Abstract
Lysophosphatidic acid (LPA, 1-acyl-2-hemolytic-sn-glycerol-3-phosphate) extracted from membrane phospholipid is a kind of simple bioactive glycophospholipid, which has many biological functions such as stimulating cell multiplication, cytoskeleton recombination, cell survival, drug-fast, synthesis of DNA and ion transport. Current studies have shown that six G-coupled protein receptors (LPAR1-6) can be activated by LPA. They stimulate a variety of signal transduction pathways through heterotrimeric G-proteins (such as Gα12/13, Gαq/11, Gαi/o and GαS). LPA and its receptors play vital roles in cancers, nervous system diseases, cardiovascular diseases, liver diseases, metabolic diseases, etc. In this article, we discussed the structure of LPA receptors and elucidated their functions in various diseases, in order to better understand them and point out new therapeutic schemes for them.
Keywords: lysophosphatidic acid, LPAR, structure, physiology and pathology, cancer
Introduction
Lysophosphatidic acid (LPA, 1-acyl-2-hemolytic-sn-glycerin-3-phosphate), a small glycerophosphatidic acid, widely exists in human body. It has many different biological functions, such as promoting cell growth, differentiation, movement, survival and cytoskeleton morphological change 1. LPA combines with a variety of known G-protein coupled receptors (GPCRs) to perform a wide range of biological functions.
The six lysophosphatidic acid receptors (LPAR) currently known can be subdivided according to their homology. LPA1-3 receptor belongs to the endothelium differentiation gene (EDG) receptor, which has 50-57% amino acid identity with each other, while LPA4-6 receptor, which is a non EDG receptor with a long phylogenetic distance, has 35-55% amino acid identity with each other 2,3. Since the LPA receptors currently known are all G-protein coupled receptor families, they are all rhodopsin-like and have seven transmembrane domains, three extracellular loops (extracellular loop-ECL1, ECL2, ECL3) and three intracellular loops (intracellular loop-ICL1, ICL2 , ICL3). It also has an N-terminus within the cell and a C-terminus outside the cell 4. At least two Gα subunits (Gα12/13, Gαq/11, Gαi/o and GαS) are used by the LPA receptors to signal, thereby activating different downstream pathways, and under different environments and cell types produce different results 5. Numerous studies have shown that the role of LPA and its receptors is crucial in neurological diseases, tumors, metabolic diseases, liver diseases and cardiovascular diseases 6,7. In recent years, the role of LPA and its receptors in diseases has been paid more and more attention, and research into their mechanisms to find new disease treatment programs has become the focus of current research.
In this review, we hope to provide some new ideas and directions for the prevention and treatment of related diseases by summarizing the different functions of LPA receptors in different diseases.
Structure of lysophosphatidic acid receptor
LPAR1
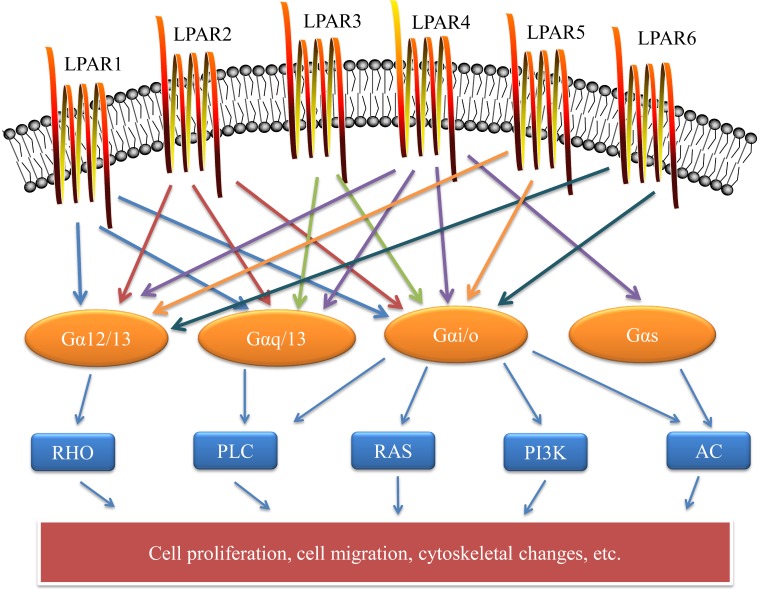
LPAR1, the earliest LPA receptor, was found in the neuroproliferative ventricular zone (VZ), superficial marginal zone, and meninges in a brain study in 1996 and identified as a receptor mediating LPA action 8. LPA1 is widely expressed in various tissues and organs of human body, in which the mRNA levels in brain, heart, colon, small intestine and placenta are higher, but relatively lower in other organs and tissues 9. LPAR1, a 41 kDa protein, consists of 364 amino acids. Its human chromosome gene is located on chromosome 9 (9q31.3), and has 7 transmembrane domains like other LPA receptors (Figure 1) 10. Three extracellular loops and three intracellular loops were formed by patterning 7 times on the plasma membrane. The transmembrane span III (TMIII) and transmembrane spanning V (TMV) and transmembrane spanning VII (TMVII) linkages to ECL1 and ECL2 are key regions for LPA binding to LPA1. The activation and desensitization of intracellular signals is associated with the ICL2 region, while the smallest ICL1 allows the cytoplasmic organelle to correctly process the receptor and express it on the cell surface. Among several intracellular and extracellular loops, ICL3, which has 33 amino acids, is the largest. It is a key region for LPA1 signal transduction and attenuation 11. It was also found that LPA1 preferred to receive ligands in extracellular environment 12. The receptor activates downstream pathways such as Akt, rho, mitogen-activated protein kinase (MAPK) and phospholipase C (PLC) by coupling with Gαi/o, Gαq/11 and Gα12/13. LPA mediates a great diversity of functions through LPA1 coupled to the G protein, including cell survival, cell proliferation, cell adhesion, cell migration, cytoskeletal changes, Ca2+ mobilization, immune function, and myelination 5. Such as promoting astrocyte proliferation and neuronal differentiation, proliferation of oligodendrocytes and smooth muscle cells, migration and anti-apoptosis of Schwann cells, mineralization and osteogenic transformation of valve interstitial cell (VIC) 13,14. Approximately 50% of LPA1-deficient mice presented neonatal mortality, which may be due to the lack of olfactory agent detection or olfactory processing in these mice, resulting in their inability to locate nipples for breast-feeding. These mice also showed changes characterized by nose shrinkage, eye spacing enlargement, cerebral cortex development changes and weight loss. Besides, some mice also experienced frontal cranial hemorrhage 5.
Figure 1.
Cell surface LPA receptors and their downstream signaling pathways. LPA signaling is mediated through six known G protein-coupled receptors. The LPA receptor is a 7-TM structure that is coupled to at least two G protein family members to activate downstream pathways that mediate a variety of cellular responses. These include cell proliferation, cell migration, and cytoskeletal changes. LPAR: lysophosphatidic acid receptor; RHO: rho protein; PLC: phospholipase C; RAS: ras protein; PI3K: phosphatidylinositol 3-kinase; AC: adenylyl cyclase.
LPAR2
LPA2 was identified from a gene library homology search of orphan GPCR genes, which has approximately 60% amino acid similarity to LPA1 15. LPAR2 is a receptor encoding 348 amino acids with a molecular weight of about 39 kDa. The human gene is located on chromosome 9 (19p12) 9. The coding region of the mouse LPA2 gene containing three exons is located in exons 2 and 3 16. LPA2 receptor mRNA expression in kidney, uterus, testis and leukocytes was higher than that in thymus, pancreas and spleen. LPA2 is the same as LPA1, is also coupled with Gαi/o, Gαq/11 and Gα12/13 in the heterotrimeric G protein family. In turn, through Ras, MAPK, phosphatidylinositol 3-kinase (PI3K), Rac, PLC, diacylglycerol (DG) and Rho and other downstream molecules transmit signals that mediate cell survival and cell migration 5,9. The PSD-95/DlgA/ZO-1 (PDZ) binding motif is located at the carboxy terminus of the LPA2 receptor and interacts with a variety of PDZ scaffold proteins. These scaffold proteins include Na+/H+ exchange regulatory factor-1 (NHERF-1), Na+/H+ exchange regulatory factor-2 (NHERF-2), inverted orientation-2 (MAGI-2), inverted orientation-3 (MAGI-3), neurabin, PDZ-RhoGEF (PRG) and leukemia-associated Rho GEF (LARG) proteins. The interaction between NHERF-2 and LPA2 enhanced LPA mediated cell proliferation and cell migration, while the interaction between MAGI-3 and LPA2 resulted in the opposite effect of NHERF-2. MAGI-3 had a negative regulatory effect on LPA2 mediated cell function 17. Studies have shown that spatial organization of PDZ motif-mediated LPA2 receptor macromolecular complex assembly mediates LPA gradient sensing in fibroblasts 18. LPA mediates anti-apoptosis of cells through LPA2 receptor, and the damage repair effect of LPA2 receptor on DNA can protect cells from radiation to some extent 19. There was no apparent phenotypic abnormality in LPA2-deficient mice, but in LPA1 and LPA2-deficient mice, the incidence of frontal lobe hematoma increased during perinatal period 9.
LPAR3
LPA3 was discovered similarly to LPA2 and cloned by a cloning method based on degenerate polymerase chain reaction 20. LPAR3, a human chromosomal gene, is located at 1p22.3-p31.1 and consists of 353 amino acids, with a molecular weight of approximately 40kDa. LPA3, located on the X chromosome, is similar to LPA1 and LPA2 in that it has about 50% amino acids 9,21. LPAR3 is coupled to Gαi/o and Gαq of the heterotrimeric G protein, mediating PLC activation, Ca2+ mobilization, inhibition and activation of adenylate cyclase (AC), and protein kinase activation that mediates mitogen activation 22. The mRNA level of LPAR3 is higher in human heart, lung, pancreas, brain, testis, prostate and ovary. However, in mice, the mRNA levels of LPAR3 in testis, kidney, thymus, small intestine, lung, stomach and brain are higher 9. Under the action of LPA3, immature mouse dendritic cells can be chemotaxis to LPA 23. The LPA3 receptor can also participate in the mobilization and recruitment of smooth muscle progenitor cells (SPC) and mediate the development of central post-stroke pain (CPSP) along with the LPA1 receptor 14,24. In contrast to mice lacking LPA1 and LPA2, the absence of LPA3 resulted in delayed embryo implantation and changes in embryo spacing, which dramatically reduces embryo crowding and litter size. The effect of LPA3 on embryo implantation is related to cyclooxygenase-2 (COX-2) and its derived prostacyclin (PGI2), as the lack of COX-2 leads to reproductive failure in multiple female mice 25,26. The lack of LPA3 receptor alone did not show a definite change in male reproduction, but the LPA1 receptor, LPA2 receptor and LPA3 receptor combined with defective male mice showed decreased germ cell survival and increased azoospermia prevalence 26. The above studies suggest that LPA3 is closely related to reproduction.
LPAR4
The LPA4 receptor discovered by ligand screening differs from the amino acid sequence of LPA1-3. Its amino acid similarity with LPAR1-3 is only 20-24%. LPA4 is structurally distinct from previously identified EDG family receptors (LPA1, LPA2, LPA3) and more similar to P2Y purinergic receptors 27. In humans, the 370 amino acid LPA4 gene is located in the q13-q21.1 region of the X chromosome and contains a 1113 bp intron-free open reading frame with a molecular weight of about 40 kDa 9,27. LPAR4 mRNA levels are higher in mouse skin, heart, ovary, thymus, developing brain and embryonic fibroblasts, and levels in the ovary are more pronounced 7,9. LPA4 binds primarily to Gαq, Gαi, Gα12/13 and Gαs to activate downstream pathways 28. LPA4 induces intracellular cyclic adenosine monophosphate (cAMP) accumulation by Gαs 27, cell aggregation and adhesion by N-cadherin 29, as well as neurite contraction and stress fiber formation by activating the Rho/ROCK pathway 30. Other studies have shown that it can affect the differentiation of immortalized hippocampal progenitor cells 31. In contrast to the LPA1 receptor, LPA-mediated migration and invasion of the cell are inhibited by the LPA4 receptor; for example, studies have found that continued progression of rat neuroblastoma and human colon cancer can be inhibited by LPA4 32,33. Different degrees of subcutaneous hemorrhage and vasodilation, impaired vascular endothelial cell wall cell coverage, and lymphatic dysplasia were observed in LPA4-deficient mouse embryos 34. It indicates that LPA4 receptor can promote angiogenesis and vascular development. In addition, the regulatory factor Yes-associated protein (YAP) and the transcriptional coactivator with PDZ-binding motif (TAZ) can also be activated by LPA4/LPA6 via the Gα12/Gα13 signaling pathway 35. YAP and TAZ can accelerate the progression of cancer by promoting the proliferation and migration of cancer cells, such as accelerating liver cancer, bladder cancer and lung cancer 36-38. Also, the volume, number and thickness of trabeculae increased in adult LPA4-/-mice, contrary to those observed in LPA1-/-mice, suggesting that LPA4 has a negative regulatory effect on bone formation and can counteract LPA1-induced bone formation 39.
