TEACHING POINTS.
-
•
Technological developments in molecular biology are showing that known respiratory viruses are more prevalent than previously thought across the childhood age range. New viruses continue to be discovered in association with acute respiratory tract infections.
-
•
The study of morbidity and mortality that is due to acute lower respiratory tract infection is hampered by lack of precise definitions and specific, practical diagnostic methods.
-
•
Children in developing countries account for at least 70% of the global burden and mortality from acute lower respiratory tract infection.
-
•
Malnutrition, poverty, indoor smoke, and comorbid conditions, including malaria, tuberculosis, and human immunodeficiency virus infection, greatly increase the risks of children acquiring and dying from acute lower respiratory tract infection.
-
•
Emerging respiratory diseases present an ongoing risk for children in an era of globalization.
This chapter deals with three aspects of the epidemiology of respiratory infections in children: common respiratory viruses, acute lower respiratory tract infection (ARI), and emerging/reemerging organisms.
COMMON RESPIRATORY VIRUSES
Respiratory tract infections are among the most frequent diseases in early life. Many viruses are known to be associated with symptomatic respiratory tract infections, the most common being respiratory syncytial virus (RSV), influenza viruses types A and B, parainfluenza viruses, adenoviruses, and rhinoviruses. Viral infections are consistently more commonly found in younger children,1, 2 among whom viruses are the cause of as many as 90% of all lower respiratory tract infections (LRIs).3 Increasingly, these viruses are also being associated with a substantial burden of respiratory disease in adults, including elderly persons, and in immunocompromised persons. The advent of molecular technologies has improved laboratory detection of virus in clinical samples and extended the ability to characterize the epidemiology of respiratory virus infections, but it has also led to the identification of subtypes and genotypes of known respiratory viruses and to the discovery of novel respiratory viruses.
Laboratory Detection
CONVENTIONAL DIAGNOSTIC METHODS
Conventional virologic methods, including antigen detection, serology, and culture, have clearly identified the viruses most frequently causing respiratory illnesses in children and associated with both upper respiratory tract (URI) and LRI infections. Table 31-1 shows the relative frequency of viruses identified within the Tecumseh, Michigan, community study, from 1976 to 1981.4
Table 31-1.
Annual Isolation Rates of Respiratory Viruses, Tecumseh, Michigan, 1976-1981
| Numerator |
Annual Isolation Rate, Adjusted by Proportion of Illnesses Sampled. By age group |
Percentage Isolated |
Annual Number of Attributable Illnesses |
Percentage of Illnesses Resulting in Consultation |
|
|---|---|---|---|---|---|
| Denominator | 100 Person-years | All Respiratory Illnesses |
10,000 Population |
Illnesses Attributable to Specific Agent |
|
| All ages: children and adults |
|||||
| Age group | 0-4 yr | 5-19 yr | % | n | % |
| Rhinoviruses | 59.6 | 13.2 | 54 | 8325 | 17.6 |
| Coronaviruses | 14 | 3428 | 17.6 | ||
| Influenza viruses | 14.6 | 30.4 | 9 | 2204 | 37.9 |
| Parainfluenza viruses | 28.3 | 7.5 | 4 | 979 | 26.2 |
| Respiratory syncytial viruses | 29.3 | 3.7 | 4 | 979 | 55.6 |
| Adenoviruses | 16.6 | 3.4 | 2 | 490 | 43.2 |
| Other viruses | 4.8 | 2.7 | 2 | 490 | 27.8 |
| Bacterial | 8 | 1959 | 48.6 | ||
| Unknown and/or noninfectious | 23 | 5650 | 21.5 | ||
| Total or average | 154.3 | 61.1 | 100 | 24,484 | 25.4 |
Adapted from Monto AS: Epidemiology of viral respiratory infections. Am J Med, 2002 Apr 22;112 Suppl 6A:4S-12S.
© 2008
MOLECULAR DIAGNOSTIC METHODS
The inclusion of nucleic acid technologies (e.g., polymerase chain reaction [PCR]) for the detection of respiratory virus infections in children has substantially increased the diagnostic sensitivity for most respiratory viruses.5, 6, 7 This technology has allowed the recovery of viruses that have been difficult to detect by conventional methods.8 For example, PCR is three to five times as sensitive as cell culture for detecting rhinoviruses.9, 10 Figure 31-1 shows the viral identification breakdown in three recent studies of children5, 11, 12 in different countries over the winter viral season using PCR techniques. This figure illustrates the differences between studies in patient sampling and methods and the consequent differences in results. However, in all three studies, rhinovirus and RSV are the dominant viruses, with the relative proportions of each differing even between the two studies in infants (studies A and B). Mixed viral infections, particularly with rhinovirus, are common. Finally, studies find only the organisms they look for—only study C tested for bocavirus.
Figure 31-1.
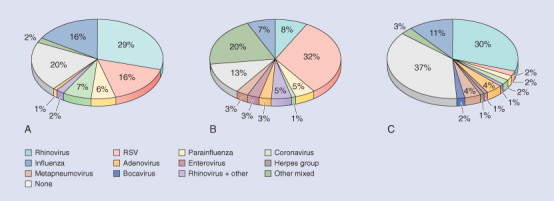
Three studies of virus isolation in childhood respiratory tract infection (RTI) over winter using molecular virology. Data sources and details are as follows: A, Legg et al.11Denominator: 123 RTIs in a cohort of 88 infants with at least one atopic parent, followed in Southampton, UK. Specimens: nasal lavage. Virology methods: individual RT-PCR for six virus groups including picornavirus (not specifically identified as rhinovirus), M. pneumoniae, and C. pneumoniae.B, Jennings et al.5Denominator: 75 children (65 under 12 months old) presenting to hospital in Christchurch, New Zealand, with acute respiratory illness suggesting lower RTI. Specimens: nasopharyngeal swabs. Virology methods: PCR/RT-PCR for 11 viruses as well as immunofluorescence and viral culture. C, Arden et al.12 and personal communication, Ian Mackay. Denominator: 315 respiratory specimens obtained from people (1 day to 80 years old, 79% under 5 years old) presenting with RTIs to Brisbane hospitals, selected from all four seasons in 2003-2004. Specimens: not described. Virology: immunoassay, culture, and 17 PCR/RT-PCR assays.
Molecular methods have also allowed the rapid identification and characterization of previously unknown viruses causing respiratory illness. The human metapneumovirus (hMPV) was first identified in the Netherlands in 200013 and shows a wide geographic distribution.14, 15 Five new human coronaviruses have been discovered,16 including severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV),17, 18 human coronavirus NL63 (hCoV-NL63),19 and human coronavirus HKU1 (hCo-HKU1).20 Another virus, the human bocavirus (hBoV), has also been identified in association with respiratory illness in hospitalized patients.21
MULTIPLE INFECTIONS
Studies using conventional virology have detected multiple respiratory viruses in 1.8% to 15.8% of acute LRIs.22 While more recent studies using nucleic acid technologies have identified multiple viruses in up to 27% of hospitalized children with LRI.5 All of the common respiratory viruses have been identified as contributing to multiple infections. They have been recognized more frequently in younger children (≥4 years) and in hospitalized children. The presence of more than one virus may result in more severe or prolonged infection.15, 23, 24 Alternately, because young children are immunologically naïve and experience a median of 4.425 to 5.526 respiratory illnesses per year and because viral shedding is commonly prolonged in young children, a continuum of residual virus or viral nucleic acid may be being detected.
Epidemiology
PERIODICITY AND SEASONALITY
Both the periodicity and the seasonality of viruses that cause respiratory tract infections are well established in temperate climates3, 11, 26 (Fig. 31-2 ; also Fig. 31-1). Most viruses circu late in a community every year; however, some, especially influenza type B and the parainfluenza viruses, may cause epidemics at biennial or longer intervals.3 Different viruses also predominate in a community during different seasons of the year; overlap invariably occurs and the actual timing and severity of each outbreak or epidemic can vary from year to year. In tropical climates, seasonality also exists. In these regions, RSV occurs predominantly in the rainy season, whereas influenza activity occurs throughout the year with a marked peak in activity during the dry season and a lesser peak during the rainy season.27, 28 Seasons of both higher and lower temperature have been associated with these viruses in different countries.27 The reasons for the seasonality of respiratory virus infections and their variation by latitude are not clear. More important than the environmental conditions themselves may be their effect on behavior. Seasons when people spend more time indoors, in closer contact with other people (colloquially called “closed-in season” in some regions), will favor more rapid person-to-person transmission.
Figure 31-2.

Monthly rates per 100 children of defined virus-specific episodes in the Finnish Otitis Media Cohort Study (red lines). This was a population cohort study. Corresponding virus infection rates (cases per 10,000 person-years) that were reported in the general population in Finland are also shown (yellow lines), although data for rhinoviruses and parainfluenza viruses were not available.
(From Vesa S, Kleemola M, Blomqvist S, et al: Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J 20:574-581, 2001. Used with permission of Lippincott Williams & Wilkins.)
© 2008 Lippincott Williams & Wilkins
AGE
Respiratory viruses cause infections in children at specific ages. RSV causes the most severe LRI in children less than 1 year of age, with a peak occurring at a mean age of 3 months.29 The peak age for hMPV infection is later than that for RSV,30 but the majority of children have had clinical or subclinical infection by 2 years of age. Similarly, hPIV infections occur at an older peak age than RSV. Human parainfluenza virus-1 causes LRIs predominantly in infants aged 7 to 36 months, with peak infection occurring in the second and third years of life. The peak infection of hPIV-2 occurs in the second year of life, but hPIV-3 differs in that it infects infants younger than 6 months and most children experience infection in the first year of life. All age groups are at risk of infection from influenza type A and B viruses, but preschool-age children in day care and school-age children have the highest infection rates.31 The very young, the elderly, and those with chronic conditions experience more severe disease. Rhinoviruses and coronaviruses commonly cause URI and infect individuals many times throughout their lives. Rhinoviruses are also clearly associated with LRI in children, but their role remains to be elucidated.32
Signs and Symptoms
The common respiratory viruses are often associated with specific clinical syndromes, although most can cause infection at any level in the human respiratory tract.
RSV is clearly the most important pathogen associated with bronchiolitis and pneumonia in infancy,33 but it can infect all age groups, causing influenza-like illness.34
Infection of young children with hMPV, a newly described virus, causes illness that resembles hRSV bronchiolitis but also upper respiratory tract disease and diarrhea with fever.13, 35, 36, 37, 38 Metapneumovirus now surpasses the parainfluenza viruses as the second most common cause of bronchiolitis and pneumonia among young children.
