Abstract
This chapter presents the pathology of cetaceans, a diverse group of mammals restricted exclusively to aquatic habitats. The taxa include the largest mammals on earth, the baleen whales, as well as marine and freshwater toothed whales, dolphins, and porpoises. Pathologies of these species include infectious, toxic, and other disease processes, such as ship strike and entanglements in free-ranging animals. In animals under managed care, concerns include nutritional, degenerative and geriatric processes, such as formation of ammonium urate renal calculi. Due to potential population level effects and individual animal health concerns, viral agents of interest include morbilliviruses, pox virus, and herpes viruses. Both free ranging and captive animals have important neoplasms, including a variety of toxin-related tumors in beluga whales from the St. Lawrence Estuary and oral squamous cell carcinomas in bottlenose dolphins in managed care.
Keywords: bacteria, cetacean, dolphin, fungi, parasites, porpoise, ship strike, squamous cell carcinoma, viruses, whale
Introduction
This chapter presents the pathology of cetaceans, a diverse group of mammals exclusive to aquatic habitats. The taxa include the largest mammals on earth, baleen whales, as well as marine and freshwater toothed whales, dolphins, and porpoises (the odontocetes). The taxonomy of the cetacean infraorder is presented in the Supplemental Materials in Table e1, Table e2. There are approximately 89 living species of cetaceans. Of these, 15 species comprise the baleen whales and there are more than 70 species of toothed whales. Odontocetes include species with diverse ecological (including foraging strategies, prey selection and habitat use), anatomical and physiological differences ranging from the freshwater river dolphins to deep diving beaked whales. This subgroup includes sperm whales, porpoises, dolphins, beluga, narwhal, and beaked whales. These adaptations can have important implications when evaluating gross pathology and histopathologic findings. Free-ranging whales and dolphins may have global distributions but many ecotypes have localized habitats with or without a specific migratory range. A limited number of odontocetes, primarily delphinids, are maintained in captivity. For this reason, the published material of cetacean pathology is heavily biased towards odontocetes using material from captive and stranded animals.
Table e1.
Taxa of the Baleen Whalesa
| Family | Common Names | Genus and Species |
|---|---|---|
| Balaenidae | Bowhead whale | Balaena mysticetus |
| North Atlantic right whale | Eubalaena glacialis | |
| North Pacific right whale | Eubalaena japonica | |
| Southern right whale | Eubalaena australis | |
| Cetotheriidae | Pygmy right whale | Caperea marginata |
| Balaenopteridae | Common minke whale | Balaenoptera acutorostrata |
| Antarctic minke whale | Balaenopterabonaerensis | |
| Sei whale | Balaenoptera borealis | |
| Bryde’s whale | Balaenoptera brydei | |
| Eden’s whale | Balaenoptera edeni | |
| Omura’s whale | Balaenoptera omurai | |
| Blue whale | Balaenoptera musculus | |
| Fin whale | Balaenoptera physalus | |
| Humpback whale | Megaptera novaeangliae | |
| Gray whale | Eschrichtius robustus |
Reynolds, J.E. III, Rommel, S.A. (Eds.), 1999. Biology of Marine Mammals. Smithsonian Institution Press, Washington, D.C., USA.
Table e2.
Taxa of Toothed Whalesa
| Family | Common Names | Genus and Species |
|---|---|---|
| Delphinidae | Commerson’s, Chilean, Heaviside’s, Hector’s dolphin | Cephalorhyncus commersonii, C. eutropia, C. heavisidii, C. hectori |
| Long-beaked, short-beaked, Arabian common dolphin | Delphinus capensis, D. delphis, D. tropicalis | |
| Pygmy killer whale | Feresa attenuate | |
| Short-finned, long-finned pilot whale | Globicephala macrorhynus, G. melas | |
| Risso’s dolphin | Grampus griseus | |
| Fraser’s dolphin | Lagenodelphis hosei | |
| Atlantic white-sided, white-beaked, Peale’s, Hourglass, Pacific white-sided, Dusky dolphin | Lagenorhynchus acutus, L. albirostris, L. australis, L. cruciger, L. obliquidens, L. obscurus | |
| Northern, southern right whale dolphin | Lissodelphis borealis, L. peronii | |
| Irrawaddy, Australian snubfin dolphin | Orcaella brevirostris, O. heinsohni | |
| Killer whale | Orcinus orca | |
| Melon-headed whale | Peponocephala electra | |
| False killer whale | Pseudorca crassidens | |
| Tucuxi, Guiana dolphin | Sotalia fluviatilis, S. guianensis | |
| Pacific, Indian, Atlantic humpback dolphin | Sousa chinensis, S. plumbea, S. teuszii | |
| Pantropical spotted, Clymene, striped, Atlantic, Spinner dolphin | Stenella attenuata, S. clymene, S. coeruleoalba, S.frontalis, S. longirostris | |
| Rough-toothed dolphin | Steno bredanensis | |
| Indian Ocean bottlenose, Burrunan, common bottlenose dolphin | Tursiops aduncus, T. australis, T. truncatus | |
| Monodontidae | Beluga | Delphinapterus leucas |
| Narwhal | Monodon monoceros | |
| Phocoenidae | Finless porpoise | Neophocaena phocaenoides |
| Spectacled, harbor, Vaquita, Burmeister’s porpoise | Phocoena dioptrica, P. phocaena, P. sinus, P. spinipinnis | |
| Dall’s porpoise | Phocoenoides dalli | |
| Physeteridae | Sperm whale | Physeter catodon |
| Kogiidae | Pygmy, Dwarf sperm whale | Kogia breviceps, K. sima |
| Plantanistidae | South Asian river dolphin | Platanista gangetica |
| Iniidae | Amazon, Bolivian, Araguaian river dolphin | Inia geoffrensis, I. boliviensis, I. araguaiaensis |
| Pontoporiidae | La Plata dolphin | Pontopoaria blainvillei |
| Ziphidae | Arnoux’s, Baird’s beaked whale | Berardius arnuxii, B. bairdii |
| Northern, Southern bottlenose whale | Hyperoodon ampullatus, H. planifrons | |
| Indo-Pacific beaked whale | Indopacetus pacificus | |
| Sowerby’s, Andrew’s, Hubb’s, Blainville’s, Gervais’, Ginkgo-toothed, Gray’s, Hector’s, Strap-toothed, True’s, Perrin’s, Pygmy, Stejneger’s, Spade-toothed, Deraniyagala, Shepherd’s, Cuvier’s beaked whale | Mesoplodon bidens, M. bowdoini, M. carlhubbsi, M. densirostris, M. europaeus, M. ginkgodens, M. grayi, M. hectori, M. layardii, M. mirus, M. perrini, M. peruvianus, M. stejnegeri, M. traversii, M. hotaula |
Reynolds, J.E. III, Rommel, S.A. (Eds.), 1999. Biology of Marine Mammals. Smithsonian Institution Press, Washington, D.C., USA.
Unique features
Important clinical pathology of the cetaceans includes a modification of the clotting pathways based on the absence of factor XII and thus a loss of the intrinsic arm of the clotting cascade. This condition is more thoroughly presented in Chapter 4. This altered clotting system does not lead to pathologic bleeding tendencies but can impede collection of serum as part of routine clinical chemistry evaluations. To manage this, routine clinical blood draws typically involve three different blood collection tubes containing anticoagulant: one orange-topped (thrombin), one light blue-topped (sodium citrate), and one purple topped (potassium EDTA) tube (Fig. 22.1 ). This assortment of tubes facilitates clinical chemistry with minimal hemolysis, electrolytes and acid-base determinations, hematology, clotting profiles, whole blood sedimentation times, and plasma fibrinogen determination. For genomic assays, a green-topped (lithium heparin) tube should be added to the collection set.
Figure 22.1.
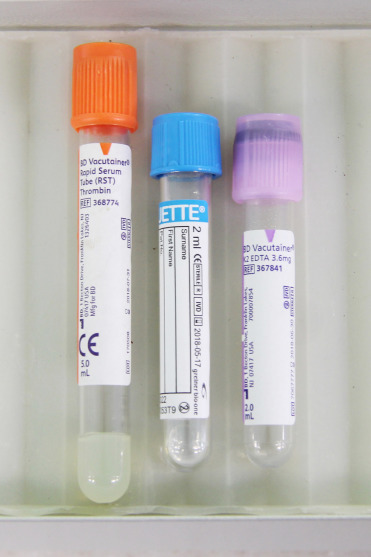
Blood collection tube set for cetacean blood.
The orange-topped tube is a serum separator tube and contains thrombin to induce clotting. The pale blue-topped tube contains 3.2% sodium citrate and is useful for coagulation assays and determination of plasma fibrinogen concentrations. The lavender-topped tube contains potassium EDTA and is useful for hematology and sedimentation rate determinations.
Extensive review of cetacean special anatomy is beyond the scope of this chapter. The reader is therefore referred to marine mammal peer-reviewed literature and texts. Anatomy of Dolphins: Insights into Body Structure and Function is specifically recommended (Cozzi et al., 2017). A number of particularly notable gross and histologic features as well as special features that impact interpretation by pathologists are noted below.
The anatomy of cetacea varies from domestic mammal species in many ways. The most obvious difference is musculoskeletal, with the lack of hind limbs and evolutionary transformation of the forelimb to a flipper appendage. With the loss of hindlimbs there is an associated reduction of the pelvis. Vestigial hip bones with occasional articulations (pseudoarthroses) remain and can be located deep in the muscle of the ventrolateral caudal abdominal wall. The presence of these bones has been linked to male sexual performance (Dines et al., 2014). The altered gravitational effects of a fluid environment, hydrodynamic configurations, and buoyancy enhanced by a thick blubber layer and lung capacity, have resulted in lower bone densities than would be expected if cetacean bones had the gravitational forces of terrestrial mammalian counterparts. As long bones of the forelimbs have no medullary cavity, bone marrow is best extracted from vertebral bodies or ribs. The torso is enveloped by a thick axial fibroelastic sheath which stores kinetic energy with each fluke stroke to enhance swimming efficiency.
The skull of cetaceans varies by species but in general it has a marked elongation with formation of a cranial “beak” or rostrum. The skull elongation is asymmetrical with the bony margins of the blow hole off center. Above the elongated maxilla, is the melon, a structure designed for forward propagation of echolocation clicks. Returning sound or echolocation vibrations enter the head via the soft tissues and are specifically focused towards the mandibular fat body within the caudal, “pan” section of the mandibles. Cetaceans have no external ears and have a rudimentary ear canal that does not connect to the tympanoperiotic capsule containing the bones of the middle and inner ears. In contrast to the axial and appendicular skeleton, these bones are remarkably dense and have ligamentous attachments to the adjoining skull with expanded accessory tympanoperiotic air sinuses, homologous to guttural pouches in horses. At necropsy, ear wax may be collected from eustachian tubes from some large cetaceans, and hormonal and chemical analyses provide valuable insights into temporal reproductive and stress hormone levels, as well as contaminant exposure through the life of the animal.
The gastrointestinal morphology of cetaceans varies by genera/species. Instead of teeth, oral cavities of the baleen (mysticete) whales contain multiple plates of keratin with a fibrous fringe extending from the upper jaw to the lower gums. To resist forces of water engulfment and prey extraction associated with filter feeding, the plates are secured by zwischensubstanz to the gingiva. The number and length of plates vary throughout the baleen suborder. On cross section, they present thick cortical plates encasing 3–5 layers of horny tubules (Slijper, 1979). Isotopic analysis of baleen provides valuable insights into life history endocrine levels and prey selection (Hunt et al., 2014) and foreshortening, abrasion, fraying, and erosion of plates have been attributed to mechanical forces associated with lunge or ram feeding and tongue movements. At necropsy, thorough examination of the oral cavity for foreign debris is imperative. The teeth of the odontocetes vary by family and prey-type. Tooth shape of the delphinids is either conical or spade-shaped; each tooth has only one root. There are no incisors or molars—all teeth are more or less identical (homodont). The squid-eating sperm whale has one row of conical teeth on both sides of the lower jaw but none in the upper jaw. The Kogiadae—pygmy and dwarf sperm whales similarly only have lower teeth. The beaked whales have no more than one or two erupted teeth in the lower jaw. In one species of beaked whale, the strap-toothed whale, the lower teeth erupt and extend to encircle the maxilla so that the jaw can only open a few centimeters. A unique dental modification of the odontocetes is found in the narwhal. Narwhal gums typically have only a single erupted tooth. The male’s tusk is a giant canine tooth with a distinct left-handed spiral. It can grow as long as three meters and is covered in cementum rather than enamel. Rarely, both canines can erupt and form tusks. The canines of females can erupt and produce a shortened tusk. The porosity of the tusk suggests that the structure acts as a sensory device for measuring water salinity, temperature and pressure (Nweeia et al., 2014), as well as for social interactions (dominance) and hunting (striking) of prey species.
Histologically, the odontocete tooth is composed of a central pulp layer surrounded by dentin with an outer layer of cementum and an enamel cap. This cap wears over time (see conditions of aged animals below). Annular layers are enumerated for age estimation of odontocetes but must be validated with animals of know age. As with the narwhal, the sperm whale, Kogidae, and beaked whales lack the enamel covering on the teeth.
Stomach morphology varies between species but generally consists of a large, usually conical and muscular forestomach with no distinct sphincter at the entry from the esophagus. The main fundic stomach is a smaller, generally round structure with a deep red-brown folded, glandular mucosa. Gastric glands containing neck cells, parietal cells, and chief cells line the surface of this chamber. The third, pyloric chamber of the stomach is “U-shaped” with a prominent sphincter that regulates the passage of ingesta into the small intestine. There is a sacular dilation of the proximal duodenum to form an ampulae. The size, length, and number of saculations in the connecting chamber vary considerably between species. The saculations are created by a series of valves without distinct sphincters. Histologically, the mucosa of the connecting chambers and pylorus is the same and features deep branching tubular pits producing primarily mucus along with gastrin and lysozyme. In contrast, the duodenal ampula is identified histologically by the regular components of the duodenal wall—villous mucosa, submucosa, tunica muscularis, and serosa. All cetaceans lack a gall bladder. Likewise, the cecum is notably absent in the odontocetes with the exception of river dolphins, making gross distinction between small and large intestine difficult. The rectum of odontocetes has lymphoid nodules within the mucosa and submucosa termed the “colonic tonsil.” Dwarf and pygmy sperm whales (Kogia spp.) have a sacular dilation of the distal colon colloquially called the “ink sac” (Fig. 22.2 ). This structure can hold up to 12 L of dark, fluid, fecal material. When forcibly expelled, this liquid creates a dense cloud to distract predators, allowing the whale to swim away. In sperm whales a unique compound, ambergris is produced by consumption and partial digestion of squid beaks.
Figure 22.2.
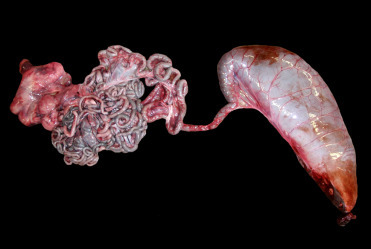
The normal “ink sac” of a pygmy sperm whale.
Dilation of the distal colon/rectum (on the right side of this image) is normal for this species. The reservoir of fluid fecal material it contains is voluntarily released in response to danger. It provides a “cloud-screen” that allows escape from dangerous situations.
The respiratory tract of cetaceans has a variety of modifications that must be understood to interpret gross and histologic observations and to comprehend the special vulnerability of cetaceans to lung disease. The blow hole is analogous to the nostrils and is located on the dorsal aspect of the head. There are two external openings in mysticetes and a single blowhole in odontocetes. The blowhole is closed as the associated muscles are relaxed. In either group, the underlying skull has a nasal septum so all cetceans have dual entry into the upper respiratory tract. There are no turbinates to filter inhaled particles in any cetaceans. With large tidal volumes and respiratory exchange rates of less than 1–2 s, deposition of particles and pathogens deep into the respiratory tract is easier than with terrestrial mammals. Because of this, respiratory disease is a much more significant cause of morbidity and mortality in cetaceans (St Leger et al., 2009). A nasal air sac system with analogous anatomy and function to the paranasal sinuses in terrestrial mammals extends from the cranial respiratory tract rostral to the integration of the larynx. These sacs are not enclosed in bone like sinuses (Reidenberg and Laitman, 2008). An elongated larynx called a “goosebeak” extends through the esophagus to the bony nasal passage and is held in place by the muscular palatopharyngeal sphincter (Reynolds and Rommell, 1999). While the sphincter and larynx form a tight seal, the larynx can still be displaced through esophageal pressure associated with swallowing large objects or voluntarily by the animal contracting the hyoepiglottic and associated laryngeal muscles. This behavior has been associated with cases of fatal aspiration and asphyxiation. At the base of the larynx, are numerous trabeculae that entrap foreign particulate material and overlie microscopic lymphoglandular elements; the latter are, homologous to human adenoids. The trachea has a large vascular venous plexus with prominent lacunae in the subepithelium. This structure has a critical role in fungal tracheitis (presented under fungal infections).
Modifications in cetacean pulmonary anatomy are reflected both grossly and histologically. First, cartilage extends from the trachea all the way to the level of distal bronchioles and on gross examination, are visible as cartilage “nodules” throughout the parenchyma. Within the bronchioles, smooth muscle and elastic sphincters create a series of compartments along the airway to the level of the alveoli (Fig. 22.3). These changes are a diving adaptation. However, the valves can obstruct pulmonary clearance of parasites and secondary bacterial involvement can result in bronchiectasis and multifocal pulmonary abscesses. Additionally, granular to tophic, basophilic calcific deposits can be present in the epithelium and subepithelial tissues of the bronchioles. These deposits likely reflect prior areas of bronchiolar damage or inspissated material. Lastly, the alveolar walls of the cetacean lung contain both type I and type II pneumocytes, but type III (brush border cells) are absent. The alveolar walls contain double (as opposed to single in terrestrial mammals) rows of capillaries (Fig. 22.3 ). A common age-related change is an increase in fibrous tissue expanding the alveolar interstitium and pleura. This change likely reflects an element of a chronic immune response. Areas with severe chronic pulmonary fibrosis likely reflect resolution of prior inflammation.
Figure 22.3.
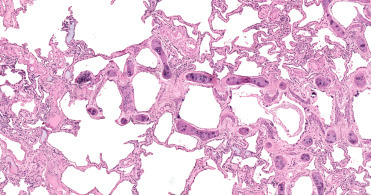
Normal lung histology in a bottlenose dolphin.
Cartilage extends to the level of the bronchioles. Crescent-shaped, myoelastic sphincters create small gates within the small airways. Multifocally, there are scattered small basophilic foci of mineralization in the subepithelium. The alveolar interstitium contains a double row of capillaries.
The blood supply to the cetacean head is notable. Not only are there a number of countercurrent vascular exchange units, but the rete mirabile is the primary source of blood to the brain. The internal carotid arteries are vestigial and supply blood to the eyes and ears and provide little to no oxygenated blood to the brain. Instead, the brachiocephalic trunk and descending aorta branch off to feed the intercostal and dorsal thoracic arteries. These vessels supply blood to the thoracic (Fig. 22.4 ) and spinal rete mirabile. Retes are structures in which the arteries branch into a number of small vessels that finally reconstitute into a single vessel. The smaller arterioles may be intertwined or bathed in a sinus of cooler venous blood. The blood supply to the head of the dolphin derives from the spinal meningeal arteries and enters the cranium at the occipital foramen. The unique vascular modifications of cetaceans are many and may predispose these animals to spontaneous intravascular gas bubble formation and embolization (Caisson’s disease). The reader is referred to anatomical and biological texts for additional information on this topic.
Figure 22.4.
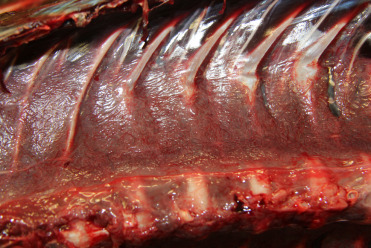
Normal thoracic rete mirabile in a bottlenose dolphin.
A mat of small vessels is visible at the dorsal aspect of the thorax. The interwoven mesh of small vessels is postulated to serve as a trap for intravascular bubbles to protect the brain from gas embolism as the result of diving.
Cetaceans are highly tactile and their skin not only provides sensory input, but also streamlines the animal for efficient hydrodynamics. Callosities are normal, large patches of irregularly raised epithelial tissue particular to right (Eubalaena) and bowhead (Balaena) whales (Fig. 22.5 ). They are composed of hyperplastic, cornified skin above the eyes, along the jaw and around the lips. With time, this tissue becomes infested with light colored cyamids (whale lice) that contrast with the surrounding black skin. To date, Cyamus ovalus and C. gracilis have been found on both northern and southern right whale; C. erraticus has been reported only from southern right whales.
Figure 22.5.

Normal callosities in the skin of a North Atlantic right whale.
These areas of irregular epidermal hyperplasia are normal in right (Eubalaena) and bowhead (Balaena) whales. They remain, generally unchanging, throughout an animal’s life; because each animal has a unique pattern, they are useful for identifying individuals. Cyamid amphipods reside within these protrusions, give the callosities their light coloration, and consume sloughed epithelium and algae that grows in the skin.
(Photo Courtesy of Hubbs-SeaWorld Research Institute)
Similar irregular proliferations in the skin can be seen in other baleen whales. These are composed of attached barnacles and cyamids. The irregular patchy white spots on gray whales are barnacles. As larvae, the whale barnacles swim freely in the nursery lagoons where the grey whales calve. The gray whale barnacle, Cryptolepas rhachianecti, attaches to calves and mothers. Once the crustacean has settled, it spends its entire life cycle attached to the whale. Barnacle clusters, similar to callosities can be used to identify individual whales. Also as with the callosities, species specific cyamids lodge within these proliferative areas to feed on whale skin. These parasites spread from dams to their calves during birth, nursing, and general contact. Microscopically, affected skin is markedly hyperplastic with hyperkeratosis, pseudoepitheliomatous hyperplasia, and spongiosus with superficial bacterial colonization and varying degrees of inflammation in the superficial dermis.
Accessory spleens (splenules) (Fig. 22.6 ) adjacent to the primary spleen or scattered within the adjoining mesentery are smaller spherical to oblong structures that are commonly observed in small odontocetes. These structures appear to arise through embryogenesis, may have a role in extramedullary hematopoiesis, and should not be interpreted as neoplasia or an indication of prior abdominal trauma.
Figure 22.6.
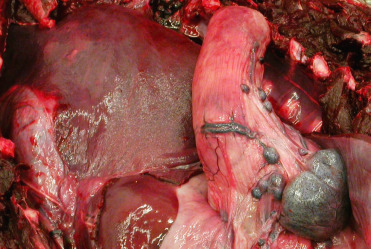
Multiple normal accessory spleens in the abdomen of a stranded Atlantic spotted dolphin.
These are a common incidental finding and do not necessarily indicate prior abdominal trauma. They are thought to play a role in extramedullary hematopoiesis.
(Photo Courtesy of Hubbs-SeaWorld Research Institute)
A common histologic observation in stranded dolphins and whales is hyaline intracytoplasmic inclusions in hepatocytes (Fig. 22.7 ). The inclusions are round to oval, refractile, periodic acid–Schiff (PAS) positive, and range from 4 to 20 μm in diameter. They often compress and peripherally displace the nucleus. Inclusions are randomly scattered through the hepatic parenchyma and are usually solitary. By transmission electron microscopy, these structures are electron dense with occasional central or eccentric more highly electron-dense cores, and are morphologically consistent with aggregates of microtubules. From a diagnostic perspective, microtubular aggregates may be differentiated from viral inclusions by the lack of associated inflammatory infiltrate and necrosis. Electron microscopy, special stains, molecular studies, and virus isolation may prove valuable as ancillary diagnostics for differentiation.
Figure 22.7.
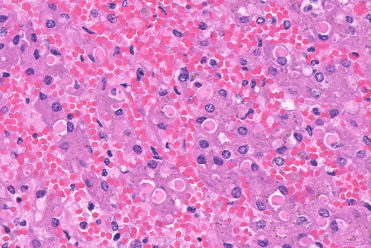
Hyaline intracytoplasmic inclusions in the liver of a bottlenose dolphin.
These inclusions are common in stranded cetaceans. Without evidence of a viral infection, it is most likely that they are aggregates of consolidated microtubes and not viral inclusions.
(Photo Courtesy of D. Gasper, Pacific Zoo & Wildlife Diagnostics)
Non-infectious diseases
Nutritional
Limitations in prey availability can result in inanition to the point of cessation of reproductive activity and even starvation. Because the changes in nutritional status are critical from a management standpoint and population level health perspective, blubber depths as well as multiple measurements of an animal’s girth are critical information to collect during a health assessment or necropsy examination. However, it should be noted that alternations in body condition may be physiologic and related to prolonged migration or reproductive status, or pathologic and attributed to a variety of conditions including impaired mobility due the musculoskeletal disease or verminous pneumonia. Vision or hearing deficits can also result in reduced foraging capacity. Inanition does not always mean that insufficient food was available and natural history considerations and intercurrent pathology should be thoroughly investigated.
Hepatic lipidosis (Fig. 22.8 ) is a relatively common finding in stranded cetaceans, especially those with metabolic disorders produced by toxic injuries, hypoxia or nutritional deficiencies. Stranded animals suffering from starvation or that are nursing calves that have a dietary intake high in carbohydrates accumulate excessive triglycerides in hepatocytes. Hepatic lipidosis in very young animals is considered physiologic (Jaber et al., 2004). In these cases, the liver is diffusely pale tan and waxy. Microscopically, the hepatocytes are swollen with clear cytoplasmic micro and macrovesicles. In pathologic conditions, there may also be compression of the sinusoids and bile canaliculi with cholestasis. Venn-Watson et al. (2012) have associated mild cases of hepatic lipidosis in adult bottlenose dolphins with insulin resistance and metabolic syndrome.
Figure 22.8.
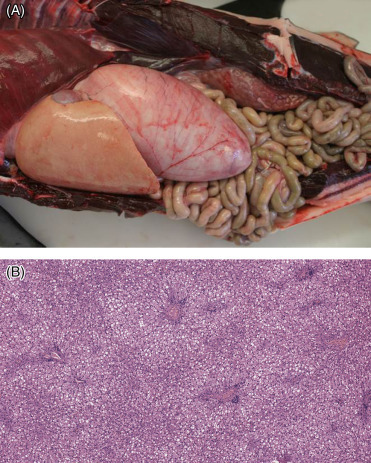
Hepatic lipidosis in a common dolphin.
