Biology
Members of the families Procyonidae and Viveridae are small- and medium-sized, mainly nocturnal members of the order Carnivora. Although the two groups are taxonomically distant, they share susceptibilities to several important infectious diseases and are handled similarly by veterinarians. Only a few procyonid and viverid species are commonly exhibited by zoos or kept as pets.
Procyonids are arctoid or canoid carnivores, more closely related to canids than felids; all but one species, the red panda, are native to the temperate and tropical New World (Table 49-1 ). Raccoons are the best known and most widely distributed member of this family. In addition to their distribution in their native North and Central Americas, feral raccoon populations have been established in Japan and Eurasia. The raccoon has been the best studied member of the Procyonidae family because of its almost ubiquitous presence in a wide variety of rural and suburban habitats.
TABLE 49-1.
Biologic Data for the Family Procyonidae
| Scientific Name | Common Name | Weight (kilogram) | Geographic Distribution |
|---|---|---|---|
| Procyon lotor | Raccoon | 2–12 | North America to Panama |
| Nasua nasua | Coatimundi | 3–6 | Arizona, United States to Argentina |
| Potos flavus | Kinkajou | 1.4–4.6 | Southern Mexico to Central Brazil |
| Olingo gabbii | Olingo | 0.9–1.5 | Central America to Northern South America |
| Bassariscus astutus | Ringtail cat, cacomistle | 0.8–1.4 | Western United States to Southern Mexico |
| Ailurus fulgens | Red panda | 4–8 | Himalayas to Northern Myanmar, Central China |
Other members of family Procyonidae are the arboreal kinkajous and olingos, the diurnal coatimundis, and the secretive ringtail cats. Red pandas are an exceptional species in this family—they are the only strictly herbivorous members of the family, and their taxonomy has been a subject of debate. The species has been variously assigned to its own family (family Ailuridae), grouped with the giant panda in the family Ailuropodidae, and assigned to the family Procyonidae. It is the only procyonid native to the Old World, living in the mountainous regions of Nepal, northern Southeast Asia, and central China.
Members of the family Viveridae are feloid carnivores; they are more closely related to felids than to canids and are predominantly forest dwellers. The family contains approximately 36 genera and 70 species, and most are small and nocturnal. They are widely distributed in the temperate and tropical regions of Eurasia and throughout Africa (Table 49-2 ). Mongooses have been introduced to the Pacific and Caribbean islands for pest control but are now considered a detrimental introduced species. Few members of this family are routinely exhibited outside of their range states. The diurnal, social meerkat is the most widely exhibited viverid species. Banded and dwarf mongooses are also popular exhibit animals, mostly in Europe. The binturong is the largest viverid and is frequently kept in captivity.
TABLE 49-2.
Biologic Data for Selected Members of the Family Viveridae
| Scientific Name | Common Name | Weight (kilogram) | Geographic Distribution |
|---|---|---|---|
| Arctictis binturong | Binturong | 9–14 | Southeast Asia |
| Genetta spp. | Genets | 1–3 | Southern Europe, Africa, and the Middle East |
| Paradoxurus hermaphrodites | Common palm civet | 1.5–4.5 | Southern and Southeast Asia |
| Cynictis penecillata | Yellow mongoose | 0.4–0.8 | Southern Africa |
| Suricata suricatta | Meerkat, suricate | 0.6–1.0 | Southern Africa |
| Cryptoprocta ferox | Fossa | 7–12 | Madagascar |
| Mungos mungo | Banded mongoose | 1.0–1.2 | Sub-Saharan Africa |
| Helogale parvula | Dwarf mongoose | 0.23–68 | Eastern and Southern Africa |
Unique Anatomy and Physiology
Most procyonids and viverids have elongated, slender bodies and long tails. Kinkajous and binturongs have prehensile tails. Both families are anatomically conservative, quadripedal mammals, with most species having five digits per limb. Several species in both families have semi-retractable claws. The digitigrade viverids have a “waltzing trot” gait, whereas plantigrade species such as the binturong have a more shuffling gait.
The soles of red pandas' feet are covered with hair and possess a central pad scent gland that may be mistaken for a skin lesion. The red panda forelimb also possesses an enlarged radial sesamoid bone, termed the “panda's thumb.” This bone is slightly movable and is used to grasp and hold bamboo, their principle food.
Kinkajous have a long, narrow tongue adapted to eating fruit and honey. Procyonids, including the red panda, lack a cecum, whereas the viverids, with the exception of Nandinia spp., have a cecum. Male viverids possess a baculum, and gender identification is not difficult in these animals, with the exception of the immature female fossa (Cryptoprocta ferox). The young female fossa undergoes a period of masculination during when the animal has an elongated clitoris, which contains an os clitoris, and may have scrotumlike swellings. The os penis disappears at maturity.
A notable anatomic feature of the viverids is their enlarged perianal scent glands. These glands vary in size and complexity among the species. The glands' secretions are used to mark territories and may also be used as a defense. The perianal glandular secretion from the genera Civetticitis, Viverra, and Viveriricula is known as “civet” and is used in the manufacture of perfume and medicines.
