Abstract
In general viruses' modus operandi to propagate is achieved by the co-opting host cell components, membranes, proteins, and machineries to their advantage. This is true for virtually every aspect of a virus' replication cycle from virus entry to the budding or release of progeny virus particles. In this chapter, we will discuss new information on the impacts of virus-mediated manipulation of Dynein motor complexes and associated machineries and factors. We will highlight how these host cell components impact on pathogenicity and immune responses, as many of the virus-mediated hijacked components also play pivotal roles in immune responses to pathogen insult. There are several comprehensive reviews that define virus–Dynein interactions including the first edition of this book that describes how viruses manipulate the host cell machineries their advantage. An updated table is included to summarize these virus–host interactions. Notably, barriers to intracellular translocation represent major hurdles to viral components during de novo infection and during active replication and the generation of progeny virus particles. Clearly, the subversion of host cell molecular motor protein activities takes advantage of constitutive and regulated membrane trafficking events and will target virus components to intracytoplasmic locales and membrane assembly. Broadening our understanding of the interplay between viruses, Dynein and the cytoskeleton will likely inform on new types of therapies. Continual enhancement of the breadth of new information on how viruses manipulate host cell biology will inevitably aid in the identification of new targets that can be poisoned to block old, new, and emerging viruses alike in their tracks.
Keywords: Dynein, Emerging viruses, Innate immunity, Intracellular trafficking, Viral pathogenesis, Virus assembly, Virus restriction, Virus–host interactions
10.1. Dynein and viral replication
The Dynein complex is a microtubule (MT)-associated protein complex that mediates retrograde transport of macromolecules in the cytoplasm [2]. It is a 1.6-MDa complex built around two copies of ATPase energy-generating subunits called Dynein heavy chains (DHCs). Two Dynein intermediate chains (DICs) and two light intermediate chains (LICs) bind directly to the DHCs. Three Dynein light chains (DLCs) serve as Dynein adapter proteins, such as DLC 1 (DYNLL1, LC8, DLC1), DLC Tctex-type 1 (DYNLT1), and p150Glued, which have been implicated in cargo recruitment to Dynein complex during retrograde transport [3], [4], [5], [6]. More details on Dynein function are found below and in accompanying chapters. Dynein involvement in virus replication has been well studied. Usually investigations have centered on major questions of viral capsid translocation in the cell. For neurotropic viruses such as herpesviruses that require transit through long distances in differentiated and extended cells this is intuitively obvious [7], [8]. In this case, herpesvirus will interact on de novo infection and after gaining entry in the cell with Dynein subunits to transit toward the nucleus. The requirements for active transport are equally substantial for several other viruses such as human immunodeficiency virus type 1 (HIV-1) and recent investigations into other retroviruses such as murine leukemia virus (MLV), and reviewed extensively in Ref. [9], [10], [11]. Previous studies have implicated DYNLL1 and DYNLT1 proteins in different aspects of virus replication (reviewed in Ref. [12]). For example, DYNLL1 interacts with a rabies virus phosphoprotein and contributes to the viral gene expression [13], [14], [15]. Also, DYNLL1 interacts with the CA protein of bovine immunodeficiency virus (BIV) and contributes to retrograde transport [3]. DYNLT1 interacts with the CA protein of human papilloma virus type-16 (HPV-16) and contributes to the HPV-16 replication at an unknown replication step(s) [16]. Unlike the DYNLL1 and DYNLT1, the potential involvement of p150Glued in the replication of viruses is currently not very clear. However, the component of the Dynein complex, Dynactin associates to p150Glued [17] and has been implicated in viral replication and the retrograde transport of viruses [6], [18], [19].
10.2. Kinesins
The activity of kinesins co-opted by viruses is worthy of note. The tug-of-war that exists opposing motors, Kinesin and Dynein motors, is a precept that is generally accepted in the cell biology field. Morphological switches mediated by specific interactions with organelles and motor proteins will likely determine trafficking polarity in cells and the winner in the Dynein–Kinesin tug-of-war [8], [20], [21] especially since both motor proteins occupy intracytoplasmic vesicles, and virus capsids too, and cooperate to determine directionality [8], [10], [22]. This is clearly the case for retroviruses such as Mason–Pfizer Simian Virus (M-PSV) that interacts with the DLC (DYNLT1) Tctex-1 to target to intracellular viral assembly sites adjacent to the nucleus, at the microtubule organizing center (MTOC) [23], [24]. In contrast to the directed capsid assembly of the lentivirus, HIV-1, at the plasma membrane, capsid assembly of M-PSV occurs near the MTOC. A morphogenetic switch, mediated by an interaction between de novo synthesized Gag and DYNLT1, allows for M-PSV to acquire bilipid envelope at the plasma membrane, likely by the activity of MT + end motors such as Kinesin [1], [24]. Interestingly, a single point mutation in the Dynein-binding domain not only prevents DYNLT1 binding, but also changes the site of assembly to that exhibited by HIV-1, by targeting viral capsid assembly to the plasma membrane. The presence of viral proteins such as retroviral Gag as well as host proteins on endosomal membranes may constitute cargo that is available for directed transport on MTs toward viral assembly sites. Interactions of viral proteins with Dynein mediators such as Lissencephaly-1 (LIS-1) and other host proteins [25], [26], [27] could also provide information to switch from a minus-end to plus-end motor directed traffic. Curiously, the HIV-1 regulatory protein, Tat, enhances MT polymerization [28] and interacts with LIS-1 protein [29], whereas HIV-1 Rev, Vaccinia virus, and ASFV were reported to break down MTs [30], [31], [32]. Recent work demonstrates in fact that PKA phosphorylates cytoplasmic Dynein at a novel site in the Dynein light intermediate chain 1 (LIC1) that is essential for Dynein binding to the hexon capsid subunit and for virus motility [33], [34]. This posttranslational modification mediates an adenovirus-derived switch that promotes late endosome/lysosome dispersal thereby inducing a tempered specific dispersal of late endosome/lysosomes. This was shown to be due to the disruption of the LIC1 interaction with the Rab7-interacting lysosomal protein (RILP). At the other end of the virus replication cycle, late events during HIV-1 replication for example are also characterized by virus-mediated disruptions in membrane trafficking. During viral egress, HIV-1 commandeers late endosome/lysosomes to promote outbound trafficking of viral components along with late endosome/lysosomes-associated factors such as mTORC1 and the viral RNA (Alessandro Cinti & A.J. Mouland, unpublished; [35], [36]). Co-opting these membranes that bear characteristics of late endosome/lysosomes, containing LAMP-1 and other integral membrane markers, may have the obvious result of promoting correct targeting to virus assembly domains. Topologically, the eventual fusion of these vesicles at the plasma membrane should result in localization to the inner plasma membrane followed by outward, virus budding (see Ref. [1]). Interestingly, another recent study demonstrated that phagosome maturation was impaired in HIV-1–infected human macrophages. Testing pathogen clearance in these cells, it was found that the accessory Vpr gene product interacted with EB1 (plus-end MT tracking proteins), p150Glued, and the DHC thereby blocking EB1 loading on Dynein motors on the plus ends of MTs that is necessary for phagosome maturation [9], [37] (Fig. 10.1 ). These observations will necessarily have a major bearing on pathogenesis but as well as on the cell’s ability to ward off other opportunistic infections, which is the case in mid- to late-stage infections. The effects of HIV-1 Vpr in this case might be reflected in patients as opportunistic coinfections take hold in largely untreated or drug-naive patients in the developing world [38]. Lastly, the RILP–Dynein interaction (Fig. 10.1) was found to be critical for HCV intracellular trafficking. Virus-mediated cleavage of RILP generates an N-terminally truncated protein that dissociates the Dynein motor from viral cargo over the course of infection. The resulting preference for outbound trafficking of viral cargo promotes the transition from the intracellular-predominant to the secretion-predominant phenotype that corresponds to the production of the cleaved RILP [39]. See Table 10.1 .
Figure 10.1.
