Abstract
Children that have experienced psychosocial neglect display impairments in self-monitoring and controlling their behavior (cognitive control) and are at broad, transdiagnostic risk for psychopathology. However, the neural underpinnings of such effects remain unclear. Event-related mediofrontal theta oscillations reflect a neural process supporting cognitive control that may relate to transdiagnostic psychopathology risk. Recent work demonstrates reduced mediofrontal theta in rodent models of neglect; however, similar findings have not been reported in humans. Here, 136 children reared in Romanian institutions were randomly assigned to either a high-quality foster care intervention and placed with families or remained in institutions; 72 never-institutionalized children served as a comparison group. The intervention ended at 54 months; event-related mediofrontal theta and psychopathology were assessed at 12- and 16-year follow-up assessments. Institutional rearing (neglect) predicted reduced mediofrontal theta by age 16, which was linked to heightened transdiagnostic risk for psychopathology (P factor); no specific associations with internalizing/externalizing factors were present once transdiagnostic risk was accounted for. Earlier placement into foster care yielded greater mediofrontal activity by age 16. Moreover, foster care placement was associated with the developmental trajectory of mediofrontal theta across the adolescent period (ages 12–16), which was, in turn, associated with greater reductions in transdiagnostic risk across this same period. These data reflect the first experimental evidence that the development of mediofrontal theta is impacted by removal from situations of neglect in humans, and further characterizes the importance of studying developmental change in mediofrontal theta during the adolescent period.
Keywords: Cognitive control, Mediofrontal cortex, Psychopathology, Transdiagnostic risk, Theta oscillations, Neglect
1. Introduction
In the majority of countries, the most common form of child maltreatment is neglect (Sedlak et al., 2010; Sheridan and McLaughlin, 2014), in which caregivers fail to provide basic physical and/or socio-emotional needs of children (Leeb et al., 2008). Neglect confers significant risk for psychopathology (Wade et al., 2018), though relatively little is known about the neurobiological processes linking neglect to psychopathology. Based on behavioral and indirect neural assessments (e.g. for a review, see: Troller‐Renfree et al., 2018), neglect is also known to impair cognitive control, which reflects the ability to monitor and control one’s behavior (also see: Wade et al., 2018, 2019a, b). Mediofrontal theta (4−8 Hz) oscillations reflect a more direct readout of a neural mechanism supporting cognitive control (Cavanagh and Frank, 2014; Narayanan et al., 2013), and recent animal work demonstrates mediofrontal theta impairments in rodent models of neglect. However, similar evidence in humans is lacking.
Rodents experiencing neglect (repeated maternal separation) during the preweaning period exhibit reductions in mediofrontal theta power during the juvenile period (Reincke and Hanganu-Opatz, 2017). During the preweaning period, local field potential (LFP) recordings from depth electrodes within mediofrontal cortex exhibit event-related reductions in theta power in response to periods of maternal separation (Courtiol et al., 2018; Sarro et al., 2014). Moreover, there is evidence for a sensitive period in the rodent, as maternal separation is less likely to influence mediofrontal theta if separations occur at older compared to younger ages (Sarro et al., 2014). Event-related changes in theta power are mediated by serotoninergic signaling (Courtiol et al., 2018), in line with work demonstrating that neglect alters serotonergic and dopaminergic innervation of mediofrontal cortex (Braun et al., 1999). Effects of neglect on these neurotransmitter systems are notable, as these systems also underlie human cognitive control ability (Jocham and Ullsperger, 2009) and have further been linked to psychiatric disorders as well (Szabo et al., 2004).
In humans, neglect predicts impaired performance on tasks requiring cognitive control (Bauer et al., 2009; Bruce et al., 2009; Hostinar et al., 2012; Pollak et al., 2010; Sheridan et al., 2017) and associated deficits in indirect markers of cognitive control (Lamm et al., 2018; Loman et al., 2013; McDermott et al., 2012, 2013; Troller-Renfree et al., 2016). Similarly, neglect leads to reduced grey matter in a number of cortical regions associated with cognitive control (Hodel et al., 2015) including the mediofrontal cortex (McLaughlin et al., 2014). However, existing work has not tested whether neglect impacts a more direct readout of a mechanism supporting cognitive control in humans (i.e. mediofrontal theta power). A common example of cognitive control is the finding that individuals sometimes increase task performance (accuracy rates) following errors (for a review, see: Danielmeier and Ullsperger, 2011). In such situations, errors of commission are thought to signal the need for an increase in cognitive control, with increases in accuracy on subsequent trials (post-error accuracy) reflecting the allocation of control (Danielmeier and Ullsperger, 2011). In line with the notion that errors signal a need for increases in control, errors of commission are also associated with increased theta power at electrodes over mediofrontal cortex (Buzzell et al., 2019; Cavanagh et al., 2009). These low-frequency theta oscillations are thought to serve as a signal that control is required, as well as to integrate information across brain regions, necessary for cognitive control (Cavanagh and Frank, 2014; Verguts, 2017). Similar increases in mediofrontal theta power are observed in response to unexpected stimuli or negative events, consistent with the prevailing theory that mediofrontal theta power and synchrony are increased in situations that require “being pulled out of autopilot” to increase control (Cavanagh and Frank, 2014; Ullsperger et al., 2014; Verguts, 2017). Additionally, the causal role of mediofrontal theta oscillations in synchronizing brain regions to support cognitive control has been demonstrated in both humans and rodents (Herrmann et al., 2016; Narayanan et al., 2013). A homolog of rodent mediofrontal theta can be non-invasively recorded via EEG scalp electrodes over mediofrontal cortex in humans (Narayanan et al., 2013), allowing a test of whether this oscillatory mechanism is similarly disrupted by neglect.
It is worth noting that while no prior work has investigated relations between neglect and event-related mediofrontal theta in humans, prior work has investigated how neglect impacts relative theta power of the human brain at rest (Debnath et al., 2020; Hevia-Orozco and Sanz-Martin, 2018; Marshall et al., 2008; McLaughlin et al., 2010; Tarullo et al., 2011; Vanderwert et al., 2010, 2016). Moreover, variations in relative theta power have most commonly been linked to disorders such as ADHD (For a review, see: Barry et al., 2003). However, relative theta power reflects a qualitatively different neural process, which is anatomically and functionally distinct from event-related mediofrontal theta (Finnigan and Robertson, 2011; Miskovic et al., 2015; Uhlhaas et al., 2010; Whitford et al., 2007). While interpretations of relative theta power recorded from the brain at rest remain unclear, event-related mediofrontal theta power has a well-defined role as a neural mechanism supporting cognitive control across mammalian species (Cavanagh and Frank, 2014), including rodents (Narayanan et al., 2013), non-human primates (Tsujimoto et al., 2006; Womelsdorf et al., 2010) and humans (Cavanagh et al., 2009).
Prior work has linked cognitive control dysfunction to specific forms of psychopathology in the context of neglect (McDermott et al., 2013; Troller-Renfree et al., 2016). Additionally, recent work demonstrates that neglect confers broad risk for psychopathology that cuts across diagnostic boundaries (Wade et al., 2018). Nevertheless, the neural processes underlying broad, transdiagnostic associations between neglect and psychopathology are largely unknown. Given work suggesting that deficits in cognitive control underlie transdiagnostic risk via effects on the regulation of emotion and cognition (Kohn et al., 2014; McTeague et al., 2016, 2017; White et al., 2017), and that mediofrontal theta reflects a more direct readout of cognitive control (Cavanagh and Frank, 2014), we propose that variation in event-related mediofrontal theta might be associated with this transdiagnostic risk.
In studying the impact of early neglect on transdiagnostic risk, Wade et al. (2018) reported on a so called “sleeper effect”, in which the benefits of removal from neglect were not fully apparent until much later in development. Specifically, removal from neglect early in life did not yield differences in transdiagnostic risk for psychopathology by mid-to-late childhood (age 8), but instead impacted the developmental trajectory of such risk across the adolescent period. Removal from neglect yielded greater decreases in transdiagnostic risk across the adolescent period, such that significant differences became apparent by age 16 (Wade et al., 2018). Therefore, if our proposal that variation in event-related mediofrontal theta is associated with transdiagnostic risk is correct, then we should observe a similar “sleeper effect” at the neural level, whereby early neglect impacts the development of mediofrontal theta across adolescence. Moreover, changes in mediofrontal theta across adolescence should inversely predict changes in transdiagnostic risk for psychopathology across the same developmental window.
In order to test associations among neglect, mediofrontal theta, and transdiagnostic psychopathology risk, we leveraged data from the Bucharest Early Intervention Project (BEIP; Zeanah et al., 2003), a longitudinal and randomized control trial (RCT) of a high-quality foster care intervention for children raised in depriving institutions. Given prior work demonstrating that neglect leads to impaired cognitive control in humans (Loman et al., 2013; McDermott et al., 2013; Pollak et al., 2010; Troller-Renfree et al., 2016) and reduced mediofrontal theta power in rodents (Reincke and Hanganu-Opatz, 2017), we hypothesized that randomization to a high-quality foster care intervention in early childhood would yield later increases in mediofrontal theta power in adolescence (relative to a comparison group who remained in institutions). Further, we hypothesized that removal of children from depriving environments at younger ages would mitigate the deleterious effect of early neglect on the developing brain, consistent with a sensitive period suggested by animal work (Sarro et al., 2014). We also tested whether effects of the foster care intervention on cognitive control were present in post-error accuracy. In order to test each of these hypotheses, our analyses focused on EEG and psychopathology data from the most recent (age 16) assessment of the BEIP sample. However, we also hypothesized that disruptions to mediofrontal theta would link early neglect to transdiagnostic risk for psychopathology. For such analyses, we examined mediofrontal theta and psychopathology concurrently at age 16, but also tested whether mediofrontal theta development across adolescence (ages 12–16) predicted changes in transdiagnostic risk across this same period.
