Abstract
Introduction
Interstitial lung disease (ILD) is a frequent extra-articular manifestation of Rheumatoid arthritis (RA), but nowadays there are no randomized controlled clinical trials to support therapeutic guidelines. RA-ILD, especially with UIP pattern, shares some similarities with idiopathic pulmonary fibrosis, suggesting a possible role of antifibrotic therapy in these patients. To date, there are no published data supporting the use of pifenidone in RA-ILD.
We describe for the first time two patients with a diagnosis of RA-ILD successfully treated with hydroxychloroquine and pirfenidone, without adverse events.
Case presentation
Patient 1 and patient 2 were first diagnosed with IPF (UIP pattern at high-resolution computed tomography, no other signs or symptoms suggesting other forms of ILD, routine laboratory examinations and immunological texts negative). Patients started pirfenidone 2403 mg daily. Few months later, they referred to our multidisciplinary outpatient for arthritis. ACPA and RF were positive. A diagnosis of RA was performed and treatment with corticosteroids and hydroxychloroquine was started, in association with pirfenidone.
In both cases we assessed the stabilization of articular and lung manifestations, without adverse events.
Discussion
In absence of randomized controlled trials, the optimal treatment of RA-ILD has not been determined and remains challenging. When considering therapeutic options for RA-ILD, both pulmonary and extra-thoracic disease manifestations and degrees of activity should be assessed and taken into consideration. Future prospective research might change RA-ILD management, moving to a more personalized approach based on the identification of different phenotypes of the disease or to a combination of immunosuppressive and antifibrotic treatment.
Keywords: Anti-fibrotic drugs, Interstitial lung disease, Pirfenidone, Rheumatoid arthritis
Highlights
-
•
Nowadays there are no randomized controlled clinical trials to support therapeutic guidelines in RA-ILD.
-
•
RA-ILD shares some similarities with IPF, suggesting a possible role of antifibrotic therapy RA-ILD patients.
-
•
We describe for the first time two patients with RA-ILD successfully treated with hydroxychloroquine and pirfenidone.
-
•
A number of trials are ongoing to assess the efficacy/safety of pirfenidone in fibrosing ILDs other than IPF.
-
•
To date, multidisciplinary approach in RA-ILD patients remains mandatory.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease, affecting 0.5%–1% of the population worldwide. It is a systemic disease, characterized by typical joint involvement with symmetrical erosive synovitis and progressive disability [1].
Interstitial lung disease (ILD) is a frequent extra-articular manifestation of RA, with negative impact on overall prognosis and utilization of healthcare resources [[2], [3], [4], [5], [6], [7]]. The real incidence of RA-ILD is unknown, but a prevalence of 7–10% for symptomatic RA-ILD has been reported, that is responsible of 10–20% of all mortality, with a mean survival of 5–8 years [[3], [4], [5], [6]].
The predominant histological/radiological pattern of RA-ILD is usual interstitial pneumonia (UIP) reported in 44–66% of cases [8]. The UIP pattern demonstrated having a poorer prognosis than other forms, sharing many analogies with idiopathic pulmonary fibrosis (IPF) [9,10]. Moreover, clinical, etiopathogenetic and genetic similarities between RA-ILD, especially with UIP pattern, and IPF have been described [7,[11], [12], [13], [14], [15], [16], [17], [18]].
Several therapeutic agents have been suggested for the treatment of RA-ILD, but nowadays there are no randomized controlled clinical trials to support therapeutic guidelines; therefore, the role of immunosuppression remains uncertain. On the other hand, antifibrotic drugs have been shown to reduce the decline in lung function in patients with IPF and their use is now recommended for the treatment of this progressive fibrosing ILD [19].
This background may suggest a plausible rationale in the use of antifibrotic therapies, such as pirfenidone, in RA-ILD patients, especially in UIP pattern. Moreover, the concomitant use of anti-fibrotic agents and biological or conventional DMARDs represents an emergent gap of knowledge.
To date, there is only one ongoing clinical trial evaluating safety and efficacy of pirfenidone in RA- ILD (TRAIL1), and no other published data are available on this topic [20].
We describe for the first time two RA-ILD patients successfully treated with pirfenidone.
