Graphical abstract
Summary of the main physiological or adverse environmental situations in higher plants where the hydrogen sulfide (H2S) participates.

Keywords: Hydrogen sulfide, Abiotic stress, Fruit ripening, Nitro-oxidative stress
Highlights
-
•
Hydrogen sulfide (H2S) plays a signaling role in higher plants.
-
•
It mediates persulfidation, a post-translational modification.
-
•
It regulates physiological functions ranging from seed germination to fruit ripening.
-
•
The beneficial effects of exogenous H2S are mainly caused by the stimulation of antioxidant systems.
Abstract
The signaling properties of the gasotransmitter molecule hydrogen sulfide (H2S), which is endogenously generated in plant cells, are mainly observed during persulfidation, a protein post-translational modification (PTM) that affects redox-sensitive cysteine residues. There is growing experimental evidence that H2S in higher plants may function as a mechanism of response to environmental stress conditions. In addition, exogenous applications of H2S to plants appear to provide additional protection against stresses, such as salinity, drought, extreme temperatures and heavy metals, mainly through the induction of antioxidant systems, in order to palliate oxidative cellular damage. H2S also appears to be involved in regulating physiological functions, such as seed germination, stomatal movement and fruit ripening, as well as molecules that maintain post-harvest quality and rhizobium–legume symbiosis. These properties of H2S open up new challenges in plant research to better understand its functions as well as new opportunities for biotechnological treatments in agriculture in a changing environment.
Introduction
The description of the gasotransmitter hydrogen sulfide (H2S), with its toxic impact on the metabolism of animal and plant cells, changed drastically when this molecule was shown to be endogenously generated in cells. However, its signaling capacity has particularly fascinated researchers in many fields of investigation [1], [2], [3], [4], [5]. A number of studies in the field of plants began to show that H2S is directly or indirectly involved in a wide range of physiological processes including seed germination [6], root organogenesis [7], [8], photosynthesis [9], stomatal movement [10], [11], [12], [13], fruit ripening [14], [15], as well as senescence in leaves, flowers and fruits [16], [17]. H2S has also been shown to be involved in the mechanism of response to adverse biotic and abiotic environmental conditions [18], [19]. Research has shown a significant correlation between the functions of H2S and nitric oxide (NO), another simple molecule, whose metabolisms appear regulate each other [4]. Fig. 1 summarizes the principal functions of H2S in higher plants. The main aim of this review is to provide a broad overview of the major role played by H2S in higher plants, with particular attention paid to the beneficial effects of its biotechnological application in crop plants, especially under adverse stressful conditions.
Fig. 1.
Summary of the main physiological or adverse environmental situations in higher plants where the endogenous or exogenous H2S seems to participate which could also have biotechnological applications.
Plant biochemistry of H2S: An overview
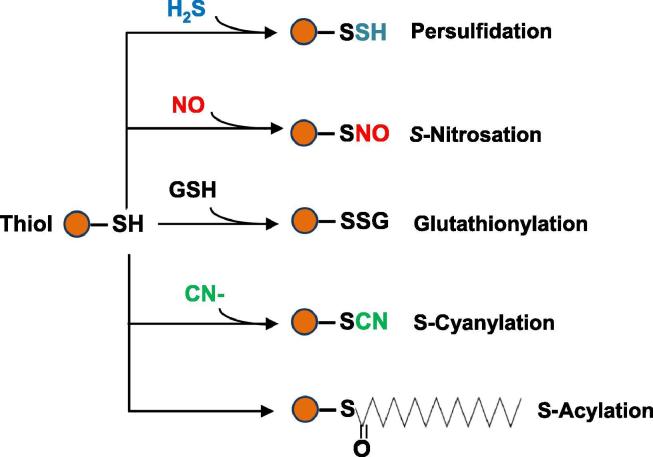
The study of H2S as a signaling molecule has focused on its capacity to interact with thiol (-SH) groups present in protein cysteine residues through the post-translational modification (PTM) persulfidation [4], [20]. It is important to point out the major regulatory role played by protein thiol groups involved in multiple interactions which can activate or inhibit the function of the target proteins [21], [22]. H2S competes with other molecules, such as nitric oxide (NO), glutathione (GSH), cyanide and fatty acids, which generate the PTMs S-nitrosation [4], [23], S-glutathionylation [24], [25], S-cyanylation [26] and S-acylation [27], [28], [29], respectively. Fig. 2 shows a simple model of these PTMs involving protein thiol groups. However, fewer studies have explored the potential protein targets of persulfidation, previously known as S-sulfhydration, and how this PTM affects up-regulates and down-regulates these proteins.
Fig. 2.
Protein thiol (-SH) modifications mediated by either the incorporation of H2S (persulfidation), NO (S-nitrosation), glutathione (GSH) (S-glutathionylation), cyanide (S-cyanylation) or fatty acid (S-acylation).
Information garnered from initial plant proteomic analyses focusing on the model plant Arabidopsis thaliana [30], [31] and that obtained from animal cells [32], [33], as well as complementary studies, have facilitated the evaluation of the in vitro effect of H2S on a specific plant protein using different H2S donors [15], [34], [35]. Table 1 shows a list of plant proteins, which have been observed to undergo persulfidation, and how their protein function is modulated [36], [37]. In some cases, a specific purified protein can behave differently under in vitro conditions depending on whether the H2S donor is applied to the whole plant, added to the nutrient solution or growth media or sprayed on the aerial part of the plant. This is due to the complex action of H2S characterized by its functional interaction/competition in whole cells with other molecules including nitric oxide (NO) [4], melatonin [38] and phytohormones such as ethylene, auxin and abscisic acid [39], [40].
Table 1.