(A) Typical gross presentation is an enlarged, pale liver, often with rounded edges. (B) There is diffuse expansion of hepatocytes with cytoplasmic clear vacuoles. This change may be physiologic in neonates but is also seen as a pathologic change in association with inanition.
Iron overload disease has been described in captive bottlenose dolphins. These animals generally demonstrate high circulating levels of serum iron (values greater than 600–700 μg/dL; reference range 92–300 μg/dL) and elevated transferrin saturation. Histologically, livers demonstrate accumulation of iron in hypertrophied Kupffer cells as well as diffuse cytoplasmic iron accumulations within hepatocytes. The cytoplasmic granules are positive with Prussian blue staining. In the absence of hemochromatosis (hepatic necrosis, hepatitis and fibrosis), which is very rare, the diagnosis of hemosiderosis is appropriate. The condition has been associated with diabetes and metabolic syndrome (a condition in humans and suggested in dolphins associated with hepatic iron accumulation and resting hyperglycemia). The etiology and pathogenesis of this condition are poorly understood; however, chronic inflammation, iron sequestration secondary to acute sepsis, excessive dietary iron consumption or supplementation, emaciation, inborn errors of metabolism, and a maladaptive type syndrome may be considerations (Venn Watson et al. 2012).
Metabolic
Renal calculi associated with ammonium urate nephroliths (Fig. 22.9 ) are rarely seen in stranded animals but are occasionally detected in bottlenose dolphins under managed care. A study by Smith et al. (2014) suggests that dolphins are susceptible, in part, because a high dietary load of acid and purines results in a transient but marked increase in the urinary supersaturation of the sparingly soluble ammonium urate salt. The presence of nephroliths in advanced cases is associated with clinical hematuria and nephritis as well as secondary renal atrophy. When dislodged from the kidney, stones can obstruct the ureters or urethra and cause subsequent hydroureter, hydronephosis, as well as possible unilateral shutdown of kidneys.
Figure 22.9.
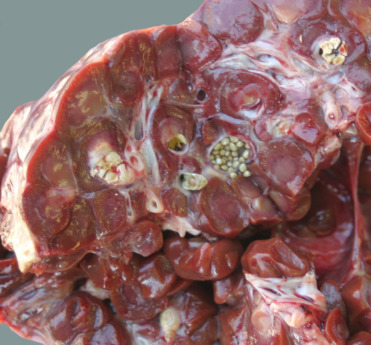
Ammonium urate nephroliths in the kidney from a captive pilot whale.
The liths present as miliary concretions scattered within the renal parenchyma. They can grow to 1–2 cm in diameter and are associated with hematuria, nephritis, ureteritis, and ureteral obstruction.
Massive urolithiasis of the penile urethra was reported in an adult pygmy sperm whale. The urolith was composed of 100% struvite (magnesium ammonium phosphate) and on culture yielded Klebsiella oxytoca, a urease positive bacterium occasionally associated with struvite urolith formation in domestic animals. Histologically, there was moderate multifocal to coalescing plasmacytic balanitis and penile urethritis. No associated bladder or kidney damage was identified (Harms et al., 2004). We have seen a case of vaginal struvite urolithiasis in a common dolphin. While these cases are uncommon, they demonstrate the need for urolith composition analysis to better understand the pathogenesis of the condition. Struvite urolithiasis is likely related to urinary tract infection as opposed to a metabolic etiology for the ammonium urate stones.
A lysosomal storage disease has been seen in multiple beluga whales (Fig. 22.10A, B). The condition is associated with reduced cognitive function. The lesions include neuron expansion and filling of the neurons with lysosomal lamellar bodies. To date, affected animals have all been adults. Investigations focus on determination of the enzyme deficit and determination of the etiology as genetic or acquired.
Figure 22.10.
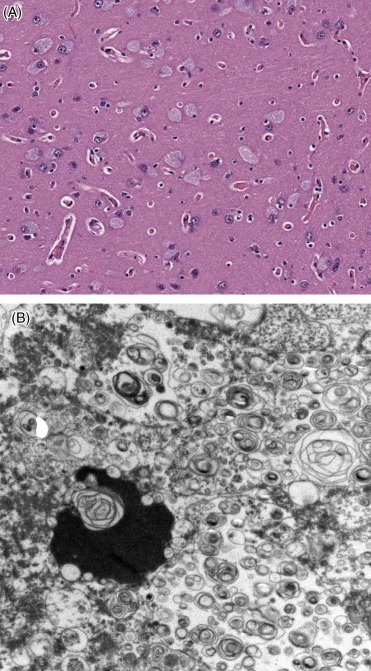
Lysosomal storage disease in a beluga whale.
(A) Neurons are distended with granular cytoplasmic content and nuclei are marginated. (B) Storage material consists of lysosomes converted into concentric lamellar bodies, scanning electron micrograph.
As with many animals, amyloidosis occurs in cetaceans. The product is most likely AA amyloid and reflects part of the inflammatory process. Amyloid can be found expanding the vascular walls of organs throughout the body. It has been specifically described additionally in the corticomedullary regions of the kidneys, around acini of the palatal salivary gland and the thyroid gland (Cowan, 1995).
Toxic
Harmful algal blooms (HABs) include a number of toxins that are elaborated by predominantly free swimming unicellular dinoflagellates (Table 22.1 ). These organisms occur in deep offshore waters and near-shore bays, lagoons and estuaries. As part of their life cycle, dinoflagellates can sexually reproduce, and then encyst for varying periods of time on substrates, and under appropriate environmental cues they reemerge and produce toxins. The geographic and ecologic distribution of these organisms parallels those of cetaceans and, depending on location as well as species of dinoflagellate and toxin produced; blooms can result in significant mortality events involving marine mammals, birds, fish, and invertebrates (Landsberg, 2002). Terrestrial species, including man, can be similarly affected; the importance of algal blooms is universal. It is important to note that toxin levels can vary between isolates within a bloom and not all algae recovered from a bloom may produce HABs. From a pathologic perspective, mass strandings of cetaceans alone or in combination with other aquatic or avian species should prompt an inquiry into a HAB event with collection of appropriate diagnostic and environmental samples. There are few pathognomonic lesions related to HAB exposure in cetaceans and sublethal, long terms effects are largely unknown. Some dinoflagellates bioaccumulate or concentrate in invertebrate or fish (foodweb) prey resulting in more protracted strandings, often with supervening or secondary conditions. Other toxins can be inhaled or ingested and are directly lethal, resulting in a pulse of mortalities. The life cycle and inciting causes of HABs is still under intensive investigation.
Table 22.1.
Notable Algal and Toxic Pollutants
| Disease | Toxin and Algae | Vectors or Exposure in | Clinical Signs and Gross Pathology | Histopathology | Confirmatory testing | Mechanism |
|---|---|---|---|---|---|---|
| Amnestic shellfish poisoning | Domoic acid (DA) Primary DA producing algal diatom Pseudo-nitzschia australis | Bivalves, scallops, mussels, clams, oysters and northern anchovy, benthic and pelagic fish Birds (pelicans and cromorants) and marine mammals | Mortality with peracute exposure may lack apparent clinical signs, gross or histopathology Piriform lobe malacia, myocardial necrosis, bronchopneumonia, reproductive loss (abortion) | In cetaceans, contribution to morbidity and mortality not fully resolved: Alga and toxins transit the bowel, whales tend to be asymptomatic, but there may be an association with ship strikes | LC-MS/MS on urine and feces ELISA on serum, urine, and feces SEM of gastric content and feces, to detect diatom frustules Cytology, gastric fluid, and feces | Glutamate receptor binding and activation |
| Diarrheic shellfish poisoning | Okadaic acid, (DTX-1 and DTX-2) and dinophysis toxin | Bivalve shellfish, scallops, mussels, clams, oysters | Unknown | Unknown | Gastric fluid and feces—assays still to be validated | Inhibitor of protein phosphatase activity Serine/threonine phosphatases 1 and 2A |
| Neurotoxic shellfish poisoning | Brevitoxin | Bivalve shellfish, scallops, mussels, clams, oysters, fish | Pulmonary and nasopharyngeal edema and hemorrhage, rhinitis, multiorgan hemosiderosis, nonsuppurative leptomeningitis | Unknown | Clinical history gross, histopathology, IHC on lungs and lymph nodes ELISA, RBA, RIA and LC-MS/MS on blood, liver, serum, urine, feces, and gastric content | Binds voltage dependent sodium channels, results in prolonged activation |
| Paralytic shellfish poisoning | Saxitoxin | Scallops, mussels, butter clams, oysters, cockles, herbivorous fish | Humpback whales, birds, nonspecific effects | Unknown | ELISA, RBA, LC-MS/MS on urine, gastric content, and feces | Binds voltage-dependant sodium channels, inhibits channel conductance and blocks neuronal activity, respiratory paralysis |
| Ciguatera fish poisoning | Ciguatoxin, Maitotoxin, Scaritoxin Gambierdiscus toxicus | Large reef fish, grouper, red snapper, amberjack, barracuda | Unknown | Unknown | LC-MS/MS RBA, RIA on liver and feces | Similar to brevitoxin, bind to voltage dependent sodium channels |
| Blue-green algae (cyanobacteria) Hepatotoxic shellfish poisoning (microcystin) | Anatoxin, Microcystin, Anabaena, Nodularins (New Zealand and Baltic), Cylindrospermopsin | Direct contact with water, aerosol inhalation, drinking water, shellfish bioaccumulation and possibly fish | Jaundice with acute hepatic hemorrhage and necrosis | Hepatocellular vacuolation, necrosis and apoptosis, cholestasis, hemorrhage hepatocellular necrosis, hemorrhage, karyocytomegaly with pseudoinclusions, biliary ductular hyperplasia and renal tubular necrosis | Histopathology and IHC HPLC, LC-MS/MS, protein phosphatase inhibition assay, ELISA On fungal mats and water Methodology in development for liver, and GI contents | Some toxins with “fast death factor” and peracute death, phospholipase inhibitor, phosphatase 1 and 2A inhibitor |
| POPs | PCBs, DDT, Aldrin, Chlordane, Dieldrin, Endrin, Haptachlor, Hexachlorobenzene, Mirex, Toxaphene, Dioxin, Polychlorinated dibenzofurans, and others | Ingestion, absorption, percutaneous, respiratory, in utero | Immune deficiency, potential reproductive failure | Thymic and splenic lymphoid depletion associated with starvation and chronic inflammation lipofuscinosis in the liver and kidney with hepatocellular hydropic change, central necrosis, and lymphocytic infiltrates | MS on blubber, liver, or serum | |
| Heavy metals | Lead, Mercury, Zinc, Iron, Manganese, Copper, Tin, Vanadium | Ingestion, respiratory, in utero | Potential CNS and vascular disease. Concerns differ with differing metals | LC-MS on liver quantify mercury with selenium levels |
CNS, Central nervous system; DA, domoic acid; DDT, dichlorodiphenyltrichloroethane ELISA, enzyme-linked immunosorbent assay; GI, gastrointestinal tract; HPLC, high-performance liquid chromatography; IHC, immunohistochemistry; LC, liquid chromatography; MS, mass spectroscopy; PCB, polychlorinated biphenyl; POP, persistent organopollutants; RBA, receptor binding assay; RIA, radioimmunoassay; SEM, scanning electron microscopy.
Saxitoxins are neurotoxic and effect voltage sensitive sodium channels resulting in impaired nerve impulse propagation and paralysis. In addition to direct, lethal effects, sublethal exposure may result in impaired reproductive success and impede an animal’s ability to successfully forage, maintain condition and possibly avoid marine vessel traffic. A disproportionate number of animals that present with evidence of ship strike or entanglement have detectable levels of saxitoxin and domoic acid; however, the contribution of these toxins to predisposing animals to vessel or other anthropogenic impact remains unknown.
Karenia brevis has a geographic distribution limited to the Gulf of Mexico, Atlantic coast of Florida and the Caribbean. As the organism is unarmoured, it tends to be more pleomorphic and labile; toxins are released on rupture of the cell membranes. The pathogenesis of brevitoxicosis has been attributed to prolonged opening of voltage gated sodium channels in neurons. Manatee die-offs have been attributed to ingestion of contaminated seagrass. Sporadic large scale stranding events with bottlenose dolphins have been associated with ingestion of heavily contaminated menhaden and aerosol exposure. Aerosolization and inhalation of the toxin is associated with pulmonary and nasopharyngeal edema and hemorrhage as well as multisystem congestion. Upper and lower respiratory tract lesions may be attributed to direct chemical irritation. Immunohistochemistry demonstrates brevitoxin in pulmonary alveolar lymphocytes and histiocytes as well as in pulmonary lymph nodes. Localized immunosuppression has been postulated as a contributing factor in secondary infections (Twiner et al., 2012).
While domoic acid has been detected in the gastrointestinal contents and urine of a number of cetaceans, a direct causal link to clinical disease and pathology has not yet been resolved. Based on extrapolation from California sea lions, acute to subacute gross lesions may include pyriform lobe necrosis, myocardial degeneration and necrosis, pulmonary edema with occasional pneumonia, gastrointestinal hemorrhage, and reproductive failure; chronic lesions may include hippocampal atrophy and emaciation. Degenerative cardiomyopathy with pericardial serous effusion has also been reported with acute and chronic manifestations of domoic acid exposure. Microscopically, the acute phase is characterized by multifocal myocardial cytoplasmic vacuolation with variable interstitial accumulation of edema fluid. With more advanced lesions, there was more generalized myocardial vacuolation with multifocal necrosis, interstitial fibrosis, and scant mononuclear infiltrates with replacement of myocardial fibers by adipocytes.
Cetaceans are particularly vulnerable to the effects of oil spills, which may occur as large scale catastrophic events such as the Deep Water Horizon or Exxon Valdez spills, or smaller and often recurrent localized spills, such as with industrial sites. Nevertheless, even small regional spills may pose a risk if critical habitats are involved, particularly to threatened or endangered populations. Exposure may be through direct contact with the skin, eyes, oral cavity, blowhole, or through ingestion and prey contamination so an awareness of pre-existing stressors in the habitat is essential. The lack of baseline data on ecosystem components, animal health, demographics, and population trends within an oiled area may significantly impede interpretation of pathology and ancillary diagnostic findings and confound delineation of long term lethal and sublethal effects of spills. Although oil exposure laboratory studies were conducted in the 1980s there are few investigations to assess the pathogenesis of exposure. Moreover, as petroleum products may include a number of inorganic and organic compounds, it is difficult to extrapolate the effects of one spill to another. As a result, the pathogenesis of oil exposure is often delineated from review of findings with oiling spill events. In the Deep Water Horizon spill, adrenocortical atrophy, pneumonia, and reproductive loss where significant findings in exposed animals and long term follow up studies may provide valuable insights into the consequences of exposure and population recovery. Detection of visible oil on the surface or within the upper respiratory tract or digestive system should prompt notification of appropriate regional and national authorities and, if the scale of the spill is sufficient, an Emergency Response with Incident Command Activation may occur. (Venn-Watson, 2015)
Although banned in many regions of the world, persistant organopollutants (POPs) (Table 22.1) are still detected in many marine mammal populations (Reif et al., 2017). This phenomenon is due to aerosolization and widespread dispersal, environmental persistence, and longevity of many cetacean species. Individual and synergistic effects of POPs may potentiate toxicity of some compounds. Although numerous hematology, clinical chemistry and in vitro and in vivo immune function studies have been conducted in cetaceans and surrogate pinnipeds, there are few descriptive gross or histopathology descriptions. In addition to impacting immune function, endocrine disruption also occurs. Long term necropsy results linked to contaminant loads of stranded cetaceans have been analyzed and identify a reduction in splenic and thymic lymphoid populations with increasing POP levels and immunosuppression is inferred. However, it is important to place contaminant loads in context of post mortem state and nutritional status of the animal as artifactual increases may occur with peripheral mobilization of adipose tissue and post mortem dissolution of fat stores.
Environmental accumulation of heavy metals by cetaceans has long been recognized with significantly increased levels of methylmercury identified relative to terrestrial species (Table 22.1). Cetaceans have protein complexes (metallothioneins and possibly other chelators) that bind copper, zinc, mercury and selenium, and sequester metals in the liver and skin with little to no untoward effect. Elevated methylmercury is implicated in peripheral and central neuropathies in terrestrial species and lab animals, but there is no indication of similar changes in cetaceans to date. In a case series of South Australia adult bottlenose dolphins, increased liver, cadmium, copper and zinc were associated with renal pathology (Lavery et al., 2009). Distention of Bowman’s space and proteinuria and bone lesions consistent with osteoporosis of the ribs were identified with elevated liver cadmium levels. Lower mineral densities of vertebrae were noted in some animals, but not statistically associated with liver metal concentrations, and may reflect impaired renal function.
Congenital/Genetic
Multiple single cases of aberrant white coloration in cetaceans have been reported but genetic analysis has not been reported. Overall, 21 species of whales, dolphins, and porpoises have been observed with anomalously white individuals. The etiology of this coloration is typically presumed to be genetically based albinism. A single case of a white killer whale was attributed to congenital Chediak-Higashi syndrome (Taylor and Farrell, 1973).
Cardiac anomalies reported are diverse. These include transposition of the pulmonary artery and aorta, as well as a ventricular septal defect (VSD), persistent ductus arteriosus (PDA), atrial septal defect (ASD), and right ventricular hypertrophy (RVH) and subvalvular pulmonic stenosis with a hypoplastic pulmonary artery and mitral valve (Powell et al., 2009).
The thyroid glands of cetaceans are distinctly lobulated. Lobulation increases with age. The average follicle diameter of the thyroid gland in wild dolphins is larger than that of captive dolphins. Congenital hyperplastic goiter has been associated with perinatal captive bottlenose dolphin deaths (Garner et al., 2002). Histologic changes included reduced follicular luminal diameter, markedly reduced or absent luminal colloid, hypertrophy of follicular epithelium, micropapillary proliferations, and follicular dysplasia.
Skeletal malformations including craniofacial abnormalities such as prognathism, brachygnathism, incomplete separation resulting in a double-faced monster, and scoliosis have been reported (Eissa and Abu-Seida, 2015). Care must be taken when diagnosing skeletal changes because early infections and traumatic events can cause skeletal changes in very young calves (Fig. 22.11A, B). Many alterations in bones such as incomplete fusion of the dorsal processes of cervical vertebrae occur with great variety in severity. Spina bifida in humpback whales is reported with large gaps of multiple cervical vertebrae (Groch et al., 2012). In killer whales, the same lesion has been seen but has only been reported to impact single cervical vertebrae with dorsal gaps varying from less than 1 to 4 cm (Fig. 22.12 ). In both species, determination of clinical impact involves assessing the entire skeleton to determine the number of animals within populations that have these changes.
Figure 22.11.

Scoliokyphosis in a young stranded common dolphin.
(A) The animal has pronounced downward and lateral deviations in the vertebral column. (B) The vertebral changes may be congenital but early trauma to the spine can cause similar vertebral damage. Computed tomography reconstruction. The animal is robust so an inability to catch prey is unlikely associated with this lesion.
Figure 22.12.

Incomplete fusion of the dorsal process of a cervical vertebra in a killer whale.
This congenital defect is similar to the changes seen in spina bifida but likely was not associated with clinical disease. Much more severe lesions have been reported in humpback whales that lived to adult ages.
Age-Related/Degenerative
Multiple age-related and degenerative changes can be seen in cetaceans. Cardiac, dental, ocular, musculoskeletal, renal and endocrine geriatric conditions have been described. Cardiac disease is very common in both captive and free ranging aged cetaceans (St. Leger, pers. obs.). Changes include multifocal myocardial degeneration and fibrosis, endocardiosis, valvular fibrosis, papillary fibro-elastomas, mitral leaflet thickening, and left ventricular hypertrophy. Atherosclerosis is uncommon but has been seen in both captive and free ranging bottlenose dolphins. Degenerative arthritis, spondylitis, and spondylosis occur and are sometimes linked to inanition perhaps secondary to reduced mobility. However, they can also occur unrelated to the proximate cause of death. A holistic evaluation of cases helps to keep pathologic findings in perspective. Tooth wear, fracture, laxity, and attrition are associated with advanced age in many odontocetes (Fig. 22.13A, B). The limited or lack of enamel covering odontocete teeth increases their vulnerability to wear and damage. Wear intensity varies by species and diet, with killer whales and pseudorcas having the highest prevalence (Loch and Simões-Lopes, 2012). As patterns of dental wear in the killer whale can reflect dietary specializations (elasmobranchs versus salmonids or other marine mammal prey species) and learned behaviors, tooth wear is not always the best indicator of age in some odontocete species.
Figure 22.13.
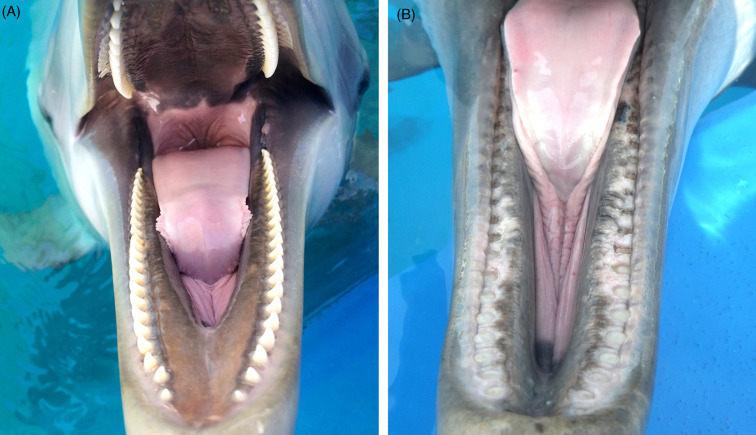
Teeth wear in dolphins.
(A) Normal conical teeth in the bottlenose dolphin. Teeth are present in both jaws and interdigitate. (B) Tooth wear in the lower jaw of a geriatric bottlenose dolphin. Teeth are worn to the level of the gingiva. Wear and breakage of teeth are not uncommon in aged odotocetes.
Both adenomatous and cystic thyroid changes are common in older bottlenose dolphins and are generally considered incidental, geriatric lesions. Changes from mild to severe adenomatous thyroid hyperplasia are commonly seen in beluga and harbor porpoises. In beluga, the total volume of the lesions adenomatous hyperplasia is also positively correlated with age (Lair et al., 1997). These changes have not been sufficiently investigated to determine an etiology or the physiologic impact, but may have important implications when assessing contaminant loads on thyroid hormone levels or gene expression.
Adrenal gland hyperplasia is a common change in older delphinids (Fig. 22.14 ). It is seen in both free-ranging and display animals and consists of variation in the dimensions of the adrenal gland (corticomedullary ratios). Increased thickening associated with either diffuse or nodular (cortical, medullary, or extracapsular) hyperplasia as well as cortical cyst formation are recognized in some species (Lair et al., 1997). The zona fasciculata and glomerulosa are primarily expanded but all three cortical zones can be involved. Medullary expansion may be difficult to distinguish from pheochromocytomas except that cells demonstrate no atypia or increased mitoses. Chlorinated hydrocarbons as a cause for this lesion have been investigated; to date a link has not been found (Kuiken et al., 1993). In general, adrenal hyperplasia and cyst formation are not associated with systemic pathology. They may represent compensatory physiology adaptations and typically appear incidental with few sequelae. This is in contrast to adrenocortical atrophy, which has been reported in animals exposed to petroleum products and likely has profound effects associated with hypoadrenocortism, possibly predisposing or exacerbating secondary bronchopneumonia in affected animals.
Figure 22.14.
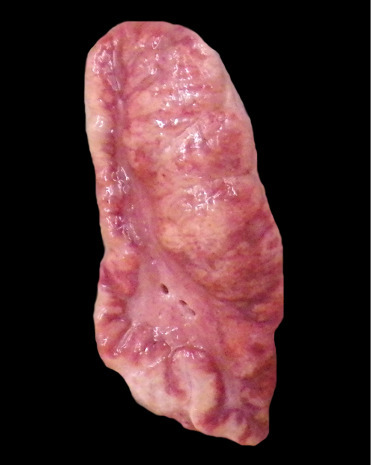
Adrenocortical hyperplasia in a killer whale.
Irregular expansion of all layers of the adrenal cortex is present and more pronounced on the right side of the gland. The hyperplastic cortical change bulges at the cut surface.
Cardiomyopathy (CMP) was first described in pygmy and dwarf sperm whales in from the southeastern United States (Bossart et al., 1985). This condition is seen in animals from both the Atlantic and Pacific Oceans. Cardiomyopathy and myocardial degeneration (MCD) lesions are identified predominantly among adult whales. Males are over-represented (up to 75%) in both species. The etiopathogenesis remains unknown but investigations are ongoing. Case evaluations suggest this is a chronic and progressive disease initially presenting as myocardial degeneration and advancing to dilated cardiomyopathy (Bossart et al., 2007). Gross findings include enlarged hearts with pale, dilated right ventricle and thin interventricular septum (Fig. 22.15 ). The condition presents as both a hypertrophic and dilated cardiomyopathy—with dilation of the right ventricle an impressive final stage of cardiac degeneration. Systemic lesions associated with the cardiomyopathy have not been recorded. Microscopically, lesions are characterized by moderate to extensive myocardial cellular degeneration, including anisokaryosis with karyomegaly and nuclear rowing as well as myocardial atrophy and loss. There is concurrent interstitial edema and fibrosis. Infrequently, cases have limited associated mononuclear myocarditis. As expected, secondary concerns like pulmonary and hepatic congestion are common.
Figure 22.15.
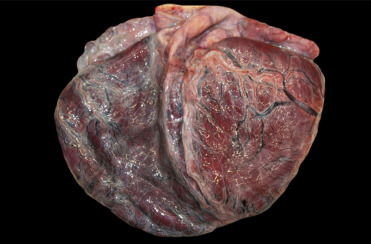
Dilated cardiomyopathy in a pygmy sperm whale.
The heart is diffusely enlarged. Both ventricles are dilated with thin and flabby walls.
