragiku T., Nakagiri N., Ito N. Nonclinical safety profile of tolvaptan. Cardiovasc. Drugs Ther. 2011;25(Suppl. 1):S91–S99. doi: 10.1007/s10557-011-6356-y. [DOI] [PubMed] [Google Scholar]
- Padilla-Carlin D.J., McMurray D.N., Hickey A.J. The Guinea pig as a model of infectious diseases. Comp. Med. 2008;58:324–340. [PMC free article] [PubMed] [Google Scholar]
- Panda A., Tatarov I., Masek B.J., Hardick J., Crusan A., Wakefield T., Carroll K., Yang S., Hsieh Y.H., Lipsky M.M., McLeod C.G., Levine M.M., Rothman R.E., Gaydos C.A., Detolla L.J. A rabbit model of non-typhoidal Salmonella bacteremia. Comp. Immunol. Microbiol. Infect. Dis. 2014;37:211–220. doi: 10.1016/j.cimid.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Neuroevolutionary sources of laughter and social joy: modeling primal human laughter in laboratory rats. Behav. Brain Res. 2007;182:231–244. doi: 10.1016/j.bbr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Parasuraman S., Raveendran R., Kesavan R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010;1:87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasana A.J., Li N., Brown T.H. Positive and negative ultrasonic social signals elicit opposing firing patterns in rat amygdala. Behav. Brain Res. 2012;226:77–86. doi: 10.1016/j.bbr.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos L.F., Mello H.E., Young R.J. Enriching tortoises: assessing color preference. J. Appl. Anim. Welf. Sci. 2014;17:274–281. doi: 10.1080/10888705.2014.917556. [DOI] [PubMed] [Google Scholar]
- Patterson-Kane E.G. Shelter enrichment for rats. Contemp. Top. Lab. Anim. Sci. 2003;42:46–48. [PubMed] [Google Scholar]
- Pena J.L., Debello W.M. Auditory processing, plasticity, and learning in the barn owl. ILAR J. 2010;51:338–352. doi: 10.1093/ilar.51.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggiagliolmi S., Crowell-Davis S.L., Alworth L.C., Harvey S.B. Environmental enrichment of New Zealand White rabbits living in laboratory cages. J. Vet. Behav. Clin. Appl. Res. 2011;6:343–350. [Google Scholar]
- Popesko P., Rajtová V., Horák J.R. Wolfe Publishing; London: 1992. A Colour Atlas of the Anatomy of Small Laboratory Animals. [Google Scholar]
- Pough F.H. Recommendations for the care of amphibians and reptiles in Academic Institutions. ILAR J. 1991;33:S1–S21. [Google Scholar]
- Pough F.H. Amphibian biology and husbandry. ILAR J. 2007;48:203–213. doi: 10.1093/ilar.48.3.203. [DOI] [PubMed] [Google Scholar]
- Pritt S. Guinea pigs: taxonomy and history. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press; London: 2012. [Google Scholar]
- Proctor N.S., Lynch P.J. Yale University Press; New Haven: 1993. Manual of Ornithology: Avian Structure & Function. [Google Scholar]
- Quesenberry K.E., Carpenter J.W. Elsevier/Saunders; St. Louis: 2012. Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery. [Google Scholar]
- Rao N., Song F., Jhamb D., Wang M., Milner D.J., Price N.M., Belecky-Adams T.L., Palakal M.J., Cameron J.A., Li B., Chen X., Stocum D.L. Proteomic analysis of fibroblastema formation in regenerating hind limbs of Xenopus laevis froglets and comparison to axolotl. BMC Dev. Biol. 2014;14:1–27. doi: 10.1186/1471-213X-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R. Inner workings: 1885, the first rabies vaccination in humans. Proc. Natl. Acad. Sci. U.S.A. 2014;111:12273. doi: 10.1073/pnas.1414226111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie B.W., Harrison G.J., Harrison L.R. Wingers Publishing; Lake Worth: 1994. Avian Medicine: Principles and Application. [Google Scholar]
- Robbins T.W., Jones G.H., Wilkinson L.S. Behavioural and neurochemical effects of early social deprivation in the rat. J. Psychopharmacol. 1996;10:39–47. doi: 10.1177/026988119601000107. [DOI] [PubMed] [Google Scholar]
- Roberts A., Lamirande E.W., Vogel L., Baras B., Goossens G., Knott I., Chen J., Ward J.M., Vassilev V., Subbarao K. Immunogenicity and protective efficacy in mice and hamsters of a beta-propiolactone inactivated whole virus SARS-CoV vaccine. Viral Immunol. 2010;23:509–519. doi: 10.1089/vim.2010.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronisz A., Delcroix M., Quarck R. Measurement of right ventricular pressure by telemetry in conscious moving rabbits. Lab. Anim. 2013;47:175–183. doi: 10.1177/0023677213483725. [DOI] [PubMed] [Google Scholar]
