Abstract
Purpose:
The purpose of this study is to evaluate intraoperative OCT (iOCT) utility and outcomes during retinal detachment (RD) repair.
Design:
The DISCOVER intraoperative OCT study is a prospective IRB-approved study.
Subjects:
Subjects in the DISCOVER study undergoing surgical repair for RD.
Methods:
This was a post-hoc analysis of eyes in the DISCOVER study undergoing surgical repair for retinal detachments. Inclusion criteria included iOCT following perfluorocarbon liquid (PFO) placement and at least 6 months follow-up. Exclusion criteria included severe retinal pathology unrelated to RD. Surgeons completed standardized questionnaires after each case evaluating the iOCT instrument’s utility. Functional and surgical outcome data was collected at the latest available time point between 6 or 12 months. Outcomes were evaluated in 2 groups: uncomplicated primary and complex cases.
Main Outcome Measures:
Intraoperative OCT utility, single-surgery success, visual acuity outcomes
Results:
One hundred three eyes were included in this study, 51 primary and 52 complex cases. Intraoperative OCT provided valuable information in 36% of cases. In 12% of cases, iOCT data directly altered surgical decision making. There was a significantly higher rate of valuable iOCT feedback in complex cases compared to primary cases (50% vs 22%, p<0.01). Among primary cases, 48 (94%) had successful single surgery repair with a mean postoperative visual acuity of 20/47 compared to the complex group’s 75% single surgery success (n=39) and mean postoperative visual acuity of 20/92.
Conclusions:
This study affirms the potential impact of iOCT in assisting select cases of RD repair, particularly with complex pathology. The single surgery success rate was good with over 80% of cases successfully repaired with one surgery.
Keywords: Intraoperative OCT, retinal detachment, optical coherence tomography
Précis:
Intraoperative OCT demonstrates significant value for surgical decision-making during retinal detachment repair, particularly with complex cases. Single surgery success was high with intraoperative OCT-assisted surgical repair.
Introduction
Successful anatomic and functional outcome for retinal detachment repair remains challenging to predict. In particular, long-term visual prognosis following anatomically successful repair of retinal detachments remains elusive.1,2 However, some factors have correlated with outcomes: age, preoperative vision, duration of detachment, and height of macular detachment.3,4 Optical coherence tomography (OCT) imaging has become integrated into routine clinical care with enhanced visualization of retinal structures, permitting beneficial modifications to surgical planning especially for more complex cases.5,6 Findings from OCT imaging studies have emphasized that disruption to the outer retina such as ellipsoid zone disruption and presence of retinal corrugations correlate with visual outcomes.7
Intraoperative OCT (iOCT) permits real-time retinal visualization during surgery offering additional operative information to surgeons.8 The technique has been found in prior studies to benefit surgical decision making in both anterior and posterior segment cases.9–13 iOCT during retinal detachment repair has been demonstrated to be feasible.12,14,15 Similar to the OCT in clinic, iOCT has provided unique insights into intraoperative anatomic dynamics. In particular, studies have reported altered foveal configurations, such as occult full-thickness macular holes, and persistent submacular fluid following perfluorocarbon liquid tamponade.12,15 To date, few studies have examined the impact of iOCT on surgical decision-making in RD repair.
The purpose of this study is to evaluate the utility iOCT in retinal detachment surgery for retinal detachments in the DISCOVER study and to assess surgical outcomes.
Methods
The DISCOVER study is an IRB-approved prospective, single-center, multi-surgeon consecutive case series evaluating the potential role of microscope-integrated iOCT during ophthalmic surgery. This study adhered to the tenets of the Declaration of Helsinki and complied with HIPAA regulations. Written informed consent was obtained from each patient. The study design and methods for the DISCOVER study have been described previously.12 For this analysis, subjects with retinal detachments from the DISCOVER study were included. Inclusion criteria included surgical repair of retinal detachment with use of iOCT. Exclusion criteria included absence of follow-up data (visual outcomes, surgical complications, reoperations) and severe retinal pathology unrelated to retinal detachment such as retinal vasculitis, retinal tumors, or trauma significant enough to cause globe rupture. Surgical cases were also divided into two groups, (1) uncomplicated primary cases and (2) complex cases. Complex cases were defined as those eyes with notable additional retinal pathology including but not limited to preoperative proliferative vitreoretinopathy (PVR), presence of a giant retinal tear, panuveitis, and recurrent detachments.
