Abstract
Non-toxic herpes simplex virus (HSV) vectors can be generated by functional deletion of all immediate-early (IE) genes, providing a benign vehicle with potential for gene therapy. However, deletion of multiple IE genes raises manufacturing concerns and thus limits clinical application of these vectors. To address this issue, we previously developed a novel production cell line, called U2OS-ICP4/27, by lentiviral transduction of human osteosarcoma U2OS cells with two essential HSV IE genes, ICP4 and ICP27. To optimize the process of vector manufacturing on this platform, we evaluated several cell culture parameters of U2OS-ICP4/27 for high-titer and -quality production of non-toxic HSV vectors, revealing that the yields and functionality of these vectors can be significantly influenced by culturing conditions. We also found that several chemical compounds can enhance the replication of non-toxic HSV vectors and their release from producer cells into the supernatants. Notably, the vector produced by our optimized protocol displayed a greatly improved vector yield and quality and showed elevated transgene expression in cultures of primary dorsal root ganglion neurons. Taken together, our optimized production approach emerges as a relevant protocol for high-yield and high-quality preparation of non-toxic HSV-based gene therapy vectors.
Graphical Abstract
Non-cytotoxic herpes simplex virus (HSV)-based vector is a high-capacity vehicle for large and multiple therapeutic gene delivery, whereas the vector manufacturing is still problematic. Kuroda and colleagues demonstrate a cost-effective and reproducible protocol for high-yield and high-quality production of non-cytotoxic HSV vectors.
Introduction
The potential of engineered herpes simplex virus (HSV) vectors as gene transfer vehicles has been studied for years and these vectors are now emerging as promising gene therapy and oncolytic agents.1, 2, 3, 4, 5, 6 A major drawback of the HSV vector system as a gene transfer tool has been its toxicity for a variety of cell types, due mainly to cell-cycle arrest and apoptosis induced by viral gene products.7 Several groups have attempted to reduce the cytopathic effects of the HSV vector by genetic manipulation. Originally, recombinant HSV vectors had single immediate-early (IE) genes deleted, such as ICP4, which encodes a major transcription factor, or ICP27, which encodes an essential post-transcriptional regulator,8, 9, 10, 11, 12 to block lytic replication. However, these mutants still exhibited cytotoxic effects on many cell types in vitro.13, 14, 15 Subsequently, double and multiple IE gene-deletion mutants in various combinations were developed to further reduce vector cytotoxicity and their safety was evaluated.8,12,16 Most of these recombinants still showed residual cytotoxic effects and their use as gene therapy vectors has therefore been limited. To eliminate these barriers, we recently developed a genetically engineered non-cytotoxic HSV vector by ablation of all IE gene expression,17 causing heterochromatinization of the viral genome upon entry into the cell and shut-down of all toxic viral gene expression; only the latency-associated transcript (LAT) locus remains transcriptionally active, allowing the expression of therapeutic genes.17 We have demonstrated that large and multiple transgene expression cassettes can be inserted into the HSV vector genome and expressed under different regulatory sequences.17,18 The engineered non-toxic HSV has successfully provided robust transgene expression without vector-mediated cytotoxicity in rat brain, indicating that the vector can be used for transduction in vivo.19 Thus, this non-toxic HSV vector platform can be expected to become a new safe and useful tool for efficient gene therapy.
Since non-toxic HSV vectors lack the ability to express any IE gene products, they cannot replicate in normal cells. Therefore, to produce the highly defective virus particle, we generated a specific producer cell line, U2OS-ICP4/27, by lentiviral transduction of human osteosarcoma U2OS cells with two essential HSV IE genes, ICP4 and ICP27.17 Because U2OS cells naturally complement the growth-promoting activity of the toxic IE protein ICP0,20 our IE gene-disabled HSV vectors could be propagated substantially more efficiently on U2OS-ICP4/27 cells than on traditional ICP4/ICP27-complementing (e.g., Vero-based 7B) cells.8,17
In the present study, we evaluated different cell-culture parameters in order to optimize the production of a prototype non-toxic HSV vector, JΔNI5,17 on U2OS-ICP4/27 cells. We demonstrate that modification of each of the culture parameters significantly affected the physical vector yield and its biological activity. Additionally, several chemical agents, including histone deacetylase (HDAC) and bromodomain and extra-terminal (BET) inhibitors, were tested for their ability to enhance the replication of non-toxic HSV. Furthermore, we examined two other chemicals, sodium chloride and cesium chloride, for their ability to increase the release of the HSV vector from its U2OS-ICP4/27 producer cells. Finally, we examined the differences in the results obtained from the conventional production method compared with an optimized protocol using the elements with the highest impact on virus yield.
Results
Optimization of Culture Conditions for JΔNI5 Vector Production
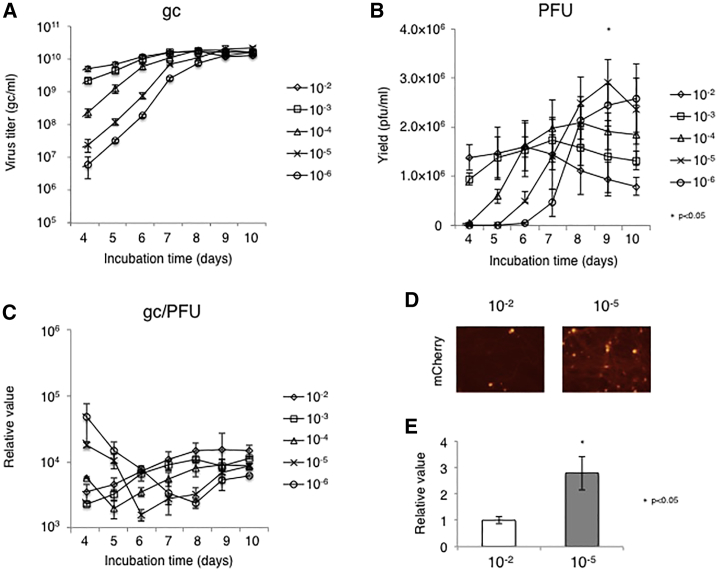
We first determined the optimal multiplicity of infection (MOI) for efficient production of JΔNI5 vector, a non-toxic HSV vector previously described by one of our labs.17 The JΔNI5 genome carries an mCherry expression cassette as a transgene in place of the terminal ICP4 locus. In previous reports, MOIs of 10−2 to 10−4 were considered suitable for recombinant HSV vector production.21 In our system, the physical titers at these MOIs increased rapidly and reached peak values of 1.66–1.79 × 1010 genome copies (gc)/mL at 7–8 days post infection (dpi) (Figure 1A). At input MOIs of 10−5 or 10−6, the titers peaked at 9 or 10 dpi, respectively, with the highest titer (2.1 × 1010 gc/mL) achieved by MOI = 10−5 at 9 dpi. Likewise, the peak biological titers (in plaque-forming units [PFU]/mL) also depended on the input MOI (Figure 1B). The highest biological titer (2.9 × 106 PFU/mL) was once again achieved using MOI = 10−5 at 9 dpi. The gc/PFU ratio was also examined to estimate the quality of the virus (Figure 1C). The minimum gc/PFU ratio was achieved at 4–6 dpi, except for MOI = 10−6 (8 dpi), and a modest increase was generally observed at later time points. The gc/PFU ratio was not significantly different among the conditions at 9 dpi. Together, these results indicated that an input MOI of 10−5 was optimal, establishing it as the standard input for optimization of other culture parameters. To verify that the gc/PFU ratio represented an accurate measure of the functionality of the vector preparation, we infected fetal rat dorsal root ganglion (rDRG) neurons in culture with 5,000 gc/cell of the 9 dpi MOI = 10−2 and MOI = 10−5 and the transduction efficiencies were examined 4 d later. As expected, the HSV vector produced by MOI = 10−5 showed a more widespread mCherry expression than to the vector produced by MOI = 10−2 (Figure 1D). This result was confirmed by quantitative reverse transcriptase PCR (qRT-PCR) measurement of mCherry mRNA levels at 7 dpi (Figure 1E). The mRNA level of mCherry from the HSV vector produced by MOI = 10−5 at 9 dpi was approximately 2.7-fold higher than that of the vector produced by MOI = 10−2 at 9 dpi (Figure 1E). These observations validated the gc/PFU ratio as an informative measure of the quality of our vector stocks. We also produced a closely related vector, JΔNI8, which was derived from JΔNI5 by deletion of the virion host shutoff (vhs) gene,22 and compared its yields with those of JΔNI5 over time at the optimal input MOI (10−5; Figure S1). Both vectors grew at a comparable rate in U2OS-ICP4/27 cells.
