Abstract
Whereas active immunity refers to the process of exposing the individual to an antigen to generate an adaptive immune response, passive immunity refers to the transfer of antibodies from one individual to another. Passive immunity provides immediate but short-lived protection, lasting several weeks up to 3 or 4 months. Passive immunity can occur naturally, when maternal antibodies are transferred to the fetus through the placenta or from breast milk to the gut of the infant. It can also be produced artificially, when antibody preparations derived from sera or secretions of immunized donors or, more recently, different antibody producing platforms are transferred via systemic or mucosal route to nonimmune individuals. Passive immunization has recently become an attractive approach because of the emergence of new and drug-resistant microorganisms, diseases that are unresponsive to drug therapy and individuals with an impaired immune system who are unable to respond to conventional vaccines. This chapter addresses the contributions of natural and artificial acquired passive immunity in understanding the concept of passive immunization. We will mainly focus on administration of antibodies for protection against various infectious agents entering through mucosal surfaces.
Keywords: Antibodies, Antibody fragments, Antibody platforms, Infection, Mucosa, Passive immunity, Pathogens
Passive Immunity: A Historical Perspective
In 1890, Emil von Behring and Shibasaburo Kitasato, working in Robert Koch’s laboratory in Berlin, Germany, initially reported that injections of toxin from diphtheria or tetanus bacilli led animals to produce in their blood substances capable of neutralizing the toxins. Furthermore, injections of blood serum from an animal that had been given a chance to develop antitoxins to tetanus or diphtheria could confer immunity to the disease on other animals and even cure animals that were already sick. From this, von Behring concluded that immunity was conferred by protective substances in the blood, which he called antitoxins, and that these substances were specific, protecting against only one particular disease (Winau et al., 2004). In the same period in Robert Koch’s laboratory, Paul Ehrlich showed that acquired immunity in mice can be transferred to the offspring from the mother by supplying antibodies to the fetus through the circulation and to the neonate through colostrum (Silverstein, 1996). He was the first to use the term “Antikörper” in one of his articles and to define the differences between active and passive immunity.
On the suggestion of Koch, Behring and Ehrlich worked on the optimization of large-scale production of the diphtheria antitoxin for human use. Clinical tests with diphtheria serum in early 1894 were successful and in August the chemical company Hoechst started to market “Diphtheria remedy synthesized by Behring-Ehrlich.” In 1904, Paul Ehrlich started developing the imaginative idea that it should be possible to find chemicals that target and kill disease-causing organisms while leaving normal body cells unharmed, which he named “magic bullets.” He used the term first to describe an antibody and later, a chemical that binds to and specifically kills microbes or tumor cells. Von Behring won the first Nobel Prize in Physiology or Medicine in 1901 for his work on serum therapy, while Ehrlich received a Nobel Prize (together with Elie Metschnikoff) in 1908 for his “side-chain” theory, a chemical theory to explain the formation of antibodies by circulating cells. Their work led to the development of a theory of humoral immunity and opened the door for specific treatment of infections using antisera and antibodies.
Serum therapy was largely used at the beginning of the century but was abandoned owing to hypersensitivity reactions and the introduction of antibiotics in the 1940s. In the second half of the twentieth century, the inability to treat certain viral diseases and the development of antibiotic resistance in bacteria drove efforts to develop antibody preparations suitable for oral and systemic administration (Casadevall, 1999, Good and Lorenz, 1991). The development of hybridoma technology for the production of monoclonal antibodies (mAbs) provided the means to produce large amount of antibodies with one specificity and isotype (Kohler and Milstein, 1975). Technological advances, such as chimerization and humanization of mAbs, DNA shuffling, and phage display technology led to the generation of antibodies or antibody fragments (scFv and Fab fragment) with improved efficiency and reduced immunogenicity (Chan et al., 2009). New methods of production of antibodies were developed, including recombinant bacteria, insect cells, transgenic plants, and transgenic animals. The discovery of camelidae heavy chain antibody lacking light chains resulted in the development of single domain antibody fragments or nanobody technology (Hamers-Casterman et al., 1993). Meanwhile, a new generation of receptor proteins derived from small and robust non-immunoglobulin “scaffolds” was born (Binz et al., 2005).
Role of Antibodies in Protection Against Pathogens
The vast majority of infectious agents invade the human body through the epithelial barrier of the gastrointestinal, genitourinary, and respiratory tracts. The mucosal immune system responds by producing large amount of immunoglobulins in mucosal secretions that act as a first line of defense against these pathogens. In humans, secretory IgA (SIgA) represents the most abundant immunoglobulin in body secretions such as saliva, tears, colostrum, and gastrointestinal; together with a variety of innate mucosal defense mechanisms, the function of secretory antibodies is to perform immune exclusion of exogenous antigens (Brandtzaeg, 2007). If the pathogens cross the epithelium and invade the body, locally produced antibodies will no longer determine the fate of the host and systemic immunity will take over. Serum immunoglobulin (particularly immunoglobulin [Ig]G) will then function as a second line of defense by eliminating pathogens that have breached the mucosal barrier. Patients with primary immunodeficiencies involving IgA are known to be highly susceptible to respiratory and gastrointestinal tract infections, which supports the notion of a crucial role for antibodies in the mucosal defense and suggests a useful role of passive immunity in the management of mucosal infections (Weiner et al., 1999).
Antibodies that bind to antigens may mediate various different biological effects (Casadevall et al., 2004). In bacterial infections they can block adhesins, promote agglutination, neutralize enzyme activity and toxins, facilitate opsonization, and, together with complement, promote bacteriolysis; in viral disease, antibodies block viral entry into uninfected cells, promote antibody-directed cell-mediated cytotoxicity by natural killer cells, and neutralize virus directly or with the participation of complement.
Naturally Acquired Passive Immunity
Maternal passive immunity is a type of naturally acquired passive immunity and refers to antibody-mediated immunity. Transfer of maternal antibodies from mother to fetus or the newborn is essential for the development of the immune system and the protection of young animals from various pathogens in their early lives. The transfer of passive immunity from mother to young is a feature of most if not all orders of mammals. This generally occurs by transfer of maternal serum IgG from the mother to the offspring either in utero or after birth, by ingestion of immunoglobulin-rich colostrum by the neonate.
The pathways for transfer of passive immunity vary in different animals. Maternal IgG is transferred mainly through the placenta before birth in guinea pigs, rabbits, nonhuman primates, and humans, whereas in ruminant (sheep, cattle, and goats), horses, and pigs, newborns are born agammaglobulinemic and receive maternal antibodies exclusively through colostrum (Lu et al., 2007). In rodents, cats, and dogs, maternal antibodies are transported to the offspring both antenatally and neonatally.
A pivotal molecule responsible for the transfer of maternal IgG in mammals is the neonatal Fc receptor (FcRn) (see also Chapter 20). Neonatal Fc receptor is a major histocompatibility complex class I-related molecule consisting of an α-chain and β2-microglobulin, and was first identified as the protein that mediated transfer of maternal, milk-born IgGs across the neonatal rodent intestine (Ghetie and Ward, 2000, Jakoi et al., 1985). Further studies revealed a similar receptor in humans, where it was found to facilitate transport of maternal IgG to the growing fetus (Leach et al., 1996). FcRn has since been identified in other mammals including nonhuman primates (Spiekermann et al., 2002) and ruminants such as sheep and cows (Mayer et al., 2002, Mayer et al., 2004). Neonatal Fc receptor also mediates transport of IgG through multiple mucosal barriers and protects both IgG and albumin from intracellular catabolic degradation and thus extends their half-lives.
In birds, maternal IgY, the homolog of IgG, is transferred across the yolk sac to immunize chicks passively during gestation and early life. The chicken yolk sac IgY receptor (FcRY) is the ortholog of the mammalian phospholipase A2 receptor, a mannose receptor family member, rather than an FcRn or major histocompatibility complex homolog (West et al., 2004).
Milk Antibodies
In humans, the main role of colostrum consumption is to provide protection for the gastrointestinal tract because the IgG required to provide systemic immunity is transferred across the placenta before birth. This probably explains the high proportion of SIgA (90%) and low proportion of IgG (2%) in human colostrum and milk. Immunoglobulin A antibodies in breast milk reflect antigenic stimulation of gut-associated lymphoid tissue and nasopharynx-associated lymphoid tissue such as the tonsils. Breast milk thus contains antibodies directed against infectious agents and other exogenous antigens in the mother’s environment, which are those likely to be encountered by the infant. Human breast milk may contain SIgA antibodies against a variety of infectious agents such as Vibrio cholera, Campylobacter jejuni, Escherichia coli (ETEC), Shigella, Salmonella, and rotavirus that protect the infected child from developing diarrhea (Lawrence and Pane, 2007).
In animals, the content of Igs in colostrum and milk highly depends on the species (Hurley and Theil, 2011). In ungulate species such as cattle, sheep, goats, and pigs, the young are born essentially agammaglobulinemic and rely entirely on uptake of colostral Igs, especially IgG, for systemic immune protection. Colostral IgG content in these species is typically greater than 75% of the total Ig content. The total amount of Igs may also differ between colostrum and milk and bovine colostrum contains levels of Igs much higher (several 100-fold) than ordinary bovine milk (Hurley and Theil, 2011). Immunoglobulins found in mammary secretions arise from systemic and local sources. In the case of IgG in milk, the major portion comes from the serum and enters via a selective receptor-mediated intracellular route through receptors (FcRn) on the surface of epithelial cells (Mayer et al., 2005). The uptake of milk-derived IgG by the intestine also varies between species. In rats and mice, there is FcRn-mediated uptake of IgG from the colostrum and milk in the neonate intestine. In newborn artiodactyls, all proteins (including IgG) are nonselectively absorbed in the first 12 h after birth and the ability of the enterocytes to pinocytically transport IgG is lost after 24 h (Quigley, 2002). Macromolecules so transported are released into the lamina propria and are then absorbed into the lymphatic or portal circulation.
Vaccination or natural immunization of the pregnant cow, ewe, or sow against enterotoxigenic E. coli or intestinal viruses can provide a degree of protection for the newborn (Hurley and Theil, 2011). In neonatal calves and piglets, maternal Ig is transferred into respiratory tract secretions and contributes to local protection against infections caused by viruses including bovine respiratory syncytial virus (RSV) or porcine respiratory coronavirus (Belknap et al., 1991, Sestak et al., 1996).
These results on the transfer of antibodies have been crucial in understanding passive immunity against pathogens and developing passive immunotherapy in the management of such infections.
Artificial Induction of Passive Immunity
Advantages and Disadvantages
Unlike active immunity, which can take days or weeks to develop, passively administered antibodies have the ability to provide rapid and immediate protection: for example, against agents of bioterrorism (Casadevall et al., 2004). Unlike vaccination, with which protective immunity depends on the host’s ability to mount an immune response, passive antibody is independent of the recipient’s immune status. Passive immunization may thus represent the therapy of choice in highly endemic areas where vaccine responses may be poor, or in selected groups of patients such as hospitalized individuals or those suffering from malnutrition and immunodeficiency, or in individuals in whom vaccination is contraindicated.
However, protection conferred by passive immunization is of short duration and might need repeated administration. Furthermore, when given at the mucosal surface, such as oral delivery, antibodies may be degraded by gastric acid and proteolytic enzymes. Production and purification of large amount of antibodies can thus result in high costs. Another disadvantage for mAbs is the emergence of variants that lack the determinant that the antibody recognizes, such as viral escape mutants. Recent research makes use of technologies for engineering more resistant antibodies that can react to multiple epitopes.
Source of Antibodies
The choice of an antibody used for therapy against an infectious agent may depend on the route of administration, the status of the host, the microorganism that is targeted, or economic factors. Various antibody isotypes may confer different biological properties to the antibody molecules. Certain classes of Igs, such as IgA, appear to be more resistant to proteolytic degradation than other classes and therefore have some advantages for oral administration. Whereas eradication of an infection may require a full antibody to mediate phagocytosis, complement activation, or antibody-dependent cellular cytotoxicity, an antibody fragment may be sufficient for neutralization of a toxin or a virus. Immunoglobulin A or antibody fragments not interacting with the host immune system might also be more desirable for protection of mucosal surfaces to promote immune exclusion without inducing inflammation.
Furthermore, humanized mAbs are more appropriate for intravenous administration than a polyclonal antibody because they are less likely to transmit infectious diseases and cause toxicity. However, polyclonal antibody preparations are usually less costly to produce and may be a better choice for oral administration.
Polyclonal Serum Antibodies
Protection against certain infections or a reduction in the severity of the illness they cause can be achieved by administering polyclonal antibodies derived from human or animal serum. The preparations available may be standard Ig of human origin, sometimes referred to as immune serum globulin or gammaglobulin, or special preparations of either human or animal sera containing high titers of specific antibodies to a particular microorganism or its toxin (Orange et al., 2006). Products of human origin are preferred over those of animal origin because of the high incidence of adverse reactions to animal sera and the longer-lasting protection conferred by human Igs.
Immunoglobulin, often referred to as gammaglobulin, is produced by combining the IgG antibody fraction from thousands of adult donors. It is based on the assumption that a large pool will contain protective levels of antibodies of different specificities including antibodies against many common diseases such as hepatitis A, measles, and rubella. The Ig product is sterile and contains more than 95% purified IgG with small amounts of IgA and IgM. It is used primarily for post-exposure prophylaxis for hepatitis A, rubella, and measles and treatment of certain primary immunodeficiency disorders (X-linked α-gammaglobulinemia and hypogammaglobulinemia), secondary immunodeficiency (such as B-cell chronic lymphocytic leukemia), or autoimmune diseases. Historically, Igs were given subcutaneously or intramuscularly because the IgG preparations contained aggregated IgG and impurities that often caused serious reactions when administered intravenously. Today, intravenous Ig (IVIG) and subcutaneous Ig (SCIG) replacement therapy is generally accepted as treatment for selected patients with immunodeficiencies. Currently, the accepted therapy for IgG deficiency is the intravenous administration of 300–600 mg/kg IgG once every 3–4 weeks or 100–200 mg/kg/week subcutaneously (Orange et al., 2006). Immunoglobulin therapy, given by both the intravenous and subcutaneous routes, is equally efficacious in infection reduction (Gardulf et al., 2006, Chapel et al., 2000) and decreases the frequency and severity of otitis and respiratory tract infection (Stiehm et al., 2010).
Human hyperimmuneglobulins are made from the donated plasma of humans with high levels of the antibody of interest. Some preparations of Ig are harvested from selected individual donors who either recently recovered from the disease or have been deliberately immunized against it. Hyperimmuneglobulins are used for postexposure prophylaxis for several diseases, including hepatitis B, rabies, tetanus, cytomegalovirus, and varicella (Stiehm et al., 2010). Heterologous hyperimmune serum is produced in animals, usually horses (equine), against toxins from Clostridium botulinum, Clostridium tetani, and Corynebacterium diphtheriae (Casadevall et al., 2004). A problem with this product is serum sickness, an immune reaction to horse proteins.
Polyclonal Antibodies from Cows and Chicken
There are a number of advantages to using cows and chickens for the production of antibodies. Bovine colostrum and IgY production are less invasive, requiring only the collection of eggs or milk compared with blood collection in mammals, and represent a relatively inexpensive source of antibodies.
Colostrum from immunized animals may have more than a 100-fold increase in antibody titers compared with colostrum from nonimmunized animals (Janson et al., 1994). Colostrum contains between 30 and 200 mg of immunoglobulin per milliliter, most (75%) of which is IgG1 (Berghman et al., 2005). The cow produces about 1–1.5 kg of immunoglobulins in the first few days after calving; it is thus attractive for large-scale antibody production. Immunization protocols vary and generally, repeated immunizations are administered during late pregnancy or during the dry period. Many of these studies have used intramuscular or subcutaneous immunization, although some also have incorporated oral or intramammary immunizations but with less success. Mammary secretions are then collected either at the first milking or are pooled from the first four to six milkings or from the first six to 10 days after calving (Hurley and Theil, 2011).
Immunization of chickens requires only small amounts of antigens to obtain high and long-lasting IgY titers in the egg yolk (Tini et al., 2002). The most common injection route is intramuscular because it results in higher levels of specific IgY antibodies than subcutaneous immunization (Chang et al., 1999). The concentration of IgY in egg yolk can reach 25 mg/mL. Because a hen can lay up to 250 eggs in a year, the yield of hyperimmunized IgY could be large. Because 160 mg of IgY could be obtained from a single egg, one immunized hen could produce 40 g of IgY in a year, of which 1–10% can be expected to be antigen-specific (Hatta et al., 1997). In contrast to mammalian serum, egg yolk contains only a single class of antibody (IgY), which can easily be isolated from the yolk by precipitation techniques, and IgY does not activate mammalian complement or interact with mammalian Fc receptors that could mediate an inflammatory response (Kovacs-Nolan and Mine, 2012).
The resistance of bovine IgG1 and IgY to low pH and luminal proteolysis makes them functionally similar to human IgA and suitable for oral administration (Kovacs-Nolan and Mine, 2012). Ruminants transmit maternal immunity only postnatally through colostral antibodies (Quigley, 2002), so colostrum and egg yolk antibodies are routinely used for prophylaxis and therapy of infectious diseases in farm animals (Berghman et al., 2005). Bovine immunoglobulin preparations against rotavirus and E. coli ETEC have been commercially available for use in farm animals for decades owing to their low cost and easy administration.
A high number of controlled clinical studies using hyperimmune bovine colostrum or egg yolk antibodies have shown both prophylactic and therapeutic effects against oral and gastrointestinal pathogens in humans, including enterotoxicogenic E. coli, Helicobacter pylori, the dental caries causing Streptococcus mutans, and rotaviral infections (Hurley and Theil, 2011, Kovacs-Nolan and Mine, 2012, Weiner et al., 1999). The ability of bovine and egg yolk antibodies to provide protection against a specific disease continues to be an area of interest (Ng et al., 2010). For example, bovine IgG with gp140 reactivity and able to neutralize human immunodeficiency virus (HIV) has been developed as a potentially efficacious and affordable topical microbicide (Kramski et al., 2012).
Monoclonal Antibodies by Hybridoma Technology
The first generation of mAb was produced by a single hybridoma clone obtained by the fusion of a myeloma cell with plasma cells from an immunized mouse. The use of therapeutic mAbs of animal origin was compromised by immunologic responses to the mAbs themselves. The creation of chimeric mAbs composed of human constant domains (CH and CL) and rodent variable domains (VH and VL) led to a reduction of human anti-chimeric antibody response. Humanization of mAbs consisting of CDRs of a rodent mAb cloned into the framework regions of a human mAb reduced the immunogenicity further, but not as much as expected (Chan et al., 2009). Furthermore, humanization is often accompanied by a loss in affinity and some residues in the framework regions need to be back-mutated to achieve acceptable affinity.
To circumvent the immunogenicity issue, full human mAbs can be generated from transgenic mice (Chan et al., 2009, Lonberg, 2005). This relies on the generation of transgenic mice in which the murine immunoglobulin genes have been disrupted and replaced with human Ig gene clusters. The transgenic mice produce human antibodies in response to immunization with an antigen and the clone can be isolated through hybridoma technology or phage antibody libraries.
Nearly 30 therapeutic mAbs have been approved by the Food and Drug Administration for marketing in the United States today (Zhang, 2012). Most of them originated from hybridomas and are in the full-length antibody molecular format, including the murine, chimeric, humanized, and human antibody category. Only one mAb, palivizumab (humanized mAb), has been licensed for an infectious disease (respiratory syncytial virus infection) (Buss et al., 2012, Huang et al., 2010). Monoclonal Abs against HIV, viral hepatitis, rabies, Clostridium difficile toxins, and Pseudomonas aeruginosa are under clinical development (Buss et al., 2012, Nelson et al., 2010). Raxibacumab, a human mAb against the protective antigen (PA) of Bacillus anthracis, significantly increased survival in monkeys (64% vs 0% for placebo) after inhalation of anthrax spores (Migone et al., 2009). The license, resubmitted in 2012, contains additional validation of previous data and evaluation of potential added benefit of using raxibacumab with antibiotics versus antibiotics alone.
Recombinant Antibodies Using Phage Display
Limitations in hybridoma technology and advances in molecular biology led several research groups to investigate the production of antibody in a recombinant form. Because the full antibody cannot be easily expressed in bacteria owing to impaired folding, a technology in which only antigen-binding parts of the antibody molecule (Fab or Fv fragments) are expressed in E. coli has been introduced (Skerra and Pluckthun, 1988). It was later shown that an scFv fragment can be displayed on the phage surfaces as a functional protein that retains an active antigen-binding domain capability (Finlay et al., 2011). In the past decade, the phage display technique has been routinely used for generation and selection of scFv, Fab, and single VL or VH domain derived from conventional antibodies and variable domain of camelidae heavy chain antibodies (VHH) (Figure 1 ). There is growing interest in producing new formats of antibodies to be tailored as desired and produced in large quantities, employing bacterial and yeast cultures.