Intraoperative Imaging and iOCT Feedback
Briefly, the study involved a prespecified intraoperative protocol for imaging patients during or after surgical milestones, or both, as determined by the operating surgeon. A microscope-integrated OCT system was used for intraoperative imaging [the RESCAN 700 prototype (Carl Zeiss Meditec, Inc., Oberkochen, Germany) or the EnFocus prototype (Bioptigen/Leica Microsystems, Wetzlar, Germany)].12 In the primary scleral buckle cases, a chandelier illumination system with concurrent use of the iOCT-enabled microscope with widefield visualization was used. Intraoperative imaging data were reviewed by the surgeon during surgery and also were reviewed independently after surgery.
Standardized surgeon questionnaires were completed immediately after surgery for all subjects, focusing on several specific areas related to the microscope-integrated system and retinal detachment repair. This included demographic information such as patient age, race, gender, iOCT system, scans obtained, the perceived value of iOCT to the procedure, the specific impact on surgical decision-making, and whether use of iOCT interfered with surgery.
Statistical Analysis
Surgical and functional outcome data were collected at postoperative month 6 and/or year 1. Outcome measures included visual acuity, surgical complications, reoperations, and outer retinal layer architecture changes. Count fingers (CF) and hand motion (HM) visual acuities were estimated as 20/2000 and 20/20000 respectively when included in outcome averages. Functional outcomes between the complex and uncomplicated cases were compared using 2-sample t-tests with LogMAR conversions and iOCT utility evaluated through comparison of two proportions. All statistics were completed in JMP Pro 14.
Results
Patient Demographics and Clinical Characteristics
A total of 103 eyes from 103 patients were identified from the DISCOVER study that had underwent retinal detachment repair for retinal detachments. Of the 103 eyes (Table 1), the mean age was 60.0 ± 13.0 years (mean and SD). There were 67 men (65%) and 36 women (35%). Preoperative mean VA was 20/500 including the 36 CF, HM, and LP cases (range 20/20-LP). Notable comorbidities included proliferative vitreoretinopathy (PVR) (22 eyes, 21%) and FTMH (4 eyes, 4%). Nearly half of the cases were phakic (50 eyes, 48%), while pseudophakic (49 eyes, 48%), and aphakic eyes (4 eyes, 4%) made up the remainder. Sixty-five eyes (63%) were macula-involving detachments. Complex cases comprised 52 of the 103 repairs (50%). Fifty-one cases had vitrectomy (49%), 11 had scleral buckle (11%), and 41 had scleral buckle and vitrectomy (40%).
Table 1.
Baseline Demographic and Clinical Characteristics.
| All (n = 103) | Uncomplicated (n = 51) | Complex (n = 52) | |
|---|---|---|---|
| Age (mean +SD) | 60 ± 13 | 60 ± 12 | 60 ± 14 |
| Sex | |||
| Male | 67 (65%) | 33 (65%) | 34 (65%) |
| Female | 36 (35%) | 18 (35%) | 18 (35%) |
| Lens status | |||
| Phakic | 50 (48%) | 29 (57%) | 21 (40%) |
| Pseudophakic | 49 (48%) | 21 (41%) | 28 (54%) |
| Aphakic | 4 (4%) | 1 (2%) | 3 (6%) |
| Macula-involving detachments | 65 (63%) | 38 (75%) | 29 (56%)* |
| PVR | 22 (21%) | 0 | 22 (42%)* |
| FTMH | 4 (4%) | 0 | 4 (8%) |
PVR = Proliferative pitreoretinopathy, FTMH = Full thickness macular hole,
= p < 0.05 comparing two proportions between uncomplicated and complex cases.
