Summary
Tracheal intubation in coronavirus disease 2019 (COVID-19) patients creates a risk to physiologically compromised patients and to attending healthcare providers. Clinical information on airway management and expert recommendations in these patients are urgently needed. By analysing a two-centre retrospective observational case series from Wuhan, China, a panel of international airway management experts discussed the results and formulated consensus recommendations for the management of tracheal intubation in COVID-19 patients. Of 202 COVID-19 patients undergoing emergency tracheal intubation, most were males (n=136; 67.3%) and aged 65 yr or more (n=128; 63.4%). Most patients (n=152; 75.2%) were hypoxaemic (Sao2 <90%) before intubation. Personal protective equipment was worn by all intubating healthcare workers. Rapid sequence induction (RSI) or modified RSI was used with an intubation success rate of 89.1% on the first attempt and 100% overall. Hypoxaemia (Sao2 <90%) was common during intubation (n=148; 73.3%). Hypotension (arterial pressure <90/60 mm Hg) occurred in 36 (17.8%) patients during and 45 (22.3%) after intubation with cardiac arrest in four (2.0%). Pneumothorax occurred in 12 (5.9%) patients and death within 24 h in 21 (10.4%). Up to 14 days post-procedure, there was no evidence of cross infection in the anaesthesiologists who intubated the COVID-19 patients. Based on clinical information and expert recommendation, we propose detailed planning, strategy, and methods for tracheal intubation in COVID-19 patients.
Keywords: airway management, ARDS, consensus recommendations, COVID-19, critical care, infection prevention and control, pneumonia, respiratory failure, tracheal intubation
Editor's key points.
-
•
Data from a series of 202 coronavirus disease 2019 (COVID-19) patients undergoing tracheal intubation in two hospitals in Wuhan, China were analysed and used to guide expert consensus recommendations from an international panel.
-
•
Using rapid sequence induction, first-pass intubation occurred in 89%, with hypoxaemia and hypotension common during intubation.
-
•
Other adverse outcomes included cardiac arrest (2%), pneumothorax (6%), and death within 24 h (10%).
-
•
Operators wore at least Level 3 personal protective equipment, and none became infected.
-
•
A detailed strategy and methods for tracheal intubation in COVID-19 patients are proposed.
On April 10, 2020, the World Health Organization (WHO) characterized COVID-19 disease as a pandemic, with more than 1,700,000 confirmed patients in more than 210 countries/territories/areas,1 with an estimated 2.3% of patients that need tracheal intubation.2 The mortality in critically ill patients with COVID-19 ranges from 16.7% to 61.5%.3 , 4 Given the highly contagious nature of the causative virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its transmission by droplet5, 6, 7 or even aerosol infection,8 , 9 tracheal intubation carries a high risk to the intubator.10, 11, 12 There is a lack of data on these patients regarding presenting characteristics, procedural success rates, and subsequent complications. There are also few data on the risk of disease transmission to healthcare workers after tracheal intubation of acutely ill COVID-19 patients.10, 24 These data would be useful for future planning and management for these patients and precautions for staff.11, 12, 13
We report clinical data on presenting patients' characteristics, procedural processes, complications, and healthcare worker infection after tracheal intubation in COVID-19 patients. Additionally, the data were reviewed by an international panel of experts, and recommendations are made to optimise tracheal intubation success, reduce patient complications and mortality, and minimise the risk of infection of healthcare workers during tracheal intubation.
This retrospective observational case series was approved by the Huazhong University of Science and Technology (TJ-C20200148 and 20200097). Written informed consent was waived, as this study was a retrospective observational study without patient interventions. Data were provided by the authors based in the two study hospitals, and were interpreted by all authors. The review panel of international experts in airway management discussed the clinical data and the problems encountered during and after intubation using two web-based teleconferences and social media. The experts provided suggestions to address problems encountered clinically, and developed a consensus agreement on a safe and adequate approach to perform tracheal intubation in COVID-19 patients. This was used to create a simple flow chart for tracheal intubation in COVID-19 patients.
Data were obtained from two major hospitals in Wuhan, China, where the COVID-19 outbreak originated: Tongji Hospital (from February 4 to March 10, 2020) and Union Hospital (from February 13 to March 12, 2020), Huazhong University of Science and Technology, Wuhan, China. All patients had SARS-CoV-2 infection confirmed by reverse transcription–polymerase chain reaction (RT–PCR) testing for viral ribonucleic acid in respiratory samples, in combination with pulmonary chest CT findings. Clinical and outcome data were obtained from hospital records, and were reviewed and approved by the authors based in the two hospitals. Some of the basic clinical information may have been stated elsewhere in narrative form,14 , 15 but detailed clinical data for these patients have not been presented previously. The survey data were summarised and analysed by survey organisers at the University of Pennsylvania (Philadelphia, PA, USA).
Assessment of airway difficulty was predicted by patient history, clinical assessment of neck length and circumference, mandible size, and clinician judgement. Mallampati score16 was usually not evaluated because of the risks of aerosol viral spreading. Hypoxaemia was defined as oxygen saturation (Sao 2) <90% or Pao 2/F io 2 <150 mm Hg, tachypnoea with ventilatory frequency >30 bpm, arterial hypotension with blood pressure <90/60 mm Hg, tachycardia with HR >120 beats min−1, and unconsciousness with a negative response to purposeful physical stimulation (likely equivalent to Glasgow coma score17 <8). Difficult laryngoscopy was defined as a Grade III–IV Cormack and Lehane18 view at laryngoscopy.
Transmission of infection to tracheal intubators was monitored and assessed continuously by clinical symptoms and signs of COVID-19 during a 14 day quarantine in a private hotel room. Anaesthesiologists without clinical symptoms after quarantine were tested with RT–PCR in respiratory samples. Chest CT examination was performed at anaesthesiologists' request. After confirmed negative PCR test results, anaesthesiologists were allowed to work in the hospital again for the next 14 day duty shift.
