INTRODUCTION
Respiratory viral infections are among the most common diseases of humans. These illnesses are caused by a heterogeneous group of viruses commonly known as human respiratory viruses (RVs). Over the last decade new molecular diagnostics have expanded the range of viruses detected in respiratory samples and several novel RVs have been identified. Real-time polymerase chain reaction (PCR) has been established as a powerful tool to diagnose and quantify RVs. The characteristic illnesses associated with the different respiratory viruses and the populations at higher risk are presented in Table 162.1 . Other viruses, such as some of the enteroviruses, influenza viruses and herpesviruses, can also cause respiratory disease but they are discussed in separate chapters.
Table 162.1.
Human respiratory viruses: characteristic illnesses and populations at higher risk
| Virus | Associated illnesses [most common virus subtype] | Patient population at higher risk |
|---|---|---|
| Adenovirus | URI [1–3, 5–7] Pharyngoconjunctival fever [3, 7, 14] URI, pneumonia [3, 4, 7, 14, 21] Pertussis-like syndrome [5] Pneumonia [1, 2, 3, 4, 7] |
Infants, young children School-aged children Military recruits Infants, young children Infants, young children, immunocompromised |
| Coronavirus | Common cold Otitis Pneumonia Exacerbation of asthma-COPD Severe acute respiratory syndrome |
All ages Children Military recruits, elderly, immunocompromised Persons with asthma-COPD All ages |
| Reovirus | Common cold (?) Enteritis (?) |
All ages Children |
| Rhinovirus | Common cold URI Exacerbation of asthma-COPD Pneumonia |
All ages All ages Persons with asthma-COPD Infants, young children, elderly, immunocompromised |
| Respiratory syncytial virus | URI Pneumonia Bronchiolitis Otitis |
All ages Infants, young children, elderly, immunocompromised Infants, young children Children |
| Parainfluenza virus | URI Croup Otitis media Pneumonia |
All ages Children Children Children, immunocompromised |
| Metapneumovirus | URI Pneumonia Bronchiolitis |
All ages Infants, young children, elderly, immunocompromised Infants, young children |
| Human bocavirus | URI (?) Pneumonia (?) |
Children Children |
COPD, chronic obstructive pulmonary disease; URI, upper respiratory tract infection.
Respiratory viral infections are largely benign and self-limited, although newer studies using molecular amplification approaches have highlighted the importance of respiratory viruses as common pathogens in adults with pneumonia. They can exacerbate underlying chronic cardiopulmonary diseases, increase visits to health-care providers and result in unnecessary prescription of antibiotics. Among patients at the extremes of age with underlying cardiopulmonary diseases or those with primary or secondary immunodeficiencies, these illnesses can be associated with serious, potentially fatal complications.
The most common methods for the laboratory diagnosis of viral respiratory infections are summarized in Table 162.2 and Fig. 162.1, Fig. 162.2, Fig. 162.3, Fig. 162.4, Fig. 162.5 .
Table 162.2.
Human respiratory viruses
Most common methods for laboratory diagnosis: culture, direct assay and serology
| Virus | Culture cell lines | Characteristic findings | Direct assay | Serology |
|---|---|---|---|---|
| Adenovirus | Primary human embryonic kidney (HEK) and the human lung carcinoma (A549) cell lines are sensitive for a broad range of adenoviruses. Some adenoviruses may require up to 28 days to grow. The continuous epithelial lines, such as Hep-2 and HeLa, are sensitive but more difficult to maintain for a long period of time as required by some serotypes | Enlarged, refractile, rounded cells forming grape like clusters (Fig. 162.1), with some isolates forming a lattice-type arrangement of rounded cells | IF and EIA | Genus-specific EIA |
| Coronavirus | Culture not performed in most laboratories. SARS-CoV and HCoV-NL63 can be cultured in monkey epithelial cells | Cytocidal coronavirus infections may form multinucleated syncytia, lysis or both | EIA used in research settings | IF, EIA |
| Reovirus | Rarely isolated in the routine diagnostic setting. PRMK, LLC-MK2, HNK, HeLa, MDBK and H292 cell lines may support the growth of this virus | CPE is slow to develop. CPE may be nonspecific with increased granularity and progressive degeneration. HI and IFA can be used for typing | IF used in research settings | CF, NT, EIA used in research settings |
| Rhinovirus | Human embryonic kidney and human fibroblast cell lines have been used most extensively: WI-38, HFF and MRC-5. HeLa is also sensitive | CPE is usually detected within the first week. CPE consists of rounded, highly refractile cells in loose clusters (Fig. 162.2). CPE permits a presumptive diagnosis of picornavirus infection. Demonstration of lability at pH 3 is used for differentiation from enteroviruses | EIA used in research settings | NT, CF, EIA used in research settings |
| Respiratory syncytial virus | Hep-2 is the preferred cell line. Alternatives include HeLa, A549, MRC-5, RhMK and Vero. Calcium and glutamine in the culture medium are important for optimal replication and cytopathic effect | CPE requires 3–7 days or more. Syncytia develop along with nonspecific granular degeneration (Fig. 162.3). Confirmation by means of IF (Fig. 162.3) or EIA | IF (Fig. 162.3), EIA, IP | CF, IF, EIA, NT |
| Parainfluenza virus | PRMK or MDCK are the preferred cell lines. LLC-MK2, a rhesus kidney heteroploid cell line and NCI_H292, a human lung carcinoma line, are also sensitive | CPE is variable and nonspecific. HPIV-2 and HPIV-3 may induce syncytia formation. Growth may be detected by means of HAd (Fig. 162.4) with confirmation and typing (Fig. 162.5), EIA, HAdI or HI | IF, EIA | HI, CF, EIA, NT |
| Metapneumovirus | Many strains replicate in tertiary monkey kidney cells/LLCMK-2 cells. Vero cell clone 118 seems to be permissible for all four lineages | CPE usually occurs after 10–21 days as syncytia formation or rounding of cells | IF | EIA |
| Human bocavirus | No cell culture model available | – | – | – |
CF, complement fixation; CPE, cytopathic effect; EIA, enzyme immunoassay; HAd, hemadsorption inhibition; HAdI, hemadsorption assay; HI, hemagglutination inhibition; IF, immunofluorescence; IP, immunoperoxidase staining; LA, latex agglutination; NT, neutralization test; SARS, severe acute respiratory syndrome; PRMK, primary rhesus monkey kidney.
Fig. 162.1.


Cytopathic effect caused by adenovirus on Hep-2 cell line culture. (a) Uninoculated cell line. (b) Enlarged, refractile, rounded cells forming grape-like clusters.
Fig. 162.2.


Cytopathic effect caused by rhinovirus on human foreskin fibroblasts (HFF) cell line culture. (a) Uninoculated cell line. (b) Formation of small teardrop- to oval-shaped highly refractile cells.
Fig. 162.3.


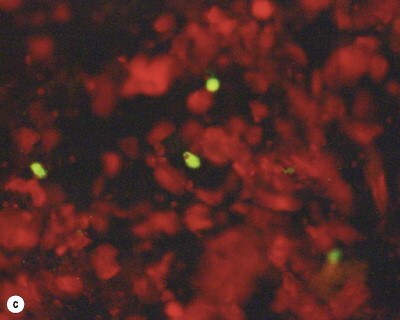
Cytopathic effect of RSV on Hep-2 cell line culture and identification of RSV antigen by means of IFA. (a) Uninoculated cell line. (b) Syncytia formation in cell line culture. (c) Positive cells coloring green under IF microscope.
Fig. 162.4.