Intraoperative OCT characteristics and utility
In 37 out of 103 cases (36%), the iOCT provided feedback the surgeons deemed valuable. Specific iOCT findings that were described as valuable included confirmation of vitreous detachment, identification and evaluation of residual fluid post-PFO, confirmation of retinal reattachment, need for retinectomy, occult macular hole, presence of preretinal membrane, and retinal cyst identification. In 13 cases (12%), the use of iOCT specifically altered the surgical procedure (Table 2). Specific surgical tasks that were altered by iOCT included additional subretinal fluid drainage, revision of retinectomy, selecting optimal drainage site, identification of subretinal PFO, and additional membrane peeling. Among uncomplicated primary cases, iOCT provided valuable feedback in 11 of 51 surgeries (22%), while in 4 cases (8%) the instrument modified surgery. In 26 of 52 complex repairs (50%) the surgeon reported useful feedback gained from the iOCT which was significantly higher than in primary repairs (p<0.05). In 9 cases (17%) its imaging output positively altered surgical maneuvering. In 4 cases, the iOCT process interfered with surgery due to delays from software malfunction.
Table 2.
Feedback that resulted in surgical changes from iOCT in DISCOVER RD
| Eye | iOCT feedback | iOCT based procedure changes |
|---|---|---|
| 1 | Identified residual preretinal PFO | Aspirated preretinal PFO |
| 2 | Showed no SRF under PFO | No additional maneuvers required |
| 3 | No residual SRF on buckle | No additional maneuvers required |
| 4 | Identified subretinal PFO | Removed PFO |
| 5 | Area of SRF plan for drainage retinotomy | Placement of drain |
| 6 | Identified preretinal membrane | Prompted peeling |
| 7 | Identified retinal cyst | Confirmed no need for further intervention |
| 8 | Identified hole | Additional laser performed |
| 9 | Limited retinal flattening | Prompted retinectomy |
| 10 | Identified preretinal membrane | Additional peeling |
| 11 | Confirmed flat retinectomy edge | No additional maneuvers required |
| 12 | SRF and associated pigment | Prompted more drainage |
| 13 | No SRF in macula | Posterior retinotomy avoided |
Abbreviations: PFO: perfluorocarbon liquid; iOCT: intraoperative optical coherence tomography; SRF: subretinal fluid.
Clinical and Surgical Outcomes Non-Complex Primary Retinal Detachment Repair
In uncomplicated primary retinal detachment repairs, 48 of the 51 eyes (94%) had successful single surgery reattachment. In 3 cases, recurrent or new detachments occurred (6%). Six cases required postoperative surgical intervention for other complications including ERM, macular hole, and vitreous hemorrhage. Nonsurgical complications included cystoidmacular edema in (7 eyes, 14%) and epiretinal membrane (6 eyes, 12%). Postoperative mean visual acuity was 20/47 (range 20/20–20/300). Mean follow-up time was 10 months.
Complex cases
In complex retinal detachment repairs, 39 of the 52 eyes (75%) had successful single surgery detachment repair. Thirteen cases (25%) required subsequent repair of a recurrent or new detachment. Three cases required additional surgical intervention for postoperative ERM (2 eyes) and scleral buckle removal due to orbital inflammation (1 eye). The most common nonsurgical complication was ERM (6 eyes, 12%). Final visual acuity was 20/55 (range 20/2020/400) with an additional 6 cases of HM or CF, while inclusion of these cases resulted in a mean visual acuity of 20/92. This was a significantly lower visual acuity outcome compared to the noncomplex primary cases (p = 0.01). Mean follow-up time was 9.6 months.
Discussion
In this study, we report on the outcomes and utility of iOCT in retinal detachment repair and the sequalae of retinal detachment on outer retinal architecture following surgical repair. The use of iOCT was deemed valuable for providing feedback in a large proportion of cases (36%) and altered surgical decision-making in a non-trivial number as well (12%). There was a wide range of reported information that surgeons utilized through iOCT imaging intra-operatively such as diagnosing or confirming retinal pathology such as macular holes to retinal membranes or visualizing pockets of subretinal fluid or subretinal perfluorcarbon liquid. In several cases, this enabled surgeons to have greater understanding of the underlying anatomy and understanding of the achievement of surgical objectives.
Interestingly, iOCT provided useful information in a notably higher percentage of complex cases (50%) than in primary repairs (22%) (p<0.05), while similar results were seen in adjusting surgery based on iOCT in complex cases 17% vs 8%. Our findings thus suggest that iOCT most benefits retinal detachment repairs in higher complexity surgeries where the real-time retinal imaging is more likely to be utilized. Additionally, integrating the workflow of iOCT into the surgical procedure appeared to be well received. Surgeons reported that in the vast majority of cases (96%) the iOCT did not interfere with surgery. Software malfunction resulting in delay to surgical case was the primary issue resulting in interference. In those small number of the total cases where interference was noted, unlikely instrument malfunction such as imaging freezing was culpable.
