Abstract
Introduction:
The vast majority of patients who undergo a diagnostic evaluation for microscopic hematuria (MH) do not have occult bladder cancer. Identifying patients with MH at high risk of harboring bladder cancer can allow for a risk adjusted approach to diagnostic interventions with the goal of safely reducing unnecessary evaluations.
Methods:
Patients with a new diagnosis of microhematuria during an 8.5 year period were retrospectively identified. All patients who had a complete MH evaluation were randomized to a training or a validation cohort. Logistic regression analysis was performed in the training cohort to identify factors related to a bladder cancer diagnosis and to develop our model. ROC curves to identify bladder cancer were constructed for the training and validation cohort and tested for their ability to discriminate true cases. A nomogram to predict a bladder cancer diagnosis was created.
Results:
In 4,178 patients split into training and validation cohorts, those diagnosed with bladder cancer were shown to be older, have a greater degree of MH (more RBC/hpf), and were former or current smokers. A nomogram created using this model was able to predict risk of a bladder cancer diagnosis with good discrimination (AUC 0.79, 95% CI 0.75–0.83). A cutoff of 0.01 probability demonstrated a sensitivity of 99.1% and a negative predictive value of 99.7%.
Conclusion:
A nomogram can accurately predict the risk of bladder cancer diagnosed during the evaluation of MH and can potentially be used avoid a significant number of work ups in those at the lowest risk.
Keywords: microhematuria, hematuria, bladder cancer, cystoscopy
INTRODUCTION:
Microhematuria (MH) is a common finding that may be a sign of occult bladder cancer. Practice guidelines suggest urologic consultation and diagnostic evaluation with abdominopelvic imaging and cystoscopy for most adults with hematuria.[1, 2] These recommendations are largely based on expert opinion and low-level evidence.[1, 2] However, the vast majority of patients who undergo evaluation for microscopic hematuria do not have occult bladder cancer.[3, 4] In the absence of risk stratification, evaluating all patients with microhematuria leads to many low-yield, invasive, and costly workups.[5] Identifying those patients with MH at highest risk of bladder cancer can allow for more focused evaluations and may potentially avoid unnecessary diagnostic interventions.
We hypothesized that factors associated with a diagnosis of bladder cancer are identifiable at the time of a microhematuria evaluation and can be used to categorize individual patients’ risk of harboring bladder tumors. Using a risk stratified approach to evaluate patients may help to improve the current MH diagnostic paradigm. Therefore, the goal of this study was to identify objective clinical factors associated with a bladder cancer diagnosis and to use these factors to create a nomogram that accurately predicts risk of bladder cancer at the time of microhematuria evaluation. This nomogram can then be used to better counsel patients about their risk of occult bladder cancer and ideally avoid the pursuit of invasive diagnostic evaluations in those at lowest risk.
METHODS:
Patient cohort
Patients with a new diagnosis of microhematuria during the study period (8-1-2007 to 12-31-15) were included in the analysis. A new diagnosis of MH was defined as 3 or more red blood cells per high powered field (RBC/hpf) on a urinalysis (UA) with microscopy in the absence of an obvious benign cause.[2, 6] Patients were excluded for benign cause if they were pregnant, had a concomitant urinary infection (defined as a positive or equivocal urine culture from the given urine sample), or had pre- or co-existing urologic or medical renal disease as defined by documented ICD9 diagnosis code (Supplement 1). Patients with existing or prior diagnoses of urothelial cancer (bladder, 188.X; upper tract urothelial cancer (UTUC) 189.1–2), kidney cancer (189.X), prostate cancer (185.X), BPH (600.X), or urolithiasis (592.X and 594.X) were also excluded. A “new diagnosis” was contingent on the patient having no prior diagnoses of hematuria (599.7x) and no prior urinalyses positive for hematuria (≥3 RBC/hpf) at any time in the EMR before the study period. Patients with gross hematuria on urinalysis were excluded by urine color (pink or red) or by encounter ICD9 diagnosis code (599.70, 599.71). Patients younger than 35 years of age were excluded in order to reflect the population currently recommended to undergo a urologic evaluation by national specialty guidelines.[2] Only urinalyses collected during outpatient encounters were included to limit risk of instrumentation induced MH.
Variables and outcomes
Baseline demographic data was collected on each patient including age, sex, race/ethnicity, and smoking status. Race was categorized as white non-Hispanic (WNH), white Hispanic (WH), black, Asian, and other/unknown. Smoking status was categorized as never, former, current, or unknown. Urinalysis microhematuria severity was categorized into 5 groups according to RBC/hpf: 0–2 (no hematuria), 3–10, 11–50, 51–100, and >100. These categories were chosen based on the standard reporting intervals of our institution’s central lab.