Suggestions were made by expert consensus. Given the novelty of COVID-19, there is a relative lack of specific evidence-based information. As a result, expert consensus was supplemented with evidence-based support whenever feasible.
Descriptive statistics were used to characterise the clinical features of the patients in the case series. Categorical variables were expressed as number (%) and compared by χ2 test or Fisher's exact test between different hospitals at a two-sided significance level of 0.05. Statistical analysis was performed with PASW® Statistics 18 (SPSS Inc., Chicago, IL, USA).
Between February 4 and March 12, 2020, 202 patients with COVID-19 underwent tracheal intubation at the two study hospitals. The clinical features of these patients and data relating to peri-procedural physiology and outcomes are summarised in Table 1, Table 2 .
Table 1.
Clinical characteristics of patients infected with coronavirus disease 2019 from two hospitals in Wuhan, China. Data are presented as n (%). Proportions were analysed using χ2 test or Fisher's exact test. RSI, rapid sequence induction intubation technique.
| Patient characteristics | Total (n=202) | Hospital A (n=137) | Hospital B (n=65) | P-value |
|---|---|---|---|---|
| Gender | ||||
| Female | 66 (32.7) | 43 (31.4) | 23 (35.4) | 0.571 |
| Male | 136 (67.3) | 94 (68.6) | 42 (64.6) | |
| Age ≥65 yr | 128 (63.4) | 90 (65.7) | 38 (58.5) | 0.319 |
| Difficult airway history | 0 (0) | 0 (0) | 0 (0) | — |
| Suspected difficult airway | 45 (22.3) | 41 (29.9) | 4 (6.2) | <0.001 |
| Unanticipated difficult airway | 3 (1.5) | 3 (2.2) | 0 (0) | 0.553 |
| Modified RSI | 202 (100) | 137 (100) | 65 (100) | — |
| Awake intubation | 0 (0) | 0 (0) | 0 (0) | — |
Table 2.
Airway management of patients infected with coronavirus disease 2019 from two hospitals in Wuhan, China. Data are presented as n (%). Proportions were analysed using χ2 test or Fisher's exact test. PAPR, powered air-purifying respirator.
| Characteristics | Total (n=202) | Hospital A (n=137) | Hospital B (n=65) | P-value |
|---|---|---|---|---|
| Before intubation | ||||
| Physical status during oxygen therapy | ||||
| Sao2 <90% | 152 (75.2) | 106 (77.4) | 46 (70.8) | 0.310 |
| Pao2/Fio2 <150 mm Hg | 194 (96.0) | 130 (94.9) | 64 (98.5) | 0.407 |
| Ventilatory frequency >30 bpm | 109 (54) | 69 (50.4) | 40 (61.5) | 0.137 |
| BP <90/60 mm Hg | 16 (7.9) | 14 (10.2) | 2 (3.1) | 0.079 |
| HR >120 beats min−1 | 49 (24.3) | 27 (19.7) | 22 (33.8) | 0.029 |
| Unconsciousness | 26 (12.9) | 14 (10.2) | 12 (18.5) | 0.102 |
| Oxygen therapy technique | ||||
| Regular nasal cannula | 8 (4.0) | 6 (4.4) | 2 (3.1) | 0.954 |
| Mask with reservoir bag | 21 (10.4) | 14 (10.2) | 7 (10.8) | 0.905 |
| High-flow nasal cannula | 28 (13.9) | 16 (11.7) | 12 (18.5) | 0.192 |
| Noninvasive ventilation | 143 (70.8) | 101 (73.7) | 42 (64.6) | 0.184 |
| Operator personal protective equipment | ||||
| Respirator (N95 or equivalent, inside) | 202 (100) | 137 (100) | 65 (100) | — |
| Surgical mask (outside) | 202 (100) | 137 (100) | 65 (100) | — |
| Goggles | 202 (100) | 137 (100) | 65 (100) | — |
| Face shield | 22 (10.9) | 7 (5.1) | 15 (23.1) | <0.001 |
| Full hood without a PAPR | 130 (64.4) | 130 (94.9) | 0 (0) | <0.001 |
| PAPR | 50 (24.8) | 0 (0) | 50 (76.9) | <0.001 |
| Intubation hampered by mask fog | 11 (5.4) | 11 (8.0) | 0 (0) | 0.044 |
| Anti-fog treatment | 197 (97.5) | 132 (96.4) | 65 (100) | 0.282 |
| Anti-fog method | N/A | Liquid soap | Iodophor | — |
| Necessary individuals | N/A | 2 | 2 | — |
| Operator infection | 0 (0) | 0 (0%) | 0 (0) | — |
| Intubation | ||||
| Induction | ||||
| Bolus of i.v. fluid | 0 (0) | 0 (0) | 0 (0) | — |
| Prophylactic vasopressor | 41 (20.3) | 41 (29.9) | 0 (0) | <0.001 |
| Preoxygenate with 100% Fio2 for 5 min | 202 (100) | 137 (100) | 65 (100) | — |
| Preoxygenate via prior oxygen therapy | 107 (53.0) | 92 (67.2) | 15 (23.1) | <0.001 |
| Preoxygenate via face mask | 95 (47.0) | 45 (32.8) | 50 (76.9) | <0.001 |
| Propofol | 194 (96.0) | 135 (98.5) | 59 (90.8) | 0.024 |
| Etomidate | 6 (3.0) | 5 (3.6) | 1 (1.5) | 0.702 |
| Midazolam | 27 (13.4) | 27 (19.7) | 0 (0) | <0.001 |
| Sufentanil | 99 (49) | 94 (68.6) | 5 (7.7) | <0.001 |
| Fentanyl | 60 (29.7) | 6 (4.4) | 54 (83.1) | <0.001 |
| Rocuronium | 200 (99.0) | 137 (100) | 63 (96.9) | 0.102 |
| Mask ventilation after induction | 188 (93.1) | 123 (89.8) | 65 (100) | 0.018 |
| Intubation device at first attempt | ||||
| Macintosh laryngoscope | 21 (10.4) | 21 (15.3) | 0 (0) | 0.001 |
| Videolaryngoscope with disposable blade | 181 (89.6) | 116 (84.7) | 65 (100) | 0.001 |
