There are an enormous number of unknowns in the management of individuals with cancer who may be at risk, or may in fact be infected with SARS-CoV-2. In such a setting, treatment decisions need to be made on a case-by-case basis and patient stratification is needed considering the prevailing situation. Our evidence to date, which rely on our anecdotal experiences, suggests that the vast majority of patients with cancer are concerned about contracting COVID-19 and ask both us and our colleagues for advice. This is occurring at a time when hospital attention is understandably diverted away from planned, elective care, and such individuals are increasingly scared to come to hospitals, regarded understandably as infection epicentres.
Liang et al. [1] reported patients with cancer have a higher likelihood of being infected, but our view is that these data are insufficient to conclude that patients with cancer have a higher risk, the reported sample size being too small and heterogenous to draw such conclusions. Thus far, the majority of confirmed COVID-19 cases are mild and the limited evidence from China and elsewhere suggests that there are no particular steps that people with cancer should take to protect themselves although they are clearly at risk, often being older. Although there are specific issues, for example, the radiologic manifestations of COVID-19 pneumonia are similar in some cases to pneumonitis caused by checkpoint inhibitors [2], the main concern we have is that once infected, patients with cancer may be at higher risk for the more severe form of COVID-19 requiring intensive care treatment [1]. Thus, for those infected, it seems reasonable to suggest that regular surveillance including monitoring oxygen saturations should be provided and perhaps if an infection occurs during chemotherapy-induced neutropenia, hospital admission would seem appropriate. Whether patients who have confirmed COVID-19 infection should stop their anti-cancer therapy or not remains debated; one reported patient with lung cancer diagnosed with COVID-19 continued targeted therapy during the course of virus infection [3]. Intriguingly, patients with cancer co-infected with HIV-1 and hepatitis B do not have viral re-activation during chemotherapy [4], suggesting here that treatment does not need to stop, although of course, data may be different for different viruses and symptoms of COVID-19 may not correlate with SARS-CoV-2 levels.
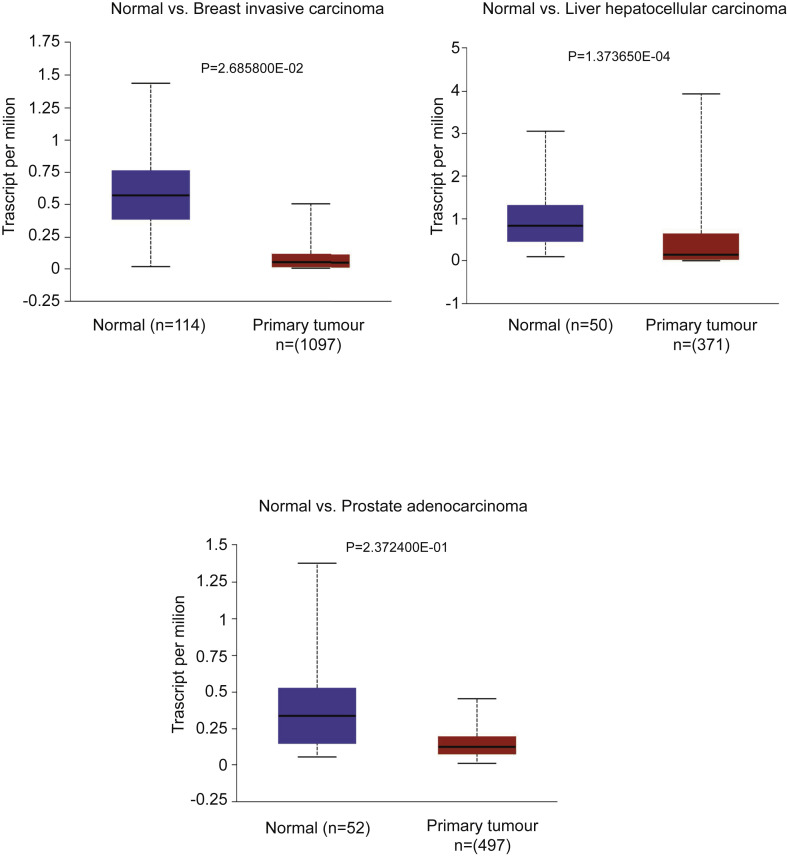
Starting with its biology, its cellular entry receptor, angiotensin-converting enzyme 2 (ACE2) [5] may be over-expressed on some cancers including cervical, pancreatic and renal carcinomas based on one study [6]. By contrast, our analysis of data from TCGA (Fig. 1 ) indicates expression of ACE2 to be significantly decreased in breast, liver and prostate cancer compared with normal adjacent tissues.
Fig. 1.
