Abstract
Single bouts of aerobic exercise can modulate cortical excitability and executive cognitive function, but less is known about the effect of light intensity exercise, an intensity of exercise more achievable for certain clinical populations. Fourteen healthy adults (aged 22 to 30) completed the following study procedures twice (≥7 days apart) before and after 30-minutes of either light aerobic exercise (cycling) or seated rest: neurocognitive battery (multitasking performance, inhibitory control and spatial working memory), paired pulse TMS-measures of cortical excitability. Significant improvements in response times during multitasking performance and increases in intracortical facilitation (ICF) were seen following light aerobic exercise. Light aerobic exercise can modulate cortical excitability and some executive function tasks. Populations with deficits in multitasking ability may benefit from this intervention.
Keywords: Exercise, Neuroplasticity, Cortical excitability, Executive function
Graphical Abstract
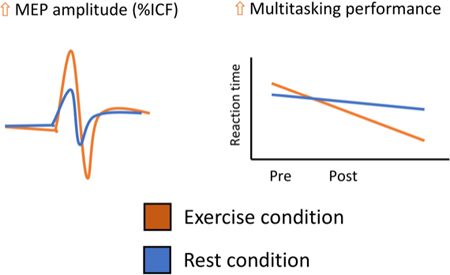
Light intensity aerobic exercise, suited to populations who may be unable to exercise at higher intensities can modulate multitasking performance and cortical excitability in a facilitative direction. Consistent with previous research however, this intensity of exercise does not appear to modulate widespread executive functions.
1. Introduction
There is a vast amount of evidence showing that participation in regular aerobic exercise has many positive effects on brain and cognitive functions, particularly on those dependent on mechanisms of neuroplasticity (Cabral et al., 2019; Cotman, Berchtold, & Christie, 2007; Gomes-Osman et al., 2018; Hillman, Erickson, & Kramer, 2008). Domain specific improvements in cognitive function have been reported and much focus has been placed on exercise-induced improvements in executive functions (Etnier & Chang, 2009). The majority of the evidence generated to date is based on moderate intensity exercise, in studies spanning various age groups (Donnelly et al., 2009; Gomes-Osman et al., 2018; Nanda, Balde, & Manjunatha, 2013; Peruyero, Zapata, Pastor, & Cervelló, 2017). It is worth noting however that certain populations, such as sedentary individuals, older adults or those with a disability or within rehabilitation may not be capable of exercising at higher exercise intensities (Franco et al., 2015; Pinto, Newman, & Hirsch, 2018), and therefore it is important to explore the effects of exercise performed at lower intensities. In one previous study, 4 weeks of light intensity exercise in previously sedentary individuals improved performance on the Stroop cognitive test (Gomes-Osman et al., 2017), suggesting that this intensity may have modulatory properties in certain populations. Single bouts of moderate intensity aerobic exercise have been shown to enhance different executive functions, such as planning, task switching, response inhibition and working memory (Chang et al., 2011; Hung, Tsai, Chen, Wang, & Chang, 2013; Kamijo et al., 2004; Kamijo, Nishihira, Higashiura, & Kuroiwa, 2007; Pontifex, Hillman, Fernhall, Thompson, & Valentini, 2009). Of these studies Kamijo and colleagues also found that light aerobic exercise increased reaction time latencies on a Flanker task (Kamijo et al., 2007) but not after a go/no-go task (Kamijo et al., 2004).
Cortical adaptations following injury to the brain (for example after stroke or traumatic brain injury) are a key goal of rehabilitation, and aerobic exercise has been associated with short term changes in cortical excitability within the motor cortex (Mooney et al., 2016; Singh, Duncan, Neva, & Staines, 2014; Smith, Goldsworthy, Garside, Wood, & Ridding, 2014). Exercise may facilitate learning-based rehabilitation via adaptive modulation of cortical excitability (Singh et al., 2014a). Accordingly, the immediate effect of exercise on executive function tasks, specifically those measured via response times (multitasking, inhibitory control), may be driven, in part, by neuroplastic changes related to neurotransmitter signalling (glutamate and gamma-aminobutyric (GABA)) (Kujirai et al., 1993; Maddock, Casazza, Fernandez, & Maddock, 2016). Transcranial magnetic stimulation (TMS) paradigms provide a means to characterize cortical excitability balance in the motor cortex (Pascual-Leone et al., 2011). Paired-pulse TMS (ppTMS) can be applied with different inter-stimulus intervals to provide an understanding of excitatory and inhibitory GABAergic and glutamatergic systems (Kujirai et al., 1993; Valls-Solé, Pascual-Leone, Wassermann, & Hallett, 1992). Studies have shown that moderate intensity exercise can modulate TMS measures of intracortical facilitation (ICF) and inhibition, including short interval intracortical inhibition (SICI) and long interval intracortical inhibition (LICI) (Lulic, El-Sayes, Fassett, & Nelson, 2017; Mooney et al., 2016; Singh et al., 2014; Smith et al., 2014). Of these studies, Smith and colleagues were the only previous study to assess the effect of light aerobic exercise on TMS measures of cortical excitability. Therefore, less evidence exists on how intracortical circuits are modulated by a single session of light aerobic exercise. Whilst studies have evaluated the effect of exercise on motor learning (Tunovic, Press, & Robertson, 2014) and procedural memory (Ostadan et al., 2016), and one previous study associated improvements in motor learning with cortical excitability and plasticity (Mang, Snow, Campbell, Ross, & Boyd, 2014), no studies have assessed the relationship between the effect of light intensity exercise on both executive function tasks and TMS measures of cortical excitability. Kamijo and colleagues did demonstrated that the P300 amplitude of an event-related potential was increased after both light and moderate-intensity exercise indicating increased executive control (Kamijo et al., 2007), however in a previous study only moderate intensity exercise lead to an increase in P300 amplitude, which was not concomitant with increased reaction times (Kamijo et al., 2004).
Further characterization of the cognitive effects and mechanistic underpinnings of single bouts of light aerobic exercise, an intervention suited to the rehabilitation setting, therefore is especially pertinent for populations who cannot exercise at higher intensities and of interest to the growing body of literature on the effects of acute exercise on executive function.
The present study was designed to explore the effects of a single bout of light aerobic exercise on several executive function tasks and cortical excitability. We hypothesized that a single bout of light aerobic exercise would improve executive function constructs (multitask performance, inhibitory control and spatial working memory) and modulate cortical excitability, as measured by TMS. An exploratory aim was to assess the relationship between these two outcome measures.