Special Housing Requirements
All procyonid and most viverid species are good climbers and should be housed in enclosures with climbing structures. Most are hardy animals and adapt to a variety of climates, but the tropical species should be provided with indoor enclosures and heat during harsh winters. The red panda is native to high mountain habitats, so enclosures should be provided with cool areas or air conditioning in regions with hot, humid summers. Pregnant and parturient red pandas should have a variety of denning boxes, as some females frequently move cubs between boxes.
Many mongooses are good burrowers and will spend considerable time digging. Natural substrate should be provided for these species.
Feeding
Procyonids are generally omnivorous, eating a wide variety of food items. Commercial dog kibble is the basis of most captive raccoon diets and is given along with a variety of fruits and vegetables. Obesity caused by overeating and lack of exercise is also a common problem in captive raccoons. The kinkajou is mainly frugivorous but also eats insects and small vertebrates. The ringtail cats are the most carnivorous of the procyonids, and the red panda eats almost exclusively bamboo in the wild. In captivity, red pandas are typically fed a mixture of commercial “primate” biscuits, fruits, vegetables, and bamboo. If at all possible, bamboo should make at least 50% of the diet. Viverids are mostly carnivorous but, depending on the species, will eat varying amounts of vegetable matter. The binturong is the most frugivorous member of this family.
Restraint and Handling
Captive procyonids and viverids may be trained to enter tubes or small kennels for capture and transport. Some small species may be briefly restrained with nets and heavy gloves, but chemical restraint is necessary to safely perform physical examinations and diagnostic procedures (Figure 49-1 ). The largest issue surrounding restraint of meerkats, and possibly other small, social viverids, is aggression associated with reintroduction of animals into a group, especially if they had been kept separate from the group overnight. Several strategies have been employed, including ensuring return of an immobilized individual to the group the same day, immobilizing several members of the colony at the same time (not just the animal requiring medical attention), and dusting animals to be reintroduced and others in the colony with talcum powder.
FIGURE 49-1.
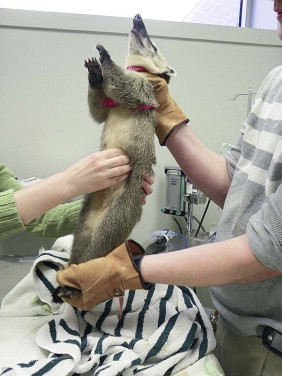
Hand restraint and examination of an adult coatimundi.
(Photo courtesy of Cheryl Greenacre.)
Chemical Restraint, Anesthesia, and Surgery
Most studies of procyonid and viverid immobilization have used a combination of a dissociative agent (ketamine or tiletamine) and either an α2-adrenergic agonist (xylazine or medetomidine) or a benzodiazepine (zolazepam) injected intramuscularly (Table 49-3 ). With the disappearance of medetomidine from the North American market, dexmedetomidine has been substituted into protocols at approximately 50% the dosage of medetomidine. The wide range of dosages listed for immobilizing members of both families most likely reflects differences in immobilization of captive and free-living (trapped) animals.
TABLE 49-3.
Chemical Restraint Agents and Intramuscular Dosages for Procyonids and Viverids
| Species | Induction Agents | Reversal Agents |
|---|---|---|
| Raccoon | 20 ketamine mg/kg + 4 xylazine mg/kg | Yohimbine 0.125 mg/kg |
| 3 mg/kg tiletamine and zolazepam + 2 mg/kg xylazine | ||
| Coatimundi | 20 mg/kg ketamine + 1 mg/kg xylazine | Yohimbine 0.125 mg/kg |
| Kinkajou | 5.5 mg/kg ketamine + 0.1 mg/kg medetomidine | Atipamizole 0.5 mg/kg |
| Olingo | 5 mg/kg tiletamine and zolazepam | |
| Red panda | 6.6 mg/kg ketamine + 0.08 mg/kg medetomidine | Atipamizole 0.4 mg/kg |
| Binturong | 2 mg/kg ketamine + 0.04 mg/kg medetomidine + 0.2 mg/kg butorphanol | Atipamizole 1.0 mg/kg |
| 19.7 mg/kg ketamine + 1.3 mg/kg xylazine | Yohimbine 0.125 mg/kg | |
| 2 mg/kg tiletamine and zolazepam | ||
| Civet | 4.4–8.8 mg/kg ketamine | |
| 10–15 mg/kg ketamine + 0.5–1.5 mg/kg xylazine | Yohimbine 0.125 mg/kg | |
| 4.4–8.8 mg/kg tiletamine and zolazepam | ||
| Genet | 5.7 mg/kg ketamine + 9.8 mg/kg xylazine | |
| Mongoose | 6 mg/kg ketamine + 6 mg/kg xylazine | Yohimbine 0.125 mg/kg |
| 4.4–5.5 mg/kg tiletamine and zolazepam | ||
| Fossa | 10.5–20 mg/kg ketamine + 2.5–5.0 mg/kg xylazine | Yohimbine 0.125 mg/kg |
| 5 mg/kg ketamine + 0.1 mg/kg medetomidine | Atipamizole 0.5 mg/kg | |
Chamber induction with isoflurane in oxygen is also a widely used induction protocol for all species weighing less than 10 kilograms (kg). Anesthesia is typically maintained by inhalation agents such as isoflurane following endotracheal intubation. Surgical procedures are performed similar to protocols used for domestic carnivores.
