Abstract
We performed an overview of systematic reviews and meta-analyses to summarize available data regarding the association between frailty and all-cause mortality. Medline, Embase, CINAHL, Web of Science, PsycINFO, and AMED (Allied and Complementary Medicine) databases were searched until February 2020 for meta-analyses examining the association between frailty and all-cause mortality. The AMSTAR2 checklist was used to evaluate methodological quality. Frailty exposure and the risk of all-cause mortality (hazard ratio [HR] or relative risk [RR]) were displayed in forest plots. We included 25 meta-analyses that pooled data from between 3 and 20 studies. The number of participants included in these meta-analyses ranged between <2000 and >500,000. Overall, 56%, 32%, and 12% of studies were rated as of moderate, low, and critically low quality, respectively. Frailty was associated with increased risk of all-cause mortality in 24/24 studies where the HR/RRs ranged from 1.35 [95% confidence interval (CI) 1.05–1.74] (patients with diabetes) to 7.95 [95% CI 4.88–12.96] (hospitalized patients). The median HR/RR across different meta-analyses was 1.98 (interquartile range 1.65–2.67). Pre-frailty was associated with a significantly increased risk of all-cause mortality in 7/7 studies with the HR/RR ranging from 1.09 to 3.65 (median 1.51, IQR 1.38–1.73). These data suggest that interventions to prevent frailty and pre-frailty are needed.
Keywords: frailty, mortality, evidence synthesis, meta-analyses, umbrella review
1. Introduction
Frailty, defined as a geriatric syndrome characterised by increased vulnerability to even minor stressors and decreased physiological reserve [1,2], is common among older people. Globally, it has been estimated that about 4.3% of older adults aged 60 years and older will develop frailty per year [3]. However, the prevalence of frailty varies widely among older people: 4.0–59.1% in community-dwelling older adults [4], 10.4–37.0% in general surgery patients [5], 6.0–86.0% in cancer patients [6], and 19.0–75.6% in nursing home residents [7].
Numerous studies have shown frailty to be associated with many negative health outcomes including increased risk of hospitalizations [8], disability [9], falls [10], fractures [11], delirium [12], and institutionalization [13]. Owing to these adverse effects, frailty is associated with higher healthcare use and cost burden [14,15,16].
Moreover, frailty has been associated with increased risk of mortality and premature death [1,17,18], and this effect has been suggested to be stable across different generational cohorts [19]. In particular, Shamliyan and colleagues have suggested that 3–5% of deaths among community-dwelling adults could be averted if there are concerted efforts to prevent frailty [20]. The exact mechanisms underpinning increased mortality among people who are frail remains unclear, although, there are suggestions that reduced physical and cognitive functions associated with frailty leads to poor prognosis [21,22]. Others have also indicated that it is the underlying clinical conditions associated with frailty that are ultimately the cause of death [23,24]. For example, an underlying physical or even psychological condition can give rise to weight loss, which contributes to the development of frailty [25]. Whether or not the effects of frailty on mortality risk are ubiquitous or more profound in selected subgroups is also unclear.
Thus, this umbrella review summarizes evidence generated from systematic reviews and meta-analyses that examined the association between frailty and all-cause mortality across different population groups and settings.
2. Methods
This review was performed according to the recommendations outlined in the Meta-analysis of Observational Studies in Epidemiology [26], the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statements [27] (Supplementary Table S1), and the Cochrane Collaboration Handbook [28].
2.1. Search Strategy and Study Selection
A systematic literature search of Medline, Embase, CINAHL, Web of Science, PsycINFO, and AMED (allied and complementary medicine) databases for systematic reviews and meta-analyses examining the association between frailty and the risk of all-cause mortality. The search was first performed in August 2019 and was last updated in February 2020. The main keywords used included “frailty OR frailty syndrome OR frail elderly OR geriatric syndrome OR geriatric disorder” AND “systematic review OR meta-analysis OR review”. The full search strategy is presented in Supplementary Table S2. In addition, the reference lists of included systematic reviews and meta-analyses were manually screened. The literature search was independently performed by two reviewers (RO and KLC), and disagreements were resolved by consensus involving a third author (BWS).
2.2. Selection Criteria
The inclusion criteria were as follows: (1) participants: any population; (2) study design: meta-analyses that reported pooled risk estimates (hazard ratios [HRs] or risk ratios [RRs]; (3) exposure: frailty or pre-frailty as defined by any criteria; and (4) outcome: risk of all-cause mortality. We restricted the review to only studies reporting HRs and RRs as these are measures are largely comparable, and in other reviews have been pooled together [28]. The exclusion criteria were as follows: (1) studies published in languages other than English; (2) meta-analyses that did not follow the methodology of a systematic review; (3) meta-analyses that pooled frailty scores without categorisation; and (4) systematic reviews without meta-analysis.
2.3. Data Extraction
Two researchers (KLC and BWS) independently extracted the following data from the original studies using a standardized data collection form: (1) author identification and year of publication; (2) databases searched for systematic review; (3) search date; (4) number of studies included; (5) age of the participants; (6) length of follow-up; (7) number of frail and non-frail populations; (8) frailty assessment tool used; (9) reported risk estimates; (8) heterogeneity reported; and (9) AMSTAR2 risk of bias rating [29]. We also extracted age- or sex-specific risk estimates if reported. Where studies reported effect of frailty at different follow-up periods, we prioritized the data on long-term mortality effects. Disagreements in data collection were resolved by discussion involving a third author (RO).
2.4. Quality Assessment
Two investigators (BS and KLC) independently assessed the methodological quality of each study after concealment of information about the authors, affiliations, date and source. A standardized checklist, the AMSTAR2 appraisal tool, for reporting systematic reviews and meta-analyses was used [29]. This checklist includes 16 criteria, each referring to a relevant methodological aspect of the study. For each review, we applied a rating of high, moderate, low, or critically low for the overall confidence in the results. The ratings were performed by two reviewers (BS and KLC) and any disagreements were resolved via consensus involving third reviewer (RO).
