Abstract
Decades of research into the biological mechanisms of PTSD suggests that chronic activation of the stress response leads to long-lasting changes in the structure and function of the nervous and endocrine systems. While the prevalence of PTSD is twice as high in females as males, little is known about how sex differences in neuroendocrine systems may contribute to PTSD. In response to the paucity of research on sex-related mechanisms, the National Institutes of Health created a policy that asks researchers to consider sex as a biological variable (SABV). This review provides an overview of the current understanding of nervous and endocrine dysfunction in PTSD (e.g., neural circuitry, autonomic arousal, hormonal response), highlighting areas where the influence of sex has been characterized and where further research is needed. We also provide recommendations for using the SABV policy to address specific gaps in PTSD neuroscience research.
Keywords: PTSD, imaging, physiology, endocrine, sex, SABV
1. Introduction
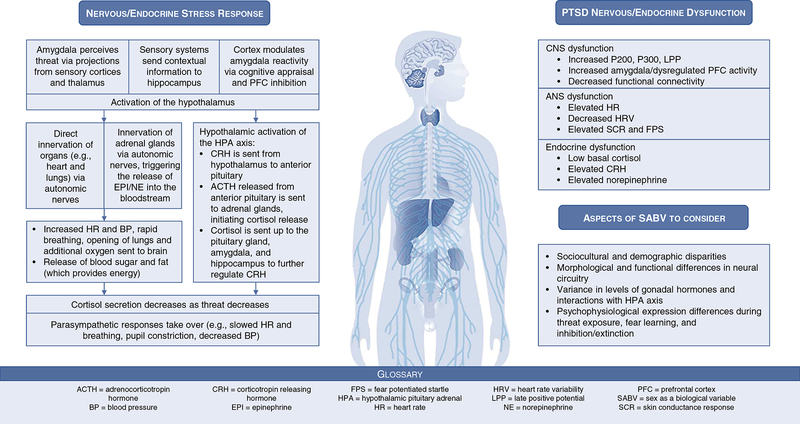
Upon perceiving threat, a complex interplay of nervous and endocrine system activity commences, ultimately leading to a cascade of hormone release and physiological changes that prepare an organism to respond (i.e., the stress response). In the case of posttraumatic stress disorder (PTSD), individuals experience these reactions long after the immediate threat has passed, leading to long-lasting changes in the structure and function of the nervous and endocrine systems (1–5). While epidemiological research consistently finds that PTSD is about twice as prevalent in females compared to males (6), the field is still nascent in its understanding of how these alterations differ among females. In response to the paucity of this research across disciplines, the National Institutes of Health have issued an institute-wide policy that asks researchers to consider sex as a biological variable (SABV). Application of the SABV policy may include sex-based research designs, analyses, and/or reporting in animal and human studies. This review provides an overview of the current understanding of nervous and endocrine dysfunction in PTSD (e.g., neural circuitry, autonomic arousal, hormonal response; Figure 1), highlighting areas where sex-based factors have been characterized and where further research is needed (Figure 2). We also provide recommendations for using the SABV policy to address specific gaps in PTSD neuroscience research.
Figure 1:
Nervous and endocrine stress response and dysfunction in PTSD
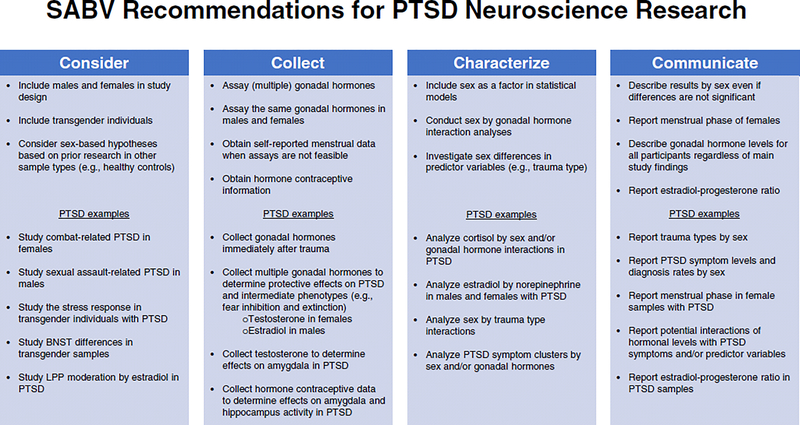
Figure 2:
SABV recommendations for PTSD neuroscience research
2. Central nervous system dysfunction
2a. Functional correlates with neuroimaging
The neural circuitry that mediates defensive responses to threat has been heavily implicated in PTSD (7–11). Prior research has noted increased amygdala and decreased ventromedial prefrontal cortex (vmPFC) activity and decreased functional connectivity among males and females with PTSD compared to trauma- and non-trauma-exposed controls (7, 10, 12–15). These patterns suggest that PTSD is associated with decreased regulation of defensive responses, leading them to be over-expressed. Further, reduced hippocampal activation and dysconnectivity within both males and females has been associated with PTSD during retrieval, such that individuals with PTSD fail to recall when a situation is effectively safe compared to trauma-exposed controls (3, 16). Recent work has also implicated the locus coeruleus (a brain stem region important for norepinephrine production) in PTSD, as it may play a role in hyperarousal (17, 18). Together, this research suggests that the neural circuitry involved in defensive behavior is more easily engaged and not sufficiently inhibited in individuals with PTSD (indicative of exaggerated fear responding in safe situations; see Figure 1).
Few studies have considered the impact of SABV on these phenomena. A recent meta-analysis reported that in response to emotional visual cues, females show greater amygdala, hippocampus, and locus coeruleus activity, but reduced mPFC and anterior cingulate cortex (ACC) activity, compared to males (19). In PTSD, females have demonstrated heightened locus coeruleus activation to masked fearful faces (20) and decreased dorsal ACC activation during extinction recall (21) compared to males. Prior work suggests the structure of the locus coeruleus is sexually dimorphic and has downstream functional differences due to interactions with sex hormones, such that estrogen increases norepinephrine production and decreases its degradation rate (22). These findings may suggest that PTSD in females involves greater activation of brainstem arousal systems, specifically the locus coeruleus, that has downstream effects on threat-related brain circuitry.
2b. Structural correlates with neuroimaging
Individuals with PTSD show altered structural morphology of brain regions important for emotional processes. Reduced hippocampal volume was initially observed in combat-exposed males with PTSD (23), and males and females with childhood abuse-related PTSD compared to controls (24), and this finding has been replicated across mixed-sex and mixed-trauma meta-analyses (25–27). In addition, gray matter density in the dorsomedial PFC has been shown to be reduced in those with PTSD in conjunction with reduced thickness of the vmPFC, both in combat-exposed males (28, 29), mixed-trauma samples (30), and meta-analyses (25). Alterations in white matter pathways interconnecting brain regions critical for fear learning have also been implicated in PTSD. Prior work has demonstrated reduced fractional anisotropy in females (31, 32) and combat-exposed males with PTSD (33) within the cingulum bundle, a white matter tract that connects the hippocampus and cingulate cortex, compared to trauma-exposed controls. Further, reduced microstructure of the uncinate fasciculus (34–36), a white matter tract that connects the mPFC to amygdala and other temporal lobe structures, has been found in males and females with PTSD compared to trauma-exposed controls. The white matter microstructure of this tract has been found to be predictive of posttraumatic stress (37) and posttraumatic anhedonia (38) severity in recently traumatized individuals. Further, microstructural differences in other white matter tracts, such as the anterior corona radiata and inferior longitudinal fasciculus have also been observed in PTSD (33, 36). Taken together, this suggests that PTSD is related to alterations in the structural morphology of key aspects of fear circuitry.