Diagnostics
Physical examination, radiology, and other diagnostic procedures are similar to those performed in domestic carnivora. Blood samples may be obtained from the jugular, cephalic, femoral, or saphenous vein, but obtaining blood samples from obese individuals may be difficult. Hematology and clinical chemistry values for procyonid and viverid species are generally similar to those for dogs and cats, with a few exceptions (Tables 49-4 and 49-5 ). Red pandas commonly have lower serum or plasma sodium concentrations (130–135 milliequivalents per liter [mEq/L]) and chloride concentrations (100–105 mEq/L) than those seen in domestic carnivores. In the author's experience, healthy red pandas also may have low hematocrits (30%–35%). Enzyme activities for aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and creatinine kinase (CK) are generally greater in procyonids and viverids than those observed in domestic carnivores (see Table 49-5). The cause for this is unclear but may be a result of the procyonids and viverids being restrained and immobilized prior to the blood samples being obtained.
TABLE 49-4.
Hematology of Selected Members of the Families Procyonidae* and Viveridae
| Raccoon | Kinkajou | Red Panda | Slender-Tailed Meerkat | Binturong | |
|---|---|---|---|---|---|
| RBC (106/µL) | 8.74 ± 1.2 | 8.76 ± 2.6 | 8.49 ± 1.2 | 9.56 ± 1.5 | 7.49 ± 1.5 |
| Hemoglobin (g/dL) | 12.2 ± 1.5 | 14 ± 2.8 | 12.3 ± 1.7 | 13.0 ± 1.9 | 16.4 ± 7.4 |
| Hematocrit (%) | 36.8 ± 5.4 | 40.5 ± 8.1 | 39.3 ± 5.1 | 41.0 ± 6.2 | 45.9 ± 8.5 |
| MCH (pg/cell) | 14.2 ± 1.4 | 17 ± 4.4 | 14.9 ± 1.7) | 13.7 ± 1.4 | 21.7 ± 1.9 |
| MCHC (g/dL) | 32.9 ± 2.2 | 35.3 ± 3.0 | 31.6 ± 3.2 | 31.6 ± 2.7 | 36.5 ± 15.3 |
| MCV (fL) | 42.8 ± 5.2 | 48.8 ± 14.2 | 46.3 ± 5.2 | 43.5 ± 3.4 | 62.9 ± 7.8 |
| WBC (103/µL) | 9.84 ± 4.1 | 8.41 ± 3.1 | 7.42 ± 3.0 | 6.65 ± 3.6 | 12.7 ± 5.0 |
| Segs (103/µL) | 4.84 ± 3.7 | 4.76 ± 2.3 | 3.79 ± 2.5 | 4.39 ± 3.0 | 7.65 ± 4.0 |
| Bands (103/µL) | 0.44 ± 0.7 | 0.08 ± 0.01 | 0.16 ± 0.5 | 0.12 ± 0.12 | 0.11 ± 0.36 |
| Lymphocytes (103/µL) | 3.94 ± 2.0 | 2.85 ± 1.5 | 3.1 ± 2.0 | 2.07 ± 1.4 | 3.68 ± 2.3 |
| Monocytes (103/µL) | 0.33 ± 0.3 | 0.27 ± 0.2 | 0.27 ± 0.3 | 0.22 ± 0.2 | 0.57 ± 0.5 |
| Eosinophils (103/µL) | 0.78 ± 0.5 | 0.64 ± 0.7 | 0.15 ± 0.2 | 0.13 ± 0.1 | 0.41 ± 0.6 |
| Basophils (103/µL) | 0.06 ± 0.04 | 0.1 ± 0.06 | 0.13 ± 0.11 | 0.08 ± 0.01 | 0.05 ± 0.14 |
| Platelet count (103/µL) | 470 ± 160 | 449 ± 76 | 576 ± 189 | 389 ± 169 | 333 ± 115 |
fL, Femtoliter; g/dL, gram per deciliter; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; µL, microgram; pg, picogram; RBC, red blood cells; Segs, neutrophils; WBC, white blood cells.
Values are means ± standard deviations.16
TABLE 49-5.