2. Cases presentation
2.1. Patient 1
In 2014, a 70-year-old man presented to the pneumological unit of our Hospital for persistent dry cough. His past clinical history revealed the presence of metabolic syndrome (type 2 diabetes mellitus, increased blood pressure, high levels of cholesterol and triglyceride, and a BMI of 32) and a post-surgical hypothyroidism for a diffuse multinodular goiter. He was a current smoker (60 pack/years), and before retiring he worked as a construction worker and foundry worker.
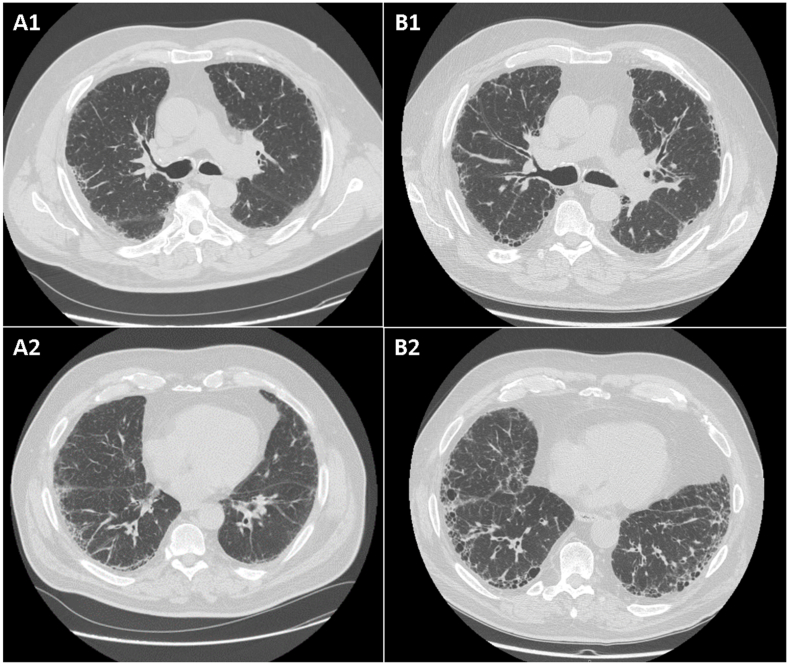
Chest X-ray showed a diffuse thickening of the lung, and a subsequent high-resolution computed tomography (HRCT) was diagnostic for a diffuse ILD with typical UIP pattern: basal and subpleural reticular opacities, honeycombing and traction bronchiectasis, associated with some areas of pleural thickening of the right lung, were recorded (Fig. 1 A).
Fig. 1.
Chest high-resolution computed tomography images of patients 1 (A: at baseline; B: at follow-up).
Pulmonary function tests (PFT) described a mild restrictive ventilatory defect with slight reduction of forced vital capacity (FVC) and normal value of single-breath diffusing capacity of the lung for carbon monoxide (DLCOsb) (FVC 81%, DLCOsb 80%).
With the exception of bilateral velcro crackles at pulmonary clinical evaluation, the patient's physical examination was unremarkable: no arthralgias or arthritis, no Raynaud phenomenon, no sicca syndrome or other signs or symptoms suggestive for connective tissue diseases (CTD) were detected. Schirmer's test was negative.
Routine laboratory examinations and immunological texts, including anti-nuclear antibodies (ANA), rheumatoid factor (RF) and anti-cyclic citrullinated peptide antigen (ACPA) were negative.
After a multidisciplinary discussion including pulmonologist, rheumatologists and radiologists, in April 2014 lung environmental exposures (in particular possible exposure to asbestos) were excluded and a diagnosis of idiopathic pulmonary fibrosis (IPF) was made. The patient started an antifibrotic therapy with pirfenidone 2403 mg daily. Afterwards, the treatment was reduced to 2136 mg/die for symptoms of gastrointestinal intolerance. Dry cough and general clinical conditions improved gradually.
During the following two years, PFT showed an improvement of FVC greater than 10% of the baseline value and a slight decrease of DLCOsb (FVC 94%, DLCOsb 65%). Chest HRCT images showed a stabilization of the lung fibrosis. No oxygen desaturation at 6-min walking test was reported (520 m of walking).
