Viral infections pose a potential threat to the health of laboratory and zoological colonies of nonhuman primates as well as the personnel involved in their care. This chapter discusses those viral diseases of importance to nonhuman primates and their caregivers by taxonomic family to which the causative agent is classified. A doctrine of comparative virology is that infection of the immunocompetent, appropriate host often is associated with minimal disease, whereas infection of the inadvertent susceptible host can have devastating consequences. The likelihood of such transmission is increased when changes in the environment place different species in close proximity. Similarly, transmission of viral agents from nonprimate host to primate host may result in severe disease. Perhaps of equal importance is the realization that experimental manipulations may inadvertently expose animals to unrecognized pathogens with lethal consequences. For instance, a number of minimally pathogenic viruses may cause severe disease in animals immunosuppressed from pharmacologic manipulation or immunodeficient from concurrent infection with viruses that target the immune system. Numerous examples of each of these scenarios are documented within this chapter.
Introduction
Viral infections pose a potential threat to the health of (1) laboratory and zoological colonies of nonhuman primates and (2) the personnel involved in their care. This is particularly true at facilities where there is frequent turnover or movement of animals or where animals recently imported from natural habitats are introduced into colonies of highly susceptible colony-born animals.
This chapter discusses those viral diseases of importance to captive primates or to the health of personnel involved in their care by taxonomic family to which the causative agent is classified. The rationale for this is that there is considerable overlap in the clinical and pathologic expression of diseases caused by viruses in the same taxonomic family regardless of the species affected. Obvious exceptions exist, but these will be noted.
A doctrine of comparative virology is that infection of the immunocompetent appropriate host often is associated with minimal disease, whereas infection of the inadvertent susceptible host can have devastating consequences. The likelihood of such transmission is increased when changes in the environment, either natural or imposed by humans, place different species in close proximity. An early example of this phenomenon comes from the family Herpesviridae in which infection of the natural reservoir host usually results in minimal clinical disease, whereas infection of other closely related species may result in an acutely lethal cytolytic or neoplastic process.
Because interspecies transmission of viruses may have such devastating consequences, direct contact between different nonhuman primate species should be prevented. Although isolation of primate species is the norm in well-managed modern facilities, such may not be the case with recently imported animals. Substandard separation of species, coupled with the stress of capture and movement, puts these animals at increased risk. Similarly, transmission of viral agents from nonprimate host to primate host may result in severe disease. Examples include transmission of lymphocytic choriomeningitis virus from rodents to callitrichids and the transmission of various orthopoxviruses such as monkeypox or cowpox from rodent vectors to nonhuman primates. Increasingly zoological collections have adapted multiple species exhibits and more naturalist settings. While such exhibits have many benefits, it should be remembered that they may promote inadvertent cross-species transmission of infectious agents.
Perhaps of equal importance is the realization that experimental manipulations may inadvertently expose animals to unrecognized pathogens with lethal consequences. Tissue homogenates and cell culture derivatives have transmitted simian immunodeficiency virus (SIV) and simian virus 40 in this fashion (Gormus et al., 1989, Mansfield et al., 1995). Moreover, the use of xenografts in both experimental and clinical settings is a potential route by which existing or novel pathogens may be introduced to a new population (Smith, 1993).
While infection of the natural host is often associated with minimal disease due to extensive host pathogen co-evolution, such may not be the case if the host is immunocompromised (Wachtman and Mansfield, 2008). A number of minimally pathogenic viruses may cause severe disease in animals immunosuppressed from pharmacologic manipulation or immunodeficient from concurrent infection with viruses that target the immune system. Examples of opportunistic infections that may cause disease in these circumstances include simian virus 40, cytomegaloviruses, and lymphocryptoviruses.
Finally, it should be recognized that outbred populations of nonhuman primates differ substantially from inbred rodent lines. Nonhuman primate populations have evolved with a spectrum of infectious agents for millions of years, and host adaptations have likely occurred to minimize the effects of these agents. Removal of ubiquitous, largely nonpathogenic infections from these populations may have unintended consequences.
Enveloped DNA Viruses
Poxviridae
Poxviruses are large (220–450 × 140–260 nm), brick- to ovoid-shaped enveloped viruses that replicate exclusively in the cytoplasm of infected cells. Their envelope is composed of lipid and tubular or globular protein structures that surround one or two lateral bodies and a dumbbell-shaped core containing a single molecule of double-stranded DNA. Virions have large genomes encoding approximately 200 polypeptides, at least one of which has homology with epidermal growth factor. This latter peptide acts to stimulate mitosis in neighboring cells and likely accounts for the proliferative epidermal and/or dermal lesions that characterize most poxvirus infections. The ability to induce cellular actin polymerization enhances cell-to-cell spread of virus. Poxviruses demonstrate independence from host cell transcriptional machinery due to the presence of virally encoded enzymes that are involved in transcription and modification of nucleic acids and proteins.
Five members of the poxvirus family belonging to Orthopoxvirus, Yatapoxvirus, and Molluscipoxvirus genera have been associated with naturally occurring epizootics in nonhuman primates (Table 1.1 ). Variola and vaccina infections of nonhuman primates represent significant experimental models.
TABLE 1.1.
Poxviridae
| Genus | Virus |
|---|---|
| Avipoxvirus | |
| Capripoxvirus | |
| Cervidpoxvirus | |
| Leporipoxvirus | |
| Molluscipoxvirus | Molluscum contagiosum |
| Orthopoxvirus | Monkeypox |
| Variola (smallpox) | |
| Vaccinia | |
| Cowpox | |
| Parapoxvirus | |
| Suipoxvirus | |
| Yatapoxvirus | Yaba monkey tumor virus |
| Yaba-like disease virus |
Monkeypox
Introduction
The first reported outbreaks of monkeypox in nonhuman primates occurred in June 1958 at the Statens Seruminstitut in Copenhagen, Denmark, and shortly thereafter at the Biological Development and Control Laboratories of Merck Sharp and Dohme in West Point, Pennsylvania (von Magnus et al., 1959, Prier et al., 1960, Sauer et al., 1960). At that time, both institutes were importing large numbers of macaque monkeys for use in polio vaccine production. In both outbreaks, cynomolgus monkeys (Macaca fascicularis) were primarily affected, but at Merck a small number of rhesus monkeys (Macaca mulatta) also exhibited signs of the disease. Since then, there have been several additional outbreaks of monkeypox infection involving both New and Old World monkeys (Arita and Henderson, 1968, Arita and Henderson, 1976).
Etiology
Monkeypox virus is a member of the family Poxviridae, subfamily Chordopoxvirinae, and genus Orthopoxvirus. This genus includes variola (smallpox virus), vaccinia (smallpox vaccine virus), cowpox virus, and several other mammalian poxviruses. Immunologically, there is a close antigenic relationship among monkeypox virus, variola, and vaccinia. Monkeypox isolates are classified in two clades with distinct differences in virulence: the West African clade and the Congo Basin clade originating in Central Africa (Esposito and Knight, 1985, Likos et al., 2005).
Epizootiology
Unlike many other poxviruses, monkeypox virus has a wide range of permissive host species. This virus exists naturally in the tropical rain forests of western and central Africa (Brennan et al., 1980, Mutombo et al., 1983), where it causes subclinical endemic infections in several nonhuman primate species and a serious, sometimes fatal, smallpox-like disease in young people in these regions.
Ironically, despite the fact that the original outbreaks of monkeypox were in macaques imported from Malaysia, a subsequent serologic survey of 481 Malaysian monkeys failed to reveal a single animal seropositive to monkeypox virus (Arita et al., 1972). While rodents, particularly African squirrels, are thought to serve as the major viral reservoir, African green monkeys (Chlorocebus aethiops) have a high prevalence of antibodies to monkeypox virus with no evidence of clinical disease (Gispen et al., 1976, Khodakevich and Szczeniowski, 1987). It is likely that the macaques involved in the original outbreaks may have been incidentally exposed to infected African green monkeys during shipment from Asia. Monkeypox virus has a relatively broad natural host range that includes humans, anthropoid apes, and Old and New World monkeys. Primates in which monkeypox infections have been shown to occur include cotton-top tamarins (Saguinus oedipus), squirrel monkeys (Saimiri sciureus), African green monkeys, owl-faced monkeys (Cercopithecus hamlyni), rhesus monkeys, cynomolgus monkeys, hanuman langurs (Semnopithecus entellus), white-handed gibbons (Hylobates lar), orangutans (Pongo pygmaeus), gorillas (Gorilla gorilla), chimpanzees (Pan troglodytes), and human beings (Homo sapiens) (von Magnus et al., 1959, Peters, 1966, McConnell et al., 1968, Marennikova et al., 1976). In addition, a variety of rodent species, anteaters, pangolins, and birds from endemic areas of Africa have been shown to have antibodies to the virus.
Pathogenesis and Pathology
In the reported epizootics of monkeypox, transmission was thought to have occurred via aerosols, although the disease may more commonly be spread by direct contact and by biting insects (Mutombo et al., 1983). Viremia develops 3–4 days following experimental infection, at which time the virus disseminates to multiple sites, including skin, lung, mucous membranes, spleen, and gastrointestinal tract (Zaucha et al., 2001). Lesions within the skin initially appear as papules consisting of proliferative acanthocytes and progress to vesicles. Intracytoplasmic eosinophilic inclusions are apparent within acanthocytes. Vesiculation is followed by umbilication to form the classic pock lesion. Progressive dermal changes take place over a 4- to 14-day period. Lesions within the lung are similar, consisting of irregular foci of hemorrhagic necrosis. These may be responsible for more serious clinical sequelae and transmission of the virus by the aerosolized route. Paralleling epidemiologic observations in humans, experimental infections in cynomolgus macaques have demonstrated that isolates from the Congo Basin clade are associated with increased lesion severity, elevated levels of virus, and greater mortality relative to isolates from the West African clade (Chen et al., 2005, Saijo et al., 2009).
Clinical Findings
Clinical manifestations of monkeypox vary with the species affected. In general, a vesicular exanthema appears 6–7 days following experimental inoculation. Lesions are more often seen on the hands, feet, and face. The skin rash may be accompanied by constitutional signs and, in severe cases with respiratory tract involvement, the disease may be fatal.
Treatment
There is no specific treatment for monkeypox, but supportive therapy may prevent the death of animals with severe systemic disease. Most cases recover spontaneously after several weeks and animals are immune to subsequent infection by the same or related viruses.
Prevention
Vaccination against smallpox confers immunity to monkeypox infection in most cases (McConnell et al., 1968).
Zoonotic Potential
Human beings are susceptible to infection by monkeypox (Arita et al., 1985). The disease is seen sporadically in Africa and is usually not associated with direct nonhuman primate contact (Hutin et al., 2001, Meyer et al., 2002). The well-publicized 2003 outbreak in the Midwestern United States was the first documentation of human monkeypox in the western hemisphere (CDC, 2003, Reed et al., 2004b). Viral transmission in these cases was from contact with ill prairie dogs comingled with rodents imported from West Africa for the pet trade. In general, a febrile prodrome precedes development of lympadenopathy and characteristic pock lesions that disseminate over the entire body in a centrifugal pattern. Human-to-human transmission is inefficient with reintroduction of virus from animal reservoirs required to sustain infection (Fine et al., 1988, Hutin et al., 2001). Fatalities have been reported, and are more common in children.
Animal Models
Monkeypox has been used as a model organism in cynomolgus and rhesus macaques to support development of therapeutics and vaccines targeting variola (Hooper et al., 2004, Edghill-Smith et al., 2005b, Stittelaar et al., 2005, Stittelaar et al., 2006, Earl et al., 2008, Marriott et al., 2008). Studies have included the use of SIV-inoculated macaques as a model of response to orthopoxvirus vaccination in the face of immunosuppression (Edghill-Smith et al., 2003, Edghill-Smith et al., 2005a).
Variola
Variola is the prototypic Orthopoxvirus. Intensive vaccination programs resulted in eradication of the virus in the late 1970s although potential for use as a biological weapon has prompted continued research and classification of this virus, along with monkeypox, as a Department of Health and Human Services (HHS) select agent. To satisfy requirements for drug and vaccine licensure, nonhuman primate models of variola infection have been described. In a study by Jahrling et al., intravenous administration of high-dose virus to cynomolgus macaques resulted in an acutely lethal infection resembling hemorrhagic smallpox in man (Jahrling et al., 2004). Symptoms included fever, anorexia, cough, and viral exanthema followed by development of a severe hemorrhagic diathesis, multisystem organ failure, and death within 6 days of inoculation. Clinicopathologic findings included leukocytosis, thrombocytopenia, increased D-dimers, and elevated serum creatinine. Dermal lesions were characterized by erythema and hemorrhage with progression to typical vesiculopustlar lesions. Virus was disseminated systemically via a monocytic cell-associated viremia to lymphoid, gastrointestinal, hepatic, and renal tissues. Additional findings included significant cytokine elaboration and depletion of T-cell dependent regions of lymphoid tissue.
Vaccinia
Vaccinia is the live attenuated Orthopoxvirus used in the smallpox vaccine. Attenuated strains, such as modified vaccinia virus Ankara, have been widely used in nonhuman primate animal models as vectors for recombinant vaccines designed to prevent diseases such as human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), influenza, and viral encephalitides. While attenuated, ocular exposure in laboratory workers can lead to severe keratoconjunctivitis and vision loss and appropriate personal protective equipment should be worn. Following intradermal injection a localized proliferative dermatitis develops and the host remains infectious until the crust is lost in 14–28 days (Figure 1.1 ).
FIGURE 1.1.

Poxviridae infections.
Intradermal inoculation of vaccinia virus produces a typical pox lesion in the skin (A) which heals within 21 days (B). Histologically eosinophilic intracytoplasmic inclusions are typical of pox virus infections (C). Ultrastructurally viral particles have a “dumbbell” appearance by electron microscopy (D).
Cowpox
Cowpox is an Orthopoxvirus reported in both New and Old World primates (Martina et al., 2006, Matz-Rensing et al., 2006). The first report in common marmosets (Callithrix jacchus) may actually be a 1982 epizootic originally attributed to a Yatapoxvirus. Clinical disease was characterized by the appearance of erythematous papules progressing through vesiculation and umbilication over a 4- to 6-week course (Gough et al., 1982). Lesions were concentrated on the face, scrotum, and palmar or plantar surfaces. Identification of the etiologic agent was based on ultrastructural examination. A more recent report describes development of hemorrhagic dermal lesions with an identical distribution in a colony of marmosets and Saguinus species (Matz-Rensing et al., 2006). Viral etiology was determined using molecular techniques to be a poxvirus with close homology to cowpox. Skin lesions from both reports were characterized by acanthosis with epidermal necrosis, ulceration, and typical pox-like intracytoplasmic inclusions. Disease may be fatal in New World monkeys. Transmission occurs via contact with rodent vectors which have a geographic distribution over much of Europe, Asia, and Africa but are not present in North America. Cowpox is considered a zoonotic disease resulting in localized, self-limiting infection in immunocompetent individuals.
Yaba Monkey Tumor Virus
Introduction
In 1957 an outbreak of subcutaneous tumors was observed in a group of captive macaques housed in Yaba, Nigeria (Bearcroft and Jamieson, 1958). A viral etiology was subsequently demonstrated and confirmed as a poxvirus. Yaba monkey tumor virus (YMTV) has been shown to naturally infect macaques (M. mulatta, M. fascicularis, and M. arctoides) and baboons (Papio anubis) (Downie, 1972). Experimentally, the pig-tailed macaque (M. nemestrina), stump-tailed macaque (M. arctoides), African green monkey, sooty mangabey (Cerocebus atys), and the Patas monkey (Erythrocebus patas) are susceptible (Kupper et al., 1970). Accidental and experimental human infection have been demonstrated. New World primates are resistant.
Etiology
YMTV is included in the genus Yatapoxvirus with tanapoxvirus and Yaba-like disease virus.
Epizootiology
Relatively few naturally occurring episodes have been documented. Captive-born African monkeys appear susceptible to experimental infection, whereas wild-caught animals are resistant, suggesting that widespread infection with YMTV or closely related virus(es) occurs in the wild, conferring life-long immunity to those individuals at an early age. The method of transmission is unknown, but arthropod vectors, tattoo needles, and trauma have been suggested as possible mechanisms.
Clinical Findings
The original outbreak of YMTV disease in rhesus macaques was characterized by multiple subcutaneous masses, often on the hands and feet, varying in size from small papules to nodules several centimeters in diameter. Larger masses would occasionally ulcerate and all masses invariably regressed by 6 weeks. Animals often developed new lesions as old lesions regressed. Subsequent cases and outbreaks have had a similar clinical course (Bruestle et al., 1981, Walker et al., 1985, Whittaker and Glaister, 1985, Schielke et al., 2002). Oral masses have been described in baboons (Bruestle et al., 1981). Intravenous inoculation may produce lesions in many organs, including lung, muscle, heart, and pleura. Because masses spontaneously regress, they are often called “pseudotumors.” Aerosol transmission of YMTV has been demonstrated experimentally in rhesus and cynomolgus macaques (Wolfe et al., 1968). Inoculated animals developed nasal, pulmonary, and pleural tumors but did not transmit the virus horizontally to cagemates.
Pathogenesis and Pathology
The characteristic histopathologic lesion consists of large pleomorphic histiocytic cells forming a nonencapsulated and infiltrative mass. These cells have hyperchromatic nuclei and prominent nucleoli. Mitotic figures are frequent. Large eosinophilic intracytoplasmic inclusion bodies may be evident. Regression is associated with erosion, ulceration, and formation of multinucleated cells.
Zoonotic Potential
Both experimentally induced and spontaneous diseases have been recognized in humans. In spontaneous human cases, lesions were most often noted on hands and feet and were associated with lymphadenopathy and fever. As in nonhuman primates, regression occurred within weeks.
Yaba-Like Disease Virus
Introduction
In 1967 an outbreak of a contagious skin disease of macaques and human handlers was observed at three primate facilities in Oregon, Texas, and California (Or-Te-Ca) and was traced to a single primate importer (Hall et al., 1967, Crandell et al., 1969, Downie et al., 1971). Affected species in the original outbreak were rhesus macaques, pig-tailed macaques, Japanese macaques (M. fuscata), and Sulawesi-black macaques (Macaca nigra).
Etiology
Yaba-like disease virus (YLDV) is classified in the genus Yatapoxvirus. This virus was initially thought to be tanapox, a relatively benign cutaneous infection of humans responsible for periodic outbreaks of illness in East Africa (Jezek et al., 1985). It has been documented that these viruses have 98.6% identity of genomic sequences. Current understanding is that these are two different strains of the same virus with tanapox representing the human virus and YLDV representing the nonhuman primate virus (Brunetti et al., 2003). Or-te-ca poxvirus and benign epidermal monkeypox (BEMP) are also names used historically to denote this virus.
Epizootiology
The unique circumstances responsible for the initial outbreak of YLDV infection in macaques are unrecognized. Serologic surveys indicate natural infection of African but not New World primates (Downie, 1972, Downie and Espana, 1974).
Pathogenesis and Pathology
Histologically, papules are composed of focally extensive regions of epidermal proliferation and ballooning degeneration and arise after a 4- to 6-day incubation period (Casey et al., 1967, Downie and Espana, 1972). Hair follicles and sebaceous glands may be involved. Eosinophilic, intracytoplasmic viral inclusions may be present. Nuclei are variably distended by large eosinophilic cytoplasmic invaginations. Ultrastructurally mature viral particles are 370 × 150 μm in size and have an outer coat consisting of seven distinct layers and an inner dumbbell-shaped core. Morphologically, these are indistinguishable from YMTV (Casey et al., 1967). Resolution is characterized by necrosis, ulceration, and infiltration of the dermis by a variety of inflammatory cells.
Clinical Findings
Following a 4- to 5-day incubation period, small red papules form and progress by 14 days to circular, firm raised foci up to 1 cm in diameter. These lesions may ulcerate and become umbilicated before resolving in 3–4 weeks and are often surrounded by a hyperemic border.
Treatment
No treatment is available.
Prevention
Previous infection with YLDV is protective against subsequent challenge. Vaccination with vaccinia does not produce protective immunity.
Zoonotic Potential
The related human virus, tanapox, occurs naturally in regions of Kenya and Congo. The initial outbreaks in 1957 were associated with flooding of the Congo River. The definitive host and vector(s) involved in these epidemics are unknown. Transmission of YLDV from infected monkeys to humans has been documented. In humans, infection is characterized by a short febrile illness accompanied by constitutional signs. As these signs abate, small nodules and papules arise eventually, forming the classic pock lesion.
Molluscum Contagiosum
Molluscum contagiosum is a chronic, mildly contagious skin disease of humans characterized by multiple small, pinkish skin nodules from which a waxy material may be extruded. Although it is caused by a pox virus, it is difficult to culture in vitro, and attempts at experimental infection of a variety of nonhuman primates have been unsuccessful. Histologically, the lesion is composed of a flask-shaped proliferative nodule of epidermal cells in which centrally enlarged acanthocytes contain homogeneous, intracytoplasmic, eosinophilic viral inclusions. These molluscum bodies are shed along with keratin debris through a central pore. A single outbreak with similar clinical and histopathologic findings has been described in chimpanzees (Douglas et al., 1967). It is unknown whether this represented a similar or identical viral agent to that seen in the human disease.
Herpesviridae
The family Herpesviridae is divided into three distinct subfamilies: Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae (Table 1.2 ). Specific viruses will be discussed herein according to their subfamily grouping. Additionally, there are a number of herpesviruses that have yet to be assigned to a subfamily. The classification of herpesviruses has been recently updated by the International Committee of the Taxonomy of Viruses (ICTV) resulting in a number of name changes (Davison et al., 2009). Names for the nonhuman primate herpesvirus species are based on the host genus with the name ending in –ine (i.e. Macacine herpesvirus 1 replaces Cercopithicine herpesvirus 1). Current taxonomic designations with the former and common names are presented in Table 1.2. Although the viral subfamilies are distinctly different, as a group the herpesviruses do share certain genetic and biologic properties. These include (1) a complex double-stranded DNA genome encoding a number of enzymes involved in protein processing, DNA synthesis, and nucleic acid metabolism; (2) DNA synthesis and capsid formation in the nucleus; (3) requisite destruction of the host cell to complete the viral replicative process; and (4) viral persistence in a latent form within the host.
TABLE 1.2.
Herpesviridae
| Classification | Former Name | Common Name |
|---|---|---|
| Subfamily: Alphaherpesvirinae | ||
| Genus: Simplexvirus | ||
| Ateline herpesvirus 1 | Spider monkey herpesvirus | |
| Cercopithicine herpesvirus 2 | SA8 | |
| Human herpesvirus 1, 2 | Herpes simplex 1, 2 | |
| Macacine herpesvirus 1 | Cercopithicine herpesvirus 1 | Herpes simiae; B virus |
| Papiine herpesvirus 2 | Cercopithicine herpesvirus 16 | Herpesvirus papio 2 |
| Saimirine herpesvirus 1 | Herpesvirus tamarinus | Herpes T |
| Genus: Varicellovirus | ||
| Cercopithicine herpesvirus 9 | Cercopithicine herpesvirus 6,7,9 | Simian varicellovirus |
| Human herpesvirus 3 | Varicella-zoster virus | |
| Subfamily: Betaherpesvirinae | ||
| Genus: Cytomegalovirus | ||
| Cercopithicine herpesvirus 5 | African green monkey CMV | |
| Human herpesvirus 5 | Human cytomegalovirus | |
| Macacine herpesvirus 3 | Cercopithicine herpesvirus 8 | Rhesus monkey CMV |
| Panine herpesvirus 2 | Pongine herpesvirus 4 | Chimpanzee CMV |
| Aotine herpesvirus 1, 3∗ | Herpesvirus aotus types 1, 3 | |
| Subfamily: Gammaherpesvirinae | ||
| Genus: Lymphocryptovirus | ||
| Callitrichine herpesvirus 3 | Marmoset lymphocryptovirus | |
| Cercopithicine herpesvirus 14 | African green monkey EBV-like virus | |
| Gorilline herpesvirus 1 | Pongine herpesvirus 3 | Gorilla herpesvirus |
| Human herpesvirus 4 | Epstein-Barr virus | |
| Macacine herpesvirus 4 | Cercopithicine herpesvirus 15 | Rhesus lymphocryptovirus |
| Panine herpesvirus 1 | Pongine herpesvirus 1 | Chimpanzee herpesvirus |
| Papiine herpesvirus 1 | Cercopithicine herpesvirus 12 | Herpesvirus papio |
| Pongine herpesvirus 2 | Orangutan herpesvirus | |
| Genus: Rhadinovirus | ||
| Ateline herpesvirus 2, 3 | Herpesvirus ateles | |
| Human herpesvirus 8 | Kaposi’s sarcoma-assoc. herpesvirus | |
| Macacine herpesvirus 5 | Cercopithecine herpesvirus 17 | Rhesus rhadinovirus |
| Saimirine herpesvirus 2 | Herpesvirus saimiri | |
| Unassigned Species in Subfamily | ||
| Saguinine herpesvirus 1 | Callitrichine herpesvirus 1 | Herpesvirus saguinus |
| Unassigned Viruses in Family | ||
| Callitrichine herpesvirus 2 | Marmoset CMV | |
| Cebine herpesvirus 1 | Capuchin herpesvirus (AL-5) | |
| Cebine herpesvirus 2 | Capuchin herpesvirus (AL- 18) | |
| Cercopithicine herpesvirus 3 | SA-6 | |
| Cercopithicine herpesvirus 4 | SA-15 | |
| Macacine herpesvirus 6 | Cercopithicine herpesvirus 10 | Rhesus leukocyte-assoc. herpesvirus |
| Macacine herpesvirus 7 | Cercopithicine herpesvirus 13 | Herpesvirus cyclopis |
Tentative species within the genus.
Alphaherpesvirinae
Macacine Herpesvirus 1: Herpes B Virus
Introduction
Herpes B virus occurs as a common, latent, and usually asymptomatic infection of Asian macaques and has been demonstrated by viral isolation or serology to occur in rhesus macaques, bonnet macaques (M. radiata), Japanese macaques, stump-tailed macaques, Formosan rock macaques (M. cyclopis), pig-tailed macaques, lion-tailed (M. silenus), and cynomolgus macaques (Hunt and Blake, 1993a, Anderson et al., 1994, Thompson et al., 2000). Although rarely responsible for disease in the natural host, inadvertent infection of humans results in a disseminated viral infection characterized by ascending paralysis and a high case fatality rate. The increased incidence of human cases in the late 1980s and early 1990s combined with the 1997 death of a primate center research assistant has spurred continued interest in the prevention and treatment of this disease. Infection of non-macaque species, including the Patas monkey, black and white colobus (Colobus abyssinicus), DeBrazza’s monkey (Cercopithecus neglectus), capuchin monkey (Cebus apella), and common marmoset, has reportedly produced fatal disease (Gay and Holden, 1933, Loomis et al., 1981, Wilson et al., 1990, Thompson et al., 2000). Persistent and asymptomatic B virus infection in a colony of capuchin monkeys housed near but not in direct contact with rhesus macaques has been reported and indicates that safe practices should be employed when working with all nonhuman primate species (Coulibaly et al., 2004).
Etiology
Herpes B virus is a member of the subfamily Alphaherpesvirinae and genus Simplexvirus. The 157-kb-long viral genome encodes approximately 70 proteins. Homologs of all of these genes occur in related viruses of humans, such as Human herpesvirus 1 and 2 (HHV1 and HHV2; Herpes simplex virus 1 and 2), and of primates, such as Papiine herpesvirus 2 (Herpesvirus papio 2) and Cercopithecine herpesvirus 2 (Ohsawa et al., 2002a, Ohsawa et al., 2003, Perelygina et al., 2003b). Notably, herpes B virus lacks a homolog of the HHV neurovirulence gene γ134.5 suggesting alternate strategies to promote replication of B virus within neurons (Perelygina et al., 2003b). The viral envelope contains at least nine glycoproteins that are targets of the immune response and help to define cellular tropism. Glycoproteins B and D have 80% and 56% identity with the respective HHV1 glycoproteins, while glycoproteins G and C demonstrate significant sequence variation (Ohsawa et al., 2002a, Perelygina et al., 2002). Genomic sequencing and restriction fragment length polymorphism analysis have demonstrated that distinct genotypes of herpes B virus occur in different species of macaques including rhesus, cynomolgus, pig-tailed, lion-tailed, and Japanese macaques (Smith et al., 1998, Thompson et al., 2000, Ohsawa et al., 2002b). The parallel arrangement of phylogenic trees for B virus isolates and mitochondrial DNA sequences from the respective macaque species indicates probable co-speciation. Viral replication occurs rapidly, with enveloped capsids present 8–10 h after infection. In cell culture, syncytial cells and Cowdry-type A intranuclear inclusions are readily apparent.
Epizootiology
The incidence of infection in immature rhesus macaques is low and increases rapidly with sexual maturity, approaching 80–90% in some colonies by 3–4 years of age (Weigler et al., 1993, Andrade et al., 2003, Sariol et al., 2006). The percentage of animals with active oral lesions is much less and in one large study of 14 400 macaques was found to be 2.3% (Keeble, 1960). The virus is transmitted through sexual or biting behavior and by fomites. In overcrowded or unsanitary conditions, animals may become infected at an earlier age and the seropositive rate may be higher. Animals remain infected for life and may periodically shed the virus in oral and genital secretions. The greatest risk of primary infection occurs during the breeding season in sexually adolescent animals 2–3 years of age (Weigler et al., 1990, Weigler et al., 1993).
Pathogenesis and Pathology
The pathogenesis of herpes B infection in macaques is similar to HHV1 infection in humans. Primary infection results in an initial round of replication at the site of inoculation. Histologically, this is characterized by the ballooning degeneration of keratinocytes with progression to vesiculation. Multinucleated, syncytial cells and eosinophilic to basophilic, intranuclear viral inclusions may be prominent. Immunohistochemistry utilizing antibodies against HHV1 can be used to demonstrate viral antigen in equivocal lesions. Inflammatory cells may be found within vesicles, epidermis, and subjacent dermis. Endothelial cell necrosis with intranuclear viral inclusions may be seen. In disseminated disease, there is widespread, hemorrhagic necrosis within the liver, lung, brain, adrenal gland, and lymphoid organs (Espana, 1973, Simon et al., 1993, Anderson et al., 1994, Carlson et al., 1997). Herpes B virus should be included in the list of viral agents responsible for multifocal, necrotizing hepatitis in macaques.
Seroconversion occurs soon after primary infection and is associated with the resolution of clinical signs. These antibodies may be detected by enzyme-linked immunosorbent assay (ELISA) and western blot methods (Ward and Hilliard, 1994, Ward and Hilliard, 2002). False-negative tests and latently infected immunologically unreactive individuals complicate the interpretation of results on single samples. Various strategies have been explored to increase the sensitivity and specificity of serodiagnosis and improve biosafety including assays based on recombinant glycoproteins and surrogate antigens such as HHV1, Papiine herpesvirus 2, and Cercopithicine herpesvirus 2 (Ohsawa et al., 1999, Takano et al., 2001, Yamamoto et al., 2005). The variable antigenic cross-reactivity of certain glycoprotein epitopes may also allow for discrimination of antibodies to closely related alphaherpesviruses (Perelygina et al., 2002, Perelygina et al., 2005, Fujima et al., 2008). A number of strategies for virus speciation via polymerase chain reaction technology have also been described (Hirano et al., 2002, Perelygina et al., 2003a, Oya et al., 2004, Miranda et al., 2005). PCR detection has decreased sensitivity and utility due to the infrequency of virus shedding.
Following initial viral replication, the virus (virion or capsid) is transported by retrograde axonal flow to the sensory ganglion where a latent infection is established for the life of the animal. Centrifugal spread may contribute to the enlargement of lesions or generalization during primary infection. Factors contributing to recrudescence are poorly understood, but stress, fever, ultraviolet light, tissue or nerve damage, and immunosuppression have been identified clinically as contributing factors in the reactivation of HHV in humans and may play a similar role with B virus in macaques. It is generally thought that stress related to changing social dynamics, transportation, or relocation can result in reactivation of herpes B virus (Mitsunaga et al., 2007, Elmore and Eberle, 2008). Detection of shedding, although uncommon, appears to be most strongly associated with the breeding season (Weigler et al., 1993, Huff et al., 2003). Other studies have shown that viral shedding in seropositive macaques was not commonly associated with common laboratory procedures or occurrences such as quarantine, parturition, and chair restraint (Weir et al., 1993, Huff et al., 2003). Reactivation of oral lesions has been described in macaques treated with immunosuppressive agents and with solid organ transplantation drug regimens (Chellman et al., 1992). Interestingly, reactivation is not commonly recognized in macaques experimentally inoculated with SIV and dying with acquired immunodeficiency syndrome (AIDS) (Simon et al., 1993) (Figure 1.2 ).
FIGURE 1.2.

Macacine herpesvirus 1 (B virus (BV)).
BV infection of mucosal surfaces may be observed with primary infection or reactivation and often appear first as small vesicles (A). In severe cases these may spread to adjacent haired skin (B) and disseminate to the gastrointestinal tract (esophagus, C). The virus may be sexually transmitted and lesions appear on the penile (D) and vaginal mucosa.
(Photographs courtesy of Drs. Dan Anderson and Sherry Klumpp, Yerkes National Primate Research Center, with permission.)
Clinical Findings
Infection of Asian macaques is usually mild and self-limiting. Characteristic vesicular lesions occur on oral and genital mucosae, which progress to ulceration and resolve within 10–14 days. Disseminated infection in macaques is rare, but when it occurs, it is usually fatal. Viral dissemination to the lung, liver, spleen, bone marrow, and adrenal cortex has been documented (Wilson et al., 1990, Simon et al., 1993). In these instances, the clinical course may vary from peracute to slowly progressive, and B virus is often not suspected as an underlying etiologic agent, thereby increasing the risk of human exposure. A respiratory form has been recognized in bonnet macaques (Espana, 1973, Scharf et al., 2008). During the 1973 epizootic, animals exhibited coryza, rhinorrhea, cough, and conjunctivitis. Both morbidity and mortality were high, and hemorrhagic interstitial pneumonia and hepatic necrosis were described at necropsy.
Treatment
Animals actively infected and shedding virus should not be treated as this entails considerable risk to the attending personnel. Recommendations for postexposure prophylaxis and treatment of clinical disease in humans have been reviewed and published (Holmes et al., 1995, Cohen et al., 2002) (Figure 1.3 ).
FIGURE 1.3.

Macacine herpesvirus 1 (B virus (BV)).
Viral infection may disseminate and cause multifocal hepatic necrosis (A and B). It may also be carried and shed asymptomatically from mucosal surfaces such as the tonsilar epithelium (C and D). Immunohistochemistry may be used for viral localization (B and D). Histologically multinucleated syncytial cells with intranuclear inclusions may be evident in epithelial cells (insert C).
Prevention
Guidelines used to establish B virus specific pathogen-free (SPF) colonies have been published (Ward and Hilliard, 1994, Hilliard and Ward, 1999). Animals are initially screened by titration ELISA and western blot. Negative animals should be kept in single cage housing or in small groups and be periodically tested by a modified ELISA for at least one year. Animals that are repeatedly negative by these criteria can then be moved to larger groups. Once in these breeding groups, animals should be periodically tested for seroconversion. Repeated testing during the quarantine period is required because animals may be (1) chronically infected and immunologically unreactive or (2) in the early stages of disease prior to seroconversion. Serologic testing on an annual or semi-annual basis should be continued as a component of colony management as seropositive macaques have been detected as late as 7 years post establishment of a SPF program (Hilliard and Ward, 1999). Because the positive predictive value of screening tests decreases concomitantly with the reduced prevalence of disease, false-positive test results are more likely during the maintenance phase and require pursuit of confirmatory testing. While antibody testing is useful for colony management, the value of a single test in an individual animal is limited.
Breaks in the SPF barrier status may occur from introduction of new animals, contact with contaminated fomites, or reactivation of latent infection in seronegative animals. Ideally, SPF colonies should be self-sustaining and not require introduction of new animals. When animals are introduced, appropriate testing and quarantine are required. If non-SPF animals are housed in the same facility, precautions should be taken to prevent transmission of the virus via fomites.
In smaller facilities, the acquisition of seronegative young animals and the subsequent individual housing of those animals have been shown to greatly reduce the occurrence of primary active infections (DiGiamoco and Shah, 1972, Olson et al., 1991). Although inappropriate for large colonies, this method may be adequate for small numbers of animals that are kept for short periods.
Implementation of SPF programs appears to be effective at reducing the risk of B virus exposure. Examination of 4 years of data from six SPF colonies indicated a greater than 20-fold reduction in probability of non-negative serologic results (Hilliard and Ward, 1999). It must be remembered that all macaque species should be treated as if they may be infected with herpes B virus regardless of the source and that this is only one of many potentially dangerous zoonotic agents that macaques may harbor.
Zoonotic Potential
Despite the widespread use of macaques and the common occurrence of B virus infection in these animals, fewer than 50 documented human cases have been described (Holmes et al., 1995). Evidence from recent outbreaks indicates that previous infection with HHV is not protective. The majority of human cases have developed following macaque-induced injury. Rarely has human-to-human contact, respiratory spread, needle stick injury, laboratory exposure, or unknown exposure been recorded as the means of transmission (Holmes et al., 1990, Weigler, 1992). The most recent fatality was due to an ocular splash with biologic material from a rhesus macaque prompting guidance on mechanisms of ocular protection (CDC, 1998). Herpes B virus is listed as a HHS select agent.
In humans, a vesicular dermatitis at the site of inoculation develops as soon as 3–5 days or as late as 24 days post-exposure, which is followed by lymphangitis and secondary lymphadenopathy. Pruritus may be intense at the site of inoculation. Neurologic signs appear 3–7 days after the initial cutaneous lesion and are characterized by an ascending myelitis. Fever, paresthesia, muscle weakness, and conjunctivitis may precede these findings. In some cases, premonitory signs and a clinical history of exposure have not been recognized. The case fatality rate in humans is approximately 70–80%, with death ensuing within 10–14 days. Although less frequent, asymptomatic infection and infections characterized by recurrent vesicular rash and respiratory signs have been identified in humans. Evidence suggests that asymptomatic infection is rare (Freifeld et al., 1995). Early recognition of clinical signs is critical as the administration of nucleoside analogs (acyclovir, valacyclovir, ganciclovir, or famciclovir) may be beneficial during the initial stages of infection.
Recommendations for the prevention and treatment of injuries inflicted by macaques have been published (Cohen et al., 2002). Education of animal care and laboratory personnel on the prevention and risks of herpes B virus infection is critical. An epidemiologic investigation following cases of human herpes B virus infection at a single animal research facility revealed that only 41% of the employees with at least weekly contact with macaques had prior knowledge of herpes B virus (Davenport et al., 1994). Advanced preparation of bite/wound kits and detailed standard operating procedures should be available at all institutions housing macaques and handling their tissues. Following exposure, thorough and vigorous cleansing of the wound with detergent and water for at least 15 min should be initiated, followed by risk assessment by an infectious disease specialist. Close clinical follow-up is warranted. Post-exposure prophylaxis with valacyclovir may also be warranted with high-risk exposures.
Although herpes B virus infection of man is rare, protection from and prevention of this zoonosis is of paramount importance. All facilities that house macaque species should implement a comprehensive B virus prevention and control plan. The basic elements of this program should include: (1) standard operating procedures for handling macaques and their tissues; (2) education and training of all personnel having potential contact with macaques; (3) the presence of supplies for immediate patient first aid; (4) maintenance of nonhuman primate-related injury database; (5) the required use of appropriate personal protective equipment; (6) access to medical care staff with expertise in herpes B virus risk assessment, diagnosis, treatment, and follow up; (7) periodic review of existing procedures and policies to ensure employee safety and compliance.
Cercopithecine Herpesvirus 2: Simian Agent 8
Cercopithecine herpesvirus 2 (SA8) is a simplexvirus of the subfamily Alphaherpesvirinae. It was originally isolated from neural tissue of an African green monkey and is found indigenously in this species. The viral genome is organized similarly to other simplexviruses, and all identified genes are homologous and collinear with those of herpes B virus (Tyler et al., 2005). Due to the close antigenic similarities, SA8 has been proposed as a surrogate antigen for diagnosis of B virus seropositivity (Malherbe and Harwin, 1958, Takano et al., 2001). SA8 is also closely related to Papiine herpesvirus 2 (previously Herpesvirus papio 2, HVP2) (Eberle et al., 1995). Although these are two distinct viruses, the original HVP2 isolates were identified as SA8 leading to some confusion in early publications. Infection in African green monkeys is usually subclinical. The virus has no known zoonotic potential.
Papiine Herpesvirus 2: Herpesvirus Papio-2
Introduction
Papiine herpesvirus 2 (HVP2) is a common infection of baboons. This virus is a member of the simplexvirus genus of the subfamily Alphaherpesvirinae. Genomic organization is identical to other simplexviruses (Bigger and Martin, 2003, Tyler and Severini, 2006). HVP2 is most closely related to SA8 and demonstrates an 85% homology to the SA8 genome. Two regions of the genome, the UL41–44 genes and the UL36 gene, demonstrate closer homology to herpes B virus, suggesting a recombination event between an SA8-like progenitor and a virus closely related to herpes B virus (Tyler and Severini, 2006). Like B virus and SA8, the HVP2 genome lacks the γ34.5 HHV neurovirulence gene (Tyler et al., 2005, Tyler and Severini, 2006). Antigenic similarities to herpes B virus have allowed HVP2 to be used as a surrogate antigen for diagnosis of B virus seroconversion. Study of HVP2 in a murine model has identified two HVP2 clades, one classified as apathogenic and the other classified as neurovirulent (Rogers et al., 2003, Rogers et al., 2006).
Epizootiology
Seropositivity in both captive and wild-caught adult baboons nears 100% (Eberle et al., 1997; Payton et al., 2004). Although virus can be spread venerally in adult baboons, oral infection prior to sexual maturity is the predominant mode of transmission (Levin et al., 1988, Eberle et al., 1998; Payton et al., 2004). As with other alphaherpesvirus infections, the virus may persist latently within sensory ganglia (Kalter et al., 1978). Recrudescence may occur periodically. With reactivation, the virus is shed in oral and genital secretions, although it is detected in less than 5% of animals sampled (Eberle et al., 1998).
Pathogenesis and Pathology
The pathogenesis of HVP2 is most similar to HHV-1 and -2 and recapitulates many aspects of B virus pathogenesis in macaques. Gross lesions include vesicular eruptions in the oral cavity or on genitalia. Vesicles or pustules coalesce, ulcerate, and resolve within 2 weeks of appearance (Rogers et al., 2005). Lesions may occasionally spread to adjacent skin. Genital lesions are occasionally severe, involving the vulvar, penile, or perineal tissues. Secondary bacterial infections and fibrosis may contribute to vaginal or urethral obstruction (Singleton et al., 1995, Martino et al., 1998). Disseminated disease has not been described in adult baboons and inoculation of juveniles with neurovirulent strains does not result in clinical disease (Rogers et al., 2005). Severe disease is possible in infant baboons. Experimental inoculation of infantile baboons (though described as an SA8 inoculation, the original inoculum was isolated from an infant baboon) produced a fibrinonecrotic pulmonary alveolitis that was accompanied by multifocal hepatic necrosis. Intranuclear inclusions were noted (Eichberg et al., 1976). A similar case of severe necrotizing pneumonia secondary to natural transmission of HVP2 from a dam to an infant baboon was recently described by Wolf and colleagues (Wolf et al., 2006b).
Clinical Findings
Many animals carry the virus asymptomatically. During primary infection or recrudescence, small vesicles and pustules may be found on the genital and oral mucous membranes (Levin et al., 1988, Martino et al., 1998). There has been one report of natural transmission to a non-host species that has resulted in severe disease (Troan et al., 2007). In this case, a black and white colobus monkey (Colobus guereza) from a zoological park demonstrated ataxia and death. Multifocal necrosis and hemorrhage of the central nervous system and adrenal gland were identified on gross and histological examination. Virus was identified using molecular techniques. Transmission via enrichment items shared with an adjacently housed troop of baboons was suspected.
Zoonotic Potential
Despite its close relatedness to herpes B virus, zoonotic transmission of HVP2 has not been reported. There is ongoing study of the comparative pathology of HHV, herpes B virus, HVP2, and SA8 in rodent models and cell culture systems with the goal to elucidate the molecular basis of alphaherpesviral neurovirulence (Ritchey et al., 2002, Ritchey et al., 2005, Rogers et al., 2006).
Saimiriine Herpesvirus 1: SaHV1, Herpevirus Tamarinus
Introduction
Saimiriine herpesvirus 1 (SaHV1; previously named Herpesvirus tamarinus or Herpes T) infection has many similarities with human herpesvirus infection of New World primates. The virus is carried asymptomatically by squirrel monkeys (S. sciureus) but induces an acutely lethal disease in owl monkeys (Aotus spp.) and several species of marmosets and tamarins (Holmes et al., 1964, Melnick et al., 1964, Hunt and Melendez, 1966, King et al., 1967, Morita, 1981).
Epizootiology
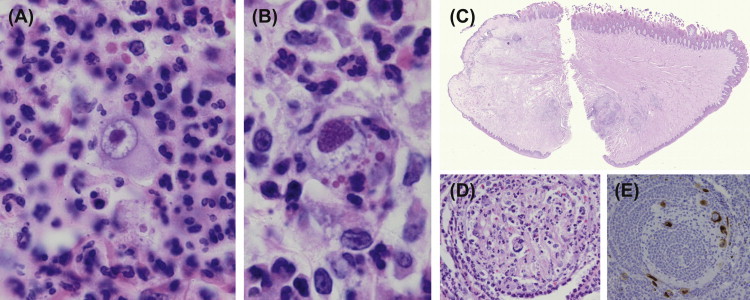
Squirrel monkeys become infected at an early age and harbor the virus asymptomatically. Viral persistence within sensory ganglia has been documented. Periodic reactivation and shedding of the virus in oral secretions represent the primary reservoir and source of infection. Antibodies to SaHV1 have been detected in asymptomatic spider monkeys (Ateles spp.), capuchin monkeys, and woolly monkeys (Lagothrix spp.), and these animals may represent additional natural reservoir hosts or, more likely, carry antigenically related simplexviruses (Hull et al., 1972, Mou et al., 1986). Initial infection of tamarins, owl monkeys, and marmosets occurs through inadvertent exposure to carrier species. Once established, intraspecies transmission results in an epizootic with high mortality. Surviving animals may continue to shed virus and represent a continued source of infection (Murphy et al., 1971a) (Figure 1.4 ).
FIGURE 1.4.

Saimiriine herpesvirus 1 (SaHV-1) or herpesvirus tamarinus.
SaHV-1 rarely causes disease in the squirrel monkeys (the natural host) but may be associated with stomatitis (A) or glossitis. Transmission to callitrichids or owl monkeys results in severe disseminated disease associated with necrotizing ulcers at mucosal surfaces (B, stomatitis) and multifocal hepatic necrosis (C).
Pathogenesis and Pathology
Following a 7- to 10-day incubation period, viral infection causes a disseminated necrotizing process involving the skin, oral mucosa, and numerous parenchymal organs. In sections of skin and mucosa, intraepithelial vesicles progress to full-thickness epidermal necrosis. Within these regions a few viable epithelial cells may remain beneath a mass of degenerate eosinophilic material admixed with pyknotic debris. Sebaceous glands, hair follicles, and apocrine glands are relatively spared. There is mild parakeratosis and intercellular edema within the adjacent epidermis, and scattered multinucleated giant cells may be present, often containing intranuclear viral inclusions. Because of the acutely lethal nature of this process, inflammatory reactions within the dermis may be minimal, consisting only of scattered neutrophils.
Foci of full-thickness necrosis similar to that present within the skin are found in the mucosa of the oral cavity and in the small and large intestine. Large sections of oral mucosa may develop necrotic plaques and slough. Multifocal necrosis is noted in the liver, spleen, lung, kidney, and adrenal gland. Hepatic lesions occur multifocally and randomly and are composed of acute hepatocellular necrosis ranging in size from small clusters of two to five cells to large coalescent foci 2–3 mm in diameter. Large numbers of Cowdry-type A intranuclear inclusions may be present in these regions. If present, encephalitis is minimal. The lesions are essentially identical to herpes simplex infection in these species, and viral isolation or molecular techniques are required to distinguish them.
Clinical Findings
In carrier species, infection is usually not associated with clinical signs and only rarely are oral vesicles and ulcers present (King et al., 1967). In susceptible species (tamarins, owl monkeys, and marmosets), inadvertent infection results in an epizootic of high mortality with variable oral, labial, and dermal lesions. Clinical signs include pruritus, anorexia, and depression. Progression to death occurs within 24–48 h.
Treatment and Prevention
Contact between susceptible and carrier species should be prevented. A live vaccine that reduces natural infection in owl monkeys has been developed; however, infrequent episodes of vaccine-induced disease have occurred, which have been characterized by a rapidly progressive disseminated infection similar to that caused by natural disease (Daniel et al., 1967).
Zoonotic Potential
There are no published reports of zoonotic transmission. A murine model of SaHV1 infection has been established to study comparative aspects of alphaherpesvirus neuropathogenesis (Breshears et al., 2005).
Human Herpesvirus 1 and 2: HHV1 and HHV2, Herpes Simplex Virus 1 and 2
Introduction
Human herpesvirus 1 (HHV1) is a common infection of humans. Inadvertent infection of gibbons, gorillas, tree shrews (Tupaia glis), orangutans, and chimpanzees has been described (Smith et al., 1969, Emmons and Lennette, 1970, McClure et al., 1972, Marennikova et al., 1973, McClure et al., 1980, Kik et al., 2005). In these species, infection usually results in mild, self-limiting oral vesicular lesions. Conversely, infection of New World species, including owl monkeys, callitrichids, and one report in a group of white-faced saki monkeys (Pithecia pithecia pithecia), results in a lethal disseminated disease similar to that caused by SaHV1 from which it must be distinguished (Hunt and Melendez, 1966, Melendez et al., 1969, Huemer et al., 2002, Matz-Rensing et al., 2003, Schrenzel et al., 2003, Lefaux et al., 2004). Disseminated disease is also reported secondary to experimental intravaginal inoculation of tamarins (Saguinus oedipus and S. fuscicollis) with HHV2 (Felsburg et al., 1973). Experimental inoculation of capuchin monkeys produced localized disease (Nahmias et al., 1971, Felsburg et al., 1972).
Etiology
HHV1 and HHV2 are members of the alphaherpesvirus subfamily. HHV1 is most often responsible for oral lesions and encephalitis in adults, whereas HHV2 is responsible for a sexually transmitted disease causing a genital infection in adults and a disseminated infection in infants. Both types are equally pathogenic in New World species and no difference in clinical outcomes has been noted with experimental infections. Serologic evidence of an alphaherpesvirus related to HHV2 has been demonstrated in healthy, free-ranging mountain gorillas (Gorilla gorilla beringei) (Eberle and Hilliard, 1989, Eberle, 1992).
Epizootiology
Nonhuman primates are not naturally infected with HHV in the wild and likely acquire the infection through human contact. Once established within New World primate colonies, the virus spreads rapidly and results in high morbidity and mortality (Matz-Rensing et al., 2003, Schrenzel et al., 2003, Lefaux et al., 2004). A natural epizootic in a research colony of gibbons was characterized by a more limited spread (Smith et al., 1969).
Pathogenesis and Pathology
The pathogenesis of human herpesviruses in owl monkeys and callitrichids is essentially identical to that caused by SaHV1 with the exception that encephalitis may be a more frequent sequela. A multifocal necrotizing and vesicular dermatitis is often most severe on facial skin and is accompanied by blepharitis and stomatitis. In gibbons, a multifocal acute meningoencephalitis may be evident in the pons and cerebral cortex. These changes may be accompanied by necrosis, reactive gliosis, and typical Cowdry-type A inclusions (Figure 1.5 ).
FIGURE 1.5.
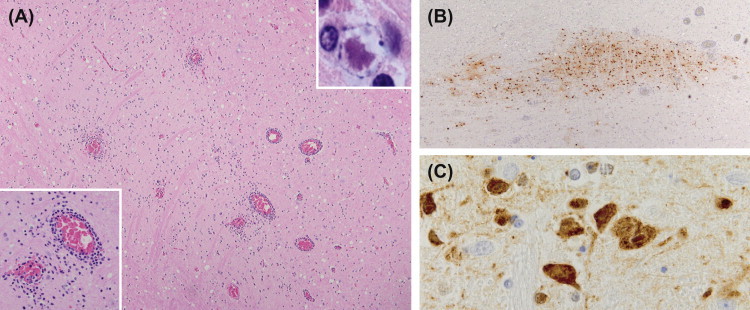
Herpesvirus simplex1 (HSV1).
HSV commonly disseminates to the CNS in neotropical primates and may occur without mucosal lesions. Within the CNS multifocal necrotizing encephalitis (A) is characteristic with perivascular infiltrates (insert A left) and intranuclear inclusions (insert A right). Immunohistochemistry often reveals more infected cells than evident with routine stains (B and C).
Clinical Findings
In gorillas, chimpanzees, and gibbons, infection is usually self-limiting with clinical signs restricted to vesiculation and ulceration of mucosal surfaces including the oral cavity, genitalia, and conjunctiva. In gibbons, viral encephalitis may occur rarely as a result of recrudescence (Landolfi et al., 2005). Generalized disease in susceptible species is identical to that induced by SaHV1. Posterior synechia and dyscoria has been reported in owl monkeys with an alphaherpesvirus infection suspected to be HHV due to a history of close human contact (Gozalo et al., 2008) (Figure 1.6 ).
FIGURE 1.6.
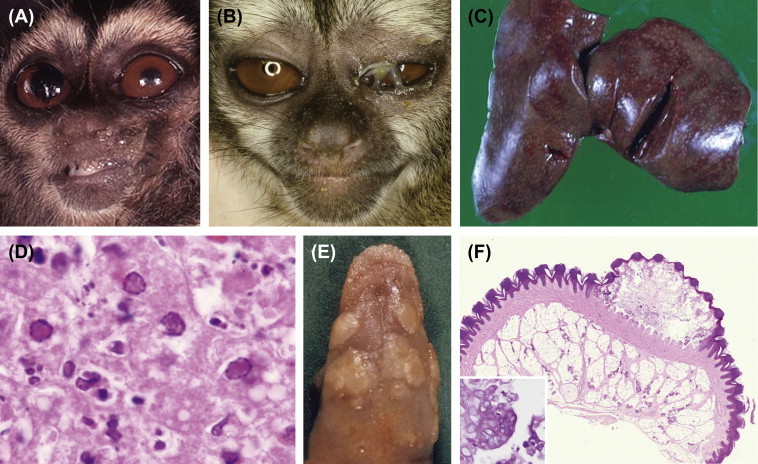
Herpesvirus simplex 1 (HSV1).
HSV1 may be transmitted to callitrichids and owl monkeys resulting in a severe disseminated viral infection. Common clinical signs include anisocoria (A), conjunctivitis (B), multifocal hepatic necrosis (C) with hepatocytes containing intranuclear inclusions (D), and stomatitis and glossitis (E and F). Multinucleated viral syncytial cells are often evident within mucosal vesicles (insert F).
Treatment and Prevention
Protective clothing and face masks should be worn by animal care personnel. A modified live vaccine was developed and found to be protective in owl monkeys (Daniel et al., 1978).
Cercopithecine Herpesvirus 9: SVV, Simian Varicella Virus
Introduction
Cercopithecine herpesvirus 9 (simian varicella virus, SVV) is a member of the Alphaherpesvirinae in the genus Varicellovirus. This virus causes a highly contagious infection of a variety of Old World nonhuman primates that can result in significant morbidity and mortality. SVV has a high degree of antigenic relatedness to human herpesvirus 3 (varicella-zoster virus, VZV), the etiologic agent of chickenpox. The SVV genome is 124 kb in length with 69 open reading frames, each of which shares homology with the corresponding VZV gene for an overall 70–75% homology and collinear arrangement (White et al., 1997, Gray et al., 2001). Experimental infection with SVV has been used as a surrogate model to investigate aspects of VZV pathogenesis, latency, and therapy.
Etiology
Original isolates were named for the facilities in which epizootics occurred and included Liverpool vervet virus, Patas herpesvirus, Medical Lake macaque virus, and Delta herpesvirus. These viruses were found to be antigenically related, and each caused a similar exanthematous viral disease. Subsequent investigation of restriction endonuclease patterns indicated that epizootics were associated with differing species of the same virus (Gray and Gusick, 1996). Infection of African green monkeys, Patas monkeys, pig-tailed macaques, Japanese macaques, cynomolgus macaques, and Formosan rock macaques has been demonstrated. SVV antibodies have also been detected in baboons with a 40% seroprevalence although clinical disease has not been reported in this species (Payton et al., 2004).
A VZV-like herpesvirus has been isolated from chimpanzees, gorillas, and orangutans with mild, self-limiting vesicular dermatitides (Heuschele, 1960, McClure and Keeling, 1971, White et al., 1972, Myers et al., 1987). Antigenically, this virus is more closely related to VZV than to SVV (Harbour and Caunt, 1979). Although not definitively confirmed with genomic sequencing, restriction endonuclease examination of viral DNA from one case suggests identity to VZV (Myers et al., 1987).
Epizootiology
Between 1966 and 1970, epizootics of SVV occurred at the Liverpool School of Tropical Medicine in African green monkeys (Liverpool vervet virus), at the Delta Regional Primate Research Center in Patas monkeys (Delta herpesvirus), and at the Medical Lake field station of the Washington Regional Primate Research Center in cynomolgus, Japanese, and pig-tailed macaques (Medical Lake macaque virus) (Clarkson et al., 1967, Blakely et al., 1973, Felsenfeld and Schmidt, 1975, Wenner et al., 1977). The epizootiology and origin of these early outbreaks is poorly understood. In several instances they occurred in recently imported animals. Serologic evidence suggests that the Medical Lake macaque virus originated in Malaysia. Transmission from an unidentified reservoir host to aberrant primate hosts may be one factor related to the establishment of epizootics. The natural host of SVV has not been identified. Because disease is less severe in Asian macaque species and neutralizing antibodies have been detected in asymptomatic stump-tailed macaques, it is possible that macaque species may play a role in transmitting the virus to more susceptible species. Reactivation from a latent carrier state also plays an important role in transmission of the virus (Soike et al., 1984, Mahalingam et al., 1992). Recent cases of SVV in animals undergoing total body irradiation or other forms of experimental immunosuppression are thought to be associated with recrudescence in clinically affected animals or their contacts (Kolappaswamy et al., 2007, Schoeb et al., 2008, Hukkanen et al., 2009). Reactivation may also occur secondary to stress of shipment or movement within a facility. Once established within a colony, the virus may spread rapidly via the respiratory route. Transmission via direct contact with skin lesions is also possible.
Clinical Findings
Clinical signs recorded in natural outbreaks and in experimental disease are similar, varying only in the extent of lesions and associated mortality. The disease in macaques may be slightly less severe than in African green monkeys and Patas monkeys. The clinical course in natural outbreaks is characterized by the eruption of a disseminated, hemorrhagic vesicular exanthema accompanied by fever and, in severe cases, progression to pneumonia and hepatitis. Vesicular eruption is often observed first in the inguinal region and spares the palms and soles. Animals may demonstrate spontaneous resolution of disease or a subclinical course while, in other instances, the case fatality rate may be high. The incubation period is 7–14 days (Figure 1.7 ).
FIGURE 1.7.