Clinical Chemistry Reference Values for Selected Procyonids and Viverids*
| Raccoon | Kinkajou | Red Panda | Slender-Tailed Meerkat | Binturong | |
|---|---|---|---|---|---|
| Glucose, milligram per deciliter (mg/dL) | 65 ± 22 | 99 ± 36 | 124 ± 39 | 122 ± 33 | 127 ± 63 |
| BUN (mg/dL) | 20 ± 7 | 13 ± 5 | 27 ± 9 | 25 ± 7 | 18 ± 10 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.6 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.3 | 1.3 ± 0.4 |
| Uric acid (mg/dL) | 1.2 ± 0.5 | 0.8 ± 0.4 | 1.2 ± 0.9 | 0.7 ± 0.4 | 1.2 ± 0.9 |
| Calcium (mg/dL) | 9.0 ± 0.7 | 9.3 ± 0.7 | 9.1 ± 0.9 | 9.7 ± 0.9 | 10.0 ± 0.9 |
| Phosphorus (mg/dL) | 4.7 ± 1.2 | 5.2 ± 1.1 | 4.7 ± 1.1 | 5.3 ± 1.3 | 5.6 ± 1.9 |
| Sodium, milliequivalent per liter (mEq/L) | 146 ± 4 | 141 ± 4 | 134 ± 5 | 149 ± 5 | 141 ± 5 |
| Potassium (mEq/L) | 4.3 ± 0.4 | 4.6 ± 0.5 | 4.4 ± 0.6 | 4.2 ± 0.4 | 4.8 ± 0.5 |
| Chloride (mEq/L) | 110 ± 3 | 105 ± 3 | 103 ± 5 | 114 ± 7 | 104 ± 5 |
| Iron, microgram per liter (µg/dL) | 146 ± 29 | 278 ± 0 | 150 ± 91 | 218 ± 136 | 190 ± 93 |
| Magnesium (mg/dL) | 3.05 ± 0.07 | 2.95 ± 0.35 | 2.34 ± 0.54 | 2.5 ± 1.12 | 2.97 ± 0.27 |
| Bicarbonate, millimole per liter (mmol/L) | 21 ± 0 | 25 ± 4.2 | 16.3 ± 4.4 | 18.0 ± 0 | 17.3 ± 0.8 |
| Cholesterol (mg/dL) | 211 ± 63 | 106 ± 50 | 199 ± 59 | 369 ± 139 | 74 ± 31 |
| Triglycerides (mg/dL) | 33 ± 17 | 36 ± 20 | 41 ± 23 | 41 ± 33 | 108 ± 54 |
| Total protein, gram per deciliter (g/dL) | 7.2 ± 0.7 | 8.0 ± 0.8 | 6.6 ± 0.7 | 6.6 ± 0.9 | 7.2 ± 0.7 |
| Albumin (mg/dL) | 3.4 ± 0.3 | 4.0 ± 0.4 | 3.20.5 | 3.3 ± 0.5 | 4.2 ± 0.6 |
| Globulins (mg/dL) | 3.7 ± 0.7 | 3.8 ± 0.7 | 3.3 ± 0.7 | 3.3 ± 0.8 | 2.9 ± 0.5 |
| AST, international unit per liter (IU/L) | 85 ± 26 | 195 ± 72 | 70 ± 37 | 91 ± 38 | 39 ± 20 |
| ALT (IU/L) | 121 ± 36 | 49 ± 45 | 69 ± 58 | 104 ± 62 | 21 ± 27 |
| Total bilirubin (mg/dL) | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.2 |
| Amylase, unit per liter (Unit/L) | 3119 ± 917 | 4468 ± 2191 | 914 ± 670 | 552 ± 330 | 1674 ± 398 |
| ALP (IU/L) | 60 ± 31 | 58 ± 30 | 28 ± 21 | 36 ± 32 | 190 ± 197 |
| LDH (IU/L) | 1299 ± 673 | 336 ± 344 | 506 ± 602 | 623 ± 215 | 341 ± 353 |
| CPK (IU/L) | 306 ± 198 | 402 ± 365 | 286 ± 306 | 350 ± 307 | 509 ± 754 |
| GGT (IU/L) | 4 ± 2 | 6 ± 4 | 3 ± 3 | 4 ± 3 | 4 ± 3 |
| TT4 (µg/dL) | 2.4 ± 0.4 | na | 7.3 ± 18.3 | na | 0.5 ± 0 |
ALP, Alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CPK, creatinine phosphokinase; GGT, glutamyltransferase; LDH, lactate dehydrogenase; na, not available TT4, total thyroxine.
Values are mean ± standard deviation.16
Infectious Diseases
Serologic evidence of a wide range of infectious diseases has been documented in wild procyonids and viverids. For example, diseases and agents that have been identified by serology or isolation in raccoons but have not been reported to cause clinical disease include Borrelia burgdorferi, Brucella spp., Aleutian mink disease virus, Ehrlichia chaffeensis, hemorrhagic disease of deer, and raccoon poxvirus.37 A number of other diseases or agents have caused clinical disease in raccoons, including leptospirosis, pseudorabies virus, canine adenovirus, Salmonella spp., snowshoe hare virus, St. Louis encephalitis virus, Tyzzer's disease, and yersiniosis, but these are rarely seen in captive animals.
Several important diseases commonly cause clinical disease in procyonids and viverids and merit discussion. One of these, canine distemper, may infect most, if not all, procyonids and viverids. The disease is best described in raccoons, and epizootics of canine distemper regularly occur in wild North American populations. Distemper has also been reported in coatimundis, kinkajous, red pandas, palm civets, and binturongs.3, 35, 37 Vaccine-induced canine distemper has been seen in kinkajous and red pandas vaccinated with modified-live virus domestic dog distemper vaccines.2, 18
Clinical signs in raccoons resemble those seen in domestic dogs but commonly include diarrhea in addition to upper respiratory signs. Hyperkeratosis of foot pads (“hard pad”) is also a typical sign. Canine distemper in raccoons seems to progress to central nervous system (CNS) disease more rapidly than in domestic dogs. This is important, as distemper-induced neurologic signs in raccoons are indistinguishable from signs of rabies, and rabies may only be ruled out after death. Wild raccoons may act as vectors for both distemper and rabies to susceptible captive animals such as red pandas. Pathologic lesions of distemper in raccoons are similar to those seen in distemper-infected dogs. On the other hand, masked palm civets with canine distemper showed neurologic lesions but no GI lesions.35
Rabies is endemic in the wild raccoon population in eastern United States. The disease was disseminated by relocation of raccoons from southern United States to the upper Atlantic seaboard states for hunting, and the range extended rapidly. Extensive oral vaccination campaigns have been undertaken to limit the westward extention of raccoon rabies. Rabies is rare in captive raccoons, most likely because of their being protected from wild vector species.
