Abstract
Background
Intestinal fibrosis is a hallmark of Crohn’s disease. Here, we investigated the impact of several putative antifibrotic compounds on the expression of fibrosis markers using murine precision-cut intestinal slices.
Methods
Murine precision-cut intestinal slices were cultured for 48 hours in the presence of profibrotic and/or antifibrotic compounds. The fibrotic process was studied on gene and protein level using procollagen 1a1 (Col1α1), heat shock protein 47 (Hsp47), fibronectin (Fn2), and plasminogen activator inhibitor-1 (Pai-1). The effects of potential antifibrotic drugs mainly inhibiting the transforming growth factor β (TGF-β) pathway (eg, valproic acid, tetrandrine, pirfenidone, SB203580, and LY2109761) and compounds mainly acting on the platelet-derived growth factor (PDGF) pathway (eg, imatinib, sorafenib, and sunitinib) were assessed in the model at nontoxic concentrations.
Results
Murine precision-cut intestinal slices remained viable for 48 hours, and an increased expression of fibrosis markers was observed during culture, including Hsp47, Fn2, and Pai-1. Furthermore, TGF-β1 stimulated fibrogenesis, whereas PDGF did not have an effect. Regarding the tested antifibrotics, pirfenidone, LY2109761, and sunitinib had the most pronounced impact on the expression of fibrosis markers, both in the absence and presence of profibrotic factors, as illustrated by reduced levels of Col1α1, Hsp47, Fn2, and Pai-1 after treatment. Moreover, sunitinib significantly reduced Hsp47 and Fn2 protein expression and the excretion of procollagen 1.
Conclusions
Precision-cut intestinal slices can successfully be used as a potential preclinical screening tool for antifibrotic drugs. We demonstrated that sunitinib reduced the expression of several fibrosis markers, warranting further evaluation of this compound for the treatment of intestinal fibrosis.
Keywords: antifibrotic compounds, intestinal fibrosis, platelet-derived growth factor inhibitors, precision-cut intestinal slices, transforming growth factor-β1 inhibitors
We demonstrate that precision-cut intestinal slices are a potential suitable screening platform for antifibrotic drugs. We tested the efficacy of a broad range of putative antifibrotic drugs and identified that sunitinib reduces the expression of several fibrosis markers, warranting further evaluation of this compound for the treatment of intestinal fibrosis.
INTRODUCTION
Crohn’s disease (CD), an inflammatory bowel disease (IBD), is often associated with intestinal fibrosis resulting in the formation of strictures, which will obstruct the intestinal lumen. These strictures are characterized by transmural condensed collagen layers in the intestinal wall.1–3 It is reported that intestinal fibrosis is initiated by severe and chronic tissue damage due to recurrent inflammation,4 as observed in CD patients. During CD, various cytokines are elevated in inflamed regions, including the archetypical profibrotic factors, transforming growth factor β (TGF-β) and platelet-derived growth factor (PDGF).1, 5, 6 These cytokines increase the expression of a variety of genes, including connective tissue growth factor (Ctgf), plasminogen activator inhibitor-1 (Pai-1) and C-myc.7 It has been reported that TGF-β is a key player during intestinal wound healing and during stricture development in CD patients.1 Activation of the TGF-β signaling pathway augments the expression of procollagen 1a1 (Col1α1), fibronectin (Fn2) and heat shock protein 47 (Hsp47).8, 9 Therefore, TGF-β is an interesting target for the treatment of fibrosis. In a previous study, we evaluated the therapeutic potential of a myriad of TGF-β pathway inhibitors in liver fibrosis using a unique ex vivo/in vitro model, namely precision-cut liver slices (PCLSs).10–13 Using this model, we demonstrated that tetrandrine (Tet), valproic acid (Val), pirfenidone (Pir), and rosmarinic acid have potential for the treatment of liver fibrosis, in line with previous studies.14–17
The other profibrotic growth factor, PDGF,18 induces cell proliferation and fibroblasts migration9, 19 but also activates intestinal myofibroblasts to increase collagen synthesis.20 Several groups successfully decreased fibrogenesis by inhibiting the PDGF pathway via PDGF receptor inhibitors.14, 21 Also, our group successfully used PCLS to study the efficacy of several PDGF inhibitors,13 including imatinib (Ima), sorafenib (Sor) and sunitinib (Sun). Despite these promising results, there are no drugs currently registered for the treatment of intestinal fibrosis, and the only available therapy is surgical intervention.22
Various animal models have been used to evaluate antifibrotic compounds in multiple organs.23, 24 Good translational animal models for intestinal fibrosis are scarce, and as a result, elucidating the mechanism of intestinal fibrosis and testing the efficacy of therapeutic compounds is hampered. Precision-cut tissue slices (PCTSs) are widely used to study drug metabolism, drug toxicity, drug efficacy, and fibrosis.25 To improve upon the applicability of the model as a drug-screening tool, slices could also be prepared from tissue obtained from animal models of IBD (eg, the mouse model of dextran sodium sulfate–induced colitis). Recently, we established a novel model for the onset of intestinal fibrosis using precision-cut intestinal slices (PCISs).10, 26 The objective of the current study was to use this model to investigate the antifibrotic effect of several putative antifibrotic compounds in the intestine, including TGF-β pathway–related inhibitors (Pir, Val, Tet, and LY2109761), p38 MAPK inhibitor (SB203580), and PDGF related–pathway inhibitors (Ima, Sor, and Sun).
MATERIALS AND METHODS
Preparation Mouse Intestinal Cores
Adult nonfasted male C57BL/6 mice were used (Harlan PBC, Zeist, the Netherlands). The mice were housed on a 12-hour light/dark cycle in a temperature and humidity-controlled room with food (Harlan chow no. 2018, Horst, the Netherlands) and water ad libitum. The animals were allowed to acclimatize for at least 7 days before the start of the experiment. The experiments were approved by the Animal Ethical Committee of the University of Groningen (DEC 6416AA).
