Abstract
This chapter reviews common diseases of pinnipeds, including species in the Otariidae (fur seals and sea lions), Phocidae (true seals), and Odobenidae (walrus) families. Much of the knowledge on pathologic conditions of pinnipeds comes from necropsies of stranded animals and those housed in captivity. As such, disease knowledge is biased toward species frequently housed in zoos and aquaria, those that strand more commonly, or those in which free-ranging populations are more easily accessible. Though historically systematic evaluations of wild populations have rarely been accomplished, in the past 10 years, with advances in marine mammal medicine and anesthesia, biologists and veterinarians more frequently completed live animal health field investigations to evaluate health and disease in free-ranging pinniped populations.
Keywords: pinniped, walrus, seal, sea lion, otariid, phocid, domoic acid, urogenital carcinoma, lung worm
Introduction
The pinnipeds consist of 33 species of mammals inhabiting marine/terrestrial interfaces around the globe. They are the Otariidae (fur seals and sea lions), Phocidae (true seals), and Odobenidae (walrus) families. Common and scientific names of species described in this chapter are presented in Supplemental Table e1. Much of the knowledge on pathologic conditions of pinnipeds comes from necropsies of stranded animals and those housed in captivity. As such, disease knowledge is biased toward species frequently housed in zoos and aquaria, those that strand more commonly, or those in which free-ranging populations are more easily accessible.
Table e1.
Scientific Names of Pinnipeds
| Common Name | Scientific Name |
|---|---|
| Phocidae | |
| Harbor seal | Phocavitulina |
| Northern elephant seal | Mirounga angustirostris |
| Gray seal | Halichoerus grypus |
| Hawaiian monk seal | Neomonachus schauinslandi |
| Baikal seal | Pusasibirica |
| Ringed seal | Pusahispida |
| Harp seal | Pagophilus groenlandicus |
| Hooded seal | Cystophora cristata |
| Ribbon seal | Histriophoca fasciata |
| Otariidae | |
| Northern fur seal | Callorhinus ursinus |
| California sea lion | Zalophus californianus |
| South American fur seal | Arctocephalus australis |
| Australian fur seal | Arctocephalus pusillu |
| Stellar sea lion | Eumetopias jubatus |
| South American/Southern sea lion | Otaria flavescens |
| New Zealand sea lion | Phocarcto shookeri |
| Odobenidae | |
| Walrus | Odobenus rosmarus |
Unique features
Pinnipeds generally demonstrate sexual dimorphism with males being larger, possessing a large sagital crest or tusks depending on species. Their organ systems broadly resemble those of the terrestrial carnivores from which they evolved, with modifications for their aquatic lifestyle. The most pronounced differences are in the lungs, skin, and vasculature, with minor modifications in the spleen and liver. Many adaptations are associated with the physiological demands of prolonged diving, including an increased requirement for oxygen stores, protection from pressure effects, and insulation/thermoregulation at low temperatures. The main differences between terrestrial carnivores and pinnipeds are described below; specific differences between phocids and otariids are listed in Supplemental Table e2.
Table e2.
Comparative Anatomy of Phocids and Otariids
| Organ System | Phocids | Otariids |
|---|---|---|
| Respiratorya,b,c | Small airways reinforced by smooth muscle | Small airways reinforced by cartilage |
| Fewer lung lobes | Similar to canidlobation (2 left lobes, 3 right) | |
| Predominantly serous glands in large airway mucosa | Predominantly mucous glands in large airway mucosa | |
| Single layer of capillaries in alveolar walls | Double layer of capillaries in some species | |
| Tracheal bifurcation at hilus | Tracheal bifurcation at thoracic inlet | |
| Renalc | Fibrous septae between renicules in some species | No fibrous separation between renicules |
| Cutaneousb,b,e | Compound hair follicles have few or no secondary hairs except for ice-dwelling species | Usually one or more secondary hairs with abundant intermediate and underfur hairs |
| Sebaceous glands prominent | Apocrine sweat glands prominent | |
| “Catastrophic molt” in some species, with distinct seasonal basilar mitosis, development of granular cell layer and sloughing of surface epithelium over days to weeks | Prolonged cyclical molt, with permanent well-developed stratum granulosum | |
| Cutaneous arteriovenous anastamoses diffusely over body | Cutaneous arteriovenous anastamoses predominantly in flippers | |
| Teethf | Dental formula: i 2-3/1-2, c 1/1, pm 4/4, m 0-2/0-2 X 2 | Dental formula: i 3/2, c 1/1, pm 4/4, m 1-2/1 X2 |
Denison, D.M., Kooyman, G.L., 1973. Structure and function of small airways in pinniped and sea other lungs. Resp. Physiol. 17, 1–10.
Rommel S.A., Lowenstine, L.J., 2001. Gross and microscopic anatomy, In: Dierauf, L.A., Gulland, F. M.D. (Eds.), CRC Handbook of Marine Mammal Medicine, 2nd Edition, CRC Press, Boca Raton, Florida, pp. 129–158.
Gray, R., Canfield, P., Rogers, T., 2006. Histology of selected tissues of the leopard seal and implications for functional adaptations to an aquatic lifestyle. J. Anat. 209(2), 179–199.
Ling, J.K., 1970. Pelage and molting in wild mammals with special reference to aquatic forms. Q. Rev. Biol. 45, 16–54.
Ponganis, P.J., Kreutzer, U., Stockard, T.K., Lin, P.C., Sailasuta, N., Tran, T.K., Hurd, R., Jue, T., 2008. Blood flow and metabolic regulation in seal muscle during apnea. J. Exp. Biol. 211(20), 3323–3332.
Nowak, R.M., 1999. Walker’s mammals of the world (Vol. 1). JHU Press.
Pinnipeds have proportionally larger livers and kidneys than terrestrial species, and the small intestine is proportionally longer. The lungs and liver are lobated, with variation in the extent of lobation between species, and the kidneys are reniculate. The heart is broad and flat. Vascular adaptions, particularly pronounced in deep diving species, include an enlarged hepatic sinus, caudal vena cava, proximal aorta (aortic bulb) and spleen. The hepatic sinus in species, such as elephant seals is very expansive and should not be confused with an aneurysmal dilation. A muscular sphincter cranial to the hepatic sinus and the thick splenic muscular capsule and trabeculae enhance blood storage. Thermoregulatory adaptations include arterio-venous anastomoses in the skin and reproductive organs (Ponganis, 2008). The lungs have prominent fibrous interlobular septae, and smaller airways are reinforced by either cartilage (otariids), smooth muscle (phocids), or a combination of the two (odobenids) (Denison and Kooyman, 1973). The reniculate kidneys have a thick capsule and prominent outer cortical veins (Stewardson et al., 1999). Pinniped epidermis is thick with no arrector pili muscles, increased numbers of sebaceous glands, and a thick adipose rich hypodermis (Ling, 1970). Brown discoloration of teeth occurs with age. Walrus upper canines develop into large, ventrally directed tusks.
Large nerve ganglia can be seen histologically near the adrenal gland capsule of some species and should not be confused with neoplasia. Vacuolation of cells within the adrenal zona glomerulosa is a common finding in California sea lions (CSLs).
Pinnipeds have a zonary, endotheliochorial placenta. Hormone induced morphologic changes are noted in the reproductive tract during different periods of the reproductive cycle and have been reviewed in CSLs by Colegrove et al. (2009c). Accumulations of hemosiderin laden macrophages are found in regions of the uterus associated with previous placental attachment. These “placental scars” can be noted up to 12 months following pregnancy. Hyperplasia of subsurface epithelial structures within the ovary can be noted in aged female CSLs.
Non-infectious diseases
Nutritional
Malnutrition is commonly observed in young pinnipeds entering rehabilitation settings. Increased numbers of malnourished animals can strand when oceanic conditions, such as El Niño events affect prey availability for both pups and dams (Colegrove et al., 2005a). In addition to a thinning and loss of blubber, lesions can include hepatocellular, muscle and gastric mucosal atrophy, and pancreatic zymogen depletion. Bile stasis and hepatic lipidosis can also be noted. Secondary bacterial infections are common. Poor juvenile survival related to emaciation and low prey availability threatens recovery of the endangered Hawaiian monk seal.
Toxic Toxins
Domoic acid (DA) is an amino acid excitatory neurotoxin produced by diatom species, especially in the genus Pseudo-nitzschia. DA has a high affinity for the α-amino-5-hydroxy-3-methyl-4-isoxazole propionic acid (AMPA) and kainite subclasses of glutamate ionotropic receptors. Receptor binding of DA causes neuronal depolarization, endogenous glutamate release, and activation of voltage gated calcium channels resulting in cell dysfunction and death (Jeffery et al., 2004). Toxicity due to DA exposure was first identified in CSLs in 1998, following a bloom of Pseudo-nitzschia australis in central California (Scholin et al., 2000). Since then there has been an increasing incidence of DA-producing diatoms along the California coast and increased frequency of strandings of CSLs with neurologic signs and lesions associated with exposure to DA (Goldstein et al., 2008, Gulland et al., 2012). Though CSLs are by far the most common species affected by DA, toxicosis has also been diagnosed in harbor seals and northern fur seals (Lefebvre et al., 2010, McHuron et al., 2013). Neurologic abnormalities with acute DA toxicosis may be continuous and include ataxia, head weaving, abnormal scratching, seizures, and coma. Acute cases often strand in clusters temporally associated with blooms of toxin-producing diatoms (Scholin et al., 2000, Goldstein et al., 2008). Animals with chronic toxicosis have more intermittent seizures, unusual behavior, vomiting, and apparent blindness and they may be clinically normal between neurologic events. Chronic cases often strand individually and may not be temporally associated with specific blooms. Eosinophilia and low serum cortisol can be noted in CSLs with both acute and chronic toxicosis (Gulland et al., 2012). Sea lions with chronic DA toxicosis may have abnormalities detected on electroencephalogram and hippocampal atrophy can be detected by magnetic resonance imaging.
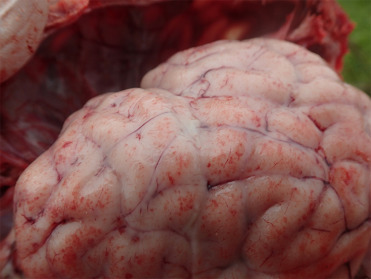
Brain lesions associated with DA exposure are centered on the hippocampus and parahippocampal gyrus and can be unilateral or bilateral; therefore, adequate histologic sections of both sides of the ventral mid-hippocampus are essential for diagnosis. In acute DA toxicosis there is acute neuronal necrosis affecting the granular cells of the dentate gyrus, pyramidal neurons of the hippocampus and neurons in the amygdala and piriform lobe (Fig. 23.1 ). Within the hippocampus, pyramidal cells in cornu ammonis (CA) sectors CA1, CA3, and CA4 are affected often with apparent sparing of CA2 cells. Occasionally in animals dying very acutely following exposure, lesions are minimal with only rare neuronal necrosis or central hippocampal neuropil vacuolation. Neuropil vacuolation should be interpreted with caution as autolysis and specimen handling can cause artifactual vacuolation along the edges of the hippocampus. In chronic cases, grossly evident hippocampal atrophy may be seen unilaterally or bilaterally (Fig. 23.2 ). Histologically, there is parenchymal atrophy in the hippocampal formation, mild to severe loss of granular cells in the dentate gyrus and hippocampal neurons, astrocytosis, oligodendrogliosis, and mild lymphocytic perivascular cuffing (Figs. 23.3 and 23.4 ). Scattered acute neuronal necrosis may be noted. In severe acute and chronic cases there may be malacia in the adjacent piriform lobe associated with neuronal necrosis, loss, and gliosis. Occasional perivascular cuffing in the piriform lobe and scattered meningeal aggregates of lymphocytes and plasma cells can be seen in chronic disease (Goldstein et al., 2008, Silvangi et al., 2005). In more recent years, occasional cases have been noted with gliosis and neuronal necrosis in the amygdala and only relatively mild or no lesions in the hippocampus, though the cause of these lesions is unknown. Also, some CSLs with DA-associated hippocampal lesions may have multifocal lymphohistiocytic encephalitis, though whether this is due to a concurrent infectious process or to DA exposure has not been elucidated.
Figure 23.4.
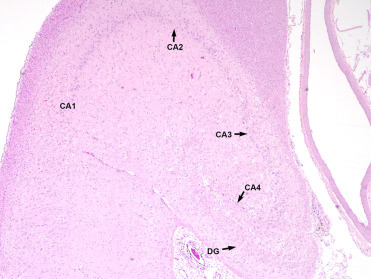
Chronic lesions of domoic acid toxicosis in the hippocampus of a California sea lion.
There is parenchymal collapse, neuronal loss throughout the dentate gyrus (DG) and cornu ammonis (CA) sectors 1-4, and gliosis.
Figure 23.1.
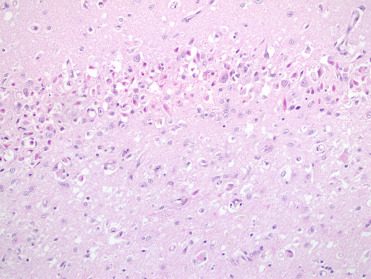
Acute domoic acid toxicosis in the brain of a California sea lion.
There is neuronal necrosis and neuropil vacuolation.
Figure 23.2.
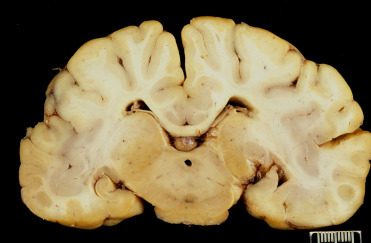
Domoic acid toxicosis in a California sea lion.
Unilateral hippocampal atrophy due to chronic domoic acid toxicosis (formalin fixed).
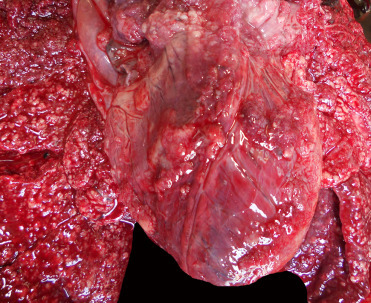
Cardiac lesions associated with DA toxicosis can manifest grossly as regions of myocardial pallor or streaks along the epicardial surface, pericardial effusion, or a globally flaccid heart. Histologically, lesions affect the interventricular septum and left ventricle with the earliest lesions appearing at the base of the septum. Acute lesions include interstitial edema, rowing of cardiomyocyte nuclei, cardiomyocyte vacuolation, and, less frequently, necrosis. In subacute to chronic lesions, there is myocyte loss and replacement by adipocytes or loose fibrous connective tissue and areas can contain occasional macrophages with cytoplasmic lipofuscin (Fig. 23.5 ). Lesions are suspected to be primarily due to direct toxin reaction with cardiac glutamate receptors causing myocyte apoptosis (Silvangi et al., 2005, Zabka et al., 2009.).
Figure 23.5.
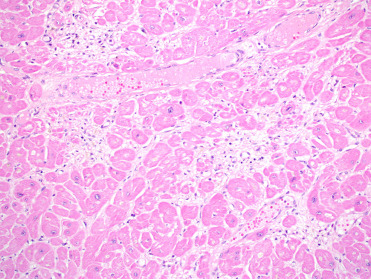
Domoic acid cardiomyopathy in a California sea lion.
Note the loss of myocytes and replacement by loose fibrous connective tissue and adipocytes, with reactive cardiomyocyte nuclei surrounding lesions.
Domoic acid may also cause abortion and premature parturition in affected pinnipeds and the toxin can be detected within amniotic fluid, fetal stomach contents and urine. Cerebral edema is rarely identified in fetuses and placental abruption can occur in dams due to seizure activity (Goldstein et al., 2009). Abnormal behavior can be noted in pup and yearling CSLs thought to have been exposed to DA in utero, though brain lesions in these animals are often not identified. Domoic acid can be detected in feces, serum, urine, and gastric contents using tandem mass spectrometry coupled with liquid chromatographic separation. Due to the short half-life, especially in serum, it will only be present in animals very recently exposed to the toxin (Fire et al., 2011, Silvangi et al., 2005).
Additional diseases of vitamin deficiency and toxic conditions are presented in Table 23.1 .
Table 23.1.
Miscellaneous Toxic and Nutritional Conditions of Pinnipeds
| Cause | Lesions | Species Affected |
|---|---|---|
| Thiamine deficiencya | Polioencephalomalcia especially in the cerebellum | Harp and harbor seals |
| Vitamin E deficiencyb | myopathy, steatitis | California sea lion |
| Scrombroid poisoningb | Nonspecific respiratory tract congestion and edema, mucus membrane irritation | Multiple |
| Lead toxicosisc | Erythrophagocytosis and hemosiderosis in lymph nodes and liver, myocardial necrosis, cerebral, and intramyelinic edema | Harbor seal |
| Organochlorinesd, e | Claw deformities, uterine stenosis, still birth, thyroid abnormalities, skull bone lesions, adrenal hyperplasia | Gray seals, harbor seals, ringed seals |
| Oil/PAHsd | fouling of pelage, impaired thermoregulation, epidermal acanthosis and hyperkeratosis, intramyelinic edema, neuronal necrosis, axonal swelling, conjunctivitis, keratitis | Harbor seals, fur seals, ringed seals |
Wohlsein, P., Peters, M., Geburek, F., Seeliger, F., Böer, M., 2003. Polioencephalomalacia in captive harbour seals (Phoca vitulina). Transbound. Emerg. Dis. 50(3), 145–150.
Worthy, G.A., 2001. Nutrition and energetics. In: Dierauf, L and Gulland, F.M.D. (eds). CRC Handbook of Marine Mammal Medicine, 2nd Edition, CRC Press, Boca Raton, FL, pp. 791–817.
Zabka, T.S., Haulena, M., Puschner, B., Gulland, F.M., Conrad, P.A., Lowenstine, L.J., 2006. Acute lead toxicosis in a harbor seal (Phoca vitulina richardsi) consequent to ingestion of a lead fishing sinker. J. Wildl. Dis. 42(3), 651–657.
O'Hara, T.M. Hart, L. 2018. Environmental Toxicology. In: Gulland, F.M.D., Dierauf, L.A., Whitman, K.L. (eds.). CRC Handbook of Marine Mammal Medicine, 3rd Edition, CRC Press, Boca Raton, FL, pp. 285-306.
Bergman, A., Olsson, M., 1985. Pathology of baltic grey seal and ringed seal females with special reference to adrenocortical hyperplasia: is environmental pollution the cause of a widely distributed disease syndrome. Finn. Game Res. 44, 47–62.