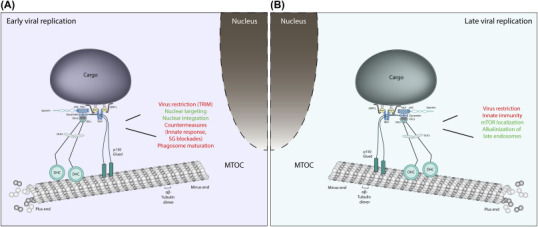
Early and late virus replication steps are characterized by virus subversion of Dynein and associated factors.
During the replication cycle, viral particles (i.e., viral proteins and complexes) need to be transported within host cells. In the early (A) and late (B) (postreplication) stages, the trafficking of viral components relies on the host cell cytoskeleton and molecular motors. Viruses require minus-end–directed Dynein motor complex for retrograde transport on microtubules (from cell surface toward the nucleus) (A) and Dynein in addition to kinesins for the anterograde transport toward the plasma membrane (B). By interacting with different Dynein complex factors, viruses can positively (in green) or negatively (in red) regulate a number of cellular processes including but not limited to viral restriction, nuclear targeting and integration, autophagosome maturation, innate immunity, alkalinization, and localization of late endosome/lysosomes and mTOR positioning. Details on the specific viral–host (Dynein complex) interactions are discussed/provided in the text and listed in Table 10.1.
Table 10.1.
Viruses that interact with Dynein components during replication to 2017
| Virusa | Virus interaction domain | Dynein component | References |
|---|---|---|---|
| Adeno-Associated virus | Capsid | Dynein | [95] |
| Adenovirus | Capsid | Dynein light and light intermediate chain IC and LIC1 | [95], [96], [97], [98], [99], [100], [101] |
| FIP-1 | TCTEL1 | ||
| African Swine Fever virus | P54 (13 amino acid motif) | DLC-8 | [19], [102] |
| Bovine Immunodeficiency virus | Capsid | DLC-8 | [3] |
| Ebolavirus | VP35 | DLC-8 | [103] |
| Hantaan virus | Unknown | Unknown | [104] |
| Hepatitus B virus | Unknown | Unknown | [105] |
| Hepatitus C virus | Unknown | Unknown | [106]. |
| Hepatitus E virus | Vp13 | Dynein | [107] |
| Herpes Simplex Virus 1 | VP26 capsid | RP3, Tctex-1 | [6], [8], [48], [108], [109], [110], [111], [112] |
| Equine Herpesvirus | Unknown | Unknown (acetylated tubulin) | [82] |
| Kaposi’s Sarcoma Herpesvirus (HHV-8) | Unknown | Unknown (acetylated tubulin) | [83] |
| Mason-Pfizer Simian virus | Matrix | DYNLT1 (Tctex-1) | [23], [24], |
| Bovine Immunodeficiency virus | Capsid | LC8 | [3] |
| Human Foamy Virus | Capsid | LC8 | [49] |
| Human immunodeficiency virus type 1 | Preintegration complex | Dynein | [35], [50], [113] |
| Human papillomavirus (HPV) | MINOR CAPSID L2 | DYNLT1 and DYNLT3 | [114], [115] |
| Poliovirus | Receptor CD155 | Tctex-1 | [116], [117], [118], [119] |
| Rabies virus | P Phosphoprotein | DYNLL (LC8) | [13], [14], [15], [120], [121], [122] |
| Sirevirus | Hopie Gag extension | DLC-8 | [123] |
| Vaccinia virus | Unknown | Unknown | [32], [124], [125]; |
| Rabies virus | polymerase L | DLC-1 | [126] |
| Murine leukemia virus (MLV) | Preintegration complex | p50 | [56], [127] |
| Porcine circovirus (PCV2) | capsid (Cap) | DIC-1, Dynein light chain, DYNLL1 | [60], [128] |
| Ebola virus (EBOV) | VP35 | Dynein light chain (LC8) | [103] |
| Human immunodeficiency virus type 1 | Vpr | Dynein light chain protein, DYNLT1, | [129] |
| Human immunodeficiency virus type 1 | Vpr | EB1, p150Glued, and DHC | [37] |
| Human immunodeficiency virus type 1 | Integrase (IN) | Dynein light chain 1 (DYNLL1) | [78] |
| Hepatitus C virus | Unknown | RILP | [39] |
| Influenza A virus | Unknown | Dynein and dynactin | [130] |
| Foot-and-mouth disease virus (FMDV) | Nonstructural protein 3A | Dynactin 3 | [131] |
| Mouse Polyomavirus (MPyV) | Unknown | Dynein | [132] |
| Rhesus rhadinovirus (RRV) | Unknown | Dynein | [133] |
| Pseudorabies virus (PRV) | Viral protein 1/2 (VP1/2) | DIC and p150Glued | [7] |
Highlighted columns indicate listings since first edition of this chapter (1). Most viruses are discussed in text.
10.3. Innate immunity, the Rabs, Rab7-interacting lysosomal protein, and vesicular transport
Following acute infection, the host cells mount a formidable innate response to infection that is not specific and usually leads to a burst of antiviral cytokine and interferon gene expression. This response includes the activity of well-described pattern recognition receptors and ensuing signaling to transcriptional activation of interferon stimulated genes, cytokines, and interferons. However, as immune-cell tropic viruses target T, B, and myeloid cell types, immunity is compromised [40]. Moreover, responses to acute infection in macrophages as well as other host cells such as dendritic cells appear to be compromised by a variety of mechanisms. One is a virus-mediated defect in viral antigen processing. By preventing autophagosome peptide generation and the presentation of antigen at the cell surface represents one way in which HIV-1, and likely other viruses, evade the host recognition [41]. Small Rab GTPases generally spike and define vesicles in the cell from early to late endosomes. Early endosomes will acidify following endocytosis and late endosomes that are generally actively directed to juxtanuclear positions carry Rab5 and then exchange with Rab7. Rab7-associated proteins such as RILP play particular roles in mediating the interaction of cargo with the Dynein motor via its association to p150Glued. RILP appears to be central to a number of processes involving intracellular trafficking of late endosome/lysosomes, membrane fusion events (e.g., endoplasmic reticulum (ER)–late endosome) and as described below, in immune responses and antigen presentation during virus infection. It remains a valuable tool in cell biology studies as the overexpression leads to the redistribution of late endosomes/lysosomes to the juxtanuclear region, the MTOC. This observed phenotype is evidence of a direct role in tethering the Dynein motor via the Dynein subunit p150glued and Rab7 expressed on late endosome/lysosomes. Expression of Dynein-interacting mutants leads to the release of late endosome/lysosomes with evidence that virus cargo is affected by RILP mutagenesis, to likely involve Dynein motor function. This was brought to the forefront of immunology in light of the involvement of Dynein in the presentation of HLA Class II antigen presentation. In multiple sclerosis for instance in which there is a susceptibility locus, late endosomal vesicle biogenesis, and HLA Class antigen presentation were shown to be dependent on CLEC16. CLEC16’s interaction with RILP and the homotypic fusion and protein sorting–tethering complex [42] and perturbed Dynein retrograde transport led to a blockade of the recruitment of MHC Class II antigens to the perinuclear region. This is mentioned here to demonstrate that while a defect in the functioning of the host gene leads to human disease, MHC Class II antigen presentation is also crucial to mount a defense against HIV-1 and other viruses. Antigens derived from within infected cells, in contrast to extracellular delivery of virus-specific antigens from virally produced cells, are also shuttled intracellularly. Defects induced by virus infection lead to intracellular trafficking deficiencies and in this type of scenario, a role for autophagic vesicles was brought to light in dendritic cells to activate HIV-1 specific CD4+ T cells [41]. While autophagy was not critical to Ag presentation to CD4+ T cells, targeting of HIV-1 specific antigens to autophagosomes nevertheless enhances virus-specific T cell antigen presentation. The MT-associated dendritic cell protein L chain (LC3) played a key role in this event, likely propelled by the Dynein motor for recruitment to juxtanuclear membrane fusion and autophagy processes.
