![]()
Definition
Zoonoses are classically, though not without controversy, defined as infections or diseases naturally transmitted between humans and vertebrate animals. There are approximately 1400 human pathogens, of which about 800 species are zoonotic. Of the approximate 180 emerging or reemerging pathogens in the past 3 decades, 130 are known to be zoonotic, with a disproportionate number of the new zoonoses being caused by RNA viruses. As a recent editorial states, this is a microbial world that humans are barely allowed to live in, and nothing microbes do or become should surprise us. We have little ability to predict how an emerging zoonotic pathogen will act. Most zoonotic pathogens, like rabies, enter a human and cause disease, but in these cases, humans are dead-end hosts and are unlikely to transmit the disease to others. Less commonly, pathogens such as human immunodeficiency virus (HIV) or recombinant swine H1N1 enter the human population with great difficulty but are then successfully transmitted to cause global epidemics solely from human-to-human transfer. The recent emphasis on emerging infections, which are predominantly zoonoses, and the potential for new pandemics markedly enhance the need for human medicine to be integrated with veterinary medicine and veterinary research.
Epidemiology
During the course of evolution, humans have sought the company of vertebrates for many reasons, including their companionship as pets. The more than 100 million dogs and cats in the United States facilitate the transmission of more than 250 different infectious species and cause more than 1 million bite injuries each year.
Almost all arthropod-transmitted infectious agents acquired from vertebrates in the United States are due to either ticks or mosquitoes. Ticks are the more common villain, and Lyme disease is the most common arthropod-transmitted infectious disease in the United States. Not all arthropod-transmitted diseases are zoonotic. For instance, arthropod-transmitted infections such as West Nile fever, Schistosoma japonicum, and Trypanosoma rhodesiense are zoonoses, whereas Schistosoma mansoni, Trypanosoma gambiense, Wuchereria bancrofti, Onchocerca volvulus, and vivax malaria are not; these non-zoonoses cause more than a billion infections per year, are transferred from human to human, and do not depend on vertebrate reservoirs. Human infestations with ectoparasites are not considered zoonoses.
The risk of contracting a zoonosis is increased by direct animal contact; in meat handlers, poachers, hunters, exotic animal smugglers, and international travelers; by exposure to and inhalation of infectious air particles; by insect bites, contact with previously infected human blood products, and contact with and ingestion of infectious agents transmitted by animal-contaminated water; and by insufficiently cooked meat, eggs, dairy products, and fish. Raw shellfish, which are the “garbage filters” of the ocean, can transmit at least 25 different infectious or toxic diseases to humans, but shellfish are invertebrates, and such diseases are not zoonoses. Ticks can introduce more than one pathogen while feeding; Lyme disease, ehrlichiosis, and babesiosis can all be transmitted by a single tick bite. Threats from mosquito bites include St. Louis encephalitis and West Nile virus. Patrons of petting zoos, pet owners, farmers, hunters, laboratory researchers, cave explorers, hikers, and veterinarians, among others, are at higher risk for a zoonosis than is the general population. Infectious agents transmitted by these routes from animal sources essentially include members of all microbial classes: viruses and prions, bacteria and rickettsia, fungi, helminths, and protozoa. Immunocompromised hosts, such as splenectomized patients, transplant recipients, patients with acquired immunodeficiency syndrome (AIDS), and pregnant women and their fetuses, are at higher risk for clinical disease when exposed to these various infectious agents. Chagas’ disease, a protozoal infection, is a zoonosis in South America but is not endemic to the United States; however, immigration from Latin America has led to an estimated 300,000 persons with Trypanosoma cruzi infection, usually subclinical, in the United States and the potential for transmission of the infection through blood or organ donations. Leishmania donovani is almost always a small-animal reservoir zoonosis, but in India, it has set up shop with just human-to-human insect transfer.