Examples of plant protein targets which function is affected by H2S and consequently they undergo persulfidation.
| Enzyme | Function | Effect | Ref. |
|---|---|---|---|
| RuBISCO | Photosynthesis | Activity up-regulated | [9] |
| O-acetylserine(thiol)lyase (OAS-TL) | Sulfur metabolism | Activity up-regulated | [9] |
| L-cysteine desulphydrase (LCD) | Sulfur metabolism | Activity up-regulated | [9] |
| Ascorbate peroxidase (APX) | Antioxidant | Activity up-regulated | [30] |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | Energy production in the glycolysis | Activity up-regulated | [30] |
| Glutamine synthetase (GS) | Metabolism of nitrogen | Activity down-regulated | [30] |
| Actin | Involved in organelle movement, in cell division and expansion | Inhibite actin polymerization | [36] |
| 1-aminocyclopropane-1-carboxylic acid oxidase (ACO) | Ethylene biosynthesis | Activity down-regulated | [37] |
| NADP-isocitrate dehydrogenase (NADP-ICDH) | Provides NADPH as a reducing agent | Activity down-regulated | [15] |
| NADP-malic enzyme (NADP-ME) | Provides NADPH as a reducing agent | Activity down-regulated | [34] |
| Catalase | Antioxidant | Activity down-regulated | [35] |
| SNF1-RELATED PROTEIN KINASE2.6 (SnRK2.6) | Promote ABA signaling. | Promote ABA-induced stomatal closure | [12] |
| Respiratory burst oxidase homolog protein D (RBOHD) | Generation of superoxide radical | Activity up-regulated | [13] |
Although the precise mechanisms involved remain unknown, H2S has been shown to regulate gene expression [41], [42]. Exogenous applications of H2S to grapevine (Vitis vinifera L.) plants trigger gene expression involved in the synthesis of secondary metabolites as well as various defensive compounds which boosts plant development and abiotic resistance [43]. In addition, microarray analysis of differentially expressed genes of tomato plants supplemented with NaHS has shown that 5349 genes were up-regulated, while 5536 were down-regulated [44].
However, any precise biochemistry of endogenous H2S in plant cells, as well as how and where H2S is produced and its metabolic interactions with other molecules, is still in its infancy. In higher plant systems, several enzymes involved in cysteine metabolism present in subcellular compartments (the cytosol, chloroplasts, mitochondria and peroxisomes) are available for the production of H2S [35], [45], [46]. These enzymes include L-cysteine desulfhydrase (L-DES), L-cysteine desulfhydrase 1 (DES1), previously known as Cys synthase-like (CS-LIKE), and cysteine synthase (CS) in the cytosol; D-cysteine desulfhydrase (D-DES) and cyano alanine synthase (CAS) in mitochondria; and sulfite reductase (SiR) in the chloroplast [3], [46], [47], [48]. However, given its highly lipophilic nature, the H2S molecule can spread with ease throughout the lipid bilayer of cell membranes [49]. New promising data also show how activities, such as cysteine desulfhydrases, in some of these enzymes are up-regulated under red light and down-regulated by blue and white light [50].
Potential biotechnological applications of exogenously applied H2S
Although further basic research on H2S is required, sufficient experimental data show that the exogenous application of H2S to different plant species at different stages of development can palliate damage caused by abiotic stress and enhance physiological features such as seed germination, root development and post-harvest preservation of vegetables [4], [51], [52]. However, an empirical evaluation of how H2S is to be applied and appropriate dosages is also required. Up to now, exogenous applications have been carried out using chemicals capable of delivering H2S. In animal research on biomedical applications, different families of chemicals, with the capacity to slowly release H2S into cells, have been developed. This has led to the development of water-soluble molecules such as (p-methoxyphenyl)morpholino-phosphinodithioic acid (GYY4137) and a family of cysteine-activated H2S donors (5a, 8l, and 8o) [53]. Few plant studies have used these chemicals [54] which are comparatively more expensive to produce than standard chemicals such as sodium hydrosulfide (NaHS) and inorganic sodium polysulfides (Na2Sn) such as Na2S2, Na2S3, and Na2S4. Thus, in aqueous solutions, delivery of H2S by these polysulfides depends on medium pH and the corresponding pKa [55]. In plant research, the cheaper NaHS is exogenously added to hydroponic solutions and in vitro growth media or is sprayed directly on plants. NaHS, which is a short-lived donor and does not mimic the slow continuous process of H2S generation in vivo, is used in a wide range of concentrations. The chemical dialkyldithiophosphate, which is capable of slowly releasing H2S [56], has recently been demonstrated to increase corn plant weight by up to 39% after 4.5 weeks of treatment. Other compounds, which are capable of releasing NO combined with H2S, are being used in anti-inflammatory pharmaceutical treatments [57].
H2S and abiotic stress
Many adverse external conditions are well known to negatively affect plant growth, development and productivity [58]. To palliate these effects, plants have developed various strategies which differ according to the type of stress and plant species involved. In many cases, these stresses are associated with unregulated overproduction of reactivate oxygen and nitrogen species (ROS/RNS) which can trigger nitro-oxidative stress [59] characterized by an increase in key parameters such as lipid peroxidation, protein tyrosine nitration and oxidative damage to proteins and nucleic acids. Table 2 shows different examples of the beneficial effects of the exogenous application of H2S through the use of different donors on a wide range of agronomically important plants affected by stresses such as heavy metals (cadmium, aluminum, chromium, copper, iron, zinc), metalloids (arsenic), salinity, drought, as well as high and low temperatures [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84]. Apart from certain specific responses, in most cases, the application of exogenous H2S appears to cause an increase in the different components of antioxidant systems, such as catalase, superoxide dismutase (SOD) isozymes, as well as enzymatic and non-enzymatic components of the ascorbate-glutathione cycle, which enables H2O2 levels and lipid peroxidation content to be reduced.
Table 2.
Main effects of the exogenous application of H2S to plants exposed to diverse environmental stresses. ABA, abscisic acid. APX, ascorbate peroxidase. AsA, ascorbate. CAT, catalase. GR, glutathione reductase. GSH, reduced glutathione. GSNOR, S-nitrosoglutathione reductase. HT, high temperature. MDA, malondialdehyde. POD, peroxidase. NaHS, sodium hydrosulfide. PIP, plasma membrane intrinsic proteins. PM, plama membrane. SOD, superoxide dismutase.
