Abstract
Context
Polycystic ovary syndrome (PCOS) is associated with decreased health-related quality of life (HRQoL), but longitudinal data beyond the reproductive years are lacking, and the impact of isolated PCOS symptoms is unclear.
Objective
To study generic HRQoL using the 15D questionnaire, life satisfaction, and self-reported health status in women with PCOS symptoms at ages 31 and 46 years.
Design
A longitudinal assessment using the Northern Finland Birth Cohort 1966.
Setting
General community.
Participants
The 15D data were available for women reporting isolated oligo-amenorrhea (OA; at age 31 years, 214; and 46 years, 211), isolated hirsutism (H; 31 years, 211; and 46 years, 216), OA + H (PCOS; 31 years, 74; and 46 years, 75), or no PCOS symptoms (controls; 31 years, 1382; and 46 years, 1412). Data for life satisfaction and current health status were available for OA (31 years, 329; and 46 years, 247), H (31 years, 323; and 46 years, 238), PCOS (31 years, 125; and 46 years, 86), control (31 years, 2182; and 46 years, 1613) groups.
Intervention(s)
None.
Main Outcome Measure(s)
15D HRQoL, questionnaires on life satisfaction, and self-reported health status.
Results
HRQoL was lower at ages 31 and 46 in women with PCOS or H than in the controls. PCOS was an independent risk factor for low HRQoL, and the decrease in HRQoL in PCOS was similar to that of women with other chronic conditions, such as asthma, migraine, rheumatoid arthritis, and depression. The risk for low HRQoL in PCOS remained significant after adjusting for body mass index, hyperandrogenism, and socioeconomic status. Mental distress was the strongest contributing factor to HRQoL. PCOS was also associated with a risk for low life satisfaction and a 4-fold risk for reporting a poor health status.
Conclusions
Women with PCOS present with low HRQoL, decreased life satisfaction, and a poorer self-reported health status up to their late reproductive years. Assessments and interventions aiming to improve HRQoL in PCOS should be targeted beyond the fertile age.
Keywords: PCOS, hirsutism, QoL, testosterone, FAI, aging
Polycystic ovary syndrome (PCOS) is a common yet underdiagnosed syndrome affecting 6% to 18% of women at reproductive age (1,2). Although the syndrome has been characterized primarily as a reproductive and metabolic disorder, its effects extend beyond metabolic and fertility-related issues (3–5). Indeed, based on recent study results, it has become evident that women with PCOS have a high prevalence of psychological morbidity into the late reproductive years (6,7). Three main components are thought to contribute to the ill health of PCOS-affected women, decreasing their health-related quality of life (HRQoL): (1) physical health issues, such as adverse body composition, increased fat accumulation, diabetes, metabolic syndrome, irregular menstrual cycles, and infertility; (2) esthetic concerns, such as acne, hirsutism, female pattern hair loss, and obesity; (3) psychological issues, such as depression, anxiety, body image, and disordered eating (8,9).
Previous studies have focused on women with PCOS during their reproductive years, reporting lower HRQoL in affected women than non-PCOS control groups (10–12). Low HRQoL has been associated with psychological morbidity and health problems in the general female population (13). Similarly, in PCOS, lower HRQoL has been associated with depression and anxiety (5,10,11,14). Recent studies have, however, revealed that psychological distress is not commonly associated with PCOS or ranked highly among other PCOS symptoms by either physicians or patients, which may delay further symptom screening, diagnoses, and treatments (15,16). As for other causes for lower QoL in PCOS, obesity seems to drive low HRQoL in affected women (17–19). As the body mass index (BMI) trajectories in PCOS begin deviating from those in other women very early in childhood or at puberty, resulting in long-term obesity, it is no wonder women experience impaired health and high stress levels (20–22). Moreover, the esthetic burden of hirsutism should not be overlooked in PCOS, as it has been identified as one of the strongest components related to psychological distress in affected women (11,23,24). This is no surprise, considering the limited effectiveness of current treatment outcomes for this symptom and the social pressure to conform to cultural ideals. Finally, infertility has been shown to decrease QoL in the general female population (25), including in PCOS (26).
Most of the previously conducted studies assessing QoL in PCOS have been small and have primarily included women of reproductive age selected from PCOS clinics. These studies have not included follow-up. Moreover, no studies on the impact of individual PCOS symptoms exist. In the present study we utilized the Northern Finland Birth Cohort 1966 (NFBC1966). This is a unique longitudinal dataset comprising all individuals born in 1966 in Northern Finland area (n = 5889 females). For the present study, the follow-up data at ages 31 and 46 were analyzed. Women with PCOS or the isolated PCOS symptoms oligo-amenorrhea (OA) or hirsutism (H) have also been identified, enabling the evaluation of the impact of PCOS, as well as the isolated symptoms, on QoL.
As some PCOS-related issues and symptoms resolve over time (infertility and menstrual irregularities) while hormonal and metabolic morbidity as well as psychological distress remain, the dataset provides a unique opportunity to assess HRQoL until the late reproductive years. The data set also allowed investigation of the factors previously suggested to aggravate HRQoL in women with PCOS (ie, obesity, biochemical hyperandrogenism, infertility, anxiety, and depressive symptoms). To estimate the health burden related to PCOS, the mean HRQoL scores in women with PCOS were compared with the mean HRQoL of women with other chronic conditions, such as asthma, migraine, rheumatoid arthritis, and depression, which have been shown to reduce HRQoL (13). A second aim for this research was to assess the self-reported life satisfaction and health statuses at ages 31 and 46 in women with PCOS symptoms in order to determine whether these factors were strongly associated with poor HRQoL.
Materials and Methods
Study population
The NFBC1966 population and the validation of characteristics related to PCOS diagnosis have been described previously (6,20,27,28). In brief, the cohort population comprises all individuals in the Northern Finland area with expected birth in 1966 (total n = 12 058, females n = 5889), representing 96.3% of all births in this region (29). This study utilized the data from follow-up at ages 31 and 46. The characteristics of the study groups are presented in Table 1. At both points, the data were gathered by sending a questionnaire to all cohort subjects and by inviting them to clinical examinations.
Table 1.
Clinical characteristics of women with oligo-amenorrhea (OA), hirsutism (H), and PCOS (OA + H) at ages 31 and 46 compared with women without PCOS symptoms.
