I. INTRODUCTION
A. Major Taxonomic and Historical Considerations
The laboratory rat, Rattus norvegicus, is within the order Rodentia and family Muridae. The genus Rattus contains more than 130 species; however, the Norway rat, R. norvegicus, and the black rat, R. rattus, are the 2 species most commonly associated with the genus. Rattus rattus preceded R. norvegicus in migration from Asia to Europe and the Americas by several hundred years. The former species reached Europe in the twelfth century, and the Americas in the sixteenth century; whereas R. norvegicus emerged in the eighteenth century in Europe and in the nineteenth century in the Western Hemisphere. Globally, the Norway rat has largely displaced the black rat, probably because of the Norway rat's larger size and aggressiveness. The domestication and introduction of the albino R. norvegicus is rooted by its use in Europe and America in the 1800s as prey for a sport (rat baiting) in which individuals would wager on which terrier dog would most swiftly kill the largest number of rats confined to a pit. Because of the need for large numbers of rats for this sport, wild rats were purpose-bred, and albinos were selected out by some people as a hobby (Robinson, 1965). The first reported use of the rat in an experiment, in 1856, has been credited to J. M. Philipeaux, who studied the effects of adrenalectomy in albino rats (Richter, 1954). Rats were used in experiments only sporadically in Europe and the United States until about 1890. Pivotal to the development of the rat for use in research was H. H. Donaldson, who at the Wistar Institute did much to produce and define early stocks of laboratory rats (Lindsey, 1979).
B. Uses in Research
The rat is second only to the mouse as the most frequently used mammal in biomedical and behavioral research. Characteristics such as a short gestation and a relatively short life span, a docile behavior, and ready availability of animals with well-defined health and genetic backgrounds are responsible for the importance of the rat as a laboratory animal. The rat is a standard species for toxicological, teratological, and carcinogenesis testing by the pharmaceutical industry and governmental agencies. Its early use in behavioral, neurological, nutritional, and endocrinology studies continues today. The size of the rat enables it to be used for surgical procedures, varying from organ transplantation to vascular techniques. Although the number of commonly used inbred strains is dwarfed by those of the mouse, inbred rat strains do represent an important repertoire of disease models (Table I ).
Table I.
Commonly Used Rat Strains
| Inbred strains | Usefulness as models |
|---|---|
| ACI | Congenital genitourinary anomalies, prostatic adenocarcinomas |
| BB/Wor | Juvenile insulin-dependent diabetes mellitus |
| BN (Brown Norway) | Inducible, transplantable myeloid leukemia, hydronephrosis, bladder carcinoma |
| BUF (Buffalo) | Spontaneous autoimmune thyroiditis, host for transplantable Morris hepatoma |
| COP (Copenhagen) | Prostate adenocarcinoma |
| F-344 (Fischer 344) | Inbred rat model for National Toxicology Program's |
| Carcinogen Bioassay Program and the National | |
| Institute on Aging | |
| LEW (Lewis) | Multiple sclerosis, various experimentally induced autoimmune diseases |
| LOU/C | Myeloma, production of IgG autoantibody |
| SHR (spontaneous hypertensive rat) | Hypertension, cardiovascular research |
| WF (Wistar-Furth) | Mononuclear cell leukemia |
| Zucker | Obesity |
| Mutant strains | Characteristics |
| Brattleboro | Diabetes insipidus (autosomal recessive) |
| Gunn | Jaundice, kernicterus (autosomal recessive) |
| Nude | T cell deficient (autosomal recessive) |
| Obese SHR | Type 4 hyperlipoproteinemia (autosomal recessive) |
C. Sources
Commercial sources for outbred and inbred rats have been significantly consolidated during the past decade or two, resulting in a high percentage of rats being sold in the United States by several companies. Many of the small firms that had regional niches have been acquired by companies that have multiple divisions within the United States and internationally. Concomitantly, there has been a raising of the bar regarding the health status and genetic integrity of laboratory rats available to investigators.
D. Summary of Laboratory Management and Husbandry
1. Secondary Enclosures
Rooms in which rats are to be housed should meet the guidelines of the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996a). Wall, ceiling, and floor surfaces should be made of materials that allow for effective sanitation and that are resistant to damage from normal use and manipulation of equipment. The environment of the room should be well controlled, to ensure animal well-being and to help limit variables to those of the experimental design. Although rats, like most other species, can adapt to changes in temperature and humidity, room temperatures within a range of 70°–76° F and with a relative humidity of 30–70% are typically accepted as being appropriate. Twenty-four-hour temperature/humidity recorders, either located in animal rooms or as a component of an electronic environmental management system, are useful in detecting changes in environmental conditions. Practice over many years has shown that, in general, ventilation rates of 10–15 air changes/hr of fresh air are sufficient to compensate for heat load and the generation of NH3 and CO2 from animals. A stable photoperiod is necessary to avoid changes in reproductive behavior, food intake, and weight gain. A cycle of 12 to 14 hr light and 10 to 12 hr dark is typically used for rats. Rats are particularly susceptible to phototoxic retinopathy. There is sufficient evidence to indicate that light intensity at cage level should be between 130 and 300 lux to prevent retinopathy (National Research Council, 1996a,b).
Prevention and control of infectious diseases are partially a function of the location, size, and environmental conditions of a rat housing room. Strategies for limiting the transfer of pathogens will vary according to the potential impact that infectious agents may have on a particular group of rats and the study in which they are being used. For instance, an appropriate-sized room or cubicle may reflect the space necessary to separate rats by such criteria as pathogen status, immunological status, vendor, protocol, or investigator. Modifications such as the incorporation of Class 100 flexible wall enclosures may be useful to help ensure the specific pathogen status of rats over an extended period of time. Because of stress produced by noise, rat rooms should be located distant from mechanical rooms, cage washing centers, and species that are apt to produce noise (National Research Council, 1996b).
2. Primary Enclosures
The amount of cage space needed for rats, whether group or individually housed, is a function of animal weight and the specific physiological or protocol requirements of the animal(s) (National Research Council, 1996a). Unless there is an experimental need, rats should be housed in solid-bottom rather than in wire-bottom cages. This will help prevent pododermatitis and injuries that are more frequently associated with wire floors (National Research Council, 1996b), and bedding within solid-bottom cages provides environmental enrichment. The most frequently used materials for solid-bottom cages are polycarbonate and polypropylene. The former plastic is often preferred because it may be repeatedly autoclaved without damage and because its translucency allows for observation of animals. Various contact bedding materials are appropriate for rats (e.g., hardwood chips, ground corncob, cellulose sheets).
3. Sanitation
Solid-bottom cages should typically be sanitized at a frequency of 1–2 times per week. A less frequent cycle may be appropriate if cage density is very low, if there are perinatal considerations, or if ventilated cages are used; and a more frequent cycle, if cage density is high or if pathophysiological considerations exist (e.g., diabetes). A detailed description of appropriate sanitation for rodent housing is given in “Laboratory Animal Management—Rodents” (National Research Council, 1996b).
II. BIOLOGY
A. Morphophysiology
This section provides a summary of some of the morpho-physiological characteristics of the rat that may be useful to the reader. For more comprehensive descriptions, see the references cited in this section.
1. General Appearance
The Norway rat has small, thick ears and a tail that is about 85% of the length of the body (in contrast, R. rattus has larger ears and a tail that is distinctly longer than the body). The hair coat is composed of two classes—long and short hair shafts, with the former being more sparse. Hair growth in the young rat is cyclic, with the resting period and the growing period each being 17 days. In the female, there are usually 12 teats, with 3 pairs in the pectoral and 3 pairs in the abdominal region (Greene, 1963). Body weights and growth rates are dependent on the stock, strain, and source of rats. Of the two most commonly used outbred stocks, the Sprague-Dawley is larger than the Wistar, and the inbred Fischer 344 rat is smaller than either of the outbreds.
2. Sensory Organs
Rat eyes are exophthalmic, which increases the risk of injury from trauma and drying during anesthesia. The eyelids are well developed, and only the cornea is visible. The Harderian gland, located medially to the orbit, and the lacrimal glands moisten the cornea. The Harderian gland secretes porphyrin in excessive amounts, termed chromodacryorrhea, when the animal is stressed (e.g., because of malnutrition, dehydration, disease, or environmental factors). Accordingly, a reddish secretion or crust located periorbitally and at the nares may be a useful indicator of illness or a husbandry problem (Moore, 1995). The orbital venous sinus beneath the medial aspect of the orbit is a useful site for blood collection in the anesthetized animal. It has been accepted that rats lack color vision; however, one recent study suggests that rats may have dichromatic color vision (Koolhass, 1999).
Olfactory signals are strong determinants for behavior in the rat. Male rats recognize the social status of other males, females in estrus, and kinship by olfactory cues. Rats also detect alarm pheromones from other rats (Koolhass, 1999).
The hearing frequency range for rats at 70 dB is 250 Hz to 70–80 kHz, with 8 kHz to 32 kHz being the most sensitive range. Except for the rat's high-frequency sensitivity, its hearing capability corresponds closely to that of other mammals (Kelly and Masterson, 1977). This high-frequency sensitivity corresponds to the 22–80 kHz vocalizations emitted by pups left alone by their dam, or by adults during sexual and aggressive behavior (Koolhass, 1999).
3. Skeleton
The skull is composed of the following bones: paired nasal, premaxillary, maxillary, zygoma, palatine, lacrimal, frontal, parietal, squamosal, periotic capsule, tympanic bulla, and mandible; 6 auditory ossicles; 4 turbinates; and single vomer, ethmoid, basisphenoid, presphenoid, occipital, interparietal, and hyoid bones.
The vertebral column consists of 7 cervical, 13 thoracic, 6 lumbar, 4 sacral, and 27–30 caudal vertebrae. The ribs consist of ventral calcified and dorsal ossified segments without true costal cartilages. The humerus, ulna, and radius are similar to those of other mammalian species. The carpus consists of 9 bones. The pelvis is formed by 2 ossa coxae, which articulate with the first 2 sacral vertebrae. The bones of the hindlimb are the femur, the tibia, and the fibula, which articulates proximally with the tibia but is fused distally. The tarsus is composed of 8 bones (Greene, 1963).
4. Digestive System
The dental formula of the rat is 2(I 1/1, C 0/0, PM 0/0, M 3/3). Incisors grow continuously. If the incisors are not worn evenly or are misaligned due to gingivitis or congenital defects, the resulting malocclusion may lead to nonfunctional, spiral elongation of the incisors, injury to the palate, and reduced food intake. The salivary glands are paired and consist of the parotid, the submandibular, and the smaller sublingual glands. The parotid gland is serous and consists of three or four lobes located ventrolaterally from the caudal border of the mandible to the clavicle. The submandibular glands are mixed glands situated ventrally between the caudal border of the mandibles and the thoracic inlet. The sublingual glands are mucous and located at the rostral pole of the submandibular glands. Multilocular adipose tissue, referred to as brown fat or the hibernating gland, is located in the ventral and lateral portions of the neck and can be confused with salivary glands. Figure 1 depicts the location of the salivary glands, cervical lymph nodes, and adipose tissue (Greene, 1963).
Fig. 1.
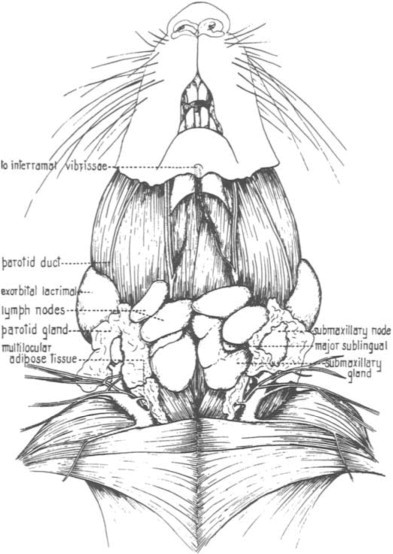
Salivary glands, cervical lymph nodes, and adipose tissue.
(From Greene, 1963.)
© 2002
The stomach is divided into two parts: the forestomach, or cardiac portion, which is nonglandular; and the corpus, or pyloric portion, which is glandular. A ridge separates the two portions, with the esophagus entering at the lesser curvature of the stomach through a fold of the ridge. This fold is responsible for the inability of the rat to vomit.
The small intestine consists of the duodenum (8 cm), jejunum (80 cm), and ileum (3 cm). The comma-shaped cecum is thin-walled, with a prominent mass of lymphoid tissue in its apical portion. The colon consists of the ascending colon, with prominent oblique mucosal ridges, and the transverse and descending portions, with longitudinal mucosa folds.
The liver has four lobes: the median, which has a deep fissure for the hepatic ligament; the right lateral, which is partially divided; the left, which is large; and the caudate, which is small and surrounds the esophagus. The rat does not have a gallbladder, and bile ducts from each lobe form the common bile duct, which enters the duodenum about 25 mm from the pyloric sphincter.
The pancreas is a very diffuse and lobulated organ that can be differentiated from adjacent adipose tissue by its darker color and firm consistency. Numerous excretory ducts fuse into 2–8 large ducts, which empty into the common bile duct (Bivin et al., 1979). Figure 2 depicts the abdominal and thoracic viscera in situ (Greene, 1963).
Fig. 2.
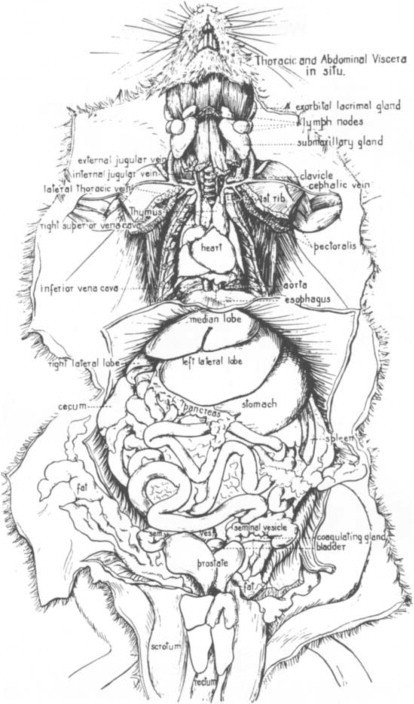
Abdominal and thoracic viscera in situ.
(From Greene, 1963.)
© 2002
5. Respiratory System
The rat has a maxillary recess (sinus) located between the maxillary bone and the lateral lamina of the ethmoid bone. The recess contains the lateral nasal gland (Steno's gland), which secretes a watery product discharged at the rostral end of the nasal turbinate. It has been postulated that this secretion may act to regulate the viscosity of the mucous layer overlying the nasal epithelium.
The lungs consist of the left lung, which is single-lobed, and the right lung, which is divided into the cranial, middle, accessory, and caudal lobes. The pulmonary vein has cardiac striated muscle fibers within its wall that are contiguous with those in the heart. The rat does not have an adrenergic nerve supply to the bronchial musculature, and bronchoconstriction is controlled by vagal tone (Bivin et al., 1979).
6. Genitourinary System
The male rat has a number of highly developed accessory sex glands (Fig. 3 ). The paired bulbourethral glands (Cowper's glands) at the base of the penis open into the dorsal surface of the urethral flexure. Within the abdominal cavity and surrounding the bladder are the large seminal vesicles and the prostate gland, which is composed of the dorsocranial (coagulation gland), ventral, and dorsolateral lobes. The female rat has a bicornate uterus, and although the uterine horns appear fused distally, there are two distinct ossa uteri and cervices.
Fig. 3.
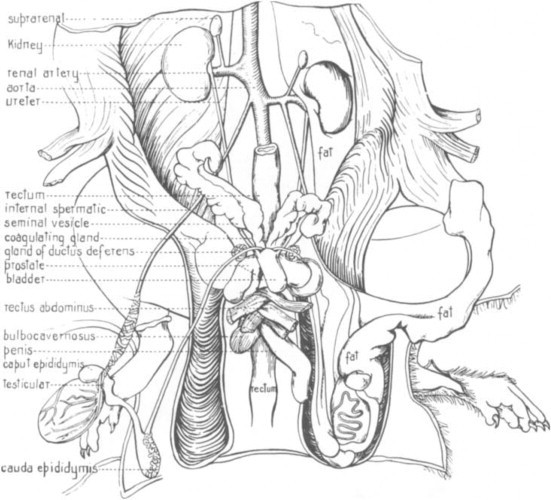
Male urogenital system.
(From Greene, 1963.)
© 2002
The right kidney is more craniad than the left and has its cranial edge at the L1 vertebra and its caudal edge at the level of L3. Like the kidneys of other rodents, the rat kidney is unipapillate, making the rat useful for studies in which cannulization of the kidney is done. The rat is also widely used as a model for investigating nephron transport in an in vivo micropuncture system, because of the presence of superficial nephrons in the renal cortex.
7. Central Nervous System
The brain is characterized by large olfactory bulbs, a smooth cerebrum, and the two parafloccular lobes of the cerebellum, which lie in deep sockets of the periotic capsule of the skull. The hypophysis lies behind the optic chiasma and is attached to the base of the brain by a thin hollow stalk, the infundibulum. The ventricular system is similar to that of other animals, but the rat lacks a foramen of Magendie. The spinal cord ends at the fourth lumbar vertebra, with the filum terminale ending at the level of the tail beyond the third caudal nerves (Greene, 1949).
8. Cardiovascular System
The heart is located on a midline in the thorax, with its apex near the diaphragm and its lateral aspects bounded mainly by the lungs. The heart is exposed to the left thoracic wall between the third and fifth ribs, making it a useful site for cardiac blood collection. The blood supply to the atria of the rat, unlike that of higher mammals, is largely extracoronary from branches of the internal mammary and subclavian arteries.
B. Normal Physiological Values
Many of the normal values determined for a specific group of rats may be accurate for only that source and stock/strain. Other factors such as age, pathogen status, sample collection methods, and husbandry conditions of the colony are also important variables (Suber and Kodell, 1985; Dameron et al., 1992; Perez et al., 1997). Selected physiological, clinical chemistry, and hematological values are listed in Table II, Table III, Table IV .
Table II.
Selected Normative Data for the Rata
| Adult weight | |
| Male | 300–500 gm |
| Female | 250–300 gm |
| Life span | 2.5–3 years |
| Body temperature | 37.5°C |
| Basal metabolism rate (400 gm rat) | 35kcal/24hr |
| Chromosome number (diploid) | 42 |
| Puberty | 50 ± 10 days |
| Gestation | 21–23 days |
| Litter size | 8–14 |
| Birth weight | 5–6gm |
| Eyes open | 10–12 days |
| Weaning | 21 days |
| Food consumption/24 hr | 5 gm/100gm body weight |
| Water consumption/24hr | 8–11 ml/100 gm body weight |
| Cardiovascular | |
| Arterial blood pressure | |
| Mean systolic | 116mm Hg |
| Mean diastolic | 90mmHg |
| Heart rate | 300–500beats/min |
| Cardiac output | 50ml/min |
| Blood volume | 6 ml/100 gm body weight |
| Respiratory | |
| Respirations/min | 85 |
| Tidal volume | 1.5 ml |
| Alveolar surface area (400 gm rat) | 7.5 m2 |
| Renal | |
| Urine volume/24hr | 5.5 ml/100 gm body weight |
| Na+ excretion/24 hr | 1.63 mEq/lOOgm body weight |
| K+ excretion/24 hr | 0.83mEq/100gm body weight |
| Urine osmolarity | 1659mOsm/kgofH20 |
| Urine pH | 7.3–8.5 |
| Urine specific gravity | 1.04–1.07 |
Data from Baker (1979) and Bivin et al. (1979).
Table III.
Clinical Chemistry Reference Ranges for Adult Ratsa
| Sprague – Dawkyb |
Fisher 344d |
||||
|---|---|---|---|---|---|
| Analyte | Units | M | F | M | F |
| Serum | |||||
| Glucose | mg/dl | 115 ± 16.9 | 111 ± 17.2 | 115 ± 12.5c | |
| Urea nitrogen | mg/dl | 19 ± 2.2 | 21 ± 3.4 | 15 ± 2.5c | |
| Creatinine | mg/dl | 0.70 ± 0.11 | 0.70 ± 0.13 | ||
| Sodium | mEq/liter | 150 ± 3.4 | 148 ± 3.5 | 149 ± 3.0c | |
| Potassium | mEq/liter | 7.00 ± 0.65 | 6.1 ± 0.67 | 4.80 ± 0.35c | |
| Chloride | mEq/liter | 103.0 ± 1.90 | 104.0 ± 2.4 | 106 ± 3.0c | |
| Calcium | mg/dl | 12.0 ± 0.94 | 12.1 ± 0.71 | 10.5 ± 0.50c | |
| Phosphorus | mg/dl | 7.30 ± 1.5 | 5.80 ± 1.10 | ||
| Magnesium | 3.12 ± 0.41f | 2.60 ± 0.21′ | |||
| Iron | μg/dl | 152 ± 70 | 220 ± 130 (19–21 weeks) | ||
| Total iron binding capacity | μg/dl | 368c | |||
| Alanine aminotransferase | IU/liter | 49 ± 24.1 | 69 ± 44.9 | 78 ± 11 | 49 ± 8 |
| Aspartate aminotransferase | IU/liter | 95 ± 31.7 | 99 ± 54.5 | ||
| Alkaline phosphatase | IU/liter | 130 ± 43.7 | 117 ± 41.7 | 49.5 ± 9.25c | |
| Lactate dehydrogenase | IU/liter | 275 ± 112 | 650 ± 75 | 650 ± 75 (20 weeks) | |
| Sorbitol dehydrogenase | IU/liter | 20±5c (32 weeks) | 20.0 ± 7.5c | ||
| γ-Glutamyl transpeptidase | IU/liter | 2.5 ± 1.25c | |||
| Creatinine kinase | IU/liter | 275 ± 112.5c | 400 ± 50c | ||
| Protein, total | gm/liter | 70 ± 5.0 | 75 ± 5 | 70.5 ± 4.75c | |
| Albumin | gm/liter | 34 ± 2.0 | 40 ± 2.5 | 42.5 ± 3.75c | |
| Cholesterol | mg/dl | 119 ± 51.3 | 119 ± 29.0 | 96.5 ± 14.25 | 130 ± 10.0 |
| Triglycerides | mg/dl | 266 ± 121.4 | 249 ± 159.7 | 122 ± 21.25 | 62.5 ± 11.25 |
| Bilirubin | mg/dl | 0.3 ± 0.16 | 0.4 ± 0.27 | 0.3 ± 0.1c | |
| Bile acids | μmol/liter | 40 ± 10c (20 weeks) | 30 ± 10c | ||
| Uric acid | mg/dl | 1.52 ± 0.30 | 1.25 ± 0.36 | ||
| Urine | |||||
| Volume | ml/16hr | 9.5 ± 4.0 | 9.3 ± 5.6 | ||
| ml/22 hr | 15.7 ± 6.7 | 11.0 ± 5.0 | |||
| Specific gravity | 1.022 ± 0.007 | 1.017 ± 0.007 | |||
| Osmolality | mOsm/kg | 943 ± 327 | 995 ± 367 | ||
| PH | 7.8 ± 0.5 | 7.7 ± 0.5 | 6.0–6.5c | ||
| Chloride | mmol/liter | 148 ± 36 | 151 ± 51 | ||
| Sodium | mmol/liter | 31 ± 11 | 50 ± 22 | ||
| Potassium | mmol/liter | 121 ± 31 | 110 ± 45 | ||
| Phosphorus | mg/dl | 142 ± 34 | 156 ± 62 | ||
| Creatinine | mg/dl | 136 ± 40 | 116 ± 41 | 80 ± 28 | 54 ± 25 |
| Glucose | mg/dl | 9.9 ± 3.9 | 5.5 ± 2.3 | ||
| Protein | mg/dl | 98.8 ± 54.4 | 11.2 ± 5.5 | ||
| Alkaline phosphatase | IU/liter | 152 ± 61 | 73 ± 37 | ||
| Lactate dehydrogenase | IU/liter | 28 ± 15 | 16 ± 8 | ||
| N-Acetyl-β-glucosaminidase | IU/liter | 12.2 ± 7.8 | 5.9 ± 2.7 | ||
| Aspartate aminotransferase | 14.4 ± 6.5 | 3.6 ± 2.5 | |||
| γ-Glutamyl transpeptidase | IU/liter | 4964 ± 780 | 1873 ± 215 | ||
| Insulin clearance | μI/min/lOOgm | 857 ± 178 | |||
| Glomerular filtration rate | μI/min | 275 ± 33c | |||
| Urine flow | μI/min/100gm | 5.2 ± 2.0 | |||
| Analyte | Units | Male | Female |
|---|---|---|---|
| Hormonee | |||
| Luteinizing hormone | ng/ml | 0.16–0.64 | 0.32–0.64 (basal) |
| 24.6–32.8 (late proestrus) | |||
| Follicle stimulating hormone | ng/ml | 5.56–11.1 (light period) | 2.22–4.44 (basal) |
| 11.1–20.0 (dark period) | 8.85–13.3 (preovulatory, estrus) | ||
| Prolactin | ng/ml | 28.6 (dark period) | 5.4–10.7 (basal) |
| 1.8–10.7 (light period) | 71.4–107 (late proestrus) | ||
| 71.4 (after coitus) | |||
| Growth hormone | ng/ml | 0.4–80c | |
| Thyroid stimulating hormone | ng/ml | 2.27–3.4 | 0.57 (basal) |
| 1.14 (early light period) | |||
| Adrenocorticotropic hormone | pg/ml | 30–100 | |
| Vasopressin | pg/ml | 1–8c | |
| Oxytocin | pg/ml | 4–10.5c | |
| Thyroxine (T4) | μβ/Λ | 5.1 ± 0.4 | 4.9 ± 0.1 (Long-Evans) |
| Triiodothyronine (T3) | ng/dl | 66 ± 3.5 | 83 ± 3 (Long-Evans) |
| Free T4 | ng/dl | 2.212 ± 0.055c | |
| Free T3 | pg/dl | 208.49 ± 8.55c | |
| Calcitonin | pg/ml | 200–1000c (F-344/9 months) | |
| Parathyroid hormone | pg/ml | 140–180 | <50–400 (Sprague-Dawley) |
| 1,25-Dihydroxy vitamin D | pg/ml | 120 ± 24 | 96 ± 17 (Wistar) |
| Corticosterone | μg/dl | 15–23 (late light period) | 70 (late light period) |
| 1–6 (late dark period) | 17 (late dark period) | ||
| Aldosterone | ng/dl | 12–35 (late light period) 4–11 (middle light period) | 25–35 (9–10 AM) |
| Epinephrine | pg/dl | 253 ± 30c (awake, undisturbed; Sprague-Dawley) | |
| 460 ± 60c (handled) | |||
| 180 ± 24c (asleep) | |||
| Norepinephrine | pg/dl | 710 ± 110c (awake, undisturbed; Sprague-Dawley) | |
| 830 ± 130c (handled) | |||
| 460 ± 80c (asleep) | |||
| Progesterone | ng/ml | 1–5 (early proestrus) | |
| 40–50 (estrus) | |||
| 20–30 (first diestrus day) | |||
| Estradiol | pg/ml | < 10 (basal) | |
| 20–30 (2nd diestrus day) | |||
| 40–50 (proestrus) | |||
| Testosterone | ng/ml | 3 (1330–1600hr) <1 (2130hr) | |
| pg/ml | 500–600 (proestrus) | ||
| 100 (estrus) |
Values are for 12-month-old animals, unless noted otherwise, as summarized from Loeb and Quimby (1999).
6–18 months old.
Gender not specified.
58-l 12 weeks old.
Species not specified.
Wistar strain.
Table IV.
Hematological Parameters in the Rata
| Erythrocyte parameters (mean values) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythrocyte (X106mm3) |
PCV(%b) |
Hemoglobin (gm/dl) |
MCV (fl)c |
MCHC (%)d |
|||||||
| Stock/strain | Age (weeks) | M | F | M | F | M | F | M | F | M | F |
| Crl.CD(SD)BR | 8 | 7.98 | 7.44 | 43 | 41 | 14.9 | 14.2 | 54 | 55 | 35 | 35 |
| 12 | 8.32 | 7.6 | 44.5 | 41.5 | 15.3 | 14.5 | 53 | 55 | 34 | 35 | |
| Crl:(WI)BR | 6–8 | 6.46 | 6.92 | 36 | 38 | 13.5 | 14.1 | 56 | 55 | 37 | 36 |
| 19–21 | 8.31 | 7.81 | 41 | 40 | 16.0 | 15.6 | 53 | 50 | 38 | 39 | |
| 32–34 | 8.4 | 7.8 | 42 | 40.7 | 15.5 | 16.3 | 50 | 52 | 37 | 40 | |
| CDF(F-344)CrlBR | 6–8 | 5.38 | 5.35 | 43 | 40 | 14.9 | 14.0 | 79 | 76 | 35 | 35 |
| 19–21 | 7.62 | 7.0 | 46 | 50 | 15.3 | 16.4 | 61 | 72 | 33 | 36 | |
| 32–34 | 5.51 | 5.05 | 49 | 42 | 16.6 | 15.0 | 89 | 83 | 34 | 36 | |
| Hsd:SD | 11–12 | 7.05 | 6.68 | 41.7 | 39.9 | 15.1 | 15.0 | 59.2 | 59.7 | 36.3 | 37.8 |
| Hsd:WI | 11–12 | 7.85 | 7.06 | 43.8 | 39.5 | 16.2 | 14.7 | 55.8 | 56.0 | 37.2 | 37.1 |
| F-344/NHsd | 11–12 | 8.61 | 7.32 | 44.9 | 40.1 | 16.7 | 14.7 | 52 | 49.8 | 37.4 | 36.7 |
| Tac:N(SD)fBR | 6 | 7.1 | 6.9 | 15.5 | 14.6 | ||||||
| 10–12 | 7.82 | 8.1 | 16.4 | 16.65 | |||||||
| Tac: Sim(LE) | 11 | 8.0 | 7.3 | 45.2 | 40.7 | 14.5 | 13.4 | 56 | 56 | 32.0 | 32.9 |
| Leukocyte parameters (mean values) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (× 103) |
Neutrophil |
Lymphocyte |
Monocyte (differential %) |
Eosinophil |
Basophil |
||||||||
| Stock/strain | Age (wee ks) | M | F | M | F | M | F | M | F | M | F | M | F |
| Crl:CD(SD)BR | 8 | 11.82 | 9.28 | 10 | 10 | 84 | 86 | 2.6 | 2.3 | 1 | 1 | <1 | <1 |
| 12 | 11.25 | 8.79 | 11 | 9 | 83 | 85 | 2.6 | 2.3 | 1 | 1 | <1 | <1 | |
| Crl:(WI)BR | 6–8 | 8.66 | 6.96 | 12 | 13 | 85 | 84 | 2 | 2 | 1 | 1 | 0 | 0 |
| 19–21 | 9.37 | 8.43 | 15 | 17 | 82 | 80 | 2 | 2 | 1 | 1 | 0 | 0 | |
| 32–34 | 7.8 | 6.0 | 18 | 23 | 80 | 75 | 0 | 0 | 2 | 2 | 0 | 0 | |
| CDF(F-344)CrlBR | 6–8 | 6.4 | 5.5 | 17 | 14 | 78 | 83 | 4 | 3 | 1 | 1 | 0 | 0 |
| 19–21 | 8.2 | 8.3 | 30 | 43 | 68 | 52 | 1 | 5 | 1 | 1 | 0 | 0 | |
| 32–34 | 4.7 | 6.9 | 52 | 38 | 46 | 56 | 2 | 4 | 1 | 1 | 0 | 0 | |
| Hsd:SD | 11–12 | 4.5 | 4.9 | 29 | 30 | 65 | 64 | <3 | <3 | <2 | <2 | <3 | <2 |
| Hsd:WI | 11–12 | 15.2 | 7.7 | 12 | 12 | 87 | 83 | <1 | <4 | <1 | <1 | <1 | <1 |
| F-344:NHsd | 11–12 | 5.8 | 5.4 | 17 | 15 | 81 | 79 | <2 | <4 | <1 | <2 | <1 | <1 |
| Tac:N(SD)fBR | 6 | 6.8 | 4.8 | 8.2 | 9.7 | 91.6 | 89.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10–12 | 5.24 | 6.71 | 11.33 | 13.88 | 88.11 | 85.75 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tac: Sim(LE) | 11 | 9.3 | 7.4 | 17.2 | 21.8 | 80.8 | 75.9 | 1.2 | 1.2 | 0.9 | 1.1 | 0 | 0 |
Derived from vendor data.
