Abstract
Goal:
To investigate associations of pre- and post-diagnosis use of statins and metformin on overall survival of patients with diabetes who later develop HCC
Background:
Statins and metformin have received considerable interest as potential chemopreventive agents against hepatocellular carcinoma (HCC) in individuals with type 2 diabetes mellitus (T2DM); however, their impact on overall survival of patients with T2DM who later develop HCC (diabetic HCC patients) is unclear.
Study:
Data on 2,499 elderly diabetic HCC patients obtained from the SEER-Medicare program (2009–2013) were analyzed. Patients were categorized based on use of statins only, metformin only, both, or neither (reference for all comparisons). The patients were further categorized based on: (1) metformin dose: ≤1,500 or >1,500 mg/day; (2) statins functional form: hydrophilic (pravastatin and rosuvastatin) or lipophilic (atorvastatin, fluvastatin, lovastatin, and simvastatin); (3) statins potency: high (atorvastatin, rosuvastatin, and simvastatin) or low (fluvastatin, lovastatin, and pravastatin); and (4) individual statins type. Multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CIs) were calculated using Cox proportional hazard models.
Results:
Pre-diagnosis use of metformin dose ≤1,500 mg/day was associated with lower risk of death after HCC diagnosis in patients with T2DM (HR=0.72, 95% CI: 0.58–0.91), adjusting for post-diagnosis metformin dose, diabetes severity, Charlson comorbidity index, tumor characteristics, and other relevant factors. No association was found for pre-diagnosis metformin dose >1500 mg/day or post-diagnosis metformin use. Further, no association was found for either pre- or post-diagnosis statins use.
Conclusions:
Pre-diagnosis use of metformin dose ≤1,500 mg/day could improve overall survival of elderly diabetic HCC patients.
Keywords: Liver cancer, hepatocellular cancer, HCC, statins, metformin
Introduction
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer and accounts for about 80% of all liver cancer diagnoses worldwide.1 In the United States (US), liver cancers, including HCC and the less common cholangiocarcinoma, are projected to become the third leading cause of cancer death by 2030.2 Major risk factors for HCC include chronic hepatitis C virus (HCV) and hepatitis B virus (HBV) infections, alcoholic liver disease and metabolic disorders, such as type 2 diabetes mellitus (T2DM) and metabolic syndrome.3 HCC currently has a 5-year survival rate of 18%, which is due mostly to delayed diagnosis.4 When diagnosed at an early stage, HCC is often treated with surgical resection, liver transplantation, or radiofrequency ablation, all of which are associated with improved prognosis. Systemic medications, such as sorafenib, regorafenib and niolumab, have also shown some modest benefits for management of advanced HCC.5–7 However, there remains a pressing need to identify modifiable factors that can help further prolong survival of HCC patients.
T2DM is a global epidemic.8 Incidence of T2DM has been increasing in the US in parallel with the increasing incidence of obesity.8,9 Many patients with T2DM develop HCC after the diagnosis of T2DM (diabetic HCC patients)10,11, and pre-existing T2DM is now recognized as not just a risk factor for HCC but also an independent predictor of shorter survival after diagnosis of HCC.12 T2DM is often managed with metformin, a first-line oral antihyperglycemic agent. Studies have shown that metformin is commonly taken simultaneously with statins (cholesterol lowering medications) because most patients with T2DM have also hyperlipidemia.13,14 Experimental studies suggest that both statins and metformin may have therapeutic potential for prolonging survival of HCC patients.15,16 The proposed mechanisms for the role of statins in cancer survival include cell-cycle arrest, apoptosis induction, and posttranslational modification of RAS proteins.17–19 Postulated anticancer effects of metformin also include attenuation of metabolic abnormalities20 and suppression of the mammalian target of rapamycin (mTOR) signaling pathway that is known to enhance cancer cell proliferation.21–23 Nonetheless, findings from epidemiologic association studies are mixed.24–27
At least two epidemiological studies have examined the association between use of statins and overall survival of HCC patients and have reported conflicting results.24,25 The same is the case for metformin, with at least two studies reporting a beneficial association26,27 and one study reporting a null association.28 Although many patients taking metformin for T2DM take also statins for hyperlipidemia29, none of the existing studies examined associations for combined use of statins and metformin on overall survival of diabetic HCC patients. Additionally, no study has examined associations for pre- and post-diagnosis use of statins or metformin while adjusting for the effect of the other. Pre- and post-diagnosis statins or metformin use may have differential association with survival in HCC patients as seen in other malignancies.30 These gaps in knowledge necessitate further investigations to delineate the roles of statins and metformin in survival of diabetic HCC patients. Thus, we investigated associations of pre- and post-diagnosis use of statins and metformin, both independently and jointly, on overall survival after HCC diagnosis in a nationally representative sample of elderly patients with T2DM using data from the Surveillance, Epidemiology, and End Results (SEER) registry linked to Medicare claims information (SEER-Medicare).
