Key features.
-
•
Bacterial pneumonias are major causes of death in the tropics
-
•
Symptoms and physical examination remain crucial for diagnosis and management
-
•
Parasitic infections can manifest as wheezing, eosinophilic pneumonia or a pleural effusion
-
•
Analysis of pleural fluid can help in management decisions
-
•
Common diseases like chronic obstructive pulmonary disease (COPD) can have a different epidemiology and etiology in the tropics than in the developed world
Introduction
The term “tropics” refers to the region of the earth lying between the Tropic of Cancer and the Tropic of Capricorn. In the tropics, warm climate, poverty, lack of education, and poor sanitation provide an ideal environment for pathogens, vectors and intermediate hosts to flourish [1]. In this vast landmass, respiratory infections are a major cause of morbidity and mortality in children and adults [2]. In a typical tropical clinic, 20–40% of outpatients have respiratory complaints, and 20–30% of inpatients have lung disease (Table 1-1 ) [2].
TABLE 1-1.
Incidence of Pneumonia Cases and Pneumonia Deaths Among Children Under 5 Years of Age, by UNICEF Region, 2004*
| UNICEF regions | Number of children under 5 years of age (in thousands) | Number of childhood pneumonia deaths (in thousands) | Incidence of pneumonia cases (episodes per child per year) | Total number of pneumonia episodes (in thousands) |
|---|---|---|---|---|
| South Asia | 169,300 | 702 | 0.36 | 61,300 |
| Sub-Saharan Africa | 117,300 | 1,022 | 0.3 | 35,200 |
| Middle East and North Africa | 43,400 | 82 | 0.26 | 11,300 |
| East Asia and Pacific | 146,400 | 158 | 0.24 | 34,500 |
| Latin America and Caribbean | 56,500 | 50 | 0.22 | 12,200 |
| CEE/CIS | 26,400 | 29 | 0.09 | 2,400 |
| Developing countries | 533,000 | 2,039 | 0.29 | 154,500 |
| Industrialized countries | 54,200 | 1 | 0.03 | 1,600 |
| World | 613,600 | 2,044 | 0.26 | 158,500 |
Modified from UNICEF/WHO. Pneumonia: the forgotten killer of children. Geneva: UNICEF/ WHO; 2006, p.13.
Many tropical patients suffer from lung diseases that are found worldwide, e.g. asthma, bronchiectasis, chronic obstructive lung disease, HIV infection-related lung disease, and lung cancer. Numerous dust diseases, e.g. silicosis, asbestosis, byssinosis, hypersensitivity pneumonitis, and diseases due to microbial contamination of agricultural products, remain under-recognized. Diseases associated with pulmonary symptoms and infection that are concentrated in the tropics include malaria, pulmonary schistosomiasis, melioidosis, paragonimiasis, echinococcal cysts, tropical eosinophilia, and diseases related to nutritional deficiencies [3]. In addition, individuals who come in contact with birds or animals may develop zoonoses such as tularemia, psittacosis, Q fever and leptospirosis [4]. In the tropics, indoor air pollution caused by biomass fuels used for cooking and heating of the homes and huts is an important cause of obstructive lung disease and chronic lung infections [5].
The following are the common tropical pulmonary conditions:
-
•
pneumonia: typical and atypical
-
•
eosinophilic pneumonias and tropical pulmonary eosinophilia
-
•
bronchiectasis, asthma and chronic obstructive pulmonary disease (COPD)
-
•
pleural effusion
-
•
nontuberculous granulomatous lung disease
-
•
occupational lung diseases.
A reasonable approach to the patient with lung disease in the tropic starts with age, occupational exposure, physical examination, HIV status, chest x-ray and blood tests. In children, bacterial pneumonia is the most common and life-threatening disorder. Known immunodeficiency suggests tuberculosis, fungi and opportunistic pathogens. Peripheral blood eosinophilia with either a pleural effusion or diffuse parenchymal consolidation may suggest a parasitic infection, or, when combined with wheezing, tropical pulmonary eosinophilia. Worldwide diseases like COPD may affect nonsmoking individuals due to indoor pollutants.
Pneumonia
Streptococcus pneumoniae is the most common bacterial cause of pneumonia. Upper respiratory involvement often precedes the onset of pneumococcal pneumonia, which is characterized by fever, chills, malaise and sweating. The patient is flushed and febrile with a rapid pulse and respiratory rate. Dyspnea is associated with a nonproductive cough, and sputum, if present, may be thick, tenacious or “rusty”. Severe pleuritic chest pain causing tachypnea and grunting respiration is often present. Such symptoms are abrupt in young, immunocompetent patients (Fig.1.1 ) [6].