Evaluation status for each patient was dichotomized as either complete or incomplete. A patient was considered to have a complete evaluation only if both imaging and a cystoscopy were performed within 1 year of initial MH diagnosis. For imaging, any computed tomography (CT), MRI or ultrasound test that could evaluate the GU organs was considered appropriate. Completion of a cystoscopy was determined via CPT/ICD9 procedure code (Supplement 2). Studies and procedures completed outside of our healthcare system were not available. Patients were assessed for a subsequent bladder cancer diagnosis using EMR-documented ICD9 diagnosis coding (Supplement 2).
Statistical Analysis
All patients with a new diagnosis of microhematuria were split equally and randomly into a training set and a validation set in a 1:1 fashion Patients who had completed a full evaluation were then sub-grouped within each set as the “training cohort” and “validation cohort” (Supplemental Figure 3). Patients with missing data were excluded (n=107). Student’s t-test was used to compare age and chi-squared testing was used to compare categorical variables.
A logistic regression analysis was performed on the final training cohort with bladder cancer diagnosis as the dependent variable, and with age, sex, race, urinalysis RBC/hpf and smoking status as independent variables. Given that our outcome of interest is bladder cancer and this is generally diagnosed purely via cystoscopy, a sensitivity analysis was performed for patients with just a cystoscopy rather than a complete evaluation (cystoscopy and imaging).
Receiver operating characteristic (ROC) curves were constructed for the training and validation cohorts based on the model and areas under the curve (AUC) were calculated. A calibration plot was calculated for each cohort to assess how well each calculated probability corresponded to the reality of a bladder cacner diagnosis for each cohort (Supplement Figure 4).
The nomogram was constructed using the coefficients from the training set. An ideal cutoff for the nomogram score was calculated using Youden’s index (sensitivity – [1 – specificity]) from the ROC analysis.[7] A classification table was created for the training and validation curves at various levels of outcome probability which are associated with nomogram scores. All tests were designated to have significance at p<0.05. Statistical analyses were completed using STATA version 13.
Data Source
The Northwestern Medicine Enterprise Data Warehouse (EDW) is a continuously updated repository of over 100 billion data points on more than 8 million unique patients that has been previously used for several clinical research endeavors.[8] The EDW contains systematically extracted clinical data such as vital signs and lab values as well as physician entered information, diagnosis coding, and billing information. There is a rigorous institutional quality assurance protocol in place. The data is sourced from multiple hospitals and clinical sites within the Northwestern Medicine healthcare system. For this data set, approximately 1% of all charts had a confirmatory manual chart review to assure accuracy of the abstracted data. This study was approved by the Northwestern University IRB (Study STU00201732).
RESULTS:
The initial data set was comprised of 52,321 patients found to have a new diagnosis of microscopic hematuria in the absence of a benign cause over the study period. These patients were then randomized in a 1:1 fashion to one of two possible preliminary cohorts. Of all 52,321 patients, 8.0% (n=4,178) underwent a complete evaluation with cystoscopy and imaging. From the preliminary cohorts, only patients who had a complete evaluation and no missing data were subsequently included in the final training (n=2126) or validation (n=2052) cohorts. The two evaluated groups were well matched for patient demographics, microhematuria severity on urinalysis, smoking status, and bladder cancer diagnosis (Table 1). Median follow-up time for all patients was 846 days [IQR 290–1743 days].
