Summary
Sickle cell trait (SCT) is the benign heterozygous carrier state for the sickle variant of the HBB gene. Most of the ~300 million people with SCT worldwide will not experience any significant complications. However, accumulating evidence finds SCT associated with increased risk for the common conditions of chronic kidney disease and venous thromboembolism, and severe but rare renal medullary carcinoma and exercise-induced rhabdomyolysis. The mechanism is uncertain, but probably involves pathological rheology of sickle trait blood in regions of low oxygen tension, resulting from sickle haemoglobin polymerization in SCT red cells and leading to reduced blood flow and further tissue hypoxia and damage. Here, we used an in vitro microfluidic flow system to study the oxygen-dependent rheology of SCT blood and show that 5-(hydroxymethyl)furfural (5HMF), a natural breakdown product of glucose and fructose-containing foods, such as fruit juices, can reduce the effects of hypoxia on sickle trait blood rheology in vitro, restoring near-normal flow velocities at very low oxygen. While opinions regarding the clinical significance of the risks associated with SCT are still evolving, these results suggest that a compound present in some food may provide a potential approach for managing risks that may be associated with SCT.
Keywords: sickle cell trait, blood flow, clinical risk reduction, rheology, microfluidics, 5HMF
Introduction
Sickle cell trait (SCT) is a heterozygous genetic state consisting of one variant sickle HBB allele and one normal HBB allele. About 1.5% of newborns in the United States have SCT, with a higher rate (7.3%) among African-Americans. SCT leads to a sickle haemoglobin (HbS) fraction in red blood cells (RBCs) of roughly 40%, lower than the ~80% or more typical for sickle cell disease (SCD) (Bain 2006, Lu, et al 2018, Noguchi, et al 1981). This lower HbS fraction, and thus lower HbS concentration, in SCT RBCs reduces haemoglobin polymerization and cell sickling until the oxygen tension drops much lower than is necessary for significant polymer to form in SCD RBCs (Barabino, et al 2010, Bunn 1997, Eaton and Bunn 2017, Higgins, et al 2007, Lu, et al 2018, Lu, et al 2017, Lu, et al 2016, Noguchi, et al 1981, Wood, et al 2012). SCT provides protection against severe outcomes in malaria infection, with one study finding that malaria patients with SCT had about a 4-fold lower risk of requiring hospitalization than those with two normal (AA) HBB alleles (Uyoga, et al 2019). While SCT RBCs do form some polymer at physiological oxygen tensions, people with SCT rarely experience symptoms or clinical consequences. Nevertheless, accumulating epidemiological evidence finds that SCT is associated with increased risk for some medical problems (Conn 1954, Goldsmith, et al 2012, Gupta, et al 1991, Harris, et al 2012, Hu, et al 2019, Jones, et al 1970, Key, et al 2015, Mitchell 2007, Mitchell 2018, Naik, et al 2014, Naik, et al 2018, Nelson, et al 2016, Pecker and Naik 2018, Skinner, et al 2018, Tsaras, et al 2009), including modestly increased risk for chronic kidney disease (CKD) and venous thromboembolism (VTE).
The mechanism by which SCT increases risk for these outcomes is uncertain, but epidemiological evidence and clinical observations suggest that polymerization of HbS in SCT RBCs under physiological conditions of low oxygen is an important factor (Pecker and Naik 2018). HbS concentrations are high enough in SCT RBCs for polymerization to occur in the low oxygen and acidic environment of the renal medulla or muscle under the most extreme exercise conditions (Lu, et al 2018, Noguchi, et al 1981). Previous work has shown that urine concentrating ability in SCT correlates with HbS fraction in individuals (Gupta, et al 1991), as would be expected if HbS polymerization played a causal role in increasing the risk of chronic renal dysfunction in SCT. The mechanism for the link between SCT and VTE is less clear, but one reported explanation is that hypoxia in regions of venous valves leads to polymerization and an increased risk of embolus formation (Hamer, et al 1981, Pecker and Naik 2018).
While multiple recent studies do find that people with SCT have statistically significant increased risks compared to people without SCT, the assessment of the clinical significance of these risks is evolving (Goldsmith, et al 2012, Hu, et al 2019, Naik, et al 2018, Pecker and Naik 2018). The extremely benign expectation for SCT in general means that only the safest approaches, like lifestyle or dietary modification, would probably ever be considered for potentially trying to manage these risks. Many current and experimental SCD treatments appear to reduce polymerization but have risk- or side-effect profiles that are far from acceptable for SCT: transfusion, hydroxycarbamide, L-glutamine, GBT440 (Dufu, et al 2016), for which a clinical trial recently completed (Vichinsky, et al 2019), other SCD treatments in development (Demirci, et al 2019), and former SCD drug candidates, such as senicapoc, which was previously studied for SCD and withdrawn (Ataga, et al 2011). 5-(hydroxymethyl)furfural (5HMF) has been shown to reduce HbS polymerization in SCD RBCs at low oxygen and is a naturally-occurring breakdown product of glucose and fructose present in many foods, including fruit juices (Abdulmalik, et al 2005, Abraham, et al 1991, Lin, et al 2008, Shapla, et al 2018a). 5HMF has been studied for SCD (NCT01597401, NCT01871142, NCT01987908), though detailed peer-reviewed reports of these studies are not available. 5HMF is an aromatic aldehyde that allosterically shifts the haemoglobin oxygen equilibrium curve, increasing Hb-oxygen affinity. The increased Hb-oxygen affinity reduces HbS polymerization along with its downstream complications (Abdulmalik, et al 2005, Abraham, et al 1991, Lin, et al 2008). The toxicity profile of 5HMF is low (Abdulmalik, et al 2005), with the average human regularly consuming 30–150 mg per day from food (Shapla, et al 2018b). 5HMF is currently available for human consumption, for instance as a “food grade” compound (https://www.sigmaaldrich.com/catalog/product/aldrich/w501808) or as a “flavour ingredient” (https://www.treatt.com/products/chemicals/high-impact-aroma-chemicals). Previous human studies of 5HMF involving consumption of dried plums, plum juice or honey have achieved plasma concentrations of 5HMF and metabolites comparable to those evaluated here (Prior, et al 2006).