Figure 1.
JΔNI5 Vector Growth at Different MOIs
Confluent U2OS-ICP4/27 cells in T225 flasks were infected with JΔNI5 vector at 10−2–10−6 plaque formation unit (PFU)/cell. (A and B) Physical titers in gc/mL (A) and biological titers in PFU/mL (B) in the supernatants were measured daily by quantitative real-time PCR and standard plaque assay, respectively. (C) gc/PFU ratios were calculated based on the results of (A) and (B). (D and E) Cultures of rat primary dorsal root ganglia (rDRGs) were infected with JΔNI5 vector preparations (5,000 gc/cell) produced at MOIs of 10−2 or 10−5. At 4 d post-infection (dpi), vector-mediated mCherry fluorescence was photographed (D) and relative mCherry mRNA levels were measured by qRT-PCR with normalization to 18S rRNA (E). Group comparisons were performed with Student’s t test or one-way ANOVA with post hoc Dunnett’s multiple comparison test. Differences between an input MOI of 10−5 and 10−2 were statistically highly significant (∗p < 0.05). Quantitative data are presented as means ± SD (n = 3).
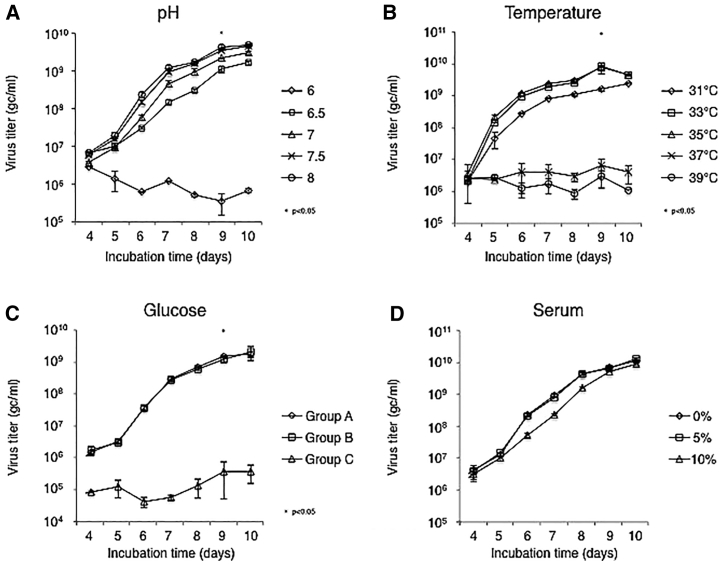
To explore whether the cell culture conditions influence the yield of JΔNI5 virus, we tested variations in pH, temperature, glucose, and serum concentration23 (Figure 2). A media pH of 7.5–8.0 resulted in the highest vector yield, whereas pH = 6 significantly inhibited virus production (Figure 2A). Virus growth at 33°C or 35°C resulted in the highest virus titers, reached at 9 dpi (Figure 2B), while significantly lower yields were obtained at higher temperatures (37°C–39°C). To examine the effect of glucose concentration, we first recorded the changes in its concentration in the media during vector production (Figure S2A). The glucose concentration decreased over time in our system, consistent with previous observations.20 Daily supplementation of glucose then enabled us to maintain the glucose concentration throughout the period of vector production (Figure S2B). To determine the glucose requirement during virus growth, we propagated JΔNI5 virus under naturally decreasing levels of glucose (group A), at a constant glucose concentration due to daily supplementation (group B), or in the absence of glucose in the culture medium (group C). The supplementation of glucose did not influence virus growth, whereas its absence clearly inhibited viral replication (Figure 2C). To examine whether serum concentration affects virus production, we tested three different serum concentrations (0%, 5%, and 10%). As shown in Figure 2D, no significant differences in virus growth kinetics or yield were observed, suggesting that serum can be eliminated from the culture medium in our system.
Figure 2.
Effects of Cell Culture Conditions on JΔNI5 Vector Production
Confluent U2OS-ICP4/27 cells in T75 flasks were infected with JΔNI5 virus at an MOI of 10−5 PFU/cell for 2 h. (A–D) The infected cells were then incubated in media at various pH values (A), temperatures (B), glucose concentrations (C), or serum concentrations (D). Supernatant titers in gc/mL were determined daily by quantitative real-time PCR. Group comparisons were performed with one-way ANOVA with post hoc Dunnett’s multiple comparison test. Statistically significant differences between pH 7.5 and pH 6 are indicated by asterisks (∗p < 0.05). Differences between 33°C and 37°C were statistically highly significant (∗p < 0.05). Statistically significant differences between 4.5 g/L and 0 g/L glucose are indicated (∗p < 0.05). Group A, glucose levels decreasing from the initial 4.5 g/L concentration; group B, glucose levels maintained at ~4.5 g/L; group C, glucose-free media. Data are presented as means ± SD (n = 3).