2. Methods
2.1. Participants
The Institutional Review Board of the Beth Israel Deaconess Medical Center (BIDMC) approved this study and participants signed informed consent prior to participating in any research procedures. Participants were recruited via an internal repository of previous research participants from the Berenson-Allen Center for Non-Invasive Brain Stimulation at BIDMC. Interested participants were screened for eligibility using the following criteria: right-handed (confirmed by the modified version of the Edinburgh Handedness questionnaire (Milenkovic & Dragovic, 2013), between the ages of 18 and 60 years, without neurological or physical conditions that might affect performance on testing procedures or known contraindications to TMS (Rossi et al., 2009). Contraindications to exercise were screened via the Physical Activity Readiness Questionnaire (PAR-Q) (Adams, 1999). Fourteen healthy adults (9 females) with a mean (±SD) age of 26 (±3) years completed all study procedures. An initial sample size calculation based on a previous meta-analysis (Etnier, Nowell, Landers, & Sibley, 2006) calculated that we needed a sample size of 13 to detect a similar effect (effect size of .34 with a standard deviation of .30, assuming a type I error rate of 0.05 with 95% power).
2.2. Protocol and study design
Participants completed two study visits in a randomized counterbalanced order design with at least 7 days between visits. This interval was chosen to minimize carry-over effects from the first visit. Study visits consisted of the following procedures (Figure 1): cognitive testing, a TMS session, either a 30-minute aerobic exercise (cycling) or control rest intervention followed by a repeat of the cognitive tasks and finally the TMS session. Study visits were scheduled so that each procedure was undertaken at roughly the same time of day over both visits. A random number sequence generated by Microsoft Excel (Microsoft, USA) determined the order in which each participant completed the study to minimize practice effects of the cognitive tasks. A series of 20 random numbers was generated and participants were allocated an order (exercise first or rest first) based on their corresponding number being odd or even.
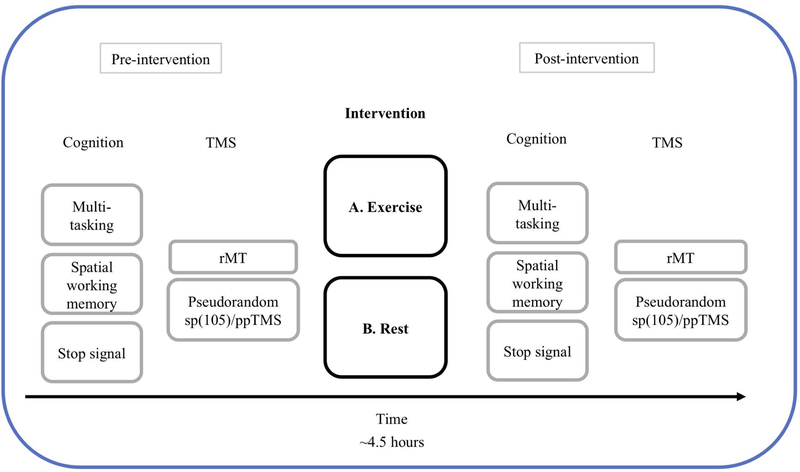
Figure 1.
Timeline of study procedures. The study employed a 2*2 (intervention by block) within-subjects A-B randomized protocol whereby participants were randomized to either perform the exercise intervention or rest control first, followed by the remaining intervention ≥7days later. The post-intervention cognitive tasks and TMS sessions were identical to the pre-intervention sessions. The time between the end of the pre-intervention TMS session the start of the post-intervention TMS session was 3 hours.
2.3. Intervention
The aerobic exercise intervention consisted of 30 minutes of light aerobic exercise on a Monarch 928 G3 static cycle electronic ergometer (Monarch exercise AB, Vansbro, Swenden). Prior to the intervention, a nurse recorded baseline vital signs (resting heart rate, blood pressure, oxygen saturation, respiratory rate). A Polar H7 heart rate strap (Polar, Kemple, Finland) was worn measuring second-by-second heart rate (HR), recorded via the cycle ergometer with an ANT+ / 5KHz receiver. HR data was also collected using an iPad (Apple Inc, California) and commercial software (Polar Flow, Kemple, Finland). The ergometer was then fitted to each participant who subsequently undertook a 5-minute warm-up consisting of passive cycling with no resistance. After the warm-up, participants undertook 30-minutes of light intensity cycling. Intensity was calculated based on the Karvonen equation and the target heart rate reserve (HRR) zone was 40 and 60% HRR:
| (1) |
This exercise intensity was chosen based on prior research with TMS and cortisol, which suggests higher intensity exercise interventions abolished the neuromodulatory effects of repetitive TMS, possibly related to exercise-induced increases in cortisol (McDonnell, Buckley, Opie, Ridding, & Semmler, 2013; A. E. Smith et al., 2018). Resistance of the cycle ergometer was adjusted by study researchers to ensure participants reached the exercise intensity zone. Upon completion of the intervention, participants cooled-down for 2-minutes (no resistance cycling), after which, post-intervention vital signs were recorded by the nurse. The control intervention consisted of seated rest for 30 minutes. During this time, participants could interact with study staff, use the mobile phones or read, but were seated and made no whole-body movements during the 30 minutes. HR was also recorded during the rest intervention using the same Polar strap.
2.4. Cognitive tasks.
A battery of three tablet-based executive function tasks was completed before and after each intervention using the Food and Drug Administration (FDA)-approved CANTAB cognitive testing software (Cambridge cognition, Cambridge, UK) using an iPad Pro (Apple Inc, California) (Luca et al., 2003). The CANTAB battery has been shown to be well correlated with traditional pen and paper neuropsychological tests (Smith, Need, Cirulli, Chiba-Falek, & Attix, 2013) and demonstrates moderate to high test-retest reliability (Gonçalves, Pinho, & Simões, 2016; Lowe & Rabbitt, 1998). Participants were given verbal instructions by the CANTAB software as well as practice trials prior to each test. The tasks were identical at each time point. The following tasks were chosen to measure inhibitory control, processing of conflicting information (multitasking) and spatial working memory (Figure 2).
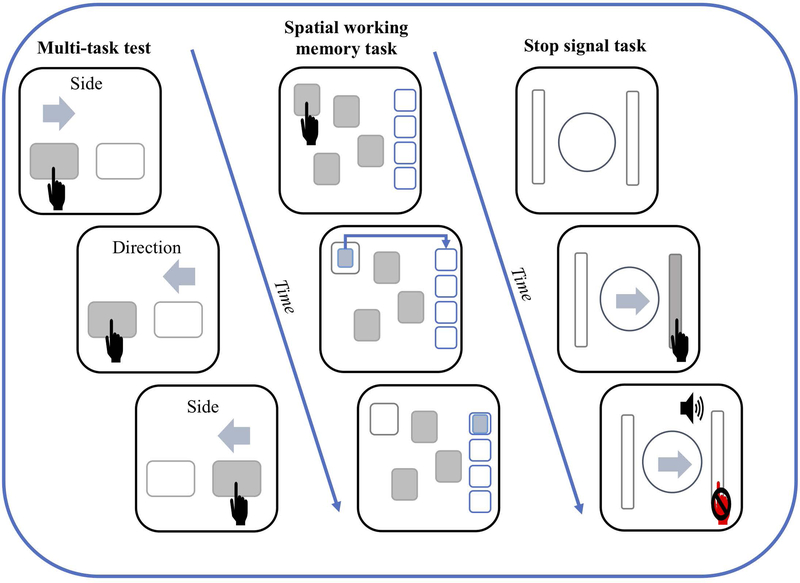
Figure 2.
