Summary
Objective
To better inform efforts to treat and control the current outbreak with a comprehensive characterization of COVID-19.
Methods
We searched PubMed, EMBASE, Web of Science, and CNKI (Chinese Database) for studies published as of March 2, 2020, and we searched references of identified articles. Studies were reviewed for methodological quality. A random-effects model was used to pool results. Heterogeneity was assessed using I2. Publication bias was assessed using Egger's test.
Results
43 studies involving 3600 patients were included. Among COVID-19 patients, fever (83.3% [95% CI 78.4–87.7]), cough (60.3% [54.2–66.3]), and fatigue (38.0% [29.8–46.5]) were the most common clinical symptoms. The most common laboratory abnormalities were elevated C-reactive protein (68.6% [58.2–78.2]), decreased lymphocyte count (57.4% [44.8–69.5]) and increased lactate dehydrogenase (51.6% [31.4–71.6]). Ground-glass opacities (80.0% [67.3–90.4]) and bilateral pneumonia (73.2% [63.4–82.1]) were the most frequently reported findings on computed tomography. The overall estimated proportion of severe cases and case-fatality rate (CFR) was 25.6% (17.4–34.9) and 3.6% (1.1–7.2), respectively. CFR and laboratory abnormalities were higher in severe cases, patients from Wuhan, and older patients, but CFR did not differ by gender.
Conclusions
The majority of COVID-19 cases are symptomatic with a moderate CFR. Patients living in Wuhan, older patients, and those with medical comorbidities tend to have more severe clinical symptoms and higher CFR.
Keywords: COVID-19, Clinical characteristics, Meta-analysis, Systematic review
Introduction
In December 2019, a cluster of pneumonia cases of unknown cause appeared in Wuhan, China.1 The National Health Commission (NHC) of the People's Republic of China later announced that a novel coronavirus, now named COVID-19 by the World Health Organization (WHO),2 was responsible for the outbreak.3 High-throughput sequencing identified COVID-19 as a betacoronavirus. This novel virus is genetically similar to bat coronaviruses, and shares about 79% and 50% of its genetic sequence with the coronaviruses responsible for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), respectively.4 Although epidemiological evidence suggests most of the initial patients were exposed to the Huanan Seafood Market in Wuhan, the animal source of COVID-19 has not yet been identified.1 Human-to-human transmission is now responsible for most new infections, including those among family members and health care workers.5, 6, 7
Pneumonia caused by 2019-nCOV, known as COVID-19, is of huge global concern, with confirmed cases in 34 Chinese provinces and nearly 30 countries across five continents. The WHO's International Health Regulations Emergency Committee declared this outbreak constitutes a Public Health Emergency of International Concern (PHEIC) on 30 January 2020.2 As of 2 March 2020 the cumulative number of confirmed cases and deaths of COVID-19 in China has reached 80,302 and 2947, respectively. Outside of China, a total of 10,449 cases have been confirmed, including 170 deaths.8
Only one published systematic review and meta-analysis summarized clinical characteristics of COVID-19.9 It reported a case-fatality rate (CFR) of 4.3% and that fever, sore throat, and muscle soreness or fatigue were the most common symptoms. In that review the incidence of abnormal chest computer tomography (CT) was 96.6%. However, this article analysed results from only ten studies, including one Chinese Center for Disease Control and Prevention (CDC) report that provides epidemiological data only, and four preprint articles (one was already withdrawn) that are not peer reviewed.10 This article failed to report any clinical laboratory findings, treatments and geographical distribution of COVID-19 which are essential to a thorough understanding of clinical characteristics. Many cases have emerged inside and outside Wuhan over the past month.1 , 5 , 6 , 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Recent publications suggest there may be significant differences between clinical outcomes for COVID-19 between patients inside and outside Wuhan. Xu, et al. found that patients outside of Wuhan experienced milder illness and less pronounced laboratory abnormalities compared to counterparts inside Wuhan.24
Although the number of COVID-19 cases continues to grow worldwide, little attention has been paid to summarizing the clinical signs, risk factors, laboratory and chest CT findings, complications, and treatments of COVID-19. We performed a systematic review and meta-analysis to provide a comprehensive characterization of COVID-19 to better inform efforts to treat and control the current outbreak.
Methods
Search strategy and selection criteria
Our systematic review and meta-analysis was undertaken according to PRISMA and MOOSE guidelines.51 , 52 We searched four databases, PubMed, EMBASE, Web of Science and CNKI (Chinese Database), to identify studies reporting COVID-19. Articles published on or before March 2, 2020 were eligible for inclusion. We used the following search terms: “coronavirus” or “nCoV” or “SARS-CoV-2″ or “COVID-19″. References of all retrieved studies were screened for additional eligible publications. Primary studies were eligible if they reported any information on COVID-19 patients in China without restriction on study type or study design. We excluded studies that focused on infection in infants, did not report original data or clear diagnostic criteria, and no reliable clinical data as well as research outside mainland China.
Two independent reviewers (LF and BW) screened the literature search and assessed each study for inclusion. Any disagreement was solved by consulting a senior investigator (HZ).
Data analysis
Four authors (TY, XC, BW, and LF) independently extracted relevant information, including first author, publication time, study designs, city, number of COVID-19 patients, mean or median age of patients, maximum follow-up duration (days), history of exposure in Wuhan, smoking history, diagnostic criteria of COVID-19, presence of medical comorbidities, clinical symptoms, radiologic findings, laboratory findings, complications, supportive treatment, and clinical outcome of COVID-19 patients. We also extracted the original author's guidelines for defining severe case and screened them according to Guidelines of Diagnosis and Treatment Of COVID-19 (Sixth Edition) from the NHC.8 We classified patients admitted to intensive care units (ICU) as severe cases when authors did not report diagnostic criteria for disease severity. Studies that only reported data for critically ill patients were excluded in the overall meta-analysis but were included in the meta-analysis restricted to severe cases.
We used the quality assessment tool for case series studies published by the National Institutes of Health (NIH) to assess the methodological quality of included studies.53 We scored 0 or 1 point for each item according to the criteria and added scores for all items to generate an overall quality score that ranged from 0 to 9. Based on the overall score, we classified studies as low (≥7), moderate (5–6), or high risk of bias (≤4). Any disagreement was resolved through discussion by all investigators.