Material and Methods
Data Source and Study Population
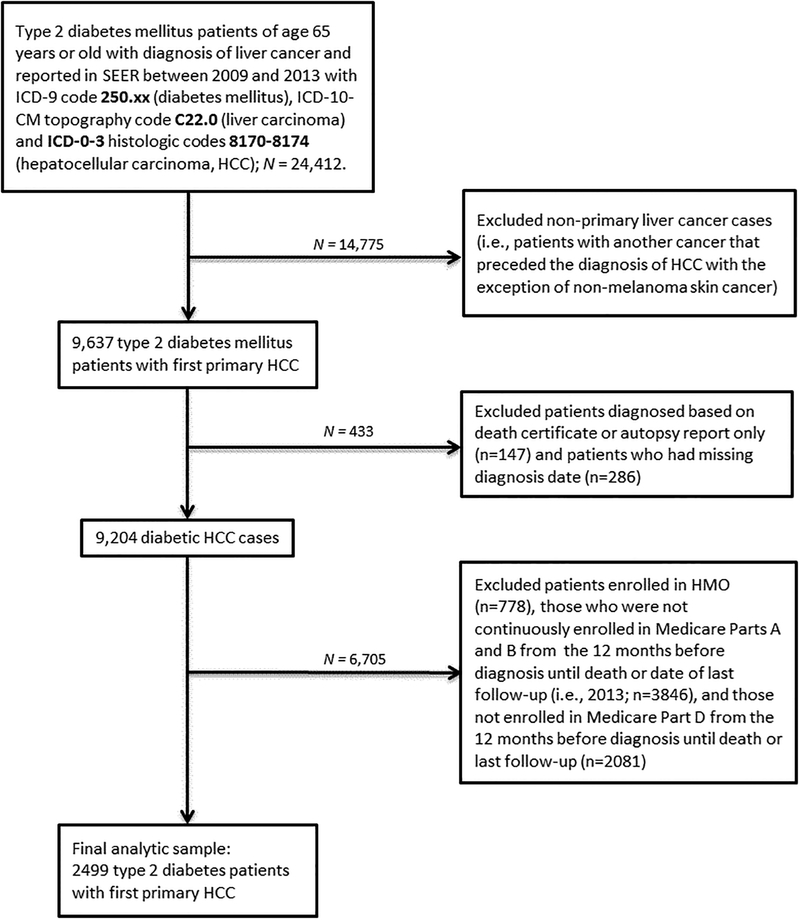
Following approval by the Mayo Clinic Institutional Review Board (IRB), we obtained data from the SEER-Medicare program. A detail description of the SEER-Medicare program has been provided.31 In brief, the SEER program is supported by the National Cancer Institute and includes data collected from population-based cancer registries that cover 18 geographic regions: Atlanta, Georgia; Connecticut; Detroit, Michigan; Hawaii; Iowa; New Mexico; San Francisco-Oakland, California; Seattle-Puget Sound, Seattle Washington; Utah; Los Angeles, California; San Jose-Monterey, California; Rural Georgia; Alaska; Greater California; Greater Georgia; Kentucky; Louisiana; and New Jersey. The Medicare program provides payments for hospital, physician, and outpatient medical services for about 97% of US citizens of age 65 years or older.31 Data on cancer patients reported to SEER who are 65 years or older are linked to Medicare claims data of the respective patients for SEER-Medicare studies. The areas covered by the SEER-Medicare program represents about 25% of the general US population.31 The Medicare claims files include information on medical diagnosis, medical procedures, and prescription drug claims. The Medicare Part A file contains claims for hospital, skilled nursing facility, and hospice services. The Medicare Part B file covers claims for physician and outpatient services, and the Medicare Part D file covers prescription drug claims. As of June 2018, data on SEER-Medicare Parts A and B were available through December 2014. Complete data on Medicare Part D prescription drug coverage were available from 2008 to 2013. To allow for a 1-year window for assessment of baseline comorbidities and utilization of Medicare Part D prescription drug coverage, analyses were restricted to patients diagnosed with HCC from January 1, 2009 to December 31, 2013. The International Classification of Disease, 9th Revision (ICD-9) code 250.xx was used to identify patients with T2DM in the SEER-Medicare data, following which the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) topography code C22.0 was used to identify the T2DM cases with diagnosis of liver cancer. We then used the International Classification of Diseases for Oncology, 3rd revision (ICD-O-3) histologic codes 8170 to 8174 to subset to the liver cancers to HCC only. The HCC diagnosis date had to have occurred after T2DM diagnosis date for inclusion in the study. Details of the sample selection process are provided in Figure 1. In Brief, we identified 9,204 patients with T2DM with first primary histologically-confirmed HCC cases diagnosed between January 1, 2009 and December 31, 2013. We then excluded patients co-enrolled in Medicare and Private Health Maintenance Organizations (n=778) and those who were not continuously enrolled in Medicare Parts A and B in the 12 months preceding HCC diagnosis until death or last follow-up (n=3846). We further excluded patients not enrolled in Medicare Part D prescription drug coverage in the 12 months before diagnosis until death or last follow-up (n=2,081), leaving a final sample of 2,499 patients with T2DM with incident HCC for analyses (Figure 1).
Figure 1:
Flow chart of study sample selection process.
Sociodemographic Information, Clinic Characteristics, and Outcome Variable
Sociodemographic information, including age, sex, race/ethnicity, marital status, body mass index (BMI), neighborhood level income, and percentage of persons with a 4-year college education in neighborhood were abstracted from the Patient Entitlement and Diagnosis Summary File (PEDSF). Information on tumor characteristics, including stage, grade and size were obtained from the SEER file. Data on comorbid conditions (e.g., cardiovascular disease, diabetes, HBV, and HCV) were identified using ICD-9 codes from the PEDSF, the Medicare Provider Analysis and Review (MEDPAR), Carrier data, and Outpatient Standard Analytical File. Comorbidities had to have existed at least 1-year before HCC diagnosis and we calculated the Charlson comorbidity index, as described.32 Because all participants had diagnosis of T2DM, we further calculated diabetes severity index as described in detail by Young et al.33 Data on cancer treatment received by the patients were derived from the Medicare Provider Analysis and Review, the Carrier, Outpatient, and the Standard Analytical Files using ICD-9 codes. The outcome of interest was overall survival. Survival time was calculated as the number of completed months from the date of diagnosis to the date of death, date last known to be alive, or date of last follow-up (December 31, 2013), whichever occurs first. Individuals who were alive at the end of follow-up were censored at that time.
Statins and Metformin Use
Data on statins and metformin use were abstracted from the Medicare Part D prescription drug file. Statins and metformin use before and after diagnosis of HCC were determined by comparing the prescription date to the date of HCC diagnosis. Pre-diagnosis medication use was defined as medication use initiated at least a year before HCC diagnosis and post-diagnosis medication use defined as medication use initiated at least one day after diagnosis. Medication use was initially categorized as statins only, metformin only, both statins and metformin, or neither. Average daily metformin dose was calculated as the total milligrams of metformin dispensed divided by the total number of days of prescription and the metformin users were categorized into ≤1,500 mg/day or >1,500 mg/day.34 Statins users were categorized further based on (1) the functional form of the statins: hydrophilic (pravastatin and rosuvastatin) or lipophilic (atorvastatin, fluvastatin, lovastatin, and simvastatin)35, (2) statins potency: high potency (atorvastatin, rosuvastatin, and simvastatin) or low potency (fluvastatin, lovastatin, and pravastatin)36, and (3) the individual statins types.