Illustrations of the tablet-based (Cantab software) executive function tasks. The left column depicts the multi-tasking test with the top and middle boxes showing a congruent trail whereas the bottom box illustrates an incongruent trail. Participants must tap the corresponding virtual button as fast as the can. The middle column illustrates a 4 box spatial working memory task. Participants must tap covered boxes to unveil hidden tokens and place them in the stack on the right once they are found. This task has trials where 4, 6 and 8 boxes are displayed. The far-right column displays the stop signal task where by the participant must press the virtual button corresponding to the direction of the arrow as fast as they can. However, upon hearing a auditory beep, the participant must inhibit their response (as shown in the bottom box).
The multitasking test presented two virtual buttons on either side of the screen and a cue (side, direction) with an arrow above either button (left or right) indicating which button to select. Cues appeared (for the full duration of the trial) in consistent (single task) and in-consistent (multitask) trials and both congruent (arrow on right side pointing right) and incongruent trials (arrow on right side pointing left) were presented. The distribution of the trials was randomly ordered within the following constraints: if multiple trials are presented then 50% must be switch trials, 25% switch trials that are congruent and 25% which are switch trials that are incongruent. Outcome measures consisted of reaction times, errors and multitasking cost (mean latency of single blocks subtracted by mean latency on multitasking blocks).
The inhibitory control task (stop signal task) required participants to respond to an arrow stimulus pointing in a given direction. The first set consisted of 16 trials where the participant practiced the response. In the second set, the participant was told to inhibit their response if they heard an auditory signal (a beep). An adaptive staircase was employed for the stop signal delay allowing the task to adapt to the performance of the participant to narrow in on a 50% success rate. An inter-stimulus interval of 1000ms was applied. The outcome measure was stop signal reaction time, the estimate of when an individual can successfully inhibit their response 50% of the time. This is inferred as the time before all actions become ballistic and the person is no longer able to stop the action.
A spatial working memory task required participants to find tokens hidden behind covered boxes and transfer them to empty boxes on the right-hand side of the screen without re-opening a box that has previously been selected. This task displayed four, six or eight boxes and outcome measures consisted of errors (trials when a participant revisits a box in which a token has been previously found) and strategy. It has been suggested that an efficient strategy to complete this task is to follow a predetermined sequence beginning with a given box and once a token has been found return to the same box to begin the next search (Owen, Sahakian, Semple, Polkey, & Robbins, 1995). Participants were not informed of this strategy. To estimate how well this strategy was utilized, the number of times a subject begins a new search with the same box was calculated. A high score represents poor use of this strategy and a low score, effective use.
2.6. Transcranial magnetic stimulation (TMS) and Electromyography (EMG)
To measure the amplitude of TMS-induced motor evoked potentials (MEPs), surface electrodes were placed in a belly-tendon montage on the right first dorsal interosseus (FDI; target muscle) and the abductor pollicis brevis (APB; reference muscle) with a ground on the ulnar styloid process. Electrodes were connected to a PowerLab 4/25T data acquisition device (ADInstruments, Colorado Springs, CO, USA). EMG data epochs (100 ms pre-trigger to 500 ms post-trigger) were digitized at 1 kHz and amplified with a range of ±10 mV (band-pass filter 0.3–1000 Hz) and peak-to-peak MEP amplitude of the non-rectified signal was calculated on individual waveforms using LabChart 8 software (ADInstruments).
All TMS parameters used in this study conform to the guidelines of the International Federation of Clinical Neurophysiology (Rossi, Hallett, Rossini, Pascual-Leone, & Safety of TMS Consensus Group, 2009). In accordance with these guidelines the following TMS procedures were applied before and after each intervention: The optimal spot for the maximal responses of the right FDI muscle was localized and deemed the “motor hotspot.” Resting motor threshold (rMT) was obtained and used to set the intensity of subsequent TMS. rMT was defined as the lowest stimulation intensity required to evoke MEPs ≥ 50 μV in the relaxed right FDI muscle, in five out of ten trials. TMS was applied to the left primary motor cortex using a passive-cooled handheld MagPro MC-B70 Butterfly Coil (outer diameter: 97 mm) connected to a MagPro X100 stimulator (MagVenture A/S, Farum, Denmark). The coil was placed tangential to each participant’s head with the handle oriented approximately 45° relative to the mid-sagittal axis. A monophasic current flowing anterior-posterior (AP) through the coil center was used to induce a posterior-anterior (PA) current approximately orthogonal to the central sulcus. Consistent targeting of the motor hotspot throughout the experiment was achieved by means of a Polaris infrared optical tracking system (Northern Digital Inc., Waterloo, ON, Canada) and a Brainsight TMS neuronavigation system (Rogue Research Inc., Montreal, QC, Canada) using the Montreal Neurological Institute structural MRI template brain. The head-tracker (headband) was removed between each TMS session and at the beginning of each subsequent session, the motor hotspot and rMT were re-checked. Anatomical landmarks (fiducial points) were used to register the subjects head into the frameless stereotaxic system that allowed accurate targeting of the motor hotspot during each of the TMS sessions. If the RMT changed (see results), the TMS protocols were subsequently applied as percentages of the new RMT value.
After determining the motor hotspot and rMT, interleaved single pulse TMS (spTMS) and ppTMS were applied over the course of three separate blocks. Each block consisted of spTMS (5 trials each at 80% rMT and 120%rMT), 10 trials of SICI (80%-rMT conditioning stimulus, 120%-rMT test stimulus, 3ms interval), 10 trials of ICF (80%-rMT conditioning stimulus, 120% test stimulus, 12ms interval), and 10 trials of LICI (120%-rMT conditioning stimulus, 120%-rMT test stimulus, 100ms interval). The trial order and the inter-trial interval were pseudorandomized to avoid any block effects or train effects, respectively. Unconditioned cortico-motor reactivity was determined by combining trials of spTMS at 120% with the conditioning stimulus of LICI. Conditioned MEPs were averaged across each ppTMS protocols. Like protocols were averaged across the three blocks. Outcome measures consisted of all ppTMS protocols, baseline MEP amplitudes and RMT values.
2.8. Statistical analysis
All statistical analyses were performed using JMP Pro (v 13.0, The SAS Institute Inc., Cary, North Carolina, USA). Following a within-subjects design, data corresponding to cognitive function scores and TMS measures were each entered into separate 2*2 random-effects linear models, with intervention (exercise, rest) and block (pre-intervention, post-intervention) as main factors and an interaction term of intervention*block. TMS measures consisted of rMT (% of maximum stimulator output; %MSO), unconditioned cortical reactivity (spTMS at 120% and the LICI conditioning pulse), and ppTMS measures of SICI, LICI and ICF (% change of conditioned MEP from unconditioned cortical reactivity [TS-CS/CS*100]). As practice effects have been evidenced for the cognitive tasks (Cacciamani et al., 2018), our main hypothesis was that exercise would improve cognitive test scores more so than rest. Accordingly, planned pairwise comparisons using Bonferroni correction to correct for multiple comparisons were used on the executive function outcomes. For the TMS data, post-hoc pairwise comparisons using Bonferroni corrections were used when an intervention by block interaction was found. All p values from the pairwise comparisons are the corrected values. The effect size for each main effect was presented as partial eta squared () for significant effects. As an exploratory step, simple bivariate correlations (Pearson’s R coefficient) were performed on variables highlighted by the linear models to show significant changes across and within interventions. We also performed a power analysis (using STATA version 15.1, StataCorp, USA) on our sample size to ensure sufficient power was gained to detect a difference. Given our sample size (n=14) and an assumed type 1 error rate of 0.05 we calculated an estimated power of 99.8% to detect a true difference of Δ = −1.402 with a standard deviation of 49.7 from a two sample paired means test on one of our main outcome measures (multitasking performance).