Simian varicella virus (SVV).
SVV causes a cutaneous exanthema characterized by vesicles (A and B) which may appear hemorrhagic (left insert A) and be associated with a necrotizing vasculitis (A insert right). Intranuclear inclusions and multinucleated syncytial cells (B insert). The disease may disseminate causing widespread lymphoid necrosis in spleen (C) and lymph nodes as well as multifocal hepatic necrosis (D). Intranuclear inclusions are often found along the margins of necrosis (insert D).
Pathogenesis and Pathology
Experimental infections of African green monkeys have provided insight into disease progression (Roberts et al., 1984, Dueland et al., 1992, Gray, 2004, Gray, 2008). A transient viremia occurs by day 3 post-inoculation with cell-associated virus likely transported in B and T cells (White et al., 2002, Gray, 2004). A vesicular dermatitis appears by day 10 post-inoculation and is often hemorrhagic in appearance. Lesions progress from papule to vesicle to crust and appear as successive crops such that lesions of all stages may be present. Cutaneous lesions are characterized histologically by the formation of multiple vesicles within the epidermis that contain cell debris, erythrocytes, and rarely syncytial cells. Hyperplasia of the basal cell layer is associated with these vesicles. Characteristic eosinophilic, Cowdry-type A, intranuclear inclusions may be found in cells adjacent to the vesicle. A necrotizing vasculitis often exists within the subjacent dermis with viral inclusions evident in endothelial and adjacent cells.
Viral antigen is found widely disseminated by 8 days and may be localized in the liver, lungs, spleen, adrenal gland, kidney, lymph node, skin, and trigeminal ganglion. Within the liver, there is multifocal to coalescing hepatocellular necrosis. Cowdry-type A inclusions may be found within hepatocytes bordering foci of necrosis. Similar necrotizing lesions may be found in the lung and throughout the gastrointestinal tract. Hepatic and pulmonary lesions may be the most severe lesions in affected animals. Neuropathological changes are not associated with SVV infections. Latency is established within neural ganglia. Competing hypotheses suggest hematogenous versus transaxonal transport to ganglia (Mahalingam et al., 2001, Kennedy et al., 2004). Investigation of genomic transcription during latency has gathered much interest and is thought to have some similarity to VZV (Ou et al., 2007, Messaoudi et al., 2009).
Laboratory Findings
Following experimental inoculation, there is marked neutrophilic leukocytosis accompanied by a decrease in platelet numbers, and elevations in alanine aminotransferase, aspartate aminotransferase, and blood urea nitrogen (BUN) levels.
Treatment
Treatment has not been attempted in natural outbreaks. Acyclovir and interferon have shown some efficacy in experimental models (Arvin et al., 1983, Soike and Gerone, 1995). Because SVV is less sensitive to acyclovir, higher doses may be required (Pumphrey and Gray, 1996, Sienaert et al., 2004). Identification of latently infected monkeys and strict species separation may prevent epizootics. Immunization with VZV has been shown to be protective in experimental infections (Felsenfeld and Schmidt, 1979, Soike et al., 1987).
Zoonotic Potential
Transmission of SVV to human beings has not been documented.
Betaherpesvirinae
Cytomegalovirus
Introduction
Cytomegalovirus (CMV) is a common asymptomatic infection of humans and many nonhuman primates. The rhesus cytomegalovirus (RhCMV), recently renamed Macacine herpesvirus 3, is the most thoroughly characterized of the simian cytomegaloviruses. In addition to a variety of macaque species, CMV infection has been demonstrated in capuchin monkeys, woolly monkeys, squirrel monkeys, chimpanzees, baboons, drill monkeys (Mandrillus leucophaeus), saddleback tamarins (Saguinus fuscicollis), and African green monkeys (Nigida et al., 1979, Rangan and Chaiban, 1980, Blewett et al., 2001, Blewett et al., 2003;). A notable exception is the Gibraltar population of Barbary macaques (M. sylvanus) which shows no evidence of CMV infection based on serosurvey (Engel et al., 2008). Viruses within this group are generally believed to have a narrow host range although interspecies transmission does occur. Current names of ICTV classified simian cytomegaloviruses are presented in Table 1.2.
Etiology
Cytomegaloviruses resemble other herpesviruses ultrastructurally, but differ from alphaherpesviruses in several aspects. They are slowly cytolytic and tend to cause enlargement of the nucleus and cytoplasm (cytomegaly) of infected cells both in vivo and in vitro. During viral replication, enveloped virions accumulate in large cytoplasmic vacuoles instead of being readily released into intercellular spaces. This feature accounts for their relative cell-associated nature and explains the fact that cytoplasmic inclusion bodies can also be found in infected cells. Unlike many of the cytolytic herpesviruses, cytomegaloviruses also tend to be restricted in terms of their host range with each viral species thought to have coevolved with its host. Finally, latent infections tend to persist in glandular tissue, lymphoreticular cells, and kidneys rather than in neurons.
The RhCMV genome is 221 kb in length and contains approximately 230 open reading frames (Hansen et al., 2003). The virus encodes a variety of proteins important for immune evasion including peptides that prevent host MHC-1 expression and a viral homolog of IL-10 that may limit inflammatory responses (Spencer et al., 2002, Powers and Fruh, 2008).
Epizootiology
Cytomegalovirus infection is common with seroprevalence in adult macaques approaching 100% in most colonies. Infection is usually not associated with disease. The virus is spread horizontally in a variety of body secretions, including saliva, blood, urine, milk, and semen (Asher et al., 1974). In contrast to herpes B, virus may be shed for extended periods and can be detected in a large number of animals at any given time (Huff et al., 2003). Macaques become infected within the first year of life (Vogel et al., 1994). Prior immunity also does not prevent re-infection, and animals can be infected with multiple genetic variants. For instance, rhesus macaques naturally infected with RhCMV are susceptible to infection by an antigenically distinct African green monkey CMV and commonly become infected with both strains during captivity (Swack and Hsiung, 1982).
Clinical Findings
Infection of immunocompetent animals is usually asymptomatic although a transient leukocytosis has been demonstrated in healthy animals following experimental infections (Lockridge et al., 1999). Immunosuppressed animals may experience reactivation, dissemination of the virus, and evidence of disease. In these individuals, clinical signs relate to the anatomic site(s) involved and may include dyspnea, diarrhea, melena, and neurologic signs.
Pathogenesis and Pathology
Cytomegalovirus persists as a latent infection and may periodically be shed in body secretions. In immunosuppressed macaques, reactivation of the virus may be associated with disseminated lesions in the brain, lymph nodes, liver, spleen, kidney, small intestine, nervous system, and arteries. Disseminated CMV may be initiated by a variety of immunosuppressive events, including viral infection (SIV or type D retrovirus) and drug therapy (cyclophosphamide, cortisone, and antithymocyte globulin).
In SIV-infected macaques, reactivation typically occurs as a terminal opportunistic infection associated with suppression of both humoral and cellular CMV-specific immune responses (Kaur et al., 2002, Kaur et al., 2003). Disease is manifest by a necrotizing enteritis, encephalitis, lymphadenitis, and/or pneumonitis (Baskin, 1987). Pulmonary lesions are common and consist of a multifocal to coalescent interstitial pneumonia. The alveolar septa are thickened and lined by hypertrophied type II pneumocytes. Alveolar spaces contain fibrin, alveolar macrophages, and neutrophils. Cytomegaly and large, intranuclear Cowdry-type A inclusion bodies may be evident in alveolar septa and septal lining cells. Similar lesions may be found in the liver, spleen, kidney, and testes. In situ hybridization often reveals many more cells to be infected than would be anticipated on routine stains and may be useful for diagnosis when only equivocal changes (i.e., mild cytomegaly) are present. Smaller amphophilic, intracytoplasmic inclusions are less frequent.
Central nervous system (CNS) lesions are multifocal, involve primarily the leptomeninges and subjacent neuropil, and are characterized by neutrophilic infiltrates with necrosis and fibrinous exudates. These findings are accompanied by characteristic viral inclusions and often by a nonsuppurative, perivascular meningoencephalitis. In the gastrointestinal tract, hemorrhage, particularly neutrophilic infiltrates, may be prominent. In all locations, these findings may be accompanied by a necrotizing and proliferative vasculitis. The pathogenesis of CMV infection in SIV-inoculated macaques shares many similarities with the disease in human patients with AIDS.
Intrauterine CMV infection of the human fetus may occur if primary infection of the mother coincides with pregnancy. This is most frequently observed when maternal infection coincides with conception or occurs during first trimester. In contrast, vertical transmission of CMV has not been demonstrated in the rhesus macaque (Yue and Barry, 2008). The high prevalence of exposure and preexisting immunity in animals of breeding age likely contributes to the lack of congenital infection in this species. Intrauterine infection has been reproduced experimentally in rhesus macaques and squirrel monkeys (London et al., 1986, Ordy et al., 1981, Barry et al., 2006).
Association between CMV and accelerated graft vs. host rejection of heart and kidney transplants in humans is well established (Streblow et al., 2007). More controversial is the role of the virus in producing arteriosclerosis in the human population at large (Stassen et al., 2006). Long-standing immune upregulation associated with persistent CMV infection is also thought to contribute to immune senescence in aging populations (Koch et al., 2007) (Figure 1.8 ).
FIGURE 1.8.
Cytomegalovirus (CMV).
Histologically CMV produces both intranuclear and intracytoplasmic inclusions accompanied by neutrophilic infiltrates (A and B). The virus may target multiple sites including the lung, gastrointestinal tract, vessels, lymphoid tissue, central nervous system and peripheral nervous system (PNS). Targeting of nerves in the PNS (D, lingual nerve) may be associated with necrosis of the overlying epithelium (C, tongue). Viral antigen may be demonstrated in infected cells with immunohistochemistry (E).
Treatment
Certain immunosuppressive regimens are associated with reactivation of latent CMV and must be coupled with prophylactic therapy to prevent severe and often fatal disease. CMV is susceptible to ganciclovir, foscarnet, and benzimidazole nucleosides (North et al., 2004, Yue and Barry, 2008). Strategies for derivation of RhCMV and baboon CMV SPF breeding colonies have been published (Wolf et al., 2006b, Barry and Strelow, 2008).
Zoonotic Potential
NHP and human CMV genomes are similar in size and organization, however disease associated with simian CMV infection of humans has not been reported. Transient detection of baboon CMV has been reported in a human xenograft recipient (Michaels et al., 2001).
Gammaherpesvirinae
Macacine Herpesvirus 4: RhLCV, Rhesus Lymphocryptovirus
Introduction
Macacine herpesvirus 4 (rhesus lymphocryptovirus, RhLCV) is a member of the Lymphocryptovirus genus. This genus contains more than 50 distinct simian lymphocryptoviruses isolated from a host of Old World and New World primate species suggesting significant co-speciation (Ehlers et al., 2010). Most of these viral species have not been formally recognized by the ICTV and are catalogued in a recent publication by Lacoste and colleagues (Lacoste et al., 2010). Viruses recognized by the ICTV include those of chimpanzees, orangutans, gorillas, baboons, macaques, African green monkeys, and marmosets (Table 1.2) (Levy et al., 1971, Falk et al., 1976, Gerber et al., 1976, Gerber et al., 1977, Rabin et al., 1980, Payton, 2004). Human herpesvirus 4 (Epstein-Barr virus, EBV) is the type species in the genus. In humans, infection with EBV is usually asymptomatic but may cause infectious mononucleosis when primary infections occur after puberty. This illness is characterized by lymphadenopathy, fever, pharyngitis, and circulating atypical lymphocytes in adolescents and young adults. EBV has been associated with Burkitt’s lymphoma, hemophagocytic syndrome, T cell lymphomas, and nasopharyngeal carcinoma in immunocompetent persons and oral hairy leukoplakia, non-Hodgkins lymphoma, and post-transplantation lymphoproliferative disorders in immunocompromised patients.
Etiology
Lymphocryptoviruses have a complex genome consisting of approximately 170 kb. The RhLCV genome has been completely sequenced and contains 80 open reading frames, an identical repertoire of lytic and latent genes found in EBV, and an overall nucleotide sequence homology to EBV of approximately 65% (Rivailler et al., 2002b). While the lytic genes are well conserved, the latent genes demonstrate a modest homology of 27–50% with the corresponding EBV genes. Despite this, the genes maintain remarkable similarity of function. Like EBV, two distinct lineages of RhLCV (RhLCV1 and RhLCV2) have been identified based on significant genomic heterogeneity of the EBNA genes (Cho et al., 1999). Each of the variants is isolated with similar frequencies, and animals may harbor both simultaneously. Lineage specific pathology has not been recognized.
Epizootiology
Epizootiology in Old World primates closely parallels that in human populations. Infection occurs primarily in the young through contact with infected oral secretions and is usually not associated with clinical signs. Maternal immunity disappears at approximately 4–6 months of age after which juvenile macaques seroconvert at 6–12 months of age. By 2 years of age virtually all animals harbor the virus (Fujimoto and Honjo, 1991). The nearly 100% prevalence in macaque colonies reflects the 85–95% prevalence of EBV in humans. The virus infects B lymphocytes and persists for life, usually in a latent form. Critical to the regulation of the latent state is the expression of EBV-encoded RNAs (EBER-1, and -2, EBERs), EBV-encoded nuclear antigens (EBNA-1, -2, -3a, -3b, -3c, and -LP), and latent membrane proteins (LMP 1, -2a, and -2b). Reactivation and transition to a lytic cycle of viral replication occurs periodically during which animals may shed virus.
Pathogenesis and Pathology
Lymphocryptovirus infection of Old World species is common and usually not associated with disease. Following experimental RhLCV infection macaques demonstrate a self-limiting lymphadenopathy and atypical lymphocytosis characterized by activated B lymphocytes (Moghaddam et al., 1997). These animals resolve the acute viremia, seroconvert within 45–60 days, and remain persistently seropositive. In contrast, SIV-inoculated animals with simian AIDS fail to develop a RhLCV-specific humoral response and demonstrate greater RhLCV viral loads indicating the importance of the CD4+ T cell response to control of acute viremia (Rivailler et al., 2004).
RhLCV has been associated with proliferative squamous epithelial lesions resembling oral hairy leukoplakia in SIV-inoculated rhesus macaques succumbing to AIDS (Baskin et al., 1995, Kutok et al., 2004). Lesions occur on the tongue, esophagus, penis, hand, and thorax and are usually not visible grossly. Histologically, focal regions of hyperkeratotic parakeratosis with or without acanthosis or pseudoepitheliomatous hyperplasia are noted. Epithelial cells demonstrate ballooning degeneration. Basophilic intranuclear viral inclusions are present in most cases and are composed of typical herpesvirus virions as observed by electron microscopy. Detection of the small viral capsid antigen (sVCA) and BZLF1 protein suggests an active lytic infection.
Development of RhLCV-associated non-Hodgkin lymphomas (NHL) in SIV-inoculated macaques has also been described (Baskin et al., 2001, Blaschke et al., 2001, Marr-Belvin et al., 2008). The disease course and pathologic characteristics parallel those observed in HIV-infected persons in whom the development of NHL represents an AIDS-defining illness. SIV-infected rhesus macaques have an incidence of NHL ranging from 4–19% while SIV-infected cynomologus macaques have an incidence of up to 40% (Rezikyan et al., 1995, Habis et al., 1999, Matz-Rensing et al., 1999). Lymphocryptovirus transcripts are frequently detected in tumor cells while SIV transcripts or proteins are not. These neoplasms usually develop in extranodal locations including the nasal cavity, periorbital tissues, gastrointestinal tract, urogenital tract, myocardium, and central nervous system and are classified as centroblastic, immunoblastic, large cell, or Burkitt-like lymphomas (Baskin et al., 2001). Tumors are comprised of clonal expansions of CD-20-positive B cells and express a full spectrum of latent gene products (Blaschke et al., 2001). While NHL typically develops in conjunction with progression to AIDS/SAIDS, T cell depletion is not sufficient for pathogenesis (Rivailler et al., 2004). The ability of lymphocryptoviruses to immortalize B cells may allow for increased risk of mutation and subsequent lymphomagenesis. Variable expression of the oncogenes p53, p21, cMyc, and bcl2 in neoplastic tissue has been described (Kahnt et al., 2002). There is one report of a mycosis fungoides-like cutaneous T cell lymphoma consisting of CD3–/CD8+ lymphocyte and characteristic Pautier microabcesses in a pig-tailed macaque. The lymphocryptovirus isolated from tumor material was designated as Macaca nemistrina herpesvirus (MneLCV1) and appears to have a unique T cell tropism (Rivadeneira et al., 1999).
A B cell lymphoproliferative disorder has also been observed in association with latent lymphocryptovirus infection in macaques assigned to solid organ transplantation protocols (Schmidtko et al., 2002). This post-transplant lymphoproliferative disorder (PTLD) reflects the condition observed in human transplant patients. An atypical and polymorphic lymphocyte population consisting of EBER-positive CD20+ B cells infiltrates lymph nodes and effaces normal architecture. Cell populations may also be observed in extranodal sites including the liver, lung, heart, spleen, and renal allograft or native kidney.
Diagnosis of RhLCV may be accomplished via a number of techniques. Antibody detection of sVCA by ELISA allows demonstration of seropositivity while PCR detection of EBERs in peripheral blood cells demonstrates persistent infection. Immunohistochemistry utilizing cross-reactive antibodies directed at EBV proteins including the lytic protein BZLF, sVCA, and EBNA-2 allows detection of viral infection in nonhuman primate tissue. In situ hybridization using an RNA probe directed against RhLCV EBER is an additional technique that has increased sensitivity for detection (Carville and Mansfield, 2008) (Figure 1.9 ).
FIGURE 1.9.

Lymphocryptovirus (LCV).
LCV is a gammaherpesvirus that may cause epithelial or lymphoproliferative disorders in immunocompromised hosts. Leukoplakia (A) is an epithelial proliferative disorder that may be observed in the oral, esophageal or genital mucosa as well as the haired skin. It is characterized by multifocal epithelial cell hyperplasia with hydropic degeneration. Intranuclear inclusions are evident and the virus may be demonstrated by immunohistochemistry for the latent antigen EBNA2 (B) or lytic antigen BZLF1. Post transplantation lymphoproliferative disorder (PTLD) is seen in animals on immunosuppressive therapy and is often extranodal invading sites such as the liver (C). Neoplastic cells first appear in the hepatic sinusoids (D) and are EBNA2-positive (E).
Zoonotic Potential
Infection of humans with simian EBV-like agents has not been reported. Due to many strong similarities, the RhLCV-infected macaque has been proposed as a model to examine aspects of EBV transmission, pathogenesis, and prevention. In support of this model a number of institutions have developed SPF macaque populations that have excluded this virus (Carville and Mansfield, 2008).
Callitrichine Herpesvirus 3: CalHV3, Marmoset Lymphocryptovirus
Many early reports suggest that lymphocryptoviruses have a host spectrum restricted to Old World primates and humans. In 2000, an investigation of B cell lymphomas observed in common marmosets housed at the Wisconsin National Primate Research Center led to the discovery of Callitrichine herpesvirus 3 (CalHV3) (Ramer et al., 2000). Marmosets demonstrated weight loss, anorexia, diarrhea, and palpable enlargement of mesenteric lymph nodes. Histologically, the masses were comprised of sheets of CD20+ neoplastic round cells effacing lymph node architecture and infiltrating the large and small intestine, liver, kidney, and lung. CalHV3 was detected in tumor material and circulating peripheral blood mononuclear cells using molecular techniques. The virus was subsequently isolated (Cho et al., 2001). Seroprevalence of CalHV3 in wild-caught and captive marmosets from various colonies ranges from 35–65% (Fogg et al., 2005). Despite similar levels of seroprevalence, not all colonies observe a high frequency of B cell lymphomagenesis suggesting that additional factors are involved in the pathogenesis. Infection in the majority of marmosets is asymptomatic.
The CalHV3 genome has been completely sequenced and demonstrates an overall homology with the EBV genome of 43% (Rivailler et al., 2002a). The genome contains at least seven open reading frames with no homology to known viral or cellular genes (Rivailler et al., 2002a, Fogg et al., 2005). These genes appear to be precursors to EBV latency genes due to their similar position within the genome and predicted protein structure. The CalHV3 genome is also missing 14 genes found in EBV and RhLCV. The absence of these genes may contribute to inefficient viral transmission and the lower seroprevalence of CalHV3 observed in marmoset colonies. These genetic differences suggest that CalHV3 may be more closely related to a primitive lymphocryptovirus from which it, EBV, and the other the Old World lymphocryptoviruses evolved (Rivailler et al., 2002a, Fogg et al., 2005, Lacoste et al., 2010). Lymphocryptoviruses have since been detected in various New World primate species including the squirrel monkey, white-faced saki, white-fronted capuchin (Cebus albifrons), black spider monkey (Ateles paniscus), golden handed tamarin (Saguinus midas), and black-pencilled marmoset (C. pencillata) (Cho et al., 2001, de Thoisy et al., 2003b, Ehlers et al., 2003).
Human Herpesvirus 4: EBV, Epstein-Barr Virus
Because EBV is the first identified human virus to demonstrate oncogenic properties there has been much interest in developing animal models with which to study aspects of pathogenesis including mechanisms of lymphogenesis and latency programs. In addition to the natural model of RhLCV infection in macaques, a number of induced models have been described in the literature. Rhesus macaques seronegative to RhLCV are susceptible to experimental inoculation with EBV and develop a resolving atypical lymphcytosis and lymphadenopathy. Cotton-top tamarins are susceptible to experimental infection with human EBV and develop malignant B-cell lymphomas (Shope et al., 1973, Miller et al., 1977, Niedobitek et al., 1994). Common marmosets develop an antibody response and persistent infection but do not consistently develop lymphoma (Emini et al., 1986). Macaques coinfected with SIV and EBV have been proposed as a model of AIDS-associated Burkitt’s lymphoma (Feichtinger et al., 1992).
Macacine Herpevirus 5: RRV, Rhesus Rhadinovirus
Introduction
Macacine herpesvirus 5 (Rhesus Rhadinovirus, RRV) is a member of the Rhadinovirus genus. Species in this genus are closely related to Kaposi’s sarcoma herpesvirus (KSHV, human herpesvirus 8). Rhadinoviruses are thought to be species specific and have been reported in rhesus and other macaque species as well as in African green monkeys, baboons, mandrills, drills, gibbons, chimpanzees, and gorillas (Greensill et al., 2000a, Greensill et al., 2000b, Lacoste et al., 2000a, Lacoste et al., 2000b, Lacoste et al., 2001, Whitby et al., 2003, Duprez et al., 2004, Bruce et al., 2005). Rhadinoviruses are also well characterized in spider monkeys and squirrel monkeys. The ICTV-recognized Rhadinovirus species are presented in Table 1.2, and additional identified species are cataloged elsewhere (Lacoste et al., 2010). Members of this genus are classified into two distinct lineages; the Rhadinovirus-1 lineage containing KSHV and the primate Retroperitoneal Fibromatosis Herpesvirus (RFHV) and the Rhadinovirus-2 lineage which includes rhesus Rhadinovirus (RRV) (Desrosiers et al., 1997, Rose et al., 1997, Schultz et al., 2000). The RV-1 lineage will be discussed separately below.
Etiology
RRV isolates were identified independently at the New England (strain H26-95) and Oregon (strain 17795) primate research centers (Desrosiers et al., 1997, Wong et al., 1999). Closely related viruses have been recognized in cynomolgus (CRV) and pig-tailed (PRV) macaques. The RRV genome is 130 kb in length and encodes 84 open reading frames (Alexander et al., 2000, Searles et al., 1999). The genome is collinear with the KSHV genome and most KSHV open reading frames have representative homologs in the RRV genome, and vice versa (Lacoste et al., 2010). Rhadinoviruses, including RRV, encode several homologs of cellular cytokines including a viral IL-6 that has proinflammatory activity and may contribute to pathogenesis (Kaleeba et al., 1999). The ORF73 gene encodes the latency-associated nuclear antigen (LANA) which is essential for maintaining the viral DNA episome tethered to mitotic host cell chromosomes, allowing the virus to cosegregate with daughter cells during cell division (Wen et al., 2009). This protein is also thought to suppress lytic viral replication (DeWire and Damania, 2005).
Epizootiology
Transmission likely occurs via oral secretions. Seropositivity rates for RRV approach 90% by 1 year of age and 98% by 2 years of age (Desrosiers et al., 1997). RRV-like sequences have been identified in recently imported Indonesian macaques suggesting that the virus circulates in wild populations as well (Strand et al., 2000). Seroconversion can readily be detected by ELISA, and PCR can be performed on blood and tissue specimens. These viruses demonstrate two phases of infection; a latent or non-productive phase and a lytic or productive phase. RRV demonstrates a tropism for CD20+ B-lymphocytes with these cells likely serving as the primary site of RRV latency (Bergquam et al., 1999). While animals may cycle between both phases of infection, natural infections are generally asymptomatic. The factors associated with a change from latent infection to reactivation remain unclear.
Clinical Findings
RRV infections are generally asymptomatic. Experimental RRV inoculation of immunocompetent animals produced a mild febrile response, antibody production, mild B cell lymphocytosis, and self-limiting peripheral lymphadenopathy (Mansfield et al., 1999). In contrast, animals coinfected with SIV developed a marked B cell lymphocytosis with an activated phenotype. Virus-specific antibody responses to both RRV and SIV were attenuated (Wong et al., 1999).
Pathogenesis and Pathology
Experimental RRV inoculation has been associated with a lymphoproliferative disorder with characteristics resembling multicentric Castleman’s disease (MCD) (Mansfield et al., 1999). Depending on the strain, lesions may resemble either the hyaline vascular variant or the plasma cell variant of MCD. In the hyaline vascular variant, changes in lymph nodes are initially characterized by paracortical lymphocytic hyperplasia and a vascular proliferation characterized by hypertrophied and hyperplastic endothelial cells. Upon regression of lymphadenopathy, lymph nodes acquire features typical of MCD including hyalinized follicles surrounded by layers of loosely concentric lymphocytes. The plasma cell variant is characterized by infiltration of lymphoid tissue with sheets of CD20+ B-lymphocytes. There is one report in the literature demonstrating an association between RRV and NHL in SIV-inoculated macaques (Orzechowska et al., 2008).
Zoonotic Potential
While the level of zoonotic risk is thought to be low, RRV is closely related to KSHV, the causative agent of Kaposi’s sarcoma. Kaposi’s sarcoma is a highly vascularized neoplasm derived from cells of endothelial origin that is diagnosed in both immunocompetent and immunocompromised individuals. KSHV is also involved in the pathogenesis of the lymphoproliferative disorders multicentric Castleman’s disease and pleural effusion lymphoma seen most commonly in AIDS patients. The ability to readily propagate RRV in culture enables its use as a model for the study of the transcription, virus structure and assembly, and protein interactions of gammaherpesviruses. Recapitulation of aspects of human disease upon inoculation of macaques makes RRV a suitable in vivo model for the study of KSHV pathogenesis.
Retroperitoneal Fibromatosis Herpesvirus: RFHV
Etiology
RFHV was identified in 1997 in rhesus (RFHVmmu) and pig-tailed (RFHVmne) macaque retroperitoneal fibromatosis (RF) specimens using degenerate PCR primers directed at the herpesviral DNA polymerase gene (Rose et al., 1997). While the virus has been difficult to isolate and propagate in culture, investigators have been able to sequence 7.7 kb of the viral genome (Rose et al., 2003). This virus has a high degree of colinearity and sequence homology (~85%) with KSHV, placing it in the RV1 lineage. Additional members of the RV1 lineage have been identified in a number of Old World primates and apes (Lacoste et al., 2010).
Epizootiology
To date serologic assays have not been developed, limiting epidemiologic investigations of the prevalence of this virus in macaque colonies. PCR analysis of blood samples can be performed and has allowed determination of a 44% prevalence of infection in one large macaque breeding colony (White et al., 2009b). Interestingly, RFHV was only detected in animals co-infected with RRV in this colony.
Pathogenesis and Pathology
Retroperitoneal fibromatosis is a multinodular to coalescent infiltrative process originating from the ileocecal junction and involving the root of the mesentery, mesenteric lymph nodes, and gastrointestinal tract. Prior to the identification of RFHV, RF was most frequently associated with Simian Retrovirus 2 (SRV2). While the detection of RFHV sequences is most often observed in SRV2 co-infected macaques, there are limited reports of SRV-negative/SIV-positive RFHV-associated mesenchymoproliferative lesions (Bielefeldt-Ohmann et al., 2005, Bruce et al., 2006). The RF lesion rarely infiltrates parenchymal organs but may produce small nodules or plaques disseminated across mesothelial surfaces. In severe cases, the entire gastrointestinal tract may be encased in a large fibrotic mass. Sclerotic and proliferative patterns of fibromatosis have been recognized and may coexist within the same animal. Histologically, the proliferative lesion is composed of spindle-shaped cells arranged in intersecting fascicles that infiltrate along serosal surfaces and surround normal structures. IHC using a cross-reactive anti-HHV8 LANA antibody demonstrates immunoreactivity within nuclei and large numbers of RFHV-infected cells in RF tissue (Bruce et al., 2006, Burnside et al., 2006). A variable lymphoplasmacytic inflammatory reaction is usually present. The tissue is highly vascular and cells are embedded within a stromal network composed of collagen and reticulin. Absence of nuclear anaplasia and mitotic figures distinguish the lesion from fibrosarcoma. These cells are vimentin, desmin, and smooth muscle α-actin-positive and reveal variable positivity for the factor VIII-related antigen (Tsai et al., 1995). An origin from vascular smooth muscle (pericyte) is favored. RF lesions have not been recognized in all large macaque colonies suggesting that RFHV is required but not sufficient for RF pathogenesis and that additional cofactors are required. Recognition of RF cases has declined in recent years and may be associated with elimination of SRV2 from SPF colonies (Figure 1.10 ).
FIGURE 1.10.

Retroperitoneal fibromatosis-associated herpesvirus (RFHV).
RFHV is a gammaherpesvirus that has been associated with mesenchymal proliferative disorders including retroperitoneal fibromatosis (RF) (A and insert), subcutaneous fibromatosis and intestinal stroma cell tumors. RF is found in association with simian retrovirus type 2 and often originates near the ileal–cecal lymph node and colonic mesentery (B). It is composed of pleomorphic spindle cells (C) and RFHV LANA can be identified by immunohistochemistry showing a characteristic punctuate staining pattern (D).
Zoonotic Potential
There are similarities in the histologic appearance and pathogenesis of RF and Kaposi’s sarcoma lesions suggesting that RFHV may be a model system with which to study aspects of pathogenesis. The inability to propagate the virus in culture limits the utility of such a model. There is no known zoonotic risk associated with RFHV.
Ateline Herpesvirus 2 and 3 and Saimiriine Herpesvirus 2
Introduction
Saimiriine herpesvirus 2 (Herpesvirus saimiri, HVS) and Ateline herpesviruses 2 and 3 (AtHV; AtHV2, AtHV3) are members of the Gammaherpesvirinae subfamily and the genus Rhadinovirus. Although a common asymptomatic infection of their natural hosts, inoculation of other species of primates results in the rapid development of malignant lymphoma or leukemia. Evidence of the ability of these viruses to induce disease in naturally infected hosts is more limited (Hunt et al., 1973).
Etiology
Like other Rhadinoviruses, HVS is an enveloped, double-stranded DNA virus with a 155-kb genome having approximately 75 open reading frames (Albrecht et al., 1992, Ensser et al., 2003). The genes encoding the “saimiri transformation-associated protein” (STP) and the “tyrosine kinase interacting protein” (Tip) have been extensively studied due to their roles in oncogenicity and cell transformation (Jung and Desrosiers, 1992, Jung et al., 1999).
Epizootiology
A large percentage of wild squirrel monkeys are infected with HVS and nearly all animals are seropositive by 1.5–2 years of age. Likewise, approximately 50% of spider monkeys are seropositive for AtHV strains. Although squirrel and spider monkeys represent the natural reservoir host for their respective viruses, little is known about the natural history and epidemiologic factors that govern transmission in these species.
Clinical Findings
Infection of the natural host is not associated with clinical signs. Experimental inoculation of HVS into owl monkeys, several species of tamarins and marmosets (Saguinus oedipus, S. fuscicollis, S. nigricollis, S. mystax, and Callithrix jacchus), howler monkeys (Alouatta caraya), and spider monkeys results in lymphoma or lymphocytic leukemia. Following inoculation, the time to development of lymphoma is variable but may be as short as 3 weeks. Generally one of three patterns occurs: (1) survival less than 40 days with development of disseminated lymphoma often associated with extensive necrosis and replacement of vital organs; (2) survival from 50–150 days with a less aggressive form of lymphocytic lymphoma involving multiple organs and associated with lymphocytic leukemia; and (3) survival over 150 days with localized, well-differentiated lymphocytic lymphoma. Limited studies indicate that certain HVS strains may be oncogenic in African green monkeys and macaques (Melendez et al., 1970, Knappe et al., 2000).
Experimental inoculation of AtHV into tamarins, marmosets, and owl monkeys induces lymphoma and leukemia. Although the virus has not been studied as extensively, the oncogenic properties of AtHV are similar to HVS.
Pathogenesis and Pathology
Microscopically, cells comprising the neoplastic infiltrate vary considerably in their degree of differentiation. In the most aggressive form the neoplastic cells are large, pleomorphic, and resemble reticulum cells or histiocytes. Many are polygonal to stellate and have a moderate amount of granular cytoplasm. In less aggressive forms the cells are more differentiated, resembling lymphocytes. Neoplastic cells are polyclonal and are of T lymphocyte origin. Ultrastructurally, bizarre convoluted nuclei are seen and viral particles are not visible. Virus may be recovered in cell culture from tumor explants.
Three different subtypes of HVS are recognized (A, B, and C) based on homology within the left end of the L-DNA (Jung et al., 1991). A and C types are highly oncogenic and transform peripheral blood lymphocytes of the common marmoset in vitro. The STP is encoded in the left terminus of the L-DNA of A and C strains and confers oncogenicity on individual isolates (Jung and Desrosiers, 1991, Jung and Desrosiers, 1992). It does not share sequence homology with other known viral oncogenes or cellular protooncogenes.
Treatment and Prevention
Natural infection of callitrichids with HVS has been reported infrequently. Exposure to tissue or blood products is probably necessary to produce infection in the inappropriate host.
Zoonotic Potential
HVS induces malignant lymphoma when inoculated into a variety of New World primate species and rabbits (Melendez et al., 1970). Its oncogenicity in other species has not been fully explored. HVS will infect and transform human T lymphocytes in vitro. These T cells have a mature, activated phenotype and maintain the antigen-specific reactivity of the parental cell. The transformed cells have cytotoxic capabilities and can continue to provide B cell help. Due to these capabilities, HVS transformed T cell lines have been investigated as a potential means to confer adoptive T-cell-mediated immunotherapy targeting diseases such as HIV/AIDS (Meinl et al., 1997, Kumar et al., 1999). The ability of HVS to persist as a high copy number episome and provide sustained gene expression has also prompted study of the virus as a potential gene vector (Griffiths et al., 2006).
Hepadnaviridae
Hepatitis B Virus
Introduction
Hepadnavirus B (hepatitis B virus, HBV) belongs to the genus Orthohepadnavirus of the family Hepadnaviridae and is an important cause of viral hepatitis in humans worldwide. Other important viruses within this family are woodchuck hepatitis B virus, duck hepatitis virus, and ground squirrel hepatitis B virus. Strains of HBV that are distinct from human strains have been described in both captive and wild populations of chimpanzees, gibbons, orangutans, and gorillas (Maynard et al., 1971, Zuckerman et al., 1978, Thung et al., 1981, Linneman et al., 1984, Norder et al., 1996, Verschoor et al., 2001). A HBV strain has also been described in one New World species, the woolly monkey (Lagothrix lagotricha) (Lanford et al., 1998). While most surveys have not been able to demonstrate infection in Cercopithecidae, there is one report of HBV seropositivity associated with clinical disease in cynomolgus macaques (Kornegay et al., 1985).
Etiology
Hepatitis B viruses are small (42 nm) enveloped DNA viruses with an icosahedral nucleocapsid containing 180 capsomeres. The genome is 3200 kb in length and consists of a single strand of circular DNA encoding the complete genome with a shorter incomplete complementary strand. The core consists of a DNA polymerase, the hepatitis B-core antigen (HBcAg), and the hepatitis B e-antigen (HBeAg). Additional proteins encoded include the surface antigen (HBsAg) and regulatory X protein. Detection of these antigens in the peripheral blood of infected human patients occurs in a predictable and sequential fashion and may be used in the diagnosis and staging of clinical disease.
There are eight human HBV genotypes described (A–H) and two additional, tentative genotypes (I and J) (Robertson and Margolis, 2002, Tran et al., 2008, Tatematsu et al., 2009). These genotypes are based on a greater than 8% divergence from one another and are found to cluster based on geographic region. Phylogenetic analyses of ape HBV genomes indicate that these viruses are genetically distinct from human HBV. As seen with human strains, genomic variants isolated from orangutans, chimpanzees, and gibbons cluster based on the geographic origin of the animals (Grethe et al., 2000, Hu et al., 2001, Verschoor et al., 2001, Starkman et al., 2003). The woolly monkey strain is the most divergent of the HBV viruses identified. These phylogenetic relationships are of great interest to researchers interested in determining the origins of human HBV infection.
Epizootiology
Transmission of HBV among humans occurs through intimate contact, blood-borne contamination, or vertical transfer. Vertical transmission from mother to child may represent the most significant route of exposure. The epizootiology of nonhuman primate transmission is poorly understood; however, as in humans, mother to infant transmission may play a critical role. Prolonged incubation and a chronic carrier state may be important in propagating the virus in nonhuman primate populations. Seroprevalence in gibbons and orangutans is estimated to range between 40–75% with approximately 15–25% of animals demonstrating a chronic carrier state (Noppornpanth et al., 2003, Sall et al., 2005, Sa-nguanmoo et al., 2008).
Pathogenesis and Pathology
In humans, a self-limiting hepatitis, fulminant hepatitis, and chronic carrier state are recognized. Chronic HBV infection is associated with a significant increased risk for the development of chronic active hepatitis and hepatocellular carcinoma. The histopathology of naturally occurring and experimental HBV infection of chimpanzees has been described (Dienstag et al., 1976). The pathology is similar to that described in humans with the exception that chronic active hepatitis and hepatocellular carcinoma have not been recognized as sequelae. Findings vary from essentially normal liver to the occurrence of moderate nonsuppurative inflammatory cell infiltrates centered within portal tracts and extending variably into the hepatic parenchyma. These latter findings are compatible with so-called “chronic persistent infection” of humans and in such specimens the typical “ground glass” hepatocytes containing viral antigen and scattered hepatocellular necrosis may be evident (Dienstag et al., 1976). The reported lesions described in cynomolgus monkeys were similar but qualitatively more severe and were accompanied by hepatocellular fatty change and Ito cell proliferation (Kornegay et al., 1985).
Clinical Findings
Infection of chimpanzees and gorillas demonstrated by serology is often asymptomatic. Experimental infection of chimpanzees produces mild anorexia, lethargy, and jaundice.
Laboratory Findings
Alterations in alanine aminotransferase and the presence of hepatitis B virus surface antigen (HBVsAg), anti-HBVsAg, and anti-HBVcAg have been described following experimental inoculation of chimpanzees (Dienstag et al., 1976). In these animals, HBVsAg peaked 3 months following infection and coincided with initial elevations in alanine aminotransferase. It was at this time that the initial histopathologic alterations were seen, which generally resolved with the apparent rise in anti-HBVc between 8 and 12 months.
Zoonotic Potential
Transmission of ape HBV strains to humans has not been documented. However, chimpanzees can be infected with human HBV strains and spider monkeys are susceptible to experimental infection with the woolly monkey strain (Lanford et al., 2003). These findings combined with the common occurrence of experimental and inadvertent exposure of chimpanzees to human HBV suggest that the risk of infection should not be underestimated. Bite wounds and needle stick injuries represent possible routes of transmission.
Nonenveloped DNA-Containing Viruses
Adenoviridae
Introduction
The family Adenoviridae encompasses a large number of ubiquitous viruses that affect a wide range of species worldwide. The family is divided into five genera. The genus Mastadenovirus contains the human and nonhuman primate isolates. The family name is derived from the fact that the original isolates were obtained from adenoids (diffuse lymphoid tissue of the nasopharynx) and tonsils of military recruits suffering from acute upper respiratory tract infection and conjunctivitis. Greater than 50 serotypes of adenoviruses have been isolated from nonhuman primate species, including macaques, African green monkeys, baboons, chimpanzees, gorillas, orangutans, squirrel monkeys, owl monkeys, and cotton-topped tamarins (Bullock, 1965, Kim et al., 1967, Eugster et al., 1969, Shroyer et al., 1979, Davis et al., 1992, Roy et al., 2009). Although adenoviruses have been confirmed as causing a mild to moderately severe respiratory or enteric disease of monkeys and apes, many isolates have been obtained from oral or anal swabs or from cell cultures derived from clinically healthy animals, suggesting that subclinical and possibly persistent infections of these species are common. A more severe disease has been described in animals whose immune systems are compromised either by immunosuppressive drugs or by concomitant infections, such as SIV and Betaretrovirus infections.
Etiology
Adenoviruses are nonenveloped, iscosahedral viruses, 70–90 nm in diameter with a capsid composed of 252 capsomeres. At the vertices of the icosahedron there are 12 capsomeres that are pentagonal in cross-section (pentons) and have filamentous glycoprotein projections with knobbly ends that protrude from their surfaces. The remaining 240 capsomeres are hexagonal in cross-section and are referred to as hexons. The adenovirus genome consists of a single linear molecule of double-stranded DNA that ranges from 26–45 kbp in length. Human adenoviruses are classified into six established species, A–F, and a recently identified seventh species, G (Jones et al., 2007). While genetic data on nonhuman primate adenoviruses are forthcoming, current evidence suggests that ape viruses group with human species B and E while those isolated from Old World monkeys group with human species A and F (Kidd et al., 1995, Kovacs et al., 2004, Roy et al., 2004). Included in the early region of the genome designated E1 are two genes, E1A and E1B, that encode proteins capable of transforming cells in vitro and causing neoplasms in hamsters and rats in vivo. These proteins have been shown to bind to and presumably inactivate the two well-known tumor suppressor genes, p53 and Rb, thereby causing malignant transformation. Despite the well-recognized ability of certain adenoviruses to experimentally transform cells in vitro and in vivo, these same agents have not been incriminated as the cause of naturally occurring neoplasia in any species.
Epizootiology
Adenoviruses occur worldwide where they cause sporadic and epidemic forms of disease in human beings and animals. In general, adenoviruses tend to be rather species specific, but, in some cases, closely related species may be infected. Initial infections are generally transmitted through aerosols generated by sneezing and coughing or through pharyngeal secretions of infected individuals. The fecal–oral route is also a significant route of transmission due to the persistence of infection in tonsilar tissue and intestinal epithelium long after the clinical illness has subsided. Healthy apes and macaques shed large amounts of virus in the stool with virus detected in 36–90% of nonhuman primates maintained in captivity depending on the species and facility type (Roy et al., 2009). Housing individuals under crowded conditions clearly promotes the spread of infection. Children and neonatal nonhuman primates are more susceptible to clinically apparent adenovirus infections than adults. The severity of infection varies widely depending on the serotype of the virus, the age and immune status of the patient, and the host species. As mentioned previously, many such infections remain clinically inapparent even though the patient is shedding large amounts of virus.
Pathogenesis and Pathology
Adenoviruses bind to specific receptors on susceptible cells by the filamentous projections that protrude from the penton capsomeres and are taken into the cell by endocytosis. In endosomes, the filament-bearing penton capsomeres are stripped from the particle, the pH within the endosome drops, and the endosome ruptures, spilling the partially degraded virion into the cytoplasm. These free particles bind to microtubules and are transported to the nuclear pores where the viral DNA is discharged into the nucleus, leaving the capsid in the cytoplasm. During the course of viral replication, cellular DNA and protein synthesis is severely disrupted, as is the normal processing of host messenger RNAs. These processes are incompatible with cell survival. Nuclei of cells containing actively replicating adenovirus develop large, amphophilic to basophilic inclusion bodies that either completely fill the nucleus or are surrounded by a prominent halo. Using electron microscopy, these inclusion bodies show prominent crystalline arrays of virions as well as unassembled viral capsid proteins and unencapsidated viral DNA.
The respiratory airways and lungs are common sites of adenovirus infection (Boyce et al., 1971, Umemura et al., 1985). Grossly, the affected portions of the lungs have patchy areas of firmness and gray–white or red discoloration that do not collapse on opening the thorax. Microscopically, epithelial cells of the trachea, bronchi, bronchioles, and alveoli are variably necrotic and contain basophilic, intranuclear inclusion bodies. These areas of necrosis and associated alveolar spaces typically are infiltrated by variable numbers of neutrophils and macrophages.
The conjunctiva and cornea may also be affected and appear edematous and congested with variable amounts of conjunctival exudate. Microscopically, the conjunctival epithelium contains areas of necrosis and intranuclear inclusion body formation.
The gastrointestinal tract is the second most common organ system affected by adenoviruses. Infection may be associated with diarrhea. Grossly, the mucosa of the stomach and small intestine may appear normal or somewhat congested and edematous. Microscopically, the mucosa contains focal and confluent areas of erosions or ulcerations in which necrotic enterocytes contain typical adenovirus inclusions (Baskin and Soike, 1989). There are also several reports of adenovirus pancreatitis in various macaque species (Chandler et al., 1974, McClure et al., 1978, Martin et al., 1991). It would appear from the literature that this lesion occurs most often in young macaques that have been severely immunocompromised by naturally occurring or experimentally induced infections including, SRV-1, SRV-2, and SIV (Chandler and McClure, 1982). The pancreas of affected individuals contains either white or red foci that correspond to areas of necrosis and hemorrhage microscopically. Histologically, this chronic active pancreatitis is characterized by lobular fibrosis and extensive necrosis often centered on intralobular ducts and infiltrated by large numbers of neutrophils. These foci are often dispersed among more normal-appearing lobules. The acinar and ductal epithelial cells may contain adenovirus inclusions.
Less commonly recognized forms of disease include necrotizing hepatitis and hemorrhagic cystitis and tubulointerstitial nephritis (Davis et al., 1992, Zoller et al., 2008). Intranuclear inclusions must be differentiated from those present in CMV, B virus, SV40, and measles virus infections. As with both CMV and SV40, some strains of adenovirus can be associated with significant nucleomegaly and cytomegaly (Figure 1.11 ).
FIGURE 1.11.

Simian adenoviruses.
Simian adenovirus infections are generally asymptomatic but may target pancreas (A and B), intestine (D and E) and liver (F) in immunocompromised hosts. Lesions are necrotizing (C, pancreas) and intranuclear inclusions are evident (C, D, and F). Immunohistochemistry may be used to demonstrate viral antigen in hepatocytes (E).
Clinical Findings
Monkeys and apes with primary adenovirus infections may appear clinically healthy and asymptomatic or may exhibit a variety of clinical signs, depending on the tropism of the virus. Clinically apparent infections of the respiratory tract are characterized by cough and hyperpnea and, when severe, by dyspnea and cyanosis. There may be an associated keratoconjunctivitis. Most animals, but especially adults, recover within a week to 10 days. Except in neonates, mortality is generally low and is often the result of secondary bacterial infection. Other animals may experience diarrhea as the result of viral replication in the enterocytes of the small intestine. As with the respiratory tract, recovery generally occurs within 2 weeks, but virus may continue to be shed in the feces for many weeks after the animal becomes asymptomatic. This persistent shedding of virus in the feces serves as the source of infection for other susceptible individuals. In rare cases, immunocompromised macaques may experience a severe necrotizing pancreatitis that may be associated with diarrhea and death.
Laboratory Findings
Virus may be detected using molecular techniques. Serological tests exist but detect only specific serotypes of adenovirus. Ultrastructural microscopy, immunohistochemistry, or in situ hybridization techniques may be used to demonstrate virus in tissue samples. Viral isolation may be performed on a variety of cell lines, including A549 (human lung carcinoma), Hep-2, HeLa, and KB (epidermoid carcinoma) cells. Clinically healthy animals may yield as many positive samples as animals that are clinically ill.
Treatment
There are no specific antiviral drugs commercially available for adenovirus infection. Consequently, treatment regimens are focused on preventing secondary bacterial infections and dehydration in the case of diarrheal illness. Supportive therapy to maintain caloric intake in animals that are severely anorexic is also beneficial (Figure 1.12 ).
FIGURE 1.12.
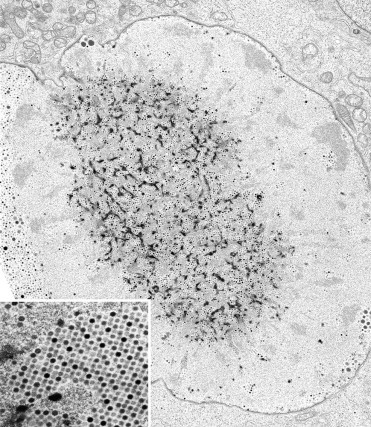
Adenovirus.
Enterocyte with intranuclear virions, rhesus macaque.
Prevention
Vaccines do not exist for specific serotypes of nonhuman primate adenoviruses. Accordingly, preventive measures to control or eliminate adenovirus infection in nonhuman primate colonies must be directed toward minimizing the exposure of susceptible populations to potentially infected aerosols or feces. Mixing of animals from different sources during the quarantine period should be avoided so as to reduce the risk of exposure of noninfected animals to those that may have been infected at their previous location. Minimizing overcrowding also reduces the risk of exposure by minimizing the concentration of virus in the environment. Measures to prevent cross-contamination of cages by feces that may contain infectious virus should also be in place.
Zoonotic Potential
Recombinant strains of human adenovirus are of interest as vectors for gene therapy and vaccines. Recombinant vaccines based on human serotype 5 generate robust cellular and humoral immunity although prior natural infection, and presence of neutralizing antibodies may lead to reduced vaccine efficacy (Shiver et al., 2002, Santra et al., 2005). Because of this limitation, there has been interest in the use of engineered simian strains as vectors (Roy et al., 2006, Tatsis et al., 2006). Recent surveys for pre-existing immunity to simian strains have demonstrated presence of antibodies to chimpanzee adenoviruses within human populations from sub-Saharan Africa (Xiang et al., 2006, Dudareva et al., 2009). While it is possible that human and chimpanzee antibodies may be cross-reactive, the presence of these antibodies suggests potential for cross-species transmission.
Polyomaviridae
Viruses within the family polyomaviridae have been of considerable scientific interest because of their oncogenic and transforming effects on mammalian cells and because of their ability to induce disease in immunocompromised hosts. These viruses are small (45 nm) and have a naked icosahedral capsid with 72 capsomeres and 420 structural subunits. Members of the family recognized to infect primates include Simian virus 40 and cynomolgus polyomavirus, baboon polyomavirus 1 (SA-12 or polyomavirus papionis 1) and 2 (polyomavirus papionis 2) and JC and BK virus in man. Novel and not yet fully characterized viruses have also been recognized in chimpanzees and squirrel monkeys (Johne et al., 2005, Verschoor et al., 2008a).
Polyomavirus Macacae: Simian Virus 40
Introduction
Simian virus 40 (SV40) and closely related polyomaviruses are common latent viral infections of feral and captive Asian monkeys, including rhesus, cynomolgus, Japanese, and Formosan rock macaques (M. cyclopis). Based on limited sequence data Cynomolgus polyomavirus (CPV) is a distinct genotype reported in normal and immunosuppressed cynomolgus macaques (van Gorder et al., 1999). Considerable genetic heterogeneity has been observed in SV40 isolates from naturally infected animals (Lednicky et al., 1998). Simian virus 40 was originally isolated from normal rhesus kidney cell cultures and subsequently from cell lines used for the production of killed poliovirus vaccine from 1954 to 1963. Because of its ability to readily transform cells in vitro and to produce malignant neoplasms in vivo, SV40 is one of the most extensively studied nonhuman primate viruses. Although its capacity to induce neoplasms in suckling hamsters is well established, its association with human disease is controversial.
Etiology
Simian virus 40 is classified in the polyomavirus genus of the family Polyomaviridae. Its genome consists of double-stranded circular DNA approximately 5000 bp in length, encompassing early- and late-coding regions and a noncoding regulatory region. Two DNA-binding proteins coded within the early region are referred to as the large and small T (tumor) antigens and bind to viral regulatory sequences to facilitate the transcription of late viral genes. These genes have been studied extensively and are used in the construction of transgenic animals due to their transcriptional promoting activity. Evidence from macaques suggests that distinct subtypes of SV40 may exist that differ in their genomic sequence and ability to induce disease. Based on the genetic analysis of the SV40 regulatory region, a protoarchetypal strain of SV40 has been identified in neotropical Goeldi’s monkeys (Callimico goeldii) suggesting that the host range of viral genotypes may be more extensive than previously recognized (Zdziarski et al., 2004).
Epizootiology
Although serologic evidence indicates that infection of captive macaques with SV40 is common, clinical disease is rare and is usually associated with some immunosuppressive disease or immunomodulatory therapy. While common in captive colonies, surveys of wild or feral populations indicate markedly different infection rates. For example examination of Mauritian cynomolgus macaques reveals low seroprevalence rates (8.8%), while serosurveys of rhesus macaques in Nepal reveals that more than 90% of the animals are positive as adults (Jones-Engel et al., 2006, Verschoor et al., 2008b). The principal mode of transmission has not been investigated but the identification of infected renal epithelial cells in immunologically normal animals suggests that urinary excretion may play a role. Pathology results from reactivation of latent infection or primary infection during periods of immunosuppression. Concurrent natural or experimental infection with SIV has been demonstrated in multiple cases (Horvath et al., 1992).
Clinical Findings
The vast majority of animals infected with SV40 do not show clinical signs and harbor the virus asymptomatically. The occurrence of clinical disease should prompt a search for an underlying immunosuppressive agent (most likely SIV). When disease does occur, most animals are in the terminal stages of AIDS with severe depletion of CD4+ T lymphocytes. At this time, clinical signs may relate to CNS or renal involvement or to a host of other opportunistic infections often present at death. Due to the slowly progressive nature of the disease in SIV-infected animals, the CNS lesions in the white matter may be advanced before clinical signs are evident (Figure 1.13 ).
FIGURE 1.13.

Simian virus 40 (SV40).
SV40 causes progressive multifocal leukoencephalomalacia (PML) (A through D) in SIV-infected macaques characterized by demyelinization and gliosis within white matter tracts. Virus may be demonstrated by in situ hybridization (C) or immunohistochemistry and intranuclear inclusions may be observed in oligodendrocytes (D). SV40 may also cause an interstitial nephritis (E) and pneumonitis.
Pathogenesis and Pathology
Lesions are confined to the brain, lung, and kidney. The brain lesion consists of multifocal to confluent regions of demyelination scattered throughout the cerebral white matter and subependymal regions. Demyelination can be confirmed by Luxol fast blue staining and results from direct viral injury to oligodendroglial cells. Large basophilic inclusions fill and enlarge nuclei of oligodendrocytes and astrocytes. As the lesion progresses, gitter cells and astrocytes predominate. These lesions resemble progressive multifocal leukoencephalopathy (PML) of humans with JC virus reactivation. Immunohistochemistry or in situ hybridization can be used to confirm SV40 infection.
Renal lesions are found primarily within the inner cortex and medulla. Collecting tubules are lined by hypertrophic/hyperplastic and occasional dysplastic epithelial cells containing typical viral inclusions. These inclusions are often found in the desquamated epithelial cells present in tubular casts. Their large size and deep basophilic color often make them visible under the lowest magnification. These findings are accompanied by a chronic nonsuppurative tubulointerstitial nephritis, fibrosis and glomerular sclerosis, and atrophy. It is postulated that renal lesions may predominate in macaques acquiring infection during immunosuppression, whereas CNS lesions are more likely to occur following a recrudescence of latent infection (Horvath et al., 1992). Disease phenotype may be dependent on viral genotype; for example CPV has been associated exclusively with interstitial nephritis. Pulmonary lesions are seen less frequently and consist of a proliferative interstitial pneumonia with intracellular viral inclusions present within hypertrophied type 2 pneumocytes.
A distinct SV40-induced meningoencephalitis without demyelination has been recognized in SIV-inoculated macaques with AIDS (Simon et al., 1995). In these animals, polyomavirus infection of astrocytes rather than oligodendrocytes predominates. Sequencing data suggest that this manifestation may be the result of a viral strain of SV40 distinct from that which causes PML. A SV40 molecular clone has recently been developed and with a unique tropism for neurons (Dang et al., 2008).
SV40 sequences have been detected in a malignant astrocytoma from an SIV-infected macaque (Hurley et al., 1997). However, an etiologic relationship to the neoplasm could not be determined (Figure 1.14 ).
FIGURE 1.14.

Simian virus 40 (SV40).
Electron photomicrograph of cytoplasmic and intranuclear viral particles, rhesus macaque.
Laboratory Findings
In SIV-infected macaques with renal lesions, clinicopathologic findings included a mild hypochromic microcytic anemia and moderate elevations in BUN and creatinine. Because SV40 is found ubiquitously in macaques, the ability to isolate this virus from tissue is not definitive evidence of its role as a pathogen in an individual animal. The demonstration of polyomavirus virions by electron microscopy or polyomavirus DNA/RNA by in situ hybridization in typical lesions is diagnostic.
Treatment and Control
No specific treatment for SV40 is available. Use of seronegative donors and recipients may be required to prevent disease in solid organ transplantation protocols. Specific pathogen-free breeding colonies have been developed.
Zoonotic Potential
The zoonotic potential of SV40 is controversial (Bergsagel et al., 1992). Although large numbers of individuals have been exposed to SV40 through contaminated poliovirus vaccine, a clear relationship to any human disease has not been demonstrated (Melnick and Butel, 1988). Moreover, although SV40 has been isolated from human neoplasms, the widespread contamination of cell lines by SV40 has interfered with the interpretation of these results. Serological responses to SV40 have been detected in zoo workers with primate contact consistent with its zoonotic potential (Engels et al., 2004).
Baboon Polyomavirus 1 (Polyomavirus Papionis-l; SA12)
SA12 is a papovavirus closely related to, but distinct from, the human papovavirus, BK virus. While initially isolated from a vervet monkey, only natural infection of baboons (Papio sp.) has been demonstrated (Valis et al., 1977). Serological responses have also been detected in Patas monkeys and macaques (Braun et al., 1980). Like SV40, SA12 transforms cells and has produced neoplasms in hamsters. Complete viral sequences have been described (Cantalupo et al., 2005). While the virus circulates in baboon colonies, natural disease in normal or immunosuppressed animals has not been reported. This virus has not been associated with natural disease and is more closely related to BK virus than to JC virus or SV40 (Cunningham and Pipas, 1985).
African Green Monkey Polyomavirus (Polyomavirus Cercopitheci; Lymphotropic Papovavirus)
Lymphotropic virus (LPV) is a papovavirus originally isolated from a B-lymphoblastic African green monkey cell line and is unique in its B-cell tropism (zur Hausen et al., 1979). It is related to, but distinct from, other primate polyomaviruses and appears to be most closely related to the recently recognized human Merkel cell tumor virus (Feng et al., 2008). Serological surveys indicate that humans and a variety of nonhuman primates are immunoreactive for LPV or an antigenically related virus (Takemoto et al., 1982). This virus induces tumors in hamsters but has not been associated with naturally occurring disease in primates.
Recent evidence has demonstrated a zoonotic potential for LPV (Delbue et al., 2010). LPV genomic sequences have been detected in the peripheral blood mononuclear cells of both HIV-infected and -noninfected individuals.
Polyomavirus Hominis-2 and Polymomavirus Hominis-1: JC Virus and BK Virus
Infection of humans with JC virus and BK virus is a common occurrence in childhood and while not associated with clinical disease, the virus persists for life. Recrudescence may occur with immunosuppression, leading to viral tubulointerstitial nephritis in the case of BKV and progressive multifocal leukoencephalopathy in the case of JCV. A closely related virus (SV40) causes a progressive demyelinating disorder in SIV-inoculated macaques with many clinicopathologic similarities to PML. Despite the fact that a variety of nonhuman primates are susceptible to infection with SV40, there is a paucity of information concerning the occurrence of JCV and BKV in these species. Natural infection of nonhuman primates with these viruses reportedly does not occur (Shah, 1990). Owl monkeys were susceptible to intracerebral inoculation of JCV and developed primitive neural tumors after a prolonged incubation (London et al., 1978, Miller et al., 1984, Major et al., 1987).
Papillomaviridae
Introduction
Over 100 different papillomaviruses have been recognized in human beings, many with strict site specificity and different tendencies to promote the malignant transformation of keratinocytes. Fewer, approximately 47 at the time this chapter was written, have been recognized in nonhuman primates. It is likely that more will be identified and that the spectrum of disease with which they are associated will increase. Papillomavirus infection has been demonstrated in the Colobus monkey (Colobus guerza), spider monkey, howler monkey (Alouatta fusca) rhesus macaque, cynomolgus macaque, chimpanzee, pygmy chimpanzee (P. paniscus), and gorilla (Tate et al., 1973, Kloster et al., 1988, Sundberg et al., 1992, Sa et al., 2000, Antonsson and Hansson, 2002). A viral etiology has been suspected in a number of benign and malignant proliferative disorders in a variety of species, including cebus monkeys, baboons, and a squirrel monkey (Sundberg and Reichman, 1993).
Etiology
Papillomaviruses are spherical, double-stranded DNA viruses 50–55 nm in diameter with a capsid composed of 72 capsomeres. This capsid consists of two proteins: the major capsid protein, which comprises approximately 80% of the total viral protein, and a slightly larger minor capsid protein. Their genome is approximately 8 kb in size and encodes ten open reading frames. Research has focused on transregulatory factors (El and E2) and early viral proteins (E6 and E7) and their role in host cell growth dysregulation. Most papillomaviruses identified in nonhuman primates are of the Alphapapillomavirus genus although recently a Betapapillomavirus was identified in cynomolgus macaques (Joh et al., 2009). Papillomaviruses cannot be successfully grown using traditional cell culture methods.
Epizootiology
The epizootiology of papillomavirus infection of nonhuman primates is unknown. In other species, initial infection occurs primarily in the young and is thought to require defects in the superficial layers of the dermis. The virus is spread by fomites, by direct contact, or as a sexually transmitted disease. Lesions may be multiple and generally resolve with the mounting of an effective immune response. Not all primate papillomaviruses show strict species specificity with some strains identified in both cynomolgus and rhesus macaques (Wood et al., 2007, Chen et al., 2009b).
Pathogenesis and Pathology
Viral infection of skin or oral mucosa produces an exophytic mass that is often described grossly as “cauliflower like” and may reach 1–2 cm in diameter. Histologically, there is massive hyperplasia of the stratum spinosum and corneum. Basophilic intranuclear inclusions are occasionally found in the stratum spinosum. Acidophilic intracytoplasmic inclusions likely represent keratohyaline granules. The formation of rete ridges that invest the mass with blood vessels and contain scattered inflammatory cells often accompany epidermal proliferation. Infection with some viruses may induce massive fibroblastic proliferation (fibropapilloma) in which viral antigen may be detected.
Specific strains of human papillomavirus (HPV) including HPV 16 and 18 are associated with an increased risk of cervical carcinoma. Viral DNA in these carcinomas and in viral-induced dysplastic lesions is present in an integrated form. If integration disrupts the E2 open reading frame, the normal control of early viral genes (E6/E7 products) is disrupted. These gene products can promote unregulated cell growth by two mechanisms: E7 protein binds to and deactivates the tumor suppressor protein (pRb) and E6 protein facilitates the degradation of another tumor-suppressing protein (p53). HPV types associated with cervical carcinoma apparently contain early viral proteins with high affinity for pRb and p53, whereas HPV types associated with low cancer risk are not. Other genetic and environmental factors likely act in conjunction with HPV infection to complete carcinogenesis. Papillomavirus DNA with similarities to HPV 16 was detected in a penile squamous cell carcinoma with lymph node metastasis in a rhesus macaque (Kloster et al., 1988, Ostrow et al., 1991). Female macaques that had mated with this male or were exposed via intermediate sexual contacts demonstrated a 71% incidence of papillomavirus infection that was detected clinically, histologically, or via molecular methods (Ostrow et al., 1990). Of the females surveyed, one presented with an adenosquamous carcinoma of the endocervix and another with a squamous cell carcinoma of the cervix. These initial reports indicated that genital infection with this genus of viruses may be carcinogenic for nonhuman primates, as is the case in humans. Subsequent surveys by Wood and colleagues demonstrated up to a 19% overall prevalence of cervical intraepithelial neoplasia (CIN) in cynomolgus macaques (Wood et al., 2004, Wood et al., 2007). Lesions were highly associated with presence of papillomavirus infection and included benign vaginal papillomas, mild to severe intraepithelial dysplasia, and occasional invasive cervical carcinomas. Key morphologic features included koilocytosis, nuclear atypia, anisokaryosis, expansion of the basal epithelium, and presence of epithelial pearls. Transfer of exfoliated cervicovaginal cells propagated infection and initiated lesion development in a subset of animals (Wood et al., 2007).
Focal epithelial hyperplasia has been described as a distinct entity in chimpanzees (P. troglodytes and P. paniscus) although a recent report describes a similar pathology in a howler monkey (Hollander and van Noord, 1972, Tate et al., 1973, Van Ranst et al., 1991, Glad and Nesland, 1995, Sa et al., 2000). Multiple, sessile, well-circumscribed proliferative structures 0.2–0.5 cm in diameter are described within the oral mucosa. These may persist for extended periods and undergo spontaneous regression. Histologically, the most striking feature is marked irregular acanthosis with the formation of anastomosing rete ridges. Typical papillomavirus virions have been demonstrated in some cases.
A recent report describes “butcher’s warts” with a flat, verrucous appearance on the hands and feet of a cynomolgus macaque (Joh et al., 2009). These lesions were associated with the first nonhuman primate papillomavirus identified as belonging to the Betapapillomavirus genus (MfPV-1). Viruses of this genus have a predilection toward growth in cutaneous tissue and are often associated with persistent wart-like lesions with potential for malignant transformation in immunosuppressed human patients. Presence or a cause of immune compromise was not identified in the affected animal.
Treatment and Prevention
Formalin-inactivated autologous vaccines have been used in the treatment and prevention of papillomavirus infection in cattle and dogs. Their effectiveness is not established.
Parvoviridae
Simian Parvovirus
Introduction
Parvoviridae represent some of the smallest known vertebrate viruses. They are nonenveloped, measuring 18–26 nm in diameter with icosahedral symmetry and a genome consisting of a single strand of DNA. This simple genome is only 5 kb in length and encodes three proteins, a nonstructural protein NS-1 and two structural proteins, VP1 and VP2. In addition to nonhuman primates, parvovirus infections have been recognized in cats, dogs, swine, mink and ferrets, rats, mice, and humans.
Etiology
Simian parvovirus (SPV) was first described in cynomolgus macaques concurrently infected with the simian-type D retrovirus (O’Sullivan et al., 1994). SPV resides within the erythrovirus genus and shares 65% homology at the DNA level with human B19 parvovirus within the major capsid protein. Subsequently, similar parvoviruses have been recognized in several other species of macaques including rhesus and pig-tailed macaques. Systematic surveys of other nonhuman primate species have not been conducted.
Epizootiology
Infection of a cohort of five cynomolgus monkeys and two additional contacts was demonstrated in an initial report (O’Sullivan et al., 1994). Serological surveys indicate that SPV and related erythroviruses may be common and yet unrecognized infections in captive macaque colonies. As in human patients with parvovirus B19, it is suspected that infection with SPV is usually asymptomatic and clinical disease is rarely seen. The mode of natural transmission is unknown, although it appears direct contact may not be required. Intranasal inoculation does result in experimental transmission (O’Sullivan et al., 1997).
Pathogenesis and Pathology
Human infection with parvovirus B19 has been associated with a variety of clinical syndromes, including profound anemia, polyarteritis, fetal loss, and erythema infectiosum (Pattison, 1994). The pathogenesis of these disorders is poorly understood; however, a propensity for the virus to infect rapidly dividing cells likely contributes to clinical manifestations.
Infected macaques have a variety of gross and microscopic lesions, some of which may have been attributable to an underlying immunosuppressive process. Bone marrow reveals marked dyserythropoiesis with a loss of mature erythroid elements and increased numbers of atypical erythroid precursors (pronormoblasts). Large intranuclear inclusion bodies are evident and typical 24-nm-diameter viral particles are observed ultrastructurally. While initially described in SRV-infected cynomolgus macaques, disease has now been reported in animals on solid organ transplantation studies receiving immunosuppressive therapy and in SHIV/SIV-infected macaques (Foresman et al., 1999, Green et al., 2000, Schroder et al., 2006, Simon, 2008).
Experimental inoculation of cynomolgus macaques with SPV by the intravenous or intranasal route results in a transient subclinical anemia with reticulocytopenia (O’Sullivan et al., 1997). In these immunologically normal animals these alterations rapidly resolved (Figure 1.15 ).
FIGURE 1.15.

Simian parvovirus (SPV).
SPV may be associated with anemia and in the bone marrow dyserythropoiesis (A). Brightly eosinophilic intranuclear inclusions may be observed (B) and infection confirmed by in situ hybridization (C).
Clinical Findings
Clinical signs in infected macaques are varied and have included diarrhea, dehydration, and moderate to severe normocytic/normochromic anemia often with reticulocytopenia. Animals may present in acute anemic crisis or may have periods of anemia that may appear to resolve spontaneously.
Laboratory Findings
Affected macaques may have a severe normocytic normochromic anemia which may appear regenerative or nonregenerative. In initial reports, parvoviral DNA was demonstrated in the serum of affected animals by dot-blot hybridization using radiolabeled B19 human parvovirus genomic probes. PCR and serological assays have been developed and work in cynomolgus macaques suggests that both antibody-positive nonviremic and antibody-negative viremic states may occur. Definitive diagnosis may require both serological assays and PCR to determine the true viral status. Typical 24-nm parvovirus particles could also be found by electron microscopy within bone marrow and histologic findings can be confirmed by in situ hybridization.
Treatment
No antiviral treatment is available. Use of corticosteroids may help alleviate signs associated with acute hemolytic crisis. If animals are to be used in transplantation studies, screening by serology and PCR to assure that animals are free of SPV may be beneficial.
Zoonotic Potential
SPV can replicate in human bone marrow stromal cells and serological responses to SPV have been detected in individuals with and without primate contact (Brown et al., 2004). The zoonotic potential of SPV remains controversial as the specificity of serological assays and ability to distinguish SPV from B19 infection in man is uncertain (Simon, 2008).
Enveloped RNA-Containing Viruses
Rhabdoviridae
The family Rhabdoviridae encompasses a diverse group of over 100 viruses that infect a number of mammals, plants, reptiles, fish, and crustaceans. They are linked by a common rod-shaped (Rhabdo) ultrastructural appearance. Genera Lyssavirus and Vesiculovirus, respectively, contain the agents of rabies and vesicular stomatitis, the viruses of importance to nonhuman primates.
Rabies Virus
Introduction
Rabies has been reported in whitelipped tamarins, golden lion tamarins (Leontopithecus rosalia), common marmosets, squirrel monkeys, capuchin monkeys, cynomolgus macaques, rhesus macaques, and chimpanzees (Fiennes, 1972, Richardson and Humphrey, 1971, Favoretto et al., 2001). Given the wide host range of this virus, its occurrence in other nonhuman primate species would not be unexpected.
Etiology
The occurrence of rabies in captive-bred nonhuman primates is extraordinarily rare, and present-day housing practices do not facilitate exposure to carrier species. Nonetheless, potential exposures do occur (Smith et al., 1987) and the serious zoonotic potential of this agent dictates that it be considered in sporadic cases of encephalitis. In regions of the world where rabies is endemic, populations of wild nonhuman primates may represent important vectors in the transmission of the virus to domestic species and humans. A report from the state of Ceará, Brazil attributed at least eight cases of human rabies deaths to transmission by rabid common marmosets (Favoretto et al., 2001). Viral isolates did not correspond to previously identified variants of rabies virus and were suggested to represent a unique strain. The tendency in this region to capture and raise marmosets as pets as well as the close proximity of marmoset habitats to urban centers are factors thought to contribute to the emergence of this cycle.
Epizootiology
Although nonhuman primates obviously become infected by contact with reservoir or inadvertent host species, the viral strains, vectors, and factors governing this transmission are unknown. Evidence suggests that several New World species and baboons may become infected when vaccinated with attenuated strains (Bingham et al., 1992, Potkay, 1992).
Pathogenesis and Pathology
Following experimental infection of rhesus macaques, furious rabies develops in 15–35 days, whereas in the dumb or paralytic form this period may extend to 105 days. The incubation in natural cases is unknown. Evidence in humans suggests that the incubation period may be as long as 6 years or more in unusual cases (Smith et al., 1991).
Following exposure, initial replication of the virus occurs within myocytes at the site of inoculation and the virus is transported through peripheral nerves to the CNS where it spreads rapidly and exclusively infects neurons. Transmission between cells is mediated by the passage of ribonucleoprotein across synaptic junctions and exocytosis of complete virions. Centrifugal spread to peripheral organs occurs near the time of the onset of clinical signs, resulting in widespread dissemination of the virus at the death.
Microscopically, lesions vary from few recognized changes to marked formation of glial nodules, perivascular, nonsuppurative encephalitis, and neuronal degeneration. Negri bodies, which may consist of prominent, eosinophilic intracytoplasmic inclusions within nerve bodies, likely represent the defective assembly of precursor proteins. The changes are qualitatively similar to those seen in other species. Fluorescent antibody testing may be used to confirm the diagnosis on frozen neural tissue (Figure 1.16 ).
FIGURE 1.16.

