Abstract
COVID-19 has now been declared a pandemic. To date, COVID-19 has affected over 2.5 million people worldwide, resulting in over 170,000 reported deaths. Numerous preventative strategies and non-pharmaceutical interventions have been employed to mitigate the spread of disease including careful infection control, the isolation of patients, and social distancing. Management is predominantly focused on the provision of supportive care, with oxygen therapy representing the major treatment intervention. Medical therapy involving corticosteroids and antivirals have also been encouraged as part of critical management schemes. However, there is at present no specific antiviral recommended for the treatment of COVID-19, and no vaccine is currently available. Despite the strategic implementation of these measures, the number of new reported cases continues to rise at a profoundly alarming rate. As new findings emerge, there is an urgent need for up-to-date management guidelines. In response to this call, we review what is currently known regarding the management of COVID-19, and offer an evidence-based review of current practice.
Keywords: SARS-CoV-2, COVID-19, Pandemic, Management guidelines
Highlights
-
•
COVID-19 has recently been declared a pandemic by WHO.
-
•
Increased cases globally have highlighted the need for updated management guidelines.
-
•
Currently, supportive management is the first-line treatment.
-
•
New medical therapies are currently in phase 1 and 2 trials.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), initially named novel coronavirus or 2019-nCoV, is a single-stranded RNA virus which forms one of the seven coronaviridae - 229E, OC43, NL63, HKU1, severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV) [1] - now known to infect humans. It is the virus responsible for causing coronavirus disease 2019 (COVID-19), a type of lower respiratory tract infection with the potential to cause severe and possibly fatal atypical novel coronavirus (2019-nCoV)–infected pneumonia (NCIP) in humans [[2], [3], [4]].
1.1. Prevalence
Now labelled a pandemic [5], COVID-19 has affected over 2.5 million people worldwide, with the majority of cases (n = 793,505) seen in the USA alone, followed by Spain (n = 200,210) and in third place Italy (n = 181,228). There have been over 170,000 reported deaths and at least 663,400 recovered cases [6,7]. Sohrabi et al. highlighted the extent of the outbreak with the World Health Organization (WHO) declaring the COVID-19 outbreak as a global emergency on January 30, 2020 [2].
1.2. Mode of evolution and transmission
To facilitate the characterisation of SARS-CoV-2, comparisons have been made with the better known structure of SARS-CoV. Both viruses share an amino acid sequence similarity of 76.5% [8] and utilise angiotensin-converting enzyme 2 (ACE2) receptors as a mode of entry into healthy cells. Variations between the receptor-binding domain of the two brought about by mutations [9], genetic recombination [10], and natural selection [9] enable SARS-CoV-2 to bind to the receptor more effectively [8]. Furthermore, evidence of genetic recombination as a mechanism of viral evolution has sparked concerns regarding the misdiagnosis of infections by SARS-CoV-2, inaccurate tracking of transmission rates, adaptation of the virus to human immunity, as well as increasing severity of the infection with time [10].
A study looking into the first 425 confirmed cases of NCIP has provided evidence to support that the main method of viral transmission is human-to-human [3]. To date, a confirmed case of NCIP is defined as at least one of the following obtained from the respiratory tract (ie. pharyngeal swabs, sputum samples, and alveolar lavage) or serological samples [1,11]:
-
●
Isolation of SARS-CoV-2
-
●
≥2 positive real time RT-PCR assays of SARS-CoV-2
-
●
Genetic sequence matching of SARS-CoV-2
Recovery and clearance of the virus is thought to be achieved when ≥2 negative oral swabs are confirmed in an infected individual. Emerging evidence, however, has speculated the complete clearance of the virus in such cases as anal swabs and blood cultures may remain positive despite having negative oral swabs [12] and supports that the main modes of spread of the virus include respiratory droplets, bodily fluids, fecal-oral, direct contact, and transmission through environmental surfaces [13]. Current evidence supports that there is no vertical transmission of the virus [14].
Alarmingly, despite extensive efforts by governments to contain the virus, a recent Chinese study has demonstrated that although 80.9% of sufferers had subclinical or mild symptoms of the disease they still possessed the potential to spread the virus further [15]. Interestingly, they also possessed the same viral load to patients who exhibited symptoms of the disease [16]. As it stands, it has been estimated that an infected individual is likely to spread the disease to an average of 2.2 people [4].
1.3. Course of disease
Emerging evidence has been collated in an attempt to delineate the course of the disease. The World Health Organisation (WHO) estimates that the incubation time from infection to presentation of symptoms is 5.2 days, with a range of 1–14 days [17]. Furthermore, the mean time from presentation of symptoms to seeking medical advice is 5.8 days, and to hospital admission is 12.5 days [4]. The stages of the disease from onset of symptoms have been classified based on non-contrast enhanced chest computed tomography (CT) findings and can be divided into early (0–4 days), progressive (5–8 days), peak (9–13 days), and absorption stages (≥14 days) [18]. Early stage disease consists of subpleural ground glass opacities (GGO) located in the lower lung lobes. The progressive stage demonstrates bilateral distribution of the infective process and diffuse GGO. Presence of dense consolidation, crazy-paving pattern and residual parenchymal bands indicates transition into the peak stage. Finally, the absorption stages, which may last more than 26 days, appears to demonstrate a better controlled disease process on CT, gradual resolution, and signs of recovery [18].
2. Presentation
2.1. Signs and symptoms
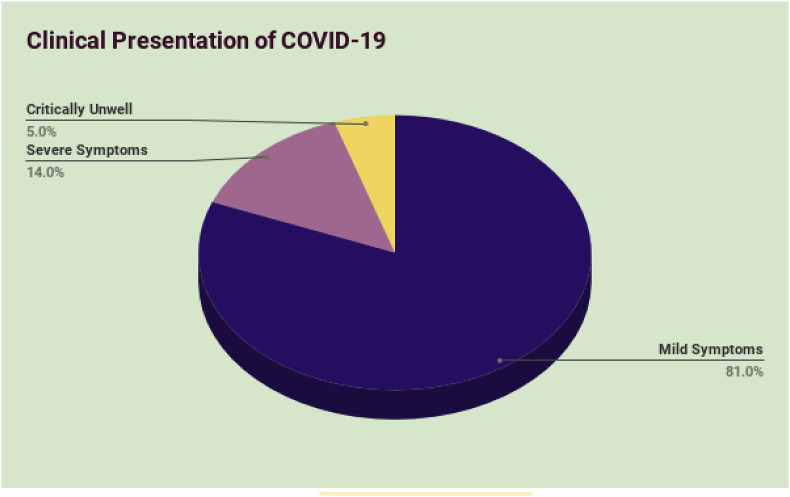
Data from a report of 72,314 cases published by the Chinese Center for Disease Control and Prevention has revealed that the severity of clinical symptoms can vary between individuals [19]. 81% of cases were described as mild (i.e. non-pneumonia and mild pneumonia). 14% of cases were severe (i.e. dyspnea, respiratory frequency ≥30/min, blood oxygen saturation ≤93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, and/or lung infiltrates >50% within 24–48 h), and 5% were critical (i.e. respiratory failure, septic shock, and/or multiple organ dysfunction or failure) [20] (Fig. 1 ). Published data from this early, single chinese study does not seem to represent current global percentages. With emerging information, clinicians will more reliably be able to characterise the disease process and clinical presentation of COVID-19. Other studies indicate that patients with multiple comorbidities are prone to severe infection and may also present with acute kidney injury (AKI) and features of ARDS.