| Character | Age | Ctrl at age 31 N = 1382–2182 at age 46 N = 1412–1613 | OA at age 31 N = 214–329 at age 46 N = 211–247 | H at age 31 N = 211–323 at age 46 N = 216–238 | PCOS at age 31 N = 74–125 at age 46 N = 75–86 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 31 | 23.8 ± 4.3 | 24.8 ± 5.3** | 24.3 ± 4.6 | 27.3 ± 6.9*** | ||||||||
| 46 | 26.4 ± 5.3 | 27.3 ± 5.7* | 27.3 ± 6.0** | 29.1 ± 6.0*** | |||||||||
| ≥25kg/m 2 | ≥30 | ≥25 | ≥30 | ≥25 | ≥30 | ≥25 | ≥30 | ||||||
| BMI (%) | 31 | 21.8 | 8.1 | 26.1** | 12.5** | 20.2 | 12.1 | 28.1*** | 25.6*** | ||||
| 46 | 31.9 | 21.8 | 32.0 | 26.6 | 38.2 | 22.7 | 24.3*** | 43.3*** | |||||
| BMI change (kg/m2) | 14–31 | 4.4 ± 3.5 | 5.2 ± 4.4** | 4.4 ± 3.5 | 6.3 ± 4.9*** | ||||||||
| 31–46 | 2.8 ± 3.2 | 2.7 ± 3.6 | 2.9 ± 3.3 | 2.0 ± 4.1* | |||||||||
| Waist (cm) | 31 | 78.8 ± 11.8 | 80.9 ± 13.1** | 79.1 ± 11.8 | 88.0 ± 17.5*** | ||||||||
| 46 | 86.2 ± 13.1 | 88.9 ± 13.8** | 90.0 ± 14.1* | 93.3 ± 15.2** | |||||||||
| Testosterone (nmol/L) | 31 | 0.98 (0.75;1.23) | 1.11 (0.85;1.45)*** | 1.04 (0.82;1.38)** | 1.40 (1.17;1.80)*** | ||||||||
| 46 | 0.83 (0.63;1.05) | 0.81 (0.65;1.04) | 0.87 (0.69;1.16)** | 0.93 (0.72;1.17)* | |||||||||
| FAI | 31 | 2.10 (1.49;2.97) | 2.79 (1.77;4.11)*** | 2.50 (1.64;3.71)*** | 4.38 (2.75;6.79)*** | ||||||||
| 46 | 1.51 (1.06;2.15) | 1.62 (1.22;2.32)** | 1.70 (1.21;2.54)** | 1.89 (1.41;2.51)*** | |||||||||
| Age at menarche | 12.9 ± 1.26 | 13.2 ± 1.51** | 12.9 ± 1.34 | 12.4 ± 1.39 | |||||||||
| CHC use (%) | 46 | 4.1 | 3.6 | 2.5 | 4.8 | ||||||||
| 0 | ≥1 | 0 | ≥1 | 0 | ≥1 | 0 | ≥1 | ||||||
| Parity (%) | 31 | 29.3 | 70.3 | 26.6 | 73.4 | 32.5 | 67.5 | 36.8 | 63.2 | ||||
| 46 | 16.7 | 83.3 | 12.3 | 87.7 | 21.1 | 78.9 | 11.9 | 88.1 | |||||
| Infertility (%) | 31 | 15.0 | 21.2** | 15.2 | 42.6*** | ||||||||
| 46 | 16.7 | 23.0* | 21.9 | 37.0*** | |||||||||
| ≦9 | 9–12 | ≥12 | ≦9 | 9–12 | ≥12 | ≦9 | 9–12 | ≥12 | ≦9 | 9–12 | ≥12 | ||
| Education (years,%) | 31 | 1.4 | 46.3 | 52.3 | 1.8 | 41.4 | 56.7 | 2.8 | 57.3 | 39.9*** | 3.2 | 47.6 | 49.2 |
| 46 | 1.7 | 55.7 | 42.6 | 2.0 | 50.6 | 47.4 | 2.9 | 62.8 | 34.3* | 4.7 | 54.7 | 40.7 | |
| Marital status (%) | 31 | 77.7 | 76.0 | 69.4** | 75.0 | ||||||||
| 46 | 77.7 | 76.1 | 74.3 | 82.6 | |||||||||
| Smoking (regular, %) | 31 | 25.0 | 21.6 | 27.3 | 26.6 | ||||||||
| 46 | 18.4 | 11.7* | 19.4 | 23.3 | |||||||||
| Alcohol consumption (g/day) | 31 | 2.20 (0.60;5.90) | 1.70 (0.50;4.80)** | 1.95 (0.60;5.50) | 2.10 (0.65;6.55) | ||||||||
| 46 | 2.90 (0.57;8.51) | 2.10 (0.24;6.28)* | 2.27 (0.49;7.57) | 2.21 (0.11;9.24) | |||||||||
| Depressive symptoms (%) | 31 | 15.6 | 17.2 | 22.9** | 24.0* | ||||||||
| 46 | 23.4 | 25.8 | 34.5*** | 33.7* | |||||||||
| Anxiety symptoms (%) | 31 | 8.2 | 7.6 | 16.1*** | 16.1** | ||||||||
| 46 | 8.3 | 7.7 | 14.8** | 12.8 |
Abbreviations: FAI, free androgen index, CHC combined hormonal contraceptives. The data are presented as mean ± SD or median with (25% lower quartile and 75% upper quartile). ***P < .001, **P < 0.01, and *P < .05 compared with controls. The differences between the controls and different PCOS symptom groups were analyzed using Student’s t-test or Mann–Whitney U test when appropriate with the continuous variables and the differences of prevalence in women with PCOS symptoms compared to controls were analyzed using Pearson chi-square test. Number of subjects for each measurement may vary due to data availability. The missing measurement data of weight and height were displaced with by self-report of the follows proportion at age 31 34.1% and at age 46 14,4%.
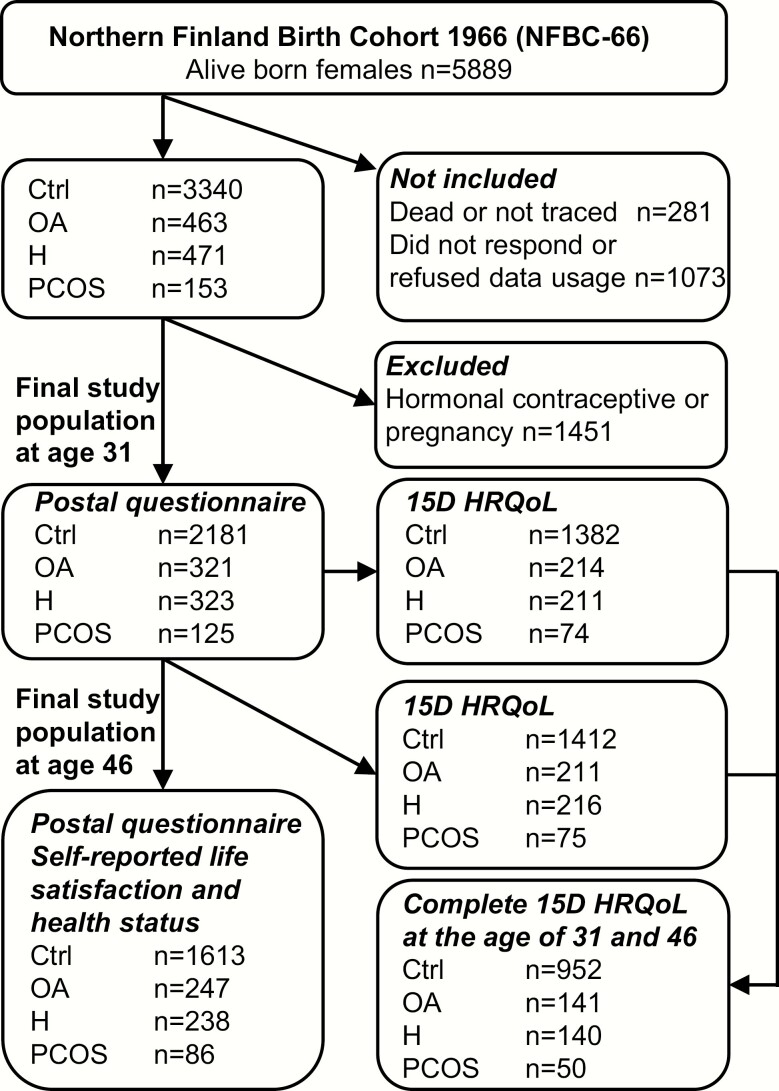
The questionnaire was sent to all 5608 women in the cohort at age 31 (1996–1997), and of these 4523 (81%) responded. The questionnaire included questions on weight, height, and screening for PCOS symptoms: (1) Is your menstrual cycle longer than 35 days more than twice a year? (considered as having OA), and (2) Do you have excessive bothersome body hair? (considered as having H). Of all women, 463 reported OA, 471 H, and 153 reported having both symptoms (considered to be indicative of PCOS). Women who were pregnant or using hormonal contraceptives were excluded from the final study population. The final analysis group at age 31 consisted of 2181 asymptomatic women (considered controls), 331 (11.2%) women with OA, 323 (10.9%) women with H, and 125 (4.2%) with PCOS. The flow chart of the study has been previously published (6) and is shown in Fig. 1. Clinical examinations, including measurements for weight, height, serum testosterone (T), and free androgen index (FAI) were performed in 3127 (76%) women.
Figure 1.
Flow chart of the study population in the Northern Finland Birth Cohort 1966 (NFBC1966). Including the numbers of participants in study groups controls (Ctrl) and women with oligo-amenorrhea (OA), hirsutism (H), or polycystic ovary syndrome (PCOS) and for measurements self-reported life-satisfaction, health status and 15D health-related quality of life (HRQoL).
The questionnaires and clinical examinations (including the same measurements as at age 31) were replicated in 2013, when the participants were 46 years old. The questionnaire was sent to 5123 women (alive and address known) with an answer rate of 72% (n = 3760). Furthermore, 3280 (64%) women participated in the clinical examinations. All participants provided informed consent, and the study was approved by the Ethics Committee of the Northern Ostrobothnia District (EETTMK 94/2011).
15D Quality of life measure
The 15D questionnaire is a generic, standardized, well-validated, self-administered measure of HRQoL including 15 different dimensions: mobility, vision, hearing, breathing, sleeping, eating, speech, excretion, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity. The questionnaire can be found in the article by Sintonen (30). The 15D score can be used as a pooled variable of all 15 dimensions (mean score), or they can be presented separately. The dimensions are ranked into 5 different levels describing the current health status of the respondent. The 15D score and the dimensional level values (on a 0–1 scale) are calculated from the health state descriptive system by using a set of population-based preference or utility weights; the higher the score, the higher the HRQoL. As for the improvement or deterioration of HRQoL, the minimum clinically significant change for the 15D score is estimated to be 0.015, and this can also be regarded as a significant difference between groups in cross-sectional analysis (31). In this work, we divided the mean 15D scores into quartiles for logistic regression analysis to identify the risks associated with the lowest quartile. Given that the 15D data were collected at the clinical study visit, the 15D score was available at age 31 for 214 women with OA, 211 women with H, 74 women with PCOS, and 1382 controls; and at age 46 for 211 women with OA, 216 women with H, 75 women with PCOS, and 1412 controls. For both data collections, 141 women with OA, 140 with H, 50 women with PCOS, and 952 controls had complete 15D data available.
