Abstract
The Suidae and Tayassuidae live on all continents except Antarctica. True wild boars were indigenous to Europe and Asia and are the ancestors to the domestic pig; with whom they share the same scientific name Sus scrofa. Wild boars have been introduced to the Americas and many islands. Because of the close genetic relationship, in many areas they have interbred with domestic pigs and formed considerable populations of feral suids that represent wild boar and feral pig crosses. Wild suid populations are relatively hardy and most disease research has been focused on their potential as a reservoir for diseases of concern for commercial pig production. The Togian Island babirusa, pygmy hog, Visayan warty pig, Javan warty pig, and Chacoan peccary are endangered. For all species, hunting, habitat loss, and hybridization are important threats to conservation.
Keywords: suidae, tayassuidae, boar, peccary, pig, tuberculosis, African swine fever, disease, pathology
Introduction
The suids, Suidae and Tayassuidae, primarily species of the tropics and subtropics, have a worldwide distribution on all continents except Antartica. The true wild boars were indigenous to Europe and Asia and are the ancestor to the domestic pig with which it shares the same scientific name, Sus scrofa. Wild boars can also survive in temperate climates and have been introduced to the Americas and many islands. Because of the close genetic relationship, in many areas they have interbred with domestic pigs and formed considerable populations of feral suids that represent wild boar and feral pig crosses.
Wild suid populations are relatively hardy and most disease research has been focused on their potential as a reservoir for diseases of concern for commercial pig production. High densities and gregarious behavior increase the chance of disease transmission. Many wild boar populations have overlapping ranges with commercial swine operations, which increases the risk for transmission among these groups. Within the Suidae, the Togian Island babirusa, pygmy hog, Visayan warty pig, and the Javan warty pig are listed as endangered or critically endangered. Within the Tayassuidae, only the Chacoan peccary is endangered. For all suids, hunting, habitat loss, and hybridization are important threats to conservation.
Unique features
Nondomestic suids share many anatomic and clinical pathologic features with domestic pigs. Placentation is diffuse, epitheliochorial and in warthogs the villi are short and blunt. (www.placentation.ucsd.edu). The dental formula is variable (I 1-3/3, C 1/1, P 2-4/2-4, M 3/3) (Sutherland-Smith, 2015). Desert warthogs lack upper incisors. In babirusa, the canine tusks erupt dorsally curving caudally (Fig. 8.1 ). In rare cases, when they do not have the opportunity to grind them down, the tusks can pierce the skin and grow into the skull. Most suids are omnivores and diet varies with local availability, for example, including cactus in the diets of some peccaries. Most suids have a simple stomach similar to the domestic pig. Babirusa has an enlarged stomach with a diverticulum lined by mucus glands for fermentation; reduced fermentation occurs in the large colon. Peccaries have a four-chambered stomach with three nonglandular and one glandular stomach. Peccaries lack a gall bladder (Gottdenker and Bodmer, 2004). Along the dorsum cranial to the tail base, peccaries have prominent scent gland. In Chacoan peccaries, prominent salivary gland papillae have been described (Sutherland-Smith, 2015).
Figure 8.1.

Normal babirusa tusks.
The canine teeth are modified in this species into dorsally erupting curved tusks, which, if not worn through use, can pierce the skin and skull.
(Photo Courtesy of the Wildlife Conservation Society)
Histologically, wild suids share many features with domestic pigs. This includes distinct hepatic lobules bridged by fibrous connective tissue (Fig. 8.2 ) and an “inside-out” lymph node in which follicles are present centrally and surrounded by sinusoids.
Figure 8.2.
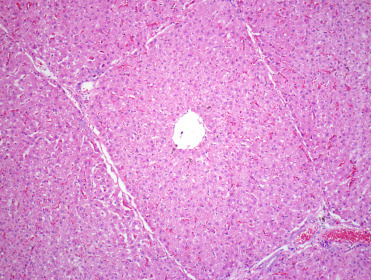
Normal liver from a warthog.
Note the thin fibrous connective tissue septae separating the liver into lobules.
Non-infectious diseases
Nutritional
Obesity is not uncommon in captive suids. It predisposes to arthritis and may negatively impact reproduction (Sutherland-Smith, 2015).
Metabolic
Hyperthermia is not uncommon in both the suidae and tayassuidae, and can be fatal. Beyond elevated environmental temperatures in areas with inadequate ventilation, shipping, anesthesia, and stressful situations can precipitate hyperthermia. Capture and restraint are common causes of mortality, possibly exacerbated by hyperthermia, as is capture myopathy (Batista et al., 2008, Batista et al., 2014, Sutherland-Smith, 2015).
Piglets are sensitive to hypothermia. Unlike other mammals, suids appear to lack brown adipose hindering their ability to thermoregulate when young. Domestic pigs, European wild boars, Bornean bearded pigs, warthogs, and red river hogs all have deletions within the uncoupling protein 1 (UCP1) gene (Berg et al., 2006). UCP1 is expressed exclusively in brown fat and located along the inner membrane of mitochondria where it catalyzes protein leakage that results in release of heat. The European wild boar shivers when cold and builds farrowing nests, which may be behavioral adaptations to more temperate climates.
Gastric ulceration in the glandular stomach is not uncommon, especially during periods of stress.
Congenital/Genetic
Rare congenital anomalies have been described. These include duplication of the pelvis and rear extremities suggestive of ischiopagus, a type of conjoined twin, in Chacoan peccaries (Benirschke et al., 1995), and cyclopia and limb deformities in collared peccaries (Hellgren et al., 1984).
Age-Related/Degenerative
In both captivity and in the wild, nondomestic suids are generally hardy animals and develop common age-related degenerative conditions. Arthritis is most common in joints of the distal appendicular skeleton although intervertebral disc disease also occurs. In captivity, hoof problems are also common. Periodontal disease can also occur and primarily affects the cheek teeth. Studies of wild red river hog and warthog skulls noted an increase in prevalence of periodontal disease with age in red river hogs but not warthogs, presumably due to their more abrasive diet (Woodall, 1989). Polycystic kidney disease has also been reported in peccaries and is presumed to be acquired rather than genetic (Batista et al., 2014). Similar to domestic species, cystic endometrial hyperplasia (Fig. 8.3 ) can develop in nondomestic suids (Thompson et al., 2015). Cyst epithelium lacks vacuoles typical of progesterone stimulation, thus the pathogenesis is presumed similar to that of domestic pigs where cystic endometrial hyperplasia is associated with prolonged estrogen stimulation (endogenous or exogenous).
Figure 8.3.
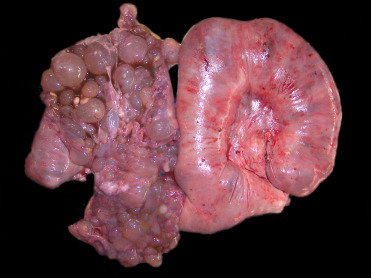
Cystic endometrial hyperplasia in a minipig.
Bilaterally the uterine horns are enlarged and dilated. The left uterine horn is opened and contains numerous, variably sized, cystic structures that expand the endometrium. In suids, this condition is associated with prolonged estrogen stimulation.
Miscellaneous
Both intestinal and gastric volvulus, occur in a variety of suids (Fig. 8.4 ) (Batista et al., 2014). Trichobezoars are somewhat common in captive collared peccaries (Batista et al., 2014). Most cases are incidental findings at necropsy but they may be associated with hyporexia and weight loss. Bezoars occur in adults without apparent sex predilection. They tend to be single, round to stomach-shaped (Fig. 8.5 ), and up to 5−7 cm diameter and 14 cm long and filling most of the stomach’s lumen. Bezoars are composed almost entirely of compacted hair but may contain areas of mineralization. Mild to severe erosions of the gastric mucosa can occur. Low fiber diets may predispose to the formation of bezoars, and excessive grooming due to stress is contributory.
Figure 8.4.
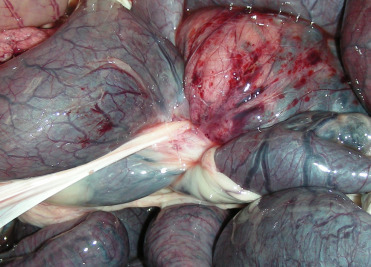
Intestinal volvulus in a red river hog.
Torsion at the root of the mesentery occurs in multiple species of suids. In this case, 360 degree torsion around the cranial mesenteric artery (center of the image) is associated with distension and congestion of the small intestine and multifocal serosal hemorrhage.
Figure 8.5.

Trichobezoars from the stomachs of collared peccaries.
Note that some bezoars take on the shape of the stomach.
Neoplastic
Neoplasms are infrequently described in suids and include lymphosarcoma, intestinal carcinoma, squamous cell carcinoma, uterine adenocarcinoma, uterine leiomyosarcoma, pheochromocytoma, plasmacytomas, hemangiomas, and mammary carcinomas (Batista et al., 2014, Cole et al., 2008, Olinda et al., 2016, Sutherland-Smith, 2015).
Infectious diseases
Much of the research on infectious disease in wild suids is focused on diseases of concern for domestic pigs or potential zoonoses. Wild suids are important reservoirs for maintenance and transmission of several viruses and bacteria of concern to livestock and human health (Meng et al., 2009, Ruiz-Fons, 2017). Supplemental Table e1 contains a list of several infectious diseases to which wild suids may be infected and serve as either a reservoir or part of the multihost disease ecology but for which associated disease has not been described or is mild.
Table e1.
Additional Infectious Diseases of Domestic Suids and Evidence for Infection in Wild Suids
| Pathogen | Disease in Domestic Pig | Wild Host | Disease in Wild | Evidence in Wild Suids |
|---|---|---|---|---|
| DNA Virus | ||||
| Porcine parvovirus | Stillbirth, abortion, fetal death, and loss | Bushpig, Wild boar | None described | Metagenomic (Blomström et al., 2012); Serology (Vengust et al., 2006; Montagnaro et al., 2010) |
| Torque teno sus virus | Uncertain; possibly increases pathogenicity of porcine circovirus | Bushpig, Wild boar | None described | Metagenomic (Blomström et al., 2012); PCR (Martínez et al., 2006) |
| RNA Virus | ||||
| Hepatitis E | Unknown | Wild boar | None described; zoonotic concerns | Serology and PCR (Sonoda et al., 2004; Michitaka et al., 2007; de Deus et al., 2008; Kaba et al., 2010) |
| Transmissable gastroenteritis virus (coronavirus) | Gastroenteritis, diarrhea | Unknown | None described | |
| Porcine respiratory coronavirus | Interstitial pneumonia, mild | Wild boar | None described | Serology (Vengust et al., 2006) |
| Porcine epidemic diarrhea virus (coronavirus) | Gastroenteritis, watery diarrhea, vomiting | Wild boar | None described | Serology (Lee et al., 2016) |
| Porcine reproductive and respiratory syndrome virus (arterivirus) | Subclinical to abortions, stillbirths, and cyanosis of ears and vulva in adults; pneumonia in neonatal pigs | Wild boar | None described | Serology (Montagnaro et al., 2010; Choi et al., 2012); Serology and RT-PCR (Stankevicius et al., 2016) |
| Vesicular stomatitis virus (Rhabdoviridae; vesiculovirus) | Vesicular lesions along the snout and coronary bands | Feral swine, peccaries | None to mild similar to domestic (Dardiri, 1969) | Serology (Fletcher et al., 1985; Stallknecht et al., 1986; Corn et al., 1987; Stallknecht et al., 1993) |
| Bacteria | ||||
| Lawsonia intracellularis | Proliferative enteritis | Wild boar | None described | Serology and/or PCR (Tomanova et al., 2002; Dezorzova-Tomanova et al., 2006; Yeh et al., 2014) |
| Leptospira interrogans (multiple serovars) | Often subclinical; can cause sepsis, nephritis, hepatitis, meningitis, and abortion | Peccaries, Wild boar | None described | Serology (Clark et al., 1983; Corn et al., 1987; Vale-Gonçalves et al., 2015; Zmudzki et al., 2016; Ruiz-Fons, 2017) |
| Enterotoxigenic Escherichia coli(Shiga- toxin and O157 strains) | Neonatal and postweaning diarrhea | Wild boar | None described | Culture (Miko et al., 2009; Wahlstrom et al., 2003; Wacheck et al., 2010; Ruiz-Fons, 2017) |
Blomström, A-L., Stååhl, K., Masembe, C., Okoth, E., Okurut, A.R., Atmnedi, P., Kemp, S., Bishop, R., Belák, S., Berg, M., 2012. Viral metagenomic analysis of bushpigs (Potamochoerus larvatus) in Uganda identified novel variants of Porcine parvovirus 4 and Torque teno sus virus 1 and 2. Virol. J. 9, 192.
Clark, R.K., Jessup, D.A., Hird, D.W., Ruppanner, R., Meyer, M.E., 1983. Serologic survey of California wild hogs for antibodies against selected zoonotic disease agents. J. Am. Vet. Med. Assoc. 183(11), 1248–1251.
Corn, J.L., Lee, R.M., Erickson, G.A., Murphy, C.D., 1987. Serologic survey for evidence of exposure to vesicular stomatitis virus, pseudorabies virus, brucellosis and leptospirosis in collared peccaries from Arizona. J. Wildl. Dis. 23, 551–557.
Choi, E.J., Lee, C.H., Hyun, B.H., Kim, J.J., Lim, S.I., Song, J.Y., Shin, Y.K., 2012. A survey of porcine reproductive and respiratory syndrome among wild boar populations in Korea. J. Vet. Sci. 13, 377–383.
de Deus, N., Peralta, B., Pina, S., Allepuz, A., Mateu, E., Vidal, D., Ruiz-Fons, F., Martín, M., Gortázar, C., Segalés, J., 2008. Epidemiological study of hepatitis E virus infection in European wild boars (Sus scrofa) in Spain. Vet. Microbiol. 129, 163–170.
Dezorzova-Tomanova, K., Smola, J., Trcka, I., Lamka, J., Pavlik, I., 2006. Detection of Lawsonia intracellularis in wild boar and fallow deer bred in one game enclosure in the Czech Republic. J. Vet. Med. Ser. B 53, 42–44.
Fletcher, W.O.; Stallknecht, D.E., Jenney, E.W., 1985. Serologic surveillance for vesicular stomatitis virus on Ossabaw Island, Georgia. J. Wildl. Dis. 21, 100–104.
Kaba, M., Davoust, B., Marié, J-L., Colson, P., 2010. Detection of hepatitis E virus in wild boar (Sus scrofa) livers. Vet. J. 186, 259–261.
Lee, D.U., Kwon, T., Je, S.H., Yoo, S.J., Seo, S.W., Sunwoo, S.Y., Lyoo, Y.S., 2016. Wild boards harboring porcine epidemic diarrhea virus (PEDV) may play an important role as a PEDV reservoir. Vet. Microbiol. 192, 90–94.
Montagnaro, S., Sasso, S., De Martino, L., Longo, M., Iovane, V., Ghiurmino, G., Pisanelli, G., Nava, D., Baldi, L., Pagnini, U., 2010. Prevalence of antibodies to selected viral and bacterial pathogens in wild boar (Sus scrofa) in Campania region, Italy. J. Wildl. Dis. 46, 316–319.
Martínez, L., Kekarainen, T., Sibila, M., Ruiz-Fons, F., Vidal, D., Gortázar, C., Segalés J., 2006. Torque teno virus (TTV) is highly prevalent in the European wild boar (Sus scrofa). Vet. Microbiol. 118, 223–229.
Michitaka, K., Takahashi, K., Furukawa, S., Inoue, G., Hiasa, Y., Horiike, N., Onji, M., Abe, N., Mishiro, S., 2007. Prevalence of hepatitis E virus among wild boar in the Ehime area of western Japan. Hepatol. Res. 37, 214–220.
Miko, A., Pries, K., Haby, S., Steege, K., Albrecht, N., Krause, G., Beutin, L. 2009. Assessment of shiga toxin-producing Escherichia coli isolates from wildlife meat as potential pathogens for humans. Appl. Environ. Microbiol. 6462–6470.
Ruiz Fons, F., 2017. A review of the current status of relevant zoonotic pathogens in wild swine (Sus scrofa) populations: changes modulating the risk of transmission to humans. Transbound. Emerg. Dis. 64, 68–88.
Sonoda, H., Abe, M., Sugimoto, T., Sato, Y., Bando, M., Fukui, E., Mizuo, H., Takahasi, M., Nishizawa, T., Okamoto, H., 2004. Prevalence of hepatitis E virus (HEVV) infection in wild boars and deer and genetic identification of a genotype 3 HEV from a boar in Japan. J. Clin. Microbiol. 42, 5371–5374.
Stallknecht, D.E., Nettles, V.F., Erickson, G.A., Jessup, D.A., 1986. Antibodies to vesicular stomatitis virus in populations of feral swine in the United States. J. Wildl. Dis. 22, 320–325.
Stallknecht, D.E., Kavanaugh, D.M., Corn, J.L., Eernisse, K.A., Comer, J.A., Nettles, V.F., 1993. Feral swine as a potential amplifying host for vesicular stomatitis virus New Jersey serotype on Ossabaw Island, Georgia. J. Wildl. Dis. 29, 377–383.
Stankevicius, A., Buitkuveine, J., Sutkiene, V., Spancerniene, U., Pampariene, I., Pautienius, A., Oberauskas, V., Zilinskas, H., Zymantiene, J., 2016. Detection and molecular characterization of porcine reproductive and respiratory syndrome virus in Lithuanian wild boar populations. Acta Vet. Scand. 58, 51.
Tomanova, K., Bartak, P., Smola, J. 2002. Detection of Lawsonia intracellularis in wild pigs in the Czech Republic. Vet. Rec. 151(25), 765–767.
Vale-Gonçalves, H.M., Cabral, J.A., Faria, M.C., Nunes-Pereira, M., Faria, A.S., Veloso, O., 2015. Prevalence of Leptospira antibodies in wild boars (Sus scrofa) from northern Portugal: risk factor analysis. Epidemiol. Infect. 143, 2126–2130.
Vengust, G., Valencak, Z., Bidovec, A., 2006. A serological survey of selected pathogens in wild boar in Slovenia. J. Vet. Med. Ser. B 53, 24–27.
Wahlstrom, H., Tys, E., Engvall, E.O., Brandstrom, B., Eriksson, E., Morner, T., Vagsholm, I., 2003. Survey of Campylobacter species, VTEC 0157 and Salmonella species in Swedish wildlife. Vet. Rec. 153, 74–80.
Wacheck, S., Fredriksson-Ahomaa, M., Koenig, M., Stolle, A., Stephan, R., 2010. Wild boars as an important reservoir for food-borne pathogens. Foodborne Pathog. Dis. 7(3), 307–312.
Yeh, J.Y., 2014. Seroprevalence of porcine proliferative enteropathy among wild boars in the Republic of Korea. BMC Vet. Res. 10, 5.
Żmudzki, J., Jabłoński, A., Nowak, A., Zębek, S., Arent, Z., Bocian, L., Pejsak, Z., 2016. First overall report of Leptospira infections in wild boars in Poland. Acta Vet. Scand. 58, 3.
DNA Viruses
African swine fever (ASF) is a devastating, OIE listed, notifiable systemic disease in domestic pigs that causes up to 100% morbidity and mortality in naive populations. The disease is caused by a DNA arbovirus (family Asfaviridae) with multiple genotypes from different geographic regions. ASF virus (ASFV) is endemic in most of sub-Saharan Africa and Sardinia, and the virus is spreading through the Caucasus, Eastern Europe, and Baltic countries (Guinat et al., 2016, Sanchez-Vizcaino et al., 2015). ASFV is transmitted by direct contact with infected animals, contaminated fomites, feces, ingestion of contaminated feed, and ticks of the genus Ornithodorus (Guinat et al., 2016, Ravamoana et al., 2010).
Warthogs, bushpigs, and Ornithodorus species of ticks are natural hosts of ASFV. A sylvatic cycle involving wild suids and soft ticks of the Ornithodorus moubata complex has been established in East and Southern Africa, and possibly the Indian Ocean region (Jori et al., 2013). However, the tick/warthog sylvatic cycle is questionable in other regions of Africa where the disease is also endemic (Guinat et al., 2016, Jori and Bastos, 2009, Jori et al., 2013). Warthogs are susceptible to infection but do not develop clinical signs of disease (Kleiboeker and Scoles, 2001). The majority of warthogs are infected during the first few weeks of life and develop a generalized viremia, sufficient to infect ticks that feed on them. The virus then remains in high numbers for a limited period in systemic lymph nodes. Adult warthogs remain infected but remaining viral load is low. Ticks in warthog burrows feed on infected animals and become infected (Jori and Bastos, 2009, Jori et al., 2013). Transmission to domestic pigs in endemic areas is by tick bites; maintenance of ASFV in domestic pigs and pig to pig transmission also occurs (Guinat et al., 2016, Sanchez-Vizcaino et al., 2015).
Bushpigs are also suspected to be natural reservoirs of ASFV, but the role they play in the ecology of ASF is considered of lesser importance than the warthog (Ravamoana et al., 2010, Ravamoana et al., 2011). Natural infection is known to occur without clinical signs, and blood levels in infected bushpigs are high enough to infect both ticks and domestic pigs. However, unlike warthogs, bushpigs do not live in burrows where contact with ticks usually takes place. In Madagascar, where ASF was introduced in the late 1990s and evolved into an enzootic disease, bushpigs were sporadically infected but, to date, do not appear to play a role in maintenance and transmission of ASF (Ravamoana et al., 2010, Ravamoana et al., 2011, Roger et al., 2001). Although the frequency and prevalence of infection is lower than in warthogs, bushpigs should be considered possible reservoirs and a source of transmission of ASFV in endemic countries in east and southern Africa (Jori et al., 2013). Peccaries were not susceptible to ASFV in experimental studies (Dardiri et al., 1969).
The role of other wild pigs in the epidemiology of ASF is often obscure and variable, particularly because of differences between the four wild suid genera, Phacochoerus, Potamochoerus, Hylochoerus and Sus in taxonomy, distribution, ecology, ASF clinical signs, and potential contact with domestic pigs, among other factors (Jori and Bastos, 2009). For instance, though ASF infection in warthogs and bushpigs in endemic regions is asymptomatic, several experimental studies suggest that the Eurasian wild boar is highly susceptible to acute disease similar to domestic pigs. Additional experimental studies demonstrated transmission occurring directly between wild boars and domestic pigs (Gabriel et al., 2011). Furthermore, infection of free-ranging wild boars and deaths caused by ASF has been observed in Russia (Gogin et al., 2013, Guinat et al., 2016). However, it is unclear whether ASFV can be sustained in these wild populations, and persistence of ASF may be self-limiting (Guinat et al., 2016, Lange et al., 2014).
