Abstract
In healthcare settings, Candida spp. cause invasive disease with high mortality. The overwhelming majority of cases are associated with the use of critically-needed medical devices, such as vascular catheters. On the surface of these indwelling materials, Candida forms resilient, adherent biofilm communities. A hallmark characteristic of this process is the production of an extracellular matrix, which promotes fungal adhesion and provides protection from external threats. In this review, we highlight the medical relevance of device-associated Candida biofilms and draw attention to the process of Candida-biofilm-matrix production. We provide an update on the current understanding of how biofilm extracellular matrix contributes to pathogenicity, particularly through its roles in the promoting antifungal drug tolerance and immune evasion.
Keywords: Candida, matrix, biofilm, immunity, drug resistance
1. What Is the Health Impact of Candida Biofilms?
Candida spp. are the leading cause of invasive fungal infection in the clinical setting [1]. A recent multistate point prevalence survey of hospitalized patients found Candida spp. to be the group of microorganisms accounting for the greatest number of bloodstream infections (22%) [1]. As invasive candidiasis carries the highest mortality rate among all nosocomial pathogens (with up to 47% attributable mortality), these infections often lead to devastating consequences [2,3]. Epidemiological studies have linked the presence of medical devices, such as indwelling vascular catheters, to the development of Candida bloodstream infection (candidemia) and invasive candidiasis [4]. On artificial devices, Candida attaches to the surface and proliferates as adherent microbial communities [5,6,7]. Fungal cells within these biofilms exhibit resistance to all available drug therapies and impair host defenses [8,9,10,11,12,13,14,15,16]. Ultimately, cells from the communities disperse into the bloodstream and disseminate to other organs, including the eyes, heart valves, joints, spleen, kidneys, and liver, too commonly leading to patient’s death [3,17]. Data from the Centers for Disease Control and Prevention show Candida spp. to be the most frequent cause of vascular-device-associated bloodstream infection in the hospital setting [1,4,18]. Device removal is recommended for patients with Candida-infected medical devices, as mortality rates are even higher if vascular catheters are retained [3,19]. However, removal of vascular access can be difficult for critically-ill patients, and mortality rates remain high even after removal [19].
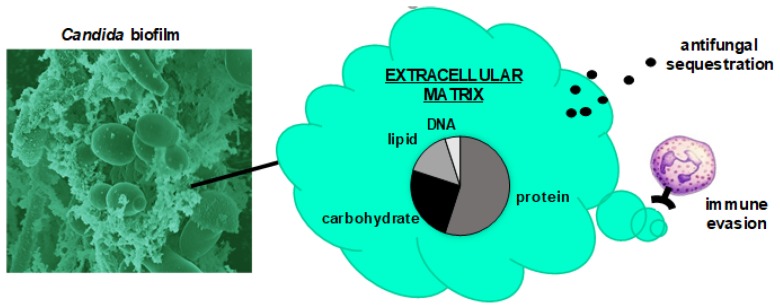
Candida albicans, the most prevalent species of Candida, has been employed as the model organism for the study of fungal biofilms [20,21,22,23,24,25,26,27,28,29,30,31,32]. As biofilms form, C. albicans frequently undergoes filamentation, producing elongated pseudohyphae and hyphae, in addition to maintaining cells in the yeast morphology [33]. The extent of this morphologic transition appears to vary among C. albicans strains and clinical niches. In addition to C. albicans, the importance of non-albicans species, including C. tropicalis, C. parapsilosis, and C. glabrata has become increasingly recognized [7,21,33,34,35,36,37,38]. Similarly, the recently emergent species C. auris readily forms biofilms on artificial materials, which likely accounts for the predilection of this species to cause disease in patients with indwelling medical devices [39,40,41,42,43,44,45]. Many of the non-albicans Candida spp. lack the capacity for filamentation and instead form biofilms comprised entirely of yeast cells [46]. However, biofilms formed by all Candida spp. involve the production of a matrix, which is an extracellular adherent polymeric material that defines biofilms [20,47,48,49]. In addition to its roles in adhesion and cohesion, this material also accounts for the vast majority of the tolerance of antifungals and resistance to host defenses that has been observed for Candida biofilms (Figure 1) [11,13,20,50].
Figure 1.