Fig. 1.
Percentage of individuals from a Chinese study presenting with mild, severe, or critical symptoms of COVID-19 [20].
There are reports of both adult and paediatric patients being infected with COVID-19. Data pooled from three large case series indicate the following results [21,22]; The majority of adult patients present with fever (92.8%; n = 258), cough (69.8%; n = 194), dyspnoea (34.5%; n = 96), myalgia (27.7%; n = 77), headache (7.2%; n = 20), diarrhoea (6.1%; n = 17), rhinorrhoea (4.0%), a sore throat (5.1%), and pharyngalgia (17.4%). Data from an European multicentre study indicates that sudden gustatory (88.8%; n = 342) and olfactory (85.6%; n = 357) dysfunctions were also other important symptoms in patients infected with COVID-19 [70]. Analysis of data of 1.5 million users from the COVID Symptoms Tracker app developed by King's College London shows that ageusia and anosmia are stronger predictors of positive COVID-19 diagnosis than self-reported fever [71]. In the paediatric population, symptoms may include fever, fatigue, cough, nasal congestion, runny nose, expectoration, diarrhoea, and headache. As the disease progresses, signs of dyspnoea, cyanosis, in addition to systemic toxic symptoms, including malaise or restlessness, poor feeding, bad appetite and reduced activity may also present. In the most severe situations, these younger patients may progress into respiratory failure unresponsive to conventional oxygen therapy, septic shock, metabolic acidosis, irreversible bleeding, and coagulation dysfunction [23].
2.2. Diagnosis and differential diagnosis
Clinical symptoms must be assessed to aid in the diagnosis of COVID-19. Both the WHO and United States Centers for Disease Control and Prevention (CDC) have issued guidance for key clinical and epidemiological findings suggestive of COVID-19 [24,25]. Extensive laboratory tests should be requested to confirm diagnosis of COVID-19. RT-PCR should be performed in isolated samples of throat swabs, sputum, stool, and blood samples.
Key laboratory results on admission include leucocytes below or above the normal range; neutrophils above the normal range; lymphocytes, haemoglobin and platelets below the normal range. Key liver findings may include elevated alanine aminotransferase, aspartate aminotransferase, C-reactive protein, creatine kinase, lactate dehydrogenase, blood urea nitrogen, and serum creatinine levels. Regarding the infection index, procalcitonin levels may be above the normal range [26].
Radiological findings may also aid the diagnosis of pneumonia in virally infected patients. Bilateral and multi-lobe lung involvement were common in over 75% and 71% of adult patients, respectively [21,22]. In paediatric patients, the following criteria for rapid respiratory rate should be followed for diagnosis of COVID-19 associated pneumonia: ≥60 times/min for less than 2 months old; ≥50 times/min for 2–12 months old, ≥40 times/min for 1–5 years old, ≥30 times/min for >5 years old (after ruling out the effects of fever and crying) [23].
Differential diagnosis can include other viral respiratory infections caused by SARS virus, influenza virus, parainfluenza virus, adenovirus, respiratory syncytial virus and metapneumovirus [23]. These patients present with similar clinical presentations, except for normal or decreased leukocyte count in some patients. Patients may also present with pneumonia due to bacterial causes, which may be accompanied by high fever and moist rale cough [23]. Mycoplasmal pneumonia is another common type of false presentation. Chest X-ray images for such patients may indicate reticular shadows and small patchy or large consolidations. Mycoplasma-specific IgM are helpful for this differential diagnosis. Epidemiological exposure and blood or sputum culture will be helpful for ensuring the correct diagnosis of COVID-19 [23].
2.3. High risk groups
Current reports suggest that all demographics of the global population could be susceptible to infection of COVID-19, however there are some groups that are at higher risk of severe disease [22,26,27]. According to the CDC, older adults - further classified as over 65 years of age - are more at risk of severe disease than younger people. Furthermore, patients with serious chronic underlying medical conditions, namely cardiovascular disease, diabetes, cancer (especially of the lung), chronic obstructive pulmonary disease, and hypertension are at an increased risk of severe complications [28,29]. There is currently no evidence to suggest that children are more susceptible to infection [30], however, there now appears to be an association between male gender and a severer form of the disease [72]. In one study of patients with confirmed COVID-19 infection, 85.9% (n = 67) stabilised whereas 14.1% (n = 11) continued to deteriorate despite treatment. Of the 14.1% who deteriorated, when compared to the stabilised group (median age 37; range = 32–41), the patients were significantly older (median age of 66; range = 51–70), had a history of smoking, and presented with a higher maximum body temperature on admission [31].
Occupational risks have also been identified by various authorities. During the preliminary stages of the COVID-19 outbreak, employees of seafood and wet animal wholesale markets in Wuhan were most at risk of contracting the virus in addition to any customers who had visited these markets [32]. This was closely followed by the subsequent epidemic which posed a high risk to healthcare workers who regularly came into contact with patients with suspected COVID-19. As a result, healthcare workers with pre-existing risks such as an increased age or chronic respiratory disease are advised to ask colleagues who are not in high risk groups to care for patients with potential COVID-19 where possible [29].
2.4. Complications
Various mild and severe clinical syndromes have been associated with the SARS-CoV-2 infection. Mild uncomplicated illnesses include non-specific symptoms including fever, cough, sore throat, nasal congestion, headache, and muscle pain. Elderly and immunosuppressed individuals may present with atypical findings. Mild and severe pneumonia have also been associated with COVID-19. In adults, the latter is characterised by fever or suspected respiratory infection plus one of either respiratory rate >30 breaths/min, severe respiratory distress, or SpO2 <90% on room air. In children, severe pneumonia is indicated by cough or difficulty in breathing plus at least one of either central cyanosis or SpO2 <90%, severe respiratory distress (e.g. grunting, very severe chest indrawing), signs of pneumonia with a general danger sign (e.g. inability to breastfeed or drink, unconsciousness, or convulsions). ARDS presents with new or worsening respiratory symptoms within one week of known clinical insult, and chest imaging reveals bilateral lung opacities. Sepsis and septic shock are further complications of COVID-19. Notably, long-term complications amongst SARS-CoV-2 survivors are not yet available. The mortality rate for cases globally remains between 1 to 2%.