The parainfluenza viruses (hPIV 1-4) are also an important cause of LRIs and regularly cause pneumonia, bronchiolitis, and croup among infants and young children. The most frequent are hPIV-1 and hPIV-2 infections. hPIV-1 is most commonly associated with croup, whereas hPIV-3 is an important cause of bronchiolitis and pneumonia in young infants. hPIV-2 is most commonly associated with croup, but all respiratory syndromes have been described. Studies rarely identify hPIV-4, although this virus has also been associated with all respiratory syndromes.39
Influenza viruses can cause any of the typical respiratory syndromes. Very young infants often present with fever only and no specific lower respiratory tract symptoms. School-age children and adolescents most often present with symptoms of classic influenza (i.e., febrile tracheobronchitis with myalgia and cough).1
The most common lower respiratory syndrome caused by adenoviruses is pneumonia, but all syndromes can occur.40 Damage to bronchial architecture can occur with certain adenovirus strains, leading to life-threatening infections, and to bronchiolitis obliterans and bronchiectasis.
Rhinoviruses are the most ubiquitous respiratory pathogens. They cause the majority of cases of the common cold and may also commonly be associated with LRIs in children and adults.32
The coronaviruses (hCoV-229E and hCoV-OC43) are a frequent cause of URIs in children and adults.41 Human bocavirus 42 and the newer coronaviruses (hCoV-NL63 and hCo-HKU1)16, 19, 43 have been associated with respiratory tract infection in children, but their epidemiology remains to be fully elucidated.
ACUTE LOWER RESPIRATORY TRACT INFECTION
Scope and Limitations of Epidemiologic Study of Acute Lower Respiratory Tract Infection
DEFINITIONS AS USED IN THIS SECTION
Pneumonia: Inflammation of the lung with consolidation.44 The term is usually used to indicate infection (most commonly bacterial or viral) of the lung parenchyma resulting in obliteration of alveolar air space by purulent exudate.
ARI or clinical pneumonia: These terms are commonly used in studies in developing countries for a clinical diagnosis of infection of the lower respiratory tract (below the larynx) based on the three signs of fever, cough, and rapid breathing. Other signs, such as grunting, indrawing, bronchial breathing, auscultatory crackles, etc., may or may not be present.
Community-acquired pneumonia (CAP): This term is most commonly used in studies in developed countries and usually includes a radiological finding of pneumonia. Used in distinction to hospital-acquired pneumonia, which follows injury, surgery, immobility, or immunosuppression or is due to unusual hospital pathogens.
To prove a diagnosis of “pneumonia” according to the first, pathologic definition requires proof that there is infection of the lung parenchyma and proof that there is airspace consolidation. The definition of ARI or clinical pneumonia, on the other hand, relies on clinical signs only and requires no such proof. The chief difficulty in studying cases of pneumonia, especially for epidemiologists, is that of reconciling these two definitions. Why is this important? When we describe the epidemiology of clinical pneumonia we need to know:
-
•
What is the relationship between the condition we are describing and the more objective pathologic entity of pneumonia? (Validity)
-
•
How confident are we that the studies we collect in our description are identifying the same condition? (Reliability)
The difficulties inherent in answering these two questions include the following.
-
1.
Limitations of identifying the organism: Clinical signs and symptoms provide only a limited guide to the many possible organisms causing a respiratory infection. Different organisms require different types of sample and different specialized laboratory identification methods, few of which are in routine use, even in developed countries. For example, sensitive detection of RSV, Streptococcus pneumoniae, and Mycobacterium tuberculosis requires, respectively, immunofluorescent antigen detection on nasopharyngeal swab or aspirate, bacterial culture of lung biopsy tissue, and inoculation of bronchoalveolar lavage or gastric aspirate specimens into liquid broth with subsequent confirmation by PCR speciation.
-
2.
Limitations of identifying consolidation: Chest radiography accurately identifies established pathologic consolidation but may miss early infection. However, as with microbial diagnosis, radiology is not routinely necessary for clinical management and is not consistently performed during respiratory infections.
-
3.
Limitations of availability of facilities and expertise: Clinical, laboratory, and radiology facilities and diagnostic expertise are distributed in a very patchy fashion throughout the world. In many parts of the developing world, diagnosis and case management rely on poorly resourced village health workers with the support of a central clinic. Often, these are the regions with the highest incidence, morbidity, and mortality from childhood respiratory infection.
THE WORLD HEALTH ORGANIZATION CASE MANAGEMENT GUIDELINE
The difficulties and limitations of identifying ARI by microbial cause and radiologic consolidation, particularly in developing countries, have forced epidemiologists to develop tools using clinical pattern recognition to estimate the burden of respiratory disease. The World Health Organization (WHO) guideline for the management of ARIs and pneumonia (Fig. 31-3 ) was developed by Shann and others45, 46 in Papua, New Guinea, from 1980 onward. The aim was a simple step-by-step instruction in recognizing and treating respiratory infection, requiring minimal training, and usable by health workers in developing countries. By 1994, 130 developing countries were using the protocol with or without modification.47 The guideline has been incorporated into the global Integrated Management of Childhood Illness (IMCI) program,48 which had been adopted by 81 developing countries by 2000. For these reasons, the WHO guideline has become an important tool for measuring the incidence and mortality due to ARI.
Figure 31-3.

The modified WHO case management guideline for acute respiratory illness.
(Used with permission. This chart and its explanation can be found at http://www.who.int/child-adolescent-health/Emergencies/ARI_chart.pdf.)
STRENGTHS AND LIMITATIONS FOR EPIDEMIOLOGY STUDIES IN THE WHO GUIDELINES
The strengths of the WHO case management guideline for epidemiological study of ARI are as follows:
-
•
Minimal training required, needing no specialized facilities; therefore, low cost and wide applicability across geopolitical and socioeconomic boundaries
-
•
Proved effective as a basis for management and reducing mortality
A meta-analysis of nine community-based trials49, 50 has shown that application of the WHO case management system reduced pneumonia mortality by 36% and total mortality by 24% in the 0- to 4-year-old age group (Fig. 31-4 ). The impact is most likely due to improved recognition of antibiotic treatable pneumonia due to S. pneumoniae and H. influenzae, but identification of malnutrition and other health factors may also contribute.
Figure 31-4.

Risk ratio and 95% confidence interval for reduction in total and pneumonia mortality in children 0 to 4 years old, showing estimates in individual studies and random effects, pooled estimates, and weights for each study.
Letters in study column refer to study locations (G, Gadchiroli, India; J, Jumla, Nepal; N; Navangwal, India). See source article for complete study references. (From Sazawal S, Black RE: Effect of pneumonia case management on mortality in neonates, infants, and preschool children: A meta-analysis of community-based trials. Lancet Infect Dis 3:547-556, 2003.)
Compared to a radiologic definition of pneumonia, the WHO guideline lacks specificity due to:
-
1.
Lack of distinction between viral bronchiolitis, virus-associated asthma, and viral or bacterial pneumonia
-
2.Possible inclusion of the following:
- •
- •
-
•Any other cause of rapid breathing such as cardiac failure57
-
3.
Respiratory rate, a key element of the WHO criteria, may be raised by increased body temperature53, 58 and high altitude59 and lowered by malnutrition.47, 60
In two studies in The Gambia61 and Peru,62 radiographs confirmed pneumonia in 24% and 36% of cases, respectively, identified by WHO criteria.
THE “VERBAL AUTOPSY”: USE AND LIMITATIONS IN MORTALITY ESTIMATES
The “verbal autopsy,” commonly used for gathering mortality data in developed countries, consists of a questionnaire (including the WHO criteria for ARI) administered to families about the final illness. This is a simple method of obtain ing circumstantial data regarding causes of death without the cultural problems often associated with medical autopsy. Verbal autopsies have a range of reported sensitivity for pneumonia of 28% to 72% and specificity of 60% to 90%.49 Limitations of the verbal autopsy are similar to those listed for the WHO guideline; such as possible inclusion of deaths from malaria (where prevalent) among deaths from ARI63 and frequent confounding of cause of death by comorbidities such as malnutrition, measles, or human immunodeficiency virus (HIV) infection in poor countries.49 ARI is an associated cause or final mode of death even more often than it is a direct cause of death.64
LIMITATIONS IN COMPARING EPIDEMIOLOGY IN THE DEVELOPING VERSUS THE DEVELOPED WORLD
We should bear in mind the sensitivity and specificity of the tool used when comparing data. Compared with children in developed countries, children in developing countries have high exposure to risk factors, high rates of bacterial infection, low access to medical care, and high incidence of and mortality from pneumonia. The WHO criteria for ARI have been deliberately chosen to have high sensitivity, accepting the cost of lower specificity. This may have the effect of exaggerating differences in estimates of ARI between developed and developing countries. On the other hand, many researchers state that their best estimates of ARI in developing countries remain conservative and are likely to underestimate the rates. These differences in method should be kept in mind when discussing the estimates below.
OTHER LIMITATIONS OF METHODS
Global estimates and national estimates of ARI incidence and mortality usually depend on data derived indirectly by curve fitting, extrapolation, and applying one proportion to another. Studies at different time points and using different methods may be included. Many of the incidence studies go back to the 1980s,65 whereas mortality estimates are based on more recent total and cause-specific mortality data.
LIMITATIONS OF AGE GROUPS STUDIED
The majority of international studies of the epidemiology of ARIs deal with children under the age of 5 years. There are fewer data for older age groups; nonetheless, several studies66, 67 have shown a decreasing incidence with age. For the remainder of this section, we confine our discussion to the child less than 5 years of age.
Incidence of Acute Lower Respiratory Tract Infection
Rudan and associates65 recently estimated worldwide incidence for ARI in children under 5 years of age. They performed a meta-analysis of 28 studies completed mostly in the late 1980s in developing countries. They sought to confirm the directly reported rates in these studies with indirectly calculated rates using case-fatality, rates of severe pneumonia, and case-fatality of severe pneumonia. Their best estimate for the incidence of ARI in the developing world was 0.29 events per child-year, or 150.7 million new cases of ARI per year, of which 11 to 20 million are severe enough to require hospital admission.
Rudan and associates were unable to find studies for the developed world that used active surveillance and WHO criteria for ARI. They based their estimate of 0.026 event per child-year (or 2.1 million cases of pneumonia per year) on four large population-based studies of community-acquired pneumonia in the United States and Europe.