10.4. Dynein, viruses, and the innate immune response
Many viruses hijack the cellular machinery for their retrograde transport during the early stages of their replication cycle, as was reviewed previously by us and others [1], [10], [43], [44]. In particular, multiple viruses subvert the MT network and MT-associated molecular motors; examples include adenoviruses [45], [46], parvoviruses [47], herpes simplex virus [8], [48], and retroviruses [49]. HIV-1 interactions with MTs and Dynein in the early infection stages have been relatively thoroughly investigated. A landmark live cell microscopy study that made use of fluorescently labeled individual viral particles yielded fascinating movies of HIV-1 traveling along MTs en route toward the MTOC [50]. Following MT-dependent transport, HIV-1 cores accumulate in the vicinity of the MTOC and then at nuclear pores [50], [51], [52].
Inspired by previous findings with other viruses, HIV-1 retrograde transport was proposed early on to be mediated by the Dynein motor, as evidenced by an accumulation of viral particles in the cell periphery following microinjection of anti-Dynein antibodies [50]. However, these results were descriptive in nature, offering no link between Dynein and MT functions and the infectivity of incoming HIV-1. Indeed, when functional studies were undertaken, they yielded rather conflicting results. In HeLa cells, for instance, none of several genetic or pharmacological approaches to interfere with Dynein or MTs had a significant impact on infectivity, even when they had a clear effect on HIV-1 intracellular movements [53], [54], [55]. Treatment with nocodazole and depletion of the DHC similarly did not affect the infectivity of another lentivirus, SIVmac, in human and nonhuman primate cells [53], [54], and also had no effect on the infectivity of MLV, a gammaretro virus. We take these sobering observations as evidence that identifying the specific cytoskeleton and transporter subunit components involved is necessary to fully understand the mechanisms of retrograde transport for any given virus. Indeed, a specific DLC, DYNLRB2, was recently found to mediate MLV retrograde transport, and its expression levels correlated with MLV infectivity [56]. Likewise, a kinesin-1 adaptor, Fasciculation and Elongation Factor zeta 1 was recently found to be important for HIV-1 retrograde transport and infectivity [57]. In addition, not all MTs are alike, and HIV-1 seems to promote the formation of and then use “stable” MTs, characterized by a state of decreased polymerization/depolymerization, which renders them resistant to nocodazole (explaining why multiple investigators saw little to no effect of nocodazole on HIV-1 infectivity, and see below) [58]. Human herpes simplex virus 1 is transported on long distances toward the nucleus of sensory neurons through an association with MTs initiated by binding to protein complexes called “+TIPs,” which comprise the Dynein interactor dynactin-1 [59]. The capsid protein of circoviruses interacts with the intermediate chain 1 of Dynein complexes for its retrograde transport [60]. Thus, viruses accomplish retrograde transport by interacting with Dynein motors, and possibly other motor complexes, using a variety of molecular mechanisms. Some viruses may modulate the dynamics and functions of MTs themselves, in addition to hijacking molecular motors.
The reliance of viruses on MT-dependent retrograde transport provides a weak spot that may be exploited by innate immune mechanisms to target these intracellular parasites. Indeed, several type 1 interferon-induced innate effectors act in the early postentry stages of viral infections [61]. One would expect some of these effectors to intercept viruses while they associate with MTs. This idea has been explored in the context of the inhibition of retroviruses by the tripartite motif (TRIM) protein family member TRIM5α. This E3 ubiquitin ligase forms cytoplasmic bodies in which inhibition-sensitive viruses are trapped, disassembled, and degraded [62]. Because these large cytoplasmic structures resemble aggresomes, their interactions with MTs were investigated soon after the discovery of their antiretroviral properties. It was found that indeed, TRIM5α cytoplasmic body subcellular localization is dependent on functional MTs [63]. Accordingly, the presence of functional MTs and Dynein motors was shown to be important for the inhibition of retroviruses by TRIM5α [54], [55]. TRIM proteins form a large family of proteins of which many have antiviral properties [64] and, on the other hand, often show an affinity for MTs [65]. Therefore, it is entirely possible that TRIM5α is the tip of the iceberg and that more examples of molecular motors and MTs being essential to the antiviral roles of the TRIM proteins will be uncovered in the future.
10.5. IFITM3 and VAP-A
An additional restriction factor, that is also interferon-inducible, whose function depends on Dynein and Dynein-associated factors, is the interferon-inducible transmembrane protein 3 (IFITM3). IFITM3 has antiviral activity and depends on lipid metabolism in the cell. While many viruses depend on lipid scaffolds such as lipid rafts for assembly, viruses also depend on cholesterol dynamics for early and late infection. Interestingly, perturbation of cholesterol homeostasis can block viral entry steps (e.g., Ref. [66]). IFITM3 was shown to interact with VAP-A, a membrane-associated protein on the ER, on tight junctions and synaptic vesicles. Humans contain two VAP genes, VAP-A and VAP-B. VAP-A, as well as B, has an MSP domain at the N terminus, followed by a coiled coil region and ending in transmembrane domain that anchors the protein into the ER membrane. The MSP region has been shown to interact with FFAT domains commonly found on oxysterol binding proteins cholesterol sensor proteins, such as ORP1L. VAP-A has also been shown to interact with viral proteins from both HCV and Norwalk viruses and has been shown to modulate viral transport [67], [68]. Prosser et al. determined that VAP-A creates a block in vesicular trafficking from the ER to the Golgi network and resolved the block by overexpressing VAP-A as well as mutants of this protein [69]. The overexpression also had dramatic effects on IFITM3 restriction activity, in that this blocked its interaction with ORP1L [70] thereby preventing the fusion of intraluminal virion-containing vesicles with endosomal membranes and thereby blocking virus release.
Interestingly, Rocha et al. [71] found that purified VAP-A was able to remove the C25 fragment of p150glued from preassembled, and purified, Rab7–RILP–ORP1L complexes on metal affinity beads. Through in vivo experiments they determined that late endosome/lysosomes were often in contact with the ER membrane and the conformation of ORP1L could dictate whether there were more or less membrane contact sites. When cholesterol is high, ORP1L takes on a conformation where the FFAT domain is sequestered and therefore cannot interact with ER-bound VAP-A. As a consequence, Dynein–p150glued–RILP promotes juxtanuclear localization of late endosome/lysosomes. In contrast, Dynein function is blocked when ORP1L is in a low cholesterol conformation with its FFAT domain exposed to interact with the MSP domain of the ER-associated, VAP-A. Importantly, HIV-1 subverts the cholesterol sensing mediated by ORP1L by maintaining peripheral localization of late endosomes/lysosomes not only impacting on HIV-1 protein and RNA localization but also on mTORC1 positioning and activity [71a] (Fig. 10.1).
As an integral component of late endosome/lysosomal membranes, the small GTPase, Rab7, which associates to RILP and the Dynein p150Glued, was shown to play a critical role in viral restriction mediated by the bone marrow stromal antigen 2 or Tetherin host protein. Tetherin, a lipid raft-associated protein that is encoded by the bst2 gene, is the last restriction factor discussed in this chapter. Tetherin, as the name implies promotes tethering of mature virus particles to the outer plasma membrane. Interestingly, HIV-1 and other viruses counter the innate activity of this host factor to allow for budding viruses to be released from the cell surface. While intense research into this factor has yielded almost a complete understanding of structure and mechanism of action, recent work has now identified a role of the Dynein effector, Rab7a, in enhancing Tetherin restriction activity in virus release and budding [34]. Similar to findings for HCV, this discovery suggests that endosomal sorting somehow influences the virus restriction activity of Tetherin, mediated by Dynein, thereby promoting both egress of late endosome/lysosomes and mature virus particle release from the cell surface, but this relationship will require further substantiation.
10.6. Dyneins and nuclear integration of viral DNA
On entry into the host cell, viruses translocate within the cytoplasm to sites of replication, to a perinuclear region and can use this as a scaffold to enter the nucleus in viruses that require a nuclear intermediate. In a manner identical to host cell vesicles or macromolecular complexes that are transported intracellularly, viral translocation is not achieved by passive diffusion but by energy-dependent, active transport mechanisms. Many viruses are transported along MTs and interact with various cellular cofactors including factors associated to the Dynein motor complex, as described herein. During the early stages of HIV-1 replication following acute infection for instance, the viral genomic RNA is reverse-transcribed into a complementary DNA and forms a preintegration complex, which undergoes intracytoplasmic retrograde transportation and nuclear import, and subsequently integrates into the host cell genome (reviewed in Ref. [72], [73]). During these processes, different viral proteins interact with and utilize various cellular proteins for replication. Genome-wide si/shRNA screening and other functional studies have uncovered a large number of host proteins with putative roles in HIV-1 replication (reviewed in Ref. [74], [75], [76]). However, molecular events associated with HIV-1 retrograde transport in the cytoplasm are still not well understood. Interestingly, several studies have indicated that a functional Dynein complex or intact MT network is essential for efficient HIV-1 uncoating following entry [53], [77], [78] and retrograde transport toward the nucleus prior to nuclear integration [50], [77], [79].