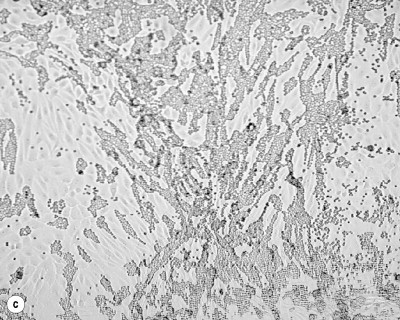
Identification of hemadsorbing viruses. (a) Uninoculated primary rhesus monkey kidney (PRMK) cell line. (b) Nonspecific rounding or clumping of PRMK cells. (c) Positive hemadsorption of guinea-pig red blood cells.
Fig. 162.5.

Differentiation of hemadsorbing viruses by means of IFA. Negative control.
ADENOVIRUS
(See healthmap.org for outbreaks of Adenovirus)
Nature
Adenoviruses were first isolated in the 1950s from human adenoids and later from respiratory secretions of military recruits with acute febrile respiratory illnesses. Human adenoviruses belong to the family Adenoviridae. More than 50 serotypes have been described and are being grouped into six species (A–F). Approximately one-half of these serotypes are known human pathogens. Virions are non-enveloped icosahedral particles measuring 80–100 nm in diameter and containing a linear double-stranded DNA genome. The capsid consists of 252 capsomers, of which 240 are hexons and 12 are pentons (at the vertices). A fiber with a knob at the end projects from every penton.
Epidemiology
Although epidemiologic characteristics of the adenoviruses vary by type, the route of transmission is either via the respiratory route, fecal–oral or water-borne. Infections are most common during early childhood and nearly 100% of adults have serum antibodies against multiple serotypes. Adenoviruses have been found to account for 3–5% of acute respiratory infections in children and up to 2% in adults. Close contact in crowded institutions increases the risk for adenovirus infections and outbreaks have been described in day-care centers, hospitals, shipyards and military quarters. Adenovirus infections can occur throughout the year but outbreaks of adenovirus-associated respiratory disease have been more common in the late winter, spring and early summer.
Pathogenesis
Adenoviruses causing respiratory disease can be spread via contact transmission (direct or indirect) and droplet transmission. Droplet transmission occurs when droplets containing infectious viral particles generated from the infected person are propelled a short distance (<1 meter) and deposited on the host's conjunctivae, nasal mucosa or mouth. Droplet particles (>5 μm diameter) are produced during coughing, sneezing or talking and during the performance of certain procedures such as suctioning and bronchoscopy.
Virions attach to human cells via interaction of the fibers with the CAR receptor (coxsackievirus and adenovirus receptor) on the cell surface and enter the cell via endocytosis. Virus uncoating occurs in the cytoplasm and the virus core is imported into the nucleus. The transcription of viral DNA depends in part on host cell machinery.
The replicative cycle in the host cells may be lytic or may result in the establishment of a latent infection, primarily in lymphoid tissue. The symptoms of adenovirus disease are due to the lysis of infected epithelial cells and the resulting inflammatory response. The initial immune response is probably the recognition of infected cells by natural killer cells and monocytes, which evokes a cytokine response, followed by induction of cytotoxic T cells and B cells. Prolonged shedding of adenoviruses in stools has been recognized even in immunocompetent individuals. Some adenovirus serotypes (especially 12, 18 and 31) can induce oncogenic transformation in cultured cell lines. In recent years, intensive research has focused on the use of adenoviruses as gene vectors.1
Prevention
An effective oral enteric-coated vaccine has been used to prevent adenovirus serotypes 4 and 7 infections in military recruits; however, this vaccine is not used in the general population. Contact and droplet precautions are indicated for controlling nosocomial transmission of adenovirus infection. Contact precautions include the isolation or cohorting of infected patients, the use of personal protective equipment (gloves, gowns), hand washing and the use of dedicated patient care equipment when possible. Droplet precautions include the additional use of masks by health-care workers within the patient's room and the use of masks by the patient when outside the room. Maintaining adequate levels of chlorination is necessary to prevent swimming pool-associated outbreaks of adenovirus conjunctivitis.
Diagnostic microbiology
Virus isolation, antigen detection, PCR assay and serology can be used to identify adenovirus infections. Adenovirus serotyping is usually accomplished by hemagglutination inhibition and/or neutralization with type-specific antisera. The isolation of adenoviruses from body secretions is not sufficient to establish a diagnosis of adenovirus disease as prolonged asymptomatic shedding of adenoviruses can occur. New molecular diagnostics such as quantitative PCR have improved the rapid diagnosis of adenovirus infections and monitoring of viral loads in blood has been shown to correlate with clinical progression of disease.2
Clinical manifestations
Respiratory illnesses associated with adenovirus infections include undifferentiated upper respiratory illness, pharyngoconjunctival fever, pertussis-like syndrome and pneumonia. The various adenovirus and pneumonia. serotypes associated with respiratory disease are shown in Table 162.1. In addition, adenoviruses may also cause gastroenteritis, keratoconjunctivitis, cystitis, meningoencephalitis and hepatitis, depending on the serotype. The incubation period for the endemic serotypes ranges from 5 to 10 days and the symptoms last approximately 1 week. Chronic lung damage in the form of bronchiolitis obliterans has been reported after infections with serotypes 1, 3, 4, 7 and 21.
The incidence of severe adenovirus disease is increasing with the rising number of immunomodulatory treatments used in modern medicine. Pediatric patients are more susceptible to adenovirus infection and disease than adults, probably due to the higher rate of primary infections occurring among children. However, cases of severe adenovirus disease have also been reported in apparently normal hosts. Immunocompromised patients, particularly hematopoietic stem cell transplant (HSCT) and solid-organ transplant recipients, are susceptible to severe and potentially fatal adenovirus disease,3., 4., 5. such as protracted hemorrhagic cystitis, encephalitis, nephritis, hepatitis, enteritis, pneumonia or a combination of these illnesses (disseminated disease).
Management
Most infections are mild and require only symptomatic treatment. Ribavirin has in-vitro activity against several adenovirus serotypes but the clinical experience in immunocompromised individuals has produced conflicting results (see Chapter 147).
CORONAVIRUS
(See healthmap.org for outbreaks of Coronavirus)
Nature
Human coronavirus (HCoV) was first isolated in 1965 from a patient with symptoms of the common cold. Three new coronaviruses have been detected since 2001: the severe acute respiratory syndrome (SARS)-associated coronavirus in 2003, coronavirus NL63 in 2004 and coronavirus HKU1 in 2005. Coronaviruses are classified into three groups: group 1 consists of HCoV-229E and HCoV-NL63, group 2 harbors HCoV-OC43 and HCoV-HKU1, and SARS-CoV is classified independently and is thought to be of animal origin.6 Virions are pleomorphic and measure 80–200 nm in diameter. The outer envelope bears distinctive ‘club-shaped’ peplomers that correspond to the S (long spike) glycoprotein. The resulting ‘crown-like’ appearance under electron microscopy gives the family its name. The S glycoprotein is involved in receptor binding and cell fusion and is a major target for neutralizing antibodies. Other structural proteins include the M (matrix) protein, the E (envelope) protein and the N (nucleocapsid) phosphoprotein. In some types, there is also a glycoprotein HE (hemagglutinin-esterase, short spike). Entry occurs via endocytosis and membrane fusion. Replication of the linear single-stranded positive-sense RNA genome takes place in the cytoplasm.