Figure 1.
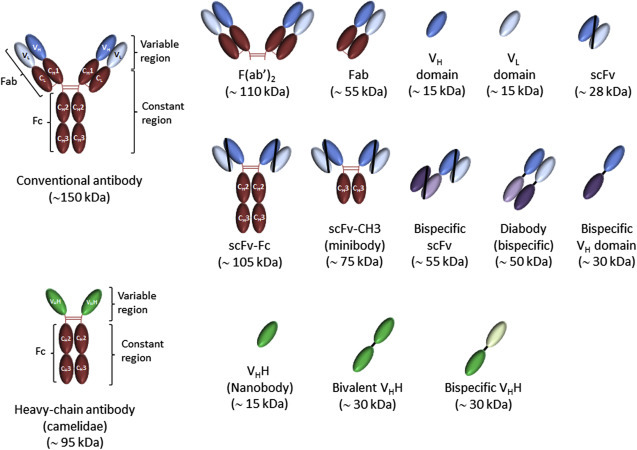
Schematic representation of antibodies and antibody fragments.
The conventional antibody contains two variable regions (each composed of VH and VL domains) that confer antigen-binding specificity of antibody and an Fc fragment in the constant region that recruits effecter functions of the immune system. Camelidae heavy chain antibodies lack both constant and variable light chains (CL and VL) and the first heavy chain constant (CH1) domain, and the antigen-binding site is formed only by the heavy chain variable domain (VHH or nanobody).
Antibody Fragments Derived from Conventional Antibodies (scFv, Fab, VH, and VL)
A single chain fragment variable (scFv) is about 30 kDa and consists of variable regions of heavy (VH) and light (VL) chains that are joined together by a flexible peptide linker. In the scFv, the order of the domains can be either VH-linker-VL or VL-linker-VH and in both orientations. The peptide linker is usually a 15-aa linker with the sequence (Gly4Ser)3 (Finlay et al., 2011). The larger Fab is a heterodimer consisting of the variable and first constant domains of heavy (VH-CH) and light chain (VL-CL) segments linked by disulfide bonds. These fragments show similar binding specificities as the original antibodies and a low degree of immunogenicity and are more easily manipulated than the bivalent parent antibody. The smaller size of the fragments permits penetration into tissues inaccessible to full-size mAbs (Yokota et al., 1992). On the downside, fragments demonstrate short circulating half-lives in humans, most likely owing to kidney clearance. The Fab or Fv fragments can be successfully generated from hybridoma cells (Kruger et al., 2002). However, to select antibody fragments with different specificities and a high binding affinity, it is generally preferable to select the fragments directly from a phage library.
The selected scFv, Fab, VH, or VL can be directly used as fragments or reconverted into different antibody formats such as full-length antibodies, scFv-CH3 (minibody), scFv-Fc, or diabodies, among others (Holliger and Hudson, 2005, Nelson et al., 2010) (Figure 1). The phage display technology has been used to generate at least 35 full-length human antibodies that are in clinical development, including CR6261 (influenza virus) and KB001 (Ps. aeruginosa) (Nelson et al., 2010).
Camilidae VHH Antibody
Members of the Camelidae family (i.e., Camelus dromedarius, Camelus bactrianus, Lama glama, Lama guanaco, Lama alpaca, and Lama vicugna) produce heavy chain-only antibodies (HCAbs), a type of antibody that lacks the first constant domain (Chapter 1) and light chains (Hamers-Casterman et al., 1993, Rahbarizadeh et al., 2011). The antigen-binding fragments of such HCAbs are composed in a single-domain, referred to as VHH (variable domain of llama heavy chain antibodies) or nanobody. Camelidae heavy chain antibodies are small (12–15 kDa), stable molecules with superior solubility and similar affinities as conventional antibodies (Harmsen and De Haard, 2007).
Camelidae heavy chain antibodies exhibit several advantages over conventional antibodies and derived fragments because they are markedly more acid- and heat-resistant than conventional antibodies, and because they are formed by a single polypeptide, they are easier to express in a functional recombinant form (Harmsen and De Haard, 2007, Vanlandschoot et al., 2011). These properties make them suitable for therapy at mucosal sites such as the gastrointestinal tract, where the acidic pH can limit the functionality of conventional antibodies. In addition, owing to their small size and naturally longer CDR3 regions, llama VHH antibody fragments are superior to conventional antibodies at accessing clefts such as active sites of enzymes and canyons on virus capsids (Lauwereys et al., 1998). Their small size also allows them to penetrate tissues and tumors more rapidly and deeply than mAbs. Camelidae heavy chain antibodies have low immunogenicity and are unlikely to exhibit untoward side effects during chronic application (Coppieters et al., 2006). Moreover, camel milk contains heavy chain antibodies and is regularly consumed in African/Arabic countries without adverse effects.
Camelidae heavy chain antibodies are usually generated by polymerase chain reaction cloning of the variable domain repertoire from blood, lymph nodes, or spleen cDNA obtained from immunized animals into a phage display vector. Large-scale production of VHH in the yeast Saccharomyces cerevisiae is highly efficient and results in the secretion of functional antibody fragments (VHH) in the growth medium (Frenken et al., 2000). Several VHH are currently being studied for use in various areas, including infectious diseases (Harmsen and De Haard, 2007). Camelidae heavy chain antibodies have been generated against protozoa (Trypanosoma evansi), bacteria (Str. mutans, Cl. difficile, and Cl. botulinum), and viruses (respiratory syncytial virus, rotavirus, rabies virus, influenza, and HIV) (Rahbarizadeh et al., 2011, Vanlandschoot et al., 2011). Camelidae heavy chain antibodies against RSV and rotavirus are in clinical development (Vanlandschoot et al., 2011). Oral consumption of VHH against rotavirus has also been confirmed to be safe in human safety studies (Sarker et al., 2013).
The small size (gene and domain) and the strict monomeric and soluble behavior render VHH ideal to generate multivalent or multispecific constructs, or for integration in more complex assemblies by fusing to an Fc, an enzyme, or a toxin (Vanlandschoot et al., 2011). Bivalent or bispecific VHH constructs can be assembled to target multiple antigens or epitopes on the same cell or antigen, greatly improving neutralization potencies and reducing the risk of escape mutants (Hultberg et al., 2011).
Engineered Protein Scaffolds
With the emergence of protein engineering techniques, new binding proteins based on alternative scaffolds have been designed as therapeutic agents. New scaffold proteins should have more attractive physical and chemical properties than conventional antibodies such as higher solubility, affinity, and stability, or an absence of disulfide bridges for optimal expression in microorganisms. The diversity of potential applications has led to the investigation of more than 40 scaffolds including affibodies, fibronectins, DARPins, and variable lymphocyte receptors (VLR) of lamprey, among others (Alder et al., 2008, Boersma and Pluckthun, 2011, Hackel et al., 2008, Nygren, 2008, Tasumi et al., 2009). To confer effector functions on these proteins, one can also fuse these scaffolds to the Fc region of antibodies. Several alternative binding proteins are now under preclinical investigation, but few data exist on the serum half-life, tissue penetration, or immunogenicity of most alternative binding molecules.
Production System for Recombinant Antibodies
Several expression systems are available for the production of antibodies and antibody fragments, including bacteria, yeast, plant, insect, and mammalian cells as well as transgenic animals (Schirrmann et al., 2008). Mammalian cell cultures, predominantly Chinese hamster ovary cells and the murine myeloma cell line NSO, are the dominant production platforms for therapeutic mAb whereas yeast is generally used for VHH fragments.
There is a need to develop technology to produce antibodies at lower costs. Although tobacco was used in the early studies (Hiatt et al., 1989), a large number of different crops can now be used to produce antibodies and antibody fragments, including cereals, legumes, and fruits (Schirrmann et al., 2008) (see also Chapter 66). Plantibodies to combat infectious diseases include SIgA that recognizes the surface antigen I/II of Str. mutans for protection against caries produced in tobacco, a humanized anti-CCR5 antibody (Mapp66) produced in tobacco and an anti-gp120 human mAb produced in maize for use as a topical microbicide to prevent HIV infection, and a VHH fragment against rotavirus produced in rice (Mucorice-ARP1) (Tokuhara et al., 2013, Yusibov et al., 2011). Plant bioreactors are expected to yield over 10 kg of therapeutic antibody per acre of tobacco, maize, soybean, and alfalfa and costs at least 10 times less compared with steel-tank bioreactors using mammalian cells or microorganisms (Larrick and Thomas, 2001). Antibody-containing cereal such as rice, wheat, and pea seeds can be stored at room temperature, a major advantage for use in developing countries.
The use of transgenic farm animals for production of mAbs in milk and egg yolk was investigated previously (Castilla et al., 1998, Demain and Vaishnav, 2009, Houdebine, 2009, Kawabe et al., 2006, Lillico et al., 2005, Pollock et al., 1999, Zhu et al., 2005). The advantages of transgenic animals for antibody production would be the low cost production as well as the high quality of the proteins. Farm animals could also be used for the production of polyclonal human antibodies (Houdebine, 2011). Transgenic cattle have been generated by transferring a human artificial chromosome vector carrying the entire unrearranged, human Ig heavy (hIGH) and κ-light (hIGK) chain loci to bovine fibroblasts in which two endogenous bovine IgH chain loci were inactivated (Kuroiwa et al., 2009). The production of only human antibodies awaits for the complete inactivation of the corresponding cow Ig genes. A similar approach is also currently being performed in chickens (Houdebine, 2011).
Lactobacilli are gram-positive bacteria that are normal residents of the oro-gastrointestinal and vaginal tract and are also used in food fermentation and preservation. Their generally regarded as safe for humans status has led to reports in which lactobacilli producing antibody fragments (i.e., lactobodies), either secreted or displayed on their surface, are directly used for oral administration and treatment of mucosal infections (Andersen et al., 2014, Kruger et al., 2002, Kruger et al., 2005, Marcotte et al., 2006, Martin et al., 2011, Pant et al., 2006). Stable and contained expression systems have been developed allowing transformed lactobacilli to be administered to humans (Martin et al., 2011). The antibody fragment can be expressed in lactobacilli isolated from the oral, gastrointestinal, or vaginal tract to protect different mucosal surfaces. The use of genetically engineered antibody fragments produced and locally delivered by bacteria in the mucosal sites could thus provide an efficient therapy at low cost, particularly in developing countries.
Passive Administration of Antibodies
Passive immunization has been used for more than 100 years against various pathogens in both animals and humans. We will summarize published data on the use of antibodies for prophylaxis and therapy against selected pathogens that infect the oral, pulmonary, gastrointestinal, and vaginal tracts (Table 1, Table 2, Table 3, Table 4 ). We also want to highlight the protection conferred by different forms of antibodies that are being developed, produced, and delivered using different approaches and technologies.
Table 1.
Passive Immunization Against Oral Pathogens
| Pathogen | Antibody Source | Targeted Antigen | Subject | Administration | Outcome | References |
|---|---|---|---|---|---|---|
| Streptococcus mutans | Cow milk | Str. mutans | Rat | Diet supplemented with immune milk | Decrease in caries and count of Str. mutans in dental plaque | Michalek et al. (1987) |
| Cow milk | Str. mutans | Human | Daily mouth rinse with immune milk | Reduced counts of Str. mutans in dental plaques | Filler et al., 1991, Loimaranta et al., 1999a | |
| IgY | Str. mutans or Gtfs | Rat | Diet supplemented with IgY | Inhibition of dental plaque accumulation and caries development | Hamada et al., 1991, Kruger et al., 2004, Otake et al., 1991 | |
| IgY | Str. mutans | Human | Daily mouth rinse containing IgY | Decreased number of Str. mutans in the saliva of healthy volunteers | Hatta et al. (1997) | |
| Murine mAb | AgI/II | Rhesus monkey | Repeated topical application of mAbs (Guy’s 13, IgG1) onto teeth | Prevented colonization by Str. mutans and caries development over a period of 1 year | Lehner et al. (1985) | |
| Murine mAb | AgI/II | Human | Topical application (3 times) of mAbs Guy’s 13 onto teeth | Reduced the colonization of exogenously applied Str. mutans | Ma et al. (1987) | |
| Murine mAb | AgI/II | Human | Topical application (6 times) of mAbs Guy’s 13 onto teeth | Prevented the recolonization of resident Str. mutans for up to 2 years | Ma et al., 1989, Ma et al., 1990 | |
| Plant-SIgA/G | AgI/II | Human | Topical application (6 times) of SIgA/G (CaroRx™) onto teeth | Prevented the recolonization of resident Str. mutans for up to 4months | Ma et al., 1995, Ma et al., 1998 | |
| Lactobacilli- scFv | AgI/II | Rat | Repeated topical application of lactobacilli-scFv anchored in the oral cavity | Reduced Str. mutans counts and caries scores | Kruger et al., 2002, Kruger et al., 2005 | |
| VHH | AgI/II | Rat | Orally daily pipetting of VHH (S-36) | Reduced development of caries | Kruger et al. (2006) | |
| Porphyromonas gingivalis | IgY | Gingipains | Human | Single subgingival application of IgY gel | Prevented recolonization by Po. gingivalis for a period of 4 weeks | Yokoyama et al. (2007b) |
| Mouse mAb | Gingipain RgpA | Human | Subgingival application (4 times) of mAb 61BG1.3 (IgG1) | Prevented recolonization of deep pocket with Po. gingivalis for up to 9 months | Booth et al. (1996) | |
| Candida albicans | IgY | Candida | Mouse | Daily oral application of a IgY gel | Reduced oral candidiasis and systemic dissemination in immunosuppressed mice | Ibrahim et al. (2008) |
| Bovine colostrum | Candida + mannan | Human | Daily oral administration of bovine antibodies in a chocolate drink | Reduction in Candida count in saliva of bone marrow–transplanted patients | Tollemar et al. (1999) | |
| Human scFv | hsp90 | Mouse | Single intravenous dose of efungumab (Mycograb®) in combination with amphotericin B | Combination more efficient than amphotericin B only in killing Can. albicans | Matthews et al. (2003) | |
| Human scFv | hsp90 | Human | Intravenous doses of efungumab (Mycograb®) (1 mg/kg) for 5 days in combination with amphotericin B | Combination more efficient than amphotericin B only in patients with invasive candidiasis | Pachl et al. (2006) |
Table 2.
Passive Immunization Against Lung Pathogens
| Pathogens | Antibody Source | Antigen | Model | Administration | Outcome | References |
|---|---|---|---|---|---|---|
| Various lung infections | Human purified Ig | Human | IVIG and SCIG | Reduced incidence of pneumonia in patients with PAD | Chapel et al., 2000, Fried and Bonilla, 2009, Wood and Swanson, 2007 | |
| Haemophilus influenzae Hib | Human hyperimmune globulin | Polysaccharide | Human (infants) | Intramuscular administration of Ig | Protection against Hib bacteremia and meningitis in infants | Santosham et al., 1987, Siber et al., 1992 |
| Human hyperimmune globulin | Polysaccharide | Human (infants) | Intramuscular administration of Ig | Decreased the frequency of acute otitis media in infants | Shurin et al. (1993) | |
| Streptococcus pneumoniae | Human mAb | Capsular polysaccharide | Mouse | Intraperitoneal administration of mAb A7 (IgM) | Protective against a systemic lethal challenge with Str. pneumoniae serotype 3 | Chang et al., 2002, Fabrizio et al., 2010b, Fabrizio et al., 2010a |
| Murine mAb | Capsular polysaccharide | Mouse | Single intraperitoneal dose of mouse mAbs (IgG1) | Prophylactically protective against a lethal intranasal challenge with Str. pneumoniae serotype 3 | Tian et al., 2009, Weber et al., 2012 | |
| Pseudomonas aeruginosa | Human mAb derived from scFv | Exopolysaccharide Psl | Mouse | Single intraperitoneal dose of mAb Cam-003 (IgG1) | Prophylactically protective in a lethal Ps. aeruginosa pneumonia mouse model | Digiandomenico et al. (2012) |
| Human mAb | Alginate | Mouse | Single intranasal administration of mAb (IgG1) | Prophylactically protective in a lethal Ps. aeruginosa pneumonia mouse model | Pier et al. (2004) | |
| Human mAb | Flagellin type b | Mouse | Single intravenous injection of mAb LST-007 (IgG1) | Therapeutically protective in a lethal Ps. aeruginosa pneumonia mouse model | Adawi et al. (2012) | |
| Human mAb | Type 3 secretion system (PcrV) | Mouse | Single intravenous injection of mAb KBPA101 (IgM) | Prophylactically protective in a lethal Ps. aeruginosa pneumonia mouse model | Horn et al. (2010) | |
| Human Fab | Type 3 secretion system (PcrV) | Mouse | Intratracheal administration of Fab fragments (IA8) | Protective in a lethal Ps. aeruginosa pneumonia mouse model | Baer et al. (2009) | |
| Human mAb | LPS O- polysaccharide moiety | Human | Three infusion of panobacumab (IgM) (1.2 mg/kg) | High rate of clinical cure and survival in patients developing nosocomial Ps. aeruginosa O11 pneumonia | Lu et al. (2011) | |
| Human PEGylated Fab’ | Type 3 secretion system (PcrV) | Human | Single intravenous infusion of mAb KB001 (3 mg/kg) | Reduction in Ps. aeruginosa pneumonia incidence in mechanically ventilated patients | Francois et al. (2012) | |
| IgY | Ps. aeruginosa | Human | Mouth rinse containing IgY twice daily on a continuous basis | Reduction or prevention of Ps. aeruginosa colonization | Nilsson et al. (2008) | |
| H5N1 | Human plasma | A/H5N1 | Human | Transfusion with plasma from humans recovering from H5N1 infection | Recovery from H5N1 influenza A viral infection in two case studies | Kong and Zhou, 2006, Zhou et al., 2007 |
| F(ab’)2 (equine) | A/H5N1 | Mouse | Single intraperitoneal injection of F(ab’)2 | Therapeutically protective against a lethal dose of H5N1 | Lu et al. (2006) | |
| IgY | A/H1N1 | Mouse | Single intranasal administration of IgY | Prophylactically and therapeutically protective against a lethal dose of H1N1 | Nguyen et al. (2010) | |
| Bovine IgG and F(ab’)2 | A/H1N1 | Mouse | Single intranasal administration of IgG or F(ab’)2 | IgG prophylactically protected mice from a sublethal H1N1 dose more efficiently than F(ab’)2 | Ng et al. (2010) | |
| Humanized mAbs | HA (H5) | Mouse | Intraperitoneal administration of mAbs | Prophylactically and therapeutically protective against a lethal infection with H5N1 | Prabakaran et al., 2009, Prabhu et al., 2009 | |
| Murine mAb | HA (H5) | Mouse | Single intranasal administration of mAb DPJY01 (IgA) | Prophylactically and therapeutically protective against a sublethal dose of H5N1 | Ye et al. (2010) | |
| Human mAb | HA (H1) | Mouse | Single systemic injection of mAb 6F12 (IgG2b) | Prophylactically and therapeutically protective against a lethal challenge with H1N1 | Tan et al. (2012) | |
| Human mAb | HA (H1, H2, H5, H6, H8, H9) | Mouse | Single systemic injection of mAb CR6261(IgG1) | Prophylactically and therapeutically protective against a lethal challenge with H1N1 and H5N1 | Ekiert et al., 2009, Throsby et al., 2008 | |
| VHH | HA (H5) | Mouse | Single intranasal administration of mono- and bivalent VHH | Bivalent VHH prophylactically and therapeutically more protective against lethal infection with H5N1 | Ibanez et al. (2011) | |
| RSV | Hyperimmune human IgG | RSV | Rats | Single intraperitoneal injection of IgG | Prophylactically and therapeutically protective against an intranasal challenge with RSV | Prince et al. (1985) |
| Human polyclonal | RSV | Human (infants) | Intravenous injection of RSV-IVIG | Reduced incidence of RSV hospitalization among high-risk infants and children by 30–60% | Morris et al. (2009) | |
| Humanized mAb | F Glycoprotein | Human (infants) | Intramuscular injection Palivizumab (Synagis®) (IgG1) | Reduced incidence of RSV hospitalization among high-risk infants and children by 30–60% | Morris et al. (2009) | |
| Humanized mAb | F Glycoprotein | Human | Intramuscular injection Palivizumab (Synagis®) | Prophylactically protected hematopoietic stem cell transplant patients from RSV infection | Kassis et al. (2010) | |
| VHH | F Glycoprotein | Rats | Intranasal administration of trimeric (identical) VHHs (ALX-0171) | Prophylactically and therapeutically protective against intranasal challenge with RSV | www.ablynx.com | |
| VHH | F Glycoprotein | Human | Trimeric VHHs (ALX-0171) administered to volunteers via nebulization | No adverse events in a phase I study | www.ablynx.com |
Table 3.
