Abstract
MYB transcription factors (TFs), as one of the largest gene families in plants, play important roles in multiple biological processes, such as plant growth and development, cell morphology and pattern building, physiological activity metabolism, primary and secondary metabolic reactions, and responses to environmental stresses. The function of MYB TFs in crops has been widely studied, but few studies have been done on medicinal plants. In this review, we summarized the MYB TFs that play important roles in secondary metabolism and emphasized the possible mechanisms underlying how MYB TFs are regulated at the protein, posttranscriptional, and transcriptional levels, as well as how they regulate the downstream target gene networks related to secondary metabolism in plants, especially in medicinal plants.
Keywords: MYB TFs, secondary metabolism, plants transcription
1. Introduction
Plant secondary metabolites include many types of compounds, such as flavonoids (phlobaphenes, isoflavonoids, flavanones, flavones, flavonols, proanthocyanidins, anthocyanins), stilbenes, various phenolic acids, and monolignols. The functions of these compounds have been determined and include mechanical strength (cell wall components), signaling molecules, pigments, ultraviolet (UV) light protectants, and phytoalexins. v-Myb myeloblastosis viral oncogene homolog (MYB) transcription factors (TFs) represent one of the largest families of a transcription factor in plants. These MYB TFs have important functions in the regulation of the biosynthesis of secondary metabolites in plants.
The MYB TF is named by its conserved MYB domain at the N-terminus and is an evolutionarily conserved present in nearly all eukaryotes [1,2]. v-Myb is the first MYB TF identified in avian myeloblastosis virus (AMV) [3]. Subsequently, many MYB TFs have been found in fungi, slime mold, animals, and plants [4,5,6]. Compared with MYB TFs in yeast and animals, the functions and structures of those are conserved in plants [5]. The MYB TF is one of the largest TF families in plants, accounting for about 9% of the total TFs family in Arabidopsis thaliana [7]. In A. thaliana, researchers have identified more than 1600 TFs, representing about 6% of the whole genome [7,8]. The MYB TF family members typically contain one, two, three, or four imperfect repeats [4,6]. The MYB TFs are classified into four subfamilies that are called 1R-MYB, 2R-MYB, 3R-MYB, and 4R-MYB, based on the structure of the DNA-binding domain. In plants, COLORED1 (C1) is the first gene identified to encode an MYB domain protein, and it has been confirmed to participate in anthocyanin synthesis in the kernels aleurone layer of Zea mays [9]. In A. thaliana, AtMYB2 shows considerable homology with C1 from Z. mays [10]. The large number of MYB proteins encoded in the plant genome indicates that they may each involve unique functions, which has been confirmed by a large number of published papers [2,11]. The MYB TFs are involved in different biological processes, such as circadian rhythm, defense and stress responses, cell fate and identity, seed and floral development, and regulation of primary and secondary metabolism in plants [2,11,12,13,14]. The secondary metabolites are affected by many factors in medicinal plants [15,16,17]. The MYB TFs also play important roles in the secondary metabolism of medicinal plants [13,18,19,20], which is also an important part of the research and deserves further investigation. Here, we reviewed and discussed the potential functions and emphasized the role and potential regulatory mechanisms of MYB TFs in the secondary metabolism of medicinal plants.
2. Diversity and Structure of the MYB TFs
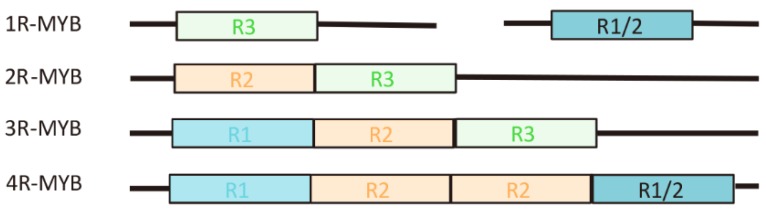
It is now clear that the common characteristic of MYB TFs is the conserved DNA-binding domain (MYB domain), which interacts with DNA [1,2,15]. In general, the MYB domain usually consists of 1-4 imperfect repeats in plants [1,2,15]. Each repeat encodes three α-helices and contains about 50-53 amino acids [1]. Among these three helices, the second and third helices form a helix-turn-helix (HTH) structure [2,21]. The MYB TFs can be inserted into the major groove of the target DNA through the HTH structure and then combine with the target DNA to regulate the expression of the target gene [22]. In general, each MYB repeat domain contains three conserved tryptophan residues, which are separated by 18 to 19 amino acids, forming a secondary structure. According to the number of MYB domains (Figure 1), MYB TF of medicinal plants can be divided into four subfamilies: 1R-MYB, which contains one MYB domain and plays an essential role in regulating plant transcription and maintaining the structure of chromosomes. This subfamily is clustered into several subgroups, including I-box-binding-like, TBP-like, TRF-like, CPC-like, CCA1-like, and other MYB-related proteins [8]. MybSt1 is the first identified MYB-related protein in plants and can be used as a transcriptional activator in potato [23]. As the second-largest subfamily of the MYB TFs, 1R-MYB is widely distributed in plants. There are 64 members in model plants Oryza sativa, and 68 members in A. thaliana [2]. The Dendrobium candidum genome also encodes 42 1R-MYB genes. 1R-MYB TFs involve in flower and fruit development, response to phosphate starvation, circadian clock control, and phenylpropanoid-derived compounds [24].
Figure 1.
Illustration of classification and structure of MYB TFs in plants. MYB TFs with one to four MYB domain repeats that are identified in plants.
2R-MYB, which contains two MYB domains, is the most widely existed protein, regulates the transcription of secondary metabolic processes in medicinal plants, and regulates cell differentiation. At the same time, recent studies have shown that 2R-MYB acts as a regulatory protein involved in the metabolic pathways of flavonoids and phenylpropanoids in medicinal plants [20]. The 2R-MYB subfamily, which is probably evolved from an R1-MYB by duplication or from a 3R-MYB ancestor by loss of R1 [5,25], can be grouped into 28 subgroups (Figure 2) on the basis of the conserved amino acid sequence motifs present in their most C-terminal MYB domain and phylogenetic analyses [2,4,26]. There are more than 90 members in the model plants O. sativa and 120 members in A. thaliana [2]. In medicinal plants, the 2R-MYB subfamily also is the largest group of the MYB family. For example, the Dendrobium candidum genome encodes 117 2R-MYB TFs, and the Jatropha curcas genome contains 123 2R-MYB TFs [27]. The expansion of 2R-MYB TFs suggests that they may contribute to play a key role in plant-specific processes, which is consistent with the findings of published articles. 2R TFs have been clarified to participate in the determination of cell fate and identity, regulation of development, hormone signal transduction, primary and secondary metabolism, and response to abiotic and biotic stresses. For example, 2R-MYB TFs, such as AtMYB123 (TT2), production of anthocyanin pigment 1–4 (PAP1–4) (i.e., AtMYB114, AtMYB113, AtMYB90, and AtMYB75), VvMYBPA2, VvMYBA2, and ZmC1 regulate proanthocyanidin and anthocyanin pathways [9,28,29,30,31]; AtMYB7 can be downregulated by AtMYB4, which functions as a repressor of flavonol synthesis [32].
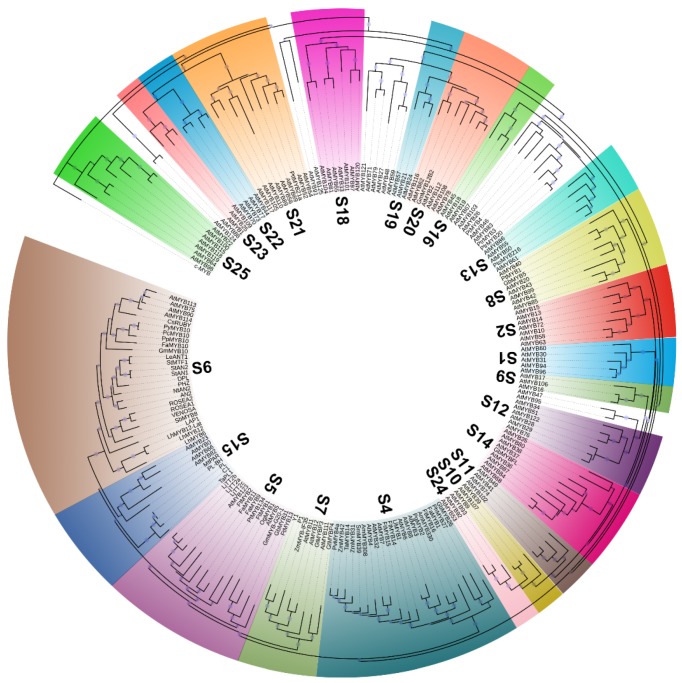
Figure 2.