TABLE 1:
PATIENT DEMOGRAPHICS AND RISK FACTORS
| TRAINING COHORT (N=2126) | VALIDATION COHORT (N=2052) | ||||
|---|---|---|---|---|---|
| N | MEAN (SEM) | N | MEAN(SEM) | ||
| Age (years) | 2126 | 58.8 (13.0) | 2052 | 59.1 (13.1) | |
| N | % | N | % | ||
| Sex | |||||
| Female | 1050 | 49.4 | 1064 | 51.9 | |
| Male | 1076 | 50.6 | 988 | 48.2 | |
| Race | |||||
| White | 1248 | 58.7 | 1196 | 58.3 | |
| Black | 432 | 20.3 | 441 | 21.5 | |
| Asian | 67 | 3.2 | 82 | 4.0 | |
| Other | 230 | 10.8 | 205 | 10.0 | |
| Unknown | 149 | 7.0 | 128 | 6.2 | |
| Urinalysis (RBC/hpf) | |||||
| 4–10 | 949 | 44.6 | 885 | 43.1 | |
| 11–50 | 587 | 27.6 | 556 | 27.1 | |
| 51–100 | 133 | 6.3 | 142 | 6.9 | |
| >100 | 457 | 21.5 | 469 | 22.9 | |
| Smoking status | |||||
| Never | 707 | 33.3 | 722 | 35.2 | |
| Former | 438 | 20.6 | 415 | 20.2 | |
| Current | 135 | 6.4 | 122 | 6.0 | |
| Unknown | 846 | 39.8 | 793 | 38.7 | |
| Bladder cancer diagnosis | |||||
| No | 2019 | 95.0 | 1968 | 95.9 | |
| Yes | 107 | 5.0 | 84 | 4.1 | |
In the training and validation cohorts of patients, all of whom were completely evaluated, there were 107 (5.03%) and 84 (4.09%) bladder cancer diagnoses, respectively. There were significant demographic and urinalysis differences seen among patients with and without a bladder cancer diagnosis in the training set (Table 2). Patients who were subsequently found to have bladder cancer after a microhematuria diagnosis were older (69.1 vs. 58.2 years old, p<0.0001), more commonly male (68.2% vs. 49.7%, p=0.0002), had more RBC/hpf on urinalysis (p<0.0001), as well as a had a stronger history of smoking (p=0.001).
TABLE 2:
COMPARISON OF DEMOGRAPHICS AND RISK FACTORS BETWEEN PATIENTS WITH AND WITHOUT A BLADDER CANCER DIAGNOSIS IN TRAINING COHORT
| NO BLADDER CANCER DIAGNOSIS (N=2019) | BLADDER CANCER DIAGNOSIS (N=107) | ||||
|---|---|---|---|---|---|
| N | MEAN(SEM) | N | MEAN(SEM) | p-value | |
| Age (years) | 2019 | 58.2 (12.8) | 107 | 69.1 (11.7) | <0.01 |
| N | % | N | % | ||
| Sex | <0.01 | ||||
| Female | 1016 | 50.3 | 34 | 31.8 | |
| Male | 1003 | 49.7 | 73 | 68.2 | |
| Race | 0.04 | ||||
| White | 1172 | 58.1 | 76 | 71.0 | |
| Black | 421 | 20.9 | 11 | 10.3 | |
| Asian | 63 | 3.1 | 4 | 3.7 | |
| Other | 221 | 11.0 | 9 | 8.4 | |
| Unknown | 142 | 7.0 | 7 | 6.5 | |
| Urinalysis (RBC/hpf) | <0.01 | ||||
| 4–10 | 924 | 45.8 | 25 | 23.4 | |
| 11–50 | 564 | 27.9 | 23 | 21.5 | |
| 51–100 | 124 | 6.1 | 9 | 8.4 | |
| >100 | 407 | 20.2 | 50 | 46.7 | |
| Smoking status | <0.01 | ||||
| Never | 688 | 34.1 | 19 | 17.8 | |
| Former | 405 | 20.1 | 33 | 30.8 | |
| Current | 128 | 6.3 | 7 | 6.5 | |
| Unknown | 798 | 39.5 | 48 | 44.9 | |
A logistic regression model with bladder cancer diagnosis as the outcome demonstrated significant associations of age (OR 1.06 for each year, 95% CI 1.04–1.08), increasing severity of microhematuria (RBC/hpf), and smoking status with bladder cancer (Table 3). Sensitivity analysis performed with all patients who completed a cystoscopy (vs. a complete evaluation with cystoscopy AND imaging) demonstrated no significant differences in the model effects.