In experimental studies, the Eurasian wild boar suffers peracute disease and 100% mortality after infection with ASFV (Blome et al., 2012, Gabriel et al., 2011). Clinical signs in boar include lethargy, depression, anorexia, diarrhea, respiratory distress, and high fever; less commonly, epistaxsis, and neurological signs may be observed. In experimental studies, boars died within 7–9 days postinfection regardless of age or sex (Blome et al., 2012). Lesions include acute hemorrhagic lymphadenitis, hemorrhagic gastritis, pulmonary edema, splenomegaly, petechiae, and ecchymoses (Figs. 8.6 and 8.7 ). Skin lesions have not, to date, been reported in natural or experimental disease in boar; however, because their skin is dark colored, they are difficult to assess. Domestic pigs with ASF have concurrent thrombocytopenia and leukopenia. The pathogenesis in boar is unknown but in domestic pigs both viral and host factors impact disease development (reviewed in Blome et al., 2013). In general, the monocyte/macrophage lineage is a key viral target, and subsequent production of cytokines, such as TNF-α, IL-1α, IL-1β, and IL-6 are critical to the development of disease. It is the production of these proinflammatory cytokines, rather than direct viral damage, that is associated with clinical signs and activation of endothelial cells resulting in activation of the clotting cascade as well as lymphocyte apoptosis. In more chronic infections, immune complexes may be important in the pathogenesis of vascular damage. Infection needs to be differentiated from classical swine fever infection. PCR for the p72 gene and immunofluorescent antigen detection in tissue smears offer rapid diagnostic options. Sections of tonsil, lymph node, kidney, and/or spleen as well as blood samples are the most useful for diagnosis.
Figure 8.6.

Experimental African swine fever virus (ASFV) infection in a European wild boar.
(A) Splenomegaly; while not pathognomonic, is more common in ASFV than with classical swine fever infections. (B) Renal petechiae can occur in infections with both African swine fever and classical swine fever virus infections and cannot therefore be used as a differentiating feature (see also Fig. 8.10C). (C) Hemorrhagic lymphadenitis.
(Photos Courtesy of the Friedrich Loeffler Institute)
Figure 8.7.
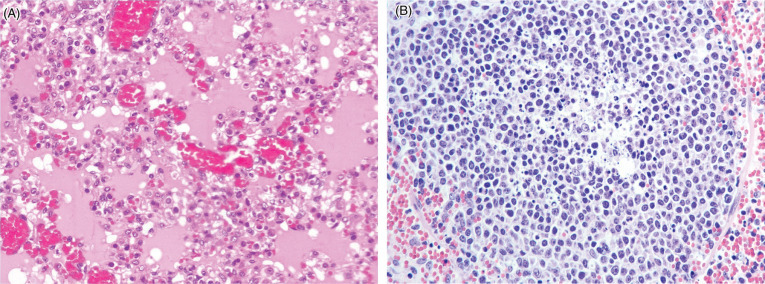
African swine fever virus infection in a domestic pig.
(A) Diffuse pulmonary edema with flooding of alveolar air spaces by edema and foamy macrophages. There is hemorrhage within alveolar septae. (B) Necrosis of lymphocytes within a lymph node with central cellular debris and apoptotic bodies. Surrounding the follicle are extravasated erythrocytes.
(Photos Courtesy of the Department of Compared Anatomy and Anatomic Pathology. Veterinary Faculty of the University of Cordoba, Spain [Archive].)
Suid herpesvirus 1 (SuHV1) (syn. Pseudorabies virus [PrV]) is an Alphaherpesvirus and the causative agent of Aujeszky’s disease or pseudorabies. This disease is OIE listed and notifiable. It has been eradicated from domestic pigs in North America, New Zealand, and some countries in Europe. However, it remains an important disease in domestic pigs in other areas of the world and is a disease for which wild boars are considered reservoir hosts. Seroprevalence in wild boars varies geographically and in some regions viral infection may be endemic (Köppel et al., 2007, Montagnaro et al., 2010, Müller et al., 2011, Ruiz-Fons et al., 2006, Vengust et al., 2006, Župancˇic´ et al., 2002). Rare seropositivity has also been noted in peccaries in the southwestern United States and SuHV-1 has been identified by PCR in postmortem samples from white-lipped and collared peccaries in Brazil (Corn et al., 1987, De Castro et al., 2014). Multiple genotypes are present in wild boar populations, some of which suggest transmission from domestic or feral pigs while others are distinct from strains found in domestic pigs inhabiting the same geographic region. Oral, nasal, and venereal shedding are suggested based on virus isolation studies with higher viral DNA loads in genital swabs (Müller et al., 2011, Romero et al., 1997). Piglets may have both anti-PrV antibodies and virus suggesting vertical transmission (Verin et al., 2014). Taken together, these findings support the importance of venereal transmission in adult boars.
The pathogenesis of PrV infection in wild boars is incomplete as outcome of experimental infection has varied and disease has generally been milder than what is noted in domestic pigs. To what extent this is due to PrV strain factors, host adaptation or earlier maturation of CD8 T cells in wild boars is uncertain (Müller et al., 2011, Page et al., 1992). While young domestic piglets (<7 days) are highly susceptible, high mortality has not been described in similar age wild suids; disease in young wild suids may be underrepresented due to the difficulty in recovering carcasses from very young wild animals. In domestic pigs, primary replication occurs in nasal and oropharyngeal mucosa with viral latency within ganglia. Clinical disease and lesions, when described, appear to be more common and more severe in juvenile boars (4–18 months) than adults (Gortázar et al., 2002, Schulze et al., 2010). Similar to domestic pigs, neurologic signs and lesions are more common in younger boars. Gross lesions in natural outbreaks include lymphadenomegaly and meningeal congestion. Histologically, nonsuppurative encephalitis with neuronal necrosis, lymphoplasmacytic perivascular cuffs and edema have been described (Fig. 8.8 ). Intranuclear inclusions are variably noted but virus can be demonstrated by immunohistochemistry (IHC) and immunofluorescence in sections of tonsil and brain. In adult domestic pigs, respiratory signs are a hallmark of PrV infection but they have not been described in natural infections in boars. Mild sneezing, nasal discharge, and conjunctivitis have been described after experimental infection of wild boar with strains of PrV isolated from wild boar (Müller et al., 2001). However, wild boar treated with immunosuppressant drugs develop severe pleuropneumonia as do wild boar inoculated experimentally with some domestic pig PrV strains. Further research is needed to better understand the pathogenesis of these varying strains in the differing hosts.
Figure 8.8.
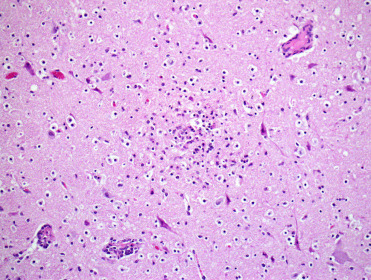
Suid herpesvirus infection encephalitis in a pig.
Necrotizing nonsuppurative encephalitis is associated with neuronal necrosis and perivascular cuffing. Eosinophilic intranuclear inclusions may be present but are generally more easily seen with immunohistochemical labeling.
Porcine circoviruses (PCV) occur as two genotypes, PCV1 which is considered nonpathogenic in domestic pigs and PCV2, the cause of post weaning multisystemic wasting syndrome (PMWS). PCV1 has been detected by PCR and serology in wild boar populations but not associated with disease, similar to domestic pigs (Cságola et al., 2008, Tischer et al., 1986). PCV2 seroprevalence in European wild boar populations is generally high suggesting the virus is endemic (Cano-Manuel et al., 2014, Ruiz-Fons et al., 2006). Some populations have evidence of exposure without evidence of disease (Reiner et al., 2010a), but other populations have had histologic lesions and increased piglet mortality. In some cases, disease has followed confinement of wild animals suggesting potential cofactors in pathogenesis (Ellis et al., 2003). Affected animals are generally young (4–10 m).
Lesions in wild boars are similar to those described in domestic pigs and include emaciation with adipose and skeletal muscle atrophy, lymphoid depletion with sinus histiocytosis in lymph nodes (Fig. 8.9 A) and Peyer’s patches, lymphoplasmacytic interstitial pneumonia, and mild erosive enterocolitis associated with mononuclear inflammatory cell infiltrates (Ellis et al., 2003, Hohloch et al., 2015, Lipej et al., 2007, Schulze et al., 2003, Sofia et al., 2008, Vicente et al., 2004). Characteristic basophilic, botryoid intracytoplasmic and less common intranuclear inclusions are present within macrophages (Fig. 8.9B). Diagnosis can be confirmed by immunohistochemical labeling of PCV antigen in macrophages, lymphatic endothelial cells, and in gut associated lymphoid tissue. PCR is also available, although as the virus appears to be endemic in wild suids in some areas, identification of viral DNA may not imply causality. Furthermore, in some studies, PCV2 DNA could not be detected even in seropositive boars with histologic lesions of PMWS (Hohloch et al., 2015). Other lesions, such as hyperplasia of bronchial-associated lymphoid tissue may be due to secondary infections with Mycoplasma sp. or Pneumocystis sp. (Borba et al., 2011). Populations of boar with higher PCV2 prevalence also have higher prevalence of Mycobacterium bovis infection, and more severe and more generalized disease (Risco et al., 2013, Risco et al., 2014). However, findings in other studies have been less clear suggesting that the interaction between PCV2 infection and potential copathogens may be more complex than simply PCV2 induced immune suppression (Díez-Delgado et al., 2014). In addition to boars, PCV2 has been identified by PCR in tissues from white-lipped and collared peccaries in Brazil but no lesions have been described (De Castro et al., 2014). Collared peccaries lack antibodies to PCV2 (Gerber et al., 2012); however, absence of detectable titers can be noted in domestic pigs as well and in peccaries this could be due to viral or host factors or potential geographic differences.
Figure 8.9.
Porcine circovirus type 2 infection in a lymph node in a pig.
(A) Sinuses contain increased numbers of histiocytes and there is diffuse lymphoid depletion. (B) Intrahistiocytic, basophilic, intracytoplasmic inclusions have a characteristic botryoid appearance.
A novel papillomavirus, Sus scrofa papillomavirus 2 (SsPV2) has been associated with cutaneous fibropapillomas in a wild boar (Link et al., 2017). The distribution of this virus in wild suids is unknown.
RNA Viruses
Classical Swine Fever (CSF), also called hog cholera, is caused by an enveloped RNA virus of the Pestivirus genus (family Flaviviridae) and is OIE notifiable. The natural hosts for CSF virus (CSFV) are members of Suidae. The disease causes major economic losses in domestic pig populations. The Eurasian wild boar is also susceptible and plays an important role in the transmission and epidemiology of the disease in Europe (Artois et al., 2002, Rossi et al., 2005). The wild boar is not a classic reservoir but is capable of maintaining infection over long periods of time (Artois et al., 2002). Wild African Suidae, such as the common warthog and the bushpig have been experimentally infected (Everett et al., 2011, Gers et al., 2011).
Transmission routes are similar in the domestic pig and wild boar. The virus is spread by direct contact between infected animals and indirectly by contact with contaminated food, feces and carcasses. Naïve populations are infected accidentally by indirect or direct contact with infected domestic pigs or wild boars (Moennig, 2015). CSF outbreaks with highly virulent strains of CSFV in small populations of wild boars can be self-limiting (Artois et al., 2002, Moennig, 2015). However, there is concern in Europe about the wild boar becoming a dangerous reservoir in large populations where CSFV tends to become endemic and persist for years (Artois et al., 2002, Moennig, 2015, Penrith et al., 2011). Animal density also plays an important role in maintenance of the virus, since a higher turnover rate increases the number of younger susceptible pigs (Moennig, 2015). A study in France supported the idea that denser populations with more young favored the persistence of CSF. Additionally, populations of wild boar occupying larger forests had a higher incidence of CSF than populations occupying smaller spaces (Rossi et al., 2005).
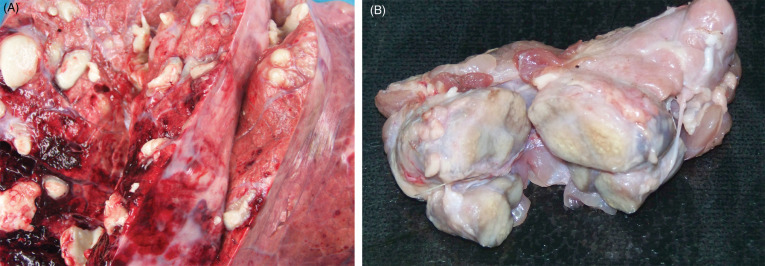
The pathogenesis is not fully understood but involves cytopathic effects of the virus on lymphoreticular cells and macrophages, the vascular endothelium, and epithelial cells. Virus enters through mucous membranes, replicates in the tonsillar epithelium, and spreads to cervical lymph nodes. Acute infections of CSF cause hemorrhagic fever, but subacute, chronic and subclinical forms of the disease also occur depending on virulence of the strain and the immune response of the pig (Penrith et al., 2011). Clinically, animals with acute disease show anorexia, lethargy, fever, and leukopenia (Penrith et al., 2011, Robinson and Robinson, 2015). Hemorrhages in the periphery of lymph nodes and renal petechiae are common findings. Experimental studies in common warthogs and bushpigs demonstrated intra-species transmission (Everett et al., 2011, Gers et al., 2011). Bushpigs developed similar clinical signs and lesions as domestic pigs. Consistent lesions included necrotizing and ulcerative enteritis, suppurative rhinitis and pneumonia (Fig. 8.10 ). Renal petechiae can also be present, similar to ASFV infection (Fig. 8.10c). Histologically, affected animals also had lymphoid necrosis and depletion. Perivascular cuffs in multiple organs were observed in surviving animals. In contrast, infected warthogs may be clinically normal. Histologically, warthogs may have rare or subtle lesions consisting of lymphoplasmacytic infiltrates in various organs and rare perivascular cuffing (Gers et al., 2011).
Figure 8.10.
Experimental classical swine fever virus infection in a European wild boar.
(A) Necrotizing tonsillitis. (B) Necrotizing enterocolitis with button ulcers due to infarction of capillaries. (C) Renal petechiae are similar in gross appearance to those in ASFV infection (see also Fig. 8.6B).
(Photos Courtesy of the Friedrich Loeffler Institute)
Control measures for domestic pigs in the European Union (EU) are based on culling infected or suspicious animals and temporary animal movement restrictions; emergency vaccinations may be employed when uncontrollable spread of CSF is suspected/occurs (Moennig, 2015). In wildlife, detection of infection is more difficult and control measures are focused on reduction of population densities, intensive diagnostics, and good hygiene practice during hunting season. In some parts of Germany, these measures have been supplemented by oral vaccination combined with hunting efforts that target young wild boars (Penrith et al., 2011).
In addition to carnivores, canine distemper virus (CDV) (genus Morbillivus) can cause encephalitis in collared peccaries (Appel et al., 1991). Clinically affected animals can appear blind, reluctant to move and develop myoclonus. Lesions are restricted to the brain with variable neuronal necrosis, astrocytosis, and perivascular cuffs. Intracytoplasmic eosinophilic inclusions are present within neurons. CDV antigen can be demonstrated within neurons by IHC. Serologic surveys suggest peccaries are enzootically infected (Noon et al., 2003) although much is unknown about disease epidemiology. Experimental studies show peccaries as also being susceptible to another morbillivirus, rinderpest virus (RPV) (Dardiri et al., 1969).
Rinderpest virus has caused fatal disease in wild suids. Natural infection has been reported in warthogs, bush pigs (red river hogs), and giant forest hogs in Africa (Anderson et al., 1996), and been experimentally reproduced in peccaries (Dardiri et al., 1969). Lesions include extensive ulceration with overlying diphtheritic membranes in the stomach and over Peyer’s patches in the small intestine and cecum, although they can occur in other areas within the gastrointestinal tract. Congestion is common in the gastrointestinal tract, kidneys, and urinary bladder. Regions of cyanosis or mucosal ulceration may be seen in the skin, tongue, trachea, and larynx. The myocardium may be pale. Rinderpest was eradicated in the wild in 2011 but is still an OIE listed notifiable disease.
Foot and mouth disease (FMD), caused by an Apthovirus, foot and mouth disease virus, is a severe, highly contagious disease of a wide variety of cloven-hoofed species including suids. The disease has been eradicated in some countries and the geographic distribution of disease has decreased; however, it is still present in South America, Africa, Asia and parts of the Middle East and Eastern Europe. There are seven different serotypes but it is unknown whether there are differences in pathogenicity among wild suids. While the virus can survive within the oropharynx in persistently infected hoofstock, viral persistence has not been shown in swine. Some serologic surveys have identified seroconversion in wild boar closely related to outbreaks in livestock while others have failed to show spill-over to wild boar populations (Alexandrov et al., 2013, Elbers et al., 2003). Classic FMD lesions are cutaneous and mucosal vesicles with ulceration. Associated clinical signs depend on site. In suidae, the most common clinical sign is lameness due to vesicles and ulceration along the coronary band between the claws (MacLachlan and Dubovi, 2011). Secondary bacterial infection is common. Vesicles also occur on the snout and to a lesser extent in the mouth. Disease in wild boars appears to be less severe than in domestic pigs based on experimental studies (Breithaupt et al., 2012).
Ebola viruses (EBOV), family Filoviridae, can cause fatal hemorrhagic diseases in humans and nonhuman primates (Chapters 14 and 151415). In 2008, investigation into an unusually severe outbreak of respiratory disease and abortions in domestic pigs suggestive of a pathogenic strain of porcine reproductive and respiratory syndrome virus identified coinfection with Reston EBOV (REBOV) (Barrette et al., 2009). In this initial study, REBOV antigen localized to the capsular surface of lymph nodes while PRRSV was present within germinal centers. Both viruses co-localized to the lung and were associated with interstitial pneumonia and necrotic debris within alveoli. Subsequent studies have found PRRSV and REBOV coinfections in other populations of domestic pigs (Pan et al., 2014). In experimental studies in pigs, Reston EBOV replicates within internal organs and can be shed from the nasopharynx without evident clinical signs (Marsh et al., 2011); experimental infection with Zaire EBOV (ZEBOV) causes severe neutrophilic and histiocytic pneumonia and respiratory distress (Kobinger et al., 2011, Nfon et al., 2013).
Fruit bats are thought to be the natural reservoir for EBOV. However, given the known susceptibility of domestic pigs to EBOV and the sympatric distribution of wild suids in regions where previous EBOV outbreaks have occurred, some have suggested suids as either an incidental host or potentially an important part of EBOV disease ecology. Serosurveys demonstrate wide variation in seroprevalence from 3% to 70% for REBOV (Sayama et al., 2012). REBOV can be transmitted from suids to humans and experimental studies suggest that suids can also transmit ZEBOV to nonhuman primates (Weingartl et al., 2012, Weingartl et al., 2013). Virus neutralization assays to test for ZEBOV have recently been optimized for use in swine (Pickering et al., 2017).
Suids are important hosts for influenza A viruses because they can be infected with both avian and human viruses. Coinfection increases the opportunity for viral reassortment and the development of increased pathogenicity. Confirmed infection is notifiable to OIE. Consistent with this concern is the identification in wild suids of both avian and human influenza virus infections including pandemic H1N1 2009, classical H1N1, H1N2, H3N2 (Biondo et al., 2014, Cano-Manuel et al., 2014, Cho et al., 2015, Foni et al., 2013, Kaden et al., 2008 Perera et al., 2013, Shimoda et al., 2017, Touloudi et al., 2015, Vicente et al., 2002). In one study, H1N2 virus was associated with bronchopneumonia in boars; however, it is unknown if the virus was contributory, as IHC was negative for influenza A and positive for Mycoplasma hyopneumoniae (Biondo et al., 2014).
Bacteria
The Mycobacterium tuberculosis complex (MTC) includes mycobacteria capable of causing tuberculosis (TB) in countless wildlife species including suids. The wild boar is generally considered as a spillover or dead-end host for tuberculosis and similar observations have been noted in other wild suids (Corner et al., 1981, Keuling et al., 2013). Generalized lesions and mortality due to tuberculosis are low and infection usually occurs in wild boar populations in contact with livestock and wild ungulates (Keuling et al., 2013, Naranjo et al., 2008). Recent epidemiological studies show this is true for wild boar in central and northern Europe. However, the scenario differs in the Spanish Mediterranean ecosystems where evidence suggests wild boars are natural reservoirs able to maintain TB infection and transmit disease to other species (Barasona et al., 2016, Martin-Hernando et al., 2007, Naranjo et al., 2008).
Mycobacterium bovis (bTB) and other genotypes of the MTC are prevalent in wild boar in the Spanish Mediterranean region (Barasona et al., 2016, Naranjo et al., 2008). Infection is high in dense populations and juvenile piglets play an important role in disease epidemiology. Studies show that up to one third of juvenile wild boars may become infected during the first months of life and are more likely to develop larger, generalized lesions (Barasona et al., 2016, Martin-Hernando et al., 2007). Additionally, bTB was found to be the cause of death in approximately 30% of the wild boars. The proportion of wild boars with generalized bTB lesions decreased with age as did deaths associated with bTB (Barasona et al., 2016). These results contrast with results obtained in other parts of Europe, where the death rate due to bTB is 3% (Keuling et al., 2013).
In wild boar, bTB lesions can either be localized in one anatomical region or found affecting several organ systems. In one study, head lymph nodes (LNs), particularly the mandibular, were the sites most frequently and consistently affected, often being the only site of disease. Other affected organs include lung, thoracic LNs, liver, spleen, and mammary gland (Martin-Hernando et al., 2007). Both respiratory and food-borne infections seem plausible in this species given the distribution of lesions. In most cases, mycobacteria enter the oral mucosa and disseminates to tonsils and mandibular LNs. Wild boar with extensive lesions and systemic infection are potentially capable of excreting mycobacteria through several routes (Barasona et al., 2016, Martin-Hernando et al., 2007).
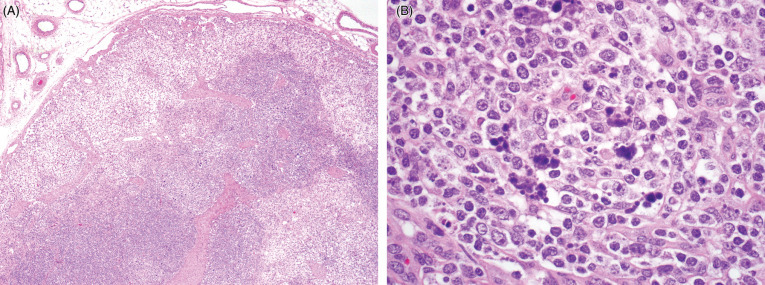
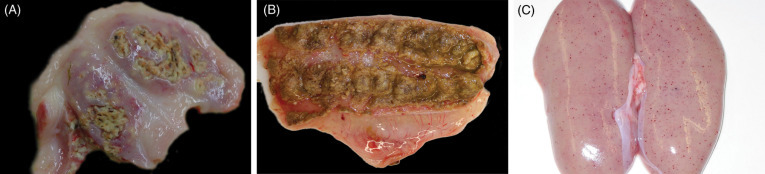
Gross lesions in boar with bTB resemble those of tuberculosis in other species, and consist of focal to multifocal to miliary, variably sized granulomas (Fig. 8.11 ). Microscopically, granulomas are characterized by nodular aggregates of epithelioid macrophages, multinucleated giant cells, surrounded by lymphocytes, plasma cells and macrophages (Fig. 8.12 ). Larger or more chronic granulomas are additionally surrounded by fibrosis and contain central areas of necrosis with multifocal mineralization. Necrosis and mineralization are more extensive in generalized cases of TB, when more than one organ is affected. Acid fast bacilli are consistently found in larger numbers in pulmonary granulomas, in contrast to lymph nodes where intralesional mycobacteria are scant to absent.
Figure 8.11.
Tuberuclosis due to Mycobacterium bovis in a European wild boar.
