Abstract
Acquired immune function shows recognizable changes over time with organismal aging. These changes include T-cell dysfunction, which may underlie diminished resistance to infection and possibly various chronic age-associated diseases in the elderly. T-cell dysfunction may occur at distinct stages, from naive cells to the end stages of differentiation during immune responses. The thymus, which generates naive T cells, shows unusually early involution resulting in progressive reduction of T-cell output after adolescence, but peripheral T-cell numbers are maintained through antigen-independent homeostatic proliferation of naive T cells driven by the major histocompatibility complex associated with self-peptides and homeostatic cytokines, retaining the diverse repertoire. However, extensive homeostatic proliferation may lead to the emergence of dysfunctional CD4+ T cells with features resembling senescent cells, termed senescence-associated T (SA-T) cells, which increase and accumulate with age. In situations such as chronic viral infection, T-cell dysfunction may also develop via persistent antigen stimulation, termed exhaustion, preventing possible immunopathology due to excessive immune responses. Exhausted T cells are developed through the effects of checkpoint receptors such as PD-1 and may be reversed with the receptor blockade. Of note, although defective in their regular T-cell antigen-receptor-mediated proliferation, SA-T cells secrete abundant pro-inflammatory factors such as osteopontin, reminiscent of an SA-secretory phenotype. A series of experiments in mouse models indicated that SA-T cells are involved in systemic autoimmunity as well as chronic tissue inflammation following tissue stresses. In this review, we discuss the physiological aspects of T-cell dysfunction associated with aging and its potential pathological involvement in age-associated diseases and possibly cancer.
Keywords: age-associated diseases, homeostatic proliferation, senescence-associated T cells, T-cell dysfunction, T-cell exhaustion
The cellular basis of T-cell senescence
Introduction
Aging is not a disease per se, but it does result in physiological changes of organismal functions in all animals. Over time, however, aging may result in the increased incidence of various common chronic diseases, such as visceral adiposity and diabetes, atherosclerotic cardiovascular diseases, rheumatic arthritis, renal sclerosis, and degenerative brain diseases, which are often called age-associated diseases (1). Whereas the phenotypes of organismal aging comprise complex changes in most vital organs, age-associated diseases are characterized by chronic low-grade inflammatory reactions in various tissues (2–4). These diseases may be triggered by distinct etiological factors, either genetic or environmental, but aging apparently prompts the overt manifestations of these chronic diseases.
Accumulating evidence indicates that cellular senescence in tissues may underlie these chronic diseases, at least in part (5). Originally, cellular senescence was viewed as an irreversible cell-cycle arrest mechanism (6) and, as such, it was proposed as a safeguard against malignant transformation of cells (7). However, more recent studies revealed that cell senescence includes diverse cellular states after the initial growth arrest, with progression involving multiple steps including profound chromatin changes (8), and cell senescence may play broader roles in age-related pathologies (5).
Among vital organ systems, the immune system also shows significant changes in overall function over time with age, including a diminished acquired immune capacity, increasing pro-inflammatory traits and a higher risk for autoimmunity (9). Immune system aging may reflect functional alterations in diverse cell components in the immune system as well as their dynamic networks with age. While it seems likely that cellular senescence may play a part in it, the cellular basis leading to immune aging phenotypes is poorly understood. In this review, we summarize the features and cellular basis of immune system aging from the perspective of T cells that control the immune response, and discuss their potential involvement in age-associated pathology.
Age-associated changes in T-cell dynamics
One of the most prominent and earliest visible changes in the immune system that occurs with age is shrinkage of the thymus. Thymic involution is already evident in adolescence and the organ is almost entirely replaced by fat tissue in middle age and later (10, 11). Accordingly, the rate of thymic T-cell output (around 2 × 106 cells per day at the peak) declines over time, with an estimated half-life of about 16 years in humans (12). Although the primary cause of thymic involution was initially thought to be the decreased capacity of T-cell progenitors to home and develop in the thymus, recent studies strongly suggest that age-dependent deterioration of the thymic epithelial cells (TECs) also plays an important part (13, 14).
We recently reported that the clonogenic activity of TEC stem cells rapidly diminishes soon after birth, leading to the defective replenishment of TECs at the adult stage (15). However, the self-renewing activity of TEC stem cells was maintained well after birth in mice that had impaired T-cell development due to genetic defects in T-cell progenitors such as Rag2−/− mice. This finding suggests that robust proliferation of T-cell progenitors followed by massive cell death during the selection processes, which are crucial for generating functional T cells with a diverse repertoire, may prompt the deterioration of TEC turnover (15). As such, it appears that thymic involution may be an inevitable cost for the robust thymopoiesis that occurs during the perinatal stage (16).
Despite the progressive decline of thymic naive T-cell output because of thymic involution, total T-cell numbers in the periphery are largely maintained with age in healthy adults. However, while T cells in infants show a naive phenotype in the quiescent state, those with a memory phenotype (MP) progressively increase in proportion over time and become predominant at midlife and later (17, 18). Human studies also noted that, with age, increasing proportions of naive T cells were in a cycling state (19, 20). Subsequent studies have suggested that such an autonomous proliferation of naive T cells represents antigen-independent proliferation, comparable to the T-cell homeostatic proliferation (HP) that occurs when the host becomes moderately T-lymphopenic, for instance, after sub-lethal γ-ray irradiation (17, 21, 22) or thymectomy (19, 23). Notably, naive T cells that have undergone HP also eventually show phenotypic conversion to MP, with the effect being more prominent in mice than in humans (17, 21, 22).
It was reported that naive T cells showed marked proliferation in γ-ray-irradiated young mice, whereas these T cells barely proliferated in γ-ray-irradiated old mice (24). However, we have indicated that naive T cells from young mice hardly proliferated when transferred into intact young mice, but showed remarkable cell divisions in untreated aged mice followed by a phenotypic conversion to MP (25). Hence, it is suggested that the lymphoid tissue environment of aged mice allows extensive HP of the introduced naive T cells, although the supporting capacity of HP per se is much more radio-sensitive in aged mice than in young mice; the effect may reflect the age-dependent changes in stroma cells providing homeostatic cytokines (see below). In any case, it appears that maintenance of the peripheral T-cell pool size becomes increasingly dependent on the HP of peripheral naive T cells over time with age; the situation may be more prominent in humans than in mice probably because of humans’ much longer life span (26).