HRQoL in PCOS and in other chronic conditions
The HRQoL measures were also assessed in women presenting with other chronic conditions. The questionnaire included the following question at ages 31 and 46: “Do you currently have, or have you ever had, any of the following symptoms, diseases, or injuries diagnosed or treated by a doctor?” Asthma, migraine, rheumatoid arthritis, and depression were some of the answer options, and the prevalence of these diseases in our study population were asthma at age 31 11.2%, and age 46 12.7%; migraine at age 31 19.5%, and age 46 26.1%; rheumatoid arthritis at age 31 1.3%, and age 46 1.6%; and depression at age 31 5.7%, and age 46 15.4%. The mean 15D for the women with different conditions was compared with the mean 15D score of women with PCOS.
Self-reported current life satisfaction and health status
Questions about the self-reported current life satisfaction and health status were included in the postal questionnaires. The data were available for women with OA (at ages 31, 329; and 46, 247), H (at ages 31, 323; and 46, 238), PCOS (at ages 31, 125; and 46, 86), and the control group (at ages 31, 2182; and 46, 1613). At ages 31 and 46, the women were asked, “How do you feel about your current life situation?” The answer options were (1) extremely satisfied, (2) somewhat satisfied, (3) somewhat dissatisfied, (4) extremely dissatisfied, and (5) cannot say. The answer option 5 was excluded from the analysis due to the very small number of people choosing it (at age 31, n = 14 [0.6%]; and at age 46, n = 43 [1.5%]). Answers 3 and 4 were merged due to the low number of responses. Logistic regression analysis was performed to estimate the risk for being somewhat or extremely dissatisfied.
The questionnaires also included the question, “What is your estimate about your health status?” with the answer options of (1) very good, (2) good, (3) moderate, (4) poor, and (5) very poor. As the number of responses was low in the extreme answer options, options 1 and 2 and options 4 and 5 were merged for the analysis. Logistic regression analysis was performed to estimate the risk of experiencing a poor or very poor health status. To further assess life satisfaction and health status, relation analysis using the 15D HRQoL instrument was performed.
Confounding variables
Body mass index.
In the clinical examinations, weight (kg) was measured on a digital scale that was calibrated regularly. Height (cm) was measured twice, using a standard and calibrated stadiometer. BMI was calculated in kg/m2. When a measurement was missing, the self-reported values were used. No statistical difference was observed between the measured and the self-reported BMIs (20). Change in BMI from 14 to 31 years and from 31 to 46 years was also calculated. Waist circumference (WC; cm) at ages 31 and 46 was measured midway between the lowest rib margin and the iliac crest.
Testosterone and FAI.
Serum T and the sex hormone binding globulin (SHBG) were assayed at ages 31 and 46, as previously described (6). Testosterone levels were assayed using Agilent triple quadrupole 6410 liquid chromatography/mass spectrometry equipment with an electrospray ionization source operating in positive-ion mode (Agilent Technologies). At 31 years, SHBG was assayed using fluoroimmunoassay (Wallac, Inc. Ltd, Turku, Finland), and at 46 years SHBG was assayed using chemiluminometric immunoassay (IMMULITE 2000, Siemens Healthcare, Llanberis, UK). To adjust for level differences in the assays used at the 2 time points, the values at age 31 were amended as follows: 0.7615 × (SHBG at age 31) + 0.7088. FAI was calculated as follows: 100 × T (nmol/liter)/SHBG (nmol/L).
Socioeconomic status (SES)/education.
Socioeconomic status was based on education. The variable was classified into 3 groups by the number of education years: ≤9, 9–12, and >12 years.
Marital status.
The postal questionnaires asked about marital status at ages 31 and 46. At age 31, the group “with partner” consisted of those who were married or cohabitating. At age 46, this group also included registered partnerships.
Regular smoking.
Smoking at ages 31 and 46 was considered regular if the study subject reported smoking at least once per week.
Alcohol consumption.
Alcohol consumption at ages 31 and 46 was reported in grams per day (g/day) based on answers to the questionnaire.
Depression and anxiety.
Current psychological distress was assessed using the Hopkins Symptoms Checklist-25 (HSCL-25) at ages 31 and 46, as previously described (6). At ages 31 and 46, the study subjects were also asked, “Do you currently have, or have you ever had, any of the following symptoms, diseases, or injuries diagnosed or treated by a doctor?” “Depression” was 1 of the answer options. In the present analysis, clinically relevant depressive symptoms were determined to be present if the HSCL score for depressive symptoms was ≥1.75 and if the subject self-reported being diagnosed with depression. For anxiety symptoms, a HSCL anxiety score of ≥1.75 was used (32).
Infertility.
At ages 31 and 46, the subjects were asked, “Have you ever experienced infertility?” to assess fertility-related problems. The data from women reporting “No” and “I have never tried to get pregnant” were pooled. Infertility was diagnosed if the woman reported “Yes, I am currently, or I have previously been affected by infertility.”
Statistical analysis
Statistical analyses were performed using SPSS system version 24 for Windows. The 15D mean scores were analyzed using analysis of variance (ANOVA) and post hoc analyses (ANOVA, Tukey). The change in the 15D score between 31 years and 46 years was analyzed using a paired sample t-test. Individual 15D items and adjustments with BMI were performed using nonparametric analysis of covariance analysis (Rank ANCOVA). Differences between the groups regarding life satisfaction and self-estimated health status were analyzed using the chi-square test. Logistic regression analysis was performed to explore the association between PCOS symptoms and low HRQoL. In multivariate analyses, the following possible confounding variables were used in different models. In Model 1, the adjustments were done for BMI, BMI change between ages 14–31 years (at age 31) and 31–46 (at age 46), waist circumference, serum testosterone, and FAI. In Model 2, the adjustments included education, marital status, alcohol consumption, smoking, depression, anxiety, and infertility. In Model 3, the adjustments were done only for the significant variables from Models 1 and 2.
We used generalized linear mixed models to account for both within-subject (fixed effects) and between-subject (random effects) variability using the repeated measures data. We specified a binomial distribution with a logit link. For within-subject effects, we reported the odds ratios obtained from fixed effects and adjusted the odds ratios for PCOS for body mass index, waist circumference, smoking, alcohol consumption, depression, and anxiety.
The results are reported as means, percentages (%), and unadjusted (crude) and adjusted odds ratio (OR) with 95% confidence interval (CI). P < .05 was considered statistically significant.
Drop-out analysis and missing data
The drop-out rate between the ages 31 and 46 was not extensive. As reported previously, drop-outs had more depression and psychological morbidity (6,33). Also, when compared with women participating in the follow-up, drop-outs displayed worse scores in socioeconomic variables, such as education, marital status, smoking, alcohol use, and BMI (data not shown). The women with PCOS who dropped out after the data collection at age 31 were less likely to be married than the PCOS subjects who participated in the follow-up. However, likely due to the small number of women in the analysis, the differences did not reach statistical significance (data not shown).
The 15D score, life satisfaction, and health status of the follow-up participants were also compared with drop-outs at age 31 and showed no statistical differences in 15D mean sum scores (15D, follow-ups 0.948; drop-outs 0.941, P = .061). Additionally, weight analysis was done using the following weighted variables: education, marital status, and depression. The analysis did not result in any considerable changes when correcting for the bias of drop-outs (data not shown). There were some missing data that were considered when forming the variables for the analysis. If the person did not complete the 15D score, the mean score was not calculated. However, the individual items were taken into consideration. Altogether there were very few sporadically missing data and when going through the answers of women with PCOS, there was only one uncompleted 15D questionnaire at age 46. Thus, no imputation was applied.
Results
15D health-related quality of life
Individual dimensions at ages 31 and 46, and the 15D mean score.