The recent UNICEF/WHO report on pneumonia68 indicates that in 2004, 15 countries accounted for three quarters of childhood pneumonia cases worldwide, amounting to approximately 113 million cases. These countries, and the estimated number of cases in 2004, were India (44 million), China (18 million), Nigeria and Pakistan (7 million each), Bangladesh and Indonesia (6 million each), Brazil and Ethiopia (4 million each), Democratic Republic of the Congo and Philippines (3 million each), followed by Afghanistan, Egypt, Mexico, Sudan, and Vietnam (2 million each).
Mortality Due to Acute Lower Respiratory Tract Infection
By country, the percentage of annual deaths of children under 5 years that are due to ARI is related in a logarithmic fashion to the total under 5-year mortality (Fig. 31-5 ).49 Figure 31-6 shows the estimated percentage range of deaths from lower ARI among children of each country in 2000.49 These data were estimated by extrapolating to the year 2000 the 1999 WHO total under-5 mortality data by country, and using the blue fitted curve in Figure 31-5 to estimate the percentage of deaths due to ARI. The authors estimated that between 1.6 and 2.2 million children died of ARI in 2000 and that 40% of these deaths occurred in Africa and 30% occurred in Southeast Asia. Mortality from ARI in excess of 25% of total under-5 deaths was estimated for Afghanistan, Sierra Leone, and Niger. The regions with the lowest proportions of deaths due to ARI were in Western Europe, North America, and Australasia. Other respiratory deaths in young children, estimated for 1998, fell into the following categories: upper respiratory infection and otitis media—37,000, neonatal pneumonia—215,500, pertussis—287,000, measles—594,700, and AIDS-associated ARI—87,500.69
Figure 31-5.

Percentage of deaths due to acute lower respiratory tract infection, adjusting for bias in verbal autopsies. The blue curve is a weighted log-linear fit to the data points, which are assembled from a number of studies. The red and green curves are (respectively) expected values and their 95% confidence intervals after correcting for the calculated underestimation and variability of reporting deaths from acute lower respiratory tract infection because of the use of verbal autopsies in many of the studies.
(From Williams BG, Gouws E, Boschi-Pinto C, et al: Estimates of world wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2:25-32, 2002.)
Figure 31-6.

Estimates of the percentage of childhood deaths that are attributable to acute lower respiratory tract infection by country in 2000. The last category includes values up to 36%.
(From Williams BG, Gouws E, Boschi-Pinto C, et al: Estimates of world wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2:25-32, 2002.)
Table 31-2 gives an updated summary of mortality due to ARI from the WHO 2005 report70 by region averaged for the years 2000 to 2003. The numbers have slightly increased but are otherwise similar to those of Williams and associates.49 Table 31-3 gives a summary of global statistics for ARI in children under 5 years of age, divided into those for the developing and those for the developed world. These figures are based on Rudan and colleagues65 and the recent UNICEF/WHO report.68
Table 31-2.
WHO Report 2005: Data for 2000-2003 on Deaths Due to Acute Respiratory Infection (ARI) under 5 Years of Age
| ARI Deaths by Region | ARI as Proportion of All Deaths in Each Region | Distribution of Global ARI Deaths by Region | |
|---|---|---|---|
| World | 2,027,000 | 19% | 100% |
| Africa | 924,000 | 21% | 45% |
| The Americas | 54,000 | 12% | 3% |
| Canada, United States | 1000 | 2% | |
| Rest of the Americas | 53,000 | 14% | |
| Southeast Asia | 590,000 | 19% | 29% |
| Europe | 32,000 | 12% | 1.6% |
| Low-mortality countries* | < 1000 | 2% | |
| Rest of Europe | 31,000 | 13% | |
| Eastern Mediterranean | 292,000 | 21% | 14% |
| Western Pacific | 137,000 | 13% | 7% |
| Australia, Japan, New Zealand | < 1000 | 4% | |
| Rest of Western Pacific | 137,000 | 13% |
Andorra, Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, the Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, and the United Kingdom.
Adapted from van Lerberghe W, World Health Organization: Make Every Mother and Child Count. Geneva, World Health Organization, 2005.
© 2008 World Health Organization
Table 31-3.
Acute Respiratory Infection (ARI) under 5 Years of Age: Epidemiology at a Glance
| Incidence 2004: Millions of Cases per Year | Incidence: Events per Child-Year | Case-Fatality Rate: Median | Mortality 2004: Deaths per Year | ||
|---|---|---|---|---|---|
| Developing countries | All ARI | 154.5 | 0.29 | 2% | 2,039,000 |
| Severe ARI* | 11-20 | 0.03 | 10% | ||
| Developed countries | 1.6 | 0.026 | — | 1,000 | |
| World | 158.5 | 0.26 | — | 2,044,000 |
Severe ARI is ARI severe enough to require hospital admission.
From UNICEF/WHO: Pneumonia: The Forgotten Killer of Children. New York, UNICEF/WHO, 2006; and Rudan I, Tomaskovic L, Boschi-Pinto C, et al: Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Org 82:895-903, 2004.
© 2008 UNICEF/WHO
If for the moment we ignore the potential errors due to the limitations mentioned above, it would seem that 90% of the child deaths from ARI occur in the developing world, with 75% of them in Africa and Southeast Asia. ARIs constitute about 20% of total mortality in the developing world but only 2% of total mortality in the developed areas of Europe, the Western Pacific, and the Americas. The differences in estimation and comparison are unlikely to have caused such large discrepancies.
Risk Factors for Acute Lower Respiratory Tract Infection
Table 31-4 shows well-established risk factors for developing ARI or pneumonia. Risk factors such as malnutrition are prevalent in developing countries, whereas vehicle emissions may be higher in developed countries.
Table 31-4.
Risk Factors for Getting Acute Respiratory Infection
| Personal Health Factors | Local Environmental Factors |
|---|---|
| Small Child | Poverty |
| Low birth weight/prematurity | Low family income/parent education level |
| < 5 years old, and especially <1 year old | ↓ Access to clean water, sanitation, clothing, housing, health care, immunizations |
| Lack of Breastfeeding | Crowding |
| Malnutrition | Large families, late–birth order children, early child care groups, peri-urban slums |
| Kwashiorkor/marasmus | Indoor Air Pollution |
| Micronutrient deficiency (zinc, vitamin A) | Use of biomass fuel (wood products, refuse, dung) |
| Underlying Heart or Lung Disease | Tobacco smoke exposure |
| Left-to-right shunt/heart failure | Outdoor Air Pollution |
| Bronchiectasis/cystic fibrosis | Vehicle emissions/CO |
| Asthma/ciliary dyskinesia | Geographic Factors |
| Immunodeficiency | High rainfall? |
| HIV infection | High altitude? |
| Immunosuppression | Wet or cold season |
| Congenital immunodeficiency |
Risk factors for dying from ARI (almost all from pneumonia) include most of the same factors, but comorbid diseases (especially diarrhea, malaria, measles, tuberculosis, and HIV infection) further increase the mortality risk.49, 71 Lack of adequate health care is also an important associated factor in deaths from pneumonia. Geography, education, access, and accessibility are all factors in the adequacy of health care. Where there is access to health care, the cost of life-saving treatment for pneumonia is relatively small.
According to a Lancet editorial in 2003,72 26% of the world's children under the age of 2 do not receive basic diphtheria, tetanus, and pertussis vaccination, 58% do not receive exclusive breastfeeding for the first 4 months of life, 25% have malnutrition, and 40% do not receive appropriate antibiotic treatment for pneumonia. These risk factors are of course bound together by many strands within communities. As Bawaskar73 has pointed out, illiteracy is a major factor in infant malnutrition, and low income and illiteracy are enmeshed with impoverished, unsanitary and crowded living conditions, high rates of HIV infection, and lack of access to health care.
MALNUTRITION AND ACUTE LOWER RESPIRATORY TRACT INFECTION
Black and colleagues71 have estimated that as many as 50% of the deaths from ARI/pneumonia in children are attributable to being underweight (Fig. 31-7 ). A study in Costa Rica in the early 1970s documented a 12-fold increased risk (71% versus 6%, respectively) of pneumonia among malnourished compared with normally nourished children.74
Figure 31-7.
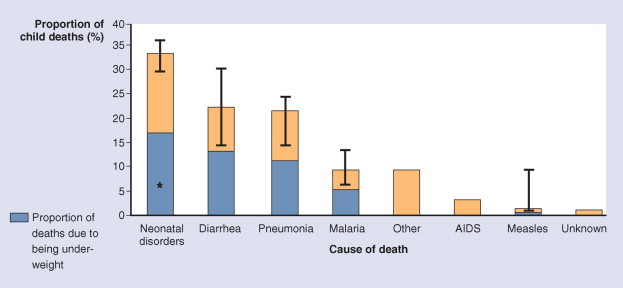
Distribution of global deaths by cause. Bars indicate uncertainty bounds. *Work in progress to establish the cause-specific contribution of being underweight to neonatal deaths.
(From Black RE, Morris SS, Bryce J: Where and why are 10 million children dying every year? Lancet 361:2226-2234, 2003.)
Rice and coworkers75 presented a comprehensive review of the recent literature regarding the effects of malnutrition on death from diarrhea, ARI, malaria, and measles. They summarized three community studies and 12 facility-based studies. The results were consistent across all studies showing a major association between death from ARI and low weight-for-age (W/A) Z-scores or median W/A less than 80% of median. The odds ratios, risk ratios, or relative risks of dying from respiratory infection compared with that for normally nourished children ranged very widely from single figures up to an odds ratio of 26 for W/A Z-score = -2.0 (compared with >0) in a Brazilian study and an adjusted relative risk of 27 for children whose W/A was less than 60% of the local reference median (compared to ≥90%) in a Philippines study. All studies that analyzed risks by degree of malnutrition found a dose-response effect.
Figure 31-8 , taken from Victora and colleagues76 and based on Kirkwood and colleagues,77 shows that 84% of deaths from pneumonia occur in the first 18 months of life and indicates the potential impacts of nutritional problems at different ages. Victora and colleagues' estimated reduction in pneumonia mortality by region and by intervention is shown in Table 31-5 .
Figure 31-8.