HP and senescence-associated T cells
All naive T cells that have been positively selected in the thymus bear weak yet measurable reactivity to major histocompatibility complex (MHC) associated with self-peptides, and the T cells may be under constant tonic signals from surrounding cells expressing self-MHC (17). Although the tonic T-cell antigen-receptor (TCR) signal alone may be insufficient for triggering their proliferation, naive T cells can be induced to proliferate in the presence of sufficient amounts of IL-7 and IL-15, known as homeostatic cytokines, which are increased in T-lymphopenic lymphoid tissues (17, 27). As such, the HP of naive T cells is largely non-clonal and instead crucially depends on the availability of homeostatic cytokines in the microenvironment. The proliferation rate is relatively slow, one cell division per 3–4 days, as compared with antigen-driven clonal T-cell proliferation with one cell division or more per day.
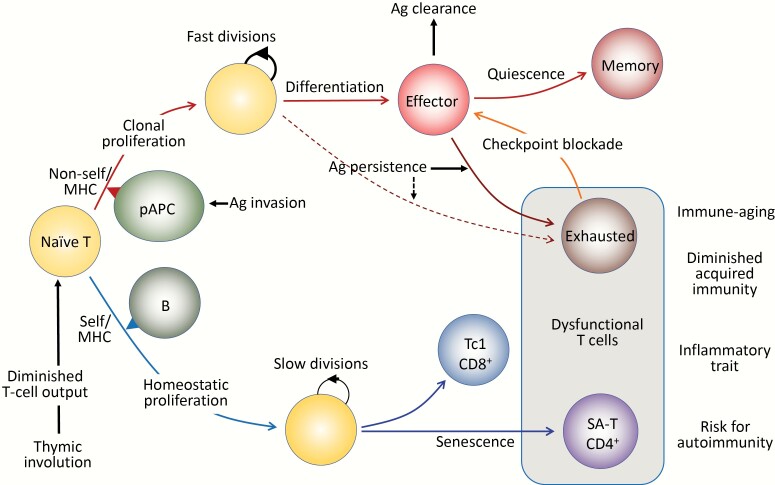
Eventual cell fates of HP of naive T cells may be different from those of antigen-driven proliferation (Fig. 1). In response to specific antigens, the initial clonal proliferation via an optimal TCR signal combined with proper costimulatory signals from professional antigen-presenting cells is linked to the programmed differentiation into effector cells, which is followed by activation-induced cell death or conversion to quiescent memory cells as antigens are cleared. To avoid immunopathology due to excessive immune responses, however, some of the effector T cells, particularly those of the CD8+ cell lineage, may become dysfunctional when the antigen stimulation persists, such as in chronic viral infection and possibly cancer, which is known as T-cell exhaustion (28, 29). Exhausted T cells are characterized by the constitutive expression of inhibitory immunoreceptors called checkpoint receptors, such as PD-1 and LAG3, and the function may be reverted by checkpoint blockade (30) (Fig. 1, upper).
Fig. 1.
Antigen (Ag)-driven and antigen-independent generation of dysfunctional T cells. (Upper) In response to the optimal TCR stimulation via foreign antigens presented by professional antigen-presenting cells (pAPCs) expressing proper costimulatory molecules, specific naive T cells initiate robust clonal proliferation with fast cell divisions, followed by functional differentiation to various effector cells. As the antigens are cleared, the effector cells may die off, but a portion of them become quiescent and are maintained as central memory T cells. However, when antigen stimulation persists, the effector cells may go into a dysfunctional state via constitutive expression of checkpoint receptors such as PD-1 and LAG3 to prevent immunopathology due to excessive immune responses, called exhausted T cells. The exhausted T cells may also be derived from unique progenitor cells (pre-exhausted T cells). The function of exhausted T cells may be reverted with checkpoint blockade, although these T cells may eventually become refractory. (Lower) Naive T cells that developed through positive selection in the thymus have intrinsic affinity to self-MHCs and are under tonic TCR stimulation, mainly through B cells for CD4+ T cells. Although the tonic TCR signal per se is insufficient for causing proliferation, naive T cells initiate HP with slow cell divisions in the presence of sufficient amounts of homeostatic cytokines (IL-7, IL-15), which are increased in the lymphoid milieu in T-lymphopenic conditions. Thymic involution begins early in life with a progressive reduction of naive T-cell output over time, which increasingly drives HP of naive T cells with age to maintain the size of the T-cell pool in the periphery. Sustained HP of CD8+ T cells leads to the generation of CXCR3+ cells with increased capacity of IFNγ/TNFα production, predisposed to Tc1-type pro-inflammatory T cells. In CD4+ T cells, continuous HP results in the generation of dysfunctional T cells bearing the features resembling cell senescence, termed senescence-associated T (SA-T) cells. The SA-T cells are also characterized by the constitutive expression of PD-1 and LAG3, and partly CD153, although there is so far no evidence that the function is restored by PD-1 checkpoint blockade. SA-T cells are defective in proliferation and regular differentiation in response to optimal TCR stimulation, but these T cells secrete abundant pro-inflammatory cytokines, such as osteopontin and chemokines directed to innate inflammatory cells (Ccl3, 4), reminiscent of SASP. SA-T cells are progressively increased in proportion with age, but in addition, these T cells may remarkably accumulate in the GCs of lymphoid tissues or various other tissues under stresses or insults, predisposing to systemic autoimmunity or age-associated chronic inflammatory diseases.
The sustained T-cell HP causes heterogeneity of the naive T-cell compartment (31), and the consequences may differ in CD4+ and CD8+ lineages. Naive CD8+ T cells that had undergone HP tended to show an up-regulated expression of CXCR3 with an increased capacity to produce IFNγ and TNFα, leading to a predisposition to become Tc1 cytotoxic/inflammatory T cells (32). On the other hand, CD4+ T cells become progressively dysfunctional after extensive cell divisions during persistent HP in mice, resulting in a markedly reduced capacity to proliferate and differentiate even via optimal TCR signaling (25). These CD4+ T cells also exhibit constitutive PD-1 expression, and to a lesser proportion CD153, although there is so far no evidence of the involvement of PD-1 in their dysfunction (33). It is mostly PD-1+ CD153−/+ cells among CD4+ MP T cells that increase over time with age; the effects were barely observed in aged mice deficient in B cells, which are the major population to provide tonic signals for naive CD4+ T cells via self-MHC class II (33) (Fig. 1, lower).