Rabies virus (RV).
RV intracytoplasmic inclusions (Negri body).
Clinical Findings
Both the furious and the paralytic forms of rabies have been recognized in nonhuman primates. Reported clinical signs include self-mutilation, irritability, and paralysis of pharyngeal and pelvic muscles (Bougler, 1966, Fiennes, 1972). These findings are often not suggestive of the diagnosis.
Prevention
Facilities that house nonhuman primates in indoor/outdoor configurations may include rabies vaccination as a component of preventative health care regimens (Nieves et al., 1996). Vaccination with killed vaccines induces a neutralizing antibody response although the efficacy of such vaccination for preventing transmission in nonhuman primates is unknown. The use of attenuated vaccines is contraindicated as they have been implicated in the occurrence of vaccine-induced disease. An inactivated hamster diploid cell vaccine has been used for post exposure prophylaxis in white-handed gibbons potentially exposed to a rabid bat (Smith et al., 1987). A killed vaccine combined with human rabies immune globulin has also been used prophylactically in capuchin monkeys exposed under similar circumstances (Kenny et al., 2001). Similar protocols might be considered in valuable animals if proper quarantine facilities exist and with the realization that the natural incubation period in primate species is unknown. Nonhuman primates are frequently utilized as animal models for the development and efficacy testing of novel vaccines and vaccine regimens for the prevention of rabies (Cenna et al., 2009, Franka et al., 2009).
Zoonotic Potential
Rabies is a significant zoonotic threat, and nonhuman primate to human transmission has been documented.
Vesicular Stomatitis Virus
Antibodies to vesicular stomatitis virus have been identified in Geoffroy’s marmoset (Saguinus geoffroyi) in Panama (Srihongse, 1969). No clinical signs were associated with seropositivity. Phlebotomine sandflies may carry the virus, which causes vesicular lesions in the oral mucosa of a variety of mammalian species. The role of New World primates in the arborial cycle of this virus is unknown.
Recombinant vesicular stomatitis virus has shown considerable promise as a vaccine vector for expression of recombinant proteins of HIV, Ebola virus, Lassa fever virus, and other viruses (Clarke et al., 2006). Replication competent vaccines must undergo stringent safety testing including assessments of neurovirulence. Intranasal or intramuscular inoculation has not been associated with adverse effects. However, depending on the strain, intrathalamic inoculation in cynomolgus macaques may result in moderate to severe necrotizing menigoencephalitis (Johnson et al., 2007). This has created recent interest in strategies to further attenuate vaccine strains of this virus.
Vesicular stomatitis virus infection in cattle can result in significant morbidity and is clinically indistinguishable from foot-and-mouth disease. Because of the significant impact to agriculture, it is included in the list of select agents compiled by the United States Department of Agriculture. Vesicular stomatitis virus is transmissible to humans and may present as a flu-like illness (Brandly and Hanson, 1957).
Filoviridae
Filoviruses
Introduction
In 1967, an outbreak of hemorrhagic fever was identified in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia (Smith et al., 1967). The disease was seen in laboratory workers preparing cell lines derived from African green monkey tissue. Twenty-five primary cases with seven deaths were recorded. In addition, the agent spread to an additional six contacts. Reportedly the shipment of African green monkeys from which tissue had been obtained was normal and the source of infection in these animals remains unknown. An interesting account of the management practices concerning the procurement of these animals has been given and suggests that animals were infected in Uganda prior to shipment (Smith, 1982). The first filovirus, Marburg virus, was isolated and characterized from this outbreak. Since that time, isolated and small clusters of cases caused by an antigenically identical virus were identified in Zimbabwe, Kenya, and Uganda. In 2005 a more extensive outbreak occurred in Angola with over 200 infection-associated fatalities (WHO, 2005).
The first members of a second group of closely related viruses (Ebola subtype Zaire and Ebola subtype Sudan) were identified in 1976 as the etiologic agent of an epidemic of hemorrhagic fever in Zaire (present day Democratic Republic of the Congo (DRC)) and Sudan, respectively. During these simultaneous outbreaks, 500 human cases with over 430 deaths were recognized. The virus was named Ebola virus after the Ebola river in northwest DRC. As with the Marburg virus, secondary and tertiary cases occurred in contacts. In these contact cases, lower mortality suggested attenuation of the virus. A distinct viral strain, Ebola Côte-d’Ivoire, was isolated from a 34-year-old female patient with a dengue-like syndrome after she necropsied a chimpanzee (Guenno et al., 1995). Ebola hemorrhagic fever resulted in over 250 deaths during 1995 in Kikwit, DRC, due to Ebola Zaire and over 200 deaths in Gulu, Uganda, due to Ebola Sudan in 2000 (CDC, 1995, WHO, 2001). Since 1995 smaller outbreaks have also occurred in Central and Western Africa. The most recent outbreak in 2007 in Uganda was associated with an Ebola virus distantly related to Ebola Côte-d’Ivoire that was tentatively designated Ebola Bundibugyo (Towner et al., 2008).
A distinct filovirus subtype (Ebola Reston) was identified in 1989 and 1990 during an outbreak of hemorrhagic fever in newly imported Asian macaques. The animals originated from the Philippines and were housed at primate quarantine facilities in Virginia, Pennsylvania, and Texas. The virus caused a contagious, hemorrhagic fever with high mortality in cynomolgus monkeys. Although no clinical signs or deaths were recorded in human contacts, inadvertent infection of animal handlers in both United States and Philippine facilities did occur (CDC, 1990a, CDC, 1990b). In 1992, a similar virus was identified in Siena, Italy, in macaques obtained from the same Philippine source (WHO, 1992). The virus resurfaced again in Texas in 1996 after which the Philippine facility was depopulated and permanently closed (CDC, 1996).
Etiology
The family Filoviridae contains one genus (Filovirus) with two distinct viruses: Marburg and Ebola. Four species of Ebola virus are currently recognized by the ICTV (Zaire, Sudan, Reston, and Côte-d’Ivoire). One species of Marburg virus is recognized (Lake Victoria) although this species is comprised of at least nine genetically distinct strains with up to 21% divergence at the nucleotide level (Bausch et al., 2006, Towner et al., 2006). These viruses have a single-stranded antisense RNA genome 12.7 kb in size. Morphologically, the virus forms distinctive filamentous, sometimes branching, structures 800–1000 nm in length and 80 nm in diameter. This structure is surrounded by a closely adherent host membrane in which 10-nm peplomers are found. The virus replicates by budding, and nucleocapsids accumulate in the cytoplasm, forming closely packed arrays of bundled filaments that are visible microscopically as viral inclusions (Murphy et al., 1971b).
Epizootiology
Factors that allow filoviruses to become established in primate hosts are unknown. A reservoir host has not yet been definitively identified although reports implicate fruit bats as a potential reservoir for both Ebola and Marburg viruses (Leroy et al., 2005, Leroy et al., 2009, Towner et al., 2009). Viral antibodies to both Marburg and Ebola have been detected in healthy, free-roaming and wild-caught chimpanzees, gorilla, African green monkeys, drills, mandrills, and baboons (Johnson et al., 1982, Leroy et al., 2004) indicating that infection is not always lethal for these species. Seroprevalence is reportedly as high as 12.9% in wild-born chimpanzees and 6.7% in gorillas (Leroy et al., 2004). Ongoing outbreaks of Ebola have reportedly resulted in the death of up to 90% of gorillas from certain regions of Western Africa (Bermejo et al., 2006). Human outbreaks often coincide with outbreaks in ape populations. Once established in the human population, Marburg, Ebola Sudan, and Ebola Zaire viruses were transmitted between close contacts and to medical personnel. The Ebola Reston virus was transmitted between macaques by direct contact, fomites, and aerosolization.
The Reston outbreak was associated with concurrent infection with an arterivirus (simian hemorrhagic fever virus (SHFV)) (Dalgard et al., 1992). This infection causes a fatal hemorrhagic fever of macaques, but is carried asymptomatically by a number of African primates, principally the Patas monkey. This suggests that while macaques involved in the Reston outbreak were obtained from the Philippines, they may have become infected by inadvertent contact with African species. Inappropriate husbandry procedures such as reuse of needles and failure to organize flow of work may have contributed to the spread of virus within the Philippine facility (Miranda et al., 2002). Recently, Reston Ebolavirus has been isolated in the Philippines from pigs in association with a severe outbreak of porcine reproductive and respiratory disease syndrome (Barrette et al., 2009). These findings suggest that concurrent arterivirus infection may exacerbate the severity of Reston Ebolavirus disease.
Pathogenesis and Pathology
The pathogenicity of filoviruses is dependent on the viral strain and species affected. Generally, the African filovirus, Ebola Zaire, is considered the most pathogenic, followed by Ebola Sudan, Marburg, and Ebola Reston (Fischer-Hoch et al., 1992). Certain strains of Marburg, specifically the Angola strain, may demonstrate increased pathogenicity. Macaques are thought to be more susceptible to filovirus infection than African green monkeys.
The pathophysiology of filovirus infection is most thoroughly elucidated for Ebola virus with many of the pathways determined from study of disease progression in macaque models. Macrophages and monocytes are the initial targets of infection and distribute virus to secondary sites of replication. Mediators released from monocytes and macrophages are thought to recruit additional macrophages thus increasing the number of viral target cells. These mediators additionally may cause endothelial cell activation and breakdown of endothelial integrity contributing to hypovolemic shock. Disseminated intravascular coagulation results in microvascular thrombosis and secondary multi-organ failure. Tissue factor release from infected monocytes is thought to be a key mediator in the induction of DIC in Ebola virus infections (Geisbert et al., 2003). Upregulation of tissue factor and subsequent fibrin deposition is a less prominent feature of Marburg viral infections (Geisbert et al., 2007).
Declines in lymphocyte numbers are observed and thought to be due to bystander apoptosis. Experimental infection of cynomolgus macaques with Ebola Zaire resulted in a 60–70% decrease in CD4+ and CD8+ T cell counts with the loss of CD8+ cells most prominent in the natural killer (NK) population (Reed et al., 2004a). Loss of these cell populations is thought to contribute to failure to mount an effective immune response. Marburg virus infection of cynomolgus macaques is associated with a more robust but delayed immune response. Loss of circulating CD4+ and CD8+ T cells was not observed in this model and, while NK cell populations in the blood declined, an increase was observed in the spleen suggesting trafficking of this cellular population to tissues (Fritz et al., 2008). Both viral infections are associated with a massive cytokine elaboration and abrogation of the host interferon response.
Lesions within the spleen, gastrointestinal tract, lymphoid organs, and kidney are similar to those described in SHFV infection (Dalgard et al., 1992). There is extensive lymphoid necrosis and deposition of fibrin within splenic white pulp. Depletion of germinal centers is evident in lymphoid tissues. A characteristic lesion present within the liver is scattered hepatocellular necrosis accompanied by a mild, mononuclear inflammatory cell infiltrate. Multifocal necrosis is similarly present within the zona glomerulosa of the adrenal gland. In both locations, large amphophilic to eosinophilic intracytoplasmic inclusions may be evident. Immunohistochemical staining for viral antigen identifies presence of virus within hepatocytes, Kupffer cells, monocytes/macrophages, endothelial cells, and fibroblasts. Hepatocellular necrosis and necrosis within the adrenal cortex serve to distinguish filovirus infection from SHFV infection. An additional finding described in both Marburg and Ebola Reston viruses is the presence of a mild interstitial pneumonia accompanied by more diffuse evidence of disseminated microvascular thrombosis.
Evidence suggests that an epizootic among chimpanzees in the Tai National Forest was responsible for the transmission of Ebola Côte-d’Ivoire to a single human subject (Guenno et al., 1995). This troop experienced episodes of increased mortality in November 1992 and 1994. Although many animals were found in a state of advanced decomposition, tissues examined from one chimpanzee revealed lesions compatible with Ebola hemorrhagic fever, including multifocal necrotizing splenitis and hepatitis. Infrequent acidophilic intracytoplasmic inclusions were identified and were immunoreactive for Ebola-specific antigen. The magnitude of the impact of Ebola virus on ape morbidity and mortality in Africa has since been recognized (Figure 1.17 ).
FIGURE 1.17.

Filoviridae.
Marburg (A) and Ebola (B) virus infection cause widespread lymphoid necrosis (A), multifocal hepatic necrosis (B), and adrenocortical necrosis. Fibrin deposition is often widespread in the spleen and characteristic intracytoplasmic inclusions are evident in hepatocytes (B insert).
Clinical Findings
Following experimental inoculation with Ebola Reston, there is a variable incubation period of 7–14 days. Once clinical signs appear, progression to death is rapid (<24 h). Animals become anorexic, lethargic, and hypothermic. Cardiovascular collapse is followed by severe depression and coma. Petechiae are noted on the face, chest, and medial aspects of arms and thighs. A separate study of Ebola Zaire in rhesus macaques indicated prolonged partial thromboplastin time, increased fibrinogen degradation products, and normal prothrombin time (Fischer-Hoch et al., 1983).
Interpretations of clinical signs observed during the occurrence of natural disease in Reston were complicated by the concurrent infection with SHFV. This outbreak was associated with a rapidly progressive and fatal disseminated viral infection. Animals would abruptly become anorexic and lethargic and were often found dead in the morning without premonitory clinical signs.
Laboratory Fingings
Experimental inoculation with filoviruses was associated with significant increases in lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) (Fischer-Hoch et al., 1983). These values peaked earlier following inoculation with African Ebola viruses than with Asian strains and quickly returned to normal in those animals that survived infection. Hematologic changes included thrombocytopenia, neutrophilia, and lymphopenia. A reactive lymphocytosis was noted in survivors. Filovirus antigen ELISA, PCR, and virus isolation may be utilized for definitive diagnosis although these assays are best outsourced to laboratories with biosafety level 4 capabilities if index of suspicion for disease is high.
Treatment and Prevention
Because of the serious zoonotic potential associated with filovirus infection, affected animals should be euthanized. Experimental inoculation of animals requires biosafety level 4 conditions. Animals surviving infection with filoviruses may not be protected against subsequent challenge with a heterotypic virus. Recommendations for biocontainment and management of the various hemorrhagic fever-inducing agents have been published by the Centers for Disease Control and Prevention (CDC, 1988). Importation of Ebola Reston to the United States also led to additional guidance and oversight of primate importation and quarantine activities (DeMarcus et al., 1999).
Zoonotic Potential
The zoonotic potential with these agents is high. The initial outbreak of Marburg virus infection was associated with the handling of infected African green monkey tissue. Human Ebola Côte-d’Ivoire virus infection resulted from contact with chimpanzee tissue but was nonlethal and did not spread to secondary contacts. Experience with Ebola Reston indicates that while this virus appears less pathogenic in humans, transmission occurred readily to animal handlers (CDC, 1990a, CDC, 1990b). Both Ebola and Marburg viruses are HHS select agents.
Orthomyxoviridae
Influenza Viruses
Introduction
Influenza viruses are an important cause of respiratory illness in humans and occasionally infect nonhuman primates. Worldwide pandemics occurred in 1918, 1957, and 1968, and an estimated 20 000 000 humans died as a result of influenza between 1918 and 1919 (Acha and Szyfres, 1980). Animal infections (swine and fowl) are believed to have played a critical role in the generation of influenza viral strains responsible for these pandemics. More recently, highly pathogenic avian strains (H5N1) and swine origin strains (H1N1) have emerged as significant public health threats and nonhuman primates have played an important role as biomedical research models of these infections.
Etiology
Influenza viruses are 80–120 nm in size with a single-stranded segmented RNA genome encoding 9–10 proteins. Based on soluble (S) internal antigens, three virus types are recognized (A, B, and C). Types B and C infect only humans, have limited antigenic variation, and usually produce only sporadic cases. Type A strains infect a variety of animal species (humans, nonhuman primates, foals, fowl, swine, and seals), are more virulent, and have been responsible for the great pandemics. Two protein subunits within the viral envelope (hemagglutinin (H) antigen and neurominidase (N) antigen) define viral subtypes. The H antigen mediates adhesion of the virion to sialic acid residues on respiratory epithelial cells. The N antigen facilitates viral entrance to the cytosol following fusion and endosome formation.
Infection with a particular strain produces strong immunity, preventing reinfection. Unfortunately, genetic reassortment between genes coding for H and N subunits occurs with striking regularity, producing new subtypes that pre-existing immunity does not protect against. It is the emergence of these new strains that are sufficiently different from previously circulating subtypes and for which pre-existing immunity is not protective that has been associated with widespread human infection. Infection of fowl and/or swine is believed to be critical in the production of many of these strains.
Epizootiology
Evidence shows that gorillas, chimpanzees, orangutans, gibbons, macaques, baboons, African green monkeys, marmosets, tamarins, squirrel monkeys, owl monkeys, and capuchin monkeys are susceptible to infection (Kalter et al., 1969, Kalter et al., 1974, Murphy et al., 1972, Kalter and Heberling, 1978, Renegar, 1992). The virus is highly contagious by the aerosolized route and nonhuman primates likely become infected through contact with humans or wildlife species. Once established within a cohort, transmission may occur among nonhuman primate members. Evidence cited from an epizootic in gibbons suggests that adaptation of the virus in a naive population may be associated with increased virulence.
Pathogenesis and Pathology
In mild cases of upper respiratory tract infection, changes noted grossly may be minimal, consisting of hyperemia of mucous membranes and overproduction of mucoid secretions. Depending on the strain, experimental inoculation of macaque species results in acute bronchointerstitial pneumonia with diffuse alveolar damage characterized by alveolar spaces filled with edema and inflammatory infiltrates (Baskin et al., 2009, Chen et al., 2009a, Itoh et al., 2009). An exuberant inflammatory response may be an important contributor to the observed pathology (Cilloniz et al., 2009). Most strains replicate efficiently in the lungs with variable replication noted within the trachea, nasal mucosa, oropharynx, and tonsils (Rimmelzwaan et al., 2003, Kobasa et al., 2007, Chen et al., 2009a, Itoh et al., 2009). Variation in tropism for Type I versus Type II pneumocytes may significantly impact degree of pathology with the more pathogenic strains preferentially targeting Type II pneumocytes. Resolution of severe pulmonary involvement is characterized by marked septal thickening and hyperplasia of type II pneumocytes. Natural cases may be complicated by secondary bacterial infection and, in these instances, inflammation may be more purulent. Histologically, a desquamative alveolitis with the formation of hyaline membranes and microvascular thrombi is evident.
Clinical Findings
Clinical signs are nonspecific and consist of fever, oculonasal discharge, anorexia, lethargy, and gastrointestinal signs. Changes in respiratory rate and pulse oximetry measures may be observed. The incubation period is 1–3 days and the illness may last 3–6 days. Occasionally, nonhuman primate illnesses will coincide with human cases, suggesting a diagnosis.
Treatment
No specific treatment is available; however, the prevention of secondary bacterial infections may be beneficial (Johnsen et al., 1971). Demonstration of seroconversion to the S antigen by the hemagglutination–inhibition test is evidence of recent infection. Nasopharyngeal swabs or lung tissue collected at necropsy may be used for viral isolation on primary monkey kidney cell culture or embryonated chicken eggs. CPE is usually not observed and virus is first demonstrated by hemadsorption techniques. Immunofluorescent staining may be used for viral identification.
Paramyxoviridae
The family Paramyxoviridae contains two subfamilies: Paramyxovirinae and Pneumovirinae. The former is divided into three genera known to infect nonhuman primates: Respirovirus, Rubulavirus, and Morbillivirus. Pertinent species from each genus are presented in Table 1.3 . The subfamily Pneumovirinae contains two genera, Pneumovirus, which includes respiratory synctial viruses (chimpanzee coryza agent), and the recently identified Metapneumovirus. Specific viruses will be discussed herein according to their subfamily grouping.
TABLE 1.3.
Paramyxoviridae
| Subfamily | Genus | Virus |
|---|---|---|
| Paramyxovirinae | Morbillivirus | Measles virus |
| Paramyxovirus saguinus | ||
| Respirovirus | Human parainfluenza virus 1 | |
| Human parainfluenza virus 3 | ||
| Sendai virus | ||
| Simian virus 10 | ||
| Rubulavirus | Human parainfluenza virus 2 | |
| Human parainfluenza virus 4 | ||
| Parainfluenza virus 5 (formerly Simian virus 5) | ||
| Simian virus 41 | ||
| Pneumovirinae | Metapneumovirus | Human metapneumovirus |
| Pneumovirus | Human respiratory syncitial virus |
Paramyxovirinae
Members of the subfamily Paramyxovirinae are pleomorphic, occasionally filamentous, enveloped, RNA-containing viruses with a diameter of 150–200 nm. Their genome consists of a single molecule of single-stranded, mostly negative-sense RNA, but some contain a positive strand. Unenveloped nucleocapsids consist of long tubular structures and are assembled in the nucleus. These acquire a fuzzy, protein coat once they are released into the cytoplasm. These coated tubular structures then align themselves beneath the cell membrane through which they bud and acquire an envelope. The envelope consists of host cell membrane lipids with inserted viral-encoded proteins and glycoproteins. The latter protrude from the surface of the virions as 8- to 12-nm projections. Members of the genera Respirovirus and Rubulavirus contain a hemagglutinin-neuraminidase protein while members of the genus Morbillivirus lack neuraminidase. Members of the genus Pneumovirus, however, exhibit neither neuraminidase nor hemagglutinin activity. The surface spikes of all Paramyxoviridae members contain two glycosylated proteins that are responsible for the attachment and fusion of the virus to susceptible cells. The hemagglutinin or hemagglutinin-neuraminidase functions as an attachment protein in the case of the Paramyxovirinae subfamily. Within the Pneumovirinae subfamily, the attachment protein is termed protein G. Both subfamilies have a fusion protein (F) that is responsible for membrane fusion. The F protein contributes to fusion of infected cells with noninfected cells and formation of multinucleated syncytial cells.
Parainfluenza Viruses
Epizootiology
Because of the potential for significant impact on nonhuman primate colonies, members of the Morbillivirus genus are discussed separately below. Here we will address the members of the Respirovirus and Rubulavirus genera. The term parainfluenza virus will be used broadly to refer to viruses within these genera. Parainfluenza viruses have a global distribution where they are highly contagious and responsible for upper and lower respiratory ailments of a wide variety of species. Serological surveys indicate that most species of monkeys and apes have been infected with one or more parainfluenza viruses either in their natural habitats, as in the case of newly imported animals, or after being maintained in laboratory colonies. Many of these infections are mild and may go clinically undetected. The principal mode of transmission is by aerosols, although direct contact with infected secretions can also be a mode of transmission. Parainfluenza viruses appear to be less species specific than adenoviruses, as cross-species transmission among nonhuman primates, human beings, rodents, and possibly even dogs and cattle seems likely based on the antigenic relatedness of homologous serotypes isolated from these species. Reinfections with the same or related viruses occur with some frequency.
Pathogenesis and Pathology
Once in the respiratory tract, parainfluenza viruses bind to epithelial cells of the respiratory mucosa in the nasal cavity, nasopharynx, trachea, or bronchi and bronchioles. The virus enters susceptible cells by binding to and fusing with the cell membranes. Its negative-strand RNA is transcribed to form multiple molecules of messenger RNA that code for various viral proteins and as an entire transcript of positive-strand RNA that serves as the template for the synthesis of entire molecules of negative-strand viral progeny RNA. During the replicative process, infected cells develop both intranuclear and intracytoplasmic, eosinophilic viral inclusion bodies corresponding to the naked, intranuclear, tubular nucleocapsid and the fuzzy coated cytoplasmic nucleocapsids, respectively. Infected cells, bearing viral-encoded fusion proteins, often fuse with adjacent noninfected cells to form multinucleated syncytial giant cells. Thus, the hallmark of infection by viruses of the family Paramyxoviridae is the presence of single or multinucleated syncytial cells bearing both intranuclear and intracytoplasmic, eosinophilic inclusion bodies. Infected cells undergo virus-induced lysis and desquamate from the mucosa, thereby predisposing the host to secondary bacterial infection. Coinfecting bacterial species include Klebsiella pneumoniae, Streptococcus pneumonia, and Pasteurella multocida (Sutherland et al., 1986, Kondgen et al., 2008, Szentiks et al., 2009). The disease produced by parainfluenza viruses varies from a mild upper respiratory infection (coryza) to a rhinotracheobronchitis or croup-like illness to frank pneumonia. Most animals recover from these infections unless secondary bacterial infection results in death. Most respirovirus and pneumovirus infections do not result in a viremia.
Clinical Findings
Clinical signs associated with parainfluenza infections are not specific for any particular genus of virus. Depending on the portion of the respiratory tract most affected, nonhuman primates with active infection may exhibit no signs of clinical illness or may have nasal discharge and sneezing, wheezing, coughing, and even severe hyperpnea, dyspnea, and cyanosis. Epizootics of parainfluenza type 3 virus have been described in Patas monkeys and gibbons whereas parainfluenza type 1 has resulted in disease in marmosets (Churchill, 1963, Flecknell et al., 1983, Martin and Kaye, 1983). Sendai virus, the murine counterpart to human parainfluenza type 1, replicates at high levels in the upper and lower respiratory tracts of experimentally inoculated African green monkeys and chimpanzees but has not been associated with clinical disease (Skiadopoulos et al., 2002).
Laboratory Findings
Serological tests that demonstrate a rise in antibody titer to a specific viral agent in a convalescent serum are a practical method to determine which, if any, parainfluenza virus is responsible for the respiratory illness. Detection of virus using PCR or IHC techniques is also useful for diagnosis. One must be aware, however, that there are numerous viral agents in addition to members of the paramyxovirinae that can cause respiratory disease.
Treatment
As most simians recover naturally from parainfluenza virus infections, treatment is largely supportive in nature and is directed toward minimizing the risk of secondary bacterial infection. Parainfluenza 3 virus infection has been shown to predispose to invasive pneumococcal disease in chimpanzees (Jones et al., 1984).
Prevention
Although there are effective vaccines available commercially for canine parainfluenza virus (a host variant of Parainfluenza virus 5) and bovine parainfluenza virus 3, these have not been tested nor are they approved for the prevention of infection by simian isolates of these serotypes. Thus, preventative measures should be similar to those indicated for adenovirus infections, namely the prevention of crowding and minimizing exposure to other species that might harbor similar agents.
Measles (Rubeola) Virus
Introduction
Measles virus infection is a common viral disease with the potential to significantly impact the health and management of nonhuman primate colonies. This virus is a member of the genus Morbillivirus which contains many viruses of veterinary importance including canine and phocine distemper viruses, Rinderpest virus, virus of Pestes des petites ruminants, and the recently documented equine morbillivirus. These agents produce illnesses in a diverse group of animals with remarkable clinical and histopathologic similarities.
Measles virus infection has been described previously in rhesus macaques, cynomolgus macaques, bonnet macaques, Formosan rock macaques, silvered leaf monkeys, African green monkeys, squirrel monkeys, colobus monkeys, chimpanzees, marmosets, cotton-top tamarins, white-lipped tamarins, and owl monkeys (Scott and Keymer, 1975, Albrecht et al., 1980). Historically, this disease was once widespread in newly imported macaques and responsible for significant morbidity in animals stressed by importation and quarantine procedures. While largely controlled in the United States and Europe, recent outbreaks have occurred in Southeast Asia and infected animals have been recognized following importation. Measles virus infection should be considered a serious threat to nonhuman primate colonies, and appropriate preventative measures should be taken.
Etiology
The measles virus is grouped with the viruses of canine distemper and rinderpest in the genus Morbillivirus and family Paramyxoviridae. The virus is spherical to pleomorphic and 120–270 nm in diameter. The core consists of single-stranded negative-sense RNA and virion assembly occurs at the plasma membrane with the formation of spherical particles. Six gene products have been identified: N (nucleoprotein), P (polymerase protein), L (large protein), M (matrix protein), H (hemagglutinin), and F (fusion factor). Neuraminidase, a protein of other Paramyxoviridae, is not present.
Epizootiology
Serological evidence indicates that while measles can be a common infection in captive nonhuman primates, it does not occur in their natural habitats. Historically, seroconversion was often observed to occur within weeks of first human contact, leading to endemic infection within groups or, if combined with stress of capture, quarantine, or shipping, to epizootics characterized by high morbidity and mortality. Roberts et al. documented an epizootic with high mortality in a group of immunosuppressed macaques inoculated with SIV (Roberts et al., 1988). In a naive population, the disease spreads rapidly by the aerosolized route. With the prohibition of importation of many primate species and the advent of widespread vaccination, serious epizootics have become less frequent. In recent years unfounded concerns over side effects associated with human vaccination have increased the number of unvaccinated individuals in the United States and Europe. Such individuals have been associated with measles cases and pose a risk to nonhuman primate colonies. Measles remains problematic in human populations throughout Southeast Asia and serious epizootics have been recognized in captive macaque populations. Such animals may be infected prior to exportation and pose a significant threat to colonies.
Clinical Findings
Clinical signs vary, depending on the species infected. In macaques the disease is usually mild or asymptomatic unless animals are stressed or immunosuppressed. The incubation period varies from 6–10 days and is followed by fever and a maculopapular exanthema. This rash is most pronounced on the ventral body surface and generally spares plantar and palmar surfaces. It then progresses to a dry or scaly desquamative dermatitis and may continue for up to 2–3 weeks (Hall et al., 1971). Animals may be infectious prior to the onset of overt clinical signs.
While considered pathognomonic, Koplik’s spots are present inconsistently. These small, white foci are rimmed by a raised red border and are found within the oral mucosa. In some cases, respiratory signs, including cough and conjunctivitis, may be present. Infected animals may be more susceptible to enteric bacterial infections, such as that caused by Shigella flexneri, and these animals may present with primarily gastrointestinal signs (Roberts et al., 1988, McChesney et al., 1989). Such concurrent bacterial infections may adversely impact morbidity and mortality. Abortion and neurologic signs may occur in some individuals (Renne et al., 1973, Steele et al., 1982).
In marmosets, owl monkeys, and colobines the disease is more severe. In these species the disease is characterized by a rapidly progressive course and a predominance of gastrointestinal signs. The characteristic exanthema may be lacking. Edema of the periorbital region may be pronounced. Mortality may approach 100%, and lesions are centered within the gastrointestinal tract (Figure 1.18 ).
FIGURE 1.18.
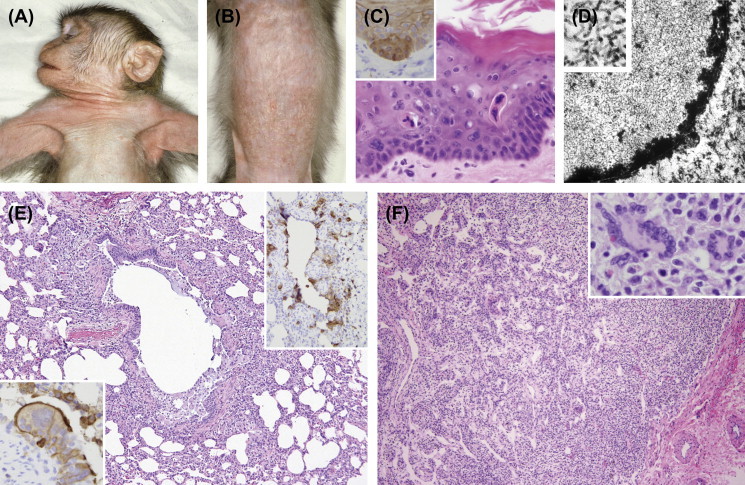
Measles virus (MV).
MV causes a viral exanthema often appearing first on the face and axilla (A), spreading to the abdomen (B). Multinucleated viral syncytial cells may be present in the epithelium (C) and viral infection may be demonstrated by immunohistochemistry for the measles matrix antigen (C, insert). Intranuclear inclusions composed of viral nucleocapsids (D) may be observed. A bronchointerstitial pneumonia may be observed (E) with antigen-positive cells centered on airways (E, insert right). Multinucleated syncytial cells (E, insert left) are often evident. The virus targets lymphoid tissue causing marked lymphoid depletion (F). Large multinucleated syncytial (Warthin-Finkeldey) cells may be observed in lymph node (F insert).
Pathogenesis and Pathology
The pathogenesis of measles virus infection is similar to that of rinderpest and canine distemper viruses. Following infection by the aerosolized route, there is an initial round of replication within the regional lymph nodes. The resulting viremia leads to dissemination of the virus to lymphoreticular organs and epithelial surfaces. It is in the terminal phases of this viremia that the characteristic skin rash appears. This exanthema coincides with rising neutralizing antibodies and may, in part, represent an Arthus-type (antibody–antigen complex) reaction.
Histologically, there is mild erythema and parakeratotic hyperkeratosis of the skin. Multinucleated syncytial cells may occasionally be found (Hall et al., 1971). Lesions are most pronounced within the hair follicles where follicular necrosis is a characteristic change. In more severe cases there is often a proliferative and necrotizing bronchointerstitial pneumonia. Large syncytial cells are often present and careful inspection may reveal intranuclear and intracytoplasmic eosinophilic inclusions present within these cells, as well as type II pneumocytes and histiocytes. A purulent bacterial bronchopneumonia may be superimposed on these findings.
The latter stages of infection may be associated with significant viral-induced immunosuppression, which arises from lymphopenia and the destructive effect of the virus on thymic tissue, and may predispose animals to bacterial infections of the respiratory and gastrointestinal tracts. Morphologically marked depletion of lymphoid tissue in the lymph nodes and spleen may be evident. Viral inhibition of interferon-γ-induced upregulation of MHC class II antigen expression may further compromise immunologic function (Leopardi et al., 1993). These effects on the immune system of the host may induce hyporesponsiveness to tuberculin antigen, resulting in a false-negative skin test in sensitized animals. Immunodysfunction may be so severe that animals develop opportunistic infections such as disseminated reactivation of CMV and candidiasis. The resolution of infection correlates with the appearance of cytotoxic CD8+ T lymphocytes.
Giant cells lacking viral inclusions (Warthin–Finkeldey cells) may be found within lymphoid tissue. In the most severe cases, typical viral inclusions may be found in a variety of epithelial surfaces. In marmosets, these changes may be accompanied by a necrotizing gastroenteritis. Ultrastructurally, the virus may be identified in tissues in two forms: (1) nucleocapsids that form tangles of filamentous tubules with a diameter of 20 nm and (2) paracrystalline arrays of filamentous rods 20 nm in diameter surrounded by an outer rim of less electron-dense material, giving the structure an overall diameter of 40–50 nm (Raine et al., 1969). These latter structures can occasionally be found subjacent to the cell membrane in various stages of budding. Immunohistochemistry is useful in confirming the etiology of the viral infection.
A well-characterized postinfectious syndrome in human is subacute sclerosing panencephalitis (SSPE), which occurs in a small minority of patients 5–10 years after primary infection, and is characterized by progressive gliosis, demyelination, and neuronal loss (Tellez-Nagel and Harter, 1966). Intranuclear inclusions composed of viral nucleocapsid are prominent and result from defective viral replication. One or more defects in the M, H, or F antigens prevent the formation of mature virons. Although an acute measles encephalitis in macaques and other species is well recognized (Baringer and Griffith, 1970), it is less clear whether reported cases of SSPE in nonhuman primates critically fulfill all diagnostic requirements of SSPE in humans (Kim et al., 1970, Albrecht et al., 1972).
Laboratory Findings
Presumptive diagnosis may be made on seroconversion, characteristic clinical, histopathologic, and ultrastructural findings. Further support may be gathered by demonstrating seroconversion. Definitive diagnosis requires viral isolation and identification. Isolation is best performed on human or rhesus monkey kidney cells from nasopharyngeal secretions, whole blood, or buffy coat preparations. Cultures should be maintained for 4–6 weeks with weekly transfers of supernatant to fresh cultures. Characteristic CPE include multinucleated syncytial cells and eosinophilic intracytoplasmic and intranuclear viral inclusions.
Treatment
No specific treatment is available. Epizootics in macaques have been controlled by the infusion of exposed animals with human γ-globulin and vaccination with modified live vaccine (Roberts et al., 1988). Supportive therapy for dehydration and secondary bacterial infections may be beneficial.
Prevention
Infant macaques should be vaccinated at 3 months of age or older with a modified live measles vaccine design for human use. A second dose given no sooner than 6 weeks produces protective antibody levels. Use of a commercial canine distemper measles modified live vaccine has also been reported and protects against wild-type challenge. In recent years availability of these products has been intermittent.
Zoonotic Potential
A macaque-to-human transmission has been demonstrated (Roberts et al., 1988).
Paramyxovirus Saguinus
Introduction
A single outbreak of infectious gastroenteritis was diagnosed in cotton-topped tamarins, red-chested moustached tamarins (S. labiatus), black-chested moustached tamarins (S. mystax), and common marmosets at the New England Primate Research Center in 1977 (Fraser et al., 1978).
Etiology
A virus isolated from the spleen of a terminally ill tamarin was identified and used to infect four cotton-topped tamarins that later developed characteristic lesions and died. The virus was closely related to, but antigenically distinct from, measles virus. Although the exact relationship to measles virus is unknown, the causative agent likely represents a variant of measles virus (Hunt and Blake, 1993b).
Epizootiology
The origin of infection in these animals is unknown. Virus was shed in the feces and a fecal–oral route of transmission seems likely.
Pathogenesis and Pathology
Pathogenesis likely parallels measles virus infection in nonhuman primates. Affected animals had moderate to severe necrotizing colitis and typhilitis with multinucleated syncytial cells present within crypts. Similar syncytial cells were present in pancreatic acini, renal tubules, and hepatic cords. A striking change was the presence of large syncytial cells containing up to 20–25 nuclei and intracytoplasmic viral inclusions within bile duct epithelium. Lymphoid necrosis within germinal centers was evident. No exanthema was recognized, and in contrast to measles virus infection of macaques, lesions were absent in the lungs and brain.
Clinical Findings
Clinically there was acute onset of anorexia, dehydration, and diarrhea, which progressed rapidly to death. Colony mortality of S. oedipus was approximately 10% and approached 100% in S. mystax and S. labiatus.
Treatment
No specific treatment is available.
Pneumovirinae
Respiratory Syncytial Virus
Introduction
Respiratory syncytial virus (RSV) was first isolated from a chimpanzee with coryza in 1956 (Blount et al., 1956). Subsequently, this agent has been shown to be an important cause of mild to severe respiratory disease in children worldwide.
Etiology
Respiratory syncytial virus, of the genus Pneumovirus, is a single-stranded RNA virus 90–130 nm in diameter. Individual isolates of RSV, in general, have a broad host range.
Epizootiology
The virus is highly contagious and is spread through aerosols. Anti-RSV antibodies are widespread in human and nonhuman primate populations (Richardson-Wyatt et al., 1981). Nonhuman primates likely become infected through human contact. Naturally occurring infections have almost exclusively been reported in apes, particularly gibbons and chimpanzees, although African green monkeys and owl monkeys have been experimentally infected with demonstrated signs of clinical disease (Koff et al., 1983, Kakuk et al., 1993).
Pathogenesis and Pathology
Neutralizing antibodies (IgG) are nonprotective and may predispose individuals to more severe disease through the deposition of immune complexes within pulmonary vessels. Conversely, IgA is protective. The disease is often more severe in children within the first months of life due to the persistence of maternal antibodies (IgG not IgA). Similarly, in a clinical study of children, parenteral inoculation with killed vaccine was found to exacerbate and prolong infection as a consequence of the production of neutralizing antibodies.
In more severe cases, disease is characterized by a necrotizing bronchiolitis to bronchopneumonia. Multinucleated syncytial cells with intracytoplasmic inclusion bodies may be apparent. Lethal episodes are invariably associated with diffuse alveolar damage and microvascular thrombosis, which are characteristic of the acute respiratory distress syndrome (ARDS).
Clinical Findings
In nonhuman primates, the disease is often mild and is characterized by nonspecific upper respiratory signs, including coughing, sneezing, and mucopurulent oculonasal discharge. Fatalities have been described in infant or juvenile chimpanzees (Clarke et al., 1994, Kondgen et al., 2008, Szentiks et al., 2009). Epizootics in wild populations of apes may be associated with high morbidity of greater than 90% (Kondgen et al., 2008).
Laboratory Findings
Rapid detection of RSV antigen in nasopharyngeal secretions of affected human infants using a direct immunofluorescent test has been described (Choa et al., 1979). Viral isolation may be made on Hep-2 cells or rhesus monkey kidney cells and identified by immunofluorescent staining techniques. CPE is characterized by syncytial formation 1–14 days postinoculation. Molecular diagnostic techniques may also be employed.
Prevention
Vaccination by the parenteral route is not recommended because neutralizing antibodies (IgG) may predispose to more severe disease.
Human Metapneumovirus
Human metapneumovirus was first identified in 2001 as a cause of respiratory disease in children from the Netherlands and is a cause of community-acquired influenza-like illness (van den Hoogen et al., 2001). A 61% seroprevalence has been demonstrated in captive-bred chimpanzees with exposure likely to have occurred secondary to contact with human handlers (Skiadopoulos et al., 2004). Clinical signs following experimental infection of chimpanzees included nasal discharge and decreased appetite. African green monkeys were also able to support a high level of viral replication following experimental infection although there was no evidence of clinical disease (Skiadopoulos et al., 2004). Human metapneumovirus has been implicated as a cause of respiratory disease in wild populations of chimpanzees (Kaur et al., 2008, Kondgen et al., 2008).
Arteriviridae
The family Arteriviridae contains one genus, Arterivirus, which includes the viral species simian hemorrhagic fever virus, equine arteritis virus, lactate dehydrogenase-elevating virus of mice, and porcine reproductive and respiratory syndrome virus. These viruses are positive-stranded RNA viruses with genomes that vary from 12–15 kb in length.
Simian Hemorrhagic Fever Virus
Introduction
Simian hemorrhagic fever virus (SHFV) is a highly contagious, fatal infectious disease of rhesus macaques. It was first recognized in 1964 simultaneously at the National Institutes of Health (Bethesda, MD) and at the Sukhumi Institute (USSR) (Allen et al., 1968, Palmer et al., 1968, Abildgaard et al., 1975). It must be distinguished from other viruses capable of causing hemorrhagic fevers.
Etiology
SHFV is a single-stranded RNA virus 40–45 nm in diameter. It can be propagated in vitro on primary macaque macrophage cultures and on the MA-104 embryonic African green monkey kidney cell line (Tauraso et al., 1968). Diagnostic ultrastructural changes become apparent after 24–72 h in these cells and include the formation of unique lamellar replicative structures.
Epizootiology
SHFV has been shown to naturally infect several African species, including Patas monkeys, African green monkeys, and baboons (Papio anubis and P. cynocephalus). Of these, the Patas monkey represents the most important reservoir and the greatest risk to macaques. In these species, infection is usually asymptomatic and reportedly animals may be viremic and seronegative (Gravell et al., 1980b). Several strains of SHFV have been identified in Patas monkeys, which vary in their ability to cause clinical disease, persistent infection, and an antibody response (Gravell et al., 1986b). Interestingly, Patas monkeys persistently infected and unable to mount an antibody response are able to clear infection when inoculated with a more virulent strain capable of inducing acute disease (Gravell et al., 1986a).
Transmission to Asian macaques results in a fulminant and fatal infection characterized by a bleeding diathesis and rapid progression to death. Initial infection of macaques appears to require parenteral exposure to infected blood or tissue from carrier species. Once established the virus is highly contagious and may then be spread from macaque to macaque by aerosol, direct contact, or fomites (Renquist, 1990). Mortality may be as high as 100%.
Pathogenesis and Pathology
Macaques
At necropsy there is striking locally extensive congestion, hemorrhage, and necrosis of the proximal duodenum. Similar foci are found randomly distributed throughout the gastrointestinal tract and in the renal capsule, retrobulbar tissue, subcutis, and lung. The spleen is enlarged two to three times normal, and the white pulp may not be visible grossly.
Microscopically, characteristic lesions are found in lymphoid tissue. Extensive lymphoid necrosis and congestion exist within the spleen. Prominent perifollicular hemorrhages are often separated from the subjacent follicular mantle by a fibrinous exudate (Allen et al., 1968). Sinuses may be distended by fibrin and plasma. Necrosis of germinal centers in other lymphoid tissues is prominent. Complete cortical thymic necrosis with sparing of the medulla is reportedly unique to SHFV infection (Zack, 1993). Lesions elsewhere are compatible with those associated with disseminated intravascular coagulation and include deposition of fibrin thrombi within glomeruli, hepatic sinusoids, and lung. Lesions within the small intestine consist of extensive hemorrhage within the lamina propria accompanied by intestinal epithelial cell necrosis. As with other arteriviruses, fetal loss is common. A lymphohistiocytic meningoencephalitis is present in a minority of animals. Macrophages represent the principal target cell of SHFV infection, and the unique sensitivity of macaques to this viral infection relates to the propensity of their macrophages to support viral growth (Gravell et al., 1980a).
The absence of hepatic and/or adrenal necrosis in conjunction with the previously described findings is highly suggestive of SHFV. Their presence suggests possible infection with Ebola virus, Marburg virus, or Kyasanur Forest disease virus. Additional distinguishing features of Ebola Reston virus are intracytoplasmic inclusion bodies and patchy interstitial pneumonia (Figure 1.19 ).
FIGURE 1.19.

Simian hemorrhagic fever virus (SHFV).
In macaques simian hemorrhagic fever targets lymphoid tissue causing widespread lymphoid necrosis and deposition of fibrin.
African Monkeys
Simian hemorrhagic fever virus infection of Patas monkeys, African green monkeys, and baboons is generally asymptomatic. In Patas monkeys, the viral strain appears to play a critical role in determining the disease course (Gravell et al., 1986a). Viral strains LVR and P180, both of which induce lytic infection of Patas monkey peritoneal macrophages, may induce an acute and rarely fatal disease in this species. Animals frequently were febrile and showed anorexia, lethargy, facial edema, and dehydration. Small subcutaneous hemorrhages were noted occasionally. These viral strains induced a strong antibody response and did not result in persistent infection. In contrast, viral strains that did not result in lytic infection of Patas monkey peritoneal macrophages were more likely to result in asymptomatic infection, poor antibody response, and persistent infection.
Clinical Findings
Following experimental inoculation of macaques there is an incubation period of 3–7 days. Initially a fever without other clinical signs is recognized followed shortly by a bleeding diathesis (epistaxis, hematuria, melena, ecchymoses and petechiae). In the terminal stages, depression, dehydration, photophobia, and cyanosis are present. The entire clinical course may last from 1–7 days (Palmer et al., 1968). An alternative presentation is sudden death without obvious clinical signs.
Laboratory Findings
Fibrin degradation products may become elevated as early as 24–48 h postexperimental inoculation (Abildgaard et al., 1975). There is an increase in aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase at the onset of clinical signs. The coagulopathy is characterized by thrombocytopenia and prolonged partial thromboplastin times. Proteinuria and hematuria may be noted on urinalysis.
Treatment
No treatment is available. A summary of control measures has been published and should be instituted at the first evidence of an epizootic hemorrhagic disease in macaques (Gravell et al., 1986b, Zack, 1993). This multifaceted approach includes (1) elimination of clinically ill and exposed animals, (2) quarantine of remaining animals, (3) disinfection of premises, (4) diagnostic efforts to identify the etiologic agent, (5) notification of appropriate government agencies, and (6) monitoring of human contacts.
Prevention
Exposure of macaques to African primates and their blood and tissue products should be prevented. When Patas monkeys and macaques are housed within the same facility, special precautions should be taken to prevent inadvertent exposure. Although ELISA assays have been developed, serology may be of limited value in determining which African primates carry the virus (Godeny, 2002).
Zoonotic Potential
There is no evidence that simian hemorrhagic fever virus is a zoonosis. A major concern is that the clinical signs of this disease in macaques mimic several other diseases (Ebola virus, Marburg virus, yellow fever virus, and Kyasanur Forest virus) that may infect human beings with lethal consequences. As such, all macaques exhibiting these clinical signs should be treated as if infected with a potentially lethal zoonotic agent.
Togaviridae
Togaviridae are single-stranded, positive-sense RNA viruses that are 70 nm in diameter with a lipid envelope and peplomers composed of a heterodimer of two glycoproteins. The Togaviridae family contains two genera (Alphavirus and Rubivirus). The Alphavirus genus consists of approximately 30 species of arthropod-borne viruses including the significant human pathogens eastern equine encephalitis, western equine encephalitis, Sindbis virus, Chikungunya virus, and Mayaro virus. These viruses are grouped into seven serocomplexes based on serological data and three phylogenetic clusters. These viruses may also be categorized into Old World and New World viruses based on geographic distribution. Free-roaming populations of both Old and New World nonhuman primates have been proposed as vertebrate hosts and potential reservoirs for Mayaro and Una viruses, prevalent in South America, and Chikungunya virus, distributed in Africa and Asia (Hoch et al., 1981, Seymour et al., 1983, Talarmin et al., 1998, de Thoisy et al., 2003a, Inoue et al., 2003, Diaz et al., 2007). Clinical disease associated with Mayaro and Una virus has not been reported in nonhuman primates.
Chikungunya Virus
Introduction
Chikungunya virus was first isolated in 1952, reemerged in Kenya in 2004, and has since contributed to significant human morbidity in over 18 countries (Ross, 1956, Staples et al., 2009). Genetic mutations in viral strains have allowed adaptation to more widely distributed mosquito vectors likely contributing to spread of the epidemic (Tsetsarkin et al., 2007). Disease in humans is characterized by fever, maculopapular rash, myalgia, and arthralgia that may persist in a portion of patients.
Epizootiology
Nonhuman primates are susceptible to infection and likely act as a contributor to the viral reservoir in Africa and Asia. Free roaming populations of red tail monkeys (Cercopithecus ascanius), African green monkeys, baboons, chimpanzees, and colobus monkeys have demonstrated seropositivity (McCrae et al., 1971). Seropositivity rates for IgM and IgG antibodies were 14.8% and 59.3% respectively in wild-caught cynomolgus macaques in the Philippines (Inoue et al., 2003). Interestingly, there currently does not appear to be strong evidence to indicate that the large population of cynomolgus macaques residing on the island of Mauritius has contributed to the significant epidemic within this region (Higgs and Ziegler, 2010).
Pathogenesis and Pathology
Naturally occurring disease has not been reported in nonhuman primates, however, experimental infection of cynomolgus macaques recapitulates human symptomatology and pathology (Labadie et al., 2010). Depending on dose of inoculum, macaques demonstrated evidence of fever, morbilliform skin rash, increased hepatic transaminases, and detectable levels of circulating virus. Macaques inoculated with a high dose demonstrated evidence of swollen joints, menigoencephalitis, and variable mortality. Histopathological lesions included extensive histiocytic infiltration of spleen and lymph nodes. Infiltrates were present as long as 6 months postinoculation suggesting that macrophages act as the main cellular reservoir for persistent Chikungunya virus.
Zoonotic Potential
Zoonotic transmission of Chickungunya virus from animals housed in research settings has not been reported.
Flaviviridae
The family Flaviridae (Table 1.4 ) encompasses greater than 70 viruses, many of which are important pathogens of humans and animals. In humans, pathogenic flaviruses characteristically produce meningoencephalitis (e.g., Japanese encephalitis, St. Louis encephalitis, West Nile virus) or a hemorrhagic fever syndrome (e.g., yellow fever, dengue). Although many of these viruses are transmitted by arthropod vectors (ticks and mosquitoes), some may be transmitted directly among mammalian hosts. The Flavivirade family is comprised of three genera including Flavivirus, Hepacivirus, and Pestivirus. Members of the Flavivirus genus can be grouped serologically into distinct groups based on differences within the envelope protein, the most significant of which are the Dengue, Japanese encephalitis virus, and yellow fever virus subgroups. The number of identified flaviruses is continually expanding, and the pathogenicity of most for nonhuman primates is unknown.
TABLE 1.4.
Flaviviridae
| Family | Genus | Virus |
|---|---|---|
| Flaviridae | Flavivirus | Dengue virus |
| Japanese encephalitis virus | ||
| Kyasanur Forest disease virus | ||
| St. Louis encephalitis virus | ||
| West Nile virus | ||
| Yellow fever virus | ||
| Zika virus | ||
| Pestivirus | ||
| Hepacivirus | Hepatitis C virus | |
| GB virus A, B, and C |
Yellow Fever Virus
Introduction
Yellow fever, a member of the genus Flavivirus, is a devastating viral disease of humans and New World primate species that is transmitted by a variety of mosquito vectors. It is thought that yellow fever virus originated in Africa and was spread to the New World in the 16th and 17th centuries along trans-Atlantic trade routes. Once established in native mosquitoes, its introduction was associated with devastating epidemics and epizootics in humans and indigenous nonhuman primates.
Epizootiology
Yellow fever virus infects forest and bush-living mosquitoes, including Aedes spp., Haemagogus spp., and Sabethes spp., with which it commensally exists. In A. aegypti the virus may persist through the dry season by transovarian transmission. The mosquito represents the reservoir host and largely determines the geographic distribution and persistence of the virus in nature.
Yellow fever virus has been shown to infect a variety of primate species. In African species, infection is associated with viremia but few clinical signs and is eliminated with rising neutralizing antibodies generally within 6 days. Wild hosts that support this sylvatic cycle reportedly include baboons, mangabeys, chimpanzees, red colobus monkeys (Pilocolobus badius), African green monkeys, and Patas monkeys. Viremia in these animals serves to propagate the virus in vector species and may initiate the urban cycle when transmission to peridomestic mosquitoes such as A. aegypti occurs. Once established in mosquito species that feed on humans, epidemics may become established in human populations.
In New World primates, the disease is more severe and epizootics have been described in howler monkeys, spider monkeys, and squirrel monkeys and may coincide with cases in adjacent human populations (Sallis et al., 2003, Rifakis et al., 2006). Capuchin monkeys and wooly monkeys may be less susceptible. In tropical South America, human infection generally results from inadvertent exposure of individuals to the sylvatic cycle. Other than a small urban outbreak in Bolivia in 1999, the reduction of A. aegypti populations has limited the urban cycle within South America (Van der Stuyft et al., 1999).
Pathogenesis and Pathology
Although yellow fever is not found naturally in Asia, experimental inoculation of macaques is associated with a rapidly lethal disease that models many of the features present in fatal human cases. As such, much of what is known about the pathogenesis of yellow fever virus infection has been determined in this experimental setting (Hudson, 1928, Klotz and Belt, 1930a, Klotz and Belt, 1930b, Monath et al., 1981).
Following inoculation, initial rounds of replication occur in the regional lymph nodes, with rapid dissemination to multiple organs. While neurotropic and viscerotropic strains are recognized, the viscerotropism of the virus accounts for the cardinal clinical signs. During the initial phase of viremia, virus is visualized first within Kupffer cells and subsequently with hepatocytes. Disseminated intravascular coagulation and depletion of vitamin-K-dependent clotting factors contribute to the recognized bleeding diathesis.
Histologically, lesions in the liver are characterized by multifocal hepatocellular necrosis with the formation of Councilman bodies and Torres bodies (Bearcroft, 1960, Bearcroft, 1962). These changes are invariably accompanied by the fatty degeneration of the remaining hepatocytes. Lymphoid depletion within germinal centers and splenic arterial sheaths may be prominent. Although not a natural disease of macaques, clinical and pathologic findings following experimental inoculation are remarkably similar to syndromes caused by other hemorrhagic fever agents such as simian hemorrhagic fever virus and filoviruses.
Clinical Findings
Infection of African species is asymptomatic. Natural infection of New World nonhuman primates is generally recognized by an increased mortality in susceptible species and may accompany human cases in the geographical vicinity.
Prevention
Yellow fever is primarily a disease of wild nonhuman primates. Because there is a short incubation period and no carrier state is established, the risk of capture and importation of infected animals is relatively low. Nevertheless it should be considered in the differential diagnosis of hemorrhagic fever in endemic regions and in recently imported primates.
West Nile Virus
Introduction
West Nile virus, first documented in Uganda in 1937, emerged in the United States in 1999, gradually spread across the continent, and is now endemic within North America (Lanciotti et al., 1999). The single-stranded RNA virus of the Flavivirus genus is a member of the Japanese encephalitis virus serogroup. The mosquito-borne virus causes clinically evident disease primarily within human, avian, and equine hosts although it can also infect numerous vertebrate species as incidental hosts. Avian species are considered the primary reservoir host. Disease in humans is often a mild, self-limiting febrile illness although meningoencephalitis is a rare complication observed primarily in aged or immunocompromised individuals.
Epizootiology
West Nile virus seropositivity has been demonstrated within outdoor-housed nonhuman primates. A survey of baboons, rhesus, and pig-tailed macaques at the Tulane National Primate Research Center demonstrated a 36% overall seroprevalence, while a similar survey at the Yerkes National Primate Research Center demonstrated a lack of seroprevalence within outdoor-housed rhesus macaques and a limited 6.6% seroprevalence within Sooty Mangabeys (Ratterree et al., 2003, Cohen et al., 2007). This difference was suspected to be associated with the variability in the abundance of mosquito vectors between the two sites. The short duration and low level of viremia are thought to exclude nonhuman primates as a potential source of transmission of the virus.
Pathogenesis and Pathology
The majority of natural and experimental West Nile virus infections in nonhuman primates are subclinical (Ratterree et al., 2004, Hukkanen et al., 2006, Wolf et al., 2006a). Intracerebral inoculation of rhesus macaques and intranasal inoculation of Bonnet macaques reportedly results in a variable incidence of encephalitis (Pogodina et al., 1983, Goverdhan et al., 1992). Naturally acquired and clinically evident disease has been reported in a Barbary macaque housed outdoors within a zoological setting (Olberg et al., 2004). Clinical signs observed in this case included ataxia, tremors, cranial nerve deficits, and nystagmus. Histopathology revealed a nonsuppurative meningoencephalitis characterized by gliosis and glial nodules as well as perivascular lymphoplasmacytic cuffing. Mononuclear infiltrates and edema were observed in the meninges. West Nile virus as the etiology was confirmed with IHC and molecular techniques.
Prevention
Control of exposure to mosquito vectors may be indicated for outdoor-housed animals.
Kyasanur Forest Disease Virus
Kyasanur Forest disease, also of the genus Flavivirus, was first recognized in March of 1957 as a cause of increased mortality in langurs (Presbytis entellus) and bonnet macaques (Iyer et al., 1959, Iyer et al., 1960). Subsequent investigations demonstrated a concurrent illness in adjacent human populations. The virus has been isolated from a variety of ixodid ticks, including Haemaphysalis spinigera, H. turturis, and loxdes petauristae (Singh et al., 1963). In addition, the virus has been isolated from rodents and bats from the endemic region.
The disease in nonhuman primates is characterized by multifocal hepatocellular necrosis accompanied by hemorrhages within the adrenal gland, brain, kidney, and lung. There is prominent leukopenia, anemia, and thrombocytopenia (Webb and Chaterjea, 1962). In humans, the disease may produce mild nonspecific signs or a fulminant hemorrhagic disease with high mortality. This virus is a HHS select agent.
Dengue Viruses; Serotypes 1–4
Dengue viruses (DNV) are important causes of human hemorrhagic fever in tropical and subtropical regions worldwide. These viruses are members of the genus Flavivirus. There are four serotypes each with approximately three to five genotypes. Free-ranging nonhuman primate populations, including cynomolgus monkeys, Toque macaques (Macaca sinica), and brow-ridged langurs (Trachypithecus (Presbytis) cristatus and T. obscura), may have a high rate of seroreactivity, indicating widespread exposure (Rudnik, 1965, Rodhain, 1991, Peiris et al., 1993). Neutralizing antibodies have been demonstrated in nonhuman primates in association with epidemics in Nigeria, Sri Lanka, and other regions (de Silva et al., 1999). As with yellow fever, a sylvatic cycle involving Aedes spp. mosquitoes and nonhuman primates and an urban cycle in which the disease is spread among the human population are postulated to occur.
Experimental infection of macaques, African green monkeys, mangabeys (Cercocebus spp.), owl monkeys, and baboons produces viremia and antibody response but has been associated with only minimal clinical signs (Halstead, 1981, Schiavetta et al., 2003, Martin et al., 2009). This has allowed species such as the rhesus macaque and owl monkey to be useful for evaluation of Dengue vaccination strategies and study of virologic and immunologic responses (Halstead et al., 1973, Halstead and Palumbo, 1973, Blair et al., 2006, Raviprakash et al., 2008, Simmons et al., 2010). However, the absence of significant clinical disease has limited the utility of such models for the study of the pathogenesis of Dengue hemorrhagic fever and Dengue shock syndrome. A recent publication suggests that high-dose intravenous inoculation with DNV2 was associated with a hemorrhagic phenotype accompanied by modest thrombocytopenia, increased D-dimer, and increased thrombin–antithrombin complexes (Onlamoon et al., 2010). The significance of dengue virus infection of wild nonhuman primates is unknown.
Hepatitis C Virus (HCV)
Introduction
Hepatitis C virus is the most common bloodborne pathogen recognized in the United States and in man causes a persistent viral infection leading to chronic hepatitis and hepatocellular carcinoma. HCV-related deaths are expected to triple in the next decade in this country and currently there are limited treatment options and no effective vaccine. HCV is a member of the tentative genus Hepacivirus. Like other members of the flaviridae, HCV is a single-stranded positive sense RNA virus. It was identified in 1989 through work largely made possible through transmission of infectious serum from human patients with non-A non-B hepatitis to chimpanzees (Choo et al., 1989). Like many other RNA viruses, HCV has an error-prone replicase resulting in a high mutation rate that leads to the production of progeny virions exhibiting extensive sequence variation. There are at least six major genotypes and more than 50 serotypes defined. Vaccines and therapeutic strategies must address this diversity to be successful.
Etiology
The HCV genome itself is a single-stranded positive-sense RNA of about 9.5 kb in length. Highly conserved non-translated regions flank a single open reading frame encoding a polyprotein of about 3000 amino acids. In the 5’NTR is an internal ribosome entry site (IRES) which functions to initiate translation of the viral protein. This polyprotein is processed by cellular and viral proteases to produce specific viral gene products. Structural proteins including the capsid and envelop proteins are found at the N-terminus of the polyprotein while the nonstructural proteins (the proteases and proteins forming the replicase complex) are found at the C-terminus. The IRES, NS3 protease and RNA-dependent polymerase are under intense investigation as potential therapeutic targets.
Epizootiology
HCV is not a natural infection of nonhuman primates and attempted experimental inoculation of a number of species has failed to result in transmission (Abe et al., 1993). Chimpanzees are susceptible to infection and remain an important animal model of this condition (Choo et al., 1989).
Pathogenesis and Pathology
According to the World Health Organization there are approximately 170 million individuals chronically infected with HCV worldwide. Only 4 million of these individuals are found in North America with the vast majority located in Asia and Africa. These are areas that also have a high prevalence of HIV, and co-infection of patients with HCV and HIV is increasingly a concern. The natural history of disease is prolonged with average time to the development of chronic hepatitis being 10 years, 20 years for cirrhosis, and 30 years for hepatocellular carcinoma.
Following exposure there are two disease patterns in individuals infected with HCV: one characterized by control and recovery and the other with chronic infection and progression. In the first pattern there is evidence of viral replication and hepatic damage in the first few months following infection. The rise in ALT may be associated with clinical signs; however, the host mounts an effective immune response and the appearance of anti-HCV antibodies is associated with resolution of the viremia and abatement of hepatic disease. In the second pattern primary infection is associated with clinical signs but the immune response is only partially effective in controlling viral replication.
The chimpanzee has been the primary animal model involved in HCV research and was in fact instrumental in the initial recognition of HCV as a distinct clinical entity and molecular characterization of the virus (Tabor et al., 1979, Choo et al., 1989). Experimental inoculation recapitulates the course of human infection and the model has several advantages including: (1) susceptibility to infection with HCV; (2) similarities in onset of disease, level of viremia and timing of serologic responses and elevations in liver enzymes; and (3) the ability to obtain liver tissue and examine early time points. The model continues to find important uses and recently demonstrated the efficacy of inhibition of miRNA122 in lowering viral load in chronically infected animals (Lanford et al., 2010).
GB Agent Viruses: GBV-A, GBV-B, and GBV-C
Introduction
Two novel viruses have been identified using subtractive PCR methodology on tissue samples from tamarins infected with the serially passaged “GB agent” (Schlauder et al., 1995a, Simons et al., 1995). This agent was first identified in the mid-1960s when serum from a human patient with acute hepatitis was inoculated into four marmosets that subsequently developed a nonsuppurative portal hepatitis (Deinhardt et al., 1967). At the time, investigators questioned whether the agent identified in marmosets represented a human pathogen or reactivation of an indigenous marmoset virus (Parks and Melnick, 1969).
Subsequent molecular techniques have identified two distinct viruses (designated GBV-A and GBV-B) in tamarins infected with pooled marmoset plasma from the original studies. Furthermore, a third virus (GBV-C) with close homology to GBV-A has been found in human hepatitis patients; however, the relationship of this virus to clinical disease in humans remains to be determined (Zuckerman, 1995). Finally, PCR using primers directed against the 5’ end of GBV-A has identified GBV-A sequences within tamarins not exposed to the GB agent, suggesting that the virus may be more widespread than originally thought (Schlauder et al., 1995b).
Etiology
These GB agents, of the genus Hepacivirus, are closely related to hepatitis C virus of humans and share similar genomic organization and replication strategies. They are single-stranded, positive-sense RNA viruses with a 9.5-kb genome encoding a single polyprotein that is cleaved by cellular and viral proteases. Structural proteins are encoded within the N-terminus of the polyprotein and the homologous nonstructural proteins encoded within the C-terminus. GBV-A and -C are unique among the flaviviridae in that they lack a well-defined capsid protein. As with all flaviridae translation of the viral proteins is mediated by an internal ribosome entry site (IRES), a highly structured RNA sequence within the 5’ NTR. The IRES binds to the host 40S ribosomal subunit and the eukaryotic initiation factor to precisely position the start codon at the ribosome decoding site. One of the critical elements of the IRES is the IIIc stem loop which is highly conserved not only among HCV genotypes but GBV-B and pestiviruses. Deletion of the IIIc loop results in a significant reduction of IRES-initiated translation and thus has been an attractive target for small molecule inhibitors. The structure and function of the IIIc stem loop have been compared between HCV and GBV-B and have revealed extensive homology suggesting that GBV-B may represent an appropriate model to study such inhibitors.
Epizootiology
GBV-A and now additional variants have been identified in a number of New World nonhuman primate species. Distinct GBV-A viral sequences have been detected in moustached tamarins (Sanguinus labiatus and S. mystax), cotton-topped tamarins, owl monkeys (Aotus trivirgatus), and common marmosets. The origin and importance of GBV-like agents to wild and captive marmosets is presently unclear. Samples from wild-caught primates in South America have found animals to be naturally infected with these agents and distinct sequence differences have been observed between isolates from captive colonies and those in wild animals. There are significant variations in these isolates with 52% to 72% identity at the nucleotide level among the different strains. Cross-species transmission studies suggest high host specificity. Together this evidence suggests that GBV-A variants circulate in many wild populations of neotropical primates.
The epizootiology of GBV-B infection is less clear and the agent has not been recognized outside of strains derived from passage 11 GB serum. The natural host and associated disease processes are unknown.
Pathogenesis and Pathology
No clear disease process has been associated with GBV-A or -C. GBV-C, also known as hepatitis G virus, was initially associated with fulminant hepatitis in humans; however, additional work revealed that if GBV-C caused hepatic disease this was an infrequent occurrence. The initial association with hepatic disease is probably due to the fact that GBV-C is often co-transmitted with HCV as a bloodborne pathogen. GBV-C viremia is common in the US with about 1.5% of normal blood donors infected. The peripheral viremia is cleared with the development of the virus-specific antibody response. Similar viruses have been detected in wild chimpanzee populations (Birkenmeyer et al., 1998).
GBV-B produces a nonsuppurative hepatitis following intravenous inoculation of tamarins or marmosets and has been proposed as a small primate surrogate model of HCV infection. Tamarins, owl monkeys, and common marmosets appear to be susceptible and develop increases in liver function test values after intravenous inoculation. In marmosets hepatic pathology and peripheral viremia could be quantified biochemically, immunophenotypically and morphologically, and persisted for periods of up to 6 months in some animals (Jacob et al., 2004). Hepatitis was characterized by a marked influx of CD3+ CD8+ T lymphocytes and CD20+ B cells within the first 2 months of primary infection. In vitro systems to cultivate the virus in primary marmoset hepatocytes have also been developed (Jacob et al., 2007).
Arenaviridae
Arenaviruses are pleomorphic, enveloped viruses 110–130 μm in diameter containing two segments of RNA that encode at least three gene products. A characteristic biological feature of arenaviruses is life-long viral persistence within the definitive rodent host. The persistently infected host sheds the virus in urine and body secretions which then contaminate the environment and play a critical role in the transmission to the inadvertent (primate or human) host. A number of arenaviruses have been identified, with at least 23 species recognized by the ICTV. Viruses are classified according to antigenic properties and genetic differences into two groups: the Tacaribe or New World serocomplex and the Lassa-lymphocytic choriomeningitis (LCM) or Old World serocomplex (Table 1.5 ). Other than lymphocytic choriomeningitis virus (LCMV) which has a worldwide distribution, members of the Arenaviridae family demonstrate a restricted geographic distribution determined primarily by the range of the reservoir species. Several arenaviruses including Machupo, Junin, Guanarito, Sabia, and Lassa fever viruses cause hemorrhagic fever in humans. These viruses are all HHS select agents.
TABLE 1.5.
Arenaviridae
| Complex | Virus |
|---|---|
| Old World | Lymphocytic choriomeningitis virus |
| Lassa fever virus | |
| New World | Junin virus (Argentine hemorrhagic fever) |
| Machupo virus (Bolivian hemorrhagic fever) |
Lymphocytic Choriomeningitis Virus
Introduction
A rapidly progressive viral hepatitis occurring in zoological collections has been characterized (Montali et al., 1989). Outbreaks have been reported in golden lion tamarins, emperor marmosets (Saguinus imperator), pygmy marmosets (Cebuella pygmaea), common marmosets, cotton-topped tamarins, white-fronted tamarins (S. nigricollis), saddle-backed tamarins (S. fuscicollis), and Goeldi’s monkeys (Potkay, 1992, Montali, 1993, Asper et al., 2001). Due to the predilection for disease in these species, the original reports termed this virus Callitrichid Hepatis Virus.
Etiology
The etiologic agent is LCMV. This arenavirus is pleomorphic and 85–105 nm in diameter. The genome is a two-segmented RNA genome consisting of the S segment that encodes the glycoprotein precursor and nucleoprotein and the L segment that encodes the viral polymerase and small zinc-binding protein. The virus has been identified within cytoplasmic vesicles of affected animals (Montali, 1993). Although reports of natural infections in nonhuman primates are limited to the above species, macaques are susceptible to experimental infection with LCMV and therefore wider species susceptibility may be anticipated. LCMV infection in macaques is similar in presentation to and used as a surrogate model for Lassa fever.
Epizootiology
Animals become infected through contact with or ingestion of infected rodents. The virus may be introduced to colonies by either wild mice or the intentional feeding of neonatal laboratory mice (“pinkies”) (Montali et al., 1995b). The incubation period is 1–2 weeks. The incidence and mortality rate may be high. Serologic evidence of infection of captive marmosets without recognized clinical signs has been demonstrated (Potkay, 1992).
Clinical Findings
Clinical signs include dyspnea, anorexia, weakness, and lethargy. Animals may be jaundiced and evidence of coagulopathy may be apparent. In many cases there is sudden death without clinical signs.
Pathogenesis and Pathology
At necropsy, hepatosplenomegaly, pleural and pericardial effusions, jaundice, and subcutaneous and intramuscular hemorrhages are characteristic (Montali, 1993). Histopathologic lesions within the liver consist of multifocal hepatic necrosis with infiltration by lymphocytes and neutrophils. Acidophilic bodies representing apoptotic hepatocytes are prominent and may be found in hepatic sinusoids or within Kupffer cells (Montali, 1993). The LCMV antigen and mRNA have been identified within hepatocytes, suggesting that direct viral infection may be responsible for the observed hepatocellular necrosis (Montali et al., 1995a). In addition, necrosis may be evident in the abdominal lymph nodes, adrenal gland, spleen, and gastrointestinal tract (Montali et al., 1989). Lymphoid tissue demonstrates hyperplasia of follicular and parafollicular regions with depletion of germinal centers. Immunohistochemistry for viral antigen demonstrates presence of virus within macrophages and intrafollicular reticular cells although not in lymphocytes. Viral antigen is also present within both epithelial and endothelial cells from pulmonary and renal tissues (Montali et al., 1995a) (Figure 1.20 ).
FIGURE 1.20.