| Environmental stress | H2S donor(μM) | Plant species | Effects | Ref. |
|---|---|---|---|---|
| Aluminum | NaHS(2) | Rice (Oryza sativa L.) | Increases root elongation and decrease Al contents in rice root tips. Increase antioxidant enzyme activities. Decrease MDA and H2O2 content in roots | [60] |
| NaHS(50) | Soybean (Glycine max L.) | Reduce Al accumulation. H2S function downstream of NO and induce citrate secretion through the upregulation of PM H+-ATPase-coupled citrate transporter cotransport systems | [61] | |
| Cadmium (Cd) | NaHS(100) | Alfafa (Medicago sativa L.) | Reduces the accumulation of MDA and H2O2. Increase the content of GSH and the activity of antioxidant enzymes (SOD, CAT and POD) | [62] |
| NaHS(500) | Bermudagrass (Cynodon dactylon L) | Alleviates Cd damages by modulating enzymatic and non-enzymatic antioxidants. | [63] | |
| NaHS(200) | Barley (Hordeum vulgare L.) | Reduces the accumulation of H2O2 and superoxide ions in roots | [64] | |
| NaHS(200) | Wheat (Triticum aestivum) | Increases the activities of antioxidant enzymes. Inhibits Cd uptake and reduce proline content | [65] | |
| Endogenous H2S | Arabidopsis (Arabidopsis thaliana) | Overexpression of D-Cysteine desulfhydrase (DCD) decreases Cd and ROS content | [66] | |
| Chromium(Cr) | NaHS(500) | Maize (Zea mays L.) | Alleviate chromium toxicity and enhances antioxidant activities (CAT, SOD, APX) | [67] |
| NaHS(200) | Caulifower (Brassica oleracea L.) | Decreases Cr content, H2O2 and MDA concentrations. Increases activity of antioxidant enzymes | [68] | |
| Copper (Cu) | NaHS(1,400) | Wheat (Triticum aestivum L.) | Lowers levels of MDA and H2O2 in germinating seeds. Increases SOD and CAT activities, and decreases lipoxygenase | [6] |
| Iron deficiency | NaHS(200) | Strawberry (Fragaria × ananassa) | Reduces electrolyte leakage, and content of H2O2 and MDA. Upregulate activities of antioxidant enzymes. Improved Fe uptake | [69] |
| Zinc (Zn) | NaHS (200) | Pepper (Capsicum annuum L.) | Increases plant growth, fruit yield, water status and proline content. Enhances the activity of antioxidant enzymes | [70] |
| Arsenic (As) | NaHS(100) | Pea (Pisum sativum L. | Increases of AsA and GSH contents and activities of the AsA–GSH cycle enzymes | [71] |
| Salinity | NaHS(50) | Rice (Oryza sativa L.) | Decreases the uptake of Na+ and the Na+/K+ ratio | [72] |
| NaHS(50) | Wheat (Triticum aestivum L.) | Suppresses ROS accumulation by increasing antioxidant defense | [73] | |
| NaHS(20) | Cucumber (Cucumis sativus L.) | Keeps Na+ and K+ homeostasis by the gene expression of plasma membrane Na+/H + antiporter (SOS1). Decrease lipid peroxidation content and ROS generation. Increases activity of antioxidant system | [74] | |
| NaHS(200) | Mangrove plant (Kandelia obovata) | Enhances the quantum efficiency of photosystem II (PSII) and the membrane lipid stability | [75] | |
| Drought | NaHS(500) | Wheat (Triticum aestivum L.) | Increases antioxidant enzyme activities, reduces MDA and H2O2 contents in both leaves and roots. Increases of the transcription levels of genes encoding ABA receptors. | [40] |
| NaHS(400) | Wheat (Triticum aestivum L.) | Induction of genes that code for antioxidant enzymes | [76] | |
| NOSH(1) compounds (100) | Alfalfa (Medicago sativa L.) | Lowers MDA. Induce Cu/ZnSOD, FeSOD genes | [77] | |
| Osmotic stress | NaHS(150) | Arabidopsis (Arabidopsis thaliana) | Increase phospholipase Dα1 and the antioxidant enzyme system. Reduce ROS and MDA content and reduce electrolyte leakage | [78] |
| Low temperature | NaHS(50) | Cucumber (Cucumis sativus L.) | Increases GSH and cucurbitacin C content | [79] |
| NaHS(500) | Lowbush blueberry (Vaccinium angustifolium) | Alleviate the degradation of chlorophyll and carotenoids and reduce the photoinhibition of PSII and PSI. | [80] | |
| High temperature | NaHS(100) | Strawberry (Fragaria × ananassa cv. 'Camarosa') | Induction of gene expression ocoding for antioxidant enzymes (cAPX, CAT, MnSOD, GR), heat shock proteins (HSP70, HSP80, HSP90) and aquaporins (PIP) | [81] |
| NaHS(500) | Maize (Zea mays L.) | Improves seed germination and increases antioxidant enzymes. Accumulation of proline | [82] | |
| NaHS (50) or MGYY4137 (10) | Poplar (Populus trichocarpa) | Increases GSNOR activity and reduce HT-induced damage to the photosynthetic system | [83] | |
| NaHS (100) or GYY4137 (10) | Arabidopsis thaliana | Enhances seed germination rate under HT.Increases gene expression of ABI5 (ABA-INSENSITIVE 5). | [84] |
Resulted in the Utility Patent Pub. No.: WO/2015/123273.
H2S in fruit ripening and post-harvest damage to fresh produce
Information available on endogenous H2S metabolism in fruits and vegetables is highly limited. Recently, endogenous H2S content in non-climacteric sweet pepper (Capsicum annumm L) fruits was reported to increase during the transition from green immature to red ripe [15]. However, the number of studies focusing on the economic impact of biotechnological applications of H2S on fruit ripening and post-harvest storage, which prevent the loss of fresh produce caused by fungi, bacteria, viruses and low temperatures used to store fruits and vegetables, has increased over the last ten years. Given that all these factors are usually associated with oxidative stress, many studies have shown that the exogenous application of H2S could have a beneficial effect on the shelf life of a diverse range of fruits, vegetables and flowers [14], [16], [85], [86], [38]. Table 3 provides representative examples of the exogenous application of H2S to fruits and vegetables [87], [88], [89], [90], [91], [92], [93], [94] which enables their quality to be maintained. Another common effect observed following exogenous treatment with H2S is an increase in antioxidant systems which prevent ROS overproduction and consequently oxidative damage.
Table 3.