Further analysis has revealed that the PD-1+ MP CD4+ T cells in aged mice exhibit characteristic features resembling cell senescence, including markedly enhanced expression of senescence-associated β-galactosidase (SA-β-Gal) and negative cell-cycle regulators (Cdkn1a, Cdkn2b), increased heterochromatin foci (SAHF) and increased expression of a DNA-damage-repairing complex (H2A.X) (33) as well as a histone subtype unique for senescent cells (H2A.J) (34).
Notably, despite the defective TCR-mediated proliferation and differentiation, they secreted abundant osteopontin along with other potentially pro-inflammatory cytokines, which is reminiscent of a senescence-associated secretory phenotype (SASP) (33, 35). Hence, we termed these CD4+ T cells senescence-associated T (SA-T) cells. Among the SA-T cells, a minor CD153+ cell fraction that experienced more cycles of cell divisions exhibited even more profound senescence-related features, suggesting that the dysfunctional state progressed along repeated cell cycles during HP. The features of SA-T cells in aged mice were indeed indistinguishable from those that developed from naive CD4+ T cells following extensive HP in sub-lethally γ-ray-irradiated young hosts (25). The accelerated SA-T cell generation by extensive HP in mice may be consistent with the observation of premature T-cell aging in thymectomized human patients (36).
Together, these findings strongly suggest that sustained HP of naive CD4+ T cells with age intrinsically results in the increasing accumulation of dysfunctional CD4+ T cells bearing senescence-related features. Supporting this notion, transplantation of fetal thymi at midlife caused reduced T-cell HP and delayed the increase in SA-T cells at later stages (25).
Multiple mechanisms underlying age-associated T-cell dysfunction
Recent reports have revealed that the profile of chromatin modifications regulating epigenetic phenotypes in human immune cells including T cells shows remarkable age-associated alterations with increased heterogeneity among individual cells (37–39). Notably, the effects are predominantly attributable to non-heritable influences (39). Although the mechanisms remain to be seen, such increased epigenetic variations may underlie age-associated dysfunction of T cells. For instance, in mice, deficiency of Menin resulted in reduced antigen responsiveness of CD4+ T cells in association with some features reminiscent of cell senescence including a SASP-like phenotype, such as abundant osteopontin secretion (40). The effects were attributed to the defective epigenetic regulation of Bach2, which encodes a transcription factor involved in various immune responses. However, it remains to be investigated whether such a mechanism is indeed involved in the ‘physiological’ progression of age-associated dysfunction of CD4+ T cells.
Human CD4+ T cells showed a progressive decline of the expression of a particular micro-RNA, miR-181a, with age in the naive, but not the MP, population (41). The CD4+ T cells with reduced miR-181a expression showed a decreased sensitivity, or an increased threshold, for TCR-induced activation of extracellular signal-regulated kinase because of the enhanced expression of a dual specific phosphatase (DUSP6), a target of miR-181a, and the dysfunction was reverted by re-expression of miR-181a. The naive CD4+ T cells with reduced miR-181a may show a biased differentiation toward Th2 over Th1 cells, since Th2 differentiation requires a lower TCR signal strength than Th1 does, possibly leading to a pro-inflammatory trait in the elderly (41). We also noted a profound decline of miR-181a in murine SA-T cells (33), but it remains to be clarified whether the entire senescence-related features of SA-T cells can be accounted for solely by this effect.
In humans, CD4+ T cells show progressive loss of costimulatory receptors, such as CD27 and CD28 during end-stage differentiation following antigen stimulation, and the numbers of CD27− CD28− CD4+ T cells increase in the elderly (42–44). The CD27− CD28− CD4+ T cells show a reduced TCR-induced proliferation capacity with shortened telomeres and an increased DNA-damage response, referred to as senescent T cells, but they exhibit potent effector functions and may not be viewed as dysfunctional cells per se (45). More recently, CD27− CD28− CD4+ T cells were reported to show a markedly increased expression of sestrins, a family of stress-inducible metabolic regulators (46), forming a novel immune-inhibitory complex with mitogen-activated protein kinases that was responsible for the unique features of senescent CD4+ T cells (47).
These so-called senescent T cells have been considered in the context of antigen-driven terminal differentiation of T cells and are increased during chronic viral infection, resembling the aforementioned exhausted effector T cells, both of which are potentially reversible effects. Bone fide naive T cells in elderly humans also show shorter telomeres than the equivalent cells in young individuals (48), possibly via HP, but it remains unclear whether such naive T cells with shorter telomeres also become dysfunctional due to the sestrin-based immune-inhibitory complex.
SA-T cells derived from naive CD4+ T cells in an antigen-independent manner show high basal levels of transcripts for diverse pro-inflammatory genes including Spp1 encoding osteopontin, being consistent with increased transcriptional noise as a signature of aging (39). Curiously, upon optimal TCR stimulation, the SA-T cells exhibited a further increase in Spp1 expression followed by abundant osteopontin secretion, whereas production of regular T-cell cytokines, such as IL-2 and IL-4 remained negligible (33). Further, PD-1 ligation, which suppressed even the minimal residual IL-4 production, did not affect the TCR-mediated osteopontin production by SA-T cells at all (33). These results imply that an atypical pro-inflammatory gene expression reminiscent of SASP in SA-T cells may somehow be linked to TCR signaling via unconventional downstream pathways distinct from main pathways mediating the proliferation and T-cell cytokine production.
CD153 ligation enhances TCR-induced osteopontin secretion in SA-T cells (33), suggesting that CD153 signaling may play a role in the unique cross-talk of TCR signaling and SASP-like gene expression. CD153 mediates signals via ligation with CD30, so-called reverse signaling where the ligand-bearing cell is stimulated (49), and examination of a possible role for CD153 in the progression and/or maintenance of SA-T cells is currently underway. It seems also possible that increased variation of chromatin marks with stochastic chromatin opening at otherwise repressed sits is associated with repeated cell divisions in SA-T cells.
