Summary
In the throes of the current coronavirus disease-2019 (COVID-19) pandemic, interest has burgeoned in the cardiovascular complications of this virulent viral infection. As troponin, a biomarker of cardiac injury, often rises in hospitalized patients, its interpretation and actionability require careful consideration. Fulminant myocarditis due to direct viral infection can certainly occur, but in patients with increased oxygen demands due to tachycardia and fever and reduced oxygen delivery due to hypotension and hypoxemia, COVID-19 disease can cause myocardial injury indirectly. Cytokines released during the acute infection can elicit activation of cells within pre-existing atherosclerotic lesions, augmenting thrombotic risk and risk of ischemic syndromes. Moreover, microvascular activation by cytokines can cause not only myocardial injury but can also harm other organ systems commonly involved in COVID-19 infections including the kidneys. Dealing with the immense challenge of COVID-19, confronted with severely ill patients in dire straits with virtually no rigorous evidence base to guide our therapy, we must call on our clinical skills and judgment. These touchstones can help guide us in selecting patients who might benefit from the advanced imaging and invasive procedures that present enormous logistical challenges in the current context. Lacking a robust evidence base, pathophysiologic reasoning can help guide our choices of therapy for individual clinical scenarios. We must exercise caution and extreme humility, as often plausible interventions fail when tested rigorously. But act today we must, and understanding the multiplicity of mechanisms of myocardial injury in COVID-19 infection will help us meet our mission unsupported by the comfort of strong data.
Key Words: atherosclerosis, cytokines, endothelial cells, inflammation, sepsis, vascular biology
In the throes of the current pandemic, intense interest has burgeoned in cardiovascular involvement by novel coronavirus disease-2019 (COVID-19). Cardiologists as well as other practitioners who care for those with this virulent viral infection, and indeed the general public as well, share curiosity and concern in this regard. The torrent of published reports on this nascent topic contain clear-cut descriptions of fulminant myocarditis in certain individuals (1,2), as ably reviewed in the State-of-the-Art Review paper on cardiac involvement in COVID-19 by Atri et al. (3) in this issue of JACC: Basic to Translational Science. Indeed, the human myocardium can express the receptor that COVID-19 uses to infect host cells, angiotensin-converting enzyme-2, which is the counter-regulatory cousin of the more familiar angiotensin-converting enzyme-1. Thus, no doubt, in some cases, a viral myocarditis due to this agent can occur (Figure 1, far left). Yet, troponin rise seems nearly ubiquitous in patients requiring intensive care, an indication of cardiac involvement in many cases and a marker of poor prognosis as in many other circumstances. But can we, and should we, attribute all rises in troponin to direct myocardial infection by this virus?
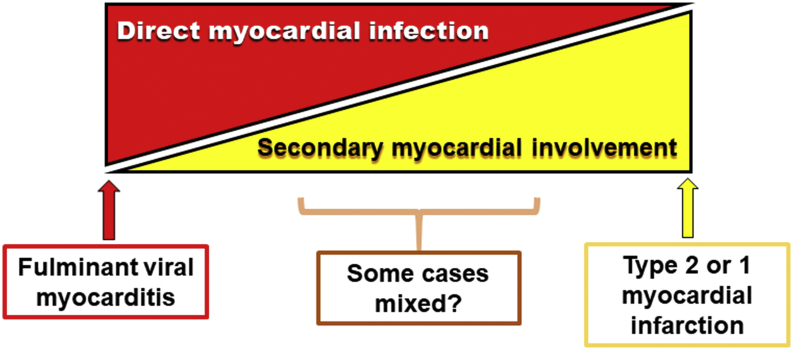
Figure 1.
Hypothetical Spectrum of Myocardial Involvement in COVID-19
This diagram represents the hypothetical spectrum of myocardial involvement in coronavirus disease-2019 (COVID-19). On the extreme left, a case of fulminant myocarditis could occur in an individual with no coronary artery atherosclerosis. On the extreme right, an individual could have an acute coronary syndrome because of severe pre-existing lesions triggered to cause an event due to the consequences of infection described in the text.
To approach this question, we need to distinguish myocarditis due to infection of cardiac cells from myocardial ischemic injury. Flow embarrassment to the heart muscle can result from lesions in epicardial coronary arteries or in the heart’s microvasculature. Cardiac ischemia can also arise from an imbalance between oxygen supply and demand, a type 2 acute coronary syndrome, a situation that can prevail in acute infections, particularly those that affect the lungs like COVID-19 does. Several of these pathophysiologic pathways to myocardial ischemia may affect those without substantial or obstructive coronary artery atherosclerosis. Hence, the distinction between these various mechanisms has important clinical consequences. The need for arduous imaging studies and invasive evaluation may vary considerably in these different scenarios, an issue of great import in acute care facilities stretched to or beyond their limits during a pandemic with a readily contagious and virulent infectious agent such as COVID-19. Considering the pathophysiologic paths to cardiac injury can inform judgment regarding the necessity of transport of severely ill patients and the performance-invasive procedures.