3. Management
3.1. Prevention
Although spread via an airborne route, air disinfection of communities is currently not known to be effective in halting further viral transmission and spread. Human-to-human transmission should be limited in order to prevent transmission amplification events. The use of personal protective equipment should be carefully considered since resources are currently in short supply. Surgical masks in particular are utilised widely within the general population, but have not been clinically proven to reduce or prevent the acquisition of COVID-19. Within the hospital setting, however, high-filtration masks including N95, goggles, and gowns should be worn by healthcare professionals working in direct contact (within 1–2 m) of infected patients [33]. If an infected individual has been identified, rapid isolation and the administration of optimised care should be provided. Suspected patients should also be given a medical mask and placed in an isolation room if available. Wherever possible, the use of adequately ventilated single rooms when performing aerosol-generating procedures should be employed. All patients should be instructed to cover their nose and mouth during coughing or sneezing with tissue. Hand hygiene after contact with respiratory secretions should be enforced. If possible, use either disposable or dedicated equipment (e.g. stethoscopes, blood pressure cuffs, and thermometers) for suspected cases, and avoid contaminating environmental surfaces (e.g. door handles).
In mid-January of 2020, Chinese authorities implemented an array of unprecedented containment strategies, including the restriction of human movement, Hubei province lockdown, and the suspension of flights and trains. These time-critical measures have contributed grossly to the decline in reported cases, and the WHO have since congratulated China on a “unique and unprecedented public health response that reversed escalating cases” [34]. Moreover, between 16th and 30th January 2020, the number of people infected by an individual host dropped to an estimated 1.05 [35], and data from other cities having implemented lockdown measures reported approximately 37% fewer cases in comparison to cities without [36]. Notably, the implementation of control measures a week earlier could have prevented approximately 67% of all Chinese cases according to a model simulation from the University of Southampton, UK [37].
Non-pharmaceutical interventions (NPIs) have been addressed in an attempt to suppress and/or mitigate the disease, with suppression defined as a reduction in the Reproduction Number (R0) - the average number of individuals one infected person can infect - to less than 1, and mitigation defined as a reduction of the effects of the pandemic on health, ultimately reducing mortality and morbidity.
NPIs comprise of strict social isolation and distancing measures and include:
-
1.
Case isolation at home:
Symptomatic cases to remain at home for 7 days which is expected to reduce the number of contacts outside the household by 75% during this timeframe [38]. All forms of social contact must be avoided by symptomatic individuals [39].
-
2.
Voluntary home quarantine:
If a symptomatic case is identified in the household, the entire household must remain at home for 14 days. This is thought to decrease contacts outside of the household by 75% and household contact to increase two-fold [38].
-
3
Social distancing for those above 70 years old:
Individuals over 70 years of age are to practice social distancing i.e. must maintain a 2 m distance from other individuals when possible and to avoid gatherings or congregations [40]. This measure is targeted to reduce contacts by 50% in the workplace and decrease other contacts by 75%, while inadvertently increasing household contacts by 25% [38].
-
4.
Social distancing for the entire population:
All individuals are to practice social distancing as described above, this way reducing all household contacts by 75% and workplace contacts by 25%. School contact rates remain the same and household contacts increase by 25% [38]. Non-essential use of public transport must be avoided and, if possible, arrangements to work from home should be made [39]. Individuals should use remote technology to keep in touch with friends and family, as all large and small gatherings must be avoided. Telephone and online services should be used to contact healthcare professionals and other essential services [39].
-
5.
Closure of schools and universities:
All schools to remain closed and only 25% of universities to remain open, in essence increasing household contact for families of students by 50% and community contacts by 25% during the time of closure [38].
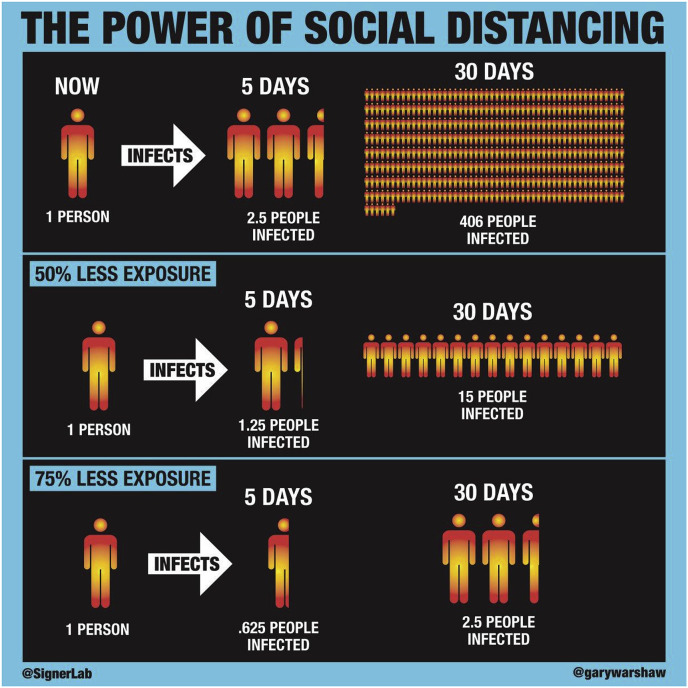
The overall effects of these measures are illustrated in Fig. 2 .
Fig. 2.
The power of social distancing. Attribution: Robert A.J. Signer, Ph.D, Assistant Professor of Medicine, University of California San Diego and Gary Warshaw, Art Director @SignerLab @GaryWarshaw.
Additionally, strict handwashing habits and respiratory hygiene must be followed by individuals to curb the spread of respiratory viruses, including COVID-19 [39].
Ferguson et al. predict that without control measures, the deaths in the UK and US will reach 510,000 and 2.2 million respectively due to the coronavirus alone with 81% of the UK and US populations becoming infected (R0 = 2.4). The number of Intensive Care Unit (ICU) beds required is expected to reach 30 times the available number in both countries. With an aim to reach R0≤1, the study recommends a combination of social distancing and case isolation in combination with household quarantine or school and University closures for a duration of 5 months, with maximum effects felt if all four interventions plus complete lockdown (i.e. individuals prevented from going to work) are implemented. Such measures are predicted to result in a decrease in the number of critical beds required by two-thirds, a figure which equates to 8 times the number of available ICU beds at present. With the above measures, they also estimate that the number of deaths will decrease by one-half - 250,000 and 1.1–1.2 million deaths in the UK and US respectively.
Of note, Ferguson et al. warn that lifting these measures in the absence of a vaccine is likely to lead to a second peak of infection due to the absence of or insufficient herd immunity, with cases reaching the predicted figures in the no-intervention scenario mentioned above. In order to minimise this effect, social distancing policies must be in place until a vaccine is made readily available - a timeframe of at least 18 months. As a response to this predicted, prolonged period of social distancing, the study examined an ‘adaptive triggering’ strategy with ‘on’ and ‘off’ thresholds (Fig. 3 ). ‘On’ triggers are to include the implementation of social distancing and closures of schools and Universities. Case isolation and household quarantine are to be implemented throughout the on/off periods. It is proposed that an ‘on’ trigger be set as 100 cases requiring ICU admission per week to keep below the UK's ICU bed capacity (reached when 200 ICU cases are admitted per week). An ‘off’ trigger to be set as 50 ICU cases per week [38].
Fig. 3.
Graph by Ferguson et al. illustrating the ‘adaptive triggering’ strategies in the UK with use of 100 cases admitted to ICU as an ‘on’ trigger and 50 ICU admissions as an ‘off’ trigger [41].