FIGURE 1.1.
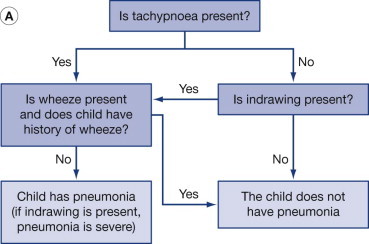
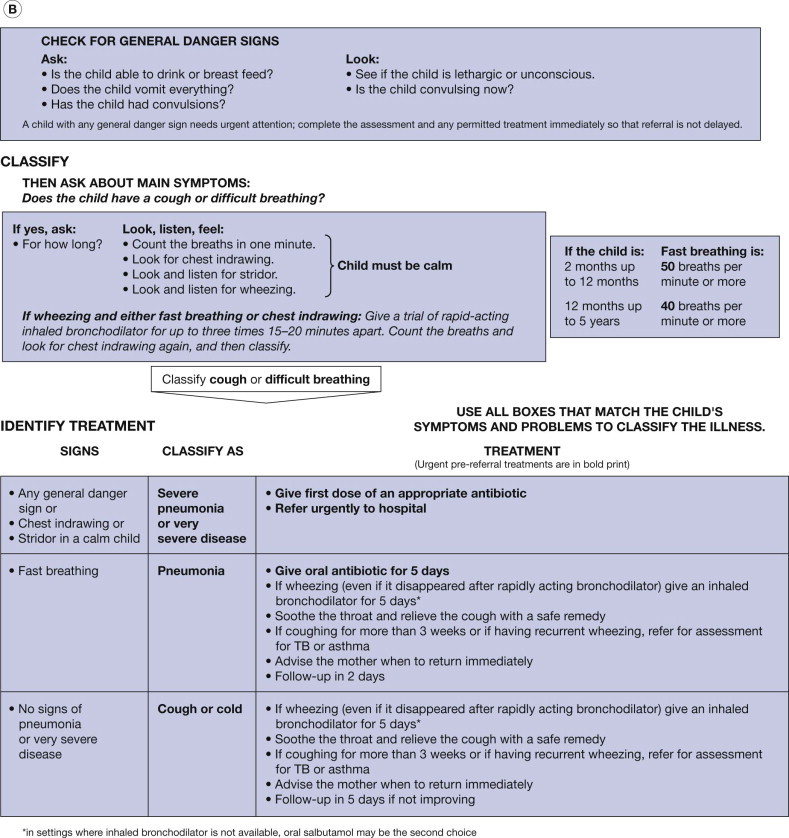
(A) World Health Organization algorithm for diagnosing pneumonia in children (modified with permission from World Health Organization, Family and Community Health Cluster, Department of Child and Adolescent Health and Development. Consultative meeting to review evidence and research priorities in the management of acute respiratory infections (ARI). Meeting report. Geneva: WHO; 2003:1–30.). (B) IMCI (Integrated Management of Childhood Illness) guidelines for treating pneumonia (modified with permission from WHO/UNICEF. Integrated Management of Childhood Illness (IMCI) for high HIV settings. Geneva: WHO; 2009;11:2.)
In elderly patients, symptoms may be few and can be dominated by confusion, delirium and prostration [7]. Physical examination of the affected lung, usually the lower lobe, reveals diminished lung expansion, impaired percussion note, decreased breath sounds, crepitations (crackles/rales) and bronchial breath sounds. Cyanosis is common and a herpes simplex eruption may be seen on the lips. With proper treatment, most patients with pneumococcal pneumonia improve clinically and radiographically within 1–2 weeks. When resolution occurs, fever subsides within a week as the temperature decreases following a crisis pattern (Fig. 1.2A ). Delayed resolution is seen in smokers, the elderly, and in those with poor nutrition, diabetes or other comorbid illnesses.
FIGURE 1.2.

Various patterns of fever. (A) In lobar pneumonia, fever subsides by crisis within a week. (B) In bronchopneumonia, fever comes down slowly by lysis and takes longer. (C) In tuberculosis, fever is remittent. (D) In malaria, fever is typically intermittent.