LPAR5
As the fifth reported LPA receptor, LPA5 was identified as a member of the LPA receptor by using reverse transcription in an unbiased screening method. LPAR5 shares 35% homology with LPAR4, but with LPAR1-3 only 22% homology 40. The molecular weight of LPAR5 consisting of 372 amino acids is about 41 kDa. The human gene is located at 12p13.31. Large amounts of LPAR5 are expressed in the spleen, small intestine, and colon, but are relatively low in most other tissues 7,40,41. LPAR5 has multiple endogenous ligands including LPA, geranylgeranyl diphosphate (GGPP), farnesyl monophosphate, farnesyl pyrophosphate, N-arachidonoyl Glycine alkyl glycerophosphate and cyclic phosphatidic acid 41,42. Stress fiber formation and neurite contraction are associated with the LPA-LPA5-Gα12/Gα13 pathway, whereas increased intracellular calcium levels and cAMP accumulation are associated with the LPA-LPA5-Gαq pathway 7,9,40. LPA binds to LPA5 expressed on mouse and human CD8+ T cells, inhibited T cell antigen receptor (TCR)-induced intracellular calcium mobilization, extracellular regulated protein kinases (ERK) activation and Nur77 expression, thereby reduced granular exocytosis and cytotoxicity 43. LPA5 is also expressed in the spinal cord and dorsal root ganglia (DRG) and can promote the signaling of extensive pain in the spinal cord 42. In intestinal epithelium, LPA5 is the primary LPA receptor regulating Na+/H+ Exchanger 3 (NHE3). It co-expressed with NHERF-2, induces Na+-dependent water absorption, and recruits NHE3 to intestinal microvilli 41,44. Studies have shown that the transmission of pruritus signals caused by LPA is mainly transmitted through LPA5, PLD; transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1) 45. Whether LPA5 deficiency leads to disease-related changes is currently unclear.
LPAR6
As the sixth prion-coupling receptor for LPA; LPAR6, formerly known as P2Y5, was recognized as an LPA receptor in 2008 and is thought to be closely related to hair growth. The location of LPAR6 on chromosome is 13 (13q14), which encodes 344 amino acids with a molecular weight of about 39 kDa 5. Like LPA4, it belongs to the P2Y receptor family. The ligand-binding pattern of LPA6 is quite different from that of LPA1, and the ligand binding pocket of LPA6 is transverse to the membrane opening, and the acyl chain of the lipid used for crystallization is incorporated therein 46. LPAR6 binds to Gαi and Gα12/13 and activates downstream pathways for multiple functions. Activation of the Gα12/13 family induces expression of SRE regulatory genes by activating Rho and ROCK 47. LPA6-mediated Rho-dependent morphological changes and cAMP accumulation were detected by using chimeric Gα13 protein 48. LPA6 mostly binds to 2-acyl-LPA instead of l-acyl-LPA. When the promiscuous Gα protein is co-expressed with LPA6, LPA activates LPA6 to increase intracellular Ca2+ and reduce cAMP and ERK1/2 activation which stimulated by forskolin 9,47. As already mentioned, the transcriptional regulators YAP and TAZ can be activated by the LPA-LPA4/LPA6-Gα12/Gα13 signaling pathway, and both YAP and TAZ are involved in tumor progression 35. Moreover, another article mentions the binding of LPA to the LPA6 receptor to regulate vascular permeability 49. LPA6 also regulates the formation of hair follicles, and the loss of LPA6 leads to congenital alopecia 46,50. Research on the role and mechanism of LPAR6 in different systems is still relatively rare, which may be a future research direction.
The role of LPAR in diseases
LPAR and cancer
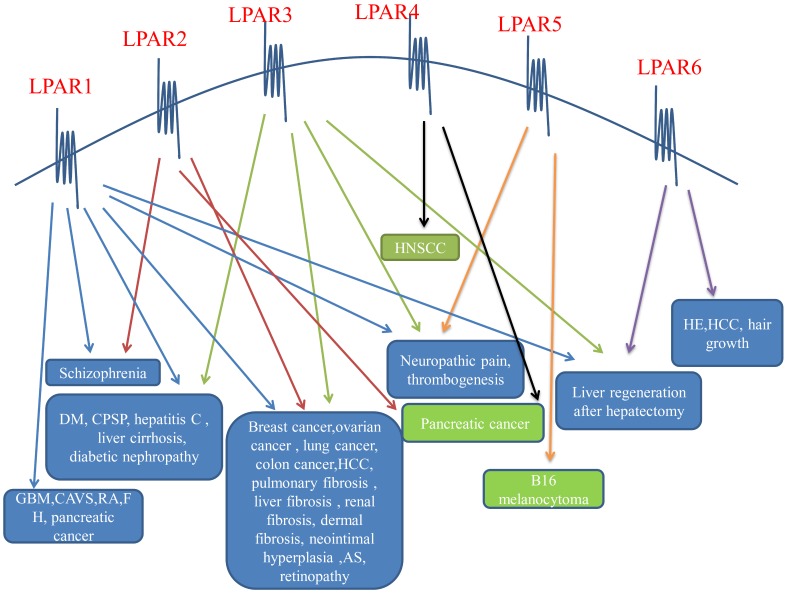
LPA induces cell proliferation, migration and survival. LPA affects cell morphology, mobility, chemotaxis and invasiveness by stimulating cell cycle, increasing cell viability, and promoting the production of interleukin (IL)-6, IL-8 and vascular endothelial growth factor (VEGF), interleukin (IL)-6, and IL-8. There is a lot of evidence that LPA plays an indispensable part in the occurrence and development of cancer because of its functions 5,51,52. The purpose of LPA in cancer is mainly achieved through its G protein coupled receptor. EDG receptors (LPA1-3) play a significant character in various cancers, but the character of non-EDG receptors (LPA4, LPA5, LPA6) in cancer is currently less studied. Different receptors of LPA receptors play different roles in various tumors to push the proliferation, migration, and anti-apoptosis of tumor cells and play a role in preventing the advancement of tumors (Figure 2).
Figure 2.
LPA receptors on the cell surface and their effects on diseases. The six LPA receptors have different effects on diseases of different systems, and both promote and inhibit the disease. The blue text box in the above figure indicates that the LPA receptors promotes it, and the green text box indicates that the LPA receptors inhibits it. LPAR: lysophosphatidic acid receptor; DM: diabetes mellitus; CPSP: central post-stroke pain; GBM: glioblastoma multiforme; CAVS: calcified aortic stenosis; RA: rheumatoid arthritis; FH: fetal hydrocephalus; HCC: hepatocellular carcinoma; AS: atherosclerosis; HNSCC: headneck squamous cell carcinoma; HE: hepatic encephalopathy.
LPAR and ovarian cancer
A large amount of LPA is present in the ascites of ovarian cancer. Overexpression of LPA2 and LPA3 in ovarian cancer cells stimulate proliferation and migration of ovarian cancer cells 53. The level of LPAR1 protein was significantly higher in lymph node metastasis and relapsed more in OSC tissues than in primary tumor lesions, and high levels of LPAR1 protein suggested poor prognosis 54. The LPA2 receptor induces the expression of VEGF by participating in the production of IL8 and induces the expression of cyclin D1 through transcriptional activation, thereby stimulating the growth of ovarian cancer and enhancing the invasiveness of ovarian cancer 51,52,55,56. The study also found that LPA stimulated COX-2 expression and cell movement through the LPA2-Gαi-Src-epidermal growth factor receptor (EGFR)-ERK signaling cascade in ovarian cancer cells 57. The expression of LPA3 receptor is mainly related to malignant cells. LPA3 selectively increases in ovarian cancer cells, and LPA with unsaturated fatty acyl chains is preferred in sn-LPA (common in ascites of ovarian cancer) 56. Yu et al. reported that the tumor volume and invasiveness of mice injected with SKOV-3 cells expressing LPA receptors increased, and the concentrations of IL-6, IL-8 and VEGF in mouse ascites and serum increased significantly. And tumor volume and invasiveness of LPA2 and LPA3 were significantly increased. HIF-1α is closely related to the occurrence and development of tumors 56. Ha et al. found that the LPA-LPAR-Gαi2 axis can induce pseudo hypoxic response through the Rac-NOXROS-HIF1α pathway, which ultimately leaded to metabolic reprogramming of ovarian cancer cells. This conclusion was verified by establishing two independent mouse models of ovarian cancer cell line xenograft (CDX) 58. PI3K/Akt pathway is critical in the process of ovarian cancer. LPAR1 plays a paramount role in the invasion, proliferation, migration, and development of ovarian serous cystadenocarcinoma (OSC) intratumoral heterogeneity (ITH) by regulating the activity of PI3K/AKT signaling pathway 54. When miR-15b was up-regulated, the proliferation of ovarian cancer cells and their apoptosis and senescence were prohibited by the LPAR3-PI3K/Akt pathway 59.
LPAR and breast cancer
In breast cancer (BC), the autotaxin (ATX)-LPA axis promotes BC cell invasion, proliferation and anti-apoptosis through high ATX expression and binding to LPA1-3, thereby brings about mammary gland inflammation and tumor formation. This is related to with activation of the LPA receptors by the ATX-LPA axis, resulting in downstream pathways PI3K/Akt, p38-PI3K and ERK/MAPK, wnt/β-catenin, estrogen receptor (ER), and cytokine activation 60. The metastasis of cancer cells mainly involves LPA1 receptors. Debio-0719 (LPA1 receptor inhibitor) can prohibit the metastasis of cancer cells to the lung and liver in 4T1 mouse breast cancer model, and Debio-0719 can also hinder the metastasis of cancer cells to the lung in MDA-MB-231T human breast cancer model. LPA1 siRNA does not alter primary tumor size, but also inhibits cancer cell metastasis 61. LPA1 receptor can also promote xenograft bone metastasis of breast cancer 62. All these indicate that metastasis of breast cancer is closely linked to LPA1 receptor. In the previous discussion of ovarian cancer, we mentioned that the effect of LPAR on the transcription factor hypoxia-inducible factor-1α (HIF-1α) is a factor in the development of ovarian cancer. Li et al. examined the tissue samples of 156 BC patients and found that the expression of LPA2 in BC tissues was higher than that in adjacent tissues, and the expression of LPA2 was more in postmenopausal women. This indicates that the overexpression of LPA2 is closely related to the canceration of postmenopausal BC. In vitro experiments have also shown that LPA2 is positively correlated with HIF-1α expression, and can also promote the proliferation, migration and invasion of BC cells 63. Liu et al. found that transgenic mice overexpressing ATX and LPAR1-3 had an increased risk of breast cancer, and LPAR3 transgenic mice had the highest metastasis rate 62. Popnikolov and others confirmed that 87 patients with invasive human breast cancer were more prone to lymph node metastasis by immunohistochemical analysis. This suggests that LPA mediates tumor aggressiveness primarily through the LPA3 receptor 64. In addition, LPA3 can contribute to the growth of triple-negative breast cancer cell lines and is of great significance for the early diagnosis of triple-negative breast cancer 65,66. Tao et al. tested 98 clinical BC and adjacent tissues and found that the expression of LPAR6 in BC was much lower than that in adjacent tissues, and the results were consistent with those obtained from the database. Knockdown of LPAR6 in cell experiments enhanced tumor cell proliferation and invasion. It is suggested that LPAR6 may play a role in inhibiting tumor development in BC 67.
LPAR and colon cancer
In colon cancer, LPA makes human colon cancer cell DLD1 more susceptible to metastasis by binding to LPA1. LPA enhances colon cancer cell proliferation and angiogenesis factor production via LPA2 68. Earlier studies found that LPA2 receptors were important receptors for LPA in the colon and mediated mitogenic signals in human colon cancer cells 69. Lin et al. found that in LPA2-/-mice, mucosal damage in the colon was reduced and tumor growth was suppressed 70. LPA2 and LPA3 are targets of LPA-induced proliferation of HCT116 and LS174T colon cancer cells by specific RNA interference (RNAi), and LPA2 and LPA3 promote the proliferation of colon cancer cells through classical protein kinase C (cPKC)-mediated activation of the β-catenin pathway 71. LPA2 receptor signaling regulates the function of colon cancer cells and is also associated with membrane-associated guanylate kinases, including MAGI-3 and NHERF-2. NHERF-2 competes with MAGI-3 for binding to LPA2, which has the opposite effect on the function of the LPA2 receptor to regulate colon cancer cells. The migration and invasion of colon cancer cells was inhibited when LPA2 binds to MAGI-3, and the opposite effect to NHERF-2 17. Beyond that, it was found that LPA4 and LPA6 can inhibit the locomotor activity of colon cancer cells 33.