Representative examples of the main beneficial effects of the exogenous application of H2S in fruits and vegetables.
| Fruit/vegetable | H2S donor | Effects | Ref. |
|---|---|---|---|
| Strawberry (Fragaria × ananassa Duch.) | 0.8 mM NaHS | Prolongs postharvest shelf life and reduces fruit rot disease | [87] |
| Broccoli (Brassica oleracea) | 2.4 mM NaHS | Alleviates senescent symptoms | [88] |
| Grape (Vitis vinifera L. × V. labrusca L. cv. Kyoho) | 1 mM NaHS | Alleviates postharvest senescence of grape and maintain high fruit quality | [89] |
| Banana (Musa acuminata, AAA group) | 1 mM NaHS | Alleviates fruit softening. Antagonizes ethylene effects | [14] |
| Tomato (Solanum lycopersicum L.) ‘Micro Tom’ | 0.9 mM NaHS | Postpones ripening and senescence of postharvest tomato fruits by antagonizing the effects of ethylene | [90] |
| Hawthorn (Crataegus oxyacantha) fruit | 1.5 mM NaHS | Confers tolerance to chilling. Triggers H2S accumulation, increase antioxidant enzyme activities of and promote phenolics accumulation | [91] |
| Avocado (Persea americana Mill, cv. 'Hass') | 200 µMNaHS | Protects against frost and day high light | [92] |
| Kiwifruit (Actinidia chinensis) | 20 µM H2S | Delays ripening and senescence. Inhibits ethylene production. Increases antioxidant activities. Regulates the cell wall degrading enzyme gene | [42] |
| Daylily (Hemerocallis fulva) | 4 mMNaHS | Delays senescence of postharvest daylily flowers. Increases antioxidant capacity to maintain the redox balance | [93] |
| Tomato (Solanum lycopersicum L.). | 1 M NaHS | Inhibits ethylene-induced petiole abscission | [94] |
Implication of H2S in rhizobium–legume symbiosis
In agriculture and natural ecosystems, a major source of nitrogen-fixation is throughout the nodule formation during the plant-rhizobia interaction [95]. As happened with the NO that was seen to be involved in the interaction rhizobium–legume symbiosis [96], [97], [98], H2S seems to be also involved in different ways in this process. A recent report indicates that exogenous H2S promotes plant growth, nodulation and nitrogenase activity in the functional symbiosis between rhizobium (Sinorhizobium fredii) and soybean (Glycine max) plants [99]. Furthermore, the synergy between H2S and rhizobia allowed the increase of soybean nitrogen contents by the regulation of related enzymes at different levels (activity, protein, and gene expression) as well as senescence-associated genes which were also regulated [100]. Moreover, new data obtained during the Mesorhizobium–Lotus symbiosis indicate that this interaction is regulated by the cross-talk among H2S with other signaling molecules including NO and ROS [101].
Conclusions and future perspectives
H2S, which is part of the plant sulfur metabolism, is a new signal molecule whose regulatory function acts through redox interactions, especially the protein post-translational modification persulfidation. The application of exogenous H2S, involving a signaling mechanism, causes an increase in different components of the antioxidant system at both the gene and protein level. Nevertheless, the precise biochemical and molecular mechanisms involved in these processes need to be further investigated in future research. However, the exogenous application of H2S undoubtedly has a beneficial effect on different plant species, especially those of considerable agronomic interest under adverse environmental conditions. Therefore, the use of H2S alone or combined with other molecules, such as nitric oxide, melatonin, thiourea, silicon, chitosan and calcium, which appear to beneficially affect crop plants, needs to be explored in light of climate change [102], [103], [104], [105], [106], [107], [108]. Thus, additional research is necessary in order to decipher the unknowns of H2S and its interaction with the metabolism of ROS and RNS under physiological and stressful conditions [109], as well as to establish biotechnological strategies to combat these stresses, which are responsible for major losses in plant yield and crop productivity.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
FJC and JMP research is supported by a European Regional Development Fund cofinanced grant from the Spanish Ministry of Economy and Competitiveness (AGL2015-65104-P and PID2019-103924GB-I00), the Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI 2020) (P18-FR-1359) and Junta de Andalucía (group BIO192), Spain.
Biographies

Francisco J. Corpas is Research Professor of the Spanish National Research Council (CSIC) which has more than 28 years of research experience in the metabolism of Reactive Oxygen, Nitrogen and Sulfur Species (ROS, RNS and RSS, respectively) in higher plants under physiology and environmental stress conditions. Special interests are the implications of these reactive species in fruit ripening and the nitro-oxidative metabolism of plant peroxisome. He was the Head of the Department of Biochemistry, Cell and Molecular Biology of Plants (2014–2018) at Research Institute named “Estación Experimental del Zaidín”-CSIC, Granada Spain. He already published more than 203 refereed research papers/review articles in peer reviewed journals (according with Scopus database with h-index: 58) and edited seven books.

José Manuel Palma is Research Professor with expertise on antioxidants and free radicals in plant systems. With more than 120 peer-reviewed research papers published, he has also been editor of five books and several special issues of diverse international journals. At present, he is involved in the investigation of the interaction between nitric oxide and antioxidants during fruit ripening. He leads the research group “Antioxidants, Free Radicals and Nitric Oxide in Biotechnology, Food and Agriculture” at Estación Experimental del Zaidín (EEZ), CSIC, Granada, Spain. He was also Deputy Director and Acting Director of the EEZ (CSIC) in the period 2007–2014.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lisjak M., Teklic T., Wilson I.D., Whiteman M., Hancock J.T. Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ. 2013;36(9):1607–1616. doi: 10.1111/pce.12073. [DOI] [PubMed] [Google Scholar]
