Abstract
Washington was the first US state to have a patient test positive for COVID-19. Before this, our children’s hospital proactively implemented an incident command structure that allowed for collaborative creation of safety measures, policies, and procedures for patients, families, staff, and providers. Although the treatment and protective standards are continuously evolving, this commentary shares our thoughts on how an institution, and specifically, surgical services, may develop collaborative process improvement to accommodate for rapid and ongoing change. Specific changes outlined include early establishment of incident command; personal protective equipment conservation; workforce safety; surgical and ambulatory patient triage; and optimization of trainee education. Please note that the contents of this manuscript are shared in the interest of providing collaborative information and are under continuous development as our regional situation changes. We recognize the limitations of this commentary and do not suggest that our approaches represent validated best practices.
Regional Context of Coronavirus-2019 Exposure
The Coronavirus Disease 2019 (COVID-19) pandemic has been recognized by the World Health Organization as an international public health emergency.1 COVID-19 is an acute respiratory disease caused by the β-coronavirus SARS-CoV-2, or 2019 Novel Coronavirus. COVID-19 is thought to spread primarily through the respiratory tract by droplets, secretions, and direct contact.2 In addition, there is evidence suggesting that COVID-19 may be spread via aerosols and fecal-oral transmission.3 There is uncertainty if healthcare workers are at higher risk of exposure to 2019 Novel Coronavirus during procedures involving the upper airway or gastrointestinal tract.4
The first case of COVID-19 in North America was announced on January 21, 2020 in the State of Washington. The first death in North America was also in the State of Washington on February 29, 2020. As a countermeasure, the first Washington State school district closed on March 4, and all school districts closed on March 11. The World Health Organization declared COVID-19 a pandemic on March 11, 2020. Currently, there are more than 4,800 positive cases for COVID-19, with 195 deaths in Washington State (Table 1 ).
Table 1.
Washington State Novel Coronavirus Exposures and Deaths as of April 7, 2020, 3:30 PM (Source: Washington State Department of Health)
| Testing | Individuals tested, n | % of tests | % of cases | % death |
|---|---|---|---|---|
| Result | ||||
| Negative | 87,818 | 91 | – | – |
| Positive | 8,682 | 9 | – | – |
| Age group | ||||
| <19 y | – | – | 3 | 0 |
| 20–39 y | – | – | 27 | 1 |
| 40–59 y | – | – | 35 | 7 |
| 60–79 y | – | – | 25 | 39 |
| ≥80 y | – | – | 10 | 53 |
Children account for between 1% and 2% of the population testing positive for the virus.5 It has been reported that children tend to be asymptomatic or have mild symptoms.6 It is also suspected that children may be more predisposed to spread of the virus through fecal oral means. Information learned from China, as well as our experience in Washington State, has helped to shape our policies.
Setting
Seattle Children’s Hospital is a licensed 407-bed, free-standing children’s hospital established in 1908 with the mission of providing hope, care, and cure to help every child live the healthiest and most fulfilling life possible, regardless of their ability to pay. The hospital serves as a quaternary referral center for the states of Alaska, Idaho, Montana, Washington, and Wyoming. The referral population for these 4 states is approximately 11 million people, with a pediatric population (<18 years) of 2.6 million. The hospital has 1 inpatient site of care and 47 regional care sites, with a workforce of more than 10,000 employees.
Lessons Learned
During the development of our policies and guidelines, we have closely followed the Centers for Disease Control (CDC) recommendations. We also acknowledge that the information shared in this manuscript is in the interest of providing collaborative information to assist other health systems develop their response plan. The material presented is continuously subject to change based on the information received and assessment of resource availability, specifically, personal protective equipment (PPE), hospital beds, intensive care beds, mechanical ventilators, and a healthy workforce, and is not intended to suggest a best practice.