Passive Immunization Against Gastrointestinal Pathogens
| Pathogens | Antibody Source | Antigen | Model | Administration | Outcome | References |
|---|---|---|---|---|---|---|
| Helicobacter pylori | Monoclonal (mouse) | H. pylori cells or urease | Mouse | Oral administration of mAbs at the time of the initial challenge | Protected mice from H. pylori infection | Blanchard et al., 1995, Czinn et al., 1993 |
| Bovine colostrum | H. pylori | Human | Oral administration of immune colostrum daily for 28 days | Infection eradicated in all 11 patients | Ando and Nakamura (1991) | |
| Bovine colostrum | H. pylori | Human | Oral administration of immune colostrum daily for 28 days | Attenuation of symptoms and decreased in H. pylori counts in most patients | Tarpila (1994) | |
| Bovine colostrum | H. pylori | Human | Oral administration of HBC daily for 28 days | Eradication of infection in two of eight patients | Casswall et al. (2002) | |
| Bovine colostrum | H. pylori, urease or adhesin | Human | Oral administration of BIC for 2 days (4–8 g) | No eradication of H. pylori | Opekun et al. (1999) | |
| Bovine colostrum | H. pylori | Human (infants) | Oral administration of HBC daily for 30 days | No decrease in H. pylori infection in infants in rural Bangladesh | Casswall et al. (1998) | |
| Bovine colostrum | H. pylori | Human (children) | Oral administration of BIC daily for 28 days | No decrease in H. pylori infection | den Hoed et al. (2011) | |
| IgY | H. pylori or urease | Gerbils | Oral administration of IgY | Attenuation of H. pylori colonization and gastric mucosal inflammation | Nomura et al., 2005, Shimamoto et al., 2002, Shin et al., 2002 | |
| IgY | Urease | Mouse | Oral administration of IgY | Reduction in H. pylori colonization and inflammation in the stomach | Malekshahi et al. (2011) | |
| IgY | Urease | Human | Oral administration of IgY 3 times daily for 4 weeks | Decrease in urea breath test values | Suzuki et al. (2004) | |
| IgY | Urease | Human | Functional drinking yogurt containing 1% IgY 3 times daily for 4 weeks | Decreased urea breath test values and reduced H. pylori antigen detection in feces | Horie et al. (2004) | |
| Rotavirus | Mouse mAbs | VP4, VP6, VP7 | Mouse | Subcutaneous injection of hybridoma producing IgG and IgA mAbs | Prophylactically protective in the murine hybridoma backpack tumor model | Burns et al., 1996, Ruggeri et al., 1998 |
| Bovine colostrum | G1-G4 serotypes | Mouse | Oral administration of HBC | Prophylactically decreased diarrhea and reduced viral load in mouse pups challenged with rotavirus | Pant et al. (2007) | |
| Bovine colostrum | G1-G4 serotypes | Human (children) | Oral administration of HBC for 14 days | Prophylactically protected children admitted to hospital against rotavirus infection | Davidson et al. (1989) | |
| Bovine colostrum | G1-G4 serotypes | Human (children) | Oral administration of 10 g HBC for 4 days | Therapeutically reduced total stool output and frequency | Sarker et al. (1999) | |
| IgY | G1-G4 serotypes | Mouse | Oral administration of IgY | Therapeutically reduced prevalence and duration of diarrhea in mouse pups challenged with rotavirus | Sarker et al. (2007) | |
| IgY | G1-G4 serotypes | Human (children) | Oral administration of 10 g IgY for 4 days | Therapeutically reduce stool output | Sarker et al. (2001) | |
| VHH | VP6 (various serotypes) | Mouse | Oral administration of VHHs | VHH fragment 2B10 (or ARP1) reduced morbidity of rotavirus-induced diarrhea | Van Der Vaart et al. (2006) | |
| VHH | VP6 (various serotypes) | Human (children) | Oral administration of VHH ARP1 | Therapeutically reduced severity in phase II clinical trial conducted in Bangladesh | Sarker et al. (2013) | |
| Lactobacilli-VHH | VP6 (various serotypes) | Mouse | Oral administration of Lactobacillus expressing surface-anchored VHH against rotavirus (ARP1 and ARP3) | Prophylactically and therapeutically decreased diarrhea and viral load in mouse pups challenged with rotavirus | Pant et al., 2006, Pant et al., 2011, Martin et al., 2011 | |
| Plant-VHH | VP6 (various serotypes) | Mouse | Oral administration of rice-produced ARP1 (Mucorice-ARP1) | Prophylactically and therapeutically decreased diarrhea and viral load in mouse pups challenged with rotavirus | Tokuhara et al. (2013) | |
| Clostridium difficile | Human polyclonal (IVIG) | Toxin A and B | Human | Intravenous infusion of human Ig | Resolution of diarrhea, abdominal tenderness, and distention in patients with severe pseudomembranous colitis | Salcedo et al., 1997, Wilcox, 2004 |
| Human polyclonal (IVIG) | Toxin A and B | Human | Intravenous infusion of human Ig | Three of five cases with recurrent Cl. difficile infections responded successfully | Salcedo et al. (1997) | |
| Human and humanized mAbs | Toxin A and B | Hamster | Intraperitoneal injection of mAbs CDA1 and CDB1 | Prophylactically and therapeutically prevented mortality in hamsters challenged with Cl. difficile spores | Babcock et al., 2006, Marozsan et al., 2012 | |
| Human mAb | Toxin A and B | Human | Single intravenous infusion of mAbs CDA1 (actoxumab) and CDB1 (bezlotoxumab) (10 mg/kg) | Reduced rate of recurrent infection as adjunct to treatment with either vancomycin or metronidazole | Lowy et al. (2010) | |
| IgY | Toxin A and B | Hamster | Daily oral administration of IgY | Prophylactically and therapeutically prevented mortality in hamsters challenged with Cl. difficile spores | Kink and Williams (1998) | |
| IgY | FliD | Hamster | Daily oral administration of IgY | Therapeutically prevent mortality in hamsters challenged with Cl. difficile spores | Mulvey et al. (2011) | |
| Bovine colostrum | Toxin A and B | Hamster | Daily oral administration of BIC | Prophylactically prevented mortality in hamsters challenged with Cl. difficile spores | Lyerly et al. (1991) | |
| Bovine colostrum | Cl. difficile + toxin A and B | Human | Oral administration of BIC for 2 weeks (10 g/day) after antibiotic therapy | None of the patients had another episode of Cl. difficile diarrhea | Numan et al. (2007) | |
| Bovine colostrum | Cl. difficile + toxin A and B | Human | Oral administration of BIC for 2 weeks (5 g/day) after antibiotic therapy | Reduction of relapse rate compared with contemporary controls | Van Dissel et al. (2005) | |
| Lactobacilli-VHH | Toxin B | Hamster | Daily oral administration of lactobacilli expressing surface-anchored VHH | Delayed the death of hamsters after challenge with spores from toxin A-deleted Cl. difficile strain | Andersen et al. (submitted) | |
| Escherichia coli | Human mAb | Stx2 of EHEC |
Piglet | Single intraperitoneal injection of human mAbs against Stx2 | Protected against systemic complication after onset of diarrhea | Mukherjee et al., 2002, Sheoran et al., 2005 |
| IgY | Stx2 of EHEC |
Mouse | Oral administration of anti-Stx-2 IgY | Therapeutically reduced mortality of mice infected intestinally with EHEC O157:H7 | Neri et al. (2011) | |
| IgY | Fimbrial antigen of ETEC | Piglet | Diet containing IgY for period of 8 days | Reduce incidence and severity of diarrhea | Marquardt et al. (1999) | |
| IgY | Pilus of ETEC | Calf | Fed milk containing IgY | Prophylactically protected neonatal calves from challenged with ETEC (K99+) | Ikemori et al. (1992) | |
| Cow milk | EPEC strains | Human (infants) | Oral administration of milk Ig concentrate for 10 days (1 g/kg/day) | Therapeutically protective against EPEC-induced diarrhea in hospitalized infants | Mietens et al. (1979) | |
| Bovine colostrum | ETEC colonization factor | Human | Oral administration of HBC 3 times daily for 2 days | Prophylactically protected against oral experimental challenge with ETEC (O78:H11) | Freedman et al. (1998) | |
| Bovine colostrum | ETEC and EPEC strains | Human (children) | Oral administration of HBC for 4 days (20 g/day) | No therapeutic benefit against E. coli-induced diarrhea | Casswall et al. (2000) | |
| Bovine colostrum | ETEC strains | Human | Tablets containing 400 mg HBC, 2 times daily for 7 days | Protected volunteers against ETEC-induced diarrhea | Otto et al. (2011) |
BIC, hyperimmune bovine immunoglobulin concentrate; HBC, hyperimmune bovine colostrum.
Table 4.
Passive Immunization Against Vaginal Pathogens
| Pathogens | Antibody Source | Antigen | Model | Administration | Outcome | References |
|---|---|---|---|---|---|---|
| SHIV | Human mAbs | gp120 or gp41 | Monkey | Intravenous infusion of mAbs (b12, 2G12, 2F5, 4E10) | Prophylactically protective in macaques vaginally challenged with SHIV | Hessell et al., 2009, Hessell et al., 2010, Parren et al., 2001, Mascola et al., 2000, Moldt et al., 2012 |
| Human mAb | gp120 | Monkey | Vaginally administered mAbs | Prophylactically protected 9 of 12 macaques vaginally challenged with SHIV | Veazey et al. (2003) | |
| HIV | Human mAb | gp120 or gp41 | Mouse (RAG-hu) | Intravaginal application of VRC01 or mix of b12, 2F5, 4E10, and 2G12 mAbs | Prophylactically protective in RAG-hu mice challenged vaginally with HIV-1 | Veselinovic et al. (2012) |
| Human mAb | gp120 or gp41 | Human | Infusions of 2G12, 2F5, and 4E10 | Delay in viral rebound in chronically HIV-1 patients off ART | Trkola et al. (2005) | |
| Humanized mAb | CD4 | Human | Infusions of mAb ibalizumab | Reductions in HIV-1 RNA levels in 20 of 22 HIV-1 patients off ART | Bruno and Jacobson (2010) | |
| Humanized mAb | CCR-5 | Human | Single intravenous infusions of anti-CCR5 PRO 140 (5–10 mg/kg) | Reduction in the viral load in 31 subjects infected with CCR5-tropic (R5) HIV-1 | Jacobson et al. (2010) | |
| Lactobacilli- scFv | ICAM-1 | Mice (SCID) | Intravaginal administration of lactobacilli secreting scFv | Reduced transmission of cell-associated HIV in Hu-PBL-SCID mouse model | Chancey et al. (2006) | |
| Candida albicans | Mouse mAb | MP65 or Sap-2 | Rat | Intravaginal administration of mAbs AF1 (IgM) or GF1 (IgG1) | Therapeutically protective against vaginal challenge with Can. albicans | De Bernardis et al. (1997) |
| Human VH and Vκ | MP65 or Sap-2 | Rat | Intravaginal administration of mono- or bispecific domain antibodies | Prophylactically and therapeutically protective against Can. albicans infection equivalent to treatment with fluconazole | De Bernardis et al. (2007) | |
| Monoclonal (mouse) | β-glucan | Rat | Intravaginal administration of mAb 2G8 (IgG2b) | Prophylactically and therapeutically protective against Can. albicans | Torosantucci et al. (2005) | |
| Plant-chimeric mAb and plant-scFv-Fc | β-glucan | Rat | Intravaginal administration of two chimeric antibody (complete IgG or scFv-Fc) derivatives of mAb 2G8 | Therapeutically protective in vaginal rat model of Cl. difficile infection | Capodicasa et al. (2011) | |
| mAb and scFv anti-idiotypic | Idiotype of anti-killer toxin mAb | Rat | Intravaginal administration of mAb K10 and scFv-H6 fragments (yeast killer like-toxin) | Therapeutically protective in vaginal rat model of Cl. difficile infection | Magliani et al. (1997) | |
| Streptococcus gordonii-scFv anti-idiotypic | Idiotype of anti-killer toxin mAb | Rat | Intravaginal inoculation of Str. gordonii expressing secreted or surface-anchored scFv-H6 | Therapeutically protective in vaginal rat model of Cl. difficile infection | Beninati et al. (2000) |
ART, antiretroviral therapy.
Oral Pathogens
Streptococcus mutans
Streptococcus mutans is a normal resident of the human oral cavity recognized as one of the major etiologic agents of caries. Passive immunization against caries was developed using oral administration of antibody preparation against whole cells of Str. mutans or associated virulence factors involved in bacteria adherence (Table 1). Oral administration of hyperimmune bovine milk or chicken antibodies against Str. mutans and glucosyltransferases resulted in a reduction in Str. mutans in dental plaque and caries formation in rats and reduced counts of Str. mutans in saliva or dental plaque in humans (Filler et al., 1991, Hamada et al., 1991, Hatta et al., 1997, Kruger et al., 2004, Loimaranta et al., 1999a, Michalek et al., 1987, Otake et al., 1991). These antibody preparations may protect against Str. mutans by different mechanisms, such as inhibiting the formation of extracellular polysaccharides (glucan and fructan) and preventing the adherence of Str. mutans to salivary coated hydroxyapatite and aggregation of Str. mutans (Loimaranta et al., 1998, Loimaranta et al., 1999b).
Another approach is the use of mAbs against the cell surface antigen I/II (SAI/II) adhesion molecule of Str. mutans (Lehner et al., 1985, Ma et al., 1987, Ma et al., 1989, Ma et al., 1990). Human volunteers receiving a treatment consisting of oral chlorhexidine disinfection followed by repeated topical applications of anti-AgI/II mAb (Guy’s 13) onto the teeth showed a lack of re-colonization by indigenous Str. mutans for up to 2 years (Ma et al., 1989, Ma et al., 1990). Because the functional mAb was only detected on the teeth for only up to 3 days after application, it was speculated that the ecological niche vacated by Str. mutans was filled by other bacteria (Ma et al., 1990). This mAb was subsequently re-engineered as a chimeric IgA/IgG Ab with a rabbit secretory component for expression in tobacco plants. The plant-derived SIgA/G (CaroRx Planet Biotechnology) was reported to be effective in passive immunization trials in humans (Ma et al., 1995, Ma et al., 1998) although another study reported only a trend in reducing colonization by Str. mutans (Weintraub et al., 2005). The product is currently undergoing phase II clinical trials in the United States.
An scFv was also derived from the mAb Guy’s 13 and expressed in lactobacilli (Kruger et al., 2002). Administration of fresh lactobacilli expressing surface anchored scFv in drinking water reduced Str. mutans bacterial counts and caries development in rats (Kruger et al., 2002, Kruger et al., 2005). Modified lactobacilli could prevent caries by different mechanisms such as blocking the SAI/II adhesion and aggregation of Str. mutans in combination with the production of inhibitory substances (e.g., bacteriocin) (Kruger et al., 2005). A VHH antibody fragment against the SAI/II adhesin (S36-VHH) also reduced the development of smooth surface caries in the desalivated rat caries model (Kruger et al., 2006).
Porphyromonas gingivalis
Porphyromonas gingivalis, a gram-negative anaerobe present in subgingival plaque, was identified as a major etiologic agent of chronic periodontitis (Marcotte and Lavoie, 1998). The factors by which Po. gingivalis might express its virulence include lipopolysaccharides, hemagglutinin, fimbriae, and the Arg-X-specific (Rgp) and Lys-X-specific (Kgp) cysteine proteinases (the gingipains) (Andrian et al., 2004).
Immunoglobulin Y against the Po. gingivalis 40-kD outer membrane protein and hemagglutinin (HagA) were found to inhibit aggregation and hemagglutination in vitro (Hamajima et al., 2007, Tezuka et al., 2006). Egg yolk antibodies against Po. gingivalis gingipains decreased bacterial adhesion and hydrolytic activity in vitro (Yokoyama et al., 2007b) and reduced levels of Po. gingivalis when applied to the teeth of periodontitis patients (Yokoyama et al., 2007a).
The anti-Po. gingivalis mAb 61BG1.3 is reactive with the adhesion-associated epitope contained in the beta fragment of gingipain RgpA and has been shown to inhibit hemagglutination of human red blood cells by Po. gingivalis (Booth and Lehner, 1997). Topical application of the mAb in patients with periodontitis prevented recolonization with Po. gingivalis for up to 9 months (Booth et al., 1996). Modified lactobacilli expressing scFv derived from 61BG1.3 on the cell surface were also shown to aggregate Po. gingivalis and inhibit its growth in vitro (Marcotte et al., 2006).
Candida albicans
Oropharyngeal candidiasis is mostly associated with Candida albicans and is a major cause of morbidity in immunocompromised patients such as HIV or organ- and bone marrow-transplanted patients (Dongari-Bagtzoglou and Fidel, 2005, Hung et al., 2005). The narrow range of antifungal agents, the toxicity of some of the drugs, and the emergence of resistant strains of Can. albicans highlight the need for new therapeutic approaches against invasive candidiasis (Espinel-Ingroff, 2009). Antibodies may have an important role by blocking the adhesion of Can. albicans to tissues, inhibiting germ tube formation, and promoting phagocytosis (Cabezas et al., 2010, Coleman et al., 2009, De Bernardis et al., 2007, Wellington et al., 2007).
Polyclonal IgY antibodies prepared against Can. albicans have been shown to prevent the growth, adherence to epithelial cells, and biofilm formation of Can. albicans in vitro (Fujibayashi et al., 2009, Wang et al., 2008) and reduced oral candidiasis and systemic dissemination when administered orally to immunosuppressed mice (Ibrahim et al., 2008). Antibodies from cows immunized with whole Candida organisms and purified mannan prevented the adherence of Can. albicans to oral buccal cells in a dose-dependent manner (Weiner et al., 1999) and reduced Candida counts in saliva when administered orally to bone marrow-transplanted patients (Tollemar et al., 1999).
Efungumab (Mycograb®) was developed as a human recombinant scFv antibody fragment against a heat shock protein (hsp90) for the treatment of invasive Candida infection in combination with amphotericin B (Matthews and Burnie, 2001, Matthews et al., 2003, Pachl et al., 2006). Despite efficacy of the combination treatment in an animal model and a clinical trial, Mycograb® did not receive licensure from the European Medicines Agency for clinical use owing to safety concerns mainly regarding autoaggregation of the compound. Novartis AG then discontinued the development of this antibody. Despite these issues, efungumab demonstrated an important proof of principle for the development of mAbs with antifungal activity.
Lung Pathogens
Streptococcus pneumoniae and Haemophilus influenzae Type b
Almost all patients with primary antibody deficiency (PAD) have upper and lower respiratory tract bacterial infections, predominantly otitis media, sinusitis, and pneumonia. Life-threatening infections in PAD patients are caused mainly by encapsulated bacteria, especially Streptococcus pneumoniae and Haemophilus influenzae type b (Hib). Furthermore, in infants and young children, Hib causes bacteremia, pneumonia, and acute bacterial meningitis. In the past 60 years, several studies have demonstrated that replacement Ig therapy (IVIG and SCIG) is beneficial for PAD patients (Baris et al., 2011, Chapel et al., 2000, Fried and Bonilla, 2009, Wood and Swanson, 2007) (Table 2). Furthermore, a human hyperimmunoglobulin termed bacterial polysaccharide immune globulin (BPIG) was prepared from plasma of donors immunized with Hib, pneumococcal, and meningococcal capsular PS vaccines. This product, administered intramuscularly, was protective against Hib bacteremia and meningitis, and pneumococcal otitis in infants (Santosham et al., 1987, Shurin et al., 1993, Siber et al., 1992).
The main virulence factor of Str. pneumoniae and H. influenzae is their polysaccharide (PS) capsule, which reflects the important role of antibodies in opsonization of encapsulated bacteria. One option is thus to develop mAbs that target the capsular polysaccharide of the serotypes causing most infection and to give the mAbs as a mixture. Intraperitoneal administration of mAbs to the capsular polysaccharide protects mice against a lethal systemic or intranasal challenge with Str. pneumoniae serotype III (Chang et al., 2002, Fabrizio et al., 2010a, Fabrizio et al., 2010b, Tian et al., 2009, Weber et al., 2012). Some of these protective mAbs do not promote phagocytosis in vitro. Their efficacy may depend on their capacity to agglutinate and alter the expression of gene involved in quorum sensing, tricking the bacteria to kill each other (fratricide) (Fabrizio et al., 2007, Tian et al., 2009, Yano et al., 2011).
Pseudomonas aeruginosa
The gram-negative bacterium Ps. aeruginosa is an opportunistic nosocomial pathogen in immunocompromised patients and the major cause of morbidity and mortality in cystic fibrosis patients (Muller-Premru and Gubina, 2000). Already intrinsically resistant to many antibiotics, reports of Ps. aeruginosa acquisition of multidrug resistance to late-generation antibiotics are common (Jovcic et al., 2011).
