Abstract
Objective:
Catheter-directed interventions (CDIs) are increasingly performed for acute pulmonary embolism (PE) as they are presumed to provide similar therapeutic benefits to systemic thrombolysis (ST) while decreasing the associated complications. The purpose of this study was to compare outcomes between CDI and ST.
Methods:
Consecutive patients who underwent CDIs or ST for massive or submassive PE between 2006 and 2016 were identified. Clinical and echocardiographic parameters at baseline and after treatment were recorded. Clinical success was defined as decompensation resolution (or prevention) without major bleeding, stroke, other major treatment-related event, or in-hospital death. The χ2 test and t-test were used for between-groups comparisons.
Results:
There were 213 patients who received CDIs (standard catheter thrombolysis in 56, ultrasound-assisted thrombolysis in 146, suction thrombectomies in 10, and pharmacomechanical thrombolysis in 1) and 104 patients who received ST (94 high dose [100 mg], 10 low dose [50 mg]). At baseline, CDI and ST groups had comparable echocardiographic parameters, demographics, and comorbidities, except for PE type (massive PE, 8.5% for CDIs vs 69.2% for ST; P < .001), age (60.2 ± 14.9 years for CDIs vs 55.9 ± 17.3 years for ST; P =.023), and renal function (glomerular filtration rate, 78.1 ± 33.7 mL/min/1.73 m2 for CDIs vs 64.1 ± 35.2 mL/min/1.73 m2 for ST; P =.001). Without stratifying per PE type, CDIs had a higher clinical success rate (87.8% vs 66.3%; P < .001) and a lower rate of major bleed (8.0% vs 19.2%; P =.003), stroke (1.4% vs 4.8%; P = .120), and death (1.4% vs 13.5%; P < .001). On stratifying by PE type, there was no difference in clinical success between groups. The mean reduction in right ventricular/left ventricular diameter ratio between baseline and the first post-treatment echocardiographic examination (within 30 days) was significantly higher for CDI (0.27 ± 0.20 vs 0.18 ± 0.15; P =.037). Beyond 30 days, there was no echocardiographic difference between groups. There was no significant difference in clinical outcomes and echocardiographic parameters between standard and ultrasound-assisted CDIs.
Conclusions:
CDIs provide improved recovery of right ventricular function compared with ST. Major bleeding and stroke complications may be lower, but larger studies are needed to validate this. CDIs are complementary to ST, and their use should be individualized on the basis of the patients’ clinical presentation, risk profile, and local resources.
Acute pulmonary embolism (PE) is a leading cause of in-hospital morbidity and mortality with a broad spectrum of severity.1 Its increased incidence during the past two decades, in part due to the higher diagnosis rate and the aging population, has driven practice toward newer treatment modalities.2 The goal of treatment is focused primarily on preventing mortality and secondarily on preventing PE recurrence and late-onset chronic thromboembolic pulmonary hypertension. PE risk classification has evolved to reflect the severity and subsequent mortality of an acute episode. Standard of care guidelines recommend anticoagulation for low-risk PE; systemic thrombolysis (ST) is reserved for high-risk (massive) PE associated with hypotension.3–5 In selected patients without hypotension but with evidence of cardiopulmonary deterioration, such as right ventricular (RV) strain and elevated cardiac biomarkers (intermediate-risk or submassive PE), the risk-benefit ratio may also favor thrombolytic therapy to prevent decompensation.3–5 Because of the high associated risks, mainly those of major bleed and stroke, and because of a wide spectrum of contraindications, only 30% of patients who need it are actually receiving ST.6 The inherent limitations with ST use have driven contemporary practice toward catheter-directed interventions (CDIs) that are presumed to provide similar therapeutic benefits while decreasing complication rates as a result of the lower doses or even the absence of lytics.7–10 The Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) randomized trial, A Prospective, Single-arm, Multicenter Trial of EkoSonic Endovascular System and Activase for Treatment of Acute Pulmonary Embolism (SEATTLE II), the Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT) registry, and multiple case series, including our institutional experience, have demonstrated a relative safety and efficacy of CDI, triggering more interest in CDI as first-line treatment of acute massive and high-risk submassive PE.8,11–16
Whereas both CDI and ST have been shown to improve and to reverse RV dysfunction, comparative studies investigating the degree of RV function improvement for each modality are lacking.8,17 The significance of RV function was assessed in a recent meta-analysis showing an increase in short-term mortality in hemodynamically significant PE patients with RV dysfunction.18 Studies directly comparing clinical outcomes of CDI vs ST are few and controversial. The U.S. National Inpatient Sample (NIS) analysis has led to conflicting results regarding mortality differences yet matching results regarding the consistently lower stroke rates for CDI.7,19
The objective of this study was to compare the clinical and echocardiographic outcomes between CDI and ST for acute massive and submassive PE.
METHODS
The study protocol was approved and exempted from informed consent by the Quality Review Board of the University of Pittsburgh Medical Center.
Study design.