Although prior research has found sex differences in the structural morphology of the brain (39, 40), limited PTSD research to date has investigated SABV in brain structure. Research in children with PTSD has found that boys show greater insular volume compared to girls (41), as well as smaller brain volumes overall (42). Few prior studies have reported on white matter microstructure differences in PTSD, and one recent investigation of uncinate fasciculus and cingulum microstructure did not find sex differences when comparing individuals with PTSD to trauma-exposed controls (35). These data may suggest that white matter microstructure does not differ between sexes in PTSD, but with such limited data it is difficult to draw firm conclusions.
2c. Electrophysiological correlates of brain activity
To date, most event-related potential (ERP)-based electroencephalography (EEG) research in PTSD has focused on the P300 and late positive potential (LPP), which are implicated in emotional salience. Individuals with PTSD demonstrate increased P300 and LPP amplitudes for unpleasant and trauma-related stimuli, although prior reviews identified inconsistencies such that a minority of studies reported opposing or null findings (43, 44). Studies in healthy females have demonstrated that LPP amplitudes were greater among those in the follicular phase of the menstrual cycle compared to those in the luteal phase (45), and that greater LPP amplitudes were significantly associated with decreased progesterone levels among women in the early follicular phase (46). Thus, the estradiol-to-progesterone ratio may be an important factor influencing how menstrual phase confers differential emotional responses (47).
EEG findings regarding resting asymmetry and power spectrum analyses are scarce and inconsistent (48, 49). There is a general trend for increased trait asymmetry in males and females with PTSD compared to trauma-exposed controls (50) and this may be associated with worse cluster D symptoms among males (negative alterations in cognition and mood; 51). Given that cluster D symptoms closely resemble those of depression, this may suggest that resting asymmetry is not a specific indicator of PTSD. State-dependent asymmetry findings have shown that PTSD symptoms are associated with greater activation of right frontal regions in response to trauma-related stimuli among males and females (52, 53). While no studies have examined sex and asymmetry in PTSD, two studies demonstrated that decreased left frontal activation was associated with depression among females but not males (54, 55). High-density EEG studies have explored functional connectivity in PTSD but findings require replication. While one study reported decreased connectivity in the beta and gamma bands among those with PTSD (56), another found that PTSD was associated with increased alpha connectivity (57). Decreased network strength in delta, theta, and beta bands has also been reported (58, 59). While high-density EEG is a promising tool for neuroscience research in PTSD, the influence of SABV on EEG-based connectivity measures remains unknown.
3. Autonomic nervous system dysfunction
3a. Heart rate and heart rate variability
Individuals with PTSD demonstrate stress response deficits across sympathetic and parasympathetic systems, indicative of increased arousal and decreased arousal regulation, respectively (see Figure 1). One of the most-frequently reported indices of increased arousal in PTSD is elevated heart rate (HR) at rest and in response to negative stimuli (60–64). Elevated HR in the peritraumatic period has also been associated with an increased probability of later developing PTSD among males and females (65). Although most studies suggest that HR is elevated among those with PTSD, this may depend on one’s level of cardiac vagal control, which refers to the influence of the vagus nerve on the heart’s sinoatrial node and is associated with emotion regulation and psychological health (66, 67). Heart rate variability (HRV) is a common measure of vagal control and represents the variability in time between heartbeats. Among individuals with PTSD, elevated resting HR may only be present among those with low HRV, suggesting that high HRV is a protective factor against the negative effects of elevated HR (66). Other studies in male, female, and mixed samples have demonstrated that HRV tends to be lower at rest and during challenge or provocation among those with PTSD compared to trauma-exposed controls, providing further evidence of decreased vagal control in PTSD (68–73).
While a recent meta-analysis found that females generally demonstrate greater HRV reactivity than males (74), few studies have examined sex in the relationship between PTSD and HR or HRV. Among male and female assault survivors, females who demonstrated increased HR in response to script-driven imagery had worse PTSD symptoms compared to males and compared to females who didn’t have an increased HR response (75). These females were also three times more likely to have a PTSD diagnosis six months later (75). A study of motor vehicle accident survivors reported no difference in HR between males and females at the emergency department, and although the authors found sex differences in PTSD at six-months, they did not test differences in HR because of the null findings at baseline (76). Among a sample of highly traumatized individuals, HRV was elevated among males with PTSD compared to those without PTSD (contrary to prior HRV findings in PTSD), but the effect was not observed in females (77).
3b. Skin conductance and startle response
Another aspect of sympathetic activation that has received significant attention is skin conductance response (SCR), which reflects sweat glad activity caused by arousal. SCR appears to be stronger at rest and in response to aversive stimuli among those with PTSD compared to controls (78–80). In fear conditioning studies, males and females with PTSD exhibit greater SCRs to conditioned stimuli paired with aversive unconditioned stimuli during both fear acquisition and extinction phases compared to trauma- and non-trauma-exposed controls (81–83). Skin conductance during trauma recall in the immediate aftermath of trauma has recently been shown to predict future PTSD development among a sample of both sexes (84). In terms of SABV, one study found that females demonstrated stronger fear acquisition indexed by SCR compared to males (85). Among healthy females, higher estradiol has been associated with decreased SCR during extinction recall and SCR levels in this group were comparable to males (86). In a female trauma-exposed sample, those with PTSD in the mid-luteal phase demonstrated worse extinction retention, while those without PTSD demonstrated better extinction retention in this phase (where estradiol and progesterone are both high; 87). Among those with PTSD, higher progesterone levels were associated with worse extinction retention, and subsequent research suggested that deficient synthesis of the progesterone metabolites, allopregnanolone (ALLO) and pregnanolone, may be a mechanistic explanation for this finding (88). A recent follow-up study reported similar findings in males, such that ALLO levels were negatively related to PTSD symptoms (89). However, different precursors were implicated in deficient ALLO synthesis compared to those found in females, suggesting that there may be sex-specific differences in ALLO synthesis that are problematic in PTSD (89).