Recent studies have demonstrated that the inhibition of DHC (DYNC1H1) by siRNA or the disruption of the intact MTs by nocodazole treatment delayed the uncoating process during HIV-1 infection [53], [77]. This suggests that the Dynein complex and intact MT network facilitate the HIV-1 uncoating [53], [77]. Another study showed that the DYNLL1-KD or the disruption of HIV-1 integrase/DYNLL1 interaction resulted in a significant loss of reverse transcription and an increase in the rate of HIV-1 uncoating [78]. However, it is still unclear whether it is the Dynein complex–associated DYNLL1 that promotes HIV-1 reverse transcription and uncoating. Interestingly, a previous study suggested that DYNLL1 may not be able to mediate cargo recruitment to the Dynein complex [80]. Therefore, further investigation is required to elucidate the roles of DYNLL1 and Dynein complex or MT network in HIV-1 replication.
The requirement of HIV integrase/DYNLL1 for the proper uncoating of HIV-1 and its reverse transcription has also been demonstrated [78]. However, the molecular mechanisms of this virus–host interaction that contributes to the proper uncoating of HIV-1 and reverse transcription will require further characterization. DYNLL1 has been shown to bind to a number of cellular proteins and facilitates protein complex formation [81]. Therefore, it can be speculated that cellular proteins may be recruited to the reverse transcription complex via DYNLL1 interaction, which helps HIV-1 RTC reorganization and/or stabilization, and consequently contributes to proper uncoating and/or efficient reverse transcription. Conversely, the disruption of HIV-1 integrase/DYNLL1 interaction could lead to aberrant uncoating and the formation of unstable RTC, resulting in low levels of HIV-1 reverse transcription. These findings provide evidence for a possible alternative mechanism by which HIV-1 integrase facilitates the proper uncoating and efficient viral reverse transcription.
10.7. Posttranslationally modified microtubules and Dynein
A major theme in virus-cell biology is the ability of the virus to commandeer factors to enable all aspects of the replication cycle. A variety of studies have focused attention on how viruses traffic on cytoskeletal elements. Notable interactions include motor protein translocation on MTs with cargo, including vesicles and viral components such as RNPs and incoming capsids. Indeed, these are critical to the establishment of infection. Nevertheless, recent work has uncovered that viruses will utilize more stable MTs that are modified posttranslationally, either by acetylation or tyrosination to confer enhanced stability and perhaps secure efficient viral targeting. This would contribute to the delivery of viral components to destinations in the cells during both ingress and egress. For example, resistance to the effects of the MT destabilizing agent nocodazole as mentioned above led to investigations on characterizing subpopulations of MTs that resisted nocodazole treatment. Indeed, several herpesviruses were found associated to acetylated MTs [82], [83]. Recent evidence for retroviruses also demonstrates that there is a preference for acetylated and detyrosinated stable MTs early in infection [58], for which Dynein activity was shown to be critical [50]. As such, recent work on the specific roles of these posttranslationally modified MTs [84], [85] highlights earlier work from several groups that demonstrate in vivo and in live cell experiments that retroviruses require late endosome/lysosomes for intracellular trafficking [35], [36], [86]. Future work focusing on the selectivity achieved by viruses for subpopulations of MTs during both ingress and egress should be informative.
10.8. Emerging viruses and co-opting of Dynein
Recent global threats to human health from emerging viruses have fueled intense efforts into the understanding of virus molecular and cellular biology. Emerging viruses include Ebola, severe acute and Middle East respiratory syndrome Coronavirus (SARS/MERS-CoV), Zika, and Chikungunya viruses, among others [87]. Despite new vaccines that are in the pipeline [88], [89], [90], scant new information on these viruses exist, especially in regard to how they commandeer host Dynein and associated machineries. For example, Ebola virus was shown to interact with the highly conserved 8 kDa cytoplasmic light chain (LC8), like many viruses ([91]; reviewed in Ref. [1]) but few details at the time were available. Current evidence indicates that this interaction indeed enhances RNA replication of this highly pathogenic virus [91]. Furthermore, Ebola replication was found to be sensitive Dynein as the expression of mutants of the cholesterol sensor, ORP1L, which acts as a regulator of Dynein motor binding to RILP, dramatically affected virus output [92]. In contrast, scant evidence for an association between SARS-CoV and Dynein motor complexes is available. Indeed, the SARS-CoV Envelope membrane–associated Envelope protein brought down the DHC, among other proteins; but little else is understood about this potential interaction [93]. Members of the flaviviridae including West Nile, HCV, Dengue, and Zika among others have each their own prevalence worldwide, the last two of which are considered as the current emerging threats to human health [94], in North America and elsewhere. There are little data on virus–host interactions between Zika and components of the Dynein machinery in mid-2017. Nevertheless, as Zika infects several cell types including neuronal cell lineages, and its study does not require high-level biosafety containment, a role for Dynein, as is the case for HCV [39], in executing virus-mediated programs will likely be revealed through current and intense further investigation.
Major outstanding questions
In light of the characterized interplay between viruses and molecular motor proteins such as Dynein and Kinesin, several questions remain, but are not necessarily readily answered. These include:
-
•
Are virus–Dynein interactions druggable in selective and specific ways?
-
•
Can the targeting of virus–Dynein interactions help in enhancing immune responses to infection?
-
•
In what other ways do viruses commandeer intracellular trafficking machineries for early and late replication events?
-
•
Is the selective commandeering of stable populations of microtubules a general feature of virus-mediated subversion of the host cell?
Acknowledgments
We would like to thank Shringar Rao for editorial input and Michael Sacher for critical contributions. This work was supported in part by grants from the Canadian Institutes of Health Research to X.Y., L.B., and A.J.M.
