Abstract
Background
Ejection fraction is the principal parameter used clinically to assess cardiac mechanics and provides prognostic information. However, significant abnormalities of myocardial deformation can be present despite preserved ejection fraction. Cardiac-Magnetic-Resonance (CMR) feature-tracking techniques now allow assessment of strain from routine cine-images, without specialized pulse sequences. Whether abnormalities of strain measured using CMR feature-tracking have prognostic value in patients with preserved ejection fraction is unknown.
Objectives
To evaluate the prognostic value of CMR feature-tracking derived global longitudinal strain (GLS) in a large multicenter population of patients with preserved ejection fraction.
Methods
Consecutive patients with preserved ejection fraction (EF≥50%) and a clinical indication for CMR at four US medical centers were included in this retrospective study. Feature-tracking GLS was calculated from 3 long-axis-cine-views. The primary endpoint was all-cause death. Cox proportional hazards regression modeling was used to examine the independent association between GLS and death. The incremental prognostic value of GLS was assessed in nested models.
Results
Of the 1274 patients in this study, 115 died during a median follow-up of 6.2years. By Kaplan-Meier-analysis, patients with GLS≥median (−20%) had significantly reduced event free survival compared to those with GLS<median (log-rank p<0.001). By Cox multivariable regression modeling, each 1% worsening in GLS was associated with a 22.8% increased risk-of-death after adjustment for clinical and imaging risk factors (HR=1.228 per %; p<0.001). Addition of GLS in this model resulted in significant-improvement in the global-chi-square (94 to 183;p<0.0001) and Harrell’s C-statistic (0.75 to 0.83;p<0.0001).
Conclusions
CMR feature-tracking derived GLS is a powerful independent predictor of mortality in patients with preserved ejection fraction, incremental to common clinical and imaging risk factors.
Keywords: cardiac magnetic resonance imaging, prognosis, mortality, left ventricular function, global longitudinal strain, feature tracking
INTRODUCTION
Ejection fraction is the principal parameter used clinically to assess cardiac mechanics. It is frequently used to diagnose myocardial dysfunction and provides prognostic information. However, echocardiographic strain imaging has shown that significant abnormalities of myocardial deformation may be present despite preserved ejection fraction, and can be associated with adverse prognosis(1,2). Cardiac-Magnetic-Resonance (CMR) feature-tracking techniques now allow assessment of strain from routinely acquired cine-images, without specialized pulse sequences. We and others have shown that feature-tracking derived global longitudinal strain (GLS) is a powerful independent predictor of adverse outcomes in patients with reduced ejection fraction(3–5).
Whether abnormalities of strain measured using CMR feature-tracking have prognostic value in patients with preserved ejection fraction is unknown. We hypothesized that feature tracking derived GLS may provide prognostic information incremental to clinical and CMR derived parameters in this patient group.
Therefore, the aim of this study was to evaluate the prognostic value of CMR feature-tracking derived GLS in a large multicenter population of patients with preserved ejection fraction undergoing CMR.
METHODS
Study Design
Four geographically diverse medical centers in the United States participated in this retrospective, observational, multicenter study. The University of Illinois in Chicago served as the data-coordinating center using a cloud-based database (CloudCMR, www.cloudCMR.com) containing de-identified searchable data from consecutive patients with full DICOM datasets from the participating centers. Institutional review board approval was obtained at each center.
Study Population
Consecutive patients with preserved ejection fraction (EF≥50%) and a clinical indication for CMR who had undergone CMR in 2011 with both cine and late gadolinium enhancement (LGE) imaging formed the study population of 1274 patients. Exclusion criteria included uninterpretable image quality for GLS assessment, severe valvular disease, as well as hypertrophic and infiltrative cardiomyopathies (total excluded=111). Baseline demographics (age, gender, BMI, history of diabetes, history of hyperlipidemia, history of hypertension, history of smoking, history of MI, cardiac medications) were obtained by local site investigators at the time of the clinical study. History of diabetes, history of hyperlipidemia, history of hypertension, history of smoking, and history of MI were assessed based on documentation of the diagnosis in the electronic medical record at the time of the CMR exam
CMR Acquisition
Images were acquired with phased-array receiver coils according to the routine scan protocol at each site using a variety of scanners from all three major vendors (Siemens, Philips and General Electric) at both 1.5 and 3 Tesla. A typical protocol included steady-state free-precession cine images acquired in multiple short-axis and three long-axis views with short-axis views obtained every 1cm to cover the entire left ventricle. Typical temporal resolution of cine images was <45msec. LGE imaging was performed 10–15 minutes after Gadolinium contrast (0.15 mmol/kg) administration using a 2D segmented gradient echo inversion-recovery sequence in the same views used for cine-CMR. Inversion delay times were typically 280 to 360 ms.