Public health education must be based on validated scientific evidence in order to adequately inform the public of the current situation as well as to reduce anxiety levels and distress. Misinformation may inadvertently spread panic amongst the general population. As such, epidemiological findings should be reported promptly to ensure accurate assessment and interpretation.
Although there is currently no known effective treatment for COVID-19, reports of the use of oseltamivir, lopinavir/ritonavir, and antibiotics have been reported despite the WHO making no recommendation for the use of antiviral drugs, antibiotics, or glucocorticoids [22]. Care should therefore be taken to not administer medication with unknown efficacy to patients of critically-ill status. Consequently, efforts to prevent and control COVID-19 require an evidence-based and likely multifactorial approach. Fundamentally, successful prevention requires an in-depth understanding of the clinical severity of COVID-19, extent of transmission and infection, and the efficacy of treatment options in order to accelerate the development of diagnostics and therapeutic modalities.
3.2. Supportive management
Supportive management received by a patient is dependent upon the observed severity of disease, feasibility of quarantine, and possible need for hospitalisation.
For asymptomatic neonates and young children with suspected COVID-19 infection, monitoring and supportive care in a quarantined ward are essential. Vital observations including heart rate, respiration rate, SpO2 should be closely monitored. Neonatal feeding should be considered if the mother is COVID-19 positive. For symptomatic neonates, medical management and intervention are necessary [42].
For adults with mild infection - typically characterised by an uncomplicated illness with absence of a severe acute respiratory infection (SARI) - management at home is deemed appropriate and a patient may be isolated in an outpatient setting. Key aspects of delivered care involve monitoring for any clinical deterioration that may require hospitalisation as well as preventing the transmission to other people in the household [43,44].
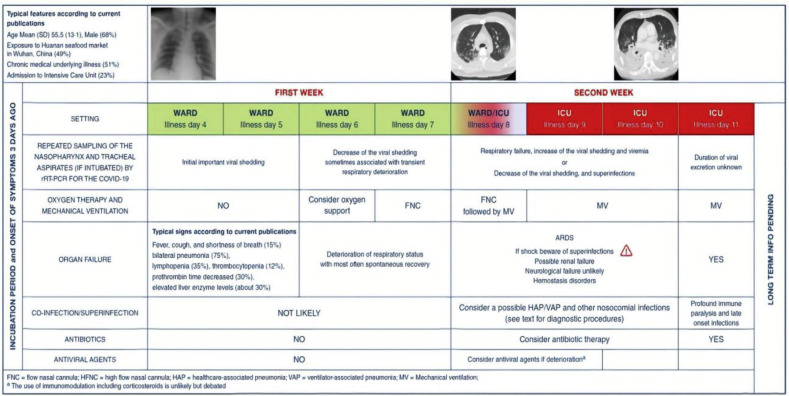
Fig. 4 summarises the likely course of the disease in patients with severe COVID-19. For patients with severe disease, the WHO defines early supportive therapy and monitoring as follows:
- Intravenous (IV) Fluid Administration
-
●Use conservative fluid management in patients with SARI with no evidence of shock.
-
○Treat carefully with IV fluids as aggressive resuscitation can impact oxygenation where mechanical ventilation availability is limited.
-
○
-
●
- Oxygen Therapy
-
●Provide supplemental oxygen therapy immediately if patients present with SARI, hypoxaemia or shock.
-
○Give oxygen therapy at 5L/min to reach target SpO2 of at least 90% in non-pregnant adults (over 92% in pregnant patients).
-
○Children with severe breathing difficulties should have a target SpO2 of over 94%.
-
○
-
●Closely monitor patients with SARI in case of rapid respiratory failure or sepsis and intervene immediately.
-
○This is of utmost importance for patients with COVID-19.
-
○Patients with increased work of breathing or hypoxaemia despite oxygen therapy may be developing hypoxemic respiratory failure seen in ARDS. Clinicians should consider mechanical ventilation at this point [25].
-
○
-
●Appreciate a patient's comorbidities to enable management to be tailored and prognosis realised. Communicate this early with both the patient and relatives [25].
-
●
- Corticosteroids
-
●Routinely administer corticosteroids in the treatment of viral pneumonia unless in a clinical trial or if steroids are indicated for another condition.
-
○Their use in studies on influenza have been found to exacerbate the infection and increase mortality rates [25].
-
○
-
●
Fig. 4.
Illustration by Bouadma et al. demonstrating the progression of severe COVID-19 cases requiring ICU admission [56].
3.3. Management of critical COVID-19
3.3.1. Admission to ICU
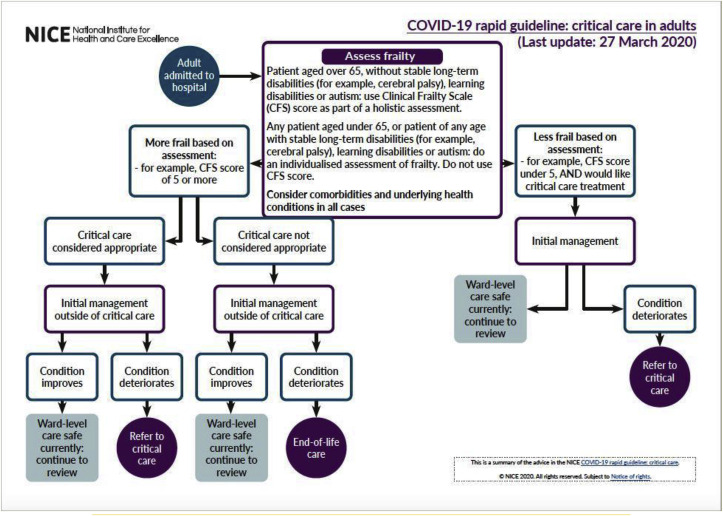
With 5% of all COVID-19 cases becoming seriously or critically unwell and 20–30% [45] of hospitalised patients requiring intensive care support, it is imperative that up-to-date guidelines are in place to aid management. Patients with failing standard oxygen therapy are likely to require advanced oxygen therapy or ventilatory support [25]. With hospital admissions overwhelming healthcare systems worldwide, the National Institute of Health and Care Excellence (NICE) has published an algorithm to ensure appropriate ICU admissions (Fig. 5). Factors taken into consideration when making such decisions are age over sixty-five, frailty - assessed via the Clinical Frailty Scale (CFS) - and comorbidities. Special considerations should be made in patients with long-term disabilities, learning disabilities and autism. In such cases an individual assessment of frailty must be performed [46].
Fig. 5.
NICE algorithm for appropriate critical care referrals [46].
NICE encourages intensivists to start critical care therapy with clear targets or outcomes from the start. It recommends frequent reviews with simultaneous assessment of response to treatment. Critical care treatment should be withdrawn when the outcomes set at initiation of treatment are not reached and the patient fails to improve. Decisions must be communicated with the patient when possible and their family, carers and/or independent mental capacity advocate, if appropriate [47].