Lymphocytic choriomeningitis virus (LCMV).
LCMV can be transmitted to callitrichids causing severe hepatitis. Grossly jaundice, hepatomegaly and serosal hemorrhages are observed (A). (Photograph courtesy of Dr. Richard Montali and Smithsonian National Zoological Park.) In the liver brightly eosinophilic apoptotic (Councilman) bodies are evident (B) as well as intracytoplasmic eosinophilic cellular inclusions (C).
Laboratory Findings
Elevations in liver enzymes, bilirubin, and serum alkaline phosphatase have been demonstrated.
Treatment
Treatment directed at correcting hypovolemia and electrolyte disturbances may be of some benefit. Ribavirin therapy in macaques inoculated with the related Junin, Machupo, and Lassa fever viruses has shown some efficacy (Jahrling et al., 1980). The pharmacokinetics and safety of these drugs in marmosets are unknown.
Prevention
Screening of food source rodent colonies for LCMV and preventing contact with wild rodents should prevent disease transmission. Biologics of murine origin should be screened for the presence of LCMV before use in tamarins or marmosets.
Zoonotic Potential
Lymphocytic choriomengingitis virus may cause disease in humans. The most common presentation is a subclinical or a self-resolving influenza-like illness. Severe disease presenting as meningitis or meningoencephalitis may occur. The virus is considered a teratogenic pathogen resulting in fetal loss or developmental defects. Seroconversion of veterinarians in contact with infected marmosets has been demonstrated (Montali et al., 1995a).
Lassa Fever Virus
Lassa fever virus is a member of the Old World Arenaviridae serogroup and is responsible for outbreaks of human disease in western Africa. Manifestation of disease in humans varies from asymptomatic to a fatal hemorrhagic fever. Experimental inoculation of rhesus and cynomolgus macaques results in disease that recapitulates that observed in humans (Callis et al., 1982, Baize et al., 2009). Clinical symptoms include fever, anorexia, and an acute respiratory syndrome. On histopathologic examination an interstitial pneumonia and alveolitis may be identified as well as a multifocal, necrotizing hepatitis with minimal to moderate periportal, mononuclear infiltrates. Dendritic cells, macrophages, hepatocytes, and endothelial cells are the cellular targets of infection. Earlier and more robust humoral and cellular immune responses are associated with control of viral replication and survival in the cynomolgus model (Baize et al., 2009). Severe, fatal disease associated with experimental Lassa virus infection in common marmosets has also been described (Carrion et al., 2007). Animals developed fever and weight loss accompanied by high levels of viral replication. At necropsy, the characteristic multifocal hepatic necrosis was present in addition to evidence of hepatocyte proliferation as indicated by KI67 immunostaining. Animals demonstrated lymphoid depletion characterized by loss of T and B cells. Depleted regions were replaced with histiocytic infiltration. Such changes were likely associated with impairment of the adaptive immune response and progression of disease.
Bolivian Hemorrhagic Fever Virus: Machupo Virus
Machupo virus is an arenavirus from the New World serogroup that has contributed to epidemics of hemorrhagic fever in Central and South America. The susceptibility and importance of nonhuman primates in natural disease are unknown. Rhesus macaques, Geoffrey’s marmosets, and African green monkeys may be experimentally infected with the Machupo virus. In these species it causes a severe, disseminated infection involving the central nervous system, gastrointestinal tract, and lungs. Hemorrhages are found in the skin, liver, oral cavity, and adrenal cortex. The virus has been isolated from rodents within the Muridae and Cricetidae families.
Retroviridae
The family Retroviridae consists of two subfamilies; the Orthoretrovirinae containing six genera (including the four that infect nonhuman primates: Betaretrovirus, Deltaretrovirus, Gammaretrovirus, and Lentivirus) and the Spumaretrovirinae containing one genus; Spumavirus. These viruses are single-stranded RNA viruses that possess the enzyme reverse transcriptase (RT) that transcribes RNA into double-stranded DNA and the enzyme integrase that enables the DNA provirus to integrate into the host genome. The retrovirus genome consists of four major genes including gag encoding the structural capsid proteins, pol encoding the RT and integrase enzymes, env encoding the transmembrane and envelope proteins, and pro encoding a protease enzyme. Additional genes encoding regulatory proteins vary among specific viruses (Figure 1.21 ).
FIGURE 1.21.

Ultrastructural morphology of simian retroviridae.
Simian retrovirus (A), simian immunodeficiency virus (B) and simian foamy virus (C).
Orthoretrovirinae
Simian Retrovirus (SRV)
Introduction
During the 1960s and 1970s, several regional primate research centers located in the United States experienced epizootics of malignant lymphoma and immunosuppressive disease (Stowell et al., 1971, Smith et al., 1973, Henrickson et al., 1983, Hunt et al., 1983, King et al., 1983). At the time these epizootics were suspected to be the result of an underlying simian viral infection. The issue remained unresolved until the early 1980s when, spurred by a burgeoning epidemic of immunodeficiency in homosexual men, investigators simultaneously identified two agents capable of inducing immunosuppressive disease in macaques. The first virus, a member of the Betaretrovirus genus, was identified and designated simian retrovirus type D (SRV/D) (since designated simian retrovirus [SRV]) (Daniel et al., 1984, Gravell et al., 1984, Marx et al., 1984). The second, a member of the Lentivirus genus, was designated simian immunodeficiency virus of macaques (SIVmac) (Daniel et al., 1985, Letvin et al., 1985). This primate lentivirus is closely related to the etiologic agent of human AIDS and has subsequently been the subject of intensive investigation as an animal model of human immunodeficiency virus infection. Because SRV is more distantly related to the human immunodeficiency viruses than SIV, it has received far less attention than its lentivirus cousin. Although SIV may be of greater scientific interest as an animal model of human AIDS, SRV is clearly of greater significance in the management and care of captive macaques. As experience has shown, not only is SRV more difficult to eliminate from macaque colonies, it is also responsible for most cases of spontaneous viral-induced immunodeficiency in captive members of these species.
Etiology
Simian Betaretroviruses (formerly Type D retroviruses) exist as both endogenous and exogenous forms (Table 1.6 ). Endogenous virus sequences have been recognized in squirrel monkeys, spectacled langurs, members of the Cercopithecinae, and are suspected in several species of colobines (Heberling et al., 1977, Todaro et al., 1978, van der Kuyl et al., 1997). SRV was originally isolated in 1970 from the mammary neoplasm of a female rhesus macaque and was designated the Mason–Pfizer monkey virus (MPMV) (Chopra and Mason, 1970). Subsequent work indicated that this virus caused a wasting syndrome and thymic atrophy when inoculated into infant rhesus macaques and that approximately 25% of all macaques housed at US regional primate research centers were seroreactive to MPMV or a closely related virus (Fine et al., 1975, Fine and Arthur, 1981). Several SRV serotypes have since been reported and include SRV type 1 (SRV-1), type 2 (SRV-2), type 4 (SRV-4), and type 5 (SRV-5). More recently SRV-Tsukuba (SRV-T) was isolated from macaques housed at the Tsukuba Primate Center in Japan while SRV-6 and SRV-7 were identified in free-ranging rhesus macaques and langurs (Semnopithecus entellus) from India (Nandi et al., 2000, Nandi et al., 2006, Hara et al., 2005). These three novel viruses remain untyped by antibody neutralization assays. Significant genetic variation exists among the serotypes. The prototypic retrovirus, MPMV (SRV-3), is most closely related to SRV-1 and SRV-2, which are the viruses that serve as the major causes of immunosuppressive disease in captive macaques. While SRV-1 is most typically detected in rhesus macaques and SRV-2 more commonly isolated from cynomolgus or pig-tailed macaques, animals housed in research environments may carry any of the serotypes or possibly multiple serotypes (Lerche and Osborn, 2003, Montiel, 2010).
TABLE 1.6.
Simian Betaretroviruses
| Classification | Host | Ref. |
|---|---|---|
| Endogenous | ||
| Squirrel monkey retrovirus | S. saimiri | Heberling et al. (1977) |
| Langur virus (PO-1-Lu) | Presbytis obscurus | Todaro et al. (1978) |
| Simian endogenous retrovirus | Cercopithecinae | Van der Kuyl et al. (1997) |
| Exogenous | ||
| Simian retrovirus type 1 (SRV-1) | Macaca sp. | |
| Simian retrovirus type 2 (SRV-2) | Macaca sp. | |
| Mason–Pfizer monkey Virus (SRV-3) | Macaca sp. | Chopra and Mason (1970) |
| Simian retrovirus type 4 (SRV-4) | M. fascicularis | |
| Simian retrovirus type 5 (SRV-5) | M. mulatta | |
| Simian retrovirus type 6 (SRV-6) | Semnopithecus entellus | Nandi et al. (2000) |
| Simian retrovirus type 7 (SRV-7) | M. mulatta and S. entellus | Nandi et al. (2006) |
| Simian retrovirus Tsukuba (SRV-T) | Hara et al. (2005) |
Epizootiology
SRV appears to be transmitted readily among captive macaques. SRV-seropositive rates vary widely among colonies and likely relate to husbandry, housing practices, and efforts to eliminate the virus. Investigation of an epizootic of SRV in captive Celebes black macaques (Macaca nigra) revealed an increase in the seropositive status from 0% to greater than 90% during an 8-year period following the introduction of several infected animals to the colony (Lerche et al., 1986, Shiigi et al., 1989). New World primates appear to be resistant to SRV infection in part due to species-specific variability in the TRIM5α protein that is a major factor in determining host range of retroviral infections (Diehl et al., 2008). This protein facilitates premature dissociation of virus cores and subsequent proteasomal degradation.
SRV-1 may be isolated from the saliva of healthy carrier animals, and biting with inoculation of saliva or blood is the most likely method of horizontal transmission (Lerche et al., 1986). Wilkinson and colleagues determined that exposure to blood from viremic animals carried a higher risk of infection while blood from non-viremic animals with provirus integrated in circulating mononuclear cells was less likely to transmit infection (Wilkinson et al., 2003). Vertical transmission may play an additional role in the propagation of the virus within macaque colonies. Cesarean-derived infants raised in isolation may be infected (Tsai et al., 1990). Moreover, mother-to-infant transmission may occur in the perinatal or postnatal period. Of fundamental importance to the design and implementation of control strategies is the fact that animals may be persistently infected, healthy, and shedding virus while seronegative for antibodies against SRV. It is these seronegative virus-positive individuals that frustrate control strategies. Evidence suggests that in utero infection may be more likely to result in this seronegative, virus-positive status than transmission at other times (Moazed and Thouless, 1993). These animals may play a key role in viral persistence within colonies.
Pathogenesis and Pathology
Superficially there are many similarities between the immunosuppressive diseases induced in macaques by SRV and simian lentiviruses. There are, however, fundamental differences in their molecular biology and genomic organization that impart differences in the resulting clinical syndrome (Table 1.7 ) (Lackner, 1988). SRV has a wider tissue tropism than simian lentiviruses and readily infects T cells (CD4+ and CD8+), B cells, macrophages, epithelial cells, and cells of the choroid plexus (Maul et al., 1988, Lackner et al., 1989).
TABLE 1.7.
Characteristic Differences of SIVmac and SRV Infection of Macaques
| Characteristic | SIVmac | SRV |
|---|---|---|
| Natural host range | Related viruses endemic in several species of African monkeys | Endemic in several species of Asian macaques |
| Significance as natural disease in captive macaques |
Serologic surveys indicate low prevalence | Most significant viral agent of acquired immunodeficiency in captive macaques |
| Occurrence of seronegative, viral positive infection | Uncommon in natural host |
Common |
| Viral tropism | CD4+ T lymphocytes and macrophages | B and T lymphocytes, macrophages, and epithelial cells |
| Uniquely associated diseases | Giant cell pneumonia and encephalitis; SIV arteriopathy; lymphoma; Mycobacterium avium | Retroperitoneal and subcutaneous fibromatosis in association with RFHV; noma |
The mechanism by which SRV produces the severe immunosuppression characteristic of this disease is unknown. Animals may harbor the virus asymptomatically for extended periods with few or no clinical signs. In asymptomatic animals there may be generalized lymphadenopathy characterized by varying proportions of follicular and paracortical lymphocytic hyperplasia consisting of immature CD4+ and CD8+ T lymphocyte subsets. With the onset of severe immunosuppression, eventually the spleen, thymus, and lymph nodes evidence marked lymphoid depletion and complete effacement of normal architecture. In these nodes the paracortex is often depleted of lymphocytes and plasma cells, which are largely replaced by histiocytes and contain small postreactive follicles with hyalinized arterioles (Osborn et al., 1984). Hyperplastic or hypoplastic bone marrow may be present and contributes to the frequently observed anemia and granulocytopenia.
The hallmark of the acquired immunodeficiency in macaques induced by both SRV and SIVmac is the occurrence of unusual opportunistic infections. Despite some similarities, there are, for reasons that are presently unclear, differences in the propensity of these viruses to induce disease by specific opportunistic organisms. SRV-infected animals appear to be more susceptible to infection by pyogenic bacteria and less susceptible to infection by Mycobacterium avium, Pneumocystis carinii, SV40, and adenovirus. Moreover, SIV-specific lesions are lacking and the occurrence of overt lymphoma is uncommon in SRV-infected animals. A rapidly progressive necrotizing gingivitis and stomatitis known as noma (cancrum otis) is most often described in SRV-infected animals while other common opportunistic infections such as disseminated CMV, oral and esophageal candidiasis, intestinal cryptosporidiosis can be observed in either SRV or SIV-infected animals (Lowenstine, 1993).
In addition to a syndrome of immunosuppressive disease, a unique fibroproliferative disorder termed retroperitoneal fibromatosis (RF) was recognized in association with SRV infection. In 1997 RFHV was identified in rhesus and pigtail macaque RF specimens. These lesions have not been recognized in all large macaque colonies suggesting that RFHV is required but not sufficient for RF pathogenesis and that additional cofactors, possibly SRV coinfection and associated immunosuppression, are required. This syndrome is discussed in more detail in the section on RFHV. A localized subcutaneous fibromatosis has also been reported characterized by multiple proliferative nodules within the subcutis and oral cavity in pig-tailed macaques (Tsai et al., 1985b).
Clinical Findings
SRV infection of rhesus macaques may be responsible for the induction of several phenotypes, including (1) the occurrence of severe, rapidly progressive disease accompanied by high viremia and no antibody response, (2) the occurrence a low-grade viremia and a transient antibody response with or without disease, and (3) no clinical disease with a high antibody response and transient viremia.
The clinical features and AIDS definition of SRV infection are presented in Table 1.8 . Young animals often present for small body stature and failure to thrive. Body weight may be 40–50% of normal cagemates and these animals are often physically traumatized through intraspecies aggression. Intermittent to chronic gastroenterocolitis is common and results from direct viral infection of mucosal cells or damage induced by a variety of opportunistic organisms. Because the occurrence of an antibody-negative viremia is common in these young animals, diagnosis is best achieved through viral isolation or PCR. Alternatively, in cases in which serum or frozen tissue is not available, the virus may be visualized in formalin-fixed, paraffin-embedded tissue through the use of SRV-specific probes and in situ hybridization. In older animals the illness is more insidious and while wasting may be conspicuous, clinical signs often relate to the specific opportunistic infections present.
TABLE 1.8.
Case Definition of Simian Retrovirus-Induced Simian AIDSa
| Generalized lymphadenopathy and/or splenomegaly accompanied by at least four of the following clinical and laboratory findings: |
| Weight loss (>10%) |
| Fever (>103°F) |
| Persistent refractory diarrhea |
| Chronic infections unresponsive to therapy |
| Opportunistic infections |
| Noma (cancrum oris) |
| Retroperitoneal or subcutaneous fibromatosis |
| Hematologic abnormalities |
| Anemia (PCV <30%) |
| Neutropenia (<1700) |
| Lymphopenia (<1600) |
| Thrombocytopenia (<50,000) |
| Pancytopenia |
| Bone marrow hyperplasia |
| Characteristic lymph node lesions |
From Lackner (1988).
Prevention
Because of the potential for SRV to cause clinical and subclinical disease as well as serve as a significant research confounder, efforts to establish SRV-free macaque colonies are paramount to the continued use of these animals in biomedical research. To date these efforts have been very successful despite the difficulties in detecting infection. Because macaques may be seronegative but harbor virus, viral isolation or PCR to confirm virus-free status must be used in conjunction with serological methods of detection (White et al., 2009a). Successful algorithms used to establish retrovirus-free breeding colonies have been published (Schroder et al., 2000, Lerche and Osborn, 2003). These protocols utilize PCR detection of viral nucleic acid or viral isolation combined with antibody detection (EIA and Western blot) to screen all animals. Once established, colonies should be self-propagating and remain closed with periodic retesting to verify continued virus-free status.
Zoonotic Potential
The risk of zoonotic transmission of SRV is not completely elucidated. The most convincing evidence for human SRV infection is presented in a case report describing the isolation of virus and SRV transcripts in lymphoma tissue from an HIV/AIDS patient. This individual was additionally seropositive for antibodies directed against multiple viral proteins although they had no known nonhuman primate exposure (Bohannon et al., 1991). Serological and molecular techniques were also used to demonstrate possible SRV exposure with a relatively high frequency in healthy individuals from Guinea (Morozov et al., 1996). However, a survey of over 200 individuals occupationally exposed to nonhuman primates demonstrated a very low seroprevalence of 0.9% and virus was not able to be detected via viral isolation or molecular techniques (Lerche et al., 2001). Based on this evidence zoonotic exposure should be considered a possibility although the risk may be quite low.
Simian T-Cell Leukemia Virus
Introduction
Simian T-cell leukemia virus (STLV), human T-cell leukemia virus (HTLV), and bovine leukemia viruses are species within the Deltaretrovirus genus of the subfamily Orthoretrovirinae. The viruses within this genus share a common ancestry, genomic organization, and the propensity to induce lymphoproliferative disease in the host animal. The association between HTLV and various disease processes has raised interest in closely related simian viruses. There are three viral subtypes, numbered 1 through 3, for both STLV and HTLV. Analysis of a STLV-1 isolates obtained from a variety of nonhuman primate species demonstrate high degrees of homology with human isolates. This combined with a clustering by geography rather than by host species strongly suggests cross-species transmission events and a nonhuman primate origin of HTLV-1 (Sakesena et al., 1994, Mahieux et al., 1998, Van Dooren et al., 2001, Sintasath et al., 2009).
HTLV-1 has a worldwide distribution and is endemic in Japan and the Caribbean basin, as well as regions within subSaharan Africa, South America and the Middle East. While usually asymptomatic, in approximately 2–5% of cases infection may be associated with adult T-cell leukemia/lymphoma (ATLL), tropical spastic paresis (TSP), and HTLV-1-associated myelopathy (HAM). As in human patients with HTLV-1, STLV-1 has been associated with lymphoproliferative disease in baboons, gorillas, macaques, and African green monkeys (Lee et al., 1985, McCarthy et al., 1990, Traina-Dorge et al., 1992). Although overt neoplastic disease is an uncommon sequela to infection of some species, STLV-1 agents immortalize T cells in vitro and their presence may complicate the interpretation of experimental protocols.
Etiology
Seroreactivity to STLV-1 or STLV-like agents has been demonstrated in at least 33 species of captive and wild African and Asian nonhuman primate species, including Cercopithecus spp., macaque species, Patas monkey, olive baboon (Papio anubis), mandrill (Mandrillis sphinx), gorilla, and siamang (Symphalangus syndactylus) (Hayami et al., 1984, Ishikawa et al., 1987, Sakakibara et al., 1986). Restriction patterns of initial STLV-1/HTLV-1 isolates suggested that a number of distinct but related viruses were contained within the STLV grouping. Sequencing data have since confirmed the existence of seven clusters or clades. The C clade contains Asian STLV/HTLVs, whereas the A, B, and D–F clades contain African STLV/HTLV isolates. The seventh clade G is populated by STLV-1 species isolated from Papio sp. (Koralnik et al., 1994). It is postulated that this diversity is due to repeated cross-species transmission within restricted geographic localities. Moreover, it suggests that STLV-1 has been present in nonhuman primate populations longer than HTLV-1 has been in humans and a likely STLV origin of HTLV-1. Differences in the ability of the various isolates to induce lymphoproliferative disease are largely unknown.
Epizootiology
Unless steps have been taken to exclude the virus from colonies, serologic surveys may indicate high rates of infection in captive African green monkeys, macaques, sooty mangabeys, and baboons (Traina-Dorge et al., 2005, Sariol et al., 2006, d’Offay, 2007). Seroprevalence may be high in free-ranging populations. These surveys indicate an increasing prevalence with age and while the mechanism of natural transmission is unknown, parenteral and sexual routes are suspected of being of greater importance than perinatal transmission.
Pathogenesis and Pathology
STLV-1 is a cell-associated virus that is spread via breeding, breast feeding, transfusion with infected blood cells, and blood contamination during wounding episodes (d’Offay et al., 2007). The virus has been demonstrated to infect both CD4+ and CD8+ cells with proviral loads significantly higher within CD4+ cells (Souquiere et al., 2009). Virus replicates within the host via a clonal expansion of infected cells contributing to the low genetic variability of this virus compared to other retroviruses that rely on continuous, error-prone reverse transcription (Gabet et al., 2003).
Although STLV-1 is a common and usually asymptomatic infection, it has been associated with lymphoproliferative disease in several nonhuman primate species. This has perhaps been best characterized in baboons (Voevodin et al., 1985, McCarthy et al., 1990, Hubbard et al., 1993). In this species, STLV-1 infection has been linked to the development of an ATLL-like syndrome characterized by non-Hodgkin’s lymphoma and leukemia. Involvement of the lymph nodes, spleen, liver, skin, and especially lung is common. Overt leukemia has been documented in greater than 50% of the cases and is occasionally associated with the presence of circulating multilobulated neoplastic lymphocytes, a clinical feature of ATLL (McCarthy et al., 1990). Unlike human patients with ATLL, hypercalcemia is uncommon. Within the lung, the earliest changes may be present in a perivascular and/or peribronchiolar distribution. Although a variety of cell types have been recognized, most cases have been of T-cell lineage (CD2+ CD4+). Interestingly, an ATLL-like disease consisting of cells expressing CD8+ was identified in an African green monkey. Spontaneously transformed cell lines from STLV-1 infected African greens also consisted of CD8+ cells suggesting that STLV-1 may preferentially transform CD8+ cells in this species (Akari et al., 1998). Histologically, infiltrates vary from a monomorphic population of neoplastic lymphocytes to a more pleomorphic population accompanied by multinucleated giant cells, necrosis, and inflammatory cells.
Although STLV-1 is clearly associated with lymphoproliferative disease, the mechanism involved is not completely understood. The tax gene product stimulates transcription of viral mRNA by acting on the 5′ long terminal repeat (LTR) and is highly conserved among all STLV/HTLV isolates, suggesting a fundamental role in viral replication and disease pathogenesis (Sakesena et al., 1994). In addition to stimulating the 5′ LTR, tax may activate host genes responsible for controlling T-cell proliferation, including c-fos, c-sis, interleukin-2 (IL-2), IL-2r, and GM-CSF, thereby leading to polyclonal T-cell expansion. It is postulated that secondary events are required for monoclonal neoplastic transformation. Monoclonal integration of STLV-1 has been demonstrated in African green monkeys with non-Hodgkin’s lymphoma and preneoplastic lymphoproliferative disease (Tsujimoto et al., 1987). Such integration is considered definitive evidence of the etiologic role of PTLV in lymphomagenesis. Coinfection with other viral agents such as SIV or Epstein–Barr-like viruses may promote neoplastic transformation (Voevodin et al., 1985, Traina-Dorge et al., 1992).
Clinical Findings
STLV-1-associated lymphoma/leukemia in baboons is clinically characterized by depression, anorexia, regional or generalized lymph node enlargement, and hepatosplenomegaly. As in humans with ATLL, radiographic and histologic evidences of pulmonary infiltrates are frequent. Cutaneous involvement, hypercalcemia, and effusions may be noted less commonly. Leukocytosis and multilobulated neoplastic cells within peripheral blood smears are present with the majority but not all cases.
Prevention
Altered cytokine profiles secondary to STLV-1 infection indicate potential for significant confounding of research protocols prompting efforts to eliminate this virus from breeding colonies (Lerche and Osborn, 2003). A two-stage testing algorithm to evaluate macaque sera for the presence of antibodies to STLV-I has been published (Lerche and Osborn, 2003). Samples are initially screened by enzyme immunoassay (EIA), and negative results indicate a provisional virus-free status. Positive results are further tested by confirmatory Western blot, and reacting animals are culled or segregated from the colony. Additionally, PCR should be used to test seronegative or seroindeterminant animals. Occasionally there may be long intervals to seroconversion indicating the need for continual monitoring for virus during the establishment of SPF colonies (Lerche and Osborn, 2003).
Other Simian T-Cell Leukemia Viruses
A virus closely related to HTLV-2 and designated STLV-2 has been identified in bonobo chimpanzees (P. paniscus) (Van Brussel et al., 1998). HTLV-2 is present within regions of North America, South America, and Africa and shows approximately 75% identity to the STLV-2 sequences. STLV-2 has not been linked to disease in nonhuman primates. A reported STLV-2 isolate from a single spider monkey (Ateles fusciceps) has not been confirmed and likely represents a laboratory contaminant (Chen et al., 1994).
HTLV-3 has been identified in human populations although currently there are no known disease associations. Simian counterparts to HTLV-3 have been identified in a variety of nonhuman primate species including red-capped managbys (Cercocebus torquatus), Hamadryas baboons (Papio hamadryas), Cercopithecus sp., and agile mangabeys (Cercocebus agilus) (Meertens et al., 2002, Courgnaud et al., 2004). Coinfections with STLV-1 and STLV-3 have been documented.
HTLV-4 is rare, having only been identified in a hunter from Cameroon (Wolfe et al., 2005). A simian counterpart to HTLV-4 is yet to be identified.
Simian Sarcoma Virus and Gibbon Ape Leukemia Virus
These viruses are members of the genus Gammaretrovirus. Simian sarcoma virus was isolated from a spontaneous fibrosarcoma of a pet woolly monkey (Wolfe et al., 1972). Further investigation revealed the isolate to be composed of two agents; a defective transforming virus (simian sarcoma virus, SSV-1) and a replication competent helper virus (simian sarcoma-associated virus, SSAV-1) (Theilen et al., 1971, Wolfe et al., 1972). When injected intracerebrally into newborn marmosets, animals developed gliomas from which virus could be recovered and identified (Johnson et al., 1975).
Gibbon ape leukemia virus (GALV) has been isolated from a number of captive gibbons with spontaneous hematopoietic neoplasms. Various isolates have been identified (GALV, GALV-1, GALV-SEATO, GBr-1, and Gbr-3) with differing abilities to induce malignant lymphoma and leukemia. Clinical aspects of a spontaneous epizootic in white-handed gibbons have been reported and are characterized by a prolonged clinical course, a marked elevation in the peripheral granulocyte count, and involvement of bone marrow, liver, lymph nodes, and spleen (DePaoli et al., 1973). As described above for SRV infection of macaques, gibbons may not seroconvert following persistent infection with this virus.
Simian Immunodeficiency Virus
Introduction
Simian immunodeficiency viruses (SIVs) are a group of closely related viruses within the Lentivirus genus of the family Retroviridae, which infect a variety of Old World nonhuman primates. The striking similarity between SIV-induced disease in macaques and HIV-induced disease in humans makes the SIV-infected macaque an extremely valuable model for the study of human HIV infection. Macaques infected with SIV develop many of the same clinical and pathologic abnormalities that occur in AIDS patients and die from the same array of opportunistic infections (OI). Using SIV-infected macaques as a model of human AIDS has produced a volume of research concerning all aspects of lentiviral biology and pathogenesis that cannot begin to be summarized here. The lessons to be reinforced from a standpoint of colony management and conservation are that (1) well-adapted viruses may be carried asymptomatically and silently by one primate species and yet have devastating consequences when introduced into another species and (2) the occurrence of unusual opportunistic infections should spur a vigorous search for an underlying immunosuppressive etiologic agent.
Etiology
SIVs are indigenous viral agents of African nonhuman primate species and are enzootic in many populations. Prevalence varies by species and geographical region although it can be quite high in some populations (Hayami et al., 1994). Other than in rare instances, these viruses do not cause disease in their natural hosts and only manifest as an immunodeficiency syndrome when transferred to a different species. Over 40 different SIV isolates from various African species have been recognized. Viral strains are designated by a three-letter suffix to indicate the common name of the species from which they were originally isolated (e.g., SIVcpz from chimpanzees). When distinct viruses have been isolated from different host subspecies, the subspecies is designated with a second suffix (SIVcpzPtt from P. troglodytes troglodytes and SIVcpzPts from P. troglodytes schweinfurthii). The relatedness of SIV isolates correlates most closely with speciation of the host (Franchini and Reitz, 1994). Phylogenetic analysis reveals that isolates from each nonhuman primate species form monophyletic lineages. While this suggests that there has been significant co-speciation of virus and host, there are also numerous instances of cross-species transmission and viral recombination. With the continued survey of African primates and discovery of novel SIVs, further genetic characterization of these isolates will only add to the complexity of the phylogenetic classification. Currently the monophyletic lineages can be grouped into seven major lineages or clades (Table 1.9 ).
TABLE 1.9.
Major Simian Immunodeficiency Virus Lineages
| Lineage | Representative Isolates | Nonhuman Primate Species |
|---|---|---|
| Chimpanzee group | SIVcpzPtt | Pan troglodytes troglodytes |
| SIVcpzPts | P. troglodytes schweinfurthii | |
| SIVgor | Gorilla gorilla | |
| Arboreal guenon group | SIVsyk | Cercopithecus albogularis |
| SIVdeb | Cercopithecus neglectus | |
| SIVgsn | Cercopithecus nictitans | |
| SIVmon | Cercopithecus mona | |
| SIVmus-1 and SIVmus-2 | Cercopithecus cephus | |
| SIVery | Cercopithecus erythotis | |
| SIVden | Cercopithecus denti | |
| SIVblu | Cercopithecus mitis | |
| SIVasc | Cercopithecus ascanius | |
| SIVtal | Miopithecus ogouensis | |
| Red-capped mangabey group | SIVrcm | Cercocebus torquatus |
| Sooty mangabey group | SIVsmm | Cercocebus atys |
| SIVmac | Macaca mulattaa | |
| SIVmne | M. nemestrinaa | |
| SIVstm | M. arctoidesa | |
| African green monkey group | SIVagmVer | Chlorocebus pygerythrus |
| SIVagmGri | Chlorocebus aethiops | |
| SIVagmTan | Chlorocebus tantalus | |
| SIVagmSab | Chlorocebus sabaeus | |
| L’Hoest and mandrill group | SIVlho | Cercopithecus lhoesti |
| SIVsun | Cercopithecus solatus | |
| SIVpre | Cercopithecus preussi | |
| SIVmnd-1 | Mandrillus sphinx | |
| SIVwrcPbb | Piliocolobus badius badius | |
| SIVwrcPbt | Piliocolobus badius temminckii | |
| Mantled colobus group | SIVcol | Colobus guereza |
These animals are not natural hosts and demonstrate progressive disease with viral inoculation.
The chimpanzee group contains SIVcpzPtt, SIVcpzPts, and SIVgor, the simian viruses most closely related to HIV-1 (Peeters et al., 1989). Similarities in the viral genomes combined with opportunities for geographic comingling and exposure via hunting provides strong evidence that the emergence of HIV-1 resulted from at least three cross-species transmission events between apes and man (Desrosiers, 1990, Gao et al., 1999 ; Hahn et al., 2000). HIV-1 isolates are organized into three major groups, M–O. Viruses in group M have spread globally thereby causing the HIV-1 pandemic. Viruses from groups N and O have remained confined to patients living in Cameroon and surrounding regions. The origins of HIV-1 groupings M and N have been traced to troops of Pan troglodytes troglodytes residing in southeastern and south central Cameroon where the prevalence of infection in apes can range as high as 35% (Keele et al., 2006). A divergent SIVcpzPtt lineage is thought to have given rise to SIVgor which shares closest homology to HIV-1 group O. Evidence suggests two possibilities for a source of HIV-1 group O: independent transmission events resulting in transfer of virus from Pan troglodytes troglodyes to both gorillas and humans or, alternatively, western lowland gorillas serving as an intermediate host and transmitting virus directly to humans (Van Heuverswyn et al., 2006, Takehisa et al., 2009). As of yet there is no known human counterpart to the SIV harbored by P. troglodytes schweinfurthii. P. troglodytes versus and P. troglodytes vellerosus have not been found to harbor SIV. The origin of SIVcpz is thought to be the result of a recombination event involving viruses from two distinct lineages; an ancestor of SIVrcm of red-capped mangabeys and an ancestor of the arboreal guenon clade (Bailes et al., 2003). Chimpanzees exposed to these primate species through hunting likely contracted both SIVrcm and SIVgsn ancestral viruses which led to recombination within the chimpanzee host and subsequent emergence of SIVcpz.
The arboreal guenon clade contains viruses isolated from the Cercopithecus genus and represents the group with the largest number of nonhuman primate species known to be infected with SIV. Viral sequences have been detected from at least ten species including the Sykes’s monkey (SIVsyk; Cercopithecus albogularis), DeBrazza’s monkey (SIVdeb; C. neglectus), greater spot-nosed guenon (SIVgsn; C. nictitans), Mona money (SIVmon; C. mona), mustached monkey (SIVmus; C. cephus), red-eared guenon (SIVery; C. erythrotis), Dent’s mona monkey (SIVden; C. denti), Blue monkey (SIVblu; C. mitis), Schmidt’s guenon (SIVasc; C. ascanius) and talopin monkeys (SIVtal; Miopithecus ogouensis) (Emau et al., 1991, Bibollet-Ruche et al., 2004, Verschoor et al., 2004, Liegeois et al., 2006). Two co-circulating variants have been described in mustached monkeys ranging over a small region of Cameroon indicating that it is not necessarily geographic separation that results in divergent viral variants (Aghokeng et al., 2007). Four viruses in this group (SIVgsn, SIVmon, SIVmus, and SIVden) have a vpu gene. This accessory gene encodes a protein thought to enhance virion release and viral pathogenicity (Stephens et al., 2002). Originally it was believed that only HIV-1 and SIVcpz strains encoded a vpu gene. Identification of the vpu gene in SIVs of the arboreal guenon lineage provides further evidence that one of these viruses contributed the 3’ segment to the SIVcpz genome during an ancient recombination event (Courgnaud et al., 2002, Barlow et al., 2003).
The red-capped mangabey group currently contains one isolate found circulating in populations of the group’s namesake (Beer et al., 2001). The red-capped mangabey is closely related to sooty mangabeys, and viruses from both species contain a vpx gene. This would suggest that these viruses should cluster together. However, the pattern of clustering depends on the region of the SIVrcm genome examined. This genomic mosaicism indicates that the SIVrcm strain arose from multiple recombination events. The virus has thus been designated its own lineage. As mentioned previously, SIVcpz resulted from a recombination event between a SIVrcm-like virus and a guenon virus. SIVrcm uses the CCR2b co-receptor for viral entry rather than the more commonly used co-receptor CCR5.
The sooty mangabey group is the group from which the highest numbers of SIV isolates have been made (Fultz et al., 1986). These isolates demonstrate a high degree of diversity and are the simian strains most closely related to HIV-2. As with SIVcpz and HIV-1, several cross-species transmission events between sooty mangabeys and man are thought to have contributed to the emergence of HIV-2 (Hirsch et al., 1989, Gao et al., 1992). HIV-2 isolates are arranged in groups A–H with groups A and B representing epidemic strains. Sooty mangabeys from the Ivory Coast harbor SIVs related to HIV-2 groups A and B while those from Sierra Leone and Liberia harbor SIVs related to groups C–H (Santiago et al., 2005). Like HIV-2, representatives from the sooty mangabey group have genomes containing a vpx gene. SIVsmm is ancestral to SIVmac from rhesus macaques, SIVmne from pig-tailed macaques, and SIVstm from stump-tailed macaques (Daniel et al., 1985, Benveniste et al., 1986, Murphey-Corb et al., 1986, Lowenstine et al., 1992, Apetrei et al., 2005). With a prevalence of disease close to 60%, sooty mangabeys represent a substantial reservoir of SIV (Fultz et al., 1990, Santiago et al., 2005).
The African green monkey group contains four related viruses recognized in the four African green species including vervets (SIVagmVer; Chlorocebus pygerythrus), grivets (SIVagmGri; C. aethiops), tantalus species (SIVagmTan; C. tantalus), and sabaeus species (SIVagmSab, C. sabaeus) (Ohta et al., 1987). There are multiple variants of each virus. While prevalence of infection in wild African populations can range as high as 60%, African green monkeys residing in the Caribbean islands demonstrate 0% prevalence (Daniel et al., 1988, Phillips-Conroy et al., 1994, Jolly et al., 1996). This founder effect is likely due to the transport of uninfected animals captured at a young age to these locales.
The L’Hoest and mandrill group contains isolates from a variety of unrelated nonhuman primate species including three Cercopithecus species, the L’Hoest’s monkey (SIVlho; C. l’hoesti), the sun-tailed monkey (SIVsun; C. solatus), and the Preuss’s monkey (SIVpre, C. preussi). L’Hoest’s monkeys have a 57% prevalence of SIV representing a significant reservoir (Beer et al., 2000). Western red colobus (SIVwrcPbb; Piliocolobus badius badius and SIVwrcPbt; P. badius temminckii) and olive colobus (SIVolc; Procolobus verus) isolates align with the L’Hoest lineage when examining homology across the entire viral genome although certain regions of the genome such as the gag and 5’ end of the pol sequences cluster with the Colobus lineage (Locatelli et al., 2008, Liegeois et al., 2009). SIVmnd-1 isolated from mandrills (Mandrillus sphinx) residing in central and southern Gabon also clusters with the L’Hoest lineage (Tsujimoto et al., 1988, Souquiere et al., 2001). Interestingly, a second, highly divergent isolate (SIVmnd-2) has been identified in mandrills from northern and western Gabon. SIVmnd-2, unlike SIVmnd-1, has a vpx gene. SIVmnd-2 shares some homology with SIVrcm suggesting that a recombination event between SIVrcm and SIVmnd-1 may have given rise to SIVmnd-2 (Souquiere et al., 2001). SIVdrl from drills (Mandrillus leucophaeus) also shows homology to SIVmnd-2 (Hu et al., 2003).
The colobus lineage contains one strain: SIVcol from the mantled guereza (Colobus guereza). This is one of the most divergent of SIV isolates with an average amino acid identity to other known SIV isolates of 40% for the gag encoded proteins, 50% for the pol encoded proteins, and 28% for the env encoded proteins (Courgnaud et al., 2001). SIVcol was the first isolate to be discovered from the Colobinae family. The SIV infection status of Asian Colobinae species is not known.
Natural SIV infection of New World nonhuman primates has not been identified. Common marmosets and cotton-top tamarins were susceptible to experimental infection with HIV-2 (McClure et al., 1989). Although animals seroconverted and remained healthy, HIV-specific nucelotide sequences could be demonstrated in tamarin peripheral blood lymphocytes by PCR for extended periods. It is likely that host restriction factors prevent viral infection from progressing.
The lentivirus genome is approximately 10 kb in length and is diploid with two identical positive-sense RNA genomes contained within each viral core. The open reading frames include three genes encoding structural proteins (gag, pol, and env), two genes encoding regulatory proteins (tat, rev), and the genes encoding accessory proteins (vif, nef, vpr, vpu, vpx). The compliment of accessory genes varies by the viral isolate. The function of the viral proteins and process of lentiviral replication have been extensively reviewed and will only be discussed briefly here (Freed, 2001, Voevodin et al., 2009). The three structural protein genes are each expressed as a polyprotein that is subsequently cleaved into multiple functional proteins. The SIV gag gene encodes the p55 precursor protein that is cleaved into the capsid (p27 protein homologous to the HIV major core protein p24), matrix (p17), nucleocapsid (p8), and p6 proteins. These proteins not only make up the viral capsid but are also integral to viral assembly. The pol gene encodes the viral enzymes protease, reverse transcriptase, and integrase. The protease enzyme is involved in cleaving precursor peptides, the reverse transcriptase enzyme catalyzes the conversion of the RNA genome to a double-stranded DNA copy, and the integrase enzyme catalyzes the insertion of the viral DNA into the host cell chromosome. Each of these enzymes is a target for antiretroviral therapeutics. The env gene encodes two viral glycoproteins: the surface glycoprotein gp120 and the transmembrane glycoprotein gp41. The gp41 anchors gp120 and is involved in mediating fusion of the virus with host cell membranes during viral entry. The gp120 interacts with the CD4 molecule and a coreceptor to bind virus to the target cell and induces a conformational change in gp41 that promotes fusion of the virion envelope and the host cell plasma membrane. Most SIVs utilize the β-chemokine receptor CCR5 as the co-receptor for viral entry (Zhang et al., 2000).
All SIV genomes contain two regulatory genes: tat and rev. tat is the transactivator of SIV gene expression. It is comprised of two exons encoding a protein that binds to the transactivation response region (TAR) of the viral genome to initiate and increase the rate of transcription. rev encodes a highly conserved protein that binds to the rev response element (RRE) on unspliced or partially spliced viral mRNAs to promote their export from the nucleus to the cytoplasm. There are two additional accessory proteins commonly present in complex retroviruses. The vif gene encodes the viral infectivity factor that aborts the antiviral action of the host APOBEC protein system. The nef gene encodes a 27-kDa protein termed “negative factor.” This protein has a variety of functions including downregulation of the CD3–T cell receptor (CD3–TCR) complex and MHC class I expression at the host cell surface thus impairing the ability of cytotoxic T cells to recognize infected cells.
The remaining accessory genes are variably present depending on the SIV isolate. Three types of genomes are recognized: the vpr bearing, the vpr-vpx bearing, and the vpr-vpu bearing. HIV-1, SIVcpz, and certain members of the arboreal guenon lineage have the vpu gene. HIV-2, SIVsmm, SIVrcm, SIVmnd-2, and SIVdrl have the vpx gene. The vpr encoded protein (viral protein R) is involved in nuclear import of preintegration complexes and modulation of host cell functions. The vpx gene is the result of a duplication of the vpr gene. The function of this gene product is not yet fully elucidated. The vpu product (viral protein U) promotes degradation of CD4 and enhancement of virion release.
Epizootiology
SIV infection in natural hosts likely occurs in both a horizontal and a vertical fashion. Epidemiologic assessment of SIVagm in Ethiopian grivet monkeys (Cercopithecus aethiops aethiops) suggests that sexual transmission is a significant mode of transmission in wild populations (Phillips-Conroy et al., 1994). The infection rate increases with sexual maturity in both captive and wild sooty mangabeys, further supporting this route as a major mechanism in the propagation of the virus in nonhuman primate populations (Fultz et al., 1990, Chen et al., 1996). Transmission via wounding is also likely a significant mode of transmission. Microsatellite analysis of isolates from sooty mangabey dams and infants confirms that vertical transmission does occur although this is not thought to be a major contributor to transmission (Santiago et al., 2005).
History has shown that inadvertent transmission of virus from a natural host species to a susceptible species within the research setting is also a potential mode of SIV transmission. Retrospective analysis of tissues achieved from outbreaks of opportunistic infections and malignant lymphoma in captive macaques that occurred in the late 1960s and early 1970s demonstrated underlying SIV infection (Daniel et al., 1988, Lowenstine et al., 1992, Mansfield and Lackner, 1994, Mansfield et al., 1995). The close homology between one of the isolates (SIVstm) and SIVsmm suggested the source was sooty mangabeys. The virus was propagated within the captive macaque populations and resulted in significant morbidity and mortality. In another instance, macaques were inadvertently infected when inoculated with sooty mangabey-derived tissue containing Mycobacterium leprae (Gormus et al., 1989). Xenobiotic inoculation or transplantation of tissue carries the risk of introducing unsuspected or unknown agents into the recipient animal. Although such protocols clearly play an important role in biomedical research, and in this case led to the serendipitous establishment of the SIV model of HIV/AIDS, they should only be undertaken with realization of the potential risks involved. Recipient animals should be housed in an appropriate fashion to prevent the further transmission of such agents.
Pathogenesis and Pathology
Simian immunodeficiency viruses have marked tropism for cells that express the CD4 molecule on their surface. These cells include the helper–inducer subset of T lymphocytes, monocyte macrophages, and antigen-presenting dendritic cells (Spira et al., 1996). Viruses enter these permissive cells through an interaction between the viral envelope glycoprotein gp120, the CD4 molecule, and the CCR5 co-receptor. Once inside the cell, the single-stranded viral RNA is transcribed via reverse transcriptase into DNA copies of itself, which ultimately become integrated into the host cell DNA. Transcription of this proviral DNA results in the production of progeny virus that buds primarily from the surface of infected lymphocytes.
The most common presentation in African nonhuman primates is a persistent, nonpathogenic SIV infection. In contrast, macaque monkeys with naturally acquired or experimentally induced SIV infection demonstrate a profound depletion of CD4+ T lymphocytes that leads to severe immune dysfunction and death from opportunistic infections or viral-induced syndromes. The pathogenesis and clinical presentation of SIV infection in African and Asian nonhuman primate species will each be discussed separately below.
SIV in African Species
Understanding how natural hosts maintain chronic SIV infection without disease progression is of great interest to scientists and may give insight to disease processes in man. The pathophysiology of SIV infection in African species, most thoroughly studied in African green monkeys, sooty mangabeys, and mandrills, has recently been the subject of several review articles (Silvestri et al., 2007, Pandrea et al., 2008, Paiardini et al., 2009, Sodora et al., 2009). Aspects of the course of disease in these species will be presented here.
Despite a high prevalence of SIV infection in African species, other than in a few reported instances, these animals do not develop immunodeficiency or the accompanying spectrum of pathology observed following SIV infection of Asian macaques. The acute phase of infection in African species is similar in many respects to that observed in macaques. Peak virus production occurs within 1–3 weeks post-inoculation, innate and adaptive immune responses are initiated, and a partial control of viral replication enables achievement of the set point of viral replication. Virus replicates to high titer in both Asian and African species targeting the gut and lymphoid tissue and resulting in a depletion of CD4+ T cells at mucosal sites (Gordon et al., 2007, Pandrea et al., 2007). Asian species demonstrate peak viral loads of 107–109 RNA copies/ml of plasma in line with or higher than those observed in normal rhesus macaque progression profiles (Broussard et al., 2001, Holzammer et al., 2001, Pandrea et al., 2006, Paiardini et al., 2009). In both species, acute infection is accompanied by modest declines in CD4+ T cell counts (Rey-Cuille et al., 1998). Humoral and cell-mediated immune responses of African primates appear to be comparable if not slightly diminished when compared to macaques (Zahn et al., 2008). From acute infection onward, distinct differences in pathophysiology between Asian and African species begin to emerge. In African species, CD4+ T cell numbers remain stable throughout chronic infection despite continued elevations in viral load that result in high set points of 104–106 RNA copies/ml of plasma. Further loss of mucosal T cell populations does not occur in African species and mucosal immunity is preserved. In contrast, loss of both circulating and mucosal CD4+ T cells is progressive in rhesus macaques.
There are a number of mechanisms suspected to enable African species to maintain disease resistance in the face of high viral loads. A key factor may be the fact that there is a marked reduction in the degree of immune activation following transition from the acute to the chronic phase of infection. Activation of CD4+ and CD8+ T cells resulting in apoptosis of uninfected, bystander cells is believed to contribute substantially to the observed depletion of uninfected CD4+ and CD8+ T cells occurring with HIV/SIV progression to AIDS (Hazenberg et al., 2000, Hazenberg et al., 2003, Deeks et al., 2004, Holm and Gabuzda, 2005). By maintaining an anti-inflammatory phenotype, SIV-infected African species experience reduced activation-induced cell death and preservation of T cell homeostasis (Kornfeld et al., 2005, Cumont et al., 2008, Meythaler et al., 2009). The ability of the nef product of SIVsmm and SIVagm to more efficiently down-regulate the CD3-TCR from the surface of infected CD4+ T cells may contribute to reductions in immune activation (Brenchley et al., 2008). An additional contributing factor to the maintenance of CD4+ T cell counts in African species is the preservation of T cell regenerative capacity of bone marrow, thymus, and lymphoid compartments.
On rare occasions progression to simian acquired immunodeficiency syndrome (SAIDS) has been reported in African monkeys. A classic SAIDS presentation was observed in a sooty mangabey naturally infected with SIVsmm for 18 years (Ling et al., 2004). During the final year of life, this animal developed a 100-fold increase in viral load, progressive CD4+ T cell decline, and disseminated B cell lymphoma. Similar cases of increased viral load and CD4+ T cell loss have been reported in mandrills and African greens with longstanding SIV infection (Pandrea et al., 2009). Reports such as this illustrate that, given a long enough incubation period, host or environmental factors may influence disease occurrence in these species.
Recent findings suggest that SIVcpz infection in chimpanzees may be associated with disease (Keele et al., 2009). Over a 9-year observation period, a 10–16-fold increase in death rate was observed in SIV-infected chimpanzees in two Tanzanian communities. Spleen and lymph node samples collected from a subset of animals demonstrated CD4+ T cell depletion and high levels of viral replication. Histopathological findings consistent with SAIDS were observed in one female. It is suggested that co-evolution of host and virus has allowed for development of a state of disease resistance in African hosts. The more recent SIVcpz emergence on an evolutionary time scale may not have allowed for sufficient virus-host adaptation.
SIV in Asian Species of Macaques
In contrast to the phenomena observed in African hosts, SIV infection of macaques commonly results in a progressive loss of CD4+ T cells and onset of SAIDS. The disease phenotype replicates the clinical course of HIV/AIDS in man enabling the SIV-inoculated macaque to serve as an animal model critical for elucidating aspects of HIV pathogenesis and prevention. Primary infection occurs 1–3 weeks post-inoculation and is characterized by viremia, transient leukopenia, and prodromal signs such as fever, lymphadenopathy, diarrhea, rash, anorexia, and malaise. It is during this phase that the SIV-specific antibody response develops. The asymptomatic phase of infection is characterized by a stabilization of viral load, termed the viral set point (Lifson et al., 1997). Circulating CD4+ T cell numbers rebound slightly followed by a gradual and progressive decline. SAIDS, the final phase of infection, is characterized by profound CD4+ T cell depletion and onset of opportunistic infections and SAIDS-associated pathologies.
There are three infection profiles: the normal progression profile observed in 75% of animals, the rapid progression profile observed in 20% of animals, and the elite controller profile observed in 5% of animals. Normal progression is characterized by a peak viral load of approximately 107 RNA copies/ml of plasma at 2 weeks post-inoculation. The humoral and cellular immune responses control viral replication to a set point of approximately 105 RNA copies/ml. The disease course averages about 18 months at which point CD4+ T cell counts typically drop below 300 cells/mm3 and opportunistic infections develop. Elite controllers have a vigorous cell-mediated and humoral immune response and are able to limit viral replication. Viral load in these animals peaks at approximately 105 RNA copies/ml. During chronic infection viral replication remains below the level of detection and CD4+ T cell counts are within normal limits. In contrast, rapid progressors have high viral loads that peak at 108 RNA copies/ml and remain high throughout the course of infection. These animals demonstrate a reduced antibody response and a survival of only 3–4 months.
A combination of host, viral, and environmental factors likely influence the progression profile observed in a given animal. Host factors include species or subspecies, age, and genotype. Cynomolgus macaques inoculated with SIVmac251 have lower viral loads, higher CD4+ T cell counts, stronger virus-specific immune responses, and prolonged survival when compared to similarly inoculated rhesus macaques (Reimann et al., 2005). Likewise, Chinese origin rhesus macaques demonstrate significantly lower viral loads, less prominent depletion of intestinal lymphocyte populations, stronger antibody response, and prolonged survival when compared to similarly inoculated Indian origin rhesus macaques (Ling et al., 2002, Trichel et al., 2002). Genotype also plays a significant role. The major histocompatibility complex (MHC) class I and II gene products are cell surface glycoproteins involved in the presentation of peptides to CD8+ and CD4+ T cells, respectively. The rhesus macaque MHC region, designated by the prefix Mamu, demonstrates significant diversity due to the expression of multiple genes per haplotype (Otting et al., 2005). Certain MHC class I alleles such as Mamu-A∗01, Mamu-B∗17, and Mamu-B∗08 have been associated with slower SIV disease progression, lower viral set points, or an increased likelihood of expressing an elite controller phenotype (Sauermann, 2001). Additional genetic polymorphisms are an area of scientific interest. Particular alleles encoding the cytoplasmic tripartite motif protein 5α (TRIM5α) are associated with a reduced efficiency of binding to viral capsid in vitro and a significant lower viral load set points in vivo (Lim et al., 2010). Polymorphisms in genes coding for chemokine receptors (CCR5, CXCR6, GPR15), chemokine receptor ligands (RANTES), proteins serving as disruptors to viral life cycle (APOBEC3 family), and cytokines (IL-10, TNFα) have also been investigated as potential host factors (Yu et al., 2004, Weiler et al., 2006).
Viral factors may also attenuate, accelerate or alter disease phenotype. SIVsmm and SIVmac strains vary from those that are minimally to highly pathogenic in macaque species (Hirsch and Lifson, 2000). Viral factors include source, strain, and tissue culture passage history. Uncloned isolates tend to be more pathogenic than molecularly cloned viruses. There are exceptions; the molecularly cloned SIVmac239 is highly pathogenic in rhesus macaques. Modification of certain regions of the viral genome can result in variable virulence. Deletion of nef reduces the pathogenic potential of SIVmac239 in rhesus macaques as evidenced by decreased viral load, a strong and persistent antibody response, and decreased risk of SAIDS (Kestler et al., 1991). Deletion of the vif sequence has also been shown to result in significant attenuation of virulence (Desrosiers et al., 1998). Targeted mutation of the viral genome to produce attenuated strains represents an important avenue of investigation in the search for a HIV vaccine.
Lastly, environmental factors may influence viral pathogenicity. The presence of opportunistic infections, abuse of alcohol or illicit drug use, and stress have all been shown to negatively influence HIV outcome in humans and have been modeled using the SIV-infected macaque (Zhou et al., 1999, Bagby et al., 2006, Capitanio et al., 2008). Diet composition may also impact SIV progression. Macaques fed a high-fat/high-cholesterol diet prior to infection with SIVmac239 demonstrated a significantly increased risk of SIV-related death relative to those animals receiving a standard primate diet (Mansfield et al., 2007).
The mechanism(s) by which CD4+ T cell depletion occurs in context of SIV infection is not fully understood but likely includes a combination of the following: (1) accumulation of toxic quantities of viral nucleic acids or structural proteins in the cytoplasm of infected cells; (2) lysis of CD4+ T lymphocytes bearing viral-encoded antigens on their surface by virus-specific cytotoxic T-lymphocytes or natural killer cells; (3) lysis of infected CD4+ T lymphocytes by an antibody-dependent cellular cytotoxicity reaction; (4) enhancement of apoptosis of CD4+ T lymphocytes (activation induced cell death, AICD); and (5) destruction of lymph node and thymic architecture leading to reduced regenerative capacity (McCune, 2001). In contrast, infected macrophages, in which viral assembly occurs primarily within cytoplasmic vacuoles rather than on the surface of infected cells, are seemingly resistant to lysis and in fact may be responsible for dissemination of the virus to nonlymphoid tissue such as the brain.
Simian AIDS is characterized by the development of opportunistic infections with viral, bacterial, and parasitic pathogens. These agents are described elsewhere within this volume. In addition to these characteristic opportunistic infections, SAIDS may be accompanied by a number of viral induced lesions in a variety of organ systems including the skin and gastrointestinal, cardiopulmonary, nervous, renal, and lymphoid systems. These syndromes will be discussed here.
Lymphoid System
Not unexpectedly, lymphoid tissues are a major target of viral infection and six distinct microscopic patterns of change have been recognized: (1) normal morphology; (2) follicular hyperplasia; (3) follicular involution with normal or expanded paracortical regions; (4) depletion of follicular and paracortical regions; (5) distinctive granulomatous (giant cell) lymphadenitis; and (6) a generalized lymphoproliferative syndrome. These morphologic criteria are not mutually exclusive and various patterns may coexist in different lymphoid tissues within the animal at any one time. In situ hybridization demonstrates large numbers of infected cells within the first week after experimental inoculation. These positive cells may temporarily disappear with the emergence of an appropriate immunologic response, only to reappear with progressive CD4+ T lymphocyte depletion and viral destruction of follicular dendritic cells.
Giant cell disease is a relatively common manifestation of SIV infection. A survey of cynomolgus macaques inoculated with SIVsmm revealed a 48% prevalence (Li et al., 1991). Large multinucleate syncytial cells are observed in multiple organs including lymphoid tissue, lungs, liver, intestine, and central nervous system. Presence of lesions correlates with degree of immunosuppression (Li et al., 1991). Expression of the cell surface marker CD68 indicates that these cells are of the macrophage/monocyte lineage. The expression of viral env glycoproteins on the surface of infected cells leads to fusion with adjacent CD4-expressing cells. Alterations in the env gene may alter syncytium inducing capacity and impact strain pathogenicity (Etemad-Moghadam et al., 2000).
Nervous System
SIV-inoculated macaques may develop a characteristic meningoencephalitis that resembles the encephalopathy of human patients with AIDS. SIV encephalitis affects the gray and white matter of the spinal cord and brain and is composed of multifocal perivascular aggregates of giant cells and histiocytes with smaller numbers of lymphocytes and rare neutrophils. Surrounding these foci are evidence of myelin degeneration and the formation of scattered glial nodules. In situ hybridization shows that giant cells and histiocytes contain large amounts of replicating virus. Evidence shows that the CNS becomes uniformly infected during primary infection with pathogenic strains of SIVmac and yet only a small percentage of animals develop SIV encephalitis in the chronic phase of the disease (Lackner et al., 1991). The reason for this paradox is unknown, however, alterations in brain endothelium are likely critical because (1) SIV encephalitis is associated with an increased expression of VCAM-1 on brain endothelium and (2) inoculation of “endothelial tropic” viral strains accelerate and promote the occurrence of lesions within the CNS (Sasseville et al., 1992, Sasseville et al., 1994, Mankowski et al., 1994). Pathogenesis involves the recruitment and activation of SIV-infected macrophages to the CNS. These cells elaborate mediators of neuroinflammation resulting in neuronal apoptosis and loss.
Peripheral neuropathy is the most frequent neurologic complication associated with HIV. A ganglionitis has been described in the trigeminal ganglion and the myenteric plexus of SIV infected macaques (Laast et al., 2007, Orandle et al., 2007). This lesion is characterized by the presence of multifocal mononuclear infiltrates, primarily macrophages and lesser numbers of lymphocytes, accompanied by neuronophagia and neuronal loss. Nageotte nodules are typically present and represent replacement of neurons with satellite cells and infiltrating inflammatory cells. The presence of trigeminal ganglionitis is not necessarily associated with concurrent encephalitis (Laast et al., 2007). Ganglionitis of the myenteric plexus may compromise intestinal innervation and contribute to the pathogenesis of SIV/HIV enteropathy (Orandle et al., 2007). Additional contributors to virus-associated peripheral neuropathies in HIV patients include toxic neuropathy from antiretroviral drugs, a diffuse infiltrative lymphocytosis syndrome (DILS), and inflammatory polyneuropathies associated with OI.
Gastrointestinal System
Chronic diarrhea and wasting are the most common clinical signs in SIV-infected macaques (Baskin et al., 1988). Although several opportunistic infections such as Mycobacterium avium, Cryptosporidium parvum, Entamoeba spp., Enterocytozoon bieneusi, and cytomegalovirus may be responsible, in many instances secondary opportunistic agents are lacking. In these cases symptoms are attributed to a direct SIV enteropathy equivalent to AIDS enteropathy in humans. Experimental evidence now indicates that the gastrointestinal tract is a major target organ during primary infection due in large part to the number of CCR5+ CD4+ T lymphocytes that are normally present in mucosal tissue. These cells are rapidly depleted during primary infection resulting in compromise of mucosal immunity (Veazey et al., 1998). CD4+ T cell loss, as well as loss of intestinal epithelial cells, appears to result from apoptosis during the first 7–14 days post-inoculation (Li et al., 2008). Lesions are characterized by villous blunting and atrophy, crypt hyperplasia, and presence of infiltrating macrophages. Compromise of the intestinal mucosa is thought to result in microbial translocation which may then enhance the systemic immune activation observed to correlate with SIV/HIV progression (Brenchley et al., 2006).
A distinct SIV isolate, SIVmacPbj, induces a fulminant necrohemorrhagic gastroenteritis when inoculated into pig-tailed macaques (Fultz and Zack, 1994). Death results within 7–9 days. Similarly, a molecular clone of SIVmac239 (designated SIVmac239YE) produces nearly identical lesions and differs from its parent strain by two amino acids within the nef gene product (Zhenjian et al., 1995). These changes apparently affect a tyrosine kinase that indiscriminately causes activation of infected macrophages and lymphocytes and the elaboration of a host of cytokines. The outcome of SIV infection of the gastrointestinal tract (i.e., fulminant, chronic, or asymptomatic infection) likely involves an interplay between host and viral factors, much as is seen in the CNS.
Cardiopulmonary System
Simian immunodeficiency virus arteriopathy is a unique lesion of unknown etiology described in macaques experimentally inoculated with SIVmac (Chalifoux et al., 1992). Histologically the lesion resembles a chronic obliterative arteriopathy induced by the virus of malignant catarrhal fever in cattle. It is characterized by extensive medial and intimal proliferation of medium- and large-sized pulmonary arteries. The lesion is often associated with thrombosis of vessels and hemorrhage, consolidation, and infarction of pulmonary parenchyma. The vessels are infiltrated by moderate numbers of CD68+ macrophages and rare CD2+ lymphocytes. Although CMV antigen can rarely be localized to the lesion, it is unknown whether the arteriopathy is the direct result of SIV infection or another agent. Lesions are not limited to the pulmonary tissue. Disseminated lesions have been reported in the kidney, liver, pancreas, intestine, heart, lymphoid tissue, and testis (Yanai et al., 2006).
SIV-associated myocarditis has been reported (Yearley et al., 2006) and consists of multifocal cardiomyocyte degeneration and necrosis with infiltrating mononuclear inflammatory cells. SIV viral protein can be demonstrated although it is present within macrophages and not cardiomyocytes.
Lymphocytic interstitial pneumonia is a common sequela of pediatric HIV infection and consists of peribronchial and interstitial lymphoplasmacytic infiltrates accompanied by hyperplasia of bronchial lymphoid tissue. The syndrome in SIV rhesus macaques differs in that macrophages are a significant contributor to inflammatory populations (Mankowski et al., 1998). Multinucleated giant cells are frequently observed. Alveolar septae are expanded with infiltrating mononuclear cells and, in severe cases, alveolar spaces may be filled with fibrin, inflammatory infiltrates, and cellular debris (Baskin et al., 1991).
Skin
As with many systemic viral infections, a disseminated cutaneous eruption occurs in macaques inoculated with pathogenic strains of SIVmac (Ringler et al., 1987). The rash generally appears within 1–2 weeks following inoculation involving the trunk, groin, medial thighs, and face. Complete resolution is apparent within 1–7 weeks. Histologically the exanthema is characterized by a nondescript, superficial, and perivascular lymphocytic dermatitis with variable swelling and degeneration of the epidermis. Immunohistochemistry has revealed these inflammatory cells to be predominantly CD8+ lymphocytes and cytotoxic activity directed at epidermal Langerhans cells.
Urinary System
HIV nephropathy is characterized by a focal segmental glomerulosclerosis accompanied by a variable interstitial nephritis. HIV can be demonstrated in renal epithelial cells. Similar lesions have been described in 5–20% of rhesus macaques infected with SIV depending on the strain (Alpers et al., 1997, Stephens et al., 2000). Inoculation with macrophage tropic strains results in a high incidence (Stephens et al., 2000). Lesions consist of a focal segmental to global glomerulosclerosis with infiltrating macrophages. Viral protein can be demonstrated within glomerular epithelial cells. Mild tubulointerstitial inflammation may be present. Crescentric glomerulonephritis characterized by hypercellular glomeruli, expansion of the mesangial matrix, and immunoglobulin deposition has also been reported (Borda et al., 2004). Azotemia and proteinuria may be observed clinically.
Prevention and Control
Asian macaques should not be allowed direct contact with African species or their tissue products. Control of host-adapted enzootic SIV strains is more problematic. In contrast to Asian macaques in which natural infection is usually followed by seroconversion, African species may harbor the virus and not seroconvert.
Zoonotic Potential
SIVmac is a known zoonotic agent but has not yet been associated with disease in humans (CDC, 1992a, CDC, 1992b, Khabbaz et al., 1992, Khabbaz et al., 1994). In at least two instances, accidental exposure of humans to the virus has resulted in seroconversion and/or infection. Following a needle stick injury, one individual seroconverted, but the virus could not be isolated or demonstrated by PCR techniques (Khabbaz et al., 1992). In the second instance, the virus was isolated and could be demonstrated by PCR in the seropositive individual (Khabbaz et al., 1994). This laboratory worker had a history of working with the virus without gloves while receiving corticosteroids for dermatitis. Whether exposure occurred at this time is unknown. In both instances CD4+ T cell counts remained stable suggesting non-progressive disease. Seroconversion of a laboratory worker was also discovered during an anonymous serologic survey (CDC, 1992a). Whether this represents a third instance of SIV transmission to a human being or retesting of sera from one of the previously mentioned individuals is unknown.
The most likely route of exposure is through the parenteral route as a bloodborne pathogen. Facilities housing potentially infected animals should develop a prevention and control plan based on the bloodborne pathogen standards published by the US Occupational Safety and Health Administration (OSHA). This plan should include: (1) implementation of universal precautions; (2) medical surveillance; (3) use of personal protective equipment; (4) provision of appropriate training; (5) implementation of a sharps injury prevention plan including annual review of safety devices; and (6) development of a post exposure plan that is based on risk assessment and stratification. Evaluation of SIV-associated exposures should be performed by a physician experienced in HIV/SIV risk assessment, and, when indicated, post exposure prophylaxis should be provided. First aid kits should be close at hand in the event of an exposure to allow for immediate cleansing of wounds. Strategies for exposure avoidance should be based on eliminating or reducing the use of needles and other sharps whenever possible, utilizing safety equipment such as retractable needles, and training employees on safe work practices.
Spumaretrovirinae
Simian Foamy Viruses
Simian foamy viruses (SFV) of the genus Spumavirus are, in many respects, typical of complex retroviruses. They share a similar genomic organization having gag, pol, and env sequences flanked by LTRs. However, a key difference lies in the timing of reverse transcription. Foamy virus reverse transcription occurs during viral assembly or budding such that the process is complete before the virus infects new cells. Subsequently, the functional nucleic acid of SFV consists of double-stranded linear DNA rather than single-stranded RNA (Moebes et al., 1997, Yu et al., 1999). SFV also have two unique nonstructural proteins; Tas is a transcriptional activator, and Bet is involved in latency regulation.
SFV have been isolated from a number of Old and New World nonhuman primate species (Rustigian et al., 1955, Hooks et al., 1972, Neuman-Haefelin et al., 1983, Thumer et al., 2007). Originally classified by serotype, viruses are currently named according to the host species of origin (i.e. SFVcpz). Phylogenetics demonstrates significant co-evolution of virus and host species (Switzer et al., 2005). Prevalence in all nonhuman primate species is high and, depending on the species, colony, or habitat, often approaches 100% (Blewett et al., 2000, Hussain et al., 2003, Liu et al., 2008). Proviral DNA can be isolated from most organs and peripheral blood mononuclear cells indicating widespread latent infection (Falcone et al., 1999). In contrast, viral RNA is produced at high levels primarily in tissues of the oropharynx suggesting that this is the major site of SFV replication (Murray et al., 2006, Murray et al., 2008). Shedding of virus at high levels within saliva is thought to contribute to efficient transmission.
The virus appears to be nonpathogenic in nonhuman primates. However, these viruses can replicate in a wide variety of cultured cells and may serve as a significant confounder in certain experimental protocols. In cell culture, SFV infection produces CPE characterized by vacuolization of cytoplasm and syncytia formation and superficially may resemble other retroviruses. SFV-free animals should be used for long-term culture of macrophages derived from bronchoalveolar lavage fluid or peripheral blood mononuclear cells.
There is no human-specific foamy virus. To date all human isolates have originated from nonhuman primates and, despite the apathogenic nature of infection, SFV should be considered a significant zoonotic organism. Previously described human foamy virus is genetically identical to SFV-6 isolated from chimpanzees. Reported prevalence of SFV in humans occupationally exposed to nonhuman primates ranges from 1–5% with many subjects reporting a history of sustaining bite wounds (Heneine et al., 1998, Switzer et al., 2004). Investigators continue to search for associations with naturally occurring diseases in humans. Foamy viruses have been proposed as vectors for gene transfer due to the stable integration into host cells, wide cellular tropism, lack of pre-existing immunity in most patients, and presumptive apathogenicity (Bastone et al., 2007).
Nonenveloped RNA-Containing Viruses
Reoviridae
Rotavirus
Introduction
Rotaviruses are a common cause of contagious enteritis in young children, piglets, calves, and lambs. Rotaviruses have been isolated from macaques, but their association with disease is less clear.
Etiology
Rotaviruses are of the family Reoviridae, subfamily Sedoreovirinae, and genus Rotavirus. These nonenveloped viruses have an icosahedral capsid that is arranged in three layers with a core containing an 11-segmented, double-stranded RNA genome. Rotaviruses are divided into seven groups (A–G) based on antigenic differences of the inner capsid protein VP6. Viruses may be further characterized into P and G serotypes based on the spike protein VP4 and the major surface glycoprotein, respectively. Viruses may also be classified according to genotype.
Type A viruses are most often associated with disease in humans. Despite the ubiquitous nature of these viruses, relatively few simian isolates have been well characterized. SA11 and rhesus rotavirus are two identified nonhuman primate strains. More recently identified strains include PTRV isolated from a pig-tailed macaque with diarrhea, YK-1 also isolated from a pig-tailed macaque with chronic diarrhea, and TUCH (for the Tulane University and Cincinnati Children’s Hospital isolate) isolated from an asymptomatic rhesus macaque (McNeal et al., 2005, Hoshino et al., 2006, Westerman et al., 2006). Phylogenetic analysis of these isolates by Matthijnssens and colleagues indicates that the PTRV genome is of bovine origin (Matthijnssens et al., 2010). The rhesus rotavirus and TUCH genomes are of canine or feline origin although they also demonstrate evidence of reassortment with bovine, human, or other rotavirus strains. SA11 shares little genetic similarity to the other identified simian strains (Matthijnssens et al., 2010).
Epizootiology
The virus is transmitted by the fecal–oral route. Infant and juvenile rhesus macaques are susceptible to inoculation with human and/or simian rotavirus isolates (Wyatt et al., 1976, Chege et al., 2005, McNeal et al., 2005). There has been less work documenting the extent and clinical importance of spontaneous rotavirus infection in nonhuman primates. Screening of captive macaque colonies with evidence of diarrhea demonstrates a 0% to 88% infection rate (Jiang et al., 2004, Wang et al., 2007). Although surveys such as this do not establish a causal relationship, experience from other species suggests that rotaviruses may be a common cause of mild, self-limiting diarrhea during infancy. Maternal antibody is thought to be protective (Westerman et al., 2005). No carrier state has been identified. In fact, shedding of virus following experimental infection usually resolves within 14 days (McNeal et al., 2005). However, the virus will survive in the environment for extended periods.
Pathogenesis and Pathology
Virus entry occurs via binding of the outer capsid proteins to cell surface molecules followed by endocytosis and decapsidation of the virion. The virus replicates in the epithelium of the distal one-third of the intestinal villus tip, resulting in epithelial cell necrosis and villous atrophy. Defects caused by epithelial cell loss are closed within hours by reconstitution; however, full repair may take several days and require maturation of newly formed enterocytes. Villous atrophy is associated with diarrhea, which peaks 72 h after infection.
Clinical Findings
Following a short incubation period of 24–48 h, viral infection causes profuse watery diarrhea, which persists for several days. Infection is usually self-limiting and usually does not require clinical attention.
Laboratory Findings
Although antibodies to SA11 were demonstrated in 15 of 16 species of New and Old World nonhuman primates studied, electron microscopy did not reveal SAll viral particles in 123 random fecal samples from baboons, macaques, squirrel monkeys, and capuchin monkeys (Kalter et al., 1979, Kalter, 1982). The virus may be identified in diarrheic stool following ultracentrifugation by negative-staining electron microscopy. Commercially available ELISA and latex agglutination kits can also be used to detect type A rotaviruses within stool samples. Immunoelectron microscopy techniques utilizing antisera against rotaviruses will increase the sensitivity of viral visualization considerably. Molecular techniques may be utilized as well.
Treatment and Prevention
The ubiquitous nature of rotaviruses makes their prevention difficult. Therapy in most cases is not required.
Orthoreovirus
Baboon orthoreovirus, of the family Reoviridae and subfamily Spinareovirinae, was identified as the cause of a nonsuppurative meningoencephalomyelitis in eight captive-housed baboons that demonstrated evidence of acute, progressive hemiparesis and paresis (Duncan et al., 1995, Duncan, 1999, Leland et al., 2000). Histopathologic examination revealed lymphoplasmacytic perivascular cuffing, microglial nodules, demyelination, and axonal degeneration. Certain species within the Reoviridae family are fusogenic, including baboon orthoreovirus, representing the only nonenveloped viruses capable of inducing syncytium formation in cell culture.
Picornaviridae
Picornaviruses (pico = small, rna = ribonucleic acid) are small, single-stranded, nonenveloped RNA-containing viruses. The family Picornaviridae contains many viruses of importance to human medicine and agriculture, including members of the Hepatovirus, Enterovirus, Apthovirus, and Cardiovirus genera. Picornoviruses reported in nonhuman primates are listed in Table 1.10 .
TABLE 1.10.
Picornaviridae
| Genus | Virus |
|---|---|
| Hepatovirus | Hepatitis A virus |
| Cardiovirus | Encephalomyocarditis virus |
| Enterovirus | Poliovirus |
| Coxsackievirus A and B | |
| Simian enteroviruses | |
| Sapelovirus | SV2 |
Hepatitis A Virus
Introduction
Hepatitis A virus (HAV) is classified as a member of the Hepatovirus genus within the family Picornaviridae. It is responsible for a self-limiting viral hepatitis in human beings and may be transmitted by the fecal–oral route during acute infection or by the ingestion of uncooked contaminated shellfish. The availability of vaccination programs within the United States has resulted in a 92% decline in reported cases between 1995 and 2007 (Daniels et al., 2009). The virus does remain endemic within many developing countries and poses a risk for travelers to these regions. It is well accepted that chimpanzees, tamarins, cynomolgus macaques, and owl monkeys are susceptible to HAV. Serologic evidence exists for the infection of rhesus macaques, stump-tailed macaques, Celebes black macaques, baboons, African green monkeys, capuchins (Cebus albifrons), marmosets, spider monkeys (Ateles geoffroyi), gibbons (Hylobates lar), mandrills, and patas monkeys with the same or similar viruses (Eichberg and Kalter, 1980, Anonymous, 1981; Brack, 1987a; Lankas and Jensen, 1987, Shevstsova et al., 1988, Potkay, 1992).
Etiology
The hepatitis A virus is a small, 25- to 30-nm-diameter RNA virus with a dodecahedral configuration composed of 12 pentamers. The 7.4-kb genome consists of a single open reading frame encoding a large polypeptide that is post-translationally cleaved into 11 functional proteins. Six HAV genotypes are recognized with genotypes I–III containing primarily human isolates and genotypes IV–VI containing simian isolates. A variety of nonhuman primates may be infected experimentally with human HAV isolates (Pinto et al., 2000, Amado et al., 2010). A great wealth of information exists on the experimental infection of tamarins. These animals have served as an important animal model of the human disease and were critical in the initial characterization of the virus and subsequent vaccine development.
Epizootiology
Transmission likely occurs by the fecal–oral route. Serologic evidence of infection has been demonstrated in both wild-caught and captive nonhuman primates. In several instances, seroconversion has occurred during the initial period of captivity, suggesting that the combination of stress and environmental factors at this time may promote transmission of the virus. Most experimental work indicates that animals shed virus and are contagious for only short periods (Cohen et al., 1989). A single report suggests that under some circumstances animals may remain viremic and shed virus for extended periods (Lapin and Shevtsova, 1990).
Pathogenesis and Pathology
Pathogenesis and histopathology are similar in all species studied to date. Following fecal–oral transmission there is a prolonged incubation of 20–50 days. Abnormalities of liver enzymes may be noted at this time. Virus is shed in feces for 10–30 days and onset of this shedding usually precedes detectable clinicopathologic alterations.
Hepatocellular injury is mediated by cytotoxic CD8+ T lymphocytes and is not the direct cytolytic effect of the virus itself. Characteristic histopathologic changes are the activation of sinusoidal cells, focal hepatocellular necrosis, and portal, nonsuppurative inflammatory cell infiltrates. Hyperplasia of bile ducts and bile duct epithelial cell necrosis have been described in chimpanzees. The occurrence of these findings closely parallels elevations in liver enzymes (Dienstag et al., 1976).
Clinical Findings
Clinical signs are uncommon and nonspecific. Anorexia and diarrhea have been noted in some chimpanzees infected with HAV (Brack, 1987).
Laboratory Findings
Elevations of alanine aminotransferase and aspartate aminotransferase 2–10 times above normal, as well as mild increases in bilirubin, have been documented and coincide with the development of humoral and cellular immunity. Anti-HAV IgM and anti-HAV IgG increase and may be used to confirm infection. Although the disease associated with HAV is often mild, infection with this virus in several instances has interfered with the interpretation of clinicopathologic data collected during toxicology studies (Lankas and Jensen, 1987, Slighter et al., 1988).
The hepatitis A virus is difficult to grow in cell culture and serologic evidence is often utilized in clinical diagnosis. Commercially available immunoassays have been used in a number of Old and New World primate species and may demonstrate acute-phase IgM or convalescent-phase IgG anti-HAV antibodies (Eichberg and Kalter, 1980, Lankas and Jensen, 1987).
Treatment
In most nonhuman primates, infection is usually self-limiting. Previous infection with HAV is protective.
Zoonotic Potential
Numerous cases of human HAV infection contracted from nonhuman primates have been documented (Brack, 1987). The vast majority of these cases have been transmitted from recently imported chimpanzees. Hepatitis A virus infection of human beings is often asymptomatic in young children while a majority of adults present with a febrile prodome associated with non-specific gastrointestinal symptoms followed by self-limiting acute hepatitis. Fulminant fatal hepatitis is a rare outcome. In contrast to hepatitis B virus, chronic infection and carrier states are not seen.
Encephalomyocarditis Viruses
Introduction
Encephalomyocarditis viruses (EMCV) are a group of closely related viruses 30 nm in diameter with a genome characteristic of Picornaviridae. They are members of the genus Cardiovirus. The protype strain, EMCV Rueckert, was isolated in 1945 from a chimpanzee with fatal myocarditis (Helwig and Schmidt, 1945). As with other members of the Picornaviridae, they are relatively resistant to a number of environmental factors, including desiccation and freezing.
Epizootiology
Encephalomyocarditis viruses infect a variety of wild rodents and have been infrequently implicated in causing disease in rhesus macaques, marmosets, owl monkeys, squirrel monkeys, mandrills, and chimpanzees (Gainer, 1967, Blanchard et al., 1987, Baskin, 1993, Reddacliff et al., 1997, Canelli et al., 2010). Several epizootics have been recognized in baboons, suggesting a unique susceptibility (Hubbard et al., 1992). Animals likely become infected when rodents contaminate food or other surfaces with feces. Although mice represent the natural reservoir host, rats are often implicated in transmission of the virus to nonhuman primates and swine. Horizontal intraspecies transmission has been documented in swine and rodents and such transmission in primates is suspected.
Pathogenesis and Pathology
Clinical disease is due primarily to the destructive effect of viral replication and host immunologic response within the myocardium. Ultrastructurally, viral particles may be found within myocytes and endothelium. At necropsy, pulmonary congestion, pericardial effusion, and mottling of the myocardium may be noted. Histologically, there is a multifocal to coalescing, necrotizing, nonsuppurative myocarditis (Tesh and Wallace, 1978, Hubbard et al., 1992). Neurologic lesions may additionally be observed and consist of perivascular lymphohistiocytic infiltrates within the cerebrum and cerebral cortex. Pathologic findings may be suggestive, but definitive diagnosis requires viral isolation. Differential diagnosis should include other agents such as coxsackie virus, toxoplasmosis, and trypanosomiasis (Chagas’ disease) (Figure 1.22 ).
FIGURE 1.22.