(A) Multiple pulmonary granulomas are characteristic lesions in tuberculous mycobacterial infections. (B) Granulomatous lymphadenitis.
(Photos Courtesy of José Ángel Barasona García-Arévalo, SUAT-VISAVET, Department of Animal Health of the Complutense University of Madrid)
Figure 8.12.
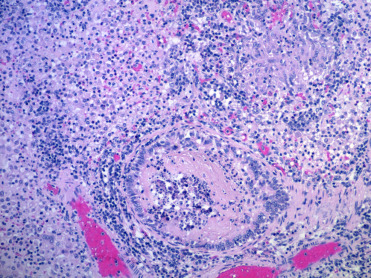
Tuberculosis due to Mycobacterium bovis in a free-ranging warthog.
Granulomatous pneumonia with both more diffuse granulomatous inflammation as well as organizing granulomas.
(Photo Courtesy of E. Mitchell, National Zoological Gardens of South Africa)
Diagnosis of bTB is made by detection and identification of mycobacteria of the MTC in affected organs. Bacterial culture, molecular diagnostics, and enzyme-linked immunosorbent assays (ELISAs) are invaluable tools for diagnosis and detection of exposure (Boadella et al., 2011a, Bollo et al., 2000, Gómez-Laguna et al., 2010). Histochemical detection of acid-fast bacteria or immunohistochemical labeling of intracellular or intralesional mycobacteria within or near granulomas or necrosis, are suggestive of the disease; however, false negative results may occur when bacteria are rare. Additionally, classic and real-time polymerase chain reaction (PCR), with genome reamplification are sensitive, specific, and fast diagnostic tools for TB and are of use in the wild boar (Gómez-Laguna et al., 2010).
Brucellosis in wild swine is typically caused by Brucella suis although B. abortus and B. melitensis are also reported to cause mild disease. B. suis biovar 1 has been detected in populations of collared peccaries in Venezuela and wild boars in Croatia. In these populations, few animals develop disease but the populations are considered an important reservoir for infection. In contrast, biovar 2 is associated with disease outbreaks in European wild boars (Godfroid et al., 2013, Lord and Lord, 1991). Disease in wild swine is similar to that in domestic swine with oral or venereal transmission followed by lymphadenitis and bacteremia. Granulomatous lesions with central coagulative necrosis surrounded by suppurative exudate are typical and can be found in male and female genitalia (Fig. 8.13 ), bone, joints, mammary gland, lymph nodes, spleen, liver, kidney, bladder, and brain. Uterine lesions consist of focal to coalescent/miliary abscesses and granulomas in pregnant and nonpregnant endometrium and placenta. Histologically, uterine glands are dilated and filled with neutrophils and mucus, and there is a loss of endometrium. The fetal placenta is congested and there is multifocal to coalescing hemorrhage, edema, and a mucopurulent exudate with numerous bacteria. Aborted fetuses are usually autolyzed with serosanguinous fluid in body cavities, subcutaneous tissues, and abnormal stomach content. Vertebral lumbar osteomyelitis and fibrinopurulent synovitis are common lesions in juveniles and in chronic disease. Serological cross-reaction has been reported with Yersinia O:9, Escherichia coli O:17, and some serobiovars of Salmonella, which complicates diagnosis based solely on serology (Godfroid et al., 2013, MacMilan, 1999, Schlafer and Foster, 2016).
Figure 8.13.
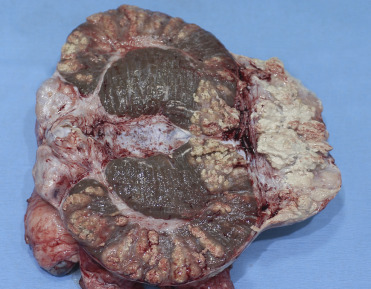
Brucella suis epididymitis in a domestic pig.
The epididymis contains numerous coalescing granulomas and granulomatous inflammation.
(Photo Courtesy of T. LaBranche, University of Georgia)
Erysipelothrix rhusopathiae is a Gram-positive bacillus with a wide geographic distribution and host range. It is the cause of porcine erysipelas and is capable of causing outbreaks in other domestic and wild animals. Pigs are a reservoir of infection, particularly for other pigs, since E. rhusopathiae can persist for long periods in lesions and persists in the tonsils, bone marrow, and intestine of healthy swine (Robinson and Robinson, 2015). Aggregation and high wild boar density can influence spread (Boadella et al., 2011b, Risco et al., 2011, Ruiz-Fons, 2017). Seroprevalence has been detected in wild boars in Japan and coastal Mediterranean countries, such as Greece and Spain; it is also likely to be present in other populations of wild boars (Closa-Sebastia et al., 2011, Marinou et al., 2015, Risco et al., 2011, Shimizu et al., 2016, Vicente et al., 2002).
Porcine erysipelas occurs in swine of all ages but young animals (up to 12 months) and pregnant sows are most susceptible. Disease can be acute, causing septicemia and death. Mild and chronic forms are characterized by skin necrosis, endocarditis, and polyarthritis (Robinson and Robinson, 2015). Gross lesions in acute or chronic disease include multifocal to diffuse petechiae, erythema, and/or cyanosis. Histologically, vasculitis with microthrombi, intralesional bacteria and associated leucocytic infiltrates, mural fibrinoid necrosis, and hemorrhage occur throughout the body but are most remarkable in the kidneys, urinary bladder, and skin (Fig. 8.14 ). Multifocal mononuclear infiltrates may be seen in the kidneys (Risco et al., 2011), and lymphoplasmacytic infiltrates, microthrombi as well as alveolar edema and congestion may be seen in alveolar septae. Corneal edema and panuveitis with fibrinoid vasculitis has also been described. Lesions in wild boar reported by Risco et al. (2011) were similar to those of porcine erysipelas and erysipelas in other species. Septicemia in wild boars due to erysipelas has also been reported in Japan (Yamamoto et al., 1999).
Figure 8.14.
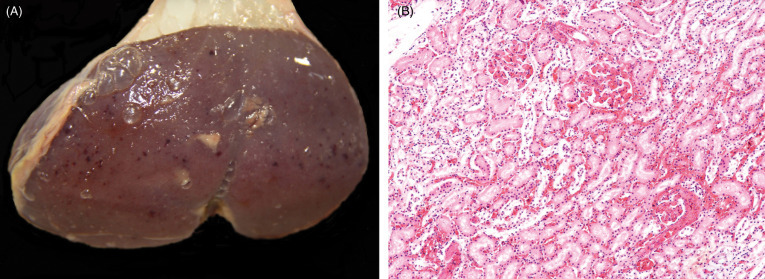
Erysipelothrix rhusopathieae nephritis in a wild boar.
(A) Renal petechiae cannot be differentiated from petechiae due to other causes in suids. (B) Hemorrhage is present in the renal interstitium and in glomeruli. Proximal tubules adjacent to hemorrhage are degenerate and necrotic due to ischemia. Glomerular capillaries contain fibrin thrombi.
(Photos Courtesy of David Risco Perez, INGULADOS S.L.)
Brachyspira are Gram negative, anaerobic spirochetes, adapted to occupy specialized nitches in the large intestines of animals, including swine. Two important entities in domestic swine are caused by Brachyspira hyodisenteriae and B. pilosicoli, known as swine dysentery (bloody scours) and porcine intestinal spirochetosis, respectively (Hampson, 2012). B. hyodisenteriae is a highly hemolytic bacterium, capable of causing episodes of mucohemorrhagic diarrhea with a serious impact in morbidity and mortality, and thus the economy of domestic swine herds. Information about Brachyspira infection in wild boars or in other wild suid genera is limited. Several studies in free-ranging wild boar in Sweden and Spain failed to demonstrate Brachyspira in fecal samples, which seemed to indicate that these spirochetes are not a natural part of the intestinal flora (Jacobson et al., 2005, Osorio et al., 2013, Vadillo et al., 2017). However, in one study in Spain that included 40 wild boars from several intensive growth farms, B. intermedia, B. pulli, and B. hyodysenteriae were isolated and associated with clinical disease in 3 animals (Vadillo et al., 2017). In addition to documenting infection, this study also supported the hypothesis that weakly hemolytic species, such as of B. intermedia and B. pulli have pathogenic potential in nondomestic suids. Details of the lesions in wild boar were not specified. Several species of Brachyspira, including a highly hemolytic strain of B. hyodysenteriae, have also been identified in feral pigs in Western Australia (information on associated disease or lesions has not been described) (Phillips et al., 2009).
Mycoplasma hyopneumoniae is the cause of mycoplasmal pneumonia, also known as enzootic pneumonia, of swine. M. hyopneumoniae is a small bacterium that lacks a cell wall and adheres to the ciliated epithelium of large airways and to a lesser extent, the bronchioles. Most mycoplasmas contain superantigens in their cell membranes that induce polyclonal lymphoid proliferation and characteristic lymphoid aggregates adjacent to airways (Caswell and Williams, 2016). Seroprevalence of M. hyopneumoniae has been reported in wild boars in several parts of Europe, such as Switzerland, Greece, and Spain (Chiari et al., 2013, Kuhnert and Overesch, 2014, Kuhnert et al., 2011, Sibila et al., 2010, Vengust et al., 2006). Most of these studies have not associated serology with disease. However, in one comprehensive study on the prevalence of M. hyopneumoniae in wild boars in Switzerland, mild to moderate lymphoplasmacytic and histiocytic bronchointerstitial pneumonia, primarily affecting the cranioventral region of the lung, was identified (Fig. 8.15 A) (Batista Linhares et al., 2015). Lesions were consistent with the acute and chronic stages of the disease described in domestic pigs. These include acute disease with multilobular, patchy red to dark red areas affecting less than 50% of the lungs versus more chronic lesions in which a higher percentage of the pulmonary parenchyma is affected by dark red to gray, atelectatic areas. Histologically, infected boars had hyperplastic bronchial associated lymphoid tissue (BALT) with reactive lymphoid follicles, interstitial infiltrates of plasma cells; lymphocytes and histiocytes, alveolar and bronchial neutrophilic infiltrates, and alveolar edema were also, but less commonly, seen (Fig. 8.15B). The amount of inflammation, number of neutrophils, and hyperplastic BALT increased with the chronicity. Juvenile and young adult boars are more susceptible to infection, which corresponds to serological reports of wild boars in Italy, and also to the age range of susceptibility in the domestic pig. While wild boars are frequently viewed as the reservoir for disease in domestic pigs, in this study it was instead more likely for wild boars to become infected by contact with domestic pigs. Based on this, the role of wild boars as reservoirs for M. hyopneumoniae transmission to domestic pigs is considered minor.
Figure 8.15.
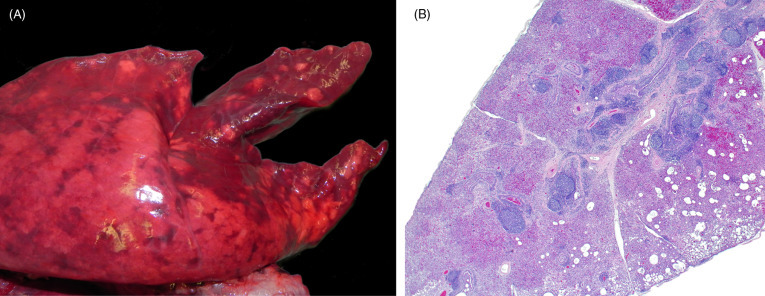
Mycoplasma hyopneumoniae infection (enzootic pneumonia) in the lungs of a wild boar.
(A) Infection causes bronchopneumonia with consolidation that often has a cranioventral pattern. Affected parenchyma will be firm, often discolored dark red, and demarcation between affected and less or unaffected adjacent tissue may be apparent. (B) Infection is airway oriented and regionally, the airways and the alveolar interstitium contain numerous inflammatory cells. Bronchial associated lymphoid tissue is hyperplastic. With chronicity, the severity of infection increases.
(Photos Courtesy of the Centre for Fish and Wildlife Health, University of Bern)
Several species of Chlamydophila have been reported both serologically and histologically in wild boars (Cano-Manuel et al., 2014, Hotzel et al., 2004); however, its significance as a pathogen is uncertain. In one study C. psittaci was the most prevalent species; C. abortus and C. suis were also identified but less commonly. Chlamydial inclusions in the lung were present in smooth muscle cells around bronchioli, the endothelium and interstitium of alveolar walls as well as the myometrium of the uterus (Hotzel et al., 2004). The significance of chlamydial infection in wild boars is currently uncertain.
Clostridia are large, spore forming, strictly anaerobic to oxygen-tolerant anaerobic bacteria. Clostridium perfringens types A and C, and C. difficile are the principal enteric clostridial pathogens of domestic swine. All subtypes of C. perfringens can be commensals in the intestines of many animal species; therefore, diagnosis must be made by detecting the toxin in association with consistent lesions and in the absence of other potential pathogens.
C. perfringens type A is part of the normal intestinal flora in pigs but can cause enteric disease in neonatal and occasionally weaned pigs. The pathogenesis of C. perfringens type A in pigs is poorly understood but it is likely to be multifactorial. C. perfringens is a known cause of enteritis in a variety of wild animal species and has been reported in wild suidae, such as wild boar and pygmy hogs (Das et al., 2008, Glenn Songer and Uzal, 2005, Li et al., 2017, Shome et al., 2010). In an outbreak occurring in wild boar in China, C. perfringens type A and C were isolated by serological and molecular assays, and found to be the cause of death. Gross lesions consisted of extensive hemorrhage in lungs, intestine, and inguinal lymph nodes (Li et al., 2017). Hemorrhagic enteritis associated with C. perfringens type A has also been reported in a research and breeding center for pygmy hog in Assam, India. Lesions were similar to those reported in the domestic pig (Shome et al., 2010). Hemorrhagic and necrotizing enteritis with diffuse small and large intestinal edema occurred in another adult, female pygmy hog at the Assam State Zoo, in Assam, India. Molecular assays confirmed C. perfringens type A infection (Das et al., 2008). C. perfringens type C occurs worldwide and causes necrotizing and hemorrhagic enteritis that is often fatal in domestic swine. Hallmark histologic lesions are deep mucosal necrosis and hemorrhage with emphysema in small intestine that sometimes also affects the colon. It is a primary pathogen and usually causes more severe disease in young animals.
C. difficile is a cause of enteritis in neonatal pigs that usually develops between 1 and 7 days of age. Hallmark lesions include edema of the mesocolon, suppurative inflammation of the colonic lamina propria, and mesenteric lymph node edema (Glenn Songer and Uzal, 2005). C. difficile has been isolated from feral pig feces in areas with intensive commercial swine production in Canada, and feral pigs could be reservoirs (Thakur et al., 2011).
Wild suids are important reservoirs for Salmonella species and infection is reported worldwide, particularly in wild boars and pygmy hogs. Wild boars may be asymptomatic carriers and maintenance reservoirs in areas with sufficient boar density (Cano-Manuel et al., 2014, McGregor et al., 2015). Transmission occurs directly or indirectly by contact with contaminated water, food, feces, or other infected individuals. Several acute, fatal outbreaks have occurred in both adult and young (<1 year of age) pygmy hogs (Rahman et al., 2001). Only adults showed clinical signs, which included generalized loss of body condition, anorexia, high fever, loose feces that contained mucus and blood, weakness, and tremors. All animals had necrohemorrhagic enterocolitis with multifocal, discrete areas of necrosis in the cecum and colon. Additionally, the liver, epicardium, and pericardium contained areas of hemorrhage, and the spleen was enlarged. Cerebral congestion was also a consistent feature. Enterotoxigenic Salmonella Enteritidis (Salmonella enterica enteritidis) that carried fimbrial genes for colonization of enterocytes was isolated in other pygmy hog outbreaks, Salmonella typhimurium has been isolated (Rahman et al., 2005).
Haemophilus parasuis, the cause of Glasser’s disease or porcine polyserositis and arthritis syndrome, causes fibrinosuppurative inflammation of one to several serosal surfaces and joints; it may also cause pneumonia or meningoencephalitis (Craig et al., 2015). Antibodies to H. parasuis have been reported in wild boars in Slovenia, Spain, and Germany (Olvera et al., 2007, Reiner et al., 2010b, Vengust et al., 2006), but information on disease development in this species or other wild suids is limited. Fatal infections have been reported in free-ranging wild boars in central Spain. Clinical signs included respiratory distress and weakness; however, there was only one documented fatality suggesting a low mortality rate (Cuesta Gerveno et al., 2013). The one young boar that died had a fibrinous bursitis, interstitial bronchopneumonia, interstitial nephritis with hemorrhage, and mild meningitis. H. parasuis was cultured and confirmed with molecular methods. The polyserositis and fibrinous serosal exudates usually associated with infection in domestic pigs were lacking in the boar (Craig et al., 2015, Cuesta Gerveno et al., 2013).
Pasteurella multocida is a Gram-negative bacillus with five known serovars, A, B, C, D, and E. Coinfection with toxin producing strains of P. multocida and other bacterial pathogens are the cause of progressive atrophic rhinitis (PAR) in domestic pigs and sporadic outbreaks of septicemia can occur. The disease usually affects 6–12 week-old pigs and causes sneezing, nasal discharge, unilateral epixtaxis, and nasal deformity (Caswell and Williams, 2016). Serovars A and D are known causes of pneumonia in weaner and finisher pigs, but are rarely primary pathogens. Predisposing factors, such as environmental conditions or coinfection with Mycoplasma hyopneumoniae, porcine herpesvirus 1, or other respiratory pathogens are considered necessary for disease development in domestic pigs (Caswell and Williams, 2016, Register et al., 2012). Serologic evidence of P. multocida infection without disease has been reported in peccaries (Martins Gomes de Castro et al., 2014). However, both PAR and systemic pasteurellosis have been reported in wild boars (Kaden et al., 2001, Risco et al., 2013). In cases of systemic pasteurellosis, boars were found dead with epistaxis but had no clinical signs prior death. Gross lesions consisted of subcutaneous edema in the ventral region of the neck, generalized congestion, and fibrin within the peritoneal cavity. Histologically, multiple organs were congested and multifocal hemorrhage and thrombosis were seen (Fig. 8.16 ).
Figure 8.16.
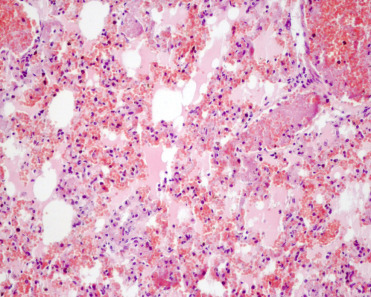
Systemic pasteurellosis in a wild boar.
Several capillaries are ocluded by fibrin thrombi. Alveolar septae are hemorrhagic and capillaries contain fibrin, neutrophils, and numerous erythrocytes. Alveolar spaces or filled with edema fluid.
(Photo Courtesy of David Risco Perez, INGULADOS S.L.)
Streptococcus suis is an important bacterial pathogen in swine that has worldwide distribution. Infection causes a variety of diseases including sepsis, meningitis, polyarthritis, bronchopneumonia, and endocarditis. Over 30 serotypes are recognized, but serotypes 1 and 2 are generally of greater significance in domestic pigs. Serotype 1 infects suckling pigs and is not pathogenic for other species, while serotype 2 causes disease in weaner and feeder pigs and humans. Serotype 7 can also be zoonotic (Craig et al., 2015). Feral swine and wild boars are known carriers of several S. suis serotypes, some of which are potentially zoonotic (Baroch et al., 2015, Baums et al., 2007, Sánchez del Rey et al., 2014, Seol et al., 1998, Risco et al., 2015). Several cases of S. suis infection in hunters in contact with wild boars have occurred (Baums et al., 2007). However, not much is known about the clinical and pathological significance of infection in wild boars. A single case of fatal S. suis serotype 2 infection was reported in a wild boar in Spain. The animal was young (approximately 2 weeks old) and had suppurative bronchopneumonia and meningitis with hemorrhage (Fig. 8.17 ), which is similar to lesions that are described in domestic pigs (Risco et al., 2015). Other organs, such as the kidneys had congested vessels, microthrombi, and hemorrhages, consistent with septicemia.
Figure 8.17.
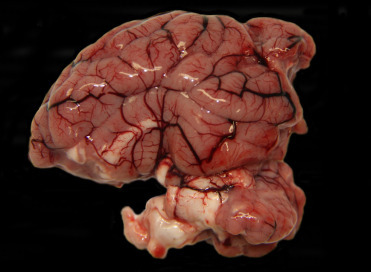
Streptococcus suis in a wild boar.
Acute meningitis and hemorrhage with meningeal vascular congestion.
(Photo Courtesy of David Risco Perez, INGULADOS S.L.)
Fungi
Coccidioides immitis is a dimorphic fungus that is endemic to the southwestern United States (US), Northern Mexico, and some countries in Central and South America. Coccidioidomycosis has been reported in collared peccaries but, despite the species rooting behavior and the range overlap with the agent, few exposed individuals develop clinical signs and lesions. Nodular to milliary pyogranulomatous or granulomatous pneumonia is most common, but lesions may be multisystemic and involve regional lymph nodes. Microscopically, necrotic foci contain fungal spherules in different stages of maturation and are surrounded by pyogranulomatous or granulomatous inflammation that matures into organized granulomas or pyogranulomas. Intralesional basophilic, 10–80 μm diameter, round to oval and double contour walled spherules with multiple, round to oval, 2–5 μm, basophilic, PAS and GMS positive endospores supports the diagnosis. Infection has also caused a neurological-like disorder (incoordination, muscle tremors, and convulsive seizures) in the absence of gross or microscopic brain lesions in Texan Peccaries (Fowler, 1996, Lochmiller et al., 1985). High antibody titers may be suggestive of active disease. Mature sporangia may be up to 200 μm diameter, a feature that is helpful in differentiating them from blastomycosis and histoplasmosis (both are smaller fungi) (Caswell and Williams, 2016).
Metazoa
A wide variety of parasites have been described in free-ranging suids. Many of these are important pathogens of domestic swine but appear to be asymptomatic in wild suids. Supplemental Table e2 contains additional parasites known to infect wild suids.
Table e2.