Mice were anesthetized with isoflurane/O2 (Nicholas Piramal, London, UK). Mouse jejunum (about 15 cm distal from the stomach and 10 cm in length) were excised and preserved in ice-cold Krebs-Henseleit buffer (KHB), supplemented with 25 mM of D-glucose (Merck, Darmstadt, Germany), 25 mM of NaHCO3 (Merck), 10 mM of HEPES (MP Biomedicals, Aurora, OH, USA), saturated with carbogen (95% O2/5% CO2), and adjusted to pH 7.4.
The jejunum was cleaned by flushing KHB through the lumen and subsequently divided into 2-cm segments. These segments were filled with 3% agarose (w/v) solution in 0.9% NaCl at 37oC and embedded in an agarose core-embedding unit.27
Preparation of Precision-cut Intestinal Slices
Precision-cut intestinal slices were prepared in ice-cold KHB by the Krumdieck tissue slicer (Alabama Research and Development, Munford, AL, USA). The slices with a wet weight of 3 to 4 mg have an estimated thickness of 300 to 400 μm. Slices were stored on ice-cold KHB until the start of the experiments.27
Profibrotic and Antifibrotic Compounds
Transforming growth factor β-1 (5 ng/mL; hTGF-β1; Roche Applied Science, Mannheim, Germany) and PDGF-BB (50 ng/mL; Recombinant Human PDGF-BB; Peprotech, Bioconnect, Huissen, the Netherlands) were used as profibrotic stimuli.
Different antifibrotic compounds were tested: TGF-β inhibitors such as valproic acid (1 mM; Sigma Aldrich, Zwijndrecht, the Netherlands), tetrandrine (5 μM; Sigma Aldrich), pirfenidone (2.5 mM; Sigma Aldrich), and LY2109761 (10 µM; Selleck Chemicals, Houston, TX, USA); PDGF inhibitors such as imatinib (10 μM; Novartis, Basel, Switzerland), sorafenib (4 μM; LC laboratories, Woburn, USA), and sunitinib (5 μM; LC laboratories); and p38 MapK inhibitor SB203580 (5 μM; Bioconnect, Huissen, the Netherlands).
Incubation of Intestinal Slices
Slices were incubated in 24-well plates for mouse PCIS (mPCIS). Mouse PCISs were incubated individually in 0.5 mL of Williams Medium E with L-glutamine (Invitrogen, Paisly, UK) supplemented with 25 mM of glucose, 50 μg/mL of gentamycin (Invitrogen) and 2.5 μg/mL of amphotericin-B (Invitrogen). During incubation (at 37oC and 80% O2/5% CO2) in an incubator (MCO-18M, Sanyo), the plates were horizontally shaken at 90 rpm (amplitude 2 cm). Mouse PCISs were incubated up to 48 hours in the absence or presence of human TGF-β1 (5 ng/mL) or PDGF-BB (50 ng/mL). Precision-cut intestinal slices were incubated up to 48 hours, during which time slices were exposed to profibrotic and/or antifibrotic compounds.
Viability
Viability was assessed by measuring the adenosine triphosphate (ATP) content of the PCIS using the ATP bioluminescence kit (Roche Diagnostics, Mannheim, Germany), as previously described.27 We have previously demonstrated that ATP levels significantly correlate with the morphological integrity of PCIS, indicating that ATP values can be used as a proxy for viability.26 Adenosine triphosphate values (pmol) were normalized to the total protein content (μg) of the PCIS estimated by the Lowry protein assay (Bio-rad RC DC Protein Assay, Bio-Rad, Veenendaal, the Netherlands). Values are displayed as relative values compared with the related controls.
Gene Expression
After incubation, slices were snap frozen in liquid nitrogen and stored at −80ºC until use. Total RNA of 3 to 6 pooled snap frozen slices was isolated using the Qiagen RNAeasy mini kit (Qiagen, Venlo, the Netherlands). The amount of isolated RNA was measured with the BioTek Synergy HT (BioTek Instruments, Winooski, VT, USA). Afterward, 1 μg of RNA was reverse transcribed using the Reverse Transcription System (Promega, Leiden, the Netherlands). The RT-PCR reaction was performed in the Eppendorf mastercycler with the following gradient: 25ºC for 10 minutes, 45ºC for 60 minutes, and 95ºC for 5 minutes.
The expression of several fibrosis genes (eg, Col1α1, αSma, Hsp47, and Fn2; Table 1) and 3 pathway-specific genes (C-myc, Pai-1, and Ctgf; Table 1) were determined by SYBR green method. The real-time PCR reaction was performed in a 7900HT Real-Time PCR (Applied Biosystems, Bleiswijk, the Netherlands) with 45 cycles of 10 minutes at 95ºC, 15 seconds at 95ºC, and 25 seconds at 60ºC, followed by a dissociation curve. Ct values were corrected for the Ct values of the housekeeping gene Gapdh (∆Ct) and compared with the control group (∆∆Ct). Results are presented as fold induction (2-∆∆Ct).
TABLE 1.
