Abstract
Lactic acid bacteria (LAB) isolates possessed functional probiotic attributes, such as high hydrophobicity and autoaggregation ability, coaggregation capability with bacterial pathogens, antimicrobial activity, antioxidant potential, and hypocholesterolemic effects. Selected potential probiotic LAB, i.e. Lactobacillus paracasei M3, L. casei M5, L. paracasei M7, and few others were studied for their ability to lower cholesterol using a number of methods viz. cholesterol assimilation, bile salt deconjugation, cholesterol co-precipitation, cholesterol adhesion to probiotic cell wall, and miceller sequestration of cholesterol. L. casei M5 showed maximum bile salt hydrolase (BSH) activity, and released 57.63 nmol of glycine/min, and was closely followed by LAB isolate M9 which generated 52.12 nmol of glycine/min. Sodium glycocholate was deconjugated by L. casei M5 to produce 27.77 μmol/mL of cholic acid, while other isolates produced 20–26 μmol/mL of cholic acid. Cholesterol was assimilated significantly by isolate M6 (82.15%) and L. casei M5 (76.51%). L. casei M5 showed higher cholesterol co-precipitation ability (50.16 μg/mL) as compared to other LAB isolates (33–44 μg/mL). Miceller cholesterol concentration was reduced maximally by LAB isolate M8 (87.5%), followed by isolates M5 (84.75%), M9 (84%), M10 (80%), and M37 (79%). Higher cell wall adhesion of cholesterol was realized by L. casei M5 (42.48 μg/mL) than other LAB isolates (30–40 μg/mL). Selected LAB probiotics demonstrated short chain fatty acid (acetate, propionate, and butyrate) producing ability, yet another way of probiotics-mediated cholesterol lowering.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02183-8) contains supplementary material, which is available to authorized users.
Keywords: Probiotics, Hypocholesterolemia, Lactobacillus casei, Lactobacillus paracasei, Bile salt hydrolase
Introduction
Probiotics have gained considerable research attention in recent years due to their multifarious health-promoting benefits like immune system stimulation, anticancer, antidiabetic, anti-inflammatory, and anti-allergic properties, and alleviation of different types of diarrhea (Song et al. 2015; Andrabi et al. 2016; Bhat et al. 2017). As health benefits of probiotics are highly specific with respect to species and strains, and as such cannot be generalized (Park et al. 2018), bioprospecting for new/novel strains of efficacious probiotics has been a continuous practice (Bajaj et al. 2014). Probiotics have been isolated and characterized from a wide range of econiches (Andrabi et al. 2016; Bhat et al. 2017; Gupta and Bajaj 2017; Sharma et al. 2017). Lactic acid bacteria (LAB) together with Bifidobacteria are the most investigated probiotics (Ding et al. 2017).
Lactic acid bacteria (LAB) are Gram-positive, facultative anaerobic rods or cocci that are generally acid and salt-tolerant. They produce either lactic acid alone as the main product of fermentation (homofermentative), or produce lactic acid along with several other products (heterofermentative). LAB share common biochemical/metabolic and physiological attributes, and are ubiquitously associated with plants, animals, foods, decomposing biomaterials, soil, and water. Historically, LAB have been associated with production of several traditional fermented foods, and enjoy ‘generally recognized as safe’ (GRAS) status (Andrabi et al. 2016). LAB due to their ability to produce a wide range of metabolites may help enhancing organoleptic, textural, nutritional, sensorial, and technological properties of fermented foods (Chlebowska-Smigiel et al. 2017; Chlebowska-Śmigiel et al. 2019; Ruiz Rodriguez et al. 2019). Furthermore, LAB possess desired fermentation attributes, such as ability to survive over a wide pH range, tolerance to high salt concentrations, and resistance to drying/desiccation. Additionally, LAB can ferment a broad range of carbohydrates and other macromolecules present in food matrices, and may produce several bioactive metabolites viz., bacteriocins, lactic acid, extracellular enzymes, and exopolysaccharides (Bajaj et al. 2015; Bhat and Bajaj 2018) and non-digestible polysaccharides, such as pullulan, a low-calorie food for diabetics (Chlebowska-Smigiel et al. 2017). Thus, LAB due to their desired functional attributes may have application potential in various industries like food, pharmaceutical, dairy, and beverage (de Melo Pereira et al. 2019; Llamas-Arriba et al. 2019).
LAB have been explored widely as potential probiotics (Gupta and Bajaj 2017). Prospective probiotics must have ability to survive the harsh gastrointestinal tract (GIT) environment (acidic pH, high salt, and bile concentrations), and must colonize GIT in sufficient numbers. Probiotics are desired to have autoaggregation potential, coaggregation ability with pathogens, antimicrobial activity against pathogens, sensitivity to commonly used antibiotics, and ability to survive during fermentation processes (Bajaj et al. 2014; Owusu-Kwarteng et al. 2015; Ding et al. 2017). LAB probiotics have been isolated and characterized from various sources, such as fermented milk products (Gupta and Bajaj 2017), infant feces (Razdan et al. 2012), poultry gut (Andrabi et al. 2016), and a variety of other sources (Bhat and Bajaj 2018, 2019). Most of the probiotic strains are of Western origin which may not efficiently colonize the gut of Indian subjects due to different gut ecology/food habits of Indian populations (Kumar et al. 2012a).
Cholesterol lowering ability is an important functional attributes of probiotics (Ding et al. 2017). Probiotic LAB strains with high-cholesterol lowering potential have been isolated and characterized from various sources, but fewer reports are available on such strains from human breast milk (Sharma et al. 2017). The current study explored the cholesterol lowering characteristics of potential probiotic LAB isolates from human breast milk. High-serum cholesterol level is generally considered as a main risk factor for cardiovascular diseases (CVDs), obesity, coronary heart diseases, and other metabolic disorders (Park et al. 2018). CVDs and correlated complications are the leading causes of global deaths, and may account for high mortality by 2030 (Bhat and Bajaj 2019). Elevated serum cholesterol concentrations could be lowered by dietary intervention, behavior adjustment, regular physical exercise, and therapeutic approaches. Although effective cholesterol lowering drugs are available, they are known to cause adverse side effects, such as muscular pain and liver toxicity (Park et al. 2018). Therefore, non-pharmacological approaches, particularly application of probiotics, have gained attention for regulation of consistent cholesterol level rise, and thereby management of CVDs (Kumar et al. 2012a; Saikia et al. 2018).
Cholesterol lowering potential of probiotic strains may be manifested through several mechanisms viz., bile salt deconjugation ability and bile salt hydrolase (BSH) activity (Tsai et al. 2014), cholesterol assimilation ability (Kumar et al. 2011), co-precipitation of cholesterol with bile acids (Anandharaj et al. 2015), adhesion of cholesterol to probiotic cell wall (Choi and Chang 2015), miceller sequestration of cholesterol (Tsai et al. 2014), conversion of cholesterol to an insoluble compound coprostanol (Park et al. 2018), and short chain fatty acid-mediated cholesterol lowering (Kumar et al. 2012b). BSH activity is closely correlated with cholesterol lowering ability of probiotics, and can be explored as a functional probiotic biomarker for selection of hypocholesterolemic strains (Kumar et al. 2012a, b). Several probiotic species and strains, such as Lactobacillus, Bifidobacterium, Enterococcus, and Saccharomyces boulardii have been reported to possess BSH activity (Saikia et al. 2018).
The analysis of available research data shows that probiotic intervention could be a potent non-pharmacological approach to reduce cardiovascular risk factors by lowering the raised serum cholesterol level (Wang et al. 2018). Considering that probiotics of Western origin show poor colonization and adaptation in the gut of Indian subjects (Kumar et al. 2012a), there is an immense focus on targeting indigenous probiotics. Furthermore, human breast milk has mostly remained as an unexplored ecological niche for probiotic isolation. Therefore, the current study aimed to explore the functional probiotic attributes of LAB isolated indigenously from human breast milk. Selected LAB probiotic isolates were further investigated for their cholesterol lowering potential using multifarious methods.
Materials and methods
Chemicals/reagents, media/medium components, and lactic acid bacteria
All chemicals, reagents, media, and media components used were of analytical grade obtained from Sigma-Aldrich Chemicals Ltd, St. Louis MO, USA; HiMedia Laboratories Ltd, Mumbai, India; Qualigens Fine Chemicals Ltd, Mumbai, India; and Merck and Co. Inc., White House Station, NJ, USA.
Isolation of lactic acid bacteria, and their survival in simulated gastric juice (SGJ)
Lactic acid bacteria were isolated from human breast milk samples collected from Department of Pediatrics, Sri Maharaja Gulab Singh (SMGS) Hospital, Jammu, with proper consent from mothers. Samples were inoculated (1%, v/v) in De Man, Rogosa, and Sharpe (MRS) broth for enrichment, and incubated for 18 h under shaking (180 rpm). Enriched broth was spread plated on MRS agar, and the plates were incubated in an anaerobic chamber for 24–48 h. Pin head sized presumptive LAB colonies were selected, and pure cultured on MRS agar. Presumptive LAB isolates were subjected to morphological and biochemical tests (Andrabi et al. 2016). Stock of each culture was maintained in 20% glycerol at − 80 °C.
Survival of selected LAB isolates was examined in simulated gastric juice (SGJ) (Gupta and Bajaj 2017). LAB were grown under anaerobic conditions in MRS broth (pH 6.0–6.2) for 18 h at 37 °C (180 rpm). Cell pellet obtained by centrifugation at 5000×g for 10 min (Eppendorf 5804R) was washed twice with sterile saline, and again suspended in the same to obtain a uniform cell suspension (108–109 cells/mL). One mL of cell suspension was centrifuged (12,000×g , 5 min, 4 °C), and cell pellet was suspended in 1.0 mL of filter sterilized SGJ (pH 3.0), and incubated at 37 °C for 3 h. Cells were spread plated on MRS agar, and plates were incubated at 37 °C for 48 h. Survival of LAB in SGJ was determined by colony counting, and expressed as log cfu/mL.
