1. INTRODUCTION
Results from laboratory testing are an important source of information for biosurveillance systems. Clinical laboratory tests are vital for the correct diagnosis and treatment of individuals. Clinical laboratories analyze blood, urine, mucus, saliva, respiratory secretions, cerebrospinal fluid, semen, vaginal secretions, sweat, feces, fluid aspirated from joints, and tissues from humans and animals. The tests performed include cell counts; analytical chemistries, including drug and toxin tests; and examinations to detect and identify microbes and markers of current and past infection. Environmental testing is critical to the detection of outbreaks, the prevention of disease, and the monitoring of the environment. Environmental laboratories analyze samples of water, food, air, soil, plant material, and unknown powders, as well as samples taken from surfaces for evidence of bacterial, viral, toxin, or chemical contamination.
Data produced by laboratories are important for biosurveillance of virtually every disease caused by biological agents, chemicals, or toxins. Data collected during the preanalytical, analytical, and postanalytical phases of testing can be captured and incorporated directly into biosurveillance systems. Preanalytical data, such as the type of test ordered and the reason for a test, can provide an early clue to the existence of an outbreak. Similarly, analytic results, such as the initial Gram stain of a cerebrospinal fluid specimen, can potentially confirm a diagnosis when combined with other clinical information, as it did in the first case of inhalational anthrax in the 2001 postal attack. The actual results of tests are obviously foundational to biosurveillance.
The range of tests offered by an individual laboratory varies significantly among laboratories in the United States. Many small laboratories perform a limited number of tests that are needed on an urgent basis or for screening purposes. Larger laboratories provide more complex confirmatory analyses. The majority of clinical laboratories in the United States are small laboratories located in physician offices; however, the larger laboratories account for a high volume of all tests performed.
Table 8.1 describes the clinical laboratory tests that contribute to the diagnosis of inhalational anthrax. Anthrax, as well as many other infectious diseases, is diagnosed after the performance of presumptive and confirmatory tests in combination with the clinical presentation. Clinical specimens, such as blood, cere-brospinal fluid, urine, sputum, throat swabs, and skin scrapings, are used to isolate a causative agent that is later subjected to further testing with confirmatory procedures to make the final identification. Preliminary tests results are sometimes released before the confirmatory tests results become available. When preliminary results are reported, the report often contains a statement about when final results will be available.
TABLE 8.1.
Clinical Laboratory Tests that Contribute to the Diagnosis of Anthrax
| Type of Test | Specimen | Expected Result |
|---|---|---|
| Nonspecific | ||
| White blood count | Whole blood | Elevated count |
| Cerebrospinal fluid (CSF) analysis | CSF | Normal |
| Presumptive | ||
| Growth on sheep blood | Blood, CSF, lesion | Growth within 24 hours |
| Colony morphology | Bacterial growth | Gray-white colonies, flat or convex, ground glass appearance |
| Gram stain | Bacterial growth | Large Gram-positive rods |
| Hemolysis | Bacterial growth | Clear hemolysis |
| Motility | Bacterial growth | Motile |
| Sporulation | Bacterial growth | Visual spores with malachite green stain |
| Confirmatory | ||
| Capsular stain | Bacterial growth | Visual capsules with M'Faydean stain |
| Gamma phage | Bacterial growth | Lysis by gamma phage |
| Direct fluorescent antibody (DFA) | Bacterial growth | Positive fluorescence |
| Polymerase chain reaction (PCR) | Bacterial growth | Positive PCR |
| Time-resolved fluorescence (TRF) | Bacterial growth | Positive TRF assay |
| Molecular characterization | Bacterial growth | Positive match with control materials |
Laboratories that produce the types of data most useful for biosurveillance include clinical laboratories operated by the human or animal health systems, commercial laboratories, and governmental laboratories. Laboratories typically specialize in either human or animal testing. Commercial laboratories are free-standing laboratories that are not associated with hospitals or healthcare facilities and that often provide a broad range of services over a wide geographical area. Governmental laboratories exist at the federal, state, and local level and often provide testing that is not readily available from other laboratories. We describe each of these types of laboratories in this chapter.
Laboratories that test for biologic agents are classified as biosafety level 1, 2, 3, or 4, with biosafety level 4 providing the highest degree of protection to personnel and the environment. Most clinical laboratory work is performed at level 2. These biosafety levels combine the use of laboratory safety practices, safety equipment, and laboratory facilities to provide greater levels of safety for the more dangerous organisms. Each level is specifically appropriate for handling various biologic agents (CDC, 1999).
2. CLINICAL LABORATORIES
There are more than 186,000 clinical laboratories in the United States in which clinical laboratory scientists, pathologists, medical technologists, and laboratory technicians perform 7 billion or more diagnostic tests annually (Centers for Medicare and Medicaid Services, 2004). The American Society for Clinical Pathology (ASCP) currently certifies more than 280,000 laboratory professionals who primarily work in clinical diagnostic and research laboratories. Clinical laboratory services in the United States are delivered either by commercial clinical laboratories or by “in-house” laboratories at healthcare facilities (hospitals, clinics, physician offices), departments of health, veterinary hospitals, and clinics. Individual veterinarians and physicians and the staff within their offices also conduct laboratory testing and produce results that are important for biosurveillance.
Professional laboratorians provide services that include simple, rapid screening tests; more advanced diagnostic tests; and complex confirmatory analyses. Clinicians use the information provided by laboratories to establish diagnoses and to make treatment decisions on virtually every patient. The demand for testing is increasing as the population ages and requires more health care, including analytical services. New tests are frequently introduced that improve diagnosis and care. The emergence of new diseases, the threat of bioterrorism, and the need for better biosurveillance systems have increased the demand for qualified laboratory professional in all fields, especially infectious disease testing. Although the demand for more laboratory professionals is increasing, the number of established laboratory professional training programs is decreasing.
The Centers for Medicare and Medicaid Services (CMS) registers all clinical laboratories in the United States that examine materials derived from the human body for diagnosis, prevention, or treatment. CMS administers the program for the Secretary of Health and Humans Services in conjunction with the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA). CMS regulates laboratories and establishes criteria for other organizations, such as state health departments, that also regulate laboratories to ensure compliance with the federal Clinical Laboratory Improvement Act (CLIA). CLIA was first enacted by Congress in 1967 and set guidelines for large independent laboratories. In 1988, Congress amended CLIA 67 to expand the type of laboratories that must comply; CLIA 88 further established quality standards for laboratories to ensure accuracy, reliability, and timeliness of test results.
In August, 2004, 186,734 laboratories were registered with the CMS (Centers for Medicare and Medicaid Services, 2004). Table 8.2 shows the distribution of these laboratories by type. More than 55% of these laboratories are located in physician offices. Skilled nursing facilities (7.9%), hospitals (4.6%), and home health agencies (4.4%) accounted for an additional 20% of laboratories. The remaining clinical laboratories are found in community health clinics, health maintenance organizations, blood banks, industrial facilities, and health departments. All of these laboratories are frequent sources of biosurveillance data.
TABLE 8.2.