References
- 1.Mouland A.J., Milev M.P. Role of dynein in viral pathogenesis. In: King S.M., editor. Dynein: Structure, Biology and Disease. Elsevier, Inc.; 2012. pp. 561–583. [Google Scholar]
- 2.Mallik R., Gross S.P. Molecular motors: strategies to get along. Curr. Biol. 2004;14(22):R971–R982. doi: 10.1016/j.cub.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Su Y., Qiao W., Guo T., Tan J., Li Z., Chen Y. Microtubule-dependent retrograde transport of bovine immunodeficiency virus. Cell Microbiol. 2010;12(8):1098–1107. doi: 10.1111/j.1462-5822.2010.01453.x. [DOI] [PubMed] [Google Scholar]
- 4.Navarro C., Puthalakath H., Adams J.M., Strasser A., Lehmann R. Egalitarian binds dynein light chain to establish oocyte polarity and maintain oocyte fate. Nat. Cell Biol. 2004;6(5):427–435. doi: 10.1038/ncb1122. [DOI] [PubMed] [Google Scholar]
- 5.Lo K.W., Kogoy J.M., Pfister K.K. The DYNLT3 light chain directly links cytoplasmic dynein to a spindle checkpoint protein, Bub3. J. Biol. Chem. 2007;282(15):11205–11212. doi: 10.1074/jbc.M611279200. [DOI] [PubMed] [Google Scholar]
- 6.Dohner K., Wolfstein A., Prank U., Echeverri C., Dujardin D., Vallee R. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell. 2002;13(8):2795–2809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaichick S.V., Bohannon K.P., Hughes A., Sollars P.J., Pickard G.E., Smith G.A. The herpesvirus VP1/2 protein is an effector of dynein-mediated capsid transport and neuroinvasion. Cell Host Microbe. 2013;13(2):193–203. doi: 10.1016/j.chom.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radtke K., Kieneke D., Wolfstein A., Michael K., Steffen W., Scholz T. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 2010;6(7):e1000991. doi: 10.1371/journal.ppat.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudin R., de Alencar B.C., Arhel N., Benaroch P. HIV trafficking in host cells: motors wanted! Trends Cell Biol. 2013;23(12):652–662. doi: 10.1016/j.tcb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Dodding M.P., Way M. Coupling viruses to dynein and kinesin-1. EMBO J. 2011;30(17):3527–3539. doi: 10.1038/emboj.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slonska A., Polowy R., Golke A., Cymerys J. Role of cytoskeletal motor proteins in viral infection. Postepy Hig. Med. Dosw. (Online) 2012;66:810–817. doi: 10.5604/17322693.1016360. [DOI] [PubMed] [Google Scholar]
- 12.Merino-Gracia J., Garcia-Mayoral M.F., Rodriguez-Crespo I. The association of viral proteins with host cell dynein components during virus infection. FEBS J. 2011;278(17):2997–3011. doi: 10.1111/j.1742-4658.2011.08252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan G.S., Preuss M.A., Williams J.C., Schnell M.J. The dynein light chain 8 binding motif of rabies virus phosphoprotein promotes efficient viral transcription. Proc. Natl. Acad. Sci. U.S.A. 2007;104(17):7229–7234. doi: 10.1073/pnas.0701397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raux H., Flamand A., Blondel D. Interaction of the rabies virus P protein with the LC8 dynein light chain. J. Virol. 2000;74(21):10212–10216. doi: 10.1128/jvi.74.21.10212-10216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poisson N., Real E., Gaudin Y., Vaney M.C., King S., Jacob Y. Molecular basis for the interaction between rabies virus phosphoprotein P and the dynein light chain LC8: dissociation of dynein-binding properties and transcriptional functionality of P. J. Gen. Virol. 2001;82(Pt 11):2691–2696. doi: 10.1099/0022-1317-82-11-2691. [DOI] [PubMed] [Google Scholar]
- 16.Schneider M.A., Spoden G.A., Florin L., Lambert C. Identification of the dynein light chains required for human papillomavirus infection. Cell Microbiol. 2011;13(1):32–46. doi: 10.1111/j.1462-5822.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 17.Schroer T.A. Dynactin. Annu. Rev. Cell Dev. Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 18.Engelke M.F., Burckhardt C.J., Morf M.K., Greber U.F. The dynactin complex enhances the speed of microtubule-dependent motions of adenovirus both towards and away from the nucleus. Viruses. 2011;3(3):233–253. doi: 10.3390/v3030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso C., Miskin J., Hernaez B., Fernandez-Zapatero P., Soto L., Canto C. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 2001;75(20):9819–9827. doi: 10.1128/JVI.75.20.9819-9827.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller M.J., Klumpp S., Lipowsky R. Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc. Natl. Acad. Sci. U.S.A. 2008;105(12):4609–4614. doi: 10.1073/pnas.0706825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kural C., Kim H., Syed S., Goshima G., Gelfand V.I., Selvin P.R. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308(5727):1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno N., Toba S., Edamatsu M., Watai-Nishii J., Hirokawa N., Toyoshima Y.Y. Dynein and kinesin share an overlapping microtubule-binding site. Embo J. 2004;23(13):2459–2467. doi: 10.1038/sj.emboj.7600240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sfakianos J.N., LaCasse R.A., Hunter E. The M-PMV cytoplasmic targeting-retention signal directs nascent gag polypeptides to a pericentriolar region of the cell. Traffic. 2003;4(10):660–670. doi: 10.1034/j.1600-0854.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 24.Vlach J., Lipov J., Rumlova M., Veverka V., Lang J., Srb P. D-retrovirus morphogenetic switch driven by the targeting signal accessibility to Tctex-1 of dynein. Proc. Natl. Acad. Sci. U.S.A. 2008;105(30):10565–10570. doi: 10.1073/pnas.0801765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torisawa T., Nakayama A., Furuta K., Yamada M., Hirotsune S., Toyoshima Y.Y. Functional dissection of LIS1 and NDEL1 towards understanding the molecular mechanism of cytoplasmic dynein regulation. J. Biol. Chem. 2010;286(3):1959–1965. doi: 10.1074/jbc.M110.169847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada M., Toba S., Takitoh T., Yoshida Y., Mori D., Nakamura T. mNUDC is required for plus-end-directed transport of cytoplasmic dynein and dynactins by kinesin-1. Embo J. 2010;29(3):517–531. doi: 10.1038/emboj.2009.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada M., Toba S., Yoshida Y., Haratani K., Mori D., Yano Y. LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. Embo J. 2008;27(19):2471–2483. doi: 10.1038/emboj.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Mareuil J., Carre M., Barbier P., Campbell G.R., Lancelot S., Opi S. HIV-1 Tat protein enhances microtubule polymerization. Retrovirology. 2005;2:5. doi: 10.1186/1742-4690-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epie N., Ammosova T., Sapir T., Voloshin Y., Lane W.S., Turner W. HIV-1 Tat interacts with LIS1 protein. Retrovirology. 2005;2:6. doi: 10.1186/1742-4690-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts N.R., Sackett D.L., Ward R.D., Miller M.W., Wingfield P.T., Stahl S.S. HIV-1 rev depolymerizes microtubules to form stable bilayered rings. J. Cell Biol. 2000;150(2):349–360. doi: 10.1083/jcb.150.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jouvenet N., Wileman T. African swine fever virus infection disrupts centrosome assembly and function. J. Gen. Virol. 2005;86(Pt 3):589–594. doi: 10.1099/vir.0.80623-0. [DOI] [PubMed] [Google Scholar]