| Results of intubation | ||||
| Successful intubation at first attempt | 180 (89.1) | 116 (84.7) | 64 (98.5) | 0.003 |
| Total successful intubation | 202 (100) | 137 (100) | 65 (100) | — |
| Duration of intubation ≤3 min | 187 (92.6) | 123 (89.8) | 64 (98.5) | 0.040 |
| Duration of intubation >3 min | 12 (5.9) | 11 (8) | 1 (1.5) | 0.108 |
| Duration of intubation >5 min | 3 (1.5) | 3 (2.2) | 0 (0) | 0.553 |
| Adverse events during intubation | ||||
| Hypoxaemia (Sao2 <90%) | 175 (73.3) | 110 (80.3) | 38 (58.5) | 0.001 |
| Hypotension (BP <90/60 mm Hg) | 36 (17.8) | 14 (10.2) | 22 (33.8) | <0.001 |
| After intubation | ||||
| Physical status | ||||
| Hypoxaemia (Sao2 <90%) | 36 (17.8) | 16 (11.7) | 20 (30.8) | 0.001 |
| Hypotension (BP <90/60 mm Hg) | 18 (27.7) | 27 (19.7) | 18 (27.7) | 0.203 |
| Cardiac arrest | 4 (2.0) | 0 (0) | 4 (6.2) | 0.017 |
| Ventilation and adverse events | ||||
| Prone ventilation | 67 (33.2) | 55 (40.1) | 12 (18.5) | 0.002 |
| Pneumothorax | 12 (5.9) | 6 (4.4) | 6 (9.2) | 0.296 |
| All-cause mortality within 24 h | 21 (10.4) | 11 (8.0) | 10 (15.4) | 0.110 |
Clinical characteristics and personal protection equipment preparation before tracheal intubation
Patients were predominantly males (n=136; 67%) and aged 65 yr or more (n=128; 63%). Forty-five (22%) patients had a predicted anatomically difficult airway, and all patients were anticipated to have physiologically difficult airway as a result of severe hypoxaemia.19
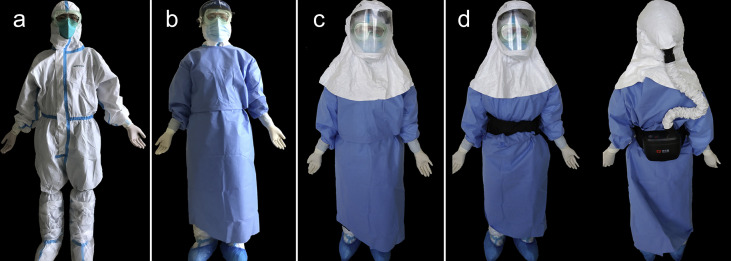
All intubations were undertaken by two trained operators. For personal protective equipment (PPE), all intubating clinicians wore N95 respirators (medical particulate respirator; Winner Medical Co., Shenzhen, Guangdong, China), surgical masks (covering the N95 respirator), eye protection goggles, and a protective coverall with hood and foot covers as inner layer protection (Fig. 1 a). The outer layer of protection comprised a water-resistant full gown and either a face shield (11%; Fig. 1b) or a full hood, either without a powered air-purifying respirator (PAPR) (64%; Fig. 1c) or with a PAPR (25%; Fig. 1d) with double pairs of gloves used in all intubations. Donning and doffing were checked by a nurse, and the two clinicians checked each other. The number of anaesthesiologists involved in the 202 intubations was 36 in Hospital A and 16 in Hospital B.
Fig 1.
Two layers of personal protective equipment. (a) Inner layer. (b) Outer layer with a face field. (c) Outer layer with a hood without a powered air-purifying respirator (PAPR). (d) Outer layer with a hood PAPR.
Before tracheal intubation, most patients showed gross physiological abnormalities, including hypoxaemia, tachypnoea, hypotension, tachycardia, and unconsciousness (Table 2). Supplemental oxygen or ventilation therapy was administered to all patients, most commonly by noninvasive mask ventilation (NIV; 70.8%) (Table 2).
Clinical characteristics during tracheal intubation
Before induction of general anaesthesia, preoxygenation was performed for 5 min in all patients either using a face mask supplying 100% oxygen (47%) or by continuing the previous oxygen therapy (53%). Propofol was used for induction in 194 (96%) cases with rocuronium for neuromuscular block in 200 (99%); other drugs used at induction are shown in Table 2. Mask ventilation after induction and before intubation was undertaken in 93% of intubations. Laryngoscopy was performed with either a UESCOPE® videolaryngoscope (TD-C model with a disposable sheath; UE Medical Devices, Inc., Taizhou, Zhejiang, China) (89.6%) or a standard Macintosh direct laryngoscope, Zhejiang Sujia Medical Device Co., Ltd., Jiaxing, Zhejiang, China (10.4%).
Intubation time was generally ≤3 min (92.6%). The first time and overall intubation success rates were 89% and 100%, respectively (Table 2). During intubation, hypoxaemia occurred in 73% and hypotension in 18% (Table 2). There were three (1.5%) cases of unexpected difficult laryngoscopy. Eleven intubators reported vision hampered by fogging of their mask, all from the centre where a full hood without PAPR was used despite routine use of anti-fog treatment.
Clinical characteristics after tracheal intubation
Hypoxaemia, which was often prolonged, occurred in 16% of patients and hypotension in 22% (Table 2). Pneumothorax was identified in 5.9% of patients. There were four cardiac arrests during intubation only at Hospital B; all four patients were successfully resuscitated. Prone position ventilation was used in 40% of patients within 24 h after tracheal intubation. All-cause mortality within 24 h after tracheal intubation was 10.4%.