2. Materials and methods
2.1. Participants and study design
Participants were from the BEIP study, which began in the Spring of 2001 (Zeanah et al., 2003). One-hundred thirty-six children aged 6–31 months (M age = 21.6 months) residing for at least half their life in Romanian institutions were assessed; half of these children (n = 68, 33 boys) were randomly assigned to receive “care-as-usual” (CAUG), which here meant remaining in the institutions, and the other half (n = 68, 34 boys) were randomly assigned to be placed into high-quality foster-care with families that received training on proper care (foster care group; FCG). The investigators achieved randomization by drawing names from a hat; given the nature of the study, masking of group assignment to children, caregivers, and investigators was not possible (i.e., individuals were aware of children’s placements). By design, the RCT ended at 54 months, however, these two groups were then followed longitudinally, with the current study presenting data from the 12- and 16-year assessments of these groups, when behavioral, EEG and mental health symptom measures were collected (see supplement for the complete experimental protocol). Throughout the duration of the study, a non-interference policy was followed; therefore, although most CAUG children remained in institutional care through 54 months, many were removed from institutional care at some point by the 16-year assessment. A group of age- and gender-matched youth that had never experienced prior institutionalization (n = 72) were additionally recruited from Bucharest, Romania, in order to serve as a comparison group (never-institutionalized group; NIG); similar measures assessed for this group at age 16 are also reported in the current study. See the supplement for a complete flow diagram of participant enrollment/exclusion and study design.
2.1.1. RCT groups (CAUG and FCG)
Primary analyses of 16-year mediofrontal theta in the current report utilize an intent-to-treat analytic approach, focusing on 40 CAUG (M age = 16.72, sd = 0.44; 21 boys; 19 Romanian, 16 Roma, 5 other/unknown) and 40 FCG (M age = 16.64, sd = 0.61; 20 boys; 24 Romanian, 10 Roma, 6 other/unknown) who had sufficient behavioral and EEG data. An additional 4 CAUG and 5 FCG, were analyzed for analyses of 16-year behavioral data as these participants either did not have EEG data collected or the EEG contained too few trials per condition following preprocessing. Analyses within the FCG to test whether age-of-placement impacted 16-year mediofrontal theta and post-error accuracy were performed for all participants on which placement age was available (one participant within the FCG intent-to-treat group was adopted prior to foster-care placement and was therefore not included in this additional analysis). For analyses involving measures of psychopathology at age 16, 49 CAUG and 52 FCG had available psychopathology data for analyses. For modeling of mediofrontal theta and psychopathology development across adolescence, 43 CAUG and 45 FCG had sufficient behavioral and EEG data at the 12-year visit; 56 CAUG and 56 FCG had psychopathology data at the 12-year visit.
2.1.2. Comparison group (NIG)
In addition to the intent-to-treat analyses (comparing CAUG and FCG), 44 NIG (M age = 17.06, sd = .65; 15 boys; 39 Romanian, 2 Roma, 3 other/unknown) with sufficient behavioral and EEG data at age 16, were analyzed for comparison with each of the intent-to-treat groups. An additional 4 NIG were analyzed for analyses of behavioral data as these participants either did not have EEG data collected or the EEG contained too few trials per condition following preprocessing. For analyses involving measures of psychopathology, 48 NIG had available psychopathology data for analyses at age 16. For modeling of mediofrontal theta development across adolescence, 44 NIG had sufficient behavioral and EEG data at the 12-year visit.
2.1.3. Ethical considerations
For complete details of the BEIP study design and implementation see Zeanah and colleagues 2003. Care was taken to ensure ethical integrity of this study, with IRB approval from Boston Children’s Hospital protocol number 10-04-0185; University of Maryland relied on this protocol and Tulane University protocol number 196,018. Parents or guardians provided consent at each assessment and children assented to participate in the reported 12- and 16-year visits and to each procedure. For a more complete discussion of the ethical aspects of this study, see prior publications e.g., Miller, 2009; Zeanah et al., 2012).
2.2. Go/Nogo task
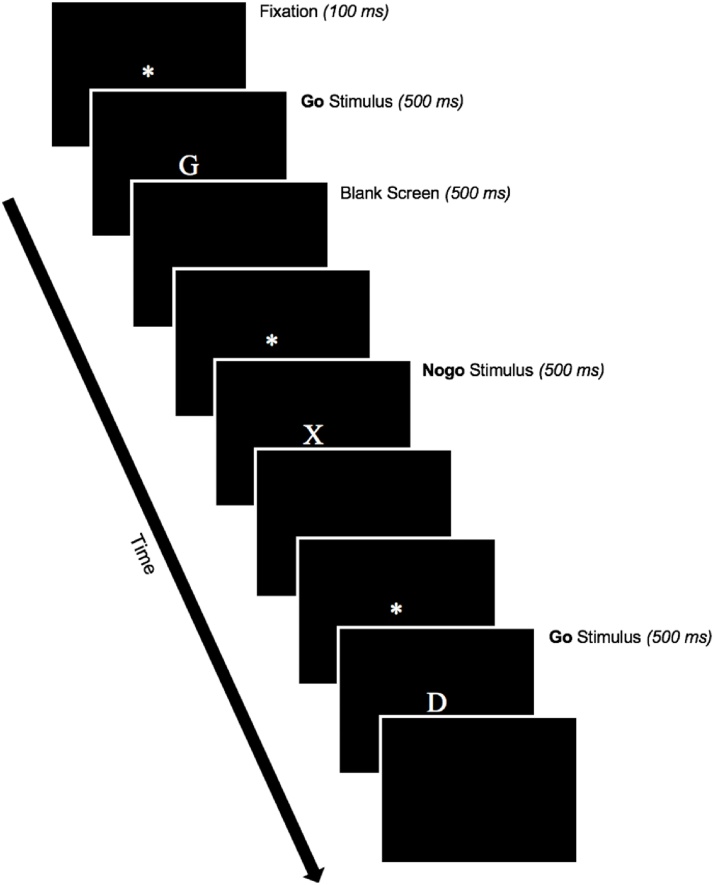
At both the 12- and 16-year visits, participants completed a modified go/nogo task (Lamm et al., 2018) in which responses to frequently presented go stimuli (any letter other than “X”) had to be made, while withholding responses to infrequently presented nogo stimuli (the letter “X”); the letter “K” was never presented due to its perceptual similarity to the nogo letter “X”. On each trial, a white fixation asterisk was presented for 100 ms on a solid black background, followed by an uppercase letter (white, 170 pt., Times New Roman font) for 500 ms, and then a 500 ms blank screen; see Fig. 1). Participants were required to respond within 1000 ms via an EGI response pad (Electrical Geodesic, Inc., Eugene, OR). Following a practice block of 10 trials, participants completed two 140-trial experimental blocks that consisted of 70 % go and 30 % nogo stimuli. Go and nogo stimuli were presented in a pseudo-random order, such that all nogo trials were preceded by at least one go trial. Additionally, each block of 140 trials was initiated by a stream of an additional 20 consecutive go trials in order to build up a prepotent response tendency towards go stimuli. The go/nogo task was presented on a 17” monitor using E-Prime software (Psychological Software Tools, Pittsburgh, PA). Note that a go/nogo task was also completed at the 8-year visit (McDermott et al., 2012), however, these data are not included due to differences task parameters and the EEG system employed.
Fig. 1.
Go/nogo task. On each trial, a fixation asterisk was presented for 100 ms, followed by an uppercase letter for 500 ms, and then a 500 ms blank screen. Participants were required to respond within 1000 ms to frequently presented “go” stimuli (any letter other than “X”) and withhold responses to infrequently presented “nogo” stimuli (the letter “X”). Go/nogo stimuli were presented in a 70/30 ratio in a pseudo-random order, with all nogo trials preceded by at least one nogo trial; each experimental block was initiated by a stream of an additional 20 consecutive go trials in order to build up a prepotent response tendency towards go stimuli.
2.3. Assessment of transdiagnostic risk for psychopathology
Caregivers and teachers completed the MacArthur Health and Behavior Questionnaire (HBQ), which provides dimensional assessment of physical and mental health in youth (Luby et al., 2002). Consistent with recent transdiagnostic formulations of general psychopathology (Caspi et al., 2014), we used the HBQ to assess general psychopathology by employing a latent bifactor model in which a single factor captured the shared variance across domains of psychopathology (i.e., “P factor”), and two additional factors captured variance in internalizing and externalizing symptoms that were not accounted for by the general factor (for complete details of this approach, see Wade et al., 2018). The factor scores for general psychopathology, internalizing, and externalizing described by Wade et al. (2018) were saved and used as manifest variables to assess relations with mediofrontal theta. In the supplement, we present a more traditional set of analyses that employ the HBQ Internalizing, Externalizing, and ADHD composite variables. These analyses yield results that are consistent with those presented in the main text.
2.4. EEG acquisition and preprocessing
At ages 12 and 16, EEG data were collected via a 64-channel HydroCel Geodesic Sensor Net and EGI software (Electrical Geodesic, Inc., Eugene, OR). All EEG analyses were performed using the EEGLAB toolbox (Delorme and Makeig 2004), custom MATLAB scripts (The MathWorks, Natick, MA) and additional MATLAB scripts provided by other researchers (Bernat et al., 2005). Electrode impedances were lowered to < 50 kΩ prior to beginning recording, given that a high input-impedance system was used. Online, data were sampled at 250 Hz and referenced to the vertex. Following acquisition, a systematic marker offset of 18 ms was corrected for the EGI system. Data were high-pass filtered at .3 Hz; a low- pass filter with a 39 Hz passband and 49 Hz stopband was used. FAST tools (Nolan et al., 2010) were employed to identify and remove bad channels. To identify and remove artifacts, ICA decomposition was run on a copy of the dataset with an additional 1 Hz high-pass filter (Viola et al., 2010). The 1 Hz filtered data set was epoched into arbitrary 1 s epochs; noisy epochs were detected and removed if amplitude was +/- 1000 u V or power within the 20-40hz band (after Fourier analysis) was greater than 30 dB. Further, if a channel led to > 20 % of data being rejected, this channel was instead rejected. ICA was run on the 1 Hz high-pass filtered dataset and ICA weights were copied back to the original (continuous) .3 Hz high-pass filtered dataset (for details of this approach, see Viola et al., 2010); all subsequent processing was performed on the .3 Hz high-pass filtered dataset. ICA components capturing artifacts (e.g. blinks, saccades) were automatically detected via the ADJUST toolbox (Mognon et al., 2011), followed by manual inspection of ICA components, before being subtracted from the data.