TABLE 3:
MULTIVARIATE ANALYSIS OF RISK FACTORS FOR BLADDER CANCER DIAGNOSIS IN FINAL TRAINING COHORT
| Risk Factor | Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Age (each year) | 1.06 | 1.04 | 1.08 | <0.01 |
| Sex: male vs female | 1.38 | 0.88 | 2.16 | 0.16 |
| RBC/hpf on UA 11–50 vs 4–10 | 1.37 | 0.76 | 2.47 | 0.29 |
| RBC/hpf on UA 51–100 vs 4–10 | 2.41 | 1.07 | 5.43 | 0.03 |
| RBC/hpf on UA >100 vs 4–10 | 3.28 | 1.93 | 5.57 | <0.01 |
| Smoking status: former vs never | 2.06 | 1.13 | 3.77 | 0.02 |
| Smoking status: current vs never | 2.70 | 1.07 | 6.83 | 0.04 |
| Smoking status: unknown vs never | 2.05 | 1.17 | 3.60 | 0.01 |
| Race: Black vs White | 0.54 | 0.28 | 1.06 | 0.07 |
| Race: Asian vs White | 1.05 | 0.36 | 3.12 | 0.92 |
| Race: Other vs White | 0.70 | 0.34 | 1.46 | 0.34 |
| Race: Unknown vs White | 0.91 | 0.40 | 2.08 | 0.83 |
A receiver operating curve (ROC) was generated for the training cohort and demonstrated good ability to discriminate (AUC 0.79, 95% CI 0.75–0.83) cases of bladder cancer (true positives, true negatives) from incorrectly predicted (false positives, false negatives). An ROC curve for the validation set was then created which also demonstrated good discrimination (AUC 0.74, 95% CI 0.67–0.80). Both ROC curves were plotted together in Figure 1. Calibration plots (supplemental Figure 4) shows that the model adequately predicts the probability of bladder cancer diagnosis for patients associated with a probability of 0.3 or less, after which it appears to plateau. These results indicate that the model works best for a range of probabilities of (0–0.30), which is the vast majority of patients clinically and in our data. Caution should be used when interpreting risk above these levels given the poor calibration past this point.
Figure 1-.
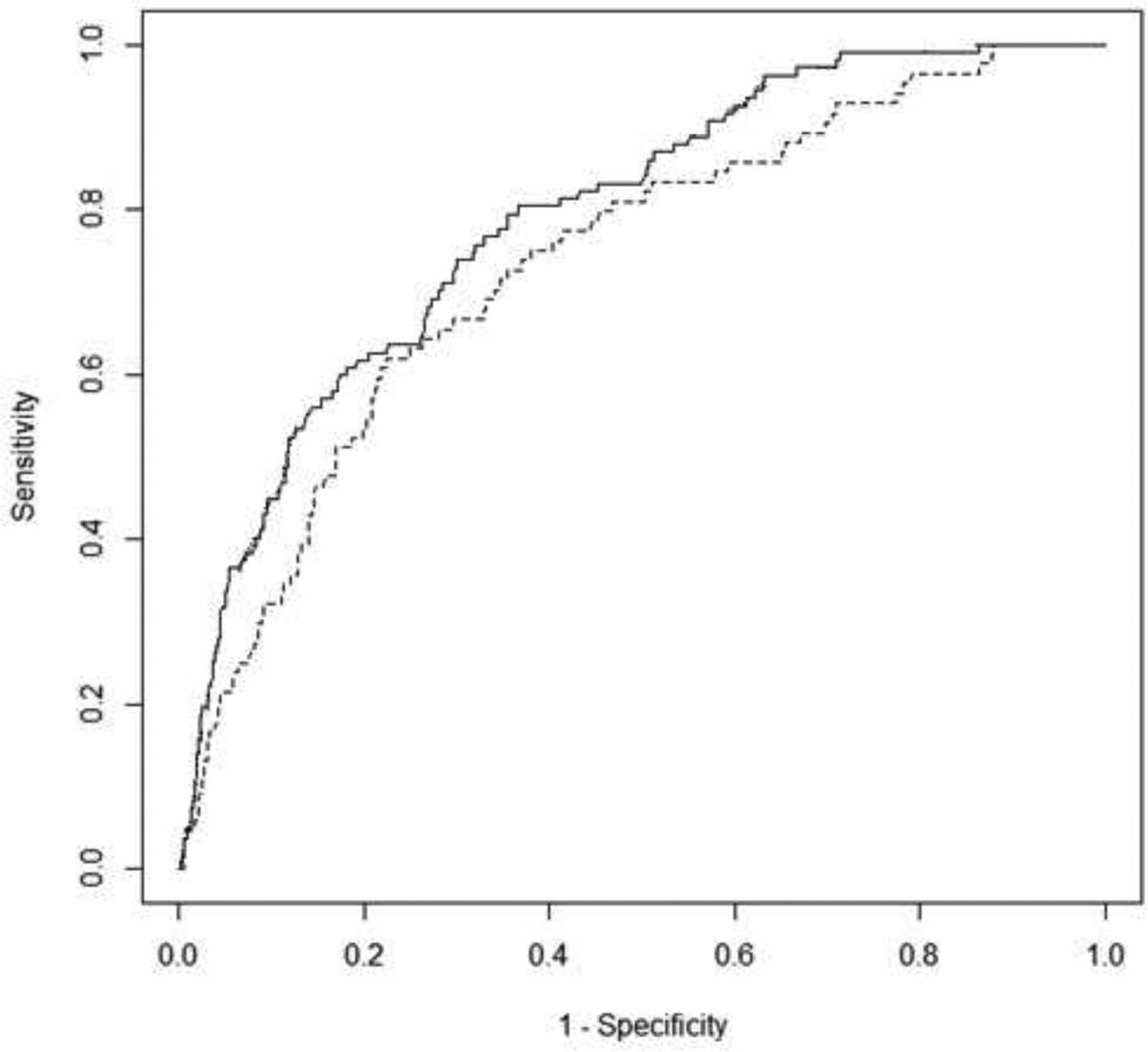
Receiver operating curves (ROC) for training and validation models predicting bladder cancer. Solid line = training cohort (n=2126 patients with 107 malignancies) with an AUC=0.79, 95% CI 0.75–0.83. Dashed line = validation cohort (n=2052 patients with 84 malignancies) with an AUC=0.74, 95% CI 0.67–0.80.