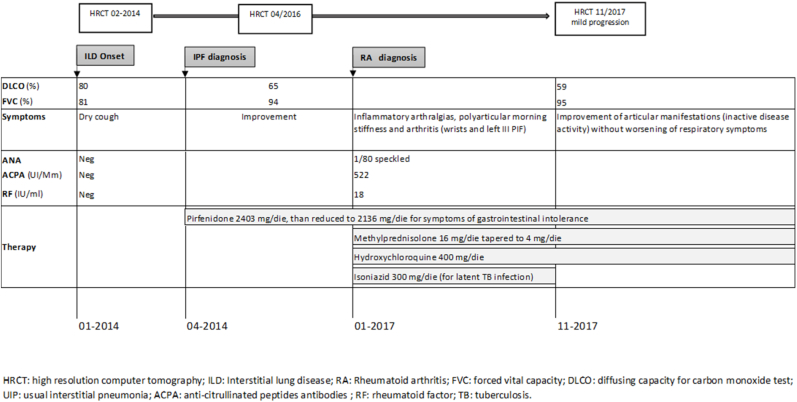
In January 2017, the patient referred to our multidisciplinary outpatient of the university-based Center for Rare Pulmonary Diseases for the occurrence of inflammatory arthralgias, polyarticular morning stiffness and bilateral swelling of the wrists. The ultrasound sonography confirmed an arthritis involving wrists and III proximal interphalangeal joint of the left hand.
Laboratory test were repeated and revealed: increased erythrocyte sedimentation (57 mm) and C-reactive protein (2.05 mg/dl), ANA 1:80 speckled, RF 18 U/ml (normal value < 14) and ACPA 522 U/ml (normal value < 20). Extractable nuclear antigens (ENA), anti-synthetase antibodies and antineutrophil cytoplasmic antibodies were negative; C3 and C4 were normal. Screenings for hepatitis virus C and B were negative, while QuantiFERON TB Gold test was compatible with latent tuberculosis infection, in absence of X-Ray signs of disease.
Patient satisfied the EULAR/ACR classification criteria for RA and treatment with methylprednisolone 16 mg/die, gradually tapered to 4mg/die, and hydroxychloroquine 400 mg/die was started. Considering the clinical, functional and radiological stabilization of the ILD with the use of pirfenidone, the antifibrotic therapy was maintained in association with anti-rheumatic therapy. Finally, a treatment regimen for latent TB infection using isoniazid 300 mg/die was started.
Few months later, the patients experienced an improvement of articular manifestations and reached an inactive disease activity without worsening of respiratory symptoms. In November 2017, a chest HRCT was repeated, showing mild progression of the lung fibrosis, with interstitial subpleural thickening of the lower lung lobes, traction bronchiectasis and multiple cysts (Fig. 1 B). Simultaneously, PFTs confirmed a stabilization of the lung function (FVC 95% and DLCOsb 59%) and no major changes in 6-min walking test were detected (510 m of walking).
Clinical history of patient 1 is summarized in Fig. 2.
Fig. 2.
Clinical case summary of patient 1.
2.2. Patient 2
In 2015, a 69-year-old man referred to the pneumological unit of our Hospital for dyspnea.
He reported a past clinical history of type II diabetes mellitus, hypertension and at least 3 cardiac surgery procedures of coronary artery bypass. He worked as a mail carrier and no environmental exposure were detected during the clinical interview. He was a former smoker (45 pack/years until 1995).
Patient's physical examination revealed fine velcro crackles in bilateral lower lung fields. Routine laboratory examinations and immunological texts, including anti-nuclear and anti-neutrophil cytoplasmic antibodies, RF and ACPA were negative.