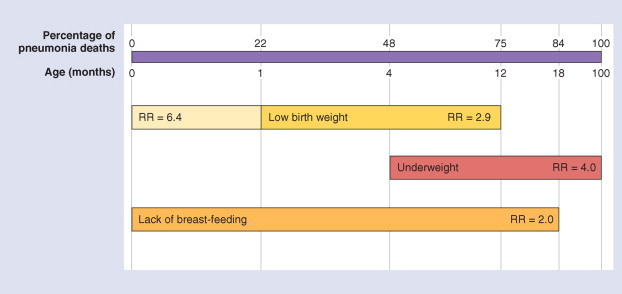
Relations between nutritional risk factors for pneumonia mortality and distribution of pneumonia deaths by age. RR, relative risk.
(Redrawn from Victora CG, Kirkwood BR, Ashworth, A, et al: Potential interventions for the prevention of childhood pneumonia in developing countries: Improving nutrition. Am J Clin Nutr 70:309-320, 1999.)
© 2008
Table 31-5.
Hypothetical Reductions in Pneumonia Mortality According to Different Nutritional Interventions, Assuming a 40% Reduction in Prevalence of the Risk Factor
| Risk Factor to Be Reduced* |
|||
|---|---|---|---|
| Region | Low Birth Weight | Malnutrition | Non–breastfeeding |
| Sub-Saharan Africa | 9.0 | 10.0 | 0.5 |
| Middle East and North Africa | 6.5 | 7.0 | 4.3 |
| South Asia | 14.0 | 13.3 | — |
| East Asia and the Pacific | 6.9 | 9.1 | 2.2 |
| Latin America and the Caribbean | 6.9 | 5.1 | 7.0 |
| All developing countries | 10.1 | 10.7 | 3.3 |
Values given in percent reduction in pneumonia mortality rates.
Adapted with permission from the American Journal of Clinical Nutrition from Victora CG, Kirkwood BR, Ashworth A, et al: Potential interventions for the prevention of childhood pneumonia in developing countries: Improving nutrition. Am J Clin Nutr 70:309-320, 1999.76
© 2008
HUMAN IMMUNODEFICIENCY VIRUS INFECTION AND ACUTE LOWER RESPIRATORY TRACT INFECTION
Graham and coworkers78 have summarized recent data for the respiratory effects of HIV infection in African children. Approximately 90% of the HIV-infected children in the world come from sub-Saharan Africa. HIV infection greatly increases the incidence of and mortality from pulmonary disease, mostly in children under 5 years. Conversely respiratory disorders are the most common cause of death in HIV-infected African children. For children hospitalized for severe pneumonia, HIV-infected children in Malawi were three times as likely to die, and in South Africa six times as likely to die, as were non–HIV-infected children.
The high incidence of opportunistic infections (such as Pneumocystis jirovecii—formerly known as Pneumocystis carinii) of the respiratory tract in HIV infection is well known. The common pathogens found in HIV-infected children with pneumonia are, in the United States, S. pneumoniae and Salmonella spp., and in Africa, S. pneumoniae, H. influenzae, Staphylococcus aureus, Escherichia coli, Klebsiella spp., and nontyphoidal Salmonella spp.78
HIV infection is commonly associated with other risk factors for pneumonia including poverty and malnutrition, such as children with marasmus.78 As a result, it can be difficult to determine the relative importance of the HIV infection compared with other risk factors in determining the nature and outcome of pneumonia in regions with high HIV prevalence.
Epidemiology and Prevention of Pneumonia Due to Streptococcus pneumoniae and Haemophilus influenzae
Among causes of ARIs in young children, the two organisms responsible for the largest number of cases (and 70% of the deaths)57 are S. pneumoniae and H. influenzae, the latter including nontypable strains, type B (Hib), and sometimes other typable strains.79 We now have the means to prevent pneumonia due to Hib and to many strains of S. pneumoniae. Madhi and colleagues80 demonstrated that Soweto children (both HIV positive and negative) immunized with pneumococcal conjugate vaccine had 31% fewer episodes of virus-associated pneumonia compared with placebo controls. This seems to imply that virus-associated pneumonia commonly involves mixed infections with bacteria.
STREPTOCOCCUS PNEUMONIAE
Isolation rates of S. pneumoniae in pneumonia are very dependent on sample and identification method and are highly variable in studies in developing and developed countries.79, 81 Berman82 reported an overall isolation rate of 27% in 14 studies, and 60% of the studies reported isolation rates greater than 30%. The estimated incidence of pneumonia due to S. pneumoniae also varies greatly. Incidence rates from 18.1 to almost 200 per 100,000 children per year have been described in developing countries.79, 83, 84 Incidence rates estimated in Southern California were 17 per 100,000 per year in 1996.67, 85 Serotypes 1, 2, 5, 7, 9, 14, 15, 18, 19, and 23 are common in both developing and developed countries.79 Despite the frequency of isolation of S. pneumoniae, there are few specific estimates of pneumonia mortality due to this organism.
The peak season for pneumococcal pneumonia in developed countries is winter66, 86 (Fig. 31-9 ), closely corresponding to peak isolation rates of RSV and influenza. In tropical countries, studies have reported either little seasonal variation84 or small peaks in the hot season and the monsoon season83 (Fig. 31-10 ). The peaks observed could be explained either by secondary infection on the heels of seasonal viral infections or (in parallel to virus infections) by increased interpersonal transmission of bacteria during seasons when people spend more time indoors.
Figure 31-9.

Weekly isolation of winter respiratory viruses RSV (red line) and influenza (blue line) and frequency of invasive pneumococcal disease (green line) from January 1, 1995, through June 30, 2002.
(From Talbot TR, Poehling KA, Hartert TV, et al: Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation. Am J Med 118:285-291, 2005.)
© 2008
Figure 31-10.

Seasonal distribution of pneumococcal pneumonia in 103 children in Upper River Division, The Gambia, 1989 to 1991.
(Data from O'Dempsey TJ, McArdle TF, Lloyd-Evans N, et al: Pneumococcal disease among children in a rural area of west Africa. Pediatr Infect Dis J 15:431-437, 1996. Used with permission of Lippincott Williams & Wilkins.)
© 2008 Lippincott Williams & Wilkins
Studies in developing countries have indicated that both total child mortality and mortality due to pneumonia could be lowered by the use of pneumococcal vaccines. Black and coworkers,87 in a double-blind randomized controlled trial of heptavalent pneumococcal conjugate vaccine in California, reported a 32.2% reduction in pneumonia with positive radiographs in children under 1 year, 23.4% in children under 2 years, and 9.1% in children over 2 years old. Cutts and coworkers88 have reported a randomized double-blind placebo controlled trial of nine-valent pneumococcal conju gate vaccine in Gambian infants less than 1 year old. They estimated (based on intention-to-treat analyses) a reduction in first episodes of pneumonia of 6%, reduction of admissions to hospital of 13%, reduction of admissions due to potentially invasive pneumococcal disease of 19%, and reduction in all-cause mortality of 14%. Vaccine efficacy was 77% against invasive pneumococcal disease due to vaccine-related serotypes and 50% against invasive disease due to all serotypes.
HAEMOPHILUS INFLUENZAE
Levine and coworkers,89 in a 1998 review, estimated that H. influenzae (all strains) caused 16% of all pneumonia deaths not due to measles or pertussis or 482,000 deaths from pneumonia each year in children under 5 years in developing countries. Peltola90 conservatively estimated the annual prevaccination incidence of Hib pneumonia in children under 5 years in developing countries at 300 per 100,000 based on Gambian studies in the 1990s.90, 91 This represents 1.7 million cases and 220,00 to 400,000 deaths with a case-fatality rate of 13% to 24%. In developed countries, Peltola estimated a prevaccination incidence of 6 per 100,000, representing 5000 cases, 250 deaths, and a case-fatality rate of 5%.
Nontypable strains have been found to be as commonly or more commonly associated with pneumonia in several studies,79, 90 but only in developing countries. Levine and colleagues89 reported that Hib accounted for a mean of 35% of all H. influenzae pneumonia cases in three studies of all H. influenzae pneumonias but for a mean of 71% of bacteremic H. influenzae pneumonias in eight studies. If most deaths occur in bacteremic pneumonia, as is supposed, then Hib may be responsible for up to 70% of the deaths from H. influenzae pneumonia in under-5 children.
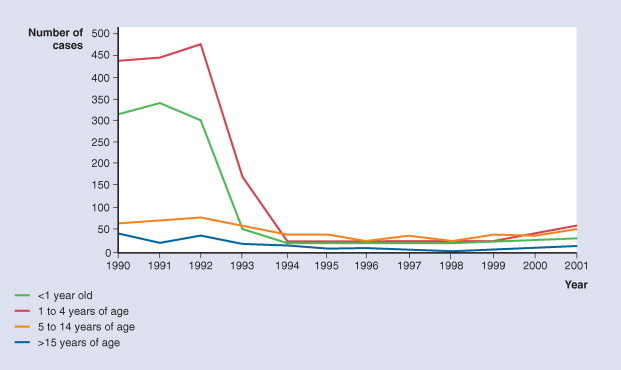
Vaccination for H. influenzae type B has dramatically reduced all causes of invasive Hib in the developed world92 (Fig. 31-11 ).
Figure 31-11.
Invasive Hib infections by age, 1990 to 2001, England and Wales, combined PHLS HRU/CDSC data.
(From McVernon J: Hib surveillance and remaining HIB disease in the UK. Paper presented at Global Reduction of Hib Disease Meeting, September 22-25, 2002, Scottsdale, Arizona.)
© 2008
Trials of Hib conjugate vaccine in developing countries have included prospective case-control studies in Chile93 and Brazil94 and a double-blind randomized placebo controlled trial in The Gambia.95 Vaccine efficacy in young children ranges from 21% to 31% against radiology-confirmed pneumonia from all causes in these studies. The Gambian study showed reduction of oropharyngeal carriage of Hib.96
Levine and co-workers in 199889 estimated the potential impact of routine immunization of infants with 3 doses of Hib conjugate vaccine in developing countries (Table 31-6 ). The estimates include all Hib disease (the majority being pneumonia and meningitis). They suggested that 60% of deaths and 67% of cases would be prevented if no herd immunity were operative. In the presence of herd immunity over 300,000 deaths (83% of deaths) and 2.5 million cases (85% of cases) might be prevented. Miller97 estimated that among Asian countries, about 136,000 (87%) Hib deaths could be prevented annually with incorporation of Hib vaccine into the immunization programs at a cost of between 0.1% and 3.0% of per capita gross national product per child younger than 5 years. However, a recent Hib vaccine probe trial among 55,073 children in Lombok, Indonesia,98 found no protective effect against radiology-confirmed pneumonia despite a protective effect against clinically defined pneumonia and against meningitis. Various reasons are discussed in the paper and the accompanying editorial.99
Table 31-6.