Fibrotic Primers Gene Expression
| Primer | Forward Sequence | Reverse Sequence |
|---|---|---|
| Gapdh | ACAGTCCATGCCATCACTGC | GATCCACGACGGACACATTG |
| Col1α1 | TGACTGGAAGAGCGGAGAGT | ATCCATCGGTCATGCTCTCT |
| αSma | ACTACTGCCGAGCGTGAGAT | CCAATGAAAGATGGCTGGAA |
| Hsp47 | AGGTCACCAAGGATGTGGAG | CAGCTTCTCCTTCTCGTCGT |
| Fn2 | CGGAGAGAGTGCCCCTACTA | CGATATTGGTGAATCGCAGA |
| C-myc | GCTGTAGTAATTCCAGCGAGAGACA | CTCTGCACACACGGCTCTTC |
| Pai-1 | GCCAGATTTATCATCAATGACTGGG | GGAGAGGTGCACATCTTTCTCAAAG |
| Ctgf | CAAAGCAGCTGCAAATACCA | GGCCAAATGTGTCTTCCAGT |
Western Blot
Heat shock protein 47, Fn2 and PDGF-β-receptor protein expression were determined by Western blot. The Western blot analyses were performed as described by Luangmonkong et al.12 Snap frozen PCIS in liquid nitrogen and stored until analyses at −80˚C. Tissue lysate was prepared in 200 μL Pierce RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA), PhosSTOP (Roche, Mannheim, Germany), and protein inhibitor cocktail (Sigma-Aldrich, Zwijndrecht, the Netherlands). The tissue was homogenized on ice and centrifuged for 30 minutes at 4°C at 13,000 rpm. Protein concentrations were determined in the supernatant using a Bio-Rad DC protein assay according to the manufacturer’s protocol. As much as 50 µg of protein samples were prepared in 4x Laemmli sample buffer (Bio-Rad, USA), supplemented with 10% ß-mercaptoethanol, boiled for 15 minutes at 100°C, and loaded on a 1.5-mm 10% stain-free gel to be separated by SDS-PAGE. Precision Plus protein standard dual color (Bio-Rad, Hercules, CA, USA) was used as a reference marker on the gel. Gels were blotted using the Bio-Rad semi-dry trans-blot turbo mini PVDF system (1x minigel, 25 A, 10 min) and blocked in Tris-buffered saline, supplemented with blotting-grade blocker (Bio-Rad, USA) and 0.1% Tween-20 for 1 hour. Subsequently, membranes were incubated with rabbit-α-heat shock protein 47 (1:1000, Abcam, Cambridge, UK), mouse-α-fibronectin (IST-9) (1:1000, Abcam, UK), rabbit-α-PDGF-β-receptor (1:1000, Cell Signaling Technology, Danvers, MA, USA) and mouse-α-Gapdh (1:5000, Sigma, Saint Louis, MO, USA). For detection, horseradish peroxidase (HRP, conjugated secondary antibodies rabbit-α-mouse immunoglobulins) and secondary goat-α-rabbit (Dako, Glostrup, Denmark) were used in combination with Clarity Western ECL Substrate (Bio-Rad, USA) chemiluminescence reagent kit and Chemidoc MP imaging system (Bio-Rad, USA). Results are displayed as relative values compared with the control and normalized with Gapdh protein expression.
ELISA
Murine procollagen I levels were assessed using ELISA (Abcam) according to the instructions provided by the manufacturer. Briefly, 96 well plate strips were supplied and ready to use. It was not necessary to rinse the plate before adding reagents. First, 50 μL of all sample or standard was added to appropriate wells, and 50 μL of the antibody cocktail was added to each well. After that, the plate was sealed and incubated for 1 hour at room temperature on a plate shaker set to 400 rpm. Each well was then washed with 3 × 350 μL 1X Wash Buffer PT. Then, 100 μL of tetramethylbenzidine (TMB) substrate was added to each well and incubated for 10 minutes in the dark on a plate shaker set to 400 rpm. Finally, 100 μL of stop solution was added to each well, and the plate was shaken on a plate shaker for 1 minute to mix (record the OD at 450 nm). All concentrations were calculated using a standard curve made from mouse procollagen I alpha 1 provided by the manufacturer.
Statistics
Statistics were performed using GraphPad Prism 6.0. The data represent the mean of the biological replicates (ie, the number of mice used, reflected by the n in the figure legend). Moreover, we used 3 and 6 technical replicates for ATP analysis and RNA isolation, respectively. Differences were determined using a paired, 1-tailed Student t test or a 1-way ANOVA followed by Dunnett or Tukey multiple comparisons test, as appropriate. A P value <0.05 was considered significant. Statistical differences were determined on the relative value of ATP, ∆∆Ct value for real-time PCR results, and relative signal intensity of the proteins.
RESULTS
Gene Expression of Fibrosis Markers
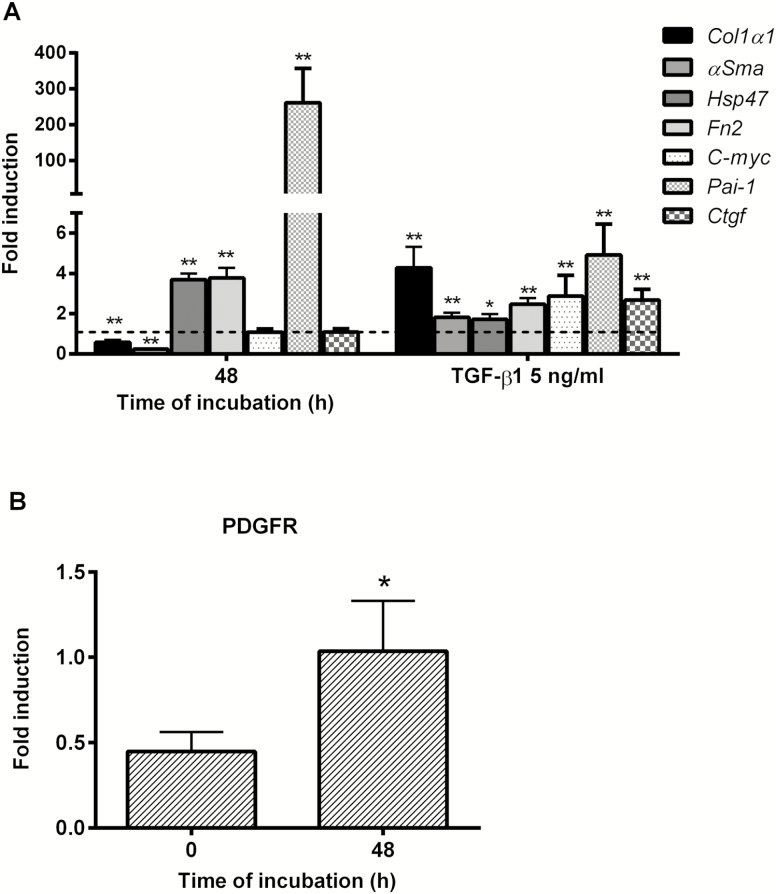
After 48 hours of incubation, there was no significant difference in the ATP content of PCIS compared with the 0-hour time point (Supplementary Fig. S1), indicating that the viability and morphological integrity of the slices was maintained, as demonstrated previously.26 During culture, gene expression of Hsp47 and Fn2, early markers of fibrosis, were increased significantly compared with 0 hour. However, Col1α1 and αSma were significantly decreased compared with directly after slicing (Fig. 1A). Exposure of PCIS to TGF-β1 and PDGF-BB did not affect the viability of the slices (Supplementary Fig. S1). Gene expressions of the fibrosis markers Col1α1, αSma, Hsp47, and Fn2 were upregulated at least 2-fold in the presence of TGF-β1 (Fig. 1A).