Epidemiology
HCoVs are ubiquitous. Serologic studies with the initial two coronaviruses OC43 and 229E have suggested that these viruses are a major cause of respiratory disease and account for 10–30% of all common colds. Serologic studies have also suggested that one-half of the infections with coronaviruses are asymptomatic.7 The identification of a coronavirus as the underlying pathogen for SARS was published in 2003 and this was the first time that a coronavirus was definitely demonstrated to cause severe human disease.8, 9 The SARS epidemic was contained through global infection control efforts organized by the World Health Organization (WHO) and currently there is no evidence that SARS coronavirus is active in human populations. More than 8000 cases were reported in the course of the SARS epidemic in 29 affected countries, leading to more than 800 deaths worldwide.10 Since the discovery of SARS two newer coronaviruses HKU1 and NL63 have been identified. These HCoVs are similar to their older cousins in being mostly associated with common cold symptoms but severe clinical disease can be caused in young children, the elderly and in immunocompromised patients.11
Pathogenesis
Intranasal inoculation of volunteers with these viruses results in common colds with disruption of the ciliated epithelium and ciliary dyskinesia.12 Aminopeptidase N (APN), the receptor for HCoV-229E, is expressed on the apical surfaces of respiratory and intestinal epithelium, myelocytic cells, kidney tubular epithelium and synaptic junctions. HCoV-OC43 binds to cells by means of the HE glycoprotein or the viral S glycoprotein, which can recognize 9-O-acetylated sialic acid on the cell surface.
Prevention
No specific antiviral drugs or vaccines are currently available. The major transmission modes for SARS are the contact and/or droplet route, although airborne transmission over a limited distance had been suggested.13 The outbreak of SARS was successfully controlled through early detection of cases by screening of persons with symptoms of a respiratory infection and implementing strict contact and droplet precautions. The Centers for Disease Control and Prevention (CDC) currently recommends contact precautions and airborne precautions with N95 or higher respirator masks13 although in Hong Kong the use of contact and droplet precautions alone was sufficient to protect health-care workers.14
Diagnostic microbiology
Diagnosis of infection is achieved by nucleic acid extraction, reverse transcription and PCR. Coronaviruses are included in multiplex assays that have been developed for the diagnosis of respiratory viral infections. Some of the coronaviruses can be cultured in monkey epithelial cells (SARS-CoV and NL63). Most of the population studies of HCoVs have used serologic methods, including complement fixation, hemagglutination inhibition (OC43 only) and recently enzyme immunoassay (EIA).
Clinical manifestations
Apart from SARS-HCoV, HCoVs are predominantly associated with mild upper respiratory tract infections and occasionally severe disease is observed in immunocompromised patients. In a study of adult volunteers the mean incubation period was 3.3 days, with a range of 2–4 days. The symptoms lasted for a mean of 7 days, with a range of 3–18 days.15 In contrast, SARS-HCoV was capable of causing a multisystem disease with infection of the gastrointestinal tract, liver, kidney and brain.16
The clinical course is mainly characterized by fever, dyspnea, lymphopenia and lower respiratory tract infection; 30–40% of patients had diarrhea.10 Compared to the other coronaviruses SARS was causing a much more severe disease with a mortality rate of nearly 10%.17 Coronavirus particles have also been seen (via electron microscopy) in the stools of children and adults with diarrhea and gastroenteritis. Earlier speculations that coronavirus could participate in the etiology of multiple sclerosis were not confirmed.10 However, the importance of human coronaviruses as a cause of diseases outside the respiratory tract remains to be determined.
Management
No effective antiviral therapy against coronaviruses, including SARS, has been found so far. In-vitro ribavirin is capable of inhibiting viral replication but there are no in-vivo data supporting its therapeutic efficacy.10 Steroids were used to treat SARS-infected patients with the aim of modulating cytokine levels. Interferons were found to possess antiviral activity in vitro and in animal models.10 A protective vaccine against SARS has not yet been generated.
REOVIRUS
Nature
Isolation of the first reovirus was made in 1951, but it was in 1959 that Sabin coined the term reovirus to emphasize the fact that these viruses were isolated from the respiratory and enteric tracts and that they were not associated with any known human disease (Respiratory Enteric Orphan viruses). Reoviruses are nonenveloped viruses of about 65–75 nm in diameter. Viruses of the genus Orthoreovirus are composed of two concentric icosahedrically symmetric protein capsids, containing 10 discrete segments of genomic double-stranded RNA. Orthoreoviruses have been isolated from a wide variety of mammalian, avian and reptilian hosts, and over recent years several previously unknown reoviruses (especially of bat origin) have been reported.18
Epidemiology
A study using enzyme linked immunosorbent assay (ELISA) as the diagnostic method showed that the prevalence of antibodies against reoviruses increased steadily throughout life from about 35% in infants to 60% in teenagers and to more than 85% among those older than 60 years of age.19
Diagnostic microbiology
Trying to diagnose reovirus infection on a routine basis is not recommended. In the research setting, a variety of techniques are available.
Clinical manifestations
In a study of adult volunteers, intranasal inoculation of reovirus was followed by an increase in serotype-specific antibodies, enteric virus isolation and development of respiratory illness in a third of the individuals infected with reovirus T1L.20 Two newer reoviruses, Melaka virus and Kampar virus, were isolated from patients with acute respiratory tract disease, raising the possibility that the clinical importance of this group of viruses was underestimated in the past.
RHINOVIRUS
Nature
Human rhinovirus (HRV) was first isolated in the 1950s from nasopharyngeal secretions of patients with the common cold. HRV belongs to the Picornaviridae family, which includes the genera Enterovirus and Hepatovirus (hepatitis A virus). Rhinoviruses are small (30 nm), nonenveloped particles that contain a linear single-stranded positive-sense RNA genome. The icosahedral capsid is composed of 60 subunits arranged as 12 pentameres. Each subunit is composed of one of each of four structural proteins (VP1 to VP4). VP1–3 have exterior projections that are major epitopes for neutralizing antibodies.
More than 100 serotypes have been identified to date. Phylogenetic analysis of genome sequences revealed two different species of rhinovirus A and B, HRV-A and HRV-B. Recently, a third genetic cluster was proposed (HRV-C) by analysis of viruses amplified from children with exacerbations of asthma.21
Epidemiology
Rhinoviruses are ubiquitous and infections occur in all age groups. They cause about half of all common colds and over the last few years an increasing number of publications have linked HRVs to more severe upper and lower respiratory tract infections in children, the elderly and immunocompromised patients.22., 23., 24. HRV infections occur throughout the year, with peaks in the fall and spring, and with multiple serotypes circulating simultaneously in a given geographic location. Preschool children commonly introduce the virus to the rest of the family, with a secondary attack rate of about 50% after an interval of 2–5 days. Extensive studies in susceptible individuals have shown that 95% of challenged subjects become infected and about 75% of them become ill. The major factor determining the risk of illness is the prechallenge level of neutralizing antibodies.
Pathogenesis
In spite of the extensive literature on the transmission of rhinoviruses, controversy still exists about the predominant mode of transmission. Most data support the notion that contact transmission, primarily as hand shaking followed by inoculation of the nasal mucosa and lacrimal canals, is the most efficient mode of transmission. HRVs can survive from a few hours to as long as 4 days on nonporous surfaces and for over 2 hours on human skin. Although droplet or airborne transmission of rhinovirus infection is possible, prolonged and close exposure is apparently required. There is no convincing clinical evidence to support the common notion that exposure to cold plays a role in the genesis of HRV illness.
Rhinoviruses attach to respiratory epithelium and spread locally. The leukocyte binding protein CD54/intercellular adhesion molecule 1 (ICAM-1) is the receptor for the majority of rhinovirus serotypes. The receptor-binding site resides in a depression of the capsid surface that is not accessible to antibodies. After endocytosis, a conformational change of the capsid allows the release of the viral RNA into the host cell cytoplasm, where viral replication and assembly take place. A polyprotein precursor is processed mainly by two viral proteases designated 2A and 3C. The 2A protease makes the first cleavage between the structural and nonstructural proteins, while the 3C protease catalyzes most of the remaining internal cleavages.
Infection is followed by activation of several inflammatory mechanisms including the release of cytokines, particularly interleukin (IL)-8, bradykinins, prostaglandins and histamine, as well as stimulation of parasympathetic reflexes.
