Objective
To raise awareness for possible benefits of examining known COVID-19 patients presenting sudden clinical worsening with CT pulmonary angiography instead of standard non-contrast chest CT.
Keywords: COVID-19: coronavirus disease 2019, lung infection, pulmonary thromboembolism, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, computed tomography pulmonary angiography
Highlights
-
•
Patients having COVID-19 pneumonia are at risk of coagulopathy and pulmonary embolism
-
•
In the absence of contraindications, patients admitted for COVID-19 should receive prophylactic low molecular weight heparin
-
•
Elevated D-dimer levels on admission or sudden clinical deterioration should raise suspicion of pulmonary embolism
-
•
Selected patients may benefit from CT pulmonary angiography to confirm pulmonary embolism and initiate appropriate therapy
1. Background
Since December 2019, the world is facing a rapidly expanding pandemic of lower respiratory tract infection by a novel coronavirus SARS-CoV-2 (severe respiratory syndrome coronavirus 2). In some patients, this viral infection causes a clinical syndrome referred to as coronavirus disease 2019 (COVID-19), but the heterogeneity of the disease course poses a challenge to healthcare providers and optimal management of patients. The use of CT imaging in the diagnosis and follow-up has rapidly grown, and radiological patterns along the disease course are increasingly understood. While COVID-19-related lung injury shares some radiological findings with other viruses from the coronaroviridae family, some differences emerge already. To date, most, if not all the available literature regarding SARS-CoV-2 infection relies on non-contrast CT, which is considered the first-line imaging tool [1] and has even proven useful to diagnose COVID-19 pneumonia when initial polymerase chain reaction screening is negative [2].
Nevertheless, the exact role of CT imaging in the management of COVID-19 is still being debated, and evidence-based guidance regarding acquisition protocols is lacking. Current guidelines advocate the use of non-contrast chest CT for the diagnosis, severity assessment, and monitoring of COVID-19 [3]. Generally, when CT is indicated, the examination should be carried out with as little harm as possible to the patient; this implies that contrast agent injection should be performed only when needed to prevent possible complications, such as acute renal insufficiency or allergic reactions. Though detection of typical lung imaging features of COVID-19 does not require intravenous contrast agent use, patients with known COVID-19 and sudden onset clinical deterioration with unexplained worsening of dyspnea or chest pain, may benefit from vascular enhancement to be appropriately diagnosed and managed.
2. Case presentation and discussion
While most patients having COVID-19 pneumonia will have a mild disease course, some patients will develop severe respiratory distress, sepsis, and septic shock. Coagulopathy commonly occurs in sepsis and may predict outcomes in severe COVID-19 [4]. Han et al. reported disturbed coagulation function in patients infected with SARS-CoV-2 as compared to healthy controls, including elevated D-dimer, fibrin/fibrinogen degradation products, and fibrinogen levels [5]. Additionally, two different studies by Zhou et al. [6] and Tang et al. [7] recently reported a positive correlation between elevated D-dimer levels on admission and in-hospital COVID-19 mortality, raising questions regarding potentially unknown pulmonary embolism and outlining the possible role of CT pulmonary angiography in patients with COVID-19 and rapid clinical worsening. On the other hand, taking the same patients to the CT suite several times to perform non-contrast and subsequently contrast-enhanced CT may be logistically challenging for radiology departments since time-consuming infection control measures are required.