Statistical Analysis
Distributions of sociodemographic and clinical characteristics of the participants were compared by pre-diagnosis use of statins only, metformin only, both, or neither, using chi-square tests for categorical variables and Student’s t-test for continuous variables. Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Prior to fitting the Cox proportional models, the proportional hazard assumption was assessed by creating interaction variables between the predictors and a log transformation of survival time37 and was determined to have been satisfied. Association analyses were performed in minimally-adjusted and fully-adjusted models. The minimally-adjusted model included age, sex, and pre- and post-diagnosis medication use. In the fully-adjusted model, additional adjustment for year of diagnosis, race/ethnicity, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, presence of chronic obstructive pulmonary disease (COPD), dyslipidemia, and hepatitis status (HBV, HCV, both, none) was performed as categorized in Table 1. Associations for pre- and post-diagnosis medication use were initially assessed among users of statins only, metformin only, both, and neither (i.e., non-users; reference group). Associations for use of pre- and post-diagnosis hydrophilic and lipophilic statins and for use of high or low potency statins were assessed compared with non-users. We assessed also associations for individual statins type (pravastatin, rosuvastatin, atorvastatin, fluvastatin, lovastatin, and simvastatin) compared with non-users. Finally, we examined associations for average daily metformin dose (≤1,500 mg/day, >1,500 mg/day vs. non-users) used pre- and post-diagnosis of HCC. All statistical tests were two-sided and a p-value lower than 0.05 was considered statistically significant. Analyses were performed in SAS® version 9.4 (SAS Institute, Cary, North Carolina).
Table 1:
Demographic and clinical characteristics of diabetic HCC patients by pre-diagnostic statin and/or metformin use groups; N = 2499.
| Non-users (N=1,193) | Statins only (N=582) | Metformin only (N=295) | Both (N=429) | p value | |
|---|---|---|---|---|---|
| Age at diagnosis, years | <0.0001 | ||||
| N | 1,193 | 582 | 295 | 429 | |
| Mean (SD) | 75.0 (7.2) | 77.3 (6.7) | 73.5 (6.3) | 75.6 (6.1) | |
| Range | (65.0–100.0) | (65.0–94.0) | (65.0–96.0) | (65.0–94.0) | |
| Sex | 0.0873 | ||||
| Male | 717 (60.1%) | 384 (66.0%) | 185 (62.7%) | 276 (64.3%) | |
| Female | 476 (39.9%) | 198 (34.0%) | 110 (37.3%) | 153 (35.7%) | |
| Marital status | 0.1650 | ||||
| Married/Cohabitation | 542 (45.4%) | 287 (49.3%) | 144 (48.8%) | 216 (50.3%) | |
| Separated/Divorced/Widowed | 423 (35.5%) | 211 (36.3%) | 101 (34.2%) | 149 (34.7%) | |
| Single | 178 (14.9%) | 64 (11.0%) | 41 (13.9%) | 43 (10.0%) | |
| Unknown | 50 (4.2%) | 20 (3.4%) | 9 (3.1%) | 21 (4.9%) | |
| Race | 0.0023 | ||||
| Non-Hispanic White | 676 (56.7%) | 335 (57.6%) | 163 (55.3%) | 240 (55.9%) | |
| Non-Hispanic Black | 125 (10.5%) | 57 (9.8%) | 24 (8.1%) | 24 (5.6%) | |
| Asian | 209 (17.5%) | 126 (21.6%) | 54 (18.3%) | 84 (19.6%) | |
| Hispanic | 93 (7.8%) | 35 (6.0%) | 36 (12.2%) | 48 (11.2%) | |
| Unknown/other | 90 (7.5%) | 29 (5.0%) | 18 (6.1%) | 33 (7.7%) | |
| Neighborhood median income level | 0.2888 | ||||
| <$35,000 | 378 (31.7%) | 177 (30.4%) | 91 (30.8%) | 119 (27.7%) | |
| $35,000–49,000 | 292 (24.5%) | 117 (20.1%) | 64 (21.7%) | 93 (21.7%) | |
| $50,000–74,999 | 181 (15.2%) | 105 (18.0%) | 55 (18.6%) | 82 (19.1%) | |
| ≥75,000 | 101 (8.5%) | 48 (8.2%) | 21 (7.1%) | 30 (7.0%) | |
| Unknown | 241 (20.2%) | 135 (23.2%) | 64 (21.7%) | 105 (24.5%) | |
| Percent with 4-year college education in Neighborhood | 0.8009 | ||||
| <10% | 267 (22.4%) | 120 (20.6%) | 69 (23.4%) | 98 (22.8%) | |
| 10–19% | 272 (22.8%) | 126 (21.6%) | 69 (23.4%) | 82 (19.1%) | |
| 20–29 | 150 (12.6%) | 77 (13.2%) | 37 (12.5%) | 55 (12.8%) | |
| ≥30% | 263 (22.0%) | 124 (21.3%) | 56 (19.0%) | 89 (20.7%) | |
| Unknown | 241 (20.2%) | 135 (23.2%) | 64 (21.7%) | 105 (24.5%) | |
| Tumor grade | 0.2243 | ||||
