Abstract
Targeted protein degradation using small chimeric molecules, such as proteolysis-targeting chimeras (PROTACs) and specific and nongenetic inhibitors of apoptosis protein [IAP]-dependent protein erasers (SNIPERs), is a promising technology in drug discovery. We recently developed a novel class of chimeric compounds that recruit the aryl hydrocarbon receptor (AhR) E3 ligase complex and induce the AhR-dependent degradation of target proteins. However, these chimeras contain a hydrophobic AhR E3 ligand, and thus, degrade target proteins even in cells that do not express AhR. In this study, we synthesized new compounds in which the AhR ligands were replaced with a hydrophobic adamantane moiety to investigate the mechanisms of AhR-independent degradation. Our results showed that the compounds, 2, 3, and 16 induced significant degradation of some target proteins in cells that do not express AhR, similar to the chimeras containing AhR ligands. However, in cells expressing AhR, 2, 3, and 16 did not induce the degradation of other target proteins, in contrast with their response to chimeras containing AhR ligands. Overall, it was suggested that target proteins susceptible to the hydrophobic tagging system are degraded by chimeras containing hydrophobic AhR ligands even without AhR.
Keywords: protein degradation, chimeric compound, hydrophobic tagging, adamantane
1. Introduction
A technology for selectively degrading a target protein is expected not only to elucidate the physiological function of proteins but also to develop a therapeutic drug for a disease caused by the aberrant protein. Previous studies reported a series of chimeric compounds that recruit E3 ubiquitin ligases to specifically degrade target proteins via the ubiquitin-proteasome system [1,2]. These chimeras, which are called specific and nongenetic inhibitors of apoptosis protein [IAP]-dependent protein erasers (SNIPERs) and proteolysis-targeting chimeras (PROTACs), contain two ligands connected with a linker: one ligand specific to an E3 ubiquitin ligase and another ligand specific to the target protein. These chimeric compounds are designed to cross-link the E3 ligase with the target protein within the cells, and thereby, induce ubiquitination and subsequent degradation by proteasomes. Several chimeric degraders have successfully been employed to degrade several proteins such as nuclear receptors [3,4,5,6,7], oncogenic kinases [8], epigenetic regulators [9,10], transcription factors [11], and others [12,13]. Some compounds can also degrade target proteins in vivo, suggesting the possibility of clinical applications [5,9,10,14,15]. In contrast to traditional inhibitor-based pharmaceuticals, such as reversible/irreversible inhibitor and antagonists, the chimeras require only the transient binding to any surface of the target to catalytically induce its ubiquitination. Thus, this technology has emerged as a novel therapeutic approach known as “undruggable” proteins.
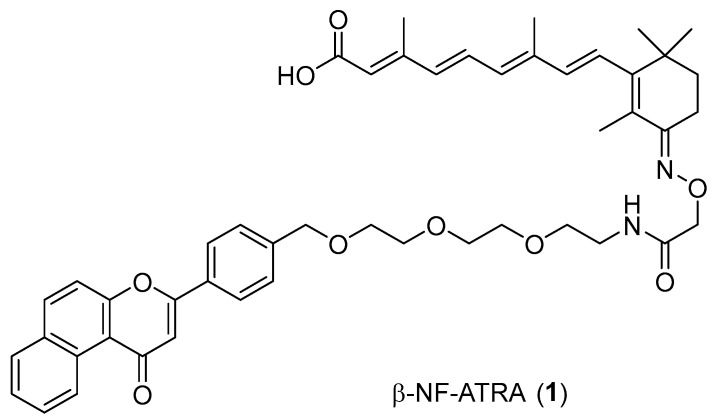
Recently, we developed a new class of small molecule chimeras that recruit the aryl hydrocarbon receptor (AhR) E3 ligase and induce the AhR-dependent degradation of target proteins [16]. β-NF-ATRA, 1 (Figure 1), a chimeric compound directed against cellular retinoic acid-binding proteins (CRABPs), induced the AhR-dependent degradation of CRABP-1 and CRABP-2 in MCF-7 and IMR-32 cells expressing AhR [16]. Interestingly, the 1-induced significant reduction of CRABP-2, but not of CRABP-1, was also observed in SH-SY5Y cells, which do not express AhR (Figures S1 and S2). Similar results were also obtained by the treatment of the ITE-ATRA [16], in which an alternative AhR ligand was used (Figure S3). The ligand-bound AhR forms a CUL4B-based E3 ligase complex [17]. The NEDD8-activating E1 enzyme inhibitor MLN4924 [18,19] completely inhibited the 1-induced reduction of CRABP-1 but partly that of CRABP-2 (Figure S4). These results suggested that these chimeric compounds degrade CRABP-1 and CRABP-2 via an AhR-dependent mechanism, whereas CRABP-2 is also degraded via an AhR-independent mechanism.
Figure 1.
Chemical structure of β-NF-ATRA (1).
In this study, we synthesized several compounds to investigate the mechanisms of AhR-independent degradation and evaluate their activities. These results will provide useful information for the development of chimeric compounds using a highly hydrophobic E3 ligand and their pharmaceutical applications.
2. Results and Discussion
2.1. Design and Synthesis of Compounds
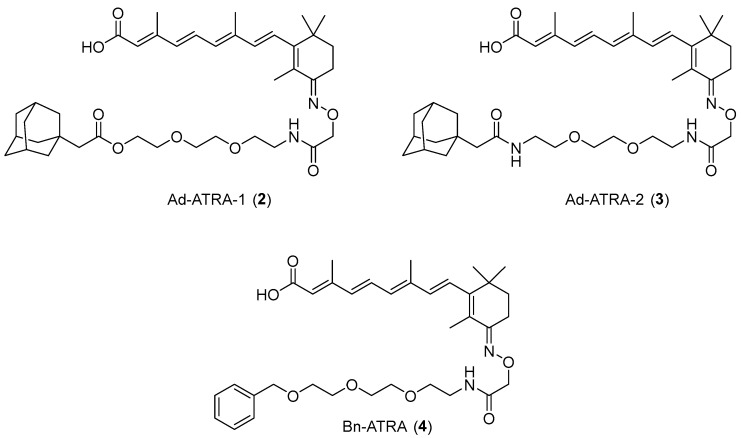
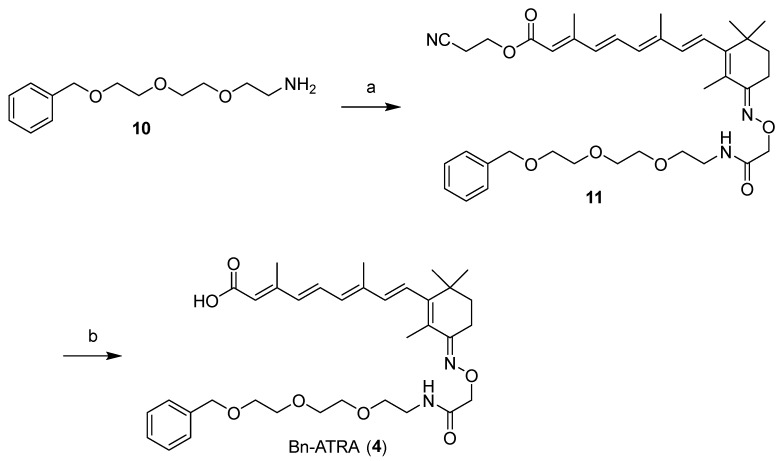
Since CRABP-2 was degraded by 1 and ITE-ATRA in SH-SY5Y cells that do not express AhR (Figures S1–S3), we assumed that the high hydrophobicity of the β-NF ligand module might play a role in the AhR-independent degradation of CRABP-2. Appending a hydrophobic moiety to a protein surface induces protein degradation; the hydrophobic moiety mimics a state of partial unfolding, inducing the degradation of the target protein via an intracellular quality control system [20,21,22]. Boc3-Arg- and adamantane-based hydrophobic tagging (HyT) strategies have been applied to a range of exogenous [23,24] and endogenous proteins [25,26]. To investigate whether CRABP proteins are degraded by the HyT system, we designed and synthesized 2 (Ad-ATRA-1) and 3 (Ad-ATRA-2) by conjugating an adamantane moiety (Figure 2). Besides, we designed 4 that lacked the benzochromene moiety of 1 because we expected that the hydrophobicity of 4 would be smaller than that of 1.