EEG data were epoched to the response markers from -1000 to 2000 ms and baseline corrected using the -400 to −200 ms period preceding response. A final rejection of +/- 125 u V was used to identify and remove bad epochs in the data that might have been missed by other methods; if > 20 % of the data were rejected, the channel was rejected instead. All missing channels were interpolated using a spherical spline interpolation and then referenced to the average of all electrodes. Given a focus on theta band activity, data were down-sampled to 32 Hz to improve computational speed with no loss to the signal of interest (i.e. theta = ∼4−8 Hz; Nyquist =16 Hz).
Deciding how many clean EEG trials each participant should have, in order to be included in error-related analyses, involves balancing reliability of the EEG signal on the one hand, and risk of creating a biased sample on the other. Creating a biased sample through participant exclusion is particularly problematic within the context of an RCT follow-up and/or a unique and valuable sample. Moreover, simulation studies have yet to identify the optimal number of trials necessary for calculating reliable error-related theta signals. However, two independent simulation studies of the associated “error-related negativity” (ERN), a time domain EEG signal that theta is known to contribute substantially to (Trujillo and Allen, 2007), suggest that either 4–6 (Steele et al., 2016) or 6–8 (Pontifex et al., 2010) trials are needed to identify a reliable ERN. Therefore, we initially conducted analyses of the age 16 data utilizing an inclusion criterion of 4 trials to maximize participant inclusion and guard against a biased sample. Subsequently, we re-ran all analyses after removing participants (1 CAUG, 2 FCG, 2 NIG) with less than 6 trials per condition and found qualitatively similar results for all reported effects. Given that all statistical analyses yielded qualitatively similar results regardless of whether a 4 or 6 trial threshold was used, the main text reports results reflecting the more conservative inclusion threshold of 6 trials, and the supplement reports the same analyses when using the more liberal inclusion threshold of 4 trials.
As part of a standard preprocessing pipeline of time-frequency data that allows meeting additional assumptions necessary for synchrony-based analyses, a subsampling approach was implemented (Buzzell et al., 2018). However, given that the subsampling procedure was conducted only as a preprocessing step for analyses of synchrony (not reported here) and do not serve to improve the reported power-based analyses, details of the subsampling procedure are described in the supplement.
2.5. Time-frequency and principle components analysis
For the age 16 data, we employed a multi-step procedure to optimize extraction of mediofrontal theta oscillations associated with cognitive control. First, we employed Cohen’s class reduced interference distributions (RID), which yield superior time-frequency resolution (Bernat et al., 2005), to decompose a time- frequency representation of response-locked average power; delta band activity was filtered out using a 2 Hz high-pass filter prior to TF decomposition to isolate theta activity. Next, we subjected the average power time-frequency surface to principal components analysis (TF-PCA; Bernat et al., 2005) to isolate separate sources of theta activity peri-response. Investigation of the scree plot suggested a 2-factor solution described the data best. This 2-factor solution identified a clear mediofrontal theta band factor maximal immediately following the response, consistent with prior work investigating post-error theta (Cavanagh et al., 2009). After identifying the mediofrontal theta band factor using the average power time-frequency surface, we applied these factor loadings to a time-frequency decomposition of total power, again using Cohen’s Class RID and pre- filtering out delta. Identifying factor loadings first within the TF surface for average power improves separation of TF events; applying these loadings to a TF decomposition of total power incorporates both phase-locked and non-phase-locked data (Cohen 2014), the most commonly employed metric for studying mediofrontal theta associated with cognitive control (Cohen 2014). All subsequent analyses and references to “theta power” in the text refer to the total power measure weighted by the average power TF-PCA loading. For analyses and plotting, mediofrontal theta power was averaged within each condition of interest for a cluster of electrodes that included E4 (approximately equal to FCz) and the two immediately adjacent electrodes to the left and right of E4 (E7 and E54); see supplement for a diagram of electrode locations. This cluster of electrode locations is consistent with a review of studies investigating mediofrontal theta (Cavanagh and Frank, 2014).
For the longitudinal analyses of mediofrontal theta power, the 12-year EEG data was analyzed using identical procedures as those described for the 16-year data. Participants with less than 6 trials per condition (3 CAUG and 1 FCG) were not included in the longitudinal EEG analyses. Given that a TF-PCA approach was employed for the 16-year data, the same TF-PCA loadings were applied to the 12-year data, to ensure comparable neural processes were analyzed at each timepoint and to facilitate the interpretation of any longitudinal changes.
2.6. Analysis Software
Data reduction and statistical analyses were performed using Matlab 2014b (The MathWorks, Natick, MA), SPSS version 25.0 (IBM Corp., Armonk, NY) and Mplus version 7.3 (Muthñn and Muthñn, 2012). Prior to conducting independent-samples t-tests, Levine’s test for equality of variances was conducted; the Welch t statistic (Welch, 1947) was substituted for the traditional independent-samples t-tests, where appropriate, when equal variances between groups could not be assumed (for simplicity, raw degrees of freedom are reported for all t-tests).
2.7. Statistical Analysis of Behavioral data at age 16
2.7.1. Overall go/nogo accuracy and RT
Percentage of correct go and nogo trials were calculated; participants were required to have a go accuracy above 60 % to be included in further behavioral and EEG analyses (all participants at the 16-year visit met this criteria; at the 12-year visit, one participant from the FCG was removed from the longitudinal EEG analyses based on these criteria). Mean RT was calculated for trials with a response (correct-go and error-nogo). Using an intent-to-treat RCT analysis approach, an ANOVA model tested the effect of RCT group (CAUG, FCG) and trial type (go, nogo) on accuracy. Similarly, an ANOVA model tested for RCT group and trial type (go-correct, nogo-error) RT effects. See supplement section for behavioral analyses of the NIG.
2.7.2. Post-error accuracy
In line with the notion that cognitive control reflects the ability to monitor and adapt behavior in order to achieve task goals, a classic behavioral index of cognitive control is post-error accuracy (PEA; Danielmeier and Ullsperger, 2011), which captures the degree to which participants adapt their behavior following errors. PEA was calculated as go-trial accuracy following error responses relative to go-trial accuracy following correct responses. In this way, PEA reflects the differential performance on go trials, as a function of whether the prior trial was an error-of-commission or not; relatively more positive values for PEA are interpreted as reflecting greater cognitive control.
To determine whether the RCT foster care intervention led to overall changes in cognitive control at the behavioral level, we employed an intent-to-treat comparison of PEA values for the CAUG and the FCG via an independent-samples t-test; see supplement for analyses of PEA involving the NIG. In order to determine whether the timing of foster care placement influenced the magnitude of relations between the foster care intervention and later PEA, we tested whether timing (age) of foster care placement for the FCG correlated with PEA; to isolate effects of foster care placement timing from potential effects of the overall duration of time spent in foster care (earlier foster care placement, on average, is associated with a longer duration of time spent in foster care) we controlled for the percentage of time a child spent in foster care by age 16. Additionally, we tested whether individual differences in mediofrontal theta were correlated with PEA.
2.8. Statistical analysis of mediofrontal theta power at age 16
2.8.1. Intent-to-treat RCT analysis
Leveraging the RCT design and an intent-to-treat analysis approach, an ANOVA model with RCT group (FCG, CAUG) and trial type (error, correct) as factors assessed the impact of early experience on PC-weighted mediofrontal theta total power.
2.8.2. Comparisons with NIG
To determine whether improvements observed for the FCG reflect normative neurocognitive functioning, or only relative improvement compared to the CAUG, we incorporated a control group of never-institutionalized adolescents residing in Bucharest, Romania (NIG). Towards this end, we employed a 2 trial type (error, correct) X 3 group (CAUG, FCG, NIG) ANOVA to analyze mediofrontal theta across the three groups. Follow-up analyses employed error-related mediofrontal theta difference scores (error minus correct) to isolate error-related mediofrontal theta effects across groups.
2.8.3. Timing of foster care placement
We also tested whether the age of foster care placement (within FCG) was correlated with error-related mediofrontal theta difference scores. To isolate effects of foster care placement timing from potential effects of the overall duration of time spent in foster care, we controlled for the percentage of time a child spent in foster care by age 16 when testing whether placement timing predicted mediofrontal theta.
2.8.4. Indirect neural pathway to general psychopathology at age 16
A series of Pearson’s product-moment correlation tests assessed whether error-related mediofrontal theta power related to the general psychopathology factor, as well as the externalizing and internalizing factors. Next, the potential for error-related mediofrontal theta to serve as an indirect neural pathway linking early neglect to adolescent psychopathology was tested within a path analytic framework. Indirect effects of RCT group on general, externalizing, and internalizing psychopathology through error-related mediofrontal theta difference scores were tested using a maximum likelihood (ML) estimator, and the significance of the indirect effects were evaluated across 10,000 bootstrap samples. A similar model was also estimated among the FCG by supplementing the binary RCT group predictor with a continuous measure of age at foster care placement. Given that psychopathology and mediofrontal theta were both assessed at age 16, we also re-ran the models when reversing the order of these variables; these additional analyses allowed us to determine if the indirect effects were only significant when mediofrontal theta was employed as the intervening variable (see supplement).