Effect of Frequency of Media Collection on JΔNI5 Vector Production
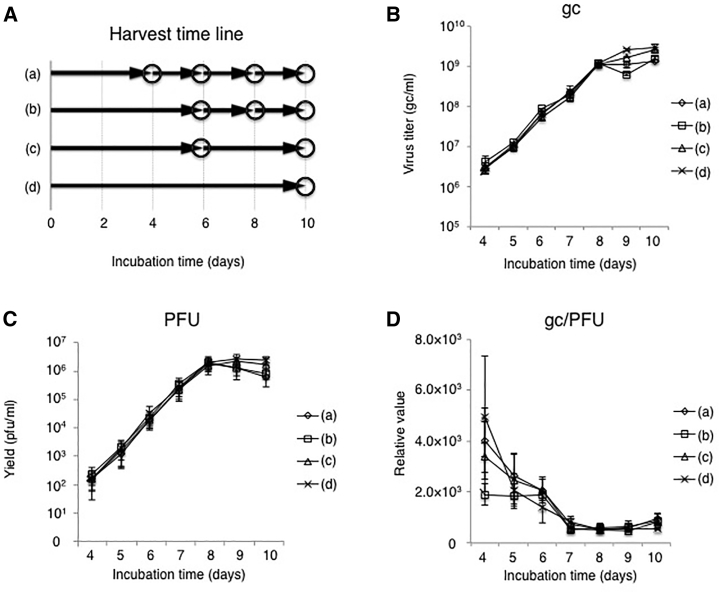
Next, we asked whether the frequency of culture media collection could impact virus production. Four media collection protocols were tested (Figure 3A). In each protocol, whole culture supernatant was collected on the days indicated by circles in the figure and replaced with fresh medium, namely, culture supernatant was collected 4 times at 4, 6, 8, and 10 dpi in protocol (a), and 3 times at 6, 8, and 10 dpi in protocol (b). Alternatively, we harvested culture supernatant 2 times at 6 and 10 dpi in protocol (c) and only once at 10 dpi in protocol (d). The virus growth curves (Figures 3B and 3C) and accompanying changes in gc/PFU ratios (Figure 3D) were very similar between the protocols, even protocol (a) involving multiple media replacements. Total virus yields and gc/PFU ratios are listed in Table 1. The total gc of protocols (a) and (b) were higher relative to protocol (d) and the total PFU of protocol (b) was approximately 2-fold higher than that of protocol (d). In addition, the total gc/PFU ratio of protocol (b) was superior to that of all other groups. Together, these results indicated that frequent media collection can improve vector yield and quality.
Figure 3.
Effect of Media Exchange Frequency on JΔNI5 Vector Production
(A) Timeline of media changes in 4 experimental groups (a–d). Circles in the diagram represent complete media changes. (B and C) Confluent U2OS-ICP4/27 cells in T75 flasks were infected with JΔNI5 virus at an MOI of 10−5 PFU/cell and viral titers in the media were measured daily by quantitative real-time PCR (B) and standard plaque assay (C). (D) gc/PFU ratios were calculated from the results of (B) and (C). Data are presented as means ± SD (n = 3).
Table 1.
Summary of Vector Yields Using Different Media Replacement Protocols (n = 3)
| (a) | (b) | (c) | (d) | |
|---|---|---|---|---|
| Total gc | 7.8 ± 0.91 × 1010 | 6.0 ± 0.24 × 1010 | 4.9 ± 0.44 × 1010 | 4.9 ± 0.10 × 1010 |
| Total PFU | 4.3 ± 1.5 × 107 | 6.0 ± 1.7 × 107∗ | 3.2 ± 0.75 × 107 | 2.9 ± 0.93 × 107 |
| Total gc/PFU | 1,816 ± 607 | 990 ± 371 | 1520 ± 267 | 1,665 ± 506 |
Group comparisons were performed with one-way ANOVA with post hoc Dunnett’s multiple comparison test. ∗p < 0.05.
Enhancement of Non-toxic HSV Vector Production by Treatment with Chemical Agents
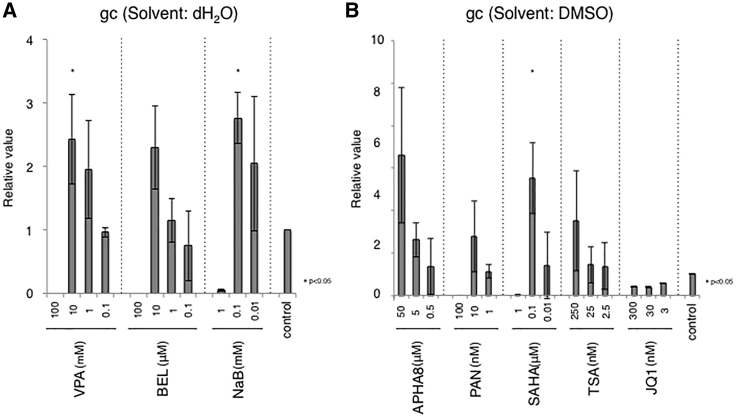
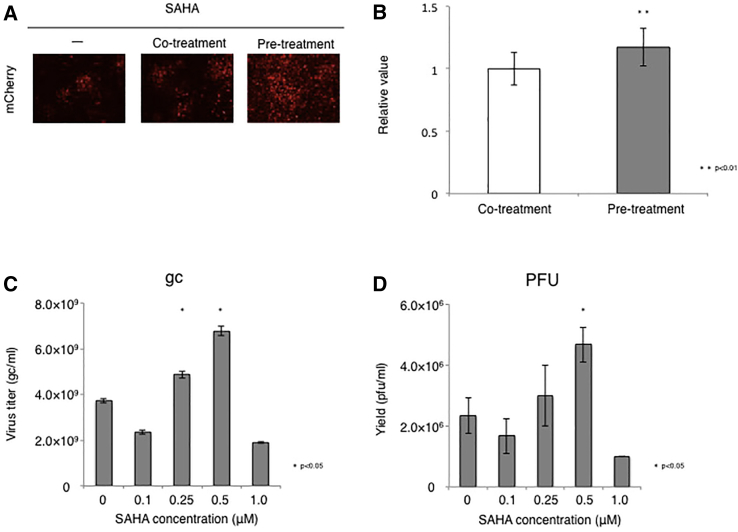
It has been shown that HDAC inhibitors and BET motif inhibitors enhance oncolytic HSV replication in tumor cells but not normal cells.24, 25, 26, 27, 28 Therefore, we evaluated whether these agents can also promote non-toxic HSV replication in U2OS-ICP4/27 cells, which are derived from an osteosarcoma tumor cell line. Based on previous reports, we selected the nine agents listed in Table 2 for initial analysis. We infected U2OS-ICP4/27 cells with JΔNI5, treated the cells with the indicated agents at different doses, and measured the virus titers at 6 dpi. For accurate comparison to negative controls, experiments were divided into two groups based on the solvent (Table 2; Figures 4A and 4B). All HDAC inhibitors showed dose-related increases in vector yields compared with the control while BET inhibitor JQ1 did not enhance JΔNI5 virus production. In particular, significant increases in vector yields were observed with Valproic acid (VPA, 10 mM), Belinostat (BEL, 10 μM), sodium butyrate (NaB, 0.1 mM), and suberoylanilide hydroxamic acid (SAHA, 0.1 μM). Because SAHA showed the highest vector yield among these agents, we focused on SAHA for further experiments. Previous reports have suggested that pre-treatment of the cells with HDAC inhibitors followed by infection in the absence of inhibitor (“pre-treatment”) is more effective in enhancing virus replication than in the continued presence of inhibitor (“co-treatment”).28, 29, 30 We therefore compared these two conditions in our system. As illustrated in Figure 5A, SAHA removal prior to infection resulted in more efficient viral spread. Furthermore, the vector yield was slightly higher using this protocol compared to co-treatment (Figure 5B). We also explored whether concentrations of SAHA between 0.1 and 1 μM analyzed in Figure 4B would increase vector yield further (Figures 5C and 5D). Both the physical and the biological titer reached a peak at 0.5 μM SAHA (1.82-fold compared to no-SAHA control, p < 0.05), while the gc/PFU ratio was similar to that of the control (Table 3), indicating that SAHA can increase the yield of non-toxic HSV vector without reducing its quality.