Carbohydrate fermentation pattern of LAB isolates
LAB isolates (M2, M3, M5, M6, M7, M8, M9, M10, and M37) which survived successfully in SGJ were subjected to carbohydrate fermentation tests using Hicarbohydrate™ kit (HiMedia Laboratories Ltd, Mumbai). The tests were conducted by following the manufacturer’s protocol.
Hydrophobicity analysis of LAB isolates
Cell surface hydrophobicity assay for LAB isolates was performed on the basis of bacterial adhesion to solvents, such as chloroform, xylene, and ethylacetate. Freshly cultivated LAB were inoculated in MRS broth (1%, v/v), and grown under shaking (180 rpm) for 18 h at 37 °C. Cultural broth was centrifuged (7500×g, 10 min, 4 °C), and cell pellet was washed twice with phosphate buffered saline, PBS (1.54 mM KH2PO4, 0.1 mM NaCl, 2.71 mM Na2HPO4·7H2O, pH 7.4), and again suspended in PBS. Further, 1.0 mL of either of the solvents was added to 3.0 mL of cell suspension (108 cfu/mL), and the reaction contents were preincubated for 10 min at room temperature (25 °C), and then analyzed for initial absorbance (A0) at 580 nm. Subsequently, reaction contents were incubated for 30 min at 25 °C for phase separation. Aqueous layer was separated and examined for absorbance (A580). Cell surface hydrophobicity (%) was determined (Ramos et al. 2013) according to the following Eq. 1:
| 1 |
where Ai is final absorbance after incubation of 30 min, while A0 is initial absorbance of cell suspension.
Autoaggregation and coaggregation ability of LAB isolates
Autoaggregation (similar cell aggregates) analysis of LAB was performed as described by Andrabi et al. (2016). LAB were inoculated in MRS broth and grown under shaking (180 rpm) for 18 h at 37 °C. Cultural broth was centrifuged (5000×g, 10 min, 4 °C), and the cell pellet was washed twice with sterile PBS, and suspended again in PBS to obtain a uniform cell density (108–109 cells/mL). A 4.0 mL of LAB cell suspension was vortexed for 10 s and allowed to stand for different time intervals (1–5 h). After appropriate time intervals, 0.1 mL of upper layer of cell suspension was withdrawn, mixed with 3.9 mL of PBS, and examined for absorbance (A600). Auto-aggregation ability (AAg%) was determined using following Eq. 2:
| 2 |
where At is absorbance at different time intervals (At) = 1, 3, and 5 h, and A0 is absorbance at 0 h.
Coaggregation (aggregates of different cells) ability assay of LAB isolates was performed with various Gram-positive (Bacillus cereus PSI, Staphylococcus aureus, Micrococcus sp., Enterococcus faecalis, B. subtilis, Streptococcus pneumoniae), and Gram-negative pathogens (Escherichia coli, Campylobacter sp., Alcaligenes denitrificans, and Klebsiella sp.) as described previously (Andrabi et al. 2016). Coaggregation (%) was determined using the Eq. 3:
| 3 |
where Apath and Aprobio represent absorbance at 600 nm of individual pathogenic or probiotic bacterial suspension (control), and A(path+probio) represents absorbance of mixed bacterial suspension (pathogen and probiotic).
Antibiotic susceptibility analysis of LAB isolates
Antibiogram of LAB was evaluated towards eleven commonly used antibiotics, i.e. penicillin G, gentamycin, vancomycin, erythromycin, ampicillin, chloramphenicol, fusidic acid, clindamycin, kanamycin, and tetracycline. Standard disc diffusion assay was carried out using modified Kirby–Bauer method (Sharma et al. 2017). The results were depicted as susceptible (S) and resistant (R).
Antimicrobial activity of LAB isolates
For antibacterial activity assay (Andrabi et al. 2016), LAB were grown in MRS broth for 24 h at 37 °C. Cultural broth was centrifuged (5000×g for 10 min), and cell-free cultural supernatant (CFCS) of each LAB strain was collected, and filter sterilized through 0.45 µm filter (Millipore). CFCS (adjusted to pH 6.5) was used to determine the antimicrobial activity against various pathogenic bacteria. Pathogenic bacteria were cultivated in Luria–Bertani (LB) broth (18 h at 37 °C), and spread plated on Mueller–Hinton (MH) agar. Wells of 6.0 mm diameter were cut on MH agar plates, and 50 μL of LAB CFCS was poured into each well, and the plates were incubated for 24–48 h at 37 °C. Formation of zone of inhibition around the wells was suggestive of antimicrobial activity.
Extracellular enzymatic activity of LAB isolates
Selected LAB strains were evaluated for extracellular cellulase, xylanase, lipase, amylase, protease, and phytase activity (Gupta and Bajaj 2017). LAB cultures were grown for 24 h at 37 °C under shaking. Cultural broth was centrifuged, and CFCS was considered as crude extracellular enzyme preparation. Cellulase and xylanase activity was examined on carboxymethylcellulose and xylan agar plates, respectively. Lipase activity was detected on tributyrin agar plates, whereas amylolytic activity was examined using starch agar plates. Protease activity was studied using skimmed milk agar plates, whereas phytase activity was measured using sodium phytate agar.
Antioxidant activity of CFCS of LAB isolates
Antioxidant potential of CFCS of LAB was analyzed based on 2, 2-diphenyl-1-picrylhydrazyl (DPPH)-free radical scavenging ability. LAB cultures were inoculated in MRS broth and grown under shaking conditions (180 rpm) for 18 h at 37 °C (Kalisz et al. 2020). Cultural broth was centrifuged (5000×g, 10 min, 4 °C), and CFCS was used to analyze DPPH-free radical scavenging activity (Li et al. 2012) using following Eq. 4:
| 4 |
where Asample is absorbance of CFCS with DPPH, Ablank shows absorbance of reaction mixture where CFCS was replaced by equal volume of deionized water, and Acontrol is absorbance of reaction mixture where CFCS was replaced by equal volume of ascorbic acid (0.5%, w/v).
Similarly, hydroxyl radical scavenging assay of LAB CFCS was evaluated (Li et al. 2012), and measured by Eq. 5:
| 5 |
where Asample is absorbance of sample (experimental), Acontrol is absorbance of control in absence of sample, and Ablank is absorbance of reaction mixture where sample was replaced with deionized water.
Bile salt hydrolase (BSH) and deconjugation ability of LAB isolates
Potential probiotic LAB isolates were assessed for their BSH activity by qualitative plate assay (Kumar et al. 2011). Briefly, freshly prepared LAB cultures were spotted on MRS agar plates supplemented with bile salt taurodeoxycholic acid (0.5%, w/v) and CaCl2 (0.37 g/L). Plates were incubated under anaerobic conditions at 37 °C for 72 h. MRS agar plates without taurodeoxycholic acid were used as controls. Presence of precipitated bile acid around culture spots was indicative of BSH activity.
For assessing BSH activity of resting cells, exponentially grown LAB cultures were centrifuged at 10,000×g for 10 min at 4 °C. Cell pellet was washed thrice with peptone saline (pH 7.2) and suspended in sodium acetate buffer (0.1 M) to get cell count of 108–1010 cfu/mL (Kumar et al. 2011). Reaction mixture (200 µL) containing 100 µL of resting cell suspension and 100 µL of conjugated bile salt glycocholic acid (20 mM) was incubated at 37 °C for 30 min. Reaction was terminated by adding 200 µL of trichloroacetic acid (TCA) (15%, w/v), and content was centrifuged (10,000×g for 10 min), and the supernatant was examined for glycine content at 575 nm (UV–VIS 1800, Shimadzu, Japan) using a glycine standard curve. One unit of BSH activity (U/mL) was defined as the amount of enzyme that liberated 1 nmol of glycine from substrate per min.
For bile salt deconjugation ability assay, LAB were grown anaerobically at 37 °C for 24 h in modified-MRS broth supplemented with sodium glycocholate (6.0 mM). Cultures were centrifuged (10,000×g, 4 °C, 10 min), and CFCS was analyzed for released free cholic acid content (Anandharaj et al. 2015). Uninoculated MRS was used as a control. One mL of cultural supernatant (CFCS) was mixed with 3 mL of ethyl acetate. Reaction mixture was vortexed, and phase separation was allowed to take place at room temperature. Top layer (1.0 mL) of ethyl acetate was collected, transferred to clean test tube, and evaporated at 60 °C under nitrogen atmosphere. Then, 1.0 mL of NaOH (0.1 N) was added, followed by the addition of 2.0 mL of H2SO4 (16 N), and 1.0 mL of furfuraldehyde (1%). The reaction mixture was incubated at 65 °C for 10 min. After cooling to room temperature (25 °C), the reaction mixture was added with glacial acetic acid (5.0 mL), and analyzed for absorbance (A660) for estimation of cholic acid (µmol/mL) using a standard curve.
Cholesterol assimilation
Selected BSH-positive probiotic LAB isolates were examined for their ability to assimilate cholesterol. Freshly MRS-grown LAB culture was inoculated in modified-MRS (1%, v/v) that was supplemented with filter-sterilized cholesterol (100 µg/mL) and oxgall 0.3% (w/v). LAB culture was incubated anaerobically at 37 °C for 24 h at 180 rpm. Cultural broth was centrifuged (10,000×g, 4 °C for 10 min), and CFCS was examined for residual cholesterol concentration (Rudel and Morris 1973). To 1.0 mL of supernatant, 3.0 mL of ethanol (95%, v/v), and 2 mL of KOH (30%, w/v) were added, and reaction contents were incubated at 60 °C for 10 min after mixing. After cooling to room temperature, the reaction mixture was added with 3 mL of hexane, and allowed to stand for 15 min for phase separation. A 1.0 mL of top hexane layer was transferred to a clean test tube and evaporated at 60 °C under nitrogen atmosphere. Then, 2 mL of o-phthalaldehyde reagent (0.5 mg/mL in glacial acetic acid) and 2 mL of deionized water were added. Reaction contents were mixed well, and kept at room temperature for 10 min, followed by addition of 2 mL of concentrated sulphuric acid. The reaction mixture was examined spectrophotometrically (A560) after cooling to room temperature, and cholesterol assimilation was determined from cholesterol standard curve using Eq. 6:
| 6 |
where C1 and C2 represent concentration of total cholesterol in the uninoculated and inoculated medium, respectively.