Overall clinical features and outcome
This study summarises patient, physiological, and outcome data around the time of tracheal intubation in 202 COVID-19 patients. The investigating authors and a group of international experts identified the problems encountered and their possible causes, and made recommendations for prevention. Previous publications have made recommendations regarding airway management in COVID-19 patients.20, 21, 22, 23 This study bases management recommendations for COVID-19 patients on relevant clinical data.
The high rate of first-pass and overall intubation success in a group of patients who are likely to present both physiological and logistical difficulties is notable. Intubation occurred promptly in all cases. There was worsening of already-deranged physiology with four cases of cardiac arrest, all successfully resuscitated. Pneumothorax after intubation and early mortality were notable major adverse outcomes. There was no evidence of disease transmission to intubating medical personnel.
Personnel for tracheal intubation
All personnel for tracheal intubations were anaesthesiologists. It is likely that the high rates of success and speed reflect clinician experience. Tracheal intubation has been reported in 12 COVID-19 patients by pulmonologists in another hospital in China.24 We suggest that the intubation team should consist of at least two personnel to minimise risks of healthcare worker infection.11 , 12 A third person may stand by as an additional assistant if needed. The most skilled airway manager should perform tracheal intubation with a second operator assisting. The airway plan, including backup techniques, should be agreed upon before starting the procedure. Where tracheal intubation is undertaken by a non-anaesthesiologist, these individuals should be previously well trained before attempting airway management in a COVID-19 patient, and whenever feasible, an anaesthesiologist or ear, nose, and throat surgeon should be immediately available to assist in the event of unexpected difficulty in airway management.12
PPE preparation and outcome
Powered air-purifying respirators were the PPE of choice in both hospitals. However, availability may be limited to some hospitals during a worldwide pandemic,25 and no PAPR was available in 137 cases from Hospital A. When face shields or full hoods without PAPR were substituted, there were no instances of infection of operators. To estimate the confidence interval of the transmission rate from these ‘zero numerator’ data, we used the ‘rule of three’ statistical method.26 With no events in a series of 202 cases, the upper 95% confidence limit of the transmission rate is unlikely to be >1.5%. A larger series is necessary to give greater confidence. Recent narrative publications are also reassuring that, with similar PPE to that described here, the risk of disease transmission to healthcare workers is very low.14 , 15 There remains uncertainty and variable practice regarding PPE globally, and some recommend lower levels of PPE (e.g. either face shield or eye goggles rather than both).27, 28, 29 Outcome data, or the association between level of PPE and coronavirus transmission from the current epidemic, are lacking and require further investigation. During the SARS epidemic, besides non-compliance with appropriate precautions and lack of trained and monitored practices in the use of PPE, the recommended practices themselves were considered to have contributed to healthcare worker infection. As it had become so complicated, errors were likely unavoidable opportunities for transmission through contamination during donning or doffing of PPE.30
There is uncertainty whether an N95/FFP3 respirator should be worn if a PAPR is used. Intubators from the two hospitals in this study chose to wear N95/FFP3 to protect them from self-contamination during the doffing of PPE. The PPE may have had an impact on the logistical ease of intubation, despite using anti-fogging measures: 80% of operators from Hospital A complained of fogging of their eye goggles when using a full hood without PAPR, which impaired technical efficiency during tracheal intubation. Measures to prevent fogging in eye goggles (e.g. liquid soap and iodophor) should be used to prevent interference with vision during airway management if PAPR devices are unavailable.
Because of the high risk of disease transmission during tracheal intubation,11 we suggest that highly protective levels of PPE are worn (Fig. 1). The zero rate of transmission to intubating healthcare workers in our study suggests maximal airborne and droplet precautions are useful in preventing transmission of infection. The risk of virus exposure attributable to self-contamination is high during the removal of PPE. Therefore, educational training for proper donning and doffing of PPE, and monitoring for compliance are crucial.31 Each intubator should receive individualised training and practice on donning and doffing of PPE by an institution-approved instructor until he or she is qualified to use PPE properly. Special attention should be paid to prevention of self-contamination during doffing of PPE. Intubators should be trained in PPE use by instructors and, if conditions permit, simulation before they undertake tracheal intubation in COVID-19 patients.
Induction drugs
Drug choices differed between the two hospitals (Table 2). Propofol was used in almost all patients, often combined with other sedative agents. Considering the high incidence of hypotension during tracheal intubation, propofol may have been overused because of its ease of availability. Midazolam and etomidate were used in only a small portion of patients. Ketamine was unavaialble in either hosptial. A single low dose of etomidate is not considered to impair adrenal or immune function significantly.32 , 33 Midazolam causes less interference with cardiovascular function and has the benefit of a strong amnesic effect. Ketamine, which can stimulate the cardiovascular function through its sympathomimetic effects, was not used because of its low availability in China. Neuromuscular blocking agents were used in all 202 patients.
Propofol use should be minimised if other induction agents with lower risks of hypotension are available. A combination of etomidate (0.2–0.6 mg kg−1) or ketamine (1–2 mg kg−1) with low-dose midazolam is recommended. There should be immediate availability and appropriate use of prophylactic cardiovascular-stimulating agents at the time of tracheal intubation to minimise hypotension. Rocuronium (e.g. 1.2 mg kg−1) is the recommended neuromuscular blocking agent because of its rapid onset of action and favourable side-effect profile compared with succinylcholine. The longer duration of rocuronium reduces the risk of coughing compared with succinylcholine if intubation attempts are prolonged.