Because 5HMF may meet the stringent safety requirements for any viable approach for managing risks associated with SCT, and because it has a potentially effective mechanism of action, we studied its effect on the low-oxygen rheology of SCT blood in vitro using a microfluidic platform (Figure 1) that we previously showed can recapitulate the low-oxygen rheological behaviour of blood from individuals with SCT and SCD (Higgins, et al 2007, Lu, et al 2018, Lu, et al 2017, Lu, et al 2016, Wood, et al 2012). It is important to emphasize that this study does not alter the currently evolving assessment of the clinical significance of the risks associated with SCT.
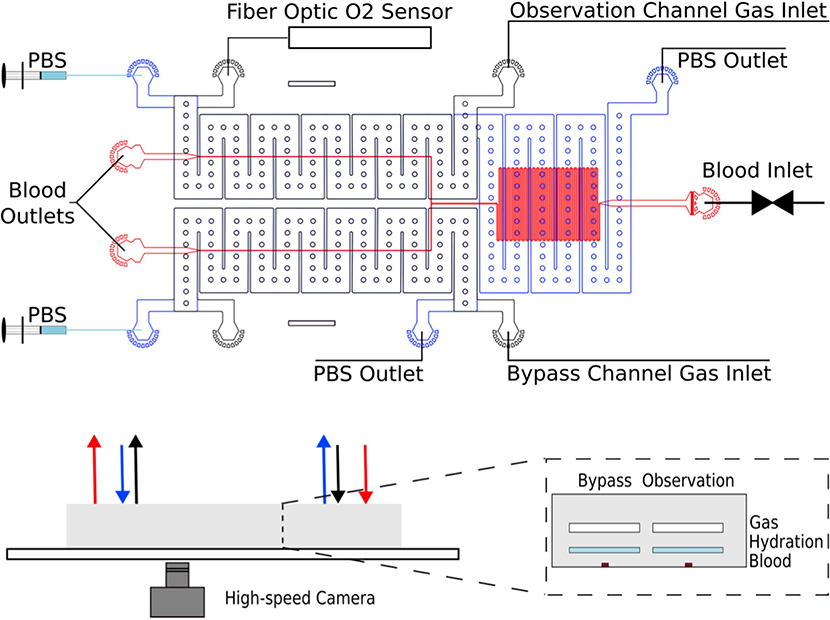
Figure 1. Microfluidic device layout and experimental setup for measuring oxygen-dependent blood rheology.
The device consists of three layers of poly(dimethylsiloxane) (PDMS), as shown above in the bottom right: 1) a top gas layer, 2) a middle hydration layer and 3) a blood layer bonded to a glass slide. During an experiment, blood flows from the inlet (“Blood Inlet”) at a constant pressure controlled by a pressure regulator and collected at the outlet ports. A high-speed camera captures images of blood flowing through the device. The oxygen concentration of the gas passing through the observation channel is controlled by an upstream mixing system and measured by a fibre optic oxygen sensor at the outlet. Separate pressure regulators are used to drive gas through the observation and bypass channels. PBS is driven countercurrent to the flow of gas through the device at a rate of 1000μ/h to prevent dehydration from the blood channel.
Materials and Methods
Blood Sample Collection
Whole blood samples were collected from individuals with SCT at the Massachusetts General Hospital and studied under protocols approved by the Institutional Review Boards at Partners Healthcare and the University of Minnesota. SCT status was confirmed by high performance liquid chromatography. Blood samples were stored in 5 ml EDTA vacutainers at 4°C until testing. The blood samples were centrifuged and washed three times with phosphate-buffered saline (PBS) to remove all plasma. Packed red cells were then combined with PBS to achieve a target haematocrit of 25%. Previous studies have shown that storage under these conditions for up to 4 days and resuspension with PBS do not significantly alter rheological properties in this type of microfluidic system (Lu, et al 2018, Lu, et al 2016, Wood, et al 2012). The samples were treated with a stock solution of 0.25 M 5HMF in PBS to achieve a concentration of 1 mM 5HMF and incubated at 37°C for 1 h. After incubation, the samples were washed a final time and stored at 4°C until ready for use. To ensure that any 5HMF effects were not mediated by treatment-induced changes in the mean corpuscular haemoglobin concentration (MCHC), we measured full blood count indices in a representative SCT specimen before and after treatment and found no significant changes. See Table S1 for details.
Microfluidic Device Fabrication
The microfluidic device design consisted of three poly(dimethylsiloxane) (PDMS) layers: 1) A top gas layer to control the oxygen concentration in the device, 2) a middle hydration layer for PBS circulation to prevent dehydration of the blood channel, and 3) a bottom blood channel plasma bonded to a glass slide. Each master mould was created using soft lithography techniques as described previously (Wood, et al 2012).