Consecutive patients who received treatment for acute PE between January 2006 and September 2016 were identified from our institution’s electronic medical records. Patients with low-risk PE on presentation were excluded from the analysis, leaving patients with acute high-risk (massive) and intermediate-risk (sub-massive) PE treated with CDI and ST. This classification of PE types is in accordance with published guidelines.3,5 High-risk PE is defined as sustained hypotension for at least 15 minutes or requiring vasopressors. Intermediate-risk PE is defined as RV dysfunction on echocardiography or computed tomography scans or presence of cardiac biomarkers but without hypotension.
Records were reviewed for demographics, risk factors, laboratory markers such as glomerular filtration rate (which was calculated using the Modification of Diet in Renal Disease method) and cardiac biomarkers (troponin and brain natriuretic peptide), lower extremity venous duplex ultrasound studies, intraprocedural data, and periprocedural complications as well as longer term data when available. Echocardiographic parameters were collected for each group at baseline, after CDI or ST, and at follow-up. The post-CDI or post-ST echocardiography was performed within 30 days of procedure or treatment, respectively. Follow-up echocardiographic parameters were obtained from the most recent report beyond the initial 30-day period after treatment. Patients were stratified into intermediate-risk vs high-risk PE groups per our definition.
Our primary outcome was 30-day clinical success, a composite outcome defined as decompensation resolution for massive PE (or prevention of decompensation for submassive PE) without major bleeding, stroke, other major treatment-related adverse event (eg, heart or valve injury), need for surgical embolectomy, or in-hospital death. Decompensation was defined as persistent hypotension (and need for pressor support) for patients with massive PE and as development of hypotension for patients with submassive PE despite treatment.
Major bleeding events were defined according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) classification, whereby both GUSTO moderate and GUSTO severe constituted major bleeding.20 GUSTO moderate includes bleeding requiring transfusions without hemodynamic compromise, whereas GUSTO severe includes bleeding that causes hemodynamic instability and requires an intervention and bleeding that is intracranial.
Absolute and relative contraindications to thrombolysis were defined per the American Heart Association guidelines.21
Echocardiographic parameters for assessing RV function before and after treatment were blindly and independently reviewed by two raters (A.N.A.A. and N.L.L.), and mean values were used for analysis. Echocardiographic measurements were made in accordance with the ULTIMA protocol.8
Treatment protocol.
Patients not receiving heparin at the time of admission were given an intravenous bolus of 80 IU/kg, followed by an infusion of 18 IU/kg/h. If the patient was receiving heparin at admission, infusion was continued to maintain an activated partial thromboplastin time of 68 to 106 seconds per institutional protocol. Between 2006 and 2009, ST was the only treatment modality. Since 2009, CDI procedures gradually entered our practice on a physician-based preference; and since 2014, the decision to undergo CDI vs ST vs surgical thrombectomy vs anticoagulation alone for PE treatment was determined by the institution’s multidisciplinary PE response team consisting of pulmonary, critical care, cardiology, vascular surgery, and cardiothoracic services.
The PE response team’s decision is based on an evolving protocol that considers intermediate- to high-risk (echocardiographic RV dysfunction and presence of cardiac biomarkers [troponin >0.1 ng/mL and brain natriuretic peptide >100 pg/mL]) and high-risk (systolic blood pressure <90 mm Hg for at least 15 minutes or requiring inotropic support) PE patients potential candidates for CDI. RV dysfunction is defined as echocardiographic or computed tomographic RV/left ventricular (RV/LV) diameter ratio >0.9. The majority of the patients (>90%) receive baseline echocardiography. In our current practice, CDIs are favored in submassive PE cases eligible for thrombolysis, whereas massive PE treatment is individualized on the basis of the patient’s risk profile.
An inferior vena cava filter was generally used in patients with a contraindication to anticoagulation and selectively in high-risk patients with low cardiopulmonary reserve. An institutional protocol for inferior vena cava filter retrieval is in place; patients are followed up and contacted by dedicated staff and asked to return for filter retrieval.
Systemic lysis protocol.
All PE patients receiving ST are monitored and treated in an intensive care unit setting. Barring any contraindication, PE patients received an ST dose of 50 mg or 100 mg of recombinant tissue plasminogen activator infused during 2 hours. Unfractionated heparin infusion is typically held within this time interval.
Catheter intervention technique.
Ultrasound-guided femoral or internal jugular vein access is used in all patients. Dual-lumen jugular or femoral sheaths or two single-lumen femoral sheaths are used for bilateral PEs. If necessary, an inferior vena cava filter is inserted before pulmonary artery catheterization. A wire is then navigated toward the pulmonary arteries. As the wire crosses from the right atrium into the right ventricle, it may go through the chordae tendineae of the tricuspid valve. As long as the catheter or device used is small bore (#8F), this will be uneventful. When larger catheters are used, the chordae can rupture, leading to tricuspid valve insufficiency. To prevent this, when large devices are considered, crossing of the tricuspid valve should be done either with a pigtail or with an inflated balloon catheter (eg, Swan-Ganz). A pulmonary arteriogram is obtained to assess the clot’s location. Standard CDI protocol includes placement of unilateral or bilateral 5-cm multi-side hole catheters (5F Cragg-McNamara [Boston Scientific, Marlborough, Mass] or UniFuse [AngioDynamics, Latham, NY]) across the heaviest clot burden (unilateral or bilateral). On-table infusion of 2 to 4 mg of tissue plasminogen activator is given if it is deemed necessary, followed by the initiation of lysis at a rate of 0.5 to 1 mg/h. Another frequent CDI alternative involves the use of ultrasound-assisted thrombolysis with the EkoSonic catheter (EKOS Corp, Bothell, Wash). In a few cases, aspiration thrombectomy using the AngioVac device (AngioDynamics) or the Arrow-Trerotola thrombolytic device (PTD; Arrow, Reading, Pa), rheolysis using the AngioJet device (Boston Scientific), catheter-based mechanical fragmentation, and on-table only catheter-directed infusion (without initiation of continuous infusion) were used per the physician’s preference. Patients are monitored in the intensive care unit.