LPAR and liver cancer
If factors such as hepatitis are not removed in time, the liver will continue to be damaged, and fibrosis will intensify, which may develop into cirrhosis and liver cancer. Hepatocellular carcinoma (HCC) is a type of primary hepatic carcinoma, accounting for 90% of primary liver cancer. In HCC tissues, LPA expression was higher and LPA receptors were also highly expressed. In liver cancer, studies have found that the expression of ATX and LPA1 receptors in HCC was higher than that in normal tissues. LPA1 receptor inhibition and inhibition of phosphoinositide 3-kinase (PI3K)/Akt and protein kinase Cd (PKCd)/p38-MAPK pathways all result in decreased MMP-9 activity and invasiveness of HCC. This demonstrates that LPA enhances MMP-9 expression and HCC invasion through LPA1 receptor and synergistic activation of the PI3K and p38MPAK signaling cascades 4,72,73. The migration ability of SKHep1 cells can be enhanced by LPA3-Gαi-ERK-MAPK signaling pathway, indicating that LPAR3 can promote the development of liver cancer 74. Okabe et al. also suggested that LPAR3 was related to the migration of liver cancer cells 75. Mazzocca et al. confirmed in vitro experiments that LPAR6 promoted the proliferation and tumorigenic phenotype of HCC cells, and established a relevant model to prove that LPAR6 promotes tumor growth, and the higher the expression level of LPAR6 in tumor tissues of liver cancer patients, the worse the prognosis of patients. Abnormally expressed LPAR6 can activate the protooncogene pim-3 via the signal transducers and activators of transcription 3 (STAT3) binding site, thereby maintaining the tumor proliferative capacity and tumorigenic capacity of HCC 76. LPAR1/3/6 mRNA expression was more elevated than non-tumor liver tissue, and LPA6 mRNA expression was highest. Regarding the role of LPA and its receptors in hepatocellular carcinoma, the researchers suggest that on the one hand, LPA produced by other pathways promotes the development of HCC. On the other hand, HCC produces LPA and can continue to promote HCC progress in combination with LPAR. For example, the growth and progression of HCC are maintained along with the autocrine loop produced by LPAR6 in tumor cells 77,78. These results indicate that the function of LPA and its receptors in the development of HCC cannot be ignored. In addition, Enooku et al. analyzed liver cancer tissues and adjacent tissues of 58 patients with HCC and found that the high expression of LPAR2 and LPAR6 often indicated that the tumor has a higher degree of malignancy 79.
LPAR and pancreatic cancer
In pancreatic cancer, the progress of PANC-1 cells can be promoted by LPA1 and LPA3, suggesting that LPAR1 and LPAR3 contribute to the movement and development of pancreatic cancer cells 80, while LPA actively inhibits the action of pancreatic cancer cells through LPAR2-Gα12/13-Rho signal transduction pathway 81. Several other studies have also shown that LPAR1 can promote the metastasis and invasion of pancreatic cancer cells, while LPAR2 has the opposite effect 81,82. Komachi et al. used oral administration of Ki16198 (specific inhibitors of LPAR1 and LPAR3) in nude mice modeled with pancreatic cancer cells, which inhibited tumor growth and reduced invasion and metastasis of other organs 83. Yang et al. proposed that YAP promoted the migration and invasion of pancreatic cancer cells by up-regulating LPAR3 in cells 84. Furthermore, by using shRNA to establish knockdown models-PANC-sh4, PANC-sh5, PNAC-sh6, LPA4 was found to inhibit the development of lung cancer, while LPA5-6 promoted pancreatic cancer 65.
LPAR and glioblastoma
Glioblastoma (GBM) is one of the most aggressive brain tumors. Earlier research discovered that LPA1 was highly expressed in the brain and participated in various activities of the nervous system 5. Recently, LPA1 receptor was discovered related to the proliferation and migration of GBM. In three GBM cell lines, LPA induces PKCα activation by LPA1, leading to nuclear translocation of kinases, increasing cell number and increasing cell viability 85,86.
LPAR and melanoma
LPAR1, LPAR2 and LPAR5 play essential roles in the invasion and metastasis of melanoma. Host LPA1 and LPA5 receptors promote B16F10 melanoma cell-derived lung metastasis. The expression of LPA2 in tumor cells promotes invasion, but LPA5 inhibits invasion. Jongsma et al. also proposed that LPA5 receptors inhibited melanoma cell migration and were related to downstream cAMP 87. It was worth noting that LPA5 receptor on cytotoxic CD8 + T cells acts contrary to LPA5 receptor in tumor cells. Its activation inhibits the activation and proliferation of T cells, thus facilitating the escape of host immunity 72. In another study, by using LPAR1/3 antagonists, it was suggested that LPAR3 was related to the activity of B16F10 metastatic melanoma cells and may be an important target for the treatment of melanoma 88.
LPAR and lung cancer
In A549 lung tumor cells, LPA inhibits P53 activity via LPA1-3 receptor, reduces p53-dependent transcription, promotes loss of p53 protein, and protects tumor cells from actinomycin D-induced apoptosis 89. It is suggested that the expression of LPA1-3 receptor contributes to the development of lung cancer. However, it was found that increased migration of rat lung tumor cells may be related to the loss of LPAR3 90. Yamada et al. proposed that mutations in the LPAR1 gene promoted the development of rat lung adenomas into lung adenocarcinomas 91. Magkrioti et al. also proposed that gene deletions of LPAR1 and nucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2) slowed the progression of lung fibrosis and lung cancer 92.
In the above discussion, we can see that EDG receptors are immeasurable in the progress of cancer, and the role of the same LPA receptor in different cellular environments was not the same. Future research can focus on the study of anticancer drugs based on LPA receptors.
LPAR and nervous system disease
Lysophosphatidic acid (LPA) is essential for brain development and nervous system function, signaling through six different G-protein coupled receptors (LPAR1-6). The role of LPA receptors in the nervous system is one of the earliest research findings. The LPA1 receptor was first discovered in the VZ, and the LPA1 receptor was most widely distributed in the nervous system 8.
LPAR and neuropathic pain
Through pharmacological and genetic studies, it was found that in mice lacking the LPA1 receptor, due to the inability to activate the Rho-ROCK pathway, abnormal mechanical pain, thermal hyperalgesia and demyelination, up-regulation of PKCγ and Caα2δ1 expression was inhibited, and the same phenomenon occurred after intrathecal injection of BoTXC3 into peripheral nerves 93. In a novel CPSP model developed by photochemically induced thrombosis (PIT) and tissue plasminogen activator, LPA1/3 antagonist Ki-16425 treatment can reverse established heat or mechanical hyperalgesia, blocking established CPSP. This also indicates that LPAR1 and LPAR3 are involved in neuropathic pain 24. Microglia are involved in the physiological and pathological processes of the nervous system and are permanent immune cells in the central nervous system (CNS). LPA may activate macrophages/microglia via LPAR1 and LPAR3 and promote self-amplification of LPA, increasing microglial migration and pro-inflammatory phenotype through the LPAR5/protein kinase D (PKD) axis. Activation and migration of microglia are associated with demyelination in the spinal cord after injury, and microglia are more involved in the initiation of neuropathic pain (NP) 94,95. In the cuprizone (CPZ)-induced multiple sclerosis (MS) model, LPA5 signaling mediates pain allergy induced by A-delta fibers and demyelination produced by CPZ. At the same time, phosphorylation of cAMP response element binding protein may lead to LPA5-mediated hyperalgesia in peripheral nerve injury in mice. This indicates that LPA5 signaling is related to neuropathic pain mediated by multiple sclerosis 96. It was found that intravenous LPA and GGPP (LPA5 agonists) induced allodynia, but GGPP-induced allodynia did not show up in LPA5-KO mice, indicating pain signals in the spinal cord by LPA5 transfer 94-96.
LPAR and fetal hydrocephalus
There is a common neurological disease in the newborn, fetal hydrocephalus (FH), and its occurrence is closely related to LPA and its receptors. In a mouse model of intracranial hemorrhage, by exposing the mouse embryonic brain to blood or LPA, the LPA1 receptor, which is dependent on the expression of neural progenitor cells (NPC), is over-activated, resulting in disruption and thinning of the cortical layer, ultimately leading to FH. FH was also found to be staged, suggesting that LPA receptors modulators can be used in the short-term to improve hydrocephalus without affecting LPA-mediated normal cortical development 97. Park et al. demonstrated that LPA may be involved in the development of fetal hydrocephalus by regulating the expression of the downstream factor Yap 98. When LPA was used to induce the production of posthemorrhagic hydrocephalus (PHH) by LPAR1-5 single gene knockout mice, LPAR1 and LPAR3 were found to be the main receptors of LPA-induced PHH production. When Ki16425 (LPAR1/3 inhibitor) was used in mice without gene knockout, the probability and severity of PHH production were reduced. This indicates that LPAR1 and LPAR3 may be key receptors for fetal hydrocephalus 99.
LPAR and hepatic encephalopathy
In hepatic encephalopathy mice, elevated serum ATX activates the LPA6-associated Gα12/13-Rho pathway in cerebral capillary vessel endothelial cells, resulting in enhanced blood-brain barrier (BBB) permeability and brain edema. The role of the LPA6 receptor in hepatic encephalopathy is suggested 100, 101. In addition, LPA and the tricyclic antidepressant amitriptyline (TCA) signaled LPAR1 to cut down P-glycoprotein transport in the BBB, thereby increasing drug delivery in blood-brain therapy 102.
LPAR and Alzheimer's disease
Alzheimer's disease (AD) is a chronic neurodegenerative disease distinguished by cognitive deterioration and behavioral abnormalities. LPA receptor-mediated Aβ accumulation, tau hyperphosphorylation and neuronal dysfunction are associated with AD. At the same time, traumatic brain injury, metabolic syndrome and chronic hypoperfusion are all mediated by LPA receptors and can be further developed into AD 103.
LPAR and retinopathy
The retina was an extension of the nervous system, and studies have shown that LPA was associated with retinopathy. In adult rats, the expression of LPA1 and LPA2 in retinal ganglion cells increased significantly after retinal ischemia, and LPA1 mediated retinal ganglion cell death in retinopathy of premature infants. In contrast, LPAR1-3 expressed by retinal pigment epithelial cells can promote retinal healing. It suggests that LPA has neuroprotective effects on the retina by binding to different LPA receptors on different cells, or has a neurodegenerative effect 104. LPAR1 and LPAR2 were also associated with schizophrenia 100,104.
In summary, considering the role of LPA in the nervous system, we can develop corresponding drugs to treat nervous system diseases by studying the mechanism of LPA and its receptors in the nervous system.
LPAR and cardiovascular system disease
With lifestyle changes and an aging population, cardiovascular disease (CVD) has become a major threat to the health. In China, the prevalence of CVD is high, with about 290 million people affected by CVD, and the number is still growing 105. LPA and its receptors are critical for the development of cardiovascular disease. First, as mentioned earlier, the LPA4 signaling pathway in the vascular endothelium was essential for vascular development. Furthermore, the process of atherosclerosis and thrombosis was also associated with LPA and its receptors 34.
LPAR and atherosclerosis
When blood vessels are damaged, phenotypic regulation of vascular smooth muscle cells (SMCs) (including dedifferentiation, proliferation, and migration) and CXC motif ligand 12 (CXCL12)-dependent SPCs mobilization, as well as promotion of intimal hyperplasia, occur. These are the critical causes of atherosclerosis and stenosis. The ability of LPA1 and LPA2-deficient mouse vascular SMC to migrate is reduced, and the intimal hyperplasia caused by vascular injury is reduced, but only LPA1-deficient mice may have an effect of enhancing vascular injury. This may be related to the increased migration of vascular smooth muscle cells caused by concurrent LPAR3 compensatory upregulation. It was worth noting that although LPA was associated with blood pressure regulation, it was not associated with LPAR1 and LPAR2 106. The use of Ki16425 (LPA1 and LPA3 antagonists) after vascular injury significantly reduced neointimal expression of CXCL12 and hypoxia-inducible factor (HIF)-1α, mobilization of CXCL12-dependent SPCs, and also inhibited neointimal hyperplasia. These indicate that LPA1, LPA2 and LPA3 mediate the formation of neointimal after vascular injury 14. In addition, the long-term use of Ki16425 can also inhibit the recruitment of monocytes that can lead to atherosclerosis. Studies have shown that LPA promotes the adhesion of monocytes induced by CXCL1 through the release of endothelial cell CXCL1 mediated by LPA1 and LPA3 receptors 107. The occurrence of atherosclerosis was also associated with inflammation. In recent studies, it was found that in LDLr-/-mice, Ki16425 induced a systemic anti-inflammatory response by inhibiting CCL2-CCR2 signaling. This enhanced the anti-inflammatory innate and adaptive immune response and lowered plasma cholesterol levels, ultimately damaging the progression of atherosclerosis 108. In summary, LPA1-3 was a critical aim for the cure of atherosclerosis and neointimal formation after stent implantation.
LPAR and thrombogenesis
Thrombogenesis is another risk factor for cardiovascular disease. In one aspect, LPA mediates platelet activation by stimulating LPAR1 and LPAR3, and LPA was a crucial thrombogenic component of the plaque lipid core. Selective antagonists of LPAR1 and LPAR3 dioctylphosphatidic acid [PA (8:0)] and dioctylglycerol pyrophosphate [DGPP (8:0)] can selectively suppress induction by LPA and mox-LDL Changes in platelet shape (inhibition of platelet activation by LPA), thereby reducing the formation of intravascular thrombus 109. On the other hand, plaque rupture caused by the further development of atherosclerotic plaques can lead to acute thrombotic occlusion of the arterial lumen, and there was a risk of cardiovascular diseases such as myocardial infarction and stroke. Studies have shown that after rupture of atherosclerotic plaques, LPA exposed from lipid nucleus induces platelet shape changes by binding to LPA5 and synergizes with adenosine diphosphate (ADP) to stimulate platelet aggregation and thrombosis 110. In another study on thrombosis, it was also suggested that LPA can enhance the assembly of fibrin and thus promote thrombosis 111.