2.9. Longitudinal analysis of mediofrontal theta and psychopathology across ages 12–16
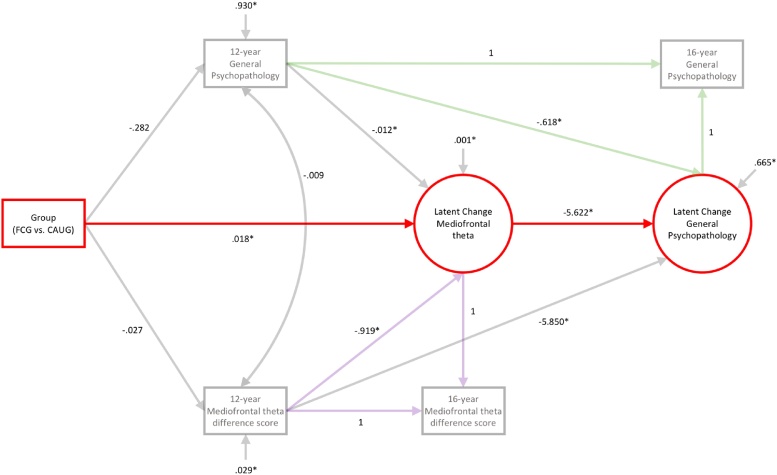
While the current study focuses on the most-recent, age 16 data, we also reanalyzed EEG data from the 12-year assessment in order to further explore whether error-related mediofrontal theta serves as an indirect neural pathway linking early neglect to adolescent psychopathology. Specifically, we tested whether RCT group predicted developmental changes in mediofrontal theta (across ages 12–16), and if such changes further predicted developmental changes in general psychopathology across the same period of adolescence. Towards this end, we fit a bivariate latent change score model (Kievit et al., 2018) that allows for isolating 12-year (baseline) mediofrontal theta and general psychopathology symptoms, as well as 12–16-year development (latent change scores) for each construct. The bivariate latent change score model described by Kievit et al. (2018) was modified to allow for testing the indirect effect of RCT group on developmental changes in general psychopathology (latent change score) through developmental changes in mediofrontal theta (latent change score). The model also tested whether RCT group predicted baseline differences in mediofrontal theta or general psychopathology, and possible indirect effects associated with these variables. In Fig. 9, the indirect effect of RCT group on developmental changes in general psychopathology (latent change score) through developmental changes in mediofrontal theta (latent change score) is depicted in red; the subset of the model capturing latent change (and baseline levels) of mediofrontal theta are drawn in purple, latent change (and baseline levels) of general psychopathology are drawn in green. All effects were tested using an ML estimator and significance was evaluated across 10,000 bootstrap samples. An additional bivariate latent change score model was run when reversing the order of the latent change score factors to determine if the indirect effect was only significant when latent change in mediofrontal theta was employed as an intervening factor (see supplement). Further, we fit a latent change score model for the NIG in order to determine the typical pattern of mediofrontal theta development over the 12–16-year period (see supplement).
Fig. 9.
Bivariate Latent Change Score Model. Unstandardized effects are reported; * indicates significance using bias-corrected, bootstrapped 95 % confidence intervals. The significant indirect effect of RCT group on developmental changes in general psychopathology (latent change score) through developmental changes in mediofrontal theta (latent change score) is depicted in red; latent change (and baseline levels) of mediofrontal theta are drawn in purple, latent change (and baseline levels) of general psychopathology are drawn in green. Foster care group (FCG); Care as usual group (CAUG).
3. Results
3.1. Behavioral performance at age 16
3.1.1. Accuracy and RT
Consistent with prior go/nogo studies (Lamm et al., 2018; McDermott et al., 2012), participants were more accurate for go compared to nogo trials [F(1, 87) = 269.59, p < .001], and faster on nogo-error compared to go-correct trials [F(1, 87) = 166.09, p < .001]. No main effect of RCT group emerged for overall accuracy [F(1, 87) = 1.51, p = .223] or RT [F(1, 87) = 0.002, p = .961], and no interactions between trial and group emerged for overall accuracy [F(1, 87) = 0.09, p = .763] or RT [F(1, 87) = 3.23, p = .076]. However, we report below significant differences in post-error accuracy, a classic index of cognitive control, as a function of RCT group. Table 1 presents behavioral data as a function of trial type and RCT group; see supplement for further behavioral analyses of the never-institutionalized group (NIG).
Table 1.
Accuracy and RT as a function of trial type and group.
| Accuracy (%) |
RT (ms) |
|||
|---|---|---|---|---|
| Go | Nogo | Go-correct | Nogo-error | |
| CAUG | 96.06 | 72.81 | 332.42 | 288.37 |
| (4.14) | (14.27) | (37.57) | (42.12) | |
| FCG | 97.70 | 75.29 | 326.63 | 293.37 |
| (2.69) | (13.99) | (34.49) | (44.74) | |
Note. Means (and standard deviations) for accuracy and response time (RT), as function of group (CAUG, care-as-usual group; FCG, foster-care group) and trial type (Go, Nogo).
3.1.2. Post-error accuracy
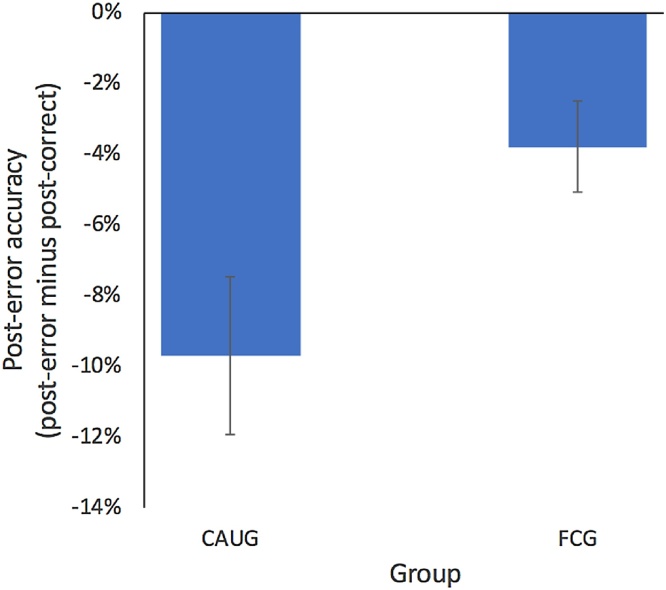
The CAUG exhibited significantly lower (relatively more negative) PEA compared to the FCG [t(1, 87) = -2.29 p = .025], suggesting that the CAUG were less likely to positively adapt their behavior following errors and, instead, were more likely to commit additional errors [CAUG M PEA = -9.70 % (SE = 2.23 %) vs. FCG M PEA = -3.79 % (SE = 1.29 %)]; see Fig. 2. Similar results were obtained after removing a potential outlier from the FCG in this analysis [t(1, 86) = -2.93 p = .005]. See the supplement for further analyses of PEA that incorporate the NIG.
Fig. 2.
Post-error accuracy as a function of RCT group.
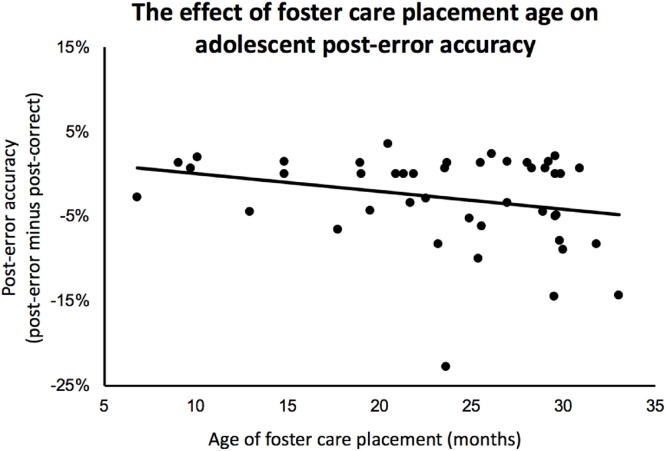
In addition to RCT group-level improvements in PEA, we tested whether timing of the foster care intervention (controlling for the overall duration of time spent in foster care) was related to PEA. Indeed, placement into foster care at younger ages was associated with greater (relatively more positive) PEA (partial r = -.323, df = 41, p = .035); this effect was largely the same after removing a potential outlier (Fig. 3; partial r = -.299, df = 40, p = .055). Moreover, no association between foster care duration and PEA was identified (either with the potential outlier included: r = -.114, p = .457; or without the potential outlier: r = -.079, p = .636), reinforcing the notion that placement timing, as opposed to the overall duration of time spent in foster care, influenced the degree to which the foster care intervention led to improvements in PEA, a classic index of cognitive control. Although we describe similar patterns of change for error-related mediofrontal theta below, individual differences in PEA were not correlated with mediofrontal theta (r = -.02, p = .864).
Fig. 3.
Effect of foster care placement age on post-error accuracy.
3.2. Effects of early experience on 16-year mediofrontal theta
3.2.1. Foster care placement increases error-related mediofrontal theta
Analyses focused on response-locked changes in mediofrontal theta power on nogo-error and go-correct trials, as each involve a response, but errors are known to elicit theta power increases reflecting cognitive control (Cavanagh et al., 2009). Following data reduction via time-frequency PCA (Bernat et al., 2005), we used an intent-to-treat analysis comparing RCT groups (FCG versus CAUG) to assess whether removal from an institution and placement in a family (FCG) yielded increased
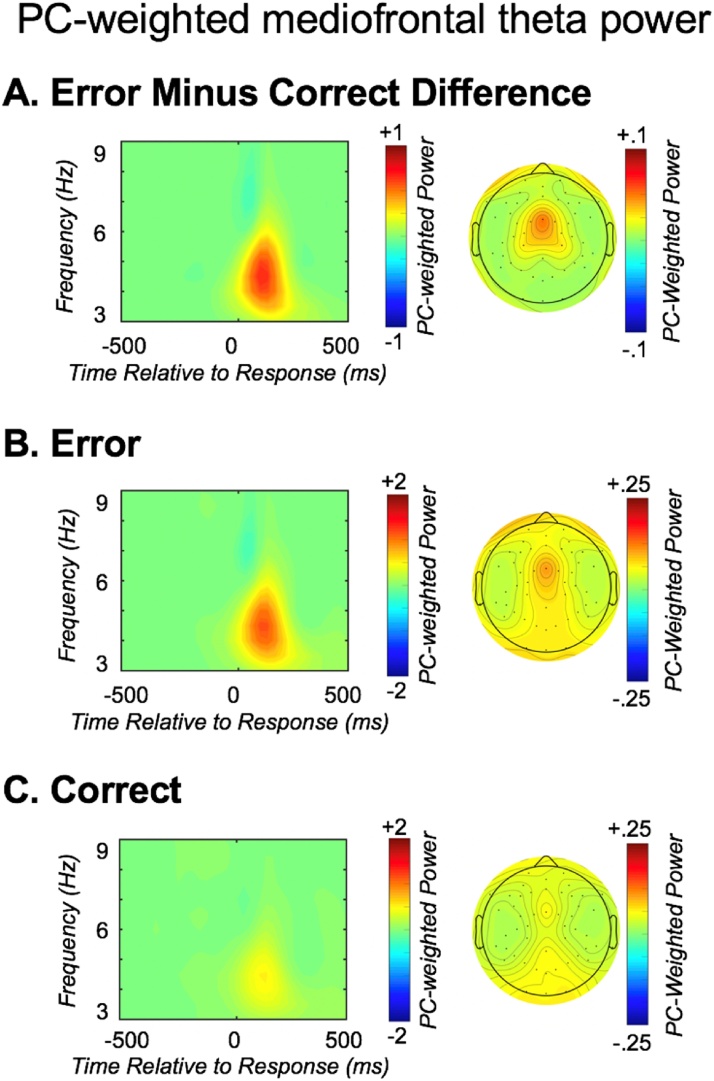
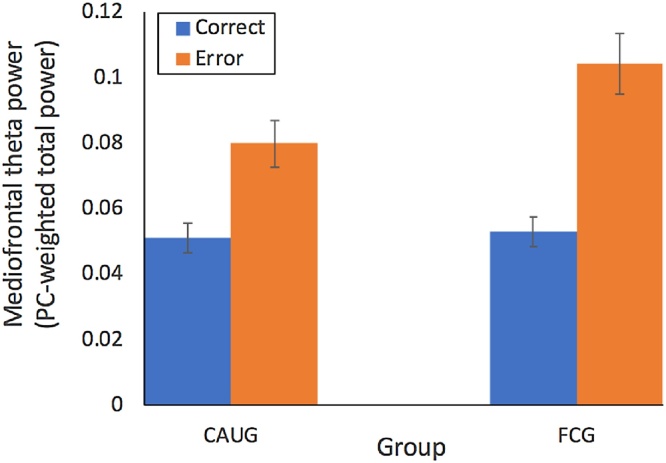
mediofrontal theta power (PC-weighted total power) for events that trigger cognitive control (errors). Consistent with prior work (Buzzell et al., 2018; Cavanagh et al., 2009), errors yielded increased mediofrontal theta power compared to correct trials [F(1, 75) = 73.65, p < .001]; see Fig. 4. No main effect of group was identified [F(1, 75) = 2.57, p = .113]; however, RCT group interacted with trial type [F(1, 75) = 5.81, p = .018] – the nature of this interaction was such that the FCG exhibited increased mediofrontal theta compared to the CAUG on error trials [t(1, 75) = 2.08 p = .041], but not correct trials [t(1, 75) = .30 p = .764]; see Fig. 5. This pattern suggests that, within the foster care group, there was a selective improvement in mediofrontal theta associated with cognitive control (in response to errors), as opposed to nonspecific increases in overall theta power. Therefore, subsequent analyses focused on an error-related mediofrontal theta difference score (error minus correct; Fig. 6).