Our heparinization protocol during catheter-directed lysis shifted from subtherapeutic dosing early in our experience to low therapeutic dosing (activated partial thromboplastin time of 40–60 seconds). After intervention, all patients remained on full systemic anticoagulation, after which they were transitioned to long-term oral anticoagulation. No adjunct medications (eg, prostanoids) are used for pulmonary hypertension. Termination of catheter-directed thrombolysis gradually evolved through operating room lysis check to bedside catheter removal based on improvement of clinical (oxygen requirements, oxygen saturation, shortness of breath, chest pain), hemodynamic (blood pressure, heart rate), and echocardiographic (RV strain) parameters or any complication that necessitates discontinuation of treatment. Pulmonary artery pressures are transduced before catheter removal at bedside and compared with the intraoperative ones. Filter retrieval is performed at a later date, if applicable.
Statistical analysis.
Descriptive characteristics are reported as mean ± standard deviation or as number of cases and percentages. Dichotomous covariates between the two groups (CDI and ST) were compared using the χ2 and Fisher exact tests; continuous covariates were assessed using independent and paired t-tests. A univariate χ2 analysis was performed between the clinical outcomes and mode of treatment (CDI vs ST) stratified according to PE type.22 This model was repeated using inverse propensity score weighting, with propensity scores computed using the variable “age,” “PE type,” and “contraindication for lytics.” Interobserver agreement for the echocardiographic parameters between the two investigators’ measurements was assessed by Lin’s concordance correlation and by Bland-Altman analysis. Results were considered statistically significant if P<.05. Data analysis was performed using Statistical Package for the Social Sciences, version 22 (IBM Corp, Armonk, NY) and Stata 14 (including Pscore by S.O. Becker and A. Ichino; StataCorp LP, College Station, Tex).
RESULTS
During the study period, 213 patients received CDI and 104 patients received ST for the treatment of acute PE. The mean age of the cohort was 58.8 ± 15.8 years, and 152 (47.9%) were male. Baseline characteristics are listed in Table I. The CDI group was older (60.2 ± 14.9 vs 55.9 ± 17.3 years; P = .023), with better renal function (glomerular filtration rate, 78.1 ± 33.7 vs 64.1 ± 35.2 mL/min; P = .001) and with a preponderance of submassive PEs (91.5% vs 30.8%) compared with ST, which was mainly used in massive PEs (8.5% vs 69.2%; P < .001). Although not statistically significant, catheter interventions were used more frequently in cases with absolute contraindications to thrombolytics (5.2% vs 2.9%; P = .239).
Table I.
Characteristics of study population by treatment type
| Overall (N = 317) | CDI (n = 213) | ST (n = 104) | P value | |
|---|---|---|---|---|
| Age, years | 58.8 ± 15.8 | 60.2 ± 14.9 | 55.9 ± 17.3 | .023 |
| Male sex | 152 (47.9) | 106 (49.8) | 46 (44.2) | .354 |
| PE type, massive | 90 (28.4) | 18 (8.5) | 72 (69.2) | <.001 |
| sPESI score (=1) | 294 (92.7) | 191 (89.7) | 103 (99.0) | .003 |
| Acute DVT | 192 (65.8) | 132 (64.4) | 60 (69.0) | .451 |
| Hypercoagulable state | 21 (6.7) | 11 (5.2) | 10 (9.6) | .141 |
| Recent surgery | 71 (22.4) | 41 (19.2) | 30 (28.8) | .054 |
| Recent trauma | 13 (4.1) | 7 (3.3) | 6 (5.8) | .304 |
| Malignant disease | 54 (17.0) | 35 (16.4) | 19 (18.3) | .683 |
| Contraceptives | 18 (5.7) | 10 (4.7) | 8 (7.7) | .288 |
| Recent travel | 22 (7.0) | 19 (9.0) | 3 (2.9) | .045 |
| Previous DVT | 51 (16.2) | 38 (18.0) | 13 (12.6) | .224 |
| Previous PE | 43 (13.7) | 32 (15.2) | 11 (10.6) | .265 |
| Previous PE or DVT | 43 (13.7) | 34 (16.1) | 9 (8.7) | .070 |
| Previous stroke | 4 (1.3) | 3 (1.4) | 1 (1.0) | .732 |
| No contraindications | 261 (82.3) | 178 (83.6) | 83 (79.8) | .239 |
| Major contraindications | 14 (4.4) | 11 (5.2) | 3 (2.9) | – |