2.5. Data Synthesis
To depict the relationship between frailty and all-cause mortality, the risk estimates (HR/RR) and corresponding 95% confidence intervals (CIs) for each meta-analysis were displayed in a forest plot [30,31,32]. Using the point HR/RR from each study, we also calculated the median risk estimate along with corresponding interquartile range (IQR) to describe the distribution of the reported effect of frailty on all-cause mortality. The level of heterogeneity across individual meta-analysis was assessed via the I2 statistic, and depending on its values heterogeneity was considered as the following: might not be important (0–40%), may represent moderate heterogeneity (30–60%), substantial heterogeneity (50–90%), or considerable heterogeneity (75–100%) [28]. Forest plots were produced using StataSE software version 16 (StataCorp, TX, USA), which displayed data from original meta-analyses without including any additional analyses.
3. Results
3.1. Characteristics of Included Studies
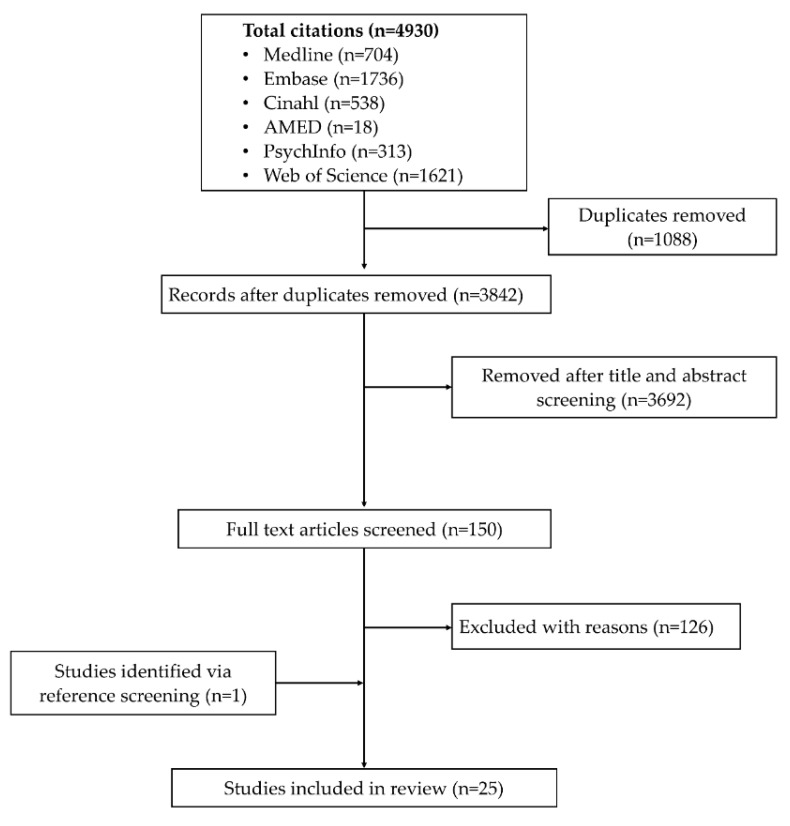
Our search identified 4930 citations. A total of 150 articles were selected for full text examination while others were removed following initial screening based on titles and abstract or duplicates. Of the 150 articles, 24 were eligible for analysis. One additional study was retrieved via reference screening. The studies’ selection process is summarised in Figure 1.
Figure 1.
Flow chart of studies’ selection process.
The descriptive characteristics of the included studies are presented in Table 1. The meta-analyses were published between 2015 and 2019 and included between three and 24 studies with sample sizes ranging from <2000 to >500,000 participants. All the systematic reviews and meta-analyses conducted their search in two or more databases. The meta-analyses focused on community-dwelling older adults (n = 3); patients who had undergone transcatheter aortic valve implantation or replacement (n = 3), percutaneous coronary intervention (PCI; n = 1), or left ventricular assist device implantation (n = 1); vascular surgery patients (n = 1); patients with chronic kidney disease or end-stage renal disease (n = 1); patients with heart failure (n = 4), diabetes (n = 1), acute coronary syndrome (ACS; n = 3), or multiple myeloma (n = 1); patients who had been admitted to an intensive care unit (ICU; n = 1), nursing home residents (n = 1); patients who had undergone a range of other surgical interventions (n = 3) or those hospitalized for a range of medical conditions (n = 1).
Table 1.
Descriptive characteristics of the included meta-analyses.
| Author | Databases Searched | Search Period | No. of Studies Pooled | Population/Setting | Age | % Women | Countries of Included Studies | Follow up Duration |
Total Sample (% Frail) | Exposures Assessed | Pre-Defined Frailty Measurement Criteria | Frailty Mortality HR/RRs (95% CI) |
Model used | I 2 | AMSTAR Quality Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anand et al. [33] | Medline, Embase and Cinahl | 1 January 2000 to 1 June 2015 | 7 | TAVI patients | Mean age: 81–86 y | 48–66% | Switzerland, Canada, Germany, Netherlands, Italy, USA | >30 days | 3159 (23.2%) | Frailty | Any | 1.63 (1.34–1.97) a | RE | 66.0% | Moderate |
| Huang et al. [34] | PubMed, Embase and Cochrane library | Inception to January 2018 | 10 | TAVR patients | NR | NR | USA, Poland, Germany, Spain, Japan, Switzerland | >6 months | 2992 (32.5%) | Frailty | Any | 2.81 (1.90–4.15) b | RE | 84.0% | Moderate |
| Ida et al. [35] | Medline, Cochrane central and Clinicaltrials.gov | Inception to 1 December 2018 | 4 (Frail vs. non-frail) 2 (pre-frail vs. non-frail) |
Patients with diabetes | Mean age: 56–76 y | 46–67% | Italy, USA, Spain, Taiwan | 3–12 y | 563,761 (NR) |
Frailty, Pre-frailty | Any | Frailty: 1.35 (1.05–1.74) a Pre-frailty: 1.09 (1.01–1.17) a |
RE | 92.0% (Frailty) 89.0% (Pre-frailty) |
Moderate |
| Kojima et al. [36] | Embase, Scopus, Medline, Cinahl PsycINFO and Google scholar | Inception to March 2018 | 3 | Community-dwelling middle-aged and older adults | Mean age: ≥59.9 y | 0–53.4% | UK, Mexico, Italy, Belgium, Denmark, France, Greece, Italy, Netherlands, Spain, Sweden, Switzerland | 2.4–4.3 y | 9273 (NR) | Frailty, Pre-frailty | FRAIL scale | Frailty: 3.53 (1.66–7.49) a** Pre-frailty: 1.75 (1.14–2.70) a |
RE | 79.0% (Frailty) 64.0% (Pre-frailty) |