Additional Parasites of Suids
| Pathogen | Disease in Domestic Pig | Wild Host | Disease in Wild | Evidence in Wild Suids |
|---|---|---|---|---|
| Protozoan Parasites | ||||
| Microsporidia | ||||
| Enterocytozoon bieneusi | Infection rates are highly variable in domestic pigs. Infection is typically subclinical. Zoonotic risk | Wild boar, warthogs, Visayan warty pigs | Subclinical, zoonotic risk. | Fecal PCR (Nemejc et al., 2014; Slodkowicz-Kowalska et al., 2007). |
| Encephalitozoon cuniculi | Infection rates are highly variable in domestic pigs. Infection is typically subclinical. | Wild boar | Subclinical. | Fecal PCR. (Nemejc et al., 2014; Slodkowicz-Kowalska et al., 2007). |
| Encephalitozoon intestinalis | Rarely identified in domestic pigs. Infection is typically subclinical. | Wild boar | Subclinical. | Fecal microscopy, fecal PCR (Nemejc et al., 2014; Slodkowicz-Kowalska et al., 2007). |
| Neospora caninum | Natural disease not reported in domestic pigs. | Wild boar, warthogs | Disease not reported | Serology. (ELISA, IFAT) (Buxton et al., 2002; Donahoe et al., 2015; Reiterova et al., 2016). |
| Sarcosporidia | ||||
| Sarcocystis dubeyella | Infrequently reported in domestic pigs. Typically, subclinical. White-tan parasitic cysts up to 12 mm in length and 1 mm wide within skeletal and cardiac muscle. Cyst encapsulated by host cell membrane. | Warthogs | Subclinical. Similar to domestic pigs. *Ddx Cysticercus cellulosae/Taenia solium. | Histopathology (Junker et al., 2015; Stolte et al., 1998). |
| Sarcocystis phacochoeri | Not reported in domestic pigs. | Warthogs | Subclinical. White-tan parasitic cysts up to 1–4 mm length 0.2 mm width in skeletal and cardiac muscle. Not encapsulated by host cell membrane. *Ddx Cysticercus cellulosae/Taenia solium. | Histopathology (Junker et al., 2015; Stolte et al., 1998). |
| Sarcocystis spp. | Common in domestic pigs. Generally nonpathogenic. Parasitic cysts within skeletal and cardiac muscle. DDx for cysicercosis. | Wild boar | Parasitic cysts within the right ventricle. | Histopathology (Comeaux et al., 2016). |
| Piroplasms | ||||
| Anaplasma phagocytophilum | Low infection rate in domestic pigs. Infection is typically subclinical, Potential zoonosis. | Wild boar | Disease not reported. | Serology, DNA PCR (Reiterova et al., 2016; Silaghi et al., 2014). |
| Babesia bigemina | Regionally common in domestic pigs. Infection results in hemolytic anemia, petechial hemorrhages, edema. Disease is generally mild. | Wild boar | Disease not reported. | DNA PCR (Zanet et al., 2014). |
| Babesia trautmanni | Uncommon in domestic pigs. Most frequently occurs in Africa. Infection results in pyrexia, inappetence, listlessness, followed by spontaneous recovery. | Warthogs, bushpigs | Similar to domestic pigs. | Walker et al. (2005) |
| Eperythrozoon sp. (likely Mycoplasma sp) | Regionally common in domestic pigs. Mycoplasma suis (E. suis) causes potentially hemolytic anemia in naïve animals. Gross lesions include splenomegaly and icterus. Parasites identified on the surface of erythrocytes. Arthropod vector. | Wild boar (rare), South American peccaries (captive) | Parasite species not identified. Subclinical disease. Possible risk to livestock. | Hemocytology (Castellano Margardio and Mangini, 2001; Hannon et al., 1985). |
| Theileria sp. | Clinical disease not reported in domestic pigs. Arthropod vector. | Warthog, Wild boar | Clinical disease not reported. Risk to livestock. | Hemocytology (Fowler, 1996; Junker et al., 2015; Zanet et al., 2014). |
| Coccidia | ||||
| Eimeria spp. | E. scabra, E. spinose, E. perminuta, E. cerdonis, E debliecki, and E neodebliecki cause self-limiting diarrhea in 1–3 month-old pigs. Direct life cycle. Gross and microscopic lesions similar to I. suis. | All suids susceptible | Infrequently reported. E. pecari, E. chaparralensis, and E. dicotylensis are only reported in collared peccaries. | Fecal microscopy (oocysts), histopathology (Fowler, 1996; Wilber et al., 1996). |
| Isospora (Cystisospora) suis | Severe, occasionally fatal enteritis in neonatal domestic pigs. Direct life cycle. Gross lesions include white-yellow diarrhea. Microscopic ileal and jejunal lesions include villus atrophy, ulcerative to fibrinonecrotic enteritis with intraepithelial coccida. | All suids susceptible, reported in wild boar, warthogs, peccaries. | Infrequently reported. Similar to domestic pigs. | Fecal microscopy (oocysts), histopathology (Fowler, 1996). |
| Ciliates | ||||
| Balantidium coli | Ubiquitous in domestic pigs (definitive host). Direct fecal-oral life cycle. Ingestion of cyst, excyst in small intestine, trophozoites in cecum, colon. Resident in lumen, may be invasive into mucosa. Invasive disease results in necrotizing and ulcerative typhlocolitis with intralesional trophozoites. Zoonotic. | Wild boar, warthogs, peccaries, likely all suids. | All suids susceptible. Episodic diarrhea, disease similar to domestic pigs. | Fecal microscopy. Cysts and trophozoites (Castellano Margardio and Mangini, 2001; Mansouri et al., 2016; Samuel and Low, 1970; Solaymani-Mohammadi et al., 2004). |
| Flagellates | ||||
| Chilomastix mesnili (hominis; suis) | Primarily a commensal of primates, also infects domestic pigs. Infects cecum and colon, generally nonpathogenic. Pyriform flagellates in feces, increased in number with other protozoal infections. Direct fecal-oral life cycle. | Wild boar | Disease not reported. | Fecal microscopy (Solaymani-Mohammadi et al., 2004). |
| Dientamoeba fragilis | Infects domestic pigs, disease not reported. Zoonotic, causes diarrhea in humans. Direct fecal-oral life cycle. | Wild boar | Disease not reported. | Fecal analysis; PCR (Crotti et al., 2012). |
| Giardia sp. | Ubiquitous in domestic pigs, all ages may be infected. Direct fecal-oral life cycle. May be associated with mild diarrhea, no specific gross or microscopic lesions. | Wild boar, peccaries | Similar to domestic pigs. | Fecal microscopy. Cysts and trophozoites (Rodriguez-Rivera et al., 2016). |
| Trypanosoma pecarii | Not reported. | Collared peccaries | Disease not reported. | Hemocytology (Castellano Margardio and Mangini, 2001). |
| Tritrichomonas suis (T. foetus) | Commensal in nasal cavity and lower digestive tract in domestic pigs. Incidental tissue invasion may occur secondary to infection with other pathogens (e.g., Brachyspira pilosicoli colitis) | Wild boar | Disease not reported. | Fecal microscopy (Solaymani-Mohammadi et al., 2004). |
| Amoebae | ||||
| Iodamoeba butschlii | Generally nonpathogenic in domestic pigs. May be associated with diarrhea. Zoonotic. | Wild boar | Nonpathogenic | Fecal microscopy (Solaymani-Mohammadi et al., 2004). |
| Entamoeba polecki | Generally nonpathogenic in domestic pigs. Some isolates have been associated with diarrhea. Zoonotic. | Wild boar | Nonpathogenic | Fecal microscopy (Solaymani-Mohammadi et al., 2004). |
| Entamoeba suis | Generally nonpathogenic in domestic pigs. Rare isolates may be associated with diarrhea and hemorrhagic colitis. Zoonotic. | Wild boar | Not reported. | Fecal microscopy (Solaymani-Mohammadi et al., 2004). |
| Metazoan Parasites | ||||
| Nematodes | ||||
| Upper GI | ||||
| Diplogaster parasiticus (taxonomy unresolved) | Not reported. | Bushpig (Potamochoerus sp.) | Poorly described rhabditid nematodes colonizing buccal pouch. Lesions not reported. | Necropsy (Marlow, 1955; Round, 1968). |
| Eucoleus (Capillaria) garfiai | Infest lingual epithelium. Generally nonpathogenic. | Wild boar | Similar to domestic pigs. | Necropsy (Fernandez-de-Mera et al., 2003). |
| Gonglyonema pulchrum, G. spp. | Infest esophageal mucosa. Mild esophagitis. Zoonotic. | Wild boar, collared peccaries | Similar to domestic pigs. | Necropsy (Fernandez-de-Mera et al., 2003). |
| Stomach | ||||
| Capillaria sp. (not C. hepatica or C. (Eucoleus) garfiai) | Rarely reported. | Wild boar | Gastritis, not otherwise described. | Necropsy (Lowenstein and Kutzer, 1989). |
| Gnathostoma hispidum, G. spp. | Gastric spiruid, nodular, and ulcerative gastritis. Freshwater crustacean (e.g., cyclops) intermediate host. | Wild boar, warthogs, bushpigs (Potamochoerus sp.) | Similar to domestic pigs. | Necropsy (Belem, 2012; Round, 1968). |
| Parabronema peccariae | Not reported. | Peccaries | Parasite ID only, disease not reported. | Necropsy (Samson and Donaldson, 1968). |
| Physocephalus sexalatus | Primarily infests lumen, generally noninvasive. Often subclinical, heavy infestation associated with catarrhal gastritis, anemia, and melena. Dung beetle intermediate host. | Wild boar, warthogs, bushpigs (Potamochoerus sp.), peccaries | Similar to domestic pigs. | Necropsy (Fernandez-de-Mera et al., 2003; Horak et al., 1988; Junker et al., 2015; Round, 1968; Samuel and Low, 1970; Van Wyk and Boomker, 2011). |
| Parostertagia heterospiculum | Rarely reported, poorly described. Disease similar to Hyostrongylus rubidus, less severe. | Peccaries | Parasite ID only, disease not reported. | Necropsy (Samuel and Low, 1970). |
| Simondsia paradoxa | Chronic gastritis. Occurs concurrently with Hyostrongylus rubidus (see text), disease is similar, but less severe. | Wild boar, warthogs | Similar to pigs | Fecal microscopy, necropsy (Belem, 2012; Fernandez-de-Mera et al., 2003). |
| Trichostrongylus falcatulus, T. instabilis, T. thomasi, T. deflexus | Nodular gastritis. Infrequently reported. Direct life cycle. Rarely found in small intestine. | Wild boar, warthogs, peccaries, bushpigs (Potamochoerus sp.) | Similar to domestic pigs. | Necropsy (Boomker et al., 1991; Junker et al., 2015; Round, 1968; Samson and Donaldson, 1968). |
| Small intestine | ||||
| Ascaris phacochoeri | Not reported. | Warthogs, bushpigs (Potamochoerus sp.) | Similar to A. suum. | Fecal examination, necropsy (Belem, 2012; Horak et al., 1988; Junker et al., 2015). |
| Capillaria sp. (not C. hepatica or C. (Eucoleus) garfiai) | Rarely reported. Disease not described. | Wild boar, Pygmy hogs | Enteritis (pygmy hogs) | Small intestine mucosal scraping. Microscopy (Kakati et al., 2015). |
| Cooperia hungi | Not reported. | Warthogs | Infestation rarely reported, primarily a parasite of impala. | Necropsy (Van Wyk and Boomker, 2011). |
| Texicospirura turki | Not reported. | Collared peccaries; | Nematode species originally erroneously described in stomach of collared peccaries, Disease not described. | Necropsy (Fowler and Cubas, 2001; Samson and Donaldson, 1968; Samuel and Low, 1970). |
| Cecum, colon | ||||
| Morgascaridia sellsi, M. kugii | Not reported. | Wild boar, bushpigs (Potamochoerus sp.) | Rare. Clinical disease not reported. | Necropsy (Round, 1968; Sato et al., 2008). |
| Osesophagostomum dentatum, O. aethiopicum; O. eurycephalum, O. goodeyi, O. mocambiquei, O. mpwapwae, O. mwanzae, O. oldi, O. roubaudi, O. santosdiasi, O. simpsoni, O. yorkei | Chronic colitis, with nodular to diffuse thickening and mixed inflammation in the colon wall. Ova hatch in large intestine, migrate into submucosa, return to mucosa. Deep penetration to serosa associated adhesions. | Wild boar, warthogs, bushpigs (O. aethiopicum) | Similar to domestic pigs. | Fecal examination, necropsy, histopathology (Belem, 2012; Horak et al., 1988; Junker et al., 2015; Round, 1968; Van Wyk and Boomker, 2011). |
| Morgascaridia sellsi, M. kugii | Not reported. | Wild boar, bushpigs (Potamochoerus sp.) | Rare. Clinical disease not reported. | Necropsy (Round, 1968; Sato et al., 2008). |
| Murshidia hamata, M. pugnicaudata | Uncommon. Primarily infest the colon. Clinical disease not reported. | Warthogs | Similar to domestic pigs. May be present in large numbers in free-ranging warthogs, but clinical disease not reported. | Necropsy (Horak et al., 1988). |
| Probstmayria vivipara | Small equine pinworm. Infests colon, rectum. Clinical disease not reported. | Warthogs | High prevalence and heavy burdens in free-ranging warthogs. Clinical disease not reported. | Necropsy (Belem, 2012; Junker et al., 2015; Van Wyk and Boomker, 2011). |
| Trichuris suis | Whipworms primarily infest the cecum, large burdens extend to colon. Primarily disease of young animals. Parasites embedded in mucosa, cause catarrhal enteritis, may progress to necrotizing and hemorrhagic enteritis. Direct life cycle. | Wild boar, Warthogs, all suids likely susceptible | Similar to domestic pigs. | Necropsy (Fowler, 1996). |
| Lungs | ||||
| Metastrongylus elongatus (apri), M. pudendotectus, M salmi | Indirect, possibly direct life cycle. Earthworm intermediate host. Gross lesions include thread-like 4–7 cm long adult nematodes within airways, severe pulmonary congestion, edema, and consolidation. Larvae may migrate through liver. | Wild boar, all suids likely susceptible. | Similar to domestic pigs. Secondary bacterial pneumonia. | Necropsy, histopathology (Fowler, 1996; Mansouri et al., 2016). |
| Liver | ||||
| Capillaria hepatica (Calodium heptaicum) | Chronic-active eosinophilic hepatitis. Zoonotic. | Collared peccaries | Similar to domestic pigs. | Castellano Margardio and Mangini, (2001) |
| Kidneys | ||||
| Stephanurus dentatus | Kidney worm of domestic swine. Direct life cycle. Eggs passed in the urine and infection by ingestion of larvae or percutaneous larval invasion. Earthworm paratenic host. Larval migration to the liver via the portal system causes a chronic hepatitis, and subsequent peritoneal migration may cause peritonitis. Aberrant migration is common, and larvae may be found in the visceral adipose tissue, lungs, epaxial muscles, or spinal cord. Adults are typically found in the kidneys, ureters, and perirenal tissue, and are often enclosed in a fibrous capsule. | Wild boar. | Similar to domestic pigs. | Necropsy (Kaufmann, 2014; Sutherland-Smith, 2015). |
| Other | ||||
| Dirofilaria acutiuscula | Not reported. | Collared peccaries | Filariae in subcutaneous dorsolumbar fascia. Disease limited to localized inflammation. | Necropsy (Samuel and Low, 1970). |
| Impalaia tuberculate | Primarily infests African antelope, accidental infection in domestic pigs. Disease not reported. | Warthogs | Disease not reported. | Necropsy (Junker et al., 2015; Van Wyk and Boomker, 2011). |
| Parabronema pecariae | Not reported. | Peccaries | Adult spiruids within the mucosa of the stomach. Mild gastritis. | Necropsy (Samuel and Low, 1970; Vicente et al., 2000). |
| Setariaspp. (S. congolensis, S. bernardi, S. thomasi, S. castroi) | Domestic pigs (S. congolensis). Mosquito vector. Adults in peritoneal cavity, microfilariae in microvasculature may cause ocular and neurological lesions. | Warthogs, bushpigs | Some species, microfilariae free in abdominal cavity. Incidental; not associated with disease. | Necropsy (Junker et al., 2015; Khin and Win, 1981; Round, 1968; Van Wyk and Boomker, 2011). |
| Cestodes (larva) | ||||
| Echinococcus granulosus | Larvae in lungs, other viscera. Canids are definitive hosts. | Wild boar, bush pigs (red river hogs) | Similar to domestic species, zoonotic risk | Histopathology, DNA PCR (Horak et al., 1988; Mansouri et al., 2016; Van Wyk and Boomker, 2011). |
| Moniezia benedeni, M. mettami, M. spp. | Uncommon (M. expansa). Incidental adults in small intestine. | Warthogs, Collared peccaries (Moniezia benedeni) | Adults in anterior small intestine. Disease not reported. | Necropsy (Boomker et al., 1991; Junker et al., 2015; Round, 1968; Samuel and Low, 1970). |
| Paramonezia phaochoeri | Not reported. | Warthogs | Adults in small intestine. | Necropsy (Van Wyk and Boomker, 2011). |
| Stilesia globipunctata | Adults in small intestine. Uncommon, generally nonpathogenic. | Warthogs | Similar to domestic pigs. | Necropsy (Belem, 2012). |
| Taenia acinonyxi | Visceral cysticercosis. Cheetahs are definitive hosts. | Warthogs, wild boar | Similar to domestic pigs. | Necropsy (Sutherland-Smith, 2015). |
| Taenia crocutae | Visceral cysticercosis. Hyenas are definitive hosts. | Warthogs | Similar to domestic pigs. | Necropsy (Sutherland-Smith, 2015). |
| Taenia hyaenae | Visceral cysticercosis. Hyenas are definitive hosts. | Warthogs | Similar to domestic pigs. | Necropsy (Sutherland-Smith, 2015). |
| Taenia hydatigena (Cysticercus tenuicolis) | Visceral cysticercosis. Many canids and felids are definitive hosts. | Wild boar | Cysticerci in mesentery, lungs | Necropsy (Mansouri et al., 2016). |
| Taenia regis | Visceral cysticercosis. Lions are definitive hosts. | Warthogs | Similar to domestic pigs. | Necropsy (Sutherland-Smith, 2015). |
|
Taenia solium (Cysticerus cellulosae) |
Cysticerci in the central nervous system (brain, meninges), heart, skeletal muscle, and liver. | Wild boar | cysticerci isolated from muscle and heart. Zoonotic risk. | Histopathology, DNA PCR (Mansouri et al., 2016). |
| Trematodes | ||||
| Alaria alata | Rare. Mesocercaria in cheek, skeletal muscle, not associated with clinical disease. Indirect life cycle. Carnivore definitive host. Gastropod 1st intermediate host. Amphibian 2nd intermediate host. | Wild boar | Similar to domestic pigs. Zoonotic risk. | Necropsy; alaria migration technique (Paulsen et al., 2013). |
| Dicrocoelium dendriticum | Indirect life cycle. Gastropod 1st intermediate host, ant 2nd intermediate host. Infection by ingestion of ants. Lesions similar to Fasciola hepatica. | Wild boar | Similar to domestic pigs. | Necropsy. (Mansouri et al., 2016). |
| Gastrodiscoides hominis (Gastrodiscus aegyptiacus) | Infest cecum, colon. May result in chronic necrotizing and eosinophilic and lymphoplasmacytic typhlocolitis with goblet cell. Associated with severe cecal edema and catarrhal typhlitis/diarrhea. Pig definitive host. Metacercaria on aquatic plants and in intermediate host crayfish, tadpoles, frogs. Zoonotic. | Wild boar, warthogs, bushpigs (Potamochoerus sp.) | Similar to domestic pigs. Zoonotic risk. | Fecal analysis, necropsy (Round, 1968; Yu and Mott, 1994). |
| Fasciola gigantica | Lifecycle and lesions similar to F. hepatica. More common in Africa, Asia. | Wild boar | Similar to domestic pigs. | Necropsy (Mansouri et al., 2016). |
| Fasciola hepatica | Indirect life cycle. Gastropod intermediate host, infective metacercaria on aquatic plants. Larval migration to liver and bile ducts results in necrotizing hepatitis, and maturation in bile ducts results in chronic cholangiohepatitis, portal fibrosis. Larval trematodes may be present in parenchymal lesions, adults present within bile ducts. More common in Europe, North and South America. | Wild boar (esp. Nebrodi Black pigs) | Similar to domestic pigs. | Necropsy (Thompson et al., 2009). |
| Fascioloides magna | Life cycle and lesions similar to Fasciola hepatica, but do not invade bile ducts. Adults are encapsulated in liver parenchyma. | Wild boar, Collared peccaries | Similar to domestic pigs. | Necropsy (Samuel and Low, 1970). |
| Ornithobilharzia turkestanicum | Larval schistosomes migrate through liver, adults in mesenteric veins. Eggs deposited in walls of mesenteric vessels provoke intense inflammation and fibrosis, may have nodular lesions on gross examination. Chronic eosinophilic and granulomatous phlebitis and mesenteritis centered on ova. Egg migration into liver may result in cirrhosis. Indirect life cycle. Gastropod intermediate host. | Wild boar | Adults in mesenteric veins. Disease similar to domestic pigs. | Necropsy (Mansouri et al., 2016; Maxie and Robinson, 2007). |
| Paragonimus westermani | Uncommon. Larvae in skeletal muscle not associated with clinical disease. Canid definitive host (lung). Gastropod 1st intermediate host. Crustacean (crab, crayfish) 2nd intermediate host. Zoonotic. | Wild boar (paratenic host) | Similar to domestic pigs. Zoonotic risk. | Histology. Larvae in muscle tissue digestion, PCR (Sugiyama et al., 2015). |
| Pentastomes | ||||
| Linguatula nuttalli | Not described in domestic pigs. Lion, other carnivores are definitive hosts. | Warthog | Nymphs recovered at necropsy, tissue not identified (common in liver in African antelope) | Necropsy (Horak et al., 1988; Junker et al., 2015). |
| Arthropod Ectoparasites | ||||
| Mites | ||||
| See text | ||||
| Fleas | ||||
| Ctenocephalides felis | “Cat flea” | Collared peccaries | Clinical disease not reported. | Parasite identification (Gruver and Guthrie, 1996). |
| Echidnophaga larina | Not reported. | Warthog (may be host-specific) | Stick-tight fleas, favor ventrum. Clinical disease not reported. | Parasite identification (Horak et al., 1988; Matthee et al., 2013). |
| Moeopsylla sjoestedti | Not reported. | Warthog (may be host-specific) | Jumping fleas, favor head and neck. Clinical disease not reported. | Parasite identification (Horak et al., 1988; Matthee et al., 2013). |
| Phacopsylla (Echidnophaga) inexpectata, P. spp. | Not reported. | Warthog (may be host-specific) | Stick-tight fleas, favor ventrum. Clinical disease not reported. | Parasite identification (Horak et al., 1988; Junker et al., 2015; Matthee et al., 2013). |
| Pulex porcinus | “Javalina flea,” or “Peccary flea.” | Collared peccaries, wild boar | Clinical disease not reported. | Parasite identification (Samuel and Low, 1970). |
| Ticks | ||||
| Ixodidae | ||||
| Amblyomma americanum | “Lone star tick.” 3-host life cycle, with a wide host range in all lifestages | Wild boar (USA) | Clinical disease not reported. | Parasite identification (Allan et al., 2001; Helcel et al., 2016). |
| Amblyomma boeri | Not reported in domestic pigs. 1-host life cycle. | Chacoan peccaries | All life stages of tick present on peccaries, may be host-specific | Parasite identification (Nava et al., 2009). |
| Amblyomma cajenesse | “Cayenne tick.” 3-host life cycle with a wide host range in all life stages. | All peccaries, wild boar (USA) | Opportunistic | Parasite identification (Fowler and Cubas, 2001; Helcel et al., 2016). |
| Amblyomma hebraeum | “South African Bont Tick,” 3-host life cycle. Immature and adult ticks may infest livestock. Vector for Ehrlichia ruminantium infection in livestock (but not suids) and Rickettsia africae infection in humans Prefer ventrum, axillae, tail. | African warthogs (primary host), “bushpigs” (red river hogs) | Opportunistic is common, tick-bite wounds may be severe and are predilection sites for secondary bacterial dermatitis and old-world screwworm infection. | Horak et al. (1988); Horak et al. (1991); Levin (2016); Matthee et al. (2013) |
| Amblyomma inornatum | Not reported in domestic pigs. Life cycle incompletely characterized, likely 3-host life cycle. | Collared peccary | Clinical disease not reported. | Parasite identification (Gladney et al., 1977). |
| Amblyomma maculatum | “Gulf coast tick.” 3-host life cycle, adults on medium and large animals | Wild boar (USA) | Tick-bite wounds may be severe, with secondary bacterial dermatitis and new-world screwworm infection. | Parasite identification (Allan et al., 2001; Helcel et al., 2016; Levin, 2016). |
| Amblyomma neumanni | Not described in domestic pigs. 1-host life cycle. Primarily a parasite of cattle. | All peccaries | Clinical disease not reported. | Parasite identification (Nava et al., 2009). |
| Amblyomma parvum | Not reported in domestic pigs. Life cycle incompletely characterized, likely 3-host life cycle. | All peccaries, wild boar | Clinical disease not reported. | Parasite identification (Nava et al., 2009; Ramos Vdo et al., 2014). |