Clinical Laboratories Registered by Center for Medicare and Medicaid Services (CMS) by Type of Facility, August 2004
| Type of Laboratory | Number | Percentage |
|---|---|---|
| Ambulatory surgical centers | 3,229 | 1.7 |
| Community clinic | 6,717 | 3.6 |
| Comprehensive outpatient rehabilitation facility | 205 | 0.1 |
| Ancillary testing site in healthcare facility | 2,712 | 1.5 |
| End-stage renal disease dialysis facility | 3,657 | 2.0 |
| Health fair | 482 | 0.3 |
| Health maintenance organization | 657 | 0.4 |
| Home health agency | 8,308 | 4.4 |
| Hospice | 1,285 | 0.7 |
| Hospital | 8,749 | 4.6 |
| Independent | 5,162 | 2.8 |
| Industrial | 1,647 | 0.9 |
| Insurance | 49 | 0.02 |
| Intermediate care facility for mentally retarded | 856 | 0.5 |
| Mobile laboratory | 1,096 | 0.5 |
| Pharmacy | 2,423 | 1.3 |
| School/student health facility | 1,771 | 0.9 |
| Skilled nursing facility/nursing facility | 14,792 | 7.9 |
| Physician office | 103,378 | 55.4 |
| Other practitioner | 2,239 | 1.2 |
| Tissue bank/repositories | 36 | 0.02 |
| Blood banks | 361 | 0.2 |
| Rural health clinic | 982 | 0.5 |
| Federally qualified health center | 247 | 0.1 |
| Ambulance | 1,993 | 1.1 |
| Public health laboratories | 119 | 0.06 |
| Other | 13,582 | 7.3 |
| 186,734 | 100 |
Over 58% of the 186,734 laboratories registered with CMS only perform simple tests. These simple tests, often referred to as waived tests, usually are based on commercially available test kits determined by the FDA to be sufficiently simple to perform that there is little risk of operator error. Laboratories that perform waived tests must enroll in the CLIA program, pay certification fees, and follow the manufacturers' test instructions. However, laboratories that perform only waived tests do not undergo inspections or need to comply with other CLIA requirements for larger laboratories. Laboratories that perform tests that use a microscope to examine specimens that are not easily transportable during the course of a patient visit are required to enroll in a CLIA program, pay applicable fees, and maintain certain quality and administrative requirements. These laboratories, known as provider-performed microscopy providers (PPMPs), represent 22% of the registered laboratories and are not subject to routine inspections. The remaining clinical laboratories must either be accredited by 1 of 6 approved clinical laboratory accrediting organizations (American Association of Blood Banks, American Osteopathic Association, American Society of Histocompatibility and Immunogenetics, College of American Pathologists; Commission on Office Laboratory Accreditation, Joint Commission on Accreditation of Healthcare Organizations) or obtain a compliance certificate directly from CMS.
In August 2004, these six organizations had accredited 15,667 (8.7%) laboratories, and CMS had certified 20,758 (11.5%) laboratories. The balance of the clinical laboratories (144,022) were either waived test providers or PPMPs (Centers for Medicare and Medicaid Services, 2004). Accreditation of clinical laboratories helps ensure that laboratories meet or exceed clinical standards established by governmental and nongovernmental associations. CMS, insurance companies, and healthcare plans require laboratories to be certified or accredited by these organizations for reimbursement of laboratory services.
Table 8.3 shows the annual test volume of the 20,758 clinical laboratories that were certified by CMS in August 2004. Over 84% of these laboratories perform fewer than 25,000 tests per year. Only 125 of these laboratories performed 500,000 or more tests per year. These 125 laboratories represent only 0.6% of all laboratories, yet they perform approximately 20% of all tests.
TABLE 8.3.
Clinical Laboratories Registered by the Center for Medicare and Medicaid Services (CMS) by Annual Test Volume, August 2004
| Annual Test Volume | Total Number of Laboratories | Percentage of Laboratories | Physician Office Laboratories | Percentage of Physician Office Laboratories |
|---|---|---|---|---|
| ≤2000 | 8,955 | 43.1% | 6,998 | 51.1% |
| 2001–10,000 | 6,212 | 29.9% | 4,469 | 32.7% |
| 10,001–25,000 | 2,347 | 11.4% | 1,206 | 8.7% |
| 25,001–50,000 | 1,303 | 6.3% | 475 | 3.5% |
| 50,001–75,000 | 643 | 3.1% | 207 | 1.5% |
| 75,001–100,000 | 384 | 1.8% | 111 | 0.8% |
| 100,001–500,000 | 789 | 3.8% | 221 | 1.5% |
| 500,001–1,000,000 | 74 | 0.4% | 19 | 0.1% |
| >1,000,000 | 51 | 0.2% | 1 | 0.1% |
| 20,758 | 100% | 13,697 | 100% |
Two state health departments have developed and currently administer state clinical laboratory improvement programs that CMS deems equivalent to the CMS program. Laboratories in Washington and New York must meet the standards of these state programs. Approximately 25 additional states have laboratory licensure programs. They receive funding from CMS to implement the federal CLIA program. In states that do not have clinical laboratory regulatory programs, the laboratories must choose between accreditation through one of the six approved organizations or submitting to inspection and certification by CMS.
3. ENVIRONMENTAL LABORATORIES
Environmental testing laboratories perform physical, chemical, and microbiological analysis of specimens collected in the environment. For example, a water sample may undergo physical testing (temperature, turbidity, odor, color), chemical testing (nitrates, sulfates, pesticides, metals), and microbiological testing (total plate counts, coliforms, Giardia, cryptosporium). Environmental testing laboratories provide a wide range of testing that is in many ways similar to the testing performed in clinical laboratories. Sanitarians or water quality technicians often perform basic tests (e.g., for temperature, pH, volatility, and physical appearance) at the site where samples are collected. They transmit the results of these simple tests to the laboratory along with the samples, where chemists and micro-biologists perform additional presumptive and confirmatory testing. Results from the simple tests may suggest the need for more definitive testing using instruments such as atomic adsorption spectrophotometers, gas chromatographs, and mass spectrometers. Laboratories perform much of the routine environmental testing in batches of 10 to 50 samples on semiautomated or fully automated instruments. The raw analytical data are captured, processed, and reported by using software that interfaces directly with the instrument and the laboratory's data management system.
Environmental laboratories are certified by accreditation authorities recognized by the Environmental Protection Agency (EPA) as part of the National Environmental Laboratory Accreditation Program (NELAP). At least 12 states currently are recognized by the EPA as environmental laboratory accrediting authorities. These state programs apply nationally recognized standards to the laboratories that they accredit so that there is some consistency in the quality of tests performed by accredited laboratories.
4. COMMERCIAL LABORATORIES
Commercial laboratories are an important component of the medical delivery system in the United States. A commercial laboratory is a laboratory that is free-standing; that is, it is not associated with a hospital or other healthcare organization. Commercial laboratories may specialize in clinical specimens, environmental specimens, or both.
Commercial laboratories can be important partners for organizations that wish to develop biosurveillance systems because of size of the laboratories and their use of information technology. Commercial laboratories have grown significantly in size during the past decade as a result of mergers and consolidations, and they may offer tests that are not readily available. Many of these laboratories began as specialized reference laboratories, offering tests that could not be economically provided by smaller laboratories. Small laboratories merged with larger laboratories that were, in turn, purchased by large laboratory corporations. Regional consolidation of clinical laboratories that provided services to healthcare organizations led to the formation of large commercial laboratories. These commercial laboratories, such as Lab Corp, ARUP, and Quest, have developed service systems that allow them to provide clinical testing at their headquarters and at distributed sites around the country.
Large commercial laboratories have established elaborate courier systems that collect samples locally for overnight distribution to the appropriate laboratory in their network. Although a sample may be transported to a distant laboratory, the results, nevertheless, frequently become available overnight. The commercial laboratories use information systems referred to as laboratory information management systems (LIMSs) to track tests and results. These systems monitor test requests, capture test results, and electronically report the results, often within hours of the sample being received.