Moderate |
| Man et al. [37] | PubMed and Embase | Inception to October 1 2018 | 5 (Frailty) 3 (Pre-frailty) |
Patients with ACS | ≥65 y | 32.8–48.9 | Canada, Spain, France, Sweden | ≥12 months | 1270 (38.4%) | Frailty, Pre-frailty | Any | Frailty: 2.44 (1.92–3.12) a Pre-frailty:1.65 (1.01–2.69) a |
FE | 0.0% (Frailty), 0.0% (pre-frailty) |
Critically low |
| Muscedere et al. [38] | Cochrane central, Medline, Embase, PubMed, Cinahl and Clinicaltrials.gov | Inception to April 2017 | 6 | Patients admitted to the ICU | Mean age: 57.1–84.0 y | NR | USA, Canada, Turkey, France | >6 months | 2484 (42.8%) | Frailty | Any | 1.53 (1.40–1.68) b | RE | 0.0% | Moderate |
| Panayi et al. [39] | PubMed and Cochrane central | Inception to 1 January 2018 | 11 | Surgical patients (All surgeries) | NR | NR | USA, Canada | NR | 523,598 (70.7%) | Frailty | Modified frailty index (mFI) | 4.19 (2.96–5.12) b | RE | 91.0% | Moderate |
| Salazar et al. [40] | Medline, Embase, Scopus, Cochrane Database of Systematic Reviews, Cochrane Central, and Clinicaltrials.gov | Inception to August 2018 |
3 | Patients with multiple myeloma | Median age: 58–74 | NR | Italy, Czech Republic, Netherlands, China, Germany | 13–28 months | 1622 (NR) | Frailty | Any | 2.17 (1.00–2.34) a | RE | 33.7% | Critically low |
| Sandini et al. [41] | Medline, Embase, PubMed, Cochrane and Scopus libraries | 1990 to January 2017 | 8 | Elective major abdominal surgery patients | NR | NR | USA, Korea, Norway, Netherlands | Up to 1 y | 16,825 (NR) | Frailty | Any | 2.71 (1.63–4.49) a | RE | 88.3% | Moderate |
| Shu-Fang et al. [42] | Medline and CINAHL databases and the Cochrane Library | January 2001 to July 2014 | 11 | Community-dwelling older adults | ≥65 y | NR | France, Spain, USA, Israel, Finland | 1.5–10 y | 35538 (NR) | Frailty, Pre-frailty | Fried (CHS) criteria | Frailty:2.00 (1.73–2.32) a Pre-frailty: 1.34 (1.26–1.41) a |
RE | 61.7% (Frailty) 0.0% (Pre-frailty) |
Moderate |
| Thongprayoon et al. [43] | Medline, Embase, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews | Inception to November 2016 | 8 | TAVR patients | NR | NR | USA, Germany, France | NR | 10,498 (NR) | Frailty | Any | 2.01 (1.44–2.80) b | RE | 58.0% | Moderate |
| Tse et al. [44] | PubMed and EMBASE | Inception to July 23 2017 | 8 | Patients who have undergone PCI | Mean age 69 y | 32 | Spain, Indonesia, UK, Japan, USA | Mean 2.5 y | 2332 (NR) | Frailty | Any | 2.97 (1.56–5.66) a | RE | 79.0% | Low |
| Tse et al. [45] | PubMed and Embase | Inception to September 11, 2017 | 7 | Advanced Heart Failure Patients Undergoing Left Ventricular Assist Device Implantation | Mean age 48–69 y | 20.8 | Mean 13 months | 2942 (13.5%) | Frailty | Any | 1.44 (1.15–1.80) a | FE | 0.0% | Moderate | |
| Vermeiren et al. [46] | PubMed, Web of Knowledge and PsycINFO | Inception to January 2016 | 17 | Community-dwelling older adults | ≥65 y | NR | USA, France, Spain, Cuba, Dominican Republic, Venezuela, Mexico, Peru, India, China, Italy, Finland | 10–120 months | 150,763 (NR) | Frailty | Any | 1.83 (1.68–1.98) a/b | n.s | 98.0% | Low |
| Wang et al. [47] | Medline, Embase, Cochrane, Scopus | Inception to September 2017 | 9 | Patients who have undergone major vascular surgery | Mean age ≥59.1 y | NR | Canada, USA, Japan | 1–8.4 y | 1957 (35.5%) | Frailty | Any | 2.22 (1.81–2.73) a | RE | 0.0% | Moderate |
| Wang et al. [48] | PubMed, Embase, Cochrane, Web of science | Inception to November 8 2017 | 6 | Patients with heart failure | Mean age: 82.6 y | 17.6 | USA, Italy, Spain | >30 days | 1747 (53.0%) | Frailty | Any | 1.70 (1.41–2.04) a | FE | 0.0% | Low |
| Yang et al. [49] | Medline, Embase, Cochrane Central | January 1966 to March 2018 | 8 | Patients with chronic heart failure | Mean age: 73.4 y | 40.1 | USA, Italy, Australia, Spain | NR | 2645 (50.7%) | Frailty | Any | 1.54 (1.34–1.75) a | RE | 0.0% | Moderate |
| Zhang et al. [50] | Medline, Embase, Cochrane Central | Inception to October 2018 | 14 | Nursing home residents | Mean age:84.9 | 72.1 | France, Spain, Canada, Belgium, USA, Japan, Poland, China, Australia | 0.5–9 y | 9076 (53.9%) | Frailty | Any | 1.88 (1.57–2.25) a | RE | 47.8% | Moderate |
| Zhang et al. [51] | Medline, Embase, Cochrane Central | Inception to October 2018 | 3 | Heart failure patients | NR | NR | USA, Spain | NR | NR | Frailty | Any | Pre-frailty: 1.51 (0.99–2.31) a | RE | 0.0% | Critically low |