SA-T cells and systemic autoimmunity
Aging may be associated with an increased risk of autoimmunity (9, 20), which can be either central or peripheral. Thymic involution, in particular the diminution in numbers of medullary TECs, which play an essential role in establishing central T-cell self-tolerance, may increase the potential risk for the breakdown of self-tolerance (50). On the other hand, studies have suggested that HP of naive T cells in the periphery also favors the development of autoimmunity, partly because persistent HP may cause a biased increase in the T-cell population bearing higher intrinsic affinity to self-MHC molecules (51–54). It was reported, for instance, that the development of insulin-dependent diabetes mellitus in non-obese diabetic mice, which show intrinsic T-lymphopenia, was delayed by suppressing the endogenous T-cell HP with continuous infusion of normal T cells (55).
In addition, SA-T cells in aged mice tend to localize in the splenic follicular region, often in association with spontaneous germinal centers (GCs) (33). Robust spontaneous GC reactions of self-reactive B cells are a characteristic feature of lupus disease (56), and genetically lupus-prone female BWF1 mice spontaneously develop abundant GCs at an early stage, which contain CD4+ T cells with features indistinguishable from SA-T cells in aged mice (33). These T cells shared the high expression of several genes, such as Pdcd1, Bcl6, and Ascl2 in common with follicular helper T cells induced by antigen immunization, but follicular helper T cells rarely expressed CD153, and overall transcriptomes were quite distinct from each other (33). Functionally, the SA-T cells in female BWF1 mice barely produce IL-4, a principal cytokine produced by follicular helper T cells, but instead produce abundant osteopontin in response to autologous GC-B cells in a TCR/MHC II-dependent manner (33). As such, GC reactions in regular immune responses and systemic autoimmunity appear to involve different types of ‘follicular’ T cells.
B-cell intrinsic Toll-like receptor 7 (TLR7) signaling plays a crucial role in spontaneous GC reactions and anti-nuclear antibody production in mice (57), and TLR7-ligand administration in vivo also induces a rapid increase and accumulation of SA-T cells in the spontaneous GCs (58), suggesting that the increased SA-T-cell numbers are associated with TLR7-signaled B cells. Robust proliferation of B cells in GCs is accompanied by their massive apoptosis, but normally the apoptotic cells are swiftly engulfed and digested by professional phagocytic cells called tingible body macrophages (TB-Mφ) (59). However, in lupus disease, it appears that the disposal of apoptotic bodies in GCs is defective for unknown reasons, allowing sustained exposure of non-tolerized nuclear components to the GC B cells and eventual anti-nuclear antibody production (60).
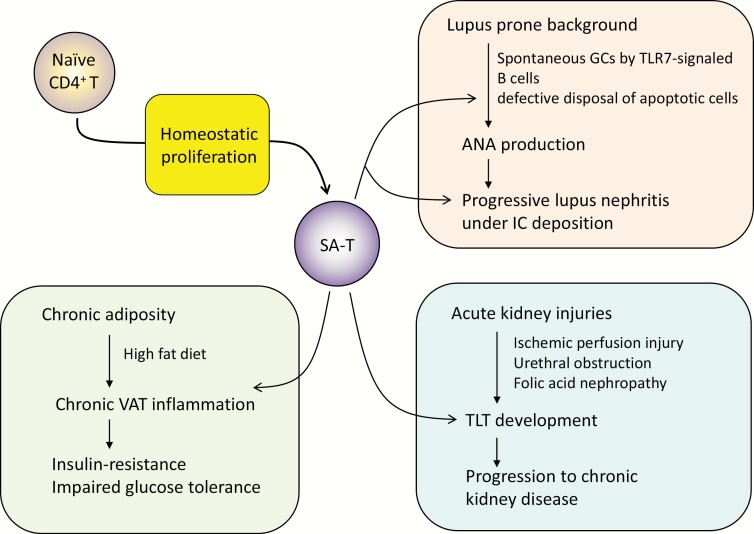
Osteopontin secreted by SA-T cells in GCs inhibits the engulfment of apoptotic bodies by TB-Mφ through sustained Rac1 GTPase activation in them, and treatment with anti-osteopontin antibody delayed the development of anti-nuclear antibodies and lupus nephritis in female BWF1 mice (33, 58). Thus, besides aging, SA-T cells are increased robustly and prematurely in mice with a genetic predisposition to lupus with abnormal B cells and play a crucial role in the development of systemic autoimmune diseases. We noted that PD-1+ CD153+ SA-T cells also accumulated in the lymphoid follicular structures of affected kidneys of female BWF1 mice (33), suggesting that these T cells may play a role in the progression of lupus nephritis with immune-complex deposition as well (Fig. 2).
Fig. 2.
Involvement of SA-T cells in systemic autoimmune disease and chronic inflammatory diseases. Detailed explanation may be found in the text. SA-T, senescence-associated CD4+ T cells; GC, germinal center; TLR7, Toll-like receptor 7; ANA, anti-nuclear antibody; IC, immune complex; TLT, tertiary lymphoid tissue; VAT, visceral adipose tissue.
Impacts of SA-T cells on age-associated diseases
Chronic low-grade tissue inflammation in various tissues underlies common age-associated diseases (2–4). For instance, chronic inflammation in visceral adipose tissue (VAT) in association with obesity predisposes metabolic and cardiovascular diseases of the elderly (61). In a mouse model, a sustained high fat diet (HFD) caused obesity with VAT inflammation and impaired glucose tolerance due to insulin-resistance. We recently discovered that SA-T cells were robustly increased in the VAT along with macrophages and B cells in HFD mice (35). As anticipated, the SA-T cells in VAT, in particular the PD-1high CD153+ population, were defective in TCR-mediated proliferation but secreted remarkably abundant osteopontin along with other pro-inflammatory cytokines, such as TNFα and IL-6 (35) (Fig. 2).