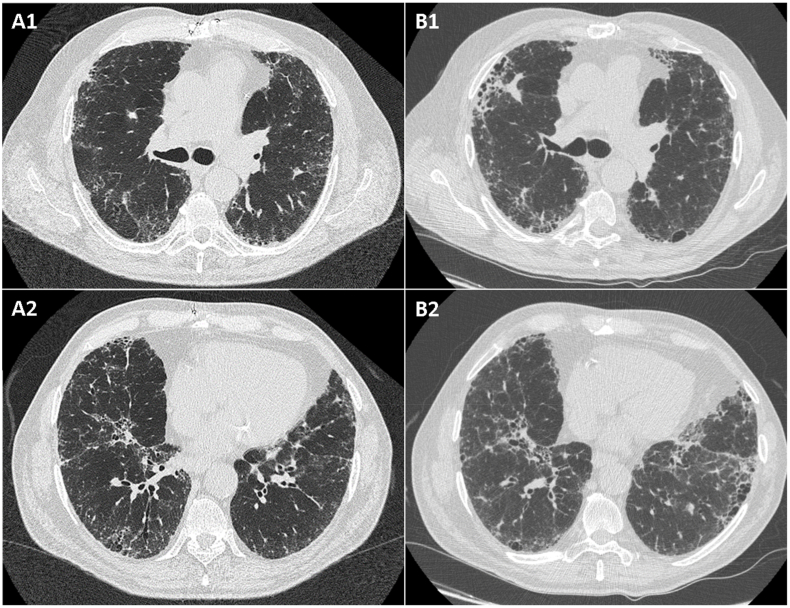
HRCT showed parenchymal consolidation areas, cysts, ground-glass opacities and interlobular septal thickening, as well as bronchiectasis. These abnormalities were consistent with an UIP pattern (Fig. 3 A), and a diagnosis of IPF was made.
Fig. 3.
Chest high-resolution computed tomography images of patients 2 (A: at baseline; B: at follow-up).
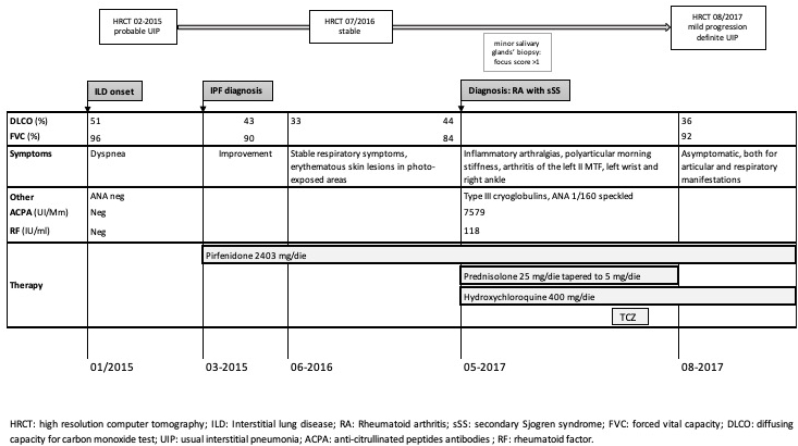
PFT confirmed a mild restrictive ventilatory defect (FVC 96%, TLC 71%) with reduction of DLCOsb (51%). His 6-min walking test was normal (550 m without oxygen desaturation). In march 2015, the patient started pirfenidone 2403 mg/die, without adverse events.
At 6-month follow-up, the patient reported a clinical improvement and PFTs also revealed stability of pulmonary function (FVC 90%, TLC 80%, DLCOsb 43%). An echocardiogram excluded the presence of indirect signs of pulmonary hypertension.
However, at 18-month follow-up, a decrease of DLCOsb was recorded (33%) without worsening of clinical symptoms. The patient also reported erythematous skin lesions in photo-exposed areas of the body, possible side effect of pirfenidone. Clinicians suggest to repeat a chest HRCT and PFTs, they informed the patient about the importance of sun protection during the assumption of pirfenidone, and the antifibrotic treatment was continued. A stabilization of lung function (FVC 84%, TLC 71% and DLCO sb 44%) and of the ILD at chest HRCT was assessed.
In May 2017, for persistent arthralgias and polyarticular morning stiffness, the patient referred to our rheumatologic unit. He reported a past clinical history of inflammatory arthralgias with anecdotal episodes of swelling joints. He denied sicca syndrome, Raynaud's phenomenon, purpuric skin lesions or photosensitivity skin reactions and oral ulcers. Physical articular examination revealed an arthritis of the II metatarsophalangeal joint of the left foot, left wrist and right ankle, confirmed by articular ultrasound.
A positivity for type III cryoglobulins, RF (118 U/l) and ACPA (7579 U/l) was detected. ANA were also positive with a speckled pattern and a title of 1:160, while ENA and anti-Synthetase antibodies were negative.