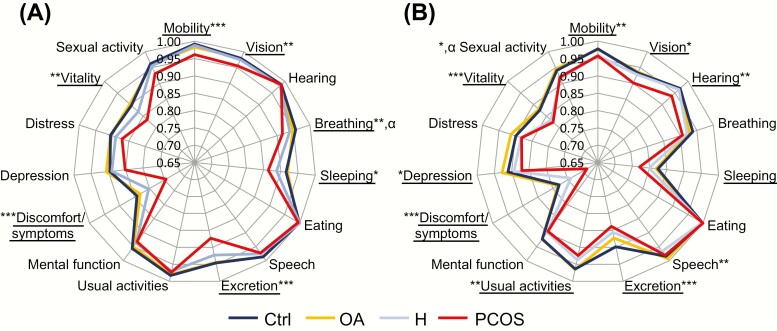
The individual items with a lower 15D score in women with PCOS symptoms (also isolated symptoms) than in the controls at age 31 were mobility, vision, breathing, sleeping, excretion, discomfort/symptoms, and vitality. At age 46, the items with a lower score in women with PCOS symptoms than in the controls were mobility, vision, hearing, speech, excretion, usual activities, discomfort/symptoms, depression, vitality, and sexual activity (Fig. 2). In the post hoc analysis, most of the differences were observed between PCOS and the control group, whereas no differences were observed between women with OA and the controls (Fig. 2). BMI explained the differences between the groups for some of the individual items, including breathing at age 31 and sexual activity at age 46 (Fig. 2).
Figure 2.
15D health-related quality of life (HRQoL) instrument in 15 individual dimensions among the study groups: control women (Ctrl, dark blue), women with oligo-amenorrhea (OA, yellow), women with hirsutism (H, light blue), or polycystic ovary syndrome (PCOS, red) at ages 31 (A) and 46 (B). The closer the value to the center, the lower the score for this individual 15D dimension. The women with PCOS presented with the lowest individual dimension scores. *P < .05 **P < .01 ***P < .011 crude P-value between the study groups. α means the significance disappeared after adjusting for BMI. Underlined individual items represent significant differences between women with PCOS and the control group.
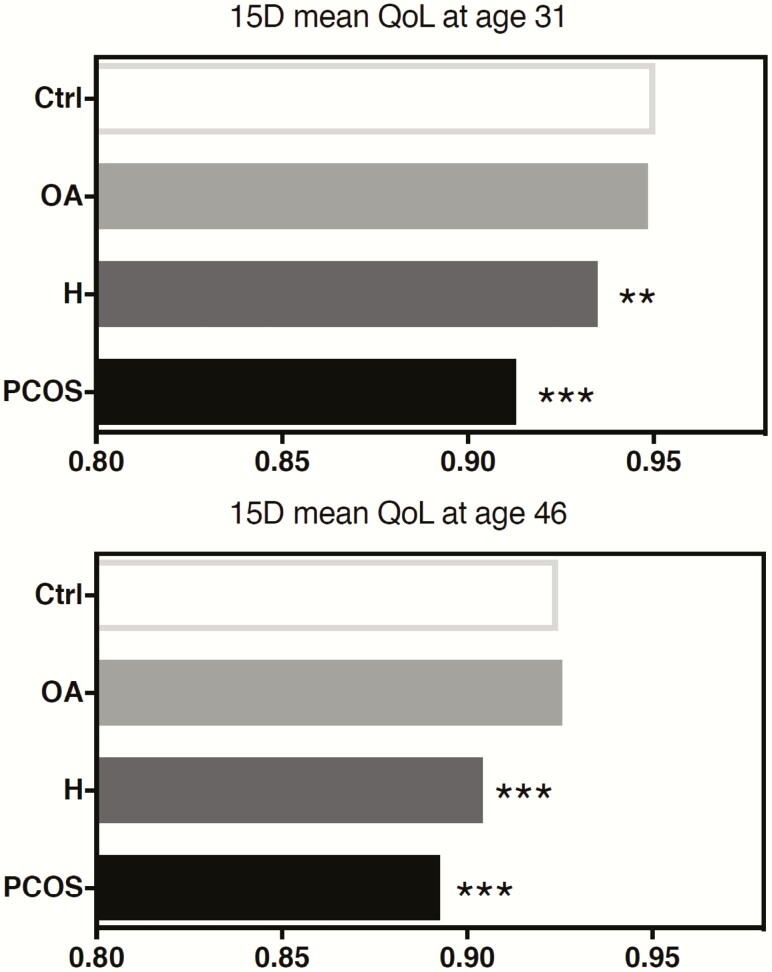
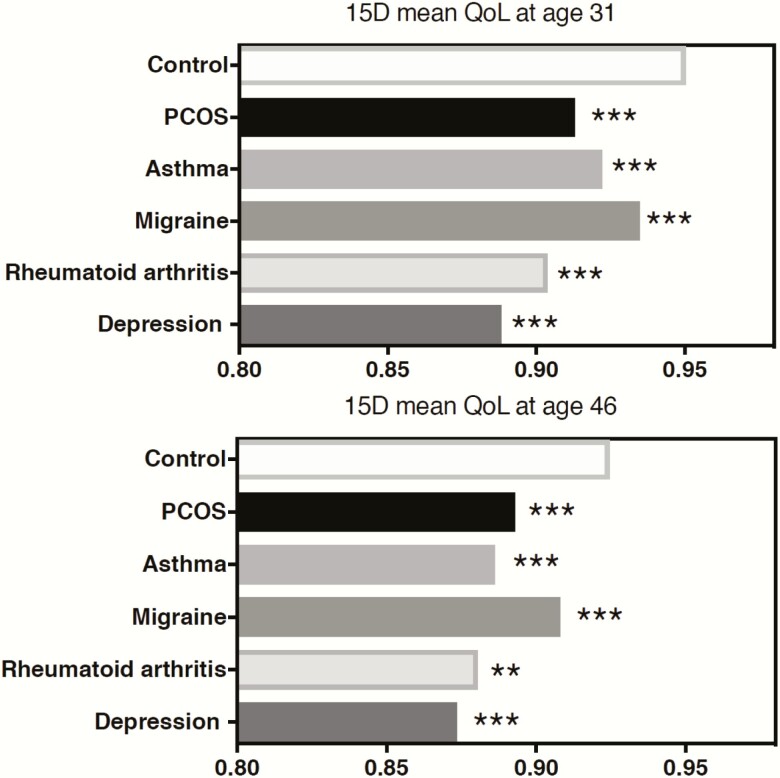
Among women with PCOS symptoms and the controls, the mean 15D score, including all 15 items, decreased from ages 31 to 46 (mean 15D score 0.947 vs 0.920, respectively); the decrease was of a similar extent in all study groups. Women with PCOS or isolated H showed a decreased mean score at age 31 (PCOS 0.913 and H 0.935 vs Ctrl 0.950 P < .001 and P = .000) and at age 46 (PCOS 0.893 and H 0.904 vs Ctrl 0.925 P < .001) compared with the controls, whereas the OA group did not differ from the controls (Fig. 3). Importantly, the mean 15D score was similar to scores observed in women with other chronic conditions like asthma, migraine, rheumatoid arthritis, and depression at ages 31 and 46 years (Fig. 4).
Figure 3.
15D health-related quality of life (HRQoL) mean sum scores at ages 31 and 46 in the control group (Ctrl) and women with oligo-amenorrhea (OA), hirsutism (H), or polycystic ovary syndrome (PCOS). At ages 31 and 46, the 15D mean sum scores were lower in the PCOS and H groups when compared with the control group. (*P < .05, **P < .01, ***P < .001 compared with the controls).
Figure 4.
15D health-related quality of life (HRQoL) mean sum scores of women with polycystic ovary syndrome (PCOS) or with self-reported diagnoses of asthma, migraine, rheumatoid arthritis, or depression compared with the controls. The number of women with self-reported diagnoses at age 31 were asthma n = 158, migraine n = 374, rheumatoid arthritis n = 23, and depression n = 99; and at age 46 were asthma n = 162, migraine n = 529, rheumatoid arthritis n = 32, and depression n = 303. The 15D mean sum scores were lower in all groups with the condition when compared with the controls without the condition. The range of 15D HRQoL varied in the control group at ages 31 (0.947–0.950) and 46 (0.921–0.929) depending on the condition chosen for the comparison. HRQoL for the controls in the figure represents the mean 15D compared with PCOS group. (*P < .05, **P < .01, ***P < .001 compared with the controls).
15D risk analysis at ages 31 and 46, and confounding variables.
