Normal lung function depends on efficient gas exchange between the capillary network in alveolar septae and gases in the alveolar spaces. Gas exchange is compromised if fluid accumulates in alveolar spaces or if there is an increased amount of connective tissue in alveolar septae. In different pathologic conditions, impaired gas exchange may affect either oxygenation or removal of CO2 more severely. Normal lung compliance (the stiffness of lung tissue) is important for normal pulmonary function. If lung tissue is abnormally stiff (as a result of fibrosis), the work of breathing increases markedly so that individuals may not be able to adequately oxygenate or remove carbon dioxide. Lung compliance can also be affected by intra-alveolar fluid accumulations or loss of surfactant (either can increase surface tension and increase the work of breathing). The same pathologic processes often affect both gas exchange and lung compliance. Loss of lung parenchyma (e.g., in emphysema) has a direct impact on the amount of surface area available for gas exchange but also reduces radial tension on airways that normally helps keep them patent during expiration. Premature collapse of these airways during expiration causes air trapping and can greatly reduce effective gas exchange (the dead space is larger). Because of these interactions, some relatively mild pathologic changes can result in significant pulmonary function abnormalities.
Since the lung communicates directly with the external environment, host defense mechanisms are essential to maintain normal pulmonary function and resist infection. Bronchi and bronchioles are lined by a ciliated columnar epithelium with interspersed goblet cells that secrete mucin. The cilia on the surface of these cells beat rhythmically, causing the directional movement of the mucin outward to the trachea, where it can be expectorated. Bacteria and particulates that make contact with the mucociliary blanket are effectively cleared from the lung. Individuals with genetic abnormalities of cilia and cigarette smokers have defective mucociliary blanket function and are at increased risk for infection. Particles that are small enough to reach the alveoli (beyond the mucociliary blanket) may not be effectively cleared from the lung. The size of particulate air pollutants and aerosolized infectious agents is a key factor in determining whether they reach alveoli to cause disease or infection. The normal gag reflex is also an important host defense mechanism that prevents aspiration of oral contents into the lower respiratory tract. Individuals with a deficient gag reflex (e.g., chronic alcoholics) are at risk for aspiration of oral secretions, which contain numerous microorganisms. Inherited and acquired immunodeficiency syndromes are significant risk factors for pulmonary infection.
PULMONARY EDEMA
Pulmonary edema can result from abnormalities in the cardiovascular system that increase pulmonary arterial or venous pressure. High pressure in the pulmonary circulation favors the net movement of water into lung tissue. Since the volume of the lung interstitium is minimal, transudates rapidly move into alveolar spaces. Accumulation of a significant amount of fluid in alveolar spaces increases the work of breathing and blocks effective gas exchange. The amount of plasma proteins carried across with water in edema fluid is an index of the severity of the pulmonary edema. In severe cases, dense pink, frothy proteinaceous material is visible in alveolar spaces in tissue sections (Fig. 8-1A ). Lungs with pulmonary edema are markedly increased in weight, and their cut surface appears frothy and exudes fluid. Long-standing pulmonary edema in patients with congestive heart failure results in microhemorrhages into alveoli. Red cells are ingested by macrophages and iron derived from hemoglobin is converted to hemosiderin, which is only slowly cleared from alveoli. Inflammation associated with the small hemorrhages causes incremental fibrosis in alveolar septae, and chronic pulmonary edema can result in significant interstitial pulmonary fibrosis. Hemosiderin-containing alveolar macrophages are excellent markers of chronic congestive heart failure (so-called heart failure cells) but can also result from other types of pulmonary hemorrhage (see Fig. 8-1B). Edema fluid can also collect in the pleural spaces (i.e., pleural effusion), resulting in progressive collapse of the lungs and diminished gas exchange.
Figure 8-1.

A, Pulmonary edema. Microscopic section of lung tissue showing dense eosinophilic proteinaceous material filling alveolar spaces. A few hemosiderin-containing macrophages are present. B, Pulmonary hemosiderosis. Microscopic section of lung tissue with numerous hemosiderin-containing macrophages from a patient with long-standing congestive heart failure.
DIFFUSE ALVEOLAR DAMAGE
Diffuse alveolar damage (DAD) is a stereotyped response to injury in lung tissue. DAD consists of an intra-alveolar exudate (often described as hyaline membrane) in association with marked hyperplasia of type II pneumocytes that may appear cytologically bizarre and pleomorphic (Fig. 8-2 ). Hyaline membranes consist of a mixture of proteinaceous exudate, surfactant from type II pneumocytes, and cellular debris. Diffuse alveolar damage can progress to organizing pneumonia (granulation tissue organizes the exudate), which may result in permanent pulmonary fibrosis or resolve with the restoration of normal lung architecture and function. The outcome depends on host factors, the severity of lung injury, and whether or not the inciting cause of DAD is corrected. Many different types of injury can produce diffuse alveolar damage including shock, some types of infection, chemotherapeutic agents, irradiation, and oxygen toxicity. Free radical damage from high levels of oxygen often required in an ICU setting is common cause of DAD in severely ill, hospitalized patients.
Figure 8-2.

A, Adult respiratory distress syndrome. Gross cross-section of the lower lobe of the lung showing diffuse consolidation and hemorrhage. B, Adult respiratory distress syndrome. Microscopic section of lung tissue showing prominent hyaline membranes (eosinophilic exudate composed of fibrin and cellular debris) lining alveolar spaces. Scattered inflammatory cells are present.
PNEUMONIA
Pneumonia usually means an inflammatory process within the alveolar spaces and often implies an infectious etiology (e.g., bacteria, Mycoplasma, or viruses). The term pneumonitis is typically used for inflammatory diseases of lung tissue that result in significant interstitial inflammation and fibrosis and are immune mediated.
Viral Pneumonia
Many different types of viruses can produce pneumonia, but some viruses are more commonly associated with clinical pneumonia than others. Influenzavirus typically results in moderate to severe pulmonary involvement with a predominantly lymphoid infiltrate and exudation of proteinaceous fluid into alveolar spaces. Emerging agents of viral pneumonia include the so-called severe acute respiratory syndrome (SARS). SARS can rapidly produce a DAD-like pattern of injury that may result in death or permanent lung dysfunction.
Most forms of viral pneumonia do not cause death by themselves but can significantly predispose to the development of bacterial pneumonia, particularly in elderly patients or in patients with comorbid diseases such as congestive heart failure. Diminished lung function with protein-rich fluid in alveolar spaces strongly predisposes to colonization and infection by bacterial organisms present in the normal flora or in the environment. Some types of viruses tend to produce pneumonia only in immunocompromised hosts or neonates (e.g., cytomegalovirus [CMV]) but can produce a life-threatening pneumonia in these susceptible individuals.
Bacterial Pneumonia
Bacterial pneumonia can occur as a primary infection of the lungs or as a complication of sepsis. The former is more common in the outpatient setting. Streptococcus pneumoniae is the prototypic organism of community-acquired pneumonia. S. pneumoniae is spread by airborne droplets and has a well-formed capsule that resists phagocytosis by innate immune cells. S. pneumoniae tends to produce a bronchopneumonia in which intra-alveolar exudate is centered around bronchi and bronchioles that have been inoculated with bacteria. Individuals without functional spleens are at substantially higher risk for developing life-threatening sepsis during S. pneumoniae infection (the spleen can normally remove the small number of organisms circulating in the bloodstream in patients with pneumonia to prevent generalized infection).
MICROBIOLOGY.
Severe Acute Respiratory Syndrome (SARS)
SARS was first recognized in late 2002 as a life-threatening respiratory disease in Guandong, China, with subsequent cases identified in Hong Kong and Canada, apparently as a result of transmission by airline passengers.
The SARS virus was initially cultured at the Centers for Disease Control and Prevention (CDC), and broad-spectrum RT-PCR primers homologous to the coronavirus polymerase gene were found to amplify viral RNA from patient samples. These PCR products showed a unique coding sequence that is most closely related to type II coronaviruses (approximately 60% sequence conservation). Serologic assays were subsequently developed for SARS.
Coronaviruses are enveloped RNA viruses with helical symmetry that are usually spread from person to person via aerosol and usually produce a common cold-like illness. The SARS coronavirus measures 80 to 140 nm in diameter by electron microscopy and has 20- to 40-nm complex surface projections at its periphery. Public health measures effectively contained the initial outbreak of this highly virulent new human pathogen.