3.3.2. Non-invasive ventilation (NIV)
Initial reports did not favour the use of NIV in COVID-19, over fears of large tidal volumes and high transpulmonary pressures causing further lung damage [25]. NIV methods - continuous positive airway pressure (CPAP) or Bilevel Positive Airway Pressure (BiPAP) - were also not recommended as they are aerosol-generating medical procedures and therefore increase the risk of spread of COVID-19 [48]. There is now emerging evidence to support CPAP use during the pandemic [49]. Reports from Italy and China claim that many patients benefited from receiving non-invasive mechanical ventilation. Specifically in Italy, 50% of patients who received CPAP did not require invasive ventilation [[50], [51], [52]]. The WHO recommends that patients receiving CPAP should be supervised by experienced clinicians who are able to perform endotracheal intubation if the patient fails to improve or rapidly deteriorates [25]. Table 1 offers an escalation plan in cases where NIV is being trialled.
Table 1.
Treatment and escalation plan issued by NHS England for adult COVID-19 patients [53].
Teams from University College London (UCL) and University College London Hospital (UCLH) have collaborated with Mercedes Formula One and using reverse engineering methods, have adapted the existing CPAP model making it better suited for mass production. This will make the machines readily available for COVID-19 patients [51].
National Health Service (NHS) England has specified that CPAP should be used for hypoxaemic respiratory failure and BiPAP for hypercapnic states in cases of acute on chronic respiratory failure. Indications include [53]:
-
●
As a ceiling of care option
-
●
In an attempt to avoid intubation
-
●
In an attempt to aid extubation
3.3.3. Endotracheal intubation
If endotracheal intubation is deemed appropriate, the WHO recommends endotracheal intubation to be performed by an experienced clinician using protective equipment.
-
●
Preoxygenate the patient with 100% Fi02 for 5 min using a face mask, bag-valve mask, High-flow nasal oxygen (HFNO) or NIV prior to attempting intubation [25].
3.3.4. Invasive mechanical ventilation
Summation of evidence from a recent review by King's College Hospital NHS Trust as well as guidelines issued by the WHO conclude that severe cases requiring mechanical ventilation may benefit from the following principles:
-
1)Usage of low tidal volumes (4–8 ml/kg predicted body weight (PBW)) and target plateau pressure <30 cmH20 (<28 cmH20 in children):
-
a)Adults: Initial tidal volume 6 ml/kg PBW (may be increased to 8 ml/kg PBW if initial tidal volume not tolerated)
-
b)Children: Target tidal volume 3–6 ml/kg PBW (may be increased to 5–8 ml/kg PBW in cases with well preserved respiratory compliance) [25].
-
a)
-
2)
As a general rule, titration of positive end-expiratory pressure (PEEP) should be guided by the Fraction of Inspired Oxygen (Fi02) required to achieve a desired arterial oxygen saturation (Sp02). The settings presented in Table 2 have been derived from the ARDSnet trial and can be used to achieve an Sp02 >90% [25,54].
-
3)
Early airway pressure release ventilation should be considered in certain patients [55].
-
4)
Consideration of early prone ventilation in patients where there is no improvement observed after 12 h of ventilator optimisation (i.e. PaO2/FiO2 <150). Prone ventilation should last 12–16 h a day [25,55].
-
5)
Permissive hypercapnia may be considered if haemodynamically satisfactory parameters are maintained as opposed to forms of ventilation which may cause further lung damage [25,55].
Table 2.
[54]
| FiO2 | PEEP |
|---|---|
| 0.3 | 5 |
| 0.4 | 5–8 |
| 0.5 | 8–10 |
| 0.6 | 10 |
| 0.7 | 10–14 |
| 0.8 | 14 |
| 0.9 | 14–18 |
| 1.0 | 18–24 |
3.3.5. Extracorporeal membrane oxygenation (ECMO)
Cases of COVID-19 with refractory hypoxemia despite lung protective ventilation should receive ECMO if an extracorporeal life support (ECLS) service is available [25].
3.3.6. Fluid resuscitation and vasopressors
In adults, fluid resuscitation should be administered as 250–500 ml crystalloid fluid boluses over 15–30 min followed by assessment for fluid overload after each bolus. Vasopressors can be used if septic shock (Table 3 ) persists despite fluid resuscitation to maintain a mean arterial pressure (MAP) ≥65 mmHg. In patients over 65, a MAP of 60–65 mmHg is acceptable, a recent study suggests [25,57]. In adults, norepinephrine is the drug of choice, which can be supplemented by epinephrine or vasopressin to maintain MAP targets.
Table 3.
[58]
| SEPTIC SHOCK | |
|---|---|
| ADULTS | CHILDREN |
|
|
Vasopressor requirement to maintain a mean arterial pressure (MAP) ≥ 65 mmHg
Systolic Blood Pressure <5th percentile or >2 SD below normal for age.
In infants Heart Rate(HR) <90 bpm or >160 bpm and children HR <70 bpm >150 bpm.
In children, fluid resuscitation should be administered as 10–20 mL/kg crystalloid fluid boluses over 30–60 min followed by assessment for fluid overload after each bolus. Vasopressors may be used if signs of septic shock (Table 3) and/or fluid overload are observed or if there is an inability to maintain age-appropriate blood pressure parameters. In children, epinephrine is the drug of choice, with norepinephrine supplementation if septic shock persists [25].
3.4. Medical management
The CDC echoes the WHO's comments regarding the use of corticosteroids and further explains that their use can prolong replication of the virus when used in similar viruses to COVID-19 such as MERS-CoV [59]. When patients present with SARI, the WHO advises to administer empiric antimicrobials that can treat any likely causative agent within 1 h of assessment with confirmed sepsis. This treatment should be based on clinical diagnosis - whether community or hospital acquired pneumonia - and treated according to local guidelines [25]. Conversely, Wang et al. have suggested that the inappropriate use of broad-spectrum antibiotics should be avoided unless there is evidence of secondary bacterial infection [42].
More recently, pioneering laboratory tests have suggested that there may be drugs already used for other viruses that could be applicable to COVID-19. Remdesivir - a broad spectrum antiviral agent - is an adenosine analogue capable of interrupting the nascent viral RNA chains to cause premature chain termination, and has previously been tested for treatment of the Ebola virus. When remdesivir is injected into Vero E6 cells infected with COVID-19, the antiviral effectively inhibits the virus. When tested in human cell lines (Huh-7 cells) the virus is effectively inhibited [60]. This supports the CDC's statement that remdesivir has in vitro activity against COVID-19 [61]. Further studies are needed to confirm Remdesivir's use against COVID-19, however the National Institute of Allergy and Infectious Diseases is currently conducting a double-blind randomised controlled trial on the use of remdesivir in patients with COVID-19 infection, with results pending [62].