The resulting process includes vasodilatation of nasal blood vessels, transudation of plasma, glandular secretion and stimulation of nerve fibers, triggering sneeze and cough reflexes. Nasal mucociliary transport is reduced markedly during the illness and may be impaired for weeks. Immunity to individual rhinoviruses is type specific and not long lasting. Protection correlates more with the level of locally synthesized IgA antibodies, which decline within months of infection, rather than with IgG levels in serum, which may persist for a few years.
Prevention
Hand washing after personal contact with a symptomatic person or after touching potentially contaminated objects is strongly recommended. Alcohol-based antiseptics are also effective for this purpose. Symptomatic patients should be advised to wash hands frequently and to use disposable tissues. The CDC recommends droplet precautions for the prevention of nosocomial HRV infections.
The diversity of serotypes and the specificity of the immune response have precluded the development of an effective vaccine. A number of drugs have been studied for the prophylaxis of HRV infection, including interferon alpha (IFN-α), pirodavir and the recombinant soluble CD54/ICAM-1 molecule. However, the practical utility of these drugs remains to be demonstrated.
Diagnostic microbiology
In clinical practice HRV infections are diagnosed by viral RNA detection with RT-PCR. RT-PCR assays are more rapid and sensitive than isolation techniques for the detection of HRV RNA in clinical specimens. Historically, HRV infections have been detected by virus isolation in cell culture followed by acid lability testing to distinguish them from enterovirus infections. Virus isolation typically requires 2–14 days. Presumptive identification of a cell culture isolate as rhinovirus is based on a combination of enterovirus-like cytopathic effects and clinical information. Further differentiation from enteroviruses is based on the diminished replication of rhinoviruses at 98.6°F (37°C) compared to growth at 91.4°F (33°C) and at pH 3.0 compared with pH 7.0, respectively
ELISA has been developed for the detection of rhinovirus antigens. The use of serologic assays is limited by the diversity of serotypes and the lack of a group-specific antigen.
Clinical manifestations
Symptoms of the common cold include profuse watery rhinorrhea, nasal congestion, sneezing and quite often headache, sore throat and cough. The symptoms begin within 8–10 hours of infection and peak around 1–3 days. There is little or no fever. By days 3–5 of the illness, nasal discharge may become mucopurulent from polymorphonuclear leukocytes that have migrated to the infection site in response to IL-8. Resolution of symptoms generally occurs within a week. Complications include sinusitis, otitis media and exacerbation of asthma or chronic obstructive pulmonary disease (COPD). Secondary bacterial infections may also occur. Studies using CT of the sinuses have shown that HRV infections can cause the accumulation of secretions in the sinuses, possibly from the passage of nasal secretions into the sinuses during nose blowing.25, 26
Recent published evidence has confirmed the association of HRVs with severe airway disease in immunocompromised patients, children and the elderly.21, 22 A novel HRV species (HRV-C) has been found in a significant proportion of children with exacerbations of asthma.
Management
Symptomatic treatment with decongestants, antihistamines and antitussives is currently the mainstay of therapy. The efficacy of zinc lozenges is controversial and their practical utility is limited because of their metallic taste. A variety of candidate drugs have been studied.27
Interferon alpha 2b has been found to be effective for the prevention of rhinovirus-associated colds but ineffective for the treatment of established colds.28 Recent therapeutic advances include the development of protease 3C inhibitors, recombinant soluble ICAM-1 antagonist and capsid-function inhibitors (pleconaril), all of which exhibit potent antirhinoviral activity in vitro and varying activity in clinical trials.27, 29 Recombinant soluble ICAM-1 molecule (Tremacamra) has been found to be effective in reducing the symptoms of experimental common colds in randomized controlled trials when given either as a preventative or a therapeutic agent.30
Pleconaril binds to a hydrophobic pocket in the viral capsid, inducing conformational changes that lead to altered receptor binding and viral uncoating. Pleconaril is orally bioavailable and achieves serum concentrations in excess of those required to inhibit 90% of clinical rhino- and enteroviral isolates in vitro. In a placebo-controlled study of rhinovirus infections, pleconaril-treated patients had a 1.5-day reduction in time to resolution of symptoms, significant reduction in symptom severity within 12–24 hours after initiation of therapy and a significant reduction in median viral titers in nasal mucus.31 The US FDA Advisory Committee has not yet recommended the approval of pleconaril for treatment of the common cold.
RESPIRATORY SYNCYTIAL VIRUS
(See healthmap.org for outbreaks of Respiratory Syncytial VIRUS)
Nature
Respiratory syncytial virus (RSV) was first isolated in the mid-1950s from a symptomatic laboratory chimpanzee during an outbreak of illness resembling the common cold. This virus derives its name from the characteristic formation of multinucleated giant cells in tissue culture.
RSV is a linear single-stranded, negative-sense genomic RNA of the Paramyxoviridae family of viruses. Virions are pleomorphic, with both spherical and filamentous forms averaging 120–300 nm in diameter. The envelope consists of host-derived plasma membrane and three virally encoded transmembrane surface glycoproteins: the attachment protein G, the fusion protein F and the small hydrophobic SH protein. The F protein can mediate fusion with neighboring cells to form syncytia. The G glycoprotein plays a major role in the process of attachment. Two major types of RSV – A and B – are distinguished serologically based on the antigenic characteristics of the surface glycoprotein G. Other viral proteins include the matrix proteins (M and M2), the major nucleocapsid (N) protein, a phosphoprotein (P) and the major polymerase (L).
Epidemiology
RSV is the most common cause of bronchiolitis and pneumonia among infants and young children worldwide.32 Virtually all children are infected by the time they reach 3 years of age. Natural immunity is transient and re-infection is common. Crowding in households and day-care centers increases the attack rate of RSV. A large CDC-initiated study found that among children with acute respiratory infection admitted to hospital or attending outpatient services, 18% of specimens were positive for RSV.33 Extrapolating this figure to the entire US population, 2 million children under 5 years of age would require medical attention due to RSV infection on a yearly basis.33 In older children and immunocompetent adults, RSV infections are seldom severe and are usually manifested as upper respiratory infection (URI) or tracheobronchitis. Individuals at high risk for severe RSV infection include premature infants (particularly those born at less than 34 weeks of gestation), young infants with chronic lung disease or a hemodynamically significant congenital heart defect, elderly individuals and immunocompromised subjects. Recent studies indicate that the disease burden caused by RSV in elderly and high-risk adults had previously been underestimated and is currently thought to be similar to that of endemic influenza A.34 Development of an effective RSV vaccine is therefore required for both young children and the elderly.
RSV activity follows a seasonal pattern in temperate zones of the world. In urban centers, annual outbreaks occur during the fall, winter and early spring. In the northern hemisphere most outbreaks peak in February or March. In tropical or subtropical areas, epidemics peak during the rainy season.
Pathogenesis
RSV is present in large numbers in the respiratory secretions of symptomatic infected persons and viral titers remain high for about 1 week, followed by a gradual decline over 3–4 weeks. The portal of entry is usually the conjunctiva or the nasal mucosa. Although transmission can occur via large droplets, contact transmission predominates.35 Virus can be viable for approximately 30 minutes on impervious surfaces such as bed rails. Thus, caregivers can contaminate their hands during routine care and transmit the virus by contact to other patients. Neutralizing antibodies to the surface glycoproteins F and G correlate with resistance to re-infection, but protection is not complete and is of short duration.36
The pathology of RSV bronchiolitis is characterized by necrosis of ciliated bronchiolar epithelial cells, mononuclear infiltrates and edema of the peribronchial space. Bronchiolar obstruction from increased mucus production results in air trapping and total obstruction results in atelectasis. Pathologic examination of lungs from patients with RSV pneumonia has revealed marked interstitial inflammation and edema of the lung parenchyma, along with the findings of bronchiolitis.