Untreated bacterial pneumonia progresses through several morphologic stages of development, but these classical pathologic findings are now only rarely observed because of intercurrent antibiotic therapy. The inflammatory response to bacteria results in acute inflammatory exudate filling alveolar spaces. Inflammation progresses through a stage of “gray hepatization” with organization of the intra-alveolar exudate. The cut surface of involved lungs has nodular pattern of consolidation (solid areas in which alveoli are filled with acute inflammatory exudate) (Fig. 8-3A ). If untreated, bronchopneumonia may progress to diffuse involvement of the entire lung lobe (lobar pneumonia; see Fig. 8-3B). The severity of acute respiratory compromise depends on the volume of lung tissue affected. If the patient survives, effective humoral and cellular immunity eventually clears the infection. Areas of consolidation progress to “red hepatization” because of hemorrhage that typically occurs during the organization of the intra-alveolar exudate. Usually, there is complete resolution with the restoration of normal lung architecture. Pneumonia in hospitalized patients treated with multiple antibiotics shows some of the pathologic changes described above but often has a DAD-like pattern resulting from combined infectious, immune, and oxygen free radical-mediated injury.
Figure 8-3.
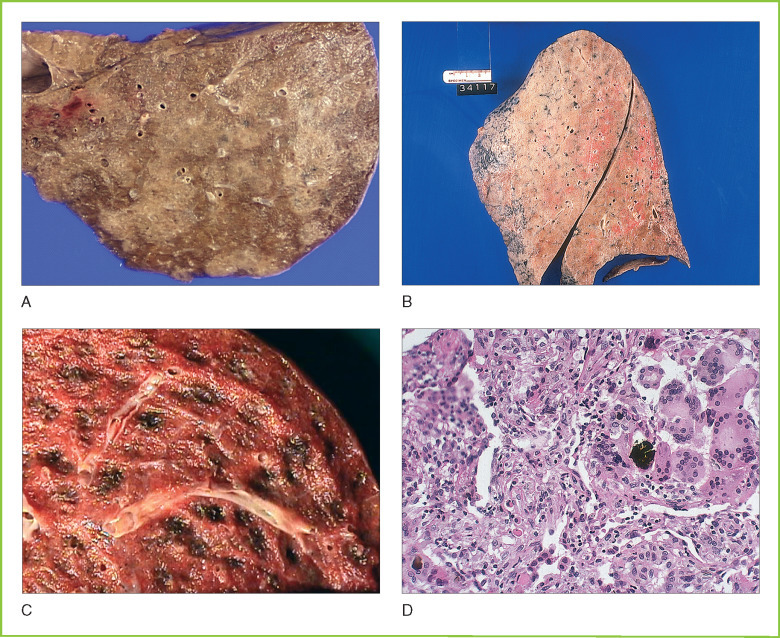
A, Bronchopneumonia. Gross cross-section of fixed lung tissue showing peribronchial consolidation. Consolidated areas appear slightly whiter and denser than aerated lung tissue. B, Lobar pneumonia. Gross cross-section of lung tissue showing diffuse consolidation of the entire lobe. C, Aspiration pneumonia. Gross cross-section of lung tissue showing focal early abscess formation in evolving aspiration pneumonia. D, Aspiration pneumonia. Microscopic section of lung tissue showing foreign body material with associated multinucleated giant cells and acute inflammatory exudate.
Pneumonia caused by more destructive bacteria, such as Staphylococcus aureus, tends to destroy the extracellular matrix supporting lung parenchyma, precluding the restoration of fully normal lung architecture and function. Lung abscess is a relatively common complication of Staphylococcal pneumonia.
Legionnaires' Disease
Legionella pneumophila is the causative agent of legionnaires' disease, which is an uncommon but potentially serious pneumonia, occurring predominantly in older individuals with comorbid disease. Legionella is named for a disease outbreak that occurred in association with an American Legion convention. Legionella is a free-living organism that grows in water supplies of rooftop air-conditioning units. Large numbers of bacteria can be aerosolized from contaminated water, resulting in epidemic infections, usually in urban settings.
The incidence of legionnaires' disease has greatly diminished because of effective public health measures including environmental monitoring of cooling units. Legionella can cause pneumonia in normal individuals but is more likely to produce disease in persons with compromised lung function (e.g., chronic obstructive pulmonary disease or congestive heart failure).
Atypical Pneumonia
Mycoplasma pneumoniae is a prokaryote lacking a cell wall that causes so-called atypical pneumonia in immunocompetent hosts. M. pneumoniae is typically self-limited but may rarely be associated with complications, such as thrombotic thrombocytopenia purpura (see Chapter 11).
Aspiration Pneumonia
Aspiration pneumonia is usually caused by the aspiration of gastric contents that introduces bacteria and foreign material (including gastric acid) into alveolar spaces (see Figs. 8-3C and 8-3D). Acid causes direct injury to pneumocytes and disrupts normal host defenses, facilitating bacterial growth. The presence of foreign material also interferes with effective innate and acquired immunity so that most patients who aspirate will develop bacterial infection. Aspiration pneumonia is often polymicrobial, and gram-negative enteric organisms are common pathogens. These infections tend to be quite destructive (similarly to staphylococcal pneumonia) and frequently progress to lung abscess. Aspiration pneumonia is much more common in debilitated patients and in alcoholics with a diminished gag reflex. Aspiration of other materials such as barium in the setting of radiographic procedures or meconium during a complicated delivery can also cause a severe inflammatory reaction that irreversibly damages lung tissue.
MICROBIOLOGY.
Legionella pneumophila
Legionella is a free-living gram-negative bacillus (2-20 μm) that resides in surface and drinking water and can cause pulmonary infection if aerosolized. L. pneumophila is the usual agent of legionnaires' disease, but any of the 39 species of Legionella can cause human disease.
L. pneumophila is a facultative intracellular parasite that proliferates in alveolar macrophages and induces a marked acute inflammatory response to produce a destructive pneumonia that responds to erythromycin (legionnaires' disease). A less common clinical syndrome (Pontiac fever) caused by L. pneumophila produces a self-limited, influenza-like illness.
Effective host defense is largely cell mediated, and activated macrophages can kill or contain bacteria. Individuals with compromised adaptive or innate immunity are at increased risk for disease. Infection can be confirmed by culture on specialized agar plates. Decontamination of contaminated water that may be aerosolized (i.e., rooftop air-conditioning units) has reduced the frequency of human infection.
Lung Abscess
Lung abscess is usually a complication of pneumonia with aggressive organisms such as S. aureus or gram-negative enteric organisms. Aspiration of foreign material also favors lung abscess formation. Hydrolytic enzymes released from bacteria and neutrophils digest the extracellular matrix in alveolar septae to form an abscess cavity within lung tissue (Fig. 8-4 ). Rupture of an abscess cavity into the pleural space can cause empyema (see below). Host immune responses are usually incapable of eradicating the infection unless the abscess cavity is drained (the abscess is an anaerobic environment in which inflammatory cells are minimally effective in killing bacteria).
Figure 8-4.

Lung abscess. Gross cross-section of lung tissue with a large abscess cavity. Surrounding lung tissue is fibrotic.
Bronchiectasis
Bronchiectasis means the presence of abnormally large bronchi at the periphery of lung. Large bronchi are seen to extend to the pleural surface in cross-sections of lung tissue. Bronchiectasis usually results from repeated cycles of pulmonary infection during childhood. Repeated injury results in the loss of normal parenchyma and in the creation of abnormal, ectatic airspaces that then undergo bronchial metaplasia. The peripheral location and abnormal architecture of these air spaces results in impaired clearance of secretions, causing mucus plugging and new cycles of infection. Bronchiectasis is typical in patients with cystic fibrosis in which inspissated mucus causes bronchial obstruction and predisposes to infection and destruction of normal bronchial architecture. Bronchiectasis is a risk factor for pneumothorax and predisposes to empyema if pneumonia develops.