FIGURE 1.
A, Gene expression of fibrosis and fibrosis-related pathway markers after 48 hours: Col1α1, αSma, Hsp47, Fn2, C-myc, Pai-1, and Ctgf with or without TGF-β1(5ng/mL). The broken line is the normalized control at 0 hours (value = 1) for the 48 hour bars; the broken line is also the normalized control of 48 hours without TGF-β1 (value = 1) for the 48 hours with TGF-β1 (5ng/mL) bars. B, Protein expression of PDGFR during culture. Data are expressed as mean +/- SEM (n = 3–8). One-tailed Student t test; *P < 0.05, **P < 0.01 vs control 0 hours or 48 hours.
Plasminogen activator inhibitor-1 expression dramatically increased, whereas the other pathway related genes did not change (Fig. 1A). These results are in line with previous studies using PCIS from various species.10, 26 Moreover, TGF-β1 significantly increased the expression of all 3 fibrosis-related pathway genes (C-myc, Pai-1, and Ctgf; Fig. 1A). Also, the PDGF receptor was present during culture (Fig. 1B), which is prerequisite for the activation and inhibition of the PDGF pathway during culture. Next, we evaluated the efficacy of multiple putative antifibrotics using the markers mentioned previously.
Antifibrotic Effect of TGF-β-related Inhibitors
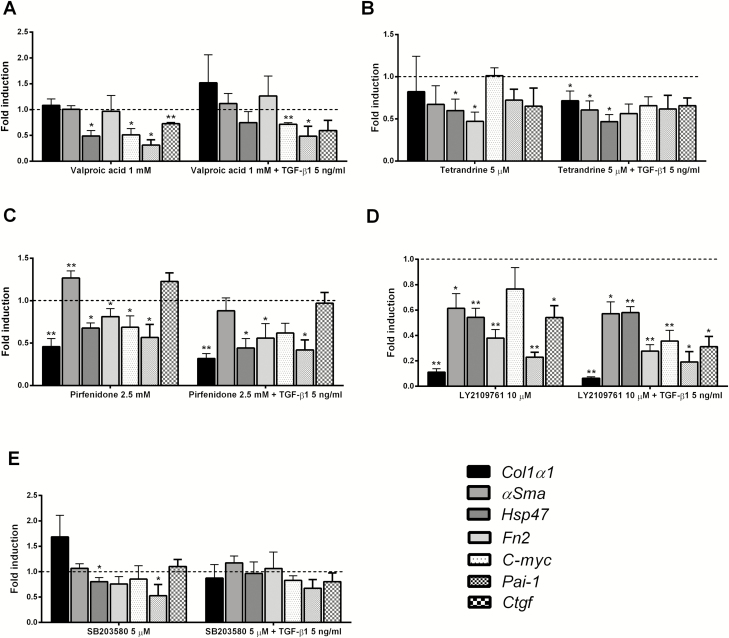
Drugs, mainly acting on the TGF-β pathway, were studied for 48 hours in the presence or absence of TGF-β1. The selected concentrations of the studied compounds were nontoxic for PCIS, as illustrated by the ATP content of the slices after treatment (Supplementary Figs. S2A, S2B). In the absence of TGF-β1, all tested inhibitors (eg, Val, Tet, and Pir) significantly decreased the gene expression of Hsp47 (Figs. 2A, 2B, 2C). Also, Tet and Pir also downregulated Fn2 expression. Moreover, Pir was the TGF-β pathway–associated drug that was able to decrease the gene expression of Col1α1 (Fig. 2C).
FIGURE 2.
Gene expression of Col1α1, αSma, Hsp47, Fn2, C-myc, Pai-1, and Ctgf in PCIS after treatment with antifibrotic compound with or without TGF-β1: (A) Val; (B) Tet; (C) Pir; (D) LY2109761; (E) SB203580. The broken line is the normalized control 48 hours (value = 1) for PCIS without TGF-β1 bars, and the broken line is also the normalized control of 48 hours + TGF-β1 (value = 1) for PCIS with TGF-β1 bars. Data are expressed as mean +/- SEM (n = 3–6). One-way ANOVA followed by Dunnett multiple comparisons test. *P < 0.05, **P < 0.01 vs control 48 hours or 48 h + TGF-β1.
Among TGF-β specific inhibitors, LY2109761 decreased the expression of all fibrosis-related genes—but especially reduced the gene expression of Col1α1 by 80%, a level that was even lower than the expression of Col1α1 directly after slicing (Fig. 2E). Meanwhile, the p38 MapK inhibitor, SB203580, only slightly downregulated the gene expression of Hsp47 in slices (Fig. 2D).
Next, PCISs were exposed to the putative antifibrotic compounds in the presence of TGF-β1. Under these conditions, Val did not change the gene expression of any of the fibrosis markers studied (Fig. 2A). However, Tet showed a clear antifibrotic effect, as it significantly reduced the expression of most of the studied genes, except for Fn2, compared with PCISs incubated with only TGF-β1 (Fig. 2B). Also, Pir significantly decreased the gene expression of Col1α1, Hsp47, and Fn2 as compared with PCIS incubated with TGF-β1 alone (Fig. 2C). Moreover, LY2109761 markedly decreased Col1α1 expression (Fig. 2E). In contrast, SB203580 did not affect any of the fibrosis-related genes (Fig. 2D).