| Amblyomma paulopunctatum | Not reported in domestic pigs. Life cycle incompletely characterized, likely a 1-host life cycle. | Red river hogs, wild boar, warthogs, Giant forest hog (Hylochoerus sp.) | Primarily found in Africa. Clinical disease not reported. All suids likely susceptible. | Parasite identification (Keirans, 1985; Leslie and Huffman, 2015). |
| Amblyomma sculptum | Domestic pigs are primary host. Can maintain a 1-host life cycle in livestock. Disease not reported, vector for multiple rickettsial pathogens. | Wild boar (South America) | Nymphs and adults on wild boar. Disease not reported. | Parasite identification (Ramos Vdo et al., 2014). |
| Amblyomma triguttatum | “Ornate kangaroo tick.” Not reported in domestic pigs. 1-host life cycle, broad host range. | Wild boar (Australia) | Nymphs infest the ears of wild boar, other life stages not identified. | Parasite identification (Guglielmone, 1990). |
| Dermacentor albipictus | “Winter tick.” 1-host life cycle prefers large animals | Wild boar (USA), | Clinical disease not reported. | Parasite identification (Helcel et al., 2016). |
| Dermacentor halli | Not reported in domestic pigs. Life cycle incompletely characterized. | Peccaries, Wild boar (USA) | Clinical disease not reported. | Parasite identification (Helcel et al., 2016). |
| Dermacentor variabilis | “American dog tick.” 3-host life cycle, adults prefer medium-large animals | Wild boar (USA) | Common in Southern USA, disease not described. | Parasite identification (Allan et al., 2001; Helcel et al., 2016). |
| Haemaphysalis leporis-palustris | Wide distribution in North America. 3-host life cycle. Disease Primarily parasitizes lagomorphs and birds. not reported, vector for rickettsial agents. | Peccaries, likely all suidae susceptible due to broad host range. | Clinical disease not reported. | Samuel and Low (1970) |
| Haemaphysalis longicornis | Disease in domestic pigs not reported. 3-host life cycle. Vector for rickettsial agents, other piroplasms. | Wild boar (Korea, Asia) | Clinical disease not reported. | Chae et al. (2017) |
| Haemaphysalis flava | Rarely reported in domestic pigs. 3-host life cycle. | Wild boar (Korea, Asia) | Clinical disease not reported. | Chae et al. (2017) |
| Haemaphysalis parmata | Bushpigs | Parasite identification (Horak et al., 1991). | ||
| Ixodes cumulatimpunctatus | Not reported in domestic pigs. Life cycle incompletely characterized. | Red river hogs | Clinical disease not reported. | Parasite identification (Leslie and Huffman, 2015). |
| Ixodes nipponensis | Not reported in domestic pigs, primarily parasitizes cattle, hares. 3-host life cycle. | Wild boar (Korea) | Clinical disease not reported. | Parasite identification (Chae et al., 2017). |
| Ixodes scapularis | “Black-legged tick,” or “Deer tick.” 3-host tick. Uncommon in domestic pigs. Vector for Borrelia burgdorferi and multiple Ehrlichia spp., Babesia spp. others. | Wild boar (USA) | Common in Southern USA, disease not described. | Parasite identification (Allan et al., 2001; Helcel et al., 2016). |
| Rhipicephalus appendiculatus | Poorly described in domestic pigs, primarily parasitizes cattle. 3-host tick. Prefers to infest head and hears, may cause severe skin lesion around ears. | Warthogs, “bushpigs” (red river hogs) | Clinical disease not reported. | Parasite identification (Horak et al., 1988; Horak et al., 1991; Matthee et al., 2013; Walker et al., 2005). |
| Rhipicephalus camicasi | Not described in domestic pigs. Life cycle incompletely characterized. Disease not reported. | Warthogs | Clinical disease not reported. | Parasite identification (Walker et al., 2005). |
| Rhipicephalus complantus | Regionally common in African domestic pigs. Disease not reported. Life cycle incompletely characterized. | Wild boar, red river hogs, bushpigs (P. larvatus), warthogs | Clinical disease not reported. | Parasite identification (Leslie and Huffman, 2015; Pourrut et al., 2011; Walker et al., 2005). |
| Rhipicephalus compositus | Poorly described in domestic pigs. Disease not reported. Life cycle incompletely characterized. | “Bushpigs” (red river hogs) | Parasite identification (Walker et al., 2005). | |
| Rhipicephalus congolensis | Not described in domestic pigs. | Red river hogs | Clinical disease not reported. | Parasite identification (Apanaskevich et al., 2013). |
| Rhipicephalus cuspidatus | Not described in domestic pigs. Life cycle incompletely characterized. Disease not reported. | Red river hogs, warthogs | Clinical disease not reported. | Parasite identification (Leslie and Huffman, 2015; Walker et al., 2005). |
| Rhipicephalus (Boophilus) decoloratus | “African blue tick.” Infrequently reported in domestic pigs, primarily parasitizes African cattle and antelope. 1-host life cycle. Vector for Babesia bigemina and multiple other piroplasms. | Warthogs | Clinical disease not reported. | Parasite identification (Horak et al., 1988; Horak et al., 2007; Matthee et al., 2013; Walker et al., 2005). |
| Rhipicephalus evertsi | Not reported in domestic pigs. 2-host life cycle. | Warthogs, “bushpigs” (red river hogs) | Clinical disease not reported. | Parasite identification (Horak et al., 1988; Horak et al., 2007; Matthee et al., 2013). |
| Rhipicephalus follis | Not described in domestic pigs. Life cycle incompletely characterized. Disease not reported. | “Bushpigs” (red river hogs) | Clinical disease not reported. | Parasite identification (Horak et al., 1991; Walker et al., 2005). |
| Rhipicephalus gertrudae | Not described in domestic pigs. Life cycle incompletely characterized. Disease not reported. | Warthogs | Clinical disease not reported. | Parasite identification (Matthee et al., 2013). |
| Rhipicephalus longiceps | Poorly described in domestic pigs. 3-host life cycle. Primarily parasitizes African ruminants, but uncommon. | Warthogs | Clinical disease not reported. | Parasite identification (Horak et al., 2007; Matthee et al., 2013; Walker et al., 2005). |
| Rhipicephalus longus | Regionally common in African domestic pigs. Disease not reported. Life cycle incompletely characterized. | Wild boar, “bushpigs” (red river hogs) | Clinical disease not reported. | Parasite identification (Leslie and Huffman, 2015; Sugiyama et al., 2015; Walker et al., 2005). |
| Rhipicephalus lunulatus | Uncommon in domestic pigs. More common in cattle, African antelope. Disease not reported. 3-host life cycle. | Wild boar, “bushpigs” (red river hogs) | Clinical disease not reported. | Parasite identification (Leslie and Huffman, 2015; Walker et al., 2005). |
| Rhipicephalus maculatus | Poorly described in domestic pigs. | “Bushpigs” (red river hogs) | Clinical disease not reported. | Parasite identification (Horak et al., 1991; Walker et al., 2005). |
| Rhipicephalus muehlensi | Not described in domestic pigs. Life cycle incompletely characterized. Disease not reported. | “Bushpigs” (red river hogs) | Clinical disease not reported. | Parasite identification (Horak et al., 1991; Walker et al., 2005). |
| Rhipicephalus muhsamae | Poorly described in domestic pigs. | Red river hogs | Clinical disease not reported. | Parasite identification (Leslie and Huffman, 2015). |
| Rhipicephalus simpsoni | Not described in domestic pigs. Life cycle incompletely characterized. Disease not reported. | Red river hogs | Clinical disease not reported. | Parasite identification (Leslie and Huffman, 2015). |
| Rhipicephalus simus | Infrequently reported in domestic pigs. 3-host life cycle. Clinical disease not reported. Vector for transmission of Babesia trautmani from warthogs to domestic pigs. | Warthogs, “bushpigs” (red river hogs) | Clinical disease not reported. | Parasite identification (Horak et al., 1991; Horak et al., 2007; Matthee et al., 2013; Walker et al., 2005). |
| Rhipicephalus zambeziensis | Poorly described in domestic pigs, primarily parasitizes African cattle. 3-host tick. | Warthogs, “bushpigs” (red river hogs) | Clinical disease not reported. Likely to cause skin lesion in/around ears similar to domestic cattle. | Parasite identification (Horak et al., 1988; Matthee et al., 2013; Walker et al., 2005). |
| Rhipicephalus (aurantiacus) ziemanni | Poorly described in domestic pigs, life cycle incompletely characterized. Disease not reported. | Red river hogs | Clinical disease not reported. | Parasite identification (Leslie and Huffman, 2015; Pourrut et al., 2011). |
| Rhipicephalus zumpti (reichnowi; planus) | Not described in domestic pigs. Life cycle incompletely characterized. Disease not reported. | “Bushpigs” (red river hogs), warthogs | Clinical disease not reported. | Parasite identification (Horak et al., 1991; Walker et al., 2005). |
| Argasidae | ||||
| Hyalomma truncatum | Rarely reported in domestic pigs. 2-host life cycle. Typically parasitizes African ruminants. | Warthogs | Clinical disease not reported. | Parasite identification (Horak et al., 1988; Horak et al., 2007; Matthee et al., 2013). |
| Ornithodoros moubata | Infrequently described in African domestic pigs, but may serve as a significant vector for transmission of African swine fever from wildlife to livestock. Multihost life cycle. Also vector for Borellia duttoni, the cause of relapsing fever in humans. | Warthogs | Clinical disease not reported. Zoonotic risk. Vector for Borellia duttoni, the cause of relapsing fever in humans. | Parasite identification (Sanchez-Vizcaino et al., 2015). |
|
Ornithodoros porcinus (part of O. mubata spp. complex) |
Infrequently described in domestic pigs. Multihost life cycle. Vector for African swine fever, predominantly found in Africa. | Warthogs, bushpigs (Potamochoerus larvatus), red river hogs | Clinical disease not reported. | Parasite identification (Horak et al., 1988; Matthee et al., 2013; Roger et al., 2001; Sanchez-Vizcaino et al., 2015). |
| Ornithodoros erraticus | Vector for African swine fever, predominantly found in Europe. but role for ASF transmission unclear in many European countries, | Wild boar | Clinical disease not reported. | Parasite identification (Pietschmann et al., 2016; Sanchez-Vizcaino et al., 2015). |
|
Ornithodoros turicata |
Feed on wild boar exposed to animal burrows. Short feeding time (20–30 min), do not remain on animals. Vector for transmission of Borrelia turicatae, a cause of relapsing fever in humans. | Wild boar (USA) | Feed on wild boar exposed to animal burrows. Short feeding time (20–30 min), do not remain on animals. Vector for transmission of relapsing fever in humans. | |
| Orthinodoros rostratus | Wild boar (South America) | Clinical disease not reported. | Parasite identification (Ramos Vdo et al., 2014). | |
| Lice | ||||
| Hematopinus apri | Sucking louse. Uncommon in domestic pigs, common in wild boar. | Wild boar (Europe, Asia) | Clinical disease not reported. | Manning and Graham (1997) |
| Hematopinus latus | Sucking louse. Uncommonly reported in domestic pigs. | Wild boar, warthogs, bushpigs (Potamochoerus larvatus), red river hogs | Clinical disease not reported. | Parasite identification (Horak et al., 1988, 1991; Junker et al., 2015). |
| Haematopinus oliveri | Sucking louse. Not reported in domestic pigs. | Pygmy hogs (possibly host restricted) | Clinical disease not reported. | Parasite identification (Mishra and Singh, 1978). |
| Hematopinus phacochoeri | Not reported in domestic pigs. | Wild boar, warthogs | Sucking louse. | Parasite identification (Horak et al., 1988; Junker et al., 2015; Matthee et al., 2013). |
| Hematopinus suis | “Common sucking louse.” | Wild boar (worldwide) | Parasite identification (Manning and Graham, 1997). | |
|
Linognathida spp. |
Not reported in domestic pigs. | Collared peccaries | Clinical disease not reported. | Gruver and Guthrie (1996) |
|
Linognathus vituli |
Not reported in domestic pigs. Primarily parasitizes cattle. | Wild boar (Europe) | Clinical disease not reported. | Manning and Graham (1997) |
| Pecaroecus javalii | “Giant sucking louse.” Not reported in domestic pigs. | Collared peccaries | Collected from periocular skin. Clinical disease not reported. | Parasite identification (Samson and Donaldson, 1968; Samuel and Low, 1970). |
| Flies/bots | ||||
| Rhinoestrus spp. | Infrequently reported in domestic pigs. Myiasis sinusitis caused by developing larvae. | Red river hogs, warthogs | Chronic catharral and eosinophilic and lymphoplasmacytic rhinitis and sinusitis. | Parasite identification (Leslie and Huffman, 2015). |
Gastric metazoa include Hyostrongylus rubidus, a trichostrongylid nematode that infects wild boars and warthogs, and Ascarops strongylina, a spirurid nematode that infects wild boar and pygmy hogs (Belem, 2012, Fernandez-de-Mera et al., 2003, Kakati et al., 2015, Mansouri et al., 2016). The life cycle for both is indirect and they utilize an arthropod intermediate host (dung beetle for Ascarops). Hyostrongylus burrows into the stomach wall causing ulceration and gastric nodules with increased mucus production while Ascarops causes a catarrhal gastritis. Hemorrhage due to infection with either parasite may cause anemia, anorexia, and melena. Diagnosis is by identification of eggs in feces or examination of mucosal scrapings at necropsy for immature larvae.
The most important small intestinal parasite is Ascaris suum, which has been identified in wild boars and collared peccaries (Fernandez-de-Mera et al., 2003, Mansouri et al., 2016). Outbreaks have been reported in captive peccaries with clinical signs of respiratory distress and coughing. This nematode parasite has a direct life cycle. Pigs are infected by ingesting eggs with second stage larvae or infected paratenic hosts (e.g., earthworms). The larvae migrate to the liver via the portal circulation, molt into third stage larvae that then travel via the venous system to the lungs where they are coughed up, swallowed, and mature into adults. Visceral migration of larvae can be associated with mucosal necrosis, hemorrhage, healing fibrosis, and lymphoplasmacytic inflammation (white areas called “milkspots”); heavy larval infection in the lungs is associated with pulmonary edema and acute necrosuppurative pneumonia. Diagnosis is by identification of eggs or worms in feces or by the presence of larvae within the lungs.
Globocephalus sp. are globally distributed hookworms. G. urosubulinatus is common in domestic pigs in the western hemisphere and Europe; it has also been reported in free-ranging feral populations of wild boars and peccaries. G. versteri more commonly occurs in African bushpigs, and G. samoensis and G. longemucronatus are more common in wild boars in southeast Asia and Japan (Fernandez-de-Mera et al., 2003, Seguel and Gottdenker, 2017, Van Wyk and Boomker, 2011). The life cycle is direct. Following larval ingestion or percutaneous infection, migration can be extensive and cause myocardial and or pulmonary inflammation before returning to the gastrointestinal tract. The adults are hematophagus and utilize specialized mouthparts to adhere to and cause ulceration of the small intestinal mucosa resulting in enteritis and blood loss. Diagnosis is by the identification of eggs in the feces by flotation.
Strongyloides ransomi infects the intestine of wild boars and is pathogenic in suckling piglets. Vertical transmission occurs during suckling after which the parasites tunnel into base of small intestinal villi resulting in blood loss, anemia, and diarrhea in piglets. Infection in adults is asymptomatic. Diagnosis is most commonly made by identification of eggs in feces collected from the rectum, mucosal scrapings at necropsy, or recovery of immature worms using the Baermann technique.
Macrocanthorhyncus hirudinaceus, the thorny-headed worm, infects the ileum and caudal small intestine of wild boar (Fernandez-de-Mera et al., 2003, Mansouri et al., 2016, Sutherland-Smith, 2015). The life cycle of this nematode parasite is indirect with arthropod intermediate hosts. While eggs are passed in the feces, diagnosis via floatation is difficult because the eggs do not float. Infection may cause multifocal chronic granulomatous enteritis with the formation of intramural granulomas that can be identified along the serosal surface. Nodules rarely perforate.
Onchocerciasis dermatitis and lymphadenitis has been described in free-ranging suidae in many countries; however, descriptions are largely limited to parasite morphology without description of gross or microscopic lesions (Uni et al., 2001, Uni et al., 2015). Transmission is typically mechanical via arthropod vectors including Simulium flies. In O. dewittei japonica infection of Japanese wild boars, adult filarial worms are encysted in fibrous subcutaneous nodules in the distal limbs and the fat bodes of the foot pads. Microfilariae are more commonly found in the skin of the ears, back, tail, and less frequently on limbs. In contrast, O. tacaokai microfilariae and filarial adults infest the skin of ears, neck, and back; these adult parasites are not associated with cutaneous nodules. Coinfection with O. dewittei japonica and O. tacaokai has been reported in Japanese wild boars. In O. ramachandrini infection of African warthogs, microfilariae are present in skin of the neck, back, and feet, and filarial adults are found coiled in the subcutis of the distal limbs. Diagnosis is by identification of larvae or adults in skin sections. Larvae will emerge from sections of skin placed into saline.
Trichinellosis is OIE listed, reportable disease with worldwide distribution. While several species of Trichinella may infect wild suids, T. spiralis and T. pseudospiralis are most commonly reported (Nöckler et al., 2006, Zamora et al., 2015). No gross lesions and only mild histologic inflammation has been noted in infected wild boars, and infection is of importance because of the zoonotic potential. Real-time PCR of muscle tissue is a sensitive diagnostic tool (Guenther et al., 2008).
Protozoa
Trypanosomiasis is recognized as a clinically significant vector-borne, protozoal disease of humans and livestock, with distinct agents and disease entities recognized in Africa and Central and South America. Subclinical infection may be regionally common in free-ranging Suidae, and warthogs, bushpigs, red river hogs, giant forest hogs, peccaries, and wild boars are important in the epidemiology due to the preference of insect vectors to feed on them. These suid species are reservoirs for trypanosomes pathogenic to humans and domestic animals including T. congolense, T. simiae, T. suis, T. evansi, and T. brucei. Warthogs are probably the main wild reservoirs of T. simiae, which causes acute fulminating infection in domestic pigs in Africa; peccaries are important in the maintenance of T. cruzi and T. evansi in Brazil; and red river hogs are important in the epidemiology of human sleeping sickness in Africa caused by T. brucei (Claxton et al., 1992, Herrera et al., 2008, Leak, 1999, Pollock, 1982).
Suids generally do not develop severe disease as a result of Trypanosome infection but they can maintain parasitemia for a long period of time (Claxton et al., 1992). In white lipped peccaries, T. evansi causes severe anemia, weight loss, and immunosuppression that predisposes to opportunistic infections. In juvenile peccaries, disseminated intravascular coagulation develops secondary to the deposition of immune complexes in the heart, liver, brain and kidneys (Herrera et al., 2008). Encephalitis due to T. suis infection has been reported in some species (Leak, 1999). Disease severity in suids is variable with T. brucei and T. congolense typically only causing mild disease, and T. suis and T. simiae often causing more clinically significant disease in suids (Pollock, 1982). Presence of sarcocysts in the myocardium with or without mild myositis and inflammation can occur with T. cruzi (Comeaux et al., 2016). Intralesional trypanosomes, hemorrhages and extravascular hemolysis may be seen in some organs. The splenic parenchyma may contain large numbers of histiocytes with marked periarteriolar sheath lymphoid hyperplasia, lymphoid depletion, and/or hemosiderosis due to extravascular hemolysis. Cerebral endothelial hypertrophy and intralesional organisms may also be present.
Free-ranging wild boars, warthogs, and bushpigs are a reservoir for Babesia trautmanni and B. perroncitoi; however, clinical diseases are best described in wild boars. Babesia spp. are typically transmitted between Suidae and Tayssuidae by ticks in the genera Rhipicephalus, Boophilus, and Dermacentor. B. trautmanni in domestic pigs and wild boars results in a chronic hemolytic disease with clinical signs including emaciation, pyrexia, anorexia, icterus, and hemoglobinurea. Gross lesions may be limited to poor body condition or emaciation, splenomegaly, and hepatomegaly, with or without polycavitary effusion, and multisystemic hemorrhages. Microscopic lesions reflect hemolytic disease with hemoglobin nephrosis, and degeneration and necrosis with a pattern suggestive of hypoxia in multiple tissues. Diagnosis is based on identification of the parasite in erythrocytes. B. gibsoni-like organisms have been detected by PCR in wild boar in Asia, but have not yet been linked to disease (Masatani et al., 2017).
Cryptosporidium suis and C. scrofarum are ubiquitous in domestic pigs and wild boar, and all Suidae and Tayssuidae appear to be susceptible to infection (Fowler, 1996, Kaupke et al., 2017, Kvac et al., 2013, Nemejc et al., 2013, Rodriguez-Rivera et al., 2016, Ryan et al., 2004). Cryptosporidium spp. have a direct life cycle, and while most naturally occurring infections are subclinical, infection has infrequently been associated with mild enteritis and diarrhea in suids. The protozoa are most commonly identified in young animals; adults appear to have a moderate degree of resistance to infection. Zoonotic transmission is the most significant feature of infection in these species, and transmission to human and nonhuman primates by wild boar, warthogs, and bushpigs is a primary concern (Parsons et al., 2015).
Additional pathogenic and commensal protozoa are presented in Supplemental Tables e2 and S3.
Table e3.
Commensal Parasites of Suids
| GI Commensals | Disease in Domestic Pig | Wild Host | Disease in Wild | Evidence |
|---|---|---|---|---|
| Telamodinium onyx | Species not reported in domestic pigs. | Warthog | None. Inhabit cecum and colon. | Booyse and Dehority (2012) |
| Megadinium aethiopieum | Species not reported in domestic pigs. | Warthog | None. Inhabit cecum and colon. | Booyse and Dehority (2012) |
| Teratodinium sphaeredon | Species not reported in domestic pigs. | Warthog | None. Inhabit cecum and colon. | Booyse and Dehority (2012) |
| Blepharoconus krugerensis | Nonpathogenic. | Warthog | None. Inhabit cecum and colon. | Booyse and Dehority (2012) |
| Blepharosphaera intestinalis | Nonpathogenic. | Warthog, bushpigs | None. Inhabit cecum and colon. | Booyse and Dehority (2012) |
| Charonina equi | Nonpathogenic. | Warthog | None. Inhabit cecum and colon. | Booyse and Dehority (2012) |
Allan, S.A., Simmons, L.A., Burridge, M.J., 2001. Ixodid ticks on white-tailed deer and feral swine in Florida. J. Vect. Ecol. 26, 93–102.