A nomogram using weights to the independent variables based on the regression coefficients was created from the training model (Figure 2). A characteristic table (Table 4) was generated for various levels of bladder cancer probability that can be assigned after calculating the total nomogram score. Using Youden’s index (J=0.418), an ideal cut off was determined to be at the 0.05 probability threshold. However, to maximize negative predictive value (reduce false negatives) a threshold of 0.01 should be chosen. Not evaluating anyone below this threshold would lead to 1 missed cancer while sparing 335 people from an evaluation. Detailed classification tables at each specific cut point associated with each level of the nomogram can be found in the supplement.
Figure 2.
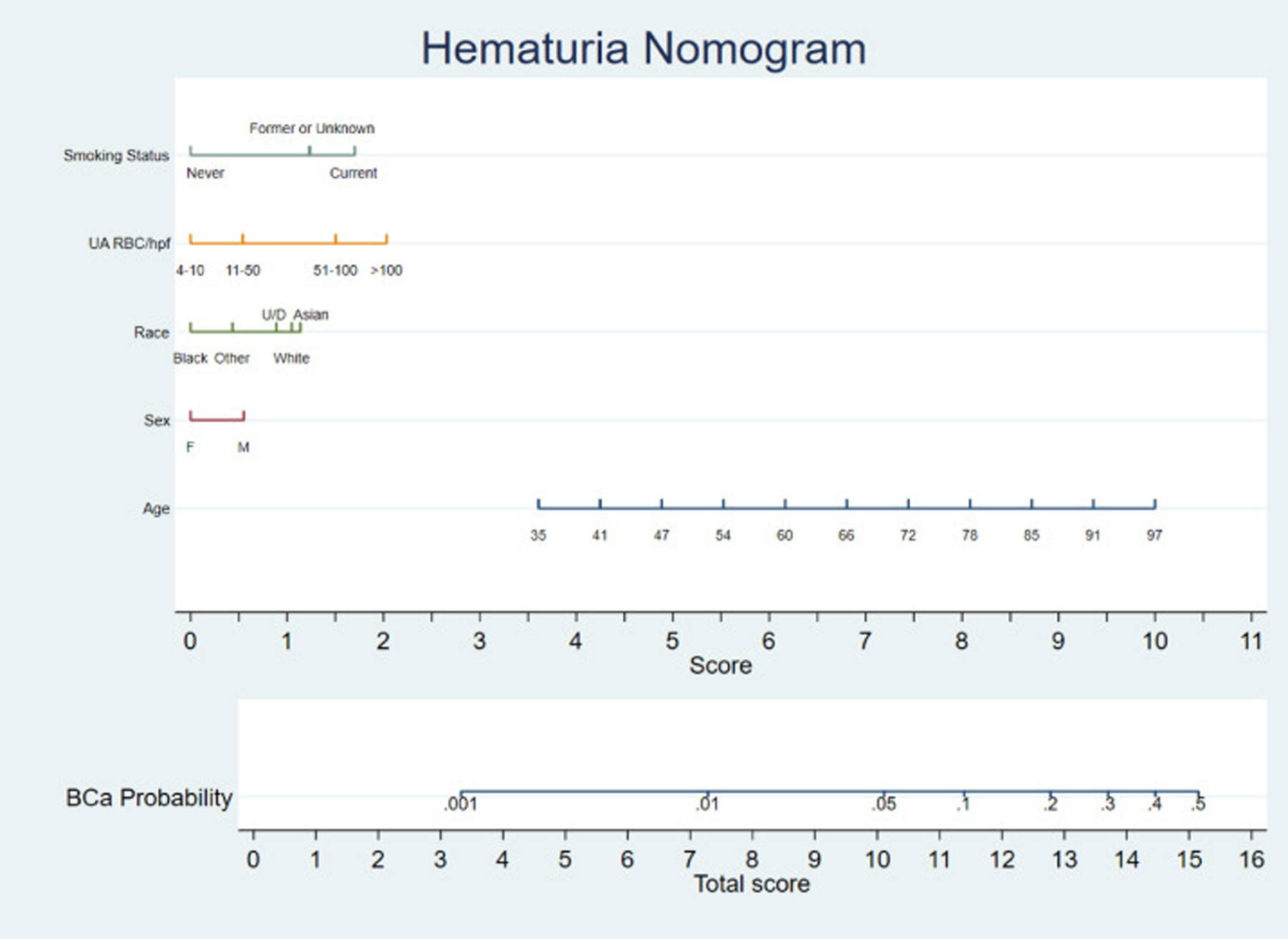
Predictive nomogram. Draw a line perpendicular from the corresponding category of each risk factor until it reaches the bottom line labeled “Score.” Total the number of points across all risk factors and locate this total on the line labelled ‘Total Score’ to calculate the predicted probability of having bladder cancer diagnosed during an evaluation with those risk factors – ‘BCa (Bladder cancer) probability.
Table 4-.
Classification table for training and validation ROC curves and corresponding probabilities from nomogram
| Training Cohort | Validation Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Cut Point | Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV |
| 0.001 | 100.0 | 0.0 | 5.0 | 0.0 | 100.0 | 0.0 | 4.1 | 0.0 |
| 0.01 | 99.1 | 16.5 | 5.9 | 99.7 | 97.6 | 19.9 | 5.0 | 99.5 |
| 0.05* | 70.1 | 71.7 | 11.6 | 97.8 | 67.9 | 75.0 | 10.4 | 98.2 |