(Photo Courtesy of Hubbs-SeaWorld Research Institute)
Trauma
Human interactions are a significant contributor to mortality in marine mammals; we continue to have long term impacts on marine mammal population status, recruitment and declines. Unfortunately, without direct field observations or photodocumentation for confirmation, many cases of human interactions are defined as probable or suspect, which may have important implications from a management, enforcement and legal perspective. Moreover, post mortem change (autolysis), scavenging and mutilation can impede recognition and interpretation of gross and microscopic findings in examined animals. However, advanced imaging studies, chemical analysis of gas emboli, and an increasing knowledge about sharp and blunt force injuries, have all contributed significantly to our improved detection and confirmation of anthropogenic insults. It is important to note that these categories are not mutually exclusive and detection and interaction of more than one entity may occur. Conversely, a single insult may result in multiple pathologic manifestations. As these incidents may trigger regulatory enforcement or legal investigation, review and use of appropriate forensic diagnostic protocols is imperative. Moore et al. (2013) is an excellent primer and includes downloadable forms as well as case definitions and gross and microscopic images of different serious injury entities.
Lesions consistent with decompression sickness are most commonly identified in deep diving cetaceans, particularly beaked whales, but have also been reported in common dolphins, Risso’s dolphins and harbor porpoises. As with “the bends” in humans, change in the dive profile is a prime contributor to gas bubble formation and can result in subsequent embolization, impaired vascular perfusion, ischemia, and infarction. The most common anthropogenic activities associated with this process include deployment of mid-range tactical naval sonar and net entanglement due to fisheries interaction resulting in abrupt ascent from depth to the surface. With acute presentations, gas bubbles may be seen in the mesenteric (Fig. 22.16A), renal, pulmonary, hepatic, coronary, meningeal, and spinal epidural rete vasculature; subserosal and mesenteric vasculature should be closely scrutinized for intravascular gas. Associated hemorrhage is most commonly identified in visceral pleura, epicardium, and acoustic fat. Histopathology typically features occlusion and variable vascular distention by clear microcavitations with acute perivascular hemorrhage and occasional infarction (Fig. 22.16B). In the acute phase of decompression, fat emboli may also be seen in the pulmonary vasculature as well as the epidural rete, subcapsular sinuses of lymph nodes, and renal vasculature (Fernández et al., 2005). Should animals survive the initial insult, lesions may resolve through vascular resorption and exhalation or offloading of gases. Progession to more chronic stages is characterized by fibrous encapsulation of pseudocysts with variable granulomatous infiltrates and Langhan’s type multinucleated giant cells. These cavitations are most commonly recognized within the liver, kidney, spleen, and stomach wall. Dysbaric osteonecrosis has also been reported in larger cetaceans. In the past, gas production associated with putrefaction, and hemorrhage associated with substrate in live stranded animals may have confounded diagnoses of decompression disease; however, with computed tomography imaging studies and mass spectrophotometric analysis of gas samples, nitrogen may be differentiated from those gases attributed to decomposition artifact.
Figure 22.16.
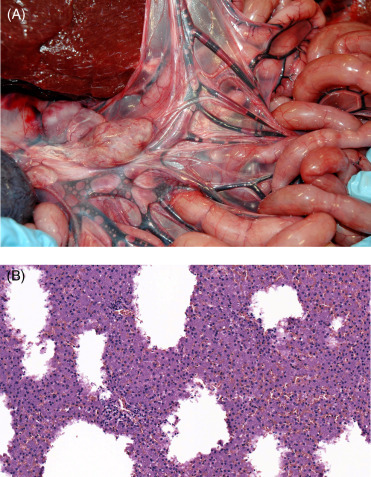
Blast-induced intravascular gas bubbles in a common dolphin.
(A) Variably sized gas bubbles are present in the mesenteric veins. (B) Multifocally, the hepatic parenchyma is replaced by clear spaces with no defined capsule, inflammation or gas forming bacteria. Similar spaces often contain hemorrhage.
High pressure blast and sonar related pathology represent a spectrum of barotrauma. Due to the unique physical properties of the aquatic environment and substrate, lesions in cetaceans are typically more profound in aquatic than in terrestrial mammals. With detonation of an explosive, there is rapid conversion of the liquid or solid to a gas under high pressure and temperature, which generates a blast front and immediate increase in underwater static pressure. This energy is transmitted and propagated radially as alternating positive and negative pressure waves. On impact with an animal, there are implosive and pressure differentials, which can generate up to a ninefold increase in pressure. In contrast to detonations on the ground or in the air, underwater explosions are more lethal due to the incompressibility of water, resulting in shock wave propagation up to three times farther than on land, as well as reflection of shock waves along the substrate and air surface interface resulting in more complex blast waves (Cox et al., 2006, Jepson et al., 2003).
Pathology related to direct exposure to blast waves is contingent on the magnitude of the explosion, distance and direction of travel of the animal from the blast, and environmental surroundings (sloping substrate versus underwater canyons). Typically, the respiratory, alimentary, circulatory, and auditory systems are involved. On gross examination, hemorrhage may be apparent in the trachea and lungs with pulmonary hematomas, edema, and emphysema tend to be more pronounced on the side of impact. Pulmonary rents with pneumothorax or hemothorax may also occur and microscopically, bronchoalveolar spaces may have frank hemorrhage admixed with exfoliated type I pneumocytes and rafts of detached and aspirated ciliated respiratory epithelia. With shearing of blood vessels in the lung and blubber, intravascular gas and fat may be apparent and embolization may further compromise homeostasis. Although the blast wave may impact the abdomen with no apparent external effect, due to gas content within the lumen of the gastrointestinal tract, this system is particularly prone to injury. Gross lesions may include segmental hemorrhage and tearing or perforation of different levels of the digestive system. Ear pathology may include peribullar hemorrhage and fractured tympanic bullae (Jepson et al., 2013). Recent advancements in acoustic mapping of the cochlea have provided valuable insights to potential sources of acoustic injury. If ears are harvested and perfused within 18–24 h after death, ultrastructural evaluation of stereocilia and immunofluorescence studies of afferent nerves may provide further evidence of barotrauma. With any suspect barotrauma related injury, examination of acoustic fats, auditory canals, eyes, and nasal sinuses should be undertaken.
Over the last 60 years, advances in vessel design and operating speeds of 14–15 knots or more have been associated with a significant increase in dead floating and live moribund whales with catastrophic lesions consistent with ship strike. Although small cetaceans and larger whales can be impacted, larger slower moving whales tend to be more frequently involved, particularly when congregated in shipping lanes. Depending on the point of contact with the vessel, animals may present with blunt force or sharp injury that may be directly lethal or contribute to sustained injuries, which may resolve or can persist for extended periods of time. Case definitions and examples of traumatic lesions are available (Moore et al., 2013). Although animals affected with blunt force injury may have no apparent external lesions, on reflection of the skin and blubber, subcutaneous hemorrhage and edema at the impact site is usually noted. If the fibroelastic sheath is intact, focally extensive bulging may be observed and on incision, massive hematoma formation with shearing and necrosis of the subjacent skeletal musculature may be evident. Rupture of the sheath at the time of impact is more typically associated with extensive subcutaneous hemorrhage, which may track dependently in the hypodermis. Inspection of the abdominal and thoracic cavities may also reveal organ herniation, transposition, rupture, and associated hemorrhage. Histologicaly, acute myocellular degeneration and necrosis with edema and hemorrhage at the impact site and in muscle samples remote to the site of impact are seen and flocculent, granular, discoid and hyalinized segmental myodegeneration are attributed to agonal catecholamine surge. Fat embolization may be apparent in the pulmonary and renal microvasculature. Depending on the circumstances, animal impacts with stationary structures, such as piers, moorings, wind farms or tidal hydroelectric turbines, may present with similar gross lesions to those of ship strike (Moore et al., 2013).
In contrast to blunt force injury, animals with propeller injuries have incised and percutaneous wounds that may be curvilinear to sigmoidal, linear, or fenestrated. Catastrophic injuries are associated with incision into the thoracic or abdominal cavities as well as in those cases with amputation of flippers, fins, or even the peduncle. Involvement of larger caliber blood vessels will result in hypovolemic shock and acute death. Histopathology of the wound margins typically feature subcutaneous edema, hemorrhage, shearing at the site of impact, necrosis and retraction. As these wounds are exposed to the marine environment, imbibed water results in dissolution of erythrocytes, tissue maceration and leaching as well as microbial and ciliate colonization and proliferation. In nonlethal cases, wound healing may resolve with primary and secondary intention repair.
Southern right whales at Peninsula Valdés, Argentina, show wounds produced by kelp gull predation (Fig. 22.17 ). Kelp gulls normally scavenge sloughed whale skin. However, an unusual form of kelp-gull predation of skin in living, free-swimming whales that was first noted in the 1980s has become a locally important problem. Adult whales have developed several strategies to avoid gulls, for example by keeping their backs under water; calves that have not developed this behavior remain highly vulnerable. The attacks can last for hours at a time and interfere with normal calf resting and nursing. The kelp gull population near Peninsula Valdés has grown dramatically in recent decades, and the percentage of mothers and calves with gull lesions has correspondingly increased from an average of 2% in the 1970s to 99% in the 2000s.Gull harassment is considered a possible contributing factor in calf mortality (McAloose et al., 2016). Skin wounds can vary in size from small, focal lesions covering approximately 0.1% of the dorsal back surface to large, coalescing wounds over 8% of the dorsal body wall. The wound patterns vary from round to oval and often coalesce. Histologically, the injuries exhibit dermal congestion, suppurative dermatitis and panniculitis, necrotizing vasculitis and vascular thrombosis. Two calves with wounds forming discrete rhomboid shapes have been described. Bacterial culture and PCR confirmed Erysipelothrix rhusiopathiae from lesions in these two calves (Fiorito et al., 2016). However, these were isolated cases and bacterial, viral or other infectious diseases that predispose the skin to predation have, to date, not been identified. Physiologic and metabolic factors associated with extensive skin loss and energetic costs associated with prolonged flight/avoidance activities are additional topics of active investigation.
Figure 22.17.

Kelp gull trauma in the skin of Southern right whales.
Southern right whale adult female and calf with dorsal skin wounds typical of kelp gull predation. The wounds are much more extensive in the calf than the adult.
(Photo Courtesy of M. Sironi, Instituto de Conservación de Ballenas/Ocean Alliance)
A final traumatic condition of note is the effect of cetacean on cetacean trauma. Attacks on porpoises by bottlenose dolphins have long been recognized in the United Kingdom (Ross and Wilson, 1996) and are increasingly seen on the Pacific coast of the United States. In these cases, harbor porpoises are pursued and killed but not eaten by bottlenose dolphins. Lesions include massive blunt force traumatic lesions, such as hemorrhage, bruising, and bone fractures with or without abundant skin lacerations from teeth (rakes). Infanticide has been reported in bottlenose dolphins and killer whales.
Inflammatory Non-infectious
Gastritis and gastric ulcerations impact both captive and free-ranging cetaceans. Parasitic infections are often associated with crateriform ulcers in the nonglandular compartment of the stomach (see the parasitic disease section); however, many cases of gastritis and gastric ulceration are not attributed to helminths. Helicobacter cetorum has been identified in numerous delphinid species with gastritis (Davison et al., 2014). Histopathology reveals mild to moderate lymphoplasmacytic gastritis in the superficial mucosa of the main and pyloric compartments with superficial to deep ulcerations and occasional hemorrhage. Ulceration in the pylorus can perforate and result in fatal chemical or septic peritonitis. Spiral to fusiform bacteria have been detected in the gastric mucosa by Warthin Starry staining. Despite these reports, the role of Helicobacter spp. in gastric ulcerations has not yet been fully resolved. Geraci (1966) associated non-parasitic gastric ulcerations with consumption of high levels of histamine from fish. Elevated histamine levels result from the freeze/thaw process so it is unlikely that this mechanism occurs in free-ranging dolphins. The possibility of physiologic or psychogenic stressors related to social dynamics or environmental concerns in the development of gastric ulcers in wild or display animals cannot be discounted.
Miscellaneous
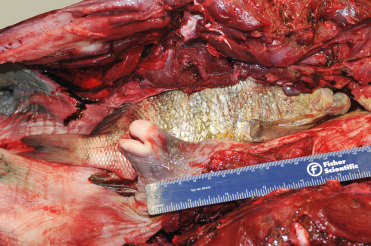
Bycatch refers to the entanglement of cetaceans in fishing gear and affects both small and large cetaceans worldwide. It is considered the most significant threat to marine mammal populations. Critically endangered species including the vaquita and baiji have largely been extirpated by fisheries interactions that may ultimately result in their extinction. Derelict and active fisheries netting, commercial monofilament lines, crab-trap lines and rope impact cetaceans directly through entanglement or entrapment. External evidence of net entanglement may include gear impressions along the surface of the skin or oral mucosa (hatched or linear furrows), abrasions, incisions, lacerations, and subcutaneous hemorrhage (Fig. 22.18 ). However, surface evidence of entanglement may not always be apparent, particularly when animals are entrapped in a trawl with large numbers of fish that prevent direct contact with the netting. In these instances, animals may present in good nutritional condition with stable white tracheobronchial froth, hyperinflated lungs with generalized pulmonary edema, congestion and subpleural emphysema. Pleural and pulmonary petechiae and ecchymoses may also be noted. Diagnosis may be contingent on the circumstances of death. In those animals that are entrapped at depth, gas bubble formation and embolization may occur with rapid accent and the pulmonary and renal microvasculature should be closely evaluated for possible fat emboli (Moore et al., 2009).
Figure 22.18.
Fishing gear trauma in a dolphin.
Fishing line laceration and wounds in the tail stock of a bottlenose dolphin. Items like fishing line and nets can lacerate tissue and create deep wounds.
(Photo Courtesy of Hubbs-SeaWorld Research Institute)
With nets or longline and crab trap lines that are tethered to the substrates, once entangled small cetaceans may be trapped under water, die from asphyxiation, and present with gross lesions consistent with entanglement. With large and smaller cetaceans that are released or may breach the line or netting, subsequent wrapping or entanglement of the gear may occur. There is a predilection for rope and net entanglement of the peduncle or tail stock, fins, flippers and oral cavity. Depending on the degree of constriction or compression and anatomic location, vascular perfusion may be impaired with hypoxia and ischemic necrosis of distal extremities. In severe cases, amputation of an appendage may occur either acutely with incision on impact or more chronic ischemia. With more protracted entanglements, animals typically lose condition and may have varying degrees of granulation tissue, fibrosis with osseous metaplasia, periosteal bone proliferation, and embedded fishing gear. If hemorrhage is apparent, a time line may be delineated by differential observation of erythrocytes, hematoidin or hemosiderosis, and margins of bone fractures should be closely evaluated to assess the extent of necrosis, inflammation, and callous formation. Exertional myopathy and myoglobinuria may also be apparent, and with more chronic cases, adrenocortical hyperplasia with lipoidal degeneration may be evident. Once gear is released, wound resolution may occur with primary and secondary intention healing.
At necropsy, it is important to photo-document and diagrams the course of the fishing line or rope throughout the carcass (wrapping pattern) and note any knots that may be present. In animals with chronic entanglement with advanced autolysis with generalized skin sloughing, the raised linear ridges of fibrosis associated with entanglement are noted and may feature superficial plaques or plugs of adhered epidermis due to adhesions. Based on long-term data analysis, it is suggested that persistent organic pollutants and harmful algal blooms (toxins) may be associated with an increased risk of entanglement. If feasible, samples to screen for these compounds should be harvested at necropsy. In some instances, the force of initial impact may be so severe, that fracture of the rostrum or mandible with tooth loss may be evident. An indirect consequence of fisheries interaction is depredation, a learned behavior recognized predominantly in killer whales, false killer whale, pilot whales, and sperm whales characterized by removal of fish from long line hooks. In rare instances, hooks have been ingested and penetrated the oropharynx, resulting in secondary septicemia. This behavior may also result in whale harassment and possibly gunshot, further contributing to the loss of these animals. Less common forms of entrapment may include interactions with aquaculture net-pen facilities, piers, and boat harbors (Read and Murray, 2000). Due to the significance of by-catch to cetaceans, pathologists should harbor a level of suspicion of entanglement and be diligent to assess all carcasses for evidence of net or line entanglement.
As with other forms of anthropogenic impacts, ingestion of lost or discarded marine debris and plastics is increasingly recognized as a significant cause of morbidity in cetaceans. Foreign material may be observed along varying levels of the alimentary canal of marine mammals and can be associated with generalized emaciation. Pica and terminal ingestion of foreign debris is noted in live, moribund stranded dolphins and may reflect a behavioral effect; whereas, in ram or lunge feeding baleen whales, ingestion of floating or suspended debris may be inadvertent. Other cetaceans with more discriminate feeding habits appear to selectively ingest material that may accumulate to sufficient quantity to impair normal digestive processes. Postmortem findings include distention of the stomach by foreign debris (including netting up to 16 m2 in sperm whales, rope, plastic bags, fishing line, and other materials), which in more severely affected animals has resulted in obstruction, localized ulceration, perforation, and secondary peritonitis (Moore et al., 2013). In additional to the physical consequences of ingested debris, manufactured plastics also incorporate polychlorinated biphenyls (PCBs), persistent organic pollutants, and other additives, which over time, may be assimilated and have a direct immunotoxicologic consequence to animals. This process may be exacerbated with inadvertent ingestion of microplastics or may result from mechanical breakdown of larger plastic items in the marine environment to secondary microparticles (Simmonds, 2012). The current understanding of the distribution and impacts of marine debris on cetaceans is poorly understand and global initiatives to better document affected species, stranding location, debris composition, and dispersal may help better define this entity.
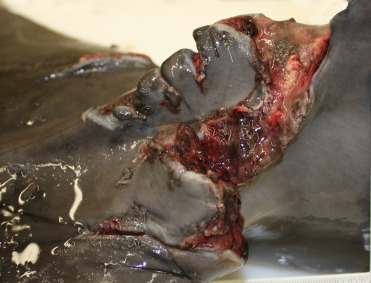
As with terrestrial species, asphyxiation is a diagnosis related to the circumstances of animal’s death and with little opportunity to observe submerged marine mammals confirmation of this diagnosis can be a challenge. Determination of a cause of death may be further confounded due to the different types of asphyxiation, including cellular, vascular, and ventilatory or mechanical obstruction. At present, there are no pathognomonic lesions or biochemical markers for asphyxiation and diagnosis typically relies on circumstances associated with recovery of the carcass. In marine mammals a presumptive diagnosis of asphyxiation is most commonly associated with bycaught individuals where animals present or are recovered entrapped in gill nets or trawls (see by-catch section for additional details). An additional form of asphyxiation is more sporadic and related to ingestion of foreign debris, such as fishing line which may obstruct or displace the larynx. The same effect is achieved when cetaceans consume prey that is sufficiently large to cause displacement of the larynx and secondarily obstruct the exposed airway (Fig. 22.19 ). Cases in animals in managed care have been seen where regurgitated objects have subsequently lodged in the displaced larynx and resulted in fatal asphyxiation. A recent case of phytobezoar asphyxiation in a bottlenose dolphin demonstrates that material need not be swallowed to be fatal (Wright et al., 2017).
Figure 22.19.
Asphyxiation in a bottlenose dolphin.
A prey species with prominent dorsal spines became lodged in the esophagus, causing dislocation of the larynx and subsequent laryngeal obstruction.
(Photo Courtesy of Hubbs-SeaWorld Research Institute)
Angiomatosis (Fig. 22.20 ) is a common finding among the stranded bottlenose dolphins in the Gulf of Mexico. The lesion has also been seen in stranded Atlantic bottlenose dolphins from eastern Florida and occasionally in common dolphins from Southern California. An evaluation of common dolphin strandings in the Canary Islands showed a majority of animals with pulmonary angiomatosis (Díaz-Delgado et al., 2012); a correlation with lung worm infections was seen in these cases. Alternate etiologic considerations include bartonellosis (see section, Bacteria). The condition is characterized by a proliferation of small, thick-walled blood vessels focally, multifocally, or diffusely throughout the lungs without inflammation, exudation, or alveolar hemorrhage. The vascular proliferation concurrently occurs in lung-associated and other visceral lymph nodes. The vascular proliferation reduces alveolar airspace and may occlude small airways. The increasing frequency of this diagnosis suggests dynamic factors in the etiology.
Figure 22.20.
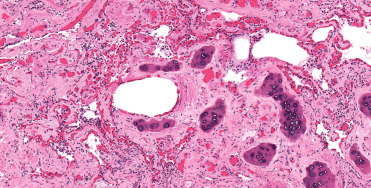
Pulmonary angiomatosis in the lung of a bottlenose dolphin.
A moderate to marked proliferation of small vessels with thick walls arranged in a haphazard pattern disrupt the bronchiole and replace the pulmonary parenchyma.
Lesions of ocular disease in cetaceans are similar to those of domestic animals but reports as scarce. Corneal ulceration commonly affects marine mammals, and corneal defects may develop secondary bacterial or fungal infections. Scarring is a common consequence and visual deficits can result. Corneal opacities that progress to keratomycosis caused by Candida albicans have been described in bottlenose dolphins (Simeone et al., 2017).
Sunburn is a common sequel to stranding for many cetaceans. As with other species, darker pigmentation is advantageous to preventing sun damage. Grossly, the condition presents as dorsal body skin edema and sloughing with patchy to diffuse ulcerative dermatitis and pronounced erythema. In stranded animal with exposed lateral surfaces for periods of time, the damage may be present on the upward side as opposed to the dorsal distribution. Skin sloughing also occurs as the result of postmortem decomposition and the two should be differentiated. Histologic changes in the epidermis in humans and many animals associated with sunburn include dyskeratotic and vacuolated keratinocytes (sunburn cells). In cetaceans, keratinocytes are not a feature of the epidermis. Degeneration of the cells in the stratum spinosum includes individual cell contraction and dissociation (Martinez-Levasseur et al., 2011). In these instances, the burn damage is coupled with damage from drying/dehydration of the skin. This results in the degeneration, fragmentation, and lifting of the stratum externum and superficial layers of the stratum spinosum. In more severe cases, clefts extend below the stratum germinativum lifting the entire epidermis. The dermis contains hemorrhage, vascular dilation, and perivascular edema. Damage is commonly deep in the dermis. Healing occurs through epidermal migration and scarring. Areas of prior damage can often be identified grossly by white cicatricial change in the skin that remains for life.
Skin trauma can also result from changes in the osmolality of the aquatic environment. Marine cetaceans in fresh-water environments suffer from osmotic skin damage (Hart et al., 2012). Differentiation primary from secondary changes can be very difficult. Lesions include patchy to diffuse areas of cutaneous edema and discoloration, excessive sloughing of the superficial epidermis, erosions, and ulcerations along with cutaneous cracking. Secondary infections are common.
Neoplastic
A comprehensive review of marine mammal tumors was compiled by Newman and Smith (2006); additional reports of neoplasia in cetaceans published since his 2006 review are listed in Table 22.2 . Three neoplastic conditions that warrant special attention are gastrointestinal neoplasia in belugas from the St. Lawrence Estuary, urogenital papillomas in both free-ranging and captive dolphins, and oral squamous cell carcinoma in bottlenose dolphins in human care.
Table 22.2.
Neoplasia in Cetaceans Since the Comprehensive Review in 2006*
| Species | Tumor Type | Location |
|---|---|---|
| Atlantic spotted dolphin | Astrocytomam | Brain |
| Leiomyoman | Uterus | |
| Pheochromocytomao | Adrenal gland | |
| Seminoma, sertoli cell tumoro | Testicle | |
| T-cell lymphomap | Uterus | |
| Beluga whale | Adenocarcinomaa | Stomach |
| Intestine | ||
| Uterus | ||
| Adenocarcinoma/lymphagiosarcoma | Adrenal gland | |
| Carcinoma | Thyroid gland | |
| Mammary gland | ||
| Lung | ||
| Dysgerminoma | Ovary | |
| Squamous cell carcinoma | Urethra | |
| Lung (metastasis) | ||
| Vertebra (met) | ||
| Bottlenose dolphin | Immunoblastic lymphomaq | Hepatosplenic |
| Leiomyomar | Stomach | |
| Papillomas | Tongue | |
| Penis | ||
| Seminomao | Testicles | |
| Squamous cell carcinomas, t | Oral cavity | |
| Harbor porpoise | Granulosa cell tumorj | Ovary |
| Squamous cell carcinomak | Stomach | |
| Long-finned pilot whale | Carcinomag | Lung |
| Pygmy sperm whale | Leiomyosarcomai | Gastrointestinal tract |
| Risso’s dolphin | Luteomah | Ovary |
| Short beaked common dolphin | Hemangiomab | Lung |
| Meningiomac, d | Brain | |
| Sertoli cell tumor, interstital (Leydig) cell tumor, seminomae | Testicles | |
| T-cell lymphomae | Central nervous system | |
| Transitional cell carcinomaf | Urinary bladder | |
| Striped dolphin | Primitive neuroectodermal tumorl | Brain |
Lair, S., Martineau, D., Measures, L.N., 2014. Causes of mortality in St. Lawrence Estuary beluga (Delphinapterus leuca), from 1983 to 2012. Ottawa, Canada: Canadian Science Advisory Secretariat.
Diaz-Delgado, J., Arbelo, M., Sacchini, S., Quesada-Canales, O., Andrada, M., Rivero, M., Fernandez, A., 2012a. Pulmonary angiomatosis and hemangioma in common dolphins (Delphinus delphis) stranded in Canary Islands. J. Vet. Med. Sci. 74(8), 1063–1066.
Miclard, J., Mokhtari, K., Jouvion, G., Wyrzykowski, B., Van Canneyt, O., Wyer, M., Colle, M. A., 2006. Microcystic meningioma in a dolphin (Delphinus delphis): immunohistochemical and ultrastructural study. J. Comp. Pathol. 135, 254–258.
Díaz-Delgado, J., de los Monteros, A. E., Fernandez-Maldonado, C., Arbelo, M., Quesada-Canales, O., Andrada, M., Fernandez, A., 2012b. Mixed testicular neoplasia in a short beaked common dolphin Delphinus delphis. Dis. Aquat. Organi. 101, 257–260.
Arbelo, M., de los Monteros, A.E., Herraez, P., Suarez-Bonnet, A., Andrada, M., Rivero, M., Grau-Bassas, E.R., Fernandez, A., 2014. Primary central nervous system T-cell lymphoma in a common dolphin (Delphinus delphis). J. Comp. Pathol. 150, 336–340.