Further, transfer of the SA-T cells isolated from the VAT of wild-type mice under HFD into the VAT of mice under normal diet caused a VAT inflammatory reaction and impaired glucose-tolerance even in the absence of obesity, indicating that SA-T cells initiate the VAT inflammation and resulting insulin resistance; transfer of SA-T cells isolated from Spp1−/− mice under HFD barely led to any such changes, suggesting a role of osteopontin derived from SA-T cells (35). Curiously, mice that had been under HFD continued to show high levels of SA-T cells in VAT and exhibited a residual insulin-resistance for some periods even after the shift to normal diet with reduced body weight, suggesting that the SA-T cells are quite stable and long-lived in the tissues (62). The increase in SA-T cells in the VAT of HFD mice also depended on B cells as anticipated, but it remains to be investigated whether the SA-T cells are generated in situ in VAT or recruited systemically from outside.
Chronic kidney disease is another common disease associated with aging, which often follows acute kidney injury such as ischemic perfusion injury, urethral obstruction, and folic acid nephropathy and may progress into end-stage renal disease (63, 64). Indeed, in mouse models, the progression to chronic kidney disease following transient acute kidney injury is frequently observed in aged mice, although the tissue damage is completely cleared in young mice (65). Such a chronic kidney disease in aged mice is characterized by the development of tertiary lymphoid tissues (TLTs) consisting of T cells including PD-1+ CD4+ T cells, B cells and stromal fibroblasts secreting abundant Cxcl13, and CD4+ T cells play a crucial role in the TLT development (65). Importantly, TLTs with comparable cellular and molecular components were often observed in kidneys of the aged, but rarely young, humans (65). The detailed features of pathogenic PD-1+ CD4+ T cells remain to be investigated, but it seems likely that SA-T cells persisting in the TLTs in much a similar manner in the splenic follicles take an important part in the progression of chronic kidney disease (Fig. 2).
Human counterparts of murine SA-T cells remain to be carefully investigated. Recent reports indicate the increase in unconventional CD4+ T cells in various chronic inflammatory diseases in humans, which may play a pathogenic role (66–68). Although the origins as well as major driving forces and signals for these ‘pathogenic’ T cells remain to be clarified, murine SA-T cells seem to share features with these human T cells, at least partly, including constitutive high PD-1 expression and atypical cytokine production. It was also reported that improved immune function of the elderly with mTOR inhibitor administration was associated with reduced PD-1+ T cells in the blood (69).
T-cell aging and cancer
The incidence of cancer also increases with age, and this has been attributed to the accumulation of somatic cancer driver-gene mutations over time with age (70). However, recent genetic analyses have indicated that increasing proportions of ‘normal cells’ in various tissues of the elderly also show accumulation of driver-gene mutations in the absence of cancer and, thus, driver mutations may co-exist in the non-cancerous cells (71, 72). These findings may challenge the classical idea that the accumulation of driver-gene mutations is directly linked to the increased cancer incidence with age. Rather, such ‘normal cells’ with driver-gene mutations may constitute a part of the aging phenotype, including oncogene-induced senescence (73).
Research indicates that host acquired immunity plays a crucial role in suppressing spontaneous tumor development in mice (74) and controlling the progression of cancer in both mice and humans (75, 76). Thus, the diminution of immune surveillance may also contribute to the increase in cancer incidence with age. Supporting this notion, a recent immune-epidemiological analysis showed that the age-related increase in the clinical incidence of many types of human cancers can be well modeled based on thymic involution and the resulting reduced T-cell output (77).
On the other hand, it was reported that senescent cells characterized by high Cdkn2a (p16Ink4a) expression increasingly accumulate throughout the tissues with age in a mouse model (78), and the genetic clearance of p16Ink4a-positive cells in tissues starting at midlife resulted in the improved function of vital organs at a later stage (79). Most notably, these mice showed significantly reduced cancer-related death even though rates of incidence and spectrum of macroscopically detectable tumors are unchanged at autopsy, suggesting that senescent cells in cancer tissues may shorten the cancer latency.
These results may highlight the importance of the tissue microenvironment in cancer growth and progression. Effects of tissue stroma cells on cancer cells may be largely mediated through host local immunity, either positively or negatively. Recent research indicates distinct types of stromal cells in cancer tissues; certain stromal reactions that cause an inflammatory tissue environment by recruiting various innate immune cells may often favor tumor growth, whereas others may effectively recruit specific T-cell immunity to restrain tumor progression (80–82). It seems likely that increased numbers of senescent tissue cells may exaggerate the inflammatory environment favoring tumor progression. In mouse models, we found that the pro-inflammatory activity of cancer stroma cells was little affected or instead increased with age, whereas stromal function capable of recruiting host primed T cells in tumor tissues was highly sensitive to aging, being markedly reduced with age (Y. Xu and N. Minato, unpublished data). These findings suggest that distinct functions of different stroma cell types are differentially affected by aging (Fig. 3).
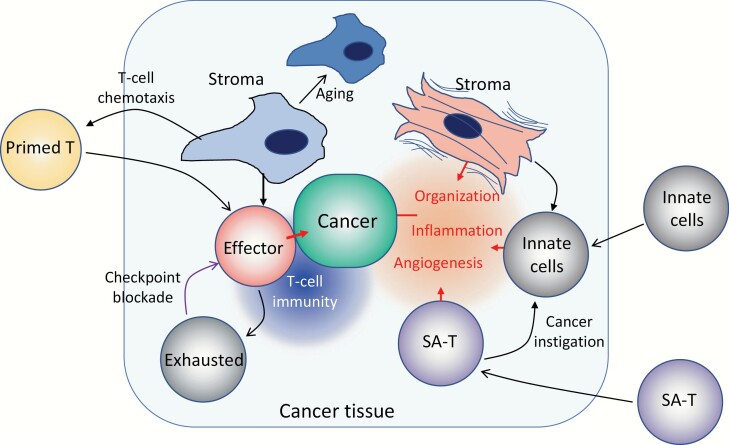
Fig. 3.