To date, there had been no study with a significant cohort of patients evaluating functional and surgical outcomes in iOCT-assisted retinal detachment repair. Mean visual acuity among uncomplicated primary repairs showed improved outcomes to the more complex cases (average of 20/47 vs 20/92). This study found among all eyes a high rate of successful single surgery repair (84%) within 12 months of follow-up that remained high with uncomplicated, primary repairs (94%) but dropped when evaluating more complex case outcomes (75%) corresponding to 6% and 25% reoperation rates respectively. The most common causes for reoperation included recurrent detachment, PVR, and ERM. This success rate of primary RD repair was excellent when compared to other studies of primary RD repair.17–24 In one large cohort assessing 6-month outcome data from 5857 primary retinal detachment cases nonstratified for case-complexity, reoperation rates were at 13.9%, close to historically reported figures of 13–17%.17,23 Another more recent retrospective cohort analysis similarly yielded a redetachment rate of 14.7% which was notable for only including uncomplicated cases.21 Rates of primary repair failure have varied significantly in the literature, however. A nationwide analysis from Denmark reported a 22% reoperation rate from 6522 eyes, while a randomized control trial comparing SB vs PPV for primary RD in medium complexity cases reported an average failure of 36.8%19,22. Several other studies have identified reoperation rates between 2030%.18,20 A number of factors have been reported to account for this wide variation including case complexity, follow-up duration, definition of primary RD failure, and inclusion vs exclusion of silicone oil use.17
Several factors have been associated with reduced rates of surgical success, particularly related to complexity of surgical case and clinical features. In a recent studies of RD with PVR at baseleline, patients visual acuity at 6 months postoperatively was 20/200 or better in only 24% of patients, while 238 of 555 (43%) required reoperation.25 A small case series looking into retinal detachment repair outcomes in patients with acute retinal necrosis found that 46% required reoperation,26 while in another series giant retinal tears were associated with a 27% reoperation rate post-vitrectomy.27 In this study, only 25% pf complex cases required reoperation. The high rate of surgeon-perceived value of iOCT data (50%) suggests that iOCT may have contributed to this relatively low reoperation rate for a complex group. However, as several other factors may contribute to surgical success including variability in surgeon skill, patient postoperative positioning compliance, and complexity of the cases themselves, a randomized comparative prospective trial would be needed to more definitively evaluate the role of iOCT for surgical success.
As with any study, our work has important limitations that must be considered. One significant limitation is the lack of a control group that underwent standard RD repair without iOCT assistance to compare long-term outcomes with the same group of surgeons using a randomized design. The feasibility of a prospective randomized comparative study is currently being evaluated. Relatedly, the reported single surgery success of this study in primary RD repair is comparable to the prior studies with some of the best outcomes; however, it is difficult to determine the true iOCT impact in these cases without a randomized control group. Although the largest study to date, the sample size for the study is still relatively small. This study also did not include comparative evaluation of the different iOCT systems. Given the vast majority of cases were performed on one device, such comparative assessment was not possible. This limitation highlights the need for potential future comparative investigations on comparative iOCT device outcomes. Additional future work will need to assess the cost benefit ratio of implementing iOCT, critical for any new technology with unclear definitive benefit, in a prospective randomized trial. Strengths of the work include its relative novelty, particularly in relation to iOCT utility and outcomes in retinal detachment repair.
In summary, this study affirms iOCT feedback may provide surgeon-perceived valuable feedback and may modify surgical decision-making, particularly in complex cases. Further research may focus on a prospective randomized design to assess iOCT utility, comparatively evaluate iOCT devices, and determine the cost-benefit ratio of iOCT in retinal detachment repair.