PCV, packed cell volume
MCV, mean corpuscular volume
MCHC, mean corpuscular hemoglobin contact
C. Nutrition
Nutritionally adequate diets for rats are readily available from commercial sources. However, the refinement of ingredients within diet formulations may vary according to classifications of commercially available products. The three classifications of diets are (1) natural-ingredient, (2) purified, and (3) chemically defined. The most commonly used type for most research applications is the natural-ingredient diet, composed of agricultural products and by-products. This class of diet can be either an open-formula diet, in which the information on the amount of each ingredient is available, or a closed-formula diet, in which such information is held confidential by the producer. The nutrient composition of ingredients in natural-ingredient diets varies from batch to batch because of various factors (e.g., relative costs of grains, weather conditions, harvesting and storage conditions, and concentrations of contaminants). Certified, natural-ingredient diets are used for toxicological and other Good Laboratory Practice (GLP) studies because each lot is assayed and certified not to exceed established maximum concentrations of a set list of contaminants (e.g., pesticides, heavy metals, mycotoxins, and estrogens) that could influence study results.
The nutrient concentrations in purified diets are less variable because defined ingredients, each composed of a single nutrient or nutrient class (e.g., casein, sugar, starch, vegetable oil, cellulose), are used in their formulation. A frequently used purified diet for rats is AIN-76. The downside of this class of diets is that they are more expensive and often less palatable. Chemically defined diets are formulated with very basically defined ingredients (e.g., specific amino acids, sugars, triglycerides, and essential fatty acids). These diets are costly and tend to lack palatability (National Research Council, 1996b). Table V summarizes the nutrient requirements of rats (National Research Council, 1995).
Table V.
Recommended Composition of Diet for Growing Ratsa
| Main components and amino acids | Minerals and trace elements | Vitamins | |||
|---|---|---|---|---|---|
| Digestible energy (kJ/gm) | 16.0 | Calcium (gm/kg) | 5 | Retinol (mg/kg) | 1.2 |
| Fat (gm/kg) | 50 | Chloride (gm/kg) | 0.5 | Cholecalciferol μg/kg) | 25 |
| Fiber (gm/kg) | r.u.b | Magnesium (gm/kg) | 0.4 | DL-α-Tocopheryl acetate (mg/kg) | 27 |
| Protein (gm/kg) | 120 | Phosphorus (gm/kg) | 4 | ||
| Arginine (gm/kg) | 6 | Potassium (gm/kg) | 3.6 | Menadione (mg/kg) | 0.05 |
| Asparagine (gm/kg) | 4 | Sodium (gm/kg) | 0.5 | Thiamin (mg/kg) | 4 |
| Glutamic acid (gm/kg) | 40 | Sulfur (gm/kg) | 0.3 | Riboflavin (mg/kg) | 3 |
| Histidine (gm/kg) | 3 | Chromium (mg/kg) | 0.3 | Pyridoxine (mg/kg) | 6 |
| Isoleucine (gm/kg) | 5 | Copper (mg/kg) | 5 | Cyanocobalamin μg/kg) | 50 |
| Leucine (gm/kg) | 7.5 | Fluoride (mg/kg) | 1 | Nicotinic acid (mg/kg) | 20 |
| Lysine (gm/kg) | 7 | Iodine (mg/kg) | 0.15 | Folic acid (mg/kg) | 1 |
| Methionine + cystine (gm/kg) | 6 | Iron (mg/kg) | 35 | Biotin (mg/kg) | s.u.c |
| Phenylalanine + tyrosine (gm/kg) | 8 | Manganese (mg/kg) | 50 | Pantothenic acid (mg/kg) | 8 |
| Proline (gm/kg) | 4 | Selenium (mg/kg) | 0.1 | Choline (mg/kg) | 1000 |
| Threonine (gm/kg) | 5 | Zinc (mg/kg) | 12 | Inositol (mg/kg) | n.rd |
| Tryptophan (gm/kg) | 1.5 | Ascorbic acid (mg/kg) | n.r.d | ||
| Valine (gm/kg) | 6 | ||||
| Glycine (gm/kg) | s.u.c | ||||
r.u., required but degree unknown.
s.u., status unknown.
n.r., not required.
In most instances, rats are fed ad libitum. However, there are numerous reports that demonstrate that unlimited feeding of rats on long-term carcinogenesis and toxicological studies reduces longevity and increases the incidence of neoplasia relative to rats fed at 70–80% of the ad libitum food amount. These effects have been found in Sprague-Dawley, Wistar, and F-344 rats and have caused increased variability among 2-year carcinogenicity and safety assessment studies, compromising the usefulness of bioassays in risk assessment (Keenan et al., 1996). For instance, Wistar rats fed 80% of ad libitum beginning at 16 weeks of age had very significant reductions in the incidence of lung, mammary, pancreatic islet cell, and pituitary tumors relative to controls fed ad libitum. The overall incidence of malignant tumors was 16% in the feed-limited group and 37% in the ad libitum group, even though the feed-limited group had a greater longevity. There was also a reduction in chronic inflammation and fibrosis of the heart, acute inflammation of the prostate, radiculoneuropathy, and acinal hyperplasia of the mammary gland in feed-limited animals (Roe et al., 1995).
D. Reproduction
1. Reproductive Physiology
In the rat, the vagina is closed at birth by compact epithelium, referred to as the vaginal plate (Del Vecchio, 1992). This begins to degenerate and cornify at 20–35 days of age and is completely open between 40 and 80 days of age. Persistence of the vaginal plate is an occasional cause of infertility.
Puberty is defined as the onset of sexual maturity, the ability to bear viable young, and occurs before full body size and weight are attained. As in most species, puberty occurs in females earlier than in males and also varies with stock or strain. Puberty most often occurs at 2–3 months of age in the rat (Fox and Laird, 1970), although considerable variation exists in reported values. Kohn and Barthold (1984) report 40–60 days, and Bennett and Vickery (1970) report 50–72 days. More recently, Ayala et al. (1998) report 45–47 days. Estrus, which should be distinguished from puberty, begins before full reproductive competency is reached and has been reported to occur at 36 days in the Wistar rat (Eckstein et al., 1973). However, some authors report successfully breeding Wistar BH rats at 35 days of age (Rosen et al., 1987).
The estrous cycle of rats is most often 4–5 days in length and occurs throughout the year, as well as postpartum. Seasonal variation is not observed in laboratory colonies. For a 4-day estrous cycle, approximately 1 day is spent in each of the four stages: estrus, metestrus, diestrus, and proestrus. However, cycles of up to 6 days are not uncommon, with the additional time in diestrus or proestrus (Peluso, 1992). In proestrus, the uterus can appear “ballooned” with fluid, especially in a peripubertal rat; this condition should not be mistaken for hydrometra.
Ovulation occurs approximately 8–11 hr after the onset of estrus, usually between midnight and 2 a.m. (Peluso, 1992), although this would obviously depend on timing of the light cycle. Ova remain viable for approximately 10–12 hr (Fox and Laird, 1970).
Testes descend from the abdomen into the scrotum at approximately 15 days of age (Russell, 1992). Sperm are first produced at about 45–46 days of age, but fertility (puberty) does not occur until approximately 62–65 days of age, and sperm production is not maximal until 75 days of age (Russell, 1992). Interestingly, on histologic examination of young rat testes, more degenerate germ cells are noted prior to 75 days of age than afterward, indicative of the poor efficiency of spermatogenesis at early ages in the rat (Russell et al., 1987). Male sexual behavior is, in part, dependent on age and experience. Prepubertal males have no preference for females in estrus, and female-oriented sexual behavior is reported to decrease after 150 days of age (Matuszczyk et al., 1994; Smith et al, 1992). Decreased serum testosterone may be partially responsible for age-related decreases in mating behavior, but this does not appear to completely explain the phenomenon.
Coitus occurs more frequently during dark periods than light periods, and more frequently during the latter portion of the dark cycle than during the early portion (Mercier et al., 1987). Multiple intromissions (5–15), each lasting 0.3–0.6 seconds and with 2–9 pelvic thrusts (Bennett and Vickery, 1970), precede the first ejaculation, which lasts about 1 second. This first series of intromissions is called the ejaculatory latency and lasts about 10 minutes, followed by a refractory period (Dewsbury, 1970). A single ejaculation with fewer intromissions is less likely to impregnate the female. Multiple series of intromissions and ejaculations occur, usually about seven, with increasing refractory periods between successive episodes. Coitus can be confirmed in rats by detection of spermatozoa on a vaginal smear, by observation of a vaginal plug (the pale coagulum formed by seminiferous fluid visible in the vagina), or by direct observation of sexual behavior.
Implantation of the blastocyst into the endometrium occurs between 4 and 7 days after fertilization and actually represents a process that requires 12–24 hours for completion (Peluso, 1992; Enders, 1970; Garside et al., 1996). Parturition occurs 21–23 days after the time of coitus, although it can occur as early as 19 days (Bennett and Vickery, 1970; Baker, 1979). Pregnant rats whose time of coitus is known are therefore of known gestational length and are called timed pregnant.
2. Detection of Estrus and Pregnancy
Several methods can be used to determine if a rat is in estrus, which is useful in production of timed pregnant rats. Rats in estrus often exhibit specific behavior, including ear quivering when the back or head are stroked, and lordosis (“sway-back” posture) when the pelvic area is stimulated (Blandau et al., 1941). Additionally, the vulva becomes swollen, and the vaginal wall appears dry in estrus, instead of the moist pink appearance during metestrus or diestrus. This is due to cornification of the vaginal epithelium during estrus (Baker, 1979). These changes in the vaginal epithelium can be assessed by cytologic examination of vaginal smears (Montes and Luque, 1988). In estrus, 25–100% of the epithelial cells are cornified (Bennett and Vickery, 1970). Changes in vaginal fluids and cytology also lead to changes in the electrical impedance in the vagina during estrus. This has been widely exploited as a more precise method of estrus detection (Koto et al., 1987a, b), using a device referred to as an impedance meter in which an electrical probe is inserted into the vagina.
Pregnancy is difficult to detect early in gestation, but conception rates of 85% or more are often observed for outbred rat stocks, somewhat less in inbred strains. After approximately 10 days, careful palpation can detect the developing fetuses; this is especially accurate after 12 days of gestation. By 14 days of gestation, mammary gland and nipple development are evident (Bennett and Vickery, 1970).
3. Husbandry Needs
Inbred rats are generally bred monogamously or in trios, with one male and two females in each cage. Outbred rats may also be bred monogamously but are more often bred polygamously by commercial breeders for reasons of economy. Pregnant females are removed to separate cages a few days before parturition to minimize cannibalism or abandonment of litters.
A number of variables have been identified that may influence the husbandry requirements of a reproducing population of laboratory rats. Despite the lack of seasonal variation in estrous cycles in the rat, both ovarian function and the estrous cycle are influenced by light cycles. Continuous light has been reported to cause persistent estrus and cystic follicles in the ovaries, without formation of corpora lutea (Fox and Laird, 1970). Chronic exposure to even low intensity light during the dark cycle has been reported to result in earlier vaginal opening and ovarian atrophy (Beys et al., 1995; Fox and Laird, 1970). Caloric restriction, a 15–30% decrease from ad libitum caloric intake, may cause cessation of estrous cycles and delayed sexual maturation (Fox and Laird, 1970).
High ambient temperatures can result in male infertility (Pucak et al., 1977) by causing irreversible degeneration of the seminiferous epithelium. Significantly, the damage may occur in rats as young as 4 days and in rats with prolonged exposure to temperatures as low as 26.6°C.
4. Parturition
Female rats increase nest-building activity approximately 5 days prepartum and continue through lactation (Bennett and Vickery, 1970). Females will use any material available, but it is not necessary to add material for this purpose to the cages. Approximately 1.5–4 hours before the first pup is born, clear mucoid fluid discharges from the vagina. In early labor, the female walks about the cage and stretches. This behavior becomes more exaggerated as events progress; then the female will lie on her abdomen with rear legs extended off the cage floor. As pups are born, the female pulls the placenta from the birth canal and eats it. Parturition averages 1–3.5 hours, varying with litter size. Nursing usually begins only after all pups are born.
Litter size varies with stock, strain, source, and maternal age. The following are examples of the effect of maternal age. Wistar BH rats had an average of 1.69 more pups (11.80 vs. 10.11) when first mated at 105 days than when first mated at 35 days of age (Rosen et al., 1987). The second litter is usually the largest (Bennett and Vickery, 1970). After 9 months of age, litter size is further decreased, and the pregnancy rate declines after 12 months of age (Niggeschulze and Kast, 1994). Loss of fetuses, termed pregnancy wastage, occurs as a function of age (Mattheij and Swarts, 1991), with less than 5% wastage in 4-month-old rats, 30% in 9-month-old rats, and 65% at 11 months of age. Wastage is primarily due to preimplantation and early postimplantation mortality. In contrast to the decremental effects of aging on litter size, maternal behavior in virgin rats is enhanced at 19–20 months, when compared with those at 3–4 months of age (Gonzalez and Deis, 1990). In addition, at least some maternal stressors can lead to fetal wastage. An increase in fetal wastage was reported to be due to an earthquake that occurred when the dams were at 7, 8, or 9 days of gestation, although no difference was noted in the number of live births, fetal weight, or incidence of runts (Fujinaga et al., 1992). This raises the possibility of similar fetal loss when rats are shipped at this stage of gestation, although such has not been documented in the peer-reviewed literature. Strenuous maternal exercise, i.e., running on a treadmill—has also been reported to result in decreased litter size and decreased fetal weight (Mottola et al., 1992). Dystocia is rare in rats. Cannibalism is not frequently encountered and is an indicator of maternal stress.
5. Early Development of the Newborn
Rat pups are altricial and nidicolous; they are hairless and blind, with poorly developed limbs, short tails, and closed ear canals (Baker, 1979). There is an inverse relationship between fetal or birth weight and litter size (Romero et al., 1992). This phenomenon is significant for the reproductive toxicologist because the tendency of a test substance to cause decreased fetal weight may be masked if it also causes fetal loss. Other factors also influence birth weight and weaning weight, including the age of the dams. Pups of dams mated at 105 days of age weighed more at weaning than pups of dams mated at 35 or 70 days (Rosen et al., 1987). The external acoustic meati open between 2.5 and 3.5 days of age. Internally, the cochlea and organ of Corti are immature at birth but develop rapidly to approximately adult morphology by the time of weaning. Rats appear to first be able to hear at about 9 days of age, although they are able to vocalize from the time of birth (Feldman, 1992). Incisors erupt at 6–8 days of age, although molars do not erupt until 16 (molar 1), 18 (molar 2), and 32–34 days of age (molar 3) (Brown and Leininger, 1992). The retina is poorly developed at birth, equivalent to a human fetus of 4–5 months. The eyelids open at about 14–17 days of age, although the retina does not fully mature until 30–40 days of age, and the final components in the angle of the anterior chamber are not fully formed until 60 days of age (Weisse, 1992). Some hairs may be present on the trunk at birth, usually associated with touch domes, indicating that they are guard hairs (English and Munger, 1992). Pups are considered fully haired at about 7–10 days of age. Maternal antibody is transferred passively across the yolk sac in utero (Laliberte et al., 1984). Antibody can also be transferred across the intestinal mucosa from maternal colostrum and milk in the suckling rat. This transfer occurs at low rates shortly after birth, reaches maximal rates at day 14, and ceases by the 21 days, when gut closure is said to be complete (Martin et al., 1997).
6. Sexing
Sex is readily determined in mature rats by direct observation of the perineal region. Males have a distinct scrotum located between the anus and the preputial opening. The penis is often visible and is larger than the urethral papilla of the female. In addition the distance between the anus and the genital opening, called the anogenital distance, is greater in the male than in the female.
Sex discrimination is more difficult in prepubertal rats but is possible even in neonates. Comparative evaluation will reveal that neonatal males have a greater anogenital distance than their female littermates, although the distinction is more subtle than in adults. More recently, a technique for sex determination of preblastocyst embryos has been described (Utsumi et al., 1991). Male embryos ceased development in the presence of antibody to the HY antigen, and resumed development only after the antibody was washed off. In contrast, 80% of the embryos that developed into blastocysts in the presence of the HY antibody produced female pups after the blastocysts were implanted.
7. Weaning
Rats are weaned at 20–21 days of age, although they may be weaned successfully as early as 17 days. Prior to 17 days, the pups may not be fully capable of urination without maternal stimulation, and weaning may result in obstructive urinary tract disease.
8. Synchronization of Estrus
Synchronization of estrus in the rat can be accomplished by administration of 40 mg methoxyprogesterone in the drinking water for 6 days (in 200 ml ethanol/liter water, prepared fresh daily), followed by intramuscular injection with 1 IU of pregnant mare's serum (Baker, 1979). Although synchronization of estrus may be useful in the production of large numbers of timed pregnant rats, use of the impedance meter, as described above, may be more practical in most circumstances. More recently, synchronization of estrus to prepare recipients in embryo transfer has been reported. Mature females were administered 40 μg of luteinizing hormone releasing hormone agonist (Rouleau et al., 1993). Five days later estrus was confirmed by vaginal cytology.
9. Artificial Insemination and Embryo Transfer
Artificial insemination (AI) in rats is complicated by the rapid coagulation of semen, especially when the semen is obtained by electroejaculation, due to the contributions of the coagulating glands and seminal vesicles (Bennett and Vickery, 1970). These glands may be surgically removed without compromising fertility rates from AI. Alternatively, sperm may also be collected by stripping directly from the epididymis, although probably not more than twice from each male rat. Sperm from the proximal portion of the cauda epididymidis are reported to have greater fertility than sperm for the middle or caudal portions (Moore and Akhondi, 1996). Once collected, sperm may be surgically introduced directly into the uterus of estrous females (Orihuela et al., 1999). An essential step in assuring the success of AI is the induction of pseudopregnancy in the recipient female by prior mating with a vasectomized male, by mechanical stimulation of the vagina, or by electrical stimulation of the cervix (Bennett and Vickery, 1970; Rouleau et al., 1993).
Embryo transfer in rats is becoming more widely used as an alternative to cesarean rederivation in order to eliminate pathogens from breeding lines. Embryo transfer can also be used to investigate whether specific characteristics are due to, or modified by, the uterine environment, in contrast to being solely determined by genetic factors (Kubisch and Gomez-Sanchez, 1999; Rouleau et al., 1993). Additionally, embryo collection is the first step in cryopreservation. In embryo transfer, embryos are collected 2–4 days after the females are bred. Embryos are usually washed in phosphate-buffered saline (PBS) and bovine serum albumin (BSA), with or without added trypsin. Trypsin may more effectively remove pathogens than PBS alone. Embryos are then suspended in PBS with BSA and fetal calf serum and surgically transferred into the uterus or oviduct of the pseudopregnant recipient (Kubisch and Gomez-Sanchez, 1999; Rouleau et al., 1993). Nonsurgical implantation of embryos through the cervix, using an otoscope, has also been reported (Bennett and Vickery, 1970) but has not found wide use. In vitro fertilization (IVF) is performed in the rat but is used primarily as a research tool for events in fertilization and early development rather than as a colony management tool (Gaddum-Rosse et al., 1984; Vanderhyden et al., 1986). One form of IVF, the microinjection of spermatids into individual oocytes, is, however, used in mice to rescue or maintain strains that do not produce motile spermatozoa (Tanemura et al., 1997; Songsasen and Leibo, 1998). The same techniques will probably become more common in rats.
10. Cryopreservation
Cryopreservation has not been performed in rats as often as it has in mice, but the technique is becoming more widespread, for the same reasons that it is used widely in mice (Tada et al., 1995). Cryopreservation can be an efficient method of maintaining the potential of raising live mice of the thousands of genetically modified genotypes currently available (Songsasen and Leibo, 1998). It can serve as a fail-safe measure, should a strain become genetically contaminated. In addition to being used for murine reproductive purposes, frozen embryos are also used to test culture reagents and environments for human IVF (Meyer et al., 1997). Although embryos, two-cell through morula, are most frequently cryopreserved, the techniques for cryopreservation of mouse sperm have recently been developed (Songsasen and Leibo, 1998; Tanemura et al., 1997). Cryopreservation of sperm has not yet been reported for rats.
E. Behavior
Relatively little space in many laboratory animal medicine texts, including the previous edition of this volume, has been devoted to the behavior of laboratory rats, especially as it relates to experimental design or disease status. Although this unfortunate, perhaps unavoidable, lack may lead some readers to conclude that rat behavior is not an important aspect of laboratory animal science, quite the contrary is actually true. Many aspects of the rat's normal behavior may affect scientific use of rats in biomedical research and should be investigated by researchers prior to initiating studies in those specific areas. Aspects of rat behavior relevant to experimental design and disease status may be considered in two broad and overlapping categories: normal behavior, and stressors and stress responses. Only a few examples will be cited here.
Laboratory rats of all stocks and strains have been selected for many years for a variety of traits, among which is docility. Nonetheless, strain differences exist. Sprague-Dawley rats, such as the CD and SD, and Lewis rats are generally more docile than Brown Norway or F-344 rats. Frequent gentle handling will increase docility, whereas infrequent or rough handling will evoke fear responses. Gentle handling not only reduces the likelihood of occupational injury for animal workers but also avoids stress for the rats. Handling-induced stress can lead to altered responses in behavioral studies (Hirsjarvi and Valiaho, 1995; Shalev et al., 1998). Handling also leads to vocalization, much of which is ultrasonic, in the range of 22 kHz (Brudzynski et al., 1993; Brudzynski and Chiu, 1995; Brudzynski and Ociepa, 1992). Stress-induced vocalization can make handling more difficult for other rats within hearing range. An additional interesting fact regarding rat vocalization, illustrative of its importance in rat behavior, is that rat pups vocalize in the ultrasonic range, probably to signal their mothers, even before their ears are sufficiently developed for them to be capable of hearing (Feldman, 1992).
Rats are most active at night but will also move and feed some during the day; they are also more active in the mornings than in afternoons (Saibaba et al., 1996). This circadian rhythm is relevant to a broad range of behavioral measurements. For example, pain threshold is often determined in a tail flick test. Female rats have shorter tail flick response times in the middle of the dark period, as well as during estrus and metestrus (Martinez-Gomez et al., 1994).
Rats, as are other rodents, are coprophagic and vary considerably between individuals in the percentage of feces consumed. This may be of significance when measuring fecal output volume or intestinal absorption of some agents. However, it appears to have no effect on iron absorption (Tidehag et al., 1988).
Rats may be housed singly or in groups. In general, males are less likely to fight when housed together than are male mice, but they also do well when housed singly, as is the norm in many toxicology and safety assessment studies. Temporary single housing of female Wistar: Han rats accustomed to group housing resulted in elevated glucose levels, although the same was not observed in males (Perez et al., 1997). It is not clear if the change in glucose levels was due to stress of being alone, was just a generic response to any change in environment, or was a result of the higher food consumption recorded in the singly housed females. Also, given a report that transportation stress reduced blood glucose in Wistar:WU rats (Van Ruiven et al., 1988), it is not clear whether the higher or lower blood glucose levels are more “normal.”
When afforded the choice, rats have shown preferences for solid flooring, bedding consisting of large particles of aspen wood chips, and nest boxes (Manser et al., 1995a,b; Blom et al., 1995; Manser et al., 1998a,b), although the consequences of being deprived of the preferred housing factors have not been reported. When provided with objects as part of an environmental enrichment program, rats will chew on inanimate objects such as wooden blocks and nylon bones and balls (Watson, 1993; Chmiel and Noonan, 1996). No deleterious effects of these objects have been found; neither have benefits been measured.
III. DISEASES
A. Infectious Diseases
1. Bacterial, Mycoplasmal, and Rickettsial Infections
a. Streptococcosis
Streptococcosis is disease caused by infection with Streptococcus spp: Several species of Streptococcus are opportunistic pathogens in rats (i.e., they can cause clinical disease under at least some circumstances). Streptococcus pneumoniae, which is α-hemolytic, is the Streptococcus species of most historic concern in the rat, although various members of the β-hemolytic group also occasionally cause opportunistic infection. In addition, Enterococcus spp., which are not truly streptococci, are often considered together with Streptococcus spp.
Pneumonia caused by S. pneumoniae has historically been referred to as streptococcosis. However, because the term streptococcosis could be used to describe any streptococcal infection, it is inherently nonspecific and should be avoided. Streptococcus pneumoniae is rarely present today in commercially obtained rats and is now considered to be a pathogen of low significance in laboratory animals (National Research Council, 1991). Humans are the natural host of S. pneumoniae, with both adults and children frequently colonized. Streptococcus pneumoniae is transmitted primarily via aerosol, although fomites may play a minor role. Disease due to S. pneumoniae has been infrequently reported in rats, but infection is usually asymptomatic. In asymptomatic rats the organism colonizes the nasopharynx. Numerous serotypes of S. pneumoniae exist; disease is predominantly associated with infection by more pathogenic serotypes, especially 2, 3, 8, 16, and 19 (Fallon et al., 1988).
Infection in rats resembles that in both human and nonhuman primates, characterized by suppurative inflammation in the upper respiratory tract, which spreads to the lung to cause bronchopneumonia (Kohn and Barthold, 1984) and sometimes fibrinosuppurative pleuritis. Affected rats may become bacteremic and may develop fibrinopurulent inflammation of other serous surfaces (e.g., peritoneum, synovium) and other tissues.
Monitoring for S. pneumoniae infection is conducted by nasopharyngeal culture onto blood agar. Differentiation of S. pneumoniae from other α-hemolytic streptococci is most often performed by the optochin inhibition test. Optochin inhibition is greater for most S. pneumoniae strains than for other α-hemolytic streptococci. However, because of the occurrence of nonpathogenic isolates (Fallon et al., 1988), isolation of S. pneumoniae from rats, even if a respiratory problem is present in the colony, does not necessarily provide a diagnosis, nor does isolation of S. pneumoniae from asymptomatic rats necessarily indicate a colony health threat. Action to eliminate S. pneumoniae is indicated in the presence of characteristic lesions or detection of known pathogenic serotypes.
β-Hemolytic streptococci are also present in many rats but rarely cause disease. β-Hemolytic streptococci are divided into groups based on Lancefield antigens, with Lancefield groups B and G most commonly isolated from rats. Infrequently, they may be isolated from abscesses, but exclusion from most colonies is neither necessary nor practical, for humans are often carriers.
So-called streptococcal enteropathy is actually due to nonhemolytic (γ-hemolytic) Lancefield group D enterococci, including Enterococcus hirae, E. faecium-durans 2, and E. faecalis 2 (Barthold, 1997).
Streptococcal enteropathy is a disease that affects only suckling rats, not postweaning animals. Affected litters develop diarrhea or soft stool, with bright yellow pasty feces. Mortality can be high. Microscopically, the villi of the small intestine are carpeted with gram-positive cocci. Disease is clearly associated with some strains of enterococci and not with others, but the factors determining the pathogenic potential have not been elucidated. They may, however, involve the ability of pathogenic isolates to adhere to the surface of the microvilli.
Control of Streptococcus spp. and Enterococcus spp. is problematic, because the organisms are virtually ubiquitous, including being present in a high percentage of the human population (Del Vecchio, 1992). Some Enterococcus spp. have even been considered autochthonous flora of the rat (Savage, 1971). Streptococci can be excluded by aseptic microisolator technique or by use of isolators (Pleasants, 1974), yet the low incidence of disease may not warrant the additional time, expense, or other resources that such housing techniques would require.
b. Pseudotuberculosis
Pseudotuberculosis is caused by Corynebacterium kutscheri, which can infect rats, mice, guinea pigs, and hamsters, although in the last two there is only bacteriological evidence. Infections with C. kutscheri are usually clinically silent (Suzuki et al., 1988; Amao et al., 1995). Nonspecific clinical signs may be observed, such as ruffled fur, hunched posture, dyspnea and rales, porphyria, mucopurulent ocular and nasal discharges, lethargy, and lameness. These are usually followed by death in 1 to 7 days.
In infected colonies, C. kutscheri will typically cause latent infections and may be cultured from submaxillary (cervical) lymph nodes, oropharynx and nasopharynx, middle ears, and preputial glands. Latent infections may be triggered to become clinical by a variety of stressors that can cause immunosuppression in the host. These include poor husbandry, overcrowding, shipping, malnutrition, intercurrent infections, irradiation, and treatment with immunosuppressive drugs (Barthold and Brownstein, 1988). As with other persistent infections, such as mycoplasmosis, disease is more frequent in older animals.
Transmission is probably through direct contact or oronasal exposure. Lesions are due to septic emboli becoming trapped in organs or tissues with an extensive capillary network, such as lung, liver, kidney, and synovium. Although any organs and tissues may be involved, the lung is the organ most frequently involved in the rat.
Gross lesions of C. kutscheri infection consist of solitary or multiple randomly distributed abscesses in the lung, liver, kidney, skin, and joints. Suppurative inflammation may also be found in the preputial gland and tympanic bullae.
Histopathologically, the lesions are generally as expected from the gross findings. Interstitial inflammation in the lung is due to the hematogenous seeding of the lung with bacteria, although bronchi and bronchioles may also contain suppurative exudate. Caseous necrosis is often prominent, and epithelioid macrophages and multinucleated giant cells may be present in areas of abscessation. Large areas of caseous necrosis may also be present in the liver. Septic embolic glomerulitis may be present in the kidneys, as may abscesses with or without pyelonephritis. Abscesses and caseous necrosis may also be observed in virtually any tissue.