Fig. 4.
Error-related mediofrontal theta power. From left to right, each row depicts: the response-locked total power time-frequency distribution weighted by the PC factor capturing mediofrontal theta; the corresponding topographic plot. The three rows depict: A) the difference between error and correct activity; B) error activity; C) correct activity.
Fig. 5.
Mediofrontal theta power as a function of trial type and RCT group.
Fig. 6.
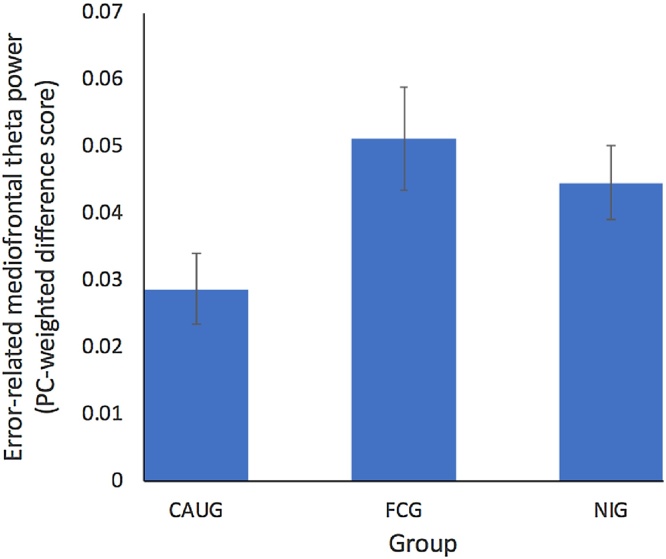
Error-related mediofrontal theta difference scores as a function of group.
3.2.2. Comparisons of error-related mediofrontal theta with the NIG
The 2 trial type (error, correct) X 3 group (CAUG, FCG, NIG) ANOVA revealed a main effect of trial type, with increased mediofrontal theta power for errors compared to correct trials [F(1, 116) = 133.39, p < .001]. No main effect of group (CAUG, FCG, NIG) was identified [F(2, 116) = 1.40, p = .252]; however, group (CAUG, FCG, NIG) interacted with trial type [F2116 = 3.36, p = .038]. Follow-up paired comparisons demonstrated that error-related mediofrontal theta was lower for the CAUG compared to the NIG [t(1, 79) = -2.06, p = .043], as well as for the CAUG compared to the FCG [t(1, 75) = -2.40, p = .019]. In contrast, the FCG yielded levels of mediofrontal theta that were comparable to the NIG [t(1, 78) = 0.70, p = .487].
3.3. Placement timing is associated with greater error-related mediofrontal theta
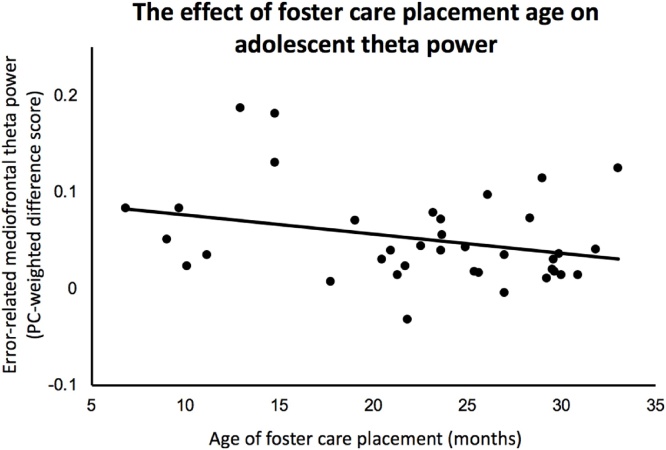
Within the FCG, we tested whether age of placement into the foster care intervention (controlling for the overall duration of time spent in foster care) was associated with mediofrontal theta. Consistent with a sensitive period in early childhood suggested by animal work (Sarro etal., 2014), placement into foster care at younger ages was associated with greater error related mediofrontal theta difference scores (partial r = -.365, df = 34, p = .029; see Fig. 7). No direct effect of foster care duration on mediofrontal theta was identified (r = -.105, n = 38, p = .532), reinforcing the notion that placement timing, as opposed to the overall duration of time spent in foster care, influenced the magnitude of the foster care intervention effect. See Table 2 for bivariate correlations amongst the study variables.
Fig. 7.
Effect of foster care placement age on error-related mediofrontal theta difference scores.
Table 2.
Bivariate correlations at age 16.
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|
| 1. Correct Theta | .598** | .077 | −.097 | .094 | .068 | −.120 | −.068 |
| 2. Error Theta | .845** | −.291^ | −.039 | .068 | −.053 | −.344** | |
| 3. Error Minus Correct Theta | −.296^ | −.105 | .039 | .014 | −.380** | ||
| 4. Age of Placement | −.362** | −.008 | .001 | −.004 | |||
| 5. Percent Time in Foster Care | .007 | −.008 | −.024 | ||||
| 6. Internalizing (I) | −.244* | .085 | |||||
| 7. Externalizing (E) | .170 | ||||||
| 8. General Psychopathology (P) |
Note. Correlations involving variables 4 and 5 only include the Foster Care Group (FCG).
p < .1.
p < .05.
p < .01.
3.3.1. Mediofrontal theta as an indirect neural pathway to general psychopathology
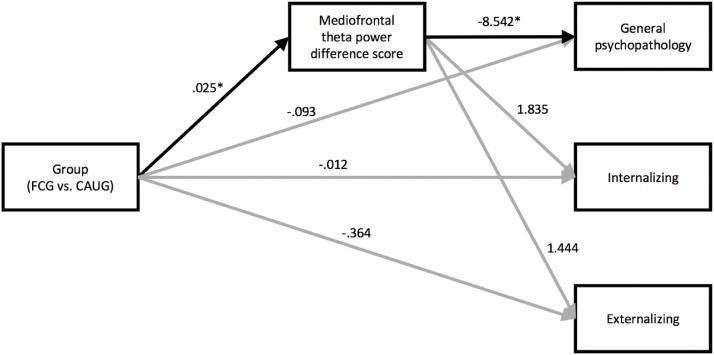
We next tested whether disruptions to mediofrontal theta reflected an indirect neural pathway to psychopathology. Here, we followed recent work that models psychopathology in terms of both general (i.e., transdiagnostic) and specific psychopathology risk (Caspi et al., 2014; Wade et al., 2018). At age 16, reductions in error-related mediofrontal theta difference scores were associated with increased general psychopathology (r = -.380, n = 76, p = .001) but were unrelated to specific internalizing (r = .039, n = 76, p = .735) or externalizing (r = .014, n = 79, p = .865) factors after accounting for general psychopathology; see Table 2. Using path analysis, there was a significant indirect effect from RCT group to the general psychopathology factor through error-related mediofrontal theta difference scores (B = -.212, 95 % CI = -.416 – -.059), but not the externalizing or internalizing factors (Fig. 8 and Table 3). See the supplement for consistent results when using a more traditional set of analyses of the HBQ Internalizing, Externalizing, and ADHD composite variables, such that mediofrontal theta formed a significant indirect effect linking the intervention and all three psychopathology scales. Similarly, an exploratory analysis within only the FCG revealed an indirect effect from timing of foster care placement to general psychopathology through error-related mediofrontal theta difference scores (B = .019, 95 % CI = .001–.053); see supplement for further details. When mediofrontal theta was used as the outcome variable and psychopathology was the intervening variable (i.e., variable order was reversed), the same indirect effects were not observed, providing further support for the direction of effects.
Fig. 8.
Bootstrapped indirect effects of RCT group on general psychopathology through error-related mediofrontal theta. Unstandardized effects are reported; * indicates significance using bias-corrected bootstrapped 95 % confidence intervals. RCT group (FCG vs. CAUG) exhibited a significant indirect effect on general psychopathology through error-related mediofrontal theta.