| I (well differentiated) | 153 (12.8%) | 74 (12.7%) | 38 (12.9%) | 54 (12.6%) | |
| II (moderately differentiated) | 190 (15.9%) | 109 (18.7%) | 51 (17.3%) | 83 (19.3%) | |
| III,IV (poorly differentiated) | 82 (6.9%) | 56 (9.6%) | 22 (7.4%) | 54 (12.6%) | |
| Unknown | 768 (64.4%) | 343 (59.0%) | 184 (62.4%) | 238 (55.5%) | |
| Stage | 0.0008 | ||||
| Stage I | 445 (37.3%) | 219 (37.6%) | 116 (39.3%) | 145 (33.8%) | |
| Stage II | 185 (15.5%) | 57 (9.8%) | 44 (14.9%) | 40 (9.3%) | |
| Stage III, IV | 209 (30.2%) | 123 (35.0%) | 58 (31.9%) | 109 (41.5%) | |
| Unknown | 202 (16.9%) | 102 (17.5%) | 38 (12.9%) | 66 (15.4%) | |
| Tumor size | <0.0001 | ||||
| < 5cm | 526 (44.1%) | 159 (27.3%) | 126 (42.7%) | 123 (28.7%) | |
| >= 5cm | 441 (37.0%) | 308 (52.9%) | 116 (39.3%) | 214 (49.9%) | |
| Unknown | 226 (18.9%) | 115 (19.8%) | 53 (18.0%) | 92 (21.4%) | |
| Chemotherapy | 0.5669 | ||||
| No | 1102 (92.4%) | 542 (93.1%) | 274 (92.9%) | 405 (94.4%) | |
| Yes | 91 (7.6%) | 40 (6.9%) | 21 (7.1%) | 24 (5.6%) | |
| Radiation | 0.1299 | ||||
| No | 996 (83.5%) | 493 (84.7%) | 254 (86.1%) | 378 (88.1%) | |
| Yes | 197 (16.5%) | 89 (15.3%) | 41 (13.9%) | 51 (11.9%) | |
| Charlson comorbidity index score | <0.0001 | ||||
| 0 | 217 (18.2%) | 51 (8.8%) | 6 (2.0%) | 4 (0.9%) | |
| 1 | 258 (21.6%) | 88 (15.1%) | 48 (16.3%) | 83 (19.3%) | |
| 2 | 189 (15.8%) | 114 (19.6%) | 73 (24.7%) | 92 (21.4%) | |
| 3 – 5 | 411 (34.5%) | 236 (40.5%) | 124 (42.0%) | 177 (41.3%) | |
| >5 | 118 (9.9%) | 93 (16.0%) | 44 (14.9%) | 73 (17.0%) | |
| Diabetes severity index | <0.0001 | ||||
| 0 | 227 (19.0%) | 70 (12.0%) | 61 (20.7%) | 69 (16.1%) | |
| 1 | 188 (15.8%) | 54 (9.3%) | 46 (15.6%) | 54 (12.6%) | |
| 2 | 293 (24.6%) | 88 (15.1%) | 46 (15.6%) | 48 (11.2%) | |
| ≥3 | 485 (40.7%) | 370 (63.6%) | 142 (48.1%) | 258 (60.1%) | |
| Obese (BMI ≥30kg/m2) | <0.0001 | ||||
| No | 1005 (84.2%) | 449 (77.1%) | 235 (79.7%) | 306 (71.3%) | |
| Yes | 188 (15.8%) | 133 (22.9%) | 60 (20.3%) | 123 (28.7%) | |
| COPD | <0.0001 | ||||
| No | 699 (58.6%) | 286 (49.1%) | 193 (65.4%) | 259 (60.4%) | |
| Yes | 494 (41.4%) | 296 (50.9%) | 102 (34.6%) | 170 (39.6%) | |
| Dyslipidemia | <0.0001 | ||||
| No | 483 (40.5%) | 49 (8.4%) | 98 (33.2%) | 28 (6.5%) | |
| Yes | 710 (59.5%) | 533 (91.6%) | 197 (66.8%) | 401 (93.5%) | |
| Hepatitis type | <0.0001 | ||||
| None | 524 (43.9%) | 365 (62.7%) | 154 (52.2%) | 311 (72.5%) | |
| Hepatitis B | 23 (1.9%) | 8 (1.4%) | 4 (1.4%) | 8 (1.9%) | |
| Hepatitis C | 584 (49.0%) | 182 (31.3%) | 127 (43.1%) | 93 (21.7%) | |
| Hepatitis B & C | 48 (4.0%) | 17 (2.9%) | 7 (2.4%) | 13 (3.0%) | |
| Unspecified type | 14 (1.2%) | 10 (1.7%) | 3 (1.0%) | 4 (0.9%) |
Abbreviations: COPD, chronic obstructive pulmonary disease; BMI, body mass index; HCC, hepatocellular carcinoma
Results
Descriptive characteristics of the study participants, including age, marital status, and HCC tumor stage, grade and size, were examined among pre-diagnosis users of statins only, metformin only, both statins and metformin, and non-users (Table 1). The statins only users were slightly older (mean age: 77 years) and included a higher proportion of non-Hispanic Whites (58%) than users of metformin only (mean age: 73 years; 55% non-Hispanic Whites), both statins and metformin (mean age: 76 years; 56 non-Hispanic Whites), or non-users (mean age: 75 years; 57% males). Users of both statins and metformin were more likely to present with stage III or IV HCC tumors (41%) compared with users of statins only (35%), metformin only (32%), and non-users (30%). Statins only users were more likely to have larger tumors (i.e., ≥5 cm; 53%) than users of metformin only (39%), both statins and metformin (50%), and non-users (37%). Additionally, users of both statins and metformin had higher Charlson comorbidity index, while users of statins only had higher diabetes severity index compared to the other groups, but no differences were observed by sex, marital status, neighborhood income or education level, tumor grade, or treatment with chemotherapy or radiation. Other findings are reported in Table 1.