When investigating the gene expression of pathway-related markers C-myc, Pai-1, and Ctgf, Val downregulated all these markers significantly (Fig. 2A). Pir only decreased the expression of C-myc and Pai-1 (Fig. 2C). But Tet did not affect any of the pathway-related markers (Fig. 2B). Furthermore, LY2109761, the TGF-β-specific inhibitor, significantly decreased Pai-1 and Ctgf gene expression in PCISs (Fig. 2E), whereas SB203580 only decreased Pai-1 gene expression significantly (Fig. 2D).
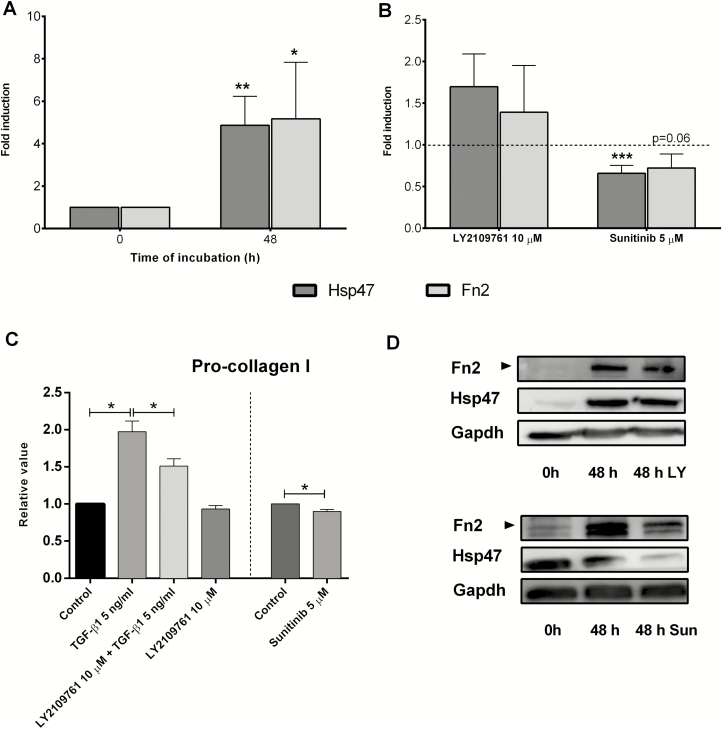
In the presence of TGF-β1, Pai-1 gene expression was downregulated by Val, Pir, and LY2109761 (Figs. 2A, 2C, 2E). Val and LY2109761 also significantly decreased C-myc gene expression (Figs. 2A, 2E). Gene expression of Ctgf was only downregulated by LY2109761 (Fig. 2E). Therefore, LY2109761 appeared to be the most effective antifibrotic compound. Therefore, we studied the impact of LY2109761 on the protein expression of Pro-collagen I, Hsp47, and fibronectin. Protein expression of those markers was significantly upregulated in PCIS under control conditions when compared with PCIS directly after slicing (Fig. 3A). In addition, protein excretion of Pro-collagen I was significantly downregulated in the culture medium in the presence of LY2109761 (Fig. 3C). However, Hsp47 and fibronectin proteins were not regulated in the presence of LY2109761 compared with control (Fig. 3B). The representative Western blots can be seen in Figure 3D.
FIGURE 3.
Protein expression/release of fibrosis markers: (A) during culture after treatment with LY2109761 and Sun; (B) Hsp47 and Fn2; (C) procollagen I; (D) representative Western blots. Data are expressed as mean +/- SEM (n = 3–18). One-way ANOVA followed by Tukey multiple comparisons test and 1-tailed Student t test; *P < 0.05, **P < 0.01, ***P < 0.001 vs control (control value = 1, depicted as a broken line).
Taken together, LY2109761 showed a significant reduction of the gene level of the investigated fibrosis markers but only downregulated the procollagen I excretion.
Antifibrotic Effect of PDGF Related Inhibitors
The impact of the PDGF inhibitors Ima, Sor, and Sun on the expression of fibrosis markers was studied in the presence and absence of PDGF-BB. Viability, as measured by the ATP-content of the slices, showed that all inhibitors were tested at nontoxic concentrations (Supplementary Fig. S2C).
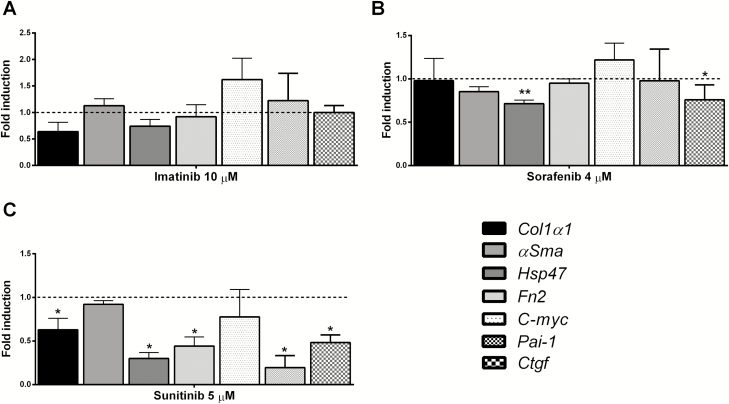
Ima did not influence gene expression of the fibrosis markers as compared with control in the presence and absence of PDGF-BB (Fig. 4A, Supplementary Fig. S3A). Sor, by itself, decreased Hsp47 expression, and in the presence of PDGF-BB, both Hsp47 and αSma levels were reduced (Fig. 4B, Supplementary Fig. S3B). Meanwhile, Sun with and without PDGF-BB downregulated not only the early markers Hsp47 and Fn2 but also the gene expression of the main fibrosis marker Col1α1 (Fig. 4C, Supplementary Fig. S3C).