As the world shrinks, warms, flattens, and remains in conflict, new infectious diseases seem inevitable, and previously rare ones are seen in unexpected places (Table 336-1 ) because of global warming, human intrusion into previously underexplored or never-explored sites, world travel, deforestation, and the increasing threat of biologic terrorism or warfare. Increasing urbanization, intermingling of humans and domestic animals, and lack of adequate sanitation can dramatically increase the incidence and severity of epidemic infectious disease.
TABLE 336-1.
NEWER ZOONOSES IN THE UNITED STATES (EMERGING INFECTIONS)
| DISEASE* | INFECTIOUS AGENT | CLINICAL FINDINGS | VECTOR/ACQUISITION |
|---|---|---|---|
| Ehrlichiosis, monocytic | Ehrlichia chaffeensis | Fever, myalgia, leukopenia, monocyte inclusions not often seen, maculopapular rash in a minority | Amblyomma (Lone Star) tick bite |
| Human granulocytic anaplasmosis (HGE) | Anaplasma phagocytophilium | Fever, myalgia, leukopenia, granulocyte inclusions often seen on blood smear | Ixodes (deer) tick bite |
| “Flu syndrome” | Ehrlichia ewingii | Immunocompromised host—fever, myalgia | Amblyomma |
| Cat-scratch disease | Bartonella spp (there are 8 identified zoonotic Bartonella spp) | Cervical lymphadenopathy in normal hosts and cutaneous and hepatic angiomatosis in AIDS patients | Cat scratch or bite |
| Hemorrhagic diarrhea | Enterohemorrhagic Escherichia coli O157:H7 (other species) | Rectal bleeding, dysentery, hemolytic-uremic syndrome | Contaminated, undercooked meat |
| Hantavirus pulmonary syndrome | Hantavirus—Sin Nombre | Noncardiac pulmonary edema, elevated hematocrit | Fomites of wild rodents |
| Cryptosporidium diarrhea | Cryptosporidium parvum | Prolonged watery diarrhea | Contaminated water—cattle and sheep |
| Dysentery | Campylobacter jejuni | Dysentery, reactive arthritis, Guillain-Barré syndrome | Contaminated chicken |
| Pyogenic skin ulcer | Capnocytophaga canimorsus | Sepsis, skin infection | Dog bites |
| West Nile fever encephalitis | West Nile virus | Encephalitis, myelitis, Guillain-Barré syndrome | Mosquito bite, birds |
| SARS | Coronavirus | Severe lower respiratory tract infection | Masked palm civet (presumed) |
| Cutaneous leishmaniasis | Leishmania mexicana | Skin nodules and ulcers | Wood rat |
AIDS = acquired immunodeficiency syndrome; SARS = severe acute respiratory syndrome.
See table of contents and index to locate a more detailed discussion of each disease.
Clinical Manifestations
Zoonoses can manifest as a variety of clinical syndromes, including respiratory disease (Table 336-2 ), central nervous system disease (Table 336-3 ), and rash or skin lesions (Table 336-4 ). Other zoonotic clinical syndromes may present as sepsis, polyarthritis, jaundice, acute renal failure, endocarditis, or diarrhea (Table 336-5 ).
TABLE 336-2.