Alonso-Farré, J.M., Gonzalo-Orden, M., Llarena-Reino, M., Monreal-Pawlowsky, T., Degollada, E., 2013. Transitional cell carcinoma in the urinary bladder of a common dolphin (Delphinus delphis) with emphasis on imaging diagnosis. Imaging 6, 1.
Suárez-Santana, C. M., Fernández-Maldonado, C., Díaz-Delgado, J., Arbelo, M., Suarez-Bonnet, A., de los Monteros, A. E., Camara, N., Sierra, E., Fernandez, A., 2016. Pulmonary carcinoma with metastasis in a long-finned pilot whale (Globicephala melas). BMC Vet. Res. 12, 229.
Nishina, H., Izawa, T., Ozaki, M., Kuwamura, M., Yamate, J., 2017. Unilateral luteoma of the ovary in a pregnant Risso’s dolphin (Grampus griseus). J. Vet. Med. Sci. 79(10), 1749–1752.
Leone, A., Dark, M., Kondo, H., Rotstein, D.S., Kiupel, M., Walsh, M.T., Erlacher-Reid, C., Gordon, N., Conway, J.A., 2013. Gastrointestinal leiomyosarcoma in a pygmy sperm whale (Kogia breviceps). J. Zoo Wildl. Med. 44, 744–748.
Seibel, H., Siebert, U., Schöpper, H., Wohlsein, P., 2012. Granulosa cell tumour in a harbour porpoise (Phocoena phocoena) from German waters. Dis. Aquat. Organ. 99, 79–83.
Siebert, U., Hasselmeier, I., Wohlsein, P., 2010. Immunohistochemical characterization of a squamous cell carcinoma in a harbour porpoise (Phocoena phocoena) from German waters. J. Comp. Pathol. 143, 179–184.
Baily, J., Morrison, L.R., Patterson, I., et al., 2013. Primitive neuroectodermal tumour in a striped dolphin (Stenella coeruleoalba) with features of ependymoma and neural tube differentiation (medulloepithelioma). J. Comp. Pathol. 149, 514–519.
Díaz-Delgado, J., Sacchini, S., Suárez-Bonnet, A., et al., 2015a. High-grade astrocytoma (glioblastoma multiforme) in an Atlantic spotted dolphin (Stenella frontalis). J. Comp. Pathol. 152, 278–282.
Díaz-Delgado, J., Fernández, A., Edwards, J., et al., 2015b. Uterine leiomyoma and prolapse in a live-stranded atlantic spotted dolphin (Stenella frontalis). J. Comp. Pathol. 153, 58–63.
Estep, J.S., Baumgartner, R.E., Townsend, F., Pabst, D.A., Mclellan, W.A., Friedlaender, A., Dunn, D.G., Lipscomb, T.P., 2005. Malignant seminoma with metastasis, Sertoli cell tumor, and pheochromocytoma in a spotted dolphin (Stenella frontalis) and malignant seminoma with metastasis in a bottlenose dolphin (Tursiops truncatus). Vet. Pathol. 42(3), 357–359.
Díaz-Delgado, J., Sierra, E., Arbelo, M., et al., 2015c. Primary uterine T-cell lymphoma with metastasis in an Atlantic spotted dolphin (Stenella frontalis), Canary islands, Spain. J. Wildl. Dis. 51, 538–541.
Jaber, J.R., Perez, J., Carballo, M., Arbelo, M., de los Monteros, A.E., Herráez, P., Munoz, J., Andrada, M., Rodriguez, F., Fernández, A., 2005. Hepatosplenic large cell immunoblastic lymphoma in a bottlenose dolphin (Tursiops truncatus) with high levels of polychlorinated biphenyl congeners. J. Comp. Pathol. 132(2), 242–247.
Rotstein, D., Harms, C., Lovewell, G., et al., 2007. Gastric leiomyoma in a free-living Atlantic bottlenosed dolphin (Tursiops truncatus). Vet. Rec. 160, 130.
Bossart, G.D., Ghim, S.J., Rehtanz, M., Goldstein, J., 2005. Orogenital neoplasia in Atlantic bottlenose dolphins (Tursiops truncatus). Aquat. Mamm. 31(4), 473.
March, D.T., Blyde, D.J., Bossart, G.D., Begg, A.P., Taylor, D.P., McClure, V., 2016. Piroxicam and doxycycline treatment for an oral squamous cell carcinoma in an inshore bottlenose dolphin (Tursiops aduncus). Aust. Vet. J. 94(6), 203–207.
Source: Newman, S.J., Smith, S.A., 2006. Marine mammal neoplasia: a review. Vet. Pathol. 43(6), 865–880 Available from: http://journals.sagepub.com/doi/abs/10.1354/vp.43-6-865.
Long-term investigations into the health of the belugas in the St. Lawrence Estuary (SLE) have demonstrated tumor rates that are among the highest percentages of neoplasia in wild cetacean populations in the world (Lair et al., 2014). Over a 30 year time frame, 222 dead animals were recovered and available for necropsy. Of these, 39 neoplasia were diagnosed in 35 adult beluga. Malignant tumors were identified as the cause of death in 14% of the beluga examined with gastrointestinal adenocarcinoma the most common, affecting 7% of mature adults. Eight cases of mammary carcinoma were identified in 10% of examined adult females. These tumors were often metastatic and in 6 of the 8 cases, mammary gland carcinoma was identified as the cause of death in affected whales. There were 2 fatal cases of thyroid carcinoma, 1 of lymphoma, and 2 benign ovarian tumors (Lair et al., 2014). No comparable prevalence of neoplasia has been found in other free-ranging cetacean populations. The hypothesis to explain this unusually high frequency of neoplasia is environmental exposure, persistence and possible foodweb bioaccumulation of carcinogens, such as polyaromatic hydrocarbons (Martineau et al., 2002).
Oral papilloma and squamous cell carcinoma (SCC) in bottlenose dolphins under managed care may reflect a progression along a spectrum of pathology. Closely associated with this condition are genital papillomas, which have been identified in both sexes of captive and free-ranging bottlenose dolphins (Fig. 22.21A, B). For this reason, these proliferative lesions are designated as orogenital papillomas with the caveat that only oral masses have been identified as transforming to either carcinoma in situ or squamous cell carcinoma at this time (Bossart et al., 2005). Oral papillomas present as raised, well-circumscribed, pale, irregular masses or plaques in the oral mucosa, mucocutaneous junction, or on the tongue. Histologically, they are composed of hyperplastic epithelium and tend to recur after excision. With time, these masses may progress to carcinoma in situ or a more aggressive squamous cell carcinoma. Grossly, malignancies present with irregular, poorly demarcated ulcerative areas admixed within neoplastic and proliferative tissue. Histologically, the presentation is classic for both concerns. The oral SCC cases bridge the mucosal basement membrane and irregular islands and fingers of proliferating cells expand from the central lesion both deeply and widely (Fig. 22.22A, B).
Figure 22.21.
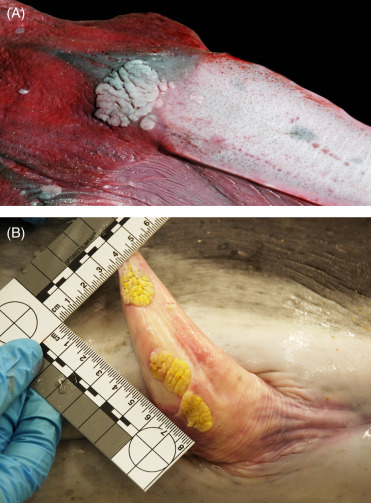
Orogenital papillomas.
(A) Oral papilloma in a stranded common dolphin. A discrete, raised, white plaque is present in the mucosa at the base of the tongue. (B) Genital papillomas in a bottlenose dolphin. Multiple, light yellow, well-circumscribed plaques are present along the mucosa of the penis. Both gamma herpes virus and papilloma virus have been associated with these lesions seen in both free ranging and captive cetaceans.
(Part B: Photo Courtesy of Hubbs-SeaWorld Research Institute)
Figure 22.22.
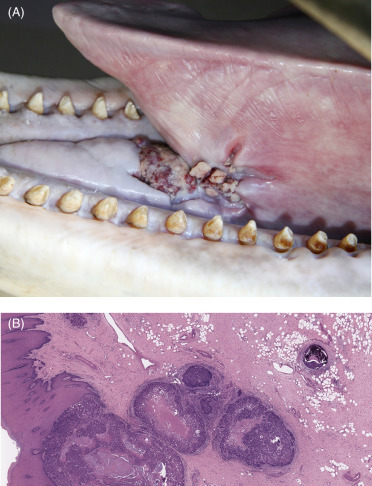
Oral squamous cell carcinoma in a bottlenose dolphin.
(A) Irregular tissue proliferation with foci of ulceration present on the floor of the oral cavity at the base of the fenulum. (B) Irregular proliferations of neoplastic epithelial cells form islands that breach and invade beneath the basement membrane of the oral mucosa. Multifocal central necrosis is present, as is intravascular metastasis in a blood vessel in the right of this image.
Oral SCCs tend to be slowly progressive but highly aggressive. The poor demarcation and wide tumor extension result in common recurrence after attempts at complete excision. Both local aggression and metastasis characterize the biological behavior of these neoplasms. Metastasis to tonsils as well as thoracic and prescapular lymph nodes and lung, have been described. Prescapular lymph node involvement is important clinically because this tissue is accessible for both ultrasound and biopsy to screen for metastatic spread. The aggressive nature of these neoplasms is reported to be enhanced during pregnancy, suggesting a role of both hormones and immune modification in the pathogenesis of these tumors. Exposure of the oral cavity to ultraviolet light associated with “heads up” feeding of captive dolphins above the water surface is suggested as having a potential role in malignant transformation of the mucosal epithelium (N. Stedman, personal communication). Both papillomaviruses and herpesviruses have been associated with genital and oral papillomas and oral squamous cell carcinomas in multiple cetacean species, both in captive and stranded animals (Rehtanz et al., 2010; Smolarek Benson et al., 2006; Van Bressem et al., 2014).
Infectious diseases
In addition to the descriptions below, see also the Supplemental Materials for a list of additional notable viral (Table e3) and parasitic (Table e4) infections in cetacea.
Table e3.
Viruses in Cetaceans
| DNA Viruses | Species | Method of Detection | Lesions | Specimen/sTested | References |
|---|---|---|---|---|---|
| Adenovirus | Beluga whale, bowhead whale, sei whale | Virus isolation | Virus detected but no associated lesions | Intestinal tissue, feces | m |
| Adenovirus | Bottlenose dolphin | PCR, serology | Gastroenteritis | Feces | t |
| Adenovirus | Harbor porpoise | PCR | Virus detected but no associated lesions | Intestinal contents | u |
| Cetacean Poxvirus | Balaenidae, Delphinidae, Phocenidea, Ziphiidae | Gross/histo | Focal or multifocal cutaneous hyperpigmentation | Skin | s |
| Cetacean Poxvirus | Atlantic white-sided dolphin, bottlenose dolphin, Burmeister’s porpoise, dusky dolphin, killer whale, long-beaked common dolphin | EM | Focal or multifocal cutaneous hyperpigmentation | Skin | m |
| Hepadnavirus | Pacific white-sided dolphin | Antigens | Chronic persistent hepatitis | Serum | m |
| Polyomavirus | Short-beaked common dolphin | EM, sequencing | Tracheobronchitis | Laryngeal mucosa | h |
| RNA Viruses | Species | Method of Detection | Lesions | Specimen/sTested | References |
|---|---|---|---|---|---|
| Calicivirus-Cetacean | Bottlenose dolphin | Virus isolation | Vesicular skin lesions | Skin | m |
| Calicivirus -SMSV, VESV, walrus, mink | Bottlenose dolphin, Bowhead whale, fin whale, Gray whale, sei whale, sperm whale | Virus isolation | Virus detected but no associated lesions | Serum | m |
| Coronavirus | Bottlenose Dolphin | PCR, sequencing | Virus detected but no associated lesions | Feces | w |
| Coronavirus | Beluga whale | Histo, EM, sequencing | Necrotizing hepatitis | Liver | x |
| Dolphin Rhabdovirus | Bottlenose dolphin, false killer whale, harbor porpoise, long-finned pilot whale, short-beaked common dolphin, striped dolphin, white beaked dolphin | Virus neutralization | Virus detected but no associated lesions | Serum | j,m |
| Enterovirus | Bottlenose Dolphin | Virus isolation, EM, sequencing, iELISA | Erosive glossitis | Throat swab, serum | z |
| Hepatitis E | Bottlenose Dolphin | RT-PCR | Virus detected but no lesions described | Serum and Liver | y |
| Influenza A | Beluga whale | ELISA | Virus detected but no associated lesions | Serum | r |
| Influenza A | Minke whale, Dall’s porpoise | ELISA, Western blot | Virus detected but no associated lesions | Serum | p |
| Morbillivirus | Atlantic white-sided dolphin, bottlenose dolphin, minke whale, long-beaked common dolphin, dusky dolphin, Fraser’s dolphin, pygmy killer whale, pygmy sperm whale, Risso’s dolphin, short-finned pilot whale, spinner dolphin | PCR, IHC | Pneumonia, encephalitis, lymphoid depletion | Multiple tissues | m,k |
| Morbillivirus | Guiana dolphin | PCR, IHC | Pneumonia, encephalitis, lymphoid depletion | Multiple tissues | n |
| Morbillivirus | Indo-Pacific beaked whale | PCR, IHC | Encephalitis, lymphoid depletion | Multiple tissues | o |
| Morbillivirus | Striped dolphin | PCR, IHC | Pneumonia, encephalitis, lymphoid depletion | Multiple tissues | b,l,m |
| Morbillivirus | Bottlenose dolphin | IHC | Pneumonia | Lung | e |
| Morbillivirus DMV | Fin whale | IHC, PCR, virus neutralization | Lymphoid depletion, encephalitis | Multiple tissues | f,m |
| Morbillivirus DMV | Short-beaked common dolphin | IHC, PCR, EM, iELISA | Pneumonia, encephalitis, lymphoid depletion necrotic stomatitis, gastroenteritis, cholangitis, | Serum | e,m,w |
| Morbillivirus DMV | White-beaked dolphin | Histology, PCR | Encephalitis | Brain | e,m |
| Morbillivirus PMV | Harbor Porpoise | IHC, iELISA | Pneumonia, encephalitis, lymphoid depletion | Serum | e,m,w |
| Morbillivirus PWMV | Long-finned pilot whale | PCR, IHC | Pneumonia, lymphoid depletion | Lung, brain | g,m |
| Norovirus | Harbor Porpoise | PCR | Virus detected but no associated lesions | Intestinal tissue, feces | v |
| Orthomyxovirus | Long-finned pilot whale | Virus isolation | Hemorrhagic lungs, enlarged hilar lymph node | Lung, hilar lymph node | m |
| Orthomyxovirus | Rorquals | Virus isolation | Virus detected but no associated lesions | Lung, liver | m |
| Papillomavirus Phocenaspinipinnis | Burmeister’s porpoise, dusky dolphin | Histology,IHC, molecular studies | Genital papilloma | Genital papilloma | m |
| Papillomavirus | Dusky dolphin, Guiana dolphin, Burmeister’s porpoise, Striped dolphin, Short-beaked common dolphin | Gross/histo | Genital papilloma | Genital papilloma | s |
| Papillomavirus | Harbor porpoise, long-beaked common dolphin, sperm whale, bottlenose dolphin | Histology, molecular studies | Genital papilloma | Genital papilloma | m |
| Papillomavirus type 2 | Bottlenose dolphin | PCR | Genital papilloma | Genital papilloma | i |
| Parainfluenza | Bottlenose dolphin | ELISA | Virus detected but no associated lesions | Serum | a |
| Parainfluenza | Bottlenose dolphin | PCR, IHC, EM | Tracheitis and pneumonia | Lung, mediastinal lymph node | c,d |
| West Nile virus | Killer whale | PCR, IHC | Encephalitis | Brain | q |
ELISA, Enzyme-linked immunosorbent assay; EM, electron microscopy; iELISA, immune enzyme-linked immunosorbent assay; IHC, immunohistochemistry; PCR, polymerase chain reaction; RIA, radioimmunoassay; RT-PCR, reverse transcription polymerase chain reaction.
Venn-Watson, S., Rivera, R., Smith, C.R., Saliki, J.T., Caseltine, S., Leger, J.S., Yochem, P., Wells, R.S., Nollens, H., 2008. Exposure to novel parainfluenza virus and clinical relevance in 2 bottlenose dolphin (Tursiopstruncatus) populations. Emerg. Infect. Dis. 14(3), 397.
Raga, J.A., Banyard, A., Domingo, M., Corteyn, M., Van Bressem, M.F., Fernández, M., Aznar, F.J., Barrett, T., 2008. Dolphin morbillivirus epizootic resurgence, Mediterranean Sea. Emerg. Infect. Dis. 14(3), 471.
Venn-Watson, S., Daniels, R., Smith, C., 2012. Thirty year retrospective evaluation of pneumonia in a bottlenose dolphin Tursiops truncatus population. Dis. Aquat. Organ. 99(3), 237–242.
Nollens, H.H., Wellehan, J.F., Saliki, J.T., Caseltine, S.L., Jensen, E.D., Van Bonn, W., Venn-Watson, S., 2008. Characterization of a parainfluenza virus isolated from a bottlenose dolphin (Tursiops truncatus). Vet. Microbiol. 128(3), 231–242.
Wohlsein, P., Puff, C., Kreutzer, M., Siebert, U., Baumgärtner, W., 2007. Distemper in a dolphin. Emerg. Infect. Dis. 13(12), 1959.
Mazzariol, S., Marcer, F., Mignone, W., Serracca, L., Goria, M., Marsili, L., Di Guardo, G., Casalone, C., 2012. Dolphin Morbillivirus and Toxoplasma gondii coinfection in a Mediterranean fin whale (Balaenoptera physalus). BMC Vet. Res. 8(1), 20.
Taubenberger, J.K., Tsai, M.M., Atkin, T.J., Fanning, T.G., Krafft, A.E., Moeller, R.B., Kodsi, S.E., Mense, M.G., Lipscomb, T.P., 2000. Molecular genetic evidence of a novel morbillivirus in a long-finned pilot whale (Globicephalusmelas). Emerg. Infect. Dis. 6(1), 42.
Anthony, S.J., St. Leger, J.A., Navarrete-Macias, I., Nilson, E., Sanchez-Leon, M., Liang, E., Seimon, T., Jain, K., Karesh, W., Daszak, P., Briese, T., 2013. Identification of a novel cetacean polyomavirus from a common dolphin (Delphinus delphis) with Tracheobronchitis. PloS One, 8(7), 68239.
Rehtanz, M., Ghim, S.J., Rector, A., Van Ranst, M., Fair, P.A., Bossart, G.D., Jenson, A.B., 2006. Isolation and characterization of the first American bottlenose dolphin papillomavirus: Tursiopstruncatus papillomavirus type 2. J. Gen. Virol. 87(12), 3559–3565.
Siegers, J.Y., van de Bildt, M.W., van Elk, C.E., Schürch, A.C., Tordo, N., Kuiken, T., Bodewes, R., Osterhaus, A.D., 2014. Genetic relatedness of dolphin rhabdovirus with fish rhabdoviruses. Emerg. Infect. Dis. 20(6), 1081.
Soto, S., González, R., Alegre, F., González, B., Medina, P., Raga, J.A., Marco, A., Domingo, M., 2011. Epizootic of dolphin morbillivirus on the Catalonian Mediterranean coast in 2007. Vet. Rec. 169(4), 102.
Di Guardo, G., Cocumelli, C., Scholl, F., Di Francesco, C.E., Speranza, R., Pennelli, M., Eleni, C., 2011. Morbilliviral encephalitis in a striped dolphin Stenellacoeruleoalba calf from Italy. Dis. Aquatic Organ. 95(3), 247–251.
Van Bressem, M.F., Van Waerebeek, K., Raga, J.A., 1999. A review of virus infections of cetaceans and the potential impact of morbilliviruses, poxviruses and papillomaviruses on host population dynamics. Dis. Aquat. Organ. 38(1), 53–65.
Groch, K.R., Colosio, A.C., Marcondes, M.C., Zucca, D., Díaz-Delgado, J., Niemeyer, C., Marigo, J., Brandão, P. E., Fernández, A., Catão-Dias, J. L., 2014. Novel cetacean morbillivirus in Guiana dolphin, Brazil. Emerg. Infect. Dis. 20(3), 511.
West, K. L., Sanchez, S., Rotstein, D., Robertson, K. M., Dennison, S., Levine, G., Davis, N., Schofield, D., Potter, C. W., Jensen, B., 2013. A Longman’s beaked whale (Indopacetus pacificus) strands in Maui, Hawaii, with first case of morbillivirus in the central Pacific. Mar. Mamm. Sc. 29(4), 767–776.
Ohishi, K., Maruyama, T., Ninomiya, A., Kida, H., Zenitani, R., Bando, T., Fujise, Y., Nakamatsu, K., Miyazaki, N., Boltunov, A.N., 2006. Serologic investigation of influenza A virus infection in cetaceans from the Western North Pacific and the Southern Oceans. Mar. Mamm. Sci. 22(1), 214–221.
St. Leger, J. A., Wu, G., Anderson, M., Dalton, L., Nilson, E., Wang, D., 2011. West Nile virus infection in killer whale, Texas, USA, 2007. Emerg. Infect. Dis. 17(8), 1531.
Nielsen, O., Clavijo, A., Boughen, J. A., 2001. Serologic evidence of influenza A infection in marine mammals of arctic Canada. J. Wildl. Dis. 37(4), 820–825.
Van Bressem, M. F., Raga, J. A., Di Guardo, G., Jepson, P. D., Duignan, P. J., Siebert, U., Barrett, T., de Oliveira Santos, M. C., Moreno, I. B., Siciliano, S., Aguilar, A., 2009. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 86(2), 143–157.
Rubio-Guerri, C., García-Párraga, D., Nieto-Pelegrín, E., Melero, M., Álvaro, T., Valls, M., Crespo, J.L. and Sánchez-Vizcaíno, J.M., 2015. Novel adenovirus detected in captive bottlenose dolphins (Tursiops truncatus) suffering from self-limiting gastroenteritis. BMC Vet. Res. 11(1) 53.
van Beurden, S. J., IJsseldijk, L. L., van de Bildt, M. W., Begeman, L., Wellehan, J. F., Waltzek, T. B., de Vrieze, G., Gröne, A., Kuiken, T., Verheije, M. H., Penzes, J. J., 2017. A novel cetacean adenovirus in stranded harbour porpoises from the North Sea: detection and molecular characterization. Arch. Virol. 1–6.
de Graaf, M., Bodewes, R., van Elk, C. E., van de Bildt, M., Getu, S., Aron, G. I., Verjans, G. M., Osterhaus, A. D., van den Brand, J. M., Kuiken, T., Koopmans, M. P., 2017. Norovirus infection in harbor porpoises. Emerg. Infect. Dis. 23(1), 87.
Woo, P.C., Lau, S.K., Lam, C.S., Tsang, A.K., Hui, S.W., Fan, R.Y., Martelli, P., Yuen, K.Y., 2014. Discovery of a novel bottlenose dolphin coronavirus reveals a distinct species of marine mammal coronavirus in Gammacoronavirus. J. Virol. 88(2), 1318–1331.
Mihindukulasuriya, K.A., Wu, G., St Leger, J., Nordhausen, R.W., Wang, D., 2008. Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J. Virol. 10, 5084–5088.
Montalvo Villalba, M.C., Cruz Martínez, D., Ahmad, I., Rodriguez Lay, L.A., Bello Corredor, M., Guevara March, C., Martínez, L.S., Martínez-Campo, L.S., Jameel, S., 2017. Hepatitis E virus in bottlenose dolphins Tursiops truncatus. Dis. Aquat. Organ. 123(1), 13–18.
Nollens, H.H., Rivera, R., Palacios, G., Wellehan, J.F., Saliki, J.T., Caseltine, S.L., Smith, C.R., Jensen, E.D., Hui, J., Lipkin, W.I., Yochem, P.K., Wells, R.S., St Leger, J., Venn-Watson, S., 2009. New recognition of Enterovirus infections in bottlenose dolphins (Tursiops truncatus).Vet. Microbiol. 139(1–2), 170–175.
Table e4.