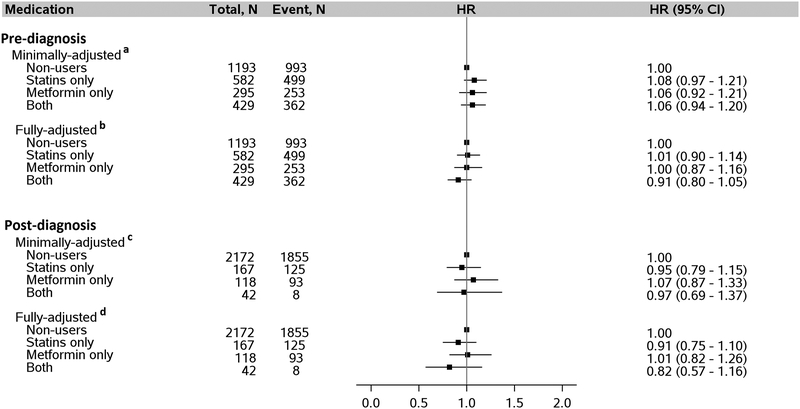
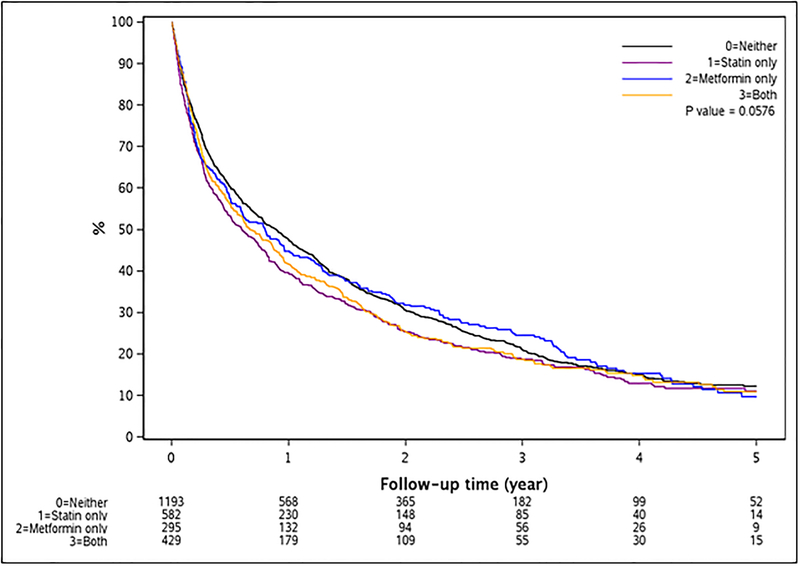
Median survival in the entire population was 9.2 months; it was highest among non-users (10.7 months), followed by pre-diagnosis users of metformin only (9.5 months), both metformin and statins (8.0 months), and lowest among statins only users (7.1 months). Results for associations of statins and/or metformin use and overall survival after HCC diagnosis did not show any significant association for either pre- or post-diagnosis use of statins only, metformin only, or both when compared with non-users (Figure 2). Kaplan-Meier survival curves for pre-diagnosis use of statins only, metformin only, both statins and metformin, and non-use did not show any differences in overall survival across follow-up (Log-rank p-value = 0.06) (Figure 3).
Figure 2:
Association between use of statins and/or metformin by patients with diabetes pre- and post-diagnosis of HCC and risk of death after HCC diagnosis. a Adjusted for age (continuous), sex, and post-diagnosis medication use (statins only, metformin only, both, neither). b Adjusted for everything in “a” plus year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, presence of COPD, dyslipidemia, and hepatitis as categorized in Table 1. c Adjusted for age, sex, and pre-diagnosis medication use (statins only, metformin only, both, neither). d Adjusted for everything in “c” with additional adjustment for year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, COPD, dyslipidemia, and hepatitis as categorized in Table 1. Abbreviation: HCC, hepatocellular carcinoma.
Figure 3:
Kaplan-Meier survival curve for overall survival by pre-diagnosis medication use.
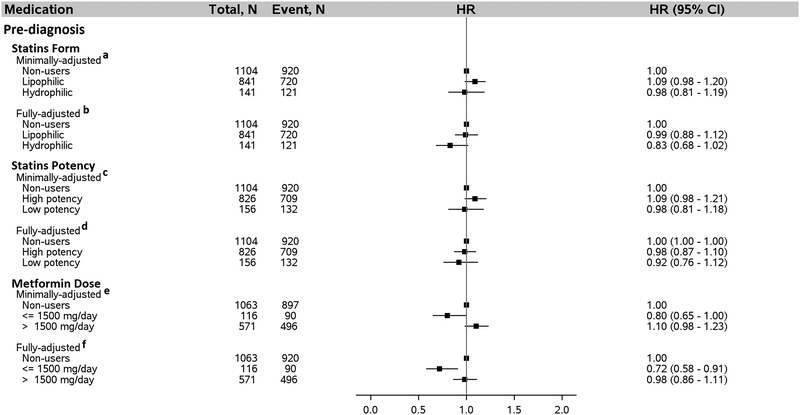
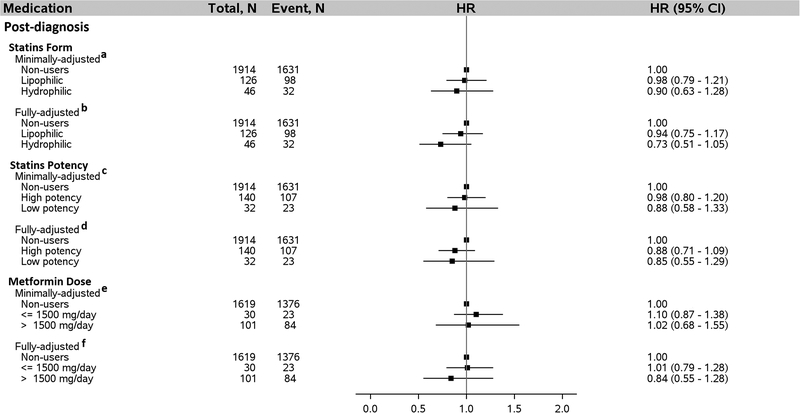
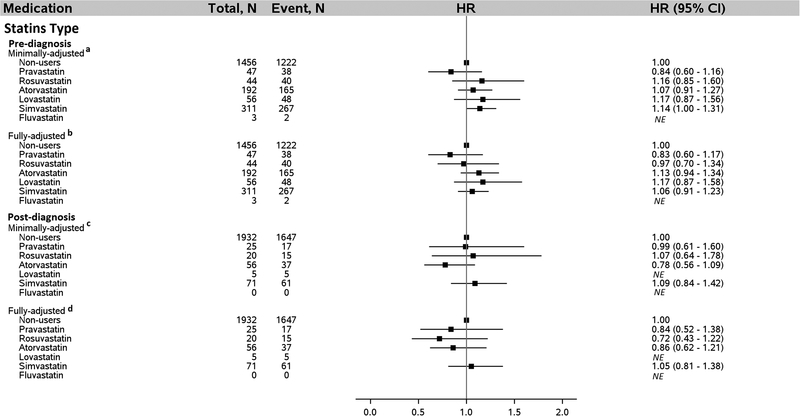
We evaluated additional details about statins and metformin use regarding functional form of the statins, statins potency, and average daily dose of metformin (Figure 4). In the fully-adjusted model that included adjustment for post-diagnosis metformin dose, pre-diagnosis average daily metformin dose ≤ 1,500 mg was associated with a 28% lower risk of death after HCC diagnosis (HR = 0.72, 95% CI: 0.58–0.91; ≤ 1,500 mg/day vs. non-users). By contrast, average pre-diagnosis metformin dose in excess of 1500 mg/day was not associated with survival after HCC diagnosis (HR = 0.98, 95% CI: 0.86–1.11; >1,500 mg/day vs. non-users), after adjusting for prost-diagnosis metformin dose and other covariates. Similarly, no association was found for either pre- or post-diagnosis statins form, statins potency, or post-diagnosis metformin dose (Figures 4 and 5). We further examined associations for individual statins type used either pre- or post-diagnosis of HCC and no association was found for any of the individual statins type (Figure 6).