Definitive diagnosis is accomplished by bacteriologic culture (Fox et al., 1987). The best site, other than lesions, to culture is probably the submandibular (cervical) lymph nodes. The oral cavity, cecum, colon, and rectum may also harbor the organism. Microscopic evaluation may reveal the characteristic irregularly branching arrays of gram-positive rods in tissue sections (Brown and Brenn stain) or impression smears (Gram stain). However, if possible, histopathology should always be confirmed by bacteriology. Disease provocation tests, often called stress tests, have also been employed to activate latent infections with C. kutscheri, as with Pneumocystis carinii and Clostridium piliforme. Adequate culture techniques should obviate the need for stress tests. Serology has also been widely employed for detection of C. kutscheri infection in immunocompetent rats (Fox et al., 1987). As with other serologic assays, especially serologic assays for agents more antigenically complex than viruses, false positives and false negatives occasionally occur, so positive results should always be confirmed by culture.
Differential diagnosis for the presence of multiple abscesses in rats should include streptococcosis, streptobacillosis, mycoplasmosis (pulmonary abscesses), CAR bacillus infection (pulmonary abscesses), or other miscellaneous bacteria. Of these, only mycoplasmosis and CAR bacillus infection would be found predominantly in older animals.
c. Tyzzer's Disease
Tyzzer's disease, first discovered by Tyzzer in Japanese Waltzing mice (Tyzzer, 1917), is caused by Clostridium piliforme (Duncan et al., 1993), formerly known as Bacillus piliformis. The host range is protean among mammals, including numerous rodent species, rabbits, carnivores, horses, and both nonhuman and human primates (DeLong and Manning, 1994; Skelton et al., 1995).
Clostridium piliforme infection is usually clinically silent (Motzel and Riley, 1992; Hansen et al., 1992a). Overt disease in rats, as in other species, is most likely to be observed in young, recently weaned animals. In these, the clinical signs are nonspecific (anorexia, lethargy, emaciation, ruffled fur) and may include acute death without clinical signs. Diarrhea may be noted and may contain mucus and blood. Particularly in the rat, a distended abdomen has been observed in weanlings with Tyzzer's disease, albeit at a very low incidence (Hansen et al., 1992b).
Clostridium piliforme is transmitted horizontally in rats by spores through fecal–oral contamination. The spores are highly resistant to desiccation and some disinfectants (Ganaway, 1980). The delicate vegetative form, however, survives only inside of cells.
After being ingested, C. piliforme spores produce a vegetative form, which is actively phagocytosed by mucosal epithelial cells covering the gut-associated lymphoid tissue, or Peyer's patches (Tyzzer, 1917; Duncan et al., 1993). Multiple, pale foci, pinpoint or larger, of necrosis are often visible on the surface of and within the liver. Megaloileitis—a greatly dilated, flaccid, and hyperemic ileum—may be present (Fig. 4 ). Hyperemia, edema, hemorrhage, and ulceration may affect any part of the intestine, especially the terminal ileum, cecum, and colon. Secondary to intestinal involvement, mesenteric lymph nodes may be enlarged, hyperemic, and edematous. In the heart, pale circumscribed areas may be visible on the epicardium. Myocardial necrosis due to Tyzzer's disease may also appear as pale linear streaks or areas in the heart, especially near the apex.
Fig. 4.
Tyzzer's disease in an adolescent rat with the skin reflected. Note enlargement of ileal loops in situ.
(Courtesy of Dr. Steven Weisbroth.)
Histopathologically, characteristic lesions may be observed in the liver, ileum, cecum, and colon, and, less frequently, the heart. In the intestinal tract, there may be necrotizing enteritis, typhlitis, and colitis. Coagulative necrosis in the liver is the hallmark lesion and is often accompanied by a moderate leukocytic infiltrate, usually neutrophils and mononuclear cells, at the periphery of the lesions. Acute lesions may be hemorrhagic, and mineralization may occur with time. In the heart, myocardial degeneration and necrosis occurs in a minority of cases, often with a mixed leukocytic infiltrate and dystrophic calcification.
Histopathologic evaluation is diagnostic if the characteristic bacilli are observed (Tyzzer, 1917; Duncan et al., 1993). The vegetative form of the organism is a filamentous bacillus, 8–20 μm long and 0.3–0.5 μm wide (Fig. 5 ). Bacilli are intracellular, are often numerous, and may appear as either a jumbled array (pickup stick) or parallel arrangement, as dictated by the shape of the cell. The vegetative form may rarely be visible in hepatocytes in tissue sections stained with hematoxylin and eosin, but usually special stains are necessary, including Warthin–Starry silver (best), Giemsa, and methylene blue stains. Although gram-negative, C. piliforme stains very poorly with gram stains. In the liver, the organisms are most often observed in surviving hepatocytes at the periphery or within lesions. In the intestine, normal gut flora within mucosal crypts and superimposed upon the mucosal epithelial cells may complicate evaluation. Organisms may also occasionally be observed in cardiac myocytes or myocytes of the tunica muscularis of the intestine.
Fig. 5.
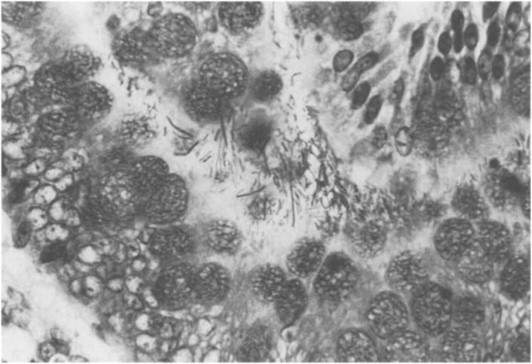
Tyzzer's disease in rat ileum. Clostridium piliforme in villous submucosa at base of crypt. Giemsa stain. Magnification: X800.
(Courtesy of Dr. Steven Weisbroth.)
Differential diagnoses for necrotizing hepatitis in the rat should include other bacterial septicemias, such as Corynebacterium kutscheri, as well as infection with rat virus. Diagnosis of clinical disease depends on demonstration of the organism in tissue. Tissue smears may facilitate rapid diagnosis; Giemsa-stained smears of suspicious liver lesions are especially useful (Percy and Barthold, 1993a).
Colony screening for latent infection is problematic. Serologic screening is rapid and technically simple (Motzel and Riley, 1991) but is subject to false positives, yielding results that can be difficult to put into context. Disease provocation tests, or stress tests, to exacerbate latent infections are widely used and are recommended as a follow-up test when serologic positive results are obtained. However, there is some doubt as to efficacy of stress tests that rely on chemical immunosuppression, usually with cyclophosphamide (Boivin et al., 1990), followed by histopathologic evaluation. The doubt arises because test animals may have already cleared the C. piliforme infection and therefore may no longer be susceptible to activation of “latent” infection. Alternatively, sentinel animals can be placed on soiled bedding, but this may require sentinels to be of the same species (to avoid species specificity's causing false negatives), for not even gerbils are susceptible to all strains of C. piliforme (Motzel and Riley, 1992; Franklin et al., 1994).
Interference of Clostridium piliforme with research has primarily been attributed to the morbidity and mortality, although effects on coagulation and leukokines have also been reported (Van Andel et al., 1996).
d. Pasteurellosis
Pasteurella pneumotropica is a gram-negative coccobacillus. It grows aerobically on sheep blood agar without producing hemolysis, but producing smooth, gray translucent colonies (Carter, 1984). It has been isolated from numerous mammalian species, including humans, and is generally considered to be of low significance in immunocompetent rats (National Research Council, 1991d).
Pasteurella pneumotropica has a high prevalence in infected colonies and is most often isolated from the nasopharynx, cecum, vagina, uterus, and conjunctiva during routine monitoring (National Research Council, 1991). The vast majority of animals are asymptomatic, with only rare instances of conjunctivitis, metritis, and mastitis (Percy and Barthold, 1993b). Histologically, lesions are characterized by necrotizing, suppurative inflammation.
Control of the agent may not be necessary in immunocompetent animals, because of the rarity of P. pneumotropica–induced disease. However, treatment with enrofloxacin has been described (Goelz et al., 1997). Rederivation by either cesarean section or embryo transfer will also eliminate the agent (SultanDosa et al., 1983). Antibiotic treatment of infected dams prior to cesarean section has been recommended by at least one major rodent vendor (C. Clifford, unpublished observations, 1998), because P. pneumotropica can be present in the uterus. The probability of successful elimination of P. pneumotropica by cesarean section can be further increased by culturing all uteri after the pups are removed and eliminating any offspring from a culture-positive uterus. Offspring should also be held in strict isolation—i.e., not mixed in with a breeding colony—until repeatedly cultured negative for P. pneumotropica. Pasteurella pneumotropica is not transmitted to a significant degree by fomites, does not persist or multiply in the environment, and only rarely colonizes humans. Therefore, once a colony is free of the agent, there is relatively little risk of reinfection except through introduction or incursion of infected animals.
e. Salmonellosis
Salmonellosis is the disease caused by bacteria of the genus Salmonella. The taxonomic classification and subdivision of the genus are controversial and, like much taxonomy, subject to change (see Chapter 3). However, for clarity in communication, it is useful to refer to all salmonellas that one is likely to encounter in rats as belonging to S. enteritidis (Percy and Barthold, 1993a; Le Minor, 1984). Salmonella enteritidis is composed of more than 2400 serovars. These vary greatly in pathogenicity and geographic distribution, which makes serovar classification of epizootiologic significance. However, this discussion will treat S. enteritidis as a single entity. Salmonellosis may be virtually nonexistent in laboratory rats in the United States, but because infection is thought to be prevalent among many other species of vertebrates, including wild rodents, the potential for introduction remains.
In rats, as in most species, clinical signs of infection with S. enteritidis are rare but may include a hunched posture, ruffled fur, lethargy, weight loss, and conjunctivitis. Soft stools and diarrhea may also be observed, usually in less than 20% of animals.
Salmonella enteritidis is transmitted by ingestion of contaminated materials, including feed, bedding, or water. Incursion of wild or feral rodents into a laboratory facility poses a further risk. In addition, salmonellosis is an anthropozoonosis (Wray, 1994); humans not only are at risk of infection from rodents but also may serve as a source of the agent.
In rats with subclinical infections, gross and microscopic lesions will usually be absent. Rats with clinical disease may have evidence of gastrointestinal involvement and septicemia, including mural thickening and mucosal ulcers in the cecum and ileum, as well as splenomegaly. Microscopically, enteric lesions are characterized by edema of the lamina propria, leukocyte infiltration in areas of ulceration, and reactive hyperplasia of crypt epithelial cells. Lymphoid hyperplasia, with focal necrosis and neutrophil infiltration, may be observed in Peyer's patches, as well as in the spleen and mesenteric lymph nodes. Septicemic rats will have necrosis in the spleen and liver, with emboli composed of fibrin, bacteria, and debris present in liver, spleen, and lymph nodes (Percy and Barthold, 1993b).
Salmonellosis is most often diagnosed by culture of feces, mesenteric lymph nodes, liver, spleen, or blood. Material is placed in enrichment broth and then inoculated onto selective growth medium. Although symptomatic animals should be culture-positive, an infected colony may have only a low incidence of asymptomatic carriers, perhaps less than 5%. Detection of S. enteritidis in these colonies may require repeated testing of significant numbers of samples. Using a probability formula (National Research Council, 1976), 58 animals would have to be tested to provide a 95% confidence of finding at least 1 positive if the true prevalence of positive samples was 5%. Differential diagnoses for diarrheal disease in rats include Tyzzer's disease, rotavirus infection, enterococcal enteropathy, cryptosporidiosis, and problems with feed and/or water.
Salmonellosis is prevented by rigorous pest control and by ensuring that food and bedding are not contaminated. Good personal hygiene of employees will prevent them from serving as a source of Salmonella or other enteric pathogens to the colony.
Once S. enteritidis is detected in a colony, all animals are usually destroyed, and all surfaces and materials either sterilized or safely discarded. Strict quarantine of a small group of animals may be practical in some situations, prior to rederivation by embryo transfer or cesarian section. This may be most feasible in a flexible film or semirigid isolator. Treatment is not recommended, because a chronic carrier state may result and there is the potential for zoonotic disease.
Rats infected with S. enteritidis should not be used in research, because of the zoonotic potential and the risk they animals pose to other animals. Research complications from salmonellosis have primarily been reported in mice (National Research Council, 1991c).
f. Pseudomoniasis
Pseudomoniasis refers to clinical disease caused by Pseudomonas aeruginosa, a gram-negative bacillus of the order Eubacteriales, family Pseudomonadaceae. Pseudomonas aeruginosa is motile, aerobic, oxidase-positive, and widely distributed in water, soil, sewage, and the skin and gastrointestinal tract of many animals. It is considered as part of the common commensal flora of humans, domestic animals, and laboratory rodents and is more frequently isolated from animals and humans receiving antibiotics (Kiska and Gilligan, 1999).
Despite its near ubiquity, P. aeruginosa is rarely implicated in disease except in mammals with specific and severe host defense deficits, particularly hosts or tissues deficient in functional phagocytes (i.e., macrophages and neutrophils, and their serum opsonins). Thus, athymic nude mice are not subject to a high incidence of pseudomoniasis unless irradiated or treated with myelosuppressive agents. In general, pseudomoniasis is considered to be of low significance in rats (National Research Council, 1991a) but should be suspected when rats that are irradiated or treated with radiomimetic agents die earlier than expected (Percy and Barthold, 1993b).
In particular, pseudomoniasis has been reported as a consequence of infection of indwelling jugular catheters (Wyand and Jonas, 1967). Signs were those of septicemia. Necropsy findings included vegetative valvular endocarditis and multifocal hemorrhagic pneumonia. Histologically, fibrin emboli, leukocytes, and gram-negative bacteria were observed in the heart, lung, and occasionally other organs.
Pseudomoniasis is diagnosed by cultural identification of the organism. However, caution should be exercised in attributing observed morbidity and mortality to an organism as nearly ubiquitous as P. aeruginosa.
Although Pseudomonas will grow on blood agar, isolation is enhanced by the use of selective media, such as Pseudomonas isolation agar, or Pseudomonas P agar. The use of selective media is particularly recommended when screening clinically healthy animals, because only low numbers of organisms may be harbored in the common sites for isolation, the cecum, and nasopharynx.
Exclusion of P. aeruginosa is rarely justified in a research setting (National Research Council, 1991c). Where appropriate, exclusion requires gnotobiotic methods and sterilization of all water reaching the animals, as well as sterilization of cages, feed, and bedding. Animals must be maintained in isolators or microisolators and must be routinely monitored. All possible sources of contamination from human skin or any wet surface must be strictly prohibited.
Control of P. aeruginosa infection often begins with the watering system. Pseudomonas aeruginosa is one of a group of organisms that forms biofilms, layers of bacteria, usually with reduced metabolic activity, embedded in a dense glycocalyx. Bacteria in a biofilm are extraordinarily resistant to chlorine (150–3000 times more resistant than free-floating bacteria) and monochloramine (2- to 100-fold) (LeChevallier et al., 1988) and may be inaccessible to antibiotics. Nonetheless, chlorination (10–13 ppm) or acidification (pH 2.5–3.0) can significantly reduce the colonization of mice with P. aeruginosa but will not eliminate infection. Rederivation by cesarian section or embryo transfer is required to eliminate P. aeruginosa from an infected colony. Treatment with gentamycin in the animal drinking water, at 1 gm liter, has been reported to eliminate the infection in mice but is probably not practical for large groups of rats (Urano et al., 1977).
g. Streptobacillosis
One cause of rat-bite fever, Streptobacillus moniliformis is primarily of historic interest. This zoonotic agent is virtually nonexistent in modern laboratory animals but nonetheless bears brief mention because of the potentially serious consequences of infection (Anderson et al., 1983; Wullenweber, 1995). The agent is a gram-negative pleomorphic bacillus, which will grow nonhemolytically on sheep blood agar, although trypticase soy agar enriched with 20% horse serum is preferred (Weisbroth, 1982; Savage, 1984).
Streptobacillus moniliformis is commensal in wild rats, inhabiting the nasopharynx, middle ear, and respiratory tract. It is present in blood and urine of infected rats and is transmitted to humans by bite wounds, aerosols, and fomites (Will, 1994). The organism is nonpathogenic in rats. Clinical signs in humans follow a 3- to 10-day incubation period and include fever, vomiting, arthralgia, and rash. Disease is treated with antibiotics, and mortality is low.
Colonies of laboratory rats are monitored by culture of blood and nasopharyngeal swabs for Streptobacillus moniliformis, and any colony in which the organism is confirmed should immediately be terminated. Because wild rats are the reservoir for S. moniliformis, its detection in a laboratory rat colony would indicate exposure to infected wild rats.
h. Helicobacteriosis
Several Helicobacter spp. have been found as natural infections of rats in the last few years. Helicobacter muridarum, one of the first helicobacters identified in rodents, was first reported in 1992 (Lee et al., 1992). More recently, H. trogontum has been identified as a naturally occurring intestinal helicobacter (Mendes et al., 1996), and H. bilis has been reported from the large bowel of immunodeficient rats (Haines et al., 1998). Organisms with the ultrastructural morphologic appearance of “H. heilmannii” (H. bizzozeroni) have also been found in the stomach of wild rats (Giusti et al., 1998).
All currently identified helicobacters of laboratory rodents are microaerophilic, gram-negative flagellated bacteria that may be spiral, slightly curved, or straight. Coccoid forms have also been described for H. bilis (and H. pylori) (Fox et al., 1995).
Although the pathogenic potential of H. pylori in human gastritis and ulcers is widely accepted, less work has been completed to confirm suspicions of similar pathogenicity of rodent helicobacters. Koch's postulates have been fulfilled for H. hepaticus as a causative agent of enterocolitis and hepatitis in mice (Fox et al., 1996b), but no experimental reproduction of disease by natural routes of infection has been performed for Helicobacter spp. in rats. Lesions reported in athymic nude rats infected with H. bilis are similar to those reported in immunodeficient mice inoculated with H. bilis or H. hepaticus and include proliferative and ulcerative typhlitis, colitis, and proctitis (Haines et al., 1998), although no causal role was confirmed. No lesions have been reported in immunocompetent laboratory rats from any naturally occurring Helicobacter species.
No studies have been published on the transmission of naturally occurring Helicobacter infections in rats. In mice, horizontal transmission by soiled bedding, probably fecal-oral transmission, has been demonstrated (Livingston et al., 1998). Fecal–oral transmission is also probable in rats.
The host range of rat Helicobacter species is not fully elucidated. Clearly, H. bilis has been found in rats and mice, and there is also an additional report of it in a dog (Eaton et al., 1996). Helicobacter muridarum has been reported in rats and mice. No host range has been reported for H. trogontum. Many other Helicobacter species, however, are able to colonize a phylogenetically wide range of mammalian hosts.
The only lesions in rats reported due to natural Helicobacter infection are in a small series of 11 male athymic nude rats, 5–8 months of age, infected with H. bilis (Haines et al., 1998). In these rats, gross lesions consisted of focal or diffuse thickening of the cecal wall, with normal-appearing colon and rectum. Cystic mesenteric lymph nodes were also noted in 8 of the 11 rats. Histologically, all 11 rats had proliferative typhlitis, with 8 of the animals also having similar lesions in the colon and rectum. Crypt epithelium was hyperplastic, with cytoplasmic basophilia, increased mitoses, and fewer goblet cells than normally observed. The lamina propria was infiltrated by lymphocytes, plasma cells, and a few eosinophils. Mucosal erosion and ulceration were observed in the cecum of the most severely affected rats. The authors experimentally reproduced many aspects of the disease by intraperitoneal injection of approximately 5 × 108 H. bilis bacteria in phosphate-buffered saline.
Once established, infection by any Helicobacter species is typically lifelong. Infection, or colonization, should be distinguished from disease. Helicobacter muridarum and H. trogontum may be nonpathogenic in rats, although H. muridarum has been reported to cause lymphocytic gastritis in aged mice, possibly associated with a loss of parietal cell mass leading to increased gastric pH. Key pathogenic factors for H. pylori include urease, a vacuolating cytotoxin (vacA), and the presence of a pathogenicity island. All three Helicobacter spp. currently identified in rats—H. bilis, H. trogontum, and H. muridarum—are urease-positive (Fox and Lee, 1997). Other virulence factors have not been reported.
Helicobacter infection should be a prime differential diagnosis when proliferative lesions of the large bowel are observed in rats. Spontaneous chronic ulcerative colitis has also been reported in athymic nude rats (Thomas and Pass, 1997). The authors were unable to culture Helicobacter spp. from affected animals, although no molecular techniques were employed.
Diagnosis of Helicobacter infection in laboratory rats is best accomplished by polymerase chain reaction (PCR) (Riley et al., 1996; Fox and Lee, 1997). A common approach is to use genus-specific primers capable of detecting any Helicobacter spp., as well as primers specific for a single species, such as H. bilis, or to follow the initial amplification with restriction endonuclease digestion and gel separation to identify digestion product banding patterns characteristic of various species. Samples are most often fecal pellets, although cecal mucosal scrapings or tissue may also be used.
Helicobacter spp may also be cultured from a variety of sources. Culture from contents of the large intestine is greatly complicated, however, by the rich flora of the site. Fox et al. (1997) recommend passage of cecal contents through a 0.65-μm filter, then culture on Brucella agar with antibiotics (trimethoprim, vancomycin, polymyxin) to suppress growth of unwanted organisms that are not removed by the filter (Fox and Lee, 1997).
In general, it is easy to exclude from animal colonies those rodent-specific organisms that do not persist or multiply in the environment. However, given the uncertainty as to the full host range of rat helicobacters, the possibility of transmission by humans is difficult to exclude. The usual source of infection for Helicobacter infection in rats is nonetheless expected to be contaminated rodents or other laboratory animals. No indication of transmission by feed, bedding, water, or aerosols has been reported.
Once a colony is infected, treatment of small groups of animals may be possible. Several antibiotic regimens have been reported to be successful for mice and might similarly be attempted in rats. These have primarily involved oral dosing by gavage several times each day or incorporation of antibiotics into the diet. A triple therapy combination of amoxicillin or tetracycline, with metronidazole and bismuth, administered by oral gavage 3 times each day for 2 weeks has been the most effective (Orcutt, 1980). Elimination of infection from large groups of rats would be less likely to be 100% effective, even if practical obstacles of dosing could be overcome, because even a single rat retaining any viable Helicobacter could lead to reinfection of the entire colony.
Research complications due to infection by Helicobacter spp. in rats have not been reported.
i. Cilia-Associated Respiratory Bacillus
Usually referred to as CAR bacillus, the cilia-associated respiratory bacillus is not taxonomically classified in the genus Bacillus. Rather, it has recently been tentatively placed in a group of bacteria known as “gliding bacteria,” based on the fact that they are motile but without visible means for such motility, and may be related to Flavobacterium or Flexispira, based on 16S rRNA sequencing (Cundiff et al., 1995a). Final identification, however, is still pending.
CAR bacillus has been identified in rats, mice, and rabbits among common laboratory animals (Van Zwieten et al., 1980; MacKenzie et al., 1981; Waggie et al., 1987; Griffith et al., 1988). In rats, infection is usually asymptomatic, although nonspecific clinical signs such as weight loss and dyspnea may occur.
Transmission is primarily via direct contact with infected animals. Fomites probably do not play a significant role in natural transmission of CAR bacillus, and bedding does not transmit the infection well (Matsushita et al., 1989). Airborne exposure is not an important means of transmission (Itoh et al., 1987).
CAR bacillus infection may not always present gross lesions, although translucent gray cystic lesions, representing dilated, mucus-filled airways may be visible on the pleural surface (Itoh et al., 1987). Coinfection with Mycoplasma pulmonis or other pathogens may occur, resulting in suppurative bronchio-pneumonia.
Histopathologically, hyperplastic peribronchial and peribronchiolar mononuclear cell cuffs are observed in the lungs (Itoh et al., 1987; Matsushita and Joshima, 1989). A thin basophilic layer may be observed on the surface of the airway epithelium in hematoxylin and eosin–stained sections, giving the impression that the cilia are more basophilic than normal, but this is not specific and should not be used as a definitive diagnostic feature. With Warthin–Starry or methenamine silver stain, filamentous bacilli are readily observed among cilia of respiratory epithelium from the nasal cavity to the bronchioles (Fig. 6 ). The upper respiratory tract is involved earlier in the course of infection than the lower tract and should be included in histologic examinations for CAR bacillus infection.
Fig. 6.
Warthin–Starry silver stain of rat bronchus. Innumerable filamentous bacteria are densely clustered at the ciliated surface of the columnar epithelium. Note the polymorphonuclear cell exudate in the bronchial lumen. Magnification: X100.
CAR bacillus infection should be distinguished from murine respiratory mycoplasmosis, pneumonia due to other bacteria (i.e., Streptococcus pneumoniae, Corynebacterium kutscheri, etc.), and viruses. Detection of CAR bacillus infection should also raise the suspicion of coinfection with other pathogens (Van Zwieten et al., 1980; MacKenzie et al., 1981) (Fig. 7 ).
Fig. 7.
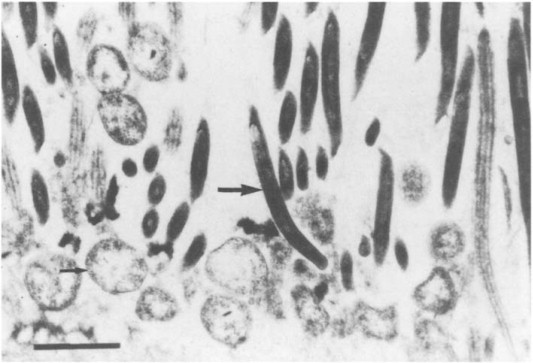
Electron micrograph showing filamentous CAR bacillus organisms (large arrow) at the surface of a ciliated bronchial epithelial cell. Note coinfection with Mycoplasma pulmonis (small arrow). Bar: 1 μm.
Colonies are best screened for CAR bacillus infection by serologic techniques, such as enzyme-linked immunosorbent assay (ELISA) or immunofluorescence assay (IFA) (Matsushita et al., 1987; Shoji et al., 1988; Lukas et al., 1987). Because false-positive reactions can occur (Hook et al., 1998), any positive results should be confirmed by a Steiner stain of tracheal mucosal scraping or histopathology with use of special stains, or by PCR. Interestingly, because infection is not readily transmitted by soiled bedding (Cundiff et al., 1995b), many sentinel programs may fail to detect CAR bacillus infection. Infection is lifelong, and the organisms are readily retrievable by tracheal lavage or scraping (Medina et al., 1998). Therefore, CAR bacillus may be readily detected by PCR (Cundiff et al., 1994), which may serve as an important confirmatory test to follow positive serologic results. PCR may be positive prior to serologic conversion, and samples for PCR may be collected as nasal swabs as a nonterminal procedure (Franklin et al., 1999).
CAR bacillus infection is prevented by exclusion of infected animals. No effective treatment has been described. As an alternative to elimination and rederivation of entire infected colonies, the requirement for direct contact for transmission may possibly be exploited to advantage. If individual animals or cages are monitored by serology, and then negative individuals are monitored by PCR, all rats that are positive (or all cages that have a positive rat) by either test may be eliminated or quarantined. Because the infection is not transmitted well by aerosol or fomites, it may be possible to control the spread of infection. However, the expense, the labor, and the consequences of possible failure would have to be weighed against the value of possibly saving some of the rats.
The interference of CAR bacillus with research is unknown. Interference with ciliary function has been suspected but not measured. Effects of CAR bacillus on other respiratory functions and on the immune response have also been postulated but not documented in the scientific literature.
j. Mycoplasmosis
Murine respiratory mycoplasmosis (MRM), also known as chronic respiratory disease, is caused by Mycoplasma pulmonis (Kohn and Kirk, 1969; Lindsey et al., 1971). Clinical signs are usually observed only in older animals; M. pulmonis infection is clinically silent in young animals. Clinical signs are nonspecific, referable to the respiratory and auditory involvement, and include rales and dyspnea, snuffling and chattering, and ocular and nasal discharges, as well as chromodacryorrhea, rubbing of eyes, and head tilt. Rats with severe middle ear involvement may spin when held up by the tail. Decreased reproductive efficiency has also been reported in rats (Leader et al., 1970).
Mycoplasma pulmonis is transmitted horizontally by direct contact and aerosol and vertically by in utero transmission (Lindsey et al., 1982). Venereal transmission may also be possible. The disease outcome depends on a complex interaction of factors relating to host, pathogen, and environment (Lindsey et al., 1985). Host factors include age, strain (Davis and Cassell, 1982), immune status and lymphoreticular function, and the presence of intercurrent infections such as Sendai virus (Schoeb et al., 1985); host nutritional deficiencies such as vitamin A and E deficiencies may exacerbate disease (Tvedten et al., 1973). Mycoplasma isolates may also vary in virulence (Davidson et al., 1988). Environmental factors may include intracage ammonia, temperature, humidity, etc. (Schoeb et al., 1982).
Mycoplasma pulmonis possibly damages host cells by causing dysfunction and/or loss of cilia (Kohn, 1971) (Fig. 8 ), which is a likely cause of the accumulation of exudate, opportunistic bacterial infections, and impaired transport of ova (infertility). Mycoplasma pulmonis competes for the host cell nutrients and metabolites (Cassell et al., 1986) and may also produce toxic metabolites, such as peroxides and nonspecific mitogens (Naot et al., 1979a,b). The latter may cause proliferation of autoreactive clones of lymphocytes, leading to the host's becoming a victim of its own immune system.
Fig. 8.
Electron micrograph of bronchial epithelial cell. Note the numerous M. pulmonis organisms cytadsorbed to the cell surface and the associated cytopathology.
Mycoplasma pulmonis successfully evades the host's immune defenses, so infection and some lesions (especially those in the upper respiratory tract) are persistent and often progressive. The exact mechanism by which M. pulmonis evades the host immune system, however, is unknown.
Gross lesions of MRM (Percy and Barthold, 1993b), listed in decreasing order of frequency, include suppurative rhinitis, otitis media, laryngitis, and tracheitis in the upper respiratory tract. In the lung, suppurative bronchopneumonia with or without atelectasis, bronchiectasis, and abscesses may occur; widespread bronchiectatic abscesses lead to the appearance referred to as “cobblestone” lung, primarily seen in adults with endstage disease. This classic lesion of MRM is rare in recent years. Arthritis may rarely be observed. No genital tract lesions are usually observed, but occasionally partially resorbed fetuses and suppurative salpingitis may be found.
Histopathologically (Van Andel et al., 1996), airway lesions in the respiratory tract are usually characterized by suppurative exudate, hyperplasia (squamous metaplasia) of mucosal epithelium, and often striking hyperplasia of the bronchial-associated lymphoid tissue (BALT). Other respiratory tract lesions include pseudoglandular hyperplasia of the nasal epithelium in chronic cases, and hyperplasia of peribronchial alveolar type II pneumocytes. CAR bacillus and/or secondary bacterial pneumonias also frequently accompany MRM. Lesions in the female genital tract of rats with mycoplasmosis may include suppurative oophoritis and salpingitis, or hydrosalpingitis, and chronic suppurative endometritis or pyometra.
Differential diagnoses for MRM include other bacterial pneumonias, such as Corynebacterium kutscheri infection, streptococcosis, cilia-associated respiratory (CAR) bacillus infection, and (rarely) mycotic pneumonia. Viral infections are less likely to be mistaken for MRM, but intercurrent infections are common, including Sendai virus, pneumonia virus of mice, and others.