Table 2.
Chemical Agents Used in This Study
| Inhibitor | Solvent | Concentration | |
|---|---|---|---|
| HDACa | VPA (Valproic acid) | dH2O | 100~0.1 mM |
| BEL (Belinostat) | dH2O | 100~0.1 μM | |
| NaB (Sodium butyrate) | dH2O | 1~0.01 mM | |
| APHA8 (APHA compound 8) | DMSO | 50~0.5 μM | |
| PAN (Panobinostat) | DMSO | 100~1 nM | |
| SAHA (Suberoylanilide hydroxamic acid, Vorinostat) | DMSO | 1~0.01 μM | |
| TSA (Trichostatin A) | DMSO | 250~2.5 nM | |
| BETb | JQ1 | DMSO | 300~3 nM |
Histone deacetylase.
Bromodomain and extra-terminal.
Figure 4.
Effect of Chemical Agents on JΔNI5 Vector Production
Confluent U2OS-ICP4/27 cells in 24-well culture dishes were treated with HDAC or BET inhibitors for 2 h prior to infection with 10−5 viral PFU/cell. At 6 dpi, supernatants were harvested and viral vector titers determined by quantitative real-time PCR. (A and B) Agents were analyzed in 2 groups according to their solvent, dH2O (A) or dimethyl sulfoxide (DMSO) (B); controls were the respective solvents alone. Group comparisons were performed with one-way ANOVA with post hoc Dunnett’s multiple comparison test. (∗p < 0.05). Data are presented as means ± SD (n = 3).
Figure 5.
Comparison of Viral Yields under Different SAHA Treatment Conditions
Confluent U2OS-ICP4/27 cells in T225 flasks were treated with 0.5 μM SAHA for 2 h. Cells in the “co-treatment” group were then infected with JΔNI5 virus. Cells in the “pre-treatment” group were washed with PBS and fresh medium without SAHA was added prior to viral infection. All infections were performed with 10−5 viral PFU/cell. (A and B) Fluorescence was recorded at 7 dpi (A) and the titers of supernatant samples were analyzed by quantitative real-time PCR at 9 dpi (B). (C and D) Confluent U2OS-ICP4/27 cells in T225 flasks were treated with the indicated concentrations of SAHA for 2 h and infected with JΔNI5 vector in SAHA-free media. Supernatant samples were collected at 9 dpi and viral titers determined by quantitative real-time PCR (C) and standard plaque assay (D). Differences between pairs were analyzed by Student’s t test for (B), and group comparisons (C and D) were performed with Student’s t test or one-way ANOVA with post hoc Dunnett’s multiple comparison test. Data are presented as the means ± SD (n = 3; ∗p < 0.05, ∗∗p < 0.01 compared to solvent).
Table 3.
gc/PFU Yields at Different SAHA Concentrations (n = 3)
| Concentration (μM) | 0 | 0.1 | 0.25 | 0.5 | 1.0 |
|---|---|---|---|---|---|
| gc/PFU | 1,695 ± 398 | 1,580 ± 491 | 1,799 ± 542 | 1,474 ± 185 | 1,906 ± 39 |
Induction of Non-toxic HSV Vector Release from Producer Cells by Salt Treatment
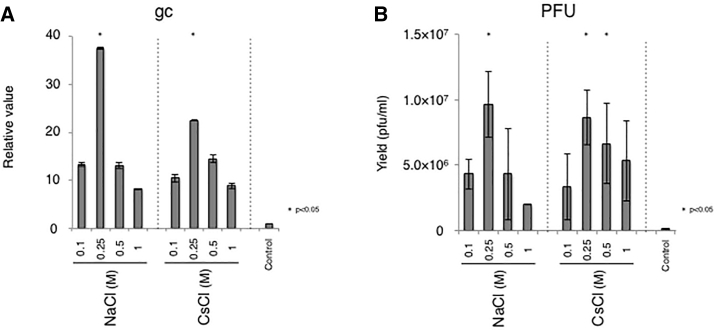
Viral vectors can be recovered from the surface of producer cells by treatment with sodium chloride31,32 or cesium chloride.33 Hence, we next evaluated whether these salts can function to promote the release of trapped virus also in our system. Culture supernatants were harvested at 9 dpi and fresh media with or without the indicated salt was added to the cells (Figures 6A and 6B). Consistent with previous results, both sodium chloride and cesium chloride induced the release of the JΔNI5 virus from its producer cells. The physical and biological titers peaked at 0.25 M sodium chloride treatment with a 37-fold increase in gc compared to the control (p < 0.05). The released virus was infectious and superior in gc/PFU ratio to the control (Table 4), showing that both salts can improve the recovery of active virus also in our system.
Figure 6.
Induction of JΔNI5 Virus Release from Producer Cells by Salts
Confluent U2OS-ICP4/27 cells in T75 flasks were infected with JΔNI5 virus at 10−5 PFU/cell. At 9 dpi, supernatants were replaced with media supplemented with NaCl or CsCl at the indicated concentrations or media alone (control). (A and B) Cultures were then incubated at 33°C for 6 h and supernatants were collected and titered by quantitative real-time PCR (A) and standard plaque assay (B). Group comparisons were performed with one-way ANOVA with post hoc Dunnett’s multiple comparison test. Data are presented as means ± SD (n = 3; ∗p < 0.05 compared to control).