- 32.Ploubidou A., Moreau V., Ashman K., Reckmann I., Gonzalez C., Way M. Vaccinia virus infection disrupts microtubule organization and centrosome function. Embo J. 2000;19(15):3932–3944. doi: 10.1093/emboj/19.15.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scherer J., Vallee R.B. Conformational changes in the adenovirus hexon subunit responsible for regulating cytoplasmic dynein recruitment. J. Virol. 2015;89(2):1013–1023. doi: 10.1128/JVI.02889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherer J., Yi J., Vallee R.B. PKA-dependent dynein switching from lysosomes to adenovirus: a novel form of host-virus competition. J. Cell Biol. 2014;205(2):163–177. doi: 10.1083/jcb.201307116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehmann M., Milev M.P., Abrahamyan L., Yao X.J., Pante N., Mouland A.J. Intracellular transport of human immunodeficiency virus type 1 genomic RNA and viral production are dependent on dynein motor function and late endosome positioning. J. Biol. Chem. 2009;284(21):14572–14585. doi: 10.1074/jbc.M808531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molle D., Segura-Morales C., Camus G., Berlioz-Torrent C., Kjems J., Basyuk E. Endosomal trafficking of HIV-1 gag and genomic RNAs regulates viral egress. J. Biol. Chem. 2009;284(29):19727–19743. doi: 10.1074/jbc.M109.019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumas A., Le-Bury G., Marie-Anais F., Herit F., Mazzolini J., Guilbert T. The HIV-1 protein Vpr impairs phagosome maturation by controlling microtubule-dependent trafficking. J. Cell Biol. 2015;211(2):359–372. doi: 10.1083/jcb.201503124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Low A., Gavriilidis G., Larke N., B-Lajoie M.R., Drouin O., Stover J. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low- and middle-income countries: a systematic review and meta-analysis. Clin. Infect. Dis. 2016;62(12):1595–1603. doi: 10.1093/cid/ciw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wozniak A.L., Long A., Jones-Jamtgaard K.N., Weinman S.A. Hepatitis C virus promotes virion secretion through cleavage of the Rab7 adaptor protein RILP. Proc. Natl. Acad. Sci. U.S.A. 2016;113(44):12484–12489. doi: 10.1073/pnas.1607277113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scully E.P., Lockhart A., Garcia-Beltran W., Palmer C.D., Musante C., Rosenberg E. Innate immune reconstitution with suppression of HIV-1. JCI Insight. 2016;1(3):e85433. doi: 10.1172/jci.insight.85433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanchet F.P., Moris A., Nikolic D.S., Lehmann M., Cardinaud S., Stalder R. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32(5):654–669. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Luijn M.M., Kreft K.L., Jongsma M.L., Mes S.W., Wierenga-Wolf A.F., van Meurs M. Multiple sclerosis-associated CLEC16A controls HLA class II expression via late endosome biogenesis. Brain. 2015;138(Pt 6):1531–1547. doi: 10.1093/brain/awv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radtke K., Dohner K., Sodeik B. Viral interactions with the cytoskeleton: a hitchhiker’s guide to the cell. Cell Microbiol. 2006;8(3):387–400. doi: 10.1111/j.1462-5822.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- 44.Cohen S., Au S., Pante N. How viruses access the nucleus. Biochimica Biophys. Acta. 2011;1813(9):1634–1645. doi: 10.1016/j.bbamcr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Suomalainen M., Nakano M.Y., Keller S., Boucke K., Stidwill R.P., Greber U.F. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 1999;144(4):657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leopold P.L., Kreitzer G., Miyazawa N., Rempel S., Pfister K.K., Rodriguez-Boulan E. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum. Gene Ther. 2000;11(1):151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 47.Xiao P.J., Samulski R.J. Cytoplasmic trafficking, endosomal escape, and perinuclear accumulation of adeno-associated virus type 2 particles are facilitated by microtubule network. J. Virol. 2012;86(19):10462–10473. doi: 10.1128/JVI.00935-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolfstein A., Nagel C.H., Radtke K., Dohner K., Allan V.J., Sodeik B. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic. 2006;7(2):227–237. doi: 10.1111/j.1600-0854.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 49.Petit C., Giron M.L., Tobaly-Tapiero J., Bittoun P., Real E., Jacob Y. Targeting of incoming retroviral gag to the centrosome involves a direct interaction with the dynein light chain 8. J. Cell Sci. 2003;116(Pt 16):3433–3442. doi: 10.1242/jcs.00613. [DOI] [PubMed] [Google Scholar]
- 50.McDonald D., Vodicka M.A., Lucero G., Svitkina T.M., Borisy G.G., Emerman M. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 2002;159(3):441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arhel N., Genovesio A., Kim K.A., Miko S., Perret E., Olivo-Marin J.C. Quantitative four-dimensional tracking of cytoplasmic and nuclear HIV-1 complexes. Nat. Methods. 2006;3(10):817–824. doi: 10.1038/nmeth928. [DOI] [PubMed] [Google Scholar]
- 52.Zamborlini A., Lehmann-Che J., Clave E., Giron M.L., Tobaly-Tapiero J., Roingeard P. Centrosomal pre-integration latency of HIV-1 in quiescent cells. Retrovirology. 2007;4:63. doi: 10.1186/1742-4690-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pawlica P., Berthoux L. Cytoplasmic dynein promotes HIV-1 uncoating. Viruses. 2014;6(11):4195–4211. doi: 10.3390/v6114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pawlica P., Dufour C., Berthoux L. Inhibition of microtubules and dynein rescues human immunodeficiency virus type 1 from owl monkey TRIMCyp-mediated restriction in a cellular context-specific fashion. J. Gen. Virol. 2015;96(Pt 4):874–886. doi: 10.1099/jgv.0.000018. [DOI] [PubMed] [Google Scholar]
- 55.Pawlica P., Le Sage V., Poccardi N., Tremblay M.J., Mouland A.J., Berthoux L. Functional evidence for the involvement of microtubules and dynein motor complexes in TRIM5α-mediated restriction of retroviruses. J. Virol. 2014;88(10):5661–5676. doi: 10.1128/JVI.03717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Opazo T., Garces A., Tapia D., Barraza F., Bravo A., Schwenke T. Functional evidence of the involvement of the dynein light chain DYNLRB2 in murine leukemia virus infection. J. Virol. 2017;91(10) doi: 10.1128/JVI.00129-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malikov V., da Silva E.S., Jovasevic V., Bennett G., de Souza Aranha Vieira D.A., Schulte B. HIV-1 capsids bind and exploit the kinesin-1 adaptor FEZ1 for inward movement to the nucleus. Nat. Commun. 2015;6:6660. doi: 10.1038/ncomms7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabo Y., Walsh D., Barry D.S., Tinaztepe S., de Los Santos K., Goff S.P. HIV-1 induces the formation of stable microtubules to enhance early infection. Cell Host Microbe. 2013;14(5):535–546. doi: 10.1016/j.chom.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jovasevic V., Naghavi M.H., Walsh D. Microtubule plus end-associated CLIP-170 initiates HSV-1 retrograde transport in primary human cells. J. Cell Biol. 2015;211(2):323–337. doi: 10.1083/jcb.201505123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao J., Lin C., Wang H., Wang L., Zhou N., Jin Y. Circovirus transport proceeds via direct interaction of the cytoplasmic dynein IC1 subunit with the viral capsid protein. J. Virol. 2015;89(5):2777–2791. doi: 10.1128/JVI.03117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merindol N., Berthoux L. Restriction factors in HIV-1 disease progression. Curr. HIV Res. 2015;13(6):448–461. doi: 10.2174/1570162x13666150608104412. [DOI] [PubMed] [Google Scholar]
- 62.Lukic Z., Hausmann S., Sebastian S., Rucci J., Sastri J., Robia S.L. TRIM5α associates with proteasomal subunits in cells while in complex with HIV-1 virions. Retrovirology. 2011;8:93. doi: 10.1186/1742-4690-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diaz-Griffero F., Li X., Javanbakht H., Song B., Welikala S., Stremlau M. Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology. 2006;349(2):300–315. doi: 10.1016/j.virol.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 64.Rajsbaum R., Garcia-Sastre A., Versteeg G.A. TRIMmunity: the roles of the TRIM E3-ubiquitin ligase family in innate antiviral immunity. J. Mol. Biol. 2014;426(6):1265–1284. doi: 10.1016/j.jmb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cox T.C. The microtubule-associated C-I subfamily of TRIM proteins and the regulation of polarized cell responses. Adv. Exp. Med. Biol. 2012;770:105–118. doi: 10.1007/978-1-4614-5398-7_8. [DOI] [PubMed] [Google Scholar]
