Abstract
We report a case of unusually aggressive behavior of a mucinous tubular and spindle cell carcinoma (MTSCC) of the kidney with no sarcomatoid changes. A 43-year-old man was referred to our hospital for a mass on his left kidney. Computed tomography revealed a tumor at the upper pole of the kidney and swollen lymph nodes. Left radical nephrectomy with lymph node dissection was performed and the tumor was diagnosed as MTSCC. Peritoneal dissemination was detected 4 months after the surgery. The patient received systemic treatments, which were not effective. He finally died of the disease 12 months after the surgery.
Keywords: Sunitinib, Renal cell carcinoma, Mucinous tubular and spindle cell carcinoma, Metastasis
Introduction
Mucinous tubular and spindle cell carcinoma (MTSCC) of the kidneys is a rare subtype of renal cell carcinoma (RCC). Although it is associated with indolent clinical behavior and carries a favorable prognosis, there have been a few cases of nodal and distant metastases or local recurrence after resection, both of which can result in death.1 Because of the rarity of such advanced MTSCCs, no standard management has yet been proposed.
We herein report a case of MTSCC of the kidney that presented with lymph node metastases at the time of presentation and progressed rapidly even after surgery and systemic therapy.
Case presentation
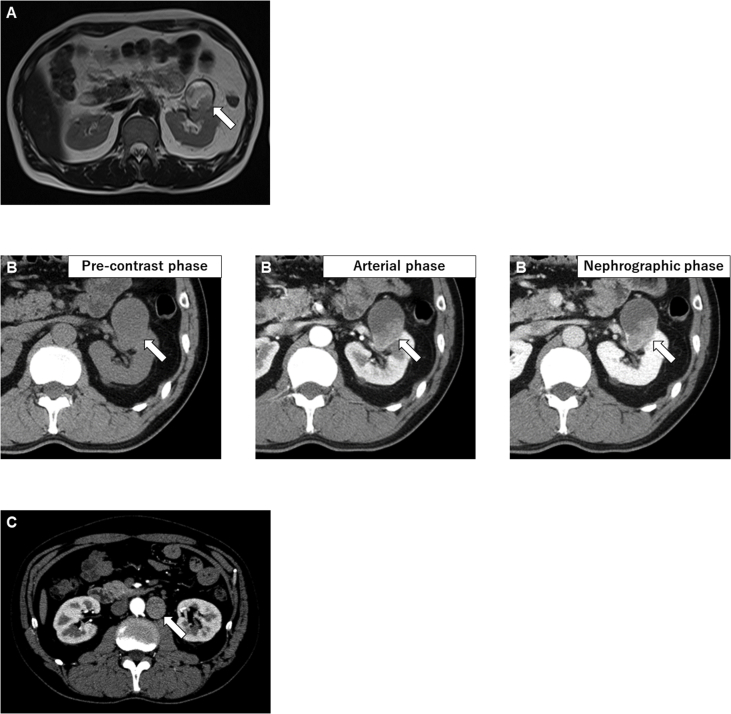
A 43-year-old Japanese man consulted our hospital for a cystic mass on his left kidney observed on ultrasonography performed as part of a complete medical checkup at another hospital. He had no symptoms and no past medical history. Magnetic resonance imaging (MRI) revealed a 50-mm cystic tumor in the superior pole of the left kidney (Fig. 1A). Dynamic computed tomography (CT) revealed a lesion that was slightly enhanced in the nephrographic and excretory phases but did not show strong enhancement in the arterial phase, as is usually observed in clear cell RCC (Fig. 1B). The renal hilar lymph nodes and the paraaortic lymph nodes were swollen. On CT performed after 1 month, the lymph nodes were markedly enlarged (Fig. 1C). Considering a preoperative diagnosis of RCC with lymph node metastases, radical nephrectomy with lymph nodes resection was performed. The lymph nodes adhered to the aorta. The left kidney and the lymph nodes were resected en bloc.
Fig. 1.
Abdominal CT and MRI at presentation.
T2-weighed MRI showing a tumor in the superior pole of the left kidney (A, arrow). CT showing a mass of the left kidney (B, arrows). The paraaortic lymph node were markedly swollen (C, arrow).