Phylogeny of MYB TFs associated with the secondary metabolic pathway. Multiple sequence alignment of full-length amino acid is carried out using MAFFT 7.0 software (https://mafft.cbrc.jp/alignment/software/) with default parameters. The best substitution model of all amino acid sequences is determined by ModelFinder, and VT+F+R9 is found as the best model fit for these proteins. Maximum likelihood (ML) phylogenetic analysis is performed using IQ-tree software (http://www.iqtree.org/) with an SH-aLRT test for 1000 random addition replicates and a bootstrap test for 10,000 replicates [33,34]. The Figtree software (http://tree.bio.ed.ac.uk/software/figtree/) and iTOL (Interactive Tree of Life) website are used to visualize the phylogenetic trees [35]. The human protein c-MYB is used as an outgroup. The subfamilies (S1–S28) are designated as previously published papers. Accession numbers in DDBJ/EMBL/NCBI databases are as follows: LAP1 (ACN79541); MtPAR (ADU78729); LlMYB1 (ADY38393); LhMYB6 (BAJ05399); LhMYB12 (BAJ05398); LhMYB12-Lat (BAO04194); LjTT2a (BAG12893); LjTT2b (BAG12894); LjTT2c (BAG12895); C1 (AAA33482); PL (AAA19820); PL-BH (AAA33492); P1 (ABM21535); ZmMYB31 (NP_001105949); ZmMYB42 (ADX60106); ZmMYB-IF35 (AAO48737); GmMYB10 (ACM62751); PpMYB10 (ABX79945); CsRUBY (AFB73913); OgMYB1 (ABS58501); PcMYB10 (ABX71487); PyMYB10 (ADN26574); AN2 (AAF66727); DPL (ADW94950); PHZ (ADW94951); PhMYB27 (AHX24372); PtMYB1 (AAQ62541); PtMYB4 (AAQ62540); PtMYB134 (ACR83705); PtrMYB3 (AGT02395); PtrMYB20 (AGT02396); PttMYB21a (CAD98761); PtMYB134 (ACR83705); PtoMYB216 (AFI80906); StAN1 (AGC31676); StAN2 (AAX53089); StMTF1 (ABY40370); TaMYB14 (AEG64799); AmMYB308 (P81393); AmMYB330 (P81395); ROSEA1 (ABB83826); ROSEA2 (ABB83827); VENOSA (ABB83828); Y1 (AAX44239); GmMYB12B2 (AEC13303); GmMYB-G20-1 (BAK24100); FaMYB1 (AAK84064); FaMYB9 (AFL02460); FaMYB10 (ABX79947); FaMYB11 (AFL02461); PvMYB4a (AEM17348); NtAN2 (ACO52470); LeANT1 (AAQ55181); FtMYB1 (AEC32973); FtMYB2 (AEC32975); FtMYB13 (KY290579); FtMYB14 (KY290580); FtMYB15 (KY290581); FtMYB16 (KY290582); SbMYB8 (KF008657); TaPL1 (AK358937); SmMYB39 (KC213793); GbMYB5 (KY703716); GbMYB26 (KY703737); GbMYB31 (KY703742); GbMYBFL (KY678611); PgMYB2 (API61854.1); GtMYBP3 (AB733016); GtMYBP4 (AB289446); c-MYB (X52125).
The 3R-MYB subfamily, whose members contain three MYB domains, is an evolutionarily conserved group in plants. Genes encoding 3R-MYB proteins have been found amongst plant genomes. There are only five members in model plants O. sativa and five members in A. thaliana [2]. This phenomenon is also found in medicinal plant genomes. For example, the D. candidum genome only encodes four 3R-MYB genes, and the Jatropha curcas genome only contains four 3R-MYB TFs. Their role, albeit divergent, is largely related to regulate cell differentiation and participate in cell cycle control [32]. The smallest subfamily is the 4R-MYB group in plants. Each number of 4R-MYB subfamily has four R1/R2 domains. Only one or two 4R-MYB TF has been identified in some plant genomes, for example, only two 4R-MYB members in A. thaliana and one member in O. sativa [36]. In medicinal plants, the 4R-MYB subfamily is also the smallest group of the MYB family. For example, the D. candidum genome only encodes two 4R-MYB TFs, and the J. curcas genome only contains one 4R-MYB TF. The function of the members of this 4R-MYB subfamily needs further study.
3. Biological Function of MYB TFs
The MYB TFs have a wide range of biological functions and participate in many life processes in plants, such as regulating plant growth and development, involved in cell morphogenesis, regulating primary and secondary metabolic reactions, and responding to abiotic and biotic stresses [22,37,38,39,40,41,42,43]. The MYB TFs also play a very important role in medicinal plants. For example, the D. candidum genome encodes 117 2R-MYB genes, and only nine of them are regulated by low temperature. DoMYB28, DoMYB29, DoMYB54, DoMYB75, DoMYB78, DoMYB81, and DoMYB111 have been up-regulated under salinity stress [20]. Effector-reporter co-expression assays and chromatin immunoprecipitation (ChIP) in Nicotiana tabacum has confirmed the binding of Betula platyphylla BplMYB46 to the E-box, GT-box, and TC-box motifs in the promoters of the superoxide dismutase (SOD), peroxidase (POD), and phenylalanine ammonia-lyase (PAL) genes, which function in secondary wall biosynthesis and abiotic stress tolerance [44]. Gossypium hirsutum GhMYB109, a homolog of AtMYBGL1 in Arabidopsis thaliana, is specifically expressed in cotton elongating fibers and fiber cell initials, indicating that this gene may be involved in the development of seed trichome in cotton [45,46]. Huang et al. (2016) confirmed EsMYBF1 as a flavonol-specific R2R3-MYB regulator, which participated in biosynthesis regulation of the flavonol-derived bioactive components in Epimedium sagittatum [47]. Heterologous expression of Dendrobium officinale DoMYB75 in A. thaliana can significantly increase seed water-soluble polysaccharide content [20]. Panax Ginseng PgMYB2, a nucleus localization protein, may play a key regulatory role in ginsenoside synthesis [48].
4. MYB TF Regulation of Secondary Metabolic Pathways
Plants will interact with their living environment during evolution. This result is the secondary metabolite, which can accumulate in the plant, resist the invasion of pathogenic microorganisms, and play an important role in the entire metabolic activity of the plant. Previous studies on the regulation of secondary metabolism by MYB TFs have mainly focused on common crops, fruits, and vegetables, such as soybean, rice, pear, and apple [6,8,49,50]. For example, MYB TFs from Chrysanthemum morifolium (CmMYB1), Leucaena leucocephala (LlMYB1), Panicum virgatum (PvMYB4a), Eucalyptus gunnii (EgMYB1), and Zea mays (ZmMYB31, ZmMYB42) are able to repress lignin biosynthesis [51,52,53,54,55,56] (Figure 2). In Malus domestica, MdMYB3, MdMYBA, and MdMYB1 can control the red-pigmented anthocyanins biosynthesis in the peel [57,58,59]. MdMYB110a is associated with the red coloration of the fruit cortex during the later stages of fruit maturity, and MdMYB10 can participate in the biosynthesis of anthocyanins in foliage, flesh, and peel [60,61]. In Petunia hybrida, 2R-MYB TFs PHZ (purple haze), DPL (deep purple), and AN2 (anthocyanin2) are responsible for vegetative pigmentation, flower tube bud-blush/venation, and full petal color, respectively [62,63,64]. In lily, the expression of the 2R-MYB TFs LhMYB12-Lat, LhMYB12, and LhMYB6 produces, respectively, tepal splatter-type spot, epal, filament and style pigmentation, tepal, tepal leaf/spot pigmentation [65,66]. In A. thaliana, AtMYB111, AtMYB12, and AtMYB11 (Figure 2) are all independently capable of activating the genes encoding flavonol synthase (FLS), flavanone 3-hydroxylase (F3H), chalcone isomerase (CHI), and chalcone synthase (CHS), which together determine the content of flavonol [67,68,69]. In strawberry, FaTTG1, FabHLH3, FaMYB11/FaMYB9 (homologs of TTG1, TT8, and TT2, respectively) form a complex that up-regulates the expression of genes encoding leucoanthocyanidin reductase (LAR), anthocyanidin reductase (ANR), anthocyanidin synthase (ANS), thereby increasing the content of proanthocyanidin [70]. However, there are few reports on the regulation of secondary metabolism by MYB TFs in medicinal plants. The study on the secondary metabolic pathways of MYB TFs regulating medicinal plants by reviewing the literature on MYB TFs is summarized in Table 1. We found that the regulation of MYB on the secondary metabolism of medicinal plants is mostly concentrated in the synthesis of flavonoids and organic acids.