Schirmer's Test was negative, and no major abnormalities were detected at nailfold videocapillaroscopy. However, minor salivary glands' biopsy showed a lymphocytic sialadenitis with 2 lymphocytic foci (focus score >1 [21]).
A diagnosis of Rheumatoid arthritis and secondary Sjogren syndrome was performed by our rheumatology-pulmonology multidisciplinary team, according to clinical and laboratory data. We started prednisone 25 mg/die, progressively tapered to 5 mg/die, and hydroxychloroquine 400 mg/die. According to the stability of the interstitial pulmonary disease, we also decided to continue pirfenidone.
Few months later, because of an articular exacerbation of the joint disease, tocilizumab 162 mg/week was started and quickly (after 4 weeks) withdrawn for leukopenia till 2400/mmc white cells.
Subsequently, our patient remained asymptomatic, both for articular and respiratory manifestations, despite the withdrawal of corticosteroid therapy. Of interest, corticosteroids were administered only for a few months; although we cannot exclude a synergic effect on the lung, the progression of lung disease preceded the diagnosis of rheumatoid arthritis and the pulmonary function was stable at the time of corticosteroid treatment. A chest HRCT in August 2017 showed a slight progression of the fibrosing ILD with a definite UIP pattern (Fig. 3 B). Nevertheless, no decrease of FVC or DLCO (FVC 92% and DLCO sb 36%) as well as no desaturation at 6-min walking test were reported.
Clinical history of patient 2 is summarized in Fig. 4.
Fig. 4.
Clinical Case summary of patient 2.
3. DISCUSSION and CONCLUSIONS
In absence of randomized controlled trials, the optimal treatment of RA-ILD has not been determined. Treatment of RA-ILD is furtherly complicated by the association between almost all DMARDs and lung toxicity; however, their ability to improve lung function and improve pulmonary symptoms have also been described in anecdotal reports [[22], [23], [24], [25]].
Moreover, treatment of RA-ILD patients with active articular disease should be continued to achieve low disease activity, and the use of any DMARDs to control joint symptoms remains mandatory [26].
RA-ILD shares some similarities with IPF, first of all the predominant UIP pattern and its association with a genic variant of MUC5B [[8], [9], [10],13,14]. It also shows a similar clinical behavior, often with a progressive fibrosing phenotype, and a comparable prognosis and survival [[2], [3], [4], [5], [6], [7],11,12].
These analogies between RA-ILD and IPF may suggest a possible role of antifibrotic therapy in these patients, in order to treat the fibrotic process, improve outcomes and reduce lung disease progression [27,28]. Antifibrotic therapy recently showed some efficacy in other ILD related to rheumatologic disorders, such as systemic sclerosis [29].
Moreover, some Authors questioning if new trajectories in the treatment of ILD will focus to treat the disease or to treat the underlying pattern [27].
In this regard, antifibrotic drugs are recently supposed to have relevance across various ILD subtypes, not only in UIP pattern (INBUILD). The INBUILD study assessed the efficacy and safety of nintedanib versus placebo in 663 patients with a diagnosis of ILD other than IPF, including RA, despite the radiological or histological pattern [30].
Pirfenidone is an oral antifibrotic and anti-inflammatory drug approved for the treatment of mild to moderate IPF. It demonstrated its efficacy in reducing the rate of absolute decline of percent predicted FVC and the decline in 6MWT distance from baseline, and also in reducing the risk of all-cause mortality [[31], [32], [33]].
Interestingly, Pirfenidone reduces the levels of IL6 and TNF-alpha, both key-cytokines in RA pathogenesis [34] and recently, an inhibitory effect on transition from fibroblast to myofibroblast has been also showed in RA-ILD [35].
Therefore, a potential therapeutic role of pirfenidone in RA-LD is supposable.
A number of trials are planned or ongoing to assess the efficacy and safety of pirfenidone in the treatment of fibrosing ILDs other than IPF, including CTD-associated lung fibrosis, unclassifiable PF-ILD, fibrotic idiopathic NSIP, IPAF [36,37], pulmonary fibrotic sarcoidosis and ILD related to ANCA antibodies or dermatomyositis [[38], [39], [40]].