LPAR and calcified aortic stenosis
Calcified aortic stenosis (CAVS) is also one of the common chronic CVD, and its main feature is progressive mineralization of the aortic valve. The research discovered that Ki1642 was given to the IGFII mouse high-fat and high-sucrose (HF-HS) diet for 6 months, and the rate of progression of aortic stenosis was reduced by a factor of three. Since LPAR3 was not expressed in VICs, LPAR1 was suggested to encourage the progress of aortic stenosis. Moreover, studies have shown that the mechanism may be that oxidative transformation of low-density lipoprotein (OxLDL)-LPA promotes the mineralization and osteogenic transformation of VIC by activating the LPAR1-RhoA-NF-κB pathway, thereby improving the development of CAVS 112.
In summary, LPA receptors (especially LPAR1 and LPAR3) play a vital part in the treatment of CVD. LPAR1 and LPAR3 can be used as the next target for the study of cardiovascular drugs.
LPAR and Fibrosis
Fibrosis is closely associated with end-stage organ failure, leading to severe morbidity and mortality 113. Here we describe the role of LPA and its receptors in the fibrosis progression in order to provide direction for the treatment of disease.
LPAR and renal fibrosis
LPA promotes the advancement of renal fibrosis through the LPA1 receptor. In the mice with renal interstitial fibrosis (TIF) induced by unilateral ureteral obstruction (UUO), the concentration of ATX and LPA were increased, and real-time reverse transcription polymerase chain reaction (RT-PCR) showed that LPA1 receptor was significantly up-regulated and LPA3 receptor was significantly down regulated 114. Fibroblast migration and proliferation can be stimulated by ATX-LPA-LPA1 receptor signaling 115. The study also found that renal fibrosis was significantly attenuated in LPA1-/-mice and mice treated with Ki16425. Moreover, when the LPA1 receptor was blocked, profibrotic cytokines (connective tissue growth factor and transforming growth factor-β) are significantly down-regulated 114. In one study, it was confirmed that LPA-LPA1 signaling directly induces connective tissue growth factor (CTGF) expression in primary proximal tubular epithelial cells via the myocardin-related transcription factor-serum response factor pathway 116. It was worth noting that the glomerular LPA1 receptor does not participate in the role of TIF caused by UUO 114. Geng et al. proposed that after renal ischemia-reperfusion injury, LPA mainly induced TGF-β activation through the LPA2-Gαq-Rho / ROCK pathway, thereby promoting the development of renal fibrosis 117. Besides, increased expression of LPA1 receptor was also detected in TIF induced by a mouse model of nephrotoxic serum (NTS) nephritis, which was more similar to the progression of human kidney disease, and this model is mainly associated with glomerulonephritis 118. Mirzoyan et al. also found that LPA is involved in subtotal nephrectomy (SNx) -mediated renal fibrosis. However, the specific LPA receptors and mechanisms are unclear 119.
LPAR and pulmonary fibrosis
Fibroblast accumulation and vascular leakage are principal factors in the pathogenesis of pulmonary fibrosis. In previous studies, LPA levels were remarkably elevated in bronchoalveolar lavage (BAL) fluid collected from patients with idiopathic pulmonary fibrosis (IPF) compared to the normal control group 120,121. In order to study the effect of LPA and LPA receptors in pulmonary fibrosis, bleomycin was used to attack mice lacking LPA1. It was found that the excessive accumulation of fibroblasts in damaged lungs and the persistent vascular leakage caused by injury were significantly reduced 121,122. Secondly, AM966, an effective antagonist of LPA1 receptor, was used in bleomycin-induced fibrosis model in mice. It not only dwindled vascular leakage, tissue damage, inflammation, and fibrosis but also dwindled the concentration of several pro-fibrosis and pro-inflammatory cytokines in bronchoalveolar lavage fluid (BALF) 123. In a randomized, double-blind, placebo-controlled clinical trial, the use of the LPA1 receptor antagonist BMS-986020 significantly slowed the decline of FVC (forced vital capacity) in patients with IPF, and alleviated clinical symptoms 124. It can be seen from the above studies, LPA contributes to the progress of pulmonary fibrosis through the LPA1 receptor, especially IPF. In bleomycin-induced pulmonary fibrosis, the progression of fibrosis can also be suppressed by knocking out other LPA receptors. Another study showed that bleomycin-induced lung injury, fibrosis and death can also be alleviated by knocking out the LPA2 receptor. Moreover, these phenomena and knockdown of LPA2 attenuated LPA-induced expression of transforming growth factor β1 (TGF-β1) and differentiation of lung fibroblasts. Hence LPA2 receptor was also important in the process of pulmonary fibrosis 125. Pulmonary fibrosis is a common and serious complication of radiation therapy for lung cancer. LPA1/LPA3 inhibitor VPC12249 can inhibit the expression of fibroblast-promoting cytokines TGFβ1 and CTGF in vivo, leading to a decrease of fibroblast proliferation and the slow progress of radiation-induced pulmonary fibrosis in mice 126. Regarding the mechanism of LPA and its receptors promoting pulmonary fibrosis, a study suggested that LPA-LPA1 pathway could induce bone marrow-derived mesenchymal stem cells (BMSC) to differentiate into myofibroblasts and promote the secretion of extracellular matrix (ECM), thereby promoting pulmonary fibrosis. Moreover, LPA1 antagonist Antalpa1 can inhibit these phenomena. After lung injury, myofibroblasts accumulate and activate, secrete excessive ECM, and eventually form fibrotic foci 127.
LPAR and liver fibrosis
Inflammation and other hepatic damaging factors activate hepatic stellate cells, make myofibroblasts accumulate and secrete excessive ECM, and then lead to liver fibrosis. The process of fibrosis is also mentioned earlier in this article. Regarding liver damage, studies have shown that when LPA can increase GSH levels, sustained phosphorylation of c-Jun N-terminal kinase (JNK) caused by acetaminophen (APAP) administration is blocked. Furthermore, blocking the production of inflammatory cytokines (TNF-α and IL-1β) protects against APAP-induced acute liver injury. But this process was independent of LPA receptors 128. Hepatitis was a key factor in the development of liver fibrosis. Make use of vitro hepatocytes and in vitro liver culture systems, as well as human liver chimeric mice and HCC tissues, the researchers demonstrated that the ATX-LPA signal axis activates PI3K and stabilizes HIF-1 α, which positively regulates hepatitis C virus (HCV) RNA replication, and this process may be related to LPA1 and LPA3 receptors, promoting disease progression to liver fibrosis and hepatocellular carcinoma 129. In another study, Silymarin, caffeine and their combinations significantly improved liver fibrosis induced by thioacetamide (TAA) by downregulating LPAR1, and downregulated α-SMA, CTGF and TGF-β1, suggesting that LPAR1 may promote the progress of liver fibrosis through α-SMA, CTGF and TGF-β1 130. This is also consistent with the mechanism of pulmonary fibrosis mentioned above. In addition, hepatitis C virus infection promotes liver fibrosis, and when ATX and LPA signals are suppressed, it reduces hepatitis C virus replication 129. The expression of serum ATX was also closely related to liver fibrosis, which also suggests that the ATX-LPA axis plays an important role in liver injury 131.
LPAR and other fibrosis
In addition to renal, pulmonary and liver fibrosis, studies have shown that LPA1 receptors can also promote the development of dermal fibrosis in bleomycin-induced skin fibrosis models, and is also related to the expression of TGF-β1 and CTGF 132. In a study on ventricular remodeling, it was found that OGN (osteoglycin) in the heart can bind to LPAR3 and attenuate the activation of EGFR signals through the Gα12/13/Rho/ROCK pathway, thereby inhibiting myocardial fibroblast (CMF) proliferation And migration. This suggests that LPAR3 may have a role in regulating fibrosis of the heart 133. LPA and its receptors are also involved in adipose tissue fibrosis. Rancoule et al. injected intraperitoneal injection of Ki16425 (LPAR1/LPAR3 antagonist) to db/db mice to reduce AT (adipose tissue) fibrosis, and experimentally found that LPAR1 may be the main receptor for LPA-promoting fibrosis 134.
LPAR and diabetes
LPAR and diabetes
Diabetes has become a disease that cannot be neglected. The occurrence of diabetes was related to many factors. Here we mainly discuss the effects of LPA and its receptors on diabetes. Current research indicates that LPA and its receptors have different regulatory effects on diabetes and its complications. Studies have shown that LPA boosts glucose uptake by stimulating skeletal muscle and fat cells, thereby reducing blood glucose levels in diabetic mice treated by streptozotocin (STZ). Moreover, the effect of LPA on glucose uptake can be entirely inhibited by Ki16425 (LPA1/3 receptor antagonist) 135. LPA also modulated NHE3 through an insulin-independent pathway that improves diarrhea in diabetic patients 136. From the above studies, it can be seen that LPA and its receptors can effectively balance blood sugar and improve some diabetic complications, but in other studies, they have found their effects to be opposite.
LPAR and diabetic nephropathy
Diabetic nephropathy is a common complication of diabetes. Its occurrence was related to the angiogenesis and fibrosis promoted by protease, growth factor, cytokines and chemokines released from renal cells 137,138. Studies have found that after using LPA1/3 receptor antagonist Ki16425 or BMS002 in db/db mice, kidney damage was significantly reduced, and the progression of diabetic nephropathy was slowed down. This phenomenon was associated with a decrease in GSK3b (Ser9) phosphorylation and SREBP1 (sterol-regulatory element binding proteins 1) activation caused by inhibition of LPAR1, and a subsequent diminish in TGF-β expression. As mentioned earlier in this paper, LPA can induce TGF-β to promote the development of renal fibrosis 137. Beyond that, it may also be related to the decrease in GFR caused by LPAR inhibition 138,139. Since Ki16425 and BMS002 are antagonists of the LPA1 receptor and the LPA3 receptor, another study used the LPAR1-specific antagonist AM095 to research the function of the LPAR1 in diabetic nephropathy. The study found that in a mouse model induced by STZ, AM095 inhibited renal cell inflammatory signaling cascade and reduced renal damage by inhibiting the TLR4/NF-κB and NADPH oxidase systems. The role of LPA1 receptor in the development of diabetic nephropathy was more clearly defined 140.
LPAR and obesity
As we all known, obesity is one of the risk factors for diabetes. It was found that after six weeks of the LPA1/3 receptor antagonist Ki16425, the fat mass and white fat cell size of HFHS-fed C57B16 mice increased significantly. Moreover, the ATX-LPA axis can cause obesity-induced insulin resistance by impairing PPARγ expression and activity. The above content showed that LPA receptors played a crucial part in the development of obesity and diabetes 141. After LPAR1-KO mice were fed high-fat and low-fat diets, compared with wild-type mice, no significant changes in body weight and fat were observed. It was suggested that LPAR1 may play a key role in lipid uptake 142.
Current studies on the relationship between diabetes and LPA and its receptors are limited to LPA and LPAR1 and LPAR3. It is still unclear whether other LPA receptors affect the development of diabetes and its complications. Future studies can focus on related drug studies and other receptor effects targeting LPAR1 and LPAR3.
LPAR and other diseases
LPAR and rheumatoid arthritis
Rheumatoid arthritis (RA) is a common chronic inflammatory disease, which is characterized by synovial proliferation, fibroblast-like synoviocytes (FLS) proliferation, angiogenesis, inflammatory cell infiltration and bone destruction of various joints. Miyabe et al. demonstrated that the expression of LPAR1 in the synovium of RA patients was high, and in LPA1-/-mice or mice using LPA1 antagonists, inflammatory cell infiltration and joint destruction in bones were reduced. In vitro experiments it was also shown that LPA1 can promote the formation of osteoclasts 143. In the K/BxN serum metastatic arthritis model, after LPA1/3 receptor antagonists were used, apoptosis increased, inflammatory mediators decreased, and bone remodeling protein decreased. These ultimately weaken the severity of arthritis. It was found that the LPA-LPA1 signaling pathway promoted the development of rheumatoid arthritis by enhancing the proliferation and migration of RA FLS and the production of inflammatory mediators 144, 145. Orosa et al. also confirmed that LPAR1 and LPAR2 were mainly expressed in RA FLS, and when LPAR1 was inhibited, tumor necrosis factor (TNF)-induced FLS proliferation was also reduced 146. Another study suggested that LPA-LPAR1 was involved in SF-stimulated hBMSC migration in RA patients 147.
LPAR and Sjogren's syndrome
Sjogren's syndrome (SS) is a chronic autoimmune disease that has not yet been developed to cure it, but studies have shown that treatment with the LPAR1/3 antagonist Ki16425 improves spontaneous development in SS and nonobese diabetic (NOD) mice in adoptive transfer models. SS. Tip LPA and its receptor may be valid targets for SS therapy 148.