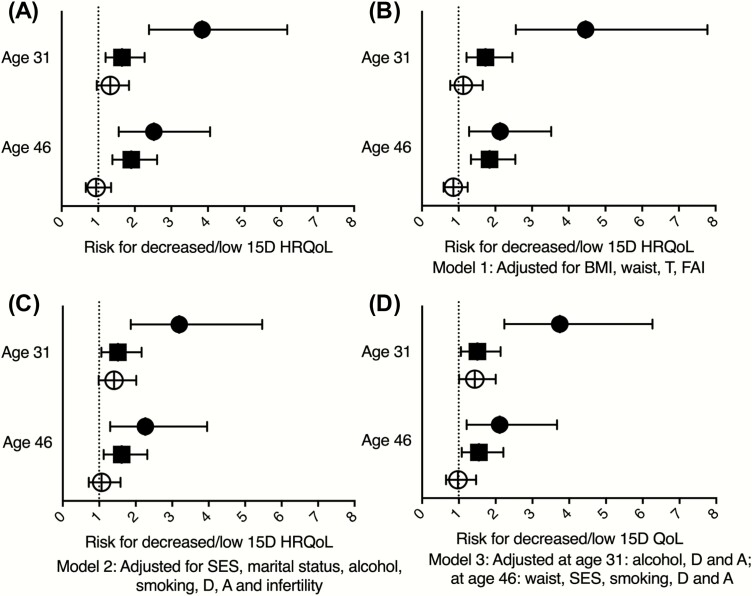
The 15D mean score was divided into quartiles, and a logistic regression model was used to estimate the risk factors associated with low HRQoL. Compared with the controls, women with PCOS presented with 2.54-fold higher risk for having low HRQoL at ages 31 and 46 years (Fig. 5A). The adjusted models showed significant risk for PCOS even after adding confounding factors to these analyses (Models 1–3, Figs. 5B–5D). The confounders that were included in Model 3 at age 31 (95% CI) were alcohol consumption (1.00–1.00), depression (3.18–5.75), and anxiety (2.46–5.27); and at age 46 were waist circumference (1.01–1.03), education (1.07–1.78), smoking (1.11–2.08), depression (2.91–4.93), and anxiety (2.05–4.52). Depression and anxiety contributed the most to low HRQoL in Model 3, being significant for depression at age 31 (OR 4.27, 95% CI 3.18–5.75) and at age 46 (OR 3.79, 95% CI 2.91–4.93), and for anxiety at age 31 (OR 3.60, 95% CI 2.46–5.27) and at age 46 (OR 3.05, 95% CI (2.05–4.52) (data not shown). Other confounding variables, such as BMI, testosterone, or infertility, were not linked to low HRQoL. During the 15-year follow-up, the risk for HRQoL differences between the study groups did not change.
Figure 5.
Logistic regression model showing risk for the lowest quartile of 15D health-related quality of life (HRQoL) mean scores at ages 31 and 46 in women with oligo-amenorrhea (OA, clear circle), hirsutism (H, black square), or polycystic ovary syndrome (PCOS, black circle). (A) Crude OR (95% CI). B) Adjusted for BMI, waist circumference, testosterone (T), and free androgen index (FAI) (Model 1). (C) Adjusted for depression (D), anxiety (A), socioeconomic status (SES), smoking, alcohol consumption, and infertility (Model 2). (D) Adjusted for significant confounding factors at age 31: alcohol consumption, D, and A; and at age 46: waist circumference, SES, alcohol consumption, D, and A (Model 3).
Self-reported current life satisfaction
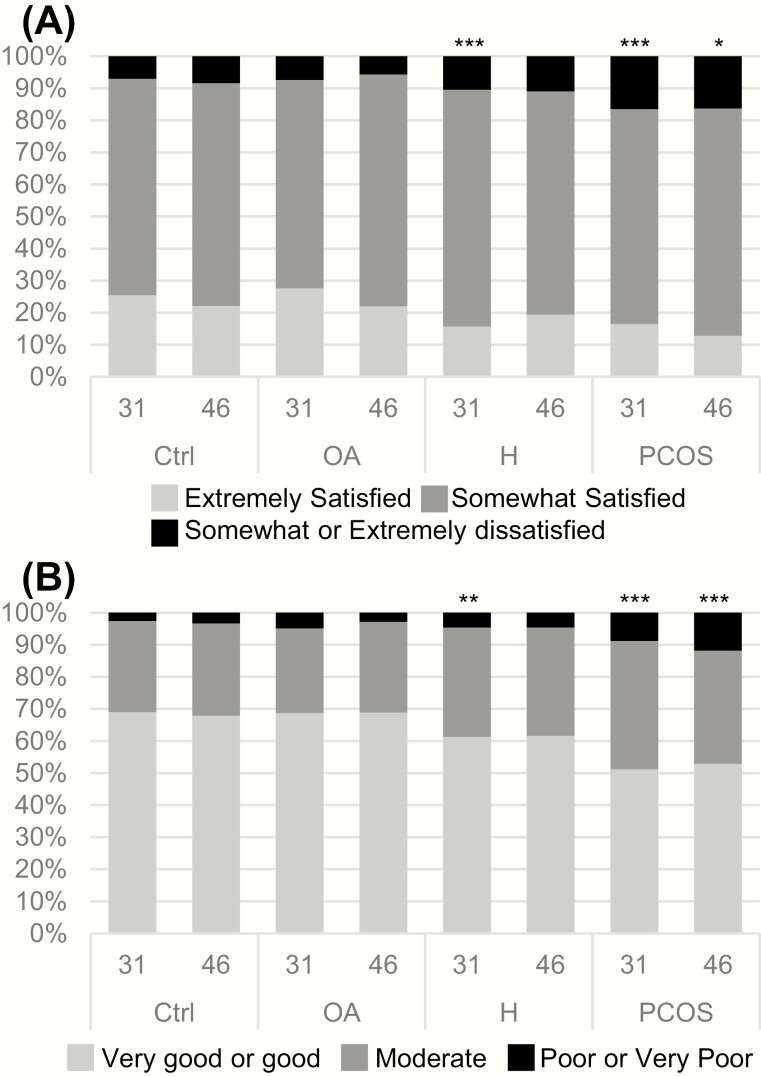
Self-reported current life satisfaction was lower in women with PCOS and isolated H at age 31 than in the controls (P < .001, Fig. 6A). Of the women with PCOS and isolated H, 16.5% and 10.5%, respectively, reported being somewhat or extremely dissatisfied with their life situation at age 31 compared with 7.0% of the control group (P < .001, P = .024, respectively). In the OA group, the proportion of dissatisfied women was similar to that of the controls (7.4% vs 7%, P = NS). At age 46, life satisfaction was again lower in women with PCOS; 16.3% reported being somewhat or extremely dissatisfied with their life situation when compared with 8.4% of the controls (P = .011, Fig. 6A). No differences were observed between the OA group and the controls.
Figure 6.
(A) The proportion (%) of control women and women with oligo-amenorrhea (OA), hirsutism (H), or polycystic ovary syndrome (PCOS) reporting life satisfaction at ages 31 and 46. Of the women with PCOS, 16.5% reported being somewhat or extremely dissatisfied with their life situation at age 31, compared with 7.0% in the control group (P < .001). (*P < .05, **P < .01, ***P < .001 compared with the controls). (B) The proportion (%) of control women and women with OA, H, or PCOS reporting their current health status at ages 31 and 46. At age 31, 8.8% of women with PCOS and 4.6% of women with isolated H reported a poor or very poor health status compared with 2.60% of the controls (P = .001, P = .041, respectively). At age 46, 11.8% of the women with PCOS reported having a poor or very poor health status compared with 3.3% in the control group. (*P < .05, **P < .01, ***P < .001 compared with the controls).
Risk analysis for decreased life satisfaction at ages 31 and 46, and confounding factors.
The logistic regression model indicated that at age 31, PCOS and H were significant risk factors for being dissatisfied with one’s current life situation (Table 2). The risk remained in women with PCOS even after adjusting for confounding variables (Models 1–3, Table 2). At age 46, the logistic regression model showed PCOS as carrying a 2-fold risk for being dissatisfied with one’s current life situation (Table 2). When adding significant confounders (95% CI) in Model 3—at age 31: marital status (1.91–3.47), smoking (1.01–1.89), and depression (5.53–9.98); and at age 46: marital status (1.36–2.67), smoking (1.69–3.37), depression (3.09–6.24), and anxiety (1.48–3.35)—the 2-fold risk remained for women with PCOS at age 31 but did not reach significance at age 46 (Table 2).
Table 2.