Apanaskevich, D.A., Horak, I.G., Mulumba-Mfumu, L.K., 2013. A new species of Rhipicephalus (Acari: Ixodidae), a parasite of red river hogs and domestic pigs in the Democratic Republic of Congo. J. Med. Entomol. 50, 479–484.
Belem, A.M.G., 2012. Gastro-intestinal parasites of warthogs (Phacochoerus Africanus) from the Nazinga Game Ranch Burkina Faso. Bull. Anim. Health Prod. Afr. 60, 199–204.
Boomker, J., Horak, I.G., Booyse, D.G., Meyer, S., 1991. Parasites of South African wildlife. VIII. Helminth and arthropod parasites of warthogs, Phacochoerus aethiopicus, in the eastern Transvaal. Onderstepoort J. Vet. Res. 58, 195–202.
Booyse, D.G., Dehority, B.A., 2012. Gastrointestinal protozoa and digestive tract parameters of wild South African warthogs (Phacochoerus aethiopicus). Afr. Zoo. 47, 169–177.
Buxton, D., McAllister, M.M., Dubey, J.P., 2002. The comparative pathogenesis of neosporosis. Trends Parasitol. 18, 546–552.
Castellano Margardio, T.C., Mangini, P.R., 2001. Order artiodactyla, family tayassuidae (Peccaries), In: Fowler, M.E., Cubas, Z.S. (Eds.), Biology, Medicine, and Surgery of South American Wild Animals, first ed. Iowa State University Press, Ames, pp. 377–391.
Chae, J.B., Kang, J.G., Kim, H.C., Chong, S.T., Lee, I.Y., Shin, N.S., Chae, J.S., 2017. Identification of tick species collected from wild boars and habitats of wild boars and domestic pigs in the Republic of Korea. Kor. J. Parasitol. 55, 185–191.
Comeaux, J.M., Curtis-Robles, R., Lewis, B.C., Cummings, K.J., Mesenbrink, B.T., Leland, B.R., Bodenchuk, M.J., Hamer, S.A., 2016. Survey of Feral Swine (Sus scrofa ) infection with the agent of chagas disease (Trypanosoma cruzi ) in Texas, 2013–14. J. Wildl. Dis. 52, 627–630.
Crotti, D., Crotti, S., Sensi, M., Salamida, S., Manuali, E., Caccio, M., Pozio, E., 2012. Dientamoeba fragilis detection in suid populations: an emerging zoonosis hypothesized in Central Italy. Microbiol. Med. 27, 137–142.
Donahoe, S.L., Lindsay, S.A., Krockenberger, M., Phalen, D., Slapeta, J., 2015. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int. J. Parasitol. Parasit. Wildl. 4, 216–238.
Fernandez-de-Mera, I.G., Gortazar, C., Vicente, J., Hofle, U., Fierro, Y., 2003. Wild boar helminths: risks in animal translocations. Vet. Parasitol. 115, 335–341.
Fowler, M.E., 1996. Husbandry and diseases of captive wild swine and peccaries. Rev. Sci. Tech. 15, 141–154.
Fowler, M.E., Cubas, Z.S., 2001. Biology, Medicine, and Surgery of South American Wild Animals, first ed. Iowa State University Press, Ames, pp. 1 online resource (x, 536 p.).
Gladney, W.J., Dawkins, C.C., Price, M.A., 1977. Amblyomma inornatum (Acarina: ixodidae): natural hosts and laboratory biology. J. Med. Entomol. 14, 85–88.
Gruver, K.S., Guthrie, J.W., 1996. Parasites and selected diseases of collared peccaries (Tayassu tajacu) in the trans-pecos region of Texas. J. Wildl. Dis. 32, 560–562.
Guglielmone, A.A., 1990. Sites of attachment in Amblyomma triguttatum triguttatum Koch (Acari: Ixodidae) on natural hosts. Ann. Parasitol. Hum. Comp. 65, 145–148.
Hannon, P.G., Lochmiller, R.L., Mellen, J.W., Craig, T.M., Grant, W.E., 1985. Eperythrozoon in captive juvenile collared peccaries in Texas. J. Wildl. Dis. 21, 439–440.
Helcel, J., Teel, P., Tyson, M., Cash, J., Hensley, T., Cathey, J.C., 2016. Wild Pigs and Ticks: Implications for Livestock Production, Human and Animal Health, In: Extension, T.A.M.U.A. (Ed.). Texas A&M University Agrilife Extension, p. 14.
Horak, I.G., Boomker, J., de Vos, V., Potgieter, F.T., 1988. Parasites of domestic and wild animals in South Africa. XXIII. Helminth and arthropod parasites of warthogs, Phacochoerus aethiopicus, in the eastern Transvaal Lowveld. Onderstepoort J. Vet. Res. 55, 145–152.
Horak, I.G., Boomker, J., Flamand, J.R., 1991. Ixodid ticks and lice infesting red duikers and bushpigs in north-eastern Natal. Onderstepoort J. Vet. Res. 58, 281–284.
Horak, I.G., Golezardy, H., Uys, A.C., 2007. Ticks associated with the three largest wild ruminant species in southern Africa. Onderstepoort J. Vet. Res. 74, 231–242.
Junker, K., Horak, I.G., Penzhorn, B., 2015. History and development of research on wildlife parasites in southern Africa, with emphasis on terrestrial mammals, especially ungulates. Int. J. Parasitol. Parasites Wildl. 4, 50–70.
Kakati, P., Sarmah, P.H., Bhattacharjee, K., Bora, D.P., Baruah, K., Deka, P.J., 2015. Gastrointestinal nematodiasis in a captive bred critically endangered pygmy hog (Porcula salvania). Intl. J. Recent Sci. Res. 6, 4231–4234.
Kaufmann, J., 2014. Parasitic Infections of Domestic Animals : A Diagnostic Manual. Springer Basel AG, Basel.
Keirans, J.E., 1985. Amblyomma paulopunctatum Neumann (Ixodoidea, Ixodidae) - redescription of the male and female, with an account of its known hosts and distribution. In: Proc. Entymol. Soc. Washington 87, 489–497.
Khin, T., Win, K.K., 1981. Setaria worms from the domestic pigs in Burma. Ann. Parasitol. Hum. Comp. 56, 241–244.
Leslie, D.M., Huffman, B.A., 2015. Potamochoerus porcus (Artiodactyla: Suidae). Mamm. Species 47, 15–31.
Levin, M.L., 2016. Amblyomma spp, In: Integumentary System—Ticks, In: Aiello, S.E., Moses, M.A., Allen, D.G. (Eds.), Merck Veterinary Manual, eleventh ed. Merck & Co, Kenilworth, NJ, USA.
Lowenstein, M., Kutzer, E., 1989. Capillaria (Nematoda, Trichuridae) of wild swine (Sus scrofa) in Austria. Angew Parasitol. 30, 221–237.
Manning, P.A., Graham, O.H., 1997. Chewing and sucking lice as parasites of mammals and birds. Technical Bulletin #1849, In: Agricultural Research Service, U.S.D.o.A. (Ed.). USDA.
Mansouri, M., Sarkari, B., Mowlavi, G.R., 2016. Helminth Parasites of Wild Boars, Sus scrofa, In: Bushehr Province, Southwestern Iran. Iran J. Parasitol. 11, 377–382.
Marlow, B.J.G., 1955. A commensal nematode in the African bush pig. J. Mammal. 36, 147.
Matthee, S., Swanepoel, M., van der Mescht, L., Leslie, A.J., Hoffman, L.C., 2013. Ectoparasites of a non-indiginous warthog population, Pahcochoerus africanus, in the Free State Province, South Africa. Afr. Zool. 48, 259–265.
Maxie, M.G., Robinson, W.F., 2007. Cardiovascular system, In: Maxie, M.G., Jubb, K.V.F. (Eds.), Pathology of Domestic Animals, fifth ed. Elsevier Saunders, Edinburgh ; New York, pp. 1–127.
Mishra, A.C., Singh, K.N., 1978. Description of Haematopinus oliveri sp. nov. (Anoplura: Hematopinidae) parasitizing Sus salvanius in India. Bull. Zool. Survey India 1, 167–169.
Nava, S., Mangold, A.J., Mastropaolo, M., Venzal, J.M., Oscherov, E.B., Guglielmone, A.A., 2009. Amblyomma boeroi n. sp. (Acari: Ixodidae), a parasite of the Chacoan peccary Catagonus wagneri (Rusconi) (Artiodactyla: Tayassuidae) in Argentina. Syst. Parasitol. 73, 161–174.
Nemejc, K., Sak, B., Kvetonova, D., Hanzal, V., Janiszewski, P., Forejtek, P., Rajsky, D., Kotkova, M., Ravaszova, P., McEvoy, J., Kvac, M., 2014. Prevalence and diversity of Encephalitozoon spp. and Enterocytozoon bieneusi in wild boars (Sus scrofa) in Central Europe. Parasitol. Res. 113, 761–767.
Paulsen, P., Forejtek, P., Hutarova, Z., Vodnansky, M., 2013. Alaria alata mesocercariae in wild boar (Sus scrofa, Linnaeus, 1758) in south regions of the Czech Republic. Vet. Parasitol. 197, 384–387.
Pietschmann, J., Mur, L., Blome, S., Beer, M., Perez-Sanchez, R., Oleaga, A., Sanchez-Vizcaino, J.M., 2016. African swine fever virus transmission cycles in Central Europe: evaluation of wild boar-soft tick contacts through detection of antibodies against Ornithodoros erraticus saliva antigen. BMC Vet. Res. 12, 1.
Pourrut, X., Emane, K.A., Camicas, J.-L., Leroy, E., Gonzales, J.P., 2011. Contribution to the knowledge of ticks (Acarina: Ixodidae) in Gabon. Acarologica 51, 465–471.
Ramos Vdo, N., Piovezan, U., Franco, A.H., Osava, C.F., Herrera, H.M., Szabo, M.P., 2014. Feral pigs as hosts for Amblyomma sculptum (Acari: Ixodidae) populations in the Pantanal, Mato Grosso do Sul, Brazil. Exp. Appl. Acarol. 64, 393–406.
Reiterova, K., Spilovska, S., Blanarova, L., Derdakova, M., Cobadiova, A., Hisira, V., 2016. Wild boar (Sus scrofa) - reservoir host of Toxoplasma gondii, Neospora caninum and Anaplasma phagocytophilum in Slovakia. Acta Parasitol. 61, 255–260.
Rodriguez-Rivera, L.D., Cummings, K.J., McNeely, I., Suchodolski, J.S., Scorza, A.V., Lappin, M.R., Mesenbrink, B.T., Leland, B.R., Bodenchuk, M.J., 2016. Prevalence and diversity of Cryptosporidium and Giardia identified among feral pigs in Texas. Vect. Borne Zoo. Dis. 16, 765–768.
Roger, F., Ratovonjato, J., Vola, P., Uilenber, G., 2001. Ornithodoros porcinus ticks, bushpigs, and African swine fever in Madagascar. Exp. Appl. Acarol. 25, 263–269.
Round, M.C., 1968. Check list of the helminth parasites of African animals of the orders Carnivora, Tubulidentata, Proboscidae, Hyracoidea, Artiodactya, and Perissodactyla.
Samson, K.S., Donaldson, B.R., 1968. Parasites of the javelina in New Mexico. Bull. Wildl. Dis. Assoc. 4, 131.
Samuel, W.M., Low, W.A., 1970. Parasites of the collared peccary from Texas. J. Wildl. Dis. 6, 16–23.
Sanchez-Vizcaino, J.M., Mur, L., Bastos, A.D., Penrith, M.L., 2015. New insights into the role of ticks in African swine fever epidemiology. Rev. Sci. Tech. 34, 503–511.
Sato, H., Suzuki, K., Yokoyama, M., 2008. Visceral helminths of wild boars (Sus scrofa leucomystax) in Japan, with special reference to a new species of the genus Morgascaridia Inglis, 1958 (Nematoda: Schneidernematidae). J. Helminthol. 82, 159–168.
Silaghi, C., Pfister, K., Overzier, E., 2014. Molecular investigation for bacterial and protozoan tick-borne pathogens in wild boars (Sus scrofa) from southern Germany. Vect. Borne Zoo. Dis. 14, 371–373.
Slodkowicz-Kowalska, A., Graczyk, T.K., Tamang, L., Girouard, A.S., Majewska, A.C., 2007. Asymptomatic Enterocytozoon bieneusi microsporidiosis in captive mammals. Parasitol. Res. 100, 505–509.
Solaymani-Mohammadi, S., Rezaian, M., Hooshyar, H., Mowlavi, G.R., Babaei, Z., Anwar, M.A., 2004. Intestinal protozoa in wild boars (Sus scrofa) in western Iran. J. Wildl. Dis. 40, 801–803.
Stolte, M., Odening, K., Quandt, S., Bengis, R.G., Bockhardt, I., 1998. Sarcocystis dubeyella n. sp. and Sarcocystis phacochoeri n. sp. (Protozoa: Sarcocystidae) from the warthog (Phacochoerus aethiopicus) in South Africa. J. Eukaryot. Microbiol. 45, 101–104.
Sugiyama, H., Shibata, K., Kawakami, Y., Arakawa, K., Morishima, Y., Yamasaki, H., Gokuden, M., Iwakiri, T., Fukumori, J., 2015. Paragonimiasis due to the consumption of wild boar meat in japan: contamination levels of lung fluke larvae in muscle samples of wild boars caught in Kagoshima prefecture. Jpn. J. Infect. Dis. 68, 536–537.
Sutherland-Smith, M., 2015. Suidae and Tayassuidae (Wild Pigs, Peccaries), In: Miller, R.E., Fowler, M.E. (Eds.), Fowler’s Zoo and Wild Animal Medicine, eighth ed. Elsevier, pp. 568–583.
Thompson, H., Irvine, R.M., Philbey, A.W., 2009. Fasciola hepatica infection in wild boar in the UK. Vet. Rec. 165, 697–698.
Van Wyk, I.C., Boomker, J., 2011. Parasites of South African wildlife. XIX. The prevalence of helminths in some common antelopes, warthogs and a bushpig in the Limpopo province, South Africa. Onderstepoort J. Vet. Res. 78, 308.
Vicente, J.J., Muniz-Pereira, L.C., Noronha, D., Pinto, R.M., 2000. Description of males of Parabronema pecariae Ivaschkin, 1960 (Nematoda, Habronematoidea) parasitizing peccaries (Mammalia, Tayassuidae) in Brazil. Mem. Inst. Oswaldo Cruz. 95, 849–851.
Walker, J.B., Keirans, J.E., Horak, I., 2005. The genus Rhipicephalus (Acardi, Ixodidae) : A Guide to the Brown Ticks of the World. Cambridge University Press, Cambridge ; New York.
Wilber, P.G., Hellgren, E.C., Gabor, T.M., 1996. Coccidia of the collared peccary (Tayassu tajacu) in southern Texas with descriptions of three new species of Eimeria (Apicomplexa: Eimeriidae). J. Parasitol. 82, 624–629.
Yu, S., Mott, K.E., 1994. Epidemiology and morbidity of food-borne intestinal trematode infections. World Health Organization Schistosomiasis Control Unit.
Zanet, S., Trisciuoglio, A., Bottero, E., de Mera, I.G., Gortazar, C., Carpignano, M.G., Ferroglio, E., 2014. Piroplasmosis in wildlife: Babesia and Theileria affecting free-ranging ungulates and carnivores in the Italian Alps. Parasit. Vectors 7, 70.
Ectoparasites
Sarcoptes scabei var suis, the cause of sarcoptic mange, are global pests of domestic pigs that have also been reported in free-ranging wild boars, warthogs, and peccaries (Fowler, 1996, Haas et al., 2015). Lesions are similar to those described in other species and include alopecia and crusting dermatitis with lichenification that primarily affects the ventrum and extremities. Histologically, parakeratotic hyperkeratosis with intralesional mites and serocellular crusts are common findings (Fig. 8.18 ). Dermal inflammation is primarily eosinophilic. Diagnosis is by skin scrape.
Figure 8.18.
Scabies due to Sarcoptes scabeii infection in the skin of a babirusa.
Marked epidermal hyperplasia and hyperkeratosis are associated with the intracorneal mites.
(Photo Courtesy of E. Mitchell, National Zoological Gardens of South Africa)
Free-ranging Suidae and Tayassuidae are hosts to numerous tick species, and infestations are not uncommon in captive animals. In free-ranging animals, tick burdens may be extremely high, and most Suidae and Tayassuidae have developed behavioral adaptations, such as wallowing that rubs ticks off of the skin and commensal relationships with other animals that scavenge ticks. Tick infestation is most commonly associated with chronic multifocal dermatitis; it is not generally associated with systemic disease. Ticks are more important due to their roles as vectors for infectious disease transmission, such as in the pathogenesis of ASF.
Additional information on ticks and other ectoparasites is provided in Supplemental Table e2.