Experimental Setup
Devices were mounted in a Zeiss Axio Vert.A1 microscope in a 37°C environment. Blood flow through the device was driven at a constant pressure by a pressure regulator with shear rates matched across all samples. Syringe pumps pushed PBS through the hydration layer at a rate of 1000 μl/h to prevent dehydration of the cells. Specific oxygen concentrations in the device were achieved using a previously described mixing setup (Lu, et al 2017, Lu, et al 2016) supplied by air (21% O2, 5% CO2, 74% N2) and nitrogen (95% N2, 5% CO2) tanks and controlled by a pressure regulator (Omega, Norfolk, CT). A fibre optic oxygen sensor (NeoFox-GT, Ocean Optics, Dunedin, FL) recorded the oxygen concentration in the gas channel of the device.
Rheological Measurements
Rheological data was collected using a high-speed camera and a tracking algorithm to determine blood velocity within the micro-channel, as described previously (Lu, et al 2018, Wood, et al 2012). A high-speed camera captures images at 700 frames per second. Blood velocities are obtained by applying a feature-tracking algorithm to the videos based on Lucas-Kanade-Tomasi feature tracking in MATLAB (The MathWorks, Natick, MA). Figure 2 shows the typical difference in the flow velocity as a function of oxygen, as measured in the device for a healthy blood sample and a SCT blood sample. Reported velocities are based on the average velocity in the middle 50% of the channel width. Data from the beginning of each new oxygen cycle was excluded to allow for the stabilization of the blood flow to the new steady state velocity. Therefore, only the steady state velocity data in each cycle was used for analysis.
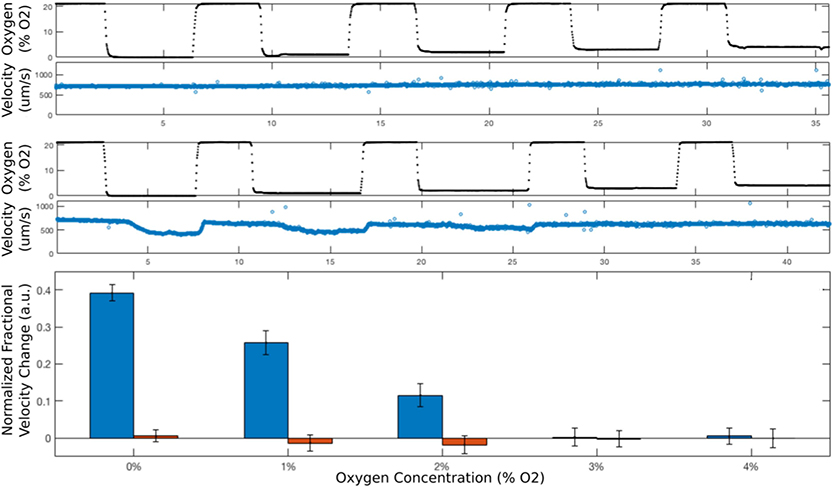
Figure 2. Flow velocity of SCT blood is oxygen-dependent at low oxygen.
(Top) For a normal blood sample (AA) containing only HbA, when the oxygen concentration (black dots in top panel) is cycled between 21% and low concentrations, first 0%, then 1%, 2%, 3%, 4%, the flow velocity (blue circles in the second panel from the top) does not change. In contrast, when the same oxygen concentration cycle (black dots in the third panel from the top) is applied to a flowing SCT blood sample (36% HbS), the flow velocity (blue circles in fourth panel from the top) drops at the lower oxygen concentrations because of sufficient hemoglobin polymerization and RBC sickling. The bottom panel compares the relative velocity changes of SCT and AA blood and shows that hemoglobin polymerization and RBC sickling are sufficient to reduce SCT flow velocity at oxygen concentrations at least as high as 2%. Error bars indicate one standard deviation.
Results
SCT Blood Flow Velocity is Oxygen-dependent at Low Oxygen Concentration
We find that the steady state in vitro flow velocity of SCT blood begins to fall when oxygen tension drops below about ~3% because haemoglobin in SCT RBCs will form significant polymer, and RBCs will sickle, increasing the effective viscosity. This behaviour is in contrast to normal (AA) blood, and our results are consistent with prior studies (Lu, et al 2018). For instance, the flow velocity of a representative SCT blood sample drops by about 40% when the oxygen concentration is lowered from 21% to 0% (Figure 2).
To quantify the effect of treatment with 5HMF on low-oxygen rheology, we focused on the maximum possible change and the sensitivity to oxygen: (1) the maximum potential rheological response to low oxygen is quantified by the relative change in flow velocity at 0% oxygen compared to that at 21%, and (2) the sensitivity of the blood sample is defined as the lowest oxygen concentration where there is no statistically significant reduction in flow velocity.