RESPIRATORY TRACT ZOONOSES
| DISEASE* | MICROORGANISM† | CLINICAL FINDINGS | RESERVOIR AND/OR VECTOR |
|---|---|---|---|
| Psittacosis‡ | Chlamydophila psittaci | Pneumonia, often severe | Aerosols from parrots, ducks, turkeys |
| Q fever | Coxiella burnetii | Pneumonia, hepatitis, myocarditis | Airborne from soil contaminated by sheep, goats, and cats, particularly if parturient |
| Tularemia | Francisella tularensis | Cutaneous ulcer and regional node, pneumonia and hilar node; pleural effusion | Rabbit contact (winter) and tick bites |
| Plague | Yersinia pestis | Inguinal nodes, bubonic plague (basilar pneumonia develops in 10%); hilar node enlargement | Fleas from prairie dogs, rock squirrels, rats |
| Hantavirus syndrome | Hantavirus | Upper respiratory to lower respiratory to adult respiratory distress syndrome (ARDS) to death | Deer mouse fomites: urine, feces, saliva |
| Rhodococcus pneumonia | Rhodococcus equi | Pneumonia often cavitates in those with AIDS and other immunosuppressed patients | Horse manure, soil |
| Mycoplasma arginini pneumonia | Mycoplasma arginini | Pneumonia, sepsis, neutropenia | Sheep, goats |
| Foot-and-mouth disease | Aphthovirus | Nonspecific upper respiratory tract infection, oral vesicles | Cloven-footed mammals |
| Whooping cough | Bordetella bronchiseptica | Pneumonia, bronchitis, whooping cough | Dogs |
| Histoplasmosis | Histoplasma capsulatum | Pneumonia or fever of unknown origin | Bats |
| Anthrax | Bacillus anthracis | Mediastinal widening with CT scan; pneumonia often absent | Herbivore mammals |
| Glanders | Burkholderia mallei | Pneumonia, erosive tracheobronchitis | Horses, mules |
AIDS = acquired immunodeficiency syndrome; CT = computed tomography.
See table of contents and index to locate a more detailed discussion of each disease.
Because of the fastidious nature of some organisms, the rapid development of diagnostic tools, and the risk some agents pose to laboratory workers, a clinical microbiologist should be consulted if these agents are considered in a patient's differential diagnosis.
Occurs in more than 1000 animal species.
TABLE 336-3.
CENTRAL NERVOUS SYSTEM ZOONOSES
| DISEASE* | ORGANISM | CLINICAL FINDINGS | ACQUISITION |
|---|---|---|---|
| Listeriosis | Listeria monocytogenes | Purulent meningitis during pregnancy, in patients older than 65 yr, in neonates, and in immunosuppressed | Unpasteurized cheese and other dairy products; cattle, goats |
| Leptospirosis | Leptospira interrogans | Aseptic meningitis, hepatorenal syndrome | Asymptomatic dogs, cattle; common water source |
| Herpes B encephalitis | Cercopithecine herpesvirus (herpes B virus) | Diffuse, progressive encephalitis | Macaca monkey bites or scratches |
| Lyme disease | Borrelia burgdorferi | Lymphocytic meningitis, motor-sensory neuropathy, facial palsy | Tick bite |
| Lymphocytic choriomeningitis | Lymphocytic choriomeningitis virus | Lymphocytic meningitis, occasionally with pneumonia | Inhalation of mouse secretions: urine, feces, saliva |
| Mosquito-borne encephalitis (U.S.) | Eastern, western equine, St. Louis, California encephalitis; West Nile virus | Diffuse encephalitis, least severe; California encephalitis, most severe; eastern equine encephalitis, myelitis, meningitis | Mosquito-borne from horses, birds |
| Rabies encephalitis | Rabies virus | Encephalitis; almost always fatal | Bites from dogs, skunks, bats, raccoons, foxes |
| Toxoplasmosis | Toxoplasma gondii | Multiple brain masses in AIDS patient | Cat feces or ingestion of undercooked lamb or pork |
| Cerebral cysticercosis | Taenia solium | Epilepsy, CNS cysts, eosinophilic meningitis, hydrocephalus | Fecal-oral; contamination of food with pork tapeworm eggs |
| New-variant Creutzfeldt-Jakob disease | Prion (proteinaceous infectious particle) | Dementia, ataxia, myoclonus | Beef from cattle fed scraps from contaminated sheep carcasses |
| Nipah virus | Paramyxovirus | Acute encephalitis, death | Pig contact |
| Hendra virus | Paramyxovirus | Pneumonitis, encephalitis | Horses, fruit bats |
AIDS = acquired immunodeficiency syndrome; CNS = central nervous system.
See table of contents and index to locate a more detailed discussion of each disease.
TABLE 336-4.