Estimated Impact of Routine Immunization of Infants with Hib Conjugate Vaccine in Developing Countries
| Three-Dose Vaccine Regimen |
|||
|---|---|---|---|
| Outcome Measure | No Immunization | Without Herd Immunity | With Herd Immunity |
| No. of Hib deaths | |||
| Expected | 377,470 | 150,670 | 65,120 |
| Prevented | 226,800 | 312,350 | |
| Percent of deaths prevented | 60% | 83% | |
| No. of Hib cases | |||
| Expected | 2,915,000 | 957,800 | 423,750 |
| Prevented | 1,957,200 | 2,491,250 | |
| Percent of cases prevented | 67% | 85% | |
Adapted from Levine OS, Schwartz B, Pierce N, Kane M: Development, evaluation and implementation of Haemophilus influenzae type b vaccines for young children in developing countries: Current status and priority actions. Pediatr Infect Dis J 17(9 suppl):S95-S113, 1998. Used with permission of Lippincott Williams & Wilkins.
© 2008 Lippincott Williams & Wilkins
Conclusion
ARI and pneumonia contribute a massive burden of death and morbidity to young children and much more so in developing countries than in developed countries. Vaccination strategies to prevent disease are well proved for single-agent diseases with obligate human hosts. However, vaccine implementation, acceptance, and coverage are always going to offer challenges when different political, economic, health, and cultural systems have to be engaged. Other major factors that will be required to decrease the morbidity and mortality due to ARI in children must include the reduction or eradication of poverty, malnutrition, and HIV infection and improve ments in air quality through reduction of smoke exposure from cigarettes and biomass fuels.
EMERGING AND REEMERGING DISEASES
Infectious diseases are the leading cause of death among young people under the age of 50 years.100 The complex matrix of these diseases from endemic diseases, which pose an ongoing threat, through to new emerging and reemerging diseases that present new challenges101 is shown in Figure 31-12 . Respiratory pathogens, including SARS, influenza, and tuberculosis, are some of the emerging and reemerging threats that have recently received or are receiving ongoing attention as global public health concerns. These infections, many of which are zoonotic in origin, are creating unprecedented challenges, and spurring the development of new approaches. The application of epidemiology simulation modeling to identify the best public health responses under a variety of conditions102 and for specific interventions with pandemic influenza103 are examples.
Figure 31-12.
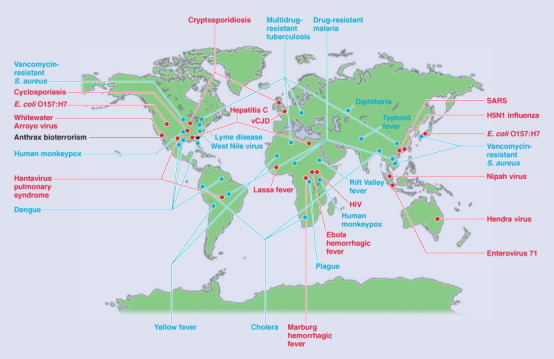
Global examples of emerging and reemerging infectious diseases. Red represents newly emerging diseases; blue, reemerging/resurging diseases; black, a “deliberately emerging” disease.
(From Morens DM, Folkers SK, Fauci AS: The challenge of emerging and re-emerging infections diseases. Nature 430:242-249, 2004.)
© 2008
Influenza
GENERAL EPIDEMIOLOGY
Of all the viruses that infect the human respiratory tract, influenza viruses cause the predominant number of serious acute respiratory tract illnesses.104 There are three types of influenza virus, A, B, and C, of which the influenza A and B viruses are clinically relevant in humans. Influenza B viruses have a human reservoir, whereas influenza A viruses have a reservoir in aquatic birds and are antigenically diverse. Influenza A viruses have two major antigenic surface proteins, the hemagglutinin (H) and neuraminidase (N). We recognize 16 different H antigens (named H1–16) and nine different N antigens (N1–9), and unique combinations of H and N antigens are called influenza A subtypes. All of these subtypes have been found circulating among wild aquatic birds, their primary natural reservoir. From time to time, these viruses cross the species barrier, with some becoming established in another avian or animal species. In the past century, the influenza A subtypes H1N1, H2N2, and H3N2 have been able to infect and establish sustained transmission among humans, whereas the H5N1 subtype has become established in domestic poultry (Fig. 31-13 ). The constant evolution of influenza A and B viruses occurs through the accumulation of mutational changes in the H and N antigens. This antigenic drift leads to the selection of new variants and regular epidemics of disease, while reassortment of the H and N genes of different influenza A subtypes (antigenic shift) leads to the emergence of a novel virus and pandemic influenza.105 However, it is becoming clear that the emergence of human influenza virus lineages can occur through avenues other than drift and shift.106
Figure 31-13.

Time course of global spread of human influenza virus A subtypes and human outbreaks of avian influenza viruses.
(Redrawn from Fauci AS: Emerging and reemerging infections diseases: The perpetual challenge. Acad Med 80:1079-1085, 2005.)
© 2008
EPIDEMIC INFLUENZA
Epidemics of influenza occur almost every year. They are caused by new variants of influenza A and B viruses that have evolved through antigenic drift, allowing them to evade the host's immune defenses. This requires regular updates of the composition in influenza vaccines. In countries with temperate climates influenza activity peaks during the winter months with influenza A epidemics occurring every 1 to 2 years and influenza B circulating every 2 to 4 years.
ATTACK RATES IN CHILDREN
Influenza incidence and illness are high in children. Attack rates can be 40% or more in preschool-aged children and 30% in school-age children, which is higher than the 10% to 20% rate commonly observed in young adults.26, 33, 107, 108, 109
DISSEMINATION
Children have an important role in spreading influenza. School provides an ideal environment for the spread of respiratory viruses, and school-age children serve as the main channels through which influenza A and B virus infections are introduced into households.107, 109, 110 Exacerbations of COPD in adults were found to be correlated with epidemics of viral infections including influenza A and B in school children and to decline during school holidays.111 In young children, influenza virus is detectable 1 to 3 days after infection and shedding often persists for 10 days to 3 weeks.112
HOSPITALIZATIONS
During childhood, influenza is the most significant cause of acute respiratory illness leading to hospitalization,113 outpatient visits,108 and courses of antibiotics in children of all ages.114 Healthy children younger than 1 year are hospitalized at rates similar to those for adults at high risk for influenza.115 The rate of hospitalization decreases with age. Similarly high hospitalization rates have been reported among children in the subtropics compared with those reported in temperate regions.116
PANDEMIC INFLUENZA
Human pandemics of influenza have been reliably described since the 16th century, with an average of three occurring every 100 years.117 Over the 20th century, there have been the 1918-1919 “Spanish” H1N1 pandemic, which caused an estimated 20 to 50 million deaths, mainly in previously healthy persons aged 20 to 40 years old, and the 1957 “Asian” H2N2 and 1968 “Hong Kong” H3N2 pandemics, which caused large numbers of cases and a combined mortality estimated to be more than 3 million deaths, mostly in the very young, the elderly, and people with underlying chronic conditions (see Fig. 31-13).
Recovery of lung tissue from victims of the 1918 pandemic has allowed the isolation of viral RNA and the reconstruction of the complete 1918 pandemic virus in the laboratory.118, 119 These experiments support the hypothesis that the 1918 H1N1 virus was of avian origin and adapted to human infection and transmission. In contrast, the influenza viruses that caused the 1957 and 1968 pandemics are human-avian reassortant viruses, and this difference may be relevant to the severity of the 1918 pandemic.120
AVIAN INFLUENZA ASSOCIATED WITH HUMAN CASES
Human infections and outbreaks following interspecies transmission of highly pathogenic avian influenza viruses have rarely been reported before 1997.121 Reports are increasing of human infections associated with direct or indirect contact with infected birds. In 1997, the infection of 18 humans, of whom 6 died, with an avian H5N1 virus raised the level of global concern of a possible human influenza pandemic. In 1999, H9N2 avian influenza infected two children in Hong Kong and there were other cases in mainland China. In 2003, H5N1 and H9N2 infections were confirmed in Hong Kong, while in the Netherlands, a large avian influenza outbreak involved an H7N7 virus. Up to 1000 cases among farmers and poultry workers occurred. Since late 2003, outbreaks of avian H5N1 have been reported among poultry in Southeast Asia.122 Human infections and deaths were initially reported in Vietnam and Thailand. This virus has become endemic in domestic poultry in Asia and has spread globally after infecting migratory waterfowl.123 Subsequently, there have been reports of human infections in an increasing number of countries. Clusters of human infection are small, suggesting that if human-to-human transmission is occurring, it is very inefficient.
As this H5N1 virus continues to circulate in and be spread by domestic and migratory avian species, there is an ongoing risk of human infection and a threat of the emergence of a human pandemic virus. Whether the H5N1 virus or one of the other potential pandemic subtypes (H2, H5, H7, or H9 viruses) will adapt to efficient human-to-human transmission remains unknown. Currently, no mechanism of prediction of the emergence of novel influenza A viruses exists, highlighting the importance of the ongoing global surveillance of animal and human influenza viruses by the WHO.
Severe Acute Respiratory Syndrome
GENERAL EPIDEMIOLOGY
SARS is an acute viral respiratory syndrome caused by a novel coronavirus, the SARS coronavirus (SARS-CoV), and rec-ognized as a global threat in mid-March 2003. The virus is an animal virus that has crossed the species barrier to humans.124, 125 The first known cases are believed to have occurred in Guangdong Province, China, in November 2002.126 Early cases were associated with occupational exposure to infected animals127; however, once the number of cases started to increase, health care workers and their close contacts were at greatest risk of infection. The epidemic was characterized by “superspreading events,”128, 129, 130 which seeded outbreaks in Canada, China, Hong Kong, Taiwan, Singapore, and Vietnam (Fig. 31-14 ). By July 2003, the international spread of SARS-CoV presented a global public health threat resulting in 8098 SARS cases in 26 countries and 774 deaths.131 The epidemic caused social and economic disruption in areas with sustained transmission of SARS, and on the travel industry internationally, in addition to the impact on health services directly.
Figure 31-14.