Influence of Candida biofilm extracellular matrix on pathogenicity. The scanning electron micrograph shows a Candida albicans biofilm consisting of yeast and hyphae encased in an extracellular matrix. This material contains a combination of polysaccharides, proteins, DNA, and lipids. Extracellular matrix contributes to pathogenicity by enhancing drug tolerance and promoting immune evasion.
2. What Is the Composition of Candida Biofilm Matrix?
The most detailed characterizations of Candida biofilm matrix have examined C. albicans biofilms during in vitro growth [29,51,52]. These investigations show that the matrix of mature biofilms contains a variety of macromolecules, including protein (55%), carbohydrate (25%), lipid (15%), and DNA (5%). Although the most abundant matrix component is protein, very little is known about the function of the individual proteins [29,32,53]. The finding of various metabolism-related proteins suggests that the matrix may function to degrade extracellular biopolymers as an energy source or as a mechanism of dispersion [32,53]. Although these hypotheses have not been tested specifically for Candida, the role of biofilm matrix as an extracellular digestive system has been described for bacterial biofilms [54,55,56,57,58].
The polysaccharide component of Candida biofilms contains building blocks similar to those found in the cell wall [29,43,59]. However, the macromolecular structures of the polysaccharides vary considerably between the cell wall and the extracellular matrix. For example, in the extracellular matrix of C. albicans, one abundant high-molecular-weight component consists of approximately 12,000 residues of α-1,2-branched α-1,6 mannan. These polysaccharide residues are nearly 10-fold greater than the mannans found in the C. albicans cell wall. Furthermore, they assemble with linear β-1,6 glucan in the extracellular space, forming a mannan–glucan complex [27,29]. Other Candida spp., including C. tropicalis, C. parapsilosis, C. glabrata, and C. auris, also produce a polysaccharide-rich matrix during biofilm growth [43,59]. Similar to C. albicans, these biofilms also contain mannan complexed with glucan. These polysaccharides sequester drugs and contribute to the resistance of biofilms to antifungals, as described in the following section [27,43].
Less abundant macromolecules of the biofilm matrix include lipids and nucleic acid [29,60,61]. The extracellular matrix of C. albicans biofilms contains a variety of lipids, including phospholipids (predominately phosphatidylcholine and phosphatidylethanolamine), sphingolipids, and eicosanoids [29,60,61]. The role of these lipids during Candida biofilm formation remains largely unexplored. The nucleic acid component of C. albicans biofilms appears to be comprised of non-coding DNA, which is anticipated to provide a structural scaffold and participate in protection from external insults, including some antifungal drugs [50,60,62].
Maybe not surprisingly, the extracellular matrix of C. albicans biofilm varies considerably between in vitro and in vivo conditions [28,29]. For example, during in vivo conditions, host proteins contribute to the extracellular biofilm matrix, accounting for >95% of the matrix proteome [28]. The composition of incorporated proteins varies by environmental niche (e.g., saliva, blood, urine). Little is known about the role of many of these host proteins. However, a subset of proteins appears to represent the host’s immune response to the biofilm [28]. Given the abundance of host proteins in biofilm matrix, further understanding of their role in biofilm pathogenicity will be of interest.
3. How Is Candida Biofilm Matrix Produced?
C. albicans has served as the model organism for discovery of pathways and regulators of biofilm matrix biogenesis [27,31,63,64,65,66]. During biofilm formation, C. albicans produces extracellular vesicles that are distinct from those manufactured during planktonic growth [66]. These 30-200 nm structures consist of lipid bilayers encasing protein, nucleic acid, lipid, and carbohydrate cargo. As C. albicans biofilms mature, cells release vesicles into the extracellular space and the accompanying cargo incorporates in the matrix. The importance of vesicle delivery in matrix biogenesis is highlighted by analysis of C. albicans mutants with disruption of key components of this pathway. Mutants defective in orthologs of endosomal sorting complexes required for transport (ESCRT) subunits fail to produce characteristic extracellular vesicles and do not manufacture a mature extracellular matrix during biofilm growth [66].