Parasites of Cetaceans
| Type Parasite | Name | Location in Host | Associated Disease (if any) | Species Affected |
|---|---|---|---|---|
| Metazoaa,b,c | ||||
| Nematode |
Anisakis (multiple species) Contracaecum (multiple species) Pseudoterranova (multiple species) |
Gastrointestinal tract | Mild infections—no clinical signs Heavy infections—gastritis and ulceration |
Multiple species |
| Nematode | Odontobiusceti | Baleen plates | Nonpathogenic | Mysticetes |
| Trematode |
Leucasiella (multiple species) Onthosplanchnus (multiple species) Synthesium (multiple species) Echinichasmus (multiple species) Galactosonum (multiple species) Ogomogaster (multiple species) |
Gastrointestinal tract | Irritation | Multiple species |
| Trematode | Odhnerilla (multiple species) | Gastrointestinal tract, liver, pancreas | Irritation, hepatitis, pancreatitis | Multiple species |
| Trematode | Pholetergastrophilus | Gastrointestinal tract | Irritation, fibrous capsule in wall of stomach | Multiple species |
| Trematode | Hadweniusmironovi, H. nipponicus | Gastrointestinal tract | Irritation | Harbor porpoise |
| Trematode | Braunina cordiformis | Gastrointestinal tract | Irritation, fibrous capsule in wall of stomach | Multiple species |
| Trematode |
Campula (multiple species) Oschmarinella (multiple species) Zalophotrema (multiple species) |
Heptatic and pancreatic ducts | Weight loss, decreased liver function, predisposition to bacterial disease, hepatic trauma, death | Odontocetes |
| Trematode | Lecithodesmus (multiple species) | Heptatic and pancreatic ducts | Weight loss, decreased liver function, predisposition to bacterial disease, hepatic trauma, death | Mysticetes |
| Trematode | Nasitrema (multiple species) | Respiratory system, sinuses, brain | Eighth cranial neuropathy, encephalitis, cerebral necrosis | Multiple species |
| Trematode | Hunterotrema (multiple species) | Lungs | Obstruction of passages ways with mucoid exudate | Amazon river dolphin |
| Trematode | Halocercus (multiple species) | Respiratory system | Pneumonia, bronchitis, cough, dyspnea, lethargy, death | Phocoenidae, Delphinidae, Monodontidae, Ziphiidae |
| Trematode | Pharurus (multiple species) | Lungs, pulmonary blood vessels, auditory spaces, air sinuses | Cough, dyspnea, lethargy, death, mucosal inflammation, purulent sinusitis, pneumonia, bronchitis | Monodontidae, Phocoenidae |
| Trematode | Pseudaliusinflexus | Respiratory system, heart, pulmonary blood vessels | Cough, dyspnea, lethargy, death, endocarditis, vasculitis, thrombosis, pneumonia, bronchitis | Phocoenidae, Delphinidae |
| Trematode | Torynurus convolutus, T. dalli | Lungs, pulmonary blood vessels, auditory spaces, air sinuses | Cough, dyspnea, lethargy, death, mucosal inflammation, purulent sinusitis, pneumonia, bronchitis | Phocoenidae, Delphinidae |
| Trematode | Pseudostenurus sunameri | Lungs, pulmonary blood vessels, auditory spaces, air sinuses | Cough, dyspnea, lethargy, death, mucosal inflammation, purulent sinusitis, pneumonia, bronchitis | Phocoenidae |
| Trematode | Skrjabinalius cryptocephalus, S. guevarai | Lungs, pulmonary blood vessels, air sinuses | Cough, dyspnea, lethargy, death, mucosal inflammation, purulent sinusitis, pneumonia, bronchitis | Delphinidae |
| Trematode | Stenurus (multiple species) | Lungs, pulmonary blood vessels, auditory spaces, air sinuses | Cough, dyspnea, lethargy, death, mucosal inflammation, purulent sinusitis, pneumonia, bronchitis | Phocoenidae, Delphinidae, Monodontidae |
| Trematode | Crassicauda (multiple species) | Air sinuses; Mammary tissue, kidneys, urogenital system | Mucosal inflammation, purulent sinusitis, osteitis, bone erosion, reduce populations, decrease milk production | Small odontocetes |
| Trematode | Placentonema gigantisma | Reproductive tract | Fetal death | Sperm whale |
| Cestode | Phyllobothrium, and Monorygma (multiple species) | Peritoneum, blubber, connective tissue | Nonpathogenic | Multiple species |
| Cestode |
Diplogonoporus (multiple species) Diphyllobothrium (multiple species) Hexagonoporus (multiple species) Plicobothrium (multiple species) Tetrabothrius (multiple species) Trigonocotyle (multiple species) Priapocephalus (multiple species) |
Gastrointestinal tract | Can cause blockage due to compression of adjacent organs | Multiple species |
| Cestode | Strobilocephalus triangularis | Gastrointestinal tract | Necrotic ulcers in colon | Small odontocetes |
| Acanthocephalans | Corynosoma and Bolbosoma (multiple species) | Gastrointestinal tract | Slight inflammation, fibrosis | Multiple species |
| Acanthocephalans | Bolbosoma balanae | Gastrointestinal tract | Abscesses | Gray whale |
| Protozoaa,d | ||||
| Ciliates | Haematophagus megapterae | Baleen plates | n/a (feeds on red blood cells—nonpathogenic) | Humpback, fin, blue |
| Ciliates | Kyaroikeus cetarius | Blowhole, skin lesions, lymph nodes | Opportunistic infections, unknown if pathogenic | Bottlenose dolphin, killer whale, false killer whale, beluga |
| Ciliates | Chilodonella sp. | Blowhole mucus, skin scrapings | Opportunistic infections, unknown if pathogenic | Bottlenose dolphin |
| Apicomplexans | Sarcocystis balaenopteralis | Skeletal muscle | n/a | Sei whale |
| Apicomplexans | Sarcocystis sp. | Skeletal muscle | n/a | Beluga, northern right whale dolphin, pilot whale, striped dolphin, sperm whale |
| Apicomplexans | Sarcocytis neurona | CNS, cardiac muscle, skeletal muscle | Encephalitis, CNS signs | |
| Apicomplexans | Toxoplasma gondii | CNS, muscle | Encephalitis | Atlantic bottlenose, Risso’s, striped, spinner dolphins |
| Apicomplexans | Cystoisospora delphini | Digestive tract | Enteritis | Bottlenose dolphin |
| Flagellates | Kinetoplastid | Blowhole mucus | Clinical significance unclear | Bottlenose dolphin, pygmy sperm whale |
| Sarcodina | Entamoeba sp. | Colon | Clinical significance unknown | Bowhead whale |
| Ectoparasitee,f | ||||
| Whale lice |
Cyamus (multiple species) Isocyamus (multiple species) Syncyamu (multiple species) |
Skin, genital folds, nostrils, eye | Dermatitis, can cause skin wounds to have delayed healing | Primarily mysticetes |
| Neocyamus physeteris | Skin, genital folds, nostrils, eye | Dermatitis, can cause skin wounds to have delayed healing | Sperm whale (females and calves) | |
| Platycyamus flaviscutatus, P. thompsoni | Skin, genital folds, nostrils, eye | Dermatitis, can cause skin wounds to have delayed healing | ||
| Scutocyamus antipodensis, S. parvus | Skin, genital folds, nostrils, eye | Dermatitis, can cause skin wounds to have delayed healing | ||
| Barnacles | Coronula (multiple species) | External | n/a | Humpback, southern right, blue, fin, sperm,Sei, minke, northern bottlenose whale |
| Cetopirus complanatus | External | n/a | Southern right whale | |
| Cryptolepas rhachianecti | External | n/a | Gray, killer, beluga, whale | |
| Tubicinella major | External | n/a | Southern right whale | |
| Xenobalanus globicipitis | External | n/a | Multiple species | |
| Arthopoda | Pennella balaenopterae | Muscle, blubber | Sei, minke, Cuiver’s beaked, fin whale, Risso’s, bottlenose dolphin, Harbor porpoise | |
| Arthopoda | Balaenophilus unisetus | Blubber | Mysticetes |
Dierauf, L., Gulland, F.M. (Eds.), 2001. CRC Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation. CRC press.
Dailey, M.D., 1985. Diseases of mammalia: Cetacea. Dis. Mar. Anim. 4(2), 805–847.
Measures, L.N., 2001. Lungworms of marine mammals. Parasitic Diseases of Wild Mammals, second ed., pp. 279–300.
Dold, C., 2001. Cetacea (Whales, Dolphins, Porpoises). In: Fowler’s Zoo and Wild Animal Medicine Current Therapy, Vol. 7. Elsevier Health Sciences, pp. 422–436.
Hayashi, R., 2013. A checklist of turtle and whale barnacles (Cirripedia: Thoracica: Coronuloidea). J. Mar. Biol. Assoc. 93(1), 143–182.
Aznar, F.J., Balbuena, J.A., Fernández, M., Raga, J.A., 2002. Living together: the parasites of marine mammals. In: Marine Mammals, Springer, US, pp. 385–423.
DNA Viruses
A unique pattern of disease colloquially called “ tattoo ” typifies poxvirus infection in cetaceans. Phylogenetic studies have identified six separate species or clusters of cetacean poxviruses. These sequences cluster together but are distinctly separate from the adjacent orthopoxviruses, suggesting that cetacean poxviruses form a separate genus. This unique phylogeny fits the unusual biologic behavior of cetacean pox in creating non-proliferative skin lesions that are present for extended periods of time. No pattern regarding species-specificity of viruses has been delineated. Both toothed and baleen whales can be infected, and typical lesions have been identified in bottlenose dolphins, killer whales, harbor porpoise, short and long-beaked common dolphins, Commerson dolphins, striped dolphins, white-beaked dolphins, Pacific white-sided dolphins, dusky dolphins, Burmeister’s porpoise, both short and long finned pilot whales, Southern right whales, and a bowhead whale (Barnett et al., 2015).
The “tattoo” lesions are foci of hyperpigmented skin variously presenting as pinpoint, ring, and serpiginous patterns (Fig. 22.23A, B). Lesions are generally contiguous with the healthy skin surface. Pinpoint lesions have a dark center, which may be depressed relative to adjacent skin), and may be surrounded by a pale margin forming a target lesion. Lesions can occur anywhere on the body but the flippers and flukes are generally less affected. They often wax and wane over many months and years. Studies in captive bottlenose dolphins demonstrate a marked reduction in lesion intensity to the point of clearing the skin with warming water temperatures (S. DiRocco, personal communication). Work by Van Bressem et al. (2009) with free-ranging animals strongly suggests anthropogenic factors as promoting expression of infection. Tattoo lesions commonly occur adjacent to or directly associated with areas of skin damage from conspecific trauma (rakes). Histologically, there is an increase in melanocytes in the basal layer, cytoplasmic vacuolation in the stratum intermedium, and mild hyperplasia within the stratum externum of the skin. Scattered within the stratum intermedium are small round, eosinophilic, intracytoplasmic viral inclusion bodies that displace the nucleus. Occasionally, viral inclusions are present within nuclei. Unless there is associated skin damage (as with rakes), inflammation is not a feature of these lesions. In spite of the hyperplasia of the stratum externum, these lesions are not raised.
Figure 22.23.
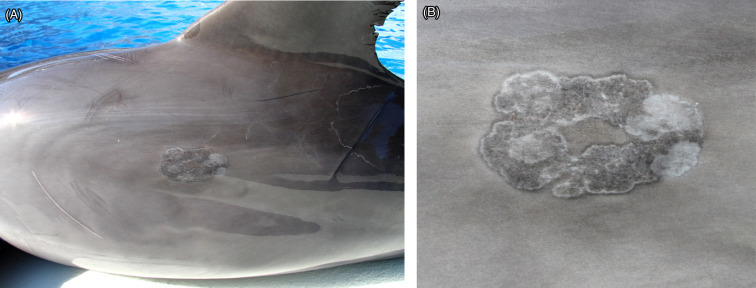
Poxvirus infection in the skin of a bottlenose dolphin.
(A) Infection takes the form of anastomosing cords and rings of pigmented skin in an irregular pattern (tattoo lesions), seen here, or as pinpoint dark foci along the trunk. The lesions typically come and go over time and are generally not raised. (B) Higher magnification.
Scanning and transmission electron microscopy (SEM and TEM, respectively) reveals particles with ultrastructural characteristics of both parapox and orthopoxviruses. Cetacean pox particles are arranged haphazardly within the epithelial cytoplasm. They are oval with a central core and an outer membrane and measure approximately 150–200 μm by 250–300 μm consistent with the phenotype of parapoxviruses. The surface morphology resembling a ball of yarn (as opposed to the orderly crisscross patterns of the parapoxviruses) is characteristic of the disorderly structure of an orthopoxvirus (Barnett et al., 2015, Geraci et al., 1979).
In both mysticetes and odontocetes, gammaherpesvirus infection has been associated with skin lesions, genital lesions, nephritis, encephalitis, and disseminated infections. Viral infections can also be detected in the absence of lesions. Cetacean herpesvirus infections can result in host immunosuppression and latent viral infections may be reactivated and possibly exacerbated by generalized debilitation or localized immunosuppression due to a number of supervening non-infectious and infectious disease processes. While many lytic and proliferative conditions have a clear association with herpesvirus infection, a specific causation has not yet been determined. Both herpes and papilloma virus have been identified in benign orogenital proliferative lesions and the contribution of these viruses not only to the development of benign papillomas but also progression to oral squamous cell carcinomas discussed under neoplasia.
Alphaherpes virus infections are seen much less frequently than gammaherpes viruses. Associated lesions vary from incidental and asymptomatic to acute, systemic necrotizing inflammation in multiple organ systems. Current herpes viral concerns and associated lesions in cetaceans are presented in Table 22.3 .
Table 22.3.
Herpes Virus Infections in Cetaceans
| Species | Herpes Subfamily | Location | Lesions |
|---|---|---|---|
| Beluga whale | Alpha | Genital/oral mucosa, skin | Necrotizing dermatitisa, j |
| Atlantic bottlenose dolphin | Alpha | Skin | No lesions describedb |
| Atlantic bottlenose dolphin | Alpha | Multiorgan | Necrotizing inflammationl |
| Striped dolphin | Alpha | Lymphoid System | Lymphoid depletionh |
| Blainville’s beaked whale | Alpha | Kidneys | Nephritise |
| Cuvier’s beaked whale | Alpha | Lymphoid System | Lymphoid necrosisg |
| Harbor porpoise | Alpha | Brain | Encephalitisf, i |
| Striped dolphin | Gamma | Genital mucosa/skin | Epithelial hyperplasiad |
| Atlantic bottlenose dolphin | Gamma | Genital and oral mucosa/skin | Epithelial hyperplasiab, c |
| Risso’s dolphin, dwarf sperm whale, Blainville’s beaked whale | Gamma | Genital mucosa/skin | Epithelial hyperplasiab, c, k |
Martineau, D., Lagacé, A., Béland, P., Higgins, R., Armstrong, D., Shugart, L.R., 1988. Pathology of stranded beluga whales (Delphinapterus leucas) from the St. Lawrence Estuary, Québec, Canada. J. Comp. Pathol. 98, 287–311.
Smolarek Benson, K.A., Manire, C.A., Ewing, R.Y., Saliki, J.T., Townsend, F.I., Ehlers, B., et al., 2006. Identification of novel alpha- and gammaherpesviruses from cutaneous and mucosal lesions of dolphins and whales. J. Virol. Methods. 136, 261–266.
Van Elk, C.E., van de Bildt, M.W., de Jong, A.A., Osterhaus, A.D., Kuiken, T., 2009. Herpesvirus in bottlenose dolphins (Tursiops truncatus): cultivation, epidemiology, and associated pathology. J. Wildl. Dis. 45, 895–906.
Sierra, E., Díaz-Delgado, J., Arbelo, M., Andrada, M., Sacchini, S., Fernández, A., 2015. Herpesvirus-associated genital lesions in a stranded striped dolphin (Stenella coeruleoalba) in the Canary Islands. J. Wildl. Dis. 51, 696–702.
Arbelo, M., Bellière, E.N., Sierra, E., Sacchinni, S., Esperón, F., Andrada, M., et al., 2012. Herpes virus infection associated with interstitial nephritis in a beaked whale (Mesoplodon densirostris). BMC Vet. Res. 8, 243.
Kennedy, S., Lindstedt, I.J., McAliskey, M.M., McConnell, S.A., McCullough, S.J., 1992. Herpesviral encephalitis in a harbor porpoise (Phocoena phocoena). J. Zoo Wildl. Med. 23, 374–379.
Arbelo, M., Sierra, E., Esperón, F., Watanabe, T.T.N., Bellière, E.N., Espinosa de los Monteros, A., et al., 2010. Herpesvirus infection with severe lymphoid necrosis affecting a beaked whale stranded in the Canary Islands. Dis. Aquat. Organ. 89, 261–264.
Soto, S., González, B., Willoughby, K., Maley, M., Olvera, A., Kennedy, S., et al., 2012. Systemic herpesvirus and morbillivirus co-infection in a striped dolphin (Stenella coeruleoalba). J. Comp. Pathol. 146, 269–273.
Van Bressem, M.F., Van Waerebeek, K., Raga, J.A., 1999. A review of virus infections of cetaceans and the potential impact of morbilliviruses, poxviruses and papillomaviruses on host population dynamics. Dis. Aquat. Org. 38, 53−65.
Bellehumeur, C., Lair, S., Romero, C.H., Provost, C., Nielsen, O., Gagnon, C.A., 2015. Identification of a novel herpesvirus associated with a penile proliferative lesion in a beluga (Delphinapterus leucas). J. Wildl. Dis. 51, 244–249.
Saliki, J.T., Cooper, E.J., Rotstein, D.S., Caseltine, S.L., Pabst, D.A., McLellan, W.A., et al., 2006. A novel gammaherpesvirus associated with genital lesions in a Blainville’s beaked whale (Mesoplodon densirostris). J. Wildl. Dis. 42, 142–148.
Blanchard, T.W., Santiago, N.T., Lipscomb, T.P., Garber, R.L., McFee, W.E., Knowles, S., 2001. Two novel alphaherpesviruses associated with fatal disseminated infections in Atlantic bottlenose dolphins. J. Wildl. Dis. 37, 297–305.
Papilloma viruses (PV) are associated with both proliferative mucosal and cutaneous lesions in free-ranging and captive cetaceans. Based on viral sequencing, currently there are at least five PV variants. As with many viruses of cetaceans, cetacean PVs demonstrate phylogenetic relatedness to PVs of artiodactylids (Gottschling et al., 2011). Serologic positivity to PV is high in free-ranging dolphins (90%) and common in captive dolphins (51%); ELISA reactivity is higher in males than females. The mean age of free-ranging dolphins with papillomas is ∼11 years; it is ∼30 years in captive dolphins. PV infection in bottlenose dolphins is common and the main route of transmission is likely horizontal with orogenital papilloma development early in life in certain free-ranging bottlenose dolphins (Rehtanz et al., 2010). PV-associated mucosal lesions are typical and an orogenital predilection with a tropism for vulvar and vaginal mucosa, penile mucosa, and oral or esophageal mucosa and frequent involvement of the frenulum of the tongue. While far less common, papillomatous proliferations in the skin of the body, have been seen in a variety of cetaceans including harbor porpoises, killer whales, and pilot whales. Lesions can be warty and/or plaque-like, singular or multiple, and coalescing. Histologic findings consist of epithelial hyperplasia producing finger-like or warty projections along a fibrovascular core. Within the hyperplastic epithelium, there is variable acanthosis and thickening of the stratum spinosum. The basement membrane remains intact.
RNA Viruses
Cetacean morbilliviruses (CeMV) are RNA viruses that were first recognized in 1988 following a series of mass cetacean and pinniped mortalities in Northwestern Europe. There are at least six distinct viral strains each of which is associated with a particular cetacean species. They include porpoise morbillivirus (PMV), first isolated from harbor porpoises, dolphin morbillivirus (DMV), first isolated from Mediterranean striped dolphins, and pilot whale morbillivirus (PWMV). Closely related to these three strains is beaked whale morbillivirus (BWMV). Additional, less well-characterized strains include isolation of CeMVs from a long-finned pilot, Longman’s beaked whale, Guiana dolphin and, Indo-Pacific bottlenose dolphin. Cetacean morbilliviruses have also caused several mass mortality events in odontocetes and mysticetes (Birkun et al., 1999; Di Guardo and Mazzariol, 2016, Van Bressem et al., 2014).
Morbillivirus transmission is thought to occur mostly by horizontal dissemination after inhalation of aerosolized virus shed by infected individuals. The close association of animals in cetacean pods makes transmission within a group through aerosols highly effective. The migratory behavior of cetaceans promotes transmission between groups (Rowles et al., 2011). CeMV infected females may also transmit the virus transplacentally to fetuses and to neonates during lactation. Chronically infected animals likely serve as reservoirs for the virus (Bossart, 2010). Morbilliviruses are lymphotropic, epitheliotropic, and neurotropic and they replicate in the lymphoid tissue before dissemination and infection of other cell types and organ systems. All are highly contagious and generally cause pneumonia and encephalitis with marked immunosuppression. Infection rates vary among populations.
Epidemiology of morbillivirus infections illustrates the episodic and contagious nature of the infections. Large unusual mortality events related to cetacean morbillivirus have occurred in many areas on repeated occasions (Duignan et al., 1996, Kemper et al., 2016, Lipscomb et al., 1994). DMV infection in the northern Gulf of Mexico (GoM) is sporadic and present at low levels. Confirmation of DMV infections and existing DMV titers demonstrate continued exposure to morbillivirus among the northern GoM cetaceans (Fauquier et al., 2010). A review of unusual mortality events in the GoM demonstrates a continued clinical impact on the population from this viral infection (Litz et al., 2014).
Gross lesions include pneumonia with pulmonary consolidation and congestion, and enlarged lymph nodes. Secondary infections as a consequence of profound immunosuppression are common and thus the presentation can vary based on the role of these additional pathogens. Secondary infections include Toxoplasma gondii, herpesviruses, opportunistic bacteria, such as Photobacterium damselae, Streptococcus phocae, and fungi including Aspergillus spp. Histologically, CeMV infection can be pancytopathic, but is primarily associated with lesions of the respiratory, lymphoid and central nervous system (CNS) organ systems. Interstitial pneumonia is characterized by necrosis of type I pneumocytes and bronchiolar epithelial cells, interstitial edema, type II pneumocyte hyperplasia, and formation of large syncytia in the alveolar and bronchiolar air spaces. Intracytoplasmic and intranuclear, eosinophilic viral inclusion bodies can be found in respiratory epithelia, bronchiolar gland epithelia, and syncytial cells. Generalized lymphoid depletion and edema with germinal center necrosis is common and syncytial cells (Warthin-Finkeldey type) are often prominent. The cortex of lymph nodes is generally the best site to identify inclusions within syncytia (Fig. 22.24 ); virus-laden cells are often difficult to identify in the face of opportunistic bacterial or fungal infections. Immunohistochemistry is very helpful in detecting these cells. Multifocal nonsuppurative meningoencephalitis with prominent perivascular cuffs, expansion of the meninges with edema, fibrin, hemorrhage and a mix of lymphocytes and plasma cells, and multifocal areas of demyelination characterizes brain lesions. Immunohistohemial (IHC) labeling using monoclonal antibodies (MoAb) to the hemagglutinin (H) glycoprotein of phocine distemper or neuraminidase (N) protein of canine distemper virus, or a rabbit polyclonal antiserum to rinderpest virus have been successfully used to diagnose CeMVs, and are valuable when tissue decomposition impedes histologic examination (Van Bressem et al., 2014).
Figure 22.24.
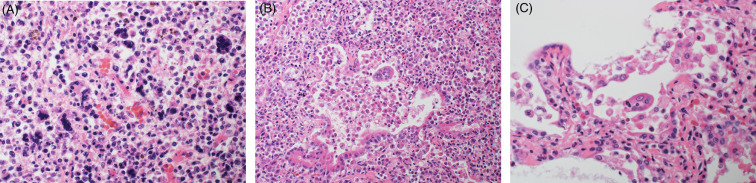
Cetacean morbillivirus in a bottlenose dolphin.
(A) Lymph node with marked disruption of normal architecture. Multiple syncytia are scattered throughout the tissue. (B) Lung with marked mononuclear inflammation. Within alveoli there is a mix of inflammatory cells, sloughed epithelium, and syncytia. (C) Higher magnification of alveolar debris demonstrating syncytia and an eosinophilic intranuclear inclusion.
(Photos Courtesy of D. Rotstein, Marine Mammal Pathology Service)
In cases in which epidemiology, clinical history, and gross necropsy and histologic examination are highly suggestive of CeMV infection, virus isolation using VERO or SLAM cells is confirmatory, and remains the gold standard diagnostic test. RT-PCR followed by sequencing can provide rapid CeMV confirmation and clade determination. Real-time RT-PCR (rtRT-PCR) targeting the hypervariable C terminal domain of the N gene provides rapid and reliable detection of dolphin and porpoise morbilliviruses. This test is very sensitive and specific for either DMV or PMV and does not crossreact with a number of other important morbilliviruses (Van Bressem et al., 2014). Serological studies including virus neutralization (VN) tests, plaque reduction (PR) assays and indirect enzyme-linked immunosorbent assays (iELISAs) are the main platforms for detecting antibodies against CeMV. Serology is useful for studying CeMV epidemiology, assessing population immune status before and after events and to identify new epidemics early in the disease course.
An atypical and more chronic CeMV presentation is characterized by profound lymphoid depletion and fatal secondary infections without typical morbillivirus lesions in the lungs and brain is recognized in stranded cetaceans. In these animals, morbillivirus can be detected in lymphoid tissues as well as in the hepatic sinusoidal endothelial cells and Kupffer cells, biliary epithelium, and myocytes in the tunica media blood vessels in the liver and mesenteric lymph nodes but not in the lungs or brain. If the pathogenesis of CeMV is similar to that of measles virus in humans, cetaceans that survive acute and subacute infection may show prolonged viral RNA persistence in the blood and lymphoid organs. Additionally, cetaceans that have cleared systemic DMV infection may develop lesions that localize only in the brain. Eosinophilic inclusions are only occasionally detected and syncytial cells are not observed. Neuronal processes have patchy positive immunostaining; some zones demonstrating almost no staining. In these cases, CeMV spread may be the result of cell-to-cell transmission rather than blood-borne infection. This CNS form shares histological characteristics with subacute sclerosing panencephalitis (SSPE) and old dog encephalitis (ODE), chronic latent localized infections in humans and dogs caused by defective forms of MV and CDV infections, respectively. Lesions are localized predominantly in the cerebral cortex, subcortical white matter, and the thalamus. Perivascular cuffing, diffuse gliosis, and glial nodules with neurophagia are the most prominent changes. Demyelination is less prominent than in dolphins with the acute or sub-acute infection. As in the human and canine presentations, antigen and viral RNA can be detected in dolphin brains but the virus is difficult to isolate. The mechanism of demyelination is undetermined but delayed antigen and RNA clearance from the CNS may be related to the reduced immune surveillance (Van Bressem et al., 2014).
The close phylogenetic genetic relationship between cetacean and ruminant morbilliviruses has led to the hypothesis that these pathogens may be derived from a common source. Cetaceans belong to the phylogenetic clade Cetartiodactyla. Several species in this clade are susceptible to rinderpest and PPR. It is therefore possible that interspecies transmission between a cetacean and another member of the Cetartiodactyla led to the development of distinct virus species that cycle exclusively in the marine environment. However, the presence of similar host proteins and cell receptors in cetaceans and artiodactyls may favor cross-species transmission.
Endogenous gammaretroviruses have been studied in both killer whales and bottlenose dolphins. While no direct disease associations have been made, anecdotal increases in the incidence of cancer and immunodeficiency states suggest a potential for viral activation and associated pathology. A survey of multiple cetacean species by PCR for gag, pol, and env gene sequences showed homologs of this virus in the DNA of eight species of delphinids, pygmy and dwarf sperm whales, and harbor porpoises, but not in screened beluga or fin whales. Analysis of the bottlenose dolphin genome revealed two full-length proviral sequences with 97.4% and 96.9% nucleotide identity to the killer whale gammaretrovirus (Lamere et al., 2009).