For cholesterol co-precipitation assay by LAB isolates, modified-MRS was prepared afresh that either contain cholesterol at 100 µg/mL (experimental set 1) or cholesterol (100 µg/mL) and 6 mM sodium taurocholate (experimental set 2). Both modified-MRS sets (set 1 and 2) were inoculated with freshly grown cells of LAB at 1%, v/v (108–1010 cfu/mL), and incubated at 37 °C for 24 h. Control set contained uninoculated MRS supplemented with only cholesterol. LAB culture was centrifuged at 10,000×g, 4 °C for 10 min, and CFCS was examined for cholesterol concentration by spectrophotometric assay as described above (Rudel and Morris 1973). Co-precipitation of cholesterol with cholic acid released due to deconjugation of bile salt, was determined by calculating the difference between cholesterol level in experimental sets 1 and 2.
Cholesterol lowering potential by cell wall of LAB was studied (Choi and Chang 2015). For cell wall content preparation, 20 mL of exponentially grown cells were harvested by centrifugation (10,000×g, 4 °C), washed twice with PBS (pH 7.4), and suspended in 20 mL of PBS. Ice jacketed LAB cell suspensions were sonicated twice (Amp 60, 10 min, pulse 2 s, SANYO), and centrifuged (5000×g, 5 min, 4 °C). Cell pellet was re-suspended in 5% (w/v) sodium dodecylsulfate and incubated at 60 °C for 30 min. Crude cell wall fraction was centrifuged (10,000×g, 30 min, 4 °C), washed thrice with PBS, and suspended in 10 mL of Tris–HCl (10 mM, pH 7.5) containing 10 mM MgCl2. Cell wall fractions were subsequently incubated with an enzyme mixture that contained RNase A, DNase, and trypsin, each at 0.01 g/mL, for 6 h at 37 °C. Finally, cell wall fraction was centrifuged, and pellet was washed thrice with PBS, suspended in 20 mL of PBS and then homogenized twice by sonication (Amp 60, 10 min, pulse 2 s).
Cell wall fraction of LAB culture was used for examining its cholesterol lowering potential. Cell wall fraction at different concentrations (1.0 and 3.0 mg/mL) was suspended in MRS containing taurodeoxycholic acid (0.5%) and water soluble cholesterol (0.1 g/L), and the suspension was incubated at 37 °C for 24 h. Cell wall suspension was centrifuged at 10,000×g, 10 min, 4 °C, and the remaining cholesterol concentration in the supernatant was measured (Rudel and Morris 1973).
Ability of LAB isolates to utilize cholesterol from mixed micelles was determined (Tsai et al. 2014). Mixed micelles were prepared, and then sequestration of cholesterol from mixed micelles by LAB was assayed. Mixed micelles were prepared by sonication (130 W, 20 kHz, SANYO) of the MRS broth supplemented with 6.6 mmol/L taurocholic acid, 2.4 mmol/L lecithin, and 0.5 mmol/L cholesterol. The micellar dispersion was filtered through a 0.45 μm filter, and stored at 4 °C for 24 h. Freshly MRS-grown LAB culture was inoculated (1%, v/v) into micellar dispersion, and incubated at 37 °C for 18 h. Cholesterol concentration was measured as described above (Rudel and Morris 1973).
For analysis of short chain fatty acid (SCFA) production (Kumar and Dhillon 2015), LAB were grown anaerobically in MRS broth at 37 °C for 18 h and centrifuged to get cell-free cultural supernatant (CFCS). For SCFA analysis, a 10 mL of supernatant was acidified with 0.6 M HCl and added with 5 mL of ether. Reaction contents were mixed well, centrifuged (5 min at 2000×g), and top layer of ether containing SCFA was collected, and dried at 60 °C. Then, derivatizing agent N-tert-Butyldimethylsilyl-N-methyltrifluoroacetamide with 1% tert-butyldimethylchlorosilane was added, and the reaction mixture was heated at 70 °C for 4 h. Then, ethyl acetate was added, and the reaction content was analyzed by GC/MS-QP2010 Ultra system (Shimadzu, Japan).
Molecular identification of selected LAB
LAB with significant hypocholesterolemic potential were identified using cultural and microscopic analysis (Gupta and Bajaj 2017), and by 16S rDNA sequencing. For 16S rDNA sequence analysis, genomic DNA was extracted (HiPurA, Himedia kit), and PCR-amplified using universal 16S rDNA primers (forward primer 5′-AGRGTTTGATCCTGGCTCAG-3′, reverse primer 5′-CGGCTAC CTTGTTACGACTTT-3′). Amplified product was eluted (Axygen DNA gel extraction kit) and sequenced (SciGenom Labs Pvt. Ltd, Chennai, India). The DNA sequence data was analyzed to establish the closest homology of the LAB isolates using BLAST analysis. Phylogenetic tree was constructed using MEGA 7 (https://www.megasoftware.net) (Andrabi et al. 2016).
Statistical analysis
All the analytical experiments were executed in triplicates as three independent runs, and the results were expressed as mean ± SD. Statistical analysis was performed using IBM SPSS software version 25. The level of statistical significance was estimated using either Student’s t test or analysis of variance where ever appropriate. The p value (< 0.05) was used to statistically validate the data. Differences in values were considered significant when the p value was < 0.05.
Results and discussion
Isolation of presumptive probiotic lactic acid bacteria (LAB) and their survival in simulated gastric juice
Due to wide variations among probiotic lactic acid bacteria with respect to their health benefitting attributes, and fermentation/technological characteristics, quest for isolation, and characterization of new/novel strains of LAB from exotic habitats has been a vital research area (Chlebowska-Smigiel et al. 2017; Ruiz Rodriguez et al. 2019). In the current study, LAB isolates from human breast milk samples were first screened for their ability to withstand the GIT environment (simulated gastric juice, SGJ), and then selected ones were studied for functional probiotic attributes including hypocholesterolemic effects. Survival in GIT environment is the foremost requirement for a probiotic (Andrabi et al. 2016). Adverse GIT conditions, such as oxidative and osmotic stress, and low pH have detrimental effect on survival of probiotics. LAB isolates survived under SGJ conditions to varying levels (Supplementary Fig. 1). The LAB isolate M5 displayed highest survival rate (98.82%), and was followed by isolates M2, M3, M8, and M37 (survival rate 90–97%), and M6, M7, and M10 (survival rate 80–87%). Similarly, a high survival rate of 90% was reported for novel LAB isolates, i.e. Lactobacillus plantarum ZF06-1, ZF06-3, IF13, and L. acidophilus 05–172 (Zeng et al. 2016). LAB isolates from a variety of ecological niches were reported to have high survival (70–90%) under GIT conditions (Gupta and Bajaj 2017). However, Lactobacillus plantarum, L. sakei, and L. plantarum LGG showed low survival (2.6–19.38%) in simulated stomach and duodenal conditions (Park et al. 2016).
Survival of LAB in harsh gut environment is a strain specific phenomenon that depends upon several factors including H+-ATPase activity. Multi-subunit FoF1-ATPase proton pump utilized by Gram-positive bacteria plays a crucial role in squeezing of protons from cytoplasm by proton motive force (Zeng et al. 2016). Nevertheless, survival under gastrointestinal conditions is the key requirement for any potential probiotic for their successful application.
Carbohydrate fermentation profile
Potential capability of LAB to ferment a wide variety of carbohydrates (carbohydrate fermentation pattern) can be used not only for differentiating among various bacterial species/strains, but to assess their metabolic diversity (Oguntoyinbo and Narbad 2015). Versatile biochemical potential of LAB helps them to endure well under a variety of ecological niches including various food matrices, as they can utilize varied substrates as sources of carbon and energy. In the current study, LAB isolates M2, M3, M5, M6, M8, M9, and M37 successfully utilized all the carbon sources examined. LAB isolate M7 did not ferment adonitol, arabitol, and erythritol, while the isolate M10 could not ferment maltose and fructose. Gupta and Bajaj (2017) reported that LAB isolates from dairy-based fermented products had ability to utilize a wide range of carbohydrates; however, a few could not utilize inositol, esculin, citrate, and malonate. Similarly, Lactobacillus plantarum subsp. argentoratensis DSM16365 and L. plantarum subsp. plantarum DSM20174 could not ferment L-arabinose, and strains ULAG11, DSM16365, and DSM20174 did not ferment L-rhamnose; whereas, L. plantarum ULAG24 fermented L-arabinose, L-rhamnose, D-turanose, and D-arabitol sugars (Oguntoyinbo and Narbad 2015). A wide variation among different probiotic LAB isolates with respect to their preferences for carbohydrates may be attributed to the diversity in their metabolic potential, and differential expression of putative genes involved in the uptake and utilization of particular carbohydrates in their respective environments (Koryszewska-Bagińska et al. 2019).