Table 4.
gc/PFU Ratios of Viruses Released by Different Salt Concentrations (n = 3)
| Concentration (M) | gc/PFU | |
|---|---|---|
| NaCl | 0.1 | 1,462 ± 392 |
| 0.25 | 1,841 ± 480 | |
| 0.5 | 1,443 ± 358 | |
| 1.0 | 1,945 ± 29 | |
| CsCl | 0.1 | 2,343 ± 1133 |
| 0.25 | 1,303 ± 296 | |
| 0.5 | 1,251 ± 478 | |
| 1.0 | 1,052 ± 457 | |
| Control | – | 4,227 ± 1545 |
Comparison of Optimal and Conventional Production Procedures
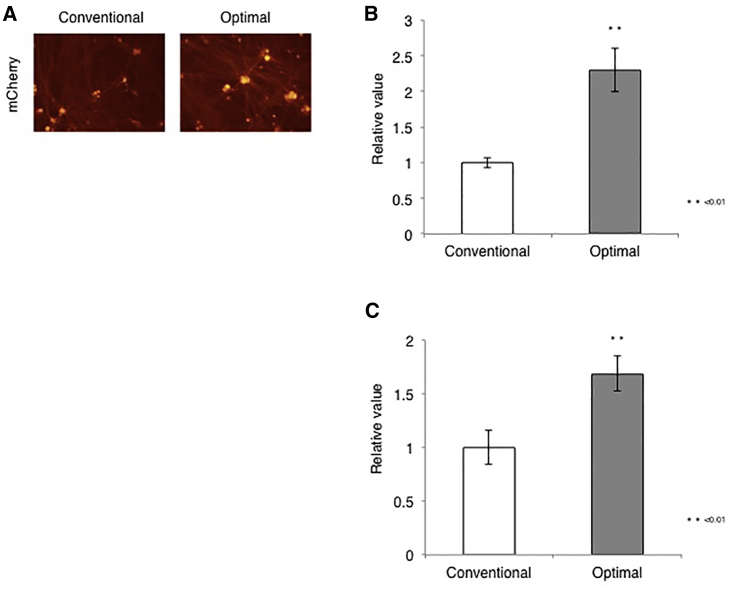
We combined the optimal parameters established above for infection and culture conditions and virus harvest to maximize virus yield and quality in what we refer to as the optimal protocol (Table 5). To evaluate its performance, we performed JΔNI5 vector production in parallel using the optimal protocol and the conventional vector production method (Table 5, conventional protocol) and the vector yields and quality were compared (Table 6). The vector yield reached by the optimal protocol was higher than that accomplished by the conventional protocol (approximately 2.8-fold in gc titer, 3.8-fold in PFU titer). Furthermore, the gc/PFU ratio obtained using the optimal protocol was superior to that achieved with the conventional protocol (approximately 1.3-fold). To confirm the superior quality of the optimal protocol vector stock, we compared the transduction efficiencies of equal gc of the two preparations in rDRG cultures (Figure 7). As expected, rDRG cultures infected with the optimal-protocol virus displayed more robust transgene expression than cultures infected with the conventional-protocol virus (Figure 7A). Lastly, we compared intracellular viral DNA levels and transgene mRNA levels between cultures infected with either virus stock. Total cellular DNA and mRNA were harvested at 4 dpi and analyzed by quantitative real-time PCR for the viral gD gene (Figure 7B) and qRT-PCR for the mCherry transgene (Figure 7C), respectively, as previously described.17 The results demonstrated that both virus entry and transgene expression were increased in cultures infected with the optimal-protocol virus compared with cultures infected with conventional-protocol virus, confirming that the optimized protocol improved not only the yield, but also the specific activity of our virus stocks.
Table 5.
Summary of Optimal and Conventional JΔNI5 Vector Production Methods
| Optimal Protocol | Conventional Protocol | |
|---|---|---|
| MOI | E-5 | E-4 |
| Temperature | 33°C | 33°C |
| Serum | 0% | 0% |
| Chemical agent | SAHA (0.5 μM, pre 2 h) | none |
| Vector release | NaCl (0.25 M, 2 h) | none |
| Medium collection | days 6, 8, 9 | day 9 |
Table 6.
Summary of Results from JΔNI5 Vector Production Runs using the Conventional and the Optimal Protocol (n = 3)
| Total gc | Total PFU | gc/PFU | |
|---|---|---|---|
| Conventional protocol | 6.8 ± 0.35 × 1010 | 4.7 ± 2.3 × 107 | 1,827 ± 730 |
| Optimal protocol | 1.9 ± 0.17 × 1011 | 1.8 ± 0.99 × 108 | 1,408 ± 710 |
Figure 7.
rDRG Transduction by JΔNI5 Viruses Produced by Optimal and Conventional Protocols
(A) rDRGs were infected with each JΔNI5 virus preparation at 5,000 gc/cell and mCherry fluorescence was photographed at 4 dpi. (B and C) Relative amounts of viral genomes (B) and mCherry mRNA (C) in rDRGs at 4 dpi as determined by quantitative real-time PCR and qRT-PCR, respectively. Intracellular viral genome numbers and mCherry mRNA levels were normalized to cellular 18S rRNA genes and RNA levels, respectively. Differences between pairs were analyzed by Student’s t test. Data are presented as means ± SD (n = 3; ∗∗p < 0.01).
Discussion
Non-toxic HSV vectors are harmless high-capacity vehicles for therapeutic gene delivery, but they replicate extremely inefficiently in traditional complementing cell lines because of the functional deletion of all IE genes, which severely limits their clinical applicability. To address these manufacturing concerns, we previously generated a novel producer cell line, U2OS-ICP4/27,17 and the experiments described in this paper were aimed at optimizing the conditions for high-yield and high-quality production of stocks of our non-toxic HSV vectors by these cells.
Our study demonstrates that several infection and cell culture parameters significantly affect the yield and quality of JΔNI5 virus preparations (Figures 1 and 2). A previous report showed that an MOI of 0.02 was the most suitable for replication-defective HSV vector production.20 However, here we found that the optimal MOI for JΔNI5 vector production by our ICP4/ICP27-complementing U2OS cell line is 10−5. The difference is most likely related to the nature of the producer cells; in the past, complementing producer cells were typically based on Vero cells, which intrinsically restrict HSV infection and spread and do not support significant replication of ICP0 null HSV mutants.34, 35, 36 In contrast, U2OS cells are highly permissive for HSV infection37,38 and inherently complement ICP0 null HSV mutants.20 These properties appear to favor the use of a very low MOI for the production of our ICP0 null JΔNI5 vector and most likely of other similarly defective vectors. As shown here, JΔNI5 infection at higher MOIs provided less robust virus replication (Figure 1A) and lower maximum PFU titers, resulting in higher gc/PFU ratios (Figures 1B and 1C), suggesting a relative increase in defective, non-infectious particle formation. The optimal cell culture parameters, including temperature, pH, and serum and glucose concentrations during JΔNI5 vector production were also different from those reported in other systems.21,39 Hence, our results demonstrate the value of varying the input MOI and cell-culture parameters when using a non-standard virus production protocol.