As discussed in Chapter 5, clinical laboratories are required to report notifiable diseases to local health departments. The large, multistate commercial laboratories face challenges in complying with notifiable disease reporting requirements that vary by state. Further, reporting requirements frequently change as new diseases of public health interest are identified and many states have expanded laboratory reporting requirements to include suspected cases of notifiable diseases. Commercial laboratories are increasingly using electronic laboratory reporting to satisfy these complex and changing requirements.
Commercial laboratories may specialize in environmental testing. These commercial environmental laboratories provide testing on a variety of samples, such as water, air, hazardous materials, dust, and soil. These laboratories often contract with governmental agencies, such as EPA, Department of Defense (DoD), and U.S. Department of Agriculture (USDA) at the federal level and with environmental and regulatory agencies at the state and local level.
5. GOVERNMENTAL LABORATORIES
Federal, state, and local government agencies, such as health departments, operate laboratories or contract with commercial laboratories for testing related to diagnosis, regulatory compliance, investigations, and environmental monitoring. Since the early 1800s, governmental laboratories have performed testing that led to the identification of outbreaks of diphtheria, cholera, smallpox, and typhoid fever. During the 20th century, these laboratories, in conjunction with academic laboratories, helped develop vaccines and contributed to the detection of polio, rubella, measles, and whooping cough. The response to West Nile fever in the United States and the release of viable Bacillus anthracis in 2001 required these laboratories to develop procedures quickly for diagnosis and identification of agents that they had not previously encountered. Governmental laboratories are key to the recognition of new and emerging infectious diseases and are vital to surveillance efforts.
5.1. Federal Laboratories
The Department of Health and Human Services (DHHS), USDA, Department of Energy (DOE), DoD, and Departments of Commerce and Justice, and the EPA operate or fund clinical, environmental, forensic, and research laboratories. Federal laboratories provide reference testing and are often involved with the development of new technologies, as well as the transfer of these technologies to other laboratories. Many federal laboratories collaborate with international partners and serve as reference centers for specialized testing. Federal laboratories often provide training, confirmatory testing, and reference materials for other governmental laboratories.
The DHHS laboratories that are associated with disease detection, control, and surveillance activities are found at the National Institutes of Health (NIH), CDC, and FDA. The NIH, headquartered in Bethesda, Maryland, conducts research on acute and chronic diseases, develops new therapies and immunizations, and develops new laboratory and diagnostic technologies for many infectious and noninfectious diseases and health conditions. Much of the NIH work is done through extramural grants and contracts with universities, private companies, and other governmental organizations. The CDC's laboratories focus on infectious diseases, occupational diseases, and environmental causes of diseases. The CDC specialized laboratories are in Colorado, Ohio, West Virginia, and Puerto Rico, in addition to its headquarters in Atlanta, Georgia. The CDC has led the development of rapid national laboratory reporting systems that have been successfully used to identify multistate outbreaks of diseases (Bean et al., 1992; Hutwagner et al., 1997). CDC-based scientists have developed and used new technologies to identify outbreaks of disease in cooperation with state and local public health laboratories. One such example of the successful application of a new technology is known as PulseNet (discussed in Chapters 3 and 5; http://www.cdc.gov/pulsenet/). PulseNet is a national network of public health laboratories that perform DNA “fingerprinting” of foodborne pathogens and clinical isolates to allow matching of isolates. This epidemiological typing method is the basis for detecting clusters of disease that are geographically diffuse, and for linking bacteria found in a specific food to bacteria found in one or more persons with a particular disease. PulseNet permits recognition of outbreaks that previously went undetected. A multistate outbreak of listeriosis in 2000 was identified only after the isolates of Listeria monocytogenes were tested by using pulsed-field gel electrophoresis and determined to all have a common PulseNet pattern (CDC, 2000).
FDA laboratories focus primarily on monitoring the food supply and ensuring the purity and potency of drugs and other pharmaceuticals. These regulatory laboratories frequently become involved in the investigation of food contamination (including ground beef, poultry) and the adulteration of drugs. FDA maintains regional laboratories in Washington, New York, Colorado, Michigan, Kansas, California, Georgia, and Arkansas, as well as specialized laboratories in Ohio, Pennsylvania, Puerto Rico, and Massachusetts. The FDA laboratories provide testing to support investigation and compliance activities. FDA's Electronic Laboratory Exchange Network (eLEXNET) is a Web-based system for real-time sharing of laboratory data derived from foods. This system allows public health officials to compare laboratory findings and to identify outbreaks earlier.
The USDA operates laboratories that support many of their regulatory, monitoring, and investigative programs. USDA laboratories conduct research on animal and plant diseases and provide testing of animals and agricultural products. The USDA's National Veterinary Services Laboratories (NVSL) located in Ames, Iowa, tests for domestic and foreign animal diseases, and function as the primary animal disease reference laboratory. The NVSL provides diagnostic support for disease control and eradication programs, import and export testing of animals, and laboratory certification for selected animal diseases. Diseases, such as anthrax, rabies, brucellosis, and bovine spongiform encephalopathy (mad cow disease), may impact both animals and humans and, therefore, constitute priorities at this laboratory. A former USDA laboratory, the Foreign Animal Disease Diagnostic Laboratory (FADDL), which is located on Plum Island off Long Island, New York, was recently transferred to the Department of Homeland
Security after supporting years of research on some of the most dangerous animal pathogens.
The DOE oversees the operation of 25 DOE national laboratories, many of which were established to support the production, use, and response to nuclear materials. After the end of the Cold War, the focus of some of the DOE laboratories shifted to other projects, including the Human Genome Project and the development of technologies and assays to support homeland security initiatives. The DOE national laboratories develop new technologies for countering biologic and chemical threats, including systems for the detection, modeling, and response to terrorist attacks (see www-ed.fnal.gov/doe/doc_labs.html).
The DoD has established laboratories worldwide in locations such as Peru, Indonesia, Egypt, Thailand, and Kenya. DoD laboratories serve the needs of the armed forces and function as screening or sentinel laboratories for infectious diseases. The U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) has the capability to detect unusual biological agents that often require advanced testing techniques. This laboratory, located in Maryland, is a member of the Laboratory Response Network (LRN; discussed later) and one of a few laboratories worldwide that can isolate and identify the most dangerous human agents, such as Ebola, smallpox and Marburg viruses.
The EPA operates 10 regional environmental testing laboratories across the United States. These laboratories have a research and environmental monitoring mission: they analyze air, drinking water, ground water, surface water, soil, sediment, and hazardous materials for biological, chemical, and radiological materials that are toxic to the environment. EPA develops standard methods for the analysis of environmental samples. EPA maintains large databases of environmental monitoring data produced by its own laboratories and others. Its Office of Research and Development (ORD) directs laboratory activities at 12 locations, including the National Center for Environmental Assessment in Research Triangle Park, North Carolina.
Although the capability and capacity of the federal laboratories described above is large, this capacity was challenged by the volume of environmental and clinical samples generated during the 2001 anthrax postal attack. The distribution of anthrax spores in mail during October 2001 led to an unprecedented demand for quality testing throughout the United States owing to discovery of real and suspected contaminations. Although few of the more than 125,000 environmental samples tested contained B. anthracis, the existing network of public and commercial laboratories was barely able to meet the demands for testing, and there were significant delays caused by the sheer volume of samples. The concept of a high-throughput laboratory capable of testing thousands of biologic, chemical, or radiological samples would require the laboratory to be equipped with the latest automated instrumentation and supported with an efficient LIMS (Layne and Beugelsdijk, 2003). The establishment of high-throughput laboratories to support the nation's homeland security needs is a reasonable concept, especially if the major federal partners were to colocate resources on a national interagency homeland security laboratory campus.