Table 1.
MYB TFs involved in secondary metabolism in plants.
| Plant Name | Transcription Factor | Biological Functions | References |
|---|---|---|---|
| Fagopyrum tataricum | FtMYB1, FtMYB2, | Overexpression enhances the synthesis and accumulation of anthocyanins | [19] |
| FtMYB13, FtMYB14, FtMYB15, FtMYB16 | Inhibition of biosynthesis of rutin | [71] | |
| FtMYB123L | Regulation of flavonoid biosynthesis | [72] | |
| FtMYB11 | Repress phenylpropanoid biosynthesis | [42] | |
| Lilium brownii var | LhsorMYB12 | Participate in the synthesis of anthocyanidins in leaves | [66] |
| Arabidopsis thaliana | AtMYBL2 | Inhibition of anthocyanin synthesis | [73] |
| AtMYB21, AtMYB24 | Promote the accumulation of anthocyanins | [74] | |
| AtMYB3, AtMYB4, AtMYB32 | Inhibit the accumulation of anthocyanins | [29] | |
| AtMYB34 | Regulation of the synthesis of glucosinolates | [75] | |
| Triticum aestivum | TaPL1, | Enhance the synthesis and accumulation of anthocyanins | [43] |
| Salvia miltiorrhiza | SmMYB39 | Inhibit the accumulation of phenolic acids | [76] |
| SmMYB4 | Affect the biosynthesis of rosmarinic acid | [77] | |
| Antirrhinum majus | AmMYB308, AmMYB330 | Reduce phenolic acid | [18] |
| Rosea1, Rosea2 and Venosa | Regulation of anthocyanin production | [78] | |
| Ginkgo biloba | GbMYB5, GbMYB26, GbMYB31 | Can participate in the biosynthesis of flavonoids under adverse conditions | [79] |
| GbMYBFL | Enhance the accumulation of flavonoids and anthocyanin | [80] | |
| Dendranthema morifolium | DmMYB1 | Negative regulation of the synthesis of flavonoids | [56] |
| Panax Ginseng | PgMYB2 | Play a key regulatory role in ginsenoside synthesis | [48] |
| Epimedium sagittatum | EsMYBF1 | Increased flavonol content and the decreased anthocyanin content in flowers | [47] |
| EsMYBA1 | Regulate anthocyanin biosynthesis | [81] | |
| EsAN2 | Regulate anthocyanin biosynthesis | [82] | |
| Scutellaria baicalensis | SbMYB8 | Regulate flavonoid biosynthesis | [83] |
| Gentiana triflora | GtMYBP3, GtMYBP4 | Activate the expression of flavonol synthesis genes | [84] |
| Perilla frutescens | MYB-P1 | Determined factor of the anthocyanin forma | [85] |
| Medicago truncatula | MtPAR | Regulate proanthocyanidin (PA) biosynthesis | [86] |
Note. The subfamilies and accession numbers of table MYBTFs are given in Figure 2.
4.1. The Role of MYB TFs in the Biosynthesis of Flavonoids Secondary Metabolism
The basic structure of flavonoids is that two benzene rings (A and B) are connected in the middle by a heterocyclic pyran or pyran (with double bond) ring (C) [87,88]. The carbon atoms are identified with “primed numerals” for the B-ring and ordinary numerals for A-ring and C-ring, although a modified number system is used for chalcones. The six major subclasses of flavonoids include the isoflavones (e.g., genistein, daidzein), anthocyanidins (cyanidin, pelargonidin), catechins or flavanols (epicatechin, gallocatechin), flavanones (naringenin, hesperidin), flavonols (quercetin, myricetin), and flavones (e.g., apigenin, luteolin) [87,89]. Each type of flavonoid is further modified, such as rhamnosylation, glucosylation, acylation, methylation, or hydroxylation, leading to the colors and enormous diversity of flavonoids scanned in nature. Flavonoids are a major component of most plant pigments. These compounds are closely related to the attraction of pollinators and the spread of seeds. They also participate in the determination of male fertility, signaling during the formation of nodule nodules, and regulation of auxin transport. Additionally, they help plants resist abiotic and biotic stresses. They are also important as nutritional, medical, and pharmaceutical compounds [90].
The reaction and biosynthesis of flavonoids are shown in Figure 3. In addition to the regulation of key functional genes, the biosynthesis of flavonoids is also regulated by MYB TFs [38,89]. The MYB TFs activate multiple genes in the secondary metabolic synthesis pathway to coordinate their expression, thereby initiating secondary metabolism in medicinal plants. MYB TFs have great significance in the secondary metabolism of flavonoids, the most significant is the discovery of the first related gene C1 gene in maize [9], and then P1 is also found to be very significant in maize [91]. Studies have shown that MYB transcription factors can increase the expression of chalcone synthase, chalcone isomer, dihydroflavone alcohol reductase, and other enzyme genes in the metabolic process. The maize C1 gene mainly controls the color of the aleurone layer and the pigmentation embryo, while P1 mainly exists in vegetative tissues to regulate the anthocyanin synthesis and accumulation [9]. C1 has a broader DNA-binding specificity than P1 in maize. From more similar studies, it can be concluded that MYB TFs play a similar role in the secondary metabolism of medicinal plants. For example, Yuan et al. (2015) found that overexpression of Scutellaria baicalensis SbMYB8 in tobacco could change the expression level of some flavonoid synthesis-related genes and ultimately regulate the biosynthesis of flavonoids [83]. Li et al. (2017) demonstrated that the Camellia sinensis CsMYB4a negatively regulated the synthesis of lignin, phenolic acids, phenylalanine, and flavonoids [92]. The CsMYB4a could inhibit the promoter activity of five phenylpropanoid pathway genes (CsANR, CsLAR, CsCHS, Cs4CL, and CsC4H) and two shikimate pathway genes (CsAROC and CsAROF) [92]. Wang et al. (2018) confirmed that CsMYB2 and CsMYB26 were involved in flavonoid biosynthesis by regulating the expression of CsF3′H and CsLAR in Camellia sinensis, respectively [93]. Huang et al. (2015) cloned and isolated Epimedium sagittatum’s two TFs (EsMYBA1 and EsMYBF1) and twelve structural genes. The transcriptional analysis suggested that EsMYBF1 and EsMYBA1, together with EsTTG1 (a WD40 TF) and EsGL3 (a bHLH TF), were probably involved in the coordinated regulation of synthesis of the flavonol-derived bioactive components and anthocyanins [81]. Thakur et al. (2020) demonstrated that the MYB family of TFs was an interacting partner of SlCOS1 (costunolide synthase gene), which catalyzed the final key step of costunolide (sesquiterpene lactone) biosynthesis in Saussurea lappa [94]. In Antirrhinum majus, AmMYB305 activates the gene encoding phenylalanine ammonia-lyase (PAL) when it is co-expressed in tobacco protoplasts [95]. Both the Gentiana triflora TFs—GtMYBP4 and GtMYBP3—can activate the expression of flavonol biosynthesis genes (such as CHS, ANR, LAR, and ANS) and, when heterologously expressed in A. thaliana, increase the content of flavonol [84]. In A. maju, VENOSA determines vein-associated anthocyanin patterning, whereas ROSEA1 and ROSEA2 are required for bud-blushed patterns, pale pink, bull’s-eye, and full red [78,96].
Figure 3.
Simplified pathways involved in the biosynthesis of flavonoids in plants. The biosynthetic pathway is adapted from previous studies [88,89,97]. PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumaric acid:coenzyme A ligase; CHI, chalcone flavanone isomerase; F3H, flavanone 3-hydroxylase; CHS, chalcone synthase, DFR, dihydroflavonol 4-reductase; AS, anthocyanin synthase; IFS, isoflavonoid synthase; FLS, flavonol synthase; UF3GT, UDP-glucose: flavonoid 3-0-glucosyl-transferase.