| Minor contraindications | 42 (13.2) | 24 (11.3) | 18 (17.3) | – |
| Hypertension | 157 (49.7) | 116 (54.7) | 41 (39.4) | .011 |
| Coronary disease | 40 (12.7) | 29 (13.5) | 11 (11.0) | .664 |
| Heart failure | 18 (5.7) | 11 (5.2) | 7 (6.7) | .585 |
| Pulmonary disease | 61 (19.4) | 47 (22.3) | 14 (13.5) | .063 |
| Oxygen on admission | 19 (6.0) | 13 (16.2) | 6 (5.8) | .891 |
| Pulmonary hypertension | 11 (3.5) | 5 (2.4) | 6 (5.8) | .122 |
| Current smoker | 65 (20.6) | 40 (18.9) | 25 (24.3) | .266 |
| Vena cava filter | 85 (26.8) | 56 (26.3) | 29 (27.9) | .746 |
| CFR >60 mL/min | 207 (65.5) | 153 (71.8) | 54 (52.4) | <.001 |
| GFR 30–59 mL/min | 87 (27.5) | 56 (26.3) | 31 (30.1) | – |
| CFR <30 mL/min | 22 (7.0) | 4 (1.9) | 18 (17.5) | – |
| CFR, mL/min | 73.5 ± 34.8 | 78.1 ± 33.7 | 64.1 ± 35.2 | .001 |
| Troponin | 1.1 ± 3.8 | 0.6 ± 1.0 | 2.2 ± 6.5 | .020 |
| BNP | 440.1 ± 478.4 | 423.0 ± 484.1 | 492.4 ± 461.3 | .361 |
| Lysis dose | 47.0 ± 36.5 | 23.2 ± 10.7 | 95.9 ± 16.6 | <.001 |
BNP, Brain natriuretic peptide; CDI, catheter-directed intervention; DVT, deep venous thrombosis; GFR, glomerular filtration rate; PE, pulmonary embolism; sPESI, simplified Pulmonary Embolism Severity Index; ST, systemic thrombolysis.
Categorical variables are presented as number (%). Continuous variables are presented as mean ± standard deviation.
Procedural data.
Data are summarized in Table II. The majority of CDI patients received ultrasound-assisted thrombolysis (146 [68.5%]); 56 (26.3%) received standard catheter-directed thrombolysis, 10 (4.7%) patients received aspiration thrombectomy, and 1 (0.5%) patient underwent rheolytic thrombectomy. The mean alteplase dose was 23.2 ± 10.7 mg (median, 23 mg; range, 0–63 mg), and the mean infusion time was 17.0 ± 9.1 hours (range, 0–51 hours). Interventions were bilateral in most CDI cases (196 [92.0%]). An inferior vena cava filter was inserted in 56 (26.3%) patients; 171 (80.3%) patients required a single trip to the angiography suite, with catheters removed at bedside and termination of infusion based on clinical and echocardiographic parameters. In the ST group, mean alteplase dose was 95.8 ± 16.3 mg (range, 40–142 mg); 94 (90.4%) patients received the high thrombolytic dose (≥100 mg), whereas 10 (9.6%) patients received the low thrombolytic dose (range, 40–50 mg).
Table II.
Procedural and treatment characteristics of catheter-directed intervention (CDI) and systemic thrombolysis (ST)
| CDI | |
|---|---|
| Femoral access | 167 (78.4) |
| Bilateral lysis | 196 (92.0) |
| Type of intervention | |
| Standard catheter lysis | 56 (26.3) |
| Ultrasound-assisted thrombolysis | 146 (68.5) |
| Suction thrombectomy | 10 (4.7) |
| Pharmacomechanical | 1 (0.5) |
| Lysis fragmentation | 5 (2.3) |
| On-table only infusion | |
| Mean tissue plasminogen activator, mg | 23.2 ± 10.7 |
| Operating room trips | |
| 1 | 171 (80.3) |
| 2 | 32 (15.0) |
| 3 | 9 (4.2) |
| 4 | 1 (0.5) |
| ST | |
| High alteplase dose (100 mg) | 94 (90.4) |
| Low alteplase dose (50 mg) | 10 (9.6) |
Categorical variables are presented as number (%). Continuous variables are presented as mean ± standard deviation.
In-hospital outcomes.
Clinical success was observed in 187 (87.8%) patients in the CDI group and in 69 (66.3%) patients in the ST group (P < .001). In-hospital mortality rates (CDI, 1.4%; ST, 13.5%; P < .001), major bleeding rates (CDI, 8.0%; ST, 19.2%; P = .003), and stroke rates (CDI, 1.4%; ST, 4.8%; P = .120) were lower in the CDI group. Two major CDI procedural events included a coronary sinus perforation leading to cardiac tamponade and tricuspid valve injury when the AngioVac was used (both included in the major bleed rate). Both patients underwent open heart surgery with good outcomes.
Multivariate analysis and propensity matching for clinical success confirmed PE type as the most powerful predictor, abolishing the effect of all other confounders including the type of treatment.