The logistic regression model assessing risk for self-reported dissatisfaction towards the current life situation at ages 31 and 46 in women with oligo-amenorrhea (OA), hirsutism (H), or PCOS (OA + H).
| Model | Age | OA, OR (95% CI) | H, OR (95% CI) | PCOS, OR (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Crude | 31 | 1.07 | (0.69–1.68) | 1.57 | (1.06–2.34) | 2.65 | (1.59–4.39) |
| 46 | 0.66 | (0.37–1.16) | 1.34 | (0.86–2.09) | 2.11 | (1.16–3.85) | |
| Model 1 | 31 | 0.92 | (0.48–1.73) | 1.38 | (0.81–2.36) | 2.43 | (1.17–5.05) |
| 46 | 0.92 | (0.51–1.65) | 1.30 | (0.78–2.16) | 1.98 | (0.96–4.07) | |
| Model 2 | 31 | 1.07 | (0.65–1.74) | 1.36 | (0.87–2.11) | 2.30 | (1.25–4.23) |
| 46 | 0.69 | (0.38–1.27) | 1.15 | (0.71–1.86) | 1.67 | (0.80–3.45) | |
| Model 3 | 31 | 0.98 | (0.60–1.59) | 1.23 | (0.80–1.91) | 2.38 | (1.36–4.17) |
| 46 | 0.67 | (0.37–1.20) | 1.13 | (0.69–1.84) | 1.77 | (0.91–3.44) |
Model 1. Adjusted for body mass index (BMI), BMI change between ages 14–31 years (at age 31) and 31–46 (at age 46), waist circumference, serum testosterone, free androgen index (FAI).
Model 2. Adjusted for education, marital status, alcohol consumption, smoking, depression, anxiety, and infertility.
Model 3. Significant adjustments at age 31: marital status, smoking, depression and at age 46: marital status, smoking, depression and anxiety.
Self-reported health status
At age 31, the self-reported health status was decreased in women with PCOS and isolated H (Fig. 5B). In women with PCOS and isolated H, 8.8% (P = .001) and 4.6% (P = .041), respectively, reported a poor or very poor state of health compared with 2.6% of the controls. Self-reported health status was still decreased at age 46 in women with PCOS and isolated H. A higher number of women with PCOS and H reported having a poor or very poor health status than the controls (11.8% and 4.8% vs 3.3%, P < .001, respectively) (Fig. 6B).
Risk analysis for decreased health status at ages 31 and 46, and confounding factors.
At age 31, PCOS was associated with a 3-fold risk, and H and OA with a 2-fold risk for a poor or very poor health status (Table 3). After including confounding variables, women with PCOS still reported a decreased health status compared with the controls at ages 31 and 46 (Models 1–3, Table 3). The significant variables in Model 3 at age 31 (95% CI) were smoking (1.55–3.57), depression (2.04–5.29), and anxiety (1.53–4.28); and at age 46 (95% CI) were smoking (1.94–5.90), depression (1.85–6.10), anxiety (1.01–3.95), and waist circumference (1.03–1.07).
Table 3.
The logistic regression model assessing the risk for poor or very poor self-reported health status at ages 31 and 46 in women with oligo-amenorrhea (OA), Hirsutism (H), or PCOS (OA + H).
| Model | Age | OA (OR (95% CI)) | H (OR (95% CI)) | PCOS (OR (95% CI)) | |||
|---|---|---|---|---|---|---|---|
| Crude | 31 | 1.91 | (1.08–3.36) | 1.82 | (1.02–3.25) | 3.60 | (1.84–7.05) |
| 46 | 0.84 | (0.38–1.87) | 1.40 | (0.72–2.73) | 3.85 | (1.89–7.85) | |
| Model 1 | 31 | 1.53 | (0.68–3.41) | 1.86 | (0.87–3.96) | 4.13 | (1.66–10.31) |
| 46 | 0.93 | (0.38–2.27) | 0.97 | (0.41–2.26) | 3.71 | (1.55–8.86) | |
| Model 2 | 31 | 2.08 | (1.15–3.75) | 1.52 | (0.83–2.78) | 2.76 | (1.30–5.88) |
| 46 | 1.02 | (0.45–2.35) | 1.20 | (0.59–2.41) | 3.75 | (1.70–8.27) | |
| Model 3 | 31 | 1.96 | (1.09–3.52) | 1.19 | (0.82–2.71) | 2.92 | (1.44–5.92) |
| 46 | 0.89 | (0.35–2.25) | 0.82 | (0.35–1.93) | 2.46 | (1.01–6.03) |
Model 1. Adjusted for body mass index (BMI), BMI change between ages 14–31 years (at age 31) and 31–46 (at age 46), waist circumference, serum testosterone, free androgen index (FAI).
Model 2. Adjusted for education, marital status, alcohol consumption, smoking, depression, anxiety, and infertility.
Model 3. Significant adjustments at age 31: smoking, depression, anxiety and at age 46: waist circumference, smoking, depression, and anxiety.
Longitudinal analysis for QoL, life satisfaction and health status
In the longitudinal analysis HRQoL between ages 31 and 46 showed that women with PCOS had significantly lower HRQoL for the follow-up period than the controls (adjusted OR = 2.69, 95% CI 1.68–4.30, Table 4). Moreover, women with PCOS were more often dissatisfied with their life situation (adjusted OR = 2.10, 95% CI 1.29–3.44) and more commonly reported poor or very poor self-rated health status (adjusted OR = 1.75, 95% CI 1.00–3.08). Hirsutism associated only with low HRQoL (adjusted OR = 1.75, 95% CI 1.24–2.46), but not life dissatisfaction or poor health status. Furthermore, OA did not predict any of the outcomes (Table 4).
Table 4.
The generalized linear mixed model analyzing longitudinally throughout ages 31 and 46 for decreased health-related quality of Life (HRQoL), life dissatisfaction and poor or very poor health status in women with oligo-amenorrhea (OA), hirsutism (H) or PCOS (OA + H).
| Decreased 15D HRQoLa | Dissatisfaction with lifea | Poor or very poor self-rated healtha | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| OA | 1.24 | 0.87–1.78 | 0.85 | 0.54–1.32 | 1.10 | 0.75–1.62 |
| H | 1.75 | 1.24–2.46 | 1.15 | 0.79–1.66 | 1.01 | 0.66–1.55 |
| PCOS | 2.69 | 1.68–4.30 | 2.10 | 1.29–3.44 | 1.75 | 1.00–3.08 |
a The odds ratios were obtained from the fixed effects and adjusted for body mass index, waist circumference, smoking, alcohol consumption, depression, and anxiety.
Discussion
This study provides the longest longitudinal follow-up to date on HRQoL in women with PCOS. It shows that affected women have a lower HRQoL, of similar magnitude to the decrease in HRQoL in women diagnosed with other chronic conditions like asthma, migraine, rheumatoid arthritis, and depression. Reflecting on existing literature, the differences in HRQoL 15D sum scores at ages 31 and 46 between the subjects with PCOS and the controls can be considered clinically relevant (31). The persistently decreased 15D scores in PCOS are observed in functions related to sleeping, excretion, discomfort and symptoms, and vitality. In the whole population, depression and anxiety contribute the most to low HRQoL, even though after the adjustment PCOS still remains as an independent contributing factor. The longitudinal analysis showed 2.7-fold risk for low HRQoL compared with controls between ages 31 and 46 years. Moreover, women with PCOS are more likely to report decreased life satisfaction and health status at ages 31 and 46 when compared with women without PCOS symptoms.
Women with isolated hirsutism had lower HRQoL at ages 31 and 46 years than asymptomatic women and they also reported lower life satisfaction and health status. Isolated OA did not seem to decrease HRQoL, but at age 31, it was associated with a poorer health status. It is notable that BMI and biochemical hyperandrogenism did not account significantly for the risk of having low HRQoL, life satisfaction, or a perceived poor health status among women with PCOS.
Considering that some PCOS symptoms, such as obesity and hirsutism, are already evident during adolescence, they can potentially affect HRQoL early on and with long-term consequences (17,34). Most previous studies have focused on women at reproductive age and, to date, no data are available on HRQoL in women with PCOS during their late reproductive years. In line with previous results, women with PCOS presented with a 3.8-fold risk of decreased HRQoL compared with the non-PCOS controls. More importantly, the risk for low HRQoL in PCOS persisted at the end of fertile life, indicating long-term HRQoL impairment in affected women. Several individual items like (sleep, vitality, discomfort and symptoms, and excretion) were lower both at ages 31 and 46 years in women with PCOS, with the first 3 representing an impairment in general wellbeing. Interestingly, sleep functions seemed to be impaired in women with PCOS already from age 31 onwards, a finding also supported by other investigators (35,36). Given these results, it is important to understand the factors contributing to lower HRQoL in women with PCOS when developing effective treatments, to target improving HRQoL. Recent studies have suggested that a positive HRQoL outcome may be achieved by lifestyle modification, hormonal contraceptive use, or the treatment of hirsutism in women with PCOS (18,37,38).