| Zhang et al. [52] | PubMed and Embase | Inception to December 3 2017 | 20 | Patients with heart failure | Mean age: 79.9 y | 11.1–60.9 | USA, Spain, Italy, Australia, Canada, UK, Hong Kong | 0.5–12 y | 17201 (9.2–76%) | Frailty | Any | 1.59 (1.39–1.82) a |
RE | 55.0% | Low |
| Dou et al. [53] | PubMed and Embase | Inception to July 1 2018 | 7 | Patients with ACS | ≥65 y | NR | Spain, France, Sweden, Canada, Japan, South Africa, Scandinavia, India, East Asia, Australia, New Zealand | In hospital to 56.4 months | 6658 (12.3%) | Frailty | Any | Frailty: 2.65 (1.81–3.89) a Pre-frailty: 1.41 (1.19–1.66) a |
RE | 60.2% (Frailty) 0.0% (Pre-frailty) |
Low |
| Zhang et al. [54] | PubMed, Web of Science, Embase and Cochrane Central databases | Inception to June 2019 | 11 | Patients with CKD or end-stage renal disease | Mean age: >45.0 y | USA, South Korea, India | 1–17 y | 127037 (7.9–82.0%) | Frailty | Fried (CHS) | 1.95 (1.50–2.53) a | RE | 82.0% | Low | |
| Houghton et al. [55] | Cinahl, PsycInfo, and Scopus | inception to September, 2018 | 9 | Vascular surgery patients | NR | USA, Japan, UK | NR | 2904 | Frailty | Any | 1.85 (1.31–2.62) a | RE | 74.0% | Low | |
| Cunha et al. [56] |
PubMed, Embase, Web of Science, Lilacs, Cinahl, PsycInfo and Google Scholar | Inception to March 2019 | 4 (Frailty); 3 (Pre-frailty) |
Hospitalized patients | NR | NR | 7–12 months | 2119 (86.6%) | Frailty, Pre-frailty | Any | Frailty: 7.95 (4.88–12.96)a, Pre-frailty: 3.65 (1.41–9.43) a | RE | 0.0% (Frailty), 28.0% (pre-frailty) | Moderate | |
| Zhang et al. [57] | Medline, Embase, and Cochrane Central | Inception to December 2018 | Patients with ACS | NR | NR | 15–60 Months | 4665 (12.8%) | Frailty | Any | 1.66 (1.35–2.05) a | FE | 11.0% | Low |
a = hazard ratio, b = risk ratio, HF = heart failure, ACS = acute coronary syndrome, CI = confidence interval, CKD = chronic kidney disease, FE = fixed effect model, ICU = intensive care unit; n.s. = not stated, RE = random effect model, TAVI = transcatheter aortic valve implantation patients; TAVR = transcatheter aortic valve replacement, LVAD = left ventricular assist device implantation;** in this study the reference group was only robust people.
The heterogeneity (I2) across the individual meta-analyses was variable and ranged from 0% to 100%. Fifty-six percent (14/25) of studies reported substantial to a considerable level of heterogeneity. In the studies which reported frailty prevalence, the baseline prevalence of frailty ranged from 7.9% to 86.6%.
After evaluation of the risk of bias by the AMSTAR 2 tool, 56% (14/25), 32% (8/25), and 12% (3/25) were rated to be of moderate, low, and critically low quality, respectively. The critical domains that were most frequently lacking were justification for exclusion of individual studies (22 out of 25), lack of protocol being registered before review commencement (11 out of 25) and assessment of present and likely impact of publication bias (eight out of 25). The breakdown of the AMSTAR 2 assessment for each study is presented in Supplementary Table S3.
3.2. Association Between Frailty and All-Cause Mortality
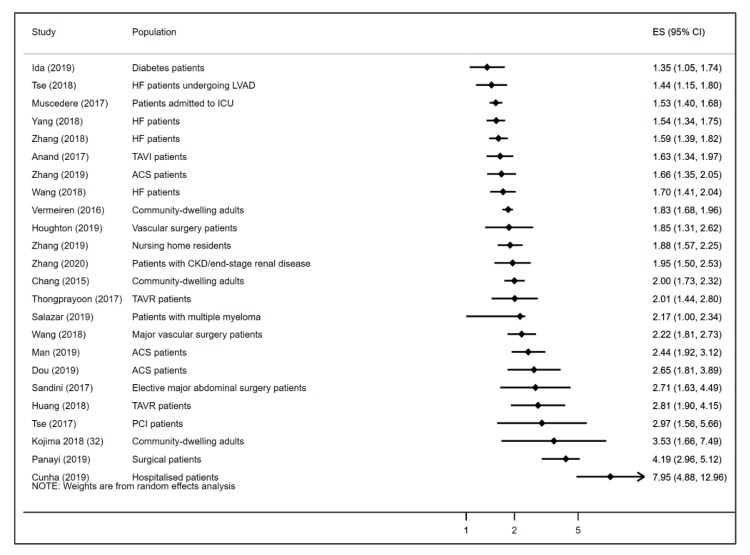
A total of 24 meta-analyses reported the association between frailty and the risk of all-cause mortality. All 24 studies found that frailty was associated with increased risk of all-cause death. The reported HR/RRs of the effect of frailty on all-cause mortality ranged from 1.35 to 7.95. The lowest HR/RR was reported among diabetes patients and the highest among hospitalized patients. The median HR/RR estimate of frailty on all-cause mortality across the included studies was 1.98 (IQR 1.65–2.67). Figure 2 displays a forest plot of the of the reported HR/RR of the association between frailty and all-cause mortality in the included studies. None of the studies reported age- or sex-specific pooled data.
Figure 2.
Forest plot showing the reported HR/RR of the association between frailty and all-cause mortality across the included meta-analyses. HF = heart failure; ACS = acute coronary syndrome; PCI = percutaneous coronary intervention; TAVI = TAVI = transcatheter aortic valve implantation patients; TAVR = transcatheter aortic valve replacement, LVAD = left ventricular assist device implantation; ICU = intensive care unit.