ZOONOSES CAUSING RASH OR SKIN NODULE-ULCER
| DISEASE* | MICROORGANISM† | CLINICAL FINDINGS | RESERVOIR AND/OR VECTOR |
|---|---|---|---|
| Ehrlichiosis (monocytic) | Ehrlichia chaffeensis | Macular rash (seen in <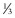 of patients) with a central distribution; prevalent in south-central U.S. of patients) with a central distribution; prevalent in south-central U.S. |
Tick bite |
| Leptospirosis | Leptospira interrogans | Central macular rash in 20%, with occasional enanthem, conjunctival suffusion, hepatorenal syndrome | Urine-contaminated water; dogs, cattle |
| Lyme disease | Borrelia burgdorferi | Erythema migrans; 20% have multiple lesions | Mouse reservoir—tick bite |
| Rocky Mountain spotted fever | Rickettsia rickettsii | Acral or peripheral distribution of maculopapular to hemorrhagic rash to gangrenous lesions—no eschar | Tick bite |
| Typhus (epidemic) | Rickettsia prowazekii | Macular rash with a central distribution (can be hemorrhagic) | Flying squirrel fleas or fomites |
| Cat-scratch disease | Bartonella spp | Bacillary angiomatosis, peliosis hepatis in HIV patients, cervical adenopathy, subacute bacterial endocarditis, fever of unknown origin | Cat scratch or bite |
| Tularemia | Francisella tularensis | Ulcer and node, typhoidal syndrome, pneumonia | Rabbit contact and tick bite |
| Anthrax | Bacillus anthracis | Painless, edematous, nonpurulent ulcer that becomes black and necrotic over days; mediastinitis | Herbivore animal products, including animal hides |
| Rickettsialpox (spotted fever Rickettsia) | Rickettsia akari, Rickettsia conorii, Rickettsia africae (8 others found on all continents) | Fever, rash, eschar (multiple), tache noire (R. africae, often multiple) | Mouse mite, tick |
| Monkeypox | Orthopoxvirus | Multiple maculopustular lesions with lymphadenopathy | Prairie dog (U.S.), African rodents |
| Erysipeloid | Erysipelothrix rhusiopathiae | Red, tender, swollen finger; subacute bacterial endocarditis | Skin pricked while cleaning fish, domestic meat animals |
| Seal finger | ? | Tender, swollen fingertip | Seal bite? |
See table of contents and index to locate a more detailed discussion of each disease.
Because of the fastidious nature of some organisms, the rapid development of diagnostic tools, and the risk some agents pose to laboratory workers, a clinical microbiologist should be consulted if these agents are considered in a patient's differential diagnosis.
TABLE 336-5.
OTHER ZOONOTIC CLINICAL SYNDROMES
| DISEASE* | ORGANISM |
|---|---|
| Sepsis syndrome | Streptococcus suis, Capnocytophaga canimorsus, Yersinia enterocolitica, Pasteurella multocida, Rickettsia rickettsii, Streptobacillus moniliformus, Rickettsia conorii, Leptospira icterohaemorrhagiae |
| Polyarthritis | Streptobacillus moniliformus, Borrelia burgdorferi, Spirillum minus, Brucella spp, alpha virus—six groups (Ross River, Sindbis, Mayaro, chikungunya, o’nyong-nyong, igbo-ora) |
| Jaundice | Coxiella burnetii, Leptospira icterohaemorrhagiae, yellow fever (sylvatic), hepatitis E, echinococcosis, Fasciola hepatica |
| Renal failure (acute) | Leptospira, Hantavirus, Rickettsia rickettsii, Rickettsia conorii, Escherichia coli O157 |
| Endocarditis | Bartonella, Coxiella burnetii, Erysipelothrix rhusiopathiae, Brucella, Streptococcus suis, Listeria monocytogenes, Chlamydia psittaci |
| Diarrhea | Salmonella, Campylobacter jejuni, Shiga toxin–positive E. coli, Giardia intestinalis, cryptosporidia, Yersinia pseudotuberculosis, Yersinia enterocolitica |
See table of contents and index to locate a more detailed discussion of each disease.