Diagnosis of mycoplasmosis in an individual rat is usually based on cultural isolation (especially exudate in the upper respiratory tract and middle ears). Surveillance of infections in colonies, however, is most effectively accomplished by ELISA (Cassell et al., 1981; Lussier, 1991). Pathology, including gross examination, and histopathology should not be considered diagnostic by themselves but may provide guidance in selecting more definitive tests.
Mycoplasma pulmonis interferes with research by its effects on the immune system, the respiratory system, and the reproductive system and by being a primary cause of early mortality in infected colonies (Cassell et al., 1986; Swing et al., 1995; Lindsey et al., 1971, 1982).
k. Hemobartonellosis
Haemobartonella muris is a gram-negative bacterium, order Rickettsiales, family Anaplasmataceae, that parasitizes erythrocytes of rats (Ristic and Kreier, 1984). Like other members of the family, it is an obligate parasite and cannot be grown in vitro.
Clinical signs are typically observed only if the normally latent infection is activated by immunosuppression or splenectomy (National Research Council, 1991e). Signs are due to erythrocyte destruction and may include weight loss, hemoglobinuria, pallor, and dyspnea. Clinical pathology demonstrates anemia, reticulocytosis, increased coagulation times, decreased plasma proteins, and increased serum immunoglobulins (IgG and IgM).
Because H. muris is transmitted by the spiny rat louse, Polyplax spinulosa, which is very rare in modern laboratory animal facilities, hemobartonellosis is also correspondingly rare (National Research Council, 1991b). However, the potential exists for infection of biological materials, which would provide a route of introduction into rat colonies. In addition, both the agent and the vector are still extant in North America and presumably elsewhere, indicating a continuing, albeit low-level, threat.
Necropsy of rats with hemobartonellosis is unrewarding except in the case of active infections, when anemia, hemoglobinuria, and splenomegaly may be observed. Blood films are likely to show parasitemia only in active infections.
Hemobartonellosis should be suspected whenever lice are found in a rat colony or whenever anemia and hemoglobinuria are observed. Diagnosis should be based on detection of the organisms on erythrocytes, where they appear as round (coccoid), elongate (rod), or dumbbell-shaped densities on the erythrocyte surface.
Hemobartonellosis is readily prevented by excluding Polyplax spinulosa and controlling biologic materials being introduced into a colony. Once the disease is confirmed in a colony, rederivation by embryo transfer or cesarian section is warranted, although treatment with antirickettsial compounds such as tetracyclines or arsenicals may be appropriate for small groups of rats (Ristic and Kreier, 1984).
Hemobartonellosis exerts its effects on research by virtue of its parasitism of erythrocytes. It reduces the half-life of erythrocytes, can alter function of the mononuclear phagocyte system, and can increase rejection of transplantable tumors, as well as interfering with research in other blood-borne parasitic diseases such as malaria and trypanosomiasis.
2. Viral Infections
a. Sendai Virus Infection
Sendai virus is an RNA virus (genus Paramyxovirus, species parainfluenza 1) comprised of strains that are antigenically homologous (National Research Council, 1991a). Unlike Sendai virus–induced disease in mice, an asymptomatic and self-limiting disease is usually induced by Sendai virus in rats. Clinical signs associated with the virus may include reduced production and litter sizes, as well as retarded growth of young within breeding colonies. Infrequently, clinical respiratory signs occur (Makino et al., 1972).
It has been shown in Lewis rats, inoculated intranasally with Sendai virus, that draining lymph nodes of the upper respiratory tract are the initial and major site of antibody production. Development of serum immunoglobulin G (IgG) antibodies coincides with clearance of respiratory tract infection and recovery from viral infection (Liang et al., 1999).
Coinfection with other respiratory pathogens such as Mycoplasma pulmonis, Flexibacter sp. (CAR bacillus), Pasteurella pneumotropica, and pneumonia virus of mice (PVM) increases the severity of clinical disease and pulmonary lesions (Besch-Williford et al., 1987; Carthew and Aldred, 1988). Although Sendai virus was once very prevalent in commercial sources of mice and rats, today it rarely occurs in barrier-maintained commercial sources. However, commercial and institutional sources that are conventionally maintained may still be sources for introduction of the virus to naive colonies of rats or mice. Sendai virus is highly contagious, with transmission occurring through the respiratory tract either by aerosol or direct contact.
After exposure, the initial tropism in the upper respiratory tract induces a rhinitis characterized by focal to diffuse necrosis of the epithelial cells, and a leukocytic infiltrate composed of neutrophils, lymphocytes, and plasma cells. Within the lungs there is a hyperplastic to suppurative bronchitis and focal alveolitis. Alveolar septa are hypercellular, with infiltrates of alveolar macrophages, neutrophils, and lymphocytes. Viral replication occurs in bronchial epithelial cells, type I and type II pneumocytes, and alveolar macrophages. Later, there is pronounced perivascular and peribronchial cuffing with a lymphocytic and plasmacytic infiltrate that may remain 7 months after the acute phase of the infection (Burek et al., 1977; Percy and Barthold, 1993b). Based upon experimental infection, lesion severity has been shown to be more severe in Brown Norway and LEW rats than in F-344 rats (Sorden and Castleman, 1991; Liang et al., 1995).
Due to the low prevalence of clinical signs, diagnosis is best achieved by detection of antibodies to the virus and demonstration of typical lesions in the respiratory tract. The ELISA is the test of choice for diagnosis of Sendai virus infections in rats. Comparison with Complement fixation (CF) and hemagglutination inhibition (HAI)) tests indicates that ELISA is the most sensitive, particularly in detection of early antibody to Sendai virus and detection of small amounts of antibody (Rottinghaus et al., 1986).
Prevention of Sendai virus introduction into an existing colony requires knowledge of the pathogen status of the source and, in some cases, quarantine with serological testing of incoming rats and mice. Regular and periodic serologic testing within colonies of rats and mice should be done to help prevent and control infection within rodent housing facilities. Mouse and rat antibody production (MAP, RAP) testing should be done on all transplantable tumors, cell lines, and other biological materials to prevent transmission of Sendai virus from infected materials to recipient animals. Recently, the use of PCR testing for the presence of Sendai virus (and other viral pathogens) in tumors and cell lines has been shown to be more sensitive than MAP testing (Riley et al., 1999).
If Sendai virus is introduced into a rat colony of immunocompetent rats, neutralizing antibody in infected rats renders the infection self-limiting. Accordingly, if antibody-naive rats are not introduced and if pregnant and preweanling rats are killed and breeding is halted, the virus will be eliminated from the colony within 4–8 weeks (Jacoby et al., 1979).
In addition to research complications associated with the respiratory tract tropisms of the virus, it may modulate some immunological responses, e.g., reducing the severity of adjuvant arthritis (Garlinghouse and Van Hoosier, 1978) and depressing T cell and thymocytotoxic autoantibody (Takeichi et al., 1988).
b. Rat Coronavirus Infection
The two prototype coronaviruses in rats are Parker's rat coronavirus (RCV-P) and sialodacryoadenitis virus (RCV-SDA). In addition to these two coronavirus strains, there are others that have been isolated and found to differ antigenically from either RCV-P or RCV-SDA. Historically, RCV-P and RCV-SDA were considered to induce two rather distinct sets of clinical signs and types of lesions in rats (Jacoby et al., 1979). More recently, however, the clinical signs, pathogenicity, and histological lesions are considered to be variable but similar for both RCV-P and RCV-SDA, and defining the neutralization group of a new RCV isolate is not useful in predicting its pathogenic potential (Compton et al., 1999). Accordingly, infection with either RCV-SDA or RCV-P cannot be differentiated by comparison of clinical signs or lesions. The antigenic differences between RCV-P and RCV-SDA are significant enough to allow cross-infection with either virus. Probably the most important point to be made from a clinical perspective is that neutralizing antibodies to one virus prototype will not offer significant cross protection from the other virus strain, thus allowing viral shedding and recurrence of clinical signs and lesions, albeit diminished (Percy and Barthold, 1993b; Jacoby, 1986; Bihun and Percy, 1994; Kojima and Okaniwa, 1991; Weir et al., 1990).
Rat coronaviruses may induce either asymptomatic infections or transient clinical infections (sialodacryoadenitis) associated with tissue tropisms for the salivary glands, lacrimal glands, Harderian glands, and respiratory epithelium. There are two distinctive types of clinical disease associated with the virus. The first is associated with breeding colonies in which the virus is endemic with mature rats immune to infection, and in which clinical disease is primarily associated with preweanling, nonimmune animals that display ocular signs associated with conjunctivitis. These signs are transient, lasting for a week or less. The second type of clinical picture is associated with a sudden onset of clinical signs in naive postweanling-to-adult rats that have been exposed to infected rats. Signs include cervical swelling due to inflammation and edema of submaxillary salivary glands (Fig. 9 ), nasal and ocular discharges that are usually porphyrin stained, photophobia, corneal opacities, and corneal ulcers. In most animals the signs last for less than 2 weeks. However, in some animals a chronic keratitis and megaloglobus may persist (Jacoby et al., 1979).
Fig. 9.
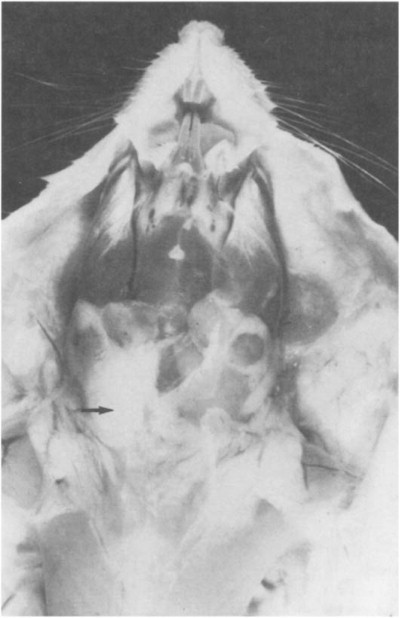
Edematous pale submaxillary gland (arrow) and moderately enlarged cervical lymph nodes.
(Courtesy of Dr. Robert Jacoby.)
Rat coronaviruses are very contagious, with transfer to susceptible rats by direct contact with infected rats, and indirectly by aerosol and fomites (La Regina et al., 1992). Virus is present in target tissues for about 1 week after exposure, at which time heightened antibody levels render the infection self-limiting. However, immunity is not lifelong. Under experimental conditions, it has been shown that rats are susceptible to reinfection as early as 6 months after initial infection and that such rats are able to transfer infection to naive rats by cage contact. However, the severity of lesions in reinfected rats is minimal compared with those associated with primary infections (Percy et al., 1990; Weir et al., 1990). Differences in pathogenicity have been reported among a few rat strains (Jacoby et al., 1979; Carthew and Slinger, 1981).
Histological changes associated with sialodacryoadenitis (SDA) in affected salivary and lacrimal glands include coagulation necrosis of ductal and acinar epithelial cells during the acute stages of the disease, followed by squamous metaplasia during the reparative period that begins 7–10 days after infection. There is a mixed leukocyte infiltrate. Regeneration of the epithelial cells occurs in about 4 weeks postinfection. However, focal lesions may persist an additional several weeks in the Harderian glands.
The microscopic changes associated with rhinitis, tracheitis, and focal bronchitis during the acute stage of the disease include a mononuclear and polymorphonuclear cell infiltration, hyperplastic respiratory epithelia with loss of ciliated surfaces, and focal alveolitis. The lesions within the lower respiratory tract abate in about 7–10 days, and those in the nasopharynx remain somewhat longer (Percy and Barthold, 1993b).
Diagnosis of SDA is best achieved by serological means using the ELISA method and histological examination of the Harderian glands and the submaxillary and parotid salivary glands (National Research Council, 1991). Because the disease is often subclinical, typical signs associated with salivary gland and Harderian gland tropisms may not be useful. Differential diagnoses include Mycoplasma, Sendai virus, and pneumonia virus of mice (PVM) infections, and stress-associated factors that induce chromodacryorrhea (Percy and Barthold, 1993).
Preventing transfer of this highly contagious coronavirus to naive colonies is predicated upon preventing entry of infected rats into a facility through knowledge of the pathogen status of vendor colonies and an effective quarantine program. Control of infection within a colony or facility is based upon the fact that rats shed the virus for only about 1 week, after which they are immune and not latently infected. The virus is not transmitted vertically. Eliminating rat coronavirus from a colony is achieved by allowing the virus to spread quickly to all animals, preventing entry of susceptible rats to the room, and suspension of breeding and removal of preweanlings. The rapidity in which all animals will seroconvert and no longer shed the virus will determine the period of time needed before susceptible animals can be safely introduced or breeding resumed. In most instances, a 6- to 8-week period should be allowed (Jacoby et al., 1979). Alternatively, if suspension of breeding cannot be done, a method to continue breeding and eliminate SDA is to define a subset of the breeding colony that is seropositive and to relocate these breeding animals to a separate room, allow litters to be born in the original colony until the relocated breeders are in late gestation, and then kill all animals in the original colony (Brammer et al., 1993).
Research complications associated with SDA reflect tropisms for the lacrimal and salivary glands, the eye, vomeronasal organ, and respiratory epithelium (Percy and Barthold, 1993b). Except for long-term ocular lesions, research complications would be expected to be linked to the period of active infection and the 2- to 3-week reparative period. During this period, food intake frequently decreases if cervical swelling occurs.
c. Rat Parvovirus Infection
Parvoviruses are single-stranded DNA viruses that have a predilection for mitotically active host cells. Parvoviruses that infect rats include (Kilham's) rat virus (RV), (Toolan's) H-1 virus, and rat parvovirus (RPV). The first two viruses were initially isolated from a transplantable tumor (RV) and a tumor cell line passed in rats (H-1). In the 1980s, testing of rat sera indicated the presence a parvovirus that was neither RV nor H-1. This virus, which was initially referred to as rat orphan parvovirus (OPV), is now designated rat parvovirus (RPV) (Jacoby et al., 1996).
Clinical signs associated with RV infection occur very sporadically in colonies showing serological evidence of infection and are usually seen only in preweanling animals. In such colonies, reduced litter size, runted litters, and fetal and neonatal death may be observed. Although subclinical infections in postweanling rats are the rule, an outbreak characterized by hemorrhage and necrosis of the brain, testes, and epididymides has been reported in young adult rats (Coleman et al., 1983). The ability of RV to cross the placenta appears to depend on the virus strain, dose, and time of gestation. Resistance to lethal infection develops during the first postpartum week (Jacoby et al., 1988; Gaertner et al., 1996). Serological surveys of rat colonies have indicated a rather high prevalence of antibody to H-1 virus; however, clinical disease is not associated with this virus (National Research Council, 1991b). Although the pathogenesis of RPV needs further definition, it appears that RPV infections are characterized as being subclinical (Weisbroth et al., 1998).
Rat virus is excreted in urine and milk and is transmitted by aerosol through direct contact or fomites (Jacoby et al., 1996). RV-contaminated bedding, stored at room temperature for up to 5 weeks, is capable of inducing seroconversion of rats for up to 5 weeks (Yang et al., 1995). Rats may harbor and transmit RV long after seroconversion occurs, with the frequency of persistent infection during natural outbreaks being RV strain-dependent (Gaertner et al., 1996). After experimental inoculation of RV into neonatal rats, the virus persists in tissues for up to 14 weeks, and the duration of infectivity to cage contacts up to 10 weeks. If weanling rats are inoculated, the duration of viral recovery and infectivity is decreased to 7 and 3 weeks, respectively (Paturzo et al., 1987; Jacoby et al., 1988). In another study, RV was recoverable for up to 6 months from tissue explant cultures derived from newborn rats (Jacoby et al., 1991). In persistent infections, DNA and antigenic evidence of RV is most likely to be observed in lymphoid tissues, endothelium, vascular muscle tunics, and renal tubular epithelium (Gaertner et al., 1996; Jacoby et al., 1991).
The correlation of age and RV pathogenicity is thought to be due to the decreased complement of target cells in the S phase of division needed for productive infection. The immune status of the host is also significant to the outcome of RV infection. Rat virus in athymic rats induces a more severe and persistent infection than in euthymic rats (Gaertner et al., 1995; Gaertner et al., 1989).
Diagnosis of RV, RPV, and H-1 virus can be accomplished by testing sera by ELISA or IFA, using RV, H-1, or recombinant NS1 as the antigen. A positive response does not delineate which rat parvovirus antibody exists but only indicates that antibody to a rodent parvovirus is present. Positive ELISA or IFA sera are then tested by hemagglutination inhibition (HAI) for RV and H-1. Samples negative by HAI tests are interpreted to be positive for RPV (Weisbroth et al., 1998). PCR assays for RV, H-1, and RPV have been developed that provide a rapid, specific and sensitive means for detecting viral DNA in tissue (Besselsen et al., 1995a,b) or the environment (W. R. Shek, personal communication).
Research complications induced by RV are associated with its tropism for mitotically active cells of fetuses, neonates, cell cultures, and tumors. Rat virus has been shown to modulate immune function through its tropism for T-cell lymphocytes (McKisic et al., 1995). Rat virus infection in the diabetes-resistant BioBreeding rat increases the expression of macrophage cytokines, leading to to an autoimmune diabetes (Chung et al., 1997). The effect of RV on the immune system has been shown to be rat strain dependent for natural killer cell activity. Natural killer cell-mediated cytotoxicity is increased in Brown Norway rats, whereas it is decreased in Wistar-Furth rats (Darrigrand et al., 1984). The effects, if any, that RPV may have on research are essentially unknown.
d. Pneumonia Virus of Mice Infection
Pneumonia virus of mice (PVM) is a pneumovirus in the family Paramyxoviridae. Contrary to the virus's name, serological evidence indicates infectivity in mice, rats, hamsters, gerbils, guinea pigs, and rabbits. The prevalence of seropositive rat colonies was reported in 1982 to exceed 50%; however, today serological evidence of PVM infection is infrequently observed in rats. Diagnosis is typically accomplished by ELISA or HAI testing (National Research Council, 1991a). The virus does not cause clinical disease, but multifocal, nonsuppurative vasculitis and interstitial pneumonitis with necrosis are prominent lesions seen in the acute phase of the disease. These lesions persist for several weeks. The virus may be a significant copathogen with other respiratory agents such as Mycoplasma pulmonis, and cross species transmission is a potential concern (Percy and Barthold, 1993b).
e. Rotavirus-Like Agent Infection
Diarrhea in suckling rats has been associated with a virus morphologically identical but antigenically and genomically distinct from group A rotaviruses (Eiden et al., 1985). The authors who first reported this disease named it infectious diarrhea of infant rats (Vonderfecht et al., 1984). Affected infant rats excreted feces that varied from liquid to being poorly formed, and the animals displayed erythema and bleeding of the perianal skin. Pathology associated with infection included small intestinal villous atrophy, villous epithelial necrosis, and syncytial cell formation. This same agent was found to be associated with diarrhea in humans and has been shown by enzyme immunoassay inhibition assay to be prevalent in children and adults. Human isolates were shown to induce diarrhea in infant rats (Eiden et al., 1985; Vonderfecht et al., 1985). This suggests that under nonexperimental conditions there may be cross-infectivity between humans and rats.
f. Hantavirus Infection
Hantaviruses are enveloped RNA viruses of the genus Hantavirus, family Bunyviridae. Rodents serve as the natural reservoirs for hantaviruses, with each virus in the genus being associated with a specific rodent species. Hantavirus infections in rodents are characterized by being chronic and subclinical, with virus being shed persistently in the feces and urine. Hantaviruses pose as significant zoonotic agents. Rattus norvegicus is the natural host for the Seoul Hantavirus, causing hemorrhagic fever with renal syndrome (HFRS) in humans. Hantavirus has been isolated from wild rats in Baltimore and several other cities in the United States. One report cites evidence of human infection with a rat-associated Hantavirus (Childs et al., 1987). Cotton rats, Sigmodon hispidus, are reservoirs for a Hantavirus that has induced hantavirus pulmonary syndrome in individuals living in Florida (Hutchinson et al., 1998). Transmission of Hantavirus from laboratory rats to laboratory personnel has been reported in Japan, Belgium, and the United Kingdom (Desmyter et al., 1983; Lloyd et al., 1984). In both reports, multiple cases occurred that resulted in hemorrhagic fever with renal syndrome.
g. Unidentified Viral Agent Associated with Lymphohistiocytic Lung Lesions
Lymphohistiocytic lung lesions probably attributable to a virus etiology have been reported in barrier-maintained commercial breeding colonies. The lesions appear as multiple small gray to tan foci on the pleural surface of the lung. Histologically, mild to moderate multifocal histiocytic alveolitis and perivascular cuffing are observed. The lesions occur in 8- to 18-week-old animals with the most severe lesions seen at 8–12 weeks. Bacteriological culturing and PCR assays for Mycoplasma, CAR bacillus, and eubacteria genera suggest that the lesions are not associated with bacteria. A viral etiology is suggested, because inoculation of lung tissue homogenates that pass through bacteriologic filters induces cytopathic effects in tissue culture (Riley and Franklin, 1997; Slaoui et al., 1998; C. Clifford personal communication, 1999).
h. Other Viral Infections
There are several rodent viruses for which there is serological evidence of infection in the rat, but for which there are negligible data demonstrating any clinical or pathological importance. These viruses include mouse adenovirus, mouse encephalomyelitis virus, reovirus 3, parainfluenza virus 3, and endogenous retroviruses (Kohn and Barthold, 1984; National Research Council, 1991; Percy and Barthold, 1993b).
3. Parasitic Infections
a. Protozoa
Protozoa are of little consequence in laboratory rats in recent decades (National Research Council, 1991a; Kohn and Barthold, 1984). Reasons for this are several. First, no spontaneous disease due to any naturally occurring enteric protozoa of laboratory rats has been reported. Second, parenteral infections are rare in laboratory rats because of absence of vectors. Third, there is almost universal use of high-quality diets, which are generally subjected to heat disinfection prior to use. The days of giving rats fresh produce are happily slipping into the past. Protozoa of potential significance in rodent facilities include Encephalitozoon cuniculi and two enteric flagellates, Spironucleus muris and Giardia muris, which may produce disease and alter immune responses in mice.
Encephalitozoon cuniculi, formerly Nosema cuniculi, is a microsporidian parasite of a wide variety of mammalian hosts, including rodents, lagomorphs, carnivores, and primates, including humans. It has also been reported in birds (Poonacha et al., 1985; Reetz, 1993).
Encephalitozoonosis is common in conventional rabbit colonies and most guinea pig colonies but is rare in rats. It is transmitted by ingestion, and possibly inhalation, of spores shed in urine (Wilson, 1979). Vertical transmission has been proposed in primates, foxes, mice, rabbits, and guinea pigs but not in rats (Boot et al., 1988; Liu et al., 1988).
Resistance to infection and the outcome of infection are dependent on T-cell function, which is strain dependent (Liu et al., 1989; Niederkorn et al., 1981). Athymic nude mice, and presumably athymic nude rats, are more susceptible to lethal infection than are euthymic animals. Encephalitozoon cuniculi has also been recovered from transplantable ascites tumors in rats (Petri, 1969).
Clinical signs and gross lesions of E. cuniculi infection are not reported in rats. On histopathologic examination (Majeed and Zubaidy, 1982), rats with E. cuniculi infection may have nonsuppurative or granulomatous meningoencephalitis in any or all parts of the brain and occasionally the spinal cord. Interstitial nephritis may also be observed Less frequently, similar lesions may be observed in other tissues. Spores may be observed in, or more frequently adjacent to, any of the lesions. Spores stain poorly with hematoxylin and eosin but are strongly gram-positive.
Diagnosis of E. cuniculi infection is usually based on serology (Pakes et al., 1984). Screening of colonies by ELISA is probably the most efficient method, because infected colonies normally have a high prevalence (Gannon, 1980). As with all serologic assays, positive serologic results should be confirmed by a second method or by repeating the assay on groups of animals to establish a pattern of positive results. Histopathologic observation of the organism is definitive.
The primary histopathologic differential diagnosis for E. cuniculi infection in rats is toxoplasmosis. Encephalitozoon cuniculi measures 1× 2 μm, stains well with Gram stain and poorly with hematoxylin and eosin. Toxoplasma gondii measures 2 × 4 μm, stains well with hematoxylin and eosin, and poorly with Gram stain (Wilson, 1979).
Encephalitozoonosis is controlled by purchasing only animals that are free of Encephalitozoon and by maintaining them away from infected animals. There is currently no effective treatment. Research complications of E. cuniculi infections have not been reported in rats, although it is potentially a confounding factor if histopathologic evaluation of the central nervous system and kidney is part of the study (Majeed and Zubaidy, 1982).
Toxoplasmosis is a zoonotic disease caused by Toxoplasma gondii. Toxoplasmosis in rats is usually subclinical. The definitive host is the domestic cat and other felids, which shed oocysts in the feces. Rats, like many other vertebrates, serve as intermediate hosts. Transmission to rats is via ingestion of cat feces. Ingestion of infected intermediate hosts might also horizontally transmit the infection, although it would not be expected as a mode of transmission in a well-managed rat colony. Infected rats can transmit T. gondii vertically, but only very poorly. Therefore, in order for a rat colony to remain infected with T. gondii, cat feces would need to be repeatedly introduced. As a result, T. gondii is an organism is of little current significance in research facilities, and routine monitoring for toxoplasmosis in rats continues only in some geographic areas (Rehbinder et al., 1996).
Numerous enteric flagellates have been reported in laboratory rats over the years, but none are of significance. The life cycle of all flagellates, and Entamoeba muris, is direct (Flynn, 1973c; Levine, 1961), with fecal-oral transmission. Trophozoites, the feeding form, are present in the gastroinestinal tract. Reproduction is asexual and produces resistant cyst forms, which are shed in the feces (Kunstyr, 1977).
Spironucleus muris colonizes mice, rats, and hamsters, where it inhabits glandular crypts and the lumen of the small intestine (Gruber and Osborne, 1979; Wagner et al., 1974). Age-infection relationships have not been reported for rats but are probably similar to that of mice, in which animals under 6 weeks of age are more susceptible to infection. Transmission of cloned S. muris between rats and mice has been attempted (Schagemann et al., 1990). An isolate from rats was not infective to hamsters, immunocompetent mice, or athymic nude mice. Similarly, rats were not persistently colonized by isolates from mice or hamsters.
Cysts of S. muris are resistant to drying (room temperature for 14 days), freezing (–20°C for 6 months), pH 2.2 for 1 day, or 0.1% glutaraldehyde for 1 hr (Kunstyr and Ammerpohl, 1978). Spironucleus muris infection is diagnosed by examination of wet mounts of duodenal scrapings of weanling rats. Phase-contrast microscopy is especially helpful in observing the trophozoites. Identification is usually based on the size, 3–4 × 10–15 μm, and characteristic rolling motion of the flagellated trophozoites. Cysts may be observed in wet mounts or in fecal smears. These measure 4× 7 μm and are reported to have a characteristic banded pattern (Kunstyr, 1977).
Giardia spp. are ancient, with one of the most highly conserved genomes of all eukaryotes (Yu et al., 1996, 1998). Giardia also has its own microflora, including mycoplasma-like particles and bacteria (Feely et al., 1988) and viruses (Tai et al., 1991, 1996). Giardia muris colonizes a wide variety of mammalian hosts, including rats, mice, hamsters, and humans (Levine, 1961). Recent evidence has suggested that G. muris may actually be a form of G. duodenalis (Sharma and Mayrhofer, 1988b). Although this conclusion seems to have implications for zoonotic potential, because G. duodenalis is a human pathogen, there does appear to be some host specificity, albeit incomplete. Giardia muris isolated from mice and hamsters, for example, did not produce infection when inoculated into rats (Kunstyr et al., 1992). Trophozoites were previously reported to attach to the surface of intestinal epithelial cells by means of a flat suction disk (Levine, 1985a). It is now thought that attachment is via a surface membrane mannose-binding lectin and can occur via any point on the parasite surface, without requiring the disk (Inge et al., 1988). Cysts stored in liquid feces have remained infective for at least 1 year (Craft, 1982).
No naturally occurring clinical disease has been reported in rats infected with G. muris. Experimental infection with G. lamblia and G. duodenalis has resulted in secretion of specific immunoglobulin A into bile (Loftness et al., 1984; Sharma and Mayrhofer, 1988a).
Giardiasis is diagnosed similarly to spironucleosis. Trophozoites, measuring 7–13 × 5–10 μm (Levine, 1961), have a characteristic piriform or teardrop shape, with a broad, rounded anterior tapering to a pointed posterior end (Levine, 1985a). The trophozoites have a slight curvature toward the ventral side, which causes the motion of their multiple flagella to impart a rolling motion to the organisms in wet mounts (National Research Council, 1991a). In stained preparations, the darkly stained dual nuclei are prominent. Two small dark median bodies are also visible, immediately posterior to the nuclei. Cysts may also be identified on fecal smears or with fecal flotation methods.
Entamoeba muris is a nonpathogenic commensal amoeba of rats, mice, and hamsters (National Research Council, 1991a; Levine, 1985b). Trophozoites, measuring 8–30 μm in length, are found in wet-mount preparations of contents from the cecum and colon, where they feed on bacteria (Levine, 1961). Cysts 9–20 μm in diameter have eight nuclei and can be observed in feces.
Control measures in rats for all intestinal flagellates and Entamoeba muris are similar. Rederivation, either by cesarean section or by embryo transfer, is effective. Contaminated animal rooms should be thoroughly cleaned, then disinfected with chlorine dioxide solutions or other suitable disinfectants (Wickramanayake and Sproul, 1991), prior to repopulation introduction. All materials brought into the room, which may have had prior exposure to rodents or rodent feces should be autoclaved. All animals should be monitored for infection prior to introduction. This should include examination of rats of appropriate age, i.e., 3–6 weeks.
Treatment of animals to eliminate infection with intestinal flagellates has met with limited success. Metronidazole (Flagyl) or dimetridazole (National Research Council, 1991a) can be added to the drinking water but is ineffective against cysts in the environment. Other authors have reported success in eliminating Giardia spp., using metronidazole in rats and mice (Sharma and Mayrhofer, 1988b). Significantly, however, metronidazole has been shown to be carcinogenic in rats and mice (Goldman, 1980).
b. Nematodes
i. Oxyuriasis
Three species of oxyurid nematodes (pinworms)—Syphacia muris, S. obvelata, and Aspicularis tetraptera—occur in the laboratory rat. Their continued occurrence, despite the dramatic progress in eliminating viral and bacterial pathogens, is due both to the persistence of the eggs in the environment and to the low degree of attention paid to these parasites.
Syphacia muris is the most common oxyurid of the rat (National Research Council, 1991a; Owen, 1992a). Syphacia obvelata is more frequently found in mice, hamsters, and gerbils but is also occasionally found in the rat, especially when housed in the same room with infested mice.