In addition to depositing in the extracellular matrix and providing a structural function, vesicle cargo proteins also remain enzymatically active. During biofilm formation, enzymes classically involved in cell wall biosynthesis are transported to the extracellular space and contribute to the assembly of mannans and glucans in the matrix [27]. Disruption of genes involved in either mannan pathways (ALG11, MNN9, MNN11, VAN1, MNN4-4, PMR1, and VRG4) or β-1,6 glucan pathways (BIG1 and KRE5) similarly impairs the deposition of both types of polysaccharides into the matrix. However, the ability of these mutants to complement each other during biofilm co-culture shows that these enzymes are active in the extracellular matrix and are critical for matrix assembly. Biofilms formed by other Candida spp., including C. tropicalis, C. parapsilosis, C. glabrata, and C. auris, also involve extracellular synthesis and modification of polysaccharides [43,59]. However, the genes involved in extracellular modification of mannan and glucan varies among species, likely accounting for their observed differences in polysaccharide side chains [59].
Several investigations have identified and characterized regulators of matrix production during C. albicans biofilm growth. These pathways appear to interface with stress response pathways during planktonic growth. For example, the molecular chaperone Hsp90 is required for mature matrix production [9]. However, in contrast to its role in stabilizing calcineurin under planktonic stress conditions, Hsp90 modulates matrix production through a separate, calcineurin-independent pathway during biofilm growth. Studies have also revealed two transcription factors involved in regulating matrix production for C. albicans biofilms, ZAP1 and RLM1 [31,63]. Zap1 serves as a negative regulator of matrix production, as genetic deletion leads to increased extracellular matrix production, presumably due to the upregulation of glucoamylases [63]. Rlm1 acts as positive regulator of extracellular matrix production, likely through the transcription of SMI1, which modulates extracellular glucan production during biofilm growth. Other transcriptional regulators of C. albicans biofilm development are also likely to influence extracellular matrix production [67].
4. How Does the Extracellular Matrix of Biofilm Influence Resistance to Antifungals?
One hallmark of C. albicans biofilm formation is the capacity of these communities to withstand antifungal concentrations many fold greater than the levels needed to kill Candida during planktonic growth [8,9,10,11,12]. As delivery of antifungals at these high concentrations is often unsafe or not feasible, device-associated biofilm infections pose a high risk for treatment failure [3]. Al-Fattani and Douglas first described how the relative abundance of the extracellular matrix correlated with antifungal drug resistance for Candida biofilms [22]. By altering the flow conditions, they modulated biofilm matrix production and linked biofilm resistance to abundant matrix production for C. albicans and C. tropicalis. Additional investigations have since identified correlations between extracellular matrix and tolerance of antifungals for additional Candida spp., including C. parapsilosis, C. glabrata, and C. auris [43,59].
Studies to date have shown that antifungal tolerance of Candida biofilms correlates most closely with the polysaccharides of the extracellular matrix. The presence of β-1,3 glucan, β-1,6 glucan, and α-1,2-branched α-1,6 mannan contribute to the resistance of Candida biofilms to anti-infectives [11,25,27,43,59,68]. The mechanism of the drug––polysaccharide interaction was first described for C. albicans biofilm matrix and the azole drug, fluconazole [25]. The glucan and mannan components for the extracellular matrix form a complex that sequesters drugs, likely through non-covalent interactions [27]. Subsequent studies have revealed the involvement of mechanisms of drug sequestration for other Candida spp., including C. tropicalis, C. parapsilosis, C. glabrata, and C. auris [23,43,59]. In addition, the matrix polysaccharides of Candida biofilms also sequester other commonly used antifungals, such as amphotericin B, anidulafungin, and flucytosine [11,68]. Furthermore, the abundance of matrix polysaccharides correlates with the ability of Candida biofilms to withstand disinfectants and oxidative stressors [69].
For many sites of infection, Candida spp. form polymicrobial biofilms with bacteria or other Candida spp. [70,71,72]. Examples include biofilms formed in the oropharynx and those found on many indwelling medical devices. In these settings, the extracellular matrix produced by one of the organisms may contribute to collectively protect other organisms within the biofilm. For example, in polymicrobial biofilms formed by C. albicans and Staphylococcus aureus, the latter exhibits increased resistance to antibiotics, when compared to monomicrobial Staphylococcus biofilms [73]. The polysaccharide-rich matrix produced by Candida appears to encase both organisms and this material contributes to impaired penetration of the vancomycin, ultimately conferring antibiotic resistance for Staphylococcus [74]. Similarly, Candida biofilm extracellular matrix also appears to enhance antibiotic resistance for Escherichia coli during polymicrobial biofilm growth [75]. However, in other polymicrobial biofilm models, polysacchardides of bacterial origin drive resistance for Candida [76]. An example of this scenario is Streptococcus mutans–C. albicans biofilms, where bacterially-produced α-glucan enhances the antifungal resistance for C. albicans [76].