Figure 2.
Chemical structures of designed compounds.
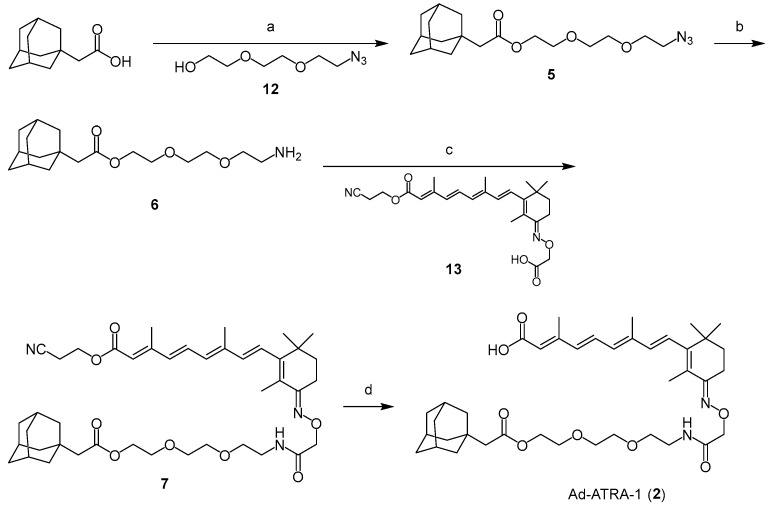
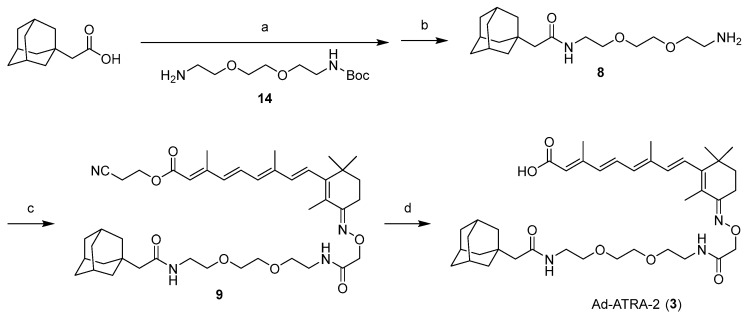
Scheme 1 presents the synthetic route of 2. 1-Adamantanecarboxylic acid was condensed with 12 [27] to obtain 5. Adamantane-ATRA, with an ester bond (Ad-ATRA-1, 2), was obtained after the reduction of the azide group in 5, the condensation with 13 [12], and the deprotection of 7. Adamantane-ATRA, with an amide bond (Ad-ATRA-2, 3) was also prepared via the condensation of 8 with 13, following a similar procedure (Scheme 2). Bn-ATRA (4) was obtained by condensing the corresponding glycol amino linkers with 13, followed by deprotection (Scheme 3).
Scheme 1.
(a) 12, EDC-HCl, DMAP, CH2Cl2; (b) H2 gas, 10% Pd/C, EtOAc; (c) 13, EDC-HCl, DMAP, CH2Cl2; (d) TBAF, THF.
Scheme 2.
(a) 14, EDC-HCl, HOBt, CH2Cl2; (b) 4M HCl/1,4-dioxane; (c) 13, HATU, DIPEA, DMF; (d) TBAF, THF.
Scheme 3.
(a) 13, HATU, DIPEA, DMF; (b) TBAF, THF.