Schematic representation of T-cell dysfunction and tissue aging in cancer. In cancer tissues, various tissue reactions with often conflicting effects on cancer progression may occur, where tissue stroma cells play important roles. Certain stroma cell types may promote effective recruitment of primed T cells into the tissue via secretion of T-cell chemokines and forming a proper scaffold for their migration. Such T cells at a close enough vicinity to cancer cells proliferate and are activated to become effector cells destroying them. Sustained activation of the T cells, however, may lead to a dysfunctional state, or exhaustion, through checkpoint receptors, such as PD-1, the effects being pronounced when cancer cells express the ligands for checkpoint receptors. The process can be reverted with checkpoint-receptor blockade to resume effective T-cell immunity. The T-cell recruiting activity of stroma cells is radio-sensitive and is also diminished with age possibly through cellular aging. On the other hand, it is also known that stroma cells with potent pro-inflammatory activity are also increased in the tissues of certain types of cancers, often called myofibroblasts with contractile features. Such stroma cells recruit various innate immune cells to cause inflammatory reactions as well as neoangiogenesis and tissue organization. It is also likely that SA-T cells, which secrete abundant chemoattractants for inflammatory cells, such as osteopontin and Ccl3/4 as part of SASP, accumulate, exaggerating the formation of the inflammatory microenvironment around cancer cells. It is reported that osteopontin acts as a potent tumor-instigating factor recruiting myeloid cells directly from bone marrow. Overall, such an inflammatory microenvironment favors the progression of cancer, via damage to normal tissue integrity as well as suppression of T-cell immunity.
As such, it appears that age-associated changes in the function of both T cells per se and the tissue microenvironment may be involved in the clinically increased incidence or shortened latency of cancer. T-cell dysfunction proceeds over time via multiple mechanisms as discussed above, including the increase in exhausted T cells and SA-T cells. Besides being dysfunctional for regular TCR-mediated effects, SA-T cells may accumulate in cancer tissues and induce chronic tissue inflammation, thereby favoring tumor growth and progression, similar to the situation in tissues under the aforementioned tissue stresses or insults. For instance, osteopontin was shown to act as a potent tumor-instigating factor to recruit inflammatory bone marrow-derived cells into tumor tissues, thus enhancing tumor aggressiveness (83). While clinical success of PD-1 checkpoint blockade therapy, if not always, revealed the crucial involvement of T-cell exhaustion in cancer progression (76), possible involvement of pro-inflammatory SA-T cells in cancer tissues remains to be carefully investigated. Safe and selective elimination of SA-T cells in the cancer tissue environment may provide a potential means to control cancer progression in humans.
Conclusion and perspectives
T cells, which play a central role in immune responses and regulation, show functional alterations over time with organismal aging. Some of these alterations occur in the course of specific antigen-driven responses, in particular, during persistent antigen stimulation, such as chronic viral infection and cancer. This T-cell exhaustion represents a dysfunctional state of specific effector T cells characterized by the constitutive expression of checkpoint receptors. The function of exhausted T cells may be reversed, if not always, by the blockade of checkpoint signals, and this has provided a highly successful means of controlling cancer in humans.
Naive T cells may also become intrinsically dysfunctional with age. T cells undergo physiological HP in association with thymic involution and consequent reduction of T-cell output with age, and sustained HP results in the emergence of dysfunctional CD4+ T cells via repeated cell divisions, called SA-T cells. The SA-T cells show features similar to senescent cells and have a profound defect of TCR-induced proliferation and differentiation capacity. SA-T cells also exhibit constitutive expression of checkpoint receptors, but there is so far no evidence that they are involved in maintenance of the dysfunctional state. SA-T cells increase in proportion over time and show a potent pro-inflammatory secretory phenotype reminiscent of SASP. In addition to aging, SA-T cells are robustly accumulated in GCs of autoreactive B cells and promote anti-nuclear antibody production and systemic autoimmune disease. SA-T cells may also be increased in various tissues under stresses or insults leading to the progression of chronic inflammatory diseases in relevant tissues.
Such dysfunctional T cells are derived as a physiological rationale to avoid immunopathology due to excess immune responses or as a consequence of homeostatic maintenance of the T-cell population and repertoire, yet may eventually include immune-aging phenotypes over time predisposing various age-associated diseases. Recent evidence indicates a crucial role of tissue senescent cells in the aging phenotypes of vital organs as well as tumor latency and, as such, senescent cells in tissues are emerging as therapeutic targets for age-associated diseases (84). Age-associated dysfunctional T cells including SA-T cells may be certainly included as potential targets, and further understanding of molecular signaling driving the T-cell dysfunction should provide a clue for controlling various age-associated chronic inflammatory diseases and possibly cancer in humans.