Recently, in a double-blind, randomized, placebo-controlled, phase 2 trial, pirfenidone showed an acceptable safety and tolerability profile in 253 patients with unclassifiable progressive fibrosing ILD. Over 24 weeks predicted mean change in FVC was lower in patients treated with pirfenidone compared to placebo (p = 0.002). Compared with the placebo group, patients treated with pirfenidone were less likely to have a decline in FVC of more than 5% (p = 0.001) or more than 10% (p = 0.011) [37].
At last, to underline the increasing interest on other possible therapeutic potentiality of this drugs, pirfenidone is currently under investigation as a therapeutic option for patients with RA-ILD (TRAIL1) [20].
Nowadays, with the exception of one case report describing the successful use of nintedanib, there are no published data supporting the use of antifibrotic therapies in RA-ILD [41].
Our case report describes for the first time two patients with a diagnosis of RA-ILD treated with pirfenidone in association with hydroxychloroquine. The association of these two drugs allowed a stabilization of both articular and lung manifestations, without adverse events.
When considering therapeutic options for RA-ILD, both pulmonary and extra-thoracic disease manifestations and degrees of activity should be assessed and taken into consideration.
Further, considering both fibrotic and inflammatory components of this systemic disease, the combination of immunosuppressive and antifibrotic treatment can potentially be the future approach to this spectrum of the disease.
Moreover, developing guideline treatment for RA-ILD has proven challenging given the undefined natural history of the disease, the unknown real prevalence of asymptomatic/subclinical RA-ILD, the lack of proven risk factors and biomarkers predictive of disease progression.
Future prospective research might change RA-ILD management, moving to a more personalized approach based on the identification of different phenotypes of the disease (underlying pattern, clinical behavior, genetic and biomarkers risk factors, etc.).
Anyhow, the heterogeneity in disease presentation, the multiple manifestation that may be present, and the broad range of disease severity, makes it difficult to speculate about one milestone therapeutic strategy, so a multidisciplinary approach including rheumatologists, pulmonologists, and other health care providers is essential [42,43].
Multidisciplinary cooperation may be advisable also for developing further prospective studies to clarify the best treatment approach in RA-ILD patients.
Efforts to develop a research network comprising dedicated centers with both pneumological and rheumatological expertise in RA-ILD also should be made, in order to fill the gap of our knowledge of such challenging disease.
Patient consent
The patients signed a written informed consent allowing us to write these Case reports.
CRediT authorship contribution statement
Giulia Cassone: Conceptualization, Investigation, Data curation, Project administration, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Marco Sebastiani: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Caterina Vacchi: Data curation, Validation, Visualization, Writing - review & editing. Stefania Cerri: Data curation, Validation, Visualization, Writing - review & editing. Carlo Salvarani: Supervision, Validation, Visualization, Writing - review & editing. Andreina Manfredi: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare they have no conflicts of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmcr.2020.101051.
Contributor Information
Giulia Cassone, Email: giulia.cassone@unimore.it.
Marco Sebastiani, Email: marco.sebastiani@unimore.it.
Caterina Vacchi, Email: caterina.vacchi@unimore.it.
Stefania Cerri, Email: stefania.cerri@unimore.it.
Carlo Salvarani, Email: carlo.salvarani@unimore.it.
Andreina Manfredi, Email: andreina.manfredi@gmail.com.