Emerging zoonotic infectious diseases include Nipah virus and Hendra virus encephalitis, Hantavirus pneumonia and Hantavirus fever with renal failure, the coronavirus that causes severe acute respiratory syndrome (SARS), West Nile encephalitis (which spread coast to coast in the United States over 3 years and is now apparently a permanent resident), monkeypox (an outbreak in the upper Midwest was caused by pet prairie dogs), Leishmania mexicana in Texas, and at least 8 to 10 newly identified tick-borne rickettsial spotted fevers worldwide, including the flea-borne Rickettsia felis in California. Avian or “bird flu” (H5N1), SARS, monkeypox, H1N1 (swine) influenza, and West Nile fever have recently exposed the fragility of our world village. Nevertheless, exotic zoonoses remain a less frequent cause of fever in travelers than the well-known, ordinary gastrointestinal and pulmonary pathogens.
Diagnosis
Non–animal-associated environment- or travel-related infectious diseases can be confused with zoonoses. The vast majority of clinical diseases caused by Legionella pneumophila, Entamoeba histolytica, Giardia lamblia, Burkholderia pseudomallei, Chromobacterium violaceum, Aeromonas hydrophila, and airborne fungi such as Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum are acquired through environmental exposure and are only rarely related to animal hosts. Sporothrix schenckii, almost always an environmentally acquired pathogen stemming from vegetation-related injuries, has also been transmitted from cats with draining cutaneous ulcers to owners and animal handlers. Histoplasmosis has been acquired by explorers (spelunkers) in caves contaminated by bat guano. Other noninfectious toxin-induced diseases acquired from animals and insects, such as tick paralysis and the fish toxin illnesses, do not represent zoonoses.
Unfortunately, some descriptive disease titles can be misleading to clinicians and interfere with reaching the correct diagnosis. The transmission of tick-borne Rocky Mountain spotted fever actually occurs much more commonly in the southeastern United States than in the Rocky Mountains and has even occurred in the middle of New York City. Urban New York City continues to be a major source of rickettsialpox, where it was first described 60 years ago. Vegetarians and other strict non–pork-eating persons have been seriously infected with the pig tapeworm Taenia solium as a result of fecal contamination of food from infected human food-handlers.
Until recently, human influenza A was not well recognized as a zoonosis; however, interspecies spread and mixing of swine, avian (H5N1 virus), and human influenza viruses can occur in unique geographic areas, such as southern China or Mexico (2009 novel H1N1 virus), where dense concentrations of ducks, pigs, and people cohabit. Viral incubation of the three influenza species in the pig, with a reassortment of antigens and subsequent spread of virulent “new” influenza strains to humans, can lead to massive influenza pandemics that in sheer numbers (billions) surpass any past epidemics of smallpox or plague. Initially, HIV-1 and HIV-2 were transmitted as simian immunodeficiency viruses from chimpanzees and mangabey primates, respectively, to humans. As with influenza, the subsequent 33 million (and counting) cases of HIV no longer require an animal reservoir for continuing transmission. Because these pandemics of AIDS and influenza now spread human to human without the help of the initiating animal host, the World Health Organization no longer considers them zoonoses.
Leprosy, an illness of biblical notoriety transmitted from human to human, is endemic in at least three animal species, including the armadillo. This animal has rarely been implicated in the transmission of leprosy to humans in the United States.
Despite the large number of zoonoses described, clinicians evaluating an individual patient usually need to consider only a limited number of historical details to arrive at an appropriate differential diagnosis:
-
1
A history of direct contact with animals or animal products, animal bites, arthropod exposure, and food ingestion may offer clues to the correct cause.
-
2
The patient's travel history must be considered because a number of zoonoses are still quite limited in geographic distribution.
-
3
Occupational and recreational high-risk activities must be ascertained.