5. Does Biofilm Extracellular Matrix Impact Immune Responses?
Host immune cells respond differently to Candida when it is growing as a biofilm or under planktonic conditions [8,13,14,15,16,77,78,79,80]. For example, upon encounter with C. albicans biofilm, peripheral blood mononuclear cells exhibit poor antifungal activity and release a cytokine profile distinct from the profile observed in response to planktonic C. albicans [16,80]. Similarly, cell culture macrophages appear to have impaired migration in the presence of C. albicans biofilms when compared to their motility in response to planktonic Candida [79]. Like mononuclear cells, human neutrophils display poor activity against C. albicans biofilms, with biofilms exhibiting an up to 5-fold higher resistance to killing when compared to their planktonic counterparts [13,14,15,80]. Similar patterns of resistance to killing by neutrophils have also been observed for biofilms formed by C. glabrata and C. parapsilosis [77].
As extracellular matrix encases Candida biofilm structures, components of this material are among the first to be encountered by leukocytes. Studies examining the role of matrix on leukocyte responses have primarily focused on the interaction of neutrophils with C. albicans biofilms [13,14]. Disruption of matrix, through either physical or genetic means, leads to increased killing of C. albicans biofilms by neutrophils [13]. The finding that biofilms formed by a mutant strain (pmr1∆/∆) deficient in the production of extracellular mannan–glucan are more susceptible to killing by neutrophils suggests a role for these polysaccharide components in immune evasion. The matrix of C. albicans biofilms prevents neutrophils from forming neutrophil extracellular traps (NETs), structures of DNA studded with histones and antimicrobial peptides that are important for the killing of many fungi, including C. albicans [13,81]. Inhibition of NET formation appears to occur in upstream events, as biofilms also dampen the production of reactive oxygen species, key signaling molecules for NET production [13,14,82]. The C. albicans pmr1∆/∆ mutant has also been employed to examine the influence of extracellular matrix on biofilm–macrophage interactions [79]. The finding that genetic disruption of this mannan pathway does not impact macrophage behavior suggests that other matrix components or biofilm properties may be involved in the impaired response of macrophages to biofilm.
6. Conclusions
Biofilms formed by Candida spp. present a major obstacle for the treatment of invasive candidiasis. During this mode of growth, fungal communities withstand high concentrations of antifungals and resist host responses. The production of extracellular matrix, a defining property of biofilm formation, is critical for providing this protection. Recent studies have defined key matrix polysaccharide components involved in both drug tolerance and immune evasion. Further understanding of these processes may reveal new strategies to combat biofilm infection. While C. albicans has served as the model species for many biofilm studies, investigation of other species will be of great interest in light of emerging and drug-resistant Candida spp.