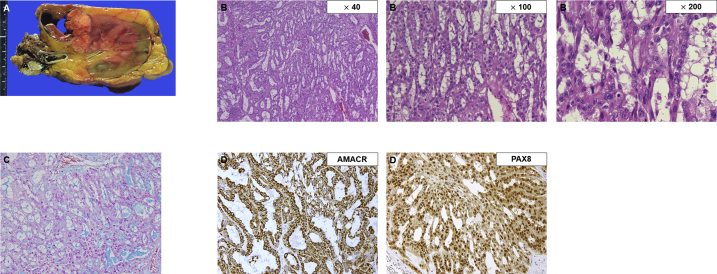
On gross examination, the tumor on the left kidney comprised yellowish multi-nodules with fibrous capsules/septa and hemorrhage (Fig. 2A). Microscopic examination revealed an admixture of non-clear cuboidal cells arranged in a various size of tubules and long cords with abrupt transition to spindle tumor cells (Fig. 2B). A subset of tumor cells showed nuclear pleomorphism with prominent nucleoli, of which the Fuhrman nuclear grade was 4. Mucin was observed in the interstitium of the tumor on alcian blue staining (Fig. 2C). Immunohistochemical analysis revealed that the tumor cells were diffusely positive for alpha-methylacyl-CoA racemase (AMACR) and PAX8 (Fig. 2D), and negative for CK7, CD10, and S100 protein. The morphological appearance and immunohistochemical profile were consistent with MTSCC of the kidneys (pT3aN2). No sarcomatoid area was identified in the specimen. Moreover, the specimen from the metastatic lymph nodes revealed a tumor with an identical appearance to the primary mass.
Fig. 2.
Histopathological features of MTSCC of the kidneys.
Macroscope image of the surgically resected specimen (A). Microscopic findings showing neoplastic tubules and cords embedded in a mucinous background (B, magnification: × 40, × 100, × 200). A subset of tumor cells showing nuclear pleomorphism with prominent nucleoli (B, magnification: × 200). Mucin production of the tumor cells observed via alcian blue staining (C, magnification: × 200). Immunohistochemical staining showing that the tumor was positive for AMACR (D, left, magnification: × 200) and PAX8 (D, right, magnification: × 200). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
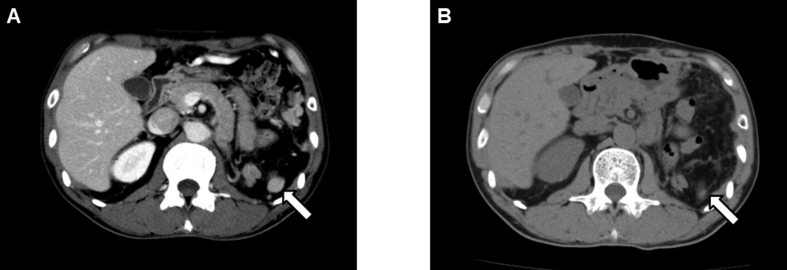
Peritoneal dissemination was detected via CT performed 4 months after surgery, following which the patient received systemic treatment with a tyrosine kinase inhibitor, sunitinib (Fig. 3A). The disseminated tumors shrunk in response to sunitinib, and the disease status was stable for 3 months (Fig. 3B). However, the patient showed ascites formation 9 months after the surgery and experienced abdominal distention. Therefore, another tyrosine kinase inhibitor, axitinib, was administered to treat the disseminated lesions, but it was not effective. Nivolumab was subsequently administered, but the patient finally died due to the disease 12 months after the surgery.
Fig. 3.
CT after surgery.
CT showing peritoneal dissemination 4 months after surgery (A, arrow) and the disseminated tumor shrinkage after sunitinib administration (B, arrow).
Discussion
MTSCC of the kidney is a rare disease. It is morphologically characterized by tubules of cords of cuboidal cells that transition to sheets of spindle cell components, separated by variable amounts of mucinous stroma.2 Extracellular mucinous/myxoid matrix is usually abundant in many cases, and alcian blue staining can highlight the scant mucin in the tumor.3 Histologically, it is very challenging to distinguish MTSCC with high-grade nuclear features from papillary renal cell carcinoma with solid architecture or other subtypes of RCC with tubular architecture and spindle cells.