We determined the gc/PFU ratios of viruses produced under varying conditions (Figures 1C and 3D; Tables 1, 3, 4, and 6) because these ratios reflect the proportion of functional HSV particles in vector stocks; the higher the ratio, the lower the percentage of active particles. Indeed, JΔNI5 virus preparations exhibiting a lower gc/PFU ratio showed higher transduction efficiencies in rDRG cultures than preparations with a higher gc/PFU ratio (Figures 1D, 1E, and 7). The presence of excess defective particles in vector stocks can cause adverse events in gene therapy, including undesired immune responses.40,41 Thus, monitoring of the gc/PFU ratio is essential for the establishment of optimal procedures to generate high-quality HSV stocks.
We evaluated the effect of HDAC inhibitors on virus production since these agents have been reported to enhance the replication of oncolytic HSV in cancer cells.24,25,28 Consistent with previous research, most HDAC inhibitors tested in this study increased virus yield in our system (Figures 4 and 5). In addition, cell conditioning with the HDAC inhibitor SAHA prior to infection followed by virus growth in SAHA-free media (“pre-treatment”) was more effective than infection and growth in the continued presence of the drug (“co-treatment”) (Figure 5). Previous studies have shown that HDAC inhibitor pretreatment, but not cotreatment, promotes gene expression of HSV and cell-cycle regulators such as p16 and p21, whereas it inhibits expression of interferon responsive genes including STAT1 and PKR.24 Thus, our results revealed that HDAC inhibitor-mediated modifications of gene expression contribute to the molecular mechanisms for high yield non-toxic HSV production. Importantly, SAHA treatment had no discernible impact on the gc/PFU ratio of the product (Table 3), indicating that the drug is capable of increasing vector yield without reducing vector functionality. Because SAHA is a simplified structural analogs of TSA and suitable for mass-production42 and SAHA has already been used to treat cutaneous T cell lymphoma in clinical practice and its safety has been confirmed,43 its use in the production of non-toxic HSV gene therapy vectors for potential future patient trials should not cause safety or regulatory concerns. However, the use of SAHA in large-scale vector production runs will require careful adjustment of its concentration since a doubling of the optimal dose in our small-scale runs was counter-productive (Figures 5C and 5D).
Efficient viral vector release from producer cells simplifies vector purification and minimizes contamination of the product with cellular impurities. Sodium chloride,31,32 cesium chloride,33 heparin,44 and dextran sulfate44,45 have all been used previously to promote virus detachment from producer cells and membrane debris. Here, we documented productive recovery of JΔNI5 virus from our U2OS-based producer cells using either sodium chloride or cesium chloride treatment and determined the concentration of both salts for optimal vector release in this system (Figure 6). Moreover, our results revealed that those treatment conditions did not adversely affect the specific infectious activity of the product (Table 4).
In conclusion, we developed an efficient method for the production of our novel class of non-toxic HSV vectors by the optimization of infection parameters, including the MOI and the composition and exchange frequency of the culture media, and achieved an important improvement in the quality and yield of our prototype of this class of vectors. In addition, we demonstrated that judicious use of HDAC inhibitors and sodium chloride increase the recovery of active virus. These results will contribute to the establishment of a cost-effective and reproducible protocol for efficient production of highly defective, non-toxic HSV vectors for pre-clinical and clinical studies as well as in vitro transduction applications.
Materials and Methods
Cells and Viruses
U2OS-ICP4/27 cells were as described previously18 and were grown in DMEM (Thermo Fisher) with 10% (vol/vol) fetal bovine serum (FBS, Thermo Fisher), penicillin-streptomycin (P/S, Sigma), puromycin (1 μg/mL), and blasticidin (5 μg/mL). All animal care and use procedures were carried out in agreement with the Animal Experiments Ethical Review Committee, and approved by the President of Nippon Medical School (Approval number 28-037). Fetal rat dorsal root ganglia (rDRGs) were microdissected from day 21 rat embryos, dissociated with 5 mg/mL collagenase A (Roche), 1 mg/mL Dispase II (Roche) in PBS for 30 min at 37°C with constant shaking, and then treated with 0.125% Trypsin/EDTA (Sigma) for 30 min at 37°C with constant shaking. After dissociation, rDRGs were washed twice with DMEM/F12 (Thermo Fisher) and plated on poly-D-lysine/laminin-coated coverslips (BD Biosciences) at 5 × 104 cells per well in 24-well dishes in 500 μL of rDRG culture medium (Neurobasal-A medium, Thermo Fisher) with 2% B-27 supplement (Thermo Fisher), P/S, 50 ng/mL nerve growth factor-7S (Sigma), and 2 ng/mL GDNF (R&D Systems). At 1 d post-plating, rDRGs were treated with arabinofuranoside hydrochloride (Sigma) in the above media for 7 d to remove dividing cells. Cells were then incubated with fresh rDRG culture media as above. HSV transduction was performed 7 d after plating. JΔNI5 and JΔNI8 vectors were amplified and titered on U2OS-ICP4/27 cells as described.18,23
Virus Growth Curves
When using T225 flasks, triplicate flasks of 2 × 107 U2OS-ICP4/27 cells were infected with JΔNI vector at a MOI (in PFU/cell) indicated in the respective figure legends for 2 h at 37°C and 5% CO2 prior to incubation at 33°C and 5% CO2. For culture parameter optimization, triplicate T75 flasks of 6.7 × 106 U2OS-ICP4/27 cells were used. The supernatants were collected daily for virus titration. Viral titers were determined by quantitative real-time PCR for the gD gene or standard plaque assay on U2OS-ICP4/27 cells. For optimization of the glucose concentration, cells were grown in D-MEM (high glucose) with L-glutamine and phenol red (044-29765, Wako) or D-MEM (no glucose) with L-glutamine and phenol red (042-32255, Wako). In order to keep the concentration of glucose at ~4.5 g/L, D(+)-glucose (049-31165, Wako) was added to the culture media. For induction of vector release, cells were infected as described above and supernatants were harvested 9 d later. Fresh media supplemented with NaCl or CsCl to different final concentrations were added to the cultures and incubation was continued for 6 h at room temperature. Supernatants were then collected and their physical and biological viral titers determined.