CMR Analysis and GLS Assessment
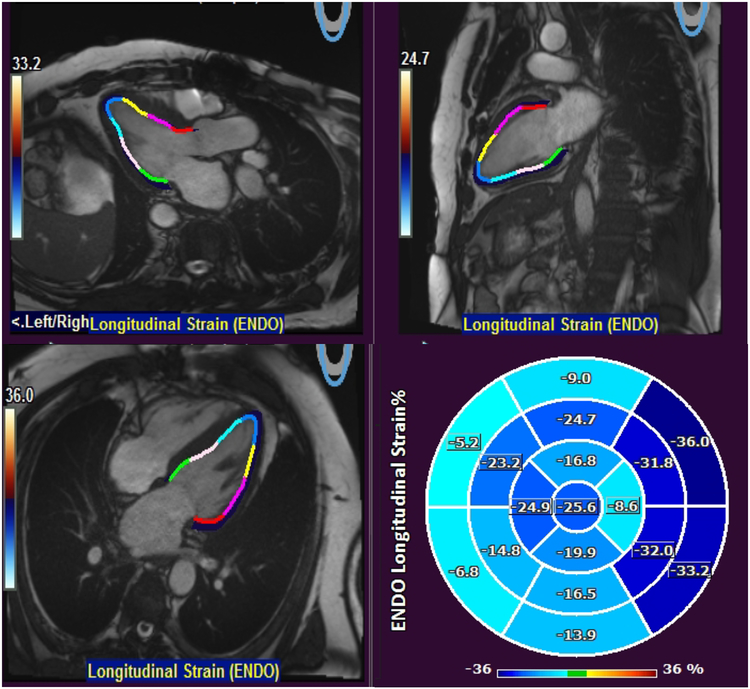
The study site investigators analyzed images on locally available workstations and were blinded to follow-up data. Late gadolinium enhancement and feature tracking GLS was assessed as described previously(3,6–10). For feature tracking analysis, endocardial left ventricular contours were manually traced (by a single physician who was blinded to patient information and outcomes) in all 3 long-axis cine views to derive 2D GLS using the Qstrain package (Medis Medical Imaging Systems, Leiden, the Netherlands) (Figure 1).
Figure 1. Measurement of GLS.
Endocardial left ventricular contours were manually traced in all 3 long-axis cine views to derive 2D GLS using the Qstrain package (Medis Medical Imaging Systems, Leiden, the Netherlands). GLS in this patient was −16.5%.
Follow-up
Patients were followed for the primary outcome of all cause mortality using the United States Social Security Death Index. Time to event was calculated as the period between the CMR study and death. Patients who did not experience the primary outcome were censored at the time of assessment.
Statistical Analysis
Normally distributed data were expressed as mean ± SD. Differences in baseline characteristics were compared with the Student’s t-test for continuous variables and the chi-squared test for dichotomous variables. Kaplan-Meier methods were used to evaluate the relationship between GLS and time to the primary outcome of all cause mortality. We used Cox proportional hazards regression modeling to examine the association between GLS and all cause mortality. All models were assessed for collinearity and proportional hazards assumption. For the multivariable models, clinical and imaging risk factors which were univariate predictors (at p≤0.10) were considered as covariates.
The incremental prognostic value of GLS was assessed in nested-models. Model discrimination was compared by calculating the C-index(11). Risk reclassification analyses were conducted with calculation of continuous net reclassification improvement (NRI)(12). A p value of <0.05 was considered statistically significant. Analyses were performed using STATA (StataCorp, TX).
RESULTS
Patient Characteristics
Table 1 summarizes baseline patient characteristics stratified by GLS above and below the median (−20%). The mean age of the study population was 57.1(±15.9) years. Fifty-three percent of patients were male and 19% had diabetes mellitus. The mean ejection fraction was 63.0 ± 6.6% and LGE was present in 18.4% of patients. Mean LGE extent was 1.3(±4.5)% of myocardium. Of the patients with LGE, 60% had an ischemic pattern (i.e. involving the subendocardium), while 40% had a non-ischemic pattern (i.e. mid myocardial or epicardial, without subendocardial involvement). Atrial fibrillation was present in 56 patients (4.4%) at the time of the CMR scan. The primary indications and suspected diagnoses for CMR are shown in table 2. The commonest symptoms were: dyspnea (29%), chest pain (23%), and palpitations (20%).