-
4
The patient's clinical manifestations (course and organ involvement) are used to focus on the most likely cause (see TABLE 336-2, TABLE 336-3, TABLE 336-4, TABLE 336-5).
-
5
Though neither specific nor sensitive, the inoculation nodule (eschar, tache noire, chancre, necrotic ulcer), with or without fever, rash, or local lymphadenopathy, is the only general clue from the physical examination that might alert the physician to a zoonosis when the history does not.
-
6
Significant unilateral hilar adenopathy in the presence of acute pneumonia can be a clue to anthrax, plague, or tularemia.
Treatment  .
.
The approach to treatment of the various zoonoses is presented in their respective chapters.
Prevention
Guidelines have been published to help prevent nosocomial transmission of zoonotic diseases. Preventive measures to decrease infection in compromised hosts include the routine immunization of pets, neutering of pets, use of caution when handling pet fomites, rigorous handwashing practices, and avoidance of ingesting undercooked meat, fish, shellfish, and eggs.
Isolation is recommended for anthrax, Andes Hantavirus disease, herpes B, monkeypox, Q fever, rabies, plague, SARS, influenza, and the hemorrhagic fever illnesses caused by Argentine, Bolivian, Crimean-Congo, Ebola, Lassa, and Marburg viruses.
Prognosis
The prognosis of zoonoses varies widely, but a number of these disease have very high case-fatality rates (Table 336-6 ).
TABLE 336-6.
HIGHLY FATAL ZOONOSES
| DISEASE* | FATALITY RATE (%) |
|---|---|
| Creutzfeldt-Jakob disease (new variant) | 100 |
| Rabies | 100 |
| Anthrax, inhalational | 80-90 |
| Herpes simiae | 50-75† |
| Ebola virus | 70 |
| Eastern equine encephalitis | 50-70 |
| Hantavirus pulmonary syndrome (U.S.) | 60‡ |
| H5N1 influenza | 15-50 |
| Yellow fever (sylvatic) | 20-50§ |
| Lassa fever | 15-25§ |
| Plague | 50-80† |
| Rocky Mountain spotted fever | 20-60† |
| East African sleeping sickness | 20-30† |
| Anthrax, cutaneous | 20† |
| Tularemia, pneumonic | 30-60† |
| Tularemia, cutaneous | 2-10 |
| Visceral leishmaniasis | 5-25† |
| Louse-borne relapsing fever | 5-40† |
See table of contents and index to locate a more detailed discussion of each disease.
If untreated.
Case mortality of hospitalized patients.
If jaundiced.
Suggested Readings
- Akritidis N. Parasitic, fungal and prion zoonoses: an expanding universe of candidates for human disease. Clin Microbiol Infect. 2011;17:331–335. doi: 10.1111/j.1469-0691.2010.03442.x. Review. [DOI] [PubMed] [Google Scholar]
- Altizer S, Bartel R, Han BA. Animal migration and infectious disease risk. Science. 2011;331:296–302. doi: 10.1126/science.1194694. Review. [DOI] [PubMed] [Google Scholar]
- Christou L. The global burden of bacterial and viral zoonotic infections. Clin Microbiol Infect. 2011;17:326–330. doi: 10.1111/j.1469-0691.2010.03441.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional Suggested Readings
- Kahn RE, Clouser DF, Richt JA. Emerging infections: a tribute to the one medicine, one health concept. Zoonoses Public Health. 2009;56:407–428. doi: 10.1111/j.1863-2378.2009.01255.x. Symposium summary of the new research models of animal-to-human transfer of emerging and reemerging infections and the potential for interhuman epidemic spread. [DOI] [PubMed] [Google Scholar]
- Woolhouse M, Gaunt E. Ecological origins of novel human pathogens. Crit Rev Microbiol. 2007;33:231–242. doi: 10.1080/10408410701647560. Overview of the interaction between the environment and animals and the processes that can lead to the emergence of new human pathogens. [DOI] [PubMed] [Google Scholar]