We performed data analyses using meta packages in R (version 3.6.0). Random-effects meta-analysis was used to calculate pooled estimated prevalence with 95% confidence intervals of clinical symptoms, laboratory findings, chest CT findings, complications, treatment, and fatality of COVID-19 patients.54 To minimize the impact of studies with extremely small or extremely large prevalence estimates on overall estimates, Freeman-Tukey double arcsine transformation was used to stabilize the variance of specific prevalence rates before using random-effects meta-analysis models to pool data.54
We assessed heterogeneity between studies using I2, with values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively.55 If substantial heterogeneity (I2>75%) was detected, we further explored the possible source of heterogeneity through subgroup analysis and used the following grouping variables: age, sex, region, and underlying medical comorbidities. We also performed subgroup analyses to explore whether the prevalence of outcomes differed by these subgroups. If a meta-analysis included more than three studies, publication bias was assessed by Egger's test.56
Results
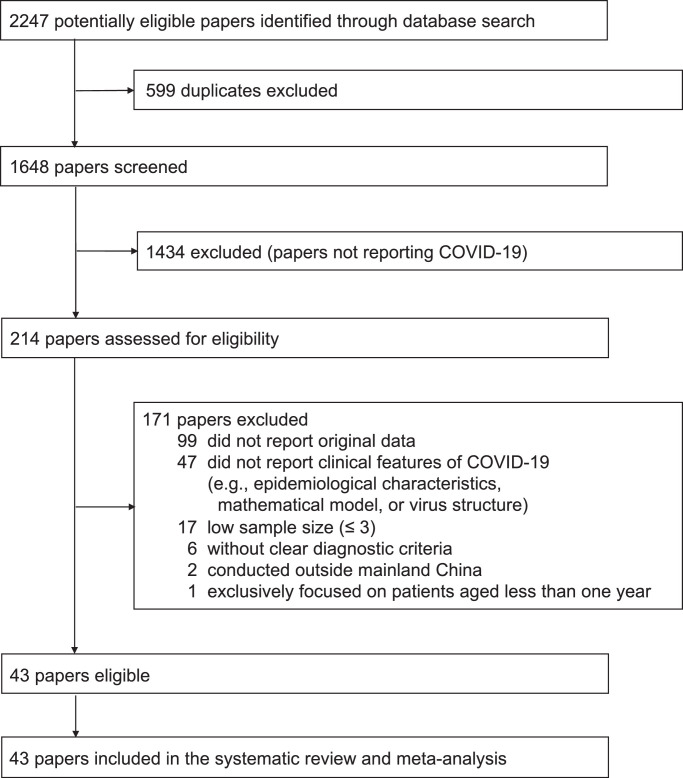
Our search produced 2247 publications. Of these, 1648 were unique records, from which 1434 records were excluded after screening their titles and abstracts (Fig. 1 ). We assessed the eligibility of 214 full-text papers, of which 99 did not report original data, 47 did not report clinical features of COVID-19 (e.g., epidemiological characteristics, mathematical models, virus structure), six did not include clear diagnostic criteria, 17 had a sample size smaller than four, two were conducted outside mainland China, and one focused on patients aged less than one year. After excluding these studies, 43 eligible studies with 3600 patients were included. Among included studies, one study only reported data on critically ill patients and was excluded from the overall meta-analysis but was included in the meta-analysis restricted to patients with severe illness.1 , 5 , 6 , 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50
Fig. 1.
Flow diagram of publication selection
*Figure legend: COVID-19: Corona Virus Disease 2019.
Table 1 summarizes characteristics of included studies. Included studies were published between 24 January 2020 and 28 February 2020, among which 25 (58.1%) were in Chinese and the remaining was in English. The earliest enrollment time was 16 December 2019 and the latest was 27 January 2020. One publication was a letter, and the remainder were journal articles. Most included studies were retrospective case series (40 [90.3%]), 27 (62.8%) were from cities outside Wuhan, and 34 (79.0%) only included patients with laboratory confirmed COVID-19. The number of patients enrolled in each study ranged from 4 to 1099. Mean or median age of patients varied from 39 to 72 years (median 41 years; 43 studies). The proportion of male patients ranged from 29.0% to 77.0% (median 56.5%; 42 studies). The proportion of patients who had ever traveled to or were resident of Hubei Province varied from 28.5% to 100.0% (median 91.0%; 36 studies). The number of family-clusters ranged from 1 to 5 (10 studies). The proportion of patients who were current smokers ranged from 0.0% to 18.0% (median 7.2%; 9 studies), and health workers ranged from 0.0% to 29.0% (median 4.0%; 5 studies). The proportion of patients with hypertension ranged from 0.0% to 48.0% (median 16.0%;27 studies), diabetes ranged from 0.0% to 50.0% (median 10.1%; 26 studies), cancer ranged from 0.0% to 17.0% (median 1.0%; 15 studies), chronic respiratory/lung diseases ranged from 0.0% to 17.0% (median 2.0%; 16 studies), having any coexisting medical comorbidity ranged from 12.0% to 67.0%. The proportion of patients diagnosed with severe COVID-19 varied from 0.0% to 100.0% (median 26.5%; 21 studies), and the most commonly used diagnostic criteria was The Guidelines on 2019-nCoV Treatment and Prevention issued by the NHC (70.6) (17 studies). 9 (20.9%) of 43 studies were rated as low risk of bias, 30 studies (69.8%) as moderate, and all remaining studies rated as high risk of bias (supplementary Table 1).