Table 1. Baseline characteristics of study population stratified by GLS above and below the median (−20%).
BMI=Body Mass Index, LA=Left Atrial, LGE=Late Gadolinium Enhancement, LVEDV =Left Ventricular End Diastolic Volume, LVEF=Left Ventricular Ejection Fraction, LVESV =Left Ventricular End Systolic Volume Index, MI=Myocardial Infarction, RVEDV = Right Ventricular End Diastolic Volume, RVEF=Right Ventricular Ejection Fraction, SD=standard deviation.
| CHARACTERISTICS | Total | GLS <median | GLS ≥median | P Value |
|---|---|---|---|---|
| CLINICAL HISTORY | ||||
| Age (±SD), years | 57.1 (±15.9) | 56.6 (±16.0) | 57.5 (±15.8) | 0.287 |
| Male % | 53.5 | 48.2 | 58.9 | <0.001 |
| BMI (±SD), kg/m2 | 28.8 (±8.0) | 28.6 (±6.4) | 28.9 (±9.4) | 0.504 |
| Diabetes % | 19.2 | 17.6 | 20.9 | 0.139 |
| Hyperlipidemia % | 41.5 | 42.7 | 40.3 | 0.375 |
| Hypertension % | 57.0 | 54.8 | 59.7 | 0.074 |
| Smoking % | 5.7 | 5.5 | 5.8 | 0.808 |
| History of MI % | 5.7 | 5.2 | 6.2 | 0.466 |
| Aspirin % | 42.3 | 40.3 | 44.2 | 0.175 |
| Statin % | 37.4 | 37.8 | 36.9 | 0.753 |
| ACE inhibitor % | 26.4 | 25.6 | 27.3 | 0.494 |
| Beat Blocker % | 22.5 | 20.8 | 24.1 | 0.176 |
| CMR VARIABLES | ||||
| Heart Rate (±SD), beats/min | 71.8 (±13.9) | 70.3 (±13.1) | 73.4 (±14.5) | <0.001 |
| Systolic BP (±SD), mm Hg | 133(±20) | 133(±19) | 133(±21) | 0.644 |
| Diastolic BP (±SD), mm Hg | 75(±23) | 74(±11) | 76(±31) | 0.060 |
| LA volume | 50.9 (±40.0) | 49.9 (±34.2) | 51.8 (±44.0) | 0.383 |
| LVEDV index (±SD), ml/m2 | 60.6 (±19.7) | 61.5 (±19.6) | 59.8 (±19.8) | 0.118 |
| LVESV index (±SD), ml/m2 | 22.1 (±11.4) | 20.7 (±11.0) | 23.4 (±11.8) | <0.001 |
| LVEF (±SD), % | 63.0 (±6.6) | 64.6 (±6.6) | 61.3 (±6.1) | <0.001 |
| LGE present % | 18.4 | 14.3 | 22.4 | <0.001 |
| RVEDV index (±SD), ml/m2 | 80.6 (±12.7) | 81.6 (±11.9) | 79.7 (±13.4) | 0.006 |
| RVEF (±SD), % | 54.8 (±3.2) | 54.9 (±2.1) | 54.6 (±3.0) | 0.039 |
Table 2.
Main Indications and Suspected Diagnoses for Performance of CMR.
| INDICATION | Prevalence |
|---|---|
| Suspected Myocardial Involvement or Cardiomyopathy | 28% |
| Known or suspected CAD | 23% |
| Known or suspected arrhythmias | 13% |
| Known or suspected aortic disease | 12% |
| Evaluation prior to possible ablation of atrial fibrillation | 7% |
| Known or suspected cardiac mass | 6% |
| Known or suspected valve disease | 6% |
| Others (including poor echo windows, syncope, pericardial disease, coronary anomaly, pulmonary hypertension, abnormal ECG) | 5% |
Primary Outcome
Of the 1274 patients in this study, 115 died during a median follow-up of 6.2 years (interquartile range: 5.6–6.7 years).