Administration of polyclonal antibody preparations derived from healthy human donors immunized with Ps. aeruginosa (Ps-IVIG) was associated with both greater and prolonged improvement in pulmonary function in patients with moderate cystic fibrosis (Cryz et al., 1991). The potential of mAbs targeting virulence factors was demonstrated in an animal model of Ps. aeruginosa pneumonia using systemic or intratracheal administration of mAbs targeting Ps. aeruginosa O-antigen lipopolysaccharide (LPS), flagella, alginate, exopolysaccharide, and components of the type 3 secretion system (pcrV) (Adawi et al., 2012, Baer et al., 2009, Digiandomenico et al., 2012, Faure et al., 2003, Horn et al., 2010, Pier et al., 2004, Secher et al., 2011).
In phase IIa clinical trials, infusion of the mAbs panobacumab (Kenta Biotech, Switzerland) and KB001 (KaloBios Pharmaceuticals, Inc, US), targeting the O-antigen LPS and pcrV, respectively, support the use of passive immunization against Ps. aeruginosa pneumonia in humans (Francois et al., 2012, Lu et al., 2011). Furthermore, a mouth rinse containing purified anti-Ps. aeruginosa IgY given twice daily on a continuous basis could significantly reduce or prevent Ps. aeruginosa colonization in patient with cystic fibrosis (Nilsson et al., 2007, Nilsson et al., 2008). The IgY preparation (PsAer-IgY) has received an orphan drug designation by the European Medicines Agency (EMEA) and a phase III, double-blind, randomized, controlled trial is under way.
Influenza Type A
Influenza type A viruses are subdivided into subtypes based on the antigenic properties of the two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA) (Daniels et al., 2013). The multiple clades and subclades of influenza viruses and high mutation rate during infection constitute a considerable problem for human vaccine development (Stephenson et al., 2004).
Systemic administration of polyclonal antibodies against influenza virus has been shown to be protective in human and mouse models of infection (Kong and Zhou, 2006, Lu et al., 2006, Zhou et al., 2007). Furthermore, a single intranasal administration of IgY or hyperimmune bovine colostrum (HBC) anti-H1N1 is both prophylactically and therapeutically protective against an intranasal challenge with influenza virus (Ng et al., 2010, Nguyen et al., 2010). The HA protein is responsible for receptor binding to host cells and for viral entry and elicits the most robust neutralizing antibodies during vaccination or natural infection. Several groups reported that systemic or intranasal administration of mAbs against HA provides protection of mice against a lethal intranasal challenge with the virus (Prabakaran et al., 2009, Prabhu et al., 2009, Tan et al., 2012, Throsby et al., 2008, Ye et al., 2010). Antigenic drift, caused by periodic amino acid changes on the globular head of hemagglutinin (HA), is one of the hallmarks of influenza A virus immune evasion and the synergistic action of two or more mAbs in combination may be required to prevent the generation of escape mutants (Prabhu et al., 2009, Prabakaran et al., 2009). An alternative is to develop antibodies targeting the conserved region of the HA and capable of recognizing and neutralizing a diverse number of influenza A virus subtypes (Ekiert et al., 2009, Tan et al., 2012). A broadly neutralizing mAb (CR6261) was shown to bind to the highly conserved helical region in the HA stem/stalk domain (Ekiert et al., 2009, Throsby et al., 2008). The antibody, developed by Crucell (The Netherlands), is currently undergoing a phase I clinical trial fusing a single intravenous dose.
Camelidae heavy chain antibody fragments against H5N1 have also been developed and multivalent VHHs have been produced through genetic fusion of two or three neutralizing VHHs (Hultberg et al., 2011). The bivalent H5N1-HA-specific VHH was at least 60-fold more effective at suppressing virus replication in the lungs than its monovalent counterpart and protected mice against a lethal H5N1 virus challenge both in a prophylactic and therapeutic setting (Ibanez et al., 2011). Crosslinking of the spike subunit by multimerized VHHs may increase avidity, mediate agglutination of the viruses, or inhibit conformational changes of the viruses, thereby preventing fusion to host cell membranes (Vanlandschoot et al., 2011).
Respiratory Syncytial Virus
Respiratory syncytial virus is the leading viral pathogen responsible for lower respiratory tract infection (LRTI) requiring hospitalization in infants and young children worldwide. Respiratory syncytial virus has recently been found to have a high prevalence in the elderly and in immunocompromised adults, particularly bone marrow and lung transplant recipients, leading to considerable morbidity and mortality (Hynicka and Ensor, 2012). The two glycosylated surface proteins, F and G, are crucial for the infectivity and pathogenesis of the virus and elicit production of neutralizing antibodies by the host (Hall, 2000). The lack of effective therapy and a vaccine against RSV infection has led to studies examining the effectiveness of passive immunity (Prince et al., 1985).
Two RSV passive antibody preparations were originally licensed. Respiratory syncytial virus–IGIV is a hyperimmune polyclonal human IgG preparation derived from plasma donors with high RSV neutralizing antibody titers, approved in 1996 for use in infants and children younger than 24 months with chronic lung disease or a history of premature birth. Palivizumab (Synagis, MedImmune, Gaithersburg, MD) is a humanized IgG1 mAb directed against the antigenic site II of the F glycoprotein, and blocks virus fusion, most likely through inhibiting the conformational changes in the F protein required for this process (Huang et al., 2010). Palivizumab is licensed for the reduction of severe lower respiratory tract RSV infection in high-risk infants and children. Both palivizumab and RSV-IGIV similarly decrease the incidence of RSV hospitalization (by 30–60%) and intensive care unit admission but RSV-IGIV results in more adverse effects and requires intravenous access (Morris et al., 2009). Palivizumab might also be a viable option for prophylaxis against RSV in patients undergoing allogeneic stem cell transplantation, pre-engraftment, during graft-versus-host disease, and when treated with high-dose steroids (Kassis et al., 2010).
A VHH antibody fragment (VHH RSV-D3) directed against the antigenic site II of the F protein and competing with palivizumab has been isolated. Remarkably, linking two identical RSV-D3 VHH improved in vitro neutralization about 4000-fold (Hultberg et al., 2011). A trivalent molecule consisting of three identical anti-RSV VHHs (ALX-0171, Ablynx) was also constructed and shown to be protective by intranasal instillation in the cotton rat model even 2 days after infection (www.ablynx.com). A phase I study in healthy volunteers indicated that ALX-0171 could be successfully administered via nebulization directly into the lung and induced no significant clinically relevant adverse events (www.ablynx.com).
Gastrointestinal Pathogens
Helicobacter pylori
Helicobacter pylori infection is responsible for chronic gastritis and peptic ulcer disease and is associated with an increased risk of developing stomach cancer (Suerbaum and Michetti, 2002). Although antimicrobial therapy is available, the cost of antibiotic therapy and the appearance of antibiotic resistance suggest that an effective alternative prevention strategy would be of great benefit, particularly in developing countries. The most prominent H. pylori virulence factors are the blood group antigen-binding adhesins (BabA, BabB, and BabC), the sialic acid-binding adhesin (SabA), the cytotoxin-associated antigen (CagA), the vacuolating cytotoxin (VacA), and urease, which is involved in gastric acid neutralization (Backert and Clyne, 2011). The delayed acquisition of H. pylori by Gambian infants corresponding to their mothers’ levels of breast milk IgA specific for H. pylori suggests that oral administration of antibodies might be used to prevent or reduce infection by H. pylori (Gorrell and Robins-Browne, 2009, Thomas et al., 1993). Furthermore, mice given mouse mAbs against Helicobacter or virulence factor (urease) at the time of the initial challenge are protected from infection (Blanchard et al., 1995, Czinn et al., 1993) (Table 3).
Antibodies from cows or chickens immunized with whole cell lysates or urease inhibit the adherence of H. pylori to the gastric mucosa and growth and urease activity in vitro (Casswall et al., 1998, Casswall et al., 2002, Shimamoto et al., 2002, Shin et al., 2002). Oral administration of these antibody preparations also reduces colonization by H. pylori and associated gastritis in a rodent model (Casswall et al., 1998, Casswall et al., 2002, Malekshahi et al., 2011, Nomura et al., 2005, Shimamoto et al., 2002, Shin et al., 2002). However, clinical studies on the effect of oral administration of colostrum antibodies and IgY antibodies against H. pylori in humans are rare and show contradictory results. Some studies thus showed a decrease in colonization and symptoms (Ando and Nakamura, 1991, Casswall et al., 2002, Tarpila S et al., 1994) whereas other studies have shown no effect of colostrum-derived antibodies (Casswall et al., 1998, Casswall et al., 2002, Den Hoed et al., 2011, Opekun et al., 1999) or only a moderate effect by anti-urease IgY (Horie et al., 2004, Suzuki et al., 2004).
Few antibodies against H. pylori adhesins have been developed, although the latter have been identified and well characterized (Aspholm-Hurtig et al., 2004, Ilver et al., 1998, Mahdavi et al., 2002). Recently, an scFv antibody fragment against BabA adhesin (Abba3) was isolated from a phage library derived from PBMC of H. pylori infected humans (Schmidt et al., in preparation). A complete human antibody derived from the scFv (Abba3-IgG) prevented the adherence of H. pylori to stomach sections.
Rotavirus
Rotavirus is the most important etiologic agent of severe diarrhea in young children, accounting for an estimated 500,000 deaths each year mainly in the developing world. The two licensed vaccines (Rotarix™ and Rotateq™) have reduced efficacy (39.3–61.2%) in developing countries in Africa and Asia (Armah et al., 2010, Madhi et al., 2010) compared with developed countries (>85%) (Ruiz-Palacios et al., 2006, Vesikari et al., 2006). There is thus a need for alternative strategies to complement vaccination strategies in situations where efficacy of vaccination alone may not be sufficient (Marcotte et al., 2008).
Studies in humans and mice suggest a role of secretory IgA and serum IgG antibodies in protection against rotavirus (Coulson et al., 1992, Istrate et al., 2008, Velazquez et al., 2000). The outer capsid proteins VP7 and VP4 and the intermediate layer protein VP6 are likely to be the most important targets of the host neutralizing response (Aladin et al., 2012, Burns et al., 1996, Corthesy et al., 2006, Ruggeri et al., 1998). Monoclonal Abs against VP4 inhibit infection by blocking attachment (Ruggeri and Greenberg, 1991), antibodies against VP7 inhibit decapsidation (Aoki et al., 2011), and VP6 antibodies inhibit genome transcription and viral transcription in vitro (Feng et al., 2002).
Several studies have shown that passive immunization using hyperimmune polyclonal bovine colostrum or hyperimmunized chicken egg yolk Ig is protective in animal models and humans (Davidson et al., 1989, Hammarstrom and Weiner, 2008, Pant et al., 2007, Sarker et al., 1998, Sarker et al., 2001, Sarker et al., 2007, Weiner et al., 1999). Novel approaches to the prevention and treatment of rotavirus-induced diarrhea are being developed to reduce the medical and economic burden in both developed and developing countries. One isolated VHH, named ARP1 (also referred to as 2B10 or VHH1), was shown to be broadly cross-reactive against different human rotavirus serotypes and provided protection in a mouse pup model of rotavirus infection (Aladin et al., 2012, Van Der Vaart et al., 2006). Most important, oral administration of ARP1 was safe and effective in reducing severity of diarrhea in children in a phase II therapeutic clinical trial conducted in Bangladesh (Sarker et al., 2013).
Based on these studies, modified lactobacilli producing surface anchored VHH antibody fragments directed against rotavirus (ARP1) were constructed and shown to bind to rotavirus and to be protective against rotavirus-induced diarrhea in mouse pups (Martin et al., 2011, Pant et al., 2006, Pant et al., 2011). The ARP1 fragment was also expressed at high level in rice (MucoRice-ARP1) using RNAi technology to suppress the production of major rice endogenous storage proteins (Tokuhara et al., 2013). MucoRice-ARP1 can be applied orally as rice powder or rice water by simply dissolving the MucoRice-ARP1 rice powder in water.
Clostridium difficile
Clostridium difficile is a spore-forming, anaerobic bacterium that is a leading cause of antibiotic-associated diarrhea in hospitals and long-term care facilities (Redelings et al., 2007). The primary treatment regime of Cl. difficile infection is discontinuation of the instigating antibiotic and administration of vancomycin or metronidazole but 10–20% of patients experience relapse when antibiotic therapy is halted. Furthermore, the incidence and severity of Cl. difficile infection increased markedly over the past decade, in part because of the emergence of unusually virulent, antibiotic-resistant strains (Redelings et al., 2007).
The main strategy for passive immunization against Cl. difficile is based on antibodies neutralizing the two toxins responsible for the disruption of colonic epithelial tight junctions (Babcock et al., 2006, Cloud and Kelly, 2007, Marozsan et al., 2012, Salcedo et al., 1997, Wilcox, 2004). Two human mAbs directed against the receptor-binding domain of toxin A (mAb CDA1 or actoxumab) or toxin B (mAb CDB1, MDX-1388 or bezlotoxumab), have been shown to neutralize the cytotoxic effects of either and prevent Cl. difficile-induced mortality in a hamster model (Babcock et al., 2006). In a phase II human trial, a single infusion of these two mAbs as an adjunct to treatment with either vancomycin or metronidazole was highly successful in reducing the rate of recurrent infection (Lowy et al., 2010). The two mAbs licensed to Merck will be tested in a phase III study.
The toxins could conceivably be neutralized within the lower gastrointestinal tract, thereby preventing the critical first step in Cl. difficile-associated disease pathogenesis. Oral administration of chicken IgY and bovine antibody directed against toxin A and B was protective in the hamster model of infection (Kelly et al., 1996, Kink and Williams, 1998, Lyerly et al., 1991, Roberts et al., 2012). Targeting other Cl. difficile virulence factors involved in adherence, such as the flagellar cap protein (FliD), may also be effective in preventing Cl. difficile infection (Mulvey et al., 2011).
There have been limited reports on Cl. difficile-associated diarrhea therapy with orally delivered antibodies in humans (Kelly et al., 1997, Warny et al., 1999). Bovine antibody-enriched whey prepared from cow’s milk directed against Cl. difficile toxins and whole cells was shown to prevent relapse after standard antibiotic therapy, while being safe and well tolerated (Van Dissel et al., 2005, Numan et al., 2007, Young et al., 2007). The limited evidence in oral immunotherapy in clinical settings has likely been hampered by the high Ig dose requirements and the associated costs. As an alternative, neutralizing single-domain antibodies derived from llamas are currently being developed against toxins A and B (Hussack et al., 2011). Furthermore, Lactobacillus producing surface-anchored VHH fragment against toxin B delay the death of hamsters after a challenge with spores from a toxin A-deleted Cl. difficile strain (Andersen et al., submitted).
Escherichia coli
Diarrheagenic E. coli has been classified into five well-described groups based on the distinct virulence characteristics clinical disease and phylogenetic profiles. Among them are enterohemorrhagic E. coli (EHEC) strains, which are associated with hemorrhagic colitis and hemolytic-uremic syndrome in humans; enteropathogenic E. coli (EPEC) strains, which cause diarrhea in children; and enterotoxigenic E. coli (ETEC) strains, which are associated with traveler’s diarrhea and cause diarrhea and at least 300,000 deaths annually in preschool children in developing countries (Brunser et al., 1992, Buchholz et al., 2011, Svennerholm and Lundgren, 2012). Whereas ETEC and EHEC are characterized by the production of distinct toxins, the hallmark phenotype of EPEC is the ability to produce an attaching and effacing (A/E) lesion. Many antibiotics have been used to treat EPEC and ETEC, but this treatment option is threatened by the rise in multiple antibiotic-resistant strains of the bacteria (Horne et al., 2002).
The fact that secretory Igs in breast milk protect neonates and young infants against diarrhea is important for considering and testing passive immunotherapy to manage this infection (Loureiro et al., 1998). Intraperitoneal injection of mAbs against Shiga toxin reduces systemic complications and prolongs survival in mice and gnotobiotic pigs intestinally infected with E. coli EHEC (Krautz-Peterson et al., 2008, Mukherjee et al., 2002, Sheoran et al., 2005). Oral administration of anti-Stx-2 IgY reduced the mortality of mice infected intestinally with EHEC O157:H7 (Neri et al., 2011). In addition, Ig preparations from cows and chickens immunized against EPEC, ETEC, and EHEC are effective as prophylaxis against infections in farm animals (Ikemori et al., 1992, Marquardt et al., 1999, Rabinovitz et al., 2012).
Supplemental bovine milk-derived antibody preparations in infant feeding formulas were successfully used to treat active EPEC disease in infants in one of the earliest controlled studies using antibodies (Mietens et al., 1979). In addition, a hyperimmune bovine milk antibody product with specific activity against purified colonization factor antigens (CFAs) protected volunteers challenged with ETEC (Freedman et al., 1998). On the contrary, oral administration of a bovine Ig milk concentrate (BIC) from cows hyperimmunized with ETEC and EPEC strains gave no significant therapeutic benefit in a randomized, placebo-controlled study in children with E. coli-induced diarrhea in Bangladesh (Casswall et al., 2000). This could be because of differences in the specificity of the antibody preparations. A better approach might thus be to generate antibodies specific for virulence factors.
Vaginal Tract
Human Immunodeficiency Virus
According to estimates by the World Health Organization and the Joint United Nations Programme on HIV/Acquired Immunodeficiency Syndrome, more than 34 million people were living with HIV-1 infection at the end of 2011. Most HIV-1 transmission in women occurs on the mucosal surface of the endocervix, cervix, and vagina during unprotected vaginal intercourse (Chancey et al., 2006, Shattock and Moore, 2003).
Despite the extraordinary genetic diversity of the virus, a few broadly neutralizing mAbs isolated from HIV-1-infected patients define conserved epitopes on the HIV Env (Hoxie, 2010, Walker and Burton, 2010, Wu et al., 2010). The most extensively studied mAbs are 4E10 and 2F5, which recognize the membrane proximal external region (MPER) of gp41, 2G12 targeting the carbohydrate-specific epitope on the outer domain of gp120, and b12 directed against the CD4 binding site (CD4bs) on gp120 (Walker and Burton, 2010, Wu et al., 2010). More potent mAbs have been isolated, such as VRC01, which targets the CD4bs and neutralized 91% of a 190-virus panel (Wu et al., 2011).
Passive intravenous transfer of human neutralizing mAbs targeting gp120 (b12, 2G12) and gp40 (2F5, 4E10) provides dose-dependent protection to macaques vaginally challenged with the R5 strain of SHIV, which suggests that the antibodies may diffuse into the mucosal and submucosal layers (Hessell et al., 2009, Hessell et al., 2010, Mascola et al., 2000, Moldt et al., 2012, Parren et al., 2001) (Table 4). Furthermore, vaginally administered b12 and VRC01 protect against mucosal SHIV challenge in animal models, which suggests the use of antibodies as potential vaginal microbicides (Veazey et al., 2003, Veselinovic et al., 2012). In humans, infusion with the neutralizing mAbs 4E10, 2F5, and 2G12 reduced viral rebound in HIV-1-infected individuals (Trkola et al., 2005). The same three mAbs are being assessed as a gel-formulated microbicide in a phase I clinical trial (Morris et al., 2010). High concentrations of neutralizing mAbs show promise for microbicide formulations but they are prohibitively expensive to produce in the amounts required. Consequently, the anti-HIV-neutralizing antibodies 2F5 and 2G12 have been expressed at high levels in plants and excellent results have been obtained in terms of production and neutralizing activity (Marusic et al., 2009, Rademacher et al., 2008, Ramessar et al., 2008).
The humanized mAbs ibalizumab (formerly TNX-355 and Hu5A8, TaidMed Biologics, Taiwan) and PRO 140 (Progenics Pharmaceuticals, New York), targeting the receptors CD4 and CCR5, respectively, have shown promising results in early clinical trials when given as an intravenous infusion (Bruno and Jacobson, 2010, Jacobson et al., 2010). These antibodies might be used as a mixture with antibodies targeting the virus in a microbicide formulation.
Camelidae heavy chain antibody fragments reacting with the gp120 CD4bs, which neutralize a variety of HIV-1 strains, have also been isolated (Forsman et al., 2008, Jahnichen et al., 2010, Mccoy et al., 2012). The leading VHH candidate (J3) neutralizes 94% of 65 HIV-1 strains of various clades, showing that broadly neutralizing VHHs can be generated against HIV-1 by immunization (McCoy et al., 2012). Camelidae heavy chain antibodies have a high potential for HIV-1 microbicide application because of their low production costs, high stability, favorable release from a vaginal ring device, and tissue penetration properties (Gorlani et al., 2012).