Appendix A. Supplementary data
References
- 1.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.Prete M., Racanelli V., Digiglio L., Vacca A., Dammacco F., Perosa F. Extra-articular manifestations of rheumatoid arthritis: an update. Autoimmun. Rev. 2011;11:123–131. doi: 10.1016/j.autrev.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Bongartz T., Nannini C., Medina-Velasquez Y., Achenbach S.J., Crowson C.S., Ryu J.H. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62:1583–1591. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson A.L., Swigris J.J., Sprunger D.B., Fischer A., Fernandez-Perez E.R., Solomon J. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am. J. Respir. Crit. Care Med. 2011;183:372–378. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raimundo K., Solomon J.J., Olson A.L., Kong A.M., Cole A.L., Fischer A. Rheumatoid arthritis-interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J. Rheumatol. 2019;46:360–369. doi: 10.3899/jrheum.171315. [DOI] [PubMed] [Google Scholar]
- 6.Hyldgaard C., Hilberg O., Pedersen A.B., Ulrichsen S.P., Løkke A., Bendstrup E. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann. Rheum. Dis. 2017;76:1700–1706. doi: 10.1136/annrheumdis-2017-211138. [DOI] [PubMed] [Google Scholar]
- 7.Spagnolo P., Lee J.S., Sverzellati N., Rossi G., Cottin V. The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheum. 2018;70:1544–1554. doi: 10.1002/art.40574. [DOI] [PubMed] [Google Scholar]
- 8.Balbir-Gurman A., Guralnik L., Yigla M., Braun-Moscovici Y., Hardak E. Imaging aspects of interstitial lung disease in patients with rheumatoid arthritis: literature review. Autoimmun. Rev. 2018;17:87–93. doi: 10.1016/j.autrev.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Nurmi H.M., Purokivi M.K., Kärkkäinen M.S., Kettunen H.P., Selander T.A., Kaarteenaho R.L. Variable course of disease of rheumatoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm. Med. 2016;16:107. doi: 10.1186/s12890-016-0269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N., Varghese J., England B.R., Solomon J.J., Michaud K., Mikuls T.R. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: a systematic literature review and meta-analysis. Semin. Arthritis Rheum. 2019;49(3):358–365. doi: 10.1016/j.semarthrit.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Paulin F., Doyle T.J., Fletcher E.A., Ascherman D.P., Rosas I.O. Rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis: shared mechanistic and phenotypic traits suggest overlapping disease mechanisms. Rev. Invest. Clin. 2015;67:280–286. [PMC free article] [PubMed] [Google Scholar]
- 12.Kolb M., Vašáková M. The natural history of progressive fibrosing interstitial lung diseases. Respir. Res. 2019;20:57. doi: 10.1186/s12931-019-1022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seibold M.A., Wise A.L., Speer M.C., Steele M.P., Brown K.K., Loyd J.E. A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juge P.A., Lee J.S., Ebstein E., Furukawa H., Dobrinskikh E., Gazal S. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N. Engl. J. Med. 2018;379:2209–2219. doi: 10.1056/NEJMoa1801562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton C.A., Oldham J.M., Ley B., Anand V., Adegunsoye A., Liu G. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur. Respir. J. 2019:53–54. doi: 10.1183/13993003.01641-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolters P.J. A recurring theme in pulmonary fibrosis genetics. Eur. Respir. J. 2017;49 doi: 10.1183/13993003.00545-2017. [DOI] [PubMed] [Google Scholar]
- 17.Doyle T.J., Patel A.S., Hatabu H., Nishino M., Wu G., Osorio J.C. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am. J. Respir. Crit. Care Med. 2015;191:1403–1412. doi: 10.1164/rccm.201411-1950OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J., Doyle T.J., Liu Y., Aggarwal R., Wang X., Shi Y. Biomarkers of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2015;67:28–38. doi: 10.1002/art.38904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghu G., Rochwerg B., Zhang Y., Garcia C.A., Azuma A., Behr J. An Official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am. J. Respir. Crit. Care Med. 2015;192:3–19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 20.Solomon J.J., Danoff S.K., Goldberg H.J., Woodhead F., Kolb M., Chambers D.C. The design and rationale of the Trail1 trial: a randomized double-blind phase 2 clinical trial of pirfenidone in rheumatoid arthritis-associated interstitial lung disease. Adv. Ther. 2019;36:3279–3287. doi: 10.1007/s12325-019-01086-2. [DOI] [PubMed] [Google Scholar]
- 21.Baldini C., Talarico R., Tzioufas A.G., Bombardieri S. Classification criteria for Sjogren's syndrome: a critical review. J. Autoimmun. 2012;39:9–14. doi: 10.1016/j.jaut.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Olivas-Flores Eva M., Bonilla-Lara David, Gamez-Nava Jorge I., Rocha-Muñoz Alberto D., Gonzalez-Lopez Laura. Interstitial lung disease in rheumatoid arthritis: current concepts in pathogenesis, diagnosis and therapeutics. World J. Rheumatol. 2015;5:1–22. [Google Scholar]