Staphylococcal pneumonia (Staphylococcus aureus), accounts for 2–10% of acute community-acquired pneumonias. It is an important cause of pneumonia in children, the elderly, patients recovering from influenza, people with diabetes mellitus, and those who are immunocompromised. Methicillin-resistant Staphylococcus aureus (MRSA) causes illness in 1% of cases of upper or lower respiratory tract infection in the community and in 10% of patients who are hospitalized. Patients with staphylococcal pneumonia are usually ill with high fever, shaking chills, chest pain, cough and purulent sputum. Chest x-ray films show patchy consolidation and cavities.
Investigations and Management
Sputum examination is an important aid in the diagnosis of pneumonia. Color, amount, consistency and odor are helpful: mucopurulent sputum is commonly found in bacterial pneumonia or bronchitis; scanty watery sputum is often noted in atypical pneumonia; “rusty” sputum is seen in pneumococcal pneumonia; and currant-jelly or dark-red sputum suggests Klebsiella pneumoniae. Foul-smelling expectoration is associated with anaerobic infections due to aspiration, lung abscess and necrotizing pneumonia. The presence of Gram-positive diplococci indicates pneumococcal pneumonia; small Gram-negative coccobacillary forms are typical of H. influenzae, and staphylococcal organisms appear in tetrads and grapelike clusters. In mycoplasma, viral and legionella pneumonia, typical bacterial organisms are not seen. If sputum is not available, a specimen can be obtained by tracheobronchial suction.
A blood count usually reveals leukocytosis in bacterial pneumonia, leukopenia in viral infection, and eosinophilia in parasitic infestation. When available, chest x-ray is extremely helpful (Table 1-2 ). Tuberculosis is omnipresent in the tropics; upper lobe lesions with or without cavities strongly suggest tuberculosis.
TABLE 1-2.
Clinical Features of Typical and Atypical Common Community-Acquired Pneumonias
| Pneumococcal | Mycoplasma | Viral | |
|---|---|---|---|
| Causative agent | Streptococcus pneumoniae | Mycoplasma pneumoniae | Influenza virus A and B |
| Fever | Sustained 102–103°F | 100–103°F | 100–103°F unremitting |
| Chills | Abrupt onset | In 25% | Chilly sensation not rigors |
| Cough | Productive | Dry, nonproductive | Nonproductive to productive |
| Sputum | Purulent, blood-stained, rusty | Mucoid, if present | Scant to purulent, may be blood-stained |
| Gram stain | Diplococci, lance-shaped, often intracellular | No organisms, many alveolar macrophages | No organisms |
| Chest x-ray | Lobar consolidation | Diffuse perihilar, patchy infiltrates | Diffuse interstitial, perihilar infiltrates |
| Typical patient | All ages, high incidence in the elderly and alcoholics | Usually children and young adults | Severe illness in elderly |
| Complications | Meningitis, empyema | Anemia, Steven–Johnson syndrome, myringitis | Staphylococcal infection |
In children, the Integrated Management of Childhood Illness (IMCI) guidelines for treating pneumonia are recommended (see Fig. 1.1) [8]. Nevertheless, a patient's illness has to be assessed based on geography, prevalence of potential etiologies, virulence of the organism, and the drug sensitivity pattern (Box 1.1 ). In some areas, particularly Papua New Guinea, South Africa and Spain, resistance of the pneumococcus to penicillin is common. For children with non-severe pneumonia, the World Health Organization (WHO) recommends oral trimethoprim–sulfamethoxazole (TMP-SMX) or oral amoxicillin for 5 days [9]. In severe pneumonia in hospitalized children, the policy in low-income countries is to first give benzylpenicillin injections, changing the therapy to oral amoxicillin when the child responds. In very severe pneumonia, in children in low-income settings, chloramphenicol may be given first with benzylpenicillin and gentamicin in combination as an alternative 10, 11.
Box 1.1. Key Facts: Acute Respiratory Infections (ARIs) in Children.
-
•
20% of all deaths in children under 5 years are due to ARIs
-
•
90% of all deaths due to ARIs are due to pneumonia
-
•
Streptococcus pneumoniae and Haemophilus influenzae are two top causes of pneumonia
-
•
Low-birthweight, malnourished and non-breastfed children are at high risk of having pneumonia
-
•
High fever, rapid breathing, and retraction of the chest are indicators for hospitalization
-
•
Children with malnutrition and edema should be admitted to hospital
Atypical Pneumonia
Atypical pneumonia is caused by Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella spp., viruses, tuberculosis, fungi and parasites. This syndrome is not extensively studied in the tropics because of the expense involved in culturing and isolating various organisms and obtaining serologic and immunologic tests.