- 66.Poh M.K., Shui G., Xie X., Shi P.Y., Wenk M.R., Gu F. U18666A, an intra-cellular cholesterol transport inhibitor, inhibits dengue virus entry and replication. Antiviral Res. 2012;93(1):191–198. doi: 10.1016/j.antiviral.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Xu G., Xin X., Zheng C. GPS2 is required for the association of NS5A with VAP-A and hepatitis C virus replication. PLoS One. 2013;8(11):e78195. doi: 10.1371/journal.pone.0078195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ettayebi K., Hardy M.E. Norwalk virus nonstructural protein p48 forms a complex with the SNARE regulator VAP-A and prevents cell surface expression of vesicular stomatitis virus G protein. J. Virol. 2003;77(21):11790–11797. doi: 10.1128/JVI.77.21.11790-11797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prosser D.C., Tran D., Gougeon P.Y., Verly C., Ngsee J.K. FFAT rescues VAPA-mediated inhibition of ER-to-Golgi transport and VAPB-mediated ER aggregation. J. Cell Sci. 2008;121(Pt 18):3052–3061. doi: 10.1242/jcs.028696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amini-Bavil-Olyaee S., Choi Y.J., Lee J.H., Shi M., Huang I.C., Farzan M. The antiviral effector IFITM3 disrupts intracellular cholesterol homeostasis to block viral entry. Cell Host Microbe. 2013;13(4):452–464. doi: 10.1016/j.chom.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rocha N., Kuijl C., van der Kant R., Janssen L., Houben D., Janssen H. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 glued and late endosome positioning. J. Cell Biol. 2009;185(7):1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71a.Cinti A., Le Sage V., Milev MP., Valiente-Echeverría F., Crossie C., Miron MJ., Panté N., Olivier M., Mouland AJ. HIV-1 enhances mTORC1 activity and repositions lysosomes to the periphery by co-opting Rag GTPases. Sci Rep. 2017 Jul 14;7(1):5515. doi: 10.1038/s41598-017-05410-0. doi: 10.1038/s41598-017-05410-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jayappa K.D., Ao Z., Yao X. The HIV-1 passage from cytoplasm to nucleus: the process involving a complex exchange between the components of HIV-1 and cellular machinery to access nucleus and successful integration. Int. J. Biochem. Mol. Biol. 2012;3(1):70–85. [PMC free article] [PubMed] [Google Scholar]
- 73.Nisole S., Saib A. Early steps of retrovirus replicative cycle. Retrovirology. 2004;1:9. doi: 10.1186/1742-4690-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bieniasz P.D. An overview of intracellular interactions between immunodeficiency viruses and their hosts. Aids. 2012;26(10):1243–1254. doi: 10.1097/QAD.0b013e328353bd04. [DOI] [PubMed] [Google Scholar]
- 75.Kok K.H., Lei T., Jin D.Y. siRNA and shRNA screens advance key understanding of host factors required for HIV-1 replication. Retrovirology. 2009;6:78. doi: 10.1186/1742-4690-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Friedrich B.M., Dziuba N., Li G., Endsley M.A., Murray J.L., Ferguson M.R. Host factors mediating HIV-1 replication. Virus Res. 2011;161(2):101–114. doi: 10.1016/j.virusres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 77.Lukic Z., Dharan A., Fricke T., Diaz-Griffero F., Campbell E.M. HIV-1 uncoating is facilitated by dynein and kinesin 1. J. Virol. 2014;88(23):13613–13625. doi: 10.1128/JVI.02219-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jayappa K.D., Ao Z., Wang X., Mouland A.J., Shekhar S., Yang X. Human immunodeficiency virus type 1 employs the cellular dynein light chain 1 protein for reverse transcription through interaction with its integrase protein. J. Virol. 2015;89(7):3497–3511. doi: 10.1128/JVI.03347-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arhel N. Revisiting HIV-1 uncoating. Retrovirology. 2010;7:96. doi: 10.1186/1742-4690-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams J.C., Roulhac P.L., Roy A.G., Vallee R.B., Fitzgerald M.C., Hendrickson W.A. Structural and thermodynamic characterization of a cytoplasmic dynein light chain-intermediate chain complex. Proc. Natl. Acad. Sci. U.S.A. 2007;104(24):10028–10033. doi: 10.1073/pnas.0703614104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rapali P., Szenes A., Radnai L., Bakos A., Pal G., Nyitray L. DYNLL/LC8: a light chain subunit of the dynein motor complex and beyond. FEBS J. 2011;278(17):2980–2996. doi: 10.1111/j.1742-4658.2011.08254.x. [DOI] [PubMed] [Google Scholar]
- 82.Frampton A.R., Jr., Uchida H., von Einem J., Goins W.F., Grandi P., Cohen J.B. Equine herpesvirus type 1 (EHV-1) utilizes microtubules, dynein, and ROCK1 to productively infect cells. Vet. Microbiol. 2010;141(1–2):12–21. doi: 10.1016/j.vetmic.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naranatt P.P., Krishnan H.H., Smith M.S., Chandran B. Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J. Virol. 2005;79(2):1191–1206. doi: 10.1128/JVI.79.2.1191-1206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pu J., Guardia C.M., Keren-Kaplan T., Bonifacino J.S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 2016;129(23):4329–4339. doi: 10.1242/jcs.196287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guardia C.M., Farias G.G., Jia R., Pu J., Bonifacino J.S. BORC functions upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep. 2016;17(8):1950–1961. doi: 10.1016/j.celrep.2016.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Basyuk E., Galli T., Mougel M., Blanchard J.M., Sitbon M., Bertrand E. Retroviral genomic RNAs are transported to the plasma membrane by endosomal vesicles. Dev. Cell. 2003;5(1):161–174. doi: 10.1016/s1534-5807(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 87.Scully C., Samaranayake L.P. Emerging and changing viral diseases in the new millennium. Oral Dis. 2016;22(3):171–179. doi: 10.1111/odi.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de La Vega M.A., Stein D., Kobinger G.P. Ebolavirus evolution: past and present. PLoS Pathog. 2015;11(11):e1005221. doi: 10.1371/journal.ppat.1005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marzi A., Hanley P.W., Haddock E., Martellaro C., Kobinger G., Feldmann H. Efficacy of vesicular stomatitis virus-ebola virus postexposure treatment in rhesus macaques infected with Ebola virus Makona. J. Infect. Dis. 2016;214(suppl 3):S360–S366. doi: 10.1093/infdis/jiw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roques P., Ljungberg K., Kummerer B.M., Gosse L., Dereuddre-Bosquet N., Tchitchek N. Attenuated and vectored vaccines protect nonhuman primates against Chikungunya virus. JCI Insight. 2017;2(6):e83527. doi: 10.1172/jci.insight.83527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luthra P., Jordan D.S., Leung D.W., Amarasinghe G.K., Basler C.F. Ebola virus VP35 interaction with dynein LC8 regulates viral RNA synthesis. J. Virol. 2015;89(9):5148–5153. doi: 10.1128/JVI.03652-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van der Kant R., Fish A., Janssen L., Janssen H., Krom S., Ho N. Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L. J. Cell Sci. 2013;126(Pt 15):3462–3474. doi: 10.1242/jcs.129270. [DOI] [PubMed] [Google Scholar]
- 93.Alvarez E., DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeno J.M., Marcos-Villar L., Enjuanes L. The envelope protein of severe acute respiratory syndrome coronavirus interacts with the non-structural protein 3 and is ubiquitinated. Virology. 2010;402(2):281–291. doi: 10.1016/j.virol.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ippolito G., Rezza G. Preface – emerging viruses: from early detection to intervention. Adv. Exp. Med. Biol. 2017;972:1–5. doi: 10.1007/5584_2017_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelkar S.A., Pfister K.K., Crystal R.G., Leopold P.L. Cytoplasmic dynein mediates adenovirus binding to microtubules. J. Virol. 2004;78(18):10122–10132. doi: 10.1128/JVI.78.18.10122-10132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gazzola M., Burckhardt C.J., Bayati B., Engelke M., Greber U.F., Koumoutsakos P. A stochastic model for microtubule motors describes the in vivo cytoplasmic transport of human adenovirus. PLoS Comput. Biol. 2009;5(12):e1000623. doi: 10.1371/journal.pcbi.1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y., Shevchenko A., Shevchenko A., Berk A.J. Adenovirus exploits the cellular aggresome response to accelerate inactivation of the MRN complex. J. Virol. 2005;79(22):14004–14016. doi: 10.1128/JVI.79.22.14004-14016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bremner K.H., Scherer J., Yi J., Vershinin M., Gross S.P., Vallee R.B. Adenovirus transport via direct interaction of cytoplasmic dynein with the viral capsid hexon subunit. Cell Host Microbe. 2009;6(6):523–535. doi: 10.1016/j.chom.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suomalainen M., Nakano M.Y., Boucke K., Keller S., Greber U.F. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. Embo J. 2001;20(6):1310–1319. doi: 10.1093/emboj/20.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lukashok S.A., Tarassishin L., Li Y., Horwitz M.S. An adenovirus inhibitor of tumor necrosis factor alpha-induced apoptosis complexes with dynein and a small GTPase. J. Virol. 2000;74(10):4705–4709. doi: 10.1128/jvi.74.10.4705-4709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mabit H., Nakano M.Y., Prank U., Saam B., Dohner K., Sodeik B. Intact microtubules support adenovirus and herpes simplex virus infections. J. Virol. 