E-Slides
-
8.e1
Cystic and adenomatous endometrial hyperplasia, babirusa, uterus. The endometrium is moderately, multifocally expanded by numerous endometrial glands, some of which are mildly to moderately dilated. (see Fig. 8.3). eSlide: VM05031
-
8.e2
Uterine adenocarcinoma, Ossabow Island hog, uterus. An unencapsulated, non-demarcated proliferation of neoplastic cells and glandular or tubular structures that is supported by a dense neoplastic stroma expands the uterine wall. Neoplastic cells are polygonal to columnar, multifocally piled up, show mild to moderate anisocytosis and pleomorphism, have a prominent nucleolus to granular chromatin, and contain abundant lightly basophilic cytoplasm. Mild multifocal lymphoplasmacytic inflammation and hemorrhage are seen throughout the tumor. eSlide: VM05074
References
- Alexandrov T., Stefanov D., Kamenov P., Miteva A., Khomenko S., Sumption K., Meyer-Gerbaulet H., Depner K. Surveillance of foot-and-mouth disease (FMD) in susceptibe wildlife and domestic ungulates in Southeast of Bulgaria following a FMD case in wild boar. Vet. Microbiol. 2013;166:84–90. doi: 10.1016/j.vetmic.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Anderson J., Barret T., Scott G.R., editors. Manual on the Diagnosis of Rinderpest. second ed. Food and Agriculture Organization of the United Nations; Rome: 1996. [Google Scholar]
- Appel M.J.G., Reggiardo C., Summers B.A., Pearce-Kelling S., Maré C.J., Noon T.H., Reed R.E., Shively, Örvell C. Canine distemper virus infection and encephalitis in javelinas (collared peccaries) Arch. Virol. 1991;119:147–152. doi: 10.1007/BF01314331. [DOI] [PubMed] [Google Scholar]
- Artois M., Depner K.R., Guberti V., Hars J., Rossi S., Rutili D. Classical swine fever (hog cholera) in wild boar in Europe. Rev. Sci. Tech. 2002;21(2):287–303. doi: 10.20506/rst.21.2.1332. [DOI] [PubMed] [Google Scholar]
- Barasona J.A., Acevedo P., Díez-Delgado I., Queiros J., Carrasco-García R., Gortazar C., Vicente J. Tuberculosis-associated death among adult wild boars, Spain 2009-2014. Emerg. Infect. Dis. 2016;22(12):2178–2180. doi: 10.3201/eid2212.160677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroch J.A., Gagnon C.A., Lacouture S., Gottschalk M. Exposure of feral swine (Sus scrofa) in the United States to selected pathogens. Can. J. Vet. Res. 2015;79:74–78. [PMC free article] [PubMed] [Google Scholar]
- Barrette R.W., Metwally S.A., Rowland J.M., Xu L., Zaki S.R., Nichol S.T., Rollin P.E., Towner J.S., Shieh W.J., Batten B., Sealy T.K., Carrillo C., Moran K.E., Bracht A.J., Mayr G.A., Sirios-Cruz M., Catbagan D.P., Lautner E.A., Ksiazek T.G., White W.R., McIntosh M.T. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325:204–206. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- Batista J.S., Bezerra F.S.B., Lira R.A., Orpinelli S.R.T., Dias C.E.V., Oliveira A.F. Stress syndrome in collared pecarys (Tayassu tajacu) submitted to capture and containment in different hours of the morning at Mossoro-RN-Brazil. Ciência Animal Brasileira. 2008;9:170–176. [Google Scholar]
- Batista J.S., Olinda R.G., Rodrígues C.M.F., Silva T.M.F., Vale R.G., Viana G.A., Oliveira A.F., Oliveira M.F. Postmortem findings in collared peccaries raised in captivity in northeastern Brazil. Pesquisa Veterinária Brasileira. 2014;34:1101–1108. [Google Scholar]
- Batista Linhares M., Belloy L., Origgi F.C., Lechner I., Segner H., Ryser-Degiorgis M.P. Investigating the role of free-ranging wild boar (Sus scrofa) in the re-emergence of enzootic pneumonia in domestic pig herds: A Pathological, prevalence and risk-factor study. PlosOne. 2015;10(3):e0119060. doi: 10.1371/journal.pone.0119060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baums C.G., Verkühlen G.J., Rehm T., Silva L.M., Beyerbach M., Pohlmeyer K., Valentin-Weigand P. Prevalence of Streptococcus suis genotypes in wild boars of Northwestern Germany. Appl. Environ. Microbiol. 2007;73(3):711–717. doi: 10.1128/AEM.01800-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belem A.M.G. Gastro-intestinal parasites of warthogs (Phacochoerus Africanus) from the nazinga game ranch burkina faso. Bull. Anim. Health Prod. Afr. 2012;60:199–204. [Google Scholar]
- Benirschke K., Hager D.A., Edwards D.K., III Observations on neonatal mortality of the Chacoan peccary, Catagonus wagneri. Vet. Pathol. 1995;32:532–534. doi: 10.1177/030098589503200511. [DOI] [PubMed] [Google Scholar]
- Berg F., Gustafson U., Andersson L. The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet. 2006;2:e129. doi: 10.1371/journal.pgen.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondo N., Schaefer R., Gava D., Cantão M.E., Silveira S., Mores M.A., Ciacci-Zanella J.R., Barcellos D.E. Genomic analysis of influenza A virus from captive wild boars in Brazil reveals a human-like H1N2 influenza virus. Vet. Microbiol. 2014;168:34–40. doi: 10.1016/j.vetmic.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Blome S., Gabriel C., Beer M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 2013;173:122–130. doi: 10.1016/j.virusres.2012.10.026. [DOI] [PubMed] [Google Scholar]
- Blome S., Gabriel C., Dietze K., Breithaupt A., Beer M. High virulence of African swine fever virus Caucasus isolate in European wild boars of all ages. Emerg. Infect. Dis. 2012;18:708. doi: 10.3201/eid1804.111813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boadella M., Lyashchenko K., Greenwald R., Esfarandiari J., Jaroso R., Carta T., Garrido J.M., Vicente J., de la Fuente J., Gortazar C. Serologic tests for detecting antibodies against Mycobacterium bovis and Mycobacterium avium subspecies paratuberculosis in Eurasian wild boar (Sus scrofa scrofa) J. Vet. Diagn. Invest. 2011;23(1):77–83. doi: 10.1177/104063871102300111. [DOI] [PubMed] [Google Scholar]
- Boadella M., Ruiz-Fons J.F., Vicente J., Martın M., Segales J., Gortazar C. Seroprevalence evolution of selected pathogens in Iberian wild boar. Trans. Emerg. Dis. 2011;59:395–404. doi: 10.1111/j.1865-1682.2011.01285.x. [DOI] [PubMed] [Google Scholar]
- Bollo E., Ferroglio E., Dini V., Mignone W., Biolatti B., Rossi L. Detection of Mycobacterium tuberculosis complex in lymph nodes of wild boar (Sus scrofa) by a target-amplified test system. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2000;47(5):337–342. doi: 10.1046/j.1439-0450.2000.00354.x. [DOI] [PubMed] [Google Scholar]
- Borba M.R., Sanches E.M., Correa A.M., Spanamberg A., de Souza Leal J., Soares M.P., Guillot J., Driemeier D., Ferreiro L. Immunohistochemical and ultra-structural detection of Pneumocystis in wild boar (Sus scrofa) co-infected with porcine circovirus type 2 (PCV2) in Southern Brazil. Med. Mycol. 2011;49:172–175. doi: 10.3109/13693786.2010.510540. [DOI] [PubMed] [Google Scholar]
- Breithaupt A., Depner K., Haas B., Alexandrov T., Polohronova L., Georgiev G., Meyer-Gerbaulet H., Beer M. Experimental infection of wild boar and domestic pigs with a foot and mouth disease virus strain detected in the southeast of Bulgaria in December of 2010. Vet. Microbiol. 2012;159:33–39. doi: 10.1016/j.vetmic.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Cano-Manuel F.J., López-Olvera J., Fandos P., Soriguer R.C., Pérez J.M., Granados J.E. Long-term monitoring of 10 selected pathogens in wild boar (Sus scrofa) in Sierra Nevada National Park, southern Spain. Vet. Microbiol. 2014;174:148–152. doi: 10.1016/j.vetmic.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Caswell J.L., Williams K.J. Respiratory system. In: Maxie M.G., editor. sixth ed. Vol. 3. Elsevier; St. Louis, MO: 2016. pp. 534–535. (Jubb, Kennedy Palmeŕs Pathology of Domestic Animals). [Google Scholar]
- Chiari M., Ferrari N., Zanoni M., Alborali L. Mycoplasma hyopneumoniae temporal trends of infection and pathological effects in wild boar populations. Eur. J. Wildl. Res. 2013;60:187–192. [Google Scholar]
- Cho Y.Y., Lim S.I., Jeoung H.Y., Kim Y.K., Song J.Y., Lee J.B., An D.J. Serological evidence for influenza virus infection in Korean wild boars. J. Vet. Med. Sci. 2015;77:109–112. doi: 10.1292/jvms.14-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton J.R., Faye J., Rawlings P. Trypanosome infections in warthogs (Phacochoerus aethiopicus) in the Gambia. Vet. Parasitol. 1992;41:179–187. doi: 10.1016/0304-4017(92)90077-m. [DOI] [PubMed] [Google Scholar]
- Closa-Sebastia F., Casas-Dıaz E., Cuenca R., Lavın S., Mentaberre G., Marco I. Antibodies to selected pathogens in wild boar (Sus scrofa) from Catalonia (NE Spain) Eur. J. Wildl. Res. 2011;57:977–981. [Google Scholar]
- Cole G., Suedmeyer W.K., Johnson G. Pheochromocytoma in an African warthog (Phacochoerus aethiopicus) J. Zoo Wildl. Med. 2008;39:663–666. doi: 10.1638/2008-0009.1. [DOI] [PubMed] [Google Scholar]
- Comeaux J.M., Curtis-Robles R., Lewis B.C., Cummings K.J., Mesenbrink B.T., Leland B.R., Bodenchuk M.J., Hamer S.A. Survey of feral swine (Sus scrofa) infection with the agent of chagas disease (Trypanosoma cruzi ) in Texas, 2013–14. J. Wildl. Dis. 2016;52:627–630. doi: 10.7589/2015-08-208. [DOI] [PubMed] [Google Scholar]
- Corn J.L., Lee R.M., Erickson G.A., Murphy C.D. Serologic survey for evidence of exposure to vesicular stomatitis virus, pseudorabies virus, brucellosis and leptospirosis in collared peccaries from Arizona. J. Wildl. Dis. 1987;23:551–557. doi: 10.7589/0090-3558-23.4.551. [DOI] [PubMed] [Google Scholar]
- Corner L.A., Barrett R.H., Lepper A.W.D., Lewis V., Pearson C.W. A survey of mycobacteriosis of feral pigs in the Northern Territory. Aust. Vet. J. 1981;57:537–542. doi: 10.1111/j.1751-0813.1981.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Craig L.E., Dittmer K.E., Thomson K.G. Bones and joints. In: Grant Maxie, editor. Vol. 1. Elsevier; St. Louis Missouri: 2015. pp. 17–146. (Jubb, Kennedy, and Palmeŕs Pathology of Domestic Animals). [Google Scholar]
- Cságola A., Kiss I., Tuboly T. Detection and analysis of porcine circovirus type 1 in Hungarian wild boars: short communication. Acta Vet. Hung. 2008;56:139–144. doi: 10.1556/AVet.56.2008.1.15. [DOI] [PubMed] [Google Scholar]
- Cuesta Gerveno J.M., Risco Pérez D., Gonçalves Blanco P., García Jiménez W.L., Gil Molino M., Fernandez-Llario P., Hermoso de Mendoza Salcedo J., Gómez Gordo L.J. Fatal infection due to Haemophilus parasuis in a young wild boar (Sus scrofa) J. Vet. Diagn. Invest. 2013;25(2):297–300. doi: 10.1177/1040638713479348. [DOI] [PubMed] [Google Scholar]
- Dardiri A.H., Yedloutschnig R.J., Taylor W.D. Clinical and serologic response of American white-collared peccaries to African swine fever, foot-and-mouth disease, vesicular stomatitis, vesicular exanthema of swine, hog holera, and rinderpest viruses. In: Proceedings of the 73rd Annual Meeting of US Animal Health Association, pp. 1969:437–452. [PubMed] [Google Scholar]
- Das A., Mazumder Y., Dutta B.K., Shome B.R., Bujarbaruah K.M., Sharma G.D. Clostridium perfringens type A beta2 toxin in elephant (Elephas maximus indicus) and pygmy hog (Sus salvanius) with haemorrhagic enteritis in Assam India. Afr. J. Microbiol. Res. 2008;2:196–201. [Google Scholar]
- De Castro A.M., Brombila T., Bersano J.G., Soares H.S., Silva S.O., Minervino A.H., Ogata R.V., Gennari S.M., Richtzenhain L.J. Swine infectious agents in Tayassu pecari and Pecari tajacu tissue samples from Brazil. J. Wildl. Dis. 2014;50:205–209. doi: 10.7589/2013-01-021. [DOI] [PubMed] [Google Scholar]
- Díez-Delgado I., Boadella M., Martín-Henando M., Barasona J.A., Beltrán-Beck B., González-Barrio D., Sibila M., Vicente J., Garrido J.M., Segalés J., Gortazar C. Complex links between natural tuberculosis and porcine circovirus type 2 infection in wild boar. Biomed. Res. Int. 2014;2014:765715. doi: 10.1155/2014/765715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers A.R., Dekkar A., Dekkers L.J. Serosurveillance of wild deer and wild boar after the epidemic of foot-and-mouth disease in The Netherlands in 2001. Vet. Rec. 2003;153:678–681. doi: 10.1136/vr.153.22.678. [DOI] [PubMed] [Google Scholar]
- Ellis J., Spinato M., Yoon C., West K., McNeilly F., Meehan B., Kennedy S., Clark E., Krakowka S., Allan G. Porcine circovirus 2-associated disease in Eurasian wild boar. J. Vet. Diagn. Invest. 2003;15:364–368. doi: 10.1177/104063870301500411. [DOI] [PubMed] [Google Scholar]
- Everett H., Crooke H., Gurrala R., Dwarka R., Kim J., Botha B., Lubisi A., Pardini A., Gers S., Vosloo W., Drew T. Experimental infection of common warthogs (Phacochoerus africanus) and bushpigs (Potamochoerus larvatus) with classical swine fever virus I: susceptibility and transmission. Trans. Emerg. Dis. 2011;58(2):128–134. doi: 10.1111/j.1865-1682.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-Mera I.G., Gortazar C., Vicente J., Hofle U., Fierro Y. Wild boar helminths: risks in animal translocations. Vet. Parasitol. 2003;115:335–341. doi: 10.1016/s0304-4017(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Foni E., Garbarino C., Chiapponi C., Baioni L., Zanni I., Cordioli P. Epidemiological survey of swine influenza A virus in the wild boar population of two Italian provinces. Influenza Other Respir. Viruses. 2013;7(Suppl. 4):16–20. doi: 10.1111/irv.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler M.E. Husbandry and diseases of captive wild swine and peccaries. Rev. Sci. Tech. 1996;15:141–154. doi: 10.20506/rst.15.1.913. [DOI] [PubMed] [Google Scholar]
- Gabriel C., Blome S., Malogolovkin A.S., Parilov S., Kolbasov D., Teifke J., Beer M. Characterization of African swine fever virus Caucasus isolate in European wild boars. Emerg. Infect. Dis. 2011;17:2342–2345. doi: 10.3201/eid1712.110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P.F., Galinari G.C., Cortez A., Paula C.D., Lobato Z.I., Heinemann M.B. Orbivius infections in collared peccaries (Tayassu tajacu) in southeastern Brazil. J. Wildl. Dis. 2012;48:230–232. doi: 10.7589/0090-3558-48.1.230. [DOI] [PubMed] [Google Scholar]
- Gers S., Vosloo W., Drew T., Lubisi A.B., Pardini A., Williams M. Experimental infection of common warthogs (Phacochoerus africanus) and bushpigs (Potamochoerus larvatus) with classical swine fever virus II: a comparative histopathological study. Trans. Emerg. Dis. 2011;58(2):135–144. doi: 10.1111/j.1865-1682.2010.01191.x. [DOI] [PubMed] [Google Scholar]
- Glenn Songer J., Uzal F.A. Clostridial enteric infections in pigs. J. Vet. Diagn. Invest. 2005;17:528–536. doi: 10.1177/104063870501700602. [DOI] [PubMed] [Google Scholar]
- Godfroid J., Garin-Bastuji B., Saegerman C., Blasco J.M. Brucellosis in terrestrial wildlife. Rev. Sci. Tech. 2013;32:27–42. doi: 10.20506/rst.32.1.2180. [DOI] [PubMed] [Google Scholar]
- Gogin A., Gerasimov V., Malogolovkin A., Kolbasov D. African swine fever in the North Caucasus región and the Russian Federation in years 2007–2012. Virus Res. 2013;173:198–203. doi: 10.1016/j.virusres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Gómez-Laguna J., Carrasco L., Ramis G., Quereda J.J., Gómez S., Pallarés F.J. Use of real-time and classic polymerase chain reaction assays for the diagnosis of porcine tuberculosis in formalin-fixed, paraffin-embedded tissues. J. Vet. Diagn. Invest. 2010;22(1):123–127. doi: 10.1177/104063871002200126. [DOI] [PubMed] [Google Scholar]
- Gortázar C., Vicente J., Fierro Y., Leon L., Cubero M.J., Gonzalez M. Natural Aujeszky’s disease in a Spanish wild boar population. Ann. NY Acad. Sci. 2002;969:210–212. doi: 10.1111/j.1749-6632.2002.tb04380.x. [DOI] [PubMed] [Google Scholar]
- Gottdenker N., Bodmer R. In: second ed, Gale, Farmington Hills, Michigan, pp. 291-301. Hutchins M., editor. Vol. 16. 2004. (Grizimek’s Animal Life Encyclopedia). [Google Scholar]
- Guenther S., Nöckler K., von-Nichisch-Roseneqk M., Landgraf M., Ewers C., Wieler L.H., Schierack P. Detection of Trichinella spiralis, T. britovi and T. pseudospiralis in muscle tissue with real-time PCR. J. Microbiol. Methods. 2008;75:287–292. doi: 10.1016/j.mimet.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Guinat C., Gogin A., Blome S., Keil G., Pollin R., Pfeiffer D.U., Dixon L. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Vet. Rec. 2016;178:262–267. doi: 10.1136/vr.103593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C., Origgi F.C., Akdesir E., Batista Linhares M., Giovannini S., Mavrot F., Cassaubon J., Ryser-Degiorgis M.P. First detection of sarcoptic mange in free-ranging wild boar (Sus scrofa) in Switzerland. Schweiz Arch Tierheilkd. 2015;157:269–275. doi: 10.17236/sat00020. [DOI] [PubMed] [Google Scholar]
- Hampson D.J. Brachyspiral colitis. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., Oxford U.K., editors. Diseases of Swine. tenth ed. Wiley-Blackwell; 2012. pp. 680–696. [Google Scholar]
- Hellgren E.C., Lochmiller R.L., Thomas M.W., Grant W.E. Cyclopia, congenital limb deformity, and osteomyelitis in the collared peccary, Tayassu tajacu (L.) J. Wildl. Dis. 1984;20:354–357. doi: 10.7589/0090-3558-20.4.354. [DOI] [PubMed] [Google Scholar]
- Herrera H.M., Abreu U.G., Keuroghlian A., Freitas T.P., Jansen A.M. The role played by sympatric collared peccary (Tayassu tajacu), white-lipped peccary (Tayassu pecari), and feral pig (Sus scrofa) as maintenance hosts for Trypanosoma evansi and Trypanosoma cruzi in a sylvatic area of Brazil. Parasitol. Res. 2008;103:619–624. doi: 10.1007/s00436-008-1021-5. [DOI] [PubMed] [Google Scholar]
- Hohloch C., Reiner G., Bronnert B., Willems H., Reinacher M. Detection of porcine circovirus type 2 and its association with PMWS in wild boars and domestic pigs in Germany: a histopathological, immunohistochemical and molecular biological study. Berliner und Munchener tierarztliche Wchenschrift. 2015;128:200–203. [PubMed] [Google Scholar]
- Hotzel H., Berndt A., Melzer F., Sachse K. Occurrence of Chlamydiaceae spp. in a wild boar (Sus scrofa L) population in Thuringia (Germany) Vet. Microbiol. 2004;103:121–126. doi: 10.1016/j.vetmic.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Gerth Löfstedt M., Holmgren N. The prevalences of Brachyspira spp. and Lawsonia intracellularis in Swedish piglet producing herds and wild boar population. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2005;52:386–391. doi: 10.1111/j.1439-0450.2005.00865.x. [DOI] [PubMed] [Google Scholar]
- Jori F., Bastos A.D. Role of wild suids in the epidemiology of African swine fever. Ecohealth. 2009;6(2):296–310. doi: 10.1007/s10393-009-0248-7. [DOI] [PubMed] [Google Scholar]
- Jori F., Vial L., Penrith M.L., Pérez-Sánchez R., Etter E., Albina E., Michaud V., Roger F. Review of the sylvatic cycle of African swine fever in sub-Saharan Africa and the Indian ocean. Virus Res. 2013;173(1):212–227. doi: 10.1016/j.virusres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Kaden V., Lange E., Starick E., Bruer W., Krakowski W., Klopries M. Epidemiological survey of swine influenza A viruses in selected wild boar populations in Germany. Vet. Microbiol. 2008;131:123–132. doi: 10.1016/j.vetmic.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Kaden V., Teifke J.P., Polster U. Progressive atrophische rhinitis—Eine seltene erkrankung beim schwarzwild (Sus scrofa scrofa L 1758) [Progressive atrophic rhinitis—a rare disease in wild boar] Eur. J. Wildl. Res. 2001;47:17–25. [Google Scholar]
- Kakati P., Sarmah P.H., Bhattacharjee K., Bora D.P., Baruah K., Deka P.J. Gastrointestinal nematodiasis in a captive bred critically endangered pygmy hog (Porcula salvania) Int. J. Recent Sci. Res. 2015;6:4231–4234. [Google Scholar]
- Kaupke A., Gawor J., Rzezutka A., Gromadka R. Identification of pig-specific Cryptosporidium species in mixed infections using Illumina sequencing technology. Exp. Parasitol. 2017;182:22–25. doi: 10.1016/j.exppara.2017.09.020. [DOI] [PubMed] [Google Scholar]
- Keuling O., Baubet E., Duscher A., Ebert C., Fischer C., Monaco A., Podgórski T., Prevot C., Ronnenberg K., Sodeikat G., Stier N., Thurfjell H. Mortality rates of wild boar (Sus scrofa L.) central Europe. Eur. J. Wildl. Res. 2013;59:805–814. [Google Scholar]
- Kleiboeker S.B., Scoles G.A. Pathogenesis of African swine fever in ornithodorus ticks. Anim. Health Res. Rev. 2001;2(2):121–128. [PubMed] [Google Scholar]
- Kobinger G.P., Leung A., Neufeld J., Richardson J.S., Falzarano D., Smith G., Tierney K., Patel A., Weingartl H.M. Replication, pathogenicity, shedding, and transmission of Zaire ebolavirus in pigs. J. Infect. Dis. 2011;204:200–208. doi: 10.1093/infdis/jir077. [DOI] [PubMed] [Google Scholar]
- Köppel C., Knopf L., Ryser M.-P., Miserez R., Thür B., Stärk K.D.C. Serosurveillance for selected infectious disease agents in wild boars (Sus scrofa) and outdoor pigs in Switzerland. Eur. J. Wildl. Res. 2007;53:212–220. [Google Scholar]
- Kuhnert P., Overesch G. Molecular epidemiology of Mycoplasma hyopneumoniae from outbreaks of enzootic pneumonia in domestic pig and the role of wild boar. Vet. Microbiol. 2014;174:261–266. doi: 10.1016/j.vetmic.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Kuhnert P., Overesch G., Belloy L. Genotyping of Mycoplasma hyopneumoniae in wild boar lung samples. Vet. Microbiol. 2011;152:191–195. doi: 10.1016/j.vetmic.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Kvac M., Kestranova M., Pinkova M., Kvetonova D., Kalinova J., Wagnerova P., Kotkova M., Vitovec J., Ditrich O., McEvoy J., Stenger B., Sak B. Cryptosporidium scrofarum n. sp (Apicomplexa: Cryptosporidiidae) in domestic pigs (Sus scrofa) Vet. Parasitol. 2013;191:218–227. doi: 10.1016/j.vetpar.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M., Siemen H., Blome S., Thulke H.H. Analysis of spatio-temporal patterns of African swine fever cases in Russian wild boar does not reveal an endemic situation. Prev. Vet. Med. 2014;117:317–325. doi: 10.1016/j.prevetmed.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Leak S.G.A. CABI Publishing and ILRI; Oxfordshire, England: 1999. Tsetse Biology and Ecology: Their Role in the Epidemiology and Control of Trypanosomosis. pp. 118–121. [Google Scholar]
- Li M., Zhang X., Zhu L., Wang H., Zhao N., Luo J., Wang C., Wang Y., Liu Y., Zhou W., Zhang B., Guo H., He H. Identification, isolation, and phylogenetic analysis of Clostridium perfringens type A and type C from wild boar (Sus scrofa) in the People’s Republic of China. J. Wildl. Dis. 2017;53(3):642–648. doi: 10.7589/2016-08-199. [DOI] [PubMed] [Google Scholar]
- Link E.K., Hoferer M., Strobel B., Riqbers K., Langenmayer M.C., Sutter G., Fux R. Sus scrofa papillomavirus 2—genetic characterization of a novel suid papillomavirus from wild boar in Germany. J. Gen. Virol. 2017;98:2113–2117. doi: 10.1099/jgv.0.000868. [DOI] [PubMed] [Google Scholar]