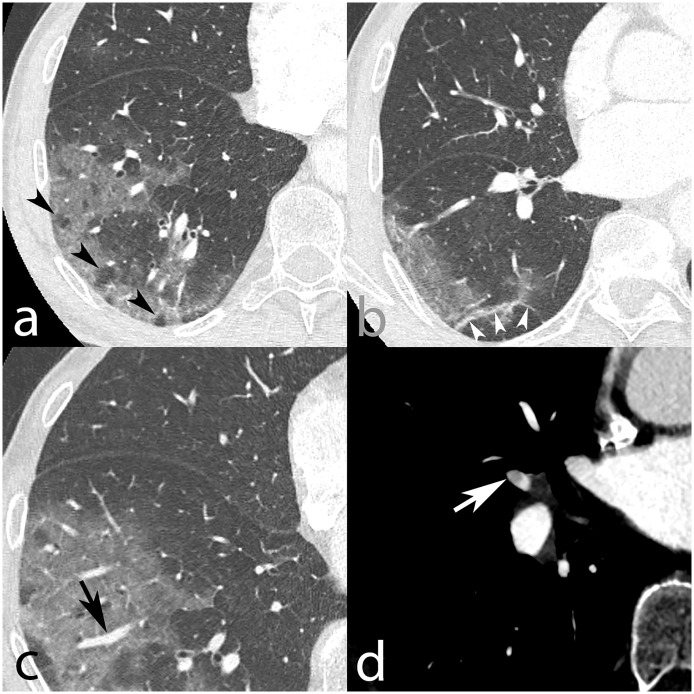
Fig. 1 shows an example of a 75-year-old patient admitted at our institution due to low-grade fever, asthenia, chills, and odynophagia. Upon physical examination, coarse end-inspiratory crackles were noted at the right lung base. Respiratory rate was 16/min, and oxygen saturation 95% on room air. Blood sampling showed leukopenia (3.3 G/L), lymphopenia (0.79 G/L), thrombopenia (129 G/L), and normal hemoglobin levels (157 g/L). Nasopharyngeal sampling was positive for SARS-CoV-2. He suddenly developed dyspnea and tachypnea on day 4 after admission, prompting further investigation with CT pulmonary angiography. The CT signs were those of multifocal ground-glass opacities predominantly located at the periphery of all five lobes, right basal subpleural fibrotic streaks, vacuolar sign, and vascular dilation sign consistent with COVID-19 pneumonia. Still, the CT pulmonary angiography – an established diagnostic tool [8] – also revealed right middle lobar segmental acute pulmonary embolism. The patient received prophylactic low molecular weight heparin (LMWH) from day 3 after admission (Enoxaparin 40 mg once daily by subcutaneous injection). Even though the etiology of acute pulmonary embolism associated with COVID-19 pneumonia remains unclear, adequate treatment can be initiated immediately considering the outcome of untreated pulmonary embolism. The incidence of acute pulmonary embolism in patients diagnosed with COVID-19 is currently unknown; however, the available biological and clinical data raise concerns about unsuspected pulmonary embolism and calls for research on this specific topic. Perhaps the only report on SARS-CoV-2 infection and CT pulmonary angiography that has come out recently is by Chen et al., and clearly shows that patients with COVID-19 are at risk of acute pulmonary embolism [9]. Given the large number of COVID-19 patients seeking medical care, the international society on thrombosis and haemostasis (ISTH) advocates the use of laboratory tests, including D-dimers, prothrombin time, and platelet count to stratify patients at risk of adverse outcome and who need hospital admission [10]. According to the same interim guidance proposed by the ISTH, all in-patients should receive antithrombotic prophylaxis with LMWH, unless there is a contraindication.
Fig. 1.
Axial CT pulmonary angiography in lung window, from a 75-year-old man, who was diagnosed with COVID-19. Images show multifocal predominantly peripheral ground-glass opacities in the right lung base (a–c), with associated vacuolar sign (black arrowheads, a), fibrous streaks (white arrowheads, b), and vascular dilation sign (black arrow, c) suggestive of SARS-CoV-2 infection. In the soft tissue window, a filling defect partially outlined by contrast agent was found in the lateral branch of the right middle lobar artery, indicating acute pulmonary embolism (white arrow). Acute pulmonary embolism was unlikely to be caused by in-situ thrombosis due to interstitial COVID-19 injury since the parenchyma in the right middle lobe was normal (b).
In summary, patients requiring hospital admission for COVID-19 pneumonia should receive prophylactic LMWH to prevent thromboembolism, in the absence of contraindication. Furthermore, CT has quickly become a cornerstone in both the diagnostic workup and follow-up of SARS-CoV-2 infection and is usually performed without intravenous contrast agent injection. Though, patients with known COVID-19 disease may have acute pulmonary embolism. In the case of elevated D-dimer levels on admission or sudden clinical worsening, CT pulmonary angiography should be considered since pulmonary embolism is a life-threatening but potentially treatable condition.
Funding statement
No funding was received for the writing of this letter.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Dr. Rotzinger has nothing to declare.
Dr. Beigelman-Aubry has nothing to declare.
Prof von Garnier has nothing to declare.
Prof Qanadli has nothing to declare.
References
- 1.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;200642 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing [published online ahead of print, 2020 Feb 12] Radiology. 2020:200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. China medical treatment expert group for covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. (Feb28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han H., Yang L., Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. S0140-6736 (20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia [published online ahead of print, 2020 Feb 19] J Thromb Haemost. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qanadli S.D., Hajjam M.E., Mesurolle B. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology. 2000;217(2):447–455. doi: 10.1148/radiology.217.2.r00nv01447. [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Wang X., Zhang S. Findings of acute pulmonary embolism in COVID-19 patients. The Lancet Infectious Diseases. 3/1/2020 doi: 10.2139/ssrn.3548771. https://ssrn.com/abstract=3548771 (preprint. Available at SSRN) [DOI] [Google Scholar]
- 10.Thachil J., Tang N., Satoshi G. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]