Hydrophobicity test for LAB isolates
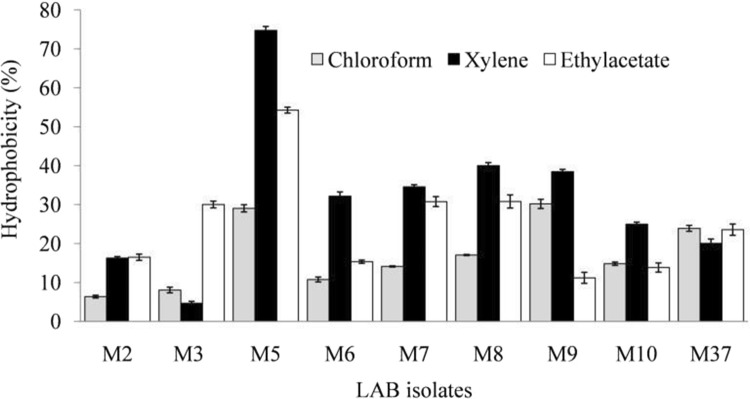
Cell surface hydrophobicity indicates the capability of probiotics to adhere to host–gut epithelium (Gupta and Bajaj 2017). Thus, high hydrophobicity shows adequate adhesion potential of probiotics. LAB isolates M9 and M5 showed high hydrophobicity of 30.17%, and 29.04%, respectively, towards chloroform (Fig. 1). Moderate to low adhesion towards chloroform specifies the hydrophobic nature of bacterial cell surface. Singh et al. (2016) have also demonstrated hydrophobicity level of 25.30–49.37% by several strains of Lactobacillus reuteri towards chloroform. LAB isolate M5 exhibited the highest hydrophobicity potential towards xylene (74.73%) and ethylacetate (54.26%). However, other LAB isolates showed low to moderate hydrophobicity. Lactobacillus kefiranofaciens XL10, an isolate from Tibetan kefir grains has been reported to exhibit 79.9% hydrophobicity towards xylene (Xing et al. 2017).
Fig. 1.
Hydrophobicity of lactic acid bacteria (LAB) with different organic solvents after 5 h of incubation at 37 °C
Adhesion potential of LAB probiotic strains depends upon their cell surface properties such as presence of various cell surface extracellular proteins and carbohydrates. Widely diverse hydrophobicity level of different LAB strains indicates that the adhesion potential of LAB might be a strain-specific property. Gupta and Bajaj (2017) reported a varied level of hydrophobicity of LAB isolates from fermented milk products towards different solvents. Furthermore, cell-surface hydrophobicity potential of probiotics may also contribute towards their cell aggregation ability (Koryszewska-Bagińska et al. 2019). Cell aggregates due to their increased density are likely to spend more time in GIT, and thereby, imparting more health benefits to the host. Hydrophobic nature of human cell surface promotes the binding of hydrophobic bacteria to GIT epithelial cells (Koryszewska-Bagińska et al. 2019). Thus, surface characteristics of probiotic LAB and gut mucosal surfaces determine the hydrophobicity potential of a probiotic.
Autoaggregation and coaggregation assay for LAB
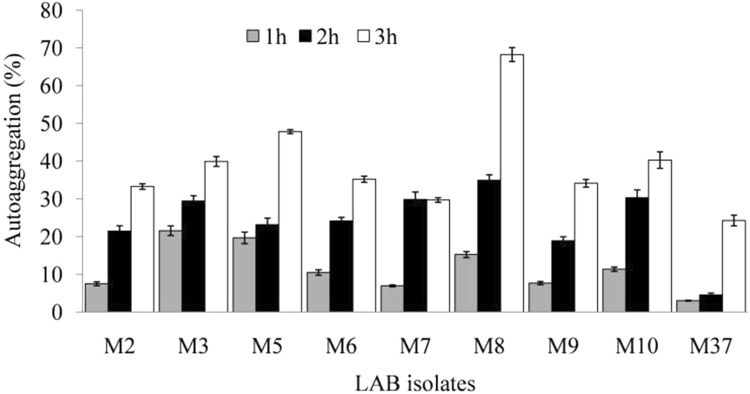
Autoaggregation shows interaction among microorganisms that are genetically equal (Sahoo et al. 2015). In this study, LAB isolates showed a varied level of autoaggregation and generally it increased with increase of incubation time (Fig. 2). An autoaggregation of 68.24 and 47.81%, respectively, was observed for LAB isolates M8 and M5, after 5 h of incubation. Similar results have been reported wherein autoaggregation ability of 62% was observed for LAB isolates from gut of Labeo rohita and Catla catla, after 6 h of incubation (Sahoo et al. 2015) Similarly, Lactobacillus plantarum SAU96 and L. fermentum CH58 showed autoaggregation of 61.9 and 55.1%, respectively (Ramos et al. 2013). Studies suggest a direct relationship between time period of incubation and autoaggregation, and the latter helps probiotics to extend their time of stay within GIT, thereby, positively influencing the hosts’ gastrointestinal microflora (Gajbhiye et al. 2015).
Fig. 2.
Autoaggregation potential of lactic acid bacterial (LAB) isolates after different time intervals of incubation at 37 °C
Adhesion potential of genetically distinct bacteria is determined by coaggregation assay. Current study examined the LAB isolates for their coaggregation ability with six Gram-positive pathogens (Supplementary Fig. 2a). LAB isolate M2 displayed highest coaggregation potential of 76.63% with Staphylococcus aureus, while other LAB isolates showed a varied coaggregation potential (37–59%). LAB isolate M3 showed coaggregation ability of 65.51% against Bacillus cereus, while the isolates M7 and M5 displayed coaggregation of 57.62 and 56.25%, respectively, with Micrococcus sp. Isolate M2 exhibited a high coaggregation potential of 76.92% with bacterial pathogen Streptococcus pneumoniae, while most of other LAB isolates showed coaggregation potential of 40–50% against this pathogen. Coaggregation ability of LAB was also studied with Gram-negative bacterial pathogens (Supplementary Fig. 2b). LAB isolates M10 and M5 exhibited a strong coaggregation potential of 63.06 and 61.19%, respectively, with Escherichia coli, while other LAB isolates manifested a relatively low degree of coaggregation (45–58%). Similarly, LAB isolate M6 displayed coaggregation potential of 63.63% against Klebsiella sp. LAB isolate M7 exhibited coaggregation of 59.18% with Alcaligenes denitrificans. LAB isolates M6, M37, and M9 exhibited almost similar coaggregation ability of 55.65, 55.96, and 55.45%, respectively, with Campylobacter sp.
Coaggregation potential of LAB probiotics has been studied by different researchers. A high coaggregation rate of 87–90% has been reported for several LAB isolates from different sources, with various pathogens like Staphylococcus aureus P07, Campylobacter sp. J1 and Micrococcus sp. SP1 (Andrabi et al. 2016). Similarly, substantial coaggregation potential (74–95%) was reported for probiotic LAB isolates with different pathogens (Gupta and Bajaj 2017). The current study interprets that coaggregation potential is a strain-dependent phenomenon, and is a function of surface properties of probiotics and pathogens. Variations in coaggregation potential may be due to differences in the surface bound protein and carbohydrate moieties. Aggregation, coaggregation, and adhesiveness are generally related properties, and are determined through cell surface proteins, as removal of such surface proteins leads to alteration in aggregation and adhesiveness (Kumar et al. 2020).
Antibiotic susceptibility of LAB isolates
Antibiotic or drug resistance in probiotics is a grave concern as it may lead to horizontal gene transfer from probiotics to gut microbiota (commensals or pathogens), and subsequent emergence of antibiotic resistance amongst deadly bacterial pathogens (Sharma et al. 2017). Thus, it must be ensured that probiotics do not carry antibiotic resistance (antibiotic resistance genes). Antibiotic susceptibility analysis showed that most of the LAB isolates were susceptible to commonly used antibiotics, such as ampicillin, clindamycin, tetracycline, penicillin G, erythromycin, and gentamycin. Furthermore, all LAB strains except M7 were sensitive to erythromycin. However, LAB isolates M2 and M3 displayed resistance towards fusidic acid and vancomycin. LAB isolate M7 showed resistance towards erythromycin and kanamycin, and isolate LAB M10 did so against penicillin G and kanamycin, while the isolate M37 exhibited resistance to vancomycin.
Similarly, several species of Lactobacillus and Enterococcus isolated from the aerial surfaces of pomegranate, were reported to be sensitive to erythromycin, however, a few were resistant to fusidic acid (Gajbhiye 2016). LAB isolates from Indonesian kefir grains were reported to exhibit susceptibility to a wide range of antibiotics (Yusuf et al. 2020). Thus, antibiotic resistance analysis of probiotics is an essential prerequisite prior to their commercial application. LAB have been reported to possess natural (intrinsic) resistance or acquired resistance to chloramphenicol, gentamicin, ampicillin, erythromycin, and tetracycline which is usually non-transferable, and poses no risk for probiotic usage (Jose et al. 2015). Variations in antibiotic sensitivity pattern among LAB probiotic isolates may be attributed to differences in their genetic makeup, and a varied level of antibiotic exposure of LAB in their natural habitats.
Extracellular enzymatic activity of LAB isolates
Extracellular enzymes produced by LAB probiotics may help in digestion of food by the host, and contribute towards energy generation (Andrabi et al. 2016). LAB isolates produced extracellular enzymes such as lipase, protease and amylase; however, no isolate showed xylanase, cellulase, and phytase activity. Similarly, LAB isolates (SI-1, SI-3, R-3, PF-1, and PF-2) from different sources demonstrated a good lipase activity, and varying level of phytase activity, but none showed extracellular protease or amylase producing ability (Andrabi et al. 2016). However, LAB isolates from Catla catla gut were reported to produce a substantial amount of several extracellular enzymes including protease, xylanase, amylase, lipase, cellulase, and phytase (Mukherjee and Ghosh 2016). Extracellular enzyme producing potential of putative probiotics might contribute to catalytic digestion of macromolecules present in foods, and may also enhance the organoleptic properties of fermented foods. Additionally, extracellular enzyme producing probiotics might help in utilizing the wide range of nutrients present in food or feed matrices.
Antioxidant potential of LAB isolates
Antioxidant activities of probiotic LAB could beneficially influence health status of consumers. LAB probiotics may provide natural and dietary source of antioxidants which can prevent the oxidative stress related damages (Li et al. 2012). In case of cellular pro- and antioxidant imbalance, i.e. weakening of enzymatic antioxidative mechanisms, and depletion of low-molecular non-enzymatic antioxidant resources, LDL lipoproteins may act as antioxidants, and protect the integrity of cell membranes (Brites et al. 2017). In the current study, cell-free cultural supernatant (CFCS) of LAB M3 and M7 showed the highest DPPH-free radical scavenging activity of 59.82 and 49.72%, respectively (Fig. 3). Free radical scavenging activity is generally examined in CFCS, but may also be associated with cells as well. Similarly, Lactobacillus plantarum C88 exhibited DPPH-free radical scavenging activity of 53.05% at 109–1010 cfu/mL of CFCS (Li et al. 2012).