Congenital/Genetic
Congenital abnormalities have most often been identified in harbor and northern elephant seals and northern fur seals, with sporadic case reports in other species (Colegrove, 2018, Spraker and Lander, 2010, St. Leger and Nilson, 2014, Trupkiewicz et al., 1997). In harbor seals, the most common abnormalities identified are hernias, skeletal malformations, and proliferative conditions. In harbor seals there may be functional patency of the foramen ovale and ductus arteriosus up to 6–7 weeks of age, therefore, these should not be considered congenital defects in very young pups without additional evidence of cardiac disease (Dennison et al., 2011). In elephant seals, hydrocephalus and cardiac abnormalities are most common. Low genetic diversity likely plays a role in the high prevalence of congenital defects noted in elephant seals (Trupkiewicz et al., 1997).
Trauma
Trauma in pinnipeds can include anthropogenic factors, such as gunshot, vessel strike, and entanglement in marine debris and fishing gear (Goldstein et al., 1999, Moore et al., 2013). Entanglement in fishing gear, plastic, or metal rings can cause chronic infections and respiratory problems. Peracute underwater entrapment or by-catch is a major source of mortality. Lesions in affected animals include evidence of a struggle, net marks, or changes suggestive of hypoxia including pulmonary congestion, edema, or emphysema. Gas-bubbles may also be detected in multiple tissues (Moore et al., 2009). Systematic detailed, postmortem evaluation is essential to diagnosing anthropogenic trauma and criteria have been reviewed by Moore et al. (2013).
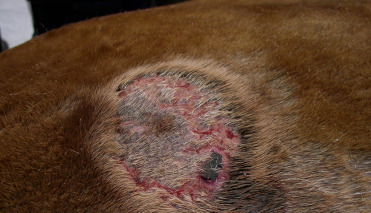
Predation by sharks and killer whales is a recognized major cause of trauma. Spiral lacerations and wounds have been associated with gray seal predation with gray seals predating upon harbor seals, other gray seals, and harbor porpoise (Fig. 23.6 ) (Brownlow et al., 2016). Conspecific trauma can be associated with breeding and crowding on rookeries.
Figure 23.6.
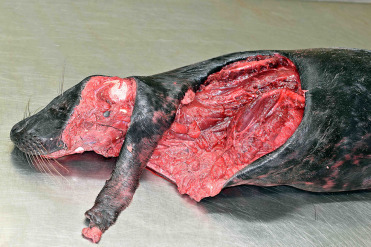
Conspecific predation lesions in a gray seal.
Lesions include large spiral blubber defects, straight wound margins, and long strips of tissue remaining on the body.
(Photo Courtesy of Institute for Terrestrial and Aquatic Wildlife Research)
Inflammatory Non-infectious
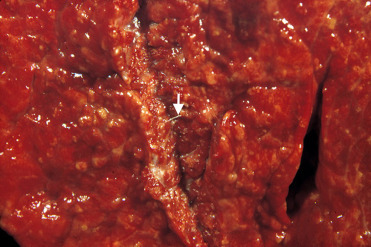
Ocular disease affecting the lens and cornea is common in pinnipeds, especially those in captivity. In otariids, progressive keratitis is characterized by corneal edema, keratitis, corneal ulceration, pigmentation, vascularization, and in severe cases, stromal thinning and secondary bacterial and fungal infections (Fig. 23.7 ). Exposure to low salinity water, oxidants (ozone), and concurrent lens disease (lens induced keratitis), and exposure to ultraviolet radiation are also potential factors (Colitz et al., 2010a). Cataracts and lens luxation also occur frequently in pinnipeds in captivity (Fig. 23.8 ). Exposure to UV light, concurrent or previous keratitis and fighting are risk factors for lens disease (Colitz et al., 2010b).
Figure 23.7.
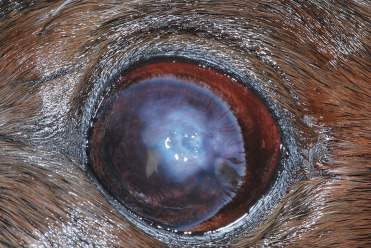
Active otariid keratopathy in a California sea lion.
Lesions are characterized by corneal edema, fibrosis, stromal loss and ulceration, and vascular and pigment migration.
(Photo Courtesy of C. Colitz)
Figure 23.8.
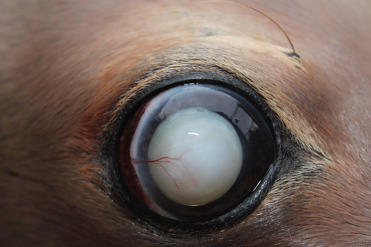
Cataract, anterior lens luxation, and keratopathy in a California sea lion.
(Photo Courtesy of C. Colitz)
Miscellaneous
Cutaneous microabsesses and ulcerations can occur due to sepsis and vasculitis. Folliculitis secondary to bacterial infection has been reported in captive walrus (Mulcahy and Fravel, 2018). A skin disease characterized by alopecia, ulceration, and hyperkeratosis was described in northern elephant seals though diagnosis has been less frequent in more recent years (Beckmen et al., 1997). Alopecia or abnormal molts can be noted on occasion, most commonly in phocids. This may be related to environmental, endocrine, dietary, or stress mediated processes, however, the pathogenesis has not been elucidated.
Myocardial interstitial fibrosis is seen occasionally in aged CSLs, most often in males. This condition has been associated with acute death during anesthetic procedures in several animals. Age-related arteriosclerosis can be noted in pinnipeds. Myocardial necrosis can be secondary to hypoxia in pinnipeds that develop complications during anesthetic events. Valvular endocarditis due to bacterial infection has been reported in CSLs (Kim et al., 2002).
Acute death due to cerebral infarction has been reported occasionally in aged pinnipeds and may be related to age-associated arteriosclerosis. Few scattered meningeal lymphocytes may be seen incidentally in free-ranging CSLs. Cerebral edema and meningitis are common lesions identified in septic phocid pups.
Systemic AA amyloidosis has been reported in CSLs and likely develops secondary to chronic inflammatory conditions. The kidney, thyroid gland, and vessels are most commonly affected. In the kidney, amyloid deposition occurs within the interstitium and glomeruli and often in a distinct band-like region along the corticomedullary junction (Colegrove et al. 2009a).
Urolithiasis is occasionally diagnosed in pinnipeds, though an underlying cause for urolith development is unknown (Colegrove, 2018 ; Dennison et al., 2007, Colegrove, 2018). Multifocal papillary foci of urothelial hyperplasia are noted occasionally within the urinary bladder of CSLs and are not related to sea lion UGC (Fig. 23.9 ). The cause is unknown and lesions are not typically associated with significant cystitis.
Figure 23.9.
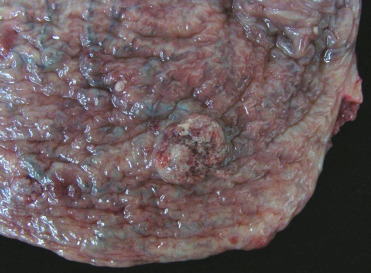
Epithelial hyperplasia in the urinary bladder of a California sea lion.
Spiral bacteria morphologically consistent with Helicobacter sp. have been identified in the stomach of CSLs; however, often they are not associated with gastritis. Spiral to filamentous bacteria can be seen within colonic crypts of debilitated CSLs with dysbiosis. Protozoa morphologically compatible with trichomonads rarely can be found within gastric glands of free-ranging CSLs and also may be a form of dysbiosis.
Acute perforating proximal duodenal/pyloric ulceration is a condition identified in free-ranging, often malnourished pup and yearling CSLs. Lesions often lead to acute death. Histologically, there is inflammation and necrosis at the site of ulceration. Ulceration is not thought to be related to parasitism, bacterial infection, or any specific extra-gastrointestinal disease. Instead ulcers may develop due to alterations in gastric emptying, abnormal gastrointestinal motility, or acid hypersecretion (Zabka et al., 2005).
Intestinal volvulus and intussusception have been reported in multiple species and may be related to bacterial enteritis (Siebert et al., 2007).
Hepatic hemosiderosois is seen frequently in several pinniped species including young northern elephant and harbor seals, Hawaiian monk seals, northern fur seals, and CSLs. Mild chronic cholecystitis and portal hepatitis are common findings in wild pinnipeds secondary to trematode infection and trematode-associated pigment accumulation can occur. Cystic hyperplasia of the gall bladder is a common finding in older sea lions. This change may or may not have associated inflammation or infection. Small, randomly distributed granulomas in hepatic parenchyma can be noted secondary to parasite migration.
Dental disease, abnormal tooth wear, gingivitis, secondary bacterial infections, and osteomyelitis have been reported in multiple pinniped species (Colegrove, 2018). Tusk pulp bacterial infections have been an occasional problem in walruses in captivity secondary to wear. These infections can extend into the overlying dorsal bone causing osteomyelitis and fistulous tracts into the nasal cavity. Preventative measures aimed at preventing worn tusks have helped to manage this condition (Mulcahy and Fravel, 2018).
Neoplastic
There have been a number of case reports describing neoplasia in pinnipeds and reviews detailing reported neoplasms have been previously published. Among the pinnipeds, neoplasms are most frequently diagnosed in free-ranging and captive CSLs (Colegrove, 2018, Newman and Smith, 2006). In captive CSLs, mammary carcinoma, lymphoma, and laryngeal squamous cell carcinoma are among the most common. Neoplasia is less common in captive harbor and gray seals, though Flower et al. (2014) documented esophageal squamous cell carcinoma in six aged harbor seals possibly associated with regurgitation. Uterine leiomyomas can be seen in aged pinnipeds.
Urogenital carcinoma (UGC) in CSLs is the most extensively studied neoplastic disease affecting pinnipeds over the past 2 decades and is considered an important wildlife model of carcinogenesis (Browning et al., 2015). A high prevalence of UGC occurs in adult CSLs stranding along the west coast of North America, with 26% of adult deceased CSLs affected at one rehabilitation facility over a 15 year period. Occasional cases of UGC have been diagnosed in captive CSLs, most of which were wild-born. Carcinogenesis is considered multifactorial with infectious agents, genetic factors, and high levels of organochlorine contaminants all possibly playing a role in tumor development. Otarine herpes virus-1 (OtHV-1), a gammaherpesvirus that is sexually transmitted, has been associated with UGC (Buckles et al., 2007). Alterations in p53, the heparanase 2 (HPSE2) gene, endogenous hormones, and contaminants that interact with steroid hormone receptor have all been postulated as other potential factors involved in UGC (Browning et al., 2015, Colegrove et al., 2009b).
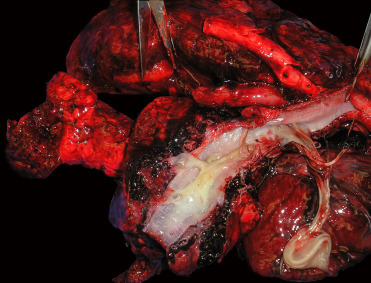
Sea lions with UGC can have perineal edema, hind limb paresis, abdominal effusion, and penile prolapse with necrosis (Gulland et al., 1996a). Tumors originate in the cervix, vagina, penis, or prepuce and often there is widespread metastasis at the time of death. Subtly raised plaques, thickening, roughing, or dulling of the mucosa, and/or tan firm masses can be noted within affected urogenital epithelium (Figs. 23.10 and 23.11 ). Metastatic tumors often are found in the inguinal and sublumbar lymph nodes as well as many other organs, such as liver, kidney, and lung. Hydronephrosis is a common sequela due to ureter compression by sublumbar lymph node masses and can be used to diagnosis UGC antemortem via ultrasound.
Figure 23.10.
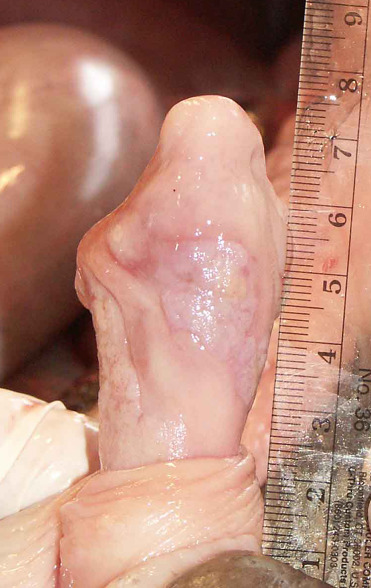
Urogenital carcinoma in the penis of a California sea lion.
Figure 23.11.
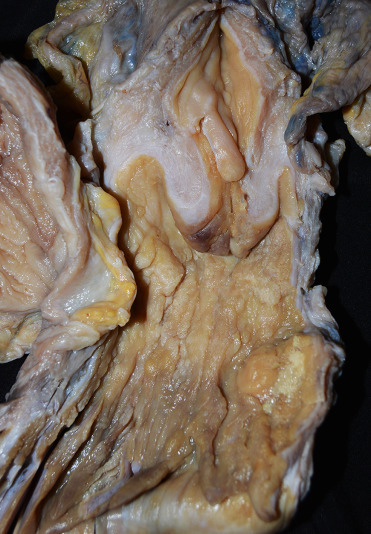
Urogenital carcinoma in the cervix and vagina of a California sea lion (formalin fixed).
There is thickening of the vaginal and cervical mucosa and a polypoid mass in the vagina.
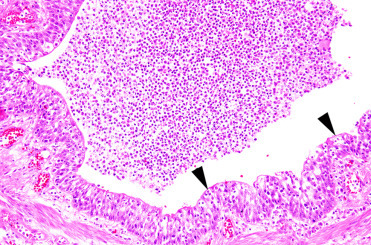
Carcinoma in situ in the reproductive tract is often very difficult to discern grossly. The in situ lesions may be seen incidentally in CSLs that die from other causes without metastatic disease. As such, it is critically important to routinely perform gross and histologic examination of the entire reproductive tract of male and female CSLs to identify these tumors (Colegrove et al., 2009b, Gulland et al., 1996a). Histologically, intraepithelial lesions may be observed multifocally along cervical, vaginal, penile, or preputial epithelium and often large regions of epithelium may be affected. Penile or prostatic urethral epithelium is rarely affected. Intraepithelial lesions may extend into the uterus along the endometrial surface; however, this is thought to be a form of intraepithelial metastasis and not primary transformation of uterine epithelium. Affected genital epithelium is often markedly thickened with dysplastic cells and disordered maturation involving up to the entire thickness of the epithelium (Fig. 23.12 ). Increased numbers of mitotic figures can be seen. Direct infiltration of the underlying submucosa is difficult to identify, even in animals with widely metastatic disease. Multiple distinct morphologic patterns including squamous, cribiform, comedone, or basaloid can be observed in metastases. Neoplastic cells are polygonal with large nuclei and there is often a high mitotic rare with bizarre mitotic figures or multinucleated neoplastic cells (Fig. 23.13 ). Central necrosis within masses is common and lymphoplasmacytic inflammation is adjacent to both intraepithelial lesions and surrounding metastases. Despite the association between UGC and OtHV-1, intranuclear inclusions are noted infrequently. When present, they are most common in genital intraepithelial lesions in which there is superficial ballooning degeneration (Fig. 23.14 ). Inclusions are extremely rare in metastatic masses.
Figure 23.12.
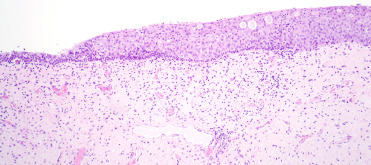
In situ urogenital carcinoma in a California sea lion.
Transition zone between normal cervical epithelium (left) and carcinoma in situ (right).
Figure 23.13.
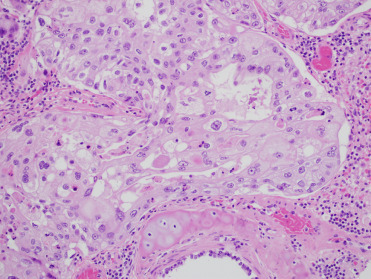
Metastatic urogenital carcinoma in the lung of a California sea lion.
Note the marked anisocytosis and anisokaryosis.
Figure 23.14.
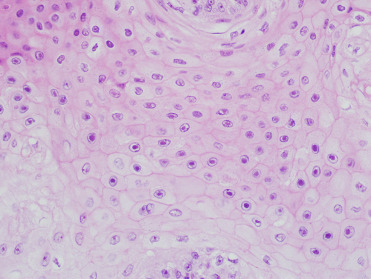
Urogenital carcinoma in a California sea lion.
Numerous Otarine herpes virus-1 intranuclear inclusions in neoplastic genital epithelium.
Infectious diseases
DNA Viruses
Phocine herpesvirus-1 (PhHV-1) is classified in the subfamily Alphaherpesvirinae and has been isolated from harbor and gray seals in Europe and from both coasts of North America. Serosurveys have documented titers in free-ranging phocids in both the southern and northern hemispheres (Goldstein et al., 2003). PhHV-1 can be associated with high morbidity and mortality and outbreaks have been documented in rehabilitation facilities (Gulland et al., 1997b). Clinical disease and fatal generalized infection are most common in neonates and young pups. Horizontal transmission via direct contact and aerosols is most common though vertical transmission has been identified (Goldstein et al. 2004).
In Pacific harbor seals, multifocal adrenocortical and hepatic necrosis are the most common lesions associated with infection. Within and adjacent to areas of necrosis, cells may have smudgy eosinophilic intranuclear inclusions (Fig. 23.15 ). Adrenal necrosis may be associated with mineralization (Fig. 23.16 ). Thymic atrophy is often noted in affected pups and lesions secondary to bacterial infection and septicemia, including omphalophlebitis, pneumonia, and meningoencephalitis, may be concurrently identified (Gulland et al., 1997b). Oral ulceration and small intestinal crypt dilation and necrosis are rare lesions. In infected European seals, interstitial pneumonia and massive liver necrosis have been reported (Borst et al., 1986). Virus isolation, PCR, IHC, and serology (ELISA) are used to diagnose infections.
Figure 23.15.
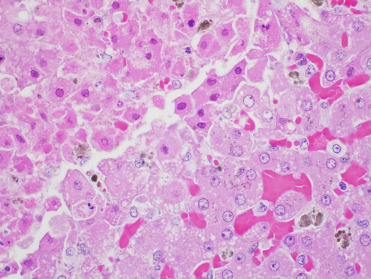
Phocine herpesvirus-1 infection in the liver of a harbor seal.
There is hepatic necrosis with intranuclear inclusions that often fill the nucleus.