We want to highlight key lessons learned so far in our journey to support our patients and community in managing this crisis. We would like to highlight the following areas:
-
1)
Incident command structure
-
2)
PPE conservation
-
3)
Workforce safety
-
4)
Surgical and ambulatory care triage
-
5)
Resident and fellow education
Incident command structure
Our incident command team was initiated on January 22, 2020. Incident command systems are well described and exist in many health systems. The incident command structure enables the institution to have a clear escalation path, decision-making structure, project management, and communication. Our incident command structure was organized into the following categories: clinical operations, logistics (facilities, supply chain, etc), planning, finance, patient safety, and communication. A section chief who is a rotating member of the executive or clinical leadership team leads each of these areas. A routine huddle structure is followed by each area in which standard work and reporting of issues are created. All processes, policies, and standard work are well documented, and cascading communications occur daily to the frontline staff and providers.
For the institutional clinical operations, representatives from each site of care or clinical area are present to discuss issues related to the COVID-19 response. In addition, local sites of care or clinical areas will have regular operational huddles, where work is prioritized and new issues identified and either resolved or escalated to the institutional clinical operation huddle. For instance, the surgical team has daily operational huddles to discuss how decisions made by the institutional clinical operations team are to be implemented in their area. Clear and regular cascading communication and documentation are necessary for a successful incident command structure. In institutions that may not have an existing incident command structure, we recommend establishment of clear and regular communication lines between front line workers, area leaders, and hospital leadership.
Personal protective equipment conservation
Given concerns for dwindling PPE supply, the majority of strict isolation work is performed with reusable gowns and a controlled air-purifying respirator (CAPR). The CAPR consists of an over-the-head helmet with single-person, reusable, ventilated face shield (reuse can occur after wiping with an antiseptic cloth). We have developed a guideline for judicious PPE use (Fig. 1 ). Before the COVID-19 outbreak, our institution had made a decision to add CAPRs to our armamentarium for respiratory protection of healthcare workers. This has allowed us to be in a position to balance types of PPE to be worn by our staff based on availability of supplies. Preferential use of CAPR devices for inpatient and surgical care of strict isolation patients may reduce the use of disposable N-95 masks.
Figure 1.
Personal protective equipment algorithm for procedures.
For intraoperative cases, a CAPR system with shroud is used unless a surgeon requires loupes, headlight, or microscope, in which case an N-95 mask would be used. The shroud allows air to be vented through the gown below the level of the surgical field. The CAPR system may also have some exposure advantage through slightly higher filtration specifications compared with an N-95. For institutions without CAPR availability, N-95 masks along with eye shields are another option.
Workforce safety
Surgical teams have reduced their in-hospital presence by 50% to 80% by re-engineering deployment schedules to minimize time in the hospital. All meetings are conducted via virtual platforms. We are now enforcing all essential workforce with any COVID-19 symptoms (eg anosmia, cough, fever, malaise, myalgia, or shortness of breath) to have a negative test for COVID-19 and be symptom-free for 72 hours before being allowed within the hospital. Access to COVID-19 testing was limited initially until it was allowed to take place outside the public health setting. We were fortunate in that the University of Washington Medical Center was able to operationalize their testing platform relatively quickly. Through a strong collaborative relationship between our institutions, Seattle Children’s Hospital followed soon after with a platform and we expect access to commercial platforms in the near future.
Our COVID-19 polymerase chain reaction (PCR) test uses 2 probes/targets. Given the absence of a gold standard at this time, it is difficult to know the true diagnostic sensitivity and specificity of the assay in a clinical setting. However, when assessed using laboratory standards, the assay is at least 95% sensitive and 95% specific. Access to COVID-19 testing is currently adequate for symptomatic patients and workforce. We have initiated an off-site, drive-through, swab testing option with results available in less than 24 hours. That system is accessible to patients as part of our surgical screening process described below.