2.2. Evaluation of Protein Degradation Activity
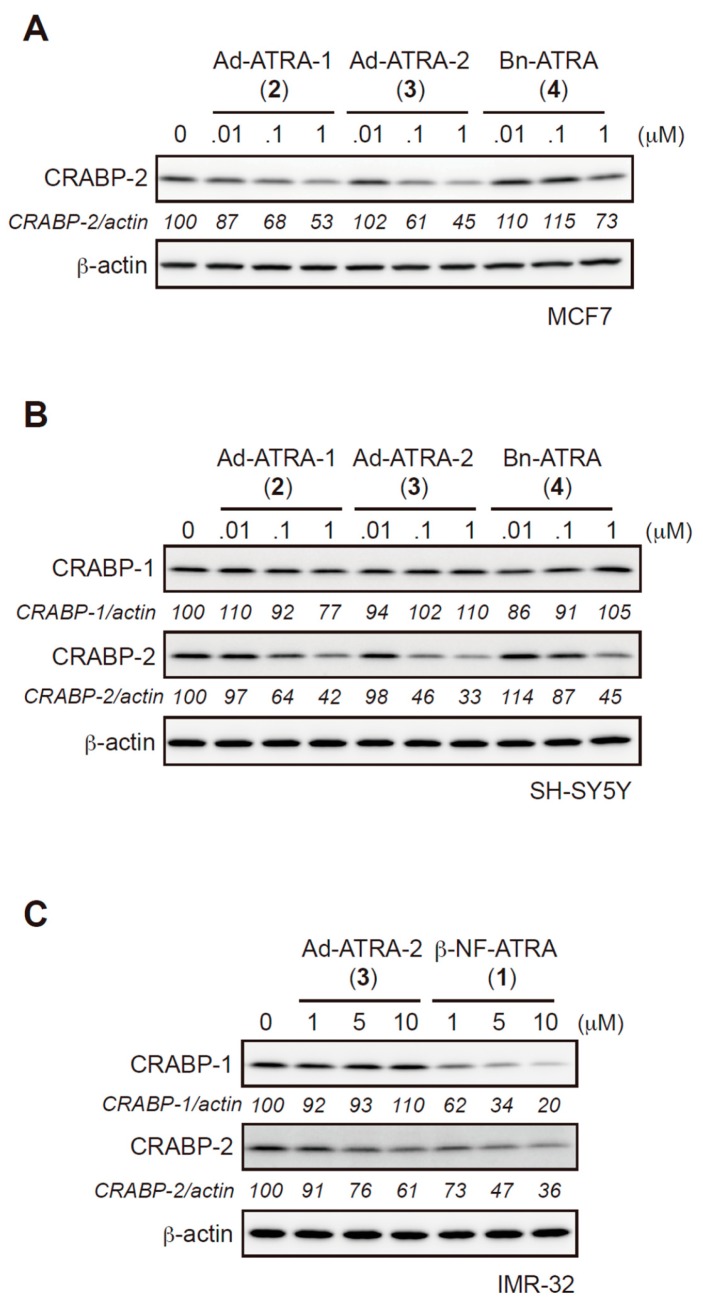
We examined the protein reduction activity of 2, 3, and 4 using various cell lines. In MCF-7 cells; 2 and 3 effectively reduced the CRABP-2 protein level, whereas 4, which contained the less hydrophobic benzyl moiety, showed attenuated protein knockdown activity (Figure 3A and Figure S5). The CRABP2 reduction activity of 3 was similar to that of 1 in MCF7 cells (Figure S6). The CRABP2 knockdown activities of 2, 3, and 4 in SH-SY5Y cells were similar to their activities in MCF-7 cells (Figure 3B). The protein level of CRABP-1 was not significantly reduced by 2, 3, and 4 in SH-SY5Y cells (Figure 3B). Besides, 3 tended to reduce CRABP-2 in IMR-32 cells that expressed AhR but not CRABP-1 in contrast to 1 (Figure S1 and Figure 3C). These results indicated that CRABP-2, but not CRABP-1, was susceptible to HyT-mediated degradation. Thus, 1, which contains the highly hydrophobic β-NF ligand, is likely to degrade CRABP-2 in the absence of AhR via the HyT mechanism.
Figure 3.
HyT compounds induce the degradation of CRABP-2 but not of CRABP-1. (A–C) Protein knockdown activities of HyT 2, 3, and 4 or β-NF-ATRA 1 in MCF-7 (A), SH-SY5Y (B), or IMR-32 cells (C). Cells were treated with the indicated compounds for 24 h. Whole-cell lysates were analyzed by western blotting. The numbers below the CRABP-1 and CRABP-2 panels represent CRABP-1/actin and CRABP-2/actin ratios, respectively, normalized by designating the expression from the vehicle control condition as 100%.
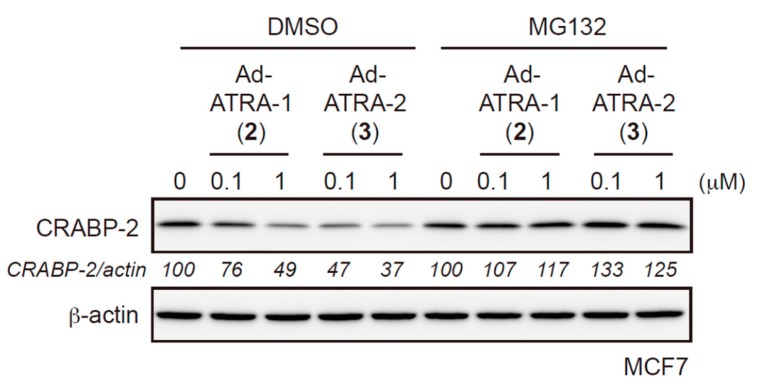
We also examined the involvement of the proteasome in HyT-mediated degradation. The 2 and 3-induced reductions of CRABP-2 were completely inhibited in the presence of the proteasome inhibitor MG132, suggesting that 2 and 3 induce the proteasomal degradation of CRABP-2 (Figure 4). This result clearly indicated the involvement of proteasome activity for the reduction of the target protein. However, this result could not deny the other possibilities, such as lysosomal degradations, or insolubilization by Lys63-linked ubiquitination. It is important to reveal the complexed pathway leading to the proteasome.
Figure 4.
Proteasomal inhibitor MG132 inhibits the reduction of CRABP-2 by HyT compounds. MCF-7 cells were incubated with the indicated concentrations of HyT compounds in the presence or absence of 10 µM MG132 for 18 h. Whole-cell lysates were analyzed by western blotting.
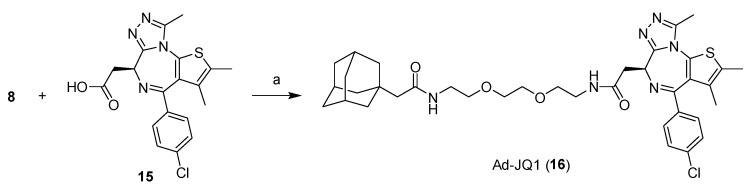
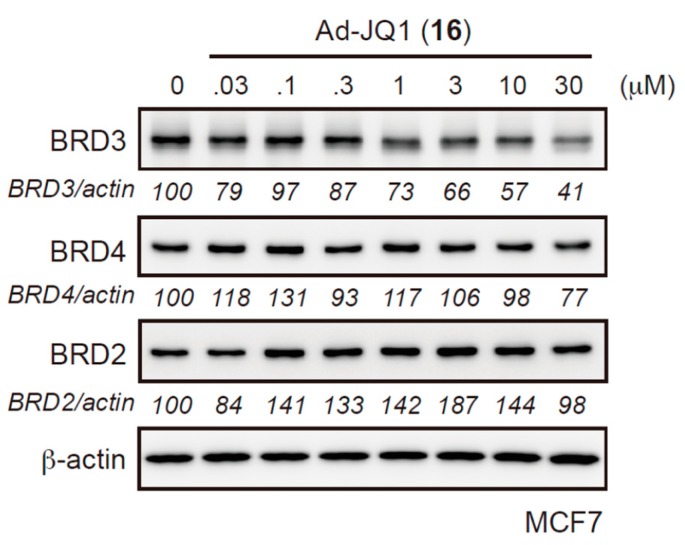
Next, we expanded the investigation to other target proteins. We previously developed the chimeric compound β-NF-JQ1 that is directed against bromodomain-containing (BRD) proteins using β-NF or (+)-JQ1 as an AhR or BRD ligand, respectively. β-NF-JQ1 induced the AhR-dependent degradation of BRD proteins in MCF-7 cells expressing AhR [16]. However, the β-NF-JQ1-induced reduction of BRD3, but not of BRD2 or BRD4, was also observed in SH-SY5Y cells, which do not express AhR (Figure S7), suggesting that BRD3 is also degraded by the AhR-independent mechanism. Therefore, we synthesized Ad-JQ1 (15) that conjugated an adamantane moiety to JQ1 (Scheme 4). Figure 5 presents the significant Ad-JQ1-induced reduction of BRD3, but not of BRD2 or BRD4 (Figure 5). These results suggested that BRD3, but not others, is susceptible to HyT-mediated degradation.