Mycoplasma pneumoniae infections occur worldwide, affecting mostly school-aged children and young adults. A typical patient with mycoplasma pneumonia is an older child or young adult with an insidious onset of fever, malaise, tightness of the chest, and dry brassy cough. Constitutional symptoms are out of proportion to the respiratory symptoms. Hemoptysis, pleural pain and gastrointestinal symptoms are uncommon. The tropical physician should be aware of the non-respiratory manifestations of mycoplasma infection, including anemia, myringitis, Stevens–Johnson syndrome, hepatitis and neuritis [12] (see Table 1-2).
Other Conditions Associated with Pulmonary Infection
Leptospirosis is common in tropical areas where sanitation is poor and water supply primitive. Epidemics of leptospirosis occur after high rainfall in monsoon seasons when the water supply is contaminated by sewage or animal urine. About half of the patients with leptospirosis have fever, cough, hemoptysis and pneumonitis [13]. Other features are jaundice, conjunctivitis and impaired renal function.
Melioidosis, caused by Burkholderia pseudomallei, is endemic in Southeast Asia (Vietnam, Cambodia, Myanmar), northern Australia and West Africa. Melioidosis is hyperendemic in northern Australia, and in parts of northeastern Thailand it is an important cause of fatal community-acquired pneumonia [14]. Patients become infected while wading through fields, paddies, and flooded roads. Clinical presentation is protean and nonspecific. The radiologic picture of upper lobe infiltration and cavity formation can be indistinguishable from tuberculosis [15]. Diagnosis requires isolation of the organism. The mortality rate ranges from 20% to 50% but is higher in HIV-infected and immunocompromised hosts.
Respiratory symptoms of cough and chest pain in typhoid are present in up to 50% of cases at the onset of the disease. Pulmonary infiltrates may be associated with positive sputum cultures for Salmonella typhi. A fever chart showing continuous fever is highly suggestive of enteric fever. Diagnosis may be difficult without blood and stool culture facilities.
In brucellosis, the lungs are involved in about 5% to 10% of cases, usually following inhalation of organisms. Abnormalities include bronchopneumonia, solitary or multiple lung nodes, miliary interstitial lung disease, lung abscess and pleural effusion. Organisms can be identified on stains or sputum cultures.
Tularemia is a generalized infection caused by Francisella tularensis and occurs after skin or mucous membrane contact with infected mammals or through the bite of an arthropod, usually a tick or biting fly. Diagnosis should be considered in the presence of a skin ulcer associated with fever, generalized lymphadenopathy, cough and signs of pneumonia. Pneumonia, either primary from inhalation of an infected aerosol or secondary to systemic infection, occurs in about 20% of cases.
Pneumonic plague is less common than either bubonic or septicemic disease. Nevertheless, fatal bronchopneumonia can occur without lymphadenopathy and is characterized by watery, bloody sputum. A sputum Gram stain can show bipolar stunted rods. Pneumonic plague and tularemic pneumonia should be considered when a severe, rapidly progressive bronchopneumonia is reported in an endemic area, and “typical” bacterial pneumonias have been ruled out.
In slaughterhouses, meat-processing plants, and areas with sheep and goat husbandry, Q fever (Coxiella burnetii) can cause epidemics of atypical pneumonia. Inhalation of dried infected material is the chief source, and fever, headache and dry cough are the main symptoms. Occasionally, the sputum is blood-streaked.
Bornholm disease (caused by coxsackieviruses and occasionally other enteroviruses), also known as epidemic pleurodynia or Devil's grip, causes chest discomfort and cough. Widespread epidemics of Bornholm disease occur in the Pacific islands and South Africa.
In 2002–2003, an unusual coronavirus was responsible for more than 8000 cases of a severe acute respiratory syndrome (SARS) that spread via international travel across continents from its origin in Guandong Province, China. The SARS coronavirus was previously unknown in humans; a possible reservoir was identified in civet cats and raccoons. After droplet inhalation of the virus, there was an incubation period of 2–7 days, then fever, cough, malaise and headache occurred. Pulmonary inflammation was characterized by desquamation of pneumocytes, hyaline membrane formation and acute respiratory distress syndrome (ARDS). The chest x-ray showed diffuse opacities or consolidation, especially in the lower lung fields. Recovery could be slow and some patients developed fibrosis. Mortality was 10–20%, with the elderly and those with cardiovascular problems being especially at risk.