Research focusing on psychological distress in women with PCOS has resulted in several studies demonstrating higher rates of depression and anxiety symptoms and eating disorders in affected women (6,7,39,40). In the present study, depression and anxiety symptoms were the strongest individual explanatory factors for decreased HRQoL. The effect of psychological distress on decreasing QoL in PCOS has also been reported by other investigators(10,12,14). These same factors were also shown to explain low life satisfaction and a poor self-reported health status in women with PCOS. The underlying biochemical or psychological mechanism responsible for increased mental distress in women with PCOS is not yet fully understood; however, causes ranging from in utero exposure to androgens, biochemical hyperandrogenism, insulin resistance, or clinical manifestations like obesity and hirsutism have been suggested (6,41–43), indicating the complex nature and variable manifestations of the syndrome. The recently published international PCOS guidelines also underline the importance of screening at an individual level for the most burdensome manifestations of PCOS, especially mental distress. For women with the most severe symptoms and manifestations, interventions aimed to improve HRQoL should be initiated early to ensure effective care (4).
Obesity affects 50% to 70% of women with PCOS, resulting in adverse health effects. Interestingly, in the present study, low HRQoL was not BMI dependent in women with PCOS, supporting the findings from previous studies (12,44), although not all studies agree (17,19). The discrepancy between these findings may have occurred because the mean BMI was not very high in the NFBC1966 data set compared with previous studies reporting association between BMI and low HRQoL.
Even though PCOS is the most common cause for anovulatory infertility, it was not observed that infertility per se affects HRQoL in these women. Contradicting our result, some studies have reported infertility as a factor that decreases QoL (26,45). On the other hand, several population-based studies have indicated that women with PCOS are as likely to have at least 1 child as non-PCOS women (including pregnancies achieved with the help of assisted reproductive technology), that is, the women with PCOS seemed to be subfertile rather than infertile (9,46,47). Women should be informed of this good prognosis, as it may reduce stress and anxiety related to the stigma of infertility.
As for isolated PCOS symptoms, hirsutism has been shown to decrease QoL and self-esteem, most likely due to treatments that are long-lasting and often only partially effective (11,23,24). In the present data, similar to PCOS, isolated hirsutism was associated with low HRQoL and low life satisfaction. These findings emphasize the need to target esthetic health issues more effectively by using both medical and cosmetic approaches. Interestingly, this study, in line with several previous studies (11,48), did not find any association between biochemical hyperandrogenism and HRQoL, suggesting that it is important to treat the clinical manifestations of hyperandrogenism effectively, rather than only focusing on biochemical hyperandrogenism.
Strengths and limitations
This study has several strengths, but also some limitations. This unique NFBC1966 population-based cohort data provide a rare opportunity to study HRQoL in a nonselected PCOS population with long-term follow-up, whereas most earlier studies involved sample groups of women attending endocrine or infertility clinics. 15D is a well-validated tool for estimating HRQoL (30,31). Our analysis may have a small risk for overadjusting regarding depression and anxiety, as 15D also includes questions on psychological distress. Commonly, PCOSQ has been used to measure HRQoL in women with PCOS; however, the 15D tool enables comparison between cases and controls.
The data have minimal ethnic, geographic, and age variations, and socioeconomic diversity is low, largely as a result of the availability of free health care and education in Finland. The cohort also includes women at the late reproductive age, and the participation and response rates throughout the follow-up remained high in this cohort. There was some drop-out during the 15-year follow-up. The available data for both time points were more limited for the longitudinal approach than the cross-sectional one, however, also the mixed model showed a significantly increased risk for low HRQoL in PCOS. The drop-out analysis indicated that the women who dropped out were in a worse psychosocial situation, possibly having some bias on the results by underestimating the effects of psychosocial distress. Additionally, extensive data on clinical and biochemical measures were available, allowing for the control of several confounding factors.
Self-reporting PCOS symptoms, without data on ultrasonography, has limitations, but the validity of the PCOS diagnosis and the hormonal, metabolic, and psychological profiles of these women have been previously published (6,20,27,28) and successfully used by other research groups (7,49). In the present study we used questionnaire data built in 1996 to identify women with OA and H. At that time the Rotterdam criteria had not been established. Moreover, there are still no specific validated questions to identify women with PCOS suffering from these symptoms. In fact, the present study expands our previous findings, showing that by asking 2 simple questions about PCOS symptoms, identifying a female population experiencing low HRQoL comparable to the decrease in HRQoL related to well-established chronic conditions like asthma, migraine, rheumatoid arthritis, and depression was possible. These diagnoses of comparator diseases were also self-reported, adding to the limitations of the study.
Conclusions
The present study shows that women with PCOS present with a long-term decrease in HRQoL. Given that the decrease is of similar magnitude than the one in women with other chronic conditions like asthma, migraine or rheumatoid arthritis, the affected women warrant targeted resources and interest. Moreover, women with PCOS are less satisfied with their current life situation and health status than non-PCOS counterparts. In line with the recently published international PCOS guidelines, the importance of screening for the most burdensome factors related to the syndrome, especially mental distress, is emphasized. For example, hirsutism was shown to be a significant factor in lowering QoL, indicating that more attention in clinical care is warranted for this symptom. The symptoms and worries of women with PCOS should be discussed and treated effectively, favoring a multidisciplinary approach. Most importantly, further studies investigating and developing the most effective means to improve HRQoL in women with PCOS are necessary.
Acknowledgments
We thank Doctor Kobra Falah Hassani for skillful statistical assistance.
Financial Support: The Finnish Medical Foundation, the North Ostrobothnia Regional Fund, the Academy of Finland 315921, 321763, 104781, 120315, 129269, 1114194, 24300796, Center of Excellence in Complex Disease Genetics and SALVE), the Sigrid Juselius Foundation, National Institute for Health Research (UK), NHLBI grant 5R01HL087679-02 through the STAMPEED program (1RL1MH083268-01), NIH/NIMH (5R01MH63706:02), ENGAGE project and grant agreement HEALTH-F4-2007–201413, EU FP7 EurHEALTHAgeing (277849), the Medical Research Council UK (G0500539, G0600705, G1002319, G0802782, PrevMetSyn/SALVE).
