Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has spread rapidly across the globe to cause a pandemic. Although it is known to be transmitted via droplets, alternative modes of transmission remain unknown. Transmission through infected ocular tissue or fluid has been a controversy.1 , 2 It is hypothesized that the nasolacrimal system can act as a conduit for viruses to travel from the upper respiratory tract to the eye. Hence, ocular tissue and fluid may represent a potential source of SARS-CoV-2. In this study, we attempted to determine the possibility of transmission through tears by assessing for the presence of SARS-CoV-2 with viral isolation and quantitative reverse-transcription polymerase chain reaction (RT-PCR) analysis. As patients were being monitored clinically via routine nasopharyngeal swabs, these results were compared with those of tears to understand further patterns of viral shedding.
Seventeen coronavirus disease 2019 (COVID-19) patients were recruited for this prospective study in Singapore after obtaining informed consent. This study was carried out in accordance with the tenets of the Declaration of Helsinki and with ethics approval from the Domain Specific Review Board of the National Healthcare Group Singapore. Nasopharyngeal swab samples were collected routinely for clinical monitoring of patient conditions, whereas tear samples were collected purely for research purposes. On some days, both tears and nasopharyngeal swab samples were collected at the same time. These samples were delivered to different labs for processing.
The COVID-19 patients showed positive results by RT-PCR of nasopharyngeal swab samples in a clinical diagnostic laboratory. Nasopharyngeal swab samples were collected in universal viral transport media, and RNA extraction was carried out using the NucliSENS easyMAG system (bioMérieux, Marcy l’Etoile, France). Then, using the A∗STAR Fortitude kit (Accelerate Technologies Pte. Ltd., Singapore, Republic of Singapore), 55 μl of the elute was used to perform RT-PCR analysis according to the manufacturer’s instructions. The limit of detection was estimated to be fewer than 25 copies of RNA.
Tears were sampled by a senior consultant ophthalmologist (R.A.) using a Schirmer test strip at varying time points between days 3 and 20 after the initial development of symptoms. Caution was taken to prevent contamination of samples. The Schirmer strip tear collection method was validated previously in other studies.3 Samples from both eyes were obtained and analyzed separately. Collected strips were placed into individual falcon tubes of universal viral transport media. Samples were delivered to a research laboratory for processing. Samples were used to inoculate Vero-E6 cells (American Type Culture Collection [ATCC] CRL-1586TM). After 4 days of incubation, cells were observed for the presence of cytopathic effect. Total RNA was extracted from all samples using E.Z.N.A. Total RNA Kit I (Omega Bio-Tek, Inc, Norcross, GA) according to the manufacturer’s instructions, and samples were analyzed by real-time quantitative RT-PCR for the detection of SARS-CoV-2 as described previously.4
Clinical data, including age, gender, symptoms, and nasopharyngeal swab results, were collected from electronic health records and were correlated with RT-PCR results. Ocular symptoms that were assessed include red eye, tearing, blurring of vision, discharge, and color desaturation. These symptoms were chosen based on the ocular manifestations of other coronaviruses known to infect humans and animals.2 Other symptoms of COVID-19 assessed included fever, cough, shortness of breath, rhinorrhea, and sore throat.
Of the 17 patients recruited, none demonstrated ocular symptoms. However, 1 patient developed conjunctival injection and chemosis during the stay in the hospital (Table S1, available at www.aaojournal.org). Fourteen patients showed upper respiratory tract symptoms at presentation, including cough, rhinorrhea, and sore throat.
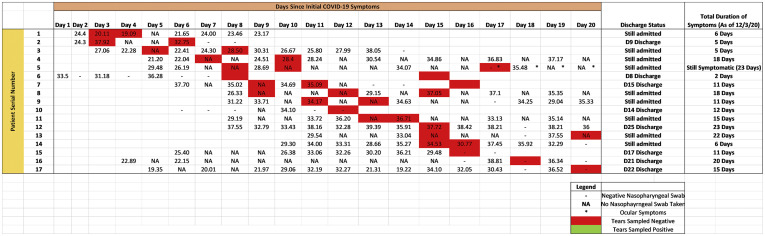
A total of 64 samples were obtained over the study period, with 12, 28, and 24 samples obtained from the first, second, and third week of initial symptoms, respectively. All samples showed negative results for SARS-CoV-2 on viral isolation and RT-PCR. Tear results were compared with nasopharyngeal swab sample results, as shown in Figure 1 . Cycle threshold values of nasopharyngeal swab samples were featured.
Figure 1.
Table showing comparison of tear samples and nasopharyngeal swab samples over the course of coronavirus disease 2019 (COVID-19) illness. Cycle threshold results of all nasopharyngeal swabs are displayed. All tear samples showed negative results on both viral isolation and reverse-transcription polymer chain reaction analysis. These results are labelled by a red box.