Table 1.
Characteristics of studies reporting clinical characteristics of COVID-19.
| Study | Publication date | Enrolment duration | Maximum follow-up duration (days) | Duration between onset of symptoms and hospitalizati–on (median [range], days) | Study design (RCS/SD/PS) | City | No. of cases | Diagnosis method | Age (median/ mean [range/ IQR], years) | Males (%) | Traveled to or resident of Hubei Province (%) | No. Family -cluster (family) | Current Smokers (%) | Health workers (%) | Underlying diseases |
Severe Cases (%) | Diagnosis of severity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyper tension (%) | Diabetes (%) | Cancer (%) | Chronic respiratory /lung diseases (%) | Having any coexisting medical condition (%) | |||||||||||||||||
| Guan et al | Feb-06 | NA | NA | NA | PS | Multi-city* | 1099 | L | 47† (35–58) | 640 (58.2) | 676 (61.5) | NA | 137 (12.4) | 32 (2.9) | 164 (15.0) | 81 (7.4) | 10 (0.9) | 12 (1.1) | 255 (23.2) | 173 (15.7) | ATS |
| Chang et al | Feb-07 | NA | NA | NA | RCS | Beijing | 13 | NA | 34† (34–48) | 10 (77.0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Zhang et al | Feb- | Jan 18 -Feb 3 | NA | NA | RCS | Beijing | 9 | L | 36 (15–48) | 5 (55.0) | 7 (78.0) | 2 | NA | 1 (11.0) | NA | 1 (11.0) | 0 | NA | NA | NA | NA |
| Yu et al | Feb-17 | Jan 21 | NA | NA | RCS | Beijing | 40 | NA | 40 (21–57) | 26 (65.0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Zhuang et al | Feb-19 | Jan 1 -Feb 18 | 49 | NA | RCS | Beijing | 26 | L | 39.77† (3–79) | 18 (77.0) | 14 (54.0) | NA | NA | NA | 4 (15.0) | 3 (12.0) | NA | NA | 9 (35.0) | NA | NA |
| Li et al | Feb-10 | Jan 22 -Feb 10 | 20 | NA | RCS | Dazhou | 17 | L | 45 (22–65) | 9 (53.0) | 11 (65.0) | NA | 3 (18.0) | NA | 1 (6.0) | 0 | 0 | 0 | 3 (18.0) | NA | NA |
| Chung et al | Feb-06 | Jan 18 -Jan 27 | NA | NA | RCS | Guangzhou | 21 | L | 51† (29–77) | 13 (62.0) | 18 (86.0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Zhang et al | Feb-19 | Jan 19 -Feb 5 | 17 | NA | RCS | Nanjing | 42 | L | 43.02† (19–96) | 23 (55.0) | 23 (55.0) | 5 | NA | NA | NA | NA | NA | NA | 5 (12.0) | 0 | NA |
| Wang et al | Jan-30 | Jan 21 -Jan 24 | 14 | 4 (1–11) | RCS | Shanghai | 4 | L | 47.5 (19–63) | 3 (75.0) | 3 (75.0) | NA | NA | NA | NA | 0 | 0 | 0 | 1 (25.0) | 2 (50.0) | NA |
| Song et al | Feb-02 | NA | NA | NA | RCS | Shanghai | 51 | NA | 49 (16–76) | 25 (49.0) | 50 (98.0) | NA | NA | NA | 1 (2.0) | 3 (6.0) | NA | 1 (2.0) | NA | NA | NA |
| Lu et al | Feb-3 | NA | NA | NA | RCS | Shanghai | 50 | L | 50 (NA) | 28 (56.0) | 37 (74.0) | NA | NA | NA | 8 (16.0) | 3 (6.0) | NA | 4 (8.0) | 18 (36.0) | NA | NA |
| Chan et al | Jan-24 | Jan 10 -Jan 15 | 14 | 7 (6–10) | RCS | Shenzhen | 6 | L | 50 (10–66) | 3 (50.0) | 5 (83.3) | 1 | NA | NA | 2 (33.0) | 1 (17.0) | 1 (17.0) | 1 (17.0) | 4 (67.0) | NA | NA |
| Liu et al | Feb-09 | Jan 11 -Jan 20 | 10 | 8.5 (5–16) | RCS | Shenzhen | 12 | L | 63 (10–66) | 8 (67.0) | 11 (91.7) | 2 | NA | NA | 3 (25.0) | 2 (16.7) | 0 | 1 (8.0) | 7 (58.0) | 5 (42.0) | Guidelines |
| Wang et al | Feb-07 | Jan 1 -Jan 28 | 34 | 7 | RCS | Wuhan | 138 | L | 56 (22–92) | 75 (54.3) | 138 (100.0) | NA | NA | 40 (29.0) | 43 (31.2) | 14 (10.1) | 10 (7.2) | 4 (2.9) | 61 (44.2) | 36 (26.1) | ICU |
| Huang et al | Jan-24 | Dec 16 -Jan 2 | 37 | 7 (4–8) | PS | Wuhan | 41 | L | 49 (41–58)‡ | 30 (73.0) | 41 (100.0) | 1 | 3 (7.3) | NA | 6 (14.6) | 8 (19.5) | 1 (2.4) | 1 (2.4) | 13 (31.7) | 13 (31.7) | ICU |
| Liu et al | Jan-24 | Jan 10 -Jan 15 | 15 | 7 (1–20) | RCS | Wuhan | 137 | L | 57 (20–83) | 61 (44.0) | 137 (100.0) | NA | NA | NA | 13 (10.0) | 14 (10.0) | 2 (2.0) | 2 (2.0) | NA | NA | NA |
| Li et al | Feb-09 | NA | NA | NA | SD | Wuhan | 425 | L | 59 (15–89) | 240 (56.0) | 21 (50.0) | NA | NA | 15 (4.0) | NA | NA | NA | NA | NA | NA | NA |
| Chen et al | Jan-29 | Jan 1-Jan 20 | 25 | NA | RCS | Wuhan | 99 | L | 55.5 (21–82) | 67 (68.0) | 49 (49.0) | 1 | NA | NA | 0 | 13 (13.0) | 1 (1.0) | 1 (1.0) | 50 (51.0) | 23 (23.0) | ICU |
| Pan et al | Feb-6 | Dec 30 -Jan 31 | 31 | NA | RCS | Wuhan | 63 | L | 44.9† (NA) | 33 (52.0) | 63 (100.0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Pan et al | Feb-13 | Jan 12-Feb 6 | 26 | NA | RCS | Wuhan | 21 | L | 40 (25–63) | 6 (29.0) | 21 (100.0) | NA | NA | NA | NA | NA | NA | NA | NA | 0 | NA |