Outcomes and GLS
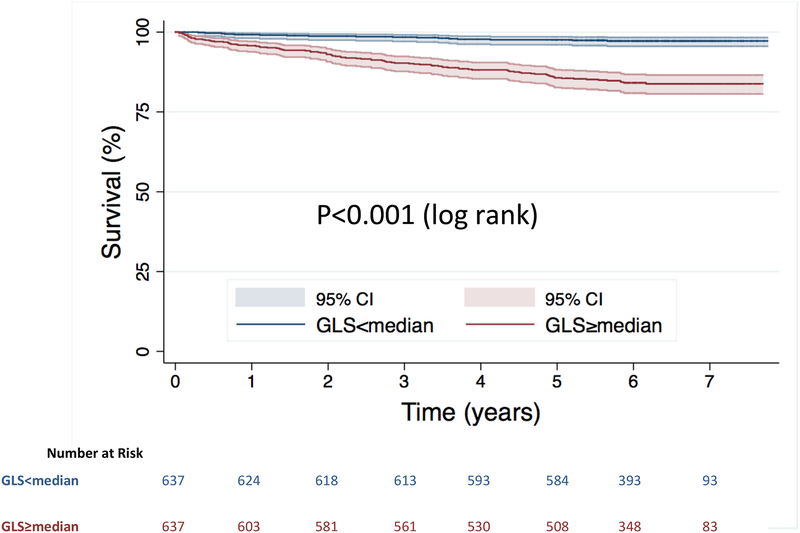
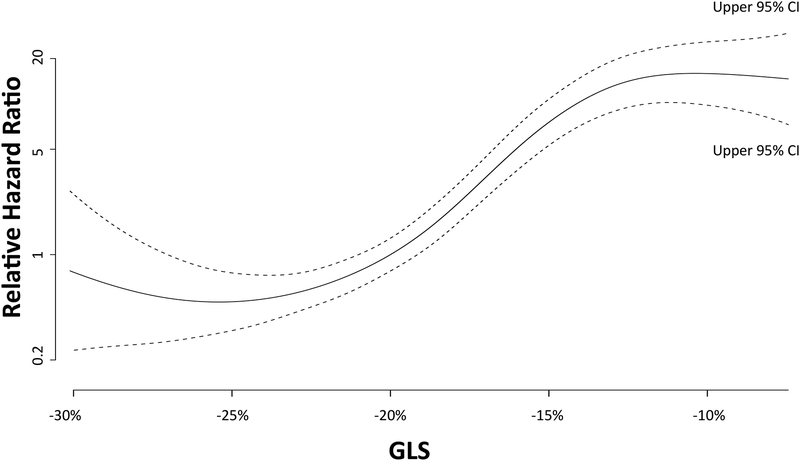
When stratified by the median value of GLS (−20%), Kaplan-Meier analysis showed significantly increased risk of death in those with GLS≥median (log-rank p<0.001) (Central Illustration). The continuous relationship between GLS and the hazard of death is shown in the cubic spline in figure 2.
Central Illustration. Kaplan-Meier survival curves.
Stratified by GLS above and below the median value.
Figure 2. Relationship between GLS and hazard of death (with 95% confidence intervals).
Hazard ratios are relative to those with median GLS.
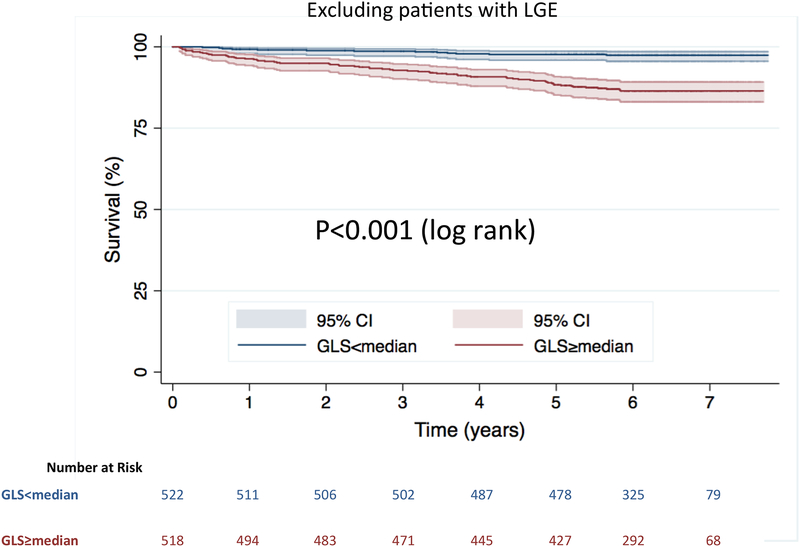
In addition, amongst the subgroup of patients without LGE (n=1040, 80 deaths) Kaplan-Meier analysis similarly showed significantly increased risk of death in those with GLS≥median (log-rank p<0.001) (Figure 3).