In this study, viral shedding in tears was compared with nasopharyngeal swab sample results during the course of COVID-19 infection. A previous study showed positive SARS-CoV-2 RT-PCR results from a patient’s tears, but isolation of the virus was unsuccessful.5 In this study, no evidence was found of SARS-CoV-2 shedding in tears through the course of the disease. Viral load detected in nasal and throat swabs have been shown to be elevated for a period of approximately 2 weeks from the onset of COVID-19 symptoms.6 In this study, the tear sampling time points cover these 2 weeks of active infection, providing a good representation of the full disease course. All tear samples showed negative results, even when nasopharyngeal swab samples continued to show positive results. Furthermore, patients with symptoms of upper respiratory tract infections did not demonstrate any viral shedding in tears, suggesting that the hypothesis of the lacrimal duct as a viral conduit may not be true. Most importantly, only 1 patient showed ocular symptoms during the disease course, and no evidence of SARS-CoV-2 could be found in the tear samples. This suggests that transmission through tears regardless of the phase of infection likely is low.
The study had several limitations. First, the samples were analyzed in different laboratories using 2 different assays. Because the nasopharyngeal swab samples were used in the clinical setting to monitor disease progression, they were analyzed in a clinical diagnostics laboratory, whereas the tear samples were analyzed in a research laboratory. Although the limit of detection for the research laboratory was not assessed because of logistical limitations, it should be noted that the tear samples were incubated with Vero-E6 cells for 4 days before obtaining the RNA for RT-PCR. If SARS-CoV-2 existed in the samples, the cytopathic effect would have been observed even in the context of false-negative RT-PCR results. We observed neither a cytopathic effect nor positive RT-PCR results; thus, the likelihood of SARS-CoV-2 being found in the tear samples remains low. Second, only tears were sampled, rather than conjunctival tissue. In the pandemic setting, COVID-19 patients already are emotionally distraught in light of their diagnosis. Hence, conjunctival tissue sampling was avoided to reduce patient distress. Despite this, we believe that our results do highlight a low risk of ocular transmission. In the acute infection of conjunctival cells, cells die through viral-mediated lysis or as a result of immune reactions. Cell death will release viral material into tears that still can be detected via RT-PCR. Third, the study had a small sample size because of the logistical limitations of the outbreak response. These patients also usually seek treatment a few days after symptom development, making sampling during early infection difficult. Finally, only 1 patient showed ocular symptoms in our study. Studying patients with ocular symptoms can be difficult. In a study of 1099 COVID-19 patients, only 0.8% showed conjunctival congestion.7
The results from this study suggests that the risk of SARS-CoV-2 transmission through tears is low. However, further definitive mechanistic studies are required. Severe acute respiratory syndrome coronavirus-2 has been known to infect cells via ACE2 receptors. More studies are required to prove definitely the presence of ACE2 on corneal and conjunctival cells. Future studies involving more patients with ocular symptoms are also required. Finally, future studies should consider the association between serum viral load and viral shedding in tears. Unfortunately, no blood samples were analyzed for this experiment because they were not part of the routine clinical investigation in the management of patients.
Acknowledgments
The authors thank all scientific staff who assisted with processing clinical samples in the Duke-NUS Medical School ABSL3 facility, especially Velraj Sivalingam and Randy Foo. The study was supported by the team of physicians, researchers, and nursing staff at the National Centre for Infectious Diseases. In particular, the authors thank research assistants Ding Ying and Shiau Hui Dong, who assisted with clinical data collection.
Footnotes
See Commentary onpage 980.
Financial Disclosure(s): The author(s) have made the following disclosure(s): B.E.Y.: Financial support – Sanofi, Roche.
This project was funded as part of the Duke-NUS Signature Research Programme, funded by the Ministry of Health, Singapore, Republic of Singapore, and the National Medical Research Council under its COVID-19 Research Fund (NMRC Project No. COVID19RF-001).
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at the Domain Specific Review Board of the National Healthcare Group Singapore approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Seah, Anderson, Wang, Young, Lye, Agrawal
Analysis and interpretation: Seah, Anderson, Kang, Wang, Rao, Young, Lye, Agrawal
Data collection: Seah, Anderson, Kang, Wang, Rao, Young, Lye, Agrawal
Obtained funding: Anderson, Wang, Young, Lye
Overall responsibility: Seah, Anderson, Young, Lye, Agrawal
Supplementary Data
References
- 1.Lu C.W., Liu X.F., Jia Z.F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seah I., Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020:1–5. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S.Y., Kim M.J., Kim M.K., Wee W.R. Comparative analysis of polymerase chain reaction assay for herpes simplex virus 1 detection in tear. Korean J Ophthalmol. 2013;27(5):316–321. doi: 10.3341/kjo.2013.27.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):23–30. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia J., Tong J., Liu M. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020; Feb 26 doi: 10.1002/jmv.25725. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;Feb 28 doi: 10.1056/NEJMoa2002032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.