Figure 4:
Association between statins form, statins potency, and metformin dose taken by patients with diabetes pre-diagnosis of HCC and risk of death after HCC diagnosis, N = 2499. a Adjusted for age (continuous), sex, and post-diagnosis statins form (lipophilic, hydrophilic, neither). Metformin only users were excluded from this analysis. b Adjusted for everything in “ a” plus year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, COPD, dyslipidemia, and hepatitis status as categorized in Table 1. Metformin only users were excluded from the analysis. c Adjusted for age, sex, and post-diagnosis statins potency (high potency, low potency, non-users). Metformin only users were not included in the analysis. d Adjusted for everything in “c” plus year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, COPD, dyslipidemia, and hepatitis as categorized in Table 1. Metformin only users were excluded from the analysis. e Adjusted for age (continuous), sex, and post-diagnosis metformin use dose (≤1,500 mg/d, >1,500 mg/d, non-users). Statins only users were excluded from this analysis. f Adjusted for everything in “e” plus year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, COPD, dyslipidemia, and hepatitis as categorized in Table 1. Statins only users were excluded from the analysis. Abbreviation: HCC, hepatocellular carcinoma; COPD, chronic obstructive pulmonary disease. Lipophilic statins: atorvastatin, fluvastatin, lovastatin, and simvastatin. Hydrophilic statins: pravastatin and rosuvastatin. High potency statins: atorvastatin, rosuvastatin, and simvastatin. Low potency statins: fluvastatin, lovastatin, and pravastatin.
Figure 5:
Association between statins form, statins potency, and metformin dose taken by patients with diabetes post-diagnosis of HCC and risk of death after HCC diagnosis, N = 2499. a Adjusted for age (continuous), sex, and pre-diagnosis statins form (lipophilic, hydrophilic, neither). a Adjusted for age (continuous), sex, and pre-diagnosis statins form (lipophilic, hydrophilic, neither). Metformin only users were excluded from this analysis. b Adjusted for everything in “ a” plus year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, COPD, dyslipidemia, and hepatitis status as categorized in Table 1. Metformin only users were excluded from the analysis. c Adjusted for age, sex, and pre-diagnosis statins potency (high potency, low potency, non-users). Metformin only users were not included in the analysis. d Adjusted for everything in “c” plus year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, COPD, dyslipidemia, and hepatitis as categorized in Table 1. Metformin only users were excluded from the analysis. e Adjusted for age (continuous), sex, and pre-diagnosis metformin use dose (≤1,500 mg/d, >1,500 mg/d, non-users). Statins only users were excluded from this analysis. f Adjusted for everything in “e” plus year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, COPD, dyslipidemia, and hepatitis as categorized in Table 1. Statins only users were excluded from the analysis. Abbreviation: HCC, hepatocellular carcinoma; COPD, chronic obstructive pulmonary disease. Lipophilic statins: atorvastatin, fluvastatin, lovastatin, and simvastatin. Hydrophilic statins: pravastatin and rosuvastatin. High potency statins: atorvastatin, rosuvastatin, and simvastatin. Low potency statins: fluvastatin, lovastatin, and pravastatin.
Figure 6:
Association between individual statins type used by diabetics both pre- and post-diagnosis of HCC and risk of death after diagnosis of HCC. a Adjusted for age (continuous), sex, and post-diagnosis statins type (pravastatin, rosuvastatin, atorvastatin, lovastatin, simvastatin, fluvastatin, and non-users). b Adjusted for everything in “a” plus year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, diabetes severity index, obesity, presence of COPD, dyslipidemia, and hepatitis as categorized in Table 1. c Adjusted for age, sex, and pre-diagnosis statins type (pravastatin, rosuvastatin, atorvastatin, lovastatin, simvastatin, fluvastatin, and non-users). d Adjusted for everything in “c” plus year of diagnosis, race, marital status, neighborhood income level, percent with 4-year college in neighborhood, tumor grade, tumor stage, chemotherapy, radiation, Charlson comorbidity index, obesity, COPD, dyslipidemia, diabetes, diabetic comorbidities index, and hepatitis status as categorized in Table 1. Abbreviation: HCC, hepatocellular carcinoma; NE, not estimated due to small numbers (less than 10 individuals in the group).
Discussion
In this population-based study of elderly patients with diabetes, we examined associations of independent and joint use of statins and metformin both pre- and post-diagnosis of HCC on overall survival. The results show that pre-diagnosis use of average daily metformin dose ≤ 1,500 mg is associated with a 28% lower risk of death after HCC diagnosis in T2DM patients. No associations was found for pre-diagnosis metformin dose >1500 mg/day or post-diagnosis metformin use, irrespective of dose. Pre- and post-diagnosis use of statins also was not associated with risk of death in patients with T2DM after HCC diagnosis. In summary, the study suggests that pre-diagnosis metformin dose ≤ 1,500 mg/day may improve survival of elderly diabetic HCC patients.