5HMF Significantly Reduces the Maximal Rheological Response to Low Oxygen in SCT Blood
We compared treated and untreated SCT samples by flowing them through the microfluidic device at constant pressure before and after incubation with 5HMF. The velocity response to oxygen was quantified as a fractional change in steady state velocity measured after lowering the oxygen concentration. Figure 3 shows that six untreated SCT blood samples had baseline rheological responses to 0% oxygen that varied from about a 7% reduction in velocity to about a 60% reduction. When treated with 1 mM 5HMF, velocity reductions at low oxygen were dramatically reduced, ranging from only 0.5% to 11%. The effect was dramatic regardless of the SCT sample’s baseline rheological response, with the sample that showed a baseline 60% reduction in velocity showing only about a 10% reduction following incubation with 1 mM 5HMF. These rheological effects are consistent with a dramatically increased haemoglobin-oxygen affinity for SCT blood treated with 5HMF. Figure S1 shows that the oxygen tension at which haemoglobin is 50% saturated (P50) of a representative SCT blood sample was left-shifted from a baseline of about 4.1% oxygen to about 0.95% oxygen following treatment with 5HMF. The effect of 5HMF, even when oxygen is undetectable, is surprising. This finding may partly reflect a direct effect on polymer stability previously described for allosteric effectors like 5HMF (Eaton and Bunn 2017). It is also possible that, despite an absence of oxygen in the source gas, a small amount of oxygen below the limit of detection of the oxygen sensor is diffusing into the system from the environment. The greatly increased oxygen affinity of SCT blood treated with 5HMF (Figure S1) suggests that oxygen tensions even below 0.5% may lead to detectable haemoglobin-oxygen saturation. Previous investigators have found that another allosteric effector, GBT440, also seems to inhibit polymer formation at near-zero oxygen concentration (Oksenberg, et al 2016), reporting that GBT440 reduces the fraction of sickled RBCs at 0% measured oxygen.
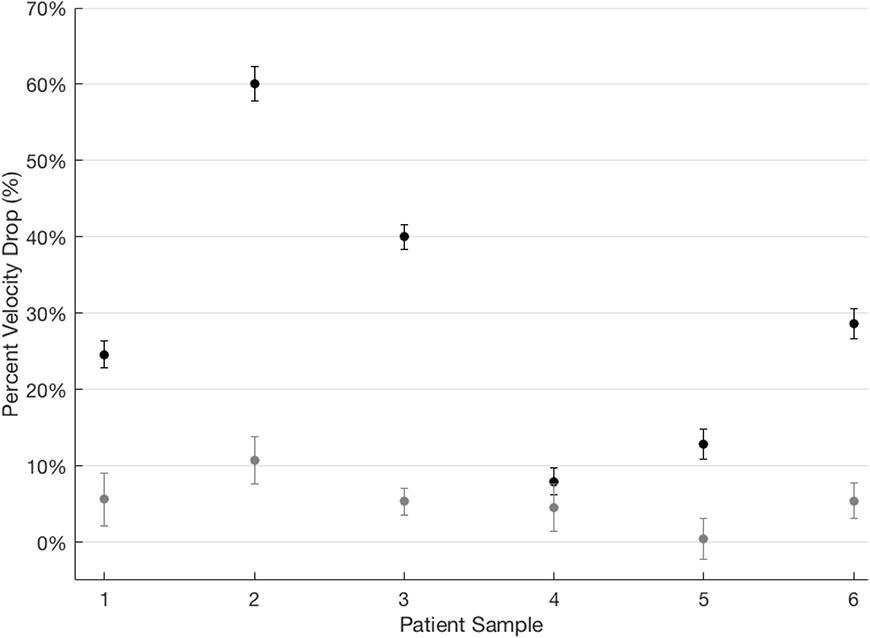
Figure 3. 5HMF significantly reduces the effect of 0% oxygen on SCT blood flow velocity.
The steady state flow velocity was measured for 6 SCT blood samples at 21% oxygen and then at 0% oxygen. (Because oxygen tension is controlled by diffusion, it is possible that there is a small nonzero oxygen concentration from ambient diffusion even when 0% oxygen is flowing into the system.) The percent reduction in flow velocity at 0% varied from 7% to 60% (black dots). After treatment with lmM 5HMF, the maximum velocity reduction at 0% oxygen was about 11% (grey dots). Responses represent the percent change in mean, steady state velocity after a drop from atmospheric to 0% oxygen concentration in the device. Differences are significant based on a Kruskal-Wallis one-way analysis of variance. Error bars represent one standard deviation of the propagated uncertainty.
5HMF Significantly Reduces the Rheological Sensitivity to Low Oxygen in SCT Blood
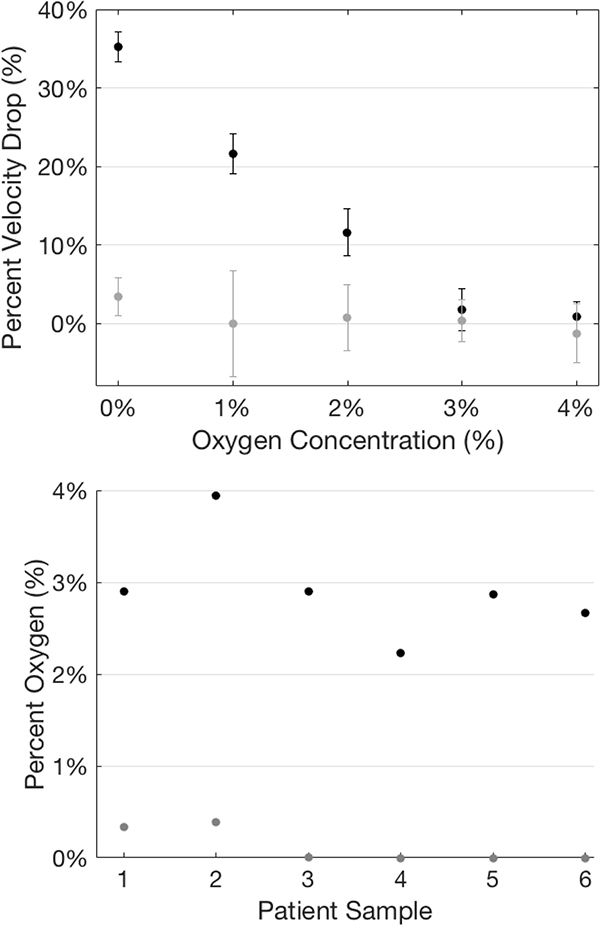
Another factor that may be important in vivo is the oxygen threshold below which pathological rheology will be seen. If that threshold oxygen concentration is below levels reached, even in hypoxic tissues under extreme conditions, then no complications due to these rheological changes would be expected. We therefore determined the lowest oxygen concentration at which each SCT blood sample showed a flow velocity indistinguishable from that at full oxygenation. Figure 4A compares the percent velocity change for a representative SCT sample before and after treatment with 1 mM 5HMF. At 4% and 3% oxygen, the flow velocity was not distinguishable from the fully oxygenated velocity, but somewhere below 3% oxygen, the rheology of this blood sample began to change, and its flow velocity dropped (black error bar no longer intersects 0% velocity drop). Following treatment with 1 mM 5HMF (grey dots), the flow velocity did not drop in response to falling oxygen concentration until the oxygen had been lowered almost to 0%. This sample’s untreated and treated thresholds were typical of the other five samples. Figure 4B shows that before treatment, all SCT samples showed rheological impairment above 2% oxygen. In contrast, after treatment, no sample showed rheological change until the oxygen concentration was lowered below 1% and almost to 0%. These untreated and treated distributions were significantly different based on a Kruskal-Wallis analysis variance.
