Abstract
Background
The emergence of many infectious diseases has been of serious public health implication in the 21st century. Hospital preparedness is a key step in strengthening a country’s ability to address any public health emergency of international concern caused by these diseases. In India, because 80% of the health-care utilization happens in the private hospitals, it is of at most importance to assess the preparedness level of these hospitals against emerging infectious diseases.
Methods
The study was a cross-sectional study, and hospitals which provided consent were included. The estimated participants for the study were 54.
Results
The results were expressed in a descriptive manner. For the purpose of analysis, the questionnaire was redistributed based on the monitoring and evaluation framework of International Health Regulations and its core capacities. It was found that there was a need to enhance the preparedness of the hospitals in the response against emerging infectious diseases. There were gaps in the implementation of various plans and protocols for staff training, risk communication, surge capacity, laboratory capacity, and infection control in the hospitals.
Conclusion
The findings were suggestive of a need for preparedness of the hospitals against the upsurge of emerging infectious diseases.
Keywords: Health-care facilities, Hospital preparedness, Emerging infectious disease, Pandemic planning
Introduction
The world today is impacted by emerging infectious diseases on an unprecedented scale. The emerging infectious diseases have the potential to be a public health emergency of international concern and can have major impact on the health and well-being of communities, health systems, steadiness of national economies, and progress toward the sustainable development goals. “Emerging infectious disease (EID) is the one that has appeared and affected the population for the first time or has existed previously or rapidly increasing either in terms of the number of population affected or its spread to the new geographical area”.1, 2, 3 They are considered to be a major public health problem because of its potential to be used as biological warfare agents and can have a devastating effect on animal and environmental health.4,5 One of the other major challenges in combating these diseases is their unpredictable nature and their ability to assume pandemic proportions.2 Their unpredictable nature also makes it difficult to control the diseases and can cause the spread of infection even across national boundaries.1 The emerging infectious disease accounts for 26% of the death worldwide and 28% of the global burden of infectious disease.1,3 In South East Asia, out of the 14 million deaths annually, 40% is due to EIDs.1 In India, infectious diseases are responsible for half of the total disease burden.3 For many centuries, infectious diseases such as plague and smallpox have claimed millions of lives.6 These lead to the adoption of various measures of public health importance after many years.6 Series of incidents in succession to these events lead to the establishment of International Health Regulations (IHRs) in 1969.7,8 But, the requirement for the transformation of IHR was accelerated after the 2002 severe acute respiratory syndrome infection because of its transcontinental transmission.7 Hence in 2005, IHR was evolved into a full-scale framework with the purpose of building international coordination in the face of a public health emergency of international concern.9
In Indian context, one of the major events of public health concern occurred in Surat, 1994, which triggered a mass alarm at the national level, which created severe economic loss and disrupted social life.6 This was followed by many large outbreaks of cholera, diphtheria, scrub typhus, Nipah virus encephalitis, Chandipura virus, H5N1, H1N1, Crimean-Congo hemorrhagic fever, dengue, chikungunya, and Japanese Encephalitis across the country.2 The risks of infectious disease outbreaks exhibit the need for more effective collaboration on health security and pandemic preparedness. Hence to enhance the infectious disease surveillance and epidemiological capacity in the country, the government established the Integrated Disease Surveillance Project (IDSP) in 2004.10,2 The project, in the long run, got transformed into a program which was decentralized so that there could be immediate detection and response to an outbreak.10,2
During an epidemic or pandemic scenario, hospitals will usually be in the frontline to provide necessary care to the community, and in case of an extended or combined outbreak, there could be overwhelming of the health systems, if the institutions are not adequately prepared.11 Other complexities involved if the hospitals are inadequately prepared is that they might not be able to meet the health-care requirements and control the disease because of various reasons such as overwhelming demand, limited time to coordinate with other stakeholders, and integration of each individual hospital into the response strategy.12 Hence to prevent these complex consequences, it is important to have prior preparedness plans, response protocols, and standards which is the first step needed to enhance countries preparedness toward an event of public health importance.13,14
Material and methods
The design of the study was cross-sectional in nature and was conducted between January 2019 and May 2019. Private hospitals in the district were approached for the study, and hospitals which granted permission were included. Purposive sampling was used, and the participants included in the study were hospital administrators, doctors with experience in treating infectious diseases, nursing superintendent, microbiologist, and lab technician. A total of 6 participants were estimated to be included from each of the 9 hospitals; therefore, the sample size was determined as 54. All the hospitals which granted consent to be included in the study were secondary care hospitals. Before the initiation of the study, ethical clearance was sought from the institutional ethics committee. Before the data collection appointments were taken from the participants, the participant information sheet was provided, and informed consent was taken. A predesigned, semistructured, and validated questionnaire was administered, and responses were recorded. The questionnaire had a separate section of questions, one for all the participants (n = 49) and the others specific for administrative staff and doctors (n = 36) and for laboratory personnel's (n = 13). The data analysis was undertaken with the help of Microsoft Excel and was expressed in percentages and frequencies.