Scheme 4.
(a) HBTU, DIPEA, and DMF.
Figure 5.
Ad-JQ1 (16) induces the degradation of BRD3 but not of BRD2 or BRD4. Protein knockdown activities of Ad-JQ1 in MCF-7 cells. Cells were treated with the indicated concentration of Ad-JQ1 for 48 h. Whole-cell lysates were analyzed by western blotting.
In the present study, we designed and synthesized compounds with adamantane moiety to investigate whether the AhR-independent degradation of CRABP-2 and BRD3 by the chimeric compounds using AhR ligands are induced by the HyT system. We observed that 2, 3, and 16 induced significant degradation of CRABP-2 and BRD3, but not of other target proteins, by the proteasome. Besides, Bn-ATRA 4, which is less hydrophobic than 1, 2, and 3, induced a weaker activity than 1, 2, and 3. In conclusion, these results indicated that CRABP-2 and BRD3 are susceptible to HyT-mediated degradation and that the AhR-independent reduction of these proteins was caused by the HyT system. These results also provided useful information for drug development using chimeric compounds with a highly hydrophobic E3 ligand, which may occasionally induce targeted protein degradation by the HyT system. In addition to our findings, it is important to clarify the types of ubiquitin chains on target proteins and the specific E3 ligases involved in the ubiquitination.
3. Materials and Methods
All reagents and solvents were purchased from Sigma-Aldrich, Wako Pure Chemical, and Tokyo Chemical Industry and used without purification. Analytical TLC was conducted using Merck silica gel 60 F254 pre-coated plates, and visualized with a 254 nm UV lamp using phosphomolybdic acid, p-anisaldehyde, or ninhydrin stains. Column chromatography was performed using silica gel (spherical, neutral) purchased from Kanto Chemical. 1H and 13C NMR spectra were measured using a Varian AS 400 spectrometer or a JEOL ECZ 600R spectrometer, and measurements were carried out using deuterated solvents. Chemical shifts are expressed in ppm downfield from a solvent residual peak or the internal standard TMS. Mass spectra were measured using a Shimadzu IT-TOF MS equipped with an electrospray ionization source.
(2E,4E,6E,8E)-9-((E)-3-(((14-((3r,5r,7r)-adamantan-1-yl)-2,13-dioxo-6,9,12-trioxa-3-azatetradecyl)oxy)imino)-2,6,6-trimethylcyclohex-1-en-1-yl)-3,7-dimethylnona-2,4,6,8-tetraenoic acid (Ad-ATRA-1, 2)
EDC-HCl (120 mg, 0.63 mmol), followed by DMAP (15 mg, 0.12 mmol), was added to a mixture of 1-adamantaneacetic acid (100 mg, 0.52 mmol) and 2-(2-(2-azidoethoxy)ethoxy)ethan-1-ol (12) [27] (108 mg, 0.62 mmol) in CH2Cl2 (2 mL). After continuous stirring for two days at room temperature, the reaction mixture was diluted with EtOAc and washed with 2M HCl, saturated with aqueous NaHCO3 and brine. The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexanes/EtOAc = 7:3) to obtain 5 as a colorless oil (47 mg, 26%). 1H NMR (400 MHz, CDCl3) δ 4.23–4.21 (m, 2H), 3.72–3.66 (m, 8H), 3.40–3.38 (m, 2H), 2.10 (s, 2H), 1.97 (br. s, 3H), 1.72–1.62 (m, 12H); 13C NMR (150 MHz, CDCl3) δ 171.8, 70.6, 70.5, 70.0, 69.2, 62.8, 50.5, 48.7, 42.2, 36.6, 32.7, 28.5. ESI-HRMS calcd for C18H30N3O4 [M+H]+: 352.2231, found: 352.2194.
A mixture of 5 (12 mg, 0.03 mmol) in EtOAc (10 mL) with 10% Pd/C (7.3 mg) was stirred for 13 h under H2 gas at room temperature and then, filtered through Celite to remove the catalyst. The filtrate was concentrated under reduced pressure to obtain the crude product (10.5 mg), which was used in the following step without further purification. EDC-HCl (14.6 mg, 0.076 mmol), followed by DMAP (9.3 mg, 0.076 mmol), was added to the mixture of the crude product (10.5 mg as 0.03 mmol) and 13 [12] (14.4 mg, 0.033 mmol) in CH2Cl2 (2 mL) After continuous stirring for 23 h at room temperature, the reaction mixture was diluted with CH2Cl2 and washed with brine. The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexanes/EtOAc = 3:1) to obtain 7 as a yellow oil (7.9 mg, 31% for two steps). 1H NMR (400 MHz, CDCl3) δ 7.03 (dd, J = 15.0, 15.0 Hz, 1H), 6.67 (t, J = 5.4 Hz, 1H), 6.35 (d, J = 15.6 Hz, 1H), 6.33–6.30 (m, 1H), 6.22 (d, J = 15.0 Hz, 1H), 6.21 (d, J = 15.6 Hz, 1H), 5.82 (s, 1H), 4.59 (s, 2H), 4.33 (t, J = 6.0 Hz, 2H), 4.21 (t, J = 6.0 Hz, 2H), 3.67 (t, J = 4.8 Hz, 2H), 3.62–3.60 (m, 4H), 3.58 (t, J = 4.8 Hz, 2H), 3.54–3.51 (m, 2H), 2.74 (t, J = 6.0 Hz, 2H), 2.67 (t, J = 6.6 Hz, 2H), 2.37 (s, 3H), 2.09 (s, 2H), 2.03 (s, 3H), 1.96 (s, 3H), 1.87 (s, 3H), 1.73–1.69 (m, 2H), 1.63–1.61 (m, 12H), 1.10 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 171.6, 170.1, 166.1, 158.6, 154.4, 150.0, 139.1, 139.1, 135.9, 131.3, 127.0, 125.0, 117.6, 117.0, 73.1, 70.4, 70.3, 70.0, 69.2, 62.8, 58.0, 48.8, 42.3, 38.6, 38.6, 36.7, 36.0, 34.8, 32.7, 28.6, 27.6, 20.2, 18.1, 14.8, 14.0, 12.8. ESI-HRMS calcd for C43H62N3O8 [M+H]+: 748.4531, found: 748.4529.