| Chen et al | Feb-4 | Jan 14 -Jan 29 | NA | NA | RCS | Wuhan | 29 | NA | 56 (26–79) | 21 (72.0) | 29 (100.0) | NA | 2 (7.0) | NA | 8 (28.0) | 5 (17.0) | 1 (3.0) | NA | 16 (55.0) | 14 (48.0) | Guidelines |
| Gong et al | Feb-18 | Dec 20 -Jan 22 | NA | NA | RCS | Wuhan | 33 | L | 51 (23–79) | 13 (39.0) | 33 (100.0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Zhong et al | Feb-13 | NA | NA | NA | RCS | Wuhan | 30 | L | 50 (22–81) | 18 (60.0) | 30 (100.0) | NA | NA | NA | NA | NA | NA | NA | 10 (30.0) | 8 (26.7) | Guidelines |
| Xia et al | Feb-18 | Jan 15 -Feb 8 | NA | (7.44±2.99) | RCS | Wuhan | 52 | L | 54 (23–82) | 24 (46.0) | 52 (100.0) | NA | NA | NA | 25 (48.0) | 26 (50.0) | NA | NA | NA | 12 (23.0) | Guidelines |
| Yang et al | Feb-21 | Dec 24 -Jan 26 | NA | NA | RCS | Wuhan | 52 | L | 59 (13.3) | 35 (67.0) | 52 (100.0) | NA | 2 (4.0) | NA | NA | 9 (17.0) | 2 (4.0) | 2 (4.0) | 21 (40.0) | 52 (100.0) | ICU |
| Du et al | Feb-9 | Jan 27 -Feb 1 | NA | NA | RCS | Xian | 7 | NA | 40 (24–55) | 4 (57.0) | 2 (28.5) | 3 | 0 | 0 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al | Feb-6 | NA | NA | NA | RCS | Xian | 10 | L | 41.8† (22–70) | 6 (60.0) | 9 (90.0) | NA | NA | NA | NA | NA | NA | NA | NA | 0 | NA |
| Liu et al | Feb-18 | NA | NA | NA | RCS | Xiaogan | 41 | L | 48 (19–64) | 32 (78.0) | 28 (68.0) | NA | NA | NA | 5 (12.0) | 2 (5.0) | NA | NA | NA | 5 (12.0) | NA |
| Xu et al | Feb-20 | Jan 10 -Jan 26 | NA | 2 (1–4) | RCS | Zhejiang | 62 | L | 41† (32–52) | 32 (58.0) | 62 (100.0) | NA | NA | NA | 5 (8.0) | 1 (2.0) | NA | 1 (2.0) | 20 (32.0) | 1 (2.0) | Guidelines |
| Yu et al | Feb-03 | Jan 21 -Feb 2 | NA | 5.5 (3–13) | RCS | Beijing | 25 | L | 37.9† (3–79) | 16 (64.0) | 23 (92.0) | 3 | NA | NA | 1 (4.0) | 3 (12.0) | NA | NA | NA | NA | NA |
| Huang et al | Feb-16 | Jan 23 -Feb 24 | NA | NA | RCS | Guangzhou | 35 | L | 44 (12–74) | 19 (54.0) | 20 (57.0) | NA | 5 (14.0) | NA | 1 (3.0) | 2 (6.0) | NA | 1 (3.0) | NA | NA | NA |
| Wang et al | Feb-15 | Jan 19 -Feb 3 | NA | NA | RCS | Zhejiang | 52 | L | 44 (13–73) | 29 (56.0) | 16 (30.0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Fang et al | Feb-25 | Jan 22 -Feb 18 | NA | NA | RCS | Hefei | 79 | L | 45.1† (5–91) | 18 (75.0) | NA | NA | NA | NA | 11 (46.0) | NA | NA | NA | NA | 24 (30.0) | Guidelines |
| Chen et al | Feb-19 | Jan 24 -Feb 8 | NA | 7 (4–9.5) | RCS | Wuhan | 54 | L | 58.5 (43–69) | 27 (50.0) | NA | NA | NA | NA | 13 (24.0) | NA | NA | NA | NA | 31 (57.0) | Guidelines |
| Xian et al | Feb-17 | Jan 21 -Jan 27 | NA | NA | RCS | Nanchang | 49 | L | 42.0† (18–78) | 33 (67.0) | 46 (94.0) | NA | 3 (6.0) | NA | 6 (12.0) | 2 (4.0) | NA | NA | NA | 9 (18.0) | Guidelines |
| Cao et al | Feb-28 | Jan 1 -Feb 15 | NA | NA | RCS | Wuhan | 36 | L | 72.5† (61–82) | 19 (55.5) | NA | NA | NA | NA | 17 (47.2) | 8 (22.2) | NA | 0.583 | NA | NA | NA |
| Li et al | Feb-24 | Jan 26 -Feb 6 | NA | NA | RCS | Anhui | 12 | L | 37 (21–71) | 8 (66.7) | 12 (100.0) | NA | 0.333 | NA | 2 (16.7) | NA | NA | NA | NA | 0 | NA |
| Sun et al | Feb-24 | Jan 21 -Feb 8 | NA | NA | RCS | Tianjin | 88 | L | 48.5† (9–91) | 49 (55.7) | 26 (29.5) | NA | NA | NA | 22 (25.0) | 10 (11.4) | NA | NA | NA | 32 (36.4) | Guidelines |
| Ji et al | Feb-24 | Jan 19 -Feb 1 | NA | NA | RCS | Jingzhou | 45 | L | 45.4† (21–67) | 27 (60.0) | 37 (82.2) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Wang et al | Feb-24 | Jan 1 -Feb 14 | NA | NA | RCS | Wuhan | 159 | L | 45.5† (20–84) | 66 (41.5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Yu et al | Feb-26 | Jan 17 -Jan 28 | NA | NA | RCS | Wenzhou | 40 | L | 45.9† (23–67) | 22 (55.0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| XIAO et al | Feb-27 | Jan 23 -Feb 8 | NA | NA | RCS | Chongqing | 143 | L | 45.1† | 73 (51.0) | 76 (53.0) | NA | NA | NA | 17 (12.0) | 10 (7.0) | NA | 4 (3.0) | NA | 36 (25.0) | Guidelines |
| Wu et al | Feb-28 | Jan 22 -Feb 14 | NA | NA | RCS | Jiangsu | 80 | L | 46.1† | 39 (49.0) | 80 (100.0) | 5 | NA | NA | 25 (31.0) | 5 (6.0) | 1 (1.0) | 1 (1.0) | NA | 3 (4.0) | Guidelines |