Figure 4. 5HMF lowers the oxygen threshold at which velocity becomes oxygen-dependent.
(Top) Representative data from a single sample demonstrating the difference in velocity response to falling oxygen, both the treated and untreated conditions. (Bottom) Summary of oxygen threshold data for treated (Median = 0%) and untreated (Median = ~3%) samples. Threshold oxygen concentrations were defined as the concentration at which a change in velocity exceeded two standard deviations was observed in response to a sudden drop in oxygen concentration.
Discussion
We here report that 1 mM 5HMF dramatically reduces the low-oxygen rheological impairment seen for SCT blood flowing in a microfluidic device. Additional study is required to determine whether 5HMF has in vivo effects that may help alleviate any of the complications related to impaired rheology. It is important to emphasize that the results of this study do not alter the currently evolving assessment of the clinical significance of the risks associated with SCT (Conn 1954, Goldsmith, et al 2012, Gupta, et al 1991, Harris, et al 2012, Hu, et al 2019, Jones, et al 1970, Key, et al 2015, Mitchell 2007, Mitchell 2018, Naik, et al 2014, Naik, et al 2018, Nelson, et al 2016, Pecker and Naik 2018, Skinner, et al 2018, Tsaras, et al 2009). Instead, we focus narrowly on in vitro evidence suggesting that 5HMF is a treatment candidate worthy of further investigation. In general, the clinical decision to treat or to manage risk is made by weighing three factors: (i) an assessment of the risks of not treating, (ii) an assessment of the likelihood of benefit from a particular treatment or management approach and (iii) an assessment of the probability of side effects from that treatment or management approach (Pauker and Kassirer 1980). SCT is extremely benign, but the baseline risk of some complications for people with SCT is clearly different than for people without SCT, and this clinical decision process may therefore reach a different conclusion for SCT. While our study does not clarify the risk of not treating people with SCT, it does provide new and important in vitro evidence in support of the hypothesis that 5HMF might provide benefit for individuals with SCT. This study also does not directly clarify the risk of side effects that might be associated with 5HMF.
The mechanism for the reported associations between SCT and increased risk for clinical complications is not clearly established, but previous authors pointed to pathological low-oxygen rheology as a likely important factor, and additional epidemiological observations lend support (Gupta, et al 1991, Pecker and Naik 2018). Rheology of SCT blood is impaired in vitro at oxygen concentrations below 3% (Lu, et al 2018), and these oxygen concentrations seem to be achieved regularly in relevant tissues in vivo. Previous studies report oxygen concentrations below 2% in the human kidney (Brezis, et al 1984) and below 2.5% in many tissues of different model animals (Vanderkooi, et al 1991),(Spencer, et al 2014). The link between polymerization, sickling and kidney dysfunction is supported by a previous study of urine concentrating deficits in individuals with SCT (Gupta, et al 1991). Because HbS% is positively associated with polymer fraction levels and rheological change in SCT (Lu, et al 2018, Noguchi, et al 1981), this association between HbS% and kidney dysfunction is consistent with a mechanistic link between rheological impairment and kidney dysfunction in SCT.
This study demonstrates a novel application of this microfluidic experimental platform in the assessment of rheological responses to pharmacological interventions for SCT. This experimental system has previously been used to investigate SCD pathophysiology, including rheological mechanisms of vaso-occlusion (Higgins, et al 2007, Higgins, et al 2009, Lu, et al 2017, Lu, et al 2016), correlations of in vitro and in vivo phenotypes (Wood, et al 2012), and the use of in vitro SCT phenotypes as a treatment target for SCD (Lu, et al 2018). There are currently no good in vitro biomarkers for the management of SCD (Kalpatthi and Novelli 2018), and the need for their development has increased dramatically recently with the advent of experimental therapies for SCD whose optimization and comparative assessment crucially depends on the availability of validated in vitro markers (Benz, et al 2019).