Figure 23.16.
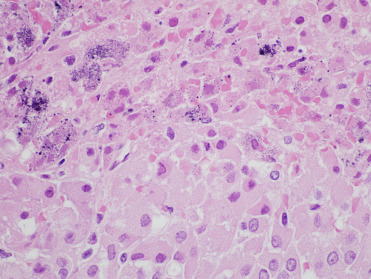
Phocine herpesvirus-1 infection in the adrenal gland of a harbor seal.
Note the cortical necrosis with intranuclear inclusions and prominent mineralization.
Otarine herpesvirus-1 (OtHV-1) is in the subfamily Gammaherpesvirinae and genus Rhabinovirus. The virus has been amplified via PCR from CSLs with UGC (see Neoplasia section) and is considered a likely factor in tumor development. Viral DNA can be amplified from the urogenital tract and, less frequently, the oral cavity of sea lions with no clinical evidence of UGC and prevalence of virus in the genital tract is higher in adults than in juveniles. Sexual transmission is thought to occur and females may transmit the virus to pups during birth (Browning et al., 2015, Buckles et al., 2007). The virus has also been associated with a single case of genital carcinoma in a South American fur seal and detected in a Steller sea lion without evidence of neoplasia (Dagleish et al., 2013).
Pox viruses have been identified in skin and mucosal lesions from numerous pinniped species including CSLs, South American sea lions, Stellar sea lions, northern fur seals, and harbor and gray seals. Pinniped poxviruses are in the genus Parapoxvirus and viruses from Atlantic and Pacific pinnipeds are phylogenetically distinct (Nollens et al., 2006c). There is widespread exposure of free-ranging pinnipeds and transmission occurs through direct contact, such as from conspecific rubbing or biting behaviors. Outbreaks have occurred in rehabilitation facilities, especially in young animals, and lesions may develop during times of stress or concurrent disease (Nollens et al., 2006a).
Poxvirus lesions appear as raised and firm, sometimes ulcerated nodules along the head, neck, and thorax (Fig. 23.17 ). Nodular or plaque-like lesions may also occur along the tongue and oral commissure (Fig. 23.18 ). Histologically, there is marked epidermal and follicular hyperplasia with ballooning degeneration in the stratum spinosum. Moderately sized, round to chunky eosinophilic cytoplasmic inclusions are noted within epidermal and follicular keratinocytes (Fig. 23.19 ). There is variable epidermal necrosis and ulceration and often moderate associated mixed inflammation. Dermal fibrosis is occasionally noted (Nollens et al., 2006b). Lesions will often spontaneously regress. Sealpox is a zoonotic disease that causes nodular cutaneous lesions in people and transmission likely occurs when virus enters broken skin (Waltzek et al. 2012).
Figure 23.17.

Poxviral skin lesions along the face and neck of a California sea lion.
(Photo Courtesy of J. St. Leger, SeaWorld)
Figure 23.18.
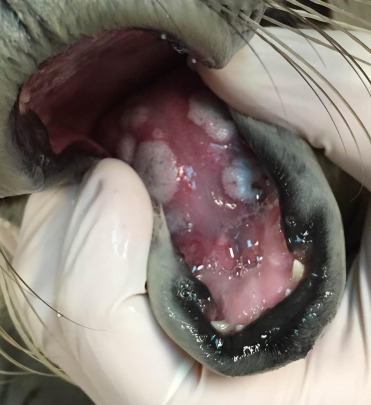
Poxviral glossal lesions in a harbor seal.
(Photo Courtesy of The Marine Mammal Center)
Figure 23.19.
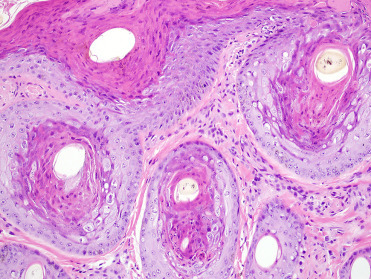
Poxviral infection in skin from a California sea lion.
Note the epidermal and follicular hyperplasia, hyperkeratosis, and eosinophilic intracytoplasmic inclusions.
Adenovirus infections have been reported sporadically in CSLs and recently been associated with an outbreak involving multiple otariids in managed care (Britt et al., 1979, Inoshima et al., 2013). Viral sequence analysis has determined that California sea lion adenovirus-1 (CSLAdV-1) is unique from canine adenovirus-1 (CAV-1) and represents a new member of the genus Mastadenovirus (Goldstein et al. 2011). Stellar sea lions have antibodies to CAV-1 and canine adenovirus-2 (CAV-2); however, canine adenoviral antibodies have not been detected in one infected CSL (Burek et al., 2005). Multifocal necrotizing hepatitis with hepatocellular intranuclear inclusions is the most common lesion reported in CSL infections (Fig. 23.20 ). In 1 case, endothelial infection was identified in the lung, lymph nodes, and eye in the absence of hepatic lesions. Ocular lesions included corneal edema, keratitis, and iridocyclitis. Several cases of necrotizing enteritis in CSLs have also been attributed to adenoviral infection; one CSL exhibited diarrhea prior to death (Fig. 23.21 ). CSLAdV1 has been amplified via PCR from liver and feces in affected individuals and adenoviral particles have been identified via transmission electron microscopy (TEM) in several cases. The virus can also be amplified from feces of individuals without evidence of clinical disease, suggesting that some animals may be subclinical shedders of the virus.
Figure 23.20.
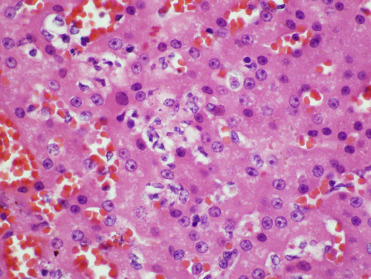
Adenovirus infection in the liver of a California sea lion.
There is hepatic necrosis with large intranuclear inclusions.
Figure 23.21.
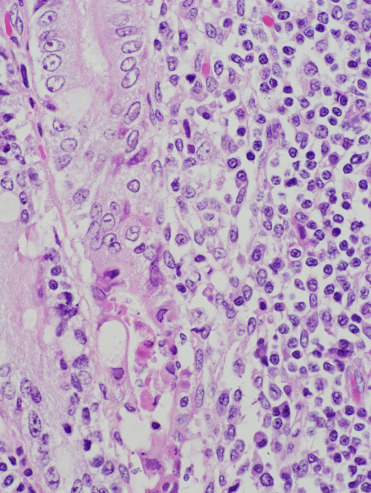
Adenovirus infection in the intestine of a California sea lion.
Infection is characterized by necrosis of intestinal crypt and mid mucosal epithelium, mild to moderate inflammation, and intranuclear inclusions.
RNA Viruses
Morbillivirus infection in pinnipeds was first observed in Baikal Seals in 1987. The infections were likely due to spread of a canine distemper virus (CDV) outbreak in terrestrial mammals (Grachev et al., 1989). In 1988, the first large-scale epizootic of phocine distemper virus (PDV) in harbor seals followed in European waters of the North and Baltic Seas causing 23,000 deaths, and in 2002 another outbreak occurred in the same region and affected 30,000 seals. Several stranding peaks in pinnipeds on the Atlantic coast of the United States have also been linked to PDV infections in harbor, harp, hooded, and gray seals but these have never caused significant die-offs as in European waters. Positive morbillivirus antibody titers are regularly found in Arctic seal species and provide evidence of widespread distribution of PDV in seal populations (Duignan et al., 2014, Härkönen et al., 2006).
Morbilliviruses are most commonly transmitted through respiratory, nasal, or ocular secretions. Clinical findings with PDV include oculonasal discharge, conjunctivitis, keratitis, coughing, dyspnea, diarrhea, abortion, head tremors, convulsions, and increased buoyancy. Pathological findings include bronchointerstitial pneumonia, pulmonary atelectasis, congestion, and edema as well as pulmonary, mediastinal and subcutaneous emphysema, nonsuppurative encephalitis, and lymphocyte depletion in different lymphoid tissues (Fig. 23.22 ). Intracytoplasmic and intranuclear eosinophilic inclusion bodies can be found in the respiratory epithelium, biliary and pancreatic ducts, renal pelvis, urinary bladder, gastrointestinal epithelium, neurons, and astrocytes (Duignan et al., 2014, Kennedy, 1998, Siebert et al., 2010). Due to viral-induced immunosuppression, PDV is often associated with secondary parasitic (pulmonary nematodiasis), bacterial (e.g., Bordetella bronchiseptica) and concurrent viral infections (Herpesvirus, Influenza virus). The diagnosis of CDV or PDV is accomplished by immunohistochemistry using cross-reactive monoclonal antibodies for the morbillivirus nucleoprotein and by the detection of morbillivirus-specific nucleic acid in tissues by RT-PCR. Antibody titers allow assessment of exposure and susceptibility to PDV (Duignan et al., 2014, Ludes-Wehrmeister et al., 2016).
Figure 23.22.
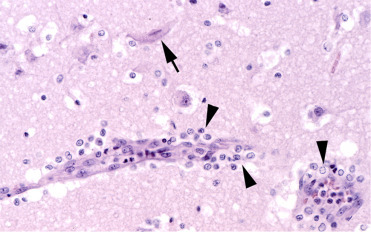
Phocine distemper virus infection in the brain of a harbor seal.
Encephalitis is characterized by mild perivascular lymphohistiocytic inflammation (arrowheads) and neuronal necrosis (arrow).
(Photo Courtesy of P. Wohlsein, Department of Pathology, University of Veterinary Medicine Hannover, Germany)
Mortality events associated with Influenza A have occurred in harbor seals in the United States in 1979/1980 (H7N7) and 1982/1983 (H4N5), when large numbers of seals were found dead in the northeast (Geraci et al., 1982, Hinshaw et al., 1984, Webster et al., 1981). In 1991/1992 two additional isolates of influenza subtypes, H4N6 and H3N3, were obtained from harbor seals from the northeast United States (Callan et al., 1995). In Europe, harbor seal mortality due to influenza A (H10N7) infection occurred in 2014/2015, starting in waters around Sweden and Denmark, and subsequently spreading to the Wadden Sea of Germany and the Netherlands resulting in large numbers of dead seals (Bodewes et al., 2015, Zohari et al., 2014). Avian influenza A (H3N8) was also associated with a 2011 harbor seal die-off in the northeast United States (Anthony et al., 2012). Influenza B, believed to be an exclusively human pathogen, was only reported from a single case, a young seal from the Dutch Wadden Sea (Osterhaus et al., 2000).
Clinical findings of influenza are similar to PDV and include dyspnea, nasal discharge, lethargy, and emphysema. Pathological findings include partially collapsed lungs, pulmonary congestion, necrotizing bronchitis and bronchiolitis, hemorrhagic alveolitis, bronchial gland adenitis, and occasionally interstitial pneumonia (Fig. 23.23 ). Additional concurrent respiratory tract lesions caused by parasitic or bacterial infections are often present (Bodewes et al., 2015, Geraci et al., 1982, Hinshaw et al., 1984, Siebert et al., 2010). Diagnosis is accomplished by ELISA, immunohistochemistry using influenza A virus nucleoprotein-specific monoclonal antibody and RT-PCR in lung and throat swabs (Bodewes et al., 2015; de Boer et al., 1990; Vahlenkamp and Harder, 2006).
Figure 23.23.
Influenza A H10N7 infection in the lung of a harbor seal characterized by acute necrotizing bronchitis (arrowheads).
(Photo Courtesy of P. Wohlsein, Department of Pathology, University of Veterinary Medicine Hannover, Germany)
Marine caliciviruses are within the vesivirus genus and include over 40 serotypes including San Miguel San Lion viruses (SMSV-1-17) and Walrus calicivirus (WCV) (McClenahan et al., 2009). Isolates and serologic evidence of exposure is documented in multiple species and in both clinically affected and healthy individuals. An unusually wide variety of marine and terrestrial species including fish, birds, and other mammals may be infected, indicating that these viruses have an ability to infect many species of marine animals, some of which may act as reservoirs for others. Opal eye perch are a likely natural host. These viruses are indistinguishable from caliciviruses causing swine vesicular exanthema, and feeding swine uncooked garbage containing marine mammals and fish was a likely route of introduction into the swine population. Caliciviruses are zoonotic, with rare cases of human vesicular dermatitis and flu-like illness after laboratory and field exposures (Smith et al., 1998, Waltzek et al., 2012).
Gross findings include vesicular dermatitis that progresses to ulceration primarily of the nonhaired surfaces of the flippers (Fig. 23.24 ). Occasionally, necrosis of underlying digits can occur. Ulcerative stomatitis and ulcerative and nodular dermatitis of the lips, nasal planum, and chin have also been reported in CSLs. Abortion and premature birth in CSLs have been attributed to calicivirus based on positive viral isolation and serologic evidence of exposure, but causation has not been proven. Lesions begin as spongiosis of the stratum spinosum followed by subcorneal vesicle formation without formation of inclusion bodies (Moeller, 2003). The vesicles are very transient and ulcers with secondary bacterial infections are common. Diagnosis is by culture or RT-PCR (Moeller, 2003, Smith et al., 1998). Because of the importance in differentiating these viruses from foreign animal diseases, a real-time reverse transcription PCR has recently been developed that can identify most serotypes and differentiate them from reportable vesicular diseases (McClenahan et al., 2009).
Figure 23.24.

Calicivirus infection in the skin of a Stellar sea lion. Infection causes vesicles that may rupture and ulcerate.
Miscellaneous viral diseases are reported on Supplemental Table e3.
Table e3.
Miscellaneous Viral Diseases of Pinnipeds
| Etiology | Virus Family | Lesions | Species Affected | Diagnosis |
|---|---|---|---|---|
| West Nile Virusa | Flavvivirus | Polioencephalomyelitis | Harbor seal | Histopathology, PCR, IHC |
| B19-like Parvovirusb | Parvoviridae | Meningoencephalitis | Harbor seal | Histopathology, PCR, viral culture |
| Coronavirusc,d | Coronaviridae | Hemorrhagic and necrotizing pneumonia with concurrent Pseudomonas sp. infection | Harbor seals | Histopathology, PCR |
| Polyomaviruse,f | Polyomaviridae | Glossal fibropapilloma; placental inclusions | California sea lions; Northern fur seal | Histopathology, PCR |
| Papillomavirusg | Papillomaviridae | Axilllary and inguinal papillomas | California sea lions | Histopathology, PCR |
| Herpesvirush,i | Gammaherpesvirinae | Tongue, oral ulcers; inclusions in tonsil epithelium | Elephant seals | Histopathology, PCR |
| Herpesvirusj | Gammaherpesvirinae | None | Monk seals | Histopathology, PCR |
| Herpesvirusk | Unknown | Inclusions in stomach, esophagus, and oral epithelium | California sea lions | Histopathology, PCR |
delPiero, F.D., Stremme, D.W., Habecker, P.L., Cantile, C., 2006. West Nile flavivirus polioencephalomyelitis in a harbor seal (Phocavitulina). Vet. Pathol. 43(1), 58–61.
Bodewes, R., García, A.R., Wiersma, L.C., Getu, S., Beukers, M., Schapendonk, C.M., van Run, P.R., van de Bildt, M.W., Poen, M.J., Osinga, N., Contreras, G.J.S., 2013. Novel B19-like parvovirus in the brain of a harbor seal. PLoS One 8(11) e79259.
Nollens, H.H., Wellehan, J.F., Archer, L., Lowenstine, L.J., Gulland, F.M., 2010. Detection of a respiratory coronavirus from tissues archived during a pneumonia epizootic in free-ranging Pacific harbor seals Phoca vitulina richardsii. Dis. Aquat. Organ. 90(2) 113–120.
Gaffney, P., Colegrove, K., Gulland, F., Byrne, B., Jang, S., Edgar, K., Lowenstine, L.J., 2008. Pathologic, microbiologic and epidemiologic characterization of Pseudomonas sp. In: California sea lions (Zalophus californianus) and Pacific harbor seals (Phocavitulina). In: Proceedings of the 39th Annual Conference of the International Association for Aquatic Animal Medicine, pp. 10–14.
Colegrove, K.M., Wellehan Jr., J.F., Rivera, R., Moore, P.F., Gulland, F.M., Lowenstine, L.J., Nordhausen, R.W., Nollens, H.H., 2010. Polyomavirus infection in a free-ranging California sea lion (Zalophus californianus) with intestinal T-cell lymphoma. J. Vet. Diagn. Invest. 22(4), 628–632.
Duncan, C., Goldstein, T., Hearne, C., Gelatt, T., Spraker, T., 2013.Novel polyomaviral infection in the placenta of a northern fur seal (Callorhinusursinus) on the Pribilof Islands, Alaska, USA. J. Wildl. Dis. 49(1), 163–167.
Rivera, R., Robles-Sikisaka, R., Hoffman, E.M., Stacy, B.A., Jensen, E.D., Nollens, H.H., Wellehan, J.F., 2012.Characterization of a novel papillomavirus species (ZcPV1) from two California sea lions (Zalophus californianus). Vet. Microbiol. 155(2), 257–266.
Maness, H.T., Nollens, H.H., Jensen, E.D., Goldstein, T., LaMere, S., Childress, A., Sykes, J., Leger, J.S., Lacave, G., Latson, F.E., Wellehan, J.F., 2011.Phylogenetic analysis of marine mammal herpesviruses. Vet. Microbiol. 149(1), 23–29.
Goldstein, T., Lowenstine, L.J., Lipscomb, T.P., Mazet, J.A., Novak, J., Stott, J.L., Gulland, F.M., 2006. Infection with a novel gammaherpesvirus in northern elephant seals (Mirounga angustirostris). J. Wildl. Dis. 42(4), 830–835.
Goldstein, T., Gulland, F.M., Braun, R.C., Antonelis, G.A., Kashinsky, L., Rowles, T.K., Mazet, J.A., Dalton, L.M., Aldridge, B.M., Stott, J.L., 2006. Molecular identification of a novel gamma herpesvirus in the endangered Hawaiian monk seal (Monachus schauinslandi). Mar. Mammal Sci. 22(2), 465.