| Xu et al | Feb-19 | Jan 23 -Feb 4 | NA | NA | RCS | Guangzhou | 90 | L | 50 (18–86) | 39 (43.0) | 86 (96.0) | NA | NA | NA | 17 (19.0) | 5 (6.0) | 2 (2.0) | 1 (1.0) | 45 (50.0) | NA | Guidelines |
NA = Not available. RCS = Retrospective case series. SD = Surveillance data. PS = Prospective study. L = Laboratory-confirmed. Guideline = Guidelines of 2019-nCoV infection from the National Health Commission of the People's Republic of China. ICU = Being admitted to ICU. ATS = American Thoracic Society guideline on admission. All studies were published in 2020. December belongs to 2019. If there is no mark, the median and range were used to represent age. *All cases originated from 31 provinces, municipalities and autonomous regions other than Hubei province. †These values are average values. ‡These data are interquartile range.
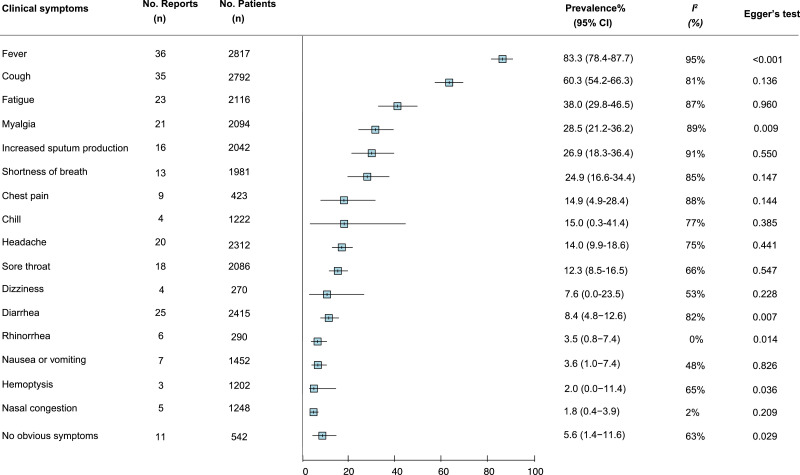
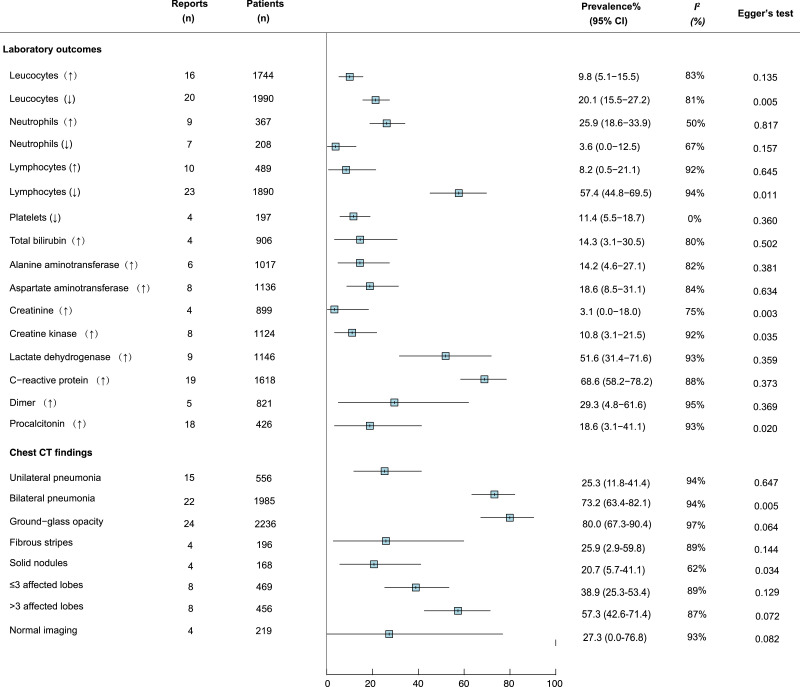
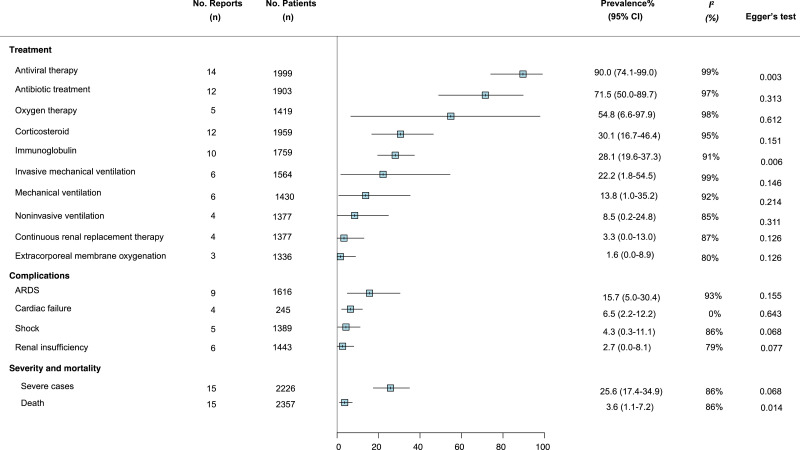
We meta-analysed the prevalence of 16 clinical symptoms among COVID-19 patients (Fig. 2 ). Fever (83.3% [95% CI 78.4–87.7]), cough (60.3% [54.2–66.3]), and fatigue (38.0% [29.8–46.5]) were the most common, followed by increased sputum production, shortness of breath, and myalgia, with estimated prevalence just under 30% for each, respectively. Eleven studies reported the proportion of COVID-19 patients who did not exhibit obvious symptoms, and the pooled estimated prevalence was 5.6% (1.4–11.6). Among 16 commonly reported laboratory findings (Fig. 3 ), the most common laboratory abnormalities were elevated C-reactive protein (68.6% [58.2–78.2]) and decreased lymphocyte count (57.4% [44.8–69.5]), as well as increased lactate dehydrogenase (51.6% [31.4–71.6]). Ground-glass opacities (80.0% [67.3–90.4]) and bilateral pneumonia (73.2% [63.4–82.1]) and were the most frequent chest CT findings (Fig. 3). The vast majority of patients received antiviral therapy (90.0% 74.1–99.0]), antibiotic treatment (71.5% [50.0–89.7]), and oxygen therapy (71•5% [28•0-99•7]). Acute respiratory distress syndrome (ARDS) was the most common complication (15.7% [5.0–30.4]). The overall estimated prevalence of severe case and death was 25.6% (17.4–34.9) and 3.6% (1.1–7.2), respectively (Fig. 4 ).
Fig. 2.
Meta-analysis of the prevalence of clinical symptoms among COVID-19 patients.
Fig. 3.
Meta-analysis of the prevalence of laboratory findings among COVID-19 patients.
Fig. 4.
Meta-analysis of the prevalence of chest CT findings, complications, severe cases, and mortality among COVID-19 patients
*Figure legend: ARDS=Acute Respiratory Distress Syndrome.
In subgroup analysis (supplementary Tables 2–5), studies from Wuhan had significantly higher prevalence of death, fever, fatigue, headache, elevated leukocyte count, and elevated lactate dehydrogenase, and elevated aspartate aminotransferase compared to patients from other cities (all p<0.05). Similarly, the prevalence of death, ARDS, headache, increased leukocyte count, and increased lactate dehydrogenase were significantly higher in studies in which the proportion of older patients was larger (all p<0.05), and the prevalence of diarrhea, and elevated lactate dehydrogenase were significantly higher in studies in which the proportion of patients with any coexisting medical condition was larger (all p<0.05). The prevalence of fatigue, myalgia, decreased leucocyte count were significantly higher in studies in which the proportion of male patients was smaller, whereas the reverse was true for the prevalence of elevated aspartate aminotransferase and lactate dehydrogenase (all p<0.05), though fatality did not differ by gender.
A total of eight studies reported separate results for severe cases and non- severe cases. Overall, the existence of clinical symptoms, abnormalities in laboratory and chest CT findings, and complications were higher among patients with severe illness compared to patients without severe illness (Table 2 ), however these differences were not statistically significant due to limited sample size and statistical power (data not shown).
Table 2.
Outcomes comparing severe cases and non-severe cases of COVID-19.