Prevention
In the household setting, the transmission of RSV may be decreased by frequent hand washing and not sharing household items such as cups, glasses and utensils. The CDC recommends contact precautions for the prevention of nosocomial RSV infections. Some institutions also observe droplet isolation precautions. Implementation of multifaceted infection control strategies resulted in a reduction of the incidence of nosocomial RSV infections.37 Passive immunoprophylaxis is indicated for high-risk children.
RSV intravenous immunoglobulin (RSV-IVIG, RespiGam) is a human polyclonal immunoglobulin with high RSV microneutralization titers. In placebo-controlled studies, monthly intravenous doses of 750 mg/kg during the RSV season significantly reduced the incidence of RSV hospitalizations and the severity of illness among premature infants and infants with bronchopulmonary dysplasia.38, 39 Palivizumab is a humanized monoclonal antibody that specifically inhibits an epitope at the A antigenic site of the F protein of RSV subtypes A and B. In a large multicenter placebo-controlled trial involving high-risk infants, palivizumab prophylaxis resulted in a 55% reduction in hospitalization rates and number of hospital days ascribed to RSV infection.40 Both agents have been well tolerated, with few adverse effects; however, their high cost necessitates strict guidelines for their use. RSV-IVIG or palivizumab is indicated for the prophylaxis of RSV infections among infants with chronic lung disease (formerly designated bronchopulmonary dysplasia) and premature infants. In children with a hemodynamically significant congenital heart defect only palivizumab is indicated for prophylaxis.41 Cost-saving benefit has not been observed uniformly,42., 43., 44., 45., 46. perhaps as a result of major differences in the prevalence of RSV lower respiratory tract infections in different countries and regions, suggesting the need for locally adapted recommendations. Palivizumab is easier to administer and does not interfere with the measles, mumps, rubella (MMR) vaccine or varicella vaccine. RSV-IVIG, however, provides additional protection against other respiratory viral illnesses and may be preferred for children receiving replacement IVIG because of underlying immune deficiency.40
The role of passive immunoprophylaxis for the prevention of RSV infections in high-risk adults remains to be defined. Development of an RSV vaccine is ongoing but none is yet available.
Diagnostic microbiology
The gold standard diagnostic method is isolation of the virus from respiratory secretions, but increasingly nonculture diagnostic methods are used for RSV.
RSV was the first respiratory virus to be readily identified by means of rapid diagnostic tests based on antigen detection. Current methods for rapid detection of RSV antigen include EIA, immunoperoxidase staining, direct and indirect immunofluorescence tests and RT-PCR. Serologic diagnosis requires a convalescent-phase RSV IgG antibody titer at least four times higher than the acute-phase titer.
Clinical manifestations
The incubation period for RSV ranges from 2 to 8 days. Illness begins most frequently with rhinorrhea, sneezing and cough. When fever is present it is generally low grade. Most children experience recovery from illness after 8–15 days. During their first RSV infection, 25–40% of infected infants and young children have signs or symptoms of bronchiolitis or pneumonia and 0.5–2% require hospitalization. In contrast, hospitalization will be required in up to 25% of high-risk children such as those with a history of prematurity or chronic lung disease. RSV bronchiolitis or pneumonia should be suspected when an infant or young child presents with progressive cough, wheezing and dyspnea during the RSV season.
Radiographic findings in patients with bronchiolitis include hyperlucency, diaphragmatic flattening and outward bowing on the intercostal spaces. Findings compatible with pneumonia include interstitial infiltrates and, less frequently, consolidation, particularly in the right upper and middle lobes.
In neonates, the clinical presentation may be atypical with lethargy, irritability or poor oral intake. RSV has been detected in lung tissue of infants with sudden death syndrome, but a causal relationship has not been confirmed. Prolonged pulmonary function deficit and airway hyperreactivity have been reported following RSV infection but it remains unclear whether these abnormalities were present before the infection.47 Otitis media has also been associated with RSV infection.
Among immunocompetent adults, RSV infection usually manifests with rhinorrhea, pharyngitis, cough, bronchitis, headache, fatigue and fever. In older persons, particularly the institutionalized elderly and those with chronic cardiopulmonary illnesses, severe pneumonia may occur, leading to respiratory failure. Among immunocompromised individuals, RSV has been the most frequently isolated viral respiratory pathogen in surveillance studies performed at large cancer centers.48 In this setting RSV infection typically begins as a URI and may evolve into lower respiratory tract disease in up to 50% of cases. A particularly high rate of progression to pneumonia (70–80%) has been reported among adult HSCT recipients developing RSV infection within the period prior to engraftment. In such settings RSV pneumonia resulted in 60–80% mortality, regardless of whether antiviral drugs were used for treatment.
Management
For patients with mild disease, only symptomatic treatment is necessary. Children with severe disease may require hospitalization for supplemental oxygen therapy, hydration and nutrition, or for mechanical ventilation. Improvements in supportive care have made a significant impact on the mortality from RSV bronchiolitis and pneumonia. The use of bronchodilators is likely beneficial for patients with significant bronchospasm. Corticosteroids are commonly used as anti-inflammatory therapy, although there is no supportive data demonstrating the benefits of such intervention. Aerosolized ribavirin (Virasole) was approved in 1986 for the treatment of infants and children with severe RSV disease. The treatment is cumbersome to deliver and its impact on mortality remains controversial.49, 50
A combination of immune therapy and ribavirin has been reported to be well tolerated and to improve the outcome of severe RSV infection among bone marrow transplant recipients.51, 52 In one open trial the mortality was only 22% among nine adults with pneumonia in whom therapy was initiated prior to the onset of profound respiratory failure.52 Pre-emptive treatment strategies are being investigated primarily in immunocompromised individuals. Delay of immunosuppressive chemotherapy should be considered when severely immunosuppressed individuals, such as HSCT recipients, develop acute RSV infection.
PARAINFLUENZA VIRUS
Nature
Human parainfluenza viruses (HPIVs) were first described in the 1950s, originally as croup-associated viruses; they belong to the Paramyxoviridae family.
The virions are pleomorphic and range in average diameter from 150 to 200 nm. They are enveloped particles with a linear single-stranded, negative-sense RNA genome. The HPIVs encode two surface glycoproteins: an attachment protein called HN (hemagglutinin-neuraminidase) that binds to sialic acid-containing receptors on host cell surfaces, and a fusion protein F that is involved in the fusion of the viral membrane with the cellular plasma membrane. The HN protein is a multifunctional molecule with binding activity (binding to sialic acid containing surface glycoproteins and glycolipids), neuraminidase activity and fusion promotion activity. The attachment activity can be measured by hemagglutination assay. The neuraminidase activity prevents aggregation of virus particles by removing sialic acid from the virion glycoproteins and facilitates infection in the respiratory tract by digesting the mucin coating of epithelial cells.
These viruses also encode three nucleocapsid associated proteins, NP, P and L, and the nonglycosylated internal protein M. The four HPIVs are segregated into two genera, the Respirovirus (HPIV-1 and HPIV-3) and the Rubulavirus (HPIV-2 and HPIV-4), based on antigenic and structural characteristics, including additional accessory proteins (V, D, W, I, X, C and SH).
Epidemiology
HPIVs are distributed worldwide. Each of the four HPIVs can cause a full spectrum of acute respiratory tract illnesses. HPIV-1 and HPIV-2 are the most common pathogens associated with croup or laryngotracheobronchitis.53 HPIV-1, -2 and -3 are also important causes of bronchiolitis and pneumonia among infants and young children;53, 54 less is known about HPIV-4. Most children have serologic evidence of infection by 5 years of age. Most HPIV infections are detected during seasonal epidemics. In a study on an outpatient pediatric population,55 HPIV-1 occurred in the fall of odd-numbered years, HPIV-2 was less predictable and HPIV-3 appeared yearly with peak activity in spring or summer.