3.3. Association between Pre-Frailty and All-Cause Mortality
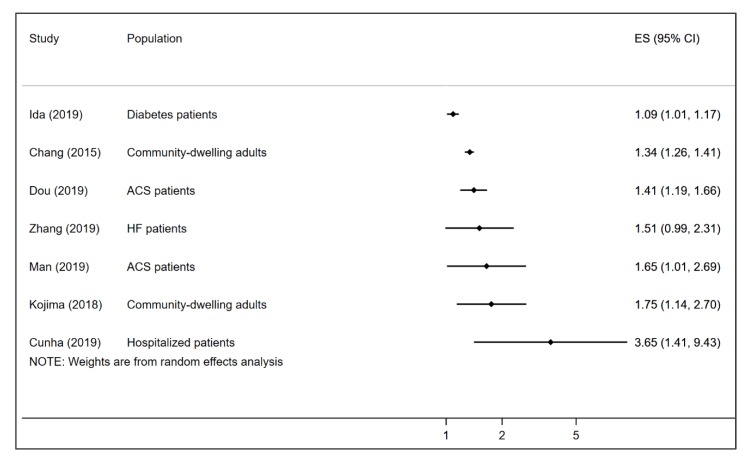
All seven meta-analyses that reported the association between pre-frailty and all-cause mortality found that pre-frailty was associated with increased risk of all-cause mortality. The reported HR/RRs of the effect of pre-frailty on all-cause mortality ranged from 1.09 among diabetes patients to 3.65 among hospitalized patients. The median HR/RR estimate of frailty on all-cause mortality across the included studies was 1.51 (IQR 1.38–1.73). Figure 3 displays a forest plot of the association between pre-frailty and all-cause mortality in the included studies. None of the studies reported age- or sex-specific pooled data.
Figure 3.
Forest plot showing the reported HR/RR of the association between pre-frailty and all-cause mortality across the included meta-analyses. ACS = acute coronary syndrome; HF = heart failure.
4. Discussion
Increasingly, frailty has been recognized as a marker of poor ageing [58], and this syndrome is associated with many negative health outcomes [9,10,11,12,15]. However, the absence of a lack of a universally accepted definition creates clinical and research conundrum [59,60]. Moreover, while frailty is expressed over a broad spectrum of severity, defining the point at which frailty begins is challenging [1]. An equally important issue also relates to identification of the tipping point at which the progression of frailty begins to exert negative effects on survival [20].
With the above in mind, this overview of systematic reviews and meta-analyses provides a synthesis of the current available evidence of the association between frailty and the risk of all-cause mortality. We found consistent data across different populations and settings suggesting that both frailty and pre-frailty are associated with increased risk of all-cause mortality. Across the included studies, frailty was on average associated with a two-fold increased risk of death, although the risk of mortality associated with pre-frailty appeared to be much lower.
Although all the included studies suggested frailty and pre-frailty to be associated with increased risk of all-cause mortality, there was significant heterogeneity in the effect sizes. For example, while one study reported frailty to be associated with about two times increased risk of death in community-dwelling adults [42], another study reported frailty to be associated with a nearly eight-fold increase in risk of mortality among patients hospitalized for a range of conditions [56]. These data suggest that the mortality effect of frailty across different populations may be graded and could also be related to other underlying pathological risk factors/stressors that exacerbate the impact of frailty to varied extents in different populations [21,22].
As frailty is associated with increased risk of mortality, screening for frailty may be useful for all older adults. However, frailty screening remains a highly debated issue especially in relation to screening eligibility, as well as where and when it should be performed [3,61]. Regardless, within specialized geriatric ambulatory care settings and long-term care facilities, the use of comprehensive geriatric assessments that incorporate the deficit accumulation definition of frailty may be more feasible to screen for frailty [62,63], whereas the frailty phenotype may be easier adopt within primary care settings to identify frail individuals [20].
Healthcare providers need to recognize frailty both as an important baseline condition and an outcome among older adults. To this end, improved understanding of the risk factors of frailty is important. Several factors are associated with increased risk of frailty including individual chronic morbidities such as diabetes, cancers, or depression or their co-existence [3,64,65]. Psychosocial (e.g., social isolation) and lifestyle factors also play important roles in the development of frailty [3,66,67]. Regardless, studies also suggest that both frailty and pre-frailty can be reversed [68,69]. Thus, to minimize the risk of frailty development, delay its progression or promote reversal, multidisciplinary interventions that aim to improve physical, cognitive, and social functioning are required [20,70,71,72]. In the context of primary care settings, relative effectiveness and ease of implementation of programs at preventing or slowing frailty progression, ranked from highest to lowest, are strength training, protein supplementation, comprehensive geriatric assessments, home visits and behavioral change interventions [72].
Although the present overview provides important insights, some limitations should be considered. First, the criteria for ascertaining death varied across the primary studies of the meta-analyses, and therefore misclassification may have affected the estimates of the association between frailty and the risk of mortality. Secondly, some primary studies may have been included in more than one meta-analysis and thus the influence of these studies would have been inflated. Thirdly, some meta-analyses involved primary studies that showed substantial heterogeneity, which may limit the validity of their, and hence our, conclusions. Furthermore, although the prevalence of frailty and its mortality effects could vary by age, sex or other characteristics [73,74,75], we could not examine this in detail due to limited data. We could also not examine the presence of publication bias due to the design of our study and the methodology used. Moreover, some meta-analyses were based on small number of studies showed large effect sizes, which may be suggestive of publication bias in reviews. Lastly, further bias may have arisen from our limitation of component studies to English, although the extent of this bias, if present, would likely have been small.
5. Conclusions
Across different populations and settings, frailty defined using multiple criteria is associated with two-fold increased risk of all-cause mortality. Thus, public health interventions to prevent or slow the progression of frailty may prevent avoidable deaths.
Supplementary Materials
The following are available online at https://www.mdpi.com/2308-3417/5/1/17/s1, Table S1: PRISMA Checklist; Table S2: Search strategy for Ovid Medline which was adapted to other databases; Table S3: AMSTAR2 grading for each study.