Metformin and statins are two of the most commonly prescribed medications among the elderly in the US13,14 and both have shown some promise for potential use as chemopreventive agents or adjuvant therapy for HCC.38,39 Metformin is the most widely used oral hypoglycemic agent for treatment of T2DM.13 In patients with T2DM, metformin improves insulin sensitivity, suppresses hepatic gluconeogenesis, and reduces glycogenolysis.40 Pre-existing T2DM is a known risk factor for HCC and associated with 2- to 4-fold increased risk for HCC.10,41–43 In a case-control study of diabetics, Donadon et al. reported an inverse association between metformin use and HCC risk.43 Nkontchou et al. also found inverse association between metformin use and risk for HCC in a prospective study of diabetics with HCV-induced cirhosis.44 However, the association between metformin use and survival after diagnosis of HCC in individuals with T2DM is not clear. Some26,27, but not all28, studies have reported an inverse association between metformin use and mortality after HCC diagnosis. None of the existing studies examined independent associations of pre- or post-diagnosis metformin use while accounting for the effect of the other. In the present study, we found that average daily metformin dose ≤ 1,500 mg/day taken before HCC diagnosis is associated with a 28% lower risk of death after diagnosis of HCC, adjusting for post-diagnosis metformin use, comorbidities, diabetes severity, tumor characteristics, and other relevant factors. However, pre-diagnosis metformin use in excess of 1500 mg/day and post-diagnosis metformin use were not associated with mortality after HCC diagnosis. From the perspective of cancer survival, metformin is known to inhibit cancer proliferation through induction of cell-cycle arrest and apoptosis45, promote of AMP-activated protein kinase signaling that result in inhibition of mammalian target of rapamycin (mTOR) pathway21,22, and attenuate metabolic abnormalities20, all of which could be mechanisms by which metformin may improve survival in HCC patients.
Many elderly patients with diabetes in the US take both metformin and statins for management of T2DM and hyperlipidemia.13,14 Statins, the HMG-CoA reductase inhibitors used primarily for hyperlipidemia, are thought to play a potential role in survival after HCC diagnosis.24 Experimental studies suggest that statins inhibit HCC progression by promoting G1/S cell cycle arrest and enhancing apoptosis.17 Other suggested mechanisms include inhibition of the P13K-AKT-mTOR pathway, which is important for cancer cell survival.18 It is worth noting that not all statins have hepatic-selectivity or enhanced suppression of HMG-CoA reductase. Lipophilic statins (i.e., atorvastatin, fluvastatin, lovastatin, and simvastatin) permeate both hepatic and extrahepatic cells with passive diffusion, while hydrophilic statins (i.e., pravastatin and rosuvastatin) selectively accumulate in the liver through a reaction mediated by transporters expressed in hepatocytes.24,35 The discriminatory accumulation of hydrophilic statins in the liver suggests that they may have some role in liver cancer risk and progression.24 In this context, pravastatin has been found to impede the progression and metastasis of established HCC.15 Few epidemiologic studies have examined the association between statins use and mortality after HCC diagnosis.24,25 Jeon et al. did not find an association between use of pravastatin and overall survival after diagnosis of stage I or II HCC in an observational study24, while in a clinical trial, Kawata et al. found that pravastatin use prolonged survival of patients with advance HCC.25 In the present study, overall statins use (hydrophilic and lipophilic combined) either pre- or post-diagnosis was not associated with survival after HCC diagnosis, neither was combined use of statins and metformin. No association was also found when statins were grouped into their functional forms (lipophilic vs. hydrophilic) or when individual statins were examined. Given the generally limited data on statins use and survival of HCC patients coupled with the inconclusive findings among existing studies, further studies, including from randomized controlled trials, are needed before firm conclusions can be drawn about the role of statins in overall survival of diabetic HCC patients.
Our study has several strengths and limitations. It is limited by the use of SEER-Medicare data that restricted the study population to elderly patients of age 65 years or older. Hence, the findings may not be applicable to younger patients. Information on medication use prior to age 65 years are not available in the SEER-Medicare data; this could have led to misclassification of medication use and may have biased the results to some extent. Moreover, the retrospective nature of the data precludes causal inferences about metformin exposure and mortality after HCC diagnosis. Because the Medicare claims data are designed for billing purposes, detail information on some of the study variables, such as radiation dose, chemotherapy type and dose, were not available. Thus, there is the possibility of residual confounding by these factors. Confounding by unmeasured factors, such as tobacco smoking history, extent of liver injury, and blood cholesterol levels cannot be ruled out. Severity of liver injury is often measured by circulating levels of aspartate aminotransferase (AST) and aminotransferase (ALT). Statins use is contraindicated in patients with extremely high ALT46; hence, patients with high ALT levels, for example, may not have been prescribed statins. The absence of information on circulating ALT and AST levels could have confounded the findings either toward or away from null. Additionally, low blood cholesterol level would lead to less frequent use of statins, which we were unable to assess or account for in the analysis. Despite these limitations, the data source provided comprehensive, population-based resource for investigating the impact of medication use on cancer outcomes and included detail patient information, such as comorbidities and indices of diabetes severity, which were accounted for in the analyses. Additional strengths include the ability to control for post-diagnosis medication use when examining association for pre-diagnosis use and vice versa. The large sample size and stringent HCC case definition, which excluded potential metastatic cases, add to the study strengths.