Figure 3. Kaplan-Meier survival curves.
Stratified by GLS above and below the median value for the subgroup of patients with no LGE.
Multivariable Analysis and Incremental Prognostic Value
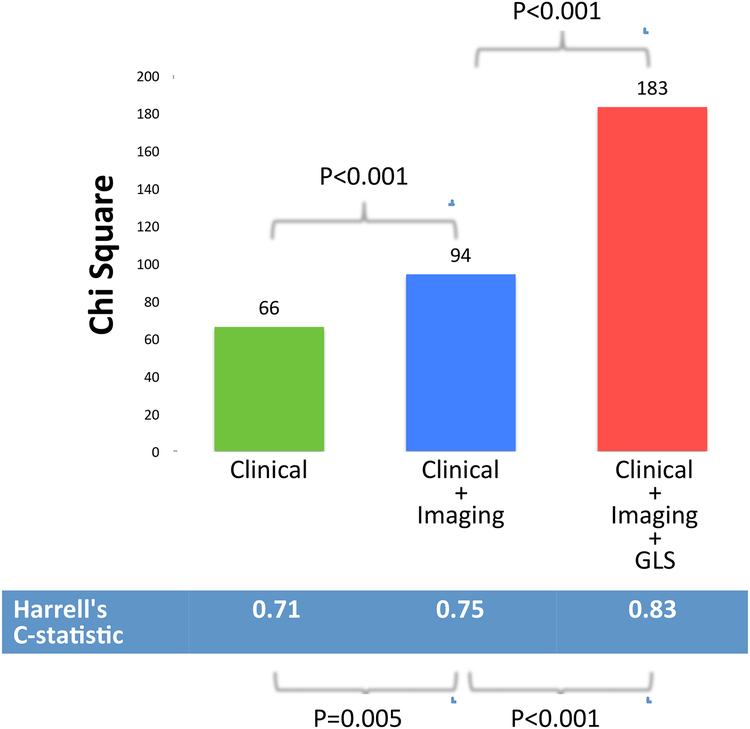
After multivariate adjustment for clinical and imaging risk factors (Age, BMI, Diabetes, Hypertension, Heart Rate, Diastolic Blood Pressure, Left Ventricular End Diastolic Volume Index, Left Ventricular Ejection Fraction, Left Atrial Volume, LGE, Right Ventricular Ejection Fraction), GLS remained a significant independent predictor of death (HR=1.228; p<0.001) i.e. each 1% worsening in GLS was associated with a 22.8% increase risk of death (Table 3). In sequential nested Cox models, a model based on clinical variables alone (Age, BMI, Diabetes, Hypertension, Heart Rate, Diastolic Blood Pressure) was significantly improved by addition of imaging variables (Left Ventricular End Diastolic Volume Index, Left Ventricular Ejection Fraction, Left Atrial Volume, LGE, Right Ventricular Ejection Fraction), and further significantly improved by adding GLS (Figure 4). Addition of GLS into the model with clinical and imaging predictors resulted in significant increase in the C-statistic (from 0.75 to 0.83 p<0.0001) and a significant increase in model Chi square value (from 94 to 183; p<0.001). This was associated with significant integrated discrimination improvement of 0.134 (95% CI, 0.078–0.199), and a continuous NRI of 0.916 (95% CI, 0.753–1.152).
Table 3. Multivariable model for death in overall population.
BMI=Body Mass Index, GLS=Global Longitudinal Strain, LA=Left Atrial, LGE=Late Gadolinium Enhancement, LVEDV=Left Ventricular Diastolic Volume, LVEF=Left Ventricular Ejection Fraction, RVEF=Right Ventricular Ejection Fraction.
| VARIABLES | Multivariable Model for Death | |
|---|---|---|
| Hazard Ratio (95% CI) | P Value | |
| Age | 1.031 (1.016–1.046) | <0.001 |
| BMI | 0.950 (0.915–0.985) | 0.006 |
| Diabetes | 1.461 (0.934–2.287) | 0.097 |
| Hypertension | 1.140 (0.742–1.751) | 0.550 |
| Heart Rate | 1.011 (0.998–1.024) | 0.107 |
| Diastolic BP | 0.988 (0.973–1.003) | 0.127 |
| LVEDV index | 0.999 (0.988–1.010) | 0.834 |
| LGE | 1.319 (0.857–2.030) | 0.207 |
| LVEF | 0.999 (0.968–1.031) | 0.938 |
| LA volume | 1.000 (0.997–1.003) | 0.912 |
| RVEF | 0.969 (0.927–1.014) | 0.176 |
| GLS | 1.228 (1.178–1.280) | <0.001 |
Figure 4. Sequential nested Cox models for death.