Pathogenesis
Although droplet transmission can occur, contact is thought to be the primary mode of transmission of HPIV infection. This can be direct person-to-person contact or indirect through intermediate objects. Inoculation is via the nasopharyngeal or oral mucosa. The pathogenesis of the ensuing laryngotracheobronchitis and croup is not completely understood. The immune response after HPIV infection is complex and includes the production of serum-neutralizing antibodies directed towards epitopes of the HN and F proteins. The mucosal immune response plays an important role in the protection against HPIVs infection, primarily through the production of IgA antibodies. In animal model studies, CD8+ T cells were critical for virus clearance.
Prevention
The CDC recommends contact precautions for the prevention of nosocomial HPIV infections. Some institutions also observe droplet isolation precautions. No specific vaccine is yet available and no drugs have been tested for prophylactic use.
Diagnostic microbiology
Definite diagnosis relies on the isolation of the virus from respiratory secretions. Other diagnostic methods include detection of viral antigens or nucleic acid, and serologic analysis. Primary isolation and identification by hemadsorption assay can be followed by typing of the virus through immunofluorescence or hemadsorption inhibition. PCR assays are more sensitive than isolation and antigen detection methods.56
Clinical manifestations
Infection in children is associated with an acute febrile illness in up to 80% of cases. Initial symptoms include coryza, sore throat, hoarseness and dry cough. In croup, a brassy or barking cough may progress to stridor and occasionally to airway obstruction. The anteroposterior radiograph of the neck shows glottic and subglottic narrowing (‘steeple sign’) which differentiates croup from epiglottitis. In cases of bronchiolitis and pneumonia, progressive cough is accompanied by wheezing, tachypnea and hypoxemia. Chest X-ray examination may reveal air trapping and interstitial infiltrates. Otitis media is a common finding in patients with HPIV infections.
In older children and adults, HPIV infections tend to be milder and present as URI. However, HPIV-1 and -3 have been diagnosed by serology in a significant number of adults admitted with acute respiratory illness.57 A few cases of HPIV meningitis have been described.58 Immunocompromised pediatric and adult individuals such as HSCT,59., 60., 61., 62., 63., 64. lung65 and renal66 transplant recipients may develop severe HPIV infections with a high rate of progression to pneumonia. The use of corticosteroids was linked with a higher risk of HPIV-3 pneumonia among HSCT recipents.61
Management
Symptomatic treatment of croup usually includes humidification of air by ultrasonic nebulizer and inhalations of racemic adrenaline (epinephrine). Adrenaline (epinephrine) provides rapid but transient relief of the airway obstruction. The anti-inflammatory effect of systemic corticosteroids is advocated to prevent or shorten the period of intubation in severe croup. Patients with bronchiolitis are additionally treated with bronchodilators. Ribavirin has exhibited in-vitro activity against HPIVs67 but data from controlled clinical trials are lacking.
HUMAN METAPNEUMOVIRUS
Nature
In June 2001, it was reported that a new respiratory virus had been isolated from nasopharyngeal aspirates of 28 young children in the Netherlands. Based on virologic data, sequence homology and gene constellation, the virus was classified as a member of the Metapneumovirus genus of the Paramyxoviridae family and was named human metapneumovirus (HMPV).68 HMPV is the first known mammalian metapneumovirus causing disease. Serologic analysis detected antibodies against the virus being present in all individuals older than 8 years of age in specimens collected since 1958.68
The genome of HMPV consists of negative-sense, nonsegmented RNA. Similar to other paramyxoviruses, virions are pleomorphic with a mean diameter of 200 nm. The lipid envelope, which is derived from the plasma membrane of the host cell, contains three viral glycoproteins, the attachment (G), fusion (F) and small SH proteins. The functions of these proteins are similar to those of their counterparts found in other paramyxoviruses (see above). There are two major genotypes of HMPV, A and B.69 Both of these major genetic lineages are further divided into two genetic subgroups termed A1 and A2 and B1 and B2. Current evidence suggests that the two genetic lineages are not distinct antigenic subtypes.70 HMPV replicates slowly in cell culture with characteristic syncytia formation
Epidemiology
HMPV infections have been diagnosed worldwide in patients of all age groups with respiratory disease. In temperate climates the virus is predominantly transmitted in late winter and spring, whereas in the subtropics infections culminate in spring/summer. Infection rates peak at the same time or shortly after the RSV infection peak.71 HPMV has been associated with upper and lower respiratory tract infections in young children and is the second most frequently isolated viral pathogen after RSV to cause bronchiolitis in early childhood.72., 73., 74., 75.
Pathogenesis
Transmission most likely occurs in a fashion similar to RSV via droplets and contaminated fomites; nosocomial transmission has been reported.76 Human respiratory infection was associated with an increase of IL-8 in respiratory secretions and with chronic inflammatory changes in the airways. Levels of inflammatory cytokines (IL-12, tumor necrosis factor, IL-6, IL-1β) found in HMPV disease were lower than levels observed with RSV infection.77
Prevention
Both droplet precautions and direct contact precautions should be observed to prevent transmission in the health-care setting.
Diagnostic microbiology
The virus is difficult to grow in cell culture. Most strains show cytopathic effect in LLC-MK2 (tertiary monkey kidney) cells; recently a Vero cell clone was reported as being permissible for all four viral lineages.70 Cytopathic effects usually occur after 10–21 days and vary from syncytia formation to rounding of cells. Direct immunofluorescence assays with commercially produced virus-specific antibodies are available, although they are probably less sensitive than RT-PCR for the detection of virus. Molecular methods have become the method of choice for the detection of the HMPV genome. Real-time RT-PCR targeting the N and/or L gene has been found to be sensitive, specific and rapid in detecting the virus in clinical specimens.78 Serologic diagnosis can be achieved by ELISA methods demonstrating a fourfold increase in antibody titers or seroconversion.
Clinical manifestations
HMPV has been associated with respiratory tract infections in all age groups: severe lower respiratory tract infections have been observed in very young children, the elderly and immunocompromised patients. HMPV infection in adults usually presents with flu-like symptoms such as rhinorrhea, sore throat and cough. Children are at higher risk for the development of lower airway disease (bronchiolitis, pneumonia), especially infants younger than 2 years of age.79 The severity varies from self-limiting mild respiratory illness to respiratory failure requiring mechanical ventilation. Clinical disease associated with HMPV is similar to RSV disease and 12% of outpatients with acute lower respiratory tract infections were harboring HMPV which was second only to RSV.72 Heikkinen et al. reported that 60% of children less than 3 years of age developed acute otitis media due to HMPV infection.79
Management
Several vaccine candidates have shown promising results in animal models but their safety and efficacy in humans remains to be determined. Antiviral treatment strategies for severe disease are derived from experiences obtained with RSV infections. Ribavirin shows equivalent antiviral potential against HMPV to that observed with RSV80 and an HMPV-neutralizing monoclonal antibody for prophylaxis in high-risk infants (similar to palivizumab in RSV) is likely to be developed.
HUMAN BOCAVIRUS
(See healthmap.org for outbreaks of Human Bocavirus)
Human bocavirus (HBoV) was identified in 2005 by random amplification and cloning of DNA followed by large scale sequencing and bioinformatic analysis.81 By sequence analysis it has been provisionally classified as a parvovirus. Since then multiple studies have confirmed the worldwide presence of HBoV in the respiratory tract of children, usually under 2 years of age.82 HBoV is often present as a co-infection with other respiratory viruses (such as RSV) raising the issue of disease association.
Determination of the pathogenicity of HBoV is complicated by the lack of an in-vitro culture system and a suitable animal model. High viral loads in respiratory specimens and detection of viral DNA in blood seem to be associated with respiratory tract symptoms, whereas the presence of low viral loads is of unclear significance.83, 84 The first serologic studies analyzing the antibody response in humans against HBoV structural proteins have now been published.85, 86 Antibodies against HBoV were detected in 71.1% of serum samples collected from people from infancy to 41 years in Japan.85 High viral load in the nasopharynx and viremia correlated with a serologic diagnosis of primary infection (IgM antibodies and/or an increase in IgG antibody level).86 Further studies are needed to clarify the pathogenesis and the natural course of infection of HBoV.