FIGURE 4.
Gene expression of Col1α1, αSma, Hsp47, Fn2, C-myc, Pai-1, and Ctgf in PCIS after treatment with antifibrotic compound without the presence of PDGF: (A) Ima; (B) Sor; (C) Sun. Data are expressed as mean +/- SEM (n = 3–6). One-way ANOVA followed by Dunnett multiple comparisons test; *P < 0.05, **P < 0.01 vs control (control value = 1, depicted as a broken line).
Although Sor only slightly decreased the gene expression of Ctgf in the absence of PDGF-BB (Fig. 4B), Sun downregulated Pai-1 and Ctgf gene expression in both the absence and presence of PDGF-BB (Fig. 4C, Supplementary Fig. S3C). Sun was the most effective PDGF inhibitor. Therefore, we only studied the effect of Sun on the protein expression of procollagen I, Hsp47, and fibronectin. Figure 3B shows that Sun also downregulated the protein expression of Hsp47 and Fn2 (P = 0.06). Furthermore, protein excretion of procollagen I was significantly downregulated in the culture medium in the presence of Sun (Fig. 3C). Thus, among PDGF inhibitors, only Sun showed potential antifibrotic effects on gene and protein level.
DISCUSSION
The present study is the first that shows the effect of potential antifibrotic drugs for the treatment of intestinal fibrosis. Gene expression of fibrosis markers was highly upregulated in PCIS after 48 hours of incubation, allowing the use of this ex vivo model to evaluate and rank the effect of potential antifibrotic drugs. A similar ex vivo model has successfully been used to evaluate antifibrotic drugs for liver fibrosis by using precision-cut liver slices.10–13
Our results demonstrate that during incubation of PCIS up to 48 hours, the gene expression of several fibrosis markers was increased. To even further induce the onset of fibrosis, PCISs were incubated with TGF-β1 or PDGF-BB. Only TGF-β1 induced fibrosis markers and pathway-related genes, which was in line with the study in isolated human intestinal fibroblasts where gene expression of Ctgf and Col1α1 is elevated after TGF-β1 stimulation.28, 29 However, a different response to PDGF-BB was observed in PCIS as compared with other in vitro models.13 In our hands, incubation of PCIS with PDGF-BB did not affect the expression of the measured fibrosis genes, despite the presence of the PDGF receptor. It might be necessary to use higher concentrations of PDGF-BB.
Several TGF-β pathway–related inhibitors were evaluated in this study, including Val, Tet, and Pir. As reported previously in an ex vivo rat PCLS model, Val reduced the gene expression of multiple fibrosis makers.30 In our PCIS model, Val did not have an antifibrotic effect. However, it affected the expression of pathway-related genes, indicating that Val inhibited the TGF-β pathway but did not alter the early onset of fibrosis. Mannaerts et al showed that Val reduced Col1α1 gene expression in mouse hepatic stellate cell (HSC) after 96 hours of culture.15 Also, Val suppressed renal fibrosis of TGF-β1–stimulated αSMA expression and induction of autophagy in unilateral ureteral obstruction (UOO) mice after 5 days.31 Therefore, an increased incubation period might be needed to fully unveil the effect of Val on the gene expression of fibrosis markers in PCIS. Furthermore, Val is a histone deacetylase inhibitor, and the effect on the pathway-related genes could also be caused by hyperacetylation of histones.32
Tet blocks the TGF-β/Smad pathway by upregulating Smad 7, which inhibits Smad2/3 phosphorylation.1, 13 In our hands, Tet did not affect the pathway related genes but attenuated the levels of several fibrosis markers. This ostensible discrepancy might also be due to timing, as the inhibition of the pathway related genes could have occurred before the 48 h sampling time. Therefore, more research is necessary to elucidate the molecular mechanisms involved in the antifibrotic effect of Tet.
Pir decreases gene expression of TGF-β, Collagen I, and Hsp47 in both cell cultures and animal fibrosis models from different organs.16, 33, 34 Pir was the first antifibrotic compound on the market, currently registered for the treatment of idiopathic pulmonary fibrosis.14 The antifibrotic properties for Tet and Pir in the intestine are in line with the results obtained in fibrosis models in other organs and PCLS.16, 30 In addition, our result showed that Pir could reduce all fibrosis markers except αSma. Schaefer et al stated that the in vitro antifibrotic activities of Pir could be divided into 3 general mechanisms: reduction of fibroblast and myofibroblast proliferation, inhibition of extracellular matrix synthesis/deposition, and reduction of fibrosis markers.16 The antifibrotic mechanism of Pir in the intestine may be mainly inhibiting the extracellular matrix synthesis/deposition. Thus, Tet and Pir could be effective for the treatment of intestinal fibrosis.
Recently, other inhibitors—albeit no marketed drugs—surfaced that are used to inhibit specific pathways in fibrosis, namely LY2109761 and SB203580. The inhibitor LY2109761 is a TGF-β inhibitor that showed promising results in blocking TGF-β signaling in cancer and fibrotic diseases.35–38 The inhibitor SB203580 is a p38 MAP Kinase inhibitor,39 which decreased the gene expression of fibrosis markers in the precision-cut liver slices. In our PCIS model, only LY2109761 showed a clear antifibrotic effect. This suggests that the TGF-β signaling pathway is instrumental during the development of intestinal fibrosis, whereas the p38 Map Kinase pathway does not play a role, that TGF-β is a key player is in line with other studies (eg, intestinal wound healing and stricture development in CD patients).1
The production, maturation, and cross-linking of collagens takes longer than our model allows for. Therefore, to investigate the early stages of collagen production in fibrosis, the excretion of procollagen I is a good marker for de novo synthesis of collagen. Our results further illustrated that LY2109761 could dampen the expression of multiple fibrosis markers on the gene and the excretion of procollagen 1, further supporting the notion that hampering the TGF-β pathway is a promising therapeutic target to treat intestinal fibrosis.