Mycobacterial Infections
Infection with Mycobacterium tuberculosis usually occurs through the respiratory tract. Patients with active tuberculosis can expectorate fomites (small particulates with viable bacteria), which can be inhaled by individuals in their close environment. If these fomites are small enough to reach beyond the mucociliary blanket, tuberculous infection may be initiated. Innate immune responses mediated by neutrophils are not effective in killing M. tuberculosis, which has a remarkably resistant cell wall. These organisms divide slowly but can grow effectively as intracellular pathogens after ingestion by macrophages. Effective killing of M. tuberculosis requires the initiation of a granulomatous inflammatory response in which stimulation of macrophages with IFN-γ (produced by TH1 T cells) induces the formation of epithelioid histiocytes that have enhanced bactericidal capacity. In many cases, activated histiocytes may successfully contain a mycobacterial infection but may not effectively kill all microorganisms. Viable mycobacteria may persist as intracellular parasites for many years. Compromise of the immune system (e.g., cancer, nutritional deficiency, and immunosuppressive therapy) can then permit reactivation of tuberculous infection.
Most individuals who develop primary pulmonary infection with M. tuberculosis have the so-called Ghon complex consisting of granulomatous lesions in lung tissue in conjunction with granulomas in draining hilar lymph nodes (Fig. 8-5A ). If the organisms are contained at this stage, granulomas become fibrotic and undergo dystrophic calcification over the course of years. A Ghon complex is often visible on routine chest radiography because of these calcifications. If the host immune response is not sufficient to contain the mycobacteria, persistent active lung infection may produce cavitation (essentially an abscess with numerous mycobacteria present). Tuberculosis preferentially involves the apices of lungs, possibly because of the higher O2 tension at this location.
Figure 8-5.

A, Calcified granuloma. Gross cross-section from lung hilum showing a calcified granuloma in an anthracotic (black) lymph node. Cross-sectioned bronchi are also visible. B, Necrotizing granuloma. Microscopic section of lung tissue showing a granuloma with central necrosis and palisading histiocytes about its periphery. C, Atypical mycobacterial infection. Microscopic section stained with Ziehl-Neelsen stain revealing large numbers of mycobacteria and histiocytes (red, rod-shaped organisms).
In individuals with weakened immune systems, initial infection with M. tuberculosis can progress to a disseminated infection with involvement of many different organs (see Fig. 8-5B). Reactivation of latent infection in immunocompromised patients often results in a pattern of miliary tuberculosis in which innumerable small foci of infection develop within lung parenchyma and other organs. These miliary lesions consist of discrete masses of mycobacteria with associated histiocytes. The emergence of multiple drug-resistant M. tuberculosis poses a significant therapeutic challenge, and the spread of these organisms in patients with AIDS is an ongoing public health problem.
Atypical mycobacteria (all mycobacterial species aside from M. tuberculosis) produce a different pattern of lung disease.
Patients with chronic obstructive pulmonary disease are at increased risk for developing infection with these lower virulence organisms. Atypical mycobacteria often produce a pneumonic pattern of involvement with areas of cavitation. In the setting of AIDS, M. avium-intracellulare can cause infection of lung tissue as well as other organs in which numerous organisms distend the cytoplasm of histocytes, producing a characteristic granularity in routine histologic sections (see Fig. 8-5C).
Fungal Pneumonia
A small number of endemic fungal organisms commonly cause pulmonary infection in immunocompetent individuals. Histoplasma capsulatum is a dimorphic fungus that is endemic to the Mississippi River valley and produces a flu-like illness in immunocompetent individuals, often resulting in the formation of calcified granulomas similarly to tuberculosis. Reactivation can occur during periods of immunosuppression with dissemination of disease. Coccidioides immitis is endemic in the southwestern United States and produces valley fever (Fig. 8-6C ). Blastomyces dermatitidis is endemic in the southern United States and can produce skin and lung infection.
Figure 8-6.
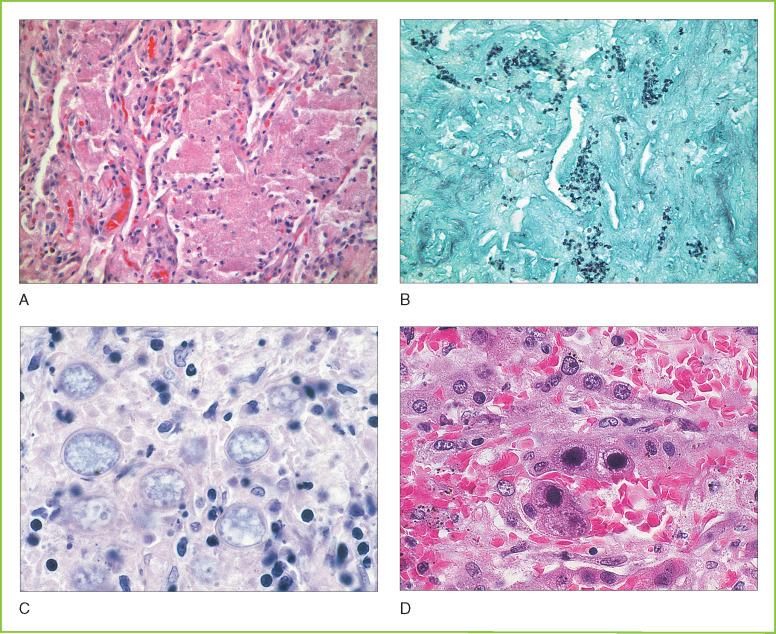
A, Pneumocystic pneumonia. Microscopic section showing lung tissue with granular eosinophilic exudate filling alveoli. B, Pneumocystic pneumonia. Microscopic section of lung tissue stained with Gomori methenamine silver stain showing numerous Pneumocystis organisms within the intra-alveolar exudate. Organisms have a characteristic cup shape. C, Coccidiomycosis. Microscopic section of lung tissue showing numerous cysts of Coccidioides immitis with refractile walls. Scattered inflammatory cells are present. D, Cytomegalovirus pneumonia. Microscopic section of lung tissue showing prominent eosinophilic intranuclear inclusions in infected pneumocytes. Smaller intracytoplasmic inclusions are also present as well as prominent hemorrhage.
MICROBIOLOGY.
Atypical Mycobacteria
Atypical mycobacteria include approximately 10 species of mycobacteria that can produce disease in humans and animals that is less aggressive than M. tuberculosis. Some atypical mycobacteria are free living (in water) and may be confused with pathogenic mycobacteria if they contaminate tissue sections.
Mycobacterium avium complex (MAC) includes two species, M. avium and M. intracellulare, and can colonize normal individuals. In patients with immunodeficiency (usually AIDS), M. intracellulare can produce a disseminated infection in which it fills and distends macrophages in many organs without producing a marked inflammatory reaction.
M. scrofulaceum is a common cause of lymphadenitis in children (scrofula) that can progress to abscess formation.
M. kansasiiis a photochromogenic organism that can produce chronic pulmonary disease similar to, but much less aggressive than, M. tuberculosis.
Skin infections can result from M. ulcerans and M. marinum acquired from aquariums or swimming pools. M. fortuitum complex is a species of free-living soil organisms that can cause wound infection.
Immunocompromised Host
Immunocompromised patients are susceptible to infection with usual pathogens as well as organisms that do not typically produce disease in immunocompetent individuals. Infection may result from organisms that are ubiquitously present, such as Pneumocystis carinii, or from reactivation of prior viral or mycobacterial infection (e.g., CMV pneumonia) (see Figs. 8-6A, 8-6B, and 8-6D). Pneumocystis produces a characteristic foamy intra-alveolar exudate that can be seen on lung biopsy or in pulmonary cytology specimens.
Fungal pneumonia is a life-threatening complication in immunocompromised patients. Aspergillus fumigatus is a common organism in this setting. It forms septate hyphae and produces an aggressive pneumonia that leads to extensive tissue destruction and necrosis. Immunocompromised patients are also at risk for pneumonia caused by more typical human pathogens, such as Histoplasma, Coccidioides, and mycobacteria. Diabetic patients are predisposed to mucormycosis infection although the lung is not the usual portal of entry for the organism. Even with effective antimicrobial treatment, some of these infections are difficult or impossible to eradicate in the setting of severe immunosuppression.