3. Results
Fourteen adults (9 female), aged 26 ± 3 years (range of 22 to 30 years) participated. Mean exercise HRR for the exercise condition was 48 ± 5% HRR and was significantly different compared to the rest condition (5 ± 4% HRR).
3.1.1. Executive functions
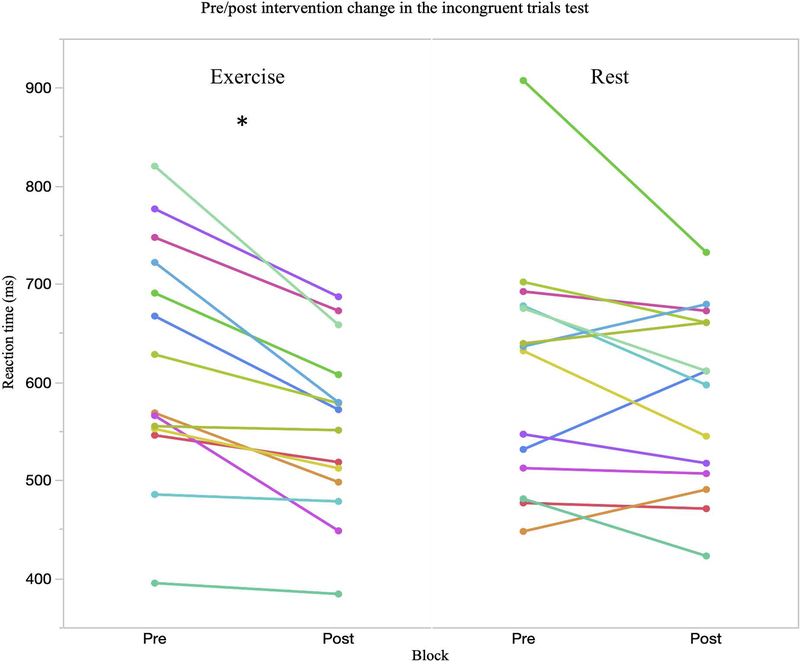
Table 1 presents mean ± SD scores for the executive function tasks at each time point. Random-effects linear models showed significant main effects of block for mean latency reaction times on the multitasking test for all congruent trials (F1,17 = 25.27, p = <.001, = .60), incongruent trials (F1,13 = 23.04, p = <.001, = .64), multitasking trials where both rules (side and direction) were used (F1,13 = 23.73, p = <.001, = .68) as well as the multitasking cost (F1,13 = 9.39, p = .009, = .42). A block*intervention interaction was observed in the incongruent trials, though it did not reach significance (F1,13 = 2.35, p = .095, = .20). Planned comparisons showed significant improvements in the exercise condition (p = .003) but not in the rest condition (p = .338). Further planned pairwise comparisons of the significant effects of block in these outcomes revealed significant pre/post differences in the exercise condition for the congruent (p = .007) and incongruent (p = .019) trials but not in the rest condition (congruent: p = .101; incongruent: p = .338). No change in either condition was seen for the multitasking cost (Figure 3).
Table 1.
Mean and SD scores for executive function tasks in healthy adults
| Task | Pre-exercise | Post-exercise | Δ | P | Pre-rest | Post-rest | Δ | P |
|---|---|---|---|---|---|---|---|---|
| Multitasking test | ||||||||
| Congruent | 555.6 ± 112.7 | 499.3 ± 78 | −47.9 ± 52.9 | .001 | 554.5 ± 102.8 | 518.6 ± 78.8 | −35.9 ± 53.2 | .083 |
| Incongruent | 622.8 ± 119.9 | 553.1 ±125.2 | −69.4 ± 49.7 | <.001 | 611.2 ± 122.4 | 584.6 ± 92.7 | −27.2 ± 65.3 | .324 |
| Multitasking | 690.3 ± 184.4 | 597.2 ±125.2 | −93.1 ± 88.3 | .007 | 682.5 ± 178.2 | 630.2 ± 116.4 | −52.3 ± 98.3 | .204 |
| Cost | 201.7 ± 150.4 | 141.9 ±100.1 | −59.8 ± 97 | .178 | 198.7 ± 139.9 | 157.9 ± 74.5 | −40.8 ± 97.8 | .437 |
| SST | ||||||||
| RT | 206.7 ± 29.7 | 221.6 ± 40 | 14.4 ± 43.4 | .174 | 211.2 ± 42.5 | 219.7 ± 34.8 | 8.5 ± 32.9 | .418 |
| SWM | ||||||||
| BE | 4.4 ± 5.2 | 5.7 ± 6.3 | 0.4 ± 1.6 | .748 | 5.1 ± 5.3 | 6.2 ± 8.1 | 0.5 ± 9.2 | .802 |
| Strategy | 5.3 ± 2.7 | 5.4 ± 3.1 | 0.1 ± 1.6 | .921 | 5.5 ± 2.8 | 5.6 ± 3.2 | 0.1 ± 3.4 | .843 |
P statistic derived from planed comparisons following 2*2 linear models. Values <.05 remained significant after Bonferroni corrections. SST= stop signal task; RT = reaction time; SWM= spatial working memory; BE= between errors.
Figure 3.
Evidence of an improvement in the multitasking test when incongruent trials were presented. At the group level a non-significant block *intervention interaction was observed (p = .095) and planned comparisons showed a significant pre/post change in the exercise condition (p = .003) but not in the rest condition (p = .338). An improvement in incongruent reaction time was seen in every subject after the exercise condition. * indicates significant post hoc change in the exercise condition at the group level.
No significant effects of block or block*intervention interaction were seen in stop signal reaction time (block: F1,13 = 4.01, p = .066, = .02; block*intervention: F1,13 = 0.12, p = .734, = .00), spatial working memory between errors (block: F1,13 = 0.24, p = .632, = .01; block*intervention: F1,13 = 0.02, p = .965, = .00) or strategy (block: F1,13 = 0.08, p = .787, = .01; block*intervention: F1,13 = 0.01, p = .953, = .00).