A panel convened by the National Heart, Lung, and Blood Institute in 1997 considered the roles of infectious agents in cardiovascular disease. The summary report of this panel explicitly considered systemic infection and the triggering of acute coronary events, and it reviewed some of the possible mechanisms (4). These considerations included cytokine responses to infection as activators of vascular cells and as inducers of the acute phase response with consequent heightened production of fibrinogen, the precursor of clots, and of endogenous inhibitors of fibrinolysis. More recent panels convened in conjunction with the National Heart, Lung, and Blood Institute re-examined this issue and highlighted the differences between direct infection and secondary responses (5). The COVID-19 pandemic elevates these pathophysiologic considerations from theoretically interesting to a level of vital clinical importance. The paper by Atri et al. (3) deals comprehensively with cardiac involvement. This commentary and the accompanying illustrations place these considerations in the context of inflammation and vascular biology
Infection at Remote Sites Can Elicit “Echoes” in the Pre-existing Atherosclerotic Lesion
The demographic most often affected by life-threatening and fatal COVID-19 has a high prior probability of established atherosclerotic lesions: they are elderly persons, predominantly male, and have pre-existing lung disease, including that associated with cigarette smoking, a risk factor for atherosclerosis. Remote infections such as the severe pneumonitis that too commonly complicates COVID-19 can elicit an acute exacerbation of the chronic smoldering inflammation that characterizes coronary atherosclerotic lesions (Figure 2). The inflammatory cells at a site of regional infection such as the lungs in COVID-19 pneumonitis can produce cytokines such as interleukin-1 and -6 and tumor necrosis factor, mediators that not only propagate local inflammation but can enter the systemic circulation. Such circulating cytokines can stimulate macrophages within the plaque to augment local cytokine production and provoke an increase in tissue factor expression that renders lesions more thrombogenic. We have referred to this local response to systemic stimuli as an “echo” phenomenon (4,6). These same systemic cytokines can stimulate leukocyte adhesion molecule expression on the endothelial cells overlying established atheroma, boosting local recruitment of these inflammatory cells. These alterations in pre-existing plaques can enhance their propensity to disrupt, be it by fibrous cap fissure or by superficial erosion, and provoke an acute coronary syndrome.
Figure 2.
Inflammatory Infectious Processes Can Produce Systemic Effects That Can Promote Atherothrombotic Events and Myocardial Ischemia
This diagram depicts how inflammatory infectious processes in remote locations including the lungs can produce systemic effects that can promote atherothrombotic events and myocardial ischemia and also evoke “echoes” within the plaque itself that can predispose toward plaque disruption or acute progression of the disease.
Reproduced with permission from Libby et al. (5).
Infection at Remote Sites Can Also Activate the Coronary Microvasculature
Even in individuals without pre-existing epicardial coronary artery disease, systemic cytokines released from sites of local infections such as in pneumonitis can affect intermural coronary vessels. They can also activate the microvascular endothelium, predisposing to vasomotor abnormalities, augmented thrombosis, reduced fibrinolysis, increased leukocyte adhesion, and other aspects of dysfunction of the microvessels of the coronary circulation. These effects wrought by distant infection can contribute to myocardial ischemia, even in the absence of epicardial atherosclerosis, and could compound cardiac injury in those with flow limitation due to plaque in the larger coronary arteries.
Infection and Pneumonia Can Worsen the Balance Between Myocardial Oxygen Supply and Demand
The cardinal signs of infection include fever and tachycardia, circumstances that increase the oxygen requirements of the myocardium (Figure 3). Hypoxemia produced by pneumonitis can decrease oxygen delivery to the myocardium. Hypotension in sepsis and in cytokine storm can impair coronary perfusion. Together these systemic effects of infection conspire to limit blood flow in the coronary arteries and reduce oxygen supply while augmenting myocardial oxygen demand. These consequences of infection predispose to myocardial ischemia. They may aggravate the peril of plaques that would not limit flow or provoke ischemia under usual conditions and could produce ischemic injury even in those with little or no coronary artery atherosclerosis.
Figure 3.
Physiologic Changes Associated With Acute Infection Affect Adversely Oxygen Supply and Demand
This figure depicts how physiologic changes associated with infection can tip the balance between myocardial oxygen supply and demand to favor myocardial ischemia. See the text for details.
Adapted with permission from Libby et al. (5).