Funding
N.M. was supported by the research grant from Dainippon Sumitomo Pharma Co. Ltd., M. H. from the research grant from Ono Pharma Co. Ltd., and Y. H. from the Takeda Science Foundation, AMED under Grant Number (JP19gm5010001), and iPS Cell Research Fund.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Franceschi, C., Garagnani, P., Morsiani, C.et al. 2018. The continuum of aging and age-related diseases: common mechanisms but different rates. Front. Med. (Lausanne) 5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chung, H. Y., Cesari, M., Anton, S.et al. 2009. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res. Rev. 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franceschi, C. and Campisi, J. 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69(Suppl. 1):S4. [DOI] [PubMed] [Google Scholar]
- 4. Ferrucci, L. and Fabbri, E. 2018. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Deursen, J. M. 2014. The role of senescent cells in ageing. Nature 509:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayflick, L. and Moorhead, P. S. 1961. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25:585. [DOI] [PubMed] [Google Scholar]
- 7. Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. and Lowe, S. W. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593. [DOI] [PubMed] [Google Scholar]
- 8. López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. and Kroemer, G. 2013. The hallmarks of aging. Cell 153:1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goronzy, J. J. and Weyand, C. M. 2012. Immune aging and autoimmunity. Cell. Mol. Life Sci. 69:1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lynch, H. E., Goldberg, G. L., Chidgey, A., Van den Brink, M. R., Boyd, R. and Sempowski, G. D. 2009. Thymic involution and immune reconstitution. Trends Immunol. 30:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaudhry, M. S., Velardi, E., Dudakov, J. A. and van den Brink, M. R. 2016. Thymus: the next (re)generation. Immunol. Rev. 271:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murray, J. M., Kaufmann, G. R., Hodgkin, P. D.et al. 2003. Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty. Immunol. Cell Biol. 81:487. [DOI] [PubMed] [Google Scholar]
- 13. Shanley, D. P., Aw, D., Manley, N. R. and Palmer, D. B. 2009. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 30:374. [DOI] [PubMed] [Google Scholar]
- 14. Abramson, J. and Anderson, G. 2017. Thymic epithelial cells. Annu. Rev. Immunol. 35:85. [DOI] [PubMed] [Google Scholar]
- 15. Sekai, M., Hamazaki, Y. and Minato, N. 2014. Medullary thymic epithelial stem cells maintain a functional thymus to ensure lifelong central T cell tolerance. Immunity 41:753. [DOI] [PubMed] [Google Scholar]
- 16. Hamazaki, Y., Sekai, M. and Minato, N. 2016. Medullary thymic epithelial stem cells: role in thymic epithelial cell maintenance and thymic involution. Immunol. Rev. 271:38. [DOI] [PubMed] [Google Scholar]
- 17. Surh, C. D. and Sprent, J. 2008. Homeostasis of naive and memory T cells. Immunity 29:848. [DOI] [PubMed] [Google Scholar]
- 18. Nikolich-Zugich, J. 2008. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. Immunol. 8:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sauce, D., Larsen, M., Fastenackels, S.et al. 2012. Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J. Immunol. 189:5541. [DOI] [PubMed] [Google Scholar]
- 20. Prelog, M. 2006. Aging of the immune system: a risk factor for autoimmunity? Autoimmun. Rev. 5:136. [DOI] [PubMed] [Google Scholar]
- 21. Sprent, J. and Surh, C. D. 2011. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat. Immunol. 12:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Younes, S. A., Punkosdy, G., Caucheteux, S., Chen, T., Grossman, Z. and Paul, W. E. 2011. Memory phenotype CD4 T cells undergoing rapid, nonburst-like, cytokine-driven proliferation can be distinguished from antigen-experienced memory cells. PLoS Biol. 9:e1001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prelog, M., Keller, M., Geiger, R.et al. 2009. Thymectomy in early childhood: significant alterations of the CD4(+)CD45RA(+)CD62L(+) T cell compartment in later life. Clin. Immunol. 130:123. [DOI] [PubMed] [Google Scholar]
- 24. Becklund, B. R., Purton, J. F., Ramsey, C.et al. 2016. The aged lymphoid tissue environment fails to support naive T cell homeostasis. Sci. Rep. 6:30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sato, K., Kato, A., Sekai, M., Hamazaki, Y. and Minato, N. 2017. Physiologic thymic involution underlies age-dependent accumulation of senescence-associated CD4+ T cells. J. Immunol. 199:138. [DOI] [PubMed] [Google Scholar]
- 26. den Braber, I., Mugwagwa, T., Vrisekoop, N.et al. 2012. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 36:288. [DOI] [PubMed] [Google Scholar]
- 27. Guimond, M., Veenstra, R. G., Grindler, D. J.et al. 2009. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat. Immunol. 10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blank, C. U., Haining, W. N., Held, W.et al. 2019. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McLane, L. M., Abdel-Hakeem, M. S. and Wherry, E. J. 2019. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37:457. [DOI] [PubMed] [Google Scholar]
- 30. Hashimoto, M., Kamphorst, A. O., Im, S. J.et al. 2018. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu. Rev. Med. 69:301. [DOI] [PubMed] [Google Scholar]
- 31. van den Broek, T., Borghans, J. A. M. and van Wijk, F. 2018. The full spectrum of human naive T cells. Nat. Rev. Immunol. 18:363. [DOI] [PubMed] [Google Scholar]
- 32. Kato, A., Takaori-Kondo, A., Minato, N. and Hamazaki, Y. 2018. CXCR3high CD8+ T cells with naïve phenotype and high capacity for IFN-γ production are generated during homeostatic T-cell proliferation. Eur. J. Immunol. 48:1663. [DOI] [PubMed] [Google Scholar]
- 33. Tahir, S., Fukushima, Y., Sakamoto, K.et al. 2015. A CD153+CD4+ T follicular cell population with cell-senescence features plays a crucial role in lupus pathogenesis via osteopontin production. J. Immunol. 194:5725. [DOI] [PubMed] [Google Scholar]
- 34. Contrepois, K., Coudereau, C., Benayoun, B. A.et al. 2017. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat. Commun. 8:14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shirakawa, K., Yan, X., Shinmura, K.et al. 2016. Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Invest. 126:4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sauce, D., Larsen, M., Fastenackels, S.et al. 2009. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Invest. 119:3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ucar, D., Márquez, E. J., Chung, C. H.et al. 2017. The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J. Exp. Med. 214:3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moskowitz, D. M., Zhang, D. W., Hu, B.et al. 2017. Epigenomics of human CD8 T cell differentiation and aging. Sci. Immunol. 2: pii: eaag0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheung, P., Vallania, F., Warsinske, H. C.et al. 2018. Single-cell chromatin modification profiling reveals increased epigenetic variations with aging. Cell 173:1385.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuwahara, M., Suzuki, J., Tofukuji, S.et al. 2014. The Menin-Bach2 axis is critical for regulating CD4 T-cell senescence and cytokine homeostasis. Nat. Commun. 5:3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li, G., Yu, M., Lee, W. W.et al. 2012. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 18:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vallejo, A. N. 2005. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol. Rev. 205:158. [DOI] [PubMed] [Google Scholar]