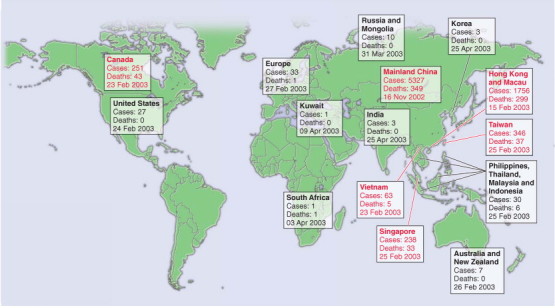
The global spread of SARS. The number of probable cases of SARS and the date of onset of the first case in each country (or group of countries) is denoted. The countries denoted in red are those where substantial local transmission occurred.
(From Peiris JS, Guan Y, Yuen KY: Severe acute respiratory syndrome. Nat Med 10(12 suppl):S88-S97, 2004.)
© 2008
Since July 2003, four sporadic community-acquired cases have occurred in Guangzhou, Guangdong Province, China,132 while three incidents have been attributed to exposures in laboratories.125, 133
RESERVOIR
The natural reservoir of SARS-CoV has been identified as the Chinese horseshoe bat.134 Although many animals have been investigated as possible reservoirs,124, 135 bats are well suited to transmit zoonotic disease, as many people in Asia eat bats and use their feces for medicines.
CHILDREN
SARS runs a more benign and shorter clinical course in young children (less than 12 years of age) during the acute phase.136 Infants born to mothers with the disease did not acquire the infection through vertical transmission. No deaths were reported in children.137 Children appear to acquire the infection by close-contact household exposure to an infected adult.138
Tuberculosis
Tuberculosis (TB) remains a major global public health problem. It is estimated that in 2004, one third of the world's population was infected with the mycobacterium bacillus that causes TB and almost 4 million were smear positive.139 A number of factors have facilitated the resurgence of tuberculosis. These include the advent of the AIDS epidemic, emigration from countries where prevalence of TB is high, noncompliance of patients, transmission in high-risk environments, and the coincident increase in the number of cases of multidrug-resistant tuberculosis (MDR-TB).
GLOBAL INCIDENCE
The estimated TB incidence globally is shown in Figure 31-15 .140 The largest number of cases occurs in the Southeast Asia region (190 cases per 100,000 population), which accounts for 33% of incident cases globally; however, in sub-Saharan Africa, the incidence per capita is nearly twice that of Southeast Asia, given by the WHO at 356 cases per 100,000 population in 2004.139
Figure 31-15.
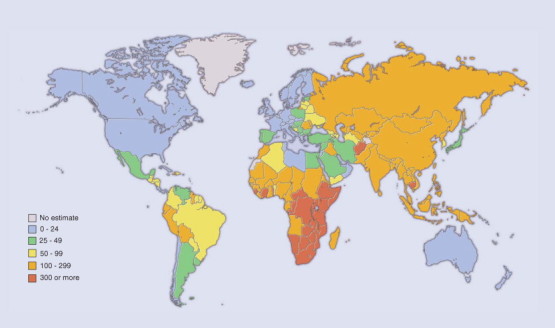
Estimated tuberculosis incidence rates, 2003.
(From the Global Plan to Stop TB 2006-2015. Geneva, World Health Organization, Actions for Life, 2006.)
© 2008 World Health Organization, Actions for Life
The WHO also estimated that 1.69 million deaths (27 deaths per 100,000 population) resulted from TB in 2004.139 As with cases of disease, the highest number of estimated deaths is in the Southeast Asia region, but the highest mortality per capita (78 deaths per 100,000 population) is in sub-Sahaian, Africa, where HIV has led to rapid increases in the incidence of TB and increases the likelihood of dying from TB (Fig. 31-16 ).140 TB accounts for about 13% of AIDS deaths worldwide.141
Figure 31-16.
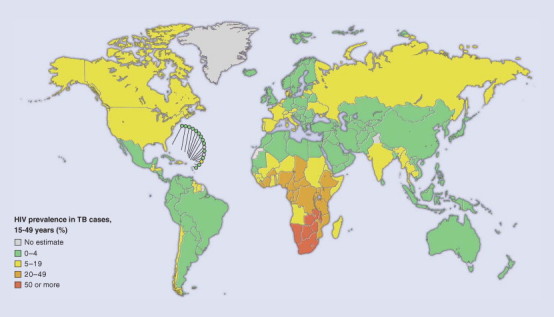
Estimated prevalence of HIV infection in TB cases, 2003.
(From the Global Plan to Stop TB 2006-2015. Geneva, World Health Organization, Actions for Life, 2006.)
© 2008 World Health Organization, Actions for Life
DRUG-RESISTANT TUBERCULOSIS
Drug-resistant TB is on the increase in many parts of the world.142 The development of resistance is a result of inconsistent or partial treatment of cases. Of particular concern is multidrug-resistant TB, which is defined as resistance to isoniazid and rifampicin, the two most effective anti-TB drugs. It is estimated that 300,000 new cases of multidrug-resistant TB are developing each year.139
SUGGESTED READINGS
Common Respiratory Viruses
- Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol. 2004;14:17–31. doi: 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson KJ. Viral pneumonia in children. Semin Pediatr Infect Dis. 1998;9:217–233. doi: 10.1016/S1045-1870(98)80035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11 suppl):S223–S227. doi: 10.1097/01.inf.0000188166.17324.60. discussion S6. [DOI] [PubMed] [Google Scholar]
- Legg JP, Warner JA, Johnston SL. Frequency of detection of picornaviruses and seven other respiratory pathogens in infants. Pediatr Infect Dis J. 2005;24:611–616. doi: 10.1097/01.inf.0000168747.94999.aa. [DOI] [PubMed] [Google Scholar]
- Zambon MC, Stockton JD, Clewley JP. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: An observational study. Lancet. 2001;358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
Acute Lower Respiratory Tract Infection
- Cashat-Cruz M, Morales-Aguirre JJ, Mendoza-Azpiri M. Respiratory tract infections in children in developing countries. Semin Pediatr Infect Dis. 2005;16:84–92. doi: 10.1053/j.spid.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Michelow IC, Olsen K, Lozano J. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- Obaro SK, Madhi SA. Bacterial pneumonia vaccines and childhood pneumonia: Are we winning, refining, or redefining? Lancet Infect Dis. 2006;6:150–161. doi: 10.1016/S1473-3099(06)70411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen Z, Pio A, Enarson P. Case management of childhood pneumonia in developing countries: Recent relevant research and current initiatives. Int J Tuberc Lung Dis. 2000;4:807–826. [PubMed] [Google Scholar]
- UNICEF/WHO . Pneumonia: The forgotten killer of children. UNICEF; New York: 2006. Available at http://www.unicef.org/publications/index_35626.html [Google Scholar]
Emerging Diseases
- Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- Fauci AS. Emerging and reemerging infectious diseases: The perpetual challenge. Acad Med. 2005;80:1079–1085. doi: 10.1097/00001888-200512000-00002. [DOI] [PubMed] [Google Scholar]
- Peiris JS, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004;10(12 suppl):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Tuberculosis. Fact Sheet No. 104. World Health Organization; Geneva: 2006. [Google Scholar]
REFERENCES
Common Respiratory Viruses
- 1.Denny FW, Clyde WA., Jr Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986;108(5 Pt 1):635–646. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- 2.Juven T, Mertsola J, Waris M. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Henrickson KJ. Viral pneumonia in children. Semin Pediatr Infect Dis. 1998;9:217–233. doi: 10.1016/S1045-1870(98)80035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112(suppl 6A):4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 5.Jennings LC, Anderson TP, Werno AM. Viral etiology of acute respiratory tract infections in children presenting to hospital: Role of polymerase chain reaction and demonstration of multiple infections. Pediatr Infect Dis J. 2004;23:1003–1007. doi: 10.1097/01.inf.0000143648.04673.6c. [DOI] [PubMed] [Google Scholar]
- 6.Kehl SC, Henrickson KJ, Hua W. Evaluation of the Hexaplex assay for detection of respiratory viruses in children. J Clin Microbiol. 2001;39:1696–1701. doi: 10.1128/JCM.39.5.1696-1701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberg GA, Erdman DD, Edwards KM. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189:706–710. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
- 8.Ieven M, Goossens H. Relevance of nucleic acid amplification techniques for diagnosis of respiratory tract infections in the clinical laboratory. Clin Microbiol Rev. 1997;10:242–256. doi: 10.1128/cmr.10.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ireland DC, Kent J, Nicholson KG. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J Med Virol. 1993;40:96–101. doi: 10.1002/jmv.1890400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston SL, Sanderson G, Pattemore PK. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993;31:111–117. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legg JP, Warner JA, Johnston SL. Frequency of detection of picornaviruses and seven other respiratory pathogens in infants. Pediatr Infect Dis J. 2005;24:611–616. doi: 10.1097/01.inf.0000168747.94999.aa. [DOI] [PubMed] [Google Scholar]
- 12.Arden KE, McErlean P, Nissen MD. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Hoogen BG, de Jong JC, Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nissen MD, Siebert DJ, Mackay IM. Evidence of human metapneumovirus in Australian children. Med J Aust. 2002;176:188. doi: 10.5694/j.1326-5377.2002.tb04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamelin ME, Abed Y, Boivin G. Human metapneumovirus: A new player among respiratory viruses. Clin Infect Dis. 2004;38:983–990. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11 suppl):S223–S227. doi: 10.1097/01.inf.0000188166.17324.60. discussion S6. [DOI] [PubMed] [Google Scholar]
- 17.Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 18.Drosten C, Gunther S, Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 19.van der Hoek L, Pyrc K, Jebbink MF. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo PC, Lau SK, Chu CM. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allander T, Tammi MT, Eriksson M. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;02:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drews AL, Atmar RL, Glezen WP. Dual respiratory virus infections. Clin Infect Dis. 1997;25:1421–1429. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papadopoulos NG, Moustaki M, Tsolia M. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 24.Waner JL. Mixed viral infections: Detection and management. Clin Microbiol Rev Apr. 1994;7:143–151. doi: 10.1128/cmr.7.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings LC, MacDiarmid RD, Miles JA. A study of acute respiratory disease in the community of Port Chalmers. I. Illnesses within a group of selected families and the relative incidence of respiratory pathogens in the whole community. J Hyg (Lond) 1978;81:49–66. doi: 10.1017/s0022172400053766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110:145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shek LP, Lee BW. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev. 2003;4:105–111. doi: 10.1016/s1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 28.Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med. 2006;3:e89. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parrott RH, Kim HW, Arrobio JO. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973;98:289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- 30.Monto AS. The Tecumseh study of respiratory illness. V. Patterns of infection with the parainfluenzaviruses. Am J Epidemiol. 1973;97:338–348. doi: 10.1093/oxfordjournals.aje.a121514. [DOI] [PubMed] [Google Scholar]