Author Contributions
Conceptualization, R.O.-A., K.L.C., B.W.S., M.M., A.R.Z. and D.L.; methodology, R.O.-A., K.L.C., B.W.S.; software, R.O.-A.; validation, R.O.-A., K.C.L, and B.S.W; formal analysis, R.O.-A., K.L.C., and B.S.W; investigation, R.O.A, K.L.C., B.S.W; resources, R.O.-A.; data curation, R.O.-A., B.S.W, and K.L.C.; writing—original draft preparation, R.O.-A.; writing—review and editing, R.O.-A., K.L.C., M.M., B.S.W, A.R.Z., D.L.; visualization, R.O.-A.; supervision, R.O.-A.; project administration, R.O.-A.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
DL reports receiving grants from Pfizer, AbbVie, AstraZeneca, and CSL-Behring and personal fees and/or other financial support from Bayer and Novartis for work unrelated to this study. All others have nothing to disclose.
References
- 1.Xue Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clegg A., Young J. The frailty syndrome. Clin. Med. 2011;11:72–75. doi: 10.7861/clinmedicine.11-1-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ofori-Asenso R., Chin K.L., Mazidi M., Zomer E., Ilomaki J., Zullo A.R., Gasevic D., Ademi Z., Korhonen M.J., LoGiudice D., et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2019;2:e198398. doi: 10.1001/jamanetworkopen.2019.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collard R.M., Boter H., Schoevers R.A., Oude Voshaar R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Amer. Geriatr. Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 5.Hewitt J., Long S., Carter B., Bach S., McCarthy K., Clegg A. The prevalence of frailty and its association with clinical outcomes in general surgery: A systematic review and meta-analysis. Age Ageing. 2018;47:793–800. doi: 10.1093/ageing/afy110. [DOI] [PubMed] [Google Scholar]
- 6.Handforth C., Clegg A., Young C., Simpkins S., Seymour M.T., Selby P.J., Young J. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann. Oncol. 2015;26:1091–1101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 7.Kojima G. Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015;16:940–945. doi: 10.1016/j.jamda.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Kojima G. Frailty as a predictor of hospitalisation among community-dwelling older people: A systematic review and meta-analysis. J. Epidemiol. Community Health. 2016;70:722–729. doi: 10.1136/jech-2015-206978. [DOI] [PubMed] [Google Scholar]
- 9.Kojima G. Frailty as a predictor of disabilities among community-dwelling older people: A systematic review and meta-analysis. Disabil. Rehabilit. 2017;39:1897–1908. doi: 10.1080/09638288.2016.1212282. [DOI] [PubMed] [Google Scholar]
- 10.Cheng M.H., Chang S.F. Frailty as a Risk Factor for Falls among Community Dwelling People: Evidence from a Meta-Analysis. J. Nurs. Scholarsh. 2017;49:529–536. doi: 10.1111/jnu.12322. [DOI] [PubMed] [Google Scholar]
- 11.Kojima G. Frailty significantly increases the risk of fractures among middle-aged and older people. Evid. Based Nurs. 2017;20:119–120. doi: 10.1136/eb-2017-102769. [DOI] [PubMed] [Google Scholar]
- 12.Persico I., Cesari M., Morandi A., Haas J., Mazzola P., Zambon A., Annoni G., Bellelli G. Frailty and Delirium in Older Adults: A Systematic Review and Meta-Analysis of the Literature. J. Am. Geriatr. Soc. 2018;66:2022–2030. doi: 10.1111/jgs.15503. [DOI] [PubMed] [Google Scholar]
- 13.Kojima G. Frailty as a Predictor of Nursing Home Placement Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. J. Geriatr. Phys. Ther. 2018;41:42–48. doi: 10.1519/JPT.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 14.Bock J.O., Konig H.H., Brenner H., Haefeli W.E., Quinzler R., Matschinger H., Saum K.U., Schottkerm B., Heider D. Associations of frailty with health care costs—Results of the ESTHER cohort study. BMC Health Serv. Res. 2016;16:128. doi: 10.1186/s12913-016-1360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kojima G. Frailty as a Predictor of Emergency Department Utilization among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2019;20:103–105. doi: 10.1016/j.jamda.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Kojima G. Increased healthcare costs associated with frailty among community-dwelling older people: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2019;84:103898. doi: 10.1016/j.archger.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Klein B.E., Klein R., Knudtson M.D., Lee K.E. Frailty morbidity and survival. Arch. Gerontol. Geriatr. 2005;41:141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Ensrud K.E., Ewing S.K., Cawthon P.M., Fink H.A., Taylor B.C., Cauley J.A., Dam T.T., Marshall L.M., Orwoll E.S., Cummings S.R., et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J. Am. Geriatr. Soc. 2009;57:492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mousa A., Savva G.M., Mitnitski A., Rockwood K., Jagger C., Brayne C., Matthews F.E. Is frailty a stable predictor of mortality across time? Evidence from the Cognitive Function and Ageing Studies. Age Ageing. 2018;47:721–727. doi: 10.1093/ageing/afy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shamliyan T., Talley K.M., Ramakrishnan R., Kane R.L. Association of frailty with survival: A systematic literature review. Ageing Res. Rev. 2013;12:719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 21.De Lepeleire J., Iliffe S., Mann E., Degryse J.M. A Frailty: An emerging concept for general practice. Br. J. Gen. Pract. 2009;59:e177–e182. doi: 10.3399/bjgp09X420653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fielding R.A. A Summary of the Biological Basis of Frailty. Karger Publishers; Basel, Switzerland: 2015. pp. 41–44. [DOI] [PubMed] [Google Scholar]
- 23.Bergman H., Ferrucci L., Guralnik J., Hogan D.B., Hummel S., Karunananthan S., Wolfson C. Frailty: An emerging research and clinical paradigm—Issues and controversies. J. Gerontol. Biol. Sci. Med. Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fougère B., Morley J.E. Weight loss is a major cause of frailty. J. Nutr. Health Aging. 2017;21:933–935. doi: 10.1007/s12603-017-0971-7. [DOI] [PubMed] [Google Scholar]
- 26.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Moher D., Liberati A., Tetzlaff J., Altman D.G. Group P: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J., Green S. Cochrane Handbook of Systematic Reviews of Interventions. John Wiley and Sons, Ltd; Hoboken, NJ, USA: 2005. [Google Scholar]