Kawasaki disease occurs in children under 5 years of age. This acute multisystem disease of unknown cause is characterized by fever of 5 days duration and four of five clinical features: non-purulent conjunctivitis; injected (or fissured) lips or pharynx or strawberry tongue; cervical adenopathy; a maculopapular rash; and changes in the extremities (erythema and edema of the palms and soles, associated with desquamation). Pneumonitis occurs in 10% of the children and coronary artery dilatation and aneurysms in 20–25% of untreated cases. In Brazil there has been a seasonal rise of the condition at the beginning and end of the monsoon season [16].
Cryptococcus neoformans and C. gatti are saprophytic fungi distributed worldwide and are particularly abundant in soil contaminated by pigeon droppings in the tropics as well as in temperate countries. Pulmonary infection results from inhalation of the organisms from environmental sources [17].
Eosinophilic Pneumonias
Systemic helminth infection usually elicits eosinophilia and increased IgE. Although eosinophilia can be a clue to a pulmonary helminth infestation, the definitive diagnosis requires demonstration of ova or larvae in sputum, bronchial alveolar lavage fluid, pleural fluid or lung biopsy [18]. Loeffler's syndrome refers to “simple” pulmonary eosinophilia with no or minimal systemic and pulmonary symptoms. In many helminth infestations (ascaris, strongyloidiasis, hookworm), the larvae migrate through the lung and can cause fever, cough, dyspnea, wheezing, hemoptysis and lung infiltrate.
Schistosomes cause two clinical syndromes. In acute disease, immature schistosomula pass through the lung, and can lead to fever, eosinophilia and pulmonary infiltrate. In chronic schistosomiasis, especially when portal hypertension has led to venous shunts, eggs can bypass the liver and plug pulmonary capillaries and arterioles, producing granuloma and pulmonary hypertension. Radiographs may show dilated pulmonary arteries (Fig. 1.3 ).
FIGURE 1.3.

Bilateral pulmonary arteries dilatation in schistosomiasis.
In paragonimiasis, the lung is the predominantly involved organ. The diagnosis must be considered in a patient from Southeast Asia with cough, hemoptysis (which is recurrent in >80% of cases), a pulmonary cavity and pleural effusion.
Tropical pulmonary eosinophilia, typically in India and other South Asian countries, causes immunologic hyperresponsiveness to Wuchereria bancrofti, Brugia malayi or other microfilariae. Clinical presentation consists of nocturnal cough, wheezing, fever and weight loss. Chest radiographs show diffuse interstitial miliary infiltrates (Fig. 1.4 ); there is a high eosinophil count. In developed countries, serum IgE and antifilarial antibodies can be used to confirm the diagnosis (Table 1-3 ).
FIGURE 1.4.

Predominant bilateral interstitial opacities affecting all lung fields, in a patient with tropical pulmonary eosinophilia.
TABLE 1-3.
Serum IgE Levels in Syndromes with Pulmonary Involvement and Eosinophilia
| Normal<150 IU | Mildly high150–500 IU | Moderately high500–1000 IU | Extremely high>1000 IU |
|---|---|---|---|
| Tuberculosis | Coccidioidomycosis | Strongyloidiasis | Allergic bronchopulmonary aspergillosis |
| Brucellosis | Drug-induced | Schistosomiasis | Tropical pulmonary eosinophilia |
| Hydatid cyst | Loeffler's syndrome | Paragonimiasis | Churg–Strauss syndrome |
| Amebiasis | Sézary syndrome | Hydatid cyst, if it leaks | |
| Sarcoidosis | Polyarteritis nodosa | ||
Bronchiectasis, Asthma, Chronic Obstructive Pulmonary Disease
Bronchiectasis is a chronic, debilitating condition. Dilatation and distortion of the airways leads to impaired mucociliary clearance, which encourages bacterial colonization and bronchial inflammation. Patients have fever, chronic cough, mucopurulent sputum, hemoptysis (Table 1-4 ), wheezing, dyspnea and malaise (Box 1.2 ). The diagnosis of bronchiectasis in developed countries is confirmed by computed tomography of the chest (Fig. 1.5 ); whereas, in the tropics, the diagnosis is mainly clinical and depends upon a compatible history, presence of finger clubbing, sputum that settles into three layers (mucoid or frothy, mucopurulent, and purulent) and a chest x-ray, if available. Treatment includes regular chest percussion, broad-spectrum antibiotics for exacerbations, and influenza and pneumococcal vaccinations.
TABLE 1-4.