Syphacia spp. have a direct life cycle, requiring 11–15 days for completion (Flynn, 1973a). Transmission is horizontal via ingestion of eggs. Eggs, which remain viable at room conditions for weeks to months, are deposited around the anus and in the colon and become infective in approximately 6 hr. They are ingested during self-cleaning and hatch in the small intestine. The larvae then mature in the cecum in 10–11 days. The morphology of adults of both species is similar, although S. muris is slightly smaller and the male has a longer tail, measured as a proportion of body width (Flynn, 1973a). Eggs vary more markedly between the species, with eggs of S. muris being 72–82 × 25–36 μm and those of S. obvelata being 118–153 × 33–55 μm. In addition, the eggs of S. obvelata are almost completely flat along one side, whereas those of S. muris are only slightly flattened on one side.
Aspicularis tetraptera is also transmitted horizontally by ingestion of eggs, which are extremely persistent in the environment (Flynn, 1973a). The direct life cycle is longer than that of Syphacia, requiring 23–25 days. Also unlike in Syphacia, Aspicularis eggs are passed in the feces and are not deposited around the anus. Adult A. tetraptera are readily recognized by the four alae present at the anterior end of the body. Eggs of A. tetraptera are approximately the same size as S. muris eggs, measuring 89–93 × 36–42 μm, and are bilaterally symmetrical.
Gross lesions of oxyuriasis are very rare (Flynn, 1973a), and histologic lesions of oxyuriasis have not been reported. Diagnosis of oxyuriasis is most practically accomplished by direct examination of macerated cecum and colon under low magnification with a stereomicroscope. This is almost as sensitive as complete direct examination of the large bowel and is significantly less time-consuming. Examination for eggs must be tailored to the infesting species suspected. The perianal tape test is effective only for Syphacia spp., and fecal flotation is effective only for A. tetraptera. Screening for oxyurid eggs is significantly less sensitive than direct examination of the bowel for the adult helminths (West et al., 1992; Klement et al., 1996).
Oxyuriasis can be eliminated in individual rats with ivermectin (Huerkamp, 1993; Klement et al., 1996; Zenner, 1988; Hasslinger and Wiethe, 1987). However, the source of the original infestation should be identified, and the premises should be thoroughly disinfected so as to prevent reinfestation. Ivermectin is not effective against eggs, which can persist for long periods in the environment. Oxyuriasis can also be eliminated by rederivation. It is readily excluded by proper adherence to modern practices of barrier room technology (Hasslinger and Wiethe, 1987).
Numerous research effects of oxyuriasis have been described. In rats, oxyuriasis has been reported to interfere with adjuvant arthritis (Pearson and Taylor, 1975), growth rate (Wagner, 1988), and intestinal electrolyte transport (Lubcke et al., 1992).
ii. Trichosomoides crassicauda
This trichurid nematode is found only in the rat (Flynn, 1973a). Although geographically widespread, Trichosomoides crassicauda is very rare in barrier-maintained rodents that have been rederived by cesarean section or embryo transfer.
Adult females, approximately 10 mm long, live in the urinary bladder, either free in the lumen or embedded in the mucosa (Flynn, 1973a; Antonakopoulos et al., 1991; Cornish et al., 1988). The males are anatomically degenerate and exist symbiotically in the vagina or uterus of the females. Embryonated eggs are laid and pass in the urine. Transmission of T. crassicauda is via ingestion of these eggs and probably occurs from dam to pups prior to weaning. The eggs hatch in the stomach, where the larvae penetrate the wall and pass through the peritoneal cavity or bloodstream to reach the lungs and other tissues. Most larvae lodge in tissues other than the kidneys and may cause hemorrhages or granulomas. Only those that reach the kidney or bladder survive and develop to maturity. The entire life cycle is 8–9 weeks, so eggs are not present in the urine until the rats are 8–12 weeks of age.
Infestation with T. crassicauda, although persistent, is usually clinically inapparent (Flynn, 1973a). Usually very few worms, perhaps averaging 3 in number (Barthold, 1996a), are present in the bladder, where they cause mild uroepithelial hyperplasia (Zubaidy and Majeed, 1981; Antonakopoulos et al., 1991). When found in the renal pelvis, they are associated with mild pyelitis and pyelonephritis.
Trichosomoides crassicauda infestation is diagnosed in live rats by filtration of urine and then examination of the filter medium for the eggs. Diagnosis in recently killed rats is by direct examination of the bladder wall, histopathology, scanning electron microscopy, or microscopic examination of cryostat sections stained with acridine orange (Barthold, 1996a; Cornish et al., 1988). The last two methods are purported to be more reliable but are probably not practical for routine, large-scale screening.
Treatment for T. crassicauda infestation has been reported, using a single dose of ivermectin (Summa et al., 1992). Follow-up found that the infestation was not eliminated in 1 of 30 rats, perhaps because of reinfection. Once a colony is free of this parasite, however, there should be little chance of reintroduction if no infected rats enter the colony.
No confirmed research effects of T. crassicauda infestation have been reported in the scientific literature, although proliferative changes in the urothelium would render these animals unsuitable for research involving the urinary system (Cohen et al., 1998). Early speculation concerning the possible etiologic role of T. crassicauda infestation in causing bladder tumors in a famous study in rats that were administered high doses of saccharin in the diet (Homburger, 1977) has not been supported by later investigators (Barthold, 1996a). However, proliferative changes in uroepithelium caused by T. crassicauda infestation are identical to those produced early in carcinogenesis by chemical compounds such as N-methylnitrosourea (MNU) (Pauli et al., 1996).
c. Cestodes
There are only two adult cestodes that are likely to be encountered in laboratory rats: Hymenolepis nana and H. diminuta. The primary differences of consequence between the two species are that H. nana is zoonotic and can have a direct life cycle, whereas H. diminuta always has an indirect life cycle, utilizing an intermediate host, and is not zoonotic. Fortunately, both are rare in laboratory rats.
Hymenolepis nana lives in the small intestine of rats, mice, hamsters, and primates, including humans (Hsu, 1979). It is primarily the ability to parasitize humans that gives H. nana significance, for it causes little damage in rats or mice (National Research Council, 1991a). Hymenolepis nana averages 20–40 mm long but can vary greatly. It is slender and less than 1 mm wide. The scolex has four suckers, and a rostellum armed with 20–27 hooks. Mature proglottids are trapezoidal and contain as many as 200 eggs, which are thin-shelled, oval, and colorless and have six visible polar filaments. Within the eggs, the embryo, or oncosphere, has three pairs of hooklets within an inner envelope. The eggs are approximately 30–56 μm × 44–62 μm and do not persist for long periods outside the host.
In the direct life cycle (Hsu, 1979), which requires 14–16 days, embryonated eggs are ingested and hatch in the small intestine. The oncospheres penetrate villi and develop into cysticercoid larvae in 4–5 days. These larvae reenter the lumen, the scolex evaginates, and they attach to the mucosa. An additional 10–12 days are required before mature proglottids are formed. Adults live only a few weeks. Infection normally results in some level of immunity, which prevents autoinfection. When autoinfection occurs, eggs hatch in the small intestine and develop, without being passed in the feces, and can result in very high worm burdens. In the indirect life cycle, grain beetles (Tenebrio molitor and T. obscurus) and fleas (Pulex irritans, Ctenocephalus canis, Xenopsylla cheopis) serve as intermediate hosts. Rats and other definitive hosts are infected by ingesting the intermediate host.
Hymenolepis diminuta has a similar host range: mice, rats, hamsters, and primates, including humans (Hsu, 1979). Hymenolepis diminuta is larger than H. nana, 20–60 mm long and 3–4 mm wide. The scolex of H. diminuta also has four suckers but the rostellum is unarmed—it has no hooks. Eggs of H. diminuta are 60–88 × 52–81 μm, and the oncosphere has three pairs of hooks, but no polar filaments. The life cycle of H. diminuta is always indirect and is similar to the indirect life cycle of H. nana.
Both Hymenolepis nana and H. diminuta are pathogenic in rats only in severe infections, where retarded growth, weight loss, impaction, and death have been reported in the older literature (Hsu, 1979), although no recent reports excluding the contributions of other potential pathogens have been published. In humans (Jueco, 1982), infection is common in some geographic areas but is usually subclinical. As in rats, the adult worms live 25–60 days before dying, but human cases may persist as long as 22 months because of autoinfection.
Infection is diagnosed by detection of the adult cestodes on direct examination of the small intestine, by observation of the eggs in feces (smear or fecal flotation), or by histopathologic detection of the cysticercoid in the small intestine (Hsu, 1979). Infection is most common in recently weaned rats and young adults, probably because of acquired immunity in older animals.
Hymenolepis nana and H. diminuta infection is prevented by purchase of clean stocks of rodents, by adequate disinfection of barrier room supplies, and thorough insect control and exclusion of wild rodents (National Research Council, 1991a). Treatment of infected animals is not generally recommended, because of the zoonotic implications of this disease. No indirect interference with research has been reported for hymenolepiasis in rats.
In addition to the adult cestodes, one may occasionally encounter larvae of Taenia taeniaformis, also called cysticercus fasciolaris. The cysts are found in the livers of rats, mice, and hamsters and are up to several centimeters in diameter. They are readily identified by the presence in the cyst of a scolex, strobila, and bladder (Hsu, 1979; Wescott, 1982). Although considered nonpathogenic (Hsu, 1979), the cyst may be associated with the development of hepatic sarcomas, probably in a mechanism similar to the induction of sarcomas in the rat by a variety of foreign bodies (Altman and Goodman, 1979). Because the definitive host is the cat, detection of the cysticercus is evidence that materials in the animals’ immediate environment, usually feed, were contaminated with unsterilized feces from an infected cat. Control, therefore, is simple.
d. Trematodes
Numerous trematodes have been reported in wild rats, including Plagiorchis muris, P. philippinensis, and P. javensis. Some are zoonotic (Hong et al., 1996), but none are significant in laboratory rats (Wescott, 1982).
e. Mites
Radfordia ensifera is the only ectoparasite of rats likely to be encountered in a laboratory animal environment, although other acarids, such as Radfordia affinis or Myobia musculi, could possibly be harbored on the pelage.
Acariasis, or mite infestation, is transmitted by eggs, which can persist in the environment for long periods. The eggs hatch in 7–8 days, and females can begin to lay eggs after another 16 days. Infestation can result in pruritus, self-excoriation, and secondary bacterial infection. In mice (Weisbroth, 1979), acariasis has also been associated with increased mitotic activity in the skin, immunologic alterations, and amyloidosis.
Acariasis is most practically diagnosed by direct examination of the animals with a dissecting microscope (Flynn, 1973b). As an alternative, dead rats or their pelts can also be placed in a sealed clear glass or plastic container and refrigerated overnight, then examined against a black background.
Control of acariasis is similar to that for other parasitic metazoa, and acariasis can be eliminated in individual rats with ivermectin (West et al., 1992). However, the source of the original infestation should be identified, and the premises thoroughly disinfected, so as to prevent reinfestation. Ivermectin is not effective against eggs, which can persist for long periods in the environment. Infestation can also be eliminated by rederivation and is readily excluded by proper adherence to modern practices of barrier room technology (Weisbroth, 1979).
f. Lice
Pediculosis in the laboratory rat is currently rare and is attributed to only one species, Polyplax spinulosa (Flynn, 1973f; Owen, 1992b). Polyplax spinulosa females are approximately 0.6–1.5 mm long; females are larger than males. Like all insects, they have six legs. The female lays eggs, called nits, which are cemented to hairs. The eggs have a distinct operculum, with a row of pores near the operculated end. The eggs hatch by a pneumatic mechanism in 5–6 days; the larvae ingest air through the pores, pass it through the body, and then use that pressure to force open the operculum (Owen, 1992b). The young nymphs are paler than the yellow-brown adults but are morphologically similar. After three ecdyses, or molts, they become adults. Depending on environmental conditions, the ecdyses require 1–3 weeks. The entire life cycle is completed in 2–5 weeks. Adults live only 25–28 days. Transmission is by direct contact (Hsu, 1979).
Pediculosis is usually inapparent, although heavily parasitized animals may appear unthrifty and pruritic. Polyplax spinulosa is also the vector of Haemobartonella muris (National Research Council, 1991b). Diagnosis is by direct examination of the pelt for adults, nymphs, and eggs (Hsu, 1979). Any time that infestation with P. spinulosa is detected, blood smears should be screened for Haemobartonella muris.
Pediculosis is prevented by introducing only animals free of the condition. Pyrethrins or organophosphates may be used effectively to treat infestations (Hsu, 1979) but would probably be advisable only in especially valuable rats in the absence of significant intercurrent infections.
4. Fungal Infections
Fungal infections in the rat have been infrequently reported and are associated with predisposing factors that reduce immunocompetence. In one report, about one-fifth of Wistar rats on a 2-year carcinogenesis study had chronic rhinitis associated with Aspergillus fumigatus (Rehm et al., 1988). The predisposing factor in these animals was thought to be Sendai virus infection. Clinical signs included sniffing and nasal exudation. At necropsy, yellowish, friable material was present either unilaterally or bilaterally in the nasal cavities, and in the most severe cases, the nasal cavities were completely blocked. The A. fumigatus–induced rhinitis was, in most cases, limited to the naso- and maxilloturbinates. A bronchial abscess containing hyphae and multiple fruiting heads occurred in one rat.
Tracheobronchial aspergillosis was reported in an aged F-344 rat with concomitant large granular-cell leukemia. Immunodeficiency due to the leukemia was thought to be involved with the multifocal, transmural necrotic lesions of the trachea and bronchi (Hubbs et al., 1991).
Pneumocystis carinii is classified as a fungus based upon DNA base sequences in genes encoding ribosomal RNAs (Feldman et al., 1996). This agent is latently present in the lungs of immunocompetent laboratory rats and humans, causing pneumonitis in hosts that are severely immunosuppressed. The immunocompromised rat is commonly used as a model of P. carinii pneumonitis that occurs in AIDS patients (Oz and Hughes, 1996). The agent is naturally acquired by rats through airborne transmission (Hughes, 1982) and is commonly present latently in rats from both conventional and barrier-maintained commercial sources. Diagnosis of infection in immunocompetent rats usually requires at least 6 weeks of treatment with a corticosteroid or cyclophosphamide to elicit a histologically detectable level of infection. Special stains such as methenamine silver demonstrate the fungal cysts within the alveoli. More recently, polymerase chain reaction (PCR) has been used to detect P. carinii infection in rat lungs consistently after only 1 week of treatment with corticosteroids or cyclophosphamide (Feldman et al., 1996). Pneumocystis carinii has a life cycle consisting of four morphologically distinct stages: trophozoite (1.5–2 μm), precyst (2–4 μm), cyst (5–7 μm), and sporozoites (1–1.7 μm) that develop within cysts (National Research Council, 1991a). Histopathology may vary from multifocal alveolar aggregates of cysts with interstitial and perivascular infiltrates in less severe cases to pulmonary consolidation with foamy, eosinophilic, honeycombed alveolar exudation and severe interstitial fibrosis (GV-SOLAS, 1999). Control of the disease in immunocompromised rats can be achieved by treatment with trimethoprim-sulfamethoxazole.
Royals et al. (1999) reported 2 cases of fungal-induced rhinitis in rats that had no known immunosuppression. Corncob and hardwood bedding from 2 sources were tested to determine if the source of the Aspergillus infection was bedding material. A range of 700 to 5400 fungal spores per gram of nonautoclaved corncob bedding was found. Six genera of fungi (Cladosporidium, Acremonium, Penicillium, Aspergillus, Fusarium, and Scolobasidium) were isolated from the samples of corncob bedding, whereas only negligible counts were isolated from hardwood bedding samples. The authors suggested that either the use of autoclaved or γ-irradiated corncob bedding should be considered as a means to eliminate fungal contamination of bedding.
Dermatomycosis (ringworm) due to Trichophyton mentagrophytes has been reported in wild and laboratory rats. However, it has not been reported in laboratory rats for many years. In rats, dermatomycosis may be presented clinically by patchy hair loss and scurfy or erythematous papular-pustular lesions (Weisbroth, 1979).
B. Noninfectious Diseases
1. Metabolic and Nutritional Diseases
a. Genetic Anomalies
One investigator's meat (desirable trait) is another investigator's poison. Every stock and strain of laboratory rat has been carefully selected for specific genetic traits for decades. Common among these are albinism, behavioral characteristics, such as docility and willingness to breed in captivity, and certain tumor profiles.
Overt metabolic diseases such as obesity (Zucker rat), diabetes (BB rat), and hypertension (SHR and fawn-hooded and Dahl rats) make these strains valuable models in biomedical research, whereas spontaneous appearance of the same characteristics in outbred stocks may complicate other research studies. More subtle strain-related tendencies, such as immunologic responsiveness characteristics in Brown Norway and Lewis rats, are exploited by researchers in particular areas of research. In recent years, genetic manipulation has allowed further development of specifically tailored metabolic disease to model critical human defects. It is beyond the scope of this chapter to catalog the innumerable genetic traits or strain-related variations that occur in laboratory rats, and the reader is encouraged to consult large electronic databases, such as the National Library of Medicine, for specific and current information on particular genes, strains, and conditions.
In addition to known and characterized, spontaneous or induced, genetic variation in rats, isolated colonies of breeding rats inevitably experience some degree of genetic drift. Although this may be monitored to some degree in inbred rats by molecular techniques such as restriction fragment length polymorphisms, it is more difficult to assess the degree to which it has occurred in outbred stocks, where expected interindividual variation may obscure intercolony differences. Nonetheless, any two colonies started from the same source will vary increasingly with time unless there is a sufficient and ongoing exchange of breeders between the colonies. Genetic drift can also be reduced by careful adherence to specific outbreeding programs, such as line breeding with systematic exchange of breeders between multiple lines.
The inevitability of some degree of genetic drift should not, however, blind researchers to the large role played by environmental, husbandry, dietary, and experimental variables in apparent differences between succeeding groups of animals. These extraneous factors can also have a major impact on the expression of underlying genetic traits. An example of modification of lesion prevalence is the impact of ad libitum overfeeding on increasing the incidence of progressive renal disease (Keenan et al., 1996, 1998).
b. Nutritional deficiencies.
Frank dietary deficiencies are uncommon, probably for several reasons. First, high-quality commercial diets are in almost universal use. Second, rats store fat-soluble vitamins and vitamin B12, manufacture vitamin C, and can fulfill many of their requirements for other B vitamins by coprophagy. However, heat and moisture, such as are associated with autoclaving, can reduce vitamin levels, particular lysine, vitamin A, vitamin E, riboflavin, and thiamin. Prolonged storage can have similar effects. In addition, diets designed for maintenance of adult rodents may be too low in protein and fat for optimal growth of young animals or successful reproduction. Clinical evidence of dietary insufficiency may include decreased reproductive performance, litter loss, poor growth, and sparse hair coat. Signs of severe deficiencies of specific vitamins are rare. If they occur, they would include squamous metaplasia of salivary ducts with hypovitaminosis A, disseminated hemorrhage with hypovitaminosis K, and embryonic death and testicular degeneration with hypovitaminosis E. Nutritional deficiencies can also alter disease susceptibility and severity.
In addition, feed qualities, aside from total levels of calories and specific nutrients, must be considered, including contaminating chemicals, microbes, and the size and hardness of pellets. For example, feeding a powdered diet will result in an increased incidence of malocclusion.
2. Management-Related Diseases: Nonnutritional
There is essentially no limit to the number of health problems that may be caused by suboptimal care and management, including those relating to experimental manipulations. Only a few of the most common will be mentioned. Sanitation of the animal's cage, bedding, water, and feed, as well as of experimental equipment, is critical. High moisture content in bedding leads to rapid growth of bacteria, which can increase the incidence of urinary tract infections and, possibly, mastitis and skin lesions. Some softwood bedding materials emit aromatic compounds that may increase hepatic microsomal levels, although these compounds are usually completely sublimated during the drying phase in bedding manufacture. Some organisms are airborne and can enter or spread within a facility via air currents. Considerations of air quality might also include factors such as ammonia, other bedding gases, dusts, fungal spores, disinfectant vapors, and pollutants. Management consideration must be given to organisms carried by human caretakers and investigators. Humans are commonly colonized by, and can transmit, Streptococcus spp, Staphylococcus spp., Escherichia coli, Klebsiella spp., Pseudomonas spp., Campylobacter spp., and so on. In addition, humans can transmit viruses that may result in serologic cross-reactions, if not outright infection, and can also serve as fomites for many other organisms.
Rats are sensitive to temperature, humidity, noise, light, room activity, and generally to any changes in their environment. Low humidity, considered to be relative humidity of less than 40%, together with high temperature, has been linked to the poorly characterized condition known as ringtail. Unfortunately, experimental reproduction of ringtail has not been reported, nor has the pathogenesis of the condition been elucidated. Ringtail is primarily a condition of young rats, usually sucklings, characterized by the formation of prominent annular constrictions of the tail and occasionally of the digits. Portions of affected extremities distal to the constrictions often become necrotic and are sloughed. This condition should not be confused with bite wounds or the normal, more subtle annulations of rat tails which develop with age.
The hearing range of rats has been given as 0.25–76 kHz (Sales and Milligan, 1992); correspondingly many vocalizations of rats also are in the ultrasonic range. Therefore, low audible noise levels for humans do not indicate that noise levels are acceptable to rats. Rats, especially albino rats are susceptible to retinal degeneration when exposed to ambient light levels above a threshold of between 130 and 270 lux (Semple-Rowland and Dawson, 1987). As a result, recommended room light levels are 325–400 lux (National Research Council, 1996b). Note that these recommended levels are as measured 1 m above the floor and do not necessarily reflect actual light levels to which rats are exposed in individual cages at various levels in racks at varying distances from light fixtures. Exposure to very high light levels of 1600 lux for 12 hours each day for 8 days resulted in necrosis in Harderian glands (Kurisu et al., 1996). In addition, exposure to constant light may cause anestrus and other breeding problems as described in Section II,D,3. Exposure (contamination) during the dark phase of the light cycle, with light levels as low as 0.21 lux, has been reported to interfere with growth and metabolism of tumors (Dauchy et al., 1997).
C. Traumatic and Iatrogenic Diseases
Traumatic lesions are uncommon in the rat. Rats housed in wire-bottom cages may develop pododermatitis or lesions on their hocks if housed long-term in such caging. Occasionally, wire-grid floors will allow a rat's foot to become entrapped in the wire grid causing severe edema and injury to the foot and leg. One of the authors (DK) has observed this most often when rats are allowed to recover from anesthesia in a wire-bottom cage. Group housing of rats is much less likely to result in traumatic injuries due to fighting than is seen in mice. Ulcerative dermatitis, associated with Staphylococcus aureus and self-induced trauma from scratching, has been reported (Fox et al., 1977; Wagner, 1977). In one report (Fox et al., 1977), the skin lesions were observed only in rats originating from two breeding colonies of one commercial vendor, leading to the hypothesis that the lesions may have been associated with specific Staphylococcus phage types or host susceptibility factors.
Adynamic ileus, sometimes leading to death, may occur subsequent to intraperitoneal administration of chloral hydrate. Clinical signs occur several days after anesthesia and include lethargy, anorexia, and abdominal distension. The most prominent dilatation occurs in the jejunum, ileum, and cecum. The usual anesthetic dose of chloral hydrate is 400 mg/kg; however, the concentration of the drug, not the dosage, appears to be correlated with the induction of ileus (Fleischman et al., 1977).
D. Neoplastic Diseases
The prevalence of neoplastic disease in the rat is well defined because this species has been routinely used for decades in large-scale carcinogenic, aging, and toxicological studies. Stock and strain-specific differences in the prevalence of some types of tumors are well documented (MacKenzie and Garner, 1973; Burek, 1978; Goodman et al., 1979, 1980). However, the overall prevalence of neoplasia and that of specific tumor types may vary considerably within stocks or strains because of genetic variation, environmental influences, and differences in laboratory methodologies and diagnostic criteria (MacKenzie and Garner, 1973; Altman and Goodman, 1979). The age at which rats are surveyed is also important, because most tumors, other than mammary gland fibroadenomas in many stocks and testicular tumors in F-344 rats, occur in animals greater than 18 months old (Kohn and Barthold, 1984). Table VI compares the incidence of the most frequently occurring tumors in Sprague-Dawley and F-344 rats.
Table VI.
Incidence of Most Prevalent Tumors in Two-Year-Old Rats a
| Incidence (%) |
||||
|---|---|---|---|---|
| Sprague-Dawley |
F-344 |
|||
| (Crl:CDBR) |
(CDF/CflBR) |
|||
| Organ/tissue | Male | Female | Male | Female |
| Testes | ||||
| Interstitial cell tumor | 4.8 | − | 78.3 | |
| Uterus | ||||
| Endometrial stromal polyp | − | 4.1 | − | 14.3 |
| Ovary | ||||
| Granulosa cell/theca cell tumor | − | 1.0 | − | 0.8 |
| Mammary gland | ||||
| Fibroadenoma | 2.0 | 31.4 | 2.5 | 12.0 |
| Carcinoma | 1.0 | 17.7 | 0 | 1.5 |
| Liver | ||||
| Hepatocellular adenoma/carcinoma | 6.8 | 2.6 | 1.4 | 1.0 |
| Lymphoreticular | ||||
| Large granular lymphocytic leukemia | 0.2 | 0.3 | 16.5 | 10.4 |
| Histiocytic sarcoma | 1.6 | 1.5 | 0.6 | 0 |
| Pituitary | ||||
| Adenoma/carcinoma, pars distalis | 67.1 | 82.6 | 16.3 | 19.7 |
| Adrenal gland | ||||
| Cortical adenoma | 2.9 | 6.0 | 0.4 | 1.0 |
| Pheochromocytoma, benign | 15.0 | 3.9 | 6.7 | 0.9 |
| Pheochromocytoma, malignant | 1.9 | 0.6 | 0.5 | 0.3 |
| Pancreas | ||||
| Islet cell adenoma | 8.3 | 3.8 | 9.3 | 2.0 |
| Islet cell carcinoma | 2.0 | 1.4 | 0.6 | 0 |
Adapted from Lang (1990, 1992).
Among the environmental influences, diet has been found to be an extremely important factor in modulating tumor prevalence. A 20% reduction in food intake was found to significantly reduce the overall tumor incidence in male and female Wistar rats, primarily to differences in the incidence of pituitary and mammary gland tumors (Tucker, 1979). In another study (Morris and Bras, 1971), tumor incidence in Sprague-Dawley rats was compared among 3 groups: rats fed ad libitum, rats food-restricted throughout life, and rats food-restricted between 21 and 70 days of age. Both food-restricted groups had a significantly reduced prevalence of tumors. The proportion of rats in the nonrestricted group with multiple tumor types was 16.7%, about twice that as for each of the other two groups. Interestingly, the group that was food-restricted up to day 70 had a persistent reduction in food consumed thereafter on an ad libitum diet.
Another environmental influence on the prevalence of tumors is the pathogen or disease status of the rats in a particular report. Data on tumor risk can be significantly influenced by the effect that some infectious diseases may have on longevity, preneoplastic changes, and masking of small tumors (Kohn and Barthold, 1984).
1. Mammary Gland Tumors
Mammary gland tumors are the most frequently occurring tumors in most stocks and strains of rats. Sprague-Dawley stocks often have an incidence of 50% in aged female animals, whereas F-344 have a relatively low incidence of about 15% (Goodman et al., 1979; Lang, 1990, 1992). Most mammary tumors are benign fibroadenomas, with carcinomas occurring less frequently. Both types can occur in aged males; however, the incidence is usually less than 1%. The tumors may arise in mammary tissue at any point from the neck to the inguinal area, and they tend to attain a large size and become ulcerated unless surgically excised. On gross examination, fibroadenomas are freely movable in subcutaneous tissues, circumscribed, firm, and lobulated. Histologically, they are characterized by well-differentiated acinar epithelial components surrounded by inter- and intralobular connective tissue components (Altman and Goodman, 1979; Percy and Barthold, 1993).
2. Testicular Tumors
Interstitial cell tumors occur in about 80% of aged F-344 rats (Goodman et al., 1979; Lang, 1990). They are discrete, soft, and yellow to brown, with areas of hemorrhage, and may occur in multiple sites unilaterally or bilaterally. Histologically, their Leydig's cell origin is apparent. Tumors have two cell types that are arranged in solid sheets or in an organoid pattern. The cell types are (1) polyhedral to elongated cells with granular to vacuolated cytoplasm and (2) smaller cells with hyperchromatic nuclei and scanty cytoplasm (Altman and Goodman, 1979; Percy and Barthold, 1993). The incidence of interstitial cell tumors in most other stocks and strains is quite low.
3. Pituitary Tumors
Pituitary tumors occur frequently in aged rats of some stocks and strains, most notably in Sprague-Dawley and Wistar rats (Percy and Barthold, 1993). Most pituitary tumors are classified as chromophobe adenomas, originating from the pars distalis. Carcinomas of the pars distalis are reported with much less frequency; however, their reported prevalence may vary considerably because of differences in classification protocols by pathologists. Surveys reflect an incidence in F-344 rats of about 20% (Goodman et al., 1979; Lang, 1990), and a 75% incidence in Sprague-Dawley rats (Lang, 1992). In some reports, the prevalence of pituitary tumors is greater in female F-344 and Sprague-Dawley rats. As was previously noted, diet restriction significantly reduces the incidence of pituitary tumors in rats.
Chromophobe adenomas vary in size, often reaching 0.5 cm in diameter. Grossly, the tumors are soft and dark red due to prominent hemorrhagic areas (Fig. 10 ). They are well circumscribed and, because of their size, often compress adjacent brain tissue and induce hydrocephalus. Microscopically, they consist of large polygonal cells with prominent vesicular nuclei and eosinophilic cytoplasm. The architecture of the tumors consists of cells arranged in nests, cords, or sheets separated by vascular sinusoids (Altman and Goodman, 1979).
Fig. 10.
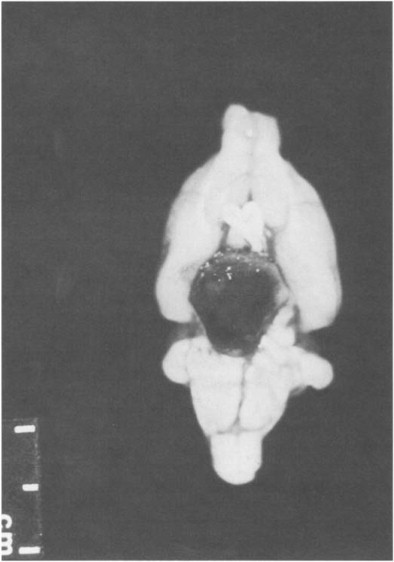
Chromophobe adenoma of the brain of a rat. The large hemorrhagic mass is visible on the ventral aspect of the brain.
(Courtesy of Dr. Robert Jacoby.)
4. Adrenal System
Lang (1990, 1992) reported a cortical adenoma incidence of 2.9% in male and 6% in female Sprague-Dawley rats, and a benign pheochromocytoma incidence of 15% in males and 3.9% in females of this stock. The incidence in F-344 rats was less than half that seen in Sprague-Dawley rats (Table VI).
5. Pancreas
Pancreatic islet cell tumors are relatively common in some stocks of rats. An incidence of 10.3% was reported in male, and
5.2% in female Sprague-Dawley rats, and the incidence in F-344 was somewhat less (Lang, 1990, 1992). Grossly, islet cell tumors may be either single or multiple and are circumscribed and reddish brown. Islet cell carcinomas are distinguished from adenomas by capsular invasion and metastases. Tumors of the exocrine pancreas are rare (Altman and Goodman, 1979).