TBAF (1 M in THF solution, 43 µL, 0.043 mmol) was added to a solution of 7 (8 mg, 0.010 mmol) in THF (0.19 mL). After continuous stirring for 1.5 h at room temperature, the reaction mixture was diluted with CHCl3, containing 0.1% AcOH and then, concentrated under reduced pressure. The residue was purified by flash silica gel column chromatography (CHCl3/MeOH = 99:1 to 98:2) to obtain 2 as a yellow solid (4.0 mg, 54%, containing ca. 10% of the 13-cis isomer). 1H NMR (400 MHz, CDCl3) δ 7.03 (dd, J = 15.6, 15.6 Hz, 1H), 6.68 (t, J = 5.7 Hz, 1H), 6.36 (d, J = 15.0 Hz, 1H), 6.31 (d, J = 16.2 Hz, 1H), 6.23–6.21 (m, 2H), 5.83 (s, 1H), 4.59 (s, 2H), 4.21 (t, J = 5.1 Hz, 2H), 3.67 (t, J = 4.8 Hz, 2H), 3.61–3.52 (m, 8H), 2.67 (t, J = 6.6 Hz, 2H), 2.37 (s, 3H), 2.03 (s, 3H), 1.96 (br., 4H), 1.87 (s, 3H), 1.69 (d, J = 12.0 Hz, 3H), 1.63–1.61 (m, 12H), 1.10 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 171.7, 170.6, 170.2, 158.7, 154.7, 150.1, 139.2, 139.0, 136.2, 131.4, 131.2, 127.0, 125.0, 118.1, 73.1, 70.4, 70.3, 69.9, 69.3, 62.8, 48.8, 42.3, 38.7, 36.7, 36.0, 34.9, 32.8, 28.6, 27.6, 20.2, 14.9, 14.0, 12.9. ESI-HRMS calcd for C40H59N2O8 [M+H]+: 695.4266, found: 695.4226.
(2E,4E,6E,8E)-9-((E)-3-(((14-((3r,5r,7r)-adamantan-1-yl)-2,13-dioxo-6,9-dioxa-3,12-diazatetradecyl)oxy)imino)-2,6,6-trimethylcyclohex-1-en-1-yl)-3,7-dimethylnona-2,4,6,8-tetraenoic acid (Ad-ATRA-2, 3)
EDC-HCl (144 mg, 0.75 mmol) was added to a mixture of 1-adamantaneacetic acid (97 mg, 0.50 mmol), N-(tert-butoxycarbonyl)-2,2′-(ethylenedioxy)diethylamine 14 (149 mg, 0.60 mmol) and HOBt-H2O (0.75 mmol) in CH2Cl2 (1.0 mL). After continuous stirring for 1.5 h at room temperature, the reaction mixture was diluted with EtOAc and washed with 1M HCl, saturated aqueous NaHCO3 and brine. The organic layer was dried over Na2SO4 and concentrated under reduced pressure to give a colorless oil (238 mg). This crude product (200 mg, as 0.47 mmol) was treated with 4M HCl/1,4-dioxane (1 mL) for 8 h at room temperature. The volatiles were removed by evaporation under reduced pressure and subsequent co-evaporation with CH3CN (1 mL × 2) under reduced pressure. HCl salt was obtained as a colorless oil (8), which was used in the following reaction without further purification. HATU (76 mg, 0.20 mmol), followed by DIPEA (104 µL, 0.60 mmol), was added to a mixture of 13 (44 mg, 0.10 mmol) and 8 (54 mg, as 0.15 mmol) in DMF (0.3 mL). After continuous stirring for 12 h at room temperature, the reaction mixture was diluted with EtOAc and washed with 1 M HCl, saturated with aqueous NaHCO3 and brine. The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3/MeOH = 100:0 to 99:1) to obtain 9 as a yellowish amorphous solid (56 mg, 75%). 1H NMR (400 MHz, CDCl3) δ 7.04 (dd, J = 15.6, 15.0 Hz, 1H), 6.67 (t, J = 5.4 Hz, 1H), 6.35 (d, J = 15.0 Hz, 1H), 6.32 (t, J = 15.6 Hz, 1H), 6.24–6.21 (m, 2H), 5.91 (br. s, 1H), 5.83 (s, 1H), 4.59 (s, 2H), 4.33 (t, J = 6.0 Hz, 2H), 3.62–3.52 (m, 10H), 3.47–3.42 (m, 2H), 2.74 (t, J = 6.0 Hz, 2H), 2.67 (t, J = 6.6 Hz, 1H), 2.37 (s, 3H), 2.04 (s, 3H), 1.96 (s, 3H), 1.93 (s, 2H), 1.87 (s, 3H), 1.71–1.69 (m, 3H), 1.63–1.62 (m, 12H), 1.11 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 170.9, 170.2, 166.1, 158.7, 154.3, 150.1, 139.1, 139.0, 135.9, 131.3, 131.2, 126.9, 124.8, 117.5, 116.9, 73.0, 70.2, 70.1, 70.0, 69.8, 58.0, 51.6, 42.5, 38.9, 38.6, 36.7, 35.9, 34.8, 32.6, 28.6, 27.5, 20.1, 18.0, 14.8, 13.9, 12.8. ESI-HRMS calcd for C43H63N4O7 [M+H]+: 747.4691, found: 747.4655.
TBAF (1 M in THF solution, 117 µL, 117.0 µmol) was added to a solution of 9 (29 mg, 0.039 mmol) in THF (0.7 mL). After continuous stirring for 1.5 h at room temperature, the reaction mixture was concentrated under reduced pressure. The residue was purified by flash silica gel column chromatography (CHCl3/MeOH = 100:0 to 98:2) to obtain 3 as a yellowish amorphous solid (11 mg, 42%, containing 10% of the 13-cis isomer). 1H NMR (400 MHz, CDCl3) δ 7.02 (dd, J = 15.0, 15.0 Hz, 1H), 6.69 (t, J = 6.0 Hz, 1H), 6.34 (d, J = 15.0 Hz, 1H), 6.32 (d, J = 16.8 Hz, 1H), 6.29–6.19 (m, 2H), 5.93 (br. t, 1H), 5.83 (s, 1H), 4.60 (s, 2H), 3.62–3.53 (m, 10H), 3.45 (t, J = 5.6 Hz, 2H), 2.67 (t, J = 6.6 Hz, 2H), 2.37 (s, 3H), 2.03 (s, 3H), 1.96 (br. s, 3H), 1.94 (s, 2H), 1.87 (s, 3H), 1.69 (d, J = 12.0 Hz, 3H), 1.63–1.61 (m, 11H), 1.10 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 171.1, 170.8, 170.4, 158.8, 154.3, 150.2, 139.2, 138.9, 136.3, 131.5, 131.1, 126.8, 124.8, 118.5, 73.0, 70.2, 70.1 (1C overlapped), 69.9, 51.7, 42.6, 39.0, 38.7, 36.7, 35.9, 34.8, 32.7, 28.6, 27.6, 20.2, 14.9, 14.0, 12.8. ESI-HRMS calcd for C40H60N3O7 [M+H]+: 694.4426, found: 694.4391.