Parvovirus infections have also been recognized in raccoons and many viverids, but the taxonomy of the etiologic agents has been the subject of some debate. Early raccoon cases were thought to be caused by feline parvovirus (feline panleukopenia virus) or “raccoon parvovirus.”37 However, recent outbreaks in wild raccoons in southeastern United States have been caused by canine parvovirus 2.1 Morbidity was largely restricted to juveniles and neonates, and mortality was high. Serologic evidence of parvovirus infections has been observed in red pandas, but clinical disease has not been described in this species. Feline parvovirus has caused deaths in Asian palm civets.6
Several viral diseases of viverids have caused more limited outbreaks. Recently, pandemic flu (H1N1) caused clinical disease in a binturong.34 An outbreak of cowpox virus in captive banded mongoose showed high morbidity (100%) and mortality (30%), and the virus was later transmitted to humans.22 Masked palm civets (Paguma larvata) were found to be serologically positive for the coronavirus that caused severe acute respiratory syndrome (SARS) in people and were implicated as the source of the infection in humans. Experimental infection of palm civets resulted in clinical disease, suggesting that civets were not natural reservoirs for this agent. Subsequent investigations have shown that fruit bats are the natural reservoirs for this agent.24
In young red pandas, dermatophytosis is an important disease.19 The disease is uniformly caused by Microsporum gypseum and typically affects cubs less than 4 months of age. Signs include small areas of hair loss and crusting on the face, limbs, chest, and tail. Pruritus may be present. The lesions on the face and paws are usually not severe and respond to clipping, cleaning, and application of topical antifungal agents. Infections on the chest or tail may be severe and may progress rapidly to a purulent lesion covered with a crust (“kerion”). Lesions in these areas require more aggressive treatment. In addition to local treatment of lesions, as described above, systemic antifungal agents such as itraconazole (5–10 milligrams per kilogram [mg/kg], orally [PO], every 12–24 hours [q12–24h]), should be considered.
Tyzzer disease is a systemic infection caused by Clostridium piliformis and has been reported in two red pandas.23 This species may be especially susceptible to this bacterium.
Vaccination
Procyonids and viverids may be safely vaccinated with a canarypox-vectored canine distemper vaccine manufactured for domestic ferrets (Purevax, Merial, Duluth, MN). The immunogenicity of this vaccine has been shown in mustelids and giant pandas. Although challenge studies have not been performed in procyonids or viverids, the author is unaware of clinical distemper in any of animals vaccinated with this product. Protocols for vaccination are similar to those for domestic dogs.
Captive procyonids and viverids may be vaccinated with a killed rabies virus vaccine. Recommendations regarding vaccination for parvovirus infection are more variable. Raccoons and palm civets should definitely be vaccinated, with a killed virus vaccine, if available. Vaccination of other procyonids and viverids for parvovirus is the decision of the attending clinician. Routine vaccination of red pandas against canine or feline parvovirus is not recommended at the time of writing this text.
Parasites
The most important parasite of the procyonids is the zoonotic ascarid Baylisascaris procyonis, which infects raccoons and kinkajous. This worm is rarely symptomatic in procyonids, but it may cause severe morbidity in humans and other hosts (e.g., parrots) because of larval migrans. Baylisascaris larva may cause impaired vision and blindness if it migrates to the eye in humans. Neurologic signs and even death may occur in people and other species if larvae migrate through the CNS. Most people affected by CNS larval migrans are children under 5 years of age or mentally impaired individuals with a propensity to geophagia.
Only a few cases of canine heartworm infections have been reported in raccoons, and they appear to be aberrant hosts for this parasite. Red pandas, however, do appear to be susceptible to infection with the canine heartworm Dirofilaria immitis. The infection is usually asymptomatic and typically discovered by routine serologic screening. In areas with significant incidence of canine heartworm, most zoos put their red pandas on a routine heartworm prevention program. A monthly oral dose of ivermectin (0.05 mg/kg) is used at the author's zoo, throughout the year. Treatment of occult heartworm infections with melarsomine, at the recommended canine regimen, has been fatal in red pandas. No data could be found on canine heartworm infection in other procyonids or viverids.