5.2. State Laboratories
Approximately 200 of the more than 186,000 laboratories in the United States are classified as state public health laboratories. Included in this number are about 150 regional or branch laboratories that are administered as part of the state public health laboratory. More than 6,500 laboratory professionals are employed by state public health laboratories.
Each state and five territories operate a state public health laboratory. One major function of theses laboratories is to provide diagnostic and analytical services for surveillance of infectious, communicable, genetic, and chronic diseases. State (and local) public health laboratories provide testing to support many public health programs (tuberculosis, sexually transmitted diseases, HIV, immunizations, and newborn screening). Areas of analysis include clinical and environmental chemistry, immunology, pathogenic microbiology, virology, and parasitology. State laboratories serve as reference laboratories, and they provide confirmatory testing of specimens submitted from other laboratories. The state public health laboratories frequently measure toxicants in human samples to document exposures to chemicals found in the diet or environment. This specialized testing requires expensive equipment (mass spectrometers, chromatographs) and well-trained chemists.
The capabilities, responsibilities, and practices of the state public health laboratories vary. During recent years, many of these laboratories have received substantial federal funding to increase staff, equipment, and capabilities to respond to biologic and chemical threats, as part of the DHHS' Cooperative Agreement on Public Health Preparedness and Response for Bioterrorism. A large percentage of the state public health laboratories have used some of the federal funding to build, remodel, and upgrade facilities. Funding for state laboratories is generally a mix of state and federal funds. Many states rely on fees, reimbursements, and service contracts to carry out their mission. Some states have regional or district laboratories to provide the necessary services throughout an entire state. State public health laboratories partner with public health laboratories operated by counties or cities to meet the needs of their communities. State public health laboratories are generally better prepared to respond to an incident requiring biologic testing as opposed to an incident requiring chemical testing.
Although research is not the primary mission of public health laboratories, several state public health laboratories have developed close ties with academic institutions. The opportunity to work on surveillance projects with faculty and students from schools of public health and academic training programs for laboratory professionals has been beneficial to these laboratories. Arrangements with academic centers afford opportunities to improve laboratory services and surveillance systems. Flexible funding and other resources, such as grants, faculty, and students, help support special research projects and training opportunities within public health laboratories.
State laboratories provide services to health departments; healthcare organizations; local, state, and federal law enforcement; local hazardous materials (hazmat) teams; civil support teams; and other private and governmental laboratories. State laboratories analyze thousands of water and air samples daily. State laboratories involved in the analysis of drinking water and other environmental samples are accredited by the NELAP, certified by EPA Office of Drinking Water Programs, or accredited by state-specific accreditation programs.
State public health laboratories are the backbone of the Laboratory Response Network (LRN), which we discuss below. The LRN also includes laboratories under the jurisdiction of federal agencies discussed in this chapter. At the time of publication, 96 state and local public health laboratories make up the 146 laboratories that are members of the LRN. Of these, 72 state, territorial, and metropolitan public health laboratories are part of the LRN's chemical testing network.
5.3. Local Public Health Laboratories
More than 1,900 local public health laboratories provide support for city and county public health programs. These laboratories offer onsite testing for child health screening, tuberculosis, refugee screening, food safety, sexually transmitted diseases, and lead poisoning prevention, as well as epidemiologic investigations and environmental monitoring. Local public health laboratories often forward specimens to their state laboratories for tests that are not available locally. Approximately 40% of the local public health laboratories only perform waived tests. Another 40% perform moderate complexity testing, and 20% perform highly complex testing (Association of Public Health Laboratories, 2004).
5.4. Other State and Local Laboratories
Many state and locally operated laboratories in addition to clinical and public health laboratories produce test results that may be useful for surveillance. Forensic laboratories and toxicology laboratories are frequently associated with departments of public safety or with state or local medical examiners. These forensic laboratories may be positioned at the federal, state, or local level, depending on the jurisdiction. Medical examiners contribute to biosurveillance by elucidating unusual causes of death (see Chapter 11). Medical examiners and forensic pathologists frequently rely on laboratories to establish the exact cause of death.
6. SERVICES PROVIDED BY LABORATORIES
Readers may understand laboratories as only providing test results after the analysis of samples that are submitted. This view of laboratories only focuses on one of their products and overlooks services provided by the professionals who work in the laboratories. Laboratory professionals provide consultation on the selection of the most useful tests for screening, diagnosis, and verification of disease. They provide information on the proper handling and transportation of samples. Development of new technologies, evaluation of technologies, and the application of technologies are other important services provided by laboratory professionals. Laboratories evaluate test kits for ease of use, storage conditions, and performance characteristics. Laboratories train individuals to perform testing and educate and inform users of laboratory services.
Laboratories may also operate laboratory-based surveillance systems for respiratory and enteric diseases. For example, several states—such as Wisconsin (www.slh.wisc.edu/labupdates.description.php), Minnesota (www.health.state.mn.us/divs/idepa/diseases/flu/avain/surveillance.html), and Nebraska (www.hhs.state.ne.us/new/o2o5nr/flu1.htm)—and the United Kingdom Health Protection Agency (www.phls.co.uk/infections) have developed laboratory-based surveillance systems that collect data on respiratory infections and make the findings available on a their Web sites daily or weekly.
7. TESTING TECHNOLOGIES
Laboratories employ many analytic technologies to provide testing for diagnosis and surveillance. These tests range from simple screening tests to presumptive diagnostic tests and confirmatory tests.
7.1. Simple Screening Tests
Simple laboratory tests are used to screen biologic samples for the presence of biological agents or other substances that might indicate a disease, an infection with a biologic agent, or a contamination with a toxin or other chemical. The classical approach to identification of biologic agents involves direct examination of stained materials by microscopic examination for the presence of agents (DHHS/CDC, n.d.; York, 2003). Microscopic examination relies on staining characteristics and the size and shape of the organisms found. With few exceptions, it is rarely possible to identify an agent based only on microscopic characteristics. Direct stains may help eliminate organisms from further consideration, but they are usually not sufficient without further testing to identify a pathogen. Wet mounts are used for the direct microscopic examination of clinical materials for fungi and parasitic agents. Additional simple assays take advantage of the ability of an organism to metabolize chemicals or produce chemical reactions that result in color changes of a substrate. Recently, many assay kits have been developed for waived tests. These waived test kits can produce reliable results in most cases; however, one must understand the limitations of these kits and the need for confirmatory testing. Field test kits, often referred to as handheld assays, have become popular with first responders who have a need to know if an unknown material contains a biologic agent or toxic chemical. Simple immunologic reactions, coupled with a colorimetric indicator, form the detection systems for many handheld assays. Validating the performance of handheld assays in comparative studies is a task generally reserved for governmental agencies or contract laboratories (Emanuel et al., 2003). The major advantage of the simple assays is that they are quick, as a result may be available in 5 to 20 minutes. The short turnaround time makes these tests ideal for use by surveillance systems provided one understands the limitations of the tests and that a method for confirmatory testing is available.
7.2. Presumptive Diagnostic Tests
Presumptive diagnostic tests are procedures that, when properly performed, may indicate the presence of a particular agent or closely related agents. Presumptive diagnostic tests for biologic and chemical agents are more complex and require more time to perform than do the simple tests described above. Most bacterial and viral agents can readily be grown in culture media or cell culture if the appropriate conditions are met. These conditions include the appropriate temperature, pH, and a source of energy (e.g., glucose). Inhibitory substances, such as antibiotics, are often placed into the growth media to prevent the growth of unwanted organisms. Growth of organisms in cell culture or artificial media takes 24 hours to 30 or more days, depending on the organism.