LPAR and asthma
Asthma is one of the most commonplace lung diseases, and acute bronchoconstriction is one of the main reasons for hospitalization and sudden death from asthma. In animal models, LPA was found to trigger acute allergen and bradykinin-mediated bronchoconstriction by activating carotid body TRPV1 and LPA receptors, causing acute asthma symptoms. It was worth noting that this mechanism has not yet been confirmed in the human body, which can be used as a new research direction 149. In a previous study, a mouse model of allergic asthma with LPA2 deficiency was found to be more severe than wild-type mice with pulmonary and systemic inflammation 150. In another study, the role of LPAR2 in suppressing inflammation in asthma was also confirmed. When LPAR2 agonists were used, the inflammation of the lungs and airways of mice caused by allergen sensitization and challenge by HDM (dermatophagoides pteronyssinus) was significantly reduced. This may be related to reduced chemokine production and inhibition of cell migration 151.
LPAR and liver regeneration
The role of LPA and its receptors is essential in the overall changing process of liver disease. First, LPA can support the involvement of Rho-kinase by regulating cell morphology and attachment to the extracellular matrix, to reinforce the remodeling of collagen matrix by hepatic stellate cells (HSCs) 152. Second, the more severe the liver damage, the higher the concentration of LPA in plasma 153. Liver disease was a common digestive system disease, and severe liver diseases such as liver cancer have a poor prognosis. Liver resection or liver transplantation was an effective method for treating HCC. Using an enzyme-linked immunosorbent assay (ELISA), it was found that after partial hepatectomy (PHx), circulating LPA increased significantly after 72 hours, and LPAR1 increased in two stages of 12-24 hours and 48 hours-7 days, respectively. The LPAR1-stained cells are HSCs. Both LPAR3 mRNA and LPAR6 mRNA expression increased within 12 hours, but LPAR6 mRNA expression also increased significantly during the subsequent 48 hours to 7 day period. Moreover, LPAR6 was more widely stained than LPAR1 and LPAR3. These results suggest that LPA receptors (LPAR1, LPAR3, LPAR6) can promote liver regeneration after hepatectomy 128.
Perspectives and future directions
The growth factor-like lipid medium LPA acts as an effective signaling molecule that affects many physiological and pathological processes. So far, research on LPA receptors has progressed in many aspects, especially the most comprehensive study of LPA1 receptors, which of course was related to the broader expression of LPA1 receptors than other receptors. However, studies on non-EDG receptors, especially LPA6 receptors, are not comprehensive. It can be used as one of the future research directions. LPA signaling was associated with pathological responses, including conduction of neuropathic pain, promotion of tumorigenesis and metastasis, promotion of fibrosis, and promotion of atherosclerosis. The six G-protein coupled receptors that bind to it have become clear targets for drug development. Some drugs have entered clinical stages, such as the LPA1 receptor antagonists ONO-7300243 and ONO-0300302 are used to treat benign prostatic hyperplasia and inhibit LPA-induced increases in intraurethral pressure in rats and dogs. The LPA1 receptor antagonist BMS986020 for idiopathic fibrosis has entered phase II clinical trials but has been discontinued due to noticeable side effects. The LPA1 receptor antagonist Debio-0716 can reduce distant metastasis and lymph node metastasis in breast cancer. The LPA1 receptor antagonist ONO-8430506 can lower tumor resistance and enhance azithromycin-induced tumor cell death. LPA1/3 receptor antagonist SAR100842 can treat systemic sclerosis 65,154. In view of the wide-ranging role of LPA and its receptors in diseases, future research on drugs targeting LPA and its receptors will be of paramount importance.
Table 1.
The role of LPAR in diseases
| LPAR | Disease | Impact | Mechanism | Reference |
|---|---|---|---|---|
| LPAR1 | Ovarian cancer | Aggravate | PI3K/AKT pathway↑ | Cui et al.,2019 |
| Glioblastoma | Aggravate | PKCα↑ | Valdés-Rives et al.,2019 | |
| Lung cancer | Aggravate | P53↓ | Murph et al.,2007 | |
| Neuropathic pain | Aggravate | Rho-ROCK pathway↑, PKCγ↑,Caα2δ1↑ | Inoue et al.,2004 | |
| Neuropathic pain | Aggravate | macrophages/microglia↑ | Velasco et al.,2017 | |
| Atherosclerosis | Aggravate | CXCL12 X, HIF-1α↑, SPC↑ | Subramanian et al.,2010 | |
| Calcified aortic stenosis | Aggravate | RhoA-NF-κB pathway↑ | Nsaibia et al.,2017 | |
| Renal fibrosis | Aggravate | the myocardin-related transcription factor-serum response factor pathway↑, CTGF↑ | Sakai et al.,2017 | |
| Pulmonary fibrosis | Aggravate | BMSC to differentiate into myofibroblasts↑, ECM↑ | Tang et al.,2014 | |
| Liver fibrosis | Aggravate | α-SMA↑, CTGF↑, TGF-β1↑ | Eraky et al.,2018 | |
| Diabetic nephropathy | Aggravate | GSK3b (Ser9) phosphorylation↑, SREBP1↑, TGF-β↑ | Li et al.,2017 | |
| Diabetic nephropathy | Aggravate | TLR4 /NF-κB pathway↑, NADPH↑ | Lee et al.,2019 | |
| Rheumatoid arthritis | Aggravate | RA FLS↑ | Miyabe et al.,2014 | |
| LPAR2 | Ovarian cancer | Aggravate | Gαi-Src-EGFR-ERK pathway↑ | Jeong et al.,2008 |
| Colon cancer | Aggravate | cPKC↑, β-catenin↑ | Yang et al.,2005 | |
| Colon cancer | Attenuate | MAGI-3↑ | Lee et al.,2011 | |
| Colon cancer | Aggravate | NHERF-2↑ | Lee et al.,2011 | |
| Pancreatic cancer | Attenuate | Gα12/13-Rho pathway↑ | Komachi et al.,2009 | |
| Lung cancer | Aggravate | P53↓ | Murph et al.,2007 | |
| Atherosclerosis | Aggravate | CXCL12↑,HIF-1α↑, SPC↑ | Subramanian et al.,2010 | |
| Renal fibrosis | Aggravate | Gαq-Rho/ROCK pathway↑, TGF-β↑ | Geng et al.,2012 | |
| Pulmonary fibrosis | Aggravate | TGF-β1↑ | Huang et al.,2013 | |
| LPAR3 | Ovarian cancer | Attenuate | PI3K/Akt pathway ↑ | Li et al.,2019 |
| Colon cancer | Aggravate | cPKC↑,β-catenin↑ | Yang et al.,2005 | |
| Liver cancer | Aggravate | Gαi-ERK-MAPK pathway↑ | Zuckerman et al.,2016 | |
| Lung cancer | Aggravate | P53↓ | Murph et al.,2007 | |
| Neuropathic pain | Aggravate | macrophages/microglia↑ | Velasco et al.,2017 | |
| Atherosclerosis | Aggravate | CXCL12↑, HIF-1α↑, SPC↑ | Subramanian et al.,2010 | |
| Cardiac fibrosis | Attenuate | α-SMA↑, CTGF↑, TGF-β1↑ | Eraky et al.,2018 | |
| LPAR4 | - | - | - | - |
| LPAR5 | Melanoma | Attenuate | cAMP↓ | Jongsma et al.,2011 |
| Neuropathic pain | Aggravate | PKD↑,microglia↑ | Velasco et al.,2017; Plastira et al.,2017 | |
| LPAR6 | Liver cancer | Aggravate | pim-3↑ | Mazzocca et al.,2015 |
| Hepatic encephalopathy | Aggravate | Gα12/13-Rho pathway↑, BBB↑ | Herr et al.,2019; Masago et al.,2018 | |
| LPAR | Ovarian cancer | Aggravate | Rac-NOXROS-HIF1α pathway↑ | Ha et al.,2018 |
| Alzheimer's disease | Aggravate | Aβ accumulation↑, tau hyperphosphorylation↑, neuronal dysfunction↑ | Ramesh et al.,2018 |
PKCα: protein kinase Cα; PKCγ: protein kinase Cγ; LPAR: lysophosphatidic acid receptors; CXCL12: CXC motif ligand 12; HIF-1α: hypoxia-inducible factor-1α; SPC: smooth muscle progenitor cells; CTGF: connective tissue growth factor; BMSC: bone marrow-derived mesenchymal stem cells; ECM: extracellular matrix; SREBP1: sterol-regulatory element binding proteins 1; TGF-β1: transforming growth factor β1; NADPH: nicotinamide adenine dinucleotide phosphate; RA: rheumatoid arthritis; ERK: extracellular regulated protein kinases; cPKC: classical protein kinase C; MAGI-3: inverted orientation-3; NHERF-2: Na+/H+ exchange regulatory factor-2; MAPK: mitogen-activated protein kinase; cAMP: cyclic adenosine monophosphate; PKD: protein kinase D; BBB: blood-brain barrier.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81873076), Shanghai Rising-Star Project (15QA1403500) and Shanghai Talents development fund Project (2017090).
Abbreviations
- LPA
lysophosphatidic acid
- GPCRs
G-protein coupled receptors
- LPAR
lysophosphatidic acid receptor
- EDG
endothelial differentiation gene
- ECL
extracellular loop
- ICL
intracellular loop
- VZ
ventricular zone
- TMIII
transmembrane-spanning III
- TMV
transmembrane-spanning V
- TMVII
transmembrane-spanning VII
- VIC
valve interstitial cell
- PDZ
PSD-95/DlgA/ZO-1
- NHERF
Na+/H+ exchange regulatory factor
- MAGI-2
inverted orientation-2
- MAGI-3
inverted orientation-3
- HCC
hepatocellular carcinoma
- PI3K
phosphatidylinositol 3-kinase
- PKCd
protein kinase Cd
- STAT3
signal transducers and activators of transcription 3 (STAT3)
- PRG
PDZ-RhoGEF
- LARG
leukemia-associated Rho GEF
- SPC
smooth muscle progenitor cells
- CPSP
central post-stroke pain
- COX-2
cyclooxygenase-2
- PGI2
prostacyclin
- cAMP
cyclic adenosine monophosphate
- YAP
Yes-associated protein
- TAZ
transcriptional co-activator with PDZ-binding motif
- TCR
T cell antigen receptor
- ERK
extracellular regulated protein kinases
- DRG
dorsal root ganglia
- NHE3
Na+/H+Exchanger 3
- PLD
phospholipase D
- TRPA1
transient receptor potential ankyrin 1
- TRPV1
transient receptor potential vanilloid 1
- VEGF
vascular endothelial growth factor
- IL
interleukin
- EGFR
epidermal growth factor receptor
- OSC
ovarian serous cystadenocarcinoma
- ITH
intratumoral heterogeneity
- BC
breast cancer
- ATX
autotaxin
- MAPK
mitogen-activated protein kinase
- RNAi
RNA interference
- cPKC
classical protein kinase C
- NERF-2
Na+/H+ exchange regulatory factor
- PLC
phospholipase C
- CARMA3
caspase recruit domain and MAGUK domain containing 3
- MALT1
mucosa-associated lymphoid tissue 1
- NF-κB
nuclear factor-kappa κB
- PKCδ
protein kinase Cδ
- GBM
Glioblastoma
- PIT
photochemically induced thrombosis
- CNS
central nervous system
- PKD
protein kinase D
- NP
neuropathic pain
- CPZ
cuprizone
- MS
multiple sclerosis
- FH
fetal hydrocephalus
- PHH
posthemorrhagic hydrocephalus
- NPC
neural progenitor cells
- BBB
blood-brain barrier
- TCA
tricyclic antidepressant amitriptyline
- AD
alzheimer's disease
- CVD
cardiovascular disease
- SMCs
smooth muscle cells
- CXCL12
CXC motif ligand 12
- HIF
hypoxia-inducible factor
- DGPP
dioctylglycerol pyrophosphate
- PA
dioctylphosphatidic acid
- ADP
adenosine diphosphate
- CAVS
calcified aortic stenosis
- HF-HS
high-fat and high-sucrose
- UUO
unilateral ureteral obstruction
- TIF
ubulointerstitial fibrosis
- RT
reverse transcription
- PCR
polymerase chain reaction
- CTGF
connective tissue growth factor
- NTS
nephrotoxic serum
- OxLDL
oxidative transformation of low-density lipoprotein
- BAL
bronchoalveolar lavage
- IPF
idiopathic pulmonary fibrosis
- BALF
bronchoalveolar lavage fluid
- FVC
forced vital capacity
- TGF
transforming growth factor
- BMSC
bone marrow-derived mesenchymal stem cells
- ECM
extracellular matrix
- JNK
c-Jun N-terminal kinase
- APAP
acetaminophen
- HCV
hepatitis C virus
- TAA
thioacetamide
- STZ
streptozotocin
- PPARγ
peroxisome proliferator-activated receptor γ
- SS
Sjogren's syndrome
- RA
Rheumatoid arthritis
- FLS
fibroblast-like synoviocytes
- NOD
nonobese diabetic
- HSCs
hepatic stellate cells
- ELISA
enzyme-linked immunosorbent assay
- PHx
partial hepatectomy
References
- 1.van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Progress in Lipid Research. 2007;46(2):145–60. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Llona-Minguez S, Ghassemian A, Helleday T. Lysophosphatidic acid receptor (LPAR) modulators: The current pharmacological toolbox. Prog Lipid Res. 2015. 58,51-75. [DOI] [PubMed]
- 3.Yanagida K, Kurikawa Y, Shimizu T, Ishii S. Current progress in non-Edg family LPA receptor research. Biochim Biophys Acta. 2013;1831(1):33–41. doi: 10.1016/j.bbalip.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Peng WT, Sun WY, Li XR, Sun JC, Du JJ, Wei W. Emerging Roles of G Protein-Coupled Receptors in Hepatocellular Carcinoma. Int J Mol Sci. 2018. 19(5) [DOI] [PMC free article] [PubMed]
- 5.Kihara Y, Maceyka M, Spiegel S, Chun J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. British journal of pharmacology. 2014;171(15):3575–94. doi: 10.1111/bph.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutoh T, Rivera R, Chun J. Insights into the pharmacological relevance of lysophospholipid receptors. British journal of pharmacology. 2012;165(4):829–44. doi: 10.1111/j.1476-5381.2011.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. Journal of lipid research. 2014;55(7):1192–214. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. The Journal of cell biology. 1996;135(4):1071–83. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T. et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–86. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 10.Contos JJ, Chun J. Complete cDNA sequence, genomic structure, and chromosomal localization of the LPA receptor gene, lpA1/vzg-1/Gpcr26. Genomics. 1998;51(3):364–78. doi: 10.1006/geno.1998.5400. [DOI] [PubMed] [Google Scholar]