ASTHMA
Asthma is a form of chronic lung disease that is characterized predominantly by hyperreactivity of airways to various stimuli as well as increased mucus production by goblet cells in the bronchial mucosa. Peribronchial smooth muscle is usually hypertrophic. Asthma is often associated with eosinophilia, and Curschmann's spirals (casts of air spaces formed by the contents of eosinophil granules) may be observed in the sputum of some patients with asthma. Asthmatics frequently develop severe bronchoconstriction in response to various stimuli including heat, cold, exogenous allergens, and some chemicals including aspirin. Triggers for asthmatic attacks tend to remain relatively constant in individual patients throughout the course of their disease. The severity of symptoms in different patients with asthma varies greatly. Patients typically have wheezing and a decreased FEV1 during attacks.
Abnormal lung function may predispose to infection that further exacerbates pulmonary dysfunction.
Several products of the 5′-lipoxygenase pathway (leukotrienes) are important mediators of vasoconstriction in patients with asthma. Corticosteroid therapy (usually inhaled) has been a mainstay of therapy for severe asthma but can lead to significant metabolic and infectious complications. Theophylline is effective in diminishing bronchoconstriction in most patients with severe asthma and has been another mainstay of therapy. Recently, a specific inhibitor of 5′-lipoxygenase has become available as a treatment option, but its role in management is not yet clear.
The precise causes of asthma remain elusive and are almost certainly heterogeneous.
The prevalence of asthma is developed countries is clearly increasing at a substantial rate. Approximately half of asthmatics have atopic disease, and disease attacks are predominantly related to exposure to environmental allergens. The remaining half of asthmatic patients do not appear to have allergen-mediated disease. Population-based studies suggest that environmental allergen exposure in childhood is important (children with more limited allergen exposure have a greater likelihood of developing asthma). Polymorphisms in the IL-13 gene segregate with asthma risk, and this cytokine appears to be mechanistically involved in the persistent inflammatory reaction in many asthmatic patients.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Chronic obstructive pulmonary disease (COPD) usually results from the combination of several different architectural abnormalities of lung tissue. Many patients with COPD have significant emphysema resulting from destruction of the lung's extracellular matrix by proteases (Figs. 8-7A and 8-7B ). In emphysema, alveolar septae are lost, resulting in the consolidation of normal alveoli into larger dysfunctional airspaces. These enlarged airspaces result in increased total lung volume with a prominent increase in dead volume and decreased effective oxygen exchange. Destruction of lung parenchyma also results in a loss of external support for larger airways, causing premature airway collapse during expiration. Once developed, emphysematous changes are irreversible.
Figure 8-7.
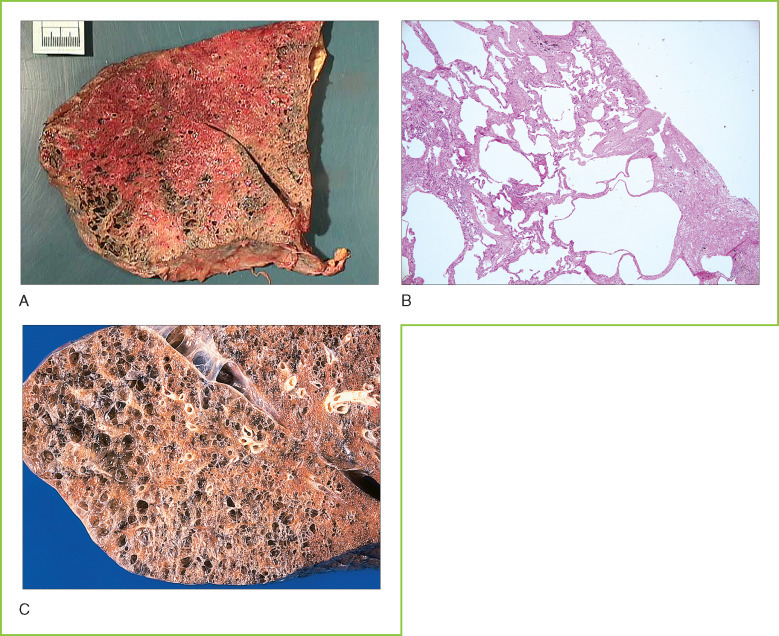
A, Pulmonary emphysema. Gross cross-section of lung tissue showing severe emphysema with massive enlargement of air spaces. B, Chronic obstructive pulmonary disease (COPD). Microscopic section of lung tissue showing abnormal air spaces (emphysema) that are more than 10 times the size of normal alveoli, with associated fibrosis in residual alveolar septae. C, End-stage lung disease. Gross cross-section of lung tissue showing honeycombing with large, abnormal air spaces.
MICROBIOLOGY.
Pneumocystis carinii
P. carinii is ubiquitously present worldwide. P. carinii produces thick-walled cysts 6-8 μm in diameter that have a characteristic cup-like shape in silver stains. Up to eight sporozoites are present within cysts, but these are not visible in routine histologic stains. An extracystic trophozoite may also be present. P. carinii has features of both a protozoan and a fungus and has not been definitively classified.
Normal individuals are colonized with P. carinii through the respiratory route early in life, and organisms remain throughout life. P. carinii never produces disease in immunocompetent individuals, but if high-level immunosuppression occurs, pneumonia may develop over the course of weeks.
Humoral immunity is not protective against P. carinii, so vaccination is not helpful. Cell-mediated immunity is effective in preventing the development of disease. Prophylactic treatment of immunocompromised patients with trimethoprim-sulfamethoxazole usually prevents pneumonia.
PHYSIOLOGY.
Pulmonary Function Tests
Pulmonary function tests are conducted by means of spirometry, which measures how the lungs move air by recording the amount of air that is breathed in and out over a set time interval. Spirometric measurements can be performed with normal breathing or with forced inspiration and expiration.
Spirograms can be used to produce flow-volume loop graphs of inspiration and expiration that can be used to assess obstructive lung disease (e.g., asthma and COPD) and restrictive lung disease (e.g., pulmonary fibrosis and chest wall abnormalities). These results can be used to calculate FEV1 (the amount of air exhaled in 1 second) and the FVC (total amount of air that can be inspired). If the ratio of FEV1 to FVC is below 0.75, significant obstructive disease is present.
Lung volume can be measured with a plethysmograph. Total lung capacity (TLC) = residual volume (RV) + FVC. TLC is markedly increased in patients with emphysema and can impede effective gas exchange.
Diffusion capacity is usually measured by breathing a small amount of carbon monoxide, which avidly binds to hemoglobin. The difference in carbon monoxide concentration between inhaled and exhaled air provides an estimate of the efficiency of gas exchange.
IMMUNOLOGY.
Asthma
Asthma incidence has increased markedly in developed countries, and its prevalence currently approaches 5% of the population. Approximately half of asthma cases develop before age 10 and more cases occur in disadvantaged persons living in urban environments.
IL-13 is necessary and sufficient to drive asthma in a mouse disease model. A complex interplay between multiple cell types and cytokine networks appears to underlie airway inflammation in most asthmatic patients.
IL-13 receptor variants have been shown to predispose to asthma, and IL-13 produced by TH2 T cells plays a key role in stimulating macrophages and bronchial epithelial cells through these receptors. A common IL-13 polymorphism (present in up to 25% of individuals in some populations) produces an IL-13 that is more active than wild-type IL-13.
Prostanoid DP receptors (PTGDRs) mediate the chemotaxis of T cells in response to mast cell degranulation (prostaglandin D synthase products). PTGDRs have also been implicated in asthma via population-based studies linking SNP haplotypes in this gene with susceptibility to asthma. Mice deficient in PTGDR are unable to initiate airway inflammation in response to allergen challenge.
Most patients with COPD also have a degree of reactive airway disease similarly to asthmatics. In some patients it may be relatively minor, while in others, reactive airway disease is a major therapeutic target of bronchodilator therapy. Inflammation and fibrosis of small airways is an important in the pathophysiology of COPD in many patients. Minute decreases in the diameter of smaller airways result in significant decreases in air flow (cross-sectional area is proportionate to the square of the diameter). Recent studies suggest that the density of B cells in small airways is a strong predictor of progressive airway disease in patients with COPD, suggesting that acquired immune mechanisms are important in the pathophysiology of COPD.