4.2. The role of MYB TFs in the Biosynthesis of Secondary Metabolism of Organic Acids
MYB TFs are also widely involved in the secondary metabolism of organic acids in medicinal plants. It has been found that the R2R3-MYB TFs of the fourth subfamily are involved in the metabolism of phenylpropanoid as a negative regulator in various medicinal plants. Tamagnone et al. (1998) found that overexpression of Antirrhinum majus AmMYB308 and AmMYB330 repressed phenolic acid metabolism in transgenic tobaccos [18]. In Salvia miltiorrhiza, SmMYB39 negatively regulates enzyme activities and transcripts of tyrosine aminotransferase (TAT) and 4-hydroxylase (C4H) [76]. SmMYB39 acts as a repressor and is participated in the regulation of the rosmarinic acid pathway [76]. Constitutive expression in Saussurea involucrate, tomato, and tobacco of the potato genes PAP1, StMTF1, StAN1, as well as that of AtMYB12, increases the content of chlorogenic acid [98,99] (Figure 2). Heterologous expression of A. thaliana AtPAP1 gene in Salvia miltiorrhiza can significantly increase the content of salvianolic acid B in transgenic plants. S. miltiorrhiza SmMYB4 gene can act as a transcriptional repressor to regulate C4H and ultimately affect the biosynthesis of rosmarinic acid [77] (Figure 2).
4.3. The Role of MYB TFs in the Biosynthesis of Lignins Secondary Metabolism
Lignin is an aromatic heteropolymer mainly derived from the monolignols sinapyl alcohol, coniferyl, and p-coumaryl, which produce syringyl (S), guaiacyl (G), and p-hydroxyphenyl (H) subunits, respectively. Lignin contributes to the rigidity and strength of stems, as well as providing most of the mechanical strength of the plant cell wall. MYB TFs are also widely involved in the secondary metabolism of lignin. For example, MYB83 and MYB46 from A. thaliana are direct targets of a cell wall-associated protein AtSND1 (NAM-ATAF-CUC [NAC] domain protein 1), and their induction triggers the expression of MYB58, MYB63, and MYB85, which, in turn, can interact with relevant promoter AC elements of the lignin synthesis genes to up-regulate these genes [100,101,102]. Geng et al., (2020) demonstrated that disruption of MYB20, MYB42, and MYB43 resulted in substantial reductions in lignin synthesis and growth development defects in A. thaliana [103]. It has been confirmed that some MYB TFs may be the positive regulators of lignin synthesis. These TFs contain EgMYB2 from Eucalyptus gunnii [102], PtoMYB216, PtrMYB20, and PtrMYB3 from Populus spp. [104,105], ZmMYB167 from Z. mays [106], PtMYB1 and PtMYB4 from Pinus taeda [107,108], and AtMYB75 and AtMYB61 from A. thaliana [28,109].
Certain MYB TFs have been implicated as repressors of the monolignol pathway. Karpinska et al. (2004) found that reducing the expression of PttMYB21a in poplar would lead to the increase of CCoAOMT transcriptional abundance and lignin content, suggesting that this TF might be a transcriptional inhibitor [110]. We also noted that other MYB TFs from Chrysanthemum morifolium (CmMYB1 and CmMYB8) [56,111], Leucaena leucocephala (LlMYB1) [55], Panicum virgatum (PvMYB4a) [54], Eucalyptus gunnii (EgMYB1) [112], Musa nana (MusaMYB31) [113], and Z. mays (ZmMYB31 and ZmMYB42) [51,52] can inhibit lignin synthesis.
5. The Regulation Mechanism of MYB TFs
The regulation network of gene expression is interrelated and mutually restrictive. MYB TF regulates the expression of downstream genes, and its expression is also regulated by upstream genes. For example, WD40 protein TTG1, as an important upstream regulator of MYB TFs, plays an important role in many regulatory pathways in MYB [114,115]. MYB TFs not only regulate functional genes but also regulate other TFs. For example, the A. thaliana NAC TFs—NST1/2/3 and VND6/7—are regulated by MYB TFs during secondary wall synthesis [102]. Recently, TFs directly regulated by MYB proteins have also been found, such as AtMYB66, which directly regulates GL2 and CPC. In addition, other TFs can directly target MYB. For example, AGL15 (AGAMOUS-Like15) can bind to about 29 MYB genes and activate its transcriptional expression [116].
MYB TFs can be divided into four different regulatory mechanisms: 1, The regulatory mechanism of protein interaction; 2, The regulatory mechanism of transcriptase; 3, The regulatory mechanism of a redox reaction. 2R-MYB is the most widely distributed MYB TF in plants [6,49]. The second MYB domain of this 2R-MYB protein contains a conserved amino acid residue composed of four cysteines (Cys). Under the condition of oxidation, in order to prevent the DNA domain of MYB TFs from being oxidized, two cysteines (Cys) will be oxidized inside the protein molecules to form the disulfide bond (S-S), so as to ensure the normal physiological activity of medicinal plants [117]. 4, Ubiquitin regulation mechanism. There are many studies on the mechanism of MYB under stress in plants, which can be generally divided into two ways: MYB protein performs its function through ABA-dependent way and some functions through ABA-dependent and non-ABA-dependent ways [118,119,120].
6. Conclusions and Perspectives
Functional, structural, and phylogenetic analyses have suggested that there are many homologs of MYB TFs with conserved MYB domains, which have similar activities and functions in divergent plant species. In a given subfamily, the biological function and primary protein structure of most MYB TFs are very conserved among angiosperm species, which means that they are evolved from a common ancestral gene. The MYB TFs play important roles in regulatory networks for flavonoid pathways in plants. As part of transcriptional networks, MYB TFs can be regulated by plant growth regulators, environmental signals, microRNAs, and other TFs. There have been more and more studies on the structure and function of MYB TFs, and it has been found that the functions of MYB TFs tend to be diversified.
To date, little is known about the role of the secondary metabolism in plants, especially in medicinal plants. Further studies should focus on detecting the bHLH-binding and promoter- binding capacity of a greater variety of MYB TFs in medicinal plants. Identifying MYB-binding targets using ChIP-seq would provide additional data on targets on a whole-genome scale. Ultimately, detailed knowledge of MYB TFs will help us to further understand the fine transcriptional regulation of the secondary metabolism in plants, especially in medicinal plants.