Tetrapetalonema sp. and Paragonimus sp. have been identified in captive binturongs in India. Coccidiosis (presumptively Eimeria procyonis) and encephalitis induced by Sarcocystis neurona have also been reported in raccoons.11
In meerkat colonies, outbreaks of toxoplasmosis and microsporidiosis have been reported. Toxoplasmosis was characterized by respiratory distress and rapid death. Toxoplasma gondii-like organisms were found widely disseminated in the tissues at necropsy.17 Toxoplasmosis has also been reported in raccoons, commonly as a concurrent infection with canine distemper. Microsporidiosis has caused neurologic signs and high mortality in meerkats. The outbreak was caused by an agent structurally similar to Nosema cuniculi.
Noninfectious Diseases
Poor hair coat is a common condition in captive red pandas and occasionally in raccoons. Animals seem to have partial seasonal sheds or patchy hair coats, with hair loss usually starting in the caudal half of the body and extending to the tail. Dermatophytes have not been found in these animals. Hypothyroidism has been reported in one red panda, but thyroid hormone concentrations in most of these animals are within the reference intervals for domestic dogs and cats. In the majority of these cases, the problem seems to be seasonal and requires no treatment.
Osteoarthritis is commonly seen in older procyonids, and chronic renal disease is frequently observed in aged red pandas. Hypertrophic cardiac disease has been reported in kinkajous and binturong.8, 14 Pancreatitis and trichobezoars have been observed in meerkats.
Thyroid pathology has been reported at an unusual frequency in raccoons.38 Early reports were from captive animals from Germany, but a recent report is of captive animals in North America.27 One European study identified 77.5% of the raccoons necropsied as having thyroid lesions, almost half of which (15 of 31) were thyroid adenocarcinomas.38 Other thyroid lesions included follicular hyperplasia (7 of 31), follicular adenomas (4 of 31), and colloid goiters (4 of 31). No thyroid pathology was found at necropsy of feral raccoons captured in the same region of the captive animals. The feral animals were, however, considerably younger than the captive animals examined. One North American raccoon with thyroid carcinoma was obese and had a palpable cervical mass when examined.27 The animal's total thyroxin (TT4) concentrations were within domestic carnivore reference intervals; thus this neoplasm was considered nonfunctional and similar to the thyroid carcinomas seen in domestic dogs. Another captive North American raccoon was obese, and a cystic thyroid gland was discovered during an attempt at jugular venipuncture. This animal had an increased TT4 concentration, and an excisional thyroid biopsy revealed adenomatous hyperplasia. The thyroid disease in this animal closely resembled that seen in older domestic cats. This animal's hyperthyroidism remained even after surgery and was successfully managed for years with a topical methimazole gel.27
Numerous neoplasms have been described in procyonids and viverids (Table 49-6 ), but as noted above, thyroid neoplasms in raccoons are notable. A large number of nasal carcinomas described in procyonids and genets are all from a group of animals housed in the same building at the Philadelphia Zoo and was, no doubt, likely the result of an environmental issue.25
TABLE 49-6.
Neoplasms Identified in Members of the Families Procyonidae and Viveridae
| Scientific Name | Common Name | Neoplasm(s) | Source(s) |
|---|---|---|---|
| Procyon lotor | Raccoon | Pancreatic adenoma | 10 |
| Astrocytoma | 13, 26 | ||
| Thyroid adenocarcinoma with pulmonary metastases | 27, 38 | ||
| Procyon cancrivorus | Crab-eating raccoon | Nasal carcinoma | 25 |
| Nasua narica | White-nosed coatimundi | Nasal carcinoma | 25 |
| Uterine adenocarcinoma | 5 | ||
| Nasua nasua | Ring-tail coatimundi | Nasal carcinoma | 25 |
| Potos flavus | Kinkajou | Nasal carcinoma | 25 |
| Bassariscus astutus | Ringtail cat | Nasal carcinoma | 25 |
| Ailurus fulgens | Red panda | Granulosa cell tumor | 7 |
| Squamous cell carcinoma | 32 | ||
| Thryoid carcinoma | 32 | ||
| Hepatocarcinoma | 32 | ||
| Lymphoma | 32 | ||
| Myelogenous leukemia | 32 | ||
| Lymphoid leukemia | 32 | ||
| Civettictis civetta | African civet | Hepatic carcinoma | 25 |
| Viverra zibetha | Large Indian civet | Urinary bladder carcinoma | 25 |
| Genetta genetta | Genet | Nasal carcinoma | 25 |
| Liver carcinoma | 25 | ||
| Cecal carcinoma | 25 | ||
| Genetta tigrina | Genet | Cholangiocarcinoma | 36 |
| Nandinia binotata | African palm civet | Hepatic angioma | 28 |
| Hepatocellular carcinoma | 28 | ||
| Lung carcinoma | 25 | ||
| Paguma larvata | White-whiskered palm civet | Lymphosarcoma | 25 |
| Arctogalidia trivirgata | Small-toothed palm civet | Hepatocellular carcinoma | 36 |
| Arctictis binturong | Binturong | Renal adenocarcinoma | 20 |
| Hepatocellular carcinoma | 20 | ||
| Pancreatic islet carcinoma | 20 | ||
| Adenoma of colon | 7 | ||
| Mammary adenocarcinoma with metastases | 7 | ||
| Sarcomatoid renal carcinoma | 4 | ||
| Cholangiocellular carcinoma | 30 | ||
| Fossa fossa | Fanaloka | Squamous cell carcinoma | 28 |
| Paradoxurus hermaphrodites | Indian palm civet | Pancreatic adenocarcinoma | 7 |
| Viverra tangalunga | Civet | Pulmonary carcinoma | 7 |
| Suricata suricatta | Meerkat | Nasal squamous cell carcinoma | 15 |
| Herpestes ichneumon | Grey mongoose | Lung carcinoma | 25 |
| Cryptoprocta ferox | Fossa | Adrenal adenocarcinoma | 28 |
| Hepatocellular carcinoma | 28 | ||
Reproduction
Temperate region species such as raccoons are seasonal animals, with breeding taking place in the late winter or early spring. Tropical species may be nonseasonal or have a seasonality based on rainfall rather than photoperiod. Red pandas in the Northern hemisphere mate in January or February and typically have their young in June or July. Breeding strategies in viverids are varied. Banded mongoose have an unusual strategy of synchronized parturition, where all young in a colony are born within a very short period.