Funding
The authors are supported by the National Institutes of Health (R01AI073289 and K08 AI108727), the Burroughs Wellcome Fund (1012299), and the Doris Duke Charitable Foundation (112580130).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gudlaugsson O., Gillespie S., Lee K., Vande Berg J., Hu J., Messer S., Herwaldt L., Pfaller M., Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 3.Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., Reboli A.C., Schuster M.G., Vazquez J.A., Walsh T.J., et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller M.A., Diekema D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Toole G.A. To build a biofilm. J. Bacteriol. 2003;185:2687–2689. doi: 10.1128/JB.185.9.2687-2689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donlan R.M., Costerton J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojic E.M., Darouiche R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee P.K., Chandra J., Kuhn D.M., Ghannoum M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003;71:4333–4340. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins N., Uppuluri P., Nett J., Rajendran R., Ramage G., Lopez-Ribot J.L., Andes D., Cowen L.E. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 2011;7:e1002257. doi: 10.1371/journal.ppat.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramage G., Bachmann S., Patterson T.F., Wickes B.L., Lopez-Ribot J.L. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 2002;49:973–980. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 11.Nett J.E., Crawford K., Marchillo K., Andes D.R. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob. Agents Chemother. 2010;54:3505–3508. doi: 10.1128/AAC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaFleur M.D., Kumamoto C.A., Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006;50:3839–3846. doi: 10.1128/AAC.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson C.J., Cabezas-Olcoz J., Kernien J.F., Wang S.X., Beebe D.J., Huttenlocher A., Ansari H., Nett J.E. The Extracellular matrix of Candida albicans biofilms impairs formation of neutrophil extracellular traps. PLoS Pathog. 2016;12:e1005884. doi: 10.1371/journal.ppat.1005884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Z., Thompson A., Sobue T., Kashleva H., Xu H., Vasilakos J., Dongari-Bagtzoglou A. Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. J. Infect. Dis. 2012;206:1936–1945. doi: 10.1093/infdis/jis607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katragkou A., Simitsopoulou M., Chatzimoschou A., Georgiadou E., Walsh T.J., Roilides E. Effects of interferon-gamma and granulocyte colony-stimulating factor on antifungal activity of human polymorphonuclear neutrophils against Candida albicans grown as biofilms or planktonic cells. Cytokine. 2011;55:330–334. doi: 10.1016/j.cyto.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Chandra J., McCormick T.S., Imamura Y., Mukherjee P.K., Ghannoum M.A. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect. Immun. 2007;75:2612–2620. doi: 10.1128/IAI.01841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards J.E., Jr., Lehrer R.I., Stiehm E.R., Fischer T.J., Young L.S. Severe candidal infections: Clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann. Intern. Med. 1978;89:91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- 18.Cleveland A.A., Farley M.M., Harrison L.H., Stein B., Hollick R., Lockhart S.R., Magill S.S., Derado G., Park B.J., Chiller T.M. Changes in incidence and antifungal drug resistance in candidemia: Results from population-based laboratory surveillance in atlanta and baltimore, 2008–2011. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012;55:1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andes D.R., Safdar N., Baddley J.W., Playford G., Reboli A.C., Rex J.H., Sobel J.D., Pappas P.G., Kullberg B.J. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012;54:1110–1122. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 20.Chandra J., Kuhn D.M., Mukherjee P.K., Hoyer L.L., McCormick T., Ghannoum M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thein Z.M., Samaranayake Y.H., Samaranayake L.P. In vitro biofilm formation of Candida albicans and non-albicans Candida species under dynamic and anaerobic conditions. Arch. Oral Biol. 2007;52:761–767. doi: 10.1016/j.archoralbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Al-Fattani M.A., Douglas L.J. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J. Med. Microbiol. 2006;55:999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell K.F., Taff H.T., Cuevas M.A., Reinicke E.L., Sanchez H., Andes D.R. Role of matrix β-1,3 glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrob. Agents Chemother. 2013;57:1918–1920. doi: 10.1128/AAC.02378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 25.Nett J., Lincoln L., Marchillo K., Massey R., Holoyda K., Hoff B., VanHandel M., Andes D. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 2007;51:510–520. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawser S.P., Baillie G.S., Douglas L.J. Production of extracellular matrix by Candida albicans biofilms. J. Med. Microbiol. 1998;47:253–256. doi: 10.1099/00222615-47-3-253. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell K.F., Zarnowski R., Sanchez H., Edward J.A., Reinicke E.L., Nett J.E., Mitchell A.P., Andes D.R. Community participation in biofilm matrix assembly and function. Proc. Natl. Acad. Sci. USA. 2015;112:4092–4097. doi: 10.1073/pnas.1421437112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nett J.E., Zarnowski R., Cabezas-Olcoz J., Brooks E.G., Bernhardt J., Marchillo K., Mosher D.F., Andes D.R. Host contributions to construction of three device-associated Candida albicans biofilms. Infect. Immun. 2015;83:4630–4638. doi: 10.1128/IAI.00931-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarnowski R., Westler W.M., Lacmbouh G.A., Marita J.M., Bothe J.R., Bernhardt J., Lounes-Hadj Sahraoui A., Fontaine J., Sanchez H., Hatfield R.D., et al. Novel entries in a fungal biofilm matrix encyclopedia. mBio. 2014;5:e01333-14. doi: 10.1128/mBio.01333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins M., Henriques M., Lopez-Ribot J.L., Oliveira R. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses. 2012;55:80–85. doi: 10.1111/j.1439-0507.2011.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nett J.E., Sanchez H., Cain M.T., Ross K.M., Andes D.R. Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot. Cell. 2011;10:1660–1669. doi: 10.1128/EC.05126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas D.P., Bachmann S.P., Lopez-Ribot J.L. Proteomics for the analysis of the Candida albicans biofilm lifestyle. Proteomics. 2006;6:5795–5804. doi: 10.1002/pmic.200600332. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn D.M., Chandra J., Mukherjee P.K., Ghannoum M.A. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 2002;70:878–888. doi: 10.1128/IAI.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin J.H., Kee S.J., Shin M.G., Kim S.H., Shin D.H., Lee S.K., Suh S.P., Ryang D.W. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: Comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 2002;40:1244–1248. doi: 10.1128/JCM.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bizerra F.C., Nakamura C.V., De Poersch C., Estivalet Svidzinski T.I., Borsato Quesada R.M., Goldenberg S., Krieger M.A., Yamada-Ogatta S.F. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008;8:442–450. doi: 10.1111/j.1567-1364.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 36.Jain N., Kohli R., Cook E., Gialanella P., Chang T., Fries B.C. Biofilm formation by and antifungal susceptibility of Candida isolates from urine. Appl. Environ. Microbiol. 2007;73:1697–1703. doi: 10.1128/AEM.02439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis R.E., Kontoyiannis D.P., Darouiche R.O., Raad I.I., Prince R.A. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an In Vitro model of Candida catheter-related bloodstream infection. Antimicrob. Agents Chemother. 2002;46:3499–3505. doi: 10.1128/AAC.46.11.3499-3505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tumbarello M., Posteraro B., Trecarichi E.M., Fiori B., Rossi M., Porta R., De Gaetano Donati K., La Sorda M., Spanu T., Fadda G., et al. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 2007;45:1843–1850. doi: 10.1128/JCM.00131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherry L., Ramage G., Kean R., Borman A., Johnson E.M., Richardson M.D., Rautemaa-Richardson R. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017;23:328–331. doi: 10.3201/eid2302.161320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams E., Quinn M., Tsay S., Poirot E., Chaturvedi S., Southwick K., Greenko J., Fernandez R., Kallen A., Vallabhaneni S., et al. Candida auris in healthcare facilities, New York, USA, 2013-2017. Emerg. Infect. Dis. 2018;24:1816–1824. doi: 10.3201/eid2410.180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lockhart S.R., Etienne K.A., Vallabhaneni S., Farooqi J., Chowdhary A., Govender N.P., Colombo A.L., Calvo B., Cuomo C.A., Desjardins C.A., et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larkin E., Hager C., Chandra J., Mukherjee P.K., Retuerto M., Salem I., Long L., Isham N., Kovanda L., Borroto-Esoda K., et al. The emerging pathogen Candida auris: Growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob. Agents Chemother. 2017;61:e02396-16. doi: 10.1128/AAC.02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dominguez E.G., Zarnowski R., Choy H.L., Zhao M., Sanchez H., Nett J.E., Andes D.R. Conserved role for biofilm matrix polysaccharides in Candida auris drug resistance. MSphere. 2019;4:e00680-18. doi: 10.1128/mSphereDirect.00680-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kean R., Delaney C., Sherry L., Borman A., Johnson E.M., Richardson M.D., Rautemaa-Richardson R., Williams C., Ramage G. Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. MSphere. 2018;3:e00334-18. doi: 10.1128/mSphere.00334-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh R., Kaur M., Chakrabarti A., Shankarnarayan S.A., Rudramurthy S.M. Biofilm formation by Candida auris isolated from colonising sites and candidemia cases. Mycoses. 2019;62:706–709. doi: 10.1111/myc.12947. [DOI] [PubMed] [Google Scholar]