On stratifying per PE type (massive and submassive), there were no significant differences between the CDI and ST groups in all outcomes, despite that ST was associated with a seemingly higher death rate in the massive PE subgroup (19.4% vs 5.6%; P = .287) and a higher stroke rate in the submassive PE subgroup (3.1% vs 1.0%; P .367; Table III). Of patients with massive PE, 5.6% did not improve on CDI compared with 19.4% of patients in the ST group (P=.287). For submassive PEs, decompensation rates were equal between the groups (3.1%). Of note, during the last 3 years of our CDI practice (168 cases), we had no procedure-related adverse events along with a reduction in CDI failure rates from 15.6% to 11.3% (P= .440), indicating our shift toward more careful selection of patients.
Table III.
Clinical outcomes by treatment modality and pulmonary embolism (PE) type
| CDI No. (%) | ST No. (%) | P value | |
|---|---|---|---|
| Overall (N = 317) | (n = 213) | (n = 104) | |
| Clinical success | 187 (87.8) | 69 (66.3) | <.001 |
| In-hospital death | 3 (1.4) | 14 (13.5) | <.001 |
| Major bleeding | 17 (8.0) | 20 (19.2) | .003 |
| Stroke | 3 (1.4) | 5 (4.8) | .120 |
| Massive PE (n = 90) | (n = 18) | (n = 72) | |
| Clinical success | 11 (61.1) | 39 (54.2) | .596 |
| In-hospital death | 1 (5.6) | 14 (19.4) | .287 |
| Major bleeding | 5 (27.8) | 19 (26.4) | .905 |
| Stroke | 1 (5.6) | 4 (5.6) | 1 |
| Submassive PE (n = 227) | (n = 195) | (n = 32) | |
| Clinical success | 176 (90.3) | 30 (93.8) | .747 |
| In-hospital death | 2 (1.0) | 0(0) | 1 |
| Major bleeding | 12 (6.2) | 1 (3.1) | .699 |
| Stroke | 2 (1.0) | 1 (3.1) | .367 |
CDI, Catheter-directed intervention; ST, systemic thrombolysis.
There was no significant difference in clinical outcomes and echocardiographic parameters between standard and ultrasound-assisted CDIs.
Echocardiographic parameters.
Baseline echocardiographic parameters (RV/LV, pulmonary artery pressures, and tricuspid regurgitant jet velocity) were comparable between the two groups (P > .05). Within 30 days, CDI lysis achieved a significantly greater improvement of RV function compared with ST (0.27 ± 0.20 vs 0.18 ± 0.15; P .037).
The echocardiographic follow up (>30 days) based on 77 CDI and 13 ST patients was 99.9 ± 82.3 days and 141.9 ± 103.4 days, respectively. There was a higher proportion of patients missing their >30-day follow-up echocardiogram in the ST group compared with the CDI group (87.5% vs 63.8%; P<.001). The mean differences between baseline and follow-up echocardiograms (beyond 30 days) were comparable. All echocardiographic parameters are summarized in Table IV.
Table IV.
Echocardiographic parameters at baseline, after treatment, and at follow-up
| Baseline | Within 30 days | Within 12 months | Difference: baseline vs 30 days | Difference: baseline vs 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CDI | ST | CDI | ST | CDI | ST | CDI | ST | CDI | ST | |
| RV/LV ratio | 1.01 ± 0.22 | 1.06 ± 0.19 | 0.83 ± 0.18 | 0.93 ± 0.18 | 0.75 ± 0.17 | 0.83 ± 0.11 | O.27± 0.20 | 0.18 ± 0.15 | 0.28 ± 0.18 | 0.35 ± 0.21 |
| No. | 150 | 70 | 103 | 45 | 72 | 13 | 61 | 31 | 48 | 9 |
| Between-group comparison, CDI vs ST | P= .161 | P = .002 | P= .105 | P = .037 | P = .306 | |||||
| Within-group comparison | – | – | – | – | – | – | P < .001 | P = .001 | P < .001 | P < .001 |
| Tricuspid regurgitant jet velocity | 3.04 ± 0.66 | 2.98 ± 0.58 | 2.77 ± 0.59 | 2.80 ± 0.62 | 2.54 ± 0.66 | 2.33 ± 0.49 | 0.42 ± 0.57 | 0.35 ± 0.49 | 0.53 ± 0.78 | 0.83 ± 0.82 |
| No. | 152 | 69 | 88 | 32 | 67 | 12 | 50 | 23 | 45 | 9 |
| Between-group comparison, CDI vs ST | P = .546 | P = .798 | P = .280 | P= .614 | P = .305 | |||||
| Within-group comparison | – | – | – | – | – | – | P = .002 | P = .157 | P < .001 | P < .001 |
| Pulmonary artery pressure | 48.3 ± 16.6 | 47.4 ± 13.8 | 39.1 ± 14.6 | 40.5 ± 13.3 | 34.9 ± 15.8 | 29.4 ± 10.9 | 11.8 ± 15.6 | 9.9 ± 11.9 | 16.7 ± 18.6 | 24.6 ± 21.6 |
| No. | 149 | 73 | 89 | 37 | 67 | 12 | 52 | 28 | 43 | 9 |
| Between-group comparison, CDI vs ST | P = .689 | P= .619 | P =255 | P = .569 | P = .267 | |||||
| Within-group comparison | – | – | – | – | – | – | P < .001 | P = .014 | P < .001 | P < .001 |
CDI, Catheter-directed intervention; RV/LV, right ventricular to left ventricular diameter ratio; ST, systemic thrombolysis.