Various types of chronic lung injury can cause or exacerbate COPD, most important of which is cigarette smoking. Cigarette smoke greatly decreases the effectiveness of the mucociliary blanket (squamous metaplasia of bronchial mucosa), increasing the likelihood of infection and decreasing effective clearance of pulmonary secretions. Over time, this can result in increased fibrosis in lung tissue, and repeated cycles of infection can worsen emphysema and interstitial fibrosis, eventually producing end-stage (honeycomb) lung disease (see Fig. 8-7C). Activation of inflammatory pathways by cigarette smoking can result in digestion of lung parenchyma by leukocyte metalloproteinases in the absence of intercurrent infection. Oxidants from cigarette smoke and inflammatory cells decrease the activity of histone deacetylase (HDAC2) in macrophages, resulting in increased histone acetylation and activation of some proinflammatory genes. IL-8 secretion recruits neutrophils that are primed by TNFα and cause additional damage to the extracellular matrix. Derepression of matrix metalloproteinases 9 and 12 in macrophages results in increased elastase activity in lung tissue, which can destroy extracellular matrix and cause premature closure of airways during expiration (air trapping).
Individuals with α1-antitrypsin deficiency have greatly accelerated development of emphysema because of genetic abnormality of this important protease inhibitor. Those with α1-antitrypsin deficiency who smoke tend to develop emphysema early in life and usually succumb to respiratory insufficiency. Polymorphisms in protease and protease inhibitor genes may underlie less severe predisposition to COPD.
PNEUMOCONIOSIS
Pneumoconiosis comprises a number of different types of occupational lung disease in which inhalation of particulates or toxic chemicals results in pulmonary fibrosis or inflammation. Many different specific forms of pneumoconiosis are recognized. Some of the most important are related to exposure to asbestos fibers. Tiny asbestos fibers that are inspired beyond the mucociliary blanket are not effectively cleared by host defense mechanisms. These fibers remain permanently in lung tissue and may spread to the pleural spaces and peritoneal cavity via lymphatics. These particulates stimulate a chronic inflammatory/fibrotic process that results in the formation of pleural fibrous plaques and causes severe interstitial fibrosis in lung tissue (Figs. 8-8A and 8-8B ). Asbestos was an important cause of pneumoconiosis in the past when heavy industrial exposure occurred, particularly in shipbuilders. Recognition of the important role of asbestos in the development of malignant mesothelioma has led to greatly reduced industrial exposure, and asbestos pneumoconiosis is now uncommon. Asbestos is also a significant risk factor for the development of bronchogenic carcinoma, particularly in individuals who smoke cigarettes.
Figure 8-8.
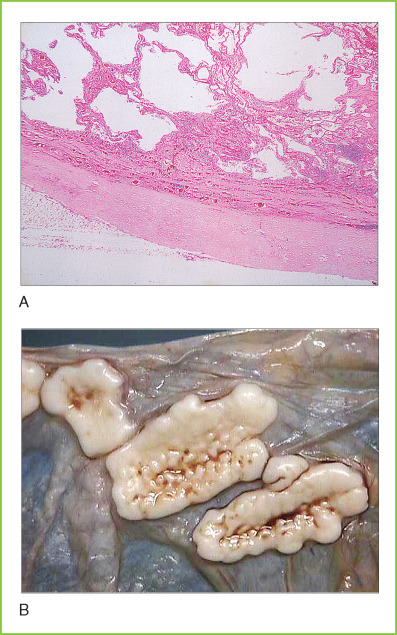
A, Asbestos pneumoconiosis. Section of lung tissue showing marked pleural fibrosis (at bottom) as well as mixed emphysema and interstitial fibrosis. B, Pleural plaques. Gross photograph of parietal pleura in a patient with asbestosis. The scalloped whitish-gray plaques are typical of heavy asbestos exposure.
Other forms of fibrogenic dust disease are associated with different kinds of occupations. Silicosis results from the inhalation of silica particles that are small enough to reach beyond mucociliary blanket, since these particles cannot be effectively cleared by host defenses. When these silica particles are ingested by inflammatory cells, they puncture their membranes to release hydrolytic enzymes and profibrotic cytokines that result in nodular fibrosis of lung tissue. Silicosis can occur in sand blasters without adequate respiratory protection and in miners of coal with high silica content.
Coal workers' pneumoconiosis is a fibrotic disease of lung tissue associated with the deposition of large amounts of carbon particles in lung (anthracosis). Anthracotic pigment is not as fibrogenic as silica or asbestos, and some anthracosis is present in all city dwellers and prominently in cigarette smokers. Coal miners can be exposed to very high concentrations of coal dust and develop dust macules (nodular lesions composed of macrophages with ingested carbon pigment) over the course of years. Fibrosis associated with these deposits eventually causes sufficient interstitial lung disease to produce respiratory failure in susceptible individuals (black lung disease).
INTERSTITIAL LUNG DISEASE
Interstitial lung disease refers to a set of inflammatory conditions in which inflammation is localized predominantly within alveolar septae rather than in alveolar spaces. There is clinical and pathologic overlap between some of these entities and many are immune mediated. Some diseases produce sufficiently characteristic pathologic changes that lung biopsy is diagnostic, but most require careful correlation between radiographic findings on high-resolution computed tomography (CT) scan and clinical course to arrive at the specific diagnosis. Many of these diseases result in progressive interstitial fibrosis and can lead to respiratory failure.
Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis is an inflammatory condition of lung tissue caused by an immune response to exogenous, inhaled allergens. Hypersensitivity pneumonitis may occur as an occupational disease or by exposure to substances in the home environment (e.g., “bird fanciers' lung,” “hot tub lung”). Exposure to respiratory allergens in sensitized individuals can cause DAD with acute pulmonary failure or chronic progressive interstitial fibrosis. A careful exposure history is critical in establishing the diagnosis; removal of the allergen terminates the inflammatory response. Bronchoalveolar lavage usually shows prominent lymphocytosis with an inversion of the CD4:CD8 ratio. Lung biopsy usually shows non-necrotizing granulomatous inflammation.
Idiopathic Interstitial Lung Disease
Idiopathic interstitial lung disease encompasses a number of different clinicopathologic entities with somewhat characteristic clinical presentations. The cause of most of these diseases is unknown although recent evidence suggests that genetic abnormalities in surfactant protein may cause some familial forms of idiopathic pulmonary fibrosis. High-resolution CT is as important as or more important than lung biopsy in diagnosing many of these entities. These diseases have recently been reclassified as nonspecific interstitial pneumonitis (NIP), usually occurring in younger patients; idiopathic pulmonary fibrosis (IPF), formerly known as usual interstitial pneumonitis (UIP); and cryptogenic organizing pneumonia (COP), formerly known as bronchiolitis obliterans organizing pneumonia (BOOP) (Fig. 8-9 ). Respiratory bronchiolitis-associated interstitial lung disease (RB-ILD) is a disease of smokers with inflammation and fibrosis of ter-minal bronchioles and prominent intra-alveolar histiocytes that share some features with desquamative interstitial pneumonia (DIP).
Figure 8-9.

Organizing pneumonia. Microscopic section of lung tissue with focal Masson bodies (intra-alveolar granulation tissue). More mature interstitial fibrosis with collagen deposition is also present.
Sarcoid
Sarcoidosis is an idiopathic inflammatory disease characterized by the formation of discrete granulomas in multiple organ systems. The lung is usually involved in sarcoidosis, and lung biopsy or biopsy of mediastinal lymph nodes may be diagnostic (Fig. 8-10 ). The granulomas in sarcoid usually contain multinucleated giant cells that often contain asteroid bodies (crystalline cytoplasmic inclusions). These granulomas are typically compact and highly organized. Central necrosis is unusual, and no microorganisms are demonstrable by staining or culture. Individuals such as fire fighters who are exposed to particulates may be at increased risk for developing sarcoid, but the detailed pathophysiology of this disease is not well understood and may be heterogeneous. Sarcoid may be progressive and lead to significant pulmonary fibrosis with compromise of lung function as well as damage to other organ systems.
Figure 8-10.

Sarcoid granulomas. Microscopic section showing numerous non-necrotizing granulomas. The tight concentric organization of granulomas is typical of sarcoidosis.