6. Lymphoreticular System
Large granular lumphocytic leukemia is a major cause of death in F-344 rats (Ward and Reynolds, 1983), with a reported incidence of 10–16% (Coleman et al., 1977; Goodman et al., 1979). The initial site of malignancy is thought to be the spleen. The neoplastic cells are transplantable to rats of the same strain. Unlike leukemia in mice, this leukemia in rats is not associated with a retrovirus. Diagnosis is based upon clinical signs of anemia, jaundice, weight loss, and laboratory findings of splenomegaly, elevated leukocyte counts of 70,000–180,000/ml, and diffuse infiltration of malignant lymphocytes in various organs (Percy and Barthold, 1993). In the Sprague-Dawley rat and most other stocks, the incidence is quite low.
Other lymphoreticular tumors include histiocytic sarcoma, which has an incidence in Sprague-Dawley rats of about 1.5% (Lang, 1992), and myelomonocytic leukemia in BN/Bi rats, with an incidence of 5% in females and 11% in males (Burek, 1978).
E. Miscellaneous Conditions
1. Congenital/Hereditary Lesions
It is beyond the scope of this chapter to catalog congenital defects in rats. The incidence of such defects is obviously influenced by administration of mutagenic and teratogenic substances, but it also varies with strain, age of mother, disease status, coincidences of statistics, and human terminology. As noted above, rats are susceptible to a wide variety of genetic diseases, some of which make them valuable models and others of which are confounding variables. Only a few spontaneous defects, involving the urinary tract, heart, and central nervous system, will be mentioned here. The growing range of genetically engineered diseases, and their unintentional side effects, will not be addressed. Researchers and supporting animal resource professionals are strongly urged to investigate, with due scientific scrutiny, background information concerning specific stocks and strains, prior to embarking on courses of research involving any laboratory animal. Large databases of defects observed in reproductive studies are available from the Middle Atlantic Reproduction and Teratology Association and Midwest Teratology Association (1996), for example, and should be consulted.
Hydronephrosis is one of the more commonly reported congenital defects of rats, characterized by unilateral or bilateral dilation of the renal pelvis. Although it may be inherited as a single dominant gene in the Gunn rat, it appears to be polygenic in the Brown Norway and Sprague-Dawley rat (Van Winkle et al., 1988). The right kidney is affected more often than the left. Severity of hydronephrosis can vary from a slight dilation of the renal pelvis to such severe dilation that the kidney appears as a transparent cystic structure. The ureter may also be affected to varying degrees. The normal renal pelvis of young animals may appear to be dilated, however, so some caution is required in identifying hydronephrosis (Maronpot, 1996). Hydronephrosis may also be mistaken for pyelonephritis, in which the material in the dilated pelvis is typically cloudy; for polycystic kidneys; and for renal papillary necrosis. Culture and histopathology of the affected site will distinguish among these conditions.
Congenital lesions of the cardiovascular system are less frequently reported but include ventricular and atrial septal defects, dextrocardia, and defects of the valves and endocardial cushion, as well as various anomalies of the great vessels. Overall incidence of cardiac defects has been estimated in one colony of Sprague-Dawley rats at 2.3% (Johnson et al., 1993).
The most common anomaly of the central nervous system of the rat is dilation of the cerebral ventricles (hydrocephalus), estimated at 2.6% in Sprague-Dawley rats (Middle Atlantic Reproduction and Teratology Association and Midwest Teratology Association, 1996). Seizures have also been reported in a variety of stocks and strains of rat but have been reported most frequently in various Wistar stocks (Nunn and MacPherson, 1995). Wistar rats are especially used in investigation of audiogenic seizures (Garcia-Cairasco et al., 1998).
Congenital and genetically determined ocular defects are very common in some strains of rats. In albino rats, the lack of a pigmented tapetum predisposes for the development of retinal atrophy. Fischer rats (F-344) have an incidence of corneal mineralization that varies from 10 to 100%, depending on subline (Bruner et al., 1992; Yoshitomi and Boorman, 1990). This is characterized by deposition of calcium salts, often visible in routinely stained sections as basophilic granules, along the interface of the corneal epithelium and the stroma. Other ocular abnormalities reported in laboratory rats include retinal degeneration, cataracts, osseous and cartilaginous metaplasia of the sclera, and colobomas.
Several abnormalities of the reproductive tract have been reported in laboratory rats, including transverse vaginal septum in female Wistar and Sprague-Dawley (Barbolt and Brown, 1989; De Schaepdrijver et al., 1995). Affected animals are functionally sterile if the septum is complete, and subfertile if the septum only partially prevents spermatozoa from entering the uterus. Pseudohermaphroditism is occasionally observed in rats, most often male pseudohermaphroditism, also known as testicular feminization; i.e., testes are present internally, but the external genitalia are approximately female. Affected rats are karyotypically XY but express the default feminine phenotype. Although mutant strains have been selected for this characteristic (Allison et al., 1965), it is also occasionally observed in other strains as well. In the testicular-feminized rat (tfm), the defect is a lack of androgen receptors due to a point mutation (Yarbrough et al., 1990), although defects in other genes could potentially result in similar syndromes.
Brown Norway rats have a high incidence of eosinophilic granulomatous pulmonary inflammation, nearing 100% incidence in both males and females at 3–4 months of age. Brown Norway rats from colonies worldwide are affected, including those maintained in isolators. Affected colonies are seronegative for all known agents, and rats of other strains maintained with the Brown Norway rats do not develop lung lesions. The lung lesions are scattered throughout the parenchyma and are characterized by generally well-organized granulomas of Langhans’ giant cells, macrophages, and eosinophils. No foreign material, fungi, or bacteria are routinely visible or can be demonstrated by polarized light or special stains.
2. Age-Related Diseases
Laboratory rats are subject to a wide range of neoplastic and nonneoplastic age-related diseases, as are most aging mammals. Because of the use of rats in 2-year carcinogenicity studies, and as models of gerontology for humans, diseases of the geriatric rat have particular significance to the laboratory animal professional. The type, incidence, and severity of these lesions vary greatly with stock or strain of rat, infectious disease status, experimental manipulation, and husbandry practices, including dietary restriction. Only a few of the most common nonneoplastic conditions will be discussed here, and readers are encouraged to consult the scientific literature, including excellent reviews for additional information concerning the particular stock or strain with which they are concerned (Mohr et al., 1992; Boorman et al., 1990). Neoplastic conditions are afforded a separate section in this chapter.
Chronic progressive nephropathy (CPN) is the most important age-related disease of rat kidneys and is among the most common causes of death in rats in lifetime studies. Synonyms abound, including chronic progressive nephrosis and old rat nephropathy. The condition is more common in males than in females and is progressive, as correctly indicated by its appellation. Gross lesions of CPN are first observed in rats more than 6 months of age and are characterized by pitting of the cortical surface. Because of cortical interstitial fibrosis, removal of the renal capsule may tear the cortical parenchyma. As it becomes more severe in rats more than 1 year of age, the cortical surface becomes increasingly irregular and may develop areas of pallor. Microscopically, glomerular changes are characterized by thickened basement membranes, thickening of the capillary tufts, adhesions to the parietal layer of Bowman's membrane, and segmental glomerulosclerosis (Short and Goldstein, 1992). As the disease advances, numerous tubules in both the cortex and medulla are often dilated and filled with eosinophilic proteinaceous casts. Secondary hyperparathyroidism may occur subsequent to renal functional compromise in advanced cases, resulting in widespread dystrophic mineralization. The etiopathogenesis of CPN is poorly understood and is probably multifactorial. However, several of the major contributing factors have been described (Barthold, 1996b; Percy and Barthold, 1993b). First, the reported incidence varies with strain. This indicates probably at least some genetic predisposition for the development of CPN. Sprague-Dawley and F-344 rats have high incidences, whereas Wistar and Long-Evans stocks have a lower incidence. Reported incidences, however, are difficult Ito interpret, because of geographic variation in use of different stocks and strains (Wistar rats have been used more predominantly in Europe, and Sprague-Dawley rats in the United States), which could lead to other factors, such as housing and diet, actually causing what otherwise appears to be a strain-related change. For example, when many of the reports of the incidence of CPN in European rats were published, rats were housed 5 per cage, which is known to result in decreased feed consumption and decreased weight gain, relative to single housing. Second, gender is a determining factor in the development of CPN. Male rats have an earlier onset, higher incidence at any given age, and greater severity of lesions than do females. Third, diet is a critical factor and is also the factor that may be the most amenable to management solutions. It is now clear that moderate dietary restriction will greatly reduce the incidence and severity of CPN at any given age, relative to ad libitum overfeeding. The mechanism is hypothesized to be that overfeeding results in prolonged increases in renal blood flow and glomerular filtration rate (Gumprecht et al., 1993). These increases cause glomerular hypertrophy, leading to macromolecule filtration deficits, mesangial damage, glomerulosclerosis, and protein leakage. Whatever the mechanism, however, 25–30% reduction in caloric intake, relative to ad libitum, results in decreased incidence and severity of CPN in female rats, and decreased severity of CPN in male rats, as well as increased survival in both sexes (Keenan et al., 1995a).
Nephrocalcinosis is defined as the deposition of calcium phosphate in renal tissue, although a variety of additional terms are sometimes employed to reflect the localization of the mineral in the cortex, medulla, and so on. In contrast to CPN, which is more common in males, nephrocalcinosis is more common in female rats. In addition to gender, the incidence varies with age and strain and may occur in F-344 rats as young as 7 weeks old. The incidence in F-344 rats may reach 50%, whereas the lower incidence of 0–7% is reported in stocks of Sprague-Dawley and Wistar rats (Montgomery and Seely, 1990). An especially high incidence is observed in BDIX rats. The incidence and severity of nephrocalcinosis may be increased by several dietary manipulations, including high levels of calcium, high phosphorus, low calcium/phosphorus ratios, or low magnesium (Percy and Barthold, 1993b). However, it is not clear if dietary levels of these minerals are a key determining factor in the background incidence of nephrocalcinosis. Histologically (Short and Goldstein, 1992), mineral deposition is observed most frequently at the corticomedullary junction, in cells of the pars recta and thin loops of Henle, as well as in the lumen of these tubules.
Chronic myocardial disease is a major cause of death in aged male rats of multiple strains, including Sprague-Dawley, when fed ad libitum (Keenan et al., 1995b). The condition is often known as cardiomyopathy, or chronic progressive cardiomyopathy, and may be observed as early as 3 months of age. Grossly, the heart is enlarged, occasionally with pale streaks visible. Increased weight of the heart correlates well with the degree of damage observed on histologic examination. Microscopically (Lewis, 1992), there is necrosis of myocardial fibers and an interstitial infiltration of mononuclear cells. Later in the course of the disease, fibrosis may be more prominent. Large reactive nuclei are also observed in myofibers. The most commonly affected myocardial sites are the papillary muscles and interventricular septum. As with chronic progressive nephropathy, the incidence of chronic progressive cardiomyopathy can be dramatically reduced at any age by moderate dietary restriction, i.e., reduction of 25–30% of total caloric intake relative to ad libitum overfed rats (Keenan et al., 1995b).
Changes in skin and pelage are often observed but rarely reported in geriatric laboratory rats, which may cause concern to the inexperienced observer. The most common change is thinning or loss of hair, especially over the back (Elwell et al., 1990). This may be observed in any stock or strain but is especially common in the Brown Norway rat. Old albino rats also have a more yellow appearance at times, because of the accumulation of sebum in the skin. The rings of scales covering the tail increase in number with age to 190 at 1 year (English and Munger, 1992). They continue to become more prominent and more yellowed with time after that. The yellowish material which accumulates on the tail and adjacent to the ear also may become black with time, probably from oxidation and/or bacterial action. In addition, male rats accumulate brown-pigmented foci on the skin, termed scales (Tayama and Shisa, 1994). These scales can be detached and can overlay skin of “normal” color. They are found on the dorsum, with some on the tail and perineum. Scale formation is abrogated by gonadectomy. The nature of the pigment is unclear, but it may be oxidized lipid or amino acids.
Alveolar histiocytosis is a very common incidental finding in the lung of aging rats of many stocks and strains (Dungworth et al., 1992). Grossly, alveolar histiocytosis is visible as white to pale tan foci, usually about 1 mm in diameter, visible on the pleural surface. The foci may extend slightly above the pleural surface in uninflated lung. Microscopically (Boorman and Eustis, 1990), clusters of alveoli, often in a subpleural locations or adjacent to a terminal bronchiole, contain increased numbers of large, pale, foamy-appearing macrophages. Occasionally, cholesterol clefts may be visible in the more dense aggregates of macrophages, and a slight infiltration of lymphocytes may be present around adjacent vessels, probably as a response to proinflammatory mediators released by the macrophages. Alveolar histiocytosis should not be mistaken for any of the viral pneumonias of rats, because affected animals are seronegative, and any lymphoid infiltrate is slight and localized to the areas of macrophage aggregation. The cause of alveolar histiocytosis is not known, but it does not appear to be infectious.
REFERENCES
- Allison J.E., Stanley A.J., Gumbreck L.G. Sex chromatin and idioigrams from rats exhibiting anomalies of the reproductive organs. Anat. Rec. 1965;153:85–91. doi: 10.1002/ar.1091530110. [DOI] [PubMed] [Google Scholar]
- Altman N.H., Goodman D.G. In: Baker H.J., Lindsey J.R., Weisbroth S.H., editors. Academic Press; New York: 1979. pp. 334–376. (“The Laboratory Rat,” Vol. 1. “Biology and Diseases”). [Google Scholar]
- Amao H., Komukai Y., Akimoto T., Sugiyama M., Takahashi K.W., Sawada T., Saito M. Natural and subclinical Corynebacterium kutscheri infection in rats. Lab. Anim. Sci. 1995;45:11–14. [PubMed] [Google Scholar]
- Anderson L.C., Leary S.L., Manning P.J. Rat-bite fever in animal research laboratory personnel. Lab. Anim. Sci. 1983;33:292–294. [PubMed] [Google Scholar]
- Antonakoipoulos G.N., Turton J., Whitefield P., Newman J. Hostparasite interface of the urinary bladder-inhabiting nematode Trichosomoides crassicauda: Changes induced in the urothelium of infected rats. Int. J. Parasitol. 1991;21:187–193. doi: 10.1016/0020-7519(91)90009-v. [DOI] [PubMed] [Google Scholar]
- Ayala M.E., Monroy J., Morales L., Castro M.E., Dominguez R. Effects of a lesion in the dorsal raphe nuclei performed during the juvenile period of the female rat, on puberty. Brain Res. Bull. 1998;47:211–218. doi: 10.1016/s0361-9230(98)00074-4. [DOI] [PubMed] [Google Scholar]
- Baker D.E.J. In: Baker H.J., Lindsey J.R., Weisbroth S.H., editors. Academic Press; New York: 1979. pp. 153–168. (“The Laboratory Rat,” Vol. 1 “Biology and Diseases”). [Google Scholar]
- Barbolt T.A., Brown G.L. Vaginal septum in the rat. Lab. Anim. 1989;18:47–48. [Google Scholar]
- Barthold S.W. In: Urinary System. 2nd ed. Jones T.C., Hard G.C., Mohr U., editors. Springer; Berlin: 1996. pp. 463–465. [Google Scholar]
- Barthold S.W. In: Urinary System. 2nd ed. Jones T.C., Hard G.C., Mohr U., editors. Springer-Verlag; Berlin: 1996. pp. 416–418. [Google Scholar]
- Barthold S.W., Brownstein D.G. The effect of selected viruses on Corynebacterium kutscheri infection in rats. Lab. Anim. Sci. 1988;38:580–583. [PubMed] [Google Scholar]
- Bennett J.P., Vickery B.H. In: Reproduction and Breeding Techniques for Laboratory Animals. Hafez E.S.E., editor. Len and Febiger; Philadelphia: 1970. pp. 299–315. [Google Scholar]
- Besch-Willford C.L., Wagner J.E., Templeman C.C. Establishment of a commercial production colony of Sprangue-Dawley rats free of Sendai virus. Lab. Anim. Sci. 1987;37:666–667. [PubMed] [Google Scholar]
- Besselsen D.G., Besch-Williford C.L., Pintel D.J., Franklin C.L., Hook R.R., Jr., Riley L.K. Detection of H-1 parvovirus and Kilham rat virus by PCR. J. Clin. Microbiol. 1995;33:1699–1703. doi: 10.1128/jcm.33.7.1699-1703.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besselsen D.G., Besch-Willford C.L., Pintel D.J., Franklin C.L., Hook R.R., Riley L.K. Detection of newly recognized rodent parvoviruses by PCR. J. Clin. Microbiol. 1995;33:2859–2863. doi: 10.1128/jcm.33.11.2859-2863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beys E., Hodge T., Nohynek G.J. Ovarian changes in Sprague-Dawley rats produced by nocturnal exposure to low intensity light. Lab. Anim. 1995;29:335–338. doi: 10.1258/002367795781088270. [DOI] [PubMed] [Google Scholar]
- Bihun C.G., Percy D.H. Coronavirus infections in the laboratory rat: Degree of cross protection following immunization with a heterologous strain. Can. J. Vet. Res. 1994;58:224–229. [PMC free article] [PubMed] [Google Scholar]
- Bivin W.S., Crawford M.P., Brewer N.R. Morphophysiology. In: Lindsey J.R., Baker H.J., Weisbroth S.H., editors. Academic Pres; New York: 1979. pp. 74–103. (“The Laboratory Rat,” Vol. 1, “Biology and Diseases”). [Google Scholar]
- Blandau R.J., Boling J.L., Young W.C. The length of heat in the albino rat as determined by the copulatory response test. Anat. Rec. 1941;79:453–463. [Google Scholar]
- Blom H.J.M., Van Tintelen G., Van Vorstenbosch C.J.A.H.V., Baumans V., Beynen A.C. Preferences of mice and rats for types of bedding material. Lab. Anim. 1995;30:234–244. doi: 10.1258/002367796780684890. [DOI] [PubMed] [Google Scholar]
- Boivin G.P., Wagner J.E., Besch-Williford C.L. Use of cyclophosphamide in diagnostic provocation of Tyzzer's disease in hamster. Lab. Anim. Sci. 1990;40:545. (Abstract) [Google Scholar]
- Boorman G.A., Eustis S.L. In: Pathology of the Fischer Rat: Reference and Atlas. Boorman G.A., Eustis S.L., Elwell M.R., Montgomery C.A., MacKenzie W.F., editors. Academic Press; San Diego: 1990. pp. 339–367. [Google Scholar]
- Boorman G.A., Eustis S.L., Elwell M.R., Montgomery C.A., MacKenzie W.F., editors. Pathology of the Fischer Rat: Reference and Atlas. Academic Press; San Diego: 1990. [Google Scholar]
- Boot R., van Knapen F., Kruijt B.C., Walvoort H.C. Serological evidence for Encephalitozoon cuniculi infection (nosemiasis) in gnotobiotic guineapigs. Lab. Anim. 1988;22:337–342. doi: 10.1258/002367788780746296. [DOI] [PubMed] [Google Scholar]
- Brammer D.W., Dysko R.C., Spilman S.C., Oskar P.A. Elimination of sialodacryoadenitis virus from a rat production colony by using seropositive breeding animals. Lab. Anim. Sci. 1993;43:633–634. [PubMed] [Google Scholar]
- Brown H.R., Leininger J.R. In: Mohr U., Dungworth D.L., Capen C.C., editors. Vol. 2. ILSI Press; Washington, D.C.: 1992. pp. 309–322. (Pathobiology of the Aging Rat). [Google Scholar]
- Brudzynski S.M., Chiu E.M. Behavioural responses of laboratory rats to playback of 22 kHz ultrasonic calls. Physiol. Behav. 1995;57:1039–1044. doi: 10.1016/0031-9384(95)00003-2. [DOI] [PubMed] [Google Scholar]
- Brudzynski S.M., Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiol. Behav. 1992;57:1039–1044. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- Brudzynski S.M., Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiol. Behav. 1992;52:655–660. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- Brudzynski S.M., Bihari F., Ociepa D., Fu X.-W. Analysis of 22 kHz ultrasonic vocalization in laboratory rats: Long and short calls. Physiol. Behav. 1993;54:221. doi: 10.1016/0031-9384(93)90102-l. [DOI] [PubMed] [Google Scholar]
- Bruner R.H., Keller W.F., Stitzel K.A., Sauers L.J., Reer P.J., Long P.H., Bruce R.D., Alden C.L. Spontaneous corneal dystrophy and generalized basement membrane changes in Fischer-344 rats. Toxicol. Pathol. 1992;20:357–366. doi: 10.1177/019262339202000306. [DOI] [PubMed] [Google Scholar]
- Burek J.D. CRC Press; Boca Raton, Florida: 1978. (Pathology of Aging Rats). [Google Scholar]
- Burek J.D., Jurcher C., Van Nunen M.C.J., Hollander C.F. A naturally occurring epizootic caused by Sendai virus in breeding and aging rodent clonies. 2. Infection in the rat. Lab. Anim. Sci. 1977;27:963–971. [PubMed] [Google Scholar]
- Carter G.R. In: Krieg N.R., Holt J.G., editors. Vol. 1. Williams and Wilkins; Baltimore: 1984. pp. 552–557. (Bergey's Manual of Systematic Bacteriology). [Google Scholar]
- Carthew P., Aldred P. Embryonic death in pregnant rats owing to intercurrent infection with Sendai virus and Pasteurella pneumotropica. Lab. Anim. 1988;22:92–97. doi: 10.1258/002367788780746566. [DOI] [PubMed] [Google Scholar]
- Carthew P., Slinger R.P. Diagnosis of sialodacryoadenitis virus infection of rats in a virulent enzootic outbreak. Lab. Anim. 1981;15:339–342. doi: 10.1258/002367781780952979. [DOI] [PubMed] [Google Scholar]
- Cassell G.H., Lindsey J.R., Davis J.K., Davidson M.K., Brown M.B., Mayo J.G. Detection of natural Mycoplasma pulmonis infection in rats and mice by an enzyme linked immunosorbent assay (ELISA) Lab. Anim. Sci. 1981;31:676–682. [PubMed] [Google Scholar]
- Cassell G.H., Davis J.K., Simecka J.W., Lindsey J.R., Cox N.R., Ross S., Fallon M.T. In: Viral and Mycoplasmal Infections of Laboratory Rodents: Effects on Biomedical Research. Bhatt P.N., Jacoby R.O., Morse H.C., New A.E., editors. Academic Press; Orlando, Florida: 1986. pp. 87–130. [Google Scholar]
- Childs J.E., Glass G.E., Korch G.W., Arthur R.R., Shah K.V., Glasser D., Rossi C., LeDuc J.W. Evidence of human infection with a rat-associated hantavirus in Baltimore, Maryland. Am. J. Epidemiol. 1987;127:875–878. doi: 10.1093/oxfordjournals.aje.a114871. [DOI] [PubMed] [Google Scholar]
- Chmiel D.J., Noonan M. Preference of laboratory rats for potentially enriching stimulus objects. Lab. Anim. 1996;30:97–101. doi: 10.1258/002367796780865790. [DOI] [PubMed] [Google Scholar]
- Chung Y.H., Jun H.S. Role of macrophages and macrophage-derived cytokines in the pathogenesis of Kilham rat virus-induced autoimmune diabetes in diaabetes-resistant BioBreeding rats. J. Immunol. 1997;159:466–471. [PubMed] [Google Scholar]
- Cohen S.M., Anderson T.A., de Oliveira L.M., Arnold L.L. Tumorigenicity of sodium ascorbate in male rats. Cancer Res. 1998;58:2557–2561. [PubMed] [Google Scholar]
- Coleman G.L., Barthold S.W., Osbaldiston G.W., Foster S.J., Jonas A.M. Pathological changes during aging in barrier-reared Fischer 344 male rates. J. Gerontol. 1977;32:258–278. doi: 10.1093/geronj/32.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman G.L., Jacoby R.O. Naturally occurring lethal parvovirus infection of juvenile and young-adult rats. Vet. Pathol. 1983;20:49–56. doi: 10.1177/030098588302000105. [DOI] [PubMed] [Google Scholar]
- Compton S.R., Smith A.L., Gaertner D.J. Comparison of the pathogenicity in rats of rat coronaviruses of different neutralization groups. Lab. Anim. Sci. 1999;49:514–519. [PubMed] [Google Scholar]
- Cornish J., Vanderwee M.A., Findon G., Miller T.E. Reliable diagnosis of Trichosomoides crassicauda in the urinary bladder of the rat. Lab. Anim. 1988;22:162–165. doi: 10.1258/002367788780864385. [DOI] [PubMed] [Google Scholar]
- Craft J.C. Experimental infection with Giardia lamblia in rats. J. Infect. Dis. 1982;145:495–498. doi: 10.1093/infdis/145.4.495. [DOI] [PubMed] [Google Scholar]
- Cundiff D.D., Besch-Williford C.L., Hook R.R., Franklin C.L., Riley L.K. Detection of cilia-associated respiratory bacillus by PCR. J. Clin. Microbiol. 1994;32:1930–1934. doi: 10.1128/jcm.32.8.1930-1934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff D.D., Besch-Williford C.L., Hook R.R., Franklin C.L., Riley L.K. Characterization of cilia-associated respiratory bacillus in rabbits and analysis of the 16S rRNA gene sequence. Lab. Anim. Sci. 1995;45:22–26. [PubMed] [Google Scholar]
- Cundiff D.D., Riley L.K., Franklin C.L., Hook R.R., Besch-Williford C.L. Failure of a soiled bedding sentinel system to detect cilia-associated respiratory bacillus infection in rats. Lab. Anim. Sci. 1995;45:219–221. [PubMed] [Google Scholar]
- Dameron G.W., Weingand K.W., Duderstdt J.M., Odioso L.W., Dierckman T.A., Schwecke W., Baran K. Effect of bleeding site on clinical laboratory testing of rats: Orbital venous plexus posterior vena cava. Lab. Anim. Sci. 1992;42:299–301. [PubMed] [Google Scholar]
- Darrigrand A.A., Singh S.B. Effects of Kilham rat virus on natural killer cell-mediated cytotoxicity in Brown Norway and Wistar-Furth rats. Am. J. Vet. Res. 1984;45:200–202. [PubMed] [Google Scholar]
- Dauchy R.T., Sauer L.A., Blask D.E., Vaughan G.M. Light contamination during the dark phase in “photoperiodically controlled” animal rooms: Effect on tumor growth and metabolism in rats. Lab. Anim. Sci. 1997;47:511–518. [PubMed] [Google Scholar]
- Davidson M.K., Davis J.K., Lindsey J.R., Cassell G.H. Clearance of different strains of Mycoplasma pulmonis from the respiratory tract of C3H/HeN mice. Infect. Immun. 1988;56:2163–2168. doi: 10.1128/iai.56.8.2163-2168.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.K., Cassell G.H. Murine respiratory mycoplasmosis in LEW and F-344 rats: Strain differences in lesion severity. Vet. Pathol. 1982;19:280–293. doi: 10.1177/030098588201900306. [DOI] [PubMed] [Google Scholar]
- DeLong D., Manning P.J. In: The Biology of the Laboratory Rabbit. 2nd ed. Manning P.J., Ringler D.H., Newcomer C.E., editors. Academic Press; San Diego: 1994. pp. 129–170. [Google Scholar]