Table 3.
Total, direct, and indirect effects of RCT group (FCG vs. CAUG) on general, internalizing and externalizing psychopathology factors through error-related mediofrontal theta.
| General | Internalizing | Externalizing | |
|---|---|---|---|
| Total effect | −.305 | .033 | −.328 |
| [-.68, .07] | [-.31, .37] | [-.76, .07] | |
| Direct effect | −.093 | −.012 | −.354 |
| [-.46, .28] | [-.38, .36] | [-.85, .09] | |
| Indirect effect | −.212* | .046 | .025 |
| [-.416, -.059] | [-.05, .21] | [-.08, .18] |
p < .05; unstandardized estimates and 95 % bias-corrected bootstrapped confidence interval.around estimate.
3.4. Longitudinal change (ages 12–16) in mediofrontal theta and psychopathology
While we report above that placement into foster care predicted higher levels of mediofrontal theta by age 16, RCT group did not significantly predict age 12 (baseline) levels of mediofrontal theta or general psychopathology (see Fig. 9 and Table 4). However, RCT group predicted developmental change in mediofrontal theta from 12 to 16 years. On average, adolescents exhibited a decrease in mediofrontal theta from ages 12–16 (negative mediofrontal theta latent change score; B = -.978, 95% CI = -.992 – -.965); a similar pattern was also observed for the NIG (see supplement). Placement into foster care yielded less of a decline from age 12-16 (positive effect of RCT group on mediofrontal theta latent change score; B = 0.018, 95% CI = .002 – -.035), leaving these adolescents with relatively higher levels of mediofrontal theta by age 16, compared to the CAUG. In turn, less of a decline in mediofrontal theta across adolescence was associated with greater reductions in general psychopathology over the same period of development (negative effect of mediofrontal theta latent change score on the general psychopathology latent change score; B = -5.622, 95% CI = -10.405 – -1.719). Ultimately, placement into foster care indirectly predicted greater reductions in general psychopathology from ages 12 to 16 through less of a decline in mediofrontal theta across the same period (indirect effect of RCT group on the general psychopathology latent change score, through the mediofrontal theta latent change score; B = -.099, 95 % CI = -.256 – -.010). See Fig. 9 and Table 4 for a complete description of the model results; no other indirect effects were significant for this model. Moreover, no indirect paths were significant when the order of the factors (latent change in mediofrontal theta and latent change in general psychopathology) were reversed, supporting the direction of effects presented here (see supplement). Additionally, further tests of moderated mediation were not significant (see supplement), reinforcing the interpretations presented here.
Table 4.
Bivariate latent change score model.
| Regression Paths | LL 2.5 % B UL 2.5 % |
|---|---|
| Group -> 12 yr. Theta | −0.098 -0.027 0.045 |
| Group -> 12 yr. P | −0.634 -0.282 0.081 |
| *Group -> Theta Change | 0.002 0.018 0.035 |
| *12 yr. Theta -> Theta Change | −0.969 -0.919 -0.873 |
| *12 yr. Theta -> P Change | −10.147 -5.850 -2.106 |
| *12 yr. P -> P Change | −0.806 -0.618 -0.459 |
| *12 yr. P -> Theta Change | −0.021 -0.012 -0.004 |
| *Theta Change -> P Change | −10.405 -5.622 -1.719 |
| Means/Intercepts | |
| *Mean Theta Change | −0.992 -0.978 -0.965 |
| *Mean P Change | −10.854 -6.164 -2.421 |
| *12 yr. Theta (intercept) | 0.121 0.173 0.230 |
| *12 yr. P (intercept) | 0.074 0.338 0.625 |
| Residual Variances | |
| *Theta Change | 0.001 0.001 0.002 |
| *P Change | 0.447 0.665 1.036 |
| *12 yr. Theta | 0.021 0.029 0.039 |
| *12 yr. P | 0.711 0.930 1.254 |
| Residual Covariances | |
| 12 yr. Theta with 12 yr. P | −0.043 -0.009 0.029 |
| Indirect Effects | |
| Group -> 12 yr. Theta -> P Change | −0.266 0.157 0.672 |
| Group -> 12 yr. P -> P Change | −0.046 0.174 0.417 |
| *Group -> Theta Change -> P Change | −0.256 -0.099 -0.010 |
| Group -> 12 yr. Theta -> Theta Change -> P Change | −0.628 -0.139 0.228 |
| Group -> 12 yr. P -> Theta Change -> P Change | −0.072 -0.020 0.001 |
Indicates significance using a 95 % confidence interval around unstandardized estimate (B). Lower level (LL); Upper level (UL); General psychopathology (P).
4. Discussion
In the current study we investigated the effect of a high-quality foster care intervention versus prolonged institutional deprivation on event-related mediofrontal theta oscillations and cognitive control behavior in humans. By age 16, adolescents who spent their early lives in institutional care but were subsequently randomized to foster care (FCG) exhibited enhanced error-related mediofrontal theta power and were more likely to adapt their behavior following errors (PEA) compared to children who experienced prolonged institutional rearing (CAUG). Earlier placement into foster care was associated with higher levels of mediofrontal theta, as well as relatively greater PEA. By age 16, the foster care intervention also yielded comparable mediofrontal theta to that of a group of never-institutionalized adolescents (NIG). Finally, reduced mediofrontal theta reflected an indirect neural pathway linking early neglect to general psychopathology, as opposed to specific associations with internalizing or externalizing factors once general psychopathology was accounted for. Whereas mediofrontal theta was found to decrease from ages 12–16 on average, placement into foster care yielded less of a decline in mediofrontal theta over this period. In turn, changes in mediofrontal theta development were predictive of greater reductions in general psychopathology across this same period. These results provide empirical evidence in humans that mediofrontal theta is impacted by the removal from situation of early neglect, thus offering a translational link between studies of neglect in animal models and non-invasive assessment of neural function in humans. The current findings bear similarity to reports of reduced mediofrontal theta in rodent models of neglect/ maternal separation (Courtiol et al., 2018; Reincke and Hanganu-Opatz, 2017; Sarro et al., 2014), but also highlight the clinical relevance of studying longitudinal changes in mediofrontal theta across the human adolescent period. Future studies may leverage assessments of mediofrontal theta in human and animal work to enhance understanding of neglect and associated transdiagnostic risk for psychopathology.
Modulation of mediofrontal theta oscillations reflects a fundamental mechanism of cognitive control across mammalian species, allowing self-monitoring and adaptive behavior (Cavanagh and Frank, 2014). In rodents, neglect impairs mediofrontal theta power (Reincke and Hanganu-Opatz, 2017) and maternal presence/absence drives event-related increases/decreases in mediofrontal theta (Courtiol et al., 2018; Sarro et al., 2014). In humans, prior work demonstrates that neglect leads to deficits in neural structures supporting cognitive control (MRI; Hodel et al., 2015; McLaughlin et al., 2014), indirect markers of cognitive control function (event-related potentials; McDermott et al., 2013; Troller-Renfree et al., 2016), and behavioral outputs of cognitive control (Bauer et al., 2009; Bruce et al., 2009; Hostinar et al., 2012; Pollak et al., 2010; Sheridan et al., 2017). The current results expand on this literature by providing experimental evidence in humans that a direct functional mechanism of cognitive control, mediofrontal theta, is also impacted by early neglect.
Assessment of mediofrontal theta predicted transdiagnostic psychopathology, as opposed to specific associations with internalizing or externalizing factors once transdiagnostic risk was accounted for. Whereas prior work has found that indirect markers of cognitive control function were associated with externalizing symptoms in the context of neglect (McDermott et al., 2013; Troller-Renfree et al., 2016), the current approach of mediofrontal theta measurement and transdiagnostic assessment of psychopathology provides a more nuanced perspective. Neglect appears to drive impairments in the cognitive control system (reduced mediofrontal theta), which is in turn associated with broad risk for psychopathology that cuts across diagnostic categories. Once this transdiagnostic risk is accounted for, no unique prediction of internalizing or externalizing factors was detectable. These findings are in line with recent work demonstrating that changes in executive functions, at the behavioral level, mediate links between early neglect and later transdiagnostic risk (Wade et al., 2019a, b); the current study provides a potential neural process associated with such relations. Moreover, associations between mediofrontal theta and transdiagnostic risk are consistent with recent work that did not assess neglect but finds the neural structures (MRI; McTeague et al., 2016), indirect markers (fMRI; Kohn et al., 2014; McTeague et al., 2017), and behavioral outputs (White et al., 2017) associated with cognitive control confer a similar association with general psychopathology. The current findings are the first evidence that mediofrontal theta confers transdiagnostic risk, in any context.
4.1. Why is theta reduced following neglect?
The current findings demonstrate that children with prolonged psychosocial deprivation exhibit impaired task-related mediofrontal theta in adolescence. This is paralleled by rodent work showing that repeated exposure to maternal separation during the preweaning period leads to lasting impairments in mediofrontal theta during the juvenile period (Reincke and Hanganu-Opatz, 2017). Additional rodent work may provide insight into why neglect leads to reductions in mediofrontal theta. During the preweaning period, maternal presence is associated with event-related increases in mediofrontal theta of rat pups, compared to reductions when the mother is absent or when the rat pup is removed from the nest (Courtiol et al., 2018; Sarro et al., 2014). Thus, chronic separation is characterized by fewer opportunities for the developing brain to generate event-related increases in theta. Neural oscillations recorded via LFP and EEG primarily reflect synchronous post-synaptic potentials across pyramidal neurons, which further increases the likelihood of these neurons firing closely together in time (Nunez and Srinivasan, 2006). A general principle of neuroscience is that synapses between neurons that regularly fire together are strengthened and less likely to undergo neuronal pruning (Hebb, 1949), increasing the likelihood of subsequent coactivation (also see: McLaughlin et al., 2017). Indeed, cortical oscillations directly influence cortical development and the maintenance of neuronal ensembles (Uhlhaas et al., 2010). Thus, repeated exposure to events that increase mediofrontal theta power early in life (e.g., caregiver stimulation) may increase the likelihood that theta-generating mediofrontal neuronal ensembles persist into the juvenile/adolescent period. In line with the experimental (RCT) findings reported here, recent correlational work has demonstrated that variations in socioeconomic status, which is associated with the quality and/or frequency of parent-child interactions, also impacts mediofrontal theta (Conejero et al., 2018). This hypothesis is further supported by work demonstrating that oscillatory stimulation causes release of brain-derived neurotrophic factor (BDNF), promoting cellular growth (Balkowiec and Katz, 2002). Therefore, it is possible that early neglect may result in lower event-related mediofrontal theta modulation early in life, which in turn reduces the likelihood that theta-generating mediofrontal neuronal ensembles persist.