3.1.2. TMS measures
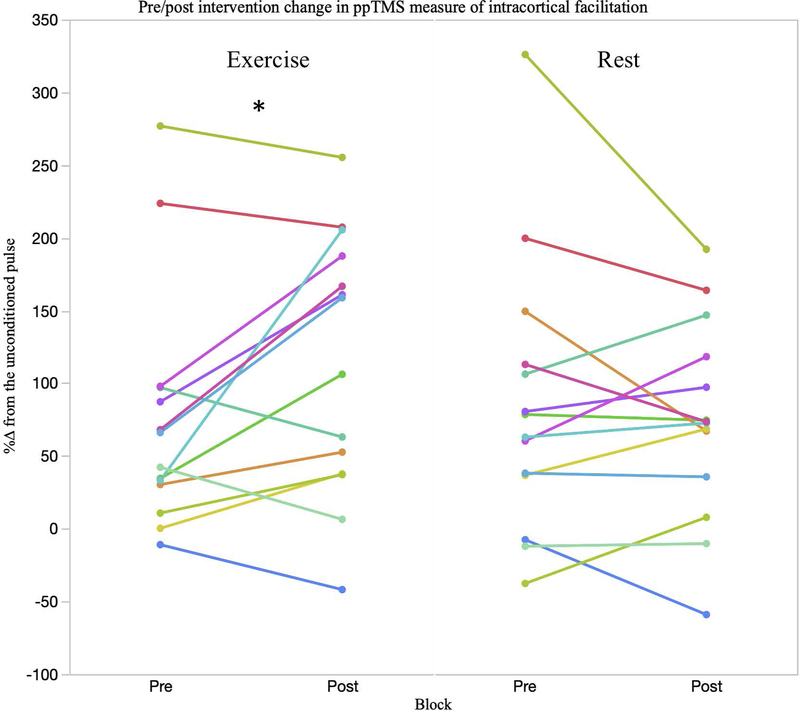
The random-effects linear model revealed a significant main effect of block (F1,13 = 7.29, p = .018 = .36) for rMT. Specifically, there was a change pre-to-post intervention of −1.12 ± .40 %MSO (95% CI’s .22, 1.99) (Table 2). A significant main effect of block (F1,12 = 5.38, p = .040 = .31) and an intervention*block interaction for ICF was found (F1,11 = 7.51, p = .018 = .41). Post hoc comparisons showed a significant increase in ICF pre-to-post exercise (p = .021). No main effects of block were seen for SICI (F1,13 = 2.44, p = .626, = .02), LICI (F1,11 = 1.56, p = .189, = .13) or MEP amplitude (F1,13 = 1.18, p = .885, = .07) (Figure 4).
Table 2.
Mean and SD scores for TMS measures
| Task | Pre-exercise | Post-exercise | Δ | P | Pre-rest | Post-rest | Δ | P |
|---|---|---|---|---|---|---|---|---|
| TMS measures | ||||||||
| rMT | 56 ± 11.5 | 55 ± 11.1 | −1 ± 2.3 | 56.6 ± 11.2 | 55.4 ± 11.2 | 1.2 ± 1.9 | ||
| MEP amplitude (uV) | 856.7 ± 570.3 | 1097.1 ± 630.7 | 191.4 ± 598.4 | 1216.4 ± 710.7 | 1191.5 ± 629.6 | −24.9 ± 438.9 | ||
| ICF | 75.5 ± 82.1 | 114.5 ± 89.2 | 40 ± 63.2 | .021 | 85.3 ± 94.2 | 74.87 ± 68.2 | −10 ± 53.5 | |
| SICI | −34.7 ± 36.6 | −43.9 ± 48.4 | −9.2 ± 60.9 | −38 ± 46.5 | −40.1 ± 31.5 | −2 ± 52.4 | ||
| LICI | −65.1 ± 33.9 | −84.5 ± 28.1 | −19.4 ± 36.5 | −74.8 ± 47.3 | −77.9 ± 19.6 | −2.1 ± 51.5 |
P statistic derived from planed comparisons following 2*2 linear models. Values <.05 remained significant after Bonferroni corrections.
Figure 4.
A significant group level block by intervention interaction (p = .018) in ICF was seen. A significant increase in ICF following exercise (p = .021) was observed. As shown in the exercise condition, 9 out of 14 subjects (~65%) demonstrated an increase %ΔICF whereas in the rest condition only 4 subjects saw larger %ΔICF after rest, with the remaining subjects either not changing or seeing a reduction in %ΔICF. * indicates significant post hoc change in the exercise condition at the group level.
3.1.3. Correlational analyses between significant outcomes and cognitive improvements
Simple linear regression yielded no relationships between %Δ in ICF and %Δ in multitask performance for any multitask outcome (congruent trials: r(12) = −.06, R2 = .004; incongruent trials: r(12) = −.04, R2 = .001 ; multitask trials: r(12) = .09, R2 = .008; multitask cost: r(12) = .21, R2 = .047).
1. Discussion
A vast majority of studies on the effects of acute bouts of exercise have assessed the effects of moderate intensity exercise (Hung, Tsai, Chen, Wang, & Chang, 2013; Lulic et al., 2017; Pontifex, Hillman, Fernhall, Thompson, & Valentini, 2009b; Singh, Duncan, Neva, & Staines, 2014b). The present study found that 30-minutes of light aerobic exercise improved response times on multiple outcomes of a multitasking task in healthy adults. Exercise-mediated increases in cortical excitability (ICF) were also observed.
Meta-analyses on the effect of single bouts of exercise on executive functions show a small but consistent improvement (Chang, Labban, Gapin, & Etnier, 2012). Nevertheless, some studies have failed to show an effect (Wang et al., 2015), suggesting exercise may not have broad widespread effects on all executive function domains. Indeed, our results show exercise enhanced several measures of the multitasking test, yet failed to modulate inhibitory control and spatial working memory. In previous research, moderate intensity aerobic exercise has shown more consistent effects on improving executive functions (Chang et al., 2011; Hung et al., 2013; Pontifex et al., 2009). Our results therefore potentially add to the debate regarding the interactions of intensity of exercise and cognitive improvements. That is, in two previous studies comparing the effects of different exercise intensities (light, moderate and intense) on inhibitory control (Go/No-Go) and response inhibition (Flanker task), light aerobic exercise improved reactions times on Flanker task (Kamijo et al., 2007) but not the Go/No-Go task (Kamijo et al., 2004), comparable to our results. It is conceivable that light aerobic exercise may not be sufficiently intense to induce an adaptive plastic response necessary to improve widespread executive functions.