- 3.Filipovic M.R., Jovanovic V.M. More than just an intermediate: hydrogen sulfide signalling in plants. J Exp Bot. 2017;2017(68):4733–4736. doi: 10.1093/jxb/erx352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corpas F.J., González-Gordo S., Cañas A., Palma J.M. Nitric oxide and hydrogen sulfide in plants: which comes first? J Exp Bot. 2019;70:4391–4404. doi: 10.1093/jxb/erz031. [DOI] [PubMed] [Google Scholar]
- 5.Kimura H. Signaling by hydrogen sulfide (H2S) and polysulfides (H2Sn) in the central nervous system. Neurochem Int. 2019;126:118–125. doi: 10.1016/j.neuint.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H., Hu L.Y., Hu K.D., He Y.D., Wang S.H., Luo J.P. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol. 2008;50:1518–1529. doi: 10.1111/j.1744-7909.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Tang J., Liu X.P., Wang Y., Yu W., Peng W.Y. Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J Integr Plant Biol. 2009;51:1084–1092. doi: 10.1111/j.1744-7909.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 8.Mei Y., Zhao Y., Jin X., Wang R., Xu N., Hu J. L-Cysteine desulfhydrase-dependent hydrogen sulfide is required for methane-induced lateral root formation. Plant Mol Biol. 2019;99(3):283–298. doi: 10.1007/s11103-018-00817-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Wu F.H., Wang W.H., Zheng C.J., Lin G.H., Dong X.J. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot. 2011;62(13):4481–4493. doi: 10.1093/jxb/err145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Mata C., Lamattina L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signaling. New Phytol. 2010;188:977–984. doi: 10.1111/j.1469-8137.2010.03465.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Zhou M., Ge Z., Shen J., Zhou C., Gotor C. ABA-triggered guard cell L -cysteine desulfhydrase function and in situ H2S production contributes to heme oxygenase-modulated stomatal closure. Plant Cell Environ. 2019 doi: 10.1111/pce.13685. [DOI] [PubMed] [Google Scholar]
- 12.Chen S., Jia H., Wang X., Shi C., Wang X., Ma P. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol Plant. 2020;S1674–2052(20):30004–30006. doi: 10.1016/j.molp.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Shen J., Zhang J., Zhou M., Zhou H., Cui B., Gotor C. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell. 2020 doi: 10.1105/tpc.19.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge Y., Hu K.D., Wang S.S., Hu L.Y., Chen X.Y., Li Y.H. Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS One. 2017;12(6):e0180113. doi: 10.1371/journal.pone.0180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz-Vargas M.A., González-Gordo S., Cañas A., López-Jaramillo J., Palma J.M., Corpas F.J. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide. 2018;81:36–45. doi: 10.1016/j.niox.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H., Hu S.L., Zhang Z.J., Hu L.Y., Jiang C.X., Wei Z.J. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol Technol. 2011;60:251–257. [Google Scholar]
- 17.Zheng J.L., Hu L.Y., Hu K.D., Wu J., Yang F., Zhang H. Hydrogen sulfide alleviates senescence of fresh-cut apple by regulating antioxidant defense system and senescence-related gene expression. HortScience. 2016;51:152–158. [Google Scholar]
- 18.Shi H., Ye T., Han N., Bian H., Liu X., Chan Z. Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J Integr Plant Biol. 2015;57(7):628–640. doi: 10.1111/jipb.12302. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee A., Tripathi D.K., Roychoudhury A. Hydrogen sulphide trapeze: Environmental stress amelioration and phytohormone crosstalk. Plant Physiol Biochem. 2018;132:46–53. doi: 10.1016/j.plaphy.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Aroca A., Gotor C., Romero L.C. Hydrogen sulfide signaling in plants: emerging roles of protein persulfidation. Front Plant Sci. 2018;9:1369. doi: 10.3389/fpls.2018.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan J., Gaffrey M.J., Qian W.J. Quantitative proteomic characterization of redox-dependent post-translational modifications on protein cysteines. Mol BioSyst. 2017;13(5):816–829. doi: 10.1039/c6mb00861e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatnagar A., Bandyopadhyay D. Characterization of cysteine thiol modifications based on protein microenvironments and local secondary structures. Proteins. 2018;86(2):192–209. doi: 10.1002/prot.25424. [DOI] [PubMed] [Google Scholar]
- 23.Astier J., Kulik A., Koen E., Besson-Bard A., Bourque S., Jeandroz S. Protein S-nitrosylation: what's going on in plants? Free Radic Biol Med. 2012;53(5):1101–1110. doi: 10.1016/j.freeradbiomed.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Zaffagnini M., Bedhomme M., Marchand C.H., Morisse S., Trost P., Lemaire S.D. Redox regulation in photosynthetic organisms: focus on glutathionylation. Antioxid Redox Signal. 2012;16(6):567–586. doi: 10.1089/ars.2011.4255. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Vivancos P., de Simone A., Kiddle G., Foyer C.H. Glutathione-linking cell proliferation to oxidative stress. Free Radic Biol Med. 2015;89:1154–1164. doi: 10.1016/j.freeradbiomed.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 26.García I., Arenas-Alfonseca L., Moreno I., Gotor C., Romero L.C. HCN Regulates cellular processes through posttranslational modification of proteins by S-cyanylation. Plant Physiol. 2019;179(1):107–123. doi: 10.1104/pp.18.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemsley P.A. An outlook on protein S-acylation in plants: what are the next steps? J Exp Bot. 2017;68(12):3155–3164. doi: 10.1093/jxb/erw497. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Qi B. Progress toward understanding protein S-acylation: prospective in plants. Front Plant Sci. 2017;8:346. doi: 10.3389/fpls.2017.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng L., Liu P., Liu Q., Wang T., Dong J. Dynamic protein S-acylation in plants. Int J Mol Sci. 2019;20(3):E560. doi: 10.3390/ijms20030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aroca Á., Serna A., Gotor C., Romero L.C. S-sulfhydration: a cysteine posttranslational modification in plant systems. Plant Physiol. 2015;168(1):334–342. doi: 10.1104/pp.15.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aroca A., Benito J.M., Gotor C., Romero L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J Exp Bot. 2017;68(17):4915–4927. doi: 10.1093/jxb/erx294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu L., Liu K., He J., Tian C., Yu X., Yang J. Direct proteomic mapping of cysteine persulfidation. Antioxid Redox Signal. 2019 doi: 10.1089/ars.2019.7777. [DOI] [PubMed] [Google Scholar]