- 11.Murph MM, Nguyen GH, Radhakrishna H, Mills GB. Sharpening the edges of understanding the structure/function of the LPA1 receptor: expression in cancer and mechanisms of regulation. Biochim Biophys Acta. 2008;1781(9):547–57. doi: 10.1016/j.bbalip.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chrencik JE, Roth CB, Terakado M, Kurata H, Omi R, Kihara Y. et al. Crystal Structure of Antagonist Bound Human Lysophosphatidic Acid Receptor 1. Cell. 2015;161(7):1633–43. doi: 10.1016/j.cell.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shano S, Moriyama R, Chun J, Fukushima N. Lysophosphatidic acid stimulates astrocyte proliferation through LPA1. Neurochemistry International. 2008;52(1-2):216–20. doi: 10.1016/j.neuint.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian P, Karshovska E, Reinhard P, Megens RTA, Zhou Z, Akhtar S. et al. Lysophosphatidic Acid Receptors LPA1 and LPA3 Promote CXCL12-Mediated Smooth Muscle Progenitor Cell Recruitment in Neointima Formation. Circulation Research. 2010;107(1):96–105. doi: 10.1161/CIRCRESAHA.109.212647. [DOI] [PubMed] [Google Scholar]
- 15.Contos JJ, Chun J. Genomic characterization of the lysophosphatidic acid receptor gene, lp(A2)/Edg4, and identification of a frameshift mutation in a previously characterized cDNA. Genomics. 2000;64(2):155–69. doi: 10.1006/geno.2000.6122. [DOI] [PubMed] [Google Scholar]
- 16.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–54. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Ritter SL, Zhang H, Shim H, Hall RA, Yun CC. MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology. 2011;140(3):924–34. doi: 10.1053/j.gastro.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren A, Moon C, Zhang W, Sinha C, Yarlagadda S, Arora K. et al. Asymmetrical macromolecular complex formation of lysophosphatidic acid receptor 2 (LPA2) mediates gradient sensing in fibroblasts. J Biol Chem. 2014;289(52):35757–69. doi: 10.1074/jbc.M114.595512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tigyi GJ, Johnson LR, Lee SC, Norman DD, Szabo E, Balogh A. et al. Lysophosphatidic acid type 2 receptor agonists in targeted drug development offer broad therapeutic potential. Journal of lipid research. 2019;60(3):464–74. doi: 10.1194/jlr.S091744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K. et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. The Journal of biological chemistry. 1999;274(39):27776–85. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 21.Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010;62(4):579–87. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii I, Contos JJ, Fukushima N, Chun J. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Molecular pharmacology. 2000;58(5):895–902. doi: 10.1124/mol.58.5.895. [DOI] [PubMed] [Google Scholar]
- 23.Chan LC, Peters W, Xu Y, Chun J, Farese RV Jr, Cases S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA) J Leukoc Biol. 2007;82(5):1193–200. doi: 10.1189/jlb.0407221. [DOI] [PubMed] [Google Scholar]
- 24.Ueda H, Neyama H, Sasaki K, Miyama C, Iwamoto R. Lysophosphatidic acid LPA1 and LPA3 receptors play roles in the maintenance of late tissue plasminogen activator-induced central poststroke pain in mice. Neurobiol Pain. 2019;5:100020. doi: 10.1016/j.ynpai.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah BH, Catt KJ. Roles of LPA3 and COX-2 in implantation. Trends Endocrinol Metab. 2005;16(9):397–9. doi: 10.1016/j.tem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Hama K, Aoki J. LPA(3), a unique G protein-coupled receptor for lysophosphatidic acid. Prog Lipid Res. 2010;49(4):335–42. doi: 10.1016/j.plipres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278(28):25600–6. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Mosier PD, Fang X, Zhang Y. Toward the three-dimensional structure and lysophosphatidic acid binding characteristics of the LPA(4)/p2y(9)/GPR23 receptor: a homology modeling study. J Mol Graph Model. 2009;28(1):70–9. doi: 10.1016/j.jmgm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanagida K, Ishii S, Hamano F, Noguchi K, Shimizu T. LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. The Journal of biological chemistry. 2007;282(8):5814–24. doi: 10.1074/jbc.M610767200. [DOI] [PubMed] [Google Scholar]
- 30.Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. The Journal of biological chemistry. 2007;282(7):4310–7. doi: 10.1074/jbc.M610826200. [DOI] [PubMed] [Google Scholar]
- 31.Rhee HJ, Nam JS, Sun Y, Kim MJ, Choi HK, Han DH. et al. Lysophosphatidic acid stimulates cAMP accumulation and cAMP response element-binding protein phosphorylation in immortalized hippocampal progenitor cells. Neuroreport. 2006;17(5):523–6. doi: 10.1097/01.wnr.0000209011.16718.68. [DOI] [PubMed] [Google Scholar]
- 32.Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A. et al. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell. 2008;19(12):5435–45. doi: 10.1091/mbc.E08-03-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi K, Fukushima K, Onishi Y, Inui K, Node Y, Fukushima N. et al. Lysophosphatidic acid (LPA) signaling via LPA4 and LPA6 negatively regulates cell motile activities of colon cancer cells. Biochem Biophys Res Commun. 2017;483(1):652–7. doi: 10.1016/j.bbrc.2016.12.088. [DOI] [PubMed] [Google Scholar]
- 34.Sumida H, Noguchi K, Kihara Y, Abe M, Yanagida K, Hamano F. et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 2010;116(23):5060–70. doi: 10.1182/blood-2010-03-272443. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda D, Kobayashi D, Akahoshi N, Ohto-Nakanishi T, Yoshioka K, Takuwa Y, Lysophosphatidic acid-induced YAP/TAZ activation promotes developmental angiogenesis by repressing Notch ligand Dll4. J Clin Invest. 2019. 130. [DOI] [PMC free article] [PubMed]
- 36.He L, Wu MZ, Wang XB, Qiu XS, Wang EH, Wu GP. Tumor Suppressor LKB1 inhibits both the mRNA Expression and the Amplification of hTERC by the Phosphorylation of YAP in Lung Cancer Cells. J Cancer. 2019;10(16):3632–8. doi: 10.7150/jca.33237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong L, Lin F, Wu W, Huang W, Cai Z. Transcriptional cofactor Mask2 is required for YAP-induced cell growth and migration in bladder cancer cell. J Cancer. 2016;7(14):2132–8. doi: 10.7150/jca.16438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Ren M, Tan X, Hu L. Distinct Changes in the Expression TAZ are Associated with Normal Cervix and Human Cervical Cancer. J Cancer. 2018;9(22):4263–70. doi: 10.7150/jca.26623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansell JP, Barbour M, Moore C, Nowghani M, Pabbruwe M, Sjostrom T. et al. The synergistic effects of lysophosphatidic acid receptor agonists and calcitriol on MG63 osteoblast maturation at titanium and hydroxyapatite surfaces. Biomaterials. 2010;31(2):199–206. doi: 10.1016/j.biomaterials.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 40.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281(33):23589–97. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 41.Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M. et al. Lysophosphatidic acid stimulates the intestinal brush border Na(+)/H(+) exchanger 3 and fluid absorption via LPA(5) and NHERF2. Gastroenterology. 2010;138(2):649–58. doi: 10.1053/j.gastro.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murai N, Hiyama H, Kiso T, Sekizawa T, Watabiki T, Oka H. et al. Analgesic effects of novel lysophosphatidic acid receptor 5 antagonist AS2717638 in rodents. Neuropharmacology. 2017;126:97–107. doi: 10.1016/j.neuropharm.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Mathew D, Kremer KN, Strauch P, Tigyi G, Pelanda R, Torres RM. LPA5 Is an Inhibitory Receptor That Suppresses CD8 T-Cell Cytotoxic Function via Disruption of Early TCR Signaling. Front Immunol. 2019;10:1159. doi: 10.3389/fimmu.2019.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkin KA, He P, Yun CC. Expression of lysophosphatidic acid receptor 5 is necessary for the regulation of intestinal Na/H exchanger 3 by lysophosphatidic acid in vivo. American journal of physiology Gastrointestinal and liver physiology. 2018;315(4):G433–G442. doi: 10.1152/ajpgi.00130.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kittaka H, Uchida K, Fukuta N, Tominaga M. Lysophosphatidic acid-induced itch is mediated by signalling of LPA5 receptor, phospholipase D and TRPA1/TRPV1. J Physiol. 2017;595(8):2681–98. doi: 10.1113/JP273961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi R, Inoue A, Sayama M, Uwamizu A, Yamashita K, Hirata K. et al. Structural insights into ligand recognition by the lysophosphatidic acid receptor LPA6. Nature. 2017;548(7667):356–60. doi: 10.1038/nature23448. [DOI] [PubMed] [Google Scholar]
- 47.Lee M, Choi S, Hallden G, Yo SJ, Schichnes D, Aponte GW. P2Y5 is a G(alpha)i, G(alpha)12/13 G protein-coupled receptor activated by lysophosphatidic acid that reduces intestinal cell adhesion. Am J Physiol Gastrointest Liver Physiol. 2009;297(4):G641–54. doi: 10.1152/ajpgi.00191.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y. et al. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem. 2009;284(26):17731–41. doi: 10.1074/jbc.M808506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takara K, Eino D, Ando K, Yasuda D, Naito H, Tsukada Y. et al. Lysophosphatidic Acid Receptor 4 Activation Augments Drug Delivery in Tumors by Tightening Endothelial Cell-Cell Contact. Cell Rep. 2017;20(9):2072–86. doi: 10.1016/j.celrep.2017.07.080. [DOI] [PubMed] [Google Scholar]
- 50.Pasternack SM, von Kügelgen I, Aboud KA, Lee Y-A, Rüschendorf F, Voss K. et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nature Genetics. 2008;40(3):329–34. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 51.Folkman J. A new link in ovarian cancer angiogenesis: lysophosphatidic acid and vascular endothelial growth factor expression. Journal of the National Cancer Institute. 2001;93(10):734–5. doi: 10.1093/jnci/93.10.734. [DOI] [PubMed] [Google Scholar]
- 52.Hu YL, Tee MK, Goetzl EJ, Auersperg N, Mills GB, Ferrara N. et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. Journal of the National Cancer Institute. 2001;93(10):762–8. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- 53.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3(8):582–91. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 54.Cui R, Cao G, Bai H, Zhang Z. LPAR1 regulates the development of intratumoral heterogeneity in ovarian serous cystadenocarcinoma by activating the PI3K/AKT signaling pathway. Cancer Cell Int. 2019;19:201. doi: 10.1186/s12935-019-0920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu YL, Albanese C, Pestell RG, Jaffe RB. Dual mechanisms for lysophosphatidic acid stimulation of human ovarian carcinoma cells. Journal of the National Cancer Institute. 2003;95(10):733–40. doi: 10.1093/jnci/95.10.733. [DOI] [PubMed] [Google Scholar]
- 56.Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J. et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008;100(22):1630–42. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeong KJ, Park SY, Seo JH, Lee KB, Choi WS, Han JW. et al. Lysophosphatidic acid receptor 2 and Gi/Src pathway mediate cell motility through cyclooxygenase 2 expression in CAOV-3 ovarian cancer cells. Exp Mol Med. 2008;40(6):607–16. doi: 10.3858/emm.2008.40.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ha JH, Radhakrishnan R, Jayaraman M, Yan M, Ward JD, Fung KM. et al. LPA Induces Metabolic Reprogramming in Ovarian Cancer via a Pseudohypoxic Response. Cancer Res. 2018;78(8):1923–34. doi: 10.1158/0008-5472.CAN-17-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li GC, Qin XL, Song HH, Li YN, Qiu YY, Cui SC. et al. Upregulated microRNA-15b alleviates ovarian cancer through inhitbition of the PI3K/Akt pathway by targeting LPAR3. J Cell Physiol. 2019;234(12):22331–22342. doi: 10.1002/jcp.28799. [DOI] [PubMed] [Google Scholar]