Colegrove, unpublished data
Bacteria
Infection with Leptospira sp. is most commonly reported in free-ranging pinnipeds including CSLs, harbor, and northern elephant seals along the Pacific coast of North America (Colegrove et al., 2005b, Gulland et al., 1996b). Sporadic infections and outbreaks have been reported in captive animals and exposure has been documented serologically in other geographic regions (Kik et al., 2006, Mackereth et al., 2005). Leptospira interrogens serovar Pomona has been implicated in disease in CSLs. Based on the long-term serosurveys utilizing the microscopic agglutination test leptospirosis is thought to be enzootic in the CSL population. Periodic epizootics occur every 3–5 years and result in large numbers of CSLs stranding with clinical leptospirosis (Gulland et al., 1996b, Lloyd-Smith et al., 2007). An asymptomatic, chronic carrier state may provide a mechanism for persistence of the bacterium in the population (Prager et al., 2013). Though cases in free-ranging CSLs can occur throughout the year they are most commonly noted between July and December (Gulland et al., 1996b). Little is known about the epidemiology of leptospirosis in other pinniped species, where infections occur more sporadically or as small outbreaks in rehabilitation situations (Colegrove et al., 2005b). Leptospirosis may also cause abortion (Gulland et al., 1996b).
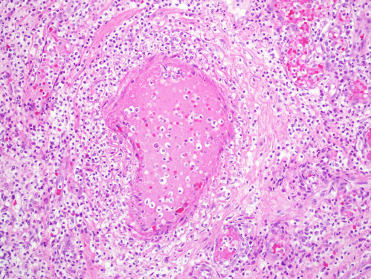
Pinnipeds with leptospirosis have clinical signs and serum biochemistry values consistent with renal failure including polydipsia and elevated BUN, creatinine, and phosphorus. Grossly, kidneys are swollen, pale tan, and exhibit lack of renule and corticomedullary differentiation (Fig. 23.25 ). Histologically, there is subacute to chronic lymphoplasmacytic tubulointerstitial nephritis, typically with a plasma cell heavy inflammatory infiltrate (Fig. 23.26 ). Leptospira-related liver lesions are not noted. In some phocids with infection, significant tubular necrosis is more severe than inflammation suggesting that the animals die in the more acute stage of infection. Infection can be confirmed histologically by demonstrating spirochetes with silver stains or IHC. IHC is particularly useful in animals with chronic infections or those treated with antibiotics since the spirochetes may be difficult to visualize; labeled antigen will be present in renal tubular epithelial cells and interstitial macrophages (Colegrove et al., 2005b, Gulland et al., 1996b). PCR can also be used for diagnosis. Given the zoonotic potential of the bacterium, appropriate personal protective measures should be undertaken when necropsying affected animals (Waltzek et al., 2012)
Figure 23.25.
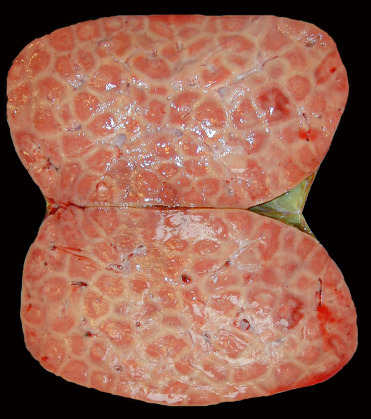
Leptospira interrogens serovar Pomona infection in the kidney of a California sea lion.
Note the swollen renules and pale cortices.
(Photo Courtesy of The Marine Mammal Center)
Figure 23.26.
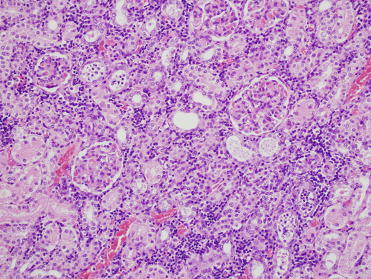
Leptospiral nephritis in a California sea lion. Lymphoplasmacytic inflammation multifocally infiltrates the interstitial parenchyma.
Mycobacteriosis was first described in a group of captive pinnipeds at a marine park in Australia (Forshaw and Phelps, 1991), and later classified as caused by a previously undescribed member of the Mycobacterium tuberculosis complex, M. pinnipedii (Cousins et al., 2003). Since then the disease has been reported in a number of captive otariid species worldwide, most frequently affecting Southern sea lions (Kriz et al., 2011). Infection in free ranging pinnipeds is confined to the southern hemisphere and transmission between species is thought to be by sub-Antarctic fur seals whose range overlaps with eight other otariid species (Bastida et al., 1999). To date, mycobacteriosis due to M. pinnipedii has not been reported for any phocid species; however, the potential host range is broad and transmission from infected fur seals and sea lions has been described for zoo species, domestic cattle, and humans (Cousins et al., 2003, Kiers et al., 2008, Loeffler et al., 2014, Moser et al., 2008, Thompson et al., 1993, Thorel et al., 1998). The most likely route of infection is aspiration of aerosolized bacteria shed in respiratory secretions of infected pinnipeds.
Pinnipeds with mycobacteriosis typically present in poor body condition. The majority of clinical infections involve the thoracic organs, supporting inhalation as the dominant route of infection, with two main gross presentations. The first involves granulomatous to pyogranulomatous pleuropneumonia, thoracic lymphadenitis and mediastinitis with pronounced fibrinous or serosanguinous effusion (Fig. 23.27 ). In the second type of presentation, there are multifocal caseating granulomas within the lungs and thoracic lymph nodes. Several cases of respiratory mycobacteriosis have included concurrent granulomatous lesions in the mesenteric lymph nodes, possibly reflecting spread into the abdominal cavity through swallowing infected respiratory secretions (de Amorim et al., 2014, Kriz et al., 2011). Meningeal, hepatic, and disseminated granulomas have also been reported (Forshaw and Phelps, 1991). Histologically, lesions are dominated by aggregates of epithelioid macrophages containing few to many intracytoplasmic acid-fast bacilli admixed with variable numbers of lymphocytes, plasma cells and neutrophils. Caseous necrosis of the centers of these granulomas is common; mineralization is generally minimal and multinucleated giant cells are rare. A presumptive diagnosis of mycobacteriosis can be made during post mortem examination based on the presence of acid-fast positive bacteria within granulomas. Confirmation of M. pinnipedii as the causative agent requires either culture and biochemical assays, which can be slow and laborious, or PCR. Population monitoring is best accomplished by necropsy of all dead pinnipeds and monitoring of live animals including lymph node cytology, thoracic radiographs, and bronchoalveolar lavage.
Figure 23.27.
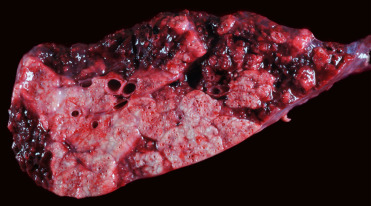
Mycobacterium pinnipedii infection in the lung of a New Zealand sea lion.
Coalescing granulomas with central caseous necrosis replace the majority of the pulmonary parenchyma.
(Photo Courtesy of S. Hunter)
Klebsiella pneumoniae are Gram-negative bacteria in the Enterobacteriaceae family. They are ubiquitous in the environment and are common inhabitants of the gastrointestinal or respiratory tract of healthy mammals. Historically, most reports of K. pneumoniae in marine mammals were of sporadic respiratory infections or isolation from tissues of healthy individuals. However, in 2002 a mass mortality event caused by K. pneumoniae occurred in New Zealand sea lion pups in the sub-Antarctic (Castinel et al., 2007). The causal isolate was later identified as a hypermucoviscous phenotype (Roe et al., 2015), but appears not to be of human origin (Castinel et al., 2007). A similar isolate has also been reported in CSLs (Jang et al., 2010; Seguel et al., 2017a). The clinical presentation of hypermucoviscous K. pneumoniae varies somewhat between host species. Affected New Zealand sea lion pups have bacteremia and fibrinosuppurative to histiocytic inflammation of the meninges, joints, lymph nodes, respiratory tract, peritoneum, and subcutaneous tissues (Fig. 23.28 ) (Roe et al., 2015). Disease has not been reported in juveniles or adults. Pleuritis, pyothorax, pneumonia, and abscesses are the most frequent lesions in CSLs and appear to occur in all ages (Jang et al., 2010; Seguel et al., 2017a), and septicemia and meningoencephalitis have recently been identified. Additionally, Spraker et al. (2007) describe a syndrome in CSLs on San Miguel Island, California, where K. pneumoniae bacteria are transferred into the abdominal cavity on the cuticle of hookworms that penetrate the intestinal tract, resulting in septic peritonitis. This has not been reported at other locations.
Figure 23.28.
Meningitis due to hypermucoviscous Klebsiella pneumoniae infection in a New Zealand sea lion pup.
Note the petechial haemorrhage over the gyri and meningeal opacity with fibrinosuppurative exudate in the subarachnoid space.
(Photo Courtesy of S. Michael)
Several different Mycoplasma spp. have been isolated from phocids and ottarids. These include M. phocicerebrale, M. phocirhinis, M. phocidea, and M. zalophi. Mycoplasma spp. are thought to be part of normal pinniped oral flora and infection is associated with skin wounds, abscesses, polyarthritis, and lymphadenitis, and necrotizing pneumonia in CSLs and skin wounds in gray and harbor seals (Ayling et al., 2011, Haulena et al., 2006, Lynch et al., 2011). In utero infection with M. phocicerebrale is associated with myocarditis and pneumonia and has caused abortion in Australian fur seal fetuses (Lynch et al., 2011). A Mycoplasma sp. was isolated from the respiratory tracts of harbor seals during an influenza outbreak, though the role of the bacteria in respiratory tract disease was undetermined (Geraci et al., 1982). Infection is diagnosed via culture or PCR. Mycoplasma sp. is the cause of “seal finger” in humans, an important zoonotic disease most often associated with the bite of a pinniped to the extremities or contact with broken skin (Waltzek et al., 2012).
Several new species in the genus Brucella have been recently described in marine mammals. Brucella pinnipedialis is the organism isolated from pinnipeds (Foster et al., 2007). Serological studies show worldwide distribution in pinnipeds with a wide variation in prevalence between geographical locations and species, although a lack of consistency in serological assays used makes valid comparisons difficult (Hueffer et al., 2013, Nymo et al., 2011). The route of infection for marine mammals has not been elucidated but a fish or invertebrate vector is possible. Brucella-infected lungworms can be found in the lungs of pinnipeds though whether the parasites serve as a primary route of transmission or are incidentally infected is unknown (Garner et al., 1997). While zoonotic transmission is a concern for humans who consume seal or whale meat as part of a traditional diet, none of the reported human cases had direct exposure to marine mammals (Waltzek et al., 2012).
The majority of B. pinnipedialis infections in pinnipeds are subclinical. However, suppurative and necrotizing placentitis in a northern fur seal was confirmed to be due to Brucella spp infection by immunohistochemistry and PCR (Duncan et al., 2014).
Nocardiosis is most commonly reported in juvenile stranded hooded seals; multiple different Nocardia spp. are associated with infection. Affected seals stranded along the east coast of the United States and Canada and have typically been found outside of their normal range. Underlying immunosuppression, environmental stressors, and decreased prey availability secondary to extralimital ranging may increase susceptibility to infection. Lesions in affected seals include pyothorax and abscesses or pyogranulomas in the lungs and thoracic lymph nodes. Infection is often systemic at death and pyogranulomatous inflammation in the skin, brain, or other tissues can occasionally be observed. Infection is diagnosed via culture or PCR, and organisms can be demonstrated using GMS and acid fast stains (St. Leger et al., 2009).
Pseudomonas sp., most commonly P. aeruginosa, can cause severe systemic infections in pinnipeds, most frequently in young animals in rehabilitation centers. Pneumonia is most common; however, meningoencephalitis, hepatitis, and splenitis can be seen with septicemia. Lesions are characterized by fibrinosuppurative inflammation with necrosis, interlobular edema, and fibrin accumulation in affected lungs. Histologically, the bacterium causes vasculitis with characteristic “swarms” of Gram-negative bacilli (Gaffney et al., 2008). P. aeruginosa infection has also been associated with hemorrhagic pneumonia in a cluster of deaths in adult free-ranging harbor seals (Nollens et al., 2010).
Additional bacterial diseases are presented in Supplemental Table e4.
Table e4.
Miscellaneous Bacterial Diseases of Pinnipeds
| Bacteria | Lesions | Species Affected |
|---|---|---|
| Coxiella burnetiia,b,c | Placental necrosis | Harbor seals, Steller sea lions, northern fur seals |
| Clostridium difficiled | Necrohemorrhagic enteritis | Harbor seals |
| Bordetella bronchisepticae,f | Pneumonia; often secondary to PDV | Harbor seals |
| Streptococcus phocae; β hemolytic Streptococcusg,h | Pneumonia; septicemia | Multiple species |
| Escherichia colig,i | Pneumonia; abscesses; septicemia; endocarditis | Multiple species |
Lapointe, J.M., Gulland, F.M., Haines, D.M., Barr, B.C., Duignan, P.J., 1999. Placentitis due to Coxiella burnetii in a Pacific harbor seal (Phoca vitulina richardsi). J. Vet. Diagn. Invest. 11(6), 541–543.
Kersh, G.J., Lambourn, D.M., Raverty, S.A., Fitzpatrick, K.A., Self, J.S., Akmajian, A.M., Jeffries, S.J., Huggins, J., Drew, C.P., Zaki, S.R., Massung, R.F., 2012. Coxiella burnetii infection of marine mammals in the Pacific Northwest, 1997–2010. J. Wildl. Dis. 48(1), 201–206.
Minor, C., Kersh, G.J., Gelatt, T., Kondas, A.V., Pabilonia, K.L., Weller, C.B., Dickerson, B.R., Duncan, C.G., 2013. Coxiella burnetii in northern fur seals and steller sea lions of Alaska. J. Wildl. Dis. 49(2), 441–446.
Anderson, C.E., Haulena, M., Zabek, E., Habing, G., Raverty, S., 2015. Clinical and epidemiologic considerations of Clostridium difficile in harbor seals (Phoca vitulina) at a marine mammal rehabilitation center. J. Zoo Wildl. Med. 46(2), 191–197.
Siebert, U., Gulland, F., Harder, T., Janiaux, T., Seibel, H., Wohlsein, P., Baumgartner, W., 2010.Epizootics in harbour seals (Phoca vitulina): clinical aspects. NAMMCO Sci. Pub. 8, 265–274.
Duignan, P.J., Van Bressem, M.F., Baker, J.D., Barbieri, M., Colegrove, K.M., De Guise, S., de Swart, R.L., Di Guardo, G., Dobson, A., Duprex, W.P., Early, G., 2014. Phocine distemper virus: current knowledge and future directions. Viruses 6(12), 5093–5134.
Thornton, S.M., Nolan, S., Gulland, F.M., 1998. Bacterial isolates from California sea lions (Zalophus californianus), harbor seals (Phoca vitulina), and northern elephant seals (Mirounga angustirostris) admitted to a rehabilitation center along the central California coast, 1994–1995. J. Zoo Wildl. Med. 29(2), 171–176.
Vossen, A., Abdulmawjood, A., Lämmler, C., Weiß, R., Siebert, U., 2004. Identification and molecular characterization of beta-hemolytic streptococci isolated from harbor seals (Phoca vitulina) and grey seals (Halichoerus grypus) of the German North and Baltic seas. J. Clin. Microbiol. 42(1), 469–473.
Kim, J.H., Lee, J.K., Yoo, H.S., Shin, N.R., Shin, N.S., Lee, K.H., Kim, D.Y., 2002. Endocarditis associated with Escherichia coli in a sea lion (Zalophus californianus). J. Vet. Diagn. Invest. 14(3), 260–262.
Fungi
Fungal disease represents a relatively small proportion of infectious diseases in pinnipeds. Systemic infections with opportunistic fungi have been reported sporadically and have been reviewed by Reidarson et al. (2018). Exposure most often is respiratory exposure but can be due to implantation. Distribution of lesions is widely variable but often involves pulmonary tissues with subsequent systemic spread. Cytology and histopathology can be used to identify characteristic fungal morphology; however, culture and/or PCR are often needed to differentiate the type and species of fungus. Burek (2001) reviewed epidemiology, gross and histopathologic features, and diagnosis of each fungal group.
Coccidioides immitis, an important human pathogen, is endemic in California and has been reported in CSLs and rarely in harbor and northern elephant seals. It is typically associated with disturbance of dry soil where it occurs in the mycelial phase and produces highly infectious arthroconidia. Transmission is generally through inhalation. In most animals C. immitis causes only mild respiratory disease, but can progress to disseminated fatal systemic infection. Animals with coccidioidomycosis are often emaciated and have pyogranulomatous pneumonia, pleuritis, peritonitis, and thoracic and abdominal lymphadenitis (Fig. 23.29 ). With systemic spread multiple miliary to large pyogranulomas, sometimes with indented necrotic centers, can be seen throughout the body. Histologically, there is intense pyogranulomatous inflammation centered on few to many, double contoured, 5–30 μm diameter immature spherules that mature into 30–200 μm diameter spherules containing a myriad of endospores. Mycelia with production of arthroconidia are rare in tissue. Because of the danger of exposure of necropsy personnel and laboratory workers to fungal spores, affected carcasses should be incinerated. Cytology, histology, and PCR are the preferred diagnostic methods (Huckabone et al. 2015). In CSLs fungal pyogranulomas may be grossly difficult to distinguish from UGC masses, though heavy involvement of the lungs and granulomatous inflammation on impression smear cytology can aid in differentiation.
Figure 23.29.
Coccidioides immitis infection in the lung of a California sea lion.
Numerous fungal granulomas are scattered throughout the lungs and epicardial surface
(Photo Courtesy of The Marine Mammal Center)
Fungal dermatitis has been reported sporadically both in captive and free-ranging pinnipeds due to a wide variety of yeasts, non-dermatophylic hyaline molds, and dermatophytes. In addition to previously described predisposing factors, mechanical disruption of the skin and water quality issues (low salinity, high temperatures) can predispose to infection. Alopecia, acanthosis, and hyperkeratosis can be associated with Candida albicans, Fusarium spp. Microsporum sp. and Trichophyton sp. infections. Commonly affected areas include mucocutaneous junctions, nail beds, and the axillae (Pollock et al., 2000, Reidarson et al., 2018). In Stellar sea lions in Alaska, “fungal patches” due to Trichophyton sp. distributed across the body are very commonly seen and can be present for extended periods of time. Affected skin is thickened with a corrugated, often alopecic, appearance, sometimes in a target pattern with either healed or raw skin in the center (Fig. 23.30 ). Histologically, dermal skin infections cause chronic dermatitis, epidermal hyperplasia, hyperkeratosis, folliculitis, and in cases of dermatophyte infection, there may be intrafollicular or superficial epithelial fungal hyphae. Special stains, such as PAS or silver stains can aid in identification and characterization of the organisms. Ulceration and suppurative dermatitis is generally related to self-trauma and secondary bacterial infection. Diagnosis can be made by skin scraping, fungal cultures, PCR; PCR is often needed to differentiate to species (Burek, 2001).