Encephalomyocarditis virus (EMCV).
EMCV may cause a multifocal necrotizing myocarditis (A and B) and be associated with dystrophic mineralizing of cardiomyocytes (C).
Clinical Findings
Affected nonhuman primates are usually found dead with no premonitory clinical signs. Following experimental inoculation, time to death in squirrel and African green monkeys was highly variable, ranging from 4 to 41 days (Blanchard et al., 1987). In less peracute cases, tachypnea, dyspnea, and frothing from the nostrils have been recorded. In a large biomedical research colony of baboons, placental infection and increased fetal loss were observed (Hubbard et al., 1992).
Treatment and Prevention
No treatment is available. Prevention and control should center on the elimination of reservoir rodent hosts. This may be difficult in outdoor housing, and the virus may persist in the environment for extended periods.
Simian Enteroviruses
The genus Enterovirus contains a large number of viruses pathogenic for humans. Species within the genus recognized by the ICTV include human enterovirus A–D, human rhinovirus A–C, and simian enterovirus A. Simian isolates are found within the human enterovirus species as well as within the species simian enterovirus A. For instance, SV19, SV43, SV46, and baboon enterovirus are members of the species human enterovirus A while SA5 is a member of the species human enterovirus B. The species simian enterovirus A includes the isolates SA4, SV4, and SV28 (Oberste et al., 2007). SV2, the first isolated simian enterovirus, has recently been designated a member of the tentative genus Sapelovirus (Oberste et al., 2003, Victoria et al., 2008). Greater than 200 serotypes within these species have been described and, since 1969, have been numbered sequentially. For instance, EV92 indicates the 92nd enterovirus isolate while SV19 indicates the 19th simian isolate. Other designations include CV indicating coxsackievirus isolates. Some isolates apparently lack strict species specificity, and the possibility that infection of an inappropriate host (including humans) may be associated with a more virulent disease should be considered.
As a group, these viruses are resistant to a variety of chemical disinfectants, including ammonia compounds and deoxycholate. They are, however, susceptible to UV light and dehydration. The pathogenesis of all enterovirus infections share certain features: (1) entry through the gastrointestinal tract; (2) a brief period of viremia associated with minimal clinical disease in the majority of those infected; (3) gastrointestinal shedding of the virus; (4) frequent antigenic mutation; and (5) infrequent dissemination of virus from the gastrointestinal tract to distant target organs. In humans, enteroviruses are associated with a number of clinical entities, including encephalomyelitis, meningitis, myocarditis, cutaneous exanthemas, respiratory disease, congenital malformations, acute hemorrhagic conjunctivitis, and diabetes mellitus. These serious sequelae are rare, and, in general, the vast majority of human beings infected have minimal clinical signs.
Because of the widespread nature of enteroviruses and the frequent asymptomatic infection of most individuals, it is often difficult to establish an etiologic relationship between a specific enterovirus and disease occurrence. The following criteria have been recommended: (1) a high rate of viral isolation from individuals with disease versus individuals without disease; (2) seroconversion during the course of illness; (3) a lack of evidence of concurrent infectious agent; and (4) virus present in significant concentrations in body fluids. While subgroups of enteroviruses are clearly associated with disease (polioviruses and coxsackieviruses) in nonhuman primates, numerous “simian” enterovirus serotypes have also been identified and their association with disease is less clear.
Species from which enteroviruses have been isolated include chimpanzees, macaques, vervet monkeys, African green monkeys, baboons, langurs, and marmosets. Many isolates have been made from animals with no clinical signs or mild diarrhea (Nix et al., 2008). An outbreak of myocarditis and meningoencephalitis in the fall of 1970 at the Lawrenceville facility of the Centers for Disease Control and Prevention illustrates the difficulty in making the association between disease and an enteroviral agent (Kaufmann et al., 1973). This outbreak was associated with high morbidity and mortality in rhesus macaques and African green monkeys. Most of the rhesus macaques were found dead without clinical signs whereas others had dysentery and experienced convulsions on handling. Lesions in these animals were inconsistent and composed of increased numbers of mononuclear cells within the cerebrospinal fluid and scattered perivascular hemorrhages. An enterovirus (simian agent 16) was cultured from four of ten brains. African green monkeys died with chronic-active myocarditis and a nonsuppurative encephalitis. Simian agent 16 is a common isolate from macaques, and this virus did not produce clinical disease when inoculated into rhesus monkeys.
Coxsackieviruses are enteroviruses that have been associated with cardiac fatalities in nonhuman primates (Miyagi et al., 1999, He et al., 2009). Multifocal lymphocytic infiltrates extending from the endocardium to the pericardium were accompanied by varying degrees of myocyte degeneration, necrosis, and fibrosis. Immunohistochemistry and molecular techniques were used to identify the virus within the cardiac tissue.
Poliovirus
Introduction
The epidemic form of human poliomyelitis has occurred since antiquity. Asian macaques have served as an important experimental model of this disease and have been critical in vaccine development and safety testing (Sabin, 1985). The experimental susceptibility of macaques was first demonstrated in 1908. In the 1950s, approximately 200 000 nonhuman primates were imported annually to support this research (Vickers, 1986). Human vaccination programs have been highly successful, reducing the 350 000 cases reported in 1988 to less than 2000 in 2007. However, the virus continues to circulate in countries such as Afghanistan, Pakistan, Nigeria, and India posing a risk for continued transmission (Fine, 2009).
Etiology
Three distinct serotypes of wild-type poliovirus are recognized. Individual isolates may vary in their virulence and invasiveness.
Epizootiology
Natural infection of the chimpanzee, gorilla, and orangutan has been demonstrated. These occurrences are exceedingly rare since the advent of widespread vaccination of human populations. The gastrointestinal tract becomes infected and animals may shed virus in feces.
Pathogenesis and Pathology
Prior to the vaccine era, polio was a disease of the young and immunosuppressed. In the face of an appropriate immune response or maternally acquired neutralizing antibodies, infection is eliminated or limited to the gastrointestinal tract. In cases in which the immune response is deficient, the virus disseminates to the central nervous system and is capable of infecting and destroying neurons.
The poliovirus infects specialized enterocytes (M cells) and initially replicates in gut-associated lymphoid tissue. The major determinant of viral tropism is the poliovirus receptor CD155. Polymorphisms in the gene encoding for this protein contribute to the host range restriction of poliovirus infection (Ida-Hosonuma et al., 2003). New World primates and rhesus macaques are not susceptible to infection via oral inoculation. Cynomolgus macaques and chimpanzees can be infected via oral routes although with reduced efficiency relative to humans (Iwasaki et al., 2002). Cell lines from tamarins and marmosets demonstrate reduced virus–receptor interaction, suggesting that a reduced ability of the CD155 receptor to bind virus contributes to this variation in infectivity by a natural route of exposure (Khan et al., 2008).
Lesions are found scattered throughout the gray matter of the central nervous system with a propensity to affect the spinal cord, cerebellar nuclei, and diencephalon. The initial inflammatory response consists of polymorphonuclear cells that are rapidly replaced by lymphocytes and plasma cells forming perivascular aggregates and infiltrating the meninges. Neuronal necrosis and glial nodules may be evident.
Rhesus macaques are experimentally susceptible to poliovirus infection and the development of the encephalomyelitis if inoculated with poliovirus parenterally (usually intracranially). Natural poliovirus infection has not been diagnosed in rhesus macaques and rhesus appear to have a specific block in the translocation of poliovirus from M cells in the gut. This block is absent in cynomolgus macaques which are susceptible oral poliovirus infection.
Clinical Findings
In many cases, no clinical signs are evident. Disseminated infection to the spinal cord may lead to paresis, paraplegia, and death. Skeletal biometric changes have been documented in Gombe chimpanzees as a long-term sequela to poliomyelitis and deinnervation atrophy of skeletal muscle.
Zoonotic Potential
Although possible, transmission from nonhuman primates to humans has not been demonstrated.
Prevention
The Sabin modified-live oral polio vaccine has reportedly been used to vaccinate and protect Gombe chimpanzees and a variety of great apes (Allmond et al., 1967, Morbeck et al., 1991). Caution is advised in using any modified-live vaccine in species in which proper testing of efficacy and biosafety have not been conducted.
Caliciviridae
Primate Calicivirus Pan paniscus Type 1: PCV-Pan 1
A calicivirus was isolated from a Pygmy chimpanzee with a mild vesicular stomatitis resembling herpes simplex infection (Smith et al., 1983). The virus was isolated on two separate occasions 6 months apart and while contact chimpanzees were seropositive, none showed clinical signs. PCV-Pan 1 had characteristic morphologic features ultrastructurally and was subsequently shown to have a high degree of genetic identity to feline calicivirus, thus placing the simian isolate in the Vesivirus genus of the Caliciviridae family (Rinehart-Kim et al., 1999). This virus has also been isolated from a silver leaf langur (Presbytis cristata), lowland gorilla, spider monkey, and douc langur (Pygathrix nemaeus) (Smith et al., 1985a, Smith et al., 1985b).
Norovirus
The Norovirus genus, of which Norwalk virus is the prototype strain, is a common cause of viral gastroenteritis in humans. While infection is self-limiting, transmission of the virus is often problematic for schools, hospitals, and the military. The virus is resistant to disinfection and is spread via ingestion of contaminated food and water. Members of the Norovirus genus have a 7.5-kb genome and are organized into five genogroups designated GI–GV. Serosurveys in mangabeys, macaques, and chimpanzees have demonstrated a high prevalence of antibodies directed against the GI and GII genogroups (Jiang et al., 2004). Presence of antibody was not associated with clinical disease. Rhesus macaques and chimpanzees were susceptible to experimental infection and, although viral shedding and seroconversion occurred, animals did not develop gastroenteritis (Wyatt et al., 1978, Rockx et al., 2005). In contrast, experimental infection of pig-tailed macaque infants resulted in a self-limiting diarrheal illness and transfer of infection to the dam (Subekti et al., 2002).
Recovirus
Using degenerate PCR primers, a previously uncharacterized calicivirus was detected in the stool of juvenile rhesus macaques housed at the Tulane National Primate Research Center (Farkas et al., 2008). The virus named Tulane virus (TV) has a genome 6.7 kb in length with a poly-A tail. Based on phylogenetic relatedness to other members of the Caliciviridae, the virus is provisionally assigned to the novel genus Recovirus (named for rhesus enteric calicivirus). Because TV was detected in stool of animals both with and without diarrhea, an association with clinical disease could not be made.
Coronaviridae
Coronaviruses have a large, positive-sense RNA genome that is approximately 28–30 kb in length. The enveloped virions are characterized by a helical nucleocapsid and large spike (S) glycoproteins. These glycoproteins form projections of the surface of the virus contributing to the classic crown- or halo-like ultrastuctral appearance from which the virus name is derived.
Coronavirus-Like Particles
Coronavirus-like particles have been detected using electron microscopy in the feces of common marmosets, cotton-topped tamarins, macaque species, baboons, and chimpanzees (Smith et al., 1982, Russell et al., 1985). These particles were identified in up to 43% of samples examined. A link to a diarrheal illness could not be established.
SARS Coronavirus
The severe acute respiratory syndrome (SARS) resulted in >8000 probable cases of pneumonitis and an associated 10% mortality rate between November 2002 and July 2003 (Peiris et al., 2003). The fulfillment of Koch’s postulates via experimental infection of nonhuman primates enabled establishment of a link between the SARS coronavirus (SARS-CoV) and this severe respiratory epidemic (Kuiken et al., 2003). Inoculation of rhesus macaques via intranasal or intratracheal routes resulted in evidence of diffuse alveolar damage characterized by the accumulation of edema, fibrin, and cellular debris within alveolar spaces and accompanied by epithelial desquamation, type 2 pneumocyte hyperplasia, hyaline membrane formation, and syncytial cells (Kuiken et al., 2003). Despite the characteristic appearance of the described lesions, most experimental infections in nonhuman primates do not reach the level of severity observed in human patients succumbing to respiratory failure (McAuliffe et al., 2004). A more recent report suggests that infection of aged macaques resulted in a stronger innate immune response and thus was associated with lesions of greater severity (Smits et al., 2010). Common marmosets also have demonstrated utility as an animal model of SARS-CoV (Greenough et al., 2005). Intratracheally inoculated marmosets developed a multifocal to coalescing pneumonitis with multinucleate syncytia and type 2 hyperplasia. Diffuse alveolar damage was not observed. Infected animals also demonstrated a multifocal hepatitis and mild diffuse colitis sharing features of extrapulmonary disease reported in human patients.
Hepeviridae
Hepatitis E Virus
Introduction
The hepatitis E virus (HEV) is an important etiologic agent of self-limiting hepatitis (non-A, non-B). Although only occasionally detected in industrialized nations, HEV may reach epidemic proportions in developing countries. Outbreaks usually occur following heavy rainfall and have been associated with sewage contamination of drinking water.
Etiology
The agent is a single-stranded RNA virus assigned to the Hepevirus genus of the family Hepeviridae. It is 34–37 nm in diameter and has a genome of approximately 7.2 kb in length. There are four major genotypes with GI found primarily in Asia and Africa, G2 in Mexico, and G3–G4 in the United States, Europe, and Asia.
Epizootiology
Humans become infected by the fecal–oral route. IgG antibody responses have been reported in wild-caught macaque species suggesting that natural infections can occur (Arankalle et al., 1994, Hirano et al., 2003, Yamamoto et al., 2008). A number of species are susceptible to experimental infection with HEV including owl monkeys, cynomolgus monkeys, rhesus macaques, moustached tamarins, African green monkeys, and chimpanzees (Bradley et al., 1987, Potkay, 1992, Ticehurst et al., 1992).
Pathogenesis and Pathology
Cynomolgus monkeys appear to be the species most susceptible to experimental inoculation. Similar to HAV infection in this species, minimal clinical signs were associated with HEV infection. Viral antigen was detected in the liver 30–37 days postinoculation and was associated with elevations in liver enzymes, a mild nonsuppurative portal hepatitis, and the appearance of an antibody response. Virus was shed in the feces through bile and was identified by electron microscopy.
Zoonotic Potential
Swine and rodent species are suspected reservoirs of infection and sources of zoonotic transmission. Given this, potential for transmission from nonhuman primates to man is possible.
References
- Abe K., Kurata T., Teramoto Y., Shiga J., Shikata T. Lack of susceptibility of various primates and woodchucks to hepatitis C virus. J. Med. Primatol. 1993;22:433–434. [PubMed] [Google Scholar]
- Abildgaard C., Harrison J., Espana C., Spangler W., Gribble D. Simian hemorrhagic fever: Studies of coagulation and pathology. Am. J. Trop. Med. Hyg. 1975;24:537–544. doi: 10.4269/ajtmh.1975.24.537. [DOI] [PubMed] [Google Scholar]
- Acha P.N., Szyfres B. Communicable Diseases Common to Man and Animals. World Health Organization; Washington, D.C: 1980. Influenza. [Google Scholar]
- Aghokeng A.F., Bailes E., Loul S., Courgnaud V., Mpoudi-Ngolle E., Sharp P.M., Delaporte E., Peeters M. Full-length sequence analysis of SIVmus in wild populations of mustached monkeys (Cercopithecus cephus) from Cameroon provides evidence for two co-circulating SIVmus lineages. Virology. 2007;360:407–418. doi: 10.1016/j.virol.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akari H., Ono F., Sakakibara I., Takahashi H., Murayama Y., Hiyaoka A., Terao K., Otani I., Mukai R., Adachi A., Yoshikawa Y. Simian T cell leukemia virus type I-induced malignant adult T cell leukemia-like disease in a naturally infected African green monkey: implication of CD8+ T cell leukemia. AIDS Res. Hum. Retroviruses. 1998;14:367–371. doi: 10.1089/aid.1998.14.367. [DOI] [PubMed] [Google Scholar]
- Albrecht J.C., Nicholas J., Biller D., Cameron K.R., Biesinger B., Newman C., Wittmann S., Craxton M.A., Coleman H., Fleckenstein B. Primary structure of the herpesvirus saimiri genome. J. Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht P., Lorenz D., Klutch M.J., Vickers J.H., Ennis F.A. Fatal measles infection in marmosets pathogenesis and prophylaxis. Infect. Immun. 1980;27:969–978. doi: 10.1128/iai.27.3.969-978.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht P., Shabo A.L., Burns G.R., Tauraso N.M. Experimental measles encephalitis in normal and cyclophosphamide-treated rhesus monkeys. J. Infect. Dis. 1972;126:154–161. doi: 10.1093/infdis/126.2.154. [DOI] [PubMed] [Google Scholar]
- Alexander L., Denekamp L., Knapp A., Auerbach M.R., Damania B., Desrosiers R.C. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi’s sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 2000;74:3388–3398. doi: 10.1128/jvi.74.7.3388-3398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A.M., Palmer A.E., Tauraso N.M., Shelokov A. Simian hemorrhagic fever. II. Studies in pathology. Am. J. Trop. Med. Hyg. 1968;17:413–421. doi: 10.4269/ajtmh.1968.17.413. [DOI] [PubMed] [Google Scholar]
- Allmond B.W., Jr., Froeschle J.E., Guilloud N.B. Paralytic poliomyelitis in large laboratory primates. Virologic investigation and report on the use of oral poliomeylitis virus (OPV) vaccine. Am. J. Epidemiol. 1967;85:229–239. doi: 10.1093/oxfordjournals.aje.a120686. [DOI] [PubMed] [Google Scholar]
- Alpers C.E., Tsai C.C., Hudkins K.L., Cui Y., Kuller L., Benveniste R.E., Ward J.M., Morton W.R. Focal segmental glomerulosclerosis in primates infected with a simian immunodeficiency virus. AIDS Res. Hum. Retroviruses. 1997;13:413–424. doi: 10.1089/aid.1997.13.413. [DOI] [PubMed] [Google Scholar]
- Amado L.A., Marchevsky R.S., de Paula V.S., Hooper C., Freire Mda S., Gaspar A.M., Pinto M.A. Experimental hepatitis A virus (HAV) infection in cynomolgus monkeys (Macaca fascicularis): evidence of active extrahepatic site of HAV replication. Int. J. Exp. Pathol. 2010;91:87–97. doi: 10.1111/j.1365-2613.2009.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.C., Swenson R.B., Orkin J.L., Kalter S.S., McClure H.M. Primary Herpesvirus simiae (B-virus) infection in infant macaques. Lab. Anim. Sci. 1994;44:526–530. [PubMed] [Google Scholar]
- Andrade M.R., Yee J., Barry P., Spinner A., Roberts J.A., Cabello P.H., Leite J.P., Lerche N.W. Prevalence of antibodies to selected viruses in a long-term closed breeding colony of rhesus macaques (Macaca mulatta) in Brazil. Am. J. Primatol. 2003;59:123–128. doi: 10.1002/ajp.10069. [DOI] [PubMed] [Google Scholar]
- Anonymous. Hepatitis A virus in primates outside captivity. Lancet. 1981;2:928–929. [PubMed] [Google Scholar]
- Antonsson A., Hansson B.G. Healthy skin of many animal species harbors papillomaviruses which are closely related to their human counterparts. J. Virol. 2002;76:12537–12542. doi: 10.1128/JVI.76.24.12537-12542.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C., Kaur A., Lerche N.W., Metzger M., Pandrea I., Hardcastle J., Falkenstein S., Bohm R., Koehler J., Traina-Dorge V., Williams T., Staprans S., Plauche G., Veazey R.S., McClure H., Lackner A.A., Gormus B., Robertson D.L., Marx P.A. Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. J. Virol. 2005;79:8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arankalle V.A., Goverdhan M.K., Banerjee K. Antibodies against hepatitis E virus in Old World monkeys. J. Viral. Hepat. 1994;1:125–129. doi: 10.1111/j.1365-2893.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Arita I., Gispen R., Kalter S.S., Wah L.T., Marennikova S.S., Netter R., Tagaya I. Outbreaks of monkeypox and serological surveys in nonhuman primates. Bull. World Health Organ. 1972;46:625–631. [PMC free article] [PubMed] [Google Scholar]
- Arita I., Henderson D.A. Smallpox and monkeypox in non-human primates. Bull. World Health Organ. 1968;39:277–283. [PMC free article] [PubMed] [Google Scholar]
- Arita I., Henderson D.A. Monkeypox and whitepox viruses in West and Central Africa. Bull. World Health Organ. 1976;53:347–353. [PMC free article] [PubMed] [Google Scholar]
- Arita I., Jezek Z., Khodakevich L., Ruti K. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am. J. Trop. Med. Hyg. 1985;34:781–789. doi: 10.4269/ajtmh.1985.34.781. [DOI] [PubMed] [Google Scholar]
- Arvin A.M., Martin D.P., Gard E.A., Merigan T.C. Interferon prophylaxis against simian varicella in Erythrocebus patas monkeys. J. Infect. Dis. 1983;147:149–154. doi: 10.1093/infdis/147.1.149. [DOI] [PubMed] [Google Scholar]
- Asher D.M., Gibbs C.J., Jr., Lang D.J., Gajdusek D.C., Chanock R.M. Persistent shedding of cytomegalovirus in the urine of healthy rhesus monkeys. Proc. Soc. Exp. Biol. Med. 1974;145:794–801. doi: 10.3181/00379727-145-37897. [DOI] [PubMed] [Google Scholar]
- Asper M., Hofmann P., Osmann C., Funk J., Metzger C., Bruns M., Kaup F.J., Schmitz H., Gunther S. First outbreak of callitrichid hepatitis in Germany: genetic characterization of the causative lymphocytic choriomeningitis virus strains. Virology. 2001;284:203–213. doi: 10.1006/viro.2001.0909. [DOI] [PubMed] [Google Scholar]
- Bagby G.J., Zhang P., Purcell J.E., Didier P.J., Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin. Exp. Res. 2006;30:1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Bailes E., Gao F., Bibollet-Ruche F., Courgnaud V., Peeters M., Marx P.A., Hahn B.H., Sharp P.M. Hybrid origin of SIV in chimpanzees. Science. 2003;300:1713. doi: 10.1126/science.1080657. [DOI] [PubMed] [Google Scholar]
- Baize S., Marianneau P., Loth P., Reynard S., Journeaux A., Chevallier M., Tordo N., Deubel V., Contamin H. Early and strong immune responses are associated with control of viral replication and recovery in lassa virus-infected cynomolgus monkeys. J. Virol. 2009;83:5890–5903. doi: 10.1128/JVI.01948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baringer J.R., Griffith J.F. Experimental measles virus encephalitis. A light, phase, fluorescence, and electron microscopic study. Lab. Invest. 1970;23:335–346. [PubMed] [Google Scholar]
- Barlow K.L., Ajao A.O., Clewley J.P. Characterization of a novel simian immunodeficiency virus (SIVmonNG1) genome sequence from a mona monkey (Cercopithecus mona) J. Virol. 2003;77:6879–6888. doi: 10.1128/JVI.77.12.6879-6888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette R.W., Metwally S.A., Rowland J.M., Xu L., Zaki S.R., Nichol S.T., Rollin P.E., Towner J.S., Shieh W.J., Batten B., Sealy T.K., Carrillo C., Moran K.E., Bracht A.J., Mayr G.A., Sirios-Cruz M., Catbagan D.P., Lautner E.A., Ksiazek T.G., White W.R., McIntosh M.T. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325:204–206. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- Barry P.A., Lockridge K.M., Salamat S., Tinling S.P., Yue Y., Zhou S.S., Gospe S.M., Jr., Britt W.J., Tarantal A.F. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J. 2006;47:49–64. doi: 10.1093/ilar.47.1.49. [DOI] [PubMed] [Google Scholar]
- Barry P.A., Strelow L. Development of breeding populations of rhesus macaques (Macaca mulatta) that are specific pathogen-free for rhesus cytomegalovirus. Comp. Med. 2008;58:43–46. [PMC free article] [PubMed] [Google Scholar]
- Baskin C.R., Bielefeldt-Ohmann H., Tumpey T.M., Sabourin P.J., Long J.P., Garcia-Sastre A., Tolnay A.E., Albrecht R., Pyles J.A., Olson P.H., Aicher L.D., Rosenzweig E.R., Murali-Krishna K., Clark E.A., Kotur M.S., Fornek J.L., Proll S., Palermo R.E., Sabourin C.L., Katze M.G. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc. Natl. Acad. Sci. U S A. 2009;106:3455–3460. doi: 10.1073/pnas.0813234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin G.B. Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. Am. J. Pathol. 1987;129:345–352. [PMC free article] [PubMed] [Google Scholar]
- Baskin G.B. Encephalomyocarditis Virus Infection, Nonhuman Primates. In: Nonhuman PrimatesJones T.C., Mohr U., Hunt R.D., editors. Vol. 2. Springer-Verlag; Berlin and New York: 1993. pp. 104–107. (Monographs on the Pathology of Laboratory Animals). [Google Scholar]
- Baskin G.B., Cremer K.J., Levy L.S. Comparative pathobiology of HIV- and SIV-associated lymphoma. AIDS Res. Hum. Retroviruses. 2001;17:745–751. doi: 10.1089/088922201750237013. [DOI] [PubMed] [Google Scholar]
- Baskin G.B., Murphey-Corb M., Martin L.N., Soike K.F., Hu F.S., Kuebler D. Lentivirus-induced pulmonary lesions in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet. Pathol. 1991;28:506–513. doi: 10.1177/030098589102800607. [DOI] [PubMed] [Google Scholar]
- Baskin G.B., Murphey-Corb M., Watson E.A., Martin L.N. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet. Pathol. 1988;25:456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- Baskin G.B., Roberts E.D., Kuebler D., Martin L.N., Blauw B., Heeney J., Zurcher C. Squamous epithelial proliferative lesions associated with rhesus Epstein-Barr virus in simian immunodeficiency virus-infected rhesus monkeys. J. Infect. Dis. 1995;172:535–539. doi: 10.1093/infdis/172.2.535. [DOI] [PubMed] [Google Scholar]
- Baskin G.B., Soike K.F. Adenovirus enteritis in SIV-infected rhesus monkeys. J. Infect. Dis. 1989;160:905–907. doi: 10.1093/infdis/160.5.905. [DOI] [PubMed] [Google Scholar]
- Bastone P., Romen F., Liu W., Wirtz R., Koch U., Josephson N., Langbein S., Lochelt M. Construction and characterization of efficient, stable and safe replication-deficient foamy virus vectors. Gene Ther. 2007;14:613–620. doi: 10.1038/sj.gt.3302890. [DOI] [PubMed] [Google Scholar]
- Bausch D.G., Nichol S.T., Muyembe-Tamfum J.J., Borchert M., Rollin P.E., Sleurs H., Campbell P., Tshioko F.K., Roth C., Colebunders R., Pirard P., Mardel S., Olinda L.A., Zeller H., Tshomba A., Kulidri A., Libande M.L., Mulangu S., Formenty P., Grein T., Leirs H., Braack L., Ksiazek T., Zaki S., Bowen M.D., Smit S.B., Leman P.A., Burt F.J., Kemp A., Swanepoel R. Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N. Engl. J. Med. 2006;355:909–919. doi: 10.1056/NEJMoa051465. [DOI] [PubMed] [Google Scholar]
- Bearcroft W.G. Cytological and cytochemical studies on the liver cells of yellow-fever-infected rhesus monkeys. J. Pathol. Bacteriol. 1960;80:19–31. doi: 10.1002/path.1700800104. [DOI] [PubMed] [Google Scholar]
- Bearcroft W.G. Studies on the livers of yellow-fever-infected African monkeys. J. Pathol. Bacteriol. 1962;83:49–58. doi: 10.1002/path.1700830107. [DOI] [PubMed] [Google Scholar]
- Bearcroft W.G., Jamieson M.F. An outbreak of subcutaneous tumours in rhesus monkeys. Nature. 1958;182:195–196. doi: 10.1038/182195a0. [DOI] [PubMed] [Google Scholar]
- Beer B.E., Bailes E., Dapolito G., Campbell B.J., Goeken R.M., Axthelm M.K., Markham P.D., Bernard J., Zagury D., Franchini G., Sharp P.M., Hirsch V.M. Patterns of genomic sequence diversity among their simian immunodeficiency viruses suggest that L’Hoest monkeys (Cercopithecus lhoesti) are a natural lentivirus reservoir. J. Virol. 2000;74:3892–3898. doi: 10.1128/jvi.74.8.3892-3898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer B.E., Foley B.T., Kuiken C.L., Tooze Z., Goeken R.M., Brown C.R., Hu J., St Claire M., Korber B.T., Hirsch V.M. Characterization of novel simian immunodeficiency viruses from red-capped mangabeys from Nigeria (SIVrcmNG409 and -NG411) J. Virol. 2001;75:12014–12027. doi: 10.1128/JVI.75.24.12014-12027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R.E., Arthur L.O., Tsai C.C., Sowder R., Copeland T.D., Henderson L.E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J. Virol. 1986;60:483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquam E.P., Avery N., Shiigi S.M., Axthelm M.K., Wong S.W. Rhesus rhadinovirus establishes a latent infection in B lymphocytes in vivo. J. Virol. 1999;73:7874–7876. doi: 10.1128/jvi.73.9.7874-7876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsagel D.J., Finegold M.J., Butel J.S., Kupsky W.J., Garcea R.L. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N. Engl. J. Med. 1992;326:988–993. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- Bermejo M., Rodriguez-Teijeiro J.D., Illera G., Barroso A., Vila C., Walsh P.D. Ebola outbreak killed 5000 gorillas. Science. 2006;314:1564. doi: 10.1126/science.1133105. [DOI] [PubMed] [Google Scholar]
- Bibollet-Ruche F., Bailes E., Gao F., Pourrut X., Barlow K.L., Clewley J.P., Mwenda J.M., Langat D.K., Chege G.K., McClure H.M., Mpoudi-Ngole E., Delaporte E., Peeters M., Shaw G.M., Sharp P.M., Hahn B.H. New simian immunodeficiency virus infecting De Brazza’s monkeys (Cercopithecus neglectus): evidence for a cercopithecus monkey virus clade. J. Virol. 2004;78:7748–7762. doi: 10.1128/JVI.78.14.7748-7762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt-Ohmann H., Barouch D.H., Bakke A.M., Bruce A.G., Durning M., Grant R., Letvin N.L., Ryan J.T., Schmidt A., Thouless M.E., Rose T.M. Intestinal stromal tumors in a simian immunodeficiency virus-infected, simian retrovirus-2 negative rhesus macaque (Macaca mulatta) Vet. Pathol. 2005;42:391–396. doi: 10.1354/vp.42-3-391. [DOI] [PubMed] [Google Scholar]
- Bigger J.E., Martin D.W. The genome of herpesvirus papio 2 is closely related to the genomes of human herpes simplex viruses. J. Gen. Virol. 2003;84:1411–1414. doi: 10.1099/vir.0.19053-0. [DOI] [PubMed] [Google Scholar]
- Bingham J., Foggin C.M., Gerber H., Hill F.W., Kappeler A., King A.A., Perry B.D., Wandeler A.I. Pathogenicity of SAD rabies vaccine given orally in chacma baboons (Papio ursinus) Vet. Rec. 1992;131:55–56. doi: 10.1136/vr.131.3.55. [DOI] [PubMed] [Google Scholar]
- Birkenmeyer L.G., Desai S.M., Muerhoff A.S., Leary T.P., Simons J.N., Montes C.C., Mushahwar I.K. Isolation of a GB virus-related genome from a chimpanzee. J. Med. Virol. 1998;56:44–51. doi: 10.1002/(sici)1096-9071(199809)56:1<44::aid-jmv8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Blair P.J., Kochel T.J., Raviprakash K., Guevara C., Salazar M., Wu S.J., Olson J.G., Porter K.R. Evaluation of immunity and protective efficacy of a dengue-3 pre-membrane and envelope DNA vaccine in Aotus nancymae monkeys. Vaccine. 2006;24:1427–1432. doi: 10.1016/j.vaccine.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Blakely G.A., Lourie B., Morton W.G., Evans H.H., Kaufmann A.F. A varicella-like disease in macaque monkeys. J. Infect. Dis. 1973;127:617–625. doi: 10.1093/infdis/127.6.617. [DOI] [PubMed] [Google Scholar]
- Blanchard J.L., Soike K., Baskin G.B. Encephalomyocarditis virus infection in African green and squirrel monkeys: comparison of pathologic effects. Lab. Anim. Sci. 1987;37:635–639. [PubMed] [Google Scholar]
- Blaschke S., Hannig H., Buske C., Kaup F.J., Hunsmann G., Bodemer W. Expression of the simian Epstein-Barr virus-encoded latent membrane protein-1 in malignant lymphomas of SIV-infected rhesus macaques. J. Med. Virol. 2001;65:114–120. [PubMed] [Google Scholar]
- Blewett E.L., Black D.H., Lerche N.W., White G., Eberle R. Simian foamy virus infections in a baboon breeding colony. Virology. 2000;278:183–193. doi: 10.1006/viro.2000.0649. [DOI] [PubMed] [Google Scholar]
- Blewett E.L., Lewis J., Gadsby E.L., Neubauer S.R., Eberle R. Isolation of cytomegalovirus and foamy virus from the drill monkey (Mandrillus leucophaeus) and prevalence of antibodies to these viruses amongst wild-born and captive-bred individuals. Arch. Virol. 2003;148:423–433. doi: 10.1007/s00705-002-0937-9. [DOI] [PubMed] [Google Scholar]
- Blewett E.L., White G., Saliki J.T., Eberle R. Isolation and characterization of an endogenous cytomegalovirus (BaCMV) from baboons. Arch. Virol. 2001;146:1723–1738. doi: 10.1007/s007050170059. [DOI] [PubMed] [Google Scholar]
- Blount R.E., Jr., Morris J.A., Savage R.E. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc. Soc. Exp. Biol. Med. 1956;92:544–549. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- Bohannon R.C., Donehower L.A., Ford R.J. Isolation of a type D retrovirus from B-cell lymphomas of a patient with AIDS. J. Virol. 1991;65:5663–5672. doi: 10.1128/jvi.65.11.5663-5672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda J.T., Pauley D.R., MacKey J.J., Alvarez X., Simon M.A., Klumpp S.A. Immunoglobulin-A nephropathy with crescentic glomerulonephritis in a pigtailed macaque (Macaca nemestrina) Vet. Pathol. 2004;41:44–49. doi: 10.1354/vp.41-1-44. [DOI] [PubMed] [Google Scholar]
- Bougler L.R. Natural rabies in a laboratory monkey. Lancet. 1966;1:941–943. doi: 10.1016/s0140-6736(66)90945-7. [DOI] [PubMed] [Google Scholar]
- Boyce J.T., Giddens W.E., Jr., Valerio M. Simian adenoviral pneumonia. Am. J. Pathol. 1971;91:259–276. [PMC free article] [PubMed] [Google Scholar]
- Brack M. Viruses. In: Brack M., editor. Agents Transmissible from Simians to Man. Springer-Verlag; New York: 1987. pp. 1–90. [Google Scholar]
- Bradley D.W., Krawczynski K., Cook E.H., Jr., McCaustland K.A., Humphrey C.D., Spelbring J.E., Myint H., Maynard J.E. Enterically transmitted non-A, non-B hepatitis: serial passage of disease in cynomolgus macaques and tamarins and recovery of disease-associated 27- to 34-nm viruslike particles. Proc. Natl. Acad. Sci. U S A. 1987;84:6277–6281. doi: 10.1073/pnas.84.17.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandly C.A., Hanson R.P. Epizootiology of vesicular stomatitis. Am. J. Public Health Nations Health. 1957;47:205–209. doi: 10.2105/ajph.47.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Kalter S.S., Yakovleva L.A., Kaschula V.R., Shah K.V. Neutralizing antibodies to simian papovavirus SA12 in Old World primates in laboratory colonies: high prevalence in baboons. J. Med. Primatol. 1980;9:240–246. doi: 10.1159/000460145. [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Paiardini M., Knox K.S., Asher A.I., Cervasi B., Asher T.E., Scheinberg P., Price D.A., Hage C.A., Kholi L.M., Khoruts A., Frank I., Else J., Schacker T., Silvestri G., Douek D.C. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J.M., Price D.A., Schacker T.W., Asher T.E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., Blazar B.R., Rodriguez B., Teixeira-Johnson L., Landay A., Martin J.N., Hecht F.M., Picker L.J., Lederman M.M., Deeks S.G., Douek D.C. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brennan J.G., Kalisa-Ruti J., Steniowsoski M., Zanotto E., Gromoyko A.I., Arita I. Human Monkeypox 1970-1979. Bull. World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- Breshears M.A., Eberle R., Ritchey J.W. Temporal progression of viral replication and gross and histological lesions in Balb/c mice inoculated epidermally with Saimiriine herpesvirus 1 (SaHV-1) J. Comp. Pathol. 2005;133:103–113. doi: 10.1016/j.jcpa.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Broussard S.R., Staprans S.I., White R., Whitehead E.M., Feinberg M.B., Allan J.S. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 2001;75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K.E., Liu Z., Gallinella G., Wong S., Mills I.P., O’Sullivan M.G. Simian parvovirus infection: a potential zoonosis. J. Infect. Dis. 2004;190:1900–1907. doi: 10.1086/425420. [DOI] [PubMed] [Google Scholar]
- Bruce A.G., Bakke A.M., Bielefeldt-Ohmann H., Ryan J.T., Thouless M.E., Tsai C.C., Rose T.M. High levels of retroperitoneal fibromatosis (RF)-associated herpesvirus in RF lesions in macaques are associated with ORF73 LANA expression in spindleoid tumour cells. J. Gen. Virol. 2006;87:3529–3538. doi: 10.1099/vir.0.82339-0. [DOI] [PubMed] [Google Scholar]
- Bruce A.G., Bakke A.M., Thouless M.E., Rose T.M. Development of a real-time QPCR assay for the detection of RV2 lineage-specific rhadinoviruses in macaques and baboons. Virol. J. 2005;2:2. doi: 10.1186/1743-422X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruestle M.E., Golden J.G., Hall A., 3rd, Banknieder A.R. Naturally occurring Yaba tumor in a baboon (Papio papio) Lab. Anim. Sci. 1981;31:292–294. [PubMed] [Google Scholar]
- Brunetti C.R., Amano H., Ueda Y., Qin J., Miyamura T., Suzuki T., Li X., Barrett J.W., McFadden G. Complete genomic sequence and comparative analysis of the tumorigenic poxvirus Yaba monkey tumor virus. J. Virol. 2003;77:13335–13347. doi: 10.1128/JVI.77.24.13335-13347.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock G. An association between adenoviruses isolated from simian tonsils and episodes of illness in captive monkeys. J. Hyg. (Lond.) 1965;63:383–387. doi: 10.1017/s0022172400045265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside K.L., Ryan J.T., Bielefeldt-Ohmann H., Gregory Bruce A., Thouless M.E., Tsai C.C., Rose T.M. RFHVMn ORF73 is structurally related to the KSHV ORF73 latency-associated nuclear antigen (LANA) and is expressed in retroperitoneal fibromatosis (RF) tumor cells. Virology. 2006;354:103–115. doi: 10.1016/j.virol.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Callis R.T., Jahrling P.B., DePaoli A. Pathology of Lassa virus infection in the rhesus monkey. Am. J. Trop. Med. Hyg. 1982;31:1038–1045. doi: 10.4269/ajtmh.1982.31.1038. [DOI] [PubMed] [Google Scholar]
- Canelli E., Luppi A., Lavazza A., Lelli D., Sozzi E., Martin A.M., Gelmetti D., Pascotto E., Sandri C., Magnone W., Cordioli P. Encephalomyocarditis virus infection in an Italian zoo. Virol. J. 2010;7:64. doi: 10.1186/1743-422X-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo P., Doering A., Sullivan C.S., Pal A., Peden K.W., Lewis A.M., Pipas J.M. Complete nucleotide sequence of polyomavirus SA12. J. Virol. 2005;79:13094–13104. doi: 10.1128/JVI.79.20.13094-13104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio J.P., Abel K., Mendoza S.P., Blozis S.A., McChesney M.B., Cole S.W., Mason W.A. Personality and serotonin transporter genotype interact with social context to affect immunity and viral set-point in simian immunodeficiency virus disease. Brain Behav. Immun. 2008;22:676–689. doi: 10.1016/j.bbi.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C.S., O’Sullivan M.G., Jayo M.J., Anderson D.K., Harber E.S., Jerome W.G., Bullock B.C., Heberling R.L. Fatal disseminated cercopithecine herpesvirus 1 (herpes B infection in cynomolgus monkeys (Macaca fascicularis) Vet. Pathol. 1997;34:405–414. doi: 10.1177/030098589703400504. [DOI] [PubMed] [Google Scholar]
- Carrion R., Jr., Brasky K., Mansfield K., Johnson C., Gonzales M., Ticer A., Lukashevich I., Tardif S., Patterson J. Lassa virus infection in experimentally infected marmosets: liver pathology and immunophenotypic alterations in target tissues. J. Virol. 2007;81:6482–6490. doi: 10.1128/JVI.02876-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carville A., Mansfield K.G. Comparative pathobiology of macaque lymphocryptoviruses. Comp. Med. 2008;58:57–67. [PMC free article] [PubMed] [Google Scholar]
- Casey H.W., Woodruff J.M., Butcher W.I. Electron microscopy of a benign epidermal pox disease of rhesus monkeys. Am. J. Pathol. 1967;51:431–446. [PMC free article] [PubMed] [Google Scholar]
- CDC Management of patients with suspected viral hemorrhagic fever. Morbid. Mortal. Wkly. Rep. 1988;37:1–16. [Google Scholar]
- CDC Update: Evidence of filovirus infection in animal caretakers in research service facility. Morbid. Mortal. Wkly. Rep. 1990;32:296. [PubMed] [Google Scholar]
- CDC Update: Filovirus infection in animal handlers. Morbid. Mortal. Wkly. Rep. 1990;39:221. [PubMed] [Google Scholar]
- CDC Anonymous survey for simian immunodeficiency virus (SIV) seropositivity in SIV-laboratory researchers – United States, 1992. Morb. Mortal. Wkly. Rep. 1992;41:814–815. [PubMed] [Google Scholar]
- CDC Seroconversion to simian immunodeficiency virus in two laboratory workers. Morb. Mortal. Wkly. Rep. 1992;41:678–681. [PubMed] [Google Scholar]
- CDC Outbreak of Ebola Viral Hemorrhagic Fever – Zaire. Morbid. Mortal. Wkly. Rep. 1995;44:381–382. [PubMed] [Google Scholar]
- CDC Ebola-Reston virus infection among quarantined nonhuman primates–Texas, 1996. Morbid. Mortal. Wkly. Rep. 1996;45:314–316. [PubMed] [Google Scholar]
- CDC Fatal Cercopithecine herpesvirus 1 (B virus) infection following a mucocutaneous exposure and interim recommendations for worker protection. Morbid. Mortal. Wkly. Rep. 1998;47:1073–1076. 1083. [PubMed] [Google Scholar]
- CDC Multistate outbreak of monkeypox – Illinois, Indiana, and Wisconsin, 2003. Morbid. Mortal. Wkly. Rep. 2003;52:537–540. [PubMed] [Google Scholar]
- Cenna J., Hunter M., Tan G.S., Papaneri A.B., Ribka E.P., Schnell M.J., Marx P.A., McGettigan J.P. Replication-deficient rabies virus-based vaccines are safe and immunogenic in mice and nonhuman primates. J. Infect. Dis. 2009;200:1251–1260. doi: 10.1086/605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux L.V., Simon M.A., Pauley D.R., MacKey J.J., Wyand M.S., Ringler D.J. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab. Invest. 1992;67:338–349. [PubMed] [Google Scholar]
- Chandler F.W., Callaway C.S., Adams S.R. Pancreatitis associated with an adenovirus in a rhesus monkey. Vet. Pathol. 1974;11:165–171. doi: 10.1177/030098587401100208. [DOI] [PubMed] [Google Scholar]
- Chandler F.W., McClure H.M. Adenoviral pancreatitis in rhesus monkeys: current knowledge. Vet. Pathol. Suppl. 1982;19(Suppl. 7):171–180. [PubMed] [Google Scholar]
- Chege G.K., Steele A.D., Hart C.A., Snodgrass D.R., Omolo E.O., Mwenda J.M. Experimental infection of non-human primates with a human rotavirus isolate. Vaccine. 2005;23:1522–1528. doi: 10.1016/j.vaccine.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Chellman G.J., Lukas V.S., Eugui E.M., Altera K.P., Almquist S.J., Hilliard J.K. Activation of B virus (Herpesvirus simiae) in chronically immunosuppressed cynomolgus monkeys. Lab. Anim. Sci. 1992;42:146–151. [PubMed] [Google Scholar]
- Chen N., Li G., Liszewski M.K., Atkinson J.P., Jahrling P.B., Feng Z., Schriewer J., Buck C., Wang C., Lefkowitz E.J., Esposito J.J., Harms T., Damon I.K., Roper R.L., Upton C., Buller R.M. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Deng W., Jia C., Dai X., Zhu H., Kong Q., Huang L., Liu Y., Ma C., Li J., Xiao C., Wei Q., Qin C. Pathological lesions and viral localization of influenza A (H5N1) virus in experimentally infected Chinese rhesus macaques: implications for pathogenesis and viral transmission. Arch. Virol. 2009;154:227–233. doi: 10.1007/s00705-008-0277-5. [DOI] [PubMed] [Google Scholar]
- Chen Y.A., Jang Y., Kanki P.J., Yu Q., Wang J., Montali R.J., Samuel K.P., Papas T.S. Isolation and characterizaton of Simian T-Cell Leukemia Virus Type II from New World monkeys. J. Virol. 1994;68:1149–1157. doi: 10.1128/jvi.68.2.1149-1157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Telfer P., Reed P., Zhang L., Gettie A., Ho D.D., Marx P.A. Isolation and characterization of the first Simian Immunodeficiency Virus from a feral Sooty Mangabey (Cercocebus atys) in West Africa. J. Med. Primatol. 1996;24:108–115. doi: 10.1111/j.1600-0684.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Chen Z., van Doorslaer K., DeSalle R., Wood C.E., Kaplan J.R., Wagner J.D., Burk R.D. Genomic diversity and interspecies host infection of alpha12 Macaca fascicularis papillomaviruses (MfPVs) Virology. 2009;393:304–310. doi: 10.1016/j.virol.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y., Ramer J., Rivailler P., Quink C., Garber R.L., Beier D.R., Wang F. An Epstein-Barr-related herpesvirus from marmoset lymphomas. Proc. Natl. Acad. Sci. U S A. 2001;98:1224–1229. doi: 10.1073/pnas.98.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.G., Gordadze A.V., Ling P.D., Wang F. Evolution of two types of rhesus lymphocryptovirus similar to type 1 and type 2 Epstein-Barr virus. J. Virol. 1999;73:9206–9212. doi: 10.1128/jvi.73.11.9206-9212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choa R.K., Fishaut M., Schwartzman J.D., McIntosh K. Detection of Respiratory Syncytial Virus in nasal secretion from infants by Enzyme Linked Immunosorbent Assay. J. Infect. Dis. 1979;139:483–486. doi: 10.1093/infdis/139.4.483. [DOI] [PubMed] [Google Scholar]
- Choo Q.L., Kuo G., Weiner A.J., Overby L.R., Bradley D.W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Chopra H.C., Mason M.M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970;30:2081–2086. [PubMed] [Google Scholar]
- Churchill A.E. The Isolation of Parainfluenza 3 Virus from Fatal Cases of Pneumonia in Erythrocebus Patas Monkeys. Br. J. Exp. Pathol. 1963;44:529–537. [PMC free article] [PubMed] [Google Scholar]
- Cilloniz C., Shinya K., Peng X., Korth M.J., Proll S.C., Aicher L.D., Carter V.S., Chang J.H., Kobasa D., Feldmann F., Strong J.E., Feldmann H., Kawaoka Y., Katze M.G. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000604. e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C.J., Watt N.J., Meredith A., McIntyre N., Burns S.M. Respiratory syncytial virus-associated bronchopneumonia in a young chimpanzee. J. Comp. Pathol. 1994;110:207–212. doi: 10.1016/s0021-9975(08)80191-0. [DOI] [PubMed] [Google Scholar]
- Clarke D.K., Cooper D., Egan M.A., Hendry R.M., Parks C.L., Udem S.A. Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector. Springer Semin. Immunopathol. 2006;28:239–253. doi: 10.1007/s00281-006-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson M.J., Thorpe E., McCarthy K. A virus disease of captive vervet monkeys (Cercopithecus aethiops) caused by a new herpesvirus. Arch. Gesamte. Virusforsch. 1967;22:219–234. doi: 10.1007/BF01240517. [DOI] [PubMed] [Google Scholar]
- Cohen J.I., Davenport D.S., Stewart J.A., Deitchman S., Hilliard J.K., Chapman L.E. Recommendations for prevention of and therapy for exposure to B virus (cercopithecine herpesvirus 1) Clin. Infect. Dis. 2002;35:1191–1203. doi: 10.1086/344754. [DOI] [PubMed] [Google Scholar]
- Cohen J.I., Feinstone S., Purcell R.H. Hepatitis A virus infection in a chimpanzee: duration of viremia and detection of virus in saliva and throat swabs. J. Infect. Dis. 1989;160:887–890. doi: 10.1093/infdis/160.5.887. [DOI] [PubMed] [Google Scholar]
- Cohen J.K., Kilpatrick A.M., Stroud F.C., Paul K., Wolf F., Else J.G. Seroprevalence of West Nile virus in nonhuman primates as related to mosquito abundance at two national primate research centers. Comp. Med. 2007;57:115–119. [PubMed] [Google Scholar]
- Coulibaly C., Hack R., Seidl J., Chudy M., Itter G., Plesker R. A natural asymptomatic herpes B virus infection in a colony of laboratory brown capuchin monkeys (Cebus apella) Lab. Anim. 2004;38:432–438. doi: 10.1258/0023677041958891. [DOI] [PubMed] [Google Scholar]
- Courgnaud V., Pourrut X., Bibollet-Ruche F., Mpoudi-Ngole E., Bourgeois A., Delaporte E., Peeters M. Characterization of a novel simian immunodeficiency virus from guereza colobus monkeys (Colobus guereza) in Cameroon: a new lineage in the nonhuman primate lentivirus family. J. Virol. 2001;75:857–866. doi: 10.1128/JVI.75.2.857-866.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courgnaud V., Salemi M., Pourrut X., Mpoudi-Ngole E., Abela B., Auzel P., Bibollet-Ruche F., Hahn B., Vandamme A.M., Delaporte E., Peeters M. Characterization of a novel simian immunodeficiency virus with a vpu gene from greater spot-nosed monkeys (Cercopithecus nictitans) provides new insights into simian/human immunodeficiency virus phylogeny. J. Virol. 2002;76:8298–8309. doi: 10.1128/JVI.76.16.8298-8309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courgnaud V., Van Dooren S., Liegeois F., Pourrut X., Abela B., Loul S., Mpoudi-Ngole E., Vandamme A., Delaporte E., Peeters M. Simian T-cell leukemia virus (STLV) infection in wild primate populations in Cameroon: evidence for dual STLV type 1 and type 3 infection in agile mangabeys (Cercocebus agilis) J. Virol. 2004;78:4700–4709. doi: 10.1128/JVI.78.9.4700-4709.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandell R.A., Casey H.W., Brumlow W.B. Studies of a newly recognized poxvirus of monkeys. J. Infect. Dis. 1969;119:80–88. doi: 10.1093/infdis/119.1.80. [DOI] [PubMed] [Google Scholar]
- Cumont M.C., Diop O., Vaslin B., Elbim C., Viollet L., Monceaux V., Lay S., Silvestri G., Le Grand R., Muller-Trutwin M., Hurtrel B., Estaquier J. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J. Virol. 2008;82:1175–1184. doi: 10.1128/JVI.00450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T.P., Pipas J.M. Simian agent 12 is a BK virus-like papovavirus which replicates in monkey cells. J. Virol. 1985;54:483–492. doi: 10.1128/jvi.54.2.483-492.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Offay J.M., Eberle R., Sucol Y., Schoelkopf L., White M.A., Valentine B.D., White G.L., Lerche N.W. Transmission dynamics of simian T-lymphotropic virus type 1 (STLV1) in a baboon breeding colony: predominance of female-to-female transmission. Comp. Med. 2007;57:105–114. [PubMed] [Google Scholar]
- Dalgard D.W., Hardy R.J., Pearson S.L., Pucak G.J., Quander R.V., Zack P.M., Peters C.J., Jahrling P.B. Combined simian hemorrhagic fever and Ebola virus infection in cynomolgus monkeys. Lab. Anim. Sci. 1992;42:152–157. [PubMed] [Google Scholar]
- Dang X., Wuthrich C., Axthelm M.K., Koralnik I.J. Productive simian virus 40 infection of neurons in immunosuppressed Rhesus monkeys. J. Neuropathol. Exp. Neurol. 2008;67:784–792. doi: 10.1097/NEN.0b013e318180f0d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M.D., Barahona H., Melendez L.V., Hunt R.D., Sehgal P.K., Marshall B., Ingalls J., Forbes M. Prevention of fatal Herpes infections in owl and marmoset monkeys by vaccination. In: Chivers D.J., Ford E.H.R., editors. Vol. 4. Academic; New York: 1978. pp. 67–69. (Recent Advances in Primatology). [Google Scholar]
- Daniel M.D., Karpas A., Melendez L.V., King N.W., Hunt R.D. Isolation of herpes-T virus from a spontaneous disease in squirrel monkeys (Saimiri sciureus) Arch. Gesamte. Virusforsch. 1967;22:324–331. doi: 10.1007/BF01242953. [DOI] [PubMed] [Google Scholar]
- Daniel M.D., King N.W., Letvin N.L., Hunt R.D., Sehgal P.K., Desrosiers R.C. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science. 1984;223:602–605. doi: 10.1126/science.6695172. [DOI] [PubMed] [Google Scholar]
- Daniel M.D., Letvin N.L., King N.W., Kannagi M., Sehgal P.K., Hunt R.D., Kanki P.J., Essex M., Desrosiers R.C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Daniel M.D., Letvin N.L., Sehgal P.K., Schmidt D.K., Silva D.P., Solomon K.R., Hodi F.S., Jr., Ringler D.J., Hunt R.D., King N.W. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int. J. Cancer. 1988;41:601–608. doi: 10.1002/ijc.2910410421. [DOI] [PubMed] [Google Scholar]
- Daniels D., Grytdal S., Wasley A. Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill. Summ. 2009;58:1–27. [PubMed] [Google Scholar]
- Davenport D.S., Johnson D.R., Holmes G.P., Jewett D.A., Ross S.C., Hilliard J.K. Diagnosis and management of human B virus (Herpesvirus simiae) infections in Michigan. Clin. Infect. Dis. 1994;19:33–41. doi: 10.1093/clinids/19.1.33. [DOI] [PubMed] [Google Scholar]
- Davis K.J., Hubbard G.B., Soike K.F., Butler T.M., Lipscomb T.P. Fatal necrotizing adenoviral hepatitis in a chimpanzee (Pan troglodytes) with disseminated cytomegalovirus infection. Vet. Pathol. 1992;29:547–549. doi: 10.1177/030098589202900612. [DOI] [PubMed] [Google Scholar]
- Davison A.J., Eberle R., Ehlers B., Hayward G.S., McGeoch D.J., Minson A.C., Pellett P.E., Roizman B., Studdert M.J., Thiry E. The order Herpesvirales. Arch. Virol. 2009;154:171–177. doi: 10.1007/s00705-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva A.M., Dittus W.P., Amerasinghe P.H., Amerasinghe F.P. Serologic evidence for an epizootic dengue virus infecting toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. Am. J. Trop. Med. Hyg. 1999;60:300–306. doi: 10.4269/ajtmh.1999.60.300. [DOI] [PubMed] [Google Scholar]
- de Thoisy B., Gardon J., Salas R.A., Morvan J., Kazanji M. Mayaro virus in wild mammals, French Guiana. Emerg. Infect. Dis. 2003;9:1326–1329. doi: 10.3201/eid0910.030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thoisy B., Pouliquen J.F., Lacoste V., Gessain A., Kazanji M. Novel gamma-1 herpesviruses identified in free-ranging new world monkeys (golden-handed tamarin [Saguinus midas], squirrel monkey [Saimiri sciureus], and white-faced saki [Pithecia pithecia]) in French Guiana. J. Virol. 2003;77:9099–9105. doi: 10.1128/JVI.77.16.9099-9105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks S.G., Kitchen C.M., Liu L., Guo H., Gascon R., Narvaez A.B., Hunt P., Martin J.N., Kahn J.O., Levy J., McGrath M.S., Hecht F.M. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- Deinhardt F., Holmes A.W., Capps R.B., Popper H. Studies on the transmission of human viral hepatitis to marmoset monkeys. I. Transmission of disease, serial passages, and description of liver lesions. J. Exp. Med. 1967;125:673–688. doi: 10.1084/jem.125.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbue S., Tremolada S., Elia F., Carloni C., Amico S., Tavazzi E., Marchioni E., Novati S., Maserati R., Ferrante P. Lymphotropic polyomavirus is detected in peripheral blood from immunocompromised and healthy subjects. J. Clin. Virol. 2010;47:156–160. doi: 10.1016/j.jcv.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarcus T.A., Tipple M.A., Ostrowski S.R. US policy for disease control among imported nonhuman primates. J. Infect. Dis. 1999;179(Suppl. 1):S281–282. doi: 10.1086/514279. [DOI] [PubMed] [Google Scholar]
- DePaoli A., Johnsen D.O., Noll M.D. Granulocytic leukemia in white handed gibbons. J. Am. Vet. Med. Assoc. 1973;163:624–628. [PubMed] [Google Scholar]