Once organisms are detected on or in growth media, various techniques are used to determine the genus and species of the organism. For bacteria, the differential growth on select media, metabolism of various carbohydrates and other chemicals, and the presence of selected enzymes are often used for identification. Special stains that incorporate florescent dyes coupled with antibodies to selected organisms are used for identification of bacteria and viruses.
Laboratories use immunologic assays to identify many biologic agents and for the subspecies typing that is needed for epidemiological purposes. Immunologic assays generally involve the use of a specific antibody that can attach to or react with the outer-surface structures of an agent. Other immunologic assays are used to detect antibodies in biological materials after infection with biological agents. After the isolation and identification of many organisms, drug-susceptibility testing determines the organism's susceptibility to drugs that are used to treat infections with the organism.
7.3. Definitive and Confirmatory Tests
A definitive or confirmatory test is a test that will identify with a very high degree of certainty the true identity of an agent. These tests have a very low likelihood of providing a false-positive result. Many of the definitive and confirmatory assays used today are molecular assays, which detect genetic material that is specific to a bacterium, virus, protozoa, or other organism. Nucleic-acid–based assays rely on the unique differences found in the structure of single strands of DNA and RNA. The unique pattern of bases is specific for a single organism or closely related organisms. The nucleic-acid–based assays involve the use of probes, which are strands of DNA or RNA that match distinctive DNA or RNA patterns of the organism being tested and will bind with that DNA or RNA if it is in the clinical specimen. Once the binding occurs, the binding can be detected by using electrochemical, colorimetric, and optical systems (Committee on Research and Development Needs for Improving Civilian Medical Response to Chemical and Biological Terrorism Incidents, 1999). This binding provides extreme selectivity between the known probes and the material found in clinical specimens. The use of a technique known as polymerase chain reaction (PCR) makes it is possible to amplify trace quantities of DNA or RNA in a clinical specimen to enable the detection of as few as 1,000 bacteria or viruses. The high specificity and sensitivity of molecular assays makes them especially suited for detecting minute quantities of biologic agents and toxins.
The use of genetic fingerprints has become a valuable tool for microbial forensics (Murch, 2003). By identifying distinct features of genes using sequencing techniques, it is possible to identify individual strains of organisms and to use this information as epidemiological markers. Molecular techniques allow investigators to link strains from various sources and to form associations that often unravel the mystery of disease outbreaks. Libraries of genetic patterns, or fingerprints, make it possible to trace the origin of many outbreaks. Not only does sequencing identify DNA or RNA patterns unique to a particular organism, but, in many cases, these probe technologies are simpler, faster, and less technology-dependent than are other traditional assays.
Many biotechnology companies are pursuing production of microarray systems that will test for 100 to 100,000 or more different DNA fragments simultaneously. The technology embeds the probes on a single glass or nylon substrate called a microchip. By using these arrays and various detection systems onto which the clinical specimen is applied, the individual components of the microchip will react with DNA fragments in the specimen and be detected. As this technology develops, it will become more common to use the microarray systems for screening and detection of diseases. Simultaneous PCR assays for multiple respiratory viruses now have sufficient sensitivity and specificity to be a valuable tool for diagnosis of respiratory viral infections (Hindiyeh et al., 2001). In the future, PCR assays will be able to screen a single specimen for a multitude of biologic agents.
8. LABORATORY INFORMATION MANAGEMENT SYSTEMS
A LIMS is a computer system that a laboratory uses to track specimens and manage analytical data. The function of a LIMS can perhaps best be explained by tracing a single test— a white blood count—from the time that a clinician orders the test until the time the clinician receives the result of the test.
8.1. Tracking a White Blood Count from Order to Result
A clinician requests (orders) that a laboratory perform a white blood count on a blood sample from a patient in one of several ways. The clinician writes the order on paper, gives a verbal order to a nurse, or enters the order directly into a computer system. A LIMS may receive this order in one of several ways: directly, if the LIMS provides an order-entry component that either the clinician uses directly or the nurse or ward clerk uses on her behalf; indirectly, if a paper order form accompanies the specimen; or electronically, from an order-entry system embedded in another information system (such a point-of-care system as discussed in Chapter 6).
We will trace the most automated path in which the LIMS receives the order electronically. The received order sets up a specimen-tracking process that is a central LIMS function. The LIMS (or a point-of-care system) controls a printer, which is often located in the clinical area from which the order originated. The printer generates a barcoded label. A phlebotomist attaches the label to a “purple top” vial (containing magnesium citrate or ethylenediaminetetraacetic acid [EDTA] to prevent the blood from coagulating). The phlebotomist draws the blood after checking carefully that the identification on the labeled tube matches the patient, and sends the sample to the laboratory. Oftentimes, the labeled specimen is transported by pneumatic chute from clinical areas, such as the emergency department, or by express overnight delivery to the laboratory. A technician in the laboratory scans the barcode of the specimen with an optical scanner, which is connected to the LIMS. If the laboratory has an automated analyzer for blood counts, the technician simply places the vial into the specimen carousel of the analyzer. The analyzer recognizes the bar code, communicates with the LIMS over an internal laboratory network to determine which tests were ordered for the specimen, runs the tests, and transmits the results to the LIMS. Other types of specimens may require preparation, such as centrifugation, before being placed into the carousel of an automatic analyzer, but the information processing and communication between the analyzer and the LIMS are otherwise identical. For tests that are done by hand, the LIMS provides user-interfaces into which medical technologists register specimens and enter intermediate and final results of tests.
Depending on the laboratory and the needs of its clinical customers, the LIMS may deliver the results as paper reports, via Web-browser interfaces, or by e-mail. A LIMS typically offers all of these options. A LIMS invariably has an outbound computer-to-computer interface that can transmit results to other clinical information and public health information systems. Although our example is of a clinical test, the process is identical for environmental tests ordered by sanitarians, water quality technicians, or outbreak investigators.
The vast majority of laboratory work in the United States is highly automated in the fashion just described. With the exception of tests done in the field or in office practices, LIMSs track and manage the analytic results of most laboratory tests performed in the United States. LIMSs are designed to make test results available to clinicians and other information systems soon after they are performed. LIMSs are highly reliable and operate in real time. LIMSs are developed within the laboratory by information technology staff, purchased from commercial vendors, or a combination of both.
8.2. Use of LIMS in Biosurveillance
Because of the importance of laboratory tests in biosurveillance, biosurveillance organizations are attempting to establish connections between LIMSs and their own biosurveillance computers. At present, however, there are significant technical barriers to connecting a LIMS located in a laboratory with a computer located in a biosurveillance organization. The most difficult technical barrier is data incompatibility. Most laboratories do not use standard coding systems to identify the names and results of laboratory tests. Data standards exist, but few laboratories use them. Most laboratories have evolved their own naming or coding systems for the tests that they perform and the results of those tests. They use these proprietary codes (or free text) to identify the laboratory test, specimen type, organism, or other results of a test. As a result, each LIMS-to-biosurveillance–computer interface requires significant effort to understand the data and to create means to translate the data into a standard format so that it can be integrated with data coming from other LIMS. We discuss standard data formats in detail in Chapter 32.
Outbound communication standards, such as HL7, also exist and we discuss them in Chapter 32. Although most LIMS support these standards, the specific implementations vary. Even if both the biosurveillance computer and the LIMS use the HL7 standard, they will not be able to communicate without significant effort to understand the specifics of the messages and to create a means for extracting the data from the messages. Importantly, a new version of HL7 (version 3.0) will solve this problem, but its penetration in the LIMS market is low at present.