- 34.Muñoz-Vargas M.A., González-Gordo S., Palma J.M. Corpas FJ. Inhibition of NADP-malic enzyme activity by H2S and NO in sweet pepper (Capsicum annuum L.) fruits. Physiol Plant. 2020;168(2):278–288. doi: 10.1111/ppl.13000. 10.1111/ppl.13000. [DOI] [PubMed] [Google Scholar]
- 35.Corpas F.J., Barroso J.B., González-Gordo S., Muñoz-Vargas M.A., Palma J.M. Hydrogen sulfide: a novel component in Arabidopsis peroxisomes which triggers catalase inhibition. J Integr Plant Biol. 2019;61(7):871–883. doi: 10.1111/jipb.12779. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Chen S., Wang X., Shi C., Liu H., Yang J. Hydrogen sulfide disturbs actin polymerization via S-Sulfhydration resulting in stunted root hair growth. Plant Physiol. 2018;178(2):936–949. doi: 10.1104/pp.18.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia H., Chen S., Liu D., Liesche J., Shi C., Wang J. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front Plant Sci. 2018;9:1517. doi: 10.3389/fpls.2018.01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee S. Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide. 2019;82:25–34. doi: 10.1016/j.niox.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Jia H., Hu Y., Fan T., Li J. Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci Rep. 2015;5:8251. doi: 10.1038/srep08251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma D., Ding H., Wang C., Qin H., Han Q., Hou J. Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS One. 2016;11:e0163082. doi: 10.1371/journal.pone.0163082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey A.K., Gautam A. Stress responsive gene regulation in relation to hydrogen sulfide in plants under abiotic stress. Physiol Plant. 2020 doi: 10.1111/ppl.13064. [DOI] [PubMed] [Google Scholar]
- 42.Lin X., Yang R., Dou Y., Zhang W., Du H., Zhu L. Transcriptome analysis reveals delaying of the ripening and cell wall degradation of kiwifruit by hydrogen sulfide. J Sci Food Agric. 2020 doi: 10.1002/jsfa.10260. [DOI] [PubMed] [Google Scholar]
- 43.Ma Q., Yang J. Transcriptome profiling and identification of functional genes involved in H2S response in grapevine tissue cultured plantlets. Genes Genomics. 2018;40(12):1287–1300. doi: 10.1007/s13258-018-0723-z. [DOI] [PubMed] [Google Scholar]
- 44.Guo Z., Liang Y., Yan J., Yang E., Li K., Xu H. Physiological response and transcription profiling analysis reveals the role of H2S in alleviating excess nitrate stress tolerance in tomato roots. Plant Physiol Biochem. 2018;124:59–69. doi: 10.1016/j.plaphy.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Gotor C., Laureano-Marín A.M., Moreno I., Aroca A., García I., Romero L.C. Signaling in the plant cytosol: cysteine or sulfide? Amino Acids. 2015;47:2155–2164. doi: 10.1007/s00726-014-1786-z. [DOI] [PubMed] [Google Scholar]
- 46.Hancock J.T., Whiteman M. Hydrogen sulfide signaling: Interactions with nitric oxide and reactive oxygen species. Ann N Y Acad Sci. 2016;1365:5–14. doi: 10.1111/nyas.12733. [DOI] [PubMed] [Google Scholar]
- 47.Calderwood A., Kopriva S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nitric Oxide. 2014;41:72–78. doi: 10.1016/j.niox.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Liu D., Lu J., Li H., Wang J., Pei Y. Characterization of the O-acetylserine(thiol)lyase gene family in Solanum lycopersicum L. Plant Mol Biol. 2019;99(1–2):123–134. doi: 10.1007/s11103-018-0807-9. [DOI] [PubMed] [Google Scholar]
- 49.Cuevasanta E., Möller M.N., Alvarez B. Biological chemistry of hydrogen sulfide and persulfides. Arch Biochem Biophys. 2017;617:9–25. doi: 10.1016/j.abb.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z., Cao C., Li Y., Yang G., Pei Y. Light regulates hydrogen sulfide signalling during skoto- and photo-morphogenesis in foxtail millet. Funct Plant Biol. 2019 doi: 10.1071/FP19079. [DOI] [PubMed] [Google Scholar]
- 51.Huo J., Huang D., Zhang J., Fang H., Wang B., Wang C. Hydrogen sulfide: a gaseous molecule in postharvest freshness. Front Plant Sci. 2018;9:1172. doi: 10.3389/fpls.2018.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng Y., Wang C., Wang N., Wei L., Li W., Yao Y. Roles of small-molecule compounds in plant adventitious root development. Biomolecules. 2019;9(9).;pii:E420. doi: 10.3390/biom9090420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y., Wang H., Xian M. Cysteine-activated hydrogen sulfide (H2S) donors. J Am Chem Soc. 2011;133(1):15–17. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamasaki H., Ogura M.P., Kingjoe K.A., Cohen M.F. D-Cysteine-induced rapid root abscission in the water fern Azolla pinnata: implications for the linkage between d-amino acid and reactive sulfur species (rss) in plant environmental responses. Antioxidants (Basel) 2019;8(9):E411. doi: 10.3390/antiox8090411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gun J., Modestov A., Kamyshny A., Ryzkov D., Gitis V., Goifman A. Electrospray ionization mass spectrometric analysis of aqueous polysulfide solutions. Microchim Acta. 2004;46:229. [Google Scholar]
- 56.Carter J.M., Brown E.M., Irish E.E., Bowden N.B. Characterization of dialkyldithiophosphates as slow hydrogen sulfide releasing chemicals and their effect on the growth of maize. J Agric Food Chem. 2019 doi: 10.1021/acs.jafc.9b04398. [DOI] [PubMed] [Google Scholar]
- 57.Kashfi K., Chattopadhyay M., Kodela R. NOSH-sulindac (AVT-18A) is a novel nitric oxide- and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Redox Biol. 2015;6:287–296. doi: 10.1016/j.redox.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mantri N., Patade V., Penna S., Ford R., Pang E. Abiotic stress responses in plants. Springer; New York: 2012. Abiotic stress responses in plants: present and future; pp. 1–19. [Google Scholar]
- 59.Corpas F.J., Barroso J.B. Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol. 2013;199(3):633–635. doi: 10.1111/nph.12380. [DOI] [PubMed] [Google Scholar]