The acoustic startle response is a defensive reflex typically measured by the magnitude of an individual’s eye blink in response to a loud noise probe. Thus, it is highly relevant to fearful and defensive responses and it is another biomarker of PTSD commonly indexed with fear conditioning paradigms. Fear potentiated startle (FPS) is the relative increase in startle when a noise probe is paired with a conditioned stimulus (e.g., a light or colored shape). FPS is generally higher among males and females with PTSD compared to trauma- and non-trauma-exposed controls, both in response to fearful and safe conditioned stimuli (demonstrating exaggerated fear responding and poor fear inhibition, respectively; 62, 1, 2, 90, 91). Individuals with PTSD exhibit increases in fear load (a term for sum total of threat responding) and deficits in fear extinction, as evidenced by high FPS to stimuli that are no longer paired with aversive unconditioned stimuli (92–94). Among females with PTSD, effects appear to be partially driven by estradiol, such that those with low estradiol levels demonstrate worse fear inhibition and extinction (indexed by FPS) compared to those with high estradiol levels (95, 96).
4. Endocrine system dysfunction
Given the prominent role of glucocorticoids and catecholamines in the stress response (see Figure 1), early endocrine research in PTSD investigated cortisol and catecholamines as potential underlying mechanisms. Although PTSD is associated with heightened stress responses, individuals with PTSD paradoxically show lower levels of basal cortisol compared to trauma-exposed and non-trauma-exposed controls in male and female samples (97, 98). This may be the product of hypersensitive glucocorticoid receptors within the HPA axis that result in a hyperresponsive negative feedback mechanism, leading to lower baseline cortisol (4, 5, 99). Glucocorticoid receptor sensitivity has been assessed using a low-dose dexamethasone suppression test, conducted across a number of samples with varying levels of trauma exposure. Though replicated in a wide range of PTSD populations, there is a high degree of variability and this endocrine finding is not consistent (5, 100). Some argue that this variation may be accounted for by factors such as sex, age at trauma exposure, and cortisol collection timing (98, 101). While several of these studies included both males and females, none investigated sex as a biological variable. Catecholamines exhibit a more expected pattern of response. A review and meta-analysis illuminated the significance of chronically elevated norepinephrine levels in males and females with PTSD compared with trauma-exposed and non-trauma-exposed controls (102). This is arguably the disorder’s most consistent endocrinological feature.
Another key substrate of the stress response is corticotropin-releasing hormone (CRH), which is released in several places throughout the brain including the amygdala and the hypothalamus, where it leads to ACTH release from the pituitary, evoking cortisol secretion by the adrenal cortex (103). Compared with trauma-exposed and non-trauma-exposed controls, males and females with PTSD demonstrate chronic hypersecretion of CRH (5, 104), which appears congruous with the finding of chronically lowered cortisol (i.e., excessive CRH release causes a downregulation of pituitary CRH receptors, decreasing the ACTH response and subsequent cortisol release; 105). However, this finding is paradoxical in nature, as the more conventional model of stress response dysfunction suggests that hypersecretion of CRH leads to hyper- rather than hypocortisolism, as seen in major depression (5). Of note, CRH’s function as a neurotransmitter in amygdala, hippocampal, and cortical CRH cerebral processing may be separately regulated from HPA-cortisol neuroendocrine regulation, which may partially account for this discrepancy (106).
The inconsistent profile of endocrinological features in PTSD challenges our understanding of its pathophysiology. We must consider the possibility that such variation and apparent contradiction may be accounted for by external factors (e.g., biological sex). While there is no evidence to suggest a global difference in gonadal hormone levels between individuals with and without PTSD, clinical reports suggest that these hormones may act as moderators of the stress response (107, 108) – the dysregulation of which has been implicated in PTSD (4). Understanding the nature of this relationship may help explain variation in PTSD prevalence rates not only between the sexes, but also within them. A thorough understanding of the effects of regular hormonal fluctuations (e.g., menstrual phase) on PTSD symptomatology within sexes is necessary to properly contrast them.
Although previous studies have identified sex differences in the healthy stress response (e.g., HPA response is higher in the luteal vs. follicular phase), there is a dearth of literature on sex differences in the dysregulated stress response in PTSD. One meta-analysis found cortisol levels to be significantly lower in females with PTSD compared with female non-traumatized controls, whereas this effect was absent in males (109). Others have observed a protective effect of testosterone, such that males with higher serum levels of testosterone are less likely to have or develop PTSD (110, 111). Although suggestive of a modulatory effect of gonadal hormones on the stress response, there is insufficient data to draw a valid conclusion about the exact nature or extent of this relationship. This paucity of data calls for a deeper look into the biological sex-driven nuances of PTSD endocrinology.
Research on HPA axis function and dysregulated stress responding in PTSD has led to an increase in studies of altered immune function (112–115). While the immune system is not central to this review, it is worth noting this emerging research on inflammation in PTSD. Trauma and PTSD have repeatedly been associated with increased inflammatory activity indicated by elevated levels of several inflammatory biomarkers, such as C-reactive protein (CRP), interleukin (IL)-1β, IL-6, interferon-γ (IFNγ), and tumor necrosis factor (TNF)-α (115–118). While several studies included female samples (119–122), only one tested sex differences and found that both males and females with PTSD demonstrated higher IL-6 and TNF-α levels at the beginning but not the end of their sleep cycles (123). Males with PTSD exhibited peak TNF-α levels at the end of the sleep cycle while those without PTSD exhibited an inverted U-shaped profile, but this difference was not observed among females. Additional research on inflammation may elucidate other mechanisms of the stress response that confer greater risk for PTSD and it will be critical to test how these are influenced by sex-based factors.
5. Sex as a Biological Variable (SABV)
A thorough understanding of the influence of sex on nervous and endocrine dysfunction in PTSD is necessary to generate complete neurobiological models of the disorder. However, there is a dearth of literature addressing this important topic in PTSD research. The NIH’s SABV policy highlights this need, encouraging researchers in all scientific arenas to address sex in some manner, and it provides four strategies for doing so: 1) Consider (sex built into study design), 2) Collect (sex-based data), 3) Characterize (analyze data by sex), and 4) Communicate (report/publish data by sex). Informed by Clayton’s initial guide (124), we will identify ways in which the SABV policy can be applied to neuroscience research in PTSD using these four strategies across the nervous and endocrine systems described above (Figure 2).
5a. SABV: Consider
Consideration of sex in PTSD research should ideally begin with study design. The SABV policy explicitly states that researchers are not required to double sample sizes in order to do this, recognizing that it is often not feasible due to logistical constraints and issues of power. However, where possible, neuroscience investigations of PTSD should include both sexes and consider experiential differences in trauma. For example, studies with Veterans have primarily included males and focused on combat trauma, but a growing number of female Veterans are entering the population and they are more likely to experience military sexual trauma (125). Similarly, most studies of sexual assault victims include only females and there is virtually no data on how neuroendocrine functions differ among males with sexual assault. These disparities make it difficult to establish generalizable markers of PTSD within trauma-types and limit our understanding of the biological underpinnings of sex differences in PTSD prevalence rates.