| 0.1 | 46.7 | 88.7 | 17.9 | 96.9 | 34.5 | 91.6 | 15.0 | 97.0 |
| 0.2 | 19.6 | 97.1 | 26.3 | 95.8 | 3.6 | 98.9 | 12.5 | 96.0 |
| 0.3 | 4.7 | 99.0 | 20.0 | 95.2 | 1.2 | 99.8 | 16.7 | 95.9 |
| 0.4** | 0.9 | 99.8 | 16.7 | 95.0 | 0.0 | 100.0 | 0.0 | 95.9 |
| 0.5** | 0.0 | 100.0 | 0.0 | 95.0 | 0.0 | 100.0 | 0.0 | 95.9 |
Ideal cutoff per Youden’s J statistic with a value of 0.418.
According to the calibration plots, cutpoints at these thresholds demonstrate poor calibration
Applying the nomogram to a hypothetical 54 year old (~5.5 points) white (~1 point) male (~0.5 points), who is a former smoker (~1.5 points) and has 50–100 RBC/hpf (~1.5 points) on his urine analysis would have a total score of approximately 10+ corresponding to roughly 5–8% chance of finding a bladder cancer during an evaluation.
DISCUSSION:
In this study we developed a nomogram that can help predict, with good discrimination, the risk of being diagnosed with bladder cancer in patients currently indicated to have an evaluation for microscopic hematuria. Using this nomogram, patients can be stratified according to their risk score and be told associated probabilities of having bladder cancer for counseling at the point of care. For patients with a score less than 8, the negative predictive value of this model is 99.7% corresponding to 1/335 missed cancers had these patients not been evaluated. Given the low likelihood of finding cancer in patients of this risk status, avoiding evaluation with invasive cystoscopic evaluations and imaging studies that typically use ionizing radiation and intravenous contrast, would represent a reduction in patient discomfort, morbidity, and cost.