- Rosania K. Barn owls: why give a hoot? Lab. Anim. (NY) 2014;43:157. doi: 10.1038/laban.538. [DOI] [PubMed] [Google Scholar]
- Roy C.S. The form of the pulse-wave: as studied in the carotid of the rabbit. J. Physiol. 1879;2:66–90. doi: 10.1113/jphysiol.1879.sp000046. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.C., Proctor S.D. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc. Pathol. 2006;15:318–330. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Russell L.D. Normal development of the testes. In: Mohr U., Dungworth D.L., Capen C.C., editors. Pathobiology of the Aging Rat. ILSI Press; Washington, DC: 1992. [Google Scholar]
- Sandmeier P., Coutteel P. Management of canaries, finches, and mynahs. In: Harrison G.J., Lightfoot T.L., editors. vol. 2. Spix Publishing; Palm Beach: 2006. (Clinical Avian Medicine). [Google Scholar]
- Santerre R.F., Wight T.N., Smith S.C., Brannigan D. Spontaneous atherosclerosis in pigeons. A model system for studying metabolic parameters associated with atherogenesis. Am. J. Pathol. 1972;67:1–22. [PMC free article] [PubMed] [Google Scholar]
- Scanes C.G. Elsevier; London: 2015. Sturke's Avian Physiology. [Google Scholar]
- Scanes C.G., McNabb F.M.A. Avian models for research in toxicology and endocrine disruption. Avian Poult. Biol. Rev. 2003;14:21–52. [Google Scholar]
- Schmidt M.F. An IACUC perspective on songbirds and their use in neurobiological research. ILAR J. 2010;51:424–430. doi: 10.1093/ilar.51.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz T.W., Dawson D.A. Housing and husbandry of Xenopus for oocyte production. Lab. Anim. (NY) 2003;32:34–39. doi: 10.1038/laban0203-34. [DOI] [PubMed] [Google Scholar]
- Scott B.B., Velho T.A., SIM S., LOIS C. Applications of avian transgenesis. ILAR J. 2010;51:353–361. doi: 10.1093/ilar.51.4.353. [DOI] [PubMed] [Google Scholar]
- Seebacher F., Franklin C.E. Physiological mechanisms of thermoregulation in reptiles: a review. J. Comp. Physiol. B. 2005;175:533–541. doi: 10.1007/s00360-005-0007-1. [DOI] [PubMed] [Google Scholar]
- Seidl A.H., Sanchez J.T., Schecterson L., Tabor K.M., Wang Y., Kashima D.T., Poynter G., Huss D., Fraser S.E., Lansford R., Rubel E.W. Transgenic quail as a model for research in the avian nervous system: a comparative study of the auditory brainstem. J. Comp. Neurol. 2013;521:5–23. doi: 10.1002/cne.23187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M., Bingman V.P., Shimizu T., Wild M., Gunturkun O. Large-scale network organization in the avian forebrain: a connectivity matrix and theoretical analysis. Front. Comput. Neurosci. 2013;7:1–17. doi: 10.3389/fncom.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheafor B., Davidson E.W., Parr L., Rollins-Smith H.,L. Antimicrobial peptide defenses in the salamander, Ambystoma tigrinum, against emerging amphibian pathogens. J. Wildl. Dis. 2008;44:226–236. doi: 10.7589/0090-3558-44.2.226. [DOI] [PubMed] [Google Scholar]
- Shiomi M., Ito T. The Watanabe heritable hyperlipidemic (WHHL) rabbit, its characteristics and history of development: a tribute to the late Dr. Yoshio Watanabe. Atherosclerosis. 2009;207:1–7. doi: 10.1016/j.atherosclerosis.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Shiomi M., Ito T., Yamada S., Kawashima S., Fan J. Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit) Arterioscler Thromb. Vasc. Biol. 2003;23:1239–1244. doi: 10.1161/01.ATV.0000075947.28567.50. [DOI] [PubMed] [Google Scholar]
- Shofner W.P., Chaney M. Processing pitch in a nonhuman mammal (Chinchilla laniger) J. Comp. Psychol. 2013;127:142–153. doi: 10.1037/a0029734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D.L., Buchman A.M. Animal models. Br. Med. Bull. 2000;56:307–317. doi: 10.1258/0007142001903238. [DOI] [PubMed] [Google Scholar]
- Smith G.D. Hamsters: taxonomy and history. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press; London: 2012. [Google Scholar]
- Smith W.R. Abnormal arrangement of the right subclavian artery in a rabbit. J. Anat. Physiol. 1891;25:325–326. [PMC free article] [PubMed] [Google Scholar]
- Sohn J., Couto M.A. Anatomy, physiology, and behavior. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press; Boston: 2012. [Google Scholar]