PTSD prevalence rates are 3–8 times higher in transgender individuals compared to the general U.S population (126), but they are tremendously underrepresented in research and it is unclear what differences may exist in their overall stress response. For example, transgender males may demonstrate a stress response akin to cis-gender females given their female sex at birth, but gender affirming hormone therapy would likely affect the response. Two recent studies implicated the X chromosome in PTSD (127, 128). Alterations in a glucocorticoid transcription factor encoded on the X chromosome were associated with PTSD risk such that greater trauma exposure was associated with higher mRNA levels and lower methylation in males but not females (127). In another study, X chromosome genes appeared to contribute to PTSD sex differences such that those which had escaped inactivation were implicated in PTSD risk among females but not males (128). These effects have not yet been tested in transgender individuals, and as evidenced by the sex-specific findings, it will be important to characterize sex chromosome effects on individuals who have undergone hormone replacement therapy to disentangle genetic versus neuroendocrine influences on sex differences in PTSD.
Regardless of whether all sexes are included, study designs that consider sex may generate sex-based hypotheses. In PTSD research, this is particularly appropriate when sex differences have been found in a biological component within a different population. For example, the LPP appears to differ among healthy females depending on menstrual cycle phase (45, 46). A study that only includes females with PTSD could therefore test the hypothesis that those with low circulating estradiol will exhibit greater LPP amplitudes compared to those with high estradiol, and future research could then test whether estradiol supplementation alters the LPP response or therapeutic outcomes for PTSD.
5b. SABV: Collect
Accounting for SABV may also include the collection of sex-based data, such as gonadal hormones (Figure 2). When trauma exposure is more recent, researchers can attempt to estimate hormone levels not only at the time of the study visit, but at the time of trauma. For example, one study found that women in the luteal phase at the time of trauma experienced more flashbacks compared to those in other phases (129). However, phase was determined by self-reported days since menstruation. Given that gonadal hormones fluctuate significantly within menstrual phases, this demonstrates how critical it is to measure actual circulating hormone levels with blood/plasma or salivary assay. Asking females to report the first day of their last menstrual period allows for some estimation of hormone levels, but reliance on this self-report should only be done when it is not feasible to collect biological samples. These kinds of analyses may help to elucidate how sex hormones contribute to initial fear learning (i.e., at the time of trauma) in addition to subsequent nervous and endocrine dysfunction.
Among studies that do collect biological assays, there is a tendency to assess only one hormone and/or one sex, such as testosterone in males or estradiol in females. This suggests that gonadal hormones are mutually exclusive, but in fact, they exist at varying levels in both males and females. Given the potential protective effects of high testosterone in males (110, 111), it would be interesting to investigate PTSD prevalence rates in females with higher circulating levels of testosterone (e.g., those with PCOS, congenital adrenal hyperplasia) to determine if they experience a comparable benefit. Prior work in healthy individuals has found that testosterone is associated with increased amygdala activity, although these findings are mixed in females (130–133), and that its administration to healthy females reduces SCR and FPS (134, 135). Similarly, estradiol is typically considered protective among healthy females and those with PTSD, such that higher levels appear to confer better fear inhibition, extinction, and extinction recall (86, 95, 96). However, there are no studies of how estradiol levels impact fear learning among males with PTSD. Overall, this makes it difficult to understand if gonadal hormones confer similar benefits for all sexes. The aforementioned research on ALLO in both females and males with PTSD highlights this important point and demonstrates the strength of collecting hormone data in both sexes (88, 89). Specifically, researchers assessed ALLO levels and ALLO synthesis in both sexes and reported similar findings in terms of the overall association between ALLO and PTSD, but differences in ALLO synthesis that were sex-specific (88, 89). These studies are critical to elucidating underlying mechanisms of sex-based hormonal contributions to PTSD.
Information should also be collected about hormonal contraceptives given that they affect hormone levels and are associated with variability in neural function. Hormone contraceptive use has been linked to reduced amygdala activity to negative stimuli (136) and hippocampal activity during fear learning (137), and emergency contraceptive use following sexual assault has been associated with reduced PTSD symptoms in women (138). However, no research has tested the effects of regular hormone contraceptive use on amygdala or hippocampus activity among females with PTSD, which is particularly important given that amygdala and hippocampal dysfunction have been routinely observed in PTSD. Thus, collection of hormone levels (or estimation of menstrual phase when assay is not feasible) and information about hormone contraceptive use (e.g., type, dose) would help to disentangle influences of sex on PTSD development and recovery.
Age of trauma exposure is a key variable that influences PTSD risk given that the stress response varies across developmental stages (139–141). For example, glucocorticoid responsivity to stress is high during puberty, making this a critical period for trauma and stress response reactivity and PTSD development (142). There are also important sex differences in the stress response across the lifespan. For example, differences in brain regions and neuroendocrine hormones involved in the stress response confer exaggerated HPA-axis responses to stress in females compared to males, and hormonal changes in females (e.g., due to menopause) cause this to vary with age (139, 140). Collecting information about trauma exposure age (in addition to current age) is therefore critical to disentangling the sex by age interaction and capturing how it affects PTSD neuroendocrine systems.
5c. SABV: Characterize
A recent meta-analysis demonstrated that combat trauma was more associated with differences in the hippocampus and ventromedial PFC, and sexual trauma was more associated with differences in dorsomedial PFC activation (143). Combat and sexual trauma are experienced disproportionately by males and females, respectively. However, as limited work has attempted to characterize sex differences in PTSD, it is unclear if these differences are trauma-type specific, influenced by sex, or a combination of the two. Characterization of these potential sex-specific influences is critical to the development of generalizable biomarkers of PTSD and effective treatment approaches.
First, sex should be considered as a factor in statistical models. When sex is not an initial factor of interest, a supplementary model should be completed that includes sex to characterize potential sex-effects. For example, findings related to cortisol in PTSD populations are often inconsistent (5). In addition to careful collection timing, researchers are encouraged to analyze data by sex and/or gonadal hormone levels to determine if some of the inconsistencies are explained by sex-based factors. While a recent meta-analysis suggested that elevated norepinephrine levels in PTSD are a more consistent finding (102), it is still unknown whether these elevated levels differ between males and females based on gonadal hormone levels. Since estradiol may increase norepinephrine and thus physiological arousal in females (22), it is important to conduct estradiol by norepinephrine analyses in females (and males) with PTSD to determine how these factors influence one another. This is particularly relevant to PTSD research because high estradiol is often associated with decreased arousal (e.g., lower SCR and FPS), and high norepinephrine is associated with increased arousal (i.e., the presence of both may cause greater arousal than the presence of high estradiol alone).
5d. SABV: Communicate
A crucial component of the SABV policy is to communicate sex-related findings to the scientific community. While some PTSD research has only included one sex, other studies included both sexes but did not provide sex-specific details on variables. Although many studies are not designed to test the role of sex in PTSD, including some information may provide preliminary insight into sex-related mechanisms suitable for guiding future research. Therefore, researchers should report main effects of sex on primary dependent variables where possible (e.g., symptom levels, neural activity, endocrine assays, physiological response; see Figure 2).