- Del Vecchio F.R. In: Mohr U., Dungworth D.L., Capen C.C., editors. Vol. 1. ILSI Press; Washington, D.C.: 1992. pp. 331–336. (Pathobiology of the Aging Rat). [Google Scholar]
- De Schaepdrijver L.M., Fransen J.L., Van der Eycken E.S., Coussement W.C. Transverse vaginal septum in the specific pathogen-free Wistar rat. Lab. Anim. Sci. 1995;45:181–183. [PubMed] [Google Scholar]
- Desmyter J., Johnson K.M., Deckers C., LeDuc J.W., Brasseur F., van Ypersele de Strihon C. Laboratory rat associated outbreak of haemorrhagic fever renal syndrome due to Hantan-like virus in Belgium. Lancet. 1983;2:1445–1448. doi: 10.1016/s0140-6736(83)90797-3. [DOI] [PubMed] [Google Scholar]
- Dewsbury D.A. In: Reproduction and Breeding Techniques for Laboratory Animals. Hafez E.S.E., editor. Lea and Febiger; Philadelphia: 1970. pp. 123–136. [Google Scholar]
- Duncan A.J., Carman R.J., Olson G.J., Wilson K.H. Assignment of the agent of Tyzzer's disease to Clostridium piliforme comb. nov. on the basis of 16S rRNA sequence analysis. Int. J. Syst. Bacteriol. 1993;43:314–318. doi: 10.1099/00207713-43-2-314. [DOI] [PubMed] [Google Scholar]
- Dungworth D.L., Ernst H., Nolte T., Mohr U. In: Mohr U., Dungworth D.L., Capen C.C., editors. Vol. 1. ILSI Press; Washington, D.C.: 1992. pp. 143–160. (Pathobiology of the Aging Rat). [Google Scholar]
- Eaton K.A., Dewhirst F.E., Paster B.J., Tzellas N., Coleman B.E., Paola J., Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: Animal and public health implications. J. Clin. Microbiol. 1996;34:3165–3170. doi: 10.1128/jcm.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein B., Golan R., Shani Mishkinsky J. Onset of puberty in the immature female rat induced by 5α-androstane-3β,17β-diol. Endocrinology. 1973;92:941–945. doi: 10.1210/endo-92-3-941. [DOI] [PubMed] [Google Scholar]
- Eiden J., Vonderfecht S. Evidence that a novel rotavirus-like agent of rats can cause gastroenteritis in man. Lancet. 1985;2:8–11. doi: 10.1016/s0140-6736(85)90057-1. [DOI] [PubMed] [Google Scholar]
- Elwell M.R., Stedham M.A., Kovatch R.M. In: Pathology of the Fischer Rat: Reference and Atlas. Boorman G.A., Eustis S.L., Elwell M.R., Montgomery C.A., MacKenzie W.F., editors. Academic Press; San Diego: 1990. pp. 261–277. [Google Scholar]
- Enders A.C. In: Reproduction and Breeding Techniques for Laboratory Animals. Hafez E.S.E., editor. Lea and Febiger; Philadelphia: 1970. pp. 137–156. [Google Scholar]
- English K.B., Munger B.L. In: Mohr U., Dungworth D.L., Capen C.C., editors. Vol. 2. ILSI Press; Washington, D.C.: 1992. pp. 363–389. (Pathobiology of the Aging Rat). [Google Scholar]
- Fallon M.T., Reinhard M.K., Gray B.M., Davis T.W., Lindsey J.R. Inapparent Streptococcus pneumoniae type 35 infections in commercial rats and mice. Lab. Anim. Sci. 1988;38:129–132. [PubMed] [Google Scholar]
- Feely D.E., Chase D.G., Hardin E.L., Erlandsen S.L. Ultrastructural evidence for the presence of bacteria, viral-like particles, and mycoplasma-like organisms associated with Giardia spp. J. Protozool. 1988;35:151–158. doi: 10.1111/j.1550-7408.1988.tb04095.x. [DOI] [PubMed] [Google Scholar]
- Feldman M.L. In: Mohr U., Dungworth D.L., Capen C.C., editors. Vol. 2. ILSI Press; Washington, D.C.: 1992. pp. 121–147. (Pathobiology of the Aging Rat). [Google Scholar]
- Feldman S.H., Weisbroth S.P., Weisbroth S.H. Detection of Pneumocystis carinii in rats by polymerase chain reaction: Comparison of lung tissue and bronchoalveolar lavage specimens. Lab. Anim. Sci. 1996;46:628–633. [PubMed] [Google Scholar]
- Fleischman R.W., McCracken D., Forbes W. Adynamic ileus in the rat induced by chloral hydrate. Lab. Anim. Sci. 1977;27:238–243. [PubMed] [Google Scholar]
- Flynn R.J. 1st ed. Iowa State University Press; Ames: 1973. pp. 203–320. (Parasites of Laboratory Animals). [Google Scholar]
- Flynn R.J. 1st ed. Iowa State University Press; Ames: 1973. pp. 435–492. (Parasites of Laboratory Animals). [Google Scholar]
- Flynn R.J. 1st ed. Iowa State University Press; Ames: 1973. pp. 3–35. (Parasites of Laboratory Animals). [Google Scholar]
- Flynn R.J. 1st ed. Iowa State University Press; Ames: 1973. pp. 376–397. (Parasites of Laboratory Animals). [Google Scholar]
- Fox J.G., Lee A. The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals (special topic overview) Lab. Anim. Sci. 1997;47:222–255. [PubMed] [Google Scholar]
- Fox J.G., Niemi S.M., Murphy J.C., Quimby F.W. Ulcerative dermatitis in the rat. Lab. Anim. Sci. 1977;27:671–678. [PubMed] [Google Scholar]
- Fox J.G., Niemi S.M., Ackerman J., Murphy J.C. Comparison of methods to diagnose an epizootic of Corynebacterium kutscheri pneumonia in rats. Lab. Anim. Sci. 1987;37:72–75. [PubMed] [Google Scholar]
- Fox J.G., Yan L.L., Dewhirst F.E., Paster B.J., Shames B., Murphy J.C., Hayward A., Belcher J.C., Mendes E.N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from the bile, liver, and intestines of aged, inbred mice. J. Clin. Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J.G., Yan L., Shames B., Campbell J., Murphy J.C., Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Contemp. Top. 1996;23:62. doi: 10.1128/iai.64.9.3673-3681.1996. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R.R., Laird C.W. In: Reproduction and Breeding Techniques for Laboratory Animals. Hafez E.S.E., editor. Lea and Febiger; Philadelphia: 1970. pp. 107–122. [Google Scholar]
- Franklin C.L., Kinden D.A., Stogsdill P.L., Riley L.K. In vitro model of adhesion and invasion by Bacillus piliformis. Infect. Immun. 1993;61:876–883. doi: 10.1128/iai.61.3.876-883.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin C.L., Motzel S.L., Besch-Williford C.L., Hook R.R., Riley L.K. Tyzzer's infection: Host specificity of Clostridium piliforme isolates. Lab. Anim. Sci. 1994;44:568–572. [PubMed] [Google Scholar]
- Franklin C.L., Pletz J.D., Riley L.K., Livingston B.A., Hook R.R., Jr., Besch-Williford C.L. Detection of cilia-associated respiratory (CAR) bacillus in nasal-swab specimens from infected rats by use of polymerase chain reaction. Lab. Anim. Sci. 1999;49:114–117. [PubMed] [Google Scholar]
- Fujinaga M., Baden J.M., Mazze R.I. Reproductive and teratogenic effects of a major earthquake in rats. Lab. Anim. Sci. 1992;42:209–210. [PubMed] [Google Scholar]
- Gaddum-Rosse P., Blandau R.J., Langley L.B., Battaglia D.E. In vitro fertilization in the rat: Observations on living eggs. Fertil. Steril. 1984;42:285–292. [PubMed] [Google Scholar]
- Gaertner D.J., Jacoby R.O. Persistence of rat parvovirus in athymic rats. Arch. Virol. 1989;105:259–268. doi: 10.1007/BF01311362. [DOI] [PubMed] [Google Scholar]
- Gaertner D.J., Jacoby R.O., Johnson E.A., Paturzo F.X., Smith A.L. Persistent rat virus infection in juvenile athymic rats and its modulation by immune serum. Lab. Anim. Sci. 1995;45:249–253. [PubMed] [Google Scholar]
- Gaertner D.J., Smith A.L., Jacoby R.O. Efficient induction of persistent and prenatal parvovirus infection in rats. Virus Res. 1996;44:67–78. doi: 10.1016/0168-1702(96)01351-2. [DOI] [PubMed] [Google Scholar]
- Ganaway J.R. Effect of heat and selected chemical disinfectants upon infectivity of spores of Bacillus piliformis (Tyzzer's disease) Lab. Anim. Sci. 1980;30:192–196. [PubMed] [Google Scholar]
- Gannon J. A survey of Encephalitozoon cuniculi in laboratory animal colonies in the United Kingdom. Lab. Anim. 1980;14:91–94. doi: 10.1258/002367780780942917. [DOI] [PubMed] [Google Scholar]
- Garcia-Cairasco N., Oliveira J.A., Wakamatsu H., Bueno S.T., Guimaraes F.S. Reduced exploratory activity of audiogenic seizures in susceptible Wistar rats. Physiol. Behav. 1998;64:671–674. doi: 10.1016/s0031-9384(98)00129-2. [DOI] [PubMed] [Google Scholar]
- Garlinghouse L.E., Van Hoosier G.L. Studies on adjuvant-induced arthritis, tumor transplantability, and serologic response to bovine serum albumin in Sendai virus-infected rats. Am. J. Vet. Res. 1978;39:297–300. [PubMed] [Google Scholar]
- Garside D.A., Charlton A., Heath K.J. Establishing the timing of implantation in the Harlan Porcellus Dutch and New Zealand White rabbit and the Han Wistar rat. Regul. Toxicol. Pharmacol. 1996;23:69–73. doi: 10.1006/rtph.1996.0010. [DOI] [PubMed] [Google Scholar]
- Giusti A.M., Crippa L., Bellini O., Luini M., Scanziani E. Castric spiral bacteria in wild rats from Italy. J. Wildl. Dis. 1998;34:168–172. doi: 10.7589/0090-3558-34.1.168. [DOI] [PubMed] [Google Scholar]
- Goelz M.F., Thigpen J.E., Mahler J., Rogers W.P., Locklear J., Wiegler B.J., Forsythe D.B. Efficacy of various therapeutic regimens in eliminating Pasteurella pneumotropica from the mouse. Lab. Anim. Sci. 1997;46:280–285. [PubMed] [Google Scholar]
- Goldman P. Drug therapy: Metronidazole. N. Engl. J. Med. 1980;303:1212–1218. doi: 10.1056/NEJM198011203032106. [DOI] [PubMed] [Google Scholar]
- Gonzalez D.E., Deis R.P. The capacity to develop maternal behavior is enhanced during aging in rats. Neurobiol. Aging. 1990;11:237–241. doi: 10.1016/0197-4580(90)90551-a. [DOI] [PubMed] [Google Scholar]
- Goodman D.G., Ward J.M., Squires R.A., Chu K.C., Linhart M.S. Neoplastic and non-neoplastic lesions in aging F-344 rats. Toxicol. Appl. Pharmacol. 1979;48:237–248. doi: 10.1016/0041-008x(79)90029-2. [DOI] [PubMed] [Google Scholar]
- Goodman D.G. Neoplastic and nonneoplastic lesions in aging Osborne-Mendel rats. Toxicol. Appl. Pharmacol. 1980;55:433–447. doi: 10.1016/0041-008x(80)90045-9. [DOI] [PubMed] [Google Scholar]
- Greene E.C. In: The Rat in Laboratory Investigation. Ferris E.J., Griffith J.Q., editors. J. B. Lippincott, Hafner Press; New York: 1949. pp. 24–50. [Google Scholar]
- Greene E.C. Hafner Publ. Co.; New York: 1963. (Anatomy of the Rat). [Google Scholar]
- Griffith J.W., White W.J., Danneman P.J., Lang C.M. Cilia-associated respiratory (CAR) bacillus infection of obese mice. Vet. Pathol. 1988;25:72–76. doi: 10.1177/030098588802500110. [DOI] [PubMed] [Google Scholar]
- Gruber H.E., Osborne J.W. Ultrastructural features of spiro-nucleosis (hexamitiasis) in X-irradiated rat small intestine. Lab. Anim. 1979;13:199–202. doi: 10.1258/002367779780937717. [DOI] [PubMed] [Google Scholar]
- Gumprecht L.A., Long C.R., Soper K.A., Smith P.F., Haschek-Hock W.M., Keenan K.P. The early effects of dietary restriction on the pathogenesis of chronic renal disease in Sprague-Dawley rats at 12 months. Toxicol. Pathol. 1993;21:528–537. doi: 10.1177/019262339302100602. [DOI] [PubMed] [Google Scholar]
- GV-SOLAS Working Group on Hygiene Pneumocystis carinii: Implications for animal experiments. Lab. Anim. 1999;33(Suppl. 1):77–78. [PubMed] [Google Scholar]
- Haines D.C., Gorelick P.L., Battles J.K., Pike K.M., Anderson R.J., Fox J.G., Taylor N.S., Shen Z., Dewhirst F.E., Anver M.R., Ward J.M. Inflammatory and large bowel disease in immunodeficient rats naturally and experimentally infected with Helicobacter bilis. Vet. Pathol. 1998;35:202–208. doi: 10.1177/030098589803500305. [DOI] [PubMed] [Google Scholar]
- Hansen A.K., Dagnaes-Hansen F., Mollegaard-Hansen K.-E. Correlation between megaloileitis and antibodies to Bacillus piliformis in laboratory rat colonies. Lab. Anim. Sci. 1992;42:449–453. [PubMed] [Google Scholar]
- Hansen A.K., Skovgaard-Jensen H.-J., Thomsen P., Svendsen O., Dagnaes-Hansen F., Mollegaard-Hansen K.-E. Rederivation of rat colonies seropositive for Bacillus piliformis and the subsequent screening for antibodies. Lab. Anim. Sci. 1992;42:444–447. [PubMed] [Google Scholar]
- Hasslinger M.A., Wiethe T. Oxyurid infestation of small laboratory animals and its control with ivermectin. Tierarztl. Prax. 1987;15:93–97. [PubMed] [Google Scholar]
- Hirsjarvi P., Valiaho T. Effects of gentling on open-field behaviour of Wistar rats in fear-evoking test situation. Lab. Anim. 1995;29:380–384. doi: 10.1258/002367795780739953. [DOI] [PubMed] [Google Scholar]
- Homburger F. Saccharin and cancer. N. Engl. J. Med. 1977;297:560–561. doi: 10.1056/nejm197709082971018. [DOI] [PubMed] [Google Scholar]
- Hong S.J., Woo H.C., Chai J.Y. A human case of Plagiorchis muris (Tanabe, 1922: Digenea) infection in the Republic of Korea: Fresh-water fish as a possible source of infection. J. Parasitol. 1996;82:647–649. [PubMed] [Google Scholar]
- Hook R.R., Franklin C.L., Riley L.K., Livingston B.A., Besch-Williford C.L. Antigenic analyses of cilia-associated respiratory (CAR) bacillus isolates by use of monoclonal antibodies. Lab. Anim. Sci. 1998;48:234–239. [PubMed] [Google Scholar]
- Hsu C.-K. In: Baker H.J., Lindsey J.R., Weisbroth S.H., editors. Academic Press; New York: 1979. pp. 307–331. (“The Laboratory Rat”, Vol. 1. “Biology and Diseases”). [Google Scholar]
- Hubbs A.F., Hahn F.F., Lundgren D.L. Invasive tracheobronchial aspirgillosis in an F-344/N rat. Lab Anim. Sci. 1991;41:521–523. [PubMed] [Google Scholar]
- Huerkamp M.J. Ivermectin eradication of pinworms from rats kept in ventilated cages. Lab. Anim. Sci. 1993;43:86–90. [PubMed] [Google Scholar]
- Hughes W.T. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J. Infect. Dis. 1982;145:842–847. doi: 10.1093/infdis/145.6.842. [DOI] [PubMed] [Google Scholar]
- Hutchinson K.L., Rollin P.E. Pathogenesis of a North American hantavirus, Black Creek Canal virus, in experimentally infected Sigmondon hispidus. Am. J. Trop. Med. Hyg. 1998;59:58–65. doi: 10.4269/ajtmh.1998.59.58. [DOI] [PubMed] [Google Scholar]
- Inge P.M., Edson C.M., Farthing M.J. Attachment of Giardia lamblia to rat intestinal epithelial cells. Gut. 1988;29:795–801. doi: 10.1136/gut.29.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kohyama K., Takakura A., Takenouchi T., Kagiyama N. Naturally occurring CAR bacillus infection in a laboratory rat colony and epizootiological observations. Exp. Anim. 1987;36:387–393. doi: 10.1538/expanim1978.36.4_387. [DOI] [PubMed] [Google Scholar]
- Jacoby R.O. Rat coronavirus. In: Bhatt P.N., Jacoby R.O., Morse H.C. III, New A.E., editors. Viral and Mycoplasmal Infections of Laboratory Rodents: Effects on Biomedical Research. Academic Press; Orlando, Florida: 1986. pp. 625–638. [Google Scholar]
- Jacoby R.O., Bhatt P.N., Jonas A.M. Viral diseases. In: Baker H.J., Lindsey J.R., Weisbroth S.H., editors. Academic Press; New York: 1979. pp. 272–306. (“The Laboratory Rat”, Vol. 1, “Biology and Diseases”). [Google Scholar]
- Jacoby R.O., Gaertner D.J., Bhatt P.N., Paturzo F.X., Smith A.L. Transmission of experimentally induced rat virus infection. Lab. Anim. Sci. 1988;38:11–14. [PubMed] [Google Scholar]
- Jacoby R.O., Johnson E.A. Persistent rat parvovirus infection in individually housed rats. Arch. Virol. 1991;117:193–205. doi: 10.1007/BF01310765. [DOI] [PubMed] [Google Scholar]
- Jacoby R.O., Ball-Goodrich L.J., Besselsen D.G., McKisic M.D., Riley F.K., Smith A.L. Rodent parvovirus infections (special topic overview) Lab. Anim. Sci. 1996;46:370–380. [PubMed] [Google Scholar]
- Johnson P.D., Dawson B.V., Goldberg S.J. Spontaneous congenital heart malformations in Sprague-Dawley rats. Lab. Anim. Sci. 1993;43:183–188. [PubMed] [Google Scholar]
- Jueco N.L. In: Handbook Series in Zoonoses. Jacobs L., Arambulo P., editors. CRC Press; Boca Raton, Florida: 1982. pp. 283–287. [Google Scholar]
- Keenan K.P., Laroque P., Ballam G.C., Soper K.A., Dixit R., Mattson B.A., Adams S.P., Coleman J.B. The effects of diet, ad libitum overfeeding, and moderate dietary restriction on the rodent bioassay: The uncontrolled variable in safety assessment. Toxicol. Pathol. 1996;24:757–768. doi: 10.1177/019262339602400620. [DOI] [PubMed] [Google Scholar]
- Keenan K.P., Soper K.A., Hertzog P.R., Gumprecht L.A., Smith P.F., Mattson B.A., Ballam G.C., Clark R.L. Diet, overfeeding, and moderate dietary restriction in control Sprague-Dawley rats: 2. Effects on age-related proliferative and degenerative lesions. Toxicol. Pathol. 1995;23:287–302. doi: 10.1177/019262339502300306. [DOI] [PubMed] [Google Scholar]
- Keenan K.P., Soper K.A., Smith P.F., Ballam G.C., Clark R.L. Diet, overfeeding, and moderate dietary restriction in control Sprague-Dawley rats: 1. Effects on spontaneous neoplasms. Toxicol. Pathol. 1995;23:269–286. doi: 10.1177/019262339502300305. [DOI] [PubMed] [Google Scholar]
- Keenan K.P., Laroque P., Dixit R. Need for dietary control by caloric restriction in rodent toxicology and carcinogenicity studies. J. Toxicol. Environ. Health, Part B. 1998;1:135–148. doi: 10.1080/10937409809524548. [DOI] [PubMed] [Google Scholar]
- Kelly J.B., Masterton B. Auditory sensitivity of the albino rat. J. Comp. Physiol. Psychol. 1977;91:930–936. doi: 10.1037/h0077356. [DOI] [PubMed] [Google Scholar]
- Kiska D.L., Gilligan P.H. In: Manual of Clinical Microbiology. 7th ed. Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolken R.H., editors. ASM Press; Washington, D.C.: 1999. pp. 517–525. [Google Scholar]
- Klement P., Augustine J.M., Delaney K.H., Lement G., Itz J.I. An oral ivermectin regimen that eradicates pinworms (Syphacia spp.) in laboratory rats and mice. Lab. Anim. Sci. 1996;46:286–290. [PubMed] [Google Scholar]
- Kohn D.F., Kirk B.E. Pathogenicity of Mycoplasma pulmonis in laboratory rats. Lab. Anim. Care. 1969;19:321–330. [PubMed] [Google Scholar]
- Kohn D.F. Bronchiectasis in rats infected with Mycoplasma pulmonis: An electron microscopic study. Lab. Anim. Sci. 1971;21:856–861. [PubMed] [Google Scholar]
- Kohn D.F., Barthold S.W. In: Laboratory Animal Medicine. Fox J.G., Cohen B.J., Loew F.M., editors. Academic Press; San Diego: 1984. pp. 91–122. [Google Scholar]
- Kojima A., Okaniwa A. Antigenic heterogeneity of sialodacryo-adenitis virus isolates. J. Vet. Med. Res. 1991;53:1059–1063. doi: 10.1292/jvms.53.1059. [DOI] [PubMed] [Google Scholar]
- Koolhass J.M. The UFAW Handbook on the Care and Management of Laboratory Animals. Blackwell Science Ltd.; Malden, Massachusetts: 1999. The laboratory rat; pp. 313–330. [Google Scholar]
- Koto M., Miwa M., Togashi M., Tsuji K., Okamoto M., Adachi J. A method for detecting the optimum for mating during the 4-day estrous cycle in the rat: measuring the value of electrical impedance of the vagina. Jikken Dobutsu. 1987;36:195–198. doi: 10.1538/expanim1978.36.2_195. [DOI] [PubMed] [Google Scholar]
- Koto M., Miwa M., Tsuji K., Okamoto M., Adachi J. Change in the electrical impedance caused by cornification of the epithelial cell layer of the vaginal mucosa in the rat. Jikken Dobutsu. 1987;36:151–156. doi: 10.1538/expanim1978.36.2_151. [DOI] [PubMed] [Google Scholar]
- Kubisch H.M., Gomez-Sanchez E.P. Embryo transfer in the rat as a tool to determine genetic components of the gestational environment. Lab. Anim. Sci. 1999;49:90–94. [PubMed] [Google Scholar]
- Kunstyr I. Infectious form of Spironucleus (Hexamita) muris: Banded cysts. Lab. Anim. 1977;11:185–188. doi: 10.1258/002367777780936747. [DOI] [PubMed] [Google Scholar]
- Kunstyr I., Ammerpohl E. Resistance of faecal cysts of Spironucleus muris to some physical factors and chemical substances. Lab. Anim. 1978;12:95–97. doi: 10.1258/002367778780953152. [DOI] [PubMed] [Google Scholar]
- Kunstyr I., Schoeneberg U., Friedhoff K.T. Host specificity of Giardia muris isolates from mouse and golden hamster. Parasitol. Res. 1992;78:621–622. doi: 10.1007/BF00936463. [DOI] [PubMed] [Google Scholar]
- Kurisu K., Sawamoto O., Watanabe H., Ito A. Sequential changes in the Harderian gland of rats exposed to high intensity light. Lab. Anim. Sci. 1996;46:71–76. [PubMed] [Google Scholar]
- Laliberte F., Mucchielli A., Laliberte M.F. Dynamics of antibody transfer from mother to fetus through the yolk-sac cells in the rat. Biol. Cell. 1984;50:255–261. doi: 10.1111/j.1768-322x.1984.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Lang P.L. Charles River Laboratories; Wilmington, Massachusetts: 1990. (Spontaneous Neoplastic Lesions in the CDF(F-344)/CrlBR Rat). [Google Scholar]
- Lang P.L. Charles River Laboratories; Wilmington, Massachusetts: 1992. (Spontaneous Neoplastic Lesions and Selected Nonneoplastic Lesions in the Crl:CDBR Rat). [Google Scholar]
- La Regina M., Woods L., Klender P., Gaertner D.J., Paturzo F.X. Transmission of sialodacryoadenitis virus from infected rats to rats and mice through handling, close contact, and soiled bedding. Lab. Anim. Sci. 1992;42:344–346. [PubMed] [Google Scholar]
- Leader R.W., Leader I., Witschi E. Genital mycoplasmosis in rats treated with testosterone propionate to produce constant estrus. J. Am. Vet. Med. Assoc. 1970;157:1923–1925. [PubMed] [Google Scholar]
- LeChevallier M.W., Cawthon C.D., Lee R.G. Inactivation of biofilm bacteria. Appl. Environ. Microbiol. 1988;54:2492–2499. doi: 10.1128/aem.54.10.2492-2499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Phillips M.W., O'Rourke J.L., Paster B.J., Dewhirst F.E., Fraser G.J., Fox J.G., Sly L.I., Romaniuk P.J., Trust T.J., Kouprach S. Helicobacter muridarum sp. nov., a microaerophilic helical bacterium with a novel ultrastructure isolated from the intestinal mucosa of rodents. Int. J. Syst. Bacteriol. 1992;42:27–36. doi: 10.1099/00207713-42-1-27. [DOI] [PubMed] [Google Scholar]
- Le Minor L. Genus III. Salmonella. In: Krieg I.N.R., Holt J.G., editors. Bergery's Manual of Systematic Bacteriology. Williams and Wilkins; Baltimore: 1984. pp. 427–448. [Google Scholar]
- Levine N.D. Burgess Publ. Co.; Minneapolis: 1961. pp. 107–128. (Protozoan Parasites of Domestic Animals and of Man). [Google Scholar]
- Levine N.D. 1st ed. Iowa State Univ. Press; Ames: 1985. pp. 109–129. (Veterinary Protozoology). [Google Scholar]
- Levine N.D. 1st ed. Iowa State Univ. Press; Ames: 1985. pp. 89–108. (Veterinary Protozoology). [Google Scholar]
- Lewis D.J. In: Pathobiology of the Aging Rat. Mohr U., Dungworth D.L., Capen C.C., editors. ILSI Press; Washington, D.C.: 1992. pp. 301–309. [Google Scholar]
- Liang S.C., Schoeb T.R., Davis J.K., Simecka J.W., Cassell G.H., Lindsey J.R. Comparative severity of respiratory lesions of sialodacryoadenitis virus and Sendai virus infections in LEW and F-344 rats. Vet. Pathol. 1995;32:661–667. doi: 10.1177/030098589503200607. [DOI] [PubMed] [Google Scholar]
- Liang S.C., Simecka J.W., Lindsey J.R., Cassell G.H., Davis J.K. Antibody responses after Sendai virus infection and their role in upper and lower respiratory tract disease in rats. Lab. Anim. Sci. 1999;49:385–394. [PubMed] [Google Scholar]
- Lindsey J.R. In: Baker H.J., Lindsey J.R., Weisbroth S.H., editors. Academic Press; New York: 1979. pp. 1–36. (“The Laboratory Rat,” Vol. 1, “Biology and Diseases”). [Google Scholar]
- Lindsey J.R., Baker H.J., Overcash R.G., Cassell G.H., Hunt C.E. Murine chronic respiratory disease: Significance as a research complication and experimental production with Mycoplasma pulmonis. Am. J. Pathol. 1971;64:675–716. [PMC free article] [PubMed] [Google Scholar]
- Lindsey J.R., Cassell G.H., Davidson M.K. In: The Mouse in Bimedical Research. Foster H.L., Small J.D., Fox J.G., editors. Academic Press; New York: 1982. pp. 21–41. [Google Scholar]
- Lindsey J.R., Davidson M.K., Schoeb T.R., Cassell G.H. Mycoplasma pulmonis-host relationships in a breeding colony of Sprague-Dawley rats with enzootic murine respiratory mycoplasmosis. Lab. Anim. Sci. 1985;35:597–608. [PubMed] [Google Scholar]
- Liu J.J., Greeley E.H., Shadduck J.A. Murine encephalitozoonesis: The effect of age and mode of transmission on occurrence of infection. Lab. Anim. Sci. 1988;38:675–679. [PubMed] [Google Scholar]
- Liu J.J., Greeley E.H., Shadduck J.A. Mechanisms of resistance/susceptibility to murine encephalitozoonosis. Parasite Immunol. 1989;11:241–256. doi: 10.1111/j.1365-3024.1989.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Livingston R.S., Riley L.K., Besch-Williford C.L., Hook R.R., Franklin C.L. Transmission of Helicobacter hepaticus infection to sentinel mice by contaminated bedding. Lab. Anim. Sci. 1998;48:291–293. [PubMed] [Google Scholar]
- Lloyd G., Bowen E.T.W., Jones N., Pendry A. HFRS outbreak associated with laboratory rats in U.K. Lancet. 1984;1:1175–1176. doi: 10.1016/s0140-6736(84)91413-2. [DOI] [PubMed] [Google Scholar]
- Loeb W., Quimby F., editors. The Clinical Chemistry of Laboratory animals. 2nd ed. Taylor and Francis; Philadelphia: 1999. [Google Scholar]
- Loftness T.J., Erlandsen S.L., Wilson I.D., Meyer E.A. Occurrence of specific secretory immunoglobulin A in bile after inoculation of Giardia lamblia trophozoites into rat duodenum. Gastroenterology. 1984;87:1022–1029. [PubMed] [Google Scholar]
- Lubcke R., Hutcheson F.A.R., Barbezat G.O. Impaired intestinal electroyte transport in rats infested with the common parasite Syphacia muris. Digest. Dis. Sci. 1992;37:60–64. doi: 10.1007/BF01308343. [DOI] [PubMed] [Google Scholar]
- Lukas V., Ruehl W.W., Hamm T.E. An enzyme-linked, immunosorbent assay to intact serum IgG in rabbits naturally exposed to cilia-associated respiratory bacillus. Lab. Anim. Sci. 1987;37:553. (Abstract) [Google Scholar]
- Lüssier G. Detection methods for the identification of rodent viral and mycoplasmal infections. Lab. Anim. Sci. 1991;41:199–225. [PubMed] [Google Scholar]
- MacKenzie W.F., Garner F.M. Comparison of neoplasms from six sources of rats. J. Natl. Cancer Inst. 1973;50:1243–1257. doi: 10.1093/jnci/50.5.1243. [DOI] [PubMed] [Google Scholar]
- MacKenzie W.F., Magill L.S., Hulse M. A filamentous bacterium associated with respiratory disease in wild rats. Vet. Pathol. 1981;18:336–839. doi: 10.1177/030098588101800616. [DOI] [PubMed] [Google Scholar]
- Majeed S.K., Zubaidy A.J. Histopathological lesions associated with Encephalitozoon cuniculi (nosematosis) infection in a colony of Wistar rats. Lab. Anim. 1982;16:244–247. doi: 10.1258/002367782780891813. [DOI] [PubMed] [Google Scholar]
- Makino S., Seko S., Nakao H., Mikazuki K. An epizootic of Sendai virus infection in a rat colony. Exp. Anim. 1972;22:275–280. doi: 10.1538/expanim1957.22.4_275. [DOI] [PubMed] [Google Scholar]
- Manser C.E., Elliot H., Morris T.H., Broom D.M. The use of a novel operant system to determine the strength of preference for flooring in laboratory rats. Lab. Anim. 1995;30:1–6. doi: 10.1258/002367796780744974. [DOI] [PubMed] [Google Scholar]
- Manser C.E., Morris T.H., Broom D.M. An investigation into the effects of solid or grid cage flooring on the welfare of laboratory rats. Lab. Anim. 1995;29:353–363. doi: 10.1258/002367795780740023. [DOI] [PubMed] [Google Scholar]
- Manser C.E., Broom D.M., Overend P., Morris T.H. Operant studies to determine the strength of preference in laboratory rats for nestboxes and nesting materials. Lab. Anim. 1998;32:36–41. doi: 10.1258/002367798780559473. [DOI] [PubMed] [Google Scholar]
- Manser C.E., Broom D.M., Overend P., Morris T.H. Investigations into the preferences of laboratory rats for nest-boxes and nesting materials. Lab. Anim. 1998;32:23–35. doi: 10.1258/002367798780559365. [DOI] [PubMed] [Google Scholar]
- Maronpot R.R. In: Urinary System. 2nd ed. Jones T.C., Hard G.C., Mohr U., editors. Springer; Berlin: 1996. pp. 306–309. [Google Scholar]
- Martin M.G., Wu S.V., Walsh J.H. Ontogenetic development and distribution of antibody transport and Fc receptor mRNA expression in rat intestine. Digest. Dis. Sci. 1997;42:1062–1069. doi: 10.1023/a:1018853506830. [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez M., Cruz Y., Salas M., Hudson R., Pacheco P. Assessing pain threshold in the rat: Changes with estrus and time of day. Physiol. Behav. 1994;55:651–657. doi: 10.1016/0031-9384(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Joshima H. Pathology of rats intranasally inoculated with the cilia-associated respiratory bacillus. Lab. Anim. 1989;23:89–95. doi: 10.1258/002367789780863600. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Kashima M., Joshima H. Serodiagnosis of cilia-associated respiratory bacillus infection by the indirect immunofluorescence assay technique. Lab. Anim. 1987;21:356–359. doi: 10.1258/002367787781363327. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Joshima H., Matsumoto T., Fukutsu K. Transmission experiments of cilia-associated respiratory bacillus in mice, rabbits, and guinea pigs. Lab. Anim. 1989;23:96–102. doi: 10.1258/002367789780863664. [DOI] [PubMed] [Google Scholar]