CONCLUSION
A better understanding of the profound impact that human respiratory viruses have on human morbidity and mortality has resulted in more resources being devoted to their study. More efficient diagnostic tools, new antiviral agents, the use of passive immunoprophylaxis, improvements in vaccination programs and in strategies to control the nosocomial transmission of these infections are among the most significant accomplishments.
Acknowledgment
Illustrations are contributed by Joseph Yarsa BS, MT(ASCP) and Xiang-Yang Han MD, PhD from the Microbiology Laboratory at The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
REFERENCES
- 1.Russell W.C. Update on adenovirus and its vectors. J Gen Virol. 2000;81(Pt 11):2573–2604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- 2.Schilham M.W., Claas E.C., van Zaane W. High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem-cell transplantation. Clin Infect Dis. 2002;35:526–532. doi: 10.1086/341770. [DOI] [PubMed] [Google Scholar]
- 3.Suparno C., Milligan D.W., Moss P.A. Adenovirus infections in stem cell transplant recipients: recent developments in understanding of pathogenesis, diagnosis and management. Leuk Lymphoma. 2004;45:873–885. doi: 10.1080/10428190310001628176. [DOI] [PubMed] [Google Scholar]
- 4.Kojaoghlanian T., Flomenberg P., Horwitz M.S. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- 5.La Rosa A.M., Champlin R.E., Mirza N. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32(6):871–896. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 6.Sloots T.P., Whiley D.M., Lambert S.B., Nissen M.D. Emerging respiratory agents: new viruses for old diseases? J Clin Virol. 2008;42:233–243. doi: 10.1016/j.jcv.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monto A.S., Lim S.K. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J Infect Dis. 1974;129:271–336. doi: 10.1093/infdis/129.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiris J., Lai S., Poon L. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;363:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ksiazek T.G., Erdman D., Goldsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1947–1958. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 10.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Micro Mol Biol Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Elden L.J., van Loon A.M., van Alphen F. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J Infect Dis. 2004;189:652–657. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chilvers M.A., McKean M., Rutman A., Myint B.S., Silverman M., O'Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J. 2001;18(6):965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- 13.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., the Healthcare Infection Control Practices Advisory Committee . Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings 2007. 2007. http://www.cdc.gov/ncidod/dhqp/gl_isolation.html Online. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seto W.H., Tsang D., Yung R.W. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradburne A.F., Bynoe M.L., Tyrrell D.A. Effects of a ‘new’ human respiratory virus in volunteers. BMJ. 1967;3:767–769. doi: 10.1136/bmj.3.5568.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu J., Gong E., Zhang B. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peiris J.S., Guan Y., Yuenk Y. Severe acute respiratory syndrome. Nature Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bing Chua K., Voon K., Crameri G. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PloS ONE. 2008;3(11):1–7. doi: 10.1371/journal.pone.0003803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selb B., Weber B. A study of human reovirus IgG and IgA antibodies by ELISA and Western blot. J Virol Methods. 1994;47:15–26. doi: 10.1016/0166-0934(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 20.Rosen L., Evans H.E., Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. doi: 10.1093/oxfordjournals.aje.a120293. [DOI] [PubMed] [Google Scholar]
- 21.Lau S.K.P., Yip C.C.Y., Tsoi H.-W. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45(11):3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser L., Aubert J.D., Pache C. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med. 2006;174:1392–1399. doi: 10.1164/rccm.200604-489OC. [DOI] [PubMed] [Google Scholar]
- 23.Miller E.K., Lu X., Erdman D.D. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner R.B. Rhinovirus: more than just a common cold virus. J Infect Dis. 2007;195:765–786. doi: 10.1086/511829. [DOI] [PubMed] [Google Scholar]
- 25.Gwaltney J.M.J., Hendley J.O., Phillips C.D., Bass C.R., Mygind N., Winther B. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis. 2000;30(2):387–391. doi: 10.1086/313661. [DOI] [PubMed] [Google Scholar]
- 26.Gwaltney J.M.J., Phillips C.D., Miller R.D., Riker D.K. Computed tomographic study of the common cold. N Engl J Med. 1994;330(1):25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 27.Patick A.K. Rhinovirus chemotherapy. Antivir Res. 2006;71:391–446. doi: 10.1016/j.antiviral.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayden F.G., Kaiser D.L., Albrecht J.K. Intranasal recombinant alfa-2b interferon treatment of naturally occurring common colds. Antimicrob Agents Chemother. 1988;32:224–230. doi: 10.1128/aac.32.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patick A.K., Brothers M.A., Maldonado F. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother. 2005;49(6):2267–2275. doi: 10.1128/AAC.49.6.2267-2275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner R.B., Wecker M.T., Pohl G. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA. 1999;281(19):1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- 31.Hayden F.G., Herrington D.T., Coats T.L. Pleconaril respiratory infection study group. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of two double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36(12):1523–1532. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1938. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 33.Breese Hall C., Weinberg G.A., Iwane M.K. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 35.Hall C.B., Douglas R.G.J. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99(1):100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 36.Hall C.B., Walsh E.E., Long C.E., Schnabel K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–778. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 37.Garcia R., Raad I., Abi-Said D. Nosocomial respiratory syncytial virus infections: prevention and control in bone marrow transplant patients. Infect Control Hosp Epidemiol. 1997;18(6):412–416. doi: 10.1086/647640. [DOI] [PubMed] [Google Scholar]
- 38.Anonymous. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997;99(1):93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 39.Atkins J.T., Karimi B.H., McDavid G., Shim S. Prophylaxis for respiratory syncytial virus with respiratory syncytial virus-immune globulin intravenous among preterm infants of thirty-two weeks gestation and less: reduction in incidence, severity of illness and cost. Pediatr Infect Dis J. 2000;19(2):138–143. doi: 10.1097/00006454-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Anonymous. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Virus Res. 1998;55(2):167–176. [PubMed] [Google Scholar]
- 41.Committee on Infectious Diseases and Committee on Fetus and Newborn Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112:1442–1446. [PubMed] [Google Scholar]
- 42.Schrand L.M., Elliott J.M., Ross M.B. A cost–benefit analysis of RSV prophylaxis in high-risk infants. Ann Pharmacother. 2001;35(10):1186–1193. doi: 10.1345/aph.10374. [DOI] [PubMed] [Google Scholar]
- 43.O'Shea T.M., Sevick M.A., Givner L.B. Costs and benefits of respiratory syncytial virus immunoglobulin to prevent hospitalization for lower respiratory tract illness in very low birth weight infants. Pediatr Infect Dis J. 1998;17(7):587–593. doi: 10.1097/00006454-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Joffe S., Ray G.T., Escobar G.J., Black S.B., Lieu T.A. Cost-effectiveness of respiratory syncytial virus prophylaxis among preterm infants. Pediatrics. 1999;104(3 Pt 1):419–427. doi: 10.1542/peds.104.3.419. [DOI] [PubMed] [Google Scholar]