Coughing can generate large droplets that fall out of the air within 3 to 6 feet of the patient. Staff may be protected from having these airborne droplets contact their mucous membranes by wearing a mask and some form of eye protection (eg face shield or goggles). These droplets can also settle on clothes and surfaces, so cleaning and regular handwashing are also essential to preventing spread by this mechanism.
Aerosol-generating procedures (AGP) are another potential mode of COVID-19 spread. Aerosol particles are smaller and can remain airborne longer and travel further than droplets. Aerosols may be inhaled by staff at a distance beyond 6 feet from the site of production and may bypass filtration capabilities of regular masks. In an effort to further protect our surgical team, guidance about what constitutes an AGP was created (appendix). In institutions where COVID-19 testing is unavailable or COVID status is unknown, we would recommend treating all high-risk AGP procedures with full PPE precautions.
Surgical case triage
All nonurgent elective cases were cancelled starting March 12, 2020. Our ambulatory surgery center was closed at the same time. Urgency of subsequent cases are being stratified into 3 categories: Green: elective, may be postponed >6 weeks; Yellow: elective, but must be done in <6 weeks; and Red: Must be done in <1 week.
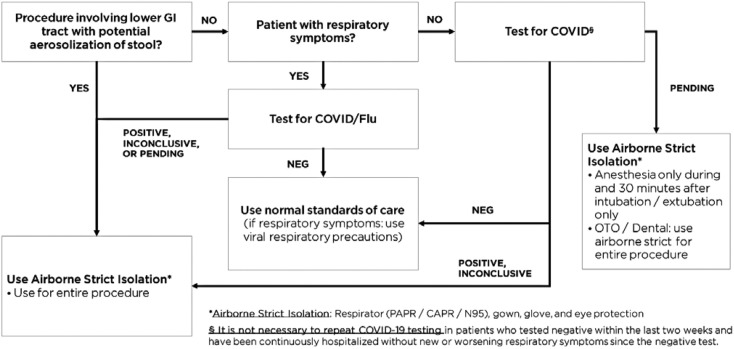
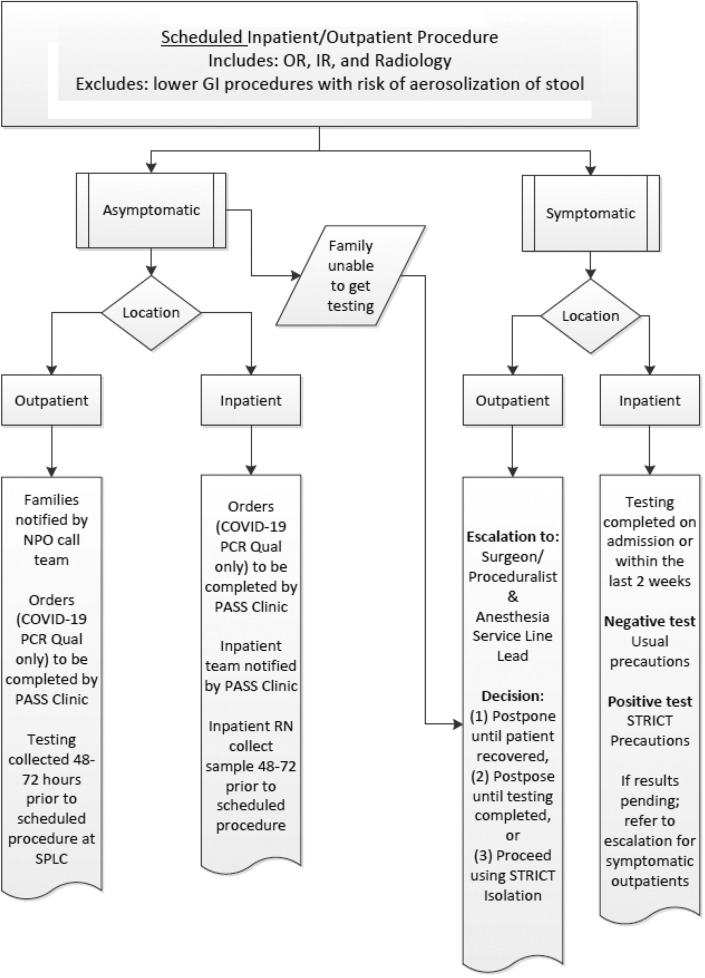
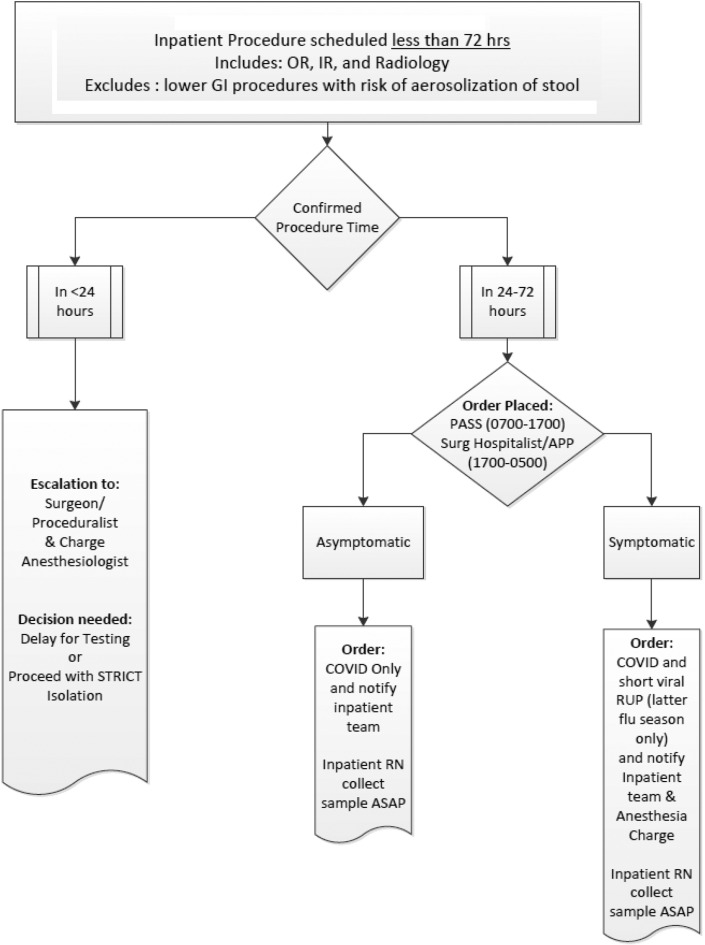
Given the uncertainty of COVID-19 transmission from asymptomatic children and potential provider exposure, several surgical, procedural, and anesthesia professional societies recommended that patients with unknown COVID-19 status undergo their procedure in airborne isolation. As a response, all nonemergent outpatient or inpatient cases are currently being screened preoperatively for COVID-19 (Figs. 2 and 3 ). In institutions where COVID testing may not be readily available, we recommend treating patients with unknown status with full PPE precautions including a respirator (N-95 mask or CAPR) and eye protection, particularly for high-risk AGP procedures.
Figure 2.
Pre-procedure COVID-19 testing guidelines.
Figure 3.
Pre-Procedure COVID-19 testing <72 hours.
Ambulatory case triage
Patients are individually screened by providers and categorized into 3 tiers: Tier 1: must be seen in person, clinical issue is urgent, and physical exam is essential; Tier 2: appropriate for a telephone or telemedicine visit; and Tier 3: visit should be rescheduled. Providers screen all outpatient visits ahead of time. Clinic nurses, medical assistants, and our call center contact families to direct their outpatient care based on stratification.