Multiple Mechanisms May Contribute to Cardiac Complications of COVID-19 in Different Degrees
At one end of the spectrum, a young individual with pristine coronary arteries might suffer severe myocardial injury due to a fulminant myocarditis caused by direct infection with COVID-19 (Figure 1, left). At the other extreme, a person with advanced coronary atherosclerosis could suffer a type 1 or type 2 acute myocardial infarction without direct viral infection of cardiac cells (Figure 1, right). Although we are early in our experience with this novel coronavirus disease, most patients affected by COVID-19 encountered by cardiologists may have more secondary cardiac involvement then primary infective myocarditis. Thus, many of our patients may fall into the zone between the bookends depicted in Figure 1.
It behooves us to consider the multiple mechanisms of cardiac injury in patients with COVID-19 (Figure 4). As in the BC (before COVID-19) era, interpretation of rises in cardiac troponin requires consideration of the context of the clinical situation. Not all rises in this biomarker of cardiac injury will result from coronary artery disease requiring invasive assessment or intervention. We urgently need randomized clinical trials to assess the value of interventions including anti-inflammatory therapies ranging from glucocorticoids to cytokine antagonism in addition to antiviral agents as outlined in the elegant exposition of Atri et al. (3).
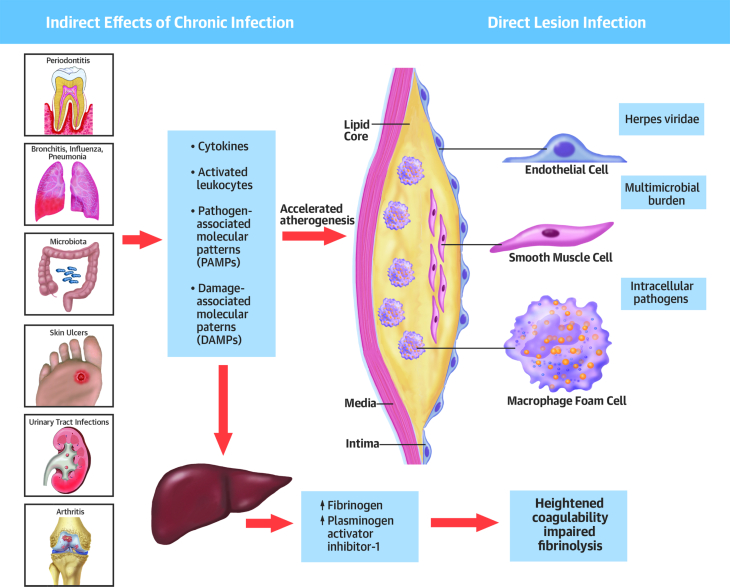
Figure 4.
Infection Has Multiple Effects That May Impinge on the Cardiovascular System and Provoke Events
In addition to the aspects detailed in Figures 1, 2, and 3, infection may predispose toward thrombosis and reduced fibrinolysis as explained in the text. The consequences of infection may also alter the function of macro- or microvascular endothelium and thus contribute to cardiovascular complications of coronavirus disease-2019.
Reproduced with permission from Libby et al. (5).
Dealing with the immense challenge of COVID-19, and confronted with severely ill patients in dire straits with virtually no rigorous evidence base to guide our therapy, we need to call on our clinical skills and judgment. These touchstones can help guide us in selecting patients who might benefit from the advanced imaging and invasive procedures that present enormous logistical challenges in the current context. In the absence of a robust evidence base, we will also need to invoke pathophysiologic reasoning to guide our choices of therapy for each individual clinical scenario. We must do so with caution and extreme humility, recognizing how often plausible interventions fail when tested rigorously. But act today we must, and the overview of Atri et al. (3) and other recent compendia will help us meet our mission unsupported by the comfort of strong data.
Footnotes
Dr. Libby has received funding support from the National Heart, Lung, and Blood Institute (grants R01HL080472 and 1R01HL134892), the American Heart Association (grant 18CSA34080399), and the RRM Charitable Fund; has provided uncompensated consulting services to, or has participated in clinical trials for Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, Pfizer, Sanofi-Regeneron, and XBiotech, Inc.; has served on the Scientific Advisory Boards for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, IFM Therapeutics, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc.; has served on the Board of XBiotech, Inc.; has received research funding in the last 2 years for his laboratory from Novartis; and participates in the Leducq Transatlantic Network on Clonal Hematopoiesis.
The author attests he is in compliance with human studies committees and animal welfare regulations of the author's institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16 doi: 10.1093/eurheartj/ehaa190. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atri D., Siddiqi H.K., Lang J., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: a state-of-the-art review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. J Am Coll Cardiol Basic Trans Science. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P., Egan D., Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095–4103. doi: 10.1161/01.cir.96.11.4095. [DOI] [PubMed] [Google Scholar]
- 5.Libby P., Loscalzo J., Ridker P.M. Inflammation, immunity, and infection in atherothrombosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2018;72:2071–2081. doi: 10.1016/j.jacc.2018.08.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P., Nahrendorf M., Swirski F.K. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease. J Am Coll Cardiol. 2016;67:1091–1103. doi: 10.1016/j.jacc.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]