Chemical Agents
HDAC and BET inhibitors and their commercial sources were as follows: APHA (3-[4-aroyl-1H-2-pyrrolyl]-N-hydroxypropenamide) compound 8 (A2478, Sigma), Belinostat (CS-0453, ChemScene), NaB (B5887, Sigma), Panobinostat (PAN; CS-0267, ChemScene), SAHA (Vorinostat; 10009929, Cayman Chemical), Trichostatin A (TSA; T-8552, Sigma), VPA (P4543, Sigma), and JQ-1 (2070-1,5, BioVision). Stock solutions of VPA, BEL, and NaB were prepared in dH2O and the remaining stock solutions (APHA8, PAN, SAHA, TSA, and JQ-1) were prepared in dimethyl sulfoxide (DMSO; 472301, Sigma). Sodium chloride (191-01665) and cesium chloride (037-19685) were purchased from Wako. For evaluation of SAHA treatment conditions, confluent U2OS-ICP4/27 cells in T225 flasks were treated with 0.5 μM SAHA for 2 h prior to viral infection. Cells were then infected with JΔNI5 virus at 10−5 (co-treatment group) or supernatants were replaced with SAHA-free media prior to infection at the same MOI (pre-treatment group). Fine-tuning of the SAHA concentration was performed in the pre-treatment protocol.
qRT-PCR and Genomic Quantitative Real-Time PCR
rDRGs were seeded in 15 wells of a 24-well tissue culture dish and infected with JΔNI5 virus at 5,000 gc/cell. At 4 dpi, cells were harvested and pooled in groups of 5, genomic DNA and total RNA were extracted from each group by RNeasy Mini kit (QIAGEN), and RNA was reverse transcribed by SuperScript IV Reverse Transcriptase (Thermo Fisher). Genomic DNA and cDNA were analyzed by quantitative real-time PCR in triplicate using the 7500 Fast Real-Time PCR System (Applied Biosystems). Results were normalized to cellular 18S rRNA genes or RNA. PCR primers used in this study were as described.18
Statistical Analyses
All values are presented as the mean ± SD. Differences between pairs were analyzed by Student’s t test or one-way analysis of variance (ANOVA) with post hoc Dunnett’s multiple comparison test using Microsoft Excel 14.7.7 or IBM SPSS statistics version 25.0. p values below 0.05 (p < 0.05) were considered statistically significant.
Author Contributions
S.K. and Y.M. conceived and designed the research. S.K., Y.M., K.A., M.Y., Y.S., and H.K. performed the experiments. S.K. and Y.M. analyzed the data. S.K. and Y.M. wrote the manuscript. Y.M., W.F.G., J.B.C., J.C.G., and T.O. supervised the research. All authors reviewed and edited the manuscript.
Conflicts of Interest
Y.M., J.B.C., and J.C.G. are co-inventors of intellectual property licensed to Coda Biotherapeutics, and J.B.C. and J.C.G. are co-inventors of intellectual property licensed to Oncorus. J.C.G. is a founder and consultant of Coda Biotherapuetics and Oncorus. W.F.G. is a consultant of Oncorus.
Acknowledgments
We are grateful to Taro Tomono (Tsukuba University) for virus production, Makoto Sukegawa (Nippon Medical School) for cell culturing, and Guillermo Posadas-Herrera (Nippon Medical School) for comments on the manuscript. This research was supported by grants to Y.M. and S.K. from Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research JP16K08249 and Nippon Medical School Grant-in-Aid for Medical Research.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.03.014.
Contributor Information
Yoshitaka Miyagawa, Email: yoshitaka-miyagawa@nms.ac.jp.
Takashi Okada, Email: t-okada@nms.ac.jp.
Supplemental Information
References
- 1.Fukuhara H., Ino Y., Todo T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373–1379. doi: 10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene laherparepvec Improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 3.Puzanov I., Milhem M.M., Minor D., Hamid O., Li A., Chen L., Chastain M., Gorski K.S., Anderson A., Chou J. Talimogene Laherparepvec in Combination With Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma. J. Clin. Oncol. 2016;34:2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington K.J., Puzanov I., Hecht J.R., Hodi F.S., Szabo Z., Murugappan S., Kaufman H.L. Clinical development of talimogene laherparepvec (T-VEC): a modified herpes simplex virus type-1-derived oncolytic immunotherapy. Expert Rev. Anticancer Ther. 2015;15:1389–1403. doi: 10.1586/14737140.2015.1115725. [DOI] [PubMed] [Google Scholar]
- 5.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 6.Artusi S., Miyagawa Y., Goins W.F., Cohen J.B., Glorioso J.C. Herpes Simplex Virus Vectors for Gene Transfer to the Central Nervous System. Diseases. 2018;6:E74. doi: 10.3390/diseases6030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobbs W.E., 2nd, DeLuca N.A. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 1999;73:8245–8255. doi: 10.1128/jvi.73.10.8245-8255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krisky D.M., Wolfe D., Goins W.F., Marconi P.C., Ramakrishnan R., Mata M., Rouse R.J., Fink D.J., Glorioso J.C. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Ther. 1998;5:1593–1603. doi: 10.1038/sj.gt.3300766. [DOI] [PubMed] [Google Scholar]
- 9.Palmer J.A., Branston R.H., Lilley C.E., Robinson M.J., Groutsi F., Smith J., Latchman D.S., Coffin R.S. Development and optimization of herpes simplex virus vectors for multiple long-term gene delivery to the peripheral nervous system. J. Virol. 2000;74:5604–5618. doi: 10.1128/jvi.74.12.5604-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preston C.M., Mabbs R., Nicholl M.J. Construction and characterization of herpes simplex virus type 1 mutants with conditional defects in immediate early gene expression. Virology. 1997;229:228–239. doi: 10.1006/viro.1996.8424. [DOI] [PubMed] [Google Scholar]
- 11.Samaniego L.A., Wu N., DeLuca N.A. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J. Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu N., Watkins S.C., Schaffer P.A., DeLuca N.A. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J. Virol. 1996;70:6358–6369. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson S.A., DeLuca N.A. Relationship of herpes simplex virus genome configuration to productive and persistent infections. Proc. Natl. Acad. Sci. USA. 2003;100:7871–7876. doi: 10.1073/pnas.1230643100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samaniego L.A., Neiderhiser L., DeLuca N.A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J. Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terry-Allison T., Smith C.A., DeLuca N.A. Relaxed repression of herpes simplex virus type 1 genomes in Murine trigeminal neurons. J. Virol. 2007;81:12394–12405. doi: 10.1128/JVI.01068-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilley C.E., Groutsi F., Han Z., Palmer J.A., Anderson P.N., Latchman D.S., Coffin R.S. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J. Virol. 2001;75:4343–4356. doi: 10.1128/JVI.75.9.4343-4356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyagawa Y., Marino P., Verlengia G., Uchida H., Goins W.F., Yokota S., Geller D.A., Yoshida O., Mester J., Cohen J.B., Glorioso J.C. Herpes simplex viral-vector design for efficient transduction of nonneuronal cells without cytotoxicity. Proc. Natl. Acad. Sci. USA. 2015;112:E1632–E1641. doi: 10.1073/pnas.1423556112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han F., Miyagawa Y., Verlengia G., Ingusci S., Soukupova M., Simonato M., Glorioso J.C., Cohen J.B. Cellular Antisilencing Elements Support Transgene Expression from Herpes Simplex Virus Vectors in the Absence of Immediate Early Gene Expression. J. Virol. 2018;92 doi: 10.1128/JVI.00536-18. E00536–E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verlengia G., Miyagawa Y., Ingusci S., Cohen J.B., Simonato M., Glorioso J.C. Engineered HSV vector achieves safe long-term transgene expression in the central nervous system. Sci. Rep. 2017;7:1507. doi: 10.1038/s41598-017-01635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao F., Schaffer P.A. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 1995;69:6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozuer A., Wechuck J.B., Goins W.F., Wolfe D., Glorioso J.C., Ataai M.M. Effect of genetic background and culture conditions on the production of herpesvirus-based gene therapy vectors. Biotechnol. Bioeng. 2002;77:685–692. doi: 10.1002/bit.10162. [DOI] [PubMed] [Google Scholar]