A model based on clinical variables alone (Age, BMI, Diabetes, Hypertension, Heart Rate, Diastolic Blood Pressure) was significantly improved by addition of imaging variables (Left Ventricular End Diastolic Volume Index, Left Ventricular Ejection Fraction, Left Atrial Volume, LGE, Right Ventricular Ejection Fraction), and further significantly improved by adding GLS.
In addition, amongst the subgroup of patients without LGE, GLS remained a significant independent predictor of death (HR=1.212; p<0.001) after adjustment to clinical and imaging risk factors (Age, BMI, Diabetes, Heart Rate, Left Ventricular Ejection Fraction) (Table 4).
Table 4. Multivariable model for death in patients without LGE.
BMI=Body Mass Index, GLS=Global longitudinal strain, LVEF=Left Ventricular Ejection Fraction
| VARIABLES | Multivariable Model for Death | |
|---|---|---|
| Hazard Ratio (95% CI) | P Value | |
| Age | 1.039 (1.022–1.056) | <0.001 |
| BMI | 0.938 (0.899–0.979) | 0.004 |
| Diabetes | 1.504 (0.891–2.540) | 0.127 |
| Heart Rate | 1.008 (0.993–1.023) | 0.282 |
| LVEF | 0.998 (0.959–1.037) | 0.901 |
| GLS | 1.212 (1.157–1.270) | <0.001 |
DISCUSSION
This study shows that feature tracking GLS is a powerful independent predictor of mortality, in a large multicenter population of patients with preserved ejection fraction undergoing CMR. We have demonstrated that GLS provides prognostic information incremental to common clinical and CMR risk factors - including late gadolinium enhancement. These findings highlight the importance of long-axis function and suggest a role for feature tracking GLS in identifying patients at highest risk of death, despite a preserved ejection fraction.
Myocardial contraction and long axis function
Long axis function plays a fundamental role in cardiac mechanics. It has been long known that the outer contour volume of the heart remains relatively constant throughout the cardiac cycle with the apex remaining still as the mitral annulus moves longitudinally(13). This results in reciprocal filling and emptying of the ventricles and atria - such that filling of one chamber occurs at the expense of emptying of the other.
Longitudinal movement of the mitral annulus is the major driver of ventricular ejection and atrial filling. Since the outer contour of the heart remains relatively constant, movement of the annulus in systole results not just in shortening of ventricular length but also increase in wall thickness (radial wall thickening) due to conservation of myocardial volume (14).
Ejection fraction and subclinical long axis dysfunction
Ejection fraction is a simple global measure reflecting the combined function of both longitudinal and circumferential fibers, without the ability to distinguish between these components. Possibly because of their subendocardial location, the more longitudinal myocardial fibers seem to be exquisitely sensitive to disturbance by various pathologies, and mitral annular motion is very rapidly reduced by ischemia in experimental models(15). This may relate to the presence of greater compressive forces and higher oxygen consumption in the subendocardium (16–19).
Thus in the early stages of many cardiac diseases, impairment in longitudinal function appears to precede reduction in circumferential contraction, giving rise to subclinical impairment of left ventricular function despite preserved ejection fraction. Early compensatory increase in circumferential function helps maintain ejection fraction despite significantly impaired longitudinal function(2).
In this study we have shown that reduction of long axis function as detected by GLS, is a powerful independent predictor of mortality in patients with preserved ejection fraction possibly because it is an early marker of subclinical pathological processes affecting the subendocardial longitudinal fibers.
CMR feature tracking GLS and prognosis
There is a growing body of literature showing the prognostic value of feature tracking GLS in patients with reduced ejection fraction and heart failure(3–5,20). In a large (n=1012) multicenter population of patients with ischemic and non-ischemic cardiomyopathy, it was shown that feature tracking derived GLS is a powerful independent predictor of mortality, incremental to common clinical and CMR risk-factors, including ejection-fraction and late-gadolinium-enhancement(4). Buss and colleagues likewise demonstrated that feature tracking derived GLS was an independent predictor of the composite endpoint of cardiac death, heart transplantation, and aborted sudden cardiac death in a single center population of 210 dilated non-ischemic cardiomyopathy patients (5). In a small population of selected patients with heart failure and preserved ejection fraction, Kammerlander reported an association between feature tracking GLS and a composite endpoint of heart failure hospitalization and cardiovascular death(21).