Fig. 3.
Antioxidant activity of lactic acid bacterial (LAB) isolates against DPPH, and hydroxyl-free radical scavenging activity
Hydroxyl radicals are harmful reactive oxygen species (ROS) that cause oxidative damage to biomolecules, therefore, their neutralization with antioxidants is very significant. LAB isolate M5 displayed highest hydroxyl radical scavenging activity of 30.48% (Fig. 3), followed by isolates M7 (19.94%), M10 (15.66%), and M6 (15.24%). Examination of LAB isolates from Cyprinus carpio gut revealed that Lactobacillus reuteri P16 possessed high antioxidant capacity (Giri et al. 2019). However, high-hydroxyl-free radical scavenging activity was shown by cells of Lactobacillus strain P22 (37.87%), and its cell-free cultural supernatant (32.14%). Furthermore, high lead tolerance of LAB isolates indicated their suitability for protection of lead-induced toxicities in aquaculture resources (Giri et al. 2019). Thus, antioxidant potential of probiotic bacteria may provide protection against oxidative damage caused by harmful ROS generated within the host due to stress, and/or multiple diseases or disorders. Probiotic LAB isolates owing to their considerable antioxidant potential could be exploited as a natural and safe source of antioxidants in contrast to the synthetic antioxidants.
Cholesterol lowering studies of LAB isolates
LAB isolates that possessed desired functional characteristics were evaluated for their hypocholesterolemic potential by several methods. Bile salt hydrolase (BSH) activity can be used as an important marker for selection of novel probiotics for functional attributes like hypocholesterolemic activity and colonization potential (Kumar et al. 2012a). BSH helps probiotics to resist bile salt during GIT transit. Several probiotic LAB isolates exhibited adequate BSH activity as examined by plate assay (Supplementary Fig. 3). Similar to the current study, Lactobacillus plantarum EM and L. sakei DC1 have also shown adequate BSH activity (Choi et al. 2015). In hosts’ gut, probiotic bacteria due to their BSH activity may help countering the antimicrobial action of the bile salt. Also, the amino acids released during BSH-mediated deconjugation of bile salts may be utilized by probiotics as the source of carbon and nitrogen (Kumar et al. 2012a; Choi et al. 2015).
LAB probiotic isolates M5 and M9 exhibited high BSH activity, and released 57.63 and 52.12 nmol/mL of glycine per min. However, LAB isolate M2 exhibited relatively low BSH activity, and produced 22.18 nmol/mL of glycine per min (Table 1). In contrast, Lactobacillus plantarum Lp91 and Lp21 have been reported to possess significantly high BSH activity, and produced 99.29 and 88.63 nmol/mL glycine per min, respectively (Kumar et al. 2011). Furthermore, it has been observed that LAB probiotics show higher preference for glycocholate as substrate than other conjugated bile salts, and this might be due to glycocholate predominance in human body (Kumar et al. 2012a). BSH activity causes deconjugation of bile salts, and resulting free bile acids are excreted via feces. To makeup this bile salt loss, dietary cholesterol is utilized for de novo synthesis of bile salts thus, deferring the cholesterol rise (Tsai et al. 2014). Therefore, application of probiotics with high BSH activity may be a vital strategy for managing serum cholesterol levels.
Table 1.
Determination of BSH activity and bile salt deconjugation ability of probiotic bacteria
| Source | Probiotic LAB isolates | Amount of glycine releaseda (nmol/mL per min) | Amount of cholic acidb released (µmol/mL) |
|---|---|---|---|
| Human milk isolates | M2 | 22.18 ± 0.017 | 24.35 ± 0.009 |
| M3 | 34.44 ± 0.011 | 24.10 ± 0.011 | |
| M5 | 57.63 ± 0.054 | 27.77 ± 0.012 | |
| M6 | 22.92 ± 0.023 | 21.07 ± 0.016 | |
| M7 | 24.80 ± 0.008 | 20.83 ± 0.015 | |
| M8 | 25.16 ± 0.047 | 26.27 ± 0.011 | |
| M9 | 52.12 ± 0.010 | 22.06 ± 0.009 | |
| M10 | 34.64 ± 0.008 | 22.52 ± 0.058 | |
| M37 | 25.19 ± 0.035 | 15.68 ± 0.015 |
aOne unit of bile salt hydrolase activity has been defined as the liberation of one nmol of glycine released from substrate per min (Absorbance600)
bCholic acid (µmol/mL) released by deconjugation of sodium glycocholate by BSH activity (Absorbance660)
Deconjugation of bile salt sodium glycocholate was examined for studying cholesterol lowering effects of LAB probiotics. BSH hydrolyses N-acyl amide bond (C-24) of the of glycine or taurine-conjugated bile salts, and the resultant deconjugated bile acids due to their poor solubility are eliminated with feces (Reis et al. 2017). Several probiotic LAB isolates deconjugated sodium glycocholate, and released cholic acid (15.68–27.77 µmol/mL). LAB isolate M5 and M8 showed considerably high-bile salt deconjugation ability, and released 27.77, and 26.27 µmol/mL of cholic acid, respectively. However, reasonably good amount of cholic acid was released by isolates M2 (24.35 µmol/mL) and M3 (24.10) as well (Table 1). Similarly, Lactobacillus and Weissella strains isolated from traditionally fermented south Indian koozh and gherkin exhibited good bile salt deconjugation ability. Weissella strains FKI13 and FKI29, deconjugated both sodium glycocholate and sodium taurocholate, but deconjugation potential for sodium glycocholate was higher. In contrast, however, Lactobacillus strain GI6 and GI15 deconjugated only sodium taurocholate. W. koreensis FKI21 released 1.94 and 0.86 mM of cholic acid upon deconjugation of sodium glycocholate and sodium taurocholate, respectively (Anandharaj et al. 2015).
Among several LAB isolates from corn silage, Lactobacillus plantarum CAAS 18,008 exhibited the highest bile salt deconjugation ability, and substantially lowered the serum LDL cholesterol, total cholesterol, and hepatic total cholesterol (Ma et al. 2019). Hypocholesterolemic potential is attributed to high-bile salt hydrolase activity, which increases daily excretion of fecal bile acid, and thereby, accelerates new bile acid synthesis from cholesterol in the liver (Ma et al. 2019). de novo synthesis of bile salts from cholesterol mainly contributes to cholesterol lowering (Kumar et al. 2012a; Tsai et al. 2014; Ding et al. 2017; Ma et al. 2019). Thus, LAB probiotics with considerable deconjugation ability might play a crucial role in serum cholesterol lowering.
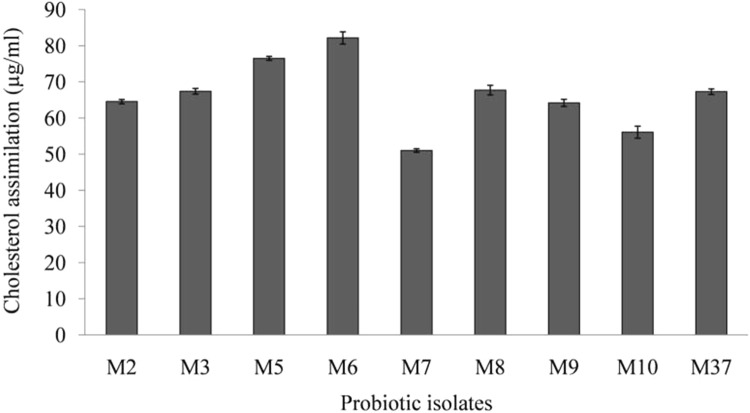
Cholesterol assimilation capability of probiotics is another important mechanism for lowering the raised cholesterol level (Kumar et al. 2012a; Ding et al. 2017). Analysis of cholesterol assimilation capabilities of LAB probiotic isolates showed that different isolates assimilated cholesterol to a varying extent (Fig. 4). The highest cholesterol assimilation was shown by LAB isolate M6 (82.15%), and it was followed by isolates M5 (76.51%), M8 (67.7%), M3 (67.4%) and M37 (67.2%). LAB isolate M7 exhibited lowest cholesterol assimilation of 51.038% (Fig. 4). Lactobacillus plantarum Lp3, an isolate from traditionally fermented Tibetan yak milk has been reported to realize cholesterol assimilation by 73.3% under in vitro conditions (Ding et al. 2017). Cholesterol lowering ability of L. plantarum Lp3 has been further established (in vivo) using rats fed with a high cholesterol diet. Administration of L. plantarum Lp3 to rats fed with a high cholesterol diet has shown considerable reduction of cholesterol, triglycerides, and lipid deposition in cytoplasm of rat’s liver tissue (Ding et al. 2017). In another study, several probiotic Lactobacillus spp. have been reported to lower cholesterol by more than 50% (Song et al. 2015).
Fig. 4.
Cholesterol assimilation potential of lactic acid bacterial (LAB) isolates
Cholesterol assimilation by probiotics helps minimizing the available cholesterol for absorption by enterocytes. Furthermore, incorporation of conjugated linoleic acid and vaccenic acid into membrane may not only favorably modify its fluidity and permeability, but also contribute towards regulation of lipid metabolism by reducing the concentration of total cholesterol, LDL cholesterol and triglycerides (Subbaiah et al. 2011). Probiotics assimilate cholesterol, and make it unavailable for absorption by enterocytes, thus, reducing overall cholesterol level. Such cholesterol assimilating probiotics indicate biotherapeutic potential for management of raised cholesterol level, and hence for combating CVD risks.