We evaluated the antifibrotic activity of the small molecule tyrosine kinase inhibitors: Ima, Sor, and Sun. All 3 drugs are used primarily in cancer therapy.40 However, there is a difference in potency between these compounds. Sun is a type 1 tyrosine kinase inhibitor, which has a higher affinity to PDGF receptor and thus potentially more effect on the PDGF signaling route than the type 2 inhibitors, such as Ima and Sor.41, 42 Our results showed that Sun had a clear effect on the gene expression of fibrosis markers and the protein levels of Hsp47 and Fibronectin, and the excretion of procollagen 1. Sun also significantly downregulated the pathway-related gene expression of Pai-1 and Ctgf, suggesting that Sun has an inhibitory effect upstream of the molecular pathogenesis of intestinal fibrosis, most likely by blocking the PDGF-α and PDGF-β receptors.43 Moreover, a recent study from Huang et al showed that Sun suppressed the degree of epithelial to mesenchymal transition induced by TGF-β in human bronchial epithelial cells and the proliferation of WI-38 human lung fibroblasts. Although the mechanism remains unknown, they showed that Sun, as a tyrosine kinase inhibitor, reduced the phosphorylation of serine residues on Smad2/3, which is induced by TGF-β.44 This is in line with our study, as Sun significantly downregulated the downstream targets of TGF-β signaling, Pai-1 and Ctgf. These results may indicate that Sun also has anti-TGF-β activity.
Westra et al used the ex vivo rat PCLS model to test Ima, Sor, and Sun.30 They demonstrated that Ima was the most effective antifibrotic compound in both the early and late stages of liver fibrosis in rat PCLS,13, 30 even though it did not become effective in human PCLS.45 Also, our results showed that Ima did not influence intestinal fibrosis in murine PCIS. Thus, it is clear that Ima elicits organ- and species-specific effects.
Recently, Qu et al reviewed the antifibrotic effect of Ima and Sor on liver fibrosis. The beneficial effects of these compounds were observed in preclinical animal models and in patients with liver fibrosis. Ima reduced the number of activated HSCs and inhibited ECM production in preclinical models only during early fibrogenesis and not in established fibrosis.46 Sor is used as the first treatment for advanced hepatocellular carcinoma cells in the clinical trials.47 In a recent multicenter, placebo-controlled randomized clinical trial of Sor, antifibrotic effects in patients with a fibrotic livers were also found.46
From the result of our study, it can be concluded that although Ima, Sor, and Sun all inhibit tyrosine kinase activity, only Sun effectively downregulated the expression of fibrosis markers in the PCIS model. From our results, it may be concluded that the dual effect on both TGF-β and PDGF signaling pathways of Sun may be beneficial in intestinal fibrosis. However, a recent case report from Boers-Sonderen et al showed that Sun treatment could cause an exacerbation of pre-existing Crohn’s disease.48 Therefore, even if our result gave an insight that Sun has a potential antifibrotic effect, more studies are necessary before Sun can be used in patients with intestinal fibrosis. Thus, a better understanding of the mechanisms of action of Sun will help to explain the observed side effects and improve its safety profile.
CONCLUSION
This study shows that PCIS could be a valuable preclinical drug-screening tool. Of the various compounds that we tested, only Sun markedly impacted the expression of fibrosis markers. This drug candidate and its mechanism of action should be further investigated to unveil its therapeutic aptitude completely. Future studies using human PCIS will establish whether these potential antifibrotic compounds are also effective in man.
Supplementary Material
Supported by: This work is supported by funding from De Nederlandse organisatie voor gezondheidsonderzoek en zorginnovatie (ZonMw); the Netherlands (Grant 114025003).
REFERENCES
- 1. Burke JP, Mulsow JJ, O’Keane C, et al. Fibrogenesis in Crohn’s disease. Am J Gastroenterol. 2007;102:439–448. [DOI] [PubMed] [Google Scholar]
- 2. Rieder F, Fiocchi C. Mechanisms of tissue remodeling in inflammatory bowel disease. Dig Dis. 2013;31:186–193. [DOI] [PubMed] [Google Scholar]
- 3. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Latella G, Rogler G, Bamias G, et al. Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis. 2014;8:1147–1165. [DOI] [PubMed] [Google Scholar]
- 5. Kumagai S, Ohtani H, Nagai T, et al. Platelet-derived growth factor and its receptors are expressed in areas of both active inflammation and active fibrosis in inflammatory bowel disease. Tohoku J Exp Med. 2001;195:21–33. [DOI] [PubMed] [Google Scholar]
- 6. Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease - Current knowledge and future perspectives. J Crohns Colitis. 2008;2:279–290. [DOI] [PubMed] [Google Scholar]
- 7. Krause C, Kloen P, Ten Dijke P. Elevated transforming growth factor β and mitogen-activated protein kinase pathways mediate fibrotic traits of Dupuytren’s disease fibroblasts. Fibrogenesis Tissue Repair. 2011;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Speca S, Giusti I, Rieder F, Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012;18:3635–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latella G, Sferra R, Speca S, et al. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur Rev Med Pharmacol Sci. 2013;17:1283–1304. [PubMed] [Google Scholar]
- 10. Iswandana R, Pham BT, van Haaften WT, et al. Organ- and species-specific biological activity of rosmarinic acid. Toxicol in Vitro. 2016;32:261–268. [DOI] [PubMed] [Google Scholar]
- 11. Luangmonkong T, Suriguga S, Adhyatmika A, et al. In vitro and ex vivo anti-fibrotic effects of LY2109761, a small molecule inhibitor against TGF-β. Toxicol Appl Pharmacol. 2018;355:127–137. [DOI] [PubMed] [Google Scholar]
