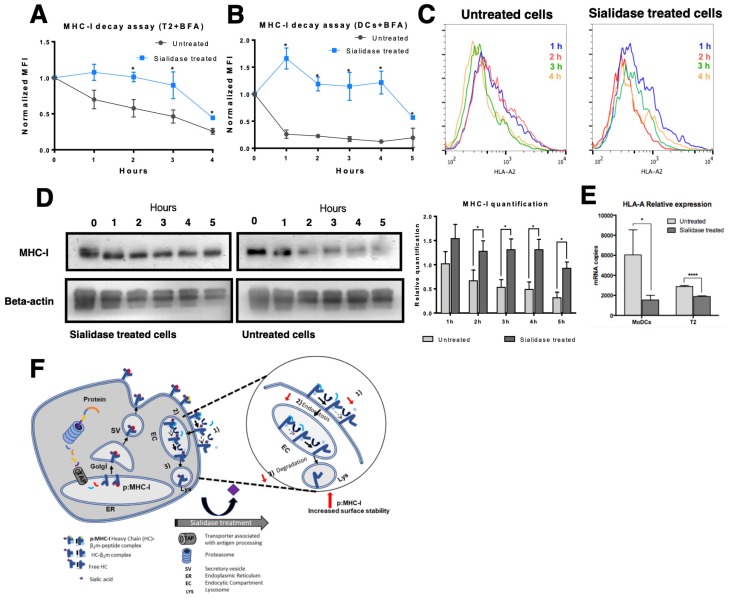
Figure 5.
Effect of sialic acid removal on MHC class I molecules internalization. Cells were incubated with Brefeldin A (BFA) and treated with sialidase for 1 h, after which sialidase was removed but BFA remained for the course of the experiment. The cell surface expression of MHC-I molecules was assessed over time by staining cells with an anti-HLA-A*02 antibody (BB7.2) and analyzed by flow cytometry in (A) T2 cells (N = 3) or (B) immature DCs (N = 5). (C) Representative histograms of antibody staining of untreated and sialidase treated human DCs over time (from 1 h to 4 h). (D) Whole cell lysates of sialidase treated and untreated DCs were obtained at each time point and probed for MHC-I (W6/32) and β-actin (loading control) by Western blot. Lysates from each experimental setup (untreated vs. sialidase treated cells) were loaded into different gels and processed in parallel. The relative quantification of MHC-I expression was calculated comparing to the expression of β-actin (N = 5). (E) HLA-A gene expression was assessed by qPCR following sialidase treatment on DCs (N = 7) and T2 cells (N = 3). (F) Schematic representation of the suggested effect of sialidase treatment on the molecular events that occur during MHC-I turnover. After synthesis and assembly in the ER, peptide: MHC-I complex (pMHC-I) is formed by a heavy chain, non-covalently bound to β2m chain and a specific peptide. In pMHC-I, the heavy chain is further glycosylated in the Golgi and then exported to cell surface. Part of the MHC-I molecules that are endocytosed is recycled back to the cell surface, while others are degraded. Upon extrinsic desialylation, we suggest that there is a slower dissociation of β2m chain and of specific peptide, probably slowing internalization by endocytose and decreasing degradation. Values presented as mean ± SEM. Statistically significant differences are indicated by asterisks (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001).