Intubation technique
The modified rapid sequence induction (RSI) with mask ventilation before intubation, in combination with videolaryngoscopy, achieved high first-pass and overall intubation success rates. Although not evaluated in comparative trials, a technique based on RSI for tracheal intubation provides the following advantages in patients with COVID-19: (i) minimises the risks of pulmonary aspiration of gastric contents; (ii) enables rapid intubation to optimise oxygenation and ventilation to correct hypoxaemia; and (iii) minimises the duration of healthcare worker exposure to patients, which in turn reduces overall exposure to SARS-CoV-2 virus. Videolaryngoscopy can extend the distance between the operator's head and the patient's mouth.34 Videolaryngoscopy improves the view at laryngoscopy, improves success when intubation is difficult, and facilitates help from the assistant.35 Awake flexible fibreoptic bronchoscopy was not used in this study. Its use should be minimised to reduce healthcare worker exposure to viral aerosolisation.11
Flexible fibreoptic bronchoscopy has been reported in patients with COVID-19 both in 12 awake patents24 and in 58 patients under general anaesthesia.23 During flexible bronchoscopic intubation with general anaesthesia, there was less hypoxaemia when high-flow nasal cannula oxygen (HFNO) was used compared with mask preoxygenation (3.6% vs 26.7%, respectively). The same group has also reported using supraglottic jet oxygenation and ventilation (SJOV) to maintain oxygenation and ventilation during fibreoptic intubation in paralysed non-COVID-19 patients.36 Compared with HFNO, SJOV may provide not only oxygenation, but also efficient ventilation in apnoeic patients.37
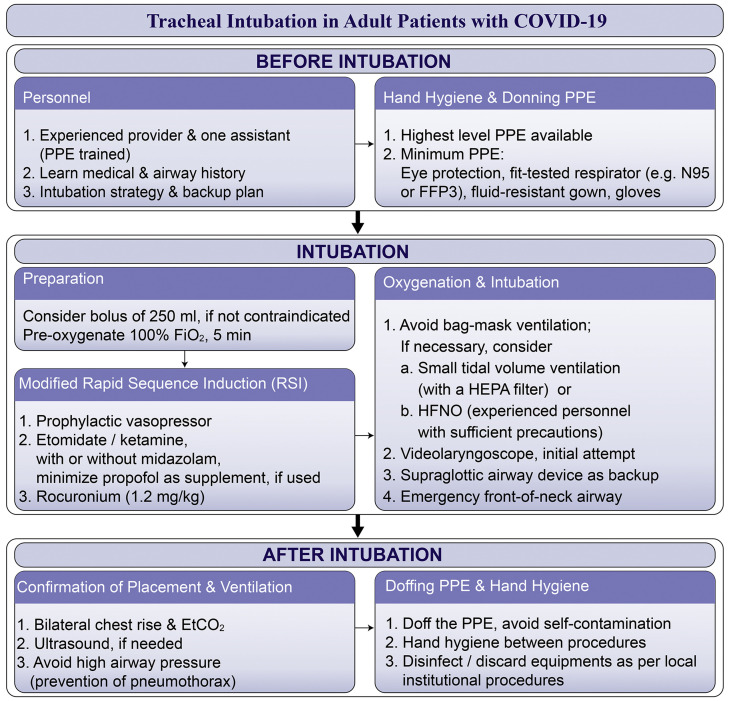
Recommendations: Based on the clinical characteristics and expert experience and opinion, we recommend head-elevated positioning before intubation to optimise intubation conditions. 38 , 39 We recommend videolaryngoscopy over direct laryngoscopy. In case of difficulty, a second-generation supraglottic airway should be available. A difficult airway cart, including emergency front-of-neck airway equipment, should be immediately available. Despite the aforementioned reports, awake fibreoptic bronchoscopy in paralysed patients is not recommended as a primary intubation technique, and should be reserved for patients with a high risk or known difficult airway. A flow chart to assist future practice on tracheal intubation in COVID-19 patients is shown in Fig. 2 .
Fig 2.
Flow chart of recommended tracheal intubation procedure in patients with coronavirus disease 2019 (COVID-19). A suggested strategy based on clinical data for tracheal intubation in 202 patients with COVID-19 from Wuhan, China, and on recommendations from a group of international experts in airway management. Etco2, end-tidal carbon dioxide; Fio2, fraction of inspired oxygen; HEPA, high-efficiency particulate air; HFNO, high-flow nasal oxygen; PPE, personal protective equipment.
Peri-procedural hypoxaemia and its prevention
Most patients were hypoxaemic before tracheal intubation, suggesting a severe intrapulmonary shunt.40 The shortage of available hospital beds during the COVID-19 pandemic may have led to delays in the decision to intubate. Some patients were profoundly hypoxaemic without signs of respiratory distress. This ‘silent hypoxia’41 may be putatively attributed to altered CNS sensation and regulation of responses to hypoxaemia.42 This may also result in delayed recognition of the severity of respiratory failure, and thus delayed tracheal intubation. Undertaking tracheal intubation before the patient is severely hypoxaemic has been recommended to reduce mortality in these patients.11 , 12 However, robust evidence that this approach reduces mortality is lacking.
More than 80% of patients in this study received NIV before tracheal intubation. Although previous studies have suggested the effective use of NIV in SARS-infected patients,43 such practice has been shown to delay tracheal intubation and decrease hospital survival in community-acquired acute pneumonia.44 Further, NIV may increase the intubation rate in patients with COVID-19.45 Based on recent studies in patients with COVID-19, prolonged NIV (>2 h) is not recommended before definitive tracheal intubation and ventilatory support.13 , 14 , 46 High-flow nasal cannula oxygen is used increasingly to treat acute respiratory failure before invasive ventilation,47, 48, 49 and has been used in COVID-19 patients.3 This approach reduces intubation rate in acute respiratory failure.48 , 50 It is still controversial whether HFNO increases virus aerosol spreading. One study using HFNO at 60 L min−1 in patients with bacterial pneumonia did not show an increase in bacterial spread in an ICU setting, which is also supported by a limited systematic review.51 , 52 Overall, HFNO is likely to have a low risk of aerosol generation.