Lactobacilli are found at about 108 colony-forming units (CFU)/mL of vaginal fluid and 10–100 CFU per single vaginal epithelial cell, and have thus been suggested as vectors for delivery of antibody fragments and other microbicides in the genital tract (Chancey et al., 2006, Lagenaur et al., 2011, Liu et al., 2006). Indeed, scFv against ICAM-1 produced by lactobacilli reduced transmission of cell-associated HIV both in vitro and in the Hu-PBL-SCID mouse model (Chancey et al., 2006).
Candida albicans
Chronic vulvovaginal candidiasis severely affects the quality of life for millions of women in the world and is frequently refractory to antifungal treatments (Sobel, 2003). Virulence factors of Can. albicans include mannoproteins that favor fungal adherence as well as aspartyl proteinase(s) (Sap) that have a crucial role in the acquisition of nutrients and invasion and destruction of the host tissue barrier (De Bernardis et al., 2007). Intravaginal administration of mAbs or human single-domain antibodies (VH or Vκ chain) recognizing the 65-kDa mannoprotein (MP65) or the secretory aspartyl proteinase-2 (SAP-2) of Can. albicans conferred protection against a vaginal challenge (Cabezas et al., 2010, De Bernardis et al., 1997, De Bernardis et al., 2007). Monoclonal antibodies targeting β1,3-glucan, a major cell wall component, inhibited hyphal growth and adherence to human epithelial cells in vitro and conferred significant protection against Can. albicans in a vaginal rat model of Can. albicans infection (Capodicasa et al., 2011, Torosantucci et al., 2005, Torosantucci et al., 2009).
The development of anti-idiotypic antibodies with yeast killer toxin (KT) activity opens a new avenue for therapeutic application (Cassone, 2008, Magliani et al., 1997). Monoclonal Abs and scFv fragments carrying the internal image of the active domain of a KT produced by Pichia anomala were elicited by idiotypic vaccination of rats with a KT-neutralizing mouse mAb KT4 (Magliani et al., 1997, Polonelli et al., 1997). Yeast killer toxin anti-idiotypic monoclonal (mAb K10) and scFv (scFv-H6) antibodies were able to exert a direct candidacidal activity in vitro and in the rat vaginitis model (Magliani et al., 1997). Furthermore, engineered Streptococcus gordonii strains producing scFv-H6 colonize the mucosal surfaces when inoculated intravaginally in rats and exert a therapeutic effect on Can. albicans vaginal infection (Beninati et al., 2000).
Passive Immunity: Toward the Future
Since Ehrlich’s recognition of the potential of antibodies as therapeutic agents in the early twentieth century, antibodies have gained importance for the treatment of a variety of diseases. Today, passive antibody administration is used to treat and prevent diseases caused by hepatitis B virus, rabies virus, RSV, Cl. tetani, Cl. botulinum, vaccinia virus, echovirus, and enterovirus (Casadevall et al., 2004). The administration of horse antitoxin remains the only specific therapy available for botulism. Antibodies can be administered as human or animal plasma or serum; as pooled human Ig for intravenous (IVIG), intramuscular (IMIG), and subcutaneous (SCIG) use; as high-titer human IVIG or IMIG from immunized or convalescing donors; or as mAb. In patients with PAD, prophylactic replacement therapy by SCIG or intravenous IMIG is the method of choice to prevent infections.
Antibody therapy against HIV, rotavirus, influenza, Bacillus anthracis, Cryptococcus neoformans, Cl. difficile, Str. mutans, Ps. aeruginosa, and Can. albicans are in clinical development. Antibodies against various other microorganisms including H. pylori and E. coli are under research development. Renewed interest in antibody-based therapies is the consequence of major advances in the technology of antibody production combined with the need for new therapeutic agents against emerging diseases (Ebola, severe acute respiratory syndrome, bird flu, West Nile virus, and bioterrorism agents) and new antibiotic-resistant microorganisms (Staphylococcus aureus, E. coli, Enterococci, and Cl. difficile). However, although antibodies have proven to be good antimicrobial agents, only one mAb, palivizumab, is licensed for an infectious disease (RSV infection). It is hoped that advances in genomics and proteomics will result in improved knowledge regarding the molecular mechanisms of pathogenicity. The possibility of generating fully human antibodies from transgenic, humanized mice, and perhaps in the near future transgenic humanized cows, as well as new developments in phage library technology will accelerate the development of antibody production. Furthermore, production of antibodies at lower cost using transgenic plants and transgenic cows as well as in situ delivery of antibody fragments by recombinant bacteria will lead to new applications of antibodies as therapeutics against infectious disease, such as mucosal delivery. Antibodies engineered in different formats and fused to other antimicrobials will result in increased potency and new molecular methods will render them non-immunogenic. All of these new developments will contribute to the realization of Erhlich’s dream: magic bullets.
Acknowledgments
The authors thank Yin Lin, Department of Laboratory Medicine, Karolinska Insitutet, for providing excellent editorial support.
References
- Adawi A., Bisignano C., Genovese T., Filocamo A., Khouri-Assi C., Neville A., Feuerstein G.Z., Cuzzocrea S., Neville L.F. In vitro and in vivo properties of a fully human IgG1 monoclonal antibody that combats multidrug resistant Pseudomonas aeruginosa. Int. J. Mol. Med. 2012;30:455–464. doi: 10.3892/ijmm.2012.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aladin F., Einerhand A.W., Bouma J., Bezemer S., Hermans P., Wolvers D., Bellamy K., Frenken L.G., Gray J., Iturriza-Gomara M. In vitro neutralisation of rotavirus infection by two broadly specific recombinant monovalent llama-derived antibody fragments. PLoS ONE. 2012;7:e32949. doi: 10.1371/journal.pone.0032949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder M.N., Herrin B.R., Sadlonova A., Stockard C.R., Grizzle W.E., Gartland L.A., Gartland G.L., Boydston J.A., Turnbough C.L., Jr., Cooper M.D. Antibody responses of variable lymphocyte receptors in the lamprey. Nat. Immunol. 2008;9:319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- Andersen K.K., Marcotte H., Alvarez B., Boyaka P.N., Hammarstrom L. In situ gastrointestinal protection against anthrax edema toxin by single-chain antibody fragment producing lactobacilli. BMC Biotechnol. 2011;11:126. doi: 10.1186/1472-6750-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.K., Strokappe N.M., Hultberg A., Truusalu K., Smidt I., Mikelsaar R., Mikelsaar M., Verrips T., Hammarstrom L., Marcotte H. 2014. Neutralization of Clostridium difficile toxin B mediated by engineered lactobacilli producing single domain antibodies. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K., Nakamura T. 1991. A Method for Producing a New Medicine for Both Treating and Preventing Peptic Ulcer Diseases and Gastritis and Thus Formulated Medicines. European Patent Application 91310049.1; 1991. [Google Scholar]
- Andrian E., Grenier D., Rouabhia M. In vitro models of tissue penetration and destruction by Porphyromonas gingivalis. Infect. Immun. 2004;72:4689–4698. doi: 10.1128/IAI.72.8.4689-4698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S.T., Trask S.D., Coulson B.S., Greenberg H.B., Dormitzer P.R., Harrison S.C. Cross-linking of rotavirus outer capsid protein VP7 by antibodies or disulfides inhibits viral entry. J. Virol. 2011;85:10509–10517. doi: 10.1128/JVI.00234-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armah G.E., Sow S.O., Breiman R.F., Dallas M.J., Tapia M.D., Feikin D.R., Binka F.N., Steele A.D., Laserson K.F., Ansah N.A., Levine M.M., Lewis K., Coia M.L., Attah-Poku M., Ojwando J., Rivers S.B., Victor J.C., Nyambane G., Hodgson A., Schodel F., Ciarlet M., Neuzil K.M. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- Aspholm-Hurtig M., Dailide G., Lahmann M., Kalia A., Ilver D., Roche N., Vikstrom S., Sjostrom R., Linden S., Backstrom A., Lundberg C., Arnqvist A., Mahdavi J., Nilsson U.J., Velapatino B., Gilman R.H., Gerhard M., Alarcon T., Lopez-Brea M., Nakazawa T., Fox J.G., Correa P., Dominguez-Bello M.G., Perez-Perez G.I., Blaser M.J., Normark S., Carlstedt I., Oscarson S., Teneberg S., Berg D.E., Boren T. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- Babcock G.J., Broering T.J., Hernandez H.J., Mandell R.B., Donahue K., Boatright N., Stack A.M., Lowy I., Graziano R., Molrine D., Ambrosino D.M., Thomas W.D., Jr. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect. Immun. 2006;74:6339–6347. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backert S., Clyne M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2011;16(Suppl. 1):19–25. doi: 10.1111/j.1523-5378.2011.00876.x. [DOI] [PubMed] [Google Scholar]
- Baer M., Sawa T., Flynn P., Luehrsen K., Martinez D., Wiener-Kronish J.P., Yarranton G., Bebbington C. An engineered human antibody fab fragment specific for Pseudomonas aeruginosa PcrV antigen has potent antibacterial activity. Infect. Immun. 2009;77:1083–1090. doi: 10.1128/IAI.00815-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baris S., Ercan H., Cagan H.H., Ozen A., Karakoc-Aydiner E., Ozdemir C., Bahceciler N.N. Efficacy of intravenous immunoglobulin treatment in children with common variable immunodeficiency. J. Investig. Allergol. Clin. Immunol. 2011;21:514–521. [PubMed] [Google Scholar]
- Belknap E.B., Baker J.C., Patterson J.S., Walker R.D., Haines D.M., Clark E.G. The role of passive immunity in bovine respiratory syncytial virus-infected calves. J. Infect. Dis. 1991;163:470–476. doi: 10.1093/infdis/163.3.470. [DOI] [PubMed] [Google Scholar]
- Beninati C., Oggioni M.R., Boccanera M., Spinosa M.R., Maggi T., Conti S., Magliani W., De Bernardis F., Teti G., Cassone A., Pozzi G., Polonelli L. Therapy of mucosal candidiasis by expression of an anti-idiotype in human commensal bacteria. Nat. Biotechnol. 2000;18:1060–1064. doi: 10.1038/80250. [DOI] [PubMed] [Google Scholar]
- Berghman L.R., Abi-Ghanem D., Waghela S.D., Ricke S.C. Antibodies: an alternative for antibiotics? Poult. Sci. 2005;84:660–666. doi: 10.1093/ps/84.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz H.K., Amstutz P., Pluckthun A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat. Biotechnol. 2005;23:1257–1268. doi: 10.1038/nbt1127. [DOI] [PubMed] [Google Scholar]
- Blanchard T.G., Czinn S.J., Maurer R., Thomas W.D., Soman G., Nedrud J.G. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect. Immun. 1995;63:1394–1399. doi: 10.1128/iai.63.4.1394-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma Y.L., Pluckthun A. DARPins and other repeat protein scaffolds: advances in engineering and applications. Curr. Opin. Biotechnol. 2011;22:849–857. doi: 10.1016/j.copbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Booth V., Ashley F.P., Lehner T. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect. Immun. 1996;64:422–427. doi: 10.1128/iai.64.2.422-427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth V., Lehner T. Characterization of the Porphyromonas gingivalis antigen recognized by a monoclonal antibody which prevents colonization by the organism. J. Periodontal Res. 1997;32:54–60. doi: 10.1111/j.1600-0765.1997.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Bruno C.J., Jacobson J.M. Ibalizumab: an anti-CD4 monoclonal antibody for the treatment of HIV-1 infection. J. Antimicrob. Chemother. 2010;65:1839–1841. doi: 10.1093/jac/dkq261. [DOI] [PubMed] [Google Scholar]
- Brunser O., Espinoza J., Figueroa G., Araya M., Spencer E., Hilpert H., Link-Amster H., Brussow H. Field trial of an infant formula containing anti-rotavirus and anti-Escherichia coli milk antibodies from hyperimmunized cows. J. Pediatr. Gastroenterol. Nutr. 1992;15:63–72. doi: 10.1097/00005176-199207000-00010. [DOI] [PubMed] [Google Scholar]
- Buchholz U., Bernard H., Werber D., Bohmer M.M., Remschmidt C., Wilking H., Delere Y., An Der Heiden M., Adlhoch C., Dreesman J., Ehlers J., Ethelberg S., Faber M., Frank C., Fricke G., Greiner M., Hohle M., Ivarsson S., Jark U., Kirchner M., Koch J., Krause G., Luber P., Rosner B., Stark K., Kuhne M. German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 2011;365:1763–1770. doi: 10.1056/NEJMoa1106482. [DOI] [PubMed] [Google Scholar]
- Burns J.W., Siadat-Pajouh M., Krishnaney A.A., Greenberg H.B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- Buss N.A., Henderson S.J., Mcfarlane M., Shenton J.M., De Haan L. Monoclonal antibody therapeutics: history and future. Curr. Opin. Pharmacol. 2012;12:615–622. doi: 10.1016/j.coph.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Cabezas J., Albaina O., Montanez D., Sevilla M.J., Moragues M.D., Ponton J. Potential of anti-Candida antibodies in immunoprophylaxis. Immunotherapy. 2010;2:171–183. doi: 10.2217/imt.09.76. [DOI] [PubMed] [Google Scholar]
- Capodicasa C., Chiani P., Bromuro C., De Bernardis F., Catellani M., Palma A.S., Liu Y., Feizi T., Cassone A., Benvenuto E., Torosantucci A. Plant production of anti-beta-glucan antibodies for immunotherapy of fungal infections in humans. Plant Biotechnol. J. 2011;9:776–787. doi: 10.1111/j.1467-7652.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- Casadevall A. Passive antibody therapies: progress and continuing challenges. Clin. Immunol. 1999;93:5–15. doi: 10.1006/clim.1999.4768. [DOI] [PubMed] [Google Scholar]
- Casadevall A., Dadachova E., Pirofski L.A. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- Cassone A. Fungal vaccines: real progress from real challenges. Lancet Infect. Dis. 2008;8:114–124. doi: 10.1016/S1473-3099(08)70016-1. [DOI] [PubMed] [Google Scholar]
- Casswall T.H., Nilsson H.O., Bjorck L., Sjostedt S., Xu L., Nord C.K., Boren T., Wadstrom T., Hammarstrom L. Bovine anti-Helicobacter pylori antibodies for oral immunotherapy. Scand. J. Gastroenterol. 2002;37:1380–1385. doi: 10.1080/003655202762671242. [DOI] [PubMed] [Google Scholar]
- Casswall T.H., Sarker S.A., Albert M.J., Fuchs G.J., Bergstrom M., Bjorck L., Hammarstrom L. Treatment of Helicobacter pylori infection in infants in rural Bangladesh with oral immunoglobulins from hyperimmune bovine colostrum. Aliment. Pharmacol. Ther. 1998;12:563–568. doi: 10.1046/j.1365-2036.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- Casswall T.H., Sarker S.A., Faruque S.M., Weintraub A., Albert M.J., Fuchs G.J., Alam N.H., Dahlstrom A.K., Link H., Brussow H., Hammarstrom L. Treatment of enterotoxigenic and enteropathogenic Escherichia coli-induced diarrhoea in children with bovine immunoglobulin milk concentrate from hyperimmunized cows: a double-blind, placebo-controlled, clinical trial. Scand. J. Gastroenterol. 2000;35:711–718. doi: 10.1080/003655200750023372. [DOI] [PubMed] [Google Scholar]
- Castilla J., Pintado B., Sola I., Sanchez-Morgado J.M., Enjuanes L. Engineering passive immunity in transgenic mice secreting virus-neutralizing antibodies in milk. Nat. Biotechnol. 1998;16:349–354. doi: 10.1038/nbt0498-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.E.Z., Chan A.H.Y., Hanson B.J., Ooi E.E. The use of antibodies in the treatment of infectious diseases. Singapore Med. J. 2009;50:663–672. [PubMed] [Google Scholar]
- Chancey C.J., Khanna K.V., Seegers J.F.M.L., Zhang G.W., Hildreth J., Langan A., Markham R.B. Lactobacilli-expressed single-chain variable fragment (scFv) specific for intercellular adhesion molecule 1 (ICAM-1) blocks cell-associated HIV-1 transmission across a cervical epithelial monolayer. J. Immunol. 2006;176:5627–5636. doi: 10.4049/jimmunol.176.9.5627. [DOI] [PubMed] [Google Scholar]
- Chang H.M., Ou-Yang R.F., Chen Y.T., Chen C.C. Productivity and some properties of immunoglobulin specific against Streptococcus mutans serotype c in chicken egg yolk (IgY) J. Agric. Food Chem. 1999;47:61–66. doi: 10.1021/jf980153u. [DOI] [PubMed] [Google Scholar]
- Chang Q., Zhong Z., Lees A., Pekna M., Pirofski L. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin Loci. Infect. Immun. 2002;70:4977–4986. doi: 10.1128/IAI.70.9.4977-4986.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapel H.M., Spickett G.P., Ericson D., Engl W., Eibl M.M., Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J. Clin. Immunol. 2000;20:94–100. doi: 10.1023/a:1006678312925. [DOI] [PubMed] [Google Scholar]
- Cloud J., Kelly C.P. Update on Clostridium difficile associated disease. Curr. Opin. Gastroenterol. 2007;23:4–9. doi: 10.1097/MOG.0b013e32801184ac. [DOI] [PubMed] [Google Scholar]
- Coleman D.A., Oh S.H., Zhao X.M., Zhao H.Y., Hutchins J.T., Vernachio J.H., Patti J.M., Hoyer L.L. Monoclonal antibodies specific for Candida albicans Als3 that immunolabel fungal cells in vitro and in vivo and block adhesion to host surfaces. J. Microbiol. Methods. 2009;78:71–78. doi: 10.1016/j.mimet.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters K., Dreier T., Silence K., De Haard H., Lauwereys M., Casteels P., Beirnaert E., Jonckheere H., De Wiele C.V., Staelens L., Hostens J., Revets H., Remaut E., Elewaut D., Rottiers P. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 2006;54:1856–1866. doi: 10.1002/art.21827. [DOI] [PubMed] [Google Scholar]
- Corthesy B., Benureau Y., Perrier C., Fourgeux C., Parez N., Greenberg H., Schwartz-Cornil I. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J. Virol. 2006;80:10692–10699. doi: 10.1128/JVI.00927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B.S., Grimwood K., Hudson I.L., Barnes G.L., Bishop R.F. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J. Clin. Microbiol. 1992;30:1678–1684. doi: 10.1128/jcm.30.7.1678-1684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S.J., Jr., Furer E., Sadoff J.C., Fredeking T., Que J.U., Cross A.S. Production and characterization of a human hyperimmune intravenous immunoglobulin against Pseudomonas aeruginosa and Klebsiella species. J. Infect. Dis. 1991;163:1055–1061. doi: 10.1093/infdis/163.5.1055. [DOI] [PubMed] [Google Scholar]
- Czinn S.J., Cai A., Nedrud J.G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- Daniels P., Wiyono A., Sawitri E., Poermadjaja B., Sims L.D. H5N1 highly pathogenic avian influenza in Indonesia: retrospective considerations. Curr. Top. Microbiol. Immunol. 2013;365:171–184. doi: 10.1007/82_2012_265. [DOI] [PubMed] [Google Scholar]
- Davidson G.P., Whyte P.B., Daniels E., Franklin K., Nunan H., Mccloud P.I., Moore A.G., Moore D.J. Passive immunisation of children with bovine colostrum containing antibodies to human rotavirus. Lancet. 1989;2:709–712. doi: 10.1016/s0140-6736(89)90771-x. [DOI] [PubMed] [Google Scholar]
- De Bernardis F., Liu H., O’mahony R., La Valle R., Bartollino S., Sandini S., Grant S., Brewis N., Tomlinson I., Basset R.C., Holton J., Roitt I.M., Cassone A. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J. Infect. Dis. 2007;195:149–157. doi: 10.1086/509891. [DOI] [PubMed] [Google Scholar]