The findings of this study suggest that pre-diagnosis use of metformin dose ≤ 1,500 mg/day may improve overall survival of elderly diabetic HCC patients. Given the sparse data on associations between pre-diagnosis metformin dose and overall survival after HCC diagnosis, verifications are warranted in larger prospective studies with adequate representation of younger patients.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- SEER
the United States Surveillance, Epidemiology, and End Results registry
- T2DM
type 2 diabetes mellitus
- US
United States
- mTOR
mammalian target of rapamycin
Footnotes
Conflict of Interest: The authors do not have any conflict of interest related to this work
References
- 1.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clinics in liver disease. 2015;19(2):223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74(11):2913–2921. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology (Baltimore, Md). 2014;60(5):1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359(4):378–390. [DOI] [PubMed] [Google Scholar]
- 6.Personeni N, Pressiani T, Santoro A, Rimassa L. Regorafenib in hepatocellular carcinoma: latest evidence and clinical implications. Drugs in context. 2018;7:212533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melero I, Sangro B, Yau TC, et al. Nivolumab dose escalation and expansion in patients with advanced hepatocellular carcinoma (HCC): The CheckMate 040 study. Journal of Clinical Oncology. 2017;35(4_suppl):226–226.28056206 [Google Scholar]
- 8.Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nature medicine. 2006;12(1):75–80. [DOI] [PubMed] [Google Scholar]
- 9.Conway BN, Han X, Munro HM, et al. The obesity epidemic and rising diabetes incidence in a low-income racially diverse southern US cohort. PloS one. 2018;13(1):e0190993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4(3):369–380. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–468. [DOI] [PubMed] [Google Scholar]
- 12.Wang YG, Wang P, Wang B, Fu ZJ, Zhao WJ, Yan SL. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PloS one. 2014;9(5):e95485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldasouqi SA, Duick DS. Safety issues on metformin use. Diabetes care. 2003;26(12):3356–3357. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson TA. NLA Task Force on Statin Safety−-2014 update. Journal of clinical lipidology. 2014;8(3 Suppl):S1–4. [DOI] [PubMed] [Google Scholar]
- 15.Taras D, Blanc JF, Rullier A, et al. Pravastatin reduces lung metastasis of rat hepatocellular carcinoma via a coordinated decrease of MMP expression and activity. Journal of hepatology. 2007;46(1):69–76. [DOI] [PubMed] [Google Scholar]
- 16.DePeralta DK, Wei L, Ghoshal S, et al. Metformin prevents hepatocellular carcinoma development by suppressing hepatic progenitor cell activation in a rat model of cirrhosis. Cancer. 2016;122(8):1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sassano A, Platanias LC. Statins in tumor suppression. Cancer letters. 2008;260(1–2):11–19. [DOI] [PubMed] [Google Scholar]
- 18.Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nature reviews Clinical oncology. 2013;10(11):625–642. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara D, Tsubaki M, Takeda T, et al. Statins induce apoptosis through inhibition of Ras signaling pathways and enhancement of Bim and p27 expression in human hematopoietic tumor cells. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39(10):1010428317734947. [DOI] [PubMed] [Google Scholar]
- 20.Moghetti P, Castello R, Negri C, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. The Journal of clinical endocrinology and metabolism. 2000;85(1):139–146. [DOI] [PubMed] [Google Scholar]
- 21.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer research. 2007;67(22):10804–10812. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Xu W, Yan Z, et al. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. Journal of experimental & clinical cancer research : CR. 2018;37(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dufour M, Dormond-Meuwly A, Demartines N, Dormond O. Targeting the Mammalian Target of Rapamycin (mTOR) in Cancer Therapy: Lessons from Past and Future Perspectives. Cancers. 2011;3(2):2478–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon CY, Goodman MT, Cook-Wiens G, Sundaram V. Statin Use and Survival with Early-Stage Hepatocellular Carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(4):686–692. [DOI] [PubMed] [Google Scholar]
- 25.Kawata S, Yamasaki E, Nagase T, et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. British journal of cancer. 2001;84(7):886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. Journal of gastroenterology and hepatology. 2011;26(5):858–865. [DOI] [PubMed] [Google Scholar]
- 27.Seo YS, Kim YJ, Kim MS, et al. Association of Metformin Use With Cancer-Specific Mortality in Hepatocellular Carcinoma After Curative Resection: A Nationwide Population-Based Study. Medicine. 2016;95(17):e3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhat M, Chaiteerakij R, Harmsen WS, et al. Metformin does not improve survival in patients with hepatocellular carcinoma. World journal of gastroenterology. 2014;20(42):15750–15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown AS. Lipid management in patients with diabetes mellitus. The American journal of cardiology. 2005;96(4a):26e–32e. [DOI] [PubMed] [Google Scholar]
- 30.Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Statin use and survival in colorectal cancer: Results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer epidemiology. 2016;45:71–81. [DOI] [PubMed] [Google Scholar]
- 31.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical care. 2002;40(8 Suppl):Iv– 3–18. [DOI] [PubMed] [Google Scholar]
- 32.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 33.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. The American journal of managed care. 2008;14(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 34.E JY, Lu SE, Lin Y, et al. Differential and Joint Effects of Metformin and Statins on Overall Survival of Elderly Patients with Pancreatic Adenocarcinoma: A Large Population-Based Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(8):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends in pharmacological sciences. 1998;19(1):26–37. [DOI] [PubMed] [Google Scholar]
- 36.Jeon CY, Pandol SJ, Wu B, et al. The association of statin use after cancer diagnosis with survival in pancreatic cancer patients: a SEER-medicare analysis. PloS one. 2015;10(4):e0121783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleinbaum DG, Klein M. Survival analysis. Vol 3: Springer; 2010. [Google Scholar]
- 38.Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62(4):606–615. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi Y, Sakamoto Y, Ito D, et al. Statin use is associated with a reduced risk of hepatocellular carcinoma recurrence after initial liver resection. Bioscience trends. 2017;11(5):574–580. [DOI] [PubMed] [Google Scholar]
- 40.Nicholson G, Hall GM. Diabetes mellitus: new drugs for a new epidemic. British journal of anaesthesia. 2011;107(1):65–73. [DOI] [PubMed] [Google Scholar]
- 41.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54(4):533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan MM, Curley SA, Li D, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116(8):1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver international : official journal of the International Association for the Study of the Liver. 2010;30(5):750–758. [DOI] [PubMed] [Google Scholar]
- 44.Nkontchou G, Cosson E, Aout M, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. The Journal of clinical endocrinology and metabolism. 2011;96(8):2601–2608. [DOI] [PubMed] [Google Scholar]
- 45.Jang JH, Song IH, Sung EG, Lee TJ, Kim JY. Metformin-induced apoptosis facilitates degradation of the cellular caspase 8 (FLICE)-like inhibitory protein through a caspase-dependent pathway in human renal cell carcinoma A498 cells. Oncology letters. 2018;16(2):2030–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. The American journal of cardiology. 2006;97(8a):77c–81c. [DOI] [PubMed] [Google Scholar]