Prior studies have made similar attempts to develop predictive models of patients with hematuria undergoing evaluation. These models demonstrate similar ability to discriminate those who will and will not be diagnosed with urologic cancer to our model.[9, 10] However, the models are limited by several factors, most importantly the inclusion of patients with gross hematuria and some using only dipstick urinalysis.[3, 4, 11] Given that the risk of genitourinary pathology significantly differs between gross and microscopic hematuria, isolating a cohort of patients with only microscopic hematuria is essential to prevent saturation of the cohort with higher risk patients with gross hematuria and therefore bias in the statistical model. There is no debate about the utility of evaluating patients with gross hematuria.[11]
The vast majority of patients undergoing evaluation for MH do not harbor occult pathology. Prior studies have demonstrated incidences of urothelial or renal cell carcinoma at or below 1%.[3, 4] Cystoscopy and imaging studies to evaluate hematuria are not without risk or cost.[12] Accordingly, the pre-test probability of evaluating the thousands of patients each year who present to urologic clinics for the evaluation of microhematuria is therefore exceedingly low and a true opportunity for improvement in value based care.[1] We demonstrate a threshold at which physicians may be able to safely eschew evaluation for bladder cancer.
It is important to address the limitations of this study and therefore the nomogram. The data used for this study were retrospectively abstracted and are subject to the traditional limitations of retrospective data, including concerns about missing data (roughly 40% of smoking status was unknown). We assessed and included a priori, the factors (gender, age, smoking status, etc) currently known to be related to bladder cancer from prior basic science and database studies.[13, 14] However, it is certainly possible that important, but unknown, causative factors have not been included in this analysis and therefore the nomogram. Additionally, other rare but known risk factors such as medications, carcinogenic dye exposure and schistosomiasis infection were not included. Use of anticoagulation has also been demonstrated to have an association with the incidence and evaluation of hematuria and was not included in this analysis.[15]
There were relatively few patients who ultimately completed an evaluation in this cohort. However, this is similar to percentages seen in prior studies. The associated limitations of this, most importantly selection bias or enrichment of high risk patients, has been previously discussed by our group and others.[16–18] Additionally, the gold standard of diagnosing bladder cancer is through cystoscopy and not by imaging studies. We only included patients with a complete evaluation (cystoscopy and imaging) in the training and validation cohorts in order to isolate the group of patients most likely to have been genuinely and completely assessed by a urologist. This strict criteria excluded some patients who underwent cystoscopy and no imaging study. Sensitivity analyses with and without this select group of patients demonstrated no significant difference in the multivariable model.
The comorbidity diagnoses as well as the diagnosis of bladder cancer were made using EMR ICD-9 coding and lack granularity on potentially important pathologic differences such as grade and stage of bladder cancer. It is possible that many of the bladder cancers diagnosed were of low stage and clinical significance. However, early detection of bladder cancer before the development of gross hematuria is associated with improved outcomes.[19] Several other urologic conditions are also related to microhematuria and would necessitate evaluation as well. This is especially important to consider in the realm of voiding dysfunction, as bladder cancer and voiding dysfunction can often mimic each other’s symptoms in presentation. Of note, in this study, the symptoms associated with microhematuria diagnosis were not captured. Symptomatology may have a role in who is referred for and who completes an evaluation. This represents an opportunity for continued work developing additional risk assessment tools for disease processes like kidney cancer, stone disease, and prostate issues.
Finally, the nomogram requires further validation to assure that it is translatable to other populations. Additionally, at the higher levels of bladder cancer nomogram probability (>0.3), due to small analytic sample size, there is poor calibration of true risk and these numbers should be interpreted and used with caution. External validation and the introduction of this nomogram at the point of care are the next logic steps in development. For microscopic hematuria evaluations, a more patient centered, shared decision making approach similar to that recommended for prostate cancer screening is sorely needed. Tools like this nomogram and other risk stratification models can help patients better understand the risks and benefits of pursuing or not pursuing evaluation.
CONCLUSION:
There are demographic, objective clinical and laboratory factors associated with an independently increased risk of occult bladder cancer in patients with microhematuria. The use of a nomogram built upon these factors may allow for risk stratification of patients to undergo invasive and costly evaluations with the potential to avoid a significant number of work ups in those at the lowest risk. External validation and continued evolution of risk stratification models are needed.
Supplementary Material
Highlights:
Patient age, degree of hematuria (RBC/hpf) on urinalysis, and smoking history are independent predictors of a bladder cancer diagnosis in a cohort of patients with microscopic hematuria
A nomogram can accurately discriminate between patients with microscopic hematuria who will and will not be diagnosed during an evaluation for bladder cancer
Patients at lowest risk of a bladder cancer diagnosis according to this nomogram may be able to safely avoid cystoscopy and genitourinary imaging
Acknowledgements:
This work is supported in part by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422 and the American Association of Medical Colleges (AAMC) Clinical Care Innovation Pilot award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Richard Matulewicz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Irene B Helenowski, PhD for her assistance with the data analysis and interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We disclose no financial or conflicts of interest.