Feature tracking GLS also appears to provide prognostic value post MI. Eitel et al, showed the incremental prognostic value of feature tracking GLS early after reperfused MI in 1235 patients (STEMI=760, NSTEMI=347) from multiple sites across Germany(22). Similarly in a single center study, Gavara et al demonstrated that feature tracking GLS was associated with a composite outcome of cardiac death, heart failure hospitalization and reinfarction in 323 patients post STEMI(23).
In this study we have now extended these prior observations by showing that feature tracking GLS is also a powerful independent predictor of mortality, in patients with preserved ejection fraction. Ultimately, better identification of high-risk patients may allow closer follow-up and more directed therapies to be applied. How this information will affect clinical care requires further investigation and future studies are warranted to explore the role of feature tracking GLS in clinical decision making. These studies will need to demonstrate that imaging driven patient management improves specific outcomes before such an approach could be advocated.
Limitations
Although this is a multicenter study, the patients in this paper may not be representative of all patients with preserved ejection fraction in the community. Since this is a CMR study, there is selection bias related to being able to undergo a CMR exam, resulting in exclusion of patients with large body size, severe renal impairment, severe claustrophobia or those with pacemakers and ICDs.
Information about downstream cardiovascular resource utilization such as revascularization, ICD implantation or cardiac surgery was not available. However, this does not detract from the main findings of this study, that feature-tracking GLS is a powerful predictor of death in these patients, independent of common clinical and imaging markers available at the time of CMR. Follow-up data was limited to the primary endpoint of all cause death and the cause of death was not known. However, many have argued that all–cause mortality is an extremely important and appropriate study endpoint because it is unbiased and clinically relevant, which is often not the case for other cardiac outcomes such as revascularization or hospitalization(1,24,25). Use of cardiac-death instead of all-cause death as an end point can be problematic for many reasons since data obtained from death certificates or from medical records are limited, biased and not necessarily accurate. In addition, determination of cause of death is often difficult due to multiple comorbidities, low autopsy rates and poor understanding of complex diseases(25). We therefore believe that all-cause mortality is a very important and valid primary end-point for this study.
It can be argued that it is unsurprising that LV ejection fraction was of limited prognostic value in this study, since by design this was a group of patients with preserved ejection fraction only. Although 23% of patients were undergoing CMR for evaluation of known or suspected CAD, information regarding the proportion of patients with non-ST segment elevation vs. ST segment elevation MI was not available. Moreover, patients in this study did not systematically undergo coronary angiography. Therefore accurate and detailed information about presence or absence of coronary artery disease was not available. A priori this was not designed as a study of patients with heart failure. Thus details such as clinical heart failure status, heart failure hospitalization, BNP and right heart catheterization were not systematically assessed or available.
T1 mapping techniques were not clinically widely available at the time of CMR image acquisition and therefore could not be performed on these clinical scans across multiple sites with different vendors and field strengths.
Conclusions
In this large multicenter study, feature tracking GLS is a significant independent predictor of mortality in patients with preserved ejection fraction – incremental to common clinical and imaging risk factors. Each 1% worsening in GLS was associated with a 22.8% increased risk-of-death after adjustment for clinical and imaging risk factors. A major strength of these findings is that they were made in a multicenter group of patients with a large number of hard events (n=115), which greatly increases the robustness of our results. Importantly, GLS remained an independent predictor of death even in the subgroup of patients without LGE, potentially allowing early identification of patients at highest risk.
Our findings highlight the importance of long-axis function and suggest that consideration may be given to measurement of GLS even in those with preserved ejection fraction. Future studies are needed to explore the role of feature tracking GLS in clinical decision making in these patients.
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
In this multicenter study, feature tracking GLS measured during cine CMR is a significant independent predictor of mortality in patients with preserved ejection fraction and a clinical indication for CMR – incremental to common clinical and imaging risk factors
Translational Outlook:
How this information will affect clinical care requires further investigation and future studies are warranted to explore the role of CMR derived feature tracking GLS in clinical decision making.