Changes in cortical excitability are a necessary precursor to sustained changes in synaptic strength underpinned by long-term potentiation and long-term depression (Daoudal & Debanne, 2003). And such increases in cortical excitability may render neuronal pools more susceptible to plasticity induction through targeted rehabilitation strategies, when preceded by a bout of exercise (Cotman et al., 2007; Griesbach, 2011). Recently TMS measures have been studied as a means to assess such exercise-mediated changes in cortical excitability (McDonnell et al., 2013; Mooney et al., 2016; Singh et al., 2014a; A. E. Smith et al., 2014) One key difference in our results from previous research is a lack of significant change in cortical inhibition measures (SICI, LICI) (Mooney et al., 2016; Singh et al., 2014a; A. E. Smith et al., 2014). Previous research has suggested that cortical excitability increases are a product of a reduction in cortical inhibition, creating a more favourable environment for potentiation-like excitability changes (Singh et al., 2014a) and whilst in our study, increases in ICF were seen, comparable to previous studies (Lulic et al., 2017; Singh et al., 2014), these were not associated with a concomitant reduction in cortical inhibition (SICI). The mechanisms of exercise-mediated changes in cortical function are not fully understood however modulation of neurotransmitter function, specifically GABA and glutamate (which mediate SICI and ICF, respectively) are thought to play a key role. An interaction between intensity of exercise and excitability changes is possible and may explain why we did not see changes in SICI however a comparison of different exercise intensities is required to fully answer such a question. In a previous study using continuous theta-burst stimulation to measure LTD-like plasticity, McDonnell and colleagues found that a preceding bout of light aerobic exercise enhanced the LTD-like inhibitory effect of cTBS (McDonnell et al., 2013). However, similar to ICF, research has suggested that the inhibitory after-effects of cTBS are modulated by NMDA receptors (Huang, Chen, Rothwell, & Wen, 2007). Consequently, our results add to the evidence that single sessions of light aerobic exercise can modulate cortical excitability in a facilitative direction. A key question that remains to be answered is whether such changes in cortical excitability are widespread or confined to the motor cortex. Previous research has suggested that exercise may exert a more widespread effect on the brain. For example increases in activation of diverse brain regions has been shown following exercise (Weng et al., 2017). Indeed, exercise can also modulate behavioural measures such as mood, pain and stress (Glass et al., 2004).
In a prior study by Ostadan and colleagues (2016), a correlation between exercise-increased cortico-spinal excitability (as measured by MEP amplitude) and procedural memory consolidation was shown, highlighting how TMS measures may be related to the effect of exercise on cognitive functions. Whilst our results add to previous research showing exercise-mediated increases in ICF (McDonnell et al., 2013; Singh et al., 2014a), the change in ICF was not correlated with the improvements in multitask performance. This finding suggests the effects of exercise on response times during processing of conflicting information and motor cortex excitability were independent. Although the motor cortex is involved in motor planning and execution (Cheney, 1985) and motor cortex excitability (as measured by ICF and SICI) is associated with voluntary movement (Christova et al., 2006; Nikolova, Pondev, Christova, Wolf, & Kossev, 2006), the ability to process conflicting information (incongruent trials and multitask cost) is dependent on higher-order cognitive regions outside of the primary motor area (Banich et al., 2000). Whereby the total response times of such tasks are a function of the sum of the encoding, decision and response output processes of task execution (Ratcliff & McKoon, 2008). Neuroimaging studies show associations between multitask performance and fronto-parietal networks, including regions such as the anterior cingulate cortex, lateral prefrontal cortices, parietal lobule and the anterior insula (Roberts & Hall, 2008). As such, the direct effect of exercise on ICF within the motor cortex may not completely reflect the more global effect exercise may exert on the brain (Weng et al., 2017). Advances in technology that allow real-time integration of TMS with electroencephalography (Farzan et al., 2016; Pascual-Leone et al., 2011; Tremblay et al., 2019) may provide a means to better assess exercise-improved cognitive performance in regions outside of the motor area. Future research characterizing the cognitive and neurophysiological effects of exercise beyond the motor cortex may benefit from this technique.
Our results should be interpreted in light of the relatively small sample of participants with a narrow age range, and so our results may not be generalizable to older populations. Regardless of the sample size however, our power calculations suggest we have sufficient power (95%) to detect an effect. Additionally, the use of the ipad during the cognitive tasks, which engages the intrinsic hand muscles, may have potentially affected the TMS results. A previous study by Classen and colleagues (Classen, Liepert, Wise, Hallett, & Cohen, 1998) showed that practice movements (repetition of a movement of an individual finger in a given direction) led to a temporary shift in the angular direction of TMS-evoked finger movements. Nevertheless, the Classen study did not report changes in excitability (i.e MEP amplitude) and their study was specific to repetitive movements of a single finger at a set frequency, which is distinct to the more-random and temporally inconsistent multi-muscle activity when using an ipad. Consequently, given no change in MEP amplitude or ppTMS measures were seen in the control condition, we believe the likelihood of this is minimal.
2. Conclusions
A greater understanding of the mechanistic underpinnings of exercise’s effect on cognitive performance will lead to the development of optimal exercise interventions for various clinical populations. Multitasking performance is modulated following light aerobic exercise as is motor cortical excitability. Consequently, patients with deficits in this domain, who cannot reach higher exercise intensities due to illness severity, may benefit from bouts of light aerobic exercise.
Acknowledgments
Funding
Dr. A. Pascual-Leone was partly supported by the Sidney R. Baer Jr. Foundation, the NIH (R01MH100186, R01HD069776, R01NS073601, R21 NS082870, R21 MH099196, R21 NS085491, R21 HD07616), the Football Players Health Study at Harvard University, and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). Dr. Santarnecchi is partially supported by the Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA), via 2014–13121700007 by the Beth Israel Deaconess Medical Center (BIDMC) via #60182 the Chief Academic Officer (CAO) grant 2017, and the Defense Advanced Research Projects Agency (DARPA) via HR001117S0030. Dr. Fried was partially supported by the NIH (R21 AG05184601). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences (NCRR and the NCATS NIH, UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the National Institutes of Health, the Sidney R. Baer Jr. Foundation, the ODNI, IARPA, or the U.S. Government
Footnotes
Disclosure of Interest
Dr. A. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Constant Therapy, Cognito, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. Dr. Santarnecchi serves on the scientific advisory boards for EBNeuro.
Data Availability
All data pertaining to this study will be made available upon request to the corresponding author.