- Desrosiers R.C. HIV-1 origins. A finger on the missing link. Nature. 1990;345:288–289. doi: 10.1038/345288a0. [DOI] [PubMed] [Google Scholar]
- Desrosiers R.C., Lifson J.D., Gibbs J.S., Czajak S.C., Howe A.Y., Arthur L.O., Johnson R.P. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R.C., Sasseville V.G., Czajak S.C., Zhang X., Mansfield K.G., Kaur A., Johnson R.P., Lackner A.A., Jung J.U. A herpesvirus of rhesus monkeys related to the human Kaposi’s sarcoma-associated herpesvirus. J. Virol. 1997;71:9764–9769. doi: 10.1128/jvi.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire S.M., Damania B. The latency-associated nuclear antigen of rhesus monkey rhadinovirus inhibits viral replication through repression of Orf50/Rta transcriptional activation. J. Virol. 2005;79:3127–3138. doi: 10.1128/JVI.79.5.3127-3138.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz L.A., Diaz Mdel P., Almiron W.R., Contigiani M.S. Infection by UNA virus (Alphavirus; Togaviridae) and risk factor analysis in black howler monkeys (Alouatta caraya) from Paraguay and Argentina. Trans. R. Soc. Trop. Med. Hyg. 2007;101:1039–1041. doi: 10.1016/j.trstmh.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Diehl W.E., Stansell E., Kaiser S.M., Emerman M., Hunter E. Identification of postentry restrictions to Mason-Pfizer monkey virus infection in New World monkey cells. J. Virol. 2008;82:11140–11151. doi: 10.1128/JVI.00269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstag J.L., Popper H., Purcell R.H. The pathology of viral hepatitis types A and B in chimpanzees. A comparison. Am. J. Pathol. 1976;85:131–148. [PMC free article] [PubMed] [Google Scholar]
- DiGiamoco R.F., Shah K.V. Virtual absence of infection with Herpesvirus Simiae in colony reared rhesus monkeys (Macaca mulatta), with a literature review on antibody prevelance in natural and laboratory rhesus populations. Lab. Anim. Sci. 1972;22:61–67. [PubMed] [Google Scholar]
- Douglas J.D., Tanner K.N., Prine J.R., Van Riper D.C., Derwelis S.K. Molluscum contagiosum in chimpanzees. J. Am. Vet. Med. Assoc. 1967;151:901–904. [PubMed] [Google Scholar]
- Downie A.W. Serologic evidence of infection with Tana and Yaba Pox viruses among several species of monkeys. J. Hyg. 1972;72:245–250. doi: 10.1017/s0022172400023445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie A.W., Espana C. Comparison of Tanapox Virus and Yaba-Like Viruses causing epidermic disease in monkeys. J. Hyg. 1972;70:23–33. doi: 10.1017/s0022172400022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie A.W., Espana C. Serologic evidence of infection with Tanapox and Yaba Virus. J. Hyg. 1974;72:245–250. [Google Scholar]
- Downie A.W., Taylor-Robinson C.H., Caunt A.E., Nelson G.S., Mansohn-Bahr P.E.C. Tanapox: A new disease caused by a Pox Virus. Br. Med. J. 1971;1:363–368. doi: 10.1136/bmj.1.5745.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva M., Andrews L., Gilbert S.C., Bejon P., Marsh K., Mwacharo J., Kai O., Nicosia A., Hill A.V. Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine. 2009;27:3501–3504. doi: 10.1016/j.vaccine.2009.03.080. [DOI] [PubMed] [Google Scholar]
- Dueland A.N., Martin J.R., Devlin M.E., Wellish M., Mahalingam R., Cohrs R., Soike K.F., Gilden D.H. Acute simian varicella infection. Clinical, laboratory, pathologic, and virologic features. Lab. Invest. 1992;66:762–773. [PubMed] [Google Scholar]
- Duncan R. Extensive sequence divergence and phylogenetic relationships between the fusogenic and nonfusogenic orthoreoviruses: a species proposal. Virology. 1999;260:316–328. doi: 10.1006/viro.1999.9832. [DOI] [PubMed] [Google Scholar]
- Duncan R., Murphy F.A., Mirkovic R.R. Characterization of a novel syncytium-inducing baboon reovirus. Virology. 1995;212:752–756. doi: 10.1006/viro.1995.1536. [DOI] [PubMed] [Google Scholar]
- Duprez R., Boulanger E., Roman Y., Gessain A. Novel gamma-2-herpesvirus of the Rhadinovirus 2 lineage in gibbons. Emerg. Infect. Dis. 2004;10:899–902. doi: 10.3201/eid1005.030964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Wyatt L.S., Espenshade O., Bassler J., Gong K., Lin S., Peters E., Rhodes L., Jr., Spano Y.E., Silvera P.M., Moss B. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. U S A. 2008;105:10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R. Evidence for an alpha-herpesvirus indigenous to mountain gorillas. J. Med. Primatol. 1992;21:246–251. [PubMed] [Google Scholar]
- Eberle R., Black D.H., Blewett E.L., White G.L. Prevalence of Herpesvirus papio 2 in baboons and identification of immunogenic viral polypeptides. Lab. Anim. Sci. 1997;47:256–262. [PubMed] [Google Scholar]
- Eberle R., Black D.H., Lehenbauer T.W., White G.L. Shedding and transmission of baboon Herpesvirus papio 2 (HVP2) in a breeding colony. Lab. Anim. Sci. 1998;48:23–28. [PubMed] [Google Scholar]
- Eberle R., Black D.H., Lipper S., Hilliard J.K. Herpesvirus papio 2, an SA8-like alpha-herpesvirus of baboons. Arch. Virol. 1995;140:529–545. doi: 10.1007/BF01718429. [DOI] [PubMed] [Google Scholar]
- Eberle R., Hilliard J.K. Serological evidence for variation in the incidence of herpesvirus infections in different species of apes. J. Clin. Microbiol. 1989;27:1357–1366. doi: 10.1128/jcm.27.6.1357-1366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edghill-Smith Y., Bray M., Whitehouse C.A., Miller D., Mucker E., Manischewitz J., King L.R., Robert-Guroff M., Hryniewicz A., Venzon D., Meseda C., Weir J., Nalca A., Livingston V., Wells J., Lewis M.G., Huggins J., Zwiers S.H., Golding H., Franchini G. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect. Dis. 2005;191:372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E., Reimann K.A., Franchini G. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y., Venzon D., Karpova T., McNally J., Nacsa J., Tsai W.P., Tryniszewska E., Moniuszko M., Manischewitz J., King L.R., Snodgrass S.J., Parrish J., Markham P., Sowers M., Martin D., Lewis M.G., Berzofsky J.A., Belyakov I.M., Moss B., Tartaglia J., Bray M., Hirsch V., Golding H., Franchini G. Modeling a safer smallpox vaccination regimen, for human immunodeficiency virus type 1-infected patients, in immunocompromised macaques. J. Infect. Dis. 2003;188:1181–1191. doi: 10.1086/378518. [DOI] [PubMed] [Google Scholar]
- Ehlers B., Ochs A., Leendertz F., Goltz M., Boesch C., Matz-Rensing K. Novel simian homologues of Epstein-Barr virus. J. Virol. 2003;77:10695–10699. doi: 10.1128/JVI.77.19.10695-10699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers B., Spiess K., Leendertz F., Peeters M., Boesch C., Gatherer D., McGeoch D.J. Lymphocryptovirus phylogeny and the origins of Epstein-Barr virus. J. Gen. Virol. 2010;91:630–642. doi: 10.1099/vir.0.017251-0. [DOI] [PubMed] [Google Scholar]
- Eichberg J.W., Kalter S.S. Hepatitis A and B: serologic survey of human and nonhuman primate sera. Lab. Anim. Sci. 1980;30:541–543. [PubMed] [Google Scholar]
- Eichberg J.W., McCullough B., Kalter S.S., Thor D.E., Rodriguez A.R. Clinical, virological, and pathological features of herpesvirus SA8 infection in conventional and gnotobiotic infant baboons (Papio cynocephalus) Arch. Virol. 1976;50:255–270. doi: 10.1007/BF01317951. [DOI] [PubMed] [Google Scholar]
- Elmore D., Eberle R. Monkey B virus (Cercopithecine herpesvirus 1) Comp. Med. 2008;58:11–21. [PMC free article] [PubMed] [Google Scholar]
- Emau P., McClure H.M., Isahakia M., Else J.G., Fultz P.N. Isolation from African Sykes’ monkeys (Cercopithecus mitis) of a lentivirus related to human and simian immunodeficiency viruses. J. Virol. 1991;65:2135–2140. doi: 10.1128/jvi.65.4.2135-2140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E.A., Luka J., Armstrong M.E., Banker F.S., Provost P.J., Pearson G.R. Establishment and characterization of a chronic infectious mononucleosislike syndrome in common marmosets. J. Med. Virol. 1986;18:369–379. doi: 10.1002/jmv.1890180410. [DOI] [PubMed] [Google Scholar]
- Emmons R.W., Lennette E.H. Natural herpesvirus hominis infection of a gibbon (Hylobates lar) Arch. Gesamte. Virusforsch. 1970;31:215–218. doi: 10.1007/BF01253755. [DOI] [PubMed] [Google Scholar]
- Engel G.A., Pizarro M., Shaw E., Cortes J., Fuentes A., Barry P., Lerche N., Grant R., Cohn D., Jones-Engel L. Unique pattern of enzootic primate viruses in Gibraltar macaques. Emerg. Infect. Dis. 2008;14:1112–1115. doi: 10.3201/eid1407.071643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels E.A., Switzer W.M., Heneine W., Viscidi R.P. Serologic evidence for exposure to simian virus 40 in North American zoo workers. J. Infect. Dis. 2004;190:2065–2069. doi: 10.1086/425997. [DOI] [PubMed] [Google Scholar]
- Ensser A., Thurau M., Wittmann S., Fickenscher H. The genome of herpesvirus saimiri C488 which is capable of transforming human T cells. Virology. 2003;314:471–487. doi: 10.1016/s0042-6822(03)00449-5. [DOI] [PubMed] [Google Scholar]
- Espana C. Herpesvirus simiae infection in Macaca radiata. Am. J. Phys. Anthropol. 1973;38:447–454. doi: 10.1002/ajpa.1330380247. [DOI] [PubMed] [Google Scholar]
- Esposito J.J., Knight J.C. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985;143:230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- Etemad-Moghadam B., Sun Y., Nicholson E.K., Fernandes M., Liou K., Gomila R., Lee J., Sodroski J. Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo. J. Virol. 2000;74:4433–4440. doi: 10.1128/jvi.74.9.4433-4440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster A.K., Kalter S.S., Kim C.S., Pinkerton M.E. Isolation of adenoviruses from baboons (Papio sp.) with respiratory and enteric infections. Arch. Gesamte. Virusforsch. 1969;26:260–270. doi: 10.1007/BF01242378. [DOI] [PubMed] [Google Scholar]
- Falcone V., Leupold J., Clotten J., Urbanyi E., Herchenroder O., Spatz W., Volk B., Bohm N., Toniolo A., Neumann-Haefelin D., Schweizer M. Sites of simian foamy virus persistence in naturally infected African green monkeys: latent provirus is ubiquitous, whereas viral replication is restricted to the oral mucosa. Virology. 1999;257:7–14. doi: 10.1006/viro.1999.9634. [DOI] [PubMed] [Google Scholar]
- Falk L., Deinhardt F., Nonoyama M., Wolfe L.G., Bergholz C. Properties of a baboon lymphotropic herpesvirus related to Epstein-Barr virus. Int. J. Cancer. 1976;18:798–807. doi: 10.1002/ijc.2910180611. [DOI] [PubMed] [Google Scholar]
- Farkas T., Sestak K., Wei C., Jiang X. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J. Virol. 2008;82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favoretto S.R., de Mattos C.C., Morais N.B., Alves Araujo F.A., de Mattos C.A. Rabies in marmosets (Callithrix jacchus), Ceara. Brazil. Emerg. Infect. Dis. 2001;7:1062–1065. doi: 10.3201/eid0706.010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feichtinger H., Li S.L., Kaaya E., Putkonen P., Grunewald K., Weyrer K., Bottiger D., Ernberg I., Linde A., Biberfeld G. A monkey model for Epstein Barr virus-associated lymphomagenesis in human acquired immunodeficiency syndrome. J. Exp. Med. 1992;176:281–286. doi: 10.1084/jem.176.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsburg P.J., Heberling R.L., Brack M., Kalter S.S. Experimental genital herpes infection of the marmoset. J. Med. Primatol. 1973;2:50–60. doi: 10.1159/000460286. [DOI] [PubMed] [Google Scholar]
- Felsburg P.J., Heberling R.L., Kalter S.S. Experimental genital infection of cebus monkeys with oral and genital isolates of Herpesvirus hominis types 1 and 2. Arch. Gesamte. Virusforsch. 1972;39:223–227. doi: 10.1007/BF01241544. [DOI] [PubMed] [Google Scholar]
- Felsenfeld A.D., Schmidt N.J. Immunological relationship between Delta herpes virus of patas monkeys and varicella-zoster virus of humans. Infect. Immun. 1975;12:261–266. doi: 10.1128/iai.12.2.261-266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld A.D., Schmidt N.J. Varicella-zoster virus immunizes patas monkeys against simian varicella-like disease. J. Gen. Virol. 1979;42:171–178. doi: 10.1099/0022-1317-42-1-171. [DOI] [PubMed] [Google Scholar]
- Feng H., Shuda M., Chang Y., Moore P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiennes R.N. Rabies. In: Fiennes R.N.T.W., editor. Pathology of Simian Primates. Karger; London: 1972. pp. 646–662. [Google Scholar]
- Fine D.L., Arthur L.O. Expression of natural antibodies against endogenous and horizontally transmitted macaque retroviruses in captive primates. Virology. 1981;112:49–61. doi: 10.1016/0042-6822(81)90611-5. [DOI] [PubMed] [Google Scholar]
- Fine D.L., Landon J.C., Pienta R.J., Kubicek M.T., Valerio M.G., Loeb W.F., Chopra H.C. Responses of infant rhesus monkeys to inoculation with Mason-Pfizer monkey virus materials. J. Natl. Cancer. Inst. 1975;54:651–658. [PubMed] [Google Scholar]
- Fine P.E. Polio: measuring the protection that matters most. J. Infect. Dis. 2009;200:673–675. doi: 10.1086/605331. [DOI] [PubMed] [Google Scholar]
- Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- Fischer-Hoch S.P., Brammer T.L., Trappier S.G., Hutwagner L.C., Farrar B.B., Ruo S.L., Brown B.G., Hermann I.M., Perez-Oronoz G.I., Goldsmith C.S., Hanes M.A., McCormick J.B. Pathogenic potential of filoviruses: role of geographic origin of primate host and virus strain. J. Infect. Dis. 1992;166:753–763. doi: 10.1093/infdis/166.4.753. [DOI] [PubMed] [Google Scholar]
- Fischer-Hoch S.P., Platt G.S., Lloyd G., Simpson D.I.H. Haemotological and biochemical monitoring of Ebola infection in rhesus monkeys: Implications for patient management. Lancet. 1983;1:1055–1058. doi: 10.1016/s0140-6736(83)91041-3. [DOI] [PubMed] [Google Scholar]
- Flecknell P.A., Parry R., Needham J.R., Ridley R.M., Baker H.F., Bowes P. Respiratory disease associated with parainfluenza Type I (Sendai) virus in a colony of marmosets (Callithrix jacchus) Lab. Anim. 1983;17:111–113. doi: 10.1258/002367783780959448. [DOI] [PubMed] [Google Scholar]
- Fogg M.H., Carville A., Cameron J., Quink C., Wang F. Reduced prevalence of Epstein-Barr virus-related lymphocryptovirus infection in sera from a new world primate. J. Virol. 2005;79:10069–10072. doi: 10.1128/JVI.79.15.10069-10072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresman L., Narayan O., Pinson D. Progressive Anemia in a Pig-tail Macaque With AIDS. Contemp. Top. Lab. Anim. Sci. 1999;38:20–22. [PubMed] [Google Scholar]
- Franchini G., Reitz M.S., Jr. Phylogenesis and genetic complexity of the nonhuman primate retroviridae. AIDS Res. Hum. Retroviruses. 1994;10:1047–1060. doi: 10.1089/aid.1994.10.1047. [DOI] [PubMed] [Google Scholar]
- Franka R., Wu X., Jackson F.R., Velasco-Villa A., Palmer D.P., Henderson H., Hayat W., Green D.B., Blanton J.D., Greenberg L., Rupprecht C.E. Rabies virus pathogenesis in relationship to intervention with inactivated and attenuated rabies vaccines. Vaccine. 2009;27:7149–7155. doi: 10.1016/j.vaccine.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Fraser E.E.O., Chalifoux L.V., Sehgal P.K., Hunt R. d., King N.W. A paramyxovirus causing fatal gastroenteritis in marmoset monkeys. In: Goldsmith E.I., Moor-Jankowski J., editors. Primates in Medicine. Karger; Basel: 1978. pp. 261–270. [PubMed] [Google Scholar]
- Freed E.O. HIV-1 replication. Somat. Cell. Mol. Genet. 2001;26:13–33. doi: 10.1023/a:1021070512287. [DOI] [PubMed] [Google Scholar]
- Freifeld A.G., Hilliard J., Southers J., Murray M., Savarese B., Schmitt J.M., Straus S.E. A controlled seroprevalence survey of primate handlers for evidence of asymptomatic herpes B virus infection. J. Infect. Dis. 1995;171:1031–1034. doi: 10.1093/infdis/171.4.1031. [DOI] [PubMed] [Google Scholar]
- Fritz E.A., Geisbert J.B., Geisbert T.W., Hensley L.E., Reed D.S. Cellular immune response to Marburg virus infection in cynomolgus macaques. Viral Immunol. 2008;21:355–363. doi: 10.1089/vim.2008.0023. [DOI] [PubMed] [Google Scholar]
- Fujima A., Ochiai Y., Saito A., Omori Y., Noda A., Kazuyama Y., Shoji H., Tanabayashi K., Ueda F., Yoshikawa Y., Hondo R. Discrimination of antibody to herpes B virus from antibody to herpes simplex virus types 1 and 2 in human and macaque sera. J. Clin. Microbiol. 2008;46:56–61. doi: 10.1128/JCM.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K., Honjo S. Presence of antibody to Cyno-EBV in domestically bred cynomolgus monkeys (Macaca fascicularis) J. Med. Primatol. 1991;20:42–45. [PubMed] [Google Scholar]
- Fultz P.N., Gordon T.P., Anderson D.C., McClure H.M. Prevalence of natural infection with simian immunodeficiency virus and simian T-cell leukemia virus type I in a breeding colony of sooty mangabey monkeys. AIDS. 1990;4:619–625. doi: 10.1097/00002030-199007000-00002. [DOI] [PubMed] [Google Scholar]
- Fultz P.N., McClure H.M., Anderson D.C., Swenson R.B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys) Proc. Natl. Acad. Sci. U S A. 1986;83:5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P.N., Zack P.M. Unique lentivirus–host interactions: SIVsmmPBj14 infection of macaques. Virus Res. 1994;32:205–225. doi: 10.1016/0168-1702(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Gabet A.S., Gessain A., Wattel E. High simian T-cell leukemia virus type 1 proviral loads combined with genetic stability as a result of cell-associated provirus replication in naturally infected, asymptomatic monkeys. Int. J. Cancer. 2003;107:74–83. doi: 10.1002/ijc.11329. [DOI] [PubMed] [Google Scholar]
- Gainer J.H. Encephalomyocarditis virus infections in Florida, 1960–1966. J. Am. Vet. Med. Assoc. 1967;151:421–425. [PubMed] [Google Scholar]
- Gao F., Bailes E., Robertson D.L., Chen Y., Rodenburg C.M., Michael S.F., Cummins L.B., Arthur L.O., Peeters M., Shaw G.M., Sharp P.M., Hahn B.H. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- Gao F., Yue L., White A.T., Pappas P.G., Barchue J., Hanson A.P., Greene B.M., Sharp P.M., Shaw G.M., Hahn B.H. Human infection by genetically diverse SIVSM-related HIV-2 in west Africa. Nature. 1992;358:495–499. doi: 10.1038/358495a0. [DOI] [PubMed] [Google Scholar]
- Gay F.P., Holden M. The herpes encephalitis problem. J. Infect. Dis. 1933;53:287–303. [Google Scholar]
- Geisbert T.W., Daddario-DiCaprio K.M., Geisbert J.B., Young H.A., Formenty P., Fritz E.A., Larsen T., Hensley L.E. Marburg virus Angola infection of rhesus macaques: pathogenesis and treatment with recombinant nematode anticoagulant protein c2. J. Infect. Dis. 2007;196(Suppl. 2):S372–381. doi: 10.1086/520608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T.W., Young H.A., Jahrling P.B., Davis K.J., Kagan E., Hensley L.E. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 2003;188:1618–1629. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- Gerber P., Kalter S.S., Schidlovsky G., Peterson W.D., Jr., Daniel M.D. Biologic and antigenic characteristics of Epstein-Barr virus-related Herpesviruses of chimpanzees and baboons. Int. J. Cancer. 1977;20:448–459. doi: 10.1002/ijc.2910200318. [DOI] [PubMed] [Google Scholar]
- Gerber P., Pritchett R.F., Kieff E.D. Antigens and DNA of a chimpanzee agent related to Epstein-Barr virus. J. Virol. 1976;19:1090–1099. doi: 10.1128/jvi.19.3.1090-1099.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen R., Brand-Saathof B.B., Hekker A.C. Monkeypox-specific antibodies in human and simian sera from the Ivory Coast and Nigeria. Bull. World Health Organ. 1976;53:355–360. [PMC free article] [PubMed] [Google Scholar]
- Glad W.R., Nesland J.M. Focal epithelial hyperplasia of the oral mucosa in two chimpanzees (Pan troglodytes) Am. J. Primatol. 1995;10:83–89. doi: 10.1002/ajp.1350100108. [DOI] [PubMed] [Google Scholar]
- Godeny E.K. Enzyme-linked immunosorbent assay for detection of antibodies against simian hemorrhagic fever virus. Comp. Med. 2002;52:229–232. [PubMed] [Google Scholar]
- Gordon S.N., Klatt N.R., Bosinger S.E., Brenchley J.M., Milush J.M., Engram J.C., Dunham R.M., Paiardini M., Klucking S., Danesh A., Strobert E.A., Apetrei C., Pandrea I.V., Kelvin D., Douek D.C., Staprans S.I., Sodora D.L., Silvestri G. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormus B.J., Murphey-Corb M., Martin L.N., Zhang J.Y., Baskin G.B., Trygg C.B., Walsh G.P., Meyers W.M. Interactions between simian immunodeficiency virus and Mycobacterium leprae in experimentally inoculated rhesus monkeys. J. Infect. Dis. 1989;160:405–413. doi: 10.1093/infdis/160.3.405. [DOI] [PubMed] [Google Scholar]
- Gough A.W., Barsoum N.J., Gracon S.I., Mitchell L., Sturgess J.M. Poxvirus infection in a colony of common marmosets (Callithrix jacchus) Lab. Anim. 1982;32:87–90. [PubMed] [Google Scholar]
- Goverdhan M.K., Kulkarni A.B., Gupta A.K., Tupe C.D., Rodrigues J.J. Two-way cross-protection between West Nile and Japanese encephalitis viruses in bonnet macaques. Acta Virol. 1992;36:277–283. [PubMed] [Google Scholar]
- Gozalo A.S., Montoya E.J., Weller R.E. Dyscoria associated with herpesvirus infection in owl monkeys (Aotus nancymae) J. Am. Assoc. Lab. Anim. Sci. 2008;47:68–71. [PMC free article] [PubMed] [Google Scholar]
- Gravell M., London W.T., Hamilton R.S., Sever J.L., Kapikian A.Z., Murti G., Arthur L.O., Gilden R.V., Osborn K.G., Marx P.A. Transmission of simian AIDS with type D retrovirus isolate. Lancet. 1984;1:334–335. doi: 10.1016/s0140-6736(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Gravell M., London W.T., Leon M., Palmer A.E., Hamilton R.S. Elimination of persistent simian hemorrhagic fever (SHF) virus infection in patas monkeys. Proc. Natl. Acad. Sci. U S A. 1986;181:219–225. doi: 10.3181/00379727-181-42244. [DOI] [PubMed] [Google Scholar]
- Gravell M., London W.T., Leon M.E., Hamilton R.S. Differences among isoltes of simian hemorrhagic fever (SHF) virus. Proc. Soc. Exp. Biol. Med. 1986;181:112–119. doi: 10.3181/00379727-181-42231. [DOI] [PubMed] [Google Scholar]
- Gravell M., London W.T., Rodriguez M., Palmer A.E., Hamilton R.S., Curfman B.J. Studies on hemorrhagic fever virus infection of patas monkeys. I. Serology. In: Montali R.J., Migaki G., editors. The Comparative Pathology of Zoo Animals. Smithsonian Institution Press; Washington, D. C: 1980. pp. 167–170. [Google Scholar]
- Gravell M., Palmer A.E., Rodriguez M., London W.T., Hamilton R.S. Method to detect asymptomatic carriers of simian hemorrhagic fever virus. Lab. Anim. 1980;30:988–991. [PubMed] [Google Scholar]
- Gray W.L. Simian varicella: a model for human varicella-zoster virus infections. Rev. Med. Virol. 2004;14:363–381. doi: 10.1002/rmv.437. [DOI] [PubMed] [Google Scholar]
- Gray W.L. Simian varicella in old world monkeys. Comp. Med. 2008;58:22–30. [PMC free article] [PubMed] [Google Scholar]
- Gray W.L., Gusick N.J. Viral isolates derived from simian varicella epizootics are genetically related but are distinct from other primate herpesviruses. Virology. 1996;224:161–166. doi: 10.1006/viro.1996.0517. [DOI] [PubMed] [Google Scholar]
- Gray W.L., Starnes B., White M.W., Mahalingam R. The DNA sequence of the simian varicella virus genome. Virology. 2001;284:123–130. doi: 10.1006/viro.2001.0912. [DOI] [PubMed] [Google Scholar]
- Green S.W., Malkovska I., O’Sullivan M.G., Brown K.E. Rhesus and pig-tailed macaque parvoviruses: identification of two new members of the erythrovirus genus in monkeys. Virology. 2000;269:105–112. doi: 10.1006/viro.2000.0215. [DOI] [PubMed] [Google Scholar]
- Greenough T.C., Carville A., Coderre J., Somasundaran M., Sullivan J.L., Luzuriaga K., Mansfield K. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am. J. Pathol. 2005;167:455–463. doi: 10.1016/S0002-9440(10)62989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greensill J., Sheldon J.A., Murthy K.K., Bessonette J.S., Beer B.E., Schulz T.F. A chimpanzee rhadinovirus sequence related to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8: increased detection after HIV-1 infection in the absence of disease. AIDS. 2000;14:F129–135. doi: 10.1097/00002030-200012010-00001. [DOI] [PubMed] [Google Scholar]
- Greensill J., Sheldon J.A., Renwick N.M., Beer B.E., Norley S., Goudsmit J., Schulz T.F. Two distinct gamma-2 herpesviruses in African green monkeys: a second gamma-2 herpesvirus lineage among old world primates? J. Virol. 2000;74:1572–1577. doi: 10.1128/jvi.74.3.1572-1577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grethe S., Heckel J.O., Rietschel W., Hufert F.T. Molecular epidemiology of hepatitis B virus variants in nonhuman primates. J. Virol. 2000;74:5377–5381. doi: 10.1128/jvi.74.11.5377-5381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R.A., Boyne J.R., Whitehouse A. Herpesvirus saimiri-based gene delivery vectors. Curr. Gene. Ther. 2006;6:1–15. doi: 10.2174/156652306775515529. [DOI] [PubMed] [Google Scholar]
- Guenno B.L., Formentry P., Wyers M., Gounon P., Walker F., Boesch C. Isolation and partial characterization of a new strain of Ebola virus. Lancet. 1995;344:1271–1274. doi: 10.1016/s0140-6736(95)90925-7. [DOI] [PubMed] [Google Scholar]
- Habis A., Baskin G.B., Murphey-Corb M., Levy L.S. Simian AIDS-associated lymphoma in rhesus and cynomolgus monkeys recapitulates the primary pathobiological features of AIDS-associated non-Hodgkin’s lymphoma. AIDS Res. Hum. Retroviruses. 1999;15:1389–1398. doi: 10.1089/088922299310098. [DOI] [PubMed] [Google Scholar]
- Hahn B.H., Shaw G.M., De Cock K.M., Sharp P.M. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- Hall A.S., McNulty W.P., Jr. A contagious pox disease in monkeys. J. Am. Vet. Med. Assoc. 1967;151:833–838. [PubMed] [Google Scholar]
- Hall W.C., Kovatch R.M., Herman P.H., Fox J.G. Pathology of measles in rhesus monkeys. Vet. Pathol. 1971;8:307–319. doi: 10.1177/030098587100800403. [DOI] [PubMed] [Google Scholar]
- Halstead S.B. Dengue and dengue hemorrhagic fever. In: Beran G.W., editor. Section B: Viral Zoonoses. CRC Press; Boca Raton, FL: 1981. pp. 421–435. [Google Scholar]
- Halstead S.B., Casals J., Shotwell H., Palumbo N. Studies on the immunization of monkeys against dengue. I. Protection derived from single and sequential virus infections. Am. J. Trop. Med. Hyg. 1973;22:365–374. doi: 10.4269/ajtmh.1973.22.365. [DOI] [PubMed] [Google Scholar]
- Halstead S.B., Palumbo N.E. Studies on the immunization of monkeys against dengue. II. Protection following inoculation of combinations of viruses. Am. J. Trop. Med. Hyg. 1973;22:375–381. doi: 10.4269/ajtmh.1973.22.375. [DOI] [PubMed] [Google Scholar]
- Hansen S.G., Strelow L.I., Franchi D.C., Anders D.G., Wong S.W. Complete sequence and genomic analysis of rhesus cytomegalovirus. J. Virol. 2003;77:6620–6636. doi: 10.1128/JVI.77.12.6620-6636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Sata T., Kikuchi T., Nakajima N., Uda A., Fujimoto K., Baba T., Mukai R. Isolation and characterization of a new simian retrovirus type D subtype from monkeys at the Tsukuba Primate Center, Japan. Microbes Infect. 2005;7:126–131. doi: 10.1016/j.micinf.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Harbour D.A., Caunt A.E. The serological relationship of varicella-zoster virus to other primate herpesviruses. J. Gen. Virol. 1979;45:469–477. doi: 10.1099/0022-1317-45-2-469. [DOI] [PubMed] [Google Scholar]
- Hayami M., Ido E., Miura T. Survey of simian immunodeficiency virus among nonhuman primate populations. Curr. Top. Microbiol. Immunol. 1994;188:1–20. doi: 10.1007/978-3-642-78536-8_1. [DOI] [PubMed] [Google Scholar]
- Hayami M., Komuro A., Nozawa K., Shotake T., Ishikawa K., Yamamoto K., Ishida T., Honjo S., Hinuma Y. Prevalence of antibody to adult T-cell leukemia virus-associated antigens (ATLA) in Japanese monkeys and other non-human primates. Int. J. Cancer. 1984;33:179–183. doi: 10.1002/ijc.2910330205. [DOI] [PubMed] [Google Scholar]
- Hazenberg M.D., Hamann D., Schuitemaker H., Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat. Immunol. 2000;1:285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- Hazenberg M.D., Otto S.A., van Benthem B.H., Roos M.T., Coutinho R.A., Lange J.M., Hamann D., Prins M., Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- He W., Lu H., Song D., Zhao K., Gai X., Wang X., Chen Q., Gao F. The evidence of Coxsackievirus B3 induced myocarditis as the cause of death in a Sichuan snub-nosed monkey (Rhinopithecus roxellana) J. Med. Primatol. 2009;38:192–198. doi: 10.1111/j.1600-0684.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- Heberling R.L., Barker S.T., Kalter S.S., Smith G.C., Helmke R.J. Oncornavirus: isolation from a squirrel monkey (Saimiri sciureus) lung culture. Science. 1977;195:289–292. doi: 10.1126/science.63993. [DOI] [PubMed] [Google Scholar]
- Helwig F.C., Schmidt C.H. A Filter-Passing Agent Producing Interstitial Myocarditis in Anthropoid Apes and Small Animals. Science. 1945;102:31–33. doi: 10.1126/science.102.2637.31. [DOI] [PubMed] [Google Scholar]
- Heneine W., Switzer W.M., Sandstrom P., Brown J., Vedapuri S., Schable C.A., Khan A.S., Lerche N.W., Schweizer M., Neumann-Haefelin D., Chapman L.E., Folks T.M. Identification of a human population infected with simian foamy viruses. Nat. Med. 1998;4:403–407. doi: 10.1038/nm0498-403. [DOI] [PubMed] [Google Scholar]
- Henrickson R.V., Maul D.H., Osborn K.G., Sever J.L., Madden D.L., Ellingsworth L.R., Anderson J.H., Lowenstine L.J., Gardner M.B. Epidemic of acquired immunodeficiency in rhesus monkeys. Lancet. 1983;1:388–390. doi: 10.1016/s0140-6736(83)91503-9. [DOI] [PubMed] [Google Scholar]
- Heuschele W.P. Varicella (chicken pox) in three young anthropoid apes. J. Am. Vet. Med. Assoc. 1960;136:256–257. [PubMed] [Google Scholar]
- Higgs S., Ziegler S.A. A nonhuman primate model of chikungunya disease. J. Clin. Invest. 2010;120:657–660. doi: 10.1172/JCI42392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard J.K., Ward J.A. B-virus specific-pathogen-free breeding colonies of macaques (Macaca mulatta): retrospective study of seven years of testing. Lab. Anim. Sci. 1999;49:144–148. [PubMed] [Google Scholar]
- Hirano M., Ding X., Tran H.T., Li T.C., Takeda N., Sata T., Nakamura S., Abe K. Prevalence of antibody against hepatitis E virus in various species of non-human primates: evidence of widespread infection in Japanese monkeys (Macaca fuscata) Jpn. J. Infect. Dis. 2003;56:8–11. [PubMed] [Google Scholar]
- Hirano M., Nakamura S., Mitsunaga F., Okada M., Shirahama S., Eberle R. One-step PCR to distinguish B virus from related primate alphaherpesviruses. Clin. Diagn. Lab. Immunol. 2002;9:716–719. doi: 10.1128/CDLI.9.3.716-719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V.M., Lifson J.D. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv. Pharmacol. 2000;49:437–477. doi: 10.1016/s1054-3589(00)49034-4. [DOI] [PubMed] [Google Scholar]
- Hirsch V.M., Olmsted R.A., Murphey-Corb M., Purcell R.H., Johnson P.R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Hoch A.L., Peterson N.E., LeDuc J.W., Pinheiro F.P. An outbreak of Mayaro virus disease in Belterra, Brazil. III. Entomological and ecological studies. Am. J. Trop. Med. Hyg. 1981;30:689–698. doi: 10.4269/ajtmh.1981.30.689. [DOI] [PubMed] [Google Scholar]
- Hollander C.F., van Noord M.J. Focal epithelial hyperplasia: a virus-induced oral mucosal lesion in the chimpanzee. Oral Surg. Oral Med. Oral Pathol. 1972;33:220–226. doi: 10.1016/0030-4220(72)90391-x. [DOI] [PubMed] [Google Scholar]
- Holm G.H., Gabuzda D. Distinct mechanisms of CD4+ and CD8+ T-cell activation and bystander apoptosis induced by human immunodeficiency virus type 1 virions. J. Virol. 2005;79:6299–6311. doi: 10.1128/JVI.79.10.6299-6311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A.W., Caldwell R.G., Dedmon R.E., Deinhardt F. Isolation and Characterization of a New Herpes Virus. J. Immunol. 1964;92:602–610. [PubMed] [Google Scholar]
- Holmes G.P., Chapman L.E., Stewart J.A., Straus S.E., Hilliard J.K., Davenport D.S. Guidelines for the prevention and treatment of B-virus infections in exposed persons. The B virus Working Group. Clin. Infect. Dis. 1995;20:421–439. doi: 10.1093/clinids/20.2.421. [DOI] [PubMed] [Google Scholar]
- Holmes G.P., Hilliard J.K., Klontz K.C., Rupert A.H., Schindler C.M., Parrish E., Griffin D.G., Ward G.S., Bernstein N.D., Bean T.W. B virus (Herpesvirus simiae) infection in humans: epidemiologic investigation of a cluster. Ann. Intern. Med. 1990;112:833–839. doi: 10.7326/0003-4819-112-11-833. [DOI] [PubMed] [Google Scholar]
- Holzammer S., Holznagel E., Kaul A., Kurth R., Norley S. High virus loads in naturally and experimentally SIVagm-infected African green monkeys. Virology. 2001;283:324–331. doi: 10.1006/viro.2001.0870. [DOI] [PubMed] [Google Scholar]
- Hooks J.J., Gibbs C.J., Jr., Cutchins E.C., Rogers N.G., Lampert P., Gajdusek D.C. Characteization and distribution of two new foamy viruses isolated from chimpanzees. Arch. Gesamte. Virusforsch. 1972;38:38–55. doi: 10.1007/BF01241354. [DOI] [PubMed] [Google Scholar]
- Hooper J.W., Thompson E., Wilhelmsen C., Zimmerman M., Ichou M.A., Steffen S.E., Schmaljohn C.S., Schmaljohn A.L., Jahrling P.B. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 2004;78:4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C.J., Simon M.A., Bergsagel D.J., Pauley D.R., King N.W., Garcea R.L., Ringler D.J. Simian virus 40-induced disease in rhesus monkeys with simian acquired immunodeficiency syndrome. Am. J. Pathol. 1992;140:1431–1440. [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Honma S., Jones R.W., Santos N., Nakagomi O., Nakagomi T., Kapikian A.Z., Thouless M.E. A rotavirus strain isolated from pig-tailed macaque (Macaca nemestrina) with diarrhea bears a P6[1]:G8 specificity. Virology. 2006;345:1–12. doi: 10.1016/j.virol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hu J., Switzer W.M., Foley B.T., Robertson D.L., Goeken R.M., Korber B.T., Hirsch V.M., Beer B.E. Characterization and comparison of recombinant simian immunodeficiency virus from drill (Mandrillus leucophaeus) and mandrill (Mandrillus sphinx) isolates. J. Virol. 2003;77:4867–4880. doi: 10.1128/JVI.77.8.4867-4880.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Javadian A., Gagneux P., Robertson B.H. Paired chimpanzee hepatitis B virus (ChHBV) and mtDNA sequences suggest different ChHBV genetic variants are found in geographically distinct chimpanzee subspecies. Virus Res. 2001;79:103–108. doi: 10.1016/s0168-1702(01)00334-3. [DOI] [PubMed] [Google Scholar]
- Hubbard G.B., Mone J.P., Allan J.S., Davis K.J., 3rd, Leland M.M., Banks P.M., Smir B. Spontaneously generated non-Hodgkin’s lymphoma in twenty-seven simian T-cell leukemia virus type 1 antibody-positive baboons (Papio species) Lab. Anim. Sci. 1993;43:301–309. [PubMed] [Google Scholar]
- Hubbard G.B., Soike K.F., Butler T.M., Carey K.D., Davis H., Butcher W.I., Gauntt C.J. An encephalomyocarditis virus epizootic in a baboon colony. Lab. Anim. Sci. 1992;42:233–239. [PubMed] [Google Scholar]
- Hudson N.P. The pathology of experimental yellow fever in the macacus rhesus: II. Microscopic pathology. Am. J. Pathol. 1928;4:407–418. [PMC free article] [PubMed] [Google Scholar]
- Huemer H.P., Larcher C., Czedik-Eysenberg T., Nowotny N., Reifinger M. Fatal infection of a pet monkey with Human herpesvirus. Emerg. Infect. Dis. 2002;8:639–642. doi: 10.3201/eid0806.010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J.L., Eberle R., Capitanio J., Zhou S.S., Barry P.A. Differential detection of B virus and rhesus cytomegalovirus in rhesus macaques. J. Gen. Virol. 2003;84:83–92. doi: 10.1099/vir.0.18808-0. [DOI] [PubMed] [Google Scholar]
- Hukkanen R.R., Gillen M., Grant R., Liggitt H.D., Kiem H.P., Kelley S.T. Simian varicella virus in pigtailed macaques (Macaca nemestrina): clinical, pathologic, and virologic features. Comp. Med. 2009;59:482–487. [PMC free article] [PubMed] [Google Scholar]
- Hukkanen R.R., Liggitt H.D., Kelley S.T., Grant R., Anderson D.M., Hall R.A., Tesh R.B., Travassos DaRosa A.P., Bielefeldt-Ohmann H. West Nile and St. Louis encephalitis virus antibody seroconversion, prevalence, and persistence in naturally infected pig-tailed macaques (Macaca nemestrina) Clin. Vaccine Immunol. 2006;13:711–714. doi: 10.1128/CVI.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R.N., Dwyer A.C., Holmes A.W., Nowakowski E., Deinhardt F., Lennette E.H., Emmons R.W. Recovery and characterization of a new simian herpesvirus from a fatally infected spider monkey. J. Natl. Cancer Inst. 1972;49:225–231. [PubMed] [Google Scholar]
- Hunt R.D., Blake B.J. Herpesvirus B infection. In: Nonhuman PrimatesJones T.C., Mohr U., Hunt R.D., editors. Vol. 1. Springer-Verlag; Berlin and New York: 1993. pp. 78–81. (Monographs on the Pathology of Laboratory Animals). [Google Scholar]
- Hunt R.D., Blake B.J. Gastroenteritis due to paramyxovirus. In: Jones T.C., Mohr U., Hunt R.D., editors. Vol. 2. Springer-Verlag; Berlin and New York: 1993. pp. 32–37. (Monographs on the Pathology of Laboratory Animals: Nonhuman Primates). [Google Scholar]
- Hunt R.D., Blake B.J., Chalifoux L.V., Sehgal P.K., King N.W., Letvin N.L. Transmission of naturally occurring lymphoma in macaque monkeys. Proc. Natl. Acad. Sci. U S A. 1983;80:5085–5089. doi: 10.1073/pnas.80.16.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R.D., Garcia F.G., Barahona H.H., King N.W., Fraser C.E., Melendez L.V. Spontaneous Herpesvirus saimiri lymphoma in an owl monkey. J. Infect. Dis. 1973;127:723–725. doi: 10.1093/infdis/127.6.723. [DOI] [PubMed] [Google Scholar]
- Hunt R.D., Melendez L.V. Spontaneous herpes-T infection in the owl monkey (Aotus trivirgatus) Pathol. Vet. 1966;3:1–26. doi: 10.1177/030098586600300101. [DOI] [PubMed] [Google Scholar]
- Hurley J.P., Ilyinskii P.O., Horvath C.J., Simon M.A. A malignant astrocytoma containing simian virus 40 DNA in a macaque infected with simian immunodeficiency virus. J. Med. Primatol. 1997;26:172–180. doi: 10.1111/j.1600-0684.1997.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Hussain A.I., Shanmugam V., Bhullar V.B., Beer B.E., Vallet D., Gautier-Hion A., Wolfe N.D., Karesh W.B., Kilbourn A.M., Tooze Z., Heneine W., Switzer W.M. Screening for simian foamy virus infection by using a combined antigen Western blot assay: evidence for a wide distribution among Old World primates and identification of four new divergent viruses. Virology. 2003;309:248–257. doi: 10.1016/s0042-6822(03)00070-9. [DOI] [PubMed] [Google Scholar]
- Hutin Y.J., Williams R.J., Malfait P., Pebody R., Loparev V.N., Ropp S.L., Rodriguez M., Knight J.C., Tshioko F.K., Khan A.S., Szczeniowski M.V., Esposito J.J. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida-Hosonuma M., Sasaki Y., Toyoda H., Nomoto A., Gotoh O., Yonekawa H., Koike S. Host range of poliovirus is restricted to simians because of a rapid sequence change of the poliovirus receptor gene during evolution. Arch. Virol. 2003;148:29–44. doi: 10.1007/s00705-002-0910-7. [DOI] [PubMed] [Google Scholar]
- Inoue S., Morita K., Matias R.R., Tuplano J.V., Resuello R.R., Candelario J.R., Cruz D.J., Mapua C.A., Hasebe F., Igarashi A., Natividad F.F. Distribution of three arbovirus antibodies among monkeys (Macaca fascicularis) in the Philippines. J. Med. Primatol. 2003;32:89–94. doi: 10.1034/j.1600-0684.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa K.I., Fukasawa M., Tsujimoto H., Else J.G., Isahakai M., Ubhi N.K., Ishida T., Takenaka O., Kawamoto Y., Spriatna Y., Abe K., Yamamoto K. Serological survey and virus isolation of simian T-cell leukemia in their native countries. Int. J. Cancer. 1987;40:233–239. doi: 10.1002/ijc.2910400219. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Shinya K., Kiso M., Watanabe T., Sakoda Y., Hatta M., Muramoto Y., Tamura D., Sakai-Tagawa Y., Noda T., Sakabe S., Imai M., Hatta Y., Watanabe S., Li C., Yamada S., Fujii K., Murakami S., Imai H., Kakugawa S., Ito M., Takano R., Iwatsuki-Horimoto K., Shimojima M., Horimoto T., Goto H., Takahashi K., Makino A., Ishigaki H., Nakayama M., Okamatsu M., Warshauer D., Shult P.A., Saito R., Suzuki H., Furuta Y., Yamashita M., Mitamura K., Nakano K., Nakamura M., Brockman-Schneider R., Mitamura H., Yamazaki M., Sugaya N., Suresh M., Ozawa M., Neumann G., Gern J., Kida H., Ogasawara K., Kawaoka Y. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Welker R., Mueller S., Linehan M., Nomoto A., Wimmer E. Immunofluorescence analysis of poliovirus receptor expression in Peyer’s patches of humans, primates, and CD155 transgenic mice: implications for poliovirus infection. J. Infect. Dis. 2002;186:585–592. doi: 10.1086/342682. [DOI] [PubMed] [Google Scholar]
- Iyer C.G., Laxmana Rao R., Work T.H., Narasimha Murthy D.P. Kyasanur Forest Disease VI. Pathological findings in three fatal human cases of Kyasanur Forest Disease. Indian J. Med. Sci. 1959;13:1011–1022. [PubMed] [Google Scholar]
- Iyer C.G.S., Work T.H., Narasimha Murthy D.P., Trapido H., Rajagopalan P.K. Pathologic findings in monkeys, Presbytis entellus and Macaca radiata found dead in the forest. Indian J. Med. Sci. 1960;48:276–286. [Google Scholar]
- Jacob J.R., Lin K.C., Tennant B.C., Mansfield K.G. GB virus B infection of the common marmoset (Callithrix jacchus) and associated liver pathology. J. Gen. Virol. 2004;85:2525–2533. doi: 10.1099/vir.0.80036-0. [DOI] [PubMed] [Google Scholar]
- Jacob J.R., Mansfield K., You J.E., Tennant B.C., Kim Y.H. Natural iminosugar derivatives of 1-deoxynojirimycin inhibit glycosylation of hepatitis viral envelope proteins. J. Microbiol. 2007;45:431–440. [PubMed] [Google Scholar]
- Jahrling P.B., Hensley L.E., Martinez M.J., Leduc J.W., Rubins K.H., Relman D.A., Huggins J.W. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc. Natl. Acad. Sci. U S A. 2004;101:15196–15200. doi: 10.1073/pnas.0405954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P.B., Hesse R.A., Eddy G.A., Johnson K.M., Callis R.T., Stephen E.L. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J. Infect. Dis. 1980;141:580–589. doi: 10.1093/infdis/141.5.580. [DOI] [PubMed] [Google Scholar]
- Jezek Z., Arita I., Szczeniowski M., Paluku K.M., Ruti K., Nakano J.H. Human tanapox in Zaire: clinical and epidemiological observations on cases confirmed by laboratory studies. Bull. World Health Organ. 1985;63:1027–1035. [PMC free article] [PubMed] [Google Scholar]
- Jiang B., McClure H.M., Fankhauser R.L., Monroe S.S., Glass R.I. Prevalence of rotavirus and norovirus antibodies in non-human primates. J. Med. Primatol. 2004;33:30–33. doi: 10.1111/j.1600-0684.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Joh J., Hopper K., Van Doorslaer K., Sundberg J.P., Jenson A.B., Ghim S.J. Macaca fascicularis papillomavirus type 1: a non-human primate betapapillomavirus causing rapidly progressive hand and foot papillomatosis. J. Gen. Virol. 2009;90:987–994. doi: 10.1099/vir.0.006544-0. [DOI] [PubMed] [Google Scholar]
- Johne R., Enderlein D., Nieper H., Muller H. Novel polyomavirus detected in the feces of a chimpanzee by nested broad-spectrum PCR. J. Virol. 2005;79:3883–3887. doi: 10.1128/JVI.79.6.3883-3887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen D.O., Wooding W.L., Tanticharoenyos P., Karnjanaprakorn C. An epizootic of A2/Hong Kong/68 influenza in gibbons. J. Infect. Dis. 1971;123:365–370. doi: 10.1093/infdis/123.4.365. [DOI] [PubMed] [Google Scholar]
- Johnson B.K., Gitau L.G., Gichogo A., Tukei P.M., Else J.G., Suleman M.A., Kimani R., Sayer P.D. Marburg, Ebola and Rift Valley Fever virus antibodies in East African primates. Trans. R. Soc. Trop. Med. Hyg. 1982;76:307–310. doi: 10.1016/0035-9203(82)90175-4. [DOI] [PubMed] [Google Scholar]
- Johnson J.E., Nasar F., Coleman J.W., Price R.E., Javadian A., Draper K., Lee M., Reilly P.A., Clarke D.K., Hendry R.M., Udem S.A. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology. 2007;360:36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L., Wolffe L.G., Whisler W.W., Norton T., Thakkar B., Deinhardt F. Induction of gliomas in marmosets by simian sarcoma virus type 1 (SSV-1) Proc. Natl. Acad. Sci. U S A. 1975;16:119. [Google Scholar]
- Jolly C., Phillips-Conroy J.E., Turner T.R., Broussard S., Allan J.S. SIVagm incidence over two decades in a natural population of Ethiopian grivet monkeys (Cercopithecus aethiops aethiops) J. Med. Primatol. 1996;25:78–83. doi: 10.1111/j.1600-0684.1996.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Jones-Engel L., Engel G.A., Heidrich J., Chalise M., Poudel N., Viscidi R., Barry P.A., Allan J.S., Grant R., Kyes R. Temple monkeys and health implications of commensalism, Kathmandu. Nepal. Emerg. Infect. Dis. 2006;12:900–906. doi: 10.3201/eid1206.060030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E.E., Alford P.L., Reingold A.L., Russell H., Keeling M.E., Broome C.V. Predisposition to invasive pneumococcal illness following parainfluenza type 3 virus infection in chimpanzees. J. Am. Vet. Med. Assoc. 1984;185:1351–1353. [PubMed] [Google Scholar]
- Jones M.S., 2nd, Harrach B., Ganac R.D., Gozum M.M., Dela Cruz W.P., Riedel B., Pan C., Delwart E.L., Schnurr D.P. New adenovirus species found in a patient presenting with gastroenteritis. J. Virol. 2007;81:5978–5984. doi: 10.1128/JVI.02650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.U., Choi J.K., Ensser A., Biesinger B. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin. Cancer Biol. 1999;9:231–239. doi: 10.1006/scbi.1998.0115. [DOI] [PubMed] [Google Scholar]
- Jung J.U., Desrosiers R.C. Identification and characterization of the herpesvirus saimiri oncoprotein STP-C488. J. Virol. 1991;65:6953–6960. doi: 10.1128/jvi.65.12.6953-6960.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.U., Desrosiers R.C. Herpesvirus saimiri oncogene STP-C488 encodes a phosphoprotein. J. Virol. 1992;66:1777–1780. doi: 10.1128/jvi.66.3.1777-1780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.U., Trimble J.J., King N.W., Biesinger B., Fleckenstein B.W., Desrosiers R.C. Identification of transforming genes of subgroup A and C strains of Herpesvirus saimiri. Proc. Natl. Acad. Sci. U S A. 1991;88:7051–7055. doi: 10.1073/pnas.88.16.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt K., Matz-Rensing K., Hofmann P., Stahl-Hennig C., Kaup F.J. SIV-associated lymphomas in rhesus monkeys (Macaca mulatta) in comparison with HIV-associated lymphomas. Vet. Pathol. 2002;39:42–55. doi: 10.1354/vp.39-1-42. [DOI] [PubMed] [Google Scholar]
- Kakuk T.J., Soike K., Brideau R.J., Zaya R.M., Cole S.L., Zhang J.Y., Roberts E.D., Wells P.A., Wathen M.W. A human respiratory syncytial virus (RSV) primate model of enhanced pulmonary pathology induced with a formalin-inactivated RSV vaccine but not a recombinant FG subunit vaccine. J. Infect. Dis. 1993;167:553–561. doi: 10.1093/infdis/167.3.553. [DOI] [PubMed] [Google Scholar]
- Kaleeba J.A., Bergquam E.P., Wong S.W. A rhesus macaque rhadinovirus related to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 encodes a functional homologue of interleukin-6. J. Virol. 1999;73:6177–6181. doi: 10.1128/jvi.73.7.6177-6181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter S.S. Enteric viruses of nonhuman primates. Vet. Pathol. Suppl. 1982;19(Suppl. 7):33–43. [PubMed] [Google Scholar]
- Kalter S.S., Heberling R.L. Serologic response of primates to influenza viruses. Proc. Soc. Exp. Biol. Med. 1978;159:414–417. doi: 10.3181/00379727-159-40360. [DOI] [PubMed] [Google Scholar]
- Kalter S.S., Heberling R.L., Cooper R.W. Serologic testing of various primate species maintained in a single outdoor breeding colony. Lab. Anim. Sci. 1974;24:636–645. [PubMed] [Google Scholar]
- Kalter S.S., Heberling R.L., Vice T.E., Lief F.S., Rodriguez A.R. Influenza (A2-Hong Kong-68) in the baboon (Papio sp.) Proc. Soc. Exp. Biol. Med. 1969;132:357–361. [PubMed] [Google Scholar]
- Kalter S.S., Smith G.C., 3rd, Heberling R.L. Electron microscopic examination of primate feces for rotaviruses. Lab. Anim. Sci. 1979;29:516–518. [PubMed] [Google Scholar]
- Kalter S.S., Weiss S.A., Heberling R.L., Guajardo J.E., Smith G.C., 3rd The isolation of herpesvirus from trigeminal ganglia of normal baboons (Papio cynocephalus) Lab. Anim. Sci. 1978;28:705–709. [PubMed] [Google Scholar]
- Kaufmann A.F., Gary G.W., Broderson J.R., Perl D.P., Quist K.D., Kissling R.E. Simian virus 16 associated with an epizootic of obscure neurologic disease. Lab. Anim. Sci. 1973;23:812–818. [PubMed] [Google Scholar]
- Kaur A., Hale C.L., Noren B., Kassis N., Simon M.A., Johnson R.P. Decreased frequency of cytomegalovirus (CMV)-specific CD4+ T lymphocytes in simian immunodeficiency virus-infected rhesus macaques: inverse relationship with CMV viremia. J. Virol. 2002;76:3646–3658. doi: 10.1128/JVI.76.8.3646-3658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A., Kassis N., Hale C.L., Simon M., Elliott M., Gomez-Yafal A., Lifson J.D., Desrosiers R.C., Wang F., Barry P., Mach M., Johnson R.P. Direct relationship between suppression of virus-specific immunity and emergence of cytomegalovirus disease in simian AIDS. J. Virol. 2003;77:5749–5758. doi: 10.1128/JVI.77.10.5749-5758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T., Singh J., Tong S., Humphrey C., Clevenger D., Tan W., Szekely B., Wang Y., Li Y., Alex Muse E., Kiyono M., Hanamura S., Inoue E., Nakamura M., Huffman M.A., Jiang B., Nishida T. Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related metapneumovirus in wild chimpanzees (Pan troglodytes) at Mahale Mountains National Park, Western Tanzania. Am. J. Primatol. 2008;70:755–765. doi: 10.1002/ajp.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble S.A. B virus infection in monkeys. Ann. N Y Acad. Sci. 1960;85:960–969. doi: 10.1111/j.1749-6632.1960.tb50016.x. [DOI] [PubMed] [Google Scholar]
- Keele B.F., Jones J.H., Terio K.A., Estes J.D., Rudicell R.S., Wilson M.L., Li Y., Learn G.H., Beasley T.M., Schumacher-Stankey J., Wroblewski E., Mosser A., Raphael J., Kamenya S., Lonsdorf E.V., Travis D.A., Mlengeya T., Kinsel M.J., Else J.G., Silvestri G., Goodall J., Sharp P.M., Shaw G.M., Pusey A.E., Hahn B.H. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B.F., Van Heuverswyn F., Li Y., Bailes E., Takehisa J., Santiago M.L., Bibollet-Ruche F., Chen Y., Wain L.V., Liegeois F., Loul S., Ngole E.M., Bienvenue Y., Delaporte E., Brookfield J.F., Sharp P.M., Shaw G.M., Peeters M., Hahn B.H. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P.G., Grinfeld E., Traina-Dorge V., Gilden D.H., Mahalingam R. Neuronal localization of simian varicella virus DNA in ganglia of naturally infected African green monkeys. Virus Genes. 2004;28:273–276. doi: 10.1023/b:viru.0000025774.19557.39. [DOI] [PubMed] [Google Scholar]
- Kenny D.E., Knightly F., Baier J., Moore S.M., Gordon C.R., Davis R.D., Heller A.C., Briggs D.J. Exposure of hooded capuchin monkeys (Cebus apella cay) to a rabid bat at a zoological park. J. Zoo. Wildl. Med. 2001;32:123–126. doi: 10.1638/1042-7260(2001)032[0123:EOHCMC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kestler H.W., 3rd, Ringler D.J., Mori K., Panicali D.L., Sehgal P.K., Daniel M.D., Desrosiers R.C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Khabbaz R.F., Heneine W., George J.R., Parekh B., Rowe T., Woods T., Switzer W.M., McClure H.M., Murphey-Corb M., Folks T.M. Brief report: infection of a laboratory worker with simian immunodeficiency virus. N. Engl. J. Med. 1994;330:172–177. doi: 10.1056/NEJM199401203300304. [DOI] [PubMed] [Google Scholar]
- Khabbaz R.F., Rowe T., Murphey-Corb M., Heneine W.M., Schable C.A., George J.R., Pau C.P., Parekh B.S., Lairmore M.D., Curran J.W. Simian immunodeficiency virus needlestick accident in a laboratory worker. Lancet. 1992;340:271–273. doi: 10.1016/0140-6736(92)92358-m. [DOI] [PubMed] [Google Scholar]
- Khan S., Peng X., Yin J., Zhang P., Wimmer E. Characterization of the New World monkey homologues of human poliovirus receptor CD155. J. Virol. 2008;82:7167–7179. doi: 10.1128/JVI.02664-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakevich L., Szczeniowski M., Manbu ma D., Jezek Z., Marennikova S., Nakano J., Messinger D. The role of squirrels in sustaining monkeypox virus transmission. Trop. Geogr. Med. 1987;39:115–122. [PubMed] [Google Scholar]
- Kidd A.H., Garwicz D., Oberg M. Human and simian adenoviruses: phylogenetic inferences from analysis of VA RNA genes. Virology. 1995;207:32–45. doi: 10.1006/viro.1995.1049. [DOI] [PubMed] [Google Scholar]
- Kik M.J., Bos J.H., Groen J., Dorrestein G.M. Herpes simplex infection in a juvenile orangutan (Pongo pygmaeus pygmaeus) J. Zoo. Wildl. Med. 2005;36:131–134. doi: 10.1638/03-004. [DOI] [PubMed] [Google Scholar]
- Kim C.S., Kriewaldt F.H., Hagino N., Kalter S.S. Subacute sclerosing panencephalitis-like syndrome in the adult baboon (Papio sp.) J. Am. Vet. Med. Assoc. 1970;157:730–735. [PubMed] [Google Scholar]
- Kim C.S., Sueltenfuss E.S., Kalter S.S. Isolation and characterization of simian adenoviruses isolated in association with an outbreak of pneumoenteritis in vervet monkeys (Cercopithecus aethiops) J. Infect. Dis. 1967;117:292–300. doi: 10.1093/infdis/117.4.292. [DOI] [PubMed] [Google Scholar]
- King N.W., Hunt R.D., Daniel M.D., Melendez L.V. Overt herpes-T infection in squirrel monkeys (Saimiri sciureus) Lab. Anim. Care. 1967;17:413–423. [PubMed] [Google Scholar]
- King N.W., Hunt R.D., Letvin N.L. Histopathologic changes in macaques with an acquired immunodeficiency syndrome (AIDS) Am. J. Pathol. 1983;113:382–388. [PMC free article] [PubMed] [Google Scholar]
- Kloster B.E., Manias D.A., Ostrow R.S., Shaver M.K., McPherson S.W., Rangen S.R., Uno H., Faras A.J. Molecular cloning and characterization of the DNA of two papillomaviruses from monkeys. Virology. 1988;166:30–40. doi: 10.1016/0042-6822(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Klotz O., Belt T.H. The pathology of the spleen in yellow fever. Am. J. Pathol. 1930;6:655–662. [PMC free article] [PubMed] [Google Scholar]
- Klotz O., Belt T.H. The pathology of the liver in yellow fever. Am. J. Pathol. 1930;6:663–687. [PMC free article] [PubMed] [Google Scholar]
- Knappe A., Feldmann G., Dittmer U., Meinl E., Nisslein T., Wittmann S., Matz-Rensing K., Kirchner T., Bodemer W., Fickenscher H. Herpesvirus saimiri-transformed macaque T cells are tolerated and do not cause lymphoma after autologous reinfusion. Blood. 2000;95:3256–3261. [PubMed] [Google Scholar]
- Kobasa D., Jones S.M., Shinya K., Kash J.C., Copps J., Ebihara H., Hatta Y., Kim J.H., Halfmann P., Hatta M., Feldmann F., Alimonti J.B., Fernando L., Li Y., Katze M.G., Feldmann H., Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Koch S., Larbi A., Ozcelik D., Solana R., Gouttefangeas C., Attig S., Wikby A., Strindhall J., Franceschi C., Pawelec G. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann. N Y Acad. Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- Koff W.C., Caplan F.R., Case S., Halstead S.B. Cell-mediated immune response to respiratory syncytial virus infection in owl monkeys. Clin. Exp. Immunol. 1983;53:272–280. [PMC free article] [PubMed] [Google Scholar]