Cystic endometrial hyperplasia has been seen spontaneously in raccoons and in a coati implanted with synthetic progestins.5, 12 Ovariohysterectomy and castration are the contraceptive methods with the best efficacy and the least adverse effects. A current area of investigation in procyonid contraception is use of gonadotropin-releasing hormone (GnRH) implants.
References
- 1.Allison AB, Harbison CE, Pagan I. Role of multiple hosts in the cross-species transmission and emergence of a pandemic parvovirus. J Virol. 2012;86:685. doi: 10.1128/JVI.06187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush M, Montali RJ, Brownstein D. Vaccine-induced distemper in a lesser panda. J Am Vet Med Assoc. 1976;169:959. [PubMed] [Google Scholar]
- 3.Chandra AMS, Ginn PE, Terrell SP. Canine distemper virus infection in binturongs (Arctictis binturong) J Vet Diagn Invest. 2000;12:88. doi: 10.1177/104063870001200120. [DOI] [PubMed] [Google Scholar]
- 4.Childs-Sanford SE, Peters RM, Morrisey JK. Sarcomatoid renal cell carcinoma in a binturong (Arctictis binturong) J Zoo Wildl Med. 2005;36:308. doi: 10.1638/02-096.1. [DOI] [PubMed] [Google Scholar]
- 5.Chittick E, Rotstein D, Brown T. Pyometra and uterine adenocarcinoma in a melangesterol actetate-implanted captive coati (Nasua nasua) J Zoo Wildl Med. 2001;32:245. doi: 10.1638/1042-7260(2001)032[0245:PAUAIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Demeter Z, Gál J, Palade EA, Rusvai M. Feline parvovirus infection in an Asian palm civet (Paradoxurus hermaphrodites) Vet Rec. 2009;164:213. doi: 10.1136/vr.164.7.213. [DOI] [PubMed] [Google Scholar]
- 7.Effron M, Griner L, Benirschke K. Nature and rate of neoplasia found in captive wild mammals, birds, and reptiles at necropsy. J Natl Cancer Inst. 1977;59:185. doi: 10.1093/jnci/59.1.185. [DOI] [PubMed] [Google Scholar]
- 8.Eschar D, Peddle GD, Briscoe J. Diagnosis and treatment of congestive heart failure secondary to hypertrophic cardiomyopathy in a kinkajou (Potos flavus) J Zoo Wildl Med. 2010;41:342. doi: 10.1638/2009-0142R3.1. [DOI] [PubMed] [Google Scholar]
- 9.Fournier P, Founrier-Chambrillon C, Vié J-C. Immobilization of wild kinkajous (Potos flavus) with medetomidine-ketamine and reversal with atipamizole. J Zoo Wild Med. 1998;29:190. [PubMed] [Google Scholar]
- 10.Fox H. JB Lippincott, Co; Philadelphia, PA: 1923. Disease in captive wild mammals and birds. [Google Scholar]
- 11.Hamir AN, Dubey JP. Myocarditis and encephalitis associated with Sarcocystis neurona infection in raccoons (Procyon lotor) Vet Parasitol. 2001;95:335. doi: 10.1016/s0304-4017(00)00400-3. [DOI] [PubMed] [Google Scholar]
- 12.Hamir AN. Spontaneous lesions in aged captive raccoons (Procyon lotor) J Am Assoc Lab Anim Sci. 2011;50:322. [PMC free article] [PubMed] [Google Scholar]
- 13.Hamir AN, Picton R, Bythe LL. Astrocytoma with involvement of medulla oblongata, spinal cord, and spinal nerves in a raccoon (Procyon lotor) Vet Pathol. 2008;45:949. doi: 10.1354/vp.45-6-949. [DOI] [PubMed] [Google Scholar]
- 14.Hollamby S, Simmons H, Bell T. Myocardial necrosis in a captive binturong (Arctictis binturong) Vet Rec. 2004;154:596. doi: 10.1136/vr.154.19.596. [DOI] [PubMed] [Google Scholar]
- 15.Howard LL, Lafortune M, Tocidlowski M. Therapy for nasal squamous cell carcinoma in a slender-tailed meerkat (Suricata suricatta) Proc Am Assoc Zoo Vet, Am Assoc Wildl Vet, Am Zool Assoc/NAG Joint Conf. 2007:141. [Google Scholar]
- 16.International Species Information System ISIS normal values, MedArks Program. http://www2.isis.org/support/MEDARKS.aspx Accessed February 11, 2013.