- Mattheij J.A.M., Swarts J.J.M. Quantification and classification of pregnancy wastage in 5-day cyclic young through middle-aged rats. Lab. Anim. 1991;25:30–34. doi: 10.1258/002367791780808202. [DOI] [PubMed] [Google Scholar]
- Matuszczyk J.V., Appa R.S., Larsson K. Age-dependent variations in the sexual preference of male rats. Physiol. Behav. 1994;55:827–830. doi: 10.1016/0031-9384(94)90067-1. [DOI] [PubMed] [Google Scholar]
- McKisic M.D., Paturzo F.X., Gaertner D.J. A nonlethal rat parvovirus infection suppresses rat T lymphocyte effector functions. J. Immunol. 1995;155:3979–3986. [PubMed] [Google Scholar]
- Medina L.V., Chladny J., Ortman J.D., Artwohl J.E., Bunte R.M., Bennett B.T. Rapid way to identify the cilia-associated respiratory bacillus: Tracheal mucosal scraping with a modified microwave Steiner silver impregnation. Lab. Anim. Sci. 1998;46:113–115. [PubMed] [Google Scholar]
- Mendes E.N., Queiroz D.M., Dewhirst F.E., Paster B.J., Moura S.B., Fox J.G. Helicobacter trogontum sp. nov., isolated from the rat in testine. Int. J. Syst. Bacteriol. 1996;46:916–921. doi: 10.1099/00207713-46-4-916. [DOI] [PubMed] [Google Scholar]
- Mercier O., Perraud J., Stadler J. A method for routine observation of sexual behaviour in rats. Lab. Anim. 1987;21:125–130. doi: 10.1177/002367728702100208. [DOI] [PubMed] [Google Scholar]
- Meyer T.K., Yurchak M.R., Pliego J.F., Wincek T.J., Dukelow W.R., Kuehl T.J. Cryopreservation of murine zygotes for use in testing culture environments. Lab. Anim. Sci. 1997;47:496–499. [PubMed] [Google Scholar]
- Middle Atlantic Reproduction and Teratology Association and Midwest Teratology Association . Charles River Laboratories; Wilmington, Massachusetts: 1996. Historical control data (1992–1994) for developmental and reproductive toxicity studies using the Crl:CD(SD)BR rat. [Google Scholar]
- Mohr U., Dungworth D.L., Capen C.C., editors. Vols. 1 and 2. ILSI Press; Washington, D.C.: 1992. (Pathobiology of the Aging Rat). [Google Scholar]
- Montes G.S., Luque E.H. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat. (Basel) 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Montgomery C.A., Seely J.C. In: Pathology of the Fischer Rat: Reference and Atlas. Boorman G.A., Eustis S.L., Elwell M.R., Montgomery C.A., MacKenzie W.F., editors. Academic Press; San Diego: 1990. pp. 127–153. [Google Scholar]
- Moore D.M. In: Rollin B.E., Kesel M.J., editors. Vol. 2. CRC Press; Boca Raton, Florida: 1995. (The Experimental Animal in Biomedical Research). Chapter 11. [Google Scholar]
- Moore H.D., Akhondi M.A. Fertilizing capacity of rat spermatozoa is correlated with decline in straight-line velocity measured by continuous computer-aided sperm analysis: Epididymal rat spermatozoa from the proximal cauda have a greater fertilizing capacity in vitro than those from the distal cauda or vas deferens. J. Androl. 1996;17:50–60. [PubMed] [Google Scholar]
- Morris R.H., Bras G.J. Lasting influence of early caloric restriction on prevalence of neoplasms in the rat. J. Natl. Cancer Inst. 1971;47:1095–1113. [PubMed] [Google Scholar]
- Mottola M.F., Plust J.H., Christopher P.D., Schachter C.L. Effects of exercise on maternal glycogen storage patterns and fetal outcome in mature rats. Can. J. Physiol. Pharmacol. 1992;70:1634–1638. doi: 10.1139/y92-234. [DOI] [PubMed] [Google Scholar]
- Motzel S.L., Riley L.K. Bacillus piliformis flagellar antigens for serodiagnosis of Tyzzer's disease. J. Clin. Microbiol. 1991;29:2566–2570. doi: 10.1128/jcm.29.11.2566-2570.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzel S.L., Riley L.K. Subclinical infections and transmission of Tyzzer's disease in rats. Lab. Anim. Sci. 1992;42:439–443. [PubMed] [Google Scholar]
- Naot Y., Merchav S., Ben-David E., Ginsburg H. Mitogenic activity of Mycoplasma pulmonis. 1. Stimulation of rat B and T lymphocytes. Immunology. 1979;36:399–406. [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Simon-Tou R., Ginsburg H. Mitogenic activity of Mycoplasma pulmonis. 2. Studies on the biochemical naure of the mitogenic factor. Eur. J. Immunol. 1979;9:149–151. doi: 10.1002/eji.1830090211. [DOI] [PubMed] [Google Scholar]
- National Research Council Long-term holding of laboratory rodents. ILAR News. 1976;19:L1–L25. [Google Scholar]
- National Research Council . Infectious Diseases of Mice and Rats. National Academy Press; Washington, D.C.: 1991. Respiratory system; pp. 33–84. [Google Scholar]
- National Research Council . Infectious Diseases of Mice and Rats. National Academy Press; Washington, D.C.: 1991. Multiple systems; pp. 236–256. [Google Scholar]
- National Research Council . In: Infectious Diseases of Mice and Rats. Lindsey J.R., Boorman G.A., Collins M.J., Hsu C.-K., Van Hoosier G.L. Jr., Wagner J.E., editors. National Academy Press; Washington, D.C.: 1991. pp. 85–163. [Google Scholar]
- National Research Council . In: Infectious Diseases of Mice and Rats. Lindsey J.R., Boorman G.A., Collins M.J., Hsu C.-K., Van Hoosier G.L. Jr., Wagner J.E., editors. National Academy Press; Washington, D.C.: 1991. pp. 187–190. [Google Scholar]
- National Research Council . In: Infectious Diseases of Rats and Mice. Lindsey J.R., Boorman G.A., Collins M.J., Hsu C.-K., Van Hoosier G.L. Jr., Wagner J.E., editors. National Academy Press; Washington, D.C.: 1991. pp. 198–221. [Google Scholar]
- National Research Council . 4th ed. National Academy Press; Washington, D.C.: 1995. (Nutritional Requirements of Laboratory Animals). [Google Scholar]
- National Research Council . National Academy Press; Washington, D.C.: 1996. (Laboratory Animal Management—Rodents). [Google Scholar]
- Niederkorn J.Y., Shadduck J.A., Schmidt E.C. Susceptibility of selected inbred strains of mice to Encephalitozoon cuniculi. J. Infect. Dis. 1981;144:249–253. doi: 10.1093/infdis/144.3.249. [DOI] [PubMed] [Google Scholar]
- Niggeschulze A., Kast A. Maternal age, reproduction, and chromosomal aberrations in Wistar derived rats. Lab. Anim. 1994;28:55–62. doi: 10.1258/002367794781065717. [DOI] [PubMed] [Google Scholar]
- Nunn G., MacPherson A. Spontaneous convulsions in Charles River Wistar rats. Lab Animal. 1995;29:50–53. doi: 10.1258/002367795780740429. [DOI] [PubMed] [Google Scholar]
- Orcutt R.P. Bacterial diseases: Agent, pathology, diagnosis, and effects on research. Lab Animal. 1980;9:28–43. [Google Scholar]
- Orihuela P.A., Ortiz M.E., Croxatto H.B. Sperm migration into and through the oviduct following artificial insemination at different stages of the estrous cycle in the rat. Biol. Reprod. 1999;60:908–913. doi: 10.1095/biolreprod60.4.908. [DOI] [PubMed] [Google Scholar]
- Owen D.G. Royal Society of Medicine Services, Ltd.; London: 1992. pp. 13–38. (Parasites of Laboratory Animals). [Google Scholar]
- Owen D.G. Royal Society of Medicine Services, Ltd.; London: 1992. pp. 89–90. (Parasites of Laboratory Animals). [Google Scholar]
- Oz H.S., Hughes W.T. Effect of sex and dexamiethasone dose on the experimental host for Pneumocystis carinii. Lab. Anim. Sci. 1996;46:109–110. [PubMed] [Google Scholar]
- Pakes S.P., Shadduck J.A., Feldman D.B., Moore J.A. Comparison of tests for the diagnosis of spontaneous encephalitozoonosis in rabbits. Lab. Anim. Sci. 1984;34:356–359. [PubMed] [Google Scholar]
- Paturzo F.Y., Jacoby R.O., Bhatt P.N., Smith A.L., Gaertner D.J., Ardito R.B. Persistence of rat virus in seropositive rats as detected by explant culture. Arch. Virol. 1987;95:137–142. doi: 10.1007/BF01311341. [DOI] [PubMed] [Google Scholar]
- Pauli B.U., Gruber A.D., Weinstein R.S. In: Urinary System. 2nd ed. Jones T.C., Hard G.C., Mohr U., editors. Springer; Berlin: 1996. pp. 381–392. [Google Scholar]
- Pearson D.J., Taylor G. The influence of the nematode Syphacia obvelata on adjuvant arthritis in rats. Immunology. 1975;29:391–396. [PMC free article] [PubMed] [Google Scholar]
- Peluso J.J. In: Pathobiology of the Aging Rat. Mohr U., Dungworth D.L., Capen C.C., editors. ILSI Press; Washington, D.C.: 1992. pp. 337–349. [Google Scholar]
- Percy D.H., Barthold S.W. 1st ed. Iowa State Univ. Press; Ames: 1993. pp. 3–69. (Pathology of Laboratory Rodents and Rabbits). [Google Scholar]
- Percy D.H., Barthold S.W. 1st ed. Iowa State Univ. Press; Ames: 1993. pp. 70–114. (Pathology of Laboratory Rodents and Rabbits). [Google Scholar]
- Percy D.H., Bond S.J., Paturzo F.X., Bhatt P.N. Duration of protection from reinfection following exposure to sialodacryoadenitis virus in Wistar rats. Lab. Anim. Sci. 1990;40:144–149. [PubMed] [Google Scholar]
- Perez C., Canal J.R., Dominguez E., Campillo J.E., Guillen M., Torres M.D. Individual housing influences certain biochemical parameters in the rat. Lab. Anim. 1997;31:357–361. doi: 10.1258/002367797780596158. [DOI] [PubMed] [Google Scholar]
- Petri M. Studies on Nosema cuniculi found in transplantable ascites tumours with a survey of microsporidiosis in mammals. Acta Pathol. Microbiol. Scand. Suppl. 1969;204:1–91. [PubMed] [Google Scholar]
- Pleasants J.R. In: Handbook of Laboratory Animal Science. Melby E.C., Altman N.H., editors. CRC Press; Cleveland: 1974. pp. 119–174. [Google Scholar]
- Poonacha K.B., William P.D., Stamper R.D. Encephalitozoonosis in a parrot. J. Am. Vet. Med. Assoc. 1985;186:700–702. [PubMed] [Google Scholar]
- Pucak G.J., Lee C.S., Zaino A.S. Effects of prolonged high temperature on testicular development and fertility in the male rat. Lab. Anim. Sci. 1977;27:76–77. [PubMed] [Google Scholar]
- Reetz J. Naturliche microsporidien-(Encephalitozoon-cuniculi-)Infektionen bei Huhnern. Tierarztl. Prax. 1993;21:429–435. [PubMed] [Google Scholar]
- Rehbinder C., Baneux P., Forbes D., Van Herck H., Nicklas W., Rugaya Z., Winkler G. FELASA recommendations for the health monitoring of mouse, rat, hamster, gerbil, guinea pig, and rabbit experimental units. Lab. Anim. 1996;30:208. doi: 10.1258/002367796780684881. [DOI] [PubMed] [Google Scholar]
- Rehm S., Waalkes M.P., Ward J.M. Aspergillus rhinitis in Wistar (Crl:(WI)BR) rats. Lab. Anim. Sci. 1988;38:162–166. [PubMed] [Google Scholar]
- Richter C.P. The effects of domestication and selection on the behavior of the Norway rat. J. Natl. Cancer Inst. 1954;15:727–738. [PubMed] [Google Scholar]
- Riley L.K., Franklin C.L. In: 2nd ed. Jones T.C., Popp J.A., Mohr U., editors. Springer-Verlag; Berlin: 1997. pp. 201–209. (Digestive System). [Google Scholar]
- Riley L.K., Franklin C.L., Hook R.R., Besch-Williford C. Identification of murine helicobacters by PCR and restriction enzyme analysis. J. Clin. Microbiol. 1996;34:942–946. doi: 10.1128/jcm.34.4.942-946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L.K., Carty A.J., Besch-Williford C.L. PS 45 PCR-based testing as an alternative to MAP testing. Lab. Anim. Sci. 1999;49:443–444. [Google Scholar]
- Ristic, M., and Kreier, J. P. (1984). “Bergey's Manual of Systematic Bacteriology” (I. N. R. Krieg, and J. G. Holt, eds.), pp. 719–739. Robinson, 1965. Reprint, Williams and Wilkins, Baltimore.
- Robinson R. Pergamon Press; New York: 1965. (Genetics of the Norway Rat). [Google Scholar]
- Roe F.J.C., Lee P.N., Conybeare G., Kelly D., Matter B., Prentice D., Tobin G. The biosure study: Influence of composition of diet and food consumption on longevity, degenerative diseases, and neoplasia in Wistar rats studied for up to 30 months post weaning. Food Chem. Toxicol. 1995;33(Suppl. 1):1S–100S. doi: 10.1016/0278-6915(95)80200-2. [DOI] [PubMed] [Google Scholar]
- Romero A., Villamayor F., Grau M.T., Sacristan A., Ortiz J.A. Relationship between fetal weight and litter size in rats: application to reproductive toxicology studies. Reprod. Toxicol. 1992;6:453–456. doi: 10.1016/0890-6238(92)90009-i. [DOI] [PubMed] [Google Scholar]
- Rosen M., Kahan E., Derazne E. The influence of the first-mating age of rats on the number of pups born, their weights, and their mortality. Lab. Anim. 1987;21:348–352. doi: 10.1258/002367787781363282. [DOI] [PubMed] [Google Scholar]
- Rottinghaus A.A., Gibson S.V., Wagner J.E. Comparison of serological tests for detection of antibodies to Sendai virus in rats. Lab. Anim. Sci. 1986;36:496–948. [PubMed] [Google Scholar]
- Rouleau A.M., Kovacs P.R., Kunz H.W., Armstrong D.T. Decontamination of rat embryos and transfer to specific pathogen-free recipients for the production of a breeding colony. Lab. Anim. Sci. 1993;43:611–615. [PubMed] [Google Scholar]
- Royals M.A., Getzy D.M., Vandewoude S. High fungal spore load in corncob bedding associated with fungal-induced rhinitis in two rats. Contemp. Top. 1999;38:64–66. [PubMed] [Google Scholar]
- Russell L.D. In: Mohr U., Dungworth D.L., Hapen C.C., editors. Vol. 1. ILSI Press; Washington, D.C.: 1992. pp. 395–405. (Pathobiology of the Aging Rat). [Google Scholar]
- Russell L.D., Alger L.E., Nequin L.G. Hormonal control of pubertal spermatogenesis. Endocrinology. 1987;120:1615–1632. doi: 10.1210/endo-120-4-1615. [DOI] [PubMed] [Google Scholar]
- Saibaba P., Sates G.D., Stodulski G., Hau J. Behavious of rats in their home cages: daytime variations and effects of routine husbandry procedures analysed by time sampling techniques. Lab. Anim. 1996;30:13–21. doi: 10.1258/002367796780744875. [DOI] [PubMed] [Google Scholar]
- Sales G.D., Milligan S.R. Ultrasound and laboratory animals. Anim. Technol. 1992;43:89–98. [Google Scholar]
- Savage D.C. In: Defining the Laboratory Animal IV Symposium, International Committee on Laboratory Animals. National Research Council, editor. National Academy of Sciences; Washington, D.C.: 1971. pp. 60–73. [Google Scholar]
- Savage N. In: Bergey's Manual of Systematic Bacteriology. Krieg I.N.R., Holt J.G., editors. Williams and Wilkins; Baltimore: 1984. pp. 598–600. [Google Scholar]
- Schagmann G., Bohnet W., Kunstyr I., Friedhoff K.T. Host specificity of cloned Spironucleus muris in laboratory rodents. Lab. Anim. 1990;24:234–239. doi: 10.1258/002367790780866146. [DOI] [PubMed] [Google Scholar]
- Schoeb T.R., Davidson M.K., Lindsey J.R. Intracage ammonia promotes growth of Mycoplasma pulmonis in the respiratory tract of rats. Infect. Immun. 1982;38:212–217. doi: 10.1128/iai.38.1.212-217.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeb T.R., Kervin K.C., Lindsey J.R. Exacerbation of murine respiratory mycoplasmosis in gnotobiotic F344/N rats by Sendai virus infection. Vet. Pathol. 1985;22:272–282. doi: 10.1177/030098588502200310. [DOI] [PubMed] [Google Scholar]
- Semple-Rowland S.L., Dawson W.W. Retinal cyclic light damage threshold for albino rats. Lab. Anim. Sci. 1987;37:289–298. [PubMed] [Google Scholar]
- Shalev U., Feldon J., Weiner L. Gender- and age-dependent differences in latent inhibition following pre-weaning non-handling: Implications for a neurodevelopmental animal model of schizophrenia. Int. J. Dev. Neurosci. 1998;16:279–288. doi: 10.1016/s0736-5748(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Sharma A.W., Mayrhofer G. Biliary antibody response in rats infected with rodent Giardia duodenalis isolates. Parasite Immunol. 1988;10:181–191. doi: 10.1111/j.1365-3024.1988.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Sharma A.W., Mayrhofer G. A comparative study of infections with rodent isolates of Giardia duodenalis in inbred strains of rats and mice and in hypothymic nude rats. Parasite Immunol. 1988;10:169–179. doi: 10.1111/j.1365-3024.1988.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Shoji Y., Itoh T., Kagiyama N. Enzyme-linked immunosorbent assay for detection of serum antibody to CAR bacillus. Exp. Anim. 1988;37:67–72. doi: 10.1538/expanim1978.37.1_67. [DOI] [PubMed] [Google Scholar]
- Short B.G., Goldstein R.S. In: Mohr U., Dungworth D.L., Capen C.C., editors. Vol. 1. ILSI Press; Washington, D.C.: 1992. pp. 211–225. (Pathobiology of the Aging Rat). [Google Scholar]
- Skelton H., Smith K., Hilyard E., Hadfield T., Tuur S., Wagner K., Decker C., Angritt P. Bacillus piliformis (Tyzzer's disease) in an HIV-1+ patient: Confirmation with 16S rRNA sequence analysis. J. Invest. Dermatol. 1995;104:687. doi: 10.1016/s0190-9622(07)80005-3. (Abstract) [DOI] [PubMed] [Google Scholar]
- Slaoui M., Dreef H.C., van Esch E. Inflammatory lesions in the lungs of Wistar rats. Toxicologic Pathology. 1998;26:712–713. doi: 10.1177/019262339802600520. [DOI] [PubMed] [Google Scholar]
- Smith E.R., Stefanick M.L., Clark J.T., Davidson J.M. Hormones and sexual behavior in relationship to aging in male rats. Horm. Behav. 1992;26:110–135. doi: 10.1016/0018-506x(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Songsasen N., Leibo S.P. Live mice derived from cryopreserved embryos derived in vitro with cryopreserved ejaculated spermatozoa. Lab. Anim. Sci. 1998;48:275–281. [PubMed] [Google Scholar]
- Sorden S.D., Castleman W.L. Brown Norway rats are high responders to bronchiolitis, pneumonia, and bronchiolar mastocytosis induced by parainfluenza virus. Exp. Lung Res. 1991;17:1025–1045. doi: 10.3109/01902149109064333. [DOI] [PubMed] [Google Scholar]
- Suber R.L., Kodell R.L. The effect of three phlebotomy techniques on hematologic and clinical chemical evaluation in Sprague-Dawley rats. Vet. Clin. Pathol. 1985;14:23–30. doi: 10.1111/j.1939-165x.1985.tb00842.x. [DOI] [PubMed] [Google Scholar]
- SultanDosa A.B., Bryner J.H., Foley J.W. Pathogenicity of Campylobacter jejuni and Campylobacter coli strain in the pregnant guinea pig model. Am. J. Vet. Res. 1983;44:2175–2178. [PubMed] [Google Scholar]
- Summa M.E.L., Ebisui L., Osaka J.T., de Tolosa E.M.C. Efficacy of oral ivermectin against Trichosomoides crassicauda in naturally infected laboratory rats. Lab. Anim. Sci. 1992;42:620–622. [PubMed] [Google Scholar]
- Suzuki E., Mochida K., Nakagawa M. Naturally occurring subclinical Corynebacterium kutscheri infection in laboratory rats: Strain and age related antibody response. Lab. Anim. Sci. 1988;38:42–45. [PubMed] [Google Scholar]
- Swing S.P., Davis J.K., Egan M.L. In vitro effects of Mycoplasma pulmonis on murine natural killer cell activity. Lab. Anim. Sci. 1995;45:352–356. [PubMed] [Google Scholar]
- Tada N., Sata M., Mizorogi T., Kasai K., Gawa S. Efficient cryopreservation of hairless mutant (bald) and normal Wistar rat embryos by vitrification. Lab. Anim. Sci. 1995;45:323–325. [PubMed] [Google Scholar]
- Tai J.H., Wang A.L., Ong S.J., Lai K.S., Lo C., Wang C.C. The course of giardiavirus infection in the Giardia lamblia trophozoites. Exp. Parasitol. 1991;73:413–423. doi: 10.1016/0014-4894(91)90065-5. [DOI] [PubMed] [Google Scholar]
- Tai J.H., Chang S.C., Chou C.F., Ong S.J. Separation and characterization of two related giardiaviruses in the parasitic protozoan Giardia lamblid. Virology. 1996;216:124–132. doi: 10.1006/viro.1996.0039. [DOI] [PubMed] [Google Scholar]
- Takeichi N., Hamada J. Depression of T cell-mediated immunity and enhancement of autoantibody production by natural infection with microorganisms in spontaneously hypertensive rats (SHR) Microbiol. Immunol. 1988;32:1235–1244. doi: 10.1111/j.1348-0421.1988.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Tanemura K., Wakayama T., Kuramoto K., Hayashi Y., Sato E., Ogura A. Birth of normal young by microinsemination with forzen-thawed round spermatids collected from aged azoospermic mice. Lab. Anim. Sci. 1997;47:203–204. [PubMed] [Google Scholar]
- Tayama K., Shisa H. Development of pigmented scales on rat skin: Relation to age, sex, strain, and hormonal effect. Lab. Anim. Sci. 1994;44:240–244. [PubMed] [Google Scholar]
- Thomas D.S., Hallmans G., Sjostrom R., Sunzel B., Wetter L., Wing K. The extent of coprophagy in rats with differing iron status and its effect on iron absorption. Lab. Anim. 1988;22:313–319. doi: 10.1258/002367788780746250. [DOI] [PubMed] [Google Scholar]
- Tucker M.J. The effect of long-term food restriction on tumors in rodents. Int. J. Cancer. 1979;23:803–807. doi: 10.1002/ijc.2910230611. [DOI] [PubMed] [Google Scholar]
- Tvedten H.W., Whitehair C.K., Langham R.F. Influence of vitamins A and E on gnotobiotic and conventionally maintained rats exposed to Mycoplasma pulmonis. J. Am. Vet. Med. Assoc. 1973;163:605–612. [PubMed] [Google Scholar]
- Tyzzer E.E. A fatal disease of Japanese Waltzing mice caused by a spore-forming bacillus (Bacillus piliformis n. sp.) J. Med. Res. 1917;37:307–338. [PMC free article] [PubMed] [Google Scholar]
- Urano T., Maejima K., Okada O., Takashina S., Syumiya S., Tamura H. Control of Pseudomonas aeruginosa infection in laboratory mice with gentamicin. Jikken Dobutsu. 1977;26:259–262. doi: 10.1538/expanim1957.26.3_259. [DOI] [PubMed] [Google Scholar]
- Utsumi K., Satoh E., Iritani A. Sexing of rat embryos with antisera specific for male rats. J. Exp. Zool. 1991;260:99–105. doi: 10.1002/jez.1402600113. [DOI] [PubMed] [Google Scholar]
- Van Andel R.A., Franklin C.L., Besch-Williford C.L., Hook R.R., Riley L.K. Cytokine expression in subclinical murine Tyzzer's disease. Contemp. Top. 1996;35(4):67. (Abstract) [Google Scholar]
- Vanderhyden B.C., Rouleau A., Walton E.A., Armstrong D.T. Increased mortality during early embryonic development after in-vitro fertilization of rat oocytes. J. Reprod. Fertil. 1986;77:401–409. doi: 10.1530/jrf.0.0770401. [DOI] [PubMed] [Google Scholar]
- Van Ruiven R., Meijer G.W., Wiersma A., Baumans V., van Zutphen L.F.M., Ritskes-Hoitinga J. The influence of transportation stress on selected nutritional parameters to establish the necessary minimum period for adaptation in rat feeding stud. Lab. Anim. 1988;32:446–456. doi: 10.1258/002367798780599893. [DOI] [PubMed] [Google Scholar]
- Van Winkle T.J., Womack J.E., Barbo W.D., Davis T.W. Incidence of hydronephrosis among several production colonies of outbred Sprague-Dawley rats. Lab. Anim. Sci. 1988;38:402–406. [PubMed] [Google Scholar]
- Van Zwieten M.J., Solleveld H.A., Lindsey J.R., de Groot F.G., Zurcher C., Hollander C.F. Respiratory disease in rats associated with a filamentous bacterium: A preliminary report. Lab. Anim. Sci. 1980;30:215–221. [PubMed] [Google Scholar]
- Vonderfecht S.L., Huber A.C. Infectious diarrhea of infant rats produced by a rotavirus-like agent. J. Virol. 1984;52:94–98. doi: 10.1128/jvi.52.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderfecht S.L., Miskuff R.L. Enzyme immunoassay inhibition assay for the detection of rat rotavirus-like agent in intestinal and fecal specimens obtained from diarrheic rats and humans. J. Clin. Microbiol. 1985;22:726–730. doi: 10.1128/jcm.22.5.726-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggie K.S., Spencer T.H., Allen A.M. Cilia associated respiratory (CAR) bacillus infection in New Zealand White rabbits. Lab. Anim. Sci. 1987;37:533. (Abstract) [Google Scholar]
- Wagner J.E. Self trauma and Staphylococcus aureus in ulcerative dermatitis of rats. J. Am. Vet. Med. Assoc. 1977;171:839–841. [PubMed] [Google Scholar]
- Wagner M. The effect of infection with the pinworm (Syphacia muris) on rat growth. Lab. Anim. Sci. 1988;38:476–478. [PubMed] [Google Scholar]
- Ward J.M., Reynolds C.W. Large granular lymphocyte leukemia. A heterogeneous lymphocytic leukemia in F-344 rats. Am. J. Pathol. 1983;111:1–10. [PMC free article] [PubMed] [Google Scholar]
- Watson D.S.B. Evaluation of inanimate objects on commonly monitored variables in preclinical safety studies for mice and rats. Lab. Anim. Sci. 1993;43:378–380. [PubMed] [Google Scholar]
- Weir E.C., Jacoby R.O., Paturzo F.X., Johnson E.A. Infection of SDAV-immune rats with SDAV and rat coronavirus. Lab. Anim. Sci. 1990;40:363–366. [PubMed] [Google Scholar]
- Weisbioth S.H. In: Foster H.L., Small D., Fox J.G., editors. Academic Press; New York: 1979. pp. 385–402. (“The Mouse in Biomedical Research”, Vol. 2. “Dissease”). [Google Scholar]
- Weishroth S.H. In: Foster H.L., Small D., Fox J.G., editors. Academic Press; New York: 1982. pp. 192–241. (“The Laboratory Rat,” Vol. 1. “Biology and Diseases”). [Google Scholar]
- Weishorth S.H., Peters R., Riley L.K., Shek W. Vol. 39. Nadonad Research Council; 1998. Microbiological assessment of laboratory rats and mice; pp. 272–290. (ILAR J.). 4. [DOI] [PubMed] [Google Scholar]
- Weisse I. In: Mohr U., Dungworth D.L., Capen C.C., editors. Vol. 2. ILSI Press; Washington, D.C.: 1992. pp. 65–119. (Pathobiology of the Aging Rat). [Google Scholar]
- Wescott R.B. In: Foster H.L., Small J.D., Fox J.G., editors. Academic Press; New York: 1982. pp. 373–384. (“The Mouse in Biomedical Research” Vol. 2 “Disease”). [Google Scholar]
- West W.L., Schofield J.C., Bennett B.T. Efficacy of the “microdot” technique for administering topical 1% ivermectin for the control of pinworms and fur mites in mice. Contemp. Top. 1992;31:7–10. [Google Scholar]
- Wickramanayake G.B., Sproul O.J. In: Disinfection, Sterilization, and Preservation. 4th ed. Block S.S., editor. Lea and Febiger; Philadelphia: 1991. pp. 72–84. [Google Scholar]
- Will L.A. In: Handbook of Zoonoses, Section A: Bacterial, Rickettsial, Chlamydial, and Mycotic. 2nd ed. Beran G.W., editor. CRC Press; Boca Raton, Florida: 1994. pp. 231–240. [Google Scholar]
- Wilson J.M. The biology of Encephalitozoon cuniculi. Med. Biol. 1979;57:84–101. [PubMed] [Google Scholar]
- Wray C. In: Handbook of Zoonoses, Section A: Bacterial, Rickettsial, Chlamydial, and Mycotic. 2nd ed. Beran G.W., editor. CRC Press; Boca Raton, Florida: 1994. pp. 289–302. [Google Scholar]
- Wullenweber M. Streptobacillus moniliformis—a zoonotic pathogen. Taxonomic considerations, host species, diagnosis, therapy, geographical distribution. Lab. Anim. 1995;29:1–15. doi: 10.1258/002367795780740375. [DOI] [PubMed] [Google Scholar]
- Wyand D.S., Jonas A.M. Pseudomonas aeruginosa infection in rats following implantation of an indwelling jugular catheter. Lab. Anim. Care. 1967;17:261–267. [PubMed] [Google Scholar]
- Yang F.C., Paturzo F.X., Jacoby R.O. Environmental stability and transmission of rat virus. Lab. Anim. Sci. 1995;45:140–144. [PubMed] [Google Scholar]
- Yarbrough W.G., Quarmby V.E., Simental J.A., Joseph D.R., Sar M., Lubahn D.B., Olsen K.L., French F.S., Wilson E.M. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J. Biol. Chem. 1990;265:8893–8900. [PubMed] [Google Scholar]
- Yoshitomi K., Boorman G.A. In: Pathology of the Fischer Rat. Reference and Atlas. Boorman G.A., Eustis S.L., Elwell M.R., Montgomery C.A., MacKenzie W.F., editors. Academic Press; San Diego: 1990. pp. 239–259. [Google Scholar]
- Yu D.C., Wang A.L., Wang C.C. Amplification, expression, and packaging of a foreign gene by giardiavirus in Giardia lamblia. J Virol. 1996;70:8752–8757. doi: 10.1128/jvi.70.12.8752-8757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D.C., Wang A.L., Botka C.W., Wang C.C. Protein synthesis in Giardia lamblia may involve interaction between a downstream box (DB) in mRNA and an anti-DB in the 16S-like ribosomal RNA. Mol. Biochem. Parasitol. 1998;96:151–165. doi: 10.1016/s0166-6851(98)00126-1. [DOI] [PubMed] [Google Scholar]
- Zubaidy A.J., Majeed S.K. Pathology of the nematode Trichosomoides crassicauda in the urinary bladder of laboratory rats. Lab. Anim. 1981;15:381–384. doi: 10.1258/002367781780952915. [DOI] [PubMed] [Google Scholar]