Researchers should also report the menstrual phase and/or hormonal levels of participants where possible. Although female participants are typically asked about their menstrual cycle and/or given pregnancy tests in the context of neuroscience research, few studies describe this information. Reporting hormonal information would inform potential hormonal effects on study findings that may be critical to developing additional research on sex-effects in PTSD. For example, progesterone levels fluctuate during the menstrual cycle and have been implicated in PTSD, but studies that analyze both estradiol and progesterone have not reported the relative ratio between the two (though it can be calculated if levels are reported). Reporting this ratio would allow PTSD researchers to compare findings on this potentially important variable as it may be more influential than the levels of estradiol and progesterone alone. Better communication of these factors will improve the ability of researchers to utilize and replicate one another’s findings as they relate to SABV in PTSD.
6. Clinical implications
Perhaps the greatest promise of neuroendocrine biomarkers is their utility in predicting PTSD risk, symptom trajectory, and treatment outcome (144, 145). While some are difficult to translate directly into clinical practice given the size and cost of the methodologies involved (e.g., MRI), others can be readily integrated (e.g., mobile SCR assessment with eSense; 146). This has the potential to bring precision medicine into the clinician’s office by offering the ability to predict which patients respond best to particular treatment approaches. Recent findings suggest that these biomarkers are already showing promise as indicators of exposure-based treatment response (147–149), but additional research is needed to understand how they might predict response to different treatments. Further, consideration of SABV will be critical in predicting outcomes based on sex and identifying sex-specific neuroendocrine predictors of treatment response.
7. Conclusions
Although prior research has identified markers of nervous and endocrine dysfunction in PTSD, limited work to date has investigated SABV in these processes. Given the higher prevalence of PTSD in females compared to males, and the discordance in trauma-types experienced between the sexes, significantly more research is needed to unravel sex-related disruption in these intermediate phenotypes. Crucial to these efforts are the recommendations of the SABV policy. While not an exhaustive list, we hope that the aforementioned recommendations will encourage and assist researchers in considering SABV when conducting neuroscience research in PTSD.
Acknowledgments
KJR supported by NIH (R21MH112956, P50MH115874, R01MH094757 and R01MH106595) the Frazier Foundation Grant for Mood and Anxiety Research and Partners Healthcare Biobank. NGH supported by NIH 8K00MH119603-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
KJR has received consulting income from Alkermes, research support from Genomind and Brainsway, and he is on scientific advisory boards for Janssen, Verily, and Resilience Therapeutics, all of which is unrelated to the present work. AVS, NGH, and JBM have no biomedical financial interests or potential conflicts of interest.
References
- 1.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ (2010): Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 27: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jovanovic T, Kazama A, Bachevalier J, Davis M (2012): Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 62: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. (2009): Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherin JE, Nemeroff CB (2011): Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. 13: 263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yehuda R (2006): Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann N Y Acad Sci. 1071: 137–166. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995): Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 52: 1048–60. [DOI] [PubMed] [Google Scholar]
- 7.Hayes JP, Hayes SM, Mikedis AM (2012): Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, et al. (1996): A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 53: 380–387. [DOI] [PubMed] [Google Scholar]
- 9.Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, et al. (2009): Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 66: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ (2013): Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. 47: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenster RJ, Lebois LAM, Ressler KJ, Suh J (2018): Brain circuit dysfunction in posttraumatic stress disorder: from mouse to man. Nat Rev Neurosci. 19: 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etkin A, Wager TD (2007): Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel R, Spreng RN, Shin LM, Girard TA (2012): Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 36: 2130–2142. [DOI] [PubMed] [Google Scholar]
- 14.Sartory G, Cwik J, Knuppertz H, Schürholt B, Lebens M, Seitz RJ, Schulze R (2013): In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD). PLoS One. 8: e58150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Helpman L, Papini S, Schneier F, Markowitz JC, Van Meter PE, et al. (2017): Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress Anxiety. 34: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazarov A, Zhu X, Suarez-Jimenez B, Rutherford BR, Neria Y (2017): Resting-state functional connectivity of anterior and posterior hippocampus in posttraumatic stress disorder. J Psychiatr Res. 94: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morey RA, Dunsmoor JE, Haswell CC, Brown VM, Vora A, Weiner J, et al. (2015): Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl Psychiatry. 5: e700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naegeli C, Zeffiro T, Piccirelli M, Jaillard A, Weilenmann A, Hassanpour K, et al. (2018): Locus Coeruleus Activity Mediates Hyperresponsiveness in Posttraumatic Stress Disorder. Biol Psychiatry. 83: 254–262. [DOI] [PubMed] [Google Scholar]
- 19.Filkowski MM, Olsen RM, Duda B, Wanger TJ, Sabatinelli D (2017): Sex differences in emotional perception: Meta analysis of divergent activation. Neuroimage. 147: 925–933. [DOI] [PubMed] [Google Scholar]
- 20.Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R (2010): Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. J Abnorm Psychol. 119: 241–247. [DOI] [PubMed] [Google Scholar]
- 21.Shvil E, Sullivan GM, Schafer S, Markowitz JC, Campeas M, Wager TD, et al. (2014): Sex differences in extinction recall in posttraumatic stress disorder: a pilot fMRI study. Neurobiol Learn Mem. 113: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bangasser DA, Wiersielis KR, Khantsis S (2016): Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 1641: 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. (1995): MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 152: 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. (1997): Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry. 41: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bromis K, Calem M, Reinders AATS, Williams SCR, Kempton MJ (2018): Meta-Analysis of 89 Structural MRI Studies in Posttraumatic Stress Disorder and Comparison With Major Depressive Disorder. Am J Psychiatry. 175: 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. (2018): Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 83: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith ME (2005): Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 15: 798–807. [DOI] [PubMed] [Google Scholar]
- 28.Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S (2006): Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 59: 582–587. [DOI] [PubMed] [Google Scholar]