Values are reported as mean ± standard deviation.
Regarding the interobserver agreement of the RV/LV ratio measurements, Lin’s concordance correlation coefficient (ρc) was 0.552 (95% confidence interval, 0.462–0.642). The Bland-Altman model gave a mean RV/LV difference between the two raters of 0.075 (95% confidence interval, 0.452 to 0.302; Fig).
Fig.
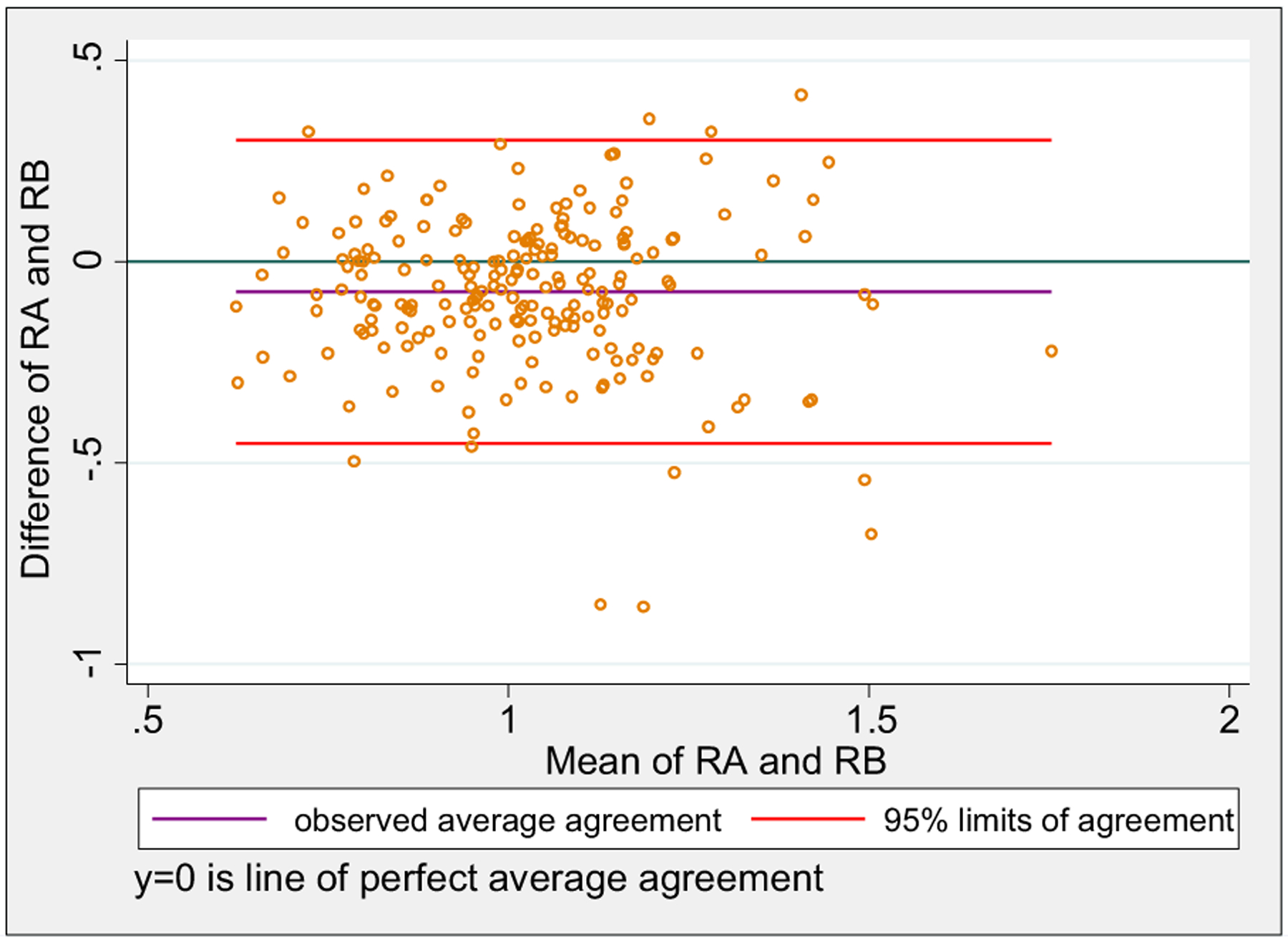
Bland-Altman interobserver agreement plot of baseline right ventricular to left ventricular (RV/LV) ratio measured independently by two raters. The x-axis depicts RV/LV ratio by rater A (RA) plus RV/LV ratio by rater B (RB) divided by 2; the y-axis depicts RV/LV ratio by RA minus RV/LV ratio by RB.
DISCUSSION
Our results suggest that CDIs might have an at least comparable if not superior effect in reversing RV dysfunction compared with ST; however, the lower lytic dose with CDI may not necessarily translate to higher clinical success rates. A potentially lower stroke or bleeding rate may be offset by procedural complications or more liberal use of CDIs against traditional contraindications.