- Song Y., Lan Z., Kohn M.H. Mitochondrial DNA phylogeography of the Norway rat. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southard T.E., Southard K.A., Krizan K.E., Hillis S.L., Haller J.W., Keller J., Vannier M.W. Mandibular bone density and fractal dimension in rabbits with induced osteoporosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000;89:244–249. doi: 10.1067/moe.2000.102223. [DOI] [PubMed] [Google Scholar]
- Spence R., Gerlach G., Lawrence C., Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Sriram S., Gibson D.J., Robinson P., Pi L., TuliI S., Lewin A.S., Schultz G. Assessment of anti-scarring therapies in ex vivo organ cultured rabbit corneas. Exp. Eye Res. 2014;125:173–182. doi: 10.1016/j.exer.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckow M.A., Brammer D.W., Rush H.G., Chrisp C.E. Biology and diseases of rabbits. In: Fox J.G., Anderson L.C., Loew F.M., Quimby F.W., editors. Laboratory Animal Medicine. second ed. Academic Press; Amsterdam: 2002. [Google Scholar]
- Swindle M.M., Nolan T., Jacobson A., Wolf P., Dalton M.J., Smith A.C. Vascular access port (VAP) usage in large animal species. Contemp. Top. Lab. Anim. Sci. 2005;44:7–17. [PubMed] [Google Scholar]
- Thomas D., Mayhew H. New York University Press; New York: 1998. The Victorian Underworld. [Google Scholar]
- Tinsley H.R. Amphibians, with special reference to Xenopus. In: Hubrecht R., Kirkwood J., editors. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. eighth ed. Wiley-Blackwell; Ames: 2010. [Google Scholar]
- Tully T.N., Dorrestein G.M., Jones A.K. second ed. Elsevier; Oxford: 2009. Handbook of Avian Medicine. [Google Scholar]
- Tysome J.R., Li X., Wang S., Wang P., Gao D., Du P., Chen D., Gangeswaran R., Chard L.S., Yuan M., Alusi G., Lemoine N.R., Wang Y. A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin. Cancer Res. 2012;18:6679–6689. doi: 10.1158/1078-0432.CCR-12-0979. [DOI] [PubMed] [Google Scholar]
- Ullman-Cullere M.H., Foltz C.J. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab. Anim. Sci. 1999;49:319–323. [PubMed] [Google Scholar]
- Van Bergeijk J.P., Van Herck H., De Boer S.F., Meijer G.W., Hesp A.P., Van Der Gugten J., Beynen A.C. Effects of group size and gentling on behaviour, selected organ masses and blood constituents in female Rivm: TOX rats. Z. Versuchstierkd. 1990;33:85–90. [PubMed] [Google Scholar]
- Varga M. Elsevier Butterworth Heinemann; Edinburgh: 2014. Textbook of Rabbit Medicine. [Google Scholar]
- Verga M., Luzi F., Carenzi C. Effects of husbandry and management systems on physiology and behaviour of farmed and laboratory rabbits. Horm. Behav. 2007;52:122–129. doi: 10.1016/j.yhbeh.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Vitt L.J., Caldwell J.P. Academic Press; Amsterdam: 2014. Herpetology: An Introductory Biology of Amphibians and Reptiles. [Google Scholar]
- Wahl-Jensen V., Bollinger L., Safronetz D., De Kok-Mercado F., Scott D.P., Ebihara H. Use of the Syrian hamster as a new model of ebola virus disease and other viral hemorrhagic fevers. Viruses. 2012;4:3754–3784. doi: 10.3390/v4123754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Zhu M., Petroll W.M., Robertson D.M. Pseudomonas aeruginosa infectious keratitis in a high oxygen transmissible rigid contact lens rabbit model. Invest. Ophthalmol. Vis. Sci. 2014;55:5890–5899. doi: 10.1167/iovs.14-14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw I.Q., Kolb B. Oxford University Press; Oxford: 2005. The Behavior of the Laboratory Rat: A Handbook With Tests. [Google Scholar]
- Wilson C. Aspects of larval rearing. ILAR J. 2012;53:169–178. doi: 10.1093/ilar.53.2.169. [DOI] [PubMed] [Google Scholar]
- Wooding F.B.P., Burton G. Springer; Berlin: 2008. Comparative Placentation: Structures, Functions and Evolution. [Google Scholar]
- Wright K.M., Whitaker B.R. Krieger Publishing; Malabar: 2001. Amphibian Medicine and Captive Husbandry. [Google Scholar]
- Xiangdong L., Yuanwu L., Hua Z., Liming R., Qiuyan L., Ning L. Animal models for the atherosclerosis research: a review. Protein Cell. 2011;2:189–201. doi: 10.1007/s13238-011-1016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Liu X.Y., Ruan Y.X., Wang L., Jiang M.M., Wu J., Chen J. Construction of corneal epithelium with human amniotic epithelial cells and repair of limbal deficiency in rabbit models. Hum. Cell. 2014;28:22–36. doi: 10.1007/s13577-014-0099-6. [DOI] [PubMed] [Google Scholar]
- Zinsser H. George Rutledge & Sons; London: 1935. Rats, Lice, and History. [Google Scholar]