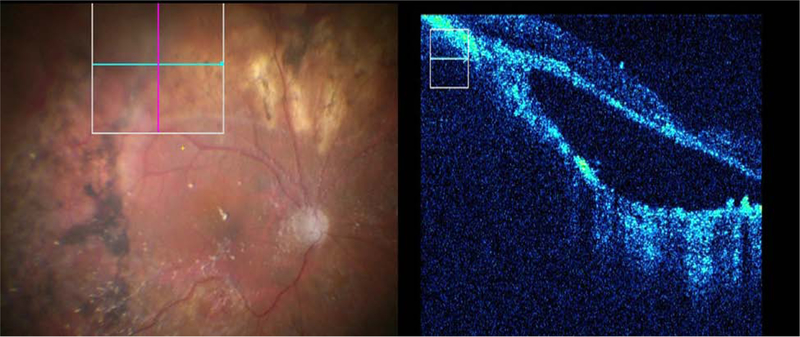
Figure 1.
Representative example of iOCT utility showing both surgical visualization and iOCT imaging. iOCT identified definitive hyperreflective subretinal membrane (arrowhead) consistent with PVR that was anatomically significant and required removal.
Figure 2.
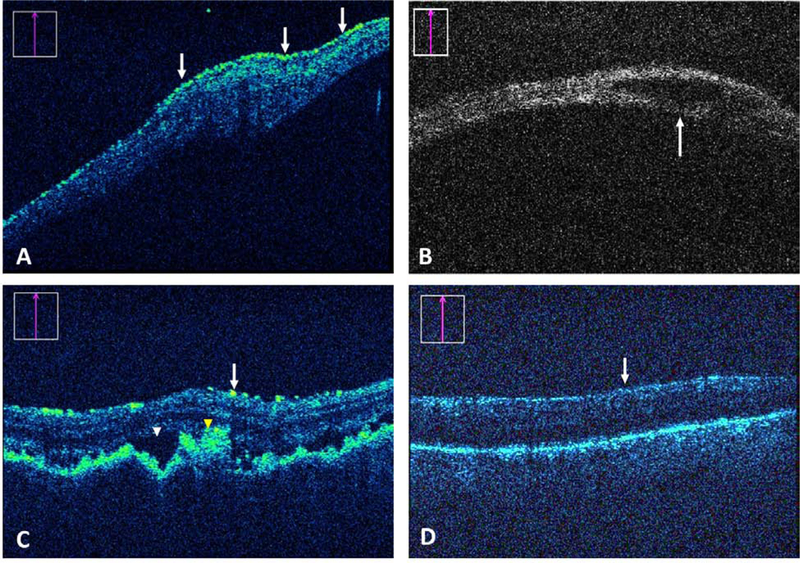
Impact of iOCT on surgical decision-making. (A) Preretinal membrane (arrows) identification by iOCT that prompted further peeling. (B) iOCT-based identification of a retinal cyst (arrow) confirming no need for additional treatment. (C) A complex combined exudative/rhegmatogenous detachment repair with suspected uveitis and choroidal folds in which SRF (white arrowhead) and abnormal pigment (yellow arrowhead) were identified by iOCT. The feedback resulted in more drainage. (D) In a possible proliferative vitreoretinopathy case, iOCT confirmed lack of preretinal membranes (arrow) that prevented unnecessary staining or membrane peel attempts.
Acknowledgments
Funding: National Institutes of Health/National Eye Institute, Bethesda, Maryland, USA, K23-EY022947–01A1 (J.P.E.); Ohio Department of Development, Columbus, Ohio, USA, TECH-13–059 (J.P.E., S.K.S.)