- 43. Fann, M., Chiu, W. K., Wood, W. H., III, Levine, B. L., Becker, K. G. and Weng, N. P. 2005. Gene expression characteristics of CD28null memory phenotype CD8+ T cells and its implication in T-cell aging. Immunol. Rev. 205:190. [DOI] [PubMed] [Google Scholar]
- 44. Lemster, B. H., Michel, J. J., Montag, D. T., Paat, J. J., Studenski, S. A., Newman, A. B. and Vallejo, A. N. 2008. Induction of CD56 and TCR-independent activation of T cells with aging. J. Immunol. 180:1979. [DOI] [PubMed] [Google Scholar]
- 45. Akbar, A. N., Beverley, P. C. and Salmon, M. 2004. Will telomere erosion lead to a loss of T-cell memory? Nat. Rev. Immunol. 4:737. [DOI] [PubMed] [Google Scholar]
- 46. Lee, J. H., Budanov, A. V. and Karin, M. 2013. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 18:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lanna, A., Gomes, D. C., Muller-Durovic, B.et al. 2017. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 18:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goronzy, J. J., Fang, F., Cavanagh, M. M., Qi, Q. and Weyand, C. M. 2015. Naive T cell maintenance and function in human aging. J. Immunol. 194:4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wiley, S. R., Goodwin, R. G. and Smith, C. A. 1996. Reverse signaling via CD30 ligand. J. Immunol. 157:3635. [PubMed] [Google Scholar]
- 50. Gray, D. H., Seach, N., Ueno, T.et al. 2006. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108:3777. [DOI] [PubMed] [Google Scholar]
- 51. Baccala, R. and Theofilopoulos, A. N. 2005. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol. 26:5. [DOI] [PubMed] [Google Scholar]
- 52. Calzascia, T., Pellegrini, M., Lin, A.et al. 2008. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc. Natl Acad. Sci. USA 105:2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ernst, B., Lee, D. S., Chang, J. M., Sprent, J. and Surh, C. D. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11:173. [DOI] [PubMed] [Google Scholar]
- 54. Zhang, B., Jia, Q., Bock, C.et al. 2016. Glimpse of natural selection of long-lived T-cell clones in healthy life. Proc. Natl Acad. Sci. USA 113:9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. King, C., Ilic, A., Koelsch, K. and Sarvetnick, N. 2004. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117:265. [DOI] [PubMed] [Google Scholar]
- 56. Vinuesa, C. G., Sanz, I. and Cook, M. C. 2009. Dysregulation of germinal centres in autoimmune disease. Nat. Rev. Immunol. 9:845. [DOI] [PubMed] [Google Scholar]
- 57. Soni, C., Wong, E. B., Domeier, P. P.et al. 2014. B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J. Immunol. 193:4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sakamoto, K., Fukushima, Y., Ito, K.et al. 2016. Osteopontin in spontaneous germinal centers inhibits apoptotic cell engulfment and promotes anti-nuclear antibody production in lupus-prone mice. J. Immunol. 197:2177. [DOI] [PubMed] [Google Scholar]
- 59. Poon, I. K., Lucas, C. D., Rossi, A. G. and Ravichandran, K. S. 2014. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 14:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Muñoz, L. E., Lauber, K., Schiller, M., Manfredi, A. A. and Herrmann, M. 2010. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 6:280. [DOI] [PubMed] [Google Scholar]
- 61. Adams, K. F., Schatzkin, A., Harris, T. B.et al. 2006. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N. Engl. J. Med. 355:763. [DOI] [PubMed] [Google Scholar]
- 62. Shirakawa, K., Endo, J., Katsumata, Y.et al. 2017. Negative legacy of obesity. PLoS One 12:e0186303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ishani, A., Xue, J. L., Himmelfarb, J.et al. 2009. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 20:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sato, Y. and Yanagita, M. 2019. Immunology of the ageing kidney. Nat. Rev. Nephrol. 15:625. [DOI] [PubMed] [Google Scholar]
- 65. Sato, Y., Mii, A., Hamazaki, Y.et al. 2016. Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney. JCI Insight 1:e87680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Caielli, S., Veiga, D. T., Balasubramanian, P.et al. 2019. A CD4+ T cell population expanded in lupus blood provides B cell help through interleukin-10 and succinate. Nat. Med. 25:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rao, D. A., Gurish, M. F., Marshall, J. L.et al. 2017. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yoshitomi, H., Kobayashi, S., Miyagawa-Hayashino, A.et al. 2018. Human Sox4 facilitates the development of CXCL13-producing helper T cells in inflammatory environments. Nat. Commun. 9:3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mannick, J. B., Del Giudice, G., Lattanzi, M.et al. 2014. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6:268ra179. [DOI] [PubMed] [Google Scholar]
- 70. Greenman, C., Stephens, P., Smith, R.et al. 2007. Patterns of somatic mutation in human cancer genomes. Nature 446:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martincorena, I. and Campbell, P. J. 2015. Somatic mutation in cancer and normal cells. Science 349:1483. [DOI] [PubMed] [Google Scholar]
- 72. Lac, V., Nazeran, T. M., Tessier-Cloutier, B.et al. 2019. Oncogenic mutations in histologically normal endometrium: the new normal? J. Pathol. 249:173. [DOI] [PubMed] [Google Scholar]
- 73. Martincorena, I., Fowler, J. C., Wabik, A.et al. 2018. Somatic mutant clones colonize the human esophagus with age. Science 362:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shankaran, V., Ikeda, H., Bruce, A. T.et al. 2001. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410:1107. [DOI] [PubMed] [Google Scholar]
- 75. Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T. and Minato, N. 2002. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99:12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Topalian, S. L., Hodi, F. S., Brahmer, J. R.et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Palmer, S., Albergante, L., Blackburn, C. C. and Newman, T. J. 2018. Thymic involution and rising disease incidence with age. Proc. Natl Acad. Sci. USA 115:1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Baker, D. J., Wijshake, T., Tchkonia, T.et al. 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baker, D. J., Childs, B. G., Durik, M.et al. 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hanahan, D. and Weinberg, R. A. 2011. Hallmarks of cancer: the next generation. Cell 144:646. [DOI] [PubMed] [Google Scholar]
- 81. Rhim, A. D., Oberstein, P. E., Thomas, D. H.et al. 2014. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xu, Y., Ikeda, S., Sumida, K., Yamamoto, R., Tanaka, H. and Minato, N. 2018. Sipa1 deficiency unleashes a host-immune mechanism eradicating chronic myelogenous leukemia-initiating cells. Nat. Commun. 9:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. McAllister, S. S., Gifford, A. M., Greiner, A. L.et al. 2008. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 133:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Serrano, M. 2017. Ageing: tools to eliminate senescent cells. Nature 545:294. [DOI] [PubMed] [Google Scholar]