- 12. Luangmonkong T, Suriguga S, Bigaeva E, et al. Evaluating the antifibrotic potency of galunisertib in a human ex vivo model of liver fibrosis. Br J Pharmacol. 2017;174:3107–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westra IM, Oosterhuis D, Groothuis GM, et al. Precision-cut liver slices as a model for the early onset of liver fibrosis to test antifibrotic drugs. Toxicol Appl Pharmacol. 2014;274:328–338. [DOI] [PubMed] [Google Scholar]
- 14. Friedman SL, Sheppard D, Duffield JS, et al. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr1. [DOI] [PubMed] [Google Scholar]
- 15. Mannaerts I, Nuytten NR, Rogiers V, et al. Chronic administration of valproic acid inhibits activation of mouse hepatic stellate cells in vitro and in vivo. Hepatology. 2010;51:603–614. [DOI] [PubMed] [Google Scholar]
- 16. Schaefer CJ, Ruhrmund DW, Pan L, et al. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yin MF, Lian LH, Piao DM, et al. Tetrandrine stimulates the apoptosis of hepatic stellate cells and ameliorates development of fibrosis in a thioacetamide rat model. World J Gastroenterol. 2007;13:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. [DOI] [PubMed] [Google Scholar]
- 19. Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs - a systematic review. Fibrogenesis Tissue Repair. 2014;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andoh A, Fujino S, Okuno T, et al. Intestinal subepithelial myofibroblasts in inflammatory bowel diseases. J Gastroenterol. 2002;37 Suppl 14:33–37. [DOI] [PubMed] [Google Scholar]
- 21. Chen YT, Chang FC, Wu CF, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. [DOI] [PubMed] [Google Scholar]
- 22. Spinelli A, Correale C, Szabo H, et al. Intestinal fibrosis in Crohn’s disease: medical treatment or surgery? Curr Drug Targets. 2010;11:242–248. [DOI] [PubMed] [Google Scholar]
- 23. Westra IM, Pham BT, Groothuis GM, et al. Evaluation of fibrosis in precision-cut tissue slices. Xenobiotica. 2013;43:98–112. [DOI] [PubMed] [Google Scholar]
- 24. Rieder F, Kessler S, Sans M, et al. Animal models of intestinal fibrosis: new tools for the understanding of pathogenesis and therapy of human disease. Am J Physiol Gastrointest Liver Physiol. 2012;303:G786–G801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stribos EG, Hillebrands JL, Olinga P, et al. Renal fibrosis in precision-cut kidney slices. Eur J Pharmacol. 2016;790:57–61. [DOI] [PubMed] [Google Scholar]
- 26. Pham BT, van Haaften WT, Oosterhuis D, et al. Precision-cut rat, mouse, and human intestinal slices as novel models for the early-onset of intestinal fibrosis. Physiol Rep. 2015;3(4):e12323. doi:10.14814/phy2.12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Graaf IA, Olinga P, de Jager MH, et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat Protoc. 2010;5:1540–1551. [DOI] [PubMed] [Google Scholar]
- 28. Beddy D, Mulsow J, Watson RW, et al. Expression and regulation of connective tissue growth factor by transforming growth factor beta and tumour necrosis factor alpha in fibroblasts isolated from strictures in patients with Crohn’s disease. Br J Surg. 2006;93:1290–1296. [DOI] [PubMed] [Google Scholar]
- 29. Mulsow JJ, Watson RW, Fitzpatrick JM, et al. Transforming growth factor-beta promotes pro-fibrotic behavior by serosal fibroblasts via PKC and ERK1/2 mitogen activated protein kinase cell signaling. Ann Surg. 2005;242:880–7, discussion 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Westra IM, Oosterhuis D, Groothuis GM, et al. The effect of antifibrotic drugs in rat precision-cut fibrotic liver slices. Plos One. 2014;9:e95462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawaoka K, Doi S, Nakashima A, et al. Valproic acid attenuates renal fibrosis through the induction of autophagy. Clin Exp Nephrol. 2017;21:771–780. [DOI] [PubMed] [Google Scholar]
- 32. Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. [DOI] [PubMed] [Google Scholar]
- 33. Hisatomi K, Mukae H, Sakamoto N, et al. Pirfenidone inhibits TGF-β1-induced over-expression of collagen type I and heat shock protein 47 in A549 cells. BMC Pulm Med. 2012;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;289:211–218. [PubMed] [Google Scholar]
- 35. Flechsig P, Dadrich M, Bickelhaupt S, et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-β and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18:3616–3627. [DOI] [PubMed] [Google Scholar]
- 36. Melisi D, Ishiyama S, Sclabas GM, et al. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7:829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hata A, Akhurst RJ. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi:10.1038/nrd3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–1878. [DOI] [PubMed] [Google Scholar]
- 40. Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. [DOI] [PubMed] [Google Scholar]
- 41. Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kufareva I, Abagyan R. Type-II kinase inhibitor docking, screening, and profiling using modified structures of active kinase states. J Med Chem. 2008;51:7921–7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faivre S, Demetri G, Sargent W, et al. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–745. [DOI] [PubMed] [Google Scholar]
- 44. Huang X, Wang W, Yuan H, et al. Sunitinib, a small-molecule kinase inhibitor, attenuates bleomycin-induced pulmonary fibrosis in mice. Tohoku J Exp Med. 2016;239:251–261. [DOI] [PubMed] [Google Scholar]
- 45. Westra IM, Mutsaers HA, Luangmonkong T, et al. Human precision-cut liver slices as a model to test antifibrotic drugs in the early onset of liver fibrosis. Toxicol in Vitro. 2016;35:77–85. [DOI] [PubMed] [Google Scholar]
- 46. Qu K, Huang Z, Lin T, et al. New insight into the anti-liver fibrosis effect of multitargeted tyrosine kinase inhibitors: from molecular target to clinical trials. Front Pharmacol. 2016;6(JAN):1–8. doi:10.3389/fphar.2015.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mejias M, Garcia-Pras E, Tiani C, et al. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–1256. [DOI] [PubMed] [Google Scholar]
- 48. Boers-Sonderen MJ, Mulder SF, Nagtegaal ID, et al. Severe exacerbation of Crohn’s disease during sunitinib treatment. Eur J Gastroenterol Hepatol. 2014;26:234–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.