Footnotes
Disclosures: Dr. Srivastava is a consultant for Bausch and Lomb, Carl Zeiss Meditec, and Leica; a researcher for Allergan and Bausch and Lomb; and has a patent licensed to Leica. Dr. Sharma is a consultant for Eyepoint. Dr. Rachitskaya is a consultant for Allergan, Alcon, and Zeiss; and is a speaker for Novartis. Dr. Ehlers is a consultant for Alcon, Allergan, Leica, Santen, Thrombogenics, Genentech, Novartis, Aerpio, Allegro, Regeneron, Roche, and Zeiss; has intellectual property licensed to Leica; and receives research support from Alcon, Genentech, Regeneron, Boehringer-Ingelheim, Novartis, Aerpio, and Thrombogenics. The following authors have no financial disclosures: Joseph Abraham, Thuy Le and Jamie Reese.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wickham L, Ho-Yen GO, Bunce C, Wong D, Charteris DG. Surgical failure following primary retinal detachment surgery by vitrectomy: risk factors and functional outcomes. British Journal of Ophthalmology. 2011;95(9):1234–1238. [DOI] [PubMed] [Google Scholar]
- 2.Silva DJD, Kwan A, Bunce C, Bainbridge J. Predicting visual outcome following retinectomy for retinal detachment. British Journal of Ophthalmology. 2008. [DOI] [PubMed] [Google Scholar]
- 3.van de Put MAJ, Croonen D, Nolte IM, Japing WJ, Hooymans JMM, Los LI. Postoperative recovery of visual function after macula-off rhegmatogenous retinal detachment. PLOS ONE. 2014;9(6):e99787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park DH, Choi KS, Sun HJ, Lee SJ. FACTORS ASSOCIATED WITH VISUAL OUTCOME AFTER MACULA-OFF RHEGMATOGENOUS RETINAL DETACHMENT SURGERY. RETINA. 2018;38(1):137. [DOI] [PubMed] [Google Scholar]
- 5.Sakata Lisandro M, DeLeon‐Ortega J, Sakata V, Girkin Christopher A. Optical coherence tomography of the retina and optic nerve – a review. Clinical & Experimental Ophthalmology. 2009;37(1):90–99. [DOI] [PubMed] [Google Scholar]
- 6.Ramos Jose Luiz B, Li Y, Huang D. Clinical and research applications of anterior segment optical coherence tomography – a review. Clinical & Experimental Ophthalmology. 2009;37(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho M, Witmer MT, Favarone G, Chan RVP, D’Amico DJ, Kiss S. Optical coherence tomography predicts visual outcome in macula-involving rhegmatogenous retinal detachment. Clin Ophthalmol. 2012;6:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlers JP, Tao YK, Farsiu S, Maldonado R, Izatt JA, Toth CA. Integration of a Spectral Domain Optical Coherence Tomography System into a Surgical Microscope for Intraoperative Imaging. Invest Ophthalmol Vis Sci. 2011;52(6):3153–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida A, Srivastava SK, Ehlers JP. Analysis of Retinal Architectural Changes Using Intraoperative OCT Following Surgical Manipulations With Membrane Flex Loop in the DISCOVER Study. Invest Ophthalmol Vis Sci. 2017;58(9):3440–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchida A, Srivastava SK, Ehlers JP. Update on the Intraoperative OCT: Where Do We Stand? Curr Ophthalmol Rep. 2018;6(1):24–35. [Google Scholar]
- 11.Ehlers JP, Tam T, Kaiser PK, Martin DF, Smith GM, Srivastava SK. Utility of Intraoperative Optical Coherence Tomography During Vitrectomy Surgery for Vitreomacular Traction Syndrome. Retina. 2014;34(7):1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehlers JP, Modi YS, Pecen PE, et al. The DISCOVER Study 3-Year Results: Feasibility and Usefulness of Microscope-Integrated Intraoperative OCT during Ophthalmic Surgery. Ophthalmology. 2018;0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers JP, Han J, Petkovsek D, Kaiser PK, Singh RP, Srivastava SK. Membrane Peeling-Induced Retinal Alterations on Intraoperative OCT in Vitreomacular Interface Disorders From the PIONEER Study. Invest Ophthalmol Vis Sci. 2015;56(12):7324–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK. NOVEL MICROARCHITECTURAL DYNAMICS IN RHEGMATOGENOUS RETINAL DETACHMENTS IDENTIFIED WITH INTRAOPERATIVE OPTICAL COHERENCE TOMOGRAPHY. RETINA. 2013;33(7):1428. [DOI] [PubMed] [Google Scholar]