Author Contributions
Y.C., Y.L., X.Z., K.L., and L.W. provided the contents and comments for the manuscript. Y.C. compiled the materials, wrote, and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Talent Research Start-up Fund of Central South University of Forestry and Technology (2019YJ012).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Martin C., Paz-Ares J.J. Myb transcription factors in plants. Trends Genet. 1997;13:67–73. doi: 10.1016/S0168-9525(96)10049-4. [DOI] [PubMed] [Google Scholar]
- 2.Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L.J. Myb transcription factors in arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Weston K., Bishop J.M.J. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell. 1989;58:85–93. doi: 10.1016/0092-8674(89)90405-4. [DOI] [PubMed] [Google Scholar]
- 4.Stracke R., Werber M., Weisshaar B.J. The r2r3-myb gene family in arabidopsis thaliana. Curr. Opin. Plant Biol. 2001;4:447–456. doi: 10.1016/S1369-5266(00)00199-0. [DOI] [PubMed] [Google Scholar]
- 5.Rosinski J.A., Atchley W.R.J. Molecular evolution of the myb family of transcription factors: Evidence for polyphyletic origin. J. Mol. Evol. 1998;46:74–83. doi: 10.1007/PL00006285. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y., Han Y., Li D., Lin Y., Cai Y.J. Myb transcription factors in chinese pear (pyrus bretschneideri rehd.): Genome-wide identification, classification, and expression profiling during fruit development. Front. Plant Sci. 2016;7:577. doi: 10.3389/fpls.2016.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riechmann J.L., Heard J., Martin G., Reuber L., Jiang C.-Z., Keddie J., Adam L., Pineda O., Ratcliffe O., Samaha R.J. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science. 2000;290:2105–2110. doi: 10.1126/science.290.5499.2105. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y., Yang X., He K., Liu M., Li J., Gao Z., Lin Z., Zhang Y., Wang X., Qiu X., et al. The myb transcription factor superfamily of arabidopsis: Expression analysis and phylogenetic comparison with the rice myb family. Plant Mol. Biol. 2006;60:107–124. doi: 10.1007/s11103-005-2910-y. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares J., Ghosal D., Wienand U., Peterson P., Saedler H.J. The regulatory c1 locus of zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987;6:3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urao T., Yamaguchi-Shinozaki K., Urao S., Shinozaki K. An arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved myb recognition sequence. Plant Cell. 1993;5:1529–1539. doi: 10.1105/tpc.5.11.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin H., Martin C.J. Multifunctionality and diversity within the plant myb-gene family. Plant Mol. Biol. 1999;41:577–585. doi: 10.1023/A:1006319732410. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., Osbourn A., Ma P.J. Myb transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant. 2015;8:689–708. doi: 10.1016/j.molp.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Ramya M., Kwon O.K., An H.R., Park P.M., Baek Y.S., Park P.H.J. Floral scent: Regulation and role of myb transcription factors. Phytochem. Lett. 2017;19:114–120. doi: 10.1016/j.phytol.2016.12.015. [DOI] [Google Scholar]
- 14.Xu W., Dubos C., Lepiniec L.J. Transcriptional control of flavonoid biosynthesis by myb–bhlh–wdr complexes. Trends Plant Sci. 2015;20:176–185. doi: 10.1016/j.tplants.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y., Meng D., Han Y., Chen T., Jiao C., Chen Y., Jin Q., Cai Y.J. Comparative analysis of b-box genes and their expression pattern analysis under various treatments in dendrobium officinale. BMC Plant Biol. 2019;19:245. doi: 10.1186/s12870-019-1851-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee P.-L., Chen J.-T.J. Plant regeneration via callus culture and subsequent in vitro flowering of dendrobium huoshanense. Acta Physiol. Plant. 2014;36:2619–2625. doi: 10.1007/s11738-014-1632-7. [DOI] [Google Scholar]
- 17.Cao Y., Li X., Jiang L.J. Integrative analysis of the core fruit lignification toolbox in pear reveals targets for fruit quality bioengineering. Biomolecules. 2019;9:504. doi: 10.3390/biom9090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamagnone L., Merida A., Parr A., Mackay S., Culianez-Macia F.A., Roberts K., Martin C.J. The ammyb308 and ammyb330 transcription factors from antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. Plant Cell. 1998;10:135–154. doi: 10.1105/tpc.10.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai Y.C., Li C.L., Zhang J.W., Li S.J., Luo X.P., Yao H.P., Chen H., Zhao H.X., Park S.U., Wu Q.J. Characterization of two tartary buckwheat r2r3-myb transcription factors and their regulation of proanthocyanidin biosynthesis. Physiol. Plantarum. 2014;152:431–440. doi: 10.1111/ppl.12199. [DOI] [PubMed] [Google Scholar]
- 20.He C., da Silva J.A.T., Wang H., Si C., Zhang M., Zhang X., Li M., Tan J., Duan J.J. Mining myb transcription factors from the genomes of orchids (phalaenopsis and dendrobium) and characterization of an orchid r2r3-myb gene involved in water-soluble polysaccharide biosynthesis. Sci. Rep. 2019;9:1–19. doi: 10.1038/s41598-019-49812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipsick J.S.J. One billion years of myb. Oncogene. 1996;13:223–235. [PubMed] [Google Scholar]
- 22.Zhou L., He Y., Li J., Liu Y., Chen H. Cbfs function in anthocyanin biosynthesis by interacting with myb113 in eggplant (Solanum melongena L) Plant Cell Physiol. 2020;61:416–426. doi: 10.1093/pcp/pcz209. [DOI] [PubMed] [Google Scholar]
- 23.Baranowskij N., Frohberg C., Prat S., Willmitzer L.J. A novel DNA binding protein with homology to myb oncoproteins containing only one repeat can function as a transcriptional activator. EMBO J. 1994;13:5383–5392. doi: 10.1002/j.1460-2075.1994.tb06873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feller A., Machemer K., Braun E.L., Grotewold E.J. Evolutionary and comparative analysis of myb and bhlh plant transcription factors. Plant J. 2011;66:94–116. doi: 10.1111/j.1365-313X.2010.04459.x. [DOI] [PubMed] [Google Scholar]
- 25.Jiang C., Gu J., Chopra S., Gu X., Peterson T.J. Ordered origin of the typical two-and three-repeat myb genes. Gene. 2004;326:13–22. doi: 10.1016/j.gene.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 26.Shelton D., Stranne M., Mikkelsen L., Pakseresht N., Welham T., Hiraka H., Tabata S., Sato S., Paquette S., Wang T.L.J. Transcription factors of lotus: Regulation of isoflavonoid biosynthesis requires coordinated changes in transcription factor activity. Plant Physiol. 2012;159:531–547. doi: 10.1104/pp.112.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng X., Liu H., Wang D., Shen S.J. Genome-wide identification of the jatropha curcas myb family and functional analysis of the abiotic stress responsive gene jcmyb2. BMC Genom. 2016;17:251. doi: 10.1186/s12864-016-2576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borevitz J.O., Xia Y., Blount J., Dixon R.A., Lamb C.J. Activation tagging identifies a conserved myb regulator of phenylpropanoid biosynthesis. Plant Cell. 2000;12:2383–2393. doi: 10.1105/tpc.12.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepiniec L., Debeaujon I., Routaboul J.-M., Baudry A., Pourcel L., Nesi N., Caboche M.J. Genetics and biochemistry of seed flavonoids. Ann. Rev. Plant Biol. 2006;57:405–430. doi: 10.1146/annurev.arplant.57.032905.105252. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez A., Zhao M., Leavitt J.M., Lloyd A.M.J. Regulation of the anthocyanin biosynthetic pathway by the ttg1/bhlh/myb transcriptional complex in arabidopsis seedlings. Plant J. 2008;53:814–827. doi: 10.1111/j.1365-313X.2007.03373.x. [DOI] [PubMed] [Google Scholar]
- 31.Heppel S.C., Jaffé F.W., Takos A.M., Schellmann S., Rausch T., Walker A.R., Bogs J.J. Identification of key amino acids for the evolution of promoter target specificity of anthocyanin and proanthocyanidin regulating myb factors. Plant Mol. Biol. 2013;82:457–471. doi: 10.1007/s11103-013-0074-8. [DOI] [PubMed] [Google Scholar]