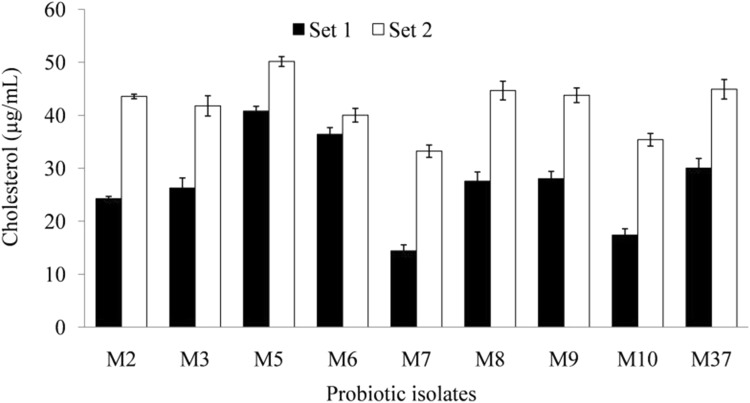
Coprecipitation of cholesterol with free bile acids released from deconjugated bile salt, represents another crucial mechanism of cholesterol lowering by probiotics. BSH causes deconjugation of bile salt, and released cholic acid lowers down medium pH (< 6.0) which in turn leads to co-precipitation of cholesterol with cholic acid at acidic pH (Kumar et al. 2012a). Probiotic LAB isolates showed a varied level of cholesterol co-precipitation ability (33.32–50.16 μg/mL) with deconjugated sodium glycocholate (Fig. 5). LAB probiotic isolate M5 exhibited high level of cholesterol co-precipitation (50.16 μg/mL), followed by isolate M37 (44.92 μg/mL). Isolates M7 and M10 showed a moderate or low level of cholesterol co-precipitation of 33.23 μg/mL and 35.39 μg/mL, respectively, as shown in Fig. 5. Shobharani and Halami (2016) studied three potential probiotic Bacillus spp. (B. flexus MCC 2458, B. flexus MCC 2427, and B. licheniformis MCC 2514) for their cholesterol removing ability by co-precipitation of cholesterol under acidic pH in the presence of ox-bile and conjugated bile acids. It revealed significant cholesterol lowering in the presence of bile salt (27.57–31.22%) than its absence (18.48–19.68%). The above results suggest that co-precipitation of cholesterol with bile acids produced due to BSH-mediated deconjugation of bile salts is an important means of cholesterol lowering by probiotics.
Fig. 5.
Co-precipitation of cholesterol with deconjugated bile acids by different lactic acid bacterial (LAB) isolates
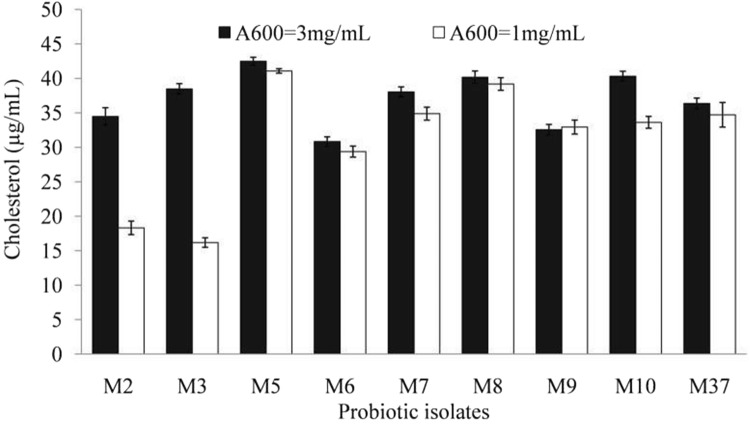
Cholesterol adsorption by cell wall of probiotics represents still another key mechanism for lowering down the elevated cholesterol level. Cell wall fractions adsorb cholesterol to their surface structures. Cell wall fractions of LAB probiotic isolates were prepared, and examined for their cholesterol lowering ability (Fig. 6). The cell wall fraction of LAB probiotic isolates removed the cholesterol in a concentration-dependent manner. Cell wall fractions (1.0 mg/mL) of LAB isolate M5 removed the cholesterol maximally (41.07%), and was followed by that of LAB isolates M8 (39.18%), M7 (34.87%) and M37 (34.71%). However, cell wall fraction of LAB isolate M3 removed cholesterol content of medium by 16.17% only. Further, cholesterol removal increased with increase in concentration of cell wall fraction (3.0 mg/mL). Cell wall fraction of LAB isolates M5, M10, and M8 removed cholesterol by 42.48, 40.32, and 40.17%, respectively, at concentration of 3.0 mg/mL (Fig. 6). Thus, cell wall of probiotics also contributes significantly to cholesterol lowering potential of probiotics. Emami and Bazargani (2014) reported significant cholesterol lowering ability (5.6 and 4.5 mg/mL, respectively) by cell wall fraction of Lactobacillus acidophilus and L. lactis, isolated from human gastrointestinal tract. In alignment with the present study, cell wall fraction of Lactobacillus plantarum EM and L. acidophilus ATCC 43,121 isolated from kimchi, removed cholesterol in a concentration-dependent manner (Choi and Chang 2015).
Fig. 6.
Cholesterol removal by probiotic cell wall fraction of lactic acid bacterial (LAB) isolates
Probiotic LAB isolates from Indonesian kefir grains have also exhibited varied levels (22.08–68.75%) of cholesterol-lowering effects (Yusuf et al. 2020). Lactobacillus kefiri JK17 reduced cholesterol maximally. Analysis of cholesterol lowering by live, resting, and dead cells of LAB showed that higher cholesterol lowering was realized by live cells than resting or dead cells. Thus, cholesterol lowering is a function of metabolic activity of probiotic organisms (Yusuf et al. 2020). The results of the current study also endorse that cholesterol removal can be accomplished even by the cell wall fraction, i.e. without the aid of any metabolic or biochemical process. Therefore, such probiotics may find application as potent hypocholesterolemic agents.
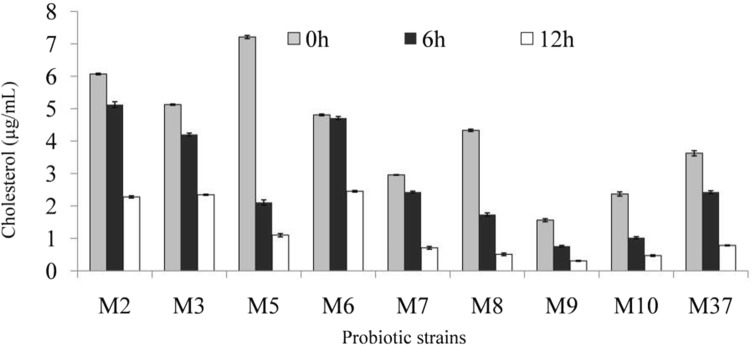
Micelles, formed of bile salts, cholesterol, and phospholipids, assist in absorption of cholesterol in intestine. Probiotics deconjugate bile salt, and thus make it unavailable for formation of micelles, and this in turn results in non-absorption of cholesterol in intestine (Kumar et al. 2012a). In addition, probiotics may influence micellar solubility, and cause sequestration of cholesterol from mixed micelles. Different LAB probiotic isolates showed a varied ability to sequester cholesterol from mixed micelles (Fig. 7). Micellar cholesterol concentrations were reduced maximally by LAB isolate M8 (87.5%), followed by isolates M5 (84.75%), M9 (84%), M10 (80%), and M37 (79%).
Fig. 7.
Lowering of micellar cholesterol concentration by different lactic acid bacterial (LAB) isolates after 12 h of incubation
LAB probiotic isolates from various plants and animal sources have been reported to exhibit good cholesterol lowering effects through BSH activity, bile acid deconjugation ability, and micellar sequestration of cholesterol (Tsai et al. 2014). LAB isolates Pediococcus acidilactici NBHK002, Bifidobacterium adolescentis NBHK006, Lactobacillus rhamnosus NBHK007, and L. acidophilus NBHK008 exhibited relatively high deconjugation ability, and reduced the miceller cholesterol concentration within 24 h. LAB probiotic isolates decreased total concentration of cholesterol, low-density lipoprotein, triglycerides, and thiobarbituric acid in serum or livers of hamsters with hypercholesterolemia (Tsai et al. 2014). Probiotic LAB isolates (from kimchi, dairy products, fermented olive and infant fecal matter), viz., Lactobacillus sp. JNU 8829, L. acidophilus KU41, L. acidophilus M23, L. fermentum NS2, L. plantarum M13, and L. plantarum NS3, were capable of reducing cholesterol level by > 50% under in vitro conditions (Song et al. 2015). Other isolates Lactobacillus spp. like L. sakei MA9, and CH decreased cholesterol level by 30% or less. Cholesterol lowering was attributed to in vitro inhibition of cholesterol micelles formation (Song et al. 2015). Thus, cholesterol lowering by probiotics through miceller means is executed in a dual way, i.e. by preventing micelles formation, and by sequestering cholesterol from mixed micelles (Kumar et al. 2012a).
Short chain fatty acid (SCFA) producing ability of probiotics has been reported to contribute towards cholesterol-lowering potential as specified by the relationship between SCFA producing ability and hypocholesterolemic effects of LAB probiotics (Kumar et al. 2012a; Ma et al. 2019). SCFA may lower the lipid content in blood by blocking synthesis of hepatic cholesterol and/or through redirecting plasma cholesterol towards liver. Probiotics ferment non-digestible carbohydrates in large intestine to derive energy, and the resultant SCFA produced may have hypocholesterolemic effects (Kumar et al. 2012a). SCFA mainly includes acetate, propionate and butyrate, and others such as succinate, formate, valerate, caproate, isobutyrate, 2-methyl-butyrate, and isovalerate (Reis et al. 2017). In this study, LAB probiotic isolates M2, M5, and M7 produced acetic acid and propionic acid, and isolate M5 produced butyric acid as well (Supplementary Fig. 4).