- De Bernardis F., Boccanera M., Adriani D., Spreghini E., Santoni G., Cassone A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 1997;65:3399–3405. doi: 10.1128/iai.65.8.3399-3405.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A.L., Vaishnav P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009;27:297–306. doi: 10.1016/j.biotechadv.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Den Hoed C.M., De Vries A.C., Mensink P.B., Dierikx C.M., Suzuki H., Capelle L., Van Dekken H., Ouwendijk R., Kuipers E.J. Bovine antibody-based oral immunotherapy for reduction of intragastric Helicobacter pylori colonization: a randomized clinical trial. Can. J. Gastroenterol. 2011;25:207–213. doi: 10.1155/2011/672093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digiandomenico A., Warrener P., Hamilton M., Guillard S., Ravn P., Minter R., Camara M.M., Venkatraman V., Macgill R.S., Lin J., Wang Q., Keller A.E., Bonnell J.C., Tomich M., Jermutus L., Mccarthy M.P., Melnick D.A., Suzich J.A., Stover C.K. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 2012;209:1273–1287. doi: 10.1084/jem.20120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A., Fidel P.L., Jr. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J. Dent. Res. 2005;84:966–977. doi: 10.1177/154405910508401101. [DOI] [PubMed] [Google Scholar]
- Ekiert D.C., Bhabha G., Elsliger M.A., Friesen R.H., Jongeneelen M., Throsby M., Goudsmit J., Wilson I.A. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinel-Ingroff A. Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005–2009) Rev. Iberoam. Micol. 2009;26:15–22. doi: 10.1016/S1130-1406(09)70004-X. [DOI] [PubMed] [Google Scholar]
- Fabrizio K., Groner A., Boes M., Pirofski L.A. A human monoclonal immunoglobulin M reduces bacteremia and inflammation in a mouse model of systemic pneumococcal infection. Clin. Vaccine Immunol. 2007;14:382–390. doi: 10.1128/CVI.00374-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio K., Manix C., Guimaraes A.J., Nosanchuk J.D., Pirofski L.A. Aggregation of Streptococcus pneumoniae by a pneumococcal capsular polysaccharide-specific human monoclonal IgM correlates with antibody efficacy in vivo. Clin. Vaccine Immunol. 2010;17:713–721. doi: 10.1128/CVI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio K., Manix C., Tian H., Van Rooijen N., Pirofski L.A. The efficacy of pneumococcal capsular polysaccharide-specific antibodies to serotype 3 Streptococcus pneumoniae requires macrophages. Vaccine. 2010;28:7542–7550. doi: 10.1016/j.vaccine.2010.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure K., Fujimoto J., Shimabukuro D.W., Ajayi T., Shime N., Moriyama K., Spack E.G., Wiener-Kronish J.P., Sawa T. Effects of monoclonal anti-PcrV antibody on Pseudomonas aeruginosa-induced acute lung injury in a rat model. J. Immune Based Ther. Vaccines. 2003;1:2. doi: 10.1186/1476-8518-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N., Lawton J.A., Gilbert J., Kuklin N., Vo P., Prasad B.V., Greenberg H.B. Inhibition of rotavirus replication by a non-neutralizing, rotavirus VP6-specific IgA mAb. J. Clin. Invest. 2002;109:1203–1213. doi: 10.1172/JCI14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler S.J., Gregory R.L., Michalek S.M., Katz J., Mcghee J.R. Effect of immune bovine milk on Streptococcus mutans in human dental plaque. Arch. Oral Biol. 1991;36:41–47. doi: 10.1016/0003-9969(91)90052-v. [DOI] [PubMed] [Google Scholar]
- Finlay W.J., Bloom L., Cunningham O. Phage display: a powerful technology for the generation of high specificity affinity reagents from alternative immune sources. Methods Mol. Biol. 2011;681:87–101. doi: 10.1007/978-1-60761-913-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman A., Beirnaert E., Aasa-Chapman M.M., Hoorelbeke B., Hijazi K., Koh W., Tack V., Szynol A., Kelly C., Mcknight A., Verrips T., De Haard H., Weiss R.A. Llama antibody fragments with cross-subtype human immunodeficiency virus type 1 (HIV-1)-neutralizing properties and high affinity for HIV-1 gp120. J. Virol. 2008;82:12069–12081. doi: 10.1128/JVI.01379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois B., Luyt C.E., Dugard A., Wolff M., Diehl J.L., Jaber S., Forel J.M., Garot D., Kipnis E., Mebazaa A., Misset B., Andremont A., Ploy M.C., Jacobs A., Yarranton G., Pearce T., Fagon J.Y., Chastre J. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trial. Crit. Care Med. 2012;40:2320–2326. doi: 10.1097/CCM.0b013e31825334f6. [DOI] [PubMed] [Google Scholar]
- Freedman D.J., Tacket C.O., Delehanty A., Maneval D.R., Nataro J., Crabb J.H. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J. Infect. Dis. 1998;177:662–667. doi: 10.1086/514227. [DOI] [PubMed] [Google Scholar]
- Frenken L.G.J., Van Der Linden R.H.J., Hermans P.W.J.J., Bos J.W., Ruuls R.C., De Geus B., Verrips C.T. Isolation of antigen specific Llama V-HH antibody fragments and their high level secretion by Saccharomyces cerevisiae. J. Biotechnol. 2000;78:11–21. doi: 10.1016/s0168-1656(99)00228-x. [DOI] [PubMed] [Google Scholar]
- Fried A.J., Bonilla F.A. Pathogenesis, diagnosis, and management of primary antibody deficiencies and infections. Clin. Microbiol. Rev. 2009;22:396–414. doi: 10.1128/CMR.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujibayashi T., Nakamura M., Tominaga A., Satoh N., Kawarai T., Narisawa N., Shinozuka O., Watanabe H., Yamazaki T., Senpuku H. Effects of IgY against Candida albicans and Candida spp. Adherence and biofilm formation. Jpn. J. Infect. Dis. 2009;62:337–342. [PubMed] [Google Scholar]
- Gardulf A., Nicolay U., Asensio O., Bernatowska E., Bock A., Carvalho B.C., Granert C., Haag S., Hernandez D., Kiessling P., Kus J., Pons J., Niehues T., Schmidt S., Schulze I., Borte M. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies–a prospective, multi-national study. J. Clin. Immunol. 2006;26:177–185. doi: 10.1007/s10875-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Ghetie V., Ward E.S. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu. Rev. Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- Good R.A., Lorenz E. Historic aspects of intravenous immunoglobulin therapy. Cancer. 1991;68:1415–1421. doi: 10.1002/1097-0142(19910915)68:6+<1415::aid-cncr2820681402>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Gorlani A., Brouwers J., Mcconville C., Van Der Bijl P., Malcolm K., Augustijns P., Quigley A.F., Weiss R., De Haard H., Verrips T. Llama antibody fragments have good potential for application as HIV type 1 topical microbicides. AIDS Res. Hum. Retroviruses. 2012;28:198–205. doi: 10.1089/aid.2011.0133. [DOI] [PubMed] [Google Scholar]
- Gorrell R.J., Robins-Browne R.M. Antibody-mediated protection against infection with Helicobacter pylori in a suckling mouse model of passive immunity. Infect. Immun. 2009;77:5116–5129. doi: 10.1128/IAI.00547-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel B.J., Kapila A., Wittrup K.D. Picomolar affinity fibronectin domains engineered utilizing loop length diversity, recursive mutagenesis, and loop shuffling. J. Mol. Biol. 2008;381:1238–1252. doi: 10.1016/j.jmb.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.B. Nosocomial respiratory syncytial virus infections: the “Cold War” has not ended. Clin. Infect. Dis. 2000;31:590–596. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- Hamada S., Horikoshi T., Minami T., Kawabata S., Hiraoka J., Fujiwara T., Ooshima T. Oral passive-immunization against dental-caries in rats by use of hen egg-yolk antibodies specific for cell-associated glucosyltransferase of Streptococcus mutans. Infect. Immun. 1991;59:4161–4167. doi: 10.1128/iai.59.11.4161-4167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamajima S., Maruyama M., Hijiya T., Hatta H., Abiko Y. Egg yolk-derived immunoglobulin (IgY) against Porphyromonas gingivalis 40-kDa outer membrane protein inhibits coaggregation activity. Arch. Oral Biol. 2007;52:697–704. doi: 10.1016/j.archoralbio.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Songa E.B., Bendahman N., Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- Hammarstrom L., Weiner C.K. Targeted antibodies in dairy-based products. Adv. Exp. Med. Biol. 2008;606:321–343. doi: 10.1007/978-0-387-74087-4_13. [DOI] [PubMed] [Google Scholar]
- Harmsen M.M., De Haard H.J. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 2007;77:13–22. doi: 10.1007/s00253-007-1142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta H., Tsuda K., Ozeki M., Kim M., Yamamoto T., Otake S., Hirasawa M., Katz J., Childers N.K., Michalek S.M. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res. 1997;31:268–274. doi: 10.1159/000262410. [DOI] [PubMed] [Google Scholar]
- Hessell A.J., Rakasz E.G., Poignard P., Hangartner L., Landucci G., Forthal D.N., Koff W.C., Watkins D.I., Burton D.R. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell A.J., Rakasz E.G., Tehrani D.M., Huber M., Weisgrau K.L., Landucci G., Forthal D.N., Koff W.C., Poignard P., Watkins D.I., Burton D.R. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A., Cafferkey R., Bowdish K. Production of antibodies in transgenic plants. Nature. 1989;342:76–78. doi: 10.1038/342076a0. [DOI] [PubMed] [Google Scholar]
- Holliger P., Hudson P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- Horie K., Horie N., Abdou A.M., Yang J.O., Yun S.S., Chun H.N., Park C.K., Kim M., Hatta H. Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. J. Dairy Sci. 2004;87:4073–4079. doi: 10.3168/jds.S0022-0302(04)73549-3. [DOI] [PubMed] [Google Scholar]
- Horn M.P., Zuercher A.W., Imboden M.A., Rudolf M.P., Lazar H., Wu H., Hoiby N., Fas S.C., Lang A.B. Preclinical in vitro and in vivo characterization of the fully human monoclonal IgM antibody KBPA101 specific for Pseudomonas aeruginosa serotype IATS-O11. Antimicrob. Agents Chemother. 2010;54:2338–2344. doi: 10.1128/AAC.01142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne C., Vallance B.A., Deng W., Finlay B.B. Current progress in enteropathogenic and enterohemorrhagic Escherichia coli vaccines. Expert Rev. Vaccines. 2002;1:483–493. doi: 10.1586/14760584.1.4.483. [DOI] [PubMed] [Google Scholar]
- Houdebine L. Production of human polyclonal antibodies transgenic animals. Adv. Biosci. Biotechnol. 2011;2:138–141. [Google Scholar]
- Houdebine L.M. Production of pharmaceutical proteins by transgenic animals. Comp. Immunol. Microbiol. Infect. Dis. 2009;32:107–121. doi: 10.1016/j.cimid.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J.A. Toward an antibody-based HIV-1 vaccine. Annu. Rev. Med. 2010;61:135–152. doi: 10.1146/annurev.med.60.042507.164323. [DOI] [PubMed] [Google Scholar]
- Huang K., Incognito L., Cheng X., Ulbrandt N.D., Wu H. Respiratory syncytial virus-neutralizing monoclonal antibodies motavizumab and palivizumab inhibit fusion. J. Virol. 2010;84:8132–8140. doi: 10.1128/JVI.02699-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultberg A., Temperton N.J., Rosseels V., Koenders M., Gonzalez-Pajuelo M., Schepens B., Ibanez L.I., Vanlandschoot P., Schillemans J., Saunders M., Weiss R.A., Saelens X., Melero J.A., Verrips C.T., Van Gucht S., De Haard H.J. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS ONE. 2011;6:e17665. doi: 10.1371/journal.pone.0017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C.C., Yang Y.L., Lauderdale T.L., Mcdonald L.C., Hsiao C.F., Cheng H.H., Ho Y.A., Lo H.J. Colonization of human immunodeficiency virus-infected outpatients in Taiwan with Candida species. J. Clin. Microbiol. 2005;43:1600–1603. doi: 10.1128/JCM.43.4.1600-1603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley W.L., Theil P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3:442–474. doi: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussack G., Arbabi-Ghahroudi M., Van Faassen H., Songer J.G., Ng K.K., Mackenzie R., Tanha J. Neutralization of Clostridium difficile toxin A with single-domain antibodies targeting the cell receptor binding domain. J. Biol. Chem. 2011;286:8961–8976. doi: 10.1074/jbc.M110.198754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynicka L.M., Ensor C.R. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann. Pharmacother. 2012;46:558–566. doi: 10.1345/aph.1Q553. [DOI] [PubMed] [Google Scholar]
- Ibanez L.I., De Filette M., Hultberg A., Verrips T., Temperton N., Weiss R.A., Vandevelde W., Schepens B., Vanlandschoot P., Saelens X. Nanobodies with in vitro neutralizing activity protect mice against H5N1 influenza virus infection. J. Infect. Dis. 2011;203:1063–1072. doi: 10.1093/infdis/jiq168. [DOI] [PubMed] [Google Scholar]
- Ibrahim El S.M., Rahman A.K., Isoda R., Umeda K., Van Sa N., Kodama Y. In vitro and in vivo effectiveness of egg yolk antibody against Candida albicans (anti-CA IgY) Vaccine. 2008;26:2073–2080. doi: 10.1016/j.vaccine.2008.02.046. [DOI] [PubMed] [Google Scholar]
- Ikemori Y., Kuroki M., Peralta R.C., Yokoyama H., Kodama Y. Protection of neonatal calves against fatal enteric colibacillosis by administration of egg yolk powder from hens immunized with K99-piliated enterotoxigenic Escherichia coli. Am. J. Vet. Res. 1992;53:2005–2008. [PubMed] [Google Scholar]
- Ilver D., Arnqvist A., Ogren J., Frick I.M., Kersulyte D., Incecik E.T., Berg D.E., Covacci A., Engstrand L., Boren T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- Istrate C., Hinkula J., Hammarstrom L., Svensson L. Individuals with selective IgA deficiency resolve rotavirus disease and develop higher antibody titers (IgG, IgG1) than IgA competent individuals. J. Med. Virol. 2008;80:531–535. doi: 10.1002/jmv.21101. [DOI] [PubMed] [Google Scholar]
- Jacobson J.M., Thompson M.A., Lalezari J.P., Saag M.S., Zingman B.S., D’ambrosio P., Stambler N., Rotshteyn Y., Marozsan A.J., Maddon P.J., Morris S.A., Olson W.C. Anti-HIV-1 activity of weekly or biweekly treatment with subcutaneous PRO 140, a CCR5 monoclonal antibody. J. Infect. Dis. 2010;201:1481–1487. doi: 10.1086/652190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnichen S., Blanchetot C., Maussang D., Gonzalez-Pajuelo M., Chow K.Y., Bosch L., De Vrieze S., Serruys B., Ulrichts H., Vandevelde W., Saunders M., De Haard H.J., Schols D., Leurs R., Vanlandschoot P., Verrips T., Smit M.J. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20565–20570. doi: 10.1073/pnas.1012865107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoi E.R., Cambier J., Saslow S. Transepithelial transport of maternal antibody: purification of IgG receptor from newborn rat intestine. J. Immunol. 1985;135:3360–3364. [PubMed] [Google Scholar]
- Janson A., Nava S., Hammarstrom L., Bruessow H., Mahalanabis D. Titers of Specific Antibodies in Immunized and Non-immunized Cow Colostrum; Implication for Their Use in the Treatment of Patients with Gastro-intestinal Infections. Indigenous Antimicrobial Agents of Milk–Recent Developments. uppusala. International Dairy Federation Press. 1994:221–228. [Google Scholar]
- Jovcic B., Lepsanovic Z., Suljagic V., Rackov G., Begovic J., Topisirovic L., Kojic M. Emergence of NDM-1 metallo-beta-lactamase in Pseudomonas aeruginosa clinical isolates from Serbia. Antimicrob. Agents Chemother. 2011;55:3929–3931. doi: 10.1128/AAC.00226-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis C., Champlin R.E., Hachem R.Y., Hosing C., Tarrand J.J., Perego C.A., Neumann J.L., Raad Ii, Chemaly R.F. Detection and control of a nosocomial respiratory syncytial virus outbreak in a stem cell transplantation unit: the role of palivizumab. Biol. Blood Marrow Transplant. 2010;16:1265–1271. doi: 10.1016/j.bbmt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Kamihira M., Ono K., Kyogoku K., Nishijima K., Iijima S. Production of scFv-Fc fusion protein using genetically manipulated quails. J. Biosci. Bioeng. 2006;102:297–303. doi: 10.1263/jbb.102.297. [DOI] [PubMed] [Google Scholar]
- Kelly C.P., Chetham S., Keates S., Bostwick E.F., Roush A.M., Castagliuolo I., Lamont J.T., Pothoulakis C. Survival of anti-Clostridium difficile bovine immunoglobulin concentrate in the human gastrointestinal tract. Antimicrob. Agents Chemother. 1997;41:236–241. doi: 10.1128/aac.41.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C.P., Pothoulakis C., Vavva F., Castagliuolo I., Bostwick E.F., Okeane J.C., Keates S., Lamont J.T. Anti-Clostridium difficile bovine immunoglobulin concentrate inhibits cytotoxicity and enterotoxicity of C. difficile toxins. Antimicrob. Agents Chemother. 1996;40:373–379. doi: 10.1128/aac.40.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kink J.A., Williams J.A. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect. Immun. 1998;66:2018–2025. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kong L.K., Zhou B.P. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med. J. 2006;12:489. [PubMed] [Google Scholar]
- Kovacs-Nolan J., Mine Y. Egg yolk antibodies for passive immunity. Annu. Rev. Food Sci. Technol. 2012;3:163–182. doi: 10.1146/annurev-food-022811-101137. [DOI] [PubMed] [Google Scholar]
- Kramski M., Center R.J., Wheatley A.K., Jacobson J.C., Alexander M.R., Rawlin G., Purcell D.F. Hyperimmune bovine colostrum as a low-cost, large-scale source of antibodies with broad neutralizing activity for HIV-1 envelope with potential use in microbicides. Antimicrob. Agents Chemother. 2012;56:4310–4319. doi: 10.1128/AAC.00453-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautz-Peterson G., Chapman-Bonofiglio S., Boisvert K., Feng H., Herman I.M., Tzipori S., Sheoran A.S. Intracellular neutralization of Shiga toxin 2 by an a subunit-specific human monoclonal antibody. Infect. Immun. 2008;76:1931–1939. doi: 10.1128/IAI.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger C., Hu Y.Z., Pan Q., Marcotte H., Hultberg A., Delwar D., Van Dalen P.J., Pouwels P.H., Leer R.J., Kelly C.G., Van Dollenweerd C., Ma J.K., Hammarstrom L. In situ delivery of passive immunity by lactobacilli producing single-chain antibodies. Nat. Biotechnol. 2002;20:702–706. doi: 10.1038/nbt0702-702. [DOI] [PubMed] [Google Scholar]
- Kruger C., Hultberg A., Marcotte H., Hermans P., Bezemer S., Frenken L.G., Hammarstrom L. Therapeutic effect of llama derived VHH fragments against Streptococcus mutans on the development of dental caries. Appl. Microbiol. Biotechnol. 2006;72:732–737. doi: 10.1007/s00253-006-0347-0. [DOI] [PubMed] [Google Scholar]
- Kruger C., Hultberg A., Van Dollenweerd C., Marcotte H., Hammarstrom L. Passive immunization by lactobacilli expressing single-chain antibodies against Streptococcus mutans. Mol. Biotechnol. 2005;31:221–231. doi: 10.1385/MB:31:3:221. [DOI] [PubMed] [Google Scholar]
- Kruger C., Pearson S.K., Kodama Y., Smith A.V., Bowen W.H., Hammarstrom L. The effects of egg-derived antibodies to glucosyltransferases on dental caries in rats. Caries Res. 2004;38:9–14. doi: 10.1159/000073914. [DOI] [PubMed] [Google Scholar]
- Kuroiwa Y., Kasinathan P., Sathiyaseelan T., Jiao J.A., Matsushita H., Sathiyaseelan J., Wu H., Mellquist J., Hammitt M., Koster J., Kamoda S., Tachibana K., Ishida I., Robl J.M. Antigen-specific human polyclonal antibodies from hyperimmunized cattle. Nat. Biotechnol. 2009;27:173–181. doi: 10.1038/nbt.1521. [DOI] [PubMed] [Google Scholar]
- Lagenaur L.A., Sanders-Beer B.E., Brichacek B., Pal R., Liu X., Liu Y., Yu R., Venzon D., Lee P.P., Hamer D.H. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 2011;4:648–657. doi: 10.1038/mi.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J.W., Thomas D.W. Producing proteins in transgenic plants and animals. Curr. Opin. Biotechnol. 2001;12:411–418. doi: 10.1016/s0958-1669(00)00236-6. [DOI] [PubMed] [Google Scholar]
- Lauwereys M., Arbabi Ghahroudi M., Desmyter A., Kinne J., Holzer W., De Genst E., Wyns L., Muyldermans S. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998;17:3512–3520. doi: 10.1093/emboj/17.13.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R.M., Pane C.A. Human breast milk: current concepts of immunology and infectious diseases. Curr. Probl. Pediatr. Adolesc. Health Care. 2007;37:7–36. doi: 10.1016/j.cppeds.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Leach J.L., Sedmak D.D., Osborne J.M., Rahill B., Lairmore M.D., Anderson C.L. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J. Immunol. 1996;157:3317–3322. [PubMed] [Google Scholar]