Abbreviations:
- CMR
Cardiac Magnetic Resonance
- EF
Ejection Fraction
- LGE
Late Gadolinium Enhancement
- LV
Left Ventricle
- GLS
Global Longitudinal Strain
Footnotes
Disclosures: None
REFERENCES
- 1.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–64. [DOI] [PubMed] [Google Scholar]
- 2.Cikes M, Solomon SD. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. European heart journal 2016;37:1642–50. [DOI] [PubMed] [Google Scholar]
- 3.Romano S, Judd RM, Kim RJ et al. Association of Feature-Tracking Cardiac Magnetic Resonance Imaging Left Ventricular Global Longitudinal Strain With All-Cause Mortality in Patients With Reduced Left Ventricular Ejection Fraction. Circulation 2017;135:2313–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romano S, Judd RM, Kim RJ et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients With Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovascular imaging 2018;11:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buss SJ, Breuninger K, Lehrke S et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. European heart journal cardiovascular Imaging 2015;16:307–15. [DOI] [PubMed] [Google Scholar]
- 6.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet 2001;357:21–8. [DOI] [PubMed] [Google Scholar]
- 7.Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol 2009;55:1–16. [DOI] [PubMed] [Google Scholar]
- 8.Indorkar R, Kwong RY, Romano S et al. Global Coronary Flow Reserve Measured During Stress Cardiac Magnetic Resonance Imaging Is an Independent Predictor of Adverse Cardiovascular Events. JACC Cardiovascular imaging 2019;12:1686–1695. [DOI] [PubMed] [Google Scholar]
- 9.Romano S, Romer B, Evans K et al. Prognostic Implications of Blunted Feature-Tracking Global Longitudinal Strain During Vasodilator Cardiovascular Magnetic Resonance Stress Imaging. JACC Cardiovascular imaging 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romano S, Judd RM, Kim RJ et al. Prognostic Implications of Mitral Annular Plane Systolic Excursion in Patients with Hypertension and a Clinical Indication for Cardiac Magnetic Resonance Imaging: A Multicenter Study. JACC Cardiovascular imaging 2019;12:1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Statistics in medicine 2004;23:2109–23. [DOI] [PubMed] [Google Scholar]
- 12.Pencina MJ, D’Agostino RB Sr., D’Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine 2008;27:157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton WFR JH Movements of the base of the ventricle and relative constancy of the cardiac olume. Am J Physiol 1932;102:559–565. [Google Scholar]
- 14.Carlsson M, Ugander M, Mosen H, Buhre T, Arheden H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. American journal of physiology Heart and circulatory physiology 2007;292:H1452–9. [DOI] [PubMed] [Google Scholar]
- 15.Sanderson JE. Left and right ventricular long-axis function and prognosis. Heart 2008;94:262–3. [DOI] [PubMed] [Google Scholar]
- 16.Henein MY, Gibson DG. Long axis function in disease. Heart 1999;81:229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henein MY, Gibson DG. Normal long axis function. Heart 1999;81:111–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chilian WM. Microvascular pressures and resistances in the left ventricular subepicardium and subendocardium. Circulation research 1991;69:561–70. [DOI] [PubMed] [Google Scholar]
- 19.Hu K, Liu D, Herrmann S et al. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. European heart journal cardiovascular Imaging 2013;14:205–12. [DOI] [PubMed] [Google Scholar]
- 20.Farzaneh-Far A, Romano S. Measuring longitudinal left ventricular function and strain using cardiovascular magnetic resonance imaging. European heart journal cardiovascular Imaging 2019. [DOI] [PubMed] [Google Scholar]
- 21.Kammerlander AA, Kraiger JA, Nitsche C et al. Global Longitudinal Strain by CMR Feature Tracking Is Associated With Outcome in HFPEF. JACC Cardiovascular imaging 2019;12:1585–1587. [DOI] [PubMed] [Google Scholar]
- 22.Eitel I, Stiermaier T, Lange T et al. Cardiac Magnetic Resonance Myocardial Feature Tracking for Optimized Prediction of Cardiovascular Events Following Myocardial Infarction. JACC Cardiovascular imaging 2018;11:1433–1444. [DOI] [PubMed] [Google Scholar]
- 23.Gavara J, Rodriguez-Palomares JF, Valente F et al. Prognostic Value of Strain by Tissue Tracking Cardiac Magnetic Resonance After ST-Segment Elevation Myocardial Infarction. JACC Cardiovascular imaging 2018;11:1448–1457. [DOI] [PubMed] [Google Scholar]
- 24.Klem I, Shah DJ, White RD et al. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging 2011;4:610–9. [DOI] [PubMed] [Google Scholar]
- 25.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 1999;34:618–20. [DOI] [PubMed] [Google Scholar]