- 60.Panupinthu N, Lee HY, Mills GB. Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression. Br J Cancer. 2010;102(6):941–6. doi: 10.1038/sj.bjc.6605588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marshall JC, Collins JW, Nakayama J, Horak CE, Liewehr DJ, Steinberg SM. et al. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J Natl Cancer Inst. 2012;104(17):1306–19. doi: 10.1093/jnci/djs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F. et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15(6):539–50. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li M, Xiao D, Zhang J, Qu H, Yang Y, Yan Y. et al. Expression of LPA2 is associated with poor prognosis in human breast cancer and regulates HIF-1alpha expression and breast cancer cell growth. Oncol Rep. 2016;36(6):3479–87. doi: 10.3892/or.2016.5206. [DOI] [PubMed] [Google Scholar]
- 64.Popnikolov NK, Dalwadi BH, Thomas JD, Johannes GJ, Imagawa WT. Association of autotaxin and lysophosphatidic acid receptor 3 with aggressiveness of human breast carcinoma. Tumour Biol. 2012;33(6):2237–43. doi: 10.1007/s13277-012-0485-1. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Sun Y, Qu J, Yan Y, Yang Y, Cai H. Roles of LPA receptor signaling in breast cancer. Expert Rev Mol Diagn. 2016;16(10):1103–11. doi: 10.1080/14737159.2016.1238763. [DOI] [PubMed] [Google Scholar]
- 66.Qiu J, Xue X, Hu C, Xu H, Kou D, Li R. et al. Comparison of Clinicopathological Features and Prognosis in Triple-Negative and Non-Triple Negative Breast Cancer. J Cancer. 2016;7(2):167–73. doi: 10.7150/jca.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tao K, Guo S, Chen R, Yang C, Jian L, Yu H. et al. Lysophosphatidic Acid Receptor 6 (LPAR6) Expression and Prospective Signaling Pathway Analysis in Breast Cancer. Mol Diagn Ther. 2019;23(1):127–38. doi: 10.1007/s40291-019-00384-3. [DOI] [PubMed] [Google Scholar]
- 68.Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T. et al. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer research. 2003;63(7):1706–11. [PubMed] [Google Scholar]
- 69.Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA. et al. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol. 2005;289(1):C2–11. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- 70.Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW. et al. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136(5):1711–20. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang M, Zhong WW, Srivastava N, Slavin A, Yang J, Hoey T. et al. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the {beta}-catenin pathway. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(17):6027–32. doi: 10.1073/pnas.0501535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SC, Fujiwara Y, Liu J, Yue J, Shimizu Y, Norman DD. et al. Autotaxin and LPA1 and LPA5 receptors exert disparate functions in tumor cells versus the host tissue microenvironment in melanoma invasion and metastasis. Mol Cancer Res. 2015;13(1):174–85. doi: 10.1158/1541-7786.MCR-14-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park SY, Jeong KJ, Panupinthu N, Yu S, Lee J, Han JW. et al. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene. 2010;30(11):1351–9. doi: 10.1038/onc.2010.517. [DOI] [PubMed] [Google Scholar]
- 74.Zuckerman V, Sokolov E, Swet JH, Ahrens WA, Showlater V, Iannitti DA. et al. Expression and function of lysophosphatidic acid receptors (LPARs) 1 and 3 in human hepatic cancer progenitor cells. Oncotarget. 2016;7(3):2951–67. doi: 10.18632/oncotarget.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okabe K, Kato K, Teranishi M, Okumura M, Fukui R, Mori T. et al. Induction of lysophosphatidic acid receptor-3 by 12-O-tetradecanoylphorbol-13-acetate stimulates cell migration of rat liver cells. Cancer Lett. 2011;309(2):236–42. doi: 10.1016/j.canlet.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 76.Mazzocca A, Dituri F, De Santis F, Filannino A, Lopane C, Betz RC. et al. Lysophosphatidic acid receptor LPAR6 supports the tumorigenicity of hepatocellular carcinoma. Cancer Res. 2015;75(3):532–43. doi: 10.1158/0008-5472.CAN-14-1607. [DOI] [PubMed] [Google Scholar]
- 77.Sokolov E, Eheim AL, Ahrens WA, Walling TL, Swet JH, McMillan MT. et al. Lysophosphatidic acid receptor expression and function in human hepatocellular carcinoma. J Surg Res. 2013;180(1):104–13. doi: 10.1016/j.jss.2012.10.054. [DOI] [PubMed] [Google Scholar]
- 78.Lopane C, Agosti P, Gigante I, Sabba C, Mazzocca A. Implications of the lysophosphatidic acid signaling axis in liver cancer. Biochim Biophys Acta Rev Cancer. 2017;1868(1):277–82. doi: 10.1016/j.bbcan.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Enooku K, Uranbileg B, Ikeda H, Kurano M, Sato M, Kudo H. et al. Higher LPA2 and LPA6 mRNA Levels in Hepatocellular Carcinoma Are Associated with Poorer Differentiation, Microvascular Invasion and Earlier Recurrence with Higher Serum Autotaxin Levels. PLoS One. 2016;11(9):e0161825. doi: 10.1371/journal.pone.0161825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fukushima K, Takahashi K, Yamasaki E, Onishi Y, Fukushima N, Honoki K. et al. Lysophosphatidic acid signaling via LPA1 and LPA3 regulates cellular functions during tumor progression in pancreatic cancer cells. Exp Cell Res. 2017;352(1):139–45. doi: 10.1016/j.yexcr.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Komachi M, Tomura H, Malchinkhuu E, Tobo M, Mogi C, Yamada T. et al. LPA1 receptors mediate stimulation, whereas LPA2 receptors mediate inhibition, of migration of pancreatic cancer cells in response to lysophosphatidic acid and malignant ascites. Carcinogenesis. 2009;30(3):457–65. doi: 10.1093/carcin/bgp011. [DOI] [PubMed] [Google Scholar]
- 82.Fukushima K, Otagaki S, Takahashi K, Minami K, Ishimoto K, Fukushima N. et al. Promotion of cell-invasive activity through the induction of LPA receptor-1 in pancreatic cancer cells. J Recept Signal Transduct Res. 2018;38(4):367–71. doi: 10.1080/10799893.2018.1531889. [DOI] [PubMed] [Google Scholar]
- 83.Komachi M, Sato K, Tobo M, Mogi C, Yamada T, Ohta H. et al. Orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo. Cancer Sci. 2012;103(6):1099–104. doi: 10.1111/j.1349-7006.2012.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang S, Zhang L, Purohit V, Shukla SK, Chen X, Yu F. et al. Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3. Oncotarget. 2015;6(34):36019–31. doi: 10.18632/oncotarget.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valdes-Rives SA, Gonzalez-Arenas A. Autotaxin-Lysophosphatidic Acid: From Inflammation to Cancer Development. Mediators Inflamm. 2017;2017:9173090. doi: 10.1155/2017/9173090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valdés-Rives SA, de la Fuente-Granada M, Velasco-Velázquez MA, González-Flores O, González-Arenas A. LPA1 receptor activation induces PKCα nuclear translocation in glioblastoma cells. The International Journal of Biochemistry & Cell Biology. 2019;110:91–102. doi: 10.1016/j.biocel.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Jongsma M, Matas-Rico E, Rzadkowski A, Jalink K, Moolenaar WH. LPA is a chemorepellent for B16 melanoma cells: action through the cAMP-elevating LPA5 receptor. PLoS One. 2011;6(12):e29260. doi: 10.1371/journal.pone.0029260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Altman MK, Gopal V, Jia W, Yu S, Hall H, Mills GB. et al. Targeting melanoma growth and viability reveals dualistic functionality of the phosphonothionate analogue of carba cyclic phosphatidic acid. Mol Cancer. 2010;9:140. doi: 10.1186/1476-4598-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murph MM, Hurst-Kennedy J, Newton V, Brindley DN, Radhakrishna H. Lysophosphatidic acid decreases the nuclear localization and cellular abundance of the p53 tumor suppressor in A549 lung carcinoma cells. Mol Cancer Res. 2007;5(11):1201–11. doi: 10.1158/1541-7786.MCR-06-0338. [DOI] [PubMed] [Google Scholar]
- 90.Hayashi M, Okabe K, Yamawaki Y, Teranishi M, Honoki K, Mori T. et al. Loss of lysophosphatidic acid receptor-3 enhances cell migration in rat lung tumor cells. Biochem Biophys Res Commun. 2011;405(3):450–4. doi: 10.1016/j.bbrc.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 91.Yamada T, Obo Y, Furukawa M, Hotta M, Yamasaki A, Honoki K. et al. Mutations of lysophosphatidic acid receptor-1 gene during progression of lung tumors in rats. Biochem Biophys Res Commun. 2009;378(3):424–7. doi: 10.1016/j.bbrc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 92.Magkrioti C, Oikonomou N, Kaffe E, Mouratis MA, Xylourgidis N, Barbayianni I. et al. The Autotaxin-Lysophosphatidic Acid Axis Promotes Lung Carcinogenesis. Cancer Res. 2018;78(13):3634–44. doi: 10.1158/0008-5472.CAN-17-3797. [DOI] [PubMed] [Google Scholar]
- 93.Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10(7):712–8. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- 94.Velasco M, O'Sullivan C, Sheridan GK. Lysophosphatidic acid receptors (LPARs): Potential targets for the treatment of neuropathic pain. Neuropharmacology. 2017;113(Pt B):608–17. doi: 10.1016/j.neuropharm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Plastira I, Bernhart E, Goeritzer M, DeVaney T, Reicher H, Hammer A. et al. Lysophosphatidic acid via LPA-receptor 5/protein kinase D-dependent pathways induces a motile and pro-inflammatory microglial phenotype. Journal of neuroinflammation. 2017;14(1):253. doi: 10.1186/s12974-017-1024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsukahara R, Yamamoto S, Yoshikawa K, Gotoh M, Tsukahara T, Neyama H. et al. LPA5 signaling is involved in multiple sclerosis-mediated neuropathic pain in the cuprizone mouse model. Journal of pharmacological sciences. 2018;136(2):93–6. doi: 10.1016/j.jphs.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 97.Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW. et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med. 2011;3(99):99ra87. doi: 10.1126/scitranslmed.3002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park R, Moon UY, Park JY, Hughes LJ, Johnson RL, Cho S-H. et al. Yap is required for ependymal integrity and is suppressed in LPA-induced hydrocephalus. Nature Communications. 2016;7(1):10329. doi: 10.1038/ncomms10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lummis NC, Sánchez-Pavón P, Kennedy G, Frantz AJ, Kihara Y, Blaho VA. et al. LPA overactivation induces neonatal posthemorrhagic hydrocephalus through ependymal loss and ciliary dysfunction. Sci Adv. 2019;5(10):eaax2011. doi: 10.1126/sciadv.aax2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herr DR, Chew WS, Satish RL, Ong WY. Pleotropic Roles of Autotaxin in the Nervous System Present Opportunities for the Development of Novel Therapeutics for Neurological Diseases. Molecular neurobiology. 2020;57(1):372–392. doi: 10.1007/s12035-019-01719-1. [DOI] [PubMed] [Google Scholar]
- 101.Masago K, Kihara Y, Yanagida K, Hamano F, Nakagawa S, Niwa M. et al. Lysophosphatidic acid receptor, LPA6, regulates endothelial blood-brain barrier function: Implication for hepatic encephalopathy. Biochem Biophys Res Commun. 2018;501(4):1048–54. doi: 10.1016/j.bbrc.2018.05.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Banks DB, Chan GN, Evans RA, Miller DS, Cannon RE. Lysophosphatidic acid and amitriptyline signal through LPA1R to reduce P-glycoprotein transport at the blood-brain barrier. J Cereb Blood Flow Metab. 2018;38(5):857–68. doi: 10.1177/0271678X17705786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramesh S, Govindarajulu M, Suppiramaniam V, Moore T, Dhanasekaran M. Autotaxin⁻Lysophosphatidic Acid Signaling in Alzheimer's Disease. Int J Mol Sci. 2018. 19(7) [DOI] [PMC free article] [PubMed]
- 104.Frisca F, Sabbadini RA, Goldshmit Y, Pébay A. Biological effects of lysophosphatidic acid in the nervous system. International review of cell and molecular biology. 2012;296:273–322. doi: 10.1016/B978-0-12-394307-1.00005-9. [DOI] [PubMed] [Google Scholar]