Results
For the purpose of analysis, the questions were redistributed under various domains of monitoring and evaluation framework of IHRs. These core capacities were further distributed under prevent, detect, and response domains. Prevent section constituted the core capacities such as national legislation, policy, and financing, IHR coordination, communication, and advocacy, antimicrobial resistance, zoonotic disease, and biosafety and security.8 The detect part of the framework included national laboratory system, surveillance, reporting, human resources, and finally, in the response, capacities such as emergency preparedness, emergency response operations, linking public health and security authorities, medical countermeasures and deployment, and risk communication were incorporated.8
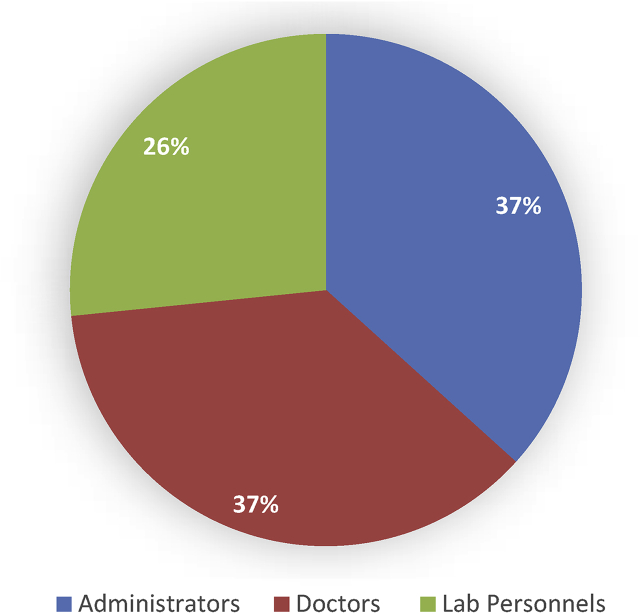
Out of the estimated participants, i.e. 54, only 49 participated in the study as 5 hospitals had the position of microbiologists vacant. The study comprised of 18 (36.7%) participants from administrative departments and 18 doctors (36.7%) and 13 participants (26.6%) from the laboratory department (Fig. 1).
Fig. 1.
Distribution of study participants based on profession (n = 49).
Prevent
In national legislation, policy, and financing, a total of 72.22% (n = 36) of administrative staff participants were not able to mention the floor area of their respective hospitals. Most participants (30.5%, n = 36) were not aware of the number of wards present in the hospital. According to maximum participants (38.33%, n = 36), their respective institutions had a bed capacity of 100–150 beds.
According to a maximum of 58.33% (n = 36) they had medical intensive care unit in the hospital, and only a minimum of 5.5% (n = 36) had postoperative intensive care unit in their respective hospitals.
In the context of IHR coordination, communication, and advocacy, IDSP was the reporting point for infectious diseases only to 4.08% (n = 49) and for 77.55% reporting point was district medical officer.
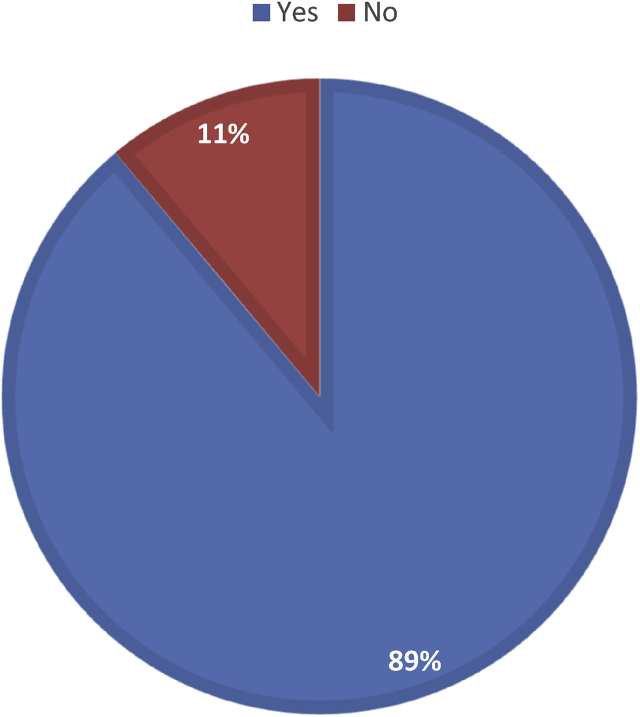
In terms of antimicrobial resistance, 11.11% hospitals (n = 9) did not have an infection control committee in place (Fig. 2), and as for zoonotic diseases, reporting pathway was the same for all infectious diseases even if it was of animal origin according to 97.95% (n = 49).