Patients with MTSCC of the kidney treated with surgical resection tend to have generally favorable outcomes.1 Recently, however, fatal cases of MTSCC with distant metastases have been reported.1 In particular, sarcomatoid changes in MTSCC can be observed in aggressive cases during the clinical course.4 On histological examination, the sarcomatoid component shows high-grade spindle cells, with marked nuclear atypia and pleomorphism, and prominent nucleoli, with necrosis and a high proliferation index, differing from the spindle cell population frequently visible in typical MTSCC.5 In our case, however, the tumor did not show any sarcomatoid changes but presented with lymph node metastases and rapid progression. The tumor contained a high nuclear grade, and invasion of the tumor cells into the lymphatic vessels and metastases to the regional lymph nodes were found histologically. These findings suggested that the MTSCC in our case was aggressive and had possibly a poor prognosis with shorter disease-free survival. Cases of MTSCC without sarcomatoid change but with metastasis are very rare and lack molecular characterization to confirm their classification.
Owing to the rarity of advanced MTSCC of the kidney, there is little consensus on adequate therapeutic management. In the current case, the patient was treated with sunitinib, one of the standard first-line treatments for advanced clear cell RCC. Although the tumor responded initially, it progressed with ascites formation after 4 months. The molecular pathology of MTSCC is not fully elucidated, and hence, it is impossible to determine the affected targets during response to therapy. Further studies including genomic molecular testing are thus needed to determine the appropriate systemic treatment for metastatic MTSCC.
Conclusion
We presented a metastatic MTSCC with no sarcomatoid changes, which showed an aggressive biological behavior, resulting in patient death. More studies with a larger number of patients with this rare disease are needed.
Consent
Written informed consent was obtained from the wife of the patient for publication of this case report and accompanying images.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
Makoto Isono: Conceptualization, Methodology, Writing- Original draft preparation.
Kenji Seguchi: Data curation, Writing- Reviewing and Editing.
Masanori Yamanaka: Data curation.
Kosuke Miyai: Data curation, Writing- Reviewing and Editing.
Kazuki Okubo: Data curation.
Keiichi Ito: Writing- Reviewing and Editing, Visualization.
Declaration of competing interest
We have no conflict of interest to declare.
Acknowledgements
The authors like to thank Akira Hebisawa, Atsushi Kitani and Tomohiko Asano for their excellent technical assistance.
Contributor Information
Makoto Isono, Email: mktisn@hotmail.com.
Kenji Seguchi, Email: seguchi-kenji@tokyo-hosp.jp.
Masanori Yamanaka, Email: yamanakam@tokyo-hosp.jp.
Kosuke Miyai, Email: mykusu228@nifty.com.
Kazuki Okubo, Email: otti1104@gmail.com.
Keiichi Ito, Email: itok@ndmc.ac.jp.
References
- 1.Ged Y., Chen Y.B., Knezevic A. Mucinous tubular and spindle-cell carcinoma of the kidney: clinical features, genomic profiles, and treatment outcomes. Clin Genitourin Canc. 2019;17:268–274. doi: 10.1016/j.clgc.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srigley J.R., Delahunt B. Uncommon and recently described renal carcinomas. Mod Pathol. 2009;22(Suppl 2):S2–S23. doi: 10.1038/modpathol.2009.70. [DOI] [PubMed] [Google Scholar]
- 3.Fine S.W., Argani P., DeMarzo A.M. Expanding the histologic spectrum of mucinous tubular and spindle cell carcinoma of the kidney. Am J Surg Pathol. 2006;30(12):1554–1560. doi: 10.1097/01.pas.0000213271.15221.e3. [DOI] [PubMed] [Google Scholar]
- 4.Pillay N., Ramdial P.K., Cooper K., Batuule D. Mucinous tubular and spindle cell carcinoma with aggressive histomorphology--a sarcomatoid variant. Hum Pathol. 2008;39(6):966–969. doi: 10.1016/j.humpath.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhao M., He X.L., Teng X.D. Mucinous tubular and spindle cell renal cell carcinoma: a review of clinicopathologic aspects. Diagn Pathol. 2015;10:168. doi: 10.1186/s13000-015-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]