Figure 23.30.
Trichophyton dermatitis in a Stellar sea lion.
Target shaped areas of alopecia with ulceration associated with infection.
Metazoa
There are two general categories of lungworms that infect pinnipeds, small lungworms, the Parafilaroides species that includes decorus, gymnus, hispida, normani, measuresae, hydrurgae, and gullandae and the large lungworms Otostrongylus circumlitus. They are metastrongyloid nematodes of the Filaroididae and Crenosomatidae families, respectively. Life cycles of marine mammal lungworms are not well understood, though fish species have been shown to be intermediate hosts (Measures, 2001, Measures, 2001).
Parafilaroides spp. infect otariids and phocid hosts in both the northern and southern hemisphere and subclinical infections are very common. Adult parasites reside in the pulmonary parenchyma, have a thin, ridged cuticle, coelomyarian polymyarian musculature, and shed embryonated eggs into the small airways where they develop into L1 larvae. L1 are coughed up, swallowed and shed in the feces. Parafilaroides spp. infect all age-classes at different intensities in different species, though infections are often more intense in younger animals. In uncomplicated infections there are small raised nodules along the pleural surface and in the parenchyma adjacent to bronchioles (Fig. 23.31 ). Parasites are fine and difficult to detect grossly; however, scraping the cut surface of lesions with a knife may enable visualization. Histologically, there are cross sections of small nematodes within alveolar spaces and bronchioles. Infection in most animals results in minimal lymphocytic interstitial and peribronchiolar inflammation and occasional granulomas may develop around degenerating parasites or larvae (Fig. 23.32 ). Heavy infections and secondary bacterial pneumonia can result in more significant inflammation, especially in young or debilitated animals. Mild bronchiole epithelial hyperplasia and lymphocytic tracheitis are also common (Measures, 2001).
Figure 23.31.
Parafilaroides decorus infection in a California sea lion.
Nodular areas of pneumonia are associated with thread-like metastrongyles (arrow).
(Photo Courtesy of The Marine Mammal Center)
Figure 23.32.
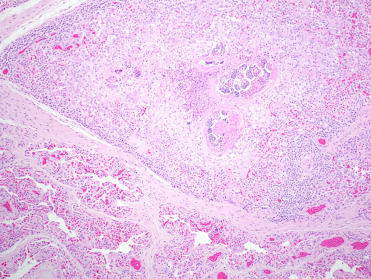
Parafilaroides decorus pneumonia in a California sea lion.
Granulomatous pneumonia with intralesional larvae and necrotic adult parasites.
Otostrongylus circumlitus is distributed in a circumpolar region and affects a variety of species. The primary hosts are ringed and harbor seals; infections have been reported in other phocids including gray, elephant, ribbon, and hooded seals (Measures, 2001, Measures, 2001). After ingestion, larvae migrate to the right ventricle, through the wall of the pulmonary artery and into the lungs. Significant infection generally occurs only in young animals. Heavy infections may impact diving performance, health and recruitment. Adult parasites reside in the bronchi and bronchioles (Fig. 23.33 ). The head of a worm is embedded in the parenchyma and the tail extends into the lumen. L1 larvae are released into airways. Infection induces variable amounts of mucus plugging of airways, mucosal cell hyperplasia, moderate lymphoplasmacytic and eosinophilic peribronchitis and mild chronic arteritis with tunica intima hyperplasia (Measures, 2001). In northern elephant seals reaction to larval parasites during migration in the pulmonary arteries can cause severe disease and death in the prepatent period. Clinical signs include harsh lung sounds, depression, neutrophilia, and disseminated intravascular coagulation (DIC). Grossly, nematodes are found in the right ventricle and pulmonary arteries; they are often associated with extensive hemorrhage in lung parenchyma. Histologically, nematodes are present within the vasculature and there is severe neutrophilic and lymphocytic arteritis, thrombosis, and interstitial pneumonia that can be complicated by secondary bacterial infection (Fig. 23.34 ) (Gulland et al., 1997a). The severe reaction in this species suggests a poorly adapted host (Measures 2001).
Figure 23.33.
Otostrongylus circumlitus in tracheitis in a ringed seal.
Note the mucous in the airways. Hemorrhage and congestion in the lung parenchyma are due to gunshot trauma.
Figure 23.34.
Otostrongylus circumlitus infection in a northern elephant seal.
Inflammation is characterized by vasculitis and interstitial pneumonia.
Hookworm infections caused by members of the genus Uncinaria have been described in a number of otariid and phocid species. Both Northern and Southern fur seals experience significant pup mortality associated with infections. In a recent study, 94% of pups examined had infections and infections were identified as the cause of death in 35% of pups (Seguel et al., 2017b). While only two distinct hookworm species, U. lucasi and U. hamiltoni, have been confirmed to infect pinnipeds, recent studies suggest that there is considerably more species diversity than currently recognized (Nadler et al., 2013). The pinniped hookworm life cycle was largely characterized in northern fur seals in the 1960s and appears to be similar for other pinniped host species. Briefly, pups are infected via the transmammary route, with ingestion of L3 larvae in the milk when a pup first suckles. Larvae mature within the pup’s intestine and eggs are shed in pup feces. Larvae develop in the environment and L3 larvae penetrate the skin of a susceptible pinniped host or are transmitted via ingestion. Larva then migrates from the intestines or subcutaneous tissues to the host ventral blubber where they lie dormant until, in pregnant females, they migrate into the milk during late pregnancy. Adult hookworms survive in the intestinal tract of infected pups from around 6 weeks (Lyons et al., 2011) to 8 months (Hernandez-Orts et al., 2013, Katz et al., 2012, Lyons et al., 2000) depending on host species. During this time, they attach to the small intestinal mucosa and feed on blood. Clinical signs are generally only seen in pups and are associated with blood loss into the intestine from parasite-induced damage. Severely affected pups have a reduced growth rate with severe fibrinohemorrhagic enteritis, anemia, and death. Typical gross lesions include pale tissues, nematodes admixed with hemorrhage in the small intestine, and numerous 2–4 mm diameter red foci on the serosal surface of the intestine that correspond to hemorrhagic and inflamed feeding sites (Fig. 23.35 ). Penetration of the wall of the intestine with subsequent peritonitis and bacteremia also occurs (Seguel et al., 2017b).
Figure 23.35.
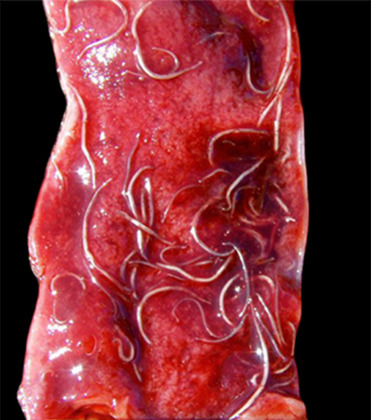
Hookworm infection in the small intestine of a New Zealand sea lion pup.
Moderate numbers of hookworms are within the small intestine admixed with hemorrhage.
Gastric and intestinal roundworm infection is extremely common in free-ranging pinnipeds, with Anisakis sp. and Contracaecum sp. most commonly reported. Within the stomach, infection results in large, “volcanic” ulcers due to embedded parasites in the mucosa and adjacent mucosal hyperplasia and submucosal fibrosis. Infection is typically considered subclinical even in cases with very large parasite loads and multiple gastric ulcers (Measures, 2018).
Heartworm, Dirofiliaria immitis, and Acanthocheilonema spirocauda, has been observed in multiple seal and sea lion species. Infection mirrors the condition in domestic dogs with congested, edematous and hemorrhagic lungs, pulmonary emphysema, interstitial and exudative pneumonia, right ventricular hypertrophy pulmonary catarrhal bronchitis, and hepatic congestion. Intimal proliferation of arteries and arterioles is observed microscopically. Worms can be found in the right ventricle, pericardial sac, pulmonary artery, and/or lung. Identification of microfilaria helps to differentiate between the two parasites; microfilaria of D. immitis are generally larger. Not all blood microfilaria signal pathogenic heartworm infection. A. odendhali microfilaria is similar in size to A. spirocauda. A. odendhali infection is generally incidental with adults located in subcutaneous, intramuscular, and cavity spaces without associated cardiovascular disease. Captive seals and some sea lions in endemic regions are routinely screened for and placed on heartworm preventative to protect from this infection (Measures, 2018).
Protozoa
Toxoplasma gondii is an intracellular apicomplexan parasite that infects many warm-blooded species, including people worldwide. Serologic evidence of exposure to T. gondii has been reported in a number of free-ranging and captive pinnipeds including artic and subartic seals and walrus with low exposure to felid definitive hosts. Parasite-associated disease is most commonly identified in free-ranging CSLs and monk and harbor seals (Miller, 2008). In CSLs with T. gondii infection, the most common lesions are necrotizing and lymphohistiocytic meningoencephalitis, myocarditis, and myositis with associated intralesional protozoa (Fig. 23.36 ). Neurologic signs including ataxia, seizures, and abnormal mentation have been observed with infection and concurrent DA toxicosis has been noted, illustrating the difficulty distinguishing protozoal disease from DA exposure on clinical signs alone. Infection has also been identified in aborted CSL pups, confirming vertical transmission of the parasite. Incidental tissue cysts can be occasionally seen within skeletal or cardiac myocytes (Carlson-Bremer et al., 2015). In harbor seals, widespread meningoencephalitis is the most common lesion of toxoplasmosis and coinfection with Sarcocystis neurona have been identified in a number of seals. In one investigation in the Pacific Northwest of the United States, T. gondii infections were most commonly caused by Type I genotypes (Gibson et al., 2011). In Hawaiian monk seals, infection is typically disseminated and very large numbers of organisms are associated with lesions. Adipose tissue is often severely affected (Barbieri et al., 2016).
Figure 23.36.
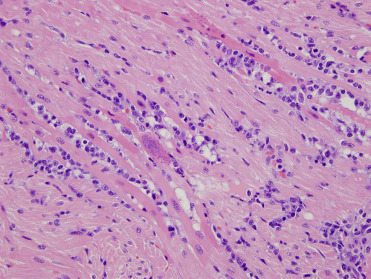
Toxoplasma gondii infection in the heart of a California sea lion.
Infection is characterized by lymphohistiocytic myocarditis with intralesional tachyzoites.
PCR and IHC can confirm infection. Occasionally, organisms are not observed with IHC in areas of protozoal-compatible inflammation, though T. gondii DNA can be amplified by PCR. Most commercially available IHC assays employ polyclonal antibodies to T. gondii, therefore, cross reaction with other protozoa is possible (Colegrove et al., 2011). Though serologic assays may be used to document exposure and rising titers have been associated with active infection, lack of pinniped specific antibodies often makes defining positive titer ranges difficult.
Mortality due to Sarcocystis neurona, an intracellular apicomplexan parasite, is most commonly identified in free-ranging pinnipeds from the Pacific coast of North America, and antibody titers reflecting exposure have been documented in harbor seals and CSLs (Carlson-Bremer et al., 2012, Greig et al., 2014, Miller, 2008). Several cases have also been identified in captive animals (Lapointe et al., 1998). Harbor seals are the most commonly reported species to develop severe fatal disease with infection, and in California subadults and adults are primarily affected (Barbosa et al., 2015, Miller, 2008). Lesions include nonsuppurative meningoencephalitis which is often most severe in the cerebellum and can be associated with numerous organisms (Lapointe et al. 1998) (Fig. 23.37 ). Rosette-form schizonts with occasionally visible residual bodies can be used to differentiate organisms from T. gondii. Barbosa et al. (2015) reported a novel genotype, Type XIII, associated with increased severity of encephalitis in multiple marine mammal species.
Figure 23.37.
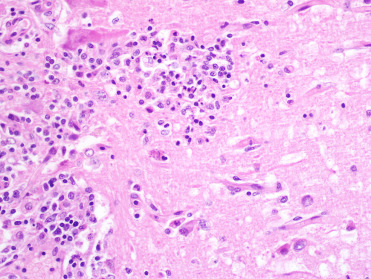
Sarcocystis neurona infection in the cerebellum of a harbor seal.
Rosette-form mature and immature schizonts characterize infection.
While incidental sarcocysts can occasionally be noted in skeletal or cardiac myocytes in multiple species, infected CSLs can develop a severe polyphasic myositis. Multiple muscle regions are affected including the esophagus and diaphragm. Grossly, affected muscle may be atrophied and/or have pale gray to tan streaks (Fig. 23.38 ). Histologically there is multifocal lymphohistiocytic myositis with myonecrosis, and evidence of regeneration (Fig. 23.39 ). Rare sarcocysts can be noted in myocytes, but often are not directly associated with lesions, suggesting a possible immune-mediated pathogenesis (Carlson-Bremer et al., 2012). Some may also develop secondary aspiration pneumonia and/or myoglobinuric nephrosis.
Figure 23.38.
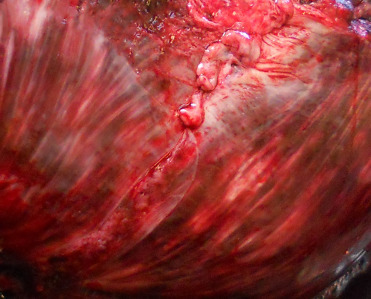
Sarcocystis neurona-associated myositis in the diaphragm in a California sea lion.
(Photo Courtesy of The Marine Mammal Center)
Figure 23.39.
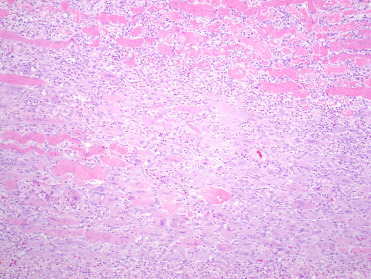
Polyphasic myositis associated with Sarcocystis neurona infection in a California sea lion.
There is myonecrosis, mononuclear inflammation, and regenerating myocytes with pale basophilic cytoplasm and rowed nuclei.
A novel Sarocystis spp. closely related to S. canis and tentatively called S. pinnipedi has been reported to cause necrotizing hepatitis sporadically in several species including CSLs, Stellar sea lions, monk, and gray seals. Ringed seals have been identified with S. canis-like muscle cysts but no apparent hepatic lesions. In affected animals schizonts and merozoites are seen in hepatocytes and Kupffer cells (Haman et al., 2006; Miller, 2008, Welsh et al., 2014).
Eimeria phoca infection in young harbor seals results in enteritis ranging from mild self-limiting inflammation to severe fatal necrotizing enteritis (Van Bolhuis et al., 2007). Several novel apicomplexan parasites with closest homology to Neospora sp. and evidence of sexual replication in enterocytes have been identified within the intestinal tracts of CSLs. Though infection in CSLs causes only mild enteritis, one of these novel coccidian species was linked to fatal infection in a neonatal harbor seal, suggesting CSLs may be a definitive host for a protozoan pathogenic to other species (Colegrove et al., 2011). Enteric coccidia have also been noted in elephant seals and South American fur seals. Both serologic evidence of exposure to Neospora sp. and infection diagnosed via PCR have been reported in several species (Miller, 2008, Gibson et al., 2011). A more extensive review of pinniped parasitic concerns is available in Supplemental Table e5.
Table e5.
Parasitic Diseases of Pinnipeds
| Type of Parasite | Name (Genus Species) | Location in Host | Associated Disease (if any) | Region Found | Species Affected |
|---|---|---|---|---|---|
| Metazoan | |||||
| Nematode (Filarid)a | Acanthocheilonema spirocauda | Right ventricle and pulmonary arteries | endarteritis of the pulmonary artery; pulmonary infarction and thrombosis; granulomatous pneumonia; possible right sided heart failure lesions | Northern hemisphere | Multiple phocids |
| Nematode (Filarid)b | Acanthocheilonema odendhali (previously Dipetalonema) | Blubber, fur, skin, intermuscular fascia | Nonpathogenic; filaremia | Northern hemisphere | Northern Fur seals and California sea lion |
| Nematode (Metastrongyle)c,d,e | Parafilaroides (Filaroides) gymnurus | Alveoli | Minor pneumonia unless secondary bacterial infections | Northern hemisphere | Atlantic harbor seal, grey, bearded seal |
| Nematode (Metastrongyle)c | P. (F.) decorus | Alveoli | Minor pneumonia unless secondary bacterial infections | Northern hemisphere | Steller sea lion, California sea lion |
| Nematode (Metastrongyle)c,f | P. (F.) hispidus | Alveoli | Mild pneumonia unless secondary bacterial infections | Northern hemisphere | Ringed seal, gray seal |
| Nematode (Metastrongyle)c,g | P. (F.) measuresae | Alveoli | Mild pneumonia unless secondary bacterial infections | Northern hemisphere | Northern Elephant seal |
| Nematode (Metastrongyle)C,G | P. (F.) gullandae | Alveoli | Mild pneumonia unless secondary bacterial infections | Northern hemisphere | Pacific harbor seal |
| Nematode (Metastrongyle)C | P. (F.) hydrurgae | Alveoli | Mild pneumonia unless secondary bacterial infections | Southern hemisphere—Antarctica | Leopard seal |
| Nematode (Metastrongyle)c | P. (F.) normani | Alveoli | Mild pneumonia unless secondary bacterial infections | Southern hemisphere—New Zealand | New Zealand fur seal, Australian fur seal, and sub-Antarctic fur seal |
| Nematode (Metastrongyle)d,h,e,i | Otostrongylus circumlitus | Right ventricle, pulmonary arteries, bronchi | Mild catarrhal bronchitis, mild arteritis, mild pneumonia; DIC, severe arteritis, thrombosis, and interstitial pneumonia in northern elephant seals | Circumpolar | Harbor, ringed, gray, northern elephant seals |
| Nematode (Hookworm)j,k | Uncinaria lucasi, U. hamiltoni | Small intestine | Anemia, hemorrhagic enteritis | Northern and southern hemispheres | Multiple ottarids, ringed seal, elephant seal. |
| Nematode (Roundworm)i,xsl | Anisakis sp., Contracaecum sp., Pseudoterranova sp., Phocoascaris sp. | Stomach, small intestine | Usually subclinical; gastric ulceration | Northern and southern hemispheres | Multiple species |
| Nematodem | Trichinella nativa | Muscle cysts | Nonpathogenic; zoonotic potential | Circumpolar | Walrus, bearded seal, grays seal, ringed seal |
| Trematodei | Multile genera; Pricetrema sp. most common | Small and large intestine | Nonpathogenic | Northern and southern hemispheres | Multiple species |
| Trematodei,n | Multiple genera; Zalophotrema hepaticum most common | Bile ducts, gall bladder | Mild biliary tract inflammation, portal fibrosis, bile duct and gall bladder hyperplasia; aberrant cerebral migration reported in CSLs | Northern hemisphere | California sea lion, harbor seal, northern elephant seal |
| Cestodei,l | Diphyllobothriidae and Tetrabothriidae (many species) | Small and large intestine | Obstruction and malabosorption with large parasite loads | Northern and southern hemispheres | Multiple species |
| Cestodei | Phyllobothrium delphini | Subcutis | Encysted larvae | Southern hemisphere | Sea lion, fur seal, leopard seal |
| Acanthocephalani | Corynosoma sp. | Small intestine | Ulcerative enteritis with occasional penetration through intestine wall | Northern and southern hemispheres | Multiple species |
| Protozoan | |||||
| Flagellatei,o,p | Giardia sp. | Intestine, feces | Unknown | Northern hemisphere | Multiple species |
| Apicomplexiao,p,q,r | Cryptosporidium | Intestine, feces | Unknown | Northern and Southern hemispheres | Multiple species |
| Ectoparasite | |||||
| Sucking Lices | Antarctophthirius sp. | Skin | With heavy infestations, anemia | Multiple species | |
| Sucking Licea | Echinophthirius horridus | Skin | With heavy infestations, anemia and weakened animals more susceptible to other infections; Focal alopecia; may transmit heartworm Acanthocheilonema spirocauda | Northern phocid seals | |
| Mitei | Demodex spp. | Skin | hyperkeratosis, alopecia, pruitus | California sea lions, northern fur seals | |
| Mitet | Halarachne miroungae | Nasal passages, nasopharynx | Mild nasal discharge, mild chronic inflammation at attachment sites | Gray seals | |
| Miteu | Orthohalarchne attenuata | Nasal passages, nasopharynx | Mild nasal discharge, mild chronic inflammation at attachment sites | Multiple ottarids and walrus | |
| Mitei,u | Orthohalarchne dimmunata | Trachea, bronchi | Mild chronic inflammation at attachment sites | Multiple ottarids | |
| Miteu | Halarchne halichoeri | Nasal passages, nasopharynx | Mild nasal discharge, mild chronic inflammation at attachment sites | Harbor and spotted seals |
Leidenberger, S., Harding, K., Härkönen, T., 2007. Phocid seals, seal lice and heartworms: a terrestrial host–parasite system conveyed to the marine environment. Dis. Aquat. Organ. 77, 235–253.