Hypoxaemia worsened after induction of anaesthesia, with 18% of patients developing hypoxaemia during tracheal intubation despite mask ventilation, likely as a result of severe lung injury. After induction of anaesthesia but before intubation, oxygenation can be supplemented by HFNO, SJOV, low-flow nasal oxygen (LFNO; i.e. oxygen flow <5 L min−1), or CPAP. When choosing a technique, the aim should be to maximise oxygenation/ventilation whilst minimising aerosol generation. Most techniques can generate aerosol, and there is a lack of evidence to guide recommendations specific to this setting. In this series, no patients continued HFNO therapy during tracheal intubation. The provision of oxygen during the apnoeic period of intubation attempt(s) is especially important in obese patients and those with a known or predicted difficult airway. Peri-procedural hypoxaemia is a significant risk.53 Most protocols for airway management for patients with COVID-19 now consider HFNO a relative contraindication.20, 21, 22 After intubation, hypoxaemia was readily corrected and persisted in only one in six patients.
Recommendations: Based on the clinical information and expert opinion, we suggest that, where possible, tracheal intubation should be performed earlier in the phase of the illness to avoid undertaking the procedure in the presence of severe hypoxaemia, which may help reduce overall mortality in COVID-19 patients. 11 , 12 Given the lack of evidence regarding the safety of HFNO and LFNO during tracheal intubation, their use should be based on the benefit/risk ratio in individual patients. In the absence of clear evidence, high-level PPE precautions should be used when HFNO is used during intubation.
Hypotension and cardiac arrest during and after tracheal intubation
Hypotension occurred in 18% of patients during and 28% of patients after tracheal intubation. Four patients developed cardiac arrest. These data are consistent with estimates of peri-intubation hypotension incidence reported previously54 , 55 and cardiac arrest of 2–3% in the critically ill, with the latter associated with increased mortality.56 , 57 Predictors of cardiac arrest in the critically ill at the time of tracheal intubation include both hypotension and hypoxaemia before intubation (odds ratio: 3.4 and 4.0, respectively).57 As with hypoxaemia, tracheal intubation earlier in the course of the disease may reduce the risk of cardiovascular collapse. All cases of cardiac arrest occurred in Hospital B. In Hospital A, prophylactic use of cardiovascular-stimulating agents was administered at the time of intubation.
Recommendations: Where possible, tracheal intubation should be performed earlier in the phase of the illness to avoid increased risk of cardiovascular collapse during anaesthesia and intubation. Despite a lack of clear evidence, we recommend consideration of the following measures to minimise hypotension: (i) a 250 ml crystalloid bolus i.v. if not contraindicated (heart failure, kidney failure with volume overload, or similar), (ii) reduction in the use or dose of propofol as an induction agent, and (iii) prophylactic use of cardiovascular-stimulating agents (e.g. phenylephrine, epinephrine, or norepinephrine).
Prevention of pneumothorax after tracheal intubation
Pneumothorax developed after tracheal intubation in 5.9% of patients, which is higher than in previous reports (∼2%).3 The lungs of late-stage COVID-19 patients are severely damaged similar to acute respiratory distress syndrome (ARDS),6 , 7 predisposing to the development of pneumothorax. Ventilatory manoeuvres that generate high airway pressures around the time of intubation (coughing during NIV or CPAP, application of large tidal volumes, and recruitment manoeuvres) may lead to increased risk of pneumothorax. Early prone ventilation is likely to improve lung compliance and has been observed anecdotally to benefit COVID-19 patients, and is recommended in those with severe ARDS.20 , 58 Prone ventilation was used more commonly in Hospital A than in Hospital B, and both pneumothorax rate and mortality were lower in the former. Whether these are related is speculative. A high percentage of patients used NIV before tracheal intubation, which has been associated with a high risk of pneumothorax (up to 15%) in SARS patients.43
Recommendations: Early intubation is expected to reduce the risk of pneumothorax. Noninvasive ventilation before intubation should be used with great caution. Large volume ventilation and recruitment manoeuvres to correct hypoxaemia immediately after tracheal intubation should be avoided. A protective ventilation strategy with small tidal volumes (e.g. 6 ml kg−1 ideal body weight) maintaining lower airway pressures is recommended. Early prone ventilation should be considered, especially where peak pressure or driving pressure is high. Methods to identify or exclude pneumothorax (e.g. chest radiography and point-of-care ultrasound) should be available immediately after tracheal intubation to enable prompt diagnosis.
Mortality for critically ill patients with COVID-19
The 24 h mortality after tracheal intubation was 10.4%. Others have reported 28 day mortality of up to 61% in critically ill patients with COVID-19.34 The 24 h mortality may be related to events at tracheal intubation, but our observational data do not allow further analysis of this. Cardiac arrest at the time of tracheal intubation of the critically ill is associated with a 3.9-fold increase in the risk of 28 day mortality. High rates of mortality in critically ill patients with COVID-19 are predominantly because of the severity and speed of the illness associated with SARS-CoV-2 and the lack of effective antiviral treatment. Limited medical resources during a pandemic when the healthcare system is overloaded likely contribute to delays in tracheal intubation and mechanical ventilation. Provision of sufficient critical care facilities and services to enable timely tracheal intubation and invasive ventilation might logically improve survival, but is unproved and is a major challenge during an epidemic surge. Research should explore whether optimal airway management at the time of intubation in critically ill patients with COVID-19 improves overall outcome.
Clinical data were obtained from only two hospitals and include relatively small patient numbers without comparators or controls. The expert opinion and recommendations were necessarily undertaken in a short time frame. Nevertheless, we believe this article provides valuable information and discussion to meet current and ongoing global needs.
Conclusions
Amongst 202 COVID-19 patients requiring urgent intubation, the majority were males and older. Hypoxaemia was almost universal and hypotension was common. A technique based on RSI and videolaryngoscopy enabled prompt tracheal intubation and was universally successful. Cardiac arrest occurred in 2%, and pneumothorax and early mortality were both observed. Despite differing approaches to PPE, there was no intubation-related healthcare worker COVID-19 infection. Based on the clinical information, analysis, and expert opinion, we provide a flow chart to facilitate tracheal intubation of adult COVID-19 patients (Fig. 2) and to improve safety of both patients and healthcare workers.
Authors' contributions
Data generation/collection: WY, FG, HZ, LX, WM, AL, TW, LW, WX, SY, XC.