- 32.Fornalé S., Lopez E., Salazar-Henao J.E., Fernández-Nohales P., Rigau J., Caparros-Ruiz D.J. Atmyb7, a new player in the regulation of uv-sunscreens in arabidopsis thaliana. Plant Cell Physiol. 2014;55:507–516. doi: 10.1093/pcp/pct187. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q.J. Iq-tree: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y., Liu W., Zhao Q., Long H., Li Z., Liu M., Zhou X., Zhang L.J. Integrative analysis reveals evolutionary patterns and potential functions of sweet transporters in euphorbiaceae. Int. J. Biol. Macromol. 2019;139:1–11. doi: 10.1016/j.ijbiomac.2019.07.102. [DOI] [PubMed] [Google Scholar]
- 35.Letunic I., Bork P.J. Interactive tree of life (itol) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katiyar A., Smita S., Lenka S.K., Rajwanshi R., Chinnusamy V., Bansal K.C.J. Genome-wide classification and expression analysis of myb transcription factor families in rice and arabidopsis. BMC Genom. 2012;13:544. doi: 10.1186/1471-2164-13-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng D.W., Abeysinghe J.K., Kamali M.J. Regulating the regulators: The control of transcription factors in plant defense signaling. Int. J. Mol. Sci. 2018;19:3737. doi: 10.3390/ijms19123737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma D., Constabel C.P.J. Myb repressors as regulators of phenylpropanoid metabolism in plants. Trends Plant Sci. 2019;24:275–289. doi: 10.1016/j.tplants.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Ge L., Dou Y., Li M., Qu P., He Z., Liu Y., Xu Z., Chen J., Chen M., Ma Y.J.I. Simyb3 in foxtail millet (setaria italica) confers tolerance to low-nitrogen stress by regulating root growth in transgenic plants. Int. J. Mol. Sci. 2019;20:5741. doi: 10.3390/ijms20225741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang Q., Wang X., Wang H., Tang X., Liu C., Yin H., Ye S., Jiang Y., Duan Y., Luo K.J. The poplar r2r3 myb transcription factor ptrmyb94 coordinates with abscisic acid signaling to improve drought tolerance in plants. Tree Physiol. 2019;40:46–59. doi: 10.1093/treephys/tpz113. [DOI] [PubMed] [Google Scholar]
- 41.Lai B., Cheng Y., Liu H., Wang Q., Wang Q., Wang C., Su R., Chen F., Wang H., Du L.J. Differential anthocyanin accumulation in radish taproot: Importance of rsmyb1 gene structure. Plant Cell Rep. 2019:1–10. doi: 10.1007/s00299-019-02485-z. [DOI] [PubMed] [Google Scholar]
- 42.Zhou M., Sun Z., Ding M., Logacheva M.D., Kreft I., Wang D., Yan M., Shao J., Tang Y., Wu Y.J. Ftsad2 and ftjaz1 regulate activity of the ftmyb11 transcription repressor of the phenylpropanoid pathway in fagopyrum tataricum. New Phytol. 2017;216:814–828. doi: 10.1111/nph.14692. [DOI] [PubMed] [Google Scholar]
- 43.Shin D.H., Choi M.-G., Kang C.-S., Park C.-S., Choi S.-B., Park Y.-I.J. A wheat r2r3-myb protein purple plant1 (tapl1) functions as a positive regulator of anthocyanin biosynthesis. Biochem. Biophys. Res. Commun. 2016;469:686–691. doi: 10.1016/j.bbrc.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y.-M., Wang C., Guo H.-Y., Wang Y.-C.J. Bplmyb46 from betula platyphylla can form homodimers and heterodimers and is involved in salt and osmotic stresses. Int. J. Mol. Sci. 2019;20:1171. doi: 10.3390/ijms20051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pu L., Li Q., Fan X., Yang W., Xue Y.J. The r2r3 myb transcription factor ghmyb109 is required for cotton fiber development. Genetics. 2008;180:811–820. doi: 10.1534/genetics.108.093070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suo J., Liang X., Pu L., Zhang Y., Xue Y.J. Identification of ghmyb109 encoding a r2r3 myb transcription factor that expressed specifically in fiber initials and elongating fibers of cotton (Gossypium hirsutum L.) Biochim. et Biophys. Acta (BBA)-Gene Struct. Expr. 2003;1630:25–34. doi: 10.1016/j.bbaexp.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Huang W., Khaldun A., Chen J., Zhang C., Lv H., Yuan L., Wang Y.J. A r2r3-myb transcription factor regulates the flavonol biosynthetic pathway in a traditional chinese medicinal plant, epimedium sagittatum. Front. Plant Sci. 2016;7:1089. doi: 10.3389/fpls.2016.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu T., Luo T., Guo X., Zou X., Zhou D., Afrin S., Li G., Zhang Y., Zhang R., Luo Z.J. Pgmyb2, a meja-responsive transcription factor, positively regulates the dammarenediol synthase gene expression in panax ginseng. Int. J. Mol. Sci. 2019;20:2219. doi: 10.3390/ijms20092219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Z.-H., Zhang S.-Z., Wang R.-K., Zhang R.-F., Hao Y.-J.J. Genome wide analysis of the apple myb transcription factor family allows the identification of mdomyb121 gene confering abiotic stress tolerance in plants. PLoS ONE. 2013;8:e69955. doi: 10.1371/journal.pone.0069955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du H., Yang S.-S., Liang Z., Feng B.-R., Liu L., Huang Y.-B., Tang Y.-X.J. Genome-wide analysis of the myb transcription factor superfamily in soybean. BMC Plant Biol. 2012;12:106. doi: 10.1186/1471-2229-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonbol F.-M., Fornalé S., Capellades M., Encina A., Tourino S., Torres J.-L., Rovira P., Ruel K., Puigdomenech P., Rigau J.J. The maize zmmyb42 represses the phenylpropanoid pathway and affects the cell wall structure, composition and degradability in arabidopsis thaliana. Plant Mol. Biol. 2009;70:283. doi: 10.1007/s11103-009-9473-2. [DOI] [PubMed] [Google Scholar]
- 52.Fornalé S., Shi X., Chai C., Encina A., Irar S., Capellades M., Fuguet E., Torres J.L., Rovira P., Puigdomènech P.J. Zmmyb31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. Plant J. 2010;64:633–644. doi: 10.1111/j.1365-313X.2010.04363.x. [DOI] [PubMed] [Google Scholar]
- 53.Legay S., Lacombe E., Goicoechea M., Briere C., Séguin A., Mackay J., Grima-Pettenati J.J. Molecular characterization of egmyb1, a putative transcriptional repressor of the lignin biosynthetic pathway. Plant Sci. 2007;173:542–549. doi: 10.1016/j.plantsci.2007.08.007. [DOI] [Google Scholar]
- 54.Shen H., He X., Poovaiah C.R., Wuddineh W.A., Ma J., Mann D.G., Wang H., Jackson L., Tang Y., Neal Stewart C.J., Jr. Functional characterization of the switchgrass (panicum virgatum) r2r3-myb transcription factor pvmyb4 for improvement of lignocellulosic feedstocks. New Phytol. 2012;193:121–136. doi: 10.1111/j.1469-8137.2011.03922.x. [DOI] [PubMed] [Google Scholar]
- 55.Omer S., Kumar S., Khan B.M.J. Over-expression of a subgroup 4 r2r3 type myb transcription factor gene from leucaena leucocephala reduces lignin content in transgenic tobacco. Plant Cell Rep. 2013;32:161–171. doi: 10.1007/s00299-012-1350-9. [DOI] [PubMed] [Google Scholar]
- 56.Zhu L., Shan H., Chen S., Jiang J., Gu C., Zhou G., Chen Y., Song A., Chen F.J. The heterologous expression of the chrysanthemum r2r3-myb transcription factor cmmyb1 alters lignin composition and represses flavonoid synthesis in arabidopsis thaliana. PLoS ONE. 2013;8:e65680. doi: 10.1371/journal.pone.0065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takos A.M., Jaffé F.W., Jacob S.R., Bogs J., Robinson S.P., Walker A.R.J. Light-induced expression of a myb gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006;142:1216–1232. doi: 10.1104/pp.106.088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ban Y., Honda C., Hatsuyama Y., Igarashi M., Bessho H., Moriguchi T.J. Isolation and functional analysis of a myb transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 2007;48:958–970. doi: 10.1093/pcp/pcm066. [DOI] [PubMed] [Google Scholar]
- 59.Vimolmangkang S., Han Y., Wei G., Korban S.S.J. An apple myb transcription factor, mdmyb3, is involved in regulation of anthocyanin biosynthesis and flower development. BMC Plant Biol. 2013;13:176. doi: 10.1186/1471-2229-13-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Espley R.V., Hellens R.P., Putterill J., Stevenson D.E., Kutty-Amma S., Allan A.C.J. Red colouration in apple fruit is due to the activity of the myb transcription factor, mdmyb10. Plant J. 2007;49:414–427. doi: 10.1111/j.1365-313X.2006.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chagné D., Lin-Wang K., Espley R.V., Volz R.K., How N.M., Rouse S., Brendolise C., Carlisle C.M., Kumar S., De Silva N.J. An ancient duplication of apple myb transcription factors is responsible for novel red fruit-flesh phenotypes. Plant Physiol. 2013;161:225–239. doi: 10.1104/pp.112.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quattrocchio F., Wing J.F., Va K., Mol J.N., Koes R.J. Analysis of bhlh and myb domain proteins: Species-specific regulatory differences are caused by divergent evolution of target anthocyanin genes. Plant J. 1998;13:475–488. doi: 10.1046/j.1365-313X.1998.00046.x. [DOI] [PubMed] [Google Scholar]