Kuzmina, T.A., Kuzmin, Y.I., Tkach, V.V., Spraker, T.R., Lyons, E.T., 2013. Ecological, morphological, and molecular studies of Acanthocheilonema odendhali (Nematoda: Filarioidea) in northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska. Parasitol. Res. 112(9), 3091–3100.
Dailey, M.D., 2009. A new species of Parafilaroides (Nematoda: Filaroididae) in three species of fur seals (carnivore: Otariidae) from the southern hemisphere. J. Parasitol. 95, 156–159.
Gosselin, J.-F., Measures, L.N., Huot, J., 1998. Lungworm (Nematoda: Metastrongyloidea) infections in Canadian Phocids. Can. J. Fish. Aquat. Sci. 55, 825–834.
Measures, L.N., 2001. Lungworms of marine mammals. In: Samuel, W.M., Pybus, M.J., Kocan, A.A. (Eds.). Parasitic Diseases of Wild Mammals, Iowa State University Press/Ames, pp. 279–300.
Kennedy, M.J., 1986. Filaroides (Parafilaroides) hispidus n. sp. (Nematoda: Metastrongyloidea) from the lungs of the ringed seal, Phocahispida (Phocidae), from the Beaufort Sea, Canada. Can. J. Zoo. 64, 864–1868.
Dailey, M.D., 2006. Restoration of parafilaroides (Dougherty, 1946) (Nematoda: Metastrongyloidea) with description of two new species form pinnipeds of eastern central Pacific. J. Parasitol. 92, 589–594.
Gulland, F.M.D., Beckmen, K., Burek, K., Lowenstine, L., Werner, L., Spraker, T., Dailey, M., Harris, E., 1997. Nematode (Otostrongylus circumlitus) infestation of northern elephant seals (Mirounga angustirostris) stranded along the central California coast. Mar. Mammal Sci. 13, 446–458.
Dailey, M.D., 2001. Parasitic diseases. In: Gulland, F.M.D, Dierauf, L.A. (Eds.), CRC Handbook of Marine Mammal Medicine, second ed. CRC Press, Boca Raton, pp. 357–379.
Lyons, E.T., Spraker, T.R., De Long, R.L., Ionita, M., Melin, S.R., Nadler, S.A., Tolliver, S.C., 2011. Review of research on hookworms (Uncinarialucasi Stiles, 1901) in northern fur seals (Callorhinus ursinus Linnaeus, 1758). Parasitol. Res. 109, 257–265.
Lyons, E.T., DeLong, R.L., Gulland, F.M., Melin, S.R., Tolliver, S.C., Spraker, T.R., 2000. Comparative biology of Uncinaria spp. in the California sea lion (Zalophus californianus) and the northern fur seal (Callorhinus ursinus) in California. J. Parasitol. 86, 1348–1352.
Lauckner, G., 1985. Diseases of Mammalia: Pinnipeda. In: Kinne, O. (Ed.). Diseases of Marine Animals, Vol 4, Pt. 2, Biologische, Anstalt, Helgoland, Hamburg, pp. 683–793.
Forbes, L.B., 2000. The occurrence and ecology of Trichinella in marine mammals. Vet. Parasitol. 93, 321–334.
Fauquier, D., Gulland, F., Haulena, M., Dailey, M., Riecheck, R.L, Lipscomb, T.P., 2004. Meningoencephalitis in two stranded California sea lions (Zalophus californianus) caused by aberrant trematode migration. J. Wildl. Dis. 40, 816–819.
Appelbee, A.J., Thompson, R.C., Measures, L.M., Olson, M.E., 2010. Giardia and cryptosporidium in harb and hooded seals from the Gulf of St. Lawrence, Canada. Vet. Parasitol. 173, 19–23.
Deng, M.A., Peterson, R.P., Cliver, D.O., 2000. First findings of Cryptosporidium and Giardia in California sea lions (Zalophus californianus). J. Parasitol. 86, 490–494.
Rengifo-Herrera, C., Ortega-Mora, L.M., Gómez-Bautista, M., García-Peña, F.J., García-Párraga, D., Pedraza-Díaz, S. Detection of a novel genotype of Cryptosporidium in Antarctic pinnipeds. Vet. Parasitol. 19, 112–118.
Greig, D.J., Gulland, F.M.D., Smith, W.A., Conrad, P.A., Field, C.L., Fleetwood, M., Harvey, J.T., Ip, H.S., Jang, S., Packham, A., Wheeler, E., Hall, A.J. 2014. Surveillance for zoonotic and selected pathogens in harbor seals Phoca vitulina from central California. Dis. Aquat. Organ. 111, 93–106.
Durden, L.A., 2001. Ectoparasites: Lice (Phthiraptera). In: Samuel, W.M., Pybus, M.J., Kocan, A.A. (Eds.), Parasitic Diseases of Wild Mammals, Iowa State University Press/Ames, pp. 3–17.
Alonso-Farré, J.M., D’Silva, J.I., Gestal, C., 2012. Naso-pharyngeal mites Halarachne halichoeri (Allman, 1847) in grey seals stranded on the NW Spanish Atlantic coast. Vet. Parasitol. 183, 317–322.
Fay, F.H., Furman, D.P., 1982. Nasal mites (Acari: Halarachnidae) in the spotted seal, Phoca largha Pallas, and other pinnipeds of Alaskan waters. J. Wildl. Dis. 18, 63–68.
E-Slides
-
23.e1
Acute domoic acid toxicosis, California sea lion, hippocampus. Acute neuronal necrosis and neuropil vacuolation within the hippocampus due to domoic acid toxicity. Neurons have the classic shrunken “red” necrotic appearance. In acute DA toxicosis, neuronal necrosis is most common in the pyramidal cells of the hippocampus, commonly most severe in sectors CA1, CA3 and CA4, the dentate gyrus, and neurons in the amygdala and pyriform lobe. (see Figure 23.1, Figure 23.2, Figure 23.3). eSlide: VM04969
-
23.e2
Domoic acid cardiomyopathy, California sea lion, heart. Multifocal vacuolation of cardiac myocytes, rare necrotic myocytes and myocyte loss associated with domoic acid toxicosis. The interstitium is edematous. In chronic lesions, With myocyte loss there is replacement by fibrosis and adipocytes. Lesions occur primarily within the interventricular septum and left ventricle. (see Fig. 23.5). eSlide: VM04970
-
23.e3
Chronic domoic acid toxicosis, California sea lion, hippocampus. In chronic domoic acid toxicosis, there is parenchymal collapse, neuronal loss throughout the dentate gyrus and cornu ammonis (CA) sectors 1-4, and gliosis. Hippocampal atrophy can be grossly evident. There is also mild lymphocytic perivascular cuffing and meningeal lymphoplasmacytic infiltrates. (see Fig. 23.6). eSlide: VM04972
-
23.e4
Pneumonia due to Parafilaroides decorus and bacterial infection, California sea lion, lung. Pneumonia due to Parafilaroides decorus with secondary bacterial infection. Adult and larval nematodes are present within the airways. Inflammation is generally mild in uncomplicated cases but can be more severe with secondary bacterial infections as in this case. (see Figure 23.31, Figure 23.32). eSlide: VM04974
-
23.e5
Vasculitis and pneumonia due to Otostrongylus infection, northern elephant seal, lung. Vasculitis with thrombosis and interstitial pneumonia due to Otostrongylus circumlitus infection in a northern elephant seal. There are no parasites within these sections of lung and they are often found in the right ventricle and pulmonary artery. (see Fig. 23.34). eSlide: VM04975
-
23.e6
Gastric ulceration due to ascarid infection, California sea lion, stomach. Volcanic ulcer in the stomach of a California sea lion with embedded ascarids, most frequently Anisakis or Contracaecum sp. Inflammation and fibrosis is present within the submucosa and muscularis. Infections are commonly considered incidental. Also present is a section of trachea with lymph node and lung infected with Parafilaroides (see also SS23.04). eSlide: VM04980
Figure 23.3.
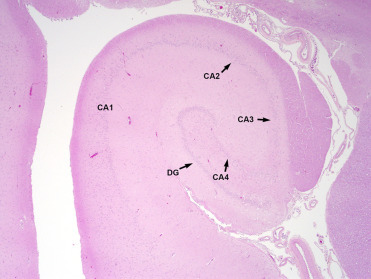
Normal hippocampus from a California sea lion.
DG, dentate gyrus; CA1-4, cornu ammonis sectors 1-4.
References
- Anthony, S.J., St. Leger, J.A., Pugliares, K., Ip, H.S., Chan, J.M., Carpenter, Z.W., Navarrete-Macias, I., Sanchez-Leon, M., Saliki, J.T., Pedersen, J., Karesh, W., Daszak, P., Rabadan, R., Rowles, T., Lipkin, W. 2012. Emergence of fatal avian influenza in New England harbor seals. Mbio. 3(4):e00166-12. [DOI] [PMC free article] [PubMed]
- Ayling R.D., Bashiruddin S., Davison N.J., Foster G., Dagleish M.P., Nicholas R.A. The occurrence of Mycoplasma phocicerebrale, Mycoplasma phocidae, and Mycoplasma phocirhinis in grey and common seals (Halichoerus grypus and Phoca vitulina) in the United Kingdom. J. Wildl. Dis. 2011;47:471–475. doi: 10.7589/0090-3558-47.2.471. [DOI] [PubMed] [Google Scholar]
- Barbieri M.M., Kashinsky L., Rotstein D.S., Colegrove K.M., Haman K.H., Margargal S.L., Sweeny A.R., Kaufman A.C., Grigg M.E., Littnan C.L. Protozoal-related mortalities in endangered Hawaiian monk seals Neoonachus schauinslandi. Dis. Aquat. Organ. 2016;47:85–95. doi: 10.3354/dao03047. [DOI] [PubMed] [Google Scholar]
- Barbosa L., Johnson C.K., Lambourn D.M., Gibson A.K., Haman K.H., Huggins J.L., Sweeny A.R., Sundar N., Raverty S.A., Grigg M.E. A novel Sarcocystis neurona genotype XIII is associated with severe encephalitis in an unexpectedly broad range of marine mammals from the northeastern Pacific Ocean. Int. J. Parasitol. 2015;45:595–603. doi: 10.1016/j.ijpara.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida R., Loureiro J., Quse V., Bernardelli A., Rodriguez D., Costa E. Tuberculosis in a wild subantarctic fur seal from Argentina. J. Wildl. Dis. 1999;35:796–798. doi: 10.7589/0090-3558-35.4.796. [DOI] [PubMed] [Google Scholar]
- Beckmen K.B., Lowenstine L.J., Nweman J., Hll J., Hanni K., Gerber J. Clinical and pathologic characterization of northern elephant seal skin disease. J. Wildl. Dis. 1997;33:438–449. doi: 10.7589/0090-3558-33.3.438. [DOI] [PubMed] [Google Scholar]
- Bodewes R., Bestebroer T.M., van der Vries E., Verhagen J.H., Herfst S., Koopmans M.P., Fouchier R.A.M., Pfankuche V.M., Wohlsein P., Siebert U., Baumgärtner W., Osterhaus A.D. Avian influenza A(H10N7) virus- associated mass deaths among harbor seals. Emerg. Infect. Dis. 2015;21:720–722. doi: 10.3201/eid2104.141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst G.H.A., Walvoort H.C., Reijnders P.J.H., van der Kamp J.S., Osterhaus A.D.M.E. An outbreak of a herpesvirus in harbor seals (Phoca vitulina) J. Wildl. Dis. 1986;22:1–6. doi: 10.7589/0090-3558-22.1.1. [DOI] [PubMed] [Google Scholar]
- Britt J.O., Nagy A.Z., Howard E.B. Acute viral hepatitis in California sea lions. J. Am. Vet. Med. Assoc. 1979;175:921–923. [PubMed] [Google Scholar]
- Brownlow A., Onoufriou J., Bishop A., Davison N., Thompson D. Corkscrew seals: grey seal (Halichoerus grypus) infanticide and cannibalism may indicate the cause of spiral lacerations in seals. Plos One. 2016;11(6) doi: 10.1371/journal.pone.0156464. e0156464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning H.M., Gulland F.M.D., Hammond J.A., Colegrove K.M., Hall A.J. Common cancer in a wild animal: the California sea lion (Zalophus californianus) as an emerging model for carcinogenesis. Philos. Transac. B Biol. Sci. 2015;19(370) doi: 10.1098/rstb.2014.0228. 20140228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckles E.L., Lowenstine L.J., DeLong R.L., Melin S.R., Vittore R., Wong H-L, Ross G.L., St.Ledger J.A., Greig D., Duerr R., Gulland F., Stott J. Age prevelance of Otarine Herpesvirus-1, a tumor associated virus, and possibility of sexual transmission. Vet. Microbiol. 2007;11(120):1–8. doi: 10.1016/j.vetmic.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Burek K. Mycotic diseases. In: Williams E.S., Barker I.K., editors. Infectious Diseases of Wild Mammals. third ed. Iowa Stae University Press; Ames: 2001. pp. 514–531. [Google Scholar]
- Burek K.A., Gulland F.M.D., Sheffield G., Beckmen K.B., Keyes E., Spraker T.R., Smith A.W., Skilling D.E., Evermann J., Stott J.L., Saliki J.T., Trites A.W. Infectious disease and the decline of the steller sea lions (Eumetopias jubatus) I Alaska, USA: insights from serologic data. J. Wildl. Dis. 2005;41:512–524. doi: 10.7589/0090-3558-41.3.512. [DOI] [PubMed] [Google Scholar]
- Callan R.J., Early G., Kida H., Hinshaw V.S. The appearance of H3N3 influenza viruses in seals. J. Gen. Virol. 1995;76:199–203. doi: 10.1099/0022-1317-76-1-199. [DOI] [PubMed] [Google Scholar]
- Carlson-Bremer D.P., Gulland F.M.D., Johnson C.K., Colegrove K.M., Van Bonn W.G. Diagnosis and treatment of Sarcocystis neuronal-induced myositis in a free-ranging California sea lion. J. Am. Vet. Med. Assoc. 2012;240:324–327. doi: 10.2460/javma.240.3.324. [DOI] [PubMed] [Google Scholar]
- Carlson-Bremer D., Colegrove K.M., Gulland F.M.D., Conrad P.A., Mazet J.A.K., Johnson C.K. Epidemiology and pathology of Toxoplasma gondii infection in free-ranging California sea lions (Zalophus californianus) J. Wildl. Dis. 2015;51:362–373. doi: 10.7589/2014-08-205. [DOI] [PubMed] [Google Scholar]
- Castinel A., Grinberg A., Pattison R., Duignan P., Pomroy B., Rogers L., Wilkinson I. Characterization of Klebsiella pneumoniae isolates from New Zealand sea lion (Phocarctos hookeri) pups during and after the epidemics on Enderby Island Auckland Islands. Vet. Microbiol. 2007;122:178–184. doi: 10.1016/j.vetmic.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Colegrove K.M., Greig D.J., Gulland F.M.D. Causes of live strandings of northern elephant seals (Mirounga angustirostris) and Pacific harbor seals (Phoca vitulina) along the central California coast 1992–2001. Aquat. Mammals. 2005;31:1–10. [Google Scholar]
- Colegrove K.M., Lowenstine L.J., Gulland F.M. Leptospirosis in northern elephant seals (Mirounga angstirostris) stranded along the California coast. J. Wildl. Dis. 2005;41 doi: 10.7589/0090-3558-41.2.426. 426–230. [DOI] [PubMed] [Google Scholar]
- Colegrove K.M., Gulland F.M., Harr K., Naydan D.K., Lowenstine L.J. Pathologic features of amyloidosis in stranded California sea lions (Zalophus californianus) J. Comp. Pathol. 2009;140:105–112. doi: 10.1016/j.jcpa.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Colegrove K.M., Gulland F.M.D., Naydan D.K., Lowenstine L.J. Tumor morphology and immunohistochemical expression of estrogen receptor, progesterone receptor, p53, and Ki67 in urogenital carcinomas of California sea lions (Zalophus californianus) Vet. Pathol. 2009;46:642–655. doi: 10.1354/vp.08-VP-0214-C-FL. [DOI] [PubMed] [Google Scholar]
- Colegrove K.M., Gulland F.M.D., Naydan D.K., Lowenstine L.J. The normal genital tract of the female California sea lion (Zalophus californianus): cyclic changes in histomorphology and hormone receptor distribution. Anat. Rec. 2009;292:1801–1817. doi: 10.1002/ar.21009. [DOI] [PubMed] [Google Scholar]
- Colegrove K.M., Grigg M.E., Carlson-Bremer D., Miller R.H., Gulland F.M., Ferguson D.J., Barr B.C., Nordhausen R., Melli A.C., Contrad P.A. Discovery of three novel coccidian parasites infecting California sea lions (Zalophus californianus) with evidence of sexual replication and interspecies pathogencity. J. Parasitol. 2011;97:868–877. doi: 10.1645/GE-2756.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegrove, K.M. 2018. Noninfectious diseases. In: Gulland, F.M.D., Dierauf, L.A. Whitman, K.L. (Eds). CRC Handbook of Marine Mammal Medicine, 3rd Edition. CRC Press, Boca Raton, pp. 255-284.