| Outcomes | Critical illness |
Non-critical illness |
||||||
|---|---|---|---|---|---|---|---|---|
| No. reports | No. patients | Prevalence% (95%CI) | I2 (%) | No. reports | No. patients | Prevalence% (95%CI) | I2 (%) | |
| Clinical symptoms | ||||||||
| Fever | 6 | 364 | 80.8 (41.1–100.0) | 97 | 6 | 1299 | 71.2 (23.8–99.9) | 98 |
| Cough | 6 | 364 | 65.6 (51.7–78.2) | 67 | 6 | 1299 | 56.7 (39.5–73.2) | 88 |
| Sore throat | 3 | 245 | 16.7 (0.0–53.2) | 77 | 3 | 1135 | 11.2 (3.5–22.4) | 63 |
| Increased sputum production | 3 | 222 | 32.1 (15.6–51.0) | 19 | 3 | 1065 | 31.4 (23.1–40.5) | 14 |
| Shortness of breath | 6 | 364 | 49.2 (21.5–77.2) | 90 | 5 | 1216 | 13.3 (2.2–30.9) | 85 |
| Myalgia | 5 | 351 | 17.6 (8.2–29.5) | 57 | 5 | 1201 | 20.8 (10.0–33.9) | 85 |
| Fatigue | 4 | 299 | 41.2 (5.2–84.0) | 92 | 5 | 1201 | 34.5 (13.2–59.6) | 93 |
| Diarrhea | 4 | 234 | 7.6 (0.0–24.0) | 55 | 3 | 1053 | 4.3 (0.1–12.5) | 54 |
| Headache | 4 | 274 | 11.3 (0.1–33.9) | 74 | 5 | 1172 | 11.9 (5.8–19.7) | 53 |
| Laboratory findings | ||||||||
| Leucocytes (↑) | 2 | 186 | 27.7 (0.0–100.0) | 91 | 3 | 838 | 9.3 (0.0–1.0) | 67 |
| Leucocytes (↓) | 3 | 216 | 33.7 (0.00–95.7) | 92 | 3 | 957 | 27.2 (24.3–30.1) | 0 |
| Lymphocytes (↓) | 3 | 203 | 81.5 (18.9–100.0) | 94 | 4 | 883 | 59.6 (32.2–84.2) | 99 |
| Platelets (↓) | 2 | 169 | 32.3 (0.0–100.0) | 93 | 3 | 740 | 16.4 (0.0–1.0) | 88 |
| Aspartate aminotransferase (↑) | 2 | 155 | 46.1 (0.0–100.0) | 56 | 3 | 653 | 15.5 (0.0–50.8) | 55 |
| Creatinine (↑) | 2 | 151 | 6.4 (0.0, 100.0) | 57 | 2 | 642 | 2.3 (0.0, 97.1) | 76 |
| Creatine kinase (↑) | 2 | 134 | 28.6 (0.0–100.0) | 76 | 3 | 563 | 16.7 (0.0–1.0) | 96 |
| Lactate dehydrogenase (↑) | 3 | 173 | 62.7 (55.7–100.0) | 83 | 3 | 818 | 28.1 (0.0, 100.0) | 99 |
| C-reactive protein (↑) | 2 | 171 | 40.3 (0.0–100.0) | 99 | 5 | 1026 | 51.2 (38.6–63.8) | 71 |
| D-dimer (↑) | 2 | 109 | 59.6 (50.2–68.7) | 0 | 1 | 451 | 43.2 (38.7–47.8) | 0 |
| Procalcitonin (↑) | 3 | 165 | 35.7 (0.0–100.0) | 95 | 4 | 660 | 55.2 (0.0–33.8) | 95 |
| Chest CT findings | ||||||||
| Bilateral pneumonia | 2 | 186 | 91.0 (0.0–100) | 83 | 1 | 926 | 39.7 (36.6–42.9) | 0 |
| Complications | ||||||||
| ARDS | 4 | 315 | 38.2 (3.2–83.0) | 96 | 2 | 130 | 4.3 (2.8, 6.0) | 0 |
| Cardiac failure | 4 | 155 | 17.1 (1.5–42.2) | 78 | 2 | 130 | 1.9 (0.0, 26.0) | 0 |
| Shock | 3 | 222 | 17.4 (0.0, 61.5) | 87 | . | . | . | . |
| Renal insufficiency | 5 | 328 | 9.8 (0.1–28.7) | 87 | . | . | . | . |
ARDS=Acute Respiratory Distress Syndrome.
Publication bias was found in the following subgroup outcomes: fever, myalgia, diarrhea, rhinorrhea, hemoptysis, decreased leucocytes, lymphopenia, increased creatine, creatine kinase, and procalcitonin, bilateral pneumonia, solid nodules, antiviral therapy, and immunoglobulin therapy (Figs. 2–4, all p<0.005 by Egger test). Substantial heterogeneity was present within most subgroups (Table 2 and Figs. 2–4).
Discussion
Our systematic review and meta-analysis of 43 studies involving 3600 patients provides the most comprehensive overview of clinical features, laboratory findings, chest imaging findings, disease severity, and CFR of COVID-19 patients. Compared with the only previous published systematic review on the subject, we included 31 additional studies performed detailed subgroup analyses. Particularly our results suggest CFR and proportion of severe cases are both declining as 2019-nCOV spreads away from Wuhan.
The dominant clinical features of COVID-19 were fever, cough, and fatigue, while congestion, rhinorrhea, sore throat and diarrhea are rare.13 , 16 , 19 , 24 The most frequently reported laboratory abnormalities were reduced lymphocyte count, elevated C-reactive protein, and elevated lactate dehydrogenase, all of which are generally consistent with previous reports of patients with COVID-19.11 , 19 , 24 However, all these laboratory markers are very non-specific, making their clinical utility limited. When evaluating suspected cases, physicians cannot rely on these laboratory abnormalities to exclude or confirm the diagnosis of COVID-19. These abnormalities are similar to those previously observed in patients with SARS and MERS.57, 58, 59 Previous research suggests these abnormalities may be related to the cytokine storm brought on by infection.22 Recently, a study suggested that COVID-19 may primarily affect T lymphocytes, especially CD4+ T cells, resulting in significant lymphopenia as well as decreased IFN- γ production.60 Additionally, by using a multiple linear regression model, a study showed that CD4+ T lymphocyte count may help predict the duration of viral RNA detection in patients’ stools (p = 0.010).61 However, the number of cases currently reported is too small to draw firm conclusions, and further studies are required. The most frequently reported finding on CT imaging was ground-glass opacities, particularly bilateral opacities impacting three or more lobes. These results are also consistent with previous studies,21 and are also frequently identified in MERS and SARS.57, 58, 59
In this systematic review and meta-analysis, we found a CFR of 3.6%, which is closer to the estimate (2.3%) in a report by the Chinese Center for Disease Control and Prevention (China CDC) that includes the epidemiological characteristics of 44,672 confirmed COVID-19 patients in mainland China (updated through February11, 2020).10 CFR may have been higher in earlier reports because of belated treatment during the earlier stages of the outbreak or a decline in fatality after sustained human-to-human transmission.1 , 14 , 19 Of note, roughly half of the studies included in our analysis were from outside Wuhan, the epicenter of the current outbreak, and our subgroup analysis found significantly lower prevalence of death among patients treated outside Wuhan. This may indicate fatality from COVID-19 is declining.
In our analysis, the proportion of severe cases (25.6%) was close to the estimate in the China CDC report (18.5%).10 This is consistent with previous studies that patients from Wuhan had significantly higher prevalence of death, fever, elevated leucocyte count, and elevated aspartate aminotransferase compared with patients from other cities in China (all p<0.05).1 , 14 , 19 Additionally, the China CDC report supports our finding that the overall CFR in Hubei (2.9%) is higher than that outside Hubei (0.4%).10 This interpretation could be supported by a study that showed lower fatality in patients who did not have direct contact with the site of the original disease.62 Similarly, the CFR, proportion of severe cases, ARDS, headache, increased leukocyte count, and increased lactate dehydrogenase were significantly higher in studies in which the proportion of older patients was larger (all p<0.05), which is consistent with previous publications.62 This finding suggests COVID-19 may disproportionately impact the elderly or people living with medical comorbidities. This is consistent with a single-center retrospective study found that older patients (>65 years) with comorbidities and ARDS were at increased risk of death.45 A multivariate Cox regression analysis results showed age and severe cases were identified as independent prognostic factors for virus clearance.62 Furthermore, a study showed that children might be less likely to become infected or, if infected, may show milder symptoms.16 Another study also confirmed that the elderly and those with comorbidities including diabetes, hypertension, cardiovascular disease, liver diseases, malignancy were more likely to develop critical illness (62.1%:25.0%, p<0.001).62
Our study did not find significant differences between men and women in terms of CFR and proportion of severe cases. This finding is similar to a previous study in which there was no difference in the proportion of men and women admitted to the intensive care unit (ICU) for treatment of COVID-19.6 However, this differs from another study which found that men are more susceptible to COVID-19 than women,63 as well as a recent publication reporting that seven of nine infant patients were female.64 There is no clear explanation as to why men and women would be at different risk of infection, however some have proposed genetic mechanisms or sex-specific effects.65 Whether there are differences in risk of infection between men and women requires further research.