References
- Adams R. (1999). Revised Physical Activity Readiness Questionnaire. Canadian Family Physician, 45, 992–1005. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2328306/ [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, … Magin R. (2000). FMRI Studies of Stroop Tasks Reveal Unique Roles of Anterior and Posterior Brain Systems in Attentional Selection. Journal of Cognitive Neuroscience, 12(6), 988–1000. 10.1162/08989290051137521 [DOI] [PubMed] [Google Scholar]
- Cabral DF, Rice J, Morris TP, Rundek T, Pascual-Leone A, & Gomes-Osman J. (2019). Exercise for Brain Health: An Investigation into the Underlying Mechanisms Guided by Dose. Neurotherapeutics. 10.1007/s13311-019-00749-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciamani F, Salvadori N, Eusebi P, Lisetti V, Luchetti E, Calabresi P, & Parnetti L. (2018). Evidence of practice effect in CANTAB spatial working memory test in a cohort of patients with mild cognitive impairment. Applied Neuropsychology. Adult, 25(3), 237–248. 10.1080/23279095.2017.1286346 [DOI] [PubMed] [Google Scholar]
- Chang YK, Labban JD, Gapin JI, & Etnier JL (2012). The effects of acute exercise on cognitive performance: A meta-analysis. Brain Research, 1453, 87–101. 10.1016/j.brainres.2012.02.068 [DOI] [PubMed] [Google Scholar]
- Chang Y-K, Tsai C-L, Hung T-M, So EC, Chen F-T, & Etnier JL (2011). Effects of Acute Exercise on Executive Function: A Study With a Tower of London Task. Journal of Sport and Exercise Psychology, 33(6), 847–865. 10.1123/jsep.33.6.847 [DOI] [PubMed] [Google Scholar]
- Cheney PD (1985). Role of cerebral cortex in voluntary movements. A review. Physical Therapy, 65(5), 624–635. [DOI] [PubMed] [Google Scholar]
- Christova MI, Pondev NG, Christova LG, Wolf W, Dengler R, & Kossev AR (2006). Motor cortex excitability during unilateral muscle activity. Journal of Electromyography and Kinesiology: Official Journal of the International Society of Electrophysiological Kinesiology, 16(5), 477–484. 10.1016/j.jelekin.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, & Cohen LG (1998). Rapid plasticity of human cortical movement representation induced by practice. Journal of Neurophysiology, 79(2), 1117–1123. 10.1152/jn.1998.79.2.1117 [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, & Christie L-A (2007). Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences, 30(9), 464–472. 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Daoudal G, & Debanne D. (2003). Long-Term Plasticity of Intrinsic Excitability: Learning Rules and Mechanisms. Learning & Memory, 10(6), 456–465. 10.1101/lm.64103 [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Greene JL, Gibson CA, Smith BK, Washburn RA, Sullivan DK, … Williams SL (2009). Physical Activity Across the Curriculum (PAAC): A randomized controlled trial to promote physical activity and diminish overweight and obesity in elementary school children. Preventive Medicine, 49(4), 336–341. 10.1016/j.ypmed.2009.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier JL, & Chang Y-K (2009). The Effect of Physical Activity on Executive Function: A Brief Commentary on Definitions, Measurement Issues, and the Current State of the Literature. Journal of Sport and Exercise Psychology, 31(4), 469–483. 10.1123/jsep.31.4.469 [DOI] [PubMed] [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, & Sibley BA (2006). A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Research Reviews, 52(1), 119–130. 10.1016/j.brainresrev.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Farzan F, Vernet M, Shafi MMD, Rotenberg A, Daskalakis ZJ, & Pascual-Leone A. (2016). Characterizing and Modulating Brain Circuitry through Transcranial Magnetic Stimulation Combined with Electroencephalography. Frontiers in Neural Circuits, 10 10.3389/fncir.2016.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco MR, Tong A, Howard K, Sherrington C, Ferreira PH, Pinto RZ, & Ferreira ML (2015). Older people’s perspectives on participation in physical activity: A systematic review and thematic synthesis of qualitative literature. Br J Sports Med, 49(19), 1268–1276. 10.1136/bjsports-2014-094015 [DOI] [PubMed] [Google Scholar]
- Glass JM, Lyden AK, Petzke F, Stein P, Whalen G, Ambrose K, … Clauw DJ (2004). The effect of brief exercise cessation on pain, fatigue, and mood symptom development in healthy, fit individuals. Journal of Psychosomatic Research, 57(4), 391–398. 10.1016/j.jpsychores.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Gomes-Osman J, Cabral DF, Hinchman C, Jannati A, Morris TP, & Pascual-Leone A. (2017). The effects of exercise on cognitive function and brain plasticity—A feasibility trial. Restorative Neurology and Neuroscience, 35(5), 547–556. 10.3233/RNN-170758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Osman J, Cabral DF, Morris TP, McInerney K, Cahalin LP, Rundek T, … Pascual-Leone A. (2018). Exercise for cognitive brain health in aging: A systematic review for an evaluation of dose. Neurology: Clinical Practice, 8(3), 257–265. 10.1212/CPJ.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves MM, Pinho MS, & Simões MR (2016). Test-retest reliability analysis of the Cambridge Neuropsychological Automated Tests for the assessment of dementia in older people living in retirement homes. Applied Neuropsychology. Adult, 23(4), 251–263. 10.1080/23279095.2015.1053889 [DOI] [PubMed] [Google Scholar]
- Griesbach GS (2011). Exercise after traumatic brain injury: Is it a double-edged sword? PM & R: The Journal of Injury, Function, and Rehabilitation, 3(6 Suppl 1), S64–72. 10.1016/j.pmrj.2011.02.008 [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, & Kramer AF (2008). Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews. Neuroscience, 9(1), 58–65. 10.1038/nrn2298 [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Chen R-S, Rothwell JC, & Wen H-Y (2007). The after-effect of human theta burst stimulation is NMDA receptor dependent. Clinical Neurophysiology, 118(5), 1028–1032. 10.1016/j.clinph.2007.01.021 [DOI] [PubMed] [Google Scholar]
- Hung T-M, Tsai C-L, Chen F-T, Wang C-C, & Chang Y-K (2013a). The immediate and sustained effects of acute exercise on planning aspect of executive function. Psychology of Sport and Exercise, 14(5), 728–736. 10.1016/j.psychsport.2013.05.004 [DOI] [Google Scholar]
- Hung T-M, Tsai C-L, Chen F-T, Wang C-C, & Chang Y-K (2013b). The immediate and sustained effects of acute exercise on planning aspect of executive function. Psychology of Sport and Exercise, 14(5), 728–736. 10.1016/j.psychsport.2013.05.004 [DOI] [Google Scholar]
- Kamijo K, Nishihira Y, Hatta A, Kaneda T, Wasaka T, Kida T, & Kuroiwa K. (2004). Differential influences of exercise intensity on information processing in the central nervous system. European Journal of Applied Physiology, 92(3), 305–311. 10.1007/s00421-004-1097-2 [DOI] [PubMed] [Google Scholar]
- Kamijo K, Nishihira Y, Higashiura T, & Kuroiwa K. (2007). The interactive effect of exercise intensity and task difficulty on human cognitive processing. International Journal of Psychophysiology, 65(2), 114–121. 10.1016/j.ijpsycho.2007.04.001 [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, … Marsden CD (1993). Corticocortical inhibition in human motor cortex. The Journal of Physiology, 471, 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C, & Rabbitt P. (1998). Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: Theoretical and practical issues. Cambridge Neuropsychological Test Automated Battery. International Study of Post-Operative Cognitive Dysfunction. Neuropsychologia, 36(9), 915–923. [DOI] [PubMed] [Google Scholar]