Causes of Hemoptysis
| Worldwide | Tropical countries |
|---|---|
| Bronchiectasis | Tuberculosis |
| Bronchogenic carcinoma | Bronchiectasis |
| Chronic bronchitis | Paragonimiasis |
| Congestive heart failure | Melioidosis |
| Blood diseases | Leptospirosis |
| Tuberculosis | Hydatid disease |
| Endemic mycosis | Endemic mycosis |
Box 1.2. Key Facts: Bronchiectasis.
-
•
Dilatation and destruction of bronchi
-
•
Cough, sputum, crackles, clubbing
-
•
Chest x-ray: increased markings, honeycombing
-
•
High-resolution CT scan: honeycombing, cysts, ring shadows
-
•
Complications: hemoptysis, cor pulmonale, amyloidosis
-
•
Treatment: antibiotics, surgery; prevention
FIGURE 1.5.

Computed tomography of the chest: cystic bronchiectasis.
The incidence of asthma in the tropics is low for unclear reasons; however, the disease remains underdiagnosed and untreated. “All that wheezes is not asthma” is a dictum that is true in the tropics, as there are many entities that cause wheezing and difficulty in breathing, including tropical eosinophilia and mitral stenosis. Asthma monitoring in the tropics can be achieved by using an inexpensive peak flow meter. Treatment should fit the frequency and severity of attacks. Beta-agonists and cromolyn sodium (sodium cromoglycate) are usually available. Oral corticosteroids in short courses can be used to control severe episodes; however, long-term use of systemic corticosteroids, without adequate monitoring, is not safe. Aerosol inhalers are of great value but they are expensive, difficult to use, and require painstaking teaching.
Chronic obstructive lung disease is a progressive disease which is characterized by airway obstruction that is only partially reversible by bronchodilator therapy. The term COPD encompasses chronic bronchitis and emphysema. Once a common disease of men, COPD is now as frequent in women because of increased tobacco use and the widespread use of dung and biomass for indoor cooking and heating in low-income countries (Box 1.3 ). The most common symptoms are dyspnea and chronic cough. The onset of dyspnea is insidious; at first it is mild and occurs only on heavy exertion. With progression of airway obstruction, patients become more short of breath and eventually cannot breathe at rest. Physical examination in the early stage is normal, but in advanced disease, prolonged expiration and expiratory wheezes are audible. In severe cases, the thoracic cage becomes barrel-shaped with increased anterior–posterior diameter; percussion note is hyperresonant. When chest x-ray and pulmonary function testing are not available, a peak-flow meter is an inexpensive device to assess severity of airway obstruction and monitor the response to treatment.
Box 1.3. Key Facts: COPD.
-
•
COPD is progressive obstructive lung disease
-
•
An estimated 210 million people have COPD worldwide and more than 3 million people die each year of COPD
-
•
90% of COPD deaths are in low- and middle-income countries
-
•
The primary cause of COPD is smoking
-
•
COPD affects men and women equally
-
•
COPD is not curable but can be prevented
Cessation of smoking is essential. Oral theophylline and beta-agonist drugs control symptoms. Antibiotics (ampicillin, tetracycline and sulfa drugs) are available to treat COPD exacerbations in the tropics.
Pleural Effusion
Pleural effusion is a frequent condition with variable clinical signs and symptoms. Small effusions can remain silent and are often detected only on chest radiography. Large effusions are associated with dyspnea and diminished chest movements on the affected side. Vocal fremitus is reduced; percussion note is stony dull; and auscultation reveals diminished breath sounds and decreased vocal resonance. Sometimes, bronchial breathing is heard at the upper level of dullness. In addition there may be a pleural friction sound.
If possible, all but the smallest effusions should be tapped. It should be established whether the fluid is serous, bloody, pus or chylous. The effusion can be further divided into transudative and exudative, according to pleural fluid characterization (Table 1-5 ). Laboratory tests that can guide the management of a pleural effusion are macroscopic appearance (Table 1-6 ), pleural fluid cell counts, biochemistry, pH and Gram stain. A simple test is centrifugation of the fluid. If an originally “milky” fluid clears with that process, it is presumably an empyema. If not, it is either a chylothorax (pleural fluid triglycerides >110 mg/dL, e.g. lymphoma, post thoracic surgery) or a cholesterol effusion (pleural fluid cholesterol >200 mg/dL).
TABLE 1-5.