2002;76(19):9962–9971. doi: 10.1128/JVI.76.19.9962-9971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hernaez B., Diaz-Gil G., Garcia-Gallo M., Ignacio Quetglas J., Rodriguez-Crespo I., Dixon L. The African swine fever virus dynein-binding protein p54 induces infected cell apoptosis. FEBS Lett. 2004;569(1–3):224–228. doi: 10.1016/j.febslet.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Kubota T., Matsuoka M., Chang T.H., Bray M., Jones S., Tashiro M. Ebolavirus VP35 interacts with the cytoplasmic dynein light chain 8. J. Virol. 2009;83(13):6952–6956. doi: 10.1128/JVI.00480-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramanathan H.N., Chung D.H., Plane S.J., Sztul E., Chu Y.K., Guttieri M.C. Dynein-dependent transport of the hantaan virus nucleocapsid protein to the endoplasmic reticulum-golgi intermediate compartment. J. Virol. 2007;81(16):8634–8647. doi: 10.1128/JVI.00418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim S., Kim H.Y., Lee S., Kim S.W., Sohn S., Kim K. Hepatitis B virus X protein induces perinuclear mitochondrial clustering in microtubule- and dynein-dependent manners. J. Virol. 2007;81(4):1714–1726. doi: 10.1128/JVI.01863-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boulant S., Douglas M.W., Moody L., Budkowska A., Targett-Adams P., McLauchlan J. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner. Traffic. 2008;9(8):1268–1282. doi: 10.1111/j.1600-0854.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 107.Kannan H., Fan S., Patel D., Bossis I., Zhang Y.J. The hepatitis E virus open reading frame 3 product interacts with microtubules and interferes with their dynamics. J. Virol. 2009;83(13):6375–6382. doi: 10.1128/JVI.02571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dohner K., Radtke K., Schmidt S., Sodeik B. Eclipse phase of herpes simplex virus type 1 infection: efficient dynein-mediated capsid transport without the small capsid protein VP26. J. Virol. 2006;80(16):8211–8224. doi: 10.1128/JVI.02528-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Douglas M.W., Diefenbach R.J., Homa F.L., Miranda-Saksena M., Rixon F.J., Vittone V. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J. Biol. Chem. 2004;279(27):28522–28530. doi: 10.1074/jbc.M311671200. [DOI] [PubMed] [Google Scholar]
- 110.Sodeik B., Ebersold M.W., Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 1997;136(5):1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Topp K.S., Meade L.B., LaVail J.H. Microtubule polarity in the peripheral processes of trigeminal ganglion cells: relevance for the retrograde transport of herpes simplex virus. J. Neurosci. 1994;14(1):318–325. doi: 10.1523/JNEUROSCI.14-01-00318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ye G.J., Vaughan K.T., Vallee R.B., Roizman B. The herpes simplex virus 1 U(L)34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J. Virol. 2000;74(3):1355–1363. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Levesque K., Halvorsen M., Abrahamyan L., Chatel-Chaix L., Poupon V., Gordon H. Trafficking of HIV-1 RNA is mediated by heterogeneous nuclear ribonucleoprotein A2 expression and impacts on viral assembly. Traffic. 2006;7(9):1177–1193. doi: 10.1111/j.1600-0854.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 114.Schneider M.A., Spoden G.A., Florin L., Lambert C. Identification of the dynein light chains required for human papillomavirus infection. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 115.Florin L., Becker K.A., Lambert C., Nowak T., Sapp C., Strand D. Identification of a dynein interacting domain in the papillomavirus minor capsid protein l2. J. Virol. 2006;80(13):6691–6696. doi: 10.1128/JVI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mueller S., Cao X., Welker R., Wimmer E. Interaction of the poliovirus receptor CD155 with the dynein light chain Tctex-1 and its implication for poliovirus pathogenesis. J. Biol. Chem. 2002;277(10):7897–7904. doi: 10.1074/jbc.M111937200. [DOI] [PubMed] [Google Scholar]
- 117.Kondratova A.A., Neznanov N., Kondratov R.V., Gudkov A.V. Poliovirus protein 3A binds and inactivates LIS1, causing block of membrane protein trafficking and deregulation of cell division. Cell Cycle. 2005;4(10):1403–1410. doi: 10.4161/cc.4.10.2041. [DOI] [PubMed] [Google Scholar]
- 118.Ohka S., Sakai M., Bohnert S., Igarashi H., Deinhardt K., Schiavo G. Receptor-dependent and -independent axonal retrograde transport of poliovirus in motor neurons. J. Virol. 2009;83(10):4995–5004. doi: 10.1128/JVI.02225-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gonzalez Duran E., del Angel R.M., Salas Benito J.S. In vitro interaction of poliovirus with cytoplasmic dynein. Intervirology. 2007;50(3):214–218. doi: 10.1159/000099221. [DOI] [PubMed] [Google Scholar]
- 120.Rasalingam P., Rossiter J.P., Mebatsion T., Jackson A.C. Comparative pathogenesis of the SAD-L16 strain of rabies virus and a mutant modifying the dynein light chain binding site of the rabies virus phosphoprotein in young mice. Virus Res. 2005;111(1):55–60. doi: 10.1016/j.virusres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 121.Jacob Y., Badrane H., Ceccaldi P.E., Tordo N. Cytoplasmic dynein LC8 interacts with lyssavirus phosphoprotein. J. Virol. 2000;74(21):10217–10222. doi: 10.1128/jvi.74.21.10217-10222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mebatsion T. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J. Virol. 2001;75(23):11496–11502. doi: 10.1128/JVI.75.23.11496-11502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Havecker E.R., Gao X., Voytas D.F. The Sireviruses, a plant-specific lineage of the Ty1/copia retrotransposons, interact with a family of proteins related to dynein light chain 8. Plant Physiol. 2005;139(2):857–868. doi: 10.1104/pp.105.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ward B.M. Visualization and characterization of the intracellular movement of vaccinia virus intracellular mature virions. J. Virol. 2005;79(8):4755–4763. doi: 10.1128/JVI.79.8.4755-4763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Herrero-Martinez E., Roberts K.L., Hollinshead M., Smith G.L. Vaccinia virus intracellular enveloped virions move to the cell periphery on microtubules in the absence of the A36R protein. J. Gen. Virol. 2005;86(Pt 11):2961–2968. doi: 10.1099/vir.0.81260-0. [DOI] [PubMed] [Google Scholar]
- 126.Bauer A., Nolden T., Nemitz S., Perlson E., Finke S. A dynein light chain 1 binding motif in rabies virus polymerase L protein plays a role in microtubule reorganization and viral primary transcription. J. Virol. 2015;89(18):9591–9600. doi: 10.1128/JVI.01298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Valle-Tenney R., Opazo T., Cancino J., Goff S.P., Arriagada G. Dynein regulators are important for ecotropic murine leukemia virus infection. J. Virol. 2016;90(15):6896–6905. doi: 10.1128/JVI.00863-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Theerawatanasirikul S., Phecharat N., Prawettongsopon C., Chaicumpa W., Lekcharoensuk P. Dynein light chain DYNLL1 subunit facilitates porcine circovirus type 2 intracellular transports along microtubules. Arch. Virol. 2017;162(3):677–686. doi: 10.1007/s00705-016-3140-0. [DOI] [PubMed] [Google Scholar]
- 129.Caly L., Kassouf V.T., Moseley G.W., Diefenbach R.J., Cunningham A.L., Jans D.A. Fast track, dynein-dependent nuclear targeting of human immunodeficiency virus Vpr protein; impaired trafficking in a clinical isolate. Biochem. Biophys. Res. Commun. 2016;470(3):735–740. doi: 10.1016/j.bbrc.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 130.Banerjee I., Miyake Y., Nobs S.P., Schneider C., Horvath P., Kopf M. Influenza A virus uses the aggresome processing machinery for host cell entry. Science. 2014;346(6208):473–477. doi: 10.1126/science.1257037. [DOI] [PubMed] [Google Scholar]
- 131.Gladue D.P., O’Donnell V., Baker-Bransetter R., Pacheco J.M., Holinka L.G., Arzt J. Interaction of foot-and-mouth disease virus nonstructural protein 3A with host protein DCTN3 is important for viral virulence in cattle. J. Virol. 2014;88(5):2737–2747. doi: 10.1128/JVI.03059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zila V., Difato F., Klimova L., Huerfano S., Forstova J. Involvement of microtubular network and its motors in productive endocytic trafficking of mouse polyomavirus. PLoS One. 2014;9(5):e96922. doi: 10.1371/journal.pone.0096922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang W., Greene W., Gao S.J. Microtubule- and dynein-dependent nuclear trafficking of rhesus rhadinovirus in rhesus fibroblasts. J. Virol. 2012;86(1):599–604. doi: 10.1128/JVI.06129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