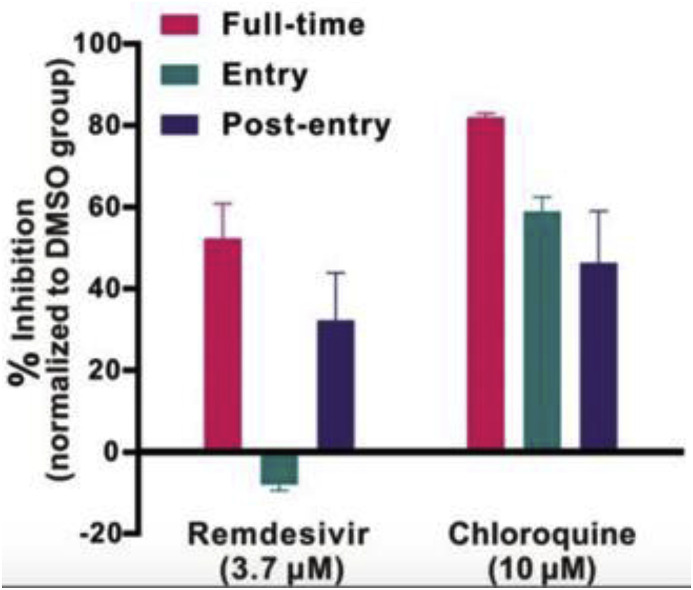
Another familiar drug that may be viable for COVID-19 is chloroquine; it is traditionally used as an antimalarial as well as against autoimmune diseases. The mechanism of action of chloroquine increases the endosomal pH above that required for virus and cell fusion whilst also interrupting the glycosylation of cellular receptors in similar viruses such as SARS-CoV. When chloroquine is introduced to Vero E6 cells infected with COVID-19, it appears to treat the infection at both entry and post-entry stages of infection. Chloroquine may also enhance immune modulation of cells, potentially increasing efficacy of the drug in vivo. In general, chloroquine is cheap and safe to use, and is widely distributed to all organ systems including the lungs when taken orally (Fig. 6 ) [63].
Fig. 6.
A graph to show the antiviral activities of test drugs against COVID-19 in vitro [63].
Oncological drugs are another class with growing interest. Some oncology drugs - whilst laboratory results are promising - cannot be tolerated by humans due to the required dose being significantly higher than the established therapeutic dose for other diseases. Conversely, anti-inflammatory drugs have been suggested due to the significant effect of inflammatory responses on lung damage and resulting mortality. Table 4 details three anti-inflammatory agents trialed on COVID-19. Their underlying mechanism of action involves inhibition of JAK-STAT signalling thus decreasing the extent of elevation of cytokines seen in patients with COVID-19 [64].
Table 4.
[64]
| Baricitinib | Ruxolitinib | Fedratinib | |
|---|---|---|---|
| Daily dose (mg) | 2–10 | 25 | 400 |
| Affinity and Efficacy: Kd or IC50, nM | |||
| AAK1 | |||
| Cell free | 17 | 100 | 32 |
| Cell | 34 | 700 | 960 |
| GAK | |||
| Cell free | 136 | 120 | 1 |
| Cell | 272 | 840 | 30 |
| BIKE | |||
| Cell free | 40 | 210 | 32 |
| Cell | 80 | 1470 | 960 |
| JAK1 | |||
| Cell free | 6 | 3 | 20 |
| Cell | 12 | 20 | 600 |
| JAK2 | |||
| Cell free | 6 | 3 | 3 |
| Cell | 11 | 21 | 100 |
| JAK3 | |||
| Cell free | >400 | 2 | 79 |
| Cell | >800 | 14 | 2370 |
| TYK2 | |||
| Cell free | 53 | 1 | 20 |
| Cell | 106 | 7 | 600 |
| Pharmacokinetics | |||
| Plasma protein binding | 50% | 97% | 95% |
| Cmax (unbound), nM | 103 | 117 | 170 |
| Safety:tolerated dose | ⩽10 mg/day | ⩽20 mg twice daily | ⩽400 mg/day |
3.5. Operative management
The first double lung transplant was successfully performed on a patient in China with irreversible bilateral lung damage secondary to COVID-19 on February 29, 2020. The 59 year old male was infected with SARS-CoV-2 on January 26, 2020, and although repeated tests confirmed the resolution and absence of ongoing infection, prolonged endotracheal intubation, ventilation, and ECMO therapy were nevertheless required. The team at Wuxi People's Hospital, led by cardiothoracic surgeon Dr Chen Jinguy, performed the 5 h operation. The operation was performed successfully with the patient requiring postoperative observation and medical therapy to avoid infection or rejection [65].
3.6. Measuring response
Due to the limited treatment options available, the ability to measure a response to treatment is challenging. When patients are tested for initial infection, a positive result is based on nucleic acid detection for SARS-CoV-2 infection. When assessing patients with deteriorating conditions, it has been noted that CRP is significantly raised and albumin is low [31]. While no clear guidelines exist on the evaluation of response to supportive treatment, a study by Cascella et al. has suggested that laboratory evaluation of samples from patients should demonstrate viral clearance prior to discharging from observation in the form of two negative respiratory tract specimens taken at least 24 h apart [66,67].
4. Outcomes
Current data has shown that there are an estimated 1,664,384 active cases worldwide, of which 97% (n = 1,623,355) display mild symptoms of the COVID-19 and 3% (n = 41,029) of currently infected patients are seriously (requiring oxygen therapy) or critically unwell (requiring mechanical ventilation). Of the closed cases (n = 834,069), 79% (n = 663,477) of infected individuals have recovered from the disease or have been successfully discharged from hospital. 21% (n = 171,017) of these cases have died of the illness or related complications [7]. As it stands, the 46th WHO situation report estimates the Crude Mortality Ratio of COVID-19 to be between 3 and 4% based on current data [68]. Median time for recovery from the onset of symptoms is approximately 2 weeks in mild cases and 3–6 weeks in severely or critically unwell individuals [34].
5. Conclusion
With a peak of 101,736 new cases confirmed on April 3, 2020 alone [69], there are fears that these findings could indicate exponential spread of the disease. Implementation and adherence to tighter restrictions of social distancing to suppress and mitigate the spread of COVID-19 will prove to be crucial in the months to come. Up-to-date, evidence-based guidelines for acute management of COVID-19 are imperative to guide clinicians through the rapidly evolving pandemic. As new evidence emerges, it is imperative that current and potential treatment options are frequently re-evaluated in order to offer the best possible care under such unprecedented circumstances.
Ethical approval
None required.
Sources of funding
None.
Author contribution
Maria Nicola: significant role in concept production and writing of manuscript, editing and approval of final draft.
Niamh O'Neill: significant role in writing of initial manuscript, editing and approval of final draft.
Catrin Sohrabi: contribution to writing of manuscript, editing and approval of final draft.
Mehdi Khan: contribution to writing of manuscript, editing and approval of final draft.
Riaz Agha: senior author, role in supervising concept production, collection of papers and approval of final draft.
Guarantor
Niamh O'Neill: Corresponding Author, niamh@ijspg.com.
Riaz Agha: Senior author, mail@riazagha.com.
Data statement
The data in this review is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature.