(2E,4E,6E,8E)-3,7-dimethyl-9-((E)-2,6,6-trimethyl-3-(((12-oxo-1-phenyl-2,5,8-trioxa-11-azatridecan-13-yl)oxy)imino)cyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoic acid) (Bn-ATRA, 4)
HATU (46 mg, 0.12 mmol), followed by DIPEA (42 µL, 0.24 mmol), were added to a mixture of 13 (44 mg, 0.10 mmol) and 10 [28,29] (48 mg, 0.20 mmol) in DMF (2 mL). After continuous stirring for 12 h at room temperature, the reaction mixture was diluted with EtOAc and washed with 1 M HCl, saturated with aqueous NaHCO3 and brine. The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3/MeOH = 100:0 to 99:1) to obtain 11 as a yellow oil (41 mg, 62%). 1H NMR (600 MHz, CDCl3) δ 7.34–7.33 (m, 4H), 7.29–7.27 (m, 1H), 7.03 (dd, J = 15.6, 15.0 Hz, 1H), 6.69 (br. t, J = 5.4 Hz, 1H), 6.33 (d, J = 15.0 Hz, 1H), 6.31 (d, J = 15.6 Hz, 1H), 6.22 (d, J = 15.0 Hz, 1H), 6.22 (d, J = 15.0 Hz, 1H), 5.82 (s, 1H), 4.58 (s, 2H), 4.56 (s, 2H), 4.32 (t, J = 6.0 Hz, 2H), 3.67–3.65 (m, 2H), 3.64–3.62 (m, 6H), 3.59–3.57 (m, 2H), 3.53–3.51 (m, 2H), 2.74 (t, J = 6.0 Hz, 2H), 2.66 (t, J = 6.6 Hz, 2H), 2.37 (s, 3H), 2.03 (s, 3H), 1.87 (s, 3H), 1.61 (t, J = 6.6 Hz, 2H), 1.09 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 170.0, 166.1, 158.5, 154.3, 149.9, 139.0, 138.0, 135.8, 131.3, 131.2, 128.3, 127.6, 127.5, 127.0, 124.9, 117.5, 117.0, 73.2, 73.0, 70.6, 70.4, 70.2, 69.8, 69.3, 58.0, 38.6, 38.5, 35.9, 34.8, 27.5, 20.1, 18.0, 14.8, 13.9, 12.8. ESI-HRMS calcd for C38H52N3O7 [M+H]+: 662.3800, found: 662.3834.
TBAF (1 M in THF solution, 99 µL, 0.099 mmol) was added to a solution of 11 (22 mg, 0.033 mmol) in THF (0.6 mL). After continuous stirring for 1.5 h at room temperature, the reaction mixture was diluted with CHCl3, containing 0.1% AcOH, and then, concentrated under reduced pressure. The residue was purified by flash silica gel column chromatography (CHCl3/MeOH = 100:0 to 98:2) to obtain 4 as a yellow solid (8.6 mg, 44%, containing ca. 10% of the 13-cis isomer). 1H NMR (400 MHz, CDCl3) δ 7.34–7.33 (m, 5H), 7.06–6.99 (m, 1H), 6.66 (br. t, J = 5.6 Hz, 1H), 6.37–6.19 (m, 4H), 5.83 (s, 1H), 4.58 (s, 2H), 4.56 (s, 2H), 3.66–3.62 (m, 8H), 3.59–3.57 (m, 2H), 3.54–3.49 (m, 2H), 2.66 (t, J = 6.8 Hz, 2H), 2.37 (s, 3H), 2.02 (s, 3H), 1.87 (s, 3H), 1.61 (t, J = 6.8 Hz, 2H), 1.10 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 171.3, 170.3, 158.7, 154.6, 150.0, 139.1, 139.0, 138.1, 136.2, 131.4, 131.2, 128.4, 127.7, 127.6, 127.0, 125.0, 118.4, 73.3, 73.1, 70.6, 70.5, 70.3, 69.9, 69.4, 38.7, 36.0, 34.8, 27.6, 20.2, 14.9, 14.0, 12.8. ESI-HRMS calcd for C35H49N2O7 [M+H]+: 609.3534, found: 609.3497.
2-((3r,5r,7r)-adamantan-1-yl)-N-(2-(2-(2-(2-((S)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetamido)ethoxy)ethoxy)ethyl)acetamide (Ad-JQ1, 16)
HBTU (21 mg, 0.055 mmol), followed by DIPEA (19.6 mg, 0.15 mmol), was added to a mixture of 8 (10.8 mg, 0.03 mmol) and 15 [10] in DMF (2 mL). After continuous stirring for 0.5 h, the reaction mixture was diluted with EtOAc and washed with 1M HCl, saturated aqueous NaHCO3, and brine. The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3/MeOH = 100:0 to 99:1) to obtain 16 as a yellow oil (6.0 mg, 34%). 1H NMR (600 MHz, CDCl3) δ 7.40 (d, J = 8.5 Hz, 2H), 7.32 (d, J = 8.9 Hz, 2H), 7.09 (dd, J = 5.4 Hz, 1H), 6.52–6.46 (m, 1H), 4.63 (dd, J = 8.1, 6.1 Hz, 1H), 3.66–3.53 (m, 10H), 3.52–3.41 (m, 3H), 3.32 (dd, J = 14.3, 6.1 Hz, 1H), 2.65 (s, 3H), 2.39 (s, 3H), 1.90 (s, 2H), 1.89–1.86 (m, 3H), 1.67 (d, J = 0.9 Hz, 3H), 1.65–1.53 (m, 12H). 13C NMR (150 MHz, CDCl3) δ 171.41, 170.57, 164.03, 155.74, 149.93, 136.94, 136.64, 132.15, 131.03, 131.00, 130.62, 129.93, 128.81, 77.32, 77.10, 76.89, 70.40, 70.37, 69.89, 54.61, 51.55, 42.71, 42.61, 39.47, 39.18, 36.87, 32.75, 28.73, 14.48, 13.19, 11.90, 0.08. ESI-HRMS calcd for C37H48N6ClO4S [M+H]+: 707.3141, found: 707.3210.
3.1. Cell Culture
Human breast carcinoma MCF-7 cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and 100 µg ml-1 kanamycin. Human neuroblastoma SH-SY5Y cells were maintained in Dulbecco’s modified Eagle’s medium, containing 10% FBS and 100 µg ml-1 kanamycin. Human neuroblastoma IMR-32 cells were maintained in Eagle’s minimum essential medium, containing 10% FBS and 100 µg ml-1 kanamycin. Cells were treated with various concentrations of compounds for the indicated duration.