- 60.Zhu C.Q., Zhang J.H., Sun L.M., Zhu L.F., Abliz B., Hu W.J. Hydrogen sulfide alleviates aluminum toxicity via decreasing apoplast and symplast al contents in rice. Front Plant Sci. 2018;9:294. doi: 10.3389/fpls.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H., Ji F., Zhang Y., Hou J., Liu W., Huang J. Interactions between hydrogen sulphide and nitric oxide regulate two soybean citrate transporters during the alleviation of aluminium toxicity. Plant Cell Environ. 2019;42(8):2340–2356. doi: 10.1111/pce.13555. [DOI] [PubMed] [Google Scholar]
- 62.Li L., Wang Y., Shen W. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometals. 2012;25(3):617–631. doi: 10.1007/s10534-012-9551-9. [DOI] [PubMed] [Google Scholar]
- 63.Shi H., Ye T., Chan Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.) Plant Physiol Biochem. 2014;74:99–107. doi: 10.1016/j.plaphy.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Fu M.M., Dawood M., Wang N.H., Wu F. Exogenous hydrogen sulfide reduces cadmium uptake and alleviates cadmium toxicity in barley. Plant Growth Regul. 2019;89:227–237. [Google Scholar]
- 65.Kaya C., Ashraf M., Alyemeni M.N., Ahmad P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plant. 2020;168(2):345–360. doi: 10.1111/ppl.13012. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Q., Cai W., Ji T.T., Ye L., Lu Y.T., Yuan T.T. WRKY13 enhances cadmium tolerance by promoting D-CYSTEINE DESULFHYDRASE and hydrogen sulfide production. Plant Physiol. 2020:01504. doi: 10.1104/pp.19.01504. pp. 01504.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kharbech O., Houmani H., Chaoui A., Corpas F.J. Alleviation of Cr(VI)-induced oxidative stress in maize (Zea mays L.) seedlings by NO and H2S donors through differential organ-dependent regulation of ROS and NADPH-recycling metabolisms. J Plant Physiol. 2017;219:71–80. doi: 10.1016/j.jplph.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 68.Ahmad R., Ali S., Rizwan M., Dawood M., Farid M., Hussain A. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plant. 2020;168(2):289–300. doi: 10.1111/ppl.13001. [DOI] [PubMed] [Google Scholar]
- 69.Kaya C., Ashraf M. The mechanism of hydrogen sulfide mitigation of iron deficiency-induced chlorosis in strawberry (Fragaria × ananassa) plants. Protoplasma. 2019;256(2):371–382. doi: 10.1007/s00709-018-1298-x. [DOI] [PubMed] [Google Scholar]
- 70.Kaya C., Ashraf M., Akram N.A. Hydrogen sulfide regulates the levels of key metabolites and antioxidant defense system to counteract oxidative stress in pepper (Capsicum annuum L.) plants exposed to high zinc regime. Environ Sci Pollut Res Int. 2018;25(13):12612–12618. doi: 10.1007/s11356-018-1510-8. [DOI] [PubMed] [Google Scholar]
- 71.Singh V.P., Singh S., Kumar J., Prasad S.M. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate-glutathione cycle: Possible involvement of nitric oxide. J Plant Physiol. 2015;181:20–29. doi: 10.1016/j.jplph.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 72.Mostofa M.G., Saegusa D., Fujita M., Tran L.S. Hydrogen sulfide regulates salt tolerance in rice by maintaining Na(+)/K(+) balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front Plant Sci. 2015;6:1055. doi: 10.3389/fpls.2015.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng Y.Q., Bao J., Yuan F., Liang X., Feng Z.T., Wang B.S. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul. 2016;79:391–399. [Google Scholar]
- 74.Jiang J.L., Tian Y., Li L., Yu M., Hou R.P., Ren X.M. H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Front Plant Sci. 2019;10:678. doi: 10.3389/fpls.2019.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y.L., Shen Z.J., Simon M., Li H., Ma D.N., Zhu X.Y. Comparative proteomic analysis reveals the regulatory effects of H2S on salt tolerance of mangrove plant Kandelia obovata. Int J Mol Sci. 2019;21(1):E118. doi: 10.3390/ijms21010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li H., Li M., Wei X., Zhang X., Xue R., Zhao Y. Transcriptome analysis of drought-responsive genes regulated by hydrogen sulfide in wheat (Triticum aestivum L.) leaves. Mol Genet Genomics. 2017;292(5):1091–1110. doi: 10.1007/s00438-017-1330-4. [DOI] [PubMed] [Google Scholar]
- 77.Antoniou C., Xenofontos R., Chatzimichail G., Christou A., Kashfi K., Fotopoulos V. Exploring the potential of nitric oxide and hydrogen sulfide (NOSH)-releasing synthetic compounds as novel priming agents against drought stress in Medicago sativa plants. Biomolecules. 2020;10(1):E120. doi: 10.3390/biom10010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao M., Liu Q., Zhang Y., Yang N., Wu G., Li Q. Alleviation of osmotic stress by H2S is related to regulated PLDα1 and suppressed ROS in Arabidopsis thaliana. J Plant Res. 2020 doi: 10.1007/s10265-020-01182-3. [DOI] [PubMed] [Google Scholar]
- 79.Liu Z., Li Y., Cao C., Liang S., Ma Y., Liu X. The role of H2S in low temperature-induced cucurbitacin C increases in cucumber. Plant Mol Biol. 2019;99(6):535–544. doi: 10.1007/s11103-019-00834-w. [DOI] [PubMed] [Google Scholar]
- 80.Tang X., An B., Cao D., Xu R., Wang S., Zhang Z. Improving photosynthetic capacity, alleviating photosynthetic inhibition and oxidative stress under low temperature stress with exogenous hydrogen sulfide in blueberry seedlings. Front Plant Sci. 2020;11:108. doi: 10.3389/fpls.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christou A., Filippou P., Manganaris G.A., Fotopoulos V. Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol. 2014;14:42. doi: 10.1186/1471-2229-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Z.H., Wang Y., Ye X.Y., Li Z.G. Signaling molecule hydrogen sulfide improves seed germination and seedling growth of maize (Zea mays L.) under high temperature by inducing antioxidant system and osmolyte biosynthesis. Front. Plant Sci. 2018;9:1288. doi: 10.3389/fpls.2018.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng T., Shi J., Dong Y., Ma Y., Peng Y., Hu X. Hydrogen sulfide enhances poplar tolerance to high-temperature stress by increasing S-nitrosoglutathione reductase (GSNOR) activity and reducing reactive oxygen/nitrogen damage. Plant Growth Regul. 2018;84:11–23. [Google Scholar]
- 84.Chen Z., Huang Y., Yang W., Chang G., Li P., Wei J. The hydrogen sulfide signal enhances seed germination tolerance to high temperatures by retaining nuclear COP1 for HY5 degradation. Plant Sci. 2019;285:34–43. doi: 10.1016/j.plantsci.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 85.Fotopoulos V., Christou A., Antoniou C., Manganaris G.A. Hydrogen sulphide: a versatile tool for the regulation of growth and defence responses in horticultural crops. J Hortic Sci Biotechnol. 2015;90:227–234. [Google Scholar]