We found the prognosis was worse among severe cases compared to non-severe cases, however these differences were not statistically significant, which is likely due to insufficient sample size. In our research, there was no significant difference in the degree of lymphocyte decline between severe cases and non-severe cases. This conclusion can be supported by this research that the expression level of lymphocyte counts has no significant correlation with the severity of the disease.22 However, some studies showed that lymphocytopenia is a prominent feature of severe cases.45 At present, it is unclear whether lymphocyte count is related to severity of disease. Further investigation is needed to establish whether lymphocytosis or lymphopenia can help predict mortality in COVID-19 patients.62
We found many patients were treated with antiviral and antibiotic therapy. Currently there is no treatment that can cure COVID-19. Supportive measures may reduce complications and fatality.14 The impact of antivirals and antibiotics on patients' prognosis remains unknown and requires further clinical evaluation. Currently, clinical trials of lopinavir / ritonavir (LPV/r) and remdesivir registered in the Chinese clinical trial registry are ongoing.
The recently published systematic review and meta-analysis on the clinical characteristics of 50,466 patients may reflect a combination of fallacies.9 Authors misuse fundamental terms. They mistake incidence for prevalence and odds ratio for proportion. They demonstrate the proportion of severe cases is 88% and case fatality rate is 42% in figures, which are misleading. PRISMA guidelines and test for heterogeneity were not mentioned. Authors state in Methods that “Only available data from published articles were collected. Data from unpublished papers were not included.” However 4 out of 10 references were from Medrxiv, a platform that publishes non-peer reviewed reports. These reports, as it clearly states on Medrxiv's website, should not be relied on to guide clinical practice or health-related behavior and should not be reported as established information. One reference providing 4021 cases was already withdrawn from publication.66 It is inappropriate to include the China CDC report providing epidemiological characteristics of 44,672 cases of COVID-19 (as of February 11, 2020) in a meta-analysis of its clinical characteristics.10 This report, based on national surveillance data, provides epidemiological data only, including spatiotemporal distribution. Albeit this report includes a large sample, data on clinical symptoms that are not systematically reported, may not be reliable. For example, 53% did not report if they have co-morbidity or not. 9 out 10 studies included in the meta-analysis were published/submitted before February 11, 2020 so cases in these 9 studies must have already been included in the China CDC report. It is inappropriate to count an individual twice. After excluding the China CDC report and the four preprint articles, only 369 patients would be reportable in that review. Authors did not list specific imaging performance in abnormal imaging, nor did they list pulmonary fibrosis and its incidence. However in Discussion they use two lengthy paragraphs to explain the content of pulmonary fibrosis, which may cause readers to mistakenly believe that the imaging abnormality is pulmonary fibrosis. Author failed to report any clinical laboratory findings and treatments of COVID-19 which are essential to a thorough understanding of clinical characteristics. They also failed to report the diagnostic criteria for abnormal chest CT detection and severe cases.
Our systematic review and meta-analysis has limitations. First, we found substantial heterogeneity between studies and significant publication bias among several subgroups. Second, this study performs an analysis during an ongoing outbreak. Many regions affected by COVID-19 haven not yet published clinical datasets, which may skew the results of this analysis. All these datasets are retrospective, which prevents us from exploring risk factors. Additionally, our meta-analysis focused on Chinese people, not those infected in other countries, so geographical and ethnic differences were not excluded. Finally, the meta-analysis was performed by comparing entire datasets against one another, therefore there was no way to analyze data on the level of individual patients.
Conclusion
This review provides a comprehensive characterization of clinical features among COVID-19 patients. Patients living in Wuhan, older patients, and those with medical comorbidities tend to have more severe clinical symptoms and higher fatality. Better therapeutics are crucial for the treatment of severe cases. Our comprehensive characterization of COVID-19 will inform healthcare providers and public health policy makers in their efforts to treat and control the current outbreak.
Contributors
HZ, YS and LL conceived the study and designed the protocol with LF and BW. LF, BW, TY and XC conducted study selection and data extraction. LF, WB, TY, XC, YA contributed to statistical analysis and interpretation of data. LF, BW, TY, XC and HZ drafted the manuscript with all authors critically revising the manuscript.
Declaration of Competing Interest
The authors declare having no conflict of interest related to this work.
Funding
This study was supported by the Natural Science Foundation of China Young Scientist Fund [81703278], the Australian National Health and Medical Research Commission (NHMRC) Early Career Fellowship (grant number APP1092621), the Precision Targeted Intervention Studies among High Risk Groups for HIV Prevention in China, National Science and Technology Major Project of China [2018ZX10721102], the Sanming Project of Medicine in Shenzhen [SZSM201811071], the Australian Research Council centre of Excellence for Mathematical and Statistical Frontiers [CE140100049], Infectious Disease Specialty of Guangzhou High-level Clinical Key Specialty (2019–2021).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.03.041.
Contributor Information
Linghua Li, Email: llheliza@126.com.
Yuelong Shu, Email: shuylong@mail.sysu.edu.cn.
Huachun Zou, Email: zouhuachun@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO main website. https://www.who.int(accessed March 2, 2020).