3.2. Western Blotting
Cells were lysed with SDS lysis buffer (0.1 M Tris-HCl at pH 8.0, 10% glycerol, 1% SDS) and immediately boiled for 10 min to obtain clear lysates. Protein concentrations were measured using the BCA method (Pierce). Lysates containing equal amounts of proteins were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, MA, USA) for western blot analysis using the appropriate antibodies. Immunoreactive proteins were visualized using the Immobilon Western chemiluminescent HRP substrate (Millipore, MA, USA) or the Clarity Western ECL substrate (Bio-Rad, CA, USA). Light emission intensity was quantified using a LAS-3000 luminous-image analyzer equipped with Image Gauge 2.3 (Fuji, Japan). The antibodies used in this study were: anti-CRABP-2 rabbit polyclonal antibody (pAb) (Bethyl, A300-809A), anti-CRABP-1 rabbit monoclonal antibody (mAb) (Cell Signaling Technology, 13206), and anti-AhR rabbit pAb (Cell Signaling Technology, 13790). All Western blotting were performed several times to confirm reproducibility. The typical results were shown here.
3.3. Cell Viability Assay
Cell viability was determined using water-soluble tetrazolium WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) in a spectrophotometric assay, according to the manufacturer’s instructions (Dojindo, Japan). Cells treated with compounds were incubated with WST-8 for 0.5 h at 37 °C in a humidified atmosphere of 5% CO2. The absorbance at 450 nm was measured using an EnVision Multilabel Plate Reader (PerkinElmer, USA).
Abbreviations
| PROTAC | proteolysis-targeting chimeras |
| SNIPER | specific and nongenetic inhibitors of apoptosis protein [IAP]-dependent protein erasers |
| AhR | aryl hydrocarbon receptor |
| β-NF | β-naphthoflavone |
| ATRA | all-trans retinoic acid |
| CRABPs | cellular retinoic acid-binding proteins |
| ITE | 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester |
| HyT | hydrophobic tagging |
| Ad | adamantane |
| HATU | 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-Oxide Hexafluorophosphate |
| DIPEA | N,N-diisopropylethylamine |
| DMF | N,N-dimethylformamide |
| TBAF | tetrabutylammonium Fluoride |
| THF | tetrahydrofuran |
| HBTU | 1-[bis(dimethylamino)methylene]-1H-benzotriazolium 3-Oxide Hexafluorophosphate |
| TLC | thin-layer chromatography |
| UV | ultra-violet |
| NMR | nuclear magnetic resonance |
| TMS | tetramethylsilane |
| IT-TOF MS | ion trap-time-of-flight mass spectrometry |
| calcd | caluculated |
| DMAP | N,N-dimethyl-4-aminopyridine |
Supplementary Materials
The following is available online at https://www.mdpi.com/1424-8247/13/3/34/s1, Figure S1: Levels of CRABP-1, CRABP-2, and AhR in the indicated cancer cells, Figure S2: Protein knockdown activity of β-NF-ATRA. SH-SY5Y cells were treated with β-NF-ATRA for 24 h. Whole-cell lysates were analyzed by western blotting, Figure S3: Chemical structure and protein knockdown activity of ITE-ATRA. SH-SY5Y cells were treated with ITE-ATRA for 8 h. Whole-cell lysates were analyzed by western blotting, Figure S4: Inhibitory effect of MLN4924 on IMR-32 cells. Cells were treated with β-NF-ATRA in the presence or absence of 10 µM MLN4924, Figure S5: Statistical analysis of protein knockdown activityof HyT 2, 3, and 4.in MCF-7 cells, Figure S6: Protein knockdown activity of the indicated compounds. MCF-7 cells were treated with the compounds for 24 h. Whole-cell lysates were analyzed by western blotting, Figure S7: Chemical structure and protein knockdown activity of β-NF-JQ1. SH-SY5Y cells were treated with β-NF-JQ1 for 24 h. Whole-cell lysates were analyzed by western blotting.
Author Contributions
T.S. and N.O. conceived and designed the research; T.S., T.F., and M.K. designed the compounds; T.S., T.F., H.I., G.T., Y.D., and M.K. synthesized the compounds; T.S., G.T., and N.O. wrote the manuscript; and T.S., N.O., Y.D., M.N., and M.K. supervised all research. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by grants from the Japan Society for the Promotion of Science (KAKENHI Grants JP19K07009 to T.S., JP18K06567 to N.O., JP17K08385 to Y.D., JP16H05090, JP16K15121 and JP18H05502 to M.N., and JP16K08340 to M.K.) and the Japan Agency for Medical Research and Development (AMED Grants JP19ak0101073 and JP19im0210616 to M.N. and JP19cm0106136 and JP19ak0101073 to N.O.).
Conflicts of Interest
M.N. received a research fund from Daiichi Sankyo Pharmaceutical Co., Ltd. All other authors declare no conflict of interest.