- 63.Quattrocchio F., Wing J., van der Woude K., Souer E., de Vetten N., Mol J., Koes R.J. Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. Plant Cell. 1999;11:1433–1444. doi: 10.1105/tpc.11.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albert N.W., Lewis D.H., Zhang H., Schwinn K.E., Jameson P.E., Davies K.M.J. Members of an r2r3-myb transcription factor family in petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J. 2011;65:771–784. doi: 10.1111/j.1365-313X.2010.04465.x. [DOI] [PubMed] [Google Scholar]
- 65.Yamagishi M., Shimoyamada Y., Nakatsuka T., Masuda K.J. Two r2r3-myb genes, homologs of petunia an2, regulate anthocyanin biosyntheses in flower tepals, tepal spots and leaves of asiatic hybrid lily. Plant Cell Physiol. 2010;51:463–474. doi: 10.1093/pcp/pcq011. [DOI] [PubMed] [Google Scholar]
- 66.Yamagishi M., Toda S., Tasaki K.J. The novel allele of the lh myb 12 gene is involved in splatter-type spot formation on the flower tepals of asiatic hybrid lilies (lilium spp.) New Phytol. 2014;201:1009–1020. doi: 10.1111/nph.12572. [DOI] [PubMed] [Google Scholar]
- 67.Pandey A., Misra P., Chandrashekar K., Trivedi P.K.J. Development of atmyb12-expressing transgenic tobacco callus culture for production of rutin with biopesticidal potential. Plant Cell Rep. 2012;31:1867–1876. doi: 10.1007/s00299-012-1300-6. [DOI] [PubMed] [Google Scholar]
- 68.Pandey A., Misra P., Khan M.P., Swarnkar G., Tewari M.C., Bhambhani S., Trivedi R., Chattopadhyay N., Trivedi P.K.J. Co-expression of arabidopsis transcription factor, at myb 12, and soybean isoflavone synthase, gm ifs 1, genes in tobacco leads to enhanced biosynthesis of isoflavones and flavonols resulting in osteoprotective activity. Plant Biotechnol. J. 2014;12:69–80. doi: 10.1111/pbi.12118. [DOI] [PubMed] [Google Scholar]
- 69.Misra P., Pandey A., Tiwari M., Chandrashekar K., Sidhu O.P., Asif M.H., Chakrabarty D., Singh P.K., Trivedi P.K., Nath P.J. Modulation of transcriptome and metabolome of tobacco by arabidopsis transcription factor, atmyb12, leads to insect resistance. Plant Physiol. 2010;152:2258–2268. doi: 10.1104/pp.109.150979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaart J.G., Dubos C., Romero De La Fuente I., Van Houwelingen A.M., de Vos R.C., Jonker H.H., Xu W., Routaboul J.M., Lepiniec L., Bovy A.G.J. Identification and characterization of myb-b hlh-wd 40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013;197:454–467. doi: 10.1111/nph.12017. [DOI] [PubMed] [Google Scholar]
- 71.Zhang K., Logacheva M.D., Meng Y., Hu J., Wan D., Li L., Janovská D., Wang Z., Georgiev M.I., Yu Z.J. Jasmonate-responsive myb factors spatially repress rutin biosynthesis in fagopyrum tataricum. J. Exp. Bot. 2018;69:1955–1966. doi: 10.1093/jxb/ery032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou M.-L., Tang Y., Zhang K.-X., Li F.-L., Yang P.-Y., Tang Y.-X., Wu Y.-M., Shao J.-R.J. Identification of tt2 gene from floral transcriptome in fagopyrum tataricum. Food Res. Int. 2013;54:1331–1333. doi: 10.1016/j.foodres.2012.10.018. [DOI] [Google Scholar]
- 73.Matsui K., Umemura Y., Ohme-Takagi M.J. Atmybl2, a protein with a single myb domain, acts as a negative regulator of anthocyanin biosynthesis in arabidopsis. Plant J. 2008;55:954–967. doi: 10.1111/j.1365-313X.2008.03565.x. [DOI] [PubMed] [Google Scholar]
- 74.Song J., Wang Z.J. Rnai-mediated suppression of the phenylalanine ammonia-lyase gene in salvia miltiorrhiza causes abnormal phenotypes and a reduction in rosmarinic acid biosynthesis. J. Plant Res. 2011;124:183–192. doi: 10.1007/s10265-010-0350-5. [DOI] [PubMed] [Google Scholar]
- 75.Celenza J.L., Quiel J.A., Smolen G.A., Merrikh H., Silvestro A.R., Normanly J., Bender J.J. The arabidopsis atr1 myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol. 2005;137:253–262. doi: 10.1104/pp.104.054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang S., Ma P., Yang D., Li W., Liang Z., Liu Y., Liu F.J. Cloning and characterization of a putative r2r3 myb transcriptional repressor of the rosmarinic acid biosynthetic pathway from salvia miltiorrhiza. PLoS ONE. 2013;8:e73259. doi: 10.1371/journal.pone.0073259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y., Yan Y.-P., Wang Z.-Z.J. The arabidopsis pap1 transcription factor plays an important role in the enrichment of phenolic acids in salvia miltiorrhiza. J. Agric. Food Chem. 2010;58:12168–12175. doi: 10.1021/jf103203e. [DOI] [PubMed] [Google Scholar]
- 78.Schwinn K., Venail J., Shang Y., Mackay S., Alm V., Butelli E., Oyama R., Bailey P., Davies K., Martin C.J. A small family of myb-regulatory genes controls floral pigmentation intensity and patterning in the genus antirrhinum. Plant Cell. 2006;18:831–851. doi: 10.1105/tpc.105.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X., Yu W., Zhang X., Wang G., Cao F., Cheng H.J. Identification and expression analysis under abiotic stress of the r2r3-myb genes in ginkgo biloba l. Physiol. Mol. Biol. Plants. 2017;23:503–516. doi: 10.1007/s12298-017-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang W., Xu F., Cheng S., Liao Y.J. Characterization and functional analysis of a myb gene (gbmybfl) related to flavonoid accumulation in ginkgo biloba. Genes Genom. 2018;40:49–61. doi: 10.1007/s13258-017-0609-5. [DOI] [PubMed] [Google Scholar]
- 81.Huang W., Sun W., Lv H., Luo M., Zeng S., Pattanaik S., Yuan L., Wang Y.J. A r2r3-myb transcription factor from epimedium sagittatum regulates the flavonoid biosynthetic pathway. PLoS ONE. 2013;8:e70778. doi: 10.1371/journal.pone.0070778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang W., Khaldun A., Lv H., Du L., Zhang C., Wang Y.J. Isolation and functional characterization of a r2r3-myb regulator of the anthocyanin biosynthetic pathway from epimedium sagittatum. Plant Cell Rep. 2016;35:883–894. doi: 10.1007/s00299-015-1929-z. [DOI] [PubMed] [Google Scholar]
- 83.Yuan Y., Qi L., Yang J., Wu C., Liu Y., Huang L.J. A scutellaria baicalensis r2r3-myb gene, sbmyb8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. (PCTOC) 2015;120:961–972. doi: 10.1007/s11240-014-0650-x. [DOI] [Google Scholar]
- 84.Nakatsuka T., Saito M., Yamada E., Fujita K., Kakizaki Y., Nishihara M.J. Isolation and characterization of gtmybp3 and gtmybp4, orthologues of r2r3-myb transcription factors that regulate early flavonoid biosynthesis, in gentian flowers. J. Exp. Bot. 2012;63:6505–6517. doi: 10.1093/jxb/ers306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gong Z., Yamazaki M., Saito K.J.M. A light-inducible myb-like gene that is specifically expressed in red perilla frutescens and presumably acts as a determining factor of the anthocyanin forma. Mol. Gen. Genet. 1999;262:65–72. doi: 10.1007/PL00008639. [DOI] [PubMed] [Google Scholar]
- 86.Verdier J., Zhao J., Torres-Jerez I., Ge S., Liu C., He X., Mysore K.S., Dixon R.A., Udvardi M.K.J. Mtpar myb transcription factor acts as an on switch for proanthocyanidin biosynthesis in medicago truncatula. Proc. Natl. Acad. Sci. USA. 2012;109:1766–1771. doi: 10.1073/pnas.1120916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Farooqui A.A. Phytochemicals, Signal Transduction, and Neurological Disorders. Springer; Berlin/Heidelberg, Germany: 2013. Beneficial effects of flavonoids on neurological disorders; pp. 83–115. [Google Scholar]
- 88.Katsumoto Y., Fukuchi-Mizutani M., Fukui Y., Brugliera F., Holton T.A., Karan M., Nakamura N., Yonekura-Sakakibara K., Togami J., Pigeaire A.J.P., et al. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007;48:1589–1600. doi: 10.1093/pcp/pcm131. [DOI] [PubMed] [Google Scholar]
- 89.Saito K., Yonekura-Sakakibara K., Nakabayashi R., Higashi Y., Yamazaki M., Tohge T., Fernie A.R.J. The flavonoid biosynthetic pathway in arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013;72:21–34. doi: 10.1016/j.plaphy.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Hichri I., Barrieu F., Bogs J., Kappel C., Delrot S., Lauvergeat V.J. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011;62:2465–2483. doi: 10.1093/jxb/erq442. [DOI] [PubMed] [Google Scholar]