This study provides only in vitro evidence and is limited to the investigation of blood samples from six individuals with SCT. The effect of 5HMF even at or near 0% measured oxygen tension is surprising but consistent with a reported direct oxygen-independent influence of allosteric effectors of haemoglobin on polymer stability (Eaton and Bunn 2017) and also consistent with prior reports that GBT440, a compound with a similar mechanism of action, reduces the sickled cell fraction at 0% measured oxygen (Oksenberg, et al 2016). Because oxygen tension in vivo rarely drops below even 1% oxygen (Spencer, et al 2014), the precise nature of 5HMF’s effects under absolute anoxic conditions may not be physiologically relevant. Safety and pharmacokinetic studies of 5HMF have been undertaken in patients with SCD (NCT01597401, NCT01871142, NCT01987908), but unfortunately there are no peer-reviewed reports of their results available. 5HMF increases the affinity of haemoglobin for oxygen at low oxygen concentration and will therefore reduce oxygen unloading. To offset this reduced oxygen unloading and to avoid exacerbating local tissue hypoxia, some improvement in flow velocity and tissue perfusion is necessary. The risks of another oxygen affinity-shifting molecule (GBT440) currently being evaluated as a drug candidate for SCD treatment have recently been discussed in detail (Hebbel and Hedlund 2018). In the case of SCD, patients are anaemic and thus have reduced tissue oxygen delivery at baseline. Increased haemoglobin oxygen affinity would further increase tissue hypoxia. For the present study, these concerns are mitigated because of differences between 5HMF and GBT440 and differences between SCT and SCD. First, 5HMF is less potent than GBT440, allowing some oxygen unloading during maximal exercise (Hebbel and Hedlund 2018). Also, individuals with SCT have haemoglobin concentrations and haematocrit equivalent to healthy non-SCT individuals (Tsaras, et al 2009) which would improve the effective haemoglobin concentration in the blood after a left-shift in the equilibrium curve. Nevertheless, experience with chronically higher levels of 5HMF in the diet or otherwise is limited, and it is unknown, for instance, if other proteins would be modified and functionally impaired. Careful follow-up investigations will be necessary for a more definitive assessment because the benign expectation for SCT makes any treatment-associated risks unacceptable.
Given the level of 5HMF present in many common foods, it is possible that some of the completed observational or retrospective cohort studies that determined the risk of complications for SCT collected enough information on subject dietary intake to explore a hypothesized protective effect derived from consumption of foods high in 5HMF (Goldsmith, et al 2012, Hu, et al 2019, Naik, et al 2018, Pecker and Naik 2018). If similar observational or natural history studies are planned in the future, this dietary intake information could be collected. Future investigators could also consider adding a study cohort encouraged to consume specified amounts of foods high in 5HMF, though adherence to recommendations for dietary modification is often limited even when evidence is much stronger than that presented here (Rosa, et al 1980), and treatment adherence is particularly challenging in individuals like those with SCT where symptoms are mild or non-existent (Nieuwlaat, et al 2014). It is possible that some SCT individuals are at higher risk of complications than others. For instance, there is in vivo evidence that the degree of hyposthenuria in SCT is associated with HbS fraction or alpha thalassaemia trait (Gupta, et al 1991) as well as in vitro evidence that the degree of oxygen-dependent rheological impairment in SCT blood is associated with HbS fraction (Lu, et al 2018). In general, if molecular or genetic markers are discovered that can identify higher risk subsets of SCT individuals, future investigations could focus on this subgroup, by both observational studies and those involving dietary modification. Any controlled clinical study of direct treatment with high-dose 5HMF would first require additional safety and pharmacokinetic studies or access to detailed reports of those previously done and may be infeasible given the duration that is probably required.
Supplementary Material
Acknowledgements
The authors were supported by NHLBI grant R01HL132906 to DKW. The authors thank Chhaya Patel and Hasmukh Patel for help processing clinical specimens and Akito Nakagawa for experimental expertise. The authors are grateful for helpful suggestions and thoughtful feedback provided by two anonymous reviewers, Robert Moloke, Jonathan Carlson, and multiple colleagues.
Footnotes
Conflicts of Interest
The authors have no known conflicts of interest related to this work.