- Lehner T., Caldwell J., Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect. Immun. 1985;50:796–799. doi: 10.1128/iai.50.3.796-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillico S.G., Mcgrew M., Sherman A., Sang H.M. Transgenic chickens as bioreactors for protein-based drugs. Drug Discov. Today. 2005;10:191–196. doi: 10.1016/S1359-6446(04)03317-3. [DOI] [PubMed] [Google Scholar]
- Liu X., Lagenaur L.A., Simpson D.A., Essenmacher K.P., Frazier-Parker C.L., Liu Y., Tsai D., Rao S.S., Hamer D.H., Parks T.P., Lee P.P., Xu Q. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob. Agents Chemother. 2006;50:3250–3259. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loimaranta V., Carlen A., Olsson J., Tenovuo J., Syvaoja E.L., Korhonen H. Concentrated bovine colostral whey proteins from Streptococcus mutans/Strep. sobrinus immunized cows inhibit the adherence of Strep. mutans and promote the aggregation of mutans streptococci. J. Dairy Res. 1998;65:599–607. doi: 10.1017/s0022029998003069. [DOI] [PubMed] [Google Scholar]
- Loimaranta V., Laine M., Soderling E., Vasara E., Rokka S., Marnila P., Korhonen H., Tossavainen O., Tenovuo J. Effects of bovine immune and non-immune whey preparations on the composition and pH response of human dental plaque. Eur. J. Oral Sci. 1999;107:244–250. doi: 10.1046/j.0909-8836.1999.eos107403.x. [DOI] [PubMed] [Google Scholar]
- Loimaranta V., Nuutila J., Marnila P., Tenovuo J., Korhonen H., Lilius E.M. Colostral proteins from cows immunised with Streptococcus mutans/S. sobrinus support the phagocytosis and killing of mutans streptococci by human leucocytes. J. Med. Microbiol. 1999;48:917–926. doi: 10.1099/00222615-48-10-917. [DOI] [PubMed] [Google Scholar]
- Lonberg N. Human antibodies from transgenic animals. Nat. Biotechnol. 2005;23:1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- Loureiro I., Frankel G., Adu-Bobie J., Dougan G., Trabulsi L.R., Carneiro-Sampaio M.M. Human colostrum contains IgA antibodies reactive to enteropathogenic Escherichia coli virulence-associated proteins: intimin, BfpA, EspA, and EspB. J. Pediatr. Gastroenterol. Nutr. 1998;27:166–171. doi: 10.1097/00005176-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Lowy I., Molrine D.C., Leav B.A., Blair B.M., Baxter R., Gerding D.N., Nichol G., Thomas W.D., Jr., Leney M., Sloan S., Hay C.A., Ambrosino D.M. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- Lu J., Guo Z., Pan X., Wang G., Zhang D., Li Y., Tan B., Ouyang L., Yu X. Passive immunotherapy for influenza A H5N1 virus infection with equine hyperimmune globulin F(ab’)2 in mice. Respir. Res. 2006;7:43. doi: 10.1186/1465-9921-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Rouby J.J., Laterre P.F., Eggimann P., Dugard A., Giamarellos-Bourboulis E.J., Mercier E., Garbino J., Luyt C.E., Chastre J., Georgescu-Kyburz V., Rudolf M.P., Gafner V., Lazar H., Koch H., Perez A., Kramer S.D., Tamm M. Pharmacokinetics and safety of panobacumab: specific adjunctive immunotherapy in critical patients with nosocomial Pseudomonas aeruginosa O11 pneumonia. J. Antimicrob. Chemother. 2011;66:1110–1116. doi: 10.1093/jac/dkr046. [DOI] [PubMed] [Google Scholar]
- Lu W., Zhao Z., Zhao Y., Yu S., Zhao Y., Fan B., Kacskovics I., Hammarstrom L., Li N. Over-expression of the bovine FcRn in the mammary gland results in increased IgG levels in both milk and serum of transgenic mice. Immunology. 2007;122:401–408. doi: 10.1111/j.1365-2567.2007.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D.M., Bostwick E.F., Binion S.B., Wilkins T.D. Passive-immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin-G concentrate. Infect. Immun. 1991;59:2215–2218. doi: 10.1128/iai.59.6.2215-2218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.K., Hiatt A., Hein M., Vine N.D., Wang F., Stabila P., Van Dolleweerd C., Mostov K., Lehner T. Generation and assembly of secretory antibodies in plants. Science. 1995;268:716–719. doi: 10.1126/science.7732380. [DOI] [PubMed] [Google Scholar]
- Ma J.K., Hikmat B.Y., Wycoff K., Vine N.D., Chargelegue D., Yu L., Hein M.B., Lehner T. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 1998;4:601–606. doi: 10.1038/nm0598-601. [DOI] [PubMed] [Google Scholar]
- Ma J.K., Hunjan M., Smith R., Kelly C., Lehner T. An investigation into the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutans. Infect. Immun. 1990;58:3407–3414. doi: 10.1128/iai.58.10.3407-3414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.K., Hunjan M., Smith R., Lehner T. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin. Exp. Immunol. 1989;77:331–337. [PMC free article] [PubMed] [Google Scholar]
- Ma J.K., Smith R., Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect. Immun. 1987;55:1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S.A., Cunliffe N.A., Steele D., Witte D., Kirsten M., Louw C., Ngwira B., Victor J.C., Gillard P.H., Cheuvart B.B., Han H.H., Neuzil K.M. Effect of human rotavirus vaccine on severe diarrhea in African infants. N. Engl. J. Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- Magliani W., Conti S., De Bernardis F., Gerloni M., Bertolotti D., Mozzoni P., Cassone A., Polonelli L. Therapeutic potential of antiidiotypic single chain antibodies with yeast killer toxin activity. Nat. Biotechnol. 1997;15:155–158. doi: 10.1038/nbt0297-155. [DOI] [PubMed] [Google Scholar]
- Mahdavi J., Sonden B., Hurtig M., Olfat F.O., Forsberg L., Roche N., Angstrom J., Larsson T., Teneberg S., Karlsson K.A., Altraja S., Wadstrom T., Kersulyte D., Berg D.E., Dubois A., Petersson C., Magnusson K.E., Norberg T., Lindh F., Lundskog B.B., Arnqvist A., Hammarstrom L., Boren T. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekshahi Z.V., Gargari S.L.M., Rasooli I., Ebrahimizadeh W. Treatment of Helicobacter pylori infection in mice with oral administration of egg yolk-driven anti-UreC immunoglobulin. Microb. Pathog. 2011;51:366–372. doi: 10.1016/j.micpath.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Marcotte H., Koll-Klais P., Hultberg A., Zhao Y., Gmur R., Mandar R., Mikelsaar M., Hammarstrom L. Expression of single-chain antibody against RgpA protease of Porphyromonas gingivalis in Lactobacillus. J. Appl. Microbiol. 2006;100:256–263. doi: 10.1111/j.1365-2672.2005.02786.x. [DOI] [PubMed] [Google Scholar]
- Marcotte H., Lavoie M.C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol. Mol. Biol. Rev. 1998;62:71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte H., Pant N., Hammarstrom L. Engineered lactobody-producing lactobacilli: a novel form of therapy against rotavirus infection. Future Virol. 2008;3:327–341. [Google Scholar]
- Marozsan A.J., Ma D., Nagashima K.A., Kennedy B.J., Kang Y.K., Arrigale R.R., Donovan G.P., Magargal W.W., Maddon P.J., Olson W.C. Protection against Clostridium difficile infection with broadly neutralizing antitoxin monoclonal antibodies. J. Infect. Dis. 2012;206:706–713. doi: 10.1093/infdis/jis416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt R.R., Jin L.Z., Kim J.W., Fang L., Frohlich A.A., Baidoo S.K. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol. Med. Microbiol. 1999;23:283–288. doi: 10.1111/j.1574-695X.1999.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Martin M.C., Pant N., Ladero V., Gunaydin G., Andersen K.K., Alvarez B., Martinez N., Alvarez M.A., Hammarstrom L., Marcotte H. Integrative expression system for delivery of antibody fragments by lactobacilli. Appl. Environ. Microbiol. 2011;77:2174–2179. doi: 10.1128/AEM.02690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusic C., Vitale A., Pedrazzini E., Donini M., Frigerio L., Bock R., Dix P.J., Mccabe M.S., Bellucci M., Benvenuto E. Plant-based strategies aimed at expressing HIV antigens and neutralizing antibodies at high levels. Nef as a case study. Transgenic Res. 2009;18:499–512. doi: 10.1007/s11248-009-9244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola J.R., Stiegler G., Vancott T.C., Katinger H., Carpenter C.B., Hanson C.E., Beary H., Hayes D., Frankel S.S., Birx D.L., Lewis M.G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- Matthews R., Burnie J. Antifungal antibodies: a new approach to the treatment of systemic candidiasis. Curr. Opin. Investig. Drugs. 2001;2:472–476. [PubMed] [Google Scholar]
- Matthews R.C., Rigg G., Hodgetts S., Carter T., Chapman C., Gregory C., Illidge C., Burnie J. Preclinical assessment of the efficacy of mycograb, a human recombinant antibody against fungal HSP90. Antimicrob. Agents Chemother. 2003;47:2208–2216. doi: 10.1128/AAC.47.7.2208-2216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B., Doleschall M., Bender B., Bartyik J., Bosze Z., Frenyo L.V., Kacskovics I. Expression of the neonatal Fc receptor (FcRn) in the bovine mammary gland. J. Dairy Res. 2005;72:107–112. doi: 10.1017/s0022029905001135. [DOI] [PubMed] [Google Scholar]
- Mayer B., Kis Z., Kajan G., Frenyo L.V., Hammarstrom L., Kacskovics I. The neonatal Fc receptor (FcRn) is expressed in the bovine lung. Vet. Immunol. Immunopathol. 2004;98:85–89. doi: 10.1016/j.vetimm.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Mayer B., Zolnai A., Frenyo L.V., Jancsik V., Szentirmay Z., Hammarstrom L., Kacskovics I. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology. 2002;107:288–296. doi: 10.1046/j.1365-2567.2002.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccoy L.E., Quigley A.F., Strokappe N.M., Bulmer-Thomas B., Seaman M.S., Mortier D., Rutten L., Chander N., Edwards C.J., Ketteler R., Davis D., Verrips T., Weiss R.A. Potent and broad neutralization of HIV-1 by a llama antibody elicited by immunization. J. Exp. Med. 2012;209:1091–1103. doi: 10.1084/jem.20112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S.M., Gregory R.L., Harmon C.C., Katz J., Richardson G.J., Hilton T., Filler S.J., Mcghee J.R. Protection of gnotobiotic rats against dental caries by passive immunization with bovine milk antibodies to Streptococcus mutans. Infect. Immun. 1987;55:2341–2347. doi: 10.1128/iai.55.10.2341-2347.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietens C., Keinhorst H., Hilpert H., Gerber H., Amster H., Pahud J.J. Treatment of infantile E. coli gastroenteritis with specific bovine anti-E. coli milk immunoglobulins. Eur. J. Pediatr. 1979;132:239–252. doi: 10.1007/BF00496847. [DOI] [PubMed] [Google Scholar]
- Migone T.S., Subramanian G.M., Zhong J., Healey L.M., Corey A., Devalaraja M., Lo L., Ullrich S., Zimmerman J., Chen A., Lewis M., Meister G., Gillum K., Sanford D., Mott J., Bolmer S.D. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 2009;361:135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- Moldt B., Rakasz E.G., Schultz N., Chan-Hui P.Y., Swiderek K., Weisgrau K.L., Piaskowski S.M., Bergman Z., Watkins D.I., Poignard P., Burton D.R. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. U.S.A. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G., Chindrove S., Woodhall S., Wiggins R., Vcelar B., Lacey C. Microbicides; Pittsburgh, PA, LB1: 2010. A prospective randomized double blind placebo-controlled phase 1 pharmacokinetic and safety study of a vaginal microbicide gel containing three potent broadly neutralizing antibodies. (2F5, 2G12, 4E10) (MabGel) p. 229. [Google Scholar]
- Morris S.K., Dzolganovski B., Beyene J., Sung L. A meta-analysis of the effect of antibody therapy for the prevention of severe respiratory syncytial virus infection. BMC Infect. Dis. 2009;9:106. doi: 10.1186/1471-2334-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J., Chios K., Fishwild D., Hudson D., O’donnell S., Rich S.M., Donohue-Rolfe A., Tzipori S. Human Stx2-specific monoclonal antibodies prevent systemic complications of Escherichia coli O157:H7 infection. Infect. Immun. 2002;70:612–619. doi: 10.1128/iai.70.2.612-619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Premru M., Gubina M. Serotype, antimicrobial susceptibility and clone distribution of Pseudomonas aeruginosa in a university hospital. Zentralbl. Bakteriol. 2000;289:857–867. doi: 10.1016/s0934-8840(00)80015-8. [DOI] [PubMed] [Google Scholar]
- Mulvey G.L., Dingle T.C., Fang L., Strecker J., Armstrong G.D. Therapeutic potential of egg yolk antibodies for treating Clostridium difficile infection. J. Med. Microbiol. 2011;60:1181–1187. doi: 10.1099/jmm.0.029835-0. [DOI] [PubMed] [Google Scholar]
- Nelson A.L., Dhimolea E., Reichert J.M. Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug. Discov. 2010;9:767–774. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- Neri P., Tokoro S., Kobayashi R., Sugiyama T., Umeda K., Shimizu T., Tsuji T., Kodama Y., Oguma K., Mori H. Specific egg yolk immunoglobulin as a new preventive approach for shiga-toxin-mediated diseases. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0026526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W.C., Wong V., Muller B., Rawlin G., Brown L.E. Prevention and treatment of influenza with hyperimmune bovine colostrum antibody. PLoS ONE. 2010;5:e13622. doi: 10.1371/journal.pone.0013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.H., Tumpey T.M., Park H.J., Byun Y.H., Tran L.D., Nguyen V.D., Kilgore P.E., Czerkinsky C., Katz J.M., Seong B.L., Song J.M., Kim Y.B., Do H.T., Nguyen T., Nguyen C.V. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS ONE. 2010;5:e10152. doi: 10.1371/journal.pone.0010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E., Kollberg H., Johannesson M., Wejaker P.E., Carlander D., Larsson A. More than 10 years’ continuous oral treatment with specific immunoglobulin Y for the prevention of Pseudomonas aeruginosa infections: a case report. J. Med. Food. 2007;10:375–378. doi: 10.1089/jmf.2006.214. [DOI] [PubMed] [Google Scholar]
- Nilsson E., Larsson A., Olesen H.V., Wejaker P.E., Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr. Pulmonol. 2008;43:892–899. doi: 10.1002/ppul.20875. [DOI] [PubMed] [Google Scholar]
- Nomura S., Suzuki H., Masaoka T., Kurabayashi K., Ishii H., Kitajima M., Nomoto K., Hibi T. Effect of dietary anti-urease immunoglobulin Y on Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2005;10:43–52. doi: 10.1111/j.1523-5378.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Numan S.C., Veldkamp P., Kuijper E.J., Van Den Berg R.J., Van Dissel J.T. Clostridium difficile-associated diarrhoea: bovine anti-Clostridium difficile whey protein to help aid the prevention of relapses. Gut. 2007;56:888–889. doi: 10.1136/gut.2006.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren P.A. Alternative binding proteins: affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J. 2008;275:2668–2676. doi: 10.1111/j.1742-4658.2008.06438.x. [DOI] [PubMed] [Google Scholar]
- Opekun A.R., El-Zaimaity H.M., Osato M.S., Gilger M.A., Malaty H.M., Terry M., Headon D.R., Graham D.Y. Novel therapies for Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1999;13:35–42. doi: 10.1046/j.1365-2036.1999.00435.x. [DOI] [PubMed] [Google Scholar]
- Orange J.S., Hossny E.M., Weiler C.R., Ballow M., Berger M., Bonilla F.A., Buckley R., Chinen J., El-Gamal Y., Mazer B.D., Nelson R.P., Jr., Patel D.D., Secord E., Sorensen R.U., Wasserman R.L., Cunningham-Rundles C., Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma, and Immunology Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J. Allergy Clin. Immunol. 2006;117:S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Otake S., Nishihara Y., Makimura M., Hatta H., Kim M., Yamamoto T., Hirasawa M. Protection of rats against dental caries by passive immunization with hen-egg-yolk antibody (IgY) J. Dent. Res. 1991;70:162–166. doi: 10.1177/00220345910700030101. [DOI] [PubMed] [Google Scholar]
- Otto W., Najnigier B., Stelmasiak T., Robins-Browne R.M. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scand. J. Gastroenterol. 2011;46:862–868. doi: 10.3109/00365521.2011.574726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachl J., Svoboda P., Jacobs F., Vandewoude K., Van Der Hoven B., Spronk P., Masterson G., Malbrain M., Aoun M., Garbino J., Takala J., Drgona L., Burnie J., Matthews R., Mycograb Invasive Candidiasis Study Group A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 2006;42:1404–1413. doi: 10.1086/503428. [DOI] [PubMed] [Google Scholar]
- Pant N., Hultberg A., Zhao Y.F., Svensson L., Pan-Hammarstrom Q., Johansen K., Pouwels P.H., Ruggeri F.M., Hermans P., Frenken L., Boren T., Marcotte H., Hammarstrom L. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. J. Infect. Dis. 2006;194:1580–1588. doi: 10.1086/508747. [DOI] [PubMed] [Google Scholar]
- Pant N., Marcotte H., Brussow H., Svensson L., Hammarstrom L. Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC Microbiol. 2007;7 doi: 10.1186/1471-2180-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant N., Marcotte H., Hermans P., Bezemer S., Frenken L., Johansen K., Hammarstrom L. Lactobacilli producing bispecific llama-derived anti-rotavirus proteins in vivo for rotavirus-induced diarrhea. Future Microbiol. 2011;6:583–593. doi: 10.2217/fmb.11.32. [DOI] [PubMed] [Google Scholar]
- Parren P.W.H.I., Marx P.A., Hessell A.J., Luckay A., Harouse J., Cheng-Mayer C., Moore J.P., Burton D.R. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G.B., Boyer D., Preston M., Coleman F.T., Llosa N., Mueschenborn-Koglin S., Theilacker C., Goldenberg H., Uchin J., Priebe G.P., Grout M., Posner M., Cavacini L. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J. Immunol. 2004;173:5671–5678. doi: 10.4049/jimmunol.173.9.5671. [DOI] [PubMed] [Google Scholar]
- Pollock D.P., Kutzko J.P., Birck-Wilson E., Williams J.L., Echelard Y., Meade H.M. Transgenic milk as a method for the production of recombinant antibodies. J. Immunol. Methods. 1999;231:147–157. doi: 10.1016/S0022-1759(99)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonelli L., Seguy N., Conti S., Gerloni M., Bertolotti D., Cantelli C., Magliani W., Cailliez J.C. Monoclonal yeast killer toxin-like candidacidal anti-idiotypic antibodies. Clin. Diagn. Lab. Immunol. 1997;4:142–146. doi: 10.1128/cdli.4.2.142-146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran M., Prabhu N., He F., Hongliang Q., Ho H.T., Qiang J., Meng T., Goutama M., Kwang J. Combination therapy using chimeric monoclonal antibodies protects mice from lethal H5N1 infection and prevents formation of escape mutants. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu N., Prabakaran M., Qian H.L., He F., Ho H.T., Qiang J., Goutama M., Lim A.P.C., Hanson B.J., Kwang J. Prophylactic and therapeutic efficacy of a chimeric monoclonal antibody specific for H5 haemagglutinin against lethal H5N1 influenza. Antivir. Ther. 2009;14:911–921. doi: 10.3851/IMP1413. [DOI] [PubMed] [Google Scholar]
- Prince G.A., Horswood R.L., Chanock R.M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J. Virol. 1985;55:517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. Passive immunity in newborn calves. West. Can. Dairy Semin. 2002;273 [Google Scholar]
- Rabinovitz B.C., Gerhardt E., Farinati C.T., Abdala A., Galarza R., Vilte D.A., Ibarra C., Cataldi A., Mercado E.C. Vaccination of pregnant cows with EspA, EspB, gamma-intimin, and Shiga toxin 2 proteins from Escherichia coli O157:H7 induces high levels of specific colostral antibodies that are transferred to newborn calves. J. Dairy Sci. 2012;95:3318–3326. doi: 10.3168/jds.2011-5093. [DOI] [PubMed] [Google Scholar]