Fig. 2.
Distribution of hospitals based on presence of infection control committee (n = 9).
Detect
According to various domains in the national laboratory system, all the hospitals (n = 13) had basic laboratory testing facilities. Lab personnel in 66.66% of hospitals (n = 9) said that they had only access to screening tests. Regarding the referral system of these hospitals, even though all the institutions had referral labs outside the district only 53.84% (n = 13), lab personnel were aware of the existence of referral laboratories being present inside the district.
In surveillance, 81.63% (n = 49) were not aware of the reporting systems for infectious diseases of animal origin at the district level (Fig. 3). Regarding the reporting capacity, all the participants (n = 49) were aware of the procedure regarding reporting of infectious diseases at the hospital level. All the participants (n = 13) mentioned that report dissemination of laboratory samples was undertaken in less than 24 hrs during an outbreak scenario.
Fig. 3.
Awareness of participants related to existence of reporting systems for infectious diseases of animal origin at the district level.
In terms of human resources, according to 63.26% (n = 49), the staff were trained in surveillance and management of the outbreak. 16.66% (n = 36) of the administrative staff was not aware of the number of departments and the number of staff present in the hospitals .
Respond
According to various contexts of emergency preparedness, infection control plan was present in the hospital according to 69.38% (n = 49). Based on 61.11% of respondents (n = 36), isolation wards were not available in their respective hospitals (Table 1). Regarding the strategic plan for maintaining medicines and vaccines in hospitals, 97.22% (n = 36) agreed to the existence of a plan. The surge capacity plan was not in place for 63.9% (n = 36). A total of 92.31% laboratory personnel (n = 13) mentioned that there was an existing protocol in place for handling infectious diseases samples at the laboratory (see Table 2).
Table 1.
Distribution of participants across the study centers (n = 49).
| Study centers | Number of participants |
|---|---|
| Hospital 1 | 6 |
| Hospital 2 | 5 |
| Hospital 3 | 5 |
| Hospital 4 | 6 |
| Hospital 5 | 6 |
| Hospital 6 | 6 |
| Hospital 7 | 5 |
| Hospital 8 | 5 |
| Hospital 9 | 5 |
Table 2.
Details of hospitals based on the facilities available (n = 9).
| Facilities | Percentage |
|---|---|
| Bed capacity | |
| <50 | 33.3% |
| 50–100 | 11.1% |
| 100–150 | 44.5% |
| 150–200 | 11.1% |
| Presence of isolation wards | |
| Yes | 39% |
| No | 61% |
In emergency response operations, the distance maintained between beds was less than 1 m according to 22.2% (8 out of 36) in wards and according to 11.11% in the intensive care unit.
As for the medical countermeasures and deployment capacity, according to 83.3% (n = 36), there was stockpiling of medicines, and 66.66% (n = 36) stated the presence of stockpiling of vaccines. The stockpiling of personal protective equipment was in place according to 97.22% (n = 36) of the respondents. However, all the laboratory personnel agreed to the presence of stockpiling of laboratory supplies in their respective hospitals. For the risk communication, 77.77% (n = 36) informed the absence of protocol in place for reporting any case of outbreak to the media.
Discussion
In a hospital, the desirable bed spacing in wards was advised to be 1 m.15 In the current study, the responses of 22.22% were discordant with the guidelines such as Clinical Establishment Act standard for hospitals and the National Accreditation Board for Hospitals and Health-Care Providers (NABH), whereas 38.88% were in concordance, and the final 38.88% were not aware of the details regarding the same.
Guidelines were suggestive of documentation of infection prevention and control program in a private health facility, whereas, in the study, it was determined that 69.38% of the administrative staff and doctors agreed for the same while 28.5% denied the presence of the same in their respective hospitals.16 Based on the guidelines, the preferred members for the infection control committee include the hospital administrator, microbiologist, physician/infection control specialist, surgeon, staff from Central Sterile Service Department, other supportive services, infection control nurse, and if required invitees from other departments.16 In the study, 24.4% were in agreement to the presence of most of the members of the infection control committee in the hospitals according to the guidelines.
According to protocol, there should be the presence of written guidelines for the handling of infectious diseases and hazardous material.16 The laboratory personnel (92.3%) suggested the presence of the protocol for infectious diseases sample, whereas 7.7% of the study participants stated its absence in the hospital.
There were certain infections that have been categorized as notifiable diseases, and according to NABH guidelines if detected, the hospitals were required to report these diseases to the appropriate authorities.16 The hierarchy of reporting differed across the participants in the study, and integrated disease surveillance program unit was the reporting point for only 4.08%, District Medical officer for 77.55%, government hospitals for 10.2%, and hospital authority for 2.04%.