Bacteria
Erysipelothrix rhusiopathiae, the agent of erysipelas, is a common clinical concern for captive cetaceans and has been identified in stranded free-ranging cetaceans. The organism is readily isolated from the slime layer of food fish and the presumptive route of exposure is oral or perhaps though rake marks or ulcers in the skin (Van Bressem et al., 2008). Two forms of the disease exist, an acute fatal septicemic form, and a milder cutaneous form (Van Bressem et al., 2008). The acute presentation is associated with sudden death and few, if any, premonitory signs. On gross examination, these animals have ascites along with mottled livers. Histologically, there is moderate multifocal necrotizing lymphadenitis and hepatitis with a hepatic capsular edema, an influx of neutrophils with fewer mononuclear cells, and numerous intralesional Gram positive bacilli (Fig. 22.25 ). The milder, cutaneous form of the disease has been seen in display beluga whales and bottlenose dolphin calves. In these cases, a clear rhomboid shaped patch of skin discoloration and ulcerative dermatitis appears in association with a loss of appetite and an inflammatory leukogram (Fig. 22.26 ). These cases are responsive to antibiotics and the lesions resolve by regression with or without sloughing of the affected skin. Additionally, development of disease in calves from vaccinated dams in enzootic populations suggests a role of species-susceptibility or antibody transfer that is protective against the acute, septicemic form of the disease.
Figure 22.25.
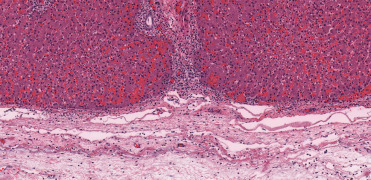
Acute erysipelas due to Erysipelothrix rhusiopathiae infection in the liver of a bottlenose dolphin.
Necrotizing periportal and capsular hepatitis is associated with marked capsular edema and expansion of the capsule and periportal regions by neutrophilic inflammation. This change is often associated acute ascites.
Figure 22.26.

Dermal erysipelas due to Erysipelothrix rhusiopathiae infection in the skin of a bottlenose dolphin.
A characteristic mild form of disease is rhomboid discolorationand/or ulceration comparable to "diamond skin disease" due to Erysipelothrix rhusiopathiae in pigs.
(Photo Courtesy of SeaWorld of Orlando)
Terrestrial Brucella spp. strains have not been identified in cetaceans; however, B. ceti, typically associated with infections in cetaceans is zoonotic and has caused bovine abortion by experimental challenge. Susceptibility to B. ceti appears to be worldwide in susceptible host populations, replicates in host macrophages and trophoblasts, and causes chronic disease in both baleen and odontocete cetaceans. B. ceti has been identified by culture or PCR in four cetacean families, and antibodies against Brucella spp. antigens have been detected in seven whale, dolphin and porpoise families. Brucella spp. exposure is common in free ranging populations, and a significant proportion of infected animals appear to overcome infection and may act as carriers and potential shedders (Guzmán-Verri et al., 2012).
As with many pathogens, the population prevalence of brucellosis in cetaceans varies by species and location. In Costa Rica, six cetacean species have antibody positivity to B. ceti and the organism was cultured from 70% of stranded striped dolphins (Hernández-Mora et al., 2017). A recent serologic survey in Italy (Profeta et al., 2015) identified no brucella antibodies in 70 cetaceans over a 15 year sampling of stranded animals. In a prior investigation in the western Mediterranean, three of three dolphins had brucellosis with a mix of lesions including neurobrucellosis in a striped dolphin, discospondylitis in a bottlenose dolphin, and a striped dolphin with no specific lesions. (Isidoro-Ayza et al., 2014).
Transmission of cetacean Brucella spp. likely involves three pathways. Brucellae are non-motile, labile in the environment, and are unlikely to persist for extended time periods outside the host. Based on extrapolation from terrestrial species, Brucella spp. may be transmitted by close physical contact between susceptible hosts by sexual intercourse, exhaled breath samples, exposure to expelled fluids and placental tissues during delivery, or during nursing. B. ceti have been isolated from the reproductive tracts and milk of infected cetaceans, and vertical transmission has been documented in fetus with in utero pneumonia (Colegrove et al., 2016). Brucella spp. have also been recovered from fish mucus and are capable of replication in these vertebrates. B. ceti also cycles in both nematodes and fish. This raise the possibility of transmission through vectors, such as Halocercus spp. and Pseudalius spp. lungworms in dolphins and porpoises, respectively, which can contain relatively large quantities of Brucella spp. Some of these parasites seem capable of crossing the placenta to affect another route of transmission from mother to the fetus (Guzmán-Verri et al., 2012).
Gross and histologic lesions of cetacean brucellosis can be wide-ranging in cases of septicemia and abscessation. However, the most important lesions are identified in the reproductive, CNS, and musculoskeletal systems. B. ceti has been isolated from the reproductive organs of both male and female cetaceans. In females, endometritis, placentitis and abortions due to brucellosis have been documented in bottlenose dolphins, harbor porpoise, and a striped dolphin. Placental lesions include multifocal necrosis, intense neutrophilic or mixed inflammatory infiltrates, submucosal edema, and multifocal, intralesional bacteria. Gram staining and IHC for Brucella antigens will highlight and localize the bacteria. Recently, metritis, placentitis, and fetal mortality due to B. pinnipedialis infection were identified in a pregnant Hector’s dolphin (Buckle et al., 2017). Additionally, Brucella spp. have been isolated from the mammary glands of sperm whales and dolphins, suggesting infection of resident macrophages in these organs.
Brucella spp.-associated epididymitis and orchitis have been described in adult male minke and Bryde’s whales, and in harbor porpoises. Infection is associated with testicular enlargement and a mix of abscesses and granulomas in the parenchyma. Histologically, granulomas are composed of macrophages admixed with and epithelioid or giant cells and contain central necrosis and mineralization. Scattered between the granulomas in a patchwork fashion are expanses of necrosis with neutrophils, fewer lymphocytes and plasma cells, and variable peripheral fibrosis (capsular formation).
Based on a case series review, the most common lesions of cetacean brucellosis were in the central nervous system. Affected species were harbor porpoises, white-beaked dolphins, white-sided dolphins, striped dolphins, bottlenose dolphins, and common dolphins. In these cases, cerebrospinal fluid (CSF) was increased in volume and cellularity, with ependymal cells and mononuclear leukocytes seen on CSF cytology; acquired hydrocephalus was a common sequela (Fig. 22.27A). Histologically, meningoencephalomyelitis was seen and characterized by nonsuppurative inflammation (Fig. 22.27B) and multifocal Purkinje cell degeneration, gliosis, satellitosis, spongiosis, and perivascular edema. Also seen multifocally were mild to moderate perivascular cuffing, fibrinoid degeneration, and necrosis with perivascular hemorrhage and edema. Expansion of inflamed tissues in the choriod plexus, leptomeninges, and the subependymal neuropil (periventriculitis) obstruct CSF flow and cause secondary hydrocephalus (Fig. 22.27A). Mononuclear choroiditis forming lymphoid follicles, periependymitis, and white matter and laminar necrosis and inflammation in the cerebrocortical gray matter have also been observed. Predilection sites are the cerebellum, brainstem, spinal cord, and medulla oblongata with less involvement of the cerebral cortex. Immunohistochemistry highlights Brucella species antigens in phagocytic cells.
Figure 22.27.
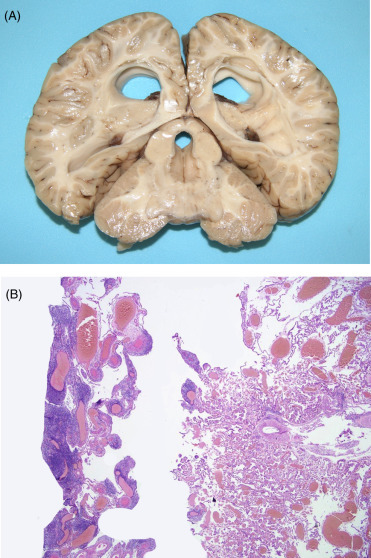
Brucellosis in the brain of a common dolphin.
(A) Obstruction with increased intracranial pressures secondary to infection and inflammation caused marked dilation of the ventricular system (hydrocephalus). (B) The meninges and choroid are markedly expanded by a mononuclear inflammatory cell infiltrate and edema; blood vessels are markedly dilated.
B. ceti has also been isolated from lesions in the bones and joints of cetaceans. Infections include a harbor porpoise with discospondylitis, a striped dolphin with fibrinopurulent osteoarthritis, and degenerative joint changes in a white-sided dolphin. Similar lesions are common in free-ranging bottlenose dolphins and in white-beaked dolphins. Additionally, cases of blubber abscesses and pneumonia have been associated with cetacean brucellosis, and vegetative valvular endocarditis with intralesional Brucella spp. positive macrophages has been described in a striped dolphin with neurobrucellosis and concurrent severe endocarditis (Guzmán-Verri et al., 2012). A level of clinical or pathologic suspicion for Brucella spp. infection in these less commonly reported lesions may focus laboratory studies to confirm infection and further characterize the natural history of cetacean brucellosis.
Atypical mycobacteriosis infections with panniculitis, pneumonia, and pleuritis have been reported in captive odontocetes; presumptive atypical mycobacterial lymphadenitis has also been reported in a stranded pygmy sperm whale. This group of bacteria is ubiquitous in nature and is associated with soil, detritus and water. Infections are via environmental exposure with percutaneous inoculation or secondary contamination of cutaneous lesions and animal to animal or animal to person infection is uncommon. Atypical mycobacteria do not have the ability to pass through the mucosa or the integument. Infections usually occur due to reduced host immunity or generalized debilitation. The unique anatomy of the cetacean respiratory tract may predispose to and possibly advance these infections.
Multiple case reports of atypical mycobacteriosis in cetceans currently exist but to date have not been compiled and reviewed. Notable cases include: a beluga with extensive ulcerative dermatitis, panniculitis, and chronic proliferative pleuritis with a marked thoracic histiocytic exudate due to Mycobacterium marinum infection (Bowenkamp et al., 2001); panniculitis with blubber pyogranulomas, necrosuppurative pneumonia and lymphadenitis due to M. chelonae in a bottlenose dolphin (Wunschmann et al., 2008); M. abscessus pyogranulomatous pneumonia and associated coughing and bloody respiratory discharge in a bottlenose dolphin (Clayton et al., 2012); and an untyped mycobacterial pyogranulomatous lymphadenitis in a pygmy sperm whale and infection in bottlenose dolphin secondary to net entanglement (Ladds, 2009). The ecologic diversity and spectrum of lesions identified in cetaceans with atypical mycobacteriosis suggest a complex natural history of the microbe, with a primary oral or respiratory route of exposure.
Nocardia spp. infections in wildlife, companion and production animals typically cause pyogranulomatous inflammation in a variety of organs, with an apparent predilection for the lung. In cetaceans, nocardiosis has been reported in Atlantic bottlenose dolphins, beluga whales and killer whales. The most common presentation is the systemic form, which involves two or more organs. Organs most frequently affect are the lung and thoracic lymph nodes, but infections can occur anywhere; infection in the brain is the site most commonly associated with death. Molecular identification and bacterial isolation have demonstrated a variety of pathogenic species in cetaceans including N. asteroides, N. farcinica, N. brasiliensis, N. cyriacigeorgica, and N. levis. Diagnosis is based on bacterial culture, gross and histologic findings, and molecular diagnostic tests. Cytology is particularly effective at identifying nocardial bacteria, which are branching, rod-shaped bacteria. The bacteria are Gram-positive, and positive with modified acid fast and Grocott’s methenamine silver (GMS) special stains, the latter of which most consistently demonstrates the characteristic organisms (Fig. 22.28 ). In both pinnipeds and cetaceans, juvenile animals are affected more often than adults (St Leger et al., 2009).
Figure 22.28.
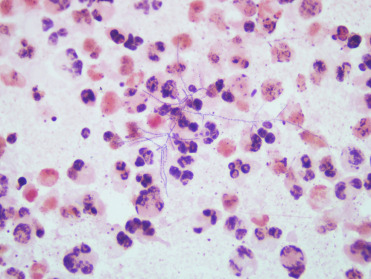
Nocardiosis in a beluga whale.
Cytology from a tissue impression demonstrating the clear beaded branching filaments of Nocardia spp. amid a sea of intact and degenerate neutrophils. Diff-Quik.
As with terrestrial animals, cetaceans harbor and occasionally develop fatal salmonellosis. Zoonotic transmission can occur and is most common in native cultures whose diets include consumption of porpoise, dolphin or whale meat. Salmonella species have been identified in the respiratory tracts of killer whales and harbor porpoises, and have been associated with perinatal mortality in a killer whale calf with sepsis (Colegrove et al., 2010). In stranded animals off of the Canary Islands, a dwarf sperm whale and common dolphin were emaciated and diagnosed with salmonellosis associated with enterotoxemic pathology (Arbelo et al., 2013). Salmonella enterica subspecies enterica is frequently found in the respiratory tract and other tissues of healthy and diseased harbor porpoises from northern European coasts. Ironically, this strain is most closely related to a strain identified in chickens in the United States in the 1950s. Reported infection rates are as high as 27% in harbor porpoises in the United Kingdom. Isolates are most commonly identified in lung tissue and transmission of via lungworm vectors has been proposed (Hasse et al., 2012).
Burkholderia pseudomallei, the agent of melioidosis, is an important pathogen of managed cetaceans and pinnipeds in Asia. Infections in Pacific bottlenose dolphins, Indo-Pacific bottlenose dolphins, killer whales, pseudorcas, and Pacific white-sided dolphins have been reported. It is likely that any marine mammal can contract this infection. The organism is indigenous to South-East Asia and Northern Australia, and is sporadically isolated in Europe, Central and South America, Africa, the Middle East, and India. The disease presents in two forms, an acute septicemic form and a chronic, debilitating granulomatous form. Clinical presentation and pathologic lesions depend on exposure load and the immune status of the animal at the time of infection. The organism has been isolated from air samples and inhalation, ingestion, or contamination of open wounds, are likely sources of infection. Reduced exposure to aerosolized bacteria in typhoon winds has been effective in mitigating clinical disease (Kinoshita, 2008).
The acute, disseminated form of meloidosis is by far the more common presentation in cetaceans. Grossly, multifocal, tan to yellow necrotic foci varying from pinpoint and military to several centimeters in diameter are present in multiple organs; concurrent splenomegaly and lymphadenomegaly with edema are also seen. These correspond histologically to small abscesses containing a mixture of hemorrhage, necrosis, and intact or degenerate neutrophils or poorly organized pyogranulomas with central necrosis, hemorrhage, fibrin, and edema admixed with intact and degenerate neutrophils surrounded by a mix of macrophages, lymphocytes and plasma cells. Acute infections in cetaceans most commonly affect the liver, spleen, and lung. In addition acute disease, chronic meliodosis with lumbar vertebral osteomyelitis has been reported in a bottlenose dolphin. In all cases, slender, Gram-negative bacteria are identified within the areas of necrosis (Kinoshita, 2008).
As with many animals, Staphlococcus spp. and Streptococcus spp. are common primary, opportunistic, or secondary infections in cetaceans. Of these, three require a brief overview. Streptococcus phocae and S.iniae are pathogenic non-β-hemolytic species. S. phocae can be cultured from many live stranded and dead pinnipeds. In cetaceans, S. phocae can be cultured from blowhole debris and fecal material of asymptomatic animals, and many isolates may reflect incidental opportunistic infection. Opportunistic infection in a case of morbillivirus coinfection was associated with fibrinonecrotic to pyogranulomatous dermatitis and panniculitis, embolic pneumonia, neutrophilic, and lymphoplasmacytic meningochoroiditis, random neutrophilic hepatitis, lymphoplasmacytic myocarditis and epicarditis, necrotizing adrenalitis, suppurative endometritis, and multicentric reactive lymphadenopathy; widespread intravascular coccoid bacterial emboli were present throughout the body (Díaz-Delgado et al., 2017).
Streptococcus iniae is a commensal and fish pathogen and exposure in free living and display cetaceans is likely from contaminated prey/feed. S. iniae is associated with generalized sepsis as well as localized infections such as pneumonia, pleuritis, pyothorax, dermatitis, myositis, and panniculitis. Lesions consist of a mix of acute necrotizing and more chronic, pyogranulomatous inflammation.
Staphlococcus aureus has caused sepsis in a killer whale and granulomatous inflammation in harbor porpoises. Lesions in stranded porpoises included pyogranulomatous myocarditis, necrotizing bronchopneumonia, pyelonephritis, osteomyelitis, leptomeningitis, and abscesses in lymph nodes and skeletal muscles. A captive porpoise had fibrinous and suppurative epicarditis and pyogranulomatous myocarditis with abscessation. Infection in porpoises has been suspected to have entered through skin lesions or the respiratory tract (Siebert et al., 2002). S. aureus associated tooth root abscessation and suppurative pneumonia and nephritis has also been described in a dead stranded killer whale (Power and Murphy, 2002). Because S. aureus has been isolated from blow samples from free-ranging killer whales, associated disease and pathologic findings to date likely represent opportunistic infection.
Mycoplasmosis has been associated with upper respiratory, oral, and otic infections in harbor seals and polyarthritis in sea lions; reports of cetacean infections are scant and infections much less well identified, characterized, or associated with disease. Mycoplasma phocicerebrale was isolated from the lungs of three harbor porpoises and the liver of one of these animals. Novel Mycoplasma spp. have been isolated from the lungs of five harbor porpoises and kidney of another, and an isolate closely related to Mycoplasma species 13CL was obtained from the kidney of a Sowerby’s beaked whale. No associated lesions were identified in any of these cases (Foster et al., 2011).
Bartonellosis has been reported in multiple cetacean species (Harms et al., 2008). These include free-ranging striped dolphins, harbor porpoises, Risso’s dolphins, a pygmy sperm whale and captive bottlenose dolphins and beluga whales. Sequencing identified a Bartonella spp. most similar to strains of B. henselae. No specific pathology has been associated with these infections. Angiomatosis, an important pathologic manifestation of Bartonella spp. infection in immunocompromised humans, has been described in stranded bottlenose dolphins and hunter harvested beluga. To date, a similar association in cetaceans has not been described.
Severe myositis due to infections with Clostridium spp. has been diagnosed in captive killer whales, pilot whales, and bottlenose dolphins. All marine mammals are likely susceptible; however, exposure is likely sporadic with a low incidence of clinical disease or pathology. The disease is characterized by acute swelling, muscle necrosis, and accumulation of gas in affected tissues and is accompanied by a severe clinical leukocytosis, serofibrinous exudate, and acute hemorrhage. Typical gross lesions include acute necrotizing myositis with dark discoloration and a general dryness of the muscle. Histologically, areas of acute muscle degeneration and necrosis often contain clear spaces due to gas production by the bacteria. Diagnosis of clostridial infection is based on detection of Gram-positive bacilli in aspirates and can be confirmed by anaerobic culture, florescent antibody screening for the bacterium, and multiplex-real time PCR. As a reminder, prosectors are reminded that microcavitations or clear bubbles in tissues is not pathognomonic for clostridial myositis and intramuscular and occasionally intravascular bubbles are often present in decomposed carcasses due to postmortem overgrowth of Clostridium spp. and do not necessarily indicate a pathologic infection or an antemortem disease process.
Fungi
Lobomycosis in humans is caused by the fungus Lacazia loboi and a morphologically similar infection in dolphins was historically attributed to this same pathogen. However, recent molecular studies have identified Paracoccidioides brasiliensis (Paracoccidioidomycosis ceti) as the causative organism (Vilela et al., 2016). This distinction may account for subtle ultrastructural distinctions in yeast morphology previously reported between dolphin and human cases. Dolphin infections are most commonly reported from Florida (United States) to the Gulf of Mexico and extend south into South America. Recent reports from Asia are suggestive of infections with a morphologically similar organism. Gross lesions tend to occur along the torso but may occur anywhere on the body, and consist of focally extensive to disseminated, smooth, cutaneous swellings that progress to papules, nodules, plaques, verrucae, and occasional ulcerations (Fig. 22.29A). Microscopically, the dermis is expanded and effaced by myriad, 6–10 μm diameter, round, thick-walled, PAS, and GMS positive (mucicarmine negative) yeast that form single to multiple budding cells or chains interconnected by a tubular isthmus. As with cryptococcosis, melanin may accumulate in or be associated with the cell wall and is considered a virulence factor, protective against oxidative stress. Yeast are embedded within lymphohistiocytic infiltrates admixed with numerous foreign body and Langhan’s type multinucleated giant cells, fewer plasma cells and occasional neutrophils (Fig. 22.29B). The yeast are slow growing and depending on the stage of infection and degree of dermal infiltrate, pseudoepitheliomatous hyperplasia, acanthosis, and parakeratotic hyperkeratosis may also be seen; expansile dermal lesions, atrophy of the overlying epidermis with attenuation or loss of rete ridges and ulceration may also occur. Opportunistic bacterial infection is associated with loss of epidermal integrity. Regional lymph node involvement has been reported, but infection is usually limited to the skin and can persist in a nonprogressive state for many years. The mode of infection is believed to be direct penetration and subsequent proliferation in the dermis.
Figure 22.29.
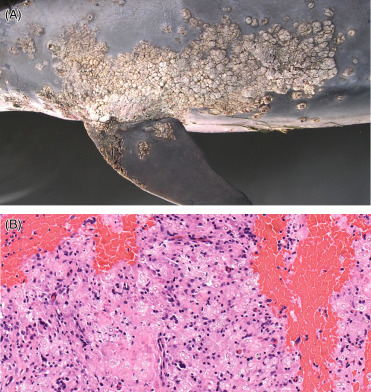
Cutaneous lobomycosis in a bottlenose dolphin.
(A) Multifocal to coalescing patches of proliferative dermatitis are present along that body wall and flipper. (B) Numerous chains of budding yeast are present within mixed pyogranulomatous inflammation.
(Photos Courtesy of Hubbs-SeaWorld Research Institute)
Zygomycosis refers to infection with members of a diverse group of fungi (class Zygomycetes) in one of its two orders, the Mucorales and Entomophthorales. The most common fungi associated with mucormycosis are Mucor spp., Rhizopus spp., Rhizomucor spp., Absidia spp., Aphophysomyces spp., and Saksenaea spp. (Abdo et al., 2012; Bossart et al., 2017; Higgins, 2000). All are distributed worldwide and typically associated with soil and detritus. Infections are most commonly acquired through inhalation, ingestion or percutaneous inoculation of spores. The fungi are angioinvasive and fungemia can result in disseminated infections. They are broad to ribbon like, have thin hyaline walls, are pauciseptate and nondichotomously branching. The hyphae are prone to twisting and folding. Vascular infections are associated with necrosis, vasculitis, perivascular hemorrhage, thromboembolism, ischemia, and infarction. An inflammatory response may be absent or consist of suppurative, pyogranulomatous, or granulomatous infiltrates. Individual case reports of localized dermatitis, cellulitis or myositis and disseminated mucormycosis (zygomycosis) have been documented in sporadic wild stranded and managed cetaceans; mucormycosis with pulmonary dissemination has also been reported secondary to satellite tag deployment in a southern killer whale. Entomophthoramycosis is generally limited to tropical regions and involves one of two genera, Basidiobolus spp. and Conidiobolus spp. Infections are more chronic, indolent and can arise through inhalation or direct inoculation of spores from decaying plant material, soil and leaves. Host animals are generally immunocompetent. In contrast to mucormycosis, fungi tend not to invade blood vessels and infections rarely disseminate from the primary site of infection. The hyphae are broad and pauciseptate with 90 degree branching. Skin and subcutaneous infections result in localized swelling, which may be fluctuant to firm. Microscopically there is granulomatous inflammation with foreign body multinucleated giant cells and Splendore-Hoeppli material. Eosinophils often accompany the inflammation and eosinophilic microabscessation may occur.
Aspergillus spp. are ubiquitous molds associated with sporadic respiratory infections in wild stranded and display managed cetaceans. In most cetacean cases, Aspergillus fumigatus has been isolated or identified by molecular studies, although A. niger and A. terreus have also been reported (Reidarson et al., 1999). Infections are generally opportunistic, acquired by inhalation with deposition of spores deep in the respiratory tract. From an epidemiologic perspective, aspergillosis in free-ranging cetaceans is often associated with morbillivirus co-infection. Initial stages of infection with airway oriented fungal hyphae are associated with invasion and necrosis in subjacent parenchyma. Gross examination reveals well delineated, grey nodules in the parenchyma with hemorrhagic margins or large cavitations composed predominantly of fungal hyphae. Dissemination to other tissues, such as the brain, kidney, and eye occur secondary to vascular invasion. Tracheal involvement with circumferential to segmental disruption and effacement of the respiratory mucosa by fibrosis and granulomatous infiltrates and variable luminal occlusion has been reported in four captive bottlenose dolphins (three with aspergillosis and one with possible zygomycosis). Severe mycotic otitis media due to A. terreus has also been described in a juvenile live stranded harbor porpoise from the United Kingdom. On gross exam, green yellow purulent exudate and caseous debris was noted in both tympanic cavities and the periotic sinuses; histologically, a large fungal aggregate was present in the tympanic cavity. Osteolytic changes were evident in the adjoining periotic bone and stapes, and small numbers of lymphocytes, plasma cells, neutrophils overlaid the scala vestibuli with granulation tissue, serous exudate, and pseudocyst formation associated with the round and oval windows. The lesions in conjunction with generalized emaciation were considered sufficiently severe to account for the live stranding of this animal. A similar presentation was identified in a by-catch harbor porpoise.
Obstructive tracheitis has been reported in four captive bottlenose dolphins (Delaney et al., 2013). The lesion consisted of severe, segmental, and circumferential fibrosing tracheitis with partial to total luminal obstruction. The tracheal cartilage, submucosa, and mucosa were distorted and replaced by extensive fibrosis and pyogranulomatous inflammation centered on fungal hyphae. The fungi associated with this lesion included Aspergillus spp. and zygomycetes identified on culture (Fig. 22.30 ).
Figure 22.30.
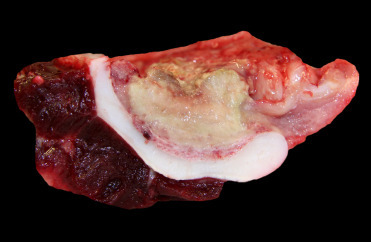
Obstructive tracheitis in a bottlenose dolphin.