Because of the customization required to connect even a single LIMS to a biosurveillance organization, there are only a few regions that have integrated data from most of the LIMSs that serve their region into biosurveillance. Ultimately, these barriers will be addressed by standardization, but as we discuss in Chapter 32, it takes many years to achieve widespread standardization of any component in a biosurveillance system.
9. NETWORKS OF LABORATORIES
The organization of laboratories into collaborative networks has been ongoing for more than a decade. The concept of laboratory networks arose from the need to ensure that critical laboratory services were available throughout the count (Gilchrist, 2000). The CDC, FDA, USDA, and state gover mental laboratories formed many of the original partnershi that grew into the laboratory networks that exist today.
Early networks included the National Laboratory Syste (NLS) of clinical, public health and federal laboratori (McDade and Hughes, 1998), and the Public Health Laborato Information System (PHLIS). PHLIS was an early DOS-base system that involved voluntary reporting of selected laborato tests directly to the CDC by 23 state and local public health la oratories and numerous military laboratories (see Chapter 3 PHLIS was one of the earliest laboratory-based surveillanc systems that became an effective tool for the identificatio of outbreaks of salmonellosis (Bean et al., 1992; Hutwagn et al., 1997).
9.1. The Laboratory Response Network
The CDC's LRN was established in 1999 in compliance with presidential directive that outlined federal agencies' counter errorism goals and responsibilities. The mission of the LRN “to maintain an integrated national and international networ of laboratories that can respond quickly to acts of chemical biological terrorism, emerging infectious diseases and oth public health threats and emergencies.” The LRN was fir tasked to address state and local public health laboratory pr paredness and response for bioterrorism. Since its inceptio its mission has expanded to include chemical terrorism. Th scope of laboratories in the LRN has expanded beyond sta and local public health laboratories in order to meet nation security needs (http://www.bt.cdc.gov/lrn/).
The LRN and its partners maintain a network of laboratori that can respond quickly to acts of chemical or biological terro ism, emerging infectious diseases, or other public health threa and emergencies. The network included 152 federal, state, an local public health; military; and international laboratories of August 1, 2005. Figure 8.1 shows the distribution of LR laboratories by type as of March 11, 2005. The LRN emplo tests that can detect biological threat agents in clinical spec mens, environmental samples, food, animals, and water. Th Association of Public Health Laboratories was a partner in th early development of the LRN and has played an importa role in ensuring that the LRN provides the training, standar ized methods, and equipment necessary for detecting biolog agents of terrorism and other threats to public health. Th Federal Bureau of Investigation (FBI) was also a key partn in establishing the network. The FBI brought its forens expertise and evidence-gathering requirements to the progra Public health and law enforcement have overlapping approach to their investigations that required collaboration between CD and law enforcement to both enhance and protect the integri of their investigations.
FIGURE 8.1.
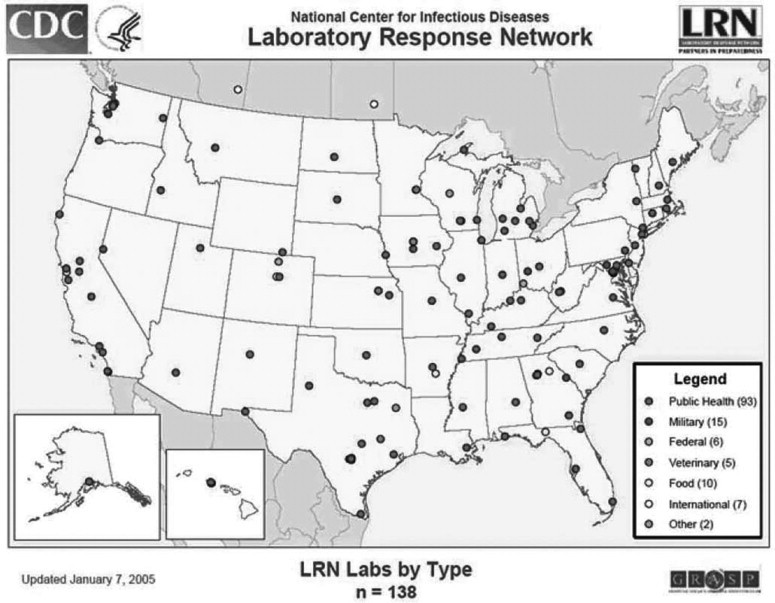
Map of Coverage of the Laboratory Response Network (LRN) in the United States, 2005.
Three levels of laboratories are recognized within the LRN f bioterrorism agent detection. LRN laboratories responsib for bioterrorism agent detection are designated as national, reference, or sentinel laboratories (Figure 8.2 ). The national laboratories include laboratories at the CDC, USAMRIID, and the Naval Medical Research Center. They have unique resources to safely identify highly infectious agents (biological safety level 4 agents) and the ability to provide definitive characterization of biologic agents. The national laboratories have developed standard tests and protocols, trained laboratory analysts, and established secure communications for the rapid sharing of laboratory results from reference laboratories.
FIGURE 8.2.
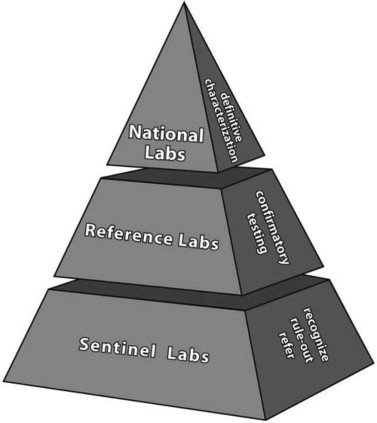
Laboratory Response Network (LRN) structure for bioter-rorism response laboratories.
Reference laboratories in the LRN perform tests to detect and confirm the presence of biological threat agents, such as those that cause anthrax, plague, tularemia, smallpox, or botulism. These laboratories ensure that state and local laboratories can have a timely response in the event of a bioterrorism incident or an emerging disease. Rather than having to rely on confirmation testing from national laboratories, reference laboratories are capable of producing conclusive results needed by local authorities for rapidly responding to emergencies. Specimens received by reference laboratories that contain threat agents are usually submitted to a national laboratory for definitive characterizations of the agent. State and local public health laboratories comprise most of the reference laboratories within the LRN.
Although sentinel laboratories are not counted among the LRN laboratories, they represent the thousands of clinical (human and veterinary) and environmental laboratories identified by the state LRN reference laboratory to serve as the front line of defense in detecting agents of terrorism. Qualification of the sentinel laboratory is based on the experience and competency of the laboratory staff, appropriateness of facilities, and completion of training provided by the LRN. Sentinel laboratories can often rule out potential bioterrorism agents based on a battery of simple tests. In a covert terrorist attack, a sentinel laboratory could be the first facility to identify a suspicious agent or an unusual cluster of diseases. A sentinel laboratory's responsibility is to rule out other diseases and refer a suspicious sample to an appropriate reference laboratory.
The LRN uses an array of presumptive and confirmatory assays to detect biological threat agents or chemical agents. Presumptive assays generally include traditional microbiological assays, such as growth on special media, use of special stains, and the use of rapid molecular diagnostic assays, such as real-time PCR. Confirmatory methods used by the national laboratories are considered the gold standard for detecting the target agent. Methods, such as time-resolved fluorescence (TRF) and molecular characterization, have replaced many of the more traditional confirmatory methods that were based on the growth and biochemical properties of the agent. Reference laboratories have access to LRN protocols through a secure Web site and are supplied standardized reagents needed to perform the necessary tests. Uniform procedures for use by sentinel laboratories have also been developed and are used for training of sentinel laboratory staff. All laboratories that are part of the LRN must provide a safe and secure environment for performing tests and must participate in a recognized proficiency testing program.