Declaration of competing interest
None.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019 doi: 10.1056/NEJMoa2001017. https://www.nejm.org/doi/10.1056/NEJMoa2001017 [Internet]. 2020 Jan 24 [cited 2020 Mar 9]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020 Apr;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. J. Am. Med. Assoc. 2020 Mar doi: 10.1001/jama.2020.3204. http://www.ncbi.nlm.nih.gov/pubmed/32125362 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia | NEJM. https://www.nejm.org/doi/full/10.1056/NEJMoa2001316 [Internet]. [cited 2020 Mar 9]. Available from: [DOI] [PMC free article] [PubMed]
- 5.Coronavirus confirmed as pandemic. 2020 Mar 11. https://www.bbc.com/news/world-51839944 BBC News [Internet] [cited 2020 Mar 11]; Available from: [Google Scholar]
- 6.Coronavirus update (live): 126,049 cases and 4,616 deaths from COVID-19 virus outbreak. https://www.worldometers.info/coronavirus/ Worldometer [Internet]. [cited 2020 Mar 11]. Available from:
- 7.Coronavirus Dashboard [Internet] https://ncov2019.live/ [cited 2020 Mar 29]. Available from:
- 8.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. [Internet] 2020 Mar 3 doi: 10.1007/s00134-020-05985-9. [cited 2020 Mar 9]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang X, Wu C, Li X, Song Y, Yao X, Wu X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. [Internet]. [cited 2020 Mar 9]; Available from:: https://academic.oup.com/nsr/advance-article/doi/10.1093/nsr/nwaa036/5775463. [DOI] [PMC free article] [PubMed]
- 10.Yi H. 2019 Novel coronavirus is undergoing active recombination. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa219. http://www.ncbi.nlm.nih.gov/pubmed/32130405 [Internet] Mar; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Y, Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): a Chinese perspective. J. Med. Virol. [Internet]. [cited 2020 Mar 9];n/a(n/a). Available from:: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.25749. [DOI] [PMC free article] [PubMed]
- 12.Novel coronavirus: study suggests multiple shedding routes. http://www.medscape.com/viewarticle/925575 [Internet]. Medscape. [cited 2020 Mar 9]. Available from:
- 13.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.3227. http://www.ncbi.nlm.nih.gov/pubmed/32129805 [Internet] Mar; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020 Mar 4 doi: 10.1016/j.jinf.2020.02.028. https://www.journalofinfection.com/article/S0163-4453(20)30109-2/abstract [Internet] [cited 2020 Mar 9];0(0). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J. Med. Virol. 2020 Mar doi: 10.1002/jmv.25748. jmv.25748-jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020 Feb 19 doi: 10.1056/NEJMc2001737. 0(0):null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Q&A on coronaviruses (COVID-19) https://www.who.int/news-room/q-a-detail/q-a-coronaviruses [Internet]. [cited 2020 Mar 9]. Available from:
- 18.Zhu Y., Liu Y.-L., Li Z.-P., Kuang J.-Y., Li X.-M., Yang Y.-Y. Clinical and CT imaging features of 2019 novel coronavirus disease (COVID-19) J. Infect. 2020 Mar 3 doi: 10.1016/j.jinf.2020.02.022. https://www.journalofinfection.com/article/S0163-4453(20)30104-3/abstract [Internet] [cited 2020 Mar 9];0(0). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi. 2020 Feb 17;41(2):145–151. [Google Scholar]
- 20.Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention | Global Health | JAMA | JAMA Network [Internet]. [cited 2020 Mar 11]. Available from:: https://jamanetwork.com/journals/jama/fullarticle/2762130. [DOI] [PubMed]
- 21.Wang F.-S., Zhang C. What to do next to control the 2019-nCoV epidemic? Lancet. 2020 Feb 8;395(10222):391–393. doi: 10.1016/S0140-6736(20)30300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China | Critical Care Medicine | JAMA | JAMA Network [Internet]. [cited 2020 Mar 11]. Available from:: https://jamanetwork.com/journals/jama/fullarticle/2761044. [DOI] [PMC free article] [PubMed]
- 23.Chen Z.-M., Fu J.-F., Shu Q., Chen Y.-H., Hua C.-Z., Li F.-B. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J. Pediatr. 2020 Feb 5 doi: 10.1007/s12519-020-00345-5. [Internet] [cited 2020 Mar 11]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC CDC. Coronavirus disease 2019 (COVID-19) - transmission. Centers for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html [Internet] [cited 2020 Mar 10]. Available from: [Google Scholar]
- 25.Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Internet]. [cited 2020 Mar 10]. Available from:
- 26.Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study - Lancet. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30211-7/fulltext [Internet]. [cited 2020 Mar 11]. Available from: [DOI] [PMC free article] [PubMed]
- 27.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China - Lancet. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30183-5/fulltext [Internet]. [cited 2020 Mar 11]. Available from: [DOI] [PMC free article] [PubMed]
- 28.CDC. Coronavirus disease 2019 (COVID-19) Centers for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/high-risk-complications.html [Internet] [cited 2020 Mar 11]. Available from: [Google Scholar]
- 29.Risk groups. https://www.fhi.no/en/op/novel-coronavirus-facts-advice/facts-and-general-advice/risk-groups---advice-and-information/ [Internet], Norwegian Institute of Public Health. [cited 2020 Mar 11]. Available from:
- 30.CDC. Coronavirus disease 2019 (COVID-19) Centers for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/children-faq.html [Internet] [cited 2020 Mar 11]. Available from: [Google Scholar]
- 31.Liu W., Tao Z.-W., Lei W., Ming-Li Y., Kui L., Ling Z. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. (Engl) 2020 Feb 28 doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.COVID-19: occupational groups that are at high risk. https://www.medicalnewstoday.com/articles/covid-19-occupational-groups-that-are-at-high-risk [Internet]. [cited 2020 Mar 11]. Available from:
- 33.国家卫生健康委办公厅关于印发<br/>新型冠状病毒感染的肺炎防控中常见医用防护用品使用范围指引(试行)的通知. http://www.nhc.gov.cn/xcs/zhengcwj/202001/e71c5de925a64eafbe1ce790debab5c6.shtml [Internet]. [cited 2020 Mar 11]. Available from:
- 34.who-China-joint-mission-on-covid-19---final-report-1100hr-28feb2020-11mar-update.pdf. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19---final-report-1100hr-28feb2020-11mar-update.pdf?sfvrsn=1a13fda0_2 [Internet]. [cited 2020 Mar 26]. Available from:
- 35.Kucharski A.J., Russell T.W., Diamond C., Liu Y., CMMID nCoV working group, Edmunds J. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Infectious Diseases (except HIV/AIDS) 2020 Feb doi: 10.1016/S1473-3099(20)30144-4. http://medrxiv.org/lookup/doi/10.1101/2020.01.31.20019901 [Internet] [cited 2020 Mar 26]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The impact of transmission control measures during the first 50 days of the COVID-19 epidemic in China | medRxiv [Internet]. [cited 2020 Mar 26]. Available from:: https://www.medrxiv.org/content/10.1101/2020.01.30.20019844v4. [DOI] [PMC free article] [PubMed]
- 37.WorldPop :: effect of non-pharmaceutical interventions for containing the COVID-19 outbreak. https://www.worldpop.org/events/COVID_NPI [Internet]. [cited 2020 Mar 26]. Available from:
- 38.Ferguson N.M., Laydon D., Nedjati-Gilani G., Imai N., Ainslie K., Baguelin M. vol. 20. 2020. (Impact of Non-pharmaceutical Interventions (NPIs) to Reduce COVID- 19 Mortality and Healthcare Demand). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guidance on social distancing for everyone in the UK. https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protecting-older-people-and-vulnerable-adults [Internet], GOV.UK. [cited 2020 Mar 22]. Available from:
- 40.Tom Inglesby answers your COVID-19 questions. https://html5-player.libsyn.com/embed/episode/id/13526585/height/90/theme/custom/thumbnail/yes/direction/forward/render-playlist/no/custom-color/ea5329/ [Internet]. [cited 2020 Mar 29]. Available from:
- 41.Ferguson . 2020. Impact of non-pharmaceutical interventions (NPIs)https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf pdf [Internet]. [cited 2020 Mar 29]. Available from: [Google Scholar]
- 42.Wang L., Shi Y., Xiao T., Fu J., Feng X., Mu D. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition) Ann. Transl. Med. 2020 Feb;8(3):47. doi: 10.21037/atm.2020.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Home care for patients with COVID-19 presenting with mild symptoms and management of their contacts. https://www.who.int/publications-detail/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts [Internet]. [cited 2020 Mar 30]. Available from:
- 44.Case management. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patient-management [Internet]. [cited 2020 Mar 11]. Available from:
- 45.CDC. Coronavirus disease 2019 (COVID-19) Centers for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html [Internet] [cited 2020 Mar 30]. Available from: [Google Scholar]
- 46.Overview | COVID-19 rapid guideline: critical care in adults | GuidanCce | NICE. https://www.nice.org.uk/guidance/ng159 [Internet]. NICE; [cited 2020 Mar 30]. Available from:
- 47.3 Starting, reviewing and stopping critical care treatment | COVID-19 rapid guideline: critical care in adults | Guidance | NICE. https://www.nice.org.uk/guidance/ng159/chapter/3-Starting-reviewing-and-stopping-critical-care-treatment [Internet]. NICE; [cited 2020 Mar 30]. Available from:
- 48.World Federation of societies of anaesthesiologists - coronavirus. https://www.wfsahq.org/resources/coronavirus [Internet]. [cited 2020 Mar 30]. Available from:
- 49.Letter regarding the use of continuous positive airway pressure (CPAP) for COVID-19 positive patients | The Faculty of Intensive Care Medicine [Internet]. [cited 2020 Mar 30]. Available from:: https://www.ficm.ac.uk/news-events-education/news/letter-regarding-use-continuous-positive-airway-pressure-cpap-covid-19.
- 50.F1 team helps build new UK breathing aid for Covid-19 patients. ISS, editor. Guardian. 2020 Mar 30 https://www.theguardian.com/world/2020/mar/30/f1-team-helps-build-new-uk-breathing-aid-for-covid-19-patients [Internet] [cited 2020 Mar 30]; Available from: [Google Scholar]
- 51.Breathing machine developed in under 100 hours to help Covid-19 patients. https://www.expressandstar.com/news/uk-news/2020/03/30/breathing-machine-developed-in-under-100-hours-to-help-covid-19-patients/ [Internet]. [cited 2020 Mar 30]. Available from:
- 52.Haichao L., Jing M., Hong Z., Yuan C., Xi W., Zhangwei H. Thoughts and practice on the treatment of severe and critical new coronavirus pneumonia. Chin. J. Tuberc. Respir. Dis. 2020 Mar 18;43 doi: 10.3760/cma.j.cn112147-20200312-00320. 00, E038–E038. [DOI] [PubMed] [Google Scholar]
- 53.NHS england and NHS improvement Guidance for the role and use of non-invasive respiratory support in adult patients with COVID19 (confirmed or suspected) https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-NIV-respiratory-support-and-coronavirus-v3.pdf [Online]. Available from.
- 54.Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N. Engl. J. Med. 2007 Sep 13;357(11):1113–1120. doi: 10.1056/NEJMct074213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.01-Kings-Critical-Care-COVID19-Evidence-Summary-9th-March-2020.pdf. https://scts.org/wp-content/uploads/2020/03/01-Kings-Critical-Care-COVID19-Evidence-Summary-9th-March-2020.pdf [Internet]. [cited 2020 Mar 29]. Available from:
- 56.Bouadma L., Lescure F.-X., Lucet J.-C., Yazdanpanah Y., Timsit J.-F. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020 Apr 1;46(4):579–582. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial | Critical Care Medicine | JAMA | JAMA Network [Internet]. [cited 2020 Mar 30]. Available from:: https://jamanetwork.com/journals/jama/article-abstract/2761427. [DOI] [PMC free article] [PubMed]
- 58.Clinical management of severe acute respiratory infection when COVID-19 is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Internet]. [cited 2020 Mar 30]. Available from:
- 59.CDC. Coronavirus disease 2019 (COVID-19) Centers for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html [Internet] [cited 2020 Mar 11]. Available from: [Google Scholar]
- 60.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020 Apr;30(4):343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.CDC. Coronavirus disease 2019 (COVID-19) Centers for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html [Internet] [cited 2020 Apr 2]. Available from: [Google Scholar]
- 62.Adaptive COVID-19 Treatment Trial - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04280705 [Internet]. [cited 2020 Mar 11]. Available from:
- 63.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Mar;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.COVID-19: combining antiviral and anti-inflammatory treatments - the Lancet Infectious Diseases. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30132-8/fulltext [Internet]. [cited 2020 Mar 11]. Available from: [DOI] [PMC free article] [PubMed]
- 65.World's First Double-Lung Transplant for COVID-19 Infection Succeeds in China - Global Times. https://www.globaltimes.cn/content/1181228.shtml [Internet]. [cited 2020 Mar 9]. Available from:
- 66.Features, evaluation and treatment coronavirus (COVID-19) - StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK554776/ [Internet]. [cited 2020 Mar 11]. Available from:
- 67.Marchand-Senécal X., Kozak R., Mubareka S., Salt N., Gubbay J.B., Eshaghi A. Diagnosis and management of first case of COVID-19 in Canada: lessons applied from SARS. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 Mar 9 doi: 10.1093/cid/ciaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Novel Coronavirus (2019-nCoV) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Internet]. [cited 2020 Mar 10]. Available from:
- 69.Coronavirus cases: statistics and charts - Worldometer. https://www.worldometers.info/coronavirus/coronavirus-cases/#daily-cases [Internet]. [cited 2020 Mar 10]. Available from:
- 70.Lechien J.R., Chiesa-Estomba C.M., De Siati Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loss of smell and taste a key symptom for COVID-19 cases [Internet]. [cited 2020 Apr 12]. Available from: https://www.kcl.ac.uk/news/loss-of-smell-and-taste-a-key-symptom-for-covid-19-cases.
- 72.Rapid risk assessment: Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – eighth update [Internet]. European Centre for Disease Prevention and Control. 2020 [cited 13 April 2020]. Available from: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-coronavirus-disease-2019-covid-19-pandemic-eighth-update.