- 29.Wrocklage KM, Averill LA, Cobb Scott J, Averill CL, Schweinsburg B, Trejo M, et al. (2017): Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol. 27: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bing X, Ming-Guo Q, Ye Z, Jing-Na Z, Min L, Han C, et al. (2013): Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with posttraumatic stress disorder. Brain Res. 1490: 225–232. [DOI] [PubMed] [Google Scholar]
- 31.Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, et al. (2015): Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex. 64: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fani N, King TZ, Shin J, Srivastava A, Brewster RC, Jovanovic T, et al. (2016): Structure and functional connectivity in posttraumatic stress disorder: associations with FKBP5. Depress Anxiety. 33: 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A (2013): Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res. 214: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu H, Zhou Y, Wang Q, Su S, Qiu Y, Ge J, et al. (2016): Association of abnormal white matter integrity in the acute phase of motor vehicle accidents with post-traumatic stress disorder. J Affect Disord. 190: 714–722. [DOI] [PubMed] [Google Scholar]
- 35.Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2017): Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: a diffusion tensor imaging study. J Psychiatry Neurosci. 42: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson EA, Cui J, Fukunaga R, Nickerson LD, Rauch SL, Rosso IM (2017): Disruption of white matter structural integrity and connectivity in posttraumatic stress disorder: A TBSS and tractography study. Depress Anxiety. 34: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harnett NG, Ference EW 3rd, Knight AJ, Knight DC (2018): White matter microstructure varies with post-traumatic stress severity following medical trauma. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fani N, Michopoulos V, van Rooij SJH, Clendinen C, Hardy RA, Jovanovic T, et al. (2019): Structural connectivity and risk for anhedonia after trauma: A prospective study and replication. J Psychiatr Res. 116: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joel D, Berman Z, Tavor I, Wexler N, Gaber O, Stein Y, et al. (2015): Sex beyond the genitalia: The human brain mosaic. Proc Natl Acad Sci USA. 112: 15468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, et al. (2014): Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA. 111: 823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klabunde M, Weems CF, Raman M, Carrion VG (2017): The moderating effects of sex on insula subdivision structure in youth with posttraumatic stress symptoms. Depress Anxiety. 34: 51–58. [DOI] [PubMed] [Google Scholar]
- 42.De Bellis MD, Keshavan MS (2003): Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neurosci Biobehav Rev. 27: 103–17. [DOI] [PubMed] [Google Scholar]
- 43.Javanbakht A, Liberzon I, Amirsadri A, Gjini K, Boutros NN (2011): Event-related potential studies of post-traumatic stress disorder: a critical review and synthesis. Biol Mood Anxiety Disord. 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lobo I, Portugal LC, Figueira I, Volchan E, David I, Garcia Pereira M, de Oliveira L (2015): EEG correlates of the severity of posttraumatic stress symptoms: A systematic review of the dimensional PTSD literature. J Affect Disord. 183: 210–220. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Zhou R, Wang Q, Zhao Y, Liu Y (2015): Progesterone mediates the late positive potentials evoked by affective pictures in high neuroticism females. Psychoneuroendocrinology. 59: 49–58. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Zhou R, Ye M (2013): Menstrual cycle modulation of the late positive potential evoked by emotional faces. Percept Mot Skills. 116: 707–723. [DOI] [PubMed] [Google Scholar]
- 47.Maeng LY, Milad MR (2015): Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 76: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shankman SA, Silverstein SM, Williams LM, Hopkinson PJ, Kemp AH, Felmingham KL, et al. (2008): Resting electroencephalogram asymmetry and posttraumatic stress disorder. J Trauma Stress. 21: 190–198. [DOI] [PubMed] [Google Scholar]
- 49.Newson JJ, Thiagarajan TC (2019): EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front Hum Neurosci. 12: 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer T, Smeets T, Giesbrecht T, Quaedflieg CWEM, Smulders FTY, Meijer EH, Merckelbach HLGJ (2015): The role of frontal EEG asymmetry in post-traumatic stress disorder. Biol Psychol. 108: 62–77. [DOI] [PubMed] [Google Scholar]
- 51.Moran JK, Crombach A, Elbert T, Nandi C, Bambonyé M, Wienbruch C, et al. (2017): The individual contribution of DSM 5 symptom clusters of PTSD, life events, and childhood adversity to frontal oscillatory brain asymmetry in a large sample of active combatants. Biol Psychol. 129: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabe S, Beauducel A, Zöllner T, Maercker A, Karl A (2006): Regional brain electrical activity in posttraumatic stress disorder after motor vehicle accident. J Abnorm Psychol. 115: 687–698. [DOI] [PubMed] [Google Scholar]
- 53.Rabe S, Zoellner T, Beauducel A, Maercker A, Karl A (2008): Changes in brain electrical activity after cognitive behavioral therapy for posttraumatic stress disorder in patients injured in motor vehicle accidents. Psychosom Med. 70: 13–19. [DOI] [PubMed] [Google Scholar]
- 54.Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB (2010): Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J Abnorm Psychol. 119: 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart JL, Allen JJB (2018): Resting frontal brain asymmetry is linked to future depressive symptoms in women. Biol Psychol. 136: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SH, Yoon S, Kim JI, Jin SH, Chung CK (2014): Functional connectivity of resting state EEG and symptom severity in patients with post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 51: 51–57. [DOI] [PubMed] [Google Scholar]
- 57.Imperatori C, Farina B, Quintiliani MI, Onofri A, Castelli Gattinara P, Lepore M, et al. (2014): Aberrant EEG functional connectivity and EEG power spectra in resting state posttraumatic stress disorder: a sLORETA study. Biol Psychol. 102: 10–17. [DOI] [PubMed] [Google Scholar]
- 58.Shim M, Im CH, Lee SH (2017): Disrupted cortical brain network in post-traumatic stress disorder patients: a resting-state electroencephalographic study. Transl Psychiatry. 7: e1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toll RT, Wu W, Naparstek S, Narayan M, Patenaude B, De Los Angeles C, et al. (in press). An electroencephalography connectomic profile of post-traumatic stress disorder. American Journal of Psychiatry. [DOI] [PubMed] [Google Scholar]
- 60.Buckley TC, Kaloupek DG (2001): A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. 63: 585–594. [DOI] [PubMed] [Google Scholar]
- 61.Ehlers A, Suendermann O, Boellinghaus I, Vossbeck-Elsebusch A, Gamer M, Briddon E, et al. (2010): Heart rate responses to standardized trauma-related pictures in acute posttraumatic stress disorder. Int J Psychophysiol. 78: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozarić-Kovacić D (2009): Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. Int J Psychophysiol. 71: 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, et al. (1998): Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: Results from a Department of Veterans Affairs Cooperative Study. J Consult Clin Psychol. 66: 914–23. [DOI] [PubMed] [Google Scholar]
- 64.Orr SP, Pitman RK, Lasko NB, Herz LR (1993): Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J Abnorm Psychol. 102: 152–9. [DOI] [PubMed] [Google Scholar]
- 65.Shalev AY, Sahar T, Freedman S, Peri T, Glick N, Brandes D, et al. (1998): A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Arch Gen Psychiatry. 55: 553–559. [DOI] [PubMed] [Google Scholar]
- 66.Hopper JW, Spinazzola J, Simpson WB, van der Kolk BA (2006): Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. J Psychosom Res. 60: 83–90. [DOI] [PubMed] [Google Scholar]
- 67.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH (2009): Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 37: 141–153. [DOI] [PubMed] [Google Scholar]