Resident and fellow education
Given the reduction in clinical volume and workforce deployment, resident and fellow exposure to cases has been reduced. There is some educational opportunity by being part of a healthcare team during a crisis, but additional educational opportunities are being actively sought by each surgical service. Countermeasures include increasing didactic lectures, increasing the frequency of telerounding on inpatients, increasing simulation exposure, and participating in national webinars.
Conclusions
COVID-19 is having a major impact on all aspects of healthcare delivery worldwide. This has created unique challenges for children’s hospitals regarding patient and provider safety, and their role in containing spread of COVID-19 through the community. We are fortunate to have more readily available screening for COVID-19, which now allows us to adequately isolate patients who enter our hospital and have front-line staff wear appropriate PPE, based on test results. The ability to pre-screen almost all patients coming to the operating room has allowed us to conserve precious PPE, especially N-95 masks, during aerosol-generating procedures. Our incident command center, along with consistent regular communication with surgical divisions, has led to rapid process improvement to optimize safety and resource use in the care of surgical patients.
Footnotes
Disclosure Information: Nothing to disclose.
Author Contributions
Study conception and design: Parikh, Avansino, Dick, Enriquez, Geiduschek, Martin, McDonald, Yandow, Zerr, Ojemann
Drafting of manuscript: Parikh, Avansino, Dick, Enriquez, Geiduschek, Martin, McDonald, Yandow, Zerr, Ojemann
Critical revision: Parikh, Avansino, Dick, Enriquez, Geiduschek, Martin, McDonald, Yandow, Zerr, Ojemann
Appendix. Aerosol-Generating Procedure (AGP) Guidelines
CDC guidance regarding what constitutes an AGP includes the following:
-
•
Open airway suctioning
-
•
Collection of respiratory specimens (such as oropharyngeal swab) except nasal swab
-
•
Resuscitation
-
•
During endotracheal intubation
-
•
Bronchoscopy/flexible laryngoscopy/tracheoscopy
-
•
Oscillating ventilation
Other procedures with evidence of aerosol generation:
-
•
Dental procedures with a drill
-
•
Lower gastrointestinal endoscopy, intussusception reductions (gastrointestinal procedures that lead to aerosol generation of stool)
Evidence is limited, but the following may be aerosol generating:
-
•
Ongoing severe coughing
-
•
Use of laryngeal mask anesthesia
-
•
Noninvasive ventilation (bilevel positive airway pressure, continuous positive airway pressure) positive pressure with mask
-
•
Extubation
-
•
Oscillatory positive expiratory pressure (OPEP) / handheld airway clearance
-
•
Cough assist
-
•
High flow nasal cannula oxygen >2 L/kg
-
•
Use of nebulized medications
-
•
Placement of nasogastric/nasoduodenal/nasojejunal tubes
-
•
Mastoid drilling
The following are not likely to be aerosol generating based on our current understanding:
-
•
Use of in-line suctioning with a ventilator
-
•
Ventilator disconnections with dry circuits and passive humidification
-
•
Spontaneous breathing
-
•
Nitrous mask (no positive pressure ventilation)
There are no data available regarding whether the following are aerosol generating:
-
•
Use of uncuffed endotracheal tubes
-
•
Use of metered dose inhaler medications
-
•
We encourage staff to use their best judgment in determining when to use CAPRs for possible aerosol-generating procedures for our patients in strict viral isolation at this time.
References
- 1.World Health Organization Novel Coronavirus (2019-nCoV), Situation Report – 11. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200131-sitrep-11-ncov.pdf?sfvrsn=de7c0f7_4 Available at:
- 2.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 Mar 3 doi: 10.1053/j.gastro.2020.02.055. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu D., Wang H., Yu R., Zhao Y. Integrated infection control strategy to minimize nosocomial infection of corona virus disease 2019 among ENT healthcare workers. J Hosp Infect. 2020 Feb 27 doi: 10.1016/j.jhin.2020.02.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020 Feb 24 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Dong Y., Mo H., Hu Y. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020 Mar [Epub ahead of print] [Google Scholar]