Common Causes of Pleural Effusion
| Worldwide | Tropical countries |
|---|---|
| Heart failure | Tuberculosis |
| Cancer | Paragonimiasis |
| Pulmonary embolism | Cryptococcosis |
| Hepatic cirrhosis | Histoplasmosis |
| Tuberculosis | Lung cancer |
TABLE 1-6.
Diagnostic Appearances of Pleural Fluid
| Appearance | Disease |
|---|---|
| Pale, straw-colored | Tuberculosis, transudate |
| Blood-tinged/frank blood | Trauma, cancer, pulmonary infarct |
| Pus | Empyema |
| Anchovy sauce | Amebiasis |
| Milky/chylous/white | Filariasis, lymphoma, lymphatic abnormality, cholesterol effusion |
Transudative pleural effusions occur in heart failure, liver disease, endomyocardial fibrosis, hypoproteinemia/malnutrition and hypothyroidism. The pleural fluid white blood cell count is typically <10,000 cells/mm3, the pH >7.2, protein <3.0 g/L, the LDH <200 IU/L and the glucose ≥60 mg/dL. A bloody effusion is caused by hemothorax, trauma, malignancy and pulmonary embolism.
Exudative effusions typically have cell counts, protein and biochemical markers opposite to those of transudates. Exudates can be further classified into neutrophilic, lymphocytic and eosinophilic. Neutrophilic exudates may be due to bacterial infection, gastrointestinal diseases, pulmonary embolism, collagen-vascular diseases (CVD) and asbestos-related benign effusion. Pleural effusion occurs in about 50% cases of pneumonia, and can progress to a complicated effusion (pleural fluid pH<7.2, positive Gram stain) or to an empyema, both necessitating pleural fluid drainage with a chest tube thoracostomy in addition to antibiotic treatment. Empyema can occur in pneumococcal, staphylococcal (most often) and Klebsiella infections. A right-sided pleural effusion may be associated with amebic liver abscess.
The disease presenting with the highest pleural fluid lymphocytosis is tuberculous pleuritis; however, early in the course, there can be a neutrophilic exudate. A large volume of pleural fluid should be obtained for examination for acid-fast bacilli. In about one-third of cases, the tuberculin skin test is negative initially and converts to positive after 2–4 weeks. Knowledge of the HIV status of a patient with pleural effusion, if positive, significantly inclines to a tuberculosis.
An eosinophilic exudate is more common in the tropics. Endemic parasitic and fungal infections are major causes of such an effusion. Ascariasis, echinococcosis and paragonimiasis are some of the causative parasitic infections. Paragonimiasis is associated with low pleural fluid glucose and low pH. Fungal diseases responsible for such an effusion are histoplasmosis, cryptococcosis and coccidioidomycosis.
Nontuberculous Granulomatous Lung Disease
In the absence of chest x-ray or biopsy evidence, it is not possible to diagnose pulmonary involvement due to sarcoidosis and other granulomatous diseases. Consequently, in the tropics, these disorders remain undiagnosed. The possibility of sarcoidosis should be considered in a patient with dyspnea, uveitis, hepatosplenomegaly, peripheral lymphadenopathy, chronic skin lesions, and a chest x-ray film showing bilateral hilar adenopathy [18].
Occupational and Dust Lung Diseases
The occupational disorders result from human social activity, and as such are preventable. The dusts that provoke occupational disorders can be classified into: those that induce granulomatous reaction (e.g. beryllium, talc and organic antigens); those that cause fibrosis (e.g. silica, asbestos and coal); and those that cause neither inflammation nor fibrosis, thus remaining inert (e.g. iron, barium and tin) (Table 1-7 ).
TABLE 1-7.
Poorly Recognized Occupational Disorders in the Tropics
| Disease | Antigen | Distribution |
|---|---|---|
| Silicosis | Silica | Widespread |
| Asbestosis, mesothelioma | Asbestos fibers | Widespread |
| Byssinosis | Cotton dust | Asia, Africa |
| Bagassosis | Sugar cane | Americas, Cuba, India |
| Hypersensitivity pneumonitis | Grain dust, vegetable matter | Widespread |
| COPD | Animal dung, biomass fuels | India, Africa, South America |
Podoconiosis is an endemic nonfilarial elephantiasis occurring in individuals exposed to red clay soil derived from alkaline rock. A chronic and debilitating disease, it exerts a large economic burden. The silica particles are found in the skin, lymph nodes and lymphatics of affected and unaffected individuals. These individuals have reduced lung function as compared with adults living in areas of low silica concentration [19].