Glossary
Abbreviations
- ANCOVA
analysis of covariance analysis
- ANOVA
analysis of variance
- BMI
body mass index
- CI
confidence interval
- FAI
free androgen index
- H
hirsutism
- HRQoL
health-related quality of life
- HSCL-25
Hopkins Symptoms Checklist-25
- OA
oligo-amenorrhea
- OR
odds ratio
- PCOS
polycystic ovary syndrome
- SHBG
sex hormone binding globulin
- T
testosterone
- WC
waist circumference
Additional Information
Disclosure Summary: The authors have no conflict of interest.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. [DOI] [PubMed] [Google Scholar]
- 2. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–2855. [DOI] [PubMed] [Google Scholar]
- 3. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. [DOI] [PubMed] [Google Scholar]
- 4. Teede HJ, Misso ML, Costello MF, et al. ; International PCOS Network . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2018;89(3):251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dokras A, Stener-Victorin E, Yildiz BO, et al. Androgen excess- polycystic ovary syndrome society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. 2018;109(5):888–899. [DOI] [PubMed] [Google Scholar]
- 6. Karjula S, Morin-Papunen L, Auvinen J, et al. Psychological distress is more prevalent in fertile age and premenopausal women with PCOS symptoms: 15-year follow-up. J Clin Endocrinol Metab. 2017;102(6):1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenwood EA, Yaffe K, Wellons MF, Cedars MI, Huddleston HG. Depression over the lifespan in a population-based cohort of women with polycystic ovary syndrome: longitudinal analysis. J Clin Endocrinol Metab. 2019;104(7):2809–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. [Published online ahead of print June 30, 2010]. BMC Med. 2010;8:41. doi:10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911–919. [DOI] [PubMed] [Google Scholar]
- 10. Li Y, Li Y, Yu Ng EH, et al. Polycystic ovary syndrome is associated with negatively variable impacts on domains of health-related quality of life: evidence from a meta-analysis. Fertil Steril. 2011;96(2):452–458. [DOI] [PubMed] [Google Scholar]
- 11. Hahn S, Janssen OE, Tan S, et al. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol. 2005;153(6):853–860. [DOI] [PubMed] [Google Scholar]
- 12. Elsenbruch S, Hahn S, Kowalsky D, et al. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(12):5801–5807. [DOI] [PubMed] [Google Scholar]
- 13. Saarni SI, Härkänen T, Sintonen H, et al. The impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Qual Life Res. 2006;15(8):1403–1414. [DOI] [PubMed] [Google Scholar]
- 14. Greenwood EA, Pasch LA, Cedars MI, Legro RS, Huddleston HG; Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network . Association among depression, symptom experience, and quality of life in polycystic ovary syndrome. Am J Obstet Gynecol. 2018;219(3):279.e1–279.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dokras A, Saini S, Gibson-Helm M, Schulkin J, Cooney L, Teede H. Gaps in knowledge among physicians regarding diagnostic criteria and management of polycystic ovary syndrome. Fertil Steril. 2017;107(6):1380–1386.e1. [DOI] [PubMed] [Google Scholar]
- 16. Gibson-Helm M, Dokras A, Karro H, Piltonen T, Teede HJ. Knowledge and practices regarding polycystic ovary syndrome among physicians in Europe, North America, and internationally: an online questionnaire-based study. Semin Reprod Med. 2018;36(1):19–27. [DOI] [PubMed] [Google Scholar]
- 17. Trent M, Austin SB, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Ambul Pediatr. 2005;5(2):107–111. [DOI] [PubMed] [Google Scholar]
- 18. Dokras A, Sarwer DB, Allison KC, et al. Weight loss and lowering androgens predict improvements in health-related quality of life in women with PCOS. J Clin Endocrinol Metab. 2016;101(8):2966–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coffey S, Bano G, Mason HD. Health-related quality of life in women with polycystic ovary syndrome: a comparison with the general population using the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the Short Form-36 (SF-36). Gynecol Endocrinol. 2006;22(2):80–86. [DOI] [PubMed] [Google Scholar]
- 20. Ollila MM, Piltonen T, Puukka K, et al. Weight gain and dyslipidemia in early adulthood associate with polycystic ovary syndrome: prospective cohort study. J Clin Endocrinol Metab. 2016;101(2):739–747. [DOI] [PubMed] [Google Scholar]
- 21. Glueck CJ, Dharashivkar S, Wang P, et al. Obesity and extreme obesity, manifest by ages 20-24 years, continuing through 32-41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol. 2005;122(2):206–212. [DOI] [PubMed] [Google Scholar]
- 22. Koivuaho E, Laru J, Ojaniemi M, et al. Age at adiposity rebound in childhood is associated with PCOS diagnosis and obesity in adulthood-longitudinal analysis of BMI data from birth to age 46 in cases of PCOS. Int J Obes (Lond). 2019;43(7):1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drosdzol A, Skrzypulec V, Plinta R. Quality of life, mental health and self-esteem in hirsute adolescent females. J Psychosom Obstet Gynaecol. 2010;31(3):168–175. [DOI] [PubMed] [Google Scholar]
- 24. Ekbäck MP, Lindberg M, Benzein E, Årestedt K. Health-related quality of life, depression and anxiety correlate with the degree of hirsutism. Dermatology. 2013;227(3):278–284. [DOI] [PubMed] [Google Scholar]
- 25. Chachamovich JR, Chachamovich E, Ezer H, Fleck MP, Knauth D, Passos EP. Investigating quality of life and health-related quality of life in infertility: a systematic review. J Psychosom Obstet Gynaecol. 2010;31(2):101–110. [DOI] [PubMed] [Google Scholar]
- 26. Amiri M, Bidhendi Yarandi R, et al. The relationship between clinical and biochemical characteristics and quality of life in patients with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2019;90(1):129–137. [DOI] [PubMed] [Google Scholar]
- 27. Taponen S, Martikainen H, Järvelin MR, et al. Hormonal profile of women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab. 2003;88(1):141–147. [DOI] [PubMed] [Google Scholar]
- 28. Taponen S, Ahonkallio S, Martikainen H, et al. Prevalence of polycystic ovaries in women with self-reported symptoms of oligomenorrhoea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod. 2004;19(5):1083–1088. [DOI] [PubMed] [Google Scholar]
- 29. Rantakallio P. The longitudinal study of the northern Finland birth cohort of 1966. Paediatr Perinat Epidemiol. 1988;2(1):59–88. [DOI] [PubMed] [Google Scholar]
- 30. Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5):328–336. [DOI] [PubMed] [Google Scholar]
- 31. Alanne S, Roine RP, Räsänen P, Vainiola T, Sintonen H. Estimating the minimum important change in the 15D scores. Qual Life Res. 2015;24(3):599–606. [DOI] [PubMed] [Google Scholar]
- 32. Veijola J, Jokelainen J, Läksy K, et al. The Hopkins symptom checklist-25 in screening DSM-III-R axis-I disorders. Nord J Psychiatry. 2003;57(2):119–123. [DOI] [PubMed] [Google Scholar]
- 33. Haapea M, Miettunen J, Läärä E, et al. Non-participation in a field survey with respect to psychiatric disorders. Scand J Public Health. 2008;36(7):728–736. [DOI] [PubMed] [Google Scholar]
- 34. Kaczmarek C, Haller DM, Yaron M. Health-related quality of life in adolescents and young adults with polycystic ovary syndrome: a systematic review. J Pediatr Adolesc Gynecol. 2016;29(6):551–557. [DOI] [PubMed] [Google Scholar]
- 35. Fernandez RC, Moore VM, Van Ryswyk EM, et al. Sleep disturbances in women with polycystic ovary syndrome: prevalence, pathophysiology, impact and management strategies. Nat Sci Sleep. 2018;10:45–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Helvaci N, Karabulut E, Demir AU, Yildiz BO. Polycystic ovary syndrome and the risk of obstructive sleep apnea: a meta-analysis and review of the literature. Endocr Connect. 2017;6(7):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cinar N, Harmanci A, Demir B, Yildiz BO. Effect of an oral contraceptive on emotional distress, anxiety and depression of women with polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27(6):1840–1845. [DOI] [PubMed] [Google Scholar]
- 38. Clayton WJ, Lipton M, Elford J, Rustin M, Sherr L. A randomized controlled trial of laser treatment among hirsute women with polycystic ovary syndrome. Br J Dermatol. 2005;152(5):986–992. [DOI] [PubMed] [Google Scholar]
- 39. Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32(5):1075–1091. [DOI] [PubMed] [Google Scholar]
- 40. Lee I, Cooney LG, Saini S, et al. Increased risk of disordered eating in polycystic ovary syndrome. Fertil Steril. 2017;107(3):796–802. [DOI] [PubMed] [Google Scholar]
- 41. Hu M, Richard JE, Maliqueo M, et al. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci U S A. 2015;112(46):14348–14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greenwood EA, Pasch LA, Cedars MI, Legro RS, Eisenberg E, Huddleston HG; Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network . Insulin resistance is associated with depression risk in polycystic ovary syndrome. Fertil Steril. 2018;110(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Greenwood EA, Pasch LA, Shinkai K, Cedars MI, Huddleston HG. Clinical course of depression symptoms and predictors of enduring depression risk in women with polycystic ovary syndrome: results of a longitudinal study. Fertil Steril. 2019;111(1):147–156. [DOI] [PubMed] [Google Scholar]
- 44. Kumarapeli V, Seneviratne Rde A, Wijeyaratne C. Health-related quality of life and psychological distress in polycystic ovary syndrome: a hidden facet in South Asian women. BJOG. 2011;118(3):319–328. [DOI] [PubMed] [Google Scholar]
- 45. Trent ME, Rich M, Austin SB, Gordon CM. Fertility concerns and sexual behavior in adolescent girls with polycystic ovary syndrome: implications for quality of life. J Pediatr Adolesc Gynecol. 2003;16(1):33–37. [DOI] [PubMed] [Google Scholar]
- 46. Joham AE, Teede HJ, Ranasinha S, Zoungas S, Boyle J. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: data from a large community-based cohort study. J Womens Health (Larchmt). 2015;24(4):299–307. [DOI] [PubMed] [Google Scholar]
- 47. West S, Vähäsarja M, Bloigu A, et al. The impact of self-reported oligo-amenorrhea and hirsutism on fertility and lifetime reproductive success: results from the Northern Finland Birth Cohort 1966. Hum Reprod. 2014;29(3):628–633. [DOI] [PubMed] [Google Scholar]
- 48. Cinar N, Kizilarslanoglu MC, Harmanci A, et al. Depression, anxiety and cardiometabolic risk in polycystic ovary syndrome. Hum Reprod. 2011;26(12):3339–3345. [DOI] [PubMed] [Google Scholar]
- 49. Tay CT, Teede HJ, Hill B, Loxton D, Joham AE. Increased prevalence of eating disorders, low self-esteem, and psychological distress in women with polycystic ovary syndrome: a community-based cohort study. Fertil Steril. 2019;112(2):353–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.