References
- Abdulmalik O, Safo MK, Chen QK, Yang JS, Brugnara C, Ohene-Frempong K, Abraham DJ & Asakura T (2005) 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. British Journal of Haematology, 128, 552–561. [DOI] [PubMed] [Google Scholar]
- Abraham DJ, Mehanna AS, Wireko FC, Whitney J, Thomas RP & Orringer EP (1991) Vanillin, a potential agent for the treatment of sickle cell anemia. Blood, 77, 1334–1341. [PubMed] [Google Scholar]
- Ataga KI, Reid M, Ballas SK, Yasin Z, Bigelow C, St James L, Smith WR, Galacteros F, Kutlar A, Hull JH, Stocker JW & Investigators ICAS (2011) Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the gardos channel blocker senicapoc (ICA-17043). British Journal of Haematology, 153, 92–104. [DOI] [PubMed] [Google Scholar]
- Bain BJ (2006) Haemoglobinopathy diagnosis. Blackwell Pub., Malden, MA. [Google Scholar]
- Barabino GA, Platt MO & Kaul DK (2010) Sickle cell biomechanics. Annu Rev Biomed Eng, 12, 345–367. [DOI] [PubMed] [Google Scholar]
- Benz EJ Jr., Mondoro TH & Gibbons GH (2019) Accelerating the Science of SCD Therapies-Is a Cure Possible? JAMA. [DOI] [PubMed] [Google Scholar]
- Brezis M, Rosen S, Silva P & Epstein FH (1984) RENAL ISCHEMIA - A NEW PERSPECTIVE. Kidney International, 26, 375–383. [DOI] [PubMed] [Google Scholar]
- Bunn HF (1997) Pathogenesis and treatment of sickle cell disease. N Engl J Med, 337, 762–769. [DOI] [PubMed] [Google Scholar]
- Conn HO (1954) Sickle-cell trait and splenic infarction associated with high-altitude flying. N Engl J Med, 251, 417–420. [DOI] [PubMed] [Google Scholar]
- Demirci S, Leonard A, Haro-Mora JJ, Uchida N & Tisdale JF (2019) CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges. Adv Exp Med Biol. [DOI] [PubMed] [Google Scholar]
- Dufu K, Lehrer-Graiwer J, Ramos E & Oksenberg D (2016) GBT440 Inhibits Sickling of Sickle Cell Trait Blood Under In Vitro Conditions Mimicking Strenuous Exercise. Hematol Rep, 8, 6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WA & Bunn HF (2017) Treating sickle cell disease by targeting HbS polymerization. Blood, 129, 2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith JC, Bonham VL, Joiner CH, Kato GJ, Noonan AS & Steinberg MH (2012) Framing the research agenda for sickle cell trait: Building on the current understanding of clinical events and their potential implications. American Journal of Hematology, 87, 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Kirchner KA, Nicholson R, Adams JG 3rd, Schechter AN, Noguchi CT & Steinberg MH (1991) Effects of alpha-thalassemia and sickle polymerization tendency on the urine-concentrating defect of individuals with sickle cell trait. J Clin Invest, 88, 1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer JD, Malone PC & Silver IA (1981) THE PO2 IN VENOUS VALVE POCKETS - ITS POSSIBLE BEARING ON THROMBOGENESIS. British Journal of Surgery, 68, 166–170. [DOI] [PubMed] [Google Scholar]
- Harris KM, Haas TS, Eichner ER & Maron BJ (2012) Sickle cell trait associated with sudden death in competitive athletes. Am J Cardiol, 110, 1185–1188. [DOI] [PubMed] [Google Scholar]
- Hebbel RP & Hedlund BE (2018) Sickle hemoglobin oxygen affinity-shifting strategies have unequal cerebrovascular risks. Am J Hematol, 93, 321–325. [DOI] [PubMed] [Google Scholar]
- Higgins JM, Eddington DT, Bhatia SN & Mahadevan L (2007) Sickle cell vasoocclusion and rescue in a microfluidic device. Proceedings of the National Academy of Sciences of the United States of America, 104, 20496–20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JM, Eddington DT, Bhatia SN & Mahadevan L (2009) Statistical Dynamics of Flowing Red Blood Cells by Morphological Image Processing. PLoS Computational Biology, 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://clinicaltrials.gov/ct2/show/NCT01597401 A Single Dose Study of the Safety, Blood Levels and Biological Effects of Aes-103 Compared to Placebo in Subjects With Stable Sickle Cell Disease.
- https://clinicaltrials.gov/ct2/show/NCT01871142 Evaluation of the Attenuation by Aes-103 of Hypoxia Mediated Decrements in Endurance Exercise Performance.
- https://clinicaltrials.gov/ct2/show/NCT01987908 Evaluation of Different Dose Regimens of Aes-103 Given for 28 Days to Subjects With Stable Sickle Cell Disease.
- Hu J, Nelson DA, Deuster PA, Marks ES, O’Connor FG & Kurina LM (2019) Sickle cell trait and renal disease among African American U.S. Army soldiers. Br J Haematol, 185, 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Binder RA & Donowho EM Jr. (1970) Sudden death in sickle-cell trait. N Engl J Med, 282, 323–325. [DOI] [PubMed] [Google Scholar]
- Kalpatthi R & Novelli EM (2018) Measuring success: utility of biomarkers in sickle cell disease clinical trials and care. Hematology-American Society of Hematology Education Program, 2018, 482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key NS, Connes P & Derebail VK (2015) Negative health implications of sickle cell trait in high income countries: from the football field to the laboratory. British Journal of Haematology, 170, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AS, Qian K, Usami Y, Lin L, Itokawa H, Hsu C, Morris-Natschke SL & Lee KH (2008) 5-Hydroxymethyl-2-furfural, a clinical trials agent for sickle cell anemia, and its mono/di-glucosides from classically processed steamed Rehmanniae Radix. J Nat Med, 62, 164–167. [DOI] [PubMed] [Google Scholar]