Severe tan/yellow, pyogranulomatous tracheal inflammation and fibrosis cause partial occlusion of the tracheal lumen (tracheal cartilage is white and crescent shaped). Mucor sp. was isolated from the lesion.
Both Cryptococcus neoformans and C gattii are yeast with prominent mucinous capsules and tropism for the lung and lymph nodes. Infections are acquired predominantly via the respiratory tract with subsequent hematogenous dissemination. C. neoformans occurs worldwide and clinical disease tends to develop in immunosuppressed or debilitated individuals; C gattii is limited to tropical and subtropical regions, largely paralleling the distribution of eucalyptus trees, and reported human infections in the northeastern Pacific tend to involve immunocompetent individuals with a history of outdoor recreational activities (Fenton et al., 2017, Kidd et al., 2004). Dead stranded cetaceans with cryptococcosis typically present with pneumonia and lymphadenitis. In dolphins and porpoises, the lack of more significant involvement of the eyes and brain may be attributed to the blowhole, vestibulum, nasal sacs, lack of paranasal bone sinuses, and nasal plugs, which regulate air flow to the larynx. The nasal cavity does not have distinct sensory epithelia or olfactory innervation, prime routes of retrograde fungal invasion to the calverium in terrestrial species.
The first multispecies outbreak of C. gattii was recognized in the Pacific Northwest and western Canada in 1998. Infections involved humans, companion animals, exotic birds, terrestrial wildlife, and among the first animals diagnosed with C. gattii infection was a harbor porpoise that stranded along the southeast coast of Vancouver Island, BC. In the Pacific Northwest, C gattii is the primary fungal pathogens recovered from stranded cetaceans and predominantly occurs in harbor porpoises, Dall’s porpoises, and rarely in Pacific white sided dolphins. Gross lesions include generalized emaciation, pulmonary consolidation, and generalized lymphadenopathy. Pulmonary lesions vary from single large, tan yellow to gray red homogeneous and occasionally centrally friable nodules that protrude above the pleural surface and extend deep into and occasionally span the entire lung lobes. In more severe infections, the nodules are miliary or become confluent with secondary bacterial and verminous pneumonia. Prominent rib impressions may be apparent. Regional lymph nodes are markedly enlarged (up to 5–10 times normal size) and on cut surface may be homogenous, glistening yellow white, and ooze clear gelatinous material or multinodular, pale gray white to brown red and associated with inflammatory infiltrates, necrosis, hemorrhage, and cavitation. Microscopically, in the more acute stages of infection, pulmonary bronchoalveolar spaces are distended and occluded by dense aggregates of encapsulated yeast with sparse to mild infiltrates of lymphocytes and histiocytes. With progression of inflammation to more chronic stages, there is nodular to diffuse accumulations of granulomatous inflammation with scattered Langhan’s type multinucleated giant cells, intra and extracellular yeast, and occasional fibrous encapsulation. Similar transition from small aggregates to variably extensive sheets of yeast is noted in the subcapsular and medullary sinuses of regional lymph nodes where yeasts are often admixed with mild lymphohistiocytic infiltrates. In more fulminant disease, evidence of multisystemic fungemia may be seen in the brain, kidney, prostate, or mammary glands, in developing feti and in other tissues. In contrast to terrestrial species, adrenal gland involvement tends to be localized to the medulla, rather than the cortex. The cell walls of organisms are best highlighted with methenamine silver and Fontana-Masson stains, and the capsule stains red with mucicarmine and blue with Alcian blue stains. Although fungal immunohistochemistry is available, fungal culture and isolation with molecular genotyping yields more valuable information.
In marine mammals, coccidiodomycosis is most frequently documented in California sea lions and southern sea otters. Sporadic cases in bottlenose and common dolphins have been identified in California. The most significant findings on necropsy are milliary nodules with central caseous necrosis throughout the lung and perihilar lymph nodes. Histologic lesions are those of pyogranulomatous pneumonia and lymphadenitis with intralesional fungal arthroconidia and immature and mature spherules, the latter of which are 60–80 μm spherule diameter with a characteristic, double contoured, refractile walls and 5–7 μm diameter intralumenal endospores (Fig. 22.31 ). Organisms can also be identified in the brain in areas of necrotizing encephalitis.
Figure 22.31.
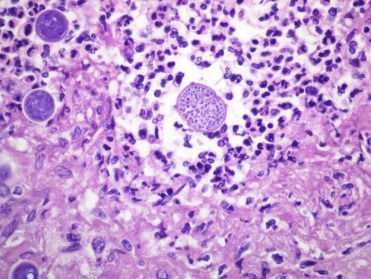
Coccidioides immitis infection in the brain of a common dolphin.
Two immature spherules are in the left of the field. A mature spherule, in the right of the field, contains numerous endospores and is surrounded by necrosis and neutrophilic inflammation.
Metazoa
As with all free-ranging wildlife, parasitic infections in cetaceans are common. While many are subclinical, secondary infections and inflammatory changes are likely related to the extent of host-parasite adaptation and the host health status. In addition to the descriptions below, an overview of cetacean parasites is presented in the Supplemental Materials Table e4. A few parasitic infections of note are discussed.
Anisakis simplex is a gastric nematode parasite of cetaceans. It is associated with crater-like ulcerations typically of the nonglandular forestomach in bottlenose dolphins from Florida and harbor porpoises throughout the western seaboard of North America and Europe. In addition to the forestomach, these parasites can be distributed within any of the three gastric compartments and can be free within the gastric and intestinal lumena. Lesions can vary from multiple ulcerations less than 1 cm in diameter to large, 3–5 cm proliferative and fibrosing nodules with superficial ulcerations and numerous intralesional nematodes embedded within the ulcerated mucosa and extending into the submucosa. Microscopically, marked gastritis with lymphocytic, plasmacytic, eosinophilic and granulomatous inflammation with giant cells, hemosiderosis, fibrosis and areas of necrosis are associated with the parasites. Degenerate and necrotic parasitic remnants with varying degrees of fibroplasia may be seen deep in the submucosa. Ulcers can be associated with gastric hemorrhage; perforation is uncommon. The larvae of this parasite are present within the muscles of numerous marine fish.
Braunina cordiformis is a digenic trematode of small delphinids. As a gastric parasite, it presents a very differently than Anisakis spp. infections. Braunina spp. trematodes are commonly found within a mat of mucus attached to the surface of the glandularcompartment (second chamber) of the stomach (Fig. 22.32A, B). Occasionally, organisms will attach to the mucosa of the gastric pylorus and duodenal ampula. The parasite produces a small focus of chronic gastritis at the site of mucosal attachment to the mucosa. While the attachment does not appear to be associated with significant disease, heavy parasitism can be so intense that extensive areas of the gastric mucosa (more than 50%) may be involved and covered by associated mucus secretion. When parasites are primarily within the second gastric chamber, animals should be evaluated for signs of maldigestion associated with a reduction in the effective gastric glands. Evaluation of prey species of affected dolphins may be helpful in determining the alternate host of this parasite.
Figure 22.32.
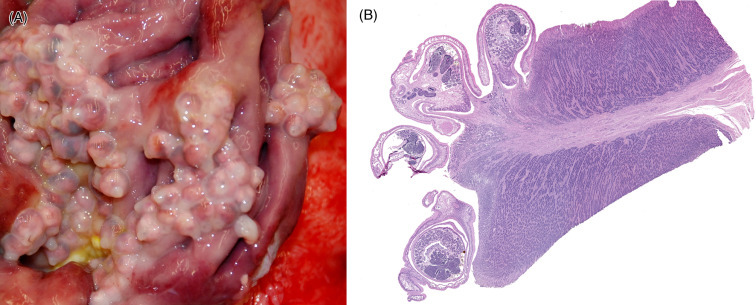
Braunina sp. infection in the glandular stomach of a bottlenose dolphin.
(A) The mucosa is covered by numerous trematodes, which appear as translucent to opaque white nodules, covered by an overlying mucous layer. (B) Braunina trematodes attached to the gastric mucosa are associated with very little, if any, associated inflammation.
(Part A: Photo Courtesy of Hubbs-SeaWorld Research Institute)
Nasitrema globicephalae and other species of this genus are trematodes that inhabit the pterygoid sinuses and tympanic cavities of odontocetes. The life cycle of this parasite is likely complex, and infection is most likely acquired through consumption of infected fish (prey items) that contain the larval stages. Aberrant migration of this parasite can result in neuritis of the eighth cranial nerves, otitis media, and marked meningoencephalitis. Migration tracts in the both gray and white matter of the brain are associated with necrosis and cavitations, extensive areas of hemorrhage, edema, and an influx of gitter cells, degenerate neutrophils and eosinophils, lymphocytes, plasma cells, and intralesional black birefringent material (fluke pigment). Within the lesions, sections of adult trematodes and thick walled, golden-brown, triangular eggs approximately 50 μm in diameter are occasionally surrounded by multinucleated giant cells. Adult trematodes are 500 μm wide with a 15 μm thick, spiny tegument and contain numerous vitellaria, paired ceca, and testes, all surrounded by parenchyma. Spongiosis and gliosis may occur within the adjacent neural tissue, and the meninges and perivascular spaces are expanded by edema, fibrin, lymphocytes, plasma cells, eosinophils, and fewer neutrophils and hemosiderin-laden macrophages (Fig. 22.33 ). A study published in the mid-1980s found a high percentage of the cetaceans stranded along the Southern Californian coastline with massive infestation of Nasitrema spp., with mature, gravid (egg-bearing) helminths in the brain tissue (Cowan et al., 1986). Because of the extent and severity of encephalitis and necrosis of the neuropil, researchers have inferred that the brain lesions were a contributing factor to the stranding of small cetaceans. Interestingly, examinations of stranded cetaceans in this same region since 2000 have demonstrated a very low incidence of this condition despite thorough neuropathology exams. This shifting prevalence may reflect a change in the dietary preferences or prey availability or distribution in this region.
Figure 22.33.
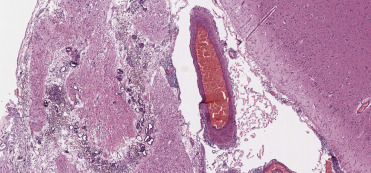
Nasitrema globicephalidae meningoencephalitis in a common dolphin.
The meninges are markedly expanded by a mixed inflammatory cell infiltrate. Within the inflammation and extending into the subjacent neuropil are scattered brown, thick shelled trematode eggs. The wide, curved pattern of inflammation and intralesional eggs suggests a migration track of an adult parasite through the brain.
Protozoa
Toxoplasmosis is due to Toxoplasma gondii is a sporadic, but significant cause of nonsuppurative meningoencephalitis, disseminated parasitemia, necrotizing placentitis, and abortion in cetaceans. Infections have been reported in a number of New Zealand Hector’s and critically endangered Maui dolphins. One study identified death in 7 of 28 (25%) dolphins due to disseminated toxoplasmosis, including two of three Maui dolphins. A further ten dolphins had one or more tissues that were positive for the presence of T. gondii DNA using PCR. Fatal cases had intralesional protozoal zoites and cysts in areas of necrotizing and hemorrhagic pneumonia, lymphadenitis, hepatitis, and adrenalitis (Fig. 22.34A, B). Tachyzoites and tissue cysts were present in other organs including the brain, heart, stomach, and uterus with minimal associated inflammatory response. One dolphin had a marked suppurative metritis with numerous intraepithelial tachyzoites (Roe et al., 2013). Two of eight striped stranded dolphins in late 2007 on the Ligurian Sea coast of Italy had nonsuppurative meningoencephalitis characterized by prominent perivascular mononuclear cell cuffing and macrophage accumulations in the neuropil. These lesions were associated with mild lymphocytic and plasmacytic inflammation in the choroid plexus in one dolphin. Toxoplasma gondii cysts and zoites, confirmed by immunohistochemical labeling, were scattered throughout the brain parenchyma (Di Guardo et al., 2009). Similarly, two Atlantic spotted dolphins were diagnosed with nonsuppurative meningoencephalitis due to toxoplasmosis in the Canary Islands (Arbelo et al., 2013). As with the Hector’s and Maui dolphins, the toxoplasmosis “hot spots” may suggest excessive marine contamination from terrestrial run-off, sexual recombination of parasites with emergence of hypervirulent clones, or possible occult morbillivirus infections.
Figure 22.34.
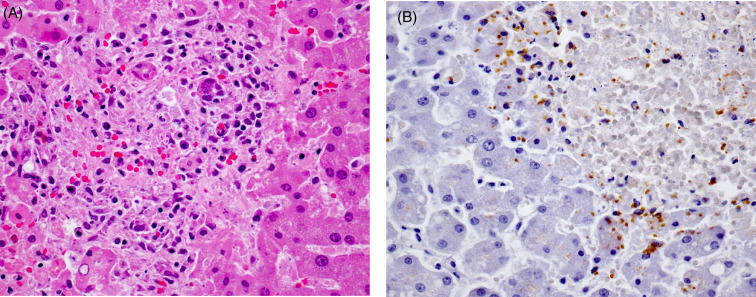
Hepatic toxoplasmosis in a Hector’s dolphin.
(A) Focal necrotizing hepatitis with a mixed inflammatory response surrounds a single enlarged hepatocyte containing a protozoal cyst with multiple tachyzoites. (B) Immunohistochemical labeling at the edge of a necrotic focus contains moderate numbers of brown, reactive zoites.
(Photos Courtesy of W. Roe, Massey University)
Sarcocystis neurona is associated with lesions and mortality in cetaceans from the Pacific Northwest. Microscopic examination of a Pacific white-sided dolphin revealed marked, nonsuppurative and necrotizing meningoencephalitis with perivascular lymphoplasmacytic cuffing, scattered gliosis, and intralesional protozoal schizonts and dispersed merozoites. In contrast, a harbor porpoise presented with moderate interstitial pneumonia, peribronchial lymphofollicular hyperplasia and florid, predominantly intravascular accumulations of protozoa, mild nonsuppurative encephalitis, cholangiohepatitis, myocarditis, and tonsillitis. In both cases, immunohistochemistry was strongly positive for Sarcocystis neurona. Tissue exams of Atlantic white-sided dolphins performed by Ewing et al. (2002) demonstrated both skeletal muscle and cardiac incidental Sarcocystis spp. cysts in 39% of animals from two mass stranding events. Similar cysts have been seen in stranded beluga.
Ectoparasites
Cyamids (Fig. 22.35 ) are external amphipod parasites of cetaceans that infest both baleen whales and much less frequently, odontocetes. They are a common commensal and do not suggest specific disease. They are referred to as “whale lice” but they are not insects. They can be found anywhere on the skin. Typically, they reside in genital folds, ventral pleats, nostrils, are associated with callosities and barnacles, and occur at the periphery of the eyes and within and around skin wounds. The primary “party fact” of cyamids is that each whale species generally has its own species of cyamid parasite. They do not experience a free-swimming phase and infections are transferred by contact. The parasites spread from dams to their calves during birth, nursing, and general contact. In the sperm whale, the parasitic relationship is sex-specific. Cyamus catodontis lives exclusively on the skin of the male, while Neocyamus physeteris is found only on females and calves. Cyamids consume the algae and sloughing superficial skin of their hosts. The rapid turnover of cetacean skin provides ample support for hundreds, even thousands of these organisms on individual whales.
Figure 22.35.

Cyamids on the skin of a North Atlantic right whale.
Often called “whale lice,” cyamids are not insects. They are amphipod parasites.
(Photo Courtesy of Hubbs-SeaWorld Research Institute)
E-Slides
-
22.e1
Normal lung, common bottlenose dolphin, lung. The top three sections are normal cetacean lung tissue. Normal cetacean lung has unique histologic features including cartilage extending into the level of the bronchioles, smooth muscle within the walls of the bronchioles forming a system of “valves” and a double layer of epithelium sandwiching the alveolar capillaries. (see Fig. 22.3). eSlide: VM04950
-
22.e2
Hepatic lipidosis, bottlenose dolphin, liver. This slide contains liver, kidney and lung from a young bottlenose dolphin. Sections of liver demonstrate diffuse clear vacuoles within the hepatic cytoplasm. (see Fig. 22.8). eSlide: VM05221
-
22.e3
Lysosomal storage disease, beluga whale, brain (cerebrum). Within the brain there is moderate to severe neuronal and glial cell distention with granular to vacuolar pale tan cytoplasmic material. Affected cells are oval to round due to cytoplasmic distention. There are a few areas of perivascular edema and scattered meningeal vessels are occluded with foamy histiocytic cells. (see Fig. 22.10). eSlide: VM05224
-
22.e4
Barotrama, Intravascular and parenchymal bubbles, common dolphin, liver and adrenal gland. Tissues demonstrate a mix of multifocal clear dilation of blood vessels and lymphatics along with clear spaces within the parenchyma with no marginal capsule. In this slide, these areas are not associated with hemorrhage although in many cases, this is an associated change. (see Fig. 22.16). eSlide: VM05225
-
22.e5
Pulmonary angiomatosis, bottlenose dolphin, lung. Pulmomary angiomatosis in the lung of a stranded bottlenose dolphin. There is a moderate to marked proliferation of small vessels with thick walls arranged in a haphazard pattern disrupting the bronchiole and replacing the pulmonary parenchyma. (see Fig. 22.20). eSlide: VM05226
-
22.e6
Genital papilloma, bottlenose dolphin, penis. There is irregular proliferation of the epithelium along papilliform fibrovascular changes in the submucosa. In these cases, the basement membrane remains intact and a change in size over time is not generally appreciated. (see Fig. 22.21). eSlide: VM05227
-
22.e7
Oral squamous cell carcinoma, bottlenose dolphin, oral mucosa. There is irregular proliferation of epithelial cells forming islands that extend beneath the basement membrane of the oral mucosa. Islands often demonstrate central necrosis and keratin formation. Blood vessels can contain neoplastic thrombus. (see Fig. 22.22). eSlide: VM05228
-
22.e8
Pox virus infection, bottlenose dolphin, skin. The skin contains a shallow, focal cavitation with surface erosions and generalized irregularity. There is prominent intracellular edema and vacuolation with nuclear margination primarily of the mid-level epithelial cells as they extend down rete pegs. Similar changes, including distinct eosinophilic cytoplasmic inclusions (Guarnieri-like) are present in epithelium at the base of epidermal pages and to adjacent epidermis. (see Fig. 22.23). eSlide: VM05229
-
22.e9
Erysipelothrix, bottlenose dolphin, liver. Within the liver isacute periportal and capsular necrotizing hepatitis. There is marked capsular edema with neutrophils expanding the capsule and periportal regions. This change is often associated a marked acute ascites. (see Figure 22.25, Figure 22.26). eSlide: VM05246
-
22.e10
Lobomycosis, bottlenose dolphin, skin. Abundant chains of budding yeast are present within a mixed pyogranumatous inflammatory reaction. (see Fig. 22.29). eSlide: VM05247
-
22.e11
Braunina, bottlenose dolphin, stomach. The gastric mucosa is covered by trematodes with an overlying mucous layer. There is very little associated inflammation. (see Fig. 22.32). eSlide: VM05250
-
22.e12
Hyaline intracytoplasmic inclusions, killer whale, liver. Without evidence of a viral infection, it is most likely that this material represents aggregates of consolidated microtubes and is not indicative of a viral infection. (see Fig. 22.7). eSlide: VM05283
-
22.e13
Adrenocortical hyperplasia, killer whale, adrenal gland. There is irregular expansion of all layers of the adrenal cortex. The hyperplastic changes result in enlargement of the gland as well as an undulating pattern to the parts of the cortex. (see Fig. 22.14). eSlide: VM05284
-
22.e14
Nocardia, Beluga whale, skin. The skin contains, moderate, diffuse epidermal hyperplasia and moderate to severe purulent tp pyogranulomatous dermatitis. There is a mix of mostly intact and some degenerate neutrophils and occasional histiocytic macrophages amidst areas of hemorrhage within the dermis. Multifocal vasculitis and necrosis are also seen. (see Fig. 22.28). eSlide: VM05285
References
- Abdo W., Kakizoe Y., Ryono M., Dover S.R., Fukushi H., Okuda H., Kano R., Shibahara T., Okada E., Sakai H., Yanai T. Pulmonary zygomycosis with Cunninghamella bertholletiae in a killer whale (Orcinus orca) J. Comp. Pathol. 2012;147:94–99. doi: 10.1016/j.jcpa.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Arbelo M., Los Monteros A.E., Herráez P., Andrada M., Sierra E., Rodríguez F., Jepson P.D., Fernández A. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999–2005) Dis. Aquat. Organ. 2013;26(2):87–99. doi: 10.3354/dao02558. [DOI] [PubMed] [Google Scholar]
- Barnett J., Dastjerdi A., Davison N., Deaville R., Everest D., Peake J. Identification of novel cetacean poxviruses in cetaceans stranded in South West England. PLoS One. 2015;10(6):e0124315. doi: 10.1371/journal.pone.0124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkun A., Jr., Kuiken T., Krivokhizhin S., Haines D.M., Osterhaus A.D. Epizootic of morbilliviral disease in common dolphins (Delphinus delphis ponticus) from the Black Sea. Vet. Rec. 1999;144:85–92. doi: 10.1136/vr.144.4.85. [DOI] [PubMed] [Google Scholar]
- Bossart G.D. Morbillivirus infection in free-ranging Atlantic bottlenose dolphins (Tursiops truncatus) from the Southeastern United States: seroepidemiologic and pathologic evidence of subclinical infection. Vet. Microbiol. 2010;143(2-4):160–166. doi: 10.1016/j.vetmic.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Bossart G.D., Ghim S., Rehtanz M. Orogenital neoplasia in Atlantic bottlenose dolphins (Tursiops truncatus) Aquatic Mammals. 2005;31(4):473–480. [Google Scholar]
- Bossart G.D., Fair P., Schaefer A.M., Reif J.S. Health and environmental risk assessment project for bottlenose dolphins Tursiops trnucatuus from the southeastern USA. I. Infectious disease. Dis. Aquat. Organ. 2017;125(2):141–153. doi: 10.3354/dao03142. [DOI] [PubMed] [Google Scholar]
- Bossart G.D., Hensley G., Goldstein J.D., Kroell K., Manire C.A., Defran R.H., Reif J.S. Cardiomyopathy in stranded pygmy (Kogia breviceps) and dwarf (Kogia sima) sperm whales. Aquat. Mamm. 2007;33(2):214–222. [Google Scholar]
- Bossart G.D., Odell D.K., Altman N.H. Cardiomyopathy in stranded pygmy and dwarf sperm whales. J. Am. Vet. Med. Assoc. 1985;187(11):1137–1140. [PubMed] [Google Scholar]
- Bowenkamp K.E., Frasca S., Jr., Draghi A., Jr., Tsongalis G.J., Koerting C., Hinckley L., De Guise S., Montali R.J., Goertz C.E., St Aubin D.J., Dunn J.L. Mycobacterium marinum dermatitis and panniculitis with chronic pleuritis in a captive white whale (Delphinapterus leucas) with aortic rupture. J. Vet. Diagn. Invest. 2001;13(6):524–530. doi: 10.1177/104063870101300613. [DOI] [PubMed] [Google Scholar]
- Buckle K., Roe W.D., Howe L., Michael S., Duignan P.J., Burrows E., Ha H.J., Humphrey S., McDonald W.L. Brucellosis in endangered hector’s dolphins (Cephalorhynchus hectori) Vet. Pathol. 2017;54(5):838–845. doi: 10.1177/0300985817707023. [DOI] [PubMed] [Google Scholar]
- Clayton L.A., Stamper M.A., Whitaker B.R., Hadfield C.A., Simons B., Mankowski J.L. Mycobacterium abscessus pneumonia in an Atlantic bottlenose dolphin (Tursiops truncatus) J. Zoo Wildl. Med. 2012;43(4):961–965. doi: 10.1638/2012-0110R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegrove K.M., St Leger J.A., Raverty S., Jang S., Berman-Kowalewski M., Gaydos J.K. Salmonella Newport omphaloarteritis in a stranded killer whale (Orcinus orca) neonate. J. Wildl. Dis. 2010;46(4):1300–1304. doi: 10.7589/0090-3558-46.4.1300. [DOI] [PubMed] [Google Scholar]