Immune-Mediated Lung Disease
Pulmonary interstitial fibrosis occurs in some patients as a manifestation of systemic autoimmune diseases (e.g., systemic sclerosis and systemic lupus erythematosus) (Fig. 8-11 ). Goodpasture's syndrome is caused by type II hypersensitivity in which anti-basement membrane antibodies react with the capillary basement membranes in lung tissue (as well as renal glomeruli) to produce pulmonary hemorrhage that may be life threatening.
Figure 8-11.

Pulmonary fibrosis in scleroderma. Gross cross-section of lung tissue showing severe septal fibrosis (strand-like whitish areas).
PULMONARY EMBOLI
Pulmonary emboli result from dislodgment of venous thrombi, which are then propelled into the pulmonary circulation. At least 90% of pulmonary emboli arise from deep venous thrombi in the thigh. Venous thrombosis in these larger deep veins is typically asymptomatic so that the diagnosis may not be suspected until pulmonary emboli develop. Small pulmonary emboli are frequently asymptomatic because of the dual pulmonary circulation but may cause hemoptysis. Involvement of more than 40% of lung parenchyma results in hypoxemia, even in normal individuals. Large pulmonary emboli that occlude the bifurcation of the pulmonary artery trunk are called “saddle emboli” (Fig. 8-12A ) and are almost instantly fatal because they cause complete blockage of pulmonary blood flow with rapid and complete loss of cardiac output.
Figure 8-12.

A, Pulmonary emboli. Gross cross-section from the lung hilum with an in situ acute pulmonary embolus blocking a large pulmonary artery trunk. B, Pulmonary infarct. Microscopic section of lung tissue showing in situ pulmonary embolus and associated hemorrhage and coagulative necrosis indicative of a pulmonary infarct.
Over time pulmonary emboli are organized by tissue repair and thrombolysis, which recanalize affected blood vessels. These vessels often retain eccentric scars or a “spider web” of fibrous tissue, which are markers of chronic pulmonary emboli. Chronic pulmonary emboli can result in increased pulmonary artery pressure with strain on the right heart that may ultimately develop into cor pulmonale in the setting of pulmonary hypertension.
Pulmonary infarcts are rare except in patients with intrinsic pulmonary vascular disease or repeated episodes of pulmonary emboli. Pulmonary infarcts are typically hemorrhagic and wedge-shaped and produce symptoms related to pleural inflammation (see Fig. 8-12B). A pleural friction rub is produced owing to fibrin deposition on the pleural surfaces. Pulmonary emboli are usually not visible in routine chest radiography, but helical CT scan with contrast can identify even small pulmonary emboli, in most cases permitting a specific diagnosis.
Pulmonary emboli are a major cause of morbidity and mortality in United States with over 200,000 deaths annually. Factors predisposing to pulmonary emboli are largely those favoring deep venous thrombosis. These include stasis, often associated with immobilization of elderly individuals for surgical procedures. Prophylactic treatment of hospitalized patients at risk with low-molecular-weight heparin can significantly reduce the occurrence of pulmonary emboli.
PULMONARY HYPERTENSION
Pulmonary hypertension is defined numerically as a mean pulmonary artery pressure greater than 40 mm Hg. Hypertension can result from recurrent pulmonary emboli, from in situ thrombosis within the pulmonary vasculature, as a consequence of pulmonary fibrosis, secondary to increased left atrial pressure (in congestive heart failure or cardiomyopathy), or as an idiopathic condition (i.e., primary pulmonary hypertension). Over time, increased pulmonary artery pressures result in remodeling of the pulmonary vasculature with thickening of the walls of pulmonary arteries. In severe cases, necrosis of vascular walls may develop, and plexiform lesions may be observed. Once the pulmonary vasculature has been remodeled to accommodate high arterial pressures, adequate lung perfusion requires these high pressures. Increased pulmonary artery pressure produces strain on the right side of the heart and can cause cor pulmonale (Fig. 8-13 ).
Figure 8-13.

Cor pulmonale. Gross cross-section of a heart with massive right ventricular hypertrophy. The left ventricle (smaller chamber) is relatively normal.
PULMONARY TUMORS AND TUMOR-LIKE CONDITIONS
Some bronchogenic tumors are highly associated with cigarette smoking while others show a weaker association.
Because of the large functional pulmonary reserve in normal lungs, tumors usually do not become symptomatic until they are large. Many lung cancers are not curable by surgery at the time they are discovered.
Bronchogenic Carcinoma
Bronchogenic carcinoma includes the most common lung cancers of adults and is classified into two major categories based on morphology and different clinical approaches to therapy. Non-small cell carcinoma includes squamous cell carcinoma and adenocarcinoma as well as large-cell carcinoma. These tumors often share morphologic features and, when feasible, are primarily treated by surgical excision. In contrast, small-cell undifferentiated carcinoma usually arises near the hilum of the lung and spreads to lymph nodes and other organs early in its course. For these reasons, small-cell carcinoma is not amenable to surgical therapy and is typically treated primarily with chemotherapy. Only a tiny fraction of tumors have features that overlap between small-cell and non-small cell carcinoma.
Non-Small Cell Carcinoma
Adenocarcinomas of the lung are less strongly associated with cigarette smoking than are other types of bronchogenic carcinoma. Adenocarcinomas tend to arise in the periphery of lung tissue and may have two different patterns of growth. Solid adenocarcinomas can arise in a preexisting scar (e.g., healed tuberculosis) or de novo (Fig. 8-14A ). Well-differentiated tumors show obvious gland formation, but poorly differentiated tumors often show morphologic overlap with large-cell undifferentiated and squamous cell carcinoma.
Figure 8-14.

A, Bronchogenic adenocarcinoma. Microscopic section of lung tissue with a solid type adenocarcinoma with extensive associated fibrosis. Gland formation is evident. B, Bronchioalveolar carcinoma. Microscopic section of lung showing tumor growth along normal alveolar septae. This lepidic growth pattern is characteristic. C, Non-small cell carcinoma. Microscopic section of lung showing focal squamous differentiation in a typical non-small cell carcinoma. D, Non-small cell carcinoma. Gross cross-section of lung tissue showing a large non-small cell carcinoma (white lesion at the top) with scattered anthracotic pigment.
Bronchioalveolar carcinoma is a distinct form of adenocarcinoma that does not form a solid mass. The malignant cells in bronchoalveolar carcinomas grow along existing alveolar septae, replacing normal pneumocytes (see Fig. 8-14B). This lepidic pattern of growth may begin as atypical alveolar hyperplasia (dysplasia of alveolar ring cells). Bronchioalveolar tumors may be small or large and are frequently multifocal. It is important to distinguish primary adenocarcinomas of lung from metastatic adenocarcinoma from other organs. Large-cell undifferentiated carcinoma does not show obvious glandular differentiation but shares many features with adenocarcinoma. Some pathologists view large-cell undifferentiated carcinoma as a form of poorly differentiated adenocarcinoma. The absence of neuroendocrine marker expression and the presence of vesicular nuclei with prominent nucleoli distinguish large-cell undifferentiated carcinoma from neuroendocrine carcinoma (see below).
Squamous cell carcinoma of lung tends to arise in the middle portion of the lung or near the carina, where carcinoma in situ develops in squamous metaplasia in larger bronchi. Squamous cell carcinoma is strongly associated with cigarette smoking. Tumors may be well differentiated with abundant keratin production or poorly differentiated (see Fig. 8-14C). Bronchogenic carcinomas with mixed squamous and glandular differentiation are common, and this mixed pattern is relatively characteristic of primary lung cancers.
BIOCHEMISTRY.
Tobacco-associated Carcinogens
Tobacco smoke predisposes to a large number of different types of cancer, and at least 60 known carcinogens are present in cigarette smoke including nitrosamines, polycyclic aromatic hydrocarbons (PAH), and aromatic amines.
Metabolic processing of most tobacco-derived compounds is required before they become active carcinogens (e.g., benzo[a]pyrene (BaP), 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone (NNK), N-nitrosodimethylamine (NDMA), N′-nitrosonornicotine (NNN), ethylene oxide, and 4-aminobipheynyl). In most cases, carcinogen activation is accomplished by cytochrome P-450 enzymes (see Chapter 3) that produce reactive electrophiles that can directly combine with DNA. Polymorphisms in cytochrome P-450 enzymes are associated with susceptibility or resistance to tobacco carcinogens and may underlie ethnic (racial) differences in susceptibility of smokers to lung cancer.