The chemical component of the LRN employs a more centralized structure, with only a few laboratories currently prepared to provide definitive analysis of specimens for chemical agents or their metabolites. LRN laboratories responsible for chemical agent detection are designated as level 1, 2, or 3 laboratories. Five laboratories located in California, Michigan, New Mexico, New York, and Virginia participate in level 1 activities. At this level, personnel are trained to detect exposure to an expanded number of chemicals in human blood or urine, including all level 2 laboratory analyses, plus analyses for mustard agents, nerve agents, and other toxic chemicals. Forty-one laboratories participate in the chemical LRN by providing level 2 activities. At this level, laboratory personnel are trained to detect exposure to a limited number of toxic chemical agents in human blood or urine. Analysis of cyanide and toxic metals in human samples are examples of level 2 laboratory activities. Each of the 62 chemical network members participates in level 3 activities. Level 3 laboratories are responsible for the following:
-
•
Working with hospitals in their jurisdiction
-
•
Knowing how to properly collect and ship clinical specimens
-
•
Ensuring that specimens used as evidence in a criminal investigation are handled properly and chain-of-custody procedures are followed
-
•
Being familiar with chemical agents and their health effects
-
•
Training on anticipated clinical sample flow and shipping regulations
-
•
Working to develop a coordinated response plan for their respective state and jurisdiction
Initial testing in a suspected chemical event will occur at CDC or 1 of 5 level 1 chemical laboratories that have been established by CDC. By use of mass spectrometry, CDC laboratories perform tests on the first 40 or more clinical specimens to measure human exposure. Results of these tests would be reported to affected states, and if needed, appropriate LRN members may be asked to test additional samples. This approach is necessary because the analytical expertise and technology resources required to respond to a chemical event is expected to be high.
The LRN supports secure communications on emerging and emergency issues, a secure mechanism for ordering reagents and testing protocols, and a system for electronically reporting test results. The LRN provided valuable testing after the release of anthrax spores in 2001, the identification of monkey pox in 2002, and the response to severe acute respiratory syndrome (SARS) in 2003.
9.2. Other Laboratory Networks
In 2001, Canada created a laboratory network, known as the Canadian Public Health Laboratory Network (CPHLN), to strengthen the linkages between federal and provincial public health laboratories. This Canadian network is modeled after the LRN in the United States. The CPHLN has been providing responses to naturally occurring infections and deliberate releases of biologic agents and toxins. The CPHLN coordinates pathogen detection and infectious disease prevention activities, as well as conducts laboratory-based surveillance and early warning systems for emerging pathogens and bioterrorism threats.
The National Animal Health Laboratory Network (NAHLN) is part of a national strategy in the United States to coordinate the nation's federal, state, and academic animal health laboratories. The USDA has taken the lead in the development of this network, which includes agriculture and animal health laboratories operated by state agricultural agencies and those associated with veterinary teaching facilities. The facilities and professional expertise of NAHLN members allows authorities to better respond to animal health emergencies that might include a bioterrorist event, the emergence of a new domestic animal disease, or the appearance of a foreign animal disease that could threaten the nation's food supply and public health. Because many of the biologic agents that cause the greatest concern as terrorist agents infect both humans and animals, the role of veterinarians in the early detection of disease is very important. The NAHLN currently consists of 44 laboratories in 37 states. An effort is underway to deploy standardized testing methods in member laboratories and to improve the information technology system used by member laboratories to track test requests and report results. The U.S. Animal Health Association (USAHA) and the American Association of Veterinary Laboratory Diagnosticians (AAVLD) have members who participate in the NAHLH and contribute expertise to protect animals and public health.
The National Plant Diagnostic Network (NPDN) is an agricultural laboratory network that provides detection, identification, and reporting of pests and pathogens that have been deliberately or accidentally introduced into agricultural systems. The primary concern of this network is food security and the economic threats to the nation's food supplies. The NPDN includes five regional centers located at Cornell University, Michigan State, Kansas State, University of Florida at Gainesville, and the University of California at Davis. The NPDN recently implemented a new database that helps with required reporting to state and national agencies. A Web-based plant diagnostic system using digital photography allows laboratories to share images with specialists in remote locations.
The Food Emergency Response Network (FERN) is a network of state and federal laboratories that are committed to analyzing food samples in the event of a biological, chemical, or radiological terrorist attack in the United States. The federal partners in the FERN include the FDA, USDA, CDC, and EPA. As of May 2005, there are 99 laboratories in FERN, representing 44 states and Puerto Rico. Twenty-six federal laboratories, 68 state laboratories, and five local laboratories are enrolled in FERN. The mission of FERN is to integrate the nation's food testing laboratories for the detection of threat agents in food while using standardized diagnostic protocols and procedures. Of the 99 laboratories in FERN, there are 64 that perform chemical tests on food, 64 that perform biological tests on food, and 25 that perform radiological tests on food. These laboratories strengthen preparedness and provide surge capacity. The FDA and USDA jointly share the leadership within FERN and have been working to obtain federal funds that can be made available to support further development of the network.
The data capture and information exchange system for FERN is the eLEXNET, is an integrated, secure system designed for use by multiple governmental agencies involved in food safety activities. Laboratories report test results and public health officials assess risks and analyze trends in food-borne diseases. eLEXNET has GIS reporting functions and uses HL7 data exchanges between laboratories. Similar to those in LRN, participants in FERN receive training on the latest equipment and are required to participate in proficiency testing programs. eLEXNET provides the necessary infrastructure for an early warning system that can identify potentially hazardous foods and share laboratory reports in a timely manner. As of January 2005, 113 federal, state, and local laboratories in all 50 states have joined the eLEXNET system. About 90 laboratories actively exchange data by using eLEXNET.
The Radiological Emergency Analytical Laboratory Network (REALnet) is a national network of radiological laboratories that are capable of responding to the needs for radiological testing after a terrorist attack. Academic, commercial, military, federal, state, and local laboratories participate in REALnet. These laboratories serve as a science and technology asset for the Department of Homeland Security. REALnet is modeled after the LRN and FERN and includes a Web-based database containing information on the capabilities, capacity, and competence of member laboratories. Information on accreditation, certification, and performance testing is also maintained. Standards, guidelines, and laboratory procedures are developed and distributed by REALnet. Gaps in the standards are being addressed by appropriate standards development organizations. Internet-based tools, such as bulletin boards and list servers, are used to promote the exchange of information. The system provides links to other resources that would be useful during an emergency caused by the release of a radioactive material.
The expansion of laboratory networks designed to produce test results in response to an act of terrorism or other public health emergency has led to increased sharing of laboratory results. Coordination and integration of the various networks has not always been a priority. Duplicate systems and overlapping missions suggest that an integrated consortium of laboratory networks could provide timely results for early detection and response to acts of terrorism. The networks need to agree on standardized tests and policies that would promote a timely response no matter which network is reporting results. An overall system to ensure that laboratory capacity will be available to test clinical (human and animal) specimens and environmental samples, including food and water, does not exist. Laboratories that can perform screening, monitoring, and definitive testing are needed for each class of specimen. Although laboratory information systems have been developed for capturing and sharing data, the diverse systems are not fully compatible with each other. Failure to standardize nomenclatures and use recognized data transmission protocols make sharing of large quantities of data for surveillance purposes problematic.