- 91.Grotewold E., Athma P., Peterson T.J. Alternatively spliced products of the maize p gene encode proteins with homology to the DNA-binding domain of myb-like transcription factors. Proc. Natl. Acad. Sci. USA. 1991;88:4587–4591. doi: 10.1073/pnas.88.11.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li M., Li Y., Guo L., Gong N., Pang Y., Jiang W., Liu Y., Jiang X., Zhao L., Wang Y.J. Functional characterization of tea (camellia sinensis) myb4a transcription factor using an integrative approach. Front. Plant Sci. 2017;8:943. doi: 10.3389/fpls.2017.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W.-L., Wang Y.-X., Li H., Liu Z.-W., Cui X., Zhuang J. Two myb transcription factors (csmyb2 and csmyb26) are involved in flavonoid biosynthesis in tea plant [camellia sinensis (l.) o. Kuntze] BMC Plant Biol. 2018;18:288. doi: 10.1186/s12870-018-1502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thakur V., Bains S., Pathania S., Sharma S., Kaur R., Singh K. Comparative transcriptomics reveals candidate transcription factors involved in costunolide biosynthesis in medicinal plant-saussurea lappa. Int. J. Biol. Macromol. 2020;150:52–67. doi: 10.1016/j.ijbiomac.2020.01.312. [DOI] [PubMed] [Google Scholar]
- 95.Sablowski R., Moyano E., Culianez-Macia F.A., Schuch W., Martin C., Bevan M. A flower-specific myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J. 1994;13:128–137. doi: 10.1002/j.1460-2075.1994.tb06242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shang Y., Venail J., Mackay S., Bailey P.C., Schwinn K.E., Jameson P.E., Martin C.R., Davies K.M.J. The molecular basis for venation patterning of pigmentation and its effect on pollinator attraction in flowers of antirrhinum. New Phytol. 2011;189:602–615. doi: 10.1111/j.1469-8137.2010.03498.x. [DOI] [PubMed] [Google Scholar]
- 97.Bradley J.M., Davies K.M., Deroles S.C., Bloor S.J., Lewis D.H.J. The maize lc regulatory gene up-regulates the flavonoid biosynthetic pathway of petunia. Plant J. 1998;13:381–392. doi: 10.1046/j.1365-313X.1998.00031.x. [DOI] [Google Scholar]
- 98.Jiang H., Wood K.V., Morgan J. Metabolic engineering of the phenylpropanoid pathway in saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005;71:2962–2969. doi: 10.1128/AEM.71.6.2962-2969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Payyavula R.S., Singh R.K., Navarre D.A.J. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. J. Exp. Bot. 2013;64:5115–5131. doi: 10.1093/jxb/ert303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhong R., Richardson E.A., Ye Z.-H. The myb46 transcription factor is a direct target of snd1 and regulates secondary wall biosynthesis in arabidopsis. Plant Cell. 2007;19:2776–2792. doi: 10.1105/tpc.107.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhong R., Lee C., Zhou J., McCarthy R.L., Ye Z.-H. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in arabidopsis. Plant Cell. 2008;20:2763–2782. doi: 10.1105/tpc.108.061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou J., Lee C., Zhong R., Ye Z.-H. Myb58 and myb63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in arabidopsis. Plant Cell. 2009;21:248–266. doi: 10.1105/tpc.108.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geng P., Zhang S., Liu J., Zhao C., Wu J., Cao Y., Fu C., Han X., He H., Zhao Q. Myb20, myb42, myb43, and myb85 regulate phenylalanine and lignin biosynthesis during secondary cell wall formation. Plant Physiol. 2020;182:1272–1283. doi: 10.1104/pp.19.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tian Q., Wang X., Li C., Lu W., Yang L., Jiang Y., Luo K. Functional characterization of the poplar r2r3-myb transcription factor ptomyb216 involved in the regulation of lignin biosynthesis during wood formation. PLoS ONE. 2013;8:e76369. doi: 10.1371/journal.pone.0076369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCarthy R.L., Zhong R., Fowler S., Lyskowski D., Piyasena H., Carleton K., Spicer C., Ye Z.-H. The poplar myb transcription factors, ptrmyb3 and ptrmyb20, are involved in the regulation of secondary wall biosynthesis. Plant Cell Physiol. 2010;51:1084–1090. doi: 10.1093/pcp/pcq064. [DOI] [PubMed] [Google Scholar]
- 106.Bhatia R., Dalton S., Roberts L.A., Moron-Garcia O.M., Iacono R., Kosik O., Gallagher J.A., Bosch M. Modified expression of zmmyb167 in brachypodium distachyon and zea mays leads to increased cell wall lignin and phenolic content. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-45225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patzlaff A., McInnis S., Courtenay A., Surman C., Newman L.J., Smith C., Bevan M.W., Mansfield S., Whetten R.W., Sederoff R.R. Characterisation of a pine myb that regulates lignification. Plant J. 2003;36:743–754. doi: 10.1046/j.1365-313X.2003.01916.x. [DOI] [PubMed] [Google Scholar]
- 108.Patzlaff A., Newman L.J., Dubos C., Whetten R.W., Smith C., McInnis S., Bevan M.W., Sederoff R.R., Campbell M.M. Characterisation of ptmyb1, an r2r3-myb from pine xylem. Plant Mol. Biol. 2003;53:597–608. doi: 10.1023/B:PLAN.0000019066.07933.d6. [DOI] [PubMed] [Google Scholar]
- 109.Newman L.J., Perazza D.E., Juda L., Campbell M.M. Involvement of the r2r3-myb, atmyb61, in the ectopic lignification and dark-photomorphogenic components of the det3 mutant phenotype. Plant J. 2004;37:239–250. doi: 10.1046/j.1365-313X.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- 110.Karpinska B., Karlsson M., Srivastava M., Stenberg A., Schrader J., Sterky F., Bhalerao R., Wingsle G. Myb transcription factors are differentially expressed and regulated during secondary vascular tissue development in hybrid aspen. Plant Mol. Biol. 2004;56:255–270. doi: 10.1007/s11103-004-3354-5. [DOI] [PubMed] [Google Scholar]
- 111.Zhu L., Guan Y., Zhang Z., Song A., Chen S., Jiang J., Chen F. Cmmyb8 encodes an r2r3 myb transcription factor which represses lignin and flavonoid synthesis in chrysanthemum. Plant Physiol. Biochem. 2020;149:217–224. doi: 10.1016/j.plaphy.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 112.Legay S., Sivadon P., Blervacq A.S., Pavy N., Baghdady A., Tremblay L., Levasseur C., Ladouce N., Lapierre C., Séguin A. Egmyb1, an r2r3 myb transcription factor from eucalyptus negatively regulates secondary cell wall formation in arabidopsis and poplar. New Phytol. 2010;188:774–786. doi: 10.1111/j.1469-8137.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 113.Tak H., Negi S., Ganapathi T. Overexpression of musamyb31, a r2r3 type myb transcription factor gene indicate its role as a negative regulator of lignin biosynthesis in banana. PLoS ONE. 2017;12:e0172695. doi: 10.1371/journal.pone.0172695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramsay N.A., Glover B.J.J. Myb–bhlh–wd40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005;10:63–70. doi: 10.1016/j.tplants.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 115.Mishra A.K., Puranik S., Prasad M.J. Structure and regulatory networks of wd40 protein in plants. J. Plant Biochem. Biotechnol. 2012;21:32–39. doi: 10.1007/s13562-012-0134-1. [DOI] [Google Scholar]
- 116.Thakare D., Tang W., Hill K., Perry S.E.J. The mads-domain transcriptional regulator agamous-like15 promotes somatic embryo development in arabidopsis and soybean. Plant Physiol. 2008;146:1663–1672. doi: 10.1104/pp.108.115832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morse A.M., Whetten R.W., Dubos C., Campbell M.M.J. Post-translational modification of an r2r3-myb transcription factor by a map kinase during xylem development. New Phytol. 2009;183:1001–1013. doi: 10.1111/j.1469-8137.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- 118.Reyes J.L., Chua N.H.J. Aba induction of mir159 controls transcript levels of two myb factors during arabidopsis seed germination. Plant J. 2007;49:592–606. doi: 10.1111/j.1365-313X.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- 119.Cui F., Brosché M., Sipari N., Tang S., Overmyer K.J. Regulation of aba dependent wound induced spreading cell death by myb 108. New Phytol. 2013;200:634–640. doi: 10.1111/nph.12456. [DOI] [PubMed] [Google Scholar]
- 120.Fujita Y., Fujita M., Shinozaki K., Yamaguchi-Shinozaki K.J. Aba-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011;124:509–525. doi: 10.1007/s10265-011-0412-3. [DOI] [PubMed] [Google Scholar]