SCFA produced by LAB probiotics play a key role in biochemical reactions involving cholesterol metabolism. Butyric acid is a strong inhibitor of 3-hydroxymethyl-3-glutaryl-CoA (HMG-CoA) reductase, while acetic acid is converted to acetyl-CoA in liver and further utilized in the process of cholesterol synthesis. Similarly, propionate inhibits cholesterol and lipid biosynthesis in general (Kumar et al. 2012a; Tsai et al. 2014). Propionic acid enhances hepatic bile salt synthesis by enhancing 7α-hydroxylase activity, which converts cholesterol to 7α-hydroxycholesterol (Reis et al. 2017). Furthermore, propionate suppresses lipogenesis and cholesterogenesis by preventing incorporation of acetate into plasma lipids, resulting in reduced cholesterol synthesis (Park et al. 2018). A varying SCFA producing ability has been documented among different LAB probiotics (Kahouli et al. 2015). Two Lactobacillus reuteri strains produced high amounts of acetic acid, whereas five strains produced propionic acid, and three other strains produced butyric acid. Inhibition of HMG-CoA reductase, the rate limiting enzyme of mevalonate pathway that produces cholesterol, by butyric acid, results in reduced pace of cholesterol synthesis. Thus, SCFA produced by probiotics may have a vital role towards their hypocholesterolemic effects.
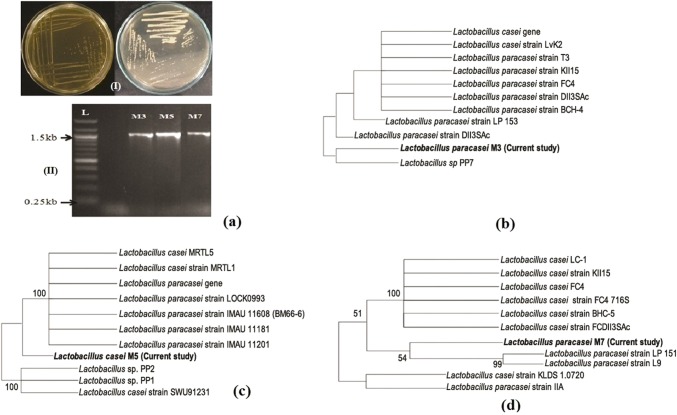
Identification of the selected probiotic isolates
Selected LAB isolates (M3, M5, and M7) which exhibited substantial probiotic functional characteristics especially hypocholesterolemic effects were identified by cultural (Fig. 8aI), microscopic, and 16S rDNA sequence analysis (Fig. 8aII, b–d). LAB isolates were analyzed for sequence homology of their 16S rDNA gene with that of available strains in National Center for Biotechnology Information (NCBI database) (Fig. 8aII, b–d). The sequences have been deposited in GenBank, and accession numbers have been assigned. Based on the results the isolates were identified, and designated as Lactobacillus paracasei M3 (Accession no. MG754429), Lactobacillus casei M5 (Accession no. MG757514), and Lactobacillus paracasei M7 (Accession no. MG597206) (Fig. 8b–d). Several probiotic LAB have been studied for cholesterol lowering potential viz. Lactobacillus plantarum Lp91 and Lp21 (Kumar et al. 2012b), Lactobacillus curvatus KFP419 and Leuconostoc mesenteroides subsp. mesenteroides KDK411 (Park et al. 2018), Pediococcus acidilactici NBHK002, B. adolescentis NBHK006, L. rhamnosus NBHK007, and L. acidophilus NBHK008 (Tsai et al. 2014), Lactobacillus fermentum strains (Owusu-Kwarteng et al. 2018), Lactobacillus plantarum (Ma et al. 2019), Lactobacillus kefiri JK17 (Yusuf et al. 2020), and even certain yeast like Saccharomyces cerevisiae ARDMC1 (Saikia et al. 2018).
Fig. 8.
Isolation of lactic acid bacteria (LAB) on MRS agar (I), PCR amplification (II) of 16 S rDNA (a), and sequence homology analysis of 16S rDNA sequence of selected LAB isolates with that of other strains available in NCBI database (b, c, d)
Conclusions
LAB isolates from human breast milk possessed numerous functional probiotic attributes including hypocholesterolemic effects. The results of the current study endorse the significance of bioprospecting of efficacious probiotics from exotic ecological niches. Cholesterol lowering was executed by LAB probiotics through various mechanisms including bile salt hydrolase activity and deconjugation of bile salts, cholesterol assimilation, adsorption of cholesterol on cell wall, inhibition of micelle formation, and miceller sequestration of cholesterol. LAB isolates with promising hypocholesterolemic potential, i.e. Lactobacillus paracasei M3, L. casei M5, and L. paracasei M7 must be investigated in vivo to fully establish their commercial prospective. Furthermore, studying the molecular mechanisms that govern probiotics-mediated cholesterol lowering may provide better understanding of the process.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Dr. Bijender Kumar Bajaj gratefully acknowledges VLIR-UOS for ‘Short Research Stay Scholarship’ (‘SRS’ Scholarship) at the Department of Bioscience Engineering, University of Antwerp, Antwerpen, Belgium; ERASMUS-MUNDUS for Invited Professorship at University of Naples, Naples, Italy; Department of Science and Technology (Government of India), for granting Research Project (Ref. SR/SO/BB-66/2007), and Department of Biotechnology (DBT), Government of India, for financial support. Ms Bilqeesa Bhat gratefully acknowledges the Council of Scientific and Industrial Research (CSIR) for CSIR-Senior Research Fellowship for Doctoral Research. Authors gratefully acknowledge the following for critically reading and correcting the MS for English language: Dr. R.S. Jayasomu, Chief Scientist, CSIR-National Institute of Science Communication and Information Resources, New Delhi, India Dr. Garima Gupta, Senior Assistant Professor, Department of English, University of Jammu, Jammu, and Mr. Hardik Bajaj, Jammu. Authors thank the Director, School of Biotechnology, University of Jammu, Jammu, for laboratory facilities.
Author contributions
BKB conceptualized the research hypothesis, and planned the experiments; BB designed and performed the experiments; BKB and BB analyzed and interpreted the data, and BB wrote the draft MS; BKB corrected and submitted the MS.
Funding
This study was funded by the Department of Science and Technology (Govt. of India) (Grant no. Ref. SR/SO/BB-66/2007) and Council of Scientific and Industrial Research, India (Grant no. SRF).
Compliance with ethical standards
Conflict of interest
All authors declare that there is no conflict of interest.
References
- Anandharaj M, Sivasankari B, Santhanakaruppu R, Manimaran M, Rani RP, Sivakumar S. Determining the probiotic potential of cholesterol-reducing Lactobacillus and Weissella strains isolated from gherkins (fermented cucumber) and south Indian fermented koozh. Res Microbiol. 2015;166:428–439. doi: 10.1016/j.resmic.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Andrabi ST, Bhat B, Gupta M, Bajaj BK. Phytase-producing potential and other functional attributes of lactic acid bacteria isolates for prospective probiotic applications. Probiotics Antimicrob Proteins. 2016;8:121–129. doi: 10.1007/s12602-016-9220-3. [DOI] [PubMed] [Google Scholar]
- Bajaj KB, Andrabi T, Claes IJJ, Lebeer S. Bioprospecting for functionally-proficient potential probiotics. Curr Nutr Food Sci. 2014;10:251–263. [Google Scholar]
- Bajaj BK, Claes IJJ, Lebeer S. Functional mechanisms of probiotics. J Microbiol Biotechnol Food Sci. 2015;4:321–327. [Google Scholar]
- Bhat B, Bajaj BK. Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei. Bioresour Technol. 2018;254:264–267. doi: 10.1016/j.biortech.2018.01.078. [DOI] [PubMed] [Google Scholar]
- Bhat B, Bajaj BK. Hypocholesterolemic potential of probiotics: concept and mechanistic insights. Indian J Exp Biol. 2019;57:73–85. [Google Scholar]
- Bhat B, Gupta M, Andrabi ST, Bajaj BK. Growth and viability of probiotic Weissella kimchi R-3 in fruit and vegetable beverages. Indian J Biochem Biophys. 2017;54:191–199. [Google Scholar]
- Chlebowska-Smigiel A, Gniewosz M, Kieliszek M, Bzducha-Wrobel A. The effect of pullulan on the growth and acidifying activity of selected stool microflora of human. Curr Pharm Biotechnol. 2017;18:121–126. doi: 10.2174/1389201017666161229154324. [DOI] [PubMed] [Google Scholar]
- Chlebowska-Śmigiel A, Kycia K, Neffe-Skocińska K, Kieliszek M, Gniewosz M, Kołożyn-Krajewska D. Effect of pullulan on physicochemical, microbiological, and sensory quality of yogurts. Curr Pharm Biotechnol. 2019;20:489–496. doi: 10.2174/1389201020666190416151129. [DOI] [PubMed] [Google Scholar]
- Choi EA, Chang HC. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT Food Sci Technol. 2015;62:210–217. [Google Scholar]
- Choi SB, Lew LC, Yeo SK, Parvathy SN, Liong MT. Probiotics and the BSH-related cholesterol lowering mechanism: a Jekyll and Hyde scenario. Crit Rev Biotechnol. 2015;35:392–401. doi: 10.3109/07388551.2014.889077. [DOI] [PubMed] [Google Scholar]
- de Melo Pereira GV, da Silva VA, de Carvalho Neto DP, Muynarsk ES, Soccol VT, Soccol CR. Lactic acid bacteria: what coffee industry should know? Curr Opin Food Sci. 2019 doi: 10.1016/j.cofs.2019.07.004. [DOI] [Google Scholar]
- Ding W, Shi C, Chen M, Zhou J, Long R, Guo X. Screening for lactic acid bacteria in traditional fermented Tibetan yak milk and evaluating their probiotic and cholesterol-lowering potentials in rats fed a high-cholesterol diet. J Funct Foods. 2017;32:324–332. [Google Scholar]
- Emami A, Bazargani A. Dual effects of lactobacilli as a cholesterol assimilator and an inhibitor of gastrointestinal pathogenic bacteria. Int J Enteric Pathog. 2014 doi: 10.17795/ijep15768. [DOI] [Google Scholar]
- Gajbhiye MH. Probiotic traits of lactic acid bacteria isolated from aerial surfaces of pomegranate. Int J Bioassays. 2016;5:4733–4738. [Google Scholar]