References
- 1.Ohoka N., Shibata N., Hattori T., Naito M. Protein Knockdown Technology: Application of Ubiquitin Ligase to Cancer Therapy. Curr. Cancer Drug Targets. 2016;16:136–146. doi: 10.2174/1568009616666151112122502. [DOI] [PubMed] [Google Scholar]
- 2.Pettersson M., Crews C.M. PROteolysis TArgeting Chimeras (PROTACs)-Past, present and future. Drug Discov. Today Technol. 2019;31:15–27. doi: 10.1016/j.ddtec.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demizu Y., Okuhira K., Motoi H., Ohno A., Shoda T., Fukuhara K., Okuda H., Naito M., Kurihara M. Design and synthesis of estrogen receptor degradation inducer based on a protein knockdown strategy. Bioorg. Med. Chem. Lett. 2012;22:1793–1796. doi: 10.1016/j.bmcl.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 4.Bondeson D.P., Mares A., Smith I.E., Ko E., Campos S., Miah A.H., Mulholland K.E., Routly N., Buckley D.L., Gustafson J.L., et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 2015;11:611–617. doi: 10.1038/nchembio.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohoka N., Okuhira K., Ito M., Nagai K., Shibata N., Hattori T., Ujikawa O., Shimokawa K., Sano O., Koyama R., et al. In Vivo Knockdown of Pathogenic Proteins via Specific and Nongenetic Inhibitor of Apoptosis Protein (IAP)-dependent Protein Erasers (SNIPERs) J. Biol. Chem. 2017;292:4556–4570. doi: 10.1074/jbc.M116.768853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata N., Nagai K., Morita Y., Ujikawa O., Ohoka N., Hattori T., Koyama R., Sano O., Imaeda Y., Nara H., et al. Development of Protein Degradation Inducers of Androgen Receptor by Conjugation of Androgen Receptor Ligands and Inhibitor of Apoptosis Protein Ligands. J. Med. Chem. 2018;61:543–575. doi: 10.1021/acs.jmedchem.7b00168. [DOI] [PubMed] [Google Scholar]
- 7.Salami J., Alabi S., Willard R.R., Vitale N.J., Wang J., Dong H., Jin M., McDonnell D.P., Crew A.P., Neklesa T.K., et al. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun. Biol. 2018;1:100. doi: 10.1038/s42003-018-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata N., Shimokawa K., Nagai K., Ohoka N., Hattori T., Miyamoto N., Ujikawa O., Sameshima T., Nara H., Cho N., et al. Pharmacological difference between degrader and inhibitor against oncogenic BCR-ABL kinase. Sci. Rep. 2018;8:13549. doi: 10.1038/s41598-018-31913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raina K., Lu J., Qian Y., Altieri M., Gordon D., Rossi A.M., Wang J., Chen X., Dong H., Siu K., et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA. 2016;113:7124–7129. doi: 10.1073/pnas.1521738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter G.E., Buckley D.L., Paulk J., Roberts J.M., Souza A., Dhe-Paganon S., Bradner J.E. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348:1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCoull W., Cheung T., Anderson E., Barton P., Burgess J., Byth K., Cao Q., Castaldi M.P., Chen H., Chiarparin E., et al. Development of a Novel B-Cell Lymphoma 6 (BCL6) PROTAC To Provide Insight into Small Molecule Targeting of BCL6. ACS Chem. Biol. 2018;13:3131–3141. doi: 10.1021/acschembio.8b00698. [DOI] [PubMed] [Google Scholar]
- 12.Itoh Y., Ishikawa M., Naito M., Hashimoto Y. Protein knockdown using methyl bestatin-ligand hybrid molecules: Design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins. J. Am. Chem. Soc. 2010;132:5820–5826. doi: 10.1021/ja100691p. [DOI] [PubMed] [Google Scholar]
- 13.Okuhira K., Ohoka N., Sai K., Nishimaki-Mogami T., Itoh Y., Ishikawa M., Hashimoto Y., Naito M. Specific degradation of CRABP-II via cIAP1-mediated ubiquitylation induced by hybrid molecules that crosslink cIAP1 and the target protein. FEBS Lett. 2011;585:1147–1152. doi: 10.1016/j.febslet.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Han Z., Bondeson J.C., Lewis J.H., Mannarino E.G., Friesen S.A., Wagar M.M., Balboni T.A., Alexander B.M., Arvold N.D., Sher D.J., et al. Evaluation of initial setup accuracy and intrafraction motion for spine stereotactic body radiation therapy using stereotactic body frames. Pract. Radiat. Oncol. 2016;6:e17–e24. doi: 10.1016/j.prro.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Toure M., Crews C.M. Small-Molecule PROTACS: New Approaches to Protein Degradation. Angew. Chem. Int. Ed. Engl. 2016;55:1966–1973. doi: 10.1002/anie.201507978. [DOI] [PubMed] [Google Scholar]
- 16.Ohoka N., Tsuji G., Shoda T., Fujisato T., Kurihara M., Demizu Y., Naito M. Development of Small Molecule Chimeras That Recruit AhR E3 Ligase to Target Proteins. ACS Chem. Biol. 2019;14:2822–2832. doi: 10.1021/acschembio.9b00704. [DOI] [PubMed] [Google Scholar]
- 17.Ohtake F., Takeyama K., Matsumoto T., Kitagawa H., Yamamoto Y., Nohara K., Tohyama C., Krust A., Mimura J., Chambon P., et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 18.Soucy T.A., Smith P.G., Rolfe M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin. Cancer Res. 2009;15:3912–3916. doi: 10.1158/1078-0432.CCR-09-0343. [DOI] [PubMed] [Google Scholar]
- 19.Soucy T.A., Smith P.G., Milhollen M.A., Berger A.J., Gavin J.M., Adhikari S., Brownell J.E., Burke K.E., Cardin D.P., Critchley S., et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 20.Lai A.C., Crews C.M. Induced protein degradation: An emerging drug discovery paradigm. Nat. Rev. Drug Discov. 2017;16:101–114. doi: 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burslem G.M., Crews C.M. Small-Molecule Modulation of Protein Homeostasis. Chem. Rev. 2017;117:11269–11301. doi: 10.1021/acs.chemrev.7b00077. [DOI] [PubMed] [Google Scholar]
- 22.Cromm P.M., Crews C.M. Targeted Protein Degradation: From Chemical Biology to Drug Discovery. Cell Chem. Biol. 2017;24:1181–1190. doi: 10.1016/j.chembiol.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neklesa T.K., Tae H.S., Schneekloth A.R., Stulberg M.J., Corson T.W., Sundberg T.B., Raina K., Holley S.A., Crews C.M. Small-molecule hydrophobic tagging-induced degradation of HaloTag fusion proteins. Nat. Chem. Biol. 2011;7:538–543. doi: 10.1038/nchembio.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long M.J., Gollapalli D.R., Hedstrom L. Inhibitor mediated protein degradation. Chem. Biol. 2012;19:629–637. doi: 10.1016/j.chembiol.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie T., Lim S.M., Westover K.D., Dodge M.E., Ercan D., Ficarro S.B., Udayakumar D., Gurbani D., Tae H.S., Riddle S.M., et al. Pharmacological targeting of the pseudokinase Her3. Nat. Chem. Biol. 2014;10:1006–1012. doi: 10.1038/nchembio.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafson J.L., Neklesa T.K., Cox C.S., Roth A.G., Buckley D.L., Tae H.S., Sundberg T.B., Stagg D.B., Hines J., McDonnell D.P., et al. Small-Molecule-Mediated Degradation of the Androgen Receptor through Hydrophobic Tagging. Angew. Chem. Int. Ed. Engl. 2015;54:9659–9662. doi: 10.1002/anie.201503720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canaria C.A., Smith J.O., Yu C.J., Fraser S.E., Lansford R. New syntheses for 11-(mercaptoundecyl)triethylene glycol and mercaptododecyltriethyleneoxy biotin amide. Tetrahedron Lett. 2005;46:4813–4816. doi: 10.1016/j.tetlet.2005.04.091. [DOI] [Google Scholar]
- 28.Zhou J., Wu J., Liu X., Qu F., Xiao M., Zhang Y., Charles L., Zhang C.C., Peng L. Cooperative binding and self-assembling behavior of cationic low molecular-weight dendrons with RNA molecules. Org. Biomol. Chem. 2006;4:581–585. doi: 10.1039/b515667j. [DOI] [PubMed] [Google Scholar]
- 29.Suthagar K., Fairbanks A.J. A new way to do an old reaction: Highly efficient reduction of organic azides by sodium iodide in the presence of acidic ion exchange resin. Chem. Commun. (Camb.) 2017;53:713–715. doi: 10.1039/C6CC08574A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.