- Lu X, Galarneau MM, Higgins JM & Wood DK (2017) A microfluidic platform to study the effects of vascular architecture and oxygen gradients on sickle blood flow. Microcirculation, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Chaudhury A, Higgins JM & Wood DK (2018) Oxygen-dependent flow of sickle trait blood as an in vitro therapeutic benchmark for sickle cell disease treatments. Am J Hematol, 93, 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XR, Wood DK & Higgins JM (2016) Deoxygenation Reduces Sickle Cell Blood Flow at Arterial Oxygen Tension. Biophysical Journal, 110, 2751–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BL (2007) Sickle cell trait and sudden death--bringing it home. J Natl Med Assoc, 99, 300–305. [PMC free article] [PubMed] [Google Scholar]
- Mitchell BL (2018) Sickle Cell Trait and Sudden Death. Sports Med Open, 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik RP, Derebail VK, Grams ME, Franceschini N, Auer PL, Peloso GM, Young BA, Lettre G, Peralta CA, Katz R, Hyacinth HI, Quarells RC, Grove ML, Bick AG, Fontanillas P, Rich SS, Smith JD, Boerwinkle E, Rosamond WD, Ito K, Lanzkron S, Coresh J, Correa A, Sarto GE, Key NS, Jacobs DR, Kathiresan S, Bibbins-Domingo K, Kshirsagar AV, Wilson JG & Reiner AP (2014) Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA, 312, 2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik RP, Smith-Whitley K, Hassell KL, Umeh NI, de Montalembert M, Sahota P, Haywood C, Jenkins J, Lloyd-Puryear MA, Joiner CH, Bonham VL & Kato GJ (2018) Clinical Outcomes Associated With Sickle Cell Trait A Systematic Review. Annals of Internal Medicine, 169, 619–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Deuster PA, Carter R 3rd, Hill OT, Wolcott VL & Kurina LM (2016) Sickle Cell Trait, Rhabdomyolysis, and Mortality among U.S. Army Soldiers. N Engl J Med, 375, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, Sivaramalingam B, Iserman E, Mustafa RA, Jedraszewski D, Cotoi C & Haynes RB (2014) Interventions for enhancing medication adherence (Review). Cochrane Database of Systematic Reviews, Issue 11. Art. No.: CD000011. DOI: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi CT, Torchia DA & Schechter AN (1981) Polymerization of hemoglobin in sickle trait erythrocytes and lysates. J Biol Chem, 256, 4168–4171. [PubMed] [Google Scholar]
- Oksenberg D, Dufu K, Patel MP, Chuang CH, Li Z, Xu Q, Silva-Garcia A, Zhou CJ, Hutchaleelaha A, Patskovska L, Patskovsky Y, Almo SC, Sinha U, Metcalf BW & Archer DR (2016) GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. British Journal of Haematology, 175, 141–153. [DOI] [PubMed] [Google Scholar]
- Pauker SG & Kassirer JP (1980) The threshold approach to clinical decision-making. New England Journal of Medicine, 302, 1109–1117. [DOI] [PubMed] [Google Scholar]
- Pecker LH & Naik RP (2018) The current state of sickle cell trait: implications for reproductive and genetic counseling. Blood, 132, 2331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior RL, Wu XL & Gu LW (2006) Identification and urinary excretion of metabolites of 5-(hydroxymethyl)-2-furfural in human subjects following consumption of dried plums or dried plum juice. Journal of Agricultural and Food Chemistry, 54, 3744–3749. [DOI] [PubMed] [Google Scholar]
- Rosa RM, Bierer BE, Thomas R, Stoff JS, Kruskall M, Robinson S, Bunn HF & Epstein FH (1980) A STUDY OF INDUCED HYPONATREMIA IN THE PREVENTION AND TREATMENT OF SICKLE-CELL CRISIS. New England Journal of Medicine, 303, 1138–1143. [DOI] [PubMed] [Google Scholar]
- Shapla UM, Solayman M, Alam N, Khalil MI & Gan SH (2018a) 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: effects on bees and human health. Chemistry Central Journal, 12, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapla UM, Solayman M, Alam N, Khalil MI & Gan SH (2018b) 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: effects on bees and human health. Chem Cent J, 12, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigma-Aldrich (2019) https://www.sigmaaldrich.com/catalog/product/aldrich/w501808. Vol. 2019. [Google Scholar]
- Skinner SC, Diaw M, Pialoux V, Mbaye MN, Mury P, Lopez P, Bousquet D, Gueye F, Diedhiou D, Joly P, Renoux C, Sow D, Diop S, Ranque B, Vinet A, Samb A, Guillot N & Connes P (2018) Increased Prevalence of Type 2 Diabetes-Related Complications in Combined Type 2 Diabetes and Sickle Cell Trait. Diabetes Care, 41, 2595–2602. [DOI] [PubMed] [Google Scholar]
- Spencer JA, Ferraro F, Roussakis E, Klein A, Wu JW, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Cote D, Vinogradov SA, Scadden DT & Lin CP (2014) Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature, 508, 269–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treatt P (2019) https://www.treatt.com/products/chemicals/high-impact-aroma-chemicals.
- Tsaras G, Owusu-Ansah A, Boateng FO & Amoateng-Adjepong Y (2009) Complications associated with sickle cell trait: a brief narrative review. Am J Med, 122, 507–512. [DOI] [PubMed] [Google Scholar]
- Uyoga S, Macharia AW, Ndila CM, Nyutu G, Shebe M, Awuondo KO, Mturi N, Peshu N, Tsofa B, Scott JAG, Maitland K & Williams TN (2019) The indirect health effects of malaria estimated from health advantages of the sickle cell trait. Nature Communications, 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi JM, Erecinska M & Silver IA (1991) Oxygen in mammalian tissue - methods of measurement and affinities of various reactions. American Journal of Physiology, 260, C1131–C1150. [DOI] [PubMed] [Google Scholar]
- Vichinsky E, Hoppe CC, Ataga KI, Ware RE, Nduba V, El-Beshlawy A, Hassab H, Achebe MM, Alkindi S, Brown RC, Diuguid DL, Telfer P, Tsitsikas DA, Elghandour A, Gordeuk VR, Kanter J, Abboud MR, Lehrer-Graiwer J, Tonda M, Intondi A, Tong B & Howard J (2019) A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- Wood DK, Soriano A, Mahadevan L, Higgins JM & Bhatia SN (2012) A Biophysical Indicator of Vaso-occlusive Risk in Sickle Cell Disease. Science Translational Medicine, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.