4.2. Why is the developmental pattern of theta impacted?
It is important to note that early neglect was found to impact mediofrontal theta by age 16, but not age 12. Moreover, early neglect predicted differences in the developmental slope of mediofrontal theta across adolescence (ages 12–16). On average, adolescents within each of the RCT groups, as well as the NIG, exhibited decreases in mediofrontal theta across adolescence; however, placement into foster care yielded a less steep decline in mediofrontal theta across this developmental window, relative to the CAUG. Limited work has explored mediofrontal theta development during the adolescent period and to our knowledge, extant research has only employed cross-sectional designs to study how mediofrontal theta changes during this period. Broadly, the emerging literature suggests that task-related theta power and synchronization generally increase from childhood to adulthood, with one exception being the adolescent period. During early to late adolescence, at least two cross-sectional studies have demonstrated that mediofrontal theta synchronization decreases, on average, which mirrors the overall pattern observed in the current longitudinal study. Crowley et al. (2014) reported that task-related mediofrontal theta power and synchronization increased from childhood to early adolescence, but then decreased from early to late adolescence. Similarly, Uhlhaas et al. (2009) showed that task-related mediofrontal theta synchronization increased from childhood to early adolescence, decreased from early to late adolescence, and then exhibited further increases from late adolescence to adulthood. Critically, the current study provides the first evidence of a longitudinal decrease in theta during the adolescent period, regardless of early caregiving context, consistent with prior cross-sectional work. It is important to note that patterns of theta recorded at rest, as opposed to in response to task events, can show alternative patterns of age-related change (Marek et al., 2018)
The current study demonstrates that adolescent decreases in mediofrontal theta are less pronounced for individuals placed into foster care early in life, relative those that remain in institutions. It currently remains unclear as to why removal from situations of neglect early in life would produce a “sleeper effect” for mediofrontal theta, with the benefits of foster care not becoming fully apparent until the adolescent period. However, adolescence is a time of dramatic social and neural change (Blakemore, 2008; Crone and Dahl, 2012), and early differences in caregiving could yield cascading effects that manifest during this unique period of human development. Moreover, we note that such delayed benefits of foster care have previously been reported by Wade et al. (2018) in relation to adolescent psychopathology. The current study extends prior work by demonstrating similar delayed benefits of foster care on mediofrontal theta development, and that adolescent changes in mediofrontal theta are inversely related to adolescent changes in transdiagnostic risk for psychopathology.
4.3. Limitations and future directions
It is important to note that the sample size for this study is relatively small. Also, while neglect is the most common form of maltreatment faced by children, this is only one form of early life stress/adversity that many children must face, and other forms of early maltreatment (e.g. physical and emotional abuse, poverty, etc.) may operate via disparate processes acting on neurodevelopmental outcomes (McLaughlin et al., 2017; Sheridan and McLaughlin, 2014). Future work should also seek to directly compare how neglect impacts mediofrontal theta in contrast to related neural measures, such as the error-related negativity (Troller-Renfree et al., 2016).
4.4. Conclusions
This study provides experimental evidence that mediofrontal theta is disrupted by neglect in humans, providing a bridge to animal work, aiding the translation of animal studies to better understand and interpret neurobiological changes as a result of neglect. Additionally, mediofrontal theta was implicated in a neural pathway associated with transdiagnostic risk for psychopathology, illustrating the relevance of this neural process. Mediofrontal theta can be assessed in humans via EEG, a non-invasive and cost-effective technique that is well-suited for conducting research in low-resource environments where neglect is most common. To clarify the neurobiological consequences of neglect and relations to psychopathology, future translational work can leverage assessment of mediofrontal theta.
Funding
This research was supported by grants from the NIMH (MH091363) to Nelson, Zeanah and Fox, and from the Binder Family Foundation to Nelson. The funding sources played no role in designing the study, data collection, analysis, interpretation of results, or the decision to submit results for publication.
Declaration of Competing Interest
All authors declare no competing financial (or other) conflicts of interest.
Acknowledgement
We thank the families and the children who participated in this study, as well as the research team and staff in Romania for their support and investment in this project. The authors acknowledge the important contributions of Alexandra Cercel, Carmen Iuga, Mariana Mitu, Iuliana Dobre, Nadia Radu, Anca Radulescu, and Florin Tibu.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100777.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Balkowiec A., Katz D.M. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. 2002;22(23):10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R.J., Clarke A.R., Johnstone S.J. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophysiol. 2003;114(2):171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Bauer P.M., Hanson J.L., Pierson R.K., Davidson R.J., Pollak S.D. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biol. Psychiatry. 2009;66(12):1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat E.M., Williams W.J., Gehring W.J. Decomposing ERP time–frequency energy using PCA. Clin. Neurophysiol. 2005;116(6):1314–1334. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9(4):267. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Braun K., Lange E., Metzger M., Poeggel G. Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience. 1999;95(1):309–318. doi: 10.1016/s0306-4522(99)00420-0. [DOI] [PubMed] [Google Scholar]
- Bruce J., Tarullo A.R., Gunnar M.R. Disinhibited social behavior among internationally adopted children. Dev. Psychopathol. 2009;21(1):157–171. doi: 10.1017/S0954579409000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell G.A., Barker T.V., Troller-Renfree S.V., Bernat E.M., Bowers M.E., Morales S., Bowman L.C., Henderson H.A., Pine D.S., Fox N.A. Adolescent cognitive control, Theta oscillations, and social motivation. BioRxiv. 2018:366831. doi: 10.1016/j.neuroimage.2019.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell G.A., Barker T.V., Troller-Renfree S.V., Bernat E.M., Bowers M.E., Morales S., Bowman L.C., Henderson H.A., Pine D.S., Fox N.A. Adolescent cognitive control, theta oscillations, and social observation. NeuroImage. 2019;198:13–30. doi: 10.1016/j.neuroimage.2019.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Houts R.M., Belsky D.W., Goldman-Mellor S.J., Harrington H., Israel S., Meier M.H., Ramrakha S., Shalev I., Poulton R. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. (Regul. Ed.) 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Cohen M.X., Allen J.J. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J. Neurosci. 2009;29(1):98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejero Á., Guerra S., Abundis‐Gutiérrez A., Rueda M.R. Frontal theta activation associated with error detection in toddlers: influence of familial socioeconomic status. Dev. Sci. 2018;21(1) doi: 10.1111/desc.12494. [DOI] [PubMed] [Google Scholar]
- Courtiol E., Wilson D.A., Shah R., Sullivan R.M., Teixeira C.M. Maternal regulation of pups’ cortical activity: role of serotonergic signaling. Eneuro. 2018;5(4) doi: 10.1523/ENEURO.0093-18.2018. ENEURO.0093-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crowley M.J., van Noordt S.J.R., Wu J., Hommer R.E., South M., Fearon R.M.P., Mayes L.C. Reward feedback processing in children and adolescents: medial frontal theta oscillations. Brain Cogn. 2014;89:79–89. doi: 10.1016/j.bandc.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C., Ullsperger M. Post-error adjustments. Front. Psychol. 2011;2:233. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath R., Tang A., Zeanah C.H., Nelson C.A., Fox N.A. The long-term effects of institutional rearing, foster care intervention and disruptions in care on brain electrical activity in adolescence. Dev. Sci. 2020;23(1) doi: 10.1111/desc.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnigan S., Robertson I.H. Resting EEG theta power correlates with cognitive performance in healthy older adults: resting theta EEG correlates with cognitive aging. Psychophysiology. 2011;48(8):1083–1087. doi: 10.1111/j.1469-8986.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- Hebb D.O. A neuropsychological theory; 1949. The Organization of Behavior. [Google Scholar]
- Herrmann C.S., Strüber D., Helfrich R.F., Engel A.K. EEG oscillations: from correlation to causality. Int. J. Psychophysiol. 2016;103:12–21. doi: 10.1016/j.ijpsycho.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Hevia-Orozco J.C., Sanz-Martin A. EEG characteristics of adolescents raised in institutional environments and their relation to psychopathological symptoms. J. Behav. Brain Sci. 2018;8(10):519–537. [Google Scholar]
- Hodel A.S., Hunt R.H., Cowell R.A., Van Den Heuvel S.E., Gunnar M.R., Thomas K.M. Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C.E., Stellern S.A., Schaefer C., Carlson S.M., Gunnar M.R. Associations between early life adversity and executive function in children adopted internationally from orphanages. Proc. Natl. Acad. Sci. 2012;109(Supplement 2):17208–17212. doi: 10.1073/pnas.1121246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G., Ullsperger M. Neuropharmacology of performance monitoring. Neurosci. Biobehav. Rev. 2009;33(1):48–60. doi: 10.1016/j.neubiorev.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Kievit R.A., Brandmaier A.M., Ziegler G., van Harmelen A.-L., de Mooij S.M.M., Moutoussis M., Goodyer I.M., Bullmore E., Jones P.B., Fonagy P., Lindenberger U., Dolan R.J. Developmental cognitive neuroscience using latent change score models: A tutorial and applications. Dev. Cogn. Neurosci. 2018;33:99–117. doi: 10.1016/j.dcn.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Troller-Renfree S., Zeanah C.H., Nelson C.A., Fox N.A. Impact of early institutionalization on attention mechanisms underlying the inhibition of a planned action. Neuropsychologia. 2018;117:339–346. doi: 10.1016/j.neuropsychologia.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb R., Paulozzi L., Melanson C., Simon T., Arias I. Centers for Disease Control and Prevention (CDC); 2008. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements [Technical Report]https://ncvc.dspacedirect.org/handle/20.500.11990/337 [Google Scholar]