- Lipej Z., Segalés J., Jemersic L., Olvera A., Roić B., Novosel D., Mihaljević Z., Manojlović L. First description of postweaning multisystemic wasting syndrome (PMWS) in wild boar (Sus scrofa) in Croatia and phylogenetic analysis of partial PCV2 sequences. Acta Vet. Hung. 2007;55:389–404. doi: 10.1556/AVet.55.2007.3.13. [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L., Hellgren E.C., Hannon P.G., Grant E.W., Robinson R.M. Coccidioidomycosis (Coccidioides immitis) in the collared peccary (Tayassu tajacu: Tayassuidae) in Texas. J. Wildl. Dis. 1985;21:305–309. doi: 10.7589/0090-3558-21.3.305. [DOI] [PubMed] [Google Scholar]
- Lord V.R., Lord R.D. Brucella suis infections in collared peccaries in Venezuela. J. Wildl. Dis. 1991;27:477–481. doi: 10.7589/0090-3558-27.3.477. [DOI] [PubMed] [Google Scholar]
- Maclachlan N.J., Dubovi E.J. fourth ed. Academic Press; Tokyo: 2011. Fenner’s Veterinary Virology. pp. 425–441. [Google Scholar]
- MacMilan A.P. In: Diseases of Swine. eighth ed. Straw B.E., D́Allaire S., Mengeling W.L., Taylor D.J., editors. Iowa State University Press; Ames, Iowa: 1999. pp. 385–393. [Google Scholar]
- Mansouri M., Sarkari B., Mowlavi G.R. Helminth parasites of wild boars, Sus scrofa, in Bushehr Province Southwestern Iran. Iran J. Parasitol. 2016;11:377–382. [PMC free article] [PubMed] [Google Scholar]
- Marinou K.A., Papatsiros V.G., Gkotsopoulos E.K., Odatzoglou P.K., Athanasiou L.V. Exposure of extensively farmed wild boars (Sus scrofa scrofa) to selected pig pathogens in Greece. Vet. Q. 2015;35:97–101. doi: 10.1080/01652176.2015.1022666. [DOI] [PubMed] [Google Scholar]
- Marsh G.A., Haining J., Robinson R., Foord A., Yamada M., Barr J.A., Payne J., White J., Yu M., Bingham J., Rollin P.E., Nichol S.T., Wang L.F., Middleton D. Ebola Reston virus infection of pigs: clinical significance and transmission potential. J. Infect. Dis. 2011;3:S804–S809. doi: 10.1093/infdis/jir300. [DOI] [PubMed] [Google Scholar]
- Martin-Hernando M.P., Höfle U., Vicente J., Ruiz-Fons F., Vidal D., Barral M., Garrido J.M., de la Fuente J., Gortazar C. Lesions associated with Mycobacterium tuberculosis complex infection in the European wild boar. Tuberculosis. 2007;87:360–367. doi: 10.1016/j.tube.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Martins Gomes de Castro A.M., Brombila T., Garcia Bersano J., Sousa Soares H., Oliveira de Souza Silva S., Hamad Minervino A.H., Akio Ogata R., Gennari S.M., Richtzenhain L.J. Swine infectious agents in Tayassu pecari and Pecari tajacu tissue samples from Brazil. J. Wildl. Dis. 2014;50(2):205–209. doi: 10.7589/2013-01-021. [DOI] [PubMed] [Google Scholar]
- Masatani T., Hayashi K., Andoh M., Tateno M., Endo Y., Asada M., Kusakisako K., Tanaka T., Gokuden M., Hozumi N., Nakadohzono F., Matsuo T. Detection and molecular characterization of Babesia, Theileria, and Hepatozoon species in hard ticks collected from Kagoshima, the southern region in Japan. Ticks Tick Borne Dis. 2017;8:581–587. doi: 10.1016/j.ttbdis.2017.03.007. [DOI] [PubMed] [Google Scholar]
- McGregor G.F., Gottschalk M., Godson D.L., Wilkins W., Bollinger T.K. Disease risks associated with free-ranging wild boar in Saskatchewan. Can. Vet. J. 2015;56:839–844. [PMC free article] [PubMed] [Google Scholar]
- Meng X.J., Lindsay D.S., Sriranganathan N. Wild boars as sources for infectious diseases in livestock and humans. Philos. Trans. R. Soc. B Biol. Sci. 2009;264:2697–2707. doi: 10.1098/rstb.2009.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moennig V. The control of classical swine fever in wild boar. Front. Microbiol. 2015;6:1–10. doi: 10.3389/fmicb.2015.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnaro S., Sasso S., De Martino L., Longo M., Iovane V., Ghiurmino G., Pisanelli G., Nava D., Baldi L., Pagnini U. Prevalence of antibodies to selected viral and bacterial pathogens in wild boar (Sus scrofa) in Campania region, Italy. J. Wildl. Dis. 2010;46:316–319. doi: 10.7589/0090-3558-46.1.316. [DOI] [PubMed] [Google Scholar]
- Müller T., Hahn E.C., Tottewitz F., Kramer M., Klupp B.G., Mettenleiter T.C., Freuling C. Pseudorabies virus in wild swine: a global perspective. Arch. Virol. 2011;156:1691–1705. doi: 10.1007/s00705-011-1080-2. [DOI] [PubMed] [Google Scholar]
- Müller T.F., Teuffert J., Zellmer R., Conraths F.J. Experimental infection of European wild boars and domestic pigs with pseudorabies viruses with differing virulence. Am. J. Vet. Res. 2001;62:252–258. doi: 10.2460/ajvr.2001.62.252. [DOI] [PubMed] [Google Scholar]
- Naranjo V., Gortazar C., Vicente J., de la Fuente J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 2008;127(1–2):1–9. doi: 10.1016/j.vetmic.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Nemejc K., Sak B., Kvetonova D., Hanzal V., Janiszewski P., Forejtek P., Rajsky D., Ravaszova P., McEvoy J., Kvac M. Cryptosporidium suis and Cryptosporidium scrofarum in Eurasian wild boars (Sus scrofa) in Central Europe. Vet. Parasitol. 2013;197:504–508. doi: 10.1016/j.vetpar.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nfon C.K., Leung A., Smith G., Embury-Hyatt C., Kobinger G., Weingartl H.M. Immunopathogenesis of severe acute respiratory disease in Zaire ebolavirus-infected pigs. PLoS One. 2013;8:e61904. doi: 10.1371/journal.pone.0061904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöckler K., Rechinger S., Pozio E. Trichinella spiralis and Trichinella pseudospiralis mixed infection in a wild boar (Sus scrofa) of Germany. Vet. Parasitol. 2006;137:364–368. doi: 10.1016/j.vetpar.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Noon T.H., Heffelfinger J.R., Olding R.J., Lynn Wesche S.L., Reggiardo C. Serologic survey for antibodies to canine distemper virus in collared peccary (Tayassu tajacu) populations in Arizona. J. Wildl. Dis. 2003;39:221–223. doi: 10.7589/0090-3558-39.1.221. [DOI] [PubMed] [Google Scholar]
- Olinda R.G., Viana G.A., Rodrigues C.M.F., Silva T.M.F., Lucena R.B., Bezerra F.S.B., Batista J.S. Extramedullary plasmacytoma in a captive collared peccary (Pecari tajacu) Pesquisa Veterinária Brasileira. 2016;36:516–519. [Google Scholar]
- Olvera A., Cerda-Cuellar M., Mentaberre G., Casas-Diaz E., Lavin S., Marco I., Aragonl V. First isolation of Haemophilus parasuis and other NAD-dependent Pasteurellaceae of swine from European wild boars. Vet. Microbiol. 2007;125:182–186. doi: 10.1016/j.vetmic.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Osorio J., Carvajal A., Naharro G., Rubio P., La T., Phillips N.D., Hampson D.J. Identification of weakly haemolytic Brachyspira isolates recovered from pigs with diarrhoea in Spain and Portugal and comparison with results from other countries. Res. Vet. Sci. 2013;95:861–869. doi: 10.1016/j.rvsc.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Page G.R., Wang F.I., Hahn E.C. Interaction of pseudorabies virus with porcine peripheral blood lymphocytes. J. Leuk. Biol. 1992;52:441–448. doi: 10.1002/jlb.52.4.441. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhang W., Cui L., Hua X., Wang M., Zeng Q. Reston virus in domestic pigs in China. Arch. Virol. 2014;159:1129–1132. doi: 10.1007/s00705-012-1477-6. [DOI] [PubMed] [Google Scholar]
- Parsons M.B., Travis D., Lonsdorf E.V., Lipende I., Roellig D.M., Collins A., Kamenya S., Zhang H., Xiao L., Gillespie T.R. Epidemiology and molecular characterization of Cryptosporidium spp. in humans, wild primates, and domesticated animals in the Greater Gombe Ecosystem Tanzania. PLoS Negl. Trop. Dis. 2015;9:e0003529. doi: 10.1371/journal.pntd.0003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrith M.L., Vosloo W., Mather C. Classical swine fever (Hog Cholera): review aspects relevant to control. Trans. Emerg. Dis. 2011;58:187–196. doi: 10.1111/j.1865-1682.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- Perera H.K., Wickramasinghe G., Cheung C.L., Nichiura H., Smith D.K., Poon L.L., Perera A.K., Ma S.K., Sunil-Chandra N.P., Guan Y., Peiris J.S. Swine influenza in Sri Lanka. Emerg. Infect. Dis. 2013;19:481–484. doi: 10.3201/eid1903.120945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N.D., La T., Adams P.A., Harland B.L., Fenwick S.G., Hampson D.J. Detection of Brachyspira hyodysenteriae, Lawsonia intracellularis and Brachyspira pilosicoli in feral pigs. Vet. Microbiol. 2009;134:294–299. doi: 10.1016/j.vetmic.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Pickering B.S., Collingnon B., Smith G., Marszal P., Kobinger G., Weingartl H.M. Detection of Zaire ebolavirus in swine: assay development and optimization. Transbound. Emerg. Dis. 2017;65(1):77–84. doi: 10.1111/tbed.12606. [DOI] [PubMed] [Google Scholar]
- Pollock J.N., editor. Vol. 1. Food and Agriculture Organization of the United Nations; 1982. (Tsetse Biology, Systematics and Distribution; Techniques). [Google Scholar]
- Rahman H., Chakraborty A., Deka P.J., Narayan G., Prager R. An Outbreak of Salmonella enteritidis Infection in Pygmy Hogs (Sus salvanius) Trop. Anim. Health Prod. 2001;33:95–102. doi: 10.1023/a:1005229412814. [DOI] [PubMed] [Google Scholar]
- Rahman H., Deka P.J., Chakraborty A., Narayan G. Salmonellosis in pigmy hogs (Sus salvanius)—a critically endangered species of mammal. Rev. Sci. Tech. 2005;24(3):959–964. [PubMed] [Google Scholar]
- Ravamoana J., Jori F., Vial L., Pérez-Sánchez R., Blanco E., Michaud V., Roger F. Assessment of interacions between African swine fever virus, bushpigs (Potamochoerus larvatus) Ornithodorus ticks and domestic pigs in north-western Madagascar. Transbound. Emerg. Dis. 2011;58(3):247–254. doi: 10.1111/j.1865-1682.2011.01207.x. [DOI] [PubMed] [Google Scholar]
- Ravamoana J., Michaud V., Jori F., Andriatsimahavandy A., Roger F., Albina E., Vial L. First detection of African swine fever virus in Ornithodorus porcinus in Madagascar and new insights into tick distribution and taxonomy. Parasit. Vectors. 2010;3(115):2–9. doi: 10.1186/1756-3305-3-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register K.B., Brockmeier S.L., de Jong M.F., Pijoan C. In: Diseases of Swine. tenth ed. Zimmermann J., Karriker L., Ramirez A., editors. Wiley; Hoboken, NJ: 2012. pp. 798–810. [Google Scholar]
- Reiner G., Bronnert B., Hohloch C., Fresen C., Haack I., Willems H., Reinacher M. Qualitative and quantitative distribution of PCV2 in wild boars and domestic pigs in Germany. Vet. Microbiol. 2010;145:1–8. doi: 10.1016/j.vetmic.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Reiner G., Fresen C., Bronnert S. Prevalence of Haemophilus parasuis infection in hunted wild boars (Sus scrofa) in Germany. Eur. J. Wildl. Res. 2010;56:815–818. doi: 10.7589/0090-3558-46.2.551. [DOI] [PubMed] [Google Scholar]
- Risco D., Llario P.F., Velarde R., García W.L., Benítez J.M., García A., Bernejo F., Cortés M., Rey J., de Mendoza J.H., Gómez L. Outbreak of swine erysipelas in a semi-intensive wild boar farm in Spain. Transbound. Emerg. Dis. 2011;58:445–450. doi: 10.1111/j.1865-1682.2011.01234.x. [DOI] [PubMed] [Google Scholar]
- Risco D., Fernández-Llario P., Cuesta J.M., García-Jiménez W.L., Gil M., Goncalves P., Martínez R., Gómez L., García A., Rey J., Hermoso de Mendoza M., Hermoso de Mendoza J.H. Fatal outbreak of systemic pasteurellosis in a wild boar (Sus scrofa) population from southwest Spain. J. Vet. Diagn. Invest. 2013;25:791–794. doi: 10.1177/1040638713504411. [DOI] [PubMed] [Google Scholar]
- Risco D., Fernández-Llario P., Cuesta J.M., García-Jiménez W.L., Gonçalves P., Martínez R., García A., Rosales R., Gómez L., de Mendoza J.H. Fatal case of Streptococcus suis infection in a young wild boar (Sus scrofa) from southwestern Spain. J. Zoo Wildl. Med. 2015;46(2):370–373. doi: 10.1638/2014-0135R1.1. [DOI] [PubMed] [Google Scholar]
- Risco D., Serrano E., Fernández-Llario P., Cuesta J.M., Goncalves P., García-Jiménez W.L., Martínez R., Cerrato R., Velarde R., Gómez L., Segalés J., Hermoso de Mendoza J. Severity of bovine tuberculosis is associated with co-infection with common pathogens in wild boar. PLoS One. 2014;9:e110123. doi: 10.1371/journal.pone.0110123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W.F., Robinson N.A. Cardiovascular system. In: Grant Maxie., editor. Vol. 3. Elsevier; St. Louis, Missouri: 2015. pp. 2–99. (Jubb, Kennedy, and Palmeŕs Pathology of Domestic Animals). [Google Scholar]
- Rodriguez-Rivera L.D., Cummings K.J., McNeely I., Suchodolski J.S., Scorza A.V., Lappin M.R., Mesenbrink B.T., Leland B.R., Bodenchuk M.J. Prevalence and diversity of cryptosporidium and giardia identified among feral pigs in Texas. Vect. Borne Zoo. Dis. 2016;16:765–768. doi: 10.1089/vbz.2016.2015. [DOI] [PubMed] [Google Scholar]
- Roger F., Ratovonjato J., Vola P., Uilenber G. Ornithodorus porcinus ticks, bushpigs, and African swine fever in Madagascar. Exp. Appl. Acarol. 2001;25(3):263–269. doi: 10.1023/a:1010687502145. [DOI] [PubMed] [Google Scholar]
- Romero C.H., Meade P., Santagata J., Gillis K., Lollis G., Hahn E.C., Gibbs E.P. Genital infection and transmission of pseudorabies virus in feral swine in Florida, USA. Vet. Microbiol. 1997;55:131–139. doi: 10.1016/s0378-1135(96)01307-7. [DOI] [PubMed] [Google Scholar]
- Rossi S., Fromont E., Pontier D., Crucière C., Hars J., Barrat J., Pacholek X., Artois M. Incidence and persistence of classical swine fever in free-ranging wild boar (Sus scrofa) Epidemiol. Infect. 2005;133(3):559–568. doi: 10.1017/s0950268804003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Fons F., Vicente J., Vidal D., Höfle U., Villanúa D., Gauss C., Segalés J., Almeria S., Montoro V., Gortázar C. Seroprevalence of six reproductive pathogens in European wild boar (Sus scrofa) from Spain: the effect on wild boar female reproductive performance. Theriogenology. 2006;65:731–743. doi: 10.1016/j.theriogenology.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ruiz-Fons F. A review of the current status of relevant zoonotic pathogens in wild swine (Sus scrofa) populations: changes in modulating the risk of transmission to humans. Transbound. Emerg. Dis. 2017;64:68–88. doi: 10.1111/tbed.12369. [DOI] [PubMed] [Google Scholar]
- Ryan U.M., Monis P., Enemark H.L., Sulaiman I., Samarasinghe B., Read C., Buddle R., Robertson I., Zhou L., Thompson R.C., Xiao L. Cryptosporidium suis n. sp. (Apicomplexa: Cryptosporidiidae) in pigs (Sus scrofa) J. Parasitol. 2004;90:769–773. doi: 10.1645/GE-202R1. [DOI] [PubMed] [Google Scholar]
- Sánchez del Rey V., Fernández-Garayzábal J.F., Mentaberre G., Briones V., Lavín S., Domínguez L., Gottschalk M., Vela A.I. Characterisation of Streptococcus suis isolates from wild boars (Sus scrofa) Vet. J. 2014;200:464–467. doi: 10.1016/j.tvjl.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vizcaino J.M., Mur L., Gomez-Villamandos J.C., Carrasco L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015;152(1):9–21. doi: 10.1016/j.jcpa.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Sayama Y., Demtria C., Saito M., Azul R.R., Taniguchi S., Fukushi S., Yoshikawa T., Iizuka I., Mizutani T., Kurane I., Malbas F.F., Jr., Lupisan S., Catbaganm D.P., Animas S.B., Morales R.G., Lopez E.L., Dazo K.R., Cruz M.S., Olveda R., Saijo M., Oshitani H., Morikawa S. A seroepidemiologic study of Reston ebolavirus in swine in the Phillipines. BMC Vet. Res. 2012;8:82. doi: 10.1186/1746-6148-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlafer D.H., Foster R.A. Female genital system. In: Maxie M.G., editor. sixth ed. Vol. 3. Elsevier; St. Louis, MO: 2016. pp. 404–405. (Jubb, Kennedy and Palmeŕs Pathology of Domestic Animals). [Google Scholar]
- Schulze C., Hlinak A., Wohlsein P., Kutzer P., Müller T. Spontaneous Aujeszky’s disease (pseudorabies) in European wild boars (Sus scrofa) in the federal state of Brandenburg, Germany. Berl. Munch. Tierarztl. Wochenschr. 2010;123:359–364. [PubMed] [Google Scholar]
- Schulze C., Neumann G., Grütze I., Engelhardt A., Mirle C., Ehlert F., Hlinak A. Case report: Porcine circovirus type 2 infection in an European wild boar (Sus scrofa) in the state of Brandenburg, Germany. Deut. Tierarztl. Wochenschr. 2003;110:426–428. [PubMed] [Google Scholar]
- Seguel M., Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int. J. Parasitol. Parasites Wildl. 2017;6:177–194. doi: 10.1016/j.ijppaw.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol B., Naglić T., Vrbanac I. Isolation of Streptococcus suis capsular type 3 from a young wild boar (Sus scrofa) Vet. Rec. 1998;143(24):664. [PubMed] [Google Scholar]
- Shimizu T., Okamoto C., Aoki H., Harada K., Kataoka Y., Ono F., Kadohira M., Takai S. Serological surveillance for antibodies against Erysipelothrix species in wild boar and deer in Japan. Jpn. J. Vet. Res. 2016;64:91–94. [PubMed] [Google Scholar]
- Shimoda H., Van Nguyen D., Yonemitsu K., Minami S., Nagata N., Hara N., Kuwata R., Murakami S., Kodera Y., Takeda T., Yoshikawa Y., Horimoto T., Maeda K. Influenza A virus infection in Japanese wild boars (Sus scrofa leucomystax) J. Vet. Med. Sci. 2017;79:848–851. doi: 10.1292/jvms.17-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shome B.R., Shome R., Bujarbaruah K.M., Das A., Rahman H., Sharma G.D., Dutta B.K. Investigation of haemorrhagic enteritis in pygmy hogs (Sus salvanius) from India. Rev. Sci. Tech. 2010;29:687–693. doi: 10.20506/rst.29.3.2010. [DOI] [PubMed] [Google Scholar]
- Sibila M., Mentaberre G., Boadella M., Huerta E., Casas-Díaz E., Vicente J. Serological, pathological and polymerase chain reaction studies on Mycoplasma hyopneumoniae infection in the wild boar. Vet. Microbiol. 2010;144(1–2):214–218. doi: 10.1016/j.vetmic.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Sofia M., Billinis C., Psuchas V., Birtsas P., Sofianidis G., Leontides L., Knowles N., Spyrou V. Detection and genetic characterization of porcine circovirus 2 isolates from the first cases of postweaning multisystemic and wasting syndrome in wild boars in Greece. J. Wildl. Dis. 2008;44:864–870. doi: 10.7589/0090-3558-44.4.864. [DOI] [PubMed] [Google Scholar]
- Sutherland-Smith M. Suidae and tayassuidae (wild pigs, peccaries) In: Miller R.E., Fowler M.E., editors. Vol. 8. Elsevier Saunders; St. Louis: 2015. pp. 568–584. (Fowler’s Zoo and Wild Animal Medicine). [Google Scholar]
- Thakur S., Sandfoss M., Kennedy-Stoskopf S., DePerno C.S. Detection of Clostridium difficile and Salmonella in feral swine population in North Carolina. J. Wildl. Dis. 2011;47:774–776. doi: 10.7589/0090-3558-47.3.774. [DOI] [PubMed] [Google Scholar]
- Thompson K.A., Niehaus A., Shellabarger W., Depenbrock S., Agnew D. Antemortem diagnosis of cystic endometrial hyperplasia and successful ovariohysterectomy in an African warthog (Phacochoerus africanus) J. Zoo Wildl. Med. 2015;46:904–908. doi: 10.1638/2015-0079.1. [DOI] [PubMed] [Google Scholar]
- Tischer I., Mields W., Wolff D., Vagt M., Griem W. Studies on epidemiology and pathogenicity of porcine circovirues. Arch. Virol. 1986;91:271–276. doi: 10.1007/BF01314286. [DOI] [PubMed] [Google Scholar]
- Touloudi A., Valiakos G., Athanasiou L.V., Birtas P., Giannakopoulos A., Papspyropoulos K., Kalaitzis C., Sokos C., Tsokana C.N., Spyrou V., Petrovska L., Billinis C. A serosurvey for selected pathogens in Greek European wild boar. Vet. Rec. Open. 2015;28:e000077. doi: 10.1136/vetreco-2014-000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni S., Bain O., Takaoka H., Miyashita M., Suzuki Y. Onchocerca dewittei japonica n. subsp., a common parasite from wild boar in Kyushu Island, Japan. Parasite. 2001;8:215–222. doi: 10.1051/parasite/2001083215. [DOI] [PubMed] [Google Scholar]
- Uni S., Fukuda M., Agatsuma T., Bain O., Otsuka Y., Nakatani J., Matsubayashi M., Harada M., Omar H., Ramli R., Hashim R., Azirun M.S., Takaoka H. Onchocerca takaokai n. sp. (Nematoda: Filarioidea) in Japanese wild boars (Sus scrofa leucomystax): description and molecular identification of intradermal females. Parasitol. Int. 2015;64:493–502. doi: 10.1016/j.parint.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Vadillo S., San-Juan C., Calderón M., Risco D., Fernández-Llario P., Pérez-Sancho M., Redondo E., Hurtado M.A., Igeño M.I. Isolation of Brachyspira species from farmed wild boar in Spain. Vet. Rec. 2017;181:145. doi: 10.1136/vr.104348. [DOI] [PubMed] [Google Scholar]
- Van Wyk I.C., Boomker J. Parasites of South African wildlife. XIX. The prevalence of helminths in some common antelopes, warthogs and a bushpig in the Limpopo province, South Africa. Onderstepoort J. Vet. Res. 2011;78:308. doi: 10.4102/ojvr.v78i1.308. [DOI] [PubMed] [Google Scholar]
- Verin R., Varuzza P., Mazzei M., Poli A. Serologic, molecular, and pathologic survey of pseudorabies virus infection in hunted wild boars (Sus scrofa) in Italy. J. Wildl. Dis. 2014;50:559–565. doi: 10.7589/2013-01-004. [DOI] [PubMed] [Google Scholar]
- Vengust G., Valencak Z., Bidovec A. A serological survey of selected pathogens in wild boar in Slovenia. J. Vet. Med. Ser. B. 2006;53:24–27. doi: 10.1111/j.1439-0450.2006.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente J., León-Vizcaíno L., Gortázar C., José Cubero M., González M., Martín-Atance P. Antibodies to selected viral and bacterial pathogens in European wild boars from southcentral Spain. J. Wildl. Dis. 2002;38:649–652. doi: 10.7589/0090-3558-38.3.649. [DOI] [PubMed] [Google Scholar]
- Vicente J., Segalés J., Höfle U., Balasch M., Plana-Durán J., Domingo M., Gortázar C. Epidemiological study on porcine circovirus type 2 (PCV2) infection in the European wild boar (Sus scrofa) Vet. Res. 2004;35:243–253. doi: 10.1051/vetres:2004008. [DOI] [PubMed] [Google Scholar]
- Weingartl H.M., Embury-Hyatt C., Nfon C., Leung A., Smith G., Kobinger G. Transmission of Ebola virus from pigs to non-human primates. Sci. Rep. 2012;2:811. doi: 10.1038/srep00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H.M., Nfon C., Kobinger G. Review of Ebola virus infections in domestic animals. Dev. Biol. 2013;135:211–218. doi: 10.1159/000178495. [DOI] [PubMed] [Google Scholar]
- Woodall P.F. Periodontal disease in southern African bushpigs (Potamochoerus porcus) and warthogs (Phacohoerus aethiopicus) J. Wildl. Dis. 1989;25:66–69. doi: 10.7589/0090-3558-25.1.66. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kijima M., Takahashi T., Yoshimura H., Tani O., Kojyou T., Yamawaki Y., Tanimoto T. Serovar, pathogenicity and antimicrobial susceptibility of Erysipelothrix rhusiopathiae isolates from farmed wild boars (Sus scrofa) affected with septicemic erysipelas in Japan. Res. Vet. Sci. 1999;67:301–303. doi: 10.1053/rvsc.1999.0311. [DOI] [PubMed] [Google Scholar]
- Zamora M.J., Alvarez M., Olmedo J., Blanco M.C., Pozio E. Trichinella pseudospiralis in the Iberian Peninsula. Vet. Parasitol. 2015;210:255–259. doi: 10.1016/j.vetpar.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Župančić Ž., Jukić B., Lojkić M., Čač Ž., Jemeršić L., Starešina V. Prevalence of antibodies to classical swine fever, Aujeszky’s disease, porcine reproductive and respiratory syndrome, and bovine viral diarrhoea viruses in wild boars in Croatia. J. Vet. Med. B. 2002;49:253–256. doi: 10.1046/j.1439-0450.2002.00562.x. [DOI] [PubMed] [Google Scholar]