DNA adducts from tobacco carcinogens typically result in missense mutation, most commonly G→T and G→A. The presence of a large number of these base changes in a tumor is suggestive of tobacco-induced carcinogenesis.
The lung is a comparatively common site for metastasis, and it may be difficult to differentiate primary from metastatic adenocarcinomas. Immunohistochemical studies may be helpful in this regard, since most primary adenocarcinomas of the lung express thyroid transcription factor 1 (TTF-1), which is not expressed in most metastatic carcinomas from other organs. On gross examination, bronchogenic carcinomas tend to be stellate in configuration and typically contain anthracotic pigment (see Fig. 8-14D). In contrast, metastatic tumor deposits in the lung tend to be spherical in configuration and often do not contain grossly visible anthracotic pigment.
Non-small cell carcinoma usually spreads to hilar and mediastinal lymph nodes before metastasizing to other sites. Many tumors extend to the pleural surface, and they may extend through the visceral pleura to involve the parietal pleura. Tumors in the apex of the lung that extend into the chest wall can produce pain and paralysis by invading the brachial plexus (Pancoast's tumor). Non-small cell carcinomas that have spread to contralateral mediastinal lymph nodes or to the parietal pleura are not curable by surgery. Combination chemotherapy and radiation therapy can effectively shrink some unresectable non-small cell carcinomas and convert them to surgically resectable lesions. Even patients without demonstrable lymph node metastases at the time of lung resection are at risk for systemic metastasis. Non-small cell lung cancer is a fairly frequent cause of singular brain metastasis.
Small-Cell Undifferentiated Carcinoma and Neuroendocrine Tumors
Neuroendocrine tumors of the lung range from well-differentiated carcinoid tumors to small-cell undifferentiated carcinoma. Peripheral carcinoid tumors are relatively common in the lung and have features similar to carcinoid tumors arising at other sites in the body. Carcinoid tumors typically show a nested pattern of growth with granular chromatin (so-called “salt and pepper” pattern) in the nuclei of tumor cells (Fig. 8-15A ). Nucleoli are inapparent, mitotic figures are inconspicuous, and tumor necrosis is not observed. Some carcinoid tumors have a predominantly spindle cell pattern of growth. All carcinoid tumors strongly express neuroendocrine markers including chromogranin A, which is a component of dense core neurosecretory granules.
Figure 8-15.

A, Pulmonary carcinoid tumor. Small peripheral carcinoid tumor composed of neuroendocrine cells with a nested growth pattern. B, Small-cell undifferentiated carcinoma. Microscopic section showing tumors cells with finely divided nuclear chromatin and prominent molding. Tumor cell cytoplasm is almost invisible in this section.
Although it is unusual for small carcinoid tumors to metastasize, all carcinoid tumors possess some metastatic potential. Larger tumors that show atypia are more likely to cause metastatic disease usually involving hilar or mediastinal lymph nodes.
Atypical carcinoid tumors are less well differentiated neuroendocrine tumors of the lung that are usually diagnosed as intermediate-grade neuroendocrine carcinoma. Morphologically, they show a less well developed nested architecture and tend to have a higher mitotic rate, often with foci of necrosis. The classification of neuroendocrine carcinoma reflects the lack of clear boundaries between well and poorly differentiated neuroendocrine tumors. Neuroendocrine carcinomas have a significantly higher potential for lymph node or hematogenous metastasis than do well-differentiated carcinoid tumors.
Small-cell undifferentiated carcinoma is a very poorly differentiated neuroendocrine carcinoma that usually arises in the central portion of the lung and involves hilar and mediastinal lymph nodes early in its course. The primary tumor itself may not be visible by radiography although enlarged lymph nodes suggest the diagnosis of metastatic disease. Small-cell undifferentiated carcinoma is almost always a disease of heavy smokers and consists of small- to intermediate-sized tumor cells with scant cytoplasm and with finely divided nuclear chromatin. Tumor cells characteristically show nuclear molding in which one tumor cell may indent the nucleus of another tumor cell (see Fig. 8-15B). The fragility of nuclear membranes in small-cell carcinoma often leads to marked crush artifact in biopsy specimens of these neoplasms, which may be a useful diagnostic finding. Mitotic activity is brisk, and tumor necrosis is almost always present. Small-cell undifferentiated carcinoma typically has poorly formed cell junctions, making tumor cells poorly cohesive in cytologic preparations. Most small-cell undifferentiated carcinomas show some evidence of neuroendocrine differentiation by immunohistochemistry, but neurosecretory granules are usually scant in these poorly differentiated tumors.
BIOCHEMISTRY.
Neurosecretory Granules
Neurosecretory granules are membrane-bound vesicles that contain various hormones and chromogranin A. Neurosecretory granules have electron-dense cores bounded by membranes and are a characteristic and easily recognizable feature of neuroendocrine cells by electron microscopy. Synaptophysin was so-named because it was discovered in neuronal synapses, but it is a component of all neuroendocrine cells.
The membranes of neurosecretory granules have v-SNAREs (vesicle-associated soluble N-ethylmaleimide-sensitive factor attachment proteins) and regulatory proteins that control their docking and fusion with the cytoplasmic membrane in response to various stimuli (e.g., Ca++ influx or phosphorylation/dephosphorylation). v-SNARE proteins interact with t-SNAREs on the inner surface of the cytoplasmic membrane to mediate membrane fusion and exocytosis. Docking proteins are then recycled from the plasma membrane via clathrin-coated pits or other mechanisms.
Patients with small-cell undifferentiated carcinoma frequently have extensive liver metastases and usually are not treated surgically because of the likelihood of metastatic spread. Chemotherapy including cisplatin and etoposide often produce objective responses in patients with small-cell undifferentiated carcinoma, but none of these cases are ultimately curable. Effective chemotherapy may extend life significantly (months), but almost all patients succumb to their tumor over the course of several years.
Other Primary Lung Tumors
Other types of primary lung tumors are relatively uncommon. Tumors of endobronchial glands (similar to salivary gland tumors) and sarcomas can occur but are rare. Pulmonary hamartomas may simulate a lung tumor on radiographic analysis. Hamartomas are usually composed predominantly of mature cartilage but may include other cell types that normally occur in normal lung tissue. Pulmonary hamartomas are probably benign neoplasms rather than true hamartomas (i.e., developmental abnormalities), since they are not found in children. Primary lymphomas of the lung are unusual with low-grade non-Hodgkin's lymphomas (NHLs) of bronchial mucosa-associated lymphoid tissue (similar to the more frequent MALT lymphomas in the gastrointestinal tract) encountered most frequently. Pulmonary pseudotumor is a poorly defined entity composed of inflammatory cells and proliferating fibroblasts that may simulate a lung tumor.
Pulmonary Sequestration
Pulmonary sequestration is a developmental abnormality in which a portion of lung tissue is supplied by a systemic artery (rather than a pulmonary artery) and is not connected to the bronchial tree in a normal fashion. Sequestrations can develop infection and present as a lung mass lesion, mimicking a lung tumor. Angiography can usually demonstrate the abnormal vascular connections that are characteristic of sequestration, and surgical excision is curative.
LARYNGEAL PATHOLOGY
The larynx is surrounded by a cartilaginous wall that is an effective barrier to the spread of laryngeal tumors. The true vocal cords lack lymphatics as a consequence of their function in producing speech. As a result, the true cords are susceptible to lymphedema, which can result in so-called vocal nodules (singer's nodules caused by voice strain) that consist of accumulations of myxoid material in the stroma (Fig. 8-16 ).
Figure 8-16.

Vocal nodule. Microscopic section of true vocal cord showing the accumulation of myxoid material in the submucosa. The overlying squamous epithelium shows mild reactive changes.
Papillomatosis
Infection of the larynx with some strains of human papillomavirus (HPV) can result in the formation of numerous papillomas that can cause dysarthria (difficulty speaking) and may produce tracheal obstruction in extreme cases (Fig. 8-17A ). Infection may be venereal in origin. A significant proportion of squamous cell carcinomas arising in the larynx contain HPV DNA, suggesting that this virus may be a predisposing factor for tumor development.