Data analysis/interpretation: all authors.
Article conception/writing/final approval: all authors.
Declarations of interest
HW is a consultant of Well Lead Medical Co., Guangzhou, Guangdong, China. EB receives research funding from Fisher & Paykel (Auckland, New Zealand) and is a member of speaker bureau for KARL STORZ Endoscopy America (El Segundo, CA, USA).
Acknowledgements
The authors thank the valuable discussion and suggestion from the following physicians: Chunchun Zhai, Qianqian Qiao, Qingjian Liu, and Zhongyuan Xia (RenMin Hospital of Wuhan University, Wuhan, China); Lize Xiong (Shanghai Fourth People's Hospital, Shanghai, China); Hongfei Zhang (Zhujiang Hospital, Southern Medical University, Guangzhou, China); Yusha Shi (Department of Anesthesiology, Lanzhou University, Gansu, China); Dan Liu (Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China); Yi Feng (Department of Anesthesiology, Peking University People's Hospital, Beijing, China); Yuhang Ai (Xiangya Hospital, Central South University, Changsha, China); and Lingzhong Meng (Yale University School of Medicine, New Haven, CT, USA).
Handling editor: Hugh C Hemmings Jr
Contributor Information
Xiangdong Chen, Email: Xiangdongchen2013@163.com.
Ailin Luo, Email: alluo@tjh.tjmu.edu.cn.
Huafeng Wei, Email: huafeng.wei@pennmedicine.upenn.edu.
collaborators:
Appendix S1.
Collaborators: Zhiyong Peng (Department of Critical Care Medicine, Zhongnan Hospital, Wuhan University, Wuhan, China), Hansheng Liang (Department of Anesthesiology, Peking University People's Hospital, Beijing, China), and Koji Nishikawa (Department of Anesthesiology and Operating Room, General Sagami Kosei Hospital, Kanagawa, Japan).
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) situation report—81. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200413-sitrep-84-covid-19.pdf Available from.
- 2.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. Advance Access Published February 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med Adv. 2020 doi: 10.1016/S2213-2600(20)30079-5. Access Published February 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [Google Scholar]
- 6.Yang W., Cao Q., Qin L. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients with Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2020 doi: 10.1016/j.jtho.2020.02.010. Advance Access Published February 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 doi: 10.1056/NEJMc2004973. Advance Access Published March 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.4756. Advance Access Published March 26. [DOI] [PubMed] [Google Scholar]
- 10.Respiratory Care Committee of Chinese Thoracic Society Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;17:E020. doi: 10.3760/cma.j.issn.1001-0939.2020.0020. [DOI] [PubMed] [Google Scholar]
- 11.Zuo M.Z., Huang Y.G., Ma W.H. Expert recommendations for tracheal intubation in critically ill patients with novel coronavirus disease 2019. Chin Med Sci J. 2020 doi: 10.24920/003724. Advance Access Published February 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020 doi: 10.1007/s12630-020-01591-x. Advance Access Published February 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.XU Kaijin, CAI Hongliu, SHEN Yihong, NI Qin, CHEN Yu, HU Shaohua, LI Jianping, WANG Huafen, YU Liang, HUANG He, QIU Yunqing, WEI Guoqing, FANG Qiang, ZHOU Jianying, SHENG Jifang, LIANG Tingbo, LI Lanjuan. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Journal of Zhejiang University (Medical Sciences) [J] 2020;49(1) doi: 10.3785/j.issn.1008-9292.2020.02.02. 0–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng L., Qiu H., Wan L. Intubation and ventilation amid the COVID-19 outbreak: Wuhan's experience. Anesthesiology. 2020 doi: 10.1097/ALN.0000000000003296. Advance Access Published March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X., Liu Y., Gong Y. Perioperative management of patients infected with the novel coronavirus: recommendation from the joint task force of the Chinese society of anesthesiology and the Chinese association of anesthesiologists. Anesthesiology. 2020 doi: 10.1097/ALN.0000000000003301. Advance Access Published March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallampati S.R., Gatt S.P., Gugino L.D. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–434. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 17.Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 18.Cormack R.S., Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–1111. [PubMed] [Google Scholar]
- 19.Mosier J.M., Joshi R., Hypes C., Pacheco G., Valenzuela T., Sakles J.C. The physiologically difficult airway. West J Emerg Med. 2015;16:1109–1117. doi: 10.5811/westjem.2015.8.27467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alhazzani W., Møller M.H., Arabi Y.M. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. Advance Access Published March 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brewster D.J., Chrimes N.C., Do T.B. Consensus statement: safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020 doi: 10.5694/mja2.50598. https://www.mja.com.au/journal/2020/consensus-statement-safe-airway-society-principles-airway-management-and-tracheal [Preprint, 1 April 2020]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020 doi: 10.1111/anae.15054. Advance Access Published March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C.N., Xia L.Z., Li K.H. High-flow nasal-oxygenation-assisted fibreoptic tracheal intubation in critically ill patients with COVID-19 pneumonia: a prospective randomised controlled trial. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.02.020. Advance Access Published March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai S.J., Wu L.L., Chen D.F. Analysis of bronchoscope-guided tracheal intubation in 12 cases with COVID-19 under the personal protective equipment with positive pressure protective hood. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E033. doi: 10.3760/cma.j.cn112147-20200222-00153. [DOI] [PubMed] [Google Scholar]
- 25.Ji Y., Ma Z., Peppelenbosch M.P., Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8:e480. doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanley J.A., Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA. 1983;249:1743–1745. [PubMed] [Google Scholar]
- 27.European Centre for Disease Prevention and Control . 2020. Infection prevention and control for COVID-19 in healthcare settings.https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-infection-prevention-and-control-healthcare-settings-march-2020.pdf Available from. [Google Scholar]