- Colitz C.M., Renner M.S., Manire C.A., Doescher B., Schmitt T.L., Osborn S.D., Croft L., Olds J., Gehring E., Mergl J., Tuttle A.D., Sutherland-Smith M., Rudnick J.C. Characterization of progressive keratitis in otariids. Vet. Ophthalmol. 2010;13:47–53. doi: 10.1111/j.1463-5224.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- Colitz C.M., Saville W.J., Renner M.S., McBain J.F., Reidarson T.H., Schmitt T.L., Nolan E.C., Dugan S.J., Knightly F., Rodriguez M.M., Mejia-Fava J.C., Osborn S.D., Clough P.L., Collins S.P., Osborn B.A., Terrell K. Risk factors associated with cataracts and lens luxation in captive pinnipeds in the United States and the Bahamas. J. Am. Vet. Med. Assoc. 2010;237:429–436. doi: 10.2460/javma.237.4.429. [DOI] [PubMed] [Google Scholar]
- Cousins D.V., Bastida R., Cataldi A., Quse V., Redrobe S., Dow S., Duignan P., Murray A., Dupont C., Ahmed N., Collins D.M., Butler W.R., Dawson D., Rodriguez D., Loureiro J., Romano M.I., Alito A., Zumarraga M., Bernardelli A. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int. J. Syst. Evol. Microbiol. 2003;53:1305–1314. doi: 10.1099/ijs.0.02401-0. [DOI] [PubMed] [Google Scholar]
- Dagleish M.P., Barrows M., Maley M., Killick R., Finlayson J., Goodchild R., Valentine A., Saunders R., Willoughby K., Smith K.C., Stidworthy M.F. The first report of otarine herpesvirus-1-associated urogenital carcinoma in a South American fur seal (Arctocephalus australis) J. Comp. Pathol. 2013;149(1):119–125. doi: 10.1016/j.jcpa.2012.10.002. [DOI] [PubMed] [Google Scholar]
- de Amorim D.B., Casagrande R.A., Alievi M.M., Wouters F., De Oliveira L.G.S., Driemeier D., Tavares M., Ikuta C.Y., Telles E.O., Ferreira-Neto J.S. Mycobacterium pinnipedii in a Stranded South American Sea Lion (Otaria byronia) in Brazil. J. Wildl. Dis. 2014;50:419–422. doi: 10.7589/2013-05-124. [DOI] [PubMed] [Google Scholar]
- de Boer G.F., Back W., Osterhaus A.D. An ELISA for detection of antibodies against influenza A nucleoprotein in humans and various animal species. Arch. Virol. 1990;115:47–61. doi: 10.1007/BF01310622. [DOI] [PubMed] [Google Scholar]
- Denison D.M., Kooyman G.L. Structure and function of small airways in pinniped and sea otter lungs. Resp. Physiol. 1973;17:1–10. doi: 10.1016/0034-5687(73)90105-9. [DOI] [PubMed] [Google Scholar]
- Dennison S.E., Gulland F., Haulena M., De Morais H., Colegrove K. Urate nephrolithiasis in a northern elephant seal (Mirounga angustirostris) and a California sea lion (Zalophus californianus) J. Zoo Wildl. Med. 2007;38:114–120. doi: 10.1638/05-121.1. [DOI] [PubMed] [Google Scholar]
- Dennison S.E., Boor M., Fauquier D., Van Bonn W., Greig D.J., Gulland F.M.D. Foramen ovale and ductus arteriosus patency in neonatal harbor seal (Phoca vitulina) pups in rehabilitation. Aquat. Mammals. 2011;37:161–166. [Google Scholar]
- Duncan C.G., Tiller R., Mathis D., Stoddard R., Kersh G.J., Dickerson B., Gelatt T. Brucella placentitis and seroprevalence in northern fur seals (Callorhinus ursinus) of the Pribilof Islands, Alaska. J. Vet. Diagn. Invest. 2014;26:507–512. doi: 10.1177/1040638714532647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duignan P., Van Bressem M.F., Baker J.D., Barbieri M., Kathleen M., Colegrovem K.M., De Guise S., de Swart R.L., Di Guardo G., Andrew Dobson A., Duprex W.P., Early G., Fauquier D., Goldstein T., Goodman S.J., Grenfell B., Groch K.R., Gulland F., Hall A., Jensen B.A., Lamy K., Matassa K., Mazzariol S., Sinead E., Morris S.E., Nielsen O., Rotstein D., Rowles T.K., Saliki J.T., Siebert U., Waltzek T., Wellehan J.F.X. Phocine distemper virus: current knowledge and future directions. Viruses. 2014;6:5093–5134. doi: 10.3390/v6125093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire S.E., Wang Z., Byrd M., Whitehead H.R., Paternoster J., Morton S.L. Co-occurrence of multiple classes of harmful algal toxins in bottlenose dolphins (Tursiops truncatus) stranding during an unusual mortality event in Texas USA. Harmful Algae. 2011;10:330–336. [Google Scholar]
- Flower J.E., Gamble K.C., Stone M., Lyons J.A., Maganti R.J., Tuomi P.A., Olds J.E., Sims M.A., Gauger P., Tuttle A.D. Esophageal squamous cell carcinoma in six harbor seals (Phoca vitulina spp.) J. Zoo Wildl. Med. 2014;45:620–631. doi: 10.1638/2012-0218R1.1. [DOI] [PubMed] [Google Scholar]
- Forshaw D., Phelps G.R. Tuberculosis in a captive colony of pinnipeds. J. Wildl. Dis. 1991;27:288–295. doi: 10.7589/0090-3558-27.2.288. [DOI] [PubMed] [Google Scholar]
- Foster G., Osterman B.S., Godfroid J., Jacques I., Cloeckaert A. Brucella ceti sp nov and Brucella pinnipedialis sp nov for Brucella strains with cetaceans and seals as their preferred hosts. Int. J. Syst. Evol. Microbiol. 2007;57:2688–2693. doi: 10.1099/ijs.0.65269-0. [DOI] [PubMed] [Google Scholar]
- Gaffney P.M., Colegrove K.M., Gulland F.M.D., Byrne B., Jang S.S., Edgar K., Lowenstine L.J. Pathologic, microbiologic, and epidmiologic characterization of Pseudomonas sp. in California sea lions (Zalophus californianus) and Pacific harbor seals (Phoca vitulina). Proceedings of the 39th Conference of the International Association for Aquatic Animal Medicine; Pomezia, Italy; 2008. p. 100. [Google Scholar]
- Garner M.M., Lambourn D.M., Jeffries S.J., Hall P.B., Rhyan J.C., Ewalt D.R., Polzin L.M., Cheville N.F. Evidence of Brucella infection in Parafilaroides lungworms in a Pacific harbor seal (Phoca vitulina richardsi) J. Vet. Diagn. Invest. 1997;9:298–303. doi: 10.1177/104063879700900311. [DOI] [PubMed] [Google Scholar]
- Geraci J.R., St. Aubin D.J., Barker I.K., Webster R.G., Hinshaw V.S., Bean W.J., Ruhnke H.L., Prescott J.H., Early G., Baker N.F., Madoff S., Schooley R.T. Mass mortality of harbor seals: pneumonia associated with influenza A virus. Science. 1982;215:1129–1131. doi: 10.1126/science.7063847. [DOI] [PubMed] [Google Scholar]
- Gibson A.K., Raverty S., Lambourn D.M., Huggins J., Magargal S.L., Grigg M.E. Polyparasitism is associated with increased disease severity in Toxoplasma gondii-infected marine sentinel species. PLoS Neglect. Trop. Dis. 2011;5:e1142. doi: 10.1371/journal.pntd.0001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein T., Johnson S.P., Phillips A.V., Hanni K.D., Fauquier D.A., Gulland F.M.D. Human-related injuries observed in live stranded pinnipeds along the central California coast 1986-1998. Aquat. Mammals. 1999;25:43–51. [Google Scholar]
- Goldstein T., Gulland F.M.D., Aldridge B.M., Harvey J.T., Rowles T., Lambourn D.M., Jeffries S.J., Measures L., Yochem P.K., Stewart B.S., Small R.J., King D.P., Stott J.L., Mazet J.A. Antibodies to phocine herpesvirus-1 are common in North American harbor seals (Phoca vitulina) J. Wildl. Dis. 2003;39:487–494. doi: 10.7589/0090-3558-39.3.487. [DOI] [PubMed] [Google Scholar]
- Goldstein, T., Mazet, J.A., Gulland, F.M., Rowles, T., Harvey J.T., Allen S.G., King, D.P., Aldridge, B.M., Stott, J.L., 2004. The transmission of phocine herpesvirus-1 in rehabilitating and free-ranging Pacific harbor seals (Phoca vitulina) in California. Vet. Microbiol. 103, 131–141. [DOI] [PubMed]
- Goldstein, T, Mazet, J.A.K., Zabka, T.S., Langlois, G., Colegrove, K.M., Silver, M., Bargu, S., Van Dolah, F., Leighfield, T., Conrad, P.A., Barakos, J., Williams, D.C., Dennison, S., Haulena, Gulland, F.M.D., 2008. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc. Biol. Sci. 275(1632), 267–276. [DOI] [PMC free article] [PubMed]
- Goldstein T., Zabka T.S., DeLong R.L., Wheeler E.A., Ylitalo G., Bargu S., Silver M., Leighfield T., Van Dolah F., Langlois G., Sidor I., Dunn J.L., Gulland F.M.D. The role of domoic acid in abortion and premature parturition of California sea lions (Zalophus californianus) on San Miguel Island. Calif. J. Wildl. Dis. 2009;45:91–108. doi: 10.7589/0090-3558-45.1.91. [DOI] [PubMed] [Google Scholar]
- Goldstein T., Colegrove K.M., Hanson M., Gulland F.M. Isolation of a novel adenovirus from California sea lions, Zalophus californianus. Dis. Aquat. Organ. 2011;94:243–248. doi: 10.3354/dao02321. [DOI] [PubMed] [Google Scholar]
- Greig D.J., Gulland F.M.D., Smith W.A., Conrad P.A., Field C.L., Fleetwood M., Harvey J.T., Ip H.S., Jang S., Packham A., Wheeler E., Hall A.J. Surveillance for zoonotic and selected pathogens in harbor seals Phoca vitulina from central California. Dis. Aquat. Organ. 2014;111:93–106. doi: 10.3354/dao02762. [DOI] [PubMed] [Google Scholar]
- Grachev M.A., Kumarev V.P., Mamaev L.V., Zorin V.L., Baranova L.V., Denikina N.N., Belikov S.I., Petrov E.A., Kolesnik V.S., Kolesnik R.S. Distemper virus in Baikal seals. Nature. 1989;338:209. [Google Scholar]
- Gulland, F.M.D., Trupkiewicz, J.G., Spraker, T.R., Lowenstine, L.J. 1996a. Metastatic carcinoma of probable transitional cell origin in 66 free-living California sea lions (Zalophus californianus), 1979-1994. J. Wildl. Dis. 32(2), 250–258. [DOI] [PubMed]
- Gulland F.M.D., Koski M., Lowenstine L.J., Colagross A., Morgan L., Spraker T. Leptospirosis in California sea lions (Zalophus californianus) stranded along the central California coast, 1981-1994. J. Wildl. Dis. 1996;32:572–580. doi: 10.7589/0090-3558-32.4.572. [DOI] [PubMed] [Google Scholar]
- Gulland F.M.D., Beckmen K., Burek K., Lowenstine L., Werner L., Spraker T., Dailey M., Harris E. Nematode (Otostronglus circumlitus) infestation of Northern elephant seals (Mirounga angustirostris) stranded along the central California coast. Mar. Mammal Sci. 1997;13:46–459. [Google Scholar]
- Gulland F.M., Lowenstine L.J., Lapointe J.M., Spraker T., King D.P. Herpesvirus infection in stranded Pacific harbor seals of coastal California. J. Wildl. Dis. 1997;33:450–458. doi: 10.7589/0090-3558-33.3.450. [DOI] [PubMed] [Google Scholar]
- Gulland F.M.D., Hall A.J., Greig D.J., Frame E.R., Colegrove K.M., Booth R.K.N., Wasser S.K., Scott-Moncrieff J.C.R. Evaluation of circulating eosinophil count and adrenal gland function in California sea lions naturally exposed to domoic acid. J. Am. Vet. Med. Assoc. 2012;241:943–959. doi: 10.2460/javma.241.7.943. [DOI] [PubMed] [Google Scholar]
- Haman, K.H., Raverty, S., Daoust, P.Y., St. Leger, J., Hammill, M., Fenton, H., Grigg, M., 2013. Novel Sarcocystis spp. associated with mortality in pinnipeds of the North Atlantic and Pacific Oceans. In: Proceedings of the 44th Annual Conference for the International Association for Aquatic Animal Medicine. Sausalito, California, USA.