REFERENCES:
- [1].Nielsen M, Qaseem A, High Value Care Task Force of the American College of P. Hematuria as a Marker of Occult Urinary Tract Cancer: Advice for High-Value Care From the American College of Physicians Annals of internal medicine. 2016. [DOI] [PubMed] [Google Scholar]
- [2].Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. The Journal of urology. 2012;188:2473–81. [DOI] [PubMed] [Google Scholar]
- [3].Jung H, Gleason JM, Loo RK, Patel HS, Slezak JM, Jacobsen SJ. Association of hematuria on microscopic urinalysis and risk of urinary tract cancer. The Journal of urology. 2011;185:1698–703. [DOI] [PubMed] [Google Scholar]
- [4].Loo RK, Lieberman SF, Slezak JM, Landa HM, Mariani AJ, Nicolaisen G, et al. Stratifying risk of urinary tract malignant tumors in patients with asymptomatic microscopic hematuria. Mayo Clinic proceedings. 2013;88:129–38. [DOI] [PubMed] [Google Scholar]
- [5].Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al. Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation. Health technology assessment. 2006;10:iii-iv, xi-259. [DOI] [PubMed] [Google Scholar]
- [6].Lambert M, American Urological A. AUA guideline addresses diagnosis, evaluation, and follow-up of asymptomatic microhematuria. American family physician. 2013;87:649, 53. [PubMed] [Google Scholar]
- [7].Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- [8].Starren JB, Winter AQ, Lloyd-Jones DM. Enabling a Learning Health System through a Unified Enterprise Data Warehouse: The Experience of the Northwestern University Clinical and Translational Sciences (NUCATS) Institute. Clin Transl Sci. 2015;8:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tan WS, Ahmad A, Feber A, Mostafid H, Cresswell J, Fankhauser CD, et al. Development and validation of a haematuria cancer risk score to identify patients at risk of harbouring cancer. J Intern Med. 2019;285:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cha EK, Tirsar LA, Schwentner C, Hennenlotter J, Christos PJ, Stenzl A, et al. Accurate risk assessment of patients with asymptomatic hematuria for the presence of bladder cancer. World journal of urology. 2012;30:847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tan WS, Feber A, Sarpong R, Khetrapal P, Rodney S, Jalil R, et al. Who Should Be Investigated for Haematuria? Results of a Contemporary Prospective Observational Study of 3556 Patients. European urology. 2018;74:10–4. [DOI] [PubMed] [Google Scholar]
- [12].Halpern JA, Chughtai B, Ghomrawi H. Cost-effectiveness of Common Diagnostic Approaches for Evaluation of Asymptomatic Microscopic Hematuria. JAMA internal medicine. 2017;177:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Janisch F, Shariat SF, Schernhammer E, Rink M, Fajkovic H. The interaction of gender and smoking on bladder cancer risks. Current opinion in urology. 2019;29:249–55. [DOI] [PubMed] [Google Scholar]
- [14].Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. European urology. 2013;63:234–41. [DOI] [PubMed] [Google Scholar]
- [15].Wallis CJD, Juvet T, Lee Y, Matta R, Herschorn S, Kodama R, et al. Association Between Use of Antithrombotic Medication and Hematuria-Related Complications. JAMA: the journal of the American Medical Association. 2017;318:1260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matulewicz RS, Demzik AL, DeLancey JO, Popescu O, Makarov DV, Meeks JJ. Disparities in the diagnostic evaluation of microhematuriaand implications for the detection of urologic malignancy. Urologic oncology. 2019. [DOI] [PubMed] [Google Scholar]
- [17].Nieder AM, Lotan Y, Nuss GR, Langston JP, Vyas S, Manoharan M, et al. Are patients with hematuria appropriately referred to Urology? A multi-institutional questionnaire based survey. Urologic oncology. 2010;28:500–3. [DOI] [PubMed] [Google Scholar]
- [18].Shinagare AB, Silverman SG, Gershanik EF, Chang SL, Khorasani R. Evaluating Hematuria: Impact of Guideline Adherence on Urologic Cancer Diagnosis. The American journal of medicine. 2014. [DOI] [PubMed] [Google Scholar]
- [19].Ramirez D, Gupta A, Canter D, Harrow B, Dobbs RW, Kucherov V, et al. Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU international. 2016;117:783–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