- 46.Silva S., Henriques M., Martins A., Oliveira R., Williams D., Azeredo J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009;47:681–689. doi: 10.3109/13693780802549594. [DOI] [PubMed] [Google Scholar]
- 47.Fox E.P., Nobile C.J. A sticky situation: Untangling the transcriptional network controlling biofilm development in Candida albicans. Transcription. 2012;3:315–322. doi: 10.4161/trns.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramage G., Mowat E., Jones B., Williams C., Lopez-Ribot J. Our current understanding of fungal biofilms. Crit. Rev. Microbiol. 2009;35:340–355. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 49.Ramage G., Saville S.P., Thomas D.P., Lopez-Ribot J.L. Candida biofilms: An update. Eukaryot. Cell. 2005;4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flemming H.C., Wingender J. The biofilm matrix. Nat. Rev. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 51.Baillie G.S., Douglas L.J. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 2000;46:397–403. doi: 10.1093/jac/46.3.397. [DOI] [PubMed] [Google Scholar]
- 52.Ramage G., Vandewalle K., Wickes B.L., Lopez-Ribot J.L. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 2001;18:163–170. [PubMed] [Google Scholar]
- 53.Martinez-Gomariz M., Perumal P., Mekala S., Nombela C., Chaffin W.L., Gil C. Proteomic analysis of cytoplasmic and surface proteins from yeast cells, hyphae, and biofilms of Candida albicans. Proteomics. 2009;9:2230–2252. doi: 10.1002/pmic.200700594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutherland I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001;9:222–227. doi: 10.1016/S0966-842X(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 55.Absalon C., Ymele-Leki P., Watnick P.I. The bacterial biofilm matrix as a platform for protein delivery. MBio. 2012;3:e00127-112. doi: 10.1128/mBio.00127-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romani A.M., Fund K., Artigas J., Schwartz T., Sabater S., Obst U. Relevance of polymeric matrix enzymes during biofilm formation. Microb. Ecol. 2008;56:427–436. doi: 10.1007/s00248-007-9361-8. [DOI] [PubMed] [Google Scholar]
- 57.Jiao Y., D’Haeseleer P., Dill B.D., Shah M., Verberkmoes N.C., Hettich R.L., Banfield J.F., Thelen M.P. Identification of biofilm matrix-associated proteins from an acid mine drainage microbial community. Appl. Environ. Microbiol. 2011;77:5230–5237. doi: 10.1128/AEM.03005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen C., Krishnan V., Macon K., Manne K., Narayana S.V., Schneewind O. Secreted proteases control autolysin-mediated biofilm growth of Staphylococcus aureus. J. Biol. Chem. 2013;288:29440–29452. doi: 10.1074/jbc.M113.502039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dominguez E., Zarnowski R., Sanchez H., Covelli A.S., Westler W.M., Azadi P., Nett J., Mitchell A.P., Andes D.R. Conservation and divergence in the Candida species biofilm matrix mannan-glucan complex structure, function, and genetic control. MBio. 2018;9:e00451-18. doi: 10.1128/mBio.00451-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martins M., Uppuluri P., Thomas D.P., Cleary I.A., Henriques M., Lopez-Ribot J.L., Oliveira R. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia. 2010;169:323–331. doi: 10.1007/s11046-009-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lattif A.A., Mukherjee P.K., Chandra J., Roth M.R., Welti R., Rouabhia M., Ghannoum M.A. Lipidomics of Candida albicans biofilms reveals phase-dependent production of phospholipid molecular classes and role for lipid rafts in biofilm formation. Microbiology. 2011;157:3232–3242. doi: 10.1099/mic.0.051086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajendran R., Williams C., Lappin D.F., Millington O., Martins M., Ramage G. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot. Cell. 2013;12:420–429. doi: 10.1128/EC.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nobile C.J., Nett J.E., Hernday A.D., Homann O.R., Deneault J.S., Nantel A., Andes D.R., Johnson A.D., Mitchell A.P. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 2009;7:e1000133. doi: 10.1371/journal.pbio.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nett J.E., Sanchez H., Cain M.T., Andes D.R. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J. Infect. Dis. 2010;202:171–175. doi: 10.1086/651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taff H.T., Nett J.E., Zarnowski R., Ross K.M., Sanchez H., Cain M.T., Hamaker J., Mitchell A.P., Andes D.R. A Candida biofilm-induced pathway for matrix glucan delivery: Implications for drug resistance. PLoS Pathog. 2012;8:e1002848. doi: 10.1371/journal.ppat.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zarnowski R., Sanchez H., Covelli A.S., Dominguez E., Jaromin A., Bernhardt J., Mitchell K.F., Heiss C., Azadi P., Mitchell A., et al. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol. 2018;16:e2006872. doi: 10.1371/journal.pbio.2006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fox E.P., Bui C.K., Nett J.E., Hartooni N., Mui M.C., Andes D.R., Nobile C.J., Johnson A.D. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol. Microbiol. 2015;96:1226–1239. doi: 10.1111/mmi.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vediyappan G., Rossignol T., d’Enfert C. Interaction of Candida albicans biofilms with antifungals: Transcriptional response and binding of antifungals to beta-glucans. Antimicrob. Agents Chemother. 2010;54:2096–2111. doi: 10.1128/AAC.01638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nett J.E., Guite K.M., Ringeisen A., Holoyda K.A., Andes D.R. Reduced biocide susceptibility in Candida albicans biofilms. Antimicrob. Agents Chemother. 2008;52:3411–3413. doi: 10.1128/AAC.01656-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peters B.M., Jabra-Rizk M.A., O’May G.A., Costerton J.W., Shirtliff M.E. Polymicrobial interactions: Impact on pathogenesis and human disease. Clin. Microbiol. Rev. 2012;25:193–213. doi: 10.1128/CMR.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harriott M.M., Noverr M.C. Ability of Candida albicans mutants to induce Staphylococcus aureus vancomycin resistance during polymicrobial biofilm formation. Antimicrob. Agents Chemother. 2010;54:3746–3755. doi: 10.1128/AAC.00573-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen A.I., Dolben E.F., Okegbe C., Harty C.E., Golub Y., Thao S., Ha D.G., Willger S.D., O’Toole G.A., Harwood C.S., et al. Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR-controlled biofilm formation as part of a cyclic relationship involving phenazines. PLoS Pathog. 2014;10:e1004480. doi: 10.1371/journal.ppat.1004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harriott M.M., Noverr M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009;53:3914–3922. doi: 10.1128/AAC.00657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kong E.F., Kucharíková T.C.S., Andes D., Van Dijck P., Jabra-Rizk M. Protection of staphylococcus aureus against antimicrobials by candida albicans biofilm matrix; Proceedings of the 13th ASM Conference on Candida and Candidiasis; Seattle, WA, USA. 13–17 April 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Brucker K., Tan Y., Vints K., De Cremer K., Braem A., Verstraeten N., Michiels J., Vleugels J., Cammue B.P., Thevissen K. Fungal beta-1,3-glucan increases ofloxacin tolerance of Escherichia coli in a polymicrobial E. coli/Candida albicans biofilm. Antimicrob. Agents Chemother. 2015;59:3052–3058. doi: 10.1128/AAC.04650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim D., Liu Y., Benhamou R.I., Sanchez H., Simon-Soro A., Li Y., Hwang G., Fridman M., Andes D.R., Koo H. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018;12:1427–1442. doi: 10.1038/s41396-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson C.J., Kernien J.F., Hoyer A.R., Nett J.E. Mechanisms involved in the triggering of neutrophil extracellular traps (NETs) by Candida glabrata during planktonic and biofilm growth. Sci. Rep. 2017;7:13065. doi: 10.1038/s41598-017-13588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katragkou A., Chatzimoschou A., Simitsopoulou M., Georgiadou E., Roilides E. Additive antifungal activity of anidulafungin and human neutrophils against Candida parapsilosis biofilms. J. Antimicrob. Chemother. 2011;66:588–591. doi: 10.1093/jac/dkq466. [DOI] [PubMed] [Google Scholar]
- 79.Alonso M., Gow N., Erwig L., Bain J. Macrophage migration is impaired within Candida albicans biofilms. J. Fungi. 2017;3:31. doi: 10.3390/jof3030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katragkou A., Kruhlak M.J., Simitsopoulou M., Chatzimoschou A., Taparkou A., Cotten C.J., Paliogianni F., Diza-Mataftsi E., Tsantali C., Walsh T.J., et al. Interactions between human phagocytes and Candida albicans biofilms alone and in combination with antifungal agents. J. Infect. Dis. 2010;201:1941–1949. doi: 10.1086/652783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urban C.F., Reichard U., Brinkmann V., Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 82.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]