- 3.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.-j., Ni Z.-y., Hu Y., et al. Clinical characteristics of 2019 novel coronavirus infection in China. 2020: 10.1101/2020.02.06.20020974. [DOI]
- 8.National Health Commission of the People's Republic of China. http://www.nhc.gov.cn(Assessed on March 2, 2020). [DOI] [PMC free article] [PubMed]
- 9.Sun P, Qie S, Liu Z, et al. Clinical characteristics of hospitalized patientswith SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol 2020. doi:10.1002/jmv.25735. [DOI] [PMC free article] [PubMed]
- 10.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chinese J Epidemiol. 2020;41(2):145–151. [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020 doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Chen X., Lu Y. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined chinese and western medicine treatment. Biosci Trends. 2020 doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 13.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kui L., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung M, Bernheim A. CT Imaging Features of 2019 Novel Coronavirus(2019-nCoV). Radiology 2020 Apr;295(1):202-207. [DOI] [PMC free article] [PubMed]
- 16.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, ofnovel coronavirus-infected pneumonia. N Engl J Med 2020.doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed]
- 17.Song F, Shi N, Shan F, et al. Emerging 2019 Novel Coronavirus(2019-nCoV) Pneumonia. Radiology 2020:200274. [DOI] [PMC free article] [PubMed]
- 18.Chang, Lin M., Wei L. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes inpatients with the novel coronavirus pneumonia (2019-nCoV): a study of 63patients in Wuhan, China. Eur Radiol 2020.doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed]
- 21.Pan F, Ye T. Time course of lung changes on chest CT during recovery from2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020:200370.doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed]
- 22.Chen L., Liu H.G., Liu W. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M.Q., Wang X.H., Chen Y.L. [Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E013. doi: 10.3760/cma.j.issn.1001-0939.2020.0013. [DOI] [PubMed] [Google Scholar]
- 24.Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patientsinfected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan,China: retrospective case series. BMJ 2020;368:m606. [DOI] [PMC free article] [PubMed]
- 25.Wu J., Liu J., Zhao X. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020 doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M., An W., Xia F. Retrospective analysis of COVID-19 patients with different clinical subtypes. Herald of Med. 2020:1–12. [Google Scholar]
- 28.H X., N L., L F. Analysis of Chinese medical characteristics of 35 patients with novel coronavirus pneumonia. J Emergen Tradit Chinese Med. 2020:1–4. [Google Scholar]
- 29.D Y.H., J C.W., Y J. Clinical features and ct signs of early family clustering novel coronavirus pneumonia. J Xi'an Jiaotong Uni. 2020:1–7. [Google Scholar]
- 30.Y X., Y H., Y S. Chest CT features of COVID⁃19. J Pract Med. 2020:1–3. [Google Scholar]
- 31.G L., Z J.P., D Y.H. CT features of patients with imported 2019-nCov-pneumonia. J Xi'an Jiaotong Uni. 2020:1–9. [Google Scholar]
- 32.G X.M., L H., S L. Preliminary explore on ct characteristics of corona virus disease 2019 (COVID-19) Radiol Pract. 2020:1–5. [Google Scholar]
- 33.X K., S L., P X. The clinical features of the 143 patients with COVID-19 in north-east of Chongqing. J Third Military Med Uni. 2020:1–5. [Google Scholar]
- 34.X F, Q M, T Y, et al. Clinical features and treatment analysis of 79 cases ofCOVID-19. Chinese pharmacological bulletin 2020:1–7.
- 35.L F.M., D H.L., G X.M. Chest CT performance and clinical characteristics of corona virus disease 2019 (COVID-19) Radiol Pract. 2020:1–3. [Google Scholar]
- 36.L Y.F., Y Z.G., W M. Analysis on chinese medical clinical characteristics of 50 patients with 2019-nCoV-infected pneumonia. Academic Journal of Shanghai University of Traditional Chinese Medicine. 2020:1–5. [Google Scholar]
- 37.T. X, J. L, F. X, et al. Analysis of clinical characteristics of 49 patients with novel coronavirus pneumonia in Jiangxi province. Chinese J Resp Critical Care Med:1–7.
- 38.J G., H M., Z Q. CT manifestations and dynamic changes of corona virus disease 2019. Chinese J Med Imaging Technol. 2020:1–6. [Google Scholar]
- 39.C J., Z J., L X. Clinical characteristics and ct signs of corona virus disease 2019 (COVID-19) in the elderly. Med J Wuhan Uni. 2020:1–4. [Google Scholar]
- 40.Y S., W Z., Q E. Analysis of clinical characteristics of 25 patients with coronavirus disease in 2019. Chinese J Integrated Trad Western Med. 2020:1–2. [Google Scholar]
- 41.W J., L J., W Y. Dynamic changes of chest ct imaging in patients with corona virus disease-19 (COVID-19) J Zhejiang Uni (Med Sci) 2020:1–13. doi: 10.3785/j.issn.1008-9292.2020.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun H., Bi Y., Zhu Z. Analysis of Chinese medical characteristics of 88 patients with novel coronavirus pneumonia in Tianjin. J Tradit Chinese Med. 2020:1–4. [Google Scholar]
- 43.X G., A C., Z C. Clinical study on treatment of 34 cases of COVID-19 with combination of traditional Chinese and western medicine. Journal of Traditional Chinese Medicine. 2020:1–7. [Google Scholar]
- 44.W Y., C J., W X. CT image features of asymptomatic patients with novel coronavirus. Med J Wuhan Uni. 2020:1–5. [Google Scholar]
- 45.Xiaobo Yang Y.Y., Xu Jiqian, Shu Huaqing, Xia* Jia'an, Liu* Hong, Wu Yongran, Zhang Lu, Yu Zhui, Fang Minghao, Yu Ting, Wang Yaxin, Shangwen Pan X.Z., Yuan Shiying, Shang You. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in wuhan, china: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Pan H., Shu J. Clinical presentations and ct features of imported corona virus disease 2019. Chinese J Med Imaging Technol. 2020:1–4. [Google Scholar]
- 47.Y S.M., C Y.F., ZX W. Analysis of the relationship between clinical features and tongue manifestations of 40 cases with novel coronavirus pneumonia. Beijing J Trad Chinese Med. 2020:1–8. [Google Scholar]
- 48.Z X., L L., D G.C. A preliminary study on the clinical characteristics and Chinese medical syndrome of 42 cases of COVID-19 in Nanjing. J Nanjing Uni Trad Chinese Med. 2020:1–5. [Google Scholar]
- 49.Z F.Y., Z H.F., W B.C. CT findings in 2019 novel coronavirus disease (COVID-19) patients. Med J Wuhan Uni. 2020:1–5. [Google Scholar]
- 50.Z Y.J., C Z., L J. Clinical and epidemiological characteristics of 26 patients diagnosed with novel coronavirus pneumonia. Chinese J Nosocomiol. 2020:1–4. [Google Scholar]
- 51.Liberati A., Altman D.G., Tetzlaff J. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-anal Observat Stud Epidemiol (MOOSE) Group. Jama. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 53.American National Institute of Health. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 54.Barendregt J.J., Doi S.A., Lee Y.Y. Meta-analysis of prevalence. J Epidemiol Commun Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 55.Higgins J.P., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egger M., Davey Smith G., Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung W.K., To K.F., Chan P.K. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peiris J.S., Chu C.M., Cheng V.C. Clinical progression and viral load in a community outbreak of coronavirus-associated Sars pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assiri A., McGeer A., Perl T.M. Hospital outbreak of middle east respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen G., Wu D., Guo W., et al. Clinical and immunologic features in severe and moderate forms of coronavirus disease 2019. 2020:medRxiv2020.02.16.20023903.
- 61.Ling Y., Xu S.B., Lin Y.X. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseaseshospitaloutside Hubei province, China. medRxiv 2020.doi: 10.1101/2020.02.17.20024018. [DOI] [PubMed]
- 63.Qian K, Deng Y, Tai Y, et al. Clinical characteristics of 2019 novel infectedcoronavirus pneumonia:a systemic review and meta-analysis. medRxiv 2020.doi: 10.1101/2020.02.14.20021535. [DOI]
- 64.Wei M., Yuan J., Liu Y. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schurz H., Salie M., Tromp G. The x chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. 2019;13:2. doi: 10.1186/s40246-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y, Lu Q, Liu M, et al. Epidemiological and clinical features of the 2019novel coronavirus outbreak in China. medRxiv 2020.doi: 10.1101/2020.02.10.20021675. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.