- Rademacher T., Sack M., Arcalis E., Stadlmann J., Balzer S., Altmann F., Quendler H., Stiegler G., Kunert R., Fischer R., Stoger E. Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly single-GlcNAc N-glycans. Plant Biotechnol. J. 2008;6:189–201. doi: 10.1111/j.1467-7652.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- Rahbarizadeh F., Ahmadvand D., Sharifzadeh Z. Nanobody; an old concept and new vehicle for immunotargeting. Immunol. Invest. 2011;40:299–338. doi: 10.3109/08820139.2010.542228. [DOI] [PubMed] [Google Scholar]
- Ramessar K., Rademacher T., Sack M., Stadlmann J., Platis D., Stiegler G., Labrou N., Altmann F., Ma J., Stoger E., Capell T., Christou P. Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3727–3732. doi: 10.1073/pnas.0708841104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelings M.D., Sorvillo F., Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg. Infect. Dis. 2007;13:1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Mcglashan J., Al-Abdulla I., Ling R., Denton H., Green S., Coxon R., Landon J., Shone C. Development and evaluation of an ovine antibody-based platform for treatment of Clostridium difficile infection. Infect. Immun. 2012;80:875–882. doi: 10.1128/IAI.05684-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri F.M., Greenberg H.B. Antibodies to the trypsin cleavage peptide Vp8-Star neutralize rotavirus by inhibiting binding of virions to target-cells in culture. J. Virol. 1991;65:2211–2219. doi: 10.1128/jvi.65.5.2211-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri F.M., Johansen K., Basile G., Kraehenbuhl J.P., Svensson L. Antirotavirus immunoglobulin a neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J. Virol. 1998;72:2708–2714. doi: 10.1128/jvi.72.4.2708-2714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios G.M., Perez-Schael I., Velazquez F.R., Abate H., Breuer T., Clemens S.C., Cheuvart B., Espinoza F., Gillard P., Innis B.L., Cervantes Y., Linhares A.C., Lopez P., Macias-Parra M., Ortega-Barria E., Richardson V., Rivera-Medina D.M., Rivera L., Salinas B., Pavia-Ruz N., Salmeron J., Ruttimann R., Tinoco J.C., Rubio P., Nunez E., Guerrero M.L., Yarzabal J.P., Damaso S., Tornieporth N., Saez-Llorens X., Vergara R.F., Vesikari T., Bouckenooghe A., Clemens R., De Vos B., O’ryan M., Grp H.R.V.S. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. New. Engl. J. Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- Salcedo J., Keates S., Pothoulakis C., Warny M., Castagliuolo I., Lamont J.T., Kelly C.P. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366–370. doi: 10.1136/gut.41.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosham M., Reid R., Ambrosino D.M., Wolff M.C., Almeido-Hill J., Priehs C., Aspery K.M., Garrett S., Croll L., Foster S. Prevention of Haemophilus influenzae type b infections in high-risk infants treated with bacterial polysaccharide immune globulin. N. Engl. J. Med. 1987;317:923–929. doi: 10.1056/NEJM198710083171503. [DOI] [PubMed] [Google Scholar]
- Sarker S.A., Casswall T.H., Juneja L.R., Hoq E., Hossain I., Fuchs G.J., Hammarstrom L. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J. Pediatr. Gastroenterol. Nutr. 2001;32:19–25. doi: 10.1097/00005176-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Sarker S.A., Casswall T.H., Mahalanabis D., Alam N.H., Albert M.J., Brussow H., Fuchs G.J., Hammarstrom L. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrum. Pediatr. Infect. Dis. J. 1998;17:1149–1154. doi: 10.1097/00006454-199812000-00010. [DOI] [PubMed] [Google Scholar]
- Sarker S.A., Pant N., Juneja L.R., Hammarstrom L. Successful treatment of rotavirus-induced diarrhoea in suckling mice with egg yolk immunoglobulin. J. Health Popul. Nutr. 2007;25:465–468. [PMC free article] [PubMed] [Google Scholar]
- Sarker S.A., Jakel M., Sultana S., Alam N.H., Bardhan P.K., Chisti M.J., Salam M.A., Theis W., Hammarstrom L., Frenken L.G. Anti-rotavirus protein reduces stool output in infants with diarrhea: a randomized placebo-controlled trial. Gastroenterology. 2013;145:740–748. doi: 10.1053/j.gastro.2013.06.053. [DOI] [PubMed] [Google Scholar]
- Schirrmann T., Al-Halabi L., Dubel S., Hust M. Production systems for recombinant antibodies. Front. Biosci. 2008;13:4576–4594. doi: 10.2741/3024. [DOI] [PubMed] [Google Scholar]
- Secher T., Fauconnier L., Szade A., Rutschi O., Fas S.C., Ryffel B., Rudolf M.P. Anti-Pseudomonas aeruginosa serotype O11 LPS immunoglobulin M monoclonal antibody panobacumab (KBPA101) confers protection in a murine model of acute lung infection. J. Antimicrob. Chemother. 2011;66:1100–1109. doi: 10.1093/jac/dkr038. [DOI] [PubMed] [Google Scholar]
- Sestak K., Lanza I., Park S.K., Weilnau P.A., Saif L.J. Contribution of passive immunity to porcine respiratory coronavirus to protection against transmissible gastroenteritis virus challenge exposure in suckling pigs. Am. J. Vet. Res. 1996;57:664–671. [PubMed] [Google Scholar]
- Shattock R.J., Moore J.P. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- Sheoran A.S., Chapman-Bonofiglio S., Harvey B.R., Mukherjee J., Georgiou G., Donohue-Rolfe A., Tzipori S. Human antibody against shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infect. Immun. 2005;73:4607–4613. doi: 10.1128/IAI.73.8.4607-4613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto C., Tokioka S., Hirata I., Tani H., Ohishi H., Katsu K. Inhibition of Helicobacter pylori infection by orally administered yolk-derived anti-Helicobacter pylori antibody. Hepatogastroenterology. 2002;49:709–714. [PubMed] [Google Scholar]
- Shin J.H., Yang M., Nam S.W., Kim J.T., Myung N.H., Bang W.G., Roe I.H. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin. Diagn. Lab. Immunol. 2002;9:1061–1066. doi: 10.1128/CDLI.9.5.1061-1066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin P.A., Rehmus J.M., Johnson C.E., Marchant C.D., Carlin S.A., Super D.M., Vanhare G.F., Jones P.K., Ambrosino D.M., Siber G.R. Bacterial polysaccharide immune globulin for prophylaxis of acute Otitis-Media in high-risk children. J. Pediatr. 1993;123:801–810. doi: 10.1016/s0022-3476(05)80865-0. [DOI] [PubMed] [Google Scholar]
- Siber G.R., Thompson C., Reid G.R., Almeidohill J., Zacher B., Wolff M., Santosham M. Evaluation of bacterial polysaccharide immune globulin for the treatment or prevention of Haemophilus-Influenzae type-B and pneumococcal disease. J. Infect. Dis. 1992;165:S129–S133. doi: 10.1093/infdis/165-supplement_1-s129. [DOI] [PubMed] [Google Scholar]
- Silverstein A.M. Paul Ehrlich: the founding of pediatric immunology. Cell. Immunol. 1996;174:1–6. doi: 10.1006/cimm.1996.0286. [DOI] [PubMed] [Google Scholar]
- Skerra A., Pluckthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988;240:1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Sobel J.D. Management of patients with recurrent vulvovaginal candidiasis. Drugs. 2003;63:1059–1066. doi: 10.2165/00003495-200363110-00002. [DOI] [PubMed] [Google Scholar]
- Spiekermann G.M., Finn P.W., Ward E.S., Dumont J., Dickinson B.L., Blumberg R.S., Lencer W.I. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson I., Nicholson K.G., Wood J.M., Zambon M.C., Katz J.M. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect. Dis. 2004;4:499–509. doi: 10.1016/S1473-3099(04)01105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehm E.R., Orange J.S., Ballow M., Lehman H. Therapeutic use of immunoglobulins. Adv. Pediatr. 2010;57:185–218. doi: 10.1016/j.yapd.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suerbaum S., Michetti P. Medical progress: Helicobacter pylori infection. New. Engl. J. Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nomura S., Masaoka T., Goshima H., Kamata N., Kodama Y., Ishii H., Kitajima M., Nomoto K., Hibi T. Effect of dietary anti-Helicobacter pylori-urease immunoglobulin Y on Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2004;20:185–192. doi: 10.1111/j.1365-2036.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- Svennerholm A.M., Lundgren A. Recent progress toward an enterotoxigenic Escherichia coli vaccine. Expert Rev. Vaccines. 2012;11:495–507. doi: 10.1586/erv.12.12. [DOI] [PubMed] [Google Scholar]
- Tan G.S., Krammer F., Eggink D., Kongchanagul A., Moran T.M., Palese P. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J. Virol. 2012;86:6179–6188. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpila S., Korhonen H., Salminen S. 1994. Immune Colostrum in the Treatment of Helicobacter pylori Gastritis. 24th. International Dairy Congress, Australia, Melbourne. pp. 293. [Google Scholar]
- Tasumi S., Velikovsky C.A., Xu G., Gai S.A., Wittrup K.D., Flajnik M.F., Mariuzza R.A., Pancer Z. High-affinity lamprey VLRA and VLRB monoclonal antibodies. Proc. Natl. Acad. Sci. U.S.A. 2009;106:12891–12896. doi: 10.1073/pnas.0904443106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka A., Hamajima S., Hatta H., Abiko Y. Inhibition of Porphyromonas gingivalis hemagglutinating activity by IgY against a truncated HagA. J. Oral Sci. 2006;48:227–232. doi: 10.2334/josnusd.48.227. [DOI] [PubMed] [Google Scholar]
- Thomas J.E., Austin S., Dale A., Mcclean P., Harding M., Coward W.A., Weaver L.T. Protection by human milk IgA against Helicobacter pylori infection in infancy. Lancet. 1993;342:121. doi: 10.1016/0140-6736(93)91327-i. [DOI] [PubMed] [Google Scholar]
- Throsby M., Van Den Brink E., Jongeneelen M., Poon L.L.M., Alard P., Cornelissen L., Bakker A., Cox F., Van Deventer E., Guan Y., Cinatl J., Ter Meulen J., Lasters I., Carsetti R., Peiris M., De Kruif J., Goudsmit J. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM(+) memory B cells. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Weber S., Thorkildson P., Kozel T.R., Pirofski L.A. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect. Immun. 2009;77:1502–1513. doi: 10.1128/IAI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tini M., Jewell U.R., Camenisch G., Chilov D., Gassmann M. Generation and application of chicken egg-yolk antibodies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;131:569–574. doi: 10.1016/s1095-6433(01)00508-6. [DOI] [PubMed] [Google Scholar]
- Tokuhara D., Alvarez B., Mejima M., Hiroiwa T., Takahashi Y., Kurokawa S., Kuroda M., Oyama M., Kozuka-Hata H., Nochi T., Sagara H., Aladin F., Marcotte H., Frenken L.G., Iturriza-Gómara M., Kiyono H., Hammarström L., Yuki Y. Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J. Clin. Invest. 2013;123:3829–3838. doi: 10.1172/JCI70266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollemar J., Gross N., Dolgiras N., Jarstrand C., Ringden O., Hammarstrom L. Fungal prophylaxis by reduction of fungal colonization by oral administration of bovine anti-Candida antibodies in bone marrow transplant recipients. Bone Marrow Transplant. 1999;23:283–290. doi: 10.1038/sj.bmt.1701560. [DOI] [PubMed] [Google Scholar]
- Torosantucci A., Bromuro C., Chiani P., De Bernardis F., Berti F., Galli C., Norelli F., Bellucci C., Polonelli L., Costantino P., Rappuoli R., Cassone A. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosantucci A., Chiani P., Bromuro C., De Bernardis F., Palma A.S., Liu Y., Mignogna G., Maras B., Colone M., Stringaro A., Zamboni S., Feizi T., Cassone A. Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A., Kuster H., Rusert P., Joos B., Fischer M., Leemann C., Manrique A., Huber M., Rehr M., Oxenius A., Weber R., Stiegler G., Vcelar B., Katinger H., Aceto L., Gunthard H.F. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 2005;11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- van der Vaart J.M., Pant N., Wolvers D., Bezemer S., Hermans P.W., Bellamy K., Sarker S.A., Van Der Logt C.P.E., Svensson L., Verrips C.T., Hammarstrom L., Van Klinken B.J.W. Reduction in morbidity of rotavirus induced diarrhoea in mice by yeast produced monovalent llama-derived antibody fragments. Vaccine. 2006;24:4130–4137. doi: 10.1016/j.vaccine.2006.02.045. [DOI] [PubMed] [Google Scholar]
- van Dissel J.T., De Groot N., Hensgens C.M.H., Numan S., Kuijper E.J., Veldkamp P., Van’t Wout J. Bovine antibody-enriched whey to aid in the prevention of a relapse of Clostridium difficile associated diarrhoea: preclinical and preliminary clinical data. J. Med. Microbiol. 2005;54:197–205. doi: 10.1099/jmm.0.45773-0. [DOI] [PubMed] [Google Scholar]
- Vanlandschoot P., Stortelers C., Beirnaert E., Ibanez L.I., Schepens B., Depla E., Saelens X. Nanobodies (R): new ammunition to battle viruses. Antiviral. Res. 2011;92:389–407. doi: 10.1016/j.antiviral.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Veazey R.S., Shattock R.J., Pope M., Kirijan J.C., Jones J., Hu Q.X., Ketas T., Marx P.A., Klasse P.J., Burton D.R., Moore J.P. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- Velazquez F.R., Matson D.O., Guerrero M.L., Shults J., Calva J.J., Morrow A.L., Glass R.I., Pickering L.K., Ruiz-Palacios G.M. Serum antibody as a marker of protection against natural rotavirus infection and disease. J. Infect. Dis. 2000;182:1602–1609. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- Veselinovic M., Neff C.P., Mulder L.R., Akkina R. Topical gel formulation of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 confers protection against HIV-1 vaginal challenge in a humanized mouse model. Virology. 2012;432:505–510. doi: 10.1016/j.virol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T., Matson D.O., Dennehy P., Van Damme P., Santosham M., Rodriguez Z., Dallas M.J., Heyse J.F., Goveia M.G., Black S.B., Shinefield H.R., Christie C.D.C., Ylitalo S., Itzler R.F., Coia M.L., Onorato M.T., Adeyi B.A., Marshall G.S., Gothefors L., Campens D., Karvonen A., Watt J.P., O’brien K.L., Dinubile M.J., Clark H.F., Boslego J.W., Offit P.A., Heaton P.M., Team R.S. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. New. Engl. J. Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- Walker L.M., Burton D.R. Rational antibody-based HIV-1 vaccine design: current approaches and future directions. Curr. Opin. Immunol. 2010;22:358–366. doi: 10.1016/j.coi.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.Z., Fan B., Liu L.G., Hu X.Y., Li R.Y., Wei Y., Wan Z., Deng X.L. In vitro inhibition of oral Candida albicans by chicken egg yolk antibody (IgY) Mycopathologia. 2008;165:381–387. doi: 10.1007/s11046-008-9097-0. [DOI] [PubMed] [Google Scholar]
- Warny M., Fatimi A., Bostwick E.F., Laine D.C., Lebel F., Lamont J.T., Pothoulakis C., Kelly C.P. Bovine immunoglobulin concentrate Clostridium difficile retains C. difficile toxin neutralising activity after passage through the human stomach and small intestine. Gut. 1999;44:212–217. doi: 10.1136/gut.44.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S., Tian H., Van Rooijen N., Pirofski L.A. A serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibody requires Fcgamma receptor III and macrophages to mediate protection against pneumococcal pneumonia in mice. Infect. Immun. 2012;80:1314–1322. doi: 10.1128/IAI.06081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner C., Pan Q., Hurtig M., Boren T., Bostwick E., Hammarstrom L. Passive immunity against human pathogens using bovine antibodies. Clin. Exp. Immunol. 1999;116:193–205. doi: 10.1046/j.1365-2249.1999.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub J.A., Hilton J.F., White J.M., Hoover C.I., Wycoff K.L., Yu L., Larrick J.W., Featherstone J.D. Clinical trial of a plant-derived antibody on recolonization of mutans streptococci. Caries Res. 2005;39:241–250. doi: 10.1159/000084805. [DOI] [PubMed] [Google Scholar]
- Wellington M., Dolan K., Haidaris C.G. Monocyte responses to Candida albicans are enhanced by antibody in cooperation with antibody-independent pathogen recognition. FEMS Immunol. Med. Microbiol. 2007;51:70–83. doi: 10.1111/j.1574-695X.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- West A.P., Herr A.B., Bjorkman P.J. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A(2) receptor homolog. Immunity. 2004;20:601–610. doi: 10.1016/s1074-7613(04)00113-x. [DOI] [PubMed] [Google Scholar]
- Wilcox M.H. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J. Antimicrob. Chemother. 2004;53:882–884. doi: 10.1093/jac/dkh176. [DOI] [PubMed] [Google Scholar]
- Winau F., Westphal O., Winau R. Paul Ehrlich – in search of the magic bullet. Microb. Infect. 2004;6:786–789. doi: 10.1016/j.micinf.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Wood G.C., Swanson J.M. Aerosolised antibacterials for the prevention and treatment of hospital-acquired pneumonia. Drugs. 2007;67:903–914. doi: 10.2165/00003495-200767060-00006. [DOI] [PubMed] [Google Scholar]
- Wu X., Zhou T., Zhu J., Zhang B., Georgiev I., Wang C., Chen X., Longo N.S., Louder M., Mckee K., O’dell S., Perfetto S., Schmidt S.D., Shi W., Wu L., Yang Y., Yang Z.Y., Yang Z., Zhang Z., Bonsignori M., Crump J.A., Kapiga S.H., Sam N.E., Haynes B.F., Simek M., Burton D.R., Koff W.C., Doria-Rose N.A., Connors M., Program N.C.S., Mullikin J.C., Nabel G.J., Roederer M., Shapiro L., Kwong P.D., Mascola J.R. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.L., Yang Z.Y., Li Y.X., Hogerkorp C.M., Schief W.R., Seaman M.S., Zhou T.Q., Schmidt S.D., Wu L., Xu L., Longo N.S., Mckee K., O’dell S., Louder M.K., Wycuff D.L., Feng Y., Nason M., Doria-Rose N., Connors M., Kwong P.D., Roederer M., Wyatt R.T., Nabel G.J., Mascola J.R. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M., Gohil S., Coleman J.R., Manix C., Pirofski L.A. Antibodies to Streptococcus pneumoniae capsular polysaccharide enhance pneumococcal quorum sensing. MBio. 2011;2 doi: 10.1128/mBio.00176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.Q., Shao H.X., Hickman D., Angel M., Xu K.M., Cai Y.B., Song H.C., Fouchier R.a.M., Qin A.J., Perez D.R. Intranasal delivery of an IgA monoclonal antibody effective against sublethal H5N1 influenza virus infection in mice. Clin. Vaccine Immunol. 2010;17:1363–1370. doi: 10.1128/CVI.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Milenic D.E., Whitlow M., Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52:3402–3408. [PubMed] [Google Scholar]
- Yokoyama K., Sugano N., Rahman A.K., Oshikawa M., Ito K. Activity of anti-Porphyromonas gingivalis egg yolk antibody against gingipains in vitro. Oral Microbiol. Immunol. 2007;22:352–355. doi: 10.1111/j.1399-302X.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Sugano N., Shimada T., Shofiqur R.A., Ibrahim El S.M., Isoda R., Umeda K., Sa N.V., Kodama Y., Ito K. Effects of egg yolk antibody against Porphyromonas gingivalis gingipains in periodontitis patients. J. Oral Sci. 2007;49:201–206. doi: 10.2334/josnusd.49.201. [DOI] [PubMed] [Google Scholar]
- Young K.W., Munro I.C., Taylor S.L., Veldkamp P., Van Dissel J.T. The safety of whey protein concentrate derived from the milk of cows immunized against Clostridium difficile. Regul. Toxicol. Pharmacol. 2007;47:317–326. doi: 10.1016/j.yrtph.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Yusibov V., Streatfield S.J., Kushnir N. Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Hum. Vaccin. 2011;7:313–321. doi: 10.4161/hv.7.3.14207. [DOI] [PubMed] [Google Scholar]
- Zhang C. Hybridoma technology for the generation of monoclonal antibodies. Methods Mol. Biol. 2012;901:117–135. doi: 10.1007/978-1-61779-931-0_7. [DOI] [PubMed] [Google Scholar]
- Zhou B., Zhong N., Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- Zhu L., Van De Lavoir M.C., Albanese J., Beenhouwer D.O., Cardarelli P.M., Cuison S., Deng D.F., Deshpande S., Diamond J.H., Green L., Halk E.L., Heyer B.S., Kay R.M., Kerchner A., Leighton P.A., Mather C.M., Morrison S.L., Nikolov Z.L., Passmore D.B., Pradas-Monne A., Preston B.T., Rangan V.S., Shi M., Srinivasan M., White S.G., Winters-Digiacinto P., Wong S., Zhou W., Etches R.J. Production of human monoclonal antibody in eggs of chimeric chickens. Nat. Biotechnol. 2005;23:1159–1169. doi: 10.1038/nbt1132. [DOI] [PubMed] [Google Scholar]