- 29.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Plaza B., Bermejo L.M., Santurino C., Cavero-Redondo I., Alvarez-Bueno C., Gomez-Candela C. Milk and Dairy Product Consumption and Prostate Cancer Risk and Mortality: An Overview of Systematic Reviews and Meta-analyses. Adv. Nutr. 2019;10:S212–S223. doi: 10.1093/advances/nmz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez-Bueno C., Cavero-Redondo I., Martinez-Vizcaino V., Sotos-Prieto M., Ruiz J.R., Gil A. Effects of Milk and Dairy Product Consumption on Type 2 Diabetes: Overview of Systematic Reviews and Meta-Analyses. Adv. Nutr. 2019;10:S154–S163. doi: 10.1093/advances/nmy107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole R., Kennedy O.J., Roderick P., Fallowfield J.A., Hayes P.C., Parkes J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. doi: 10.1136/bmj.j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anand A., Harley C., Visvanathan A., Shah A.S.V., Cowell J., MacLullich A., Shenkin S., Mills N.L. The relationship between preoperative frailty and outcomes following transcatheter aortic valve implantation: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes. 2017;3:123–132. doi: 10.1093/ehjqcco/qcw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang L., Zhou X., Yang X., Yu H., Baltatu O.C. The impact of preoperative frailty status on outcomes after transcatheter aortic valve replacement: An update of systematic review and meta-analysis. Medicine. 2018;97:e13475. doi: 10.1097/MD.0000000000013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ida S., Kaneko R., Imataka K., Murata K. Relationship between frailty and mortality, hospitalization, and cardiovascular diseases in diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2019;18:81. doi: 10.1186/s12933-019-0885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kojima G. Frailty Defined by FRAIL Scale as a Predictor of Mortality: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2018;19:480–483. doi: 10.1016/j.jamda.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Man C., Xiang S., Fan Y. Frailty for predicting all-cause mortality in elderly acute coronary syndrome patients: A meta-analysis. Ageing Res. Rev. 2019;52:1–6. doi: 10.1016/j.arr.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Muscedere J., Waters B., Varambally A., Bagshaw S.M., Boyd J.G., Maslove D., Sibley S., Rockwood K. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensive Care Med. 2017;43:1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panayi A.C., COrkaby A.R., Sakthivel D., Endo Y., Varon D., Roh D., Orgill D.P., Neppl R.L., Javedan H., Bhasin S., et al. Impact of frailty on outcomes in surgical patients: A systematic review and meta-analysis. Am. J. Surg. 2019;218:393–400. doi: 10.1016/j.amjsurg.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salazar A.S., Recinos L.M., Mian H.S., Stoll C., Simon L.E., Sekhon S., Colditz G.A., Wildes T.M. Geriatric Assessment and Frailty Scores Predict Mortality in Myeloma: Systematic Review and Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2019;19:488. doi: 10.1016/j.clml.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Sandini M., Pinotti E., Persico I., Picone D., Bellelli G., Gianotti L. Systematic review and meta-analysis of frailty as a predictor of morbidity and mortality after major abdominal surgery. BJS Open. 2017;1:128–137. doi: 10.1002/bjs5.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shu-Fang C., Pei-Ling L. Frail phenotype and mortality prediction: A systematic review and meta-analysis of prospective cohort studies. Int. J. Nurs. Stud. 2015;52:1362–1374. doi: 10.1016/j.ijnurstu.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Thongprayoon C., Cheungpasitporn W., Thamcharoen N., Ungprasert P., Kittanamongkolchai W., Mao M.A., Sakhuja A., Greason K.L., Kashani K. Association of frailty status with acute kidney injury and mortality after transcatheter aortic valve replacement: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0177157. doi: 10.1371/journal.pone.0177157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tse G., Gong M., Nunez J., Sanchis J., Li G., Ali-Hasan-Al-Saegh S., Wong W.T., Wong S.H., Wu W.K.K., Bazoukis G., et al. Frailty and Mortality Outcomes After Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2017;18:1097-e1. doi: 10.1016/j.jamda.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Tse G., Gong M.Q., Wong S.H., Wu W.K.K., Bazoukis G., Lampropoulos K., Wong W.T., Xia Y.L., Wong M.C.S., Liu T., et al. Frailty and Clinical Outcomes in Advanced Heart Failure Patients Undergoing Left Ventricular Assist Device Implantation: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2018;19:255–261. doi: 10.1016/j.jamda.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 46.Vermeiren S., Vella-Azzopardi R., Beckwee D., Habbig A.-K., Scafoglieri A., Jansen B., Bautmans I. Gerontopole Brussels Study g: Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016;17:1163-e1. doi: 10.1016/j.jamda.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 47.Wang J., Zou Y., Zhao J., Schneider D.B., Yang Y., Ma Y., Huang B., Yuan D. The Impact of Frailty on Outcomes of Elderly Patients After Major Vascular Surgery: A Systematic Review and Meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2018;56:591–602. doi: 10.1016/j.ejvs.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Wang X., Zhou C., Li Y., Li H., Cao Q., Li F. Prognostic Value of Frailty for Older Patients with Heart Failure: A Systematic Review and Meta-Analysis of Prospective Studies. BioMed Res. Int. 2018;2018:1–9. doi: 10.1155/2018/8739058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X., Lupón J., Vidán M.T., Ferguson C., Gastelurrutia P., Newton P.J., Macdonald P.S., Bueno H., Bayés-Genís A., Woo J., et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J. Am. Hear. Assoc. 2018;7:e008251. doi: 10.1161/JAHA.117.008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X., Dou Q., Zhang W., Wang C., Xie X., Yang Y., Zeng Y. Frailty as a Predictor of All-Cause Mortality Among Older Nursing Home Residents: A Systematic Review and Meta-analysis. J. Am. Med Dir. Assoc. 