- Giri SS, Jun JW, Yun S, Kim HJ, Kim SG, Kang JW, Kim SW, Han SJ, Park SC, Sukumaran V. Characterisation of lactic acid bacteria isolated from the gut of Cyprinus carpio that may be effective against lead toxicity. Probiotics Antimicrob Proteins. 2019;11:65–73. doi: 10.1007/s12602-017-9367-6. [DOI] [PubMed] [Google Scholar]
- Gupta M, Bajaj BK. Functional characterization of potential probiotic lactic acid bacteria isolated from kalarei and development of probiotic fermented oat flour. Probiotics Antimicrob Proteins. 2017;10:654–661. doi: 10.1007/s12602-017-9306-6. [DOI] [PubMed] [Google Scholar]
- Jose NM, Bunt CR, Hussain MA. Comparison of microbiological and probiotic characteristics of lactobacilli isolates from dairy food products and animal rumen contents. Microorganisms. 2015;3:198–212. doi: 10.3390/microorganisms3020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahouli I, Malhotra M, Tomaro-Duchesneau C, Saha S, Marinescu D, Rodes LS, Alaoui-Jamali MA, Prakash S. Screening and in-vitro analysis of Lactobacillus reuteri strains for short chain fatty acids production, stability and therapeutic potentials in colorectal cancer. J Bioequiv Availab. 2015;7:39–50. [Google Scholar]
- Kalisz S, Oszmiański J, Kolniak-Ostek J, Grobelna A, Kieliszek M, Cendrowski A. Effect of a variety of polyphenols compounds and antioxidant properties of rhubarb (Rheum rhabarbarum) LWT. 2020 doi: 10.1016/j.lwt.2019.108775. [DOI] [Google Scholar]
- Koryszewska-Bagińska A, Gawor J, Nowak A, Grynberg M, Aleksandrzak-Piekarczyk T. Comparative genomics and functional analysis of a highly adhesive dairy Lactobacillus paracasei subsp. paracasei IBB3423 strain. Appl Microbiol Biotechnol. 2019;103:7617–7634. doi: 10.1007/s00253-019-10010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Bansal P, Singh J, Dhanda S, Bhardwaj JK. Aggregation, adhesion and efficacy studies of probiotic candidate Pediococcus acidilactici NCDC 252: a strain of dairy origin. World J Microbiol Biotechnol. 2020;36:10. doi: 10.1007/s11274-019-2785-8. [DOI] [PubMed] [Google Scholar]
- Kumar S, Dhillon MK. Lipophilic metabolite profiling of maize and sorghum seeds and seedlings, and their pest spotted stem borer larvae: a standardized GC–MS based approach. Indian J Exp Biol. 2015;53:70–176. [PubMed] [Google Scholar]
- Kumar R, Grover S, Batish VK. Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague-Dawley rats. Br J Nutr. 2011;105:561–573. doi: 10.1017/S0007114510003740. [DOI] [PubMed] [Google Scholar]
- Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S, Yadav H. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res. 2012;2012:902917. doi: 10.1155/2012/902917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Grover S, Batish VK. Bile salt hydrolase (Bsh) activity screening of lactobacilli: in vitro selection of indigenous Lactobacillus strains with potential bile salt hydrolysing and cholesterol-lowering ability. Probiotics Antimicrob Proteins. 2012;4:162–172. doi: 10.1007/s12602-012-9101-3. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao Y, Zhang L, Zhang X, Huang L, Li D. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012;135:1914–1919. doi: 10.1016/j.foodchem.2012.06.048. [DOI] [PubMed] [Google Scholar]
- Llamas-Arriba MG, Hernández-Alcántara AM, Yépez A, Aznar R, Dueñas MT, López P. Functional and Nutritious Beverages Produced by Lactic Acid Bacteria. In: Grumezescu A, Holban AM, editors. Nutrients in Beverages. Cambridge, USA: Academic Press; 2019. pp. 419–465. [Google Scholar]
- Ma C, Zhang S, Lu J, Zhang C, Pang X, Lv J. Screening for cholesterol-lowering probiotics from lactic acid bacteria isolated from corn silage based on three hypothesized pathways. Int J Mol Sci. 2019;20:2073. doi: 10.3390/ijms20092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Ghosh K. Antagonism against fish pathogens by cellular components and verification of probiotic properties in autochthonous bacteria isolated from the gut of an Indian major carp, Catla catla (H amilton) Aquacult Res. 2016;47:2243–2255. [Google Scholar]
- Oguntoyinbo FA, Narbad A. Multifunctional properties of Lactobacillus plantarum strains isolated from fermented cereal foods. J Funct Foods. 2015;17:621–631. [Google Scholar]
- Owusu-Kwarteng J, Tano-Debrah K, Akabanda F, Jespersen L. Technological properties and probiotic potential of Lactobacillus fermentum strains isolated from West African fermented millet dough. BMC Microbiol. 2015 doi: 10.1186/s12866-015-0602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MY, Kim J, Kim S, Whang KY. Lactobacillus curvatus KFP419 and Leuconostoc mesenteroides subsp. mesenteroides KDK411 isolated from kimchi ameliorate hypercholesterolemia in rats. J Med Food. 2018;21:647–653. doi: 10.1089/jmf.2017.4125. [DOI] [PubMed] [Google Scholar]
- Park S, Ji Y, Park H, Lee K, Park H, Beck BR, Shin H, Holzapfel WH. Evaluation of functional properties of lactobacilli isolated from Korean white kimchi. Food Control. 2016;69:5–12. [Google Scholar]
- Ramos CL, Thorsen L, Schwan RF, Jespersen L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013;36:22–29. doi: 10.1016/j.fm.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Razdan K, Parihar J, Bajaj BK. Isolation and characterization of a lipolytic and phytase producing probiotic for potential application in poultry feed. Online J Anim Feed Res. 2012;2:369–377. [Google Scholar]
- Reis SA, Conceição LL, Rosa DD, Siqueira NP, Peluzio MCG. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutr Res Rev. 2017;30:36–49. doi: 10.1017/S0954422416000226. [DOI] [PubMed] [Google Scholar]
- Rudel LL, Morris MD. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973;14:364–366. [PubMed] [Google Scholar]
- Ruiz Rodriguez LG, Mohamed F, Bleckwedel J, Medina R, De Vuyst L, Hebert EM, Mozzi F. Diversity and functional properties of lactic acid bacteria isolated from wild fruits and flowers present in Northern Argentina. Front Microbiol. 2019;10:1091. doi: 10.3389/fmicb.2019.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo TK, Jena PK, Nagar N, Patel AK, Seshadri S. In vitro evaluation of probiotic properties of lactic acid bacteria from the gut of Labeo rohita and Catla catla. Probiotics Antimicrob Proteins. 2015;7:126–136. doi: 10.1007/s12602-015-9184-8. [DOI] [PubMed] [Google Scholar]
- Saikia D, Manhar AK, Deka B, Roy R, Gupta K, Namsa ND, Chattopadhyay P, Doley R, Mandal M. Hypocholesterolemic activity of indigenous probiotic isolate Saccharomyces cerevisiae ARDMC1 in a rat model. J Food Drug Anal. 2018;26:154–162. doi: 10.1016/j.jfda.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Gulati S, Thakur N, Singh BP, Gupta S, Kaur S, Mishra SK, Puniya AK, Gill JPS, Panwar H. Antibiotic sensitivity pattern of indigenous lactobacilli isolated from curd and human milk samples. 3 Biotech. 2017 doi: 10.1007/s13205-017-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobharani P, Halami PM. In vitro evaluation of the cholesterol-reducing ability of a potential probiotic Bacillus spp. Ann Microbiol. 2016;66:643–651. [Google Scholar]
- Singh TP, Malik RK, Kaur G. Cell surface proteins play an important role in probiotic activities of Lactobacillus reuteri. Nutrire. 2016 doi: 10.1186/s4111. [DOI] [Google Scholar]
- Song M, Yun B, Moon JH, Park DJ, Lim K, Oh S. Characterization of selected Lactobacillus strains for use as probiotics. Korean J Food Sci Anim Resour. 2015;35:551–556. doi: 10.5851/kosfa.2015.35.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah PV, Gould IG, Lal S, Aizezi B. Incorporation profiles of conjugated linoleic acid isomers in cell membranes and their positional distribution in phospholipids. Biochim Biophys Acta. 2011;1811:17–24. doi: 10.1016/j.bbalip.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Lin PP, Hsieh YM, Zhang ZY, Wu HC, Huang CC. Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. Sci World J. 2014 doi: 10.1155/2014/690752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Guo MJ, Gao Q, Yang JF, Yang L, Pang XL, Jiang XJ. The effects of probiotics on total cholesterol: a meta-analysis of randomized controlled trials. Medicine. 2018 doi: 10.1097/MD.0000000000009679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Tang W, Geng W, Zheng Y, Wang Y. In vitro and in vivo evaluation of the probiotic attributes of Lactobacillus kefiranofaciens XL10 isolated from Tibetan kefir grain. App Microbiol Biotechnol. 2017;101:2467–2477. doi: 10.1007/s00253-016-7956-z. [DOI] [PubMed] [Google Scholar]
- Yusuf D, Nuraida L, Dewanti-Hariyadi R, Hunaefi D. In vitro characterization of lactic acid bacteria from Indonesian kefir grains as probiotics with cholesterol-lowering effect. J Microbiol Biotechnol. 2020 doi: 10.4014/jmb.1910.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Luo J, Zuo F, Zhang Y, Ma H, Chen S. Screening for potential novel probiotic Lactobacillus strains based on high dipeptidyl peptidase IV and α-glucosidase inhibitory activity. J Funct Foods. 2016;20:486–495. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.