- 86.Ziogas V., Molassiotis A., Fotopoulos V., Tanou G. Hydrogen sulfide: a potent tool in postharvest fruit biology and possible mechanism of action. Front Plant Sci. 2018;9:1375. doi: 10.3389/fpls.2018.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu L.Y., Hu S.L., Wu J., Li Y.H., Zheng J.L., Wei Z.J. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J Agric Food Chem. 2012;60(35):8684–8693. doi: 10.1021/jf300728h. [DOI] [PubMed] [Google Scholar]
- 88.Li S.P., Hu K.D., Hu L.Y., Li Y.H., Jiang A.M., Xiao F. Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. J Agric Food Chem. 2014;62(5):1119–1129. doi: 10.1021/jf4047122. [DOI] [PubMed] [Google Scholar]
- 89.Ni Z.J., Hu K.D., Song C.B., Ma R.H., Li Z.R., Zheng J.L. Hydrogen sulfide alleviates postharvest senescence of grape by modulating the antioxidant defenses. Oxid Med Cell Longev. 2016;2016:4715651. doi: 10.1155/2016/4715651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao G.F., Wei Z.Z., Li T.T., Tang J., Huang Z.Q., Yang F. Modulation of enhanced antioxidant activity by hydrogen sulfide antagonization of ethylene in tomato fruit ripening. J Agric Food Chem. 2018;66(40):10380–10387. doi: 10.1021/acs.jafc.8b03951. [DOI] [PubMed] [Google Scholar]
- 91.Aghdam M.S., Mahmoudi R., Razavi F., Rabiei V., Soleimani A. Hydrogen sulfide treatment confers chilling tolerance in hawthorn fruit during cold storage by triggering endogenous H2S accumulation, enhancing antioxidant enzymes activity and promoting phenols accumulation. Sci Hortic. 2018;238:264–271. [Google Scholar]
- 92.Joshi N.C., Yadav D., Ratner K., Kamara I., Aviv-Sharon E., Irihimovitch V. Sodium hydrosulfide priming improves the response of photosynthesis to overnight frost and day high light in avocado (Persea americana Mill, cv. ‘Hass’) Physiol Plant. 2020;168(2):394–405. doi: 10.1111/ppl.13023. [DOI] [PubMed] [Google Scholar]
- 93.Liu D., Xu S., Hu H., Pan J., Li P., Shen W. Endogenous hydrogen sulfide homeostasis is responsible for the alleviation of senescence of postharvest daylily flower via increasing antioxidant capacity and maintained energy status. J Agric Food Chem. 2017;65(4):718–726. doi: 10.1021/acs.jafc.6b04389. [DOI] [PubMed] [Google Scholar]
- 94.Liu D., Li J., Li Z., Pei Y. Hydrogen sulfide inhibits ethylene-induced petiole abscission in tomato (Solanum lycopersicum L.) Hortic Res. 2020;7:14. doi: 10.1038/s41438-019-0237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahmud K., Makaju S., Ibrahim R., Missaoui A. Current progress in nitrogen fixing plants and microbiome research. Plants (Basel). 2020;9(1):97. doi: 10.3390/plants9010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Puppo A., Pauly N., Boscari A., Mandon K., Brouquisse R. Hydrogen peroxide and nitric oxide: key regulators of the Legume-Rhizobium and mycorrhizal symbioses. Antioxid Redox Signal. 2013;18:2202–2219. doi: 10.1089/ars.2012.5136. [DOI] [PubMed] [Google Scholar]
- 97.Hichri I., Boscari A., Castella C., Rovere M., Puppo A., Brouquisse R. Nitric oxide: a multifaceted regulator of the nitrogen-fixing symbiosis. J Exp Bot. 2015;66(10):2877–2887. doi: 10.1093/jxb/erv051. [DOI] [PubMed] [Google Scholar]
- 98.Berger A., Guinand S., Boscari A., Puppo A., Brouquisse R. Medicago truncatula phytoglobin 1.1 controls symbiotic nodulation and nitrogen fixation via the regulation of nitric oxide concentration. New Phytol. 2020 doi: 10.1111/nph.16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zou H., Zhang N.N., Pan Q., Zhang J.H., Chen J., Wei G.H. Hydrogen sulfide promotes nodulation and nitrogen fixation in soybean-rhizobia symbiotic system. Mol Plant Microbe Interact. 2019;32(8):972–985. doi: 10.1094/MPMI-01-19-0003-R. [DOI] [PubMed] [Google Scholar]
- 100.Zhang N.N., Zou H., Lin X.Y., Pan Q., Zhang W.Q., Zhang J.H. Hydrogen sulfide and rhizobia synergistically regulate nitrogen (N) assimilation and remobilization during N deficiency-induced senescence in soybean. Plant, Cell Environ. 2020 doi: 10.1111/pce.13736. [DOI] [PubMed] [Google Scholar]
- 101.Fukudome M., Shimada H., Uchi N., Osuki K.I., Ishizaki H., Murakami E.I. Reactive sulfur species interact with other signal molecules in root nodule symbiosis in Lotus japonicus. Antioxidants (Basel) 2020;9(2):145. doi: 10.3390/antiox9020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fuentes-Lara L.O., Medrano-Macías J., Pérez-Labrada F., Rivas-Martínez E.N., García-Enciso E.L., González-Morales S. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules. 2019;24(12):E2282. doi: 10.3390/molecules24122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Batista P.F., Müller C., Merchant A., Fuentes D., de Oliveira Silva Filho R., da Silva F.B., Costa A.C. Biochemical and physiological impacts of zinc sulphate, potassium phosphite and hydrogen sulphide in mitigating stress conditions in soybean. Physiol Plant. 2019 doi: 10.1111/ppl.13034. [DOI] [PubMed] [Google Scholar]
- 104.Waqas M.A., Kaya C., Riaz A., Farooq M., Nawaz I., Wilkes A. Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front Plant Sci. 2019;10:1336. doi: 10.3389/fpls.2019.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bianchini H.C., Marques D.J. Tolerance to hydric stress on cultivars of silicon fertilized corn crops: absorption and water use efficiency. Biosci J. 2019;35(2):527–539. [Google Scholar]
- 106.Hidangmayum A., Dwivedi P., Katiyar D., Hemantaranjan A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol Mol Biol Plants. 2019;25(2):313–326. doi: 10.1007/s12298-018-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geng B., Huang D., Zhu S. Regulation of Hydrogen sulfide metabolism by nitric oxide inhibitors and the quality of peaches during cold storage. Antioxidants (Basel) 2019;8:401. doi: 10.3390/antiox8090401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valivand M., Amooaghaie R., Ahadi A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol Biochem. 2019;143:286–298. doi: 10.1016/j.plaphy.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 109.Corpas F.J. Hydrogen Sulfide: a new warrior against abiotic stress. Trends Plant Sci. 2019;24(11):983–988. doi: 10.1016/j.tplants.2019.08.003. [DOI] [PubMed] [Google Scholar]