ACE2 expressions on different cancers were analysed using 3 TCGA data sets with the ULCAN database. The blue box indicates that ACE2 expression is significantly higher in normal tissue, i.e. adjacent tissue compared with breast, liver and prostate cancer tissue. ACE2, angiotensin-converting enzyme 2. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The likely impact of the underlying cancer varies enormously – from an early breast cancer to metastatic lung cancer. Many adjuvant patients benefit a great deal more than 5%, as much as 30% in absolute terms in patients with breast cancer at high risk, for example, but there are no data on who to treat or not during the pandemic. In terms of risk, there is no separate hazard ratio for use of chemotherapy as this will be treatment (drug, dose density and frequency), host (age, perhaps sex too), intent (palliative versus curative) and tumour (stage, type) dependent; the only direct report is three-fourth of patients receiving chemotherapy needed intensive care or died (but only a sample of 4) [7]. Immunotherapy has clearly different risks, as does underlying co-morbidities, notably hypertension or any pulmonary disease. The additional effects due to bed capacity, for example, giving chemotherapy when there is no intensive care availability, are challenging.
It appears that the host response observed during infection that probably mediates much of its pathogenesis [8] analogous to cytokine storms during CAR-T therapy. In patients with cancer infected with SARS-CoV-2, inhibiting excessive immune cell activation and cytokine production is probably central, although use of corticosteroids is controversial [9,10]. It is notable to us that one of the best prospects for treating the virus modulates the host immune response and is useful too in treating manifestations of the rare cancer, multicentric Castleman's disease, as well as its licenced rheumatoid arthritis indication [11]; targeting the IL-6 pathway using tocilizumab has led to inclusion in China's latest version of diagnosis and treatment guidelines on COVID-19 [12].
Because anti–programmed cell death 1 (PD-1) therapy has been implicated as useful in treatment of chronic infections [13], a Chinese manufactured antibody, camrelizumab, is being investigated in patients without cancer in China infected with COVID-19 (ChiCTR200002806). However, whether the possibility of PD-1 inhibitor–related pneumonia and potential risk of cytokine-release syndrome would aggravate underlying infections remain unknown [14], as does the interplay here of chemotherapy-induced neutropenia. An artificial intelligence (AI)–derived knowledge graph indicated that the JAK1 inhibitor baricitinib may help in preventing viral entry via inhibition of clathrin-mediated endocytosis [15], as well as inhibiting downstream cytokines [16]; it is notable that those data revealed a number of tyrosine kinase inhibitors as being potentially useful too, but the authors immediately considered them too toxic.
The identification of effective interventions for patients with cancer infected with COVID-19 remains a major challenge. Given the available knowledge of possible mechanisms, clinical trials of drugs are still warranted and individuals with cancer should be studied.
Conflict of interest statement
J.S.’ conflicts of interest can be found at https://www.nature.com/onc/editors. S.Z. and L.P. have nothing to declare.
References
- 1.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis:Feb. 2020;20(4) doi: 10.1016/S1473-3099(20)30086-4. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H., Huang Y., Xie C. The treatment and outcome of a lung cancer patient infected with SARS-CoV-2. J Thorac Oncol. 2020 Mar 5 doi: 10.1016/j.jtho.2020.02.025. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stebbing J., Atkins M., Nelson M. Hepatitis B reactivation during combination chemotherapy for AIDS-related lymphoma is uncommon and does not adversely affect outcome. Blood. 2004;103:2431–2432. doi: 10.1182/blood-2003-12-4222. [DOI] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. Feb 3 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia X., Yin C., Lu S. Two things about COVID-19 might need attention. Preprints. 2020 2020020315. [Google Scholar]
- 7.Yu J., Wen O., Chua M.L.K. SARS-CoV-2 transmission in cancer patients of a tertiary hospital in Wuhan. Medrxhiv. 2020 doi: 10.1101/2020.02.22.20025320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang L., Zhao J., Hu Y. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bower M., Veraitch O., Szydlo R. Cytokine changes during rituximab therapy in HIV-associated multicentric Castleman disease. Blood. 2009;113:4521–4524. doi: 10.1182/blood-2008-12-197053. [DOI] [PubMed] [Google Scholar]
- 12.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Chinavix. 2020;26 doi: 10.1073/pnas.2005615117. 202003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyck L., Mills K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47:765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 14.Rotz S.J., Leino D., Szabo S. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Canc. 2017;64 doi: 10.1002/pbc.26642. [DOI] [PubMed] [Google Scholar]
- 15.Stebbing J., Phelan A., Griffin I. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis:Feb. 2020;27 doi: 10.1016/S1473-3099(20)30132-8. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson P., Griffin I., Tucker C. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]