- 45.Thomas M., Bedford-Russell A., Sharland M. Hospitalization for RSV infection in ex-preterm infants – implications for use of RSV immune globulin. Arch Dis Child. 2000;83(2):122–127. doi: 10.1136/adc.83.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens T.P., Sinkin R.A., Hall C.B., Maniscalco W.M., McConnochie K.M. Respiratory syncytial virus and premature infants born at 32 weeks’ gestation or earlier: hospitalization and economic implications of prophylaxis. Arch Pediatr Adolesc Med. 2000;154(1):55–61. [PubMed] [Google Scholar]
- 47.McBride J.T. Pulmonary function changes in children after respiratory syncytial virus infection in infancy. J Pediatr. 1999;135(2 Pt 2):28–32. [PubMed] [Google Scholar]
- 48.Whimbey E., Champlin R.E., Couch R.B. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;22(5):778–782. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 49.Moler F.W., Steinhart C.M., Ohmit S.E., Stidham G.L. Effectiveness of ribavirin in otherwise well infants with respiratory syncytial virus-associated respiratory failure. Pediatric Critical Study Group. J Pediatr. 1996;128(3):422–428. doi: 10.1016/s0022-3476(96)70294-9. [DOI] [PubMed] [Google Scholar]
- 50.Randolph A.G., Wang E.E. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract. Cochrane Database Syst Rev. 2000;(2):CD000181. doi: 10.1002/14651858.CD000181. [DOI] [PubMed] [Google Scholar]
- 51.DeVincenzo J.P., Hirsch R.L., Fuentes R.J., Top F.H.J. Respiratory syncytial virus immune globulin treatment of lower respiratory tract infection in pediatric patients undergoing bone marrow transplantation – a compassionate use experience. Bone Marrow Transplant. 2000;25(2):161–225. doi: 10.1038/sj.bmt.1702118. [DOI] [PubMed] [Google Scholar]
- 52.Whimbey E., Champlin R.E., Englund J.A. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant. 1995;16(3):393–449. [PubMed] [Google Scholar]
- 53.Henrickson K.J., Kuhn S.M., Savatski L.L. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin Infect Dis. 1994;18(5):770–779. doi: 10.1093/clinids/18.5.770. [DOI] [PubMed] [Google Scholar]
- 54.Counihan M.E., Shay D.K., Holman R.C. Human parainfluenza virus-associated hospitalizations among children less than five years of age in the United States. Pediatr Infect Dis J. 2001;20(7):646–653. doi: 10.1097/00006454-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Knott A.M., Long C.E., Hall C.B. Parainfluenza viral infections in pediatric outpatients: seasonal patterns and clinical characteristics. Pediatr Infect Dis J. 1994;13(4):269–273. doi: 10.1097/00006454-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Fan J., Henrickson K.J. Rapid diagnosis of human parainfluenza virus type 1 infection by quantitative reverse transcription-PCR-enzyme hybridization assay. J Clin Microbiol. 1996;34(8):1914–1917. doi: 10.1128/jcm.34.8.1914-1917.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marx A., Gary H.E., Jr, Marston B.J. Parainfluenza virus infection among adults hospitalized for lower respiratory tract infection. Clin Infect Dis. 1999;29(1):134–140. doi: 10.1086/520142. [DOI] [PubMed] [Google Scholar]
- 58.Arisoy E.S., Demmler G.J., Thakar S., Doerr C. Meningitis due to parainfluenza virus type 3: report of two cases and review. Clin Infect Dis. 1993;17(6):995–997. doi: 10.1093/clinids/17.6.995. [DOI] [PubMed] [Google Scholar]
- 59.Wendt C.H., Weisdorf D.J., Jordan M.C., Balfour H.H.J., Hertz M.I. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992;326(14):921–926. doi: 10.1056/NEJM199204023261404. [DOI] [PubMed] [Google Scholar]
- 60.Cortez K.J., Erdman D.D., Peret T.C. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis. 2001;184(9):1093–1097. doi: 10.1086/322041. [DOI] [PubMed] [Google Scholar]
- 61.Nichols W.G., Corey L., Gooley T., Davis C., Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98(3):573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 62.Hohenthal U., Nikoskelainen J., Vainionpaa R. Parainfluenza virus type 3 infections in a hematology unit. Bone Marrow Transplant. 2001;27(3):295–300. doi: 10.1038/sj.bmt.1702776. [DOI] [PubMed] [Google Scholar]
- 63.Whimbey E., Vartivarian S.E., Champlin R.E., Elting L.S., Luna M., Bodey G.P. Parainfluenza virus infection in adult bone marrow transplant recipients. Eur J Clin Microbiol Infect Dis. 1993;12(9):699–701. doi: 10.1007/BF02009383. [DOI] [PubMed] [Google Scholar]
- 64.Lewis V.A., Champlin R., Englund J. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clin Infect Dis. 1996;23(5):1033–1037. doi: 10.1093/clinids/23.5.1033. [DOI] [PubMed] [Google Scholar]
- 65.Vilchez R.A., McCurry K., Dauber J. The epidemiology of parainfluenza virus infection in lung transplant recipients. Clin Infect Dis. 2001;33(12):2004–2008. doi: 10.1086/324348. [DOI] [PubMed] [Google Scholar]
- 66.DeFabritus A.M., Riggio R.R., David D.S., Senterfit L.B., Cheigh J.S., Stenzel K.H. Parainfluenza type 3 in a transplant unit. JAMA. 1979;241(4):384–446. [PubMed] [Google Scholar]
- 67.Browne M.J. Comparative inhibition of influenza and parainfluenza virus replication by ribavirin in MDCK cells. Antimicrob Agents Chemother. 1981;19(5):712–715. doi: 10.1128/aac.19.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van den Hoogen B.G., de Jong J.C., Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biacchesi S., Skiadopoulos M.H., Boivin G. Genetic diversity between human metapneumovirus subgroups. Virology. 2003;315:1–9. doi: 10.1016/s0042-6822(03)00528-2. [DOI] [PubMed] [Google Scholar]
- 70.van den Hoogen B.G., Herfst S., Sprong L. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10:658–666. doi: 10.3201/eid1004.030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sloots T.P., Whiley D.M., Lambert S.B., Nissen M.D. Emerging respiratory agents: new viruses for old diseases? J Clin Virol. 2008;42:233–243. doi: 10.1016/j.jcv.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams J.V., Harris P.A., Tollefson S.J. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams J.V., Wang C.K., Yang C.F. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sasaki A., Suzuki H., Saito R. Prevalence of human metapneumovirus and influenza virus infections among Japanese children during two successive winters. Pediatr Infect Dis J. 2005;24:905–908. doi: 10.1097/01.inf.0000180984.61778.1e. [DOI] [PubMed] [Google Scholar]
- 75.Chano F., Rousseau C., Laferriere C., Couillard M., Charest H. Epidemiological survey of human metapneumovirus infection in a large pediatric tertiary care center. J Clin Microbiol. 2005;43:5520–5525. doi: 10.1128/JCM.43.11.5520-5525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peiris J.S., Tang W.H., Chan K.H. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laham F.R., Israele V., Casellas J.M. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J Infect Dis. 2004;189:2047–2056. doi: 10.1086/383350. [DOI] [PubMed] [Google Scholar]
- 78.Mackay I.M., Jacob K.C., Woolhouse D. Molecular assays for detection of human metapneumovirus. J Clin Microbiol. 2003;41:100–105. doi: 10.1128/JCM.41.1.100-105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heikkinen T., Osterback R., Peltola V., Jartti T., Vainionpaa R. Human metapneumovirus infections in children. Emerg Infect Dis. 2008;14:101–106. doi: 10.3201/eid1401.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wyde P.R., Chetty S.N., Jewell A.M., Boivin G., Piedra P.A. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res. 2004;60:51–59. doi: 10.1016/s0166-3542(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 81.Allander T., Tammi M.T., Eriksson M. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allander T. Human bocavirus. J Clin Virol. 2008;41:29–33. doi: 10.1016/j.jcv.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 83.Allander T., Jartti T., Gupta S. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fry A.M., Lu X., Chittaganpitch M. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Endo R., Ishiguro N., Kikuta H. Seroepidemiology of human bocavirus in Hokkaido prefecture, Japan. J Clin Microbiol. 2007;45:3218–3223. doi: 10.1128/JCM.02140-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kantola K., Hedman L., Allander T. Serodiagnosis of human bocavirus infection. Clin Infect Dis. 2008;46:540–546. doi: 10.1086/526532. [DOI] [PMC free article] [PubMed] [Google Scholar]