- 31.Frank AL, Taber LH, Glezen WP. Reinfection with influenza A (H3N2) virus in young children and their families. J Infect Dis. 1979;140:829–836. doi: 10.1093/infdis/140.6.829. [DOI] [PubMed] [Google Scholar]
- 32.Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol. 2004;14:17–31. doi: 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glezen P, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 34.Zambon MC, Stockton JD, Clewley JP. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: An observational study. Lancet. 2001;358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 35.Boivin G, De Serres G, Cote S. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowe JE., Jr Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr Infect Dis J. 2004;23(11 suppl):S215–S221. doi: 10.1097/01.inf.0000144668.81573.6d. [DOI] [PubMed] [Google Scholar]
- 37.Greensill J, McNamara PS, Dove W. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–375. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peiris JS, Tang WH, Chan KH. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindquist SW, Darnule A, Istas A. Parainfluenza virus type 4 infections in pediatric patients. Pediatr Infect Dis J. 1997;16:34–38. doi: 10.1097/00006454-199701000-00008. [DOI] [PubMed] [Google Scholar]
Acute Lower Respiratory Infection
- 40.Kajon AE, Wadell G. Molecular epidemiology of adenoviruses associated with acute lower respiratory disease of children in Buenos Aires, Argentina (1984-1988) J Med Virol. 1992;36:292–297. doi: 10.1002/jmv.1890360411. [DOI] [PubMed] [Google Scholar]
- 41.Isaacs D, Flowers D, Clarke JR. Epidemiology of coronavirus respiratory infections. Arch Dis Child. 1983;58:500–503. doi: 10.1136/adc.58.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold JC, Singh KK, Spector SA. Human bocavirus: Prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43:283–288. doi: 10.1086/505399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Hoek L, Pyrc K, Berkhout B. Human coronavirus NL63, a new respiratory virus. FEMS Microbiol Rev. 2006;30:760–773. doi: 10.1111/j.1574-6976.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorland's Illustrated Medical Dictionary. 30th ed. WB Saunders; Philadelphia: 2003. [Google Scholar]
- 45.Clinical management of acute respiratory infections in children. A WHO memorandum. Bull World Health Org. 1981;59:707–716. [PMC free article] [PubMed] [Google Scholar]
- 46.Shann F, Hart K, Thomas D. Acute lower respiratory tract infections in children: Possible criteria for selection of patients for antibiotic therapy and hospital admission. Bull World Health Org. 1984;62:749–753. [PMC free article] [PubMed] [Google Scholar]
- 47.Pio A. Standard case management of pneumonia in children in developing countries: The cornerstone of the acute respiratory infection programme. Bull World Health Org. 2003;81:298–300. [PMC free article] [PubMed] [Google Scholar]
- 48.Gove S. Integrated management of childhood illness by outpatient health workers: Technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bull World Health Org. 1997;75(suppl 1):7–24. [PMC free article] [PubMed] [Google Scholar]
- 49.Williams BG, Gouws E, Boschi-Pinto C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 50.Sazawal S, Black RE. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: A meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 51.English M, Punt J, Mwangi I. Clinical overlap between malaria and severe pneumonia in Africa children in hospital. Trans R Soc Trop Med Hyg. 1996;90:658–662. doi: 10.1016/s0035-9203(96)90423-x. [DOI] [PubMed] [Google Scholar]
- 52.Perkins BA, Zucker JR, Otieno J. Evaluation of an algorithm for integrated management of childhood illness in an area of Kenya with high malaria transmission. Bull World Health Org. 1997;75(suppl 1):33–42. [PMC free article] [PubMed] [Google Scholar]
- 53.O'Dempsey TJ, Laurence BE, McArdle TF. The effect of temperature reduction on respiratory rate in febrile illnesses. Arch Dis Child. 1993;68:492–495. doi: 10.1136/adc.68.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graham SM, Walsh AL, Molyneux EM. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg. 2000;94:310–314. doi: 10.1016/s0035-9203(00)90337-7. [DOI] [PubMed] [Google Scholar]
- 55.O'Dempsey TJ, McArdle TF, Lloyd-Evans N. Importance of enteric bacteria as a cause of pneumonia, meningitis and septicemia among children in a rural community in The Gambia, West Africa. Pediatr Infect Dis J. 1994;13:122–128. doi: 10.1097/00006454-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Shann F. The management of pneumonia in children in developing countries. Clin Infect Dis. 1995;21(suppl 3):S218–S225. doi: 10.1093/clind/21.supplement_3.s218. [DOI] [PubMed] [Google Scholar]
- 57.Adegbola RA, Obaro SK. Diagnosis of childhood pneumonia in the tropics. Ann Trop Med Parasitol. 2000;94:197–207. doi: 10.1080/00034980050006366. [DOI] [PubMed] [Google Scholar]
- 58.Campbell H, Byass P, O'Dempsey TJ. Effects of body temperature on respiratory rate in young children. Arch Dis Child. 1992;67:664. doi: 10.1136/adc.67.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lozano JM, Steinhoff M, Ruiz JG. Clinical predictors of acute radiological pneumonia and hypoxaemia at high altitude. Arch Dis Child. 1994;71:323–327. doi: 10.1136/adc.71.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falade AG, Tschappeler H, Greenwood BM. Use of simple clinical signs to predict pneumonia in young Gambian children: The influence of malnutrition. Bull World Health Org. 1995;73:299–304. [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell H, Byass P, Lamont AC. Assessment of clinical criteria for identification of severe acute lower respiratory tract infections in children. Lancet. 1989;1:297–299. doi: 10.1016/s0140-6736(89)91308-1. [DOI] [PubMed] [Google Scholar]
- 62.Lanata CF: Incidence and evolution of pneumonia in children at the community level. Washington, DC, Pan American Health Organization [PAHO], Division of Disease Prevention and Control, Communicable Diseases Program, Integrated Management of Childhood Illness, 1999.
- 63.Todd JE, De Francisco A, O'Dempsey TJ. The limitations of verbal autopsy in a malaria-endemic region. Ann Trop Paediatr. 1994;14:31–36. doi: 10.1080/02724936.1994.11747689. [DOI] [PubMed] [Google Scholar]
- 64.Puffer RR, Serrano CV. Other diseases and external causes: Diseases of respiratory system. In: Puffer RR, Serrano CV, editors. Patterns of Mortality in Childhood: Report of the Inter-American Investigation of Mortality in Childhood. Pan American Health Organization; Washington, DC: 1973. pp. 229–231. [Google Scholar]
- 65.Rudan I, Tomaskovic L, Boschi-Pinto C. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Org. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy TF, Henderson FW, Clyde WA., Jr Pneumonia: An eleven-year study in a pediatric practice. Am J Epidemiol. 1981;113:12–21. doi: 10.1093/oxfordjournals.aje.a113061. [DOI] [PubMed] [Google Scholar]
- 67.Jokinen C, Heiskanen L, Juvonen H. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am J Epidemiol. 1993;137:977–988. doi: 10.1093/oxfordjournals.aje.a116770. [DOI] [PubMed] [Google Scholar]
- 68.UNICEF/WHO . Pneumonia: The Forgotten Killer of Children. UNICEF/WHO; New York: 2006. [Google Scholar]
- 69.Rasmussen Z, Pio A, Enarson P. Case management of childhood pneumonia in developing countries: Recent relevant research and current initiatives. Int J Tuberc Lung Dis. 2000;4:807–826. [PubMed] [Google Scholar]
- 70.van Lerberghe W, World Health Organization . Make Every Mother and Child Count. World Health Organization; Geneva: 2005. [Google Scholar]
- 71.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–2234. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 72.The world's forgotten children. Lancet. 2003;361:1. [PubMed] [Google Scholar]
- 73.Bawaskar HS. The world's forgotten children. Lancet. 2003;361:1224–1225. doi: 10.1016/s0140-6736(03)12931-5. [DOI] [PubMed] [Google Scholar]
- 74.James JW. Longitudinal study of the morbidity of diarrheal and respiratory infections in malnourished children. Am J Clin Nutr. 1972;25:690–694. doi: 10.1093/ajcn/25.7.690. [DOI] [PubMed] [Google Scholar]
- 75.Rice AL, Sacco L, Hyder A. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull World Health Org. 2000;78:1207–1221. [PMC free article] [PubMed] [Google Scholar]
- 76.Victora CG, Kirkwood BR, Ashworth A. Potential interventions for the prevention of childhood pneumonia in developing countries: Improving nutrition. Am J Clin Nutr. 1999;70:309–320. doi: 10.1093/ajcn/70.3.309. [DOI] [PubMed] [Google Scholar]
- 77.Kirkwood BR, Gove S, Rogers S. Potential interventions for the prevention of childhood pneumonia in developing countries: A systematic review. Bull World Health Org. 1995;73:793–798. [PMC free article] [PubMed] [Google Scholar]
- 78.Graham SM, Coulter JB, Gilks CF. Pulmonary disease in HIV-infected African children. Int J Tuberc Lung Dis. 2001;5:12–23. [PubMed] [Google Scholar]
- 79.Cashat-Cruz M, Morales-Aguirre JJ, Mendoza-Azpiri M. Respiratory tract infections in children in developing countries. Semin Pediatr Infect Dis. 2005;16:84–92. doi: 10.1053/j.spid.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 80.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heath PT. Epidemiology and bacteriology of bacterial pneumonias. Paediatr Respir Rev. 2000;1:4–7. doi: 10.1053/prrv.2000.0001. [DOI] [PubMed] [Google Scholar]
- 82.Berman S. Epidemiology of acute respiratory infections in children of developing countries. Rev Infect Dis. 1991;13(suppl 6):S454–S462. doi: 10.1093/clinids/13.supplement_6.s454. [DOI] [PubMed] [Google Scholar]
- 83.O'Dempsey TJ, McArdle TF, Lloyd-Evans N. Pneumococcal disease among children in a rural area of west Africa. Pediatr Infect Dis J. 1996;15:431–437. doi: 10.1097/00006454-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 84.Usen S, Adegbola R, Mulholland K. Epidemiology of invasive pneumococcal disease in the Western Region, The Gambia. Pediatr Infect Dis J. 1998;17:23–28. doi: 10.1097/00006454-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Zangwill KM, Vadheim CM, Vannier AM. Epidemiology of invasive pneumococcal disease in southern California: Implications for the design and conduct of a pneumococcal conjugate vaccine efficacy trial. J Infect Dis. 1996;174:752–759. doi: 10.1093/infdis/174.4.752. [DOI] [PubMed] [Google Scholar]