- Luca CRD, Wood SJ, Anderson V, Buchanan J-A, Proffitt TM, Mahony K, & Pantelis C. (2003). Normative Data From the Cantab. I: Development of Executive Function Over the Lifespan. Journal of Clinical and Experimental Neuropsychology, 25(2), 242–254. 10.1076/jcen.25.2.242.13639 [DOI] [PubMed] [Google Scholar]
- Lulic T, El-Sayes J, Fassett HJ, & Nelson AJ (2017). Physical activity levels determine exercise-induced changes in brain excitability. PLOS ONE, 12(3), e0173672. 10.1371/journal.pone.0173672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Casazza GA, Fernandez DH, & Maddock MI (2016). Acute Modulation of Cortical Glutamate and GABA Content by Physical Activity. Journal of Neuroscience, 36(8), 2449–2457. 10.1523/JNEUROSCI.3455-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Snow NJ, Campbell KL, Ross CJD, & Boyd LA (2014). A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. Journal of Applied Physiology (Bethesda, Md.: 1985), 117(11), 1325–1336. 10.1152/japplphysiol.00498.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Buckley JD, Opie GM, Ridding MC, & Semmler JG (2013). A single bout of aerobic exercise promotes motor cortical neuroplasticity. Journal of Applied Physiology (Bethesda, Md.: 1985), 114(9), 1174–1182. 10.1152/japplphysiol.01378.2012 [DOI] [PubMed] [Google Scholar]
- Milenkovic S, & Dragovic M. (2013). Modification of the Edinburgh Handedness Inventory: A replication study. Laterality: Asymmetries of Body, Brain and Cognition, 18(3), 340–348. 10.1080/1357650X.2012.683196 [DOI] [PubMed] [Google Scholar]
- Mooney RA, Coxon JP, Cirillo J, Glenny H, Gant N, & Byblow WD (2016). Acute aerobic exercise modulates primary motor cortex inhibition. Experimental Brain Research, 234(12), 3669–3676. 10.1007/s00221-016-4767-5 [DOI] [PubMed] [Google Scholar]
- Nanda B, Balde J, & Manjunatha S. (2013). The Acute Effects of a Single Bout of Moderate-intensity Aerobic Exercise on Cognitive Functions in Healthy Adult Males. Journal of Clinical and Diagnostic Research : JCDR, 7(9), 1883–1885. 10.7860/JCDR/2013/5855.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova M, Pondev N, Christova L, Wolf W, & Kossev AR (2006). Motor cortex excitability changes preceding voluntary muscle activity in simple reaction time task. European Journal of Applied Physiology, 98(2), 212–219. 10.1007/s00421-006-0265-y [DOI] [PubMed] [Google Scholar]
- Ostadan F, Centeno C, Daloze J-F, Frenn M, Lundbye-Jensen J, & Roig M. (2016). Changes in corticospinal excitability during consolidation predict acute exercise-induced off-line gains in procedural memory. Neurobiology of Learning and Memory, 136, 196–203. 10.1016/j.nlm.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, & Robbins TW (1995). Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia, 33(1), 1–24. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, … Rotenberg A. (2011). Characterizing Brain Cortical Plasticity and Network Dynamics Across the Age-Span in Health and Disease with TMS-EEG and TMS-fMRI. Brain Topography, 24(3–4), 302–315. 10.1007/s10548-011-0196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruyero F, Zapata J, Pastor D, & Cervelló E. (2017). The Acute Effects of Exercise Intensity on Inhibitory Cognitive Control in Adolescents. Frontiers in Psychology, 8 10.3389/fpsyg.2017.00921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto SM, Newman MA, & Hirsch MA (2018). Perceived Barriers to Exercise in Adults with Traumatic Brain Injury Vary by Age. Journal of Functional Morphology and Kinesiology, 3(3), 47 10.3390/jfmk3030047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Hillman CH, Fernhall B, Thompson KM, & Valentini TA (2009a). The Effect of Acute Aerobic and Resistance Exercise on Working Memory. Medicine & Science in Sports & Exercise, 41(4), 927–934. 10.1249/MSS.0b013e3181907d69 [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Hillman CH, Fernhall B, Thompson KM, & Valentini TA (2009b). The Effect of Acute Aerobic and Resistance Exercise on Working Memory. Medicine & Science in Sports & Exercise, 41(4), 927–934. 10.1249/MSS.0b013e3181907d69 [DOI] [PubMed] [Google Scholar]
- Ratcliff R, & McKoon G. (2008). The Diffusion Decision Model: Theory and Data for Two-Choice Decision Tasks. Neural Computation, 20(4), 873–922. 10.1162/neco.2008.12-06-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KL, & Hall DA (2008). Examining a Supramodal Network for Conflict Processing: A Systematic Review and Novel Functional Magnetic Resonance Imaging Data for Related Visual and Auditory Stroop Tasks. Journal of Cognitive Neuroscience, 20(6), 1063–1078. 10.1162/jocn.2008.20074 [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, & Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 120(12), 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AM, Duncan RE, Neva JL, & Staines WR (2014a). Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Science, Medicine & Rehabilitation, 6, 23 10.1186/2052-1847-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AM, Duncan RE, Neva JL, & Staines WR (2014b). Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Science, Medicine & Rehabilitation, 6, 23 10.1186/2052-1847-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Goldsworthy MR, Garside T, Wood FM, & Ridding MC (2014). The influence of a single bout of aerobic exercise on short-interval intracortical excitability. Experimental Brain Research, 232(6), 1875–1882. 10.1007/s00221-014-3879-z [DOI] [PubMed] [Google Scholar]
- Smith AE, Goldsworthy MR, Wood FM, Olds TS, Garside T, & Ridding MC (2018). High-intensity Aerobic Exercise Blocks the Facilitation of iTBS-induced Plasticity in the Human Motor Cortex. Neuroscience. 10.1016/j.neuroscience.2017.12.034 [DOI] [PubMed] [Google Scholar]
- Smith PJ, Need AC, Cirulli ET, Chiba-Falek O, & Attix DK (2013). A comparison of the Cambridge Automated Neuropsychological Test Battery (CANTAB) with “traditional” neuropsychological testing instruments. Journal of Clinical and Experimental Neuropsychology, 35(3), 319–328. 10.1080/13803395.2013.771618 [DOI] [PubMed] [Google Scholar]
- Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R, … Daskalakis ZJ (2019). Clinical utility and prospective of TMS-EEG. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 130(5), 802–844. 10.1016/j.clinph.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Tunovic S, Press DZ, & Robertson EM (2014). A Physiological Signal That Prevents Motor Skill Improvements during Consolidation. The Journal of Neuroscience, 34(15), 5302–5310. 10.1523/JNEUROSCI.3497-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, & Hallett M. (1992). Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 85(6), 355–364. 10.1016/0168-5597(92)90048-G [DOI] [PubMed] [Google Scholar]
- Wang C-C, Shih C-H, Pesce C, Song T-F, Hung T-M, & Chang Y-K (2015). Failure to identify an acute exercise effect on executive function assessed by the Wisconsin Card Sorting Test. Journal of Sport and Health Science, 4(1), 64–72. 10.1016/j.jshs.2014.10.003 [DOI] [Google Scholar]
- Weng TB, Pierce GL, Darling WG, Falk D, Magnotta VA, & Voss MW (2017). The Acute Effects of Aerobic Exercise on the Functional Connectivity of Human Brain Networks. Brain Plasticity, 2(2), 171–190. 10.3233/BPL-160039 [DOI] [PMC free article] [PubMed] [Google Scholar]