The increased use of CDI has been attributed both to the increased incidence of PE and to a presumed better efficacy and safety profile over ST.7,16 This current appeal for CDI comes as an extrapolation from the Pulmonary Embolism Thrombolysis (PEITHO) trial and a subsequent meta-analysis showing superior mortality and decompensation prevention for ST compared with anticoagulation alone, at the cost of high bleeding and stroke rates.9,10 Given the concern for these risks and the standard contraindications to thrombolytics, ST is eventually used in approximately 30% of eligible patients.10 The prospective ULTIMA trial and PERFECT registry exemplified the CDI safety profile with no major bleeding or stroke events.8,15 However, the SEATTLE II trial along with retrospective series demonstrated that CDIs are not bleeding risk-free procedures, even if stroke rates appear to be very low.14,16,23 The SEATTLE II trial had a CDI success rate of 85.3% with an in-hospital mortality of 2.7%, adverse events related to device rate of 2.0%, and major bleeding rate of 10.0% with only one (0.7%) GUSTO severe bleeding.14 Nonetheless, CDI has been shown to improve early RV function recovery compared with anticoagulation, with higher reductions in the RV/LV ratio.16
Our results are consistent with the literature in showing comparable clinical success rates, in-hospital mortality, and major bleeding rates for CDI and ST. Apart from one randomized study, real-world studies have revealed a CDI success rate ranging between 86% and 94%, comparable to our 87.8%.7,13–15 The NIS study revealed a CDI success rate of 83.4% with an in-hospital mortality rate between 9.2% and 13.4% and a major bleeding rate between 3.2% and 8.5%; the ST group had a success rate of 72.0% with an in-hospital mortality of 10.3% to 21.8% and a major bleeding rate of 4.6% to 8.8%.7,19
Comparative analyses between CDI and ST, however, have yielded controversial results.7,19 The NIS sample was queried twice; one study showed lower mortality rates (13.4% vs 21.8%; P = .007) and intracranial hemorrhage rates (0% vs 1.4%;P = .08) with CDI with comparable major bleeding rates (3.2% vs 4.6%; P = .380),7 whereas the other analysis revealed comparable mortality rates (9.2% vs 10.3%; P =.300) and major bleeding rates (8.5% vs 8.8%; P =.700) with consistently lower intracranial hemorrhage rates (1.3% vs 2.8%; P = .010).19 The mortality discrepancy, apart from potential methodologic flaws, highlights the difficulty in eliminating the inherent patient selection bias favoring better outcomes for CDI over ST. The favorable unadjusted mortality benefit for CDI over ST, both in the NIS data analysis and in our study, appears to reflect patient selection rather than treatment efficacy; propensity matching and PE type stratification eliminate the presumed CDI mortality benefit.19 The absence of mortality benefit is not surprising, given the rapid improvement in RV function seen with both modalities in multiple studies and in ours.8,24 Still, head-to-head comparison of the effect of treatment modality on RV function was lacking, and our study is the first one to demonstrate a potentially faster recovery in favor of CDI. This should be interpreted with caution as ST can be administered immediately at the bedside, whereas a CDI requires time-consuming operating room preparation, and if it involves catheter thrombolysis, it will still need time for the lytics to take effect (typically 6–12 hours). This may be of importance for the higher risk unstable patients.
Consistent with the NIS analysis, our data showed lower major bleeding (8.0% vs 19.2%; P .003) and intracranial hemorrhagic stroke rates (1.4% vs 4.8%; P =.120) with CDI, even though stroke differences were not significant. Significance was lost for major bleeding events in stratifying for PE type, so no definite conclusions can be made. The ST stroke rate appears higher than the one reported in recent literature, potentially related to our aggressive treatment strategies in all comers or the small sample. The comparative trends in favor of CDI are otherwise consistent with a recent meta-analysis showing that the major bleed and stroke rate with CDI is 4.7% and 0.4% compared with 9.2% and 1.5% with ST, repectively.7,10,19,25 We should not overlook the procedure-related complications that may offset these potential bleeding reduction benefits. Yet as experience improves and the decisions on selection of patients mature, procedural complications and overall failures are anticipated to decrease. Our institutional clinical failure (decompensation or major bleeding/stroke or other major treatment-related adverse event) rates have decreased during the last 3 years from 15.6% to 11.3% (P= .440).
This study is among the first analyses to compare linical and echocardiographic outcomes between CDI and ST for the management of acute PE; however, the results should be interpreted with caution, given the retrospective nature of the study. The study was limited by the number of outcome events. Selection bias favoring a cohort of higher risk patients in the ST group cannot be ruled out despite stratifying the clinical outcomes by PE type. The echocardiographic parameters had several missing data. The unsatisfactory correlation between the echocardiographic raters prevents a comprehensive assessment of the differential impact of CDI and ST on RV function at this stage. In addition, there was a between-group differential loss to echocardiographic follow-up beyond 30 days, which precludes solid conclusions regarding the long-term impact of either treatment on RV function. This, however, was not our primary end point, whereas prior studies have demonstrated that RV function normalizes after 30 days irrespective of treatment modality, and this is in agreement with our results. Finally, as there is no standardized CDI protocol within our institution, the ideal CDI technique, thrombolytic dose, and duration remain heterogeneous and unclear.