Figure 8-17.
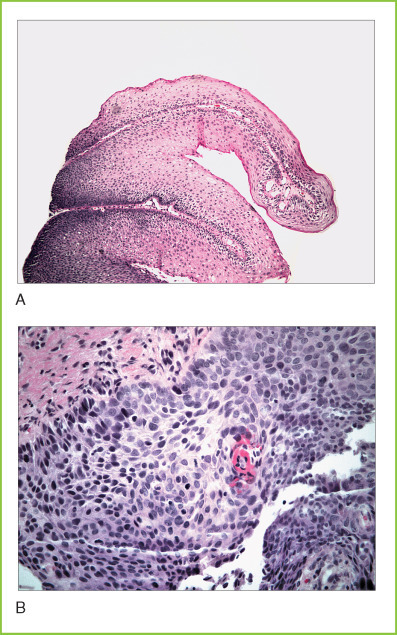
A, Laryngeal papilloma. Microscopic section of a papilloma showing features similar to condyloma of the uterine cervix. B, Squamous dysplasia in the larynx. Microscopic section of high-grade squamous dysplasia with loss of normal maturation pattern and pleomorphic preneoplastic cells. No invasion is evident in this section.
Carcinoma
The predominant malignant tumor of the larynx is squamous cell carcinoma, strongly associated with heavy alcohol consumption, cigarette smoking, or both. Tumors often develop in areas of epithelial dysplasia, and biopsy specimens of laryngeal mucosa before the development of an invasive tumor often show varying degrees of epithelial dysplasia (see Fig. 8-17B). Some carcinomas are associated with evidence of HPV infection. Invasive squamous cell carcinomas of the larynx tend to be confined by the laryngeal and thyroid cartilages (cartilage is hypoxic and is a poor substrate for tumor growth) so that spread into surrounding soft tissues occurs relatively late in their course. Since most tumors produce vocal symptoms early, patients often come to medical attention before there is extensive tumor spread. Primary radiation therapy is curative in some patients, and hemilaryngectomy is adequate for small tumors and allows preservation of relatively normal speech. Metastatic spread is typically to draining lymph nodes.
NASOPHARYNGEAL PATHOLOGY
Most nasopharyngeal pathology is related to tumors, and by far the most common is squamous cell carcinoma (either primary or by extension from the oral cavity or larynx). Mantle cell lymphoma can present around Waldeyer's ring (tonsillar and adenoid lymphoid tissue). A few tumors described below are unique to the nasopharynx.
Nasopharyngeal Carcinoma
Nasopharyngeal carcinoma has several different morphologic subtypes. The most characteristic is the so-called lymphoepithelial type in which tumor cells are intimately associated with infiltrating small lymphocytes. These tumors are usually associated with Epstein-Barr virus (EBV) infection and are relatively common in individuals from Asia. Tumor cells express epithelial markers but do not show overt evidence of squamous differentiation. These tumors often present at an advanced stage but are highly responsive to radiation therapy, which often is curative. The precise role of EBV in the genesis of these neoplasms is not fully defined. Other subtypes of nasopharyngeal carcinoma that show overt keratinization are usually not curable with radiation therapy and behave similarly to squamous cell carcinoma.
Other Tumors
Angiofibroma is a vascular neoplasm of the nasopharynx that typically occurs in adolescent boys and can produce nasal obstruction or bleeding that may be severe. Angiofibromas are usually hormone (androgen) responsive, and excision is curative.
Aesthesioneuroblastoma is a malignant tumor of adults that arises from olfactory nerves in the nasopharynx, and its morphologic features and neuroendocrine differentiation are quite similar to neuroblastoma occurring in the adrenal gland.
PLEURAL PATHOLOGY
Pleural Fluid Accumulations
Patients with pulmonary edema can accumulate fluid in the pleural spaces as well, resulting in progressive collapse of the lungs and diminished gas exchange. Prolonged lung collapse may be irreversible. Effusions resulting from congestive heart failure are usually bilateral and are characteristically transudates. Exudative pleural effusions can form in response to inflammation or neoplasia in the lung. Malignant pleural effusions are almost always exudates with increased protein concentration and specific gravity, and many are hemorrhagic. Cytologic examination may reveal clusters of tumor cells. Lung cancer frequently involves the pleura by direct extension and may lead to malignant effusion or chest wall involvement. Malignant effusions may be intractable, requiring therapeutic obliteration of the pleural space by sclerosing agents to maintain lung inflation. Patients with widely metastatic breast and ovarian carcinoma develop malignant pleural effusions with some frequency.
Tuberculosis can result in an exudative effusion with lymphocytes as the major cellular component. Bacterial pneumonia can produce an exudative pleural effusion with a neutrophilic infiltrate. Direct infection of pleural fluid can result in empyema that is essentially an abscess within the pleural space. Empyema most often develops in association with pneumonia caused by destructive organisms such as Staphylococcus. Empyema can also develop because of bacterial seeding of an existing pleural effusion of any cause. Empyema requires external drainage for the infection to heal. Healing usually results in extensive fibrosis with dense pleural adhesions or obliteration of the pleural space.
Pleurisy is an inflammation of the pleura that causes pleuritic chest paint (pain with breathing or coughing resulting from sliding of inflamed visceral and parietal pleura against each other). Inflammation often results in a fibrin exudate, which can produce a friction rub that can be heard on auscultation. The most common cause of primary pleural inflammation is viral infection. Patients with autoimmune disease and uremic patients may also develop clinically significant pleurisy. Pleurisy can occur secondary to pneumonia, pulmonary infarction, or malignancy.
Mesothelioma
Malignant mesothelioma is a highly aggressive neoplasm of mesothelial cells that usually arises in the pleural cavity but can arise in the peritoneum or pericardium. Tumors are often biphasic, with alternating papillary and spindle cell growth patterns (Fig. 8-18A ). Biphasic tumors are morphologically characteristic, whereas pure papillary/glandular mesotheliomas can be difficult to differentiate from adenocarcinomas. Mesothelial cells express calretinin, which is only rarely expressed in epithelial tumor cells, and this immunohistochemical marker provides a useful diagnostic test for mesothelioma in biopsy specimens. Mesotheliomas are almost universally associated with asbestos exposure.
Figure 8-18.

A, Malignant mesothelioma. Microscopic section of a predominantly papillary mesothelioma. It would be difficult to distinguish this tumor from a bronchogenic adenocarcinoma on histologic grounds. B, Malignant mesothelioma. Gross cross-section of lung with malignant mesothelioma. Whitish tumor extends along pleural surfaces and permeates lung parenchyma.
Mesotheliomas spread along pleural surfaces and also extend along vascular and lymphatic spaces into lung tissue (see Fig. 8-18B). They are usually unresectable at the time of diagnosis and tend to progress relatively rapidly, with diffuse involvement of lung parenchyma producing respiratory failure.
Solitary Fibrous Tumor
The solitary fibrous tumor of pleura is a recently recognized entity composed of spindle cells that express CD34 and show random orientation (so-called patternless pattern) in histologic sections. Since the description of solitary fibrous tumor as a pleural neoplasm of intermediate biologic potential, this tumor has been recognized at many other sites throughout the body (many of these tumors were previously diagnosed as hemangiopericytomas). The majority of these tumors are cured by local excision, but a subset is associated with more aggressive biologic behavior and metastasis.
BIOCHEMISTRY.
Asbestos
Asbestos is the name given to a collection of naturally occurring silicate minerals that can be separated to form long, flexible fibers and woven to form many useful materials. Asbestos is mechanically strong and extremely resistant to heat. These properties resulted in heavy industrial usage of asbestos until the mid twentieth century, when its biological hazards were recognized. Chrysotile asbestos is the most common form in industrial use and is still utilized in brake pads in automobiles.
Large asbestos fragments are not dangerous to humans, but small particles that are inhaled beyond the mucociliary blanket in the lung can produce pneumoconiosis and predispose to mesothelioma. Asbestos exposure need not be heavy to produce a mesothelioma, and individuals with even minor exposures are at some risk for developing this rare tumor. The exact mechanism by which asbestos produces mesotheliomas remains unclear. Asbestos exposure also greatly enhances the risk of lung cancer in cigarette smokers.