- 23.Roubille C., Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin. Arthritis Rheum. 2014;43:613–626. doi: 10.1016/j.semarthrit.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Rojas-Serrano J., González-Velásquez E., Mejía M., Sánchez-Rodríguez A., Carrillo G. Interstitial lung disease related to rheumatoid arthritis: evolution after treatment. Reumatol. Clínica. 2012;8:68–71. doi: 10.1016/j.reuma.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Jani M., Hirani N., Matteson E.L., Dixon W.G. The safety of biologic therapies in RA associated interstitial lung disease. Nat. Rev. Rheumatol. 2014;10:284–294. doi: 10.1038/nrrheum.2013.197. [DOI] [PubMed] [Google Scholar]
- 26.Smolen J.S., Landewé R., Bijlsma J., Burmester G., Chatzidionysiou K., Dougados M. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017;76:960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 27.Morisset J., Lee J.S. New trajectories in the treatment of interstitial lung disease: treat the disease or treat the underlying pattern? Curr. Opin. Pulm. Med. 2019;25:442–449. doi: 10.1097/MCP.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richeldi L., Varone F., Bergna M., de Andrade J., Falk J., Hallowell R. Pharmacological management of progressive-fibrosing interstitial lung diseases: a review of the current evidence. Eur. Respir. Rev. 2018;27:150. doi: 10.1183/16000617.0074-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Distler O., Highland K.B., Gahlemann M., Azuma A., Fischer A., Mayes M.D. For the SENSCIS trial Investigators.Nintedanib for systemic sclerosis–associated interstitial lung disease. N. Engl. J. Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 30.Flaherty K.R., Wells A.U., Cottin V., Devaraj A., Walsh S.L.F., Inoue Y. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 31.Noble P.W., Albera C., Bradford W.Z., Costabel U., Glassberg M.K., Kardatzke D. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 32.King T.E., Jr., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 33.Cottin V., Maher T. Long-term clinical and real-world experience with pirfenidone in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2015;24:545. doi: 10.1183/09059180.00011514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaefer C.J., Ruhrmund D.W., Pan L., Seiwert S.D., Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur. Respir. Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C., Lin H., Zhang X. Inhibitory effects of pirfenidone on fibroblast to myofibroblast transition in rheumatoid arthritis-associated interstitial lung disease via the downregulation of activating transcription factor 3 (ATF3) Int. Immunopharm. 2019;74 doi: 10.1016/j.intimp.2019.105700. [DOI] [PubMed] [Google Scholar]
- 36.Behr J., Neuser P., Prasse A., Kreuter M., Rabe K., Schade-Brittinger C. Exploring efficacy and safety of oral pirfenidone for progressive, non-IPF lung fibrosis (RELIEF) – a randomized, double-blind, placebo-controlled, parallel group, multi-center, phase II trial. BMC Pulm. Med. 2017;17:122. doi: 10.1186/s12890-017-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maher T.M., Corte T.J., Fischer A., Kreuter M., Lederer D.J., Molina-Molina M. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2019;S2213–2600:30341–30348. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 38.London J., Ait el Ghaz S. Pilot study of pirfenidone in pulmonary fibrosis with anti-myeloperoxydase antibodies (PIRFENIVAS) https://clinicaltrials.gov/ct2/show/NCT03385668 Date last updated: March 19, 2018. Date last accessed.
- 39.Baughman R.P., Reeves R. Pirfenidone for progressive fibrotic sarcoidosis (PirFS) https://clinicaltrials.gov/ct2/show/NCT03260556 Date last updated:
- 40.Li T., Guo L., Chen Z., Gu L., Sun F., Tan X. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci. Rep. 2016;6:33226. doi: 10.1038/srep33226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakuwa T., Izumi S., Sakamoto K., Suzuki T., Iikura M., Sugiyama H. A successful treatment of rheumatoid arthritis-related interstitial pneumonia with nintedanib. Respir. Med.Case Rep. 2018;26:50–52. doi: 10.1016/j.rmcr.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer A., Richeldi L. Cross-disciplinary collaboration in connective tissue disease-related lung disease. Semin. Respir. Crit. Care Med. 2014;35:159–165. doi: 10.1055/s-0034-1371530. [DOI] [PubMed] [Google Scholar]
- 43.Walsh S.L.F., Wells A.U., Desai S.R., Poletti V., Piciucchi S., Dubini A. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respir Med. 2016;4:557–565. doi: 10.1016/S2213-2600(16)30033-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.