- 22.Miyagawa Y., Verlengia G., Reinhart B., Han F., Uchida H., Zucchini S., Goins W.F., Simonato M., Cohen J.B., Glorioso J.C. Deletion of the Virion Host Shut-off Gene Enhances Neuronal-Selective Transgene Expression from an HSV Vector Lacking Functional IE Genes. Mol. Ther. Methods Clin. Dev. 2017;6:79–90. doi: 10.1016/j.omtm.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wechuck J.B., Ozuer A., Goins W.F., Wolfe D., Oligino T., Glorioso J.C., Ataai M.M. Effect of temperature, medium composition, and cell passage on production of herpes-based viral vectors. Biotechnol. Bioeng. 2002;79:112–119. doi: 10.1002/bit.10310. [DOI] [PubMed] [Google Scholar]
- 24.Otsuki A., Patel A., Kasai K., Suzuki M., Kurozumi K., Antonio Chiocca E., Saeki Y. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol. Ther. 2008;16:1546–1555. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 25.Liu T.C., Castelo-Branco P., Rabkin S.D., Martuza R.L. Trichostatin A and oncolytic HSV combination therapy shows enhanced antitumoral and antiangiogenic effects. Mol. Ther. 2008;16:1041–1047. doi: 10.1038/mt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren K., Zhang W., Chen X., Ma Y., Dai Y., Fan Y., Hou Y., Tan R.X., Li E. An Epigenetic Compound Library Screen Identifies BET Inhibitors That Promote HSV-1 and -2 Replication by Bridging P-TEFb to Viral Gene Promoters through BRD4. PLoS Pathog. 2016;12:e1005950. doi: 10.1371/journal.ppat.1005950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi Y. The Bromodomain and Extra-Terminal Domain (BET) Family: Functional Anatomy of BET Paralogous Proteins. Int. J. Mol. Sci. 2016;17:E1849. doi: 10.3390/ijms17111849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cody J.J., Markert J.M., Hurst D.R. Histone deacetylase inhibitors improve the replication of oncolytic herpes simplex virus in breast cancer cells. PLoS ONE. 2014;9:e92919. doi: 10.1371/journal.pone.0092919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakashima H., Kaufmann J.K., Wang P.Y., Nguyen T., Speranza M.C., Kasai K., Okemoto K., Otsuki A., Nakano I., Fernandez S. Histone deacetylase 6 inhibition enhances oncolytic viral replication in glioma. J. Clin. Invest. 2015;125:4269–4280. doi: 10.1172/JCI80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbein G., Wendling D. Histone deacetylases in viral infections. Clin. Epigenetics. 2010;1:13–24. doi: 10.1007/s13148-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goins W.F., Huang S., Hall B., Marzulli M., Cohen J.B., Glorioso J.C. Engineering HSV-1 Vectors for Gene Therapy. Methods Mol. Biol. 2020;2060:73–90. doi: 10.1007/978-1-4939-9814-2_4. [DOI] [PubMed] [Google Scholar]
- 32.Adamson-Small L., Potter M., Byrne B.J., Clément N. Sodium Chloride Enhances Recombinant Adeno-Associated Virus Production in a Serum-Free Suspension Manufacturing Platform Using the Herpes Simplex Virus System. Hum. Gene Ther. Methods. 2017;28:1–14. doi: 10.1089/hgtb.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandenberghe L.H., Xiao R., Lock M., Lin J., Korn M., Wilson J.M. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum. Gene Ther. 2010;21:1251–1257. doi: 10.1089/hum.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everett R.D., Boutell C., Orr A. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 2004;78:1763–1774. doi: 10.1128/JVI.78.4.1763-1774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Everett R.D., Parada C., Gripon P., Sirma H., Orr A. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 2008;82:2661–2672. doi: 10.1128/JVI.02308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Everett R.D. Depletion of CoREST does not improve the replication of ICP0 null mutant herpes simplex virus type 1. J. Virol. 2010;84:3695–3698. doi: 10.1128/JVI.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deschamps T., Kalamvoki M. Impaired STING pathway in human osteosarcoma U2OS Cells contributes to the growth of ICP0-null mutant herpes simplex virus. J. Virol. 2017;91:E00006–E00017. doi: 10.1128/JVI.00006-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alandijany T. 2018. Distinct Temporal Regulation of Intrinsic and Innate Intracellular Immunity to Herpes Simplex virus type 1 (HSV-1) Infection. PhD thesis, University of Glasgow, Glasgow. [Google Scholar]
- 39.Ozuer A., Wechuck J.B., Russell B., Wolfe D., Goins W.F., Glorioso J.C., Ataai M.M. Evaluation of infection parameters in the production of replication-defective HSV-1 viral vectors. Biotechnol. Prog. 2002;18:476–482. doi: 10.1021/bp010176k. [DOI] [PubMed] [Google Scholar]
- 40.Penaud-Budloo M., François A., Clément N., Ayuso E. Pharmacology of Recombinant Adeno-associated Virus Production. Mol. Ther. Methods Clin. Dev. 2018;8:166–180. doi: 10.1016/j.omtm.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goswami R., Subramanian G., Silayeva L., Newkirk I., Doctor D., Chawla K., Chattopadhyay S., Chandra D., Chilukuri N., Betapudi V. Gene Therapy Leaves a Vicious Cycle. Front. Oncol. 2019;9:297. doi: 10.3389/fonc.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S., Dong G., Sheng C. Structural simplification: an efficient strategy in lead optimization. Acta Pharm. Sin. B. 2019;9:880–901. doi: 10.1016/j.apsb.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen E.A., Kim Y.H., Kuzel T.M., Pacheco T.R., Foss F.M., Parker S., Frankel S.R., Chen C., Ricker J.L., Arduino J.M., Duvic M. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J. Clin. Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 44.Mundle S.T., Hernandez H., Hamberger J., Catalan J., Zhou C., Stegalkina S., Tiffany A., Kleanthous H., Delagrave S., Anderson S.F. High-purity preparation of HSV-2 vaccine candidate ACAM529 is immunogenic and efficacious in vivo. PLoS ONE. 2013;8:e57224. doi: 10.1371/journal.pone.0057224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Keeffe R., Johnston M.D., Slater N.K. The primary production of an infectious recombinant Herpes Simplex Virus vaccine. Biotechnol. Bioeng. 1998;57:262–271. doi: 10.1002/(sici)1097-0290(19980205)57:3<262::aid-bit2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.