- Colegrove K.M., Venn-Watson S., Litz J., Kinsel M.J., Terio K.A., Fougeres E., Ewing R., Pabst D.A., McLellan W.A., Raverty S., Saliki J., Fire S., Rappucci G., Bowen-Stevens S., Noble L., Costidis A., Barbieri M., Field C., Smith S., Carmichael R.H., Chevis C., Hatchett W., Shannon D., Tumlin M., Lovewell G., McFee W., Rowles T.K. Fetal distress and in utero pneumonia in perinatal dolphins during the Northern Gulf of Mexico unusual mortality event. Dis. Aquat. Organ. 2016;119(1):1–16. doi: 10.3354/dao02969. [DOI] [PubMed] [Google Scholar]
- Cowan D.F. Amyloidosis in the bottlenose dolphin, Tursiops truncatus. Vet. Pathol. 1995;32(3):311–314. doi: 10.1177/030098589503200314. [DOI] [PubMed] [Google Scholar]
- Cowan D.F., Walker W.A., Brownell R.L., Jr. Pathology of small cetaceans stranded along southern California beaches. In: Bryden M.M., Harrison R., editors. Research on Dolphins. Oxford Science Publications; Oxford: 1986. pp. 323–368. [Google Scholar]
- Cozzi B., Huggenberger S., Oelschläger H.A. Elsevier Publications; San Diego, CA, USA: 2017. Anatomy of Dolphins Insights into Body Structure and Function. [Google Scholar]
- Cox T.M., Ragen T.J., Read A.J., Vox R.E., Baird W. Understanding the impacts of anthropogenic sound on beaked whales. J. Cetacean Res. Manage. 2006;7:177–187. [Google Scholar]
- Davison N.J., Barnett J.E.F., Koylass M., Whatmore A.M., Perkins M.W., Deaville R.C., Jepson P.D. Helicobacter cetorum infection in striped dolphin (Stenella coeruleoalba), Atlantic white-sided dolphin (Lagenorhynchus acutus), and short-beaked common dolphin (Delphinus delphus) from the southwest coast of England. J. Wildl. Dis. 2014;50(3):431–437. doi: 10.7589/2013-02-047. [DOI] [PubMed] [Google Scholar]
- Delaney M.A., Terio K.A., Colegrove K.M., Briggs M.B., Kinsel M.J. Occlusive fungal tracheitis in 4 captive bottlenose dolphins (Tursiops truncatus) Vet. Pathol. 2013;1:172–176. doi: 10.1177/0300985812446153. [DOI] [PubMed] [Google Scholar]
- Díaz-Delgado J., Arbelo M., Sacchini S., Quesada-Canales Ó., Andrada M., Rivero M., Fernández A. Pulmonary angiomatosis and hemangioma in common dolphins (Delphinus delphis) stranded in Canary Islands. J. Vet. Med. Sci. 2012;74(8):1063–1066. doi: 10.1292/jvms.11-0573. [DOI] [PubMed] [Google Scholar]
- Díaz-Delgado J., Sierra E., Vela A.I., Arbelo M., Zucca D., Groch K.R., Fernández A. Coinfection by Streptococcus phocae and cetacean morbillivirus in a short-beaked common dolphin Delphinus delphis. Dis. Aquat. Organ. 2017;11(3):247–252. doi: 10.3354/dao03124. [DOI] [PubMed] [Google Scholar]
- Di Guardo G., Mazzariol S. Cetacean morbillivirus-associated pathology: knowns and unknowns. Front. Microbiol. 2016;7:112. doi: 10.3389/fmicb.2016.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guardo G., Proietto U., Di Francesco C.E., Marsilio F., Zaccaroni A., Scaravelli D., Mignone W., Garibaldi F., Kennedy S., Forster F., Iulini B., Bozzetta E., Casalone C. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea Coast of Italy. Vet. Pathol. 2009;47(2):245–253. doi: 10.1177/0300985809358036. [DOI] [PubMed] [Google Scholar]
- Dines J.P., Otárola-Castillo E., Ralph P., Alas J., Daley T., Smith A.D., Dean M.D. Sexual selection targets cetacean pelvic bones. Evolution. 2014;68:3296–3306. doi: 10.1111/evo.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duignan P.J., House C., Odell D.K., Wells R.S. Morbillivirus infection in bottlenose dolphins: evidence for recurrent epizootics in the western Atlantic and Gulf of Mexico. Mar. Mamm. Sci. 1996;12:499–515. [Google Scholar]
- Eissa Alaa Eldin, Abu-Seida Ashraf M. Synopsis on the most common pathologies of dolphins. J. Fish. Aqu. Sci. 2015;10:307–322. [Google Scholar]
- Ewing R., Zaias J., Stamper M.A., Bossart G.D., Dubey J.P. Prevalence of Sarcocystis sp. in stranded Atlantic white-sided dolphins (Lagenorhynchus acutus) J. Wildl. Dis. 2002;38(2):291–296. doi: 10.7589/0090-3558-38.2.291. [DOI] [PubMed] [Google Scholar]
- Fauquier D.A., Litz J., Sanchez S., Colegrove K., Schwacke L.H., Hart L., Saliki J., Smith C., Goldstein T., Bowen-Stevens S., McFee W., Fougeres E., Mase-Guthrie B., Stratton E., Ewing R., Venn-Watson S., Carmichael R.H., Clemons-Chevis C., Hatchett W., Shannon D., Shippee S., Smith S., Staggs L., Tumlin M.C., Wingers N.L., RowlesF T.K. Evaluation of morbillivirus exposure in cetaceans from the northern Gulf of Mexico. Endanger. Species Res. 2010;33:211–220. 2014. [Google Scholar]
- Fenton H., Daoust P.-Y., Forzan M.J., Vanderstichel R.V., Ford J.K., Spaven L., Lair S., Raverty S. Causes of mortality of harbor porpoises Phocoena phocoena along the Atlantic and Pacific coasts of Canada. Dis. Aquat. Organ. 2017;(3):171–183. doi: 10.3354/dao03080. [DOI] [PubMed] [Google Scholar]
- Fernández A., Edwards J.F., Rodriguez F., de los Monteros A.E., Herráez P. “Gas and fat embolic syndrome” involving a mass stranding of beaked whales (Family Ziphiidae) exposed to anthropogenic sonar signals. Vet. Pathol. 2005;42:446–457. doi: 10.1354/vp.42-4-446. [DOI] [PubMed] [Google Scholar]
- Fiorito C.D., Bentancor A., Lombardo D., Bertellotti M. Erysipelothrix rhusiopathiae isolated from gull-inflicted wounds in southern right whale calves. Dis. Aquat. Organ. 2016;121(1):67–73. doi: 10.3354/dao03041. [DOI] [PubMed] [Google Scholar]
- Foster G., McAuliffe L., Dagleish M.P., Barley J., Howie F., Nicholas R.A., Ayling R.D. Mycoplasma species isolated from harbor porpoises (Phocoena phocoena) and a Sowerby’s beaked whale (Mesoplodon bidens) stranded in Scottish waters. J. Wildl. Dis. 2011;47(1):206–211. doi: 10.7589/0090-3558-47.1.206. [DOI] [PubMed] [Google Scholar]
- Garner M.M., Shwetz C., Ramer J.C., Rasmussen J.M., Petrini K., Cowan D.F., Raymond J.T., Bossart G.D., Levine G.A. Congenital diffuse hyperplastic goiter associated with perinatal mortality in 11 captive-born bottlenose dolphins (Tursiops truncatus) J. Zoo Wildl. Med. 2002;33(4):5–350. doi: 10.1638/1042-7260(2002)033[0350:CDHGAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Geraci J.R., Gerstmann K.E. Relationship of dietary histamine to gastric ulcers in the dolphin. J. Am. Vet. Med. Assoc. 1966;149(7):90–884. [PubMed] [Google Scholar]
- Geraci J.R., Hicks B.D., St Aubin D.J. Dolphin pox: a skin disease of cetaceans. Can. J. Comp. Med. 1979;43(4):399–404. [PMC free article] [PubMed] [Google Scholar]
- Groch K.R., Marcondes M.C., Colosio A.C., Catão-Dias J.L. Skeletal abnormalities in humpback whales Megaptera novaeangliae stranded in the Brazilian breeding ground. Dis. Aquat. Organ. 2012;8;101(2):145–158. doi: 10.3354/dao02518. [DOI] [PubMed] [Google Scholar]
- Gottschling M., Bravo I.G., Schulz E., Bracho M.A., Deaville R., Jepson P.D., Van Bressem M.F., Stockfleth E., Nindl I. Modular organizations of novel cetacean papillomaviruses. Mol. Phylogenet. Evol. 2011;59(1):34–42. doi: 10.1016/j.ympev.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Guzmán-Verri C., González-Barrientos R., Hernández-Mora G., Morales J.-A., Baquero-Calvo E., Chaves-Olarte E., Moreno E. Brucella ceti and brucellosis in Cetaceans. Front. Cell. Infect. Microbiol. 2012;2:3. doi: 10.3389/fcimb.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms C.A., Lo Piccolo R., Rotstein D.S., Hohn A.A. Struvite penile urethrolithiasis in a pygmy sperm whale (Kogia breviceps) J. Wildl. Dis. 2004;40(3):588–593. doi: 10.7589/0090-3558-40.3.588. [DOI] [PubMed] [Google Scholar]
- Harms C.A., Maggi R.G., Breitschwerdt E.B., Clemons-Chevis C.L., Solangi M., Rotstein D.S., Fair P.A., Hansen L.J., Hohn A.A., Lovewell G.N., McLellan W.A., Pabst D.A., Rowles T.K., Schwacke L.H., Townsend F.I., Wells R.S. Bartonella species detection in captive, stranded and free-ranging cetaceans. Vet. Res. 2008;39(6):59–66. doi: 10.1051/vetres:2008036. [DOI] [PubMed] [Google Scholar]
- Hart L.B., Rotstein D.S., Wells R.S., Allen J., Barleycorn A., Balmer B.C. Skin lesions on common bottlenose dolphins (Tursiops truncatus) from three sites in the Northwest Atlantic, USA. PLoS One. 2012;7(3):e33081. doi: 10.1371/journal.pone.0033081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasse J.K., Brown D.J., Weill F.X., Mather H., Foster G., Brisse H., Wain J., Atchman M. Population genetic structure of 4, 12: a: Salmonella enterica strains from harbor porpoises. Appl. Environ. Microbiol. 2012;78(24):8829–8833. doi: 10.1128/AEM.02310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Mora G., Bonilla-Montoya R., Barrantes-Granados O., Esquivel-Suárez A., Montero-Caballero D., González-Barrientos R. Brucellosis in mammals of Costa Rica: an epidemiological survey. PLoS One. 2017;12(8):e0182644. doi: 10.1371/journal.pone.0182644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins R. Bacteria and fungi of marine mammals: a review. Can. Vet. J. 2000;41(2):105–116. [PMC free article] [PubMed] [Google Scholar]
- Hunt K.E., Stimmelmayr R., George C., Hanns C., Suydam R., Brower H., Rolland R.M. Baleen hormones: a novel tool for retrospective assessment of stress and reproduction in bowhead whales (Balaena mysticetus) Conserv. Physiol. 2014;2:cou030. doi: 10.1093/conphys/cou030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidoro-Ayza M., Ruiz-Villalobos N., Pérez L., Guzmán-Verri C., Muñoz P.M., Alegre F., Barberán M., Chacón-Díaz C., Chaves-Olarte E., González-Barrientos R., Moreno E., Blasco J.M., Domingo M. Brucella ceti infection in dolphins from the Western Mediterranean sea. BMC Vet. Res. 2014;17(10):206. doi: 10.1186/s12917-014-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber J.R., Pérez J., Arbelo M., Andrada M., Hidalgo M., Gómez-Villamandos J.C., Van Den Ingh T., Fernández A. Hepatic lesions in cetaceans stranded in the Canary Islands. Vet. Pathol. 2004;41(2):147–153. doi: 10.1354/vp.41-2-147. [DOI] [PubMed] [Google Scholar]
- Jepson P.D., Arbelo M., Deaville R., Patterson I.A.P., Castro P. Gas-bubble lesions in stranded cetaceans. Nature. 2003;425:575–576. doi: 10.1038/425575a. [DOI] [PubMed] [Google Scholar]
- Jepson P.D., Deaville R., Acevedo-Whitehouse K., Barnett J., Brownlow A., Brownell RL., Jr. What caused the UK’s largest common dolphin (Delphinus delphis) mass stranding event. PLoS One. 2013;8(4):e60953. doi: 10.1371/journal.pone.0060953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper C.M., Tomo I., Bingham J., Bastianello S.S., Wang J., Gibbs S.E., Woolford L., Dickason C., Kelly D. Morbillivirus-associated unusual mortality event in South Australian bottlenose dolphins is largest reported for the Southern Hemisphere. R. Soc. Open Sci. 2016;3(12):160838. doi: 10.1098/rsos.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S., Hagen F., Tscharke R.L., Huynh M., Bartlett K.H., Fyfe M., MacDougall L., Boekhout T., Swon-Chung K., Meyer W. A rare genotype of Cryptococcus gatti caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc. Natl. Acad. Sci. USA. 2004;101(49):17258–17362. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita R. Zoo and wild animal medicine current therapy. In: Miller M.E., Fowler R.E., editors. Melioidosis in Marine Mammals. 2008. pp. 299–307. 6. [Google Scholar]
- Kuiken T., Hofle U., Bennett P.M., Allchin C.R., Kirkwood J.K., Baker J.R., Appleby E.C., Lockyer C.H., Walton M.J., Sheldrick M.C. Adrenocortical hyperplasia, disease and chlorinated hydrocarbons in the harbour porpoise (Phocoena phocoena) Mar. Pollut. Bull. 1993;26:440–446. [Google Scholar]
- Ladds P. CISRO Publishers; Collingwood, Australia: 2009. Pathology of Australian Native Wildlife. [Google Scholar]
- Lair S., Beland P., DeGuise S., Martineau D. Adrenal hyperplastic and degenerative changes in beluga whales (Delphinapterus leucas) J. Wildl. Dis. 1997;33:430–437. doi: 10.7589/0090-3558-33.3.430. [DOI] [PubMed] [Google Scholar]
- Lair S., Martineau D., Measures L.N. Causes of mortality in St. Lawrence Estuary beluga (Delphinapterus leuca) from 1983 to 2012. DFO Can. Sci. Advis. Sec. Res. 2014:14. [Google Scholar]
- Lamere S.A., St Leger J.A., Schrenzel M.D., Anthony S.J., Rideout B.A., Salomon D.R. Molecular characterization of a novel gammaretrovirus in killer whales (Orcinus orca) J. Virol. 2009;83(24):12956–12967. doi: 10.1128/JVI.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002;10:113–390. [Google Scholar]
- Lavery T.J., Kemper C.M., Sanderson K., Schultz C.G., Coyle P., Mitchell J.G., Seuront L. Heavy metal toxicity of kidney and bone tissues in South Australian adult bottlenose dolphins (Tursiops aduncus) Mar. Environ. Res. 2009;67(1):1–7. doi: 10.1016/j.marenvres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Lipscomb T.P., Schulman F.Y., Moffett D., Kennedy S. Morbilliviral disease in Atlantic bottlenose dolphins (Tursiops truncatus) from 1987-1988 epizootic. J. Wildl. Dis. 1994;30:567–571. doi: 10.7589/0090-3558-30.4.567. [DOI] [PubMed] [Google Scholar]
- Litz J.A., Baran M.A., Bowen-Stevens S.R., Carmichael R.H., Colegrove K.M., Garrison L.P., Fire S.E., Fougeres E.M., Hardy R., Holmes S., Jones W., Mase-Guthrie B.E., Odell D.K., Rosel P.E., Saliki J.T., Shannon D.K., Shippee S.F., Smith S.M., Stratton E.M., Tumlin M.C., Whitehead H.R., Worthy G.A.J., Rowles T.K. Review of historical unusual mortality event (UMEs) in the Gulf of Mexico (1990-2009): providing context for the multi-year northern Gulf of Mexico cetacean UME declared in 2010. Dis. Aquat. Organ. 2014;112:161–175. doi: 10.3354/dao02807. [DOI] [PubMed] [Google Scholar]
- Loch C., Simões-Lopes P. Dental wear in dolphins (Cetacea: Delphinidae) from southern Brazil. Arch. Oral Biol. 2012;58(2):134–141. doi: 10.1016/j.archoralbio.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Martineau D., Lemberger K., Dallaire A., Labelle P., Lipscomb T.P., Michel P., Mikaelian I. Cancer in wildlife, a case study: beluga from the St. Lawrence estuary, Quebec, Canada. Environ. Health Perspect. 2002;110:285–292. doi: 10.1289/ehp.02110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Levasseur L.M., Gendron D., Knell R.J., O’Toole E.A., Singh M., Acevedo-Whitehouse K. Acute sun damage and photoprotective responses in whales. Proc. R. Soc. B. 2011;278(1711):1581–1586. doi: 10.1098/rspb.2010.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAloose D., Rago M.V., Di Martino M., Chirife A., Olson S.H., Beltramino L., Pozzi L.M., Musmeci L., La Sala L., Mohamed N., Sala J.E., Bandieri L., Andrejuk J., Tomaszewicz A., Seimon T., Sironi M., Samartino L.E., Rowntree V., Uhart M.M. Post-mortem findings in southern right whales Eubalaena australis at Península Valdés, Argentina, 2003-2012. Dis. Aquat. Organ. 2016;119(1):17–36. doi: 10.3354/dao02986. [DOI] [PubMed] [Google Scholar]
- Moore M.J., Bogomolni A.L., Dennison S.E., Early G., Garner M.M., Hayward B.A., Lentell B.J., Rotstein D.S. Gas bubbles in seals, dolphins, and porpoises entangled and drowned at depth in gillnets. Vet. Pathol. 2009;46(3):536–547. doi: 10.1354/vp.08-VP-0065-M-FL. [DOI] [PubMed] [Google Scholar]
- Moore M., van der Hoop J., Barco S.G., Costidis A.M., Gulland F.M., Jepson P.D., Moore K.T., Raverty S., McLellan W. Criteria and case definitions for serious injury and death of pinnipeds and cetaceans caused by anthropogenic trauma. Dis. Aquat. Org. 2013;103:229–264. doi: 10.3354/dao02566. [DOI] [PubMed] [Google Scholar]
- Nweeia M.T., Eichmiller F.C., Hauschka P.V., Donahue G.A., Orr J.R., Ferguson S.H., Watt C.A., Mead J.G., Potter C.W., Dietz R., Giuseppetti A.A., Black S.R., Trachtenberg A.J., Kuo W.P. Sensory ability in the narwhal tooth organ system. Anat. Rec. 2014;297:599–617. doi: 10.1002/ar.22886. [DOI] [PubMed] [Google Scholar]
- Newman S.J., Smith S.A. Marine mammal neoplasia: a review. Vet. Pathol. 2006;43(6):865–880. doi: 10.1354/vp.43-6-865. [DOI] [PubMed] [Google Scholar]
- Powell J.W., Archibald R.T., Cross C.A., Rotstein D.S., Soop V.m., McFee W.E. Multiple congenital cardiac abnormalities in an Atlantic bottlenose dolphin (Tursiops truncatus) J. Wildl. Dis. 2009;45(3):839–842. doi: 10.7589/0090-3558-45.3.839. [DOI] [PubMed] [Google Scholar]
- Power E., Murphy S. Staphylococcus aureus septicaemia in a killer whale. Vet. Rec. 2002;29(26):819. [PubMed] [Google Scholar]
- Profeta F., Di Francesco C.E., Marsilio F., Mignone W., Di Nocera F., De Carlo E., Lucifora G., Pietroluongo G., Baffoni M., Cocumelli C., Eleni C., Terracciano G., Ferri N., Di Francesco G., Casalone C., Pautasso A., Mazzariol S., Centelleghe C., Di Guardo G. Retrospective seroepidemiological investigations against Morbillivirus, Toxoplasma gondii and Brucella spp. in cetaceans stranded along the Italian coastline (1998-2014) Res. Vet. Sci. 2015;101:89–92. doi: 10.1016/j.rvsc.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Read, A.J., Murray, K.T., 2000. Gross Evidence of Human-Induced Mortality in Small Cetaceans. U.S. Department of Commerce, NOAA Tech. Memo. NMFS-OPR-15, 21 p.
- Reidarson T.H., McBain J.F., Dalton L.M., Rinaldi M.G. Diagnosis and treatment of fungal infections in marine mammals. In: Fowler M.E., London R.E.Miller, Saunders W.B., editors. Zoo and Wild Animal Medicine, Current Therapy. fourth ed. WB Saunders; Philadelphia: 1999. p. 478-485. [Google Scholar]
- Reidenberg J.S., Laitman J.T. Sisters of the sinuses: cetacean air sacs. Anat. Rec. 2008;291(11):1389–1396. doi: 10.1002/ar.20792. [DOI] [PubMed] [Google Scholar]
- Reif J.S., Schaefer A.M., Bossart G.D., Fair P.A. Health and environmental risk assessment project for bottlenose dolphins Tursiops truncatus from the southeastern USA II. Environ. Aspect. 2017;125:155–166. doi: 10.3354/dao03143. [DOI] [PubMed] [Google Scholar]
- Rehtanz M., Ghim S.J., McFee W., Doescher B., Lacave G., Fair P.A., Reif J.S., Bossart G.D., Jenson A.B. Papillomavirus antibody prevalence in free-ranging and captive bottlenose dolphins (Tursiops truncatus) J. Wildl. Dis. 2010;46(1):136–145. doi: 10.7589/0090-3558-46.1.136. [DOI] [PubMed] [Google Scholar]
- Reynolds J.E., Rommel S.A. Smithsonian Institution Press; Washington, D.C., USA: 1999. Biology of Marine Mammals. [Google Scholar]
- Roe W.D., Howe L., Baker E.J., Burrows L., Hunter S.A. An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’s dolphins (Cephalorhynchus hectori) Vet. Parasitol. 2013;192(1–3):67–74. doi: 10.1016/j.vetpar.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Ross H.M., Wilson B. Violent interactions between bottlenose dolphins and harbour porpoises. Proc. R. Soc. B. 1996;263:283–286. doi: 10.1098/rspb.1998.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowles T.R., Schwacke L.S., Wells R.S., Saliki J.T., Hansen L. Evidence of susceptibility to morbillivirus infection in cetaceans from the United States. Mar. Mamm. Sci. 2011;27(1):1–19. [Google Scholar]
- St Leger J.A., Begeman L., Fleetwood M., Frasca S., Garner M.M., Lair S., Trembley S., Linn M.J., Terio K.A. Comparative pathology of nocardiosis in marine mammals. Vet. Pathol. 2009;46(2):299–308. doi: 10.1354/vp.46-2-299. [DOI] [PubMed] [Google Scholar]
- Siebert U., Müller G., Desportes G., Weiss R., Hansen K., Baumgärtner W. Pyogranulomatous myocarditis due to Staphylococcus aureus septicaemia in two harbour porpoises (Phocoena phocoena) Vet. Rec. 2002;150(9):273–277. doi: 10.1136/vr.150.9.273. [DOI] [PubMed] [Google Scholar]
- Simeone C.A., Traversi J.P., Meegan J.M., LeBert C., Colitz C.M.H., Jensen E.D. Clinical management of Candida albicans keratomycosis in a bottlenose dolphin (Tursiops truncatus) Vet. Ophthalmol. 2017 doi: 10.1111/vop.12459. [DOI] [PubMed] [Google Scholar]
- Simmonds M.P. Cetaceans and marine debris: the great unknown. J. Mar. Biol. 2012;2012:8. [Google Scholar]
- Slijper E.J. Cornell University Press; Ithaca, NY, USA: 1979. Whales. [Google Scholar]
- Smith C.R., Poindexter J.R., Meegan J.M., Bobulescu I.A., Jensen E.D., Venn-Watson S., Sakhaee K. Pathophysiological and physicochemical basis of ammonium urate stone formation in dolphins. J. Urol. 2014;192(1):260–266. doi: 10.1016/j.juro.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolarek Benson, K.A., Manire, C.A., Ewing, R.Y., Saliki, J.T., Townsend, F.I., Ehlers, B., et al., 2006. Identification of novel alpha- and gammaherpesviruses from cutaneous and mucosal lesions of dolphins and whales. J. Virol. Methods. 136, 261–266. [DOI] [PubMed]
- Taylor R.F., Farrell R.K. Light and electron microscopy of peripheral blood neutrophils in a killer whale affected with Chediak-Higashi syndrome. Fed. Proc. 1973;32:822. [Google Scholar]
- Twiner M.J., Flewelling L.J., Fire S.E., Bowen-Stevens S.R., Gaydos J.K. Comparative analysis of three brevetoxin-associated bottlenose dolphin (Tursiops truncatus) mortality events in the Florida panhandle region (USA) PLoS One. 2012;7:1–19. doi: 10.1371/journal.pone.0042974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bressem M.F., Duignan P.J., Banyard A., Barbieri M., Colegrove K.M., De Guise S., Di Guardo G., Dobson A., Domingo M., Fauquier D., Fernandez A., Goldstein T., Grenfell B., Groch K.R., Gulland F., Jensen B.A., Jepson P.D., Hall A., Kuiken T., Mazzariol S., Morris S.E., Nielsen O., Raga J.A., Rowles T.K., Saliki J., Sierra E., Stephens N., Stone B., Tomo I., Wang J., Waltzek T., Wellehan J.F. Cetacean morbillivirus: current knowledge and future directions. Viruses. 2014;6(12):5145–5181. doi: 10.3390/v6125145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bressem M.F., Van Waerebeek K., Aznar F.J., Raga J.A., Jepson P.D., Duignan P., Deaville R., Flach L., Viddi F., Baker J.R., Di Beneditto A.P., Echegaray M., Genovo T., Reyes J., Felix F., Gaspar R., Ramos R., Peddemors V., Sanino G.P., Siebert U. Epidemiological pattern of tattoo skin disease: a potential general health indicator for cetaceans. Dis. Aquat. Organ. 2009;85:225–237. doi: 10.3354/dao02080. [DOI] [PubMed] [Google Scholar]
- Van Bressem, M.F., Van Waerebeek, K., Flach, L., Reyes, J.C., de Oliveira Santos, M.C., Siciliano, S., Echegaray, M., Viddi, F., Felix, F., Crespo, E., Sanino, G.P., Avila, I.C., Fraijia, N., Castro, C., 2008. Skin Diseases in Cetaceans. Report of the International Whaling Commission Scientific Committee (SC/60/DW8).
- Venn-Watson S., Benham C., Carlin K., DeRienzo D., St Leger J. Hemochromatosis and fatty liver disease: building evidence for insulin resistance in bottlenose dolphins (Tursiops truncatus) J. Zoo Wildl. Med. 2012;43(3):S35–S47. doi: 10.1638/2011-0146.1. [DOI] [PubMed] [Google Scholar]
- Venn-Watson S., Colegrove K.M., Litz J., Kinsel M., Terio K., Saliki J., Rowles T. Adrenal gland and lung lesions in Gulf of Mexico common bottlenose dolphins (Tursiops truncatus) found dead following the Deepwater Horizon oil spill. PLoS One. 2015;10(5):e0126538. doi: 10.1371/journal.pone.0126538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela R., Bossart G.D., St. Leger J.A., Dalton L.M., Reif J.S., Schaefer A.M., McCarthy P., Fair P.A., Mendoza L. Cutaneous granulomas in dolphins caused by novel uncultivated Paracoccidioides brasiliensis. Emerg. Infect. Dis. 2016;22(12):2063–2069. doi: 10.3201/eid2212.160860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A.K., Theilmann R.J., Ridgway S.H., Scadeng M. Diffusion tractography reveals pervasive asymmetry of cerebral whitematter tracts in the bottlenose dolphin (Tursiops truncatus) Brain Struct. Funct. 2017 doi: 10.1007/s00429-017-1525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunschmann A., Armien A., Harris N.B., Brown-Elliott B.A., Wallace R.J., Jr., Rasmussen J., Willette M., Wolf T. Disseminated panniculitis in a bottlenose dolphin (Tursiops truncatus) due to Mycobacterium chelonae infection. J. Zoo Wildl. Med. 2008;39(3):412–420. doi: 10.1638/2007-0135.1. [DOI] [PubMed] [Google Scholar]