2019;20:657–663. doi: 10.1016/j.jamda.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y., Yuan M., Gong M., Li G., Liu T., Tse G. Associations Between Prefrailty or Frailty Components and Clinical Outcomes in Heart Failure: A Follow-up Meta-analysis. J. Am. Med Dir. Assoc. 2019;20:509–510. doi: 10.1016/j.jamda.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y.P., Yuan M., Gong M.Q., Tse G., Li G.P., Liu T. Frailty and Clinical Outcomes in Heart Failure: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2018;19:1003–1008. doi: 10.1016/j.jamda.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Dou Q., Wang W., Wang H., Ma Y., Hai S., Lin X., Liu Y., Zhang X., Wu J., Dong B. Prognostic value of frailty in elderly patients with acute coronary syndrome: A systematic review and meta-analysis. BMC Geriat. 2019;19:222. doi: 10.1186/s12877-019-1242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q., Ma Y., Lin F., Zhao J., Xiong J. Frailty and mortality among patients with chronic kidney disease and end-stage renal disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2020;52:363–370. doi: 10.1007/s11255-019-02369-x. [DOI] [PubMed] [Google Scholar]
- 55.Houghton J.S.M., Nickinson A.T.O., Morton A.J., Nduwayo S., Pepper C.J., Rayt H.S., Gray L.J., Conroy S.P., Haunton V.J., Sayers R.D. Frailty Factors and Outcomes in Vascular Surgery Patients. Ann. Surg. 2019 doi: 10.1097/SLA.0000000000003642. [DOI] [PubMed] [Google Scholar]
- 56.Cunha A.I.L., Veronese N., Borges S.D.M., Ricci N.A. Frailty as a predictor of adverse outcomes in hospitalized older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2019;56:100960. doi: 10.1016/j.arr.2019.100960. [DOI] [PubMed] [Google Scholar]
- 57.Zhang S., Meng H., Chen Q., Wang X., Zou J., Hao Q., Yang M., Wu J. Is frailty a prognostic factor for adverse outcomes in older patients with acute coronary syndrome? Aging Clin. Exp. Res. 2019:1–8. doi: 10.1007/s40520-019-01311-6. [DOI] [PubMed] [Google Scholar]
- 58.Fedarko N.S. The Biology of Aging and Frailty. Clin. Geriatr. Med. 2011;27:27–37. doi: 10.1016/j.cger.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morley J.E. Frailty: diagnosis and management. J. Nutr. Health Aging. 2011;15:667–670. doi: 10.1007/s12603-011-0338-4. [DOI] [PubMed] [Google Scholar]
- 60.Sternberg S.A., Karunananthan S., Bergman H., Clarfield A.M., Schwartz A.W. The Identification of Frailty: A Systematic Literature Review. J. Am. Geriatr. Soc. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 61.Ambagtsheer R.C., Beilby J.J., Visvanathan R., Dent E., Yu S., Braunack-Mayer A.J. Should we screen for frailty in primary care settings? A fresh perspective on the frailty evidence base: A narrative review. Prev. Med. 2019;119:63–69. doi: 10.1016/j.ypmed.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Rockwood K., Abeysundera M.J., Mitnitski A. How should we grade frailty in nursing home patients? J. Am. Med Dir. Assoc. 2007;8:595–603. doi: 10.1016/j.jamda.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Searle S.D., Mitnitski A., Gahbauer E.A., Gill T.M., Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vetrano D.L., Palmer K., Marengoni A., Marzetti E., Lattanzio F., Roller-Wirnsberger R., Samaniego L.L., Rodriguez-Mañas L., Bernabei R., Onder G., et al. Frailty and Multimorbidity: A Systematic Review and Meta-analysis. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2018;74:659–666. doi: 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 65.Hanlon P., Nicholl B.I., Jani B.D., Lee D., McQueenie R., Mair F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Heal. 2018;3:e323–e332. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gale C.R., Westbury L., Cooper C. Social isolation and loneliness as risk factors for the progression of frailty: The English Longitudinal Study of Ageing. Age Ageing. 2017;47:392–397. doi: 10.1093/ageing/afx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng Z., Lugtenberg M., Franse C., Fang X., Hu S., Jin C., Raat H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE. 2017;12:e0178383. doi: 10.1371/journal.pone.0178383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ofori-Asenso R., Chin K.L., Mazidi M., Zomer E., Ilomaki J., Ademi Z., Bell J.S., Liew D. Natural Regression of Frailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Gerontologist. 2019 doi: 10.1093/geront/gnz064. [DOI] [PubMed] [Google Scholar]
- 69.Kojima G., Taniguchi Y., Iliffe S., Jivraj S., Walters K. Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Res. Rev. 2019;50:81–88. doi: 10.1016/j.arr.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Puts M.T.E., Toubasi S., Andrew M.K., Ashe M.C., Ploeg J., Atkinson E., Ayala A.P., Roy A., Rodriguez-Monforte M., Bergman H., et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46:383–392. doi: 10.1093/ageing/afw247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Apostolo J., Cooke R., Bobrowicz-Campos E., Santana S., Marcucci M., Cano A., Vollenbroek-Hutten M., Germini F., D’Avanzo B., Gwyther H., et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: A systematic review. JBI Database System Rev. Implement. Rep. 2018;16:140–232. doi: 10.11124/JBISRIR-2017-003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Travers J., Romero-Ortuno R., Bailey J., Cooney M.T. Delaying and reversing frailty: A systematic review of primary care interventions. Br. J. Gen. Pract. 2019;69:e61–e69. doi: 10.3399/bjgp18X700241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon E.H., Peel N., Samanta M., Theou O., Howlett S.E., Hubbard R.E. Sex differences in frailty: A systematic review and meta-analysis. Exp. Gerontol. 2017;89:30–40. doi: 10.1016/j.exger.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 74.Hubbard R.E. Sex Differences in Frailty. Interdiscip. Top. Gerontol. Geriatr. 2015;41:41–53. doi: 10.1159/000381161. [DOI] [PubMed] [Google Scholar]
- 75.Franse C., Van Grieken A., Qin L., Melis R.J.F., Rietjens J.A.C., Raat H. Socioeconomic inequalities in frailty and frailty components among community-dwelling older citizens. PLoS ONE. 2017;12:e0187946. doi: 10.1371/journal.pone.0187946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.