References
- 1.Zumla A, James D. Immunological aspects of tropical lung disease. Clin Chest Med. 2002;23:283–308. doi: 10.1016/s0272-5231(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 2.UNICEF/WHO . UNICEF/WHO; Geneva: 2006. Pneumonia: the forgotten killer of children. [Google Scholar]
- 3.Vijayan V. Parasitic lung infections. Curr Opin Pulm Med. 2009;15:274–282. doi: 10.1097/MCP.0b013e328326f3f8. [DOI] [PubMed] [Google Scholar]
- 4.Charoenratanakul S. Tropical infections and the lung. Arch Chest Dis. 1977;52:376–379. [PubMed] [Google Scholar]
- 5.Steinoff M. Pulmonary disease. In: Strickland GT, editor. Hunter's Tropical Medicine. 7th edn. WB Saunders; Philadelphia: 1991. pp. 1–7. [Google Scholar]
- 6.World Health Organization, Family and Community Health Cluster, Department of Child and Adolescent Health and Development . WHO; Geneva: 2003. Consultative meeting to review evidence and research priorities in the management of acute respiratory infections (ARI). Meeting report. 1–30. [Google Scholar]
- 7.Metlay J, Schults R, Li Y. Influence of age on symptoms and presentation in patients with community acquired pneumonia. Arch Intern Med. 1997;157:112–124. [PubMed] [Google Scholar]
- 8.WHO/UNICEF . WHO; Geneva: 2009. Integrated Management of Childhood Illness (IMCI) for high HIV settings. [Google Scholar]
- 9.Catchup Study Group Clinical efficacy of co-trimoxazole versus amoxicillin twice daily for treatment of pneumonia: a randomised controlled clinical trial in Pakistan. Arch Dis Child. 2002;86:113–118. doi: 10.1136/adc.86.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shann F, Barker J, Poore P. Chloramphenicol alone versus chloramphenicol plus penicillin for severe pneumonia in children. Lancet. 1985;2:684–686. doi: 10.1016/s0140-6736(85)92928-9. [DOI] [PubMed] [Google Scholar]
- 11.Duke T, Poka H, Dale F. Chloramphenicol versus benzylpenicillin and gentamicin for the treatment of severe pneumonia in children in Papua New Guinea: a randomised trial. Lancet. 2002;359:474–480. doi: 10.1016/S0140-6736(02)07677-8. [DOI] [PubMed] [Google Scholar]
- 12.Martin G. Approach to the patient with tropical pulmonary disease. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens and Practice. Churchill Livingstone Elsevier; Philadelphia: 2006. pp. 1544–1553. [Google Scholar]
- 13.Carvalho CRR, Bethlem EP. Pulmonary complications of leptospirosis. Clin Chest Med. 2002;23:469–478. doi: 10.1016/s0272-5231(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 14.Currie BJ, Fisher DA, Howard DM. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 2000;74:121–127. doi: 10.1016/s0001-706x(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 15.Kronman K, Truett A, Hale B, Crum-Cianfione N. Melioidosis after brief exposure: a serological survey in US Marines. Am J Trop Med Hyg. 2009;80:182–184. [PMC free article] [PubMed] [Google Scholar]
- 16.Magalhaes C, Vasconcelos P, Pereira M. Kawasaki disease: a clinical and epidemiological study of 70 children in Brazil. Trop Doct. 2009;39:99–101. doi: 10.1258/td.2008.080124. [DOI] [PubMed] [Google Scholar]
- 17.Luna C, Faure C. Common tropical pneumonias. In: Sharma OP, editor. Lung Biology in Health and Disease: Tropical Lung Disease. 2nd edn. Vol. 211. Taylor & Francis; New York: 2006. pp. 117–142. (Lung Biology in Health and Disease: Tropical Lung Disease). [Google Scholar]
- 18.Mihailovic-Vucinic V, Sharma O. Tropical granulomas: diagnosis. In: Sharma OP, editor. Lung Biology in Health and Disease: Tropical Lung Disease. 2nd edn. Vol. 211. Taylor & Francis; New York: 2006. pp. 173–193. (Lung Biology in Health and Disease: Tropical Lung Disease). [Google Scholar]
- 19.Morrison C, Davey G. Assessment of respiratory function in patients with podoconiosis. Trans R Soc Trop Med Hyg. 2009;103:315–317. doi: 10.1016/j.trstmh.2008.10.021. [DOI] [PubMed] [Google Scholar]