The guidelines also recommend the availability of policies regarding the storage of medications in the respective hospitals.16 Based on the response given by the administrative staff and doctors, 97.22% assured the presence of well-documented plan for maintaining of medicines at the hospitals, and it was absent for 2.78% at their respective hospitals. Personal protective equipment stockpiling is an important aspect of the hospital preparedness, and national standards suggest maintaining of adequate inventories that include gloves, goggles, mask, apron, gown, shoe covers, and head covers.16 According to all the included participants of the present study, there was stockpiling of gloves, mask, and disposable gown, but only 72.2% agreed to the presence of goggles, 77.7% informed regarding the head covers stock, and 94.4% for the shoe cover stockpiling. The limitation of the study was that only one-third of the private hospitals from the entire district were covered in the study.
The need of the hour is to enhance the focus on deployment of expert staff and material resources early in the event to ensure an effective assessment, establishing coordination with partners for collective response, and to develop evidence-based capacity which can aid in the preparedness of the hospitals. Regular assessment of the progress regarding the preparedness can assist in improving and maintaining the preparedness of hospitals against these emerging infectious diseases.
Conflicts of interest
All authors have none to declare.
Acknowledgments
The authors would like to acknowledge all the support provided by Prasanna school of Public Health, during the course of this study. They would also like to extend their thanks to Manipal Academy of Higher Education for all the logistics support.
References
- 1.Combating Emerging Infectious Diseases in the South-East Asia Region. World Health Organization; 2005. pp. 1–36. [Google Scholar]
- 2.Mukherjee S. Emerging infectious diseases: epidemiological perspective. Indian J Dermatol. 2017;62(2):459–467. doi: 10.4103/ijd.IJD_379_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dikid T., Jain S.K., Sharma A., Kumar A., Narain J.P. Emerging & re-emerging infections in India: an overview. Indian J Med Res. 2013 Jul;138(1):19–31. [PMC free article] [PubMed] [Google Scholar]
- 4.Rotz L.D., Khan A.S., Lillibridge S.R., Ostroff S.M., Hughes J.M. Public Health Assessment of Potential Biological Terrorism Agents. Emerg Infect Dis. 2002 Feb doi: 10.3201/eid0802.010164. http://www.ncbi.nlm.nih.gov/pubmed/11897082 Centers for Disease Control and Prevention; [cited 2019 Jul 1];8(2):225. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookes V.J., Hernández-Jover M., Black P.F., Ward M.P. Preparedness for emerging infectious diseases: pathways from anticipation to action. Epidemiol Infect. 2015;143(10):2043–2058. doi: 10.1017/S095026881400315X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrus J.K., Aguilera X., Oliva O., Aldighieri S. Global health security and the international health Regulations. BMC Publ Health. 2010;10(suppl 1):S2. doi: 10.1186/1471-2458-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gostin L.O., Rebecca K. The international health Regulations: the governing framework for global health security. Milbank Q. 2016;94(2):264–313. doi: 10.1111/1468-0009.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International health regulations . World Health Organization; 2005. Monitoring and Evaluation Framework; p. 20. 2005. [Google Scholar]
- 9.Garg S., Basu S., Dahiya N. International Health Regulations: Is India prepared after 10 Years of implementation? Indian J Comm Dis. 2017;3(1):23–27. [Google Scholar]
- 10.Mankar M., Pinto V. International health regulation- A review article. Indian J Prev Soc Med. 2009;40(3):120–125. [Google Scholar]
- 11.Hospital Preparedness Checklist for Pandemic Influenza: Focus on Pandemic (H1N1) 2009. World Health Organization; 2009. pp. 1–35.http://www.euro.who.int/__data/assets/pdf_file/0004/78988/E93006.pdf Internet. Available from: [Google Scholar]
- 12.Hospital Preparedness for Epidemics. World Health Organization; 2014. pp. 1–7. [Google Scholar]
- 13.Reidy M., Ryan F., Hogan D., Lacey S., Buckley C. Preparedness of hospitals in the republic of Ireland for an influenza pandemic , an infection control perspective. BMC Publ Health. 2015;15:847. doi: 10.1186/s12889-015-2025-6. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X., Huang J., Zhang H. An analysis of hospital preparedness capacity for public health emergency in four regions of China : Beijing , Shandong , Guangxi , and Hainan. BMC Publ Health. 2008;8:319. doi: 10.1186/1471-2458-8-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Establishment Act Standard for Hospital ( Level 2 ) Central Government of India; 2012. p. 36. [Google Scholar]
- 16.Guide Book to Accreditation Standards December 2015 National Accreditation Board for Hospitals and Healthcare Providers ( NABH ) National Accreditation Board for Hospitals and Healthcare Providers; 2015. p. 240. [Google Scholar]