- 68.Chang HA, Chang CC, Tzeng NS, Kuo TB, Lu RB, Huang SY (2013): Decreased cardiac vagal control in drug-naïve patients with posttraumatic stress disorder. Psychiatry Investig. 10:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y (1997): Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol Psychiatry. 41: 627–9. [DOI] [PubMed] [Google Scholar]
- 70.Hauschildt M, Peters MJ, Moritz S, Jelinek L (2011): Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biol Psychol. 88: 215–22. [DOI] [PubMed] [Google Scholar]
- 71.Keary TA, Hughes JW, Palmieri PA (2009): Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. Int J Psychophysiol. 73: 257–264. [DOI] [PubMed] [Google Scholar]
- 72.Minassian A, Geyer MA, Baker DG, Nievergelt CM, O’Connor DT, Risbrough VB et al. (2014): Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom Med. 76: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB (2015): Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiatry. 72: 979–86. [DOI] [PubMed] [Google Scholar]
- 74.Beauchaine TP, Bell Z, Knapton E, McDonough-Caplan H, Shader T, Zisner A (2019): Respiratory sinus arrhythmia reactivity across empirically based structural dimensions of psychopathology: A meta-analysis. Psychophysiology. 56: e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kleim B, Wilhelm FH, Glucksman E, Ehlers A (2010): Sex differences in heart rate responses to script-driven imagery soon after trauma and risk of posttraumatic stress disorder. Psychosom Med. 72: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irish LA, Fischer B, Fallon W, Spoonster E, Sledjeski EM, Delahanty DL (2011): Gender differences in PTSD symptoms: an exploration of peritraumatic mechanisms. J Anxiety Disord. 25: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamkwalala A, Norrholm SD, Poole JM, Brown A, Donley S, Duncan E, et al. (2012): Dark-enhanced startle responses and heart rate variability in a traumatized civilian sample: putative sex-specific correlates of posttraumatic stress disorder. Psychosom Med. 74: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McTeague LM, Lang PJ, Laplante M-C, Cuthbert BN, Shumen JR, Bradley MM (2010): Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol psychiatry. 67: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitman RK, Orr SP, Forgue DF, Altman B, de Jong JB, Herz LR (1990): Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. J Abnorm Psychol. 99: 49–54. [DOI] [PubMed] [Google Scholar]
- 80.Shalev AY, Orr SP, Pitman RK (1992): Psychophysiologic response during script-driven imagery as an outcome measure in posttraumatic stress disorder. J Clin psychiatry. 53: 324–326. [PubMed] [Google Scholar]
- 81.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH (2007): Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 45: 2019–2033. [DOI] [PubMed] [Google Scholar]
- 82.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK (2008): Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 42: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peri T, Ben-Shakhar G, Orr SP, Shalev AY (2000): Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol psychiatry. 47: 512–519. [DOI] [PubMed] [Google Scholar]
- 84.Hinrichs R, Michopoulos V, Winters S, Rothbaum AO, Rothbaum BO, Ressler KJ, Jovanovic T (2017): Mobile assessment of heightened skin conductance in posttraumatic stress disorder. Depress Anxiety. 34: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, et al. (2013): Sex differences in fear conditioning in posttraumatic stress disorder. J Psychiatr Res. 47: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM (2010): The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 168: 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pineles SL, Nillni YI, King MW, Patton SC, Bauer MR, Mostoufi SM, et al. (2016): Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. J Abnorm Psychol. 125: 349–355. [DOI] [PubMed] [Google Scholar]
- 88.Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Arditte Hall KA, et al. (2018): PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. 93: 133–141. [DOI] [PubMed] [Google Scholar]
- 89.Rasmusson AM, King MW, Valovski I, Gregor K, Scioli-Salter E, Pineles SL, et al. (2019): Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology. 102: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grillon C, Morgan CA 3rd (1999): Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 108: 134–142. [DOI] [PubMed] [Google Scholar]
- 91.Sijbrandij M, Engelhard IM, Lommen MJJ, Leer A, Baas JMP (2013): Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD). J Psychiatr Res. 47: 1991–1997. [DOI] [PubMed] [Google Scholar]
- 92.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ (2011): Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 69: 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B, et al. (2015): Fear load: The psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int J Psychophysiol. 98: 270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orcutt HK, Hannan SM, Seligowski AV, Jovanovic T, Norrholm SD, Ressler KJ, McCanne T (2016): Fear-Potentiated Startle and Fear Extinction in a Sample of Undergraduate Women Exposed to a Campus Mass Shooting. Front Psychol. 7: 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD (2012): Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 72: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, et al. (2013): Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci. 38: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daskalakis NP, Lehrner A, Yehuda R (2013): Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am. 42: 503–513. [DOI] [PubMed] [Google Scholar]
- 98.Morris MC, Compas BE, Garber J (2012): Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 32: 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW (1993): Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J psychiatry. 150: 83–86. [DOI] [PubMed] [Google Scholar]
- 100.Morris MC, Hellman N, Abelson JL, Rao U (2016): Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clin Psychol Rev. 49: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Delahanty DL, Nugent NR (2006): Predicting PTSD prospectively based on prior trauma history and immediate biological responses. Ann N Y Acad Sci. 1071: 27–40. [DOI] [PubMed] [Google Scholar]
- 102.Pan X, Kaminga AC, Wen SW, Liu A (2018): Catecholamines in Post-traumatic Stress Disorder: A Systematic Review and Meta-Analysis. Front Mol Neurosci. 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. (2011): Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 6: 603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, et al. (1999): Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J psychiatry. 156: 585–588. [DOI] [PubMed] [Google Scholar]
- 105.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB (2001): Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J psychiatry. 158: 575–581. [DOI] [PubMed] [Google Scholar]
- 106.Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP (2006): Regulation of synaptic transmission by CRF receptors. Rev Neurosci. 17: 279–307. [DOI] [PubMed] [Google Scholar]
- 107.Barel E, Abu-Shkara R, Colodner R, Masalha R, Mahagna L, Zemel OC, et al. (2018): Gonadal hormones modulate the HPA-axis and the SNS in response to psychosocial stress. J Neurosci Res. 96: 1388–1397. [DOI] [PubMed] [Google Scholar]
- 108.Stephens MAC, Mahon PB, McCaul ME, Wand GS (2016): Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 66: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BPR, Olff M (2007): Cortisol and posttraumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 191: 387–392. [DOI] [PubMed] [Google Scholar]
- 110.Mulchahey JJ, Ekhator NN, Zhang H, Kasckow JW, Baker DG, Geracioti TD Jr (2001): Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology. 26: 273–285. [DOI] [PubMed] [Google Scholar]
- 111.Reijnen A, Geuze E, Vermetten E (2015): The effect of deployment to a combat zone on testosterone levels and the association with the development of posttraumatic stress symptoms: A longitudinal prospective Dutch military cohort study. Psychoneuroendocrinology. 51: 525–533. [DOI] [PubMed] [Google Scholar]