CONCLUSIONS
Whereas current guidelines recommend ST for high-risk PE, ineligibility of the patient due to thrombolytic contraindications and the increased bleed and stroke rate make CDI a favorable alternative with efficacy (RV function improvement) comparable to if not better than that of ST. The differences lie within the complication profile of each treatment modality, with the lower thrombolytic dose with CDI being counterbalanced by its invasive procedural nature. Under this prism, both treatment modalities should be complementary as they both have a role in the appropriate setting. Careful selection of patients in high-volume centers with appropriate CDI expertise is essential. PE response teams can help in this direction.
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective comparative cohort study
Take Home Message: Comparison of results of catheter-directed intervention (CDI) in 213 patients with systemic thrombolysis (ST) in 104 patients for treatment of pulmonary embolism (PE) was performed. On stratifying by PE type, there was no difference in clinical success among groups, but CDIs improved recovery of right ventricular function compared with ST.
Recommendation: The authors suggest that CDIs are complementary to ST for treatment of PE, and their use should be individualized on the basis of the patients’ clinical presentation, risk profile, and local resources.
Footnotes
Presented at the Twenty-ninth Annual Meeting of the American Venous Forum, New Orleans, La, February 14-17, 2017.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Kahn SR, Houweling AH, Granton J, Rudski L, Dennie C, Hirsch A. Long-term outcomes after pulmonary embolism: current knowledge and future research. Blood Coagul Fibrinolysis 2014;25:407–15. [DOI] [PubMed] [Google Scholar]
- 2.Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985–2009). Am J Med 2014;127:829–39.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788–830. [DOI] [PubMed] [Google Scholar]
- 4.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinides SV. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3145–6. [DOI] [PubMed] [Google Scholar]
- 6.Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med 2012;125:465–70. [DOI] [PubMed] [Google Scholar]
- 7.Patel N, Patel NJ, Agnihotri K, Panaich SS, Thakkar B, Patel A, et al. Utilization of catheter-directed thrombolysis in pulmonary embolism and outcome difference between systemic thrombolysis and catheter-directed thrombolysis. Catheter Cardiovasc Interv 2015;86:1219–27. [DOI] [PubMed] [Google Scholar]
- 8.Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479–86. [DOI] [PubMed] [Google Scholar]
- 9.Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014;311:2414–21. [DOI] [PubMed] [Google Scholar]
- 11.Engelberger RP, Moschovitis A, Fahrni J, Willenberg T, Baumann F, Diehm N, et al. Fixed low-dose ultrasound-assisted catheter-directed thrombolysis for intermediate and high-risk pulmonary embolism. Eur Heart J 2015;36:597–604. [DOI] [PubMed] [Google Scholar]
- 12.Engelberger RP, Kucher N. Ultrasound-assisted thrombolysis for acute pulmonary embolism: a systematic review. Eur Heart J 2014;35:758–64. [DOI] [PubMed] [Google Scholar]
- 13.Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol 2009;20: 1431–40. [DOI] [PubMed] [Google Scholar]
- 14.Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015;8: 1382–92. [DOI] [PubMed] [Google Scholar]
- 15.Kuo WT, Banerjee A, Kim PS, De Marco FJ, Levy JR, Facchini FR, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest 2015;148:667–73. [DOI] [PubMed] [Google Scholar]
- 16.Avgerinos ED, Liang NL, El-Shazly OM, Toma C, Singh MJ, Makaroun MS, et al. Improved early right ventricular function recovery but increased complications with catheter-directed interventions compared with anticoagulation alone for submassive pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2016;4:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becattini C, Agnelli G, Salvi A, Grifoni S, Pancaldi LG, Enea I, et al. Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res 2010;125:e82–6. [DOI] [PubMed] [Google Scholar]
- 18.Cho JH, Kutti Sridharan G, Kim SH, Kaw R, Abburi T, Irfan A, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord 2014;14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang NL, Avgerinos ED, Singh MJ, Makaroun MS, Chaer RA. Systemic thrombolysis increases hemorrhagic stroke risk without survival benefit compared with catheter-directed intervention for the treatment of acute pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2017;5:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–82. [DOI] [PubMed] [Google Scholar]
- 21.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol 2004;44:E1–211. [DOI] [PubMed] [Google Scholar]
- 22.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 2004;66:411–21. [DOI] [PubMed] [Google Scholar]
- 23.Abou Ali AN, Liang NL, Chaer RA, Avgerinos ED. Catheter interventions for pulmonary embolism: are they really that safe? Am J Cardiol 2016;118:307–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ten Wolde M, Sohne M, Quak E, Mac Gillavry MR, Buller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004;164:1685–9. [DOI] [PubMed] [Google Scholar]
- 25.Bloomer TL, El-Hayek GE, McDaniel MC, Sandvall BC, Liberman HA, Devireddy CM, et al. Safety of catheter-directed thrombolysis for massive and submassive pulmonary embolism: results of a multicenter registry and meta-analysis. Catheter Cardiovasc Interv 2017;89:754–60. [DOI] [PubMed] [Google Scholar]