- Loman M.M., Johnson A.E., Westerlund A., Pollak S.D., Nelson C.A., Gunnar M.R. The effect of early deprivation on executive attention in middle childhood. J. Child Psychol. Psychiatry. 2013;54(1):37–45. doi: 10.1111/j.1469-7610.2012.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Heffelfinger A., Measelle J.R., Ablow J.C., Essex M.J., Dierker L., Harrington R., Kraemer H.C., Kupfer D.J. Differential performance of the macarthur HBQ and DISC-IV in identifying DSM-IV internalizing psychopathology in young children. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(4):458–466. doi: 10.1097/00004583-200204000-00019. [DOI] [PubMed] [Google Scholar]
- Marek S., Tervo-Clemmens B., Klein N., Foran W., Ghuman A.S., Luna B. Adolescent development of cortical oscillations: Power, phase, and support of cognitive maturation. PLoS Biol. 2018;16(11) doi: 10.1371/journal.pbio.2004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Reeb B.C., Fox N.A., Nelson C.A., Zeanah C.H. Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Dev. Psychopathol. 2008;20(3) doi: 10.1017/S0954579408000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott J.M., Westerlund A., Zeanah C.H., Nelson C.A., Fox N.A. Early adversity and neural correlates of executive function: Implications for academic adjustment. Dev. Cogn. Neurosci. 2012;2(Suppl 1):S59–66. doi: 10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott J.M., Troller-Renfree S., Vanderwert R., Nelson C.A., Zeanah C.H., Fox N.A. Psychosocial deprivation, executive functions, and the emergence of socio-emotional behavior problems. Front. Hum. Neurosci. 2013:7. doi: 10.3389/fnhum.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Fox N.A., Zeanah C.H., Sheridan M.A., Marshall P., Nelson C.A. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of Attention-Deficit/Hyperactivity disorder. Biol. Psychiatry. 2010;68(4):329–336. doi: 10.1016/j.biopsych.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Winter W., Fox N.A., Zeanah C.H., Nelson C.A. Widespread Reductions in Cortical Thickness Following Severe Early-Life Deprivation: A Neurodevelopmental Pathway to Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry. 2014;76(8):629–638. doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Nelson C.A. Neglect as a violation of species-expectant experience: neurodevelopmental consequences. Biol. Psychiatry. 2017;82(7):462–471. doi: 10.1016/j.biopsych.2017.02.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Goodkind M.S., Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J. Psychiatr. Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Huemer J., Carreon D.M., Jiang Y., Eickhoff S.B., Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am. J. Psychiatry. 2017;174(7):676–685. doi: 10.1176/appi.ajp.2017.16040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F.G. The randomized controlled trial as a demonstration project: an ethical perspective. Am. J. Psychiatry. 2009;166(7):743–745. doi: 10.1176/appi.ajp.2009.09040538. [DOI] [PubMed] [Google Scholar]
- Miskovic V., Ma X., Chou C.-A., Fan M., Owens M., Sayama H., Gibb B.E. Developmental changes in spontaneous electrocortical activity and network organization from early to late childhood. NeuroImage. 2015;118:237–247. doi: 10.1016/j.neuroimage.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognon A., Jovicich J., Bruzzone L., Buiatti M. ADJUST: an automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011;48(2):229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Muthñn L.K., Muthñn B.O. seventh edition. Muthñn & Muthñn; Los Angeles, CA: 2012. Mplus. Statistical Analysis With Latent Variables. User’s Guide. [Google Scholar]
- Narayanan N.S., Cavanagh J.F., Frank M.J., Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat. Neurosci. 2013;16(12):1888–1895. doi: 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H., Whelan R., Reilly R.B. FASTER: fully automated statistical thresholding for EEG artifact rejection. J. Neurosci. Methods. 2010;192(1):152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Nunez P.L., Srinivasan R. Oxford University Press; USA: 2006. Electric Fields of the Brain: the Neurophysics of EEG. [Google Scholar]
- Pollak S.D., Nelson C.A., Schlaak M.F., Roeber B.J., Wewerka S.S., Wiik K.L., Frenn K.A., Loman M.M., Gunnar M.R. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Dev. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex M.B., Scudder M.R., Brown M.L., O’Leary K.C., Wu C.-T., Themanson J.R., Hillman C.H. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47(4):767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Reincke S.A.J., Hanganu-Opatz I.L. Early-life stress impairs recognition memory and perturbs the functional maturation of prefrontal-hippocampal-perirhinal networks. Sci. Rep. 2017;7:42042. doi: 10.1038/srep42042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro E.C., Wilson D.A., Sullivan R.M. Maternal regulation of infant brain state. Curr. Biol. 2014;24(14):1664–1669. doi: 10.1016/j.cub.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak A.J., Mettenburg J., Basena M., Peta I., McPherson K., Greene A. US Department of Health and Human Services; Washington, DC: 2010. Fourth National Incidence Study of Child Abuse and Neglect (NIS-4) Retrieved on July, 9, 2010. [Google Scholar]
- Sheridan M.A., McLaughlin K.A. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn. Sci. (Regul. Ed.) 2014;18(11):580–585. doi: 10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M.A., Peverill M., Finn A.S., McLaughlin K.A. Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Dev. Psychopathol. 2017;29(5):1777–1794. doi: 10.1017/S0954579417001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele V.R., Anderson N.E., Claus E.D., Bernat E.M., Rao V., Assaf M., Pearlson G.D., Calhoun V.D., Kiehl K.A. Neuroimaging measures of error-processing: extracting reliable signals from event-related potentials and functional magnetic resonance imaging. Neuroimage. 2016;132:247–260. doi: 10.1016/j.neuroimage.2016.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S.T., Gould T.D., Manji H.K. The American Psychiatric Publishing Textbook of Psychopharmacology. 3rd ed. American Psychoanalytic Association; 2004. Neurotransmitters, receptors, Signal transduction, and second messengers in psychiatric disorders; pp. 3–52. [Google Scholar]
- Tarullo A.R., Garvin M.C., Gunnar M.R. Atypical EEG power correlates with indiscriminately friendly behavior in internationally adopted children. Dev. Psychol. 2011;47(2):417–431. doi: 10.1037/a0021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S., Nelson C.A., Zeanah C.H., Fox N.A. Deficits in error monitoring are associated with externalizing but not internalizing behaviors among children with a history of institutionalization. J. Child Psychol. Psychiatry. 2016;57(10):1145–1153. doi: 10.1111/jcpp.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller‐Renfree S., Zeanah C.H., Nelson C.A., Fox N.A. Neural and cognitive factors influencing the emergence of psychopathology: insights from the bucharest early intervention project. Child Dev. Perspect. 2018;12(1):28–33. doi: 10.1111/cdep.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo L.T., Allen J.J. Theta EEG dynamics of the error-related negativity. Clin. Neurophysiol. 2007;118(3):645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T., Shimazu H., Isomura Y. Direct recording of Theta oscillations in primate prefrontal and anterior cingulate cortices. J. Neurophysiol. 2006;95(5):2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Singer W., Haenschel C., Sireteanu R., Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc. Natl. Acad. Sci. 2009;106(24):9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Rodriguez E., Rotarska-Jagiela A., Singer W. Neural synchrony and the development of cortical networks. Trends Cogn. Sci. (Regul. Ed.) 2010;14(2):72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., Fischer A.G., Nigbur R., Endrass T. Neural mechanisms and temporal dynamics of performance monitoring. Trends Cogn. Sci. (Regul. Ed.) 2014;18(5):259–267. doi: 10.1016/j.tics.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Vanderwert R.E., Marshall P.J., Nelson C.A., Zeanah C.H., Fox N.A. Timing of Intervention Affects Brain Electrical Activity in Children Exposed to Severe Psychosocial Neglect. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert R.E., Zeanah C.H., Fox N.A., Nelson C.A. Normalization of EEG activity among previously institutionalized children placed into foster care: a 12-year follow-up of the Bucharest Early Intervention Project. Dev. Cogn. Neurosci. 2016;17:68–75. doi: 10.1016/j.dcn.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verguts T. Binding by random bursts: a computational model of cognitive control. J. Cogn. Neurosci. 2017;29(6):1103–1118. doi: 10.1162/jocn_a_01117. [DOI] [PubMed] [Google Scholar]
- Viola F.C., Debener S., Thorne J., Schneider T.R. Simultaneous EEG and FMRI: Recording, Analysis, and Application: Recording, Analysis, and Application; 2010. Using ICA for the Analysis of Multi-channel EEG Data; pp. 121–133. [Google Scholar]
- Wade M., Fox N.A., Zeanah C.H., Nelson C.A. Effect of foster care intervention on trajectories of general and specific psychopathology among children with histories of institutional rearing: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1137. doi: 10.1001/jamapsychiatry.2018.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M., Fox N.A., Zeanah C.H., Nelson C.A. Long-term effects of institutional rearing, foster care, and brain activity on memory and executive functioning. Proc. Natl. Acad. Sci. 2019 doi: 10.1073/pnas.1809145116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M., Zeanah C.H., Fox N.A., Nelson C.A. Global deficits in executive functioning are transdiagnostic mediators between severe childhood neglect and psychopathology in adolescence. Psychol. Med. 2019:1–8. doi: 10.1017/S0033291719001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch B.L. The generalization of’ Student’s’ problem when several different population variances are involved. Biometrika. 1947;34(1–2):28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- White L.K., Moore T.M., Calkins M.E., Wolf D.H., Satterthwaite T.D., Leibenluft E., Pine D.S., Gur R.C., Gur R.E. An evaluation of the specificity of executive function impairment in developmental psychopathology. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56(11):975–982. doi: 10.1016/j.jaac.2017.08.016. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford T.J., Rennie C.J., Grieve S.M., Clark C.R., Gordon E., Williams L.M. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum. Brain Mapp. 2007;28(3):228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T., Johnston K., Vinck M., Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc. Natl. Acad. Sci. 2010;107(11):5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah C.H., Nelson C.A., Fox N.A., Smyke A.T., Marshall P., Parker S.W., Koga S. Designing research to study the effects of institutionalization on brain and behavioral development: the Bucharest Early Intervention Project. Dev. Psychopathol. 2003;15(4):885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- Zeanah C.H., Fox N.A., Nelson C.A. Case study in ethics of research: the bucharest early intervention project. J. Nerv. Ment. Dis. 2012;200(3):243–247. doi: 10.1097/NMD.0b013e318247d275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.