In an effort to improve coordination among laboratory networks, the Department of Homeland Security is working to integrate these networks through the creation of the Integrated Consortium of Laboratory Networks (ICLN). The ICLN employs the LRN model for coordinating laboratory assets for terrorism among federal, state, local, and scientific partners. A memorandum of agreement was signed in 2005 by the USDA, DoD, DOE, DHHS, EPA, and the Departments of Homeland Security, State, Justice, and Interior to form the ICLN. These federal agencies will collaborate to ensure that laboratory resources available within each agency can be used to respond to terrorist events and other national emergencies.
10. SUMMARY
Clinical, commercial, and governmental laboratories are an important source of data for biosurveillance systems. Test results obtained from the analysis of human and animal specimens may be early indicators of disease within the population. Before the establishment of a definitive diagnosis, a sudden rise in the number of tests being requested by clinicians might be the first indication of an outbreak. The combination of laboratory data from environmental laboratories and the occurrence of illness in humans or animals might signal onset of an infectious disease or poisoning. The establishment of a LIMS that can rapidly capture and report laboratory data electronically will greatly contribute to the use of laboratory data in biosurveillance. Challenges still exist for integrating 190,000 laboratories into a real-time network to support biosurveillance. The multitude of laboratory networks that are being formed will enhance the analytical capabilities of laboratories as well as the ability of laboratories to distribute peak loads during emergencies and quickly and efficiently share data electronically. Enormous potential exists for the use of data that that is locked up in systems by the lack of standardization. Although much recent emphasis has been focused on building laboratory networks in preparation for a biological, chemical, or nuclear attack, many of the enhanced laboratory capabilities will assist with the response to other public health emergencies.
ADDITIONAL RESOURCES
Web Sites
American Society of Clinical Pathology (ASCP), http://www.ascp.org.
American Society for Clinical Laboratory Science (ASCLS), http://www.ascls.org.
American Society for Microbiology (ASM), http://www.asm.org.
Association of Public Health Laboratories, http://www.aphl.org.
Clinical Laboratory Management Association (CLMA), http://www.clma.org.
Clinical Laboratory Improvement Act (CLIA), http://www.cms.hhs.gov/clia.
American Association of Veterinary Laboratory Diagnosticians (AAVLD), http//www.aavld.org.
U.S. Animal Health Association (USAHA), http://www.usaha.org.
Laboratory Response Network (LRN), http://www.bt.cdc.gov/lrn/.
National Environmental Laboratory Accreditation Program (NELAP), http://www.epa.gov/nerlesd1/land-sci/nelac/.
National Animal Health Laboratory Network (NAHLN), http://www.nahln.us.
National Plant Diagnostic Network (NPDN), http://www.npdn.org.
Canadian Public Health Laboratory Network (CPHLN), http://www.cphln.ca.
National Laboratory System and Database (NLS), http://www/phppo.cdc.gov/mlp/nls.aspx.
FoodNet Surveillance, http://www.cdc.gov/foodnet.
U.S. EPA Laboratories, http://www/epa/gov/OSP/tribes/sciinf/labs.htm.
Recommended Further Reading
Cowan, D.F., ed. (2002). Informatics for the Clinical Laboratory: A Practical Guide. New York: Springer-Verlag.
Hinton, M. (1994). Laboratory Information Management Systems: Development, and Implementation for a Quality Assurance Laboratory. New York: Marcel Dekker.
Paszko, C. and Turner, E. (2001). Laboratory Information Management Systems, 2nd ed. New York: Marcel Dekker.
REFERENCES
- Association of Public Health Laboratories . 2004. Assessing America's Local Public Health Laboratory Capacity.http://www.aphl.org. December 2004. [Google Scholar]
- Bean N.H., Martin S.M., Bradford H., Jr An Electronic System for Reporting Public Health Data from Remote Sites. American Journal of Public Health. 1992;vol. 82:1273–1276. doi: 10.2105/ajph.82.9.1273. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=RetrievedbPubMeddoptCitationlist_uids=1323935. PHLIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC] Multistate Outbreak of Listeriosis, United States, 2000. Morbidity and Mortality Weekly Report. 2000;vol. 49:1129–1130. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=RetrievedbPubMeddoptCitationlist_uids=11190115. [PubMed] [Google Scholar]
- CDC . 4th ed. National Institutes of Health; Bethesda, MD: 1999. Biosafety in Microbiological and Biomedical Laboratories.http://www.cdc.gov/od/ohs. [Google Scholar]
- Centers for Medicare and Medicaid Services . 2004. CLIA Data Base.http://www.cms.hhs.gov/clia/. [Google Scholar]
- Committee on Research and Development Needs for Improving Civilian Medical Response to Chemical and Biological Terrorism Incidents . Chemical and Biological Terrorism—Research and Development to Improve Civilian Medical Response. National Academy Press; Washington, DC: 1999. Detection and Measurement of Biological Agents. [PubMed] [Google Scholar]
- Department of Health and Human Services [DHHS]/CDC. Bioterrorism Response Guide for Clinical Laboratories. http://www. bt. cdc.gov/labissues/.
- Emanuel P.A., Chue C., Kerr L., Cullin D. Validating the Performance of Biological Detection Equipment: The Role of the Federal Government. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science. 2003;vol. 1:131–137. doi: 10.1089/153871303766275808. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=RetrievedbPubMeddoptCitationlist_uids=15040191. [DOI] [PubMed] [Google Scholar]
- Gilchrist M.J. A National Laboratory Network for Bioterrorism: Evolution from a Prototype Network of Laboratories Performing Routine Surveillance. Military Medicine. 2000;vol. 165(Suppl 2):28–31. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=RetrievedbPubMeddoptCitationlist_uids=l0920634. [PubMed] [Google Scholar]
- Hindiyeh M., Hillyard D.R., Carroll K.C. Evaluation of the Prodesse Hexaplex Multiplex PCR Assay for Direct Detection of Seven Respiratory Viruses in Clinical Specimens. American Journal of Clinical Pathology. 2001;vol. 116:218–224. doi: 10.1309/F1R7-XD6T-RN09-1U6L. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=RetrievedbPubMeddoptCitationlist_uids=11488068. [DOI] [PubMed] [Google Scholar]
- Hutwagner L.C., Maloney E.K., Bean N.H., Slutsker L., Martin S.M. Using Laboratory-based Surveillance Data for Prevention: An Algorithm for Detecting Salmonella Outbreaks. Emerging Infectious Diseases. 1997;vol. 3:395–400. doi: 10.3201/eid0303.970322. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=RetrievedbPubMeddoptCitationlist_uids=9284390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne S.P., Beugelsdijk T.J. High-Throughput Laboratories for Homeland and National Security. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science. 2003;vol. 1:123–130. doi: 10.1089/153871303766275790. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=RetrievedbPubMeddoptCitationlist_uids=15040190. [DOI] [PubMed] [Google Scholar]
- McDade J., Hughes J. The U.S. Needs a National Laboratory System. U.S. Medicine. 1998;vol. 34(9) [Google Scholar]
- Murch R.S. Microbial Forensics: Building a National Capacity To Investigate Bioterrorism. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science. 2003;vol. 1:117–122. doi: 10.1089/153871303766275781. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=RetrievedbPubMeddoptCitationlist_uids=15040189. [DOI] [PubMed] [Google Scholar]
- York M. Sentinel Bioterrorism Responders: Are Hospital Labs Ready. Medical Laboratory Observer. 2003;vol. 35:12–17. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=RetrievedbPubMeddoptCitationlist_uids=12942658 19; quiz 22-23. [PubMed] [Google Scholar]