- Kolappaswamy K., Mahalingam R., Traina-Dorge V., Shipley S.T., Gilden D.H., Kleinschmidt-Demasters B.K., McLeod C.G., Jr., Hungerford L.L., DeTolla L.J. Disseminated simian varicella virus infection in an irradiated rhesus macaque (Macaca mulatta) J. Virol. 2007;81:411–415. doi: 10.1128/JVI.01825-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondgen S., Kuhl H., N’Goran P.K., Walsh P.D., Schenk S., Ernst N., Biek R., Formenty P., Matz-Rensing K., Schweiger B., Junglen S., Ellerbrok H., Nitsche A., Briese T., Lipkin W.I., Pauli G., Boesch C., Leendertz F.H. Pandemic human viruses cause decline of endangered great apes. Curr. Biol. 2008;18:260–264. doi: 10.1016/j.cub.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Koralnik I.J., Boeri E., Saxinger W.C., Monico A.L., Fullen J., Gessain A., Guo H.G., Gallo R.C., Markham P., Kalyanaraman V. Phylogenetic associations of human and simian T-cell leukemia/lymphotropic virus type I strains: evidence for interspecies transmission. J. Virol. 1994;68:2693–2707. doi: 10.1128/jvi.68.4.2693-2707.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornegay R.W., Giddens W.E., Jr., Van Hoosier G.L., Jr., Morton W.R. Subacute nonsuppurative hepatitis associated with hepatitis B virus infection in two cynomolgus monkeys. Lab. Anim. Sci. 1985;35:400–404. [PubMed] [Google Scholar]
- Kornfeld C., Ploquin M.J., Pandrea I., Faye A., Onanga R., Apetrei C., Poaty-Mavoungou V., Rouquet P., Estaquier J., Mortara L., Desoutter J.F., Butor C., Le Grand R., Roques P., Simon F., Barre-Sinoussi F., Diop O.M., Muller-Trutwin M.C. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G.M., Davison A.J., Zakhartchouk A.N., Harrach B. Analysis of the first complete genome sequence of an Old World monkey adenovirus reveals a lineage distinct from the six human adenovirus species. J. Gen. Virol. 2004;85:2799–2807. doi: 10.1099/vir.0.80225-0. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S., Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Stipp H.L., Sheffer D., Narayan O. Use of herpesvirus saimiri-immortalized macaque CD4(+) T cell clones as stimulators and targets for assessment of CTL responses in macaque/AIDS models. J. Immunol. Methods. 1999;230:47–58. doi: 10.1016/s0022-1759(99)00123-4. [DOI] [PubMed] [Google Scholar]
- Kupper J.L., Casey H.W., Johnson D.K. Experimental Yaba and benign epidermal monkey pox in rhesus monkeys. Lab. Anim. Care. 1970;20:979–988. [PubMed] [Google Scholar]
- Kutok J.L., Klumpp S., Simon M., MacKey J.J., Nguyen V., Middeldorp J.M., Aster J.C., Wang F. Molecular evidence for rhesus lymphocryptovirus infection of epithelial cells in immunosuppressed rhesus macaques. J. Virol. 2004;78:3455–3461. doi: 10.1128/JVI.78.7.3455-3461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laast V.A., Pardo C.A., Tarwater P.M., Queen S.E., Reinhart T.A., Ghosh M., Adams R.J., Zink M.C., Mankowski J.L. Pathogenesis of simian immunodeficiency virus-induced alterations in macaque trigeminal ganglia. J. Neuropathol. Exp. Neurol. 2007;66:26–34. doi: 10.1097/nen.0b013e31802c398d. [DOI] [PubMed] [Google Scholar]
- Labadie K., Larcher T., Joubert C., Mannioui A., Delache B., Brochard P., Guigand L., Dubreil L., Lebon P., Verrier B., de Lamballerie X., Suhrbier A., Cherel Y., Le Grand R., Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner A.A. University of California at Davis; 1988. Tissue distribution of simian AIDS retrovirus serotype 1 and its relationship to the pathogenesis of simian AIDS. [Google Scholar]
- Lackner A.A., Schiodt M., Armitage G.C., Moore P.F., Munn R.J., Marx P.A., Gardner M.B., Lowenstine L.J. Mucosal epithelial cells and Langerhans cells are targets for infection by the immunosuppressive type D retrovirus simian AIDS retrovirus serotype 1. J. Med. Primatol. 1989;18:195–207. [PubMed] [Google Scholar]
- Lackner A.A., Smith M.O., Munn R.J., Martfeld D.J., Gardner M.B., Marx P.A., Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am. J. Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- Lacoste V., Lavergne A., de Thoisy B., Pouliquen J.F., Gessain A. Genetic diversity and molecular evolution of human and non-human primate Gammaherpesvirinae. Infect. Genet. Evol. 2010;10:1–13. doi: 10.1016/j.meegid.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Lacoste V., Mauclere P., Dubreuil G., Lewis J., Georges-Courbot M.C., Gessain A. KSHV-like herpesviruses in chimps and gorillas. Nature. 2000;407:151–152. doi: 10.1038/35025145. [DOI] [PubMed] [Google Scholar]
- Lacoste V., Mauclere P., Dubreuil G., Lewis J., Georges-Courbot M.C., Gessain A. A novel gamma 2-herpesvirus of the Rhadinovirus 2 lineage in chimpanzees. Genome Res. 2001;11:1511–1519. doi: 10.1101/gr.158601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste V., Mauclere P., Dubreuil G., Lewis J., Georges-Courbot M.C., Rigoulet J., Petit T., Gessain A. Simian homologues of human gamma-2 and betaherpesviruses in mandrill and drill monkeys. J. Virol. 2000;74:11993–11999. doi: 10.1128/jvi.74.24.11993-11999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R.S., Roehrig J.T., Deubel V., Smith J., Parker M., Steele K., Crise B., Volpe K.E., Crabtree M.B., Scherret J.H., Hall R.A., MacKenzie J.S., Cropp C.B., Panigrahy B., Ostlund E., Schmitt B., Malkinson M., Banet C., Weissman J., Komar N., Savage H.M., Stone W., McNamara T., Gubler D.J. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Landolfi J.A., Wellehan J.F., Johnson A.J., Kinsel M.J. Fatal human herpesvirus type 1 infection in a white-handed gibbon (Hylobates lar) J. Vet. Diagn. Invest. 2005;17:369–371. doi: 10.1177/104063870501700412. [DOI] [PubMed] [Google Scholar]
- Lanford R.E., Chavez D., Barrera A., Brasky K.M. An infectious clone of woolly monkey hepatitis B virus. J. Virol. 2003;77:7814–7819. doi: 10.1128/JVI.77.14.7814-7819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R.E., Chavez D., Brasky K.M., Burns R.B., 3rd, Rico-Hesse R. Isolation of a hepadnavirus from the woolly monkey, a New World primate. Proc. Natl. Acad. Sci. U S A. 1998;95:5757–5761. doi: 10.1073/pnas.95.10.5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R.E., Hildebrandt-Eriksen E.S., Petri A., Persson R., Lindow M., Munk M.E., Kauppinen S., Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankas G.R., Jensen R.D. Evidence of hepatitis A infection in immature rhesus monkeys. Vet. Pathol. 1987;20:340–344. doi: 10.1177/030098588702400409. [DOI] [PubMed] [Google Scholar]
- Lapin B.A., Shevtsova Z.V. Persistence of spontaneous and experimental hepatitis A in rhesus macaques. Exp. Pathol. 1990;39:59–60. doi: 10.1016/s0232-1513(11)80223-9. [DOI] [PubMed] [Google Scholar]
- Lednicky J.A., Arrington A.S., Stewart A.R., Dai X.M., Wong C., Jafar S., Murphey-Corb M., Butel J.S. Natural isolates of simian virus 40 from immunocompromised monkeys display extensive genetic heterogeneity: new implications for polyomavirus disease. J. Virol. 1998;72:3980–3990. doi: 10.1128/jvi.72.5.3980-3990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.V., Prowten A.W., Satchidanand S.K., Srivastava B.I. Non-Hodgkin’s lymphoma and HTLV-1 antibodies in a gorilla. N. Engl. J. Med. 1985;312:118–119. doi: 10.1056/NEJM198501103120212. [DOI] [PubMed] [Google Scholar]
- Lefaux B., Duprez R., Tanguy M., Longeart L., Gessain A., Boulanger E. Nonhuman primates might be highly susceptible to cross-species infectivity by human alpha-herpesviruses. Vet. Pathol. 2004;41:302–304. doi: 10.1354/vp.41-3-302-a. [DOI] [PubMed] [Google Scholar]
- Leland M.M., Hubbard G.B., Sentmore H.T., 3rd, Soike K.F., Hilliard J.K. Outbreak of Orthoreovirus-induced meningoencephalomyelitis in baboons. Comp. Med. 2000;50:199–205. [PubMed] [Google Scholar]
- Leopardi R., Ilonen J., Mattila L., Salmi A.A. Effect of measles virus infection on MHC class II expression and antigen presentation in human monocytes. Cell Immunol. 1993;147:388–396. doi: 10.1006/cimm.1993.1078. [DOI] [PubMed] [Google Scholar]
- Lerche N.W., Osborn K.G. Simian retrovirus infections: potential confounding variables in primate toxicology studies. Toxicol. Pathol. 2003;31(Suppl):103–110. doi: 10.1080/01926230390174977. [DOI] [PubMed] [Google Scholar]
- Lerche N.W., Osborn K.G., Marx P.A., Prahalada S., Maul D.H., Lowenstine L.J., Munn R.J., Bryant M.L., Henrickson R.V., Arthur L.O. Inapparent carriers of simian acquired immune deficiency syndrome type D retrovirus and disease transmission with saliva. J. Natl. Cancer Inst. 1986;77:489–496. [PubMed] [Google Scholar]
- Lerche N.W., Switzer W.M., Yee J.L., Shanmugam V., Rosenthal A.N., Chapman L.E., Folks T.M., Heneine W. Evidence of infection with simian type D retrovirus in persons occupationally exposed to nonhuman primates. J. Virol. 2001;75:1783–1789. doi: 10.1128/JVI.75.4.1783-1789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy E.M., Epelboin A., Mondonge V., Pourrut X., Gonzalez J.P., Muyembe-Tamfum J.J., Formenty P. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009;9:723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P., Delicat A., Paweska J.T., Gonzalez J.P., Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- Leroy E.M., Telfer P., Kumulungui B., Yaba P., Rouquet P., Roques P., Gonzalez J.P., Ksiazek T.G., Rollin P.E., Nerrienet E. A serological survey of Ebola virus infection in central African nonhuman primates. J. Infect. Dis. 2004;190:1895–1899. doi: 10.1086/425421. [DOI] [PubMed] [Google Scholar]
- Letvin N.L., Daniel M.D., Sehgal P.K., Desrosiers R.C., Hunt R.D., Waldron L.M., MacKey J.J., Schmidt D.K., Chalifoux L.V., King N.W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Levin J.L., Hilliard J.K., Lipper S.L., Butler T.M., Goodwin W.J. A naturally occurring epizootic of simian agent 8 in the baboon. Lab. Anim. Sci. 1988;38:394–397. [PubMed] [Google Scholar]
- Levy J.A., Levy S.B., Hirshaut Y., Kafuko G., Prince A. Presence of EBV antibodies in sera from wild chimpanzees. Nature. 1971;233:559–560. doi: 10.1038/233559a0. [DOI] [PubMed] [Google Scholar]
- Li Q., Estes J.D., Duan L., Jessurun J., Pambuccian S., Forster C., Wietgrefe S., Zupancic M., Schacker T., Reilly C., Carlis J.V., Haase A.T. Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J. Infect. Dis. 2008;197:420–429. doi: 10.1086/525046. [DOI] [PubMed] [Google Scholar]
- Li S.L., Kaaya E.E., Feichtinger H., Putkonen P., Parravicini C., Bottiger D., Biberfeld G., Biberfeld P. Monocyte/macrophage giant cell disease in SIV-infected cynomolgus monkeys. Res. Virol. 1991;142:173–182. doi: 10.1016/0923-2516(91)90054-7. [DOI] [PubMed] [Google Scholar]
- Liegeois F., Courgnaud V., Switzer W.M., Murphy H.W., Loul S., Aghokeng A., Pourrut X., Mpoudi-Ngole E., Delaporte E., Peeters M. Molecular characterization of a novel simian immunodeficiency virus lineage (SIVtal) from northern talapoins (Miopithecus ogouensis) Virology. 2006;349:55–65. doi: 10.1016/j.virol.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Liegeois F., Lafay B., Formenty P., Locatelli S., Courgnaud V., Delaporte E., Peeters M. Full-length genome characterization of a novel simian immunodeficiency virus lineage (SIVolc) from olive Colobus (Procolobus verus) and new SIVwrcPbb strains from Western Red Colobus (Piliocolobus badius badius) from the Tai Forest in Ivory Coast. J. Virol. 2009;83:428–439. doi: 10.1128/JVI.01725-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson J.D., Nowak M.A., Goldstein S., Rossio J.L., Kinter A., Vasquez G., Wiltrout T.A., Brown C., Schneider D., Wahl L., Lloyd A.L., Williams J., Elkins W.R., Fauci A.S., Hirsch V.M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., Davidson W., Galloway R., Khristova M.L., Reynolds M.G., Zhao H., Carroll D.S., Curns A., Formenty P., Esposito J.J., Regnery R.L., Damon I.K. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- Lim S.Y., Rogers T., Chan T., Whitney J.B., Kim J., Sodroski J., Letvin N.L. TRIM5alpha Modulates Immunodeficiency Virus Control in Rhesus Monkeys. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000738. e1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B., Apetrei C., Pandrea I., Veazey R.S., Lackner A.A., Gormus B., Marx P.A. Classic AIDS in a sooty mangabey after an 18-year natural infection. J. Virol. 2004;78:8902–8908. doi: 10.1128/JVI.78.16.8902-8908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B., Veazey R.S., Luckay A., Penedo C., Xu K., Lifson J.D., Marx P.A. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS. 2002;16:1489–1496. doi: 10.1097/00002030-200207260-00005. [DOI] [PubMed] [Google Scholar]
- Linneman C.C., Kramer L.W., Askey P.A. Familial clustering of hepatitis B infection in gorillas. Am. J. Epidemiol. 1984;119:424–430. doi: 10.1093/oxfordjournals.aje.a113760. [DOI] [PubMed] [Google Scholar]
- Liu W., Worobey M., Li Y., Keele B.F., Bibollet-Ruche F., Guo Y., Goepfert P.A., Santiago M.L., Ndjango J.B., Neel C., Clifford S.L., Sanz C., Kamenya S., Wilson M.L., Pusey A.E., Gross-Camp N., Boesch C., Smith V., Zamma K., Huffman M.A., Mitani J.C., Watts D.P., Peeters M., Shaw G.M., Switzer W.M., Sharp P.M., Hahn B.H. Molecular ecology and natural history of simian foamy virus infection in wild-living chimpanzees. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000097. e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli S., Lafay B., Liegeois F., Ting N., Delaporte E., Peeters M. Full molecular characterization of a simian immunodeficiency virus, SIVwrcpbt from Temminck’s red colobus (Piliocolobus badius temminckii) from Abuko Nature Reserve, The Gambia. Virology. 2008;376:90–100. doi: 10.1016/j.virol.2008.01.049. [DOI] [PubMed] [Google Scholar]
- Lockridge K.M., Sequar G., Zhou S.S., Yue Y., Mandell C.P., Barry P.A. Pathogenesis of experimental rhesus cytomegalovirus infection. J. Virol. 1999;73:9576–9583. doi: 10.1128/jvi.73.11.9576-9583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London W.T., Houff S.A., Madden D.L., Fuccillo D.A., Gravell M., Wallen W.C., Palmer A.E., Sever J.L., Padgett B.L., Walker D.L., ZuRhein G.M., Ohashi T. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus) Science. 1978;201:1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- London W.T., Martinez A.J., Houff S.A., Wallen W.C., Curfman B.L., Traub R.G., Sever J.L. Experimental congenital disease with simian cytomegalovirus in rhesus monkeys. Teratology. 1986;33:323–331. doi: 10.1002/tera.1420330311. [DOI] [PubMed] [Google Scholar]
- Loomis M.R., O’Neill T., Bush M., Montali R.J. Fatal herpesvirus infection in patas monkeys and a black and white colobus monkey. J. Am. Vet. Med. Assoc. 1981;179:1236–1239. [PubMed] [Google Scholar]
- Lowenstine L.J. Springer-Verlag; Berlin and New York: 1993. Type D retrovirus infection, macaques. [Google Scholar]
- Lowenstine L.J., Lerche N.W., Yee J.L., Uyeda A., Jennings M.B., Munn R.J., McClure H.M., Anderson D.C., Fultz P.N., Gardner M.B. Evidence for a lentiviral etiology in an epizootic of immune deficiency and lymphoma in stump-tailed macaques (Macaca arctoides) J. Med. Primatol. 1992;21:1–14. [PubMed] [Google Scholar]
- Mahalingam R., Clarke P., Wellish M., Dueland A.N., Soike K.F., Gilden D.H., Cohrs R. Prevalence and distribution of latent simian varicella virus DNA in monkey ganglia. Virology. 1992;188:193–197. doi: 10.1016/0042-6822(92)90749-f. [DOI] [PubMed] [Google Scholar]
- Mahalingam R., Wellish M., Soike K., White T., Kleinschmidt-DeMasters B.K., Gilden D.H. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–342. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- Mahieux R., Chappey C., Georges-Courbot M.C., Dubreuil G., Mauclere P., Georges A., Gessain A. Simian T-cell lymphotropic virus type 1 from Mandrillus sphinx as a simian counterpart of human T-cell lymphotropic virus type 1 subtype D. J. Virol. 1998;72:10316–10322. doi: 10.1128/jvi.72.12.10316-10322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major E.O., Vacante D.A., Traub R.G., London W.T., Sever J.L. Owl monkey astrocytoma cells in culture spontaneously produce infectious JC virus which demonstrates altered biological properties. J. Virol. 1987;61:1435–1441. doi: 10.1128/jvi.61.5.1435-1441.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe H., Harwin R. Neurotropic virus in African monkeys. Lancet. 1958;2:530. [Google Scholar]
- Mankowski J.L., Carter D.L., Spelman J.P., Nealen M.L., Maughan K.R., Kirstein L.M., Didier P.J., Adams R.J., Murphey-Corb M., Zink M.C. Pathogenesis of simian immunodeficiency virus pneumonia: an immunopathological response to virus. Am. J. Pathol. 1998;153:1123–1130. doi: 10.1016/S0002-9440(10)65656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski J.L., Spelman J.P., Ressetar H.G., Strandberg J.D., Laterra J., Carter D.L., Clements J.E., Zink M.C. Neurovirulent simian immunodeficiency virus replicates productively in endothelial cells of the central nervous system in vivo and in vitro. J. Virol. 1994;68:8202–8208. doi: 10.1128/jvi.68.12.8202-8208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield K.G., Carville A., Wachtman L., Goldin B.R., Yearley J., Li W., Woods M., Gualtieri L., Shannon R., Wanke C. A diet high in saturated fat and cholesterol accelerates simian immunodeficiency virus disease progression. J. Infect. Dis. 2007;196:1202–1210. doi: 10.1086/521680. [DOI] [PubMed] [Google Scholar]
- Mansfield K.G., Lackner A.A. SIV infection in macaques at the NERPRC during 1970-72. J. Med. Primatol. 1994;23:244. [Google Scholar]
- Mansfield K.G., Lerch N.W., Gardner M.B., Lackner A.A. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J. Med. Primatol. 1995;24:116–122. doi: 10.1111/j.1600-0684.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Mansfield K.G., Westmoreland S.V., DeBakker C.D., Czajak S., Lackner A.A., Desrosiers R.C. Experimental infection of rhesus and pig-tailed macaques with macaque rhadinoviruses. J. Virol. 1999;73:10320–10328. doi: 10.1128/jvi.73.12.10320-10328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marennikova S.S., Maltseva V.I., Shelukhina E.M., Shenkman L.S., Korneeva V.I. A generalized herpetic infection simulating small pox in a gorilla. Intervirology. 1973;2:280–286. [Google Scholar]
- Marennikova S.S., Shelukhina E.M., Maltseva V.I., Ladnnyj I.D. Pox viruses isolated from clinically ill and asymptomatically infected monkeys and a chimpanzee. Bull. World Health Organ. 1976;46:613–620. [PMC free article] [PubMed] [Google Scholar]
- Marr-Belvin A.K., Carville A.K., Fahey M.A., Boisvert K., Klumpp S.A., Ohashi M., Wang F., O’Neil S.P., Westmoreland S.V. Rhesus lymphocryptovirus type 1-associated B-cell nasal lymphoma in SIV-infected rhesus macaques. Vet. Pathol. 2008;45:914–921. doi: 10.1354/vp.45-6-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott K.A., Parkinson C.V., Morefield S.I., Davenport R., Nichols R., Monath T.P. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine. 2008;26:581–588. doi: 10.1016/j.vaccine.2007.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B.J., Dysko R.C., Chrisp C.E. Pancreatitis associated with simian adenovirus 23 in a rhesus monkey. Lab. Anim. Sci. 1991;41:382–384. [PubMed] [Google Scholar]
- Martin D.P., Kaye H.S. Epizootic of parainfluenza-3 virus infection in gibbons. J. Am. Vet. Med. Assoc. 1983;183:1185–1187. [PubMed] [Google Scholar]
- Martin J., Hermida L., Castro J., Lazo L., Martinez R., Gil L., Romero Y., Puente P., Zaragoza S., Cosme K., Guzman M.G., Cardosa J., Guillen G. Viremia and antibody response in green monkeys (Chlorocebus aethiops sabaeus) infected with dengue virus type 2: a potential model for vaccine testing. Microbiol. Immunol. 2009;53:216–223. doi: 10.1111/j.1348-0421.2009.00112.x. [DOI] [PubMed] [Google Scholar]
- Martina B.E., van Doornum G., Dorrestein G.M., Niesters H.G., Stittelaar K.J., Wolters M.A., van Bolhuis H.G., Osterhaus A.D. Cowpox virus transmission from rats to monkeys, the Netherlands. Emerg. Infect. Dis. 2006;12:1005–1007. doi: 10.3201/eid1206.051513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M.A., Hubbard G.B., Butler T.M., Hilliard J.K. Clinical disease associated with simian agent 8 infection in the baboon. Lab. Anim. Sci. 1998;48:18–22. [PubMed] [Google Scholar]
- Marx P.A., Maul D.H., Osborn K.G., Lerche N.W., Moody P., Lowenstine L.J., Henrickson R.V., Arthur L.O., Gilden R.V., Gravell M. Simian AIDS: isolation of a type D retrovirus and transmission of the disease. Science. 1984;223:1083–1086. doi: 10.1126/science.6695196. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J., Taraporewala Z.F., Yang H., Rao S., Yuan L., Cao D., Hoshino Y., Mertens P.P., Carner G.R., McNeal M., Sestak K., Van Ranst M., Patton J.T. Simian rotaviruses possess divergent gene constellations that originated from interspecies transmission and reassortment. J. Virol. 2010;84:2013–2026. doi: 10.1128/JVI.02081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz-Rensing K., Ellerbrok H., Ehlers B., Pauli G., Floto A., Alex M., Czerny C.P., Kaup F.J. Fatal poxvirus outbreak in a colony of New World monkeys. Vet. Pathol. 2006;43:212–218. doi: 10.1354/vp.43-2-212. [DOI] [PubMed] [Google Scholar]
- Matz-Rensing K., Jentsch K.D., Rensing S., Langenhuyzen S., Verschoor E., Niphuis H., Kaup F.J. Fatal Herpes simplex infection in a group of common marmosets (Callithrix jacchus) Vet. Pathol. 2003;40:405–411. doi: 10.1354/vp.40-4-405. [DOI] [PubMed] [Google Scholar]
- Matz-Rensing K., Pingel S., Hannig H., Bodemer W., Hunsmann G., Kuhn E.M., Tiemann M., Kaup F.J. Morphologic and immunophenotypic characteristics of malignant lymphomas in SIV-infected rhesus macaques. J. Med. Primatol. 1999;28:318–328. doi: 10.1111/j.1600-0684.1999.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Maul D.H., Zaiss C.P., MacKenzie M.R., Shiigi S.M., Marx P.A., Gardner M.B. Simian retrovirus D serogroup 1 has a broad cellular tropism for lymphoid and nonlymphoid cells. J. Virol. 1988;62:1768–1773. doi: 10.1128/jvi.62.5.1768-1773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J.E., Hartwell W.V., Berquist K.R. Hepatitis-associated antigen in chimpanzees. J. Infect. Dis. 1971;126:660–664. doi: 10.1093/infdis/123.6.660. [DOI] [PubMed] [Google Scholar]
- McAuliffe J., Vogel L., Roberts A., Fahle G., Fischer S., Shieh W.J., Butler E., Zaki S., St Claire M., Murphy B., Subbarao K. Replication of SARS coronavirus administered into the respiratory tract of African Green, rhesus and cynomolgus monkeys. Virology. 2004;330:8–15. doi: 10.1016/j.virol.2004.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy T.J., Kennedy J.L., Blakeslee J.R., Bennett B.T. Spontaneous malignant lymphoma and leukemia in a simian T-lymphotropic virus type I (STLV-I) antibody positive olive baboon. Lab. Anim. Sci. 1990;40:79–81. [PubMed] [Google Scholar]
- McChesney M.B., Fujinami R.S., Lerche N.W., Marx P.A., Oldstone M.B. Virus-induced immunosuppression: infection of peripheral blood mononuclear cells and suppression of immunoglobulin synthesis during natural measles virus infection of rhesus monkeys. J. Infect. Dis. 1989;159:757–760. doi: 10.1093/infdis/159.4.757. [DOI] [PubMed] [Google Scholar]
- McClure H.M., Chandler F.W., Hierholzer J.C. Necrotizing pancreatitis due to simian adenovirus type 31 in a rhesus monkey. Arch. Pathol. Lab. Med. 1978;102:150–153. [PubMed] [Google Scholar]
- McClure H.M., Keeling M.E. Viral diseases noted at the Yerkes Primate Center colony. Lab. Anim. Sci. 1971;21:1002–1010. [PubMed] [Google Scholar]
- McClure H.M., Keeling M.E., Olberding B., Hunt R.D., Melendez L.V. Natural Herpesvirus hominis infection of tree shrews (Tupaia glis) Lab. Anim. Sci. 1972;22:517–521. [PubMed] [Google Scholar]
- McClure H.M., Swenson R.B., Kalter S.S., Lester T.L. Natural genital herpesvirus hominis infection in chimpanzees (Pan troglodytes and Pan paniscus) Lab. Anim. Sci. 1980;30:895–901. [PubMed] [Google Scholar]
- McClure M.O., Schulz T.F., Tedder R.S., Gow J., McKeating J.A., Weiss R.A., Baskerville A. Inoculation of New World primates with the human immunodeficiency virus. J. Med. Primatol. 1989;18:329–335. [PubMed] [Google Scholar]
- McConnell S.J., Hickman R.L., Wooding W.L., Huxsoll D.L. Monkey-pox: Experimental infection in chimpanzees and immunization with vaccinia virus. Am. J. Vet. Res. 1968;29:1675–1680. [PubMed] [Google Scholar]
- McCrae A.W., Henderson B.E., Kirya B.G., Sempala S.D. Chikungunya virus in the Entebbe area of Uganda: isolations and epidemiology. Trans. R. Soc. Trop. Med. Hyg. 1971;65:152–168. doi: 10.1016/0035-9203(71)90212-4. [DOI] [PubMed] [Google Scholar]
- McCune J.M. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–979. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- McNeal M.M., Sestak K., Choi A.H., Basu M., Cole M.J., Aye P.P., Bohm R.P., Ward R.L. Development of a rotavirus-shedding model in rhesus macaques, using a homologous wild-type rotavirus of a new P genotype. J. Virol. 2005;79:944–954. doi: 10.1128/JVI.79.2.944-954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L., Mahieux R., Mauclere P., Lewis J., Gessain A. Complete sequence of a novel highly divergent simian T-cell lymphotropic virus from wild-caught red-capped mangabeys (Cercocebus torquatus) from Cameroon: a new primate T-lymphotropic virus type 3 subtype. J. Virol. 2002;76:259–268. doi: 10.1128/JVI.76.1.259-268.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinl E., Fickenscher H., Hoch R.M., Malefyt R.D., de Waal Malefyt R., t Hart B.A., Wekerle H., Hohlfeld R., Fleckenstein B. Growth transformation of antigen-specific T cell lines from rhesus monkeys by herpesvirus saimiri. Virology. 1997;229:175–182. doi: 10.1006/viro.1996.8427. [DOI] [PubMed] [Google Scholar]
- Melendez L.V., Espana C., Hunt R.D., Daniel M.D., Garcia F.G. Natural herpes simplex infection in the owl monkey (Aotus trivirgatus) Lab. Anim. Care. 1969;19:38–45. [PubMed] [Google Scholar]
- Melendez L.V., Hunt R.D., Daniel M.D., Trum B.F. New World monkeys, herpes viruses, and cancer. In: Balner H., Beveridge B., editors. Infections and Immunosuppression in Subhuman Primates. Munksgaard; Copenhagen: 1970. pp. 111–117. [Google Scholar]
- Melnick J.L., Butel J.S. Neurologic tumors in offspring after inoculation of mothers with killed-poliovirus vaccine. N. Engl. J. Med. 1988;318:1226. [PubMed] [Google Scholar]
- Melnick J.L., Midulla M., Wimberly I., Barrera-Oro J.G., Levy B.M. A New Member of the Herpesvirus Group Isolated from South American Marmosets. J. Immunol. 1964;92:596–601. [PubMed] [Google Scholar]
- Messaoudi I., Barron A., Wellish M., Engelmann F., Legasse A., Planer S., Gilden D., Nikolich-Zugich J., Mahalingam R. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000657. e1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Perrichot M., Stemmler M., Emmerich P., Schmitz H., Varaine F., Shungu R., Tshioko F., Formenty P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meythaler M., Martinot A., Wang Z., Pryputniewicz S., Kasheta M., Ling B., Marx P.A., O’Neil S., Kaur A. Differential CD4+ T-lymphocyte apoptosis and bystander T-cell activation in rhesus macaques and sooty mangabeys during acute simian immunodeficiency virus infection. J. Virol. 2009;83:572–583. doi: 10.1128/JVI.01715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels M.G., Jenkins F.J., St George K., Nalesnik M.A., Starzl T.E., Rinaldo C.R., Jr. Detection of infectious baboon cytomegalovirus after baboon-to-human liver xenotransplantation. J. Virol. 2001;75:2825–2828. doi: 10.1128/JVI.75.6.2825-2828.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Coope D., Waters L., Pagano J., Bornkamn G., Henle W. Lymphoma in cotton-top marmosets after inoculation with Epstein-Barr virus: tumor incidence, histologic spectrum antibody responses, demonstration of viral DNA, and characterization of viruses. J. Exp. Med. 1977;145:948–967. doi: 10.1084/jem.145.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N.R., McKeever P.E., London W., Padgett B.L., Walker D.L., Wallen W.C. Brain tumors of owl monkeys inoculated with JC virus contain the JC virus genome. J. Virol. 1984;49:848–856. doi: 10.1128/jvi.49.3.848-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M.B., Handermann M., Darai G. DNA polymerase gene locus of Cercopithecine herpesvirus 1 is a suitable target for specific and rapid identification of viral infection by PCR technology. Virus Genes. 2005;30:307–322. doi: 10.1007/s11262-004-6773-0. [DOI] [PubMed] [Google Scholar]
- Miranda M.E., Yoshikawa Y., Manalo D.L., Calaor A.B., Miranda N.L., Cho F., Ikegami T., Ksiazek T.G. Chronological and spatial analysis of the 1996 Ebola Reston virus outbreak in a monkey breeding facility in the Philippines. Exp. Anim. 2002;51:173–179. doi: 10.1538/expanim.51.173. [DOI] [PubMed] [Google Scholar]
- Mitsunaga F., Nakamura S., Hayashi T., Eberle R. Changes in the titer of anti-B virus antibody in captive macaques (Macaca fuscata, M. mulatta, M. fascicularis) Comp. Med. 2007;57:120–124. [PubMed] [Google Scholar]
- Miyagi J., Tsuhako K., Kinjo T., Iwamasa T., Kamada Y., Kinju T., Koyanagi Y. Coxsackievirus B4 myocarditis in an orangutan. Vet. Pathol. 1999;36:452–456. doi: 10.1354/vp.36-5-452. [DOI] [PubMed] [Google Scholar]
- Moazed T.C., Thouless M.E. Viral persistence of simian type D retrovirus (SRV-2/W) in naturally infected pigtailed macaques (Macaca nemestrina) J. Med. Primatol. 1993;22:382–389. [PubMed] [Google Scholar]
- Moebes A., Enssle J., Bieniasz P.D., Heinkelein M., Lindemann D., Bock M., McClure M.O., Rethwilm A. Human foamy virus reverse transcription that occurs late in the viral replication cycle. J. Virol. 1997;71:7305–7311. doi: 10.1128/jvi.71.10.7305-7311.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam A., Rosenzweig M., Lee-Parritz D., Annis B., Johnson R.P., Wang F. An animal model for acute and persistent Epstein-Barr virus infection. Science. 1997;276:2030–2033. doi: 10.1126/science.276.5321.2030. [DOI] [PubMed] [Google Scholar]
- Monath T.P., Brinker K.R., Chandler F.W., Kemp G.E., Cropp C.B. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am. J. Trop. Med. Hyg. 1981;30:431–443. doi: 10.4269/ajtmh.1981.30.431. [DOI] [PubMed] [Google Scholar]
- Montali R.J. Callitrichid hepatitis. In: Jones T.C., Mohr U., Hunt R.D., editors. Vol. 2. Speringer-Verlag; Berlin and New York: 1993. pp. 61–62. (Monographs on the Pathology of Laboratory Animals: Nonhuman Primates). [Google Scholar]
- Montali R.J., Connolly B.M., Armstrong D.L., Scanga C.A., Holmes K.V. Pathology and immunohistochemistry of callitrichid hepatitis, an emerging disease of captive New World primates caused by lymphocytic choriomeningitis virus. Am. J. Pathol. 1995;147:1441–1449. [PMC free article] [PubMed] [Google Scholar]
- Montali R.J., Ramsay E.C., Stephensen C.B., Worley M., Davis J.A., Holmes K.V. A new transmissible viral hepatitis of marmosets and tamarins. J. Infect. Dis. 1989;160:759–765. doi: 10.1093/infdis/160.5.759. [DOI] [PubMed] [Google Scholar]
- Montali R.J., Scanga C.A., Pernikoff D., Wessner D.R., Ward R., Holmes K.V. A common source outbreak of callitrichid hepatitis in captive tamarins and marmosets. J. Infect. Dis. 1995;167:946–950. doi: 10.1093/infdis/167.4.946. [DOI] [PubMed] [Google Scholar]
- Montiel N.A. An updated review of simian betaretrovirus (SRV) in macaque hosts. J. Med. Primatol. 2010;39:303–314. doi: 10.1111/j.1600-0684.2010.00412.x. [DOI] [PubMed] [Google Scholar]
- Morbeck M.E., Zihlman A.L., Summner D.R., Galloway A. Poliomyelitis and skeletal asymmetry in Gombe chimpanzees. Primates. 1991;32:77–91. [Google Scholar]
- Morita M. Fatal herpesvirus tamarinus infection in cotton-topped marmosets (Saguinus oedipus) Jpn. J. Med. Sci. Biol. 1981;34:268–270. [PubMed] [Google Scholar]
- Morozov V.A., Lagaye S., Lyakh L., ter Meulen J. Type D retrovirus markers in healthy Africans from Guinea. Res. Virol. 1996;147:341–351. doi: 10.1016/s0923-2516(97)85126-6. [DOI] [PubMed] [Google Scholar]
- Mou S.W., Hilliard J.K., Song C.H., Eberle R. Comparison of the primate alphaherpesviruses. I. Characterization of two herpesviruses from spider monkeys and squirrel monkeys and viral polypeptides synthesized in infected cells. Arch. Virol. 1986;91:117–133. doi: 10.1007/BF01316733. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L.N., Rangan S.R., Baskin G.B., Gormus B.J., Wolf R.H., Andes W.A., West M., Montelaro R.C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Murphy B.L., Maynard J.E., Krushak D.H., Berquist K.R. Microbial flora of imported marmosets: viruses and enteric bacteria. Lab. Anim. Sci. 1972;22:339–343. [PubMed] [Google Scholar]
- Murphy B.L., Maynard J.E., Krushak D.H., Fields R.M. Occurrence of a carrier state for Herpesvirus tamarinus in Marmosets. Appl. Microbiol. 1971;21:50–52. doi: 10.1128/am.21.1.50-52.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F.A., Simpson D.I., Whitfield S.G., Zlotnik I., Carter G.B. Marburg virus infection in monkeys. Ultrastructural studies. Lab. Invest. 1971;24:279–291. [PubMed] [Google Scholar]
- Murray S.M., Picker L.J., Axthelm M.K., Hudkins K., Alpers C.E., Linial M.L. Replication in a superficial epithelial cell niche explains the lack of pathogenicity of primate foamy virus infections. J. Virol. 2008;82:5981–5985. doi: 10.1128/JVI.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S.M., Picker L.J., Axthelm M.K., Linial M.L. Expanded tissue targets for foamy virus replication with simian immunodeficiency virus-induced immunosuppression. J. Virol. 2006;80:663–670. doi: 10.1128/JVI.80.2.663-670.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutombo W.M., Arita I., Jezek Z. Human monkey pox transmitted by a chimpanzee in a tropical rainforest area of Zaire. Lancet. 1983;1:735–737. doi: 10.1016/S0140-6736(83)92027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M.G., Kramer L.W., Stanberry L.R. Varicella in a gorilla. J. Med. Virol. 1987;23:317–322. doi: 10.1002/jmv.1890230403. [DOI] [PubMed] [Google Scholar]
- Nahmias A.J., London W.T., Catalano L.W., Fuccillo D.A., Sever J.L., Graham C. Genital herpesvirus hominis type 2 infection: an experimental model in cebus monkeys. Science. 1971;171:297–298. doi: 10.1126/science.171.3968.297. [DOI] [PubMed] [Google Scholar]
- Nandi J.S., Bhavalkar-Potdar V., Tikute S., Raut C.G. A novel type D simian retrovirus naturally infecting the Indian Hanuman langur (Semnopithecus entellus) Virology. 2000;277:6–13. doi: 10.1006/viro.2000.0567. [DOI] [PubMed] [Google Scholar]
- Nandi J.S., Van Dooren S., Chhangani A.K., Mohnot S.M. New simian beta retroviruses from rhesus monkeys (Macaca mulatta) and langurs (Semnopithecus entellus) from Rajasthan, India. Virus Genes. 2006;33:107–116. doi: 10.1007/s11262-005-0032-x. [DOI] [PubMed] [Google Scholar]
- Neuman-Haefelin D., Rethwilm A., Bauer G., Gudat F., Hausen H. Characterization of a foamy virus isolated from Cercopithecus aethiops lymphoblastoid cells. Med. Microbiol. Immunol. 1983;172:75–86. doi: 10.1007/BF02124508. [DOI] [PubMed] [Google Scholar]
- Niedobitek G., Agathanggelou A., Finerty S., Tierney R., Watkins P., Jones E.L., Morgan A., Young L.S., Rooney N. Latent Epstein-Barr virus infection in cottontop tamarins. A possible model for Epstein-Barr virus infection in humans. Am. J. Pathol. 1994;145:969–978. [PMC free article] [PubMed] [Google Scholar]
- Nieves P., Rodriguez J.F., Kessler M.J., Bercovitch F. Subcutaneous rabies vaccination of pigtail macaques. J. Med. Primatol. 1996;25:14–16. doi: 10.1111/j.1600-0684.1996.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Nigida S.M., Falk L.A., Wolfe L.G., Deinhardt F. Isolation of a cytomegalovirus from salivary glands of white-lipped marmosets (Saguinus fuscicollis) Lab. Anim. Sci. 1979;29:53–60. [PubMed] [Google Scholar]
- Nix W.A., Jiang B., Maher K., Strobert E., Oberste M.S. Identification of enteroviruses in naturally infected captive primates. J. Clin. Microbiol. 2008;46:2874–2878. doi: 10.1128/JCM.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppornpanth S., Haagmans B.L., Bhattarakosol P., Ratanakorn P., Niesters H.G., Osterhaus A.D., Poovorawan Y. Molecular epidemiology of gibbon hepatitis B virus transmission. J. Gen. Virol. 2003;84:147–155. doi: 10.1099/vir.0.18531-0. [DOI] [PubMed] [Google Scholar]
- Norder H., Ebert J.W., Fields H.A., Mushahwar I.K., Magnius L.O. Complete sequencing of a gibbon hepatitis B virus genome reveals a unique genotype distantly related to the chimpanzee hepatitis B virus. Virology. 1996;218:214–223. doi: 10.1006/viro.1996.0181. [DOI] [PubMed] [Google Scholar]
- North T.W., Sequar G., Townsend L.B., Drach J.C., Barry P.A. Rhesus cytomegalovirus is similar to human cytomegalovirus in susceptibility to benzimidazole nucleosides. Antimicrob. Agents Chemother. 2004;48:2760–2765. doi: 10.1128/AAC.48.7.2760-2765.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M.G., Anderson D.C., Fikes J.D., Bain F.T., Carlson C.S., Green S.W., Young N.S., Brown K.E. Identification of a novel simian parvovirus in cynomolgus monkeys with severe anemia. A paradigm of human B19 parvovirus infection. J. Clin. Invest. 1994;93:1571–1576. doi: 10.1172/JCI117136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M.G., Anderson D.K., Goodrich J.A., Tulli H., Green S.W., Young N.S., Brown K.E. Experimental infection of cynomolgus monkeys with simian parvovirus. J. Virol. 1997;71:4517–4521. doi: 10.1128/jvi.71.6.4517-4521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M.S., Maher K., Pallansch M.A. Genomic evidence that simian virus 2 and six other simian picornaviruses represent a new genus in Picornaviridae. Virology. 2003;314:283–293. doi: 10.1016/s0042-6822(03)00420-3. [DOI] [PubMed] [Google Scholar]
- Oberste M.S., Maher K., Pallansch M.A. Complete genome sequences for nine simian enteroviruses. J. Gen. Virol. 2007;88:3360–3372. doi: 10.1099/vir.0.83124-0. [DOI] [PubMed] [Google Scholar]
- Ohsawa K., Black D.H., Sato H., Eberle R. Sequence and genetic arrangement of the U(S) region of the monkey B virus (cercopithecine herpesvirus 1) genome and comparison with the U(S) regions of other primate herpesviruses. J. Virol. 2002;76:1516–1520. doi: 10.1128/JVI.76.3.1516-1520.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K., Black D.H., Sato H., Rogers K., Eberle R. Sequence and genetic arrangement of the UL region of the monkey B virus (Cercopithecine herpesvirus 1) genome and comparison with the UL region of other primate herpesviruses. Arch. Virol. 2003;148:989–997. doi: 10.1007/s00705-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Ohsawa K., Black D.H., Torii R., Sato H., Eberle R. Detection of a unique genotype of monkey B virus (Cercopithecine herpesvirus 1) indigenous to native Japanese macaques (Macaca fuscata) Comp. Med. 2002;52:555–559. [PubMed] [Google Scholar]
- Ohsawa K., Lehenbauer T.W., Eberle R. Herpesvirus papio 2: alternative antigen for use in monkey B virus diagnostic assays. Lab. Anim. Sci. 1999;49:605–616. [PubMed] [Google Scholar]
- Ohta Y., Masuda T., Tsujimoto H., Ishikawa K., Kodoma T., Morikawa S., Nakai M., Honjo S., Hayami M. Isolation of simian immunodeficiency virus from African green monkeys and seroepidemiologic survey of the virus from various non-human primates. Int. J. Cancer. 1987;41:115–122. doi: 10.1002/ijc.2910410121. [DOI] [PubMed] [Google Scholar]
- Olberg R.A., Barker I.K., Crawshaw G.J., Bertelsen M.F., Drebot M.A., Andonova M. West Nile virus encephalitis in a Barbary macaque (Macaca sylvanus) Emerg. Infect. Dis. 2004;10:712–714. doi: 10.3201/eid1004.030675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L.C., Pryor W.H., Jr., Thomas J.M. Persistent reduction of B virus (Herpesvirus simiae) seropositivity in rhesus macaques acquired for a study of renal allograft tolerance. Lab. Anim. Sci. 1991;41:540–544. [PubMed] [Google Scholar]
- Onlamoon N., Noisakran S., Hsiao H.M., Duncan A., Villinger F., Ansari A.A., Perng G.C. Dengue virus-induced hemorrhage in a nonhuman primate model. Blood. 2010;115:1823–1834. doi: 10.1182/blood-2009-09-242990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orandle M.S., Veazey R.S., Lackner A.A. Enteric ganglionitis in rhesus macaques infected with simian immunodeficiency virus. J. Virol. 2007;81:6265–6275. doi: 10.1128/JVI.02671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordy J.M., Rangan S.R., Wolf R.H., Knight C., Dunlap W.P. Congenital cytomegalovirus effects on postnatal neurological development of squirrel monkey (Saimiri sciureus) offspring. Exp. Neurol. 1981;74:728–747. doi: 10.1016/0014-4886(81)90247-8. [DOI] [PubMed] [Google Scholar]
- Orzechowska B.U., Powers M.F., Sprague J., Li H., Yen B., Searles R.P., Axthelm M.K., Wong S.W. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: animal model for KSHV-associated malignancies. Blood. 2008;112:4227–4234. doi: 10.1182/blood-2008-04-151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn K.G., Prahalada S., Lowenstine L.J., Gardner M.B., Maul D.H., Henrickson R.V. The pathology of an epizootic of acquired immunodeficiency in rhesus macaques. Am. J. Pathol. 1984;114:94–103. [PMC free article] [PubMed] [Google Scholar]
- Ostrow R.S., LaBresh K.V., Faras A.J. Characterization of the complete RhPV 1 genomic sequence and an integration locus from a metastatic tumor. Virology. 1991;181:424–429. doi: 10.1016/0042-6822(91)90519-h. [DOI] [PubMed] [Google Scholar]
- Ostrow R.S., McGlennen R.C., Shaver M.K., Kloster B.E., Houser D., Faras A.J. A rhesus monkey model for sexual transmission of a papillomavirus isolated from a squamous cell carcinoma. Proc. Natl. Acad. Sci. U S A. 1990;87:8170–8174. doi: 10.1073/pnas.87.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting N., Heijmans C.M., Noort R.C., de Groot N.G., Doxiadis G.G., van Rood J.J., Watkins D.I., Bontrop R.E. Unparalleled complexity of the MHC class I region in rhesus macaques. Proc. Natl. Acad. Sci. U S A. 2005;102:1626–1631. doi: 10.1073/pnas.0409084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y., Davis K.A., Traina-Dorge V., Gray W.L. Simian varicella virus expresses a latency-associated transcript that is antisense to open reading frame 61 (ICP0) mRNA in neural ganglia of latently infected monkeys. J. Virol. 2007;81:8149–8156. doi: 10.1128/JVI.00407-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya C., Ochiai Y., Taniuchi Y., Takano T., Ueda F., Yoshikawa Y., Hondo R. Specific detection and identification of herpes B virus by a PCR-microplate hybridization assay. J. Clin. Microbiol. 2004;42:1869–1874. doi: 10.1128/JCM.42.5.1869-1874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiardini M., Pandrea I., Apetrei C., Silvestri G. Lessons learned from the natural hosts of HIV-related viruses. Annu. Rev. Med. 2009;60:485–495. doi: 10.1146/annurev.med.60.041807.123753. [DOI] [PubMed] [Google Scholar]
- Palmer A.E., Allen A.M., Tauraso N.M., Shelokov A. Simian hemorrhagic fever. I. Clinical and epizootiologic aspects of an outbreak among quarantined monkeys. Am. J. Trop. Med. Hyg. 1968;17:404–412. [PubMed] [Google Scholar]
- Pandrea I., Apetrei C., Dufour J., Dillon N., Barbercheck J., Metzger M., Jacquelin B., Bohm R., Marx P.A., Barre-Sinoussi F., Hirsch V.M., Muller-Trutwin M.C., Lackner A.A., Veazey R.S. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J. Virol. 2006;80:4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I., Silvestri G., Apetrei C. AIDS in african nonhuman primate hosts of SIVs: a new paradigm of SIV infection. Curr. HIV Res. 2009;7:57–72. doi: 10.2174/157016209787048456. [DOI] [PubMed] [Google Scholar]
- Pandrea I., Sodora D.L., Silvestri G., Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I.V., Gautam R., Ribeiro R.M., Brenchley J.M., Butler I.F., Pattison M., Rasmussen T., Marx P.A., Silvestri G., Lackner A.A., Perelson A.S., Douek D.C., Veazey R.S., Apetrei C. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W.P., Melnick J.L. Attempted isolation of hepatitis viruses in marmosets. J. Infect. Dis. 1969;120:539–547. doi: 10.1093/infdis/120.5.539. [DOI] [PubMed] [Google Scholar]
- Pattison J.R. A new parvovirus: similarities between monkeys and humans. J. Clin. Invest. 1994;93:1354. doi: 10.1172/JCI117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton M.E., d’Offay J.M., Prado M.E., Black D.H., Damania B., White G.L., Eberle R. Comparative transmission of multiple herpesviruses and simian virus 40 in a baboon breeding colony. Comp. Med. 2004;54:695–704. [PubMed] [Google Scholar]
- Peeters M., Honore C., Huet T., Bedjabaga L., Ossari S., Bussi P., Cooper R.W., Delaporte E. Isolation and partial characterization of an HIV-related virus occurring naturally in chimpanzees in Gabon. AIDS. 1989;3:625–630. doi: 10.1097/00002030-198910000-00001. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- Peiris J.S.M., Dittus W.P.J., Ratnayake C.B. Seroepidemiology of dengue and other arboviruses in a natural population of toque macaques (Macaca sinica) at Polonnaruwa Sri Lanka. J. Med. Primatol. 1993;22:240–245. [PubMed] [Google Scholar]
- Perelygina L., Patrusheva I., Hombaiah S., Zurkuhlen H., Wildes M.J., Patrushev N., Hilliard J. Production of herpes B virus recombinant glycoproteins and evaluation of their diagnostic potential. J. Clin. Microbiol. 2005;43:620–628. doi: 10.1128/JCM.43.2.620-628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelygina L., Patrusheva I., Manes N., Wildes M.J., Krug P., Hilliard J.K. Quantitative real-time PCR for detection of monkey B virus (Cercopithecine herpesvirus 1) in clinical samples. J. Virol. Methods. 2003;109:245–251. doi: 10.1016/s0166-0934(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Perelygina L., Zhu L., Zurkuhlen H., Mills R., Borodovsky M., Hilliard J.K. Complete sequence and comparative analysis of the genome of herpes B virus (Cercopithecine herpesvirus 1) from a rhesus monkey. J. Virol. 2003;77:6167–6177. doi: 10.1128/JVI.77.11.6167-6177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelygina L., Zurkuhlen H., Patrusheva I., Hilliard J.K. Identification of a herpes B virus-specific glycoprotein d immunodominant epitope recognized by natural and foreign hosts. J. Infect. Dis. 2002;186:453–461. doi: 10.1086/341834. [DOI] [PubMed] [Google Scholar]
- Peters J.C. An epizootic of monkey-pox at Rotterdam Zoo. Int. Zool. Yearb. 1966;6:274–275. [Google Scholar]
- Phillips-Conroy J.E., Jolly C.J., Petros B., Allan J.S., Desrosiers R.C. Sexual transmission of SIVagm in wild grivet monkeys. J. Med. Primatol. 1994;23:1–7. doi: 10.1111/j.1600-0684.1994.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Pinto M.A., Marchevsky R.S., Pelajo-Machado M., Santiago M.A., Pissurno J.W., Franca M.S., Baptista M.L., Gouvea A.S., Santana A.A., Bertho A.L., Schatzmayr H.G., Gaspar A.M., Kubelka C.F. Inducible nitric oxide synthase (iNOS) expression in liver and splenic T lymphocyte rise are associated with liver histological damage during experimental hepatitis A virus (HAV) infection in Callithrix jacchus. Exp. Toxicol. Pathol. 2000;52:3–10. doi: 10.1016/S0940-2993(00)80006-8. [DOI] [PubMed] [Google Scholar]
- Pogodina V.V., Frolova M.P., Malenko G.V., Fokina G.I., Koreshkova G.V., Kiseleva L.L., Bochkova N.G., Ralph N.M. Study on West Nile virus persistence in monkeys. Arch. Virol. 1983;75:71–86. doi: 10.1007/BF01314128. [DOI] [PubMed] [Google Scholar]
- Potkay S. Diseases of the Callitrichidae: a review. J. Med. Primatol. 1992;21:189–236. [PubMed] [Google Scholar]
- Powers C.J., Fruh K. Signal peptide-dependent inhibition of MHC class I heavy chain translation by rhesus cytomegalovirus. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000150. e1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prier J.E., Sauer R.M., Malsberger R.G., Sillaman J.M. Studies on a pox disease of monkeys. II. Isolation of the etiologic agent. Am. J. Vet. Res. 1960;21:381–384. [PubMed] [Google Scholar]
- Pumphrey C.Y., Gray W.L. Identification and analysis of the simian varicella virus thymidine kinase gene. Arch. Virol. 1996;141:43–55. doi: 10.1007/BF01718587. [DOI] [PubMed] [Google Scholar]
- Rabin H., Strnad B.C., Neubauer R.H., Brown A.M., Hopkins R.F., 3rd, Mazur R.A. Comparisons of nuclear antigens of Epstein-Barr virus (EBV) and EBV-like simian viruses. J. Gen. Virol. 1980;48:265–272. doi: 10.1099/0022-1317-48-2-265. [DOI] [PubMed] [Google Scholar]
- Raine C.S., Feldman L.A., Sheppard R.D., Bornstein M.B. Ultrastructure of measles virus in cultures of hamster cerebellum. J. Virol. 1969;4:169–181. doi: 10.1128/jvi.4.2.169-181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer J.C., Garber R.L., Steele K.E., Boyson J.F., O’Rourke C., Thomson J.A. Fatal lymphoproliferative disease associated with a novel gammaherpesvirus in a captive population of common marmosets. Comp. Med. 2000;50:59–68. [PubMed] [Google Scholar]
- Rangan S.R., Chaiban J. Isolation and characterization of a cytomegalovirus from the salivary gland of a squirrel monkey (Saimiri sciureus) Lab. Anim. Sci. 1980;30:532–540. [PubMed] [Google Scholar]
- Ratterree M.S., da Rosa A.P., Bohm R.P., Jr., Cogswell F.B., Phillippi K.M., Caillouet K., Schwanberger S., Shope R.E., Tesh R.B. West Nile virus infection in nonhuman primate breeding colony, concurrent with human epidemic, southern Louisiana. Emerg. Infect. Dis. 2003;9:1388–1394. doi: 10.3201/eid0911.030226. [DOI] [PubMed] [Google Scholar]
- Ratterree M.S., Gutierrez R.A., Travassos da Rosa A.P., Dille B.J., Beasley D.W., Bohm R.P., Desai S.M., Didier P.J., Bikenmeyer L.G., Dawson G.J., Leary T.P., Schochetman G., Phillippi-Falkenstein K., Arroyo J., Barrett A.D., Tesh R.B. Experimental infection of rhesus macaques with West Nile virus: level and duration of viremia and kinetics of the antibody response after infection. J. Infect. Dis. 2004;189:669–676. doi: 10.1086/381461. [DOI] [PubMed] [Google Scholar]
- Raviprakash K., Wang D., Ewing D., Holman D.H., Block K., Woraratanadharm J., Chen L., Hayes C., Dong J.Y., Porter K. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J. Virol. 2008;82:6927–6934. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddacliff L.A., Kirkland P.D., Hartley W.J., Reece R.L. Encephalomyocarditis virus infections in an Australian zoo. J. Zoo. Wildl. Med. 1997;28:153–157. [PubMed] [Google Scholar]
- Reed D.S., Hensley L.E., Geisbert J.B., Jahrling P.B., Geisbert T.W. Depletion of peripheral blood T lymphocytes and NK cells during the course of ebola hemorrhagic Fever in cynomolgus macaques. Viral Immunol. 2004;17:390–400. doi: 10.1089/vim.2004.17.390. [DOI] [PubMed] [Google Scholar]
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., Swain G.R., Olson V.A., Sargent E.K., Kehl S.C., Frace M.A., Kline R., Foldy S.L., Davis J.P., Damon I.K. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Reimann K.A., Parker R.A., Seaman M.S., Beaudry K., Beddall M., Peterson L., Williams K.C., Veazey R.S., Montefiori D.C., Mascola J.R., Nabel G.J., Letvin N.L. Pathogenicity of simian-human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgus macaques and associated with early T-lymphocyte responses. J. Virol. 2005;79:8878–8885. doi: 10.1128/JVI.79.14.8878-8885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renegar K.B. Influenza virus infections and immunity: a review of human and animal models. Lab. Anim. Sci. 1992;42:222–232. [PubMed] [Google Scholar]
- Renne R.A., McLaughlin R., Jenson A.B. Measles virus-associated endometritis, cervicitis, and abortion in a rhesus monkey. J. Am. Vet. Med. Assoc. 1973;163:639–641. [PubMed] [Google Scholar]
- Renquist D. Outbreak of simian hemorrhagic fever. J. Med. Primatol. 1990;19:77–79. [PubMed] [Google Scholar]
- Rey-Cuille M.A., Berthier J.L., Bomsel-Demontoy M.C., Chaduc Y., Montagnier L., Hovanessian A.G., Chakrabarti L.A. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J. Virol. 1998;72:3872–3886. doi: 10.1128/jvi.72.5.3872-3886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezikyan S., Kaaya E.E., Ekman M., Voevodin A.F., Feichtinger H., Putkonen P., Castanos-Velez E., Biberfeld G., Biberfeld P. B-cell lymphomagenesis in SIV-immunosuppressed cynomolgus monkeys. Int. J. Cancer. 1995;61:574–579. doi: 10.1002/ijc.2910610423. [DOI] [PubMed] [Google Scholar]
- Richardson-Wyatt L.S., Belshe R.B., London W.T., Sly D.L., Camargo E., Chanock R.M. Respiratory syncytial virus antibodies in nonhuman primates and domestic animals. Lab. Anim. Sci. 1981;31:413–415. [PubMed] [Google Scholar]
- Richardson J.H., Humphrey G.L. Rabies in imported nonhuman primates. Lab. Anim. Sci. 1971;21:1083. [PubMed] [Google Scholar]
- Rifakis P.M., Benitez J.A., De-la-Paz-Pineda J., Rodriguez-Morales A.J. Epizootics of yellow fever in Venezuela (2004-2005): an emerging zoonotic disease. Ann. N Y Acad. Sci. 2006;1081:57–60. doi: 10.1196/annals.1373.005. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., Kuiken T., van Amerongen G., Bestebroer T.M., Fouchier R.A., Osterhaus A.D. A primate model to study the pathogenesis of influenza A (H5N1) virus infection. Avian Dis. 2003;47:931–933. doi: 10.1637/0005-2086-47.s3.931. [DOI] [PubMed] [Google Scholar]
- Rinehart-Kim J.E., Zhong W.M., Jiang X., Smith A.W., Matson D.O. Complete nucleotide sequence and genomic organization of a primate calicivirus, Pan-1. Arch. Virol. 1999;144:199–208. doi: 10.1007/s007050050497. [DOI] [PubMed] [Google Scholar]
- Ringler D.J., Hancock W.W., King N.W., Letvin N.L., Daniel M.D., Desrosiers R.C., Murphy G.F. Immunophenotypic characterization of the cutaneous exanthem of SIV-infected rhesus monkeys. Apposition of degenerative Langerhans cells and cytotoxic lymphocytes during the development of acquired immunodeficiency syndrome. Am. J. Pathol. 1987;126:199–207. [PMC free article] [PubMed] [Google Scholar]
- Ritchey J.W., Ealey K.A., Payton M.E., Eberle R. Comparative pathology of infections with baboon and African green monkey alpha-herpesviruses in mice. J. Comp. Pathol. 2002;127:150–161. doi: 10.1053/jcpa.2002.0575. [DOI] [PubMed] [Google Scholar]
- Ritchey J.W., Payton M.E., Eberle R. Clinicopathological characterization of monkey B virus (Cercopithecine herpesvirus 1) infection in mice. J. Comp. Pathol. 2005;132:202–217. doi: 10.1016/j.jcpa.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Rivadeneira E.D., Ferrari M.G., Jarrett R.F., Armstrong A.A., Markham P., Birkebak T., Takemoto S., Johnson-Delaney C., Pecon-Slattery J., Clark E.A., Franchini G. A novel Epstein-Barr virus-like virus, HV(MNE), in a Macaca nemestrina with mycosis fungoides. Blood. 1999;94:2090–2101. [PubMed] [Google Scholar]
- Rivailler P., Carville A., Kaur A., Rao P., Quink C., Kutok J.L., Westmoreland S., Klumpp S., Simon M., Aster J.C., Wang F. Experimental rhesus lymphocryptovirus infection in immunosuppressed macaques: an animal model for Epstein-Barr virus pathogenesis in the immunosuppressed host. Blood. 2004;104:1482–1489. doi: 10.1182/blood-2004-01-0342. [DOI] [PubMed] [Google Scholar]
- Rivailler P., Cho Y.G., Wang F. Complete genomic sequence of an Epstein-Barr virus-related herpesvirus naturally infecting a new world primate: a defining point in the evolution of oncogenic lymphocryptoviruses. J. Virol. 2002;76:12055–12068. doi: 10.1128/JVI.76.23.12055-12068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivailler P., Jiang H., Cho Y.G., Quink C., Wang F. Complete nucleotide sequence of the rhesus lymphocryptovirus: genetic validation for an Epstein-Barr virus animal model. J. Virol. 2002;76:421–426. doi: 10.1128/JVI.76.1.421-426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E.D., Baskin G.B., Soike K., Gibson S.V. Pathologic changes of experimental simian varicella (Delta herpesvirus) infection in African green monkeys (Cercopithecus aethiops) Am. J. Vet. Res. 1984;45:523–530. [PubMed] [Google Scholar]