- 17.Juan-Salles C, Prats N, Lopez S. Epizootic disseminated toxoplasmosis in captive slender-tailed meerkats (Suricata suricatta) Vet Pathol. 1997;34:1. doi: 10.1177/030098589703400101. [DOI] [PubMed] [Google Scholar]
- 18.Kazacos KR, Thacker HL, Shivaprasad HL. Vaccination-induced distemper in kinkajous. J Am Vet Med Assoc. 1981;179:1166. [PubMed] [Google Scholar]
- 19.Kearns KS, Pollock CG, Ramsay EC. Dermatophytosis is red pandas (Ailurus fulgens fulgens): A review of 14 cases. J Zoo Wildl Med. 1999;30:561. [PubMed] [Google Scholar]
- 20.Klaphake E, Shoieb A, Ramsay EC. Renal adenocarcinoma, hepatocellular carcinoma, pancreatic islet cell carcinoma in a binturong (Arctictis binturong) J Zoo Wildl Med. 2005;36:127. doi: 10.1638/03-084. [DOI] [PubMed] [Google Scholar]
- 21.Kreeger TJ, Arnemo JM. ed 3. Sunquest; Shanghai, China: 2007. Handbook of wildlife chemical immobilization. [Google Scholar]
- 22.Kruth A, Straube M, Kuczka A. Cowpox virus outbreak in banded mongooses (Mungos mungos) and jaguarundis (Herpailurus yagouaroundi) with a time-delayed infection to people. PLoS. 2009;4:e6683. doi: 10.1371/journal.pone.0006883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langan J, Bemis D, Harbo S. Tyzzer's disease in a red panda (Ailurus fulgens fulgens) J Zoo Wildl Med. 2000;31:558. doi: 10.1638/1042-7260(2000)031[0558:TSDIAR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Shi Z, Yu M. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 25.Lombard LS, Witte EJ. Frequency and types of tumors in mammals and birds of the Philadelphia Zoological Gardens. Cancer Res. 1959;19:127–141. [PubMed] [Google Scholar]
- 26.Maurer KE, Nielsen SW. Neurologic disorders in the raccoon in Northeastern United States. J Am Vet Med Assoc. 1981;179:1095. [PubMed] [Google Scholar]
- 27.McCain SL, Allender MC, Bohling MW. Thyroid neoplasia in captive raccoons (Procyon lotor) J Zoo Wildl Med. 2010;41:121–127. doi: 10.1638/2009-0155.1. [DOI] [PubMed] [Google Scholar]
- 28.Montali RJ. An overview of tumors in zoo animals. In: Montali RJ, Migaki G, editors. The comparative pathology of zoo animals. Smithsonian Institution Press; Washington, DC: 1980. [Google Scholar]
- 29.Moresco A, Larsen RS. Medetomidine-ketamine-butorphanol anesthetic combinations in binturongs (Arctictis binturong) J Zoo Wildl Med. 2003;34:346. doi: 10.1638/03-016. [DOI] [PubMed] [Google Scholar]
- 30.Nashiruddullah N, Chakraborty A. Spontaneous neoplasms in captive wild carnivores of Assam State Zoo. Ind J Vet Pathol. 2003;27:39. [Google Scholar]
- 31.Palomares F. Immobilization of common genets, Genetta genetta, with a combination of ketamine and xylazine. J Wildl Dis. 1993;29:174. doi: 10.7589/0090-3558-29.1.174. [DOI] [PubMed] [Google Scholar]
- 32.Philippa J, Ramsay EC. Captive red panda medicine. In: Glatson A, editor. The red panda: Biology and conservation of the first panda. Elsevier; Amsterdam, The Netherlands: 2010. [Google Scholar]
- 33.Schaftenaar W. A short note on the immobilization of the red panda (Ailurus f. fulgens) In: Glatston AR, editor. The red or lesser panda studbook. Rotterdam Zoo; Rotterdam, The Netherlands: 1993. [Google Scholar]
- 34.Schrenzel MD, Tucker TA, Stalis IH. Pandemic (H1N1) 2009 virus in 3 wildlife species, San Diego, California, USA. Emerg Infect Dis. 2011;17:747. doi: 10.3201/eid1704.101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takayama I, Kubo M, Takenaka A. Pathological and phylogenetic features of prevalent canine distemper viruses in wild masked palm civets in Japan. Comp Immunol Microbiol Infect Dis. 2009;32:539. doi: 10.1016/j.cimid.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Wadsworth PF, Jones DM, Pugsley SL. Primary hepatic neoplasia in some captive mammals. J Zoo Anim Med. 1982;13:29. [Google Scholar]
- 37.Williams ES, Barker IK. ed 3. Iowa State Press; Ames, IA: 2001. Infectious diseases of wild mammals. [Google Scholar]
- 38.Wisser J. Zumm vorkommen von thyreopathien bei waschbaeren (Procyon lotor) Verh ber Erkrg Zootierre. 1995;37:435. [Google Scholar]