- 15.Abraham JR, Srivastava SK, Reese JL, Ehlers JP. Intraoperative OCT Features and Postoperative Ellipsoid Mapping in Primary Macula-Involving Retinal Detachments from the PIONEER Study. Ophthalmol Retina. 2019;3(3):252–257. [DOI] [PubMed] [Google Scholar]
- 16.Arepalli S, Srivastava SK, Hu M, et al. Assessment of inner and outer retinal layer metrics on the Cirrus HD-OCT Platform in normal eyes. PLOS ONE. 2018;13(10):e0203324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallam AB, Donachie PHJ, Yorston D, et al. ROYAL COLLEGE OF OPHTHALMOLOGISTS’ NATIONAL DATABASE STUDY OF VITREORETINAL SURGERY: Report 7, Intersurgeon Variations in Primary Rhegmatogenous Retinal Detachment Failure. Retina. 2018;38(2):334–342. [DOI] [PubMed] [Google Scholar]
- 18.Mitry D, Awan MA, Borooah S, et al. Surgical outcome and risk stratification for primary retinal detachment repair: results from the Scottish Retinal Detachment study. Br J Ophthalmol. 2012;96(5):730–734. [DOI] [PubMed] [Google Scholar]
- 19.Heimann H, Bartz-Schmidt KU, Bornfeld N, et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114(12):2142–2154. [DOI] [PubMed] [Google Scholar]
- 20.Dugas B, Lafontaine P-O, Guillaubey A, et al. The learning curve for primary vitrectomy without scleral buckling for pseudophakic retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2009;247(3):319–324. [DOI] [PubMed] [Google Scholar]
- 21.Adelman RA, Parnes AJ, Ducournau D, European Vitreo-Retinal Society Retinal Detachment Study G. Strategy for the management of uncomplicated retinal detachments: the European vitreo-retinal society retinal detachment study report 1. Ophthalmology. 2013;120(9):1804–1808. [DOI] [PubMed] [Google Scholar]
- 22.Hajari JN, Christensen U, Kiilgaard JF, Bek T, la Cour M. Reoperation for rhegmatogenous retinal detachment as quality indicator for disease management: a register study. Acta Ophthalmol. 2015;93(6):505–511. [DOI] [PubMed] [Google Scholar]
- 23.Hilton GF, Grizzard WS, Avins LR, Heilbron DC. The drainage of subretinal fluid: a randomized controlled clinical trial. Retina. 1981;1(4):271–280. [DOI] [PubMed] [Google Scholar]
- 24.Ehrlich R, Ahmad N, Welch S, Hadden P, Polkinghorne P. Vitreoretinal fellow surgical outcomeof small gauge pars plana vitrectomy for acute rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2011;249(8):1147–1152. [DOI] [PubMed] [Google Scholar]
- 25.Scott IU, Flynn HW, Murray TG, Feuer WJ. Outcomes of surgery for retinal detachment associated with proliferative vitreoretinopathy using perfluoro-n-octane: a multicenter study. American Journal of Ophthalmology. 2003;136(3):454–463. [DOI] [PubMed] [Google Scholar]
- 26.Kopplin LJ, Thomas AS, Cramer S, et al. Long-Term Surgical Outcomes of Retinal Detachment Associated With Acute Retinal Necrosis. Ophthalmic Surg Lasers Imaging Retina. 2016;47(7):660–664. [DOI] [PubMed] [Google Scholar]
- 27.Ghasemi Falavarjani K, Alemzadeh SA, Modarres M, et al. Outcome of surgery in patients with giant retinal tear: 10 years experience. Eye (London, England). 2017;31(9):1284–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gharbiya M, Grandinetti F, Scavella V, et al. CORRELATION BETWEEN SPECTRAL-DOMAIN OPTICAL COHERENCE TOMOGRAPHY FINDINGS AND VISUAL OUTCOME AFTER PRIMARY RHEGMATOGENOUS RETINAL DETACHMENT REPAIR. RETINA. 2012;32(1):43. [DOI] [PubMed] [Google Scholar]
- 29.Terauchi G, Shinoda K, Matsumoto CS, Watanabe E, Matsumoto H, Mizota A. Recovery of photoreceptor inner and outer segment layer thickness after reattachment of rhegmatogenous retinal detachment. British Journal of Ophthalmology. 2015;99(10):1323–1327. [DOI] [PubMed] [Google Scholar]
- 30.Kroll AJ, Machemer R. Experimental retinal detachment in the owl monkey. V. Electron microscopy of the reattached retina. American Journal of Ophthalmology. 1969;67(1):117–130. [DOI] [PubMed] [Google Scholar]
- 31.Guérin CJ, Lewis GP, Fisher SK, Anderson DH. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci. 1993;34(1):175–183. [PubMed] [Google Scholar]