- 112.Mellon SH, Gautam A, Hammamieh R, Jett M, Wolkowitz OM (2018): Metabolism, metabolomics, and inflammation in posttraumatic stress disorder. Biol Psychiatry. 83: 866875. [DOI] [PubMed] [Google Scholar]
- 113.Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T (2017): Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. 42: 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller MW, Lin AP, Wolf EJ, Miller DR (2018): Oxidative stress, inflammation, and neuroprogression in chronic PTSD. Harv Rev Psychiatry. 26: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. (2015): Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2: 1002–12. [DOI] [PubMed] [Google Scholar]
- 116.Fonkoue IT, Marvar PJ, Norrholm S, Li Y, Kankam ML, Jones TN, et al. (2019): Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD). Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park J, Marvar PJ, Liao P, Kankam ML, Norrholm SD, Downey RM, et al. (2017): Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. J Physiol. 595: 4893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rosen RL, Levy-Carrick N, Reibman J, Xu N, Shao Y, Liu M, et al. (2017): Elevated C-reactive protein and posttraumatic stress pathology among survivors of the 9/11 World Trade Center attacks. J Psychiatr Res. 89: 14–21. [DOI] [PubMed] [Google Scholar]
- 119.Gill JM, Saligan L, Lee H, Rotolo S, Szanton S (2013): Women in recovery from PTSD have similar inflammation and quality of life as non-traumatized controls. J Psychosom Res. 74: 301–6. [DOI] [PubMed] [Google Scholar]
- 120.Imai R, Hori H, Itoh M, Lin M, Niwa M, Ino K, et al. (2018): Inflammatory markers and their possible effects on cognitive function in women with posttraumatic stress disorder. Jof Psychiatr Res. 102: 192–200. [DOI] [PubMed] [Google Scholar]
- 121.Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, et al. (2017): Crosssectional and longitudinal associations of chronic posttraumatic stress disorder with inflammatory and endothelial function markers in women. Biol Psychiatry. 82: 875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, et al. (2018): Posttraumatic stress disorder onset and inflammatory and endothelial function biomarkers in women. Brain Behav Immun. 69: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Küffer A, Straus LD, Prather AA, Inslicht SS, Richards A, Shigenaga JK, et al. (2019): Altered overnight levels of pro-inflammatory cytokines in men and women with posttraumatic stress disorder. Psychoneuroendocrinology. 102: 114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clayton JA (2016): Studying both sexes: A guiding principle for biomedicine. FASEB J. 30: 519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klingensmith K, Tsai J, Mota N, Southwick SM, Pietrzak RH (2014): Military sexual trauma in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 75: e1133–9. [DOI] [PubMed] [Google Scholar]
- 126.Reisner SL, White Hughto JM, Gamarel KE, Keuroghlian AS, Mizock L, Pachankis JE (2016): Discriminatory experiences associated with posttraumatic stress disorder symptoms among transgender adults. J Couns Psychol. 63: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lebow MA, Schroeder M, Tsoory M, Holzman-Karniel D, Mehta D, Ben-Dor S, et al. (2019): Glucocorticoid-induced leucine zipper “quantifies” stressors and increases male susceptibility to PTSD. Transl Psychiatry. 9: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu S, Chen C, Pan Y, Kurz MC, Datner E, Hendry PL, et al. (2019): Genes known to escape X chromosome inactivation predict co-morbid chronic musculoskeletal pain and posttraumatic stress symptom development in women following trauma exposure. Am J Med Genet B Neuropsychiatr Genet. 180: 415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bryant RA, Felmingham KL, Silove D, Creamer M, O’Donnell M, McFarlane AC (2011): The association between menstrual cycle and traumatic memories. J Affect Disord. 131: 398–401. [DOI] [PubMed] [Google Scholar]
- 130.Bos PA, van Honk J, Ramsey NF, Stein DJ, Hermans EJ (2013): Testosterone administration in women increases amygdala responses to fearful and happy faces. Psychoneuroendocrinology. 38: 808–817. [DOI] [PubMed] [Google Scholar]
- 131.Buades-Rotger M, Engelke C, Beyer F, Keevil BG, Brabant G, Kramer UM (2016): Endogenous testosterone is associated with lower amygdala reactivity to angry faces and reduced aggressive behavior in healthy young women. Sci Rep. 6: 38538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, Habel U (2009): Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 34: 687–693. [DOI] [PubMed] [Google Scholar]
- 133.van Wingen GA, Zylicz SA, Pieters S, Mattern C, Verkes RJ, Buitelaar JK, Fernández G (2009): Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacology. 34: 539–547. [DOI] [PubMed] [Google Scholar]
- 134.Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J (2006): A single administration of testosterone reduces fear-potentiated startle in humans. Biol Psychiatry. 59: 872–4. [DOI] [PubMed] [Google Scholar]
- 135.Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J (2007): Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 32: 1052–61. [DOI] [PubMed] [Google Scholar]
- 136.Petersen N, Cahill L (2015): Amygdala reactivity to negative stimuli is influenced by oral contraceptive use. Soc Cogn Affect Neurosci. 10: 1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT (2012): Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm Behav. 62: 531–538. [DOI] [PubMed] [Google Scholar]
- 138.Ferree NK, Wheeler M, Cahill L (2012): The influence of emergency contraception on posttraumatic stress symptoms following sexual assault. J Forensic Nurs. 8: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hodes GE, Epperson CN (2019): Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry. 86: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Novais A, Monteiro S, Rogue S, Correia-Neves M, Sousa N (2016): How age, sex and genotype shape the stress response. Neurobiol Stress. 6: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van der Kolk BA (1997): The psychobiology of posttraumatic stress disorder. J Clin Psychiatry. 9: 16–24. [PubMed] [Google Scholar]
- 142.Romeo RD, McEwen BS (2006): Stress and the adolescent brain. Ann NY Acad Sci. 1094: 202–14. [DOI] [PubMed] [Google Scholar]
- 143.Boccia M, D’Amico S, Bianchini F, Marano A, Giannini AM, Piccardi L (2016): Different neural modifications underpin PTSD after different traumatic events: an fMRI meta-analytic study. Brain Imaging Behav. 10: 226–237. [DOI] [PubMed] [Google Scholar]
- 144.Briscione MA, Jovanovic T, Norrholm SD (2014): Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Front Psychiatry. 5: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Michopoulos V, Norrholm SD, Jovanovic T (2015): Diagnostic biomarkers for posttraumatic stress disorder: Promising horizons from translational neuroscience research. Biol Psychiatry. 78: 344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.eSense skin conductance system. Mindfield Biosystems, Inc., Berlin, Germany. [Google Scholar]
- 147.Helpman L, Marin MF, Papini S, Zhu X, Sullivan GM, Schneier F, et al. (2016): Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study. Neuroimage Clin. 12: 715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maples-Keller JL, Rauch SAM, Jovanovic T, Yasinski CW, Goodnight JM, Sherrill A, et al. (2019): Changes in trauma-potentiated startle, skin conductance, and heart rate within prolonged exposure therapy for PTSD in high and low treatment responders. J Anxiety Disord. 68: 102147. [DOI] [PubMed] [Google Scholar]
- 149.Norrholm SD, Jovanovic T, Gerardi M, Breaseale KG, Price M, Davis M, et al. (2016): Baseline psychophysiological and cortisol reactivity as a predictor of PTSD treatment outcome in virtual reality exposure therapy. Behav Res Ther. 82: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]