- 28.Public Health England . 2020. COVID-19: infection prevention and control.https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control Last updated March 13, 2020. Available from. [Google Scholar]
- 29.World Health Organization . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available from. [Google Scholar]
- 30.Nicolle L. SARS safety and science. Can J Anaesth. 2003;50(983–5):985–988. doi: 10.1007/BF03018360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbeek J.H., Rajamaki B., Ijaz S. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2019;7:CD011621. doi: 10.1002/14651858.CD011621.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu W.J., Wang F., Tang L., Liu J.C. Single-dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials and observational studies. Chest. 2015;147:335–346. doi: 10.1378/chest.14-1012. [DOI] [PubMed] [Google Scholar]
- 33.Vinclair M., Broux C., Faure P. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Med. 2008;34:714–719. doi: 10.1007/s00134-007-0970-y. [DOI] [PubMed] [Google Scholar]
- 34.Hall D., Steel A., Heij R., Eley A., Young P. Videolaryngoscopy increases ‘mouth-to-mouth’ distance compared with direct laryngoscopy. Anaesthesia. 2020 doi: 10.1111/anae.15047. Advance Access Published March 27. [DOI] [PubMed] [Google Scholar]
- 35.Lewis S.R., Butler A.R., Parker J., Cook T.M., Smith A.F. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;11:CD011136. doi: 10.1002/14651858.CD011136.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C., Wei J., Cen Q. Supraglottic jet oxygenation and ventilation-assisted fibre-optic bronchoscope intubation in patients with difficult airways. Intern Emerg Med. 2017;12:667–673. doi: 10.1007/s11739-016-1531-6. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S. Supraglottic jet oxygenation and ventilation—a novel ventilation technique. Indian J Anaesth. 2020;64:11–17. doi: 10.4103/ija.IJA_597_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsan S.E.H., Lim S.M., Abidin M.F.Z., Ganesh S., Wang C.Y. Comparison of Macintosh laryngoscopy in bed-up-head-elevated position with GlideScope laryngoscopy: a randomized, controlled, noninferiority trial. Anesth Analg. 2019 doi: 10.1213/ANE.0000000000004349. Advance Access Published on July 23. [DOI] [PubMed] [Google Scholar]
- 39.Khandelwal N., Khorsand S., Mitchell S.H., Joffe A.M. Head-elevated patient positioning decreases complications of emergent tracheal intubation in the ward and intensive care unit. Anesth Analg. 2016;122:1101–1107. doi: 10.1213/ANE.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 40.Ensinger H., Georgieff M. Is infection and septic shock caused by a global oxygen deficiency? An overview in 2 parts. 1: infection and correlation between DO2 and VO2. Anasthesiol Intensivmed Notfallmed Schmerzther. 1996;31:132–142. doi: 10.1055/s-2007-995889. [DOI] [PubMed] [Google Scholar]
- 41.Xie J., Tong Z., Guan X., Du B., Qiu H., Slutsky A.S. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05979-7. Advance Access Published March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. Advance Access Published February 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yam L.Y., Chen R.C., Zhong N.S. SARS: ventilatory and intensive care. Respirology. 2003;8:S31–S35. doi: 10.1046/j.1440-1843.2003.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrillo A., Gonzalez-Diaz G., Ferrer M. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38:458–466. doi: 10.1007/s00134-012-2475-6. [DOI] [PubMed] [Google Scholar]
- 45.Arabi Y.M., Fowler R., Hayden F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46:315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Namendys-Silva S.A. Respiratory support for patients with COVID-19 infection. Lancet Respir Med. 2020;8:e18. doi: 10.1016/S2213-2600(20)30110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koga Y., Kaneda K., Fujii N. Comparison of high-flow nasal cannula oxygen therapy and non-invasive ventilation as first-line therapy in respiratory failure: a multicenter retrospective study. Acute Med Surg. 2020;7:e461. doi: 10.1002/ams2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy S.D., Alladina J.W., Hibbert K.A., Harris R.S., Bajwa E.K., Hess D.R. High-flow oxygen therapy and other inhaled therapies in intensive care units. Lancet. 2016;387:1867–1878. doi: 10.1016/S0140-6736(16)30245-8. [DOI] [PubMed] [Google Scholar]
- 49.Weingart S.D., Levitan R.M. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59:165–175.e1. doi: 10.1016/j.annemergmed.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Huang H.B., Peng J.M., Weng L., Liu G.Y., Du B. High-flow oxygen therapy in immunocompromised patients with acute respiratory failure: a review and meta-analysis. J Crit Care. 2018;43:300–305. doi: 10.1016/j.jcrc.2017.09.176. [DOI] [PubMed] [Google Scholar]
- 51.Leung C.C.H., Joynt G.M., Gomersall C.D. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101:84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel A., Nouraei S.A. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70:323–329. doi: 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smischney N.J., Demirci O., Diedrich D.A. Incidence of and risk factors for post-intubation hypotension in the critically ill. Med Sci Monit. 2016;22:346–355. doi: 10.12659/MSM.895919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smischney N.J., Seisa M.O., Heise K.J. Predictors of hemodynamic derangement during intubation in the critically ill: a nested case-control study of hemodynamic management—part II. J Crit Care. 2018;44:179–184. doi: 10.1016/j.jcrc.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Nolan J.P., Kelly F.E. Airway challenges in critical care. Anaesthesia. 2011;66:81–92. doi: 10.1111/j.1365-2044.2011.06937.x. [DOI] [PubMed] [Google Scholar]
- 57.De Jong A., Rolle A., Molinari N. Cardiac arrest and mortality related to intubation procedure in critically ill adult patients: a multicenter cohort study. Crit Care Med. 2018;46:532–539. doi: 10.1097/CCM.0000000000002925. [DOI] [PubMed] [Google Scholar]
- 58.Bajwa A.A., Arasi L., Canabal J.M., Kramer D.J. Automated prone positioning and axial rotation in critically ill, nontrauma patients with acute respiratory distress syndrome (ARDS) J Intensive Care Med. 2010;25:121–125. doi: 10.1177/0885066609356050. [DOI] [PubMed] [Google Scholar]