- Roberts J.A., Lerche N.W., Markovits J.E., Maul D.H. Epizootic measles at the CRPRC. Lab. Anim. Sci. 1988;38:492. (abstr.) [Google Scholar]
- Robertson B.H., Margolis H.S. Primate hepatitis B viruses – genetic diversity, geography and evolution. Rev. Med. Virol. 2002;12:133–141. doi: 10.1002/rmv.348. [DOI] [PubMed] [Google Scholar]
- Rockx B.H., Bogers W.M., Heeney J.L., van Amerongen G., Koopmans M.P. Experimental norovirus infections in non-human primates. J. Med. Virol. 2005;75:313–320. doi: 10.1002/jmv.20273. [DOI] [PubMed] [Google Scholar]
- Rodhain F. The role of monkeys in the biology of dengue and yellow fever. Comp. Immunol. Microbiol. Infect. Dis. 1991;14:9–19. doi: 10.1016/0147-9571(91)90036-d. [DOI] [PubMed] [Google Scholar]
- Rogers K.M., Ealey K.A., Ritchey J.W., Black D.H., Eberle R. Pathogenicity of different baboon herpesvirus papio 2 isolates is characterized by either extreme neurovirulence or complete apathogenicity. J. Virol. 2003;77:10731–10739. doi: 10.1128/JVI.77.20.10731-10739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K.M., Ritchey J.W., Payton M., Black D.H., Eberle R. Neuropathogenesis of herpesvirus papio 2 in mice parallels infection with Cercopithecine herpesvirus 1 (B virus) in humans. J. Gen. Virol. 2006;87:267–276. doi: 10.1099/vir.0.81476-0. [DOI] [PubMed] [Google Scholar]
- Rogers K.M., Wolf R.F., White G.L., Eberle R. Experimental infection of baboons (Papio cynocephalus anubis) with apathogenic and neurovirulent subtypes of herpesvirus papio 2. Comp. Med. 2005;55:425–430. [PubMed] [Google Scholar]
- Rose T.M., Ryan J.T., Schultz E.R., Raden B.W., Tsai C.C. Analysis of 4.3 kilobases of divergent locus B of macaque retroperitoneal fibromatosis-associated herpesvirus reveals a close similarity in gene sequence and genome organization to Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2003;77:5084–5097. doi: 10.1128/JVI.77.9.5084-5097.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T.M., Strand K.B., Schultz E.R., Schaefer G., Rankin G.W., Jr., Thouless M.E., Tsai C.C., Bosch M.L. Identification of two homologs of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J. Virol. 1997;71:4138–4144. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R.W. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J. Hyg. (Lond.) 1956;54:177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Gao G., Clawson D.S., Vandenberghe L.H., Farina S.F., Wilson J.M. Complete nucleotide sequences and genome organization of four chimpanzee adenoviruses. Virology. 2004;324:361–372. doi: 10.1016/j.virol.2004.03.047. [DOI] [PubMed] [Google Scholar]
- Roy S., Vandenberghe L.H., Kryazhimskiy S., Grant R., Calcedo R., Yuan X., Keough M., Sandhu A., Wang Q., Medina-Jaszek C.A., Plotkin J.B., Wilson J.M. Isolation and characterization of adenoviruses persistently shed from the gastrointestinal tract of non-human primates. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000503. e1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Zhi Y., Kobinger G.P., Figueredo J., Calcedo R., Miller J.R., Feldmann H., Wilson J.M. Generation of an adenoviral vaccine vector based on simian adenovirus 21. J. Gen. Virol. 2006;87:2477–2485. doi: 10.1099/vir.0.81989-0. [DOI] [PubMed] [Google Scholar]
- Rudnik A. Studies of the ecology of Dengue in Malaysia. A preliminary report. J. Med. Entomol. 1965;2:203–208. doi: 10.1093/jmedent/2.2.203. [DOI] [PubMed] [Google Scholar]
- Russell R.G., Brian D.A., Lenhard A., Potgieter L.N., Gillespie D., Clapp N.K. Coronavirus-like particles and Campylobacter in marmosets with diarrhea and colitis. Dig. Dis. Sci. 1985;30:72S–77S. doi: 10.1007/BF01296981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustigian R., Johnston P., Reihart H. Infection of monkey kidney tissue cultures with virus-like agents. Proc. Soc. Exp. Biol. Med. 1955;88:8–16. doi: 10.3181/00379727-88-21478. [DOI] [PubMed] [Google Scholar]
- Sa-nguanmoo P., Thongmee C., Ratanakorn P., Pattanarangsan R., Boonyarittichaikij R., Chodapisitkul S., Theamboonlers A., Tangkijvanich P., Poovorawan Y. Prevalence, whole genome characterization and phylogenetic analysis of hepatitis B virus in captive orangutan and gibbon. J. Med. Primatol. 2008;37:277–289. doi: 10.1111/j.1600-0684.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- Sa L.R., DiLoreto C., Leite M.C., Wakamatsu A., Santos R.T., Catao-Dias J.L. Oral focal epithelial hyperplasia in a howler monkey (Alouatta fusca) Vet. Pathol. 2000;37:492–496. doi: 10.1354/vp.37-5-492. [DOI] [PubMed] [Google Scholar]
- Sabin A.B. Oral poliovirus vaccine: History of its development and use and current challenge to eliminate poliomyelitis from the world. J. Infect. Dis. 1985;151:420–436. doi: 10.1093/infdis/151.3.420. [DOI] [PubMed] [Google Scholar]
- Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H., Iizuka I., Shiota T., Sakai K., Ogata M., Fukushi S., Mizutani T., Sata T., Kurata T., Kurane I., Morikawa S. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J. Gen. Virol. 2009;90:2266–2271. doi: 10.1099/vir.0.010207-0. [DOI] [PubMed] [Google Scholar]
- Sakakibara L., Sugimoto Y., Sasawa A., Honjo S., Tsujimoto H., Nakamura H., Hayami M. Spontaneous malignant lymphoma in an African green monkey naturally infected with STLV-1. J. Med. Primatol. 1986;15:311–318. [PubMed] [Google Scholar]
- Sakesena N.K., Herve V.M., Durand J.P., Leguenno B., Diop O.M., Digoutte J.P., Mathiot C., Muller M.C., Love J.L., Dube S., Sherman M.P., Benz P.M., Erensoy S., Galat-Luong A., Galat G., Paul B., Dube D.K., Barre-Sinoussi F., Poiesz B.J. Seroepidemiologic, molecular and phylogenetic analyses of STLV-1 from various naturally infected monkey species from central and western Africa. J. Virol. 1994;198:297–310. doi: 10.1006/viro.1994.1033. [DOI] [PubMed] [Google Scholar]
- Sall A.A., Starkman S., Reynes J.M., Lay S., Nhim T., Hunt M., Marx N., Simmonds P. Frequent infection of Hylobates pileatus (pileated gibbon) with species-associated variants of hepatitis B virus in Cambodia. J. Gen. Virol. 2005;86:333–337. doi: 10.1099/vir.0.80274-0. [DOI] [PubMed] [Google Scholar]
- Sallis E.S., de Barros V.L., Garmatz S.L., Fighera R.A., Graca D.L. A case of yellow fever in a brown howler (Alouatta fusca) in Southern Brazil. J. Vet. Diagn. Invest. 2003;15:574–576. doi: 10.1177/104063870301500611. [DOI] [PubMed] [Google Scholar]
- Santiago M.L., Range F., Keele B.F., Li Y., Bailes E., Bibollet-Ruche F., Fruteau C., Noe R., Peeters M., Brookfield J.F., Shaw G.M., Sharp P.M., Hahn B.H. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d’Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J. Virol. 2005;79:12515–12527. doi: 10.1128/JVI.79.19.12515-12527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S., Seaman M.S., Xu L., Barouch D.H., Lord C.I., Lifton M.A., Gorgone D.A., Beaudry K.R., Svehla K., Welcher B., Chakrabarti B.K., Huang Y., Yang Z.Y., Mascola J.R., Nabel G.J., Letvin N.L. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 2005;79:6516–6522. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariol C.A., Gonzalez-Martinez J., Arana T., Gascot S., Suarez E., Maldonado E., Gerald M.S., Rodriguez M., Kraiselburd E.N. Differential distribution of antibodies to different viruses in young animals in the free-ranging rhesus macaques of Cayo Santiago. J. Med. Primatol. 2006;35:369–375. doi: 10.1111/j.1600-0684.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Sasseville V.G., Newman W., Brodie S.J., Hesterberg P., Pauley D., Ringler D.J. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am. J. Pathol. 1994;144:27–40. [PMC free article] [PubMed] [Google Scholar]
- Sasseville V.G., Newman W.A., Lackner A.A., Smith M.O., Lausen N.C., Beall D., Ringler D.J. Elevated vascular cell adhesion molecule-1 in AIDS encephalitis induced by simian immunodeficiency virus. Am. J. Pathol. 1992;141:1021–1030. [PMC free article] [PubMed] [Google Scholar]
- Sauer R.M., Prier J.E., Buchanan R.S., Creamer A.A., Fegley H.C. Studies on a pox disease of monkeys. I. Pathology. Am. J. Vet. Res. 1960;21:377–380. [PubMed] [Google Scholar]
- Sauermann U. Making the animal model for AIDS research more precise: the impact of major histocompatibility complex (MHC) genes on pathogenesis and disease progression in SIV-infected monkeys. Curr. Mol. Med. 2001;1:515–522. doi: 10.2174/1566524013363555. [DOI] [PubMed] [Google Scholar]
- Scharf B.A., Wan C.H., Bluth M., Eberle R., Videan E.N., Smith E., Coplan J. Lethargy, ulcers, bronchopneumonia and death in two aged female bonnet macaques presumed to be caused by Cercopithicine herpes virus I. J. Med. Primatol. 2008;37(Suppl. 1):60–64. doi: 10.1111/j.1600-0684.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- Schiavetta A.M., Harre J.G., Wagner E., Simmons M., Raviprakash K. Variable susceptibility of the owl monkey (Aotus nancymae) to four serotypes of dengue virus. Contemp. Top. Lab. Anim. Sci. 2003;42:12–20. [PubMed] [Google Scholar]
- Schielke J.E., Kalishman J., Liggitt D., Bielefeldt-Ohmann H. What is your diagnosis?: Multifocal subcutaneous tumors in a young male baboon. Contemp. Top. Lab. Anim. Sci. 2002;41:27–29. [PubMed] [Google Scholar]
- Schlauder G.G., Dawson G.J., Simons J.N. Molecular and serologic analysis in the transmission of the GB hepatitis agents. J. Med. Virol. 1995;46:81–90. doi: 10.1002/jmv.1890460117. [DOI] [PubMed] [Google Scholar]
- Schlauder G.G., Pilot-Matais T.J., Gretchen S.G., Simons J.N., Muerhoff a. S., Dawson G.J. Origin of GB-hepatitis viruses. Lancet. 1995;346:447–448. doi: 10.1016/s0140-6736(95)92821-9. [DOI] [PubMed] [Google Scholar]
- Schmidtko J., Wang R., Wu C.L., Mauiyyedi S., Harris N.L., Della Pelle P., Brousaides N., Zagachin L., Ferry J.A., Wang F., Kawai T., Sachs D.H., Cosimi B.A., Colvin R.B. Posttransplant lymphoproliferative disorder associated with an Epstein-Barr-related virus in cynomolgus monkeys. Transplantation. 2002;73:1431–1439. doi: 10.1097/00007890-200205150-00012. [DOI] [PubMed] [Google Scholar]
- Schoeb T.R., Eberle R., Black D.H., Parker R.F., Cartner S.C. Diagnostic exercise: papulovesicular dermatitis in rhesus macaques (Macaca mulatta) Vet. Pathol. 2008;45:592–594. doi: 10.1354/vp.45-4-592. [DOI] [PubMed] [Google Scholar]
- Schrenzel M.D., Osborn K.G., Shima A., Klieforth R.B., Maalouf G.A. Naturally occurring fatal herpes simplex virus 1 infection in a family of white-faced saki monkeys (Pithecia pithecia pithecia) J. Med. Primatol. 2003;32:7–14. doi: 10.1034/j.1600-0684.2003.01040.x. [DOI] [PubMed] [Google Scholar]
- Schroder C., Pfeiffer S., Wu G., Azimzadeh A.M., Aber A., Pierson R.N., 3rd, O’Sullivan M.G. Simian parvovirus infection in cynomolgus monkey heart transplant recipients causes death related to severe anemia. Transplantation. 2006;81:1165–1170. doi: 10.1097/01.tp.0000203170.77195.e4. [DOI] [PubMed] [Google Scholar]
- Schroder M.A., Fisk S.K., Lerche N.W. Eradication of simian retrovirus type D from a colony of cynomolgus, rhesus, and stump-tailed macaques by using serial testing and removal. Contemp. Top. Lab. Anim. Sci. 2000;39:16–23. [PubMed] [Google Scholar]
- Schultz E.R., Rankin G.W., Jr., Blanc M.P., Raden B.W., Tsai C.C., Rose T.M. Characterization of two divergent lineages of macaque rhadinoviruses related to Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2000;74:4919–4928. doi: 10.1128/jvi.74.10.4919-4928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G.B.D., Keymer I.F. The pathology of measles in Abyssinian colobus monkeys (Colobus guereza): A description of an outbreak. J. Pathol. 1975;117:229–233. doi: 10.1002/path.1711170405. [DOI] [PubMed] [Google Scholar]
- Searles R.P., Bergquam E.P., Axthelm M.K., Wong S.W. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 1999;73:3040–3053. doi: 10.1128/jvi.73.4.3040-3053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour C., Peralta P.H., Montgomery G.G. Serologic evidence of natural togavirus infections in Panamanian sloths and other vertebrates. Am. J. Trop. Med. Hyg. 1983;32:854–861. doi: 10.4269/ajtmh.1983.32.854. [DOI] [PubMed] [Google Scholar]
- Shah K.V. Polyomaviruses. In: Fields B.N., Knipe D.M., editors. Virology. Raven Press; New York: 1990. pp. 1609–1623. [Google Scholar]
- Shevstsova Z.V., Lapin B., Doroshenko N.V., Krilova R.I., Korzaja L.I., Lomovskaya I.B., Dzhelieva Z.N., Zairov G.G., Stahkanova V.M., Belova E.G., Sazhchenko L.A. Spontaneous and experimental hepatitis A in old world monkeys. J. Med. Primatol. 1988;17:177–194. [PubMed] [Google Scholar]
- Shiigi S., Wilson B., Leo G., MacDonald N., Toyooka D., Hallick L., Karty R., Belozer M.L., McNulty W., Wolff J. Serologic and virologic analysis of type D simian retrovirus infection in a colony of Celebes black macaques (Macaca nigra) J. Med. Primatol. 1989;18:185–193. [PubMed] [Google Scholar]
- Shiver J.W., Fu T.M., Chen L., Casimiro D.R., Davies M.E., Evans R.K., Zhang Z.Q., Simon A.J., Trigona W.L., Dubey S.A., Huang L., Harris V.A., Long R.S., Liang X., Handt L., Schleif W.A., Zhu L., Freed D.C., Persaud N.V., Guan L., Punt K.S., Tang A., Chen M., Wilson K.A., Collins K.B., Heidecker G.J., Fernandez V.R., Perry H.C., Joyce J.G., Grimm K.M., Cook J.C., Keller P.M., Kresock D.S., Mach H., Troutman R.D., Isopi L.A., Williams D.M., Xu Z., Bohannon K.E., Volkin D.B., Montefiori D.C., Miura A., Krivulka G.R., Lifton M.A., Kuroda M.J., Schmitz J.E., Letvin N.L., Caulfield M.J., Bett A.J., Youil R., Kaslow D.C., Emini E.A. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Shope T., Dechairo D., Miller G. Malignant lymphoma in cottontop marmosets after inoculation with Epstein-Barr virus. Proc. Natl. Acad. Sci. U S A. 1973;70:2487–2491. doi: 10.1073/pnas.70.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer E.L., Kelley S.T., Taylor P.C., Vanderloo P., Lester T.L. Three serologic types of adenovirus infections of owl monkeys. Am. J. Vet. Res. 1979;40:532–536. [PubMed] [Google Scholar]
- Sienaert R., Andrei G., Snoeck R., De Clercq E., McGuigan C., Balzarini J. Inactivity of the bicyclic pyrimidine nucleoside analogues against simian varicella virus (SVV) does not correlate with their substrate activity for SVV-encoded thymidine kinase. Biochem. Biophys. Res. Commun. 2004;315:877–883. doi: 10.1016/j.bbrc.2004.01.136. [DOI] [PubMed] [Google Scholar]
- Silvestri G., Paiardini M., Pandrea I., Lederman M.M., Sodora D.L. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Invest. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M., Burgess T., Lynch J., Putnak R. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology. 2010;396:280–288. doi: 10.1016/j.virol.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Simon M.A. Simian parvoviruses: biology and implications for research. Comp. Med. 2008;58:47–50. [PMC free article] [PubMed] [Google Scholar]
- Simon M.A., Daniel M.D., Lee-Parritz D., King N.W., Ringler D.J. Disseminated B virus infection in a cynomolgus monkey. Lab. Anim. Sci. 1993;43:545–550. [PubMed] [Google Scholar]
- Simon M.A., Ilyinskii P.O., Veazey R., Baskin G.B., Young H., Pauley D., Daniel M., Lackner A. Association of SV40 with a CNS lesion distinct from PML in macaques with AIDS. Vet. Pathol. 1995;32:576. [Google Scholar]
- Simons J.N., Pilot-Matais T.J., Leary T.P. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc. Natl. Acad. Sci. U S A. 1995;92:3401–3405. doi: 10.1073/pnas.92.8.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.R., Pavris K., Anderson C.R. Experimental transovarian transmission of Kyasanur forest disease virus in Haemsphysalis spinigera. Nature. 1963;199:513. doi: 10.1038/199513a0. [DOI] [PubMed] [Google Scholar]
- Singleton W.L., Smikle C.B., Hankins G.D., Hubbard G.B., Ehler W.J., Brasky K.B. Surgical correction of severe vaginal introital stenosis in female baboons (Papio sp.) infected with simian agent 8. Lab. Anim. Sci. 1995;45:628–630. [PubMed] [Google Scholar]
- Sintasath D.M., Wolfe N.D., Lebreton M., Jia H., Garcia A.D., Le Doux-Diffo J., Tamoufe U., Carr J.K., Folks T.M., Mpoudi-Ngole E., Burke D.S., Heneine W., Switzer W.M. Simian T-lymphotropic virus diversity among nonhuman primates, Cameroon. Emerg. Infect. Dis. 2009;15:175–184. doi: 10.3201/eid1502.080584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiadopoulos M.H., Biacchesi S., Buchholz U.J., Riggs J.M., Surman S.R., Amaro-Carambot E., McAuliffe J.M., Elkins W.R., St Claire M., Collins P.L., Murphy B.R. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J. Virol. 2004;78:6927–6937. doi: 10.1128/JVI.78.13.6927-6937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiadopoulos M.H., Surman S.R., Riggs J.M., Elkins W.R., St Claire M., Nishio M., Garcin D., Kolakofsky D., Collins P.L., Murphy B.R. Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology. 2002;297:153–160. doi: 10.1006/viro.2002.1416. [DOI] [PubMed] [Google Scholar]
- Slighter R.G., Kimball J.P., Barbolt A.D., Drobeck H.P. Enzootic hepatitis A infection in cynomolgus monkeys (Macaca fascicularis) Am. J. Primatol. 1988;14:73–81. doi: 10.1002/ajp.1350140107. [DOI] [PubMed] [Google Scholar]
- Smith A.L., Black D.H., Eberle R. Molecular evidence for distinct genotypes of monkey B virus (herpesvirus simiae) which are related to the macaque host species. J. Virol. 1998;72:9224–9232. doi: 10.1128/jvi.72.11.9224-9232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.W., Skilling D.E., Anderson M.P., Benirschke K. Isolation of primate calicivirus Pan paniscus type 1 from a douc langur (Pygathrix nemaeus l.) J. Wildl. Dis. 1985;21:426–428. doi: 10.7589/0090-3558-21.4.426. [DOI] [PubMed] [Google Scholar]
- Smith A.W., Skilling D.E., Benirschke K. Calicivirus isolation from three species of primates: an incidental finding. Am. J. Vet. Res. 1985;46:2197–2199. [PubMed] [Google Scholar]
- Smith A.W., Skilling D.E., Ensley P.K., Benirschke K., Lester T.L. Calicivirus isolation and persistence in a pygmy chimpanzee (Pan paniscus) Science. 1983;221:79–81. doi: 10.1126/science.6304880. [DOI] [PubMed] [Google Scholar]
- Smith C.E.G., Simpson D.I.H., Bowen E.T.W., Zlotnick E.T. Fatal human disease from Vervet monkeys. Lancet. 1967;2:1109–1112. doi: 10.1016/s0140-6736(67)90621-6. [DOI] [PubMed] [Google Scholar]
- Smith D.M. Endogenous retroviruses in xenografts. N. Engl. J. Med. 1993;328:142–143. doi: 10.1056/NEJM199301143280218. [DOI] [PubMed] [Google Scholar]
- Smith E.K., Hunt R.D., Garcia F.G., Fraser C.E., Merkal R.S., Karlson A.G. Avian tuberculosis in monkeys. A unique mycobacterial infection. Am. Rev. Respir. Dis. 1973;107:469–471. doi: 10.1164/arrd.1973.107.3.469. [DOI] [PubMed] [Google Scholar]
- Smith G.C., Lester T.L., Heberling R.L., Kalter S.S. Coronavirus-like particles in nonhuman primate feces. Arch. Virol. 1982;72:105–111. doi: 10.1007/BF01314455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.S., Fishbein D.B., Rupprecht C.E., Clark K. Unexplained rabies in three immigrants in the United States. A virologic investigation. N. Engl. J. Med. 1991;324:205–211. doi: 10.1056/NEJM199101243240401. [DOI] [PubMed] [Google Scholar]
- Smith M.W. Field aspects of the Marburg virus outbreak: 1967. Primate Supply. 1982;7:11–15. [Google Scholar]
- Smith P.C., Yuill T.M., Buchanan R.D., Stanton J.S., Chaicumpa V. The gibbon (Hylobates lar); a new primate host for Herpesvirus hominia. I. A natural epizootic in a laboratory colony. J. Infect. Dis. 1969;120:292–297. doi: 10.1093/infdis/120.3.292. [DOI] [PubMed] [Google Scholar]
- Smith R.E., Pirie G.J., England J.J. Rabies vaccination of captive white-handed gibbons potentially exposed to wild rabies virus. Lab. Anim. Sci. 1987;37:668–669. [PubMed] [Google Scholar]
- Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van I.W.F., Eijkemans M.J., van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000756. e1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora D.L., Allan J.S., Apetrei C., Brenchley J.M., Douek D.C., Else J.G., Estes J.D., Hahn B.H., Hirsch V.M., Kaur A., Kirchhoff F., Muller-Trutwin M., Pandrea I., Schmitz J.E., Silvestri G. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat. Med. 2009;15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soike K.F., Gerone P.J. Acyclovir in the treatment of simian varicella infection of the African green monkey. Am. J. Med. 1995;73:112–117. doi: 10.1016/0002-9343(82)90075-4. [DOI] [PubMed] [Google Scholar]
- Soike K.F., Keller P.M., Ellis R.W. Immunization of monkeys with varicella-zoster virus glycoprotein antigens and their response to challenge with simian varicella virus. J. Med. Virol. 1987;22:307–313. doi: 10.1002/jmv.1890220403. [DOI] [PubMed] [Google Scholar]
- Soike K.F., Rangan S.R., Gerone P.J. Viral disease models in primates. Adv. Vet. Sci. Comp. Med. 1984;28:151–199. doi: 10.1016/b978-0-12-039228-5.50011-5. [DOI] [PubMed] [Google Scholar]
- Souquiere S., Bibollet-Ruche F., Robertson D.L., Makuwa M., Apetrei C., Onanga R., Kornfeld C., Plantier J.C., Gao F., Abernethy K., White L.J., Karesh W., Telfer P., Wickings E.J., Mauclere P., Marx P.A., Barre-Sinoussi F., Hahn B.H., Muller-Trutwin M.C., Simon F. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 2001;75:7086–7096. doi: 10.1128/JVI.75.15.7086-7096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquiere S., Mouinga-Ondeme A., Makuwa M., Beggio P., Radaelli A., De Giuli Morghen C., Mortreux F., Kazanji M. T-cell tropism of simian T-cell leukaemia virus type 1 and cytokine profiles in relation to proviral load and immunological changes during chronic infection of naturally infected mandrills (Mandrillus sphinx) J. Med. Primatol. 2009;38:279–289. doi: 10.1111/j.1600-0684.2009.00356.x. [DOI] [PubMed] [Google Scholar]
- Spencer J.V., Lockridge K.M., Barry P.A., Lin G., Tsang M., Penfold M.E., Schall T.J. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 2002;76:1285–1292. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A.I., Marx P.A., Patterson B.K., Mahoney J., Koup R.A., Wolinsky S.M., Ho D.D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihongse S. Vesicular stomatitis virus infections in Panamanian primates and other vertebrates. Am. J. Epidemiol. 1969;90:69–76. doi: 10.1093/oxfordjournals.aje.a121051. [DOI] [PubMed] [Google Scholar]
- Staples J.E., Breiman R.F., Powers A.M. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- Starkman S.E., MacDonald D.M., Lewis J.C., Holmes E.C., Simmonds P. Geographic and species association of hepatitis B virus genotypes in non-human primates. Virology. 2003;314:381–393. doi: 10.1016/s0042-6822(03)00430-6. [DOI] [PubMed] [Google Scholar]
- Stassen F.R., Vega-Cordova X., Vliegen I., Bruggeman C.A. Immune activation following cytomegalovirus infection: more important than direct viral effects in cardiovascular disease? J. Clin. Virol. 2006;35:349–353. doi: 10.1016/j.jcv.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Steele M.D., Giddens W.E., Jr., Valerio M., Sumi S.M., Stetzer E.R. Spontaneous paramyxoviral encephalitis in nonhuman primates (Macaca mulatta and M. nemestrina) Vet. Pathol. 1982;19:132–139. doi: 10.1177/030098588201900204. [DOI] [PubMed] [Google Scholar]
- Stephens E.B., McCormick C., Pacyniak E., Griffin D., Pinson D.M., Sun F., Nothnick W., Wong S.W., Gunderson R., Berman N.E., Singh D.K. Deletion of the vpu sequences prior to the env in a simian-human immunodeficiency virus results in enhanced Env precursor synthesis but is less pathogenic for pig-tailed macaques. Virology. 2002;293:252–261. doi: 10.1006/viro.2001.1244. [DOI] [PubMed] [Google Scholar]
- Stephens E.B., Tian C., Dalton S.B., Gattone V.H., 2nd Simian-human immunodeficiency virus-associated nephropathy in macaques. AIDS Res. Hum. Retroviruses. 2000;16:1295–1306. doi: 10.1089/08892220050117050. [DOI] [PubMed] [Google Scholar]
- Stittelaar K.J., Neyts J., Naesens L., van Amerongen G., van Lavieren R.F., Holy A., De Clercq E., Niesters H.G., Fries E., Maas C., Mulder P.G., van der Zeijst B.A., Osterhaus A.D. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439:745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H., Niesters H.G., van Doornum G., van der Zeijst B.A., Mateo L., Chaplin P.J., Osterhaus A.D. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell R.E., Smith E.K., Espana C., Nelson V.G. Outbreak of malignant lymphoma in rhesus monkeys. Lab. Invest. 1971;25:476–479. [PubMed] [Google Scholar]
- Strand K., Harper E., Thormahlen S., Thouless M.E., Tsai C., Rose T., Bosch M.L. Two distinct lineages of macaque gamma herpesviruses related to the Kaposi’s sarcoma associated herpesvirus. J. Clin. Virol. 2000;16:253–269. doi: 10.1016/s1386-6532(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Streblow D.N., Orloff S.L., Nelson J.A. Acceleration of allograft failure by cytomegalovirus. Curr. Opin. Immunol. 2007;19:577–582. doi: 10.1016/j.coi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subekti D.S., Tjaniadi P., Lesmana M., McArdle J., Iskandriati D., Budiarsa I.N., Walujo P., Suparto I.H., Winoto I., Campbell J.R., Porter K.R., Sajuthi D., Ansari A.A., Oyofo B.A. Experimental infection of Macaca nemestrina with a Toronto Norwalk-like virus of epidemic viral gastroenteritis. J. Med. Virol. 2002;66:400–406. doi: 10.1002/jmv.2159. [DOI] [PubMed] [Google Scholar]
- Sundberg J.P., Reichman M.E. Papillomavirus infections. In: Jones T.C., Mohr U., Hunt R.D., editors. Vol. 2. Springer-Verlag; Berlin and New York: 1993. pp. 1–8. (Monographs on the Pathology of Laboratory Animals: Nonhuman Primates). [Google Scholar]
- Sundberg J.P., Shima A.L., Adkison D.L. Oral papillomavirus infection in a pygmy chimpanzee (Pan paniscus) J. Vet. Diagn. Invest. 1992;4:70–74. doi: 10.1177/104063879200400115. [DOI] [PubMed] [Google Scholar]
- Sutherland S.D., Almeida J.D., Gardner P.S., Skarpa M., Stanton J. Rapid diagnosis and management of parainfluenza I virus infection in common marmosets (Callithrix jacchus) Lab. Anim. 1986;20:121–126. doi: 10.1258/002367786780865151. [DOI] [PubMed] [Google Scholar]
- Swack N.S., Hsiung G.D. Natural and experimental simian cytomegalovirus infections at a primate center. J. Med. Primatol. 1982;11:169–177. [PubMed] [Google Scholar]
- Switzer W.M., Bhullar V., Shanmugam V., Cong M.E., Parekh B., Lerche N.W., Yee J.L., Ely J.J., Boneva R., Chapman L.E., Folks T.M., Heneine W. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J. Virol. 2004;78:2780–2789. doi: 10.1128/JVI.78.6.2780-2789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer W.M., Salemi M., Shanmugam V., Gao F., Cong M.E., Kuiken C., Bhullar V., Beer B.E., Vallet D., Gautier-Hion A., Tooze Z., Villinger F., Holmes E.C., Heneine W. Ancient co-speciation of simian foamy viruses and primates. Nature. 2005;434:376–380. doi: 10.1038/nature03341. [DOI] [PubMed] [Google Scholar]
- Szentiks C.A., Kondgen S., Silinski S., Speck S., Leendertz F.H. Lethal pneumonia in a captive juvenile chimpanzee (Pan troglodytes) due to human-transmitted human respiratory syncytial virus (HRSV) and infection with Streptococcus pneumoniae. J. Med. Primatol. 2009;38:236–240. doi: 10.1111/j.1600-0684.2009.00346.x. [DOI] [PubMed] [Google Scholar]
- Tabor E., April M., Seeff L.B., Gerety R.J. Acquired immunity to human non-A, non-B hepatitis: cross-challenge of chimpanzees with three infectious human sera. J. Infect. Dis. 1979;140:789–793. doi: 10.1093/infdis/140.5.789. [DOI] [PubMed] [Google Scholar]
- Takano J., Narita T., Fujimoto K., Mukai R., Yamada A. Detection of B virus infection in cynomolgus monkeys by ELISA using simian agent 8 as alternative antigen. Exp. Anim. 2001;50:345–347. doi: 10.1538/expanim.50.345. [DOI] [PubMed] [Google Scholar]
- Takehisa J., Kraus M.H., Ayouba A., Bailes E., Van Heuverswyn F., Decker J.M., Li Y., Rudicell R.S., Learn G.H., Neel C., Ngole E.M., Shaw G.M., Peeters M., Sharp P.M., Hahn B.H. Origin and biology of simian immunodeficiency virus in wild-living western gorillas. J. Virol. 2009;83:1635–1648. doi: 10.1128/JVI.02311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K.K., Furuno A., Kato K., Yoshiike K. Biological and biochemical studies of African green monkey lymphotropic papovavirus. J. Virol. 1982;42:502–509. doi: 10.1128/jvi.42.2.502-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarmin A., Chandler L.J., Kazanji M., de Thoisy B., Debon P., Lelarge J., Labeau B., Bourreau E., Vie J.C., Shope R.E., Sarthou J.L. Mayaro virus fever in French Guiana: isolation, identification, and seroprevalence. Am. J. Trop. Med. Hyg. 1998;59:452–456. doi: 10.4269/ajtmh.1998.59.452. [DOI] [PubMed] [Google Scholar]
- Tate C.L., Conti P.A., Nero E.P. Epithelial hyperplasia in the oral mucosa of a chimpanzee. J. Am. Vet. Med. Assoc. 1973;163:619–621. [PubMed] [Google Scholar]
- Tatematsu K., Tanaka Y., Kurbanov F., Sugauchi F., Mano S., Maeshiro T., Nakayoshi T., Wakuta M., Miyakawa Y., Mizokami M. A genetic variant of hepatitis B virus divergent from known human and ape genotypes isolated from a Japanese patient and provisionally assigned to new genotype J. J. Virol. 2009;83:10538–10547. doi: 10.1128/JVI.00462-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N., Tesema L., Robinson E.R., Giles-Davis W., McCoy K., Gao G.P., Wilson J.M., Ertl H.C. Chimpanzee-origin adenovirus vectors as vaccine carriers. Gene Ther. 2006;13:421–429. doi: 10.1038/sj.gt.3302675. [DOI] [PubMed] [Google Scholar]
- Tauraso N.M., Shelokov A., Palmer A.E., Allen A.M. Simian hemorrhagic fever. 3. Isolation and characterization of a viral agent. Am. J. Trop. Med. Hyg. 1968;17:422–431. [PubMed] [Google Scholar]
- Tellez-Nagel I., Harter D.H. Subacute sclerosing leukoencephalitis: Ultrastructure of intranuclear and intracytoplasmic inclusions. Science. 1966;154:899–901. doi: 10.1126/science.154.3751.899. [DOI] [PubMed] [Google Scholar]
- Tesh R.B., Wallace G.D. Observations on the natural history of encephalomyocarditis virus. Am. J. Trop. Med. Hyg. 1978;27:133–143. doi: 10.4269/ajtmh.1978.27.133. [DOI] [PubMed] [Google Scholar]
- Theilen G.H., Gould D., Fowler M., Dungworth D.L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J. Natl. Cancer Inst. 1971;47:881–889. [PubMed] [Google Scholar]
- Thompson S.A., Hilliard J.K., Kittel D., Lipper S., Giddens W.E., Jr., Black D.H., Eberle R. Retrospective analysis of an outbreak of B virus infection in a colony of DeBrazza’s monkeys (Cercopithecus neglectus) Comp. Med. 2000;50:649–657. [PubMed] [Google Scholar]
- Thumer L., Rethwilm A., Holmes E.C., Bodem J. The complete nucleotide sequence of a New World simian foamy virus. Virology. 2007;369:191–197. doi: 10.1016/j.virol.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Thung S.N., Gerber M.A., Purcell R.H., London W.T., Mihalik K.B., Popper H. Chimpanzee carriers of hepatitis B virus. Am. J. Pathol. 1981;105:328–332. [PMC free article] [PubMed] [Google Scholar]
- Ticehurst J., Rhodes L.L., Jr., Krawczynski K., Asher L.V., Engler W.F., Mensing T.L., Caudill J.D., Sjogren M.H., Hoke C.H., Jr., LeDuc J.W. Infection of owl monkeys (Aotus trivirgatus) and cynomolgus monkeys (Macaca fascicularis) with hepatitis E virus from Mexico. J. Infect. Dis. 1992;165:835–845. doi: 10.1093/infdis/165.5.835. [DOI] [PubMed] [Google Scholar]
- Todaro G.J., Benveniste R.E., Sherr C.J., Schlom J., Schidlovsky G., Stephenson J.R. Isolation and characterization of a new type D retrovirus from the Asian primate, Presbytis obscurus (spectacled langur) Virology. 1978;84:189–194. doi: 10.1016/0042-6822(78)90231-3. [DOI] [PubMed] [Google Scholar]
- Towner J.S., Amman B.R., Sealy T.K., Carroll S.A., Comer J.A., Kemp A., Swanepoel R., Paddock C.D., Balinandi S., Khristova M.L., Formenty P.B., Albarino C.G., Miller D.M., Reed Z.D., Kayiwa J.T., Mills J.N., Cannon D.L., Greer P.W., Byaruhanga E., Farnon E.C., Atimnedi P., Okware S., Katongole-Mbidde E., Downing R., Tappero J.W., Zaki S.R., Ksiazek T.G., Nichol S.T., Rollin P.E. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000536. e1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner J.S., Khristova M.L., Sealy T.K., Vincent M.J., Erickson B.R., Bawiec D.A., Hartman A.L., Comer J.A., Zaki S.R., Stroher U., Gomes da Silva F., del Castillo F., Rollin P.E., Ksiazek T.G., Nichol S.T. Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J. Virol. 2006;80:6497–6516. doi: 10.1128/JVI.00069-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner J.S., Sealy T.K., Khristova M.L., Albarino C.G., Conlan S., Reeder S.A., Quan P.L., Lipkin W.I., Downing R., Tappero J.W., Okware S., Lutwama J., Bakamutumaho B., Kayiwa J., Comer J.A., Rollin P.E., Ksiazek T.G., Nichol S.T. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000212. e1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina-Dorge V., Blanchard J., Martin L., Murphey-Corb M. Immunodeficiency and lymphoproliferative disease in an African green monkey dually infected with SIV and STLV-I. AIDS Res. Hum. Retroviruses. 1992;8:97–100. doi: 10.1089/aid.1992.8.97. [DOI] [PubMed] [Google Scholar]
- Traina-Dorge V.L., Lorino R., Gormus B.J., Metzger M., Telfer P., Richardson D., Robertson D.L., Marx P.A., Apetrei C. Molecular epidemiology of simian T-cell lymphotropic virus type 1 in wild and captive sooty mangabeys. J. Virol. 2005;79:2541–2548. doi: 10.1128/JVI.79.4.2541-2548.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T.T., Trinh T.N., Abe K. New complex recombinant genotype of hepatitis B virus identified in Vietnam. J. Virol. 2008;82:5657–5663. doi: 10.1128/JVI.02556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichel A.M., Rajakumar P.A., Murphey-Corb M. Species-specific variation in SIV disease progression between Chinese and Indian subspecies of rhesus macaque. J. Med. Primatol. 2002;31:171–178. doi: 10.1034/j.1600-0684.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- Troan B.V., Perelygina L., Patrusheva I., Wettere A.J., Hilliard J.K., Loomis M.R., Voe R.S. Naturally transmitted herpesvirus papio-2 infection in a black and white colobus monkey. J. Am. Vet. Med. Assoc. 2007;231:1878–1883. doi: 10.2460/javma.231.12.1878. [DOI] [PubMed] [Google Scholar]
- Tsai C.C., Follis K.E., Snyder K., Windsor S., Thouless M.E., Kuller L., Morton W.R. Maternal transmission of type D simian retrovirus (SRV-2) in pigtailed macaques. J. Med. Primatol. 1990;19:203–216. [PubMed] [Google Scholar]
- Tsai C.C., Warner T.F.C.S., Uno H., Giddens W.E., Ochs H. Subcutaneous fibromatosis associated with an acquired immune deficiency syndrome in pig-tailed macaques. Am. J. Pathol. 1985;120:30–37. [PMC free article] [PubMed] [Google Scholar]
- Tsai C.C., Wu H., Meng F. Immunocytochemistry of Kaposi’s sarcoma-like tumor cells from pigtailed macaques with simian AIDS. J. Med. Primatol. 1995;24:43–48. doi: 10.1111/j.1600-0684.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Tsetsarkin K.A., Vanlandingham D.L., McGee C.E., Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto H., Cooper R.W., Kodama T., Fukasawa M., Miura T., Ohta Y., Ishikawa K., Nakai M., Frost E., Roelants G.E. Isolation and characterization of simian immunodeficiency virus from mandrills in Africa and its relationship to other human and simian immunodeficiency viruses. J. Virol. 1988;62:4044–4050. doi: 10.1128/jvi.62.11.4044-4050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto H., Noda Y., Ishikawa K., Nakamura H., Fukasawa M., Sakakibara I., Sasagawa A., Honjo S., Hayami M. Development of adult T-cell leukemia-like disease in African green monkey associated with clonal integration of simian T-cell leukemia virus type I. Cancer Res. 1987;47:269–274. [PubMed] [Google Scholar]
- Tyler S.D., Peters G.A., Severini A. Complete genome sequence of cercopithecine herpesvirus 2 (SA8) and comparison with other simplexviruses. Virology. 2005;331:429–440. doi: 10.1016/j.virol.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Tyler S.D., Severini A. The complete genome sequence of herpesvirus papio 2 (Cercopithecine herpesvirus 16) shows evidence of recombination events among various progenitor herpesviruses. J. Virol. 2006;80:1214–1221. doi: 10.1128/JVI.80.3.1214-1221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura T., Inagaki H., Goryo M., Itakura C. Aspiration pneumonia with adenovirus infection in a Japanese macaque (Macaca fuscata fuscata) Lab. Anim. 1985;19:39–41. doi: 10.1258/002367785780890631. [DOI] [PubMed] [Google Scholar]
- Valis J.D., Newell N., Reissig M., Malherbe H., Kaschula V.R., Shah K.V. Characterization of SA12 as a simian virus-40 related papovavirus of Chacma baboons. Infect. Immun. 1977;17:247–252. doi: 10.1128/iai.18.1.247-252.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brussel M., Salemi M., Liu H.F., Gabriels J., Goubau P., Desmyter J., Vandamme A.M. The simian T-lymphotropic virus STLV-PP1664 from Pan paniscus is distinctly related to HTLV-2 but differs in genomic organization. Virology. 1998;243:366–379. doi: 10.1006/viro.1998.9075. [DOI] [PubMed] [Google Scholar]
- van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A., Osterhaus A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kuyl A.C., Mang R., Dekker J.T., Goudsmit J. Complete nucleotide sequence of simian endogenous type D retrovirus with intact genome organization: evidence for ancestry to simian retrovirus and baboon endogenous virus. J. Virol. 1997;71:3666–3676. doi: 10.1128/jvi.71.5.3666-3676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stuyft P., Gianella A., Pirard M., Cespedes J., Lora J., Peredo C., Pelegrino J.L., Vorndam V., Boelaert M. Urbanisation of yellow fever in Santa Cruz, Bolivia. Lancet. 1999;353:1558–1562. doi: 10.1016/s0140-6736(99)03291-2. [DOI] [PubMed] [Google Scholar]
- Van Dooren S., Salemi M., Vandamme A.M. Dating the origin of the African human T-cell lymphotropic virus type-i (HTLV-I) subtypes. Mol. Biol. Evol. 2001;18:661–671. doi: 10.1093/oxfordjournals.molbev.a003846. [DOI] [PubMed] [Google Scholar]
- van Gorder M.A., Della Pelle P., Henson J.W., Sachs D.H., Cosimi A.B., Colvin R.B. Cynomolgus polyoma virus infection: a new member of the polyoma virus family causes interstitial nephritis, ureteritis, and enteritis in immunosuppressed cynomolgus monkeys. Am. J. Pathol. 1999;154:1273–1284. doi: 10.1016/S0002-9440(10)65379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heuverswyn F., Li Y., Neel C., Bailes E., Keele B.F., Liu W., Loul S., Butel C., Liegeois F., Bienvenue Y., Ngolle E.M., Sharp P.M., Shaw G.M., Delaporte E., Hahn B.H., Peeters M. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444:164. doi: 10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- Van Ranst M., Fuse A., Sobis H., De Meurichy W., Syrjanen S.M., Billiau A., Opdenakker G. A papillomavirus related to HPV type 13 in oral focal epithelial hyperplasia in the pygmy chimpanzee. J. Oral. Pathol. Med. 1991;20:325–331. doi: 10.1111/j.1600-0714.1991.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Veazey R.S., DeMaria M., Chalifoux L.V., Shvetz D.E., Pauley D.R., Knight H.L., Rosenzweig M., Johnson R.P., Desrosiers R.C., Lackner A.A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Verschoor E.J., Fagrouch Z., Bontjer I., Niphuis H., Heeney J.L. A novel simian immunodeficiency virus isolated from a Schmidt’s guenon (Cercopithecus ascanius schmidti) J. Gen. Virol. 2004;85:21–24. doi: 10.1099/vir.0.19427-0. [DOI] [PubMed] [Google Scholar]
- Verschoor E.J., Groenewoud M.J., Fagrouch Z., Kewalapat A., van Gessel S., Kik M.J., Heeney J.L. Molecular characterization of the first polyomavirus from a New World primate: squirrel monkey polyomavirus. J. Gen. Virol. 2008;89:130–137. doi: 10.1099/vir.0.83287-0. [DOI] [PubMed] [Google Scholar]
- Verschoor E.J., Niphuis H., Fagrouch Z., Christian P., Sasnauskas K., Pizarro M.C., Heeney J.L. Seroprevalence of SV40-like polyomavirus infections in captive and free-ranging macaque species. J. Med. Primatol. 2008;37:196–201. doi: 10.1111/j.1600-0684.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- Verschoor E.J., Warren K.S., Langenhuijzen S., Heriyanto, Swan R.A., Heeney J.L. Analysis of two genomic variants of orang-utan hepadnavirus and their relationship to other primate hepatitis B-like viruses. J. Gen. Virol. 2001;82:893–897. doi: 10.1099/0022-1317-82-4-893. [DOI] [PubMed] [Google Scholar]
- Vickers J.H. Approaches to determining colony infections and improving colony health. In: Bernischke J., editor. Primates: The Road to Self-Sustaining Populations. Springer-Verlag; Berlin and New York: 1986. pp. 521–530. [Google Scholar]
- Victoria J.G., Kapoor A., Dupuis K., Schnurr D.P., Delwart E.L. Rapid identification of known and new RNA viruses from animal tissues. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000163. e1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voevodin A.F., Lapin B.A., Yakovleva L.A., Ponomaryeva T.I., Oganyan T.E., Razmadze E.N. Antibodies reacting with human T-lymphotropic retrovirus (HTLV-I) or related antigens in lymphomatous and healthy hamadryas baboons. Int. J. Cancer. 1985;36:579–584. doi: 10.1002/ijc.2910360511. [DOI] [PubMed] [Google Scholar]
- Voevodin A.F., Marx P.A., Jr. Simian Virology. Wiley-Blackwell; Ames: 2009. Introduction to Retroviruses; pp. 69–76. [Google Scholar]
- Vogel P., Weigler B.J., Kerr H., Hendrickx A.G., Barry P.A. Seroepidemiologic studies of cytomegalovirus infection in a breeding population of rhesus macaques. Lab. Anim. Sci. 1994;44:25–30. [PubMed] [Google Scholar]
- von Magnus P., Andersen E.K., Petersen K.B., Birch-Anderson A. A pox like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959;46:156–176. [Google Scholar]
- Wachtman L.M., Mansfield K.G. Opportunistic infections in immunologically compromised nonhuman primates. ILAR J. 2008;49:191–208. doi: 10.1093/ilar.49.2.191. [DOI] [PubMed] [Google Scholar]
- Walker D.H., Voelker F.A., McKee A.E., Jr., Nakano J.H. Diagnostic exercise: tumors in a baboon. Lab. Anim. Sci. 1985;35:627–628. [PubMed] [Google Scholar]
- Wang Y., Tu X., Humphrey C., McClure H., Jiang X., Qin C., Glass R.I., Jiang B. Detection of viral agents in fecal specimens of monkeys with diarrhea. J. Med. Primatol. 2007;36:101–107. doi: 10.1111/j.1600-0684.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J.A., Hilliard J.K. B virus-specific pathogen-free (SPF) breeding colonies of macaques: issues, surveillance, and results in 1992. Lab. Anim. Sci. 1994;44:222–228. [PubMed] [Google Scholar]
- Ward J.A., Hilliard J.K. Herpes B virus-specific pathogen-free breeding colonies of macaques: serologic test results and the B-virus status of the macaque. Contemp. Top. Lab. Anim. Sci. 2002;41:36–41. [PubMed] [Google Scholar]
- Webb H.E., Chaterjea J.B. Clinico-pathological observations on monkeys infected with Kyasanur Forest disease virus, with special reference to the haemopoietic system. Br. J. Haematol. 1962;8:401–413. doi: 10.1111/j.1365-2141.1962.tb06544.x. [DOI] [PubMed] [Google Scholar]
- Weigler B.J. Biology of B virus in macaque and human hosts: a review. Clin. Infect. Dis. 1992;14:555–567. doi: 10.1093/clinids/14.2.555. [DOI] [PubMed] [Google Scholar]
- Weigler B.J., Hird D.W., Hilliard J.K., Lerche N.W., Roberts J.A., Scott L.M. Epidemiology of cercopithecine herpesvirus 1 (B virus) infection and shedding in a large breeding cohort of rhesus macaques. J. Infect. Dis. 1993;167:257–263. doi: 10.1093/infdis/167.2.257. [DOI] [PubMed] [Google Scholar]
- Weigler B.J., Roberts J.A., Hird D.W., Lerche N.W., Hilliard J.K. A cross sectional survey for B virus antibody in a colony of group housed rhesus macaques. Lab. Anim. Sci. 1990;40:257–261. [PubMed] [Google Scholar]
- Weiler A., May G.E., Qi Y., Wilson N., Watkins D.I. Polymorphisms in eight host genes associated with control of HIV replication do not mediate elite control of viral replication in SIV-infected Indian rhesus macaques. Immunogenetics. 2006;58:1003–1009. doi: 10.1007/s00251-006-0166-6. [DOI] [PubMed] [Google Scholar]
- Weir E.C., Bhatt P.N., Jacoby R.O., Hilliard J.K., Morgenstern S. Infrequent shedding and transmission of herpesvirus simiae from seropositive macaques. Lab. Anim. Sci. 1993;43:541–544. [PubMed] [Google Scholar]
- Wen K.W., Dittmer D.P., Damania B. Disruption of LANA in rhesus rhadinovirus generates a highly lytic recombinant virus. J. Virol. 2009;83:9786–9802. doi: 10.1128/JVI.00704-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner H.A., Abel D., Barrick S., Seshumurty P. Clinical and pathogenetic studies of Medical Lake macaque virus infections in cynomolgus monkeys (simian varicella) J. Infect. Dis. 1977;135:611–622. doi: 10.1093/infdis/135.4.611. [DOI] [PubMed] [Google Scholar]
- Westerman L.E., Jiang B., McClure H.M., Snipes-Magaldi L.J., Griffin D.D., Shin G., Gentsch J.R., Glass R.I. Isolation and characterization of a new simian rotavirus, YK-1. Virol. J. 2006;3:40. doi: 10.1186/1743-422X-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman L.E., McClure H.M., Jiang B., Almond J.W., Glass R.I. Serum IgG mediates mucosal immunity against rotavirus infection. Proc. Natl. Acad. Sci. U S A. 2005;102:7268–7273. doi: 10.1073/pnas.0502437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby D., Stossel A., Gamache C., Papin J., Bosch M., Smith A., Kedes D.H., White G., Kennedy R., Dittmer D.P. Novel Kaposi’s sarcoma-associated herpesvirus homolog in baboons. J. Virol. 2003;77:8159–8165. doi: 10.1128/JVI.77.14.8159-8165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.A., Todd P.A., Rosenthal A.N., Yee J.L., Grant R., Lerche N.W. Development of a generic real-time PCR assay for simultaneous detection of proviral DNA of simian Betaretrovirus serotypes 1, 2, 3, 4 and 5 and secondary uniplex assays for specific serotype identification. J. Virol. Methods. 2009;162:148–154. doi: 10.1016/j.jviromet.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.A., Todd P.A., Yee J.L., Kalman-Bowlus A., Rodgers K.S., Yang X., Wong S.W., Barry P., Lerche N.W. Prevalence of viremia and oral shedding of rhesus rhadinovirus and retroperitoneal fibromatosis herpesvirus in large age-structured breeding groups of rhesus macaques (Macaca mulatta) Comp. Med. 2009;59:383–390. [PMC free article] [PubMed] [Google Scholar]
- White R.J., Simmons L., Wilson R.B. Chickenpox in young anthropoid apes: clinical and laboratory findings. J. Am. Vet. Med. Assoc. 1972;161:690–692. [PubMed] [Google Scholar]
- White T.M., Mahalingam R., Kolhatkar G., Gilden D.H. Identification of simian varicella virus homologues of varicella zoster virus genes. Virus Genes. 1997;15:265–269. doi: 10.1023/a:1007940822747. [DOI] [PubMed] [Google Scholar]
- White T.M., Mahalingam R., Traina-Dorge V., Gilden D.H. Persistence of simian varicella virus DNA in CD4(+) and CD8(+) blood mononuclear cells for years after intratracheal inoculation of African green monkeys. Virology. 2002;303:192–198. doi: 10.1006/viro.2002.1664. [DOI] [PubMed] [Google Scholar]
- Whittaker D., Glaister J.R. A Yaba-like condition in a young baboon (Papio anubis) Lab. Anim. 1985;19:177–179. doi: 10.1258/002367785780893557. [DOI] [PubMed] [Google Scholar]
- WHO Viral hemorrhagic fever in imported monkeys. Wkly. Epidemiol. Rec. 1992;67:142–143. [PubMed] [Google Scholar]
- WHO Outbreak of Ebola haemorrhagic fever, Uganda, August 2000–January 2001. Wkly. Epidemiol. Rec. 2001;76:41–46. [PubMed] [Google Scholar]
- WHO Marburg haemorrhagic fever, Angola. Wkly. Epidemiol. Rec. 2005;80:158–159. [PubMed] [Google Scholar]
- Wilkinson R.C., Murrell C.K., Guy R., Davis G., Hall J.M., North D.C., Rose N.J., Almond N. Persistence and dissemination of simian retrovirus type 2 DNA in relation to viremia, seroresponse, and experimental transmissibility in Macaca fascicularis. J. Virol. 2003;77:10751–10759. doi: 10.1128/JVI.77.20.10751-10759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.B., Holscher M.A., Chang T., Hodges J.R. Fatal Herpesvirus simiae (B virus) infection in a patas monkey (Erythrocebus patas) J. Vet. Diagn. Invest. 1990;2:242–244. doi: 10.1177/104063879000200321. [DOI] [PubMed] [Google Scholar]
- Wolf R.F., Papin J.F., Hines-Boykin R., Chavez-Suarez M., White G.L., Sakalian M., Dittmer D.P. Baboon model for West Nile virus infection and vaccine evaluation. Virology. 2006;355:44–51. doi: 10.1016/j.virol.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Wolf R.F., Rogers K.M., Blewett E.L., Dittmer D.P., Fakhari F.D., Hill C.A., Kosanke S.D., White G.L., Eberle R. A naturally occurring fatal case of Herpesvirus papio 2 pneumonia in an infant baboon (Papio hamadryas anubis) J. Am. Assoc. Lab. Anim. Sci. 2006;45:64–68. [PubMed] [Google Scholar]
- Wolfe L.G., Griesemer R.A., Farrell R.L. Experimental aerosol transmission of Yaba virus in monkeys. J. Natl. Cancer Inst. 1968;41:1175–1195. [PubMed] [Google Scholar]
- Wolfe L.G., Smith R.K., Deinhardt F. Simian sarcoma virus, type 1 (Lagothrix): focus assay and demonstration of nontransforming associated virus. J. Natl. Cancer Inst. 1972;48:1905–1908. [PubMed] [Google Scholar]
- Wolfe N.D., Heneine W., Carr J.K., Garcia A.D., Shanmugam V., Tamoufe U., Torimiro J.N., Prosser A.T., Lebreton M., Mpoudi-Ngole E., McCutchan F.E., Birx D.L., Folks T.M., Burke D.S., Switzer W.M. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Natl. Acad. Sci. U S A. 2005;102:7994–7999. doi: 10.1073/pnas.0501734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.W., Bergquam E.P., Swanson R.M., Lee F.W., Shiigi S.M., Avery N.A., Fanton J.W., Axthelm M.K. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi’s sarcoma-associated herpesvirus. J. Exp. Med. 1999;190:827–840. doi: 10.1084/jem.190.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C.E., Borgerink H., Register T.C., Scott L., Cline J.M. Cervical and vaginal epithelial neoplasms in cynomolgus monkeys. Vet. Pathol. 2004;41:108–115. doi: 10.1354/vp.41-2-108. [DOI] [PubMed] [Google Scholar]
- Wood C.E., Chen Z., Cline J.M., Miller B.E., Burk R.D. Characterization and experimental transmission of an oncogenic papillomavirus in female macaques. J. Virol. 2007;81:6339–6345. doi: 10.1128/JVI.00233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R.G., Greenberg H.B., Dalgard D.W., Allen W.P., Sly D.L., Thornhill T.S., Chanock R.M., Kapikian A.Z. Experimental infection of chimpanzees with the Norwalk agent of epidemic viral gastroenteritis. J. Med. Virol. 1978;2:89–96. doi: 10.1002/jmv.1890020203. [DOI] [PubMed] [Google Scholar]
- Wyatt R.G., Sly D.L., London W.T., Palmer A.E., Kalica A.R., Van Kirk D.H., Chanock R.M., Kapikian A.Z. Induction of diarrhea in colostrum-deprived newborn rhesus monkeys with the human reovirus-like agent of infantile gastroenteritis. Arch. Virol. 1976;50:17–27. doi: 10.1007/BF01317997. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Li Y., Cun A., Yang W., Ellenberg S., Switzer W.M., Kalish M.L., Ertl H.C. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 2006;12:1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Li T.C., Koshimoto C., Ito K., Kita M., Miyashita N., Arikawa J., Yagami K., Asano M., Tezuka H., Suzuki N., Kurosawa T., Shibahara T., Furuya M., Mohri S., Sato H., Ohsawa K., Ibuki K., Takeda N. Serological evidence for hepatitis e virus infection in laboratory monkeys and pigs in animal facilities in Japan. Exp. Anim. 2008;57:367–376. doi: 10.1538/expanim.57.367. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Ohsawa K., Walz S.E., Mitchen J.L., Watanabe Y., Eberle R., Origasa H., Sato H. Validation of an enzyme-linked immunosorbent assay kit using herpesvirus papio 2 (HVP2) antigen for detection of herpesvirus simiae (B virus) infection in rhesus monkeys. Comp. Med. 2005;55:244–248. [PubMed] [Google Scholar]
- Yanai T., Lackner A.A., Sakai H., Masegi T., Simon M.A. Systemic arteriopathy in SIV-infected rhesus macaques (Macaca mulatta) J. Med. Primatol. 2006;35:106–112. doi: 10.1111/j.1600-0684.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- Yearley J.H., Pearson C., Carville A., Shannon R.P., Mansfield K.G. SIV-associated myocarditis: viral and cellular correlates of inflammation severity. AIDS Res. Hum. Retroviruses. 2006;22:529–540. doi: 10.1089/aid.2006.22.529. [DOI] [PubMed] [Google Scholar]
- Yu Q., Chen D., Konig R., Mariani R., Unutmaz D., Landau N.R. APOBEC3B and APOBEC3C are potent inhibitors of simian immunodeficiency virus replication. J. Biol. Chem. 2004;279:53379–53386. doi: 10.1074/jbc.M408802200. [DOI] [PubMed] [Google Scholar]
- Yu S.F., Sullivan M.D., Linial M.L. Evidence that the human foamy virus genome is DNA. J. Virol. 1999;73:1565–1572. doi: 10.1128/jvi.73.2.1565-1572.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Barry P.A. Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus. Adv. Virus Res. 2008;72:207–226. doi: 10.1016/S0065-3527(08)00405-3. [DOI] [PubMed] [Google Scholar]
- Zack P.M. Simian hemorrhagic fever. In: Jones T.C., Mohr U., Hunt R.D., editors. Vol. 1. Springer-Verlag; Berlin and New York: 1993. pp. 118–131. (Monographs on the Pathology of Laboratory Animals: Nonhuman Primates). [Google Scholar]
- Zahn R.C., Rett M.D., Korioth-Schmitz B., Sun Y., Buzby A.P., Goldstein S., Brown C.R., Byrum R.A., Freeman G.J., Letvin N.L., Hirsch V.M., Schmitz J.E. Simian immunodeficiency virus (SIV)-specific CD8+ T-cell responses in vervet African green monkeys chronically infected with SIVagm. J. Virol. 2008;82:11577–11588. doi: 10.1128/JVI.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaucha G.M., Jahrling P.B., Geisbert T.W., Swearengen J.R., Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab. Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdziarski J.M., Sarich N.A., Witecki K.E., Lednicky J.A. Molecular analysis of SV-40-CAL, a new slow growing SV-40 strain from the kidney of a caged New World monkey with fatal renal disease. Virus Genes. 2004;29:183–190. doi: 10.1023/B:VIRU.0000036378.42136.7c. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lou B., Lal R.B., Gettie A., Marx P.A., Moore J.P. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 2000;74:6893–6910. doi: 10.1128/jvi.74.15.6893-6910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhenjian D., Lang S., Sasseville V.G., Lackner A., Ilynski P.O., Daniel M.D., Jung J.U., Desrosiers R.C. Identification of a nef allele that causes lymphocytic activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- Zhou D., Shen Y., Chalifoux L., Lee-Parritz D., Simon M., Sehgal P.K., Zheng L., Halloran M., Chen Z.W. Mycobacterium bovis bacille Calmette-Guerin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J. Immunol. 1999;162:2204–2216. [PubMed] [Google Scholar]
- Zoller M., Matz-Rensing K., Kaup F.J. Adenoviral hepatitis in a SIV-infected rhesus monkey (Macaca mulatta) J. Med. Primatol. 2008;37:184–187. doi: 10.1111/j.1600-0684.2008.00295.x. [DOI] [PubMed] [Google Scholar]
- Zuckerman A.J. The new GB hepatitis viruses. Lancet. 1995;345:1453–1454. doi: 10.1016/s0140-6736(95)91032-8. [DOI] [PubMed] [Google Scholar]
- Zuckerman A.J., Thornton A., Howard C.R., Tsiquaye K.N., Jones D.M., Brambell M.R. Hepatitis B outbreak among chimpanzees at the London Zoo. Lancet. 1978;2:652–654. doi: 10.1016/s0140-6736(78)92761-7. [DOI] [PubMed] [Google Scholar]
- zur Hausen H.L., Gissmann A., Mincheva A., Bocker J.F. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med. Microbiol. Immunol. 1979;167:137–153. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]