- 105.Jiang L, Krumholz HM, Li X, Li J, Hu S. Achieving best outcomes for patients with cardiovascular disease in China by enhancing the quality of medical care and establishing a learning health-care system. The Lancet. 2015;386(10002):1493–505. doi: 10.1016/S0140-6736(15)00343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Panchatcharam M, Miriyala S, Yang F, Rojas M, End C, Vallant C. et al. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ Res. 2008;103(6):662–70. doi: 10.1161/CIRCRESAHA.108.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Z, Subramanian P, Sevilmis G, Globke B, Soehnlein O, Karshovska E. et al. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 2011;13(5):592–600. doi: 10.1016/j.cmet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 108.Kritikou E, van Puijvelde GH, van der Heijden T, van Santbrink PJ, Swart M, Schaftenaar FH. et al. Inhibition of lysophosphatidic acid receptors 1 and 3 attenuates atherosclerosis development in LDL-receptor deficient mice. Sci Rep. 2016;6:37585. doi: 10.1038/srep37585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G. et al. Subtype-selective antagonists of lysophosphatidic Acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108(6):741–7. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- 110.Schober A, Siess W. Lysophosphatidic acid in atherosclerotic diseases. Br J Pharmacol. 2012;167(3):465–82. doi: 10.1111/j.1476-5381.2012.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho J, Mosher DF. Enhancement of thrombogenesis by plasma fibronectin cross-linked to fibrin and assembled in platelet thrombi. Blood. 2006;107(9):3555–63. doi: 10.1182/blood-2005-10-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nsaibia MJ, Boulanger MC, Bouchareb R, Mkannez G, Le Quang K, Hadji F. et al. OxLDL-derived lysophosphatidic acid promotes the progression of aortic valve stenosis through a LPAR1-RhoA-NF-κB pathway. Cardiovascular research. 2017;113(11):1351–63. doi: 10.1093/cvr/cvx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 2013;27(5):1830–46. doi: 10.1096/fj.12-219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P. et al. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol. 2007;18(12):3110–8. doi: 10.1681/ASN.2007020196. [DOI] [PubMed] [Google Scholar]
- 115.Sakai N, Bain G, Furuichi K, Iwata Y, Nakamura M, Hara A. et al. The involvement of autotaxin in renal interstitial fibrosis through regulation of fibroblast functions and induction of vascular leakage. Sci Rep. 2019;9(1):7414. doi: 10.1038/s41598-019-43576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sakai N, Chun J, Duffield JS, Lagares D, Wada T, Luster AD. et al. Lysophosphatidic acid signaling through its receptor initiates profibrotic epithelial cell fibroblast communication mediated by epithelial cell derived connective tissue growth factor. Kidney Int. 2017;91(3):628–41. doi: 10.1016/j.kint.2016.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Geng H, Lan R, Singha PK, Gilchrist A, Weinreb PH, Violette SM. et al. Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF through LPA2- and Galphaq-mediated Rho and alphavbeta6 integrin-dependent activation of TGF-beta. Am J Pathol. 2012;181(4):1236–49. doi: 10.1016/j.ajpath.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pradere JP, Gonzalez J, Klein J, Valet P, Gres S, Salant D. et al. Lysophosphatidic acid and renal fibrosis. Biochim Biophys Acta. 2008;1781(9):582–7. doi: 10.1016/j.bbalip.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mirzoyan K, Baiotto A, Dupuy A, Marsal D, Denis C, Vinel C. et al. Increased urinary lysophosphatidic acid in mouse with subtotal nephrectomy: potential involvement in chronic kidney disease. J Physiol Biochem. 2016;72(4):803–12. doi: 10.1007/s13105-016-0518-0. [DOI] [PubMed] [Google Scholar]
- 120.Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B. et al. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37(3):311–22. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 121.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z. et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14(1):45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 122.Funke M, Zhao Z, Xu Y, Chun J, Tager AM. The Lysophosphatidic Acid Receptor LPA1Promotes Epithelial Cell Apoptosis after Lung Injury. American Journal of Respiratory Cell and Molecular Biology. 2012;46(3):355–64. doi: 10.1165/rcmb.2010-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC. et al. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol. 2010;160(7):1699–713. doi: 10.1111/j.1476-5381.2010.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palmer SM, Snyder L, Todd JL, Soule B, Christian R, Anstrom K. et al. Randomized, Double-Blind, Placebo-Controlled, Phase 2 Trial of BMS-986020, a Lysophosphatidic Acid Receptor Antagonist for the Treatment of Idiopathic Pulmonary Fibrosis. Chest. 2018;154(5):1061–9. doi: 10.1016/j.chest.2018.08.1058. [DOI] [PubMed] [Google Scholar]
- 125.Huang LS, Fu P, Patel P, Harijith A, Sun T, Zhao Y. et al. Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. Am J Respir Cell Mol Biol. 2013;49(6):912–22. doi: 10.1165/rcmb.2013-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gan L, Xue JX, Li X, Liu DS, Ge Y, Ni PY. et al. Blockade of lysophosphatidic acid receptors LPAR1/3 ameliorates lung fibrosis induced by irradiation. Biochem Biophys Res Commun. 2011;409(1):7–13. doi: 10.1016/j.bbrc.2011.04.084. [DOI] [PubMed] [Google Scholar]
- 127.Tang N, Zhao Y, Feng R, Liu Y, Wang S, Wei W. et al. Lysophosphatidic acid accelerates lung fibrosis by inducing differentiation of mesenchymal stem cells into myofibroblasts. J Cell Mol Med. 2014;18(1):156–69. doi: 10.1111/jcmm.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Simo KA, Niemeyer DJ, Hanna EM, Swet JH, Thompson KJ, Sindram D. et al. Altered lysophosphatidic acid (LPA) receptor expression during hepatic regeneration in a mouse model of partial hepatectomy. HPB (Oxford) 2014;16(6):534–42. doi: 10.1111/hpb.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Farquhar MJ, Humphreys IS, Rudge SA, Wilson GK, Bhattacharya B, Ciaccia M. et al. Autotaxin-lysophosphatidic acid receptor signalling regulates hepatitis C virus replication. J Hepatol. 2017;66(5):919–29. doi: 10.1016/j.jhep.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 130.Eraky SM, El-Mesery M, El-Karef A, Eissa LA, El-Gayar AM. Silymarin and caffeine combination ameliorates experimentally-induced hepatic fibrosis through down-regulation of LPAR1 expression. Biomed Pharmacother. 2018;101:49–57. doi: 10.1016/j.biopha.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 131.Nakagawa H, Ikeda H, Nakamura K, Ohkawa R, Masuzaki R, Tateishi R. et al. Autotaxin as a novel serum marker of liver fibrosis. Clin Chim Acta. 2011;412(13-14):1201–6. doi: 10.1016/j.cca.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 132.Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS. et al. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011;63(5):1405–15. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zuo C, Li X, Huang J, Chen D, Ji K, Yang Y. et al. Osteoglycin attenuates cardiac fibrosis by suppressing cardiac myofibroblast proliferation and migration through antagonizing lysophosphatidic acid 3/matrix metalloproteinase 2/epidermal growth factor receptor signalling. Cardiovasc Res. 2018;114(5):703–12. doi: 10.1093/cvr/cvy035. [DOI] [PubMed] [Google Scholar]
- 134.Rancoule C, Viaud M, Gres S, Viguerie N, Decaunes P, Bouloumie A. et al. Pro-fibrotic activity of lysophosphatidic acid in adipose tissue: in vivo and in vitro evidence. Biochim Biophys Acta. 2014;1841(1):88–96. doi: 10.1016/j.bbalip.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 135.Yea K, Kim J, Lim S, Park HS, Park KS, Suh PG. et al. Lysophosphatidic acid regulates blood glucose by stimulating myotube and adipocyte glucose uptake. J Mol Med (Berl) 2008;86(2):211–20. doi: 10.1007/s00109-007-0269-z. [DOI] [PubMed] [Google Scholar]
- 136.He P, Zhao L, Zhu L, Weinman EJ, De Giorgio R, Koval M. et al. Restoration of Na+/H+ exchanger NHE3-containing macrocomplexes ameliorates diabetes-associated fluid loss. J Clin Invest. 2015;125(9):3519–31. doi: 10.1172/JCI79552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li HY, Oh YS, Choi JW, Jung JY, Jun HS. Blocking lysophosphatidic acid receptor 1 signaling inhibits diabetic nephropathy in db/db mice. Kidney Int. 2017;91(6):1362–73. doi: 10.1016/j.kint.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 138.Lee JH, Kim D, Oh YS, Jun HS. Lysophosphatidic Acid Signaling in Diabetic Nephropathy. Int J Mol Sci. 2019. 20(11) [DOI] [PMC free article] [PubMed]
- 139.Zhang MZ, Wang X, Yang H, Fogo AB, Murphy BJ, Kaltenbach R. et al. Lysophosphatidic Acid Receptor Antagonism Protects against Diabetic Nephropathy in a Type 2 Diabetic Model. J Am Soc Nephrol. 2017;28(11):3300–11. doi: 10.1681/ASN.2017010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee JH, Sarker MK, Choi H, Shin D, Kim D, Jun HS. Lysophosphatidic acid receptor 1 inhibitor, AM095, attenuates diabetic nephropathy in mice by downregulation of TLR4/NF-kappaB signaling and NADPH oxidase. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1332–40. doi: 10.1016/j.bbadis.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 141.D'Souza K, Paramel GV, Kienesberger PC. Lysophosphatidic Acid Signaling in Obesity and Insulin Resistance. Nutrients. 2018;10(4):399. doi: 10.3390/nu10040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dusaulcy R, Daviaud D, Pradère JP, Grès S, Valet P, Saulnier-Blache JS. Altered food consumption in mice lacking lysophosphatidic acid receptor-1. J Physiol Biochem. 2009;65(4):345–50. doi: 10.1007/BF03185929. [DOI] [PubMed] [Google Scholar]
- 143.Miyabe Y, Miyabe C, Iwai Y, Takayasu A, Fukuda S, Yokoyama W. et al. Necessity of lysophosphatidic acid receptor 1 for development of arthritis. Arthritis Rheum. 2013;65(8):2037–47. doi: 10.1002/art.37991. [DOI] [PubMed] [Google Scholar]
- 144.Orosa B, Garcia S, Martinez P, Gonzalez A, Gomez-Reino JJ, Conde C. Lysophosphatidic acid receptor inhibition as a new multipronged treatment for rheumatoid arthritis. Ann Rheum Dis. 2014;73(1):298–305. doi: 10.1136/annrheumdis-2012-202832. [DOI] [PubMed] [Google Scholar]
- 145.Miyabe Y, Miyabe C, Iwai Y, Yokoyama W, Sekine C, Sugimoto K. et al. Activation of fibroblast-like synoviocytes derived from rheumatoid arthritis via lysophosphatidic acid-lysophosphatidic acid receptor 1 cascade. Arthritis research & therapy. 2014;16(5):461. doi: 10.1186/s13075-014-0461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Orosa B, Gonzalez A, Mera A, Gomez-Reino JJ, Conde C. Lysophosphatidic acid receptor 1 suppression sensitizes rheumatoid fibroblast-like synoviocytes to tumor necrosis factor-induced apoptosis. Arthritis Rheum. 2012;64(8):2460–70. doi: 10.1002/art.34443. [DOI] [PubMed] [Google Scholar]
- 147.Song HY, Lee MJ, Kim MY, Kim KH, Lee IH, Shin SH. et al. Lysophosphatidic acid mediates migration of human mesenchymal stem cells stimulated by synovial fluid of patients with rheumatoid arthritis. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2010;1801(1):23–30. doi: 10.1016/j.bbalip.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 148.Park E, Kim D, Lee SM, Jun HS. Inhibition of lysophosphatidic acid receptor ameliorates Sjögren's syndrome in NOD mice. Oncotarget. 2017;8(16):27240–51. doi: 10.18632/oncotarget.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jendzjowsky NG, Roy A, Barioni NO, Kelly MM, Green FHY, Wyatt CN. et al. Preventing acute asthmatic symptoms by targeting a neuronal mechanism involving carotid body lysophosphatidic acid receptors. Nat Commun. 2018;9(1):4030. doi: 10.1038/s41467-018-06189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Emo J, Meednu N, Chapman TJ, Rezaee F, Balys M, Randall T. et al. Lpa2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation. J Immunol. 2012;188(8):3784–90. doi: 10.4049/jimmunol.1102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Knowlden SA, Hillman SE, Chapman TJ, Patil R, Miller DD, Tigyi G. et al. Novel Inhibitory Effect of a Lysophosphatidic Acid 2 Agonist on Allergen-Driven Airway Inflammation. Am J Respir Cell Mol Biol. 2016;54(3):402–9. doi: 10.1165/rcmb.2015-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yanase M, Ikeda H, Matsui A, Maekawa H, Noiri E, Tomiya T. et al. Lysophosphatidic acid enhances collagen gel contraction by hepatic stellate cells: association with rho-kinase. Biochem Biophys Res Commun. 2000;277(1):72–8. doi: 10.1006/bbrc.2000.3634. [DOI] [PubMed] [Google Scholar]
- 153.Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Tomiya T. et al. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 2007;81(12):1009–15. doi: 10.1016/j.lfs.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 154.Zhao Y, Hasse S, Zhao C, Bourgoin SG. Targeting the autotaxin - Lysophosphatidic acid receptor axis in cardiovascular diseases. Biochemical Pharmacology. 2019;164:74–81. doi: 10.1016/j.bcp.2019.03.035. [DOI] [PubMed] [Google Scholar]