- Härkönen T., Dietz R., Reijnders P., Teilmann J., Harding K., Hall A., Brasseur S., Siebert U., Goodman S.J., Jepson P.D., Rasmussen T.D., Thompson P. A review of the 1988 and 2002 phocine distemper virus epidemics in European harbour seals. Dis. Aquat. Organ. 2006;68:115–130. doi: 10.3354/dao068115. [DOI] [PubMed] [Google Scholar]
- Haulena M., Gulland F.M., Lawrence J.A., Fauquier D.A., Jang S., Aldridge B., Spraker T., Thomas L.C., Brown D.R., Wendland L., Davidson M.K. Lesions associated with a novel Mycoplasma sp. California sea lions (Zalophus californianus) undergoing rehabilitation. J. Wildl. Dis. 2006;42:40–45. doi: 10.7589/0090-3558-42.1.40. [DOI] [PubMed] [Google Scholar]
- Hernandez-Orts J.S., Montero F.E., Juan-Garcia A., Garcia N.A., Crespo E.A., Raga J.A., Aznar F.J. Intestinal helminth fauna of the South American sea lion Otaria flavescens and fur seal Arctocephalus australis from northern Patagonia Argentina. J. Helminthol. 2013;87:336–347. doi: 10.1017/S0022149X12000454. [DOI] [PubMed] [Google Scholar]
- Hinshaw V.S., Bean W.J., Webster R.G., Rehg J.E., Fiorelli P., Early G., Geraci J.R., St.Aubin D.J. Are seals frequently infected with avian influenza viruses? J. Virol. 1984;51:863–865. doi: 10.1128/jvi.51.3.863-865.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckabone S.E., Gulland F.M., Johnson S.M., Colegrove K.M., Dodd E.M., Pappagianis D., Dunkin R.C., Casper D., Carlson E.L., Sykes J.E., Meyer W. Coccidioidomycosis and other systemic mycoses of marine mammals stranding along the central California, USA coast: 1998-2012. J. Wildl. Dis. 2015;51:295–308. doi: 10.7589/2014-06-143. [DOI] [PubMed] [Google Scholar]
- Hueffer K., Gende S.M., O’Hara T.M. Assay dependence of Brucella antibody prevalence in a declining Alaskan harbor seal (Phoca vitulina) population. Acta Vet. Scand. 2013;55:2. doi: 10.1186/1751-0147-55-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshima Y., Murakami T., Ishiquro N., haseqawa K., Kasamatsu M. An outbreak of lethal adenovirus infection among different otariid species. Vet. Microbiol. 2013;165:455–459. doi: 10.1016/j.vetmic.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Jang S., Wheeler L., Carey R.B., Jensen B., Crandall C.M., Schrader K.N., Jessup D., Colegrove K., Gulland F.M.D. Pleuritis and suppurative pneumonia associated with a hypermucoviscosity phenotype of Klebsiella pneumoniae in California sea lions (Zalophus californianus) Vet. Microbiol. 2010;141:174–177. doi: 10.1016/j.vetmic.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Jeffery B., Barlow T., Moizer K., Paul S., Boyle C. Amnesic shellfish poison. Food Chem. Toxicol. 2004;42:545–557. doi: 10.1016/j.fct.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Katz H., Morgades D., Castro-Ramos M. Pathological and parasitological findings in South American fur seal pups (Arctocephalus australis) in Uruguay. ISRN Zoology 2012. 2012;2012:7. [Google Scholar]
- Kennedy S. Morbillivirus infections in aquatic mammals. J. Comp. Pathol. 1998;119:201–205. doi: 10.1016/s0021-9975(98)80045-5. [DOI] [PubMed] [Google Scholar]
- Kiers A., Klarenbeek A., Mendelts B., Van Soolingen D., Koeter G. Transmission of Mycobacterium pinnipedii to humans in a zoo with marine mammals. Int. J. Tuberc. Lung Dis. 2008;12:1469–1473. [PubMed] [Google Scholar]
- Kik M.J., Goris M.G., Bos J.H., Hartskeerl R.A., Dorrestein G.M. An outbreak of leptospirosis in seals (Phoca vitulina) in captivity. Vet. Q. 2006;28:33–39. doi: 10.1080/01652176.2006.9695204. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lee J.K., Yoo H.S., Shin N.S., Lee K.H., Kim D.Y. Endocarditis associated with Escherichia coli in a sea lion (Zalophus californianus) J. Vet. Diagn. Invest. 2002;14:260–262. doi: 10.1177/104063870201400315. [DOI] [PubMed] [Google Scholar]
- Kriz P., Kralik P., Slany M., Slana I., Svobodova J., Parmova I., Barnet V., Jurek V., Pavlik I. Mycobacterium pinnipedii in a captive Southern sea lion (Otaria flavescens): a case report. Vet. Med. 2011;56:307–313. [Google Scholar]
- Lapointe J.M., Duignan P.J., Marsh A.E., Gulland F.M., Barr B.C., Naydan D.K., King D.P., Farman C.A., Burek-Huntingdon K.A., Lowenstine L.J. Meningoencephalitis due to Sarcocystis neurona-like protozoan in Pacific harbor seals (Phoca vitulina richardsi) J. Parasitol. 1998;84:1184–1189. [PubMed] [Google Scholar]
- Lefebvre K.A., Robertson A., Frame E.R., Colegrove K.M., Nance S., Baugh K.A., Wiedenhoft H., Gulland F.M.D. Clinical signs and histopathology associated with domoic acid poisoning in Northern fur seals (Callorhinus ursinus) and validation of toxin detection methods. Harmful Algae. 2010;9(4):374–383. [Google Scholar]
- Ling J.K. Pelage and molting in wild mammals with special reference to aquatic forms. Q. Rev. Biol. 1970;45:16. doi: 10.1086/406361. [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith J.O., Greig D.J., Hietala S., Ghneim G.S., Palmer L., St.Leger J., Grenfell B.T., Gulland F.M. Cyclical changes in seroprevalence of leptospirosis in California sea lion: endemic and epidemic disease in one host species? BMC Infect. Dis. 2007;7(125) doi: 10.1186/1471-2334-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler S.H., de Lisle G.W., Neill M.A., Collins D.M., Price-Carter M., Paterson B., Crews K.B. The seal tuberculosis agent, Mycobacterium pinnipedii, infects domestic cattle in New Zealand: epidemiologic factors and DNA strain typing. J. Wildl. Dis. 2014;50:180–187. doi: 10.7589/2013-09-237. [DOI] [PubMed] [Google Scholar]
- Ludes-Wehrmeister E., Dupke C., Harder T.C., Baumgärtner W., Haas L., Teilmann J., Dietz R., Jensen L.F., Siebert U. Phocine distemper virus (PDV) seroprevalence as predictor for future outbreaks in harbour seals. Vet. Microbiol. 2016;183:43–49. doi: 10.1016/j.vetmic.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Lynch M., Taylor T.K., Duignan P.J., Swingler J., Marenda M., Arnould J.P., Kirkwood R. Mycoplasma in Australian fur seals (Arctocephalus pusillus doriferus): identification and association with abortion. J. Vet. Diagn. Invest. 2011;23:1123–1130. doi: 10.1177/1040638711425699. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., DeLong R.L., Gulland F.M., Melin S.R., Tolliver S.C., Spraker T.R. Comparative biology of Uncinaria spp. in the California sea lion (Zalophus californianus) and the northern fur seal (Callorhinus ursinus) in California. J. Parasitol. 2000;86:1348–1352. doi: 10.1645/0022-3395(2000)086[1348:CBOUSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Spraker T.R., De Long R.L., Ionita M., Melin S.R., Nadler S.A., Tolliver S.C. Review of research on hookworms (Uncinaria lucasi Stiles, 1901) in northern fur seals (Callorhinus ursinus Linnaeus 1758) Parasitol. Res. 2011;109:257–265. doi: 10.1007/s00436-011-2420-6. [DOI] [PubMed] [Google Scholar]
- Mackereth G.F., Webb K.M., O’keefe J.S., Duignan P.J., Kittelberger R. Serological survey of pre-weaned New Zealand fur seals (Arctocephalus forsteri) for brucellosis and leptospirosis. N Z Vet. J. 2005;53:428–432. doi: 10.1080/00480169.2005.36588. [DOI] [PubMed] [Google Scholar]
- McClenahan S.D., Bok K., Neill J.D., Smith A.W., Rhodes C.R., Sosnovtsev S.V., Green K.Y., Romero C.H. A capsid gene-based real-time reverse transcription polymerase chain reaction assay for the detection of marine vesiviruses in the Caliciviridae. J. Virol. Meth. 2009;161:12–18. doi: 10.1016/j.jviromet.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHuron E.A., Greig D.J., Colegrove K.M., Fleetwood M., Spraker T.R., Gulland F.M.D., Harvey J.T., Lefebvre K.A., Frame E.R. Domoic acid exposure and associated clinical signs and histopathology in Pacific harbor seals (Phoca vitulina richardii) Harmful Algae. 2013;23:28–33. [Google Scholar]
- Measures L.N. Lungworms of Marine Mammals. In: Samuel W.M., Pybus M.J., Kocan A.A., editors. Parasitic Diseases of Wild Mammals. Iowa State University Press; Ames: 2001. pp. 279–300. [Google Scholar]
- Measures, L.N. 2018. Helminths and parasitic arthropods In: Gulland, F.M.D., Dierauf, L.A., Whitman, K.L. (Eds.). CRC Handbook of Marine Mammal Medicine, 3rd Edition, CRC Press, Boca Raton, pp. 457–486.
- Miller M.A. Tissue-Cyst Forming Coccidian of Marine Mammals. In: Fowler M.E., editor. Zoo and Wildlife Medicine. Saunders Elsevier; Philadelphia: 2008. pp. 319–340. [Google Scholar]
- Moeller R.B. Pathology of Marine Mammals with Special Reference to Infectious Diseases. In: Vos J.G., Bossart G.D., Fournier M., O’Shea T.J., editors. Toxicology of Marine Mammals. Taylor, Francis; London: 2003. pp. 3–54. [Google Scholar]
- Moore M.J., van der Hoop J., Barco S.G., Costidis A.M., Gulland F.M., Jepson P.D., Moore K.T., Raverty S., McLellan W.A. Criteria and case definitions for serious injury and death in pinnipeds and cetaceans caused by anthropogenic trauma. Dis. Aquat. Organ. 2013;103:229–264. doi: 10.3354/dao02566. [DOI] [PubMed] [Google Scholar]
- Moore M.J., Bogomolni A.L., Dennison S.E., Early G., Garner M.M., Hayward B.A., Lentell B.J., Rotstein D.S. Gas bubbles in seals, dolphins, and porpoises entangled and drowned at depth in gillnets. Vet. Pathol. 2009;46:536–547. doi: 10.1354/vp.08-VP-0065-M-FL. [DOI] [PubMed] [Google Scholar]
- Moser I., Prodinger W.M., Hotzel H., Greenwald R., Lyashchenko K.R., Bakker D., Gomis D., Seidler T., Ellenberger C., Hetzel U., Wuennemann K., Moisson P. Mycobacterium pinnipedii: transmission from South American sea lion (Otaria byronia) to Bactrian camel (Camelus bactrianus bactrianus) and Malayan tapirs (Tapirus indicus) Vet. Microbiol. 2008;127:399–406. doi: 10.1016/j.vetmic.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Mulcahy, D.M., and Fravel, V. 2018. Walrus medicine. In: Gulland, F.M.D., Dierauf, L.A., Whitman, K.L. (Eds.). CRC Handbook of Marine Mammal Medicine, 3rd Edition, CRC Press, Boca Raton, pp. 919-932.
- Nadler S.A., Lyons E.T., Pagan C., Hyman D., Lewis E.E., Beckmen K., Bell C.M., Castinel A., DeLong R.L., Duignan P.J., Farinpour C., Huntington K.B., Kuiken T., Morgades D., Naem S., Norman R., Parker C., Ramos P., Spraker T.R., Beron-Vera B. Molecular systematics of pinniped hookworms (Nematoda: Uncinaria): species delimitation, host associations and host-induced morphometric variation. Int. J. Parasitol. 2013;43:1119–1132. doi: 10.1016/j.ijpara.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Newman S.J., Smith S.A. Marine mammal neoplasia: a review. Vet. Pathol. 2006;43:865–880. doi: 10.1354/vp.43-6-865. [DOI] [PubMed] [Google Scholar]
- Nollens H.H., Gulland F.M.D., Hernandez J.A., Condit R.C., Klein P.A., Walsh M.T., Jacobson E.R. Seroepidemiology of parapoxvirus infections in captive and free-ranging California sea lions Zalophus californianus. Dis. Aquat. Organ. 2006;69:153–161. doi: 10.3354/dao069153. [DOI] [PubMed] [Google Scholar]
- Nollens H.H., Jacobson E.R., Gulland F.M.D., Beusse D.P., Bossart G.D., Hernandez J.A., Klein P.A., Condit R.C. Pathology and preliminary characterization of a parapoxvirus isolated from a California sea lion (Zalophus californianus) J. Wildl. Dis. 2006;42:23–32. doi: 10.7589/0090-3558-42.1.23. [DOI] [PubMed] [Google Scholar]
- Nollens H.H., Gulland F.M.D., Jacobson E.R., Hernandez J.A., Klein P.A., Walsh M.T., Condit R.C. Parapoxviruses of seals and sea lions make up a distinct subclade within the genus Parapoxvirus. Virology. 2006;349:316–324. doi: 10.1016/j.virol.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Nollens H.H., wellehan J.F.X., Archer L., Lowenstine L.J., Gulland F.M.D. Detection of a respiratory coronavirus from tissues archived during a pneumonia epizootic in free-ranging Pacific harbor seals Phoca vitulina richardsii. Dis. Aquat. Organ. 2010;90:113–120. doi: 10.3354/dao02190. [DOI] [PubMed] [Google Scholar]
- Nymo I.H., Tryland M., Godfroid J. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata) Vet. Res. 2011;42:93. doi: 10.1186/1297-9716-42-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhaus A.D.M.E., Rimmelzwaan G.F., Martina B.E., Bestebroer T.M., Fouchier R.A. Influenza B virus in seals. Science. 2000;288:1051–1053. doi: 10.1126/science.288.5468.1051. [DOI] [PubMed] [Google Scholar]
- Ponganis P.J. Circulatory system. In: Perrin W.F., Wursig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. second ed. Elsevier Science; 2009. pp. 230–233. [Google Scholar]
- Pollock C.G., Rohrbach B., Ramsay E.C. Fungal dermatitis in captive pinnipeds. J. Zoo Wildl. Med. 2000;31:374–378. doi: 10.1638/1042-7260(2000)031[0374:FDICP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Prager K.C., Greig D.J., Alt D.P., Galloway R.L., Hornsby R.L., Palmer L.J., Soper J., Wu Q., Zuerner R.L., Gulland F.M., Lloyd-Smith J.O. Asymptomatic and chronic carriage of Leptospira interrogans serovar Pomona in California sea lions (Zalophus californianus) Vet. Microbiol. 2013;164:177–183. doi: 10.1016/j.vetmic.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Reidarson, T.H., García-Párraga, D., Wiederhold, N. 2018. Marine mammal mycoses. In: Gulland, F.M.D., Dierauf, L.A., Whitman, K.L. (Eds.). CRC Handbook of Marine Mammal Medicine, 3rd Edition, CRC Press, Boca Raton, pp. 377-412.
- Roe W.D., Rogers L., Pinpimai K., Dittmer K., Marshall J., Chilvers B.L. Septicaemia and meningitis caused by infection of New Zealand sea lion pups with a hypermucoviscous strain of Klebsiella pneumoniae. Vet. Microbiol. 2015;176:301–308. doi: 10.1016/j.vetmic.2015.01.019. [DOI] [PubMed] [Google Scholar]
- St.Leger J.A., Begeman L., Fleetwood M., Frasca Jr. S., Garner M.M., Lair S., Trembley S., Linn M.J., Terio K.A. Comparative pathology of nocardiosis in marine mammals. Vet. Pathol. 2009;46:299–308. doi: 10.1354/vp.46-2-299. [DOI] [PubMed] [Google Scholar]
- St. Leger J.A., Nilson E.M. Intestinal atresia in a harbor seal (Phoca vitulina) and review of congenital conditions of the species. Aquat.Mammals. 2014;40:207–212. [Google Scholar]
- Scholin C.A., Gulland F., Doucette G.J., Benson S., Busman M., Chavez F.P., Cordaro J., DeLong R., De Vogelaere A., Harvey J., Haulena M., Lefebvre K., Lipscomb T., Loscutoff S., Lowenstine L.J., Marin R., Miller P.E., McLellan W.A., Moeller P.D., Powell C.L., Rowles T., Silvagni P., Silver M., Spraker T., Trainer V., Van Dolah F.M. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature. 2000;403:80–84. doi: 10.1038/47481. [DOI] [PubMed] [Google Scholar]
- Seguel M., Gottdenker, N.L., Colegrove, K., Johnson, S., Struve, C., Howerth, E.W. 2017a. Hypervirulent Klebsiella pneumoniae in California sea lions (Zalophus californianus): pathologic findings in natural infections. Vet Pathol. 54: 846-850. [DOI] [PubMed]
- Siebert U., Wohlsein P., Lehnert K., Baumgärtner W. Seguel M., Gottdenker, N.L., Colegrove, K., Johnson, S., Struve, C., Howerth, E.W. 2017a. Hypervirulent Klebsiella pneumoniae in California sea lions (Zalophus californianus): pathologic findings in natural infections. Vet Pathol. 54: 846-850. Pathological findings in Harbour Seals (Phoca vitulina): 1996-2005. J. Comp. Pathol. 2007;137:47–58. doi: 10.1177/0300985817705172. [DOI] [PubMed] [Google Scholar]
- Siebert U., Gulland F., Harder T., Janiaux T., Seibel H., Wohlsein P., Baumgartner W. Epizootics in harbour seals (Phoca vitulina): clinical aspects. NAMMCO Sci. Pub. 2010;8:265–274. [Google Scholar]
- Silvangi S., Lowenstine L.J., Spraker T., Lipscomb T., Gulland F.M.D. Pathology of domoic acid toxicity in California sea lions (Zalophus californianus) Vet. Pathol. 2005;42:184–191. doi: 10.1354/vp.42-2-184. [DOI] [PubMed] [Google Scholar]
- Smith A.W., Berry E.S., Skilling D.E., Cherry N., Mean J.H., Matson D.O. Calicivirus emergence from ocean reservoirs: zoonotic and interspecies movements. Emerg. Infect. Dis. 1998;4:13–20. doi: 10.3201/eid0401.980103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker T.R., DeLong R.L., Lyons E.T., Melin S.R. Hookworm enteritis with bacteremia in California sea lion pups on San Miguel Island. J. Wildl. Dis. 2007;43:179–188. doi: 10.7589/0090-3558-43.2.179. [DOI] [PubMed] [Google Scholar]
- Spraker T.R., Lander M.E. Causes of mortality in northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska in 2007. J. Wildl. Dis. 2010;46:450–473. doi: 10.7589/0090-3558-46.2.450. [DOI] [PubMed] [Google Scholar]
- Stewardson C.L., Hemsley S., Meyer M.A., Canfield P.J., Maindonald J.H. Gross and microscopic visceral anatomy of the male Cape fur seal, Arctocephalus pusillus pusillus (Pinnipedia : Otariidae), with reference to organ size and growth. J. Anat. 1999;195:235–255. doi: 10.1046/j.1469-7580.1999.19520235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.J., Cousins D.V., Gow B.L., Collins D.M., Williamson B.H., Dagnia H.T. Seals, seal trainers, and mycobacterial infection. Am. Rev. Resp. Dis. 1993;147:164–167. doi: 10.1164/ajrccm/147.1.164. [DOI] [PubMed] [Google Scholar]
- Thorel M.F., Karoui C., Varnerot A., Fleury C., Vincent V. Isolation of Mycobacterium bovis from baboons, leopards and a sea-lion. Vet. Res. 1998;29:207–212. [PubMed] [Google Scholar]
- Trupkiewicz J.G., Gulland F.M.D., Lowenstine L.J. Congenital defects in northern elephant seals stranded along the central California coast. J. Wildl. Dis. 1997;33:220–225. doi: 10.7589/0090-3558-33.2.220. [DOI] [PubMed] [Google Scholar]
- Van Bolhuis G.H., Phillippa J.D., Garadhar A.A., Osterhaus A.D., Kuiken T. Fatal enterocolitis in harbor seals (Phoca vitulina) caused by infection with Eimeria phocae. Vet. Rec. 2007;160:297–300. doi: 10.1136/vr.160.9.297. [DOI] [PubMed] [Google Scholar]
- Vahlenkamp T.W., Harder T.C. Influenza virus infections in mammals. Berliner und Münchener Tierärztliche Wochenschrift. 2006;119:123–131. [PubMed] [Google Scholar]
- Waltzek T.B., Cortés-Hinojosa G., Wellehan Jr. J.F.X., Gray G.C. Marine mammal zoonoses: a review of disease manifestations. Zoonoses Public Health. 2012;59(8):521–535. doi: 10.1111/j.1863-2378.2012.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R.G., Hinshow V.S., Bean W.J., Van Wyke K.L., Geraci J.R., Aubin St. D.J., Petursson G. Characterization of an influenza A virus from seals. Virology. 1981;113:712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Welsh T., Burek-Huntington K., Savage K., Rosenthal B., Dubey J.P. Sarcocystis canis associated hepatitis in a Stellar sea lion (Eumetopias jubatus) from Alaska. J. Wildl. Dis. 2014;50:405–408. doi: 10.7589/2013-03-079. [DOI] [PubMed] [Google Scholar]
- Zabka, T.S., Lowenstine, L.J., Gulland, F.M.D., 2005. Normal gastrointestinal anatomy and perforating ulcerative gastroduodenitis in California sea lions (Zalophus californianus) 2005. In: Proceedings of the 36th Annual Conference of the International Association for Aquatic Animal Medicine. Seward, AK.
- Zabka T.S., Goldstein T., Cross C., Mueller R.W., Kreuder-Johnson C., Gill S., Gulland F.M. Characterization of a degenerative cardiomyopathy associated with domoic acid toxicity in California sea lions (Zalophus californianus) Vet. Pathol. 2009;46:105–119. doi: 10.1354/vp.46-1-105. [DOI] [PubMed] [Google Scholar]
- Zohari S., Neimanis A., Härkönen T., Moraeus C., Valarcher J. Avian influenza A(H10N7) virus involvement in mass mortality of harbour seals (Phoca vitulina) in Sweden March through October 2014. Eur. Surv. 2014;19:20967. doi: 10.2807/1560-7917.es2014.19.46.20967. [DOI] [PubMed] [Google Scholar]