- 86.Talbot TR, Poehling KA, Hartert TV. Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation. Am J Med. 2005;118:285–291. doi: 10.1016/j.amjmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 87.Black SB, Shinefield HR, Ling S. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21:810–815. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 88.Cutts FT, Zaman SM, Enwere G. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: Randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–1146. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 89.Levine OS, Schwartz B, Pierce N. Development, evaluation and implementation of Haemophilus influenzae type b vaccines for young children in developing countries: Current status and priority actions. Pediatr Infect Dis J. 1998;17(9 suppl):S95–S113. doi: 10.1097/00006454-199809001-00003. [DOI] [PubMed] [Google Scholar]
- 90.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: Global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–317. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Greenwood B. Epidemiology of acute lower respiratory tract infections, especially those due to Haemophilus influenzae type b, in The Gambia, West Africa. J Infect Dis. 1992;165(suppl 1):S26–S28. doi: 10.1093/infdis/165-supplement_1-s26. [DOI] [PubMed] [Google Scholar]
- 92.Watt JP, Levine OS, Santosham M. Global reduction of Hib disease: What are the next steps? J Pediatr. 2003;143(6 suppl):S163–S187. doi: 10.1067/s0022-3476(03)00576-6. [DOI] [PubMed] [Google Scholar]
- 93.Levine OS, Lagos R, Munoz A. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–1064. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 94.de Andrade AL, de Andrade JG, Martelli CM. Effectiveness of Haemophilus influenzae b conjugate vaccine on childhood pneumonia: A case-control study in Brazil. Int J Epidemiol. 2004;33:173–181. doi: 10.1093/ije/dyh025. [DOI] [PubMed] [Google Scholar]
- 95.Mulholland K, Hilton S, Adegbola R. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997;349:1191–1197. doi: 10.1016/s0140-6736(96)09267-7. [DOI] [PubMed] [Google Scholar]
- 96.Adegbola RA, Mulholland EK, Secka O. Vaccination with a Haemophilus influenzae type b conjugate vaccine reduces oropharyngeal carriage of H. influenzae type b among Gambian children. J Infect Dis. 1998;177:1758–1761. doi: 10.1086/517440. [DOI] [PubMed] [Google Scholar]
- 97.Miller MA. An assessment of the value of Haemophilus influenzae type b conjugate vaccine in Asia. Pediatr Infect Dis J. 1998;17(9 suppl):S152–S159. doi: 10.1097/00006454-199809001-00012. [DOI] [PubMed] [Google Scholar]
- 98.Gessner BD, Sutanto A, Linehan M. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: Hamlet-randomised vaccine-probe trial. Lancet. 2005;365:43–52. doi: 10.1016/s0140-6736(04)17664-2. [DOI] [PubMed] [Google Scholar]
- 99.Andrade AL, Silva SA, Martelli CM. Population-based surveillance of pediatric pneumonia: Use of spatial analysis in an urban area of Central Brazil. Cad Saude Publica. 2004;20:411–421. doi: 10.1590/s0102-311x2004000200008. [DOI] [PubMed] [Google Scholar]
Emerging/Reemerging Organisms
- 100.Beaglehole R, Irwin A, Prentice T, World Health Organization . Changing History. World Health Organization; Geneva: 2004. [Google Scholar]
- 101.Fauci AS. Emerging and reemerging infectious diseases: The perpetual challenge. Acad Med. 2005;80:1079–1085. doi: 10.1097/00001888-200512000-00002. [DOI] [PubMed] [Google Scholar]
- 102.Barrett CL, Eubank SG, Smith JP. If smallpox strikes Portland. Sci Am. 2005;292:42–49. [PubMed] [Google Scholar]
- 103.Longini IM, Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159:623–633. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 104.Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 105.Webster RG, Bean WJ, Gorman OT. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghedin E, Sengamalay NA, Shumway M. Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution. Nature. 2005;437:1162–1166. doi: 10.1038/nature04239. [DOI] [PubMed] [Google Scholar]
- 107.Fox JP, Hall CE, Cooney MK. Influenzavirus infections in Seattle families, 1975-1979. I. Study design, methods and the occurrence of infections by time and age. Am J Epidemiol. 1982;116:212–227. doi: 10.1093/oxfordjournals.aje.a113407. [DOI] [PubMed] [Google Scholar]
- 108.Glezen WP, Paredes A, Taber LH. Influenza in children. Relationship to other respiratory agents. JAMA. 1980;243:1345–1349. doi: 10.1001/jama.243.13.1345. [DOI] [PubMed] [Google Scholar]
- 109.Longini IM, Jr, Koopman JS, Monto AS. Estimating household and community transmission parameters for influenza. Am J Epidemiol. 1982;115:736–751. doi: 10.1093/oxfordjournals.aje.a113356. [DOI] [PubMed] [Google Scholar]
- 110.Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974-76. N Engl J Med. 1978;298:587–592. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 111.McManus TE, Coyle PV, Kidney JC. Childhood respiratory infections and hospital admissions for COPD. Respir Med. 2006;100:512–518. doi: 10.1016/j.rmed.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: Implications for control in health care settings. Clin Infect Dis. 2003;37:1094–1101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 113.Couch RB, Kasel JA, Glezen WP. Influenza: Its control in persons and populations. J Infect Dis. 1986;153:431–440. doi: 10.1093/infdis/153.3.431. [DOI] [PubMed] [Google Scholar]
- 114.Neuzil KM, Mellen BG, Wright PF. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 115.Neuzil KM, Zhu Y, Griffin MR. Burden of interpandemic influenza in children younger than 5 years: A 25-year prospective study. J Infect Dis. 2002;185:147–152. doi: 10.1086/338363. [DOI] [PubMed] [Google Scholar]
- 116.Chiu SS, Lau YL, Chan KH. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med. 2002;347:2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 117.Potter CW. Chronicle of influenza pandemics. In: Nicholson KG, Webster RG, editors. Textbook of Influenza. Blackwell Science; Ames, IA: 1998. [Google Scholar]
- 118.Taubenberger JK, Reid AH, Lourens RM. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005;437:889–893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 119.Tumpey TM, Basler CF, Aguilar PV. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 120.Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 121.World Health Organization . Avian Influenza: Assessing the Pandemic Threat. World Health Organization; Geneva: 2005. [Google Scholar]
- 122.de Jong MD, Hien TT. Avian influenza A (H5N1) J Clin Virol. 2006;35:2–13. doi: 10.1016/j.jcv.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Webster RG, Peiris M, Chen H. H5N1 outbreaks and enzootic influenza. Emerg Infect Dis. 2006;12:3–8. doi: 10.3201/eid1201.051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guan Y, Zheng BJ, He YQ. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 125.SARS . Breaking the Chains of Transmission. World Health Organizations; Geneva: 2003. [Google Scholar]
- 126.Zhong NS, Zheng BJ, Li YM. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu RH, He JF, Evans MR. Epidemiologic clues to SARS origin in China. Emerg Infect Dis. 2004;10:1030–1037. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shen Z, Ning F, Zhou W. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10:256–260. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mark IC, Loon SC, Leong HN. Understanding the super-spreading events of SARS in Singapore. Ann Acad Med Singapore. 2006;35:390–395. [PubMed] [Google Scholar]
- 130.Lai PC, Wong CM, Hedley AJ. Understanding the spatial clustering of severe acute respiratory syndrome (SARS) in Hong Kong. Environ Health Perspect. 2004;112:1550–1556. doi: 10.1289/ehp.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peiris JS, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004;10(12 suppl):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liang G, Chen Q, Xu J. Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg Infect Dis. 2004;10:1774–1781. doi: 10.3201/eid1010.040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lim PL, Kurup A, Gopalakrishna G. Laboratory-acquired severe acute respiratory syndrome. N Engl J Med. 2004;350:1740–1745. doi: 10.1056/NEJMoa032565. [DOI] [PubMed] [Google Scholar]
- 134.Hampton T. Bats may be SARS reservoir. JAMA. 2005;294:2291. doi: 10.1001/jama.294.18.2291. [DOI] [PubMed] [Google Scholar]
- 135.Cyranoski D, Abbott A. Virus detectives seek source of SARS in China's wild animals. Nature. 2003;423:467. doi: 10.1038/423467a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hui DS, Chan MC, Wu AK. Severe acute respiratory syndrome (SARS): Epidemiology and clinical features. Postgrad Med J. 2004;80:373–381. doi: 10.1136/pgmj.2004.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li AM, Ng PC. Severe acute respiratory syndrome (SARS) in neonates and children. Arch Dis Child Fetal Neonatal Educ. 2005;90:F461–F465. doi: 10.1136/adc.2005.075309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wong GW, Li AM, Ng PC. Severe acute respiratory syndrome in children. Pediatr Pulmonol. 2003;36:261–266. doi: 10.1002/ppul.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.World Health Organization . Tuberculosis. Fact Sheet No. 104. World Health Organization; Geneva: 2006. [Google Scholar]
- 140.The Global Plan to Stop TB 2006-2015. World Health Organization; Geneva: 2006. Actions for Life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corbett EL, Watt CJ, Walker N. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 142.Espinal MA. The global situation of MDR-TB. Tuberculosis (Edinb) 2003;83(1-3):44–51. doi: 10.1016/s1472-9792(02)00058-6. [DOI] [PubMed] [Google Scholar]
- 143.Michelow IC, Olsen K, Lozano J. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 144.Obaro SK, Madhi SA. Bacterial pneumonia vaccines and childhood pneumonia: Are we winning, refining, or redefining? Lancet Infect Dis. 2006;6:150–161. doi: 10.1016/S1473-3099(06)70411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vesa S, Kleemola M, Blomqvist S. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J. 2001;20:574–581. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 146.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fauci AS. Infectious diseases: Considerations for the 21st century. Clin Infect Dis. 2001;32:675–685. doi: 10.1086/319235. [DOI] [PubMed] [Google Scholar]


